Note: Descriptions are shown in the official language in which they were submitted.
CA 02577863 2007-02-21
WO 2006/027412 PCT/F12005/000380
Preparation of zinc chemicals from the minor flow of a process
The invention relates to the preparation processes of zinc and zinc chemicals.
To be more precise, the invention relates to a process arrangement and circuit
for preparing zinc chemicals in connection with a hydrometallurgical
purification
and preparation process of zinc.
In connection with zinc preparation, primary and secondary preparations are
often mentioned. The primary production refers to various dissolutions of zinc
ores, zinc concentrates and calcines and the treatment of solutions obtained
therefrom by means of various hydrometallurgical methods, including liquid-
liquid extraction. The secondary production refers to the use of various raw
ma-
terials of lesser volumes, such as particulates of electric furnaces, Waeltz
ox-
ides and galvanizing cinders, as raw material.
In the primary production of zinc, traditional original materials, such as
sulphide
zinc ores, are concentrated, i.e., a zinc concentrate, and when so desired,
also
zinc calcine are further prepared from them. Further, a concentrated zinc sul-
phate solution is often produced from the zinc concentrate and the zinc
calcine,
being well-suited for the electrolytic preparation of zinc metal. The present
in-
vention relates to the traditional hydrometallurgical primary production of
zinc.
In the traditional hydrometallurgical production of zinc, complex solution
purifi-
cation is needed after the dissolution of the raw material, comprising the
prepa-
ration of a pure zinciferous solution. The electrolytic preparation of zinc is
very
sensitive to certain impurity agents. Therefore, it is important to prepare a
suffi-
ciently pure solution for the zinc electrolysis.
For the preparation of zinc, a method is widely used, wherein zinc concentrate
is calcined to form zinc oxide, the zinc oxide is dissolved in a diluted
sulphuric-
acid solution in a so-called neutral dissolution, the zinciferous solution is
puri-
fied, and zinc is separated from the purified solution by electrolytic
separation.
CA 02577863 2007-02-21
WO 2006/027412 PCT/F12005/000380
2
At the dissolving stage of the zinc calcine, the zinc dissolves and the major
part
of the iron contained by the zinc calcine can be separated as zinc ferrite
that
does not dissolve in the diluted acid. In addition to the zinc, the solution
ob-
tained from the neutral dissolution contains bivalent iron, cadmium, copper,
co-
balt, nickel, calcium, manganese and chlorides.
To obtain a solution that is suitable for the electrolytic recovery of zinc,
the solu-
tion obtained from the neutral dissolution is purified by a multi-stage
solution
purification process. Normally, the solution purification comprises three
stages,
wherein copper, cobalt, nickel, and finally, cadmium are removed.
The zinciferous sulphate solution obtained from the neutral dissolution is
also
suitable for a feed material in the leaching method of zinc. Until recently,
the
liquid-liquid extraction has been used for zinc preparation mainly in small-
scale
processes that employ secondary raw materials. The US Patent 5,135,652, for
example, describes a solvent extraction that can be used to selectively sepa-
rate zinc from the zinc sulphate solution, which may contain zinc sulphate up
to
its saturation concentration, and one or more of a group containing the follow-
ing: bivalent iron, trivalent iron, calcium, magnesium, manganese, sodium, po-
tassium, arsenic, antimony, copper, cadmium, germanium, and indium.
The purpose of this invention is to provide a new kind of a process
arrangement
for producing pure zinc chemicals from the minor flow of a zinc preparation
process of a calcining-dissolving-electrolysis type. The invention is based on
the fact that part of the production of the zinc sulphate-containing solution
ob-
tained from the dissolving of the calcining-dissolving-electrolysis plant is
di-
rected to the preparation of zinc chemicals. As only part of the production of
the
zinc sulphate solution is directed to the preparation of zinc chemicals, the
ac-
tual recovery process of zinc that is based on the electrolysis is not
disturbed.
In the process arrangement according to the invention, the preparation process
of zinc chemicals is based on a selective separation of zinc by extraction
with
CA 02577863 2007-02-21
WO 2006/027412 PCT/F12005/000380
3
known extraction techniques. The known extraction process is based on the
use of any organically substituted phosphoric acid or organically substituted
thiophosphinic acid. According to the invention, the extraction process is con-
nected to the calcining-dissolving-electrolysis process described above.
The invention provides considerable advantages. In the conventional calcining-
dissolving-electrolysis process, the bottleneck of the production lies in the
elec-
trolysis process. Accordingly, the solution according to the invention for
taking
the minor flow to the preparation line of zinc chemicals adds value to the
actual
production process of zinc. The invention provides an advantageous process
solution for the preparation of zinc chemicals of a lesser demand. In the
extrac-
tion process of zinc used in the invention, a strong acid solution is
generated as
a by-product, which can simply be recycled to be exploited in the preparation
process of zinc. In that case, no separate investments for the final treatment
of
the acid solution are needed.
The invention comprises a process circuit and arrangement for preparing pure
zinc chemicals from a zinc sulphate-containing solution by an extraction
method, whereby the raw material flow of the extraction is separated as a
minor
flow from the calcining-dissolving-electrolysis type process line of zinc
primary
production after the neutral dissolving stage.
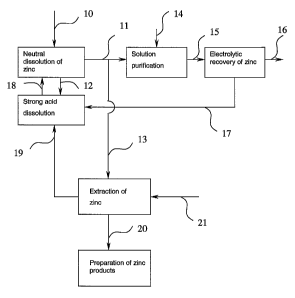
Fig. 1 shows a simplified process chart of the calcining-dissolving-
electrolysis
type preparation process of zinc and the preparation stage of zinc chemicals
connected thereto.
In the following, the invention is described in detail with reference to the
ap-
pended drawing.
In the preparation of zinc, a calcine 10 containing zinc oxide is dissolved in
a
sulphuric-acid solution in a neutral dissolution. The neutral dissolution is
typi-
cally carried out in several stages. A solution residue 12, which is not
soluble in
CA 02577863 2007-02-21
WO 2006/027412 PCT/F12005/000380
4
the dissolution and contains iron, among others, is directed to a strong acid
dis-
solution. A zinc sulphate-containing solution 11 is directed to a multi-stage
solu-
tion purification, wherein a zinc powder 14 is used to precipitate copper,
cobalt,
nickel and cadmium. Thesolution-purified zinc sulphate solution 15 is directed
to an electrolysis plant, wherein the zinc is recovered at cathodes 16 by
means
of electrolysis. The electrolyte, from which the zinc was recovered 17,
contains
a considerable amount of sulphuric acid, which is reintroduced by directing it
to
the strong acid dissolution, among others. In the strong acid dissolution, the
acid is used up in reactions. Accordingly, in addition to the recycled acid, a
cer-
tain amount of pure acid is added into the process. In the neutral
dissolution,
the pH of the solution should not be raised too high. It is reasonable to
adjust
the pH of the neutral dissolution with fresh acid and, in addition, with an
acid 18
that is obtained from the strong acid dissolution.
According to the invention, a minor flow 13 is separated from the main flow 11
of the zinc sulphate-containing solution obtained from the neutral
dissolution,
and directed to the extraction of zinc. In the extraction,
diethylhexylphosphoric
acid (DEHPA) or di-2-ethylhexylphosphoric acid (D2EHPA) is exploited. To-
wards the end of the dissolving stage, the pH of the solution that is going to
the
extraction can be raised to a level suitable for the extraction, when needed,
by
means of the calcine. Zinc is separated from the organic phase by stripping it
with an acid-containing solution 21. The raffinate 19, which is generated in
the
extraction, contains sulphuric acid, among others, and is directed to the
strong
acid stage to be reused.
In stripping, wherein pure acid is used, the pure zinc can be recovered in a
sul-
phuric-acid solution, wherein the content of zinc can be up to 150g/I. Various
pure zinc products can be prepared from this solution 20 by any known meth-
ods, such as by chemical precipitation or evaporation.