Note: Descriptions are shown in the official language in which they were submitted.
CA 02565090 2010-02-09
METHOD FOR FORMING A HARDENED SURFACE ON A SUBSTRATE
TECHNICAL FIELD
The invention pertains to metallic coatings and methods of forming metallic
coatings.
BACKGROUND OF THE INVENTION
Steel is a metallic alloy which can have exceptional strength characteristics,
and
which is accordingly commonly utilized in structures where strength is
required or
advantageous. Steel can be utilized, for example, in the skeletal supports of
building
structures, tools, engine components, and protective shielding of modern
armaments.
The composition of steel varies depending on the application of the alloy. For
purposes of interpreting this disclosure and the claims that follow, "steel"
is defined as any
iron-based alloy in which no other single element (besides iron) is present in
excess of 30
weight percent, and for which the iron content amounts to at least 55 weight
percent, and
1
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
carbon is limited to a maximum of 2 weight percent. In addition to iron, steel
alloys can
incorporate, for example, manganese, nickel, chromium, molybdenum, and/or
vanadium.
Steel alloys can also incorporate carbon, silicon, phosphorus and/or sulfur.
However,
phosphorus, carbon, sulfur and silicon can be detrimental to overall steel
quality if present in
quantities greater than a few percent. Accordingly, 'steel typically contains
small amounts of
phosphorus, carbon, sulfur and silicon.
Steel comprises regular arrangements of atoms, with the periodic stacking
arrangements forming 3-dimensional lattices which define the internal
structure of the steel.
The internal structure (sometimes called "microstructure") of conventional
steel alloys is
always metallic and polycrystalline (consisting of many crystalline grains).
Steel is typically formed by cooling a molten alloy. The rate of cooling will
determine
whether the alloy cools to form an internal structure that predominately
comprises crystalline
grains, or, in rare cases, a structure which is predominately amorphous (a so-
called metallic
glass). Generally, it is found that if the cooling proceeds slowly (i.e., at a
rate less than about
104 K/s), large grain sizes occur, while if the cooling proceeds rapidly
(i.e., at a rate greater
than or equal to about 104 K/s) microcrystalline internal grain structures are
formed, or, in
specific rare cases amorphous metallic glasses are formed. The particular
composition of the
molten alloy generally determines whether the alloy solidifies to form
microcrystalline grain
structures or an amorphous glass when the alloy is cooled rapidly. Also, it is
noted that
particular alloy compositions (not iron based) have recently been discovered
which can lead
to microscopic grain formation, or metallic glass formation, at relatively low
cooling rates
(cooling rates on the order of 10 K/s).
2
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Both microcrystalline grain internal structures and metallic glass internal
structures
can have properties which are desirable in particular applications for steel.
In some
applications, the amorphous character of metallic glass can provide desired
properties. For
instance, some glasses can have exceptionally high strength and hardness. In
other
applications, the particular properties of microcrystalline grain structures
are preferred.
Frequently, if the properties of a grain structure are preferred, such
properties will be
improved by decreasing the grain size. For instance, desired properties of
microcrystalline
grains (i.e., grains having a size on the order of 10.6 meters) can frequently
be improved by
reducing the grain size to that of nanocrystalline grains (i.e., grains having
a size on the order
of 10.9 meters). It is generally more problematic to form grains of
nanocrystalline grain size
than it is to form grains of microcrystalline grain size. Accordingly, it is
desirable to develop
improved methods for forming nanocrystalline grain size steel materials.
Further, as it is
frequently desired to have metallic glass structures, it is desirable to
develop methods of
forming metallic glasses.
SUMMARY OF THE INVENTION
In one aspect, the invention encompasses a method of forming a metallic
coating. A
metallic glass coating is formed over a metallic substrate. After formation of
the coating, at
least a portion of the metallic glass can be converted into a crystalline
material having a
nanocrystalline grain size.
In another aspect, the invention encompasses metallic coatings comprising
metallic
glass.
"'3
CA 02565090 2010-02-09
In yet another aspect, the invention encompasses metallic coatings comprising
crystalline metallic material, with at least some of the crystalline metallic
material
having a nanocrystalline grain size.
In yet a further aspect, the present invention resides in a method of forming
a
hardened surface on a substrate, comprising: providing a substrate; and
forming a
molten alloy and cooling said alloy to form a metallic glass coating on the
substrate, the
forming comprising forming a successive buildup of metallic glass layers, the
metallic
glass coating having a hardness of at least about 9.2 GPa and comprising one
or more
materials selected from the group consisting of (Feo.85Cro.i5)83B17,
(Feo.sCro.2)83B17,
(Feo.75Cro.25)83B17, (Feo.6Co0.2Cro.2)83B17, (Feo.8Cro.l5Moo.05)83B17,
(Feo.sCr'0.2)79B17C4, (Feo.8Cr'o.2)79B17Si4, (Feo.sCro.2)79B17A14,
(Feo.sCr'o.2)75B17Al4C4, (Feo.8Cro.2)75B17Si4C4, (Feo.sCro.2)75B17Si4A14,
(Feo.sCr'o.2)71B17Si4C4A14, (Feo.7Coo.1Cro.2)83B17, (Feo.sCro.2)76B17A17, and
(Feo.8Cr'o.2)80B20.
In yet another aspect, the present invention resides in a method of forming a
hardened surface on a substrate, comprising: providing a substrate; forming a
molten
alloy and cooling said alloy to form a metallic glass coating on the substrate
and having
a first hardness of at least about 9.2 GPa, the metallic glass comprising one
or more
materials selected from the group consisting of (Feo.85Cro.15)83B17,
(Feo.8Cro.2)83B17,
(Feo.75Cr'o.25)83B17, (Feo.6Coo.2Cro.2)83B17, (Feo.8Cro.15Mo0.05)83B17,
(Feo.8Cro.2)79B17C4, (Feo.8Cro.2)79B17Sr4, (Feo.8Cro.2)79B17A14,
(Feo.sCr'o.2)75B17Al4C4, (Feo.sCr'o.2)75B17Si4C4, (Feo.8Cro.2)75B17Si4A14,
(Feo.8Cro.2)71B17Si4C4A14, (Fe0.7Coo.iCro.2)83B17, (Feo.sCr'o.2)76B17A17, and
4
CA 02565090 2010-02-09
(Feo.8Cro.2)goB2o; and converting at least a portion of the metallic glass
coating to a
crystalline material having a nanocrystalline grain size and a second hardness
of at least
about 9.2 GPa.
BRIEF DESCRIPTION OF THE DRAWINGS
Preferred embodiments of the invention are described below with reference to
the
following accompanying drawings.
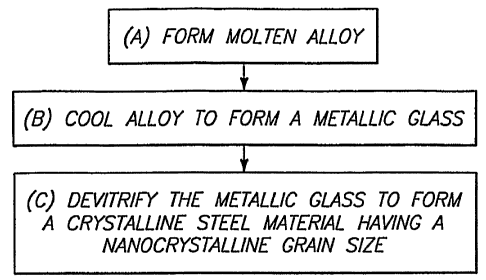
Fig. 1 is a block-diagram flowchart view of a method encompassed by the
present
invention.
Fig. 2 is a diagrammatic perspective view of a barrel being treated according
to a
method of the present invention.
Fig. 3 is a fragmentary, diagrammatic, cross-sectional view of a metallic
material
substrate at a preliminary step of a treatment process encompassed by the
present invention.
Fig. 4 is a view of the Fig. 3 fragment shown at a processing step subsequent
to that
of Fig. 3.
Fig. 5 is a view of the Fig. 3 fi-agment shown at a processing step subsequent
to that
of Fig. 4.
Fig. 6 is a view of the Fig. 3 fi-agment shown at a processing step subsequent
to that
of Fig. 5.
Fig. 7 is an optical micrograph of a metallic glass ribbon formed in
accordance with
methodology of the present invention, and formed from a composition comprising
Fe63Crg M02B17C5S11A14.
4a
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Fig. 8 is a scanning electron microscope micrograph of a cross section of a
gas
atomized powder particle formed in accordance with the present invention, and
formed from
a composition comprising Fe63Cr8Mo2B17C5Si1A14.
Fig. 9 is a graph illustrating the results of a differential thermal analysis
scan of a
ribbon produced in accordance with the present invention. The ribbon was
produced from a
composition comprising Fe63Cr8Mo2B17C5Si1A14. An exothermic glass to
crystallization
transition occurs at 550 C, and an endothermic solid to liquid melting
transition occurs at
1,150 C.
Fig. 10 is a TEM micrograph of a steel alloy produced in accordance with the
methodology of the present invention, and comprising a composition
Fe63Cr8Mo2B17C5Si1Al41
which has been heat treated for 650 C for one hour. A nanoscale nanocomposite
microstructure is visible, with phase sizes from 1 to 75 nanometers.
Fig. 11 illustrates Vickers hardness for different metallic alloys.
Specifically, the
figure compares DAR1 (Fe63Cr8Mo2B17C5SitA14) with DAR20
(Fe64Ti3Cr5Mo2B16C5Si1A12La2). The hardness is compared as a function of heat
treatment
temperature.
Fig. 12 shows examples of Vickers hardness tests using a diamond pyramid
indenter.
Specifically, a top portion of the figure shows the test relative to gas
atomized powder
particles, and a lower portion shows the test utilized for a melt-spun ribbon.
The tested
composition was Fe63Cr8Mo2B17C5Si1A14.
Fig. 13 is an optical micrograph of a steel composition which has been plasma
sprayed
onto a stainless steel substrate. The plasma-sprayed steel composition
comprises
-5
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Fe63Cr8Mo2B17C5Si1A14. The top portion of Fig. 9(a) is a cross-sectional view
of the sprayed
material, and the lower portion (b) shows a top surface of the coated
material.
Fig. 14 illustrates an x-ray diffraction scan of a plasma-sprayed deposit
having a free
surface. The plasma-sprayed composition was Fe63Cr8Mo2B17C5Si1A14.
Fig. 15 shows an x-ray diffraction scan of the plasma-sprayed composition of
Fig. 14,
and illustrates the structure at the substrate surface.
Fig. 16 illustrates a graph showing coefficient of friction versus the number
of turns
for Pin On Disk testing of a spray coating. The tested coating was
Fe63Cr8Mo2B17C5Si1A14. It
is noted that while the initial friction was low, Si3N4 deposition and buildup
caused the
friction to increase. (The sliding friction of Si3N4 on itself is 0.8).
Fig. 17 is a profile curve of a "wear-groove" on an as-sprayed steel substrate
after
2,000 cycles of Pin On Disk testing. As shown, instead of a groove developing
on the steel
substrate, the Si3N4 wore and deposited material onto the substrate. The
tested composition
was Fe63Cr8Mo2B17C5Si1A14.
Fig. 18 is an optical micrograph of an as-spun ribbon of (Fe0 8CR0 2)81B 17W2.
The
alloy exhibits high ductility, and can be bent severely without fracture.
Fig. 19 illustrates data obtained from differential thermal analysis of
(Fe0.8Cr0.2)75B17Si4Al4 (top graph) and Fe63Cr8Mo2B17C5Si1A14 (lower graph).
The graph
curves show glass to crystalline transitions and melting temperatures for the
tested alloys.
Fig. 20 shows peak crystallization temperatures measured by differential
thermal
analysis for a variety of alloys. Specifically, Fig. 20 shows the alloy
Fe63Cr8Mo2B17C5Si1A14
as 1, (Fe0.85Cr0.15)83B17 as 2, (Fe0.8Cr0.2)83B17 as 3, (Fe0.75Cr0.25)83B17 as
4, (Fe0.8Mo0.2)83B17 as 5,
(Fe0.6Co0.2Cr0.2)83B17 as 6, (Fe0.8Cr0.15Mo0.05)83B17 as 7,
(Fe0.8Cr0.2)79B17C4 as 8,
6
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
(Fe0.8Cr0.2)79B17Si4 as 9, (Fe018Cr0.2)79B17Al4 as 10, (Fe0.8Cr0.2)75B17Al4C4
as 11,
(Fe0.8Cr0.2)75B 17Si4C4 as 12, (Fe0.8Cr0.2)75B 17Si4A14 as 13, (Fe0 8Cr0.2)71B
17Si4C4A14 as 14,
(Fe07Co01Cr0.2)83B17 as 15, (Fe0.8Cr0.2)76B17Al7 as 16, (Fe0.8Cr0.2)79B17W2C2
as 17,
(Fe 0.8Cr0.2)81B17W2 as 18 and (Fe0.8Cr0.2)80B20 as 19.
Fig. 21 illustrates crystallization enthalpies measured by differential
scanning
calorimetry for various alloys encompassed by the present invention.
Specifically, Fig. 21
shows the alloy Fe63Cr8Mo2B17C5Si1Al4 as 1, (Fe0.85Cr0.15)83B17 as 2,
(Fe08Cr0.2)83B17 as 3,
(Fe0.75Cr0.25)83B17 as 4, (Fe0.8Mo0.2)83B17 as 5, (Fe0.6Co0.2Cr0.2)83B17 as 6,
(Fe0.8Cr0.15Mo0.05)83B17
as 7, (Fe0.8Cr0.2)79B17C4 as 8, (Fe0.8Cr0.2)79B17Si4 as 9,
(Fe0.8Cr0.2)79B17A14 as 10,
(Fe0.8Cr0.2)75B17A14C4 as 11, (Fe0.8Cr0.2)75B17Si4C4 as 12,
(Fe0.8Cr0.2)75B17Si4Al4 as 13,
(Fe0.8Cr0.2)71B17Si4C4A14 as 14, (Fe0.7Co0.1Cr0.2)83B17 as 15,
(Fe0.8Cr0.2)76B17Al7 as 16,
(Fe 0.8Cr0.2)79B17W2C2 as 17, (Fe0.8Cr0.2)81B17W2 as 18 and (FeO. as 19.
Fig. 22 illustrates a graph of transformation rates of glass to
crystallization
transformation for various alloys encompassed by the present invention.
Specifically, Fig. 22
shows the alloy Fe63Cr8Mo2B17C5Si1A14 as 1, (Fe0.85Cr0.15)83B17 as 2,
(Fe0.8Cr0.2)83B17 as 3,
(Fe0.75Cr0.25)83B17 as 4, (Fe0.8Mo0.2)83B17 as 5, (Fe0.6Co0.2Cr0.2)83B17 as 6,
(Fe0.8Cr0.15Mo0.05)83B17
as 7, (Fe0.8Cr0.2)79B17C4 as 8, (Fe03Cr0.2)79B17Si4 as 9, (Fe0.8Cr0.2)79B17A14
as 10,
(Fe0.8Cr0.2)75B17A14C4 as 11, (Fe0.8Cr0.2)75B17Si4C4 as 12,
(Fe0.8Cr0.2)75B17Si4A14 as 13,
(Fe0.8Cr0.2)71B17Si4C4Al4 as 14, (Fe0.7Co0.1Cr0.2)83B17 as 15,
(Fe0.8Cr0.2)76B17Al7 as 16,
(Fe0.8Cr0.2)79B17W2C2 as 17, (Fe0.8Cr0.2)81B17W2 as 18 and (Fe0.8Cr0.2)80B20
as 19.
Fig. 23 illustrates peak melting temperatures measured by differential thermal
analysis
for various alloys encompassed by the present invention. Specifically, Fig. 23
shows the
alloy Fe63Cr8Mo2B17C5Si1A14 as 1, (Fe0.85Cr0.15)83B17 as 2, (Fe0.8Cr0.2)83B17
as 3,
7
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
(Feo.75Cr0.25)83B17 as 4, (FeO.8Moo.2)83B17 as 5, (Fe0.6Coo.2Cro.2)83B17 as 6,
(Feo.8Cr0.15Moo.05)83B17
as 7, (Feo.8Cro.2)79B17C4 as 8, (Feo.8Cro.2)79B17Si4 as 9,
(Fe0.8Cr0.2)79B17Al4 as 10,
(Fe0.8Cro.2)75B17Al4C4 as 11, (FeO.8Cr0.2)75B17Si4C4 as 12,
(Fe0.8Cro.2)75B17Si4Al4 as 13,
(Fe0.8Cr0.2)71B17Si4C4Al4 as 14, (Fe0.7Co0.1Cr0.2)83B17 as 15,
(Fe0.8Cr0.2)76B17Al7 as 16,
(FeO.3CrO.2)79B17W2C2 as 17, (Fe0.8Cr0.2)81B17W2 as 18 and (Fe0.8Cr0.2)80B20
as 19.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The invention encompasses methodology for forming steel materials having
nanocrystalline scale composite microstructures, methods of utilizing such
steel materials,
and also encompasses the steel material compositions. A process encompassed by
the present
invention is described generally with reference to the block diagram of Fig.
1. At an initial
step (A) a molten alloy is formed. Such alloy comprises a steel composition.
An exemplary
alloy comprises at least 50% Fe and at least one element selected from the
group consisting of
Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W, Al, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho,
Er, Tm, Yb,
and Lu; and at least one element selected from the group consisting of B, C,
N, 0, P and S.
In particular aspects of the present invention, the alloy will be a magnetic
alloy with ultrafine
crystal grains having a composition represented by the formula: Fe(100-x-
y)M(x)B(y) (atomic
percent) wherein M represents at least one element selected from Ti, Zr, Hf,
V, Nb, Mo, Ta,
Cr, W and Mn, wherein 15>_x>_4, wherein 25>_y>_2, and wherein 35 _ (x+y) _>7.
Also, at least
50% of the alloy structure is preferably occupied by crystal grains having an
average size of
O
1000A or less, with the crystal grains being based on a bcc structure. The
alloy can further
contain X (Si, Ge, P, Ga, etc.) and/or T (Au, Co, Ni, etc.).
8
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Alloys of the present invention preferably comprise fewer than 11 elements,
and can
more preferably comprise fewer than seven elements. Additionally, the alloys
can comprise
fewer than five elements. An advantage in having fewer elements in the
compositions is that
it can be easier to reproduce a material if fewer components are utilized in
forming the
material. Generally, alloys of the present invention have from four to six
elements in their
compositions. Among such elements are iron; chromium, which can be included
for
corrosion resistance; boron and/or phosphorus which can be included to
generate a particular
glass transition temperature; and one or both of molybdenum and tungsten which
can be
included for hardness.
Exemplary alloys which can be utilized in methodology of the present invention
are:
(Feo.85Cr0.15)83B17, (Fe0.8Cr0.2)83B17, (Feo.75Cro.25)83B17,
(Fe0.8Mo0.2)83B17, (Fe0.6Co0.2Cr0.2)33B17'
(Fe0.8Cr0.15Mo0.05)83B17, (Fe0.8Cr0.2)79B17C4, (Feo.8Cr0.2)79B17Si41
(Feo.8Cro.2)79B17Al4,
(Feo.8Cro.2)75B17Al4C4, (Feo.8Cro.2)75B17S14C4, (Feo.8Cro.2)75B17Si4Al4,
(Feo.8Cr0.2)71B17Si4C4Al4,
(Feo.7Coo.1Cra2)83B17, (Feo.8Cr0.2)76B17Al71 (Feo.BCro.2)79B17W2C2,
(Feo.8Cro.2)81B17W2, and
(Feo.BCro.2)80B20.
The alloy of step (A) can be formed by, for example, melting a composition
under an
argon atmosphere.
At step (B) of Fig. 1, the alloy is cooled to form a metallic glass. Such
cooling
typically comprises a rate of at least about 104 K/s, with the rate varying
depending on the
particular composition of the molten alloy. The cooling can be accomplished by
a number of
different processes, including, for example, melt-spinning, gas atomization,
centrifugal
atomization, water atomization and splat quenching. The powder can be
consolidated by, for
example, hipping, hot pressing, hot extrusion, powder rolling, powder forging
and dynamic
9
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
compaction. In an exemplary method, the cooling of step (B) is accomplished by
centrifugal
atomization. Preferably, the melt stream leaves a centrifugal cup and is hit
by high pressure
helium gas to facilitate fast cooling (greater than 105 K/s.) The helium gas
can be collected,
purified and reused. The speed of the rotating centrifugal cup is preferably
about 40,000
RPM, and such speed can be adjusted to produce a fine powder with about a 25
micrometer
mean size.
Referring to step (C) of Fig. 1, the metallic glass of step (B) is devitrified
to form a
crystalline steel material having a nanocrystalline grain size. Such
devitrification can be
accomplished by heating the metallic glass to a temperature of from about 600
C to less than
the melting temperature of the alloy. Such heating enables a solid state phase
change wherein
the amorphous phase of the metallic glass is converted to one or more
crystalline solid
phases. The solid state devitrification of the amorphous precursor from step
(B) enables
uniform nucleation to occur throughout the metallic glass to form
nanocrystalline grains
within the glass. The metal matrix microstructure formed via the
devitrification can comprise
a steel matrix (iron with dissolved interstitials), with an intimate mixture
of ceramic
'precipitates (transition metal carbides, borides, silicides, etc.). The
nanocrystalline scale
metal matrix composite grain structure can enable a combination of mechanical
properties
which are improved compared to the properties which would exist with larger
grain sizes or
with the metallic glass. Such improved mechanical properties can include, for
example, high
strength, and high hardness coupled with significant ductility.
The particular temperature employed for devitrifying the metal glass can be
varied
depending on the particular alloy utilized in the glass, and a particular time
of application.
to
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Post treatment of the devitrified metallic material from step (C) can include
a surface
treatment utilized to transform only the surface of the material to a metallic
glass. Exemplary
surface treatment techniques are high and low pressure plasma spraying, high
velocity
oxyfuel spraying, and spray forming. The plasma spraying can be accomplished
with a
plasma spray system. The post treatment can offer improvements in, for
example, corrosion
resistance and lowering the coefficient of friction of a steel material.
Accordingly, it can be
advantageous to treat at least the surface of a crystalline steel material to
convert such surface
to a metallic glass. It is noted that a metallic glass coating can also offer
advantages over
existing coatings such as, for example, chrome, nickel and tin plating in that
the metallic
glass coating can be cheaper and can give a better metallurgical bond between
the surface and
the base metal.
Referring to Fig. 2, a specific embodiment application of the present
invention is
illustrated. Specifically, Fig. 2 illustrates a metallic barrel 50 being
sprayed with a molten
metal material 52. Molten metal material 52 is sprayed from a spraying device
54, and can
comprise, for example, one or more of the above-described exemplary alloys of
the present
invention. The molten metal can be formed by melting an alloy composition
under an argon
atmosphere and subsequently centrifugally atomizing the alloy composition. As
the melt
stream leaves a centrifugal cup, it can be hit by a high pressure helium gas
to form a fine
powder of solidified metallic alloy material with such fine powder having
about a 25
micrometer mean size. The fine powder can be fed into a plasma (high or low
pressure)
system wherein it is converted to a liquid spray which is sprayed on the
inside and outside of
metallic drum 50. In particular applications, drum 50 comprises a steel drum,
such as, for
example, a 55 gallon steel drum. It is noted that the powder may or may not be
fully melted
11
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
upon exposure to the plasma, and will be deposited into and onto the surface
of barrel 50 as a
continuous coating. In either event, the metallic material 52 sprayed onto and
within drum 50
cools rapidly to form a metallic glass. Drum 50 can be subsequently heat-
treated at a
temperature of equal to or greater than 600 C to devitrify the metallic glass.
The metallic structure formed over and within barrel 50 from material 52 can
have
greater corrosion resistance than stainless steel. Drum 50 be utilized, for
example, for storing
corrosive and otherwise dangerous materials, such as, for example, spent
nuclear fuel. If a
surface of material 52 is coated with a metallic glass, the anti-corrosive
properties and low
coefficient of friction properties associated with metallic glass can be
obtained.
Figs. 3-6 illustrate another embodiment application of the present invention.
Referring to Fig. 3, a metallic substrate 100 is provided. Such substrate can
comprise, for
example, one or more of the above-described exemplary alloys of the present
invention.
Referring to Fig. 4, a metallic melt 102 is sprayed onto substrate 100
utilizing a
sprayer 104. Melt 102 can comprise, for example, a molten alloy comprising one
or more of
(Fe0.85Cr0.15)83B171 (FeO.8Cr0.2)83B171 (Fe0.75Cr0.25)83B17,
(Fe0.8Mo0.2)83B171 (Fe0.6Co0.2Cr0.2)83B171
(Fe0.8Cr0.15Mo0.05)83B17, (Fe0.8Cr0.2)79B17C41 (Fe0.8Cr0.2)79B17S14,
(Fe0.8Cr0.2)79B17A141
(Fe0.8Cr0.2)75B17Al4C41 (Fe0.8Cr0.2)75B17S14C4, (Fe0.8Cr0.2)75B17Si4Al4,
(FeO.3Cr0.2)71B17Si4C4Al41
(Fe0.7Co0.1Cr0.2)83B17, (Fe0.8Cr0.2)76B17Al71 (Fe0.8Cr0.2)79B17W2C21
(Fe0.8Cr0.2)81B17W2, and
(FeO.8Cr0.2)80B20. Instead of being in a molten form, material 102 can
alternatively comprise a
powder material heated to a sufficient temperature to bond with the metal of
layer 100.
Material 102 deposits on substrate 100 to form a layer 106. Material 102 also
heats an
exposed surface of material 100 to form a heat-treated portion 108 of material
100. If
material 100 comprises a metallic glass, heat-treated portion 108 can comprise
a devitrified
12
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
material. Specifically, if layer 106 is formed at a temperature which heats a
surface of layer
100 to greater than 600 C, such heating can devitrify a portion of material
100 exposed to
such temperatures. In particular applications, temperatures greater than 600 C
can permeate
entirely through substrate 100 to heat-treat an entire thickness of material
100. Spray nozzle
104 is preferably resistant to the temperature and composition of material
102.
Referring to Fig. 5, substrate 100 is illustrated after layer 106 has been
formed across
an entire surface of substrate 100. Heat-treated portion 108 also extends
across an entire
surface of substrate 100. In particular embodiments, layer 106 can be formed
as a metallic
glass.
Referring to Fig. 6, subsequent treatments of the type illustrated in Fig. 4
can be
utilized to form multiple heat-treated layers 120 and an exposed outer surface
layer 124. Note
that one of the lower heat-treated layers 120 is previous layer 106. The
subsequent formation
of another metallic glass layer over layer 106 has heat-treated the entire
layer 106. In
particular embodiments wherein layer 106 comprises a metallic glass, such heat
treatment can
devitrify layer 106. Accordingly, heat treated layers 120 can comprise
devitrified metal
layers. In alternative methods of the present invention, each of the layers
106 and 120 can be
deposited as metallic glass and can remain in the metallic glass form during
deposition of
remaining layers 120. Then, if desired, some or all of the deposited layers
can be heat-treated
to at least partially devitrify the coating defined by layers 106 and 120.
Outermost layer 124 may or may not be heat-treated, and can comprise a
metallic
glass. Accordingly, the method of the present invention has enabled an
exterior coating to be
formed over layer 100, with said exterior coating comprising devitrified metal
layers 120 and
an outermost surface of metallic glass 124.
13
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
The methodology described with reference to Figs. 3-6 can have application for
a
number of uses, including military uses. Specifically, armor can be formed out
of a material
100. If the armor becomes punctured or cracked, the methodology of Figs. 3-6
can be utilized
to repair the armor and effectively build a metallic shell over the weakened
areas of the
armor. Spraying device 104 can be adapted to be utilizable in battlefield
situations.
In addition to the utilizations described above for materials of the present
invention,
the materials can also be utilized as powders for surface finishing (i.e.,
mechanical blasting)
and surface treatments such as, for example, shot peening.
The invention can be considered a method for forming a new class of steel
called
devitrified nanocomposite (DNC) steel, with DNC steel being defined as having
a primarily
nanoscale (less than 100 nanometer) microstructure grain size developed by
processing the
steel through a solid-solid transformation (specifically, glass
devitrification). Alloys are
developed having low cooling rates (less than 10' K/s) for metallic glass
formation, and
accordingly the alloy compositions form metallic glasses when rapidly
solidified by a chill
surface (such as, for example, melt-spinning, splat quenching, etc.) or
atomization (gas,
water, centrifugal, etc.) methods. The glass is utilized as a precursor stage,
and the alloy
subsequently processed through a glass devitrification transformation upon
heating above a
crystallization temperature of the alloy. Due to uniform nucleation in the
glass coupled with
a high nucleation frequency, there is little time for grain growth processes,
and nanoscale
nanocomposite microstructures (i.e., grains) result. The nanocomposite
microstructures can
lead to materials having significant increases in hardness and strength over
conventional steel
alloys.
14
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Initial studies described herein show that DNC steel formed in accordance with
methodology of the present invention has exceptional hardness and wear
resistance, and can
be used potentially for any application which involves sliding, rolling, or
rotation.
Additionally, initial studies have shown that the unlubricated DNC steel
surface has
exceptionally low coefficients of friction (in the range of lubricated steels)
which can be a
beneficial property in reducing wear resistance, frictional energy losses, and
heating between
moving surfaces. This can allow the use of DNC steel in unlubricated
applications, and can
also be useful as a fail-safe mechanism allowing additional time before
failure in some
applications, such as gasoline or diesel engines, where lubrication is
unexpectedly lost. The
high wear resistance of DNC steel, coupled with low friction, can allow
extension of the
lifetime of parts formed from DNC steel relative to parts formed from
conventional steel
alloys. Such can enable large savings in both operating energy and cost
associated with part
replacement, repair, maintenance and down-time. Exemplary applications for
utilization of
DNC steels of the present invention include bearings, gun barrel surfaces,
bearing journals,
hydraulic cylinder connecting rods, crankshafts, pistons, cylinder liners,
gears, camshafts,
universal joints, valves, gun breach boxes, missile launcher tubes, and tank
gear boxes.
Unlike conventional steel alloys which rely on manipulation of solid state
eutectoid
transformation (yso,=aso1+Fe3C), DNC steel utilizes a different approach, and
specifically
utilizes processing through a solid/solid state glass devitrification
transformation. DNC steel
alloys have been developed which have exceptionally low cooling rates (103 K/s
to 105 K/s)
for metallic glass formation. This can allow the production of metallic glass
structures during
rapid solidification via chill surface or atomization methods.
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Examples of DNC steel melt-spun ribbon and gas atomized powder are shown in
Figs.
7 and 8, respectively. Metallic glass structures are produced by both of these
rapid
solidification processing methods. The glass precursor can be devitrified into
a nanoscale
composite microstructure by heating above the crystallization temperature.
A differential thermal analysis scan for as-spun DNC steel is shown in Fig. 9.
The
glass crystallization temperature typically varies from 750K to 900K with
enthalpies of
transformation from -75 J/g to -200 J/g, and melting temperatures from 1,375K
to 1,500K for
alloys encompassed by the present invention (as described in the charts of
Figs. 20-23).
Because there is uniform nucleation and extremely high nucleation frequency
during
crystallization of alloys of the present invention, there can be little time
for grain growth
before impingement between neighboring grains and accordingly nanoscale
nanocomposite
microstructures are formed. The individual phase sizes can vary from 1 to 75
nanometers,
which is finer than conventional steels produced by conventional casting or
even when
rapidly solidified. When the microstructure is, reduced to the nanoscale
level, a high
percentage of the atoms of the material (about 30%) can be associated with
grain boundaries,
and an extremely high density of two-dimensional defect interfaces (such as
phase in grain
boundaries) reside in the microstructure. The microstructure of a devitrified
ribbon showing
the nanoscale nanocomposite microstructure is shown in Fig. 10. The
nanostructure results in
the development of extreme strength and hardness, which are significantly
higher than found
in conventional steel or other metallic based alloys.
The hardness of glass and devitrified DNC steel has been measured using both
nanoindentor and Vickers microhardness testing, and excellent agreement is
found between
the two methods. Specialized nanoindentor testing using a Berkovich indentor
was
16
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
performed on the as-atomized and heat-treated sieved (10-20 micrometer and 75-
100
micrometer) gas atomized particles from a Fe63Cr8Mo2B17C5Si,A14 alloy as a
function of depth
into the particle. The elastic modulus was found to be as high as 300 GPa,
which is
approximately 50% higher than a conventional steel (which commonly exhibits
elastic
moduli from 200 GPa to 220 GPa). This means that bonding strength is
increased, which can
be of beneficial result since it allows close tolerances to be maintained
during application of
high elastic loads, and can have additional benefits concerning wear
resistance. The hardness
was also found to be extremely high at greater than 15 GPa, which is harder
than
conventional metallic materials. Examples of various compositions which can be
utilized in
methodology of the present invention for forming hard materials are shown in
Table 1. In
referring to Table, the various compositions are given reference names
(specifically, they are
referred to as alloys DARX) to simplify reference to the compositions herein.
Table 2
contrasts hardness of various materials with the alloy DAR1.
17
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
TABLE 1
DNC Alloy Compositions
Alloy Composition
DAR1 Fe63Cr8Mo2B 17C5Si1A14
DAR2 (Fe0.85Cr0.15)83B 17
DAR3 (Fe0_8Cr0.2)83B 17
DAR4 (Fe0.75Cr0.25)83B 17
DAR5 (Fe0.8Mo0.2)83B 17
DARE (Fe0.6Co0.2Cr0.2)83B 17
DART (Fe0.8Cr0.15Mo0.05)83B 17
DAR8 (Fe0.8Cr0.2)79B 17C4
DAR9 (Fe0.8Cr0.2)79B 17Si4
DAR 10 (Fe0.8Cr0.2)79B 17A14
DAR11 (Fe0.8Cr0.2)75B 17A14C4
DAR 12 (Fe0.8Cr0.2)75B 17Si4C4
DAR 13 (Fe0.8Cr0.2)75B 17S14A14
DAR14 (Fe0.8Cr0.2)71B 17Si4C4A14
DAR15 (Fe0.7Co0.1Cr0.2)83B17
DAR 16 (Fe0.8Cr0.2)76B 17A17
DAR17 (Fe0.8Cr0.2)79B17W2C2
DAR 18 (Fe0.8Cr0.2)81B 17W2
DAR 19 (Fe0.8Cr0.2)80B20
DAR20 Fe64Ti3Cr5Mo2B16C5Si1A12La2
18
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
TABLE 2
Example Hardness of Metallic Materials
Material Hardness
18 Cr-10 Ni austenitic stainless steel 1.5 GPa
0.74 plain carbon steel 4.9 GPa
4340 ultra high strength steel 5.5 GPa
T5 W tool steel 7.5 GPa
90 WC - 10 Co cemented carbide 10.1 GPa
Fe63Cr$Mo2B17C5Si1A14 (DAR1) 15.5 GPa
From the hardness determined for DAR1, the yield strengths for the DNC steel
can be
estimated to be 725 ksi, which is significantly higher than conventional (150
ksi) or ultra high
strength (220 ksi) steels. If the plasticity is fully developed, the yield
strength can be
estimated to be 1/3 of the hardness. This gives the DNC steel a specific
strength of 0.65 x
106M which makes this material an alternate for Al in lightweight
applications. Little
hardness difference was found between the large and small heated powders
indicating that
similar microstructures were obtained independent of powder size. It is noted
that the
hardness tests described herein were relative to a material DAR1
(Fe63Cr8Mo2B17C5Si1A14)
which is not a preferred material of the present invention. Rather, preferred
materials of the
present invention would have fewer elements, and are listed as DAR2 through
DAR 19 in
Table 1.
A preferred material of the present invention (specifically DAR20) is compared
with
DAR1 in Fig. 11. Specifically, Vickers microhardness measurements with a 100
gram load
were performed on 75 micrometer to 100 micrometer powder size fractions for as-
atomized
alloys, and also as a function of heat treatment temperature. The tested
alloys exhibited
-19
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
extreme hardness from 10.1 GPa to 16.0 GPa Vickers hardness. Examples of
diamond
pyramid indentations on a melt-spun ribbon and gas-atomized powder particle
are shown in
Fig. 12. While the Rockwell C is the most common hardness measurement for
steels, it
cannot be used in the present case due to the extreme hardness of the alloys
of the present
invention (which are off of the Rockwell C scale). Note that a Vickers
Hardness number of
9.2 GPa corresponds to a Rockwell C of 68. Referring again to Fig. 11, it is
noted that little
hardness change occurs in the as-atomized state of alloys of the present
invention after
subsequent heat treating. This can be significant, as it means that optimum
microstructures
are obtained directly during solidification and the optimum structures are
stable to high
temperatures (at least to 850 C, as shown in Fig. 11).
DNC steels contain multiple combinations of elements which result in
relatively low
melting points (typically around 1,150 C) and low melt viscosities. This can
make the DNC
steels easy to process from the liquid state, and ideal feedstock materials
for forming coatings
by thermal deposition methods. Initial low plasma spraying tests have been
performed
utilizing the atomized 20 to 50 micrometer Feb3Cr8Mo2B17C5Si1A14 steel powder
as feed stock.
Several uniform DNC steel coatings of 0.1 inch in thickness were deposited
onto 4"x4" 301
stainless steel plates (shown in Fig. 13). While typical thermally deposited
coatings are only
25 micrometers to i00 micrometers thick, much thicker coatings (up to 2,500
micrometers)
were sprayed to illustrate an extreme case (in other words, thinner coatings
are easier to spray,
but thicker coatings were sprayed to illustrate operability of a method of the
present
invention).
Metallographic examinations of the coatings indicate that the percent porosity
of the
initial coatings was at least 3%. X-ray diffraction scans were performed both
on the substrate
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
side and free surface side of the coatings, and show that an amorphous
structure was obtained
through the cross-sectioning of the coatings (specifically, Fig. 14 shows an x-
ray structure of
a free surface side of the coating, and Fig. 15 shows an x-ray structure of
the substrate side of
the coating). Differential scanning calorimetry methods verified the formation
of the glass
structure in the coating which exhibited a high crystallization enthalpy (-110
J/g). This result
is surprising due to the supreme thickness of the coating which resulted from
successive
build-up of continuous layers of deposited powder, and the fact that the
substrate was not
cooled. Thus, DNC steel coatings represent a class of materials called bulk
glasses. Bulk
glasses are normally very difficult to produce, but readily form in the DNC
alloys by thermal
processing methods.
The as-sprayed DNC metallic glass coatings can be devitrified into a nanoscale
structure by heating above the crystallization temperature. However, due to
the unique
properties of the metallic glasses, the glass state itself may be useful as a
coating. Metallic
glasses are essentially super-cooled liquids, and have structures which are
very homogeneous.
Typically there are few defects, and there can be a complete absence of grains
and phase
boundaries. Hardness testing was performed on both the as-sprayed (amorphous)
and heat-
treated (800 C for one hour) nanocrystalline coatings. The Vickers hardness of
these coatings
was found to be 10.9 GPa and 13.8 GPa for the as-sprayed and heat-treated
coatings,
respectively. It is noted that while the amorphous sample is not as hard as
the crystalline
sample, it is still harder than the hardest tool steel (about 9.3 GPa), or
tungsten carbon (WC)
cemented carbide cutting tool (about 10.0 GPa).
Tribology testing experiments were done on the as-sprayed and heat-treated
(100 C
for one hour) plasma sprayed coatings using ASTM G99 Pin-on-Disk tests. The
"pin" was a
-21
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
one-half inch diameter Si3N4 ball which was rotated at a test speed of 97 RPM,
with a test
radius of 10.4 mm and with no lubrication. During the test, the coefficients
of friction were
measured (shown in Fig. 16). The coefficient of static friction for the steel
substrate in botli'
the as-sprayed and heat-treated condition was 0.22, which represents a low
value. For
example, the following coefficients of sliding friction were obtained for
specimens sliding
over a normalized steel (0.13% C, 3.42% Ni): aluminum (0.6), cartridge brass
(0.5), copper
(0.8), cast iron (0.4), and normalized steel (on itself 0.8). For conventional
steels, the
coefficients of static friction for unlubricated surfaces generally vary from
0.8 to 1.0, while
lubricated steels have much lower values (typically from 0.1 to 0.25). Thus,
the unlubricated
DNC steels have coefficients of static friction in the range of lubricated
steel surfaces.
Accordingly, utilization of DNC steel coatings in place of conventional steel
may allow the
elimination of lubrication in some applications. Note that the coefficient of
sliding friction of
the steel substrate could not be measured due to Si3N4 deposition from the
pin.
The profile of the wear surface of the steel showed that the steel experienced
no wear
during the test (Fig. 17). Instead of the expected wear groove, a raised hill
of deposited Si3N4
was found on the steel surface. Examination of the silicon nitride ball showed
that it
experienced a large ball scar as a result of wear. This was surprising due to
the hardness of
the ball material (15.4 GPa), which is used specifically for these type of
tests due to its
excessive hardness and wear resistance. Note that Si3N4 is currently the
hardest pin material
available to perform this ASTM test.
The Fe63Cr8Mo2B17C5Si1A14 steel utilized in generating the data described
above is an
exemplary DNC steel. However, it suffers from a disadvantage of having
numerous elements
included therein, which can make it difficult to produce uniform batches of
the material.
22
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
Accordingly, improved DNC alloys have been developed. Such improved alloys are
listed in
Table 1 as DAR2 through DAR19. The alloys have been designed to form metallic
glasses at
low cooling rates, and are further designed to reduce the number of elements
utilized in the
alloys.
Ingots of the 19 alloys listed in Table 1 were melt-spun at 15 m/s with the
following
melt-spinning parameters: chamber 1/3 atmosphere helium, ejection pressure 150
Torr,
ejection temperature 1,400 C, crucible up to wheel distance 6 mm, and crucible
orifice
diameter 0.81 mm to 0.84 mm.
All of the tested alloys were melt-spun with few problems. Interestingly, many
of the
preferred alloys (i.e., DAR2 through DAR19) formed uniform continuous ribbons
up to 10
meters in length. This may be due to increased glass forming ability and
increased ductility
of the glass that is produced relative to the less preferred alloy DAR1.
Qualitative inspections
of the ribbons by bending the ribbons back and forth until fracture indicated
that all of the
alloys DAR2 through DAR19 have higher ductility than the DAR1 alloy. In fact,
some of the
alloys DAR2 through DAR 19 in ribbon-form cannot be broken by bending, and had
to be
cut. An example of a melt-spun ribbon which exhibits high ductility is shown
in Fig. 18, and
was formed from the material DAR18 (Fe0.8Cr0.2)81B17W2=
Differential thermal analysis (DTA) and differential thermal calorimetry (DSC)
studies were done on each melt-spun ribbon sample in ultra-high purity argon
from 30 C to
1,375 C at a heating rate of 10 C/min. A typical DTA scan showing DAR14
((Fe08Cr02)75B17Si4A14) compared with DAR1 (Fe63Cr8Mo2B17C5Si1A14) is
illustrated in Fig.
19. From the DTA/DSC studies, the glass to crystalline transformation
temperatures,
enthalpy of transformation, transformation rate, and melting temperatures
could be
23
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
determined. The results of these studies are shown in Figs. 20-23. As shown,
all of the alloys
but one (specifically, DAR5 ((Fe0.8Mo0.2)33B17), formed a metallic glass
structure when melt-
spun at reduced cooling rate. Thus, the alloys are expected to form metallic
glass powders
when atomized.
Vickers hardness testing using a 100 gram load was done on the cross-sections
of the
melt-spun ribbons of each alloy in the as-spun and heat-treated (700 C for one
hour and
800 C for one hour) conditions. For each sample (60 samples total), 10 Vickers
hardness
tests on five ribbons were done in order to get a reportable average value. In
general, only
small variations were found to occur in hardness when the same sample was
tested.
Summaries of the completed Vickers hardness measurements are shown in Table 4.
TABLE 4
ALLOY Condition Hardness (kg/mm2) Hardness (GPa)
(Fe0.85Cr0.15)83B17 As-Spun 996 9.77
(Fe0.85Cr0.15)83B 17 700 C 835 8.19
(Fe0.85Cr0.15)53B 17 800 C 864 8.47
(Fe0.8Cr0.2)83B 17 As-Spun 1048 10.28
(Fe0.8Cr0.2)83B17 700 C 935 9.17
(Fe0.8Cr0.2)83B17 800 C 870 8.53
(Fe0.75Cr0.25)53B17 As-Spun 1065 10.45
(Fe0.75Cr0.25)83B 17 700 C 1011 9.91
(Fe0.75Cr0.25)83B17 800 C 888 8.71
(Fe0.6C00.2Cr0.2)83B17 As-Spun 980 9.61
(Fe0.6Co0.2Cr0.2)83B17 700 C 1119 10.97
(Fe0.6CO0.2Cr0.2)83B 17 800 C 984 9.65
(Fe0.8Cr0.15MO0.05)83B17 As-Spun 1062 10.41
(Fe0.8Cr0.15MO0.05)83B17 700 C 1573 15.43
24
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
(Fe0.8Cr0.15MO0.05)83B17 800 C 900 8.83
(Fe0.8Cr0.2)79B17C4 As-Spun 1103 10.82
(Fe0.8Cro.2)79B17C4 700 C 1317 12.91
(Fe0.8Cr0,2)79B 17C4 800 C 1194 11.71
(Fe0.8Cro.2)79B17Si4 As-Spun 1096 10.75
(Fe0.8Cr0.2)79B17Si4 700 C 1150 11.28
(Fe0.8Cro.2)79B175i4 800 C 885 8.67
(Fe0.8Cr0.2)79B17A14 As-Spun 1053 10.32
(Fe0.8Cr022)79B17A14 700 C 1119 10.97
(Feo.8Cro.2)79B17A14 800 C 946 9.28
(Feo.8Cr0.2)75B17A14C4 As-Spun 1098 10.76
(Feo.8Cro.2)75B17Al4C4 700 C 1380 13.53
(Fe088Cro.2)75B17Al4C4 800 C 1159 11.37
(Fe0.8Cr02)75B17S14C4 As-Spun 1184 11.61
(Fe088Cro.2)75B17Si4C4 700 C 1509 14.80
(Fe0.8Cro.2)75B17Si4C4 800 C 1245 12.21
(Fe0.8Cr0.2)75B17Si4A14 As-Spun 1063 10.42
(Feo.SCro.2)75B17Si4A14 700 C 1266 12.42
(Feo.SCro.2)75B17Si4A14 800 C 1055 10.34
(Feo.8Cr02)71B17Si4C4A14 As-Spun 1093 10.72
(Fe0.8Cr0.2)71B17Si4C4A14 700 C 1376 13.49
(Fe0.8Cr0.2)71B17Si4C4A14 800 C 1134 11.12
(Fe0.7COO.1Cro.2)83B17 As-Spun 1042 10.22
(Fe0.7Co0.1Cr0.2)83B17 700 C 1135 11.13
(Feo.7Coo.1Cro.2)83B17 800 C 885 8.68
(Feo.8Cr0 2)80B20 As-Spun 1093 10.72
(Fe0.8Cro.2)80B20 700 C 1045 10.24
(Fe0.8Cr0 .2)80B20 800 C 965 9.47
(Fe0.8Cr0.2)76B17A17 As-Spun 1028 10.08
(Fe0 .8Cr0 .2)76B17A17 700 C 1441 14.13
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
(Feo.8Cr0.2)76B 17A17 800 C 868 8.51
(Fe0.8Cr0.2)79B17W2C2 As-Spun 1124 11.02
(Feo.8Cr0.2)79B17W2C2 700 C 1653 16.21
(Fe0.8Cro.2)79B 17W2C2 800 C 1223 11.99
(Fe0.8Cr0_2)81B17W2 As-Spun 1052 10.31
(Feo88Cr0.2)81B17W2 700 C 1565 15.34
(Feo.8Cr0.2)81B17W2 800 C 1100 10.79
As indicated by the tables and Figures provided herein, materials of the
present
invention having less than 11 elements, and more preferably less than seven
elements, can
form glass compositions. It is not a trivial task to form materials having
such limited number
of elements, which are also capable of forming metallic glasses. However, such
has been
accomplished in the present invention. The present invention also has
developed improved
ductility and toughness of DNC steel alloys, while maintaining or possibly
even improving
hardness. The DNC alloys are believed to be useful for numerous services,
including military
applications, due to their strength and wear resistance. The alloys can also
be resistant to
electrochemical attack (i.e., corrosion). In general, as the scale of a
microstructure decreases,
the electrochemical resistance of a particular material is expected to
increase. Thus,
nanocrystalline scale DNC microstructures are expected to have good corrosion
resistance.
Further, metallic glass DNC structures can have improved corrosion resistance
due to high
homogeneity (short range order on a 2 nanometer length scale) and the absence
of two-
dimensional defects (such as grain or phase boundaries). Specifically, a
uniform single-phase
structure can make it difficult for sites to initiate for anodic attack and
electron transfer since
there will not be distinct anodic and cathodic sites. While the metallic glass
or nanostructure
of a certain composition can have a higher relative resistance to
electrochemical attack than
26
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
the same material in bulk form, the material's nobility will be dependent on
both the structure
and the composition. For instance, a high level of chromium can improve
resistance to
electrochemical attack.
Among the advantages of the alloys described herein is that such alloys can
have a
relatively simple composition (i.e., from four to six elements in the
composition). Also, the
alloys can contain a relatively high percentage of transition metals (from 90%
to 97%) which
can lead to improved industrial properties of the materials.
A distinction of the materials of the, present invention relative to
conventional hard
materials is that the materials of the present invention can comprise no
carbon. In
conventional steels, hardness is typically tied directly to carbon content in
a martensite. In
contrast, the extreme hardness of DNC steels arises from development of
nanoscale
nanocomposite microstructures, rather than from martensitic transformations.
An advantage
of carbon-free compositions is that the extremely hard alloys can be developed
to still be
reasonably ductile, which is typically not possible in conventional steel
alloys (i.e.,
untempered martensite and transition metal carbides are typically hard, and
also brittle).
Group VI transition metals (Cr, Mo, and W) can be particularly potent
additions to
DNC steels. Chromium, consistent with data on conventional steel alloys, is
expected to also
provide excellent corrosion resistance. Molybdenum and tungsten can be
exceptionally
potent additions to promote hardness in DNC steels. Tungsten can also be
potent at
increasing hardness while retaining or increasing ductility.
Because of its hardness and high strength (greater than 725 ksi), DNC steel
can be
difficult to process into bulk parts starting from powder and using
conventional powder
metallurgical consolidation processes. However, DNC steel can be easy to
process from the
27
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
liquid state. Alternatively, powder of DNC steel can be fed through a
conventional plasma
gun and sprayed as a coating onto metal substrates with good adhesion and with
absence of
cracking. Other methods for forming a coating of DNC steel include axial feed
plasma
spray, conventional plasma spray, high velocity oxy-fuel spray, and a
detonation gun.
When DNC steel is sprayed onto metallic substrates it can readily form a
metallic
glass structure. If consecutive layers are continuously sprayed onto a bulk
substrate
(thickness greater than 0.1 inches) metallic glasses can be formed. This may
be the most
inexpensive and easiest way to form bulk metallic glass coatings or even bulk
glass
monolithic parts.
DNC steels can be rapidly solidified into an amorphous glass precursor and
then the
rapidly solidified powders can be consolidated into a useful form.
Accordingly, the cost of
technology of the present invention can involve three items: the alloy cost,
the powder
production cost, and the consolidation cost. All three items can be estimated.
To produce
rapidly solidified powder, centrifugal atomization may be the best method, and
even at
relatively low production rates. If it is feasible to produce DNC steel powder
by water
atomization, processing cost to produce the powder could drop to a few pennies
per pound.
The powder consolidation costs will vary depending on the specific application
and the
thickness of the coating. Coatings from 5 micrometers to 2,500 micrometers in
thickness can
be readily deposited using conventional commercially available thermal
deposition methods,
such as plasma spraying or high velocity oxy-fuel spraying. The DNC steel's
cost can
compare favorably to other hard materials such as, for example, diamond and
cubic BN.
DNC steel coating may also be a direct competing technology to replace
tungsten carbide
28
CA 02565090 2006-10-30
WO 2005/116286 PCT/US2005/015365
cemented carbide coatings, since the DNC steel exhibits higher hardness and
greater tensile
ductility.
Although the invention is described herein for coating steel alloy
compositions of the
present invention on metallic substrates, it is to be understood that the
alloys of the present
invention can also be coated on non-metallic substrates, such as, for example,
ceramics, to
provide a hard and/or lubricating surface over the non-metallic substrates.
29