Note: Descriptions are shown in the official language in which they were submitted.
CA 02568646 2006-11-22
Liquid-gas-phase exposure reactor for cell culturing
Description
The invention relates to a method and a device for initiation of cell growth
and for cultivation of
cells in high densities, wherein the cells to be cultivated are located in
hollow-filament mem-
branes and are brought alternately into a liquid nutrient medium and a gas
phase present there-
above.
Prior art
Mammalian cell cultivation for the synthesis of biopharmaceutical drugs is
operated mainly in
stirred reactors. Heretofore airlift reactors have been used less frequently
and hollow-fiber reac-
tors very rarely for servicing the market with drugs based on mammalian cells.
To improve the
volumetric product yields in stirred reactors, the cell density and the
effective production time of
the cells are increased by optimizing the methods and using nutritional
regimens specific to the
cell lines in fed batch methods. The production technology is laid out in
bioreactor trains contain-
ing three to four stirred reactors, each with a volumetric capacity of
approximately five times that
of the preceding bioreactor. The largest available stirred reactor for
cultivation of mammalian
cells currently has a volumetric capacity of 20,000 liters. Fed-batch
processes in stirred reactors
are robust, can be scaled up to the cited volumes and long ago were accepted
by the authorities for
drug synthesis. Disadvantages are the long dwell times of the products in the
culture chamber, the
need for separation of cells from the harvest supernatant, the cleaning and
sterilization expenses
incurred during multiple use and the high investment and operating expenses
for plants equipped
with this technology.
For proteins such as factor VII, which are susceptible to degradation and thus
impose a short
dwell time in the bioreactor during synthesis, there have been developed
devices and systems that
permit perfusion of the culture chamber and thus continuous operation of the
stirred reactors. For
this purpose, efficient cell retention with continuous media feed and product
harvesting is neces-
sary. Spin filters are used here in the interior chamber of the stirred
reactor, while support materi-
als in the form of fluidized or stationary beds are used in the traps, where
the production cells can
CA 02568646 2006-11-22
adhere to surfaces. The continuous mode of operation of stirred reactors can
also be achieved via
external cell-retention systems, such as cell sedimentation, continuous cell
centrifugation or ultra-
sonic cell collection. Advantages of the continuous mode of operation are
short product dwell
times in the bioreactor, constant product quality during synthesis, increase
of the volumetric pro-
ductivity and greater flexibility of batch volume as a function of the
cultivation time to be defined.
Disadvantages are contamination of the harvest with residual cells, the
cleaning and sterilization
expenses incurred for multiple use and the high investment and operating costs
for the corre-
sponding plants.
Besides the hollow-fiber bioreactors of ACUSYST X Cell Generation, which have
proved effec-
tive for the synthesis of biopharmaceuticals, other reactor systems are
available in which all com-
ponents coming in contact with the cell culture are designed as disposable
components. Thus they
can be discarded once they have been used to synthesize a batch. Expensive
cleaning and steriliza-
tion procedures are not required. Commercially available systems of this type
are membrane-
based systems such as Cell-Pharm , Cellmax , Technomouse , CELLine , miniPERM
or Opti-
Cell . Membrane methods have several advantages. In perfusion operation they
can achieve very
high cell densities (10'-10g cells/ml) - by virtue of a large membrane surface
per unit volume.
Moreover, the cells are protected by the membranes from shearing forces. In
principle, they are
designed for one-time use, so that cleaning and sterilization after use are
not necessary. In the art
of disposable bioreactors, the wave bioreactor has also proved effective
heretofore in the trial
phase for the synthesis of biopharmaceuticals. In the system, the cells are
cultivated in a bag sys-
tem, which is systematically agitated in order to improve intimate mixing. One
advantage of this
reactor technology is the one-time usability of the culture system.
Disadvantages are the low
achievable densities and the limited scale-up capability.
In all cited methods and devices, uniform nutrient supply and in particular
oxygen supply at high
cell densities is problematic. Neither the attempt to solve this problem via
complex process steps
involving pressurization (1989, US Patent 4804628 A) nor the direct
introduction of oxygen into
the cell culture chamber via a further membrane system 1986, German Patent
2431450 Al and
1995, German Patent 4230194 Al) led to culture systems whose scale could be
increased as de-
sired and in which the cells could be uniformly supplied. In hollow-fiber
bioreactors, in which the
cells are cultivated between the hollow fibers and the nutrients are
transported in the lumen of the
2
CA 02568646 2006-11-22
fibers, scale-up is limited by the length of the hollow fibers. However, the
length of the hollow
fibers is limited by consumption of the oxygen from the hollow fibers. Thereby
scale-up is possi-
ble only by the use of parallel units. In practice, however, this leads to
unprofitable processes. In
other words, the scale-up capability of the hollow-fiber reactors is defeated
by the lack of ade-
quate homogeneous supply of the cells with fresh gas and liquid nutrient
components.
In International Patent Application WO 03/064586 A2, it was proposed that
cells be cultivated in
high density in compartments, the dimension of which compartments is not to
exceed 5 mm in
length. The interior chamber of the compartments forms a culture chamber,
which is partitioned
from the supply chamber by a semipermeable element. The cells are retained in
the compartments,
and oxygen exchange takes place via hollow-fiber membranes. Supply of the
cells with nutrients
and with oxygen is ensured by means of a variably adjustable mixture of gas
and cell-culture me-
dia. Although the culture device and the method solve the problem of nutrient
and oxygen supply
and guarantee scale-up capability, the method described in WO 03/064586 A2
suffers from a dis-
advantage in that cells of high density must be introduced into the
compartments. To overcome
this disadvantage, it is proposed in International Patent Application WO
03/102123 A2 that bio-
degradable gels be used to reduce the inoculation density at the beginning of
cell cultivation.
A liquid-gas-phase exposure bioreactor has been developed in principle by the
Zellwerk Co. and
is being sold by the Sartorius Co. In this bioreactor, the cells that adhere
to surfaces are immobi-
lized on disks of carrier material. The disks are disposed in series on a
shaft, and are rotated in a
cylinder that is half-filled with medium and half-filled with gas. An
advantage of this arrangement
is the cyclic exposure of these cells to both phases. Disadvantages are the
limitation of the system
and method to adhering cells, the presence of detached cells in the harvest
fluid and the limitation
of scale-up capability.
Object of the invention
The object of the invention is to further improve the method described in WO
03/064586 A2.
Achievement of the object
3
CA 02568646 2006-11-22
The object was achieved as specified in the claims. According to the
invention, the initial growth
and cultivation of cells is undertaken in a liquid-gas-phase exposure
bioreactor containing a sup-
ply chamber in which there are disposed hollow-filament membranes having an
inside diameter of
no larger than 5 mm and whose inner volume forms culture compartments. The
following process
steps take place:
- introduction of the cells into the culture compartments
- filling approximately one half of the supply chamber with nutrient medium
and the other
half with a gas mixture
- turning on perfusion of medium and gas simultaneously or separately
- cyclic exposure of the hollow-filament membranes and of the cells contained
therein in the
gas or liquid phase
According to a preferred alternative embodiment of the inventive method, the
hollow-filament
membranes are oriented horizontally in the bioreactor. After the reactor has
been filled, half of the
membranes are covered with nutrient medium. By rotating the reactor 360 in
one direction and
then in the opposite direction, cyclic exposure of the hollow-filament
membranes and thus of the
cells in the gas or liquid phase is achieved.
Rotation in one direction and then in the opposite direction prevents the
tubing connected to the
reactor from becoming twisted.
According to the invention, the rotation is stopped for a certain time after
180 in order to achieve
equal exposure times in the gas and liquid phases. The holding times can be
variably adjusted.
Thereby it is ensured that the cells are supplied sufficiently with nutrients
during the dwell time of
the membranes in liquid nutrient medium and sufficiently with oxygen during
the dwell time in
the gas phase. By varying the holding times, it is simultaneously possible to
adapt to the individ-
ual metabolic requirements of the individual cell lines.
Alternatively, the cyclic exposure of the hollow-filament membranes can be
achieved by immers-
ing the hollow-filament membranes in the nutrient medium and then lifting them
into the gas
phase. Different dwell times of the cells in the two phases can be achieved by
this procedure.
4
CA 02568646 2006-11-22
To implement the inventive method, cells of low density are first introduced
into the culture
chamber, whereupon they grow to cells of high density. By using gels -as
described in Interna-
tional Patent Application WO 03/102123 A2 -it is possible to introduce, into
the culture chamber,
cells of the lowest cell density together with gels of cross-linked
polypeptides, which have a high
glutamine content, and/or with semisolid media of viscous fluids or fluids
composed of micro-
scopically small gel fragments.
The cells are introduced into the compartments via a central charging system
outside the supply
chamber, so that simultaneous uniform input of the cells into all compartments
is possible via one
port.
The inventive method is suitable for cultivating protozoa, bacteria, yeasts,
fungi and plant or
mammalian cells.
The method is novel compared with the method described in International Patent
Application WO
03/064586 A2, since therein there is provided no exposure of the cells in two
different phases but
merely exposure of the cells in a variably adjustable mixture of gas and cell
culture media. Also,
no movement of the reactor or of the membranes was described therein. Not even
a hint that the
membranes containing the cells can be moved for certain times in the
corresponding phases is
obtained from WO 03/064586 A2. This lies in the fact that, in the cited
publication, there is
needed a device for production of the variably adjustable mixture of gas and
cell culture media,
and so movement of the membranes - especially by rotation - would necessitate
further compli-
cated provisions with respect to the connections.
The inventive device is composed of a cylindrical or spherical two-phase
supply chamber (which
can be charged with gas and medium respectively), in which -parallel to the
longitudinal axis of
the cylinder shell- polymeric, cell-retaining, micro filtering, hollow-
filament membranes having
an inside diameter of no more than 5 mill are fixed in the end plates, the
inner volumes of which
form culture compartments, in which the cells to be cultivated are disposed,
the supply chamber
containing a gas phase through which a gas mixture can flow and a liquid phase
through which a
culture medium can flow, each hollow-filament membrane having a spacing of at
least 5 mm to
the neighboring hollow-filament membrane over the length of the cylinder, the
hollow-filament
5
CA 02568646 2006-11-22
membranes being symmetrically disposed relative to an imaginary cross section
along the axis of
rotation of the cylinder and no membrane being disposed on an imaginary cross-
sectional plane
along the axis of rotation of the cylinder.
s The membranes are permeable for all substances but not for whole cells. The
culture chamber,
composed of the total volume of the compartments, is partitioned from the
supply chamber by the
membrane. This permits the supply substrates to pass into the culture chamber
and supply the
cells. The partition system also permits products to pass out of the cell
compartments into the
supply environment.
The membrane is composed of polymers, such as polysulfone, polyether sulfone
or polycarbonate.
Hollow-filament membranes that are composed of poly ether sulfone, which is a
biocompatible
material, and that have membrane wall thicknesses smaller than 300 m, water
permeabilities of
greater than 6 m3/m2*h*bar and pore diameters of 0.1 to 1.0 m have proved to
be particularly
suitable.
To prevent the formation of liquid films and thus to ensure uniform exposure
of the membranes in
the gas phase, the membranes have a minimum spacing relative to one another.
The membranes are preferably disposed in a hexagonal array. This means that
every membrane -
with the exception of those located at the outer peripheries -is surrounded by
6 membranes with
the same spacing relative to the central membrane. Thus the most uniform
possible packing den-
sity can be ensured in the supply chamber. Further space-saving devices are
not necessary.
The hollow-filament membranes are symmetrically arranged relative to an
imaginary cross sec-
tion along the axis of rotation of the cylinder -which for practical purposes
represents the phase
boundary. No membranes are located on the imaginary cross-sectional plane
along the axis of
rotation of the cylinder. In this way it is ensured that, during the holding
times, all membranes are
either completely in the gas phase or completely in the liquid phase.
The inventive device is novel compared with the device described in WO
03/064586 A2, since
two-phase operation is not provided therein. Furthermore, it does not need any
special device for
6
CA 02568646 2006-11-22
production of a mixture of gas and media or for collection of liquid from the
spent mixture of gas
and media.
For input and removal of gas, every end plate of the cylinder contains at
least two ports which are
respectively disposed above and below the imaginary cross-sectional plane, so
that continuous
supply with gas is ensured even during rotation of the cylinder around its
axis of rotation.
Furthermore, at least one tubing port for media perfusion and at least one
inlet for introduction of
seed cells into the culture chamber are disposed on the head faces.
In addition, the device contains tubings, gas humidifiers, a medium trap in
the gas line, an ultrafil-
tration unit in a product-harvesting line, a hardware unit, pumps, measuring
and control units as
well as a drive motor and a frame, to permit mounting and rotation of the
device.
is The purpose of the ultrafiltration unit in a product-harvesting line is to
concentrate the respective
product.
Surprisingly, it has been found that a higher cell density and thus a higher
yield of cell products
can be achieved with the inventive device than with the device according to WO
03/064586 A2.
This can be attributed on the one hand to the optimal use of space and on the
other hand to the
improved supply of the cells by cyclic exposure of the hollow-filament
membranes in the two
phases.
Alternatively, the ports for the gas supply are mounted not on the head faces
but on the cylinder
shell, above and below the imaginary cross-sectional plane.
The inventive use of the device lies in the cultivation of cells at high
densities and in the recovery
of cell products, cell constituents, viruses, proteins or low molecular weight
substances, such as
drugs as well as diagnostic and research reagents.
7
CA 02568646 2006-11-22
The features of the invention follow from the elements of the claims and from
the description,
both individual features and also pluralities of features in the form of
combinations representing
advantageous embodiments for which protection is applied for with this
specification.
The substance of the invention comprises a combination of known elements
(partitioning of cul-
ture and supply chambers by membranes) and new elements (arrangement of the
membranes in
the supply chamber, rotatability of the device, alternating exposure of the
cells in the gas and liq-
uid phases respectively), which influence one another mutually and in their
overall effect lead to
an advantage in use and to the desired success, which lies in the fact that
there is achieved the
capability of effective continuous cultivation of cells in high densities and
of recovery of products
from these cells with simultaneous cell retention.
The invention and its function will be explained hereinafter with reference to
figures. The purpose
of the figures is to permit better understanding, but they are not to be
construed as the only con-
structions with which the claims can be implemented.
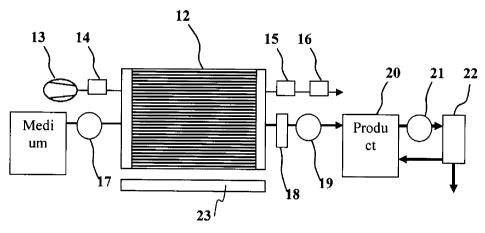
is Figure 1: Schematic diagram of the bioreactor system
The figure represents one version of the bioreactor system on the basis of a
cylindrical supply
chamber (12). The alignment but not the real dimensions and actual number of
hollow-filament
culture compartments disposed in the supply chamber is represented by the
black lines in the sup-
ply chamber. The cylindrical vessel is driven by a rotary device (23) such
that its direction of
movement alternates periodically at a suitable rhythm. The flow of gas phase
through the supply
chamber is ensured by a gas line, which is composed of a gas-mixing station
(13) and a gas hu-
midifier (14) for the gas feed into the cylinder and of a media trap (15) and
a contamination trap
(16) for the gas discharge out of the cylinder. The flow of liquid phase
through the cylinder is
ensured by a media line, which is composed of the media reservoir and a pump
(17) for the media
feed and of a pump (19) and the product-collecting vessel (20) for the
discharge from the cylinder.
Furthermore, a measuring-sensor train (18) for measuring the oxygen, pH and
temperature is inte-
grated in the discharge line. A circulation containing a pump (21) and an
ultrafiltration module
(22) is connected to the product-collecting vessel for concentration of the
product in the product-
collecting vessel (20). The product-free filtrate is discharged at the bottom
of this ultrafiltration
8
CA 02568646 2006-11-22
module and discarded, while the concentrated product is recycled to the
product-collecting vessel.
Advantageously, all elements of the bioreactor system, beginning with the port
on the gas-mixing
station, are disposable materials The pumps are designed as hose pumps.
Figure 2: Longitudinal section of the cylindrical two-phase supply chamber
During reactor operation, the cylindrical supply chamber (12) contains a gas
phase (5) in the up-
per part and a liquid phase (6) in the lower part, the two phases forming a
phase boundary (7). On
the left, the supply chamber is terminated by an end plate (1) for the feed of
gas and medium, and
on the right it is terminated by an end plate (2) for discharge thereof. On
the right terminated
which terminates. Gas is passed through ports (3, 4) in the end plates. Media
transport takes place
via central ports (8, 9) -located on the axis of rotation of the cylinder -in
the respective end plates.
The individual culture compartments for the cells are designed as identical
hollow-filament mem-
branes and are represented in the supply chamber by parallel black lines.
Input of the cell suspen-
sion takes place separately from the gas and media supply, via ports (10)
shown in solid black in
the end plates. For uniform seeding with the cells in all hollow-filament
membranes, the ports end
1s in cell-distributing chambers (11), which are in communication with the
interior space of every
individual hollow-filament membrane.
Figure 3: Top view of the end plates of the supply chamber
In the top view of the feed end plate (1) there is illustrated one of the
arrangements used for the
inlets for gas -shown as small circles -and for medium (8) into the supply
chamber. The top view
of discharge end plate (2) shows the central port for product discharge (9)
and an arrangement
used for the gas-discharge ports, which are shown as small circles. In this
example four ports,
represented by black dots, for seeding with the cell suspension are integrated
in each of the two
end plates, which can be charged via a merged tubing connection.
The invention will be explained by means of practical examples, without being
limited to these
examples.
9
CA 02568646 2006-11-22
Practical examples:
Example 1: Cells of high density
Two bioreactor systems were constructed according to the scheme illustrated in
Fig. 1. The cylin-
drical supply chamber had a total volumetric capacity of 14 liters. During the
process, the liquid
phase contained 7 liters. In both cases, 144 hollow-filament membranes each
500 mm in length
were disposed in axially symmetric arrangement in the supply chamber. Seeding
with cells in the
interior spaces of the hollow-filament membranes took place with cells of high
density in PBG1.0
basic medium containing 0.02% of added human serum albumin via the seeding
ports in the end
plates. The cell line produces a human protein, which can be isolated from the
culture supematant
by a one-step chromatographic method and then assayed exactly as to its
content. The culture time
was 10 and 23 days. Over this time, a mixture of air and 5% COZ was passed
continuously
through the gas phase of the supply chamber. In total, a quantity
corresponding to 11 liters in 10
days and 24 liters in 23 days was used to supply the cells in the runs. During
the experiment, the
liquid-phase and gas-phase exposure cycles were each 30 seconds between the
phase alternations.
After completion of culturing, the cells were harvested from the hollow-
filament membranes via
the seeding ports and the cell density and viability were determined. The
protein was isolated
from an aliquot of the cell-free product harvest and its content was assayed.
The following table
shows the cell density and viability achieved in the hollow-filament culture
compartments.
Bioreactor run 1(10 days) Bioreactor run 2 (23 days)
Total cell count in inoculum 1.5E7 1.8E7
[cells per ml of culture chamber]
Viability of inoculum [%] 67 80
Total cell count of harvest 2.4E7 2.25E7
Viability of harvest (%) 54 29
Total quantity of protein (mg) 48 168
CA 02568646 2006-11-22
High cell densities were successfully achieved in the system. Furthermore, 48
mg and 168 mg of
protein were formed during the process and were collected from the cell-free
culture supematant
into the corresponding product-collecting vessel.
Example 2: Cells of low density
Cells in living cell densities of 1.3E5 cells per milliliter of culture
chamber were used for inocula-
tion in two two-phase exposure reactors, each containing 12 hollow-filament
membrane shaving a
length of 200 mm, and were cultivated for 4 days while both plastic reactors
were being rotated.
Prior to inoculation, the cells were mixed with microscopically small gel
fragments of HSA. Me-
dia exchange was effected discontinuously. The metabolic activity was measured
via the glucose
consumption. After completion of the runs, the cell densities were determined
by harvesting the
gel together with the cells contained therein and counting via Trypan Blue.
Within the short cul-
ture time, expansion of the cells to 6E5 living cells per milliliter of
culture chamber (4.6 times)
and 5E5 living cells per milliliter of culture chamber (3.8 times) was
successfully achieved.
Example 3: Functioning principle of the bioreactor system
The supply principle of the bioreactor is based on exposing the cells
alternately in medium and in
a gas mixture, thus making it possible to improve the supply of the cells with
oxygen compared
with conventional systems.
Example 4: Construction of the system
The core piece of the bioreactor is a cylindrical plastic reactor mounted
horizontally. In this ves-
zo sel, hollow-filament membranes are clamped over the length, parallel to the
axis of rotation, as
illustrated in Fig. 2. Hereby two chambers separated from one another by the
membranes are cre-
ated in the cylinder. One is the supply chamber, which surrounds the hollow-
filament membranes
and is composed of a liquid phase and a gas phase. The boundary between these
two phases is
sketched in Figs. 2 and 3. The other is the space inside each hollow-filament
membrane. The sum
of all hollow-filament internal spaces represents the culture chamber for the
cells. Via the number
11
CA 02568646 2006-11-22
of hollow-filament membranes disposed symmetrically around the axis of
rotation, the system can
be scaled-up to any desired size in terms of its culture chamber. The two
chambers have separate
inlets, and are partitioned from one another. The only communication between
the supply cham-
ber and the culture chamber is represented by the pores of the membrane. With
an advantageous
pore diameter of 0.1 to 1.0 g per milliliter, these pores are permeable for
small molecules and
proteins, but not for the cells. The overall device cited and described in
Fig. 1 ensures that gas and
liquid can be passed continuously through the system.
The bioreactor system also includes a mobile hardware unit, the pumps and
compressors, as well
as measuring and control units. Furthermore, this unit also includes a drive
motor and.a rotary
device, which permits mounting and rotation of the plastic reactor.
Example 5: Functioning principle
By means of the rotary device, the plastic reactor is turned around its axis
of rotation in a rotation
cycle that can be adapted to the respective cell line. This rotation cycle is
advantageously repeated
without interruption over the entire bioreactor run time. The eight phases of
a rotation cycle are
1s listed below by way of example.
Phase: rotation to the right by 180
Phase: holding time
Phase: rotation to the right by 180
Phase: holding time
Phase: rotation to the left by 180
Phase holding time
Phase: rotation to the left by 180
Phase: holding time
As the result of a rotation cycle, the reactor has performed one full
revolution in one direction and
one full revolution in the opposite direction and is once again disposed in
the original starting po-
sition. The alternation of direction of rotation permits media and gas to flow
through ports, which
are integrated in fixed position in the reactor and to which plastic tubes are
fixed. In contrast to
the majority of mammalian-cell bioreactors, therefore, the system operates
effectively without
12
CA 02568646 2006-11-22
mobile structural components that project into the sterile supply or culture
zone, such as impeller
shafts or media and gas feed tubes. Thus the associated contamination risk
does not exist, and no
expenses are incurred for safeguarding corresponding rubbing surfaces, for
example by double
rotating mechanical seals.
The system construction and mode individual of operation simultaneously ensure
that every hol-
low-filament membrane is subjected to identical exposure conditions in both
phases over the en-
tire bioreactor run time, regardless of the number of such membranes in the
system. The exposure
in the gas phase primarily achieves the supply of oxygen, while the exposure
in the liquid phase
achieves primarily the uptake of dissolved nutrients and the discharge of
metabolic products. Both
nutrient medium and gas mixture can be fed continuously.
Advantages / novel features:
Reactor with integrated cell retention system as disposable article
Membrane is permeable for protein, permitting cell-free harvesting
Identical exposure conditions for every individual hollow-filament membrane in
the system
Short diffusion paths for oxygen during exposure in the gas phase
No gradient formation in the gas phase of the exposure reactor over the length
of the reactor
A plurality of gas ports, which are distributed appropriately over the end
caps and which permit
gas to flow through continuously even during rotation
Definitions:
Cells of high density: A culture with high cell density is achieved at cell
densities greater than lE7
cells per milliliter of culture chamber.
Cells of low densitv are achieved when the cell density in the culture chamber
lies between lE4
and lE7 per milliliter of culture chamber.
Cells of lowest density: A culture with the lowest cell density is achieved at
densities lower than
lE4 cells per milliliter of culture chamber.
13
CA 02568646 2006-11-22
Nutrient medium: Nutrient medium is an aqueous solution containing the
nutrients essential for
the cells, such as glucose, amino acids and trace elements.
Gas mixture: Within the meaning used here, a gas mixture advantageously
describes a mixture of
air and carbon dioxide with variable mixing ratio. Furthermore, the present
meaning of the term
gas mixture also includes variable mixing ratios of nitrogen, oxygen and COZ.
List of reference numerals:
1 Feed end plate 11 Cell-distributing chambers
2 Discharge end plate 12 Cylindrical supply chamber
3, 4 Ports for gas supply and removal 13 Gas-mixing station
5 Gas phase 14 Gas humidifier
6 Liquid phase 15 Media trap
7 Phase boundary 16 Contamination trap
8 Inlet for medium (central port) 17, 19, 21 Pumps
9 Central port for product discharge 22 Ultrafiltration module
Ports for the culture chamber 23 Rotary device
14