Note: Descriptions are shown in the official language in which they were submitted.
CA 02578767 2007-02-28
WO 2004/100897 PCT/US2004/01 1827
1
Process for the Preparation of 7-Alkyl-l0-Hydroxy-20(S)--
Camptothecin
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to an efficient three-step
process for the preparation of 7-alkyl-l0-hydroxy-20(S)-
camptothecin from the readily available natural product,
20(S)-camptothecin. The invention also demonstrates a
novel intermediate useful in this synthesis.
2. Description of the Related Art
Camptothecin derivatives have shown significant
cytotoxic activity and several have been developed into
useful pharmaceuticals. Specifically Irinotecan (Campto)
has shown excellent activity toward colon-rectal cancers
and is widely marketed. It shows considerable advantage
over other camptothecin derivatives in that it is water
soluble.
- Irinotecan is prepared in several steps from the
key intermediate, 7-ethyl- l0-hydroxy-20(S)-camptothecin.
Considerable effort has been expended to introduce both
the 10-hydroxy and the 7-ethyl functionality into 'the
camptothecin molecule. Therefore, while there is some
prior art associated with each of these individual
groups, there is very little knowledge on introduction
of both these functionality simultaneously into the
molecule.
Sawada (Chem. Pharma. Bull., 39(12), 3183(1991)
demonstrates the synthesis of 7-ethyl-l0-hydroxy-20(S)-
camptothecin through the synthesis of 7-ethyl-20(S)-
camptothecin by known means, the subsequent formation of
an N-oxide and the photochemical rearrangement to
CA 02578767 2009-05-20
2
provide 7-ethyl-l0-hydroxy-20(S)-camptothecin. However,
this synthesis suffers considerably from the
insolubility of 7-ethyl-20(S)-camptothecin in suitable
solvents and thus only small quantities can be prepared.
10-Hydroxy-20(S)-camptothecin has been prepared by
the hydrogenation of 20(S)-camptothecin to 1,2,6,7-
tetrahydro-20(S)-camptothecin and subsequent oxidation.
Thus US Pat. 5,734,056 describes the preparation through
the hydrogenation of 20(S)-camptothecin to 1,2,6,7-
tetrahydro-20(S)-camptothecin followed by the oxidation
with iodosobenzene derivatives specifically esters such
as iodobenzenediacetate. Hydrogenation of camptothecin
followed by oxidation with agents such as CAN(cerium
(IV) ammonium nitrate, chromic acid, potassium
permanganate, Fremy's salt is also known. Similarly,
Sawada, et. al. (Chem.Pharm. Bull. 39(120)3183, 1991)
describes a reduction and oxidation with lead
tetraacetate. In all these cases, the use of a 7-
substituted derivative has not been demonstrated.
The preparation of 7-ethyl-20(S)-camptothecin has
been demonstrated previously through the Fenton reaction
by employing 20(S)-camptothecin and propionaldehyde with
ferrous sulfate and sulfuric acid.
Therefore there is a need for an efficient
synthesis of 7-ethyl-l0-hydroxy-20(S)-camptothecin which
can be used in commercial scale.
SUMMARY OF THE INVENTION
The present invention provides as one
embodiment a novel process employing the formation of
the 7-ethyl-20(S)-camptothecin followed by the catalytic
reduction and subsequent oxidation to the desired 7-
CA 02578767 2009-05-20
3
ethyl -l0-hydroxy-20(S)-camptothecin, shown in Scheme I,
which is useful in the synthesis of Irinotecan.
Scheme I
H3C H3C H
H ai~N 0 0 0
N Ethylation N \ ~ Hydrogenation N H\
0 O H O
H3C OH O H3C OH O H3C OH O
I II
20-IS)-Camptothecin
Oxidation
C
HNCN-
HO III
0
H3C OH
In a broad aspect, the present invention provides a compound
of the formula:
O
H
N O
N O
O H OH
a
In another broad aspect, the present invention provides a
process for the preparation of 7-ethyl-l0-hydroxy-20(S)-
camptothecin of formula:
CA 02578767 2009-05-20
3a
O
HO
N I O
N \ O
OH
In another broad aspect, the present invention provides a
process of producing irinotecan comprising: reducing 7-ethyl-
20(S)-camptothecin to 7-ethyl-1,2,6,7-tetrahydro-20(S)-
camptothecin with hydrogen gas catalyzed by a reduction
catalyst; oxidizing the 7-ethyl-1,2,6,7-tetrahydro-20(S)-
camptothecin with an oxidizing agent to produce 7-ethyl-l0-
hydroxy-20(S)-camptothecin; and converting 7-ethyl-l0-
hydroxy-20(S)-camptothecin to the irinotecan.
In another broad aspect, the present invention provides a
compound of the formula:
R
H
0
N
N
H 0
O
wherein R is a lower alky group having 1 to 6 carbon atoms.
In another broad aspect, the present invention provides a
process for the preparation of 7-alkyl-l0-hydroxy-20(S)-
camptothecin of the formula
II CA 02578767 2011-03-08
3b
O
HO
N O
N O
OH
wherein R is an alkyl group, comprising the steps of:
reducing 7-alkyl-20(S)-camptothecin to 7-alkyl-l,2,6,7-
tetrahydro-20(S)-camptothecin with hydrogen gas catalyzed by
a reduction catalyst; and oxidizing the 7-alkyl-l,2,6,7-
tetrahydro-20(s)-camptothecin with an oxidizing agent to
produce 7-alkyl-l0-hydroxy-20(S)-camptothecin. The present
invention also provides a process for producing tecans by
this process
DETAILED DESCRIPTION OF THE PRESENTLY PREFERRED EMBODIMENTS
The formation of 7-alkyl-20(S)-camptothecin (I) was
accomplished by known methodology. It is known in the
literature that the hydrogenation of tetra-substituted
olefins is very difficult. Therefore, it was expected
that the hydrogenation of this compound to 7-alkyl-
1,2,6,7 - tetrahydro-20(S)-camptothecin (II) would be
challenging. We were surprised to learn that we could
indeed accomplish this hydrogenation in good yield and
good purity using Pt02 as the catalyst in a suitable
solvent in which the 7-alkyl-20(S)-camptothecin is
soluble. Catalysts other than Pt02 may be used, such as
reduction catalyst containing at least one of the
elements platinum, rhodium, lawrencium, and ruthenium.
Further, the hydrogenation step may be conducted with a
CA 02578767 2007-02-28
WO 2004/100897 PCT/[IS2004/014827
4
catalysis modifier, such as dimethylsulfoxide and
ammonium hydroxide. Acetic acid is a preferred solvent
for this purpose- Other solvent systems such as alcohols
and mixtures of acetic acid and alcohols can be employed
in this hydrogenation but high solubility of
camptothecin in acetic acid makes acetic acid the most
desirable solvent. By employing this catalytic
hydrogenation, the desired product can be easily
obtained in greater than 90% yield.
It was found that unlike the known 1,2,6,7-
tetrahydro-20(S)-camptothecin, 7-alkyl-1,2,6,7 -
tetrahydro-20(S)-camptothecin (II) is oxidized readily
back to 7-alkyl-20(S)-camptothecin (I) under an oxygen
atmosphere. Therefore there was a question as to whether
the oxidation would produce the 10-hydroxy derivative in
good yield- In fact, the oxidation with
iodobenzenediacetate in acetic acid/water did. produce
the desired 7-alkyl-l0-hydroxy-20(S)-camptothecin (III)
in very good yield. The reaction can be carried out in a
variety of solvent systems but again acetic acid/water
was the most convenient and preferred solvent system.
Other suitable solvents include C1-C6 ester, C1-C6 acid,
C1-C6 alcohol and water. More specifically, the C1-C6
acid may be butenic acid, propanoic acid and acetic
acid. The reaction may also be carried out with various
other oxidizing agents, including those containing
hypervalent iodine, ruthenium (VIII), manganate (VII),
osmium (VIII), lead (IV) and chromium (VI). The product
precipitates during the reaction and can be collected by
filtration. The product obtained is of sufficient purity
to be used directly or it can be purified by
CA 02578767 2007-02-28
WO 2004/100897 PCT/US2004/014827
recrystallization from organic solvents such as acetic
acid.
Therefore the present invention provides for an
efficient synthesis of 7-alkyl-i0-hydroxy-20(S)-
5 camptothecin (III).
Examples
Preparation of 7-ethyl-20(S)-camptothecin (I)
20(S)-camptothecin (60.-0 g), ferrous sulfate
heptahydrate (12.0 g) and 9N sulfuric acid (1200 ml) are
subsequently charged to a 5-L reactor equipped with a
mechanical stirrer, condenser and a thermometer under
nitrogen atmosphere. The resulting mixture is stirred at
25 C until all the suspension is dissolved, and it is
cooled to between -10 and 0 C. Propionyl aldehyde (10.0
g) is added to the cold reaction mixture- A solution of
10% hydrogen peroxide (116.9 g) and propionyl aldehyde
(15'.0 g) are simultaneously charged to the cold reaction
mixture over a period of 30-60 minutes, while
maintaining the temperature at 10 to 0 C. The resulting
mixture is stirred at the same temperature for 60 to 90
minutes. The reaction mixture was diluted with water and
neutralized with aqueous ammonium hydroxide to
precipitate out the desired product. The crude product
was crystallized from acetic acid and water to give
compound I, 49.83 g in 71.6% yield with purity of 95.16%
by HPLC. 1H-NMR (DMSO-d6) 6: 0.9 (3H, t), 1.3 (3H, t),
1.85 (2H, q), 3.2 (2H, q), 5.28 (2H, s), 5.44 (2H, s),
6.5 (1H, s), 7.32 (1H, s), 7.7 (1H, dd), 7.85 (1H, dd),
8.15 (1H, d), 8.26 (1H, d).
CA 02578767 2010-04-01
6
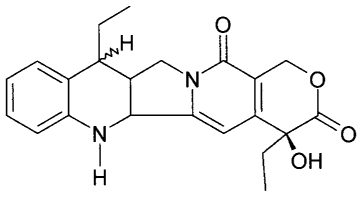
Preparation of 7-ethyl-1,2,6,7-tetrahydro-20(S)-
camptothecin (II)
7-ethyl-20(S)-camptothecin (I) (30.0 g) and acetic
acid (900 ml) were charged together and heated to 80 C
to facilitate the dissolution. The resulting solution is
then transferred to a 2-L autoclave reactor and cooled
to room temperature. Ammonium hydroxide (30% contents,
3.4 ml), platinum oxide and dimethyl sulfoxide (2.2 ml)
were added into the resulting suspension at 25 C. The
resulting mixture is then subjected to hydrogenation at
a hydrogen pressure of 5 bars until the starting
material, 7-ethyl-20(S)-camptothecin I, disappeared by
TLC analysis. The catalyst was removed by filtering
through a pad of celiteTM and washed with acetic acid,
the resulting solution is used directly for the next
reaction. The sample was characterized by HPLC, NMR, IR
and LC/MS analysis. HPLC shows three diastereoisomers in
a ratio of 6: 61: 13, which are detected by LC/MS to
have MS m/z: 380 (M+). 1H-NMR (DMSO-d6) 6: 0.78 (3H, t),
0.82 (3H, m), 1.2-1.35 (2H, m), 1.8 (3H, m), 2.65 (1H,
m), 3.12 (1H, m), 3.75 (1H, dd), 4.08 (1H, dd), 4.92
(1H, dd), 5.23 (1H, s), 6.48 (1H, s), 6.5-6.98 (4H, m),
6.62 (1H, s); IR (KBr) v: 3310, 2967, 1744, 1652, 1586,
1491, 1465 cm-1.
Preparation of 7-ethyl-10-hydroxy-20(S)-camptothecin
(III)
The hydrogenated filtrate of 7-ethyl-1,2,6,7-
tetrahydro-20(S)-camptothecin was charged to a 3-L,
four-necked round bottom flask equipped with a
mechanical stirrer, thermometer under nitrogen
atmosphere, and was cooled to 10 C. Water ( 900 ml) was
CA 02578767 2007-02-28
WO 2004/100897 PCT/US2004/014827
7
added to the solution and the resulting solution was
stirred at this temperature for 20 minutes.
Subsequently, iodobenzene diacetate (65.5 g) was added
to the solution in several small portions, while
maintaining the temperature below 10 C. The resulting
mixture was stirred at this temperature until the
complete disappearance of the starting material, 7-
ethyl-1,2,6,7-tetrahydro-20(S)-camptothecin (II), as
monitored by TLC. The reaction was quenched by the
addition of Methanol (230 ml) to facilitate the
precipitation of the product. The reaction slurry was
then filtered and the collected solids are washed with
aqueous acetic acid and methanol to give the desired
product 28.3 g (90% overall yield in two steps) . 1H-NMR
(DMSO-d6) 6: 0.9 (3H, t), 1.32 (3H, t), 1.88 (2H, q), 3.1
(2H, q), 5.28 (1H, s), 5.42 (1H, s), 6.46 (1H, s), 7.28
(1H, s) , 7 . 4 (2H, m) , 8. 0 (1H, d) , 10.5 (1H, s) .
Preparation of 7-methyl-20(S)-camptothecin
We performed a process corresponding to the above
process to make 7-ethyl-20(S)-camptothecin to provide
the product, 25.6 g in 60% yield. IH-NMR (DMSO-d6) b:
0.90 (3H, t), 1.88 (2H, m), 2.79 (3H, s), 5.29 (2H, s),
5.44 (2H, s), 6.51 (1H, s), 7.34 (1H, s), 7.73 (1H, t),
7.86 (1H, t), 8.15 (1H, d) , 8.25 (1H, d) .
Preparation of 7-methyl-1,2,6,7-tetrahydro-20(S)-
camptothecin
We performed a process corresponding to the above
process to make 7-ethyl-1,2,6,7-tetrahydro-20(S)-
camptothecin. HPLC of the product shows three
diastereoisomers in a ratio of 13: 68: 19, 1H-NMR (DMSO-
CA 02578767 2009-05-20
8
d6) b: 0.78 (3H, t), 1.02 (3H, d), 1.72 (2H, m), 1.90
(3H, m), 3.01 (1H, m), 3.17 (1H, m), 3.91 (1H, m), 4.06
(1H, m) , 4.91 (1H, m) , 5. 21 (1H, s ) , 6.30 (1H, s) , 6. 56-
6.6 (2H, m), 6.8-7.0 (2H, m).
Preparation of 7-methyl-10-hydroxy-20(s)-camptothecin
We performed a process corresponding to the above
process to make 7-ethyl-10-hydroxy-20(s)-camptothecin
(III). The HPLC of the reaction product shows 17% of
the desired product and 41% 7-methyl-20(S)-camptothecin.
Thus, while there have shown and described and
pointed out fundamental novel features of the invention
as applied to a preferred embodiment thereof, it will be
understood that various omissions and substitutions and
changes in the form and details of the process
illustrated, and in its operation, may be made by those
skilled in the art without departing from the spirit of
the invention. For example, it is expressly intended
that all combinations of those elements and/or method
steps which perform substantially the same function in
substantially the same way to achieve the same results
are within the scope of the invention. Moreover, it
should be recognized that structures and/or elements
and/or method steps shown and/or described in connection
with any disclosed form or embodiment of the invention
may be incorporated in any other disclosed or described
or suggested form or embodiment as a general matter of
design choice. It is the intention, therefore, to be
limited only as indicated by the scope of the claims
appended hereto.