Note: Descriptions are shown in the official language in which they were submitted.
Boehringer IngelhelfT'1 CA 02561210 2006-09-26 Case 1/1678
f
foreign filing text
86780fft
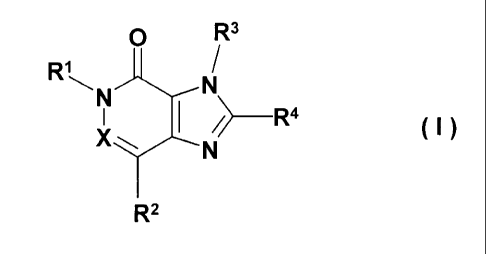
New 2-amino-imidazo[4,5-d]pyridazin-4-ones, their preparation and their use as
pharmaceutical compositions
The present invention relates to new substituted imidazo[4,5-d]pyridazin-4-
ones and
2-amino-imidazo[4,5-c]pyridin-4-ones of general formula
Rs
R~ N
~~R
'N
R2
the tautomers, the enantiomers, the diastereomers, the mixtures thereof and
the salts
thereof, particularly the physiologically acceptable salts thereof with
inorganic or
organic acids which have valuable pharmacological properties, particularly an
inhibiting effect on the activity of the enzyme dipeptidylpeptidase-IV (DPP-
IV), the
preparation thereof, the use thereof for preventing or treating illnesses or
conditions
connected with an increased DPP-IV activity or capable of being prevented or
alleviated by reducing the DPP-IV activity, particularly type I or type II
diabetes
mellitus, the pharmaceutical compositions containing a compound of general
formula
(I) or a physiologically acceptable salt thereof and processes for the
preparation
thereof.
Imidazo[4,5-d]pyridazin-4-ones and imidazo[4,5-c]pyridin-4-ones which are
substituted by cyclic groups in the 2 position are known from WO 03/104229.
In the above formula I
R' denotes an arylmethyl or arylethyl group,
a heteroarylmethyl or heteroarylethyl group,
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
2
an arylcarbonylmethyl group,
a heteroarylcarbonylmethyl group or
an arylprop-2-enyl or heteroarylprop-2-enyl group, wherein the propenyl chain
may
be substituted by 1 to 4 fluorine atoms or a cyano, C1_3-alkyloxy-carbonyl or
nitro
group,
X denotes a nitrogen atom or a C-R5 group, while R5 denotes a hydrogen atom or
a
C1_3-alkyl group,
R2 denotes a hydrogen atom,
a C1_6-alkyl group,
an aryl or heteroaryl group,
a C1_6-alkyl group substituted by a group Ra, where
Ra denotes a C3_~-cycloalkyl group wherein one or two methylene groups may
each be replaced independently of one another by an oxygen or sulphur atom, by
an -NH- or-N(C1_3-alkyl)- group or by a carbonyl, sulphinyl or sulphonyl
group,
or a trifluoromethyl, aryl, heteroaryl, cyano, carboxy, C~_3-alkoxy-carbonyl,
amino-
carbonyl, C1_3-alkylamino-carbonyl, di-(C1_3-alkyl)-amino-carbonyl, pyrrolidin-
1-
ylcarbonyl, piperidin-1-ylcarbonyl, morpholin-4-ylcarbonyl, piperazin-1-
ylcarbonyl,
4-(C1_3-alkyl)-piperazin-1-ylcarbonyl, arylcarbonyl, heteroarylcarbonyl, C1_3-
alkylsulphinyl, C1_3-alkylsulphonyl, hydroxy, C1_3-alkoxy, C1_3-
alkylsulphanyl,
amino, C1_3-alkylamino, di-(C1_3-alkyl)-amino, pyrrolidin-1-yl, piperidin-1-
yl,
morpholin-4-yl, piperazin-1-yl or 4-(C1_3-alkyl)-piperazin-1-yl group,
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
3
a trifluoromethyl, carboxy, C~_4-alkoxy-carbonyl, aminocarbonyl, C~_3-
alkylamino-
carbonyl, di-(C1_3-alkyl)-amino-carbonyl, pyrrolidin-1-ylcarbonyl, piperidin-1-
ylcarbonyl, morpholin-4-ylcarbonyl, piperazin-1-ylcarbonyl, 4-(C1_3-alkyl)-
piperazin-1-
ylcarbonyl, arylcarbonyl, heteroarylcarbonyl, C~_3-alkylsulphinyl, C~_3-
alkylsulphonyl,
C1_4-alkoxy, C~_4-alkylsulphanyl, amino, C~_3-alkylamino or di-(C1_3-alkyl)-
amino,
pyrrolidin-1-yl, piperidin-1-yl, morpholin-4-yl, piperazin-1-yl or 4-(C1_3-
alkyl)-piperazin-
1-yl group,
a C3_~-cycloalkyl group wherein one or two methylene groups independently of
one
another may each be replaced by an oxygen or sulphur atom, by an -NH- or -
N(C1_3-
alkyl)- group, or by a carbonyl, sulphinyl or sulphonyl group, or
a C3_6-alkenyl or C3_6-alkynyl group,
R3 denotes a C5_~-cycloalkenylmethyl group optionally substituted by a C1_3-
alkyl
group,
an arylmethyl or heteroarylmethyl group,
a straight-chain or branched C2_$-alkenyl group which may be substituted by 1
to 15
fluorine atoms or a cyano, nitro or C1_3-alkoxy-carbonyl group,
or a straight-chain or branched C3_$-alkynyl group which may be substituted by
1 to 9
fluorine atoms or a cyano, nitro or C~_3-alkoxy-carbonyl group,
and
R4 denotes an amino group substituted by the groups Rt5 and R16 wherein
R'5 denotes a hydrogen atom, a C1_6-alkyl, C3_6-cycloalkyl, C3_6-cycloalkyl-
Ct_3-
alkyl, aryl or aryl-C~_3-alkyl group and
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
4
R~6 denotes a R"-C2_3-alkyl group, while the C2_3-alkyl moiety is straight-
chain
and may be substituted by 1 to 4 C~_3-alkyl groups, which may be identical or
different, and the C2_3-alkyl group may be linked to Rt' from position 2
onwards,
and
R" denotes an amino or C~_3-alkylamino group,
an amino group substituted by the groups R'S and Rt8 wherein
R'5 is as hereinbefore defined and R'$ denotes a C3_~o-cycloalkyl-C~_2-alkyl-
group substituted in the 1 position of the cycloalkyl group by R'9 or a C3_~o-
cyclo-
alkyl group substituted in 1 or 2 position by a R'9-C~_2-alkyl- group, while
R'9
denotes an amino or C~_3-alkylamino group,
an amino group substituted by the groups R'5 and R2° wherein
R'5 is as hereinbefore defined and R2° denotes a C4- or C$_~o-
cycloalkyl group
wherein a methylene group is replaced by an -NH- group from position 3 onwards
of the C4- or C$_~o-cycloalkyl group,
or an amino group substituted by the groups R'S and RZ' wherein
R'S is as hereinbefore defined and R2' denotes a C3_a or C$_~o-cycloalkyl
group
substituted in the 2 or 3 position by an amino or C~_3-alkylamino group,
while the above-mentioned groups R'$, R2° and R2' by Rb may be mono- or
disubstituted, the substituents may be identical or different and Rb denotes a
fluorine
atom, a C~_3-alkyl, trifluoromethyl, cyano, amino, C~_3-alkylamino, hydroxy or
C~_3-
alkyloxy group, and wherein one or two methylene groups of the cycloalkyl
group
independently of one another may each be replaced by an oxygen or sulphur atom
or
by an -NH- or-N(C~_3-alkyl)- group, or by a carbonyl, sulphinyl or sulphonyl
group,
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
while by the aryl groups mentioned in the definition of the above groups are
meant
phenyl or naphthyl groups, which may be mono-, di- or trisubstituted
independently of
one another by Rh, while the substituents may be identical or different and Rh
denotes a fluorine, chlorine, bromine or iodine atom, a trifluoromethyl,
cyano, nitro,
5 amino, aminocarbonyl, C~_3-alkoxy-carbonyl, aminosulphonyl, methylsulphonyl,
acetylamino, methylsulphonylamino, C1_3-alkyl, cyclopropyl, ethenyl, ethynyl,
morpholinyl, hydroxy, C~_3-alkyloxy, difluoromethoxy or trifluoromethoxy
group, and
wherein additionally each hydrogen atom may be replaced by a fluorine atom,
by the heteroaryl groups mentioned in the definition of the above-mentioned
groups
is meant a pyrrolyl, furanyl, thienyl, pyridyl, indolyl, benzofuranyl,
benzothiophenyl,
quinolinyl or isoquinolinyl group,
or is meant a pyrrolyl, furanyl, thienyl or pyridyl group, wherein one or two
methyne
groups are replaced by nitrogen atoms,
or is meant an indolyl, benzofuranyl, benzothiophenyl, quinolinyl or
isoquinolinyl
group, wherein one to three methyne groups are replaced by nitrogen atoms,
and the above-mentioned heteroaryl groups may be mono- or disubstituted by
Rh, while the substituents may be identical or different and Rh is as
hereinbefore defined,
by the above-mentioned cycloalkyl groups as defined are meant both monocyclic
and
also polycyclic ring systems, while the polycyclic groups may be annelated,
spiro-
linked or bridged in structure, e.g. the polycyclic groups may be decalin,
octahydroindene, norbornane, spiro[4,4]nonane, spiro[4,5]decane,
bicyclo[2,1,1 ]hexane, bicyclo[2,2,2]octane, bicyclo[3,2,1 ]octane,
bicyclo[3,2,2]nonane, bicyclo[3,3,1 ]nonane, bicyclo[3,3,2]decane or
adamantine,
while, unless otherwise stated, the above-mentioned alkyl, alkenyl and alkynyl
groups may be straight-chain or branched,
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
6
the tautomers, enantiomers, diastereomers, the mixtures thereof, the prodrugs
thereof and the salts thereof.
The carboxy groups mentioned in the definition of the abovementioned groups
may
be replaced by a group which can be converted into a carboxy group in vivo or
by a
group which is negatively charged under physiological conditions,
and furthermore the amino and imino groups mentioned in the definition of the
abovementioned groups may be substituted by a group which can be cleaved in
vivo.
Such groups are described for example in WO 98/46576 and by N.M. Nielsen et
al. in
International Journal of Pharmaceutics 39, 75-85 (1987).
By a group which can be converted in vivo into a carboxy group is meant, for
example, a hydroxymethyl group, a carboxy group esterified with an alcohol
wherein
the alcohol moiety is preferably a C~_6-alkanol, a phenyl-C~_3-alkanol, a
C3_9-cycloalkanol, while a C5_$-cycloalkanol may additionally be substituted
by one or
two C~_3-alkyl groups, a C5_8-cycloalkanol wherein a methylene group in the 3
or 4
position is replaced by an oxygen atom or by an imino group optionally
substituted by
a C~_3-alkyl, phenyl-C~_3-alkyl, phenyl-C~_3-alkoxycarbonyl or C2_6-alkanoyl
group and
the cycloalkanol moiety may additionally be substituted by one or two C~_3-
alkyl
groups, a C4_~-cycloalkenol, a C3_5-alkenol, a phenyl-C3_5-alkenol, a C3_5-
alkynol or
phenyl-C3_5-alkynol with the proviso that no bonds to the oxygen atom start
from a
carbon atom which carries a double or triple bond, a C3_$-cycloalkyl-C~_3-
alkanol, a
bicycloalkanol with a total of 8 to 10 carbon atoms which may additionally be
substituted in the bicycloalkyl moiety by one or two C~_3-alkyl groups, a 1,3-
dihydro-3-
oxo-1-isobenzofuranol or an alcohol of formula
Rp-CO-O-(RqCRr)-OH,
wherein
Rp denotes a C~_$-alkyl, C5_~-cycloalkyl, C~_$-alkyloxy, C5_~-cycloalkyloxy,
phenyl or
phenyl-C~_3-alkyl group,
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 fOrelgn filing text
7
Rq denotes a hydrogen atom, a C~_3-alkyl, C5_~-cycloalkyl or phenyl group and
Rr denotes a hydrogen atom or a C~_3-alkyl group,
by a group which is negatively charged under physiological conditions is
meant, for
example, a tetrazol-5-yl, phenylcarbonylaminocarbonyl,
trifluoromethylcarbonylaminocarbonyl, C~_s-alkylsulphonylamino,
phenylsulphonylamino, benzylsulphonylamino, trifluoromethylsulphonylamino,
C~_s-alkylsulphonylaminocarbonyl, phenylsulphonylaminocarbonyl,
benzylsulphonylaminocarbonyl or perfluoro-C~_s-alkylsulphonylaminocarbonyl
group
and by a group which can be cleaved in vivo from an imino or amino group is
meant,
for example, a hydroxy group, an acyl group such as a phenylcarbonyl group
optionally mono- or disubstituted by fluorine, chlorine, bromine or iodine
atoms, by
C~_3-alkyl or C~_3-alkoxy groups, while the substituents may be identical or
different, a
pyridinoyl group or a C~_~s-alkanoyl group such as the formyl, acetyl,
propionyl,
butanoyl, pentanoyl or hexanoyl group, a 3,3,3-trichloropropionyl or
allyloxycarbonyl
group, a C~_~s-alkoxycarbonyl or C~_~s-alkylcarbonyloxy group, wherein
hydrogen
atoms may be wholly or partially replaced by fluorine or chlorine atoms such
as the
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl, tert.butoxycarbonyl, pentoxycarbonyl, hexoxycarbonyl,
octyloxycarbonyl, nonyloxycarbonyl, decyloxycarbonyl, undecyloxycarbonyl,
dodecyloxycarbonyl, hexadecyloxycarbonyl, methylcarbonyloxy, ethylcarbonyloxy,
2,2,2-trichloroethylcarbonyloxy, propylcarbonyloxy, isopropylcarbonyloxy,
butylcarbonyloxy, tert.butylcarbonyloxy, pentylcarbonyloxy, hexylcarbonyloxy,
octylcarbonyloxy, nonylcarbonyloxy, decylcarbonyloxy, undecylcarbonyloxy,
dodecylcarbonyloxy or hexadecylcarbonyloxy group, a phenyl-C~_s-alkoxycarbonyl
group such as the benzyloxycarbonyl, phenylethoxycarbonyl or
phenylpropoxycarbonyl group, a 3-amino-propionyl group wherein the amino group
may be mono- or disubstituted by C~_s-alkyl or C3_~-cycloalkyl groups and the
substituents may be identical or different, a C~_3-alkylsulphonyl-C2_4-
alkoxycarbonyl,
C~_3-alkoxy-C2_4-alkoxy-C2_4-alkoxycarbonyl, Rp-CO-O-(RqCRr)-O-CO-, C~_s-alkyl-
CO-
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
8
NH-(RSCRt)-O-CO- or C~_6-alkyl-CO-O-(RSCRt)-(RSCRt)-O-CO- group, wherein Rp to
Rr are as hereinbefore defined,
RS and Rt, which may be identical or different, denote hydrogen atoms or
C~_3-alkyl groups.
Moreover, unless otherwise stated, the saturated alkyl and alkoxy moieties
containing more than 2 carbon atoms mentioned in the definitions above also
include
the branched isomers thereof such as the isopropyl, tert.butyl, isobutyl
group, etc.
R' may denote for example a 2-cyanobenzyl, 3-fluorobenzyl, 3-methoxybenzyl, 4-
bromo-2-cyanobenzyl, 3-chloro-2-cyanobenzyl, 2-cyano-4-fluorobenzyl, 3,5-
dimethoxybenzyl, 2,6-dicyanobenzyl, 5-cyanofuranylmethyl, oxazolylmethyl,
isoxazolylmethyl, 5-methoxycarbonylthienylmethyl, pyridinylmethyl, 3-
cyanopyridin-2-
ylmethyl, 6-cyanopyridin-2-ylmethyl, 6-fluoropyridin-2-ylmethyl, 3-(2-
cyanophenyl)-
prop-2-enyl, 3-(pyridin-2-yl)-prop-2-enyl, 3-(pentafluorophenyl)-prop-2-enyl,
phenyl-
carbonylmethyl, 3-methoxyphenylcarbonylmethyl, naphthyl-1-methyl, 4-
cyanonaphth-
1-ylmethyl, quinolin-1-ylmethyl, 4-cyanoquinolin-1-ylmethyl, isoquinolin-1-
ylmethyl, 4-
cyanoisoquinolin-1-ylmethyl, 4-cyanoisoquinolin-3-ylmethyl, 3-
methylisoquinolin-1-
ylmethyl, quinazolin-2-ylmethyl, 4-methylquinazolin-2-ylmethyl,
[1,5]naphthiridin-2-
ylmethyl, [1,5]naphthiridin-3-ylmethyl, quinoxalin-6-ylmethyl or 2,3-dimethyl-
quinoxalin-6-ylmethyl group.
R2 may denote, for example, a hydrogen atom, a methyl, ethyl, propyl, 2-
propyl,
butyl, 2-butyl, 2-methylpropyl, tert.-butyl , cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl, 2-propen-1-yl, 2-propyn-1-yl, cyclopropylmethyl, phenyl, benzyl,
2-phenylethyl, 3-phenylpropyl, 2-hydroxyethyl, 2-methoxyethyl, 2-ethoxyethyl,
2-(dimethylamino)ethyl, pyrrolidin-1-yl, piperidin-1-yl, morpholin-1-yl,
piperazin-1-yl,
carboxy, methoxycarbonyl, ethoxycarbonyl, carboxymethyl,
(methoxycarbonyl)methyl, aminocarbonyl, methylaminocarbonyl, dimethylamino-
carbonyl, pyrrolidinocarbonyl, piperidinocarbonyl, morpholinocarbonyl,
(aminocarbonyl)methyl, (methylaminocarbonyl)methyl, (dimethylaminocarbo-
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
9
nyl)methyl, (pyrrolidinocarbonyl)methyl, (piperidinocarbonyl)methyl,
(morpholino-
carbonyl)methyl, cyanomethyl, 2-cyanoethyl or pyridinyl group.
R3 may denote for example a 2-propen-1-yl, 2-methyl-2-propen-1-yl, 1-buten-1-
yl, 2-
buten-1-yl, 3-buten-1-yl, 2-methyl-2-buten-1-yl, 3-methyl-2-buten-1-yl, 2,3-
dimethyl-2-
buten-1-yl, 3-methyl-3-buten-1-yl, 1-cyclopenten-1-ylmethyl, (2-methyl-1-
cyclopenten-
1-yl)methyl, 1-cyclohexen-1-ylmethyl, 2-propyn-1-yl, 2-butyn-1-yl, 3-butyn-1-
yl, 2-
chlorobenzyl, 2-bromobenzyl, 2-iodobenzyl, 2-cyanobenzyl, 3-fluorobenzyl, 2-
methoxybenzyl, 2-furanylmethyl, 3-furanylmethyl, 2-thienylmethyl or 3-
thienylmethyl
group.
R4 may denote for example a (2-aminocyclopropyl)amino, N-(2-aminocyclopropyl)-
N-
methyl-amino, (2-aminocyclobutyl)amino, N-(2-aminocyclobutyl)-N-methyl-amino,
N-
(3-aminocyclobutyl)-N-methyl-amino, N-(2-aminoethyl)-N-methyl-amino, N-(1-
aminoprop-2-yl)-N-methyl-amino, N-(2-aminopropyl)-N-methyl-amino, N-(1-amino-2-
methyl-prop-2-yl)-N-methyl-amino, N-(2-amino-2-methyl-propyl)-N-methyl-amino,
N-
[(1-aminocyclopropyl)methyl]-N-methyl-amino or N-(1-aminomethylcyclopropyl)-N-
methyl-amino group.
Preferred compounds of general formula I are those wherein
R' and R4 are as hereinbefore defined,
X denotes a nitrogen atom or a -CH group,
R2 denotes a hydrogen atom, a C~_4-alkyl, C3_6-cycloalkyl or phenyl group and
R3 denotes a 1-buten-1-yl, 2-buten-1-yl, 2-butyn-1-yl, cyclopent-1-enyl-
methyl,
furanylmethyl, thienylmethyl, chlorobenzyl, bromobenzyl, iodobenzyl,
methoxybenzyl
or cyanobenzyl group,
the enantiomers, the diastereomers, the mixtures thereof and the salts
thereof.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
Particularly preferred compounds of general formula I are those wherein
R' denotes a phenylmethyl, phenylcarbonylmethyl, phenylprop-2-enyl,
5 pyridinylmethyl, pyrimidinylmethyl, naphthylmethyl, quinolinylmethyl,
isoquinolinylmethyl, quinazolinylmethyl, quinoxalinylmethyl,
naphthyridinylmethyl or
benzotriazolylmethyl group which may be substituted in each case by one or two
fluorine, chlorine, bromine atoms or one or two cyano, nitro, amino, C~_3-
alkyl, C~_3-
alkyloxy and morpholinyl groups, while the substituents are identical or
different,
X denotes a nitrogen atom or a -CH group,
R2 denotes a hydrogen atom,
R3 denotes a 1-buten-1-yl, 2-buten-1-yl, 2-butyn-1-yl, cyclopent-1-enyl-
methyl,
furanylmethyl, thienylmethyl, chlorobenzyl, bromobenzyl, iodobenzyl or
cyanobenzyl
group and
R4 denotes an N-(2-aminoethyl)-N-methyl-amino group wherein the ethyl group
may
be substituted by 1 to 4 methyl groups,
the tautomers, the mixtures thereof and the salts thereof.
Most particularly preferred are those compounds of general formula I wherein
R' denotes a isoquinolinylmethyl, quinazolinylmethyl or benzyl group which may
be
substituted by a methyl or cyano group,
X denotes a nitrogen atom or a -CH group,
RZ denotes a hydrogen atom,
R3 denotes a 2-butyn-1-yl group and
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
11
R4 denotes an N-(2-aminoethyl)-N-methyl-amino, N-(2-aminopropyl)-N-methyl-
amino
or N-(2-amino-2-methylpropyl)-N-methyl-amino group,
the tautomers and the salts thereof.
The following preferred compounds may be mentioned by way of example:
(a) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(b) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-methyl-
isoquinolin-1-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(c) (S)-2-[N-(2-aminopropyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(2-cyanophenyl-
methyl)-3, 5-d ihyd ro-im idazo[4, 5-d]pyridazin-4-one
(d) 2-[N-(2-amino-2-methylpropyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(2-
cyanophenylmethyl) -3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(e) (S)-2-[N-(2-aminopropyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-yl)methyl] -3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(f) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-methyl-
isoquinolin-1-
yl)methyl]-3,5-dihydro-imidazo[4,5-c]pyridin-4-one
(g) (S)-2-[N-(2-aminopropyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-cyano-
isoquinolin-3-yl)methyl]-3,5-dihydro-imidazo[4,5-c]pyridin-4-one
and the tautomers and the salts thereof.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
12
According to the invention the compounds of general formula I are obtained by
methods known per se, for example by the following methods:
a) reacting a compound of general formula
3
RAN N
N
Rz
wherein
R' to R3 and X are as hereinbefore defined and
Z' denotes a leaving group such as a halogen atom, a substituted hydroxy,
mercapto, sulphinyl, sulphonyl or sulphonyloxy group such as a chlorine or
bromine
atom, a methanesulphonyl or methanesulphonyloxy group,
with R4-H or salts thereof, where R4 is as hereinbefore defined.
The reaction is expediently carried out in a solvent such as isopropanol,
butanol,
tetrahydrofuran, dioxane, dimethylformamide, dimethylsulphoxide, ethylene-
glycolmonomethylether, ethyleneglycoldiethylether or sulpholane, optionally in
the
presence of an inorganic or tertiary organic base, e.g. sodium carbonate,
potassium
carbonate or potassium hydroxide, a tertiary organic base, e.g. triethylamine,
or in
the presence of N-ethyl-diisopropylamine (Hianig base), while these organic
bases
may simultaneously also serve as solvent, and optionally in the presence of a
reaction accelerator such as an alkali metal halide or a palladium-based
catalyst at
temperatures between -20 and 180°C, but preferably at temperatures
between -10
and 120°C. The reaction may, however, also be carried out without a
solvent in an
excess of ethylenediamine derivative with conventional heating or in the
microwave
oven.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
13
b) deprotecting a compound of general formula
0 Rs
R~ N
~~Zz (III),
N
Rz
wherein R1, R2, R3 and X are as hereinbefore defined and
Z2 denotes one of the groups mentioned hereinbefore for R4 which contain an
amino
group not directly bound to the basic imidazopyridazinone structure which is
Boc-
protected in Z2, where Boc denotes a tert.-butyloxycarbonyl group.
The tert.-butyloxycarbonyl group is preferably cleaved by treating with an
acid such
as trifluoroacetic acid or hydrochloric acid or by treating with
bromotrimethylsilane or
iodotrimethylsilane, optionally using a solvent such as methylene chloride,
ethyl
acetate, dioxane, methanol, isopropanol or diethyl ether at temperatures
between 0
and 80°C.
In the reactions described hereinbefore, any reactive groups present such as
amino,
alkylamino or imino groups may be protected during the reaction by
conventional
protecting groups which are cleaved again after the reaction.
For example, protecting groups for an amino, alkylamino or imino group may be
a
formyl, acetyl, trifluoroacetyl, ethoxycarbonyl, tert.butoxycarbonyl,
benzyloxycarbonyl,
benzyl, methoxybenzyl or 2,4-dimethoxybenzyl group and additionally, for the
amino
group, a phthalyl group.
Any protecting group used is optionally subsequently cleaved for example by
hydrolysis in an aqueous solvent, e.g. in water, isopropanol/water, acetic
acid/water,
tetrahydrofuran/water or dioxane/water, in the presence of an acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the presence
of an alkali
metal base such as sodium hydroxide or potassium hydroxide or aprotically,
e.g. in
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
14
the presence of iodotrimethylsilane, at temperatures between 0 and
120°C,
preferably at temperatures between 10 and 100°C.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl group is cleaved, for
example, hydrogenolytically, e.g. with hydrogen in the presence of a catalyst
such as
palladium/charcoal in a suitable solvent such as methanol, ethanol, ethyl
acetate or
glacial acetic acid, optionally with the addition of an acid such as
hydrochloric acid at
temperatures between 0 and 100°C, but preferably at ambient
temperatures between
20 and 60°C, and at a hydrogen pressure of 1 to 7 bar, but preferably
from 3 to 5 bar.
However, a 2.4-dimethoxybenzyl group is preferably cleaved in trifluoroacetic
acid in
the presence of anisole.
A tert.-butyl or tert. butyloxycarbonyl group is preferably cleaved by
treating with an
acid such as trifluoroacetic acid or hydrochloric acid or by treating with
iodotrimethylsilane, optionally using a solvent such as methylene chloride,
dioxane,
methanol or diethyl ether.
A trifluoroacetyl group is preferably cleaved by treating with an acid such as
hydrochloric acid, optionally in the presence of a solvent such as acetic acid
at
temperatures between 50 and 120°C or by treating with sodium hydroxide
solution,
optionally in the presence of a solvent such as tetrahydrofuran, at
temperatures
between 0 and 50°C.
A phthalyl group is preferably cleaved in the presence of hydrazine or a
primary
amine such as methylamine, ethylamine or n-butylamine in a solvent such as
methanol, ethanol, isopropanol, toluene/water or dioxane at temperatures
between 20
and 50°C.
Moreover, the compounds of general formula I obtained may be resolved into
their
enantiomers and/or diastereomers, as mentioned hereinbefore. Thus, for
example,
cis/trans mixtures may be resolved into their cis and trans isomers, and
compounds
with at least one optically active carbon atom may be separated into their
enantiomers.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
Thus, for example, the cisltrans mixtures obtained may be separated by
chromatography into their cis and trans isomers, the compounds of general
formula I
obtained which occur as racemates may be separated by methods known per se
(cf.
5 Allinger N. L. and Eliel E. L. in "Topics in Stereochemistry", Vol. 6, Wiley
Interscience,
1971 ) into their optical enantiomers and compounds of general formula I with
at least
2 asymmetric carbon atoms may be resolved into their diastereomers on the
basis of
their physical-chemical differences using methods known per se, e.g. by
chromatography and/or fractional crystallisation, and, if these compounds are
10 obtained in racemic form, they may subsequently be resolved into the
enantiomers
as mentioned above.
The enantiomers are preferably separated by column separation on chiral phases
or
by recrystallisation from an optically active solvent or by reacting with an
optically
15 active substance which forms salts or derivatives such as e.g. esters or
amides with
the racemic compound, particularly acids and the activated derivatives or
alcohols
thereof, and separating the diastereomeric mixture of salts or derivatives
thus
obtained, e.g. on the basis of their differences in solubility, whilst the
free antipodes
may be released from the pure diastereomeric salts or derivatives by the
action of
suitable agents. Optically active acids in common use are e.g. the D- and L-
forms of
tartaric acid or dibenzoyltartaric acid, di-o-tolyltartaric acid, malic acid,
mandelic acid,
camphorsulphonic acid, glutamic acid, aspartic acid or quinic acid. An
optically active
alcohol may be, for example, (+)- or (-)-menthol and an optically active acyl
group in
amides, for example, may be a (+)- or (-)-menthyloxycarbonyl.
Furthermore, the compounds of formula I obtained may be converted into the
salts
thereof, particularly for pharmaceutical use into the physiologically
acceptable salts
with inorganic or organic acids. Acids which may be used for this purpose
include for
example hydrochloric acid, hydrobromic acid, sulphuric acid, methanesulphonic
acid,
phosphoric acid, fumaric acid, succinic acid, lactic acid, citric acid,
tartaric acid or
malefic acid.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
16
Moreover, the new compounds of formula (I), if they contain a carboxy group,
may if
desired be converted into the salts thereof with inorganic or organic bases,
particularly for pharmaceutical use into the physiologically acceptable salts
thereof.
Suitable bases for this include, for example, sodium hydroxide, potassium
hydroxide,
cyclohexylamine, ethanolamine, diethanolamine and triethanolamine.
The compounds of general formulae II and III used as starting compounds are
either
known from the literature or may be prepared by methods known from the
literature
(see Examples I to XI).
As already mentioned hereinbefore, the compounds of general formula I
according to
the invention and the physiologically acceptable salts thereof have valuable
pharma-
cological properties, particularly an inhibiting effect on the enzyme DPP-IV.
The biological properties of the new compounds were investigated as follows:
The ability of the substances and their corresponding salts to inhibit the DPP-
IV
activity can be demonstrated in an experiment in which an extract of the human
colon
carcinoma cell line Caco-2 is used as the DPP IV source. The differentiation
of the
cells in order to induce the DPP-IV expression was carried out in accordance
with the
description by Reiher et al. in an article entitled "Increased expression of
intestinal
cell line Caco-2" , which appeared in Proc. Natl. Acad. Sci. Vol. 90, pp. 5757-
5761
(1993). The cell extract was obtained from cells solubilised in a buffer (10mM
Tris
HCI, 0.15 M NaCI, 0.04 t.i.u. aprotinin, 0.5% Nonidet-P40, pH 8.0) by
centrifugation
at 35,000 g for 30 minutes at 4°C (to remove cell debris).
The DPP-IV assay was carried out as follows:
50 NI of substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin),
final
concentration 100 pM, were placed in black microtitre plates. 20 p1 of assay
buffer
(final concentrations 50 mM Tris HCI pH 7.8, 50 mM NaCI, 1 % DMSO) was
pipetted
in. The reaction was started by the addition of 30 p1 of solubilised Caco-2
protein
(final concentration 0.14 pg of protein per well). The test substances under
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
17
investigation were typically added prediluted to 20 p1, while the volume of
assay
buffer was then reduced accordingly. The reaction was carried out at ambient
temperature, the incubation period was 60 minutes. Then the fluorescence was
measured in a Victor 1420 Multilabel Counter, with the excitation wavelength
at 405
nm and the emission wavelength at 535 nm. Dummy values (corresponding to 0
activity) were obtained in mixtures with no Caco-2 protein (volume replaced by
assay
buffer), control values (corresponding to 100 % activity) were obtained in
mixtures
without any added substance. The potency of the test substances in question,
expressed as ICSO values, were calculated from dosage/activity curves
consisting of
11 measured points in each case. The following results were obtained:
Compound DPP IV inhibition
(Example No.) IC5o [nM]
1 1
1(1) 10
1 (2) 34
2 76
2(1 ) 336
3 2
The compounds prepared according to the invention are well tolerated as no
toxic
side effects could be detected in rats after the oral administration of 10
mg/kg of the
compound of Example 1, for example.
In view of their ability to inhibit DPP-IV activity, the compounds of general
formula I
according to the invention and the corresponding pharmaceutically acceptable
salts
thereof are suitable for influencing any conditions or diseases which can be
affected
by the inhibition of the DPP-IV activity. It is therefore to be expected that
the
compounds according to the invention will be suitable for the prevention or
treatment
of diseases or conditions such as type I and type II diabetes mellitus,
diabetic
complications (e.g. retinopathy, nephropathy or neuropathies), metabolic
acidosis or
ketosis, reactive hypoglycaemia, insulin resistance, metabolic syndrome,
dyslipidaemias of various origins, arthritis, atherosclerosis and related
diseases,
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
18
obesity, allograft transplantation and osteoporosis caused by calcitonin. In
addition,
these substances are suitable for preventing B-cell degeneration such as e.g.
apoptosis or necrosis of pancreatic B-cells. The substances are also suitable
for
improving or restoring the function of pancreatic cells and additionally
increasing the
size and number of pancreatic B-cells. Additionally, on the basis of the role
of the
glucagon-like peptides such as e.g. GLP-1 and GLP-2 and their link with DPP-IV
inhibition, it is expected that the compounds according to the invention will
be
suitable for achieving, inter alia, a sedative or tranquillising effect, as
well as having a
favourable effect on catabolic states after operations or hormonal stress
responses
or possibly reducing mortality and morbidity after myocardial infarct.
Moreover, they
are suitable for treating any conditions connected with the effects mentioned
above
and mediated by GLP-1 or GLP-2. The compounds according to the invention may
also be used as diuretics or antihypertensives and are suitable for preventing
and
treating acute kidney failure. The compounds according to the invention may
also be
used to treat inflammatory complaints of the respiratory tract. They are also
suitable
for preventing and treating chronic inflammatory bowel diseases such as e.g.
irritable
bowel syndrome (IBS), Crohn's disease or ulcerative colitis and also
pancreatitis. It is
also expected that they can be used for all kinds of injury or damage to the
gastrointestinal tract such as may occur in colitis and enteritis, for
example.
Moreover, it is expected that DPP-IV inhibitors and hence the compounds
according
to the invention can be used to treat infertility or to improve fertility in
humans or
mammals, particularly if the infertility is connected with insulin resistance
or with
polycystic ovary syndrome. On the other hand these substances are suitable for
influencing sperm motility and are thus suitable for use as male
contraceptives. In
addition, the substances are suitable for treating growth hormone deficiencies
connected with restricted growth, and may reasonably be used for all
indications for
which growth hormone may be used. The compounds according to the invention are
also suitable, on the basis of their inhibitory effect on DPP-IV, for treating
various
autoimmune diseases such as e.g. rheumatoid arthritis, multiple sclerosis,
thyroiditis
and Basedow's disease, etc. They may also be used to treat viral diseases and
also,
for example, in HIV infections, for stimulating blood production, in benign
prostatic
hyperplasia, gingivitis, as well as for the treatment of neuronal defects and
neuro-
degenerative diseases such as Alzheimer's disease, for example. The compounds
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
19
described may also be used for the treatment of tumours, particularly for
modifying
tumour invasion and also metastasisation; examples here are their use in
treating T-
cell lymphomas, acute lymphoblastic leukaemia, cell-based thyroid carcinomas,
basal
cell carcinomas or breast cancers. Other indications are stroke, ischaemia of
various
origins, Parkinson's disease and migraine. In addition, further indications
include
follicular and epidermal hyperkeratoses, increased keratinocyte proliferation,
psoriasis, encephalomyelitis, glomerulonephritis, lipodystrophies, as well as
psycho-
somatic, depressive and neuropsychiatric diseases of all kinds.
The compounds according to the invention may also be used in conjunction with
other active substances. Suitable therapeutic agents for such combinations
include
for example antidiabetic agents such as metformin, sulphonylureas (e.g.
glibenclamide, tolbutamide, glimepiride), nateglinide, repaglinide,
thiazolidinediones
(e.g. rosiglitazone, pioglitazone), PPAR-gamma agonists (e.g. GI 262570) and
antagonists, PPAR-gamma/alpha modulators (e.g. KRP 297), alpha-glucosidase
inhibitors (e.g. acarbose, voglibose), other DPPIV inhibitors, alpha2
antagonists,
insulin and insulin analogues, GLP-1 and GLP-1 analogues (e.g. exendin-4) or
amylin. Also, combinations with SGLT2 inhibitors such as T-1095 or KGT-1251
(869682), inhibitors of protein tyrosine phosphatase 1, substances which
influence
deregulated glucose production in the liver, such as e.g. inhibitors of
glucose-6-
phosphatase, or fructose-1,6-bisphosphatase, glycogen phosphorylase, glucagon
receptor antagonists and inhibitors of phosphoenol pyruvate carboxykinase,
glycogen
synthase kinase or pyruvate dehydrokinase, lipid lowering agents, such as HMG-
CoA-reductase inhibitors (e.g. simvastatin, atorvastatin), fibrates (e.g.
bezafibrate,
fenofibrate), nicotinic acid and its derivatives, PPAR-alpha agonists, PPAR-
delta
agonists, ACAT inhibitors (e.g. avasimibe) or cholesterol absorption
inhibitors such
as for example ezetimibe, bile acid-binding substances such as for example
cholestyramine, inhibitors of ileac bile acid transport, HDL-raising compounds
such
as for example inhibitors of CETP or regulators of ABC1 or active substances
for the
treatment of obesity, such as e.g. sibutramine or tetrahydrolipostatin,
dexfenfluramine, axokine, antagonists of the cannabinoid1 receptor, MCH-1
receptor
antagonists, MC4 receptor agonists, NPYS or NPY2 antagonists or (33-agonists
such
as SB-418790 or AD-9677 as well as agonists of the 5HT2c receptor.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 fOrelgn filing text
It is also possible to combine the compounds with drugs for treating high
blood
pressure such as e.g. All antagonists or ACE inhibitors, diuretics, f3-
blockers, Ca-
antagonists, etc., or combinations thereof.
5
The dosage required to expediently achieve such an effect is, by intravenous
route, 1
to 100 mg, preferably 1 to 30 mg, and by oral route 1 to 1000 mg, preferably 1
to 100
mg, in each case 1 to 4 times a day. For this purpose, the compounds of
formula I
prepared according to the invention, optionally combined with other active
10 substances, may be incorporated together with one or more inert
conventional
carriers and/or diluents, e.g. with corn starch, lactose, glucose,
microcrystalline
cellulose, magnesium stearate, polyvinylpyrrolidone, citric acid, tartaric
acid, water,
water/ethanol, water/glycerol, water/sorbitol, water/polyethylene glycol,
propylene
glycol, cetylstearyl alcohol, carboxymethylcellulose or fatty substances such
as hard
15 fat or suitable mixtures thereof into conventional galenic preparations
such as plain
or coated tablets, capsules, powders, suspensions or suppositories.
The Examples that follow are intended to illustrate the invention:
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
21
Preparation of the starting compounds:
Example I
Dimethyl 2-bromo-1-(2-butyn-1-yl)-1 H-imidazole-4,5-dicarboxylate
A solution of 15.0 g dimethyl 2-bromo-imidazole-4,5-dicarboxylate, 5.15 ml 1-
bromo-
2-butyne and 50 ml N,N-diisopropylethylamine in 280 ml of tetrahydrofuran is
refluxed for one hour. The mixture is concentrated by evaporation, the residue
is
combined with approx. 100 ml of water and extracted three times with 70 ml of
ethyl
acetate. The extracts are washed with 50 ml of water, dried and evaporated
down.
The crude product thus obtained is purified by column chromatography through
silica
gel with methylene chloride/ethanol (1:0->49:1) as eluant.
Yield: 13.50 g (75% of theory)
Rf value: 0.82 (silica gel, methylene chloride/ethanol = 9:1 )
Mass spectrum (ESI+): m/z = 315/317 (Br) [M+H]+
Example II
Methvl 2-bromo-3-(2-butvn-1-vl)-5-formvl-3H-imidazol-4-carboxvlate
43 ml of a 1 M solution of diisobutylaluminium hydride in tetrahydrofuran are
added
dropwise within 20 minutes to a solution of 13.5 g of dimethyl 2-bromo-1-(2-
butyn-1-
y1)-1 H-imidazole-4,5-dicarboxylate in 220 ml of tetrahydrofuran under an
argon
atmosphere at -70°C. The mixture is stirred for a further four hours at
-70°C, then 20
ml of a mixture of 1 M hydrochloric acid and tetrahydrofuran are added
dropwise.
After heating to ambient temperature approx. 200 ml of water are added and the
mixture is extracted three times with 70 ml of ethyl acetate. The combined
extracts
are dried and evaporated down. The crude product thus obtained is purified by
column chromatography through silica gel with petroleum ether/ethyl acetate
(4:1-
>1:1) as eluant.
Yield: 6.40 g (52% of theory)
Mass spectrum (ESI+): m/z = 285/287 (Br) [M+H]+
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
22
Example III
2-bromo-3-(2-butyn-1-yl)-3,5-dihydro-imidazof4,5-d]pyridazin-4-one
0.31 ml hydrazine hydrate, dissolved in 1 ml of ethanol, are added dropwise at
ambient temperature to a solution of 1.80 g methyl 2-bromo-3-(2-butyn-1-yl)-5-
formyl-
3H-imidazole-4-carboxylate in 25 ml of ethanol. Five minutes later 1.5 ml
concentrated acetic acid are added, and the mixture is refluxed for 30
minutes. After
cooling the solid precipitate is suction filtered, washed with 10 ml of
ethanol and 20
ml diethyl ether and dried.
Yield: 1.25 g (74 % of theory)
Mass spectrum (ESI+): m/z = 267/269 (Br) [M+H]+
Example IV
2-bromo-3-~2-butyn-1-yl)-5-f (4-methyl-4uinazolin-2-yl)methtrl]-3, 5-dihydro-
imidazof4,5-dlayridazin-4-one
A mixture of 365 mg 2-bromo-3-(2-butyn-1-yl)-3,5-dihydro-imidazo[4,5-
d]pyridazin-4-
one, 265 mg 2-chloromethyl-4-methyl-quinazoline and 210 mg potassium carbonate
in 6 ml acetonitrile is stirred for 17 h at ambient temperature. Then the
reaction
mixture is filtered through 5 g aluminium oxide with ethyl acetate and the
filtrate is
evaporated down. The residue is triturated in diisopropylether, separated from
the
ether and dried.
Yield: 300 mg (53% of theory)
Mass spectrum (ESI+): m/z = 423/425 (Br) [M+H]+
The following compounds are obtained analogously to Example IV:
(1) 2-bromo-3-(2-butyn-1-yl)-5-[(3-methyl-isoquinolin-1-yl)methyl]-3,5-dihydro-
imidazo[4,5-d]pyridazin-4-one
Mass spectrum (ESI+): m/z = 422/424 (Br) [M+H]+
(2) 2-bromo-3-(2-butyn-1-yl)-5-(2-cyanophenylmethyl)-3,5-dihydro-imidazo[4,5-
d]pyridazin-4-one
Mass spectrum (ESI+): m/z = 382/384 (Br) [M+H]+
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
23
(3) 2-bromo-3-(2-butyn-1-yl)-5-(4-cyano-isoquinolin-3-ylmethyl)-3,5-dihydro-
imidazo[4,5-d]pyridazin-4-one
Mass spectrum (ESI+): m/z = 463/465 (Br) [M+H]+
Example V
1S)-2-[(2-aminopropyl)aminol-3-(2-butyn-1-yll-5-(2-cyanophenylmeth~)-3,5-
dihydro-
imidazo[4,5-dl~yridazin-4-one
A mixture of 0.36 g 2-bromo-3-(2-butyn-1-yl)-5-(2-cyanophenylmethyl)-3,5-
dihydro
imidazo[4,5-d]pyridazin-4-one, 0.42 g (S)-1,2-diaminopropane dihydrochloride
and
0.52 g potassium carbonate in 6 ml N-methylpyrrolidone is stirred for 2.5 h at
120°C.
Then saturated aqueous sodium chloride solution is added and the precipitate
formed is separated off. The aqueous phase is extracted with ethyl acetate,
the
combined organic phases are dried over sodium sulphate and evaporated down.
Yield: 580 mg (approx. 60% pure)
Rf value: 0.30 (silica gel, methylene chloride/methanol/ammonium hydroxide =
90:10:0.1)
The following compound is obtained analogously to Example V:
(1) 2-[(2-amino-2-methyl-propyl)amino]-3-(2-butyn-1-yl)-5-(2-
cyanophenylmethyl)-3,5-
dihydro-imidazo[4,5-d]pyridazin-4-one
Rf value: 0.25 (silica gel, methylene chloride/methanol/ammonium hydroxide =
90:10:0.1 )
(2) (S)-2-[(2-benzyloxycarbonylamino-prop-1-yl)amino]-3-(2-butyn-1-yl)-5-[(4-
methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Product (2) was obtained by reacting 2-bromo-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one with (S)-N-
(2-
benzyloxycarbonylamino-prop-1-yl)-N-methyl-amine.
Mass spectrum (ESI+): m/z = 565 [M+H]+
(3) (S)-2-[(2-aminopropyl)amino]-3-(2-butyn-1-yl)-5-(4-cyano-isoquinolin-3-
ylmethyl)-
3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
24
Rf value: 0.30 (silica gel, methylene chloride/methanol/ammonium hydroxide =
90:10:0.1 )
Example VI
(S)-2-f(2-tert-butyloxycarbonylamino-propyl)amino]-3-r;2-burn-1-yl)-5-(2-
cyanophenylmethyl)-3,5-dihydro-imidazof4,5-dlpyridazin-4-one
0.73 g di-tert. butyl pyrocarbonate are added at ambient temperature to a
solution of
1.04 g (S)-2-[(2-aminopropyl)amino]-3-(2-butyn-1-yl)-5-(2-cyanophenylmethyl)-
3,5-
dihydro-imidazo[4,5-d]pyridazin-4-one and 0.54 ml triethylamine in 200 ml
dichloromethane. The solution is stirred for 16 h at ambient temperature and
then
evaporated down. The residue is taken up in ethyl acetate and in each case
washed
once with water, dilute citric acid, water and saturated aqueous sodium
chloride
solution. Then the organic phase is dried over sodium sulphate and evaporated
down. The residue is purified by chromatography through silica gel (petroleum
ether/ethyl acetate 1:1 ).
Yield: 0.40 g (30% of theory)
Mass spectrum (ESI+): m/z = 476 [M+H]+
The following compound is obtained analogously to Example VI:
(1) 2-[(2-tert-butyloxycarbonylamino-2-methyl-propyl)amino]-3-(2-butyn-1-yl)-5-
(2-
cyanophenylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Mass spectrum (ESI+): m/z = 490 [M+H]+
(2) (S)-2-[(2-tert-butyloxycarbonylamino-propyl)amino]-3-(2-butyn-1-y1)-5-(4-
cyanoisoquinolin-3-ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Mass spectrum (ESI+): m/z = 557 [M+H]+
Example VII
(S)-2-f N-(2-tert-butyloxycarbonylamino-propyl)-N-methyl-aminol-3-(2-butyn-1-
yl)-5-(2-
cYanophenylmethyl)-3,5-dihydro-imidazof4,5-dlpyridazin-4-one
0.96 g potassium-tert-butoxide are added to an ice-cooled solution of 0.39 g
(S)-2-
[(2-tert-butyloxycarbonylamino-propyl)amino]-3-(2-butyn-1-yl)-5-(2-
cyanophenylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one in 5 ml
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
dimethylsulphoxide. The mixture is stirred at ambient temperature, until the
solution is
clear. Then 53 p1 methyl iodide are added, and the solution is stirred for a
further 3.5
h at ambient temperature. Then water is added to the reaction solution, the
precipitate formed is separated off and washed with water. The dried
precipitate is
5 purified by chromatography through silica gel (petroleum ether/ethyl acetate
1:1 ).
Yield: 0.26 g (66% of theory)
Mass spectrum (ESI+): m/z = 490 [M+H]+
The following compound is obtained analogously to Example VII:
(1 ) 2-[N-(2-tert-butyloxycarbonylamino-2-methyl-propyl)-N-methyl-amino]-3-(2-
butyn-
1-yl)-5-(2-cyanophenylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Mass spectrum (ESI+): m/z = 504 [M+H]+
(2) (S)-2-[N-(2-tert-butyloxycarbonylamino-propyl)-N-methyl-amino]-3-(2-butyn-
1-yl)-
5-(4-cyano-isoquinolin-3-ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Mass spectrum (ESI+): m/z = 571 [M+H]+
Example VIII
Methyl2-bromo-5-(traps-2-metho~~-vinyl)-3H-imidazole-4-carboxylate
Under an argon atmosphere 38 ml of a 0.5 M solution of potassium-
bis(trimethylsilyl)amide in toluene are added dropwise to an ice-cooled
solution of
6.64 g methoxymethyltriphenyl phosphonium chloride in 140 ml of
tetrahydrofuran
over 10 min. The reaction mixture is stirred for a further 15 min in the ice
bath and
then cooled to -70°C. Then a solution of 4.41 g methyl 2-bromo-3-(2-
butyn-1-yl)-5-
formyl-3H-imidazole-4-carboxylate in 40 ml of tetrahydrofuran is added
dropwise over
min. After a further 45 min stirring at -70°C the solution is heated to
ambient
temperature and stirred for another 1 h at this temperature. Then water is
added to
the reaction solution and this is extracted with ethyl acetate. The organic
extracts are
30 dried over sodium sulphate, the solvent is removed and the residue is
chromatographed through silica gel (cyclohexane/ethyl acetate 9:1->7:3).
Yield: 2.67 g (55% of theory), pure traps compound
Mass spectrum (ESI+): m/z = 313/315 (Br) [M+H]+
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
26
Example IX
2-bromo-5-(traps-2-methoxy-vinyl)-3H-imidazole-4-carboxylic acid
A solution of 2.20 g lithium hydroxide in 175 ml of water is added to a
solution of 3.50
g methyl 2-bromo-5-(traps-2-methoxy-vinyl)-3H-imidazole-4-carboxylate in 140
ml of
tetrahydrofuran. The solution is stirred for 4 h at ambient temperature. Then
92 ml
aqueous 1 M hydrochloric acid are added, and the solution is cooled in the ice
bath.
The precipitate is separated off, washed with water and dried.
Yield: 3.30 g (99% of theory)
Mass spectrum (ESI+): m/z = 299/301 (Br) [M+H]+
Example X
2-bromo-3-(2-butyn-1-yl)-5-(traps-2-methoxy-vinyl)-3H-imidazole-4-carboxylic
acid-
(3-methyl-isoauinolin-1-ylmethyl)-amide
A solution of 3.50 g 2-bromo-5-(traps-2-methoxy-vinyl)-3H-imidazole-4-
carboxylic
acid and 1.30 g TBTU in 1.20 ml triethylamine and 30 ml of dimethylformamide
is
stirred for 15 min at ambient temperature. Then 1.09 g 3-methyl-isoquinolin-1-
yl-
methylamine are added, and the resulting suspension is stirred for 4 h at
ambient
temperature. Then ice-cooled water is added and the precipitate is separated
off. The
precipitate is dissolved in dichloromethane, the solution is dried over sodium
sulphate
and the solvent is removed.
Yield: 1.38 g (78% of theory)
Mass spectrum (ESI+): m/z = 453/455 (Br) [M+H]+
Example XI
2-bromo-3-(2-butyn-1-yl)-5-f(3-methyl-isoauinolin-1- I)Y methyll-3 5-dihydro-
imidazof4,5-clpyridin-4-one in a 1:1-mixture with 2-chloro-3-(2-butyn-1- I~[(3-
methyl-isoauinolin-1-yl)methyll-3,5-dihydro-imidazof4 5-clpyridin-4-one
0.74 g 2-bromo-3-(2-butyn-1-yl)-5-(traps-2-methoxy-vinyl)-3H-imidazole-4-
carboxylic
acid-(3-methyl-isoquinolin-1-ylmethyl)-amide are stirred in 35 ml aqueous 4 M
hydrochloric acid for 3 h at 85°C. After cooling to ambient temperature
dichloromethane is added and the solution is made alkaline with sodium
hydroxide
solution. The organic phase is separated off and the aqueous phase is
extracted
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
27
twice with dichloromethane. The combined organic phases are dried over sodium
sulphate, the solvent is removed and the residue is purified through silica
gel
(dichloromethane/methanol 99:1->95:5).
Yield: 0.39 g (1:1 mixture of the two title compounds)
Mass spectrum (ESI+): m/z = 421/423 (Br) [M+H]+ and m/z = 377/379 (CI) [M+H]+
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
28
Preparation of the end compounds:
Example 1
2-f N-(2-aminoethyl)-N-methyl-aminol-3-(2-butlm1-yl)-5-[(4-methyl-auinazolin-2-
yl)methyll-3,5-dihydro-imidazof4,5-dlpyridazin-4-one
I
I \~
N' / N O i
N
N \ I />--N
N
NH2
A mixture of 300 mg 2-bromo-3-(2-butyn-1-yl)-5-[(4-methyl-quinazolin-2-
yl)methyl]-
3,5-dihydro-imidazo[4,5-d]pyridazin-4-one, 400 mg N-methyl-ethylenediamine and
210 mg potassium carbonate in 6 ml dimethylsulphoxide is stirred for 8 h at
60°C.
Then saturated aqueous sodium chloride solution is added and the aqueous phase
is
extracted with ethyl acetate. The combined organic phases are dried over
sodium
sulphate and evaporated down. The crude product is purified by chromatography
through a silica gel column with methylene chloride/methanol (3:2) as eluant.
Yield: 85 mg (29% of theory)
Mass spectrum (ESI+): m/z = 417 [M+H]+
The following compound is obtained analogously to Example 1:
(1) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-methyl-
isoquinolin-1-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
I
/ ~N O i
N
/~-N/
N
NHZ
Mass spectrum (ESI+): m/z = 416 [M+H]+
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
29
(2) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-methyl-
isoquinolin-1-
yl)methyl]-3,5-dihydro-imidazo[4,5-c]pyridin-4-one
N
/~-N
N
NHz
Product (2) was obtained by reacting a 1:1 mixture of 2-bromo-3-(2-butyn-1-yl)-
5-[(3
methyl-isoquinolin-1-yl)methyl]-3,5-dihydro-imidazo[4,5-c]pyridin-4-one and 2-
chloro
3-(2-butyn-1-yl)-5-[(3-methyl-isoquinolin-1-yl)methyl]-3,5-dihydro-imidazo[4,5
c]pyridin-4-one with N-methyl-ethylenediamine according to the instructions
described above.
Mass spectrum (ESI+): m/z = 415 [M+H]+
Example 2
(S)-2-f N-(2-amino-propel)-N-methyl-aminol-3-(2-butyn-1-yl)-5-(2-
cyanophenylmethyl~
3,5-dihydro-imidazof4,5-dlpyridazin-4-one
~ o
N
N
N w ~ ~~ N
N
~N H2
1.4 ml trifluoroacetic acid are added dropwise to a solution of 250 mg (S)-2-
[N-(2-tert-
butyloxycarbonylamino-propyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(2-
cyanophenylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one in 5 ml
dichloromethane. The solution is stirred for 4 h at ambient temperature, then
diluted
with dichloromethane and made alkaline with saturated aqueous sodium carbonate
solution. The organic phase is separated off, washed with water, dried over
magnesium sulphate and evaporated down. The residue is stirred with tert-
butylmethylether and after separation of the ether dried in vacuo at 40-
50°C.
Yield: 161 mg (81 % of theory)
Mass spectrum (ESI+): m/z = 390 [M+H]+
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
The following compound is obtained analogously to Example 2:
(1) 2-[N-(2-amino-2-methyl-propyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(2-
cyanophenylmethyl) -3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
N
5 NH2
Mass spectrum (ESI+): m/z = 404 [M+H]+
(2) (S)-2-[N-(2-amino-propyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(4-cyano-
isoquinolin-3-ylmethyl) -3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
N
II ~
N
~N
I / ~N Nw I N N
10 ~NHZ
Mass spectrum (ESI+): m/z = 441 [M+H]+
Example 3
1S)-2-f N-(2-aminopropyl)-N-methyl-aminol-3-(2-butyn-1-yl)-5-f (4-methyl-
auinazolin-2-
15 I)~yll-3,5-dihydro-imidazof4,5-dlpyridazin-4-one
I~
I ~~
N' / N O i
N
/~- N
N
~NHz
p1 iodotrimethylsilane are added to a solution of 130 mg (S)-2-[(2-
benzyloxycarbonylamino-prop-1-yl)amino]-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one in 3 ml dichloromethane.
After
20 stirring at ambient temperature for 1 and 3 h, a further 100 p1 of
iodotrimethylsilane
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
31
are added on each occasion. The solution is stirred for a further 4 h at
ambient
temperature and then combined with 5 ml of methanol and evaporated down. Then
1
M hydrochloric acid is added and the aqueous phase is washed twice with
dichloromethane. The aqueous phase is made alkaline with sodium carbonate and
extracted three times with dichloromethane. The organic extracts are dried
over
sodium sulphate, the solvent is removed and the residue is purified through
silica gel
(dichloromethane/methanol 1:0->3:1 ).
Yield: 30 mg (30% of theory)
Mass spectrum (ESI+): m/z = 431 [M+H]+
The following compounds may also be obtained analogously to the foregoing
Examples and other methods known from the literature:
(1) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-cyano-naphth-1-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(2) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(2-cyano-
phenylmethyl)-
3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(3) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(quinoxalin-6-
ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(4) 2-[N-(2-aminocyclopropyl)amino]-3-(2-butyn-1-yl)-5-(2-cyano-phenylmethyl)-
3,5-
dihydro-imidazo[4,5-d]pyridazin-4-one
(5) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-cyano-
naphth-1-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(6) 2-[N-(2-amino-2-methyl-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-
methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(7) 2-[N-(2-amino-2-methyl-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-
(phenylcarbonylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(8) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[3-(2-
nitrophenyl)prop-2-en-1-yl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(9) 2-[N-(1-aminocycloprop-1-ylmethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-
methyl-quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(10) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-chloro-2-cyano-
phenyl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
32
(11) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-([1,5]naphthyridin-
2-
ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(12) 2-[N-(2-aminoprop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(quinoxalin-6-
ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(13) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(quinazolin-7-
ylmethyl)-
3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(14) 2-[N-(2-aminoprop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(1-cyano-
isoquinolin-3-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(15) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-morpholin-4-
ylquinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(16) 2-[N-(2-aminoprop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-phenyl-
pyrimidin-
2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(17) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(2,3-dimethyl-
quinoxalin-
6-ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(18) 2-[N-(2-aminoethyl)-N-methyl-amino]-3- (2-butyn-1-yl)-5-[(1-cyano-
isoquinolin-3-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(19) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-methoxy-
phenyl)car-
bonylmethyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(20) 2-[N-(2-aminoprop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(2-methoxy-
phenyl)-
carbonylmethyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(21) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-buten-1-yl)-5-[(3-methyl-
isoquinolin-1-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(22) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(3-methyl-but-2-en-1-yl)-5-[(3-
methyl-isoquinolin-1-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(23) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(1-cyclopenten-1-yl)-5-[(3-methyl-
iso-
quinolin-1-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(24) 2-[N-(2-amino-2-methyl-prop-1-yl)-N-methyl-amino]-3-(1-buten-1-yl)-5-[(3-
methyl-isoquinolin-1-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(25) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-[(2-chloro-phenyl)methyl]-5-[(4-
methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(26) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-[(2-bromo-phenyl)methyl]-5-[(4-
methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
33
(27) 2-[N-(2-amino-2-methyl-prop-1-yl)-N-methyl-amino]-3-[(2-iodo-
phenyl)methyl]-5-
[(4-methyl-quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(28) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-[(2-cyano-phenyl)methyl]-5-[(4-
methyl-quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(29) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(furan-2-ylmethyl)-5-[(4-
methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(30) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(thien-3-ylmethyl)-5-[(4-methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(31) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-methoxy-
naphth-1-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(32) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[3-
(pentafluorophenyl)prop-2-en-1-yl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(33) 2-[(azetidin-3-yl)amino]-3-(2-butyn-1-yl)-5-[(4-methyl-quinazolin-2-
yl)methyl]-3,5-
dihydro-imidazo[4,5-d]pyridazin-4-one
(34) 2-[N-(azetidin-3-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(35) 2-[N-(2-amino-1,1,2-trimethylprop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-
5-[(3-
methyl-isoquinolin-1-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(36) 2-[(3-methyl-azetidin-3-yl)amino]-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-yl)-
methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(37) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-methyl-
isoquinolin-1-yl)methyl]-3, 5-d ihyd ro-imidazo[4, 5-d]pyridazin-4-one
(38) 2-[N-(2-amino-1-methyl-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-
methyl-isoquinolin-1-yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(39) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-cyano-
pyridin-2-
yl)methyl]-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(40) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(3-cyano-
pyridin-2-
yl)methyl]-3,5-dihydro-imidazo[4,5-c]pyridin-4-one
(41) 2-[N-(2-aminoethyl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-
yl)methyl]-3,5-dihydro-imidazo[4,5-c]pyridin-4-one
(42) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-[(4-methyl-
quinazolin-2-yl)methyl]-3,5-dihydro-imidazo[4,5-c]pyridin-4-one
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
34
(43) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-
([1,5]naphthyridin-
2-ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
(44) 2-[N-(2-amino-prop-1-yl)-N-methyl-amino]-3-(2-butyn-1-yl)-5-(4,6-
dimethylpyrimi-
din-2-ylmethyl)-3,5-dihydro-imidazo[4,5-d]pyridazin-4-one
Ex. structure Ex. structure
(1) II (2) I w
w w , / o
N
I / / O ~ N I N\ N/
N ~ Nr
N
I /~N/ NHz
Nw N
N HZ
(3) (4) I ~
N
I / O i
N
/ O i N I ~ H
I ~N
N N ~ N
-NH2
NW N
NHZ
(5) II (6) I w
I I
/ / O ~ NYN O i
I\ N
~N I N~N/ N ~ I /~N/
N ~ N
NH2 2
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
(7) i ($) w
O O i O N. I
O i_
N N~ / \ O
I I / N
N\ N ~ N I N~N
NHZ N ~ N
NH2
(9) I w (10) ci I w
N, ~ o
NYN O % N I /~-N
N\ / N~ N
N I // N/ ~NHz
N
NHZ
(11) I ~N (12) ~N
N
NI ~ O i I ~ O i
/~- N/ N \ I /~' N
N ~ N
NHz NHz
(13) ~Nw (14) I w
II /N
N ~ ~
I ~ O i I ~~ O i
/~ N N \ I /~-N
N ~ N
NHZ NHz
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
36
X15) I \ ~o X16) \
\ ~ I\
I _ N /N 0 i
N N O ~N N
N I /~ N~ N I /~ N
w N
N
NHZ
NHZ
(17) ~1$) \
I ~ N I \ // N
N I \ I /N O i
/ O i
N
N N~ /~N
N I /~ N~ N ~ N
N ~ N ~ NHz
NHZ
/ I X20) / I
~o \ ° o ~ \ o
0 0
N
/~---N~ N I /~' N~
N
NH2 NHz
I \ \ X22) I \ \
/ /N O ~ / /N O
N N
N~NHz N \ I /~N/ NHz
N
I \ \ X24) I \ \
/ /N O ~ / /N i
O
~N N ~ ~N N
/~N NHZ N I /~---N NH2
N ~--~ N
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
37
(25) I \ (26) I \
\ c1 \ Br
I
N~N O ~ I N / N O
N N ~ ~ N
/>-N N~ /~N/
N N
NHz NHz
(27) I \ (28) I \
N
\ I \ \\
NI / N O ~ I NI / N O
N ~ N
/>-N/ N I /~-N
N ~ N
z Hz
(29) I \ (3~) I \
\
N /N O \ ~ NI /N O ~ S
N ~ N
/~-N/ N I /~N~
N N
NHz NHz
(31 ) O~ (32) F
F \ F
\ \ I
I / / O ~ F / F
N N ~ \ O i
N~ I N N N I N
NHz N ~ N
I /~-- N
NHz
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
38
(33) I \ (34) I \
I \ I \
N~N O i N ~N O i
N N ~ N
/~-N N \ I />-N
N ~ N ~NH
H
X35) ~ \ \ (36) I \
/ ~N O i \
N N ~ NI / N
O i
N \ I N>-N NHZ
N N
N ~ I /~-
N
N
H
~3~) I \ \ (3$) I \ \
/ iN O i / ~N O i
N N
I /~--N~ NHz N I /~--N~ NHz
Nw N ~ Nw N
X39) I \ X40) I \
i ~N O i ~ ~N
N, ~ O i
N
I /~ N N I /~ N
N N \ N
NHZ NHZ
~4~ ) I \ X42) I \
\ \
NI ~~
O i N ~N O i
N N ~ ~ N
\ I /~--N N\ I /~--N
N ~ N
NH2 NHZ
Boehringer Ingelheim Case 1/1678
" ' CA 02561210 2006-09-26 foreign filing text
39
(43) I w N (44)
N\ / N
O i
I
N / O ~ N I /~N
N N ~ N~ N
I I /~ N ~ H
N w N 2
NH2
Example 3
Coated tablets containingi 75 mg of active substance
1 tablet core contains:
active substance 75.0 mg
calcium phosphate 93.0 mg
corn starch 35.5 mg
polyvinylpyrrolidone 10.0 mg
hydroxypropylmethylcellulose 15.0 mg
magnesium stearate 1.5 mg
230.0 mg
Preparation:
The active substance is mixed with calcium phosphate, corn starch, polyvin-
ylpyrrolidone, hydroxypropylmethylcellulose and half the specified amount of
magnesium stearate. Blanks about 13 mm in diameter are produced in a tablet-
making machine and these are then rubbed through a screen with a mesh size of
1.5
mm using a suitable machine and mixed with the rest of the magnesium stearate.
This granulate is compressed in a tablet-making machine to form tablets of the
desired shape.
weight of core: 230 mg
die: 9 mm, convex
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
The tablet cores thus produced are coated with a film consisting essentially
of
hydroxypropylmethylcellulose. The finished film-coated tablets are polished
with
beeswax.
Weight of coated tablet: 245 mg.
5
Example 4
Tablets containing 100 mg of active substance
10 Composition:
1 tablet contains:
active substance 100.0 mg
lactose 80.0 mg
corn starch 34.0 mg
15 polyvinylpyrrolidone 4.0 mg
magnesium stearate 2.0 mg
220.0 mg
Method of Preparation:
The active substance, lactose and starch are mixed together and uniformly
moistened
with an aqueous solution of the polyvinylpyrrolidone. After the moist
composition has
been screened (2.0 mm mesh size) and dried in a rack-type drier at 50°C
it is
screened again (1.5 mm mesh size) and the lubricant is added. The finished
mixture
is compressed to form tablets.
Weight of tablet: 220 mg
Diameter: 10 mm, biplanar, facetted on both sides and notched on one side.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
41
Example 5
Tablets containin 1q 50 ma of active substance
Composition:
1 tablet contains:
active substance 150.0 mg
powdered lactose 89.0 mg
corn starch 40.0 mg
colloidal silica 10.0 mg
polyvinylpyrrolidone 10.0 mg
magnesium stearate 1.0 mg
300.0 mg
Preparation:
The active substance mixed with lactose, corn starch and silica is moistened
with a
20% aqueous polyvinylpyrrolidone solution and passed through a screen with a
mesh
size of 1.5 mm.
The granules, dried at 45°C, are passed through the same screen again
and mixed
with the specified amount of magnesium stearate. Tablets are pressed from the
mixture.
Weight of tablet: 300 mg
die: 10 mm, flat
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
42
Example 6
Hard Gelatine capsules containing 150 mg of active substance
1 capsule contains:
active substance 150.0 mg
corn starch (dried) approx. 180.0 mg
lactose (powdered) approx. 87.0 mg
magnesium stearate 3.0 mG
approx. 420.0 mg
Preparation:
The active substance is mixed with the excipients, passed through a screen
with a
mesh size of 0.75 mm and homogeneously mixed using a suitable apparatus. The
finished mixture is packed into size 1 hard gelatine capsules.
Capsule filling: approx. 320 mg
Capsule shell: size 1 hard gelatine capsule.
Example 7
Suppositories containinG 150 mg of active substance
1 suppository contains:
active substance 150.0 mg
polyethyleneglycol 1500 550.0 mg
polyethyleneglycol 6000 460.0 mg
polyoxyethylene sorbitan monostearate 840.0 mG
2,000.0 mg
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
43
Preparation:
After the suppository mass has been melted the active substance is
homogeneously
distributed therein and the melt is poured into chilled moulds.
Example 8
Suspension containinc~50 ma of active substance
100 ml of suspension contain:
active substance 1.00 g
carboxymethylcellulose-Na-salt 0.10 g
methyl p-hydroxybenzoate 0.05 g
propyl p-hydroxybenzoate 0.01 g
glucose 10.00 g
glycerol
5.00 g
70% sorbitol solution 20.00 g
flavouring 0.30 g
dist. water ad 100 ml
Preparation:
The distilled water is heated to 70°C. The methyl and propyl p-
hydroxybenzoates
together with the glycerol and sodium salt of carboxymethylcellulose are
dissolved
therein with stirring. The solution is cooled to ambient temperature and the
active
substance is added and homogeneously dispersed therein with stirring. After
the
sugar, the sorbitol solution and the flavouring have been added and dissolved,
the
suspension is evacuated with stirring to eliminate air.
5 ml of suspension contain 50 mg of active substance.
Boehringer Ingelheim Case 1/1678
CA 02561210 2006-09-26 foreign filing text
44
Example 9
Ampoules containing 10 mg active substance
Composition:
active substance 10.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 2.0 ml
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI, made
isotonic with common salt, filtered sterile and transferred into 2 ml
ampoules.
Example 10
Ampoules containing 50 mg of active substance
Composition:
active substance 50.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 10.0 ml
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI, made
isotonic with common salt, filtered sterile and transferred into 10 ml
ampoules.