Note: Descriptions are shown in the official language in which they were submitted.
CA 02576355 2008-07-29
TITLE OF THE INVENTION
TREATMENT OF WASTE USING THREE TEMPERATURE STAGES
WITHIN ONE CHAMBER
FIELD OF THE INVENTION
The invention relates generally to processes for the thermal treatment
of waste materials. In particular, the invention relates to a process for the
thermal treatment of pharmaceutical waste materials, and subsequent
recovery of oil.
BACKGROUND OF THE INVENTION
Disposal of waste materials, in particular hazardous and non-
hazardous pharmaceutical and personal consumer product waste, has
generally been completed primarily through shredding and landfilling. While
shredding removes potential product liability issues such as black market re-
selling of off-spec and expired products as well as unwanted public visibility
of corporate identifiers, the desire to reduce the volume of waste to be
landfilled has led to the development of alternative processes such as
recycling, composting and incineration. These processes have some
benefits but also present significant disadvantages. For example, not all
components of the waste stream present sufficient economic value to justify
a recycling program. Also, not all components of the waste stream are
suitable for compost production. And incineration is widely known as
requiring high levels of energy and sophisticated equipment as well as
producing airborne emissions; moreover incineration does not allow for the
recovery of hydrocarbons. On the other hand, it is desirable to recover at
least some valuable materials from the waste in order to reduce the
environmental pollutants that may result from their decomposition.
Numerous waste materials comprise medicinal ingredients that are
classified as regulated compounds, and require total destruction. Various
processes allowing for the destruction of these compounds, including
CA 02576355 2007-01-26
-2-
thermal treatment, are known in the art. For example, US 2005/0218037
discloses a process for thermal treatment of multiphase residues. The
process uses a three-stage temperature gradient, wherein the temperature
increases from about 80 to 250 C. Each stage is carried out within a
separate zone of the reactor, and the material to be treated is circulated
from one zone to the other. The process takes place under inert
atmosphere, and yields oil, water, hydrocarbons as well as solid residue. US
6,840,712 discloses a similar process with the temperature increasing from
about 316 to 649 C.
US 4,013,516 and Canadian patents 2,543,320; 2,515,431;
2,313,801; 2,251,004; and 2,423,714 also disclose similar thermal
treatment processes, at various temperature ranges.
There remains a need for simple, environmentally friendly and cost
efficient thermal treatment processes of waste materials.
SUMMARY OF THE INVENTION
The inventors have developed a process that combines desorption,
depolymerization and pyrolysis for the treatment of waste materials,
particularly pharmaceutical waste materials. The material to be treated is
placed in a reaction chamber and subjected to a three-stage gradient
temperature, without any need to circulate the material. The process
ensures residence time at such temperatures as to thermally destroy the
medicinal ingredients. The process also allows for a continuous recovery of
oil, water as well as non-condensable synthesis gas that can be used as
heating source for the process. The process yields a solid residue of
significantly reduced size that is suitable for safe landfilling.
The invention thus provides according to an aspect, for a process for
the thermal treatment of waste material in a chamber. The process
comprises the steps of: a) heating the chamber at a first temperature to
cause water in the material to desorb as water vapor; b) heating the
chamber at a second temperature higher than the first temperature to cause
CA 02576355 2007-01-26
-3-
organic ingredients in the material to desorb and decompose producing
condensable organic vapor and non-condensable synthesis gas; and c)
heating the chamber to a third temperature higher than the second
temperature to cause polymer-based components of the material to
depolymerize and decompose producing condensable hydrocarbon vapor.
The water vapor, the condensable organic vapor and the condensable
hydrocarbon vapor produced in the process can be continuously recovered
and condensed as an oil-water mixture; and the non-condensable synthesis
gas can be continuously recovered as fuel for combustion or for heating.
In a preferred embodiment of the process, the first temperature can
be about 100 C, the second temperature can be about 250 to 350 C and
the third temperature can be about 350 to 500 C.
The process according to the invention can be carried out on various
types of waste materials including but not limited to waste material derived
from industrial wastes such as pharmaceutical, petrochemical, packaging,
plastics manufacturing, mining, petroleum refining, paint and ink manu-
facturing, sludges as well as mixtures thereof. The waste material can also
be a cellulose material, a polyolefin-based resin, used tires, contaminated
soils or mixtures thereof.
In a preferred embodiment of the invention, the waste material is a
pharmaceutical waste material.
The waste materiai may be subjected to a size reduction step prior to
being placed into the chamber. The size reduction step may comprise
shredding the waste material to obtain particles having a size of less than
about 30 mm.
In a preferred embodiment, the non-condensable synthesis gas is
used as heat source for the process. Also, the process can further comprise
a step of separating oil and water from the oil-water mixture.
During the process, heat can be transmitted to the chamber through
its external surface. Optionally, the heat may stem from a heating source
CA 02576355 2007-01-26
-4-
which can be hot oil, electrical resistance heating, induction heating or a
flue
of the non-condensable synthesis gas. The material in the chamber may be
subjected to continuous agitation. The pressure inside the chamber may be
negative, and the atmosphere substantially free of oxygen or inert.
Optionally, an inert gas such as nitrogen or argon can be introduced into the
chamber.
The volume of the treated waste material recovered after the process
may be about 90% reduced.
According to another aspect, the invention provides for a process for
the thermal treatment of waste material in a chamber, the process
comprising the steps of: a) heating the chamber at a first temperature to
cause water in the material to desorb as water vapor; b) heating the
chamber at a second temperature higher than the first temperature to cause
organic ingredients in the material to desorb and decompose producing
condensable organic vapor and non-condensable synthesis gas; c) heating
the chamber to a third temperature higher than the second temperature to
cause polymer-based components of the material to depolymerize and
decompose producing condensable hydrocarbon vapor; d) continuously
recovering oil, water, hydrocarbon vapor and non-condensable synthesis
gas, the non-condensabie synthesis gas being used as heat source for the
process; and e) recovering treated waste material from the chamber, the
treated waste material having a volume which is about 90% reduced.
In a preferred embodiment of this process, the waste materiai is a
pharmaceutical waste material.
These and other aspects of the invention wiil be more clearly seen
from the detailed description of a preferred embodiment outlined below.
DESCRIPTION OF THE DRAWING
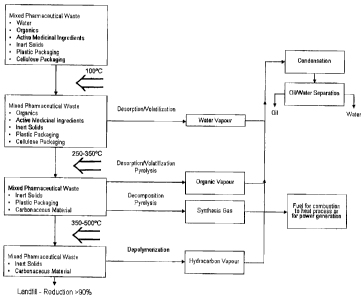
Figure 1 is a thermal process flow diagram which illustrates the
process according to the invention.
CA 02576355 2007-01-26
-5-
While the invention will be described in conjuncture with the
illustrated embodiment, it will be understood that it is not intended to limit
the invention to such embodiment. On the contrary, it is intended to cover
all alternatives, modifications and equivalents as may be included within the
spirit and scope of the invention as defined by the appended claims.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS OF THE
INVENTION
As used herein and in the claims, the following terms have the
meaning and definition set out below.
The term "pyrolysis" is meant to encompass processes wherein the
atmosphere in the pyrolysis chamber may contain a small amount of air
(oxygen), but the amount is so small that there is no visible combustion.
Although the term "pyrolysis" is generally defined as the "transformation of
a compound into one or more substances by heat alone, i.e., without
oxidation" (Hawley's Condensed Chemical Dictionary, 13 th Ed. (1997).), it is
envisaged that at least a small amount of oxygen may be present into the
chamber during the process according to the invention. Indeed, some air
may enter the chamber during the loading of the waste. Also some air may
be entrained within the waste. Moreover, as the pressure within the
chamber may be slightly negative, a small amount of air may be drawn into
the furnace through deficient seals for example.
The term "waste material" is meant to refer to any suitable product
that can be subjected to the process according to the invention, including
but not limited to pharmaceutical waste materials. Other streams for which
the process according to the invention is also suitable include but are not
limited to petrochemical, sludges, packaging, plastics manufacturing,
mining, petroleum refining, paint and ink manufacturing as well as mixtures
thereof. The waste material can also be a cellulose-based material,
polyolefin-based resin, used tires or other industrial rubbers, contaminated
soils or mixtures thereof.
CA 02576355 2007-01-26
-6-
The term "non-condensable gas" is meant to refer to a gas which is
not readily condensed, and includes gases such as methane, propane or
butane but also hydrogen, carbon dioxide and carbon monoxide.
The term "depolymerization" is meant to refer to the process of
converting a long chain hydrocarbon-based polymer into a shorter chain
hydrocarbon through a sequence of reactions at certain temperatures.
An embodiment of the process according to the invention will be
described with reference to the accompanying process flow diagram. The
process is carried out in a standard apparatus known in the art.
The waste material to be treated is fed to the reactor hopper. A
series of two dual flapper valves or rotary valves are provided at the base of
the hopper, each valve feeding the reaction chamber where the thermal
process including desorption, depolymerization and pyrolysis, takes place.
The use of dual flapper valves or rotary valves limits the ingress of oxygen
into the chamber. The waste material is introduced into the reaction
chamber at a specific rate which is determined by the valve rate.
In order to facilitate handing and also to have a consistent feedstock,
the waste material is shredded prior to being fed to the reactor hopper.
During this stage there is some degree of decomposition of the medicinal
ingredients through physical breakdown from the shredding action.
Preferably, shreds having a dimension of less than about 30 mm are
formed. Shredding can be carried out on-site or at another location.
The waste material is placed into an indirectly heated thermal
reaction chamber. The reaction chamber is heated externally with hot oil,
electrical resistance heating, induction heating, or flue gas from the
combustion of fuel. The temperature inside the extraction chamber is
controlled by a simple thermal control loop. The pressure in the reaction
chamber can be either positive or negative. Preferably, the pressure inside
the reaction chamber is negative. An inert gas such as nitrogen or argon
can be injected into the chamber such that the process takes place in an
inert atmosphere.
CA 02576355 2007-01-26
-7-
The waste material enters the reaction chamber where it is conveyed
along the length of a steel reaction tube associated with the reaction
chamber, by sloping the said tube. A rotating tube or an auger located
inside the reaction chamber continually agitates the material. Heat is
transmitted to the waste material inside the reaction chamber via
conductive transfer of energy.
Turning to the process flow diagram, at a first stage where the
temperature inside the reactor chamber is maintained at about 100 C,
water in the waste material is desorbed as water vapor, which can be
removed and condensed.
At a second stage, the temperature is increased to about 250 to
350 C, any remaining water from the waste material is desorbed. Also,
active organic ingredients are desorbed then decomposed yielding
condensable organic vapor and non-condensable synthesis gas. At this
stage the condensable organic vapor which may also include some water
vapor, is removed and condensed. And the non-condensable synthesis gas
is also removed.
A further stepped increase in temperature from 350 C to about 500 C
in the reaction chamber beyond desorption, volatilization and decomposition
temperatures results in depolymerization of plastic resins and polymers.
This depolymerization results in the release of condensable hydrocarbons
from their solid state allowing for their recovery and reuse. At this stage,
any organic ingredients that were not decomposed in the second stage, are
decomposed producing hydrocarbon vapor that can be removed through the
same means as the organic vapor. At these temperatures the medicinal
ingredients resident in the pharmaceutical waste product is thermally
decomposed. This decomposition is confirmed via the review of boiling
points and decomposition temperatures of medicinal compounds. Several
common examples are: acetylsalicylic acid (140 C), celebrex (150 C),
acetaminophen (170 C), pseudoephdrine hydrochloride (186 C), minoxidil
(78 C) and metoropol (120 C).
CA 02576355 2007-01-26
- ~ -
Indeed, water vapor, organic vapor and hydrocarbon vapors can be
removed together and condensed as a mixture of oil-water. And the
mixture of oil and water can further be subjected to a separation step to
yield oil and water. The non-condensable synthesis gas can be used as
heating source for the process.
The vapors and gases in the reaction chamber are removed either
under pressure or by vacuum. The vapors and gases are preferably
removed under vacuum. The vapors can be condensed into a liquid using a
heat exchanger, quenched with water or with any suitable type of oil
fraction similar to diesel. In the case where a recirculating water quench
system is used, the condensed oil and water from the process can be
separated in an oil water separator and the water fraction is cooled with a
heat exchanger and recycled to the quench. In the case where a
recirculating oil quench is used, the quenched oil and water can be
separated in an oil water separator and the oil fraction is cooled with a heat
exchanger and recycled to the quench. In any of these cases, excess oil
and water is continuously removed from the process approximately
proportional to the mixed waste feed content.
During the process a volume of condensable vapors and non-
condensable gases are generated. The condensable hydrocarbons vapors
are recovered in a liquid state as described above. The non-condensable
gases include methane, propane, butane as well as hydrogen, carbon
dioxide and carbon monoxide. These gases can be filtered for further
removal of particulate and fine mist and final polishing using activated
carbon. The non-condensable gases are commonly referred to as synthesis
gas and can be combusted to generate energy for the process or to
generate steam for power generation.
The hydrocarbon extracted is similar in terms of its composition to
No. 2 Fuel Oil or Diesel and is suitable for reuse or further fractional
distillation. A mass balance of the process generates the following outputs
based on the input feed characteristics described above.
CA 02576355 2007-01-26
-9-
A certain fraction of the material remains from the process as an inert
carbonaceous mass. This solid residue is removed from the reaction
chamber using a rotary paddle airlock into a water based cooling system
prior to being discharged.
Table 1 outlines an example of composition of a sample that can be
treated according to the process of the invention.
Table 1
Summary Mixed By wt
Pharmaceutical
Plastic Bottles 35%
Medicinal ingredients 10%
Paper (cellulose) 20%
Inert Solids & Foil 25%
Other Liquids 10%
100%
Table 2 outlines an example of relative amounts of the products
obtained after treatment by the process of the invention.
Table 2
Total
Byproducts
Oil 35%
Gas 16%
Carbon/Inert 32%
Solids
Water 17%
100%
Thus it is apparent that there has been provided in accordance with
the invention a process for the thermal treatment of waste materials, in
particular pharmaceutical waste materials. The process combines
desorption, depolymezation and pyrolysis; and is simple, environmentally
CA 02576355 2007-01-26
-10-
friendly and cost efficient. While the invention has been described in
conjunction with the illustrated embodiment, it is evident that many
alternatives, modifications and variations will be apparent to those skilled
in
the art in light of the foregoing description. Accordingly, it is intended to
embrace all such alternatives, modifications and variations as fall within the
spirit and broad scope of the invention.