Note: Descriptions are shown in the official language in which they were submitted.
CA 02565646 2006-10-26
- 1 -
SYSTEMS AND METHODS OF CLINICAL STATE PREDICTION UTILIZING
MEDICAL IMAGE DATA
FIELD OF THE INVENTION
The present invention relates to computer-aided method and
system predicting a clinical state of a subject via analysis of in vivo
medical
images.
BACKGROUND OF THE INVENTION
Medical imaging is widely used for diagnosis purposes and a
general approach for diagnosis is to detect subtle differences in the
composition,
morphology or other behavior in organs as can be imaged by different
techniques
and equipment (ie. modalities) and relate these differences to clinical
phenomena
of interest.
Image data can be obtained from various sources including for
example TI weighted Magnetic Resonance Imaging ("T1w MRI"), 12 weighted
MRI ("T2w MRI'), Proton Density weighted MRI ("PD MRI"), Photon Emission
Tomography ("PET"), Single Photon Emission Computer Tomography ("SPECT)
and Computer Tomography ("CT).
Diagnosis of diseases based solely on their imaging characteristics
is a challenging task for computer vision. If successful, however, diagnosis
approaches can serve multiple purposes such as disease characterization or the
morphological assessment of drug effect. Many studies have been conducted to
find correlations between image and disease some examples are provided in the
following references:
P. A. Freeborough and N. C. Fox, "MR image texture analysis
applied to the diagnosis and tracking of Alzheimer's disease," IEEE Trans Med
Imaging. vol. 17. pp. 475-9. 1998: J. P. Lerch, J. C. Pruessner, A, Zijdenbos,
H.
Hempel, S. J. Teipel, and A. C. Evans, "Focal decline of cortical thickness in
CA 02565646 2006-10-26
- 2 -
Alzheimer's disease identified by computational neuroanatomy," Cereb Cortex,
vol. 16, pp, 996-1001. 2005: Y..Liu, L. Teverovskiy, 0. Carmichael, R.
Kikinis, M.
Shenton, C. S. Carter, V. A. Stenger, S. Davis, H. Aizenstein, J. T. Becker,
0. L.
Lopez, and C. C. Meltzer, "Discriminative MR Image Feature Analysis for
Automatic Schizophrenia and Alzheimer Disease Classification," presented at
Medical Image Computing and Computer Assisted Intervention, Saint-Malo,
France, 2004; Z. Lao, D. Shen, Z_ Xue, B. Karacali, S. M. Resnick, and C.
Davatzikos, "Morphological classification of brains via high-dimensional shape
transformations and machine learning methods," Neurolmage, vol. 21, pp. 46-57,
2004; J. G. Csernansky, L. Wang, S. Joshi, J. P. miller, M. Gado, D. Kido, D.
McKee!, J. C. Morris, and M. I_ Miller, "Early DAT is distinguished from aging
by
high-dimensional mapping of the hippocampus. Dementia of the Alzheimer type,"
Neurology, vol. 65, pp. 1636-43, 2000; L. G. Apostolova, R. A. Dutton, I. 0,
Dinov, K. M. Hayashi, A. W. Toga, J. L. Cummings, and P. M. Thompson,
"Conversion of mild cognitive impairment to Alzheimer disease predicted by
hippocampal atrophy maps," Arch Neurol, vol. 63, pp. 693-9, 2006; P. Go!land,
W. E. Grimson, M. E. Shenton, and R. Kikinis, "Detection and analysis of
statistical differences in anatomical shape," Med Image Anal, vol. 9, pp. 69-
86,
2005; Psychiatry Res. 2006 Apr 30:146(3):283-7. Epub 2006 Mar 10. Predicting
conversion to dementia in mild cognitive impairment by volumetric and
diffusivity
measurements of the hippocampus, Fellgiebel A,Dellani PR,Greverus
D,Scheurich A,Stoeter P,Muller MJ. Surg Radial Anat. 2006 May;28(2):150-6.
White matter damage of patients with Alzheimer's disease correlated with the
decreased cognitive function. Duan JH,Wang HQ,Xu J,Lin X,Chen SQ,Kang
Z,Yao ZB. Acts Neurol Scand. 2006 Jan;113(1):40-5. Amygdalar volume and
psychiatric symptoms in Alzheimer's disease: an MR! analysis. Horinek
D,Petrovicky P,Hort J,Krasensky J,Brabec J,Bojar M,Vaneckova M,Seidl
Z.Neurol India. 2004 Sep:52(3):332-7. T2-weighted MRI in Parkinson's disease;
substantia nigra pars compacta hypointensity correlates with the clinical
scores,
CA 02565646 2006-10-26
- 3 -
Atasoy HT,Nuyan 0,Tunc T,Yorubulut M,Unal AE,Inan LE. Neuroradiology.
2002 Jan;44(1):43-8. Five-year retrospective changes in hippocampal atrophy
and cognitive screening test performances in very mild Alzheimees disease: the
Tajiri Project. Yamaguchi S,Meguro K,Shimada M,Ishizaki J,Yamadori A,Sekita
Y. Neuroreport, 2002 Dec 3:13(17):2299-302. Diffusion tensor in posterior
cingulate gyrus: correlation with cognitive decline in Alzheimer's disease.
Yoshiura T,Mihara F,Ogornori K,Tanaka A,Kaneko K,Masuda K. Arch Gerontol
Geriatr. 2006 May 22; Linear measures of temporal lobe atrophy on brain
magnetic resonance imaging (MRI) but not visual rating of white matter changes
can help discrimination of mild cognitive impairment (MCI) and Alzheimer's
disease (AD). Saks E,Dogan EA,Topcuoglu MA,Senol U,Balkan S. Psychiatry
Clin Neurosci, 2006 Jun;60(3):319-26. Association of minimal thickness of the
medial temporal lobe with hippocampal volume, maximal and minimal
hippocampal length: volumetric approach with horizontal magnetic resonance
imaging scans for evaluation of a diagnostic marker for neuroimaging of
Alzheimer's disease. Uotani C,Sugimori K,Kobayashi K. Cogn Behav Neurol.
2005 Sep;18(3):179-84. Predictive model for assessing cognitive impairment by
quantitative electroencephalography. OnishiJ,SuzukY,YoshikoK,HibinoS,Iguch A.
However, diagnosis approaches, while providing important
information on the state of an individual at a point in time, does not in
itself
provides an assessment of the future evolution of a particular clinical state.
Such
predictions are only based on the experience of medical practitioners and are
a
very subjective estimation of the future evolution of a clinical state.
SUMMARY OF THE INVENTION
In a broad aspect of the invention, there is provided a method for
predicting the future evolution of a clinical state of a subject based on
analysis of
imaging data. The method advantageously provides an objective approach to the
prediction of future clinical state.
CA 02565646 2006-10-26
- 4 -
In one embodiment, the method comprises providing a statistical
image-based predictive model for predicting the evolution of the state, the
model
incorporating one or more image-derived features from at least one volume of
interest (V01) comprising information related to the clinical state,
collecting image
data from the at least one VOI in the subject, deriving the one or more image
features from the collected image data from the subject, and using the one or
more derived image features from the at least one VOI of the subject and the
predictive model to predict the evolution of the clinical state.
In another embodiment of the invention the predictive model can be
obtained by deriving a set of modes of variation of the image features from a
plurality of training subjects, selecting a subset of the modes of variation
based
on a first univariate or multivariate analysis or combination thereof between
the
modes of variation and at least one clinical variable, and establishing the
model
based on a second univariate, or multivariate analysis or combination thereof
between the selected subset of modes and the at least one clinical variable.
BRIEF DESCRIPTION OF THE DRAWINGS
In drawings which illustrate by way of example only embodiments
of the present invention:
FIGS. 1 to 9 are representative of the prior art, and;
FIG. 1 is a schematic illustration of the design of an automated
classification system:
FIG. 2 is a flow chart, illustrating exemplary steps performed at a
computing device of FIG. 1;
FIG. 3 is a flow chart, further illustrating the data collection step of
FIG. 2;
FIG. 4 is a flow chart, further illustrating the VOI selection step of
FIG. 2;
CA 02565646 2006-10-26
- 5 -
FIG. 5 is a flow chart, further illustrating the intensity data
calculation step of FIG. 2;
FIG. 6 is a flow chart, further illustrating the spatial data calculation
step of FIG. 2;
FIG. 7 is a flow chart, further illustrating the variation model creation
step of FIG. 2;
FIG. 8 is a flow chart, further illustrating the classifier building step
of FIG. 2;
FIG. 9 is a flow chart, further illustrating the test patient
classification step of FIG. 2;
FIG. 10 is a flow chart illustrating exemplary steps for predicting a
clinical state of a subject;
FIG 11, is a flow chart further illustrating exemplary steps for
predicting a clinical state of a subject;
FIG_ 12 A Demographic information. B Leave-one-out, forward
stepwise linear discriminant analysis of the patient eigencoordinates in the
reference space was 100% accurate at classifying groups (decliners, stable,
improvers). The data is shown here projected on the 3 most discriminating
eigenvectors; and
FIG. 13 (A) Clinical model built from multiple regression of age, sex
and baseline MMSE against 1 year MMSE changes. (B) Residuals for the
"Clinical" model. The correlation of predicted vs actual values was r = 0.429.
(C)
MRI+Baseline MMSE model built from multiple regression Of the 10 most
correlated reference space eigenvectors plus baseline MMSE. (D) Plot of
residuals. With a correlation between predicted vs actual yearly MMSE changes
of r=0.6955 , this model was a significant improvement over the "Clinical" one
(F-stat = 2.691, P r- 0.002).
CA 02565646 2014-06-06
6
DETAILED DESCRIPTION
For explanatory purposes FIG 1 through 9 will be generally discussed
prior to the detailed description of the Invention.
A schematic illustration of the design of an automated classification
system 102 in manners exemplary of the present invention is shown in FIG. 1.
The example automated classification system 102 determines a classification
and
diagnosis of the neurological disease state of a given test subject 112, based
on
3D image data of the brain 104. The image data 104 may be mono-modal or
multi-modal. Possible types of images that may be acquired include, but are
not
limited to images based on: T1w MRI, T2w MRI, PD MRI, PET, SPECT, and CT.
As illustrated, example automated classification system 102 is
determined using a general purpose computing device, executing software
exemplary of the aspects of the present invention. The computing device may
have any suitable combination of dynamic and persistent storage memory. To
classify the disease state of a test subject 112, a plurality of 3D images 104
is
first collected from subjects in population 110 (in some embodiments,
typically
only one image is collected for each subject). The subjects within population
110
consist of three separate groups: reference subjects 126, training subjects
128
and control subjects 130. This results in a set of reference subject images
116,
training subject images 118, and control subject images 120. In a preferred
embodiment, all subject images are acquired using the same standard, one
example for which is described in Mazziotta J C, Toga A W. Evans A, Fox P,
Lancaster J, "A probabilistic atlas of the human brain: theory and rationale
for its
development", The International Consortium for Brain Mapping (ICBM),
Neuroimage 1995, 2(2):89-101.
This image data is presented to the classification system 102 to train
itself in the classification of a particular neurological disease or disorder,
CA 02565646 2006-10-26
- 7 -
The automated classification system 102, once trained, may then classify any
test subject 112 on the basis of that subjects image data 108.
The exemplary steps performed by the automated classification
system 102 are illustrated in the flow chart shown in FIG. 2. In step 8202, 3D
image data 104 is first collected from the scanner and pre-processed.
Following
image acquisition, known types of preprocessing operations are typically
executed to prepare the images for use in analysis in later steps. These
preprocessing operations may include the correction of intensity
inhomogeneities
or global re-alignment (registration) of the image into a standard reference
space.
Based on standard reference coordinates, one or more particular volumes of
interest (VOls) within the brain are manually selected in step S204, the
specific
selection of a VOI depending on the particular disease that is to be
classified.
Both intensity and spatial characteristics of the image data are calculated in
steps S206 and 8208. These steps define the features of the images that will
be
analyzed in later steps. Statistical models are created in step S210 based on
training subject images 118 and define multi-dimensional spaces within which
subjects may be represented. These statistical models are merged to create one
single, final multi-dimensional classification space or universe. In step
8212, a -
classifier is built within this classification space based on control group
image
data 120 and divides the universe of subjects into two or more regions, such
that
each region defines a space of subjects having a particular condition (or
state of
nature). This classifier is then used in step 8214 to identify and
characterize the
disease state of individuals, such as a test patient 112, based on the
location of
an individual's representation within the classification space.
The data collection step S202 is more particularly illustrated in FIG.
1 and FIG. 3. Prior to the processing of any data by the automated
classification
system 102, subjects are selected in step S302. For each selected subject,
brain
image data is acquired in step S304 using an appropriate medical imaging
device. This results in image data 116, 118, 120 for reference subjects,
training
CA 02565646 2006-10-26
- 8 -
subjects, and control subjects, respectively. This is the image data that is
required in order to build and train the automated classification system 102
to
diagnose and classify a particular neurological disease or disorder. Test
subject
image data 108 is also obtained for the individuals whose neurological disease
state is to be diagnosed and classified by the automated classification system
102. Possible types of images that may be acquired include, but are not
limited
to: 11w MRI, T2w MRI, PD MRI, PET, SPECT, and CT. The nature of the
information encoded at each voxel of the image data will depend on the
particular
imaging modality chosen, and thus the term "intensity" and "image signal" are
intended to cover the different possibilities corresponding to the different
modalities.
The subjects may be chosen in step S302 in a number of different
ways, understood by a person skilled in the art, in order to discriminate
between
groups of subjects on the hypothesis that there exists intensity and spatial
differences between brain images of individuals in the groups. Groups of
subjects
need not always include "normal" non-pathological individuals. For example,
the
classifier may be used to separate between groups of pathological individuals.
In
order to capture the variability between individual subjects within the
statistical
models, a large enough number of training subjects 128, must be selected,
Selecting a minimum of 30-40 training subjects 128 is sufficient. Similarly,
the
selection of a minimum of 30-40 control subjects 130 is sufficient for
determining
functions that divide the universe of subjects into classification regions. It
is not
necessary that the group of control subjects contain known members of each
possible condition (or state of nature), For example, pathological individuals
of a
particular condition (or state of nature) might be classified by the system on
the
basis of a control group consisting solely of known pathological subjects of
that
particular condition (in such an embodiment, a different model for the
definition of
membership within each classification region would be built than one for which
the control group contains known member of each possible condition). In a
CA 02565646 2014-06-06
9
preferred embodiment, the training subject images 118 and the control subject
images 120 are obtained from two distinct groups of subjects in order to
ensure
statistical independence.
Global intensity correction is typically performed on all of the images
in order to correct intensity inhomogeneities due to scanner variations (not
shown
in FIG. 3.) A number of standard techniques may be used to accomplish this.
Two such techniques are described in J. G. Sled, A, P. Zijdenbos, and A. C.
Evans, "A Nonparametric Method for Automatic Correction of Intensity
Nonuniformity in MRI Data", IEEE Transactions on Medical Imaging, Vol. 17, No.
1, February 1998, pp. 87-97, and Van Leemput K, Maes F, Vandermeulen 0,
Suetens P, "Automated model-based bias field correction of MR images of the
brain", IEEE Trans Med Imaging 1999, 18(10):885-96.
As illustrated in FIG. 3, after subject selection S302 and brain image
acquisition S304 there are different sets of subject images, 116, 118, 120,
and
108. Each set of subject images serves a different purpose in the automated
classification system 102. The present system does not require that all of
these
images be pre-processed in step S202 as shown in FIG. 3 at the same time (e.g.
the test subject images 108 may be pre-processed at a separate time, possibly
at
a clinic for diagnosis).
Reference subject images 116 facilitate the comparison of the image
data between different individuals by being the basis for the formation of a
single
reference image 318 against which all other images may be registered. After
the
reference image 318 is formed, the reference subject images 116 are no longer
needed. The linear registration of an image against a reference image 318 in
step
S320 will globally align the image into a standard reference space, such as
the
Talairach space (a normalized coordinate system commonly used in the field of
neuroscience). For example, the linear registration technique described in D.
L.
Collins, P. Neelin, T. M. Peters, and A. C. Evans, "Automatic
CA 02565646 2014-06-06
3D Intersubject Registration of MR Volumetric Data in Standardized Talairach
Space", Journal of Computer Assisted Tomography, Vol 18(2), March/April 1994,
5 pp. 192-205, describes a method based on a 3D cross-correlation with an
average brain image volume. An image may be quantitatively determined to be
aligned into a standard reference space through the minimization of an error
or
cost function based on the cross-correlation of image gradients. Thus,
reference
subject images 116 are first each registered with a standard reference space
in
10 step S314. A voxel-by-voxel average of all of the reference subject
images is
then taken in step S316 to create a final, single reference image 318.
Training subject images 118 are used to build the statistical model,
which are the mathematical variation models which define multi-dimensional
spaces within which subjects may be represented. Control subject images 120
are used to build mathematical functions that will identify and characterize
the
disease state of individuals. Test subject images 108 are used to represent a
test
patient 112 who is to be classified by the classification system 102. All of
these
subject images are linearly registered in step S320 against the reference
image
318. For example, a 9-degrees of freedom (3 translational, 3 rotational, 3
scaling)
linear transformation that maximizes the cross-correlation between
characteristics of a subject image and the reference image 318 at each voxel
might be employed to accomplish the linear registration in step S320. Other
linear
transformation techniques can be employed in other embodiments. Initial
processing of the subject images also includes resampling the data onto an
isotropic grid in step S322. In a preferred embodiment, an isotropic grid with
a
resolution of 1 mm3 is used. Other known pre-processing techniques that can be
employed include AIR and SPM, described in Woods R P, Grafton S T, Watson J
D, Sicottte N L, Mazziota J C, "Automated image registration: II. Intersubject
validation of linear and nonlinear models", Journal of Computer Assisted
Tomography 1998, 22(1):153-165 and described in Ashburner J, Friston K J,
CA 02565646 2014-06-06
11
"Voxel-based morphometry-the methods", Neuroimage 2000, 11(6 Pt 1):806-
2100.
After image data has been collected and pre-processed in step S202
a large, non-specific volume of interest (V01) is selected in step S204. This
will
typically be done manually by a person with sufficient experience to decide
what
is a suitable VOI in the particular circumstances. It is, however,
contemplated to
widen the search space so that even large (more than 1/3) portions of the
brain
might be sufficient to perform this task, regardless of anatomical
variability. It is
also contemplated that a computer with artificial intelligence might be
programmed to perform this task.
This step of selecting a VOI is more particularly illustrated in FIG. 4.
The present system does not require that this step be performed for all of the
subject images 104 at the same time (e.g. the test subject images 108 may be
processed at a separate time, possibly at a clinic for diagnosis). The VOI is
defined in step S402 for the purpose of extracting a specific portion of a
global
brain image for analysis. There are a number of advantages to using a
relatively
large, non-specific VOL First, the VOI is useful because particular diseases
will
affect certain areas of the brain more than others. The VOI allows a focused
analysis that reduces the noise introduced into the global analyses by parts
of the
brain outside of the VOL, however, it is not generally the case that only a
single
anatomical structure of the brain is affected by a given disease. Often there
are
complex interactions between brain components, which cannot be captured
through the analysis of a single brain structure. Thus, the selection of a
larger
VOI in step S402 that encapsulates more than one brain structure enables the
present invention to analyze characteristics of a specific volume in the brain
without restricting analysis to a single brain structure.
The VOI will typically be selected to cover a larger region of interest
than one specific brain component. Thus the VO1 can be selected to encompass
one or more specific components of the brain which are known to be associated
CA 02565646 2006-10-26
- 12 -
with a specific pathology, and will provide a boundary that extends a distance
beyond the edge of the component(s) of interest,
The VOI is also "non-specific" in the sense that absolute accuracy
in the delineation of the boundary of the VOI is not essential. Even if the
selection
of a given VOI in step 8402 is inaccurate (e.g. centimeters off from an
optimal
selection) the classification system will still likely function properly to
classify a
test subject. The larger the number of subjects used in training and building
the
system, the less precise the selection of the VOI needs to be. One practical
advantage is that the selection of the VOI in step 5402 may possibly be done
by
an individual who merely has neuroanatomical knowledge and does not
necessarily need to be an individual with special expert medical or
neuroscientific
knowledge.
The present invention also combines the analysis of different
features of both intensity and spatial shape characteristics of images. This
allows
even greater flexibility in the image analysis, since a different VOI may be
selected at step 5402 for each particular feature of interest that is to be
analyzed.
For example, one VOI may be selected for the analysis of a feature based on
intensity data, while a second VOI may be selected for the analysis of a
feature
based on spatial data. The classification system will perform its analysis
taking
into account both VOls. Thus, multiple and different VOls may be defined for
any
given application of the classification system.
Once a VOI has been defined in step 5402, that portion of the
image is extracted from the global volume based on its standard reference
(e.g.
Talairach) coordinates. This extraction is performed for a given subject image
104 as well as the reference image 318, resulting in a reference VOI image 408
and a subject VOI image 410. To further reduce any positional variations in
brain
structures due to normal inter- and intra-individual variability not
eliminated
during the linear registration step S320 (since that step is a global
registration of
the entire image and not just the selected V01)1 the subject VOI image 410 is
CA 02565646 2006-10-26
- 13 -
linearly registered against the reference Vol image 408, For example, a 12-
degrees of freedom (3 translational, 3 rotational, 3 scaling, 3 skewing)
linear
transformation that maximizes the cross-correlation between characteristics of
a
subject VOI 410 and the reference VOI 408 at each voxel might be employed to
accomplish the linear registration. Some other possibilities for this linear
registration of the subject VOI image against the VOI image include using
fewer
degrees of freedom, however a 12-degrees of freedom transform substantially
reduces the "barrel effect", due to gradient coil inhomogeneity.
In step S206. training subject images 118, control subject images
120, and test subject images 108, are intensity processed as illustrated in
FIG. 6.
The present system does not require that all of these images be processed at
the
same time (e.g. the test subject images 103 may be processed at a separate
time, possibly at a clinic for diagnosis). Intensity data for a given subject
VOI
image 506 is first intensity normalized in step $502 with respect to the
reference
VOI image 408 to reduce unwanted noise from the analysis. This produces a
normalized subject VOI image. In intensity modeling, non-linear registration
of the
VOI is not performed because it would induce conformity in all data sets,
potentially eliminating the pathological effects that are being modeled at the
same time as the normal, anatomical variability.
Training subject normalized images 118, control subject normalized
images 120, and test subject normalized images 108 are rasterized in step $510
to produce a subject intensity vector (i.e. single vector created by
"unwrapping"
the 3D image data). Subject intensity vector (g) 512 represents a particular
feature of the VOI of a given subject. For example, the feature may be the
voxel-
by-voxel difference between a subject VOI image 506 and the mean of all
subject
VOI images 506 in the training group. The resulting subject intensity vector
512
would be:
CA 02565646 2006-10-26
- 14 _
g=vSlIbi.ect¨vaverage
Other intensity based features might be determined through the use
of texture operators to calculate voxel-wise higher-order intensity features.
Spatial data is calculated for each subject VOI as well. In step
S208, training subject images 118, control subject images 120, and test
subject
images 108, are processed for spatial shape-based features, as illustrated in
FIG. 6. The present system does not require that all of these images be
processed at the same time (e.g. the test subject images 108 may be processed
at a separate time, possibly at a clinic for diagnosis). A non-linear
registration of
a given subject VOI image 608 against the reference VOI image 408 is performed
first in step S602. Non-linear registration S802 attempts to match image
features
from a source volume to those of the reference image at a local level. The
result
of the non-linear registration is a dense deformation field 608 that captures
the
displacements required to align the subject VOI image 506 to the reference VOI
image 408. A number of non-linear registration processes exist for performing
this process. One example is ANIMAL, described in D. L. Collins, C. J. Holmes,
T. M. Peters, and A. C. Evans, "Automatic 3-D Model-Based Neuroanatomical
Segmentation", Human Brain Mapping, Vol. 3,1995, pp. 190-208, the contents of
which are hereby incorporated by reference, The ANIMAL algorithm attempts to
match image grey-level intensity features at a local level in successive
blurring
steps, by minimizing the cross-correlation function of voxel intensities
between
source and reference images. For example, the non-linear transformation
(represented by a deformation field 608) may first be determined at a low
resolution (highly blurred data) with 8 mm of spacing between the nodes. The
results are refined recursively by increasing the resolution to 4 mm, then 2
mm,
and finally 1 mm. Another possible approach to non-linear registration may be
to
register the VOI using basis functions, and then perform an analysis of the
basis
function weights.
CA 02565646 2014-06-06
A series of calculations are performed in step S610 on the resulting
dense deformation field 608 to produce a rasterized vector which represents a
5 particular
feature of the VOI of a given subject such as local volume change.
Other examples might include torque or shift magnitude. A method of computing
the local volume change at each voxel by using the rate of the Jacobian change
of the deformation is described by M. K. Chung, K. J. Worsely, T. Paus, C.
Cherif,
D. L. Collins, J. N. Giedd, J. L. Rapoport, and A. C. Evans, "A Unified
Statistical
10 Approach to Deformation-Based Morphometry", Neuroimage, Vol. 14(3),2001,
pp. 595-606, If U represents the deformation field which matches homologous
points between two images by storing a 3-0 displacement vector for each voxel,
then the deformation in the Lagrangian coordinate system at time t is:
x¨OX+U(X,0
15 The local
volume change of the deformation in the neighbourhood of
any given voxel at a point x is determined by the Jacobian determinant J which
is
defined as:
i(x, t) ¨ ¨ der(! U
-Tx)
where I denotes the identity matrix and 3x3 displacement gradient matrix VU
is:
CA 02565646 2006-10-26
- 16 -
1 ath otli
()xiax ax,
au aU2 aU2 802
vu =--(x,0= ¨õ,
ax oxi 0X2 ox3
803 003 803
kôxj ax, 8x3 J
For relatively small displacements, the trace of the 3x3
displacement gradient VU is a crude yet indicative measure of local volume
change:
.1.74+tr(V
Thus, a rasterlzed subject trace vector (t) 612, calculated at step
5610, is an indicator of morphological change and represents a particular
feature
of the VOI of a given subject 506 (namely, the local volume change at each
voxel). If the feature is the voxel-by-voxel difference between a subject VOi
image 506 and the mean of all subject VOI images 606 in the training group,
the
resulting subject trace vector 612 would be:
t=V 1.! ¨V
suuject avenge
where,
V=Vlocal volume change4r070
CA 02565646 2014-06-06
17
One possible Implementation of the trace calculation is discussed in
A. L. Janke, G. de Zubicaray, S. E. Rose, M. Griffin, J. B. Chalk, and G. J.
Galloway, "4D Deformation Modeling of Cortical Disease Progression in
Alzheimer's Dementia", Magnetic Resonance in Medicine, Vol. 46, 2001, pp. 661-
666.
Another possibility for spatial modeling may be to use each of the
differential elements in the displacement gradient matrix VU for tensor-based
morphometry as described in Thompson P M. Giedd J N, Woods R P,
MacDonald D, Evans A C, Toga A W, "Growth patterns in the developing brain
detected by using continuum mechanical tensor maps", Nature 2000, 404(6774);
190-3.
The creation of variation models in step S210 is more particularly
illustrated in FIG. 7. In step S702 training subject vectors 704 are analyzed
using
Principal Components Analysis (PCA). In the intensity data and spatial data
calculation steps S206, S208 discussed previously, a set of vectors are
created
that represent particular features of the VOI of a given training subject. For
example, for each training subject there may exist a training subject trace
vector
(t) 612 and a training subject intensity vector (g) 512. Linear variation
models 706
are created for each particular feature (e.g. one for local volume change and
one
for intensity difference).
For a given feature, if there are N subjects in the entire set of training
subjects, and there are L number of voxels in the VOI, then each subject is a
point in L-dimensional space. For example, each training subject trace vector
(t)
612 is a vector of length L and the entire model training subject dataset 704
for
the trace feature may be expressed in matrix form:
CA 02565646 2014-06-06
18
r1,1 = .=
M
TN! 44,
Application of PCA in step S702 to the model training subject dataset
704 results in a set of eigenvectors that characterize the training data.
After this
stage, the training subject data is no longer needed, as the statistical model
has
now been generated. As long as N L, then the total number of non-zero
eigenvectors of the covariance matrix is N-I. These resulting eigenvectors may
then be used to create a statistical model of the appearance of the Image. For
example, a linear variation model 706 can be generated that can describe any
instance of a subject trace vector based on the training subject dataset
704.For
example, using the notation identical to that in employed in T. F. Cootes, G.
J,
forwards, and C. J. Taylor, "Active Appearance Models", IEEE Transactions on
Pattern Analysis and Machine Intelligence, Vol. 23, No.6, June 2001, pp. 681-
685:
t=t +P b
mcan t t
where t
-mean is the mean normalised trace vector, Pt is the set of
orthogonal modes of variation (eigenvectors) for the trace data and bt is a
vector
of parameters. A given subject trace vector is described by varying bt. The
upper
bound on the dimensionality of Pt and bt is the total number of eigenvectors,
which is N-I.
Similarly, a linear variation model 706 may also be generated for the
training intensity data:
CA 02565646 2006-10-26
- 19 -
g=gmean+PEP8
A linear variation model 706 is generated for each set of training
subject vectors 704 that represent a particular feature of the VOL Generalized
forms of the model training subject matrix 704 and linear variation model 706
are
6 shown below.
xi.i = = -
M
XN 71 XN ,L
X = Xmean Pxb.,
where
1Nv
xmean =
N ________________ 4
13, is the set of orthogonal modes of variation: and
b, is a vector of parameters
The ensemble of principal components from each of the linear
variation models 706 define an Allowable Domain as the space of all possible
elements expressed by the eigenvectors. For example, an Allowable Grey
Domain G is defined by the intensity eigenvectors and an Allowable Trace
Domain T is defined by the trace eigenvectors. We now wish to reduce the
dimensionality of these Allowable Domains from the upper-bound of N-I. For
example, in order to determine how each principle component contributes to the
total variance of the system, the ratio of relative importance of the
eigenvalue 4
associated with the eigenvector k might be used:
CA 02565646 2006-10-26
- 20 -
Ak
rk = -
N-I
where the fraction rk is the relative importance for eigenvalue 1,k. This
information
may be employed to reduce the dimensionality of the Allowable Domains by
retaining fewer than N-1 eigenvectors, thus defining a restricted space
Allowable
Grey Domain G' and a restricted Allowable Trace Domain T*. It is contemplated
that other types of linear variation models might also be created using other
analytical methods, such as independent component analysis.
In step S708, the restricted spaces are merged to create a single,
final classification eigenspace or universe C*. It is within this eigenspace
that
Subjects are classified, based on their expressed eigencoordinates, For
example,
classification eigenspace C may be created by merging restricted Allowable
Trace Domain T* and restricted Allowable Grey Domain G*. Individuals can thus
be represented in the space:
C*---T*UG*
The classifier building step S212 is more particularly illustrated in
FIG. 8. In this step, control subject vectors 804 are used to create
discriminant
functions 808 that divide the eigenspace into regions to classify a given test
subject 112 (e.g. one region for those test subjects likely to have a
particular
disease state and one region for those that are unlikely to have the disease
state). The control group clataset thus contains as many homogeneous groups of
individuals as necessary for the classification problem. Each individual in
the
control group is assigned a state of nature in. For example, two states of
nature
CA 02565646 2006-10-26
-21 -
may be defined in the system: wi or normal subjects and cu2 for patients. Each
control subject vector 804 is projected into the classification eigenspace C*.
In the intensity data and spatial data calculation steps 5208, S208
discussed previously, a set of vectors are created that represent particular
features of the VOI of a given control subject. The vector representing a
particular feature for each control group subject i, belonging to state w, is
projected into the corresponding restricted Allowable Domain for that feature.
For
example, if each control subject i has a control subject trace vector (t) 612
and a
control subject intensity vector (g) 512, then vector (t) 612 of each subject
i
belonging to state co is projected into Domain 1* forming eigencoordinate
vector
r. Similarly, vector (g) 512 is projected into Domain G* forming
eigencoordinate
vector y1.
A number of possible features may be calculated on the distribution
of eigencoordinate vectors. One possibility is to use the eigenposition along
the
principal component axis. If the distribution of the eigencoordinate vectors
is
assumed to be normal (Gaussian) then the formulation of feature vectors c for
each subject i within classification eigenspace C may be represented as:
(IL?,
¨ kJ let
where CO indicates which state the control subject belongs to.
Based on the control group subject data 804, a multivariate linear
discriminant analysis (LDA) classifier is built in the classification
eigenspace C*,
in step S306. Linear discriminant functions 808 are defined for this purpose.
For
example, if there are two states wi and (02, the following discriminant
function f(c)
808 might be built;
CA 02565646 2006-10-26
- 22 -
f(c)=wde+wA
where w is the weight vector, d represents the dimension of classification
eigenspace C", c is the feature vector of a subject expressed in
eigencoordinates, and wo is the bias or threshold weight. The parameters into
a
given linear discriminant function 303 (weight vector and bias/threshold
weight)
determine the orientation and location of a linear decision boundary. These
parameters are based on the control group subject data 804. For example, these
parameters may be set automatically using statistics software Such as SYSTAT,
JMP IN or MATLAB.
For a two-state classifier, the classification rule for linear
discriminant function 808 may be stated as:
decide col iff(c)>0 and (02 iff(c),.0
Though not necessary to the present invention, in an effort to
further reduce the dimensionality of the classification eigenspace C*, it is
possible to select only the most significant eigenvectors for classification
in C*,
based on the control group subject data 804. This might be done in a multi-
level
fashion, by selecting the most significant eigenvectors in each Allowable
Domain
separately (e.g. T* and G*). These spaces would be combined to form a new
classification eigenspace of reduced dimensionality. Forward stepwise
regression, backward stepwise regression and Wilks' lambda statistics are
among the numerous methods that may be used in the determination of
significant eigenvectors in this process.
The classification of a new test patient 112 in step $214 is more
particularly illustrated in FIG. 9. In the intensity data and spatial data
calculation
steps S206, S203 discussed previously, a set of vectors are created that
CA 02565646 2014-06-06
23
represent particular features of the VOI of a given test subject. The vector
representing a particular feature for the test subject 112 is projected into
the
classification eigenspace C* in step S902 in the same manner as described
above for the control group subject data 804 to formulate a feature vector for
that
subject in step S906. The resulting feature vector is then analyzed according
to
LDA discriminant functions 808 built in the classification building step S212.
Other types of classifiers that might be employed include logistic regression,
artificial neural networks and support vector machines.
The automated classification system 102 has been successfully
applied to temporal lobe epilepsy (TLE) lateralization, as described in S.
Duchesne, N. Bernasconi, A. Bernasconi, D. L. Collins, "Temporal lobe epilepsy
lateralization based on MR image intensity and registration features",
Conference
Proceedings of MICCAI, Springer Verlag, (2003), 2879(1):367-374. TLE is
defined by seizures originating in the medial temporal lobe (MTL). Since the
majority of TLE patients are resistant to anticonvulsant drugs but can be
helped
by surgery, the present invention is useful in the automated lateralization of
the
seizure focus as being left or right MTL in origin. Currently, lateralization
is
performed on the basis of volumetric analysis of hippocampal atrophy and
requires a priori segmentation of the hippocampus.
In the data collection step S202, the population subjects 110 are
selected. They consist of 150 reference subjects (taken from the International
Consortium for Brain Mapping database), 150 training subjects (in this case,
the
same set of subjects as the reference subjects), and 138 control subjects
(consisting of 51 normal subjects and 87 patients). The normal subjects in the
control group are different from those in reference and model training group.
The
patients in the control group are further subdivided into groups of patients
with left
TLE (47) and right TLE (40) as determined by manual volumetry. 3D MRI
CA 02565646 2014-06-06
24
brain images are gathered in step S304 for each subject using a 1.5 T scanner
Ti -fast field echo sequence.
Recent observations in patients with TLE, in N. Bernasconi, A,
Bernasconi, Z. Caramanos, S. B. Antel, f. Andermann, and D. L. Arnold, "Mesial
temporal damage in temporal lobe epilepsy: a volumetric MRI study of the
hippocampus, amygdala and parahippocampal region", Brain, Vol. 126(Pt 2),
February 2003, pp. 462-9, indicate that the epileptogenic zone is broad. The
research suggests that the substrate for seizure generation is distributed
over a
network of brain structures in the MTL and not just the hippocampus. Thus, in
this
application, a large non-specific VOI centred on the left MTL is selected in
step
S204, capturing the hippocampus and neighbouring structures. The VOL is
360800 voxels in size (55x82x80).
This application uses both intensity and trace vectors. The calculation
of intensity data in step S206 consists of the voxel-by-voxel difference
between a
subject VOI image and the mean of all subject VOI images in the training
group,
resulting in the following subject intensity vector 512:
g=v5ubj cci¨ Pa Image
The calculation of spatial data in step S208 consists of the trace of
the Jacobian matrix of the deformation field for a given subject VOI image,
which
is an indicator of morphological change (namely, the local volume change at
each
voxel). This results in the following subject trace vector 612:
fr-Vsutiect¨Veverege
where,
CA 02565646 2006-10-26
- 25 -
V=Viocal volume charizetrR
The creation of linear variation models 706 in step S210 is based
on intensity and trace model subject training vectors 512, 612. The first 25
eigenvectors for each model (25 trace, 25 intensity) were chosen, for a total
of 50
eigenvectors in the classification space.
Three states of nature are defined for the classifier building step
S212. Normal subjects 0)1), left TLE 0.02), and right TLE (0)3), The prior
probabilities for each state of nature are p(%)=0.37, p(w2)=-0.34, and
p(co3)=0.29.
The first classification performed distinguishes between normal (wi) and TLE
(m2,
(.03) states. A backward stepwise regression is used, which reduces the number
of eigenvectors kept from 50 to 20. The second classification performs
lateralization of the TLE. A forward stepwise regression with identical
tolerance
as previously used is employed.
To classify each test patient 112 in step S214, a feature vector is
formulated in step S908 for each test subject 112:
w-y UT (u
pi
In this example, the results of classifying each subject in the control
group as a test subject 112 are summarized below. Table 1 summarizes the
results of the first classification between normal and patient subjects
(accuracy
95%) and Table 2 summarizes the results of the TLE lateralization (accuracy
75%).
CA 02565646 2006-10-26
- 26 -
TABLE 1
True positive results on the Normals-Normals/TLE-TLE diagnonal,
shown in bold
Normals TLE
correct
Normals 45 6 88
TL E 1 86 99
Total 46 92 95
TABLE 2
True positive results on the Left-Left/Right-Right diagonal,
shown in bold
Left Right
correct
Loft 36 11 77
Right ii29 73
Total 47 40 75
Another example of the successful application of the automated
classification system 102 is its application to the successful computerized
differentiation of Alzheimer's dementia (AD) and mild cognitive impairment
(MCI)
from normal aging (NA). AD is a progressive neurodegenerative disorder.
Currently, the diagnosis of clinically probable AD can be made with high
accuracy in living subjects only once the stage of dementia has been reached,
and requires clinical, neuropsychological and imaging assessments, Early
detection of AD is therefore critical if treatment is to be effective.
In the data collection step 5202, the population subjects 110 are
selected. They consist of 152 reference subjects, 152 training subjects, and
44
CA 02565646 2014-06-06
27
control subjects (consisting of 22 normal subjects, 15 subjects with AD, and 7
subjects with MCI). 3D MRI brain images are gathered in step S304 for each
subject with T1-weighted MRI protocol on a 1.5 T scanner using a fast gradient
echo sequence.
Neuropathological studies, such as in J. R. Petrella REC, P. M.
Doraiswamy, "Neuroimaging and Early Diagnosis of Alzheimer Disease: A Look
to the Future", Radiology 2003, 226(2):315-336, have shown that brain
degeneration occurs very early in the course of the disease, even before the
first
clinical signs, in certain regions such as the medial temporal lobe (MTL). In
this
application, a large non-specific VOI centered on the left MTL is selected in
step
S204. The VOI is 55x82x80=360800 voxels in size and captures the
hippocampus and neighboring MTL structures, such as the parahippocampal
gyrus.
Both intensity and trace vectors are employed in this application.
Intensity data is calculated in step S206 by taking the voxel-by-voxel
difference
between a subject VOI image and the mean of all subject VOI Images in the
training group, resulting in the following subject intensity vector 512:
g--"Vsubject¨Vaverage
The calculation of spatial data in step S208 consists of the trace of
the Jacobian matrix of the deformation field for a given subject VOI image,
which
is an indicator of morphological change (namely, the local volume change at
each
voxel). This results in the following subject trace vector 612:
i'vsubjecrilitverage
CA 02565646 2006-10-26
- 28 -
where,
V=Vlocat volume ehange4atr(V ti)
Linear variation models 706 are created in step S210 based on
intensity and trace model subject training vectors 512, 612. The first 40
eigenvectors were chosen for the classification eigenspace.
Three states of nature are defined for the classifier building step
S212, normal subjects (col), AD subjects (w2), and MCI Subjects ((o3). The
prior
probabilities for each state of nature are p(wi)=0.50, P(o32)=0.34, and
p(w3)=0.16.
Forward stepwise regression was used to select eigenvectors that yielded
maximal discrimination between the groups. The first classification
distinguishes
between normal (o01) and AD (002) states, after reducing the number of
eigenvectors from 35 to 3 with the stepwise process. The Second classification
distinguishes between normal (col) and AD+MCI (w2, 0)3) states, after reducing
the number of eigenvectors from 40 to 2 with the regression model. The third
classification distinguishes between AD (c02) and MCI (033) states, after
reducing
the number of eigenvectors from 20 to 3 with the regression model.
Tables 3, 4 and 5 summarizes the results of the three
classifications. respectively.
TABLE 3
True positive results on the AD-AD/Normal-Normal diagonal,
shown in bold
AD Normal
correct
AD 15 0 100
Normal 0 22 100
Total 15 22 100
CA 02565646 2006-10-26
-29 -
TABLE 4
True positive results on the AD + MCI-AD + MCl/Normal-Normal
diagonal; shown in bold
AD + MCI Normal
correct
AD + MCI 22 0 100
Normal 0 22 100
Total 22 22 100
TABLE 6
True positive results on the AD-AD/MCI-MCI diagonal,
shoWn in bold
AD MCI
correct
AD 12 3 80
MCI 0 7 100
Total 12 10 90
These examples serve to illustrate the potential applicability of the
present automated classification system to the detection of neurological
diseases
or disorders. Schizophrenia is anbttier example of a neurological disorder
that
the present invention may be applied to. The system might also be applied as a
differentiator between Alzheimer's dementia and other types of dementia such
as
frontal lobe dementia, Parkinson d*entia, and vascular dementia. Studies on
movement disorders may also be conducted using the present invention.
Having described aspects of the prior art, embodiments of the
present invention will now be describ0.
=
CA 02565646 2006-10-26
- 30 -
In one aspect of the invention there is provided a method for
predicting the evolution of the state of a subject. By state of a subject it
is Meant
a clinical state which relates to the physiological or health state of the
subject.
Preferably the state of the subject refers to the physiological or health
state of a
6 particular organ or system of the subject. For example, the method can be
used
to predict the evolution of the state of a subject with regard to his or her
cardiac
system, nervous system and the like. The method is generally illustrated in
FIG
wherein image-based data from a subject is imported into a predictive model
to obtain a predictive value for the future state of the subject. Thus, one or
more
10 images are acquired at step S1001 from a volume of interest (V01) of a
subject
and at least one image feature is extracted or measured, step S1002 and
imported in the predictive model, step S1003 to generate a predictive value of
the
evolution of a state of the subject. The state of the subject may be assessed
by
known clinical scales. These clinical scales are well known in the art and are
function of the state to be assessed. A few examples of clinical scales
include but
are not limited to MMSE for assessing MCI, tumor staging for assessing the
aggressiveness of a cancer and the like.
The predictive model is a statistical image-based model that is
established by providing a correlation function between one or more image
features and a future value of a clinical variable that represents a measure
on a
given clinical scale. The establishment of the model is realized by acquiring
data
from a group of training subjects.
As mentioned previously, the image data can be acquired using
any imaging modality suitable for acquiring information from a given volume of
interest (V01). Such modalities include but are not limited to MRI, including
structural, spectroscopic, functional, diffusion, and magnetization transfer
MR1,
near infrared imaging, optical imaging, microwave imaging, X-ray, ultrasound,
PET, SPEC, CT, scintigraphy, tomosynthesis, fluoroscopy, portal imaging, and
CA 02565646 2006-10-26
- 31 -
combinations thereof, The model may incorporate variables from more than one
imaging modality.
The selection of the training subjects is in part dictated by the
particular clinical state for which the establishment of a predictive model is
desired. Thus the training subjects may include subjects that have been
assessed as presenting characteristics of a given clinical state. Thus, in one
embodiment of the invention images of one or more VOls of the subjects are
acquired and the subjects are then clinically assessed over time using an
appropriate clinical scale for the state for which a prediction is desired.
The
model is built based on a univariate or multivariate analysis of one or more
image
features and one or more clinical variable.
It will be appreciated that the model may incorporate image
features representative of various stages of evolution of a particular state.
Thus,
the model can be built based on images acquired over a period of time for each
training subject. In another alternative embodiment the images from subjects
that
are at different stages of a given clinical stage may be acquired. For example
if
the evolution of a particular state, say a neurodegenerative disorder, is age
dependent the training group can include subjects of different ages.
The image features that are derived from the images in order to
build the model and to obtain a predictive value for the evolution of a state
of
subject can be any features commonly known in the art to be extractable from
the image. As described above, the features can be Classified in two broad
categories. One related to the image signal and the other to the spatial-shape
characteristics. By image signal it is meant the information encoded at each
voxel of the image data such as the intensity of the signal. Other type of
information can be encoded in the voxel such as the lifetime of the signal,
information about its frequency (such as the wavelength of light in optical
imaging) and the like. By spatial-shape characteristics it is meant
morphologic
information about the VOI. For example, the spatial-shape characteristics of a
CA 02565646 2006-10-26
- 32 -
VOI may represent anatomical structure within the VOI. Image features from any
Combination of voxels or all voxels within a VOI can be used in a model. Image
features are also meant to include any information that Is convey by a
particular
dimensionality of the image. People skilled the art will recognize that
information
provided by an imaging modality can be expressed in single or multi-
dimensional
spaces.
The model can be built by deriving a set of modes of variation of
Image features from a plurality of training subjects and selecting a subset of
the
modes of variation based on univariate or multivariate analysis, or a
combination
of both, between the modes of variation and at least one clinical variable.
The
selected subset is then used to provide a relationship, based on univariate or
multivariate analysis, or a combination of both between one or more image
features of a test subject and one or more clinical variable. In one
embodiment
the modes of variations can be derived as described above. With reference to
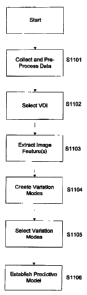
FIG. 11 the establishment of the predictive model comprises: collecting image
data from training subjects at step S1101, selecting one or more VOls at step
$1102, extracting one or more image features at step 51103, creating variation
modes at step 51104, selecting variation modes at step 51106 and establishing
the predictive model at step $1106.
The model may also comprise variables other than image features.
For example the model may comprise demographic information (age, sex, etc,.)
or results from a clinical test or any other variable that can increase the
accuracy
of the model.
It will also be appreciated that the predictive model may be
generated based on en ensemble of training subjects that have been classified
as described above. Thus while it is possible to use a clinical scale to
assess the
training subjects at different points in the evolution of the clinical state,
it is also
possible to classify the training subjects using the classification method as
described in FIG.1-9.
CA 02565646 2006-10-26
- 33 -
The predictive ability of the model can be assessed using
appropriate statistical analysis based on the predicted and actual values. For
example, the predictive ability can be assessed by computing F-statistics
based
on the residuals of predicted vs actual values for the clinical variable.
In another aspect of the invention, the predictive model can be
used in a patient management program for establishing a treatment protocol
based on a predicted evolution of a state of a subject. The predictive model
can
be also be used for selecting subjects in a clinical trial or study to improve
the
development more reliable epidemiological protocols.
In yet another aspect of the invention there is also provided a
system for predicting an evolution of a state of a subject, comprising one or
more
imaging devices for acquiring imaging data, an image processor for providing
image features measurements of at least one volume of interest (VOI) of the
subject and a predictive model calculator for providing a predictive value of
a
state,
Examples
The following examples describes the establishment of a predictive
model for Mild Cognitive Impairment but the person skilled in the art will
appreciated that other disease states as well as normal states can also be
predicted by an appropriately built predictive model. Disease states that can
be
predicted include but are not limited to temporal lobe epilepsy, general,
focal,
temporal lobe, frontal lobe_dementias alzheimer, parkinson, lewy bodies,
vascular, fronto-temporal, multiple sclerosis including primary progressive,
secondary progressive, relapse-remitting, mild cognitive impairment,
epilepsies
multiple systems atrophy, progressive supranuclear palsy, eorticobasal
degeneration.
CA 02565646 2006-10-26
- 34 -
Prediction of Mild Cognitive Impairment
MCI is widely viewed as the transition phase between normal aging
and Alzheimer's disease (AD) and amnestic MCI individuals are known to be at
risk for progression to AD. There is evidence that in those who will progress,
measurable hippocampal and entorhinal cortex atrophy, demonstrable on T1w
MRI serves as a moderate, though labor-intensive, predictor. Microscopically
the
strongest predictor of premodern cognitive dysfunction appears to be the
relative
area of entorhinal cortex occupied by beta-amyloid deposition. Existing MRI
measures that have been developped to predict decline are longitudinal, as for
example a study by Rusinek at al showing that an increased rate of atrophy in
the MR predicted future cognitive decline.
The Ethics Committee of the Montreal Neurological Institute
(Montreal, Canada) and the IRCCS San Giovanni di Die FEIF (Brescia, Italy)
approved the study and informed consent was obtained from all participants. A
total of 199 subjects were included in this study. The reference group
consisted
in 152 young, neurologically healthy individuals from the International
Consortium
for Brain Mapping database (ICBM) whose scans were used to create the non-
pathological, reference space. The training population consisted in 47 MCI
patients (23 MMSE < 30), seen at the IRCCS San Giovanni di Dia FBF
Hospital, that have been followed clinically a minimum of 12 months after
their
initial MR scan.
MRI data for our 152 ICBM subjects was collected with a T1w MRI
protocol on a 1.5 T scanner (Philips Gyroscan, Best, Netherlands) using a fast
gradient echo sequence (TR=18 ms, TE=10 ms, 1 NEX pulse sequence, flip
angle=30 , matrix size=256 x 256, FOV=256mm, slice thickness=1 mm). Data for
MCI patients were acquired on a 1.0T scanner (Philips Gyroscan, Best,
Netherlands) using an FFE sequence (TR = 19,7 ms, TE = 5.9 ms, sagittal
acquisition, 0_9365 x 0_9375 x 1.3 mm3), All global MRI data were processed to
CA 02565646 2006-10-26
- 35 -
correct for intensity non-uniformity due to scanner variations. The 152 ICBM
subjects were registered in a Talairach-like stereotaxic space in the context
of
the ICBM project Most (33/47) of the MCI data were linearly registered (9 Don
automatically into stereotaxic space while the remaining volumes were manually
registered due to high scalp brightness. All reference and training volumes
were
resampled onto a 1mm isotropic grid.
Two VOls were selected for this study, Centered on the left and
right medial temporal lobe, using Talairach coordinates (start coordinates x1-
57,+2] for the left and right side respectively, y=-53 and z=-52). Each VOI
measured n = 55 x 82 x 80 = 360800 voxels. The VOI was selected so that its
extent captured the hippocampus and neighboring MTL structures (e.g. ento and
perirhinal cortex, parahippocampal gyrus), irrespective of normal inter- and
intra-
individual variability. After extraction, each VOI was linearly registered (9
DoE) to
the reference volume to further reduce local distortions, and its mean
intensity
scaled to the mean intensity of the reference VOI, which serves to eliminate
the
first-order drift in signal measurement between patients.
Two image features at each voxel location were retained. The first
feature is the grey level intensity consisting in the rasterized data from the
intensity-scaled VOls. The second feature is the trace or the first-order
approximation of the determinant of the Jacobian matrix of a non-linear
registration-derived deformation field. The latter is calculated to map each
subject's VOI to our reference ICBM target. The trace represents an estimate
of
local volume change. Principal Components Analysis (PCA) is used to reduce
the dimensionality of the input training data and generate linear variation
models
based on the N=152 datasets from our ICBM normal subjects, The resulting four
PC models were each pz... N-1 (or 151-dimensional). Most of the variation can
usually be explained by a smaller number of modes, I, where I n and I <p. We
preceded in selecting 535 eigenvectors in total from our four models
(left/right
intensity/trace VOls),that accounted to a per-model variance of 99.7%,
CA 02565646 2006-10-26
- 36 -
The predictive model may be obtained as follows: an eigenspace
from a large training group of subjects is generated as described above. Then
test subjects image data are projected in the reference eigenspace to
calculate a
correlation coefficient between the projection coordinates and the clinical
variable
for each eigenvector. Thus rasterized vectors of the processed VOI image
features for each test subject are projected into the training space, and thus
form
eigencoordinate vectors. For each eigenvector the correlation of the
eigencoordinate distribution with the clinical variable is then computed. A
pre-
determined threshold for the correlation coefficient is selected and used to
identify eigenvectors for the predictive model. Finally, a predictive model is
then
built from those eigenvectors using multiple regression (for example: ..IMP
IN,
SAS Institute, Cary, N. Carolina). The model is then used to predict the
future
value of the clinical variable of interest.
A number of possible features can be calculated on the distribution
of the projected data. One example is to take a predictor that is based on the
position along the PC axes. The distribution of eigencoordinates along any
principal component for a given population is normally distributed as assessed
via Shapiro-Wilke statistics.
The selection of the q eigenvectors for the predictive model was
based on an arbitrarily predefined threshold for the correlation coefficient
of r
10.301.
Four experiments were completed. Experiment 1 served as a
baseline for the classification of our patient population into 3 groups based
on
their MMSE changes at 1 year follow-up from clinical variables (age, sex,
baseline MMSE). Experiment 2 attempted the same 3-group classification but
this time based on the projection eigencoordinates in the reference space.
Experiment 3 served as a baseline for the prediction of yearly MMSE decline by
building a linear model based on clinical variables ("Clinical"). Experiment 4
attempted the same prediction but with a model based on projected
CA 02565646 2006-10-26
- 37 -
eigencoordinates, as per the methodology described above ("MRI"), while in
Experiment 5 we added baseline MMSE as an additional variable to the
projected eigencoordinates ("MR1+baseline MMSE").
Results
When comparing MMSE results between baseline and 12 months
follow-up, we can separate the 47 patients in the test population into three
distinct groups: 16 decliners -1 point
negative change in MMSE or cognitive
decline), 6 improvers (> 1 point positive change in MMSE or cognitive
improvement), and 26 stable individuals (MMSE change between [-1 ,1]).
Demographic information about each group can be found in FIG 12 There was
no statistically significant age difference between either groups, as assessed
from ANOVA and Tukey-Kramer HSD P 0.05, DF=2). There was a statistically
significant baseline MMSE difference between the decliners and improvers (P =
0.0003, DF = 2), but no other significant difference between groups for
baseline
MMSE, The improvers had the lowest mean baseline MMSE of all three groups.
The classification was based on a leave-one-out, forward stepwise
linear discriminant analyses (SYSTAT 10.2, Georgia, PA; P-to-enter < 0.05 ) of
either clinical variables (age, sex and baseline MMSE) or eigencoordinates
along
the 535 reference space eigenvectors. The clinical classifier of Experiment
*Owes
53% accurate in separating the 47 patients into decliners, improvers and
stable
subjects (DF r-= 2, VVilk's klambda = 0.69 ), while the 3-way classifier based
on
projection eigencoordinates of Experiment 2 was 1001% accurate, with 31
significantly discriminant eigenvectors ( P-to-enter < 0.06 , DF = 31, VVilk's
\lambda = 0 ). FIG 12 displays the data plotted along the three most
discriminating eigenvectors.
CA 02565646 2006-10-26
- 38 -
Table 6
Model Features r r2 SD= F stat P F Stat P
To Clin. to MRI
Clinical 3 0.429 0.176 1.86
MRI 10 0.668 0.446 1.53 2.499 0.003
MRI+MMSE 11 0.696
0.484 1.48 2.691 0.002 2_555 0.002
Experiments 1 and 2 classified the data into groups. In the following
3 experiments the magnitude of the yearly MMSE change is predicted. Baseline
MMSE, age and sex were all negatively and weakly correlated with 1 year MMSE
change ( r = -0.25 r = -021 and r = 0.15 , respectively). In contrast, out of
the
535 reference space eigenvectors, 10 had a correlation ratio of r> 10.301 , We
predicted MMSE change for all patients using each linear model. The number of
input features to the model, the resulting correlation ( r) and squared
correlation
(r2) of predicted vs. actual values, the standard deviation of the predicted
score
and F-test values (against "Clinical" and "ICBM") are shown in table 6. Recall
that the first predictive model (Experiment 3) is based on the 3 clinical
variables
("Clinical"), the second (Experiment 4) on the 10 selected eigenvectors
("MRI")
and the last (Experiment 5) using the 10 eigenvectors plus the baseline MMSE
("MRI+baseline MMSE"). The linear fit for the "Clinical" and "MRI+baseline
MMSE" models are shown in FIG 13 alongside their residual plots. The best
model was the "MRI+baselineMMSE" of Experiment 5, with a correlation
between predicted and actual value of r 0.6955. It was also significantly
better
than either the "Clinical" model (Fstat = 3.39 , P = 0.0001, DF1 = 43, DF2 =
35)
or the "MRI" model ( Fstat = 2.59, P= 0,002, DF1 = 38, DF2 = 35).
The aforementioned and other features, benefits and advantages of
the present invention can be understood from this description and the drawings
by those skilled in the art. The above described exemplary embodiments of this
CA 02565646 2014-06-06
39
invention are intended to be illustrative and in no way limiting. Many
modifications
are possible in the exemplary embodiments without materially departing from
the
novel teachings and advantages of this invention. All such modifications are
intended to be encompassed within the scope of the present invention.