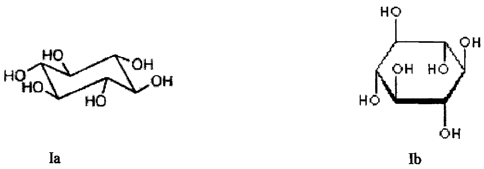Note: Descriptions are shown in the official language in which they were submitted.
CA 02579188 2007-02-19
Title: Treatment of Amyloid-Related Diseases
This application claims the benefit of priority under 35 U.S.C. 119(e) to
U.S. Provisional
Application 60/744,918, filed February 17, 2006 and U.S. Provisional
Application 60/811,587, filed June
7, 2006, incorporated herein by reference in full.
FIELD OF THE INVENTION
The invention relates generally to scyllo-inositol compounds and compositions,
and methods and
uses of the compositions, in particular methods for treating amyloid-related
diseases.
BACKGROUND OF THE INVENTION
Multiple lines of evidence suggest that the accumulation of neurotoxic
oligomeric/protofibrillar
aggregates of amyloid (3-peptide (A(3) is a central event in the pathogenesis
of Alzheimer disease (AD) [ 1,
2]. This has led to attempts to develop therapies based upon blocking the
generation of A(3 (e.g., with (3-
or y-secretase inhibitors), accelerating its removal,or preventing its
aggregation and toxicity. The
potential utility of anti-A(3 therapies for AD has received tentative support
from a clinical trial of a
vaccine, which suggested clinical and neuropathological improvement in a small
cohort of AD patients
[24, 25]. However, the anti-A(3 vaccine also induced a T-cell-mediated meningo-
encephalitis in some
patients which renders this particular vaccine unsuitable for widespread
clinical use [36]. Nevertheless,
A(3 vaccines have been shown in some mouse models to act via antibody-mediated
inhibition of A(3
fibrillogenesis and toxicity [21, 37, 38]. Thus, it would be desirable to
identify small molecule inhibitors
of A(3-aggregation that would avoid the potential risks of immunotherapy.
SUMMARY OF THE INVENTION
The invention provides a composition, in particular a pharmaceutical
composition, comprising a
scyllo-inositol compound that provides beneficial effects in the treatment of
an amyloid-related disease.
In an aspect the invention provides a pharmaceutical composition, comprising
one or more scyllo-inositol
compound that provides beneficial effects, in particular sustained beneficial
effects, following treatment.
The beneficial effects provided by a composition of the invention can include
enhanced therapeutic
effects, in particular sustained therapeutic effects.
The invention also provides a pharmaceutical composition intended for
administration to a subject
to provide beneficial effects, in particular sustained beneficial effects,
comprising a scyllo-inositol
compound, in particular a pure scyllo-inositol compound, more particularly a
substantially pure scyllo-
inositol compound, optionally together with one or more pharmaceutically
acceptable carriers, excipients,
or vehicles.
The invention also provides a pharmaceutical composition for the treatment of
a disorder and/or
disease comprising a therapeutically effective amount of a scyllo-inositol
compound to provide a
sustained beneficial effect in a pharmaceutically acceptable carrier,
excipient, or vehicle.
In an aspect, a pharmaceutical composition comprising a scyllo-inositol
compound is provided
which has been adapted for administration to a subject to provide sustained
beneficial effects to treat an
amyloid-related disease. In an embodiment, the composition is in a form such
that administration to a
1
CA 02579188 2007-02-19
subject suffering from an amyloid-related disease results in improved
cognitive function, reduced vascular
load, reduced astrogliosis, reduced amyloid burden, reduced microgliosis,
and/or improved survival. In
particular, the composition is in a form that results in improved cognitive
function, reduced vascular load,
reduced astrogliosis, reduced amyloid burden, reduced microgliosis, and/or
improved survival in the
subject, in particular for a sustained period of time after cessation of
treatment.
The present invention is directed to compositions comprising a scyllo-inositol
compound that
provides beneficial effects, in particular sustained beneficial effects, in
the treatment of an amyloid-related
disease, more particularly Alzheimer's disease.
In another aspect, the invention features a composition comprising a scyllo-
inositol compound in
a dosage effective for improving cognitive function, reducing vascular load,
reducing astrogliosis,
reducing amyloid burden, reducing microgliosis, and/or improving survival in
the subject, in particular for
a sustained period following administration of the compound. The composition
can be in a
pharmaceutically acceptable carrier, excipeint, or vehicle.
The invention additionally provides a method of preparing a stable
pharmaceutical composition
comprising one or more scyllo-inositol compound adapted to provide beneficial
effects, preferably
sustained beneficial effects, following treatment. The invention further
provides a method of preparing a
stable pharmaceutical composition comprising a therapeutically effective
amount of one or more pure, in
particular substantially pure, scyllo-inositol compound adapted to provide
beneficial effects, preferably
sustained beneficial effects, following treatment. After compositions have
been prepared, they can be
placed in an appropriate container and labelled for treatment of an indicated
condition. For administration
of a composition of the invention, such labelling would include amount,
frequency, and method of
administration.
A scyllo-inositol compound for use in the present invention may be in the form
of a prodrug that
is converted in vivo to an active compound. By way of example, a scyllo-
inositol compound may
comprise a cleavable group that is cleaved after administration to a subject
to provide an active (e.g.
therapeutically active) compound, or an intermediate compound that
subsequently yields the active
compound. The cleavable group may be an ester that can be removed either
enzymatically or non-
enzymatically.
A scyllo-inositol compound for use in the present invention may optionally
comprise a carrier
interacting with the compound. A carrier may include a polymer, carbohydrate,
or peptide, or
combinations thereof. A carrier may be substituted, for example, with one or
more alkyl, halo, thiol,
hydroxyl, or amino group.
In an aspect, the invention provides a dietary supplement composition
comprising one or more
scyllo-inositol compound or nutraceutically acceptable derivatives thereof. In
an aspect, the invention
provides a dietary supplement for mammalian consumption, particularly human
consumption for the
purpose of improving memory comprising a scyllo-inositol compound or
nutraceutically acceptable
derivatives thereof. In another aspect, the invention provides a supplement
comprising a scyllo-inositol
2
CA 02579188 2007-02-19
compound or nutraceutically acceptable derivatives thereof for slowing the
deterioration of mental
processes and improving memory, in particular short-term memory, of
individuals who have taken the
supplement. A dietary supplement of the invention is preferably pleasant
tasting, effectively absorbed into
the body and provides substantial therapeutic effects.
The invention also provides methods to make commercially available
formulations which contain
a scyllo-inositol compound.
In an aspect, scyllo-inositol compounds, in particular pure or substantially
pure scyllo-inositol
compounds, and compositions of the invention may be administered
therapeutically or prophylactically to
treat an amyloid-related disease.
The invention also contemplates the use of a composition comprising at least
one scyllo-inositol
compound for the preparation of a medicament for preventing and/or treating
any amyloid-related disease.
The invention additionally provides uses of a pharmaceutical composition of
the invention in the
preparation of medicaments for the prevention and/or treatment of an amyloid-
related disease.
The invention provides a method for treating and/or preventing amyloid-related
diseases in a
subject comprising administering to the subject a therapeutically effective
amount of one or more scyllo-
inositol compound to provide beneficial effects. In an aspect the invention
provides a treatment which
results in sustained beneficial effects following treatment.
This invention also includes a regimen for supplementing a healthy human's
diet by administering
a scyllo-inositol compound or a dietary supplement comprising a scyllo-
inositol compound or a
nutraceutically acceptable derivative thereof, and an acceptable carrier, to
the human. The invention
further includes a regimen for supplementing a healthy human's diet by
administering daily to the human a
scyllo-inositol compound or a nutraceutically acceptable derivative thereof.
The invention also provides a kit comprising one or more scyllo-inositol
compound or a
pharmaceutical composition of the invention. In an aspect, the invention
provides a kit for preventing
and/or treating a disorder and/or disease, containing a composition comprising
one or more scyllo-inositol
compound, a container, and instructions for use. The composition of the kit
can further comprise a
pharmaceutically acceptable carrier, excipient, or vehicle. In an aspect, a
pharmaceutical kit is provided
comprising one or more containers filled with a pharmaceutical composition of
the invention, and a notice
in the form prescribed by a governmental agency regulating the labeling,
manufacture, use or sale of the
pharmaceutical composition, which notice reflects approval by the agency of
manufacture, use, or sale for
human administration, in particular for human administration in the treatment
of Alzheimer's disease,
dementia, or mild cognitive impairment.
These and other aspects, features, and advantages of the present invention
should be apparent to
those skilled in the art from the following drawing and detailed description.
DESCRIPTION OF THE DRAWINGS
The invention will be better understood with reference to the drawings in
which:
3
CA 02579188 2007-02-19
Figure 1. Spatial reference memory test in six month old mice following 28
days of treatment,
beginning at five months of age (n=10 mice per treatment arm) was performed.
The performance of epi-
cyclohexanehexol treated TgCRND8 mice was not different from untreated TgCRND8
littermates
(p=0.27; Figure lA) and remained impaired with respect to non-Tg littermates
(Fl,1a=11.7, p=0.004;
Figure 1 C). In contrast, scyllo-cyclohexanehexol treated TgCRND8 mice were
significantly better than
untreated TgCRND8 littermates (p=0.01; Figure 1B) and were indistinguishable
from non-Tg littermates
(F1,13=2.9, p=0.11; Figure 1D). The probe trial, using annulus crossing index,
demonstrated that scyllo-
cyclohexanehexol treated mice were not statistically different from non-Tg
littermates (p=0.64; Figure
lE). Vertical bars represent s.e.m. After one month of scyllo-cyclohexanehexol
treatment, mice had a
lower plaque burden compared to control animals with a high plaque burden in
the hippocampus (Figure
1F, Figure 1G). Plaque burden was identified using anti-A(3 antibody (brown)
and astrocytes are labeled
using anti-GFAP antibody (red). Scale bar 300 m.
Figure 2. Dot blot analyses of soluble oligomeric A(3 in scyllo-
cyclohexanehexol and epi-
cyclohexanehexol treated and untreated TgCRND8 mice (Figure 2A). Soluble
proteins isolated from 4
representative four and six month old untreated and treated TgCRND8 mice from
the prophylactic study,
and from the five month old treatment groups, untreated and treated were
applied to nitrocellulose and
probed with oligomer-specific antibody followed by re-probing with 6E 10.
Synthetic A(342, monomeric
(bottom row: lane 1 and 2) and fibrillar (lane 3 and 4) were used as negative
controls for the oligomer-
specific antibody, which only recognizes soluble aggregates. 6E 10 recognises
all A(3 species (bottom lane,
right four lanes). Long-term potentiation is blocked by soluble A(3 oligomers
(Figure 2B; green squares)
and rescued by scyllo-cyclohexanehexol treatment (Figure 2B; blue circles).
LTP is unaffected by scyllo-
cyclohexanehexol treated 7PA2 culture medium which contains A(3 oligomers
(Figure 2C; red squares;
same data as in Figure 2B) and plain CHO medium which lacks oligomers (Figure
2C; blue circles).
Figure 3. Cyclohexanehexols improve behaviour in TgCRND8 mice. Spatial
reference memory
version of the Morris Water Maze test in TgCRND8 mice (n=8-10 per treatment
arm) was used as a
measure of cognition. At four months of age, non-treated TgCRND8 mice show
cognitive impairment
relative to AZD-102 (A) and AZD-103(B) treated mice (F2,26=3.99, p=0.03). AZD-
102 treated mice (C)
were significantly different from treated and untreated non-Tg mice
(FI118=11.7, p=0.004), whereas AZD-
103 treated mice (D) approached that of non-Tg mice (F1, = 2.89, p=0.97). At
six months of age, non-
treated TgCRND8 show cognitive impairment relative to non-Tg controls
(F1330=31.16, p<0.001) and
AZD- 102 (E) and AZD- 103 (F) treated mice (F2,36=4.1, p<0.02). The
performance of both AZD- 102
treated TgCRND8 mice (FI,21=2.35, p=0.14; G) and AZD-102 approached that of
non-Tg littermates
(F1,2Z=3.26, p=0.44; D). Non-Tg littermate behaviour was not affected by
either AZD-102 (G) or AZD-
103 (H) treatment (F2,37=0.83, p=0.45).
Figure 4. Cyclohexanehexols improve pathological characteristics in TgCRND8
mice. Vascular
A(3 burden was quantitated on serial sagittal sections in treated and
untreated TgCRND8 mice.
TgCRND8 mice have a significant vascular Ab burden that is associated with
small and medium sized
4
CA 02579188 2007-02-19
vessels; the load is decreased in AZD-103 treated TgCRND8 mice (A). AZD-103
treatment significantly
decreased the total vascular load in comparison to untreated and epi-inositol
treated TgCRND8 mice. Tg
CRND8 mice demonstrate an astrogliotic response to increased A(3 levels within
the CNS and treatment
with AZD- 102 decreased the percent brain area covered in astrogliosis at both
4- and 6-months of age (B).
AZD-103 treatment decreased the astrogliotic response to a greater extent at
both ages (B). Similarly,
microgliosis has been correlated with plaque burden in the TgCRND8 mice (C).
Treatment with both
AZD-102 and to a greater extent with AZD-103 decreased the percent brain area
covered with
microgliosis. Kaplan Meier cumulative survival plot demonstrates the increased
survival of TgCRND8
mice after treatment with AZD-103 (light gray circles; p=0.02) in comparison
to untreated TgCRND8
mice (gray circles). AZD-102 did not significantly improve survival (black
circles) (D). ANOVA *
p<0.05, ** p<0.001.
Figure 5. At six months of age, the plaque burden and astrogliosis in TgCRND8
mice untreated,
AZD-102 and AZD-103 treated mice were examined. Control animals have a high
plaque load and
astrogliosis in the hippocampus and cerebral cortex. Higher magnification
demonstrates that astrocytic
activation is not only associated with plaque load. AZD-102 treatment has a
modest effect on amyloid
burden with a decrease in astrogliosis. AZD-103 treatment significantly
decreased amyloid burden and
gliosis. Astrocytes labeled with anti-GFAP antibody (red) and plaque burden
identified with anti-Ap
antibody (brown). Scale Bar 300 m or 62.5 m.
Figure 6. Spatial reference memory test in six month old mice following 28
days of treatment,
beginning at five months of age (n= 10 mice per treatment arm) was performed.
The performance of AZD-
102 treated TgCRND8 mice was not different from untreated TgCRND8 littermates
(p=0.27;A) and
remained impaired with respect to non-Tg littermates (F1114=11.7, p=0.004; C).
In contrast, AZD-103
treated TgCRND8 mice were significantly better than untreated
TgCRND8littermates (p=0.01; B) and
were indistinguishable from non-Tg littermates (F1,13=2.9, p=0.11; D).
Figure 7. A, B, C and D. A cue test was performed at the end of the spatial
memory version of
the Morris Water Test. A flag was placed on the platform and the path length
required to reach the
platform is comparable for all treatment groups (p=0.78) indicating that
treatment does not affect visual
acuity. The open field test for duration of grooming (A), pausing (B) and
walking (C) confirms that AZD-
103 does not affect activity levels in the TgCRND8 mice or in their non-Tg
littermates.
Figure 8. Dot blot analyses of soluble oligomeric A(3 in AZD-102 and AZD-103
treated and
untreated TgCRND8 mice (A,B). Soluble proteins isolated from all four and six
month old untreated
(solid bars) and treated (hatched bars) TgCRND8 mice from the prophylactic
study (n=8-10 per
experimental arm), and from the five month old treatment groups, untreated
(solid bars) and treated
(hatched bars) (n=8-10 per experimental arm) were applied to nitrocellulose
and probed with oligomer-
specific antibody followed by re-probing with 61310. Synaptophysin reactivity
was increased after AZD-
103 treatment in both the prophylactic and treatment paradigms in the CA1
region (C) (n=3, for each
5
CA 02579188 2007-02-19
experimental arm). In contrast, synaptophysin reactivity was unchanged in the
TgTauP301 L mice after
treatment (n=3 for treated and untreated).
Figure 9. In vitro y-secretase assays in HEK293 cells transfected with human
APPswe. After
swAPP stable HEK293 cells were untreated or treated with AZD-103 or 10 nM
compound E, cell
membranes were used for APP-FL and -CTF detection and for e-stubs generation
in vitro assays at 37 C
for 1 hr. Lanes 1&2. 90 mg/ml AZD-103 treatment; lanes 3&4 900 mg/ml AZD-103
treatment; lanes
5&6 compound E treated and lanes 7&8 untreated HEK293 APPswe cells.
Figure 10. Cyclohexanehexols improve behavior in TgCRND8 mice. Spatial
reference memory
version of the Morris water maze test in TgCRND8 mice (n = 8-10 per treatment
arm) was used as a
measure of cognition. At 4 months of age, nontreated TgCRND8 mice showed
cognitive impairment
relative to mice treated with epi-cyclohexanehexol (a) and
scyllocyclohexanehexol (b; F2,26 = 3.99, P =
0.03). Epi-cyclohexanehexol-treated mice (a) were significantly different from
treated and untreated
nontransgenic mice (F 1,18= 11.7, P = 0.004), whereas scyllo-cyclohexanehexol-
treated mice (b)
approached that of nontransgenic mice (F l,17 = 2.89, P = 0.97). At 6 months
of age, nontreated
TgCRND8 mice showed cognitive impairment relative to nontransgenic control
mice (F 1,30 = 31.16, P <
0.001) and mice treated with epi-cyclohexanehexol (c) or
scyllocyclohexanehexol (d; F2,36 = 4.1, P <
0.02). The performance of both epi-cyclohexanehexol- treated TgCRND8 mice
(171,21 = 2.35, P = 0.14;
c) and scyllo-cyclohexanehexol-treated TgCRND8 mice approached that of
nontransgenic littermates
(F1,22 = 3.26, P = 0.44; d). Nontransgenic littermate behavior was not
affected by treatment with either
epi- (c) or scyllo-cyclohexanehexol (d; F2,37 = 0.83, P = 0.45). Vertical bars
represent s.e.m.
Figure 11. Cyclohexanehexols improve pathological characteristics in TgCRND8
mice.
TgCRND8 mice have a considerable vascular A(3 burden that is associated with
small and medium-sized
vessels (a). Scyllocyclohexanehexol treatment significantly decreased the
total vascular load in
comparison to untreated and epi-inositol- treated TgCRND8 mice (a). TgCRND8
mice showed an
astrogliotic response to increased A(3 levels, and treatment with epi-
cyclohexanehexol decreased the
percent brain area covered in astrogliosis at both 4 and 6 months of age (b).
Scyllo-cyclohexanehexol
treatment decreased the astrogliotic response to a greater extent than epi-
cyclohexanehexol at both ages
but had no effect in disease stage transgenic Tau (P301L) 23027 mice (b).
Similarly, microgliosis has
been correlated with plaque burden in the TgCRND8 mice (c). Treatment with
both epi- and to a greater
extent with scyllo-cyclohexanehexol decreased the percent brain area covered
with microgliosis (c).
Kaplan-Meier cumulative survival plot shows the increased survival of TgCRND8
mice after treatment
with scyllo-cyclohexanehexol (P = 0.02) in comparison to untreated TgCRND8
mice (d). Epi-
cyclohexanehexol did not significantly improve survival. Synaptophysin
immunoreactivity was used as a
measure of synaptic density and was increased after scyllo-cyclohexanehexol
treatment in both the
prophylactic and treatment paradigms in the CA1 region (e). In contrast,
synaptophysin reactivity was
unchanged in TgTau (P301L) mice after treatment (e). *P < 0.05, **P < 0.001 by
ANOVA.
6
CA 02579188 2007-02-19
Figure 12. Spatial reference memory test was performed in 6-month-old mice
after 28 d of
treatment, beginning at 5 months of age (n = 10 mice per treatment arm). The
performance of epi-
cyclohexanehexol-treated TgCRND8 mice was not different from untreated TgCRND8
littermates (P =
0.27; a) and remained impaired with respect to nontransgenic littermates (F
1,14 = 11.7, P = 0.004). In
contrast, scyllo-cyclohexanehexol-treated TgCRND8 mice were significantly
better than untreated
TgCRND8 littermates (P = 0.01; b) and were indistinguishable from
nontransgenic littermates (F1,13 =
2.9, P = 0.11). The probe trial, using annulus-crossing index, showed that
scyllo-cyclohexanehexol-
treated TgCRND8 mice were not statistically different from nontransgenic
littermates (P = 0.64; c). nTg,
nontransgenic mice; Tg, TgCRND8 mice. Vertical bars represent s.e.m.
Figure 13. Dot-blot analyses of soluble oligomeric A(3 in TgCRND81eft
untreated or treated with
epi-cyclohexanehexol (a) or scyllo-cyclohexanehexol (b). Soluble proteins
isolated from untreated (black
bars) and treated (gray bars) TgCRND8 mice from the 4- and 6-month
prophylactic study and from the 5-
month-old treatment group (n = 8-10 per experimental arm) were probed with
oligomer-specific antibody
followed by re-probing with 6E 10 (c). Synthetic A(342, monomeric A(342 and
fibrillar A(342 were used as
negative controls and aggregated A(342 was used as a positive control for the
oligomer-specific antibody
6E 10 recognizes all A(3 species and was used as an Ab positive control.
Western blot analyses of soluble
fractions from 4-month-old TgCRND8 mice untreated (n = 4) or treated (n = 4)
with scyllo-
cyclohexanehexol showed a decrease in high-molecular-weight A(3 species and a
subsequent increase in
smaller oligomers (d). The highmolecular-weight A(3 species does not comigrate
with holo- APP, as
reprobing the same blot with 22C 11 recognizing an N-terminal epitope in the
APP ectoderm identifies a
faster migrating doublet (d). The gel was reprobed with GAPDH-specific
antibody as a loading control.
Quantification of changes in high-molecular-weight Ab oligomer, trimer and
monomer bands using
densitometry confirmed the western blot analyses (e).
Figure 14. Dose-dependent effects of scyllo-cyclohexanehexol on 4-month-old
TgCRND8 mice.
Four-month-old TgCRND8 mice with signs of clinical disease were administered
scyllo-
cyclohexanehexol orally for 1 month (n = 8-9 per group). Outcome measures
indicated improvement in
cognitive deficits using the Morris water maze test of spatial memory (a),
plaque count using 6F3D
immunostaining and image analyses (b), and soluble Ab oligomers using
oligomerspecific antibody in a
dot-blot assay (c, d). To show that the dose-response effect is not specific
to younger mice, 5-month-old
TgCRND8 mice were gavaged with 0-30 mg/kg/d scyllo-cyclohexanehexol. Brain
A(342 levels were
examined and a dose-dependent decrease in both soluble and insoluble A(342
levels was detected (e,f).
Figure 15. Cyclohexanehexol stereoisomer structures. The positioning of the
hydroxyl groups on
the ring structure of myo (1), epi-(2) and scyllo-cyclohexanehexol (3) are
shown.
Figure 16. At six months of age, the plaque burden and astrogliosis in TgCRND8
mice untreated,
epi- and scyllo-cyclohexanehexol treated mice were examined. Control animals
have a high plaque load
and astrogliosis in the hippocampus (a) and cerebral cortex (b). Higher
magnification demonstrates that
astrocytic activation is not only associated with plaque load (c). Epi-
cyclohexanehexol treatment has a
7
CA 02579188 2007-02-19
modest effect on amyloid burden with a decrease in astrogliosis (d, e, f).
Scyllo-cyclohexanehexol
treatment significantly decreased amyloid burden and gliosis (g.h.i). Higher
magnification illustrates the
smaller mean plaque size in scyllo-cyclohexanehexol treated mice (i).
Astrocytes labeled with anti-GFAP
antibody (red) and plaque burden identified with anti-A(3 antibody (brown).
Scale Bar 300 m (a, b, d, e,
g, h) and 62.5 m(c,f,j).
Figure 17. Spatial reference memory version of the Morris Water Maze test in
six month old
TgCRND8 mice non-treated or treated with mannitol. Mannitol treated TgCRND8
mice (dashed line)
were not significantly different from untreated TgCRND8 mice (solid line: P =
0.89; a). The performance
of mannitol treated TgCRND8 mice (dashed line) was significantly different
from mannitol treated non-
Tg littermates (solid line: P = 0.05; b). Vertical Bars represent SEM. Plaque
burden was analysed at six
months of age by quantitative image analyses (c). Mannitol treated TgCRND8
mice were
indistinguishable from untreated TgCRND8 mice when plaque count was used as a
measure of total
plaque burden (P = 0.87). Vertical bars represent SEM. Kaplan-Meier Cumulative
survival plots for
TgCRND8 mice treated and untreated with mannitol (d). The two cohorts of
animals, n= 35 per group,
were not significantly different as determined by the Tarone-Ware statistical
test, P = 0.87.
Figure 18. A cue test was performed at the end of the spatial memory version
of the Morris Water
Test. A flag was placed on the platform and the path length required to reach
the platform is comparable
for all treatment groups (P = 0.78) indicating that treatment does not affect
visual acuity (a). The open
field test for duration of grooming (b), pausing (c) and walking (d) confirms
that scyllo-cyclohexanehexol
does not affect activity levels in the TgCRND8 mice or in their non-Tg
littermates.
DETAILED DESCRIPTION OF EMBODIMENTS
Glossary
Numerical ranges recited herein by endpoints include all numbers and fractions
subsumed within
that range (e.g. 1 to 5 includes 1, 1.5, 2, 2.75, 3, 3.90, 4, and 5). It is
also to be understood that all numbers
and fractions thereof are presumed to be modified by the term "about." The
term "about" means plus or
minus 0.1 to 50%, 5-50%, or 10-40%, preferably 10-20%, more preferably 10% or
15%, of the number to
which reference is being made. Further, it is to be understood that "a," "an,"
and "the" include plural
referents unless the content clearly dictates otherwise. Thus, for example,
reference to a composition
containing "a compound" includes a mixture of two or more compounds.
The terms "administering" and "administration" refer to the process by which a
therapeutically
effective amount of a compound or composition contemplated herein is delivered
to a subject for
prevention and/or treatment purposes. Compositions are administered in
accordance with good medical
practices taking into account the subject's clinical condition, the site and
method of administration,
dosage, patient age, sex, body weight, and other factors known to physicians.
The term "treating" refers to reversing, alleviating, or inhibiting the
progress of a disease, or one
or more symptoms of such disease, to which such term applies. Depending on the
condition of the subject,
the term also refers to preventing a disease, and includes preventing the
onset of a disease, or preventing
8
CA 02579188 2007-02-19
the symptoms associated with a disease. A treatment may be either performed in
an acute or chronic way.
The term also refers to reducing the severity of a disease or symptoms
associated with such disease prior
to affliction with the disease. Such prevention or reduction of the severity
of a disease prior to affliction
refers to administration of a compound or composition of the present invention
to a subject that is not at
the time of administration afflicted with the disease. "Preventing" also
refers to preventing the recurrence
of a disease or of one or more symptoms associated with such disease. The
terms "treatment" and
"therapeutically," refer to the act of treating, as "treating" is defined
above.
The terms "subject", "individual", or "patient" are used interchangeably
herein and refer to an
animal including a warm-blooded animal such as a mammal, which is afflicted
with or suspected of
having or being pre-disposed to a disorder and/or disease disclosed herein.
Mammal includes without
limitation any members of the Mammalia. In aspects of the invention, the terms
refer to a human. The
terms also include domestic animals bred for food or as pets, including
horses, cows, sheep, poultry, fish,
pigs, cats, dogs, and zoo animals, goats, apes (e.g. gorilla or chimpanzee),
and rodents such as rats and
mice. Typical subjects for treatment include persons susceptible to, suffering
from or that have suffered an
amyloid-related disease. A subject may or may not have a genetic
predisposition for a disorder and/or
disease disclosed herein such as Alzheimer's disease. In some aspects, a
subject shows signs of cognitive
deficits and amyloid plaque neuropathology. In embodiments of the invention
the subjects are suspectible
to, or suffer from Alzheimer's disease.
As utilized herein, the term "healthy subject" means a subject, in particular
a mammal, having no
disease, in particular no diagnosed disease, disorder, infirmity, or ailment
known to impair or otherwise
diminish memory.
The term "pharmaceutically acceptable carrier(s), excipient(s), or vehicle(s)"
refers to a medium
which does not interfere with the effectiveness or activity of an active
ingredient and which is not toxic to
the hosts to which it is administered. A carrier, excipient, or vehicle
includes diluents, binders, adhesives,
lubricants, disintegrates, bulking agents, wetting or emulsifying agents, pH
buffering agents, and
miscellaneous materials such as absorbants that may be needed in order to
prepare a particular
composition. Examples of carriers etc include but are not limited to saline,
buffered saline, dextrose,
water, glycerol, ethanol, and combinations thereof. The use of such media and
agents for an active
substance is well known in the art.
As used herein "nutraceutically acceptable derivative" refers to a derivative
or substitute for the
stated chemical species that operates in a similar manner to produce the
intended effect, and is structurally
similar and physiologically compatible. Examples of substitutes include
without limitation salts, esters,
hydrates, or complexes of the stated chemical. The substitute could also be a
precursor or prodrug to the
stated chemical, which subsequently undergoes a reaction in vivo to yield the
stated chemical or a
substitute thereof.
The term "pure" in general means better than 90%, 92%, 95%, 97%, 98% or 99%
pure, and
"substantially pure" means a compound synthesized such that the compound, as
made as available for
9
CA 02579188 2007-02-19
consideration into a composition or therapeutic dosage of the invention, has
only those impurities that can
not readily nor reasonably be removed by conventional purification processes.
"Pharmaceutically acceptable salt(s)," means a salt that is pharmaceutically
acceptable and has the
desired pharmacological properties. By pharmaceutically acceptable salts is
meant those salts which are
suitable for use in contact with the tissues of a subject or patient without
undue toxicity, irritation, allergic
response and the like, and are commensurate with a reasonable benefit/risk
ratio. Pharmaceutically
acceptable salts are described for example, in S. M. Berge, et al., J.
Pharmaceutical Sciences, 1977, 66:1.
Suitable salts include salts that may be formed where acidic protons in the
compounds are capable of
reacting with inorganic or organic bases. Suitable inorganic salts include
those formed with alkali metals,
e.g. sodium and potassium, magnesium, calcium, and aluminum. Suitable organic
salts include those
formed with organic bases such as the amine bases, e.g. ethanolamine,
diethanolamine, triethanolamine,
tromethamine, N-methylglucamine, and the like. Suitable salts also include
acid addition salts formed
with inorganic acids (e.g. hydrochloride and hydrobromic acids) and organic
acids (e.g. acetic acid, citric
acid, maleic acid, and the alkane- and arene-sulfonic acids such as
methanesulfonic acid and
benezenesulfonic acid). When there are two acidic groups present, a
pharmaceutically acceptable salt may
be a mono-acid-mono-salt or a di-salt; and similarly where there are more than
two acidic groups present,
some or all of such groups can be salified.
A "combination treatment" means that the active ingredients are administered
concurrently to a
patient being treated. When administered in combination each component may be
administered at the
same time, or sequentially in any order at different points in time.
Therefore, each component may be
administered separately, but sufficiently close in time to provide the desired
effect, in particular a
beneficial, additive, or synergistic effect. The first compound may be
administered in a regimen that
additionally comprises treatment with the second compound. In aspects the
terms refer to the
administration of a scyllo-inositol compound and a second therapeutic agent
optionally within one year,
including separate administration of medicaments each containing one of the
compounds as well as
simultaneous administration whether or not the compounds are combined in one
formulation or whether
they are in separate formulations.
"Detectable substance" includes without limitation radioisotopes (e.g., 3H,
'aC, 355, i251, 1311),
fluorescent labels (e.g., FITC, rhodamine, lanthanide phosphors), luminescent
labels such as luminol;
enzymatic labels (e.g., horseradish peroxidase, beta-galactosidase,
luciferase, alkaline phosphatase,
acetylcholinesterase), biotinyl groups (which can be detected by marked avidin
e.g., streptavidin
containing a fluorescent marker or enzymatic activity that can be detected by
optical or colorimetric
methods), predetermined polypeptide epitopes recognized by a secondary
reporter (e.g., leucine zipper
pair sequences, binding sites for secondary antibodies, metal binding domains,
or epitope tags). In some
embodiments, labels are attached via spacer arms of various lengths to reduce
potential steric hindrance.
A "beneficial effect" refers to an effect of a compound of the invention or
composition thereof in
certain aspects of the invention, including favorable pharmacological and/or
therapeutic effects, and/or
CA 02579188 2007-02-19
improved biological activity. In aspects of the invention, the beneficial
effects include without limitation
improved cognitive function, reduced vascular load, reduced astrogliosis,
reduced amyloid burden,
reduced microgliosis, and/or improved survival. In an aspect, a beneficial
effect is a favourable
characteristic of a composition/formulation of the invention includes enhanced
stability, a longer half life,
and/or enhanced uptake and transport across the blood brain barrier. In some
aspects, a beneficial effect of
a composition of the invention is rapid brain penetrance, in particular brain
penetrance within 1-6, 1-5, 1-
4, 1-3 or 1-2 hours of administration.
The beneficial effect may be a statistically significant effect in terms of
statistical analysis of an
effect of a scyllo-inositol compound versus the effects without the compound.
In an aspect, the beneficial
effect is one or more of improved cognitive function, reduced vascular load,
reduced astrogliosis, reduced
amyloid burden, reduced microgliosis, and/or improved survival. "Statistically
significant" or
"significantly different" effects or levels may represent levels that are
higher or lower than a standard. In
embodiments of the invention, the difference may be 1.5, 2, 3, 4, 5, 6, 7, 8,
9, 10, 15, 20, 25 or 50 times
higher or lower compared with the effect obtained without a scyllo-inositol
compound.
"Therapeutically effective amount" relates to the amount or dose of an active
compound or
composition of the invention that will provide or lead to one or more desired
beneficial effects, in
particular, one or more sustained beneficial effects. A therapeutically
effective amount of a substance can
vary according to factors such as the disease state, age, sex, and weight of
the individual, and the ability of
the substance to elicit a desired response in the individual. A dosage regimen
may be adjusted to provide
the optimum therapeutic response (e.g. one or more beneficial effect, in
particular a sustained beneficial
effect). For example, several divided doses may be administered daily or the
dose may be proportionally
reduced as indicated by the exigencies of the therapeutic situation.
"A scyllo-inositol compound" is understood to refer to any compound, which
fully or partially,
directly or indirectly, provides one or more beneficial effects described
herein. A scyllo-inositol
compound that can be used in the invention has the base structure of the
formula Ia or Ib:
H (D
r~H
Ho
H~ ~~H
()t;
~
~.1
NU H 0
0 H
Ia Ib
A scyllo-inositol compound includes a functional derivative of a compound of
the formula Ia or
Ib. A "functional derivative" refers to a compound that possesses a biological
activity (either functional or
structural) that is substantially similar to the biological activity of scyllo-
inositol of the formula Ia or Ib.
The term "functional derivative" is intended to include "variants" "analogs"
or "chemical derivatives" of
11
CA 02579188 2007-02-19
scyllo-inositol. The term "variant" is meant to refer to a molecule
substantially similar in structure and
function to scyllo-inositol or a part thereof. A molecule is "substantially
similar" to scyllo-inositol if both
molecules have substantially similar structures or if both molecules possess
similar biological activity.
The term "analog" refers to a molecule substantially similar in function to a
scyllo-inositol molecule. The
term "chemical derivative" describes a molecule that contains additional
chemical moieties which are not
normally a part of the base molecule.
A scyllo-inositol compound of the invention includes crystalline forms of the
compound which
may exist as polymorphs. Solvates of the compounds formed with water or common
organic solvents are
also intended to be encompassed within this invention. In addition, hydrate
forms of scyllo-inositol
compounds and their salts, are included within this invention.
A scyllo-inositol compound includes a compound of the formula Ia or lb wherein
one, two or
three hydroxyl groups are replaced by substituents, in particular univalent
substituents, with retention of
configuration. Suitable substituents include without limitation hydrogen,
alkyl, acyl, alkenyl, alkoxy, =0,
cycloalkyl, halogen, -NHRI wherein R' is hydrogen, acyl, alkyl or -R2R3
wherein R2 and R3 are the same
or different and represent acyl or alkyl; -P03H2; -SR4 wherein R4 is hydrogen,
alkyl, or -03H; and -OR3
wherein R3 is hydrogen, alkyl, or -SO3H. In aspects of the invention, a scyllo-
inositol compound does not
include scyllo-inositol substituted with one or more phosphate group.
Particular aspects of the invention utilize scyllo-inositol compounds of the
formula Ia or lb
wherein one or more of the hydroxyl groups are replaced with alkyl, acyl,
alkoxy, alkenyl, -NHR1 wherein
R' is hydrogen, acyl, alkyl or -R2R3 wherein R2 and R3 are the same or
different and represent acyl or
alkyl; -SR4 wherein R4 is hydrogen, alkyl, or -03H; and -OR3 wherein R3 is
hydrogen, alkyl, or -SO3H,
more particularly -SR4 wherein R4 is hydrogen, alkyl, or -03H or -SO3H.
Particular aspects of the invention utilize scyllo-inositol compounds of the
formula Ia or lb
wherein one or more of the hydroxyl groups are replaced with Ci-C6alkyl, CZ-
C6alkenyl, C1C6alkoxy, C3-
Clocycloalkyl, CI-C6 acyl, -NH2, -NHRI, -NR2R3, halo, haloalkyl, haloalkoxy,
hydroxyalkyl, or oxo.
Particular aspects of the invention utilize scyllo-inositol compounds of the
formula Ia or lb
wherein one or more of the hydroxyl groups is replaced with CI -C6 alkyl, C2-
C6 alkenyl, CI C6alkoxy,
CI-C6 acyl, -NH2, halo, or oxo.
In embodiments of the invention, a scyllo-inositol compound of the formula Ia
or lb is employed
wherein one or two hydroxyl groups are replaced by =0. In particular
embodiments of the invention one
hydroxyl group is replace by =0, more particularly the hydroxyl group at
position 2 is replaced by =0.
In embodiments, scyllo-cyclohexanehexol (i.e., scyllo-inositol), in particular
pure or substantially
pure scyllo-cyclohexanehexol, is used in the compositions, methods and uses
disclosed herein.
"Alkyl" refers to monovalent alkyl groups preferably having from 1 to 20 or 1
to 10 carbon atoms,
preferably from about 1 to 10, 1 to 8, 3 to 8, 1 to 6, or 1 to 3, more
preferably about 3 to 6 carbon atoms.
This term is exemplified by groups such as methyl, ethyl, n-propyl, iso-
propyl, n-butyl, iso-butyl, n-hexyl,
isopropyl, isobutyl, isopentyl, amyl, sec-butyl, tert-butyl, tert-pentyl, n-
heptyl, n-octyl, n-nonyl, n-decyl,
12
CA 02579188 2007-02-19
undecyl, n-dodecyl, n-tetradecyl, pentadecyl, n-hexadecyl, heptadecyl, n-
octadecyl, nonadecyl, eicosyl,
dosyl, n-tetracosyl, and the like, along with branched variations thereof. In
certain embodiments of the
invention an alkyl radical is a C1-C6 lower alkyl comprising or selected from
the group consisting of
methyl, ethyl, n-propyl, n-butyl, n-pentyl, n-hexyl, isopropyl, isobutyl,
isopentyl, amyl, tributyl, sec-butyl,
tert-butyl, tert-pentyl, and n-hexyl. An alkyl group can be a substituted
alkyl.
"Substituted alkyl" refers to an alkyl group, preferably of from i to 10
carbon atoms, having from
1 to 5 substituents, and preferably 1 to 3 substituents, for example, alkyl,
alkoxy in particular lower
alkoxy, cycloalkyl, acyl, amino, substituted amino, cyano, halo, hydroxyl,
carboxyl, substituted carboxyl,
carboxylalkyl, keto, thioketo, thiol, thioalkoxy, aryl, hydroxyamino,
alkoxyamino, nitro, sulfonyl,
sulfenyl, sulfinyl, sulfate, or sulfoxide.
"Alkenyl" refers to alkenyl groups preferably having from 2 to 10 carbon atoms
and more
preferably 3 to 8 carbon atoms and having at least I and preferably from 1-2
sites of alkenyl unsaturation.
Alkenyl radicals may preferably contain from about 3 to 6 or 2 to 6 carbon
atoms. Examples of suitable
alkenyl radicals include ethenyl, propenyl such as prop-l-en-l-yl, prop-l-en-2-
yl, prop-2-en-l-yl (allyl),
prop-2-en-2-yl, buten-l-yl, but-l-en-2-yl, 2-methyl-prop-l-en-l-yl, but-2-en-l-
yl, but-2-en-2-yl, buta-1,3-
dien-l-yl, buta- 1,3-dien-2-yl, hexen-l-yl, 3-hydroxyhexen-l-yl, hepten-l-yl,
and octen-l-yl, and the like.
Preferred alkenyl groups include ethenyl (-CH=CH2), n-propenyl (-CH2CH=CH2),
iso-propenyl (-
C(CH3)=CH2), and the like.
"Substituted alkenyl" refers to an alkenyl group as defined above having from
1 to 3 substituents,
for example, alkyl, alkoxy in particular lower alkoxy, cycloalkyl, acyl,
amino, substituted amino, cyano,
halo, hydroxyl, carboxyl, substituted carboxyl, carboxylalkyl, keto, thioketo,
thiol, thioalkoxy, aryl,
hydroxyamino, alkoxyamino, nitro, sulfonyl, sulfenyl, sulfinyl, sulfate, or
sulfoxide
"Acyl" refers to the groups alkyl-C(O)-, substituted alkyl-C(O)-, cycloalkyl-
C(O)-, substituted
cycloalkyl-C(O)-, aryl-C(O)-, heteroaryl-C(O)- and heterocyclic-C(O)- where
alkyl, substituted alkyl,
cycloalkyl, substituted cycloalkyl, aryl, substituted aryl, heteroaryl and
heterocyclic are as defined herein.
An acyl group may be substituted with for example a group disclosed herein for
alkyl. Illustrative
examples of "acyl" radicals are formyl, acetyl, 2-chloroacetyl, 2-bromacetyl,
benzoyl, trifluoroacetyl,
phthaloyl, malonyl, nicotinyl, and the like.
"Alkoxy" refers to a linear or branched oxy-containing radical having an alkyl
portion of one to
about ten carbon atoms, such as a methoxy radical, which may be substituted.
Particular alkoxy radicals
are "lower alkoxy" radicals having about 1 to 6, 1 to 4 or 1 to 3 carbon
atoms. An alkoxy having about 1-6
carbon atoms includes a C1-C6 alkyl-O- radical wherein C1-C6 alkyl has the
meaning set out herein.
Illustrative examples of alkoxy radicals include without limitation methoxy,
ethoxy, propoxy, butoxy,
isopropoxy and tert-butoxy. An "alkoxy" radical may optionally be further
substituted with one or more
substitutents disclosed herein including alkyl atoms (in particular lower
alkyl) to provide "alkylalkoxy"
radicals; halo atoms, such as fluoro, chloro or bromo, to provide "haloalkoxy"
radicals (e.g.
fluoromethoxy, chloromethoxy, trifluoromethoxy, difluoromethoxy,
trifluoroethoxy, fluoroethoxy,
13
CA 02579188 2007-02-19
tetrafluoroethoxy, pentafluoroethoxy, and fluoropropoxy) and "haloalkoxyalkyl"
radicals (e.g.
fluoromethoxymethyl, chloromethoxyethyl, trifluoromethoxymethyl,
difluoromethoxyethyl, and
trifluoroethoxymethyl).
"Aryl" refers to an unsaturated aromatic carbocyclic group of from 6 to 14
carbon atoms having a
single ring (e.g., phenyl) or multiple condensed (fused) rings (e.g., naphthyl
or anthryl). Preferred aryls
include phenyl, naphthyl and the like. In aspects of the invention an aryl
radical has 4 to 24 carbon atoms,
in particular 4 to 10, 4 to 8, or 4 to 6 carbon atoms. The term "aryl"
includes without limitation aromatic
radicals such as phenyl, naphthyl, indenyl, benzocyclooctenyl,
benzocycloheptenyl, pentalenyl, azulenyl,
tetrahydronaphthyl, indanyl, biphenyl, acephthylenyl, fluorenyl, phenalenyl,
phenanthrenyl, and
anthracenyl, preferably phenyl. An aryl group may be a substituted aryl group
which may include an aryl
group as defined herein having from 1 to 8, 1 to 6, 1 to 4, or I to 3
substituents, for example, alkyl,
alkoxy, cycloalkyl, acyl, amino, cyano, halogen, hydroxyl, carboxyl,
carboxylalkyl, keto, thioketo, thiol,
thioalkoxy, aryl, hydroxyamino, alkoxyamino, and nitro. Examples of
substituted aryl radicals include
benzyl, chlorobenyzl, and amino benzyl.
"Cycloalkyl" refers to cyclic alkyl groups of from 3 to 16, 3 to 15 or 3 to 12
carbon atoms having
a single cyclic ring or multiple condensed rings.
In aspects of the invention a cycloalkyl comprises one, two, three, or four
rings wherein such
rings may be attached in a pendant manner or may be fused, in particular
cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl, adamantyl, and
the like. In certain aspects of
the invention the cycloalkyl radicals are "lower cycloalkyl" radicals having
from about 3 to 10, 3 to 8, 3 to
6, or 3 to 4 carbon atoms, in particular cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl and
cyclooctyl,. A cycloalkyl group includes multiple ring structures such as
adamantanyl, and the like. The
term "cycloalkyl" also embraces radicals where cycloalkyl radicals are fused
with aryl radicals or
heterocyclyl radicals. A cycloalkyl radical may be optionally substituted with
groups as disclosed herein.
"Substituted cycloalkyl" refers to cycloalkyl groups having from 1 to 5 (in
particular 1 to 3)
substituents including without limitation alkoxy, cycloalkyl, substituted
cycloalkyl, cycloalkenyl, acyl,
acylamino, acyloxy, amino, aminoacyl, aminoacyloxy, oxyacylamino, cyano,
halogen, hydroxyl,
carboxyl, carboxylalkyl, keto, thioketo, thiol, thioalkoxy, aryl, aryloxy,
heteroaryl, heteroaryloxy,
heterocyclic, heterocyclooxy, hydroxyamino, alkoxyamino, and nitro.
"Halogen" refers to fluoro, chloro, bromo and iodo and preferably is either
fluoro or chloro.
"Heteroaryl" refers to an aromatic group of from 1 to 15 carbon atoms and I to
4 heteroatoms
selected from oxygen, nitrogen and sulfur within at least one ring (if there
is more than one ring). Such
heteroaryl groups can be optionally substituted with 1 to 5 substituents, for
example, acyloxy, hydroxy,
acyl, alkyl, alkoxy, alkenyl, alkynyl, substituted alkyl, substituted alkenyl,
substituted alkynyl, amino,
substituted amino, aminoacyl, acylamino, alkaryl, aryl, aryloxy, azido,
carboxyl, carboxylalkyl, cyano,
halo, nitro, heteroaryl, heterocyclic, aminoacyloxy, oxyacylamino, thioalkoxy,
substituted thioalkoxy,
14
CA 02579188 2007-02-19
thioaryloxy, and thioheteroaryloxy. Such heteroaryl groups can have a single
ring (e.g., pyridyl or furyl)
or multiple condensed rings (e.g., indolizinyl or benzothienyl).
"Heterocycle" or "heterocyclic" refers to a monovalent saturated or
unsaturated group having a
single ring or multiple condensed rings, from 1 to 15 carbon atoms and from 1
to 4 hetero atoms selected
from nitrogen, sulfur or oxygen within the ring. Heterocyclic groups can have
a single ring or multiple
condensed rings. Heterocyclic groups can be optionally substituted with I to 5
substituents, for example,
alkoxy, cycloalkyl, substituted cycloalkyl, cycloalkenyl, acyl, acylamino,
acyloxy, amino, aminoacyl,
aminoacyloxy, oxyacylamino, cyano, halogen, hydroxyl, carboxyl, carboxylalkyl,
keto, thioketo, thiol,
thioalkoxy, aryl, aryloxy, heteroaryl, heteroaryloxy, heterocyclic,
heterocyclooxy, hydroxyamino,
alkoxyamino, or nitro.
Examples of heterocycles and heteroaryls include, without limitation, pyrrole,
furan, imidazole,
pyrazole, pyridine, pyrazine, pyrimidine, pyridazine, indolizine, isoindole,
indole, indazole, purine,
quinolizine, isoquinoline, quinoline, phthalazine, naphthylpyridine,
quinoxaline, quinazoline, cinnoline,
pteridine, carbazole, carboline, phenanthridine, acridine, phenanthroline,
isothiazole, phenazine,
isoxazole, phenoxazine, phenothiazine, imidazolidine, imidazoline, piperidine,
piperazine, indoline,
morpholino, piperidinyl, tetrahydrofuranyl, and the like as well as N-alkoxy-
nitrogen containing
heterocycles.
Scyllo-inositol compounds can be prepared using conventional processes or they
may be obtained
from commercial sources. A scyllo-inositol compound can be prepared using
chemical and/or microbial
processes. In aspects of the invention, a scyllo-inositol compound is produced
using process steps
described by M. Sarmah and Shashidhar, M., Carbohydrate Research, 2003, 338,
999-1001; Husson, C., et
al, Carbohyrate Research 307 (1998) 163-165; Anderson R. and E.S. Wallis, J.
American Chemical
Society (US), 1948, 70:2931-2935; Weissbach, A., J Org Chem (US), 1958, 23:329-
330; Chung, S.K. et
al., Bioorg Med Chem. 1999, 7(11):2577-89; or Kiely D.E., and Fletcher, H.G.,
J. American Chemical
Society (US) 1968, 90:3289-3290; described in JP09-140388, DE 3,405,663, JP04-
126075, JP05-192163,
or W006109479, or described in WO0503577, US20060240534, EP1674578, JP9140388,
JP09140388,
JP02-184912, JP03-102492 (Hokko Chemical Industries). In particular aspects of
the compositions and
methods of the invention, a scyllo-inositol compound is prepared using the
chemical process steps
described in Husson, C., et al, Carbohyrate Research 307 (1998) 163-165. In
other aspects of the
compositions and methods of the invention, the scyllo-inositol compound is
prepared using microbial
process steps described in W005035774 (EP1674578 and US20060240534) (Hokko
Chemical
Industries). Derivatives may be produced by introducing substituents into a
scyllo-inositol using methods
well known to a person of ordinary skill in the art
A scyllo-inositol compound may additionally comprise a carrier, including
without limitation one
or more of a polymer, carbohydrate, peptide or derivative thereof. A carrier
may be substituted with
substituents described herein including without limitation one or more alkyl,
amino, nitro, halogen, thiol,
thioalkyl, sulfate, sulfonyl, sulfenyl, sulfinyl, sulfoxide, hydroxyl groups.
A carrier can be directly or
CA 02579188 2007-02-19
indirectly covalently attached to a compound of the invention. In aspects of
the invention the carrier is an
amino acid including alanine, glycine, proline, methionine, serine, threonine,
or asparagine. In other
aspects the carrier is a peptide including alanyl-alanyl, prolyl-methionyl, or
glycyl-glycyl.
A carrier also includes a molecule that targets a compound of the invention to
a particular tissue
or organ. In particular, a carrier may facilitate or enhance transport of a
compound of the invention to the
brain by either active or passive transport.
A "polymer" as used herein refers to molecules comprising two or more monomer
subunits that
may be identical repeating subunits or different repeating subunits. A monomer
generally comprises a
simple structure, low-molecular weight molecule containing carbon. Polymers
can be optionally
substituted. Examples of polymers which can be used in the present invention
are vinyl, acryl, styrene,
carbohydrate derived polymers, polyethylene glycol (PEG), polyoxyethylene,
polymethylene glycol, poly-
trimethylene glycols, polyvinylpyrrolidone, polyoxyethylene-polyoxypropylene
block polymers, and
copolymers, salts, and derivatives thereof. In particular aspects of the
invention, the polymer is poly(2-
acrylamido-2-methyl-l-propanesulfonic acid); poly(2-acrylamido-2-methyl,-l-
propanesulfonic acid-
coacrylonitrile, poly(2-acrylamido-2-methyl-l-propanesulfonic acid-co-
styrene), poly(vinylsulfonic acid);
poly(sodium 4-styrenesulfonic acid); and sulfates and sulfonates derived
therefrom; poly(acrylic acid),
poly(methylacrylate), poly(methyl methacrylate), and poly(vinyl alcohol).
A "carbohydrate" as used herein refers to a polyhydroxyaldehyde, or
polyhydroxyketone and
derivatives thereof. The simplest carbohydrates are monosaccharides, which are
small straight-chain
aldehydes and ketones with many hydroxyl groups added, usually one on each
carbon except the
functional group. Examples of monosaccharides include erythrose, arabinose,
allose, altrose, glucose,
mannose, threose, xylose, gulose, idose, galactose, talose, aldohexose,
fructose, ketohexose, ribose, and
aldopentose. Other carbohydrates are composed of monosaccharide units,
including disaccharides,
oligosaccharides, or polysaccharides, depending on the number of
monosaccharide units. Disaccharides
are composed of two monosaccharide units joined by a covalent glycosidic bond.
Examples of
disaccharides are sucrose, lactose, and maltose. Oligosaccharides and
polysaccharides, are composed of
longer chains of monosaccharide units bound together by glycosidic bonds.
Oligosaccharides generally
contain between 3 and 9 monosaccharide units and polysaccharides contain
greater than 10
monosaccharide units. A carbohydrate group may be substituted at one two,
three or four positions, other
than the position of linkage to a compound of the formula Ia or lb. For
example, a carbohydrate may be
substituted with one or more alkyl, amino, nitro, halo, thiol, carboxyl, or
hydroxyl groups, which are
optionally substituted. Illustrative substituted carbohydrates are glucosamine
or galactosamine.
In aspects of the invention, the carbohydrate is a sugar, in particular a
hexose or pentose and may
be an aldose or a ketose. A sugar may be a member of the D or L series and can
include amino sugars,
deoxy sugars, and their uronic acid derivatives. In embodiments of the
invention where the carbohydrate
is a hexose, the hexose is selected from the group consisting of glucose,
galactose, or mannose, or
substituted hexose sugar residues such as an amino sugar residue such as
hexosamine, galactosamine,
16
CA 02579188 2007-02-19
glucosamine, in particular D-glucosamine (2-amino-2-doexy-D-glucose) or D-
galactosamine (2-amino-2-
deoxy-D-galactose). Suitable pentose sugars include arabinose, fucose, and
ribose.
The term "carbohydrate" also includes glycoproteins such as lectins (e.g.
concanavalin A, wheat
germ agglutinin, peanutagglutinin, seromucoid, and orosomucoid) and
glycolipids such as cerebroside and
ganglioside.
A "peptide" for use as a carrier in the practice of the present invention
includes one, two, three,
four, or five or more amino acids covalently linked through a peptide bond. A
peptide can comprise one or
more naturally occurring amino acids, and analogs, derivatives, and congeners
thereof. A peptide can be
modified to increase its stability, bioavailability, solubility, etc. "Peptide
analogue" and "peptide
derivative" as used herein include molecules which mimic the chemical
structure of a peptide and retain
the functional properties of the peptide. In aspects of the invention the
carrier is an amino acid such as
alanine, glycine, proline, methionine, serine, threonine, histidine, or
asparagine. In other aspects the
carrier is a peptide such as alanyl-alanyl, prolyl-methionyl, or glycyl-
glycyl. In still other aspects, the
carrier is a polypeptide such as albumin, antitiypsin, macroglobulin,
haptoglobin, ceruloplasm, transferrin,
a- or (3- lipoprotein, (3- or y- globulin or fibrinogen.
Approaches to designing peptide analogues, derivatives and mimetics are known
in the art. For
example, see Farmer, P. S. in Drug Design (E. J. Ariens, ed.) Academic Press,
New York, 1980, vol. 10,
pp. 119-143; Ball. J. B. and Alewood, P. F. (1990) J Mol. Recognition 3:55;
Morgan, B. A. and Gainor, J.
A. (1989) Ann. Rep. Med. Chem. 24:243; and Freidinger, R. M. (1989) Trends
Pharmacol. Sci. 10:270.
See also Sawyer, T. K. (1995) "Peptidomimetic Design and Chemical Approaches
to Peptide Metabolism"
in Taylor, M. D. and Amidon, G. L. (eds.) Peptide-Based Drug Design:
Controlling Transport and
Metabolism, Chapter 17; Smith, A. B. 3rd, et al. (1995) J. Am. Chem. Soc.
117:11113-11123; Smith, A.
B. 3rd, et al. (1994) J. Am. Chem. Soc. 116:9947-9962; and Hirschman, R., et
al. (1993) J. Am. Chem.
Soc. 115:12550-12568.
Examples of peptide analogues, derivatives and peptidomimetics include
peptides substituted with
one or more benzodiazepine molecules (see e.g., James, G. L. et al. (1993)
Science 260:1937-1942),
peptides with methylated amide linkages and "retro-inverso" peptides (see U.S.
Pat. No. 4,522,752 by
Sisto).
Examples of peptide derivatives include peptides in which an amino acid side
chain, the peptide
backbone, or the amino- or carboxy-terminus has been derivatized (e.g.,
peptidic compounds with
methylated amide linkages).
The term mimetic, and in particular, peptidomimetic, is intended to include
isosteres. The term
"isostere" refers to a chemical structure that can be substituted for a second
chemical structure because the
steric conformation of the first structure fits a binding site specific for
the second structure. The term
specifically includes peptide back-bone modifications (i.e., amide bond
mimetics) well known to those
skilled in the art. Such modifications include modifications of the amide
nitrogen, the alpha-carbon, amide
carbonyl, complete replacement of the amide bond, extensions, deletions or
backbone crosslinks. Other
17
CA 02579188 2007-02-19
examples of isosteres include peptides substituted with one or more
benzodiazepine molecules (see e.g.,
James, G. L. et al. (1993) Science 260:1937-1942)
Other possible modifications include an N-alkyl (or aryl) substitution
([CONR]), backbone
crosslinking to construct lactams and other cyclic structures, substitution of
all D-amino acids for all L-
amino acids within the compound ("inverso" compounds) or retro-inverso amino
acid incorporation
([NHCO]). By "inverso" is meant replacing L-amino acids of a sequence with D-
amino acids, and by
"retro-inverso" or "enantio-retro" is meant reversing the sequence of the
amino acids ("retro") and
replacing the L-amino acids with D-amino acids. For example, if the parent
peptide is Thr-Ala-Tyr, the
retro modified form is Tyr-Ala-Thr, the inverso form is thr-ala-tyr, and the
retro-inverso form is tyr-ala-thr
(lower case letters refer to D-amino acids). Compared to the parent peptide, a
retro-inverso peptide has a
reversed backbone while retaining substantially the original spatial
conformation of the side chains,
resulting in a retro-inverso isomer with a topology that closely resembles the
parent peptide. See
Goodman et al. "Perspectives in Peptide Chemistry" pp. 283-294 (1981). See
also U.S. Pat. No. 4,522,752
by Sisto for further description of "retro-inverso" peptides.
A peptide can be attached to a compound of the invention through a functional
group on the side
chain of certain amino acids (e.g. serine) or other suitable functional
groups. In an embodiment of the
invention the carrier may comprise four or more amino acids with groups
attached to three or more of the
amino acids through functional groups on side chains. In another embodiment,
the carrier is one amino
acid, in particular a sulfonate derivative of an amino acid, for example
cysteic acid.
"An amyloid-related disease" includes a disease associated with the
accumulation of amyloid
fibrils, which can either be restricted to one organ, i.e. "localized
amyloidosis", or spread to several
organs, which is denoted "systemic amyloidosis". An amyloid-related disease
includes amyloid diseases
or amyloidoses. In aspects of the invention, the term include conditions
associated with the formation,
deposition, accumulation, or persistence of amyloid or amyloid fibrils,
comprising an amyloid protein
comprising or selected from the group consisting of A(3 amyloid, AA amyloid,
AL amyloid, IAPP
amyloid, PrP amyloid, a2-microglobulin amyloid, transthyretin, prealbumin, and
procalcitonin, especially
A(i amyloid and IAPP amyloid. A disorder and/or disease may be a condition
where it is desirable to
dissociate abnormally aggregated proteins and/or dissolve or disrupt pre-
formed or pre-deposited amyloid
or amyloid fibril.
In certain aspects of the invention the disease is an amyloidosis.
"Amyloidosis" refers to a diverse
group of diseases of acquired or hereditary origin and characterized by the
accumulation of one of several
different types of protein fibrils with similar properties called amyloid.
Amyloid can accumulate in a
single organ or be dispersed throughout the body. The disease can cause
serious problems in the affected
areas, which may include the heart, brain, kidneys and digestive tract. The
fibrillar composition of
amyloid deposits is an identifying characteristic for various amyloid
diseases. Intracerebral and
cerebrovascular deposits composed primarily of fibrils of beta amyloid peptide
((3-AP) are characteristic
of Alzheimer's disease (both familial and sporadic forms); islet amyloid
protein peptide (IAPP; amylin) is
18
CA 02579188 2007-02-19
characteristic of the fibrils in pancreatic islet cell amyloid deposits
associated with type II diabetes; and,
(3-2-microglobulin is a major component of amyloid deposits which form as a
consequence of long term
hemodialysis treatment. Prion-associated diseases, such as Creutzfeld-Jacob
disease, scrapie, bovine
spongiform encephalitis, and the like are characterized by the accumulation of
a protease-resistant form of
a prion protein (designated as AScr ro PrP-27).
Certain disorders are considered to be primary amyloidoses, in which there is
no evidence for
preexisting or coexisting disease. Primary amyloidoses are typically
characterized by the presence of
"amyloid light chain-type" (AL-type) protein fibrils. In secondary amyloidosis
there is an underlying
chronic inflammatory or infectious disease state (e.g., rheumatoid arthritis,
juvenile chronic arthritis,
ankylosing spondylitis, psoriasis, Reiter's syndrome, Adult Still's disease,
Behcet's Syndrome, Crohn's
disease, chronic microbial infections such as osteomyelitis, tuberculosis, and
leprosy, malignant
neoplasms such as Hodgkin's lymphoma, renal carcinoma, carcinomas of the gut,
lung, and urogenital
tract, basel cell carcinoma, and hairy cell carcinoma). Secondary amyloidosis
is characterized by
deposition of AA type fibrils derived from serum amyloid A protein (ApoSSA).
Heredofamilial
amyloidoses may have associated neuropathic, renal, or cardiovascular deposits
of the ATTR transthyretin
type, and they include other syndromes having different amyloid components
(e.g., familial Mediterranean
fever which is characterized by AA fibrils). Other forms of amyloidosis
include local forms, characterized
by focal, often tumor-like deposits that occur in isolated organs. In
addition, amyloidoses are associated
with aging, and are commonly characterized by plaque formation in the heart or
brain. Amyloidoses
includes systemic diseases such as adult-onset disabetes, complications from
long-term hemodialysis and
consequences of chronic inflammation or plasma cell dyscrasias.
Amyloid-related diseases that can be treated and/or prevented using the
compounds,
compositions and methods of the invention include without limitation,
Alzheimer's disease, Down's
syndrome, dementia pugilistica, multiple system atrophy, inclusion body
myositosis, hereditary cerebral
hemorrhage with amyloidosis of the Dutch type, Nieman-Pick disease type C,
cerebral (3-amyloid
angiopathy, dementia associated with cortical basal degeneration, the
amyloidosis of type 2 diabetes, the
amyloidosis of chronic inflammation, the amyloidosis of malignancy and
Familial Mediterranean Fever,
the amyloidosis of multiple myeloma and B-cell dyscrasias, nephropathy with
urticaria and deafness
(Muckle - Wells syndrome), amyloidosis associated with systemic inflammatory
diseases, idiopathic
primary amyloidosis associated with myeloma or macroglobulinemia; amyloidosis
associated with
immunocyte dyscrasia; monoclonal gammopathy; occult dyscrasia; local nodular
amyloidosis associated
with chronic inflammatory diseases; amyloidosis associated with several
immunocyte dyscrasias; familial
amyloid polyneuropathy; hereditary cerebral hemorrhage with amyloidosis
Alzheimer's disease and other
neurodegenerative diseases, amyloidosis associated with chronic hemodialysis,
diabetes type II,
insulinoma, the amyloidosis of the prion diseases, (transmissible spongiform
encephalopathies prion
diseases), Creutzfeldt-Jakob disease, Gerstmann-Straussler syndrome, Kuru, and
scrapie, the amyloidosis
19
CA 02579188 2007-02-19
associated with carpal tunnel syndrome, senile cardiac amyloidosis, familial
amyloidotic polyneuropathy,
and the amyloidosis associated with endocrine tumors, especially Alzheimer's
disease and type 2 diabetes.
In aspects of the invention, the amyloid-related diseases included or are
selected from the group
consisting of Alzheimer's disease, Down's syndrome, dementia pugilistica,
multiple system atrophy,
inclusion body myositosis, hereditary cerebral hemorrhage with amyloidosis of
the Dutch type, Nieman-
Pick disease type C, cerebral 0-amyloid angiopathy, dementia associated with
cortical basal degeneration,
the amyloidosis of type 2 diabetes, the amyloidosis of chronic inflammation,
the amyloidosis of
malignancy and Familial Mediterranean Fever, the amyloidosis of multiple
myeloma and B-cell
dyscrasias, the amyloidosis of the prion diseases, Creutzfeldt-Jakob disease,
Gerstmann-Straussler
syndrome, kuru, and scrapie, the amyloidosis associated with carpal tunnel
syndrome, senile cardiac
amyloidosis, familial amyloidotic polyneuropathy, and the amyloidosis
associated with endocrine tumors,
especially Alzheimer's disease and type 2 diabetes.
In other aspects of the invention, the amyloid-related diseases included or
are selected from the
group consisting of conditions of the central or peripheral nervous system or
a systemic organ that result
in the deposition of proteins, protein fragments, and peptides in beta-pleated
sheets, fibrils, and/or
aggregates or oligomers. In particular the disease is Alzheimer's disease,
presenile and senile forms;
amyloid angiopathy; mild cognitive impairment; Alzheimer's disease-related
dementia (e.g., vascular or
Alzheimer dementia); tauopathy (e.g., argyrophilic grain dementia,
corticobasal degeneration, dementia
pugilistica, diffuse neurofibrillary tangles with calcification,
frontotemporal dementia with parkinsonism,
Prion-related disease, Hallervorden-Spatz disease, myotonic dystrophy, Niemann-
Pick disease type C,
non-Guamanian Motor Neuron disease with neurofibrillary tangles, Pick's
disease, postencephalitic
parkinsonism, cerebral amyloid angiopathy, progressive subcortical gliosis,
progressive supranuclear
palsy, subacute sclerosing panencephalitis, and tangle only dementia), alpha-
synucleinopathy (e.g.,
dementia with Lewy bodies, multiple system atrophy with glial cytoplasmic
inclusions, Shy-Drager
syndrome, spinocerebellar ataxia (e.g., DRPLA or Machado-Joseph Disease);
striatonigral degeneration,
olivopontocerebellar atrophy, neurodegeneration with brain iron accumulation
type I, olfactory
dysfunction, and amyotrophic lateral sclerosis); Parkinson's disease (e.g.,
familial or non-familial);
Amyotrophic Lateral Sclerosis; Spastic paraplegia (e.g., associated with
defective function of chaperones
and/or triple A proteins); Huntington's Disease, spinocerebellar ataxia,
Freidrich's Ataxia;
neurodegenerative diseases associated with intracellular and/or intraneuronal
aggregates of proteins with
polyglutamine, polyalanine or other repeats arising from pathological
expansions oftri- or tetra-nucleotide
elements within corresponding genes; cerebrovascular diseases; Down's
syndrome; head trauma with post-
traumatic accumulation of amyloid beta peptide; Prion related disease
(Creutzfeldt-Jakob disease,
Gerstmann-Straussler-Scheinker disease, and variant Creutzfeldt-Jakob
disease); Familial British
Dementia; Familial Danish Dementia; Presenile Dementia with Spastic Ataxia;
Cerebral Amyloid
Angiopathy, British Type; Presenile Dementia With Spastic Ataxia Cerebral
Amyloid Angiopathy, Danish
Type; Familial encephalopathy with neuroserpin inclusion bodies (FENIB);
Amyloid Polyneuropathy
CA 02579188 2007-02-19
(e.g., senile amyloid polyneuropathy or systemic Amyloidosis); Inclusion Body
myositis due to amyloid
beta peptide; Familial and Finnish Type Amyloidosis; Systemic amyloidosis
associated with multiple
myeloma; Familial Mediterranean Fever; chronic infections and inflammations;
and Type II Diabetes
Mellitus associated with islet amyloid polypeptide (IAPP).
In aspects of the invention, in particular combination therapies, the amyloid-
related disease is a
neuronal disorder (e.g., Alzheimer's disease, Down Syndrome, Parkinson
disease, Chorea Huntington,
pathogenic psychotic conditions, schizophrenia, impaired food intake, sleep-
wakefulness, impaired
homeostatic regulation of energy metabolism, impaired autonomic function,
impaired hormonal balance,
impaired regulation, body fluids, hypertension, fever, sleep dysregulation,
anorexia, anxiety related
disorders including depression, seizures including epilepsy, drug withdrawal
and alcoholism,
neurodegenerative disorders including cognitive dysfunction and dementia).
In aspects of the invention, the disease is an Amyloid Polyneuropathy
including senile amyloid
polyneuropathy or systemic amyloidosis.
In embodiments of the invention, the disease is Alzheimer's disease or
Parkinson's disease
including familial and non-familial types. In particular embodiments of the
invention, the disease is
Alzheimer's disease.
In embodiments of the invention, the disease is mild cognitive impairment.
In embodiments of the invention, the disease is dementia, in particular
vascular, dementia with
Lewy bodies, mixed dementia, Alzheimer dementia, or a secondary dementia
caused by drugs, delirium,
or depression. A dementia is generally characterized by loss of integrated
central nervous system
functions, resulting in the inability to understand simple concepts or
instructions, to store and retrieve
information into memory, and in behavioral and personality changes. Diagnostic
features of dementia
according to the DSM-IV (Diagnostic and Statistical Manual for Mental
Disorders, American Psychiatric
Association) include memory impairment and at least one of the following:
language impairment
(aphasia), lost ability to execute learned motor functions (apraxia),
inability to recognize familiar objects
(agnosia), or disturbances in executive functioning or decision making.
Compositions
A scyllo-inositol compound may be formulated into a pharmaceutical composition
or dietary
supplement for administration to a subject. Pharmaceutical compositions of the
present invention or
fractions thereof typically comprise suitable pharmaceutically acceptable
carriers, excipients, and vehicles
selected based on the intended form of administration, and consistent with
conventional pharmaceutical
practices. Particular compositions of the invention can contain a scyllo-
inositol compound that is pure or
substantially pure.
Suitable pharmaceutical carriers, excipients, and vehicles are described in
the standard text,
Remington: The Science and Practice of Pharmacy, 21s' Edition. University of
the Sciences in
Philadelphia (Editor), Mack Publishing Company. By way of example, for oral
administration in the form
of a capsule or tablet, the active components can be combined with an oral,
non-toxic pharmaceutically
21
CA 02579188 2007-02-19
acceptable inert carrier such as lactose, starch, sucrose, methyl cellulose,
magnesium stearate, glucose,
calcium sulfate, dicalcium phosphate, mannitol, sorbital, and the like. For
oral administration in a liquid
form, the drug components may be combined with any oral, non-toxic,
pharmaceutically acceptable inert
carrier such as ethanol, glycerol, water, and the like. Suitable binders (e.g.
gelatin, starch, corn sweeteners,
natural sugars including glucose; natural and synthetic gums, and waxes),
lubricants (e.g. sodium oleate,
sodium stearate, magnesium stearate, sodium benzoate, sodium acetate, and
sodium chloride),
disintegrating agents (e.g. starch, methyl cellulose, agar, bentonite, and
xanthan gum), flavoring agents,
and coloring agents may also be combined in the compositions or components
thereof. Compositions as
described herein can further comprise wetting or emulsifying agents, or pH
buffering agents.
The invention provides commercially useful formulations including without
limitation pills,
tablets, caplets, soft and hard gelatin capsules, lozenges, sachets, cachets,
vegicaps, liquids, liquid drops,
elixirs, suspensions, emulsions, solutions, syrups, aerosols (as a solid or in
a liquid medium) suppositories,
sterile injectable solutions, and/or sterile packaged powders, which contain a
scyllo-inositol compound, in
particular a pure or substantially pure scyllo-compound.
A composition can be a sustained release formulation or formulated as a
suppository with
traditional binders and carriers such as triglycerides. Oral formulations can
include standard carriers such
as pharmaceutical grades of mannitol, lactose, starch, magnesium stearate,
sodium saccharine, cellulose,
magnesium carbonate, etc. Various delivery systems are known and can be used
to administer a
composition of the invention, e.g. encapsulation in liposomes, microparticles,
microcapsules, and the like.
In aspects of the invention, a pharmaceutical composition is provided for oral
administration of
one or more scyllo-inositol compound for treatment of an amyloid related
disease. In a particular aspect, a
stable oral pharmaceutical composition for treatment of a disease
characterized by abnormal protein
folding and/or aggregation, and/or amyloid formation, deposition,
accumulation, or persistence (e.g.,
Alzheimer's disease) is provided comprising a substantially pure scyllo-
inositol compound of the formula
Ia or Ib.
Formulations for parenteral administration may include aqueous solutions,
syrups, aqueous or oil
suspensions and emulsions with edible oil such as cottonseed oil, coconut oil,
almond oil, or peanut oil.
Dispersing or suspending agents that can be used for aqueous suspensions
include synthetic or natural
gums, such as tragacanth, alginate, acacia, dextran, sodium
carboxymethylcellulose, gelatin,
methylcellulose, and polyvinylpyrrolidone.
Compositions for parenteral administration may include sterile aqueous or non-
aqueous solvents,
such as water, isotonic saline, isotonic glucose solution, buffer solution, or
other solvents conveniently
used for parenteral administration of therapeutically active agents. A
composition intended for parenteral
administration may also include conventional additives such as stabilizers,
buffers, or preservatives, e.g.
antioxidants such as methylhydroxybenzoate or similar additives.
Compositions of the invention can be formulated as pharmaceutically acceptable
salts as
described herein.
22
CA 02579188 2007-02-19
In aspects of the invention, the compositions include without limitation at
least one buffering
agent or solution. Examples of buffering agents include, but are not limited
to hydrochloric, hydrobromic,
hydroiodic, sulfuric, phosphoric, formic, acetic, propionic, succinic,
glycolic, glucoronic, maleic, furoic,
citric, glutamic, benzoic, anthranilic, salicylic, phenylacetic, mandelic,
embonic, pamoic,
methanesulfonic, ethanesulfonic, pantothenic, benzenesulfonic, stearic,
sulfanilic, algenic, galacturonic
acid and mixtures thereof. Additional agents may also be included such as one
or more of pregelatinized
maize starch, polyvinyl pyrrolidone, hydroxypropyl methylcellulose, lactose,
microcrystalline cellulose,
calcium hydrogen phosphate, magnesium stearate, talc, silica, potato starch,
sodium starch glycolate,
sodium lauryl sulfate, sorbitol syrup, cellulose derivatives, hydrogenated
edible fats, lecithin, acacia,
almond oil, oily esters, ethyl alcohol, fractionated vegetable oils, methyl,
propyl-p-hydroxybenzoates,
sorbic acid and mixtures thereof. A buffering agent may additionally comprise
one or more of
dichlorodifluoromethane, trichlorofluoromethane, dichlorotetra fluoroethane,
carbon dioxide, poly (N-
vinyl pyrrolidone), poly (methylmethacrylate), polyactide, polyglycolide and
mixtures thereof. In an
embodiment, a buffering agent can be formulated as at least one medium
including without limitation a
suspension, solution, or emulsion. In other embodiments, a buffering agent may
additionally comprise a
formulatory agent including without limitation a pharmaceutically acceptable
carrier, excipient,
suspending agent, stabilizing agent or dispersing agent.
A composition of the invention may be sterilized by, for example, filtration
through a bacteria
retaining filter, addition of sterilizing agents to the composition,
irradiation of the composition, or heating
the composition. Alternatively, the compounds or compositions of the present
invention may be provided
as sterile solid preparations e.g. lyophilized powder, which are readily
dissolved in sterile solvent
immediately prior to use.
After pharmaceutical compositions have been prepared, they can be placed in an
appropriate
container and labeled for treatment of an indicated condition. For
administration of a composition of the
invention, such labeling would include amount, frequency, and method of
administration.
A scyllo-inositol compound may be in a form suitable for administration as a
dietary supplement.
A supplement of the invention may optionally include inactive ingredients such
as diluents or fillers,
viscosity-modifying agents, preservatives, flavorings, colorants, or other
additives conventional in the art.
By way of example only, conventional ingredients such as beeswax, lecithin,
gelatin, glycerin, caramel,
and carmine may be included.
A dietary supplement composition of the invention may optionally comprise a
second active
ingredient. In an embodiment, the second active ingredient is pinitol or an
active derivative or metabolite
thereof. Pinitol can be produced from plant sources, including without
limitation alfalfa, Bougainvillea
leaves, chick peas, pine trees and soy beans. Pinitol is also commercially
available, for example InzitolTM
(Humanetics Corporation, Min). Examples of derivatives and metabolites of
pinitol include without
limitation pinitol glycosides, pinitol phospholipids, esterified pinitol,
lipid-bound pinitol, pinitol
phosphates, pinitol phytates, and hydrolyzed pinitol such as d-chiro-inositol.
23
CA 02579188 2007-02-19
A dietary supplement may be provided as a liquid dietary supplement (e.g., a
dispensable liquid)
or alternatively the compositions may be formulated as granules, capsules or
suppositories. The liquid
supplement may include a number of suitable carriers and additives including
water, glycols, oils,
alcohols, flavoring agents, preservatives, coloring agents and the like. In
capsule, granule or suppository
form, the compositions of the present invention are formulated in admixture
with a pharmaceutically
acceptable carrier. In an aspect, a dietary supplement of the present
invention is formulated as a beverage,
but may be formulated in granule, capsule or suppository form.
A supplement may be presented in the form of a softgel which is prepared using
conventional
methods. A softgel typically includes a layer of gelatin encapsulating a small
quantity of the supplement.
A supplement may also be in the form of a liquid-filled and sealed gelatin
capsule, which may be made
using conventional methods.
To prepare a dietary supplement composition of the present invention in
capsule, granule or
suppository form, one or more compositions of the present invention may be
intimately admixed with a
pharmaceutically acceptable carrier according to conventional formulation
techniques. For solid oral
preparations such as capsules and granules, suitable carriers and additives
such as starches, sugars,
diluents, granulating agents, lubricants, binders, disintegrating agents and
the like may be included.
According to another aspect of the invention, a kit is provided. In an aspect,
the kit comprises a
compound or a pharmaceutical composition of the invention. The kit can be a
package which houses a
container which contains a composition of the invention and also houses
instructions for administering the
composition to a subject.
In embodiments of the invention, a pharmaceutical pack or kit is provided
comprising one or more
containers filled with one or more of the ingredients of a pharmaceutical
composition of the invention to
provide a beneficial effect, in particular a sustained beneficial effect.
Associated with such container(s)
can be various written materials such as instructions for use, or a notice in
the form prescribed by a
governmental agency regulating the labeling, manufacture, use or sale of
pharmaceuticals or biological
products, which notice reflects approval by the agency of manufacture, use, or
sale for human
administration.
Applications
The invention is related to compositions and methods that utilize one or more
scyllo-inositol
compound to provide beneficial effects. In particular, the invention
contemplates the use of a composition
of the invention for treating an amyloid-related disease, in particular
preventing, and/or ameliorating
disease severity, disease symptoms, and/or periodicity of recurrence of
disease. The invention also
contemplates preventing and/or treating in mammals, amyloid-related diseases
using the compositions or
treatments of the invention. The present invention in embodiments may provide
a composition comprising
a compound that provides beneficial effects including greater solubility,
stability, efficacy, potency, and/or
utility, in particular greater solubility and stability.
24
CA 02579188 2007-02-19
In an aspect, the invention provides a method of improving memory of a healthy
subject or the
memory of a subject with age impaired memory by administering an effective
amount of one or more
scyllo-inositol compound, or a composition comprising one or more scyllo-
inositol compound, and a
pharmaceutically acceptable carrier, excipient, or vehicle.
In another aspect, the present invention further relates to a method for
improving memory,
especially short-term memory and other mental dysfunction associated with the
aging process comprising
administering an effective amount of one or more scyllo-inositol compound, or
a pharmaceutically
acceptable salt thereof, or a composition comprising one or more scyllo-
inositol compound, and a
pharmaceutically acceptable carrier, excipient, or vehicle.
In an embodiment, a method is provided for treating a mammal in need of
improved memory,
wherein said mammal has no diagnosed disease, disorder, infirmity or ailment
known to impair or
otherwise diminish memory, comprising the step of administering to the mammal
an effective memory-
improving amount of one or more scyllo-inositol compound, a pharmaceutically
acceptable salt thereof, or
a dietary supplement comprising one or more scyllo-inositol compound or a
nutraceutically acceptable
derivative thereof.
In another aspect of the invention, a method is provided for treating in a
subject a condition of the
central or peripheral nervous system or systemic organ associated with a
disorder in protein folding or
aggregation, or amyloid formation, deposition, accumulation, or persistence,
comprising administering to
the subject a therapeutically effective amount of one or more scyllo-inositol
compound, or a
pharmaceutically acceptable salt thereof, or a composition comprising one or
more scyllo-inositol
compound and a pharmaceutically acceptable carrier, excipient, or vehicle.
In a further aspect, the invention provides a method involving administering
to a subject a
therapeutic compound of one or more scyllo-inositol compound, or
pharmaceutically acceptable salts
thereof, or a composition comprising one or more scyllo-inositol compound, and
a pharmaceutically
acceptable carrier, excipient, or vehicle which improves cognitive function,
reduces vascular load, reduces
astrogliosis, reduces amyloid burden, reduces microgliosis, and/or improves
survival.
In a further aspect, the invention provides a method involving administering
to a subject a
therapeutic compound of one or more scyllo-inositol compound, or
pharmaceutically acceptable salts
thereof, or a composition comprising one or more scyllo-inositol compound, and
a pharmaceutically
acceptable carrier, excipient, or vehicle which inhibit amyloid formation,
deposition, accumulation and/or
persistence, and/or which cause dissolution/disruption of pre-existing
amyloid. Thus, the compounds and
compositions of the invention may be used for inhibiting amyloidosis in
disorders in which amyloid
deposition occurs.
In another aspect, the invention provides a method for treating in a subject a
condition associated
with an amyloid interaction that can be disrupted or dissociated with a
compound of the invention
comprising administering to the subject a therapeutically effective amount of
one or more scyllo-inositol
CA 02579188 2007-02-19
compound, a pharmaceutically acceptable salt thereof, or a composition
comprising one or more scyllo-
inositol compound and a pharmaceutically acceptable carrier, excipient, or
vehicle.
In an aspect, the invention provides a method for preventing, reversing,
reducing or inhibiting
vascular load, astrogliosis, amyloid burden, and/or microgliosis in a subject
comprising administering a
therapeutically effective amount of one or more scyllo-inositol compound, a
pharmaceutically acceptable
salt thereof, or a composition comprising one or more scyllo-inositol
compound, and a pharmaceutically
acceptable carrier, excipient, or vehicle.
In an aspect, the invention provides a method for increasing or maintaining
synaptic function in a
subject comprising administering a therapeutically effective amount of one or
more scyllo-inositol
compound, a pharmaceutically acceptable salt thereof, or a composition
comprising one or more scyllo-
inositol compound, and a pharmaceutically acceptable carrier, excipient, or
vehicle.
The invention has particular applications in treating an amyloid-related
disease, in particular
Alzheimer's disease. Thus, the invention relates to a method of treatment
comprising administering a
therapeutically effective amount of one or more scyllo-inositol compound, a
pharmaceutically acceptable
salt thereof, or a composition comprising a scyllo-inositol compound and a
pharmaceutically acceptable
carrier, excipient, or vehicle, which upon administration to a subject with
symptoms of an amyloid-related
disease in particular Alzheimer's disease, produces beneficial effects,
preferably sustained beneficial
effects. In an embodiment, beneficial effects are evidenced by one or more of
the following: improved
cognitive function, reduced vascular load, reduced astrogliosis, reduced
amyloid burden, reduced
microgliosis, and/or improved survival.
In an aspect, the invention provides a method for amelioriating progression of
an amyloid-related
disease or obtaining a less severe stage of a disease in a subject suffering
from such disease (e.g.
Alzheimer's disease) comprising administering a therapeutically effective
amount of one or more scyllo-
inositol compound, a pharmaceutically acceptable salt thereof, or a
composition comprising one or more
scyllo-inositol compound, and a pharmaceutically acceptable carrier,
excipient, or vehicle.
In another aspect, the invention relates to a method of delaying the
progression of an amyloid-
related disease (e.g. Alzheimer's disease) comprising administering a
therapeutically effective amount of
one or more scyllo-inositol compound, a pharmaceutically acceptable salt
thereof, or a composition
comprising one or more scyllo-inositol compound, and a pharmaceutically
acceptable carrier, excipient, or
vehicle.
In a further aspect, the invention relates to a method of increasing survival
of a subject suffering
from an amyloid-related disease comprising administering a therapeutically
effective amount of one or
more scyllo-inositol compound, a pharmaceutically acceptable salt thereof, or
a composition comprising
one or more scyllo-inositol compound, and a pharmaceutically acceptable
carrier, excipient, or vehicle.
In an embodiment, the invention relates to a method of improving the lifespan
of a subject
suffering from an amyloid-related disease (e.g., Alzheimer's disease)
comprising administering a
therapeutically effective amount of one or more scyllo-inositol compound, a
pharmaceutically acceptable
26
CA 02579188 2007-02-19
salt thereof, or a composition comprising one or more scyllo-inositol
compound, and a pharmaceutically
acceptable carrier, excipient, or vehicle.
In an aspect the invention provides a method for treating mild cognitive
impairment (MCI)
comprising administering a therapeutically effective amount of one or more
scyllo-inositol compound, a
pharmaceutically acceptable salt thereof, or a composition comprising one or
more scyllo-inositol
compound, and a pharmaceutically acceptable carrier, excipient, or vehicle.
In an embodiment, the invention provides a method of reducing or reversing
amyloid deposition
and neuropathology after the onset of cognitive deficits and amyloid plaque
neuropathology in a subject
comprising administering to the subject a therapeutically effective amount of
one or more scyllo-inositol
compound, a pharmaceutically acceptable salt thereof, or a composition
comprising one or more scyllo-
inositol compound and a pharmaceutically acceptable carrier, excipient, or
vehicle.
In another embodiment, the invention provides a method of reducing or
reversing amyloid
deposition and neuropathology after the onset of cognitive deficits and
amyloid plaque neuropathology in
a subject comprising administering to the subject an amount of one or more
scyllo-inositol compound, a
pharmaceutically acceptable salt thereof, or a composition comprising one or
more scyllo-inositol
compound and a pharmaceutically acceptable carrier, excipient, or vehicle
effective to reduce or reverse
amyloid deposition and neuropathology after the onset of cognitive deficits
and amyloid plaque
neuropathology.
Aspects of the invention provide improved methods and compositions for use of
one or more
scyllo-inositol compound for sustained treatment of a disorder and/or disease
(e.g., Alzheimer's disease).
The present invention in an embodiment provides a composition comprising one
or more scyllo-inositol
compound that achieves greater efficacy, potency, and utility. For example,
the greater efficacy can be
shown by improving or reversing cognitive decline and/or survival in
Alzheimer's disease with treatment
resulting in sustained improvement and/or increased survival after ceasing
treatment.
In an aspect of the invention a compound of the formula la or lb is utilized
in the treatment of
Alzheimer's disease. Thus, Alzheimer's disease may be treated by administering
a therapeutically
effective amount of a compound of the formula Ia or formula lb. Such treatment
may be effective for
retarding the degenerative effects of Alzheimer's disease, including
specifically, but not exclusively,
deterioration of the central nervous system, loss of mental facilities, loss
of short term memory, and
disorientation.
In an embodiment, where the disease is Alzheimer's disease, dementia or MCI,
beneficial effects
of a compound or composition or treatment of the invention can manifest as at
least one, two, three, four,
five, six, seven, eight, nine, ten, twelve, thirteen, fourteen, fifteen, or
all of the following, in particular five
or ten or more, more particularly fifteen or more of the following:
a) An increase or restoration of long term potentiation relative to the level
in the absence of
a compound disclosed herein after administration to a subject with symptoms of
Alzheimer's disease. In aspects of the invention the compounds induce at least
about a
27
CA 02579188 2007-02-19
0.05%, 0.1%, 0.5%, 1%, 2%, 5%, 10%, 15%, 20%, 30%, 33%, 35%, 40%, 45%, 50%,
60%, 70%, 80%, 90%, 95%, or 99% increase in long term potentiation in a
subject.
b) An increase or maintenance of synaptic function relative to the level of
synaptic function
in the absence of a compound disclosed herein after administration to a
subject with
symptoms of Alzheimer's disease. In aspects of the invention the compounds
induce at
least about a 0.05%, 0.1%, 0.5%, 1%, 2%, 5%, 10%, 15%, 20%, 30%, 33%, 35%,
40%,
45%, 50%, 60%, 70%, 80%, 90%, 95%, 99%, 100%, 125%, 150%, 175% or 200%
increase in synaptic function in a subject.
c) An increase in synaptophysin. In aspects of the invention there is at least
about a 2%, 5%,
10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, 99%, 100%, 125%,
150%, 175% or 200% increase in synaptophysin.
d) An increase in synaptophysin reactive boutons and cell bodies. In aspects
of the
invention there is at least about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%,
70%,
80%, 90%, 95%, 99%, 100%, 125%, 150%, 175% or 200%, more particularly about a
100-150% or 140-150%, increase in synaptophysin reactive boutons and cell
bodies,.
e) A reduction, slowing or prevention of an increase in, or an absence of
symptoms of
inflammation, in particular an A(3-induced inflammatory response, after
administration to
a subject with symptoms of Alzheimer's disease.
f) A reduction, slowing or prevention of an increase in cerebral accumulation
of amyloid (3
relative to the levels measured in the absence of a compound disclosed herein
in subjects
with symptoms of Alzheimer's disease. In aspects of the invention, the
compound
induces at least about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%,
or
90% decrease in cerebral accumulation of amyloid P.
g) A reduction, slowing or prevention of an increase in deposition of cerebral
amyloid
plaques, relative to the levels measured in the absence of a compound
disclosed herein in
subjects with symptoms of Alzheimer's disease. In aspects of the invention,
the
compound induces at least about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%,
70%, 80%, or 90% decrease in deposition of cerebral amyloid plaques.
h) A reduction, slowing or prevention of an increase in plaque number. In
aspects of the
invention, a compound disclosed herein induces at least about a 2%, 5%, 10%,
15%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction in plaque number. In
particular aspects the compound induces a 5-15% or 10-15% reduction in plaque
number.
i) A reduction, slowing or prevention of an increase in plaque size. In
aspects of the
invention, a compound disclosed herein induces at least about a 2%, 5%, 10%,
15%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction in plaque size. In
particular
aspects the compound induces a 5-15% or 10-15% reduction in plaque size.
28
CA 02579188 2007-02-19
j) A reduction, slowing or prevention of an increase in percent area of the
brain covered in
plaques. In aspects of the invention, a compound disclosed herein induces at
least about a
2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction in
percent
area of the brain covered in plaques. In particular aspects the compound
induces a 5-15%
or 10-15% reduction in percent area of the brain covered in plaques.
k) A reduction, slowing or prevention of an increase in soluble A(3 oligomers
in the brain,
relative to the levels measured in the absence of a compound disclosed herein
in subjects
with symptoms of Alzheimer's disease. In aspects of the invention, the
combination
induces at least about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%,
or
90% decrease in soluble A(3 oligomers.
1) A reduction, slowing or prevention of an increase in brain levels of A(340.
In aspects of
the invention, a compound disclosed herein induces at least about a 2%, 5%,
10%, 15%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction in A(340. In particular
aspects
the compound induces a 10-50%, 20-45%, or 25-35% reduction in brain levels of
A(340.
m) A reduction, slowing or prevention of an increase in A(3421evels in a body
fluid such as
CSF or blood. In aspects of the invention, a compound disclosed herein induces
at least
about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction
in
A(342. In particular aspects the compound induces a 10-50%, 15-40%, or 20-25%
reduction in brain levels of A(342.
n) A reduction, slowing or prevention of an increase in brain levels of A(342.
In aspects of
the invention, a compound disclosed herein induces at least about a 2%, 5%,
10%, 15%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction in AP42. In particular
aspects
the compound induces a 10-50%, 15-40%, or 20-25% reduction in brain levels of
A(342.
o) A reduction, slowing or prevention of an increase in glial activity in the
brain, relative to
the levels measured in the absence of a compound disclosed herein in subjects
with
symptoms of Alzheimer's disease. Preferably, the compound induces at least
about a 2%,
5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% decrease in glial
activity
p) Maintenance of synaptic function at about normal for a prolonged period of
time, in
particular for at least 5 weeks, 6 weeks, 8 weeks, 10 weeks, 12 weeks, 14
weeks, 16
weeks, 20 weeks, 24 weeks, 30 weeks, 40 weeks, 52 weeks, or 78 weeks, more
particularly, 2 to 4 weeks, 2 to 5 weeks, 3 to 5 weeks, 2 to 6 weeks, 2 to 8
weeks, 2 to 10
weeks, 2 to 12 weeks, 2 to 16 weeks, 2 to 20 weeks, 2 to 24 weeks, 2 weeks to
12
months, or 2 weeks to 24 months following treatment.
q) A reduction or slowing of the rate of disease progression in a subject with
Alzheimer's
disease. In particular a reduction or slowing of cognitive decline in a
subject with
Alzheimer's disease.
29
CA 02579188 2007-02-19
r) A reduction in totat vascular load. In aspects of the invention, the
compounds induce at
least about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90%
reduction in total vascular load.
s) A reduction in astrogliosis. In aspects of the invention, the compounds
induce at least
about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction
in
astrogliosis.
t) A reduction in microgliosis. In aspects of the invention, the compounds
induce at least
about a 2%, 5%, 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% reduction
in
microgliosis.
u) A reduction or slowing of cognitive deficits.
v) A reduction in or slowing of amyloid angiopathy.
w) A reduction in accelerated mortality.
x) An increase in survival in a subject with symptoms of Alzheimer's disease.
Preferably,
the compounds induce at least about a 20%, 30%, 40%, 50%, 60%, 70%, 75%, 80%,
90%, or 95% increase in survival.
In aspects of the invention beneficial effects of a composition or treatment
of the invention can
manifest as (a) and (b); (a), (b) and (c); (a), (b), (e), (f) and (g); (a),
(b), (e), (f) through (h); (a), (b), (e),
(f) through (i); (a), (b), (e), (f) through (j); (a), (b), (e), (f) through
(k); (a), (b), (e), (f) through (1); (a), (b),
(e), (f) through (m); (a), (b), (e), (f) through (n); (a), (b), (e), (f)
through (o); (a), (b), (e), (f) through (p);
(a), (b), (e), (f) through (q); (a), (b), (e), (f) through (r); (a), (b), (e),
(f) through (s); (a), (b), (e), (f) through
(t); (a) through (d); (a) through (e); (a) through (f); (a) through (g); (a)
through (h); (a) through (i); (a)
through (j); (a) through (k); (a) through (1); (a) through (m); (a) through
(n); (a) through (o); (a) through
(p); (a) through (q); (a) through (r); (a) through (s); (a) through (t); (a)
through (u); (a) through (v); (a)
through (w) or (a) through (x).
Compounds, pharmaceutical compositions and methods of the invention can be
selected that have
statistically significant beneficial effects, in particular one or more of the
effects of (a) through (x) above.
Compounds, pharmaceutical compositions and methods of the invention can also
be selected that have
sustained beneficial effects, in particular statistically significant
sustained beneficial effects. In an
embodiment, a pharmaceutical composition is provided with statistically
significant sustained beneficial
effects, in particular sustained beneficial effects of one or more of (a)
through (x) above, comprising a
therapeutically effective amount of one or more scyllo-inositol compound. In
aspects of the invention, one
or more of the beneficial effects provide enhanced therapeutic effects
compared with conventional
therapies.
Greater efficacy and potency of a treatment of the invention in some aspects
may improve the
therapeutic ratio of treatment, reducing untoward side effects and toxicity.
Selected methods of the
invention may also improve long-standing Alzheimer's disease even when
treatment is begun long after
CA 02579188 2007-02-19
the appearance of symptoms. Prolonged efficacious treatment can be achieved in
accordance with the
invention following administration of a compound or composition of the
invention.
In a further aspect, the invention provides a method for treating Alzheimer's
disease in a patient in
need thereof which includes administering to the individual a composition that
provides one or more
scyllo-inositol compound in a dose sufficient to improve cognitive function,
reduce vascular load, reduce
astrogliosis, reduce amyloid burden, reduce microgliosis, and/or improve
survival. In another aspect, the
invention provides a method for treating Alzheimer's disease comprising
administering, preferably orally
or systemically, an amount of a scyllo-inositol compound to a mammal, to
improve cognitive function,
reduce vascular load, reduce astrogliosis, reduce amyloid burden, reduce
microgliosis, and/or improve
survival for a prolonged period following administration.
In an aspect, the invention relates to a method for treating Alzheimer's
disease comprising
contacting Ap, A(3 aggregates, or A(3 oligomers in particular A(340 or A(340
aggregates or oligomers
and/or A(342 or A(342 aggregates or oligomers, in a subject with a
therapeutically effective amount of one
or more scyllo-inositol compound or a composition comprising an epi-inositol
compound.
In another aspect, the invention provides a method for treating Alzheimer's
disease by providing a
composition comprising one or more one or more scyllo-inositol compound in an
amount sufficient to
disrupt aggregated A(3 or A(3 oligomers for a prolonged period following
administration.
In a further aspect, the invention provides a method for treating Alzheimer's
disease in a patient in
need thereof which includes administering to the individual a composition that
provides one or more one
or more scyllo-inositol compound in a dose sufficient to increase or restore
long term potentiation and/or
maintain synaptic function. In another aspect, the invention provides a method
for treating Alzheimer's
disease comprising administering, preferably orally or systemically, an amount
of one or more scyllo-
inositol compound to a mammal, to reduce cerebral accumulation of A(3,
deposition of cerebral amyloid
plaques, soluble A(3 oligomers in the brain, glial activity, and/or
inflammation for a prolonged period
following administration.
The invention in an embodiment provides a method for treating Alzheimer's
disease, the method
comprising administering to a mammal in need thereof a composition comprising
one or more scyllo-
inositol compound in an amount sufficient to reduce cognitive decline,
especially for a prolonged period
following administration, thereby treating the Alzheimer's disease.
The invention in an embodiment provides a method for treating Alzheimer's
disease, the method
comprising administering to a mammal in need thereof a composition comprising
one or more one or
more scyllo-inositol compound in an amount sufficient to increase or maintain
synaptic function,
especially for a prolonged period following administration, thereby treating
the Alzheimer's disease.
In another aspect, the invention provides a method for preventing and/or
treating Alzheimer's
disease, the method comprising administering to a mammal in need thereof a
composition comprising one
or more one or more scyllo-inositol compound in an amount sufficient to
disrupt aggregated A(3 or A(3
oligomers for a prolonged period following administration; and determining the
amount of aggregated A(3
31
CA 02579188 2007-02-19
or A(3 oligomers, thereby treating the Alzheimer's disease. The amount of
aggregated A(3 or A(3
oligomers may be measured using an antibody specific for A(3 or an epi-
inositol labeled with a detectable
substance.
The present invention also includes methods of using the compositions of the
invention in
combination treatments with one or more additional therapeutic agents
including without limitation beta-
secretase inhibitors, gamma-secretase inhibitors, epsilon-secretase
inhibitors, other inhibitors of beta-
sheet aggregation/fibrillogenesis/ADDL formation (e.g. Alzhemed), NMDA
antagonists (e.g. memantine),
non-steroidal anti-inflammatory compounds (e.g. Ibuprofen, Celebrex), anti-
oxidants (e.g. Vitamin E),
hormones (e.g. estrogens), nutrients and food supplements (e.g. Gingko
biloba), statins and other
cholesterol lowering drugs (e.g. Lovastatin and Simvastatin),
acetylcholinesterase inhibitors (e.g.
donezepil), muscarinic agonists (e.g. AF102B (Cevimeline, EVOXAC), AF150(S),
and AF267B), anti-
psychotics (e.g. haloperidol, clozapine, olanzapine), anti-depressants
including tricyclics and serotonin
reuptake inhibitors (e.g. Sertraline and Citalopram Hbr), statins and other
cholesterol lowering drugs (e.g.
Lovastatin and Simvastatin), immunotherapeutics and antibodies to A(3 (e.g.
ELAN AN-1792), vaccines,
inhibitors of kinases (CDK5, GSK3a, GSK3(3) that phosphorylate TAU protein
(e.g. Lithium chloride),
inhibitors of kinases that modulate A(3 production (GSK3a, GSK3(3, Rho/ROCK
kinases) (e.g. lithium
Chloride and Ibuprofen), drugs that upregulate neprilysin (an enzyme which
degrades A(3); drugs that
upregulate insulin degrading enzyme (an enzyme which degrades A(3), agents
that are used for the
treatment of complications resulting from or associated with a disease, or
general medications that treat or
prevent side effects. The present invention also includes methods of using the
compositions of the
invention in combination treatments with one or more additional treatments
including without limitation
gene therapy and/or drug based approaches to upregulate neprilysin (an enzyme
which degrades A(3), gene
therapy and/or drug based approaches to upregulate insulin degrading enzyme
(an enzyme which degrades
A(3), or stem cell and other cell-based therapies.
Combinations of a scyllo-inositol compound and a therapeutic agent or
treatment may be selected
to provide unexpectedly additive effects or greater than additive effects i.e.
synergistic effects. Other
therapeutics and therapies may act via a different mechanism and may have
additive/synergistic effects
with the present invention
A composition or method (i.e., combination treatment) comprising one or more
scyllo-inositol
compound and a therapeutic agent employing different mechanisms to achieve
maximum therapeutic
efficacy, may improve tolerance to the therapy with a reduced risk of side
effects that may result from
higher doses or longer term monotherapies (i.e. therapies with each compound
alone). A combination
treatment may also permit the use of lower doses of each compound with reduced
adverse toxic effects of
each compound. A suboptimal dosage may provide an increased margin of safety,
and may also reduce the
cost of a drug necessary to achieve prophylaxis and therapy. In addition, a
treatment utilizing a single
combination dosage unit may provide increased convenience and may result in
enhanced compliance.
Other advantages of a combination therapy may include higher stability towards
degradation and
32
CA 02579188 2007-02-19
metabolism, longer duration of action, and/or longer duration of action or
effectiveness at particularly low
doses.
In an aspect, the invention contemplates the use of a composition comprising
at least one scyllo-
inositol compound for the preparation of a medicament in treating a disorder
and/or disease. The invention
also contemplates the use of a composition comprising at least one scyllo-
inositol compound for the
preparation of a medicament for preventing and/or treating disorders and/or
diseases. The invention
additionally provides uses of a pharmaceutical composition of the invention in
the preparation of
medicaments for the prevention and/or treatment of disorders and/or diseases.
The medicaments provide
beneficial effects, preferably sustained beneficial effects following
treatment. The medicament may be in
a form for consumption by a subject such as a pill, tablet, caplet, soft and
hard gelatin capsule, lozenge,
sachet, cachet, vegicap, liquid drop, elixir, suspension, emulsion, solution,
syrup, aerosol (as a solid or in a
liquid medium) suppository, sterile injectable solution, and/or sterile
packaged powder for inhibition of
amyloid formation, deposition, accumulation, and/or persistence, regardless of
its clinical setting.
In an embodiment, the invention relates to the use of a therapeutically
effective amount of at least
one scyllo-inositol compound or a composition of the invention for preparation
of a medicament for
providing therapeutic effects, in particular beneficial effects, preferably
sustained beneficial effects, in an
amyloid-related disease.
In another embodiment the invention provides the use of one or more scyllo-
inositol compound or
composition of the invention for the preparation of a medicament for prolonged
or sustained treatment of
Alzheimer's disease.
In a further embodiment the invention provides the use of a scyllo-inositol
compound for
preparation of a pharmaceutical composition to be employed through oral
administration for treatment of a
disorder characterized by abnormal protein folding and/or aggregation, and/or
amyloid formation,
deposition, accumulation, or persistence.
Therapeutic efficacy and toxicity of compositions and methods of the invention
may be
determined by standard pharmaceutical procedures in cell cultures or with
experimental animals such as
by calculating a statistical parameter such as the ED50 (the dose that is
therapeutically effective in 50% of
the population) or LD50 (the dose lethal to 50% of the population) statistics.
The therapeutic index is the
dose ratio of therapeutic to toxic effects and it can be expressed as the
ED50/LD50 ratio. Pharmaceutical
compositions which exhibit large therapeutic indices are preferred. One or
more of the therapeutic effects,
in particular beneficial effects disclosed herein, can be demonstrated in a
subject or disease model. For
example, beneficial effects may be demonstrated in a model described in the
Examples herein, in
particular beneficial effects may be demonstrated in a TgCRND8 mouse with
symptoms of Alzheimer's
disease.
Administration
Scyllo-inositol compounds and compositions of the present invention can be
administered by any
means that produce contact of the active agent(s) with the agent's sites of
action in the body of a subject or
33
CA 02579188 2007-02-19
patient to produce a therapeutic effect, in particular a beneficial effect, in
particular a sustained beneficial
effect. The active ingredients can be administered simultaneously or
sequentially and in any order at
different points in time to provide the desired beneficial effects. A compound
and composition of the
invention can be formulated for sustained release, for delivery locally or
systemically. It lies within the
capability of a skilled physician or veterinarian to select a form and route
of administration that optimizes
the effects of the compositions and treatments of the present invention to
provide therapeutic effects, in
particular beneficial effects, more particularly sustained beneficial effects.
The compounds and compositions may be administered in oral dosage forms such
as tablets,
capsules (each of which includes sustained release or timed release
formulations), pills, powders,
granules, elixirs, tinctures, suspensions, syrups, and emulsions. They may
also be administered in
intravenous (bolus or infusion), intraperitoneal, subcutaneous, or
intramuscular forms, all utilizing dosage
forms well known to those of ordinary skill in the pharmaceutical arts. The
compositions of the invention
may be administered by intranasal route via topical use of suitable intranasal
vehicles, or via a transdermal
route, for example using conventional transdermal skin patches. A dosage
protocol for administration
using a transdermal delivery system may be continuous rather than intermittent
throughout the dosage
regimen. A sustained release formulation can also be used for the therapeutic
agents.
In aspects of the invention the compounds and compositions are administered by
peripheral
administration, in particular by intravenous administration, intraperitoneal
administration, subcutaneous
administration, intramuscular administration, oral administration, topical
administration, transmucosal
administration, or pulmonary administration.
The dosage regimen of the invention will vary depending upon known factors
such as the
pharmacodynamic characteristics of the agents and their mode and route of
administration; the species,
age, sex, health, medical condition, and weight of the patient, the nature and
extent of the symptoms, the
kind of concurrent treatment, the frequency of treatment, the route of
administration, the renal and hepatic
function of the patient, and the desired effect.
An amount of a scyllo-inositol compound or composition comprising same which
will be
effective in the treatment of a particular disorder and/or disease to provide
effects, in particular beneficial
effects, more particularly sustained beneficial effects, will depend on the
nature of the disorder and/or
disease, and can be determined by standard clinical techniques. The precise
dose to be employed in the
formulation will also depend on the route of administration, and the
seriousness of the disease, and should
be decided according to the judgment of the practitioner and each patient's
circumstances.
Suitable dosage ranges for administration are particularly selected to provide
therapeutic effects,
in particular beneficial effects, more particularly sustained beneficial
effects. A dosage range is generally
effective for triggering the desired biological responses. The dosage ranges
are generally about.5 mg to
about 2 g per kg, about 1 mg to about I g per kg, about 1 mg to about 200 mg
per kg, about 1 mg to about
100 mg per kg, about 1 mg to about 50 mg per kg, about 10 mg to about 100 mg
per kg, or about 30 mg to
70 mg per kg of the weight of a subject administered once, twice, three times
or more daily.
34
CA 02579188 2007-02-19
In some aspects of the invention, the dosage ranges of a compound disclosed
herein administered
once twice, three times or more daily, especially once or twice daily, are
about 1 to 100 mg/kg, 1 to 90
mg/kg,, 1 to 80 mg/kg, 1 to 75 mg/kg, 1 to 70 mg/kg, I to 60 mg/kg, 1 to 50
mg/kg, 1 to 40 mg/kg, 1 to 35
mg/kg, 2 to 35 mg/kg, 2.5 to 30 mg/kg, 3 to 30 mg/kg, 3 to 20 mg/kg, or 3 to
15 mg/kg.
In embodiments of the invention, the required dose of a compound disclosed
herein administered
twice daily is about 1 to 50 mg/kg, 1 to 40 mg/kg, 2.5 to 40 mg/kg, 3 to 40
mg/kg, 3 to 35 mg/kg, most
preferably 3 to 30 mg/kg. In embodiments of the invention, the required daily
dose of the compound is
about 1 to 80 mg/kg and within that range 1 to 70 mg/kg, 1 to 65 mg/kg, 2 to
70 mg/kg, 3 to 70 mg/kg, 4
to 65 mg/kg, 5 to 65 mg/kg, or 6 to 60 mg/kg.
In embodiments of the invention, the required dose of a compound disclosed
herein, administered
twice daily is about 1 to 50 mg/kg, 1 to 40 mg/kg, 2.5 to 40 mg/kg, 3 to 40
mg/kg, 3 to 35 mg/kg, most
preferably 3 to 30 mg/kg.
In other embodiments of the invention, the required daily dose of a compound
disclosed herein, is
about 1 to 80 mg/kg and within that range I to 70 mg/kg, 1 to 65 mg/kg, 2 to
70 mg/kg, 3 to 70 mg/kg, 4
to 65 mg/kg, 5 to 65 mg/kg, or 6 to 60 mg/kg.
A composition or treatment of the invention may comprise a unit dosage of at
least one scyllo-
inositol compound to provide beneficial effects, in particular one or more of
the beneficial effects (a) to (t)
set out herein. A "unit dosage" or "dosage unit" refers to a unitary i.e., a
single dose which is capable of
being administered to a patient, and which may be readily handled and packed,
remaining as a physically
and chemically stable unit dose comprising either the active agents as such or
a mixture with one or more
solid or liquid pharmaceutical excipients, carriers, or vehicles.
A subject may be treated with a scyllo-inositol compound or composition or
formulation thereof
on substantially any desired schedule. A composition of the invention may be
administered one or more
times per day, in particular 1 or 2 times per day, once per week, once a month
or continuously. However,
a subject may be treated less frequently, such as every other day or once a
week, or more frequently.
A scyllo-inositol compound, composition or formulation of the invention may be
administered to
a subject for about or at least about 1 week, 2 weeks to 4 weeks, 2 weeks to 6
weeks, 2 weeks to 8 weeks,
2 weeks to 10 weeks, 2 weeks to 12 weeks, 2 weeks to 14 weeks, 2 weeks to 16
weeks, 2 weeks to 6
months, 2 weeks to 12 months, 2 weeks to 18 months, or 2 weeks to 24 months,
periodically or
continuously.
In an aspect, the invention provides a regimen for supplementing a human's
diet, comprising
administering to the human a supplement comprising a scyllo-inositol compound,
or nutraceutically
acceptable derivatives thereof. A subject may be treated with a supplement at
least about every day, or
less frequently, such as every other day or once a week. A supplement of the
invention may be taken daily
but consumption at lower frequency, such as several times per week or even
isolated doses, may be
beneficial.
CA 02579188 2007-02-19
In a particular aspect, the invention provides a regimen for supplementing a
human's diet,
comprising administering to the human about 25 to about 200 milligrams of a
compound of the formula la
or lb, or nutraceutically acceptable derivatives thereof on a daily basis. In
another aspect, about 50-100
milligrams of a compound of the formula la or lb is administered to the human
on a daily basis.
A supplement of the present invention may be ingested with or after a meal.
Thus, a supplement
may be taken at the time of a person's morning meal, and/or at the time of a
person's noontime meal. A
portion may be administered shortly before, during, or shortly after the meal.
For daily consumption, a
portion of the supplement may be consumed shortly before, during, or shortly
after the human's morning
meal, and a second portion of the supplement may be consumed shortly before,
during, or shortly after the
human's noontime meal. The morning portion and the noontime portion can each
provide approximately
the same quantity of a scyllo-inositol compound. A supplement and regimens
described herein may be
most effective when combined with a balanced diet according to generally
accepted nutritional guidelines,
and a program of modest to moderate exercise several times a week.
In an embodiment, a regimen for supplementing a human's diet is provided
comprising
administering to the human a supplement comprising, per gram of supplement:
about 5 milligram to about
30 milligrams of one or more scyllo-inositol compound or a nutraceutically
acceptable derivative thereof.
In an embodiment, a portion of the supplement is administered at the time of
the human's morning meal,
and a second portion of the supplement is administered at the time of the
human's noontime meal.
The invention will be described in greater detail by way of specific examples.
The following
examples are offered for illustrative purposes, and are not intended to limit
the invention in any manner.
Those of skill in the art will readily recognize a variety of noncritical
parameters which can be changed or
modified to yield essentially the same results.
Example 1
The following methods were used in the studies described in this example:
Mice. Experimental groups of TgCRND8 mice [12, 13] on a C3H/B6 outbred
background were initially
treated with either epi- or scyllo-cyclohexanehexol 30 mg/day. This initial
dosage was chosen based upon
the dosage of myo-cyclohexanehexol (6-18 grams/day/adult or 86-257mg/Kg/day)
that is typically
administered to human patients for various psychiatric disorders [35]. In
these dosages, myo-
cyclohexanehexol had no toxicity in humans or animals. The studies described
herein were repeated using
doses of 5mg/Kg/day-100mg/Kg/day, and these alternate doses have generated the
same results (data not
shown). A cohort of animals (n=10 mice per treatment arm) entered the study at
five months of age, and
outcomes were then analyzed after one month of treatment. The body weight,
coat characteristics and in
cage behaviour were monitored. Mannitol was used as a negative control for
potential alterations in
caloric intake. All experiments were performed according to the Canadian
Council on Animal Care
guidelines.
Behavioural tests: Morris Water Maze testing was performed as previously
described [ 14]. After non-
spatial pre-training, mice underwent place discrimination training for 5 days
with 4-trials per day,
36
CA 02579188 2007-02-19
followed by a cued visible platform to rule out general motivational, learning
deficits and motor problems,
and a probe trial to evaluate memory. Data were subjected to a mixed model of
repeated measures
analysis of variance (ANOVA) with treatment (untreated, epi- or scyllo-
cyclohexanehexol) and genotype
(TgCRND8 versus non-Tg) as 'between-subject' factors. Open field test for
motor activity was preformed
as described previously [28]. Duration of walking, pausing and grooming were
analyzed as indices of
spontaneous locomotor activity. Sensorimotor function was examined with an
EconomexT"1 accelerating
rotarod (Columbus Instruments, Columbus, OH), as described elsewhere [29]. The
rod was set to
accelerate at a rate of 0.2 r.p.m./s, from an initial, constant speed of 5
r.p.m.. Latency to fall was recorded
in four daily trials, conducted at 30 min intervals. All mice were trained for
seven days before testing. The
test day performance score for each animal was obtained by summing its latency
to fall over the four trials
Cerebral amyloid burden. Brains were removed and one hemisphere was fixed in
4% paraformaldehyde
and embedded in paraffin wax in the mid sagittal plane. To generate sets of
systematic uniform random
sections, 5 m serial sections were collected across the entire hemisphere.
Sets of sections at 50 gm
intervals were used for analyses (10-14 sections/set). Plaques were identified
after antigen retrieval with
formic acid, and incubation with primary anti-A(3 antibody (Dako M-0872),
followed by secondary
antibody (Dako StreptABCcomplex/horseradish kit). End products were visualized
with DAB and were
counter-stained with luxol fast blue. Amyloid plaque burden was assessed with
Leco IA-3001 image
analysis software interfaced with Leica microscope and Hitachi KP-M1U CCD
video camera. Openlab
imaging software (Improvision, Lexington, MA) was then used to convert
micrographs to binary images
for plaque number and plaque area determinations. Vascular amyloid burden was
defined as amyloid
originating from or surrounding blood vessels and was analysed similarly.
Plasma and Cerebral A(3 Content. Hemi-brain samples were homogenized in a
buffered sucrose
solution, followed by either 0.4% diethylamine/ 100mM NaCl for soluble A(3
levels or cold formic acid for
the isolation of total A(3. After neutralization, the samples were diluted and
analyzed for A(340 and A(342
using commercially available kits (BIOSOURCE International). Each hemisphere
was analyzed in
triplicate and the mean values SEM reported. Western blot analyses were
performed on all fractions
using urea gels for A[3 species analyses [30]. A[3 was detected using 6E10
(BIOSOURCE International)
and Enhanced Chemiluminenscence (Amersham).
Gliosis Quantitation. Five randomly selected, evenly spaced, sagittal sections
were collected from
paraformaldehyde-fixed and frozen hemispheres of treated and control mice.
Sections were
immunolabelled for astrocytes with anti-rat GFAP IgG2a (Dako; diluted 1:50)
and for microglia with anti-
rat CD68 IgG2b (Dako; 1:50). Digital images were captured using a Coolsnap
digital camera
(Photometrics, Tuscon, Arizona) mounted to a Zeiss, Axioscope 2 Plus
microscope. Images were
analysed using Openlab 3.08 imaging software (Improvision, Lexington MA).
Survival Census: The probability of survival was assessed by the Kaplan-Meier
technique [31],
computing the probability of survival at every occurrence of death, thus
making it suitable for small
37
CA 02579188 2007-02-19
sample sizes. For the analyses of survival, 35 mice were used for each
treatment group. The Tarone-
Ware test was used to assess effects of treatments.
Analysis of APP in brain. Mouse hemi-brain samples were homogenized and spun
at 109,000 x g, in
20mM Tris pH7.4, 0.25M sucrose, 1mM EDTA and 1 mM EGTA, and a protease
inhibitor cocktail, mixed
with 0.4%DEA (diethylamine)/100mM NaCI. The supernatants were analysed for
APPs levels by Western
blotting using mAb 22C11, while the pellets were analysed for APP holoprotein
with mAb C1/6.1 as
previously described [12,13].
Soluble A(3 oligomer Analyses. The levels of soluble A(3 oligomers were
measured by a dot blot assay
with anti-oligomer specific antibodies [16]. Briefly, oligomers were
solubilised from one hemi-brain in
PBS in the presence of protease inhibitor cocktail (Sigma). After
centrifugation at 78,500 x g for 1 hr at
4 C, the supernatants were analysed. Protein content was determined by the BCA
protein assay (Pierce).
Two g of total protein was spotted onto nitrocellulose, blocked with 10% non-
fat milk in TBS before
incubation with the biotinylated oligomeric specific antibody. Blots were
incubated with streptavidin-
HRP and ECL chemiluminescence kit. Soluble and fibrillar A(342 were used as
negative controls and
synthetic oligomeric A(342 was used as a positive control [20]. Control
samples were re-identified after
oligomeric antibody was stripped and re-probing with the anti-A(3 antibody
6E10.
Long Term Potentiation. Field potentials were recorded in CA1 of mouse
hippocampus by standard
procedures [39, 40]. Swiss Webster mice between the ages of P16 and P26 were
anesthetized with
isoflurane. The brain was rapidly removed and placed in ice cold oxygenated
sucrose-CSF containing (in
mM): 248 sucrose, 2 KCI, 2 MgSO4, 1.24 NaH2PO4, 1 CaC12, 1 MgClz, 26 NaHCO3,
10 D-glucose, pH
7.4, -315 mOsmol [41]. The hippocampus from each hemisphere was isolated and
350 m coronal
sections were made. The slices were transferred to a holding chamber
containing NaCI-CSF (in mM: 124
NaCI, 2 KCI, 2 MgSO4, 1.25 NaH2PO4, 2 CaC12, 26 NaHCO3, 10 D-glucose, pH 7.4, -
310 mOsmol) and
allowed to recover for more than 1 hour. Once placed in the chamber, slices
were continuously perfused
by a closed loop containing 15 ml of ACSF to conserve the oligomeric A(3.
After 20 minutes of stable
baseline, 1 ml of 15x concentrated 7PA2 conditioned medium 1.25 M scyllo-
cyclohexanehexol was
added to the perfusion loop. A bipolar stimulating electrode (World Precision
Inst.) was placed in the
Schaffer collaterals to deliver baseline stimuli and tetani. A borosilicate
glass recording electrode (2-4
MS2) containing ACSF was positioned approximately 75 - 200 m from the
stimulating electrode. The
intensity of the stimulus (typically between 10-20 Amps) was set to obtain 25-
40% of the maximal field
potential response. Test stimuli were delivered at 0.05Hz. To induce LTP, 4
tetani (100 Hz for I second)
were delivered 5 minutes apart. Field potential responses were amplified I Ox
using an Axopatch 200B.
The data was sampled at 10 kHz and filtered at 2kHz. Traces were analyzed
using pClamp 9.2. The slope
of the field potential was estimated using approximately 10-60% of the total
response.
Synaptophysin Quantification.
Synaptophysin immunohistochemical staining was performed on 3 evenly spaced
saggital
sections of paraformaldehyde-fixed treated and control mice. Sections were
immunolabelled for
38
CA 02579188 2007-02-19
synaptophysin with anti-synaptophysin IgG (1:40; Roche, Laval, PQ). Digital
images were captured and
analyzed as described above. Within each section, three randomly chosen 100
m2 areas of the CA1
region of the hippocampus were counted for synaptophysin reactive cell bodies
and boutons. The results
are expressed as the mean of the number of reactive bodies and boutons per 100
mZ [33, 34].
Results
To assess their effectiveness in vivo, inositol compounds were administered to
a robust murine
model of Alzheimer's disease (TgCRND8) [12,13]. TgCRND8 mice express a human
amyloid precursor
protein transgene (APP695) bearing two missense mutations that cause AD in
humans (KM670/671NL and
V717F). At about three months of age, the mice display progressive spatial
learning deficits that are
accompanied both by rising cerebral A(3 levels and by increasing numbers of
cerebral extracellular
amyloid plaques [ 12]. By six months of age, the levels of A(3 and the
morphology, density and distribution
of the amyloid plaques in the brain of TgCRND8 mice are similar to those seen
in the brains of humans
with well-established AD [13]. As in human patients with AD, the biochemical,
behavioural and
neuropathological features of the mouse model are accompanied by accelerated
mortality [12, 13].
The TgCRND8 mice and non-transgenic littermates were assigned to sex- and age-
matched
cohorts that were then used to test the effectiveness of the cyclohexanehexol
stereoisomers as a
therapeutic (with treatment delayed until five months of age and continued for
one month until six months
of age). The mice were randomly assigned to receive active compound
(1,2,3,4,5/6- (epi-)
cyclohexanehexol or 1,3,5/2,4,6- (scyllo-) cyclohexanehexol administered
orally), mock therapy
(mannitol), or no therapy. The endpoints were cognitive function, brain
A(3levels, and neuropathology.
1,2,3,5/4,6-(myo-) cyclohexanehexol was not included in these studies because
prior in vitro studies [11]
had indicated that myo-cyclohexanehexol was only weakly effective, and because
pilot in vivo studies
showed no significant benefit (data not shown). Over the course of these
experiments, observers were
unaware of genotype or treatment group.
Cyclohexanehexol Stereoisomers Reverse Established Cerebral Amyloid Deposition
Most AD patients will seek treatment only after they have become symptomatic,
i.e., at a time
when A(3 oligomerization, deposition, toxicity and plaque formation are
already well advanced. To assess
whether cyclohexanehexol stereoisomers could abrogate a well-established AD-
like phenotype, the start
of treatment of the TgCRND8 mice was delayed until five months of age. At this
age, TgCRND8 mice
have significant behavioural deficits, accompanied by profuse A(3 peptide and
plaque burdens [12].
Cohorts of TgCRND8 and non-Tg littermates (10 mice per cohort) were either
treated for 28 days with
epi-cyclohexanehexol or with scyllo-cyclohexanehexol, or were left untreated.
The dosage and oral
administration of compounds, and the neurochemical and neuropathological
assays used for these
experiments were the same as those employed in the initial prophylactic
experiments. Mortality curves
were not generated for this cohort of animals because the brevity of the trial
resulted in too few deaths in
the untreated TgCRND8 mice to generate meaningful data.
39
CA 02579188 2007-02-19
Spatial learning in these mice was compared between six month old TgCRND8 mice
that had
been treated with epi-cyclohexanehexol or with scyllo-cyclohexanehexol or that
were untreated for 28
days. The performance of six month old TgCRND8 mice that had been treated with
epi-cyclohexanehexol
for 28 days was not significantly different from that of untreated TgCRND8
littermates (FI,15=3.02;
p=0.27; Figure 1A), and was significantly poorer than the performance of their
non-Tg littermates
(FI,14=11.7, p=0.004; Figure 1C). Furthermore, the probe trial confirmed that
epi-cyclohexanehexol
treated TgCRND8 mice were not statistically different from untreated TgCRND8
mice (p=0.52; Figure
1 E). Epi-cyclohexanehexol had no significant impact on brain A(340 or A(342
levels, percent area of the
brain covered with plaques, or plaque number in animals with pre-existing
disease (Table 1).
The 28-day treatment of five month old TgCRND8 mice with scyllo-
cyclohexanehexol resulted in
significantly better behavioural performance compared to untreated TgCRND8
mice (p=0.01). Indeed,
the cognitive performance of these scyllo-cyclohexanehexol-treated TgCRND8
mice was
indistinguishable from that of their non-Tg littermates (F1,13=2.9, p=0.11;
Figure 1B, D). This beneficial
effect of cyclohexanehexol treatment was not due to non-specific effects on
behavioural, motor, or
perceptual systems because cyclohexanehexol treatment had no effect on the
cognitive performance of
non-Tg mice (F2119=0.98; p=0.39). In the probe trial, the annulus-crossing
index showed a significant
improvement in memory for scyllo-cyclohexanehexol treated TgCRND8 mice that
was not statistically
different from non-Tg littermates (p=0.64; Figure 1E). In a separate cohort of
mice and using % time in
target quadrant as an alternate measure, scyllo-cyclohexanehexol treated
TgCRND8 mice were not
statistically different from non-Tg littermates (p=0.28; data not shown). The
beneficial effects of scyllo-
cyclohexanehexol were not due to alteration of sensorimotor behaviour. Scyllo-
cyclohexanehexol had no
effect on grooming or activity of TgCRND8 mice in comparison to both untreated
TgCRND8 mice
(FI,9=0.25; p=0.63) and non-Tg littermates (FI,12=0.02; p=0.89) in the open
field test (supplemental data).
Similarly, Rotarod testing revealed no difference between scyllo-
cyclohexanehexol treated and untreated
TgCRND8 mice (p=0.42) or between treated TgCRND8 and treated or untreated non-
Tg littermates
(p=0.79) in sensorimotor function. In agreement with the results of the
prophylactic study, a 28 day
course of scyllo-cyclohexanehexol at 5 months of age also: 1) reduced brain
levels of A(340 and A(342
(e.g. insoluble A(340 = 29t2.3% reduction, p<0.05; insoluble A(342 = 23 1.4%
reduction, p<0.05); and
2) significantly reduced plaque number, plaque size, and percent area of the
brain covered in plaques
(plaque number =13 0.3% reduction, p<0.05; plaque size =16f0.4% reduction, p
= 0.05; percent area of
the brain covered by plaques = 14 0.5% reduction, p<0.05; Table 1; Figure 1F-
G). These results are
comparable in effect to those of the six month prophylactic studies.
In sum the data show that scyllo-cyclohexanehexol, and to a lesser degree, epi-
cyclohexanehexol,
can prevent and reverse the AD-like phenotype in TgCRND8 mice, reducing
cognitive deficits, amyloid
plaques, amyloid angiopathy, A(3-induced inflammatory response, and
accelerated mortality. These
effects are likely direct effects of the compounds within the CNS because: 1)
the compounds are
transported across the blood brain barrier by facilitated transport [17, 19];
and 2) their presence can be
CA 02579188 2007-02-19
demonstrated in the brain tissue of treated mice by gas chromatography-mass
spectrometry [20] (data not
shown).
There was no change in the levels of APP holoprotein, APP glycosylation, APPs-
a or APPs-(3, or
A(3 speciation (i.e. A(31-3 8 levels) in brain homogenates from treated and
untreated TgCRND8 mice (data
not shown). Similarly, the peripheral distribution of A(3 as measured by
plasma A(342 levels were not
different between treated and untreated TgCRND8 mice. Plasma A(342 levels in
the cohort of TgCRND8
mice following 28 days of cyclohexanehexol therapy at five months of age were:
untreated = 1144 76
pg/ml; epi-cyclohexanehexol = 1079 79 pg/ml; scyllo-cyclohexanehexol = 990 f
73 pg/ml; p=0.87. The
absence of alterations in peripheral/plasma A(342 may be relevant because
plasma A(3 levels were also
unchanged in patients who developed a strong antibody response and an apparent
clinical improvement
following A(3 immuno-therapy [25].
To directly address the possibility that the cyclohexanehexol stereoisomers
inhibit A(3
oligomerization in the brain, an activity that they clearly have in vitro [10,
11], a dot blot immunoassay
[ 16] was used to measure levels of A(3 oligomers in the brains of treated and
untreated TgCRND8 mice.
This assay employs an antibody that selectively identifies oligomeric A(3
species [16]. The levels of
soluble A(3 oligomers were significantly reduced in the brain of treated mice,
and these reductions were
commensurate with the degree of behavioural and neuropathological improvements
induced by these
compounds (Figure 2A). A(3 oligomers were not significantly reduced after one-
month treatment with epi-
cyclohexanehexol in the five month old TgCRND8 mice with existing pathology
(56 4 pixels in
untreated TgCRND8 versus 47 f 2 pixels in epi-cyclohexanehexol treated
TgCRND8, p=0.12). Delayed
28-day treatment with scyllo-cyclohexanehexol at five months of age also
caused a 30% reduction in
soluble A(3 oligomers (63 3 pixels in untreated TgCRND8 versus 45 2 in
scyllo-cyclohexanehexol
treated TgCRND8, p=0.008). The dot blots were negative for cross-reactivity to
tau, a-synuclein and
tubulin, demonstrating specificity of the antibody for A(3 in the TgCRND8
brain homogenates. These
results directly demonstrate that scyllo-cyclohexanehexol, but not epi-
cyclohexanehexol, decreases the
amount of soluble A(3 oligomers in the brain.
To address the possibility that scyllo-cyclohexanehexol inhibits A[3 oligomer-
induced
neurotoxicity, its effects were determined on both long term potentiation
(LTP) in mouse hippocampal
slices and on synaptic density as measured by the level of synaptophysin
immunoreactivity in the brains
of TgCRND8 mice. Hippocampal LTP is a measure of synaptic plasticity, and has
been shown to be
disrupted by natural cell-derived oligomeric A(3 species [42]. As previously
reported in rat [42, 43],
soluble A(3 oligomers secreted into the conditioned media of CHO cells stably
transfected with human
APPV717F (7PA2 cells) inhibited LTP in wild-type mouse hippocampal slices
(Figure 2B). However,
when the 7PA2-conditioned medium was pretreated in vitro with scyllo-
cyclohexanehexol, there was a
significant recovery of LTP compared with 7PA2-conditioned media alone
(p=0.003; Figure 2B). Scyllo-
cyclohexanehexol had no direct effect on LTP as scyllo-cyclohexanehexol
treated culture media from
41
CA 02579188 2007-02-19
plain CHO cells that were not transfected with human APP (Figure 2C) and
untreated culture media from
these cells were indistinguishable from scyllo-cyclohexanehexol treated 7PA2
culture media, i.e., all three
samples allowed LTP. The LTP effects were not a result of altered baseline
transmission, since scyllo-
cyclohexanehexol did not change synaptic response in the absence of a
potentiating tetanus (data not
shown). In order to correlate this protection of LTP in slice cultures with in
vivo effects on synaptic
function, the level of synaptophysin immunoreactivity was measured in the CAl
region of the
hippocampus in scyllo-cyclohexanehexol-treated and untreated TgCRND8 mice.
Synaptophysin
immunoreactivity is a measure of synaptic density, which is correlated to
synaptic function. The levels of
synaptophysin were significantly increased. Thus, scyllo-cyclohexanehexol
increased the number of
synaptophysin reactive boutons and cell bodies in the CA1 region of the
hippocampus by 148% for a
prophylactic study group (1610 176/100 m2 in untreated TgCRND8 mice versus
2384 232/100 m2
in scyllo-cyclohexanehexol treated TgCRND8 mice; p=0.03) and by 150% for the
delayed treatment study
(1750 84/100 gmZ in untreated versus 2625 124/100 m2 in scyllo-
cyclohexanehexol treated
TgCRND8 mice; p<0.001). Together, the results of the LTP and synaptophysin
studies suggest that in the
brain, scyllo-cyclohexanehexol may restore the inhibition of LTP induced by
naturally secreted human A(3
oligomers, and allow maintenance of synaptic function.
Scyllo-inositol was also administered to TgCRND8 mice for 2 months, ending at
7 months of age.
Sustained effects both on cognition and pathology were observed in these
treated animals.
Example 2
Cyclohexanehexol-based inhibitors of Ab-aggregation prevent and reverse
Alzheimer-like features
in a transgenic model of Alzheimer Disease.
SUMMARY
When given orally to a transgenic mouse model of Alzheimer disease,
cyclohexanehexol
stereoisomers inhibit aggregation of amyloid 0 peptide (A(3) into high-
molecular-weight oligomers in the
brain and ameliorate several Alzheimer disease-like phenotypes in these mice,
including impaired
cognition, altered synaptic physiology, cerebral A(3 pathology and accelerated
mortality. These therapeutic
effects, which occur regardless of whether the compounds are given before or
well after the onset of the
Alzheimer disease-like phenotype, support the idea that the accumulation of
A(3 oligomers has a central
role in the pathogenesis of Alzheimer disease.
To assess their effectiveness in vivo, these compounds were administered to a
robust transgenic
mouse model of Alzheimer disease [ 12] (TgCRND8). This model expresses a human
amyloid precursor
protein transgene (APP695) bearing missense mutations that cause Alzheimer
disease in humans
(KM670/671NL and V717F). At about 3 months of age, these mice have progressive
spatial learning
deficits that are accompanied by rising cerebral A[3 levels and by increasing
numbers of cerebral amyloid
plaques [12]. By 6 months of age, the levels of A(3 and the morphology,
density and distribution of
amyloid plaques are similar to those seen in brains of people with well-
established Alzheimer disease
42
CA 02579188 2007-02-19
[12]. As in humans with Alzheimer disease, these biochemical, behavioral and
neuropathological
phenotypes are accompanied by accelerated mortality [12, 13].
TgCRND8 mice and nontransgenic littermates were assigned to sex and age-
matched cohorts that
were used to test the effectiveness of cyclohexanehexol stereoisomers in two
different treatment
paradigms. In the first paradigm, cyclohexanehexols were orally administered
prophylactically, with
treatment beginning at 6 weeks of age (that is, about 6 weeks before onset of
phenotype) and continuing
until either 4 or 6 months of age. In the second paradigm, compounds were
given therapeutically
beginning at 5 months of age (when the Alzheimer disease-like phenotype is
already well established),
and continuing until 6 months of age. Within each of these experimental arms,
mice were randomly
assigned to receive active compound (1,2,3,4,5/6-(epi) cyclohexanehexol or
1,3,5/2,4,6- (scyllo-)
cyclohexanehexol), mock therapy (mannitol, a sugar of similar molecular
weight) or no therapy. The
endpoints of these studies were: cognitive function (as measured by spatial
reference learning in the
Morris water maze test [13, 14], brain A(3 levels, neuropathology and
mortality. 1,2,3,5/4,6-(myo-)
cyclohexanehexol was not included in these studies because prior in vitro
studies [ 11 ] indicated that myo-
cyclohexanehexol was only weakly effective, and because pilot in vivo studies
showed no significant
benefit (data not shown).
METHODS
The following methods were used in the study:
Mice. Experimental groups of TgCRND8 mice on a C3H/B6 outbred background were
initially treated
with either epi- or scyllo-cyclohexanehexol. Two cohorts (n = 10 mice in each
treatment arm) entered the
study at 6 weeks of age, and outcomes were analyzed at 4 and 6 months of age.
A third cohort of mice (n
= 10 mice per treatment arm) entered the study at 5 months of age, and
outcomes were analyzed after I
month of treatment. Dose-ranging studies of 0.3 mg/kg/d to 30 mg/d were
performed by oral gavage twice
daily in mice up to 4 months of age. All experiments were performed according
to the Canadian Council
on Animal Care guidelines.
Behavioral tests. Morris water maze testing was carried out as previously
described [131. After nonspatial
pretraining, mice underwent place discrimination training for 5 d with four
trials per day, followed by a
cued visible platform trial and a probe trial. An open-field test for motor
activity was carried out as
described previously [28]. Sensorimotor function was assessed with an Economex
accelerating rotarod
(Columbus Instruments) as previously described [29].
Cerebral A(3 burden. Brains were removed and fixed one hemisphere from each in
4% paraformaldehyde
and embedded in paraffin wax in the midsagittal plane. Sets of sections at 50-
mm intervals for analyses
(10-14 sections per set) were used. Plaques with primary A(3-specific antibody
(Dako M-0872) were
identified and visualized with DAB. A(3 plaque burden was assessed with
Openlab imaging software
(Improvision) and used to convert micrographs to binary images for
determinations of plaque number and
plaque area. Vascular A(3 burden was defined as A(3 plaques originating from
or surrounding blood vessels
and was analyzed similarly.
43
CA 02579188 2007-02-19
Plasma and cerebral Ap content. Hemi-brain samples were homogenized in a
buffered sucrose solution,
followed by either a mixture of 0.4% diethylamine and 100 mM NaC1 for soluble
A(3 levels or cold formic
acid for the isolation of total A(3. After neutralization, samples were
diluted and analyzed for A040 and
A042 using commercially available kits (Biosource International). Each
hemisphere was analyzed in
triplicate and the mean s.e.m. was reported. Western blot analyses were
carried out on all fractions using
urea gels for A(3 species analyses [30]. A(3 was detected using 6E10
(Biosource International).
Quantification of gliosis. Five randomly selected, evenly spaced sagittal
sections from
paraformaldehyde-fixed and frozen hemispheres of treated and control mice were
collected. Sections were
immunolabeled for astrocytes with an antibody against rat GFAP IgG2a (Dako;
diluted 1:50) and for
microglia with an antibody to rat CD68 IgG2b (Dako; 1:50). Digital images were
captured using a
Coolsnap digital camera (Photometrics) mounted to a Zeiss, Axioscope 2 Plus
microscope. Images were
analyzed using Openlab 3.08 imaging software (Improvision).
Survival census. The probability of survival was assessed using the Kaplan-
Meier technique [31 ],
computing the probability of survival at every occurrence of death, thus
making it suitable for small
sample sizes. For the analyses of survival, 3 5 mice were used for each
treatment group. The Tarone-Ware
test was used to assess effects of treatments.
Analysis of APP processing. Mouse hemi-brain samples were homogenized and spun
at 109,000g, in 20
mM Tris pH 7.4, 0.25 M sucrose, 1 mM EDTA and 1 mM EGTA, and a protease
inhibitor cocktail, mixed
with a mixture of 0.4% DEA (diethylamine) and 100 mMNaCI. The supernatants
were analyzed for APP
levels by western blotting using the monoclonal antibody 22C 11, and the
pellets were analyzed for APP
holoprotein with the monoclonal antibody C116.1 as previously described [32].
After swAPP stable
HEK293 cells were treated (or untreated) with scyllo-cyclohexanehexol or 10 nM
compound E, a y-
secretase inhibitor, conditioned media was collected for A[3 assay by ELISA,
and cell membranes were
used for detection of full length APP, the C-terminal fragment of APP and
generation of c-stubs in vitro
(that is, incubation at 37 C for 1 h). The target protein was probed with
polyclonal APP C-terminal
fragment-specific antibody (Sigma) by western blotting [32].
Soluble A(3 oligomer analyses. Levels of soluble A(3 oligomers were measured
by a dot-blot assay with
oligomer-specific antibodies on all brain homogenates from all experimental
groups [25]. Soluble
oligomers were also examined by western blot analyses.
Synaptophysin quantification. Immunohistochemical staining was carried out for
synaptophysin on
three evenly spaced sagittal sections of paraformaldehyde-fixed treated and
control mice. Sections were
immunolabeled for synaptophysin with synaptophysin-specific IgG (1:40; Roche).
Digital images were
captured and analyzed as described above. Within each section, three randomly
chosen 100 mZ areas of
the CA 1 region of the hippocampus were counted for synaptophysin-reactive
cell bodies and boutons. The
results are expressed as the mean of the number of reactive bodies and boutons
per 100 m2 [33, 34].
Supplementary methods:
44
CA 02579188 2007-02-19
Mice. Experimental groups of TgCRND8 mice on a C3H/B6 outbred background were
initially treated
with either epi- or scyllo-cyclohexanehexo130 mg/day. This initial dosage was
chosen based upon the
dosage of myo-cyclohexanehexol (6-18 grams/day/adult or 86 - 257 mg/kg/day)
that is typically
administered to human patients for various psychiatric disorders [35]. In
these dosages, myo-
cyclohexanehexol had no toxicity in humans or animals. The studies described
here were also repeated
using doses of 5 mg/kg/day - 30 mg/day. Two cohorts (n=10 mice in each
treatment arm) entered the
study at six weeks of age and outcomes were analyzed at four and six months of
age. A third cohort of
animals (n=10 mice per treatment arm) entered the study at five months of age,
and outcomes were then
analyzed after one month of treatment. The body weight, coat characteristics
and in-cage behaviour was
monitored. Mannitol was used as a negative control for potential alterations
in caloric intake. All
experiments were performed according to the Canadian Council on Animal Care
guidelines and approved
by the Animal Care Committee, University of Toronto.
Behavioural tests. Morris Water Maze testing was performed as previously
described [13]. After non-
spatial pre-training, mice underwent place discrimination training for 5 days
with 4-trials per day,
followed by a cued visible platform to rule out general motivational, learning
deficits and motor problems,
and a probe trial to evaluate memory. The probe trial was conducted at 72 h
after the last testing, and used
an annulus-crossing index to assess spatial recall. The annulus-crossing index
measures the number of
passes over the original platform position relative to passes over the other
three quadrants. Data were
subjected to a repeated measures analysis of variance (ANOVA) with treatment
(untreated, epi- or scyllo-
cyclohexanehexol) and genotype (TgCRND8 versus non-Tg) as 'between-subject'
factors. Open field test
for motor activity was performed as described previously [28]. Duration of
walking, pausing and
grooming were analyzed as indices of spontaneous locomotor activity.
Sensorimotor function was
examined with an EconomexTM accelerating rotarod (Columbus Instruments,
Columbus, OH), as
described elsewhere [29]. The rod was set to accelerate at a rate of 0.2
r.p.m./s, from an initial, constant
speed of 5 r.p.m. Latency to fall was recorded in four daily trials, conducted
at 30 min intervals. All mice
were trained for seven days before testing. The test day performance score for
each animal was obtained
by summing its latency to fall over the four trials.
Cerebral A(i burden. Brains were removed and one hemisphere was fixed in 4%
paraformaldehyde and
embedded in paraffin wax in the mid sagittal plane. To generate sets of
systematic uniform random
sections, 5 m serial sections were collected across the entire hemisphere.
Sets of sections at 50 m
intervals were used for analyses (10-14 sections/set). Plaques were identified
after antigen retrieval with
formic acid, and incubation with primary anti-A(3 antibody (Dako M-0872),
followed by secondary
antibody (Dako StreptABCcomplex/horseradish kit). End products were visualized
with DAB and were
counter-stained with luxol fast blue. A(3 plaque burden was assessed with Leco
IA-3001 image analysis
software interfaced with Leica microscope and Hitachi KP-M1U CCD video camera.
Openlab imaging
software (Improvision, Lexington, MA) was then used to convert micrographs to
binary images for plaque
CA 02579188 2007-02-19
number and plaque area determinations. Vascular A(3 burden was defined as A[3
plaques originating from
or surrounding blood vessels and was analyzed similarly.
Soluble A[3 oligomer Analyses. The levels of soluble A[3 oligomers were
measured by a dot blot assay
with anti-oligomer specific antibodies on all brain homogenates from all
experimental groups [16].
Oligomers were solubilised from one hemi-brain in PBS in the presence of
protease inhibitor cocktail
(Sigma). After centrifugation at 78,500 x g for 1 h at 4 C, the supernatants
were analyzed. Protein
content was determined by the BCA protein assay (Pierce). Two g of total
protein was spotted onto
nitrocellulose, blocked with 10 % non-fat milk in TBS before incubation with
the biotinylated oligomeric
specific antibody. Blots were incubated with streptavidin-HRP and ECL
chemiluminescence kit. Soluble
and fibrillar A(342 were used as negative controls and synthetic oligomeric
A(342 was used as a positive
control. Control samples were re-identified after oligomeric antibody was
stripped and re-probing with the
anti-A(3 antibody 6E10.
Right hemispheres from 4 month old CRND8 Tg mice treated or untreated with
scyllo-
cyclohexanehexol were sonicated in 10 vol/wet weight Tris buffered saline
(TBS; 20 mM Tris [pH 7.3],
140 mM NaCI containing a protease inhibitor cocktail). Samples were
centrifuged (100, 000g, 20 min,
4 C), supernatant collected and frozen at -80 C until use. Proteins (20 g)
were separated by SDS-PAGE
on a 10-20 % Tris-Tricine gel, transferred to a nitrocellulose membrane,
blocked for 1 h at room temp
with 8 % non-fat milk and incubated overnight with anti-A(3 (6E10; 1:2,500).
Membranes were rinsed
with TBST, exposed to anti-mouse (1:5,000) for 1 h at room temperature, washed
with TBST (6 x 10 min)
and developed using enhanced chemiluminescence. Blots were stripped and re-
probed with either anti-
APP (22C 11; 1:1,000) or anti-GAPDH (1:10, 000) to confirm equal protein
loading.
RESULTS
Cyclohexanehexols prevent Alzheimer disease-like phenotype. In the
prophylactic trial, TgCRND8
mice were treated with cyclohexanehexol stereoisomers (Figure 15) from 6 weeks
of age until either 4 or
6 months of age. In the cohort examined at 4 months of age, TgCRND8 mice
treated with either epi-
cyclohexanehexol or scyllo-cyclohexanehexol had significant behavioral
improvement compared to
untreated TgCRND8 mice (treatment effect, P < 0.05; training day effects, P <
0.0001; Figure 3, Figure 10
a, b and Table 3). Scyllo-cyclohexanehexol-treated TgCRND8 mice were
indistinguishable from
nontransgenic littermates on days 4 and 5 of testing (treatment effect, P =
0.97; Figure 10 b). These results
were confirmed in a second cohort treated from 6 weeks until 6 months of age.
Cyclohexanehexol-treated
TgCRND8 mice again had significantly better cognitive function compared to
untreated TgCRND8
littermates (P < 0.02; Figure 10 c, d), and their performance was
indistinguishable from nontransgenic
littermates (treatment effects, P = 0.14, Figure 10c and P = 0.84, Figure lOd;
epi- and scyllo-
cyclohexanehexol, respectively).
This improvement in cognitive function could not be attributed to nonspecific
effects on
cognition: cyclohexanehexol treatment had no effect on the performance of
nontransgenic mice at any age
(treatment effect, P = 0.26 and 0.45, respectively; Figure 10 and Table 3).
The improved performance
46
CA 02579188 2007-02-19
could also not be attributed to nutritional or caloric effects because body
weight, activity and coat
condition were not different between treated and untreated cohorts.
Furthermore, treatment with mannitol
had no effect on behavior (P = 0.84). Gender effects were not significantly
different between any
treatment groups (P = 0.85, Figure 5, Figure 16). Visual acuity was not
altered by treatment; all cohorts of
mice performed equally well in a cue test (P = 0.78).
These behavioral improvements were accompanied by improvements in other
Alzheimer disease-
like phenotypes including reductions in brain Ap levels, brain amyloid
pathology, synaptic loss, glial
inflammatory reaction and reductions in mortality. Like the behavioral
improvements described above,
however, these changes showed stereoisomer-specific differences, with scyllo-
cyclohexanehexol inducing
a larger and more sustained effect. Thus, whereas prophylactic treatment with
epi-cyclohexanehexol
reduced both A(340 and A042 at 4 months of age (22-62% reduction; P = 0.02;
Table 2), by 6 months of
age, the concentrations of soluble A(340, insoluble A040 and soluble A042 rose
to levels equivalent to
those observed in untreated TgCRND8 mice (Table 2). In contrast, prophylactic
treatment with scyllo-
cyclohexanehexol caused a sustained decrease in brain concentrations of both
A040 and A(342 at both 4
and 6 months of age (20-44% reduction; P = 0.04 and P = 0.02, respectively;
Table 2).
The cyclohexanehexol stereoisomer-induced changes in brain A(3 were
accompanied by similar
reductions in Alzheimer disease-like neuropathology (plaque burden,
angiopathy, glial reaction and
synaptic loss; Table 2 and Figure 4, Figure 8 c, Figure 11). Compared with
untreated TgCRND8 mice,
TgCRND8 mice treated prophylactically with epi-cyclohexanehexol showed a
significant decrease in
mean plaque size at 4 months (P = 0.04), but reductions were not sustained at
6 months of age. In contrast,
but in agreement with behavioral data, prophylactic treatment with scyllo-
cyclohexanehexol caused a
sustained decrease in all measures of plaque burden at 4 and 6 months of age
(P < 0.001; Table 2). Similar
differential effects were observed on vascular Ap deposits (epi-
cyclohexanehexol, no change, P = 0.87;
scyllo-cyclohexanehexol, reduced brain area covered by cerebrovascular A(3, P
= 0.05; reduced mean size
of cerebrovascular deposits, P = 0.008; Figure 11 a).
Astroglial and microglial reactions are a neuropathological feature of both
human Alzheimer
disease and of transgenic models of Alzheimer disease. Epi-cyclohexanehexol
decreased the astrogliotic
response at 4 months of age (P = 0.002) and at 6 months of age (P = 0.04;
Figure 11 b), but had no effect
on microglial activation (P = 0.74 at 6 months of age). In contrast, scyllo-
cyclohexanehexol decreased
astrogliosis much more efficiently (4 months of age, P < 0.0001; 6 months of
age, P = 0.006), and also
attenuated microglial activation (P < 0.001; Figure 11 c). This effect is a
specific consequence of
inhibition of A(3 aggregation, because scyllo-cyclohexanehexol treatment did
not prevent the astrogliosis
observed in a transgenic TauP301 L mouse model of frontotemporal dementia
(Figure 11 b).
Like people with Alzheimer disease, TgCRND8 mice have accelerated mortality,
and only 50% of
untreated TgCRND8 mice survive to 6 months of age (Figure 11 d). This
accelerated mortality was
reversed by prophylactic treatment with scyllo-cyclohexanehexol (P = 0.02),
but not epi-
47
CA 02579188 2007-02-19
cyclohexanehexol (P = 0.34) or mannitol (P = 0.89, Figure 16). Scyllo-
cyclohexanehexol had no effect on
survival, weight, fur condition or cage behavior of wild-type mice.
Synaptic pruning is a feature of both human Alzheimer disease and of the
Alzheimer disease-like
illness in many transgenic models. This presumably reflects the chronic
synaptotoxic effects of A(3
oligomers, and probably represents a morphological correlate of the cognitive
impairment in these models.
Measurement of synaptophysin levels in the CA 1 region of the hippocampus
showed that scyllo-
cyclohexanehexol increased the number of synaptophysin-reactive boutons and
cell bodies by 148% in the
6-month prophylactic study (P = 0.03, Figure 11 e). Again, this effect is
likely to arise specifically from its
ability to block A[3 oligomer-induced synaptic toxicity, because
scyllocyclohexanehexol had no effect on
synaptic loss in the transgenic TauP30IL mouse model (P = 0.89, Figure 11 e).
Cyclohexanehexols reverse established disease
To assess whether cyclohexanehexols could abrogate a well-established
Alzheimer disease-like
phenotype, the start of treatment in TgCRND8 mice was delayed until 5 months
of age. At this age,
TgCRND8 mice have considerable behavioral deficits, accompanied by profuse A[3
peptide and plaque
burdens [ 12]. Cohorts of TgCRND8 and nontransgenic littermates were either
treated for 28 d with epi-
cyclohexanehexol or scyllo-cyclohexanehexol, or left untreated. The dosage and
oral administration of
compounds and the neurochemical and neuropathological assays were as used in
the prophylactic
experiments. Mortality curves were not generated because the short treatment
interval generated too few
events.
As would be expected from the ineffectiveness of epi-cyclohexanehexol at 6
months in the
prophylactic trial, epi-cyclohexanehexol was also ineffective at reversing
behavioral deficits in TgCRND8
mice when given from 5 to 6 months of age (spatial learning compared to
untreated TgCRND8, P = 0.27;
compared to nontransgenic littermates, P = 0.004; Figure 12 a; spatial
reference memory on probe trial
using the annulus-crossing index was indistinguishable from untreated TgCRND8
mice, P = 0.52; Figure
12 c). Epi-cyclohexanehexol also had no significant effect on levels of A(340
or A(342 in the brain, percent
area of the brain covered with plaques or plaque number in mice with
preexisting disease (Table 2).
In contrast, but in agreement with results from the prophylactic trial, 28-d
treatment of 5-month-
old TgCRND8 mice with scyllocyclohexanehexol resulted in improved behavioral
performance (treatment
effect compared to untreated TgCRND8 mice, P = 0.01, Table 3) that was
indistinguishable from
nontransgenic littermates (P = 0.11; Figure 7, Figure 12 b, Figure 18).
Spatial reference memory, as
measured by the annulus-crossing index in the probe trial, showed that scyllo-
cyclohexanehexol-treated
TgCRND8 mice were indistinguishable from treated or untreated nontransgenic
littermates (P = 0.64;
Figure 12 c, Table 3). This behavioral improvement was accompanied by reduced
levels of insoluble
A(340 and A(342 in the brain (for example, insoluble A040, P < 0.05; insoluble
A(342, P < 0.05), and
significantly reduced plaque number (P < 0.05), plaque size (P = 0.05) and
percent area of the brain
covered in plaques (P < 0.05; Table 2). These results are comparable in effect
size to those of the 6-month
prophylactic studies. The 28-d cyclohexanehexol treatment had no effect on
cognition or sensorimotor
48
CA 02579188 2007-02-19
behavior of nontransgenic mice (open-field test and rotarod test were
indistinguishable between scyllo-
cyclohexanehexol-treated TgCRND8 mice, untreated TgCRND8 mice (P = 0.63) and
nontransgenic
littermates (P = 0.89; Figure 6, Figure 17)).
There are three non-mutually exclusive mechanisms by which these beneficial
effects might
occur. The first and least likely mechanism is that cyclohexanehexol
stereoisomers may have altered the
expression or proteolytic processing of APP. In vitro assays, however, showed
that scyllo-
cyclohexanehexol had no effect on [3- and y-secretase enzyme activities
(Figure 9); this stereoisomer also
had no effect on the levels of APP holoprotein, APP glycosylation, APPs-a or
APPs-[3 in brain
homogenates of treated TgCRND8 mice.
A second possibility is that cyclohexanehexol stereoisomers may have simply
reduced plasma A(3
levels, thereby creating a'sink effect' that improved efflux of A(3 from the
brain into the plasma. But
plasma A(342 levels were not different between treated and untreated TgCRND8
mice in either the
prophylactic or the delayed-therapy trials (P = 0.89 and P = 0.87,
respectively), arguing against an
enhanced peripheral clearance mechanism.
Finally, in light of inhibition of A(3 aggregation and cytotoxicity by
cyclohexanehexol in vitro [10,
11], these compounds may directly inhibit A(3 oligomeric assembly and toxicity
in the brain and, by
precluding oligomeric assembly and insoluble fibril formation [15, 16], may
allow A(3 to remain in a
biophysical state that can be cleared through normal mechanisms. To address
this hypothesis, brain A(3
oligomer levels were measured using a dot-blot immunoassay with an antibody
that selectively detects
oligomeric A(3 species with a mass greater than 40 kDa16. As predicted, levels
of soluble A(3 oligomers
were significantly reduced in the brain of cyclohexanehexol-treated TgCRND8
mice and paralleled the
degree of behavioral and neuropathological improvements induced by each
compound (Figure 2A, Figure
8 a-b, Figure 13 a-c). Thus, epi-cyclohexanehexol, which was effective only
when given prophylactically,
reduced A(3 oligomers by 61% at 4 months of age (P = 0.01). This effect was
not sustained, however, in
either the 6-month prophylactic trial (P =0.1) or the 1-month treatment trial
(P = 0.12). In contrast,
treatment with scyllo-cyclohexanehexol induced a 40% reduction in soluble A(3
oligomers in both the 4-
and 6-month prophylactic trials (P = 0.03 and P = 0.004, respectively), and
caused a 30% reduction in the
28-d treatment trial (P = 0.008).
To investigate which species of A(3 oligomers were principally affected in the
brain of scyllo-
cyclohexanehexol-treated mice, western blots of soluble fractions of brain
homogenates from 4-month-
old treated and untreated TgCRND8 mice were performed. These experiments
showed that scyllo-
cyclohexanehexol reduced the abundance of high-molecular-weight A(3 oligomeric
species by 25 4.5%
(P = 0.02; Figure 13 d,e), and caused a concomitant 282 5% and 133 4%
increase in low-molecular-
weight trimeric (P < 0.00 1) and monomeric species, respectively (P < 0.001).
These results suggest that
scyllocyclohexanehexol exerts its beneficial effect through inhibition and/or
disaggregation of high-
molecular-weight A(3 oligomers.
49
CA 02579188 2007-02-19
Finally, dose-response studies, in which TgCRND8 mice treated from 6 weeks to
4 months of age
with scyllo-cyclohexanehexol (0.3-30 mg/kg/d), showed a dose-dependent
improvement in spatial
memory (P < 0.05; Figure 14 a), which was accompanied by a corresponding dose-
dependent decrease in
both plaque count (P <_ 0.02; Figure 14 b) and in brain A(3 oligomers (P 5
0.02; Figure 14 c, d). These
effects were maintained at 6 months of age, as shown by the dose-dependent
decrease in soluble A042
levels within the brain (P <_ 0.02; Figure 14 e, f, Table 2).
DISCUSSION
The results reported here show that orally administered scyllocyclohexanehexol
and, to a lesser
extent, epi-cyclohexanehexol significantly inhibit Alzheimer disease-like
behavioral deficits,
neuropathology and accelerated mortality in the TgCRND8 mouse model of
Alzheimer disease. Notably,
these effects occur regardless of whether the compounds are given during the
latent (or presymptomatic)
phase or the overt (or symptomatic) phase of the Alzheimer disease-like
illness.
Several independent lines of evidence suggest that the beneficial effects of
cyclohexanehexol
stereoisomers arise from a decrease in the accumulation of neurotoxic A(3
oligomers in brain. First, the
compounds are transported across the blood-brain barrier by facilitated
transport [17-20]; and their
presence in brain tissue of treated mice can be determined by gas
chromatography-mass spectrometry
[20]. Second, the stereoisomer-specific effects on brain A(3 oligomers levels,
cognitive deficits, amyloid
neuropathology and accelerated mortality in the TgCRND8 mouse model of
Alzheimer diseasegenerally
parallel the relative effects on blocking A(3 oligomerization and cytotoxicity
in vitro [10, 11]. Third, there
is a close correlation between the dose-response curves for the effect of
scyllo-cyclohexanehexol on brain
oligomer levels and on cognition, neuropathology and total brain A(3levels.
Finally, consistent with the
possibility that cyclohexanehexols and A(3-specific antibodies (especially
those directed to the N-terminal
residues of A(3 [21-23]) both have antiaggregation effects, the behavioral and
neuropathological effects of
cyclohexanehexol therapy are similar to those previously shown by A(3-specific
antibodies in this model
[13]. They also resemble the beneficial effects described in preliminary human
clinical trials of A(342
immunization [24, 25].
Oligomeric aggregates of misfolded proteins is an important mechanism
underlying
neurodegenerative disease. This concept necessarily predicts that attempts to
block the formation of toxic
oligomeric aggregates should also block the disease phenotype, as was shown
here. This is supported by
the observation that oral administration of another low-molecular-weight
osmolyte, trehalose, decreased
polyglutamine aggregates in brain, improved motor dysfunction and extended
lifespan of a mouse model
of Huntington disease [26]. Trehalose, cyclohexanehexols and other low-
molecular-weight osmolytes may
function by modulating the folding of proteins that are prone to misfold and
aggregate into toxic
oligomers [9]. By stabilizing these proteins as monomers and nontoxic
conformers that are unable to
further assemble (for example, (3-sheet spherical micelles [ 10, 11 ]), these
compounds would: (i) favor the
disassembly of high-molecular-weight oligomers and fibrillar deposits; (ii)
block the clinical
consequences; and (iii) by maintaining the protein in a soluble state,
encourage its clearance by normal
CA 02579188 2007-02-19
pathways. There is debate as to which species of A(3 are neurotoxic. The data
suggests that these toxic
species are likely to be higher-order oligomers. This conclusion is in general
agreement with the recent
observation that the abundance of a high-molecular-weight 12-unit oligomer
(A(3*) correlates with the
appearance of neurotoxicity in the TgAPPswe mouse [27]. It is presently
unclear whether the scyllo-
cyclohexanehexol-sensitive -40-mer peptide and A(3* are related species (for
example, as a multimer of
A(3*), whether these different apparent molecular weights reflect the
existence of multiple toxic A(3
oligomer species or whether the different apparent molecular masses simply
reflect differences in sample
preparation and electrophoretic procedures.
The present invention is not to be limited in scope by the specific
embodiments described herein,
since such embodiments are intended as but single illustrations of one aspect
of the invention and any
functionally equivalent embodiments are within the scope of this invention.
Indeed, various modifications
of the invention in addition to those shown and described herein will become
apparent to those skilled in
the art from the foregoing description and accompanying drawings. Such
modifications are intended to
fall within the scope of the appended claims.
All publications, patents and patent applications referred to herein are
incorporated by reference in
their entirety to the same extent as if each individual publication, patent or
patent application was
specifically and individually indicated to be incorporated by reference in its
entirety. The citation of any
reference herein is not an admission that such reference is available as prior
art to the instant invention.
51
CA 02579188 2007-02-19
Table 1
Cyclohexanehexol treatment for 28 days decreases brain A(340 and AP42 Levels
and amyloid plaques at 6 months of age.
A(340 A(342 Total Total Mean
(ng/gm wet brain ~ (ng/gm wet brain f Plaque Plaque Plaque
sem) sem)
Soluble Insoluble Soluble Insoluble Count Area ( mZ) Size
l m2)
Control 204 4 4965 457 426 14 14503 1071 1441 29 486002 16156 401 14
Epi-
cyclohexanchexol 264 11 3637 113* 540 14 12830 330 1342 114 459706 49966 346 6
Scyllo-
cyclohexanehexol 178 11 3527 241* 374 23 11115 647* 1260 27* 420027 14986* 336
6*
ANOVA with Fisher's PLSD, * p<0.05.
52
CA 02579188 2007-02-19
Table 2
Prophylactic and treatment with Cyclohexanehexol decreases brain
A(340 and AR42 Levels and Plaques
A040 A042 Total Nieair
(ng19m wet brain sexn) (ug"gtn wet brain se1n) Plaque P"laque
Sohible Irisoluble Soluble Itrsotzible Area (}tm?) Size (tcnr7)
4-month Prophylactic
Coxrtrol 75-6 1163= 9 273=18 5658=248 100766 7564 136 15
Epi-cyclohexanehexol 43-7* 615- 32w 85'-7+ 4059-179* 65042.~:~199 95=43
Scyllo-cyclohexanehexol 37--5* 437,80'2fl6-8* 4409--135* 63847 2895 103--4*
6-month Proplzylactic
Control 187=29 3576+172 626=87 15802-237 411288t11912 123-22
Epi-c}'clolrexaiiehexol 188-24 3668 149 665=39 13943-?77+ 380456f13498 370-9
Scyllo-cyclohexanehexol 105 -=8* 2453 251+ 475-26* 12588--82'+~ 262379 5373+
339 =10t
1 month Treatment
Control 204_-4 4965i457 426=14 14503-1071 486002 16156 401 14
Epi-cyclohexanehexol 264=11 3637=113* 540=14 12830-330 459706t49966 346=6
Scyllo-cyclohexauelrexol178 11 3527=241* 374t23 11115y647* 420027 14986* 336
6*
ANOVA with Fisher's PLSD, t p' ~O.O0 1 and * p=-'O.O5.
53
CA 02579188 2007-02-19
Table 3
Overall effect of cyclohexanehexols on cognitive function
Repeated measures ANOVA with compound (untreated, epi- and scyllo-
cyclohexanehexol), genotype
(Tg versus nTg) as between subject factors and day of testing as within
subject factors.
1. Prophylactic Study:
4-months of age test with epi-cyclohexanehexol:
Epi-cyclohexanehexol effect F(1,35) = 0.2 P = 0.89
Genotype effect F(1,35) = 42 P< 0.0001
Day effect F(4,140) = 11 P< 0.0001
Epi-cyclohexanehexol x genotype F(1,35) = 1.2 P = 0.28
Day x epi-cyclohexanehexol F(4,140) = 0.4 P = 0.81
Day x genotype F(4,140) = 0.4 P = 0.4
Epi-cyclohexanehexol x genotype x day F(4,140) = 0.62 P = 0.65
4-months of age test with scyllo-cyclohexanehexol
Scyllo-cyclohexanehexol effect F(1,35) = 8.5 P = 0.006
Genotype effect F(1,35) = 31 P< 0.0001
Day effect F(4,140) = 15.3 P < 0.0001
Scyllo-cyclohexanehexol x genotype F(1,35) = 1.1 P = 0.3
Day x scyllo-cyclohexanehexol F(4,140) = 1.0 P = 0.44
Day x genotype F(4,140) = 1.5 P= 0.2
Scyllo-cyclohexanehexol x genotype x day F(4,140) = 0.7 P = 0.63
6-months of age test with epi-cyclohexanehexol
Epi-cyclohexanehexol effect F(1,51) = 0.9 P= 0.35
Genotype effect F(1,51) = 23 P< 0.0001
Day effect F(4,204) = 13 P< 0.0001
Epi-cyclohexanehexol x genotype F(1,51) = 5.6 P = 0.02
Day x epi-cyclohexanehexol F(4,204) = 2.0 P= 0.10
Day x genotype F(4,204) = 0.6 P = 0.65
Epi-cyclohexanehexol x genotype x day F(4,204) = 1.83 P = 0.12
6-months of age test with scyllo-cyclohexanehexol
Scyllo-cyclohexanehexol effect F(1,52) = 3.2 P = 0.08
Genotype effect F(1,52) = 25 P< 0.0001
Day effect F(4,208) = 23 P< 0.0001
Scyllo-cyclohexanehexol x genotype F(1,52) = 5.9 P = 0.02
Day x scyllo-cyclohexanehexol F(4,208) = 2.1 P = 0.08
Day x genotype F(4,208) = 0.5 P = 0.75
Scyllo-cyclohexanehexol x genotype x day F(4,208) = 0.7 P = 0.6
2. Treatment Study
Epi-cyclohexanehexol
Epi-cyclohexanehexol effect F(1,46) = 0.9 P = 0.94
Genotype effect F(1,46) = 18 P = 0.0004
Day effect F(4,184) = 20 P< 0.0001
Epi-cyclohexanehexol x genotype F(1,46) = 2.0 P = 0.17
Day x epi-cyclohexanehexol F(4,184) = 1.7 P = 0.15
Day x genotype F(4,184) = 1.5 P= 0.22
Epi-cyclohexanehexol x genotype x day F(4,184) = 1.06 P = 0.38
Scyllo-cyclohexanehexol
Scyllo-cyclohexanehexol effect F(1,45) = 0.5 P = 0.5
54
CA 02579188 2007-02-19
Genotype effect F(1,45) = 16 P = 0.0008
Day effect F(4,180) = 17 P< 0.0001
Scyllo-cyclohexanehexol x genotype F(1,45) = 1.9 P = 0.18
Day x scyllo-cyclohexanehexol F(4,180) = 0.29 P = 0.88
Day x genotype F(4,180) = 0.29 P = 0.88
Scyllo-cyclohexanehexol x genotype x day F(4,180) = 0.7 P = 0.6
CA 02579188 2007-02-19
The following are citations for publications.
1. Selkoe, D.J. Deciphering the genesis and fate of amyloid beta-protein
yields novel therapies for
Alzheimer disease. J. Clin Invest. 110, 1375-1381 (2002).
2. Glabe, C.C. Amyloid accumulation and pathogenesis of Alzheimer's disease:
significance of
monomeric, oligomeric and fibrillar A. Subcell Biochem. 38, 167-177 (2005).
3. McLaurin, J. & Chakrabartty, A. Membrane disruption by Alzheimer beta-
amyloid peptides
mediated through specific binding to either phospholipids or gangliosides.
Implications for
neurotoxicity. J. Biol. Chem. 271, 26482-26489 (1996).
4. McLaurin, J. & Chakrabartty, A. Characterization of the interactions of
Alzheimer beta-amyloid
peptides with phospholipid membranes. Eur. J. Biochem. 245, 355-363 (1997).
5. Koppaka, V. & Axelson, P.H. Accelerated accumulation of amyloid beta
proteins on oxidatively
damaged lipid membranes. Biochemistry 39, 10011-10016 (2000).
6. Mizuno, T. et al., Cholesterol-dependent generation of a seeding amyloid
beta-protein in cell
culture. J. Biol. Chem. 274, 15110-15114 (1999).
7. Choo-Smith, L.P. & Surewicz, W.K. The interaction between Alzheimer amyloid
beta(1-40)
peptide and ganglioside GM1-containing membranes. FEBS Lett 402, 95-98 (1997).
8. Yanagisawa, K. et al., GM 1 ganglioside-bound amyloid beta-protein (A
beta): a possible form of
preamyloid in Alzheimer's disease. Nat. Med. 1, 1062-1066 (1995).
9. Auton, M, Bolen DW. Predicting the energetics of osmolyte-induced protein
folding/unfolding.
Proc Natl Acad Sci U S A. 102, 15065-15068 (2005).
10. McLaurin, J. Franklin, T. Chakrabartty, A. & Fraser, P. E.
Phosphatidylinositol and inositol
involvement in Alzheimer amyloid-beta fibril growth and arrest. J. Mol. Biol.
278, 183-194
(1998).
11. McLaurin, J., Goloumb, R., Jurewicz, A., Antel, J. P. & Fraser, P. E.
Inositol stereoisomers
stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and
inhibit abeta -induced
toxicity. J. Biol. Chem. 275, 18495-18502 (2000).
12. Chishti, M. A. et al., Early-onset amyloid deposition and cognitive
deficits in transgenic mice
expressing a double mutant form of amyloid precursor protein 695. J.Biol Chem
276, 21562-
21570 (2001).
13. Janus,C. et al., A(3 peptide immunization reduces behavioural impairment
and plaques in a model
of Alzheimer's disease. Nature 408, 979-982 (2000).
14. Morris, R. Development of a water-maze procedure for studying spatial
learning in the rat. J.
Neurosci Methods 11, 47-60 (1984).
15. Klein, W.L. Ap toxicity in Alzheimer's disease: globular oligomers (ADDLs)
as a new vaccine
and drug targets. Neurochem. Int. 41, 345-352 (2002).
56
CA 02579188 2007-02-19
16. Kayed, R. et al., Common Structure of Soluble amyloid oligomers implies
common mechanism
of pathogenesis. Science 300, 486-489 (2003).
17. Spector, R. Myo-inositol transport through the blood-brain barrier.
Neurochemical Research 13,
785-787 (1988).
18. Uldry, M. et al., Identification of a mammalian H(+)-myo-inositol
symporter expressed
predominantly in the brain. EMBO 20, 4467-4477 (2001).
19. Uldry, M. & Thorens B. The SLC2 family of facilitated hexose and polyol
transporters. Pflugers
Acta, 447, 480-489 (2004).
20. Shetty, H.U. & Hollway, H.W. Assay of myo-inositol in cerebrospinal fluid
and plasma by
chemical ionization mass spectrometry of the hexaacetate derivative. Biol.
Mass Spec. 23, 440-
444 (1994).
21. McLaurin, J. et al., Therapeutically effective antibodies against amyloid-
beta peptide target
amyloid-beta residues 4-10 and inhibit cytotoxicity and fibrillogenesis. Nat.
Med. 8, 1263-1269
(2002).
22. Frenkel, D., Katz, 0., & Solomon, B. Immunization against Alzheimer's (3-
amyloid plaques via
EFRH phage administration. Proc. Natl. Acad. Sci. USA 97, 11455-11459 (2000).
23. Bard, F., et al., Epitope and isotype specificities of antibodies to (3-
amyloid peptide for protection
against disease-like neuropathology. Proc. Natl. Acad. Sci. USA 100, 2023-2028
(2003).
24. Nicoll, J.A.R., Wilkinson, D., Holmes, C., Steart, P., Markham, H. &
Weller, R.O.
Neuropathology of human Alzheimer disease after immunization with amyloid-(3
peptide: a case
report. Nat. Med. 9, 448-452 (2003).
25. Hock, C. et al., Antibodies against beta-amyloid slow cognitive decline in
Alzheimer's disease.
Neuron 38, 547-554 (2003).
26. Tanaka, M. et al., Trehalose alleviates polyglutamine-mediated pathology
in a mouse model of
Huntington disease. Nat. Med. 10, 148-154 (2004).
27. Lesne, S., et al., A specific amyloid-(3 protein assembly in the brain
impairs memory. Nature
440, 352-357 (2006).
28. Vaucher, E. et al., Object Recognition Memory and Cholinergic Parameters
in Mice Expressing
Human Presenilin 1 Transgenes. Exp. Neurol. 175, 398-406 (2002).
29. Mount, H.T.J., Progressive sensorimotor impairment is not associated with
reduced dopamine and
high energy phosphate donors in a model of ataxia-telangiectasia. J Neurochem
88:1449-1454.
30. Wiltfang, J. et al., Highly conserved and disease-specific patterns of
carboxyterminally truncated
Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer's disease and in
patients with
chronic neuroinflammation J Neurochem 81, 481-496 (2002)
31. Haccou, P., & Mellis, E., Statistical Analysis of Behavioural Data, pg 120-
186, Oxford University
Press, Oxford (1995).
57
CA 02579188 2007-02-19
32. Chen, F. et al. Carboxyl-terminal fragments of Alzheimer beta-amyloid
precursor protein
accumulate in restricted and unpredicted intracellular compartments in
presenilin 1-deficient
cells. J Biol Chem. 275, 36794-36802 (2002).
33. Phinney, A. et al., No hippocampal neuron or synaptic bouton loss in
learning-impaired aged (3-
amyloid precursor protein-null mice. Neuroscience 90, 1207-1216 (1999).
34. Hu, L. et al., The impact of Ap-plaques on Cortical Cholinergic and Non-
cholinergic Presynaptic
boutons in Alzheimer's Disease-like Transgenic mice. Neuroscience 121, 421-432
(2003).
35. Levine, J. Controlled trials of inositol in psychiatry. Eur.
Neuropsychopharmacology 7, 147-155
(1997).
36. Orgogozo, J.M., et al., Subacute meningoencephalitis in a subset of
patients with AD after A(342
immunization. Neurology 61, 46-54 (2003).
37. Schenk, D, et al., Immunization with amyloid-beta attenuates Alzheimer-
disease-like pathology
in the PDAPP mouse. Nature 400, 173-177 (1999).
38. Golde, T.E. Alzheimer disease therapy: Can the amyloid cascade be halted?
J. Clin. Invest. 111,
11-18 (2003).
39. Sarvey, JM, Burgard EC & Decker G. Long-term potentiation: studies in the
hippocampal slice. J
Neurosci Methods 28,109-124 (1989).
40. Stanton, PK & Sarvey JM. Norepinephrine regulates long-term potentiation
of both the
population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res
Bull. 18, 115-119
(1987).
41. Moyer, JR Jr, & Brown TH. Methods for whole-cell recording from visually
preselected neurons
of perirhinal cortex in brain slices from young and aging rats. J Neurosci
Methods.86, 35-54
(1998).
42. Walsh, D.M. et al., Naturally secreted oligomers of amyloid beta protein
potently inhibit
hippocampal long-term potentiation in vivo. Nature 416, 535-539 (2002a)
43. Walsh, D.M., Klyubin, I., Fadeeva, J.V., Rowan, M.J. & Selkoe, D.J.
Amyloid-beta oligomers:
their production, toxicity and therapeutic inhibition. Biochem Soc. Trans. 30,
552-557 (2002b).
58