Note: Descriptions are shown in the official language in which they were submitted.
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
UTILIZING LIPOPOLYSACCHARIDE IN EXHALED BREATH CONDENSATE TO
DIAGNOSE GRAM NEGATIVE PNEUMONIA
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of, and claims priority to
provisional
U.S. Patent Application Serial No. 60/577,641, filed on June 7, 2004, which is
incorporated
hei-ein by reference in its entirety.
[0002] This application is also a continuation-in-part of U.S. Patent
Application
Serial No, 10/742,721 filed December 19, 2003, which claims the benefit of
provisional U.S.
Patent Application Serial No. 60/434,916 filed December 20, 2002 and provi-
sional U.S.
Patent Application Serial No. 60/447,581 filed February 14, 2003. The entirety
of each of the
aforementioned applications is incorporated herein by reference.
[0003] In addition, this is a continuation-in-part of U.S. Patent Application
Serial
No. 10/778,477 filed February 13, 2004, which claims the benefit of
provisional U.S. Patent
Application Serial No. 60/447,581 filed February 14, 2003. The entirety of
each of the
aforementioned applications is likewise incorporated herein by reference.
BACKGROUND OF THE PRESENT INVENTION
Field of the Present Invention
[0004] The present invention relates generally' to a method and devices for
diagnosing gram negative bacterial pneumonia and, in particular, to diagnosing
gram
negative bacterial pneumonia by detecting the presence of lipopolysaccharide
in exhaled
breath condensate.
Background
[0005] Pneumonia represents a common disease with significant morbidity and
mortality. Pneumonia is the number one cause of death by infectious disease
and the sixth
most common cause of death in the United States. The National Hospital
Ambulatory
Medical Care Survey found that in 2001, 1.48 million emergency department
visits were
related to a diagnosis of pneumonia. The National Hospital Discharge Survey
found that in
1998, 1.32 million patients were discharged after having been treated for
pneumonia.
1
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
[0006] Pneumonia can be caused by lung infection of many types of
microorganisms, including viruses, chlamydia, mycoplasma, protozoa, fungi, and
bacteria.
For a patient with suspected pneumonia, the clinician has a duty to determine
the exact cause
of infection because the identity of the infectious agent dictates the choice
of antimicrobial
' treatment. The most common cause in Western society is bacterial pneumonia;
and when
bacterial pneumonia is suspected; clinicians generally seek to categorize the
cause of
bacterial pneumonia as Gram positive, Gram negative or anaerobic.
[0007] In particular, clinicians are motivated to identify the presence of
Gram
negative bacterial infection because Gram negative lung infections are
aggressive and are
associated with higher rates of complications and death. The Gram negative
feature of bacteria refers to the color of the bacteria after a staining
protocol that will be well
understood by those skilled in the art. Gram negative bacterial infections,
including gram
negative bacterial pneumonia, require specific antimicrobial therapy, which is
different from
treatment for.other types of bacterial infections, and warrant an elevated
level of financial
reimbursement from third party payors such as Medicare.
[0008] In current clinical practice, pneumonia is diagnosed by combining
clinical,
laboratory and radiographic information. In general, features such as the
patient's complaint,
the patient's vital signs, the peripheral white blood count, and the results
of chest radiography
are used to determine the presence or absence of pneumonia. When these sources
of data fit a
typical pattern, the diagnosis can be made with reasonable clinical certainty,
-and
antimicrobial therapy can be initiated prior to the results of bacteriological
cultures.
Although nonspecific clinical data is often used to initiate antibiotic
treatment for Gram
negative infection, common practice dictates that the 'final diagnosis of Gram
negative
pneumonia requires more specific evidence of Gram negative bacterial lung
infection.
[0009] Toward this goal, a Gram's stain can be performed immediately on sputum
that is coughed from the lower airways, and microscopic analysis may reveal
bacteria with
morphology and color suggesting Gram negative infection. However, useful
sputum samples
are notoriously difficult to obtain from humans with pneumonia. When blood
specimen
cultures from a patient who has a clinical pattem consistent with pneumonia
grow a Gram
negative bacterium, this provides a specific indication of Gram negative
pneumonia.
2
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
Unfortunately, more often than not, the blood is sterile in a patient with
Gram negative
pneumonia.
[0010] In addition, a patient's blood may be examined for endotoxin
concentration using chemical assays for the endotoxin molecule. However,
endotoxin
concentrations have been found to be an iriaccurate predictor of either the
cause or severity of
the more general sepsis syndrome. Endotoxin concentrations in the blood may
fluctuate
widely over short time periods. Further, certain disease states, including
iiver disease,
polytrauma, hypertension, and hematological malignancies are associated with
chronically
elevated endotoxin concentrations in the absence of clinically significant
infection. No study
has examined whether circulating endotoxin concentrations can predict a gram
negative
source of pneumonia.
[0011] A sensitive and specific method to diagnose Gram negative lung
infection
is to perform bronchalveolar lavage and to perform a bacteriological culture
on the lavage
fluid. Another method is to chemically assay for lipopolysaccharide content '
in
bronchoalveolar lavage samples. Investigators using this method found that
high
concentrations of lipopolysaccharide are associated with concomitant growth,
of gram
negative bacteria in cultures of the bronchalveolar fluid. A complete
description of this
method may be found. in Flanagan, P.G., Jackson, S. K., Findlay, G.,
"Diagnosis of Gram
negative, ventilator associated pneumonia by assaying endotoxin in bronchial
lavage fluid", J.
Clin. Pathol. 2001, 54:107-110 and Pugin, J., Auckenthaler, R. and Delaspre,
0., "Rapid
Diagnosis of gram-negative pneumonia by assay of endotoxin in bronchoalveolar
lavage
fluid", Thorax 1992, 47:547-549. Both methods have the drawbacks that special
endoscopic
equipment and subspecialty expertise are required and that they are
relati.vely invasive and
uncomfortable procedures. Moreover, known culture methods iequire at least 24
hours to
obtain results. As such, a patient may wait for up to at least 24 hours before
receiving an
effective antibiotic treatment.
[0012] The cell wall of Gram negative bacteria comprises endotoxins.
Endotoxins are toxic materials released by bacterium on bacterial lysis. While
endotoxins
were first recognized for their ability to induce fever, they are now: known
to have a broad
spectrum of biologic activities. On bacteriolysis, endotoxins consisting of
aggregates of
lipopolysaccharides and protein and lipids, are released from the bacterium
into surrounding
3
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
medium. Endotoxins consist primarily of lipopolysaccharide ("LPS") with
various amounts
of protein and lipid. Since almost all of the biologic activities usually
attributable to bacterial
endotoxins can also be elicited with isolated chenmically pure
lipopolysaccharide, the terms
"endotoxin" and "lipopolysaccharide" are used interchangeably.
[0013] From a pathogenic standpoint, the presence of lipopolysaccharide is one
of
the most important implications of a Gram negative infection. As such,
detecting
lipopolysaccharide in a patient's bodily fluid is an indicator of Gram
negative bacterial
infection. More specifically, the presence of lipopolysaccharide in a
patient's'bodily fluid is
an important potential indicator of Gram negative pneumonia.
[0014] The present invention overcomes the above-described clinical
disadvantages of diagnosing Gram negative bacterial pneumonia by performing an
assay for
lipopolysaccharide on the liquid derived from condensation of exhaled breath.
Using novel
devices and methods described herein, exhaled breath condensate may be
obtained from a
spontaneously breathing subject via a mouthpiece, facemask or other similar
means or from a
subject breathing with the assistance of a mechanical ventilator via a
connection to the
expiratory tubing of the mechanical ventilator. An additional advantage of the
present
invention is the shorter time period for diagnosing a Gram negative bacterial
infection, such
as pneumonia, than is required for other diagnostic methods.
[0015] Alternative devices for collecting exhaled breath condensate are also
known. These devices include those disclosed in Gaston et al., U.S. Patent
Nos. '6,033,368
and 6,419,634; Hunt et al., U.S. Patent No. 6,585,661; Baddour, U.S.
Application Serial No.
10/257,912; and EU Patent No. 0,759,169 B1 to Winsel et al., all of which are
incorporated
by reference herein in their entireties. In addition, Kline, U.S. Application
Serial Nos.
10/742,721 and 10/778,477, two commonly-assigned non-provisional patent
applications,
disclose devices for collecting exhaled breath and are. incorporated by
reference herein in
their entireties.
[0016] Exhaled condensate is known to contain many molecules that can serve
as'
markers of many lung diseases, as reviewed by Kharitinov et al. in 2001
(Biomarkers 7(1):1-
32, 2002.). However, the concept of measuring lipopolysaccharide in exhaled
breath
condensate for the purpose of diagnosing Gram negative pneumonia or other Gram
negative
bacterial infections has not been disclosed previously.
4
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
SUMMARY OF THE PRESENT INVENTION
[0017] The present invention comprises a method for determining whether a
subject has Gram negative bacterial pneumonia based on the presence of
lipopolysaccharide
in exhaled breath condensate collected from the subject, The present invention
further
comprises the collection devices utilized to collect exhaled breath condensate
from both
sporitaneously breathing and mechanically ventilated subjects and the devices
utilized to
determine whether lipopolysaccharide is present in the collected exhaled
breath condensate.
[0018] Broadly defined, the present invention, according to one aspect, is a
method for diagnosing and monitoring intrapulmonary Gram negative bacterial
infection in
an air-breathing vertebrate subject, including: collecting exhaled breath
condensate from an
air-breathing vertebrate subject; measuring the concentration of
lipopolysaccharide in the
collected exhaled breath condensate; and determining whether the subject has
an
intrapulmonary Gram negative bacterial irifection based on the measured
concentration of
lipopolysaccharide in the exhaled breath condensate.
[0019] In features of this aspect, the intrapulmonary Gram negative bacterial
irifection is pneumonia; the intrapulmonary Gram negative bacterial infection
is a bronchial
infection; the method further includes selecting an antibiotic therapy in
response to a positive
determination that the subject has an intrapulmonary Gram negative bacterial
infection;
collecting exhaled breath condensate from an air-breathing vertebrate subject
includes
collecting exhaled breath condensate from a mammalian subject, and the method
further
includes monitoring the response of the mammalian subject to antibiotic
therapy; the method
further includes identifying the particular strain of the intrapulmonary Gram
negative
bacteria; identifying includes identifying the particular strain of
intrapulmonary Gram
negative bacteria based upon a determination of the O-saccharide portion of
the
lipopolysaccharide molecule; measuring the concentration of lipopolysaccharide
includes
measuring the concentration of lipopolysaccharide using the limulus amoebocyte
lysate
assay; a measured concentration of lipopolysaccharide of at least about 0.20
Endotoxin
Units/mL (EU/mL) indicates the presence of a Gram negative bacterial
infection; collecting
exhaled breath condensate from an air-breathing vertebrate subject includes
collecting the
expired breath condensate from a spontaneously breathing subject; collecting
exhaled breath
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
condensate from an air-breathing vertebrate subject includes collecting the
expired breath
condensate from a mechanically ventilated subject; and collecting exhaled
breath condensate
from an air-breathing vertebrate subject includes utilizing an exhaled breath
condensate
collection device comprising . a chamber having inner walls that may be cooled
to a
temperature of about 32 degrees Fahrenheit and below to promote condensation.
[0020] The present invention, according to another aspect, is a method for
diagnosing and monitoring intrapulmonary Gram negative bacterial infection in
an air-
breathing vertebrate subject, including: collecting exhaled breath condensate
from an air-
breathing vertebrate subject; providing a reaction chamber, wherein said
reaction chamber
has a reaction reagent disposed therein; delivering at least a portion of the
collected exhaled
breath condensate to the reaction chamber; and determining whether the subject
has an
intrapulmonary Gram negative bacterial infection based on a physical change
that occurs
when the at least a portion of the collected exhaled breath condensate 'is
delivered to the
reaction chamber, wherein said physical change is caused by the presence of
lipopolysaccharide in the exhaled breath condensate.
[0021] In features of this aspect, the reaction reagent includes a limulus
amoebocyte lysate, a chromogenic substrate and/or a fluorogenic substrate; the
reaction
reagent includes a chemical that liberates heat through an exothermic reaction
upon delivery
of the at least a portion of the exhaled breath condensate; the physical
change is ,the formation
of a gel; the method further includes visually matching a color change in; the
reaction
chamber to a standard, wherein said standard comprises a printed strip of
color patches of
increasing hue intensity, and wherein each color patch corresponds to
increasing
concentrations of lipopolysaccharide, respectively; a hue intensity
corresponding to a
concentration of 0.2 endotoxin units per milliliter indicates the presence of
an intrapulmonary
Gram negative bacterial infection; collecting exhaled breath condensate from
an air-breathing
vertebrate subject includes collecting the expired breath condensate from a
spontaneously
breathing subject; collecting exhaled breath condensate from an air-breathing
vertebrate
subject includes collecting the expired breath condensate from a mechanically
ventilated
subject; collecting exhaled breath condensate from an air-breathing vertebrate
subject
includes utilizing an exhaled breath condensate collection device comprising a
chamber
having inner walls that may be cooled to a temperature of about 32 degrees
Fahrenheit and
below to promote condensation; and delivering at least a portion of the
exhaled breath
6
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
condensate comprises injecting at least of portion of the exhaled breath
condensate into the
reaction chamber via a hypodermic needle.
[0022] The present invention, according to another aspect, is a method for
diagnosing and monitoring intrapulmonary Gram negative bacterial infection in
an air-
breathing vertebrate subject, including: collecting exhaled breath condensate
from an air-
breathing vertebrate subject; providing a reaction chamber with an interior
surface, wherein
said reaction chamber has' a material that binds lipopolysaccharide disposed
therein;
delivering at least a portion of the collected breath condensate to the
reaction chamber;
washing the reaction chamber with a buffer; delivering a reaction reagent to
the reaction
chamber; and determining whether the subject has an intrapulmonary Gram
negative bacterial
infection based on a physical change that occurs when the at least a. portion
of the collected
exhaled breath condensate is delivered to the reaction chamber, wherein said
physical change
is caused by the presence of lipopolysaccharide in the exhaled breath
condensate,
[0023] In features of this aspect, the reaction reagent comprises a
chromogenic
substrate and/or a fluorogenic substrate; the binding material is an antibody.
that binds a
specific species of lipopolysaccharide; the antibody is disposed on the
interior surface of the
reaction chamber; the binding material is an insoluble polymer; the binding
material is
embedded in a matrix surface disposed within the reaction chamber; the method
further
includes visually matching a color change in the reaction chamber to a
standard, wherein said
standard comprises a printed strip of color patches of increasing hue
intensity, and wherein
each color patch corresponds to increasing concentrations of
lipopolysaccharide; respectively;
a hue intensity corresponding to a concentration of 0.2 endotoxin units per
milliliter indicates
the presence of an intrapulmonary Gram negative bacterial infection;
collecting exhaled
breath condensate from an air-breathing vertebrate subject includes collecting
the expired
breath condensate from a spontaneously breatliing subject; collecting exhaled
breath
condensate from an air-breathing vertebrate subject includes collecting the
expired breath
condensate from a mechanically ventilated subject; collecting exhaled breath
condensate
from an air-breathing vertebrate subject includes utilizing an exhaled breath
condensate
collection device comprising a chamber having inner walls that may be cooled
to a
temperature of about 32 degrees Fahrenheit and below to promote condensation;
delivering at
least a portion of the exhaled breath condensate comprises injecting at least
of portion of the
exhaled breath condensate into the reaction chamber via a hypodermic needle.
7
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
[0024] The present invention, according to another aspect, is a method for
diagnosing and monitoring intrapulmonary Gram negative bacterial infection in
an air-
breathing vertebrate subject, including: collecting exhaled breath condensate
from an air-
breathing vertebrate subject in a collection device having a narrow tube;
inserting a fibrous
plug impregnated with enzymes that cause gelation upon exposure to
lipopolysaccharide into
the narrow tube; introducing at least a portion of the collected exhaled
breath condensate
sample into the narrow tube to wet the fibrous plug; and determining whether
the subject has
an intrapulmonary Gram negative bacterial infection based on the amount of
gelation that
occurs in the narrow tube, wherein said gelation is caused by the presence of
lipopolysaccharide in the exhaled breath condensate.
[0025] In features of this aspect, the fibrous plug is impregnated with
enzymes
from limulus amoebocyte lysate; the method further includes providing a
syringe disposed
within the collection device, said syringe having a manometer disposed on one
side thereof
for measuring hydraulic pressure; and the narrow tube is a hypodermic needle
disposed at the
end of the syringe.
[0026] The present invention, according to another aspect, is a device for
collecting exhaled breath condensate from a subject breathing. with the
assistance of a
mechanical ventilation circuit, including: a mechanical ventilation circuit
for facilitating
breathing of the subject, wherein the mechanical ventilation circuit includes
an expiratory
flow tube that serves as a conduit for removing exhaled breath of the subject;
a central
chamber having an interior, wherein said central chamber may be cooled to a
temperature of
about 32 degrees Fahrenheit and below to promote condensation; a breath input
assembly,
disposed at one end of the central chamber, in fluid communication with the
interior of the
central chamber and the expiratory flow tube, whereby the breath input
assembly connects
the expiratory flow tube and the central chamber; an exit assembly, disposed
at the other end
of the central chamber, in fluid communication with the interior of the
central chamber; and a
vacuum device connected to the exit assembly for collecting exhaled breath
condensate from
the central chamber.
[0027] The present invention, according to another aspect, is a breath
condensate
collection device, including: a central chamber having an interior and first
and second
opposing ends; a breath input assembly in fluid communication with the
interior of the'central
8
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
chamber; and an exit assembly in fluid communication with the interior of the
central
chamber, wherein the exit assembly includes a narrow tube, said narrow tube
having a fibrous
plug disposed therein, said plug being impregnated with a reaction reagent
that causes
gelation upon exposure to lipopolysaccharide.
[0028] In features of this aspect, the plug is a fiber matrix impregnated with
enzymes from a limulus amoebocyte; and the device further includes a plunger
assembly
having a piston and a handle, wherein the piston is slidably disposed in the
interior of the
central chamber and wherein the handle extends from the first end of the
central chamber so
as to permit the piston to be moved within the central chamber, whereby the
collected breath
condensate may contact the fibrous plug disposed within the narrow tube.
[0029] The present invention, according to another aspect, is a reaction
chamber
assembly for measuring the concentration of lipopolysaccharide in exhaled
breath
condensate, including: a reaction chamber; a delivery port for delivery of the
exhaled breath
condensate into the reaction chamber; a reaction reagent to react with
lipopolysaccharide
present in the exhaled breath condensate; and a viewing portion to allow for
visible detection
of a physical change in the reaction chamber..
[0030] , In features of this aspect, the delivery port is a re-sealable cover
that may
be punctured with a hypodermic needle for delivery of the exhaled breath
condensate; the
viewing portion is a transparent wall; the reaction chamber further includes
an interior
surface, and a lipopolysaccharide-immobilizing agent is bound to at least a
portion of the
interior surface. of the reaction chamber; the lipopolysaccharide-immobilizing
agent is
selected from the group consisting of monoclonal antibodies, polyclonal
antibodies,
polymyxin antibiotic, lipopolysaccharide-binding protein and limulus anti-
lipopolysaccharide
factor; the reaction chamber further includes a chemical disposed within the
reaction
chamber, wherein the chemical liberates heat upon hydration, whereby a
temperature inthe
range of 34 to 43 degrees Celsius is achieved for a period of 15 to 30 minutes
upon hydration
of the chemical; the chemical is a salt in a semipermeable matrix; the
chemical is a salt in
crystalline form; and the chemical is sodium thiosulfate pentahydrate,
10031] The present invention, according to another aspect, is a test kit
cartridge
for detecting the presence of lipopolysaccharide in exhaled breath condensate,
including: a
housing; a test modute disposed within the housing, said test module utilizing
a user-initiated
9
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
chemical reaction to detect the presence of lipopolysaccharide in the exhaled
breath
condensate; and a positive control module disposed within the housing adjacent
to tha test
module, said positive control module having lip-opolysaccharide disposed
therein for showing
a definite positive result for the presence of lipopolysaccharide for
comparison with the test
module.
[0032] In features of this aspect, a chemical disposed in the housing provides
a
controlled exothermic reaction, whereby a temperature between 34 and 43
degrees Celsius is
achieved for a period of 15 to 30 minutes; the chemical is sodium thiosulfate
pentahydrate;
each module of the test kit includes a re-sealable injection port, a reaction
well, a fluid
connection between the reaction well and the injection port, a first one-way
valve disposed in
the fluid connection, an exit tube providing an outlet for the reaction well,.
and a second one-
way valve disposed in the exit tube; each reaction well includes a clear
covering adapted to
allow visual determination of physical change; each reaction well includes an
'insoluble
polymeric lipopolysaccharide-binding matrix; the positive control module.
reaction well
includes lipopolysaccharide from a suitable species of Gram negative bacteria;
the test kit
cartridge further includes a chromogenic substrate, reconstituted from a
reaction chamber; the
test kit cartridge further includes a color strip, the color strip having
color patches of
incrementally increasing color intensity, wherein each color patch corresponds
to a prescribed
range of endotoxin units to allow for visual comparison against a color change
in the reaction
wells; the housing is formed from a polymer; the polymer is plastic.
[0033] Further areas of applicability of the present invention will become
apparent from the detailed description provided hereinafter. It should be
understood that the
detailed description and specific examples, while indicating the preferred
embodiment of the
invention, are intended for purposes of illustration only and are not intended
to limit the
scope of the invention.
.BRIEF DESCRIPTION OF THE DRAWINGS
[0034] Further, features, embodiments, and advantages of the present invention
will become apparent from the following detailed description with reference to
the drawings,
wherein:
Fig. 1 is a schematic diagram of the basic structure of lipopolysaccharide;
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
Fig. 2 is a side cross-sectional schematic view of a breath condensate.
collection device in accordance with a first preferred embodiment of the
present
invention;
Fig. 3A is a partial side cross-sectional schematic view of the breath
condensate collection device of Fig. 2 with the plunger assembly in a
partially
depressed position;
Fig. 3B is a partial side cross-sectional schematic view of the breath
condensate collection device of Fig. 2 with the plunger assembly in a fully
depressed
position;
Fig. 4 is a partial side cross-sectional view of a breath condensate
collection
device in accordance with a second preferred embodiment of the present
invention;
Fig. 5. is a side cross-sectional schematic view of a breath condensate
collection device in accordance with a third preferred embodiment of the
present
invention;
Fig. 6 is a schematic illustration of an alternative ventilation system
implementation of the preferred embodiments of the present invention;
Fig. 7 is a side cross-sectional schematic view of a reaction chamber device
suitable for use with the collection devices of Figs. 2, 4 and 5;
Fig. 8 is a top view of the reaction chamber device of Fig. 7;
Fig. 9 is a side perspective view of a first alternative reaction chamber
device;
Figs. 1OA-lOD are partial side cross-sectional views of the first alternative
reaction chamber device of Fig. 9;
Fig. 11 'is a perspective view of a test kit incorparating a second
alternative
reaction chamber device;
Fig. 12 is a side cross-sectional view of the test module of Fig. 11, taken
along
line 12-12;
11
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
Fig. 13 is a side cross-sectional view of the positive control rriodule of
Fig. 11,
taken along line 13-13;
Fig. 14 is a partial side cross-sectional view of a breath condensate
collection
device in accordance with a fourth preferred embodiment of the present
invention;
Fig. 15 is an enlarged partial side cross-sectional view of the needle of Fig.
14;
and
Fig. 16 is a scatter plot graph illustrating the measured endotoxin
concentrations for patients in each of three study groups.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0035] The method of the present invention utilizes exhaled breath condensate
to
determine whether LPS is. present in the exhaled breath condensate and thus
determine
whether a subject.has an intrapulmonary Gram negative bacterial infection. The
present
application is directed toward diagnosing Gram negative bacterial pneumonia;
however, one
of ordinary skill in the art will understand that the present invention may be
utilized to detect
any Gram negative bacterial infection, which is also useful. A patient
diagnosed with a Gram
negative bacterial infection will be treated with the same antimicrobial
therapy whether the
diagnosis is Gram negative pneumonia or another Gram negative bacterial
infection. One of
ordinary skill in the art will understand that while the methodology for.
detecting: LPS, asdescribed herein, is the limulus amoebocyte lysate assay,
any methodology for detecting the
presence of LPS, including, but not limited to the ELISA assay and the rabbit
pyrogen test,
may be utilized in the present invention.
[0036] Intrapulmonary LPS coritent can vary based upon the health of the lung
in
the subject providing a breath condensate sample. Generally, a concentration
of LPS in
exhaled breath condensate of at least about 0.2 EU/mL corresponds to a
clinical, test positive
threshold, indicating the presence of Gram negative bacterial infection in the
lungs. An EU is
an endotoxin unit. The USDA recommends reporting the concentration of
endotoxin in
endotoxin units because an EU can be standardized, unlike typical mass
concentrations,
which cannot be standardized because of the variable potency of
lipopolysaccharide, which
varies between bacteria type. An endotoxin unit refers to the amount of LPS
reactivity
contained in the unknown sample. This amount of reactivity is determined by
interpolation
12
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
from a standard curve, which is derived from ari FDA-approved strain of E-Coli
that has a
known amount of LPS reactivity.
[0037] It is possible for the device used to collect exhaled breath condensate
to
contain a "background" level of LPS due to naturally occurring LPS. In order
to account for
this background concentration, a "mock" standard may be conducted with the
device that will
be used for exhaled breath collection by instilling LPS-free water through the
device. The
LPS concentration values found in the water sample may then be subtracted from
the LPS
concentration values.found in breath condensate samples to remove the
background "noise"
of the system. The background concentration of LPS will vary depending on the
device
being used for collection.
[0038] A breath condensate collection device, several examples of which are
described herein below in further detail, may be used to collect exhaled
breath condensate
from a patient presenting symptoms synonymous with Gram negative bacterial
pneumonia
for detection of LPS content in amounts indicating infection.
[0039] Referring now to the drawings, in which like numerals represent like
components throughout the several views, the preferred embodiments of the
present invention
are next described. The following description of the preferred embodiment(s)
is merely
exemplary in nature and is in no way intended to limit the invention, its
application, or uses.
[0040] Fig. 1 is a schematic diagram of the basic structure of
lipopolysaccharide.
This structure is found in the cell wall of all Gram negative bacteria.
Lipopolysaccharide
(also referred to as endotoxin) is a complex glycolipid, weighing
approximately 10Kd. The
basic structure of lipopolysaccharide involves three relatively well defined
regions and is
similar in all gram-negative bacteria. These regions are an 0-antigen portion,
a core
polysaccharide and lipid A. The 0-antigen portion is composed of repeating
polysaccharide
units, each having 2-6 saccharides. The 0-antigen portion varies considerably
among Gram
negative species and can thus serve as a marker of individual bacterial
species, based upon
binding of specific monoclonal antibodies. The core lies between the 0-antigen
portion and
lipid A and is a branching polysaccharide having representative sugars such as
glucose, N-
acetylglucosamine and galactose. Unlike the 0-antigen portion, there is only
minor variatiori
throughout the core region with the structure being highly conserved in the
inner core region
proximal to lipid A. The most highly conserved portion of the LPS molecule is
lipid A, a
13
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
disaccharide diphosphate, to which long-chain fatty acids are attached. The
lipid A portion of
the molecule confers toxicity in virtually all complex eukariotic organisms.
In humans, this
includes induction of cytokines, fever, leukocytosis, recruitment and
activation of leukocytes,
vascular damage, vasodilation, intravascular coagulation and organ damage.
[0041] One accepted method of detecting and quantifying LPS takes advantage of
the toxic effect of LPS on the aqueous extract of amoebocytes from the
horsehoe crab,
limulus polyphemus. The term "amoebocytes," as used in the present
application, refers to
blood cells. This method, known generally as the limulus ainoebocyte lysate
("LAL") assay,
is described in U.S. Patent Nos. 4,322,217; 5,310,657 and 5,702,882. Other
recognized
methods include the in-vivo rabbit pyrogen assay and the human pyrogen assay,
which are
well known to those skilled in the art and thus, will not be discussed in
detail herein.
[0042] Briefly summarized, LAL forms a coagulen gel when incubated with LPS,
which enables the detection of small quantities of LPS. More specifically, in
the LAL assay,
LPS activates an enzyme, commonly known as factor C, which is contained in the
amoebocytes. Activated factor C, in turn, activates factor B by hydrolyzing a
specific site of
factor B. Activated factor B activates a proclotting enzyme to convert it
?into a clotting
enzyme. The clotting enzyme then cleaves coagulogen (coagulant protein,
molecular weight:
19,723) at specific sites (i.e., Arg8 - Thr19 and Arg46 - G1y47) to cause
gelation of themixture.
In addition, co-factors such as salts of calcium, magnesium and phosphate or
other organic
compounds such as hydroxymethyl ("TRIS") aminomethane buffer must be present
to
maintain a pH between 6.5 and 7.4, enabling the clotting enzyme to function,
[0043] Various techniques have been utilized to detect LPS based on the
formation of a coagulen gel in the LAL assay. Some are endpoint assays that
simply wait for
the formation of a gel to determine the presence of LPS. Others are more
complex and use
kinetic turbidimetric methods to measure the increase in turbidity. as
coagulation occurs in the
LAL assay.
[0044] An altemative assay methodology using chromogenic or fluorogenic
substrates has also been used to detect LPS in order to overcome difficulties
associated with
accurately deterinining gel form,ation. This alternative methodology relies on
the clottirig
enzyme that was formed in the above cascade reaction hydrolyzing an amide bond
in a
synthetic substrate. The synthetic substrate may be covalently attached to a
marker molecule
14
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
or compound, which is liberated when hydrolyzed by, the clotting enzyme. The
liberated
marker may then be detected by colorimetry or spectrophotometry, thus
indicating the
presence of LPS. Examples of synthetic substrates may include, but are not
limited, t-
butoxycarbonyl-leucyl-glycyl-arginine-paranitroanilide (N-t-Boc-Leu-Gly-Arg-
pNA), -N-t-
Boc-Val-Leu-Gly-Arg-pNA (SEQ ID NO: 1), benzyloxycarbonyl-leucyl-glycyl-
arginine-
paranitroanilide (Z-Leu-Gly-Arg-pNA), Boc-Ile-Glu-Gly-Arg-pNA (SEQ ID NO: 2),
Boc-
Val-Ser-Gly-Arg-pNA (SEQ ID NO: 3) or Boc-Ser-Gly-Arg-pNA, all of'which
liberate
paranitroaniline, which imparts a yellow color to the reaction mixture.
Specific examples of
chromogenic assays are described in Ling, U.S. Patent No. 6,645,724, and
Tamura et al., U.S.
Patent No. 5,702,882. .
[0045] It is also possible to synthesize the enzyme needed for the clotting
cascade
in-vitro by recombinant biotechnology rather than obtaining the enzyme from a
horseshoe
crab. Such synthesized enzyme can be used to detect and quantify LPS in an
unknown
solution using the chromogenic method.
[0046] Other alternate methods of capturing and detecting LPS are also:known.
An LPS binding material, e.g., polymyxin antibiotics or resins, in the form of
an insoluble
matrix polymer, may be affixed to a support for immobilization and subsequent
detection of
LPS by any of the above described turbidimetric, chromogenic or fluorogenic
methods. -In
addition, LPS may be immobilized by any number of antibodies with a high
affinity for LPS.
Antibodies have a Y-shaped structure and include two basic units. The first
unit is the
fragment-antigen binding portion ("Fab") and it binds an antigen, in this
situation, LPS. The
second unit is the fragment-crystallized portion ("Fc"). The Fc unit
canfunction as a handle
to dock the antibody to an immobile surface. Potentially useful antibodies
include, but are not
limited to, polyclonal or. monoclonal antibodies, such as lipid-A reactive
monoclonal
antibody ("HA-lA") and murine anti-endotoxin immunoglobulin M ("E5"),
lipopolysaccharide-binding protein ("LBP".) and related proteins,
bacteriocidal/permeability
increasing protein ("BPI") and limulus anti-LPS factor ("LALF").
[0047] A method utilizing enzyme-linked immunoassay technology is also known
in which various selected matrix-bound antibodies may be used to capture and
detect specific
endotoxins and therefore specific bacteria types. Examples of endotoxin from
bacteria that
may be detected, include, but are not limited to, Escherichia, Bordetilla,
Branhamella,
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
Salmonella, Haemophilus, Klebsiella, Proteus, Enterobacter, Pseudomonas,
Pasteurella,
Acinetrobacter, Chlamydia and Neisseria and in general any bacteria whose LPS
is capable of
binding to the selected antibody.
[0048] It is further recognized that the presence of (3-1,3 glucans (D -D-
glucans)
may also activate the above-described clotting cascade. One of ordinary skill
in the art will
understand that specific measures may be undertaken to limit this false
positive effect.
[0049] The present invention includes several collection devices designed to
allow rapid (e.g., less than 30 minutes), noninvasive collection of exhaled
breath condensate
("EBC") from a spontaneously breathing subject or a patient receiving
mechanical
ventilation, followed by one-step quantitative or semi-quantitative analysis -
of the condensate
for the concentration of LPS. In spontaneously breathing subjects, the exhaled
condensate
may generally be collected via a mouthpiece held by the lips; however, in
patients with
severe respiratory distress, the sample may be collected by fitting the
patient with an airtight,
snug-fitting facemask that allows the delivery of oxygen, while allowing the
diversion of
exhaled gases and aerosol into a condensing chamber such as those described
below.
[0050] In general, the breath condensate collection devices each comprise a
collection chamber that has sterile, LPS-free inner walls that can be cooled
to a temperature
below 32 F to allow condensation of exhaled breath. These breath condensate
collection
devices are preferably disposable and lightweight. Each includes coaxial
chambers with an
interposed area containing coolant that can be chilled externally or via an
internal
endothermic reaction. Such breath condensate collection devices are generally
described in
the aforementioned commonly-developed and commonly-assigned U.S. Patent
Application
Serial Nos. 10/742,721 and 10/778,477, However, the devices described and
illustrated
herein have additional novel and useful features not included in any prior art
devices.
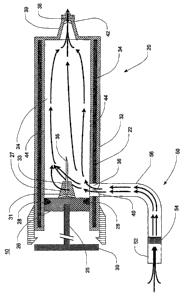
[0061] Fig. 2 is a side cross-sectional schematic view of a breath condensate
collection device 10 in accordance with a preferred embodiment of the present
invention.
This configuration may be particularly appropriate for use with cooperative
humans. The
breath condensate collection device 10 includes a double-walled syringe 20 and
a breath
input assembly 50. The inner wall 22 of the syringe 20 defines a cylindrical
central chamber
24 in which is fitted a plunger assembly 25 that includes a piston 26, a
rubber gasket 28 and a
handle 30 extending from one end of the syringe 20. The outer wall 32 is
arranged around
16
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
the inner wall 22 in such a way as to create a narrow space between the inner
and outer walls
22, 32. During manufacture, the space between the inner and outer walls 22, 32
may be filled
with a jacket of coolant material 34, and the outer wall 32 may then be sealed
to the inner
wall 22 to prevent leakage. In a preferred embodiment, water may be used as
the coolant
material 34, but it should be clear that other materials may likewise be used,
such as
polyethylene glycol ("PEG') and the like.
[0052] The syringe 20 further includes an inlet 36, an outlet 38 and a pair of
one-
way valves 40, 42. The first valve 40 is an intake valve that may be. disposed
in or adjacent
to the inlet 36, while the second valve 42 is an exit valve that may be
disposed in or adjacent
to the outlet 38 in order to facilitate the passage of exhaled air.through the
central chamber 24
in only a single direction. The outlet 38 is preferably disposed at the end
opposite the plunger
handle 30 in order to permit materials collected within the central chamber 24
to be expressed
through the outlet 38 by the piston 26. The outlet 38 may be disposed in the
end of a nozzle.
39 that is in the form of a nipple. The nozzle 39 may also include a fitting,
such as the female
portion of a luer lock, at its distal end. Such a fitting may permit a
protective cover or other
accessory to be attached to the nozzle 39. The valves 40,.42 are illustrated
only
schematically in the various drawings, but they may, for example, include two
or three self-
sealing leaves formed from plastic or another deformable polymer. The design
of such
valves would be apparent to those of ordinary.skill in the art.
[0053] The piston 26 includes a tip or protrusion 27 of dimensions and shape
suitable for.fitting snugly into the nozzle 39 when the plunger, assembly 25
is. fully depressed.
The tip 27 includes a plurality of radial conduits 31 arranged around the base
of the tip 27 and
connecting to a hollow central shaft or conduit 33 in which is disposed a
needle- 35, such as a
hypodermic needle. The radial conduits 31 and the central conduit 33 are
preferably between
about 1 and 2 mm in diameter, and the outside diameter of the needle 35 is
likewise
preferably between about 1 and 2-mm in diameter in order to fit snugly into
the central
conduit 33.
[0054] Further, because the piston 26 fills one end of the syringe 20 and the
outlet
38 is disposed in the opposite end, the inlet 36 is preferably arranged to
penetrate both the
inner and outer walls 22, 32 on the side of the syringe 20. In order to cause
the most
interaction between exhaled air passing through the central chamber 24 and the
inner surfaces
17
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
44 of the inner wall 22, the inlet 36 is preferably disposed as close to the
piston 26 as
possible; however, it will be clear.that other arrangements of these
components are likewise
possible without departing from the scope of the present invention.
[0055] The breath input assembly 50 includes a mouthpiece 52, a filter 54 and
any
tubing 56 necessary to guide exhaled breath from the mouthpiece 52 to the
inlet 36 of the
syringe 20. The mouthpiece 52 is of suitable size and shape so as to permit
comfortable
contact with the mouth area of a patient. The filter 54, which may comprise a
polymer
material having perforations or successive intrusions therein, may, be
arranged within the
tubing 56 between the mouthpiece 52 and the syringe inlet 36 to prevent saliva
and other
liquid or solid matter of a minimum size from passing therethrough and into
the syringe 20.
Saliva may be further prevented from reaching the central chamber 24 by
arranging the
breath input assembly 50 beneath the syringe 20, so that air passing through
the breath input
assembly 50 moves upward. In this arrangement, the effect of gravity on the
saliva and other
liquid or solid matter helps to prevent such matter from passing up into the
central chamber
24, as it instead tends to collect in the tubing 56.
[0056] The tubing 56 is preferably configured so as to avoid interference
between
the mouthpiece 52, or any other part of the tubing 56, and the operation of
the plunger
assembly 25, as such operation is described herein. More preferably,'the
mouthpiece 52 is
oriented to be generally parallel with the syringe 20 and the plunger assembly
25 therein, or
in other words, the mouthpiece 52 is oriented in parallel to the main axis
defined by the
syringe 20. In this orientation, exhaled breath may be received from a patient
without
causing interference to the operation of the plunger assembly 25, and
condensate formed on
the inside of the syringe 20 as the patient uses the device 10 will tend to
drain downward
toward the outlet 38.
[0057] The dimensions of the device 10 are chosen so that a sufficient volume
of
condensate may be collected in a relatively short period of time using a
device 10 that is
small and light enough to be easily held by a patient or attendant and that
does not require the
patient to change his breathing patterns. The walls 22, 32 and other
structures.of the device
are preferably constructed of a material that tends not to bind to proteins,
such as
platinum-cured silicon. Other suitable materials may include, but are not
limited to, glass,
plastic, polyethylene, polycarbonate, or polyvinyl or other synthetic
polymer., The plunger
18
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
assembly 25 is likewise preferably constructed from a non-protein-binding
material, but may
be constructed from any suitable inert material including, but not limited to,
plastic, vinyl,
polyethylene, rubber, platinum-cured silicon or a fluorine-containing polymer.
In addition,
TEFLON , which is a fluorine-containing polymer and is a registered trademark
owned by
E.I. Du Pont De Nemours and Company Corporation of Wilmington, Delaware, may
be used.
In a preferred embodiment, the syringe 20 is between 10 and 20 cm long with a
diameter of
between 2 and 5 cm. The thickness of the coolant jacket 34 may be between 1
and 10 mm,
and the sample volume, expressed from a single use, is preferably between 100
pL and 1000
p,L, although it may be possible to obtain useful results from samples as
small as 25 L.
[0058] The plunger assembly 25 is modified relative to the plunger assemblies
disclosed in prior applications through the inclusion of the hypodermic needle
35, described
previously, or a similar structure. This modification facilitates the sterile
delivery of
condensate into a specialized reaction chamber device, examples of which are
described
below. The needle 35 is centrally disposed in the central conduit 33 of the
piston tip 27, and
the interior of the needle 35 is arranged in fluid communication with the
radial conduits 31 in
the tip 27 for a purpose made evident hereinbelow.
[0059] In operation, one or more syringes 20 are first stored in a
refrigeration
device, such as a conventional household or commercial freezer, that is
capable of lowering
the temperature to approximately 0 F, thus freezing the jacket of coolant
material 34
contained between the inner and outer walls 22, 32 of the syringe 20. When a
patient is to be
examined, a single syringe 20 is first withdrawn from the freezer. If the
breath input
assembly 50 or mouthpiece 52 is stored separately from the rest of the device
10; then the
device 10 is assembled for use by coupling the various components together.
Next, the
patient positions .the mouthpiece 52 in a sealed relationship to his mouth
area and exhales into
the mouthpiece 52. The exhaled breath is guided through the tubing 56 and into
the central
chamber 24 via the inlet 36. The intake valve 40 is forced open by positive
pressure, but in
the absence of such pressure, it prevents air within the central chamber 24
from escaping
through the inlet 36. The exhaled breath then exits through the outlet 38, on
the end of the
chamber 24 opposite the intake end via the needle 35. The exit v.alve 42
permits air to pass
out of the central chamber 24 only when positive pressure exists on the
cylinder side of the
valve 42, while in.the absence of such pressure, the valve 42 prevents ambient
air from
entering the central chamber 24 via the outlet 38.
19
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
[0060] As the patient exhales through the device 10, the moisture in the
exhaled
breath begins to condense'on the inner surfaces 44 of the central chamber 24.
Because of the
depressed temperature of the coolant 34 and the syringe 20, the condensate may
freeze
immediately. on the inner surface 44. The diameters of the nozzle 39 and the
needle 35 are
small enough to cause resistance to the exhalation of the patient. The
diameters may be
preferably chosen so as to slow the rate of expiration until each exhalation
requires
approximately 5 seconds to complete or until a resistance of up to about 5 cm
of water
pressure is provided. It will be apparent to one of ordinary skill in the art
that the nozzle 39
may alternatively be fitted with a positive end-expiratory pressure valve (a
"PEEP" valve),
which has a dial to vary the resistance to exhalation. PEEP valves are
commercially available
from Life Assist Inc, Rancho Cordova, CA. This modification would increase the
amount of
time for exhaled breath to equilibrate with the inside surfaces 44 of the
central chamber 24.
[0061] As the patient continues to exhale through the device 10, the frozen
coolant 34 disposed in the space between the inner wall 22 and outer wa1132 of
the device 10
begins to melt. The composition, volume and thickness of the coolant jacket 34
surrounding
the central chamber.24 is preferably calibrated such that the coolant 34
begins to melt after
approximately 10-15 exhalations by the patient. Once the coolant 34 melts or
thaws after the
desired number of exhalations, the condensate likewise can begin to melt. Once
the
condensate is melted, the plunger assembly 25 may be depressed to express the
collected
condensate sample through the outlet 38.
[0062] Figs. 3A and 3B are partial side cross-sectional schematic views of the
breath condensate collection device 10 of Fig. 2 with the plunger assembly 25
in a partially
depressed position and a fully depressed position, respectively. As the
plunger assembly 25
is depressed, the needle 35 is forced through the outlet 38. Meanwhile, as the
volume of the
space between the piston 26 and the outlet 38 shrinks, condensate is forced
through the radial
conduits 31 to the central conduit 33 and from there into the hypodermic
needle 35. The
condensate is then ready for ejection into a suitable reaction chamber-
device, several
examples of which are described below with reference to Figs. 7-13. Ejection
may be
facilitated by complete depression of the plunger assembly 25. A clip
assembly, may be
provided at the opposite end of the syringe 20 in order to capture the handle
30 of the plunger
assembly 25, thereby providing an indication to the user that the plunger
assembly 25 has
been fully depressed. Finally, once the EBC has been collected and ejected
via=the needle 35
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
in accordance with the test procedures described hereinbelow, the entire
device 10 may be
disposed of according to conventional waste disposition procedures.
[0063] Fig. 4 is a partial side cross-sectional view of a breath condensate
collection device 60 in accordance with a second preferred embodiment of the
present
invention. Like the first embodiment, this breath condensate collection device
60 includes a
double-walled syringe 62, which includes a central chamber 24, a plunger
assembly 65, a
rubber gasket 28 and a handle 30, and a breath input assembly (not shown). The
body of the
syringe 62, including the central chamber 24 and the various components
thereof, are similar
to those of the first syringe 20 with several significant exceptions. First,
the outlet 38 of the
first embodiment is replaced with an bleed port 68, approximately 1 mm in
diameter,
penetrating the inner and outer walls 22, 32 of the syringe 62 near the distal
end thereof. The
bleed port 68 allows air to escape from the central chamber 24 as the plunger
assembly is
depressed but retains the EBC. Also, the plunger assembly 65 does not include
a nipple-
shaped tip on the face of the piston 66 or a needle extending therefrom.
However, the face .of
the piston 66 does have a slight conical shape, and the end of the central
chamber 24 is
correspondingly-shaped. Together, this configuration may guide collected
condensate to the
outlet 38 more efficiently. It will be apparent, however, that the nipple-
shaped tip 27 of the
previous device 10 and the conical shape disclosed in the device.of Fig. 4 are
not mutually
exclusive, and that the conical shape may easily be incorporated into the
device of Fig. 2, i.e.,
by combining the nipple-shaped tip 27 of Fig. 2 with the conical shape of Fig.
4.
[0064] Another difference between the first and second devices 10, 60 is that
the
second device includes a threaded nozzle 61 in which may be fixed a hypodermic
needle 35.
The needle 35 is similar to the needle 35 of the first device 10, but is -
fixed in the nozzle 61
rather than being mounted on the plunger assembly 65. The interior of the
needle 35 is
communicatively connected to the interior of the central chamber 24. The
needle 35 may be
protected by a removable plastic covering 69 having a threaded fitting that
facilitates easy
connection and removal of the covering 69 from the threaded nozzle 61. When
attached to
the syringe 62, the covering 69 preferably provides a relatively air-tight
seal for a purpose
described below.
[0065] Use of this second device 60 is somewhat similar to that of the first
device
10, although the covering 69 must be removed before the subject may breathe
through the
21
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
needle 35. However, the modifications described above may make this device 60
better.
adapted to deliver a calibrated amount of condensate than the first device 10.
Once the
subject has taken a sufficient number of breaths through the device 60 to
ensure that a
minimum volume of condensate has been collected, the covering 69 may be
replaced on the
end of the syringe 62, and the plunger assembly 65 may be carefully depressed.
Although the
covering 69 effectively prevents gas or liquid from passing through the needle
35, the bleed
port 68, which is of conventional design, permits egress of air and any excess
condensate
until the face of the piston 66 passes the calibration line 78. If desired, a
cotton ball or other
absorbent material, and an appropriate support structure if desired, may be
disposed over the
bleed port 68 in order to prevent EBC from escaping to the environment. By
holding the
device 60 with the bleed port 68 oriented in an upward direction, most or all
air may be
removed from the central chamber 24, and a calibrated volume of condensate is
left therein as
measured by the calibration line 78. As shown in Fig. 4, the syringe 62 may
use a single wall
design in the region of the calibration line 78 in order to make it easier to
see the liquid
contained therein. Preferably, the syringe 62 is calibrated to collect a
volume of between 250
and 500 p,L. The covering 69 may then be removed again and this calibrated
volume may be
delivered by the needle 35 into a suitable reaction chamber device,
[0066] Fig. 5 is a side cross-sectional schematic view of a breath condensate
collection device 80 in accordance with a third preferred embodiment of the
present
invention. It may be advantageous to fractionate exhaled breath into airway,
and alveolar
components, to allow condensation to occur only from the portion of breath
originating from
the alveoli. This partitioning step may help distinguish lower tract lung
infection from
bronchitis. As described in the aforementioned U.S. Patent Application Serial
No. '
10/778,477 to Kline, a device for collection of exhaled alveolar. breath
condensate may
incorporate a gating mechanism actuated, for example, by a rise in the partial
pressure of
exhaled carbon dioxide. The breath condensate collection device 80 illustrated
in Fig. 5 is an
example of a device suitable for use in the preferred embodiments of that
device or in
comparable devices. The device 80 of Fig. 5 is similar to the device 10 of
Fig. 2 except that
the breath input assembly 50 of the first device 10 has been removed to permit
the device 80
to be inserted into a housing such as that included in the device disclosed in
U.S. Patent
Application Serial No. 10/778,477. The direction of airflow during the
condensation process
may also be reversed, as shown in Fig. 5, either to facilitate fluid
communication from the
22
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
gating mechanism or for other purposes, as will be apparent to those of
ordinary skill in the
art. In this case, the respective positions of the outlet 36 and inlet 38, and
their respective
one-vyay valves 81, 83, are now reversed with respect to the device 10 of Fig.
2.
[0067] Although not illustrated herein, airflow reversal may also be applied
to the
device 60 shown in Fig. 4. Even with the airflow direction reversed, however,
the condensate
may still be delivered into a reaction chamber device via the hypodermic
needle 35 as
described with regard to either Figs. 3A and 3B or Fig. 4.
[0068] In the preceding embodiments, the respective devices 10, 60, 80 are
designed for the collection of EBC from spontaneously breathing subjects,
where the subject
exhales via a mouthpiece or facemask (not shown) into tubing that directs flow
into the
appropriate entry port, such as the ones diagrammed in Fig. 2 or Fig. 5. It
would also be
advantageous for the present invention to be adapted to allow collection from
patients being
ventilated mechanically. In most hospitals, humans are ventilated against
pressurized air,
often enriched with oxygen and humidified with excess water vapor. This excess
water vapor
causes condensation to accumulate in the outflow tubing that directs the
exhaled breath away
from the patient's lungs during the respiratory cycle. It is a standard
practice for respiratory
therapists to affix a small cylinder, of approximately 20-100 mL volume, to
collect this
condensate in the most dependent portion of the exhalation circuit. To take
advantage of the
present invention to diagnose Gram negative bacterial lung infection in
a,ventilated patient,
an aliquot of the collected condensate may be analyzed for LPS content. In the
simplest
embodiment, a commercially available syringe of a type used for drug delivery
(e.g., an
insulin syringe) may be used to withdraw a set volume of condensate (e.g., 100-
200
microliters) found in the condensate reservoir in the exhalation line of the
ventilation circuit,
and this volume could be injected into a reaction chamber device, examples of
which are
described hereinbelow.
[0069] Fig. 6 is a schematic illustration of an alternative ventilation system
implementation of the preferred embodiments of the present invention. Using
the device 80
of Fig. 5 as an example, collection from a ventilated patient may be
facilitated by adding a
vacuum system 92 to the outlet 36 of the device 80 and connecting a-Y- or T-
fitting 94 to the
luer lock fitting, described previously, on the end of the nozzle 39. The Y-
or T-fitting 94 is
connected inline in the middle of the outflow path from the patient 96 to a
conventional
23
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
ventilator 98. The outflow path includes two polyethylene tubes 102, 104, the
first of which
is connected from a conventional endotracheal tube 100, or other patient
interface, to the inlet
of the Y- or T-fitting 94, and the second of which is connected between the
outlet of the Y- or
T-fitting 94 and a port such as the one usually provided on the outflow track
of the ventilator
110. A third polyethylene tube 106, is connected from the inflow track of the
ventilator 110
to the endotracheal tube 100. Each polyethylene tube .102, 104, 106 may be of
approximately
10-30 mm internal diameter and 1 meter or more in length. Together, such-an
apparatus
allows the vacuum system 92 to withdraw a continuous sidestream sample of
exhaled breath
through the collection device 80 until a sufficient volume of EBC is
collected. The rate of
aspiration by the pump of the vacuum system 92 may be set at approximately 100
mL/min.
[0070] Figs.. 7 and 8 are a side cross-sectional schematic view and a top
view,
respectively, of one reaction chamber assembly 120 suitable for use with the
collection
devices 10, 60, 80 of Figs. 2, 4 and 5. The reaction chamber assembly 120 is
designed to
detect and quantify the amount of LPS present, in the EBC. The assembly 120
resembles the
general configuration of a sterile ampule that is commonly used to store drugs
for injection.
The assembly 120 may function both as a reaction chamber and a
spectrophotometer cuvette
to measure the percentage of transmission of visible light, which is
proportional to the
concentration of LPS in the sample. The assembly 120 is vacuum-sealed and
includes a
reaction chamber 122 and a plug 124, a retaining ring 126, and a protective
cap 128. The
reaction chamber 122 is preferably made of glass or another transparent
material, but may
alternatively be made of a semi-transparent (translucent or other non-opaque)
material if the
chosen color or light change or phenomenon, described below,, may be :,
readily seen
therethrough. The reaction chamber 122 preferably has a volume of
approximately 0.5-1.0
mL. Although as shown the reaction chamber 122 is round, it will be apparent
that the
reaction chamber 122 can likewise be square or have another geometric shape to
facilitate its
insertion into a spectrophotometer, or can be altered to allow "retrofitting"
into existing
commercial laboratory assay systems. The reaction chamber 122 further includes
a threaded
male luer lock fitting 132 of similar size to that of the condensate
collection devices 10, 80
described above. Of course, if a device 60 such as that disclosed in Fig. 4 is
utilized, then the
luer lock fitting 132 may not be necessary.
[0071] The plug 124, which is preferably formed from rubber, may be disposed
in
the top of the chamber 122 to maintain a sealed, sterile, pyrogen-free
environment inside the
24
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
chamber 122. The plug 124 is retained in the chamber 122 by the retaining ring
126, which is
preferably formed from aluminum and has an opening in the center to permit
delivery of
exhaled breath condensate into the reaction chamber 122 via a delivery port
130 in the plug
124. In a preferred ernbodiment, .the delivery port 130 is a re-sealable cover
that may be
punctured with a hypodermic needle. The protective cap =128, which is
preferably formed
from plastic, is temporarily attached over the top of the assembly 120 to
protect the retaining
ring 126 and plug 124 from damage or soiling.
[0072] In use, a sterile reaction chamber assembly 120 is retrieved from
storage,
and an EBC sample is collected using one of the disclosed devices 10, 60, 80
(or another
equivalent device). The protective cap 128 of the reaction chamber assembly
120 is removed
and a relatively.precise volume of the EBC sample is then delivered to the
reaction .chamber
122 by inserting the respective hypodermic needle 35 in the delivery port 130
of the plug
124. If provided, the respective luer lock fittings may be engaged,
effectively locking the
device 10, 60, 80 to the reaction chamber assembly 120. The plunger assembly
25 may then
be depressed until a sufficient volume of EBC is delivered to the assembly
120. A calibration
line 134 may be marked on the reaction chamber 122 to indicate the riecessary
volume, but if
the volume has been pre-calibrated in the collection device 60, then such a
line 134'may not
be necessary. During assembly, the reaction chamber 122 may sealed under a
vacuum to
withdraw a sufficient volume of EBC as needed for accurate measurement of
endotoxin
analyte.
[0073] The chamber 122 contains a prespecified dry mass 136 of factor C,
factor
B; proclotting enzyme and chromogenic substrate, together with salts of
calcium and
magnesium, and phosphate salts, or other organic compound, such as TRIS.
aminomethane
buffer to maintain a pH of approximately 7.4 upon hydration. The amounts and
activities of
these enzymes are precalibrated to allow detection of a clinically relevant
range of LPS in the
specified volume of EBC. The amounts will be calibrated to allow the reaction
to produce a
linear optical density reading that ranges from 0 to 1.00 when measured at 15-
30. minutes
reaction time at approximately 37 C, corresponding proportionately to a
concentration of
LPS ranging from 0 to approximately 10 EU/mL in undiluted EBC.. It is
preferred that the
temperature of the reaction be held at least about 34 C. It is more preferred
that the
temperature of the reaction be held at least about 37 C.
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
[0074] The chromogenic substrate can tolerate temperatures up to about 43 C
without loss of activity. As such, the reaction chamber may be heated by a
controlled
exothermic reaction up to about 43 C. It is preferred that the exothermic
reaction be
controlled in order to produce even incubation at 34-43 C for at least 15
minutes, and
preferably 25 minutes. Accordingly, and as described hereinbelow, steps should
be taken to
control the rate of the exotherniic hydration reaction.
[0075] Such an exothermic reaction may be initiated by 'placing a salt that
liberates heat upon hydration within the reaction chamber. An example of such
a salt
includes, but is not limited to, sodium thiosulfate pentahydrate. Placing the
salt within the
reaction chamber allows introduction of the sampled condensate to initiate the
exothermic
reaction. It is preferred to use a powdered salt, which would facilitate
immediate and
complete hydration within a short heating period. For example, the salt may be
in a semi-
permiable matrix or a crystalline form that allows controlled.hydration to
produce a reaction
temperature of 34-43 C for at least 15 minutes. Alternatively, the salt may be
disposed in a
user-initiated heating jacket that surrounds the reaction chamber. In this
embodiment, the
reaction chamber may be inserted into a double-walled jacket consisting of a
flexible
polymer. The jacket may contain dry salt capable of liberating heat upon
hydration and an
ampule of water (2-5 mL) that can easily be ruptured by squeezing with two
fingers. The
action of rupturing the ampule of water initiates hydration of the salt,
causing an exothermic
reaction, thus heating the reaction chamber to about 43 C for approximately
15-30 minutes,
at which point, the color intensity and corresponding endotoxin activity can
be quantified as
further described herein below.
[0076] Preliminary data suggest that concentrations of LPS exceeding
approximately 0.20 EU/mL (a calibrated linear optical density reading of
approximately 0.20)
will correspond to the clinical test positive threshold, indicating the
presence of Gram
negative.bacterial infection in the lungs. In the preferred embodiment, the
chromogenic
substrate may be a peptide conjugated to a dye such as para aminoanilde that
is cleaved by
the clotting enzyme to liberate a yellow color to the reaction solution with
peak-absorbance at
about 405 nm. It should be readily understood that other chromogenic
substrates could be
substituted, and other dye markers could be conjugated to the substrate which
can be detected
at different isobestic points. Additionally, the marker molecule may be a
compound that
contains the property of excitation fluorescence, whereby the molecule
liberates light-within a
26
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
narrow wavelength interval in response to stimulation by an incident beam of
monochromatic
light. One of ordinary skill in the art will understand that various
chromogenic and
fluorogenic substrates may be used as markers for detecting the presence of
the LPS.
[0077] Alternatively, a reaction chamber, .assembly may be designed to capture
LPS using antibodies -matrix-bound on the sides of the well. Fig. 9 is a
perspective view of a
first alternative reaction chamber assembly 140. In this alternative
embodiment, the
assembly 140 would function as a vertical microtiter well employing enzyme-
linked
immuhoassay technology and would have the advantage of allowing detection of
species-
specific LPS molecules 154. The assembly 140, which may function as a cuvette,
may
include a chamber 142, a plug 144 and a retaining ring or other structure 146.
Windows 143
are formed in at least a portion of the walls of the chamber 142 for a purpose
made evident
hereinbelow. Like the ring 126 of the previous device 120, the ring 146
includes an-opening
that exposes an injection target area 150 in the center of the plug 144.
[0078] Figs. l0A-lOD are partially schematic, fragmentary side cross-sectional
views of the first alternative reaction chamber assembly 140 of Fig. 9. The Fc
portion of
each antibody 152 is conjugated or bound to the windows 143 of the assembly
140 using
conventional techniques. The Fab portion of each antibody 152 is thus oriented
toward the
center of the assembly 140 to better capture the O-polysaccharide portion of
the LPS 154.
After introduction of the EBC sample, the chamber 142 may then be washed with
an
albumin-containing buffer (not shown) to remove nonspecific materials. Then, a
separate
ampule, containing a prespecified amount of chromogenic substrates 156, such
as those
described previously, is reconstituted by adding LPS-free water (not shown),
and the
chromogenic reagent sample is withdrawn by syringe and injected into the
reaction chamber
142, as shown in Fig. lOC. The chromogenic substrates 156 react with the LPS
154, thereby
liberating a dye 158. After approximately 15-30 minutes, optical density may
be read by one
of several methods. One chromogenic substrate 156 suitable for use in the
preferred
embodiments of the present invention incorporates para nitroanilide, which
when freed can
be detected at about 405-410 nm.
[0079] It will be apparent to those of ordinary skill in the art that rather.
than an
antibody with specific immunogenicity toward 0-antigen, other molecules with
specific and
high affinity for any portion of LPS may be bound to the walls of the reaction
chamber for
27
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
capture of LPS. These molecules include, but are not limited to, HA-lA and E5,
lipopolysaccharide binding protein ("LPB"), bacteriocidal/permeability
increasing protein
("BPI") ; limulus anti-lipopolysaccharide factor ("LALF") or the polymyxin
antibiotics.
[0080] In order to quantify LPS concentration, a number of embodiments may be
utilized. In one embodiment; a semi-quantitative determination may be made
using a visual
comparison to a color strip. The color strip may resemble a standard ruler
having patches of
one color displayed left to right in incrementally increasing degrees of shade
intensity, with
the lightest shade being located furthest left and the darkest shade being
located furthest right.
Each shade patch is associated with a specific range of endotoxin unit (EU)
reactivity with
increasing degrees of shade intensity signifying increasing EU reactivity, An
observer may
hold the clear reaction chamber assembly 120, 140 in ambient light next to the
plastic or
paper color strip for comparing the shade of the contents of the reaction
chamber assembly
120, 140 with the color strip patches. The observer may then select the color
patch that best
matches the color shade intensity observed in the reaction chamber. A positive
reaction
would be indicated by a specific color shade threshold, corresponding to
approximately 0.20
EU/mL or as determined from additional experiments.
[0081] More precise quantification may be obtained with a small, specially
designed single beam light spectrophotometer that is configured to accept the
reaction
chamber after incubation as described above. The spectrophotometer may contain
a heating
coil that is activated upon insertion of the reaction chamber to warm the
sample to 37 F. The
spectrophotometer may alternatively display or print the measure optical
density after 25
minutes of incubation.
[0082] Fig. 11 is a perspective view of a test kit 200 incorporating a second
alternative reaction chamber device, which would allow EBC to be delivered to
a single-use
cartridge that could be read visually without the use of a spectrophotometer.
The test kit 200
has two modules 203, 223, including a test module 203 and a positive control
module 223,
arranged in a housing 202. Fig. 12 is a side cross-sectional view of the test
module 203 of
Fig. 11, taken along line 12-12. The test module 203 includes a port 204. Port
204
preferably has a volume of approximately 100 microliters and contains dry mass
136. of.factor
C, factor B, proclotting enzyrne and chromogenic substrate, together with
EDTA, salts of
calcium and magnesium, and phosphate salts, or other organic compound, which
allows the
28
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
dry mass to maintain a pH of approximately 7.4 upon hydration and to forrri an
aqueous
reagent.
[0083] Fig 11 further shows a reaction chamber 208, a test matrix 212, a pair
of
microtubules 214, 216, a pair of one-way valves 218, 220 and an outlet 222.
Reaction
chamber 208 preferably has 'a volume of at least about 250 microliters. The
port 204 and
reaction chamber 208 are connected by the first microtubule 214, while the
reaction chamber
208 is connected to the outlet 222 by the second microtubule'216. One of
ordinary skill in
. the art will understand that microtubule 214 may include one or more
microtubules. Valves
218, 220 are disposed in the first and second microtubules 214, 216,
respectively, so as.to
facilitate one-way fluid communication from the port 204 through the reaction
chamber 208
to the outlet 222. If microtubule 214 includes a plurality of microtubules,
more than one
valve 218, 220 may be necessary to accommodate the plurality of microtubules.
The top of
the cartridge has a preprinted color strip 221 with a series of successively
darker color
patches disposed thereon and arranged such that each successively darker color
patch
corresponds to an incrementally greater interval of endotoxin activity.
[0084] The chamber 208 is covered with a clear material 210 to allow viewing
of
the matrix 212 for color change indicating the presence of LPS. The matrix
212, which is
arranged in the chamber 208, is a surface formed from an insoluble polymer or
other material
that will accept and be stained by the chromogenic substrate. Suitable
materials include, but
are not limited to, polymers derived from cellulose, agarose, methacrylate,
polystyrene,
polyphenyl, polynaphthyl, polybenzyl, nylon, silk or other fabric and the
like, The matrix
212 is absorbent but tightly woven such that it permits fluid to remain
suspended in the
chamber 208 until the fluid moves by gravity and capillary action through
microtubule 216.
As an advantage, the matrix 212 may intrinsically bind LPS itself or may
covalently or
otherwise bind one of the LPS-binding molecules, such as HA-1A and E5, LPB,
BPI, LALF
or the polymyxin antibiotics. The matrix 212, therefore, develops' a color,
intensity in
proportion to the concentration of eridotoxin in the unknown sample. Visual
comparison to
the pre-printed color-strip 221 allows a semi-quantitative determination of
endotoxin
concentration, as described above.
[0085] The interior of the test kit 200 may also contain sodium thiosulfate
pentahydrate and a sealed ampule of water that ruptures upon squeezing of the
test kit 200.
29
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
The action of rupturing the ampule of water will initiate hydration of the
sodium thiosulfate,
causing an exothermic reaction, thus heating the entire test kit 200 to about
43 C for =
approximately 25 minutes. Although the materials for the exothermic reaction
are disposed
within the test kit 200, they are separated from all areas of the test kit 200
in which the
sample is introduced and in which any LPS-related chemical reactions occur.
[0086] In operation, the reaction is initiated by injecting condensate
collected with
device 10, 60, 80 into the chamber 204 that contains the dry mass 136
described previously.
It is preferred that at least about 0.35 mL of condensate be injected into the
reaction chamber
204. The action of injecting the condensate causes a mixing action of the
condensate and dry
mass 136 in the reaction chamber 204 and the microtubule 214, thereby
initiating the LAL
reaction. The pressure differential induced by injection, coupled with the
force of capillary
action causes the reaction mixture to flow down microtubule 214 into the
reaction chamber
208, where it remains while the chromogenic reaction continues at a
temperature of 34-43 C.
Since endotoxin will be bound to the matrix 212, the colored molecule that.is
hydrolyzed
from the chromogenic substrate will stain the matrix 212 in a color hue
intensity that is
proportional to the activity of endotoxin in the condensate, the color change
can be visually
compared with the color strip 221 for quantitation.
[0087] Fig. 13 is a side cross-sectional view of the positive control module
223 of
Fig. 11, taken along line 13-13. The positive control module 223 includes a
port 224; a
reaction chamber 228; a test matrix 232; a quantity of dry LPS combined with
dry mass 136,
represented herein as 244; a pair of microtubules 234, 236; a pair of one-way
valves 238, 240
and an outlet 242. The port 224 and reaction chamber 228 are connected by the
first
microtubule 234, and the reaction chamber 228 is connected to the outlet 242
by the second
microtubule 236. The valves 238; 240 are disposed in the first and second
niicrotubules 234,
236, respectively, so as to permit one-way fluid communication from the port
224 through the
reaction chamber 228 to the outlet 242. As illustrated, the LPS 244 is
disposed ian the port
224, but it will be apparent that the LPS 244 may alternatively or
additionally be disposed in
the first microtubule 234 or on the test matrix 232.
[0088] The second alternative reaction chamber device may also be used as
follows. An EBC sample is injected from one of the previously-described breath
condensate
collection devicees 10, 60, 80 into the test module port 204, from where it
may be directed
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
via the first test module microtubule 214 to the test module reaction chamber
208. In a
preferred embodiment, the volume of the EBC sample is about 0.35 mL. The
volume of the
sample is preferably calibrated such that the well 208 containing the matrix
212 fills to
capacity, thus covering the matrix 212 with liquid and initiating LPS binding
to the matrix
212. Excess EBC may flow out through the second test module microtubule 216.
The one-
way valve 218 prevents entry of LPS from ambient air. Absorbent material may
be placed in
216 to prevent any external fluid leak.
[0089] After a relatively precise predetermined period of time, which may be
about 60 seconds, the test module 203 and the positive control module 223 may
each be
washed with a quantity of sterile (endotoxin-free) water. In a preferred
embodiment, the
volume of sterile water that is injected into each module 203, 223 is about
0,25 mL. The
water passes through the respective reaction chambers 208, 228 and is allowed
to drain
through the respective outlets 222, 242 via the respective second microtubule
216, 236.
[0090] Next, LPS-free water is added to an ampule, such as the reaction
chamber
device 120 of Figs. 7 and 8, containing a.dry mass 136 of factor C, factor B,
proclotting
enzyme and chromogenic substrate, together with EDTA, salts of calcium and
magnesium,
and phosphate salts, or other organic compound to maintain a pH of
approximately 7.4 upon
hydration to form an aqueous reagent. Once the reagent is reconstituted, it
may be withdrawn
using a syringe such as that commonly used to deliver insulin or to provide
tuberculosis.
testing antigen (often referred to as a "TB syringe") (not shown) and
injected: into both the
test module port 204 and the positive control module port 224. The test kit
200 may then be
left to develop for a prespecified time interval, e.g., 10 minutes at 23 C,
and. then visually
inspected for color or pattem change in the test well 208. The positive
control module 223 is
included to ensure activity of the chromogenic enzyme system.
[0091] In a.still further alternative; the proportion of LP'S in EBC could be
measured by allowing gel formation of the clotting cascade within a narrow
tube, as
described in Neeman et al., U.S. Patent No. 4,370,413, (the '413 patent), the
entirety of which
is incorporated herein by reference. Fig. 14 is a partial side cross-sectional
view of a breath
condensate collection device 260 in accordance with a fourth preferred
embodiment of the
present invention. This device 260 is similar to that of Fig. 4, in that it
includes a syringe 262
having double walls 22, 32, a central chamber 24, a plunger assembly 65 with.
a piston 26, a
31
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
rubber gasket 28 and a handle 30, a breath input assembly (not shown), an
outlet 68, a
threaded nozzle 61, and a removable plastic covering 69. Each of these
components is
generally similar to those of the device 60 of Fig. 4, except that the syringe
262 further
includes a manometer 265 extending from the side of the syringe in fluid
communication
with the- outlet 68. In addition, although each device 60, 260 includes a
needle 35, 275, the
needle 275 of this fourth embodiment serves as the narrow tube in which EBC is
contacted
with the LAL assay reagent. Accordingly, the needle 275 is 'plugged with a
dry, insoluble
and inert fiber matrix 270, as shown in Fig. 15, which is preloaded with the
LAL assay.
reagent for gelation if LPS is present in the EBC.
[0092] A reaction may be initiated by depressing the plunger assembly 65 until
the plug 270 is wetted with EBC. The amount of gelation in the outflow needle
275 will be
proportional to the amount of LPS in the EBC. After a specified period of
time, the. plunger
assembly 65 may be forcibly depressed again, and the extrusion pressure
measured within the
central chamber 24 using the manometer 265. The extrusion pressure is
proportional to the
LPS concentration in the EBC.
[0093] In all of the preceding embodiments, it should be recognized that the
specificity of the reaction can be enhanced by incltision of chemicals or
methods to prevent
reaction with beta glucans (factor G), including heating the sample to 75 C,:
adding
alkylglucosides, or molecules with beta 1,4 glucosidic linkages.
[0094] Once a Gram negative bacterial infection is diagnosed, antibiotic
therapy
may begin. Those skilled in the art will understand that the method of the
present invention
may be utilized to monitor the effectiveness of nntibiotic therapy. It can be
predicted by
those skilled in the art, that effective antibiotic therapy may cause an
initial increase in LPS
concentration in expired breath condensate, owing to bacterial demise with
cell inembrane
rupture and increased release of free LPS into alveolar epithelial fluid.
This, concentration
increase would be expected in the first 24 to 72 hours after antibiotic
administration. It could
further be predicted that effective antibiotic therapy would eventually cause
a decrease in the
,concentration of LPS in expired breath condensate over the subsequent 3-10
days after
antibiotic administration. Accordingly, it can be surmised that serial LPS
measurements may
be used to monitor treatment efficacy. A failure to exhibit an
initial'increase in expired LPS
32
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
content or a failure to exhibit a decline in expired LPS content in the
subsequent days could
signal resistance of the Gram negative bacteria to treatment.
[0095] The present invention is advantageous because it provides a method and
device for diagnosing a Gram negati ve bacterial infection in a relatively
easy, painless and
quick manner. The present application is directed to diagnosing Gram negative
bacterial
pneumonia by measuriing the concentration of LPS in exhaled breath condensate.
However,
the present invention may also be utilized to diagnose any intrapulmonary Gram
negative
bacterial infection, including, but not limited to, septicemia. One of
ordinary skill in the art
will understand that Gram negative bacterial infections may be treated with
the same
antibiotic treatment and thus diagnoses of any Gram negative bacterial
infection is beneficial
for a subject suffering from such infection. Further, the present invention
may also be
utilized to identify a specific strain of Gram negative bacteria causing
infection, in a situation
in which this information may be beneficial.
[0096] Examples
[0097] Example 1
[0096] LPS was detected in exhaled breath condensate samples from subjects
that
were awake, cooperative and able to breathe spontaneously in order to diagnose
whether such
subjects had Gram negative bacterial pneumonia according to the following
procedure.
[0099] The subjects for the procedure were selected according to the following
procedure. Subjects (N=8 per group) were recruited based upon three criteria:
1) diagnosis of
pneumonia, 2) healthy patients who actively smoked more than 10 cigarettes per
day and 3)
healthy nonsmokers. To obtain subjects diagnosed with pneumonia, subjects
diagnosed on
standard clinical grounds, including cough productive of colored sputum,
measured. fever >
101 F, a leukocytosis, evidenced by a peripheral total white blood,count of
>12,000 cells per
cubic microliter, and the presence of an infiltrate on chest radiograph were
selected.
Exclusion criteria for subjects included any, use of antimicrobial
medications, acute illness or
anatomical abnormality that precluded breath collection and/or suspected
pulmonary
tuberculosis.
[00100] The breath condensing device utilized for collected exhaled breath
samples
is described as follows. The device consisted of a one-meter long polyvinyl
tube (15 mm ID)
33
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
attached to a modified glass flask, which was approximately 50 cm in height.
The glass flask
had a specially designed, removable glass stopper at the top but was otherwise
similar in
appearance to a staridard laboratory volumetric (Florence) flask. The glass
stopper had two
ports, one for entry of a glass tube that projected upward and was bent at a
90 angle and
another to allow dried, exhaled air to exit into ambient air. One end of.the
glass tube was
connected to a polyvinyl tube that fit snugly over the glass tube. The other
end, of the glass
tube projected into the interior of the glass bulb of the flask by
approximately 25 cm. The
entire glass flask was submerged in a cooling slurry of frozen carbon dioxide
in ethanol.
[00101] Exhaled breath condensate was collected according to the following
procedure. A subject was allowed to hold the polyvinyl tube and breath into a
duckbill-
shaped mouthpiece that was attached to the opposite end of the polyvinyl
.tube. The subject's
breath passed through the polyvinyl tube into the condenser chamber of the
flask, where the
cooled inner side of the flask would condense and freeze.the expired water
vapor and
aerosolized droplets in the subject's breath. Subjects were asked to deliver
approximately 30
deep exhalations, which yielded approximately 1-3 mL of sample.
[00102] Prior to collection of breath samples from subjects, multiple steps
were
take to reduce sample contamination with extraneous endotoxin or beta glucan
molecules. All
components were sterilized by autoclaving at 220 C for= a minimum of 4 hours
between uses
and by using a commercially available washing solution designed to remove
endotoxin. The
polyvinyl tube was wrapped in sterile foil prior to autoclaving. The sterile
foil was left in
place during sample collection but was removed prior to removing the polyvinyl
tube. The
technician used sterile gloves to handle all 'components. However, the
terminal 6 cm of the
polyvinyl tube was able to be grasped by the subject, who did not wear sterile
gloves. After
the condensate was allowed to melt, it was. aspirated from the condenser
chamber with
pyrogen-free pipette tips and transferred to pyrogen-free cryotubes under a
laminar hood. To
examine for background endotoxin,' a "mock" standard of the glassware and
tubing was
conducted according to the following procedure. Two milliliters of endotoxin-
free water
(Sigma Chemical, St. . Louis, MO) was instilled into the washed, autoclaved
c.ondenser
chamber, agitated and then aspirated and stored in. the same manner as the
condensate
samples. Condensate saniples and the tivater run for a mock standard were
stored at -57 C
until assay.
34
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
[00103] The presence of endotoxin was detected according to the following
procedure. An assay was performed on undiluted, thawed condensate using a
chromogenic
limulus assay according to manufacturer's specifications (Pyrochrome Assay,
Associates of
Cape Cod, Falmouth, MA, USA). Reactions were carried out on 96-well microtitre
plates
(Pyrochrome microtitre plates, Associates of Cape Cod) at 37 C for 30 nvn, and
the color
change read with a microtitre plate spectrophotometer at 405 nm. All samples
were run
during one batch. The standard curve was performed using the assay endotoxin
standard and
was linear with R=0.997.
[00104] Fig. 16 is a scatter plot graph illustrating the measured endotoxin
concentrations for patients in each of three study groups. The mean (::LSD)
endotoxin
concentration in normals, smokers, and patients with pneumonia was 0.096-
0.050,
0.091:L0.040, and 0.234 0.130 EU/mL, respectively. The plot suggests that 0.20
EU/mL may
be a useful cutoff point to distinguish subjects with Gram negative pneumonia
from subjects
without Gram negative pneumonia.
[00105] Two subjects with pneumonia and test values below 0.20 EU/mL are
labeled "A" and "B". Traditional clinical tests confirmed that these subjects
did not have
Gram negative bacterial pneumonia. Patient A had blood cultures that were
positive for
streptococcus pneumonia, which was clinically presumed to be the bacterial
cause of the
patient's pneumonia; patient B had clinically suspected influenzal (viral)
pneumonia with
negative blood cultures and no other specimens submitted for analysis.
[00106] Two of the water samples from the mock standard had values of 0.10 and
0.14 EU/mL (mean 0.12 EU/mL). Accordingly, 0.12 EU/mL was considered the
background
"noise" of the system, and this value was subtracted from the cutoff value of
0.20 EU/mL
determined in uncorrected samples. Using this analysis, a sample of expired
breath
condensate containing a value above 0.08 EU/mL would be considered an abnormal
test
result. It should be.recognized that this background correction value may
differ if a different
coridenser apparatus is used in another laboratory.
[00107] Example 2
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
[00108] LPS was detected in exhaled breath condensate samples from subjects
that
were breathing with the assistance of a ventilator in order to diagnose
whether such subjects
had Gram negative bacterial pneumonia according to the following procedure.
[00109] Six subjects participated in the study. Four of the ventilated
subjects
presented clinical evidence of pneumonia and were being treated with
antibiotic therapy, and
two of the ventilated subjects presented no clinical evidence of pneumonia and
were used as
controls.
[00110] Breath condensate samples were obtained according to the following
procedure. The analyte was obtained from the exhaled breath condensate that
accumulated in
outflow tubing attached to the endotracheal tube of the ventilation system.
Analyte appeared
clear and non-turbid upon visual inspection.
[00111] The presence of endotoxin was detected according to the following
procedure. An assay was performed on undiluted and diluted condensate using a
chromogenic limulus assay according to manufacturer's specifications
(Pyrochrome Assay,
Associates of Cape Cod, Falmouth, MA, USA). The dilutions included 1:10, 1:100
and
1:1000 dilutions in endotoxin-free water. Reactions were carried out on 96-
well microtitre
plates (Pyrochrome microtitre plates, Associates of Cape Cod) at 37 C for 30
minutes, and
the color change read with a microtitre plate spectrophotometer at 405 nm..
All samples were
run during one batch. The upper limit of detection of the assay was 0,25
EU/mL.
[00112] The results of the assay are as follows. All six samples were above
limits
of detection in the undiluted samples (i.e., greater than 0.25 EU/mL). The
mean endotoxin
concentration in all the 1:10 dilution samples of subjects with pneumonia was
above the limit
of linear detection, but the mean endotoxin concentration from the two
ventilated patients
without pneumonia in the 1:10 dilutions was < 0.05 EU/mL. These data suggest
that the
presence of more than 0.25 EU/mL in samples of condensate in the outflow
tubing of a
ventilator circuit that appear clear and non-turbid upon visual inspection
would predict the
presence of a bronchoalveolar lavage sample that would be positive for gram
inegative
pneumonia.
[00113] Example 3
36
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
[00114] LPS was detected in exhaled. breath condensate according to the
following
procedure. A conunercially available 1-liter volume glass flask was prepared
for collecting
breath condensate samples according to the following procedure. The glass was
heated to
400 C for 3 hours to render its surfaces LPS-free. A sterilized, flexible
polyvinyl tube, 11
mm in internal diameter, was arranged in fluid communication to a side-arm of
the flask such that when a patient breathed into the tube, the patient's
breath passed through the glass flask
and out through an exit port. The flask was partially submerged in a dry ice
and ethanol
slurry mixture as a coolant to facilitate capture of exhaled breath condensate
in the flask. The
tube and flask were arranged in a fashion to keep the condensing flask above
the 'level of the
patient to prevent any capture of aerosolized saliva.
[00115] Exhaled breath was collected according to the following procedure.
Eight
subjects of varying health status breathed into the tube connected to the
flask. Each subject
breathed 100 deep exhalations into the flask, using a hand counter to keep
track of breaths.
This method provided a mean volume of condensate equal to 11 + 4 mL.
[00116] A chromogenic commercial LAL assay (Cape Cod, MA) was used to
detect LPS in the exhaled breath condensate according to the following
procedure. From
each subject's condensate sample, duplicate 50 L aliquots were transferred
into two wells of
a standard 96-well ELISA plate. The reagents required for the chromogenic LAL
assay were
added and the samples were incubated for 30 minutes at 37 C. The optical
density was read
at 405 nm in a commercially available plate well reader., Concentrations were
determined by
comparison to a standard curve using known amounts of LPS obtained from a FDA-
certified
source.
[00117] Results were obtained as follows. Four normal volunteers were all
found
to have concentrations of LPS below 100 pg/mL, whereas two otherwise healthy
smokers
were found to have concentrations below 200'pg/mL. Two smokers with clinical
pneumonia
were found.to have.EBC LPS concentrations above '800 pg/mL. From this
experiment, it was
concluded that LPS content of EBC samples can vary based upon the health of
the lung in the
subjects providing the samples. These data also suggest that EBC can be
sampled without
contamination from oral flora in healthy subjects.
[00118] Based on the foregoing information, it is readily understood by those
persons skilled in the art that the present invention is susceptible of broad
utility and
37
CA 02566804 2006-11-14
WO 2006/007180 PCT/US2005/018232
application. Many embodiments and adaptations of the present invention other
than those
specifically described herein, as well as many variations, modifications; and
equivalent
arrangements, will be apparent from or reasonably suggested by the present
invention and the
foregoing descriptions thereof, without departing from the substance or scope
of the present
invention. Accordingly, while the present invention has been described herein
in detail in
relation to its preferred embodiment, it is to be understood that this
disclosure is only
illustrative and exemplary of the present invention and is made merely for the
purpose of
providing a full and enabling disclosure of the invention. The foregoing
disclosure is not
intended to be construed to limit the present invention or otherwise exclude
any such other
embodiments, adaptations, variations, modifications or equivalent
arrangements; the present
invention being limited only by the. claims appended hereto and the
equivalents thereof.
Although specific terms are employed herein, they are used in a generic and
descriptive sense
only and not for the purpose of limitation.
38