Note: Descriptions are shown in the official language in which they were submitted.
CA 02571689 2007-O1-11
r
AN ADSORPTIVE-FILTRATION MEDIA FOR THE CAPTURE
OF WATERBORNE OR AIRBORNE CONSTITUENTS
This application is a divisional application of co-pending application Serial
No.
2,429,510, filed November 14, 2001.
BA-CKGROUND OF INVENTION
Technical Field
The present invention relates to the removal of water borne metal contaminants
from
water. In particular, the invention relates to a filter media which removes
metal contaminants
from water passed through the filter media.
Background Art
An area of increasing concern in the environmental sciences and engineering is
the
treatment of metals such as Cd, Cu, Zn, Ni, Pb, and Cr, which become water
borne and are
carried by rain water run-off and the like to environmentally sensitive areas.
As used herein,
metals being "water borne" means being transported by water in any manner,
whether the
metal is actually in solution, suspended in water through a particulate bond
or a colloidal
bond, or simply physically carried by the velocity of flowing water. One of
the most
common manners in which metals become water borne is through entrainment with
storm
water run off from road surfaces. The above metals are typically deposited on
the road
surface though vehicle exhaust, fluid leakage, vehicular wear, pavement
degradation and
pavement maintenance. Subsequent rainfall entrains the metals and transports
the metals to
the area in which storm water run-off accumulates. Typically, 60% to 80% of
these metals
are dissolved in the run-off water, while the remaining percentage is
suspended by other
mechanisms such as those mentioned above.
It is desirable to intercept the runoff and remove the metals prior to
allowing the water
to continue to its natural drainage areas. One method of removing the water
borne metals is
to pass the water through a sorbent filter media. One of the most common media
for
removing particulate bound metals from water is sand. However sand has very
little capacity
for removal of dissolved metals and therefore, is generally not considered
effective in
removing dissolved metals. Granular activated carbon (GAC) has long used as a
media for
removing dissolved metals. However, GAC has relatively little absorptive
capacity and thus,
absorbed metals must frequently be removed or the GAC "recharged." Also, GAC
has very
little compressive strength. Any application which
CA 02571689 2007-O1-11
places a load on the GAC material may cause crushing and zgreatly reduce
absorptive capacity
of the GAC.
A much more recently developed sorbent media is iron oxide coated sand (IOCS).
IOCS
is formed by coating silica sand with a thin layer of iron oxide and it has
been shown to be an
effective sorbent media for metals. Iron oxides and hydroxides possess little
or no permanent
surface charge, but will take on a positive or negative surface charge in the
presence of protons or
hydroxyl ions. In other words, depending on the pH of the solution in which
the iron oxide is
place, the iron oxide may take on a net positive or negative charge. A
substance which exhibits
a net positive or negative charge depending on the pH level may be referred to
as an "amphoteric"
substance.
Iron oxide typically has a neutral charge in a pH range of approximately 7 to
8. When the
pH rises above approximately 8, the iron oxide becomes more negatively
charged. Thus, positively
charged metal ions borne by water passing over the negatively charged iron
oxide will tend to bond
to the iron oxide and be sorbed from the water. Conversely, if the pH falls
below approximately
7, the iron oxide becomes positively charged and is less likely to bond with
metal ions. The pH
at which the net surface charge of a particle is zero is denominated the point
of zero charge or
"pzc".
One major disadvantage of IOCS is that the oxide coating is not sufficiently
durable. The
comparatively smooth surface of sand particles tends to result in the oxide
coating flaking off.
Attempts to avoid this flaking have led to time consuming sand preparation
efforts such as
cleaning the sand of organics and applying a scratch surface to the sand
before applying the oxide
coating. However, even with these preparation efforts, IOCS still exhibits
considerable flaking
and thus a lack of oxide coating durability.
The smooth surface of sand is also disadvantageous from the standpoint of
providing a
comparatively low specific surface area (SSA). The specific surface area of a
material is generally
defined as the surface area per unit mass with the typical unit being m2/gm.
As used herein,
specific surface area means the total area on the surface of the material in
addition to any available
porous internal surface area (such as found the GAC discussed above). The
greater the surface
area of the substrate, the greater the surface area of oxide coating which
will be exposed to water
borne metals. Thus, it is desirable to provide a substrate with as great of an
SSA as possible
considering other design restraints. The SSA of sand is typically about 0.05
to about 0.10 mz/gm.
CA 02571689 2007-O1-11
Another problem found in IOCS is the tendency of the oxide coating to
crystallize. When
the coating crystallizes, the crystals set up a uniform lattice which does not
maximize the surface
area of the coating. The surface area of the coating is much more optimal if
the oxide molecules
are randomly distributed in a non-lattice or "amorphous" fashion. For example,
the SSA of IOCS
may reach 85 m2/gm if a method of sufficiently inhibiting crystallization
could be provided.
However, a purely crystallized oxide coating may have a SSA as low as 5 m'/gm
(or even lower).
What is needed in the art is a manner to reliably inhibit crystallization in
IOCS. Even more
desirable would be a substrate other than sand which has a higher SSA than
sand and a superior
tendency to inhibit crystallization. It would also be desirable to provide
substrates which could
simultaneously act as a filter and provide other functions, such as providing
a roadway pavement
or parking pavement. Another desirable characteristic of a substrate (such as
porous concrete)
would be providing pH elevation to the fluid stream being treated.
OBJECTS OF THE INVENTION
One embodiment of the present invention is a sorptive-filtration media for the
capture of
waterborne or airborne constituents. The media comprising a granular substrate
and an amphoteric
compound bonded to the substrate in the presence of a crystal inhibiting
agent.
Another embodiment of the present invention includes a sorptive-filtration
media which
comprises a substrate having a specific gravity of less than 1.0 and an
amphoteric compound
bonded to the substrate.
Another embodiment is a pavement material for the capture of waterborne
constituents.
The pavement material comprises a porous pavement substrate and an amphoteric
compound
bonded to the substrate.
Another embodiment includes a process for producing a sorptive-filtration
media for the
capture of waterborne or airborne constituents. The process comprises the
steps of providing a
substrate with a specific surface area of greater than 0.1 mz/gm, introducing
the substrate to an
amphoteric metal solution, and drying the substrate.
Another embodiment includes a sorptive-filtration media which comprises a
substrate with
a specific surface area of greater than 0.1 m2/gm and an amphoteric compound
bonded to the
substrate.
A further embodiment includes a storm water storage basin capable of
supporting velucular
traffic. The basin comprise a layer of porous pavement having a hydraulic
conductivity of more
than 0.0001 cm/sec. The layer of porous pavement is at least 3 inches in
depth, and the layer has
CA 02571689 2007-O1-11
a length and a width wherein the ratio between the length and the width is
less than 20. In the
instance where the pavement is composed of a series of pavement sections, each
section would
have a length to width ratio of less than 20.
Another' embodiment includes a method for producing a porous, cementitious
material.
The method includes the steps of providing and thoroughly mixing cement and
aggregate, mixing
water with the cement and aggregate into a slung while maintaining a water to
cement ratio of less
than one, initiating curing of said slurry under pressure and in the presence
of steam, and
continuing the curing at ambient temperature and pressure until the
cementitious material is
substantially dry.
Another embodiment is a roadway with a gravel shoulder for the removal of
waterborne
ionic constituents. The roadway comprises a pavement section and a gravel
shoulder section
adjacent the pavement section. The gavel shoulder has a depth of at least 3
inches and includes
gravel coated with an amphoteric compound.
Another embodiment includes a method of constructing a sub-base for the
removal of
waterborne constituents. The method includes the steps of placing a layer of
uncompacted sub-
base material; distributing upon the layer a solution containing an amphoteric
compound; and
compacting the layer to a selected density.
Another embodiment is a sorptive-filtration media for the capture of
waterborne or
airborne constituents. The media comprises a flexible, planar, porous
substrate; and an amphoteric
compound bonded to said substrate.
Another embodiment is a drainage pipe capable of capturing waterborne
constituents. The
drainage pipe comprise a length of pipe having an interior surface, at least a
portion of the surface
being designed to be in contact with water. An amphoteric compound is then
applied to the
portion of the surface designed to be in contact with water.
Another embodiment of the invention includes a process for creating a
filtering media for
the capture of waterborne or airborne constituents. The process comprises the
steps of providing
a filter substrate; applying a first coating of an iron oxide compound (or
alternatively amorphous
silica) to the substrate; and applying a second coating of a manganese oxide
compound to the
substrate.
Another embodiment of the invention includes a roadway with a shoulder forming
a filter
for constituents. The roadway comprises a roadway pavement section and a
cementitious, porous,
shoulder adjacent the pavement section and the shoulder having an amphoteric
compound applied
CA 02571689 2007-O1-11
thereto.
Another embodiment provides an absorptive-filtration media having a porous
structure of
a fixed matrix and a porosity of approximately 0.05 to 0.6 and an amphoteric
compound.
Another embodiment provides a sorptive-filtration media having a granular
substrate and
an amphoteric compound formed of a manganese oxide formed on the substrate.
Another embodiment includes a method for forming a porous pavement roadway.
This
method includes the steps of providing and thoroughly mixing cement and
aggegate; mi ding water
with the cement and aggregate forming it into a slurry while maintaining a
water to cement ratio
of less than one; and placing the slurry into a roadway bed.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a diagram of an upflow filter.
Figure 2 is a chart of surface charge versus pH for certain amphoteric
compounds.
Figure 3 is a chart of aggregate distribution.
Figure 4 is a cross-section of a roadway.
BEST MODE FOR CARRYING OUT THE INVENTION
The filtering media of the present invention generally comprises a sorptive-
filtration media.
Absorption is a volumetric phenomena which relates to an intraphase solute
distribution such as
incorporating a heavy metal solute into a solid phase. Adsorption relates to
an interface solute
accumulation, for example incorporating a heavy metal solute onto a surface
such as a
heterogeneous hydrated amphoteric material. The term "sorptive" may be used to
describe both
absorption and adsorption. The media rnay also have a filtration
characteristic which relies on the
purely physical capture of contaminants which are larger than the void spaces
in the media.
In one general embodiment, the invention includes a granular substrate with an
amphoteric
compound bonded thereto in the presence of a crystal inhibiting agent. The
granular substrate
could be sand or any other granular substrate such as crushed limestone,
crushed concrete, or other
granular substances. In another general embodiment, the present invention
includes all substrates
having a specific surface area of 0.1 mz/gm or greater and having an
amphoteric compound bonded
to the substrate. In these latter embodiments with a substrate having an SSA
of greater than 0.1
m2/gm, the substrate could include a wide variety of materials such as precast
cementitious porous
pavement (CPP) discussed herein (SSA of S-10 m2/gm), wood chips, recycled
concrete chips,
recycled concrete pavement rubble, construction debris and building
rubble/debris natural
aggregates, synthetic aggregates, polymeric compounds, granular activated
carbon (SSA of 600-
CA 02571689 2007-O1-11
6
1200 m2/gm) etc.
1. Iron Oxide Coated Media.
The amphoteric compound of the present invention is intended to include any
compound
having amphoteric properties. Preferred embodiments of amphoteric compounds
include oxides
of iron, manganese, or aluminum.
In regards to iron oxide compounds, there are at least 13 iron oxide minerals,
of which
there are 8 major iron oxides. These iron oxides differ in composition, the
valence state of Fe and
in crystalline structure. However all iron oxides contain Fe and O or OH.
Table 1 summarizes the
major iron oxides with selected characteristics.
Table 1 Selected properties and attributes of major iron oxide minerals.
Mineral Formula Structural DensitySSA Color
Name system (g/cm3)(mz/g)
Hematite a-Fe~03 Trigonal 5.26 20-30 blood red
Maghemite y-Fez03 Cubic or tetragonal4.87 80-130 chocolate
Magnetite Fe30a Cubic 5.18 --4 black
Goethite a.-Fe00H Orthorhombic 4.26 20-40 mustard
Lepidocrocitey-Fe00H Orthorhombic 4.09 70-80 orange-
brown
Ferrihydrite'SFez03 9H20Trigonal 3.96 180-300 deep brown
'
Feroxyhyte 8'-Fe00H Hexagonal 4.20 190-210 brown
Akaganeite ~3-Fe00H Tetragonal 3.56 ~30 dark mustard
': ferrihydrite
& feroxyhyte
have the
only amorphous
or poorly-crystalline
structures
low SSA precipitation
from Fe(N03)39H20 with
hydrolysis, KOH
high SSA
from Fe3+
2: other
formulas
include
: Fe5H0g
4H20 and
Feb(04H3)3
point of H 7-8
zero charge
(pzc) for
all minerals
shown is
between
p
a : hexagonal
close packed
(more stable
than y)
~i : goethite
polymorph
in presence
of high
Cl- levels
y : cubic
close packed
8~ : poorly-ordered
ferromagnetic
form of
Fe00H
CA 02571689 2007-O1-11
7
From Table 1 it can be seen that the more amorphous ferrihydrite or feroxyhyte
are the
forms of iron oxide with the highest SSA. If these forms are coated onto
silica sand (or any
surface), their higher SSA, as compared to say the more crystalline hematite,
will create a more
preferable sorbent media. For this reason, a preferred embodiment of the
amphoteric compound
focuses on the use of these forms, specifically ferrihydrite. Those skilled in
the art will understand
that ferrihydrite is not produced in isolation, but is typically formed in a
solution having various
other iron oxide compounds. The ferrihydrite may transform into other, more
crystalline iron
oxide compounds (such as hematite or goethite) depending on factors such as
temperature; pH, and
whether the iron source is ferric or ferrous ions. To inhibit such
transformation to the more
crystalline compounds, inhibiting agents such as silica (Si02), silica fume or
silica gel,
microporous silica, inorganic compounds such as phosphates, polymeric
compounds whether
naturally occurnng (e.g. natural organic matter in soil) or synthetic (e.g.
polyethylene), sodium
hydroxide, oils, grease, or any other substance which inhibits
crystallization, may be introduced
in the process for synthesizing ferrihydrite or applying the iron oxide
coating. Where sand is the
substrate, a highly acidic compound, such as ferric nitrate or ferric chloride
(used to form the
amphoteric compound as described below) may soluablize silica off the sand
substrate, thereby
producing an inhibiting agent. Because so many substances may act as
inhibiting agents, it is
possible that certain impurities in the materials selected (such as grease or
oil in a sand substrate)
can be engineered to act as a sufficient inhibiting agent without the addition
of further inhibiting
agents. Careful selection of substrates with crystal inhibiting impurities is
one manner of carrying
out the present invention.
Two known methods for producing ferrihydrite follow. The first method involves
preheating 2000 mL of DI water to 75 °C in an oven and then withdrawing
the water and adding
20 g of unhydrolyzed crystals of Fe(N03)3~9H20. The solution is stirred
rapidly and reheated at
75 °C for 14 to 12 minutes. The formation of iron hydroxy polymers will
change the solution from
a dull gold color to dark reddish brown. The solution is then dialyzed for
three days to produce
approximately S g of ferrihydrite. This procedure produces a ferrihydrite of
lower SSA, in the
range of 180 to 200 m2/g.
A second method involves dissolution of 40 g of Fe(N03)3 9H20 in 500 mL of DI
water
and addition of approximately 330 mL of 1M KOH until the pH is 7 to 8 while
stirring the
solution. This procedure produces a ferrihydrite of higher SSA, in the range
of 200 to 300 m2/g.
The solution is then centrifuged and dialyzed to produce approximately 10 g of
ferrihydrite. While
CA 02571689 2007-O1-11
both of these procedures work well for a small mass of ferrihydrite (i.e. 10
g) in a laboratory
environment, they are not easily adapted to be economically feasible at
production or field scale
levels that require tons of such a coating. Rather, the above methods would
require design and
construction of a plant-sized process to produce multiple tons of
ferrihydrite.
The present invention includes another, more economical method for producing
sufficient
quantities of ferrihydrite. In this method, the source of ferric ions is
either Fe(N03)3~9H20, (ferric
nitrate (FN)) or FeCl3, (ferric chloride (FC)). Both FN and FC are available
as reagent-grade salts
or available commercially in larger quantities as bulk solutions. FC has the
additional advantage
of being more economical and being a by-product of pickling waste. When FN or
FC are dissolved
in potable water to produce an approximately 1M to approximately 3M solution,
the resulting iron
oxides in the solution will typically be approximately 50% ferrihydrite and
50% other iron oxides.
2. Anpl n~ngIron Oxide to a Substrate.
One substrate to which may be coated with the amphoteric compound may be
adhered is
sand. Sand typically has a comparatively low SSA of about 0.05 to about 0.10
m2/gm. Moreover,
this low SSA is indicative of a relatively smooth surface to which iron oxide
coatings will have
difficulty adhering. As discussed above, without some agent to inhibit
crystallization of the iron
oxide coating, the SSA may remain in the range of I to 5 mz/gm. To produce a
sand substrate
filtration media with a markedly improved SSA (at least 5, but more commonly
about 10-20
m2/gm or higher), sand was subject to a multi-step process as seen in the
following two examples.
In the first example, the sand was first cleaned and tumbled in acidic
solution (of a pH <
2), rinsed with DI water, and then cleaned and tumbled in a very dilute basic
solution before a final
rinse is made. Second, to promote bonding, an initial scratch coat applied by
immersing the sand
in an approximately 1M FN solution. The sand was then heat at about 100
degrees C until this
coating was dry and then the sand was disaggregated and rinsed in DI water to
remove any loose
coating. After this rinsing, the sand was reheated until dry and then cooled.
Third, the sand was
immersed in another solution of 1.6 M FN. In this solution, 1,000 ppm SiOz was
added (in the
range of 1% of the aqueous volume) to help inhibit the transformation of
ferrihydrite to hematite
or possibly to goethite. Fourth, the sand was again dried with drying times
minimized in order not
to promote the transformation to hematite due to dehydration. However, drying
of the sand at high
temperatures could also lead to thermal transformation of ferrihydrite to
hematite. It was
determined that drying could take place at an acceptably fast rate at
100°C if an inhibitor such as
Si02 was used to prevent crystalline bonds from forming. Once drying was
complete, the sand was
CA 02571689 2007-O1-11
y
allowed to cool and the coated media was disaggregated. As a final step, the
media was pH
conditioned to a neutral pH by passing DI water at a pH of 8 to 9 (raised with
NaOH or a similar
base) through the media until the pH of the effluent was between 7.5 and 8,
above the point of zero
charge for iron oxides. This also removed any loose iron coating. It is noted
that the above
mentioned scratch coating is necessary because the granular substrate was sand
which has a
relatively smooth surface. However, other granular substrates such as crushed
limestone have a
sufficiently rough surface that a scratch coat is not required. Additionally,
while it is preferred
to maintain the drying temperature at or below about 100 °C, higher
drying temperatures of below
about 200 °C or even below about 300 °C may be used,
particularly if there is a sufficiently
effective crystal inhibiting agent present.
The second example is provided by a large-scale field production. The above
method is
scaled up by using a larger gasoline-powered concrete mixer and a gas-fired
heater. A 3.0 M ferric
chloride (FC) solution containing 1000ppm silica solution was prepared in
sufficient volume such
that the sand could be completely immersed. Thereafter, heat was applied via
the gas-fired heater
to evaporate the liquid and attach the iron to the sand surface. Typically
greater efforts must be
made to insure dryness of the FC treated sand as opposed to the FN treated
sand since FC is
significantly more hydroscopic than FN. This method proved feasible to produce
the required 9
tons of OCS necessary for a related experiment.
For each batch, approximately 90 pounds of filter sand was placed in the
concrete mixer
with an excess of ferric chloride solution. The amount of ferric chloride
solution put into the
mixture was enough to just cover the filter sand. The mixture was stirred
vigorously and heat
applied by the a gas-fired heater. The gas-fired heater was directed into the
mouth of the concrete
mixer. The slurry was continuously stirred by the concrete mixture until the
sand was completely
dry. Typical drying time for each batch was 3 hours.
Once dry, the sand was poured from the concrete mixer into a backhoe bucket
and placed
in a tandem dump truck for cooling. In preparation for pH neutralization,
complete drying of the
sand was essential to ensure the iron coating would not be removed by the
sodium hydroxide in
the pH neutralization process. If the sand is not completely dry, the iron
coating washes off easily
when put into the NaOH solution.
Since the sand was placed in a tandem dump truck for cooling, it decided to
neutralize the
entire truckload at once to reduced handling of the OCS. The dump truck full
of OCS was parked
facing down a slope and a solution (of approximately 10 lbs. of NaOH per 55
gallons of potable
CA 02571689 2007-O1-11
water) was poured into the truck bed on top of the OCS. The idea was to create
a bathtub effect
to neutralize the sand. The truck bed did leak but the level of the solution
was kept above the
depth of the sand with continual addition of NaOH solution. Leakage of the
truck bed proved
beneficial due to the continual addition of new solution to replace loss. The
new solution was
more capable of neutralizing the OCS while the used solution was removed from
the system. The
pH was checked with a pH probe at several depths in the truck bed to ensure
complete
neutralization. Approximately 10 tons of OCS was produced, the largest known
quantity of such
material. In the above process, the inhibiting agents were formed by the
impurities found in the
mixer, the gas-fired heater, NaOH and the construction process in the field to
such a degree that
it was not necessary to add additional silica as an inhibiting agent.
3. Substrates with a specific avity less than I.O.
There are a large number of likely substrates having an specific gravity of
less than 1Ø
One family of such substrates is wood, with pine having by way of example a
specific gravity of
about 0.35. Another family of such substrates are polymeric compounds.
Polymeric compounds
may include light weight materials such as foam packing pellets, which would
form a granular
media having a specific gravity of approximately 0.2. Polymeric compounds
could also include
heavier polymers having a specific gravity of up to 0.97. Polymeric compounds
could also include
polymer-type materials which have similar weight, flexibility, and long
molecular chains. Of the
polymer family, it has been found that polyethylene (PE) or polypropylene (PP)
have many
characteristics making them suitable substrates for the present invention. PE
and PP have a
specific gravity of about 0.9. It is believed PE, PP, and other similar
polymeric compounds are
particularly useful when in the form of polymeric floating media filter beads.
Typically, the
diameter of the beads will be about 2mm to 3mm. However, bead diameters of may
range from
about Imm to about l Omm or even larger. Nor is this size range limited to
polymer beads, but is
appropriate for many other types of granular substrates disclosed herein.
Normally, polymer beads
will have a specific gravity ranging between approximately 0.50 and 0.95. One
simple example
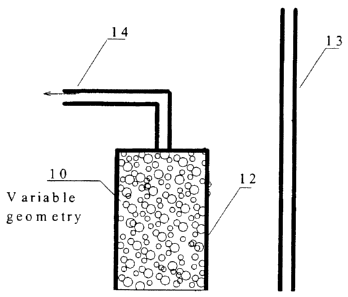
of a "filter" or "clarifier" using floating polyethylene beads can be seen in
Figure I. 1n the
embodiment of Figure l, the filter is a cylindrical geometry upflow filter,
but the filter could utilize
many geometries and flow directions depending on constraints such as media
type, coating,
specific gravity and design intentions. Filters using floating polyethylene
beads are usually upflow
filters such as seen in Figure 1, but can be downflow filters and have a
variety of geometric shapes.
In Figure l, the upflow filter 10 is filled with floating polymeric beads 12.
An influent flow I3
CA 02571689 2007-O1-11
l~
flows into filter 10, through beads 12 (where it has pollution constituents
adsorbed and~filtered),
and exits as effluent 14. While not explicitly shown in Figure 1, the upflow
filter 10 could utilize
any number of methods well known in the art for backwashing the beads. Upflow
filters have the
advantages of being easily backwashed to prevent clogging and are less likely
to hydraulically
"short-circuited" (i.e. water cutting an uninterrupted fluid path through the
beads and not having
to flow around the individual beads). It has been found that allowing a layer
of sediment to form
at the base of the filter media may actually enhance filtration as long as the
layer does not become
so thick that the layer significantly inhibits design flows. The filter media
would be backwashed
at the point design flows were significantly inhibited. It is also very
practical to direct water
through an upflow or downflow filter when the water is being drained from a
elevated grade (such
as a highway overpass or an elevated interstate).
One preferred method of applying the amphoteric compound to the polyethylene
is similar
to that used to apply iron oxide to sand and is as follows. A 1 to 3 molar
solution of FN or FC
(preferably about 1.6M) is prepared by dissolving the FC or FN in water. The
polyethylene beads
are placed in the solution and continuously stirred. The polyethylene should
remain in the solution
a sufficient time for the entire surface area of the polyethylene to become
coated with iron oxide.
An hour should be sufficient period of time under most circumstances. The
water is then
evaporated from the solution containing the polyethylene at a temperature of
approximately 90°
- 95 ° C. The drying may take place at lower temperatures, but will
unnecessarily slow the drying
process. Drying at higher temperatures is possible, but may be undesirable
from the standpoint
of the polyethylene becoming excessively plastic at temperatures above 95
° C and crystallization
of the iron oxide becoming more prevalent at higher temperatures.
One favorable characteristic of employing polyethylene as a substrate is that
polyethylene
has an inherent tendency to inhibit the crystallization of the iron oxide.
This is believed to occur
by way of polyethylene molecules detaching from the substrate surface and
becoming lodged in
the iron oxide molecules depositing on the substrate surface. As alluded to
above, this disruption
of a uniform iron oxide lattice tends to create a favorable, amorphous (thus
high specific surface
area) coating of iron oxide. In addition to taking advantage of the natural
crystallization inhibiting
character of polyethylene, when using an iron oxide as the amphoteric
compound, it also desirable
to further add an inhibitor such as the 1000 ppm SiOz solution discussed
above. The amount of
Si02 solution may vary, but an amount equal to I% of the aqueous volume is
normally considered
sufficient. If manganese oxide is the amphoteric compound, it usually is not
necessary to add an
CA 02571689 2007-O1-11
12
inhibiting agent to achieve an acceptable SSA. Significantly, it has been
found that polyethylene
beads having a specific gravity of about 0.9 maintain a specific gravity of
less than 1 (and therefore
float) even after being coated. The coating generally raises the bead's
specific gravity to about
0.95.
While the above procedure described applying an amphoteric compound to
polyethylene
beads, it will be understood that the procedure could be carried out numerous
other polymeric
materials. For example, an amphoteric compound could be applied to simple
packing material,
cheap polymeric woven and non-woven material, geosynthetics and expanded foams
as well. The
foams have to be dried at a lower temperature so they do not melt, so for the
case of expanded
foams or heat sensitive polymerics, manganese coatings are preferable to iron
coatings (which
require higher temperatures to dry).
4. Manganese Oxide Coated Media.
As mentioned above, another family of amphoteric compounds are oxides formed
from
manganese. There are a whole series of manganese oxide minerals that can be
produced that have
useful characteristics as media coatings for the treatment of storm water and
other waste streams
containing heavy metals. However, two manganese oxides groups comprise
preferred embodiments
for use with the present invention because their combination of negative
surface charge (measured
as units of charge per surface area) at nearly all environmental pH values and
because of their high
specific surface area. This results in a coated media surface with a high
surface density of negatively
charged sites for adsorption of heavy metals. These two manganese oxides are
bimessite (whose
structure is not completely understood, but is believed to be in part a
layered (Mn06) structure and
cryptomelane, (a-MnOz) which is a tunnel structure. Both are different
manganese oxide minerals
having different structures. Although not as critical as with iron oxides,
some inhibition of
crystallization may be helpful to produce poorly crystalline structures and
higher surface area.
The point of zero charge (pzc) of manganese oxides and the surface charge
density are the
keys to an important advantage of manganese oxide coatings over iron oxide
coatings in the
adsorption of heavy metals. Iron oxide coatings only have a negative charge on
their surface when
the pH of the solution surrounding the media is greater than the pzc of the
coating. For pure iron
oxides crystalline minerals, this ranges from 7 to 8 depending on the mineral
form of iron oxide (i.e.
goethite, hematite, etc.) and is a comparatively narrow range. For silica-
inhibited ferrihydrite this pzc
can be between pH values of 5.5 to 7.5. For manganese oxides the pzc values
are much lower. The
pzc occurs at a pH of less than 5. Reported values are in the range of 2 to 3.
Figure 2 illustrates the
CA 02571689 2007-O1-11
13
pzc for the manganese oxides Birnessite and Cryptomelane and the iron oxide
Goethite. Thus, for
manganese oxide coated media there is a strong negative charge at typical
environmental pH levels
of 6 to 8. This also means that pH conditioning such as rinsing with DI water
is usually not necessary
for manganese oxide coated substrates.
Those skilled in the art will recognize there are numerous methods of
producing manganese
oxides for use in the present invention. The following two methods disclose
one preferred method
of the present invention for producing both birnessite and crypotmelane.
Method A: Birnessite Coating Method (BCMI.
The disclosed binessite coating method uses a wet oxidation procedure to
precipitate the
colloid of birnessite on the media surface. In other words, a solution
containing manganese was
oxidized to create an MnOX form. Two moles of concentrated hydrochloric acid
(37.5%) were added
dropwise and continuously to a boiling solution of 0.5-M potassium
permanganate in I liter of water,
to which 0.5 liters of media was added, immersed and vigorously stirred. The
media actually used
included plastic beads, sand, GAC, concrete blocks and concrete rubble.
However, any other suitable
media (wood, etc.) could also be used. After boiling for fw-ther 10 minutes,
the media was washed
with water and dried at room temperature overnight. Under lab conditions, a
reasonably pure form
of birnessite can be produced (>80 pure). This produced a coating having a
surface area of 70-90
m2/g (i.e. surface area of coat as applied to the substrate) with a pzc at a
pH near 3. At environmental
pH values the surface charge density is very negative (-10 to -20
micromoles/mz). This coating has
an approximate mean of about 1200 micromoles of negative charge per gram of
coating.
Method B: Cryt>tomelane Coating Method (CCM~
The Cryptomelane coating method uses a wet oxidation procedure to precipitate
the colloid
of cryptomelane on the media surface. A solution of 0.35 moles KMnOa in 800m1
of water is heated
to 60 °C and dropwise continuously added into a solution 0.5 moles of
MnS04 in one liter of 2M
acetic acid. This solution was heated with 500 ml filtration media (such as
acid washed polyethylene
beads or any of the media types named above) to 80°C while vigorously
stirnng. After stirring for
15 minutes, the media was removed, filtered, washed with water and allowed to
dry at room
temperature overnight. Under lab conditions a reasonably pure form of
cryptomelane can be
produced (>80 pure). This will produce a coating having a surface area of 200
to 270 m2/g (i.e. the
swface area of the coating itself rather than applied to the substrate as
above) with a pzc at a pH near
3 to 4. At environmental pH values the surface charge density is very negative
(-2 to -5
micromoles/mz). This coating has an approximate mean of about 823 micromoles
of negative charge
CA 02571689 2007-O1-11
t-
per gram of coating. It will be understood that the most significant factor is
the combination of
specific surface area and surface charge. The difference between 1200 and 823
can be important
when these coatings are applied consistently as with a chemical process
operation. It should be noted
that at the upper end of environmental pH values, ferrihydrite (iron oxide)
has a surface area of
between 200 and 300 m2/g and a surface charge density of -0.1 to -1.0
micromoles/mz. Silicate (a
form of silica) contamination (addition of silica solution or natural silica
in clay minerals), tends to
prevent ferrihydrite from transforming to other iron oxides and thus tends to
keep the pzc at a pH of
around S.5 to 7.5, as is typical for ferrihydrite. This coating has an
approximate mean of about 113
micromoles of negative charge per gram of coating. However, the cost of an
iron oxide coating may
be somewhat lower than the cost of a manganese coating. Nevertheless, it
should be noted that the
use of iron oxide coatings is more likely to require the additional cost of pH
conditioning of the
influent, which can be significant for engineered systems.
Those skilled in the art will recognize that there is a variety of synthetic
manganese oxide
minerals as there is with iron oxide minerals. However, manganese oxides have
not been as well
studied as iron oxides. Technically, the term "bimessite" is used to refer to
a group of manganese
oxides for which the exact structures are still to a certain extent unknown.
What is known is that
these bimessite minerals are layered structures. Examples of birnessite
minerals having a valence
> +4 are vernadite, ranciete, buserite, and lithiophorite. Examples of
birnessite minerals with a
valence < +4 are magnetite and hausmannite. The other manganese oxides are
tunnel structures.
One of the more common is cryptomelane which forms a group of manganese oxides
along with
hollandite and coronadite (all having a-Mn02 structures with a large foreign
cation (K, Ba or Pb
respectively) as part of the structure). Other minerals include ramsdellite (
~3-Mn02) ,Nsutite ( p-
MnOz), romanechite ( Mn06) and todorokite. All of these minerals have negative
surface charges
and have SSA's that fall in the range of SO to 280 m'/g. Birnessite and
cryptomelane are easy to
produce and provide a good combination of negative surface charge and SSA for
adsorption of
cationic species (mainly heavy metals) when the pH is above the pzc (see
Figure 2). Naturally, it
will be understood that lowering the pH below the pzc will allow the removal
of anionic species
such as nitrite (NO2~), nitrate (N03'), or phosphates (P04-).
It will be recognized the choice between iron oxide and manganese oxide
present a typical
design choice which will be governed by the particular engineering problem
being addressed.
Additionally, different concentrations of the metal oxides have been used in
the solutions u~ which
the substrate is immersed. The concentrations may range from 0.1 M to 3.0 M
(or higher) solutions
CA 02571689 2007-O1-11
~1
of the metal oxide. Nor is the invention limited to immersing the substrate in
a metal oxide solution.
Rather, the metal oxide solution could be an aerosol which is spayed onto the
substrate. This
technique works well in a reactor that fluidizes the media using a gas such as
air. The metal oxide
coating is injected as a fine spray onto the fluidized media Once the media is
coated, the temperature
in the reactor would be raised to evaporate off the water and leave the oxide
coating on the media.
The media will continue to be fluidized throughout this process. The reactor
can be as simple as an
upflow column or a conical upflow reactor. A significant advantage of this
technique is the savings
created by the efficient use of the coating material.
Although not as generally preferred as iron oxides or manganese oxides,
aluminum oxides
may also be a viable oxide coating, especially on materials such as CPP. The
chemistry of
aluminum oxide indicates that it should be a viable material and the cost of
this material is
relatively low. Therefore, aluminum oxides (such as forms of A1203) used as
amphoteric
compounds are intended to come within the scope of the present invention.
The advantage of various alternative embodiments of the present invention will
become
apparent as those skilled in the art begin to practice the invention. For
example, using cementitious
porous pavement (CPP, discussed below) as the filter media or coating
substrate allows a unique
manner of avoiding the cost of pH conditioning of the influent. As is well
known, cement is largely
composed of alkalinity-producing substances and therefore is capable of pH
elevation. One method
is to coat only the bottom half of a CPP pavement block with iron oxide or
manganese oxide. Then,
as pavement runoff percolates down through the upper exposed cementitious
material near the
pavement surface, the pH of the percolating runoff will be elevated above the
pzc of the iron oxide
coating on the lower half of the CPP block and thus, the lower half of the CPP
block form an efficient
passive fixed adsorption matrix. Alternatively, the entire pavement block
could be coated. This
could be accomplished by immersing the entire pavement block in one of the
manganese oxide (or
iron oxide) solutions described above. In a further alternative, various
surfaces of the block could
be sprayed with an iron or manganese oxide solution. In the spray method, the
depth to which the
block is coated could be regulated in part by the total amount of solution
sprayed onto the block. A
still further alternative described below would introduce the amphoteric
compound as an admixture
in the process of mixing the concrete.
5. Cementitious Porous Pavement (CPP)
Those skilled in the art will recognize many design issues which apply to the
choice of
substrates or filter media. As discussed above, the media may be many
materials such as sand,
t
CA 02571689 2007-O1-11
16
polyethylene beads, or a fixed porous matrix such as cementitious porous
pavement (CPP).
Typically, the prior art is only concerned with making cement structures as
impervious to water as
possible. However, one aspect of the present invention is creating a cement
(or cementitious)
substrate which is quite porous. A wide range of size and gradation of
material may be used as media
and CPP blocks may be used in their block form or broken up to serve as a
rubble media. Issues such
as contact time, contact surface area, filtration ability, and hydraulic
conductivity required will
determine the choice of media or rubble size. Any of the above described
amphoteric coating
preparation techniques may be applied to CPP material either as the material
is being produced
(described below) or after the material has been produced without a coating
(in large or small blocks
or as sections). If the CPP material is not produced with the amphoteric
compound as an admixture,
the block of material will be immersed in the amphoteric coating solution of
choice and the solution
is circulated through and around the CPP block. Because of contact time
issues, one preferred
method requires the intact CPP blocks to remain in the circulating manganese
oxide solution for 60
minutes before removing and drying. Drying may take place at room temperature
for several days
under still air conditions or for 24 hours when air is being blown by both
sides of the block.
Alternatively, the porous block could be sprayed with a manganese oxide
coating, allowed to dry, and
then be used.
The CPP must be sufficiently porous to allow migration of water therethrough,
but retain
sufficient strength to withstand vehicle wheel loads typically encountered by
road-side shoulders,
parking areas or drive areas. One measure of the ability of CPP to allow the
migration of water is
saturated hydraulic conductivity (KS~,) measured in em/sec. For purposes of
the present invention,
the hydraulic conductivity of the CPP could range between 1.0 and O.OOOI
cm/see. One preferred
embodiment has a hydraulic conductivity of about 0.01. While there may be
situations where a very
high hydraulic conductivity is desirable, this must be balance against
concerns with sufficient
structural strength and sufficient surface contact between the pavement and
the fluid flowing through
it to insure mass transfer and/or filtration by the pavement. The factors
affecting the porosity of the
CPP are the water to cement ratio, whether and how much pressure is applied
during curing, and to
a lesser degree, the amount of fine aggregate in the mix.
A) Production of CPP as a-precast uncoated block
While there are many mixtures which would form the CPP of the present
invention, three
preferred mixtures are disclosed below in Table 1. The water cement ratio for
each mix design is
varied, ranging from 0.14 to 0.32. However, these water cement ratios were
used in conjunction with
CA 02571689 2007-O1-11
steam curing as described below. Those skilled in the art will recognize that
if steam curing is not
used, the chosen water cement ratio would probably be higher. Nevertheless, to
maintain a hydraulic
conductivity of between 1.0 and .0001 cm/sec., it is suggested that the water
cement ratio be
maintained below 1. When CPP is used as a cast-in-place material (i.e. not
steam cured) a water
cement ratio of 0.3 to 0.4 would be a recommended range.
Typically, the ratio of fine to course aggregates will be approximately 1 to
1. While this ratio
could vary, an excessive amount of fines may tend to reduce porosity by
filling passages in the
cement structure. As an illustrative sample, the grain size distribution of
the pea gravel and sand used
in Batch 2 is presented in Figure 3
Table 2 - Mix Designs for Porous Pavement Block
Error! Batch 1 Batch 2 Batch 3
Bookmark
not
de~ned.Co
mponent
Cement 109 kg I09 kg 109 kg
(Type In (240 lbs) (240 lbs) (240 lbs)
Water 15-20 kg 20-25 kg 30-35 kg
Coarse 472 kg 381 kg 431 kg
Sand (10401bs) (8401bs) (95016s)
# 9 Gravel336 kg ------ ------
(740 lbs)
Pea ------ 381 kg 331 kg
(8401bs) (7301bs)
------ Indicates material not used in batch
The pavement resulting from the disclosed mixes was formed in various sizes of
pre-cast
blocks, for example 24 inches x 16 inches x 4 inches thick. Naturally, this
should by no means be
considered an optimal size, but rather dimensioning of the blocks will depend
on the application. The
blocks were subject to a conventional pressurized, steam curing process. The
process incorporates
a press using hydraulic compression to press the concrete mix into the block
form. The hydraulic
press was capable of exerting up to 35 kN (4 tons) of force on the wet
cementitious mix in the form
CA 02571689 2007-O1-11
Ig
and the full 4 tons was applied in this experiment. Typically this pressure
was applied for 1 to 4
minutes. Then the precast CPP blocks were steam cured (in a kiln with over 90%
humidity) for four
days to a week to promote adequate cement hydration and then the blocks were
allowed to air dry for
two days before transport. Longer steam curing up to 28 days will produce a
higher strength material.
Of course, there is a substantial amount of flexibility in the application of
these various components
in making CPP. For example, although the experiment above used 4 tons of force
(or about 3000-
lb/ft2) applied for approximately 1-minute, both the force and duration of the
loading can vary based
on the application. Those skilled in the art will recognize many applications
that may require less
force or applications requiring more or less duration of the loading.
From each porous pavement mix design, a block was sampled at random to
determine the
strength and infiltration capacity. From each block five cores are drilled
using a 8 cm (3 in.) outside
diameter diamond tipped coring bit. This yielded cores approximately 7 cm
(2.75 in.) in diameter.
The infiltration capacity of the porous pavement blocks was evaluated by the
falling head
permeability test for soils. Each core was wrapped with an impermeable
membrane to determine
hydraulic conductivity of the block. Flow was introduced from the bottom of
the sample to ensure
complete saturation. Two trials were taken for each core resulting in ten
hydraulic conductivity
values for each porous pavement mix design. As shown in Table 3, Batch 2 has
the greatest
hydraulic conductivity. Blocks tested later as full blocks had a full block
Ksa, of approximately 0.01
cm/s.
Since the CPP on the roadway shoulder may be subject to occasional traffic
loads (or many
wheel loads in the case of parking areas), block strength is an essential
consideration in the design.
The unconfined compression strength of the blocks was evaluated. Two of the
five cores from each
mix design were tested to determine the unconfined compression strength. Since
the length to
diameter ratio of the cores was less than 1.8, the strength was reduced by
applying the appropriate
correction factor as designated in ASTM C-39. The resulting compression
strengths of the three
batches are seen in table 3.
CA 02571689 2007-O1-11
19
Table 3 - Properties of the CPP Blocks
Mix Design Unit WeightAverage HydraulicAverage Unconfined
Conductivity (cm/sec)Compressive Strength
II
Batch 1 14.8 kN/m' 0.0091 37,500 kPa
(93.9 pcf) (5440 psi)
Batch 2 14.1 kN/m' 0.0098 27,700 kPa
(89.6 pcf) (4020 psi)
Batch 3 14.6 kN/m' 0.0090 33,600
(93.0 pcf) (4880 psi)
1t is noted that these are only a few examples of measured properties of CPP
blocks. In other
blocks, it is envisioned using CPP where the hydraulic conductivity values are
designed either
higher or lower than the above values by adjusting the water to cement ratio
or adjusting the fine
to course aggregate ratio.
B) Making concrete media, cement media or CPP with an amphoteric admixture.
Previously described was a process of creating CPP blocks and then coating the
blocks with
an amphoteric compound by soaking the blocks in a solution containing the
amphoteric compound.
However, the amphoteric compound could also be incorporated in the CPP as part
of the process of
mixing the cementlaggregate slurry. An example of this method follows.
In a shallow container of large surface area compared to depth (in the lab
environment,
shallow Pyrex trays in the range of 12 x 16 inches were used), there is placed
a solution of 0.3 to 1.0
molar solution of manganese. The solution can be made by either method
described above. To this
solution, add a total of 1-kg of cement, and aggregate at the water/cement
ratio and cement/aggregate
ratio of choice to produce concrete of the strength and porosity desired.
Those skilled in the art will
understand that whatever volume of amphoteric solution is added should count
toward the total water
cement ratio. For example, using 1 kg of cement and a water cement ratio of
0.5, the adding of .25
kg of amphoteric solution will require an additional .25 kg of water to be
added. The mixture is then
dried (i.e. the cement is hydrated and the concrete mixture hardens)
approximately 12 hours. 1t
should be noted that at least part of the water in water-cement slung is
actually the solution of
manganese oxide. In effect, the entire cementitious material is coated inside
and out side with a
manganese coating. The same method could be earned out for an iron oxide
coating but with the one
CA 02571689 2007-O1-11
difference; the CPP or cementitious media must be dried at an elevated
temperature of 90 to 100 C
for at least 24 hours. As with all media discussed above, if an iron oxide
coating is not fully dry
before rinsing, some of the coating will be washed off. This typically is not
a concern with
manganese oxide coatings since manganese oxides usually bond far better to
substrates such as CPP
(and polymer beads) than iron oxides.
With cementitious material as a porous matrix (i.e. as a substrate), final pH
conditioning of
the iron oxide coating is not required because the alkaline nature of the
cement raises the pH to
acceptable levels. In fact, the acidic nature of the iron oxide solution (and
to a lesser extent the
manganese oxide solution) actually creates more internal porosity of the CPP
by consuming a portion
of the cement matrix through a neutralization reaction. However, this
increased internal porosity also
results in a reduction in the cement matrix's strength. This is problem which
is much less prevalent
when manganese oxide is the amphoteric compound.
One useful application of a CPP coated with an amphoteric compound is as a
roadway runoff
filtering shoulder. Figure 4 illustrates a cross-section of a typical roadway.
The roadway will have
driving lanes 5 with shoulders 4. In Figure 4, the shoulders are formed of a
CPP having an
amphoteric compound coating as described above. Typically, the CPP shoulder
will have a thickness
ranging from 4 to 16 inches. Rainwater run-off depicted by arrows 6 will flow
off of the driving
lanes 5 and onto the CPP shoulders 4. The runoff will percolate into the CPP
material and metal ions
will be sorbed by the amphoteric compound on the CPP material. The runoff
(with metal ions
removed) will then flow out of the side and bottom of the CPP shoulders 4.
Alternatively, the porous
pavement may employed with or without an amphoteric coating to serve as a
temporary rain water
storage basin as described in more detail below. A roadway acting as storage
basin would have a
length to width ratio of greater than 20 and typically greater than several
hundred or even several
thousand, if not more.
Another application of concrete rubble or discrete pieces of cementitious
media produced
with an amphoteric solution is use as a crushed aggregate filter media. In
other words, the object is
not to have water flow through the individual pieces of concrete, but to have
it flow around broken
up concrete rubble. To create a concrete media or cement media that is fully
impregnated with
manganese, the water-cement ratio would be higher to ensure sufficient
cohesive and adhesive
bonding within each piece of media. In this situation, the water cement ratios
are close to that of
standard concrete mixes and a preferred range would be 0.40 to 0.90. This
water cement ratio
includes the aqueous solution gained from the admixture. This will be referred
to as the "aqueous
CA 02571689 2007-O1-11
21
solution cement ratio" to imply that both water and the admixture solution are
considered in
computing the ratio. The concrete would be mixed as above and once it hardens
(from example, after
12 hours), it is broken up as rubble into media sizes of choice. Typically
these sizes can range Gom
0.2 to 10 mm. The rubble could then be coated with an amphoteric compound such
as described
above in regards to polyethylene beads.
Another preferred method of coating the CPP (or other substrates) includes
recoating the
media. One example of recoating the media was accomplished by placing the
media in a column in
which it will be fluidized with a recircalating flow of manganese solution.
Thus, I-kg of media was
placed in a vertical column (the column was approximately 2 liters in volume)
with a 6-liter
recirculating solution of 10-3 M NaHC03 and 0.035-moles/liter Mn2+
(stoichiometric amount) and
re-circulating this solution with a pump capable of handling aggressive
solutions and with a sufficient
capacity to fluidize the bed. The Mn2+ is oxidized by adding 250-mL of a 0.185
M solution of
NaOCI at a flow rate of 5 mL/minute for 1 hour to ensure complete oxidation of
the manganese. The
manganese oxide in this solution is then re-circulated for an additional 2
hours with 250-mL of 0.185
M NaOCI added in one step at the beginning of the 2 hours. After 2 hours, the
solution was drained
and then replaced with water (in the lab, it was de-ionized (DI] water) and re-
circulated for 15
minutes and then the column was drained of the water solution. The media was
then rinsed with
water (DI in lab) to a pH of 7 and then allowed to dry overnight before use.
The rising of a
manganese oxide coated media with DI water was mainly to remove impurities in
order to obtain
laboratory quality samples. In practical field applications, the final rinsing
of manganese oxide
coated media could be dispensed with.
Naturally, re-coating of the media is not limited to manganese oxide upon
manganese oxide.
Another re-coating method would include a first coating with iron oxide
followed by a second
coating of manganese oxide. If the iron oxide coated material produces a
sufficiently high SSA
substrate for the intended application, this latter method may be more
desirable since iron oxide is
normally less costly than manganese oxide. Thus, a comparatively inexpensive
substratetsuch as
sand with a low SSA may be coated with iron oxide to produce a comparatively
high SSA substrate
(i.e. a substrate with a SSA much greater than 0.1 m2/g). In other words, the
iron oxide coated sand
becomes the substrate far the final filter media which is coated with
manganese oxide.
Additionally, the increased SSA achieved by re-coating may be applied to any
of the above disclosed
substrates (CCP, wood, polymers, etc.) or with other oxides such as aluminum
or other surface active
materials of high surface area and amphoteric natwe. An alternative method of
providing an initial
CA 02571689 2007-O1-11
22
sub-coat to increase a substrate's surface area is to employ an amorphous
silica . Hydrated silica may
be applied by immersing the substrate in or spraying onto the substrate a
solution having at least
about 10,000 to 100,000 ppm silica. Additionally, various hardening agents can
be included in the
solution, such as organic esters. When the silica solution is dried onto the
substrate at temperatures
ranging between about 100 °C to 300 °C, a substantially enhanced
surface area is created. For
example, applying hydrated silica to sand which typically has a specific
surface area of about 0.1 mz/g
may raise the specific surface area to greater than 10 m2/g.
Additionally, substates could be formed from any porous structure having a
fixed matrix. A
fixed matrix is considered to be any assemblage of particles which hold a
consistent relative
position with respect to one another under shear stress. This can be
distinguished from a
deformable matrix, such as loose sand, which cannot resist shear stress. An
example of such a
porous fixed matrix would be solidified lava (or lava rock). A fixed matrix
having a porosity of
between 0.05 and 0.6 would be suitable for use in the present invention. Since
it is intended for water
to pass through the porous fixed matrix, the fixed matrix will typically have
a significantly larger size
than granular substrates. For example, a section of lava rock might have a
cross-sectional area of at
least about 200 to 300 mmz facing the direction of water flow. The thickness
of the lava rock section
could be at least about 20 mm.
6. Other embodiments of the present invention.
The present invention may be put to enumerable uses. For example, while the
above
disclosure discusses a cementitious porous pavement material, the porous
pavement material could
also be bituminous or asphaltic. Porous asphalt can be made by reducing the
asphaltic binder and,
in effect, producing a lower binder - aggregate ratio. Typically, the
amphoteric compounds described
above may also be added to the bituminous porous pavements during the mixing
stage, creating the
same type of waterborne metals filter. However, with all porous materials, an
amphoteric material
can always be added as a surface coating and much of the porous surface can be
coated by application
of a spray on the porous surface.
Large areas of porous pavements may also be used as storm water storage
basins. Parking
lots and similar large paved areas are often the source of significant volumes
of storm water runoff.
The porous pavement of the present invention provides a means of substantially
reducing the
volume of runoff from such large pavement areas. These areas may be defined as
a ratio of their
length to width. For purposes of the present invention, a storage basin may be
any pavement area
having a length to width ratio (i.e. length/width) of less than 20. A typical
parking area formed of
CA 02571689 2007-O1-11
23
porous pavement would have a porous pavement with a hydraulic conductivity of
between 0.0001
cm/sec and 1.0 em/sec and more preferably of around at least 0.0001 cm/sec.
Because it is not
necessary to transfer the water so quickly in parking areas, it preferred to
have higher strength and
lower porosity. Porous pavement having a hydraulic conductivity of between
0.0001 cm/sec. and
1.0 cm/sec will normally have a strength of between approximately 5000 psi and
3000 psi. The
void volume will be approximately 20% to 30% of the total volume of the
concrete. Such a layer
of porous pavement should be at least six to eight inches in depth and
preferably, at least twelve
to fifteen inches in depth. This depth provides both the necessary strength to
support vehicular
traffic and also provides a sufficient volume of pore space to store the water
from an average rain
storm. With a 20% to 30% pore volume, a 6 inch slab of porous pavement could
retain as much
as 1 to 1.8 inches of rainfal! upon that slab. Rather than placing further
strain on storm sewers, the
rain collected in the porous pavement will be left to evaporate during dryer
days. This method of
storing runoff from parking lots has the further benefit of tending to
immobilize parking lot
pollutants entrained by the rain water. Rather than leaving the premises of
the parking lot, such
pollutants will be retained in the porous pavement. As the water evaporates
from the porous
pavement over time, the pollutants will tend to be retained in the pavement.
Many organic
pollutants (for example poly-cyclic aromatic hydrocarbons) may be volatize
into the air during
evaporation, a process which is preferable to the volatile pollutants becoming
dissolved in water
runoff leaving the pavement. Additionally, the porous pavement may be treated
with an
amphoteric compound in order to improve the capture of waterborne ionic
constituents which are
held in the porous pavement while the retained water evaporates. It can
readily be seen how a
parking lot constructed of porous pavement will form a storm water storage
basin capable of
supporting vehicular traffic.
Another embodiment of the present invention includes a roadway gravel shoulder
capable
of capturing waterborne ionic constituents entrained in roadway rain runoff.
Roadways often have
gravel shoulders at least.four inches in depth, more typically six to eight
inches in depth and for
larger roadways, often over eight inches in depth. Commonly, the gravel for
roadways is graded
to have an average diameter of between three-fourths of an inch to one inch.
To cant' out this
aspect of the invention, the gravel may be coated with an amphoteric compound
such as one of the
iron oxides or manganese oxides disclosed above or aluminum oxide or amorphous
silica.
Preferably, this would be done prior to placing the gravel as a roadway
shoulder. Any of the
coating processes discuss above would be suitable, but the previously
described field method for
CA 02571689 2007-O1-11
2~
producing large quantities of iron oxide coated sand would be one preferred
method. The gavel
could also be subject to the multiple layer coating also described above. Once
the coating process
for the gravel was complete, the gravel would be placed along the roadside in
the normal manner
for creating a shoulder. This manner of capturing waterborne ionic
constituents is advantageous
because it can passively filter and treat pavement sheet flow directly at the
edge of the pavement
before the flow becomes concentrated.
A still further embodiment of the present invention encompasses coating a
flexible, planar,
porous substrate with an amphoteric compound. One example of a flexible
planar, porous
substrate would be geosynthetic fabrics which are well known in the art.
Geosynthetic fabrics are
generally polymeric materials which are designed to be placed in or against
soil. Often
geosynthetic fabrics are used to retain soil in place while allowing water to
pass through the fabric.
Geosynthetic fabrics may be woven or nonwoven. Woven geosynthetic fabrics are
fabrics with
filaments in warp (machine direction) and weft (cross-machine) direction.
Nonwoven fabrics have
essentially a random fabric or textile structure. For example, common felt is
a nonwoven textile.
Nonwovens are further characterized according to how fibers are interlocked or
bonded, which
is achieved by mechanical, chemical, thern~al or solvent means. Some of the
polymeric materials
used to construct geosynthetic fabrics include: polyethylenes - PE, HDPE,
LDPE, XLPE, FLPE,
CPE, CSPE; polypropylene - PP, polysulfone - PSF; polyurethane - PUR;
polycarbonate - PC;
polyvinyl chloride - PVC, polystyrene - PS; thermoplastic elastomer - TPE;
nylom- PA; polyester
- PET; nytrile; butyl; acetal - ACL; and polyamide - PA. Most typically,
geosynthetics are formed
from PE, PP, PVC, PET, PA or PS. The application of an amphoteric coating to
the geosynthetics
could be carried out by a process similar to that described above for coating
polyethylene beads.
However, rather than stirring the beads, the sheets of fabric are dipped in
solution, pulled them
out of the oxide solution, and then dried them. The sheet could be left in the
solution while dried,
but this method wastes a substantial amount of oxide solution. With fabric or
sheet material, the
preferred techniques will be to spray on the solution and dry or a dip in the
solution and dry.
Geosynthetic materials coated with amphoteric oxides can serve as more
effective filters
(higher surface area and surface roughness) which can adsorb cations (e.g.
heavy metals) or anions
(e.g. phosphates) depending on the pH of the aqueous stream, seepage, ground
water, or the like.
The filters of the present invention can be in-situ or ex-situ. An example of
an in-situ filter would
be where one has shallow contaminated groundwater or one is directing a flow
of storm water into
a trench. One can place a sheet of oxide coated geosynthetic in a trench,
backfill around it and let
CA 02571689 2007-O1-11
2~
the flow passively move through the trench and therefore move through the more
permeable
geosynthetic to provide in-situ treatment. Ex-situ filters would be all of
those cases where one
does treatment in some form of a device or reactor, like the upflow column
seen in Figure 1.
Another example of a flexible planar, porous substrate would be membrane
materials.
Membrane materials typically have much smaller pore sizes than other filters,
commercially
available on the order of 0.1 to 50 microns and can be up to 3000 or more
microns. Often
membrane materials are formed from a type of cellulose such as cellulose
acetate, cellulose esters,
cellulose nitrate, or nitrocellulose. The amphoteric coating may be applied as
described above for
oxide coated geosynthetics. The membrane substrates may be considered
"membrane filters" in
the sense that they capture constituents only on their surface. This is
distinguished from the other
substrates described herein which act as "depth filters." Depth filters
capture constituents through
some depth (even if relatively shallow) in the substrate.
The flexible planar, porous substrate could also include any number of
convention filter
materials or devices which have a larger area dimension than depth dimension.
For example,
conventional air conditioning or fiamace cartridge filters could be formed by
having an amphoteric
compound applied to the filter media within the cartridge. The filter media
will typically be a
fiberous polymeric or glass material woven or meshed together at different
densities depending
on the intended use of the filter.
A further embodiment of the present invention includes a drainage pipe capable
of
capturing waterborne ionic constituents. Most storm water runoff is carried
through conventional
concrete pipes for at least pari of the journey to its final collection point.
Thus there is the
opportunity to bring the runoff into contact with a pipe surface coated with
an amphoteric
compound and remove ionic constituents from the water. Typically, drainage
lines are sized to
accommodate a standard runoff rate which is less than the total capacity of
the drainage pipes. In
other words, drainage lines are not designed to have the average runoff
completely fill the volume
of the drainage pipe. This means that less than the entire inner circumference
of the pipe is
designed to come into contact with the runoff water. Therefore, it may not be
necessary to coat
the entire interior of the pipe with the amphoteric compound, but rather only
coat the portion of
the inner pipe surface designed to be in contact with the water. It will be
obvious that the decision
concerning how much of the inner surface of the pipe should be coated is a
engineering design
choice which will vary according to the design parameters. One manner of
applying the
amphoteric compound will simply be to immerse the section of pipe to be coated
in an amphoteric
CA 02571689 2007-O1-11
2G
compound containing solution such as disclosed above. For example, the
solution could be a 1
to 3 molar fernc nitrate or ferric chloride solution or a 0.5 to 2 molar
solution of either birnessite
or cryptomelane. Alternatively, the amphoteric solution could be applied
directly to the pipe
surface by spraying and the like.
The piping could be formed out of conventional concrete or a CPP material such
as
described above. The CPP piping would most likely be used when the pipe grade
was above the
water table or placed in soil which could otherwise readily absorb runoff. In
this manner, runoff
flowing through the water could be at least partially returned to the ground
around the run of the
pipeline. The CPP piping material would typically have a hydraulic
conductivity ranging from
about 0.0001 to about 1.0 cm/sec. Both the CPP piping and conventional
concrete piping could
have the amphoteric compound introduced in the mixing process prior to the
concrete mixture
being placed in the pipe forms. It is also in the scope of the present
invention to include
conventional fired clay piping which has been coated with an amphoteric
compound or a specially
made clay piping which has had the amphoteric compound added as part of the
clay mixture before
the pipe is fired.
Another embodiment of the present invention comprises forming a filter by
placing an
amphoteric compound in a clay liner or in a roadway sub-base. As used herein,
the term "sub-
base" is intended to include a roadway sub-base formed of clay, silt or sand
or a mixture of these
materials or recycled materials. This sub-base may be water pervious or
impervious.
Conventionally, a sub-base is formed by placing a layer of uncompacted soil or
recycled material
over the area where the sub-base is to be constructed. Water is then added to
bring the sub-base
to its optimum compacted moisture content. The layer is then compacted to a
predetermined
density. Typically, this process is carried out in layers or "lifts" as is
well known in the art. The
optimum compacted moisture content is determined by standard testing
procedures such as set out
in ASTM D698. An improved sub-base according to the present invention may be
constructed by
raising the uncompacted sub-base to its optimum compacted moisture content
with a solution
containing an amphoteric compound. It may not be necessary to add the
amphoteric solution to
all lifts, but simply the upper most 1 to 3 lifts. Clays have a wide range of
SSA values ranging
from approximately 15 m~/g for clays like kaolinite or illite up to
approximately 850 mz/g for clays
like sodium montmorrilite. Their large SSA values make clays a highly
effective substrate for
applying amphoteric compounds.
CA 02571689 2007-O1-11
27
Another geotechnical structure utilizing amphoteric cox~pounds could be water
impervious
clay liners. While clay liners are intended to be water impermeable, it is
common for liners to have
some permeability resulting in water escaping from within the liner into the
surrounding soil. IC
the clay liner is treated with an amphoteric compound, water traveling along
the liner (toward the
break) or through the liner will have ionic constituents sorbed from it. In a
similar manner, some
roadways are built with sub-bases which are intended to be water impervious.
Generally, it is also
not intended to have water flow through the pavement to the sub-base. However,
cracking in
roadways is commonplace and rainwater migrates through the cracks to the sub-
base. If the sub-
base retains its water impermeable characteristics, water will flow laterally
to the edge of the
roadway. 1f the sub-base is coated with an amphoteric compound, ionic
constituents are effectively
removed as the water travels along the sub-base toward the edge of the
roadway. If the sub-base
also forms cracks, water flowing through the sub-base will be treated.
While the foregoing invention has often been described in terms of specific
examples,
those skilled in the art will recognize many variations which are intended to
fall within the scope
of the claims. For example, while manganese and iron are two preferred
elements for from
amphoteric compounds, aluminum is a third element which may be utilized.
Furthermore, while
the above has described the media as utilized for remove heavy metals from
water, the media could
be utilized to remove many types of airborne or waterborne ionic constituents.
In particular, sand
or polyethylene beads filters could readily be adapted to treat flows of air
for ionic constituents
such as aerosols, charged particulate matter, odors, and gas emissions
containing water vapor with
anionic or cationic species. Also, many of the substrates disclosed, such as
polymer materials,
sand, lava rock, cement materials, and the like are non-metallic substrates
which have the
aluminum, iron, or manganese oxide coatings applied thereto. All of these
variations are intended
to come within the scope of the following claim.