Note: Descriptions are shown in the official language in which they were submitted.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
1
INJECTABLE PHARMACEUTICAL SUSPENSION COMPRISING POSACONAZOLE
FIELD OF THE INVENTION
The present invention relates to formulations useful for treating
infections. Specifically, these formulations include the active
pharmaceutical ingredient posaconazole in an injectable suspension that
is stable when subjected to terminal steam sterilization, and throughout
the shelf life of the product.
BACKGROUND OF THE INVENTION
Posaconazole, an anti-fungal agent, represented by the following chemical
structural formula
O H3
H NN N N S
N CH3
HO
F R 0
NsN
F \--N
is being developed as an oral suspension (40 mg/ml) under the trademark
NOXAFIL by Schering Corporation, Kenilworth, NJ. See, for example,
U.S. Patent No. 5,703,079, 5,661,151, WO 02/80678 published October
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
2
17, 2002, and EP 1 372 394 published January 2, 2004. In addition,
other formulations of posaconazole have been disclosed. A solid
(capsule/tablet) of posaconazole is disclosed in U.S. Patent Nos.
5,972,381 and 5,834,472. Lastly, a topical form of posaconazole, e.g., a
lotion, cream, ointment, or "lacquer nail polish" is contemplated based on
other similar formulations, e.g., U.S. Patent No. 4,957,730 (PENLACO
available from Dermik0).
Certain aspects of stabilization of micronized particles in pharmaceutical
compositions are addressed in the literature. For example, U.S. Patent
No. 5,858,410 discloses pharrnaceutical compositions containing particles
of active agents of average diameter less than 5 microns, having been
comrrLnuted, without prior conversion into a melt, by using a piston-gap
homogenizer. U.S. Patent Application No. 10/440,368 discloses the use of
a phospholipid surface active agent to stabilize microparticles of solid
fenofibrate in an orally administered pharmaceutical composition. U.S.
Patent No. 5,091,188 discloses the use of phospholipids, to prevent
coalescence of microcrystalline active agents in injectable pharmaceutical
compositions. Examples of disclosed phospholipids include lecithin,
phosphatidic acid, phosphatidyl ethanolamine, cholesterol, stearylamine,
glycolipids and mono-glycerides.
None of the aforementioned references however, discloses an injectable
suspension of posaconazole, that is stable when subjected to terminal
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
3
steam sterilization and throughout the shelf life of the product. There is a
need for such a formulation as it is desirable to ensure the physical
stability of the sterilized end product.
SUIVIMARY OF THE INVENTION
The present invention provides formulations of posaconazole that are
stable when subjected to terminal steam sterilization. These formulations
are useful for the treatment of infections. In particular, an aqueous
injectable suspension of posaconazole that is homogenously suspended in
vehicle with the aid of a phospholipid. In addition a thermoprotectant
agent is employed to reduce autoclave-induced particle size growth, as
well as a buffer system to stabilize the phospholipid during autoclaving.
The formulations provided remain stable after 20 minutes of autoclaving
at 121oC and after subsequent storage at 40C to 400C for at least 6
months.
The present invention provides formulations comprising a suspension of
posaconazole, stabilized by a phospholipid, in a mixture comprising a
thermoprotectant, and a buffer system.
In some embodiments, the formulation has been sterilized by autoclaving
or by irradiation.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
4
In some embodiments, the buffer system comprises sodium phosphate,
which may be provided as sodium phosphate monobasic monohydrate,
sodium phosphate dibasic anhydrous, or the combination of the two.
In some embodiments, the buffer system comprises an organic buffer.
In some embodiments, the buffer system comprises at least one of
histidine, citric acid, glycine, sodium citrate, ammonium sulfate, or acetic
acid.
In some embodiments, the buffer system maintains a pH of about 3.0 to
about 9Ø
In some embodiments, the buffer system maintains a pH of about 6.0 to
about 8Ø
In some embodiments, the buffer system maintains a pH of about 6.4 to
about 7.6.
In some embodiments, the phospholipid comprises a natural
phospholipid.
In some embodiments, the phospholipid comprises a synthetic
phospholipid.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
In some embodiments, the phospholipid comprises a natural
phospholipid and a synthetic phospholipid.
In some embodiments, the phospholipid comprises 1-palmitoyl-2-oleoyl-
sn-glycero-3-phosphocholine (POPC).
In some embodiments, the thermoprotectant comprises trehalose.
In some embodiments, the phospholipid comprises 1-palmitoyl-2-oleoyl-
sn-glycero-3-phosphocholine (POPC), the thermoprotectant comprises
trehalose, and the buffer system comprises sodium phosphate monobasic
monohydrate, sodium phosphate dibasic anhydrous, or the combination
of sodium phosphate monobasic monohydrate and sodium phosphate
dibasic anhydrous.
In some embodiments, the posaconazole has a particle size distribution
whose median value is between about 1.0 and about 8.0 microns, with
not more than about 3000 particles of 10 microns or greater size and not
more than about 300 particles of 25 microns or greater size.
In some embodiments, the posaconazole has a particle size distribution
whose median value is between about 1.0 and about 5.0 microns, with
not more than about 3000 particles of 10 microns or greater size and not
more than about 300 particles of 25 microns or greater size.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
6
In some embodiments, the posaconazole has a particle size distribution
whose median value is between about 1.2 and about 4.5 microns, with
not more than about 3000 particles of 10 microns or greater size and not
more than about 300 particles of 25 microns or greater size.
In some embodiments, the formulation has ingredients comprising:
Ingredient Concentration range
Posaconazole about 50 mg/ml
POPC about 40 mg/ml
Sodium Phosphate, 0.345 mg/ml
monobasic, monohydrate,
USP
Sodium Phosphate, dibasic, 1.065
anhydrous, USP
Trehalose 250 mg/ml
Water for Injection, USP q.s. 1 ml
ad
In some embodiments, the formulation has ingredients comprising:
Ingredient Concentration range
Posaconazole about 1 to about 100
mg/ml
POPC about 10 to about 60
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
7
mg/ml
Sodium Phosphate, about 0.01 to about 0.6
monobasic, monohydrate, mg/ml
USP
Sodium Phosphate, dibasic, about 0.04 to about 1.5
anhydrous, USP mg/m1
Trehalose about 10 to about 300
mg/ml
Water for Injection, USP q.s. about 1 ml
ad
In some embodiments, the formulation has ingredients comprising:
Ingredient Concentration ranae
Posaconazole about 40 to about 60
mg/ml
POPC about 20 to about 50
mg/ml
Trehalose about 100 to about 250
mg/ml
Water for Injection, USP q.s. about 1 ml
ad
In some embodiments, the formulation has ingredients comprising:
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
~
Ingl:edient Concentration
Posaconazole 50 mg/ml
POPC 40 mg/mi
Histidine 3 mg/rnl
Citric acid monohydrate 0.24 mg/ml
Trehalose 250 mg/ml
Water q.s. ad 1 ml
at a pH of about 6.4.
In some embodiments, the formulation has ingredients further comprising
an antioxidant.
In some embodiments, the antioxidant comprises propyl gallate at a
concentration of about 0.02 to about 0.005 mg/ml.
In some embodiments, the antioxidant comprises butylated
hydroxytoluene at a concentration of about 0.1 to about 0.02 mg/ml.
In some embodiments, the antioxidant comprises alpha-D-tocopherol at a
concentration of about 0.5 to about 0.01 mg/ml.
In some embodiments, the formulation has ingredients comprising:
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
9
Ingredient Concentration
Posaconazole 50 mg/ml
POPC 40 mg/ml
Histidine 3 mg/n-il
Citric acid monohydrate 0.24 mg/n-il
Propyl gallate 0.01 mg/ml
Butylated hydroxytoluene 0.05 mg/ml
Trehalose 250 mg/ml
Water q.s. ad 1 ml
at a pH of about 6.4.
In some embodiments, the formulation has ingredients comprising:
Ingredient Concentration
Posaconazole 50 mg/ml
POPC 40 mg/ml
Histidine 3 mg/n-il
Citric acid monohydrate 0.24 mg/ml
Alpha-D-tocopherol 0.05 mg/ml
Trehalose 250 mg/n-il
Water q.s. ad 1 ml
at a pH of about 6.5.
In some embodiments, the formulation has a wt. ratio of phospholipid to
posaconazole between about 60:1 and about 1:10.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
In some embodiments, the formulation has a wt. ratio of phospholipid to
posaconazole between about 1:1 and about 1:5.
In some embodiments, the formulation has a wt. ratio of phospholipid to
posaconazole between about 1:1 and about 4:5.
In some embodiments, the formulation has a the wt. ratio of
thermoprotectant to posaconazole between about 300:1 and about 1:10.
In some embodiments, the formulation has a wt. ratio of
thermoprotectant to posaconazole between about 1:1 and about 6:1.
In some embodiments, the formulation has a wt. ratio of
thermoprotectant to phospholipid between about 30:1 and about 1:6.
In some embodiments, the formulation has a wt. ratio of
thermoprotectant to phospholipid between about 5:4 and about 30:4.
In some embodiments, the invention encompasses a method of treating or
preventing an infection inan animal in need thereof which comprises
administering to said animal an effective amount of the formulation. In
some embodiments, the animal is a mammal, a bird, a fish, or a reptile.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
11
In some embodiments, the animal is a mammal, including but not limited
to a human.
In some embodiments, the infection is caused by a fungus or a parasite.
In some embodiments, the infection is selected from the group consisting
of:
oropharyngeal or esophageal candidiasis;
refractory oropharyngeal and esophageal candidiasis;
invasive aspergillosis, candidiasis, fusariosis, scedosporiosis, infections
due to dimorphic fungi, zygomycosis, and invasive infections due to rare
molds and yeasts;
invasive mycoses in patients who are refractory to, or intolerant of, other
therapies;
Candidiasis, invasive mould infections in patients who have undergone
intensive chemotherapy and/or radiation therapy for hematologic
malignancies, bone marrow or peripheral stem cell transplant
conditioning regimens, and patients receiving combination
immunosuppressive therapy for the treatment of acute or chronic graft-
versus-host disease or prevention of sohd organ transplantation;
Chagas disease; and,
Leishmaniasis.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
12
In some embodiments, the invention encompasses a method wherein said
formulation is administered intravenously.
In some embodiments, the invention encompasses a method wherein said
formulation is administered intramuscularly, subcutaneously,
ophthalmically, subconjuctivally, intraocularly, via anterior eye chamber
injection, intravitreally, intraperitoneally, intrathecally, intracystically,
intrapleurally, intranasally, topically, via wound irrigation, intradermally,
intrabuccally, intra-abdominally, intra-articularly, intra-aurally,
i.ntrabronchially, intracapsularly, intrameningeally, intrapulmonarilly, via
inhalation, via endotracheal or endobronchial installation, via direct
installation into pulmonary cavities, intraspinally, intrasynovially,
intrathoracically, via thoracostomy irrigation, vaginally, epidurally,
rectally, intracisternally, intravascularly,intraventricularly,
intraosseously, via irrigation of infected bone, or via application as part of
any admixture with cement for prosthetic devices.
In some embodiments, the formulation further comprises a second active
ingredient selected from one or more of the group consisting of
antifungals such as azoles; amphotericin B; deoxycholate amphotericin
B; flucytosine; terbinafine; antibacterials; antivirals; steroids;
nonsteroidal anti-inflammatory drugs ("NSAIDs"); chemotherapeutics; and
anti-emitics.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
13
In some embodiments, the invention encompasses a method further
comprising administering a second active ingredient selected from one or
more of the group consisting of: antifungals such as azoles; amphotericin
B; deoxycholate amphotericin B; flucytosine; terbinafine; antibacterials;
antivirals; steroids; nonsteroidal anti-inflammatory drugs ("NSAIDs");
chemotherapeutics; and, anti-emitics.
In some embodiments, the formulation is further characterized by
providing a mean maximum plasma concentration (Cmax) of posaconazole
of at least about 467 ng/ml at steady state, and a mean plasma Area
Under the Curve over 24 hours (AUC) value of posaconazole of at least
about 9840 ng=hr/ml at steady state, when said formulation is infused
over about 1 hour to deliver 100 mg of posaconazole, and repeated at an
interval of about 24 hours.
In some embodiments, the formulation is further characterized by
providing a mean maximum plasma concentration (Cn,ax) of posaconazole
of at least about 852 ng/ml at steady state, and a mean plasma Area
Under the Curve over 24 hours (AUC) value of posaconazole of at least
about 24,600 ng-hr/ml at steady state, when said formulation is infused
over about 1 hour to deliver 200 mg of posaconazole, and repeated at an
interval of about 24 hours.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
14
In some embodiments, the formulation is further characterized by
providing, after administration of a dosage of about 100 mg of said
posaconazole, at least one of: a mean plasma half-life in a range of about
14.9 to about 38.4 hours; and a mean plasma steady state volume of
distribution of about 200-500 L.
In some embodiments, the formulation is further characterized by
providing a mean maximum plasma concentration (Cmax) of posaconazole
of at least about 1480 ng/ml at steady state, and a mean plasma Area
Under the Curve over 24 hours (AUC) value of posaconazole of at least
about 24,600 ng=hr/ml at steady state, when said formulation is infused
over about 1 hour to deliver at least 200 mg of posaconazole, and
repeated at an interval of about 24 hours.
In some embodiments, the formulation is further characterized as
providing, after administration of a dosage of about 200 mg of said
posaconazole, at least one of: a mean plasma half-life of about 18.7 to
about 35.5 hours; and a mean plasma steady state volume of distribution
of about 200-500 L.
In some embodiments, the formulation is further characterized as
providing, after administration of a dosage of about 400 mg of said
posaconazole, at least one of: a mean plasma half-life of about 18.5 to
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
about 51.4 hours; and a mean plasma steady state volume of distribution
of about 200-500 L.
In some embodiments, the formulation is further characterized as
providing, after administration of a dosage of about 600 mg of said
posaconazole, at least one of a mean plasma half-life of about 27.2 to
about 50.6 hours; and a mean plasma steady state volume of distribution
of about 200-500 L.
In some embodiments, the formulation is further characterized as
providing a mean posaconazole blood concentration profile substantially
similar to that of Figure 1, when said formulation is infused over about 1
hour to deliver 25-600 mg of posaconazole.
In some embodiments, the formulation is further characterized as
providing a mean posaconazole plasma concentration profile
substantially similar to that of Figure 2, when said formulation is infused
over about 1 hour to deliver 25-600 mg of posaconazole.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cm. to mean posaconazole
plasma Cma,, of between about 1.5 and about 3.8, when a single dose of
said formulation is infused over about 1 hour to deliver 25-600 mg of
posaconazole.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
16
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cmax to mean posaconazole
plasma Cmax of between about 2.1 and about 3.3, when a single dose of
said formulation is infused over about 1 hour to deliver 25 mg of
posaconazole.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cmax to mean posaconazole
plasma Cmax of between about 1.9 and about 3.8, when a single dose of
said formulation is infused over about 1 hour to deliver 50 mg of
posaconazole.
In some embodiments, the formulation is further characterized as
providing a mean posaconazole blood Cmax to mean posaconazole
plasma Cma. of between about 2.2 and about 3.3, when a single dose of
said formulation is infused over about 1 hour to deliver 100 mg of
posaconazole.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cmax to mean posaconazole
plasma Cmax of between about 1.5 and about 3.2, when a single dose of
said formulation is infused over about 1 hour to deliver 200 mg of
posaconazole.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
17
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cmax to mean posaconazole
plasma Cma. of between about 1.7 and about 3.3, when a single dose of
said formulation is infused over about 1 hour to deliver 400 mg of
posaconazole.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood C,nax to mean posaconazole
plasma C. of between about 1.9 and about 3.1, when a single dose of
said formulation is infused over about 1 hour to deliver 600 mg of
posaconazole.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cm. to mean posaconazole
plasma Cm~x of between about 1.2 and about 2.5, at steady state when
said formulation is infused over about 1 hour to deliver 25-600 mg of
posaconazole, and repeated on a 24-hour basis.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cmax to mean posaconazole
plasma Cma. of between about 1.5 and about 2.3, at steady state when
said formulation is infused over about 1 hour to deliver 25 mg of
posaconazole, and repeated on a 24-hour basis.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
18
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cmax to mean posaconazole
plasma Cma., of between about 1.5 and about 2.4, at steady state when
said formulation is infused over about 1 hour to deliver 50 mg of
posaconazole, and repeated on a 24-hour basis.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cma,x to mean posaconazole
plasma Cmax of between about 1.7 and about 2.5, at steady state when
said formulation is infused over about 1 hour to deliver 100 mg of
posaconazole, and repeated on a 24-hour basis.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood C,n. to mean posaconazole
plasma Cmax of between about 1.2 and about 2.0, at steady state when
said formulation is infused over about 1 hour to deliver 200 mg of
posaconazole, and repeated on a 24-hour basis.
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood C,nax to mean posaconazole
plasma Cmax of between about 1.2 and about 2.2, at steady state when
said formulation is infused over about 1 hour to deliver 400 mg of
posaconazole, and repeated on a 24-hour basis.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
19
In some embodiments, the formulation is further characterized as
providing a ratio of mean posaconazole blood Cmax to mean posaconazole
plasma Cmx of between about 1.3 and about 1.7, at steady state when
said formulation is infused over about 1 hour to deliver 600 mg of
posaconazole, and repeated on a 24-hour basis.
In some embodiments, the water in the formulation has been removed by
lyophilization.
In some embodiments, the animal treated is human, while in other
embodiments the animal treated is non-human.
In some embodiments, the formulation is one that is bioequivalent to a
formulation disclosed herein.
In some embodiments, the method further comprises administering a
bolus loading dose of said formulation and then administering an
intravenous maintenance dose of said formulation.
In some embodiments, the method comprises administering to said
animal an effective amount of posaconazole to provide a mean maximum
plasma concentration (CmaA of posaconazole of at least about 467 ng/ml
at steady state, and a mean plasma Area Under the Curve over 24 hours
(AUC) value of posaconazole of at least about 9840 ng=hr/ml at steady
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
state, when said formulation is infused over about 1 hour to deliver 100
mg of posaconazole, and repeated at an interval of about 24 hours.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 shows posaconazole mean blood concentration-time profiles in
healthy volunteers after 1 hr intravenous infusions of 25, 50, 100, 200,
400, and 600 mg posaconazole.
FIG. 2 shows posaconazole mean plasma concentration-time profiles in
healthy volunteers after 1 hr intravenous infusions of 25, 50, 100, 200,
400, and 600 mg posaconazole.
FIG. 3 shows posaconazole mean plasma and blood concentration-time
profiles in healthy volunteers after 1 hr intravenous infusion of 25 mg
posaconazole.
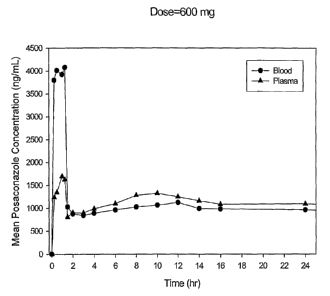
FIG. 4 shows posaconazole mean plasma and blood concentration-time
profiles in healthy volunteers after 1 hr intravenous infusion of 600 mg
posaconazole.
DETAILED DESCRIPTION OF THE INVENTION
The present invention encompasses formulations suitable for parenteral
administration, e.g., by injection, for treating an infection. These
formulations comprise a suspension of posaconazole, stabilized by a
phospholipid, in a mixture comprising water, a thennoprotectant, and a
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
L1
buffer system. Since posaconazole is minimally soluble in water, a
suspension formulation is advantageous. Phospholipids have been found
to be effective surfactants in forming stable suspensions of posaconazole
in water or an aqueous medium.
These phospholipids can degrade when subjected to the temperature
excursions experienced during terminal sterilization (e.g., autoclaving), a
step which is necessary to assure the sterility of any injectable
formulation. Thus, a thermoprotectant is used to prevent agglomeration
and crystal growth of the posaconazole particles during autoclaving.
Parenteral buffer systems are typically designed to be at physiological pH
of about 7.4. Phospholipids are known to be stable at a pH range of
about 6 to about 7. Furthermore, pH adjustment of injectable
formulations can be necessary to achieve physiological compatibility, and
thus, for example, to rr>inirn. ize injection-site irritation. In addition,
the
rate of phospholipid hydrolysis can be temperature-sensitive. Thus, in
the present formulations, the buffer systems are designed to meet
physiological pH requirements, and to maintain the temperature jpH-
dependent chemical stability of the phospholipid in the formulation
during high temperature excursions (such as experienced during
autoclaving), and throughout shelf life.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
22
In accordance with the above, it was found that POPC, an ingredient that
acts as a suspension stabilizer, was sensitive to autoclaving. Certain
buffer systems were found to control degradation of POPC-containing
posaconazole formulations during autoclaving. For example, such
formulations were found to be stable after 20 minutes of autoclaving at
121 oC. In addition, these buffer systems stabilize such formulations
during storage at 40C to 250C for at least 18 months following
autoclaving. Similarly, other phospholipids that are similar to POPC
could be used to stabilize the formulations disclosed herein. For example,
unsaturated phospholipids with an acyl chain length ranging from C12 to
C20 wherein the degree of unsaturation of the acyl chain ranges from 1 to
4; as well as saturated phospholipids with an acyl chain length ranging
from C12 to Cls are useful according to the present invention.
Examples of useful unsaturated phospholipids include:
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine ("POPC"),
1,2-Myristoleoyl-sn-Glycero-3-Phosphocholine
0
0
H +~
V~
/~ -
0
e 0
1, 2-Palmitoleoyl-sn-Glycero-3-Phosphocholine
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
23
0
Q H
0
0
1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (DOPC)
i}
0
H i~
0~~'~.~~~
1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine (DOPE)
Q
0
11 H
~ ~~"-'~O'p 0~~~'~
FI 0 O
1, 2-Linoleoyl-sn-Glycero-3-Phosphochohne
0
0
H
-P.-o r~ ~ - -
0
0
and
1-Oleoyl-2-Myristoyl-sn-Glycero-3-Phosphocholine
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
24
fl H fl
$ 0
or combinations thereof.
Examples of saturated phosphohpids include:
1,2-Dilauryl-sn-Glycero-3-Phosphocholine (DLPC)
G
0
H G
0
e 0
1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine (DMPC)
0
11 H 0
fl-'"' ~~J v
fl
1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine (DPPC)
0
0 H 0
~,,,~1,='~õ~fl'" Q
0
o p
and
1,2-Stearoyl-sn-Glycero-3-Phosphocholine (DSPC)
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
0
~ ~ 0
0- P,
0~ ~
0
or combinations thereof.
Unsaturated phospholipids are known be to prone to oxidation. To
prevent such oxidation, an antioxidant can be employed. In some
embodiments, the antioxidant comprises propyl gallate, preferably at a
concentration of about 0.02 to about 0.005 mg/ml. In other
embodiments, the antioxidant comprises butylated hydroxytoluene,
preferably at a concentration of about 0.1 to about 0.02 mg/ml. In
related embodiments, the antioxidant comprises propyl gallate, preferably
at a concentration of about 0.02 to about 0.005 mg/ml, in combination
with butylated hydroxytoluene, preferably at a concentration of about 0.1
to about 0.02 mg/ml. In yet other embodiments, the antioxidant
comprises alpha-D-tocopherol, preferably at a concentration of about 0.5
to about 0.01 mg/rnl.
The inventors have found certain ratios of components to result in
advantageous formulations. For example, the weight ratio of
phospholipid to posaconazole is preferably between about 1:0.1 and about
1:10, more preferably, between about 1:1 and about 1:5, still more
preferably, between about 1:1 and about 4:5. The weight ratio of
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
26
thermoprotectant to posaconazole is preferably between about 0.5:1 and
about 6:1, more preferably, between about 2:1 and about 6:1. The weight
ratio of thermoprotectant to phosphohpid is preferably, between about
20:1 and about 5:4, more preferably, between about 20:4 and about 30:4.
The formulations of the present invention comprise a suspension of sohd
particles of posaconazole of specific particle size distribution in an
aqueous phase. The particle size distribution displayed in the suspended
particles -is critical for physiological compatibility, syringeabihty,
physical
stability of the suspension, re-suspendability, and for pharmacokinetic
characteristics and bio-distribution (Le., sequestration within specific
bodily tissues). Since these characteristics are critical to the formulation
as dehvered to the patient, it is important that processes that contribute
to changes in particle size distribution after micronization are controlled.
Such processes can include agglomeration during autoclaving, and de-
suspension due to temperature excursions and/or agitation experienced
during shipping and storage. It is the particle size distribution in the
formulation as ready for administration to the patient that influences
phannacokinetic characteristics and bio-distribution.
The inventors of the present invention have determined that for injectable
formulations of posaconazole, these characteristics are brought within
advantageous ranges with particle size distributions whose median values
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
27
are between about 1.0 to about 8.0 microns, preferably, between about
1.0 to about 5.0 microns, more preferably between about 1.2 to about 4.5
microns. In each case, the particle size distributions display not more
than about 3000 particles of 10 microns or greater size and not more
than about 300 particles of 25 microns or greater size.
In the injectable formulations of the present invention, which include
POPC, it has been found useful to maintain a pH range of between about
3.0 and about 9.0, preferably between about 6.0 and about 8.0, and more
preferably between about 6.4 and about 7.6.
The inventors have found that certain organic buffers, e.g., histidine and
citric acid, are more advantageous in controlling the pH-related
degradation of POPC in the formulation. Components used in pH
adjustment systems can also function as components of the buffer
system, after pH adjustment has been achieved. Non-limiting examples of
pH adjustment system components that function in this way include
sodium hydroxide, hydrochloric acid, and phosphoric acid.
Anti-Infective Applications
The present invention encompasses methods of prevention and treatment
of a variety of infections caused by a broad spectrum of infectious agents.
The term "infection" is understood to include, but not be limited to, those
disease states caused by molds, yeasts and other infectious agents, such
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
28
as: Candida, dermatophytes, Dimorphics, Dematiaceous (e.g., Alternaria
and Bipolaris), Aspergillus, Acremonium, Basidiomycetes, Bjerkandera,
Coprinus, Paecilomyces, Microsporum, Trichophyton, Pseudallescheria,
Schizophyllum, Crytococcus, Histoplasma, Blastomyces, Coccidioides,
Fusarium, Exophiala, Zygomycocetes (e.g., Mucor, Rhizopus, and
Rhizomucor), Kluyveromyces, Saccharomyces, Yarrowia, Pichia,
Epidermophyton, Paracoccidioides, Scedosporium, Apophysomyces,
Curvularia, Penicillium, Fonsecaea, Wangiella, Sporothrix, Pneumocystis,
Trichosporon, Absidia, Cladophialophora, Ramichloridium,
Syncephalastrum, Madurella, Scytalidium, Leshmania, protozoa, bacteria,
gram negatives, gram positives, anaerobes, including Legionella Borrelia,
Mycoplasma, Treponema, Gardneralla, Trichomononas and Trypanosoma.
The present invention is intended to treat both opportunistic and non-
opportunistic infections, where the term "opportunistic" as used herein
denotes those infections caused by organisms capable of causing a
disease only in a host whose resistance is lowered, e.g., by chemotherapy
or H.I.V.
In particular, posaconazole is useful in the prevention and/or treatment
of the following disease states:
Initial (first line) treatment of oropharyngeal or esophageal candidiasis;
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
29
Salvage therapy of azole-refractory oropharyngeal and esophageal
candidiasis (e.g., in patients who have failed oral fluconazole and/or
itraconazole) ;
Initial treatment of invasive aspergillosis, candidiasis, fusariosis,
scedosporiosis, infections due to dimorphic fungi (e.g.; cryptococcosis,
coccidioidomycosis, paracoccidioidomycosis, histoplasmosis,
blastomycosis), zygomycosis, and invasive infections due to rare
moulds and yeasts;
Salvage therapy for invasive mycoses in patients who are refractory to
or intolerant of other therapies (e.g., amphotericin B, lipid formulations
of amphotericin B, caspofungin, voriconazole and/or itraconazole);
Prevention of invasive Candidiasis, invasive mould infections (including
zygomycosis and aspergillosis) in patients at high risk, including
patients who have undergone intensive chemotherapy and/or radiation
therapy for hematologic malignancies, bone marrow or peripheral stem
cell transplant conditioning regimens, and patients receiving
combination immunosuppressive therapy for the treatment of acute or
chronic graft-versus-host disease or prevention of solid organ
transplantation;
Chagas disease (Trypanosomiasis due to T. cruzi) including acute and
chronic forms; and,
Leishmaniasis, including visceral and localized forms.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
Administrati.on
Inmmuno-suppressant therapy (e.g., chemotherapy, radiation therapy,
myeloablative conditioning regimens) often results in one or more of the
above-referenced infections. The present invention encompasses the
administration of a posaconazole formulation adjunctive to immuno-
suppressant therapy, wherein the posaconazole formulation functions
prophylactically with regard to opportunistic infections including the
above-referenced disease states.
The present invention encompasses a variety of modes of administration
to any part, organ, interstice or cavity ofan animal's body that is subject
to an infection. A non-limiting set of examples of modes by which the
posaconasole formulations of the present invention may be administered
includes: intravenously, intramuscularly, subcutaneously, ,
ophthalmically, subconjuctivally, intraocularly, via anterior eye chamber
injection, intravitreally, intraperitoneally, intrathecally, intracystically,
intrapleurally, intranasally, topically, via wound irrigation, intradermally,
intrabuccally, intra-abdominally, intra-articularly, intra-aurally,
intrabronchially, intracapsularly, intrameningeally, intrapulmonarilly, via
inhalation, via endotracheal or endobronchial installation, via direct
installation into pulmonary cavities, intraspinally, intrasynovially,
intrathoracically, via thoracostomy irrigation, vaginally, epidurally,
rectally, intracisternally, intravascularly,intraventricularly,
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
31
intraosseously, via irrigation of infected bone, and via application as part
of any admixture with cement for prosthetic devices.
Co-formulations comprising combinations of posaconazole and at least
one other active ingredient are also within the scope of the present
invention. Non-limiting examples of such active ingredients include:
antifungals such as echinocandins (including caspofungin, micafungin,
and anidulafungin) and azoles (including voriconazole, itraconazole,
fluconazole, ketoconazole, ravuconazole); amphotericin B; deoxycholate
amphotericin B; flucytosine; and terbinafine.
Also within the scope of this invention are combinations with an
antibacterial, antiviral, steroid, or nonsteroidal anti-inflammatory drugs
("NSAIDs"), chemotherapeutics, and/or anti-emitics. Similarly, co-
administration of posaconazole with at least one of the above active
ingredients, aside from within a single formulation, is also within the
scope of the present invention.
Also within the scope of the present invention are a variety of dosing
regimens, each consisting of a frequency of dosing and a duration of
administration. Preferred frequencies of dosing include once every 12, 24,
36 and 48 hours. Preferred durations of administration are within the
range of 30 minutes to 4 hours, more preferably, 1 to 2 hours. Also
included within the scope of preferred administration is bolus dosing, at
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
32
various rates and various doses, and combinations of a bolus loading
dose, or several bolus loading doses, with an intravenous infusion
maintenance dose that provides therapeutic plasma concentration ranges
similar to or exceeding those described in Table 14 in, fra
As used herein, the following terms shall have the definitions set forth
below.
As used herein, the phrase "phospholipid" refers to a lipid compound that
yields on hydrolysis phosphoric acid, an alcohol, fatty acid and a
nitrogenous base. Examples include natural and, synthetic
phoshpholipids, which include lecithin, cephalin, sphingomyelin and 1-
palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine ("POPC").
As used herein, the phrase "natural phospholipid" refers to a
phospholipid occurring in nature, or derived from a natural source. Non-
]iuniting examples of natural phospholipids include egg phospholipids, soy
phospholipids, and animal tissue phospholipids. Combinations of more
than one natural phospholipid are within the scope of the present
invention.
As used herein, the phrase "synthetic phospholipid" refers to a man-made
phospholipid. Non-limiting examples of synthetic phospholipids include
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1, 2-oleoyl-sn-
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
33
glycero-3-phosphocholine (DOPC), 1,2-Dilauryl-sn-Glycero-3-
Phosphocholine (DLPC), 1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine
(DMPC), 1,2-Dipalrnitoyl-sn-Glycero-3-Phosphocholine (DPPC) and 1,2-
Stearoyl-sn-Glycero-3-Phosphocholine (DSPC). Combinations of more
than one synthetic phospholipid are within the scope of the present
invention.
As used herein, the phrase "buffer system" refers to a buffer comprising
one or more components that maintains a particular pH range. Non-
limiting examples of suitable buffer systems include: phosphoric acid;
glycine; sodium citrate; histidine; citric acid; acetic acid; tromethamine;
ammonium sulfate; and combinations thereof. The aforementioned
components are understood to include the salts, hydrates and solvates
thereof. Thus, for example, phosphoric acid includes the sodium
phosphate or potassium phosphate salts, among other salts. Preferred
buffer systems include sodium phosphate monobasic, sodium phosphate
dibasic, or a combination thereof. More preferred buffer systems include
sodium phosphate monobasic monohydrate, sodium phosphate dibasic
anhydrous, or a combination thereof. As used herein, the phrase "organic
buffer" refers to a buffer comprising at least one organic compound. Non-
limiting examples of suitable organic buffers include: glycin.e;, sodium
citrate; histidine; citric acid; acetic acid; and combinations thereof.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
34
As used herein, the term "antioxidant" refers to an agent that hinders
oxidation. Exemplary antioxidants include propyl gallate, butylated
hydroxytoluene, and alpha-D-tocopherol.
As used herein, the phrase "median particle size" refers to the particle size
present in the volume-weighted 50th percentile, as ascertained by
Malvern , Sympatec , or Horibe laser diffraction particle size analysis.
Particle sizes are measured throughout, and at the termination of, the
shelf life, typically up to 24 months after manufacture, when held at
either refrigerated or room temperatures. Particle sizes are also measured
and maintained when the formulation is diluted into large volume
parenterals, e.g., 5% dextrose or water for injection.
As used herein, the phrase "initial median particle size" refers to the
particle size present within 1 week after a specified timepoint. For
example, the initial median particle size after autoclaving refers to the
median particle size present within 1 week after autoclaving has been
completed.
As used herein, the term "autoclaving" refers to sterilization by the
terminal steam sterilization method. For example, autoclaving for 20
minutes at 1210C suffices to sterilize the posaconazole formulations
disclosed herein.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
As used herein, the phrase "thermoprotectant" refers to an agent that
stabilizes the phospholipid during temperature excursions. In the
present invention, a thermoprotectant is used to preserve the
phospholipid, which is necessary to control crystal growth and
aggolomeration of the posaconazole particles during autoclaving.
Thermoprotectants are typically water soluble polyhydroxyl compounds.
For example, trehalose is a thermoprotectant agent that may be used in
conjunction with posaconazole. Others include maltose, sorbitol,
dextrose, sucrose, lactose and mannitol.
As used herein, the term "prodrug" refers to a compound that is a drug
precursor which, upon administration to a subject, undergoes chemical
conversion by metabolic or chemical processes to yield posaconazole or a
salt and/or solvate thereof.
As used herein, the term."solvate" refers to a physical association between
a compound with one or more solvent molecules. This physical
association involves varying degrees of ionic and/or covalent bonding,
including hydrogen bonding. In certain instances, the solvate will be
capable of isolation, for example, when one or more solvent molecules are
incorporated in the crystal lattice of the crystalline solid. The term
"solvate" encompasses both solution-phase and isolatable solvates. Non-
limiting examples of suitable solvates include hydrates, ethanolates, and
methanolates.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
36
As used herein, the term "injectable" means adapted to parenteral
administration.
As used herein, the term "fungus" means one of the diverse morphologic
forms of yeasts and molds. Fungi include Candida, dermatophytes,
Dimorphics, Dematiaceous (e.g., Alternaria and Bipolaris), Aspergillus,
Acremonium, Basidiomycetes, Bjerkandera, Coprinus, Paecilomyces,
Microsporum, Trichophyton, Pseudallescheria, Schizophyllum,
Crytococcus, Histoplasma, Blastomyces, Coccidioides, Fusarium,
Exophiala, Zygomycocetes (e.g., Mucor, Rhizopus, and Rhizomucor),
Kluyveromyces, Saccharomyces, Yarrowia, Pichia, Epidermophyton,
Paracoccidioides, Scedosporium, Apophysomyces, Curvularia, Penicillium,
Fonsecaea, Wangiella, Sporothrix, Pneumocystis, Trichosporon, Absidia,
Cladophialophora, Ramichloridium, Syncephalastrum, Madurella,
Scytalidium, Leshmania, gram negatives, gram positives, Mycoplasma,
Treponema, Gardneralla, and Tri.chomononas.
As used herein, the term "Dematiaceous" means dark conidia and/or
hyphae, and includes as non-hmiting examples Alternaria and Bipolaris.
As used herein, the term "Zygomycocete" means a class of fungi
characterized by sexual reproduction resulting in the formation of
zygospore, and asexual reproduction by means of nonmotile spores called
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
37
sporangiospores or conidia, and includes as non-limiting examples
Mucor, Rhizopus, and Rhizomucor.
As used herein, the term "anaerobe" means a microorganism that can live
and grow in the absence of oxygen, and includes as non-limiting examples
Legionella Borrelia, Mycoplasma, Treponema, Gardneralla, and
Trichomononas.
As used herein, the term "parasite" means an organism that lives on or in
another and draws its nourishment therefrom. Parasites include
Leshmania and Trypansoma, among others.
As used herein, the term "antifungal" means an agent having activity
against one or more fungi, and includes echinocandins such as
caspofungin, micafungin, and anidulafungin.
As used herein, the term "azole" means divinyle ; ine, and includes
voriconazole, itraconazole, fluconazole, ketoconazole, ravuconazole:
As used herein, the term "mean maximum concentration (Cmax)" when
followed by the term "at steady state" means that mean maximum
concentration value that occurs after administration of a sufficient
number of repeated doses of the formulation to generate maximum blood
or plasma concentrations that are substantially equivalent to one another
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
38
in value. Thus, the subsequent maximum concentration values are no
longer rising, but rather each peak achieves substantially the same
maximum value as the previous one and the next one.
As used herein, the term "animal" is understood to include humans, non-
human manunals, fish, birds and reptiles.
As used herein, the term "bioequivalent" is understood as having that
meaning assigned to the term by the U.S. Food & Drug Administration.
"Bioequivalence means the absence of a significant difference in the rate
and extent to which the active ingredient or active moiety in
pharmaceutical equivalents or pharmaceutical alternatives becomes
available at the site of drug action when administered at the same molar
dose under similar conditions in an appropriately designed study." 21
CFR 320.1(e). Methodologies for determining bioequivalence are given in
"Guidance for Industry: Statistical Approaches to Estabhshing
Bioequivalence," U.S. Department of Health and Human Services,
Food and Drug Administration, Center for Drug Evaluation and Research
(CDER) June, 2001.
EXAMPLES
The following non-limiting examples illustrate certain aspects of the
invention.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
39
Exemplary formulations of posaconazole in conjunction with POPC and
trehalose using various buffer systems are detailed below in Tables 1-3.
These formulations provide ranges for buffer systems that maintain a
particular pH range.
Table 1.
Representative posaconazole formulations at a pH range of 6.4-7.4
Function In redient Concentration range
Active Posaconazole 50 m /ml
Stabilizer POPC 40 m /ml
Buffer Glycine 3.5 - 10.5 m/ml
Buffer Sodium citrate dih drate 4- 10.2 m/ml
Buffer Citric acid monohydrate 0.01 - 0.02 mg/nil
Stabilizer Trehalose 250 m /ml
Solvent Water g.s. ad 1 ml
Table 2.
Representative posaconazole formulations at a pH range of 6.4-6.6
Function Ingredient Concentration range
Active Posaconazole 50 m /ml
Stabilizer POPC 40 m /ml
Buffer Glycine 1.5 - 4.5 m/ml
Buffer Citric acid monohydrate 0.12 - 0.36 m/ml
Stabilizer Trehalose 250 m /ml
Solvent Water g.s. ad 1 nil
Table 3.
Representative posaconazole formulations at a pH range of 6.6-6.8
Function Ingredient Concentration range
Active Posaconazole 50 m /ml
Stabilizer POPC 40 m /ml
Buffer Histidine 1.5 - 4.5 m/ml
Buffer Ammonium sulfate 1 - 3 m/ml
Buffer Hydrochloric acid 0.1 - 0.3 m/ml
Stabilizer Trehalose 250 m /ml
Solvent Water g.s. ad 1 ml
An exemplary posaconazole formulation for each of the buffer systems
described in Tables 1-3 is provided in Examples 1-3, respectively.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
Exam le 1
Ingredient Concentration
Posaconazole 50 m /ml
POPC 40 m /ml
Glycine 7 m /ml
Sodium citrate dihydrate 8 m/ml
Trehalose 250 m /ml
Water g.s. ad 1 ml
Of note, the pH is 7.4 in Example 1.
Example 2
Ingredient Concentration
Posaconazole 50 m /ml
POPC 40 m /ml
Histidine 3 m /ml
Citric acid monohydrate 0.24 m/ml
Trehalose 250 m /ml
Water g.s. ad 1 ml
Of note, the pH is 6.4 in Example 2.
Example 3
Ingredient Concentration
Posaconazole 50 m /ml
POPC 40 m /ml
Histidine 3 m /ml
Arnmonium sulfate 2 m /ml
Hydrochloric acid 0.2 m/ml
Trehalose 250 m /ml
Water g.s. ad 1 ml
Of note, the pH is 6.6 in Example 3.
In addition, exemplary posaconazole fonnulations that include
antioxidant are described in Examples 4-6.
Example 4
Ingredient Concentration
Posaconazole 50 m /ml
POPC 40 m /ml
Glycine 7 m /ml
Sodium citrate dihydrate 8 m/ml
Propyl gallate 0.01 m/ml
Bu lated h dro oluene 0.05 m/ml
Trehalose 250 m /ml
Water .s. ad 1 ml
Of note, the pH is 7.4 in Example 4.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
41
Example 5
Ingredient Concentration
Posaconazole 50 m /rnl
POPC 40 m /ml
Histidine 3 m /ml
Citric acid monohydrate 0.24 m/ml
Propyl gallate 0.01 m/ml
Butylated h dro oluene 0.05 m/ml
Trehalose 250 m /ml
Water g.s. ad 1 ml
The pH is 6.4 in Example 5.
Example 6
Ingredient Concentration
Posaconazole 50 m /ml
POPC 40 m /ml
Histidine 3 m /ml
Citric acid monohydrate 0.24 m/ml
AI ha-D-toco herol 0.05 m/ml
Trehalose 250 m /ml
Water g.s. ad 1 ml
The pH is 6.5 in Example 6.
Example 7 is a preferred embodiment of the present invention.
Example 7
Ingredient Concentration
Posaconazole 50 m /ml
POPC 40 m /ml
Sodium Phosphate, Monobasic, 0.345 mg/ml
Monohydrate, USP
Sodium Phosphate, Dibasic, 1.065 mg/ml
Anhydrous, USP
Trehalose 250 m /ml
Sodium Hydroxide, NF (1.0 N) for pH adjustment
Phosphoric Acid, NF (20%w/w) for pH ad'ustment
Water for injection, USP gs 1 ml
The pH is 7.2 in Example 7.
The following is an exemplary placebo formulation wherein the pH is 6.4.
This exemplary placebo formulation was utilized in the comparative
stability data study described below.
In redient Concentration
Placebo
POPC 40 m /ml
Glycine 1 m /ml
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
42
Sodium citrate dihydrate 0.3 m/ml
Citric acid monohydrate 0.016 m/ml
Trehalose 250 m /ml
Water g.s. ad 1 ml
Comparatiue stability data study
The stability of POPC in formulation Examples 1-3 was compared with the
aforementioned exemplary placebo both before and after autoclaving for
20 min at 1210C. In addition, posaconazole stability, particle size, pH,
and a physical observation were ascertained for each formulation before
and after autoclaving. Each formulation was also examined following an
additional period of storage at 40C, 250C, and 40oC (Le., 4oC 20C at 60%
5% relative humidity; 250C 20C at 60% 5% relative humidity; and
400C 20C at ambient relative humidity, respectively) for 1 month, 3
months, and 6 months after autoclaving. Notably, particle size was
determined using the Malvern laser diffraction particle size analysis
technique. Particle sizes are characterized by values for median ("50th
percentile") and maximum ("100th percentile"). The stability data from
these comparative studies are compiled below for formulations reflected in
Examples 1-6, shown in Tables 4-9, respectively.
Table 4. Stability data for osaconazole formulation Example 1
Interval/ Posaconazole POPC Particle size pH Physical
Condition (mg/m1) (mg/nil) (microns) Observation
50th 100th
percentile percentile
Initial - 51.3 41.4 1.11 3.77 7.4 Milky white
Before
Autoclaving
Initial - 50.8 40.9 1.49 6.63 7.3 Milky white
After
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
43
Autoclaving
1 4 C 50.2 39.0 1.48 5.49 7.2 Milky white
month 25 C 50.7 39.2 1.48 5.49 7.2 Milky white
40 C 50.6 39.1 1.49 5.49 7.1 Milky white
3 4 C 55.1 41.8 1.47 5.49 7.2 Milky white
months 25 C 55.4 41.5 1.48 6.63 7.2 Milky white
40 C 55.4 40.0 1.49 6.63 7.1 Milky white
6 4 C 51.7 44.4 1.44 4.88 7.3 Milky white
months 25 C 50.3 42.3 1.50 5.69 7.3 Milky white
40 C 51.5 36.0 1.57 5.69 7.3 Milky white
Table 5. Stability data for osaconazole formulation Exam ple 2
Interval/ Posaconazole POPC Particle size pH Physical
Condition (mg/ml) (mg/ml) (microns) Observation
50th 1OOth
percentile percentile
Initial - 46.4 38.2 1.41 6.63 6.4 Milky white
Before
Autoclaving
Initial - 46.3 38.2 1.76 6.63 6.4 Milky white
After
Autoclaving
1 4 C 45.8 37.9 1.70 6.63 6.4 Milky white
month 25 C 45.3 37.0 1.70 6.63 6.4 Milky white
40 C 45.8 37.4 1.72 6.63 6.4 Milky white
3 4 C 44.8 36.1 1.69 6.63 6.4 Milky white
months 25 C 45.9 36.8 1.70 6.63 6.4 Milky white
40 C 45.6 35.7 1.76 35.98 6.4 Milky white
6 4 C 44.1 38.8 1.65 6.63 6.6 Milky white
months 25 C 46.1 40.1 1.71 6.63 6.6 Milky white
40 C 46.1 40.1 1.70 6.63 6.6 Milky white
Table 6. Stability data for osaconazole formulation Example 3
Interval/ Posaconazole POPC Particle size pH Physical
Condition (mg/mi) (mg/mi) (microns) Observation
50th 100th
percentile percentile
Initial - 46.1 36.7 1.39 5.49 6.6 Milky white
Before
Autoclaving
Initial - 45.9 36.2 1.75 6.63 6.6 Milky white
After
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
44
Autoclaving
1 4 C 45.3 34.6 1.76 6.63 6.5 Milky white
month 25 C 44.9 34.4 1.76 6.63 6.5 Mi1ky white
40 C 44.9 34.5 1.75 6.63 6.5 Milky white
3 4 C 46.9 35.0 1.77 6.63 6.5 Milky white
months 25 C 46.9 34.9 1.78 6.63 6.5 Milky white
400C 47.5 34.5 1.75 6.63 6.5 Milky white
6 4 C 47.0 35.3 2.18 6.63 6.6 Milky white
months 25 C 46.3 35.1 1.75 5.69 6.5 Milky white
40 C 49.4 32.5 2.03 6.63 6.6 Milky white
Table 7. Stability data for osaconazole formulation Exam le 4
Interval/ Posaconazole POPC Particle size pH Physical
Condition (mg/ml) (mg/ml) (microns) Observation
50th lOOth
percentile percentile
Initial - 65.4 50.3 1.21 5.49 7.3 Milky white
Before
Autoclaving
Initial - 65.5 50.2 1.66 6.63 7.2 Milky white
After
Autoclaving
1 4 C 65.2 50.2 1.65 6.63 7.2 Milky white
month 25 C 65.1 50.4 1.64 6.63 7.2 Milky white
40 C 67.1 50.6 1.67 6.63 7.2 Milky white
3 4 C 68.1 50.9 1.64 6.63 7.4 Milky white
months 25 C 68.4 51.0 1.64 6.63 7.4 Milky white
40 C 69.5 49.2 1.67 29.82 7.3 Milky white
6 4 C 66.7 53.3 1.61 5.68 7.2 Milky white
months 25 C 64.9 52.6 1.54 4.88 7.0 Milky gray
40 C 65.4 47.3 1.64 56.23 6.8 Milky white
Table S. Stability data for osaconazole formulation Example 5
Interval/ Posaconazole POPC Particle size pH Physical
Condition (mg/mi) (mg/ml) (microns) Observation
50th looth
percentile percentile
Initial - 50.8 39.7 1.61 6.63 6.5 Milky white
After
Autoclaving
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
1 4 C 50.9 39.6 1.61 6.63 6.5 Milky white
month 25 C 51.1 39.8 1.60 6.63 6.5 Milky white
400C 50.7 39.4 1.62 6.63 6.5 Milky white
3 4 C 53.0 40.5 1.62 6.63 6.6 Milky white
months 25 C 53.1 40.6 1.62 6.63 6.6 Milky white
40 C 51.9 39.0 1.62 6.63 6.7 Milky white
6 4 C 54.2 46.2 1.59 5.69 6.5 Milky white
months 25 C 53.2 44.6 1.59 5.69 6.5 Milky white
40 C 52.2 41.2 1.58 5.69 6.5 Milky white
Table 9. Stability data for osaconazole formulation Exam ple 6
Interval/ Posaconazole POPC Particle size pH Physical
Condition (mg/ml) (mg/ml) (microns) Observation
50tb lOOth
percentile percentile
Initial - 46.8 36.5 1.32 6.63 6.5 Milky white
Before
Autoclaving
Initial - 46.5 36.4 1.61 6.63 6.5 Milky white
After
Autoclaving
1 4 C 46.2 35.8 1.61 6.63 6.5 Milky white
month 25 C 47.6 36.9 1.60 6.63 6.5 Milky white
40 C 47.3 36.4 1.62 6.63 6.5 Milky white
3 4 C 48.3 36.8 1.63 6.63 6.5 Milky white
months 25 C 48.6 37.1 1.62 6.63 6.6 Milky white
40 C 49.1 36.4 1.61 6.63 6.4 Milky white
6 4 C 47.9 36.3 1.60 5.69 6.5 Milky white
months 25 C 47.3 36.1 1.60 5.69 6.5 Milky white
40 C 48.8 34.0 1.60 5.69 6.5 Milky white
Activities of posaconazole against a broad spectrum of infectious agents
have been tested in vitro. Tables 10 and 11 display a subset of the results
of this in vitro testing, showing some of those infectious agents against
which posaconazole is most active.
46
0
Table 10. Geometric Mean MIC and MIC 90 Values /mL for Strains Tested (n) in
Posaconazole (POS), Fluconazole (FLU) and Itraconazole (ITZ) POS FLZ ITZ
Organism n Mean MIC[90] n Mean MIC[90] n Mean MIC 90
As er illus flavus 241 0.079 0.25 94 220.898 256.0 203 0.213 1.0
As er illus fumi atus 2,158 0.118 0.5 735 247.922 512.0 1560 0.397 1.0
As er illus nidulans 33 0.055 0.25 8 76.109 (32.0-128.0) 21 0.186 0.5
As er illus niger 171 0.195 0.5 64 234.753 256.0 153 0.834 2.0
As er illus s dowii 8 0.177 (0.031-0.5) 7 115.933 (64.0-256.0) 8 0.500 (0.125-
2.0)
As er illus terreus 100 0.052 0.25 37 208.327 256.0 56 0.229 0.5
As er illus ustus 7 1.641 (0.25-8.0) 7 172.275 64.0-256.0 7 0.906 (0.125-2.0)
Candida albicans 8,847 0.037 0.25 7,879 0.415 2.0 7,686 0.064 0.25
Candida dubliniensis 339 0.062 0.25 231 0.454 32.0 197 0.107 0.5
Candida glabrata 2,507 0.672 2.0 2,197 9.719 64.0 2,188 0.853 4.0 N
Candida krusei 496 0.335 1.0 386 32.521 64.0 383 0.576 1.0 0
Candida parapsilosis 2,126 0.073 0.125 1,916 0.910 2.0 1,903 0.161 0.5 OD
Cryptococcus laurentii 5 0.095 (0.008-0.5) 3 5.040 (4.0-8.0) 3 0.397 0.25-0.5
W
Cryptococcus neoformans 1,427 0.119 0.25 1,237 1.781 8.0 1,269 0.444 4.0 0
Coccidioides immitis 50 0.304 1.0 25 16.450 32.0 50 0.198 0.25 0
Fonsecae pedrosoi 4 0.250 (0.25) 2 64.000 (64.0) 4 0.063 (0.008-0.5)
Histoplasma capsulatum 58 0.038 0.25 8 19.027 (8.0-32.0) 53 0.018 0.063
Pseudallescheria bo dii 66 0.365 1.0 41 41.237 128.0 61 0.506 1.0
Alternaria s 13 0.101 0.25 0 13 0.326 1.0
Exo hiala dermatidis 3 0.125 (0.125) 2 8.000 (8.0) 2 1.000 (1.0)
Exo hiala 'eanselmei 10 0.287 0.5 0 10 0.467 1.0
Exo hiala moniliae 2 0.016 (0.016) 0 2 0.031 (0.031)
Fusarium spp 38 2.319 16.0 27 249.512 256.0 30 13.300 16.0
(0.031-
Ramichloridium obovoideum 2 0.044 0.063 2 22.627 (16.0-32.0) 2 0.016 (0.016)
Rhizomucor s 2 0.016 (0.016) 0 2 0.016 (0.016) Mucor s 17 0.694 16.0 10
207.937 256.0 12 2.378 16.0
Rhizo us s 29 1.000 4.0 19 229.461 256.0 21 3.281 16.0
Candida famata 44 0.125 0.5 44 4.084 32.0 27 0.348 1.0
Candida guilliermondii 143 0.178 0.5 106 4.000 32.0 82 0.479 1.0
Candida lusitaniae 306 0.048 0.125 221 0.627 2.0 202 0.216 1.0
Candida kefyr 53 0.081 0.25 51 0.500 4.0 39 0.188 0.5
47
POS FLZ ITZ
Organism n Mean MIC 90 n Mean MIC[90] n Mean MIC 90
Candida ru osa 26 0.039 0.5 21 3.391 16.0 17 0.196 4.0
Candida tro icalis 1,645 0.081 0.25 1,476 0.961 4.0 1,450 0.167 0.5
Candida zeylanoides 4 0.031 ( ~ 25 4 0.354 (0.125-1.0) 4 0.105 (0.031-0.5)
Kluyveromyces marxianus 6 0.079 (0.063- 6 0.500 (0.25-1.0) 6 0.070 (0.031-
0.125)
0.25
Saccharomyces cerevisiae 86 0.249 1.0 59 2.845 16.0 54 0.418 2.0
Yarrowia li ol ica 5 0.144 (0.016-1.0) 5 1.741 (0.125-32.0) 0
Pichia anomala 13 0.689 1.0 12 2.670 4.0 12 0.375 1.0
Pichia etchel 2 0.125 (0.125) 2 0.125 (0.125) 0
Pichia ohmeri 1 0.016 0.016 1 4.000 (4.0) 0
Trichosporon spp 6 0.630 (0.5-1.0) 6 12.699 (4.0-64.0) 6 1.123 (0.5-2.0) 0
B'erkandera adusta 14 0.250 0.25 14 4.000 4.0 14 0.057 0.063 v
Blastomyces dermatitidis 43 0.053 0.125 38 2.191 16.0 38 0.045 2.0 0)
E idermo h on floccosum 70 0.029 0.125 15 1.447 2.0 18 0.088 32.0
Paracoccidioides brasiliensis 13 0.048 0.125 13 0.766 4.0 13 0.025 0.063
Scedos orium a ios ermum 32 0.173 1.0 15 84.449 256.0 26 1.341 32.0 0
S orothrix schenckii 16 0.771 2.0 0 11 0.302 0.5 0)
Wangiella dermatitidis 4 0.088 (~ 125 2 256.000 (256.0) 4 0.500 (0.063-1.0)
Absidia spp 8 0.177 (0.031-0.5) 6 143.675 (128'0- 8 0.229 (0.063-2.0)
256.0
A o h som ces spp 9 0.340 0.031-4.0 4 128.000 (128.0) 6 0.707 0.031-8.0
Bi olaris s 8 0.354 (0.125-1.0) 2 64.000 (64.0) 2 0.250 (0.25)
Curvularia spp 5 0.072 (0.031- 2 16.000
0.125) (16.0) 2 0.500 (0.5)
Microsporum audouinii 1 0.250 (0.25) 0 . . 1 0.125 (0.125)
Microsporum canis 86 0.034 0.5 11 2.000 4.0 23 0.041 2.0
Microsporum fulvum 1 0.500 (0.5) 0 . . 1 4.000 (4.0)
Microsporum gypseum 5 0.042 (0.008-0.5) 4 16.000 (4.0-128.0) 4 0.044 (0.016-
0.5)
Microsporum persicolor 1 0.250 (0.25) 1 128.000 (128.0) 1 0.500 (0.5)
Paecilomyces spp 16 0.239 0.5 14 141.323 256.0 14 0.640 2.0
48
POS FLZ ITZ
Organism n Mean MIC 90] n Mean MIC[90 n Mean MIC 90
Penicillium spp 93 0.308 1.0 66 220.996 256.0 83 0.853 2.0
Trichophyton mentagrophytes 84 0.036 0.125 30 9.190 64.0 29 0.041 0.25
Trichophyton raubitschekii 1 0.250 (0.25) 1 128.000 (128.0) 1 0.250 (0.25)
Trichophyton rubrum 148 0.047 0.25 93 4.407 32.0 91 0.074 0.25
Trichophyton soudanense 1 0.500 (0.5) 1 128.000 (128.0) 1 4.000 (4.0)
Trichophyton spp 10 0.036 0.064 0 . . 0
Trichophyton terrestre 1 0.125 (0.125) 1 1.000 (1.0) 1 0.250 (0.25)
Trichophyton tonsurans 74 0.029 0.125 24 3.775 32.0 23 0.036 0.063
0
N
Ln
0)
CD
0
W
N
0
0
0)
F-'
F-
I
N
49
Table 11. Geometric Mean MIC and MIC[90] Values ( g/mL) for # Strains Tested
(n) in Posaconazole (POS), Amphotericin (AMB) and Voriconazole (VOR) 0
POS AMB VOR
Organism n Mean MIC[90] n Mean MIC[90] n Mean MIC[90] As er illus flavus 241
0.079 0.25 177 0.910 2.0 89 0.339 1.0
As er illus fumi atus 2,158 0.118 0.5 1,567 0.683 1.0 1149 0.282 0.5
As er illus nidulans 33 0.055 0.25 20 0.758 2.0 6 0.070 0.031-0.125
As er illus niger 171 0.195 0.5 152 0.360 1.0 101 0.480 2.0
As er illus s dowii 8 0.177 (0.031-0.5) 3 1.260 (1.0-2.0) 3 0.397 (0.25-1.0)
As er illus terreus 100 0.052 0.25 54 1.759 4.0 22 0.312 0.5
As er illus ustus 7 1.641 (0.25-8.0) 7 0.673 (0.25-1.0) 4 1.189 (0.25-2.0)
Candida aibicans 8,847 0.037 0.25 6,651 0.686 1.0 3,790 0.021 0.063
Candida dubliniensis 339 0.062 0.25 211 0.513 1.0 177 0.028 0.125
Candida glabrata 2,507 0.672 2.0 1,881 0.798 1.0 1,264 0.305 2.0 N
Candida krusei 496 0.335 1.0 282 0.976 2.0 210 0.346 0.5
Candida ara silosis 2,126 0.073 0.125 1,655 0.761 1.0 1,011 0.036 0.125 0
0
Cryptococcus laurentii 5 0.095 (0.008-0.5) 3 0.794 (0.5-1.0) 1 0.250 (0.25) W
Cryptococcus neoformans 1,427 0.119 0.25 1,122 0.667 1.0 277 0.054 0.125 0
Coccidioides immitis 50 0.304 1.0 25 0.390 0.5 0 0
Fonsecae pedrosoi 4 0.250 (0.25) 2 1.000 (1.0) 1 0.500 (0.5) Histoplasma
capsulatum 58 0.038 0.25 53 0.250 0.5 0
Pseudallescheria bo dii 66 0.365 1.0 41 1.718 4.0 3 0.250 (0.125-0.5)
Alternaria s 13 0.101 0.25. 13 0.852 4.0 0
Exo hiala dermatidis 3 0.125 (0.125) 2 0.500 (0.5) 1 0.063 (0.063)
Exo hiala 'eanselmei 10 0.287 0.5 10 0.660 1.0 9 0.794 0.5-1.0
Exophiala monifiae 2 0.016 (0.016) 2 0.177 (0'125 0
0.25
Fusarium s 38 2.319 16.0 30 1.203 2.0 14 4.416 16.0
Ramichloridium obovoideum 2 0.044 (0.031-0.063) 2 1.000 1.0 0
Rhizomucor s 2 0.016 (0.016) 2 0.063 0.063 1 2.000 (2.0) Mucor s 17 0.694 16.0
15 0.274 1.0 8 24.675 1.0-128.0
Rhizopus spp 29 1.000 4.0 29 0.635 2.0 9 8.000 (1.0-32.0)
Candida famata 44 0.125 0.5 28 0.841 2.0 5 0.072 (0.008-0.5) Candida
uilliermondii 143 0.178 0.5 76 0.553 1.0 26 0.112 8.0 00
Candida lusitaniae 306 0.048 0.125 164 0.506 1.0 89 0.023 0.063
Candida kefyr 53 0.081 0.25 25 0.779 1.0 14 0.031 0.031
50
POS AMB VOR
Organism n Mean MIC[90] n Mean MIC[90] n Mean MIC[90]
Candida rugosa 26 0.039 0.5 17 0.665 1.0 15 0.072 0.5
Candida tropicalis 1,645 0.081 0.25 1,209 0.774 1.0 765 0.075 0.5
Candida ze lanoides 4 0.031 (0.008-0.25) 3 1.000 (1.0) 3 0.039 (0.008-0.125)
Klu erom ces marxianus 6 0.079 (0.063-0.25) 6 1.000 (1.0) 0
Saccharomyces cerevisiae 86 0.249 1.0 38 0.775 1.0 22 0.050 0.125
Yarrowia li ol ica 5 0.144 (0.016-1.0) 2 0.500 (0.5) 0
Pichia anomala 13 0.689 1.0 10 0.707 1.0 0
Pichia etchel 2 0.125 (0.125) 2 0.063 (0.063) 0
Pichia ohmeri 1 0.016 (0.016) 0 0
Trichos oron s 6 0.630 (0.5-1.0) 5 1.320 (1.0-2.0) 1 0.125 (0.125)
B'erkandera adusta 14 0.250 0.25 14 0.215 0.25 14 0.216 0.25
Blastomyces dermatitidis 43 0.053 0.125 38 0.153 0.5 0
E idermo h on floccosum 70 0.029 0.125 0 10 0.015 0.016 0
Paracoccidioides brasiliensis 13 0.048 0.125 13 0.096 0.25 0 Ln
Scedosporium a ios ermum 32 0.173 1.0 27 2.274 8.0 14 0.098 0.5
S orothrix schenckii 16 0.771 2.0 10 0.574 1.0 0 W
Wan iella dermatitidis 4 0.088 0.063-0.125 3 0.630 (0.25-1.0) 1 1.000 1.0
Absidia spp 8 0.177 (0.031-0.5) 8 0.545 (0.25-2.0) 6 40.318 (8.0-128.0) 0
A o h som ces s 9 0.340 (0.031-4.0) 7 0.500 (0.031-4.0) 5 42.224 (16.0-128.0)
0)
Bi olaris spp 8 0.354 (0.125-1.0) 2 0.250 (0.25) 1 1.000 (1.0) Curvularla s 5
0.072 (0.031-0.125) 2 0.500 (0.5) 1 0.125 (0.125) Microsporum audouinii 1
0.250 (0.25) 0 0
Micros orum canis 86 0.034 0.5 0 10 0.018 0.031
Microsporum fulvum 1 0.500 (0.5) 0 0
Micros orum gypseum 5 0.042 (0.008-0.5) 0 0
Microsporum persicolor 1 0.250 (0.25) 0 0
Paecilom ces s 16 0.239 0.5 14 1.104 16.0 8 0.324 (0.031-2.0)
Penicillium spp 93 0.308 1.0 83 1.025 4.0 35 0.622 2.0
Tricho h on menta ro h es 84 0.036 0.125 2 1.000 (1.0) 11 0.038 0.25
Tricho h on raubitschekii 1 0.250 (0.25) 0 0
Tricho h on rubrum 148 0.047 0.25 0 10 0.021 0.063
Tricho h on soudanense 1 0.500 (0.5) 0 0
Tricho h on s 10 0.036 0.064 0 0
Tricho h on terrestre 1 0.125 (0.125) 0 0
Tricho h on tonsurans 74 0.029 0.125 3 1.260 (0.5-8.0) 11 0.043 0.063
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
51
Pharnl.a.cokinetics
The pharmacokinetic characteristics of the posaconazole formulation were
evaluated in a Phase-1, single-site, randomized, evaluator-blinded (within
dose level), placebo-controlled, rising-single-dose study, with up to six
groups of 12 healthy subjects. The purpose of the study was to evaluate
the safety, tolerability, and pharmacokinetics of the posaconazole
intravenous drug product formulation (hereinafter referred to as "POS IV")
when delivered intravenously. Table 12 shows the POS IV formulation,
and Table 13 shows the physical characteristics of this formulation after
sterilization, but before dilution in 5% dextrose.
Table 12. POS IV Formulation, 50 mg/mL
Ingredient mg/niL
Posaconazole, micronized 50.0
1-Palrnitoyl-2-Oleoyl-sn-glycero-3-Phosphocholine,
Powder, Endotoxin tested (POPC) 40.0
Sodium Phoshate, Monobasic, Monohydrate, Crystal, USP 0.040
Sodium Phosphate, Dibasic, Anhydrous, USP 1.378
Trehalose 250.0
Sodium Hydroxide, N.F. (1.0 N) AN
Phosphoric Acid, N.F. (20% w/w) AN
Water Injection, USP, q.s. ad 1
AN = As needed for pH adjustment
Table 13. Physical Characteristics of POS IV, 50
mg/mL
Description: millky liquid
pH: -6-8
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
52
Osmolality: -875 mOsm/kg (isotonic upon
admiadng)
Particulates: meets USP Particulate Matter tests
Particle Size: Median 1.0-1.8 m 100% <_ 10 m
Sterility/ meets USP tests
Endotoxins:
Within each dose group, subjects were randomized on Day 1 according to
a computer-generated schedule provided by Schering-Plough Research
Institute.
Healthy adult males or females 18 to 45 years of age having body mass
indices (BMIs) of 19 to 27 were eligible for inclusion in Groups 1 to 4 of
the study. Healthy adult males or females 18 to 45 years of age having
BMIs of 19 to 27 and having body weights of >60 kg were eligible for
inclusion in Groups 5 and 6 of the study.
POS IV (50 mg/mL) was diluted in 5% dextrose in water (D5W) in IV bags.
Subjects assigned to active drug received in a 100-mL volume one of the
following single doses administered intravenously over 1 hour: Group 1,
25 mg; Group 2, 50 mg; Group 3, 100 mg; Group 4, 200 mg; Group 5,
400 mg; Group 6, a 125-mL volume a single dose of 600 mg administered
intravenously over 1 hour and 15 minutes.
Blood samples (10 mL each) for the determination of posaconazole
concentrations were collected immediately prior to dosing (0 hour), and at
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
53
0.25, 0.5, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 16, 24, 48, 72, 96, and
120 hours after the start of infusion, as well as on the follow-up visit on
Day 14. The blood samples were collected into two tubes containing
ethylenediaminetetraacetate salt (EDTA) with each tube containing 4 mL
to 5 mL of blood, one tube for determination of posaconazole in whole
blood and other in plasma. For determination of posaconazole in plasma,
the tube of blood (4 mL to 5 mL) was centrifuged within approximately
15 minutes of collection at approximately 4 C and 15009 for 10 minutes
to completely separate red blood cells from plasma. All blood and plasma
samples were immediately frozen to at least -20 C and maintained in the
frozen state until assayed. The blood and plasma concentrations of
posaconazole were determined using validated high performance liquid
chromatographic-mass spectrometric (LC-MS/MS) assays. The lower
limit of quantitation (LLOQ) of this assay was 5.0 ng/mL and the
calbration range was 5 to 5000 ng/mL.
The following pharmacokinetic parameters were determined: maximum
plasma concentration (Cm~.); time of maxim.um plasma concentration
(T,n~,,); the area under the plasma concentration versus time curve to
infinity (AUC[I]); the area under the plasma concentration versus time
curve to the final measurable sampling time (AUC[tfl); terminal phase
half-life (t1i2); total body clearance (CL); and, volume of distribution at
steady-state (Vdss).
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
54
Posaconazole blood and plasma concentrations above the LLOQ were used
for the non-compartmental pharmacokinetic analyses. Pharsight
Knowledgebase Server : version 2Ø1 (PKS) with WinNonlin version 4Ø1
(Pharsight Corporation, Cary, NC) was used to conduct the
phannacokinetic analysis. The Cm. and T.. were the observed values.
The terminal phase rate constant (k) was calculated as the negative of the
slope of the log-linear terminal portion of the serum concentration-time
curve using linear regression. The terminal phase half-life, t1/2, was
calculated as 0.693/k.
The area under the serum concentration-time curve from time 0 to the
time of final quantifiable sample [AUC(tf)) was calculated using the linear
trapezoidal rule. AUC(tf) was then extrapolated to infinity (I) as follows:
AUC(I) = AUC(tf) + Ces(tf)/k
where Ces(tf) is the estimated concentration determined from linear
regression at final measurable sampling time, tf.
Total body clearance, CL, was calculated by the following equation:
CL = Dose/AUC(I)
The apparent volume of distribution at steady-state, Vdss, was calculated
as:
Vdss = CL x MRT
where MRT is the mean residence time (adjusted for infusion duration)
determined from moment analysis.
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
The observed single dose plasma concentrations were used for
pharmacokinetic modeling and simulation and to project steady-state
concentrations for once-a-day (QD) dosing regimen. A nonparameteric
superposition method was used for the pharmacokinetic modeling and
simulation under the assumption of linear pharmacokinetics (see Gibaldi
M, Perrier D., Pharmacokinetics, 2nd ed., New York: Marcel Dekker, Inc.,
1982:409-17).
After cessation of infusion of POS IV, posaconazole plasma
concentrations declined unusually rapidly, and then, surprisingly,
increased subsequently, followed by a slow declining terminal phase (see
Figures 1-4). This pharmacokinetic profile is believed to be atypical and
unique among known azoles. Moreover, this pharmacokinetic pattern
was also observed after the intravenous administration of posaconazole in
animals. It is indicative of a rapid distribution of posaconazole to the liver
and spleen and subsequent slow release from these tissues. Therefore, as
noted in the literature with respect to another pharmaceutically active
agent (Townsend RW, Zutshi A, Bekersky I., "Biodistribution of 4-
[14C]cholesterol-Ambisome following a single intravenous administration
to rats", Drug Metabolism and Disposition. 2001;29:661-5), POS IV may be
initially sequestrated in tissues, such as the liver and spleen, via uptake
through the reticuloendothelial system ("RES").. Although not intended to
be limited to any single mechanism of action, it is believed that the
resulting high concentrations of posaconazole in these tissues due to
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
56
sequestration of the drug may contribute to enhanced anti-infective
activity, since these tissues are often the sites of infection.
In order to determine the target dosing for intravenous administration, it
was necessary to determine a target range for mean Cavg and mean Cmax.
Previous studies on orally administered posaconazole are instructive in
this regard. Table 14 displays pharmacokinetic data resulting from such
oral administration, arranged by quartile based on the observed range of
posaconazole plasma concentration values. For each quartile, the
response rate for apergillosis is displayed.
Table 14. Pharmacokinetic Results of Orally Administered
Posaconazole
Quartile Plasma Cmax Plasma Caõg (ng/mL) Response
n /mL (b) (%)
Mean (a) %CV Mean a %CV
1 142 51 134 45 24
2 467 27 411 21 53
3 852 15 719 12 53
4 148 16 1250 28 71
0
a: n=1 7, with the exception of Quartile 4 where n=1 6
b: Caõg = average plasma concentration across time points in the
same subject
The table shows that the target mean C. for a response rate of at least
50% should be in the range of 467 to 1480 ng/mL, or higher. The
phannacokinetic modeling and steady-state projection based on the
pharmacokinetic results of POS IV once-a-day (QD) dosing regimen show
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
57
that the projected posaconazole mean Cmax at a 100 mg POS IV QD dose
will be 714 ng/mL (see Table 17, 100 mg dose), which exceeds 467
ng/mL, the minimum clinically relevant mean plasma Cm,,. The data in
Table 17 suggest that there exists a dose between 50 and 100 mg which
will result in the minimum clinically relevant mean plasma Cma,, of about
467. However, in terminal disease states, it is desirable to treat the
patient with the maximum tolerated dose. Thus, having estabhshed that
a dose of 100 mg is projected to achieve the minimum clinically relevant
mean plasma Cma., it may be desirable to dose at higher quantities, e.g.,
200 mg, 400 mg, or 600 mg, subject to tolerability.
After intravenous administration of POS IV formulation, posaconazole was
slowly eliminated from plasma with an average terminal half-life of 21 to
39 hours. The half-life was higher at the higher dose compared to that at
lower dose groups (see Tables 15 and 16), in a range of about 15 hours
(with a 100mg dose) to about 51 hours (with a 400mg dose). A long half-
life is desirable as it provides the sustained and high plasma
concentration of antifungal agent over the entire dosing interval, likely
contributing to better antifungal activity. The systemic clearance appeared
to decrease with increasing doses and ranged from 13 to 6 L/hr (see
Tables 15 and 16). The mean volume of distribution was large (326 to
408 L) exceeding total body water volume of about 40 L. This suggests
extensive tissue distribution and penetration into the tissues, a
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
58
characteristic that likely contributes to enhanced anti-infective activity.
The range in the data for Vdss was from 219 to 516L. This is consistent
with the coefficient of variation of the data, which suggests that the
volume distribution could have a range of 200 to 500 L.
The preferable ratios of blood to plasma posaconazole Cmax and AUC
values are shown in Tables 18 and 19. Overall posaconazole exposure
(AUC) was higher in plasma compared to that in blood (see Tables 18 and
19 - AUC ratio). However, the posaconazole concentrations were greater
in blood than in plasma during the infusion and approximately up to 1 hr
post-infusion (see Figures 3 and 4; Tables 18 and 19, Cm. ratio). These
unique differences between blood and plasma concentrations may
contribute to the preferential sequestration of posaconazole in the liver
and spleen, as previously noted. The coefficient of variation of the data
suggests that the ratio of blood to plasma posaconazole Cmax could have a
range of 1.8 to 3.5 for single dose infused over 1.hour to deliver 25-600
mg of posaconazole. The coefficient of variation of the data suggests that
the ratio of blood to plasma posaconazole C. could have a range of 1.0
to 2.3 at steady state when posaconazole is infused over about 1 hour,
and repeated on a 24-hour basis, to deliver 25-600 mg of posaconazole. A
ratio different than that shown in Table 18 may provide different
distribution properties that could translate into differences in anti-
infective activity.
59
0
Table 15. Mean (n=9) Posaconazole Blood Pharmacokinetic Parameters in Subjects
after a Single-dose 1 hr IV Infusion of Posaconazole IV Formulation
Parameter 25 mg 50 m 100 m 200 mg 400 mg 600 mg Mean CV % Mean CV % Mean CV %
Mean CV % Mean CV % Mean CV %
Cmax 244 18 540 11 1130 22 2150 8 4330 14 4410 9
n/mL
Tmax (hr) 1 - 1 - 1 - 1 - 1 - 1.25 -
range 0.5-1.0 0.5-1.0 0.5-1.0 0.5-1.0 0.25-1.0 0.5-1.25
AUC(tf) 1450 28 3370 16 8110 21 19500 20 44800 23 76300 15
n .hr/mL
AUC(I) 1680 27 3620 17 8630 22 20700 21 50900 29 87300 16
n .hr/mL
t1/2 (hr) 21.1 25 22.6 19 27.8 23 26.5 19 37.5 31 39.0 22
0
CL 15.7 23 14.2 18 12.3 31 10.1 24 8.33 23 7.01 14
Uhr
CD
Vdss L 408 14 395 11 408 12 379 19 427 22 393 11 24 W
a: median
0
0
0)
F-'
F-'
I
60
0
Table 16. Mean n=9 Posaconazole Plasma Pharmacokinetic Parameters in Subjects
after a Single-dose 1 hr IV Infusion of Posaconazole IV Formulation
Parameter 25 mg 50 mg 100 mg 200 mg 400 mg 600 mg Mean CV % Mean CV % Mean CV
% Mean CV % Mean CV % Mean CV %
Cmax 103 25 206 26 426 25 898 27 1780 21 1850 21
n /m L
Tmax (hr) 1 - 1 - 1 - 1 - 1 - 1.25 -
range 0.5-1.0 0.25-4.0 0.5-1.0 0.25-1.0 0.5-1.0 1.0-10.0
AUC(tf) 1820 35 4490 18 9320 22 23300 21 49000 29 83700 16
n .hr/mL
AUC(I) 2040 34 4740 18 9890 24 24700 23 55400 36 96700 18
n .hr/mL
t'/z hr 21.4 18 21.7 23 26.5 25 27.1 22 35.4 29 39.4 23
CL 13.3 29 10.9 21 10.9 37 8.57 27 7.84 27 6.36 16 N
L/hr Ln
0)
Vdss (L) 396 20 331 12 389 13 324 18 378 22 356 23 CD
a: median W
N
0
0
0)
F-'
F-
I
N
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
61
able 17. Mean Projected Steady-state Plasma and Blood Posaconazole PK
Parameters in Subjects Receiving a Daily Dose of POS IV infusion over 1-1.25
hr
Dose Plasma Blood
(mg) Cmax AUC (0-24 hr) Cmax AUC (0-24 hr)
(ng/mL) (ng.hr/mL) (ng/mL) (ng.hr/mL)
Mean CV, % Mean CV, % Mean CV, % Mean CV, %
25 155 26 1960 36 281 18 1580 29
50 335 16 4690 19 628 10 3560 17
100 714 23 9840 24 1360 21 8570 22
200 1670 22 24600 23 2760 9 20600 21
100 3540 27 54500 35 5870 12 50000 28
600 5100 16 94900 17 7260 10 85800 16
Table 18. Ratio of Mean Blood and Plasma Posaconazole PK Parameters in
Subjects receiving a Single Dose of POS IV infusion over 1-1.25 hr
Dose Blood/Plasma Ratio
(mg) Cmax AUC (1)
Mean CV, % Mean CV, %
25 2.42 17 0.839 7
50 2.76 25 0.772 13
100 2.71 13 0.878 6
200 2.50 18 0.844 6
400 2.50 19 0.933 8
600 2.45 17 0.904 5
Table 19. Ratio of Mean Projected Steady-state Blood and Plasma
Posaconazole PK Parameters in Subjects receiving a Daily Dose of POS IV
infusion over 1-1.25 hr
CA 02567803 2006-11-23
WO 2005/117831 PCT/US2005/018945
62
Dose Blood/Plasma Ratio
(mg) Cmax AUC (r)
Mean CV, % Mean CV, %
25 1.85 12 0.822 9
50 1.90 15 0.766 13
100 1.93 12 0.877 6
200 1.70 13 0.843 7
400 1.72 19 0.932 8
600 1.44 10 0.908 5
It is to be understood that all formulations that are bioequivalent to those
disclosed herein are also within the scope of the present invention.
The present invention is not to be limited in scope by the specific
embodiments described herein. Indeed, various modifications of the
invention in addition to those described herein will become apparent to
those skilled in the art from the foregoing description. Such modifications
are intended to fall within the scope of the appended claims.
Various publications are cited herein, the disclosures of which are
incorporated by reference in their entireties.