Note: Descriptions are shown in the official language in which they were submitted.
CA 02567171 2009-09-08
TREATMENT OF BRINES FOR DEEP WELL INJECTION
RELATED PATENT APPLICATION
[0001] This application is related in part to Canadian Application
2,307,819 filed
May 8, 2000.
COPYRIGHT RIGHTS IN THE DRAWINGS
[0002] A portion of the disclosure of this patent document contains
material that
is subject to copyright protection. The applicant no objection to the
facsimile reproduction by
anyone of the patent document or the patent disclosure, as it appears in the
Patent and
Trademark Office patent file or records, but otherwise reserves all copyright
rights whatsoever.
TECHNICAL FIELD
[0003] This invention is related to the treatment of wastewater
brines prior to
disposal by underground injection, particularly where the wastewater brines
result from the
treatment of water for steam generation in operations which utilize steam to
recover oil from
geological formations.
1
CA 02567171 2006-11-03
BACKGROUND
[0004] Steam generation is necessary or desirable in many heavy oil
recovery operations, including, for example, the recovery of tar sands from
deposits in Northern Alberta, Canada, or elsewhere around the world. This is
because in order to recover heavy oil from certain geologic formations,
heating is
required to increase the mobility of the oil to be recovered from the geologic
formation. In order to produce steam for downhole use, water treatment plants
are necessary to produce high quality water meeting the applicable
specifications
for a selected high pressure steam generator system. In most cases, the
primary
source of water to be treated in order to manufacture the required steam in
the
selected high pressure steam generator is de-oiled produced water, i.e. the
water
which is brought up along with the oil by production wells when oil is removed
from the geologic formation. In such instances, oil must be separated from the
produced water in order to provide a de-oiled produced water suitable for
further
treatment, prior to steam generation.
[0005] Various processes have been heretofore utilized or proposed for
treatment of de-oiled produced waters. In those situations where the de-oiled
produced waters contain relatively high levels of silica, the wastewater
brines
produced by the required water treatment plant inevitably contain high levels
of
silica. Silica is relatively soluble at high pH, however, high pH waters may,
in
some locales, be unsuitable for disposal by underground injection. The
manufacture of wastewater brines for underground injection at neutral or near
neutral pH would be desirable in order to eliminate the necessity to
neutralize
high pH wastewater brines, as well as the necessity to reduce or effectively
eliminate from such wastewater brines the presence of silica above solubility
limits before underground injection.
[0006] Thus, it can be appreciated that it would be advantageous to
provide a produced water treatment process which minimizes the production of
high pH wastewater brine streams, and that produces a neutral or near neutral
pH wastewater brine suitable for underground injection.
2
CA 02567171 2006-11-03
BRIEF DESCRIPTION OF THE DRAWING
[0007] In order to enable the reader to attain a more complete
appreciation of the novel water treatment process disclosed and claimed
herein,
and the various embodiments thereof, and of the novel features and the
advantages thereof over prior art processes, attention is directed to the
following
detailed description when considered in connection with the accompanying
figures of the drawing, wherein:
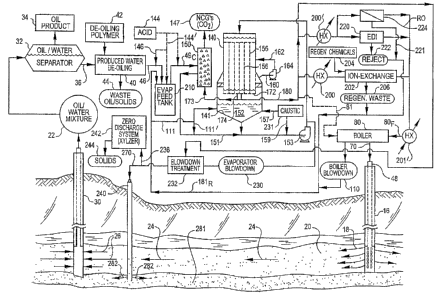
[0008] FIG. 1 illustrates one embodiment of an evaporation based water
treatment process, illustrating the use of a seeded slurry crystallizing
evaporator
based process in combination with the use of packaged boilers for steam
production, as applied to heavy oil recovery operations, and where one or more
blowdown treatment processes as disclosed and claimed herein are utilized
prior
to injection of waste brine into a geological formation.
[0009] FIG. 2 illustrates another embodiment for an evaporation based
water treatment process for heavy oil production, illustrating the use of a
seeded
slurry evaporation process in combination with the use of once-through steam
generators for steam production, as applied to heavy oil recovery operations,
wherein evaporator distillate is fed to once-through steam generators, and
where
one or more blowdown treatment processes as disclosed and claimed herein are
utilized prior to injection of waste brine into a geological formation.
[0010] FIG. 3 provides a conceptual process flow diagram for the use of
an evaporator to process de-oiled produced water, to produce a pure distillate
for
reuse, and to produce a brine that can be further treated for zero discharge
by
use of a crystallizer or for deep well injection by a brine treatment process
as
disclosed herein.
[0011] FIG. 4 shows further details of an evaporator system set up to
process de-oiled produced water and to produce an evaporator waste brine
blowdown.
[0012] FIG. 5 provides solubility characteristics of silica in water, as a
function of pH at 25 C when silica species are in equilibrium with amorphous
3
CA 02567171 2006-11-03
silica, as well as the nature of such soluble silica species (molecule or ion)
at
various concentration and pH ranges, and is provided to remind those of skill
in
the art of the need to control silica content in waste brines that are
discharged at
neutral or near neutral pH, since at such conditions silica solubility is
quite limited
in aqueous solution.
[0013] FIG. 6 illustrates certain details of a crystallizing evaporation
system that utilizes co-precipitation of calcium sulfate and silica to
minimize
and/or prevent silica scaling in the crystallizing evaporator,
[0014] FIG. 7 illustrates one embodiment for a wastewater blowdown brine
treatment system, wherein a wastewater blowdown brine comprises a seeded
slurry, and wherein the solids in the seeded slurry wastewater blowdown brine
are effectively removed by a centrifuge system, and wherein the resultant
clear
centrate is diluted with a suitable diluent such as evaporator distillate or
other
solvent such as service water (where suitable) to effectively eliminate the
tendency of any remaining scaling constituents in the resultant clear brine to
form
scale when injected into a selected geological formation.
[0015] FIG. 8 illustrates one embodiment for a wastewater blowdown brine
treatment system, wherein a wastewater blowdown brine comprises a seeded
slurry, and wherein the solids in the seeded slurry wastewater blowdown brine
are effectively removed by a filter press system, and wherein the resultant
clear
filtrate is diluted with a suitable diluent such as evaporator distillate or
other
solvent such as service water (where suitable) to effectively eliminate the
tendency of any remaining scaling constituents in the resultant clear brine to
form
scale when injected into a selected geological formation.
[0018] FIG. 9 illustrates one embodiment for a wastewater blowdown brine
treatment system, wherein a wastewater blowdown brine comprises a seeded
slurry, and wherein the solids in the seeded slurry wastewater blowdown brine
are effectively removed by a clarifier system, and wherein the resultant clear
clarifier overflow is diluted with a suitable diluent such as evaporator
distillate or
other solvent such as service water (where suitable) to effectively eliminate
the
4
CA 02567171 2006-11-03
tendency of any remaining scaling constituents in the resultant dear brine to
form
scale when injected into a selected geological formation.
[0017] FIG. 10 illustrates one embodiment for a wastewater blowdown
brine treatment system, wherein a wastewater blowdown brine comprises a
seeded slurry, and wherein the solids in the seeded slurry wastewater blowdown
brine are effectively removed by a hydrocyclone system, and wherein the
resultant clear hydrocyclone overflow is diluted with a suitable diluent such
as
evaporator distillate or other solvent such as service water (where suitable)
to
effectively eliminate the tendency of any remaining scaling constituents in
the
resultant clear brine to form scale when injected into a selected geological
formation.
[0018] The foregoing figures, being merely exemplary, contain various
elements that may be present or omitted from actual process implementations
depending upon the circumstances. An attempt has been made to draw the
figures in a way that illustrates at least those elements that are significant
for an
understanding of the various embodiments and aspects of the invention.
However, various other elements of the unique process methods, and the
combination of apparatus for carrying out the methods, are also shown and
briefly described to enable the reader to understand how various features,
including optional or alternate features or procedures, may be utilized in
order to
provide an efficient, cost effective process design which can be implemented
in a
desired throughput size and physical configuration for providing an optimum
produced water treatment plant utilizing a calcium sulfate seeded
crystallizing
evaporator having an evaporator blowdown treatment system that produces a
clear, effectively solids free treated brine suitable for downhole injection
into a
selected geologic formation.
5
CA 02567171 2006-11-03
DESCRIPTION
[0019] Many steam assisted heavy oil recovery schemes, such as a
steam assisted gravity drainage ("SAGD") heavy oil recovery process injection
and recovery well arrangements, most efficiently utilize a 100% quality steam
supply. Steam is injected downhole in a first geological formation and is used
to
heat, in situ, heavy oil deposits in the first geologic formation to decrease
the
viscosity of the oil so as to mobilize the heavy oil so that it will flow
toward
oil/water collection wells. Several workable embodiments for suitable for
heavy
oil recovery, utilizing produced water as a water makeup source to the water
treatment system for treatment of feedwater to boilers to make steam, are
depicted in FIGS. 1 and 2. Additional details for a calcium sulfate seeded
slurry
crystallizing evaporator are provided in FIGS. 4 and 6.
[0020] As depicted in FIGS. 1 and 2, high pressure steam 70 is supplied to
wellhead 48 and thence into injection well 16 for travel downhole into a
selected
first geological formation 20, and steam 70 emerges outward in the direction
of
reference arrows 18. After traveling through the first geological formation 20
as
indicated by reference arrows 24, to heat and mobilize heavy oils present
therein,
at a selected spaced apart distance, oil/water gathering wells 30 are
advantageously utilized for collecting an oil/water mixture as represented by
reference arrows 26 into the oil/water gathering wells 30. The oil/water
gathering
wells 30 collect the oil produced from the first geological formation 20, as
well as
the condensate from steam injected into the first geological formation 20, and
infiltration water to the first geological formation 20. The injection wells
16 and
the oil/water gathering wells 30 may have one or more lateral or substantially
horizontal legs, as suitable for oil production in a given formation, and, in
a typical
SAGD production environment, such lateral or substantially horizontal legs of
injection wells 16 may lie above those of the lateral or substantially
horizontal
legs of oil/water gathering wells 30, so that a gravity drainage system is
provided
for collection of an oil/water mixture 22.
[0021] As shown in FIGS. 1 and 2, an oil/water mixture 22 is pumped up
through oil gathering wells 30. The oil/water mixture 22 is sent to one or
more
6
CA 02567171 2006-11-03
oil/water separators 32. An oil product 34 is gathered for further
conditioning,
transport, and sale. The produced water 36 which has been separated from the
oil/water mixture 22 is then sent to a produced water de-oiling process unit
40,
which may be accomplished in dissolved air flotation units with the assistance
of
the addition of a de-oiling polymer 42, or by other appropriate unit
processes, to
achieve a preselected low residual oil level such as less than 20 parts per
million
of oil in the de-oiled produced water stream 46. Waste oil/solids 44 are
rejected
from the produced water de-oiling process unit 40. The de-oiled produced water
46 generated can be advantageously treated by a crystallizing evaporative
process operating in a seeded slurry mode, particularly if the oil in the de-
oiled
produced water is reduced reliably to a selected low level of less than about
20
parts per million of oil, or more preferably to less than about 10 parts per
million
of oil. Such a treatment method provides a reliable, simple, straightforward
process for produced water treatment, to produce high quality steam 70 for use
in the recovery of a heavy oil product 34.
[0022] In an embodiment of the water treatment method disclosed herein,
the de-oiled produced water stream 46 is treated and conditioned for feed to
one
or more mechanical vapor recompression evaporator units 140 (in most oil
fields,
multiple redundant units may be utilized) to concentrate the incoming de-oiled
produced water stream 46. One suitable evaporator unit 140 configuration is a
long tube vertical falling film design, wherein the feedwater from which a
portion
of water is to be evaporated is circulated on the tube side, and steam for
heating
is provided on the shell side of a vertical tube bundle, which design will be
known
to those of ordinary skill in the art and to whom this specification is
addressed.
A simplified long tube vertical falling film evaporator unit 140 system design
for
use in the treatment of de-oiled produced water stream 46 is provided in FIG.
4.
[0023] The necessary pretreatment and conditioning of the de-oiled
produced water 46 prior to the evaporator unit 140 may vary somewhat based on
feedwater chemistry - i.e. the identity and distribution of various dissolved
and
suspended solids within the de-oiled produced water 46, as well as on the
degree of concentration selected for accomplishment within the evaporator
units
7
CA 02567171 2006-11-03
=
140. In some embodiments, as shown in FIGS. 1 and 2, it may be necessary or
appropriate to add acid 144 by line 144', or at an appropriate point upstream
of
the feed tank 210, such as via line 146. A selected suitable acid (which
includes
sulfuric acid or hydrochloric acid), which should be effective to lower the pH
sufficiently so that carbonates and bicarbonates in solution are converted to
free
gaseous carbon dioxide which is removed, if not before, then at least by time
of
passage through feedwater deaerator 150. Deaerator 150 also removes, in
addition to carbon dioxide, other non-condensable gases 147 that are dissolved
in the feedwater 46, such as oxygen and nitrogen. However, use of acid 144 in
this manner may be optional, and can sometimes be avoided if feedwater
chemistry and the concentration limits of adverse scale forming species, and
in
particular alkali metal carbonates and bicarbonates, are sufficiently low at
the
anticipated concentration factor utilized in crystallizing evaporator 140.
For pH
control, it may sometimes be useful to raise the pH of operation of the
crystallizing evaporator 140 by addition of a selected base such as caustic
(sodium hydroxide) 231 to the concentrated brine recirculating in the
evaporator
140, which can be accomplished by direct injection of a selected base such as
caustic 231 into the sump 141, as indicated by line 157, or by feed of a
selected
base such as caustic 231 into the suction of recirculation pump 153, as
indicated
by line 159.
[00243 When the produced water contains an appreciable amount of silica,
and/or an appreciable amount of calcium and sulfate, the mechanical vapor
recompression evaporator 140 may in one embodiment be operated using a
calcium sulfate seeded-slurry technique. A suitable configuration for such an
evaporator is set forth in FIGS. 4 and 6. In one embodiment, the seeded slurry
technique may be operated in a near neutral pH range, i.e., from a pH of about
5.5 to a pH of about 8.0, or more preferably, from a pH of about 6.5 to a pH
of
about 7.5 or so. A calcium sulfate seeded slurry mode of operation is made
possible by the substantial elimination of non-hydroxide alkalinity before the
feedwater is introduced into the crystallizing evaporator 140. That way,
carbonate scale is not encountered when the de-oiled produced water 46 that
8
CA 02567171 2006-11-03
has been pre-treated is provided as acidified evaporator feedwater 49c that
has
been steam stripped in deaerator 150 is concentrated in the crystallizing
evaporator 140. The evaporator 140 is operated a seeded-slurry mode wherein
calcium sulfate and silica are preferentially co-precipitated on recirculating
seed
crystals, which avoids scaling of the heat transfer surfaces of the
evaporator.
[0025] As further shown in FIG. 4, at feedwater heat exchanger, the
feedwater pump 149 is used to provide sufficient pressure to send partially
pre-
treated feedwater 46A from the evaporator feed tank 210 (or feedwater 46B, if
further pre-treatment using direct acid injection is utilized) through the
feedwater
heat exchanger 148, prior to the deaerator 150. In the opposite direction, the
distillate pump 143 moves distillate 180 through the feedwater heat exchanger
148, so that the hot distillate is used to heat the feedwater stream directed
toward the deaerator 150.
[0026] The heated and conditioned evaporator feedwater 151 is sent to
the crystallizing evaporator 140. The conditioned feedwater 161 may be
directed
to the inlet of recirculation pump 153, or alternately, directed to the sump
141 of
evaporator 140 as indicated by broken tine 151' in FIG. 4. Concentrated brine
152 in the evaporator 140 is recirculated via pump 153, so only a small
portion of
the recirculating concentrated brine is removed on any one pass through the
evaporator 140. In the evaporator 140, the solutes are concentrated via
removal
of water as condensed distillate 180. As depicted in FIGS. 1, 2, 3, 4 and 6,
an
evaporator 140 is in one embodiment provided in a falling film configuration
wherein a thin brine film 164 is provided by distributors 155 and then falls
inside
one of the plurality of heat transfer elements 156, which in the embodiment
illustrated in FIGS. 3 and 4, are long tubes. A small portion of the water in
the
thin brine film 154 is extracted in the form of steam 160, after heating of
the brine
film 154 though one of the plurality of heat transfer elements 156 from the
heated,
compressed steam 162 which is condensing on the outside of the plurality heat
transfer elements 156. Thus, water is removed from the thin brine film 154 in
the
form of steam 160, and that steam is compressed through the compressor 164,
and the compressed steam 162 is condensed at one or more of the plurality of
9
CA 02567171 2006-11-03
heat transfer elements 156. The heat provided by the condensing of compressed
steam 162 produces yet more steam 160 to continue the evaporation process.
The condensed steam on the outer wall 168 of one of the plurality of heat
transfer elements 156, e.g. tubes as illustrated in FIG. 4, which those of
ordinary
skill in the evaporation arts and to which this disclosure is directed may
variously
refer to as either condensate or distillate 180, is water in relatively pure
form, that
is to say, low in total dissolved solids. In one embodiment, such distillate
contains
less than 10 parts per million of total dissolved solids of non-volatile
components.
Since, as depicted in the embodiments shown in FIGS. 3 and 4, a single stage
of
evaporation is provided, such distillate 180 may be considered to have been
boiled, or distilled, once, and thus condensed but once.
[0027] Prior to the initial startup of the crystallizing evaporator 140 in the
seeded-slurry mode, the crystallizing evaporator 140, which in such mode may
be provided in a falling-film, mechanical vapor recompression configuration,
the
fluid contents of the unit are "seeded" preferably by the addition of
anhydrous
calcium sulfate crystals 272. The seed crystals 272 circulate as solids within
the
brine slurry and serve as nucleation sites for subsequent precipitation of
calcium
sulfate 272, as well as silica 274. Those substances both are precipitated as
an
entering evaporator feedwater 46 is concentrated to an evaporator waste
blowdown 230 of desired final total dissolved solids concentration, which may
be
up to as much as twenty five percent (25%) or more. Importantly, the continued
concentrating process within crystallizing evaporator 140 produces additional
quantities of the precipitated species, and thus creates a continuing source
of
new "seed" material as these particles are broken up by the mechanical
agitation,
particularly by the action of the recirculation pump 153.
[0028] In order to avoid silica and calcium sulfate scale buildup in the
evaporator 140, calcium sulfate seed crystals 272 are continuously circulated
over the wetted surfaces, i.e., the plurality of heat transfer elements such
as
falling film evaporator tubes, as well as other wetted surfaces in the
evaporator
140. Through control of slurry concentration, seed characteristics, and system
geometry, the evaporator can operate in the otherwise scale forming
CA 02567171 2009-09-08
environment. The therm chemical operation within the evaporator 140 with
regard to the scale prevention mechanism is depicted in FIG. 6. As the water
is
evaporated from the brine film 154 inside the heat transfer elements 156 (such
long tubes), the remaining brine film becomes super saturated and calcium
sulfate and silica start to precipitate. The precipitating material promotes
crystal
growth in the slurry rather than new nucleation that would deposit on the heat
transfer surfaces; the silica crystals attach themselves to the calcium
sulfate
crystals. This scale prevention mechanism, called preferential precipitation,
has a
proven capability to promote clean inner heat transfer surfaces 260 of tubes
156.
The details of one advantageous method for maintaining adequate seed crystals
in preferential precipitation systems are set forth in U.S. Pat No. 4,618,429,
issued Oct. 21, 1986 to Howard R. Herrigel, the disclosure of which may be
referred to for further details.
[0028A] The details of one advantageous method for maintaining adequate seed
crystals
in preferential precipitation systems are set forth in U.S. Patent No.
4,618,429, issued
Oct. 21, 1986 to Howard R. Herrigel. Some of those details are further noted,
as set
forth herein below.
Both reverse osmosis and evaporation systems can be adversely affected by
precipitating salts that can deposit on the working surface(s), fouling the
system and
forming scale. The resultant layer of precipitatet reduces efficiency and can
permanently
damage the equipment by clogging or rupturing the membrane or by pitting or
forming
an insulating layer on the heat transfer surface(s).
The likelihood of scale forming depends on the nature of the solution being
treated and the operating parameters of the system. A feed solution often
contains a
variety of very soluble and slightly soluble salts, with the slightly soluble
salts being first
to reach the saturation point where they precipitate and form scale. Salts in
this category
include calcium fluoride, calcium sulfate, calcium phosphate, silica, hydrated
iron oxides,
and other hydrated metal oxides. Other salts in this category are the
fluoride, sulfate,
and phosphate salts of other alkaline earth metals such as barium and
strontium.
11
CA 02567171 2009-09-08
In preferential precipitation, scaling and fouling are avoided by allowing
solute to
. -
preferentially deposit on a slurry of seed crystals suspended in the feed
solution. When
adequate seed crystal surface area is available, precipitating salts are
deposited on the
seed and carried away from the working surfaces of the system. The surface
area of
seed crystal required to prevent scaling is preferably 5.0x106 cm2 per gram of
precipitating solute per minute (cm2 /g/min). Nucleation crystals generally
ranging from
about 1 to 100 microns in length, and preferably having an average length of
about 10
microns. are used.
The term "conoentration factor (C.F.) connotes the degree of change effected
by a solution concentration system. It is a comparison between feed solution
and
effluent, and can be defined mathematically in number of ways. For example, it
can be
defined as the concentration of system reject crwided by the concentration of
system
feed:
C.F.=[conc. sys. rejectjaconc. sys. feed]
This designation is useful when the feed solution is not saturated, or when
the
concentration factor is measured in terms of one of the highly soluble salts
or ions in a
solution saturated in less soluble salts. Using concentration to measure C.F.
for
saturated salts would result in a C.F. of 1, since the concentration of both
feed and reject
streams would be the same, with precipitate accounting for the solvent
removed.
C.F. is often expressed in volume terms as the volume of feed solution divided
by
the volume of reject solution:
C.F.=-Volume Feed]/[Volume Reject)
Expressed in volume terms, C.F. reflects the degree of change effected even in
saturated solutions, and as used in this application, concentration factor is
usually
expressed in volume terms or in terms of concentration of some very soluble
salt or ion
in solution. There are minor variations between these two methods of
expression, but as
used herein, the two methods are considered interchangeable. A system
concentrating
at a concentration factor of 1 is generally not removing solvent and the
concentration of
system reject is identical to the system feed. A system concentrating at a
C.F. of 2
produces a solution roughly twice as concentrated as Its feed solution.
11A
CA 02567171 2009-09-08
For solutions being concentrated at concentration factors greater than 2.
scaling
can be prevented by (a) determining the minimum amount of seed crystal
required to
prevent scaling for the solution being concentrated at a high concentration
factor,
C.F.mAx, of about 10 or greater, and (b) introducing into the feed solution at
least a
portion of the amount determined from step (a) according to the equation:
% introduced=0/C.F.)]x100XEC.F.mAx qc.F.mAx -1)]
The % introduced" represents a percentage of the amount of seed crystal
required at a relatively high concentration factor, "C.F.mAx." C.F., ix can be
any
concentration factor of about 10 or greater, and "C.F." is the concentration
factor at
which the feed solution will initially be concentrated. Concentration factors
"near" 1 are
those just above 1.
Accordingly, when a solution is to be concentrated at a concentration factor
near
1 and C.F.kmx is about 10, 25x(1)x(10/9)=% introduced, and at least about
27.78% of the
seed crystal that would be required at a C.F. of about 10 is introduced prior
to
concentrating. If a C.F.mAx of about 100 were used in the calculations above,
25x(1)x(100/99) would be the % introduced, and about 25.25% of the seed
required at
C.F.=100 would be the amount of seed crystal introduced into the solution
being
concentrated. It so happens that about 25.25% of the seed required at C.F.=100
and
about 27.78% of the seed required at C.F.=10 are equal, so that the amount
determined
in either case is roughly the same. Hence, regardless of the C.F.L4Ax chosen,
the amount
to be added will be approximately the same and in any event will be within the
intended
scope of the appended claims.
If the feed solution is to be initially concentrated at a C.F. of 2 and
C.F.mAx =100,
about (25)x(2)x(100/99), or at least about 50.5% of the seed required at
C.F.=100 is
added. In fact, introducing at least about 50.5% of the crystal required at a
concentration
factor of about 100 will protect against scaling over the entire range of
concentration
factors between 1 and 2. Thus, by introducing at least about 50.5% of the seed
required
at a concentration factor of about 100 or greater, the scaling sometimes
observed
between O.F=1 and C.F.=2 can be eliminated, regardless of what specific
concentration
factor the feed solution will be concentrated to.
11B
CA 02567171 2009-09-08
If the initial concentration of the feed solution will be greater than 2,
[1-(1/C.F.)]x100x(C.F.mvz /C.F.pAAx -1)] is used as the equation in step (b).
For
example, if C.F., mx is chosen as 100 and the feed solution is to be subjected
to
concentration near C.F.=5, 80.8%, or [1-(1/5)jx100x[100/(100-1)] % of the seed
required
at a concentration factor of 100 is introduced into the feed solution to
prevent scaling.
In a preferred embodiment, the percent introduced is between
(25)x(C.F.)x[C.F.mAx /(C.F.mAx -1)] and [141 /C.F.)]x100x1C.F.w,x JC.F.mAx
The method of the invention can be used in conjunction with known seed crystal
removal techniques so that a workable slurry can be maintained while the
presence of
an adequate amount of seed crystal is ensured. The method of the present
invention is
useful in numerous types of solution concentration systems, including "raced
point,"
"multi-stage," "multiple-effect," and "continuously increasing" systems. These
systems
are generally known in the art and are briefly described below.
Fixed-point systems are those designed to operate at a relatively constant
concentration factor and would ideally require a determinate amount of seed
crystal
surface area for a given feed solution. Hence, initially adding the requisite
minimum
amount of seed crystal would normally suffice throughout operation. Multi-
stage systems
employ a series of concentrating steps, often referred to as stages, to
produce a highly
concentrated product. For purposes of this invention, each step can be viewed
as a
separate unit. Multiple-effect systems often contain a series of concentrating
units
connected in tandem and cooperate to produce a highly concentrated product.
Continuously increasing systems are those that operate gradually and
continuously to increase the effective concentration factor of a given volume
of feed
solution. For purposes of this invention, the initial concentration factor of
these systems
is the concentration factor at which the system begins to concentrate.
Normally, this
value is close to 1.
The step of introducing seed crystal can be accomplished in all systems by
adding seed crystal to the feed stream from outside sources or by
recirculating seed
crystal already present in the system or by exchanging seed crystal between
units.
11C
CA 02567171 2009-09-08
.
As used herein, "solution concentration systems," "purifiers," and the
like include
systems used to concentrate, capture, or reclaim solutes as well as those
operated to
obtain solvents. The terms "requisite minimum amount of seed crystal,"
"preferred
minimum amount," "adequate amount," and the like generally refer to the amount
of
seed crystal required as disclosed. Preferably about 5.0x10 cm2/gram/min is
employed, though at least about 2.5x106 cm2 /gram/min is within the scope of
this
invention.
Additionally, it is understood to be within the scope of the present invention
that the method disclosed herein can be used in conjunction with seed crystal
removal
techniques to provide an efficient and economical means for maintaining a
workable
seed crystal slurry while assuring the presence of an adequate amount of
crystal.
[OW9] It is to be understood that a falling film evaporator 140 design is
provided only for purposes of illustration and thus enabling the reader to
understand the water treatment process(es) taught herein, and is not intended
to
limit the process to the use of such evaporator design, as those of ordinary
skill
in the art will recognize that other crystallizing evaporator designs, such
as, for
example, a forced circulation evaporator, may be alternately utilized with the
accompanying benefits and/or drawbacks as inherent in such alternative
evaporator designs.
[0030] By way of example, and not for purposes of limitation, in a falling
film evaporator embodiment, the distillate 180 descends by gravity along the
outer wall 168 of tubes 156 and accumulates above bottom tube sheet 172, from
where it as collected via condensate line 174. A small portion of steam in
equilibrium with distillate 180 may be sent via line 173 to the earlier
discussed
deaerator 150 for use in mass transfer, i.e, heating and steam stripping
descending liquids in a packed tower to remove non-condensable gases 147
such as carbon dioxide. However, the bulk of the distillate 180 is removed as
a=
liquid and may optionally be sent for further treatment in a distillate
treatment
plant, for, example such as depicted in detail in FIG. 1, or as merely
depicted in
11D
CA 02567171 2006-11-03
functional form as feed 181F for plant 181 in FIG. 2, to ultimately produce a
treated distillate product water 181p which is suitable for boiler feedwater,
such
as feedwater 80F in the case where packaged boilers 80 are utilized as
depicted
in FIG. 1. The distillate treatment plant 181 also normally produces a reject
stream 181R which may be recycled to the evaporator feed tank 210 or other
suitable location for reprocessing or reuse. In one embodiment, the reject
stream
181R may be sent directly back to the liquid sump 141 of crystallizing
evaporator
140 via line 111 In another embodiment, the reject stream 181R may be sent
back for injection via line 111' to the inlet to the recirculation pump 153.
As
shown in the embodiment set forth in FIG. 2, the distillate treatment plant
181
may be optional, especially in the case of the use of once through steam
generators 12 as depicted in FIG. 2, and in such instance the distillate 180
may
often be sent directly to once-through steam generators 12 as feedwater stream
12F. Also, as shown in FIG. 1,. a distillate treatment plant 181 may also be
optional in some cases, depending on feedwater chemistry, and in such cases,
distillate 180 may be fed directly to boiler 80 as indicated by broken line
81.
[0031] In an embodiment where steam generators (e.g., boilers 80) are
used as shown in Fig. 1, high pressure steam 70 will be generated, and a
boiler
blowdown stream 110 will be discharged as necessary to control water chemistry
within the boiler 80. Prior to feed of distillate 180 to boiler 80, it may
be
necessary or desirable to remove the residual organics and other residual
dissolved solids from the distillate 180. For example, as illustrated in FIG.
1, in
some cases, it may be necessary to remove residual dissolved solids from the
relatively pure distillate 180 produced by the evaporator 140.
[0032] In one embodiment, removal of residual dissolved solids can be
accomplished by passing the evaporator distillate 180, after heat exchanger
200,
through an ion exchange system 202. Such ion-exchange systems may be of
mixed bed type or include an organic trap, and effective to remove the salts
and/or organics of concern in a particular distillate 180 being treated. In
any
event, regenerant chemicals 204 will ultimately be required, which
regeneration
results in a regeneration waste 206 that must be further treated. Fortunately,
in
12
CA 02567171 2006-11-03
the process scheme described herein, the regeneration waste 206 can be sent
back to the evaporator feed tank 210 (along with other distillate treatment
plant
181 reject waters 181R) for a further cycle of treatment through the
evaporator
140.
[0033] In another embodiment, removal of residual dissolved solids can be
accomplished by passing the evaporator distillate 180 through a heat exchanger
200' and then through electrodeionization (EDI) system 220. The EDI reject 222
is also capable of being recycled to evaporator feed tank 210 (along with
other
distillate treatment plant 181 reject waters 181R) for a further cycle of
treatment
through the evaporator 140.
[0034] In another embodiment, when a reverse osmosis system 224 is
utilized, the reject stream includes the RO reject stream 221 which is
recycled to
be mixed with the de-oiled produced water stream 46 in the evaporator feed
tank
210 system, for reprocessing through the evaporator 140. Likewise, when ion-
exchange system 202 is utilized, the regenerant waste stream 206 is recycled
to
be mixed with the de-oiled produced water 46 in the evaporator feed tank
system,
for reprocessing through the evaporator 140. After processing in distillate
treatment plant 181, heating of the polished distillate by heat exchanger 201'
is
appropriate to produce a heated feedwater 80F for boiler 80.
[0035] In the process disclosed herein, the evaporator 140 is designed to
produce high quality distillate (typically about 2ppm to about 5 ppm non-
volatile
TDS) which, after temperature adjustment to acceptable levels in heat
exchangers 200 or 200' (typically by cooling to about 45 C, or lower) can be
fed
directly into polishing equipment (EDI system 220, or ion exchange system 202,
or reverse osmosis system 224) for removal of dissolved solids. The water
product produced by the distillate treatment plant 181 equipment just
mentioned
is most advantageously used as feedvvater for the packaged boiler 80. That is
because in the typical once-though steam generator 12 used in oil field
operations, it is normally unnecessary to incur the additional expense of
final
polishing by removal of residual total dissolved solids from the evaporator
distillate stream 180. However, in some applications, final polishing may not
be
13
CA 02567171 2006-11-03
necessary when using conventional boilers 80. It may be appropriate in some
embodiments from a heat balance standpoint that the de-oiled produced waters
46 fed to the evaporator for treatment be heated by heat exchange with the
distillate stream 180. However, if the distillate stream is sent directly to
once-
through steam generators 12, then no cooling of the distillate stream 180 may
be
appropriate. Also, in the case of once-through steam generators 12, in many
embodiments, it may be necessary or appropriate run to utilize 80% quality
steam 14 through a steam/liquid separator 130 to separate high quality steam
132 from liquid blowdown 134. Further the liquid blowdown 134 may be further
processed for heat recovery in a plurality of flash tanks F1, F2 etc., to
produce
lower pressure steam streams S1 and S2, etc., for use as suitable given the
pressure provided by the flash system, generally as shown in FIG. 2.
[0036] One of the significant economic advantages of using a vertical tube,
falling film evaporator 140 such as of the type described herein is that the
on-line
reliability and redundancy available when multiple evaporators are utilized in
the
treatment of produced water. An evaporative based produced water treatment
system can result in an increase of from about 2% to about 3% or more in
overall
heavy oil recovery plant availability, as compared to a produced water
treatment
system utilizing a conventional prior art lime and clarifier treatment process
approach. Such an increase in on-line availability relates directly to
increased oil
production and thus provides a large economic advantage over the project life
of
a heavy oil recovery plant.
[0037] The just described novel combination of process treatment steps
produces feedwater of sufficient quality, and in economic quantity, for use in
packaged boilers 80 in heavy oil recovery operations. Advantageously, when
provided as depicted in FIG. 1 a single liquid waste stream is generated,
namely
evaporator blowdown 230, which contains the concentrated solutes originally
present in de-oiled produced water 46, along with additional contaminants from
chemical additives (such as regeneration chemicals 204). In many cases, the
evaporator blowdown 230 can be further treated for disposal in an
environmentally acceptable manner, which, depending upon locale, might involve
14
CA 02567171 2006-11-03
injection in deep wells 240. Alternately, evaporation to complete dryness in a
zero discharge system 242, such as a crystallizer or drum dryer, to produce
dry
solids 244 for disposal, may be advantageous in certain locales.
[0038] It is to be appreciated-that the water treatment process described
herein for preparing boiler feedwater in heavy oil recovery operations is an
appreciable improvement in the state of the art of water treatment for oil
recovery
operations. The process eliminates numerous of the heretofore encountered
waste streams, while processing water in reliable mechanical evaporators, and
in
one embodiment, in mechanical vapor recompression ("MVR") evaporators.
Polishing, if necessary, can be accomplished in ion exchange,
electrodeionization, or reverse osmosis equipment. The process thus improves
on currently used treatment methods by eliminating most treatment or
regeneration chemicals, eliminating many waste streams, eliminating some types
of equipment. Thus, the complexity associated with a high number of treatment
steps involving different unit operations is avoided.
[0039] It should also be noted that the process described herein can be
utilized with once through steam generators, since due to the relatively high
quality feedwater¨actually treated produced water¨provided to such once
through steam generators, the overall blowdown rate of as low as about 5% or
less may be achievable in the once through steam generator. Alternately, as
shown in FIG. 2, at least a portion of the liquid blowdown 134 from the once
through steam generator 12 can be recycled to the steam generator 12, such as
indicated by broken line 135 to feedwater stream 12F.
[0040] In yet another embodiment, to further save capital and operating
expense, industrial boilers of conventional design may be utilized since the
distillate¨treated produced water¨may be of sufficiently good quality to be an
acceptable feedwater to the boiler, even if it requires some polishing. It is
important to observe that use of such boilers reduces the boiler feed system
and
evaporative produced water treatment system size by twenty percent (20%),
eliminates vapor/liquid separation equipment as noted above, and reduces the
boiler blowdown flow rate by about ninety percent (90%).
CA 02567171 2006-11-03
=
[0041] In short, evaporative treatment of produced waters using a falling
film, vertical tube evaporator is technically and economically superior to
prior art
water treatment processes for heavy oil production. It is possible to recover
ninety five percent (95%) or more, and even up to ninety eight percent (98%)
or
more, of the produced water as high quality distillate 180 for use as high
quality
boiler feedwater (resulting in only a 2% boiler blowdown stream which can be
recycled to the feed for evaporator 140). Such a high quality distillate
stream
may be utilized in SAGE) and non-SAGE) heavy oil recovery operations. Such a
high quality distillate stream may have less than 10 mg/L of non-volatile
inorganic
TDS and is useful for feed either to OTSGs or to conventional boilers.
[0042] The overall life cycle costs for the novel treatment process
described herein are significantly less than for a traditional lime softening
and ion
exchange treatment system approach. And, an increase of about 2% to 3% in
overall heavy oil recovery plant availability is achieved utilizing the
treatment
process described herein, which directly results in increased oil production
from
the facility. Since boiler blowdown is significantly reduced, by as much as
90%
or more, the boiler feed system may be reduced in size by as much as fifteen
percent (15%) or more. Finally, the reduced blowdown size results in a reduced
crystallizer size when zero liquid discharge is achieved by treating blowdown
streams to dryness.
[0043] In the improved water treatment method, the control over waste
streams is focused on a the evaporator blowdown, which can be conveniently
treated by deep well 240 injection, or in a zero discharge system 242 such as
a
crystallizer and/or spray dryer, to reduce all remaining liquids to dryness
and
producing a dry solid 244. This contrasts sharply with the prior art
processes, in
which sludge from a lime softener is generated, and in which waste solids are
gathered at a filter unit, and in which liquid wastes are generated at an ion
exchange system and in the steam generators. Moreover, this waste water
treatment process also reduces the chemical handling requirements associated
with water treatment operations.
16
CA 02567171 2006-11-03
=
. .
[0044] Evaporator blowdown 230 from the evaporator 140 is often suitable,
or may be treated in a further evaporator blowdown treatment step 232 as
indicated in FIGS. 1 and 2 and thus made suitable for disposal by injection
into a
geologic formation via deep well 240. Many produced waters encountered in
heavy oil production are high in silica, with values that may range up to
about
200 mg/I as Si02, or higher. Use of a seeded slurry operational configuration
in
evaporator 140 enables the co-precipitation of silica with precipitating
calcium
sulfate, to provide a process design which prevents the scaling of the inner
heat
transfer surfaces 260 of the plurality of heat transfer elements, namely tubes
156
with the ever-present silica. This is important, since silica solubility must
be
accounted for in the design and operation of the evaporator 140, in order to
prevent silica scaling of the inner surfaces 260 of the plurality of heat
transfer
elements 156.
[0045] Attention is now directed to FIGS. 7, 8, 9, and 10, where process
flow diagrams are provided for various exemplary embodiments 232A, 232B, 232c,
and 232D for a suitable evaporator blowdown treatment process 232. These
process units, in most embodiments alternately one of 232A, or 232B, or 232c,
or
232D, are provided prior to deep well 240 injection of clear treated brine
270.
During such periods as deep well 240 may be out of service, either a clear
treated brine 270 or untreated evaporator blowdown brine 230 may be provided
to a zero discharge system 242 (normally a crystallizer) such as via optional
line
236, for producing relatively dry solids 244 for land disposal or reuse.
[0046] In FIG. 7, a centrifuge 250 based blowdown treatment system 232A
is depicted. Evaporator blowdown 230, usually made up of a slurry of water
containing dissolved solutes and suspended solids, requires removal of
substantially all of the suspended solids therefrom, or at least those of any
significant size compared to hydrogeologic passageways of a selected
underground geologic formation 280. Thus, the waste seed from a calcium
sulfate crystallizing evaporator 140 must be captured by a selected removal
system, which as shown in FIG. 7 is centrifuge 250. The evaporator blowdown
230 is fed to centrifuge 250, where solids 252 are rejected to a hopper 254.
The
17
CA 02567171 2006-11-03
clear centrate 256 is sent to a centrate tank 261, where it may be stirred by
mixer
262. Clear centrate 256 is sent via centrate pump 258 to a brine storage tank
263. Distillate 180 and/or other suitable diluent 264 such as a service water
of
suitable composition may be added to brine storage tank 263, and utilized to
the
extent necessary or advisable to prepare a clear brine solution 270 of a
preselected composition. While further specifics will be discussed below, in
one
embodiment, the clear brine solution 270 may be of a preselected composition
substantially free of suspended solids, and wherein the dissolved solids are
provided at a level sufficiently below the solubility limit of scale forming
species
such that injection of clear brine solution 270 into well 240 will not tend to
be
adversely affected by precipitation of scale forming species in the down-hole
geologic environment 280. A high pressure pump 275 is utilized to provide
downhole pressure for injection into injection well 240, and transport of the
clear
brine 270 is provided by line 276 to well 240, where clear brine 270 is
injected
into the second geological formation 280 as indicated by reference arrows 282.
[0047] In FIG. 8, a pressure filter 279 based blowdown treatment system
232B is depicted. Evaporator blowdown 230, usually made up of a slurry of
water
containing dissolved solutes and suspended solids, requires removal of
substantially all of the suspended solids therefrom, or at least those of any
significant size compared to hydrogeologic passageways of a selected second
underground geologic formation 281. Thus, the waste seed from a calcium
sulfate seeded crystallizing evaporator 140 must be captured by a selected
removal system, which in this embodiment is pressure filter 279. The
evaporator
blowdown 230 is fed to pressure filter 279, where solids 282 are rejected to a
hopper 284. Operation of the pressure filter may require distillate 180 and
plant
air 285. The clear filtrate 286 (and liquids from catch basin drain 288) are
sent to
a filtrate tank 290, where it may be stirred by mixer 293. In some cases,
antifoam
294 and steam 296 may be added to filtrate tank 290, for foam suppression and
heating, respectively. Clear filtrate 286 is sent via filtrate pump 289 to a
brine
storage tank 263. Distillate 180 and/or other suitable diluent 264 such as a
service water of suitable composition may also be added to brine storage tank
18
CA 02567171 2006-11-03
263, and utilized to the extent necessary or advisable to prepare a clear
brine
solution 270 of a preselected composition. While further specifics will be
discussed below, the clear brine solution 270 should be of a preselected
composition substantially free of suspended solids, and wherein the dissolved
solids are provided at a level sufficiently below the solubility limit of
scale forming
species such that injection of clear brine solution 270 into well 240 will not
tend to
be adversely affected by precipitation of scale forming species in the down-
hole
environment of second geological formation 281. A high pressure pump 275 is
utilized to provide downhole pressure for injection into injection well 240,
and the
transport of clear brine 270 is provided by line 276 to well 240, where clear
brine
270 is injected into the second geological formation 281 as indicated by
reference arrows 282.
[0048] Similarly, in FIG. 9, a clarifier 310 based blowdown treatment
system 232c is depicted. Evaporator blowdown 230, usually made up of a slurry
of water containing dissolved solutes and suspended solids, requires removal
of
substantially all of the suspended solids therefrom, or at least those of any
significant size compared to hydrogeologic passageways of a selected second
underground geologic formation 281. Thus, the waste seed from a calcium
sulfate seeded crystallizing evaporator 140 must be captured by a selected
removal system, which here, is clarifier 310. The evaporator blowdown 230 is
fed to clarifier 310, where solids 312 are rejected as underflow to a sludge
pump
314. Operation of the clarifier may require addition of flocculating polymers
via
line 316, and operation of a sludge rake 318 via motor 320. The clear overflow
or clarate 326 is sent to a clarate tank 330, where it may be stirred by mixer
332.
Clear overflow or clarate 326 is sent via clarate pump 328 to a brine storage
tank
263. Distillate 180 and/or other suitable diluent 264 such as a service water
of
suitable composition may also be added to brine storage tank 263, and utilized
to
the extent necessary or advisable to prepare a clear brine solution 270 of a
preselected composition. While further specifics will be discussed below, the
clear brine solution 270 should be of a preselected composition substantially
free
of suspended solids, and wherein the dissolved solids are provided at a level
19
CA 02567171 2006-11-03
sufficiently below the solubility lirnit of scale forming species such that
injection of
clear brine solution 270 into well 240 will not tend to be adversely affected
by
precipitation of scale forming species in the down-hole environment of second
geological formation 281. A high pressure pump 275 is utilized to provide
downhole pressure for injection into injection well 240, and the transport of
clear
brine 270 is provided by line 276 to well 240, where clear brine 270 is
injected
into the second geological formation 281 as indicated by reference arrows 282.
[0049] In yet another embodiment, as shown in FIG. 10, a hydrocyclone
based blowdown treatment system 232D is depicted. Evaporator blowdown 230,
usually made up of a slurry of water containing dissolved solutes and
suspended
solids, requires removal of substantially all of the suspended solids
therefrom, or
at least those of any significant size compared to hydrogeologic passageways
of
a selected second underground geologic formation 281. Thus, the waste seed
from a calcium sulfate seeded crystallizing evaporator 140 must be captured by
a
selected removal system, which here is a system of one or more hydrocylcones
and where more than one, a series of hydrocyclones H1 through HN, where N is a
positive integer. The evaporator blowdown 230 my be stored in a concentrate
holding tank 342, where it is stirred by mixer 344 before feed via pump 346 to
the
first hydrocyclone H1. A recycle loop 347 may be provided to avoid excess
pressure on the hydrocyclones and to assist in keeping seed suspended in the
circulating blowdown 230 at tank concentrate holding tank 342. Hydrocyclone
overflow H10 from the first hydrocyclone H1, low in suspended solids, is sent
to
the next hydrocyclone H(l+i), and likewise in series until the last
hydrocyclone HN
is encountered, wherein the overflow HNO is sent to the clear overflow tank
350.
Clear overflow 356 is stored in clear overflow tank 350, and may optionally be
stirred by mixer 357, before being sent by pump 358 to the brine storage tank
263. Solids from the first hydrocyclone H1 and each subsequent hydrocyclone in
the series through HN are sent via underflow lines HiU though HNU to the seed
tank 360. Waste seed is stirred via mixer 362 and sent by pump 364 to
thickener 366, which again may be a centrifuge, pressure filter, or clarifier
system
similar to that just described above. Sludge 368 is discharged and recovered
CA 02567171 2006-11-03
=
brine 370 may be sent to centrate holding tank 342 for reprocessing in the
manner described, or otherwise purged if advisable due to the presence of
excess scale forming constituents. Distillate 180 and/or other suitable
diluent
264 such as a service water of suitable composition may also be added to brine
storage tank 263, and utilized to the extent necessary or advisable to prepare
a
clear brine solution 270 of a preselected composition. While further specifics
will
be discussed below, the clear brine solution 270 should be of a preselected
composition substantially free of suspended solids, and wherein the dissolved
solids are provided at a level sufficiently below the solubility limit of
scale forming
species such that injection of clear brine solution 270 into well 240 will not
tend to
be adversely affected by precipitation of scale forming species in the down-
hole
environment of second geological formation 281. A high pressure pump 275 is
utilized to provide downhole pressure for injection into injection well 240,
and the
transport of clear brine 270 is provided by line 276 to well 240, where clear
brine
270 is injected into the second geological formation 281 as indicated by
reference arrows 282. To assure a solids free clear brine solution 270, an
optional low pressure in-line filter 372 may be provided downstream of pump
358.
Alternately, an optional high pressure in-line filter 374 may be provided
downstream of high pressure pump 275 to provide final solids capture before
injection to well 240.
[0050] With respect to the preparation of clear brine solution 270, it must
be appreciated that the blowdown 230 will in most embodiments be saturated in
one or more scale forming species. Typical scale forming species may include
silica (both ionized and undissociated forms, depending upon the pH) calcium
sulfate, barium compounds including barium sulfate, or strontium compounds
including strontium sulfates. For example, silica, when in near neutral
aqueous
solution, may be present roughly in the range of about 200 parts per million,
depending on the exact pH and other dissolved and suspended species present.
Therefore, when the respective blowdown treatment plant prepares a
substantially solids free brine liquor, whether centrate 256 in plant 232A, or
filtrate
292 in plant 232B, or clarate 326 in plant 232, or overflow 356 in plant 232D7
it
21
CA 02567171 2006-11-03
must be recognized that the precipitation sites for removal of silica from the
solution have been eliminated by the seed removal, and thus, silica might tend
to
scale out of the silica saturated solution upon cooling or further
concentration.
Thus, such substantially suspended solids free brine liquor is generally not
suitable for direct downhole injection. Therefore, it would often be
advantageous
to dilute such substantially suspended solids free brine liquor with a
suitable
diluent, such as the relatively pure distillate stream 180 (with about 10 ppm
or
less of non-volatile dissolved solids), or even post treated distillate 180p
(with less
than 1 ppm of non-volatile dissolved solids). If suitable service waters are
available from other sources, that is, provided that the level of silica or
other
scale forming materials such as hardness and alkalinity are sufficiently low
in
such other potential diluents 264 that a clear brine solution 270 of suitable
composition may be economically prepared and injected, then use of aqueous
sources other than distillate stream 180 may be selected. However, in one
embodiment, the distillate stream 180 is useful for addition to any one of the
above mentioned substantially suspended solids free brine liquors (e.g.,
centrate
256, or filtrate 292, or clarate 326, or overflow 356), so that upon dilution
(and
thus before injection) the silica level in clear brine solution 270 is about
10%, or
further, from the level of silica in the substantially suspended solids free
brine
liquor, which for most practical purposes, represents an equivalent dilution
from
the solubility limit of silica in clear brine solution 270. Similar dilution
(i.e., 10%)
for other of the one or more scale forming species may be appropriate,
depending upon the precise chemistry in a particular application. Thus, when
silica is present at about 200 parts per million (as Si02) in the selected
substantially suspended solids free brine liquor, then dilution to a silica
level of
about 180 parts per million (as Si02) in the clear brine solution 270 is
appropriate.
In yet another embodiment, upon dilution, (and thus before injection) the
silica
level in clear brine solution 270 is about 20%, or further, from the level of
silica in
the substantially suspended solids free brine liquor, or consequently, from
the
solubility limit of silica in clear brine solution 270. Similar dilution
(i.e., 20%) for
other of the one or more scale forming species may be appropriate, depending
22
CA 02567171 2006-11-03
upon the precise chemistry in a particular application. In such embodiment,
when silica is present at about 200 parts per million (as Si02) in the
selected
substantially suspended solids free brine liquor, then dilution to a silica
level of
about 160 parts per million (as Si02) in the clear brine solution 270 is
appropriate.
in yet another embodiment, upon dilution, (and thus before injection) the
silica
level in clear brine solution 270 is about 25%, or further, from the level of
silica in
the substantially suspended solids free brine liquor, or consequently, from
the
solubility limit of silica in clear brine solution 270. Similar dilution
(i.e., 25%) for
other of the one or more scale forming species may be appropriate, depending
upon the precise chemistry in a particular application. Therefore, in such an
embodiment, when silica is present at about 200 parts per million (as Si02) in
the
selected substantially suspended solids free brine liquor, then dilution to a
silica
level of about 150 parts per million (as Si02) in the clear brine solution 270
is
appropriate. In yet another embodiment, upon dilution, (and thus before
injection) the silica level in clear brine solution 270 is about 30%, or
further, from
the level of silica in the substantially solids free brine liquor, or
consequently,
from the solubility limit of silica in clear brine solution 270. Similar
dilution (i.e.,
30%) for other of the one or more scale forming species may be appropriate,
depending upon the precise chemistry in a particular application.
Consequently,
in such an embodiment, when silica is present at about 200 parts per million
(as
Si02) in the selected substantially suspended solids free brine liquor, then
dilution to a silica level of about 140 parts per million (as Si02) in the
clear brine
solution 270 is appropriate.
Further, the solubility limit as a function of
temperature, especially as applicable to the anticipated downhole temperature
at
the second geological formation 281, should in most applications be accounted
for when calculating the anticipated silica solubility limit, as the brine
blowdown
treatment process 232 should normally provide a clear brine solution 270 that
may be injected into well 240 without fear of well plugging over time by
deposition of scale from the solution 270 being injected,
[0051] While the effect of temperature was just noted with respect to silica,
which is more soluble at higher temperature, it is important to note that the
23
CA 02567171 2006-11-03
solubility of calcium sulfate is inversely soluble, so that as a clear brine
solution
270 is cools for downhole injection, the cooling of the clear brine solution
further
aids in desaturation of the brine.
[0052] Further, it must be noted that a relatively small amount of distillate
180 may be utilized to achieve the advantageous results taught herein. For
example if 1000 US gallons per minute of de-oiled produced water 46 is treated
in a crystallizing evaporator 140, a brine blowdown stream 230 of only 10 US
gallons per minute would be produced when operating at 100 cycles of
concentration. In such a process situation, 990 US gallons per minute of
distillate 180 would be produced for generation of downhole high pressure
steam
70. Removal of suspended solids from the brine blowdown stream 230 as
described herein produces a substantially solids free clear brine liquor, of
approximately, but not quite, 10 US gallons per minute_ For purposes of
example,
however, if 10 US gallons per minute of distillate 180 is used for dilution by
addition to 10 US gallons per minute of a substantially solids free clear
brine
liquor prepared by a suitable method such as one of those set forth herein,
then
overall, the clear brine solution 270 has a volume of 20 US gallons per
minute.
Further, the degree of saturation of the scaling salts in the clear brine
solution
270 is thus cut in half, when compared with dissolved solids level in the
brine
blowdown stream 230. Thus, in this typical example, the overall recovery of
the
crystallizing evaporator system 140 is decreased only from 99% to 98%. Thus,
it
can be seen that it may be advantageous to mix a substantially suspended
solids
free clear liquor derived from brine blowdown stream 230 in a 50-50 ratio with
distillate 180, to achieve a suitable composition for a clear brine solution
270 for
injection into a selected second geological formation 281.
[0053] Although only several exemplary embodiments of this invention
have been described in detail, it will be readily apparent to those skilled in
the art
that the novel produced waste treatment process, and the apparatus for
implementing the process, may be modified from the exact embodiments
provided herein, without materially departing from the novel teachings and
advantages provided by this invention, and may be embodied in other specific
24
CA 02567171 2009-09-08
. =
forms without departing from the spirit or essential characteristics thereof.
Therefore, the
disclosures presented herein are to be considered in all respects as
illustrative and not
restrictive. It will thus be seen that the objects set forth above, including
those made apparent
from the preceding description, are efficiently attained. Many other
embodiments are also
feasible to attain advantageous results utilizing the principles disclosed
herein. Therefore, it
will be understood that the foregoing description of representative
embodiments of the
invention have been presented only for purposes of illustration and for
providing an
understanding of the invention, and it is not intended to be exhaustive or
restrictive, or to limit
the invention only to the precise forms disclosed.