Note: Descriptions are shown in the official language in which they were submitted.
CA 02569848 2006-12-07
- 1 -
SPECIFICATION
PULP BLEACHING PROCESSES
TECHNICAL FIELD
[0001]
The present invention relates to a bleaching process for
pulp characterized in that a pulp washed after an acid
treatment is irradiated with UV light and/or visible light
under alkaline conditions; a bleaching process characterized
in that a pulp washed after an acid treatment is treated by a
combination of irradiation with UV light and/or visible light
and ozone feeding, thereby promoting pulp bleaching; and a
bleaching process for chemical pulp by which a high brightness
chemical pulp can be obtained (herein collectively referred to
as the first invention); as well as a high brightness chemical
pulp with greatly improved discoloration and a paper
containing it, more specifically a novel high brightness
chemical pulp with greatly improved discoloration obtained by
further treating a bleached chemical pulp with UV light and a
paper containing it (herein collectively referred to as the
second invention).
BACKGROUND ART
[0002]
The background art of the first invention described below
is as follows.
[0003]
To meet the growing concern about the influence of waste
CA 02569848 2006-12-07
2 -
from bleaching processes of paper pulp plants on the
environment, chlorine-free (ECF) bleaching or even totally
chlorine-free (TCF) bleaching is becoming global and
mainstream in place of conventional bleaching techniques
mainly using chlorine or chlorine-based chemicals or
combinations thereof. Under such circumstances, there is a
tendency to use limited chemicals such as chlorine dioxide,
hydrogen peroxide, oxygen and ozone in ECF bleaching or TCF
bleaching. However, pulp quality, especially brightness
obtained by bleaching with only such chemicals is limited even
if they are used in combination, or large amounts of expensive
chemicals must be used to attain sufficient quality. It would
be desirable to develop chlorine-free chemicals having
unprecedentedly excellent bleaching performance or novel
bleaching techniques to solve these problems.
[0004]
It has been known that various metals from pulp promote
decomposition of oxygen-based bleaching chemicals, thereby
wasting the oxygen-based bleaching chemicals. Thus,
techniques for removing these metals to improve the bleaching
efficiency of oxygen-based bleaching chemicals were proposed,
such as acid treatments at relatively low temperatures or
chelator treatments or combinations thereof. Such acid
treatment techniques previously disclosed include a process
for delignifying a pulp prepared from a lignocellulose
material by oxygen bleaching, comprising first adding a
nitrite and an acid to the pulp to pretreat the pulp, followed
by oxygen bleaching, or a bleaching process comprising
CA 02569848 2006-12-07
3 -
subjecting a cooked chemical pulp to an acid treatment
followed by delignification with a peroxide and pressurized
oxygen in an alkaline medium (e.g., patent document 1, patent
document 2). Another bleaching process was also disclosed,
comprising subjecting a cooked chemical pulp to an oxygen
bleaching treatment at high temperature and high pressure and
then an acid treatment or a chelator treatment followed by
delignification/bleaching with a peroxide or hydrogen peroxide
and oxygen in an alkaline medium (e.g., patent document 3).
[0005]
It has become known from recent findings that not only
lignin and modified lignin but also hexenuronic acid are
responsible for discoloration of ECF or TCF bleached pulp.
This hexenuronic acid is produced by demethylation from
methylglucuronic acid in hemicellulose during the cooking
process. This hexenuronic acid is said to be responsible for
discoloration of the pulp. A method proposed to remove the
hexenuronic acid was an acid treatment technique carried out
at a relatively high temperature. This method comprises
treating unbleached pulp at a high temperature under acid
conditions, thereby acid-hydrolyzing the hexenuronic acid and
modified lignin to remove them. For example, a technique was
disclosed wherein a suspension of a cellulose pulp prepared by
the sulfate process or alkaline process is heated and treated
at about 85 to 150 C and a pH of about 2 to 5 to remove at
least about 50% of hexenuronic acid in the cellulose pulp,
thereby decreasing the Kappa number of the pulp by 2 to 9
units (see patent document 4).
CA 02569848 2006-12-07
4 -
[0006]
Bleaching techniques using irradiation were also disclosed,
such as techniques involving UV irradiation in hydrogen
peroxide bleaching of unbleached kraft pulps (e.g., see non-
patent document 1, or patent document 5), or UV irradiation in
oxygen bleaching of unbleached kraft pulps (e.g., see non-
patent document 2). UV irradiation in the presence of a
peroxide as a pretreatment for promoting normal alkaline
hydrogen peroxide bleaching was also disclosed (e.g., see
patent document 6).
[0007]
Other disclosed techniques include a pulp bleaching
process using a reducing agent involving irradiation with UV
light or visible light or a combination thereof (see patent
document 7), or a process involving irradiation with UV light
or visible light or a combination thereof in the presence of
an organic peroxide represented by ROOR' as an oxidizing agent
(see patent document 8).
[0008]
Recently, water pollution caused by industrial wastewater
or domestic wastewater is worsening and water environment
pollution has become social concerns. Under such
circumstances, water environment protection technologies such
as activated carbon treatment, membrane treatment, ozone
treatment, UV treatment, biological treatment, etc. are under
active development. Among them, the advanced oxidation
technology combining ozone and UV light (see patent document
9) is a promising comprehensive treatment capable of improving
CA 02569848 2006-12-07
-
efficiency of decomposition and deodorizing, effects of
decoloring and sterilizing as well as ensuring clarification
treatment without producing secondary waste.
Patent document 1: Japanese Patent No. 2895977.
Patent document 2: JPA No. Hei 6-101186.
Patent document 3: JPA No. Hei 6-158573.
Patent document 4: JPA No. Hei 10-508346.
Patent document 5: JPA No. 2002-88673.
Patent document 6: JPA No. Hei 6-128890.
Patent document 7: JPA No. 2002-88671.
Patent document 8: JPA No. 2002-88672.
Patent document 9: JPA No. 2004-97992.
Non-patent document 1: B. Marccia, et al. J34 - J39,
JOURNAL OF PULP AND PAPER SCIENCE: Vol. 17, No. 2, March 1991.
Non-patent document 2: J.Abbot, et al. p198 - 202, Appita
Vol. 46, No. 3, May 1993.
The background art of the second invention described below
is as follows.
[0009]
Paper products mainly made from chemical pulp, especially
communication papers such as inkjet papers and thermal
transfer papers or photographic base papers are required to
have high brightness. Normally, in order to increase
brightness of unbleached kraft pulp, materials responsible for
coloration such as lignin or polysaccharides remaining in the
unbleached pulp are removed by multistage bleaching with
chemicals such as chlorine, hypochlorites, chlorine dioxide,
oxygen, hydrogen peroxide, ozone, etc. The ISO brightness of
CA 02569848 2006-12-07
6 -
pulp obtained by chlorine bleaching with chlorine gas or more
environmentally friendly ECF bleaching with reduced production
of organic chlorine compounds using chlorine dioxide is
normally 82 - 86%. High brightness pulp bleached to a higher
brightness level than normal is typically prepared by applying
harsher cooking and/or bleaching conditions or by using easy-
to-cook and bleach wood species having low contents of
phenolic extract components.
[0010]
As a prior technique for preparing high brightness pulp, a
process for preparing a high brightness pulp was disclosed,
for example, characterized in that a pulp bleached in a
sequence including at least one chlorine bleaching stage is
treated with xylanase and further bleached in a bleaching
sequence including a hypochlorite stage and a chlorine dioxide
stage (see patent document 10). Another process for preparing
a high brightness pulp was disclosed, comprising further
bleaching a bleached pulp derived from a lignocellulose
material via a continuous sequence of a hypochlorite bleaching
stage under high temperature and highly alkaline conditions
and a chlorine dioxide bleaching stage, characterized in that
the chlorine dioxide bleaching stage is performed at a
chlorine dioxide concentration of 1-3% by weight (on the basis
of the bone dry weight of the pulp) at a high temperature of
91 C or more and less than 100 C (see patent document 11). A
photographic base paper was also disclosed, characterized in
that it uses a chemical pulp bleached in a bleaching sequence
of oxygen bleaching - ozone bleaching - alkali extraction -
CA 02569848 2006-12-07
- 7 -
hydrogen peroxide bleaching - chlorine dioxide bleaching -
hydrogen peroxide bleaching - chlorine dioxide bleaching and
that the chemical pulp is bleached with 0.1 - 1.0% by weight
of ozone on the basis of the bone dry weight of the pulp
during the ozone bleaching stage (see patent document 12). A
process for preparing a high brightness pulp with improved
discoloration for use in photosensitive materials was also
disclosed, characterized in that an unbleached kraft pulp
having a kappa number of 23 or less is bleached with oxygen at
a delignification degree of 40% or more and then bleached with
ozone at a pulp consistency of 25% or more and then adjusted
to a PN number of 2.8 or less followed by multistage bleaching
including hydrogen peroxide bleaching and chlorine dioxide
bleaching (see patent document 13). However, it was difficult
to avoid brightness loss with time or so-called discoloration
phenomenon in high brightness pulps obtained by the
conventional processes because very small amounts of potential
coloring matters liable to be darkened by heat or UV light
remain in them.
Patent document 10: JPA No. Hei 6-101185.
Patent document 11: JPA No. Hei 9-105091.
Patent document 12: JPA No. 2002-62622.
Patent document 13: JPA No. 2003-41494.
DISCLOSURE OF THE INVENTION
PROBLEMS TO BE SOLVED BY THE INVENTION
[00111
The first invention aims to further advance the acid
CA 02569848 2006-12-07
8 -
treatment or irradiation technology for pulp as described
above to develop a bleaching process using less chlorine
chemicals with higher efficiency as compared with conventional
bleaching processes, and to develop a TCF bleaching process
capable of achieving a final ISO brightness of 84% or more
while greatly shortening the irradiation period at the light
bleaching stage by inserting an acid treatment and a bleaching
stage used in normal TCF bleaching before the light bleaching
stage and an alkaline hydrogen peroxide bleaching stage after
the light bleaching stage.
[0012]
The second invention aims to overcome the drawbacks of the
conventional technology and to provide a high brightness pulp
having a low environmental impact and no discoloration as well
as a paper containing it.
MEANS TO SOLVE THE PROBLEMS
[0013]
As a result of careful studies, we found a chlorine-free
bleaching process with very high efficiency by irradiating a
pulp washed after an acid treatment with UV light and/or
visible light at a wavelength of 100 - 400 nm under alkaline
conditions, preferably in a pH range of 10 - 13, and
accomplished a first aspect of the first invention.
[0014]
We also found a pulp bleaching process with very high
efficiency by irradiating a pulp, that is washed after an acid
treatment, with UV light and/or visible light at a wavelength
CA 02569848 2010-07-16
9 -
of 100 - 400 nm in the presence of ozone, and accomplished a
second aspect of the first invention.
[0015)
We also found a very efficient totally chlorine-free
(TCF) pulp bleaching process wherein an oxygen-delignified
pulp is acid-treated and then bleached by a bleaching
process used in normal TCF bleaching, and the resulting pulp
is further subjected to a light bleaching treatment with UV
light and/or visible light at a wavelength of 100 - 400 nm
under alkaline conditions, followed by alkaline hydrogen
peroxide bleaching (a third aspect of the first invention).
As a result of careful studies to overcome the
drawbacks of conventional technology and to attain a pulp
with such a high brightness and low discoloration, we found
that a pulp having high brightness and no discoloration as
described above and also having high paper strength can be
prepared by further treating a bleached pulp with UV light,
and accomplished the second invention. Accordingly, the
second invention relates to a high brightness chemical pulp
having an ISO brightness of 88% or more and a brightness
loss of 1.0% or less in the following fading test:
a hand-made paper is prepared according to JIS P 8222
and irradiated with a xenon lamp at an intensity of 67 W/m2
for 30 minutes in an atmosphere at 30 C according to fading
test method B of J. TAPPI No.21 Paper and Paperboard (using
a xenon arc lamp light fastness tester) and then the ISO
brightness is measured and a loss from the ISO brightness
before treatment is determined.
Accordingly, in one aspect the present invention
provides a pulp bleacher for a pulp washed after an acid-
treatment, characterized in that air or oxygen or a mixture
thereof is supplied to the surroundings of an irradiation
source of UV light, visible light, or both UV light and
CA 02569848 2010-07-16
- 9a -
visible light to generate ozone and a gas containing the
ozone is fed to a pulp washed after an acid-treatment.
In another aspect, the present invention provides a
totally chlorine-free bleaching process for chemical pulp,
comprising:
treating an oxygen-delignified pulp with acid and
washing the pulp, and then
bleaching the pulp by a bleaching process used in
totally chlorine-free bleaching to give a pulp having an ISO
brightness of 70-75%, and
subjecting the resulting pulp to a light bleaching
treatment with W light, visible light or both UV light and
visible light at a wavelength of 100-400 nm under alkaline
conditions to give a pulp having an ISO brightness of 75-
80%, and then,
bleaching the pulp with alkaline hydrogen peroxide to
give a pulp having an ISO brightness of 84% or more.
CA 02569848 2006-12-07
- 10 -
BRIEF EXPLANATION OF THE DRAWINGS
[0016]
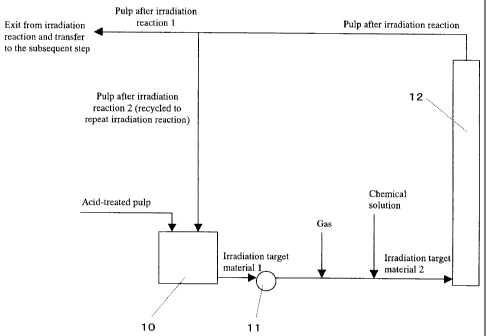
Figure 1 shows an example of a system using an irradiation
reactor in the present invention.
Figure 2 shows an irradiation reactor with an internal
light source used in the present invention.
Figure 3 shows an irradiation reactor with an internal
light source used in the examples of the present invention.
Figure 4 shows the relationship between pH and brightness
in irradiation treatments using a hardwood pulp.
Figure 5 shows the relationship between irradiation period
and brightness in irradiation treatments using a hardwood pulp.
Figure 6 shows the relationship between pH and brightness
in irradiation treatments using a softwood pulp.
Figure 7 shows an example of an experimental apparatus for
UV bleaching test.
REFERENCE NUMERALS
[0017]
10: irradiation target material conditioning tank;
11: irradiation reactor feeding pump;
12: irradiation reactor;
20: irradiation reaction chamber;
21: quartz glass tube;
22: irradiation source;
23a, 23b: three-way valve;
24: diffuser, diffuser tube;
25: pulp slurry inlet;
CA 02569848 2006-12-07
- 11 -
26: pulp slurry outlet;
27: stirrer.
PREFERRED EMBODIMENTS OF THE INVENTION
[0018]
Embodiments of the first invention are explained below.
[0019]
The first and second aspects of the first invention are
applied to pulps washed after an acid treatment. Especially,
they are applied to kraft pulps (KPs) and are well suitable
for not only unbleached KPs but also oxygen-delignified KPs,
ozone-bleached KPs, etc. The bleaching process according to
the third aspect of the first invention is applied to kraft-
cooked and oxygen-delignified chemical pulps. The pulp
materials used in the first invention and the second invention
(chemical pulp) are not specifically limited and include
hardwood and softwood as well as other plants such as kenaf,
flax, rice, bagasse, bamboo, etc. In the pulp bleaching
process according to the second aspect of the first invention,
the oxygen-delignified pulp is acid-treated and then bleached
by a bleaching process used in normal TCF bleaching, and the
resulting pulp is further subjected to an irradiation
treatment with UV light and/or visible light at a wavelength
of 100 - 400 nm under alkaline conditions, followed by
alkaline hydrogen peroxide bleaching.
[0020]
The type of the acid used in the acid treatment of the
present invention may be inorganic or organic. Inorganic
CA 02569848 2006-12-07
12 -
acids that can be used include mineral acids such as sulfuric
acid, hydrochloric acid, nitric acid, sulfurous acid, nitrous
acid, phosphoric acid, and residual acids in chlorine dioxide
generators. Sulfuric acid is preferred. Organic acids that
can be used include acetic acid, lactic acid, oxalic acid,
citric acid, formic acid, etc. The pH during the acid
treatment is in the range of 1.0 - 6.0, preferablyl.0 - 5.0,
more preferably 2.0 - 5.0, most preferably 2.5 - 3.5. If the
pH is less than 1.0, hexenuronic acid or the like and metal
ions are sufficiently removed, but the viscosity greatly
decreases because of excessive acidity. If the pH exceeds 6.0,
however, the acid concentration is so low that hexenuronic
acid or the like and metal ions are insufficiently removed.
Hardwood pulps rich in hexenuronic acid can be acid-treated at
lower temperatures in a pH range of 2.5 - 3.5, which leads to
the advantage that the acid treatment cost can be reduced.
[0021]
The acid treatment can be performed at an atmospheric
pressure or under pressure and at a temperature of 80 C - 180 C,
preferably 80 C - 130 C. Temperatures of 30 C or higher and
lower than 80 C are effective for removing metals but not
effective for removing hexenuronic acid or the like.
Temperatures lower than 100 C are advantageous in terms of
equipment costs because no pressure-tight reaction chamber is
required.
[0022]
The pulp consistency during the acid treatment is in the
range of 0.1 - 50% by weight, preferablyl.0 - 30% by weight,
CA 02569848 2006-12-07
- 13 -
more preferably 2.0 - 20% by weight.
[0023]
The extent to which hexenuronic acid or the like and
harmful metals are removed depends on the pH, reaction
temperature and reaction period during the acid treatment.
Thus, the reaction period is determined as appropriate
depending on the other two conditions, but typically the
reaction period is 1.5 - 6 hours at a reaction temperature of
90 C, the reaction period is 50 minutes - 5 hours at a
reaction temperature of 95 C, the reaction period is 30
minutes - 4.5 hours at a reaction temperature of 100 C, and
the reaction period is 5 - 50 minutes at a reaction
temperature of 120 - 130 C.
[0024]
Ozone bleaching under acidic conditions is also a form of
the acid treatment contemplated by the present invention, and
normal acidic ozone bleaching conditions can be applied. In
this connection, typical acidic ozone bleaching conditions
regarded as suitable include using ozone gas at an ozone
concentration of 1 - 20% by weight at pH 1.0 - 8.0 (the first
and third aspects of the first invention) or pH 1 - 7(the
second aspect of the first invention) at a pulp consistency of
0.1 - 50% by weight and a temperature of 25 - 95 C. The
pressure here is not specifically limited from a negative
pressure state to a pressurized state.
[0025]
A further greater bleaching reaction promoting effect is
obtained in the irradiation treatment by using chelating
CA 02569848 2006-12-07
- 14 -
agents such as EDTA, DPTA in combination with the acid
treatment.
[0026]
Although the reason why the acid treatment promotes the
bleaching effect in the subsequent irradiation treatment with
UV light and/or visible light is not clear, lignin and metal
ions, especially iron ions remaining in pulp form metal
complexes, which become colored by the irradiation treatment.
Thus, it is assumed that the bleaching effect of the
irradiation treatment is improved by removing metal ions by
the acid treatment.
[0027]
In the present invention, the pulp can be dehydrated
and/or washed by using a known dehydrator and/or washing after
the acid treatment including ozone bleaching. Washing can be
performed by using not only fresh water but also wastewater
generated from bleaching processes after the acid treatment or
wastewater generated from papermaking processes.
[0028]
In the present invention, the acid-treated pulp is
irradiated with UV light and/or visible light at a wavelength
of 100 - 400 nm under alkaline conditions, preferably at pH 10
- 13. Especially, hardwood pulps are preferably treated at pH
- 12, and softwood pulps are treated at pH 11 - 13. In the
second aspect of the first invention, the acid-treated pulp is
irradiated with UV light and/or visible light at a wavelength
of 100 - 400 nm under alkaline conditions or acidic conditions.
Preferably, the alkaline conditions are in a pH range of 10 -
CA 02569848 2006-12-07
- 15 -
13 and the acidic conditions are in a pH range of 2 - 4. The
alkalis used for this pH adjustment can be normal alkaline
chemicals, but especially preferred are sodium hydroxide,
potassium hydroxide, sodium silicate and sodium carbonate
because of easy handling.
[0029]
The pulp consistency during the irradiation treatment
according to the present invention is preferably 0.1 - 12% by
weight. Consistencies lower than 0.1% by weight are not
preferred because energy efficiency decreases though bleaching
reaction efficiency increases. Consistencies higher than 12%
by weight are not preferred because the rheology of pulp
slurry in the irradiator decreases so that bleaching reaction
efficiency decreases.
[0030]
The temperature of pulp slurry during this irradiation
treatment is preferably 20 - 95 C, and temperatures lower than
20 C are not preferred because bleaching reaction efficiency
is low while temperatures exceeding 95 C are not preferred,
either, because pulp quality may deteriorate or the pressure
in the reactor may exceed atmospheric pressure so that the
reactor must be designed to resist pressure.
[0031]
The wavelength of the light emitted in the irradiator of
the present invention is preferably 100 - 400 nm, especially
200 - 360 nm (180 - 360 nm in the second aspect of the first
invention). Wavelengths shorter than 100 nm are not preferred
because photodegradation of cellulose is promoted so that pulp
CA 02569848 2006-12-07
- 16 -
strength is greatly lowered, while wavelengths exceeding 400
nm are not preferred, either, because photosensitive coloring
matters are insufficiently photoexcited so that light
bleaching performance is greatly lowered.
[0032]
Irradiation sources that can be used include those
emitting light in a wavelength range of 100 - 400 nm,
specifically xenon short arc lamps, ultra high pressure
mercury lamps, high pressure mercury lamps, low pressure
mercury lamps, deuterium lamps, metal halide lamps, etc., [see
Kinoshita: "UV irradiators", Adhesion (2002, vol. 46, No. 7)
pp. 20 - 27; or Sugimori Akira: "Photochemistry, Chapter 8
Experimental methods of photochemistry I" (Shokabo Publishing
Co., Ltd. 1998) pp. 126 - 136], which can be used alone or by
combining two or more methods.
[0033]
The degree of irradiation to which pulp is exposed in the
irradiation reactor can be adjusted at will by controlling the
retention time of pulp in the irradiation reactor or
controlling the power of the irradiation source. Specifically,
the retention time of pulp in the irradiation reactor can be
controlled by diluting the pulp consistency in the irradiation
reactor with water or by blowing air or an inert gas such as
nitrogen into pulp slurry to control the pulp consistency.
These conditions can be selected as appropriate depending on
the pulp quality goals (brightness, etc.) after the
irradiation reaction.
[0034]
CA 02569848 2006-12-07
- 17 -
The second aspect of the first invention is characterized
in that the irradiation treatment with UV light and/or visible
light at a wavelength of 100 - 400 nm is performed in the
presence of ozone. In the case of irradiation with light in a
wavelength range of 135 - 242 nm, ozone is normally generated
due to the presence of air in the gas layer around the light
source. In the present invention, air is continuously
supplied to the surroundings of the light source while
continuously extracting the generated ozone and injecting it
into the irradiation target material, whereby the ozone can be
used as a reaction promoter without supplying ozone from any
external source. In addition, a larger amount of ozone can be
obtained by supplying air to the gas layer around the light
source. Naturally, the generated ozone can be used not only
as a promoter during the irradiation reaction but also for
regular ozone bleaching. Thus, another great advantage of the
present invention is that the ozone by-produced in the
irradiation reactor can be used. Ozone can also be generated
by supplying such air or oxygen to the pulp to be treated but
not to the surroundings of the light source. Moreover, ozone
obtained from an external source by a method not relying on
such effect of UV light can also be used as a reaction
promoter.
[0035]
The concentration of the ozone by-produced in the
irradiation reactor is 0.5 - 100 ppm, depending on the manner
of supplying air or oxygen or the concentration of oxygen. A
major feature of the second aspect of the first invention is
CA 02569848 2006-12-07
- 18 -
that a high bleaching efficiency can be obtained with even
such low-concentration ozone by combination with the
irradiation treatment.
[0036]
This concept can be further expanded to select and use
multiple light sources having a characteristic wavelength
range within 100 - 400 nm and different characteristic
wavelengths as light sources in the irradiation reactor.
Specifically, a light source having a narrow wavelength
characteristic of 135 - 242 nm providing a high ozone-
generating efficiency and a light source having an evenly
distributed wavelength range of 100 - 400 nm suitable for
irradiation reaction can be combined, for example, whereby a
further higher bleaching efficiency can be obtained.
[0037]
In the irradiation treatment of the present invention, the
irradiation reaction efficiency can be increased by using a
combination of additives such as reducing agents (e.g., NaBH4,
hydrazine, hydrogen), oxidizing agents (e.g., oxygen, ozone),
peroxides (e.g., hydrogen peroxide, peracetic acid, Na
percarbonate, Na perborate), hydrogen-donating organic
compounds (e.g., alcohols; linear amines such as ethyl amine,
diethylamine; cyclic amines such as tetramethyl piperidine),
acetyl-containing organic compounds (e.g., a-acetyl-y-
butyrolactone, acetol, acetone).
[0038]
In the third aspect of the first invention, ozone
bleaching, hydrogen peroxide bleaching and the like can be
CA 02569848 2006-12-07
- 19 -
used alone or in combination as TCF bleaching for attaining an
ISO brightness of 70 - 75% before the light bleaching stage.
[0039]
In the third aspect of the first invention, ozone
bleaching can be performed under normal ozone bleaching
conditions. That is, ozone bleaching conditions may include
using ozone gas at an ozone concentration of 1 - 20% by weight
at pH 1 - 8, a pulp consistency of 0.1 - 50% by weight, and a
temperature of 25 - 95 C. The pressure during ozone bleaching
is not specifically limited from a negative pressure state to
a pressurized state.
[0040]
In the third aspect of the first invention, hydrogen
peroxide bleaching can be performed under normal alkaline
hydrogen peroxide bleaching. That is, alkaline hydrogen
peroxide bleaching can be performed with hydrogen peroxide at
a concentration of 0.1 - 2.0% by weight based on the pulp, pH
11 - 13, a pulp consistency of 0.1 - 50% by weight, and a
temperature of 50 - 95 C.
[0041]
In the third aspect of the first invention, the ISO
brightness has been improved to 70 - 75% in advance by normal
TCF bleaching after the acid treatment, whereby the treatment
period for light bleaching can be greatly shortened, and as a
result, electric power costs required for light bleaching can
be greatly reduced. Moreover, the ISO brightness is improved
by 5% or more to reach 75 - 80% by light bleaching followed by
hydrogen peroxide bleaching at the final bleaching stage,
CA 02569848 2006-12-07
- 20 -
whereby the hydrogen peroxide bleaching reaction is promoted
so that a pulp having an ISO brightness of 84% or more can be
efficiently obtained. In terms of bleaching costs and pulp
quality, it is not preferable to increase the brightness over
75% by TCF bleaching before light bleaching because larger
amounts of chemicals are required under harsher reaction
conditions (high temperature, long period). If the ISO
brightness before light bleaching is higher than 80%, only
very small amounts of coloring components are contained in the
pulp so that the reaction efficiency of light bleaching
significantly deteriorates. Thus, the brightness is
preferably improved by hydrogen peroxide bleaching rather than
light bleaching in terms of bleaching costs. If the
brightness after TCF bleaching is lower than 70%, large
amounts of coloring components are contained in the pulp so
that a long period is required for the light bleaching
treatment to achieve a final brightness of 84% or more, which
invites an increase in electric power costs. If the
brightness after light bleaching is lower than 75%, it is
difficult to achieve a final brightness of 84% or more by
hydrogen peroxide bleaching alone because of relatively large
amounts of hard-to-bleach coloring components remaining in the
pulp.
[0042]
An example of a system using an irradiation reactor in the
present invention is shown in Figure 1.
[0043]
An acid-treated pulp is received in an irradiation target
CA 02569848 2006-12-07
- 21 -
material conditioning tank (10), where it is conditioned to a
temperature, pH and pulp consistency suitable for irradiation
reaction while stirring. The conditioned irradiation target
material 1 is sent to an irradiation reactor (12) via an
irradiation reactor feeding pump (11). Before that, an
additive such as a reducing agent in the irradiation reaction
is added as a chemical solution, if desired. The additive can
be added at the site indicated in Figure 1 or the irradiation
target material conditioning tank (10) or both at will
depending on the properties of the additive or irradiation
reaction conditions, but fast-reacting additives or highly
decomposable additives are preferably added immediately before
the entry into the irradiation reactor (12), i.e. the site
indicated in Figure 1.
[0044]
If desired, a gas can be supplied before the entry into
the irradiation reactor (12). This allows the pulp
consistency in the irradiation reactor (12) to be controlled
(in terms of% by volume here because of the low gas density),
whereby the retention time of the pulp in the irradiation
reactor (12) or the irradiation reaction period can be
controlled at will. The type of the gas used here is
preferably air or an inert gas such as nitrogen, and such gas
is used as fine bubbles dispersed in a pulp slurry. When a
gas such as hydrogen, oxygen or ozone is used among
photoreaction promoters, it can also be supplied to a site
indicated in the figure in the same manner.
[0045]
CA 02569848 2006-12-07
- 22 -
Then, the pulp having reached quality goals and leaving
the irradiation reactor (12) after the irradiation reaction
exits the irradiation reaction and is sent to the subsequent
step (Cl: pulp after irradiation reaction 1). The pulp
falling short of pulp quality goals is recycled to repeat the
irradiation reaction (C2: pulp after irradiation reaction 2).
The ratio of Cl to C2 can be determined at will in response to
pulp quality goals.
[0046]
The irradiation reactor basically consists of an
irradiation source section and a pulp slurry container section,
and the present invention is not specifically limited to
either the internal irradiation type in which the irradiation
source section exists inside the pulp slurry container section
or the external irradiation type in which the irradiation
source section exists outside the pulp slurry container
section [see Sugimori Akira: "Photochemistry, Chapter 8
Experimental methods of photochemistry I" (Shokabo Publishing
Co., Ltd. 1998) pp. 126 - 136]. It is necessary to provide a
barrier against gases such as air normally existing around the
light source section from which light is emitted to a pulp
slurry. In this regard, the choice of the barrier material is
important in order that light energy may permeate through the
barrier without being attenuated.
[0047]
In the present invention, a hard glass barrier can be used
in combination with light at a wavelength longer than e.g.,
300 nm, while a quartz glass barrier is used in combination
CA 02569848 2006-12-07
- 23 -
with light at a wavelength shorter than 254 nm. The materials
of parts of the pulp slurry container not involved in light
transmission reaction can be selected from suitable materials
less sensitive to the light wavelength used.
[0048]
An example of an irradiation reactor is shown in Figure 2.
An acid-treated pulp is conditioned to a temperature, pH and
pulp consistency suitable for irradiation reaction and
optionally combined with additives such as reducing agents if
desired, and then injected as slurry (al) into a reaction
chamber (20) via (25). The injected pulp slurry undergoes
irradiation reaction with light generated from an irradiation
source (22) and having passed through the barrier (21: quartz
glass tube) while it flows within the reactor (20), after
which it is discharged from an outlet (26) of the reactor.
[0049]
If desired, a gas can be supplied via a diffuser (24)
fitted to the irradiation reactor (20). This allows the pulp
consistency in the irradiation reactor (20) to be controlled
(in terms of % by volume because of the low gas density),
whereby the retention time of the pulp in the irradiation
reactor (20) or the irradiation reaction period can be
controlled at will. The type of the gas used here is
preferably air or an inert gas such as nitrogen, and such gas
is used as fine bubbles dispersed in a pulp slurry.
[0050]
When a gas such as hydrogen, oxygen or ozone is used among
photoreaction promoters, it can also be supplied via this
= CA 02569848 2006-12-07
- 24 -
diffuser (24).
[0051]
When light at a wavelength range of 135 - 242 nm is used
as an'irradiation source and air or oxygen is injected as a
light source cooling gas (bl) as shown in Figure 2, ozone
exists in the gas discharged from the irradiation section.
This discharged gas containing ozone can be injected into a
pulp slurry in the irradiation reactor (20) via diffuser (24),
whereby the ozone can be used as a reaction promoter without
supplying ozone from any external source. The generated ozone
can be used not only as an irradiation reaction promoter but
also for normal ozone bleaching. It can also be used in
combination with a gas effective as an irradiation reaction
promoter such as hydrogen, oxygen or ozone injected from an
external source. The use of these gases can be selected at
will by providing a three-way valve (23a, 23b).
[0052]
The irradiation reactor can be fitted at will with
accessory equipments such as temperature/pH controller, gas
concentration detector, etc., if desired.
[0053]
The irradiation treatment of the present invention can be
repeated one or more times in any manner that can be selected
as appropriate depending on a situation such as bleaching
efficiency, pulp quality goals (brightness), or the
relationship with other bleaching processes used in
combination. Examples of manners of repeating the irradiation
treatment one or more times are as follows. (1) Two or more
CA 02569848 2006-12-07
- 25 -
irradiators shown in Figure 1 can be provided. In this case,
they can be arranged in a series or in parallel. (2) Multiple
irradiation sources (which may have identical or different
characteristics) can be. placed in the irradiator shown in
Figure 1. (3) Pulp can be recycled in the system shown in
Figure 1.
[0054]
The bleaching process of the present invention can be
combined with any other known chlorine or chlorine-free
bleaching process. Specifically, another bleaching process
can be followed by the bleaching process of the present
invention, or the bleaching process of the present invention
can be followed by another bleaching process. Especially,
bleaching of the present invention is preferably followed by a
hydrogen peroxide treatment. These sequences can be repeated
multiple times, and a washing stage can be inserted between
different bleaching processes. The bleaching sequence
incorporating an irradiation system can also be repeated
multiple times. When the irradiation treatment is performed
multiple times, the irradiation treatment is preferably
followed by washing.
[0055]
Embodiments of the second invention are explained below.
The present invention relates to a high brightness
chemical pulp having an ISO brightness of 88% or more and a
brightness loss of 1.0% or less in the fading test described
below. That is, our studies revealed that the discoloration
of paper evaluated by the fading test using UV light as
CA 02569848 2006-12-07
- 26 -
described below shows a good correlation with the actual
discoloration, rather than the conventional thermal fading
test.
[0056]
A hand-made paper is prepared according to JIS P 8222 and
irradiated with a xenon lamp at an intensity of 67 W/m2 for 30
minutes in an atmosphere at 30 C according to fading test
method B of J. TAPPI No.21 Paper and Paperboard (using a xenon
arc lamp light fastness tester) and then the ISO brightness is
measured and a loss from the ISO brightness before treatment
is determined. The chemical pulp used in the present
invention can be obtained by using known cooking processes
such as kraft cooking, polysulfide cooking, sodium hydroxide
cooking or alkaline sulfite cooking, among which kraft cooking
is preferred in terms of pulp quality and energy efficiency.
Kraft cooking processes include known modified cooking
processes such as MCC, EMCC, ITC, and Lo-solids processes, any
of which can be applied to the present invention without
limitation. Wood can be kraft-cooked under known conditions,
for e.g., under conditions described below. The sulfidity of
the cooking liquor is 7 - 75%, preferably 15 - 45%, the
effective alkali content is 5 - 30% by weight, preferably 10 -
25% by weight on the basis of the bone dry weight of wood, and
the cooking temperature is 140 - 170 C. The cooking process
may be in a continuous mode or a batch mode, and the type of
cooker is not specifically limited.
[0057]
The chemical pulp used in the present invention is
CA 02569848 2006-12-07
- 27 -
obtained by washing an unbleached chemical pulp obtained by a
known cooking process, passing it through coarse screening and
fine screening steps and then subjecting it to an oxygen
delignification treatment. The oxygen delignification can be
performed under known conditions. In the case of hardwood
chemical pulps, the kappa number after oxygen delignification
is typically in the range of 5 - 15, preferably 7 - 15, more
preferably 8 - 12. This oxygen delignification treatment is
performed by a known, medium consistency process or high
consistency process. For example, typical reaction conditions
for the medium consistency process include a pulp consistency
of 10 - 18% by weight, a temperature of 100 - 110 C, a
reaction period of 60 - 120 minutes, and a pressure in the
reactor of 3 - 6 kg/m2, and the sodium hydroxide content and
the oxygen content are adjusted depending on the target kappa
number.
[0058]
In the preparation of the high brightness chemical pulp of
the present invention, it is preferable to use a pulp having
undergone oxygen delignification followed by an acid treatment.
The type of the acid used in the acid treatment of the pulp
may be inorganic or organic. Inorganic acids that can be used
include mineral acids such as sulfuric acid, hydrochloric acid,
nitric acid, sulfurous acid, nitrous acid, phosphoric acid,
and residual acids in chlorine dioxide generators. Sulfuric
acid is preferred. Organic acids that can be used include
acetic acid, lactic acid, oxalic acid, citric acid, formic
acid, etc. Hardwood pulps are desirably acid-treated in a pH
CA 02569848 2006-12-07
- 28 -
range of 1.5 - 6.0, preferably 1.0 - 5.0, more preferably 2.0
- 5.0, most preferably 2.5 - 3.5. If the pH is less than 1.0,
hexenuronic acid and metal ions are sufficiently removed, but
the pulp viscosity greatly decreases because of excessive
acidity. If the pH exceeds 6.0, however, the acid
concentration is so low that hexenuronic acid and metal ions
are insufficiently removed. Hardwood chemical pulps can be
acid-treated at lower temperatures in a pH range of 2.5 - 3.5,
which leads to the advantage that the acid treatment cost can
be reduced.
[0059]
The acid treatment can be performed at an atmospheric
pressure or under pressure. For example, the reaction
temperature during the acid treatment at an atmospheric
pressure is in the range of 80 C or higher and lower than 100 C.
Preferably, it is 80 - 95 C, more preferably 80 - 90 C.
Temperatures of 30 C or higher and lower than 80 C are
effective for removing metals but not effective for removing
hexenuronic acid.
[0060]
After the acid treatment, the pulp is continuously
bleached by a multistage bleaching process. Chemicals used
include those consisting of known bleaching agents such as
atomic chlorine (C), sodium hydroxide (E), hypochlorites (H),
chlorine dioxide (D), oxygen (0), hydrogen peroxide (P), ozone
(Z), sulfuric acid (A), and organic peracids in combination
with bleaching additives, and any combination appropriately
selected from the above list is used as bleaching chemicals.
CA 02569848 2006-12-07
- 29 -
The bleaching sequence is not specifically limited, and
examples that can be used include sequences including atomic
chlorine and chlorine bleaching chemicals such as C/D-E/O-H-D;
ECF bleaching sequences free from atomic chlorine such as D-E-
D, Z-E/O-D; and TCF bleaching sequences totally free from
chlorine chemicals such as Z-E-P, A-Z-E/O-P.
[0061]
The high brightness chemical pulp of the present invention
is preferably prepared by a process further comprising
irradiating the bleached chemical pulp obtained by the process
described above with UV light and/or visible light. The
bleached chemical pulp before light treatment preferably has
been bleached to an ISO brightness of 80% or more, preferably
86% or more. For example, very high brightness pulp can be
easily obtained by introducing a peroxide bleaching stage
after the light treatment stage.
[0062]
The irradiation treatment with UV light and/or visible
light is preferably performed under alkaline conditions. The
alkaline conditions are preferably in a pH range of 10 - 13.
The alkalis that can be used for this pH adjustment include
normal alkaline chemicals, among which sodium hydroxide is
preferred. The acids that can be used for the pH adjustment
include normal acidic chemicals, among which sulfuric acid is
preferred.
[0063]
The pulp consistency during the irradiation treatment with
UV light and/or visible light is preferably 0.1 - 12% by
CA 02569848 2006-12-07
- 30 -
weight. Consistencies lower than 0.1% by weight are not
preferred because energy efficiency decreases though bleaching
reaction efficiency increases. Consistencies higher than 12%
by weight are not preferred because the rheology of pulp
slurry in the bleacher decreases so that bleaching reaction
efficiency decreases.
[0064]
The temperature during the irradiation treatment with UV
light and/or visible light is not specifically limited, either,
but preferably 20 - 95 C. Temperatures lower than 20 C are not
preferred because bleaching reaction efficiency is low while
temperatures exceeding 95 C are not preferred, either, because
pulp quality may deteriorate or the pressure in the reactor
may exceed atmospheric pressure so that the reactor must be
designed to resist pressure.
[0065]
The irradiation period of UV light and/or visible light
can be determined as appropriate by taking into account the
structures or concentrations of potential coloring matters
contained in the material pulp.
[0066]
The UV light and/or visible light used in the present
invention is not specifically limited, but it is desirable to
use UV light and/or visible light at a wavelength of about 100
- 400 nm, preferably 200 - 360 nm. UV light at a wavelength
shorter than 100 nm is not preferred because photodegradation
of cellulose is promoted so that pulp strength and brightness
are greatly lowered, while UV light at a wavelength exceeding
CA 02569848 2006-12-07
- 31 -
400 nm is not preferred, either, because coloring matters are
insufficiently photoexcited so that light bleaching
performance is greatly lowered.
[0067]
Irradiation sources that can be used include conventional
light sources such as low-pressure mercury lamps, high-
pressure mercury lamps, and xenon lamps as well as various
excimer lamps and various lasers, but when a large amount of
pulp is to be treated, it is desirable to use a high power
ozone-generating low-pressure mercury lamp. Ozone-generating
UV lamps mainly emit UV light at a wavelength of 254 nm and
also include UV light at a wavelength of 185 nm and visible
light. The irradiation intensity of UV light at a wavelength
of 185 nm is not influenced by temperature, but the intensity
of UV light at a wavelength of 254 nm is temperature-dependent
and reaches its maximum at 20 - 40 C. Thus, high power ozone-
generating lamps having a high surface temperature are cooled
with air, and at the same time, ozone gas is generated from
oxygen in the air by UV light at a wavelength of 185 nm. In
the case of wastewater treatment, this ozone gas decomposes by
UV light at a wavelength of 254 nm to produce a highly active
oxygen species which remarkably promotes decomposition of
coloring components. In contrast to wastewater treatment in
which the treatment efficiency improves as the ozone
concentration increases, excessive ozone not only blocks UV
light at a wavelength of 254 nm, which is the most effective
UV light for pulp bleaching, to invite bleaching efficiency
loss but also accelerates damage to cellulose fibers by a lot
CA 02569848 2006-12-07
- 32 -
of active oxygen species generated from high concentration
ozone to invite significant paper strength loss. Thus, an
optimum amount of ozone should be supplied, and such an amount
is controlled as appropriate depending on the structures or
amounts of coloring matters in the pulp.
[0068]
In the present invention, all of the known reducing agents,
oxidizing agents and hydrogen-donating organic compounds can
be used as light bleaching promoters. Such reducing agents
include, for example, hydrosulfite and borohydride compounds,
etc.; oxidizing agents include hydrogen peroxide, sodium
percarbonate, peracetic acid, etc.; and hydrogen-donating
organic compounds include primary alcohols such as ethanol.
Additives in the present invention may be used alone without
using solvents, but should desirably be used as dispersions or
solutions in solvents transparent to UV/visible light.
Different additives can also be used as mixtures. Such
solvents include water, alcohols, linear or cyclic alkanes,
ethers, etc. as single solvents or mixed solvents thereof,
preferably water. The amount of each additive to be used is
not specifically limited so far as it is at or below the
saturated concentration of the additive in the solvent, but a
suitable amount is preferably 0.01 - 40% by weight, more
preferably 0.1 - 20% by weight in the solvent.
[0069]
Although the reason why the high brightness chemical pulp
obtained by the present invention shows very little
discoloration is not clear, it is assumed that discoloration
1 CA 02569848 2006-12-07
- 33 -
is not induced by relatively weak UV light emitted from the
lamp used in the fading test because materials responsible for
coloration involved in discoloration remaining in the pulp
have been preliminarily decomposed by a very intense UV light
at 254 nm and removed.
[0070]
Papers containing the high brightness chemical pulp of the
present invention can be used as not only book papers but also
offset printing papers, relief printing papers, gravure
printing papers, newsprint papers, electrophotographic papers,
or base papers for coated papers, inkjet recording papers,
thermosensitive recording papers, pressure sensitive recording
papers or the like.
[0071]
In addition to the high brightness chemical pulp of the
present invention, papers containing the high brightness
chemical pulp of the present invention may use other raw pulps
such as chemical pulps, mechanical pulps and deinked pulps
alone or in admixture at any ratio. The pH during the
papermaking process may be acidic or neutral or alkaline.
[0072]
Papers containing the high brightness pulp of the present
invention can contain paper strength enhancers. Examples of
paper strength enhancers include starches, modified starches,
polyacrylamide, polyvinyl alcohol, polyamide-polyamine resins,
urea-formalin resins, melamine-formalin resins, polyethylene
imines, etc. The paper strength enhancers are preferably
contained in an amount of 0.1% by weight or more and 2% by
CA 02569848 2006-12-07
- 34 -
weight or less on the basis of the bone dry weight of the pulp.
[0073]
Papers containing the high brightness pulp of the present
invention can contain fillers. Fillers that can be used
include known fillers such as white carbon, talc, kaolin, clay,
ground calcium carbonate, precipitated calcium carbonate,
titanium oxide, synthetic resin fillers, etc.
[0074]
Papers containing the high brightness pulp of the present
invention can further contain aluminum sulfate, sizing agents,
yield improvers, freeness improvers, colorants, dyes,
antifoaming agents, bulking agents, fluorescent whitening
agents or the like, if desired.
[0075]
Papers containing the high brightness pulp of the present
invention may not be coated or may be coated with a pigment-
free finishing agent. Non-coated papers are desirably coated
with a finishing agent based on a water-soluble polymer for
the purpose of improving surface strength or sizing
performance. Suitable water-soluble polymers include commonly
used finishing agents such as starches, modified starches,
polyacrylamide, polyvinyl alcohol, etc. alone or as mixtures
thereof. In addition to the water-soluble polymers, the
finishing agents can also contain paper strength enhancers
designed to improve water resistance or surface strength and
external sizing additives designed to provide sizing
performance. The finishing agents can be applied with coaters
such as two-roll size press coaters, gate roll coaters, blade
CA 02569848 2010-02-16
- 35 -
metering coaters, rod metering coaters, etc. The finishing
agents are preferably applied in an amount of 0.1 g/m2 or more
and 3 g/m2 or less per side.
EXAMPLES
[0076]
The following examples further illustrate the present
invention in detail without, however, limiting the invention
thereto.
<Determination of physical properties of pulp>
Determination of kappa number: performed according to JIS
P 8211.
[0077]
Determination of pulp brightness: Pulp was defibrated and
then formed into a sheet having a basis weight of 60 g/m2
according to Tappi test method T205os-71 (JIS P 8222), and
measured for pulp brightness according to JIS P 8148.
<Experimental apparatus>
The experimental apparatus used in the examples below is
shown in Figure 3.
[0078]
An irradiation reaction chamber (20) consists of a 3L glass
cylinder (100 mm f x 620 mm H). This irradiation reaction
chamber (20) is equipped with a temperature controller and a pH
meter in addition to a stirrer (27) and a diffuser tube (24)
shown in the figure. An irradiation source (16W low-pressure
mercury lamp, AY-1 from Photoscience Japan Corporation) is
placed in a quartz glass tube (45 mm f x 470 mm H, thickness 2
CA 02569848 2006-12-07
- 36 -
mm) in such a manner that air can be injected around the
irradiation source.
[0079]
[Example 1]
An oxygen-delignified hardwood kraft pulp available from
Nippon Paper Group, Inc. (kappa number 11.6, ISO brightness
45.6%) was used.
[0080]
An acid treatment was performed under the following
conditions to give a pulp having a kappa number of 5.5 and a
brightness of 47.5%.
[0081]
Acid treatment conditions: pulp consistency 10% by weight,
pH 3.0 (adjusted with sulfuric acid), temperature 95 C,
treatment period 180 minutes. After the treatment was
completed, the pulp was washed with water.
[0082]
A 5 g (bone dry weight) portion of the acid-treated pulp
thus obtained was collected and diluted to a pulp consistency
of 0.25% by weight and then prepared into pulp slurries at pHs
over an acidic to alkaline range using NaOH and H2SO4. These
slurries were injected into the experimental apparatus shown
in Figure 3, and subjected to an irradiation reaction while
stirring under conditions of a temperature of 25 C for a
treatment period of 120 minutes using a low-pressure UV lamp
having a dominant wavelength at 254 nm. After the reaction
was completed, the pulps were washed and then formed into
sheets and measured for brightness. The results are shown in
CA 02569848 2006-12-07
- 37 -
Figure 4 and Table 1.
[0083]
[Example 2]
The same oxygen-delignified hardwood kraft pulp as used in
Example 1 was treated with ozone under the following
conditions to give a pulp having a kappa number of 3.0 and a
brightness of 56.6%.
[0084]
Ozone treatment conditions: pulp consistency 10%, ozone
feed 7 kg/ADTP, temperature 50 C, treatment period 30 seconds,
pH 2.5 (adjusted with sulfuric acid).
[0085]
A 5 g (bone dry weight) portion of the ozone-treated pulp
thus obtained was collected and prepared into pulp slurries at
pHs over an acidic to alkaline range and subjected to an
irradiation reaction under similar conditions to those of
Example 1, and the resulting pulps were measured for
brightness. The results are shown in Figure 4 and Table 1.
[0086]
[Comparative example 1]
A 5 g (bone dry weight) portion of the same oxygen-
delignified hardwood kraft pulp as used in Example 1 was
collected and prepared into pulp slurries at pHs over an
acidic to alkaline range and subjected to an irradiation
reaction under similar conditions to those of Example 1, and
the resulting pulps were measured for brightness. The results
are shown in Figure 4 and Table 1.
CA 02569848 2006-12-07
- 38 -
[0087]
[Table 1]
Table 1 ISO brightness (%)
pH Example 1 Example 2 Comparative example 1
2.84 60.24 67.84
4.69 57.95 71.41
7.77 58.75 69.26
9.48 60.3 74.67 55.58
10.43 66.15 77.41 58.83
11.75 71.19 82.36 61.85
12.61 67.7 77.83 62.9
[0088]
[Example 3]
A 5 g (bone dry weight) portion of the same acid-treated
pulp as used in Example 1 was collected and diluted to a pulp
consistency of 0.25% by weight and then prepared into a pulp
slurry at pH 11.5 (adjusted with NaOH and H2SO4). This slurry
was injected into the experimental apparatus shown in Figure 3,
and subjected to an irradiation reaction while stirring under
conditions of a temperature of 25 C for varying treatment
periods using a low-pressure UV lamp having a dominant
wavelength at 254 nm. After the reaction was completed, the
pulps were washed and then formed into sheets and measured for
brightness. The results are shown in Figure 5 and Table 2.
[0089]
[Example 4]
CA 02569848 2006-12-07
39 -
A 5 g (bone dry weight) portion of the same ozone-treated
pulp as used in Example 2 was collected and subjected to an
irradiation reaction under similar conditions to those of
Example 3 for varying treatment periods and measured for
brightness. The results are shown in Figure 5 and Table 2.
[0090]
[Comparative example 2]
A 5 g (bone dry weight) portion of the same oxygen-
delignified hardwood kraft pulp as used in Comparative example
1 was collected and subjected to an irradiation reaction under
similar conditions to those of Example 3 for varying treatment
periods and measured for brightness. The results are shown in
Figure 5 and Table 2.
[0091]
[Table 2]
Table 2 ISO brightness (%)
UV
irradiation Example 3 Example 4 Comparative example 2
period (h)
0 47.5 56.57 47.5
0.5 55.57 70.97
1 62.87 79.05
2 71.19 82.36 61.85
4 78.59 85.5 66.75
8 85.69 85.86 79.59
[0092]
[Example 5]
An oxygen-delignified softwood kraft pulp available from
CA 02569848 2006-12-07
- 40 -
Nippon Paper Group, Inc. (kappa number 9.1, ISO brightness
33.3%) was used.
[0093]
An acid treatment was performed under the following
conditions to give a pulp having a kappa number of 9.1 and a
brightness of 34.3%.
[0094]
Acid treatment conditions: pulp consistency 10% by weight,
pH 3.0 (adjusted with sulfuric acid), temperature 95 C,
treatment period 180 minutes. After the treatment was
completed, the pulp was washed with water.
[0095]
A 5 g (bone dry weight) portion of the acid-treated pulp
thus obtained was collected and diluted to a pulp consistency
of 0.25% by weight and then prepared into pulp slurries at pHs
over an acidic to alkaline range using NaOH and H2SO4. These
slurries were injected into the experimental apparatus shown
in Figure 3, and subjected to an irradiation reaction while
stirring under conditions of a temperature of 25 C for a
treatment period of 120 minutes using a low-pressure UV lamp
having a dominant wavelength at 254 nm. After the reaction
was completed, the pulps were washed and then formed into
sheets and measured for brightness. The results are shown in
Figure 5 and Table 3.
[0096]
[Comparative example 3]
A 5 g (bone dry weight) portion of the same oxygen-
delignified softwood kraft pulp as used in Example 5 was
CA 02569848 2006-12-07
- 41 -
collected and prepared into pulp slurries at pHs over an
acidic to alkaline range and subjected to an irradiation
reaction under similar conditions to those of Example 5, and
the resulting pulps were measured for brightness. The results
are shown in Figure 6 and Table 3.
[0097]
[Table 3]
Table 3 ISO brightness (%)
pH Example 5 Comparative example 3
10.43 44.52 41.1
11.75 54.89 48.02
12.61 57.24 47.56
13.1 54.31 48.23
[0098]
[Example 6]
An oxygen-delignified hardwood kraft pulp available from
Nippon Paper Group, Inc. (kappa number 9.5, ISO brightness
47.5%) was used.
[0099]
An acid treatment was performed under the following
conditions to give a pulp having a kappa number of 5.5 and a
brightness of 48.6%.
[0100]
Acid treatment conditions: pulp consistency 10% by weight,
pH 3 (adjusted with sulfuric acid), temperature 85 C,
treatment period 180 minutes. After the treatment was
completed, the pulp was washed with water.
CA 02569848 2006-12-07
- 42 -
[0101]
A 5 g (bone dry weight) portion of the acid-treated pulp
thus obtained was collected and diluted to a pulp consistency
of 0.5% by weight and then prepared into a pulp slurry at pH
11.5 with NaOH. This slurry was injected into the
experimental apparatus shown in Figure 3, and subjected to an
irradiation reaction while stirring under conditions of a
temperature of 25 C for a treatment period of 120 minutes
using a low-pressure UV lamp having a dominant wavelength at
254 nm. After the reaction was completed, the pulp was washed
and then formed into a sheet and measured for brightness. The
results are shown in Table 4.
[0102]
[Comparative example 4]
A 15 g (bone dry weight) portion of the same acid-treated
pulp as used in Example 6 was collected and diluted to a pulp
consistency of 0.5% by weight and then injected into the
experimental apparatus shown in Figure 3, and subjected to an
ozone treatment while stirring at a temperature of 25 C, pH
11.5 for 120 minutes (without using an irradiation source).
The accumulated ozone feed to the pulp during the time was
0.7% by weight. The results are shown in Table 4.
[0103]
[Example 7]
A 15 g (bone dry weight) portion of the same acid-treated
pulp as used in Example 6 was collected and diluted to a pulp
consistency of 0.5% by weight and then injected into the
experimental apparatus shown in Figure 3, and subjected
CA 02569848 2006-12-07
- 43 -
simultaneously to an ozone treatment and an irradiation
reaction while stirring at a temperature of 25 C, pH 11.5 for
120 minutes using a low-pressure UV lamp having a dominant
wavelength at 254 nm. The accumulated ozone feed to the pulp
during the time was 0.7% by weight. The results are shown in
Table 4.
[0104]
[Example 8]
The same oxygen-delignified hardwood kraft pulp as used in
Example 1 (kappa number 11.6, ISO brightness 45.6%) was
treated with ozone under the following conditions to give a
pulp having a kappa number of 3.0 and a brightness of 56.6%.
[0105]
Ozone treatment conditions: pulp consistency 10%, ozone
feed 7 kg/ADTP, temperature 55 C, treatment period 30 seconds,
pH 2.5.
[0106]
A 15g (bone dry weight) portion of the ozone-treated pulp
thus obtained was collected and diluted to a pulp consistency
of 0.5% by weight and then injected into the experimental
apparatus shown in Figure 3, and subjected to an irradiation
reaction while stirring at a temperature of 25 C, pH 11.5 for
120 minutes using a low-pressure UV lamp having a dominant
wavelength at 254 nm. The results are shown in Table 4.
[0107]
CA 02569848 2006-12-07
- 44 -
[Table 4]
ISO
Treatment brightness Gain
Pulp method after after
treatment treatment
After acid Irradiation
Example 6 treatment treatment 60.4% 11.8%
with heating alone
Comparative After acid Ozone
example 4 treatment treatment 51.7% 3.1%
with heating alone
Combination
After acid of ozone
Example 7 treatment treatment and 72.2% 23.6%
with heating irradiation
treatment
After ozone Irradiation
Example 8 treatment treatment 74.7% 18.1%
[0108]
<Evaluation of physical properties of pulp>
- Determination of kappa number: performed according to
JIS P 8211.
- Determination of pulp brightness: Pulp was defibrated
and then formed into a sheet having a basis weight of 60 g/m2
according to JIS P 8222, and measured for pulp brightness
according to JIS P 8148.
<Experimental apparatus>
The experimental apparatus used in the examples below is
shown in Figure 3. An irradiation reaction chamber (20)
consists of a 4L glass cylinder (100 mm f x 620 mm H). This
irradiation reaction chamber (20) is equipped with a
temperature controller and a pH meter in addition to a stirrer
CA 02569848 2006-12-07
- 45 -
(27) and a diffuser tube (24) shown in the figure. An
irradiation source (16W low-pressure mercury lamp, AY-1 from
Photoscience Japan Corporation) is placed in a quartz glass
tube (25 mm f x 470 mm H, thickness 2 mm) in such a manner
that air can be injected around the irradiation source. In
the examples below, two such irradiation sources were used.
[Example 9]
An oxygen-delignified hardwood kraft pulp available from
Nippon Paper Group, Inc. (kappa number 11.6, ISO brightness
45.6%) was acid-treated at a pulp consistency of 10% by weight,
pH 3.0 (adjusted with sulfuric acid), at a temperature of 95 C
for a treatment period of 180 minutes. After the acid
treatment was completed, the pulp was washed with water to
give a pulp having a kappa number of 5.5 and a brightness of
47.5%.
[0109]
A 15 g (bone dry weight) portion of the acid-treated pulp
was collected and prepared into a pulp slurry having a
consistency of 0.5% by weight with water, and then the pulp
slurry was adjusted to pH 11.5 with NaOH. This pulp slurry
was injected into the experimental apparatus shown in Figure 3,
and subjected to an irradiation reaction while stirring under
conditions of a temperature of 25 C for a treatment period of
120 minutes using a low-pressure UV lamp having a dominant
wavelength at 254 nm. Simultaneously, the air injected into
the quartz glass tube shown in Figure 3 to cool the light
source was discharged via the diffuser and ozone was fed.
After the treatment was completed, the pulp was washed and
CA 02569848 2006-12-07
- 46 -
then formed into a sheet and measured for brightness. The
results are summarized in Table 5.
[Example 10]
Irradiation treatment and ozone feeding were performed
under similar conditions to those of Example 9 except that the
pulp slurry was adjusted to pH 2.9 with sulfuric acid, after
which the resulting pulp was measured for brightness. The
results are summarized in Table 5.
[Example 11]
Irradiation treatment and ozone feeding were performed
under similar conditions to those of Example 1 except that the
pulp slurry was treated at pH 6.6 without pH adjustment, after
which the resulting pulp was measured for brightness. The
results are summarized in Table 5.
[Comparative example 5]
Irradiation treatment was performed under similar
conditions to those of Example 9 except that ozone feeding was
omitted (i.e., air was not injected into the quartz glass tube
shown in Figure 3 but directly introduced into the diffuser to
feed the pulp slurry), after which the resulting pulp was
measured for brightness. The results are summarized in Table
5.
[Comparative example 6]
Reaction was performed under similar conditions to those
of Example 9 except that irradiation treatment was omitted
(i.e., the light source was not turned on) and the ozone
(concentration 50 ppm) generated from an ozone generator
(ozone spray, NS-3 available from Kankyo Kogaku Co., Ltd.) was
CA 02569848 2006-12-07
- 47 -
fed via the diffuser shown in Figure 3, after which the
resulting pulp was measured for brightness. The results are
summarized in Table 5.
(0110]
[Table 5]
Irradiation Ozone feeding pH Brightness (%)
Example 9 Yes Yes 11.5 73.8
Example 10 Yes Yes 2.9 70.9
Example 11 Yes Yes 6.6 63.3
Comparative Yes No 11.5 60.4
example 5
Comparative No Yes 11.5 53.3
example 6
[0111]
As shown in Table 5, pulps with higher brightness can be
prepared in Examples 9 - 11 involving irradiation with UV
light or visible light or both in the presence of ozone.
However, brightness was somewhat lower in Example 11 wherein
irradiation was not performed under acidic conditions of pH 2
- 4 or under alkaline conditions of pH 10 - 13.
<Determination of ISO brightness of pulp>
Determination of pulp brightness: Pulp was defibrated and
then formed into a sheet having a basis weight of 60 g/m2
according to JIP P 8222, and measured for ISO brightness
according to JIS P 8148.
<Pulp>
An oxygen-delignified hardwood kraft pulp (ISO brightness
45.6%, available from Nippon Paper Group, Inc.) was further
CA 02569848 2006-12-07
- 48 -
subjected to acid treatment - ozone bleaching under the
following conditions and the resulting pulp was used in the
examples and comparative examples below.
- Acid treatment: The oxygen-delignified hardwood kraft
pulp was acid-treated at a pulp consistency of 10% by weight,
pH 3.0 (adjusted with sulfuric acid), at a temperature of 95 C
for a treatment period of 180 minutes. After the treatment
was completed, the pulp was washed with water. At this stage,
the ISO brightness of the pulp was 47.5%.
- Ozone bleaching: The acid-treated pulp was bleached with
ozone at a pulp consistency of 10%, ozone feed 7 kg/ (lt of
air-dried pulp), at a temperature of 50 C for a treatment
period of 30 seconds at pH 2.5 (adjusted with sulfuric acid).
After the treatment was completed, the pulp was washed with
water. At this stage, the ISO brightness of the pulp was
59.7%.
[Example 12]
The ozone-bleached pulp was further bleached in a
bleaching sequence of hydrogen peroxide bleaching 1 - light
bleaching - hydrogen peroxide bleaching 2 under the following
conditions.
- Hydrogen peroxide bleaching 1: pulp consistency 10% by
weight, pH 11.5 (adjusted with sodium hydroxide), temperature
75 C, treatment period 90 minutes. After the treatment was
completed, the pulp was washed with water. At this stage, the
ISO brightness of the pulp was 75.0%.
- Light bleaching conditions: A 5 g (bone dry weight)
portion of the pulp bleached with hydrogen peroxide was
CA 02569848 2006-12-07
- 49 -
collected and diluted to a pulp consistency of 0.25% by weight
and then prepared into a pulp slurry at pH 11.5 with sodium
hydroxide. This slurry was injected into a 2L glass cylinder,
and subjected to an irradiation reaction while stirring at a
temperature of 25 C for a treatment period of 15 minutes using
a 16W low-pressure UV lamp having a dominant wavelength at 254
nm (AY-1 from Photoscience Japan Corporation). After the
treatment was completed, the pulp was washed with water. At
this stage, the ISO brightness of the pulp was 78.5%.
Hydrogen peroxide bleaching 2: The light bleached pulp
was treated under the same conditions as those of hydrogen
peroxide bleaching 1 above. The final ISO brightness was
85.0%.
[Example 13]
Bleaching treatment was performed under the same
conditions as those of Example 1 except that the treatment
period of light bleaching was 30 minutes. The ISO brightness
after light bleaching was 80.0%. The resulting light bleached
pulp was treated under the conditions of hydrogen peroxide
bleaching 2 above. The final ISO brightness was 86.1%.
[Example 14]
Treatment was performed under the same conditions as those
of Example 1 except that the treatment period of hydrogen
peroxide bleaching 1 was 45 minutes. The ISO brightness after
hydrogen peroxide bleaching 1 was 71.0%. The ISO brightness
after light bleaching was 74.5%. The resulting pulp was
treated under the same conditions as those of hydrogen
peroxide bleaching 2 above. The final ISO brightness was
CA 02569848 2006-12-07
- 50 -
84.1%.
[Example 15]
The ozone-bleached pulp was further bleached in a
bleaching sequence of light bleaching - hydrogen peroxide
bleaching. Light bleaching was performed under the same
conditions as those of Example 12 except that the treatment
period was 60 minutes. The brightness after light bleaching
was 75.4%. The resulting pulp was treated under the same
conditions as those of hydrogen peroxide bleaching 2 of
Example 12. The final ISO brightness was 84.3%.
[Example 16]
Treatment was performed under the same conditions as those
of Example 4 except that the treatment period of light
bleaching was 120 minutes. The ISO brightness after light
bleaching was 81.8%. The resulting pulp was treated under the
same conditions as those of hydrogen peroxide bleaching 2 of
Example 12. The final ISO brightness was 85.2%.
[Example 17]
Treatment was performed under the same conditions as those
of Example 12 except that the treatment period of hydrogen
peroxide bleaching 1 was 30 minutes. The ISO brightness after
hydrogen peroxide bleaching 1 was 68.2%. The ISO brightness
after light bleaching was 72.3%. The resulting pulp was
treated under the same conditions as those of hydrogen
peroxide bleaching 2 of Example 12. The final ISO brightness
was 81.7%.
[Comparative example 7]
The ozone-bleached pulp was further bleached in a
CA 02569848 2006-12-07
- 51 -
bleaching sequence of hydrogen peroxide bleaching 1 - hydrogen
peroxide bleaching 2. Bleaching treatment was performed under
the same conditions as those of Example 12 except that light
bleaching was omitted. The final ISO brightness was 79.3%.
[Comparative example 8]
The ozone-bleached pulp was further bleached in a
bleaching sequence of hydrogen peroxide bleaching 1 - light
bleaching. Light bleaching was performed under the same
conditions as those of Example 12 except that the treatment
period was 60 minutes. Hydrogen peroxide bleaching 1 was
performed under the same conditions as those of Example 12.
The final ISO brightness was 83.3%.
[Comparative example 9]
The ozone-bleached pulp was further bleached in a
bleaching sequence of hydrogen peroxide bleaching 1 - light
bleaching - hydrogen peroxide bleaching 2. Bleaching
treatment was performed under the same conditions as those of
Example 1 except that light bleaching was performed at pH 4.0
(adjusted with sulfuric acid). The brightness after light
bleaching was 75.9%. The resulting pulp was treated under the
same conditions as those of hydrogen peroxide bleaching 2 of
Example 12. The final ISO brightness was 82.6%.
[0112]
The results of Examples 12 - 17 and Comparative examples 7
- 9 are shown in Table 6.
CA 02569848 2006-12-07
- 52 -
[0113]
[Table 6]
ISO brightness ISO brightness
(M ) after ISO brightness
M after ( % ) after
hydrogen hydrogen
peroxide light
bleaching bleaching 1 blperoxide
eaching 2
Example 12 75.0 78.5 85.0
Example 13 75.0 80.0 86.1
Example 14 71.0 74.5 84.1
Example 15 - 75.4 84.3
Example 16 - 81.8 85.2
Example 17 68.2 72.3 81.7
Comparative 75.0 - 79.3
example 7
Comparative 75.0 83.3 -
example 8
Comparative
example 9 75.0 75.9 82.6
[0114]
As shown in Table 6, high brightness pulps were obtained
by treating the ozone-bleached pulp in a bleaching sequence of
hydrogen peroxide bleaching 1 - light bleaching - hydrogen
peroxide bleaching 2. However, the final brightness was
somewhat low in Example 6 wherein a pulp having an ISO
brightness of less than 70% before the light bleaching
treatment. In the sequence of Comparative example 2 wherein
hydrogen peroxide bleaching was omitted after light bleaching,
the light treatment period had to be greatly extended to
obtain an ISO brightness of 80% or more. In Comparative
CA 02569848 2006-12-07
- 53 -
example 9 wherein the pH after light bleaching was acidic,
both brightness after light bleaching and final brightness
were lower than those of Example 12.
[Example 18]
A 200 g (bone dry weight) portion of a bleached hardwood
pulp (ISO brightness 85.6%) obtained by the chlorine bleaching
process of plant A of Nippon Paper Group, Inc. was collected
and diluted to a pulp consistency of 1% and then adjusted to
pH 11.5 with sodium hydroxide. This slurry was injected into
the experimental apparatus shown in Figure 7, and subjected to
a UV light bleaching treatment while stirring at a temperature
of 25 C for a treatment period of 120 minutes. When the
treatment was completed, the pulp was washed and then formed
into a sheet and measured for brightness. The sheet measured
for brightness was then used in a fading test. A sheet was
prepared from the pulp after defibration and measured for
breaking length. The methods for these evaluations are
described below, and the results are shown in Table 1.
- Determination of freeness: A pulp slurry having a
consistency of 10% was treated at 6000 rev in a PFI mill and
then measured for freeness (CSF) according to JIS P 8121.
- Determination of pulp brightness: Pulp was defibrated
and then formed into a sheet having a basis weight of 60 g/m2
according to JIP P 8222, and measured for ISO brightness
according to JIS P 8148.
- Determination of breaking length: Pulp was defibrated
and then formed into a sheet having a basis weight of 60 g/m2
according to JIP P 8222, and measured for breaking length
CA 02569848 2006-12-07
- 54 -
according to JIS P 8113.
- Fading test: performed with a xenon lamp weatherometer.
Samples were irradiated with UV light generated from a xenon
lamp for 30 minutes and then measured for ISO brightness (JIS
P 8148). The fading test was performed at a temperature of
30 C and an intensity of 67 W/m2. In Table 1, brightness and
brightness loss are defined as follows:
0 Brightness = ISO brightness after fading test - ISO
brightness before fading test.
Brightness loss = L brightness / ISO brightness before
fading test.
- UV light bleaching experimental apparatus: The
experimental apparatus used in the example is shown in Figure
7. An ozone-generating low-pressure UV lamp (95 W, 18 mm (f)
x 1100 mm (H), SUV110D from Sen Light Corporation) is fixed at
the center of a UV light irradiation reaction chamber in the
form of a glass cylinder of 72.1 nm (f) x 1180 mm (H)
(effective capacity 2.64 L), and the ozone gas generated (540
mg/h) is introduced from the bottom of the reaction chamber
and flows upward with pulp slurry in the reaction chamber.
After light bleaching, the pulp slurry can be recycled to the
reaction chamber by a pump via a stock tank (capacity 30 L).
[Example 191
A 200 g (bone dry weight) portion of a bleached hardwood
pulp (ISO brightness 84.9%) obtained by the ozone ECF
bleaching process [acid treatment (oxygen-delignified pulp
consistency 10% by weight, pH 3 (adjusted with sulfuric acid),
temperature 85 C, treatment period 180 minutes), ozone
CA 02569848 2006-12-07
- 55 -
treatment (pulp consistency 10% by weight, pH 2.5 (adjusted
with sulfuric acid), ozone feed 7 kg/ It of air-dried pulp,
temperature 55 C, treatment period 30 seconds)] of plant B of
Nippon Paper Group, Inc. was collected and diluted to a pulp
consistency of 1% and then adjusted to pH 11.5 with sodium
hydroxide. This slurry was injected into the experimental
apparatus shown in Figure 7, and subjected to a UV light
bleaching treatment while stirring at a temperature of 25 C
for a treatment period of 120 minutes. When the treatment was
completed, the pulp was washed and then formed into a sheet
and measured for brightness. The sheet measured for
brightness was then used in the fading test. A sheet was
prepared from the pulp after defibration and measured for
breaking length. The results are shown in Table 7.
[Example 20]
A 200 g (bone dry weight) portion of a bleached hardwood
pulp (ISO brightness 84.3%) obtained by the ECF bleaching
process (chlorine dioxide treatment - hydrogen peroxide
treatment - chlorine dioxide treatment) of plant C of Nippon
Paper Group, Inc. was collected and diluted to a pulp
consistency of 1% and then adjusted to pH 11.5 with sodium
hydroxide. This slurry was injected into the experimental
apparatus shown in Figure 7, and subjected to a UV light
bleaching treatment while stirring at a temperature of 25 C
for a treatment period of 120 minutes. When the treatment was
completed, the pulp was washed and then formed into a sheet
and measured for brightness. The sheet measured for
brightness was then used in the fading test. A sheet was
CA 02569848 2006-12-07
- 56 -
prepared from the pulp after defibration and measured for
breaking length. The results are shown in Table 7.
[Comparative example 10]
A 100 g (bone dry weight) portion of a bleached hardwood
pulp (ISO brightness 86%) obtained by the chlorine bleaching
process of plant A of Nippon Paper Group, Inc. was collected
and diluted to a pulp consistency of 10% and then adjusted to
pH 11.5 with sodium hydroxide. This slurry was bleached with
3.0 kg of hydrogen peroxide / it of air-dried pulp at a
temperature of 50 C for a treatment period of 180 minutes.
When the treatment was completed, the pulp was washed and then
formed into a sheet and measured for brightness. The sheet
measured for brightness was then used in the fading test. A
sheet was prepared from the pulp after defibration and
measured for breaking length. The results are shown in
Table 7.
[Comparative example 11]
A bleached hardwood pulp (ISO brightness 89.3%) obtained
by a commercially available chlorine dioxide ECF bleaching
process was used to form a sheet and measured for brightness.
The sheet measured for brightness was then used in the fading
test. A sheet was prepared from the pulp after defibration
and measured for breaking length. The results are shown in
Table 7.
[Comparative example 12]
A bleached hardwood pulp (ISO brightness 85.6%) obtained
by the chlorine bleaching process of plant A of Nippon Paper
Group, Inc. was used to form a sheet and measured for
CA 02569848 2006-12-07
- 57 -
brightness. The sheet measured for brightness was then used
in the fading test. A sheet was prepared from the pulp after
defibration and measured for breaking length. The results are
shown in Table 7.
[Comparative example 13]
A bleached hardwood pulp (ISO brightness 84.9%) obtained
by the chlorine bleaching process of plant B of Nippon Paper
Group, Inc. was used to form a sheet and measured for
brightness. The sheet measured for brightness was then used
in the fading test. A sheet was prepared from the pulp after
defibration and measured for breaking length. The results are
shown in Table 7.
CA 02569848 2006-12-07
- 58 -
ri 4-) m %O 0 N 00 co
eN
fd m co a% .q to
r-=I in in in to to in to
4) O -ri
-P 4J
4-4 M
4 N N N N Ul)
co co
w A" M M M M M M M
U) LH
U 4)
U)
U)
O
in N co 0 O 0
O
U) 0\0 Ln .o to N O- ~O eM
010
b) r1 O O O c, N M M
r1
U)
U)
a)
C".
- - LO .O %D 01 l0 r I O'
r. dp
O O O N N M N
FI
dp
= r I ' 0 d' 0 in L- Ln O
UI 44 b O O co co 1O 1O N
co 00 co 00 co
~H 4J ON co
4-I
a,
O rl U1 in 0 %O IV M ~O a%
' .
4-4
0 (D b 41 4) c~
rn Do co 0000 co 00 co
U) M 44
N 00 O) 0 > 0 'J rl > N > M
r-I r=I N =ri* r=I =r=I r-I =r=I '-I =r=I '-I
^ N 4-) 4-+ 4-J 4J
LO r-I 4) Q) Q) R) Q) b 4) co a) (d 4)
r-I 4 r-I r-{ r-I {-I r-I $4 ri $=I r-I P H
(0
a a a ba Ada Ida Ida
x k O) O x O k O
W W W U 4) U a) U 0 U 0