Note: Descriptions are shown in the official language in which they were submitted.
CA 02569719 2012-01-03
TITLE OF THE INVENTION
ABSORBENT HYDROPHOBIC BORONATE
GALACTOMANNAN COMPLEXES AND PROCESS FOR PRODUCING
SAME.
The present application claims the benefit of
Canadian Application No. 2,507,121 filed May 12, 2005.
FIELD OF THE INVENTION
The present teachings relate to absorbent
hydrophobic galactomannan complexes. More specifically, but not
exclusively, the present teachings relates to absorbent hydrophobic
boronate galactomannan complexes and to a process for producing same.
BACKGROUND OF THE INVENTION
Water absorbent materials, such as superabsorbent
polymers, can be employed in various applications such as in disposable
sanitary products (i.e. diapers, incontinence articles, feminine hygiene
products, airlaids and absorbent dressings), household articles, sealing
materials, humectants in agricultural products for soil conditioning, in oil-
drilling fluids (i.e. lost-circulation material, fracturing fluids), anti-
condensation coatings, in agricultural, horticultural and forestry
applications
for retaining water in the soil and for the release of water to the roots of
plants and trees, in the textile industry, in printing applications, in
absorbent
paper products, in bandages and surgical pads (i.e. wound dressings), in
ore treatments, in pet litter, in water treatment, in food pads (i.e.
applications related to the transportation of fresh food and food packaging),
CA 02569719 2006-05-12
2
in detergents, in fire-fighting gels, in sealing materials, as chemical
absorbents for the cleanup of acidic and/or basic aqueous spills including
water soluble chemical spills, as polymeric gels for the slow and controlled
release of cosmetics and pharmaceuticals (also known as drug delivery
systems), as airlaids, and finally in the manufacture of artificial snow.
However, the primary use of superabsorbent polymers, also referred to a
"SAPs", is in disposable personal hygiene products. Such products
include, in increasing order of volume of superabsorbent materials used,
diapers, training pants, adult incontinence products and feminine hygiene
products.
Increased oil prices have had a negative impact on
the superabsorbent industry such that natural polysaccharide-based
superabsorbents have become an attractive alternative. Such natural
superabsorbent materials can be readily obtained from renewable sources
such as starch. Various absorbent compositions comprising
polysaccharide-based superabsorbents have been proposed by Le Group
Lysac Inc. Huppe et al. (CA 2,308,537) teach the use of biodegradable,
glass-like pregelatinized starch as absorbents for liquids. Couture et al.
(CA 2,362,006) teach the use of oligomeric polyethylene glycol crosslinked
polysaccharides, in particular polyethylene glycol crosslinked starch as
absorbent materials. Thibodeau et al. (CA 2,462,053) teach the use of
crosslinked amylopectin as absorbent materials. Bergeron et al. (CA
2,426,478) teach the use of modified starches (i.e. crosslinked
amylopectin) and mannose containing polysaccharides, ionic
polysaccharides, gelling proteins and mixtures thereof in formulating
absorbent materials. Berrada et al. (CA 2,483,049) teach the use of
phylosilicates dispersed in an absorbent polysaccharide matrix, as having
CA 02569719 2006-05-12
3
absorbent characteristics. Berrada (CA 2,519,417) teaches the use of
guanidinated polysaccharides as absorbent materials.
The use of galactomannans, essentially cross-linked
with borate, titanium or zirconium ions, as superabsorbent
polysaccharides, has been disclosed in a number of patents: US P
3,661,154; US P 3,903,889; US P 4,624,868; US P 4,333,461; US P
5,532,350; US P 5,801,116; JP 2004-089401; JP 2004-075773; JP 2004-
073370; JP 2004-066203; JP 2003-311150; JP 2003-154262; JP 2002-
253961; JP 2002-035037; JP 2001-278998; JP 2002-037924; JP 2002-
053859; JP 2001-120992; JP 2002-053859; JP 2001-226525 and JP 2001-
122905. However, these polysaccharides suffer from syneresis and gel
flowing problems. Crosslinking will seriously limit the manipulation of the
absorbent materials, especially when shear thinning behavior is desired.
Complexes of aliphatic boronates with
galactomannans have been disclosed by Bavouzet et al. (WO 97/47658).
Complexes of aromatic boronates with galactomannans were disclosed by
Bishop et al. (Dalton Transactions; 17; 2004; 2621-2634). PEG-diboronate
galactomannan complexes have been disclosed by Coveney et al.
(Molecular simulation, 2000, Vol. 25, pp. 265-299). Synthetic boronate
polymer complexes with polysaccharides have also been disclosed by
Miyazaki et al. (EP 0424168); Filipini (EP 0159521); Pelton et al. (WO
06/010268); and Destarac et al. (FR 2839723). However, these complexes
were not disclosed as being absorbent materials.
There thus remains a need for absorbent hydrophobic
boronate galactomannan complexes, as well as a process for producing
same.
CA 02569719 2012-01-03
4
The present teachings seek to meet these and other
needs.
SUMMARY OF THE INVENTION
The present teachings broadly relate to novel
absorbent or superabsorbent materials. More specifically, as broadly
claimed, the present teachings relate to boronate-galactomannan
complexes comprising a hydrophobic group, the boronate-galactomannan
complexes having absorbent properties suitable for use in personal
hygiene products. In an embodiment, the boronate-galactomannan
complexes of the present teachings are dry, solid materials having good
fluid-swelling properties and capable of gel forming upon contacting with a
liquid.
In a further embodiment, the present teachings relate
to the use of the boronate-galactomannan complexes comprising a
hydrophobic group as absorbents in disposable sanitary products (i.e.
diapers, incontinence articles, feminine hygiene products, airlaids and
absorbent dressings), household articles, sealing materials, humectants in
agricultural products for soil conditioning, in oil-drilling fluids (i.e. lost-
circulation material, fracturing fluids), anti-condensation coatings, in
agricultural, horticultural and forestry applications for retaining water in
the
soil and for the release of water to the roots of plants and trees, in the
textile industry, in printing applications, in absorbent paper products, in
bandages and surgical pads (i.e. wound dressings), in ore treatments, in
pet litter, in water treatment, in food pads (i.e. applications related to the
CA 02569719 2006-05-12
transportation of fresh food and food packaging), in detergents, in fire-
fighting gels, in sealing materials, as chemical absorbents for the cleanup
of acidic and/or basic aqueous spills including water soluble chemical
spills, as polymeric gels for the slow and controlled release of cosmetics
5 and pharmaceuticals (also known as drug delivery systems), as airlaids,
and finally in the manufacture of artificial snow.
In a further embodiment, the present teachings relate
to the use of the boronate-galactomannan complexes comprising a
hydrophobic group as absorbents for liquids, non-limitative examples of
which include water, aqueous solutions, physiological fluids and saline
solutions.
In yet a further embodiment, the present teachings
relate to compositions including at least one boronate-galactomannan
complex comprising a hydrophobic group, and a co-absorbent material.
Finally, in a further embodiment, the present
teachings relate to processes for preparing boronate-galactomannan
complexes, the boronate-galactomannan complexes comprising a
hydrophobic group.
The foregoing and other objects, advantages and
features of the present teachings will become more apparent upon reading
of the following non restrictive description of illustrative embodiments
thereof, given by way of example only with reference to the accompanying
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
In the appended drawings:
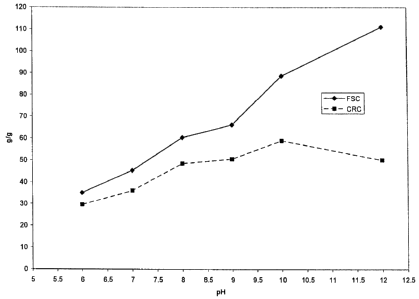
FIG. 1 is a graph illustrating the pH effect on the
CA 02569719 2006-05-12
6
performance characteristics of a boronate-galactomannan complex
comprising a hydrophobic group, according to an embodiment of the present
teachings.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
In order to provide a clear and consistent
understanding of the terms used in the present specification, a number of
definitions are provided below. Moreover, unless defined otherwise, all
technical and scientific terms as used herein have the same meaning as
commonly understood to one of ordinary skill in the art to which the present
teachings pertain.
The use of the word "a" or "an" when used in
conjunction with the term "comprising" in the claims and/or the specification
may mean "one", but it is also consistent with the meaning of "one or
more", "at least one", and "one or more than.one". Similarly, the word
"another" may mean at least a second or more.
As used in this specification and claim(s), the words
"comprising" (and any form of comprising, such as "comprise" and
"comprises"), "having" (and any form of having, such as "have" and "has"),
"including" (and any form of including, such as "include" and "includes") or
"containing" (and any form of containing, such as "contain" and "contains"),
are inclusive or open-ended and do not exclude additional, unrecited
elements or process steps.
As used in this specification and claim(s), the term
"about" is used to indicate that a value includes an inherent variation of
error for the device or the method being employed to determine the value.
As used in this specification, the term "percent" or "%"
CA 02569719 2006-05-12
7
refers to a percentage by weight (i.e. % (W/W)).
As used in this specification, the term "discrete
particle" refers to individual particles.
As used in this specification, the term "Free Swell
Capacity" (FSC), also called "Total Absorption", refers to the amount (g) of
fluid absorbed per gram of the composition. Typical fluids are saline
solutions (0.9% Weight/Weight NaCl solution, hereinafter called 0.9% NaCl
solution or saline).
As used in this specification, the term "Centrifuge
Retention Capacity" (CRC) also called "Retention", refers to the amount (g)
of fluid retained per gram of the composition, following exposure of the
composition to a centrifugation force of 250G. Typical fluids are saline
solutions (0.9% Weight/Weight NaCl solution, hereinafter called 0.9% NaCl
solution or saline).
As used in this specification, the term "Absorption
Under Load" (AUL), at 0.3 PSI, 0.7 PSI or 0.9 PSI, also called "Absorption
Against Pressure" (AAP), refers to the amount (g) of fluid absorbed per
gram of the composition under a given applied pressure. Typical fluids are
saline solutions (0.9% Weight/Weight NaCl solution, hereinafter called
0.9% NaCl solution or saline).
The present description refers to a number of
chemical terms and abbreviations used by those skilled in the art.
Nevertheless, definitions of selected terms are provided for clarity and
consistency.
As used in this specification, the term "absorbent
material" or "absorbent polymer" refers to materials in a dry, solid state,
having good fluid-swelling properties and capable of gel forming upon
CA 02569719 2006-05-12
8
contacting with a fluid. Non limiting examples of such fluids are water,
aqueous solutions, saline, or physiological fluids.
As used in this specification, the term
"superabsorbent", "superabsorbent polymer" or "SAP" refers to absorbent
materials capable of gel forming upon contacting with a fluid such as water,
aqueous solutions, saline, or physiological fluids. Such materials are
characterized a Centrifuge Retention Capacity (CRC) of at least 15 g/g.
As used in this specification, the term
galactomannan" refers to naturally occurring polysaccharides comprising a
poly 3-(1-4)-mannose backbone having varying degrees of branching (DB),
and to which single D-galactopyranosyl residues are attached via a-(1-6)
linkages. Non-limiting examples of galactomannans are guar gum, locust
bean gum, tara gum, fenugreek gum, mesquite gum and mixtures thereof.
Endosperms of coffee (US 2004/0199943 Al), alfalfa, red-clover, and
some soybeans (US 2004/0143871 Al) are also known to comprise
galactomannans.
As used in this specification, the term "diol" refers to a
pair of adjacent hydroxyl functions of galactomannans capable of reacting
with complexing agents such as a boronate. Adjacent hydroxyl functions
comprise a pair hydroxyl functions located on vicinal carbon atoms. As
reported by Bishop et al. (Dalton Transactions; (17); 2004; 2621-2634), the
3,4 cis-diols on galactopyranosyl residues and the 2,3-cis-diols on the
mannose backbone are diols capable of reacting with complexing agents
(Scheme 1).
CA 02569719 2006-05-12
9
OH
D
Galactose H
3,4-cis -diol
O
HO
OH
HO O (OH
HO
Mannose OH
2,3-cis -diol O
O
qn
Scheme 1
As used in this specification, the term "boronate" or
"boronates" refers to boron derivatives having the following general
molecular structure:
HOB,,,, B/OH
I
Ri \OH
wherein R, is a hydrophobic group selected from the group consisting of
aromatic groups, aliphatic groups and cyclic aliphatic groups.
As used in this specification, the term "complex"
refers to boron-containing materials obtained by adding a boronate to a
solution containing one or more galactomannans. The complexes of the
present teachings are derived from interactions between the 3,4 cis-diols
on galactopyranosyl residues and the 2,3-cis-diols on the mannose
CA 02569719 2006-05-12
backbone with boronates. Complexes between such 3,4 cis-diols or 2,3-
cis-diols and boronates are known as diol boronic ester linkages.
As used in this specification, the term "hydrophobic",
"hydrophobic moiety" or "hydrophobic group" refers to those compounds,
5 groups or moieties being immiscible in water.
As used in this specification, the term "hydrophilic",
"hydrophilic moiety" or "hydrophilic group" refers to those compounds,
groups or moieties being miscible in water.
As used in this specification, the term "amphiphilic",
10 "amphiphilic moiety" or "amphiphilic group" refers to those compounds,
groups or moieties having both hydrophilic and hydrophobic properties.
As used in this specification, the term "aliphatic" or
"aliphatic group" refers to, and is inclusive of, all non-aromatic acyclic or
cyclic groups. The aliphatic moieties may be saturated or unsaturated, and
may be substituted. Non-limiting examples of aliphatic groups include alkyl
groups, alkenyl groups, alkynyl groups, cycloalkyl groups, and cycloalkenyl
groups.
As used in this specification, the term "alkyl" refers to
straight, branched or substituted chain radicals having up to twenty carbon
atoms. Non-limiting examples include methyl, ethyl, propyl, butyl, pentyl,
hexyl, heptyl, octyl, nonyl, decyl, isopropyl, isobutyl, isopentyl, neopentyl,
isohexyl, isodecyl, 3-methylpentyl, 2,3,4-trimethylhexyl, sec-butyl, tert-
butyl, or tert-pentyl.
As used in this specification, the term "alkenyl" refers
to straight, branched or substituted chain radicals of 2 to 10 carbon atoms
having one or more double bonds.
CA 02569719 2006-05-12
11
As used in this specification, the term "alkynyl" refers
to straight, branched or substituted chain radicals of 2 to 10 carbon atoms
having one or more triple bonds.
As used in this specification, the term "cycloalkyl"
refers to cyclic chain radicals, optionally branched or substituted, having up
to ten carbon atoms. Non-limiting examples include cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl.
As used in this specification, the term "cycloalkenyl"
refers to cyclic chain radicals, optionally branched or substituted, of 2 to
10
carbon atoms having one or more double bonds. Non-limiting examples
include cyclopentenyl and cyclohexenyl.
As used in this specification, the term "aromatic",
"aromatic groups" or "aromatic moiety" refers to unsaturated conjugated
cyclic hydrocarbons containing one or more rings. Aromatic groups include
5- and 6-membered single-ring groups.
As used in this specification, the term "granular
material", "granules", "particles", "powders", "grains" or "dusts" refers to
particulate matter in a finely divided state. Granular material can include
highly pulverized material with very small diameters. The particles need
not be of any particular shape, but can be spherical, roughly spherical,
cubic, or non regular in shape.
As used in this specification, the term "particle size"
refers to the largest dimension of the particle. The particle size can be
directly determined using sieving methods, optical or scanning electron
microscopes as well as by other well-known methods. The particle size is
often considered the diameter of the particle.
As used in this specification, the term "alkaline" refers
CA 02569719 2006-05-12
12
to any pH greater than 7Ø
The present teachings broadly relate to absorbent
boronate galactomannan complexes comprising a hydrophobic group.
More specifically, the galactomannan is complexed with a boronate
comprising a hydrophobic moiety. It was surprisingly discovered that such
complexes exhibit absorbent characteristics similar to borax cross-linked
galactomannans.
Efficient galactomannan-based absorbent materials
are obtained by complexing the galactomannans with a boronate
comprising a hydrophobic moiety. The hydrophobic moiety is selected
from the group consisting of aromatic groups, aliphatic groups or cyclic
aliphatic groups. Efficient absorbent materials' are thus obtained without
the need for cross-linking the galactomannans.
Boronates are well documented in the art to form
complexes with the D-galactopyranosyl residues of galactomannans. Such
D-galactopyranosyl residues are also known to be at the origin of the
swelling characteristics of galactomannans. Hydrophobic pockets are
created when a boronate comprising a hydrophobic moiety is complexed
with the D-galactopyranosyl residues of galactomannans. Without being
bound by any theory, it is believed that the hydrophobic pockets will
become associated by means of weak Van der Waals interactions. Even
though weak, it is believed that such interactions are sufficient to create a
network of galactomannans having good swelling properties and efficient
absorbent characteristics.
Gels of boronate-galactomannan complexes can be
readily obtained by dispersing a galactomannan in an aqueous solution
followed by the addition of a boronate comprising a hydrophobic moiety.
CA 02569719 2006-05-12
13
The resulting reaction mixture is stirred at alkaline pH until gel formation.
In an embodiment of the present teachings, an alkaline pH of at least 8.5
was used.
The boronates of the present teachings are
amphiphilic in nature; the boronate, while bearing a hydrophobic moiety,
being hydrophilic. Because of this amphiphilic character, the boronates
only dissolve with great difficulty in water. However, the boronates readily
dissolve in non-aqueous polar solvents, a non-limiting example of which
includes tetrahydrofuran (THF).
The boronate-galactomannan complexes of the
present teachings can be prepared in accordance with a process in which
a galactomannan is dispersed in an aqueous solution, followed by the
addition of a boronate comprising a hydrophobic moiety. The resulting
boronate-galactomannan complex is then recovered by precipitation from
one or more hydrophilic organic solvents. The precipitated boronate-
galactomannan complex may then be optionally ground into a granular
material having a particle size ranging from about 80 to about 800 pm.
In an embodiment of the present teachings, the
boronate galactomannan complexes are in a dry solid state. Such dry
boronate galactomannan complexes can be easily handled and stocked.
In a further embodiment of the present teachings, the boronate
galactomannan complexes are in a dry, solid granular state. In a further
embodiment of the present teachings, the granular galactomannan
complexes comprise a particle size ranging from about 80 to about 800
pm. In yet a further embodiment of the present teachings, the granular
galactomannan complexes comprise a particle size ranging from about 150
to about 600 pm.
CA 02569719 2006-05-12
14
Dry boronate galactomannan complexes can be
obtained by precipitating the complexes using hydrophilic organic solvents.
Non-limiting examples of hydrophilic organic liquids as contemplated by the
present teachings include C1-C3 alcohols, acetone, and acetonitrile. In an
embodiment of the present teachings, the boronate galactomannan
complexes are precipitated using methanol. Once precipitated, the
boronate galactomannan complexes may further processed such as by
grinding.
Non-limiting examples of galactomannans as
contemplated by the present teachings include guar gum, locust bean gum,
tara gum, fenugreek gum, mesquite gum and mixture thereof. The
galactose to mannose ratio of galactomannans typically ranges from about
1:5 to about 1:1.
Guar gum, a typical galactomannan, is derived from
ground endosperm of the guar plant, which is grown extensively in the
semi-arid regions of Pakistan and India. As shown hereinabove in Scheme
1, the structure of guar gum comprises a random galactose to mannose
ratio of about 1:1.6. This ratio is subject to fluctuations from crop to crop
or
from subspecies to subspecies (Jasinski et al. J. Polym. Sci., part. B:
Polym. Phys. 1996, 34, 1477-1488).
Non-limiting examples of boronates as contemplated
by the present teachings include phenyl boronate, phenethyl boronate, 2-
naphtalen boronate, 3-biphenyl boronate, trans- 1 -octe n-1 -yl boronate and
cyclohexyl boronate.
By virtue of their deficient valence, boronic acids
possess a vacant p-orbital. This characteristic confers them unique
properties as mild organic Lewis acids that can coordinate basic
CA 02569719 2006-05-12
}
molecules. As such, boronates can be readily obtained from the
corresponding boronic acids under alkaline conditions as illustrated in
Scheme 2. Typical alkaline conditions comprise a pH of at least 8.5.
OH HOB, /OH
Rj \ + OH RlVol, B\
OH
OH
Boronic acid
Boronate
Boron Hybridized sP2 Boron Hybridized spa
5 Scheme 2
Boronic acid, bearing a hydrophobic group, is
dissolved in a suitable solvent such as water, aqueous alkaline solutions or
non-aqueous polar solvents such as tetrahydrofuran (THF). The solution
may be heated to increase the boronic acid solubility. As reported in the
10 literature (Bishop et al.; Dalton Transactions; (17); 2004; 2621-2634;
Pezron, E. et al. Macromolecules, 1988, 21, 1121-1125; Jasinski et al., J.
Polym. Sci. Part B: Polym. Phys., 1996, 34, 1477-1488), the type of
boronate species present in solution is directly dependent on the pH of the
solution. It was observed that an alkaline pH was particularly suitable for
15 generating boronates capable of complexing with galactomannans.
Typical alkaline conditions comprise a pH of at least 8.5.
The absorbent boronate galactomannan complexes
of the present teachings may be incorporated into absorbent personal
hygiene products such as, for example, baby diapers, incontinence
products, sanitary napkins and the like. They may be also used in
CA 02569719 2006-05-12
16
absorbent members such as absorbent cores, airlaids or foamed
structures.
The absorbent boronate galactomannan complexes
of the present teachings may also be used in other applications such as in
food pads, in agricultural and forestry applications for the retention of
water
in the soil and for the release of water to the roots of plants and trees; in
fire-fighting techniques; in bandages and surgical pads; for the cleanup of
acidic or basic solution spills, including water soluble chemical spills; as
polymeric gels for the controlled release of cosmetics and pharmaceuticals
(also known as drug delivery systems); and in artificial snow.
The absorbent boronate galactomannan complexes
of the present teachings may be mixed with other co-absorbent materials
to provide absorbent compositions. In an embodiment, the absorbent
compositions comprise from about 1 to about 99% (w/w) of boronate
galactomannan complex, and from about 99 to about 1% (w/w) of co-
absorbent material. Non-limiting examples of co-absorbent materials
include synthetic absorbent polymers, starch-based absorbents, ionic
polysaccharides, fibers and mixtures thereof. In an embodiment of the
present teachings, absorbent compositions are obtained by mixing one or
more boronate galactomannan complexes with ionic polysaccharides;
either cationic or anionic polysaccharides or mixtures thereof. In a further
embodiment of the present teachings, absorbent compositions are
obtained by mixing one or more boronate galactomannan complexes with
one or more anionic polysaccharides.
Non-limiting examples of anionic polysaccharides as
contemplated by the present teachings include carboxyalkyl
CA 02569719 2006-05-12
17
polysaccharides, carboxymethyl cellulose, carboxymethyl starch, oxidized
polysaccharides, xanthan, carrageenans, pectin and mixtures thereof.
Non-limiting examples of fibers as contemplated by
the present teachings include cellulose, viscose, rayon, cellulose acetate,
polyamides (i.e. NylonTM), polyalkylenes, polyethylene, polypropylene, bi-
component fibers, polyesters, polylactides, polypropanediols, LyocelUTM
sphagnum and mixtures thereof.
Non-limiting examples of starch-based absorbents as
contemplated by the present teachings include glass-like starches such as
disclosed by Huppe et al. (CA 2,308,537); amylopectin networks such as
disclosed by Thibodeau et al. (CA 2,462,053); starch agglomerates,
hydroxyethyl starch, hydroxypropyl starch, carboxymethyl starch, starch
nanocomposites such as disclosed by Berrada et al. (CA 2,483,049); and
mixtures thereof.
The synthetic absorbent polymers to be used as co-
absorbent materials in the absorbent compositions of the present
teachings, are generally obtained from the polymerization, typically by
radical or radical graft polymerization, of monomers, non-limiting examples
of which include acrylic acid, acrylate salts, acrylic ester, acrylic
anhydride,
methacrylic acid, methacrylate salts, methacrylic esters, methacrylic
anhydride, maleic anhydride, maleic salts, maleate esters, acrylamide,
acrylonitrile, vinyl alcohol, vinyl pyrrolidone, vinyl acetate, vinyl
guanidine,
aspartic acid, aspartic salts and mixtures thereof.
The boronate galactomannan complexes of the
present teachings, or absorbent compositions comprising such complexes,
are used in methods for absorbing liquids. In an embodiment of the
present teachings, one or more of the boronate galactomannan complexes
CA 02569719 2006-05-12
18
are contacted with a liquid to be absorbed. Non-limiting examples liquids
as contemplated by the present teachings include water, aqueous
solutions, physiological fluids and saline solutions. The boronate
galactomannan complexes, or absorbent compositions comprising such
complexes, upon contacting with the liquid(s) to be absorbed, will form a
gel trapping the liquid(s) within.
EXPERIMENTAL
Materials
Guar gum (Procol ) was obtained from Polypro
(Minneapolis, USA). Phenethyl boronic acid, phenyl boronic acid, trans-1-
octen-1-yl boronic acid, cyclohexyl boronic acid, research grade methanol
and sodium hydroxide were obtained from Sigma-Aldrich (St-Louis, USA).
Hydrochloric acid was obtained from Labmat (Quebec city, Canada).
Convection oven
Samples were dried using a Lab tray drier TY 2,
National Drying Machinery Company, (Philadelphia, USA).
Grinder
A Braun TM model KSM coffee grinder was used to
grind the samples.
Test methods
As discussed in Modern Superabsorbent Polymer
Technology (Buchholz F. L. and Graham A. T. Eds., Wiley-VCH, New York,
1998, section 4.6.1. Swelling Capacity: Theory and Practice, p. 147),
several methods of measurement are used in order to characterize the
swelling capacity of a polymer. In the field of superabsorbents, the
Gravimetric Swelling Capacity [also called the Free Swell Capacity (FSC)]
CA 02569719 2006-05-12
3 ~
19
and the Centrifuge Capacity [also called the Centrifuge Retention Capacity
(CRC)] are recommended methods. The FSC and the CRC were used to
compare the swelling capacities of the obtained absorbent products.
Tea bags for FSC and CRC measurements
Tea bags (10 X 10 cm) were made from heat sealable
AhlstromTM filter paper (16.5 0.5) g/m2.
FSC measurements
The Free Swell Capacity (FSC) in a 0.9% NaCl
solution was determined according to the recommended test method
440.2-02 from EDANA.
CRC measurements
The Centrifuge Retention Capacity (CRC) in a 0.9%
NaCl solution was determined according to the recommended test method
441.2-02 from EDANA.
EXAMPLES
COMPARATIVE EXAMPLE 1
PHENYLBORONATE GUAR COMPLEXES
A mixture comprising guar gum (6.00 g) and water
(300 ml) was prepared and left to swell for at least 45 minutes. Phenyl
boronic acid (1.34 g) was dissolved in water (40 ml) by increasing the pH of
the solution to 13.0 by the addition of an aqueous sodium hydroxide
solution (15%). The phenylboronate solution was added to the guar
suspension and the resulting suspension stirred 30 minutes. Half of the
resulting gel was blended with methanol (300 ml), triturated, and
transferred into a beaker. The pH of the suspension was adjusted to 7.9
CA 02569719 2006-05-12
using hydrochloric acid (10%), under vigorous mechanical stirring. The
final suspension was filtered, washed with methanol (3 X 50 ml), dried
overnight in a convection oven at 60 C, and crushed with a mortar to
provide a white granular material having a FSC of 51.2 g/g and a CRC of
5 39.5 g/g.
COMPARATIVE EXAMPLE 2
PHENETHYLBORONATE GUAR COMPLEXES
A mixture comprising guar gum (6.00 g) and water
(300 ml) was prepared and left to swell for at least 45 minutes. Phenethyl
10 boronic acid (1.67 g) was dissolved in water (40 ml) by increasing the pH
of
the solution to 13.0 by the addition of an aqueous sodium hydroxide
solution (15%). The phenethylboronate solution was added to the guar
suspension and the resulting suspension stirred 30 minutes. Half of the
resulting gel was blended with methanol (300 ml), triturated, and
15 transferred into a beaker. The pH of the suspension was adjusted to 7.9
using hydrochloric acid (10%), under vigorous mechanical stirring. The
final suspension was filtered, washed with methanol (3 X 50 ml), dried
overnight in a convection oven at 60 C, and crushed with a mortar to
provide a white granular material having a FSC of 62.8 g/g and a CRC of
20 45.4 g/g.
COMPARATIVE EXAMPLE 3
TRANS-1-OCTEN-1-YLBORONATE GUAR COMPLEXES
A mixture comprising guar gum (6.00 g) and water
(300 ml) was prepared and left to swell for at least 45 minutes. Trans-1-
octen-1-ylboronic acid (1.73 g) was dissolved in THE (40 ml). The trans-1-
octen-1-ylboronic acid solution was added to the guar suspension. The pH
CA 02569719 2006-05-12
21
of the suspension was increased to 10.0 by the addition of an aqueous
sodium hydroxide solution (15%) and the suspension stirred 30 minutes.
Half of the resulting gel was blended with methanol (300 ml), triturated, and
transferred into a beaker. The pH of the suspension was adjusted to 8.0
using hydrochloric acid (10%), under vigorous mechanical stirring. The
final suspension was filtered, washed with methanol (3 X 50 ml), dried
overnight in a convection oven at 60 C, and crushed with a mortar to
provide a white granular material having a FSC of 94.8 g/g and a CRC of
68.4 g/g.
COMPARATIVE EXAMPLE 4
CYCLOHEXYLBORONATE GUAR COMPLEXES
A mixture comprising guar gum (6.00 g) and water
(300 ml) was prepared and left to swell for at least 45 minutes. Cyclohexyl
boronic acid (1.43 g) was dissolved in water (40 ml) by increasing- the pH of
the solution to 13.0 by the addition of an aqueous sodium hydroxide
solution (15%). The cyclohexylboronate solution was added to the guar
suspension and the resulting suspension stirred 30 minutes. Half of the
resulting gel was blended with methanol (300 ml), triturated, and
transferred into a beaker. The pH of the suspension was adjusted to 7.9
using hydrochloric acid (10%), under vigorous mechanical stirring. The
final suspension was filtered, washed with methanol (3 X 50 ml), dried
overnight in a convection oven at 60 C, and ground to provide a white
granular material having a FSC of 112.0 g/g and a CRC of 91.0 g/g.
CA 02569719 2006-05-12
{
22
COMPARATIVE EXAMPLE 5
pH EFFECT ON THE PERFORMANCES CHARACTERISTICS OF
PHENYLBORONATE COMPLEXES
A mixture comprising guar gum (6.00 g) and water
(300 ml) was prepared and left to swell for at least 45 minutes. Phenyl
boronic acid (1.35 g) was dissolved in water (40 ml) by increasing the pH of
the solution to 10.0 by the addition of an aqueous sodium hydroxide
solution (15%). The phenylboronate solution was added to the guar
suspension and the resulting suspension stirred 30 minutes. Half of the
resulting gel was blended with methanol (300 ml), triturated, and
transferred into a beaker. The pH of the suspension was adjusted to 6.0,
7.0, 8.0, 9.0, 10.0 and 12.0 using hydrochloric acid (10%) and/or sodium
hydroxide (15%), under vigorous mechanical stirring. The final suspension
was filtered, washed with methanol (3 X 50 ml), dried overnight in a
convection oven at 60 C, and crushed with a mortar to provide a white
granular material having the FSC and CRC performance characteristics as
illustrated in Figure 1.
It is to be understood that the invention is not limited
in its application to the details of construction and parts as described
hereinabove. The invention is capable of other embodiments and of being
practiced in various ways. It is also understood that the phraseology or
terminology used herein is for the purpose of description and not limitation.
Hence, although the present teachings have been described hereinabove
by way of illustrative embodiments thereof, it can be modified, without
departing from the spirit, scope and nature of the subject teachings as
defined in the appended claims.