Note: Descriptions are shown in the official language in which they were submitted.
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
TITLE
A DESULFURIZATION SYSTEM AND METHOD FOR DESULFURIZING A
FUEL STREAM
BACKGROUND OF INVENTION
The present invention relates to a novel method for
producing a substantially desulfurized hydrocarbon fuel
stream, particularly for hydrogen generation, and more
particularly utilized within a fuel cell processing train,
by passing a nondesulfurized hydrocarbon fuel stream,
particularly natural gas, propane or liquefied petroleum gas
(LPG), through a sequential sulfur adsorbent bed system at
temperatures less than 100 C, wherein the sequential sulfur
adsorbent bed system contains a zeolite sulfur adsorbent and
at least one selective sulfur adsorbent. The, present
invention further relates to a system for generating
electricity within a fuel cell processing train from a
substantially desulfurized hydrocarbon fuel stream,
particularly desulfurized natural gas, propane or LPG,
wherein the hydrocarbon fuel stream is desulfurized using
the above-described sequential sulfur adsorbent bed system.
The present invention further includes a desulfurization
system used for hydrogen generation, particularly within a
fuel cell processing train for desulfurizing hydrocarbon
fuel streams, particularly natural gas, propane or LPG, at
1
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
temperatures as low as ambient temperatures.
For hydrogen generation, particularly for use in a
conventional low temperature fuel cell processing train,
such as a proton exchange membrane (PEM) fuel cell, which is
suitable for use in a stationary application or in a
vehicle, such as an automobile, the hydrocarbon fuel stream
can be derived from a number of conventional fuel sources
with the preferred fuel sources including natural gas,
propane and LPG. In a conventional hydrogen generation
system, particularly a fuel cell processing train, the
hydrocarbon fuel stream is passed over and/or through a
desulfurization system to be desulfurized. The desulfurized
hydrocarbon fuel stream for such fuel cell processing train
then flows into a reformer wherein the fuel stream is
converted into a hydrogen-rich fuel stream. From the
reformer the fuel stream passes through one or more heat
exchangers to a shift converter where the amount of CO in
the fuel stream is reduced. From the shift converter the
fuel stream again passes through various heat exchangers and
then through a selective oxidizer or selective methanizer
having one or more catalyst beds, after which the hydrogen
rich fuel stream flows to the fuel cell stack where it is
utilized to generate electricity.
Raw fuels, in gaseous or liquid phase, particularly
2
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
natural gas, propane and LPG, are useful as a fuel source
for hydrogen generation, particularly for fuel cell
processing trains. Unfortunately, virtually all raw fuels
contain relatively high levels, up to as high as 1,000 ppm
or so, but typically in the range of 20 to 500 ppm, of
various naturally occurring sulfur compounds, such as, but
not limited to, carbonyl sulfide, hydrogen sulfide,
thiophenes, such as tetra hydro thiophene, dimethyl sulfide,
various mercaptans, disulfides, sulfoxides, other organic
sulfides, higher molecular weight organic sulfur compounds,
and combinations thereof. In addition, because hydrocarbon
fuel streams, particularly natural gas, propane and LPG, may
have different sources of origin, the quantity and
composition of the sulfur compounds that may be present in
the fuel streams can vary substantially.
The presence of these sulfur-containing compounds in a
hydrocarbon fuel stream can be very damaging to components
of the fuel cell processing train, including the fuel cell
stack itself, and must therefore be substantially removed.
If not substantially removed, the sulfur compounds shorten
the life expectancy of components of the fuel cell
processing train.
An especially efficient desulfurization system is
necessary for use in such fuel cell processing trains as
3
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
they generally only contain a single desulfurization system.
Further, desulfurization systems for such uses must have
high capacity, as they may need to be in use for an extended
period of time before replacement.
Several processes, conventionally termed
"desulfurization," have been employed for the removal of
sulfur from gas and liquid fuel streams for hydrogen
generation. Adsorption of sulfur-contaminated compounds from
these hydrocarbon streams using a "physical" sulfur
adsorbent is the most common method for removal of sulfur
compounds from such hydrocarbon fuel streams because of
their relatively low capital and operational costs. (For
purposes of this specification, the terms "adsorption" and
"absorption" have the same, all inclusive meaning.) While
physical adsorbents are useful, they can be subject to
desorption of the sulfur compounds from the adsorbent under
certain operating conditions. In addition, there are often
limits on the quantity of sulfur compounds which can be
adsorbed by such physical sulfur adsorbents.
An additional type of adsorbent that has been useful as
a desulfurization agent is a "chemical" sulfur adsorbent.
However, chemical desulfurization normally requires the
desulfurization bed to be heated to temperatures of about
4
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
150 C to 400 C before the nondesulfurized hydrocarbon fuel
streams can be passed through the chemical adsorbent
desulfurization system. In addition, other operational
problems may occur when chemical desulfurization processes
are utilized.
While many different desulfurization processes have
been suggested for hydrocarbon fuel streams, there is still
a need for improved processes for desulfurization to achieve
enhanced adsorption of sulfur components over an extended
range of sulfur concentrations, especially at relatively low
operating temperatures and pressures, for extended periods
of time. Further, there is a need for the desulfurization
system to adsorb substantial quantities of a wide range of
sulfur compounds, including particularly hydrogen sulfide,
carbonyl sulfide, tetra hydro thiophene, dimethyl sulfide,
various mercaptans, disulfides, sulfoxides, other organic
sulfides, various higher molecular weight sulfur-containing
compounds and combinations thereof. Further, it is
important that the desulfurization system absorb this broad
range of sulfur compounds effectively for an extended period
of time to delay "breakthrough" of sulfur compounds as long
as possible. "Breakthrough" occurs when the level of any
sulfur compound remaining in the feed stream after
5
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
desulfurization is above a predetermined level. Typical
"breakthrough" levels for sulfur compounds occur at 1 ppm or
so. In addition, breakthrough by virtually any one of the
sulfur compounds present in the hydrocarbon fuel stream is
disadvantageous as substantially all sulfur compounds cause
damage to components of a hydrogen generation system,
particularly for a fuel cell processing train.
In addition, some prior art adsorbents, while effective
as adsorbents for some sulfur compounds, can synthesize the
production of additional sulfur compounds even as they are
removing some of the sulfur compounds that are present in
the hydrocarbon fuel stream. (These additional sulfur
compounds are referred to herein as "synthesized sulfur
compounds.") It is important that the desulfurization
system avoid the production of these synthesized sulfur
compounds to the greatest extent possible and for the
longest period of time possible.
These and further aspects of the invention will be
apparent from the foregoing description of preferred
embodiments of the invention.
SUMMARY OF INVENTION
The present invention is a process for supplying a
substantially desulfurized hydrocarbon fuel stream for
6
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
hydrogen generation, particularly for use in a fuel cell
processing train comprising providing a nondesulfurized
hydrocarbon fuel stream, preparing a desulfurization system
comprising a sequential sulfur adsorbent bed system
comprising a calcium exchanged zeolite sulfur adsorbent and
at least one selective sulfur adsorbent, and passing the
nondesulfurized hydrocarbon fuel stream through or over the
desulfurization system at a temperature optimally less than
100 C to produce a substantially desulfurized hydrocarbon
fuel stream with desulfurization levels as low as about 50
ppb or so. The composition and choice of the selective
sulfur adsorbent(s) and the sequence of use of the selective
sulfur adsorbent(s) and the calcium exchanged zeolite within
the desulfurization system depends on the composition of the
sulfur compounds which are present in that fuel stream.
The invention is also a system for generating
electricity from a fuel cell processing train by use of a
substantially desulfurized hydrocarbon fuel stream
comprising preparing a fuel cell processing train containing
the desulfurization system described above, passing a
nondesulfurized hydrocarbon fuel cell fuel stream through
the desulfurization system at a temperature preferably less
than 100 C, and introducing the substantially desulfurized
7
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
hydrocarbon fuel stream to the remaining components of the
fuel cell processing train.
The invention is also a desulfurization system for
hydrogen generation, particularly for use in a fuel cell
processing train, comprising an inlet for receiving a
nondesulfurized hydrocarbon fuel stream, particularly
natural gas, propane or LPG, the sequential adsorbent bed
system described above, and an outlet for passing a
substantially desulfurized hydrocarbon fuel stream
downstream to the remaining components of the hydrogen
generation system.
The invention is also a sequential sulfur adsorbent bed
system for hydrogen generation, particularly for use in a
fuel cell processing train comprising selective sulfur
adsorbent(s) and a calcium exchanged zeolite. The choice of
the particular selective sulfur adsorbent or absorbents and
the sequence of use of the selective sulfur adsorbent or
absorbents and the zeolite within the sequential sulfur
adsorbent bed depends upon the composition and quantity of
the sulfur compounds that are present in the hydrocarbon
fuel stream. One or more selective sulfur adsorbents can be
utilized with the calcium exchanged zeolite to form the
sequential adsorbent bed system of the invention. One
particularly preferred selective sulfur adsorbent comprises
8
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
one or more manganese compounds, iron oxide and a high
surface area carrier, particularly alumina. An alternative
preferred selective sulfur adsorbent comprises one or more
manganese compounds, copper oxide and a binder material.
Brief Description of Drawings
Figure 1 is a graph showing the performance of the
calcium exchanged zeolite discussed in Example 1 for the
removal of certain sulfur compounds from a synthetic natural
gas feed stream.
Figure 2 is a graph showing the performance of the
selective sulfur adsorbent of Example 2 for the removal of
the sulfur compounds of Example 1 from the synthetic natural
gas feed stream of Example 1.
Figure 3 is a graph showing the performance, as
discussed in Example 3, of a combination of the zeolite of
Example 1 with the selective sulfur adsorbent of Example 2
for the removal of the sulfur compounds of Example 1 from
the synthetic natural gas feed stream of Example 1.
Disclosure of a Preferred Embodiment of the Invention
The invention includes a method for supplying a
substantially desulfurized hydrocarbon fuel stream to a
hydrogen generation system, particularly a fuel cell
processing train. Raw fuel for use in such a hydrogen
9
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
generation system, particularly a fuel cell processing
train, such as natural gas, propane and LPG, must be
desulfurized prior to use because of the presence of
relatively high levels of naturally occurring sulfur
compounds, such as, but not limited to, hydrogen sulfide,
carbonyl sulfide, thiophenes, such as tetra hydro thiophene,
dimethyl sulfide, mercaptans (including ethyl, methyl,
propyl and tertiary butyl mercaptan), other sulfides,
various higher molecular weight organic sulfur compounds and
combinations thereof. These sulfur compounds can damage
components of the hydrogen generation system and the fuel
cell processing train. While numerous combinations and
quantities of these sulfur compounds may be present in the
fuel stream, in some situations, the sulfur compounds
present in the fuel stream may be limited to only one or two
such sulfur compounds. Where the raw fuel stream comprises
natural gas, which is in a gaseous state at operating
temperatures below 100 C, the level of sulfur compounds, such
as carbonyl sulfide, hydrogen sulfide, tetra hydro
thiophene, dimethyl sulfide, mercaptans, other organic
sulfur compounds, and combinations thereof may be as high as
about 100 ppm. The presence of such high levels of sulfur
compounds, if not removed, results in the poisoning of
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
components of the fuel cell processing train and may foul
the fuel cell stack itself. Substantially complete removal
of all of the sulfur compounds is necessary as the presence
of even modest quantities of even a single sulfur compound
can damage the components of the fuel cell processing train.
While the desulfurization system of the invention can
be utilized for a number of different hydrogen generation
processes, one particularly preferred utilization is within
a fuel cell processing train. For purposes of this
specification all hydrogen generation systems are included,
although one preferred use is within a fuel cell processing
train.
The inventors have surprisingly discovered that
substantial desulfurization of a hydrocarbon fuel stream for
fuel cell processing trains down to levels as low as 50 ppb
or so can be achieved when a sequential sulfur adsorbent bed
system is used as the desulfurization system comprising one
or more selective sulfur adsorbents used in combination with
a zeolite adsorbent, particularly a calcium exchanged
zeolite, more particularly a calcium exchanged X zeolite.
The composition and sequence of use of the components of the
sequential sulfur adsorbent bed system can be adjusted
depending on the composition and quantity of the sulfur
compounds that are present in the hydrocarbon feed stream.
11
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
The selective sulfur adsorbent(s) of the invention is
selected from a wide variety of adsorbents. As used within
this application a "selective sulfur adsorbent" is a
material that preferentially absorbs at least one of the
sulfur compounds that are commonly present in hydrocarbon
fuel cell fuel streams, particularly natural gas, propane or
LPG, such as hydrogen sulfide, carbonyl sulfide, tetra hydro
thiophene, dimethyl sulfide, mercaptans, particularly ethyl,
methyl, propyl, and tertiary butyl mercaptans and
combinations thereof, at a temperature below 100 C and
pressures of about 10-250 psig or so.
Each selective sulfur adsorbent selectively adsorbs one
or more of the sulfur compounds that are commonly present in
the hydrocarbon fuel cell fuel stream, preferably natural
gas. However, each of these adsorbents may be less or more
effective than other of the selective sulfur adsorbents for
the adsorption of particular sulfur compounds or
combinations of these compounds. Further, additional
problems can be created in the feed stream when some of the
selective sulfur adsorbents are used alone as the sulfur
adsorbent, as these selective sulfur adsorbents can
synthesize existing sulfur compounds into different, higher
molecular weight synthesized sulfur compounds that are not
removed from the fuel stream by the particular selective
12
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
sulfur adsorbent.
It has been surprisingly discovered that the
desulfurization system can be substantially enhanced by
utilizing a zeolite adsorbent particularly a calcium
exchanged zeolite, and more particularly a calcium exchanged
X zeolite, in combination with the selective sulfur
adsorbent. Further, adsorption of a broader range of sulfur
compounds from the hydrocarbon fuel cell fuel streams may
occur when more than one selective sulfur adsorbent is used
in combination with the zeolite adsorbent in the sequential
sulfur adsorbent bed system. In particular, the combination
of one or more selective sulfur adsorbents with the calcium
exchanged zeolite adsorbent performs surprisingly better
than the individual selective sulfur adsorbents or the
calcium exchanged zeolite when used individually. In
addition, the choice and arrangement of the selective sulfur
adsorbent(s) and the zeolite within the sequential sulfur
adsorbent bed system can reduce the likelihood of the
production of synthesized sulfur compounds that are
sometimes created when only a single selective sulfur
adsorbent is utilized in the desulfurization system.
It has been further discovered that the removal of
various combinations of sulfur compounds can be enhanced by
the specific arrangement of the adsorbents in the sequential
13
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
sulfur adsorbent bed system. For example, for the removal
of one type or group of sulfur compounds, it is preferable
to place the calcium exchanged zeolite in the sequential
sulfur adsorbent bed prior to the selective sulfur adsorbent
while for other sulfur compounds or combinations of sulfur
compounds, it is preferable for the non-desulfurized
hydrocarbon fuel cell fuel stream to contact one of the
selective sulfur adsorbents prior to contacting the calcium
exchanged zeolite. For other non-desulfurized hydrocarbon
fuel cell fuel streams, it may be preferable to use two or
more selective sulfur adsorbents, wherein one or more of
these selective sulfur adsorbents are placed before or after
the zeolite adsorbent in the sequential sulfur adsorbent bed
system.
Sulfur adsorption by this system is further enhanced
because some sulfur compounds, which may be synthesized to
larger and more difficult to remove sulfur compounds by a
particular selective sulfur adsorbent, are removed from the
feed stream by the zeolite adsorbent, particularly the
calcium-exchanged zeolite adsorbent, prior to synthesis by
the selective sulfur adsorbent.
Suitable selective sulfur adsorbents are selected from
a group of adsorbents including, but not limited to, a group
of manganese-based adsorbents, such as an adsorbent
14
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
comprising substantially manganese compounds, an adsorbent
which includes manganese compounds, copper oxide and a
binder and an adsorbent which includes manganese compounds,
iron oxide and a high surface area carrier, particularly
alumina. Other useful selective sulfur adsorbents for this
desulfurization system may include, but are not limited to,
zinc oxide with or without a carrier, such as alumina;
activated carbon with copper oxide; a zinc oxide/copper
oxide blend preferably containing small quantities of carbon
and alumina; copper oxide with alumina; and a copper
oxide/zinc oxide blend mixed with alumina. Other useful
selective sulfur adsorbents may include nickel on silica or
alumina and other known selective sulfur adsorbents
containing copper, zinc, molybdenum and cobalt compounds.
Various quantities of the individual components of each of
these selective sulfur adsorbents can be utilized and the
quantity of the individual components can be modified to
enhance the adsorption capacity of the overall
desulfurization system, depending on the particular sulfur
compounds that are present in the hydrocarbon fuel cell fuel
stream and the quantity thereof.
In one particularly preferred embodiment, the selective
sulfur adsorbent contains one or more manganese compounds
blended with iron oxide on a high surface area support,
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
preferably a high surface area support comprising alumina,
silica, silica-alumina, titania, and other inorganic
refractory oxides, with a more preferred support being a
high surface area alumina. By "high surface area" the
inventors are describing a support with a surface area
greater than 100 m2/g.
The inventors have surprisingly discovered that the
ability of the, manganese compound(s)/iron oxide selective
sulfur adsorbent to adsorb sulfur compounds is enhanced when
the high surface area support is a high surface area
alumina. Adsorbents comprising manganese compound(s)/iron
oxide materials with high surface area alumina perform
better and adsorb higher levels of sulfur compounds than
when the carrier comprises other inorganic materials, even
with similar surface areas. Any type of alumina with a
surface area above 100 m2/g is within the scope of the
invention. The preferred carrier comprises from 5 to 25% by
weight, preferably from 5 to 20% by weight, and most
preferably from 5 to 15% by weight of the total weight of
this selective sulfur adsorbent. The primary function of
the support material is to provide a large and accessible
surface area for deposition of the active metal compounds.
The metal compounds which are deposited on or with the
high surface area support of this selective sulfur
16
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
adsorbent, other than the one or more manganese compound(s),
include iron oxide. In a preferred embodiment the iron
oxide and manganese compound(s) together comprise at least
60% by weight, preferably at least 70% by weight and most
preferably at least 80% to 90% of this selective su l-f,ur
adsorbent, by weight.
In a preferred embodiment the quantity of iron oxide
present in this selective sulfur adsorbent exceeds the
quantity of the manganese compound(s). It is preferred that
the ratio of the iron oxide to the manganese compound(s) by
weight, should be at least 1:1 and preferably from 1:1 to
6:1. The preferred loading of iron oxide on the support is
in the range of 40 weight percent to 80 weight percent and,
more preferably from 50 to 70 weight percent of the total
weight of the selective sulfur adsorbent. Various forms of
iron oxide may be used, such as FeO and Fe203 and mixtures
thereof.
The one or more manganese compound(s) comprise from 15
weight percent to 40 weight percent, preferably from 20
weight percent to 40 weight percent of the total weight of
the selective sulfur adsorbent. Various forms of manganese
compounds can be used including Mn02, Mn203, Mn304 and Mn (OH) 4
and mixtures thereof.
A promoter or promoters may also be added to this
17
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
selective sulfur adsorbent, preferably an alkali or alkaline
earth metal oxide and more preferably calcium oxide, in
quantities from 5 to 15% by weight. While calcium oxide is
the preferred promoter, other alkali or alkaline earth metal
oxides, such as magnesium oxide, may also, or alternatively,
be utilized with the calcium oxide.
The iron oxide/manganese compound(s) selective sulfur
adsorbent according to the present invention may be prepared
by coprecipitation, decomposition, impregnation or
mechanical mixing. Preferably, this selective sulfur
adsorbent is produced by coprecipitation or decomposition.
The method chosen should guarantee that there has been an
intensive blending of the components of the selective sulfur
adsorbent.
The specific pore volume of the iron oxide/manganese
compound(s) adsorbent produced by those procedures
determined by mercury porosimetry is preferably from 0.3
cc/g to 0.6 cc/g. In addition, this selective sulfur
adsorbent preferably has a compacted bulk density of 0.4 to
1.1 g/cc. Once the material is in its preliminary product
form, it can be further processed to form the final
selective sulfur adsorbent by pelletizing or extrusion.
This selective sulfur adsorbent preferably is formed into
moldings, especially in the form of spheres or pellets,
18
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
preferably ranging in size from 0.1 cm to 1 cm in diameter.
The surface area of this selective sulfur adsorbent is at
least 100 m2/g and preferably from 100 m 2/g to 300 m2/g.
The ratio of this iron oxide/manganese compound(s) with
alumina selective sulfur adsorbent to the calcium exchanged
zeolite adsorbent is from 1:4 to 4:1, preferably 1:3 to 3:1,
by volume. The sequence of utilization of this selective
sulfur adsorbent in the sequential sulfur adsorbent bed
system with the calcium exchanged zeolite adsorbent
preferably places the calcium exchanged zeolite adsorbent
prior to this selective sulfur adsorbent.
This iron oxide/manganese compound(s) selective sulfur
adsorbent when used alone has shown especially good sulfur
adsorption when the sulfur compounds contained in a fuel
cell fuel stream comprise hydrogen sulfide, carbonyl sulfide
(COS), tertiary butyl mercaptan (TBM) and ethyl mercaptan
(EM). This selective sulfur adsorbent, when utilized with
the calcium-exchanged zeolite adsorbent, has shown enhanced
utility for adsorption of additional sulfur compounds that
are commonly present in a fuel cell fuel stream including
tetra hydro thiophene (THT) and dimethyl sulfide (DMS),
especially when the zeolite is placed in sequence before the
iron oxide/manganese adsorbent compound(s) in the sequential
sulfur adsorbent bed system. However, some common
19
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
hydrocarbon fuel streams do not contain these additional
sulfur compounds. In this circumstance use of only the iron
oxide\manganese compound(s) selective sulfur adsorbent
without the calcium-exchanged zeolite adsorbent is an
alternative preferred embodiment.
Other selective sulfur adsorbents can be utilized in
combination with this selective sulfur adsorbent and zeolite
adsorbent for the adsorption of particular sulfur compounds
from a hydrogen generation system, such as a hydrocarbon
fuel cell feed stream. For example, particularly useful
combinations contain the calcium exchanged zeolite
adsorbent with this iron oxide/manganese compound(s) with
high surface area alumina selective sulfur adsorbent and
also include a selective sulfur adsorbent containing carbon
with copper oxide or copper oxide/zinc oxide with alumina.
These selective sulfur adsorbents are described in more
detail later in this specification. The sequence of
utilization of these additional selective sulfur adsorbents
with the zeolite adsorbent preferably places the zeolite
adsorbent prior to the iron oxide/manganese compound(s) with
high surface area alumina with the carbon/copper oxide or
the copper oxide/zinc oxide with alumina selective sulfur
adsorbent placed first in the sequence of the sequential
sulfur adsorbent bed stream.
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
In one preferred embodiment of this combination, the
zeolite adsorbent preferably comprises an amount equal to,
or greater than, the quantity of the other components in the
three component system in,the sequential sulfur adsorbent
bed, with quantities of the zeolite adsorbent up to 80% of
the total sulfur adsorbents present in the sequential sulfur
adsorbent bed system with the iron oxide/manganese
compound(s) with alumina selective sulfur adsorbent
comprising up to 20% and the carbon/copper oxide or copper
oxide/zinc oxide with alumina selective sulfur adsorbent
also comprising up to 20% of the sequential sulfur adsorbent
bed system, by volume.
An additional preferred selective sulfur adsorbent that
can be utilized with the zeolite adsorbent in the sequential
sulfur adsorbent bed system is comprised of one or more
manganese compound(s), copper oxide and small quantities of
a binder. The manganese compound(s) of this selective
sulfur adsorbent may be utilized in any of the forms
previously described for the manganese compound of the
selective sulfur adsorbent described above. The manganese
compound(s) of this selective sulfur adsorbent comprise from
50 to 80% and preferably from 60 to 75% of this selective
sulfur adsorbent, by weight. The copper oxide comprises
from 15 to 40% and preferably from 15 to 30%, by weight, of
21
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
this selective sulfur adsorbent. The binder comprises from
5 to 20%, by weight, of this selective sulfur adsorbent. In
a preferred embodiment the binder may be selected from a
wide variety of clays including bentonite, diatomaceous
earth, attapulgite, kaolin, sepiolite, illite and mixtures
thereof. More preferably, the binder comprises bentonite
clay. Promoters may also be added to this selective sulfur
adsorbent to enhance its operating characteristics. This
adsorbent is prepared by conventional procedures. The
surface area of this manganese compound(s)/copper oxide with
binder selective sulfur adsorbent ranges from 100 to 300
m2/g, preferably from 200 to 300 mZ/g.
This manganese compound(s)/copper oxide/binder
selective sulfur adsorbent when used alone has shown great
utility for the adsorption of hydrogen sulfide, carbonyl
sulfide, tertiary butyl mercaptan, ethyl mercaptan and
mixtures thereof. In addition, this manganese
compound(s)/copper oxide/binder selective sulfur adsorbent,
when utilized in sequence with the zeolite adsorbent in the
sequential sulfur adsorbent bed system, has shown
significant adsorption for sulfur compounds contained in
hydrocarbon fuel cell feed streams of the same type as those
described above where the selective sulfur adsorbent
composition comprises iron oxide, manganese compound(s) and
22
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
small quantities of a high surface area alumina.
The ratio of this selective sulfur adsorbent with the
zeolite adsorbent for the removal of sulfur compounds from a
fuel cell fuel stream, particularly natural gas, propane and
LPG, is from 1:4 to 4:1 and preferably from 1:3 to 3:1, by
volume.
Other selective sulfur adsorbents, particularly of the
same type, in the same quantities,.and in the same sequence
that may be utilized with the iron oxide/manganese
compound(s) with small quantities of high surface area
alumina, may also be utilized with this selective sulfur
adsorbent and the zeolite adsorbent to form a three
component system to enhance the adsorption of particular
sulfur compounds that are present in a fuel cell fuel
stream. The choice of the particular selective sulfur
adsorbent or adsorbents used can be adjusted depending on
the particular sulfur compounds that are present in the feed
stream and their quantity.
An additional selective sulfur adsorbent that can be
utilized with the zeolite adsorbent in the sequential sulfur
adsorbent bed system in place of, or in addition to, the
above described selective sulfur adsorbents comprises zinc
oxide alone or in combination with a carrier. While alumina
is the preferred carrier, other carriers with similar
23
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
performance characteristics can be utilized. In a preferred
embodiment, the zinc oxide comprises at least 60%,
preferably from 60 to 95%, and more preferably from 70 to
90%, by weight, of the selective sulfur adsorbent with the
remaining portion preferably comprising alumina. Additives
may be added to this selective sulfur adsorbent to enhance
its capacity to absorb sulfur compounds or other performance
characteristics. The surface area of this selective sulfur
adsorbent ranges from 5 to 75 mz/g and preferably from 10 to
50 m2/g. This zinc oxide/alumina selective sulfur adsorbent
is prepared by conventional procedures.
The zinc oxide alumina selective sulfur adsorbent when
used alone as a sulfur adsorbent has shown good sulfur
adsorption when the sulfur compounds contained within the
fuel cell fuel stream comprise hydrogen sulfide and ethyl
mercaptan and mixtures thereof.
The inventors have discovered that enhanced adsorption
of sulfur compounds occurs when this zinc oxide with alumina
selective sulfur adsorbent is utilized in a sequential
sulfur adsorbent bed system with the zeolite adsorbent of
the invention. Preferably, the order of the adsorbents in
the sequential sulfur adsorbent bed system utilizes the zinc
oxide with alumina selective sulfur adsorbent after the
zeolite. In a preferred embodiment the ratio of the zinc
24
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
oxide with alumina selective sulfur adsorbent to the zeolite
adsorbent is from 1:4 to 4:1 and in a more preferred
embodiment, from 1:3 to 3:1, by volume. Although the
sequential sulfur absorbent bed system chosen may contain
only the zinc oxide with alumina selective sulfur adsorbent
with the zeolite adsorbent, depending upon the sulfur
content and composition within the fuel cell fuel stream,
additional selective sulfur adsorbents may also be utilized
as part of the sequential sulfur absorbent bed system either
prior to or after the zeolite adsorbent and this selective
sulfur adsorbent.
Another selective sulfur adsorbent that can be utilized
with the zeolite adsorbent of the invention in the
sequential sulfur adsorbent bed system is comprised of
activated carbon containing small quantities of copper
oxide. In a preferred embodiment the activated carbon
comprises from 80 to 95%, preferably 85 to 95%, by weight,
of this selective sulfur adsorbent with the remaining
portion comprising copper oxide. Additives may be added to
the composition to enhance its performance. The activated
carbon/copper oxide selective sulfur adsorbent is prepared
by conventional procedures. The surface area of the
composition ranges from 300 to 1000 m2/g, with the preferred
surface area being from 500 mz/g to 1000 mz/g. This
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
selective sulfur adsorbent is prepared by conventional
procedures.
This activated carbon with copper oxide selective
sulfur adsorbent when used alone has shown great utility for
the adsorption of tetra hydro thiophene, tertiary butyl
mercaptan, ethyl mercaptan and mixtures thereof.
The quantity of the activated carbon/copper oxide
selective sulfur adsorbent to be utilized with the zeolite
adsorbent is at a ratio of 1:4 to 4:1, preferably 1:3 to
about 3:1, by volume. Further, the preferred sequence of
1S utilization of the selective sulfur adsorbent and the
zeolite adsorbent places the zeolite adsorbent ahead of the
activated carbon/copper oxide selective sulfur adsorbent in
the sequential sulfur adsorbent bed system.
This activated carbon with copper oxide selective
sulfur adsorbent has also shown good adsorption capability
when used in combination with other selective sulfur
adsorbents and the zeolite adsorbent for the adsorption of a
broad range of sulfur compounds contained in a fuel cell
feed stream.
Another useful selective sulfur adsorbent that can be
utilized with the zeolite adsorbent in a sequential sulfur
adsorbent bed system comprises copper oxide and zinc oxide
with alumina, preferably with small quantities of carbon. In
26
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
a preferred embodiment the copper oxide comprises from 50 to
65% and more preferably from 50 to 60% of the selective
sulfur adsorbent, by weight. The zinc oxide comprises from
20 to 35% of the selective sulfur adsorbent and the alumina
comprises from 5 to 20%, preferably from 10 to 20% of the
selective sulfur adsorbent, by weight. The quantity of the
carbon, if used, should be less than 10%, preferably from 1
to 10%, by weight. The surface area of this selective
sulfur adsorbent containing copper oxide, zinc oxide,
alumina, and preferably small quantities of carbon, is from
100 to 300 mz/g and preferably from 100 to 200 m2/g. The
process for the preparation of this selective sulfur
adsorbent is conventional.
This copper oxide/zinc oxide/alumina, preferably with
small quantities of carbon, selective sulfur adsorbent when
used alone is especially useful for the adsorption of
hydrogen sulfide, tertiary butyl mercaptan, ethyl mercaptan,
carbonyl sulfide and mixtures thereof.
The ratio of this selective sulfur adsorbent to the
zeolite adsorbent when used in the sequential sulfur
adsorbent bed system is from 1:4 to 4:1, preferably from 1:3
to 3:1, by volume. When the sulfur compound(s) to be
removed from the fuel cell fuel stream include the sulfur
compounds for which this selective sulfur adsorbent is
27
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
especially useful, the sequence for utilization of this
selective sulfur adsorbent with the zeolite adsorbent
requires the zeolite adsorbent to be placed prior to this
selective sulfur adsorbent in the sequential sulfur
adsorbent bed system. In addition to the utilization of the
copper oxide/zinc oxide/alumina and preferably with carbon
selective sulfur adsorbent, other selective sulfur
adsorbents may also be utilized, either prior to or after
this selective siilfur adsorbent in the sequential sulfur
adsorbent bed system of the invention.
An additional selective sulfur adsorbent that can be
utilized with the zeolite adsorbent in the sequential sulfur
adsorbent bed system, comprises manganese compound(s), used
alone, which may be utilized in a number of forms including
Mn02, Mn203, Mn304 and Mn (OH) 4 or mixtures thereof. The
surface area of the manganese compound(s) range from 100 to
300 m2/g, and preferably from 200 to 300 m2/g. Additional
materials may be combined with the manganese compound(s)
including calcium, silver and magnesium to promote the
performance of the manganese compound(s). Conventional
methods are utilized for the formation of this selective
sulfur adsorbent.
The manganese compound(s) selective sulfur adsorbent
when used alone has shown great utility for the adsorption
28
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
of hydrogen sulfide, tertiary butyl mercaptan, ethyl
mercaptan and mixtures thereof.
When used with the zeolite adsorbent in the sequential
sulfur adsorbent bed system, the ratio of the manganese
compound(s) utilized to the zeolite adsorbent is from 1:4 to
4:1 and preferably from 1:3 to 3:1, by volume. The sequence
of utilization of this manganese compound(s) selective
sulfur adsorbent in the sequential sulfur adsorbent bed
system is preferably for the zeolite sulfur adsorbent to be
placed prior to the manganese compound(s) selective sulfur
adsorbent. In addition to the use of the manganese
compound(s) and the zeolite adsorbent, other selective
sulfur adsorbents described herein may be utilized prior to
or after the manganese compound(s) selective sulfur
adsorbent in the sequential sulfur adsorbent bed system of
the invention.
An additional selective sulfur adsorbent, that can be
utilized with the zeolite adsorbent in the sequential sulfur
adsorbent bed system, comprises copper oxide with alumina,
wherein the quantity of the copper oxide is from 5 to 25%,
preferably from 10 to 20%, by weight, and the quantity of
the alumina is from 75 to 95%, preferably from 80 to 90%, by
weight. The surface area of this selective sulfur adsorbent
is from 100 to 300 mz/g and preferably from 150 to 300 m2/g.
29
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
This selective sulfur adsorbent is prepared by conventional
procedures.
This selective sulfur adsorbent when used alone has
shown particularly usefulness for the adsorption of hydrogen
sulfide, carbonyl sulfide, tertiary butyl mercaptan, ethyl
mercaptan and mixtures thereof. In addition, this copper
oxide with alumina selective sulfur adsorbent, when utilized
in sequence with the zeolite adsorbent in the sequential
sulfur adsorbent bed system, has shown significant
adsorption for sulfur compounds contained in fuel cell feed
streams of the same type as are described above. When used
in the sequential sulfur adsorbent bed system for the
adsorption of sulfur compounds with the zeolite adsorbent,
the ratio of the selective sulfur adsorbent to the zeolite
adsorbent is from 1:4 to 4:1, preferably from 1:3 to 3:1, by
volume. The sequence of utilization of this selective
sulfur adsorbent with the zeolite adsorbent in the
sequential sulfur adsorbent bed system is preferably for the
zeolite adsorbent to be placed prior to the selective sulfur
adsorbent. Other selective sulfur adsorbents may also be
utilized with this selective sulfur adsorbent for the
absorption of sulfur compounds in the sequential sulfur
adsorbent bed system of the invention.
An additional selective sulfur adsorbent, that can be
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
utilized with the zeolite adsorbent in the sequential
adsorbent bed system, comprises copper oxide, zinc oxide and
alumina, with the quantity of copper oxide being from 15 to
25%, the quantity of the zinc oxide being from 5 to 15%, and
the quantity of the alumina being from 65 to 85%, by weight.
The surface area of this selective sulfur adsorbent is from
100 to 300 m2/g, preferably from 150 to 300 mz/g. This
selective sulfur adsorbent catalyst is prepared by
conventional procedures.
This selective sulfur adsorbent when used alone is
particularly useful for the adsorption of hydrogen sulfide,
carbonyl sulfide, tertiary butyl mercaptan, ethyl mercaptan,
and mixtures thereof.
When used with the zeolite adsorbent, the preferred
ratio of this selective sulfur adsorbent with the zeolite
adsorbent is from 1:4 to 4:1 and preferably from 1:3 to 3:1,
by volume. The sequence of use of this selective sulfur
adsorbent with the zeolite adsorbent is preferably for the
zeolite adsorbent to be placed prior to the selective sulfur
adsorbent. This selective sulfur adsorbent may be utilized
with other selective adsorbents as well as with the zeolite
adsorbent and is a particularly preferred option, as
discussed above. For example, in one particularly preferred
embodiment, this selective sulfur adsorbent is utilized with
31
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
the zeolite adsorbent and with the iron oxide, manganese
compounds and alumina selective sulfur adsorbent, as
previously described.
The inventors have surprisingly discovered that the
selective sulfur adsorbents described above work best when
utilized within a sequential sulfur adsorbent bed system
containing one or more of the selective sulfur adsorbents
and the zeolite adsorbent. While several types of ion
exchanged zeolites may be useful as the zeolite adsorbent,
the preferred ion exchange zeolite is a calcium exchanged
zeolite. While a number of calcium exchanged zeolites are
known, including calcium exchanged zeolite A, zeolite X,
zeolite Y, zeolite ZSM-5, zeolite Beta, synthetic mordenite
and blends thereof, the preferred calcium exchanged zeolite
is a calcium exchanged zeolite X. A particularly preferred
calcium exchanged zeolite X is calcium exchanged, low silica
zeolite X, known as "LSX", or calcium exchanged low silica
faujasite, known as "LSF". Zeolite X generally has a Si:Al
equivalent ratio of 1.0 to 1.25. In one common example, a
conventional, non-calcium exchanged precursor synthesized
LSF has the following anhydrous chemical composition: 2.0
Si0Z : A1203 : 0. 73 Na20: 0. 27K20, although the ratio between
sodium and potassium cations can vary, sometime
significantly, depending upon the process of manufacture of
32
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
the LSF.
For the present invention, a substantial percentage of
the cations of the zeolite X are ion exchanged with calcium
ions using conventional ion exchange procedures, such as by
treatment of the zeolite X with calcium salts, such as, but
not limited to, calcium chloride. Several methods can be
used for the ion exchange procedure with ion exchange
preferably occurring after the zeolite adsorbent has been
formed into its preferred final form, such as a bead or an
extrudate. The zeolite X is ion exchanged to a level of at
least 50%, preferably at least 60%, more preferably at least
70%, and most preferably 85 to 95% of the exchangeable metal
ions. The remaining ions may be sodium and/or potassium
ions. (For reference purposes the term "calcium exchanged
zeolite X" means a zeolite X containing at least about 50%
calcium cations.)
The calcium exchanged zeolite X of the invention
generally contains sodium or potassium ions in addition to
the calcium ions after the calcium ion exchange. However, a
portion or substantially all of these sodium/potassium ions
can be ion exchanged with other cations to enhance or modify
the performance characteristics of the calcium exchanged
zeolite X, especially for sulfur adsorption. For example,
additional cations that may be ion exchanged onto the
33
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
zeolite X to enhance its performance include zinc, cadmium,
cobalt, nickel, copper, iron, manganese, silver, gold,
scandium, lithium and combinations thereof. The percentage
of ion exchange of these additional metal ions can range
from as little as 1% up to 40% or so, depending upon the
level of calcium exchange of the zeolite X. The particular
metal ions that are ion exchanged onto the calcium exchanged
zeolite depend on the particular sulfur compounds which are
intended to be removed from the fuel cell fuel stream by the
sequential sulfur adsorbent bed system of the invention.
The calcium exchanged zeolite, when utilized above as a
sulfur adsorbent, has shown significant capability for the
adsorption of various sulfur materials, particularly tetra
hydro thiophene (THT), di-methyl sulfide (DMS), tertiary
butyl mercaptan (TBM) and ethyl mercaptan (EM).
In addition, it has been surprisingly discovered that
the capability of the selective sulfur adsorbents described
above, when used individually, and the calcium exchanged
zeolite, when used individually, can be enhanced
dramatically by the combination use of the calcium exchanged
zeolite X with the selective sulfur adsorbents to form the
sequential sulfur adsorbent bed system for the
desulfurization of a hydrocarbon fuel cell feed stream. The
use of this combination of the selective sulfur adsorbent
34
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
with the calcium exchanged zeolite permits the adsorption of
a broader range of sulfur containing compounds than has been
conventionally been adsorbed using either component alone.
For example, it has been surprisingly discovered that by the
use of the selective sulfur adsorbents mentioned above in
combination with the calcium exchanged zeolite X described
above, enhanced sulfur adsorption of a broader range of
sulfur compounds, including carbonyl sulfide, hydrogen
sulfide, tetra hydro thiophene, dimethyl sulfide, and
various mercaptans, including ethyl, methyl, propyl, and
tertiary butyl mercaptan and combinations thereof, is
possible.
It has also been surprisingly discovered that the
breakthrough time for all sulfur compounds commonly present
in a hydrocarbon fuel system can be extended by the use of
one or more selective sulfur adsorbents with the calcium
exchanged zeolite X and by arranging the order of the
components correctly within the sequential sulfur adsorbent
bed system.
It has also been surprisingly discovered that by
placement of the calcium exchanged zeolite X prior to one or
more of the selective sulfur adsorbents in the sequential
sulfur adsorption bed system, the likelihood of the
production of synthesized sulfur compounds is substantially
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
reduced.
The inventors have also surprisingly discovered that
the sequential sulfur adsorbent bed system of the invention
can be utilized at temperatures lower than normally utilized
for conventional sulfur adsorption. While conventional
chemical sulfur adsorbents require temperatures of the feed
stream of at least 150 C to 400 C, the sequential sulfur
adsorbent bed system of the invention can be utilized
effectively to adsorb the sulfur contaminants at
temperatures below 100 C and is effective for removal of
some sulfur compounds at temperatures as low as ambient
temperatures. Further, because of the lower temperature of
use, the sequential sulfur adsorption bed is easier to use
than when higher temperatures are necessary.
In addition, when the sequential sulfur adsorbent bed
system of the invention is used, the pressure on the feed
stream may be reduced to a range as low as from 1 bar to 18
bar, preferably from 1.7 bar to 7 bar, pressures lower than
normally used for adsorption of sulfur compounds in a
conventional fuel cell processing train.
The inventors have also discovered a method for
supplying a substantially desulfurized hydrocarbon fuel
stream to a fuel cell processor using the sequential sulfur
adsorbent bed system described above. In this process a
36
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
sulfur contaminated hydrocarbon fuel stream is passed over
or through the sequential sulfur adsorbent bed system of a
fuel cell processor of the invention at a temperature from
about ambient to 100 C, preferably less than 60 C, and more
preferably at ambient temperatures. By passing a
hydrocarbon fuel stream comprising, for example, natural
gas, propane or LPG, containing sulfur components at levels
up to 500 ppm, a substantial reduction in the quantity of
those sulfur compounds, preferably down to a level of less
than about 50 ppb, can be achieved.
The inventors have also discovered that the above-
described sequential sulfur adsorbent bed system of the
invention can be used in a desulfurizer, particularly for
use in a fuel cell processing train. This desulfurizer
includes an inlet for receiving the nondesulfurized
hydrocarbon fuel stream, such as natural gas, propane or
LPG, the sequential sulfur adsorbent bed system of the
invention, as described above, which is placed in a location
to desulfurize the hydrocarbon fuel stream, and an outlet
where the desulfurized hydrocarbon fuel stream is passed
down stream for further processing. For example, the
desulfurized hydrocarbon fuel stream can be passed through
the fuel cell processing train to the fuel cell stack for
37
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
the production of electricity.
The inventors have also surprisingly discovered that
this method for supplying a substantially desulfurized
hydrocarbon fuel stream is more advantageous than
conventional desulfurization systems as it permits
desulfurization of a broader range of sulfur compounds,
increases the breakthrough time for the system, reduces the
production of synthesized sulfur compounds, reduces the
required temperature and pressure of the feed stream and
permits the choice of different combinations and quantities
of selective sulfur adsorbents to be used in the sequential
sulfur adsorbent bed system depending on the sulfur
compounds that are present in the particular feed stream.
The compositions and methods of the invention also permit
the production of a substantially desulfurized hydrocarbon
fuel stream to levels of sulfur below those of conventional
desulfurizing processes.
The inventors have also discovered that the sequential
sulfur adsorbent bed system of the invention can be used in
fuel cell processors for a longer period of time than
conventional adsorbents and still achieve high levels of
sulfur absorbency.
The inventors have also discovered that the sequential
sulfur adsorbent bed system of the invention is also not
38
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
subject to desorption of the adsorbed sulfur compounds when
the conditions surrounding the catalyst bed change, as often
occurs with some conventional sulfur adsorbents.
EXAMPLES
The following examples are intended to be illustrative
of the present invention and to teach one of ordinary skill
in the art to make and use the invention. These examples
are not intended to limit the invention in any way.
In order to illustrate the operation of the invention,
the inventors have compared the performance of various
sulfur adsorbents, when used alone and in combination. In
each example, a synthetic natural gas feed stream is
utilized comprising 93% methane, 3% ethane, 2% propane, 0.2%
butane, 1% carbon dioxide and 0.75% nitrogen. Also included
in this synthetic natural gas is 10 ppm (as sulfur) of each
of either tert-butyl mercaptan or ethyl mercaptan
("mercaptan") and tetra hydro thiophene ("THT") . This
synthetic natural gas is passed through an artificial
reactor containing 10 cc of the selected sulfur adsorbent or
adsorbents in a bed. When two sulfur adsorbents are used in
combination, the quantity of the adsorbents is 7.5 cc of the
zeolite sulfur adsorbent, as described in Example 1, and 2.5
cc of the selective sulfur adsorbent, as described in
Example 2. The zeolite adsorbent is in the form of 2 mm
39
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
spheres. The selective sulfur adsorbent is a 1.18 mm x 0.85
mm mesh particulate typically produced from 1.6 mm
extrudates by grinding. The adsorbents are sized and loaded
into the reactor and the synthetic natural gas feed stream
is passed through the reactor. The temperature of the feed
stream is maintained at 38 C with a space velocity of 1500
hr-1 at a pressure of 2 bar. "Breakthrough" for this test
occurs when greater than 50 ppb of sulfur is observed in the
natural gas feed stream after passage through the adsorbent
bed. To determine the gas phase sulfur level of the feed
stream, analysis was performed using an Agilent 6890 gas
chromatograph attached to an Antek 7090 sulfur analyzer.
The gas chromatograph utilizes a 60 m X 320 micron DB-1
capillary column for sulfur compound separation. The Antek
7090 utilizes a sulfur chemiluminescense detector (SCD) for
sulfur detection. The operational detection limit for the
system is approximately 50 ppb (mole) The test unit is
controlled by automation software.
Example 1
The synthetic natural gas containing mercaptan and THT
is passed through a reactor containing only calcium
exchanged zeolite X. The zeolite X has an Si:Al equivalent
ratio of 1.17 and a calcium exchange of 70% with the
remaining metal ions comprising sodium and/or potassium.
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
The temperature of the reactor is maintained at 38 C and the
pressure is maintained at about 2 bar. The sulfur
adsorbency of the calcium exchanged zeolite is shown in
Figure 1, which shows a first breakthrough for the mercaptan
at 268 hours.
Example 2
The synthetic natural gas containing mercaptan and THT
is passed through a reactor containing only a selective
sulfur adsorbent comprising 34% by weight manganese
compounds, 54% iron oxide comprising Fe203 and 12% alumina
with a surface area of 294 m2/g. The performance of this
selective sulfur adsorbent is shown in Figure 2, wherein the
first breakthrough occurs at less than 25 hours. The sulfur
compound(s) that is produced at that time is a "synthesized
sulfur compound" as the breakthrough for THT does not occur
until after 100 hours. It is believed that the "synthesized
sulfur compounds" is at least one higher molecular weight
sulfur compound produced from the interaction of the THT
and/or the mercaptan with the selective sulfur adsorbent.
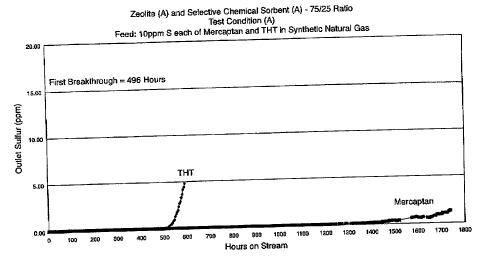
Example 3
A further test was run wherein the calcium exchanged
zeolite of Example 1 is used in combination with the
selective sulfur adsorbent of Example 2 in the reactor.
Seventy-five percent of the sulfur adsorbents by volume
41
CA 02578233 2007-02-26
WO 2006/028686 PCT/US2005/029715
comprised the zeolite and 25% comprised the selective sulfur
adsorbent. 10 ccs of the combined adsorbents are used. The
zeolite was placed ahead of the selective sulfur adsorbent
in the reactor. Otherwise, the operating conditions and the
composition of the feed stream are the same as for Examples
1 and 2. When the feed stream is passed through the
reactor, breakthrough does not occur until 496 hours as
shown in Figure 3.
As is clear from these examples, the combination of the
calcium exchanged zeolite with the selective sulfur
adsorbent increases the time of sulfur breakthrough,
prevents the formation of synthesized sulfur compounds and
extends the lifetime of the sequential sulfur adsorbent bed
system.
As many changes and variations in the disclosed
embodiments may be made without departing from the inventive
concept, the invention is not intended to be limited.
42