Note: Descriptions are shown in the official language in which they were submitted.
CA 02565915 2006-11-06
W02005/110359 1 PCT/EP2005/005078
87421pct
Inhalation powder formulations containing enantiomerically pure
beta-agonists
The present invention relates to powder formulations for inhalation,
containing
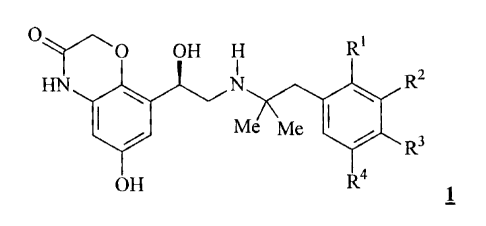
enantiomerically pure compounds of general formula 1
0 OH H R1
FIN, R2
Me Me 401
R3
OH R4 1
wherein the groups RI, R2, R3 and R4 may have the meanings given in the claims
and in the specification, optionally in the form of the pharmaceutically
acceptable
acid addition salts thereof, as well as optionally in combination with a
pharmaceutically acceptable excipient, processes for preparing them and their
use as
pharmaceutical compositions, particularly as pharmaceutical compositions for
the
treatment of respiratory complaints
Background to the invention
Betamimetics (13-adrenergic substances) are known from the prior art. For
example
reference may be made in this respect to the disclosure of US 4,460,581, which
proposes betamimetics for the treatment of a range of diseases.
For drug treatment of diseases it is often desirable to prepare medicaments
with a
longer duration of activity. As a rule, this ensures that the concentration of
the
active substance in the body needed to achieve the therapeutic effect is
guaranteed
for a longer period without the need to re-administer the drug at frequent
intervals.
Moreover, giving an active substance at longer time intervals contributes to
the
well-being of the patient to a high degree.
CA 02565915 2006-11-06
W02005/110359 2 PCT/EP2005/005078
In a particularly preferred embodiment the present invention relates to
pharmaceutical
preparations which may confer a therapeutic benefit in the treatment of
respiratory
complaints.
Particularly for treating respiratory complaints, it is useful to administer
the active
substance by inhalation. In addition to the administration of broncholytically
active
compounds in the form of metered aerosols and inhalable solutions, the use of
inhalable powders containing active substance is of particular importance.
With active substances which have a particularly high efficacy, only small
amounts
of the active substance are needed per single dose to achieve the desired
therapeutic
effect. In such cases, the active substance has to be diluted with suitable
excipients
in order to prepare the inhalable powder. Because of the large amount of
excipient,
the properties of the inhalable powder are critically influenced by the choice
of
excipient. When choosing the excipient its particle size is particularly
important. As
a rule, the finer the excipient, the poorer its flow properties. However, good
flow
properties are a prerequisite for highly accurate metering when packing and
dividing
up the individual doses of preparation, e.g. when producing capsules
(inhalettes) for
powder inhalation or when the patient is metering the individual dose before
using a
multi-dose inhaler. Moreover, the particle size of the excipient is very
important for
the emptying characteristics of capsules when used in an inhaler. It has also
been
found that the particle size of the excipient has a considerable influence on
the
proportion of active substance in the inhalable powder which is delivered for
inhalation. The term inhalable proportion of active substance refers to the
particles
of the inhalable powder which are conveyed deep into the branches of the lungs
when inhaled with a breath. The particle size required for this is between 0.5
and 10
preferably between 1 and 6 gm.
The aim of the invention is to prepare an inhalable powder containing a
betamimetic which, while being accurately metered (in terms of the amount of
active substance and powder mixture packed into each batch of powder by the
manufacturer or by the patient before use, depending on the inhaler, as well
as the
quantity of active substance released and delivered to the lungs on each
actuation by
the inhalation process) with only slight variations between batches, enables
the
active substance to be administered in a large inhalable proportion. A further
aim of
CA 02565915 2006-11-06
W02005/110359 3 PCT/EP2005/005078
the present invention is to prepare an inhalable powder containing a
betamimetic
which ensures good emptying characteristics of the capsules, whether it is
administered to the patient using an inhaler, for example, as described in WO
94/28958, or in vitro using an impactor or impinger.
The fact that betamimetics have a high therapeutic efficacy even at very low
doses
imposes further conditions on an inhalable powder which is to be used with
highly
accurate metering. Because only a low concentration of the active substance is
needed in the inhalable powder to achieve the therapeutic effect, a high
degree of
homogeneity of the powder mixture and only slight fluctuations in the
dispersion
characteristics from one batch of powder to the next are essential. The
homogeneity
of the powder mixture and minor fluctuations in the dispersion properties are
crucial in ensuring that the inhalable proportion of active substance is
released
reproducibly in constant amounts and with the lowest possible variability.
Accordingly, a further aim of the present invention is to prepare an inhalable
powder containing a betamimetic which is characterised by a high degree of
homogeneity and uniformity of dispersion. The present invention also sets out
to
provide an inhalable powder which allows the inhalable proportion of active
substance to be administered with the lowest possible variability.
The characteristics of emptying from the powder reservoir (the container from
which the inhalable powder containing the active substance is released for
inhalation) play an important part, not exclusively, but especially in the
administration of inhalable powders using capsules containing powder. If only
a
small amount of the powder formulation is released from the powder reservoir
as a
result of minimal or poor emptying characteristics, significant amounts of the
inhalable powder containing the active substance are left in the powder
reservoir
(e.g. the capsule or other container) and are unavailable to the patient for
therapeutic use. The result of this is that the dosage of active substance in
the
powder mixture has to be increased so that the quantity of active substance
delivered is sufficient to produce the desired therapeutic effect.
Against this background the present invention further sets out to provide an
inhalable powder which is also characterised by very good emptying
characteristics.
CA 02565915 2006-11-06
W02005/110359 4
PCT/EP2005/005078
Description of the invention
The present invention relates to inhalable powders containing one or more,
preferably one enantiomerically pure compound of general formula 1
0 OH H R1
2
HN R
Me Me 4101
R3
OH R4
1
wherein
denotes hydrogen, Ci-C4alkyl, Ci-C4alkoxy or halogen;
R2 denotes hydrogen, Ci-C4alkyl, Ci-C4alkoxy or halogen;
R3 denotes hydrogen, C1-C4-alkyl, Ci-C4-alkoxy, halogen, 01-1,
-0-Ci-C4-alkylene-COOH or -0-CI-C4-alkylene-000-Ci-C4-alkyl;
R4 denotes hydrogen, Cl-C4-alkyl, Ci-C4alkoxy or halogen,
optionally in the form of the pharmaceutically acceptable acid addition salts,
hydrates or solvates thereof, optionally in admixture with one or more
physiologically acceptable excipients.
Preferred inhalable powders as mentioned above are those which contain one or
more, preferably one enantiomerically pure compound of general formula 1,
wherein
RI denotes hydrogen or halogen;
R2 denotes hydrogen or halogen;
R3 denotes hydrogen, Cl-C4alkoxy or halogen;
R4 denotes hydrogen or halogen;
optionally in the form of the pharmaceutically acceptable acid addition salts,
hydrates or solvates thereof, optionally in admixture with one or more
physiologically acceptable excipients.
Preferred inhalable powders are those which contain one or more, preferably
one
enantiomerically pure compound of general formula 1 contain, wherein
RI denotes hydrogen, fluorine or chlorine, preferably hydrogen or fluorine;
R2 denotes hydrogen, fluorine or chlorine, preferably hydrogen or fluorine;
CA 02565915 2006-11-06
W02005/110359 5
PCT/EP2005/005078
R3 denotes hydrogen, methoxy, ethoxy, fluorine or chlorine,
preferably
hydrogen, methoxy, ethoxy or fluorine;
R4 denotes hydrogen, fluorine or chlorine, preferably hydrogen or
fluorine;
optionally in the form of the pharmaceutically acceptable acid addition salts,
hydrates or solvates thereof, optionally in admixture with one or more
physiologically acceptable excipients.
Preferred inhalable powders are those which contain one or more, preferably
one
enantiomerically pure compound of general formula 1 contain, wherein
Rl denotes hydrogen or fluorine;
R2 denotes hydrogen;
R3 denotes methoxy, ethoxy or fluorine;
R4 denotes hydrogen;
optionally in the form of the pharmaceutically acceptable acid addition salts,
hydrates or solvates thereof, optionally in admixture with one or more
physiologically acceptable excipients.
Of equal importance according to the invention are also inhalable powders
which
contain one or more, preferably one enantiomerically pure compound of general
formula 1, wherein
denotes hydrogen;
R2 denotes hydrogen, fluorine or chlorine, preferably hydrogen or
fluorine;
R3 denotes hydrogen;
R4 denotes hydrogen, fluorine or chlorine, preferably hydrogen or
fluorine;
optionally in the form of the pharmaceutically acceptable acid addition salts,
hydrates or solvates thereof, optionally in admixture with one or more
physiologically acceptable excipients.
Also preferred according to the invention are inhalable powders which contain
one
or more, preferably one enantiomerically pure compound of general formula 1 in
the form of the free bases thereof.
Of equal importance according to the invention are also inhalable powders
which
contain one or more, preferably one enantiomerically pure compound of general
CA 02565915 2006-11-06
W02005/110359 6
PCT/EP2005/005078
formula 1 in the form of the pharmaceutically acceptable acid addition salts
thereof,
which may be represented by general formula 1-HX.
Preferred inhalable powders contain as acid addition salts one or more,
preferably
one compound of general formula 1-HX,
_______________________________________________________ +
0 OH H H R1
\ /
HN 40 N R2
-
Me Me .
R3 X
OH R4
_ ¨ 1-HX
wherein
X-
denotes an anion with a single negative charge, preferably an anion with
a single negative charge selected from the group consisting of chloride,
bromide, iodide, sulphate, phosphate, methanesulphonate, nitrate,
maleate, acetate, benzoate, citrate, salicylate, trifluoroacetate, fumarate,
tartrate, oxalate, succinate, benzoate and p-toluenesulphonate,
and the groups RI, R2, R3 and R4 may have one of the above-mentioned meanings,
optionally in the form of the tautomers, mixtures of the tautomers, hydrates
or
solvates thereof, as well as optionally in admixture with one or more
physiologically
acceptable excipients.
Preferred inhalable powders contain one or more, preferably one compound of
formula 1-HX, wherein
X- denotes an anion with a single negative charge selected from the
group
consisting of chloride, bromide, sulphate, methanesulphonate, maleate,
acetate, benzoate, citrate, salicylate, trffluoroacetate, fumarate, tartrate
and succinate;
and the groups R', R2, R3 and R4 may have one of the above-mentioned meanings,
optionally in the form of the tautomers, mixtures of the tautomers, hydrates
or
solvates thereof, as well as optionally in admixture with one or more
physiologically
acceptable excipients.
CA 02565915 2006-11-06
W02005/110359 7
PCT/EP2005/005078
Preferred inhalable powders contain one or more, preferably one compound of
formula 1-HX, wherein
X- denotes an anion with a single negative charge selected from the
group
consisting of chloride, methanesulphonate, maleate, acetate, citrate,
salicylate, trifluoroacetate, fumarate and succinate, preferably chloride,
maleate, salicylate, fumarate and succinate, particularly preferably
chloride;
and the groups It', R2, R3 and R4 may have one of the above-mentioned
meanings,
optionally in the form of the tautomers, mixtures of the tautomers, hydrates
or
solvates thereof, as well as optionally in admixture with one or more
physiologically
acceptable excipients.
Also particularly preferred are inhalable powders which contain one or more,
preferably one enantiomerically pure compound of general formula 1. which are
selected from the group consisting of
- 6-hydroxy-8-{ (R)-1-hydroxy-2-[2-(4-methoxy-pheny1)-1,1-dimethyl-
ethylamino]-ethy1}-4H-benzo[1,4]oxazin-3-one-hydrochloride;
- 8-{ (R)-2-[2-(2,4-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl}-6-hydroxy-4H-benzo[1,4]oxazin-3-one-hydrochloride;
- 8-{ (R)-242-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl)-6-hydroxy-4H-benzo[1,4]oxazin-3-one-hydrochloride;
- 8-{(R)-2-[2-(4-ethoxy-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy11-6-
hydroxy-4H-benzo[1,4]oxazin-3-one-hydrochloride;
- 8-{(R)-2-[2-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy1}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one- hydrochloride;
- 6-hydroxy-8-{(R)-1-hydroxy-242-(4-methoxy-pheny1)-1,1-dimethyl-
ethylamino]-ethy1}-4H-benzo[1,4,]oxazin-3-one maleate;
- 6-hydroxy-8-{ (R)-1-hydroxy-2-[2-(4-methoxy-pheny1)-1,1-dimethyl-
ethylamino]-ethy1}-4H-benzo[1,4,]oxazin-3-one salicylate;
- 6-hydroxy-8-{(R)-1-hydroxy-2-[2-(4-methoxy-pheny1)-1,1-dimethyl-
ethylamino]-ethy1}-4H-benzo[1,4,]oxazin-3-one succinate;
- 6-hydroxy-8-{ (R)-1-hydroxy-2-[2-(4-methoxy-pheny1)-1,1-dimethyl-
ethylamino]-ethy1}-4H-benzo[1,4,]oxazin-3-one-fumarate;
CA 02565915 2006-11-06
W020051110359 8 PCT/EP2005/005078
- 8-{ (R)-242-(2,4-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy11-6-hydroxy-4H-benzo [1,4] oxazin-3-one-maleate;
- 8-{ (R)-242-(2,4-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-salicylate;
- 8-{ (R)-242-(2,4-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy11-6-hydroxy-4H-benzo [1,4] oxazin-3-one-succinate;
- 8-{ (R)-2- [2-(2,4-difluoro-phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-
ethyl} -6-hydroxy-4H-benzo [1,4] oxazin-3-one-fumarate;
- 8-{ (R)-2-[2-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-maleate;
- 8-{ (R)-2-[2-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl } -6-hydroxy-4H-benzo [1,4] oxazin-3-one-s alicylate;
- 8-{ (R)-2-[2-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo[1,4]oxazin-3-one-succinate;
- 8-{ (R)-2-[2-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-fumarate;
- 8-{(R)-2-[2-(4-ethoxy-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy1}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one-maleate;
- 8-{ (R)-2-[2-(4-ethoxy-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy1}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one-salicylate;
- 8-{ (R)-2- [2- (4-ethoxy-phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-
ethy1}-6-
hydroxy-4H-benzo [1,4] oxazin-3-one-succinate;
- 8-{(R)-2-[2-(4-ethoxy-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy1}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one-fumarate;
- 8-{ (R)-2-[2-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy1}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one-maleate;
- 8-{ (R)-242-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy11-6-
hydroxy-4H-benzo[1,4]oxazin-3-one-salicylate;
- 8-{ (R)-2- [2-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy11-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one-succinate and
- 8-{(R)-242-(4-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethy11-6-
hydroxy-4H-benzo[1,4]oxazin-3-one-fumarate,
optionally in the form of the tautomers, mixtures of the tautomers, hydrates
or
solvates thereof.
CA 02565915 2006-11-06
W02005/110359 9 PCT/EP2005/005078
In the inhalable powders according to the invention the enantiomerically pure
compounds of general formula 1, wherein RI, R2, R3 R4 are as hereinbefore
defined
are present in crystalline form, optionally in the form of the crystalline
tautomers,
crystalline hydrates or crystalline solvates thereof. Particularly preferred
are
enantiomerically pure, crystalline compounds of general formula 1 wherein RI,
R2,
R3 and R4 have the meanings given above, optionally in the form of the
crystalline
tautomers, crystalline hydrates or crystalline solvates thereof, which are
further
characterised in that they are crystalline compounds which occur in only one
crystal
modification.
By the expression "only one crystal modification" are meant crystalline
compounds
of formula 1 which do not constitute a mixture of any polymorphic crystal
modifications which may exist and / or mixtures of one or more crystal
modifications with the amorphous or vitreous state of the compounds according
to
formula 1.
Unless otherwise stated, the alkyl groups are straight-chained or branched
alkyl
groups having 1 to 4 carbon atoms. The following are mentioned by way of
example: methyl, ethyl, propyl or butyl. In some cases the abbreviations Me,
Et,
Prop or Bu are used to denote the groups methyl, ethyl, propyl or butyl.
Unless
otherwise stated, the definitions propyl and butyl include all the possible
isomeric
forms of the groups in question. Thus, for example, propyl includes n-propyl
and
iso-propyl, butyl includes iso-butyl, sec.butyl and tert.-butyl, etc.
Unless otherwise stated, the alkylene groups are branched and unbranched
double-
bonded alkyl bridges having 1 to 4 carbon atoms. The following are mentioned
by
way of example: methylene, ethylene, n-propylene or n-butylene.
Unless otherwise stated, the term alkyloxy groups (or -0-alkyl groups) denotes
branched and unbranched alkyl groups having 1 to 4 carbon atoms which are
linked
via an oxygen atom. Examples of these include: methyloxy, ethyloxy, propyloxy
or
butyloxy. The abbreviations Me0-, Et0-, Prop0- or BuO- are used in some cases
to denote the groups methyloxy, ethyloxy, propyloxy or butyloxy. Unless
otherwise
stated, the definitions propyloxy and butyloxy include all possible isomeric
forms of
CA 02565915 2006-11-06
W02005/110359 10 PCT/EP2005/005078
the groups in question. Thus, for example, propyloxy includes n-propyloxy and
iso-
propyloxy, butyloxy includes iso-butyloxy, sec.butyloxy and tert.-butyloxy,
etc. In
some cases, within the scope of the present invention, the term alkoxy is used
instead of the term alkyloxy. Accordingly, the terms methoxy, ethoxy, propoxy
or
butoxy may also be used to denote the groups methyloxy, ethyloxy, propyloxy or
butyloxy.
Halogen within the scope of the present invention denotes fluorine, chlorine,
bromine or
iodine. Unless stated to the contrary, fluorine, chlorine and bromine are the
preferred
halogens.
The term enantiomerically pure describes within the scope of the present
invention
compounds of formula! which are present in an enantiomerical purity of at
least 85%ee,
preferably at least 90%ee, particularly preferably > 95%ee. The term ee
(enantiomeric
excess) is known in the art and describes the optical purity of chiral
compounds.
The highly crystalline compounds of formula 1 may be obtained as illustrated
below
(Diagram 1).
CA 02565915 2006-11-06
W02005/110359 11 PCT/EP2005/005078
= o Oy^.... = o 0
OH
HN 11 HN = HN 01 Me -0'
L
OPG OPG OPG
2 3 4
H2N Fe
=
Me Me 0
n3 y'N's OH R,
= IP
HN HN R2
6 R4
110 Me
Me * ,
R"
OPG OPG R4
7
0 OH H
2
HN R
Me Me 41
R3
OH R4 1
Diagram 1:
In the compounds of formulae 2 to 5 and 7 mentioned in Diagram 1 the group
OPG denotes a hydroxyl function protected by a protective group (PG). For a
5 choice
of suitable protective groups for the hydroxyl group reference is made to the
prior art as given for example in Protective Groups in Organic Synthesis, T.W.
Greene and P.G.M. Wuts, John Wiley & Sons Inc, third Edition, 1999.
Preferably OPG denotes a group which is selected from among
-0-benzyl or -0-CO-Cl-C4-alkyl, preferably
-0-methyl, -, -0-benzyl or -0-acetyl, particularly preferably -0-methyl
or -0-benzyl, particularly preferably -0-benzyl.
In the compounds of formulae 3 and 4 mentioned in Diagram 1 the group L
denotes a leaving group. Preferably L denotes a leaving group selected from
among
chlorine, bromine, iodine, methanesulphonate,
trifluoromethanesulphonate and
CA 02565915 2006-11-06
W02005/110359 12 PCT/EP2005/005078
p-toluenesulphonate, preferably chlorine or bromine, particularly
preferably chlorine.
In the compounds of formulae 6 and 7 mentioned in Diagram 1 the groups RI, R2,
-- R3 and R4 may have the above-mentioned meanings.
Starting from 8-acetyl-6-benzyloxy-4H-benzo[1,4]oxazin-3-one (z) the compounds
of general formula 3 are prepared in the manner known from the prior art. The
compound of formula 3 is then converted enantioselectively, in the presence of
a
-- chiral transition metal catalyst, into the chiral alcohol of general
formula 4 which is
then reacted under suitable conditions to form the chiral oxiran of formula 5.
Methods of synthesising oxirans from derivatives of the compound of formula 3
are
known in the art ( cf. for example Hamada et al., Org. Letters 2002, 4, 4373-
4376).
By reacting the oxirans 5 with the amines of formula 6 the compounds of
formula 7
-- are obtained, which may be converted into the compounds of formula 1 after
cleaving of the protective group (PG). If the compounds of formula 1 are not
obtained in crystalline form by the method of synthesis outlined above, the
synthesis
may be followed by recrystallisation from suitable solvents. More detailed
comments on this subject can be found in the experimental part of the present
-- invention that follows.
In order to prepare the inhalable powders according to the invention, it is
first of all
necessary to prepare the compounds of formula 1 obtained in crystalline form
in
finely-divided (or micronised) form.
The micronising or grinding process may be carried out using conventional
mills.
Preferably, the micronisation is carried out with the exclusion of moisture,
more
preferably, using a corresponding inert gas such as nitrogen, for example. It
has
proved particularly preferable to use air jet mills in which the material is
-- comminuted by the impact of the particles on one another and on the walls
of the
grinding container. The micronising process may be carried out both by so-
called
counterflow mills, optionally with subsequent screening, and also, in
preferred
manner, using spiral air jet mills. According to the invention, nitrogen is
preferably
used as the grinding gas. The material for grinding is conveyed by the
grinding gas
-- under specific pressures (grinding pressure). Within the scope of the
present
CA 02565915 2006-11-06
W02005/110359 13 PCT/EP2005/005078
invention, the grinding pressure is usually set to a value between about 2 and
8 bar,
preferably between about 3 and 7 bar, most preferably between about 3.5 and
6.5
bar. The material for grinding is fed into the air jet mill by means of the
feed gas
under specific pressures (feed pressure). Within the scope of the present
invention
a feed pressure of between about 2 and 8 bar, preferably between about 3 and 7
bar
and most preferably between about 3.5 and 6 bar has proved satisfactory. The
feed
gas used is also preferably an inert gas, most preferably nitrogen again. The
material to be ground (crystalline compounds according to formula") may be fed
in
at a rate of about 5 - 45 g/min, preferably at about 15-35 g/min.
For example, without restricting the subject of the invention thereto, the
following
apparatus has proved suitable as a possible embodiment of an air jet mill: a 2-
inch
Microniser with grinding ring, 0.8 mm bore, made by Messrs Sturtevant Inc.,
348
Circuit Street, Hanover, MA 02239, USA. Using this apparatus, the grinding
process is preferably carried out with the following grinding parameters:
grinding
pressure: about 4.5 - 6.5 bar; feed pressure: about 4.5 - 6.5 bar; supply of
grinding
material: about 17 - 21 g/min.
Another example is the use of an air jet mill as made by Messrs Jetpharma,
type
Jetmill MC 50, which may be operated with the following process parameters:
grinding pressure: 8.0 bar (+/- 0.5 bar)
feed pressure: 8.5 bar (+/- 0.5 bar),
Note: the feed pressure is always set 0.25 to
0.5 bar higher than the grinding pressure
product feed: 20 g/min (+/- 2,0 g/min)
nozzle setting (injector): 37.2 mm (constant)
The ground material thus obtained is then further processed under the
following
specific conditions. The micronisate is exposed to water vapour at a relative
humidity of at least 40% at a temperature of 15-50 C, preferably 20-45 C, most
preferably 25-40 C . Preferably, the humidity is set to a value of 50 - 95% r.
h.,
preferably 60 - 90% r.h., most preferably 70 - 85% r.h. By relative humidity
(r.h.) is
meant within the scope of the present invention the quotient of the partial
steam
pressure and the steam pressure of the water at the temperature in question.
Preferably, the micronisate obtained from the grinding process described above
is
CA 02565915 2006-11-06
W02005/110359 14 PCT/EP2005/005078
subjected to the chamber conditions mentioned above for a period of at least 6
hours. Preferably, however, the micronisate is subjected to the chamber
conditions
mentioned above for about 12 to about 120 hours, preferably about 15 to about
96
hours, particularly preferably about 18 to about 72 hours. In an alternative
embodiment this step is followed by drying. The ground material is exposed to
an
elevated temperature. For this, the micronised material is exposed to an
elevated
temperature of at least 40 C, preferably at least 50 C and at most 70 C for a
period
of at least 0.5 hours, preferably 0.5 hours to 6 hours, particularly
preferably from
0.5 hours to 3 hours, at reduced relative humidity, i.e. a relative humidity
of less
than 60%, preferably less than 40% and particularly preferably less than 30%.
The micronised compounds of formula 1 according to the invention which may be
obtained by the method described above have a characteristic particle size X50
of
between 0.1 gm and 10gm, preferably between 0.5gm and 6 gm, particularly
preferably between 1.0 gm and 3.5gm. In addition they are characterised by the
parameter Q(5.8) of more than 60%, preferably more than 70 %, particularly
preferably more than 80%.
The characteristic value Xso denotes the median value of the particle size
below
which 50% of the quantity of particles are found, based on the volume
distribution
of the individual particles. The characteristic value Q(5.8) indicates the
quantity of
particles below 5.8 gm, based on the volume distribution of the particles. The
particle sizes were determined within the scope of the present invention by
laser
diffraction (Fraunhofer diffraction). The determination of the particle sizes
by laser
diffraction (Fraunhofer diffraction) was effected using the method described
in WO
03/078429 (page 16 ff).
The micronised compounds of general formula 1 described hereinbefore may
optionally be used for inhalation without any other excipient. Preferably,
however,
the pharmaceutical compositions according to the invention contain in addition
to
one or more, preferably one, compound of formula 1 at least one
physiologically
acceptable excipient or a mixture of physiologically acceptable excipients.
Examples of physiologically acceptable excipients which may be used to prepare
the
inhalable powders according to the invention include, for example,
monosaccharides (e.g. glucose, fructose or arabinose), disaccharides (e.g.
lactose,
CA 02565915 2006-11-06
W02005/110359 15 PCT/EP2005/005078
saccharose, maltose or trehalose), oligo- and polysaccharides (e.g.
maltodextrin,
starch, cellulose and the derivatives thereof), polylactide/glycolide
(resomer),
polyalcohols (e.g. sorbitol, mannitol, xylitol), amino acids (arginine
hydrochloride),
chitosan (particularly preferably lactose, mannitol, saccharose, sorbitol,
trehalose),
alkali metal and alkaline earth metal salts of stearic acid (e.g. Mg
stearate), salts
(e.g. sodium chloride, calcium carbonate) or mixtures of these excipients with
one
another. Preferably, mono- or disaccharides or polyalcohols are used, while
the use
of lactose, glucose, trehalose or mannitol, preferably lactose, mannitol or
glucose, is
preferred, particularly, but not exclusively, in the form of their hydrates.
For the
purposes of the invention, lactose or mannitol is the particularly preferred
excipient,
while lactose monohydrate or mannitol is most particularly preferred.
In pharmaceutical formulations according to the invention which contain in
addition to a compound of formula 1 a physiologically acceptable excipient,
the
ratio of the compound of formula 1 to the excipient is usually kept in the
range from
5:100 to 1:100000, preferably 3:1000 to 1:10000 and particularly preferably
from
1:1000 to 3:10000, the ratios given above being ratios by weight (w/w).
The inhalable powders according to the invention are usually administered in
amounts of 3-100 mg, preferably 5-50 mg for each inhalation.
If the inhalable powders according to the invention do not contain any
excipient,
only one or more, preferably one compound of formula 1 in micronised form, 1
to
30p,g, preferably 3 to 25 g and particularly preferably 5 to 2014 inhalable
powder
are usually administered per inhalation.
The inhalable powders according to the invention are preferably administered
in the
form of a pre-metered pharmaceutical preparation. Examples include an
inhalation
capsule system. It is also possible to use systems wherein the powder
preparation is
presented in single doses e.g. contained in blister wells. In another form the
preparations according to the invention are also suitable for use in inhalers
which
have a powder reservoir and wherein the quantity of powder to be administered
or
the crystalline micronisate of the active substance is not metered or divided
up until
immediately prior to use. The powder preparations described here may be
inhaled
by means of a suitable inhaler. Suitable inhalers are known from the prior
art.
CA 02565915 2012-02-24
25771-1265
16
Particularly suitable inhalers are mentioned for example in WO 03/084502.
The inhalable powders prepared according to the invention may be prepared as
described below.
The process according to the invention for preparing inhalable powders is
characterised in that N+m substantially equal portions of the physiologically
acceptable excipient and N equal portions of the micronised compound of
formula
1 are placed in alternate layers in a suitable mixing vessel and after they
have all
been added the 2N-Fm layers of the two components are mixed together using a
suitable mixer, a portion of the physiologically acceptable excipient being
put in
first, while N is an integer >0, preferably >1, and m denotes 0 or 1.
Preferably, the individual fractions are added in layers through a suitable
screening
apparatus. If desired, once the mixing process is finished, the entire powder
mixture
can be subjected to one or more additional screening processes. In the process
according to the invention, N is naturally dependent inter alia on the total
quantity
of powder mixture to be produced. When producing smaller batches, the desired
effect of high homogeneity in the sense of uniformity of content can be
achieved
with a smaller N. In principle, however, it is preferable according to the
invention if
N is at least 10 or more, more preferably 20 or more, better still 30 or more.
The
greater N is and, as a result, the greater the total number of layers of the
powder
fractions formed, the more homogeneous the powder mixture becomes in the sense
of uniformity of content.
The number m may represent 0 or 1 within the scope of the process according to
the invention. If m denotes 0 the last fraction added to the mixing apparatus,
preferably screened into it, in a layer is the last portion of the micronised
compound
of formula 1. If m represents the number 1, the last fraction added to the
mixing
apparatus, preferably screened into it, in a layer is the last portion of the
physiologically acceptable excipient. This may prove advantageous inasmuch as,
when m = 1, any residues of the last fraction of the active substance still
remaining
CA 02565915 2006-11-06
W02005/110359 17 PCT/EP2005/005078
in the screening unit can be carried into the mixing unit by means of the last
portion
of excipient.
Preferably, the first portion of the N+m portions of the excipient is put in
first, and
then the first portion of the N portions of the active substance is placed in
the
mixing container. Whereas within the scope of the process according to the
invention the individual components are normally added in roughly equal
portions,
it may be advantageous in some cases if the first of the N+m portions of
excipient
which is put into the mixing apparatus has a larger volume than the subsequent
portions of excipient.
The inhalable powders according to the invention may also be prepared by first
of
all producing a mixture of active substance and excipient according to the
method
described above and then mixing the mixture thus obtained with more excipient.
This may be done using the method described above, by mixing N batches of the
active substance I excipient mixture layer by layer with N+m batches of other
excipient.
The excipient used in the inhalable powders according to the invention
preferably
has an average particle size of 17 -120 gm, preferably about 17- 90 gm,
particularly
preferably about 20-60gm . The excipient may optionally also be a mixture of
coarser excipient with an average particle size of 17 to 75 gm and finer
excipient
with an average particle size of 1 to 9 gm, wherein the proportion of finer
excipient
in the total quantity of excipient may be 1 to 20 %. If the inhalable powders
which
may be produced using the process according to the invention contain a mixture
of
coarser and finer excipient fractions, it is preferable according to the
invention to
prepare inhalable powders wherein the coarser excipient has an average
particle size
of 17 to 50 gm, most preferably 20 to 30 gm, and the finer excipient has an
average
particle size of 2 to 8 gm, most preferably 3 to 7 gm. By average particle
size is
meant here the 50 A value of the volume distribution measured with a laser
diffractometer using the dry dispersion method. For the measurement of the
mean
particle size by this method see the disclosure of WO 03/078429 (page 21 if).
In the case of an excipient mixture of coarser and finer excipient fractions,
the
preferred processes according to the invention are those that produce
inhalable
CA 02565915 2006-11-06
W02005/110359 18
PCT/EP2005/005078
powders in which the proportion of finer excipient constitutes 3 to 15 (Yo,
most
preferably 5 to 10 % of the total amount of excipient.
The percentages given within the scope of the present invention are always
percent
by weight.
________ Ifithe __ excipient used is one of the abovementioned __ mixtures
_______ of coarser excipient and
finer excipient, it is again expedient according to the invention to produce
the
excipient mixture using the process according to the invention from N roughly
equal portions of the finer excipient fraction with N+m roughly equal portions
of
the coarser excipient fraction. In such a case it is advisable first to
generate the
above-mentioned excipient mixture from the above-mentioned excipient
fractions,
and then to produce from it the total mixture including the active substance
using
the process according to the invention.
For example, the excipient mixture may be obtained as follows, using the
process
according to the invention. The two components are preferably added through a
screening granulator with a mesh size of 0.1 to 2 mm, most preferably 0.3 to 1
mm,
even more preferably 0.3 to 0.6 mm. Preferably the first fraction of the N+m
portions of the coarser excipient is put in first and then the first portion
of the N
portions of the finer excipient fraction is added to the mixing container. The
two
components are added alternately by screening them in layer by layer.
After the preparation of the excipient mixture, the inhalable powder is
produced
from the mixture and the desired active substance using the process according
to
the invention. The two components are preferably added through a screening
granulator with a mesh size of 0.1 to 2 mm, most preferably 0.3 to 1 mm, even
more preferably 0.3 to 0.6 mm.
Preferably, the first portion of the N+m portions of the excipient mixture is
put in
and then the first portion of the N portions of the active substance is added
to the
mixing container. The two components are preferably added through a screening
unit in alternate layers, in more than 20, preferably more than 25, most
preferably
more than 30 layers. For example, with a desired total amount of powder of 30-
35
kg containing 0.3-0.5 % of active substance, for example, and using common
CA 02565915 2006-11-06
W02005/110359 19 PCT/EP2005/005078
excipients, the two components can be screened in in about 30 to 60 layers
each (N
= 30-60). As will be clearly apparent to anyone skilled in the art, the
process can
equally well be carried out with N>60 to achieve the desired effect of the
maximum
possible homogeneity of the powder mixture.
The inhalable powders according to the invention are characterised by their
multiplicity of possible applications in the therapeutic field. Particular
mention
should be made according to the invention of those applications for which the
compounds of formula 1 according to the invention are preferably used on the
basis
of their pharmaceutical activity as betamimetics.
Accordingly, in another aspect, the present invention relates to the above-
mentioned inhalable powders as pharmaceutical compositions. The present
invention also relates to the use of the above-mentioned inhalable powders for
preparing a pharmaceutical composition for the treatment of respiratory
complaints.
The present invention preferably relates to the use of the above-mentioned
inhalable powders for preparing a pharmaceutical composition for the treatment
of
respiratory complaints selected from the group comprising obstructive
pulmonary
diseases of various origins, pulmonary emphysema of various origins,
restrictive
pulmonary diseases, interstitial pulmonary diseases, cystic fibrosis,
bronchitis of
various origins, bronchiectasis, ARDS (adult respiratory distress syndrome)
and all
forms of pulmonary oedema.
Preferably, the inhalable powders according to the invention are used to
prepare a
pharmaceutical composition for the treatment of obstructive pulmonary diseases
selected from the group consisting of COPD (chronic obstructive pulmonary
disease), bronchial asthma, paediatric asthma, severe asthma, acute asthma
attacks
and chronic bronchitis, while their use for preparing a pharmaceutical
composition
for the treatment of bronchial asthma is particularly preferred according to
the
invention.
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment of pulmonary emphysema
which has its origins in COPD (chronic obstructive pulmonary disease) or al-
CA 02565915 2006-11-06
W02005/110359 20 PCT/EP2005/005078
proteinase inhibitor deficiency.
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment of restrictive
pulmonary
diseases selected from among allergic alveolitis, restrictive pulmonary
diseases
triggered by work-related noxious substances, such as asbestosis or silicosis,
and
restriction caused by lung tumours, such as for example lymphangiosis
carcinomatosa, bronchoalveolar carcinoma and lymphomas.
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment of interstitial
pulmonary
diseases selected from among pneumonia caused by infections, such as for
example
infection by viruses, bacteria, fungi, protozoa, helminths or other pathogens,
pneumonitis caused by various factors, such as for example aspiration and left
heart
insufficiency, radiation-induced pneumonitis or fibrosis, collagenoses, such
as for
example lupus erythematodes, systemic sclerodermy or sarcoidosis,
granulomatoses,
such as for example Boeck's disease, idiopathic interstitial pneumonia or
idiopathic
pulmonary fibrosis (IPF).
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment of cystic fibrosis or
mucoviscidosis.
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment of bronchitis, such as
for
example bronchitis caused by bacterial or viral infection, allergic bronchitis
and
toxic bronchitis.
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment of bronchiectasis.
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment of ARDS (adult
respiratory
distress syndrome).
CA 02565915 2006-11-06
W02005/110359 21
PCT/EP2005/005078
Also preferably, the inhalable powders according to the invention are used to
prepare a pharmaceutical composition for the treatment pulmonary oedema, for
example toxic pulmonary oedema after aspiration or inhalation of toxic
substances
and foreign substances.
Particularly preferably the present invention relates to the use of the
inhalable
powders according to the invention for preparing a pharmaceutical composition
for
the treatment of asthma or COPD. Also of particular importance is the above-
mentioned use of the inhalable powders according to the invention for
preparing a
pharmaceutical composition for once-a-day treatment of inflammatory and
obstructive respiratory complaints, particularly for the once-a-day treatment
of
asthma or COPD.
The present invention also relates to a process for the treatment of the above-
mentioned diseases, characterised in that one or more of the above-mentioned
inhalable powders according to the invention are administered in
therapeutically
effective amounts. The present invention preferably also relates to processes
for the
treatment of asthma or COPD, characterised in that one or more of the above-
mentioned inhalable powders according to the invention are administered once a
day in therapeutically effective amounts.
The examples of synthesis described below serve to illustrate the invention in
more
detail. However, they are intended only as examples of procedures to
illustrate the
invention without restricting it to the subject matter described in an
exemplifying
capacity hereinafter.
Preparation of the compounds of general formula 1:
Example 1: 6-hydroxy-8-{ (R)-1-h_ydro-2- [2-f 4-methoxy-pheriy1)-1,1-dimethyl-
ethylamino] -ethy1}-4H-benzol 1 ,41 oxazin-3-one - hydrochloride
CA 02565915 2006-11-06
W02005/110359 22
PCT/EP2005/005078
OH
HN
x HCI
Me Me 401
OMe
OH
a) 1-(5-benzvloxy-2-hydroxy-3-nitro-phenyl)-ethanone
18 mL of fuming nitric acid are added dropwise to a solution of 81.5 g (0.34
mol)
1-(5-benzyloxy-2-hydroxy-phenyl)-ethanone in 700 mL acetic acid, while being
cooled with the ice bath, in such a way that the temperature does not exceed
20 C.
Then the reaction mixture is stirred for two hours at ambient temperature,
poured
onto ice water and filtered. The product is recrystallised from isopropanol,
suction
filtered and washed with isopropanol and diisopropylether.
Yield: 69.6 g (72%); mass spectroscopy [M+H] = 288.
b) 1-(3-amino-5-benzvloxy-2-hydroxy-phenyl)-ethanone
69.5 g (242 mmol) 1-(5-benzyloxy-2-hydroxy-3-nitro-phenyl)-ethanone are
dissolved in 1.4 L methanol and hydrogenated in the presence of 14 g rhodium
on
charcoal (10%) as catalyst at 3 bar and ambient temperature. Then the catalyst
is
filtered off and the filtrate is evaporated down. The residue is reacted
further
without any additional purification.
Yield: 60.0 g (96%), Rf value = 0.45 (dichloromethane on silica gel).
c) 8-acetyl-6-benzyloxv-4H-benzo[1,4]oxazin-3-one
21.0 mL (258 mmol) chloroacetyl chloride are added dropwise to 60.0 g (233
mmol) 1-(3-amino-5-benzyloxy-2-hydroxy-phenyl)-ethanone and 70.0 g (506
mmol) potassium carbonate while being cooled with the ice bath. Then the
mixture
is stirred overnight at ambient temperature and then for 6 hours at reflux
temperature. The hot reaction mixture is filtered, then evaporated down to
approx.
400 mL and combined with ice water. The precipitate formed was suction
filtered,
dried and purified by chromatography on a short silica gel column
(dichloromethane:methanol = 99:1). The fractions containing the product are
evaporated down, suspended in isopropanol/diisopropylether, suction filtered
and
washed with diisopropylether.
Yield: 34.6 g (50%); mass spectroscopy [M+Hr = 298.
CA 02565915 2006-11-06
W02005/110359 23 PCT/EP2005/005078
d) 6-benzyloxy-8-(2-chloro-acetv1)-4H-benzol1,41oxazin-3-one
13.8 g (46.0 mmol) 8-acetyl-6-benzyloxy-4H-benzo[1,4]oxazin-3-one and 35.3 g
(101.5 mmol) benzyltrimethylammonium-dichloriodate are stirred in 250 mL
dichloroethane, 84 mL glacial acetic acid and 14 mL water for 5 hours at 65 C.
After cooling to ambient temperature 5% sodium hydrogen sulphite solution is
added and the mixture is stirred for 30 minutes. The precipitated solid is
suction
filtered, washed with water and diethyl ether and dried.
Yield: 13.2 g (86%); mass spectroscopy [M+11]+ = 330/32.
e) 6-benzyloxy-84(R)-2-chloro-l-hydroxv-ethyl)-4H-benzo[1,41-oxazin-3-one
The process is carried out analogously to a process described in the
literature (Org.
Lett. 2002, 4, 4373-4376).
8 mL of a mixture of formic acid and triethylamine (molar ratio = 5:2) are
added
dropwise at -15 C to 13.15 g (39.6 mmol) 6-benzyloxy-8-(2-chloro-acetyl)-4H-
benzo[1,4]oxazin-3-one and 25.5 mg (0.04 mmol) Cp*RhC1[(S,S)-TsDPEN] (Cr)*
= pentamethylcyclopentadienyl and TsDPEN = (1S,2S)-N-p-toluenesulphony1-1,2-
diphenylethylenediamine) in 40 mL dimethylformamide. The mixture is stirred
for
5 hours at this temperature, then 25 mg catalyst are added and the mixture is
stirred
overnight at -15 C. The reaction mixture is combined with ice water and
filtered.
The filter residue is dissolved in dichloromethane, dried with sodium sulphate
and
freed from the solvent. The residue is chromatographed
(dichloromethane/methanol-gradient) and the product is recrystallised from
diethyl
ether/diisopropylether.
Yield: 10.08 g (76%); Rf value = 0.28 (dichloromethane:methanol = 50:1 on
silica
gel).
f) 6-benzvloxv-8-(R)-oxirany1-4H-benzo[1,41oxazin-3-one
10.06 g (30.1 mmol) 6-benzyloxy-8-((R)-2-chloro-1-hydroxy-ethyl)-4H-
benzo[1,4]-oxazin-3-one are dissolved in 200 mL dimethylformamide. The
solution
is combined with 40 mL of a 2 molar sodium hydroxide solution at 0 C and
stirred
for 4 hours at this temperature. The reaction mixture is poured onto ice
water,
stirred for 15 minutes and then filtered. The solid is washed with water and
dried.
Yield: 8.60 g (96%); mass spectroscopy [M+H]+ = 298.
CA 02565915 2006-11-06
W02005/110359 24
PCT/EP2005/005078
6-benzvloxy-8-{(R)-1-hydrox_y-2-[2(4-methou-pheny1)-1,1-dimethvl-
ethylamino] -ethyl )-4H-benzo [1,4,]oxazin-3-one
5.25 g (17.7 mmol) 6-benzyloxy-8-(R)-oxirany1-4H-benzo[1,4]oxazin-3-one and
6.30 g (35.1 mmol) 2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamine are combined
with 21 mL isopropanol and stirred for 30 minutes at 135 C under microwave
radiation in a sealed reaction vessel. The solvent is distilled off and the
residue is
chromatographed (aluminium oxide; ethyl acetate/methanol gradient). The
product
thus obtained is purified by recrystallisation from a diethyl
ether/diisopropylether
mixture. Yield: 5.33 g (63%); mass spectroscopy [M+H] = 477.
h) 6-hydroxy-8-{(R)-1-hydroxy-2-[2-(4-methoxyTheny1)-111-dimethyl-
ethylaminol-ethyl)-4H-benzo ,4 Joxazin-3-one-hydrochloride
A suspension of 5.33 g (11.2 mmol) 6-benzyloxy-8-{(R)-1-hydroxy-242-(4-
methoxy-pheny1)-1,1-dimethyl-ethylamino]-ethy11-4H-benzo[1,4]oxazin-3-one in
120 mL methanol is combined with 0.8 g palladium on charcoal (10%), heated to
50 C heated and hydrogenated at 3 bar hydrogen pressure. Then the catalyst is
suction filtered and the filtrate is evaporated down. The residue is dissolved
in 20
mL isopropanol and 2.5 mL of 5 molar hydrochloric acid in isopropanol are
added.
The product is precipitated with 200 mL diethyl ether, suction filtered and
dried.
Yield: 4.50 g (95%, hydrochloride); mass spectroscopy [M+H]+ = 387.
The following compounds of formula 1 are obtained analogously by reacting the
compound 6-benzyloxy-8-(R)-oxirany1-4H-benzo[1,4]oxazin-3-one (Example 1,
Step f) with the corresponding amine.
Example 2: 8-{(R)-242-(2,4-difluoro-pheny0-1,1-dimethyl-ethylamino1-1-
hydroxy-ethy1}-6-hydroxy-4H-benzo[1,41oxazin-3-one-hydrochloride
00 OH
HN
x HCI
Me Me 401
OH
Mass spectroscopy [M+11}1- = 393.
CA 02565915 2006-11-06
WO 2005/110359 25
PCT/EP2005/005078
Example 3: 8-{ (M-242-(35-difluoro-phenv1)-1,1-dimethyl-ethylamino] -1-
hydroxy-ethy11-6-hydroxy-4H-benzol1,4]oxazin-3-one-hydrochloride
0 OH
HN
x HCI
Me Me
OH
Mass spectroscopy [M+1-1]+ = 393.
Example 4: 8-{(R)-242-(4-ethoxy-_phenv1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo[1,4]oxazin-3-one-hydrochloride
OH
HN
x HCI
Me Me 10
OMe
OH
Mass spectroscopy [M+H] = 401.
Example 5: 8-{(R)-2-[2-(4-fluoro-phenyl)-1,1-dimethyl-ethylamino1-1-hydroxy-
ethyl}-6-hydroxy-4H-benzo[1,4}oxazin-3-one- hydrochloride
O OH
HN
x HCI
Me Me 1101
OH
Mass spectroscopy [M+HP = 375.
If the compounds of formula 1 according to the method of synthesis described
above by way of example do not lead to uniform crystal modifications, it may
be
useful to recrystallise the salts of formula 1 obtained from suitable
solvents.
Moreover, other salts may be obtained from the above-mentioned Examples using
methods known per se from the prior art.
In the next section, methods of preparing uniform salts of the compounds of
formula 1 which are particularly suitable for preparing formulations for
administration by inhalation are described by way of example.
CA 02565915 2006-11-06
WO 2005/110359 26 PCT/EP2005/005078
Example 6: 6-hvdroxy-8-{(R)-1-hydroxy-242-(4-methoxv-phenyl)-1,1-dimethyl-
ethylamino]-ethyl}-4H-benzo[1,4Joxazin-3-one maleate
250 mg (0.65 mmol) 6-hydroxy-8-{(R)-1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-
dimethyl-ethylamino]-ethyl}-4H-benzo[1,4,]oxazin-3-one are combined with
sufficient ethanol to make the solid go into solution completely. Then 75 mg
(0.65
mmol) maleic acid and a crystallisation aid are added. The mixture is cooled
with
ice and the precipitated solid is filtered off and washed with ethanol and
diethyl
ether. In the salt the acid and the ethanolamine are present in the ratio 1:1.
Yield: 254 mg (78%); mass spectroscopy [M+H] = 387; melting point = 215 C.
The highly crystalline product was investigated further by X-ray powder
diffraction.
The X-ray powder diagram was recorded using the following method.
The X-ray powder diagram was recorded within the scope of the present
invention
using a Bruker D8 Advanced with an LSD (= location-sensitive detector) (CuKa
radiation, k = 1.5418 A, 30 kV, 40 mA).
For the highly crystalline compound the following characteristic values dhki
[A]were
obtained, inter alia, which give the lattice plane intervals determined in A:
d= 21.68 A; 8.62 A; 5.92 A; 5.01 A; 4.59 A; 4.36 A; 3.64 A and 3.52 A.
Example 7: 6-hydroxy-8-{(R)-1-h_ydroxy-242-(4-methoxy-phenyl)-1,1-dimethyl-
ethylaminol-ethyl}-4H-benzo [1,4d oxazin-3-one salicylate
250 mg (0.65 mmol) 6-hydroxy-8-{(R)-1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-
dimethyl-ethylamino]-ethyl}-4H-benzo[1,4,]oxazin-3-one are dissolved in a
little
ethanol and combined with 90 mg (0.65 mmol) salicylic acid. After the addition
of
a crystallisation aid the mixture is left to stand overnight, during which
time a solid
is precipitated. Diethyl ether is added and after 30 minutes the mixture is
filtered.
The white solid thus obtained is washed with diethyl ether and dried.
Yield: 295 mg (87%); mass spectroscopy [M+H] = 387; melting point = 215 C.
The highly crystalline product was investigated further by X-ray powder
diffraction.
The X-ray powder diagram was recorded using the following method.
CA 02565915 2006-11-06
W02005/110359 27
PCT/EP2005/005078
The X-ray powder diagram was recorded within the scope of the present
invention
using a Bruker D8 Advanced with an LSD (= location-sensitive detector) (CuKa
radiation, X. = 1.5418 A, 30 kV, 40 mA).
For the highly crystalline compound the following characteristic values dhki
[A]were
obtained, inter alia, which give the lattice plane intervals determined in A:
d= 9.06 A; 8.36 A; 8.02 A; 6.84 A; 6.73 A; 4.48 A; 4.35 A and 4.27 A.
Example 8: 6-hydroxy-8-{(R)-1-hydroxy-2-(2-(4-methoxv-pheny1)-1,1-dimethyl-
ethylamino] -ethyl }-4H-benzo [1,4,) oxazin-3-one succinate
500 mg (1.2 mmol) 6-hydroxy-8-{(R)-1-hydroxy-2-[2-(4-methoxy-pheny1)-1,1-
dimethyl-ethylamino]-ethy1}-4H-benzo[1,4,]oxazin-3-one hydrochloride are
combined with ethyl acetate and extracted with aqueous potassium carbonate
solution, the organic phase is dried with sodium sulphate and freed from the
solvent. The residue is dissolved in a little ethanol and combined with 140 mg
(1.2
mmol) succinic acid. After 2 hours the precipitated solid is suction filtered
and
washed with cold ethanol and diethyl ether. The ethanolamine and acid are
present
in the salt in a ratio of 1 to 0.5.
Yield: 468 mg (85%); mass spectroscopy [M+H]l- = 387; melting point = 115 C.
The highly crystalline product was investigated further by X-ray powder
diffraction.
The X-ray powder diagram was recorded using the following method.
The X-ray powder diagram was recorded within the scope of the present
invention
using a Bruker D8 Advanced with an LSD (= location-sensitive detector) (CuKa
radiation, X, = 1.5418 A, 30 kV, 40 mA).
For the highly crystalline compound the following characteristic values dui
[A]were
obtained, inter alia, which give the lattice plane intervals determined in A:
d= 14.35 A; 8.49 A; 7.37 A; 7.25 A; 5.47 A; 4.78 A; 4.14 A and 3.59 A.
Example 9: 6-hydroxv-8-{(R)-1-hydroxy-2-12-(4-methoxy-pheny1)-1,1-dimethyl-
ethylaminol-ethyl}-4H-benzo[1,4,1 oxazin-3-one-fumarate
300 mg (0.71 mmol) 6-hydroxy-8-{(R)-1-hydroxy-242-(4-methoxy-pheny1)-1,1-
dimethyl-ethylaminoi-ethy11-4H-benzo[1,4,]oxazin-3-one hydrochloride are
CA 02565915 2006-11-06
W02005/110359 28
PCT/EP2005/005078
combined with ethyl acetate and extracted with aqueous potassium carbonate
solution. The organic phase is dried with sodium sulphate and freed from the
solvent. The residue is dissolved in ethanol with the addition of a few drops
of
water. 82 mg (0.71 mmol) fumaric acid and seed crystals are added and the
mixture
is left to stand overnight. The white solid is suction filtered, washed with
diethyl
ether and ethanol and dried. The ethanolamine and acid are present in the salt
in a
ratio of 1 to 0.5.
Yield: 208 mg (63%); mass spectroscopy [M+1-1]+ = 387; melting point = 130 C.
The highly crystalline product was investigated further by X-ray powder
diffraction.
The X-ray powder diagram was recorded using the following method.
The X-ray powder diagram was recorded within the scope of the present
invention
using a Bruker D8 Advanced with an LSD (= location-sensitive detector) (CuKa
radiation, 2 = 1.5418 A, 30 kV, 40 mA).
For the highly crystalline compound the following characteristic values dmd
[A]were
obtained, inter alia, which give the lattice plane intervals determined in A:
d= 14.23 A; 5.44 A; 4.76 A; 4.57 A; 4.26 A; 4.12 A; 3.57 A and 3.48 A.
Example 10: 6-hydroxv-8-{1-hydroxv-242-(4-methoxv-pheny1)-1,1-dimethyl-
ethylamino]-ethyl}-4H-benzol114joxazin-3-one (free base)
Analogously to the preceding tests, 500 mg (1.2 mmol) 6-hydroxy-8-{ (R)-1-
hydroxy-2-[2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-ethyll-4H-
benzo[1,4,]oxazin-3-one hydrochloride are first of all combined with ethyl
acetate.
The organic phase is extracted with aqueous potassium carbonate solution,
dried
with sodium sulphate and freed from the solvent. The free base thus obtained
is
dissolved in acetonitrile with the addition of a few drops of water. The
precipitated
solid is suction filtered, washed and dried.
Yield: 168 mg (37%); mass spectroscopy [M+1-1]+ = 387; melting point = 128 C.
The highly crystalline product was investigated further by X-ray powder
diffraction.
The X-ray powder diagram was recorded using the following method.
CA 02565915 2006-11-06
W02005/110359 29 PCT/EP2005/005078
The X-ray powder diagram was recorded within the scope of the present
invention
using a Bruker D8 Advanced with an LSD (= location-sensitive detector) (CuKa
radiation, A, = 1.5418 A, 30 kV, 40 mA).
For the highly crystalline compound the following characteristic values dhid
[A]were
obtained, inter alia, which give the lattice plane intervals determined in A:
d= 14.96 A; 9.63 A; 7.05 A; 5.57 A; 5.28 A; 5.05 A; 4.63 A and 3.73 A.
Example 11: 6-hydroxy-8-{ (R)-1-hydroxv-2-12-(4-methoxy-pheny1)-1,1-dimethvl-
ethylaminol-ethyl }-4H-benzo [1,4,1oxazin-3-one-hydrochloride
300 mg (0.71 mmol) 6-hydroxy-8-1(R)-1-hydroxy-2-[2-(4-methoxy-pheny1)-1,1-
dimethyl-ethylamino]-ethyl}-4H-benzo[1,4,]oxazin-3-one hydrochloride are
dissolved by heating in 4 mL isopropanol. The solution is cooled to ambient
temperature and then placed in an ice bath for 15 minutes. The precipitated
solid is
suction filtered and dried.
Yield: 180 mg (60%); mass spectroscopy [M+H] = 387; melting point = 211 C.
The highly crystalline product was investigated further by X-ray powder
diffraction.
The X-ray powder diagram was recorded using the following method.
The X-ray powder diagram was recorded within the scope of the present
invention
using a Bruker D8 Advanced with an LSD (= location-sensitive detector) (CuKa
radiation, X = 1.5418 A, 30 kV, 40 mA).
For the highly crystalline compound the following characteristic values di
[A]were
obtained, inter alia, which give the lattice plane intervals determined in A:
d= 5.92 A; 5.81 A; 5.51 A; 5.10 A; 4.65 A; 4.50 A; 4.15 A and 4.00 A.
The following compounds may be obtained analogously using the method described
in Examples 6 to 11:
Example 12: 8-{ (R)-242-(2,4-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-
hydroxy-ethyl}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-maleate;
Example 13: 8-1(R)-2- [2-(2,4-difluoro-phenyl)-1,1-dimethyl-ethylamino] -1-
hydroxy-ethyl}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-salicylate;
CA 02565915 2006-11-06
W020051110359 30 PCT/EP2005/005078
Example 14: 8-{ (R)-2- [2- (2,4-difluoro-phenyl)-1,1-dimethyl-ethylamino] -1-
hydroxy-ethyl }-6-hydroxy-4H-benzo [1,4] oxazin-3-one-succinate;
Example 15: 8- { (R)-242-(2,4-difluoro-pheny1)-1,1-dimethyl-ethylamino] -1-
hydroxy-ethyl} -6-hydroxy-4H-benzo [1,4] oxazin-3-one-fumarate;
Example 16: 8- { (R)-2- [2-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino] -1-
hydroxy-ethyl} -6-hydroxy-4H-benzo [1,4] oxazin-3-one-maleate;
Example 17: 8-{ (R)-2- [2- (3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-
hydroxy-ethyl} -6-hydroxy-41-1-benzo [1,4] oxazin-3-one-salicylate;
Example 18: 8- { (R)-2- [2-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-
hydroxy-ethyl}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-succinate;
Example 19: 8- { (R)-2- [2- (3,5-difluoro-phenyl)-1,1-dimethyl-ethylamino] -1-
hydroxy-ethyl} -6-hydroxy-4H-benzo [1,4] oxazin-3-one-fumarate;
Example 20: 8- (R)-2- [2-(4-ethoxy-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-maleate;
Example 21: 8-{ (R)-2- [2-(4-ethoxy-phenyl)-1,1-dimethyl-ethy1amino] -1-
hydroxy-
ethyl }-6-hydroxy-4H-benzo [1,4] oxazin-3-one-salicylate;
Example 22: 8- { (R)-2- [2- (4-ethoxy-phenyl)-1,1-dimethyl-ethylamino] -1-
hydroxy-
ethy1}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-succinate;
Example 23: 8- { (R)-242- (4-ethoxy-phenyl)-1,1-dimethyl-ethylamino] -1-
hydroxy-
ethy1}-6-hydroxy-4H-benzo [1,4] oxazin-3-one-fumarate;
Example 24: 8- { (R)-242-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino] -1-hydroxy-
ethyl} -6-hydroxy-4H-benzo [1,4] oxazin-3-one-maleate;
CA 02565915 2006-11-06
W02005/110359 31
PCT/EP2005/005078
Example 25: 8-{(R)-242-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo[1,4]oxazin-3-one-salicylate;
Example 26: 8-{ (R)-2-[2-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl} -6-hydroxy-4H-benzo [1,4] oxazin-3-one-succinate;
Example 27: 8-{(R)-2-[2-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo[1,4]oxazin-3-one-fumarate;
Example 28: 8-{(R)-2-[2-(2,4-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-
hydroxy-ethy1}-6-hydroxy-4H-benzo[1,4]oxazin-3-one (free base);
Example 29: 8-{ (R)-2-[2-(3,5-difluoro-pheny1)-1,1-dimethyl-ethylamino]-1-
hydroxy-ethy1}-6-hydroxy-4H-benzo [1,4]oxazin-3-one (free base);
Example 30: 8-{ (R)-242-(4-ethoxy-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy11-6-hydroxy-4H-benzo[1,4]oxazin-3-one (free base) or also
Example 31: 8-{ (R)-2-[2-(4-fluoro-pheny1)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethy1}-6-hydroxy-4H-benzo[1,4]oxazin-3-one (free base).
Preparation of the powder formulations according to the invention:
I) Apparatus
The following machines and equipment may be used, for example, to prepare the
inhalable powders:
Mixing container or powder mixer: Turbulamischer 2 L, type 2C; manufactured
by
Willy A. Bachofen AG, CH-4500 Basel
Hand-held screen: 0.315 mm mesh size
The empty inhalant capsules may be filled with inhalable powder containing the
active substance manually or by machine. The following equipment may be used.
CA 02565915 2006-11-06
W02005/110359 32
PCT/EP2005/005078
Capsule filling machine:
MG2, type G100, manufacturer: MG2 S.r.1, 1-40065 Pian di Macina di Pianoro
(BO), Italy
Example 1:
Powder mixture:
In order to prepare the powder mixture 97.0 g excipient (lactose monohydrate
200
mesh with an average particle size of 25 - 50 m, which varies from one batch
to
another) and 3.0 g micronised compound of formula 1 are used. The proportion
of
active substance in the 100g inhalable powder obtained is 3.0 %.
The excipient is placed in a suitable mixing container through a hand-held
screen
with a mesh size of 0.315 mm. Then 3g of micronised compound of formula I. and
7g of excipient are screened in in alternate layers. The excipient and the
active
substance are added in 7 and 6 layers, respectively (premix I). The
constituents
screened in are then mixed (mixing: 30 rpm /30 min).
lOg of premix I and 90g excipient are then added in alternate layers through
the
same hand-held screen with a mesh size of 0.315 mm by screening into a
suitable
mixing container. The excipient and the premix I are added in 8 -10 layers
(final
mix). The constituents screened in are then mixed (mixing: 30 rpm / 30 min).
The following inhalable powders may be obtained according to or analogously to
the procedure described in Example 1:
Example 2:
active substance = Example 6 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 3:
active substance = Example 6 3.0g
Lactose monohydrate: 97.0g
Total: 100.0g
CA 02565915 2006-11-06
W02005/110359 33
PCT/EP2005/005078
Example 4:
active substance = Example 9 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 5:
active substance = Example 10 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 6:
active substance = Example 11 3.0g
Lactose monohydrate: 97.0g
Total: 100.0g
Example 7:
active substance = Example 13 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 8:
active substance = Example 11 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 9:
active substance = Example 10 3.0g
Lactose monohydrate: 97.0g
Total: 100.0g
Exam')le 10:
active substance = Example 13 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
CA 02565915 2006-11-06
W02005/110359 34
PCT/EP2005/005078
Example 11:
active substance = Example 14 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 12:
active substance = Example 14 3.0g
Lactose monohydrate: 97.0g
Total: 100.0g
Example 13:
active substance = Example 15 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 14:
active substance = Example 8 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 15:
active substance = Example 8 3.0g
Lactose monohydrate: 97.0g
Total: 100.0g
Example 16:
active substance = Example 7 0.6g
Lactose monohydrate: 99.4g
Total: 100.0g
Example 17:
active substance = Example 6 0.6g
Mannitol: 99.4g
Total: 100.0g
CA 02565915 2006-11-06
W02005/110359 35
PCT/EP2005/005078
Example 18:
active substance = Example 6 3.0g
Mannitol: 97.0g
Total: 100.0g
Example 19:
active substance = Example 9 0.6g
Mannitol 99.4g
Total: 100.0g
Example 20:
active substance = Example 10 0.6g
Mannitol: 99.4g
Total: 100.0g
Example 21:
active substance = Example H 3.0g
Mannitol: 97.0g
Total: 100.0g
Example 22:
active substance = Example 13 0.6g
Mannitol: 99.4g
Total: 100.0g
Example 23:
active substance = Example 11 0.6g
Mannitol: 99.4g
Total: 100.0g
Example 24:
active substance = Example 10 3.0g
Mannitol: 97.0g
Total: 100.0g
CA 02565915 2006-11-06
W02005/110359 36
PCT/EP2005/005078
Example 25:
active substance = Example 13 0.6g
Mannitol: 99.4g
Total: 100.0g
Example 26:
active substance = Example 14 0.6g
Mannitol: 99.4g
Total: 100.0g
Example 27:
active substance = Example 14 3.0g
Mannitol: 97.0g
Total: 100.0g
Example 28:
active substance = Example 15 0.6g
Mannitol: 99.4g
Total: 100.0g
Example 29:
active substance = Example 8 0.6g
Mannitol: 99.4g
Total: 100.0g
Example 30:
active substance = Example 8 3.0g
Mannitol: 97.0g
Total: 100.0g
Example 31:
active substance = Example 7 0.6g
Mannitol: 99.4g
Total: 100.0g