Note: Descriptions are shown in the official language in which they were submitted.
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
METHODS OF TREATING PFiTHALMIC CONDITIONS
by
William A. Hare, Elizabeth WoldeMussie, and Larry A. Wheeler
Cross-Reference to Related Applications
This application claims the benefit of U.S. Provisional
Application No. 60/591,423, filed July 26, 2004, the entire
contents of which are hereby incorporated by reference.
Background of the Invention
The present invention relates to methods of providing
therapeutic effects using therapeutic agents. More
particularly, this invention relates to methods of treating
ophthalmic conditions of individuals, that is humans or
animals, at early stages of the ophthalmic conditions and of
modifying responses of the central nervous system (CNS) to
ophthalmic injury or disease by administering therapeutic
agents to the individuals.
Glaucoma refers to a group of ocular disorders
characterized by disease of the retinal ganglion cell (RGC)
bodies and degeneration of the optic nerve. It is one of
the leading causes of blindness worldwide. In a patient
having glaucoma, the retinal ganglion cells slowly lose
their ability to transmit nerve impulses. As a result,
vision diminishes, often so slowly that a patient afflicted
with this disease does not notice the degradation in vision
until significant damage has occurred. Because glaucoma has
few overt symptoms, it is difficult to detect early.
One approach to testing for glaucoma is to use a
tonometer to measure intra-ocular pressure (IOP). This test
1
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
is based on the notion that high intra-ocular pressure can
damage the retinal ganglion cell layer. However, in
practice, intra-ocular pressure has not proven to be a
reliable indicator for glaucoma. In addition, some patients
with glaucoma have an IOP in the normal range. But, these
patients have visual field loss typical of glaucoma.
Another test for glaucoma is a visual field test in
which light is directed to various portions of the retina.
By asking the patient whether he sees the light, one can map
the sensitivity of the retina. Because the field vision
test measures optic nerve function more directly, it is a
more accurate indicator of glaucoma than the tonometric
test.
Treatment in individuals with hypertensive or
normotensive IOP is directed at lowering the IOP, even
though the pressure is "normal". However, existing
treatments of glaucoma do not distinguish between
asymptomatic and symptomatic types of glaucoma. This may be
due to the difficulty of diagnosing glaucoma at an early
stage.
The use of neuroprotective agents to treat retinal
cells has been disclosed. For example, U.S. Patent Nos.
5,922,773 and 6,482,854 (Lipton et al.) disclose
administration of a compound capable of reducing glutamate
induced excitotoxicity in a concentration effective to cause
reduction of such excitotoxicity. U.S. Patent No. 6,573,280
(Dreyer) discloses anti-excitotoxic agents, such as
glutamate receptor antagonists, and calcium blockers to
prevent proliferative vitreoretinopathy. U.S. Patent No.
6,573,280 discloses administration of a compound to a
patient to reduce glutamate-induced retinal cell migration
to help treat proliferative vitreoretinopathy.
2
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Neuroprotective effects of memantine are also described
in a number of articles, see Woldemussie, "Neuroprotection
of retinal ganglion cells in experimental models of
glaucoma", Minerva Oftalmol, 42(2):71-8 (2000); Wheeler,
"Experimental studies of agents with potential
neuroprotective properties", Acta Ophthalmol Scand,
77(229):27-28 (1999); Schuettauf et al., "Effects of anti-
glaucoma medications on ganglion cell survival: the DBA/2J
mouse model", Vision Res, 42(20):2333-7 (2002); WoldeMussie
et al., "Neuroprotective effects of memantine in different
retinal injury models in rats", J Glaucoma 11(6):474-480
(2002); and Hare et al., "Efficacy and safety of memantine,
an NMDA-Type Open-Channel Blocker, for reduction of retinal
injury associated with experimental glaucoma in rat and
monkey", Surv Ophthalmol 45(Suppl 3): S284-S289 (2001).
In many cases, a patient is administered a therapeutic
agent to treat glaucoma after the patient experiences a
substantial loss in vision. In these cases, it may be
difficult to prevent further vision loss or successfully
treat the glaucoma.
Thus, there remains a need for improved methods of
treating ophthalmic conditions, such as conditions
associated with ocular hypertension, including glaucoma.
Summary of the Invention
New therapeutic methods employing therapeutic agents
have been invented. The present methods involve systemic,
such as oral, administration to a human or animal of one or
more therapeutic agents to provide a desired therapeutic
effect in treating an ophthalmic condition or conditions.
The present methods can successfully prevent further vision
loss associated with the ophthalmic condition if
3
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
administered at an early stage of disease, and/or can
mitigate against a reduction in a visual response of the
central nervous system that is typically associated with the
ophthalmic condition.
In one embodiment, a method for treating an ophthalmic
condition or mitigating against an ophthalmic condition
comprises administering a therapeutic agent or therapeutic
component to an individual at a time when the individual is
not aware of visual field loss associated with the
ophthalmic condition. The therapeutic agent is effective in
treating the ophthalmic condition or mitigating against the
ophthalmic condition.
In another embodiment, a method for treating an
ophthalmic condition comprises administering a therapeutic
agent or therapeutic component to an individual with an
ophthalmic condition associated with retinal
neurodegeneration. The administering of the therapeutic
agent is effective in reducing a decrease in a central
nervous system response associated with the retinal
neurodegeneration.
The therapeutic agent of the present methods may be an
anti-excitotoxic agent, such as a glutamate receptor
antagonist. When administered systemically, the present
therapeutic agents are able to cross the blood-brain barrier
and/or blood-retinal barrier and provide a therapeutic
effect or effects with little adverse side effects or
toxicity. In certain methods, the therapeutic agent is
selected from the group consisting of memantine (1-amino-
3,5-dimethyladamantane), salts thereof, and mixtures
thereof.
Each and every feature described herein, and each and
every combination of two or more of such features, is
4
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
included within the scope of the present invention provided
that the features included in such a combination are not
mutually inconsistent. In addition, any feature or
combination of features may be specifically excluded from
any embodiment of the present invention.
These and other aspects and advantages of the present
invention are set forth in the following detailed
description, examples and claims.
Brief Description of the Drawings
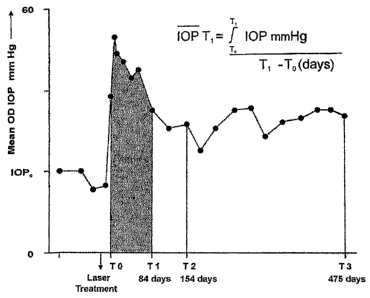
FIG. 1 is a graph of mean intraocular pressure (IOP) of
hypertensive (OD) eye of one animal as a function of time.
FIG. 2 is a graph of average OD IOP of eyes for
memantine-treated and vehicle-treated animals.
FIG. 3 provides graphs of ERG responses for a
normotensive (OS) eye. Panel A is a flash response. Panel
B is an oscillatory potential (OP) response. Panel C is a
flicker response.
FIG. 4 provides graphs of conventional ERG response
amplitude as a function of mean IOP for flash a-wave (panel
A), b-wave (panel B), OP (panel C), and flicker (panel D).
FIG. 5 provides graphs of multifocal ERG responses
obtained from one OS eye. Panel A is a trace array of first
order responses. Panel B is the average response of the
seven central traces in Panel A. Panel C is a trace array
of the second order responses. Panel D is the average
response of the second order responses in Panel C.
Calibrations are 800nV, 200 msec (panels A and C); 10
nV/deg2, 20 msec (panel B), and 5 nV/deg2, 20 msec (panel
D).
5
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
FIG. 6 provides graphs of normalized macular multifocal
ERG response amplitudes as a function of RGC count in the
RGC layer.
FIG. 7 provides graphs of multifocal ERG macular
response normalized peak amplitude as a function of mean IOP
for responses obtained at time T1.
FIG. 8 provides graphs of averaged response amplitudes
(nV/degree2) for hypertensive (OD) eyes from vehicle-
(filled squares) or memantine- (open circles) treated
animals at times Ti, T2, and T3.
FIG. 9 provides graphs of the VECP response amplitude.
Panel A is a response from stimulation of a normotensive
(OS) eye (calibration bars equal to 10 microvolts and 50
msec). Panel B is a plot of normalized (OD/OS) peak
amplitude as a function of mean IOP for both treatment
groups.
FIG. 10 is a graph of VECP response amplitude as a
function of perifoveal counts of cells in the RGC layer.
FIG. 11 provides graphs summarizing electrophysiology
measures obtained from stimulation of OS eyes of both
treatment groups at time T3.
FIG. 12 is a graph of average glutamate levels obtained
from vitreous samples from both eyes of animals in both
treatment groups at time T3.
FIG. 13 is a graph of a hypothetical model showing the
percentage of surviving RGCs as a function of time.
FIG. 14 is a graph of average IOP history for laser-
treated hypertensive (OD) eyes of memantine treated and
vehicle treated animals.
FIG. 15 is a diagram of the locations of retinal
samples used for histological analysis.
6
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
FIG. 16 provides fundus images (top panels) of
normotensive (OS) and hypertensive (OD) eyes, and
micrographs (bottom panels) of sections from the perifoveal
retinal sample region obtained from the same eye shown in
the fundus images.
FIG. 17 is a graph of RGC number as a function of IOP.
FIG. 18 is a graph of inferior RGC numbers for vehicle
treated animals and memantine treated animals.
FIG. 19 is a graph of RGC counts obtained from OS eyes
of both treatment groups.
FIG. 20 provides graphs of normalized cup measurements
from confocal laser scans at T2 for the five animals having
the highest mean IOPs in each treatment group.
FIG. 21 provides graphs of normalized neuroretinal rim
measurements at T2 from the animals in FIG. 20.
FIG. 22 provides graphs of three of the five cup
measurements shown in FIG. 20 from the hypertensive eye of
all five animals in each treatment group as a function of
time.
FIG. 23 provides graphs of three of the five
neuroretinal rim measurements shown in FIG. 21 from the
hypertensive eye of all five animals in each treatment group
as a function of time.
Detailed Description
The present methods provide desired therapeutic effects
employing certain therapeutic agents or therapeutic
components, such as anti-excitotoxic or neuroprotective
agents. The therapeutic agents are administered to an
individual, such as a human or an animal, to treat one or
more ophthalmic conditions, including disorders and diseases
of one or both eyes of the individual. The present methods
7
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
may reduce one or more symptoms associated with the
ophthalmic condition or conditions, and may prevent further
vision loss associated with the condition or conditions.
As used herein, an "anti-excitotoxic agent" is an agent
that reduces or prevents glutamate-induced cellular
toxicity.
As used herein, a "neuroprotective agent" is an agent
that reduces or prevents neuronal degeneration or neuronal
death.
As used herein, "treating" refers to the management,
prevention, reduction, and/or elimination of one or more
symptoms of one or more ophthalmic conditions. Treating
thus includes prophylactic treatment of an individual.
As used herein, an "ophthalmic condition" includes
diseases and disorders of one or more eyes of an individual.
Ophthalmic conditions typically negatively affect the health
of the individual, such as by negatively affecting the
vision of the individual, or by causing pain to the
individual. For example, an ophthalmic condition, such as
glaucoma, can be associated with vision loss, increased
intraocular pressure, retinal damage, and the like.
The present methods comprise administering one or more
therapeutic agents to an individual to treat one or more
ophthalmic conditions. The administration of the
therapeutic agent can include administering the agent
orally, topically, intraocularly, or by other systemic
routes, such as by intravenous injection, intramuscular
injection, and the like. The therapeutic agent or agents
are typically administered in compositions suitable for
pharmaceutical use, such as injectable compositions or
tablets, capsules, drops, and the like suitable for oral
and/or topical administration.
8
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
At least one of the therapeutic agents employed in the
present methods is an anti-excitotoxic agent. The anti-
excitotoxic agent may be administered with one or more
therapeutic agents that may be effective in treating
ophthalmic conditions. For example, the anti-excitotoxic
agent may be administered at approximately the same time as
an agent that is effective in reducing intraocular pressure
of an individual. The anti-excitotoxic agent may be
understood to be a neuroprotective agent since the anti-
excitotoxic agent reduces toxic effects induced by excessive
glutamate concentrations or amounts. Anti-excitotoxic
agents useful in the present methods may be agents which
prevent or reduce excessive intracellular calcium
concentrations. Thus, in accordance with the disclosure
herein, anti-excitotoxic agents include calcium channel
inhibitors, such as calcium channel blockers and
antagonists, and glutamate receptor inhibitors, such as
glutamate receptor antagonists or blockers. As used herein,
an "inhibitor" refers to an agent that reduces the activity,
such as ion flux, through a channel, or receptor-channel
complex. The inhibitor may provide its effect either by
directly binding to a channel or receptor, or may do so
indirectly by affecting one or more parameters that affect
the channel or receptor activity.
In certain of the present methods, the anti-excitotoxic
agent is an inhibitor of the N-methyl-D-aspartate (NMDA)
subtype of the glutamate receptor. Or, stated differently,
the anti-excitotoxic agent is an NMDA receptor antagonist.
An NNIDA receptor antagonist is typically an agent that
reduces neuronal damage mediated by the N.NDA receptor
complex. Examples of NMDA receptor antagonists useful in
the present methods are described in U.S. Patent Nos.
9
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
5,922,773, 6,482,854; and 6,573,280. In short, an NNIDA
receptor antagonist includes NMDA receptor channel blockers
(e.g., antagonists that operate uncompetitively to block the
NMDA receptor channel); receptor antagonists (e.g.,
antagonists that compete with NMDA or glutamate to act at
the NMDA or glutamate binding site); agents acting at either
the glycine co-agonist site or any of several modulation
sites, such as the zinc site, the magnesium site, the redox
modulatory site, or the polyamine site; or agents that
inhibit the downstream effects of NMDA receptor stimulation,
such as agents that inhibit activation of protein kinase C
activation by NMDA or glutamate stimulation, antioxidants,
and agents that decrease phosphatidyl metabolism. Some
specific examples of anti-excitotoxic agents include
amantadine derivates, salts thereof, and combinations
thereof. For example, the amantadine derivates may be
memantine, amantadine, and rimantadine. Other anti-
excitotoxic agents may include nitroglycerin, dextorphan,
dextromethorphan, and CGS-19755. Some compounds include
those in the following table
NMDA Antagonists NMDA Antagonists NMDA Antagonists
1. Competitive NMDA 2. Channel Blockers 3. Antagonists at
Antagonists (act at (Un-Competitve NMDA Glydne Site of the
agonist binding site) Antagonists) NMDA Receptor
CGS-19755 (CIBA- MK-801 (Dizocilpine) Kynurenate, 7-
GEIGY) and other and other chloro-kynurenate,
piperdine derivatives of 5,7- chloro-
derivatives, D-2- dibenzyocycloheptene kynurenate, thio-
amino-5- (Merck) derivatives, and
phosphovalerate, D-2- other derivatives.
amino-7- Sigma receptor (Merck)
phosphosoheptanoate ligands, e.g.
(AP7) CPP {[3-2- Dextrorphan, Indole-2-carboxylic
carboxypiperazin-4-y- dextromethorphan and acid
propyl-l-phosphonic morphiasn
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
acid]} derivatives (Hoffman DNQX
La Roche) such as
caramiphen and Quinoxaline or
rimcazole (which oxidiazole
also block calcium derivatives
LY 274614, CGP39551, channels) including CNQX,
CGP37849, LY233053, Ketamine, Tiletamine NBQX
LY233536 and other Glycine partial
0-phosphohomoserine cyclohexanes agonist (e.g.
Phencyclidine (PCP) Hoecht- Roussel P-
MDL100,453 and derivatives, and 9939
pyrazine compounds
4. Polyamine Site-of Memantine, 6. Other Non-
NMDA Receptor amantadine, Competitve NMDA
rimantadine and Antagonists
Arcaine and relate derivatives Hoechst 831917189
biguanidines and CNS 1102 (and
biogenic polyamines related bi- and tri- SKB Carvedilol
Ifenprodil and substituted
related drugs guanidines)
Diethylenetriamine SL Diamines
82,0715 Conantokan peptide
from Conus
1,10-diaminodecane geographus
(and related inverse Agatoxis-489
agonists)
5. Redox Site of
NMDA Receptor
Oxidized and reduced
glutathione
PQQ
(pyrroloquinoline
quinone)
Compounds that
generate Nitric
Oxide (NO) or other
oxidation states of
nitrogen monoxide
(NO+, NO-) including
those listed in the
box below
Nitroglycerin and
derivatives, Sodium
Nitroprusside, and
other NO generating
listed on p.5 of
this table
11
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Nitric oxide
synthase (NOS)
Inhibitors:
Arginise analogs
including N-mono-
methyl-L-arginine
(NMA); N-amino-L-
arginine (NAA); N-
nitro-L arginine
(NNA); N-nitro-L-
arginine methyl
ester; N-iminoethyl-
L-omithine
Flavin inhibitors;
diphenyliodinium;
Calmoduli
inhibitors,
trifluoperizine
Calcineurin
Inhibitors, e.g.,
FK-506 (inhibits
calcineurin and thus
NOS diphosphorylase)
Inhibitors of Inhibitors of Non-NMDA Receptor
Downstream Effects of Downstream Effects Antagonists
NMDA of NMDA
7. Agents to inhibit 8. Downstream 9A. Non-NMDA
protein kinase C effects from antagonists
activation by NMDA Receptor Activation (Competitive)
stimulation (Involved
in NMDA toxicity) 8a. To decrease CNQX, NBQX, YM900,
MDL 27,266 (Merrill phosphatidylinositol DNQX.
Dow) and triazoleone metabolism PD140532
derivatives kappa opioid AMOA (2-amino-3[3-
Mososialoganglioxides receptor agonist: 9carboxymethoxyl-5-
(eg GMI of Fidin U50488 (Upjohn) and methoxylisoxazol-4-
Corp.) and other dynorphan yl]propionate]
ganglioside
derivatives LIGA20, kapp opioid receptor 2-phosphophonoethyl
LIGA4 (may also agonist: PD117302, phenylalanine
affect calcium CI-977 derivatives, i.e.,
extrusion via calcium 5-ethyl, 5-methyl,
ATPase) 8b. To decrease 5-trifluoromethyl
hydrogen peroxide
and free radical 9B. Non-NMDA Non
injury, eg competitive
12
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
antioxidants 21- antagonists
aminosteroid GYK152466
(lazaroids) such as
U74500A, U75412E and Evans Blue
U74006F U74389F,
FLE26749, Trolox
(water soluble alpha
tocophenol), 3,5-
dialkoxy-4-hydroxy-
benzylamines
Compounds that
generate Nitric
Oxide (NO) or other
oxidation states of
nitrogen monoxide
(NO+, NO-) including
those listed in the
box below
Nitroglycerin and
derivatives, Sodium
Nitroprusside, and
other NO generating
listied on p.5 of
this table
Nitric oxide
Synthase (NOS)
In.hibition :
Arginine analogs
including N-mono-
methyl-L-arginine
(NMA); N-amino-L-
arginine (NAA); N-
nitro-L arginine
(NNA); N-nitro-L-
arginine methyl
ester; N-iminoethyl-
L-omithine
Agents Active at Decrease Glutamate Drugs to decrease
Metabotropic Release intracellular
Glutamate Receptors calcium following
glutamate receptor
stimulation
10a. Blockers of 11. Agents to 12a. Agents to
Metabotropic decrease glutamate decrease
Glutamate Receptors release Intracellular
13
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
AP3 (2-amino-3- calcium release
phosphonoprionic Adenosine, and Dantrolen (sodium
acid) derivatives, e.g., dantrium: Ryanodine
cyclohexyladenosine (or
10b. Agonists of CN51145 ryanodine+caffeine)
Metabotropic
Glutamate Receptors Conopeptides: SNX- 12b. Agents
(1S, 3R)-l-Amino- 111, SNX-183, SNX- Inhibiting
cyclopentane-l,3- 230 intracellular
dicarboxylic acid Calcium-ATPase
[(1S,3R)-ACPD], Omega-Aga-IVA, toxin Thaprigargin,
commonly referred to from venom of funnel cyclopiazosic acid,
as 'trans'-ACPD spider BHQ ([2,5-di-(tert
Compounds that butyl)-1,4-
generate Nitric benzohydroquinose])
Oxide (NO) or other
oxidation states of
nitrogen monoxide
(NO+, NO-) including
those listed in the
box below
Nitroglycerin and
derivatives, Sodium
Nitroprusside, and
other NO generating
listied on p.5 of
this table
Nitric oxide
Synthase (NOS)
Inhibitors:
Arginine analogs
including N-mono-
methyl-L-argini.ne
(NMA); N-amino-L-
arginine (NAA) ; N-
nitro-L arginine
(NNA); N-nitro-L-
arginine methyl
ester; N-iminoethyl-
L-omithine
Additional NO-
generating compounds
Isosorbide dinitrate
(isordil)
S-nitrosocaptopril
(SnoCap)
Serum albumin
coupled to nitric
14
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
oxide (SA-NO)
Cathepsin coupled to
nitric oxide
(cathepsin-NO)
Tissue plasminogen
activator coupled to
NO (TPA-NO)
SIN-i (also known as
SIN1 or
molsidonmine)
Ion-nitrosyl
complexes (e.g.,
nitrosyl-iron
complexes, with iron
in the Fe2+ state)
Nicorandil
Other agents which may be understood to be anti-
excitotoxic and useful in the present methods include
voltage-dependent calcium channel antagonists and
antagonists of non-NMDA receptors (glutamate receptor types
other than the NMDA receptor complex discussed above).
These non-NMDA receptor antagonists include agents which
block ionotropic glutamate receptors or interact with
metabotropic glutamate receptors, as understood by persons
of ordinary skill in the art. Other anti-excitotoxic agents
may act to limit or reduce release of glutamate from cells,
thereby acting upstream from the glutamate receptors in the
excitatory neurotoxicity process. Still other agents may
act by blocking downstream effects of glutamate receptor
stimulation, e.g., the intracellular consequences of
glutamate interaction with a cell membrane glutamate
receptor, such as agents (like dantrolene) that block the
rise in intracellular calcium following stimulation of
membrane glutamate receptors.
The therapeutic agents used in the present methods
preferably are those capable of crossing the blood-brain
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
barrier or the blood-retinal barrier; these agents may be
administered orally, intravenously, or topically and cross
intervening barriers including the blood brain barrier to
reach the retinal ganglion cells. Therapeutic agents that
do not freely cross the blood-brain barrier may be
administered intraocularly, such as by intravitreal
injection and the like so that the agent is delivered to the
retina. In the case of agents that have an intermediate
ability to cross the blood-brain barrier, the mode of
administration will depend on the dosage required and other
factors.
In certain methods of the present invention, the
therapeutic agent comprises an adamantane having the
following formula:
ZD Formula I
Adamantane
An adamantane-based amine is a compound having an amine
which is directly or indirectly bonded to or coupled with an
adamantane. In other words, the adamantane may be directly
bonded to the nitrogen of the amine, or a linking group
consisting of one or more atoms may connect the adamantane
to the amine. Additionally, the adamantane may have
additional substituents, such as a methyl group or a small
alkyl group, attached. A group comprising the basic cage
structure of adamantane and one or more substituents is
referred to as an "adamantyl" moiety. The term "amine"
should be understood as being broadly applied to both a
molecule, or a moiety or functional group, as generally
understood in the art, and may be primary, secondary, or
tertiary. While not intending to limit the scope of the
16
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
invention in any way, three compounds which are adamantane
based neuroprotective-amines, and are also neuroprotective
compounds comprising an adamantyl moiety and an amine
moiety, are amantadine, rimantadine, and memantine, as
illustrated below:
NH2 NH2 H3C NH2
H3C CH3
b --
Memantine Amantadine Rimantadine
The terms memantine, amantadine, and rimantadine as
used herein refer to the free base forms of the amine, or
any of the various salts, such as memantine hydrochloride,
which can be prepared by the addition of an acid to the free
base. The determination of the amount of memantine used in
the pharmaceutical or ophthalmic compositions is well within
the ability of one having ordinary skill in the art. An
"effective" amount of memantine is an amount which has a
detectable effect over a similar composition or method which
comprises no memantine or any other active ingredient which
would be expected to have an effect similar to that of
memantine.
In referring to concentrations of memantine herein, the
numeric value for the concentration is understood to be the
concentration of the free base, regardless of the form in
which the memantine is used. Since there is a large range
of concentrations or amounts at which memantine is
effective, the concentration or amount of memantine as used
herein may vary. In certain methods, a composition may
comprise from 0.05 to 5% memantine. Other compositions may
comprise from 0.05% to 2% memantine. Some compositions may
comprise from 0.05% to 2.5% memantine. Another composition
may comprise from 0.2% to 3% memantine. Some compositions
17
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
comprise from 0.1% to 2% memantine. Other compositions
comprise from 0.5% to 2% memantine. Other compositions
comprise from 0.5% to 3.5% memantine. Other compositions
comprise from 0.3 % to 1.5%. Another composition comprises
from 0.5% to 1.3% memantine. Other compositions comprise
from 0.1% to 1% memantine. Another compositions comprises
from about 0.5% to about 1% memantine. Other compositions
comprise about 0.5% memantine. Other compositions comprise
about 1% memantine.
With respect to the present methods, the therapeutic
agent may be an adamantane-based neuroprotective amine. For
example, the therapeutic agent may be an agent selected from
the group consisting of memantine, amantadine, rimantadine,
salts thereof, and mixtures thereof. In certain methods,
the therapeutic agent comprises memantine, salts thereof,
and mixtures thereof.
The therapeutic agents useful in the present methods
may be purchased from companies, such as Sigma Chemicals
(St. Louis, MO), or the therapeutic agents may be
synthesized using conventional chemical synthesis methods
readily known by persons of ordinary skill in the art.
Potential anti-excitotoxic agents, such as glutamate
receptor inhibitors, and the like, can be identified using
routine screening assays. For example, such agents can be
tested for binding to glutamate receptors in vitro, for
inhibition of glutamate-mediated electrical signals using
electrophysiological methods, or using the methods disclosed
in the examples herein. Anti-excitotoxic agents may be
further identified based on structural or functional
similarities with other anti-excitotoxic agents/
As discussed herein, the therapeutic agents may be
administered to an individual using any technique, including
conventional techniques, known to persons of ordinary skill
in the art, which are effective in delivering the
18
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
therapeutic agent to an eye of an individual, such as to the
retina of an eye, or to the brain or a region of the brain
of the individual. The therapeutic agent may be
administered in a liquid composition, such as a solution,
suspension, or emulsion, which may be administered by
injection or orally. For example, the therapeutic agent may
be provided in an aqueous liquid composition, a non-aqueous
liquid composition, or an oil-containing emulsion, such as
an oil-in-water emulsion or a water-in-oil emulsion. Or,
the composition may be in the form of tablets or capsules
which may be ingested to provide systemic delivery of the
therapeutic agent to the individual. Thus, the therapeutic
agent or agents used in the present methods may be provided
in formulations or compositions that may be administered by
topical, oral, rectal or parenteral (e.g. intravenous,
subcutaneous or intramuscular) routes, among others.
The compositions may be prepared using conventional
pharmaceutical techniques. Such techniques may include a
step of bringing into association the therapeutic agent, and
pharmaceutical carrier(s) or excipient(s). In general, the
formulations are prepared by uniformly combining the
therapeutic agent or agents with the carriers or excipients.
The therapeutic agents may be provided in single unit or
single dosage compositions, if desired.
Tablets may be made by compression or molding,
optionally with one or more accessory ingredients.
Compressed tablets may be prepared by compressing, in a
suitable machine, the therapeutic agent is presented as a
powder or granules, and optionally a binder, lubricant,
inert diluent, preservative, surface-active or dispersing
agent. Molded tablets may be made by molding, in a suitable
machine, a mixture of the powdered compound moistened with
an inert liquid diluent. The tablets may optionally coated
19
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
or scored and may be formulated so as to provide a slow or
controlled release of the therapeutic agent therein.
Compositions suitable for topical administration in the
mouth, include lozenges comprising the therapeutic agent in
a flavored basis, usually sucrose and acacia or tragacanth;
pastilles comprising the active ingredient in an inert basis
such as gelatin and glycerin, or sucrose and acacia.
Compositions suitable for topical administration to the
skin may be presented as ointments, creams, gels and pastes
comprising the therapeutic agent to be administered in a
pharmaceutical acceptable carrier. A preferred topical
delivery system is a transdermal patch containing the
therapeutic agent to be administered.
Compositions suitable for parenteral administration
include aqueous and non-aqueous sterile injection solutions
which may contain anti-oxidants, buffers, bacteriostats and
solutes which render the composition isotonic with the blood
of the intended recipient; and aqueous and non-aqueous
sterile suspensions which may include suspending agents and
thickening agents. The compositions may be presented in
unit-dose or multi-dose containers, for example, sealed
ampules and vials, and may be stored in a freeze-dried
(lyophilized) conditions requiring only the addition of the
sterile liquid carrier, for example, water for injections,
immediately prior to use.
Preferred unit dosage formulations are those containing
a daily dose or unit, daily sub-dose, as herein above
recited, or an appropriate fraction thereof, of the
administered ingredient.
It should be understood that in addition to the
ingredients, particularly mentioned above, the compositions
useful in the present methods may include other agents
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
conventional in the art having regard to the type of
formulation in question, for example, those suitable for
oral administration may include flavoring agents.
The exact formulation and dosage of the therapeutic
agent depends upon a number of factors known by persons of
ordinary skill in the art, including without limitation, the
route of administration, the size of the individual, and the
health of the individual. Generally, an effective daily
dose of the therapeutic agent will range from 0.01 mg/kg to
1000 mg/kg. For example, in an oral administration method,
the dose of the therapeutic agent may be from about 0.01
mg/kg/day to about 100 mg/kg/day. In certain methods, the
therapeutic agent may be administered in an amount of 0.1
mg/kg/day to about 10 mg/kg/day. In the examples described
herein, the therapeutic agent was memantine, which was
administered orally at a dose of about 4 mg/kg.
In certain embodiments, such as methods using a liquid
composition, it may be useful to include a buffer in
ophthalmic compositions to maintain the pH from about 6 to
about 8 for optimal comfort. Buffers used are those known
to those skilled in the art, and, while not intending to be
limiting, some examples are acetate, borate, carbonate,
citrate, and phosphate buffers. Tonicity agents such as
glycerin, mannitol, sorbitol, sodium chloride, and other
electrolytes may also be used in ophthalmic compositions to
adjust the concentration of dissolved material to the
desired isotonic range. Surfactants such as polysorbates,
poloxamers, alcohol ethoxylates, ethylene glycol-propylene
glycol block copolymers, fatty acid amides, alkylphenol
ethoxylates, or phospholipids may also be used in ophthalmic
compositions. Chelating agents may also be used in
ophthalmic compositions to enhance preservative
effectiveness. While not intending to be limiting, some
useful chelating agents are edetate salts, like edetate
21
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
disodium, edetate calcium disodium, edetate sodium, edetate
trisodium, and edetate dipotassium.
The administration of the therapeutic agent in
accordance with the present methods is effective in
treating, that is preventing, reducing, or eliminating one
or more symptoms, of one or more ophthalmic conditions.
Non-limiting examples of ophthalmic conditions which may be
treated with present methods include the following:
MACULOPATHIES/RETINAL DEGENERATION: Non-Exudative Age
Related Macular Degeneration (ARMD), Exudative Age Related
Macular Degeneration (ARMD), Choroidal Neovascularization,
Diabetic Retinopathy, Acute Macular Neuroretinopathy,
Central Serous Chorioretinopathy, Cystoid Macular Edema,
Diabetic Macular Edema.
UVEITIS/RETINITIS/CHOROIDITIS: Acute Multifocal Placoid
Pigment Epitheliopathy, Behcet's Disease, Birdshot
Retinochoroidopathy, Infectious (Syphilis, Lyme,
Tuberculosis, Toxoplasmosis), Intermediate Uveitis (Pars
Planitis), Multifocal Choroiditis, Multiple Evanescent White
Dot Syndrome (MEWDS), Ocular Sarcoidosis, Posterior
Scleritis, Serpignous Choroiditis, Subretinal Fibrosis and
Uveitis Syndrome, Vogt-Koyanagi-Harada Syndrome.
VASCULAR DISEASES/EXUDATIVE DISEASES: Coat's Disease,
Parafoveal Telangiectasis, Papillophlebitis, Frosted Branch
Angitis, Sickle Cell Retinopathy and other
Hemoglobinopathies, Angioid Streaks, Familial Exudative
Vitreoretinopathy.
TRAUMA.TIC/SURGICAL: Sympathetic Ophthalmia, Uveitic
Retinal Disease, Retinal Detachment, Trauma, Laser, PDT,
Photocoagulation, Hypoperfusion During Surgery, Radiation
Retinopathy, Bone Marrow Transplant Retinopathy.
PROLIFERATIVE DISORDERS: Proliferative Vitreal
Retinopathy and Epiretinal Membranes, Proliferative Diabetic
22
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Retinopathy, Retinopathy of Prematurity (retrolental
fibroplastic).
INFECTIOUS DISORDERS: Ocular Histoplasmosis, Ocular
Toxocariasis, Presumed Ocular Histoplasmosis Syndrome
(POHS), Endophthalmitis, Toxoplasmosis, Retinal Diseases
Associated with HIV Infection, Choroidal Disease Associated
with HIV Infection, Uveitic Disease Associated with HIV
Infection, Viral Retinitis, Acute Retinal Necrosis,
Progressive Outer Retinal Necrosis, Fungal Retinal Diseases,
Ocular Syphilis, Ocular Tuberculosis, Diffuse Unilateral
Subacute Neuroretinitis, Myiasis.
GENETIC DISORDERS: Systemic Disorders with Accosiated
Retinal Dystrophies, Congenital Stationary Night Blindness,
Cone Dystrophies, Fundus Flavimaculatus, Best's Disease,
Pattern Dystrophy of the Retinal Pigmented Epithelium, X-
Linked Retinoschisis, Sorsby's Fundus Dystrophy, Benign
Concentric Maculopathy, Bietti's Crystalline Dystrophy,
pseudoxanthoma elasticum, Osler Weber syndrome.
RETINAL TEARS/HOLES: Retinal Detachment, Macular Hole,
Giant Retinal Tear.
TUMORS: Retinal Disease Associated with Tumors, Solid
Tumors, Tumor Metastasis, Benign Tumors, for example,
hemangiomas, neurofibromas, trachomas, and pyogenic
granulomas, Congenital Hypertrophy of the RPE, Posterior
Uveal Melanoma, Choroidal Hemangioma, Choroidal Osteoma,
Choroidal Metastasis, Combined Hamartoma of the Retina and
Retinal Pigmented Epithelium, Retinoblastoma,
Vasoproliferative Tumors of the Ocular Fundus, Retinal
Astrocytoma, Intraocular Lymphoid Tumors.
MISCELLANEOUS: Punctate Inner Choroidopathy, Acute
Posterior Multifocal Placoid Pigment Epitheliopathy, Myopic
Retinal Degeneration, Acute Retinal Pigment Epithelitis,
23
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
cular inflammatory and immune disorders, ocular vascular
malfunctions, Corneal Graft Rejection, Neovascular Glaucoma,
closed-angle glaucoma, primary open-angle glaucoma,
pseudoexfoliation glaucoma, and the like.
In certain of the present methods, the ophthalmic
condition is associated with a loss of a visual field. For
example, the loss of the visual field, including a partial
loss or a complete loss, may be a symptom of the ophthalmic
condition, or may be a cause of the ophthalmic condition.
For example, ophthalmic conditions associated with elevated
intraocular pressure may result in a loss or a reduction in
the size of an individual's visual field. One example of
such a condition is glaucoma. Some ophthalmic conditions
treated by the present methods include retinal
neurodegenerative conditions, such as disorders or diseases.
In one embodiment of the present invention, a method
for treating an ophthalmic condition comprises administering
one or more therapeutic agents to an individual at a time
when the individual is not aware of a visual field loss.
The visual field loss is associated with the ophthalmic
condition. Thus, the administration of the therapeutic
agent may be understood to mitigate against the ophthalmic
condition.
As an example, a patient may not be aware of any visual
field loss, e.g., the patient believes he has normal vision,
but a physician may be able to diagnose the ophthalmic
condition at an early stage, before the visual field loss is
too substantial or noticeable by the patient. Thus, when
the ophthalmic condition is glaucoma, the individual or
patient may be understood to be a glaucoma suspect, that is
an individual who is suspected of being at an early stage of
glaucoma or is predisposed to developing glaucoma. High
24
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
risk glaucoma suspects, such as individuals who are ocular
hypertensive and/or exhibit suspicious optic cupping, may
have normal white on white Sita-Standard visual fields. The
therapeutic agent may be any therapeutic agents, including
the anti-excitotoxic agent, such as memantine, salts
thereof, and mixtures thereof, as described above.
In accordance with the present methods, the therapeutic
agent may be administered at a time when the individual has
less than about 80% of visual field loss. In certain
methods, the therapeutic agent is administered at a time
when the individual has less than about 40% of visual field
loss. For example, the therapeutic agent may be
administered at a time when the individual has less than
about 20%, such as less than about 10%, of visual field
loss. In certain embodiments, a method in accordance with
the disclosure herein is effective in preventing a
detectable decrease in visual field. The prevention of a
detectable decrease in visual field may be related to a
preservation of one or more visual responses within the
central nervous system, such as in the brain stem, thalamus,
and/or cortex.
As discussed herein, the ophthalmic condition may
comprise or be associated with an increased intraocular
pressure. For example, the method may be practiced to treat
glaucoma. As discussed herein, since the therapeutic agent
is administered before the patient is aware of a visual
field loss, the method may be effective in treating either
asymptomatic glaucoma or symptomatic glaucoma. The present
method may be effective in treating a retinal
neurodegenerative condition, which may or may not be
associated with glaucoma, but which typically results in a
loss or reduction of visual field.
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
The therapeutic agent may be chronically administered
to the individual. For example, the therapeutic agent may
be administered on a repeating schedule from the time of
original diagnosis by a physician until the individual no
longer requires treatment, such as when the ophthalmic
condition has been eliminated, or the patient is no longer
living. In certain methods, the administration may occur on
a daily basis, and may occur by administering one or more
units of single dose compositions disclosed herein.
Thus, the administration of the therapeutic agent may
be effective as a prophylactic to further deterioration of
the individual's vision resulting from the ophthalmic
condition. For example, the therapeutic agent may prevent
any noticeable vision loss from occurring thereby
maintaining the individual's vision and treating the
ophthalmic condition. In certain methods, the
administration of the therapeutic agent is effective in
reducing further visual field loss of the individual. For
example, an individual with a 20% loss of visual field may
be administered a therapeutic agent in accordance with the
present methods, and the individual may not experience any
greater loss of visual field.
The administration of the therapeutic agent may be
associated with reducing a decrease in a visually-evoked
cortical potential (VECP) in response to stimulation of an
eye. Additional information regarding the VECP may be found
in the examples herein. In short, a visual stimulus
activates retinal neurons which send an electrical signal
into one or more visual regions within the central nervous
system of an individual. A signal that can be recorded in
the visual cortex of the individual that receives the visual
signal is a VECP. When retinal ganglion cells are injured
26
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
or are destroyed resulting from an ophthalmic condition,
such as elevated intraocular pressure, the VECP, or one or
more components of the VECP, is decreased relative to the
VECP in an eye without the ophthalmic condition. This
decrease can be reduced by practicing the present methods.
While not wishing to be bound by any specific mechanism of
action, it is possible that the administration of the
therapeutic agent, such as the anti-excitotoxic agents
disclosed herein, prevents further retinal neuron
degeneration. The surviving retinal neurons, such as the
surviving retinal ganglion cells, may thus experience
changes and show an increase in neuronal growth, such as
axon terminal sprouting. This enhancement of interneuronal
connections (connections between neurons) may be effective
in maintaining the VECP of individuals having an ophthalmic
condition that results in a decrease of the VECP.
Thus, in one embodiment of the present methods, the
therapeutic agent is an NMDA receptor inhibitor, and chronic
administration of the NMDA receptor inhibitor is effective
in enhancing transfer of visual signals from surviving
retinal ganglion cells of the individual to at least one
central visual region of the central nervous system. The
neuronal growth may occur at one or more regions of the
visual system, such as the lateral geniculate of the
thalamus, the visual cortex, or the superior colliculus.
The therapeutic agent may be administered at or before
the onset of the ophthalmic condition, which is a time when
symptoms of the ophthalmic condition are first exhibited.
While a precise moment of onset of the ophthalmic condition
may not be determinable, with respect to glaucoma, a high
intraocular pressure at initial diagnosis of glaucoma is
27
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
indicative of a high IOP at onset of the ophthalmic
condition.
In certain embodiments, the therapeutic agent is
administered prior to an abnormal increase in glutamate
concentration in the vitreous of an eye of the individual.
For example, the therapeutic agent may be administered when
the vitreal concentration of glutamate is sub-toxic.
In certain embodiments, the therapeutic agent is
administered to the individual prior to the individual
undergoing any anti-glaucoma treatment. For example, prior
to receiving an intraocular pressure lowering drug. Or,
prior to a an individual undergoing a ophthalmic filtering
operation currently used to reduce intraocular pressure.
For example, the therapeutic agent may be administered to an
individual without operating on the individual to reduce
intraocular pressure of the individual.
Individual's who are not aware of a visual field loss
may be identified by one or more methods, which may be
conventional to persons of ordinary skill in the art.
For example, such individual's may be identified by
assessing retinal function. At least one method of
assessing retinal function is disclosed in U.S. Patent
Publication No. 2002/0133089 (Pasquale et al.).
In short, a patient having normal retinal function will
perceive an entoptic signal, which most commonly appears to
the patient as a blue arc. A patient's inability to
perceive this entoptic signal is correlated with the
likelihood that the patient's retina has experienced damage.
Thus, such an individual may be identified by selecting a
test site on the individual's retina and stimulating that
test site to cause the generation of an entoptic signal.
The patient may provide information whether he/she detects
28
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
the entoptic signal, or the entoptic signal, or the lack
thereof, may be detected using an ophthalmic instrument.
The entoptic signal is preferably detected after a period of
time in which the electrical activity of the retina has
decreased or become quiescent.
As another example, individuals who have an ophthalmic
condition and are not aware of visual field loss may be
identified using a method such as the method of diagnosing
glaucoma, as disclosed in U.S. Patent Publication No.
2003/0068632 (Garchon).
In short, the method disclosed by Garchon comprises
assessing an individual's alleles of the apolipoprotein E
(ApoE) gene, and/or assessing the individual's alleles of
the promoter of an ApoE gene, in order to determine whether
the individual has an ApoE4 allele (or two ApoE4 alleles),
and/or whether the individual has a "T" allele (or two "T"
alleles) of an ApoE gene promoter at (-491) (e.g., by
detection of the presence or absence of ApoE4 allele(s),
and/or by detection of the presence or absence of "T"
allele(s) of an ApoE gene promoter); and/or whether the
individual has an ApoE(-219G) gene promoter allele. Such
methods may be useful in individual's who have a mutation in
the gene for trabecular meshwork inducible glucocorticoid
response (TIGR) protein (a "carrier of a TIGR gene
mutation") or a mutation in the promoter of the TIGR gene (a
"carrier of a TIGR gene promoter mutation"). If it is not
known whether the individual is a carrier of a TIGR gene
mutation or a TIGR gene promoter mutation, the presence or
absence of a mutation in the TIGR gene or promoter can be
determined concurrently with the assessment of the ApoE
alleles and/or the ApoE gene promoter alleles.
29
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
In a carrier of a TIGR gene mutation, the presence of
an ApoE4 allele is indicative of an increased risk of
developing early-onset glaucoma, compared with the risk of a
carrier of a TIGR gene mutation with no ApoE4 alleles. The
presence of an ApoE4 allele in a carrier of a TIGR gene
promoter mutation is indicative of a decreased risk of
developing glaucoma with a high intraocular pressure at
onset of disease, compared with the risk of a carrier of a
TIGR gene promoter mutation with no ApoE4 alleles. The
absence of any ApoE4 alleles in a carrier of a TIGR gene
mutation is indicative of a decreased risk of developing
early-onset glaucoma, compared with the risk of a carrier of
a TIGR gene mutation with an ApoE4 allele. The absence of
any ApoE4 alleles in a carrier of a TIGR gene promoter
mutation is also indicative of an increased risk of
developing glaucoma with a high intraocular pressure at
onset of disease, compared with the risk of a carrier of a
TIGR gene promoter mutation with an ApoE4 allele.
The combination of an ApoE4 allele and a "T" allele of
a ApoE gene promoter (at -491) in an individual carrying a
mutation in the TIGR gene is also indicative of an increased
risk of developing early-onset glaucoma, compared with the
risk of a carrier of a TIGR mutation with an ApoE4 allele
but no "T" alleles of a ApoE gene promoter. The absence of
any "T" alleles of an ApoE gene promoter in a carrier of a
TIGR mutation with an ApoE4 allele is indicative of a
decreased risk of developing early-onset glaucoma, compared
with the risk of a carrier of a TIGR mutation with an ApoE4
allele and a"T" allele of an ApoE gene promoter.
The presence of a "T" allele of an ApoE gene promoter
in an individual, regardless of whether a mutation in the
TIGR gene is present or absent, is indicative of an
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
increased risk of developing glaucoma with a high
intraocular pressure at onset of disease, compared with the
risk of an individual who has no "T" alleles of an ApoE gene
promoter. The absence of a"T" allele of an ApoE gene
promoter in an individual, is indicative of a decreased risk
of developing glaucoma with a high intraocular pressure at
onset of disease, compared with the risk of an individual
who has a "T" allele of an ApoE gene promoter. Furthermore,
if the individual is a carrier of a TIGR gene promoter
mutation (e.g., a (-1000G) mutation), the presence of an
ApoE(-491T) gene promoter allele is indicative of an even
greater increased risk of developing glaucoma with a high
intraocular pressure at onset of disease, compared with the
risk of such an individual who has no ApoE(-491T) gene
promoter allele or TIGR gene promoter mutation.
The presence of an ApoE(-219G) gene promoter allele is
indicative of an increased risk of developing glaucoma with
a high visual field score, a high cup/disk ratio, or both,
compared with the risk of an individual who has no ApoE(-
219G) gene promoter allele. The absence of an ApoE(-291G)
gene promoter allele in an individual is indicative of a
decreased risk of developing glaucoma with a high visual
field score or a high cup/disk ratio, compared with the risk
of an individual who has an ApoE(-219G) gene promoter
allele.
The ApoE alleles and/or the ApoE gene promoter alleles
in an individual can be assessed by a variety of methods,
including hybridization methods (e.g., Southern or Northern
analysis), sequencing of the gene and/or the gene promoter,
allele-specific oligonucleotide analysis, analysis by
restriction enzyme digestion, or (in the case of the ApoE
alleles) by analysis of the ApoE protein(s) (e.g.,
31
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
spectroscopy, enzyme-linked immunosorbent assay,
colorimetry, electrophoresis, isoelectric focusing,
radioimmunoassay, immunoblotting (such as Western
blotting)). Several methods of assessing the ApoE alleles
are described in detail in U.S. Pat. No. 5,508,167 (Roses et
al.). Similar methods can be used to assess the alleles of
the ApoE promoter.
For example, in one method of assessing the ApoE
alleles in the individual, hybridization methods, such as
Southern analysis, can be used (see Current Protocols in
Molecular Biology, Ausubel, F. et al., eds., John Wiley &
Sons, including all supplements through 1998). For example,
a test sample containing genomic DNA, RNA, or cDNA that
includes the ApoE gene or encodes ApoE protein can be used.
Such genomic DNA, RNA and cDNA are referred to herein
collectively as "nucleic acids comprising the ApoE gene".
The test sample is obtained from an individual (the "test
individual"). The individual can be an adult, child, or
fetus. The test sample can be from any source which contains
DNA, RNA or cDNA, such as a blood sample, cerebrospinal
fluid sample, or tissue sample (e.g., from skin or other
organs). In a preferred embodiment, a test sample containing
nucleic acids comprising ApoE gene is obtained from a blood
sample, a fibroblast skin sample, from hair roots, or from
cells obtained from the oral cavity (e.g., via mouthwash).
In another preferred embodiment, a test sample containing
nucleic acids comprising the ApoE gene is obtained from
fetal cells or tissue by appropriate methods, such as by
amniocentesis or chorionic villus sampling.
To assess the ApoE alleles, a hybridization sample is
formed by contacting the test sample containing the nucleic
acid comprising the ApoE gene, with at least one nucleic
32
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
acid probe. The hybridization sample is maintained under
conditions which are sufficient to allow specific
hybridization of the nucleic acid probe to the nucleic acid
comprising the ApoE gene. "Specific hybridization", as used
herein, indicates exact hybridization (e.g., with no
mismatches). Specific hybridization can be performed under
high stringency conditions or moderate stringency
conditions, for example. "Stringency conditions" for
hybridization is a term of art which refers to the
conditions of temperature and buffer concentration which
permit hybridization of a particular nucleic acid to another
nucleic acid in which the first nucleic acid may be
perfectly complementary to the second, or the first and
second nucleic acids may share only some degree of
complementarity. For example, certain high stringency
conditions can be used which distinguish perfectly
complementary nucleic acids from those of less
complementarity. "High stringency conditions" and "moderate
stringency conditions" for nucleic acid hybridizations are
explained in chapter 2.10 and 6.3, particularly on pages
2.10.1-2.10.16 and pages 6.3.1-6 in Current Protocols in
Molecular Biology, supra, the teachings of which are hereby
incorporated by reference. The exact conditions which
determine the stringency of hybridization depend on factors
such as length of nucleic acids, base composition, percent
and distribution of mismatch between the hybridizing
sequences, temperature, ionic strength, concentration of
destabilizing agents, and other factors. Thus, high or
moderate stringency conditions can be determined
empirically. In one embodiment, the hybridization conditions
for specific hybridization are moderate stringency. In a
33
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
particularly preferred embodiment, the hybridization
conditions for specific hybridization are high stringency.
= Specific hybridization, if present, is then detected
using standard methods. If specific hybridization occurs
between the allele-specific nucleic acid probe and an ApoE
gene in the test sample, then the individual has the allele
of ApoE to which that nucleic acid probe hybridizes. More
than one nucleic acid probe can also be used concurrently in
this method (e.g., a probe that hybridizes to an ApoE2
allele and a probe that hybridizes to an ApoE3 allele).
Similar methods can also be used to assess the ApoE
gene promoter alleles, using a sample which contains nucleic
acids of the gene promoter (and amplified copies of the gene
promoter or portion of the gene promoter, if amplification
is performed) and allele-spepific nucleic acid probes that
hybridize to only one of the two ApoE gene promoter alleles.
In addition, these methods can be used to assess both the
ApoE alleles and the ApoE gene promoter alleles
concurrently, using a sample which contains nucleic acids
comprising the ApoE gene and also comprising the ApoE gene
promoters (and amplified copies of the gene and gene
promoter, or portions of the gene or gene promoter, if
amplification is performed), at least one allele-specific
nucleic acid probe that hybridizes to one of the ApoE
alleles, and an allele-specific nucleic acid probe that
hybridizes to an allele of the ApoE promoter. For example,
genomic DNA comprising the ApoE promoter and the ApoE gene
can be amplified concurrently and then assessed for the
alleles of the gene and the promoter.
Additional methods which may be used in conjunction
with or instead of the methods described above, are well
known and routine to persons of ordinary skill in the art.
34
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
In addition or alternatively, individual's having an
ophthalmic condition without a noticeable loss of visual
field may be identified using scanning laser polarimetry.
For example, the scanning laser polarimetry may be used to
evaluate the optic nerve of one of the eyes of the
individual. This method may be particularly useful in
individual's with normal-tension glaucoma. This method
examines the retinal nerve fiber layer (RNFL) using imaging
techniques. Thus, it is possible to check neural rim
integrity for thinning and notching, and assess any loss in
the RNFL. The contour, cupping, curvature of the optic
nerve, and hemorrhage on the disc margin, may suggest damage
to the individual's eye.
In another embodiment, a method of treating an
ophthalmic condition comprises administering a therapeutic
agent to an individual with an ophthalmic condition
associated with retinal neurodegeneration. The
administering of the therapeutic agent is effective in
reducing a decrease in a central nervous system response
associated with the retinal neurodegeneration.
Similar to that described above, the ophthalmic
condition of the foregoing method may comprise an increased
or elevated intraocular pressure. For example, the
ophthalmic condition may be glaucoma, including asymptomatic
or symptomatic glaucoma. Or the ophthalmic condition may be
a condition other than glaucoma. The ophthalmic condition
may be an early stage retinal neurodegenerative disorder.
The administration of the therapeutic agent may be
systemic or intraocular, such as by injection or ingestion
or topical application.
As discussed hereinabove, the administration of the
therapeutic agent may be effective in reducing a decrease in
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
the VECP in response to stimulation of the eye, the decrease
being associated with or a result of the ophthalmic
conditions.
In a further embodiment of the present invention, a
method for treating an ophthalmic condition comprises
administering a therapeutic agent to an individual with a
reduced visual field caused by the ophthalmic condition.
The administration of the therapeutic agent may be effective
in enhancing the reduced visual field. For example, after a
prolonged administration of the therapeutic agent, such as
memantine, it is believed that retinal neurons may also
exhibit neuronal growth. This neuronal growth may be
effective in increasing the size of the visual field, or in
enhancing the efficacy of transmission of visual signals
within a visual field.
The methods of the present invention may be effective
in preserving a visually-evoked cortical response without
substantially reducing retinal ganglion cell loss resulting
from the ophthalmic condition. However, as discussed above,
it may be possible to enhance the visual field with the
remaining retinal neurons. In addition, chronic
administration of the therapeutic agent may be effective in
enhancing transfer of electrical signals from surviving
retinal ganglion cells to at least one central visual region
of the central nervous system. The present methods may be
effective in enhancing adaptive response to injury within
the central nervous system.
The following non-limiting examples illustrate certain
aspects of the present invention.
EXAMPLE 1
36
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Methods of Determining Efficacy and Safety of Memantine
Treatment in Experimental Glaucoma
Eighteen young adult cynomolgous monkeys, Macaca
fascicularis, were randomly divided into two groups and base
line measures of intraocular pressure (IOP) were made. One
group of monkeys received oral doses of memantine (4 mg/kg),
and the other group received oral doses of a vehicle
control. This dose of memantine had no significant effect
on IOP in normotensive or ocular hypertensive monkey eyes.
Chronic ocular hypertension (COHT) in the right eye of
the monkeys was induced by exposure of the right eye to
argon laser, as described in Gaasterland et al.,
"Experimental glaucoma in the rhesus monkey", Invest.
Ophthalmol., 13:455-457, 1974. Using an argon laser (model
Novus 2000, Coherent, Inc., Palo Alto, CA), 30 to 40 spots
of 50 m diameter, 1 watt power, and 0.5 second duration
were applied over the superior 180 of the internal anterior
chamber angle. Two weeks later, the inferior 180 of
anterior chamber angle tissue was similarly treated.
IOP was measured under light ketamine sedation (5 mg/kg
i.p.) at regular intervals for about 16 months following the
laser treatment. IOP measurements were made between 8:00 AM
and 12:00 PM since this time of day is associated with
little diurnal variation in IOP. For IOP less than or equal
to 45 mm Hg, a Model RT pneumatonometer (Digilab, Norwell,
MA) was used, and for IOP greater than 45 mm Hg, an Alcon
pneumatonometer (Alcon, Fort Worth, TX) was used. Stable
pressure traces of approximately 4.5 seconds duration were
obtained. IOP was determined as the mean value of the
maxima and minima associated with cardiac pulsation, which
was less than 2 mm Hg peak-to-peak amplitude.
37
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Memantine concentrations were determined by obtaining
blood samples two hours after oral dosing from each animal
at two months after the onset of dosing and at two-month
intervals thereafter. Before sacrificing the treated
animals (about 16 months after laser treatment), a sample of
the vitreous humor was also obtained from each eye of each
animal. An 18-guage needle was used to obtain the sample
from the central vitreous. Vitreous samples were stored at
-700 C and were kept for less than 3 months before the
glutamate assay, described herein.
The amount of memantine in the plasma and vitreal
samples was determined. Forty microliter memantine
hydrochloride standards (0.2 M, 0.4 M, and 0.8 M) were added
to 40 microliter plasma or vitreous samples. Protein was
precipitated by adding 190 microliter acetonitrile,
vortexing for 2 minutes, followed by centrifugation at 5000g
for 4 minutes. 150 microliters of the supernatant was then
derivatized with 9-flourenylmethyl chloroform chloride
(FMOC-Cl) by mixing with 10 microliters of 15 mM FMOC-Cl in
acetonitrile and 10 microliters of 0.5 M pH 8.5 borate
buffer. Five minutes after derivitazation, 90 microliters
of the derivatized mixture was injected into a Gold HPLC
system (Beckman Instruments, Brea, CA) coupled to a Shimadzu
RF-551 fluorescence detector (Shimadzu, Sumo Sushi, Japan).
A Beckman ODS ultrasphere C-18 column (4.6 x 150 mm), a
mobile phase consisting of 60% acetonitrile, and 40% 50 mM
borate buffer, and a flow rate of 2 mL/min were used to
elute the memantine-FMOC at approximately 40 minutes. The
quantity of memantine in each plasma or vitreous sample was
determined, in triplicate, from a standard curve generated
for each individual sample.
38
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
The amount of glutamate present in the vitreous samples
was determined. Ten microliters of either a 10-, 20-, or
40-micromolar glutamate standard were added to a 90
microliter vitreous sample. Protein was precipitated by the
addition of 190 microliters of acetonitrile, followed by
vortexing for 2 minutes and centrifugation at 500g for 4
minutes. Twenty microliters of supernatant was mixed with
40 microliters of Fluoraldehyde OPA reagent (Pierce,
Rockford, IL) followed, after 2 minutes, by the addition of
100 microliters pH 7 phosphate buffer (PB). Sixty
microliters of this mixture was then injected into a Beckman
Gold HPLC system (Beckman Instruments), which was coupled to
a fluorescence detector (Shimadzu RF-551). A Beckman ODS
Ultrasphere C-18 column was used in combination with a
mobile phase gradient of 10% methanol/90% 50 mM phosphate
buffer to 75% methanol/25% phosphate buffer, a flow rate of
1.5 mL/min, and a duration of 8 minutes. Elution of 1-
alkylthio-2-alkylisoindole glutamate was observed at
approximately 6.5 minutes.
Data were collected and analyzed using software. Each
concentration was determined in triplicate, and the quantity
of glutamate in each vitreal sample was determined from a
standard curve, which was generated for each sample.
Electroretinogram (ERG) recordings were made from both
eyes of all animals at approximately 3 months (Tl), 5 months
(T2), and immediately before sacrifice at 16 months after
laser treatment (T3). Each normotensive (OS) eye was used
as an internal control for the effects of ocular
hypertension. The electrophysiological response amplitude
measures from the hypertensive eyes were normalized with
respect to the response amplitude measures obtained from the
39
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
contralateral eye of the same animal at the same recording
session.
ERG recordings were made under anesthesia and paralysis
was maintained with periodic injections of ketamine (15
mg/kg) and constant infusion of norcuronium (0.04 mg/kg/h).
A single drop of 1% tropicamide yielded pupillary apertures
of 5-6 mm diameter during recordings. Corneal voltage was
recorded using a bipolar contact lens electrode of the
Burian-Allen type (Hansen Ophthalmic Laboratories, Iowa
City, IA) while a subcutaneous (s.c.) needle placed at the
glabella was used as the indifferent electrode.
Conventional ERG responses were elicited with diffuse flash
stimuli of approximately 10 microseconds duration, which
were generated by a Grass Model P33 photostimulator (Astro
Med, West Warwick, RI). The stimulus, positioned at 10 cm
anterior to the cornea on the visual axis, subtended
approximately 50 of visual angle centered on the fovea.
Flash responses and oscillatory potentials (OPs) were
elicited with flashes of 124 photopic cd s/m2 intensity
delivered at 10-second intervals after 5 minutes of dark
adaptation at an ambient room illumination of approximately
0.05 footcandles. This initial 5-minute period of dark
adaptation was chosen to ensure that the adaptational state
during the recording was stable and consistent from one
recording session to the next. Under these conditions, the
adaptational state was determined by stimulus intensity in
combination with stimulus frequency. Response amplitude and
kinetics stabilized rapidly after onset of a stimulus
series. For flicker responses, 30 Hz stimulus trains of 512
msec duration and 78 photopic cd s/m2 intensity were
delivered every 1 second bandpass filtering from either 3-
1000 Hz (flash and flicker responses) or 100-1000 Hz (OPs)
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
used in conjunction with 60 Hz notch filtering. Flash and
OP responses obtained under these conditions likely reflect
activity of both rod and cone photoreceptors with a
relatively greater contribution from cone activity. Thirty
Hz flicker responses reflect predominantly cone-driven
activity.
For multifocal recordings, stimuli were generated on a
21 inch monitor (Radius Intercolor, Radius, Inc., San Jose,
CA) using VERIS 1 software and video driver board (Electro
Diagnostic Imaging, San Mateo, CA) and consisted of an array
of 61 hexagonal elements of equal size. The stimulus field
was positioned such that the fovea projected to the center
of the central stimulus element. At the test distance of 30
cm, the stimulus field subtended approximately 50 of visual
angle and thus illuminated the same retinal area, which was
stimulated for conventional recordings. The luminous
intensity of each stimulus element was temporally modulated
in a stepwise fashion at a frame rate of 67 Hz between a
maximum intensity of 95 cd/m2 (white) and a minimum
intensity of 5 cd/m2 (black) according to a binary m-
sequence. An m-sequence of 15 (215 stimulus frames) was
used in resulting records of approximately 8 minutes
duration. Signals were bandpass filtered from 3-300 Hz in
conjunction with 60 Hz notch filtering.
Recordings of the VECP were made using an active
electrode located on the scalp immediately anterosuperior to
the inion on the midline. The s.c. needle at the glabella
was used as the reference and an a.c. needle placed at the
base of the neck on the back was used as the indifferent
electrode. Responses were elicited using the same stimuli,
delivered at 2-second intervals, as that used for the ERG
41
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
flash and OP responses. VECP signals were bandpass filtered
from 3-1000 Hz in conjunction with 60 Hz notch filtering.
The same recording sequence was used for all animals at
all time points: 1) multifocal ERG, OD; 2) conventional ERG
(VECP), OD; 3) multifocal ERG, OS; and 4) conventional ERG
(VECP), OS. During recording, the contralateral eye was
occluded. After placement of the contact lens electrode
over the eye, retinoscopy determined the best spherical
equivalent lens power to make the retina optically conjugate
to the multifocal ERG stimulus monitor. This lens
(typically +3 to +5 diopters) was then positioned at 1 cm
anterior to the cornea. The stimulus monitor was then
positioned at 30 cm anterior to the cornea such that the
estimated visual axis projected to the center of the
stimulus field. A series of multifocal recordings of
approximately 2 minutes duration (m sequence = 13) was then
used to adjust the monitor position such that the fovea
projected to the center of the stimulus field and a clear
amplitude maximum was obtained for the first-order response
associated with the central stimulus element. Since a
prominent central macular peak was always observed, even in
eyes with severe retinal ganglion cell loss, this method
provided reliable stimulus alignment in all eyes of both
treatment groups. The precision of this method for stimulus
alignment was verified in several eyes by optically
projecting the fundus onto the stimulus monitor and noting
the location of the optic nerve head and macular image.
Stimulus alignment was also verified for each recording by
observing the location of the optic nerve head projection
(response minimum) in the first-order response trace array.
After stimulus alignment, a multifocal recording of
approximately 8 minutes duration (m sequence = 15) was made.
42
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Maintenance of neuromuscular block ensured that proper
stimulus alignment was maintained during the entire
recording session. The stimulus monitor was then covered
with a light-tight shield, the corrective lens was removed,
and the xenon flash stimulator was positioned. After a 5-
minute period of dark adaptation, conventional recordings of
the flash, OP, and flicker ERG responses were made in that
order. At the final time point (T3), the VECP response was
recorded after the flicker ERG response.
Simultaneous stereo pair fundus images were obtained
using a stereo fundus camera (Nidek Inc., Fremond, CA)
through pupils dilated approximately 6 mm diameter with 1%
tropicamide, as discussed herein. Rigid contact lenses were
used to provide an optical surface for retinal imaging.
Images were captured using Kodak Lumiere 100 ASA color slide
film in combination with a flash level (intensity) of 3.
Optic disc morphology measurements were made using a
confocal scanning laser ophthalmoscope (HRT; Heidelberg
Engineering GMBh, Heidelberg, Germany). Each image series
contained 32 transverse optic sections obtained at
consecutive height planes over a scan depth of 1.5 to 2.5
mm. For each eye, three separate 15 images were taken and
mean topography was determined using HRT software version
2.01. For each image, disc margins were manually outlined
with the aid of stereo optic disc photographs obtained at
approximately the same time. Image magnification errors
were corrected using keratometric measurements of corneal
anterior surface curvature. The standard reference plane
was positioned at 50 micrometers posterior to the mean
height of the optic disc margin contour over the temporal
segment from 350 to 356 . Scans were made under general
anesthesia induced with i.m. ketamine (10 mg/kg) in
43
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
combination with paralysis maintained with continuous IV
infusion of norcuronium bromide (0.04 mg/kg/h). Scans were
obtained at approximately 3, 5, and 10 months after laser
treatment, as shown in FIG. 14. Measurements were made on
both eyes of ten animals including the five animals with the
highest IOPs (OD) in each of the two treatment groups.
To count retinal ganglion cells (RGCs), fixed retinas
were flat mounted and 3 mm x 3 mm samples were obtained from
eight regions including one centered on the fovea. After
paraffin embedding, ten radial sections were obtained from
each sample region. For the sample centered on the fovea,
sections were obtained from the region of highest RGC
density at 500 to 700 micrometers from the center of the
foveal pit. Locations from which retinal histological
sections were obtained are illustrated in FIG. 15. After
staining with hematoxylin/eosin, the nuclei in the RGC layer
were counted along the entire 3 mm length of all ten
sections from each sample region. Glia and vascular cell
nuclei were identified using size and morphology (greater
than 4 micrometer diameter and round, respectively) and were
not counted. For sections from the perifoveal region, cell
counts were made using a Bioquant imaging system and
stereology software (R&M Biometrics, Nashville, TN).
Sections from all other sample regions were counted
manually. Examples of parifoveal (PF) sections obtained
from hypertensive (OD) and normotensive (OS) eyes of a
vehicle treated animal are shown in FIG. 16.
For each animal, OD IOP was plotted as a function of
time over the duration of the study. Heidelberg Retina
Tomograph (HRT) measurements were obtained at three time
points (T1 = 3 months; T2 = 5 months; and T3 = 10 months) .
Mean IOP elevation was estimated over each time interval (TO
44
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
to T1, TO to T2, or TO to T3) by first integrating the area
of this plot over the limits defined by the time at which
the first elevated IOP measure was obtained (soon after
laser treatment; TO) and the time at which the
electrophysiological measures were obtained (either T1, T2,
or T3). The integral was then divided by the number of days
in that interval to provide the mean IOP for that interval.
This method is illustrated in FIG. 1 for the OD IOP plot
from one animal. Values obtained from all animals for the
average of the three baseline (before laser treatment)
measures, the peak IOP measure, and the mean of the IOP
integral for all three time intervals are summarized in
Table 1, where values for normotensive (OS) eyes are shown
in parenthesis. The range and mean ( SEM) values are also
shown for each treatment group. Since IOP in the
normotensive eyes was similar to baseline at all time
points, values for the three intervals were determined for
these eyes as the average of measures over each interval.
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Table 1
I(?p
(mM ag)
I3ase3line TOP peal.c 1CO7P
1Vf.cankey # (,n.m Hg) (sum .EW T!. T2 T3
Vehicle-Trea'ted Animals
79 18.5 (I8.3) 34.5 24.0 (2C1.1) 23.4 (1.9.5)
105 19.3 td9.'1) 46.5 28.6 (20A) 26.4 M.41) 27.3 (21.8)
106 18.8 (18.7) 55.0 32.9 (26,1) 31.5 (i9,7? 28,9 (20.6)
98 21.5 (21.7) 45.0 30.8 (19.2) 30.0(19.3) 31.2 (20.3)
104 19.1(19.5) 61.0 54.5 (19.3) 49.2 C20.0) 36.3 C19.4)
gd 22.1 (21.8) 50.0 38.7 (19.4) 40.t1 C18.5) 37A (20.9)
91 18,5 (18.5) 55.0 511.0 ('19.'i) 49.6 (13.it) 49.0(19.9)
93 1.9.0 {19.03 55.9 53.4 (19.7) 52.6 ('18.5) 52.5(19.2)
92 18.7(19.1) 61,5 51.1 (20.~i 53.1 Q1,t1=) 57.7 (Z0.2)
Range 38.5-22.1 34.5-61.5 24.3-54.5 24.0-53.1 23.4-57.7
(1I3.3-21.8) (18.9-2,D.6) (18.5w22.(3) (19 2-21.8)
x t sFnl 19,5 - 0.45 51.6 2.8 4o.6 t 4.o 3916 3.9 38.1 4.0
(19.5 UA3) (19.7 (1.20) (20.t7 Q.4) (20.2 -!' 0.3)
Memantine "['r.eated A'ci.tnuls
108 19.3 (38.7) 46.5 25.4 (18.4) 24.3 (18.8) 23,9 (19.5)
102 17.5 ('17.0) 55.0 317 (18.9) 28.47 (16.8) 25.6 (17,8)
99 21.1(211.7) 31.5 25.2 t17.3..) 24.7 (x13.1i) 26.2 (20.1)
107 19.1 09.0) 48. + 35.6 (20.5) 31.4(16.0) 29.2 (18.6)
97 21. ],01.C1) 47.0 43.3 (2ll.t) 38,8 (2'J.Et) 29.3 (4.0)
100 193 (19.3) 59.0 51.5 (21.4) 42.6(20.6) 34.1. (20.0)
94 18.5 (18.5) 53.0 43.6 (j.9.6) 30.9 Cx6.5) -35.3 (18.7)
95 21.1(14.7a 60.0 56.9M,4) 53.5 (2t1.6) 42.7(21.I)
101 <.r1.2) 61.5 54.8(19.9) 58,4(17.8) 57.0 (20.0)
Range 27.5-21.1 31.5=-61.5 25.2-56.9 24.3-58.4 23.9-57.0
(1.4.7w21.2) (17.1 21.4) ('F6.0w20.6) (17.8-21.1)
x 6eM 19.i3 t 0.44 50.4 3.3 41.0-?- 4.0 37.8 :t CO 33,7 3.5
(18.9 0.70) (19.5 0.5) (18.4 0.6) (19.7 ~t 0.4)
VaEues for r.an.tr4atcra.t normotensive (OS) cpeR are shown in paro.~ntheses.
Linear regression analysis (see FIGs. 4, 6, 7, and 9)
was made using the method of least squares. To test the
hypothesis that the slopes of the regressions for two data
sets were the same, it was verified that each data set could
be reasonably well fit to a linear model. The probability
(P) that any difference in slope could have occurred by
chance was then determined by application of a general
linear models procedure for analysis of covariance. All
results of ERG and VECP measures were analyzed in this
manner and are summarized in Table 2.
46
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Table 2
TI õ- ..a........_ Tz Ta
slOpe a= Slope aN'X vs 10P. h 0.21 ~-(3.#J024~? 0.12
u'shicle f3.+U0668 0.~#t2 0.00533
Mtinaxxiizt.z --fl.tlfi:t83 0.09 O.fJUS~E~'i '0:3~. 0.00i7~ 0.06
P-value 0.404 0.311 0,973
N2-P2 tirs.lOP
tlehide -0.04054 0.76 -0.i~x8~ t3.7'6 -0.03155 0.73
~73(199 0.71
Mexnaxstiae --Q.m68 0.82 -0.0t788 0.54 --~ 0.03
;~~~al~re 0.032 0.172
P2-N3 vs IOi'
Vehlclc --0.1334192 0.97 -0,02027 0.54 -0.04142 0.66
Mexn.aindn.e 0.00057 0.03 -=[3.E0525 0.29 -4.o4596 Ã1.67
RvatÃae 0,0002 0.031 0.2%)
P-N vs IOP
Vehlcle --0.04483 0.94 -0.03IDI4 0.37 --QA034 0.80
Me.-fnalatine -0.02407 0.83 -0.E31313 0.36 -il.o5136 0.509
P-value 0.238 0.018 0.966
V-FGI'nipl vs li.7P ..-0.02835 0.89
Vehicle -{k.0750 0.38
Meniantine {]
.#1~0
P-va6ue
The method of least squares was used for linear
regression analysis of data sets which express histologic
(FIG. 16) or morphologic measurements from the two treatment
groups (vehicle or memantine) as a function of mean IOP.
This method provided measurements of slope (m) and
correlation coefficient (r) for each data set. Analysis of
covariance with both treat group and mean IOP as covariates
was used to test whether the slopes for regressions of the
two treatment groups were equivalent.
Example 2
Functional Measures of Memantine Treatment in
Experimental Glaucoma
FIG. 1 illustrates a method for estimating mean IOP of
the hypertensive (OD) eyes over three time intervals for
electrophysiological measures for the monkeys described in
Example 1. The data in FIG. 1 were obtained from monkey M94
47
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
in Table 1. TO is the first IOP measure after laser
treatment at which IOP was elevated above baseline. TO is
the same for all animals tested.
The average IOP for all eighteen OD eyes of both
treatment groups is summarized in FIG. 2. Laser treatment
was followed by an initial decrease and then an increase in
IOP. The magnitude of this initial elevation and the IOP is
summarized in Table 1 where the mean IOP is listed for each
of the three time intervals, T1, T2, and T3 for the
hypertensive eyes of all animals in the two treatment
groups. At Ti, approximately 3 months after IOP elevation,
the range and average values for the two treatment groups
are similar. Average mean IOP declines over the following
two months (T1 to T2) and the decline is greater in the
memantine treated animals at T2. From T2 to T3, average
mean pressure decreased slightly in the vehicle treated
animals while a greater decrease is seen in the memantine
treated animals. Based on these results, it can be
concluded that memantine treatment has little or no effect
on pressure in normotensive eyes.
Serum and vitreous memantine levels are summarized for
animals in the memantine-treated group in Table 3. Serum
levels of about 1 micromole were obtained at all time points
except at 2 months when values ranged from 0.03 to 0.8
micro. Vitreous memantine levels obtained a the end of the
study were also in the range of 1 micro.
Table 3
t'ltreous
Moman.ttae
Serqm Meamilne (PIM)
(1ffl)
16 mas
10 12 14 15
Monkey i 2 mos 4 mos 6 mos 8 m.os mos mos m.as nuos OLf 05
94 0.3 1.9 1.7 1.4 0.6 1.5 l.2 1.2 1.5 1.2
95 0.03 1.6 1.2 1.3 0.8 1.7 1.3 1.5 0.6 0.5
97 0.2 1.8 1.5 1.1 0.9 0.7 1.2 0.9 0.3 0.4
99 0.2 1.6 1.0 1.0 0.7 1.3 1.3 1.0 0.7 0.9
100 0.3 2.1 1.4 1.8 1.1 1.4 1.6 1.5 1.6 1.5
101 0.1. 0.9 1.5 0.8 0.8 0.8 0.7 1.1 0.5 0.8
102 0.8 1.6 2.0 1.2 0.6 0.6 0.9 0.5 0.7 0.7
107 0.8 2.1 1.6 1.1 0.7 1.7 1.4 0.6 1.8 1.7
108 0.3 1.5 1.7 1A 1.2 0.8 1.2 0.7 1.2 1.3
S 0.3 1.7 1.5 1.2 0.8 1.2 1.2 1.0 1,0 1.0
~ SF.US 0.1 0.1 0.1 0.1 0.1 0.2 0.1 0.1 0.2 0,2
Range 0.03-0.8 0.9-2.1 1,0-2.0 0.8-1.8 0.6-1.2 0.6-1.7 0.7-1.6 0.5-1.5 0.3-1.8
0.4-1.7
48
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Conventional ERG responses from a normotensive eye of
one animal are illustrated in FIG. 3. Panel A illustrates a
flash response to a stimulus of 10 microsecond duration
delivered at 0 milliseconds (average of 10 responses). The
amplitude of the a-wave and the b-wave peak voltage was
measured, as shown by the arrows. Panel B illustrates the
oscillatory potential (OP) response to the same stimulus
used to elicit the flash response in panel A (average of 25
responses). The response amplitude was measured as the RMS
voltage from 10 to 75 milliseconds after the stimulus.
Panel C illustrates the flicker response to a 30 Hz stimulus
train of 512 millisecond duration beginning at 0
milliseconds (average of 30 responses). The amplitude was
measured as the average peak-to-peak voltage of the last
three response cycles (1, 2, 3). For each response measure,
the amplitude of the hypertensive eye was normalized with
respect to the value obtained from the normotensive eye
(OD/OS). These values at time T3 are plotted in FIG. 4 as a
function of mean IOP elevation in the hypertensive eye.
Ocular hypertension had little or no effect on flash
(FIGs. 4A and 4B) or OP (FIG. 4C) responses and only a small
effect on 30 Hz flicker (FIG. 4D) response measures obtained
from either treatment group. Similar results were observed
at times Tl and T2.
The left hand panels of FIG. 5 illustrate first order
(A) and second order (C) multifocal ERG responses obtained
from a normotensive eye. In each panel, responses from
macular retina (approximately central 16 ) are highlighted
in the trace array and averaged to provide the macular
responses in the right-hand panels (B, first order; D,
second order). The peak-to-peak measures in the first order
response (N2-P2, and P2-N3) and second order response (P-N)
are correlated with the degree of COHT-induced loss of cells
in the retinal ganglion cell layer (Hare et al., Invest.
Opthalmol. Vis. Sci., 42:127-135, 2001). This correlation
49
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
is illustrated in FIG. 6, where normalized (OD/OS) macular
response amplitude measures are plotted as a function of
normalized (OD/OS) perifoveal RGC counts for seven animals
in the vehicle treated group and eight animals in the
memantine-treated group. FIGs. 6B and 6C show that RGC loss
is correlated with a decreased amplitude of these response
measures. For comparison, the amplitude of the first
negative peak (N1) in the first order response is plotted in
panel A. Even severe RGC loss had little effect on
amplitude measures of this response component. It can be
concluded that specific components of the multifocal ERG
response provided a functional measure of injury to RGCs and
these same measures may be used to determine the degree of
functional loss associated with chronic ocular hypertension.
Results from recordings made at approximately 3 months (T1)
after induction of ocular hypertension are summarized in
FIG. 7. For the measures illustrated in panels 7B and 7D,
response amplitude in the hypertensive eye of vehicle-
treated animals is inversely correlated with the mean level
of IOP exposure. Or, stated differently, higher IOP is
associated with decreased response amplitude. As shown in
panel 7A, IOP history has little or no effect on the
amplitude of peak N1 for both treatment groups. The slope
of the correlation (represented by the linear regression
lines) illustrated in panels 7B and 7D provides an
expression for the relationship between IOP elevation and
these functional measures of injury to RGCs. The effects of
memantine, or other neuroprotective agent, to reduce retinal
injury would be evident as a decrease in the slope shown in
these panels. For example, panels 7B and 7D show that the
slop for the memantine treated group is less than the slope
for the vehicle treated animals. Results for these three
measures at times T1, T2, and T3 are summarized in Table 2.
As shown in the figures and Table 2, IOP elevation is
associated with a decrease in response amplitude as
reflected in the negative slope. In addition, for times T1
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
and T2, the slope for all three measures from the memantine
treated animals is less than that obtained from the control
animals.
FIG. 8 illustrates the average amplitude (nV/degree2)
of responses from the hypertensive eye as a function of
times T1, T2, and T3. As shown in panel 8A, peak N1
amplitude changes little over time. Panels 8B and 8D
illustrate that peak N2-P2 and peak P-N increase over time.
In panel 8C, vehicle treated animals show a trend for
increasing response amplitude for peak P2-N3, and memantine
treated animals show little change over time.
Based on these results, it can be concluded that
measures of retinal function that are not correlated with
RGC injury (N1) show little effect of IOP elevation and
little or no change over time. Measures of retinal function
which are highly correlated with RGC injury (e.g., N2-P2 and
P-N) and severely attenuated by elevated IOP show a tendency
to increasing response amplitude over time in both treatment
groups.
The VECP requires transmission of the visual signal
from the retina to the visual cortex. Thus, injury to
retinal ganglion cells and their axons can directly and
indirectly affect the VECP. VECP amplitude is positively
correlated with the number of surviving cells in the
ganglion cell layer of COHT monkey eyes (Hare et al., Eur.
J. Ophthalmol., 1(suppl):30-33, 1999).
Panel A of FIG. 9 illustrates the VECP response
obtained from a normotensive (OS) eye. Panel B of FIG. 9
illustrates the normalized n1-p1 amplitudes for both
treatment groups as a function of mean IOP. The slope for
the memantine-treated group is less than the slope for the
vehicle-treated group. Thus, treatment with memantine is
associated with a preservation of the VECP response, even in
animals with highly elevated IOP. A comparison of the
slopes obtained from measures on the VECP responses of both
groups is included in Table 2.
51
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
FIG. 10 illustrates VECP amplitude measures as a
function of RGC count. As evident from FIG. 10, three
animals in each treatment group suffered a loss of about 72%
or more of the RGCs and the slope for an effect of RGC loss
on VECP response is less for the memantine treated animals
compared to the vehicle treated animals. These results
indicate that VECP responses of apparently normal amplitude
were obtained from all three memantine treated eyes, which
lost more than 30% of their RGCs.
Measures obtained at time T3 from all normotensive (OS)
eyes of the memantine-treated and vehicle-treated animals
were compared to determine whether daily oral dosing of
memantine (4 mg/kg) for 16 months had an adverse effect on
visual pathway function. Results are shown in FIG. 11.
Mean peak amplitude measures for the flash, OP, and flicker
ERG responses are shown in panel A of FIG. 11 (values
obtained from the memantine-treated group are normalized
with respect to the vehicle-treated group). FIG. 11, panel
C shows a similar comparison of time-to-peak values for the
same response measures represented in panel A. There were
no significant differences in the amplitude or timing of any
measure of conventional responses from the two treatment
groups. Panels B and D of FIG. 11 illustrate the results
for measures of amplitude and timing of peaks in the first
order macular multifocal response. No significant
differences were observed between the two treatment groups
for any measure of the conventional responses.
FIG. 12 illustrates vitreal glutamate concentrations
for vehicle and memantine-treated groups. On average,
glutamate levels for hypertensive and normotensive eyes for
memantine treated animals were similar. Vitreal glutamate
levels in the vehicle treated animals was slightly higher
for hypertensive eyes compared to normotensive eyes.
Components of the multifocal ERG response reflect the
activity of RGCs in humans and monkeys (Sutter et al., Vis.
Res., 3:419-436, 1999; Hare et al., Documenta
52
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
Ophthalmologica, 105:189-222, 2002; Hood et al., Vis.
Neurosci., 16:411-416, 1999; and Frishman et al., Doc.
Ophthalmol., 100:231-251, 2000).
The results presented herein indicate that eyes having
high mean IOPs suffered severe deficits in RGC function that
were apparent by 3 months after induction of ocular
hypertension (FIGs. 6 and 7). The RGC injury can be
distinguished from contribution of an ischemic insult based
on the observation that amplitude measures of the flash ERG
a-wave, b-wave, and OP responses were unaffected by elevated
IOP (FIG. 4).
For eyes with the highest IOPs, the amplitudes of RGC
dependent components of the multifocal ERG response were
typically larger in memantine-treated animals when compared
to responses obtained from control animals with similar IOPs
(FIG. 7 and Table 3). These observations may indicate that
in these eyes, the rate of injury to RGCs was slowed by
memantine treatment. A model potentially explaining the
apparent loss in treatment effect is illustrated in FIG. 13.
In FIG. 13, the number of surviving RGCs is plotted as a
function of time after induction of ocular hypertension.
RGCs are lost at a rate represented by the slope of the
plot. Treatment with memantine reduces the rate of RGC
injury/loss, but the magnitude of any difference in the
level of injury in the two eyes can vary with the
measurement time point. Thus, the observation that modest
levels of protection can be obtained at early time points,
and the failure of an effect of memantine treatment to
preserve ERG responses obtained at the end of the experiment
may be related to a relatively steep rate of injury as
suggested by the severe levels of injury observed at the
early measures in animals having the highest IOP elevation.
At least one unexpected result from the present results
was that the VECP responses were well preserved in memantine
treated animals (FIG. 9, panel B and FIG. 10). As discussed
herein, at this time point (T3), the ERG measures of RGC
53
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
injury were similar for memantine-treated animals and
vehicle-treated animals. One potential explanation for
these results is that memantine treatment promotes a greater
survival of RGC subtypes whose activity is not reflected in
the ERG measures but which makes a relatively large
contribution to the generation of the VECP. Another
potential explanation is that memantine treatment enhances
the ability of surviving RGCs to drive activity in the
visual cortex, for example, memantine treatment can be
associated with plastic changes occurring at more central
levels of the visual pathways, such as in the lateral
geniculate nucleus (LGN) of the thalamus, or one or more
regions of the visual cortex. Examples of plasticity may
include an enhanced axonal sprouting and/or formation of new
synapses on target neurons whose inputs may have been
reduced or lost from the ocular injury or RGC injury.
In short, the present results demonstrate that systemic
treatment with memantine, a compound that does not appear to
lower intraocular pressure, was safe and effective for
reducing a functional loss of central nervous system visual
activity associated with chronic ocular hypertension, such
as glaucoma.
Example 3
O-ahthalmic Anatomical Measures of Memantine Treatment in
Experimental Glaucoma
FIG. 14 is a graph of the average IOP history for
laser-treated hypertensive (OD) eyes of vehicle-treated and
memantine-treated monkeys described above. FIG. 14 is
similar to FIG. 1 except that FIG. 14 includes indications
of HRT measurements. FIG. 15 is an illustration of the
location of retinal samples used for histologic analysis.
Each sample was 3 mm square and cut with the use of a
transparent template from fixed flat-mounted retina-RPE-
choroid. The perifoveal (PF) sample was centered on the
54
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
fovea. Samples 1 to 3 were located on the horizontal and
vertical meridians from 3.5 to 6.5 mm from the fovea, while
samples 4 to 7 were located on the oblique meridians from
8.5 to 11.5 mm from the fovea. Sections were cut from
either the inferior edge (sample PF) or the edge facing the
fovea (samples 1 to 7) as indicated by the heavy border.
The dashed circle indicates the position of the optic nerve
head (ONH).
FIG. 16 provides photographs of the optic disc and
histologic sections from a vehicle-treated animal (M91, see
Table 5).
Table 5
IOP ('t,xxnt klg)
Monkey lfaschne toP Pea..t~~-,
(MM HR) (rardus).. tt. YM
Ve~xicte-ire.[tecl ~~<7~
96 22.1(22.8) 50.0 (2I.5) 39.9 (19.5) 3$.4(19.5)
104 19.1(13.5) 61.o .5 (23.5) 116,1 (2 t.0) 39.6(19.5)
91 18.8(18.7) 55.0 51.0 (19.0) 53..0 (1fi1.5) 49.6 (20.0)
93 19.0(19.0) 55.9 53.4(16.5) 52.2 (19.5) 53.0 (20.5)
92 18.7(19.1) 61.5 51.1(18.0) 53.3 (20.5) 54.7 (24.0)
R ume 18.7-22.1 50.0ri61.5 38.7-511.5 39.9w53.3 38.4-54.7
(1$.7-218) (I6.5-23-5) (18.5-21.0) (19.5w20.5)
t SE1L1 19.5 t 0.64 58.7 -r' 2.1 49.8 t 2.8 48.5 2.5 47.0 =!- 3.4
(19.8 0,76) (19.7 12) (19.9 0.4) (19.9 0.2)
1klexnantÃn,e-tr.eated axsimats
102 1.3 (18.7) 55.0 Y2.7 (.16.0) 281 (18,0) 26.5 (15.5)
.97 21,1(21.0) 47M 43=3(ii7.U) 37.7 ('18.5) 34.3 (1}.5)
94 18.5 G8..5) 53.0 43.6(18.0) 37.5 (175) 36.5 00.0)
95 21.1 (i4.7> 60.0 5(1.9 (215) 51.6 (:21.00) 45=E3 (23.5)
I01 20.8 (21..2) 61.5 54.8(18.5) 58.(} 09.S) 56.6 (x9.5)
Range 18.5-21.1 47.0-61.5 32.7-56.9 28.1-58.0 26.5-.56.6
(14.7-29..2) (17.5-21.0) (35.5-.23.5)
X 5E1Vi 20.1 -!- 0.53 55.3 '-" 2.6 9.6.3 4.3 42,6 " 53 39.8 t 5.1
(1$.8 1.1) (17.8 1.0) (18,9 ;..r 0A) (18.2 t 0.2)
'V'a1ues for contralateral normotensive (05) ~.t y+es are stxowa in
paa=et3theses.
The disc images were obtained approximately 5 months
after elevation of IOP (near HRT measurement time point T2)
at which point the mean IOP history for the hypertensive eye
was approximately 50 mm Hg. A single image from the stereo
pair for the hypertensive (OD) and normotensive (OS) eye is
present in the top panels of FIG. 16. The OD eye shows an
atrophic appearance of the disc and nasal deflection of
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
vessels. Below each fundus photograph is a micrograph of a
section from the PF sample region of that eye. In the OS
eye, it is evident that the RGC layer contains six or seven
layers of RGC nuclei. In the OD eye, the RGC layer contains
one layer of nuclei indicating a substantial loss of RGCs in
the hypertensive eye. The RGC loss appears to be selective
since little or no reduction was observed in the outer
nuclear layer (ONL) or inner nuclear layer (INL).
As shown in FIG. 17, counts for cells in the central
ganglion cell layer of OD eyes were highly correlated with
mean IOP. Similar effects were observed from peripheral
retinal areas. Thus, it appears that memantine treatment
does not protect the eye from RGC cell loss resulting from
increased IOP when all of the retinal regions are grouped.
However, for a subgroup of animals having a moderate mean
IOP (26 to 39 mm Hg), memantine treatment appeared to be
associated with regionally selective cell loss. For
example, inferior regions of the retina showed less cell
loss in memantine treated animals than inferior retinal
regions of vehicle-treated animals, as shown in FIG. 18. A
summary of the cell counts for the eyes represented in FIG.
18 is provided in Table 4. Table 4 also shows that the
distribution of mean IOP values obtained over the time
course from TO to death is similar among the two groups of
animals.
Table 4
Inferior SuperIor PeripBtra.l Pcrimacala PerIfaveal Total
Cell Counts Ccll Counts Cel! Counct Cell Counts Cell Counts Celt Counts
(SamPle 2,6,7) (Sample 1,4,5) (Sampte 4,5,6;'?3. '(Sample 1,2,3) (PF) (Sample
1 7,PB) iOP
MonkeyOD/OS OD/OS OD/O5 OD/OS OD/()S O0/OS (aua iig)
VC11[C1C
96 0,83 0.87 0.91 0.71 0.92 0.87 37.4
98 0.78 0,77 0.77 0.84 0.96 0.90 31.2
106 0.69 0.92 0.73 0.96 0.69 0.74 28.9
105 0,84 1.04 0.79 0.92 1.08 1.05 27.3
Average 0.78 0.90 0.80 0.86 0.91 0.88 31.2
SEM 0.04 0.06 0.04 0.06 0.08 0.06 2.20
Memautine
94 0.97 1.16 0.89 1.12 0.63 0.78 35.3
97 0.88 0.74 0.71 0.82 0.91 0.86 29.3
107 0.89 0,85 0.92 0.84 0.88 0,89 29,2
99 11.00 1.00 0.97 0.99 0.91 0.89 26.2
Avenge 0.94 0.94 0.87 0.94 0.83 0,86 30.0
SEM 0.03 0.09 0.06 0.07 0.07 0.03 1.9
P=vatue 0.01 0.69 0.36 0.38 0.49 0.69 0.15
' Compacison of two trentmeut groups: twoo-ta3le@ studetu d=test.
56
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
In view of the above, it is apparent that for eyes with
moderate IOP elevation, memantine treatment was associated
with a significant preservation of cells in the RGC layer of
the inferior retina.
To determine whether memantine treatment was associated
with histologic signs of retinal toxicity, counts of cells
in the ganglion cell layer of all OS eyes from the two
treatment groups were studied. FIG. 19 shows that RGC
counts from the memantine treated group were not
significantly different from the vehicle treated group.
This was observed for counts from all individual sample
regions and the sum of counts from all samples. Examination
of the stained tissue sections from normotensive eyes of
both groups showed no evidence of an effect of memantine
treatment on the density or appearance of any other retinal
cell layer/cell type.
Stereo fundus photographs obtained from both eyes of
all animals at approximately 5, 10, 14, 20, 48, arid 60 weeks
after IOP elevation were examined for evidence of media
opacities, disc hemorrhages, vascular nonperfusion, or overt
signs of ischemic insult to the optic nerve head and
surrounding retina. The only vascular anomaly observed was
the nasal deflection of large vessels at the disks of the
eyes with advanced cupping. In both treatment groups, eyes
having the highest IOP elevations (see Table 5) showed
evidence of moderate to severe optic nerve atrophy with
considerable loss of neuroretinal rim. Axonal loss in the
nerve fiber layer was observed at the superior and inferior
peripapillary retina.
Table 5 provides data from optic nerve head topographic
measurements derived from confocal laser tomographic scans
at about 3, 5, and 10 months after induction of chronic
ocular hypertension. Nerve head morphology was
characterized by a series of measurements summarized in
Table 6, which reflect properties of either the
57
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
physiological cup or neuroretinal rim.
Table 6
Mesnantim- Veiis.ir.l+M
S.tape$ r" S1tbpe r* P V'alaae-
Cup x [z+ea.su res
Mean cup depth t'i 0.0S0 0.85 0.206 0.61 0.282
i2 0.033 0.94 0.356 0.85 0.018
0 0.008 0.06 0.250 0:94 0.057
Cup volumc below suxf-Are tI U.o67 0,72 0.438 0.54 0.314
t2 0.038 0.87 0.756 0.74 0.059
t3 U.M) i).62 0.524 0.136 01019
GF3R tl 0.012 0.66 0.023 0.59 0.580
t2 0.008 0.52 0.033 0.77 0.217
0 ().4103 0.19 t?.029 0.83 0.116
Cup area tl 0.E155 0.98 0.305 0.56' 0.294
t2 0.071 090 0.547 0.84 0.023
0 0.072 ().75 0.57Ã3 0.94 0.004
Cup shape ti -0.073 (3.{38 -0.101 0.95 0.201
t2 --0.052 0.9$ -0.1-47 0.90 0.029
t3 -0.050 0.98 -0.076 0.89 0.261
Meutraretinal .xxnz measures
liisn area tl -0.040 0.99 -0:041 0.88 0.955
t2 --t?.U31 0.99 -0.049 0.99 0.010
t3 --Ã1.033 0.95 -0.059 0.95 0.08:5
Rim/disc t1 -0,031 0.96 --ti.{3;3t) 0.87 0.554
t2 -0.024 p .95~ 0..98 0.006
ti -{).026 0.94 --0.039 0.,94 0.039
Rim volume xi -0.057 094 -0.037 0.90 0.328
t2 -0.(335 0. 92 -0.031 0.97 0.800
0 --032 4.93 --0.039 0.95 0.553
1iNFi. thickness rl. -0.035 0.92 --0.024 0.50 0.637
C2 --0.017 0.69 --tl.042 0.64 0.409
0 -0.029 (3.134 --fl.(l6(} 0.94 0.125
liNFL crm secdprt ti -0.04-2 ().91 -0.024 0.50 0.497
t2 --0.021 0.74 -0.1139 0.50 0.564
t3 -0.033 (}.84 -d).062 0.93 0.195
" DetenufRied from linear xegxes.qiorz analysis.
Determined from attaysis =a.t =cavkarixlrcv.
YatueÃ; represent measum' fzdui =W[.tive aSainzWs .i.it each txeatment gracip
for wla3cii RRT measur,es
were made.
FIG. 20 shows results obtained for measurements of the
cup at approximately 5 months (T2) after elevation of IOP.
FIG. 21 shows results of measurements of the neuroretinal
rim obtained at the same time. In each of these plots, a
steeper slop represents a greater effect of ocular
hypertension on that measure. As shown in FIG. 20, IOP
elevation was associated with a relatively greater effect in
vehicle treated animals compared to the effect observed in
memantine-treated animals. As shown in FIG. 21,
measurements of the neuroretinal rim showed a more similar
58
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
effect of IOP elevation in the two treatment groups. A
summary of results from topographic measurements is
presented in Table 6. The results presented in FIGs. 20 and
21 show that ocular hypertension has profound effects on
measurements of the optic nerve head. In memantine treated
animals, ocular hypertension is associated with much less
effect on measurements of the cup than seen in the vehicle
treated animals, whereas effects of ocular hypertension on
measurements of the neuroretinal rim are more similar in the
two treatment groups. Non-normalized values for
measurements obtained from the hypertensive eyes of the five
animals in each treatment group were plotted at the three
measurement time points in FIG. 22 for measurements of the
cup, and FIG. 23 for measurements of the neuroretinal rim.
The left panels of FIG. 22 show results for measurements of
cup volume below the surface (A), cup shape (B), and cup
area to disc ratio (C). The right panels show results for
the same measurements from memantine treated animals.
Memantine treated animals' had, on average, smaller
measurements of cup volume and relative cup area compared to
the control animals. Most pressure-related changes in cup
morphology had occurred by approximately 3 months after IOP
elevation. As shown in FIG. 23, memantine treated animals
had, on average, larger measurements for rim area, rim
volume, and RNFL thickness.
Optic disc size remained stable over the three
measurement time points. For example, the disc size didn't
vary by more than 7% over these times.
These results demonstrate that systemic administration
of memantine for prolonged periods of time is not associated
with histologic retinal toxicity, which is consistent with
the results of the electrophysiology data described above.
Example 4
Central Nervous System Anatomical Measures of Memantine
Treatment in Experimental Glaucoma
59
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
After perfusion of the animals described in Example 1,
the brains were removed from the skulls and were fixed by
immersion in 4% paraformaldehyde in 0.1 M phosphate buffer
(pH 7.4) for at least 48 hours. The left lateral geniculate
nucleus (LGN) of the thalamus was blocked in the coronal
plane and cryoprotected by immersion in compositions
containing glycerol, dimethyl sulfoxide (DMSO), and
phosphate buffer for several days. The blocks were frozen
in isopentane cooled by a mixture of 100% alcohol and dry
eye. Coronal sections (50 micrometers) of the entire LGN
were cut serially on a sliding microtome. Every seventh
section was mounted onto a glass slid and stained with
cresyl violet.
Parvalbumin immunocytochemistry was performed on tissue
sections using a monoclonal antibody (clones PA-235, Sigma,
St. Louis, MO) and conventional techniques.
Histological examination of the tissue sections and the
determination of neuronal size and numbers were performed
using conventional techniques.
The neurons showed a normal size distribution in
memantine-treated and vehicle-treated groups for each of the
three layers. To assess cell area changes in glaucoma, the
mean neuron area for each LGN layer of each vehicle and
memantine treated animal with glaucoma was compared to the
mean neuron area for the corresponding LGN layer in normal
animals without glaucoma. The percent neuron shrinkage was
calculated from the difference between mean neuron area of
the normal group and mean neuron area of the vehicle or
memantine treated glaucoma animals, divided by the mean
neuron area in the normal group. The mean neuron areas for
the normal group for layer 1, layer 4, and layer 6 were
238.5 12.4 gm2 (mean SD) , 202.6 20.8 mZ, and 219.7
35.8 mz, respectively.
As discussed herein, in these experimental animals,
there was no significant difference in mean IOP or maximum
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
IOP between memantine-treated and vehicle-treated groups.
In addition, there was no significant difference in mean
percent optic nerve fiber loss between the two groups.
For vehicle-treated animals, cell bodies of
parvalbumin-immunoreactive neurons in layers 1, 4, and 6
appeared shrunken and ovoid compared to parvalbumin-
immunoreactive neurons in memantine-treated animals.
Overall, the memantine treated animals exhibited larger size
parvalbumin immunoreactive neurons in layers 1, 4, and 6
compared to vehicle-treated control animals. However, the
difference in layer 6 neurons was not statistically
significant.
The mean LGN neuron numbers in the memantine treated
animals and the vehicle treated control animals were not
significantly different.
In view of these results, it can be concluded that in
chronic ocular hypertension, such as experimental glaucoma,
memantine reduces transsynaptic atrophy in the LGN neurons.
Example 5
Treatment of Glaucoma With an Antiexcitoxic Agent
A 48 year old male undergoing a routine eye examination
is told by his ophthalmologist that he appears to have some
early signs of glaucoma. The patient is surprised since he
has not experienced any vision loss or increased ocular
pain. The physician suggests that the patient try a
prophylactic therapy to attempt to mitigate against further
vision loss. The physician selects an oral memantine
formulation among many potential antiexcitoxic formulations.
The patient is advised to take one tablet twice a day and to
return for an examination in six months.
Six months later, upon further examination, a
determination can be made whether the treatment was
successful. An increase in intraocular pressure and/or a
61
CA 02575204 2007-01-25
WO 2006/015075 PCT/US2005/026703
decrease in visual field can be indicative that the ocular
symptoms of glaucoma are advancing. However, functional
brain imaging of the visual cortex, such as by using an FMRI
or functional PET scan, can be used to measure electrical
activity in the visual cortex. Comparing the activity to a
control, such as the cortical activity prior to drug
therapy, can be used to evaluate whether the treatment is
ultimately successful. Similar cortical activity readings
can indicate that the patient has not experienced further
vision loss and the treatment is successful.
The present invention also includes the use of a
therapeutic agent, such as an anti-excitotoxic agent, in the
manufacture of a medicament for treating an ophthalmic
condition by administering the therapeutic agent to an
individual who is not aware of a visual field loss. In
addition, the present invention also includes the use of a
therapeutic agent, such as an anti-excitotoxic agent, in the
manufacture of a medicament for treating an ophthalmic
condition associated with retinal neurodegeneration by
administering the therapeutic agent to an individual to
reduce a decrease in a central nervous system response
associated with retinal neurodegeneration.
All patents, applications, publications and references
cited herein are incorporated by reference in their
entireties.
While this invention has been described with respect to
various specific examples and embodiments, it is to be
understood that the invention is not limited thereto and
that it can be variously practiced within the scope of the
following claims.
62