Note: Descriptions are shown in the official language in which they were submitted.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
AMINOPYRIDINE DERIVATIVES AS SELECTIVE DOPAMINE D3 AGONISTS
The present invention relates to a class of dopamine agonists, more
particularly a class of agonists that
are selective for D3 over D2. These compounds are useful for the treatment
and/or prevention of sexual
dysfunction, for example female sexual dysfunction (FSD), in particular female
sexual arousal disorder
(FSAD), hypoactive sexual desire disorder (HSDD; lack of interest in sex),
female orgasmic disorder
(FOD; inability to achieve orgasm); and male sexual dysfunction, in particular
male erectile dysfunction
(MED). Male sexual dysfunction as referred to herein is meant to include
ejaculatory disorders such as
premature ejaculation, anorgasmia (inability to achieve orgasm) or desire
disorders such as hypoactive
sexual desire disorder (HSDD; lack of interest in sex). These compounds are
also useful in treating
neuropsychiatric disorders and neurodegenerative disorders.
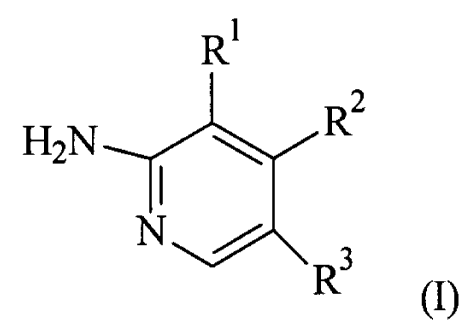
The present invention provides for compounds of formula (I):
R
H2N ~ R2
. ~ -
N / R 3
(I)
wherein:
R' is selected from H and P-C6)alkyl;
R2 is selected from H and (C1-C6)alkyl;
R3 is selected from:
A R6
1R5 (CH2)n N N. 9
R4 (II) R~ (!!i) R (IV)
wherein:
A represents 0, S or CH2;
nis1or2;
R4 is selected from, H and (CI-C6)alkyl; wherein said P-C6)alkyl may be
optionally substituted
with 1 or 2 substituents each independently selected from (Ci-C6)alkyl, OR7,
phenyl, substituted
phenyl and-heteroaryl;
R5 is selected from H and (C1-C6)alkyl; wherein said P-Cs)alkyl may be
optionally substituted
with 1 or 2 OR7 groups;
R6 is selected from H and (C1-C6)alkyl;
R7 is selected from H and (C1-CB)a(kyl; wherein said (CI-C6)alkyl may be
optionally substituted
with a phenyl, or a substituted phenyl group;
R8 is selected from H, methyl, ethyl, methoxy, and ethoxy;
R9 represents (Cl-Cs)alkyl; and
R10 is selected from H and (Ct-C6)alkyl; wherein said (Ct-C6)alkyl may be
optionally substituted
7
with I or 2 substituents each independentiy selected from OR, phenyl or
substituted phenyl;
CA 02567935 2006-11-23
69387-598
2
wherein heteroaryl means a 5 to 7 membered
aromatic ring, containing from 1 to 4 heteroatoms, said
heteroatoms each independently selected from 0, S and N;
said heteroaryl may be optionally substituted with 1 or more
substituents each independently selected from (C1-C6)alkyl,
halo and OR', each substituent may be the same or different;
and
wherein substituted phenyl means phenyl
substituted with 1 or more substituents each independently
selected from (C1-C6)alkyl, halo and OR', each substituent
may be the same or different;
with the proviso that:
when R' and R2 are H, R3 is moiety (II), A is 0, RS
is H or (C1-C6) alkyl, and R6 is H or (C1-C6) alkyl, then R4
cannot be n-propyl;
and pharmaceutically salts, solvates, polymorphs
and prodrugs thereof.
According to another aspect of the present
invention, there is provided compounds of formula (I):
Rl
R2
H2N 'Izz~z N ~
~
3
R (I~
wherein: R' is selected from H and (C1-C6) alkyl; R2 is
selected from H and (Cl-C6)alkyl; R3 is:
A R6
N RS
R4
(II)
CA 02567935 2006-11-23
69387-598
2a
wherein: A represents 0; R4 is selected from H and straight
chain (C1-C6) alkyl; wherein said straight chain (C1-C6) alkyl
may be optionally substituted with 1 or 2 substituents each
independently selected from OR7, phenyl, substituted phenyl
and heteroaryl; R5 is selected from H and (C1-C6) alkyl;
wherein said (C1-C6)alkyl may be optionally substituted with
1 or 2 OR' groups; R6 is selected from H and (C1-C6) alkyl; R'
is selected from H and (C1-C6) alkyl; wherein said (C1-C6) alkyl
may be optionally substituted with a phenyl, or a
substituted phenyl group; Ra is selected from H, methyl,
ethyl, methoxy, and ethoxy; R9 represents (C1-C6)alkyl; and
R10 is selected from H and (Cl-C6) alkyl; wherein said (C1-
C6)alkyl may be optionally substituted with 1 or 2
substituents each independently selected from OR7, phenyl or
substituted phenyl; wherein heteroaryl means a 5 to 7
membered aromatic ring, containing from 1 to 4 heteroatoms,
said heteroatom independently selected from 0, S and N; said
heteroaryl may be optionally substituted with 1 or more
substituents each independently selected from (C1-C6)alkyl,
halo and OR', each substituent may be the same or different;
and wherein substituted phenyl means phenyl substituted with
1 or more substituents each independently selected from
(C1-C6)alkyl, halo and OR', each substituent may be the same
or different; with the proviso that: when R' and R2 are H, R5
is H or (Cl-C6) alkyl, and R6 is H or (C1-C6) alkyl, then R4
cannot be n-propyl; and pharmaceutically acceptable salts,
solvates, polymorphs and prodrugs thereof.
According to still another aspect of the present
invention, there is provided compounds of formula (I):
Rl
R
H2N
N
3
R
(I)
CA 02567935 2006-11-23
69387-598
2b
wherein: Rl is selected from H and (C1-C6) alkyl; R2 is
selected from H and (C1-C6) alkyl; R3 is selected from:
A R6 Rg R10
/(CH2)n
R5 R9
14 14
R (II) R (III) (IV)
wherein: A represents S or CH2; n is 1 or 2; R4 is selected
from H and (C1-C6) alkyl; wherein said (C1-C6) alkyl may be
optionally substituted with 1 or 2 substituents each
independently selected from (C1-C6) alkyl, OR7, phenyl,
substituted phenyl and heteroaryl; RS is selected from H and
(C1-C6) alkyl; wherein said (C1-C6) alkyl may be optionally
substituted with 1 or 2 OR' groups; R6 is selected from H and
(C1-C6) alkyl; R' is selected from H and (C1-C6) alkyl; wherein
said (C1-C6)alkyl may be optionally substituted with a
phenyl, or a substituted phenyl group; R$ is selected from H,
methyl, ethyl, methoxy, and ethoxy; R9 represents
(C1-C6) alkyl; and R10 is selected from H and (Cl-C6) alkyl;
wherein said (C1-C6)alkyl may be optionally substituted with
1 or 2 substituents each independently selected from OR',
phenyl or substituted phenyl; wherein heteroaryl means a 5
to 7 membered aromatic ring, containing from 1 to 4
heteroatoms, said heteroatom independently selected from 0,
S and N; said heteroaryl may be optionally substituted with
1 or more substituents each independently selected from
(C,.-C6) alkyl, halo and OR', each substituent may be the same
or different; and wherein substituted phenyl means phenyl
substituted with 1 or more substituents each independently
selected from (C1-C6) alkyl, halo and OR', each substituent
may be the same or different; and pharmaceutically
acceptable salts, solvates, polymorphs and prodrugs thereof.
CA 02567935 2006-11-23
69387-598
2c
According to yet another aspect of the present
invention, there is provided a commercial package comprising
a compound of the invention, together with a written matter
describing instruction for the use thereof in the treatment
of a disease or condition as described herein.
Unless otherwise indicated, (C1-C6) alkyl may be
straight chain or branched.
Suitable heteroaryl groups include pyridinyl,
pyrimidinyl, pyridazinyl and pyrazinyl.
Unless otherwise indicated, the term halo means
fluoro, chloro, bromo or iodo.
Unless otherwise indicated, the term substituted
means substituted by one or more defined groups. In the
case where groups may be selected from a number of
alternatives groups, the selected groups may be the same or
different.
The pharmaceutically acceptable salts of the
compounds of the formula (I) include the acid addition and
the base salts thereof.
Suitable acid addition salts are formed from acids
which form non-toxic salts. Examples include the acetate,
adipate, aspartate, benzoate, besylate,
bicarbonate/carbonate, bisulphate/sulphate, borate,
camsylate, citrate, cyclamate, edisylate, esylate, formate,
fumarate, gluceptate, gluconate, glucuronate,
hexafluorophosphate, hibenzate, hydrochloride/chloride,
hydrobromide/bromide, hydroiodide/iodide, isethionate,
lactate, malate, maleate, malonate, mesylate,
methylsulphate, naphthylate, 2-napsylate, nicotinate,
nitrate, orotate, oxalate, palmitate, pamoate,
CA 02567935 2006-11-23
69387-598
2d
phosphate/hydrogen phosphate/dihydrogen phosphate,
pyroglutamate, saccharate, stearate, succinate, tannate,
tartrate, tosylate, trifluoroacetate and xinofoate salts.
Hemisalts of acids may also be formed, for example
hemisulphate.
For a review on suitable salts, see Handbook of
Pharmaceutical Salts: Properties, Selection, and Use by
Stahl and Wermuth (Wiley-VCH, 2002).
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
3
Pharmaceutically acceptable salts of compounds of formula I may be prepared by
one or more of three
methods:
(i) by reacting the compound of formula I with the desired acid or base;
(ii) by removing an acid- or base-labile protecting group from a suitable
precursor of the compound of
formula I or by ring-opening a suitable cyclic precursor, for example, a
lactone or lactam, using
the desired acid or base; or
(iii) by converting one salt of the compound of formula I to another by
reaction with an appropriate
acid or base or by means of a suitable ion exchange column.
All three reactions are typically carried out in solution. The resulting salt
may precipitate out and be
collected by filtration or may be recovered by evaporation of the solvent. The
degree of ionisation in the
resulting salt may vary from completely ionised to almost non-ionised.
The compounds of the invention may exist in a continuum of solid states
ranging from fully amorphous to
fully crystalline. The term 'amorphous' refers to a state in which the
material lacks long range order at the
molecular level and, depending upon temperature, may exhibit the physical
properties of a solid or a liquid.
Typically such materials do not give distinctive X-ray diffraction patterns
and, while exhibiting the
properties of a solid, are more formally described as a liquid. Upon heating,
a change from solid to liquid
properties occurs which is characterised by a change of state, typically
second order ('glass transition').
The term 'crystalline' refers to a solid phase in which the material has a
regular ordered internal structure
at the molecular level and gives a distinctive X-ray diffraction pattern with
defined peaks. Such materials
when heated sufficiently will also exhibit the properties of a liquid, but the
change from solid to liquid is
characterised by a phase change, typically first order ('melting point').
The compounds of the invention may also exist in unsolvated and solvated
forms. The term `solvate' is
used herein to describe a molecular complex comprising the compound of the
invention and one or more
pharmaceutically acceptable solvent molecules, for example, ethanol. The term
'hydrate' is employed
when said solvent is water. -
The pharmaceutically acceptable solvates of the compounds of the formula (I)
include the hydrates
thereof.
A currently accepted classification system for organic hydrates is one that
defines isolated site, channel,
or metal-ion coordinated hydrates - see Polymorphism in Pharmaceutical Solids
by K. R. Morris (Ed. H. G.
Brittain, Marcel Dekker, 1995). Isolated site hydrates are ones in which the
water molecules are isolated
from direct contact with each other by intervening organic molecules. In
channel hydrates, the water
molecules lie in lattice channels where they are next to other water
molecules. In metal-ion coordinated
hydrates, the water molecules are bonded to the metal ion.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
4
When the solvent or water is tightly bound, the complex will have a well-
defined stoichiometry independent
of humidity. When, however, the solvent or water is weakly bound, as in
channel solvates and hygroscopic
compounds, the water/solvent content will be dependent on humidity and drying
conditions. In such cases,
non-stoichiomet .ry will be the norm.
Hereinafter all references to compounds of formula I include references to the
salts and solvates thereof.
The compounds of the invention include compounds of formula I as hereinbefore
defined, including all
polymorphs and crystal habits thereof, prodrugs and isomers thereof (including
optical, geometric and
tautomeric isomers) as hereinafter defined and isotopically-labeled compounds
of formula I.
Included within the scope of the present invention are all stereoisomers,
geometric isomers and
tautomeric forms of the compounds of formula I, including compounds exhibiting
more than one type of
isomerism, and mixtures of one or more thereof. Also included are acid
addition or base salts wherein the
counterion is optically active, for example, d-lactate or I-lysine, or
racemic, for example, dl-tartrate or dl-
arginine.
A compound of the formula (I) contains one or more asymmetric carbon atoms and
therefore exists in two
or more stereoisomeric forms. Furthermore, the skilled person will understand
that moiety (II)
encompasses all stereoisomeric and distereoisomeric forms, in particular:
R6 ,, A R6
N/''~,R 5 N /"'~ R 5
R4 (IIa) R4 (Ilb)
Separation of diastereoisomers may be achieved by conventional techniques,
e.g. by fractional
crystallisation, chromatography or H.P.L.C. of a stereoisomeric mixture of a
compound of the formula (I)
or a suitable salt or derivative thereof. An individual enantiomer of a
compound of the formula (I) may also
be prepared from a corresponding optically pure intermediate or by resolution,
such as by H.P.L.C. of the
corresponding racemate using a suitable chiral support or by fractional
crystallisation of the
diastereoisomeric salts formed by reaction of the corresponding racemate with
a suitable optically active
acid or base, as appropriate.
The present invention includes all pharmaceutically acceptable isotopically-
labelled compounds of formula
I wherein one or more atoms are replaced by atoms having the same atomic
number, but an atomic mass
or mass number different from the atomic mass or mass number which
predominates in nature.
Examples of isotopes suitable for inclusion in the compounds of the invention
include isotopes of
hydrogen, such as 2 H and 3H, carbon, such as'1C,13C and 14C, chlorine, such
as 36C1, fluorine, such as
18F, iodine, such as 1231 and '251, nitrogen, such as 13 N and 15 N, oxygen,
such as 150, 1 70 and '80,
phosphorus, such as 32P, and sulphur, such as 35S.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
Certain isotopically-labelled compounds of formula I, for example, those
incorporating a radioactive
isotope, are useful in drug and/or substrate tissue distribution studies. The
radioactive isotopes tritium, i.e.
3H, and carbon-14, i.e. 14C, are particularly useful for this purpose in view
of their ease of incorporation
5 and ready means of detection.
Substitution with heavier isotopes such as deuterium, i.e. 2H, may afford
certain therapeutic advantages
resulting from greater metabolic stabiiity, for example, increased in vivo
half-life or reduced dosage
requirements, and hence may be preferred in some circumstances.
Substitution with positron emitting isotopes, such as "C, '$F, 150 and 13N,
can be useful in Positron
Emission Topography (PET) studies for examining substrate receptor occupancy.
Isotopically-labeled compounds of formula I can generally be prepared by
conventional techniques known
to those skilled in the art or by processes analogous to those described in
the accompanying Examples
and Preparations using an appropriate isotopically-labeled reagent in place of
the non-labeled reagent
previously employed.
Pharmaceutically acceptable solvates in accordance with the invention include
those wherein the solvent
of crystallization may be isotopically substituted, e.g. D20, d6-acetone, d6-
DMSO.
The following embodiments of the invention are particularly favoured:
Preferably R' is selected from H, methyl and ethyl
More preferably R' is selected from H and methyl
Most preferably R' is H.
Preferably R2 is selected from H, methyl and ethyl
More preferably R 2 is selected from H and methyl
Most preferably R2 is H.
When R3-is moiety (II):.
Moieties (Ila) and (lib) are preferred.
Preferably A is 0 or CHZ -
More preferably A is 0
Preferably R4 is (Cl-C6)alkyl optionally substituted with a phenyl or a
substituted phenyl group.
More preferably R4 is (CI-C4)alkyl optionally substituted with phenyl
Even more preferably R4 is selected from methyl, ethyl, n-propyl or n-butyl
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
6
Most preferably R4 is selected from methyl, ethyl, and n-propyl
In a first preferred embodiment, R5 is selected from H and (C,-C4)alkyl;
wherein said (Cl-C4)alkyl
may be optionally substituted with 1 or 2 OR' groups
More preferably R5 is selected from H, methyl and ethyl; wherein said methyl
and said ethyl may
be optionally substituted with an OR7 group
Most preferably R5 is selected from H, methyl and ethyl.
In a second preferred embodiment, R5 is (Cl-C4)alkyl optionally substituted
with 1 or 2 OR' groups
More preferably R5 is selected from methyl and ethyl; wherein said methyl and
said ethyl may be
optionally substituted with an OR7 group.
Most preferably R5 is selected from methyl and ethyl.
Preferably R6 is selected from H, methyl and ethyl
More preferably R 6 is selected from H and methyl
Most preferably R 6 is H
Preferably R7 is selected from H and (CI-C4)alkyl; wherein said (CI-C4)alkyl
is optionally
substituted with 1 or 2 phenyl or substituted phenyl groups
More preferably R' is selected from H, methyl and ethyl; wherein said methyl
and said ethyl are
optionally substituted with a phenyl group
Most preferably R' is selected from H and (CH2)phenyl
When R3 is moiety (III):
Preferably n is 1
Preferably R4 represents (CI-C4)alkyl, optionally substituted by 1 or 2
phenyl, substituted phenyl or
heteroaryl groups.
More preferably R4 represents ethyl, propyl or butyl, said groups being
optionally substituted by a
phenyl group.
Most preferably R4 represents ethyl or propyl, said groups being optionally
substituted by a phenyl
group.
When R3 is moiety (IV):
Preferably R8 is selected from H, methyl and methoxy. .-
More preferably Ra is selected from H and methoxy.
Most preferably Re is H.
Preferably R9 is selected from (CI-C4)alkyl.
More preferably R9 is selected from methyl, ethyl and n-propyl.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
7
Most preferably R9 is n-propyl.
Preferably R10 is selected from H and (CI-C3)alkyl; wherein said (C,-C3)alkyl
may be optionally
substituted with 1 or 2 OR' or phenyl groups.
More preferably R10 is selected from H and methyl.
Most preferably R10 is H.
Preferably R3 is selected from moieties (II) and (III)
More preferably R3 is selected from moieties (Ila), (Ilb), and (III)
Most preferably R3 is selected from moieties (Ila) and (Ilb).
Preferably heteroaryl is a 5 or 6 membered aromatic ring, containing from I to
3 heteroatoms, said
heteroatom independently selected from 0 and N;
More preferably heteroaryl is a 5 or 6 membered aromatic ring, containing from
1 to 2 nitrogen atoms.
5
Particularly preferred are compounds (and salts thereof) of the present
invention are exemplified herein;
more preferred are:
5-(Morpholin-2-yl)pyridin-2-amine (Example 2);
10 5-[4-(3-Phenylpropyl)morpholin-2-yl]pyridin-2-amine (Exampie 3);
5-[(2R, 5S)-5-Methylmorpholin-2-yl]pyridine-2-amine (Example 7a);
5-[(2S,5S)-S-Methyl-4-(3-phenylpropyl)morpholin-2-yl]pyridin-2-amine (Example
9);
5-[(2S,5S)-4-Butyl-5-methylmorpholin-2-yl]pyridin-2-amine (Example 10);
5-{(2R,5S)-5-[(Benzyloxy)methyl]-4-propylmorpholin-2-yl}pyridin-2-amine
(Example 13);
?5 [6-(6-Am i nopyridin-3-yl)-4-propyim orpholin-3-yl]m ethanol (Example 14);
4-Methyl-5-(4-Propylmorpholin-2-yl)pyridin-2-amine (Examples 18 & 19);
5-[(2S,5S)-4,5-Diethyimorpholin-2-yl]pyridin-2-amine (Example 21);
5-[(2R,5S)-4,5-Diethylmorpholin-2-yl]pyridin-2-amine (Example 22);
5-(2R,5S)-(4-ethyl-5-methylmorpholin-2-yl)-pyridin-2-yiamine (Example 25).
In an alternative embodiment, the invention additionally comprises the
compounds (+)-5-(4-
propyimorpholin-2-yl)-1,3-thiazol-2-amine and (-)-5-(4-propylmorpholin-2-yl)-
1,3-thiazol-2-amine
(Examples 26 and 27).
Compounds of the invention may be prepared, in known manner, in a variety of
ways. The routes below
illustrate methods of synthesising compounds of formula (I); the skilled man
will appreciate that other
methods may be equally as practicable.
Throughout the schemes the protected nitrogen of the 2-aminopyridine group is
signified as PGN, and Hal
represents a halogen selected from Cl, Br, or I.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
8
Compounds of formula (I) wherein R', R2, R4 and R6 are as defined above, and
R3, R5 and A are as
described herein, may be prepared according to reaction scheme 1.
N Hal
I
~ Hal PGN R 2
N (VI)
1 ~ z _ N ~ H
R (~) + (2) PGN Rz
HzN R
(V) N "' Hal Ri (VII)
~
I
N Rz
Ri (VIa) (3)
9H H OH
N N,Ra N NHz
.
PGN ~ Rz (4) PGN Rz
(5) R (IX) R1 (VIII)
R6 R6
O'l,l" O_~
N.Ra N ~ N.Ra
PGN Rz (6)
PGN I~ Rz (7)
R (X) Ri
(XI)
R6
O
N N.Ra
HZN I Rz
R
(I)
Scheme 1
Reaction Step 1. Aminopyridine protection
Compounds of formula (V), wherein, for example, NPG is the 2,5-dimethylpyrrole
system [as described in
J. Chem. Soc. Perkin Trans. 1, 1984, 2801-2807, and as illustrated by the
compound of formula (Vla)],
may be introduced through reaction of an aminopyridine of formula (V) with 1-2
equivalents of 2,5-
hexanedione in toluene at reflux with azeotropic removal of water and an acid
catalyst, such as para-
toluenesulfonic acid.
Reaction Step 2. Halide to aldehyde
Aromatic halide =of formula (VI) may be converted into aldehydes of formula
(VII) by, for example,
generation of an organometallic reagent from a halogenated pyridine of formula
(VI), followed by reaction
with a formylating agent such as dimethylformamide or morpholine-4-
carbaldehyde.
Suitable organometallic pyridine derivatives include Grignard
(organomagnesium) or organolithium
reagents, which may be prepared from the bromide (or iodide) by halogen-metal -
exchange. Typical
conditions comprise addition of isopropylmagnesium chloride (or butyllithium)
to the bromide (VI) in an
anhydrous ethereal solvent such as tetrahydrofuran at room temperature (may
require heating in certain
cases when isopropylmagnesium chloride is used as the metallating agent) or
below (e.g. -78 C when
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
9
butyllithium is used) to perform the halogen metal exchange reaction, followed
by addition of the
formylating agent at 0 C or lower.
Reaction Step 3. Conversion of aldehyde to aminoalcohol
Compounds of formula (VIII) may be prepared by reaction of an aidehyde of
formula (VII) with a cyanide
source, such as potassium cyanide or trimethylsilylcyanide, or with
nitromethane and a base, such as
sodium hydroxide, to form an intermediate adduct which may be reduced by
treatment with borane, lithium
aluminium_ hydride or hydrogenation in an ethereal solvent. Typical conditions
comprise reacting 1.0
equivalents of aldehyde in 1.5 equivalents of 3M HCI with sodium sulfite (1.5
equivalents) followed by
potassium cyanide (1.5 equivalents) at room temperature. The resulting
cyanohydrin intermediate is then
reduced by treatment with 1.2-3.0 equivalents of borane in THF at reflux,
followed by treatment with a
strong acid to hydrolyse the initially formed boron complex of the product.
The skilled person will be aware
that other non-acidic methods are available for breaking the boron complex
e.g. treatment with
5 diethanolamine.
Reaction Step 4. Reductive amination
Compounds of formula (IX) may be prepared from compounds of formula (VIII) by
employing standard
?0 amide bond forming conditions followed by reduction of the intermediate
amide with a hydride reducing
agent such as borane or lithium aluminium hydride.
For example, acid chlorides in the presence of a suitable base such as
triethylamine or 4-
methylmorpholine may be used for the amide forming stage. Typical reaction
conditions comprise 1.0
equivalents of amine (VIII), 1.2-2.0 equivalents of base (preferably
triethylamine), 1.1-1.3 equivalents of
25 acid chloride in dichloromethane at 25 C. Reducing agents such as borane or
lithium aluminium hydride
can be used for the amide reduction stage. Typical conditions comprise 1.0
equivalents of amide, 1.2-3.0
equivalents of borane in THF at reflux, followed by treatment with a strong
acid to hydrolyse the initially
formed boron complex of the product. The skilled person will be aware that
other non-acidic methods are
available for breaking the boron complex e.g. treatment with diethanolamine.
Compounds of formula (IX) can also be made by reductive amination of compounds
of formula (VIII) with
a suitable aldehyde (1 equivalent or more) in the presence of a hydride
reducing agent such as sodium
cyanoborohydride or sodium triacetoxyborohydride (1 equivalent or more) in an
alcoholic solvent such as
ethanol at room temperature.
Reaction Step 5. Morpholinone formation
Compounds of formula (X) may be prepared by reaction of compounds of formula
(IX) with chloroacetyl
chloride or 2-substituted a-chloroacyl chlorides (such as 2-chloropropionyl
chloride or 2-chlorobutyryl
chloride) in the presence of a base such as triethylamine, sodium carbonate or
potassium hydroxide.
Typical conditions comprise 1.0 equivalents of amine (IX), 1.0-1.3 equivalents
of acid chloride, 1.2-2.0
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
equivalents of triethylamine in dichloromethane at 25 C, the crude reaction
mixture is then dissolved in
IPA with 1.2-3.0 equivalents of aqueous potassium hydroxide.
Reaction Step 6. Morpholinone reduction
5
Compounds of formula (XI) may be prepared by reaction of compounds of formula
(X) with reducing
agents such as borane or lithium aluminium hydride. Typical conditions
comprise 1.0 equivalents of amide
(X), 1.2-3.0 equivalents of borane in THF at reflux, followed by treatment
with a strong acid to hydrolyse
the initially formed boron complex. The skilled person will be aware that
other non-acidic methods are
10 available for breaking the boron compiex e.g. treatment with
diethanolamine.
Reaction Step 7. Aminopyridine deprotection
Compounds of formula (I) may be prepared from compounds of formula (XI) by
deprotection. The nature
of this reaction will depend upon the protecting group selected for use.
For example, when the 2,5-dimethylpyrrole system is used to protect the
aminopyridine group it may be
deprotected by treatment with hydroxylamine. Typical conditions comprise 1.0
equivalents of compound
(XI) and 5 equivalents of hydroxylamine hydrochloride in ethanol at reflux
Alternatively, compoundsof formula (IX), wherein R1, Ra, and R4 are as defined
above, may be prepared
according to reaction scheme 2.
O o
Hal N ~ CI
PGN ' R Rz (8) PGN '~ RZ (9) PGNN
I RZ
(VI) RI (XII) RI (XIII)
(10)
OH H
N N.Ra
PGN I ~ RZ
RI (IX)
Scheme 2
Reaction Step 8. Halide to chloroketone
Chloroketones of formula (XII) may be formed from halides of formula (VI) via
generation of a reactive
organometallic intermediate. Suitable organometallic pyridine derivatives
include Grignard
(organomagnesium) or organolithium reagents, which may be prepared from the
bromide (or iodide) by
halogen-metal exchange. Thus, treatment of (VI) with 1.1 (or more) equivalents
of butyllithium in an
ethereal solvent such as tert-butylmethyl ether at low temperature (-78 C)
affords an organometallic
intermediate which can then be treated with 2-chloro-N-methoxy-N-
methylacetamide to provide
chloroketones of formula (XII).
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
11
Reaction Step 9. Chloroketone to epoxide
Chloroketones of formula (XII) may be converted in epoxides of formula (XIII)
via- reduction to an
intermediate chlorohydrin and base promoted epoxide formation. Thus, reaction
of (XlI) with sodium
borohydride (0.3 equivalents or more) in dioxan with subsequent treatment with
excess sodium hydroxide
solution affords epoxides of formula (XIII).
Enantiomerically pure, or enantiomerically enriched epoxides of formula (XIII)
may be obtained by
employing an asymmetric reducing agent. For example, reaction of chloroketones
of general formula (XIII)
with (-)-B-chlorodiisopinocampheylborane (1.5 or more equivalents) in
tetrahydrofuran at low temperature
(e.g -30 C) and subsequent treatment of the intermediate chlorohydrin with
sodium hydroxide affords
enantiomerically enriched epoxides of formula (XIII).
Reaction Step 10. Epoxide opening
Epoxides of formula (XIII) when heated with suitable primary amines in an
inert solvent such as DMSO at
90 C afford compounds of formula (IX).
Compounds of formula (I) wherein R1, R2, R4, and R5 are as defined above, and
R3, R6 and A are as
described herein, may be prepared according to reaction scheme 3.
R 5 R 5
HZN-IY O1 -,~ HN~O, Rs
O .HCI (11) OJR O (12) /~p~N~OH
(XIV) (XV) R (XVI)
1 (13)
PGN ~N I OH Rs
R~ ~ z O~ HN~,OH + (XII)
(15) R Ra Rs (14) Ra (XVII)
(XVIII) HCI
PGN ~N PGN H2N
R 1 ~ 1 OH OH - R1 ) 1 O R1 I O
R2 N r R s (16) RZ N 7) ~ RZ NJ~Rs
(XIX) - Ra (XI) Ra (1) Ra
Scheme 3
Reaction Step 11. Amide formation
Compounds of the formula (XV) may be prepared by reacting an amino acid ester
of the formula (XIV)
with acid chlorides (R=(Cl-C6)alkyl) in the presence of a suitable base such
as triethylamine and 4-
methylmorpholine (or other suitable amide bond forming conditions). Typical
reaction conditions comprise
1 equivalent amino acid ester (XIV), 1 equivalent of acid chloride and 3
equivalents of base in
dichloromethane at 25 C. Examples of compounds of formula (XV) are also
commercially available.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
12
Reaction Step 12. Amide and ester reduction then N-Boc formation
Compounds of the formula (XVI) may be prepared by reacting compounds of the
formula (XV) with
borane-THF complex, followed by treatment with a strong acid (e.g 5M HCI) to
hydrolyse the resulting
boron complexes with the product. Other non-acidic methods are available for
breaking the boron
complex e.g. treatment with diethanolamine.. This is followed by t-
butyloxycarbonyl protection of the
formed amine. Typical reaction conditions comprise 1 equivalent of the amide
(XV) with 3 equivalents of
BH3-THF in THF at reflux, cooling, cautious addition of 6M aqueous HCI, and
heating to reflux for a further
6h. Subsequent evaporation of solvent, redissolution in a methanol:water (8:1)
mix, and addition of 5
equivalents of a base such as potassium hydroxide and 1.5 equivalents of di-
tert-butyl dicarbonate, and
stirring of the mixture for 72 hours.
Reaction Step 13 N-Boc deprotection
5
Compounds of the formula (XVII) may be prepared by reacting compounds of the
formula (XVI) with an
organic solution of HCI. Typical reaction conditions comprise 1 equivalent of
the carbamate (XVI) and a 1-
10 equivalents of a 4M solution of HCI in dioxan at 25 C. Examples of
compounds of formula (XVII) are
also commercially available.
!0
Reaction Step 14. Chloroketone addition
Compounds of the formula (XVIII) may be prepared by reacting compounds of the
formula (XVII) with an
a-halo ketone of formula (XII), if necessary in the presence of a base such as
triethylamine or 4-
?5 methylmorpholine. Typical conditions comprise 1 equivalent of the
aminoalcohol (XVII) with 1-3
equivalents of triethylamine and 1 equivalent of a compound of formula (XII)
at 65 C.
Reaction Step 15. Reduction to Diol
30 Morpholinol intermediates of formula (XVIII) can be reduced to diols of
formula (XIX) by reaction with a
hydride reducing agent such as sodium borohydride (1 equivalent or more) in an
alcoholic solvent such as
ethanol at room temperature.
Reaction Step 16. Morpholine ring closure
Diol compounds of formula (XIX) can be ring-closed to morpholine compounds of
formula (XI) using a
number of methods. For example treating a dichloromethane solution of (XIX)
with excess-concentrated
sulfuric acid at room temperature will effect cyclisation.
Alternatively, the ring closure may be effected using Mitsunobu-type
conditions employing the using of 1.1
equivalents of a dialkyl azodicarboxylate reagent, such as diispropyl
azodicarboxylate (DIAD), and 1.1
equivalents of triphenylphosphine in an inert solvent such as tetrahydrofuran.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
13
A further alternative is to use a sulfonylating agent, such as p-
toluenesulfonylimidazole (1 equivalent) in
the presence of strong base such as sodium hydride in an inert solvent such as
tetrahydrofuran, as
described in Org. Lett. 2004, 6(6), 1045-1047.
Reaction step 7. Aminopyridine deprotection
Compounds of formula (I) may be prepared from compounds of formula (XI) by
deprotection. The nature
of this reaction will depend upon the protecting group selected for use.
For example, when the 2,5-dimethylpyrrole system is used to protect the
aminopyridine group it may be
deprotected by treatment with hydroxylamine. Typical conditions comprise 1.0
equivalents of compound
(XI) and 5 equivalents of hydroxylamine hydrochloride in ethanol at reflux
Alternatively, in some cases it may prove advantageous to deprotect the
aminopyridine group (PGN) prior
5 to ring closure to form the morpholine group. This is most likely to be the
case when acidic conditions are
used to effect the cyclisation. In this instance, compounds of formula (I)
wherein R1, RZ, R4, R5 and R6 are
as defined above, and R3 and A are as described herein, may be prepared from
compounds of formula
(XIX) according to reaction scheme 4.
N OH
PGN N 7R H2N
R1 ZI OHRs RI ZI HO~Rs
R2
N 6 (7) R N R6
(XIX) R4 R4
(XX)
1 (18)
H2N N
R~ O R6
R 2 N Rs
R4
(~)
a0 Scheme 4
Reaction Step 7. Amino pyridine deprotection
Compounds of formula (XX) may be prepared from compounds of formula (XIX) by
deprotection. -For
example, when the. 2,5-dimethylpyrrole system is used to protect the
aminopyridine group it may
25 deprotected by treatment with hydroxylamine. Typical conditions comprise
1.0 equivalents of compound
(XIX) and 5 equivalents of hydroxylamine hydrochloride in ethanol at reflux.
Reaction Step 18. Morpholine ring closure -30 Compounds of formula (I) may
then be prepared by cyclisation of compounds of formula (XX) by
treatment with acid. Typical conditions employ concentrated sulfuric acid and
dichloromethane as solvent
at room temperature or above.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
14
Other methods, such as those described for Reaction Step 16 in scheme 3 may
also be used to form the
morpholine ring.
Scheme 5 describes an alternative method for conversion compounds of formula
(XVIII) into compounds
of formula (XI), wherein R1, Ra, R4, R5 and R6 are as defined above.
PGN N I OH
R~~O~R
R z
N Re
R (XVIII)
(19)
PGN N
R S
z
N R
1~4
(XI)
Scheme 5
Compounds of formula (XI) may be formed from compounds of the formula (XVIII)
by reaction step 19 -
reaction of a compound of formula (XVIII) with an hydride source such as
triethylsilane and an acidic or
Lewis acidic reagent such as trimethylsilyltriflate. Typical conditions
comprise addition of 5 - 10
equivalents of triethylsilane to I equivalent of the morpholinol (XVIII) in
dichloromethane at -78 C
followed by addition of 2 equivalents of trimethy(silyltriflate.
Similarly, if the protecting group is absent from the compound of formula
(XVIII), this process step
provides an alternative route to compounds of formula (I).
An alternative procedure for the formation of compounds of formula (XIX) is
shown in Scheme 6, wherein
R', R2, R', R5 and R6 are as defined above.
0 R6
N + HNOH
PGN Rz R4 Rs (XVII)
I
Ri
(XIII) HCI
(20)
PGN N OH
HO
z
N R6
R
(XIX)
Scheme 6 Compounds of formula (XIX) may be derived by reaction step 20 -
reaction of an amine of formula (XVII)
with an epoxide of formula (XIII). The reaction is generally conducted in an
inert solvent such as toluene or
DMSO and at elevated temperature. Typical reaction conditions: involve heating
(XIII) and (XVII) together
in DMSO at 90 C.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
An alternative method for the synthesis of compounds of formula (XVIII) is
shown in Scheme 7, wherein
R1, R2, R4, RS and R6 are as defined above.
Hal OO` 'Rs
N + Jl"\
PGN I ~ RZ N R6
Ri R4
(VI) ~ (XXI)
(21)
PGN N I OH
x
R1OJ.Re
R N R
R
5 (XVIII)
Scheme 7
Compounds of formuia (XVIII) may be prepared by reaction step 21 - reaction of
an organometallic
reagent generated from a halogenated pyridine compound of formula (VI), with a
morpholinone compound
of formula (XXI) (see scheme 8). Suitable organometallic pyridine derivatives
include Grignard
10 (organomagnesium) or organolithium reagents, which may be prepared from the
bromide (or iodide) by
halogen-metal exchange. Typical conditions comprise addition of
isopropylmagnesium chloride (or
butyllithium) to the bromide (VI) in an anhydrous ethereal solvent such as
tetrahydrofuran at room
temperature (may require heating in certain cases when isopropylmagnesium
chloride is used as the
metallating agent) or below (e.g. -78 C when butyllithium is used) to perform
the halogen metal exchange
15 reaction, followed by addition of the morpholinone (XXI) at 0 C or lower.
Morpholinone compounds of formula (XXI), wherein R4, R5 and R6 are as defined
above, may be prepared
as shown in Scheme 8
HO`/Rs
0T 0~ Ji.
+ HN R5
Br R4
(XXII) (XVII)
1 (22)
OOxR6
1N R5
R4
(XXI)
Scheme 8
Morpholinone compounds of formula (XXI) may be prepared by reaction step 22 -
the- reaction of an
amino alcohol of formula (XVII) with an a-halo ester compound such as methyl
bromoacetate (XXII) in the
presence of a base such as triethylamine or 4-methylmorpholine. Typical
conditions comprise 1 equivalent
of the aminoalcohol (XVII) with 1-3 equivalents of triethylamine and I
equivalent of methyl bromoacetate
using toluene as solvent at room temperature or above. In some cases heating
with azeotropic removal of
methanol is required to achieve a good conversion to the desired product
(XXI).
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
16
An alternative method for the synthesis of epoxides of formula (XIII), wherein
R' and R 2 are as defined
above, is shown in Scheme 9.
N H
PGN RZ
R' (VII)
(23)
0
N
PGN I ~ RZ
R~
(xm)
Scheme 9
Compounds of formula (XIII) may be prepared by reaction step 23 - reaction of
an aidehyde of formula
(VII) with a sulfur ylid reagent such as that generated from
trimethylsulfonium iodide and a suitable base.
Typical reaction conditions involve: reaction of trimethylsulfonium iodide (1
equivalent) with sodium
hydride (1 equivalent) in DMSO at 0 C followed by addition of the aldehyde
(VII) and allowing the reaction
to stand at room temperature.
A further alternative method for the synthesis of epoxide compounds of formula
(XIII), wherein R' and R2
are as defined above, is shown in Scheme 10.
N ~ I
I ,
PGN (XXIII)
1(24)
0
N
PGN RZ
R~ (XIII)
Scheme 10
Compounds of formula (XIII) may be prepared by reaction step 24 - treatment of
an alkene of formula
(XXIII) with an oxidising agent such as m-chloroperbenzoic acid, or
dimethyidioxirane. Typical reaction
conditions comprise: reaction of I equivalent of alkene (XXIII) with 1-2
equivalents of m-chloroperbenzoic
acid in dichloromethane at room temperature.
Alkenes of formula (XXIII), wherein R' and R2 are as defined above, may be
prepared _according to
scheme 11.
H (25) N
PGN ~ RZ PGN I/ RZ
R~ (VII) R (XXIII)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
17
Scheme 11
Alkene compounds of formula (XXIII) may be prepared from aldehydes of formula
(VII). by reaction step
25, a Wittig or similar olefination reaction. Typical reaction conditions
involved treating 1 equivalent of
aidehyde (VII) with 1-2 equivalents of the ylid generated from the reaction of
equal molar quantities of
methyl triphenylphosphonium iodide and butyllithium, in tetrahydrofuran and
room temperature or below.
Alternatively, alkenes of formula (XXIII), wherein R' and R2 are as defined
above, may be prepared
according to scheme 12.
Hal (26)
PGN' RZ PGNN
~ RZ
R~ (VI) R, (XXIII)
Scheme 12
Alkene compounds of formula (XXIII) may be prepared by reaction step 26 - a
palladium catalysed
vinylation reaction using halide compounds of formula (VI). Typical vinyl
sources which may be used for
this process include vinyltributylstannane, ethene gas (at high pressure), or
a vinyl boronic acid. Many
Pd(0) or Pd(II) catalysts are suitable for this transformation, such as
Pd(PPh3)4. Typical conditions
comprise: reaction of a halogenated pyridine of formula (VI) (1 equivalent)
with ethylene gas (at high
pressure e.g. 120 psi) in an acetonitrile solution, in the presence of a Pd-
catalysts such as Pd(OAc)Z (1.5
mol%), a phosphine Iigand such as tri-o-tolylphosphine (5 mol%) and amine
base, such as triethylamine
(large excess) at elevated temperatures (e.g.80 C).
In the preparation of a compound of formula (I), it will be clear to those
skilled in the art that the R4 group
(as defined above) may be introduced into any of several intermediates in the
synthetic sequence. This is
most conveniently achieved by reaction step 27, a reductive amination
procedure. Examples of suitable
intermediates for use in such a transformation are shown in Scheme 13, wherein
R', R2, R5 and R6 are as
defined above. Other intermediates useful in the preparation of compounds of
formula I may be equally as
practicable.
PGN ,N OH PGN ~N
H J\R (27) i OH s .
~OYR
R Z J~
H Rs
2
R
Ra .
(XIX) (XIX)
s
Rs __1Y
~R6 (27) O R R
-- N N.Ra
NH ~
PGN / RZ
PGN RZ R2
(xi)
(xq
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
18
R6 RB
-ly R5 -1-Y R5
N~ NH (27) N\ N Re
1
H2N I/ RZ H2N ~ RZ
R~ R
Scheme 13
A typical procedure comgrises reacting 1 equivalent of secondary amine (such
as (XIX), (XI), or (I), with 1
equivalent of an aldehyde in an inert solvent such as tetrahydrofuran or
dichloromethane at room
temperature, then addition of 1 equivalent (or more) of sodium
triacetoxyborohydride or sodium
cyanoborohydride. ,
In some instances, for example in the preparation of compounds of formula (I)
wherein R4 is H, R3 and A
are as defined herein and wherein R1, R2, R5 and R6 are as defined above, it
may be advantageous to use
a protecting group PG' prior to formation of the morpholine ring. This is
illustrated in Scheme 14.
Any suitable nitrogen protecting group may be used (as described in
"Protecting Groups in Organic
Synthesis" 3rd Edition T. W. Greene and P.G. Wuts, Wiley-Interscience, 1999).
A common nitrogen
protecting group (PG') suitable for use herein is tert-butoxy carbonyl, which
is readily removed by
treatment with an acid such as trifluoroacetic acid or hydrogen chloride in an
organic solvent such as
dichloromethane.
PGN N OH PGN N OH HzN N
~ e
R s R' : I HO,/R6 R O R
N R (28) Rz NJI~Rs (16) Rz NI Rs
(XIX) H (XXIV) PG'
PG' (XXV)
(29)
H2N R ~ I O Rs
z
R N Rs
H (1)
Scheme 14
Reaction Step 28. N-protection
A compound of formula (XXIV) may be prepared by reaction step 28 - N-
protection. A compound of
formula (XIX) (in which R4 =-H) is reacted with a nitrogen protecting agent,
such as benzyl chloroformate
to produce the protected compound. Typical reaction conditions involve:
reaction of 1 equivalent of
secondary amine (XIX) with (1 equivalent, or more) of benzyl chloroformate in
an inert solvent such as
dichloromethane, together with triethylamine (I equivalent, or more) at room
temperature.
Reaction Step 16. Ring closure
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
19
Ring closure of diol (XXIV) to morpholine (XXV) may be accomplished using many
of the methods
previously described herein, see Reaction Step 16, scheme 3. Particularly
suitable in this instance is a
Mitsunobu-type ring closure reaction through the action of a dialkyl
azodicarboxylate reagent (1.1
equivalent) plus triphenylphosphine (1.1 equivalent) in an inert solvent such
as tetrahydrofuran at room
temperature.
Reaction Step 29 protecting group removal
Compounds of formula (I) may be prepared by reaction step 29 - deprotection of
morpholine (XXV), under
conditions dependent upon the nature of the protecting group used. For
example, if benzyloxycarbonyl is
used as the protecting group then it may be removed by hydrogenolysis in an
inert solvent such as ethanol
with a palladium catalyst such as palladium on carbon, under hydrogen pressure
of I atmosphere or
greater. If the morpholine nitrogen is protected with a benzyl group it can be
deprotected by transfer
hydrogenation. Typical conditions involve treating one equivalent of compound
of formula (XXV) with
ammonium formate (10 equivalents) in ethanol and the presence of 10% palladium
on carbon as catalyst
(10% by weight), at reflux for 3 hours.
Compounds of formula (XVII), wherein R4, R5 and R6 are as defined above, may
be prepared according to
scheme 15.
RS
HZN'~/OH
1R
(XVI)
(30)
R5
HN),yOH
R4 RB (XVII)
HCI
Scheme 15
Compounds of formula (XVII) (where R4 is not H) may be prepared by reaction
step 30, a reductive
amination procedure. Typical conditions involve: reaction of I equivalent of
amino alcohol of formula (XVI)
with 1.1 equivalents of an aldehyde in dichloromethane and the presence of
dried 4A molecular sieves at
room temperature. After filtration and evaporation of the reaction mixture,
the residue is redissolved in
methanol and reacted with sodium borohydride (2 equivalents or more) at room
temperature.
Alternatively the reductive amination can be accomplished in two steps via
formation and then reduction of
an intermediate amide, in a similar fashion to that described for Reaction
Step 4 (Scheme 1) and in
Reaction Steps 11 and 12 (scheme 3).
One skilled in the art will be aware that many amino alcohol compounds of
formulas (XVI) and (XVII) are
commercially available. Alternatively they may be prepared according to
numerous methods known to
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
those skilled in the art, such as those described in Tetrahedron 2000, 56,
2561-2576 and references cited
therein.
Compounds of formula (I), wherein R1, R2, R4, R5 and R6 are as defined above,
and R3, R5 and A are as
5 described herein, may be prepared from chloropyridines of formula (XXVI)
according to scheme 16.
Ph
CI N _N N
R~ O Rs (31) Ph
R~ I O R
R s
N s R
I R N~R
z z
R (XXVI) R (XXVII)
1 (32)
HZN N
R R 1 z
R N R 5
R4
(~)
Scheme 16
It will be clear to those skilled in the art that chloropyridine intermediates
of formula (XXVI) are accessible
through application of analogous synthetic methods to those previously
described herein for the
10 production of protected aminopyridine compounds of formula (XI).
Reaction Step 31. Metal catalysed amination reaction
Compounds of formula (XXVII) may be prepared by reaction step 31, reaction of
a compound of formula
15 (XXVI) with benzophenone imine, in the presence of a suitable base and a
metal catalyst, e.g. a Pd
complex. Typical reaction conditions involve: reacting chloropyridine (XXVI)
(1 equivalent) with
benzophenone imine (1.2 equivalents), sodium tert-butoxide (1.4 equivalents),
tris(dibenzylideneacetone)dipalladium(0) (1 mol%) and 2,2'-
bis(diphenylphosphino)-1.1'-binaphthyl
(BINAP) (3 mol%) in toluene at 80 to 120 C.
Reaction Step 32 Benzophenone deprotection
Compounds of formula (XXVII) may be converted to compounds of formula (I) by
hydrogenolysis (using
an inert solvent and heterogeneous catalysis such as Pd on carbon at or above
I atmosphere pressure of
hydrogen), or alternatively by treatment with an aqueous acid e.g. 2M HCI in
the presence of water and
miscible organic solvent such as tetrahydrofuran or dioxan. Transfer
hydrogenation may also be used to
effect this transformation. Typical conditions involve treating one equivalent
of compound of formula
(XXVII) with ammonium formate (10 equivalents) in ethanol and the presence of
10% palladium on carbon
as catalyst (10% by weight), at reflux for 3 hours.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
21
Compounds of formula (I) wherein R1, R2 and R4 are as defined above and R3 is
as defined herein, may
be prepared according to scheme 17.
boc H
2 boc N N
R N
.{- - -
N R~ (33) RZ (34) R~
CI I N/ R~ N '- Ra
(XXVIII) (XXIX) cl (XXX) ci (XXXI)
1 (27)
R4 R4
N N R4
N
R2 (32) R2 (31)
~- E--- R
N R~ N R~
Ph N N R6
NHZ (I) Y CI (XXXII)
Ph (XXXIII)
Scheme 17
Reaction Step 33. Zincate coupling
Compounds of formula (XXX) may be prepared by reacting compounds of the
formula (XXIX) with Zn/Cu
couple (or other activated Zn source) with sonication, followed by addition of
a 2-chloro-4-iodopyridine and
a suitable palladium catalyst and ligand, and heating to 70 C for 18 hours.
Typical conditions comprise 1
equivalent of the azetidine (XXIX) with 40 wt% Zn/Cu couple in DMF with
sonication at room temperature
for 4 hours, followed by addition of 1.05 equivalents of the halogenated
pyridine (VI), 0.05 equivalents of
tris(dibenzylideneacetone)dipalladium(0) and 0.1 equivalents of tri-o-
furylphosphine and heating to 70 C
for 18 hours. Compounds of the formula (XXIX) may be prepared as described in
Synlett, 4, 1998, 379.
Reaction Step 34 Boc deprotection
Compounds of formula (XXXI) may be prepared by reacting compounds of the
formula (XXX) with a
suitable acid, such as HCI or TFA in a suitable solvent such as
dichloromethane or diethyl ether at room
temperature or above, if necessary in the presence of a cation scavenger such
as Et3SiH. Typical
conditions comprise 1 equivalent of the azetidine (XXX) with CH2CI2 saturated
with HCI gas at 0 C then
allowing to stand at "room temperature overnight.
Reaction Step 27. Reductive amination
Compounds of formula (XXXII) may be prepared by reacting compounds of formula
{XXXI) with 1-5
equivalents of the required aldehyde in a suitable solvent at room temperature
in the presence of 1-5
equivalents of a suitable reducing agent such as sodium triacetoxyborohydride
or sodium
cyanoborohydride in a suitable solvent such as dichloromethane or
tetrahydrofuran with the optional
addition of acetic acid. Typical conditions comprise reacting 1 equivalent of
the azetidine (XXXI) with 3.1
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
22
equivalents of the aldehyde and 3.1 equivalents of sodium
triacetoxyborohydride in dichloromethane at
room temperature for 18 hours.
Reaction Step 31. Metal catalysed amination reaction
Compounds of formula (XXXII) may be converted to compounds of formula (I) via
intermediates (XXXIII).
Conversion of (XXXII) to (XXXIII) may accomplished using benzophenone imine
together with a suitable
base and a metal catalyst, e.g. a Pd(0) complex. Typical reaction conditions
involve: reacting
chloropyridine (XXXII) (1 equivalent) with benzophenone imine (1.2
equivalents), sodium tert-butoxide (1.4
equivalents), tris(dibenzylideneacetone)dipalladium(0) (1 mol%) and 2,2'-
bis(diphenylphosphino)-1.1'-
binaphthyl (BINAP) (3 mol%) in toluene at 80 to 120 C.
Reaction Step 32. Benzophenone deprotection
Compounds of formula (XXXIII) may be converted to compounds of formula (I)
either by hydrogenolysis
(using an inert solvent and heterogeneous catalysis such as Pd on carbon at or
above 1 atmosphere
pressure of hydrogen), or by treatment with an aqueous acid in the presence of
a water miscible organic
solvent such as tetrahydrofuran or dioxan. Transfer hydrogenation may also be
used for effect this
transformation. Typical conditions involve treating one equivalent of compound
of formula (XXVII) with
ammonium. formate (10 equivalents) in ethanol and the presence of 10%
palladium on carbon as catalyst
(10% by weight), at reflux for 3 hours.
Alternatively, compounds of formula (I), wherein R1, R2 and R4 are as defined
above and R3 is as defined
herein, may be prepared according to scheme 18.
boc
Hal I H
boc N N
RZ N (33) (34)
N -C Ri R2 R 2
Y(XXIX)
NPG (VI) I N Ri N Ri
NPG (XxXIV) NPG (XXXV)
(27)
Rd Ra
1 1
N N
R2 Rz
(7) N / R
N / R~
(I) NHZ NPG (XXXVI)
Scheme 18
Reaction Step 33. Zincate coupling
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
23
Compounds of formula (XXXIV) may be prepared by reacting compounds of the
formula (XXIX) with
Zn/Cu couple (or other activated Zn source) with sonication, followed by
addition of compounds of the
formula (VI) and a suitable palladium catalyst and ligand, and heating to 70 C
for 18_ hours. Typical
conditions comprise 1 equivalent of the azetidine (XXIX) with 40 wt% Zn/Cu
couple in DMF with sonication
at room temperature for 4 hours, followed by addition of 1.05 equivalents of
the halogenated pyridine (VI),
0.05 equivalents of tris(dibenzylideneacetone)dipalladium(0) and 0.1
equivalents of tri-o-furylphosphine
and heating to 70 C for 18 hours. Compounds of the formula (XXIX) may be
prepared as described in
Synlett, 4, 1998, 379.
Reaction Step 34. N-Boc deprotection
Compounds of formula (XXXV) may be prepared by reacting compounds of the
formula (XXXIV) with a
suitable acid, such as HCI or TFA in a suitable solvent such as
dichloromethane or diethyl ether at room
temperature or above, if necessary in the presence of a cation scavenger such
as Et3SiH Typical
conditions comprise I equivalent of the azetidine (XXXIV) with CH2CI2
saturated with HCI gas at 0 C then
allowing to stand at room temperature overnight.
Reaction Step 27. Reductive amination
Compounds of formula (XXXVI) may be prepared by reacting compounds of formula
(XXXV) with 1-5
equivalents of the required aldehyde in a suitable solvent at room temperature
in the presence of 1-5
equivalents of a suitable reducing agent such as sodium triacetoxyborohydride
or sodium
cyanoborohydride in a suitable solvent such as dichloromethane or
tetrahydrofuran with the optional
addition of acetic acid. Typical conditions comprise 1 equivalent of the
azetidine (XXXV) with 3.1
equivalents of the aldehyde and 3.1 equivalents of sodium
triacetoxyborohydride in dichloromethane at
room temperature for 18 hours.
Reaction Step 7. AminopVridine deprotection
Compounds of formula (XXXVI) may be converted to compounds of formula (I) by
deprotection. The
nature of this reaction will depend upon the protecting group selected for
use. For example, when the 2,5-
dimethylpyrrole system is used to protect the aminopyridine group it may be
deprotected by treatment with
hydroxylamine. Tipical conditions comprise 1.0 equivalents of compound (XXXVI)
and 5 equivalents of
hydroxylamine hydrochloride in ethanol at reflux.
SCHEMES 19-29
Compounds of formula I wherein R3 is moiety IV and unless otherwise indicated
R', R2, RB, R9 and R10 are
as defined above, may be prepared using methods described in Schemes 19-29.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
24
0
H (35) N~ ~ (36) N~ NHZ
PGN I ~ RZ ~ \
PGN RZ N PGN I~ F22
R (VII) R1 (XXXVII) R (XXXVIfI)
1 (27)
N N.R9 (7) N N.Rs
--
HZN I RZ R R' (I) R (XXXIX)
Scheme 19
Reaction Step 35. Nitrile formation
Compounds of formula (VII) may be converted to nitrile compounds of formula
(XXXVII) by reaction with
tosylmethyl isocyanide (TosMic). Typical conditions involve: treating aldehyde
(VII) (1 equivalent) with
TosMic (1 equivalent) and potassium tert-butoxide (2 equivalents) in ethylene
glycol dimethyl ether at -45
C after a period of 30 minutes methanol is added and the reaction mixture
allowed to reach room
tem perature.
Reaction Step 36. Nitrile reduction
Nitriles of formula (XXXVII) may be converted to amines of formula (XXXVIII)
by reduction of the nitrile
group. This reduction may be achieved through the action of a hydride reducing
agent, such as lithium
aluminium hydride, or sodium borohydride in the presence of a transition metal
salt, such as NiCI2 or
CoC12. Alternatively the nitrile group may be reduced by hydrogenation with a
transition metal catalyst such
as Raney Nickel or Pd on carbon.
Typical conditions involve: reacting nitrile (XXXVII) (1 equivalent) with
nickel chloride (1 equivalent) in
methanol followed by cautious addition of sodium borohydride (3 equivalents or
more) at 0 C.
Reaction Step 27. Reductive amination.
The primary amines (XXXVIII) may be converted to compounds of formula (XXXIX)
by a reductive
amination procedure, by reaction with an aldehyde and hydride reducing agent
such as sodium
triacetoxyborohydride or sodium borohydride
Typical conditions involve: reacting compounds of formula (XXXVIII) with a
suitable aldehyde (1 equivalent
or more) in the.presence of a hydride reducing agent such as sodium
cyanoborohydride or sodium
triacetoxyborohydride (1 equivalent or more) in an alcoholic solvent such as
ethanol.
Reaction Step 7 Aminopyridine deprotection
Compounds of formula (XXXIX) may be converted to compounds of formula I by
using a reaction to
deprotect the nitrogen of the aminopyridine group (PGN) to liberate the NH2 in
compounds (I). The nature
of this reaction will depend upon the protecting group selected for use. For
example, when the 2,5-
dimethylpyrrole system is used to protect the aminopyridine group, it may be
deprotected by treatment
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
with hydroxylamine. Tvpical conditions comprise 1.0 equivalents of compound
(XXXIX) and 5 equivalents
of hydroxylamine hydrochloride in ethanol at reflux.
Alternatively, nitrile compounds of formula (XXXVII) may be converted into
compounds of formula (I) as
5 shown in Scheme 20.
H H
(37) N NyR (38) N ': N.Rs
I 2
Z 2
PGN R R
PGN R ~ -~ HZ
N R
(~)
R (XXXVII) R (XL)
Scheme 20
Reaction Step 37. Reductive acylation
10 The intermediates of formula (XXXVII) may be reduced e.g. with sodium
borohydride and nickel chloride
in the presence of an acylating agent, such as a carboxylic acid anhydride to
afford amide intermediates of
formula (XL). Typical conditions involve: reacting nitrile (XXXVII) (1
equivalent) with nickel chloride (1
equivalent) and a carboxylic acid anhydride (1 equivalents or more) in
methanol followed by cautious
addition of sodium borohydride (3 equivalents or more) at 0 C.
Reaction Step 38. Amide reduction
Amides of general formula (XL) may be reduced to amines using borane or
lithium aluminium hydride.
Typical conditions comprise 1.0 equivalents of amide (XL), 1.2-3.0 equivalents
of borane in THF at reflux
followed by heating in strong aqueous acid, such as 5M HCI. The resulting
amine intermediates may then
be deprotected to give the aminopyridine compounds of formula (I), as
previously described in Reaction
Step 7.
An alternative preparation of nitrile compounds of formula (XXXVII) is shown
in Scheme 21.
N ~ HaI (39) N ~
PGN R2 PGN I~ R2
R (VI) R (XXXVII)
Scheme 21
Compounds of formula (XXXVII) may be prepared by reaction step 39 - reaction
of halogenated pyridine
(VI) with tributyl(cyanomethyl)stannane and a palladium catalyst according to
the procedure described in
Chem. Lett 1984, 1511-1512, Typical conditions involve treating 1 equivalent
of (VI) with 1.5 equivalents of
tributyl(cyanomethyl)stannane, bis(acetonitrile)dichloropalladium(II) (2.5
mol%) and tri-o-tolylphosphine (5
mol%) in xylene at 120 C.
An alternative procedure for the preparation of compounds of formula (XXXVIII)
is shown in scheme 22.
N "' Hal
PGN I ~ RZ + ~H.PG'
R
(VI) (40) (XLI)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
26
H
N Y N.PG,
PGN -:0 RZ
Ri (XLII)
~ (29)
N ~ NHz
PGN I ~ Rz
R' (XXXVIII)
Scheme 22
Reaction Step 40. B-alkyl Suzuki coupling
Protected amides of formula (XLII) are available by B-alkyl Suzuki coupling
between a vinyl carbamate
(XLI) and a halogenated pyridine (VI), in a similar fashion to that described
in J. Org. Chem. 1999, 64,
8743-8744. In a typical procedure benzyl vinyl carbamate [commercially
available, or prepared as
described in J. Org. Chem. 1999, 64, 8743-8744] was treated with I equivalent
of 9BBN solution in
tetrahydrofuran at -10 C. After completion of the hydroboration stage, the
resulting organoboron
intermediate is treated with sodium hydroxide, PdC12(dppf).CHZCI2 complex is
added together with a
halogenated pyridine of formula (VI).
Reaction Step 29 Amine deprotection
Compounds of formula (XLII) may be converted to compounds of formula (XXXVIII)
by deprotection. The
nature of this reaction will depend upon the protecting group selected for
use. For example, when
benzyloxycarbonyl is used as the protecting group then it may be removed by
hydrogenolysis in an inert
solvent such as ethanol with a palladium catalyst such as palladium on carbon.
Typical reaction conditions
involve: reacting compounds of formula (XLII) in an alcohol solvent (such as
ethanol) with hydrogen (at a
pressure of 1 atmosphere of greater) in the presence of a transition metal
catalyst such as Pd on carbon.
An alternative method for the production of compounds of formula (XXXIX) is
shown in Scheme 23.
OH H H
N N,R4 (41) N,Rs
PGN ~ RZ PGN RZ
R~ (IX) R~ (XXXIX)
Scheme 23
Compounds of formula (XXXIX) may be prepared by reaction step 41 -
deoxygenation of a compound of
formula (IX) by, for example, hydrogenation (in an inert solvent such as
ethanol, in the presence of a
transition metal catalyst, such as Pd on carbon in an atmosphere of hydrogen
(1 atmosphere or higher)).
Alternatively, a hydride source such as triethylsilane in conjunction with a
suitable acid may be used (as
described in Heterocycles 2003, 1203-1209) Typical reaction conditions
involve: -dissolving (IX) in a
mixture dichloromethane and trifluoroacetic acid at room temperature and
adding 1(or more) equivalents
of triethylsilane.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
27
A further method for the production of compounds of formula (XXXVII) is shown
in scheme 24.
o X
N H (42) N (43) N ~f'R
N
PGN I/ RZ PGN I/ RZ PGN IR' (VII) R' (XLIII) R (XXXVII)
Scheme 24
Reaction Step 42. Conversion to benzylic electrophile.
An aidehyde of formula (VII) is reduced by treatment with a hydride reducing
agent such as sodium
borohydride in an alcoholic solvent, such as ethanol at room temperature. The
resulting alcohol can be
activated towards nucleophilic displacement by conversion to a group X
(generally a halide or sulfonate
ester) to give intermediates of formula (XLIII). Typical conditions involve:
reaction of one equivalent of
methanesulfonyl chloride and one equivalent of an amine base such as
triethylamine in an inert solvent
such as dichloromethane at 0 C.
Reaction Step 43. Cyanide displacement
Intermediates of formula (XLIII) can be converted to compounds of formula
(XXXVII) by the action of a
nucleophilic source of cyanide, such as KCN, in an inert solvent, such as
dimethylformamide, at or above
room temperature, in a procedure analogous to that described in US5914319.
A further method for the production of compounds of formula (XXXIX) is shown
in Scheme 25.
0
N~ Hal (44) N~ (45) (""~O OH R1\ Rs
/
PGN I R z PGN I/ Rz PGN + H
R' (VI) RI (XLIV) R
(XLV) (XLVI)
1 (46)
Rlo R'o
N ~ N.Ro (38) N ~ N.R9
1 / 2O
PGN I 7 RZ PGN ~ R(XLVII)
R (XXXIX) R
Scheme 25
Reaction Step 44. Methyl ketone formation
Halogenated pyridines or formula (VI) can be converted to methyl ketones of
formula (XLIV) by treatment
first with butyllithium (or other agent capable of facilitating a halogen
metal exchange reaction) and
treating the resultant organometallic intermediate with a suitable acetyl
source, such as acetylmorpholine
or the Weinreb amide derived from acetic acid. Reaction Step 45. Wiligerodt-
Kindier reaction
Methyl ketones of formula (XLIV) may be converted into arylacetic acids of
formula (XLV) by treatment
with sulfur and morpholine. A typical procedure involves: reacting 1
equivalent of methyl ketone (XLIV)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
28
with sulfur (2 equivalents) and in excess morpholine at reflux (either neat or
in an alcoholic solvent such
as ethanol), followed by hydrolysis either in refluxing 2M hydrochloric acid
or 2M NaOH.
Reaction Step 46. Amide formation.
Pyridyl acetic acids of formula (XLV) may be converted to amides of formula
(XLVI) by reaction with an
amine of formula (XLVII) and a suitable amide coupling reaction, such as by
reaction with an acid chloride
or anhydride then addition of a suitable amine, or using a peptide coupling
reagent such dicylcohexyl
carbodiimide. or other carbodiimide reagent. For example, acid chlorides in
the presence 'of a suitable
base such as triethylamine or 4-methylmorpholine may be used for the amide
forming stage. Typical
reaction conditions comprise conversion of the acid (XLV) to the acid chloride
by treatment with oxalyl
chloride with a trace of dimethylformamide as catalyst in an inert solvent
such as dichloromethane. After
evaporation of solvents and excess oxalyl chloride, 1.0 equivalents of amine
(XLVII), 1.2-2.0 equivalents
of base (preferably triethylamine) are reacted with 1.0 equivalents of the
acid chloride in dichloromethane
at 25 C.
Reaction Step 38 Amide reduction
Amides of general formula (XLVI) may be converted into compounds of formula
(XXXIX) by reduction with
a hydride reducing agent, such as borane-tetrahyrdrofuran complex. Typical
conditions comprise 1.0
equivalents of amide (XLVI), 1.2-3.0 equivalents of borane in THF at reflux
then treatment with a strong
acid such as 5M HCI at elevated temperature to hydrolyse the initially formed
boron complexes with the
product. Other non-acidic methods are available for breaking the boron complex
e.g. treatment with
diethanolamine.
An alternative method for the production of compounds of formula (XLV) is
shown Scheme 26.
N _ ~_
1N (47) N r OH
PG
N IRa PGN R (XXXVII) R~ (XLV)
Scheme 26
Compouns of formula (XLV) may be prepared according to reaction step 47,
hydrolysis. A nitrile of general
formula (XXXVII) is hydrolysed by heating in a strongly acidic or basic
aqueous solution. Typical
conditions involve heating a compound of formula (XXXVII) in a 5M HCI solution
at reflux.
OH H R8
N N,R (48) N N,R4
PGN R2 I
PGN R2
R (IX) R (XLVIII)
Scheme 27
Compounds of formula (XLVIII), wherein R8 is OMe, can be formed from compounds
of formula (IX) by
reaction step 48 - methylation of alcohol (IX) with a suitable electrophilic
methyl source, such as
iodomethane. In general, a strong base, such as sodium hydride is also
required. Typical conditions
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
29
involve treating 1 equivalent of (IX) with 1.1 (or more) of sodium hydride in
an inert solvent such as
tetrahydrofuran or dimethylformamide then adding 1(or more) equivalents of
iodomethane at room
temperature.
Compounds of general formula (XLVIII) can subsequently be converted into
compounds of formula (I)
using the same methods as described for the conversion of compounds of formula
(XXXIX), as shown in
Scheme 19.
Further examples of compounds of formula (I), wherein R8 is not hydrogen, can
be prepared according to
scheme 28.
0 CN
N Hal (44) N (49) R$
11 PGN ~ Rz PGN IR~RB
PGN I Rz
R1 (VI) RI (XLIX) RI (XXXVII)
Scheme 28
Reaction Step 44. Ketone formation
Halogenated pyridine compounds of formula (VI) can be readily converted to
ketones of formula (XLIX)
using methods similar to the formation of methyl ketone compounds of formula
(XLIV) (scheme 25).
Namely, by treatment first with butyllithium (or other agent capable of
facilitating a halogen metal
exchange reaction) and treating the resultant organometallic intermediate with
a suitable acyl source, such
as acylmorpholine or Weinreb amide (both which are readily prepared using
methods well-known to the
skilled person).
Reaction Step 45. Nitrile formation
The ketone of formula (XLIX) can then be converted to nitrile (XXXVII) by
reaction with tosylmethyl
isocyanide (TosMic). Typical conditions involve: treating ketone (XLIX) (1
equivalent) with TosMic (1
equivalent) and potassium fert-butoxide (2 equivalents) in ethylene glycol
dimethyl ether at -45 C. After a
period of 30 minutes methanol is added and the reaction mixture allowed to
reach room temperature.
Nitriles of formula (XXXVII) may subsequently be converted to compounds of
formula (I) using -the
procedures previously described in scheme 19.
0 8
N'R N,Re R N.Re
PGN I~ RZ HZN I/ RZ _-
Ri (XXXIX) R, (1)
(50) (50)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
Ra Rio Ra Rto
,Re N N,R
PGN '~ RZ HZN I~ RZ
R~ (XXXIX) R
(~)
Scheme 29
Compounds of formula (XXXIX) or (I) wherein R10 = H, may be readily converted
into further compounds
5 of formula (XXXIX) or (I) wherein R10 is not H, by reaction step 50 - a
reductive amination procedure as
shown in Scheme 29. A typical procedure involves reacting 1 equivalent of a
secondary amine (such as
(XXXIX) or (I)), with 1 equivalent of an aldehyde in an inert solvent such as
tetrahydrofuran or
dichloromethane at room temperature, then addition of I equivalent (or more)
of sodium
triacetoxyborohydride or sodium cyanoborohydride.
10 Alternatively, the reductive amination may be conducted in two steps via an
intermediate amide in similar
fashion to that described for Reaction Step 4 in scheme 1.
Compounds of formula (I) wherein R1, R2, Ra and R5 are as defined above, and
R3, R5 and A are as
described herein, may be prepared according to reaction scheme 30.
H
N O H
N X HS R O(L) N~ gN~O
PGN Rz (51) PGN ~ R2 R O
R1 (XLIII) R1 (LI) (52)
H NH
(53) N S---,~N,Ra (4) N~ S'y z
PGN R
PGN I Rz R5 z R5
(LII)
R (LIII) R
OyO.R~~ 5 5
S~R S~ R
N zS R N.Ra (54) N~ N.Ra (55) N~ N.Ra
PGN R ~~ z O z
Ri (LIV) PGN R PGN R
R (LV) R _(LVI)
(7)
S--YRs
N N.Ra
HZN I R2
(I)
15 R
Scheme 30
Reaction Step 51. Thioether formation
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
31
Thioethers of the formula (LI) may be formed by reaction of a compound of
formula (XLIII), wherein X is
generally a halide or a sulfonate ester, with a compound of the formula (L)
[commercially available or
prepared a described in J.Chem.Soc Perkin l, 1987, 111-120] in the presence of
a base in an alcoholic
solvent.
5Typical conditions comprise 1.0 equivalents of alkylhalide, 1.0 equivalents
of thiol and 1.0-4.0 equivalents
of a tertiary amine base such as triethylamine in an alcoholic solvent such as
ethanol.
Reaction Step 52. N-Boc deprotection
Compounds of formula (LII) may be prepared by reacting compounds of the
formula (LI) with a suitable
acid, such as HCI or TFA in a suitable solvent such as dichloromethane or
diethyl ether at room
temperature or above, if necessary in the presence of a cation scavenger such
as Et3SiH Typical
conditions comprise adding 1 equivalent of the protected amine (LI) to CH2CI2
saturated with HCI gas at 0
C then allowing to stand at room temperature overnight.
Reaction Step 4. Reductive amination
Compounds of formula (LIII) may be prepared from compounds of formula (LII) by
employing standard
amide bond forming conditions followed by reduction of the intermediate amide
with a hydride reducing
agent such as borane or lithium aluminium hydride.
For example, acid chlorides in the presence of a suitable base such as
triethylamine or 4-
methylmorpholine may be used for the amide forming stage. Typical reaction
conditions comprise 1.0
equivalents of amine (LII), 1.2-2.0 equivalents of base (preferably
triethylamine), 1.1-1.3 equivalents of
acid chloride in dichloromethane at 25 C. Reducing agents such as borane or
lithium aluminium hydride
can be used for the amide reduction stage. Typical conditions comprise 1.0
equivalents of amide, 1.2-3.0
equivalents of borane in THF at reflux, followed by treatment with a strong
acid to hydrolyse the initially
formed boron complex of the product. Other non-acidic methods are available
for breaking the boron
complex e.g. treatment with diethanolamine.
Reaction Step 53. Carbamate formation
Compounds of formula (LIV), wherein R" is benzyl or (Cl-C6)alkyl, may be
formed by treatment of
compounds of formula (LIII) with an alkyl or benzyl chloroformate in an inert
solvent such as
dichloromethane or diethyl ether in the presence of a base.
Typical conditions comprise 1.0 equivalents of the amine (LIII), 1.0
equivalents of an alkylchloroformate
such as methyichloroformate and 1.0-3.0 equivalents of a tertiary amine base
such as triethylamine in
diethyl ether at 25 C. -
Reaction Step 54. Thiomorpholinone Ring formation
Compounds of the formula (LV) may be formed by treatment of thioether (LIV)
with a strong base such as
lithium diisopropylamide in an inert solvent such as diethyl ether or THF.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
32
Typical conditions comprise addition of 3.0 equivalents of a strong base such
as lithium diisopropylamide
to 1.0 equivalents of the thioether (LIV) at a temperature below -50 C in an
inert solvent such as THF and
allowing to warm to ambient temperature.
Reaction Step 55. Amide reduction
Compounds of formula (LVI) may be prepared by reaction of compounds of formula
(LV) with reducing
agents such as borane or lithium aluminium hydride. Typical conditions
comprise 1.0 equivalents of amide
(LV), 1.2-3.0 equivalents of borane in THF at reflux, followed by treatment
with a strong acid to hydrolyse
the initially formed boron complex. Other non-acidic methods are available for
breaking the boron complex
e.g. treatment with diethanolamine.
Reaction Step 7. Aminopyridine deprotection
Compounds of formula (LVI) may be converted to compounds of formula (I) by
deprotection. The nature
of this reaction will depend upon the protecting group selected for use.
For example, when the 2,5-dimethylpyrrole system is used to protect the
aminopyridine group it may be
deprotected by treatment with hydroxylamine. Typical conditions comprise 1.0
equivalents of compound
(LVI) and 5 equivalents of hydroxylamine hydrochloride in ethanol at reflux.
Compounds of formula (LIX) wherein R1, R2 and R5 are as defined above, may be
prepared according to
reaction scheme 31.
PGN N
OH PGN
R~ ~ OH (56) R OH X (57)
z ~
R N Rs Rz N1Rs
PG'
(XXIV) PG' (LVII)
PGN PGN N z O
OH S
RJ)CN S\ (58) Ri ~ ~ II
Rz NJI~Rs R N Rs
PG' (LIX) PG' (LVIII)
Scheme 31
Reaction Step 56. Primary alcohol activation
Compounds of the formula (LVII) may be formed from compounds of the formula
(XXIV), wherein PG' is a
carbamate protecting group such as tert-butyloxycarbonyl or benzyloxycarbonyl,
by selective conversion of
the primary hydroxyl group to a group X (generally a halide or sulfonate
ester). Typical conditions involve:
reaction of one equivalent of toluenenesulfonyl chloride and one equivalent of
an amine base such as
triethylamine in an inert solvent such as dichloromethane at 0 C.
Reaction Step 57. Thioacetate formation
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
33
Compounds of the formula (LVIII) may be formed by from compounds of the
formula (LVII), wherein PG'
is a carbamate protecting group such as tert-butyloxycarbonyl or
benzyloxycarbonyl, by reaction with a
suitable nucleophile such as thioacetic acid in an inert solvent such as
acetonitrile in the presence of a
suitable base. Typical conditions involve: reaction of one equivalent of
compounds of the formula (LVII)
with 1.0-2.0 equivalents of thioacetic acid in the presence of 1.0 - 5.0
equivalents of a suitable base such
as potassium carbonate in an inert solvent such as acetonitrile and the
mixture heated to reflux.
Reaction Step 58. Ring closure
Compounds of the formula (LVIII) may be activated towards nucleophilic
displacement at the benzylic
centre by conversion to a group X (generally a halide or sulfonate ester). In
situ ring closure may then
occur to provide compounds of the formula (LIX). Typical conditions involve:
reaction of one equivalent of
toluenenesulfonyl chloride and one equivalent of an amine base such as
triethylamine in an inert solvent
such as dichloromethane at 0 C. Evaporation of the solvent followed by
redissolution in a higher boiling
solvent such as acetonitrile with 0-5.0 equivalents of a suitable base such as
potassium carbonate and
heating of the mixture to reflux may be necessary to effect the ring closure.
Compounds of the formula (LIX), wherein PG' is a carbamate protecting group
such as
tert-butyloxycarbonyl or benzyloxycarbonyl, may be converted to compounds of
the formula (I) using
procedure analogous to those described in Scheme 18 for the conversion of
compounds of formula
(XXXIV) into compounds of formula (I).
Compounds of formula (I) wherein R1, R2 and R4 are as defined above, and R3 is
as described herein,
may be prepared according to reaction scheme 32.
(g p) NH
N (59) N N
N PGN RZ PGN IRa ~ 1a
1 PGN R
R (XXIII) R (LX) Ri (LXI)
(27)
N-R4 CN-R4
~ (7) '?~
H N N / R2 PGN RZ
2 Ri (I) R (LXII)
Scheme 32
Reaction Step 59. Cycloaddition
Compounds of the formula (LX) may be formed from an alkene of the formula
(XXIII) by reacting with
N-benzyl-N-(methoxymethyl)-trimethylsilylmethylamine and a catalytic amount of
an acid such as
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
34
trifluoroacetic acid in an inert solvent such as dichloromethane,
acetonitrile, tetrahydrofuran or toluene at -
C to the reflux temperature of the reaction mixture. Alternative catalysts
include anhydrous potassium
or cesium fluoride, tetra-n-butylammonium fluoride, trifluoromethanesulfonic
acid,
trimethylsilyltrifluoromethanesulfonate and iodotrimethylsilane. Typical
conditions involve: reaction of 1
5 equivalent of alkene (XXIII) with 1.5 equivalents of N-benzyl-N-
(methoxymethyl)-trimethylsilylmethylamine
and 0.1 equivalents of trifluoroacetic acid in dichloromethane.
Reaction Step 60. Pyrrolidine debenzylation
10 Compounds of the formula (LX) may be deprotected to secondary amines of the
formula (LXI) by
hydrogenolysis in an inert solvent such as ethanol with a palladium catalyst
such as palladium on carbon,
under hydrogen pressure of 1 atmosphere or greater. Alternatively it can be
deprotected by transfer
hydrogenation. Typical conditions involve treating one equivalent of compound
of formula (LX) with
ammonium formate (10 equivalents) in ethanol and the presence of 10% palladium
on carbon as catalyst
(10% by weight), at reflux for 3 hours.
Reaction Step 27. Reductive amination
Compounds of formula (LXII) may be prepared by reacting compounds of formula
(LXI) with 1-5
equivalents of the required aldehyde in a suitable solvent at room temperature
in the presence of 1-5
equivalents of a suitable reducing agent such as sodium triacetoxyborohydride
or sodium
cyanoborohydride in a suitable solvent such as dichloromethane or
tetrahydrofuran with the optional
addition of acetic acid. Typical conditions comprise reacting 1 equivalent of
the pyrrolidine (LX) with 3.1
equivalents of the aldehyde and 3.1 equivalents of sodium
triacetoxyborohydride in dichloromethane at
room temperature for 18 hours.
Reaction Step 7. Aminopyridine deprotection
Compounds of formula (LXI) may be converted to compounds of formula (I) by
deprotection. The nature
of this reaction will depend upon the protecting group selected for use.
For example, when the 2,5-dimethylpyrrole system is used to protect the
aminopyridine group it may be
deprotected by treatment with hydroxylamine. Typical conditions comprise 1.0
equivalents of compound
(LXI) and 5 equivalents of hydroxylamine hydrochloride in ethanol at reflux.
Compounds of formula (I) wherein R', R2, R4, R5 and R6 are as defined above
and R3 and A are as
described herein, may be prepared according to reaction scheme 33.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
R6 R6
R2 R5 Ra R5 2
Hal R' (61) N\ Ri (62) 4/N \ R
N NPG R
(VI) (Lxlli) N NPG N NPG
(LXIV)
(63)
R6 R6
R5 2 R5 RZ
R
R4,-N R (7) R4'N R
(~) N NHZ (LXV) N NPG
Scheme 33
Reaction Step 61. Reaction with 3-pyridyl borane
5
Compounds of formula (LXIII) may be prepared from compounds of formula (VI) by
reaction with 3-pyridyl
boranes (or similar boronic acid) in the presence of a suitable base and
suitable palladium catalyst.
Typical conditions comprise addition of the 3-pyridyl borane to a compound of
formula (VI) in
toluene/ethanol as solvent, in the presence of
tetrakis(triphenylphosphine)palladium(0) and sodium
10 carbonate, followed by heating to reflux. Examples of 3-pyridyl boranes (or
similar boronic acids) are
commercially available.
Reaction Step 62. Alkylation
15 Compounds of formula (LXIV) may be prepared from compounds of formula
(LXIII) by addition of an alkyl
iodide. Typical conditions comprise addition of the alkyl iodide to a compound
of formula (LXIII), in a
suitable solvent such as acetonitrile and then heating to reflux.
Reaction Step 63. Hydro eq nation
Compounds of formula (LXV) may be prepared from compounds of formula (LXIV) by
hydrogenation.
Typical conditions comprise hydrogenation of a compound of formula (LXIV), at
elevated pressure, in a
suitable solvent such as ethanol, in the presence of a suitable catalyst such
as Pt02.
Reaction Step 7. Aminopyridine deprotection
Compounds of formula (LXV) may be converted to compounds of formula (I) by
deprotection. The nature
of this reaction will depend upon the protecting group selected for use.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
36
For example, when the 2,5-dimethylpyrrole system is used to protect the
aminopyridine group it may be
deprotected by treatment with hydroxylamine. Typical conditions comprise 1.0
equivalents of compound
(LXV) and 5 equivalents of hydroxylamine hydrochloride in ethanol at reflux.
Methods for resolution of racemic compound
In cases where the above methods lead to racemic products, many methods are
available for the
separation of the racemate into its constituent enantiomers. These include:
(1) formation and selective crystallisation of diastereomeric salts produced
by salt formation between a
racemic base and an enantiomerically pure chiral acid component (or vice
versa)
(2) HPLC using a chiral stationary phase - many of which are commercially
available
(3) Formation of diastereomeric adducts by reaction of a racemic compound with
an enantiomerically pure
chiral compound or reagent, subsequent separation of the constituent
diastereoisomers by physical
methods, including crystallisation or chromatography, and splitting of the
separated adducts to release the
desired compound in enantiomerically enriched form. This is often termed a
classical resolution. For
example, a racemic alcohol may be reacted with an enantiomerically pure chiral
acid to form
diastereomeric esters using standard ester forming reactions. These esters can
then be separated e.g. by
selective crystallisation. The separated diastereomeric esters may then
separately be hydrolysed under
standard ester hydrolysis conditions to release chiral alcohols in
enantiomerically enriched form.
(4) Selective reaction of a chiral reagent (including enzymes) with one
enantiomer from a racemic mixture
- termed a kinetic resolution.
The compounds of the present invention have utility as selective D3 agonists
in the treatment of disease
states. There are a number of compounds with activity as both D2 and D3
agonists; however the use of
such compounds is associated with a large number of side effects including
nausea, emesis, syncope,
hypotension and bradycardia, some of which are a cause for serious concern.
It was previously held that the efficacy of the prior art compounds stemmed
from their ability to agonise
D2; however D2 agonism is implicated as a cause of the side effects detailed
above.
The present invention provides a class of selective D3 agonists.
Serendipitously, these have been found
to be efficacious; whilst reducing the side effects associated with
unselective prior art compounds.
Accordingly a further aspect of the invention provides a compound of formula
(I) for use as a medicament.
Compounds of present invention are particularly useful in treating sexual
dysfunction, female sexual
dysfunction, including hypoactive sexual desire disorder, female sexual
arousal disorder, female orgasmic
disorder and sexual pain disorder; male erectile dysfunction, hypertension,
neurodegeneration,
depression, and psychiatric disorders.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
37
Accordingly, the present invention provides for, the use of a compound of
formula (I) in the preparation of
a medicament for the treatment or prevention of sexual dysfunction.
The compounds of the present invention are useful in male sexual dysfunction,
particularly male erectile
dysfunction. Male erectile dysfunction (MED), otherwise known as male erectile
disorder, is defined as:
"the inability to achieve and/or maintain a penile erection for satisfactory
sexual performance"
(NIH Consensus Development Panel on Impotence, 1993)"
It has been estimated that the prevalence of erectile dysfunction (ED) of all
degrees (minimal, moderate
and complete impotence) is 52% in men 40 to 70 years old, with higher rates in
those older than 70
(Melman et a11999, J. Urology, 161, p5-11). The condition has a significant
negative impact on the quality
of life of the individual and their partner, often resulting in increased
anxiety and tension which leads to
depression and low self-esteem. Whereas two decades ago, MED was primarily
considered to be a
psychological disorder (Benet et al 1994 Comp. Ther., 20: 669-673), it is now
known that for the majority
of individuals there is an underlying organic cause. As a result, much
progress has been made in
identifying the mechanism of normal penile erection and the pathophysiologies
of MED.
Penile erection is a haemodynamic event which is dependent upon the balance of
contraction and
relaxation of the corpus cavernosal smooth muscle and vasculature of the penis
(Lerner et al 1993, J.
Urology, 149, 1256-1255). Corpus cavernosal smooth muscle is also referred to
herein as corporal
smooth muscle or in the plural sense corpus cavernosa. Relaxation of the
corpus cavernosal smooth
muscle leads to an increased blood flow into the trabecular spaces of the
corpus cavernosa, causing them
to expand against the surrounding tunica and compress the draining veins. This
produces a vast
elevation in blood pressure which results in an erection (Naylor, 1998, Br. J.
Urology, 81, 424-431).
The changes that occur during the erectile process are complex and require a
high degree of co-ordinated
control involving the peripheral and central nervous systems, and the
endocrine system (Naylor, 1998, Br.
J. Urology, 81, 424-431). Corporal smooth muscle contraction is modulated by
sympathetic noradrenergic
innervation via activation of postsynaptic a, adrenoceptors. MED may be
associated with an increase in
the endogenous smooth muscle tone of the corpus cavernosum. However, the
process of corporal
smooth muscle relaxation is mediated partly by non-adrenergic, non-cholinergic
(NANC)
neurotransmission. There are a number of other NANC neurotransmitters found in
the penis, other than
NO, such as calcitonin gene related peptide (CGRP) and vasoactive intestinal
peptide (VIP). The main
relaxing factor responsible for mediating this relaxation is nitric oxide
(NO), which is synthesised from L-
arginine by nitric oxide synthase (NOS) (Taub et a11993 Urology, 42, 698-704).
It is thought that reducing
corporal smooth muscle torie may aid NO to induce relaxation of the corpus
cavernosum. During sexual
arousal in the male, NO is released from neurones and the endothelium and
binds to and activates
soluble guanylate cyclase (sGC) located in the smooth muscle cells and
endothelium, leading to an
elevation in intracellular cyclic guanosine 3',5'-monophosphate (cGMP) levels.
This rise in cGMP leads to
a relaxation of the corpus cavernosum due to a reduction in the intracellular
calcium concentration
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
38
([CaZ+];), via unknown mechanisms thought to involve protein kinase G
activation (possibly due to
activation of Ca2+ pumps and Ca2+-activated K+ channels).
Multiple potential sites have been identified within the central nervous
system for the modulation of sexual
behaviour. The key neurotransmitters are thought to be serotonin,
norepinephrine, oxytocin, nitric oxide
and dopamine. By mimicking the actions of one of these key neurotransmitters
sexual function may be
adjusted. Dopamine D3 receptors are expressed almost exclusively in the limbic
area of the brain, regions
involved in the reward, emotional and cognitive processes.
Without being bound by any theory, it appears that "due to its role in the
control of locomotor activity, the
integrity of the nigrostriatal dopaminergic pathway is also essential for the
display of copulatory behaviour.
Somehow, more specific to sexual function, it is likely that dopamine can
trigger penile erection by acting
on oxytocinergic neurons located in the paraventricular nucleus of the
hypothalamus, and perhaps on the
pro-erectile sacral parasympathetic nucleus within the spinal cord". It now
appears that the significant site
is D3 and not as previously thought, D2.
In essence, D3 is an initiator of sexual behaviour.
Accordingly, the present invention provides for, the use of a compound of
formula (1) in the preparation of
a medicament for the treatment or prevention of erectile dysfunction.
Patients with mild to moderate MED should benefit from treatment with the
compounds according to the
present invention, and patients with severe MED may also respond. However,
early investigations suggest
that the responder rate of patients with mild, moderate and severe MED may be
greater with a selective
D3 agonist/PDE5 inhibitor combination. Mild, moderate and severe MED will be
terms known to the man
skilled in the art, but guidance can be found in The Journal of Urology, vol.
151, 54-61 (Jan 1994).
Early investigations suggest the below mentioned MED patient groups should
benefit from treatment with
a selective D3 agonist and a PDE5i (or other combination set out hereinafter).
These patient groups,
which are described in more detail in Clinical Andrology vol. 23, no.4, p773-
782 and chapter 3 of the book
by I. Eardley and K. Sethia "Erectile Dysfunction-Current Investigation and
Management, published by
Mosby-Wolfe, are as. follows: psychogenic, organic, vascular, endocrinologic,
neurogenic, arteriogenic,
drug-induced sexual dysfunction (lactogenic) and sexual dysfunction related to
cavernosal factors,
particularly venogenic causes.
Accordingly the present invention provides for the use of a compound of
formula (I) in the preparation of a
medicament in combination with a PDE5 inhibitor for the treatment of erectile
dysfunction.
Suitable PDE5 inhibitors are described herein.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
39
The compounds of the present invention are useful in the treatment or
prevention of female sexual
dysfunction (FSD), particularly female sexual arousal disorder (FSAD),
hypoactive sexual desire disorder
(HSDD; lack of interest in sex), FSAD with concomitant HSDD, and female
orgasmic disorder (FOD;
inability to achieve orgasm).
In accordance with the invention, FSD can be defined as the difficulty or
inability of a woman to find
satisfaction in sexual expression. FSD is a collective term for several
diverse female sexual disorders
(Leiblum, S.R. (1998) - Definition and classification of female sexual
disorders. Int. J. Impotence Res., 10,
S104-S106; Berman, J.R., Berman, L. & Goldstein, I. (1999) - Female sexual
dysfunction: Incidence,
pathophysiology, evaluations and treatment options. Urology, 54, 385-391.).
The woman may have lack
of desire, difficulty with arousal or orgasm, pain with intercourse or a
combination of these problems.
Several types of disease, medications, injuries or psychological problems can
cause FSD. Treatments in
development are targeted to treat specific subtypes of FSD, predominantly
desire and arousal disorders.
The categories of FSD are best defined by contrasting them to the phases of
normal female sexual
response: desire, arousal and orgasm (Leiblum, S.R. (1998) - Definition and
classification of female
sexual disorders. Int. J. Impotence Res., 10, S104-S106). Desire or libido is
the drive for sexual
expression. Its manifestations often include sexual thoughts either when in
the company of an interested
partner or when exposed to other erotic stimuli. Arousal is the vascular
response to sexual stimulation, an
important component of which is genital engorgement and includes increased
vaginal lubrication,
elongation of the vagina and increased genital sensation/sensitivity. Orgasm
is the release of sexual
tension that has culminated during arousal.
Hence, FSD occurs when a woman has an inadequate or unsatisfactory response in
any of these phases,
usually desire, arousal or orgasm. FSD categories include hypoactive sexual
desire disorder, sexual
arousal disorder, orgasmic disorders and sexual pain disorders. Although the
compounds of the invention
will improve the genital response to sexual stimulation (as in female sexual
arousal disorder), in doing so it
may also improve the associated pain, distress and discomfort associated with
intercourse and so treat
other female sexual disorders.
Hypoactive sexual desire disorder is present if a woman has no or little
desire to. be sexual, and has no or
few sexual thoughts or fantasies. This type of FSD can be caused by low
testosterone levels, due either
to natural menopause or to surgical menopause. Other causes include illness,
medications, fatigue,
depression and anxiety.
Female sexual arousal disorder (FSAD) is characterised by inadequate genital
response to sexual
stimulation. The genitalia do not undergo the engorgement that characterises
normal sexual arousal.
The vaginal walls are poorly lubricated, so that intercourse is painful.
Orgasms may be impeded. Arousal
disorder can be caused by reduced oestrogen at menopause or after childbirth
and during lactation, as
well as by illnesses, with vascular components such as diabetes and
atherosclerosis. Other causes result
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
from treatment with diuretics, antihistamines, antidepressants e.g. selective
serotonin re-uptake inhibitors
(SSRIs) or antihypertensive agents.
Sexual pain disorders (includes dyspareunia and vaginismus) is characterised
by pain resulting from
5 penetration and. may be caused by medications which reduce lubrication,
endometriosis, pelvic
inflammatory disease, inflammatory bowel disease or urinary tract problems.
As previously discussed, D3 is thought to be an initiator of sexual behaviour.
The clitoris is considered to
be a homologue of the penis (Levin, R.J. (1991), Exp. Clin. Endocrinol., 98,
61-69); the same mechanism
10 that provides provides an erectile response in the male produces an
increase in genital blood flow in the
female with an associated effect upon FSD. In addition there are changes in
proceptivity and receptivity.
Thus, in accordance with a preferred aspect of the invention, there is
provided use of a compound of
formula (I) in the preparation of a medicament for the treatment or
prophylaxis of female sexual
15 dysfunction, more particularly hypoactive sexual desire disorder, female
sexual arousal disorder, female
orgasmic disorder and sexual pain disorder.
Preferably the compounds of formula (I) are useful in the treatment or
prophylaxis of female sexual
arousal disorder (FSAD), FSAD with concomitant hypoactive sexual desire
disorder, orgasmic disorder,
?0 and hypoactive sexual desire disorder, and most preferably in the treatment
or prophylaxis of female
sexual arousal disorder.
In a preferred embodiment the compounds of formula (I) are useful in the
treatment of a subject with
female sexual arousal disorder and concomitant hypoactive sexual desire
disorder.
The Diagnostic and Statistical Manual (DSM) IV of the American Psychiatric
Association defines Female
Sexual Arousal Disorder (FSAD) as being:
"... a persistent or recurrent inability to attain or to maintain until
completion of the sexual activity adequate
lubrication-swelling response of sexual excitement. The disturbance must cause
marked distress or
interpersonal difficulty. ... ". _
The arousal response consists of vasocongestion in the pelvis, vaginal
lubrication and expansion and
swelling of the external genitalia. The disturbance causes marked distress
and/or interpersonal difficulty.
FSAD is a highly prevalent sexual disorder affecting pre-, peri- and post-
menopausal ( hormone
replacement therapy (HRT)) women. It is associated with concomitant disorders
such as depression,
cardiovascular diseases, diabetes and urogenital (UG) disorders.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
41
The primary consequences of FSAD are lack of engorgement/swelling, lack of
lubrication and lack of
pleasurable genital sensation. The secondary consequences of FSAD are reduced
sexual desire, pain
during intercourse and difficulty in achieving an orgasm.
It has recently been hypothesised that there is a vascular basis for at least
a proportion of patients with
symptoms of FSAD (Goldstein et al., Int. J. Impot. Res., 10, S84-S90,1998)
with animal data supporting
this view (Park et al., Int. J. Impot. Res., 9, 27-37, 1997).
R.J. Levin teaches us that because "... male and female genitalia develop
embryologically from the
common tissue anlagen, [that] male and female genital structures are argued to
be homologues of one
another. Thus the clitoris is the penile homologue and the labia homologues of
the scrotal sac. ..." (Levin,
R.J. (1991), Exp. Clin. Endocrinol., 98, 61-69).
Drug candidates for treating FSAD, which are under investigation for efficacy,
are primarily erectile
dysfunction therapies that promote circulation to male genitalia.
The compounds of the present invention are advantageous by providing a means
for restoring a normal
sexual arousal response - namely increased genital blood flow leading to
vaginal, clitoral and labial
engorgement. This will result in increased vaginal lubrication via plasma
transudation, increased vaginal
compliance and increased genital sensitivity. Hence, the present invention
provides a means to restore,
or potentiate, the normal sexual arousal response.
Thus, in accordance with a preferred aspect of the invention, there is
provided use of a compound of
formula (I) in the preparation of a medicament for the treatment or
prophylaxis of female sexual arousal
disorder and female sexual arousal disorder with concomitant hypoactive sexual
desire disorder.
By female genitalia herein we mean: "The genital organs consist of an internal
and external group. The
internal organs are situated within the pelvis and consist of ovaries, the
uterine tubes, uterus and the
vagina. The external organs are superficial to the urogenital diaphragm and
below the pelvic arch. They
comprise the mons pubis, the labia majora and minora pudendi, the clitoris,
the vestibule, the bulb of the
vestibule, and the greater vestibular glands" (Gray's Anatomy, C.D. Clemente,
13'h American Edition).
The compounds of the invention find application in the following sub-
populations of patients with FSD: the
young, the elderly, pre-menopausal, peri-menopausal, post-menopausal women
with or without hormone
replacement therapy.
The compounds of the invention find application in patients with FSD arising
from:-
i) Vasculogenic etiologies e.g. cardiovascular or atherosclerotic diseases,
hypercholesterolemia,
cigarette smoking, diabetes, hypertension, radiation and perineal trauma,
traumatic injury to the
iliohypogastric pudendal vascular system.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
42
ii) Neurogenic etiologies such as spinal cord injuries or diseases of the
central nervous system
including multiple sclerosis, diabetes, Parkinsonism, cerebrovascular
accidents, peripheral
neuropathies, trauma or radical pelvic surgery.
iii) Hormonal/endocrine etiologies such as dysfunction of the
hypothalamic/pituitary/gonadal axis, or
dysfunction of the ovaries, dysfunction of the pancreas, surgical or medical
castration, androgen
deficiency, high circulating levels of prolactin e.g. hyperprolactinemia,
natural menopause,
premature ovarian failure, hyper and hypothyroidism.
iv) Psychogenic etiologies such as depression, obsessive compulsive disorder,
anxiety disorder,
postnatal depressionP'Baby Blues", emotional and relational issues,
performance anxiety, marital
discord, dysfunctional attitudes, sexual phobias, religious inhibition or
traumatic past experiences.
v) Drug-induced sexual dysfunction resulting from therapy with selective
serotonin reuptake
inhibitors (SSRis) and other antidepressant therapies (tricyclics and major
tranquillizers), anti-
hypertensive therapies, sympatholytic drugs, chronic oral contraceptive pill
therapy.
The Compounds of the present invention are also useful in the treatment of
depression.
Dopamine D3 receptors are expressed almost exclusively in the limbic area of
the brain, regions involved
in reward, emotional and cognitive processes. Chronic treatment with several
classes of antidepressants
are known to increase the expression of D3 in the limbic area, and
antidepressant effects of desipramine
can be blocked by sulpride (D2/D3 antagonist) when injected to nucleus
accumbens (area rich in D3) but
not caudate-putamen (area rich in dopamine D2 receptors). In addition,
antidepressant effects were
observed preclinical models of depression and in patients treated with
pramipexole, a D3-preferring D2/D3
agonist. The available information suggests that D3 receptors mediate the anti-
depressant activity and
that selective D3 receptor agonists represent a new class of antidepressant
drugs. Since antidepressants
are known to be effective in other psychiatric disorders, D3 agonists would
have the potential to treat
psychiatric diseases.
Suitable conditions include depression (e.g. depression in cancer patients,
depression in Parkinson's
patients, postmyocardial infarction depression, subsyndromal symptomatic
depression, depression in
infertile women, major depression, child abuse induced depression, post partum
depression and grumpy
old man syndrome), single episodic or recurrent major depressive disorders,
dysthymic disorders,
depressive neurosis and neurotic depression, melancholic depression including
anorexia, weight loss,
insomnia, early morning waking or psychomotor retardation; atypical depression
(or reactive depression)
including increased appetite, hypersomnia, psychomotor agitation or
irritability, seasonal affective disorder
and pediatric depression; bipolar disorders or manic depression, for example,
bipolar I disorder, bipolar II
disorder and cyclothymic disorder; conduct disorder; disruptive behavior
disorder; trichotillomania,
kleptomania, attention deficit hyperactivity disorder (ADHD); behavioral
disturbances associated with
mental retardation, autistic disorder; borderline personality disorder;
avoidant personality disorder; anxiety
disorders such as panic disorder with or without agoraphobia, agoraphobia
without history of panic
disorder, specific phobias, for example, specific animal phobias, social
anxiety, social phobia, obsessive-
compulsive disorder, stress disorders including post-traumatic stress disorder
and acute stress disorder,
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
43
and generalized anxiety disorders; emotional lability, pathological crying;
schizophrenia and other
psychotic disorders, for example, schizophreniform disorders, schizoaffective
disorders, delusional
disorders, brief psychotic disorders, shared psychotic disorders, psychotic
disorders with delusions or
hallucinations, psychotic episodes of anxiety, anxiety associated with
psychosis, psychotic mood disorders
such as severe major depressive disorder; mood disorders associated with
psychotic disorders such as
acute mania and depression associated with bipolar disorder; mood disorders
associated with
schizophrenia; eating disorders (e.g. anorexia nervosa and bulimia nervosa),
obesity; movement disorders
such as akinesias, dyskinesias, including familial paroxysmal dyskinesias,
spasticities, Tourette's
syndrome, Scott syndrome, PALSYS and akinetic-rigid syndrome; extra-pyramidal
movement disorders
such as medication-induced movement disorders, for example, neuroleptic-
induced Parkinsonism,
neuroleptic malignant syndrome, neuroleptic-induced acute dystonia,
neuroleptic-induced acute akathisia,
neuroleptic-induced tardive dyskinesia and medication-induced postural
tremour; chemical dependencies
and addictions (e.g., dependencies on, or addictions to, alcohol, heroin,
cocaine, benzodiazepines,
nicotine, or phenobarbitol) and behavioral addictions such as an addiction to
gambling; ocular disorders
such as glaucoma and ischemic retinopathy; sleeping disorder (cataplexy) and
shock.
In a further preferred embodiment, the present invention provides for the use
of a compound of formula (I)
in the preparation of a medicament for the treatment of depression or
psychiatric disorders.
Suitable depressive conditions and psychiatric disorders are described above.
In an additional further embodiment, the invention provides for the use of
compounds of formula I in the
preparation of a medicament for the treatment of obesity.
The compounds of the present invention also have utility in the treatment of
neurodegeneration; sources
of neurodegeneration include neurotoxin poisoning; vision loss caused by
neurodegeneration of the visual
pathway, such as by a stroke in the visual pathway eg in retina, optic nerve
and/or occipital lobe; epileptic
seizures; and from impairment of glucose and/or oxygen supply to the brain.
Conditions related to neurodegeneration include Restless Leg Syndrome,
Huntington's disease, Multiple
Sclerosis, mild cognitive impairment, Down's syndrome, stroke, Hereditary
Cerebral Hemorrhage with
Amyloidosis of the Dutch-Type, cerebral amyloid angiopathy, delirium,
dementia, age-related cognitive
decline (ARCD), and amnestic and other cognitive or neurodegenerative
disorders, such as Parkinson's
disease (PD), Huntington's disease (HD), Alzheimer's disease, senile dementia,
dementia of the
Alzheimer's type, memory disorders, loss of executive function, vascular
dementia, dementias of mixed
vascular and degenerative origin, dementia associated with Parkinson's
disease, dementia associated
with progressive supranuclear palsy, dementia associated with cortical basal
degeneration, multi-infarct
dementia, alcoholic dementia or other drug-related dementia, dementia
associated with intracranial
tumors or cerebral trauma, dementia associated with Huntington's disease,
Pick's disease, Creutzfeldt-
Jakob disease, HIV or AIDS-related dementia, diffuse Lewy body type of
Alzheimer's disease,
frontotemporal dementias with parkinsonism (FTDP), head trauma, spinal cord
injury, demyelinating
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
44
diseases of the nervous system, peripheral neuropathy, pain, cerebral amyloid
angiopathy, amyotrophic
lateral sclerosis, multiple sclerosis, dyskinesia associated with dopamine
agonist therapy, mental
retardation, learning disorders, including reading disorder, mathematics
disorder; or a disorder of written
expression; age-related cognitive decline, amnesic disorders, neuroleptic-
induced parkinsonism, tardive
dyskinesias, and acute and chronic neurodegenerative disorders.
Accordingly the present invention provides for the use of a compound of
formula (I) in the preparation of a
medicament for the treatment of neurodegeneration.
Suitable neurodegenerative conditions are described above.
In addition to their role in treating Sexual dysfunction, depression,
neurodegeneration and psychiatric
disorders, the compounds of the present invention are likely to be efficacious
in a number of additional
indications.
Accordingly, the present invention provides for the use of compounds of
formula (I), in the preparation of a
medicament for the treatment of hypertension, premature ejaculation, obesity,
cluster headache, migraine,
pain, endocrine disorders (e.g. hyperprolactinaemia), vasospasm (particularly
in the cerebral vasculature),
cerebellar ataxia, gastrointestinal tract disorders (involving changes in
motility and secretion),
premenstrual syndrome, fibromyalgia syndrome, stress incontinence,
trichotillomania and chronic
paroxysmal hemicrania, headache (associated with vascular disorders).
It is to be appreciated that all references herein to treatment include
curative, palliative and prophylactic
treatment.
D3/D2 AGONIST ASSAY
Activity at the dopamine D3 receptor may be determined using the methods
described in WO
2004/052372. Using this assay, the compounds of the present invention all
exhibit a functional potency at
D3 receptor expressed as an EC50, lower than 1000nM and a 10 fold selectivity
for D3 over D2.
Selectivity is calculated as the D2 EC50 value divided by the D3 EC50 value.
Where the vaiue of the D2
EC50 was >10000, a.figure of 10000 was used in the calculation.
The compound of example 14 has a functional potency at the D3 receptor,
expressed as an EC50, of 20
nM, with an Emax (maximal response value) of 98% (relative to the maximal
effect of standard agent
pramipexole). Against the D2 receptor this compound gave only a 22% response
(relative to the maximal
effect of pramipexole) at 10000nM.
Suitable auxiliary active agents for use in the combinations of the present
invention include:
CA 02567935 2009-04-07
69387-598
1) Naturally occurring or synthetic prostagiandins or esters thereof. Suitable
prostaglandins for use
herein include compounds such as alprostadil, prostaglandin E,,prostaglandin
Fe, 13, 14 -
dihydroprosta 'glandin E,, prostagiandin E2, eprostinot, natural synthetic and
senii-synthetic
prostaglandins and derivafives thereof including those described in WO-
00033825 and/or US
5 6,037,346 issued on 14th March 2000, PGEo, PGEI, PGAI,
PGB,, PGF, a, 19-hydroxy PGA,, 19-hydroxy - PGBI, PGE2, PGt3Z, 19-hydroxy-
PGA2, 19-
hydroxy-PGB-,, PGE;a, carboprost trornethamine dinoprost, tromethamine,
dinoprostone, lipo
prost, gemeprost, metenoprost, sulprostune, tiaprost and moxisylate;
2) (x - adrenergic receptor antagonist compounds also known as a-
adrenoceptors or or-receptors or
10 a-blockers. Suitable compounds for use herein include: the a-adrenergic
receptor blockers as
described in PCT applicat-on W099130697 published on 14th June 1998, the
disclosures of which
retating to a-adrenergic receptors include, selective ai-
adrenoceptor or a27adrenoceptor blockers and non-selective adrenooeptor
blockers, suitable ar-
adrenoceptor blockers include: phentolamine, phentolamine mesylate, trazodone,
alfuzosin,
15 indoramin, naftopidil, tamsulosin, dapiprazole, phenoxybenzamine,.
idazoxan, efaraxan,
yohimbine, rauwolfa alkaloids, Recordati 15/2739, SNAP 1069, SNAP 5089,
RS17053, SL-
89.0591, doxazosin, terazosin, abanoquit and prazosin; aZ-blocker blockers
from US.6,037,346
[14th March 2000] dibenarnine, _tolazoline, trimazosin and dibenarnine; a-
adrenergic receptors as
described in US patents: 4,188,390; 4,026,894; 3,511,836; 4,315,007;
3,527,761; 3,997,666;
20 2,503,059; 4,703,063; 3,381,009; 4,252,721 and 2,599,000 each of which is
incorporated herein
by reference; a:-Adrenoceptor blockers include: clonidine, papaverine,
papaverine hydrochloride,
optionally in the.presence of a cariotonic agent such as pirxamine; .
3) NO-donor (NO-agonist) compounds. Suitable NO-donor compounds for use herein
include
25 organic nitrates, such as mono- di or tri-nitrates or organic nitrate
esters including glyceryl trinitrate
(also known as nitroglycerin), isosorbide 5-mononitrate, isosorbide dinitrate,
pentaerythritol
tetranitrate, erythrityl tetranitrate, sodium nitroprusside (SNP), 3-
morpholinosydnonlmine
molsidomine, S-nitroso- N-acetyl penicilliamine (SNAP) S-nitroso-N-glutathione
(SNO-GI-U), N-
hydroxy - L-arginine, amylnitrate, linsidomine, linsidomine chlorohydrate,
(SIN-1) S-nitroso - N-
30 cysteine, diazenium diolates,(NONOates), 1,5-pentanedinitrate, L-arginene,
ginseng, zizphi
fructus, motsidomine, Re - 2047, nitrosylated maxisylyte derivatives such as
NMI-678-11 and
NMI-937 as described in published PCT application WO 0012075;
4) Potassium channel openers or modutators. Suitable potassium channel
openers/modulators for
35 use herein include rucorandil, cromokalim, levcromakalim, lemakalim,
pinacidit, cliazoxide,
minoxidil, charybdotoxin, glyburide, 4-amini pyridine, BaCl2;
5) Vasodilator agenfs. Suitable vasodilator agents for use herein include
nimodepine, pinacidil,
cyclandelate, isoxsuprine, chloroprumazine, , Rec 15/2739, trazodone;
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
46
6) Thromboxane A2 agonists;
7) CNS active agents;
8) Ergot alkoloids; Suitable ergot alkaloids are described in US patent
6,037,346 issued on 14th
March 2000 and include acetergamine, brazergoline, bromerguride, cianergoline,
delorgotrile,
disulergine, ergonovine maleate, ergotamine tartrate, etisulergine,
lergotrile, lysergide,
mesulergine, metergoline, metergotamine, nicergoline, pergolide, propisergide,
proterguride and
terguride;
9) Compounds which modulate the action of natruretic factors in particular
atrial naturetic factor (also
known as atrial naturetic peptide), B type and C type naturetic factors such
as inhibitors or neutral
endopeptidase;
10) Compounds which inhibit angiotensin-converting enzyme such as enapril, and
combined inhibitors
of angiotensin-converting enzyme and neutral endopeptidase such as
omapatrilat.
11) Angiotensin receptor antagonists such as losartan;
12) Substrates for NO-synthase, such as L-arginine;
13) Calcium channel blockers such as amlodipine;
14) Antagonists of endothelin receptors and inhibitors or endothelin-
converting enzyme;
15) Cholesterol lowering agents such as statins (e.g. atorvastatin/ Lipitor-
trade mark) and fibrates;
16) Antiplatelet and antithrombotic agents, e.g. tPA, uPA, warfarin, hirudin
and other thrombin
inhibitors, heparin, thromboplastin activating factor inhibitors;
17) Insulin sensitising agents such as rezuiin and hypoglycaemic agents such
as glipizide; '
18) Acetylcholinesterase inhibitors such as donezipil;
19) Steroidal or non-steroidal anti-inflammatory agents;
20) Estrogen receptor modulators and/or estrogen agonists and/or estrogen
antagonists, preferably
raloxifene or lasofoxifene, (-)-cis-6-phenyl-5-[4-(2-pyrrolidin-1-yi-ethoxy)-
phenyl]-5,6,7,8-
tetrahydronaphthalene-2-ol and pharmaceutically acceptable salts thereof the
preparation of
which is detailed in WO 96/21656;
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
47
21) A PDE inhibitor, more particularly a PDE 2, 3, 4, 5, 7 or 8 inhibitor,
preferably PDE2 or PDE5
inhibitor and most preferably a PDE5 inhibitor (see hereinafter), said
inhibitors preferably having
an IC50 against the respective enzyme of less than lOOnM (with the proviso
that PDE 3 and 4
inhibitors are only administered topically or by injection to the penis);
22) Vasoactive intestinal protein (VIP), VIP mimetic, VIP analogue, more
particularly mediated by one
or more of the VIP receptor subtypes VPAC1,VPAC or PACAP (pituitory adenylate
cyclase
activating peptide), one or more of a VIP receptor agonist or a VIP analogue
(e.g. Ro-125-1553)
or a VIP fragment, one or more of a a-adrenoceptor antagonist with VIP
combination (e.g.
Invicorp, Aviptadil);
23) A melanocortin receptor (particularly of the MC3 or MC4 subtype) agonist
or modulator or
melanocortin enhance, such as melanotan II, PT-14, PT-141 or compounds claimed
in WO-
09964002, WO-00074679, WO-09955679, WO-001 05401, WO-00058361, WO-00114879, WO-
00113112, WO-09954358;
24) A serotonin receptor agonist, antagonist or modulator, more particularly
agonists, antagonists or
modulators for 5HTIA (including VML 670), 5HT2A, 5HT2C, 5HT3 and/or 5HT6
receptors,
including those described in WO-09902159, WO-00002550 and/or WO-00028993;
25) A testosterone replacement agent (including dehydroandrostendione),
testosternone (Tostrelle),
dihydrotestosterone or a testosterone implant;
26) Estrogen, estrogen and medroxyprogesterone or medroxyprogesterone acetate
(MPA) (i.e. as a
combination), or estrogen and methyl testosterone hormone replacement therapy
agent (e.g. HRT
especially Premarin, Cenestin, Oestrofeminal, Equin, Estrace, Estrofem,
Elleste Solo, Estring,
Eastraderm TTS, Eastraderm Matrix, Dermestril, Premphase, Preempro, Prempak,
Premique,
Estratest, Estratest HS, Tibolone);
27) A modulator of transporters for noradrenaline, dopamine and/or serotonin,
such as bupropion,
GW-320659; -
28) A purinergic receptor agonist and/or modulator;
29) A neurokinin (NK) receptor antagonist, including those described in WO-
09964008;
30) An opioid receptor agonist, antagonist or modulator, preferably agonists
for the ORL-1 receptor;
31) An agonist, antagonist or modulator for oxytocin receptors, preferably a
selective oxytocin agonist
or modulator;
CA 02567935 2009-04-07
69387-598
48
32) Modulators of cannabinoid receptors;
33) A SEP inhibitor (SEPi), for instance a SEPi having an ICSa at less than
100 nanomolar, more
preferably, at less than 50 nanomotar.
Preferably, the SEP inhibitors according to the present invention have greater
than 30-fold, more
preferably greater than 50-fold selectivity for SEP over neutrai endopeptidase
NEP EC 3.4.24.11
and angiotensin converting enzyme (ACE). Preferably the SEPi also has a
greater than 100-fold
selectivity over endothelin converting enzyme (ECE).
34) An antagonist or modulator for the NPY (particularly Y1 and Y5 subtype)
receptor.
35) A Sex Hormone Binding Globulin antagonist or modulator that inhibits
estrogens andlor
androgens from being bound.
5
36) An arginase II -inhibitor,
37) An agonist, antagonist or modulator for vassopressin receptors, preferably
selective for the V1a
receptor
38) A PDE5 Inhibitor. Suitable PDE5 inhibitors include:
5-[2-ethoxy-5-(4methyl-1-piperazinylsulphonyl)phenyl]-1-methyl-3-n-propyl-l,6-
dihydro-7Ft-
pyrazolo(4,3-d]pyrimidin-7-one (sildenafil), particularly sildenafil citrate;
(6R,12aR)-2,3,6,7,12, t 2a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyt)
pyrazino[2',1':6,1]pyrido[3,4b]indole-1,4-dione (IC-351 or tadalafil);
2-[2-ethoxy-5-(4-ethyl-piperazin-1-yi-9 -sulphonyl)-phenyll-5-methyl-7-propyl-
3H-imidazo[5,1-
f][1,2,41triaztn-4-one (vardenafil); 5-(5-Acetyt-2-butoxy-3-pyridinyl)-3-ethyl-
2-(1-ethyl-3=a2etidinyl)-
2,6-dihydro-7H-pyrazolo[4,3-djpyrimidin-7-one; 5-(5-Acetyi-2-propoxy-3-
pyridinyt)-3-ethyl-2-(1-
isopropyl-3-azetidinyl)-2,6-dihydro-7H-pyrazolo[4,3-dJpyrimidin-7-one ; and 5-
[2-ethoxy-5-(4-
ethytpiperazin-1-ylsulphonyl)pyridin-3-yl]-3-ethyl-2-[2-methoxyethyl]-2,6-
dihydro-7H-pyrazoki[4,3-
d]pyrimidin 7-one; 4-[(3-chloro-4-methoxybenzyl)amino}2-[(2S)-2-
(hydroxymethyl)pyrrolidin-1ryg-
N-(pyrimidin-2.ylmethyl)pyrimidine-5-carboxamide (TA-1790); 3-(1-methyl-7-oxo
3-propy!-6,7-
dihydro-1 H-pyrazolo[4,3-d]pyrimidin-5-yl)-N-[2-(1-methylpyrrolidin-2-
yl)ethyi]-4
propoxybenzenesulfonarnide (DA 8159) and pharmaceuticatly acceptable salts
thereof.
39) A selective dopamine D4 receptor agonist'such as 2-[(4-pyridin-
2ylpiperazin-1-yljmethyij-lH-
benzimidazole (ABT724).
By cross reference herein to compounds contained in patents and patent
applications which can be used
in accordance with invention, we mean the therapeuticaily acbve compounds as
defined in the claims Cn
particular of claim 1) and the specific examples.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
49
If a combination of active agents is administered, then they may be
administered simultaneously,
separately or sequentially.
The compounds of formula I should be assessed for their biopharmaceutical
properties, such as solubility
and solution stability (across pH), permeability, etc., in order to select the
most appropriate dosage form
and route of administration for treatment of the proposed indication.
Compounds of the invention intended for pharmaceutical use may be administered
as crystalline or
amorphous products. They may be obtained, for example, as solid plugs,
powders, or films by methods
such as precipitation, crystallization, freeze drying, spray drying, or
evaporative drying. Microwave or radio
frequency drying may be used for this purpose.
They may be administered alone or in combination with one or more other
compounds of the invention or
5 in combination with one or more other drugs (or as any combination thereof).
Generally, they will be
administered as a formulation in association with one or more pharmaceutically
acceptable excipients.
The term 'excipient' is used herein to describe any ingredient other than the
compound(s) of the invention.
The choice of excipient will to a large extent depend on factors such as the
particular mode of
administration, the effect of the excipient on solubility and stability, and
the nature of the dosage form.
!0
Pharmaceutical compositions suitable for the delivery of compounds of the
present invention and methods
for their preparation will be readily apparent to those skilled in the art.
Such compositions and methods for
their preparation may be found, for example, in Remington's Pharmaceutical
Sciences, 19th Edition (Mack
Publishing Company, 1995).
Accordingly the present invention provides for a pharmaceutical composition
comprising a compound of
formula (I), and a pharmaceutically acceptable diluent or carrier.
The compounds of the invention may be administered orally. Oral administration
may involve swallowing,
30 so that the compound enters the gastrointestinal tract, and/or buccal,
lingual, or sublingual administration
by which the compound enters the blood stream directly from the mouth.
Formulations suitable for oral administration include solid, semi-solid and
liquid systems such as tablets;
soft or hard capsules containing multi- or nano-particulates, liquids, or
powders; lozenges (including liquid-
35 filled); chews; gels; fast dispersing dosage forms; films; ovules; sprays;
and buccal/mucoadhesive
patches.
Liquid formulations include suspensions, solutions, syrups and elixirs. Such
formulations may be
employed as fillers in soft or hard capsules (made, for example, from gelatin
or
40 hydroxypropylmethylcellulose) and typically comprise a carrier, for
example, water, ethanol, polyethylene
glycol, propylene glycol, methylcellulose, or a suitable oil, and one or more
emulsifying agents and/or
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
suspending agents. Liquid formulations may also be prepared by the
reconstitution of a solid, for example,
from a sachet.
The compounds of the invention may also be used in fast-dissolving, fast-
disintegrating dosage forms
5 such as those described in Expert Opinion in Therapeutic Patents, 11 (6),
981-986, by Liang and Chen
(2001).
For tablet dosage forms, depending on dose, the drug may make up from 1 weight
% to 80 weight % of
the dosage form, more typically from 5 weight % to 60 weight % of the dosage
form. In.addition to the
10 drug, tablets generally contain a disintegrant. Examples of disintegrants
include sodium starch glycolate,
sodium carboxymethyl cellulose, calcium carboxymethyl cellulose,
croscarmellose sodium, crospovidone,
polyvinylpyrrolidone, methyl cellulose, microcrystalline cellulose, lower
alkyl-substituted hydroxypropyl
cellulose, starch, pregelatinised starch and sodium alginate. Generally, the
disintegrant will comprise from
1 weight % to 25 weight %, preferably from 5 weight % to 20 weight % of the
dosage form.
Binders are generally used to impart cohesive qualities to a tablet
formulation. Suitable binders include
microcrystalline cellulose, gelatin, sugars, polyethylene glycol, natural and
synthetic gums,
polyvinylpyrrolidone, pregelatinised starch, hydroxypropyl cellulose and
hydroxypropyl methylcellulose.
Tablets may also contain diluents, such as lactose (monohydrate, spray-dried
monohydrate, anhydrous
and the like), mannitol, xylitol, dextrose, sucrose, sorbitol,
microcrystalline cellulose, starch and dibasic
calcium phosphate dihydrate.
Tablets may also optionally comprise surface active agents, such as sodium
lauryl sulfate and polysorbate
80, and glidants such as silicon dioxide and talc. When present, surface
active agents may comprise from
0.2 weight % to 5 weight % of the tablet, and glidants may comprise from 0.2
weight % to I weight % of
the tablet.
Tablets also generally contain lubricants such as magnesium stearate, calcium
stearate, zinc stearate,
sodium stearyl fumarate, and mixtures of magnesium stearate with sodium lauryl
sulphate. Lubricants
generally comprise from 0.25 weight % to 10 weight %, preferably from 0.5
weight % to 3 weight % of the
tablet.
Other possible ingredients include anti-oxidants, colourants, flavouring
agents, preservatives and taste-
masking agents.
Exemplary tablets contain up to about 80% drug, from about 10 weight % to
about 90 weight % binder,
from about 0 weight % to about 85 weight % diluent, from about 2 weight % to
about 10 weight %
disintegrant, and from about 0.25 weight % to about 10 weight % lubricant.
Tablet blends may be compressed directly or by roller to form tablets. Tablet
blends or portions of blends
may alternatively be wet-, dry-, or melt-granulated, melt congealed, or
extruded before tabletting. The final
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
51
formulation may comprise one or more layers and may be coated or uncoated; it
may even be
encapsulated.
The formulation of tablets is discussed in Pharmaceutical Dosage Forms:
Tablets, Vol. 1, by H. Lieberman
and L. Lachman (Marcel Dekker, New York, 1980).
Consumable oral films for human or veterinary use are typically pliable water-
soluble or water-swellable
thin film dosage forms which may be rapidly dissolving or mucoadhesive and
typically comprise a
compound of formula I, a film-forming polymer, a binder, a solvent, a
humectant, a plasticiser, a stabiliser
or emulsifier, a viscosity-modifying agent and a solvent. Some components of
the formulation may
perform more than one function.
The compound of formula I may be water-soluble or insoluble. A water-soluble
compound typically
comprises from I weight % to 80 weight %, more typically from 20 weight % to
50 weight %, of the
5 solutes. Less soluble compounds may comprise a greater proportion of the
composition, typically up to 88
weight % of the solutes. Alternatively, the compound of formula I may be in
the form of multiparticulate
beads.
The film-forming polymer may be selected from natural polysaccharides,
proteins, or synthetic
?0 hydrocolloids and is typically present in the range 0.01 to 99 weight %,
more typically in the range 30 to 80
weight %.
Other possible ingredients include anti-oxidants, colorants, flavourings and
flavour enhancers,
preservatives, salivary stimulating agents, cooling agents, co-solvents
(including oils), emollients, bulking
25 agents, anti-foaming agents, surfactants and taste-masking agents.
Films in accordance with the invention are typically prepared by evaporative
drying of thin aqueous films
coated onto a peelable backing support or paper. This may be done in a drying
oven or tunnel, typically a
combined coater dryer, or by freeze-drying or vacuuming.
Solid formulations for oral administration may be formulated to be immediate
and/or modified release.
Modified-release formulations include delayed-, sustained-, pulsed-,
controlled-, targeted and programmed
release.
Suitable modified release formulations for the purposes of the invention are
described in US Patent No.
6,106,864. Details of other suitable release technologies such as high energy
dispersions and osmotic
and coated particles are to be found in Pharmaceutical Technology On-line,
25(2), 1-14, by Verma et al
(2001). The use of chewing gum to achieve controlled release is described in
WO 00/35298.
The compounds of the invention may also be administered directly into the
blood stream, into muscle, or
into an internal organ. Suitable means for parenteral administration include
intravenous, intraarterial,
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
52
intraperitoneal, intrathecal, intraventricular, intraurethral, intrasternal,
intracranial, intramuscular,
intrasynovial and subcutaneous. Suitable devices for parenteral administration
include needle (including
microneedle) injectors, needle-free injectors and infusion techniques.
Parenteral formulations are typically aqueous solutions which may contain
excipients such as salts,
carbohydrates and buffering agents (preferably to a pH of from 3 to 9), but,
for some applications, they
may be more suitably formulated as a sterile non-aqueous solution or as a
dried form to be used in
conjunction with a suitable vehicle such as sterile, pyrogen-free water.
The preparation of parenteral formulations under sterile conditions, for
example, by lyophilisation, may
readily be accomplished using standard pharmaceutical techniques well known to
those skilled in the art.
The solubility of compounds of formula I used in the preparation of parenteral
solutions may be increased
by the use of appropriate formulation techniques, such as the incorporation of
solubility-enhancing agents.
5
Formulations for parenteral administration may be formulated to be immediate
and/or modified release.
Modified release formulations include delayed-, sustained-, pulsed-,
controlled-, targeted and programmed
release. Thus compounds of the invention may be formulated as a suspension or
as a solid, semi-solid, or
thixotropic liquid for administration as an implanted depot providing modified
release of the active
!0 compound. Examples of such formulations include drug-coated stents and semi-
solids and suspensions
comprising drug-loaded poly(d/-Iactic-cogiycolic)acid (PGLA) microspheres.
The compounds of the invention may also be administered topically,
(intra)dermatly, or transdermally to
the skin or mucosa. Typical formulations for this purpose include gels,
hydrogels, lotions, solutions,
?5 creams, ointments, dusting powders, dressings, foams, films, skin patches,
wafers, implants, sponges,
fibres, bandages and microemulsions. Liposomes may also be used. Typical
carriers include alcohol,
water, mineral oil, liquid petrolatum, white petrolatum, glycerin,
polyethylene glycol and propylene glycol.
Penetration enhancers may be incorporated - see, for example, J Pharm Sci, 88
(10), 955-958, by Finnin
and Morgan (October 1999).
Other means of topical administration include delivery by electroporation,
iontophoresis, phonophoresis,
sonophoresis and microneedle or needle-free (e.g. PowderjectT"", BiojectT ",
etc.) injection.
Formulations for, topical administration may be formulated to be immediate
and/or modified release.
Modified release formulations include delayed-, sustained-, pulsed-,
controlled-, targeted and programmed
release.
The compounds of the invention can also be administered intranasally or by
inhalation, typically in the
form of a dry powder (either alone, as a mixture, for example, in a dry blend
with lactose, or as a. mixed
component particle, for example, mixed with phospholipids, such as
phosphatidylcholine) from a dry
powder inhaler, as an aerosol spray from a pressurised container, pump, spray,
atomiser (preferably an
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
53
atomiser using electrohydrodynamics to produce a fine mist), or nebuliser,
with or without the use of a
suitable propellant, such as 1,1,1,2-tetrafluoroethane or 1,1,1,2,3,3,3-
heptafluoropropane, or as nasal
drops. For intranasal use, the powder may comprise a bioadhesive agent, for
exampie, chitosan or
cyclodextrin.
The pressurised container, pump, spray, atomizer, or nebuliser contains a
solution or suspension of the
compound(s) of the invention comprising, for example, ethanol, aqueous
ethanol, or a suitable alternative
agent for dispersing, solubilising, or extending release of the active, a
propellant(s) as solvent and an
optional surfactant, such as sorbitan trioleate, oleic acid, or an oligolactic
acid.
Prior to use in a dry powder or suspension formulation, the drug product is
micronised to a size suitable
for delivery by inhalation (typically less than 5 microns). This may be
achieved by any appropriate
comminuting method, such as spiral jet milling, fluid bed jet milling,
supercritical fluid processing to form
nanoparticles, high pressure homogenisation, or spray drying.
Capsules (made, for example, from gelatin or hydroxypropylmethylcellulose),
blisters and cartridges for
use in an inhaler or insufflator may be formulated to contain a powder mix of
the compound of the
invention, a suitable powder base such as lactose or starch and a performance
modifier such as I-leucine,
mannitol, or magnesium stearate. The lactose may be anhydrous or in the form
of the monohydrate,
preferably the latter. Other suitable excipients include dextran, glucose,
maltose, sorbitol, xylitol, fructose,
sucrose and trehalose.
A suitable solution formulation for use in an atomiser using
electrohydrodynamics to produce a fine mist
may contain from lpg to 20mg of the compound of the invention per actuation
and the actuation volume
may vary from 1 pl to 100N1. A typical formulation may comprise a compound of
formula I, propylene glycol,
sterile water, ethanol and sodium chloride. Alternative solvents which may be
used instead of propylene
glycol include glycerol and polyethylene glycol.
Suitable flavours, such as menthol and levomenthol, or sweeteners, such as
saccharin or saccharin
sodium, may be added to those formulations of the invention intended for
inhaled/intranasal
administration.
Formulations for inhaled/intranasal administration may be formulated to be
immediate and/or modified
release using, for example, PGLA. Modified release formulations include
delayed-, sustained-, pulsed-,
controlled-, targeted and programmed release.
In the case of dry powder inhalers and aerosols, the dosage unit is determined
by means of a valve which
delivers a metered amount. Units in accordance with the invention are
typically arranged to administer a
metered dose or "puff' containing from ... to ... pg of the compound of
formula I. The overall daily dose
will typically be in the range ... pg to ... mg which may be administered in a
single dose or, more usually,
as divided doses throughout the day.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
54
The compounds of the invention may be administered rectally or vaginally, for
example, in the form of a
suppository, pessary, or enema. Cocoa butter is a traditional suppository
base, 'but various alternatives
may be used as appropriate.
Formulations for rectal/vaginal administration may be formulated to be
immediate and/or modified release.
Modified release formulations include delayed-, sustained-, pulsed-,
controlled-, targeted and programmed
release.
The compounds of the invention may also be administered directly to the eye or
ear, typically in the form
of drops of a micronised suspension or solution in isotonic, pH-adjusted,
sterile saline. Other formulations
suitable for ocular and aural administration include ointments, gels,
biodegradable (e.g. absorbable gel
sponges, collagen) and non-biodegradable (e.g. silicone) implants, wafers,
lenses and particulate or
vesicular systems, such as niosomes or liposomes. A polymer such as crossed-
linked polyacrylic acid,
polyvinylalcohol, hyaluronic acid, a cellulosic polymer, for example,
hydroxypropylmethylcellulose,
hydroxyethylcellulose, or methyl cellulose, or a heteropolysaccharide polymer,
for
example, gelan gum, may be incorporated together with a preservative, such as
benzalkonium chloride.
Such formulations may also be delivered by iontophoresis.
Formulations for ocular/aural administration may be formulated to be immediate
and/or modified release.
Modified release formulations include delayed-, sustained-, pulsed-,
controlled-, targeted, or programmed
release.
The compounds of the invention may be combined with soluble macromolecular
entities, such as
cyclodextrin and suitable derivatives thereof or polyethylene glycol-
containing polymers, in order to
improve their solubility, dissolution rate, taste-masking, bioavailability
and/or stability for use in any of the
aforementioned modes of administration.
Drug-cyclodextrin complexes, for example, are found to be generally useful for
most dosage forms and
administration routes. Both inclusion and non-inclusion complexes may be used.
As an alternative to
direct complexation with the drug, the cyclodextrin may be used as an
auxiliary additive, i.e. as a carrier,
diluent, or solubiliser. Most commonly used for these purposes are alpha-,
beta- and gamma-
cyclodextrins, examples of which may be found in International Patent
Applications Nos. WO 91/11172,
WO 94/02518 and WO 98/55148.
Inasmuch as it may desirable to administer a combination of active compounds,
for example, for the
purpose of treating a particular disease or condition, it is within the scope
of the present invention that two
or more pharmaceutical compositions, at least one of which contains a compound
in accordance with the
invention, may conveniently be combined in the form of a kit suitable for
coadministration of the
compositions.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
Thus the kit of the invention comprises two or more separate pharmaceutical
compositions, at least one of
which contains a compound of formula I in accordance with the invention, and
means for separately
retaining said compositions, such as a container, divided bottle, or divided
foil packet. An example of such
a kit is the familiar blister pack used for the packaging of tablets, capsules
and the like.
5
The kit of the invention is particularly suitable for administering different
dosage forms, for example, oral
and parenteral, for administering the separate compositions at different
dosage intervals, or for titrating
the separate compositions against one another. To assist compliance, the kit
typically comprises
directions for administration and may be provided with a so-called memory aid.
The invention is illustrated by the following non-limiting examples in which
the following abbreviations and
definitions are used:
ao optical rotation at 587nm.
Ac20 acetic anhydride
APCI atmospheric pressure chemical ionisation
Arbacel filter agent
br broad
Boc tert-butoxycarbonyl
Bu butyl
CDCI3 chloroform-dl
CD30D methanol-d4
s chemical shift
d doublet
dd double doublet
DCM dichloromethane
DMF N,N-dimethylformamide
DMSO dimethylsulfoxide
eq (molar) equivalents
ESI electrospray ionisation
Et ethyl.
EtOAc ethyl acetate
h hours
HCI hydrogen chloride
HPLC high performance liquid chromatography .-
HR M/S high resolution mass spectrum
IPA isopropylalcohol
KOAc potassium acetate
m multiplet
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
56
Me methyl
MeCN acetonitrile
M/S mass spectrum
min minutes
NMR nuclear magnetic resonance
q quartet
r.t. room temperature
s singlet
sat saturated
t triplet
td triplet of doublets
Tf trifluoromethanesulfonyl
TFA trifluoroacetic acid
THF tetrahydrofuran
TIPS triisopropylsilyl
TLC/t.I.c thin layer chromatography
'H Nuclear magnetic resonance (NMR) spectra were in all cases consistent with
the proposed structures.
Characteristic chemical shifts (S) are given in parts-per-million SH downfield
from tetramethylsilane using
conventional abbreviations for designation of major peaks: e.g. s, singlet; d,
doublet; t, triplet; q, quartet;
m, multiplet; br, broad. The following abbreviations have been used for common
solvents: CDCI3,
deuterochloroform; DMSO, dimethylsulfoxide. The abbreviation psi means pounds
per square inch and
LRMS means low resolution mass spectrometry. Where thin layer chromatography
(TLC) has been used
it refers to silica gel TLC using silica gel 60 F254 plates, Rf is the
distance travelled by a compound divided
by the distance travelled by the solvent front on a TLC plate.
Example 1
5-[(2R)-4-Benzylmorpholin-2-yl]pyridin-2-amine
N
~ ~ -
H2N N
The morpholine from. preparation 7 (2.05 g, 6 mmol) was dissolved in ethanol
(75 mL), hydroxylamine
hydrochloride (2,05 g, 30 mmol) was added and the mixture heated at 80 C
overnight (-16 h). After
cooling to room temperature, the reaction mixture was evaporated to dryness to
a yellow oily residue
which was purified by flash chromatography on silica gel eluting with
dichloromethane/methanol/ 0.880
NH3 98:2:0 increasing polarity to 95:5:0 then 95:5:0.5, then 90:10:1 to afford
the title corripound (645 mg,
40%)
'H NMR (400 MHz, CDCI3) SH 8.01 (1H, s), 7.43 (1H, d), 7.33 (5H, m), 6.46 (1H,
d), 4.45 (3H brm), 3.96
(1 H, d), 3.8 (1 H, t), 3.54 (2H, s), 2.84 (1 H, d), 2.74 (1 H, d), 2.26 (1 H,
m), 2.12 (1 H, t)
MS (APCI+) 270 (MH+)
CA 02567935 2006-11-23
WO 2005/115985 _ PCT/IB2005/001554
57
Example 2
5-[(2R)-Morpholin-2-yl]pyridin-2-amine
YDNH
H2N N
The benzyi morpholine from example 1 (990 mg, 3.7 mmol) was dissolved in
methanol (20 mL)
ammonium formate (1.16 g, 18.5 mmol) followed by 10% Pd on carbon (495 mg)
were 'added and the
mixture heated at reflux for 2 h. The cooled reaction mixture was filtered
through a plug of arbocel and
evaporated to provide an orange solid (1.49 g). This material was purified by
flash chromatography on
0 silica gel (compound pre-absorbed onto silica) eluting with
dichloromethane/methanol/ 0.880 NH3 90:10:1,
to afford the title compound as a white solid (467 mg, 70%).
1 H NMR (400 MHz, CD3OD) 8H 7.82 (1 H, s), 7.45(1 H, d), 6.58 (1 H, d), 4.34
(1 H, d), 3.95 (1 H, d), 3.72 (1 H,
t), 2.95-2.80 (3H, m) 2.7 (1H, t)
MS (APCI+) 180 (MH+)
[a]o -39.4 (c = 0.12, MeOH)
Example 3
5-[(2R)-4-(3-Phenylpropyl)morpholin-2-yl]pyridin-2-am ine
H2N N
?0 The morpholine from example 2 (80 mg, 0.45 mmol) was dissolved in
tetrahydrofuran (15 mL) and 3-
phenylpropionaldehyde (59 L, 0.45 mmol) was added as a solution in
tetrahydrofuran (15 mL) over 15
minutes. After the addition was compiete, sodium triacetoxyborohydride (227
mg, 1 mmol) was added and
the reaction mixture stirred at room temperature for 6 h. The reaction mixture
was then diluted with
saturated sodium hydrogencarbonate solution (50 mL) and extracted with ethyl
acetate (2 x 50 mL). The
combined organic fractions were dried (MgSO4), filtered and evaporated to
provide a-yellow oil.
Purification by flash chromatography on silica gel eluting with
dichloromethane/methanol/ 0.880 NH3
98:2:0.2 increasing polarity to 95:5:0.5 afforded the title compound (51 mg,
38%)
' H NMR (400 MHz, CD3OD) 5,., 7.86 (1 H, d), 7.45 (1 H, dd), 7.27-7.10 (5H,
m), 6.55 (1 H, d), 4.39 (1 H, d),
3.95 (1 H, d), 3.75 (1 H, t), 2.83 (2H, m), 2.64 (2H, t), 2.41 (2H, t), 2.20
(1 H, m), 2.05 (1 H, t), 1.84 (2H, m)
MS (APCI+) 298 (MH+) _
[a]21 +6.9 (c = 0.13, MeOH)
Example 4
5-[(2R)-4-butylmorpholin-2-yl]pyridin-2-amine
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
58
N
H2N N
The morpholine from example 2 (80 mg, 0.45 mmol) was mixed with
tetrahydrofuran (10 mL) (only partly
soluble) and butyraldehyde (40 L, 0.45 mmol) was added, resulting in a
homogeneous solution. The
reaction mixture was stirred for further 30 minutes before the addition of
sodium triacetoxyborohydride
(227 mg, 1 mmol). The reaction mixture was then stirred at room temperature
overnight (-16 h) before
diluted with saturated sodium hydrogencarbonate solution (100 mL) and
extracted with ethyl acetate (100
mL). The combined organic layer was separated, dried (MgSO4), filtered and
evaporated to provide a
yellow oil. Purification by flash chromatography on silica gel eluting with
dichloromethane/methanol/ 0.880
NH3 98:2:0.2 increasing polarity to 95:5:0.5 afforded the title compound (17
mg, 16%)
0 'H NMR (400 MHz, CD3OD) SH 7.86 (1 H, s), 7.47 (1 H, d), 6.55 (IH, d), 4.39
(1 H, d), 3.98 (1H, d), 3.76
(1 H, t), 2.86 (2H, t), 2.41 (2H, t), 2.21 (1 H, t), 2.07 (1 H, t), 1.50 (2H,
m), 1.35 (2H, m), 0.95 (3H, t)
MS (APCI+) 236 (MH+)
Example 5
5-[(2R)-4-pentylmorpholin-2-yl]pyridin-2-amine
N
H2N N
The morpholine from example 2 (80 mg, 0.45 mmol) was mixed with
tetrahydrofuran (15 mL) and
pentanal (47 L, 0.45 mmol) was added dropwise as a solution in
tetrahydrofuran (15 mL) over 15
minutes. After the addition was complete, sodium triacetoxyborohydride (227
mg, 1 mmol) was added and
the reaction mixture stirred at room temperature overnight (-16 h). The
reaction mixture was then diluted
with saturated sodium hydrogencarbonate solution (75 mL) and extracted with
ethyl acetate (100 mL). The
combined organic layer was separated, dried (MgSO4), filtered and evaporated
to provide a yellow oil.
Purification by flash chromatography on silica gel eluting with
dichloromethane/methanol/ 0.880 NH3
98:2:0.2 increasing polarity to 95:5:0.5 afforded the title compound (67 mg,
61%)
'H NMR (400 MHz, CD3OD) 8H 7.86 (1H, s), 7.46 (1H, d), 6.55 (1H, d), 4.41 (1H,
d), 3.94 (IH, d), 3.77
(1 H, t), 2.86 (2H, t), 2.39 (2H, t), 2.21 (1 H, t), 2.06 (1 H, t), 1.54 (2H,
m), 1.34 (4H, m), 0.92 (3H, t)
MS (APCI+) 250 (MH)
[a]~ +4.42 (c = 0.13, MeOH)
Example 6 _
5-[(2R)-4-(2-phenylethyl)morpholin-2-yl]pyrid in-2-am ine
-~~I
N
H2N N
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
59
The morpholine from example 2 (80 mg, 0.45 mmol) was mixed with
tetrahydrofuran (15 mL) and
phenylacetaldehyde (52 L, 0.45 mmol) was added dropwise as a solution in
tettahydrofuran (15 mL) over
15 minutes. After the addition was complete, the reaction mixture was allowed
to stir at room temperature
for 1 h before the addition of sodium triacetoxyborohydride (227 mg, I mmol).
The reaction mixture was
stirred at room temperature overnight (-16 h) and then diluted with saturated
sodium hydrogencarbonate
solution (100 mL) and extracted with ethyl acetate (100 mL). The combined
organic layer was separated,
dried (MgSO4), filtered and evaporated to provide a yellow oil. Purification
by flash chromatography on
silica gel eluting with dichloromethane/methanol/ 0.880 NH3 98:2:0.2 afforded
the title compound (31 mg,
24%)
'H NMR (400 MHz, CD3OD) SH 7.87 (IH, s), 7.47 (1H, d), 7.20 (5H, m) 6.55 (1H,
d), 4.42 (IH, d), 3.97
(1 H, d), 3.78 (1 H, t), 2.93 (2H, t) 2.82 (2H, m), 2.66 (2H, t) 2.30 (2H, t),
2.21 (1 H, t), 2.15 (1 H, t)
MS (APCI+) 284 (MH+)
Example 7a
5-[(2R,5S)-S-Methylmorpholin-2-yl]pyridin-2-amine
~ NH
~
H2N N
The material from preparation 10 (410 mg, 1.25 mmol) was dissolved in ethanol
(10 mL), 5% Pd on
carbon (40 mg) was added and the mixture hydrogenated at room temperature
overnight at 1
atmosphere. The mixture was then filtered through a plug of arbocel , washing
the plug with ethanol and
the combined filtrates and washings were evaporated to a pale yellow solid.
Purification by flash
chromatography on silica gel eluting with dichloromethane/methanol/ 0.880 NH3
93:7:0.5 afforded the title
compound as a white solid (110mg, 45%)
'H NMR: SH (400 MHz, CD3OD) 7.85 (1H, d), 7.45 (1H, dd), 6.55 (1H, d), 4.29
(1H, m), 3.90 (1H, m), 3.30
(1H, m), 2.95-2.85 (2H, m), 2.75 (1H, m), 1.01 (3H, d)
MS (ES+) 194 (MH')
Alternatively a the morpholine ring may be formed by the following conditions
to provide a mixture of
diastereoisomers`
Example 7b
5-[(5S)-5-Methylmorpholin-2-yl]pyridin-2-amine (diastereomer mixture)
0~..,,
NH
H2N N
Diol from preparation 11 (1.26 g, 5.96 mmol) was dissolved in dichloromethane
(20 mL) and treated with
concentrated sulfuric acid (8 mL) at room temperature. The mixture was stirred
for 2 h before being
quenched by cautious addition of water, basification with 880 NH3 to pH -9 and
extracted with
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
dichloromethane (2 x 150mL). The combined organics were dried over magnesium
sulfate, filtered and
evaporated to provide the title compounds as a 3:1 mixture of (R, S) and (S,
S) diastereomers
respectively.
'H NMR: SH (400 MHz, CD3OD) 7.85 (1H, m), 7.52-7.45 (1H, 2xdd), 6.60-6.52 (1H,
2xd), 4.38-4.22 (1H,
5 2xdd), 3.95-3.80 (1 H, 2xdd), 3.30 (1 H, m), 3.10-2.83 (2H, m), 2.75 (1 H,
m), 1.39-0.99 (3H, 2xd)
Examples 8 and 9
A mixture of the morpholine compounds from Example 7b (240 mg, 1.2 mmol) was
dissolved in
10 tetrahydrofuran (45 mL) and to the stirred solution was added 3-
phenylpropionaldehyde (165 L, 1.2
mmol) dropwise as a solution in tetrahydrofuran (45 mL). Once the addition was
complete, sodium
triacetoxyborohydride (270mg, 1.2 mmol) was added and the reaction mixture
left to stir at room
temperature overnight. The solvent was evaporated and the diluted with water
(30 mL) and extracted with
dichloromethane (2 x 100 mL). The combined organic fractions were dried
(MgSO4), filtered and
15 evaporated to provide a clear oil of a ca. 2:1 mixture of trans:cis
diastereoisomers.
The diastereoisomers were separated by HPLC on a Chiralcel AD-H column with a
mobile phase of
methanol:ethanol 50:50 and a flow rate of 15m1/min
Example 8 (diastereomer 1)
20 5-[(2R,5S)-5-Methyl-4-(3-phenylpropyl)morpholin-2-yl]pyridin-2-amine
O I
\ N \
HZN N
Retention time 4.80min
'H NMR: 8H (400 MHz, CD3OD) 7.83 (1 H, s), 7.44 (1 H, d), 7.29-7.08 (m, 5H)
6.52 (1 H, d), 4.40 (1 H, d),
3.79 (1 H, d), 3.30 (1 H, m), 2.91-2.78 (2H, m), 2.60-2.50 (2H, m), 2.40 (1 H,
m), 2.29(1 H, m), 2.19 (1 H, m),
25 1.88-1.68 (2H, m), 0.95 (3H, d)
MS (APCI+) 312 (MH+)
Example 9 (diastereomer 2)
5-[(2S,5S)-S-Methyl-4-(3-phenylpropyl)morpholin-2-yi]pyridin-2-amine _
. _ ' p ~= '' /
N
30 HZN N
Retention time 7.60 min
'H NMR: SH (400 MHz, CD3OD) 7.89 (1H, s), 7.49 (1H, d), 7.29-7.09 (5H, m),
6.55 (1H, d), 4.42 (1H, m),
3.87 (1H, m), 3.72 (1H, d), 2.90(IH, m), 2.70-2.62 (2H, m), 2.60-2.42 (4H, m),
1.90-1.75 (2H, m), 1.09
(3H, d)
35 MS (APCI+) 312 (MH+)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
61
Examples 10 and 11
The diol from preparation 31 (350 mg, 1.3 mmol) was dissolved in
dichloromethane (5 mL) and to the
stirred solution was added concentrated H2SO4 (2.5 mL). The reaction mixture
left to stir at room
temperature for 2 h then quenched by the cautious addition of water (10 mL)
then basified by the addition
of 0.880 NH3 to pH 8-9. The mixture was then extracted with dichloromethane (2
x 50 mL) and the
combined extracts were dried (MgSO4), filtered and evaporated to provide a
brown oil of a mixture of cis
and trans morpholine diastereomers (275 mg, 85%)
MS (ES+) 250 (MH+)
The sample of mixed diastereoisomers was subjected HPLC using a Chiralcel OD-H
column, mobile
phase was 30:70 IPA/ Hexane with diethylamine 0.1%, at a flow rate of
20mL/min.
Example 10 (diastereomer 1)
5-[(2S,5S)-4-Butyl-5-methylmorpholin-2-yl]pyridin-2-amine
0^ ,,.
H2N N
Retention time 4.90 min
'H NMR: SH (400 MHz, CD3OD) 7.86 (IH, d), 7.49 (1H, dd), 6.56 (1H, d), 4.44
(1H, m), 3.86 (IH, m), 3.39
(1 H, m), 2.99 (1 H, m), 2.88 (1 H, m), 2.52 (1 H, brm), 2.41-2.28 (2H, m),
1.60-1.27 (4H, m), 1.07 (3H, d),
0.96 (3H, t)
?0 MS (APCI+) 250 (MH+)
Example 11 (diastereomer 2)
5-[(2R,5S)-4-butyl-5-methylmorpholin-2-yl]pyridin-2-amine
~ ~
HZN N
Retention time 7.20 min
~H NMR: 5H (400 MHz, CD3OD) 7.88 (1 H, d), 7.48 (1 H, dd), 6.55 (1 H, d), 4.41
(1 H, m), 3.83 (1 H,m), 3.72
(1H, d), 2.90 (1H, m), 2.60-2.52 (2H, m), 2.48-2.40 (2H, m), 1.54-1.44 (2H,
m), 1.40-1.32 (2H, m), 1.13
(3H, d), 0.94 (3H, t)
MS (APCI+) 250 (MH+)
Example 12
5-{(2R,5S)-5-[(Benzyloxy)methyl]morpholin-2-yl}pyridin-2-amine
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
62
H O I /
H2N N
The morpholine from preparation 14 (4.4 g, 9.21 mmol) was dissolved in ethanol
(50 mL), hydroxylamine
hydrochloride (3.2 g, 46 mmol) was added and the mixture heated at 80 C
overnight (-16 h). After
cooling to room temperature the mixture was diluted with 10% aqueous K2C03
solution (100 mL) and
extracted with dichloromethane (2 x 100 mL). The combined organic fractions
were dried over magnesium
sulfate, filtered and evaporated to provide a brown oil of crude deprotected 2-
amino pyridine intermediate
(3.6 g)
This boc-protected morpholine (3.6 g, 9 mmol) was treated with 4M HCI in
dioxane (30 mL) and the
mixture stirred at room temperature for 4 h. The solvent was then evaporated
and the residue treated with
2M sodium hydroxide (100 mL) and extracted with dichloromethane (4 x 100 mL).
The combined organic
fractions were dried over magnesium sulphate, filtered and evaporated to a
light brown solid which was
purified by flash chromatography on silica gel eluting with dichloromethane/
methanol/ 880 NH3 (93:7:0.5)
to provide the title compound as a light brown solid (1.43 g, 51%)
' H NMR: SH (400 MHz, CD3OD) 7.85 (1 H, d), 7.45 (1 H, dd), 7.36-7.25 (5H, m),
6.54 (1 H, d), 4.52 (2H, s),
4.28 (1 H, m), 3.97 (1 H, m), 3.49-3.38 (3H, m), 3.06 (1 H, m), 2.96 (1 H, m),
2.76 (1 H, m)
MS (ES+) 300 (MH+)
Example 13
5-{(2R,5S)-5-[(Benzyloxy)methyl]-4-propylmorpholin-2-yl}pyridin-2-amine
O0
N
H2N N~
The morpholine from Example 11 (1.4 g, 4.8 mmol) was dissolved in THF (200 mL)
and propanal (350 L,
4.8 mmol) in THF (150 mL) was added dropwise to the stirred mixture. After the
addition was complete
NaBH(OAc)3 (1.02 g, 4.8 mmol) was added in one portion and the reaction
mixture allowed to stir at room
temperature overnight (-16 h). TLC analysis showed starting material still
remaining, so, additional
NaBH(OAc)3 (1 g) was added and the reaction mixture stirred for a further 24
h. Saturated aqueous NH4CI
(200 mL) was added 'and the organic layer was separated, dried over magnesium
sulfate, filtered and
evaporated. The residue was purified by flash chromatography on silica gel
eluting with dichloromethane/
methano1/880 NH3 (95:5:0.5) to provide the title compound as a light brown oil
(540 mg, 33%)
'H NMR: SH (400 MHz, CD3OD) 7.86 (1H, d), 7.46 (1H, dd), 7.36-7.26 (5H, m),
6.53 (IH, d), 4.52 (2H, m),
4.38 (1 H, m), 4.00 (1H, m), 3.60-3.53 (2H, m), 3.47-3.42 (IH, m), 2.89 (1 H,
m), 2.78-2.69 (1 H, m), 2.59
(1 H, m), 2.32-2.21 (2H, m), 1.60-1.37 (2H, m), 0.84 (3H, t)
MS (ES+) 342 (MH+)
CA 02567935 2009-04-07
69387-598
63
Examptes 14-17
[6-{6-Aminopyrtdin~3-yI)-4-propylmorphotin-3-yl)methanot
~~OH
N
H,N N
Dial fronrn preparation 16 (1.4g, 3.9mmol, leq) was dissolved in
dichloromethane (15 mL) and treated with
concentrated sulfuric acid (10 mL) at room temperature. The mixture was
stirred at room temperature for
2 h before the being quenched by the addition of ice, then basified with 880
NH3 to pH -9. The mixture
was extracted with dichloromethane (3 x 150 mL) and the combined organic layem
were dried over
magnesium sulfate, fiftered and evaporated. The residue was puritied by flash
chromatography on silica
gel eluting with dichloromethane/ methanoU 880 NH3 (95:5:0.5 increasing
polarity to 93:7:0.5) to afford 110
mg of a light brown oil of the title compound as a mixture of four
diastereoisomers.
The diastereoisomers were separated by HPLC on a ChiralpakTK AD column, mobile
phase 20:80
iP,4lHexane with 0.1% DEA affording four stereoisomers
I5
Example 14
Stereoisomer 1(retention time: 9.50 min) trans enantiomer 1
'H NMR: SH (400 MHz, CD,OD) 7.86 (1H, d), 7.49 (1H, dd), 6.55 (1H, d), 4.40
(1H,.m), 4.05 (1H, m), 3.71
(1 H, m), 3.55 (2H, rn), 2.93 (1 H, m), 2.82 (1 H, m), 2.47 (1 H, m), 2:34 (1
H, m), 2.26 (2H, m), 2.27-1.42
(2H, m), 0.90 (3H, t)
Example 15
5tereoisomer 2(retention time: 11.90 mEn) cis enantiorner Z
'H NMR (400MHz, CD3OD) b(ppm) : 0.9 (t, 3H), 1.5-1.7 (m, 2H), 2.5-2.8 (m, 5H),
3.7-4.0 (m, 3H), 4.05-
4.15 (m,1 H), 4.4-4.55 (m, IH), 6.6 (d, 1 H), 7.5 (d, 1 H), 7.85 (s, .1 H)
Example 16
Stereoisomer 3 (retention time: 16.60 min) cis enanfiomer 2
'H NMR (400MHz, CD3OD) b(ppm) : 0.9 (t, 3H), 1.5-1.7 (m, 2H), 2.5-2.8 (m, 5H),
3.7-4.0 (m, 3H), 4.05-
4.15 (m, 1 H), 4.4-4.55 (m, 1 H), 6.6 (d, 1 H), 7.5 (d, 1 H), 7.85 (s, 1 H)
Exampte 17
Stereoisomer 4(retenfion time: 19.70 min) trans enantiomer 2
'H NMR: SH (400 MHz, CD3OD) 7.86 (1H, d), 7.49 (1H, dd), 6.55 (1H, d), 4.40
(1H, rn), 4.05 (1H, m), 3.71
(1H, m), 3.55 (2H, m), 2.93 (1H, m), 2.82 (1H, m), 2.47 (3H, m), 2.34 (1H,
rn), 2.26 (2H, m), 2.27-1.42
(211, m), 0.90 (3H, t)
Examples 18-19
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
64
4-methy1-5-(4-Propylmorpholin-2-yl)pyridin-2-amine
~
N
OJ
H2N N
The diol from preparation 21 (950 mg, 3.7 mmol) was dissolved in
dichloromethane (15 mL) and treated
with concentrated sulfuric acid (7 mL) at room temperature and the mixture
stirred for a further 2 h. The
reaction was then quenched the addition of ice, then basified by the dropwise
addition of 880 NH3 until the
pH -9. The mixture was then extracted with dichloromethane (4 x 50 mL) and the
combined organics
dried with magnesium sulfate, filtered and evaporated to provide the title
compound as a pale brown oil
(700 mg, 79%)
'H NMR: SH (400 MHz, CD3OD) 7.85 (1 H, d), 6.38 (1 H, d), 4.60 (1 H, m), 3.99
(1 H, m), 3.78 (1 H, m), 2.92-
2.82 (2H, m), 2.38 (2H, m), 2.28-2.18 (4H, m), 2.12 (1H, m), 1.62-1.50 (2H, m)
0.93 (3H, t)
MS (APCI+) 236 (MH+)
The racemic morpholine was subjected to HPLC using a Chiralcel OD-H column
eluting with acetonitrile.
This afforded the two enantiomers.
Example 18 (enantiomer 1)
Retention time: 5.1 min
'H NMR: SH (400 MHz, CD3OD) 7.85 (1H, d), 6.38 (1H, d), 4.60 (1H, m), 3.99
(1H, m), 3.78 (1H, m), 2.92-
2.82 (2H, m), 2.38 (2H, m), 2.28-2.18 (4H, m), 2.12 (1H, m), 1.62-1.50 (2H, m)
0.93 (3H, t)
MS (APCI+) 236 (MH+)
Example 19 (enantiomer 2)
Retention time: 6.5 min
'H NMR: SH (400 MHz, CD3OD) 7.85 (1H, d), 6.38 (1H, d), 4.60 (1H, m), 3.99
(1H, m), 3.78 (1H, m), 2.92-
2.82 (2H, m), 2.38 (2H, m), 2.28-2.18 (4H, m), 2.12 (1H, m), 1.62-1.50 (2H, m)
0.93 (3H, t)
MS (APCI+) 236 (MH+)
Example 20 .
3-Methyl-5-[(5S)-5-rriethyl-4-propylmorpholin-2-yl]pyridin-2-amine
H2N N
O
N~
~
The diol from preparation 29 (200 mg, 0.74 mmol) was dissolved in
dichloromethane (4 mL) and treated
with concentrated sulfuric acid (2 mL) at room temperature and the mixture
stirred for a further 2 h. The
reaction was then quenched the cautious addition of water, then basified by
the dropwise addition of 880
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
NH3 until the pH -9. The mixture was then extracted with dichloromethane (3 x
70 mL) and the combined
organics dried with magnesium sulfate, filtered and evaporated. The residue
was purified by flash
chromatography on silica gel to provide the title compound as a clear oil as a
mixture of diastereoisomers
(35 mg, 19%)
51 H NMR: SH (400 MHz, CD30D) 7.77 (1H, d), 7.38 (1H, d), 4.41 (1H, m), 3.88-
3.70, 2.95-2.72 (3H, 2xm),
2.57 (1 H, m), 2.50-2.35 (2H, m), 2.29-2.19 (1 H, m), 2.11 (3H, 2xs), 1.61-
1.39 (2H, m), 1.18-1.00 (3H,
2xd), 0.91 (3H, m)
MS (ESI+) 250 (MH+)
10 Examples 21 and 22
The diol from preparation 24(990mg, 3.9 mmol) was dissolved in dichloromethane
(10 mL) and treated
with concentrated sulfuric acid at room temperature. The mixture was left
stirring for 2 h before being
quenched by the addition of ice and the basified by the addition of 880 NH3 to
pH -9. The mixture was
then extracted with dichloromethane (3 x 150 mL), the combined organics dried
over magnesium sulfate,
15 filtered and the solvents evaporated. The residue was purified by flash
chromatography on silica gel
eluting with dichloromethane/ methanol/ 880 NH3 (95:5:0.5) to provide the
title compound as a mixture of
diastereoisomers (470 mg, 51%).
MS (ES+) 236 (MH+)
The diastereoisomers were split using chiral HPLC on a Chiralcel OD-H column
eluting with 30% IPA in
20 hexanes with 0.1 % diethylamine. To afford:
Example 21 (diastereomer 1)
25 5-[(2S,5S)-4,5-Diethylmorpholin-2-yl]pyridin-2-amine
O
N~
H2N N
retention time: 4.1 min
'H NMR: SH (400 MHz, CD30D) 7.88 (IH, d), 7.48 (1H, dd), 6.57 (1H, d), 4.43
(1H,m), 3.98 (1H, d), 3.77
(1 H, m), 2.67-2.54 (1 H, m), 1.60 (1 H, m), 1.11 (3H, t), 0.96 (3H, t)
30 MS (ES) 236 (MH})
Example 22 (diastereomer 2)
5-[(2R,5S)-4,5-Diethylmorpholin-2-yl]pyridin-2-amine
0
N
H2N N
35 Retention time: 7.3 min
CA 02567935 2009-04-07
69387-598
66
'H NMR: 5,., (400 MHz, CD3OD) 7.87 (iH, d), 7,47 (1H, dd), 6.56 (1H, d), 4.40
(1H, m), 3.98 (1H, m), 3.43
(IH, m), 3.01-2.90 (21-1, m), 2.44 (IH, m), 2.33 (1H, m), 2.25 (1H, m), 1.78
(1H, m), 1.32 (1H, m), 1.06
(3H, t), 0.83 (3H, t)
MS (ES`) 236 (MHi)
Example 23
5-(1-Propytazetidin-3-yl)pyridin-2-am ine
H2N
The aminopyridine imine from preparation 35 (95mg, 0.267mmol, 1.Oeq) was
dissolved in EtOH (2m1),
10% Pd/C (10mg) and ammonium formate (168mg, 2.67mmol, 10eq) was added and the
mixture heated
to a gentle reflux for 3h. Further 10% PdtC (10mg) and ammonium formate
(168mg, 2_67mmol, 10eq)
added and the mixture heated to reflux for 48h. The catalyst was filtered off
through arbocel, and washed
with EtOH.'The filtrate was evaporated in vacuo to give a coiourless oil. This
material was dissolved in
THF (5m1), 2M HCI (aq) added and stirred at r.t. for 3h. The mixture was
evaporated in vacuo and basified
with t(,C03 (10% w!v aq) and extracted with CH2CI2 (3x20m1), dried (MgSO4),
filtered and evaporated to
give a colourless oil. This oil was purified by flash chromatography on silica
gel with a gradient elution from
100% CH2C12 to 90:10:1 CH2C12:MeOH:NH4OH ta give the product as a colourless
oil which solidified on
standirig (21mg,*41%)
Tic Rf=0.24 (90:10:1 CH2CI2:MeOH:NH4O14 UV visual'rcation)
MS (APCI+) 192 (MH+)
'H NMR: bsa (400 AAHz, CD30D) 7.95 (1H, s), 7.45 (1H, d), 6.5 (iH, d), 4.2-4.6
(21-1, br s), 3.5-3.8 (31-1, m),
3.05 (2H, t), 2.45 (21i, t), 1.3-1.5 (2H, m), 0.9 (31-1, t)
laatamples 24 and 25.
5-(2R,5S)-4-Ethyt-5-methylmorphotin-2 yy-pyridin 2 ytamtne & 5-(2S,5S)-4-
Ethylv-
methylmorpholin-2-yl)-pyridin-2-ylamine
o "
HZN fti H2N N
The morpholine from preparation 38 (1g, 4.17mmo1) was dissolved in CH2CIZ
(15mi) and concentrated
sulfuric acid (7.5mt) was added. The mixture was stirred at r.f_ for 2h,
bas'ified by cautious addition of
0.880 NH3i and extracted with CHZC)Z (2x200rn1), the organics combined, dried
over magnesium sulphate,
fittered and puriiied by flash chromatography on silica ge1 eluting with
CH2Ciz:MeOH:NH4OH 97:3:1 to give
the 6tte compound as a light brown oil (560mg 61%).
The d'rasbereoisomers were separated on a Chiralcel 01 OD-H column (500"50mm)
with a mobile phase of
20% IPA, 80% hexane, 0.1 % DEA at a floui rate of 80m1/min to give:
CA 02567935 2009-04-07
69387-598
67
Diasteroisomer 1- retention time S.47min (Example 24, (2S,5S) diastereoisomer)
'H NMR: S,., (400 MHz, CD30D) 7.88 (1H, s), 7.46-7.52 (1H, m), 6.58 (1H, d),
4.40-4.46 (1H, m), 3.84-3.92
(1H, m), 3.75-3.79 (1H, rn), 2.91-2.98 (1H, m), 2.47-2.60 (4H, m), 1.08-1.18
(m, 6H)
MS (APCI+) 222 (MH+)
Diasteroisomer 2 - retention time 7.96min (Example 25, (2R,5S)
diastereoisomer)
'H NMR: SH (400 MHz, CD30D) 7.88 (1H, s), 7.44-7.50 (1H, m), 6.56 (1H, d),
4.40-4.46 (m, 1H),-3.80-3.88
(1 H, m), 3.28-3.41(1 H, m), 2.88-3.00 (2H, m), 2.35-2.52 (2H, m), 2.16-2.24
(1 H, m), 1.00-1.08 (rn, 6H)
MS (APCI+) 2-22 (MH+)
Examptes26&27
(+)-5-(4-propyknorphot10-2-y1)-1,3-thiazol-2-amine & (-)-3-(4-propylmorpholin-
2 yi)-1,3 thiazol-2-
amine
H:N--{~
S N~
To 2-(2-bromo-1,3-thiazol-5-yi)-4-propyimorpholine (2.5g 8.56mmoi) in ethyiene
glycoi (60mi) at 78 C
was added Cu:O (61mg, 0.43mmol, 0.05eq) and NH3t1) (20mi) in a bomb. The
vessel was sealed, and
heated to 80 C for 18h. The vessel was allowed to coo(vented, and partitioned
between EtOAc
(2x200rnl) and water (100rrmi), organic layers combined, dried over MgSO4 and
solvent evaporated to give
a brown oil This material was chromatographed on an Isco m Companion
Combiflash autochromatography
system with a gradient elution from 99/110.1 CH2Ci2/MeOH/NH3 to 95I5/0.5 CH2C1-
4MeOH/NH3to give the
product as a brown oil (1.1g).
This material was separated by HPLC on a chiraicet OJ column (250*21.5) with a
mobile phase of 70:30
Hexane:lPA at a flow rate of 18mVmin to give two enantiomets.
Enantiomer 1 retention time 5.140min
'H NMR (400MHz, C030D) b(ppm) : 6.99 (s, 1 H), 4.63 (d, 1 H), 3.87-3.93 (m, 9
H), 3.70-3.77 (m, 1 H), .
2.95 (d. 1H), 2.78 (d, 1H), 2.31-2.39 (m, 2H), 2.10-2.23 (m, 2H), 1.48-1.60
(m, 2H), 0.92 (t, 3H)
AIUS APCI+ 228 (MH+)
Optical rotation (a) n+48.45 (c=1.45mg/ml^MeOH)
Elemental analysis +0.55 H.b Total MW=23724
Calculated C(50.63), H(7.69), N(17.71)
Actual C(50.90, 50.79), H(7.48, 7.51), N(17.35, 17.38)
Enantiomer 2 retention time 10.750min
'H NMR (400MHz, CD3OD) b(ppm) : 6.99 (s, 1H), 4.63 (d, 1H), 3.87-3.93 (m,
'iH), 3.70-3.77 (m, 1H),
2.95 (d,1H), 2.78 (d,1H), 2.31-2.39 (m, 2H), 2.10-2.23 (m, 2H), 1.48-1.60 (m,
2H), 0.92 (t. 3H)
MJS APC1+ 228 (MH+)
Optical rotation (a] 25 -43.56 (c^2.6mg1mi MeOH)
Elemental analysis +1 H20 Total MW=245.35
Calculated C(48.96), H(7.81), N(17.13)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
68
Actual C(49.05, 49.07), H(7.83, 7.85), N(17.00, 16.99)
The following preparations illustrate the synthesis of certain intermediates
used in the preparation of the
preceding examples:
Preparation I
5-Bromo-2-(2,5-dimethyl-pyrrol-1-yl)-pyridine
Br
~~ \J7
N N
2,5-hexanedione (46.2 g, 0.41 mol) was added to a suspension of 2-amino-5-
bromopyridine (50.0 g, 0.29
mol) and the reaction heated to reflux for 24 hours under Dean and Stark
conditions. para-Toluenesulfonic
acid (100 mg) was added and the reaction was refluxed for a further 18 hours.
8 mL of water was
removed, so the reaction was cooled to room temperature, washed with water
(100 mL) and passed
through a plug of silica gel, eluting with toluene. The eluent was
concentrated in vacuo and the residue
dissolved in pentane:dichloromethane (1:1 by volume) and passed through a plug
of silica gel, eluting with
pentane:dichloromethane (1:1 by volume). The eluent was concentrated in vacuo
to give a red liquid,
which solidified on standing. The solid was recrystallised (isopropanol) to
give the title compound as a pale
yellow solid (54.4 g).
'H NMR (400MHz, CDCI3): 5H 8.66 (1H, s), 7.93-7.92 (1H, d), 7.13-7.11 (1H, d),
5.91 (2H, s), 2.13 (6H, s).
LRMS (thermospray) : m/z [M+H]+ 252.
Preparation 2
2-Chloro-1-[6-(2,5-dimethyl-pyrrol-1-yl)-pyridin-3-yl]-ethanone
O
I iN CI
N
A solution of 2.5 M n-butyl lithium in hexanes (35 mL, 87.6 mmol) was added to
a solution of the bromide
from preparation 1(20.0 g, 79.7 mmol) in tert-butylmethylether (300 mL) at -78
C under nitrogen over 10
minutes. The reaction was stirred for a further 10 minutes and 2-chloro-N-
methoxy-N-methylacetamide
(12.1 g, 87.6 mmol) in tert-butylmethylether (40 mL) was added slowly. The
reaction was stirred at -78 C
for 20 minutes and then 1M hydrochloric acid (200 mL) was added. The mixture
was allowed to warm to
room temperature, stirred for 2 hours and the organic phase separated. The
aqueous phase was
extracted with tert-butyl methylether and the combined organic extracts were
washed with water (100 mL),
saturated aqueous sodium chloride (100 mL) and 1M sodium hydroxide (100 mL).
The organic phase was
dried (sodium sulfate), concentrated in vacuo and the residual oil purified by
flash column chromatography
on silica gel eluting with pentane:dichloromethane:methanol (75:25:0 changing
to 0:99:1, by volume). The
residue was recrystallised from pentane:dichloromethane to give the title
compound as a yellow solid.
(14.37g, 73%)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
69
'H NMR (400MHz, CDCI3): SH 9.11 (1H, s), 8.34-8.33 (1H, d), 7.32-7.30 (1H, d),
5.91 (2H, s), 4.66 (2H, s),
2.17 (6H, s)
LRMS (electrospray) : m/z [M-H]+ 247.
Preparation 3
2-(2,5-Dimethyl-pyrrol-l-yl)-5-[(2R)-oxiranyt]pyridine
O
N N
A solution of the chloride from preparation 2 (12.0 g, 48.1 mmol) in
tetrahydrofuran (20 ml) was slowly
added to a solution of (-)-B-chlorodiisopinocampheylborane (20.1 g, 62.5 mmol)
in tert-butylmethylether
(15 mL) and tetrahydrofuran (30 mL) at -30 C under nitrogen. The reaction was
stirred for 6 hours at -30
C and then sodium perborate tetrahydrate (7.4 g, 48.1 mmol) followed by tert-
butyimethylether (50 mL)
were added. The reaction was stirred at room temperature for 18 hours, treated
with 2M aqueous sodium
hydroxide (190 mL) and stirred for a further 6 hours. The organic phase was
separated and the aqueous
phase extracted with further tert-butylmethylether (50 mL). -The combined
organic extracts were washed
with IM aqueous sodium hydroxide (50 mL), saturated aqueous sodium chloride
(50 mL), dried (sodium
sulfate) and concentrated in vacuo. The residue was purified by flash column
chromatography on silica gel
eluting with pentane:dichloromethane (80:2 changing to 100:0, by volume) to
give the crude epoxide (65%
b/w, 11.0 g), which was used without further purification.
'H NMR (400MHz, CDCI3): SH 8.58 (1 H, brs), 7.68-7.66 (1 H, dd), 7.22-7.20 (1
H, d), 3.97-3.96 (1 H, m),
3.26-3.24 (1 H, m), 2.91-2.89 (1 H, m), 2.12 (6H, s)
LRMS (electrospray) : m/z [M+H]+ 215, [M+Nal+, 237.
Preparation 4
(1R)-2-(Benzylamino)-1-[6-(2,5-dimethyl-1H-pyrrol-l-yl)pyridin-3-I]ethanol
oH ~ I
N
/ N N
r
The epoxide from Preparation 3 (2.66 g, 12mmol) was dissolved in DMSO (30 mL),
treated with
benzylamine (1.62 mL, 15 mmol) and the mixture heated to 90 C overnight (-16
h). After cooling to room
temperature the reaction mixture was evaporated under high vacuum at 60 C to
remove most of the
DMSO. The residue was diluted with ethyl acetate (150 mL) and washed with
water (100 mL). The organic
layer was separated and the aqueous layer re-extracted with ethyl acetate (100
mL). The combined
organic fractions were dried (MgSO4), filtered and evaporated to give the
title compound as a yellow oil
(3.29 g, 84%)
' H NMR (400 MHz, CDCI3) 8.55 (1 H, s), 7.85 (1 H, d), 7.35-7.25 (5H, m), 7.2
(1 H, d), 5.9 (2H, s), 4.8 (1 H,
m), 3.89 (2H, s), 3.01 (1 H, dd), 2.78 (1 H, t), 2.1 (6H, s)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
MS (APCI+) 322 (MH+)
Preparation 5
N-Benzyl-2-chloro-N-{(2R)-2-[6-(2,5-dimethyl-1 H-pyrrol-1-yl)pyridin-3-yl]-2-
hydroxyethyl}acetamide
ci
OH
\ N \ I
LN N
5 The amino alcohol from Preparation 4 (3.22 g, 10 mmol) was dissolved in
dichloromethane (100 mL)
sodium hydroxide (2 g, 50 mmol) in an aqueous solution (35 mL) was added and
the mixture stirred
vigorously. Chloroacetylchloride (0.96 mL, 12 mmol) was added dropwise and
stirring was then continued
at room temperature overnight (-16 h). The reaction mixture was then diluted
with dichloromethane (200
0 mL) and water (100 mL). The organic layer was separated, dried (MgSO4),
filtered and evaporated to give
a brown oil (4.35 g). The 'H NMR spectrum indicated that a mixture of
chloroamide and morpholinone
(product of preparation'5)'was formed, so the mixture taken on forward without
further purification.
MS (APCI+) 398 (MH+, chloro amide), 362 (MH+, cyclised morpholinone)
5 Preparation 6
(6R)-4-Benzyl-6-[6-(2,5-dimethyl-1 H-pyrrol-l-yl)pyridin-3-yl]morpholin-3-one
p'-f 0
N
/ NI \N
The crude mixture from preparation 5 was dissolved in propan-2-ol (100 mL),
water (5 mL) was added
followed by potassium hydroxide (673 mg). The mixture was stirred vigorously
at room temperature over
?0 night. The reaction mixture was then partitioned between ethyl acetate (200
mL) and water (150 mL). The
organic layer was separated and washed with brine (150 mL), dried (MgSO4),
filtered and evaporated to
afford a dark orange oil. Purification by flash chromatography on silica gel
eluting with dichloromethane/
methanol 99:1 afforded the title compound as a yellow oil (69%)
'H NMR (400 MHz, CDC13) 8.52 (1 H, s), 7.79 (1 H, d), 7.30 (5H, m), 7.20 (1 h,
d), 5.89 (2H, s), 4.89 (1 H,
25 dd), 4.76 (1 H, d), 4.634.42 (3H, m), 3.49 (1 H, t), 3.38 (1 H, dd), 2.09
(6H, s)
MS (APCI+) 362 (MH+)
Preparation 7
(2R)-4-Benzyl-2-[6-(2,5-dimethyl-1 H-pyrrol-l-yl)pyridin-3-yl]morpholine
0'1
.~N
/ N N
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
71
The morpholinone from preparation 6 (2.47 g, 6.8 mmol) was dissolved in
tetrahydrofuran (100 mL) and
cooled (flask in ice/ water bath). Lithium aluminiumhydride (1 M in
tetrahydrofuran, 10.2 mL, 10.2 mmol)
was added dropwise and after the addition the reaction mixture was allowed to
stir at room temperature
overnight (-16h). The reaction was quenched by the cautious addition of 1M
sodium hydroxide (10 mL)
then diluted with water (150 mL) and stirred for 10 minutes. Ethyl acetate
(200 mL) was added, the
organic layer separated, dried over MgSO4i and evaporated to provide the title
compound as a yellow oil
(2.09 g, 89%)
' H NMR (400 MHz, CDC13) 8.59 (1 H, s), 7.81 (1 H, d), 7.3 (5H, m), 7.2 (1 H,
d), 5.9 (2H, s), 4.69 (1 H, d),
4.05 (1 H, d), 3.9 (1 H, t), 3.6 (2H, s), 3.0 (1 H, d), 2.8 (1 H, d), 2.35 (1
H, t), 2.19 (1 H, t), 2.1 (6H, s)
MS (APCI+) 348 (MH+)
Preparation 8
(2S)-2-({(2R)-2-[6-(2,5-Dimethyl-lH-pyrrol-1-yl)pyridin-3-yi]-2-hydroxyethyl}
amino) propan-l-ol
0H
NH
/ N N
5
The epoxide from preparation 3 (5.4 g, 20 mmol) was dissolved in DMSO (50 mL)
together with (S)-2-
aminopropan-l-ol (2.0 g, 20 mmol) and the mixture heated at 90 C overnight
(ca. 16 h). After cooling to
room temperature, the mixture was evaporated under high vacuum and the residue
purified by flash
chromatography on silica gel eluting with dich lorom ethane/ methanol (95:5
increasing polarity to 90:10) to
10 provide the title compound as a pale yellow oil (5.0 g, 75%)
Preparation 9
Benzyl (2R,5S)-2-[6-(2,5-dimethyl-1H-pyrrol-1-yl)pyridin-3-yl]-5-
methylmorpholine-4-carboxylate
~ O N, O ~ I
.~ ~
/ N N O
The diol from preparation 8 (5 g, 17.2 mmol) was dissolved in dichloromethane
(60 mL) and treated with
benzyl chloroformate (2.72 mL, 19 mmol) and triethylamine (2.65mL, 19 mmol).
The mixture was stirred
overnight (-16 h) before being quenched by the addition of 2M sodium hydroxide
(100 mL). The mixture
was extracted with dichloromethane (2 x 100 mL) and the combined organic
fractions dried (MgSO4),
filtered and evaporated. The residue was purified by flash chromatography on
silica gel eluting with a
gradient from 25% to 60% ethyl acetate in pentane to afford the CBz-protected
intermediate as a light
brown oil (2.56 g, 35%)
MS (ES+) 446 (MNa})
MS (ES") 422 (M-H+)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
72
A sample of the above CBz-proected diol (2 g, 4.7 mmol), was dissolved in
toluene (30 mL) together with
triphenylphosphine (1.5 g, 5.6 mmol). Diisopropyl azodicarboxylate (1.12 mL,
5.6 mmol) was added
dropwise and the reaction mixture was left stirring overnight (-16 h). The
reaction'mixture was diluted with
water (100 mL) and extracted with ethyl acetate (2 x 100 mL). The combined
organic fractions were dried
(MgSO4), filtered and evaporated. The residue was purified by flash
chromatography on silica gel eluting
with 20% ethyl acetate in pentane to afford the title compound as a clear oil
(1.68).1H NMR shows the
sample to contain -3 equivalents of diisopropyl hydrazine-1,2-dicarboxylate
together with the title
compound. Thus -40% by weight of material is the title compound, corresponding
to an approximate yield
of 36%.
' H NMR: 5H (400 MHz, CDCI3) 8.62 (IH, d), 7.80 (111, dd), 7.37-7.27 (5H, m),
7.11 (1 H, d), 5.88 (2H, s),
5.18 (1 H, d), 5.10 (1 H, d), 4.26 (1 H, m), 4.08 (1 H, m), 3.72 (2H, m), 3.46-
3.40 (2H, m), 2.09 (6H, s), 1.37
(2H, d)
MS (ES+) 406 (MH+)
Preparation 10
2-(6-Amino-pyridin-3-yl)-5-methyl-morpholine-4-carboxylic acid benzyl ester
N,
y
H2N N 0
Morpholine from preparation 9 (680 mg, 1. mmol) was dissolved in ethanol (12
mL) and treated with
hydroxylamine hydrochloride (600 mg, 8.4 mmol) and the mixture heated at 80 C
overnight (-16 h). After
cooling to room temperature the solvent was evaporated and the residue was
purified by flash
chromatography on silica gel eluting with dichloromethane in methanol 0%
increasing polarity to 2% to
provide the title compound as a purple coloured oil (410 mg, 95%).
'H NMR (400 MHz, CD30D) SH 7.91 (1H, d), 7.43 (1H, dd), 7.37-7.28 (5H,m), 6.52
(1H, d), 5.13 (2H, 2 x
d), 4.79 (1 H, m), 4.12 (1 H, m), 4.04 (2H, m), 3.37 (2H, m), 130 (3H, d)
MS (ES) 328 (MH+)
Preparation 11
(2S)-2-([(2R)-2-(6-Am i nopyrid i n-3-y I)-2-hyd roxyethyl]am i no}propan-l-ol
H
OH
- ~ NH
HaN N
The diol from preparation 8 (1 g, 3.35 mmol) was dissolved in ethanol and
treated with hydroxylamine
hydrochioride (1.2 g, 16.75 mmol) and the mixture heated at 80 C overnight (-
16 h). After cooling to
room temperature the soivent was evaporated and the residue was purified by
flash chromatography on
silica gel eluting with dichloromethane/ methanol/ 880 NH3 (85:15:1 increasing
polarity to 82:17:1) to
provide the title compound as a light brown oil (670 mg, 95%)
' H NMR (400 MHz, CD30D) SH 7.91 (1 H, s), 7.52 (1 H, d), 7.6 (1 H,d), 4.72 (1
H, d), 3.67 (1 H, d), 3.45 (1 H,
m), 3.1-2.85 (3H, m), 1.15 (3H, d)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
73
MS (ES+) 212 (MH+) 234 (MNa+)
Preparation 12
(2R)-3-(Benzyloxy)-2-({(2R)-2-[6-(2,5-d imethyl-1 H-pyrrol-1-yi)pyrid in-3-yl]-
2-
hydroxyethyl}amino)propan-l-ol
OH
OH~='...., .O
NH I
/ N N
The epoxide from preparation 3 (5.4 g, 25 mmol) was dissolved in DMSO (50 mL)
together with (S)-2-
amino-3-benzyloxypropan-l-oi (5.0 g, 27.6 mmol) and the mixture heated at 90
C overnight (ca. 16 h).
After cooling to room temperature, the mixture was evaporated under high
vacuum to provide a brown oil
-12g of desired title compound containing some residual DMSO but of sufficient
purity to use in the
subsequent stage without further purification.
'H NMR (400 MHz, CD3OD) 5H 8.54 (1H, d), 7.99 (1H, dd), 7.25-7.22 (6H, m),
5.81 (2H, s), 4.51 (2H, m),
3.67-3.45 (5H, m), 3.01-2.81 (3H, m), 2.03 (6H, s)
MS (ES) 396 MH+
Preparation 13
tert-Butyl [(1 R)-2-(benzyloxy)-1-(hydroxymethyl)ethyl]{(2R)-2-[6-(2,5-
dimethyl-1 H-pyrrol-l-
yl)pyridin-3-yl]-2-hydroxyethyl}carbamate
9H
OH
N-rO I
O
N
The crude diol from preparation 12 (10g, -25mmol) was dissolved in
dichloromethane (150 mL) and
treated with di-tert-butyl dicarbonate (5.52 g, 25 mmol) and the mixture
stirred at room temperature
overnight (-16 h). The reaction mixture was diluted with 10% aqueous K2C03
solution (200 mL), the
organic layer separated and the aqueous layer extracted with dichloromethane
(2 x 300 -mL). The
combined organic fractions were dried over magnesium sulfate, filtered and
evaporated. The residue was
purified by flash chromatography on silica gel eluting with 35% ethyl acetate
in pentane increasing polarity
of eluent to 50% ethyl acetate in pentane to afford the title compound as a
pale yellow oil (6.2 g, 50% yield
over 2 steps from preps 12 and 13)
'H NMR; SH (400 MHz, CD3OD) 8.55 (1H, d), 8.04-7.95 (1H, m), 7.38-7.23 (6H,
m), 5.81 (2H,_s), 5.05 (1H,
brm) 4.54 (2H, m), 3.93 (IH, brm), 3.83 (1H, brm), 3.78-3.60 (5H, m), 3.44-
3.32 (1H, m), 2.05 (6H, s),
1.44 and 1.40 (9H, two singlets)
MS (APCI+) 496 (MH')
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
74
Preparation 14
tert-Butyl (2R,5S)-5-[(benzyloxy)methyl]-2-[6-(2,5-dimethyl-lH-pyrrol-l-
yl)pyridin-3-yl]morpholine-4-
carboxylate f NJ
The diol from preparation 13 (6.2 g, 12.5 mmol) was dissolved in toluene (100
mL) and treated with
triphenylphosphine (4 g, 15 mmol) at room temperature.
Diisopropylazodicarboxylate (DIAD) (3 mL, 15
mmol) was added dropwise and the mixture allowed to stir overnight (-16 h).
The reaction mixture was
then diluted with water (200 mL), the organic layer separated and the aqueous
layer extracted with ethyl
acetate (200 mL). The combined organic layers were dried over magnesium
sulfate, filtered and
3 evaporated. The residue was purified by flash chromatography on silica gel
eluting with 10% ethyl acetate
in pentane increasing polarity to 15% ethyl acetate in pentane to provide the
title compound as a clear oil
(4.4 g, 74%)
'H NMR: SH (400 MHz, CDC13) 8.64 (1H, d), 7.88 (1H, dd)., 7.36-7.27 (5H, m),
7.22 (1H, d), 5.89 (2H, s),
4.96 (1 H, m), 4.62 (1 H, d), 4.54 (1 H, d), 4.28 (1 H, m), 4.12 (1 H, m) 3.82-
3.68 (4H, m), 3.60 (1 H, dd), 2.12
5 (6H, s), 1.44 (9H, s)
MS (APCI+) 478 MH+
Preparation 15
(2R)-3-(Benzyloxy)-2-[{(2R)-2-[6-(2,5-dimethyl-1 H-pyrrol-l-yl)pyridin-3-yl]-2-
0 hydroxyethyl}(propyl)amino]propan-l-ol
OH
OH~..,. ~O
i
nc ~
S N N N
The crude diol from preparation 12 (3g, 7.6mmol) was dissolved in
dichloromethane and propanal (1.1
mL, 15.2mmol) and NaBH(OAc)3 (3.25 g, 15.2 mmol) were added. The reaction
mixture was stirred at
room temperature overnight (-16 h) and then solvents were evaporated. The
residue was purified by flash
25 chromatography on silica gel eluting with d ich lorom ethane/ methanol/ 880
NH3 (97:3:0.5) to provide the
title compound as a light brown oil still containing -3 equivalents of DMSO
contamination from the
previous stage (4.5 g, corrected for DMSO -2.95g of product, 89% yield)
'H NMR: 5H (400 MHz, CDCI3) 8.52 (1H, d), 8.81 (IH, dd), 7.38-7.22 (5H, m),
7.17 (1H, d), 5.86 (2H, s),
4.72 (1 H, m), 4.54 (2H, s), 3.48-3.68 (4H, m), 3.16 (IH, m), 2.88-2.95 (1 H,
m) 2.82-2.55 (3H, m), 2.07
30 (6H, s), 1.50 (2H, m), 0.87 (3H, t)
MS (APCI+) 438 (MH+), 460 (MNa+)
Preparation 16
(2R)-2-[[(2R)-2-(6---Aminopyridin-3-y1)-2-hydroxyethyl](propyl)amino]-3-
(benzyloxy)propan-1-ol
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
OH
OH~......,__0
~ N ~o . . .
I , -
H2N N
The diol from preparation 15 (2.95 g, 6.7 mmol) was dissoived in ethanol (50
mL) treated with
hydroxylamine hydrochloride (2.34 g, 33.7mmol) and the mixture heated to 80 C
overnight (-16 h). After
cooling to room temperature the solvents were evaporated and the residue
purified by flash
5 chromatography on silica gel eluting with dichloromethane/ methanol/ 880 NH3
(95:5:0.5 increasing
polarity to 91:9:0.5) to afford the title compound as a light brown oil (1.4
g, 58%)
'H NMR: SH (400 MHz, CD3OD) 7.82 (IH, d), 7.46 (1H, dd), 7.38-7.22 (5H, m),
6.57 (1H, d), 4.57-4.44
(3H, m), 3.63-3.46 (4H, m), 3.07 (1 H, m), 2.77 (2H, d), 2.71-2.53 (2H, m),
1.46 (2H, m), 0.97 (3H, t)
MS (APCI+) 360 (MH+), 382 (MNa})
Preparation 17
5-Bromo-2-(2,5-d imethyl-1 H-pyrrol-l-yl)-4-methylpyridine
Br
N
2-Amino-5-bromo-4-methylpyridine [commercially available] (6 g, 32 mmol) was
dissolved in toluene (100
mL), hexane-2,5-dione (5.3 mL, 45 mmol) and para-toluene sulfonic acid
monohydrate (50 mg) were
added and the mixture heated at reflux with a Dean-Stark apparatus fitted.
When collection of water
ceased the reaction mixture was cooled and diluted with water (50 mL) and 10%
aqueous K2C03 solution
(50 mL), the organic layer was separated and the aqueous layer extracted with
ethyl acetate (200 mL).
The combined organic fractions were dried over magnesium sulphate, filtered
and evaporated. The
residue was purified by flash chromatography on silica gel eluting with 5%
ethyl acetate in pentane to
afford the title compound as a pale yellow oil (8 g, 95%)
'H NMR: SH (400 MHz, CDCI3) 8.62 (IH, s), 7.11 (IH, s), 5.90 (2H, s), 2.45
(3H, s), 2.15 (6H, s)
MS (ESI) 267 (MH+)
Preparation 18
4-Propylmorphotin-2-one
oJ
Methyl 2-bromoacetate (50 mL, 0.54 mol, 1 eq) was added slowly to N-
propylaminoethanol (62.4 ml, 0.54
mol, I eq) and Et3N (75 ml, 0.54 mol, leq) in toluene at 0 C and allowed to
stir at room temperature
overnight. Water (1 L) was added, and the mixture extracted with EtOAc (2 x
500 mL). Brine (500 mL)
was added to the aqueous layer, which was re-extracted with EtOAc (2 x 500mL).
Organic layers were
combined, dried (MgSO4), filtered and solvent removed in vacuo to give 62.7g
(81 /o) of title compound as
a clear oil.
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
76
TLC EtOAc Rf =0.5
M/S (APCI+) 144 (MH+)
'H NMR (400Mhz, CD3OD) 5H 0.9 (t, 3H), 1.4-1.6 (m, 2H), 2.3-2.4 (m, 2H), 2.6-
2.7 (m, 2H), 3.3 (s, 2H),
4.4 (m, 2H)
Preparation 19
2-[6-( 2, 5-D i m et h y I-1 H-py rro I-1-y I)-4-m et h y I py ri d i n-3 -y
I] -4-p ro py I m o r p h o I i n-2-o I
N
OJ
/ N N OH
The bromopyridine from preparation 17 (5 g, 18.8 mmol) was dissolved in THF
(80 mL) and cooled to -78
0 C. To the stirred solution was added dropwise tert-butyllithium (22 mL,
37.7 mmol). Immediately after the
addition was complete morpholinone (from preparation 18) (2.7 g, 18.8 mmol)
was added as a solution in
THF (20 mL) and the reaction mixture left stirring at -78 C for 1 h. The
reaction was then quenched the
addition on saturated aqueous NH4CI solution (100 mL), then extracted with
ethyl acetate (100 mL). The
organic fraction was dried over magnesium sulphate, filtered and evaporated.
The residue was purified by
5 flash chromatography on silica gel eluting with 35% ethyl acetate in pentane
increasing polarity of eluent to
40% ethyl acetate in pentane to afford the title compound as a pale yellow oil
(1.95 g, 32%)
'H NMR: SH (400 MHz, CDCI3) 8.78 (1H, s), 7.00 (1H, s), 5.86 (2H, s), 5.21
(1H, brs), 4.23 (1H, m), 3.85
(1 H, m), 3.03 (1 H, m), 2.82 (1 H, m), 2.62 (3H, s), 2.56-2.37 (4H, m), 2.08
(6H, s), 1.58 (2H, m), 0.97 (3H,
t)
:0 MS (ESI) 330 (MH+)
Preparation 20
1-[6-(2,5-Dimethyl-1 H-pyrrol-l-yi)-4-methylpyridin-3-yl]-2-[(2-
hydroxyethyl)(propyl)amino]ethanoi
N
OH OH
/ N N
25 The morpholinol from preparation 19 (1.95 g, 5.9 mmol) was dissolved in
ethanol (25 mL) and water (10
mL) and sodium borohydride (900 mg, 23.6 mmol) was added to the stirred
mixture at room temperature.
Stirring was maintained overnight (-16 h) before the reaction was quenched by
the addition of saturated
aqueous ammonium chloride (100 mL) and extracted with dichloromethane (2 x 100
mL), The combined
organic fractions were dried over magnesium sulfate, filtered and evaporated.
The residue was purified by
30 flash chromatography on silica gel eluting with dichloromethane/ methanol/
880 NH3 (96:4:0.5) to afford
the title compound as a yellow oil (1.4 g, 71 %)
'H NMR: SH (400 MHz, CD3OD) 8.59 (1H, s), 7.16 (1H, s), 5.80 (2H, s), 5.07
(1H, m), 3.67-3.58 (2H, m),
2.82-2.54 (6H, m), 2.47 (3H, s), 2.02 (6H, s), 1.50 (2H, m), 0.90 (3H, t)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
77
MS (ESI+) 332 (MH+)
Preparation 21
1-(6-Am ino-4-methylpyridin-3-yl)-2-[(2-hydroxyethyl)(propyl)amino]ethanol
N
fLfcOH
HaN N
The diol from preparation 20 (1.4 g, 4.22 mmol) was dissolved in ethanol (30
mL) and treated with
hydroxylamine hydrochloride (1.12 g, 16.9 mmol), and the mixture heated to
reflux over night (-16 h).
After cooling to room temperature the mixture was diluted with 10% aqueous
K2C03 solution and
extracted with dichloromethane (2 x 200 mL). The combined organic fractions
were dried over magnesium
sulfate, filtered and evaporated. The residue was purified by flash
chromatography on silica gel eluting
with d ich lorom ethane/ methanol/ 880 NH3 (93:7:1) to afford the title
compound as a pale brown oil (950
mg, 89%)
' H NMR: SH (400 MHz, CD3OD) 7.90 (1 H, s), 6.39 (1 H, s), 4.81 (1 H, m), 3.66-
3.57 (2H, m), 2.80-2.72 (1 H,
m), 2.67-2.48 (5H, m), 2.24 (3H, s), 1.58-1.46 (2H, m), 0.91(3H, t)
MS (ESI+) 254 (MH+)
Preparation 22
(2S)-2-({(2R)-2-[6-(2,5-Dimethyl-1 H-pyrrol-1-yl)pyridin-3-yi]-2-
hydroxyethyl}am ino)butan-l-ol
OH
OH~,......_"
fJ)NH
N
The epoxide from preparation 3 (10.6 g, 49.4 mmol) was dissolved in DMSO (100
mL) together with (S)-2-
aminobutan-l-ol (5.6 g, 59.4 mmol) [commercially available] and the mixture
heated at 90 C overnight
(ca. 16 h). After cooling to room temperature, the mixture was evaporated
under high vacuum to afford a
dark oil of the title compound (17.7 g) containing residual DMSO but of
sufficient purity to use in the
subsequent stage.
' H NMR: 'bH (400 "MHz, CDCI3) 8.56 (1 H, d), 7.82 (1 H, dd), 7.18 (1 H, d),
5.83 (2H, s), 4.77 (1 H, m), 3.63
(1 H, m), 3.39 (1 H, m), 3.04 (1 H, m), 2.96-2.78 (2H, brs), 2.70 (1 H, m),
2.58 (1 H, m), 2.05 (6H, s), 1.54-
1.38 (2H, m), 0.92(3H, t)
MS (ESI+) 304 (MH+)
Preparation 23
(2S)-2-[{(2R)-2-[6-(2,5-Dimethyl-1 H-pyrrol-l-yl)pyridin-3-yl]-2-
hydroxyethyl}(ethyl)amino]butan-l-ol
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
78
OH
OH~...,...\
"~
N N
The diol from preparation 22 was dissolved in dichloromethane (50 mL) and
treated with ethanal (1.66mL,
29.6 mmol) and NaBH(OAc)3 (6.3 g, 29.6 mmol) and the mixture stirred at room
temperature over night
(-16 h). The solvents were then evaporated and the residue purified by flash
chromatography on silica gel
eluting with dichloromethane/ methanol/ 880 NH3 (97:3:0.5) to afford the title
compound as a light brown
oil (2.8 g) The material was re-purified by flash chromatography on silica gel
eluting with methanol in ethyl
acetate 1% increasing polarity to 2% to afford the title compound as a clear
oil (1.42 g, 43%)
'H NMR: 8H (400 MHz, CDC13) 8.58 (1H, d), 7.95 (1H, dd), 7.21 (1H, d), 5.87
(2H, s), 4.98 (3H, brm), 3.72
(1 H, dd), 3.57 (1 H, m), 3.10 (1 H, dd), 2.95 (2H, m), 2.78 (2H, m), 2.10
(6H, s), 1.57 (1 H, m), 1.43 (1 H, m),
1.18 (3H, t), 0.97 (3H, t)
MS (ESI+) 332 (MH+)
Preparation 24
(2S)-2-[[(2R)-2-(6-Aminopyridin-3-yl)-2-hydroxyethyl](ethyl)amino]butan-1-ol
OH
OH~..,,.~".'~
H2N N
The diol from preparation 23 (1.42 g, 4.3 mmol) was dissolved in ethanol (50
mL) and treated with
hydroxylamine hydrochloride (1.5 g, 21.4 mmol) and the mixture heated to 80 C
overnight (-16 h). After
cooling to room temperature, the solvents were evaporated and the residue
purified by flash
chromatography on silica gel eluting with dichloromethane/ methanol/ 880 NH3
(91:9:0.5) to afford the title
compound as a light brown oil (990 mg, 91 %).
' H NMR: 5,., (400 MHz, CD3OD) 7.88 (1 H, d), 7.48 (IH, dd), 6.58 (IH, d),
4.50 (1 H, m), 3.42 (2H, m), 2.80
(1 H, m), 2.68 (2H, m), 1.83 (2H, m), 1.48 (1 H, m), 1.38 (m, 1 H), 1.04 (3H,
t), 0.92 (3H, t)
MS (ESI+) 254 (MH+), 276 (MNa+)
Preparation 25
5-B rom o-2-(2,5-d imethyl-1 H-pyrrol-l-yI)-3-methylpyrid ine
flBr
N N
2-Amino-3-methyl-5-bromopyridine (5.86 g, 31.3 mmol) was dissolved in toluene
(50 mL), hexane-2,5-
dione (5.15 mL, 43.9 mmol) and para-toluene sulfonic acid monohydrate (20 mg)
were added and the
mixture heated at reflux with a Dean-Stark apparatus fitted. When collection
of water ceased, the reaction
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
79
mixture was evaporated and the residue was purified by flash chromatography on
silica gel eluting with
5% ethyl acetate in pentane to afford the title compound as a pale yellow oil
(5.1 g, 61 %)
'H NMR: 5H (400 MHz, CDC13) 8.51 (1 H, d), 7.81 (1 H, d), 5.90 (2H, s), 2.01
(3H, s), 1.97 (6H, s) '
tlc Rf=0.5 (5% EtOAc:Pentane)
Preparation 26
(5S)-5-methyl-4-propylmorpholin-2-one
O
N
~
The material from preparation 36 (4g, 26mmol) was dissolved in benzene (80
mL), followed by the
addition of N-ethyldiisopropylamine (9.07 mL, 52 mmol) and methyl bromoacetate
(2.4 mL, 26 mmol). The
mixture was heated to reflux with azeotropic removal of water overnight. After
cooling to room
temperature, the solvent was removed by evaporation, the crude material
dissolved in methanol, pre-
absorbed onto Si02 and purified by flash chromatograpliy on Si02 eluting with
40% EtOAc in pentane to
afford the title compound as a clear oil (1.78 g).
'H NMR (CDC13i 400MHz) SH 0.9 (t, 3H), 1.1 (d, 3H), 1.5 (m, 2H), 2.25 (m, 1H),
2.6 (m, 1H), 2.8 (m, 1H),
3.2 (d, 1 H), 3.6 (d, 1 H), 4.05 (dd, 1 H), 4.3 (dd, 1 H)
TLC. Rf=0.18 (50% EtOAc in pentane, UV visualisation)
Preparation 27
(5S)-2-[6-(2,5-Dimethyl-1 H-pyrrol-1-yl)-5-methylpyridin-3-yl]-5-methyl-4-
propylmorpholin-2-ol
N N OH
~ O
N~'
~
Bromopyridine from preparation 25 (2.5 g, 9.4 mmol) was dissoived in THF (60
mL) and cooled to -78 C.
To the stirred sQlution was added dropwise tert-butyllithium (1.5 M in
pentane, 12.6 mL, 18.8 mmol).
Immediately after the addition was complete morpholinone (from preparation 26)
(1.5 g, 9.4 mmol) was
added as a solution in THF (10 mL) and the reaction mixture left stirring at -
78 C for I h. The reaction
was then quenched by the addition of saturated aqueous NH4CI solution (100 mL)
then extracted with
ethyl acetate (80 mL). The organic fraction was dried over magnesium sulphate,
filtered and evaporated.
The residue was purified by flash chromatography on silica gel eluting with
10% ethyl acetate in pentane
increasing polarity of eluent to 40% then 70% ethyl acetate in pentane to
afford the title compound as a
pale yellow oil (590 mg, 18%)
'H NMR: 5H (400 MHz, CDC13) 8.70 (1H, s), 7.92 (IH, s), 5.88 (2H, s), 3.77
(2H, brs), 3.0-2.37 (5H, m),
2.02 (3H, s), 1.90 (6H, s), 1.65-1.58 (2H, m), 1.10 (m, 3H), 0.99-0.84 (3H, m)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
MS (ESI+) 344 (MH+)
Preparation 28
(2S)-2-[{2-[6-(2,5-Dimethyl-1 H-pyrrol-1-yl)-5-methylpyrid in-3-yi]-2-
5 hydroxyethyl}(propyl)amino]propan-1-ol
N N
I i OH OH
N
~
The morpholinol from preparation 27 (600 mg, 1.7 mmol) was dissolved in
ethanol (6 mL) and water (3
mL) and sodium borohydride (270 mg, 7 mmol) was added to the stirred mixture
at room temperature.
Stirring was maintained overnight (-16 h) before the reaction was quenched by
the addition of saturated
0 aqueous ammonium chloride (100 mL) the basified to pH -9 with 2M NaOH
solution and extracted with
dichloromethane (2 x 200 mL). The combined organic fractions were dried over
magnesium sulfate,
filtered and evaporated to afford the title compound as a mixture of
diastereoisomers as a yellow oil (450
mg, 75%) which was used directly without further purification
MS (ESI+) 346 (MH+), 368 (MNa+)
5
Preparation 29
(2S)-2-[[2-(6-Amino-5-methylpyridin-3-yl)-2-hydroxyethyl](propyl)amino]propan-
1-ol
HZN N~
~ e OH OH
N ~--õ
~
The diol from preparation 28 (420 mg, 1.5 mmol) was dissolved in propanol (5
mL) and water (1.5 mL)
?0 treated with hydroxylamine hydrochloride (2.2 g, 31.4 mmol) and
triethylamine (2.2 mL, 15.7 mmol), and
the mixture heated to reflux for 12 h. After cooling to room temperature the
mixture was evaporated, and
the residue was purified by flash chromatography on silica gel eluting with
dichloromethane/ methanol/
880 NH3 (90:10:1) increasing polarity to (85:15:3) to afford a white solid
(1.3 g) which was triturated with
dichlorornethane- (3 x. 50 mL), the residual solvent was removed in vacuo to
give 700mg of white solid
25 which was further purified by flash chromatography on siiica gel eluting
with dichloromethane/methanoV
880 NH3 (92.5:7.5:0.) to afford the title compound as a clear oil (200 mg,
50%)
MS (ESI+) 254 (MH+)
Preparation 30
30 (2S)-2-[{(2R)-2-[6-(2,5-Dimethyl-lH-pyrrol-1-yi)pyridin-3-yi]-2-
hydroxyethyl}(butyi)amino]propan-l-
ol
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
81
OH
OH~_1111111
N
N
The diol from preparation 8(1g, 3.35mmol, leq) was dissolved in
dichloromethane (20 mL) and treated
with butanal (910 l, 10 mmol 3eq) and NaBH(OAc)3 (2.1 g, 10 mmol 3eq) and the
mixture stirred at room
temperature over night (-16 h). The reaction was quenched with water (50m1)
and extracted with CH2CI2
(2xlOOml), the organic layers combined, dried (MgSO4), filtered and
evaporated. The residue was purified
by flash chromatography on silica gel eluting with d ichlorom ethane/ methanoV
880 NH3 (95:5:0.5) to afford
the title compound as a clear oil (900 mg 86%)
' H NMR: 8H (400 MHz, CDCI3) 8.55 (1 H, d), 7.85 (1 H, dd), 7.20 (1 H, d),
6.88 (2H, s), 4.85 (1 H, m), 3.10-
2.97(1 H, m), 2.87-2.94 (1 H, m), 2.43-2.70 (3H, m), 2.10 (6H, s), 1.22-1.60
(6H, m), 0.87-1.0 (6H, m)
MS (ESI+) 346 (MH+) 368 (MNa+)
Preparation 31
(2S)-2-[[(2R)-2-(6-Aminopyridin-3-yl)-2-hydroxyethyl](butyl)amino]propan-1-ol
OH
OH õ~
( ~
HZN ~
The diol from preparation 30 (900 mg, 2.6 mmol) was dissolved in ethanol (15
mL) and treated with
hydroxylamine hydrochloride (905 mg, 13 mmol) and the mixture heated to 80
overnight (-16 h). After
cooling to room temperature, the solvents were evaporated and the residue pre-
absorbed onto silica gel
and purified by flash chromatography on silica gel eluting with a gradient of
dichloromethane/ methanol/
880 NH3 (95:5:0.5 to 92:8:0.5) to afford the title compound as a light brown
oil (330 mg)
'H NMR: 8H (400 MHz, CD3OD) 7.83 (1H, d), 7.50 (1H, dd), 6.59 (IH, d), 4.50
(IH, m), 3.25-3.40 (2H, m),
2.86-2.98 (1 H, m), 2.42-2.78 (4H, m), 1.20-1.42 (4H, m), 0.87-1.0 (6H, m)
MS (ESI) 268 (MH+), 290 (MNa+)
Preparation 32
3-(6-Chloropyridin-3-yl)-azetidine-l-carboxylic acid tert-butyl ester
O
N~01
N ~
CII
Zinc dust (127mg, 1.94mmol, 1.1eq) was dried for 18h at 100 C in vacuo,
transferred to a round bottomed
flask and heated with a hot air gun under vacuum. The flask was allowed to
cool to room temperature and
DMF (2ml) and 1,2-dibromoethane (12 l, 0.141mmol, 0.08eq) added. The mixture
was heated to 70 C for
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
82
mins, allowed to cool to r.t., and TMSCI (18 1, 0.141mmol, 0.08eq) added
dropwise. This mixture was
stirred at r.t. for 30min before dropwise addition of 3-iodoazetidine-l-
carboxylic acid tert-butyl ester (Ref
SynLett, 1998, 4, 379)(500mg, 1.766mmol, 1.Oeq) as a solution in DMF (2ml).
The mixture wes stirred at
40 C for 1 h. 2-chloro-5-iodopyridine was dissolved in DMF (2ml) and added,
followed by Pd2dba3 (32mg,
5 0.035mmol, 0.02eq) and tri-2-furylphosphine (17mg, 0.071mmol, 0.04eq) and
the mixture heated to 70 C
for 4h.
The mixture was allowed to cool, diluted with Et20(40ml) and NH4CI (40m1,
sat'd aq), layers separated, the
aqueous layer was re-extracted with Et20(20m1), organics combined, washed with
brine (2x30m1), dried
(MgSO4), filtered and evaporated to give a yellow solid.
10 This solid was flash chromatographed on silica gel with a gradient elution
from 100% CH2CI2 to 99:1
CH2CI2:MeOH to give 235mg of impure product. This material was further
purified by flsh chromatography
on silica get with a gradient elution from 100% toluene to 95:5 toluene:EtOAc
to give the title compound as
a yellow solid (193mg, 41%)
TIc Rf = 0.13 (10%EtoAc/Toluene UV visualisation)
MS (APCI+) 269 (MH+)
' H NMR: SE., (400 MHz, CDCI3) 8.3 (1 H, s), 7.7 (1 H, m), 7.35 (1 H, d), 4.35
(2H, t), 3.9 (2H, t), 3.65-3.8 (1 H,
m), 1.45 (9H, s)
Preparation 33
5-Azetidin-3-yl-2-chloropyridine dihydrochloride
H
N
N
I
CI .2HCI
3-(6-Chloropyridin-3-yl)-azetidine-l-carboxylic acid tert-butyl ester (190mg,
0.707mmol, 1.Oeq) was
dissolved in CH2CI2 (4ml), cooled to 0 C and HCI(g) bubbled through for 10min
to give a dark orange
solution. This was stirred at r.t. for 72h. The mixture was evaporated to give
a light brown solid, triturated
with Et20, the resulting solid filtered and dried at 60 C in vacuo to give the
title compound (141mg, 83%).
Tic Rf=0.15 (CH2CI2:MeOH:NH40H 90:10:1)
MS (APCI+) 169 (MH+)
MS (ESI+) 169 (MH+)
'H NMR.: SH (400 MHz, CD30D) 8.4 (1H, s), 7.95-8.05 (IH, m), 7.55 (1H, d), 4.2-
4.5 (5H, m)
Preparation 34
2-Chloro-5-(1-propylazetidin-3-yl)-pyridine
N~~ =
N
CI
5-Azetidin-3-yl-2-chloropyridine dihydrochloride (131mg, 0.542mmol 1.Oeq) was
partitioned between
CH2CI2 (10m1) and K2C03 (10mI, 10% w/v aq), the layers separated, and the
aqueous layer re-extracted
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
83
with CHaCi2 (10mi). The organic layers were combined, dried (MgSO4), filtered
and evaporated to ca 2ml
volume. Propionaldehyde (79 1, 1.084mmol, 2.Oeq) and sodium
triacetoxyborohydride (230mg,
1.084mmol, 2.Oeq) were added and the mixture stirred at r.t. for 2.5h. The
mixture was quenched with
H20(0.5m1) and partitioned between CH2CI2 (10m1) and K2CO3 (10mI, 10% w/v aq)
the layers separated,
and the aqueous, layer re-extraced with CH2CI2 (10m1). The organic layers were
combined, dried (MgSO4),
filtered and evaporated to give a dark brown oil. This oil was purified by
flash chromatography on silica gel
with a gradient elution from 100% CH2CI2 to 95:5 CH2CI2:MeOH to give the title
compound as a yellow oil
(41mg, 36%)
Tic Rf=0.39 (CH2CI2:MeOH:NH4OH 90:10:1 UV visualisation)
MS (APCI+) 211 (MH+)
'H NMR: SH (400 MHz, CDCI3) 8.25 (1 H, s), 7.65-7.75 (1 H, m), 7.25 (1 H, d),
3.6-3.75 (3H, m), 3.1 (t, 2H),
2.4 (t, 2H), 1.3-1.5 (m, 2H), 0.9 (t, 3H)
Preparation 35
Benzhydrylidene-[5-(1-ppropylazetidin-3-yl)-pyridin-2-yl]-amine
N
I ~ N
2-Chloro-5-(1-propylazetidin-3-yl)-pyridine (100mg, 0.475mmol, 1.Oeq), 1,1-
diphenylmethanimine (951A1,
0.570mmol, 1.2eq), palladium (II) acetate (4.4mg, 0.00475mmol, 0.01eq), BINAP
(8.7mg, 0.014mmol,
0.03eq) and sodium tert-butoxide (49mg, 0.665mmol, 1.4eq) were combined in
toluene (2ml) and heated
to 80 C for 16h. Further palladium (II) acetate (4.4mg, 0.00475mmol, 0.01eq)
and BINAP (8.7mg,
0.014mmol, 0.03eq) were added and the mixture heated to reflux for 4h. The
mixture was allowed to cool
to r.t. and partitioned between EtOAc (25m1) and K2C03 (20m1, 10% w/v aq), the
layers separated, and the
aqueous layer re-extracted with EtOAc (2x25mlml). The organic layers were
combined, dried (MgSO4),
filtered and evaporated to give an orange oil. This oil was purified by flash
chromatography on silica gel
eluting with a gradient on 100% CH2CI2 to 95:5:0.5 CH2CI2:MeOH:NH4OH to give
the title compound as a
yellow oil (110mg, 66%)
Tlc Rf=0.22 (90:10:1 CH2CI2:MeOH:NH40H UV visualization)
MS (APCI+) 356 (MH+)
'H NMR: SH (400 MHz, CDCI3) 8.2 (1 H, s), 7.8 (2H, m), 7.0-7.6 (9H, m), 6.55
(1 H, d), 3.65-3.8 (3H, m), 3.1
(2H, t), 2.45 (t, 2H), 1.3-1.5 (m, 2H), 0.95 (t, 3H)
CHN +0.75 H20
Calculated C(78.12) H(7.24) N(11.39)
Observed C(77.85, 78.12) H(7.04, 7.04) N(11.18, 11.28)
Preparation 36
(2S)-2-(propylamino)propan-l-ol hydrochloride
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
84
HO
HN
HCI
To (2S)-(+)-2-aminopropan-1-ol (19.6g, 0.26mo1) dissolved in CH2CI2 (500 mL)
was added
propionaldehyde (20.9 mL, 0.28 mol) followed by pre-dried, powdered 4A
molecular sieves (40g) and the
mixture stirred at room temperature overnight. The mixture was filtered
through a pad of celite (filter
agent), the pad washed with CH2CI2, and solvent evaporated from the filtrate
to give a clear oil. This oil
was dissolved in methanol (200 mL) and NaBH4 was added portionwise over 15
minutes. The mixture was
stirred at r.t. overnight, then quenched by cautious addition of 2M aqueous
HCI (200 mL), basified by
addition of 2M aqueous NaOH (200 mL) and methanol removed by evaporation. Di-
tert-butyldicarbonate
(115 g, 0.52 mol) was added to the residue followed by 1,4-dioxan (200 mL) and
the mixture stirred at r.t.
0 overnight. 1,4-dioxan was removed by evaporation and the residue diluted
with water (750 mL) and
extracted with CH2CI2 (2 x 750 mL). The combined organic fractions were dried
(MgSO4), filtered and
evaporated giving a clear oil. To this oil was added 4M HCI in 1,4-dioxan (200
mL) and the mixture stirred
at r.t. overnight. The solvent was removed by evaporation to give the title
compound as a white solid (24
g).
'H NMR (DMSO, 400MHz) 5: 0.95 (3H, t), 1.2 (3H, d), 1.6 (2H, m), 2.8 (2H, m),
3.15 (1H, m), 3.5 (IH,
brm), 3.6 (1H, m), 5.4 (1H, b), 8.6-8.9 (2H, brd)
M/S (APCI+), 118 (MH+)
Preparation 37
?0 (2S)-2-({2-[6-(2,5-Dimethyl-lH-pyrrol-1-yl)pyridin-3-yl]-2-hydroxyethyl}
amino) propan-l-ol
OH
OH õ%
NH
N N
n
The epoxide from preparation 40 (950 mg, 4.4 mmol) was dissolved in DMSO (10
mL) together with (S)-2-
aminopropan-l-ol (380 L, 4.9 mmol) and the mixture heated at 90 C overnight
(ca. 16 h). After cooling
to room temperature,.the mixture was evaporated under high vacuum and the
residue purified by flash
chromatography on silica gel eluting with dichloromethane/ methanol/ 0.880 NH3
(98:2:0 increasing
polarity to 90:10:1) to provide the title compound as a pale yellow oil (780
mg, 67% over 2 steps from
preparation 40).
'H NMR: SH (400 MHz, CDCI3) 8.61 (1H, d), 7.86 (1H, dd), 7.21 (1H, d), 5.90
(2H, s), 4.90.(1H, m), 3.68
(1 H,m), 3.46 (1 H, m), 3.26-2.72 (4H, m), 2.11 (6H, s), 1.14 (3H, 2 x d)
MS (APCI+) 290 (MH+)
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
Preparation 38
(2S)-2-[{2-[6-(2,5-Dimethyl-1 H-pyrrol-l-yl)pyridin-3-yl]-2-
hydroxyethyl}(ethyl)ami:no]propan-l-oi
(diastereomer mix)
OH
OH
I ~ N1
N
5 The diol from preparation 37 (1.5 g, 5.2 mmol) was dissolved in
dichloromethane (25 mL) and treated with
acetaldehyde (870 L, 15.5 mmol) and sodium triacetoxyborohydride (3.3 g, 15.5
mmol) and the reaction
mixture left stirring at room temperature overnight (-16 h). The reaction
mixture was diluted with saturated
ammonium chloride solution then basified by the addition of 10% aqueous K2C03
solution, and then
extracted with dichloromethane (2 x 150 mL). The combined organics were dried
(MgSO4), filtered and
10 evaporated. The residue was purified by flash chromatography on silica gel
eluting with
dichloromethane/methanol. 0.880 NH3 (93:7:0.5) to afford the title compound as
a mixture of
diastereoisomers as a slighlty impure light brown oil (2.5 g). This material
was used directly without further
purification.
'H NMR: 5H (400 MHz, CDC13) 8.56 (1H, m), 7.87 (1H, m), 7.21 (1H, d), 5.89
(2H, s), 4.82 (1H, m), 3.77-
15 3.65 (1 H, m), 3.18-3.05 (1 H, m), 2.95-2.57 (5H, m), 2.10 (6H, s), 1.18-
1.10 (2 x 3H, t), 1.00-0.92 (2 x 3H
d)
MS (ESI+) 318 (MH+)
Preparation 39
20 (2S)-2-[[2-(6-Aminopyridin-3-yl)-2-hydroxyethyl](ethyl)amino]propan-l-oi
(diastereomer mix)
OH
OH .'
N
H2N N
The diol from preparation 38 (2.5 g, 7.8 mmol) was dissolved in ethanol (80
mL) and treated with
hydroxylamine hydrochloride (2.7 g, 21.4 mmol) and the mixture heated to 80 C
overnight (--16 h). After
cooling to room temperature, the solvents were evaporated and the residue
purified by flash
25 chromatography ori silica gel eluting with d ich lorom ethane/ methanol/
880 NH3 (95:5:0.5 increasing
polarity to 90:10:1) to afford the title compound as a light brown oil (1.5 g,
80%)
'H NMR: SH (400 MHz, CDCI3) 7.86 (1H, m), 7.50 (1H, m), 6.58 (IH, m), 4.62-
4.49 (1H, m), 3.66-3.29 (2H,
m), 3.06 -2.41 (7H, m), 1.13-0.86 (6H, m)
MS (ESI+) 240 (MH+)
Preparation 40
2-(2,5-Dimethyl-pyrrol-l-yi)-5-[oxiranyl]pyridine
CA 02567935 2009-04-07
69387-598
86
O
N N
Ethanolamine (0.24mL, 4 mmol) was added dropwise to a sotution of
borane.tetrahydrofuran complex (1M
soiution in tetrahydrofuran, 8 rnL, 8 mmol) in tetrahydrofuran (5 mL) cooled
to 0 C over 15 minutes. The
mixture was allowed reach room temperature then the chloride from preparation
2 (1 g, 4 mmol) in
tetrahydrofuran was added dropwise to the stirred solution. The reaction
mixture was then stirred at room
temperature for 30 minutes then quenched by the addition of 2M sodium
hydroxide (10 mL) and the
reaction mixture stirred for a further 1 hour. The mixture was then extracted
with ethyl acetate (2 x 50 mL),
dried (MgSO4), filtered and evaporated to afford the racemic epoxide as a
yellow oil (950 mg). 'H nmr was
as for preparation 3. Thd"materiat was canied forward directly without further
purification.
Preparation 41
4-propyt-2-tFiiazol-5-yl-m orpholin-2-ol
O,-)
N
f OH 1
N
To 2-trimethytsilyl thiazole (9.5g, 60.5mmol, leq) in EtzO (200mt) at -78 C
was added dropwise n-butyl
lithium (36mi, 2.5M In hexanes, 90.7mmoi, 1.5eq) and the mixture stirred at -
78 C for 30min. 4-
propylmorpholin-2-one (prepared according to the method described in WO
2004/052372 - 8.65g,
60.5mmol, leq) in Et2O (50m1) added over 5 min (temperature increases to -55 C
during addition). The
mixture was re-cooled to 78 C and allowed to stir at !78 C for 2h. The
reaction was quenched by
dropwise addition of water, allowed to warm to r.t. and extracted with EtOAc
(2x500mf) and
dichioromekhahe (2x300m1). The organic extracts were combined, dried over
MgSO4 and solvent
evaporated to give a brown oil. The ol was chromatographed through a Si02
column on an fscoK
Companion Comtiitiash autochromatography system with a gradient elution from
9812/0.5
CHzCIa/MeOHMH,to 95/5/0.5 CHZCt~IAAeOHlNHyto give a pale brown solid (8.5g,
61%).
'H NMR (404MHz, CD3OD) b(ppm) : 8_91 (s, IH), 7.91(s,1H), 4.13-4.22 (m. IH),
3.68-3.75 (m, 1 H), 2.97
(d. 1H), 2.68-2.80 (m, 1H), 2.26-2.40 (m, 4H), 1.48-1.60 (m, 21-1), 0.93 (m,
3H)
M!S APCI+ 229 (MH+)
Tic 9515/0.5 CH2CI2INIeOH1NH3 Rf=0.35
Tlc EtOAc Rf=0.3
Preparation 42
2-[(2-hydroxyethyl)(propyl)am ino]-1-(1,3-th iazol-5-yi)ethanol
CA 02567935 2009-04-07
69387-598
87
OH
OH ~
$ N
N
To 4-propyi-2-thiazol-5-yl-morpholin-2-ol (8.5g, 37.3mmol) in EtOH (125m1) and
water (125mt) was added
NaBH4 and the mixture stirred at r.t. for 1h. The mixture was diluted with
water (200m1) and extracted with
dichloromethane (3x250mi). Organic layers combined, dried over idJgSO4 and
sotvent evaporated to give a
paie oil (6.2g).
'H NMR' (400MHz, CD3OD) b(ppm) : 8.92 (s, 1 H), 7.80 (s. I H), 5.05 (t, 1 H).
3.56-3.65- (m, 2H), 2.62-2.80
(m, 4H), 2.51-2.59 (m, 2H), 1.43-1.53 (m, 2H), 0.87 (t, 3H)
M/S APCI+ 231 (MH+). %-
tl.c. 90/10/1 CH.-C12/WOH/NFI3 Rf 0.45
Preparation 43
4-propyl-2-(1,3-thiazoi-5 yl)morphotine
S N
a N
2-1(2-hydroxyethyl){propy{)amino}-1-(1,3-thiazol-5-y1)ethanol (5.7g 24.8mmol)
in dichforomethane (20m1)
was treated with concentrated sulphuric acid (50mo . On complete addition
dichloromethane removed in
vacuo and resulting mixture heated to 140 C for 1h. The mixture was allowed to
cool to r.t, poured into
ice and cautiously quenched by addition of 0.880 NH3 with Ice cooling
maintaining T<20 C. The mixture
was extracted with dichloromethane (2x250m1) dried over MgSO4 and solvent
evaporated to give a brown
oil. This material was chromatographed on an tscomx Companion Combiflash
autochromatography system
with an eiuant of 9812l0.5 CH;CI21MeOH1NH3 to give the product as a pale brown
oil_
'H NMR (400MHz, CD30D) b(ppm) : 8.94 (s, 11i), 7.84 (s, IH), 4.93 (dd, IH),
3.93-4.0 (m, 1H), 3.76-3.84
(m, 1H), 3.07 (d,1H), 2.81 (d,1H), 2.35-2.41 (m, 2H), 2.17-2.30 (m.2H),1.50-
1.62 (m, 2H), 0.94 (t, 3H)
MIS APCI+ 213 (MH+)
Tlc 951510:5 CH2CI~lMlMeOH/NH~ Rf-0.55
Preparation 44
2-(2-bromo-1,3-thiazol-5-yl)-4-propylmorphoiine
o")
Br--~~
S N~
N
To 4-propyl-241,3-ihiazoi-5-yl)morpholine (2.9g 13.7mmol) in THF (50m1) at ?8
C was added n-butyl
iithium (5.5m1, 2.5M in hexanes, 13.7mmol, 1eq) and allowed to stir at -78 C
for 30min. A solvtion of
carbon tetrabromide (4.5g, 13.7mmol) in THF (20m1j was added maintaining T<-70
C tiur3ng the addi6on,
3nd the rmi.xiuie allflwed to stir at -70 C for 1h. The reaction was quenched
by cautious addition of water
CA 02567935 2006-11-23
WO 2005/115985 PCT/IB2005/001554
88
and allowed to warm to r.t. over 18h. The mixture was extracted with EtOAc
(3x150ml), dried over MgSO4
and solvent evaporated to give a brown oil. This material was chromatographed
on an Isco Companion
Combiflash autochromatography system with a gradient elution from 99/1/0.1
CH2CI2/MeOH/NH3 to
95/5/0.5 CH2CI2/MeOH/NH3to give the product as a brown oil (2.5g, 63%).
5'H NMR (400MHz, CD3OD) b(ppm) : 7.54 (s, 1 H), 4.83-4.89 (m, 1 H), 3.90-3.96
(m, 1 H), 3.70-3.79 (m,
1H), 3.02 (d, 1 H), 2.75 (d, 1 H), 2.35-2.41 (m, 2H), 2.17-2.30 (m, 2H), 1.50-
1.62 (m, 2H), 0.94 (t, 3H)
M/S APCI+ 293 (MH+)
Tlc 95/5/0.5 CHaCI2/MeOH/NH3 Rf=0.75