Note: Descriptions are shown in the official language in which they were submitted.
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
1
COMPOSITIONS AND METHODS FOR TREATMENT OF
NEUROPATHIC PAIN, FIBROMYALGIA AND CHRONIC FATIGUE
SYNDROME
Cross-Reference to Related Application
This application claims the benefit of copending U.S. Provisional
Application Serial No. 60/560,816, filed April 8, 2004 and copending U.S.
Patent Application Serial No. 10/999,737, filed November 30, 2004, the entire
disclosures of which are incorporated herein by reference.
Field of the Inyention
The present invention relates to methods of treatment for neuropathic
pain, fibromyalgia and chronic fatigue syndrome (CFS).
Background of the Invention
A. Tianeptine
Tianeptine, which has the systematic name 7-[(3-chloro-6,11-dihydro-6
methyl-dibenzo[c,fJ [1,2] thiazepin-11-yl) amino] heptanoic acid S,S-dioxide,
is
an antidepressant of the dibenzothiazepine type. Tianeptine is known to have
psychostimulant, antidepressive, analgesic, antitussive, antihistaminic and
gastric antisecretory properties. See, U.S. Pat. No. 3,758,528 of Malen et al.
,
the entire disclosure of which is incorporated herein by reference. Tianeptine
acts as a serotonin reuptake accelerator, in that it increases the presynaptic
uptake of serotonin. A sodium salt of tianeptine is currently marketed over-
the-
counter in Europe under the trademark STABLON~. Tianeptine is used to treat
neurotic or reactive states of depression, angiodepressive states with somatic
complaints such as digestive problems, angiodepressive states observed in
alcoholic detoxification, and asthma. The chemical structure of tianeptine is
given below:
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
2
Tianeptine
COOH
B. Tianeptine Contrasted With Tricyclic Antidepressants
Though tianeptine has been referred to in scientific literature as a
tricyclic antidepressant (TCA), both the chemical structure and the biological
mechanism of action of tianeptine are significantly different from that of
classic
TCAs. Shown below are the chemical structures of classic TCAs. The TCAs
may be divided into secondary amines (desipramine, nortryptyline and
protryptyline) and the tertiary amines (imipramine, amitriptyline and
doxepin).
The TCAs may also be characterized by whether the side chain is attached to
the
ring system via a single bond or by a double bond. However, all classic TCAs
are characterized by an amino-(C3)-alkyl sidechain, which is a significant
departure from the carboxy-(C6)-alkylamino sidechain of tianeptine.
3~2 ~C H 3~
Imipramine Amitryptyline Doxepin
\ 1~ ' \
N ~ ,s
_NHCH3 HCH3 ~NHCH3
Desipramine Nortryptyline Protryptyline
The biological activity of the classic TCAs is significantly different from
that of tianeptine. The classic TCAs act by presynaptically inhibiting
reuptake
of serotonin and norepinephrine. See, The Merck Manual, 16th Edition,
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
3
Treatment of Unipolar and Bipolar Disorders, page 1603-1604, the entire
disclosure of which is incorporated herein by reference. In contrast,
tianeptine
is a serotonin reuptake accelerator. See, Wilde et al., Cli~z.
Neuropha~macol.,
1998, 11, Suppl 2:5, page 74-82, the entire disclosure of which is
incorporated
herein by reference.
C. Known Tianeptine Analgesic Activity
Compounds according to Formula I, as defined herein, are disclosed by
Malen et al. as having been tested for analgesic activity according to the
"hot
plate" test method of Woolfe et al., J. Pha~macol. Exp. They. 80, page 300-
307,
1944, the entire disclosures of which are incorporated herein by reference.
According to the Woolf procedure, a heated platform is maintained at
temperatures of 50 to 70° C. Rats are dropped onto the platform from an
elevation of 5 cm and the latency to emit pain behaviors is recorded. The
upper
temperature limit of 70° C is used to prevent tissue damage to the paws
of
animals. Each trial lasts 30 seconds, and the time interval, termed "latency,"
for
the pain behaviors to occur decreases as the temperature of the hot-plate
increases. In these studies, longer latencies to elicit pain behaviors provide
evidence of drug-induced analgesia.
The "hot plate" test elicits nociceptive pain. Nociceptive pain represents
the healthy pain response to thermal and mechanical challenges that might
result
in tissue damage to an organism. The healthy nociceptive response requires
proper functioning of an intact nervous system.
D. Neurobathic Pain
Neuropathic pain, in contrast to nociceptive pain, comprises a
perturbation of pain signaling pathways resulting from electrophysiological
instability which may be caused by injury to nerve tissue. See, Summer, Curs.
Opih. Neurol., 2003, Oct., 16(5), page 623-628 and Krarup, Cute. Opih.
Neu~ol., 2003, Oct., 16(5), page 603-612, the entire disclosures of which are
incorporated herein by reference.
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
4
Central or peripheral nerve tissue damage may result in heightened
sensitivity to non-noxious stimuli, and/or an exaggerated response to mild to
moderately noxious stimuli. A simple focal peripheral nerve injury may
initiate
a range of peripheral and central nervous system processes that may contribute
to persistent pain and abnormal sensation. See, Dworkin et al., Arch. Neu~ol.,
2003 Nov; 60:1524-34, the entire disclosure of which is incorporated herein by
reference.
The manifestation of neuropathic pain may comprise a number of
positive and negative symptoms. See, Bonica's Management of Pain, 3rd
Edition, ISBN 06833042623. Positive sensory phenomena relate to the
exaggerated perception of stimuli (allodynia, hyperalgesia, hyperpathia),
wherein application of modest stimuli causes the false perception of a
disproportionately large stimuli. Positive motor symptoms include increased
muscle tone, tremor, dystonia, and dyskinesia. Negative sensory phenomena
include an inappropriate response to light touch, vibration, joint position,
pin
prick, or warm/cold application to the affected region. Negative motor
symptoms include hypotonia, decreased muscle strength, and decreased
endurance. The particular profile of positive and negative symptoms often
corresponds to the specific insult to the nervous system.
Various medical conditions and external factors, including diabetes, i. e.,
diabetic neuropathy (DN), hypothyroidism, uremia, nutritional deficiencies,
herpes zoster (shingles), alcoholism, stroke, HIV, multiple sclerosis, cancer
and
exposure to toxic substances, including chemotherapy (primarily chemotherapy
with vincristine, cisplatin, zalcitabine, and paclitaxel) have been associated
with
neuropathic pain. Other acquired and inherited disorders, including Guillain-
Barre syndrome (GBS), postherpetic neuralgia (PHN), Charcot-Marie-Tooth
(CMT~ disease, complex regional pain syndrome type 1 (CRPS-1), ischemic
neuropathy, painful spasticities, and other nervous system disorders that have
pain as an attendant sign and/or symptom may also be associated with
neuropathic pain. See, Carter et al., Physical Medicine ahd Rehabilitatiov~
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
Clzhics of North America 2001 May; 12(2):447-59, the entire disclosure of
which is incorporated herein by reference.
Models for neuropathic pain do not test pain response by a healthy
nervous system. Rather, neuropathic pain models test the abnormal pain
5 response resulting from damaged nerve tissue. One model produces neuropathic
pain in test animals by surgically ligating spinal nerves. See, Chung et al.,
Paih,
50, p 355-363, 1992, the entire disclosure of which is incorporated herein by
reference. The Chung et al. model provides a widely accepted model for
peripheral neuropathic pain in humans. The Chung et al. model detects
antihyperalgesic activity in rats suffering from neuropathic pain by employing
a
surgical procedure to form a spinal nerve ligature.
The spinal nerve ligature produces a constriction injury that serves to
model the perturbations associated with peripheral neuropathic injury in a
mammal. Specifically, the phenomena of thermal hyperalgesia, cold allodynia,
and tactile allodynia manifest themselves. Subsequent to the surgery to create
the constriction injury, the rats are challenged with thermal and mechanical
stinuli to determine the degree of sensitivity. Demonstration of an ability to
decrease the abnormal pain sensitivity effected by the spinal nerve ligature
is
predictive of an agent's potential efficacious treatment of neuropathic pain.
E. Fibrom, ay l~ia
Fibromyalgia is a syndrome which is a frequent cause of chronic,
widespread pain and is estimated to affect 2-4% of the population.
Fibromyalgia and CFS are thought to be related. See, Kranzler et al., LJS
Patent
6,635,675, the entire disclosure of which is incorporated herein by reference.
However, the symptom profile of fibromyalgia differs from that of CFS, i.e.,
pain is the major symptom reported in fibromyalgia while fatigue is the major
symptom reported in CFS.
Fibromyalgia is characterized by a generalized heightened perception of
sensory stimuli. Patients with fibromyalgia display abnormalities in pain
perception in the form of both allodynia (pain with innocuous stimulation) and
hyperalgesia (increased sensitivity to painful stimuli). Clinically,
fibromyalgia is
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
6
characterized by general aches or stiffness, primarily musculoskeletal in
origin,
involving three or more anatomical sites for at least three months and at
least six
typical and reproducible tender points. Other associated symptoms of
fibromyalgia include fatigue, nonrestorative sleep and memory difficulties.
Fibromyalgia is likely to be caused by dysfunction of various
components of the central nervous system. See, Yunus, J. Rheumatol., 1993,
19, page 846-850, the entire disclosure of which is incorporated herein by
reference. Evidence has accumulated that aberrant function of the autonomic
nervous system, and in particular the sympathetic nervous system, is
responsible
for the symptoms of fibrornyalgia.
Abnormal findings in fibromyalgia patients strongly indicate a
neuropathic pain syndrome, reminiscent of complex regional pain syndrome or
postherpetic neuralgia. In addition, fibromyalgia seems to share similar
characteristics with thes a neuropathic pain syndromes, including ineffective
response to many analgesics. See, Staud, Paih Med., Sept., 2(3), 208-15
(2001),
the entire disclosure of which is incorporated herein by reference.
F. Chronic Fatigue Syndrome
CFS is a disorder characterized by fatigue of an incapacitating nature
lasting at least six months. As stated above, CFS and fibromyalgia, though
manifesting different symptom profiles, are believed to be related. CFS
symptoms include, but are not limited to, mild fever or chills, sore throat,
painful lymph nodes, unexplained general muscle weakness, myalgias,
prolonged generalized fatigue after exercise previously tolerated, generalized
headaches, migratory arthralgias, neuropsychotic complaints, sleep
disturbance,
and description of a main symptom complex developing over a few hours to a
few days.
CFS diagnostic criteria have been established by the U.S. Centers for
Disease Control and Prevention. The diagnostic criteria include medically
unexplained fatigue of at least six months duration that is of new onset, not
a
result of ongoing exertion and not substantially alleviated by rest, and a
substantial reduction in previous levels of activity. In addition, diagnosis
of
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
7
CFS involves the determination of the presence of four or more of the
following
symptoms: subjective memory impairment, tender lymph nodes, muscle pain,
joint pain, headache, unrefreshing sleep, and postexertional malaise lasting
more
than 24 hours. See, Reid et al., 2000, British Medical Jourf~al 320, page 292
296, the entire disclosure of which is incorporated herein by reference.
Fibromyalgia and CFS have been treated with the same medications.
Some medications currently employed to treat CFS and/or fibromyalgia include,
but are not limited to, analgesics, hypnotics, and immune suppressants. Though
numerous agents are used to treat fibromyalgia and CFS patients, no single
pharmacological agent or combination of agents has been demonstrated to be
effective in the treatment of either.
Agents presently used to treat neuropathic pain, fibromyalgia and CFS
are not always effective. Some may produce serious side effects. Some, such as
opioid analgesics, may have serious addictive liability. There is a need for
agents which are effective in treating neuropathic pain, fibromyalgia and CFS,
as well as pain attendant to nervous system disorders. In particular, there is
a
need for agents with few side effects and low liability for addiction, that
are
appropriate for long-term use in treatment and prevention of these disorders.
Summary of the Invention
Compounds of Formula I can prevent or alleviate symptoms of
neuropathic pain, fibrornyalgia and CFS.
According to one embodiment of the invention, a method of treating
neuropathic pain, in a subject in need of such treatment, comprises
administering an effective amount of at least one compound according to
Formula I, or a pharmaceutically acceptable salt thereof, to a subject in need
of
such treatment;
wherein administration of the compound is by enteral administration or
by parenteral administration, wherein the parenteral administration is
selected
from the group consisting of intravenous, intramuscular, intraarterial,
intraperitoneal, intravaginal, intravesical, intradermal and subcutaneous
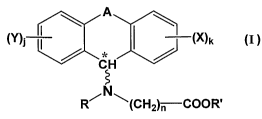
administration. Formula I is:
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
8
(Y) (X)k I
R~ ~(CH~)" COOR'
wherein:
A is diradical selected from the group consisting of -(CH2)m ,
-CH=CH-, -(CH2)pO-, -(CH~)pS-, -(CH2)pSO2-, -(CH2)pNRI- arid
-SOZNR2-; wherein:
m is 1, 2 or 3; preferably 1 yr 2;
p is 1 or 2; preferably 1;
Rl is selected from the group consisting of hydrogen and C1-Cs
alkyl; and
RZ is C1-Cs alkyl;
X and Y are independently selected from halogen;
j and k are integers independently selected from the group consisting of
0, l and 2; preferably 0 and 1;
R and R' are independently sele cted from the group consisting of
hydrogen and C1-Cs alkyl;
n is an integer from 1 to 12 inclusive, preferably from 2 to 10 inclusive,
most preferably from 4 to 8 inclusive; and
* denotes an asymmetric carbon and the bond designated by ~
indicates that the absolute conformation a..bout the asymmetric carbon may be
either (R) or (~ when all four groups attached to the asymmetric carbon are
nonequivalent.
According to one sub-embodiment the compound of Formula I is
administered parenterally by a route that is selected form the group
consisting of
intravenous, intramuscular, intraarterial, intraperitoneal, intravaginal,
intravesical, intradermal and subcutaneous administration.
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
9
According to another sub-embodiment the c ompound is administered by
enteral administration.
According to another embodiment of the invention, a method of treating
fibromyalgia or CFS, in a subject in need of such treatment, comprises
administering an effective amount of at least one compound according to
Formula I, or a pharmaceutically acceptable salt thereof, to a subject in need
of
such treatment.
According to one preferred embodiment of compounds according to
Formula I for therapeutic administration, A is -SOZ-NR2-, and/or j and k are
0.
The invention is also directed to the use of a compound according to
Formula I, or a pharmaceutically acceptable salt thereof, for preparation of a
medicament for the treatment of fibromyalgia or chronic fatigue syndrome.
The invention is further directed to the use of a compound according to
Formula I, or a pharmaceutically acceptable salt thereof, for preparation of a
medicament for enteral administration for the treatment of neuropathic pain.
The invention is also directed to the use of a compound according to
Formula I, or a pharmaceutically acceptable salt thereof, for preparation of a
medicament for parenteral administration for the treatment of neuropathic
pain;
wherein the parenteral administration is selected from the group
consisting of intravenous, intramuscular, intraarterial, intraperitoneal,
intravaginal, intravesical, intradermal and subcutaneous administration.
Definitions
The term "alkyl", by itself or as part of another substituent means a
straight, branched or cyclic chain hydrocarbon radical, including di- and
multi-
radicals, having the number of carbon atoms desigriated (i.e. C1-CS means one
to
five carbons). Straight chain alkyl groups are preferred. Examples of alkyl
groups include: methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tent-butyl,
cyclopropylmethyl, pentyl, cyclopentyl and neopentyl.
The term "halogen" means iodine, fluorine, chlorine and bromine atoms.
Preferred halogens are fluorine, chlorine and bromine atoms.
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
As used herein, "optically active" refers to a property whereby a material
rotates the plane of plane-polarized light. A compound that is optically
active
has a chemical structure which is nonsuperimposable on its mirror image. As
used herein, the property of nonsuperimposability of an object on its mirror
5 image is called "chirality." The most common structural feature producing
chirality is an asymmetric carbon atom; i. e., a carbon atom having four
nonequivalent groups attached thereto.
As used herein, "enantiomer" refers to each of the two
nonsuperimposable isomers of a pure compound thart is optically active. Single
10 enantiomers are designated according to the Cahh-I~gold Prelog system,
which
is a well-known set of priority rules for ranking the four groups attached to
an
asymmetric carbon. See, e.g., March, Advanced Organic Chemistry, 4th Ed.,
(1992), p. 109, the entire disclosure of which is herein incorporated by
reference. For example, once the priority ranking of the four groups attached
to
an asymmetric carbon of a molecule is determined, the molecule is oriented so
that the lowest ranking group is pointed away from the viewer. If the
descending rank order of the other groups proceeds clockwise, the molecule is
designated (R). If the descending rank of the other groups proceeds
counterclockwise, the molecule is designated (S). In the example below, the
Cah~-Ingold Prelog ranking sequence is A > B > C > D. The lowest ranking
atom, D is oriented away from the viewer.
A A
.",wv D .,",,w D
C B B C
(R) configuration (S7 configuration
As used herein, "racemate" or "racemic compound" refers to a 50-50
mixture of two enantiomers of a compound such that the mixture does not rotate
plane-polarized light.
By "(R)-enantiomer substantially free of the (~-enantiomer" is meant a
compound that comprises 80% or more by weight of the (R)-enantiomer, and
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
11
likewise contains 20% or less by weight of the (S~-enantiomer as a
contaminant.
By "(S~-enantiomer substantially free of the (R)-enantiomer" is meant a
compound that comprises 80% or more by weight of the (~-enantiomer, and
likewise contains 20% or less by weight of the (R)-enantiomer as a
contaminant.
Detailed Descriution of the Invention
The compounds of Formula I may be readily prepared by known
methods. For example, compounds of Formula I may be prepared according to
Scheme 1, Step B by condensation of the halogenated derivative of Formula IB
with an aliphatic w-amino ester of Formula IC. The reaction of Step B of
Scheme 1 is preferably carried out in a suitable solvent in the presence of a
suitable acid scavenger. Suitable solvents include acetonitrile, dimethyl-
formamide (DMF) and nitromethane. Suitable acid scavengers include tertiary
amines, aromatic amines such as pyridine, and inorganic bases such as alkali
metal and alkaline earth metal carbonates or bicarbonates. The acid scavenger
may comprise an excess of the aliphatic w-amino ester of Formula IB. Some
suitable acid scavengers, such as triethyl amine or pyridine may also serve as
the
solvent for the reaction of Step B. Compounds according to Formula I that are
esters may then be hydrolyzed to yield compounds of Formula I that are
carboxylic acids. The hydrolysis reaction is preferably carried out in a
suitable
aqueous solvent using either an acid or a base. Suitable aqueous solvents
include water and mixtures of water with at least one water-miscible organic
solvent such as an aliphatic alcohol, acetonitrile, tetrahydrofuran or
acetone.
A A
)j i / ( _ (X)k (Y)i i / ~ _ (X)k R~N~ COOR'
CH ~ CH .~ Ic(CHz n
IA pH Step A IB halogen
step B
A
)~ i / ~ _ (X)k 1
CH
COOR'
R~ ~(CH2 ~
Scheme 1
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
12
As shown in Scheme 1, Step A, halogenated derivatives of Formula IB
may be prepared by reaction of a hydroxy derivative of Formula IA with a
suitable halogenating reagent. The reaction of Step A is preferably carried
out
in a suitable solvent such as, for example, dichloromethane (DCM), chloroform,
acetonitrile or THF. Suitable halogenating reagents include for example, dry
HCL, and thionyl chloride.
Suitable synthetic methods are found, for example, in U.S. Pat. Nos. 4,
766,114, 3,758,528 and 3,821,249, all of Malen et al., and U. S. Pat. No.
6,441,165 of Blanchard et al., the entire disclosures of which are herein
incorporated by reference.
Certain compounds of Formula I, such as tianeptine (see, Formula II,
below), possess an asymmetric carbon. The position of the asymmetric carbon
is denoted by an asterisk (*) in Formula I. This carbon is asymmetric when
four
nonequivalent groups are attached to it. One skilled in the art can readily
determine which compounds of Formula I possess an asymmetric carbon.
Those compounds of Formula I which have an asymmetric carbon at the
position marked by an asterisk may exist as (R) and (S~ enantiomers _
Typically,
the (R) and (S~ enantiomers of a given compound of Formula Z exist as a
racemate. In the practice of the present invention, both racemates arid
individual
(R) or (S7 enantiomers of a compound of Formula I can be used to treat
neuropathic pain, fibromyalgia or CFS. According to certain embodiments of
the invention, an (R)-enantiomer of a compound of Formula I which is
substantially free of the corresponding (S~-enantiomer, or an (S~-enantiomer
of a
compound of Formula I which is substantially free of the corresponding (R)-
enantiomer, is used to treat neuropathic pain, fibromyalgia or CFS.
To isolate the individual (R)- and (S~-enantiomers of a compound of
Formula I, the racemate of that compound must be resolved. This resolution
may be achieved by converting a racemic compound of Formula I into a pair of
diastereomers, for example by covalently bonding to an optically active moiety
or by salt formation with an optically active base or acid. Either method
provides a molecule with a second chiral center, thus generating a pair of
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
13
diastereomers. The diastereomeric pair may then be separated by conventional
methods, such as crystallization or chromatography.
For example, racemic compounds of Formula I may be converted to the
(S)-dibenzoyltartaric acid salt, which is a diastereomeric mixture of (S,S)
and_
(R,S) configurations. The pairs of diastereomers (R,S) and (S,S) possess
different properties (e.g., differential solubilities) and may thereby be
separated
by conventional separation methods. Fractional crystallization of
diastereomeric salts from a suitable solvent is one such separation method.
Racemic compounds of Formula I may be separated into enantiomers
without diastereomer formation, for example, by differential absorption on a_
chiral stationary phase of a chromatography (e.g., HPLC) column. Preparative:
HPLC columns suitable for diastereomer separation are commercially available
with a variety of packing materials to suit a broad range of separation
applications. Stationary phases suitable for resolving racemic compounds of
Formula I include:
(i) macrocyclic glycopeptides, such as silica-bonded vancomycin which
contains 18 chiral centers surrounding three pockets or cavities;
(ii) chiral a1-acid glycoprotein;
(iii) human serum albumin; and
(iv) cellobiohydrolase (CBH).
Chiral al-acid glycoprotein is a highly stable protein immobilized onto
spherical silica particles that tolerates high concentrations of organic
solvents,
high and low pH, and high temperatures. Human serum albumin is especially
suited for the resolution of weak and strong acids and zwitterionic and
nonprotolytic compounds, but is also used to resolve basic compounds. CBH is
a very stable enzyme that that is typically immobilized onto spherical
silica..
particles for separating enantiomers of basic drugs from many compound
classes.
Other chromatographic techniques suitable for resolving racemic
compounds of Formula I include chiral chromatography using macrocyclic
glycopeptide as a stationary phase on a Chirobiotic VT"" column (ASTEAC~
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
14
Whippany, NJ) as described in U.S. Pat. No. 6,080,736, the entire disclosure
of
which is herein incorporated by reference, and chiral chromatography using a
chiral al-acid glycoprotein as a stationary phase on a CHIRAL-AGPT"" column
(ChromTech, Cheshire, UK), as described in Fitos et al. (J. Chr~omatogr.,
1995,
709:265, the entire disclosure of which is herein incorporated by reference.
A preferred compound of Formula I for use in the present methods is
tianeptine, or a pharmaceutically acceptable salt thereof. The structure of
tianeptine is given in Formula II:
a
HN
COOH
wherein:
* denotes an asymmetric carbon; and
the bond designated by ~ indicates that the absolute conformation
about the asymmetric carbon may be either (R) or (S~.
Tianeptine may be readily obtained by one of ordinary skill in the art, for
example by the synthetic techniques described above. Tianeptine is also sold
commercially under the trademark STABLON~.
The (R) or (~ enantiomers of tianeptine may be isolated, for example, by
the techniques discussed above. Thus, in preferred embodiments of the present
invention, the (R)-enantiomer of tianeptine which is substantially free of the
corresponding (S~-enantiomer, or the (~-enantiomer of tianeptine which is
substantially free of the corresponding (R)-enantiomer, is used in the present
methods.
In the practice of the invention, the compounds of Formula I described
above may take the form of a pharmaceutically-acceptable salt. The term
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
"salts", embraces salts commonly used to form alkali metal salts and to form
addition salts of free acids or free bases.
For example, pharmaceutically-acceptable acid addition salts may be
prepared from an inorganic acid or from an organic acid. Suitable inorganic
5 acids include hydrochloric, hydrobromic, hydroiodic, nitric, carbonic,
sulfuric
and phosphoric acid. Suitable organic acids include aliphatic, cycloaliphatic,
aromatic, araliphatic, heterocyclic, carboxylic and sulfonic classes of
organic
acids, such as formic, acetic, propionic, succinic, glycolic, gluconic,
lactic,
malic, tartaric, citric, ascorbic, glucuronic, malefic, fiunaric, pyruvic,
aspartic,
10 glutamic, benzoic, anthranilic, mesylic, salicylic, 4-hydroxybenzoic,
phenylacetic, mandelic, embonic (pamoic), methanesulfonic, ethanesulfonic,
benzenesulfonic, pantothenic, 2-hydroxyethanesulfonic, toluenesulfonic,
sulfanilic, cyclohexylaminosulfonic, stearic, alginic, beta-hydroxybutyric,
galactaric and galacturonic acid.
15 Suitable pharmaceutically acceptable base addition salts of the
compounds of Formula I, include metallic salts made from calcium, magnesium,
potassium, sodium and zinc, or organic salts made from N,N'-
dibenzylethylenediamine, chloroprocaine, choline, diethanolamine,
ethylenediamine, meglumine (N methylglucamine) and procaine. All of these
salts may be prepared by conventional means from the corresponding compound
of Formula I by reacting, for example, the appropriate acid or base with the
compound of Formula I.
The compounds of Formula I, in particular tianeptine, can be used to
treat neuropathic pain, fibromyalgia or CFS in a subject who has been
diagnosed
with either disorder. As used herein, a "subject" is includes humans and non-
human mammals. Non-human mammals include bovines, ovines, porcines,
equines, canines, felines, and rodents (e.g., rat, mouse, guinea pig and
rabbit).
Preferably, the subject is a human.
Treatment of neuropathic pain, fibromyalgia or CFS may refer to
administration of at least one compound according to Formula I to a subject
who
has been diagnosed with such a disorder. Treatment may also refer to
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
16
prophylactic administration to prevent neuropathic pain, fibromyalgia or CFS
in
a subject who is at risk of developing one or more of the disorders. Treatment
also includes administration of a compound according to Formula I to a subject
reporting one or more of the physiological symptoms of neuropathic pain,
fibromyalgia or CFS, even when the diagnosis thereof has not yet been made.
In the practice of the invention, neuropathic pain, fibromyalgia and CFS
are treated by administering an effective amount of at least one compound of
Formula I to a subject in need of such treatment.
Treatment according to the present invention may comprise
preventing, eradicating or ameliorating the neuropathic pain, fibromyalgia or
CFS. Alternately, treatment according to the present invention may comprise
preventing, eradicating or ameliorating one or more of the symptoms associated
with neuropathic pain, fibromyalgia or CFS. For example, treatment of a
subject suffering from neuropathic pain is accomplished not only when the
underlying neuropathic pain is prevented, eradicated or ameliorated, but also
when at least one symptom of the disorder is improved. Treatment may thus be
accomplished, for example, when the subject reports decreased severity,
duration, or recurrence of pain, a reduction in the number of anatomical sites
affected by pain, or an improvement in abnormally heightened sensitivity to
normally non-noxious stimuli.
Depression is often reported in subjects suffering from neuropathic
pain, fibromyalgia or CFS, and has been characterized by some health care
professionals as a symptom associated with these disorders. Tianeptine is
known in the art to be useful as an antidepressant. Accordingly, treatment of
a
subject suffering from neuropathic pain, fibromyalgia or CFS with one or more
compounds according to Formula I that causes an improvement solely in
depression but not in at least one of the physiological symptoms associated
with
the disorder is neither contemplated by, nor considered effective for purposes
of
the present invention.
As used herein, an "effective amount" of a compound of Formula I used
to treat fibromyalgia refers to the amount of the compound that prevents or
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
17
alleviates one or more symptoms of fibromyalgia. Similarly, an "effective
amount" of a compound of Formula I used to treat neuropathic pain refers to
the
amount of the compound that prevents or alleviates the neuropathic pain.
Likewise, an "effective amount" of a compound of Formula I used to treat CFS
refers to the amount of the compound that prevents or alleviates one or more
symptoms of CFS. A physician can readily determine when symptoms of
neuropathic pain, fibromyalgia or CFS are prevented or alleviated, for example
through clinical observation of a subject, or through reporting of symptoms by
the subject during the course of treatment.
One skilled in the art can readily determine an effective amount of a
compound of Formula I to be administered, by taking into account factors such
as the size, weight, age and sex of the subject, the extent of disease
penetration
or persistence and severity of symptoms, and the route of administration.
Generally, an effective amount of the compounds of Formula I administered to a
subject is from about 2 to about 100 mg per day, preferably from about 5 to
about 60 mg per day, and more preferably about 30 mg per day. Higher or
lower doses are also contemplated.
The compounds of Formula I may be administered to a subject by any
route, for example by enteral (e.g., oral, rectal, intranasal, etc.) and
parenteral
administration. Parenteral administration includes, for example, intravenous,
intramuscular, intraarterial, intraperitoneal, intravaginal, intravesical
(e.g., into
the bladder), intradermal, topical or subcutaneous administration. Also
contemplated within the scope of the invention is the instillation of the
compounds of Formula I into the body of the subject, for example in a
controlled release formulation, with systemic or local release of the compound
to occur over time or at a later time. According to some preferred
embodiments,
the compound of Formula I is localized in a depot for controlled release to
the
circulation or to a local site such as the gastrointestinal tract.
In the practice of the present methods, compounds of Formula I may be
administered in the form of a pharmaceutical composition comprising at least
one compound of Formula I and a pharmaceutically acceptable carrier.
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
18
Pharmaceutical formulations of the invention may comprise from 0.1 to 99.99
weight percent of at least one compound of Formula I. The pharmaceutical
compositions of the invention may be formulated according to standard
practices in the field of pharmaceutical preparations. See, Alphonso Gennaro,
ed., Remin~ton's Pharmaceutical Sciences, 18th Ed., (1990) Mack Publishing
Co., Easton, PA. Suitable dosage forms may comprise, for example, tablets,
capsules, solutions, parenteral solutions, troches, suppositories, or
suspensions.
By "pharmaceutically acceptable carrier" is meant any diluent or
excipient that is compatible with the other ingredients of the formulation,
and
which is not deleterious to the recipient. The pharmaceutically acceptable
carrier may be selected on the basis of the desired route of administration,
in
accordance with standard pharmaceutical practices.
Pharmaceutical compositions of the invention for parenteral
administration may take the form of an aqueous or nonaqueous solution,
dispersion, suspension or emulsion. In preparing pharmaceutical compositions
of the invention for parenteral administration, at least one compound of
Formula
I may be mixed with a suitable pharmaceutically acceptable carrier such as
water, oil (particularly a vegetable oil), ethanol, saline solutions (e.g.,
normal
saline), aqueous dextrose (glucose) and related sugar solutions, glycerol, or
glycols such as propylene glycol or polyethylene glycol. Pharmaceutical
compositions of the invention for parenteral administration preferably contain
a
water-soluble salt of at least one compound of Formula I. Stabilizing agents,
antioxidant agents and preservatives may also be added to the pharmaceutical
compositions for parenteral administration. Suitable antioxidant agents
include
sulfite, ascorbic acid, citric acid or salts thereof, and
ethylenediaminetetraacetic
acid (EDTA) or a salt thereof. Suitable preservatives include benzalkonium
chloride, methyl- or propyl-paraben, and chlorbutanol.
In preparing pharmaceutical compositions of the invention for oral
administration, at least one compound of Formula I may be combined with one
or more solid or liquid inactive ingredients to form tablets, capsules, pills,
powders, granules or other suitable oral dosage forms. For example, at least
one
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
19
compound of Formula I may be combined with at least one pharmaceutically
acceptable carrier such as a solvent, filler, binder, humectant,
disintegrating
agent, solution retarder, absorption accelerator, wetting agent absorbent or
lubricating agent. In one embodiment, at least one compound of Formula I is
combined with carboxymethylcellulose calcium, magnesium stearate, mannitol
and starch, and is formed into tablets by conventional tableting methods. In a
preferred embodiment, tianeptine is formulated into a tablet comprising
cellulose and a calcium salt, as described in U.S. Pat. No. 5,888,542, the
entire
disclosure of which is herein incorporated by reference.
Pharmaceutical compositions of the invention may also be formulated so
as to provide controlled-release of at least one compound of Formula I upon
administration of the composition to a subject. Preferably, a controlled-
release
pharmaceutical composition of the invention is capable of releasing at least
one
compound of Formula I into a subject at a desired rate, so as to maintain a
substantially constant pharmacological activity for a given period of time.
Formulation of controlled-release pharmaceutical compositions of the
invention is within the skill in the art. Controlled release formulations
suitable
for use in the present invention are described in, for example, U.S. Pat. Nos.
5,674,533 (liquid dosage forms), 5,059,595 (gastro-resistant tablet),
5,591,767
(liquid reservoir transdermal patch), 5,120,548 (device comprising swellable
polymers), 5,073,543 (ganglioside-liposome vehicle), 5,639,476 (stable solid
formulation coated with a hydrophobic acrylic polymer), the entire disclosures
of which are herein incorporated by reference.
Biodegradable microparticles may also be used to formulate controlled
release pharmaceutical compositions suitable for use in the present invention,
for example as described in U.S. Pat. Nos. 5,354,566 and 5,733,566, the entire
disclosures of which are herein incorporated by reference.
In one embodiment, controlled-release pharmaceutical compositions of
the invention comprise at least one compound of Formula I and a controlled
release component. As used herein, a "controlled-release component" is a
compound such as a polymer, polymer matrix, gel, permeable membrane,
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
liposome and/or microsphere that induces the controlled-release of the
compound of Formula I into the subject upon exposure to a certain
physiological
compound or condition. For example, the controlled-release component may be
biodegradable, activated by exposure to a certain pH or temperature, by
5 exposure to an aqueous environment, or by exposure to enzymes. An example
of a controlled-release component which is activated by exposure to a certain
temperature is a sol-gel. In this embodiment, at least one compound of Formula
I is incorporated into a sol-gel matrix that is a solid at room temperature.
This
sol-gel matrix is implanted into a subject having a body temperature high
10 enough to induce gel formation of the sol-gel matrix, thereby releasing the
active ingredient into the subject.
The practice of the invention is illustrated by the following non-limiting
example.
Example 1: Neuropathic Pain - Thermal Stimulation Model in the Rat
15 A. Overview of the Neuropathic Pain Model
The effects of tianeptine on neuropathic pain were investigated by the
method of Chung et al., Pain, 50, 355-363 (1992). The Chung et al. model
detects antihyperalgesic activity in rats suffering from neuropathic pain by
employing a surgical spinal nerve ligature and thermal stimulation to induce
20 thermal hyperalgesia.
The nerve ligature produces a constriction injury that causes a distal
perturbation in the pain signal pathways associated with the ipsilateral hind
paw.
Subsequent to the surgery, the rats are challenged with thermal stimuli to
determine the degree of sensitivity in the target hindpaw. Test compounds that
may be effective at treating neuropathic pain demonstrate an ability to
decrease
the pain sensitivity within the ipsilateral paw.
B. Animals and Test Substances
Forty male Rj: Wistar (Han) rats, 278-367 g body weight (weight at first
experiment on testing day), were used in the study. The rats were divided into
five treatment groups of 8 rats. The treatment groups received the following:
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
21
(a) Tianeptine, dispersed in carboxymethyl cellulose (CMC) (obtained
from Cooperation Pharmaceutique Fran~aise (CPF)) (1% in distilled water) at a
dose of 3.0 mg/kg, P.O.;
(b) Tianeptine, as above, 10 mg/kg, P.O.;
(c) Tianeptine, as above, 30 mg/kg, P.O.;
(d) Morphine (obtained from CPF), dissolved in distilled water, at a
dose of 128 mg/kg, P.O.; and
(e) Vehicle, 0.2% hydroxypropylmethylcellulose (HPMC) (obtained
from CPF), dissolved in distilled water.
C. Procedure for the Surgical Phase of the AssaX
The rats were anesthetized (sodium pentobarbital, obtained from Ceva
Sante Animale, at a dose of 50 mg/kg i.p.). An incision at the L4-S2 level was
performed to expose the left LS and L6 spinal nerves. A ligature was tied
tightly around each nerve. The wound was then sutured. The rats received an
i.m. injection of 50 000 IU Penicillin G and were allowed to recover. Three
weeks after the surgery, when the chronic neuropathic pain state was fully
installed, rats were submitted to thermal stimulation of both the non-lesioned
and the lesioned hindpaws.
D. Procedure for the Experimental Phase of the Assay
The experimental phase of the assay began three weeks after the surgery
phase of the model was completed. The neuropathic pain assay comprised
thermal stimulation of both the lesioned and unlesioned hindpaws of the rats.
The experiment also included tactile stimulation of the rat hindpaws. However,
the test compounds did not demonstrate activity versus tactile stimulation.
The neuropathic pain assay was performed as four separate experiments
which assessed the pain response at 30 minutes, 1 hour, 2 hours and 4 hours
following administration of the test substance. The four experiments were
performed at 3, 4, 5 and 6 weeks following the surgical phase of the model.
For
each of the four experiments, each test substance was administered P.O., in a
volume of 10 mL/kg of body weight.
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
22
The same rats were used for each of the four experiments. In each of the
four experiments, prior to administration of a test substance, the rats were
submitted to tactile stimulation and assigned to treatment groups matched on
the
basis of their pain response to tactile stimulation. The assignment of rats to
treatment groups was done in this manner independently for each of the four
experiments.
E. Apparatus and Procedure for Tactile Stimulation.
For tactile stimulation, the rat was placed under an inverted Plexiglas
box (17 x 11 x 14 cm) on a grid floor. The tip of an electronic Von Frey probe
(Model 1610 obtained from Bioseb BP 89 92370 Chaville, France) was then
applied with increasing pressure to the non-lesioned and lesioned hindpaws.
The force required to induce paw-withdrawal was automatically recorded. The
tactile stimulation procedure was carried out 3 times for each paw. The mean
force per paw was calculated to provide basic scores for each rat.
F. Apparatus and Procedure for Thermal Stimulation.
The apparatus employed for thermal stimulation consisted of 6
individual Plexiglas boxes (17 x 11 x 14 cm) placed upon an elevated glass
floor
(Model 7371, obtained from Ugo Basile Biological research apparatus Via G.
Borghi 4321025 Comerio VA - Italy). For thermal stimulation, a rat was
placed in the box and left free to habituate for 10 minutes. A mobile infrared
radiant source was then focused under the non-lesioned and lesioned hindpaws.
Paw-withdrawal by the rat interrupts the reflected radiation and switches off
the
counter and the light source, thereby automatically recording the paw-
withdrawal latencies. In the event that no reaction was elicited, the test was
terminated after 45 seconds to prevent tissue damage.
G. Results
The assay results are presented in Tables 1 and 2. The results represent
the mean ~ the standard error of the mean (SEM) for each treatment group of
eight rats. The data represent the percent change from the vehicle control.
Statistical method and significance is indicated in Table 1. All statistical
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
23
calculations were performed using commercial software (Microsoft Excel~, GB
StatC~ version 6.5), and verified test-by-test according to Porsolt & Partners
Pharmacology's standardized internal procedures. All differences were
considered statistically significant when the null hypothesis could be
rejected at
the risk a inferior to 0.05.
H. Conclusion
The data in Table 1 show that tianeptine produces a significant iricrease
in paw withdrawal latency after thermal stimulation in the lesioned pave at 30
minutes at a dose of 30 mg/leg. This response is predictive of activity of the
compound versus symptoms of neuropathic pain, fibromyalgia and CFS.
All references cited herein are incorporated by reference. The present
invention may be embodied in other specific forms without departing from the
spirit or essential attributes thereof and, accordingly, reference should be:
made
to the appended claims, rather than to the foregoing specification, as
indication
the scope of the invention.
CA 02562078 2006-10-05
WO 2005/099714 PCT/US2005/011712
24
o ~ ~ O ~ N .~N ~ O
~
~ M
U
d w n M N O r"''
O _ N ~ O O
N
~t ~ O d; d; Cy o0
p, O O O O O
V
vo d;~ Wit;~ oo~ t~~ ~,
., ~i ~ d-..mao.cSri..flv,.fl~--' II
.~ ~ .~ .r .~ o
-H -H -H -H -H 0
l~ ~' ~ ~ ~ M C/~~ C/~O dF
.~~ z N z ~,z N z v
0
I~ o
~ s ~.~ ~ s ~.s .~ s ~, ~. v
~ o ~ ~ ,-~ ~
~ ~ u
U l
p., O
U 00 01 M d' I~
~ ~
0 ~ ~ 0 0
0 0
p, 0 0 0 0 0
a
M ~ ~r-~ O ~ O r~ '1F rr
M ~ ~C,.~l~~ In.~ l0.~
.d .+.~ '-Ha -H ~'I~ .+.I '~'I O O
01 ~ C/~~-' ~ C/~l~C/~
.~
O
N z ~ ~ z ~ z ~ z
,
II v~
z
s ~ ~-~ o
N ~ ~ o ~ ~ M ~ lp~ O ~ V1~ i.~~
~
' + '~ + +
U -~- .N .N
+~ +~
U
~ M
O N 0 Q O
0 1
O M o0 O O
ai p, O O O O O
l0 M V1 M O
M ~ V1..fld'.flt~.flM ~
We ~
-1-I -I-1v -I-Iwow1_1wr -H
N 'X'M ~ ~ ~ O ~ ~n~'
~F r~- r~N
r~-i
N .~
\ \ \ ~ O
\ 00 ~ O ..~0l~_ O ~ ~ rw ~ ~''
~ wr- ..flOv~ 'u
v
~ ~p ~ v + 0 .5~, O
,
-~ -I- O U
, N
~
U ~ O ue ~ ~ ~ U
~
d ,
-
O '' O oho o O ~ O
d
M '~ O O O O O
M ~ d'.~ ~D.flV1~ ~G.fl
b
U ~n 9El~C!~N C/~M aF ~nC!~
CW/~
z ~:z M ~ M z ~,
M N
a~ a~ a~
N ~ >~ ~ ~ O O
A ,4; ~ U
N N
~
t3. ~ ~ M ~
C ~ C ~ cd
O a
ws
c c
d d
C (~ H
~