Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent: | (11) CA 2567546 |
|---|---|
| (54) English Title: | BLISTER PACKAGE ADJUNCT |
| (54) French Title: | ACCESSOIRE D'EMBALLAGE-COQUE |
| Status: | Granted and Issued |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | MACRAE & CO. |
| (74) Associate agent: | |
| (45) Issued: | 2014-03-11 |
| (22) Filed Date: | 2006-11-09 |
| (41) Open to Public Inspection: | 2008-05-09 |
| Examination requested: | 2011-09-02 |
| Availability of licence: | N/A |
| Dedicated to the Public: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | No |
|---|
| (30) Application Priority Data: | None |
|---|
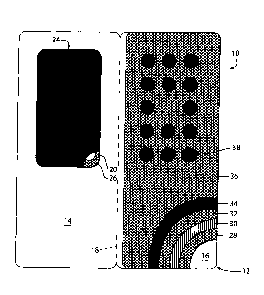
An adjunct is provided for use with a blister package having a plurality of blisters arranged in a set pattern, each blister containing a pharmaceutical object. The adjunct includes a backing sheet having first and second side portions joined along a fold line, a plurality of openings extending through the first side portion in a pattern corresponding to a pattern of blisters of the blister package, a first layer of adhesive material surrounding the openings, a second layer of adhesive material covering the second side portion of the backing sheet, a thin film of plastics material covering the second layer of adhesive material, a third layer of adhesive material covering the film of plastics material, an electronics sheet covering the third layer of adhesive material and carrying electrical traces and an electronic chip thereon, the traces extending in a pattern corresponding to the pattern of openings in the first side portion of the backing sheet, and a fourth layer of adhesive material covering the electronics sheet. Each of the first and fourth layers of adhesive material is covered prior to use of the adjunct by a sheet of release material.
Un élément annexe destiné à être utilisé avec un emballage coque comportant une pluralité d'alvéoles disposés en un motif établi, chaque alvéole contenant un objet pharmaceutique. L'élément annexe comporte une feuille de support présentant des première et seconde parties latérales assemblées selon une ligne de pliage, une pluralité d'ouvertures s'étendant à travers la première partie latérale dans un motif correspondant à un motif d'alvéoles de l'emballage coque, une première couche de matériau adhésif entourant les ouvertures, une seconde couche de matériau adhésif recouvrant la seconde partie latérale de la feuille de support, un film mince de matière plastique recouvrant la seconde couche de matériau adhésif, une troisième couche de matériau adhésif recouvrant le film de matière plastique, une feuille électronique recouvrant la troisième couche de matériau adhésif et portant des impressions conductrices d'électricité et une puce électronique, les impressions conductrices s'étendant dans un motif correspondant au motif des ouvertures dans la première partie latérale de la feuille de support, et une quatrième couche de matériau adhésif recouvrant la feuille électronique. Chacune des première et quatrième couches de matériau adhésif est recouverte préalablement à l'utilisation de l'élément annexe par une feuille de matériau de déblocage.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
2024-08-01:As part of the Next Generation Patents (NGP) transition, the Canadian Patents Database (CPD) now contains a more detailed Event History, which replicates the Event Log of our new back-office solution.
Please note that "Inactive:" events refers to events no longer in use in our new back-office solution.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Event History , Maintenance Fee and Payment History should be consulted.
| Description | Date |
|---|---|
| Inactive: IPC expired | 2023-01-01 |
| Common Representative Appointed | 2019-10-30 |
| Common Representative Appointed | 2019-10-30 |
| Inactive: Correspondence - Transfer | 2018-08-14 |
| Letter Sent | 2016-01-18 |
| Grant by Issuance | 2014-03-11 |
| Inactive: Cover page published | 2014-03-10 |
| Pre-grant | 2013-12-27 |
| Inactive: Final fee received | 2013-12-27 |
| Notice of Allowance is Issued | 2013-11-21 |
| Letter Sent | 2013-11-21 |
| Notice of Allowance is Issued | 2013-11-21 |
| Inactive: Q2 passed | 2013-11-19 |
| Inactive: Approved for allowance (AFA) | 2013-11-19 |
| Amendment Received - Voluntary Amendment | 2013-08-22 |
| Inactive: S.30(2) Rules - Examiner requisition | 2013-02-22 |
| Letter Sent | 2011-09-23 |
| Request for Examination Requirements Determined Compliant | 2011-09-02 |
| All Requirements for Examination Determined Compliant | 2011-09-02 |
| Request for Examination Received | 2011-09-02 |
| Application Published (Open to Public Inspection) | 2008-05-09 |
| Inactive: Cover page published | 2008-05-08 |
| Inactive: IPC assigned | 2008-04-29 |
| Inactive: First IPC assigned | 2008-04-29 |
| Inactive: IPC assigned | 2008-04-29 |
| Inactive: IPC assigned | 2008-04-29 |
| Inactive: IPC assigned | 2008-04-29 |
| Inactive: Declaration of entitlement - Formalities | 2007-10-11 |
| Inactive: Courtesy letter - Evidence | 2006-12-19 |
| Application Received - Regular National | 2006-12-13 |
| Inactive: Filing certificate - No RFE (English) | 2006-12-13 |
| Small Entity Declaration Determined Compliant | 2006-11-09 |
There is no abandonment history.
The last payment was received on 2013-10-30
Note : If the full payment has not been received on or before the date indicated, a further fee may be required which may be one of the following
Patent fees are adjusted on the 1st of January every year. The amounts above are the current amounts if received by December 31 of the current year.
Please refer to the CIPO
Patent Fees
web page to see all current fee amounts.
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| INTELLIGENT DEVICES SEZC INC. |
| Past Owners on Record |
|---|
| MICHAEL PETERSEN |
| MYKOLA SHERSTYUK |