Note: Descriptions are shown in the official language in which they were submitted.
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-1-
Title: METHOD FOR NICKEL AND COBALT RECOVERY FROM LATERITE
ORES BY COMBINATION OF ATMOSPHERIC AND MODERATE
PRESSURE LEACHING
Cross Reference to Related Application
[0001] This application claims the benefit of U.S. provisional patent
application No. 60/592,375 filed on August 2, 2004, the disclosure of which is
hereby incorporated herein by reference.
Field of the invention
10002] The present invention relates to the hydrometallurgical
processing of nickeliferous laterite ore, and in particular to a method for
acid
leaching both the limonite fraction and the saprolite fraction of such ores in
a
single process.
Background of the invention
[0003] Laterite ores are formed by the in-situ weathering of nickel-
bearing ultramafic rocks near or at the surface of the earth in tropical
environments by the action of naturally acidic meteoric waters over geologic
time. They consist of a variety of clay, oxide and silicate minerals, some
enriched in nickel and/or cobalt, and this distinguishes them from the other
major class of nickel ores, the sulfide ores. The latter consist typically of
sulfide minerals of iron, nickel and cobalt, often with copper and minor
precious metals, and are associated with mafic-ultramafic magmatic intrusions
in the earth's crust.
[0004] The weathering process typically creates a layered deposit, with
the products of complete or most extensive weathering occurring near the
surface and these grading into the products of lesser degrees of weathering
as depth is increased and finally terminating in unweathered rock at some
greater depth. The highly weathered layer usually contains most of its
contained nickel microscopically distributed within very finely divided
goethite
particles. Goethite is an oxyhydroxide of ferric iron with the chemical
formula
FeOOH. This layer is usually given the name limonite, and typically contains a
high proportion of iron.
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-2-
[0005] Cobalt is usually associated with the limonite layer and is usually
predominantly associated with oxidized manganese minerals (Mn(III) and/or
Mn(IV) containing oxides and hydroxides), often called asbolane or
manganese wad.
[0006] The lesser weathered layers typically contain increasing
proportions of their contained nickel in various magnesium silicate minerals,
such as, for example, serpentine. Serpentine is a silicate mineral of
magnesium which has the chemical formula 3MgO*2SiO2*2H20. It is believed
that nickel substitutes for some of the magnesium in serpentine. Magnesium
may also be substituted by other divalent metals, for example, ferrous iron
(Fe2+). There may be many other silicate minerals that also host nickel in the
incompletely weathered zones. The partially weathered, high-magnesium
bearing zone is often given the name saprolite, or garnierite. ("Garnierite"
is
also used to describe a particular apple-green colored magnesium-nickel
silicate mineral of variable composition.)
[0007] In some deposits there is another zone typically located
between the limonite and saprolite that consists predominantly of nontronite
clays; these are silicates of magnesium, iron and aluminum that may also be
nickeliferous. In most deposits located in the (current) tropics, the
nontronite
zone is largely absent.
[0008] It should be noted also that none of the weathering zones are
homogeneous in mineralogical or chemical composition, nor is the boundary
between the zones parallel to the earth's surface. However, there is usually a
fairly sharp transition from ore of high iron and relatively low magnesium
contents to ore of a relatively high magnesium content and lower, although
variable, iron content, which occurs over vertical distances of 1 to 3 m
within a
laterite deposit.
[0009] For illustration purposes only, typical chemical compositions of
limonite and saprolite are as follows:
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-3-
[0010] Limonite: 1.0-1.8 % Ni, 0.05-0.3 % Co, 35-50 % Fe, 0.2-3.5
% Mg
[0011] Saprolite: 1.2-3.5 % Ni, 0.02-0.07 % Co, 7-20 % Fe, 10-20 %
Mg
[0012] Each zone also contains typically significant concentrations of
aluminum, manganese and chromium, as well as trace concentrations of
other heavy metals such as copper and zinc in a variety of other minerals.
[0013] A challenging aspect of nickel recovery from laterite ores is that
the nickel values typically can not be concentrated substantially by physical
means, that is, so-called ore dressing techniques, prior to chemical
processing to separate the metal values. This renders the processing of
laterites expensive, and means to lower the costs of processing laterites have
been sought for many decades.
[0014] Also, because of the distinct mineralogical and chemical
composition of limonite and saprolite ores, these ores usually are not
amenable to processing by the same process technique.
[0015] One known acid leaching process for nickel laterites is the so-
called High Pressure Acid Leaching (HPAL) process (see, for example pages
437-453 in "The Winning of Nickel Its Geology, Mining and Extractive
Metallurgy," by Joseph R. Boldt, Longmans Canada Ltd. 1967). This process
was first employed at Moa Bay in Cuba in the late 1950s and additional plants
were constructed in Western Australia in the late 1990s.
[0016] The process utilizes sulfuric acid leaching at high temperature,
typically 250 C, and high pressure; the associated steam pressure at 250 C
is approx. 570 psi or 39 atmospheres. At this temperature, the nickeliferous
minerals in the ore are nearly completely solubilized. The dissolved iron is
rapidly precipitated as hematite (Fe203) at the high temperature employed
because this compound is largely insoluble even in slightly acidic solutions
at
this temperature. The nickel remains in solution and after cooling, the leach
residue containing iron is separated from the nickel-bearing solution by
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-4-
thickening in a series of wash thickeners, a so-called counter-current
decantation (CCD) circuit. Thus, the primary objective of the leaching
process,
which is separation of nickel from iron, is achieved.
[0017] A major disadvantage of the HPAL process is that it requires
sophisticated high-temperature, high-pressure autoclaves and associated
equipment which are expensive, both to install and to maintain. In addition,
the HPAL process also consumes more sulfuric acid than is required to
stoichiometrically dissolve the non-ferrous metals content of the ore because
at high temperature most of the sulfate ions provided by sulfuric acid are
tied
up as bisulfate ions (HSOa ). In other words, sulfuric acid (H2SO4) only
dissociates to release a single proton (H+) at high temperature. On cooling
and neutralization of the leach liquor the bisulfate ions decompose to sulfate
(SO4 2-) and another proton. The latter proton (acid) is therefore not
utilized
fully for leaching and results in excess sulfuric acid which must be
neutralized,
for example with limestone.
[0018] Another disadvantage of the HPAL process is that it is limited to
treating largely limonite-type feeds because the presence of saprolite will
cause a large, and often uneconomic, increase in sulfuric acid consumption
due to the leaching of magnesium from saprolite. This is exacerbated by the
bisulfate "shift" problem at high temperature, which is described above.
[0019] U.S. Pat. No. 4,097,575 describes an improvement to the HPAL
process which constitutes roasting saprolite ore below about 820 C in order
to render the ore more reactive with sulfuric acid and then using the roaster
calcine to neutralize excess acid in the discharge of an autoclave wherein
pressure leaching of limonite ore occurs. Nickel contained in the saprolite
ore
is largely dissolved during this neutralization. The reported advantages of
this
process are that it better utilizes the sulfuric acid added during pressure
leaching of limonite, it reduces the consumption of limestone or other costly
neutralizing agents to treat the autoclave discharge liquor, and it achieves
the
capability of treating both the limonite and saprolite fractions of a typical
nickel
laterite ore body. Disadvantages of the process are that it still requires the
use
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-5-
of expensive autoclaves for leaching limonite, and it requires a roasting
process for saprolite ore, which is expensive both in capital and operating
cost
terms.
[0020] U.S. Pat. No. 6,379,636 B2 describes a further improvement to
the process described in U.S. Pat. No. 4,097,575 wherein the saprolite
roasting step is eliminated and the saprolite in "raw" form is used to
neutralize
the excess acid in the autoclave discharge solution. In addition, more acid
could be added to the discharge to increase the amount of saprolite that could
be leached. However, this process still requires the use of expensive
autoclaves.
[0021] Several processes have also been described that utilize acid
leaching at atmospheric pressure only, eliminating the disadvantages of
pressure leaching described above. U.S. Patent No. 3,793,432 describes an
atmospheric leaching process for laterite ore, in which the ore is reacted
with
sulfuric acid at or below the boiling point and the precipitation of dissolved
iron
is achieved by the addition of an iron precipitating agent such as ammonium,
sodium, potassium or lithium ions. Although not stated explicitly, all of the
examples cited in the specification employed limonite ore samples, as
evidenced by the high iron content and low magnesium content of the feed
ore. While this process overcomes the disadvantages of pressure leaching, it
has other disadvantages. First, the precipitation of iron is as a jarosite
compound, which is a thermodynamically unstable compound of iron that
decomposes over time to release sulfuric acid, thus causing environmental
problems. (Although jarosite is not stated explicitly it would be apparent to
one
skilled in the art that jarosite will precipitate at the conditions outlined
in the
examples). Jarosite contains two moles of sulfate for every three moles of
iron
and thus this compound represents substantial excess consumption of sulfuric
acid to provide the necessary sulfate ions.
[0022] Second, the nickel extractions from the ore were apparently
relatively low. While extractions were not stated explicitly, based on the
nickel
content of the residue and the fact that the residue weight must be more than
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-6-
the weight of the original ore because jarosite contains a lower percentage of
iron than the original ore and virtually all of the iron remained in the
residue,
nickel extractions were usually in the 60-65 % range.
[0023] Third, there is a requirement for very long leach times, of the
order of 4-5 days. Fourth, there is a need to add relatively expensive iron
precipitating agents such as potassium carbonate, sodium carbonate or the
like.
[0024] U.S. Patent Nos. 6,261,527 B1 and 6,680,035 B2 describe
another atmospheric leaching process in which limonite ore is first "totally"
leached with strong sulfuric acid, i.e. both nickel and iron are substantially
dissolved from goethite, and then saprolite ore is leached in the resulting
limonite leach slurry while simultaneously precipitating iron as jarosite by
the
addition of a jarosite precipitating agent. This process also has the
disadvantage of producing jarosite.
[0025] WO 03/093517 Al describes an improvement to this process,
which constitutes eliminating the addition of a jarosite-forming ion such as
sodium, potassium or ammonium, and causing the iron to precipitate as a
compound other than jarosite, such as goethite. The process overcomes the
disadvantages of jarosite, but sulfuric acid consumption was 0.59 to 0.87
tonnes per tonne of ore in the examples cited, and was over 0.72 tonnes per
tonne of ore in nine of the eleven examples cited.
[0026] The processes described in U.S. Patent Nos. 6,261,527 B1 and
6,680,035 B2 and WO 03/093517 Al are based on the fact that goethite is
more refractory to acid leaching than typical saprolite minerals, such as
serpentine. This has been demonstrated by other researchers (see, for
example: John H. Canterford, "Leaching of Some Australian Nickeliferous
Laterites with Sulfuric Acid at Atmospheric Pressure," Proc. Australasian
Inst.
Min. Metall., 265 (1978), 19-26; N.M. Rice and L.W. Strong, "The Leaching of
Lateritic Nickel Ores in Hydrochloric Acid," Canadian Metallurgical Quarterly,
13(3)(1974), 485-493; and Figure 5 of U.S. Patent No. 5,571,308). Thus, only
saprolite can be used effectively in the second stage of leaching where iron
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-7-
precipitation occurs simultaneously. This is because the acidity of the
solution
must be relatively low to enable the precipitation of jarosite and even lower
to
enable the precipitation of goethite or other hydrolysis products of ferric
iron.
The goethite contained in limonite would leach very slowly under these
conditions. Limonite (principally goethite) is thus leached in an initial
stage
with a relatively high acid concentration and both iron and nickel are brought
into solution.
[0027] Another disadvantage of the processes described in U.S. Patent
Nos. 6,261,527 B1 and 6,680,035 B2 and WO 03/093517 Al is that the leach
process is slow. Greater than 10 hours leach retention time is required to
complete the reactions. Thus, many large leach reactors are required to carry
out the process and this increases the capital and operating costs of the
process compared to a leach process which has a much shorter retention
time.
[0028] The atmospheric leach processes described above address the
disadvantages of high pressure leaching but have significantly lower nickel
extraction (typically 80-85 % for atmospheric leaching versus 90-97 % for high
pressure acid leaching). The object of the present invention is to obviate or
mitigate the disadvantages of high pressure acid leaching processes while
achieving higher and faster recoveries of nickel and cobalt than the known
atmospheric leach processes.
Summary of the invention
[0029] The present invention provides a process for the efficient
leaching of nickel and cobalt from limonite and saprolite ores in two stages,
the first stage consisting of mixing and reacting a slurry of the limonite ore
with concentrated mineral acid at atmospheric pressure, and the second
stage consisting of adding saprolite ore to the resulting leach slurry and
then
leaching at a moderately elevated temperature and pressure. Iron is
efficiently
separated from nickel and cobalt in the solid leach residue primarily as an
oxide and/or hydroxide of ferric iron other than jarosite.
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-8-
[0030] The limonite leach step should be carried out at a temperature
close to the boiling point. The quantity of acid added should be approximately
that required to stoichiometrically dissolve the soluble non-ferrous metals in
the ore as well as the soluble iron, and a small excess of acid may
advantageously be used to drive the dissolution reactions. Preferably, a
reductant, such as sulfur dioxide or ferrous sulfate, is added to the limonite
leach slurry in order to enhance the dissolution of nickel, and particularly
cobalt.
[0031] In the saprolite leaching stage, the temperature should be high
enough to achieve a rapid rate of reaction and satisfactory nickel (and
cobalt)
extraction, but low enough that the resulting working pressure is within the
tolerance of a simple, low cost autoclave. The working pressure of the
autoclave is approximately equal to the pressure of saturated steam at the
working temperature employed. This pressure increases very rapidly with
increasing temperature, especially much above about 150 C. To avoid the
complexity and difficulty of operating at excessive pressures, an appropriate
range of temperature for carrying out the second stage of leaching in the
present invention is from about 120 to 160 C, and the temperature should be
kept as low as reasonably possible consistent with good process
performance. It has been found that excellent results are achieved, for
example, at 150 C. The associated pressure at this temperature
(approximately 70 psi) is low enough to enable a simple autoclave system to
be used for carrying out the leach.
[0032] In one preferred embodiment, an iron-bearing "seed" material is
added to the leach slurry at the start of the saprolite pressure leach stage
to
accelerate the precipitation of dissolved ferric iron and the extraction of
remaining nickel and cobalt from the solid phases.
[0033] Following the saprolite leaching step, the leach solution is
preferably neutralized prior to nickel and cobalt recovery.
[0034] The laterite leaching method of the present invention can
surprisingly achieve high levels of nickel extraction while avoiding the high
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-9-
cost of high-pressure autoclaves, and avoiding the production of
environmentally unfriendly, high-acid consuming jarosite compounds. While
the process does require an autoclave, the operating conditions are relatively
benign compared to the high pressure acid leaching processes (nearly ten
times or more the operating pressure required for the latter autoclave
processes). Consequently, the process of this invention permits much simpler
equipment design, and operations and maintenance are also easier than in
high pressure acid leach processes. In addition, the saprolite leach and iron
precipitation reactions occur much faster at moderately elevated temperature
and a much shorter leach retention time, compared to the previously
described atmospheric leach processes, is required. Thus, the autoclave
required to carry out the process of the current invention is much smaller
than
the atmospheric leach reactors required for the processes of U.S. Patent Nos.
6,261,527 B1 and 6,680,035 B2 and WO 03/093517 Al. In addition, the
process of the current invention achieves higher nickel extraction than can be
achieved with the aforementioned atmospheric leach processes.
[0035] It has been found that the present invention can achieve at least
about 90 %, and as high as 95 % or more, nickel extraction and as much as
95 % or more cobalt extraction, with about 5 to 10 % iron extraction.
Brief description of the drawings
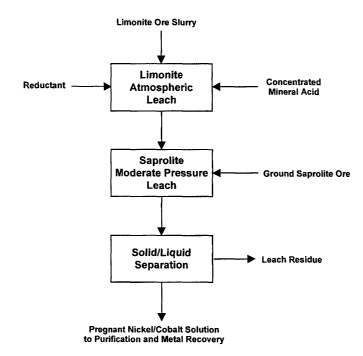
[0036] FIG. 1 is a flow sheet showing in simplified form one
embodiment of the process of the present invention.
[0037] FIG. 2 is a flow sheet showing another embodiment of the
process of the present invention in which some of the leach residue is
recycled in order to provide seed for iron precipitation.
Detailed description of the invention
[0038] Referring to FIG. 1, a slurry of limonite ore is mixed with a
concentrated mineral acid, chosen from the group sulfuric, hydrochloric and
nitric, or a mixture of any of these acids, in a suitable device such as a
stirred
tank reactor, or in the case of continuous processing, a series of stirred
tank
reactors. The limonite ore slurry is produced by conventional means, such as
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-10-
screening and pulping the ore in a drum scrubber, which will be apparent to
those skilled in the art.
[0039] The quantity of acid added is approximately that required to
stoichiometrically dissolve the soluble non-ferrous metals in the ore as well
as
the soluble iron, i.e. most of the nickel, cobalt, magnesium, aluminum,
copper,
zinc, iron present in goethite and other soluble iron hydroxyl-oxide minerals,
and a small portion of the chromium, which is usually contained primarily in
the relatively insoluble mineral chromite. A small excess of acid may be added
to drive the dissolution reactions as far as possible to completion and to
ensure maximum extraction of nickel and cobalt from the limonite ore. In
some cases, some of the magnesium and aluminum may be insoluble and
this should be taken into account to determine the precise acid addition.
[0040] The addition of concentrated acid to limonite ore slurry results in
the generation of substantial heat, raising the temperature of the mixture as
high as the boiling point of the leach solution. The atmospheric limonite
leach
step is preferably carried out close to the boiling point of the solution to
maximize the rates of the leaching reactions and thereby minimize the
residence time required to complete the reactions. Additional steam or other
energy may be added to the leach reactors in order to maintain the leach
temperature as close to the boiling point as possible. It is preferable that
the
limonite ore slurry density be as high as possible consistent with good mixing
in order to minimize the need for additional energy and to minimize the
volume of pregnant liquor that needs to be treated subsequently for nickel and
cobalt recovery.
[0041] The limonite ore slurry is leached for sufficient time to complete
the reactions. This is typically 1 to 4 hours if the limonite leach is
conducted at
approx. 95 to 105 C.
[0042] In a preferred embodiment of the process, a reductant is added
to the limonite leach slurry in order to enhance the dissolution of nickel,
and
particularly cobalt, from the ore. Most of the cobalt and a smaller proportion
of
the nickel present in limonite ore is contained typically in a variety of
oxidized
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-11-
manganese minerals, referred to collectively as "manganese wad."
Manganese is typically in the tetravalent or trivalent state in these minerals
and is refractory to acid leaching unless a reductant is added to cause the
manganese to dissolve in the divalent form. The dissolution of manganese is
necessary to allow the contained cobalt and nickel to dissolve as well, as
will
be apparent to those skilled in the art. Suitable reductants include sulfur
dioxide (SO2), either as a gas or aqueous solution of SO2 and ferrous iron, as
a soluble salt, e.g. ferrous sulfate, although many other reducing species
will
also react with oxidized manganese compounds.
[0043] The resulting limonite leach slurry is injected into an autoclave
along with the saprolite ore. The saprolite ore typically will be prepared by
crushing, grinding and screening or cycloning of the run-of-mine saprolite ore
to achieve a particle size that allows the saprolite ore particles to be
suspended in the autoclave reactor during the leaching process. The resulting
slurry is heated to the desired reaction temperature, for example in the range
of 120 to 160 C, by any appropriate means, for example by direct injection of
intermediate pressure steam into the autoclave, or by direct or indirect steam
heating of the leach slurry prior to injection into the autoclave. The
autoclave
retention time is sufficient to allow most of the iron in solution after the
limonite
atmospheric leach to hydrolyze and precipitate, and the acid regenerated by
iron hydrolysis to react with the saprolite ore, thus extracting most of the
contained nickel and cobalt, as well as magnesium and other impurity metals.
[0044] At 150 C, typically only 1 to 2 hours is required to complete the
reactions. This compares to 10 to 11 hours typically in the atmospheric
pressure leach step described in WO 03/093517 Al. Thus, although the
process of the present invention requires an autoclave, the much reduced
retention time means that this reactor will be much smaller than the
atmospheric leach reactors required in the process of WO 03/093517 Al. In
addition,,since the working pressure of an autoclave operating at up to 160 C
is equal to or less than 90 psi, this reactor is relatively simple compared to
the
autoclaves used in high pressure leaching processes at temperatures of from
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-12-
approx. 250 to 270 C and pressures of 580 to 800 psi. Overall, the moderate-
pressure autoclave systems required in the current process can be of
comparable cost to the atmospheric leach reactors required in the process of
WO 03/093517 Al and much simpler to operate and maintain than the typical
high pressure acid leach autoclaves.
[0045] It has been found that the nickel extraction obtained in the
current process is up to 10 or more percentage points greater than the nickel
extraction obtained in the process of WO 03/093517 Al, using similar acid/ore
and saprolite/limonite ratios. This is a very significant advantage of the
current
process as compared to the processes described in U.S. Patent Nos.
6,261,527 B1 and 6,680,035 B2 and WO 03/093517 Al.
[0046] The use of a temperature above the boiling point for the
saprolite leaching stage may also provide a higher nickel/iron ratio in
solution,
which is advantageous with respect to downstream processing of the leach
solution. This is because in most cases virtually all iron must be removed
from
solution before effecting nickel and cobalt recovery. Usually, the residual
iron
in solution is removed by adding a base, for example calcium carbonate, to
the leach slurry and precipitating iron oxyhydroxide compounds. Some nickel
will co-precipitate with the iron resulting in losses of the pay metal. The
neutralizing agent also represents an additional operating cost of the
process.
[0047] A further advantage of the use of higher temperature is an
improvement in the solid/liquid separation properties of the final leach
slurry,
with higher settling rates being achieved with a higher leaching temperature.
[0048] After leaching, the leach slurry is subjected to solid/liquid
separation by filtration or thickening to produce a pregnant leach solution
containing most of the nickel and cobalt contained in the ore and a solid
residue containing most of the iron in the ore. Advantageously, thickening is
carried out in a series of thickeners with counter-current flow of a wash
water
stream and the leach slurry in order to wash most of the entrained metal
values out of the leach residue, a method called counter-current decantation
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-13-
(CCD). The metal values report preponderantly to the thickener overflow of
the first thickener, which is the pregnant leach solution.
[0049] The pregnant leach solution proceeds to nickel and cobalt
recovery by methods known to those skilled in the art, such as solvent
extraction, ion exchange, sulfide precipitation using sulfiding agents, e.g.
hydrogen sulfide, or hydroxide precipitation, using for example magnesia as
the precipitating agent.
[0050] The nickel and cobalt can also be recovered from the leach
slurry without prior solid/liquid separation, using the resin-in-pulp process.
In
this process, an ion exchange resin which extracts nickel and possibly cobalt
is added directly to the leach slurry. After the extraction is complete, the
resin
is separated from the nickel-depleted leach slurry by screening. After washing
the resin to remove solids, the nickel can be eluted from the resin with a
fresh
acid solution.
[0051] Prior to or during nickel and cobalt recovery by one of the
aforementioned methods, the leach solution may be neutralized with a base,
such as calcium carbonate, magnesium oxide, sodium carbonate or the like,
to neutralize free acidity remaining from the leach process and precipitate
small amounts of ferric iron, aluminum, and chromium, while minimizing co-
precipitation of nickel and cobalt. This process may be carried out in a
single
or multiple steps separated by intermediate solid/liquid separations.
[0052] In one embodiment of the invention, the first stage of
neutralization may be carried out prior to separating the leach residue from
the leach solution. The combined leach and neutralization residue may then
be separated from the partially neutralized leach solution by filtration or
thickening, as described above. A second stage of neutralization may then
still be desirable, depending on the method selected for nickel and cobalt
recovery from the pregnant leach solution. After this second stage of
neutralization, the resulting neutralization residue may be separated from the
neutralized leach solution by filtration or thickening. This second-stage
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-14-
neutralization residue is ideally returned to the first stage neutralization
to re-
dissolve any co-precipitated nickel and cobalt.
[0053] In an alternative preferred embodiment of the invention, an iron-
bearing "seed" material is added to the leach slurry at the start of the
saprolite
pressure leach stage, as shown in FIG. 2. The purpose is to accelerate the
precipitation of dissolved ferric iron and the extraction of remaining nickel
and
cobalt from the solid phases. The surfaces of the seed particles provide low-
activation energy sites for hydrolysis and precipitation of iron, for example
as
ferric hydroxide, goethite, or hematite. This seed material is ideally a
portion
of the final leach residue itself, which contains precipitated iron compounds.
[0054] The following examples illustrate the method of the present
invention. The ore used in these examples came from a Central American
laterite deposit. The limonite and saprolite fractions of the ore had the
compositions given in Table 1. The saprolite ore was crushed and ground to
approx. -100 mesh before use in the tests.
TABLE 1
%Ni %Fe %Mg % Moisture
Limonite Ore 1.41 47.7 0.67 34.5
Saprolite Ore 3.17 8.7 17.8 21.3
[0055] EXAMPLE 1: An acid solution was prepared by adding 287.6 g
of 96 % sulfuric acid and 48.0 g of 37 % HCI (both mineral acids being
reagent grade) to 702 mL of water. The acid solution was transferred to a 2-
liter cylindrical reaction kettle equipped with a mechanical stirrer, 4
plastic
baffles, and a tight-fitting lid equipped with a water condenser open to the
atmosphere. The reaction kettle was heated by an external, electrical heating
mantle. 381.7 g (wet) of the limonite ore described in Table 1 were added to
the acid solution while heating and stirring the mixture. The temperature was
controlled at 94 to 105 C and the limonite ore was leached for 5 hours.
Liquid
samples were taken from the leach slurry at 1, 2 and 5 hours.
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-15-
[0056] After 5 hours of leaching, the leach slurry was transferred to a 2-
liter titanium autoclave, along with 344.8 g (wet) of the saprolite ore
described
in Table 1. The saprolite ore had first been wet ground to approximately -100
mesh and then filtered to form a cake containing 27.5 % moisture. 128.6 g of
technical grade hematite also was added to the autoclave to "seed" the
precipitation of iron. The autoclave was equipped with a mechanical stirrer,
thermocouple, cooling coil and external heating mantle. The leach slurry was
heated to 150 C and held at that temperature for 2 hours. A sample was
removed from the autoclave after 1 hour (6 hours total leach time, including
the limonite atmospheric leach).
[0057] The slurry was then rapidly cooled to approx. 50 C using the
water cooling coil and discharged from the autoclave. The entire slurry was
filtered. The filtercake was repulped twice in fresh water to wash out the
entrained metal values. The cake was then dried and weighed. The dry solids,
filtrate and the combined wash water were assayed separately. Based on the
weights, volumes and assays of the final residue and solutions, the
extractions of the various metals were calculated. The assays of the liquid
samples taken during the atmospheric limonite leach step and moderate
pressure saprolite leach step are shown in Table 2 and the final solution and
residue assays, as well as the calculated metal extractions, are given in
Table
3.
[0058] The limonite/saprolite weight ratio (on a dry solids basis) was
1.0 and the overall acid/ore ratio was 600 kg equivalent H2SO4 per tonne of
dry solids.
TABLE 2
Time (h) [Ni] g/L [Fe] g/L Free Acid g/L
e uiv H2SO4
1 2.84 97.2 43
2 2.78 92.0 21
5 2.61 90.1 <0.5
6 6.5 4.95 16
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-16-
TABLE 3
Ni (% or g/L) Fe (% or /L) M(% or g/L) S(%
Final Solution 9.13 6.03 36.0 --
Final Residue 0.21 40.2 0.67 1.16
Calculated 91.2 10.7 94.8 --
Extractions
* Extractions based on residue and final solutions assays, weights and
volumes.
[0059] The assays in Table 2 indicate that the nickel and iron in
limonite ore were dissolved substantially during the atmospheric leach stage
and that 1 hour was sufficient to carry the leaching reactions almost to
completion, although the further reduction in free acid at 2 and 5 hours
indicates that some further reaction occurred. The 6-hour solution assays
(Table 2, 1 hour of pressure leaching) and the final solution assays (Table 3,
2
hours of pressure leaching) indicate that the iron in solution was rapidly
hydrolyzed and precipitated at the moderate pressure and temperature
conditions in the autoclave, while dissolving most of the nickel contained in
the saprolite ore. The final solution nickel and iron assays were
significantly
higher than the 1-hour solution assays due to substantial evaporation that
occurred during vacuum filtration of the final leach liquor.
[0060] The results indicate that very high nickel extraction and low iron
extraction are features of the process of this invention. The low sulfur
content
of the final residue indicates that most of the iron could not have been
precipitated as jarosite, which has a theoretical Fe/S ratio of approx. 2.6 by
weight.
[0061] EXAMPLE 2: Another test was carried out similarly to that
described in Example 1 with the following exceptions. The limonite ore used
had the following composition: 1.55 % Ni, 48.4 % Fe, 0.47 % Mg, and 37.2 %
moisture. 398.1 g (wet) of this ore was used in the test, along with 573.7 g
water, 288.2 g of 96 % H2SO4, 46.9 g of 37 % HCI, and 285.7 g of
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-17-
MgSO4*7H20. No hematite seed material was used in the test. The
MgSO4*7H20 was dissolved in the water prior to adding the acid to the
solution. The purpose of adding soluble magnesium to the solution was to
simulate the recycle of a magnesium-rich solution which would remain after
nickel and cobalt recovery from the pregnant leach solution. The atmospheric
leach was carried out with a temperature of 96 to 101 C. No sample was
taken from the autoclave during the pressure leach stage.
[0062] The assays of the liquid samples taken during the atmospheric
limonite leach are shown in Table 4 and the final solution and residue assays,
as well as the calculated metal extractions, are given in Table 5.
[0063] The limonite/saprolite weight ratio (on a dry solids basis) was
1.0 and the overall acid/ore ratio was 600 kg equivalent HZSOa per tonne of
dry solids.
TABLE 4
Time (h) [Ni] g/L [Fe] g/L Free Acid g/L
equiv H2SO4
1 1.99 61.8 38
2 1.81 52.7 10
5 2.54 79.9 6
TABLE 5
Ni (% og/L) Fe (% o/L Mg (% og/L) S %
Final Solution 8.5 5.4 46.0 --
Final Residue 0.31 33.1 0.71 1.53
Calculated 90.2 7.7 96.0 --
Extractions *
* Extractions based on residue and final solutions assays, weights and
volumes.
[0064] The results of'this test are fairly similar to the results given in
Example 1 and illustrate that seeding is not required to achieve effective
iron
precipitation. Also, the presence of dissolved magnesium in solution initially
does not appear to impact negatively on the extraction of nickel or
precipitation of iron.
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-18-
[0065] EXAMPLE 3: For comparison, another test was done to
simulate the conditions of the process described in WO 03/093517 Al. The
atmospheric limonite leaching stage was carried out similarly to the limonite
leach stage described in Example 1. 398.1 g (wet) of the same limonite ore
used in Example 2 was added to a solution comprised of 719.9 g water, 288.2
g 96 % H2SO4 and 46.9 g of 37 % HCI. Leaching was carried out for 4 hours
at 101-104 C. After 4 hours, 310.6 g of ground saprolite containing 20.0 %
H20 and 128.6 g hematite seed were added to the leach slurry and leaching
was continued at 98-102 C for 10 additional hours. Liquid samples were
taken periodically to follow the course of leaching. The final leach slurry
was
filtered, the filtercake repulped twice with fresh water and the filtrate,
wash
solution and final washed residue were assayed as in the previous examples.
[0066] The conditions of this test were thus essentially identical to
those of Example 1, except that instead of pressure leaching at 150 C for 2
hours, atmospheric leaching at approx. 100 C for 10 hours was carried out
after the addition of saprolite ore.
[0067] The limonite/saprolite weight ratio (on a dry solids basis) was
1.0 and the overall acid/ore ratio was 600 kg equivalent H2SO4 per tonne of
dry solids.
[0068] The results of this test are given in Tables 6 and 7.
TABLE 6
Time (h) [Ni] g/L [Fe] g/L Free Acid g/L
e uiv H2SO4
1 2.25 72.6 24
2 2.39 74.0 11
4 2.39 70.4 2
5 4.74 58.2 <0.5
6 5.16 36.8 <0.5
8 4.8 13.2 <0.5
11 6.96 5.73 6
14 7.17 4.99 6
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-19-
TABLE 7
Ni (% or g/L) Fe (% or g/Q Mg (% or g/L) S(%
Final Solution 8.32 7.0 35.0 --
Final Residue 0.38 41.0 0.92 1.52
Calculated 82.9 9.4 90.2 --
Extractions
* Extractions based on residue and final solutions assays, weights and
volumes.
[0069] The results given in Tables 6 and 7 are similar to those
described in the examples of WO 03/093517 Al, as expected. However, the
nickel extraction obtained with the atmospheric leach process of WO
03/093517 Al was 8 to 9 percentage points lower than that obtained with the
combined atmospheric and pressure leaching process of the current
invention. This clearly demonstrates the advantage of the current process
compared to that of WO 03/093517 Al.
[0070] EXAMPLE 4: Another test was done to compare the results
obtained from using a combination of atmospheric and moderate pressure
leaching, as prescribed in the current invention, with the results from using
moderate pressure leaching alone. In this test, 381.7 g (wet) of the limonite
described in Table 1, 312.5 g of the ground saprolite at 20.0 % moisture,
734.4 mL of water, 288.3 g of 96 % H2SO4 and 46.8 g of 37 % HCI were
charged to a 2-liter titanium autoclave, heated to 150 C, and leached for 2
hours. After rapid cooling, the leach slurry was filtered and the leach
residue
was repulp washed as in the previous examples. The filtrate, wash solution
and solid residue were assayed as in the other examples. Metals extractions
were calculated based on the solution volumes, residue weight and assays.
[0071] The limonite/saprolite weight ratio (on a dry solids basis) was
1.0 and the overall acid/ore ratio was 600 kg equivalent H2SO4 per tonne of
dry solids.
[0072] The results are shown in Table 8.
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-20-
TABLE 8
Ni (% or g/L Fe (% or /L) M(% or g/L) S(%)
Final Solution 8.41 14.9 44 --
Final Residue 1.05 33.1 0.72 0.84
Calculated 67.9 9.2 94.0 --
Extractions * Extractions based on residue and final solutions assays, weights
and
volumes.
[0073] The very low nickel extraction achieved in this test illustrates
that direct pressure leaching of mixed limonite/saprolite ore at moderate
temperature and pressure is unlikely to result in a viable process for nickel
extraction. Whereas, by combining atmospheric leaching and moderate
pressure leaching, as in the process of the current invention, leach
extractions
approaching those of the high pressure leach processes are achieved without
the complexity of high pressure associated with the latter processes.
[0074] EXAMPLE 5: The limonite and saprolite fractions of the ore
used in this example had the compositions given in Table 9:
TABLE 9
%Ni %Fe %Mg %Si % Moisture
Limonite 1.31 47.2 0.63 2.67 41.9
Ore
Saprolite 3.13 6.0 20.0 18.8 38.9
Ore
[0075] In this case, 238 g (dry basis) of the limonite ore was slurried at
35 % solids in water and placed in a reaction kettle similar to that used in
Example 1. 338.5 g of 96 % sulfuric acid was slowly added to the reactor over
about 10 minutes. The heat of dissolution of the acid raised the temperature
to 99 C within 5 minutes of adding all of the acid. The limonite was leached
at
a temperature of 94 -102 C for 4 hours. Sulfur dioxide gas was bubbled into
the leach slurry during the limonite leaching period to control the leach
slurry
oxidation reduction potential at about 620 mV (vs. saturated Ag/AgCI
reference electrode). A sample was taken at the end of this initial leaching
period.
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-21 -
[0076] The saprolite was wet ground to -100 mesh and filtered to
produce a filtercake. 262 g (dry basis) of this filtercake was added to the
limonite leach slurry, which was then transferred to an autoclave. The
autoclave was sealed and heated to 150 C, at which temperature leaching
was allowed to continue for an additional hour before rapidly cooling the
autoclave. The slurry was sampled at the completion of the additional one-
hour leach period. The saprolite to limonite ratio was 1.1 and the sulfuric
acid
addition was 650 kg H2SO4 per tonne of ore in this test.
[0077] The assays of the leach liquor and the solid residue at the
completion of each stage of leaching are given in Table 10:
TABLE 10
Ni (% or Co (% Fe (% or Mg (% Si(% or S(%)
g/L) or g/L) g/L) or g/L) g/L)
Limonite 3.61 0.52 122.5 0.63 0.047 --
Leach
Solution
Limonite 0.18 0.024 18.6 2.64 17.6 --
Leach
Residue
Final 11.4 0.50 14.5 65.0 0.065 --
Solution
Final 0.31 0.007 31.1 0.96 16.3 1.54
Residue
Calculated 90.7 95.2 10.2 91.0
Extractions
[0078] Also shown in Table 10 are the calculated extractions of Ni, Co,
Fe and Mg. Ni, Co, and Mg extractions were calculated using a "silicon-tie"
method in which the weight of solid residue was calculated using ore and
residue silicon assays on the assumption that none of the silicon leached.
These weights and ore and residue assays were then used to calculate
extractions.
[0079] This test differs from Example 1 mainly in that only 4 hours of
limonite leaching were employed, sulfur dioxide gas was added during the
CA 02572420 2006-12-20
WO 2006/029499 PCT/CA2005/000988
-22-
limonite leach, no seed was added during the saprolite leaching phase, and
only one hour of autoclave leaching was used. The results still indicate a
very
high nickel extraction with minimal iron extraction and very good leaching of
cobalt. The latter was due to the efficacy of sulfur dioxide as a reductant
for
the manganese wad material present in the ore.
[0080] It will of course be appreciated by those skilled in the art that
many variations of the process would be possible within the broad scope of
the present invention. Those skilled in the art will appreciate that the
invention upon which the description is based may be utilized in other
embodiments that carry out the purposes and fulfill the objects of the present
invention. The above disclosure is intended to be illustrative while the scope
of the invention is defined by the following claims.