Note: Descriptions are shown in the official language in which they were submitted.
CA 02579382 2007-02-22 s
CONTROLLED RELEASE DELIVERY DEVICE
FIELD OF THE INVENTION
The present invention relates to a device for the delivery of active
ingredient(s). The present invention also relates to the use and method for
making the same.
BACKGROUND OF THE INVENTION
Many techniques have been used to provide controlled and sustained-
release pharmaceutical dosage forms in order to maintain therapeutic serum
levels of medicaments and to minimize the effects of missed doses of drugs
caused by a lack of patient compliance and the requirement of decreasing
side effects of drugs by controlling their blood concentration.
For example, there are extended release tablets which have an
osmotically active drug core surrounded by a semipermeable membrane. The
semipermeable membrane acts to delimit a reservoir chamber. These tablets
function by allowing a fluid, such as gastric or intestinal fluid, to permeate
the
coating membrane and dissolve the active ingredient so it can be released
through a passageway in the coating membrane by osmotic tension or if the
active ingredient is insoluble in the permeating fluid, pushed through the
passageway by an expanding agent such as a hydrogel. Some
representative examples of these osmotic tablet devices can be found in U.S.
Patents Nos. 3,845,770, 3,916,899, 4,034,758, 4,077,407 and 4,783,337.
The problem with these devices is that they are tedious and difficult to
fabricate. Their efficiency and precision is also in doubt as they have been
known to break up prematurely or retain some of the drug content during
transit in the gastrointestinal tract, which may lead to less drug being
released
and delivered by such devices. It is, therefore, not uncommon for such
devices to contain an overage of drug of at least 10% to account for such
inefficiencies in dose delivery. This practice is not economical and presents
a
CA 02579382 2007-02-22
danger, especially if potent drugs are used, as these devices have been
known to rupture in transit thus releasing excess dose.
There have also been reports on sustained-release devices, such as
tablets coated with a release-controlling coat, matrix tablets comprising
water
soluble polymeric compounds, matrix tablets comprising wax, matrix tablets
comprising water insoluble polymeric compounds and the like. For example,
U.S. Patent No. 3,629,393 (Nakamoto) utilizes a three-component system to
provide slow release tablets in which granules of an active ingredient with a
hydrophobic salt of a fatty acid and a polymer are combined with granules of a
hydrocolloid and a carrier and granules of a carrier and an active or a
buffering agent, which are then directly compressed into tablets.
U.S. Patent No. 3,728,445 (Bardani) discloses slow release tablets
formed by mixing an active ingredient with a solid sugar excipient,
granulating
the same by moistening with a cellulose acetate phthalate solution,
evaporating the solvent, recovering the granules and compressing under high
pressure.
U.S. Patent No. 4,704,285 (Alderman) discloses solid slow release
tablets containing 5-90% hydroxypropyl cellulose ether, 5-75% of an optional
additional hydrophilic colloid, such as hydroxypropylmethyl cellulose, an
effective amount of an active ingredient, and optional binders, lubricants,
glidants, fillers, etc.
U.S. Patent No. 6,645,528 teaches porous drug matrices and methods
of manufacture thereof.
DE Patent Application No. 3943242 discloses "matrix" type granules
comprising an active ingredient and inert excipent(s) compressible into
tablets. Each granule consists of a multitude of particles included in a
roughly
spherical matrix comprising a cellulosic polymer, a vinylic or acrylic
polymer, a
plasticizer and a lubricating agent.
There are reports in the literature of several tablets which are film-
coated with a coating material of, for example, cellulosic, acrylic, starch,
polyethylene glycol or gum type, or their derivatives. This coating functions
to
- 2 -
CA 02579382 2007-02-22
,
,
provide taste masking, protection of an active ingredient, gastro-resistance
to
physiological fluids, and also to prolong the release of the active
ingredient.
For example, U.S. Pat. No. 4,461,759 describes a coated tablet which
protects an active ingredient from the harmful effects of the acid pH of the
stomach and at the same time releasing the active ingredient at a constant
rate in the gastrointestinal tract.
The use of microporous film coating which allows the release of an
active ingredient under the effect of an osmotic pressure has also been widely
reported. One such report, PCT publication WO 91/16885 (U.S. Pat. No.
5,028,434), teaches the sustained release of an active ingredient irrespective
of the solubility of the active ingredient in the medium.
Another practice in the delivery of drugs is the use of micro-particulate
pharmaceutical systems giving a sustained release of an active ingredient.
For example, Patent EP 396,425 discloses a system intended for the
administration once daily dose of an active ingredient. To this end, the
active
ingredient is bound to the surface of inert spherules with a diameter ranging
from 250 to 2000 microns, using a known binder. The particles are then film-
coated with a cellulose compound and a plasticizer, to slow down the release
of the active ingredient.
U.S. Pat. No. 5,286,497 describes a formulation based on Diltiazem
which is designed to be taken once a day. Diltiazem is bound to the surface of
inert granules of sugar or of starch, which are then optionally film-coated.
U.S. Pat. No. 4,869,908 describes floating tablets, characterized by a
long residence time in the stomach. This system is more particularly suited to
the administration of an active ingredient having a preferential absorption at
the gastric level.
Patent FR 2,395,026 teaches a process for the preparation of a system
in which the micro-particles containing an active ingredient are in a
sustained-
release form containing, in their composition, a densifying agent which allows
a significant prolongation in the transit time, which may then exceed 24
hours.
This system was developed after observation of the fact that transit in the
small intestine is slowed down considerably when the density of the particles
- 3 -
CA 02579382 2007-02-22
exceeds 1.4 grams per cubic centimeter. The same approach of increasing
the transit time by elevation of the density is adopted in EP applications
0,080,341 and 0,173,210. However, such systems have the drawback of
requiring the introduction of a large amount of densifying agent, of the order
of
30 to 80% of the total weight of the form, which limits the content of an
active
ingredient in the system and constitutes a handicap for the manufacture of
forms requiring a large dose of active ingredient.
Another approach for controlled release consists of the development of
bioadhesive systems.
EP 0,452,268 claims a bucco-adhesive system in the form of
microparticles film-coated with a gel of xanthan/carob gums or with
ethylcellulose. The effectiveness of such a system, essentially intended for
the mouth, is not established, and all the less so since the particles are
coated
with a film of wax as an outer layer, which is intended to sustain their
release
but which makes adhesion improbable, and anyway not demonstrated in vivo.
Application EP 0,516,141 is directed towards the development of a
bioadhesive particulate system by overcoating, of any given sustained-release
form of an active ingredient, with an adhesive composition based on polymers
such as water-soluble derivatives of cellulose, acrylic polymers known under
the trade names CarbopolTM or PolycarbophilTM, alginates, gelatin or pectin.
U.S. Pat. No. 6,022,562 discloses an invention which relates to
microcapsules for the oral administration of a medicinal and/or nutritional
active ingredient, which are smaller than or equal to 1000 micrometer in size.
These microcapsules consist of particles which are coated with a coating
material consisting of a mixture of a film-forming polymer derivative, a
hydrophobic plasticizer, a functional agent and a nitrogen-containing polymer.
The invention also relates to a process for the production of the
microcapsules.
U.S. Pat. No. 6,022,562 uses multiple film forming polymers in one film
forming coating composition, i.e., the polymers are applied as an admixture to
form one or more layers of coat.
- 4 -
CA 02579382 2007-02-22
These sustained-release devices have difficulty in controlling the
release rate of water soluble or water insoluble active ingredient(s). It is
noted
that when replacing a multiple times a day dosing with once a day dosing that
the loading dose, which is represented by the first dose of an immediate
release multiple times a day product, is captured to a certain extent by the
once a day formulation via a loading dose effect which is built, ideally, into
the
formulation. Investigational studies over a long period of time were needed to
obtain devices with a desired release rate. The desired release rate being a
rate of input and extent of release that simulate a loading dose effect and an
extended release profile while using a single homogenous unit dose. The
difficulty arises because conventional and current controlled release devices
require higher amounts of polymers with high molecular weight and viscosity-
imparting or gelling properties to achieve true extended release.
Unfortunately, such high levels do not result in a loading dose effect. To
obtain a loading dose effect in such devices, a lower amount of polymer
concentration is required or a high amount of water soluble component must
be added to moderate the effect of high concentration of polymer. However,
at these levels, high variability is observed within and between lots. It is
also
difficult to obtain a product with a reproducible release rate and a loading
dose effect. Such products also present problems in quality control as precise
control and reproducibility of release profiles is difficult.
There have been reports in the literature of the use of hydrophobic
thermoplastic polymers such as ethylcellulose for the controlled release of
pharmaceutical substances. Ethylcellulose is typically applied as a coat. Drug
release is by symmetric flow (channel flow) and diffusion through the
ethylcellulose layer. Release is controlled by the layer thickness and the
rate
of channel flow or diffusion flow force. Such systems are at a disadvantage
because they allow drug delivery to be controlled via a singular property
i.e.,
coating thickness formed from use of a single film forming admixture. This
presents a high risk approach to the optimization of formulations, because the
use of coating thickness as an index for controlling rate of input presents a
narrow window to work with and limits the applicability of such systems. This
- 5 -
CA 02579382 2007-02-22
is one reason why matrix systems have superceded the use of hydrophobic
thermoplastic polymers such as ethylcellulose coats or coats consisting of a
mixture of ethylcellulose polymer and a nitrogen-containing polymer such as
polyvinylpyrolidone as means for controlling the release of drugs.
Therefore, there is a need to develop a stable drug delivery device that
can be reproducibly manufactured and have the desired effect through less
administration of the device per day.
SUMMARY OF THE INVENTION
An object of the present invention is to provide a delivery device to control
the
rate and extent of delivery of an active ingredient, for example, without
limitation an active pharmaceutical ingredient, biological, chemical,
nutraceutical, agricultural or nutritional active ingredients.
In accordance with an aspect of the present invention, there is provided a
controlled release delivery device comprising two or more polymeric coats
and at least one transition zone between the polymeric coats, wherein at least
two polymeric coats are not applied as an admixture.
In accordance with an aspect of the present invention, there is provided a
controlled release delivery device comprising: (a) two or more polymeric coats
substantially enveloping an active ingredient; and (b) at least one transition
zone formed between a pair of bordering polymeric coats.
In accordance with an aspect of the present invention, there is provided a
controlled release delivery device comprising: (a) first, second and third
polymeric coats substantially enveloping an active ingredient; (b) a first
transition zone formed between a first pair of bordering polymeric coats
comprised of the first and second polymeric coats; and (c) a second transition
zone formed between a second pair of bordering polymeric coats comprised
of the second and third polymeric coats.
- 6 -
CA 02579382 2014-05-02
In accordance with an aspect of the present invention, there is provided a
controlled release delivery device for controlled release of an active
ingredient
comprising: (a) a core particle comprising the active ingredient; (b) two or
more polymeric coats substantially enveloping the core particle comprising the
active ingredient; and (c) at least one transition zone between the polymeric
coats.
In accordance with an aspect of the present invention, there is provided a
controlled release delivery device comprising: (a) a core particle comprising
the active ingredient; (b) first, second and third polymeric coats
substantially
enveloping an active ingredient; (c) a first transition zone formed between a
first pair of bordering polymeric coats comprised of the first and second
polymeric coats; and (d) a second transition zone formed between a second
pair of bordering polymeric coats comprised of the second and third polymeric
coats.
In accordance with an aspect of the present invention, there is provided a
process for producing a controlled release delivery device for controlled
release of an active ingredient comprising:
(a) providing a core particle comprising the active ingredient;
(b) coating the core particle with a first polymeric coat comprised of a
first water insoluble polymer to substantially envelope the core particle;
(c) allowing the first polymeric coat to dry; and
(d) coating the first polymeric coat with a second polymeric polymeric
coat comprised of a second water insoluble polymer to provide a polymeric
coat that borders and substantially envelopes the first polymeric coat.
In accordance with another aspect, there is provided a controlled
delivery composition for controlled release of an active ingredient
comprising:
a core comprising the active ingredient;
at least two coatings substantially surrounding the core, wherein the
coatings comprise polymeric layers and wherein the polymers for each
- 7 -
CA 02579382 2014-05-02
coating are applied separately and are pH insensitive polymers or a
mixture of pH insensitive and pH sensitive polymers, and further wherein one
coating of the at least two coatings is made of a different polymer than
another coating of the at least two coatings; and
at least one transition zone between the at least two coatings, wherein
the flow of the active ingredient through the transition zone is at a cross
flow
relative to the flow of the active ingredient through the at least two
coatings.
The novel features of the present invention will become apparent to
those of skill in the art upon examination of the following detailed
description
of the invention. It should be understood, however, that the detailed
description of the invention and the specific examples presented, while
- 7a -
CA 02579382 2014-05-02
indicating certain embodiments of the present invention, are provided for
illustration purposes only because various changes and modifications within
the scope of the invention will become apparent to those of skill in the art
from
the detailed description of the invention and claims that follow.
BRIEF DESCRIPTION OF THE DRAWINGS
The following drawings are illustrative of embodiments of the invention and
are not meant to limit the scope of the invention as encompassed by the
claims:
FIGURE 1 shows a schematic of a controlled release delivery device
comprising three polymeric coats and two transition zones;
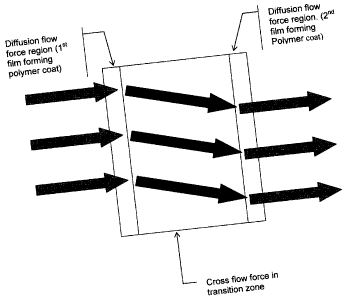
FIGURE 2 depicts a proposed or suggested mechanism of controlled release
from a transition zone bordered by two polymeric coats;
FIGURE 3 presents, in accordance with an embodiment of the invention, data
regarding the release of an active pharmaceutical ingredient from a coated
particle over a sustained-release period.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to a novel composition and to a method
of using and preparing same in order to control the rate and extent of
delivery
of an active ingredient. This is accomplished by the use of two or more
polymeric coats having a transition zone between at least two of the polymeric
coats. The active ingredient may be, without limitation, an active
pharmaceutical ingredient; or biological, chemical, nutraceutical,
agricultural
or nutritional ingredients. The active ingredient may be in any suitable
particle
known in the art, for example, without limitation, granules, tablets,
capsules,
spheroids, pellets, microspheres, nanospheres, microcapsules, or crystals.
More specifically, the novel composition of the present invention can be
used in any delivery device such as, and without being limited thereto, a
- 8 -
CA 02579382 2007-02-22
sustained release, pulsed release, delayed release and/or controlled release
device that controls the release of one or more active pharmaceutical
ingredients. The device can be a solid unit dosage form. The device can be
selected from, for example, one or more granules, one or more compressed
tablets, one or more pellets and/or one or more capsules. In a specific
embodiment, the device is a stable single homogeneous unit controlled
release device which controls the release rate, without significant
variability,
and with a reproducible controlled release rate.
The composition may be administered in any suitable manner. For
example and without being limited thereto, the composition can be in the form
of a suitable device for in vivo oral, vaginal, anal, ocular, subcutaneous,
intramuscular administration or for implantation. The composition may also
be used for in vitro or ex vivo delivery of an active ingredient.
In one example, a controlled release delivery device for sustained-
release of an active ingredient comprises: (a) a core particle comprising the
active ingredient; (b) two or more polymeric coats substantially enveloping
the
active ingredient; and (c) at least one transition zone between the polymeric
coats.
The term "active ingredient" means any compound which has
biological, chemical, or physiological utility including, without limitation,
pharmaceuticals, drugs, naturally occurring compounds, nucleic acid
compounds, peptide compounds, nutraceutical, agricultural or nutritional
ingredients or synthetic drugs.
The term "core particle" means a particle comprising an active
ingredient and which is substantially surrounded or enveloped by a polymeric
coating. The core particle can further comprise other compounds, including,
without limitation, binders, buffers, antioxidants, fliers or excipients. The
core
particle can be, without limitation, granules, tablets, capsules, spheroids,
pellets, microspheres, nanospheres, microcapsules, crystals, or suitable
mixtures thereof.
The term "polymeric coating" or "polymeric coat" means any coating
which is formed by polymerization of one or more monomers to form linear or
- 9 -
CA 02579382 2007-02-22
branched or cross-linked macromolecules. The coating may be variously
characterized as a coating, layer, membrane, shell, capsule, or the like, and
substantially surrounds or envelope a core particle. Where a device of the
present invention comprises more than one polymeric coat, a first polymeric
coat substantially surrounds or envelopes a core particle, a second polymeric
coat substantially surrounds or envelopes the first polymeric coat, and so
forth. A subsequent (for example, second) polymeric coat may be applied to
a previous (for example, first) polymeric coat in a contiguous or non-
contiguous fashion.
The term "transition zone" or "transition boundary" is a region formed
and located in between two bordering polymeric coats, where one polymeric
coat is directly applied to and substantially surrounds or envelopes another
polymeric coat. The transition zone or boundary need not be of uniform
thickness or shape along its entirety and may be interrupted by a portion of
one bordering polymeric coat leaching into another bordering polymeric coat
such that the two bordering polymeric coats appear to be contiguous at this
portion. When inspected using microscopic imaging devices portions of the
transition zone or boundary will show the polymeric coats bordering the
transition zone to be at least partially non-contiguous. The transition zone
or
boundary may be varied in width or thickness from about 1 angstrom to about
millimeter. In one example, the transition zone or boundary may be varied
in width or thickness from about 10 angstroms to about 10 micrometer. In
another example, the transition zone width or thickness may range from about
1 angstrom, 10 angstrom, 100 angstrom or any number therebetween to
25 about 500 angstrom, 1000 angstrom, 5000 angstrom, 10000 angstrom or any
number therebetween.
While not meant to be limiting and for demonstration purposes only,
Figure 1 depicts a relationship between 3 polymeric coats (first, second and
third polymeric coats) and two transition zones (first and second transition
zones). The first and second polymeric coats form a pair of bordering
polymeric coats having a first transition zone therebetween, while the second
and third polymeric coats form a pair bordering polymeric coats having a
-10-
CA 02579382 2007-02-22
second transition zone therebetween. Typically, bordering polymeric coats will
not be made of identical components. For example, without limitation, a pair
of
bordering polymeric coats may comprise two different water insoluble
polymers such as an ethylcellulose polymer and a polyvinyl acetate or an
ethylcellulose polymer and a methacrylate copolymer.
The term "sustained release", "pulsed release", "delayed release" and
"controlled release" are used interchangeably in this application and are
defined for purposes of the present invention as the release of an active
ingredient from a delivery device at such a rate that when a dose of the
active
ingredient is administered in the sustained release, pulsed release, delayed
release or controlled-release device, concentrations (levels) of the active
ingredient are maintained within a desired range but below toxic levels over a
selected period of time. In the case of in vivo administration, concentrations
(levels) of the active ingredient could be measured in blood or plasma, for
example. When administered in vivo the sustained release, pulsed release,
delayed release or controlled-release device of the present invention allows
for useful plasma concentration of an active ingredient to be maintained for
longer than in the case of immediate-release forms.
The controlled release profile may be modified on the basis of many
factors pertaining to the polymeric coats and transition zones, for example,
without limitation, through the types of polymers used, the order in which
they
are deposited, the number and or width of transition zones or boundaries, the
ratios of the polymers in the mix and the nature of their interaction at the
transition zones. The controlled-release profile can also be modified by a
variety of factors relating to the delivery device and the route of
administration
as outlined for example, in US Application No. 20070003619, published
January 4, 2007. For example, the sustained-release period will vary
depending upon the solubility of the active ingredient, the rate of clearance
of
the active ingredient from the intended site of administration, the size of
the
core particle, the amount of the active ingredient initially present in the
core
particle, the presence of other compounds within the core particle that affect
the rate of release of the active ingredient, the permeability of the
polymeric
- 11 -
CA 02579382 2007-02-22
coating(s) to the active pharmaceutical ingredient, and the rate of
degradation
of the polymeric coating(s), as well as other factors.
Prior art devices that use the singular property of coating thickness to
control release are at a disadvantage, because the use of coating thickness
as an index for controlling rate of input or drug release presents a narrow
window to work with and limits the applicability of such devices.
A controlled release delivery device of the present invention, in one
example, is formed by combining two or more polymer coats in a transition
type assembly in which the layers of coat for a select group of polymers are
deposited in a manner such that there is a transition zone from one coat to
another. A controlled release delivery device comprising two or more
polymeric coats and at least one transition zone between the polymeric coats
provides a much wider scope for formulation optimization. Moreover, it has
been found that the control of rate of input or drug release can be easier,
cost
effective or efficient with these systems in comparison to controlling rate of
release by polymeric coat thickness alone.
The mechanism of release in the transition type coating as taught in
this invention appears to be significantly different from the non-transition
type
coating taught in the prior art. Without wishing to be bound by theory, by the
use of transition coating, cross flow of drug molecules is introduced at the
transition boundary or zone as depicted, for example, in Figure 2. It is
proposed, that when placed in contact with liquid milieu, systems on which
transition coats have been applied may experience diffusion flow followed by
cross flow. The net effect may be asymmetric flow which can result in a liquid
funnel or funnel flow as drug molecules or materials migrate from the core
past a transition zone. It is the present inventors opinion that due to the
low
tortuosity factor of the pre-transition zone the flow is laminar leading to a
diffusion flow force field. Adjacent to this is the transition zone having a
higher
tortuosity factor in which a second force field is generated by the turbulent
or
funnel flow. This force field is the cross flow force field. The funnel flow
that
result helps create a velocity gradient. Regardless of mechanism, the delivery
device of the present invention comprising at least two polymeric coats and a
- 12 -
CA 02579382 2007-02-22
transition zone between the polymeric coats allows for an improved control of
release rate compared to the use of coating thickness alone.
Release control may be effected or optimized through the types of
polymers used, the order in which they are deposited, the number and or
width of transition zones or boundaries, the ratios of the polymers in the mix
and the nature of their interaction at the transition zones.
There are no specific restrictions as to the methods of manufacture of
the composition, device or excipient of the present invention. Typically, the
device can be easily prepared, for instance, by the dry or wet granulation of
an active ingredient. Optional components may be added such as, and
without being limited thereto, silicone dioxide, one or more excipients, one
or
more oil components, and/or the like. The granules thus obtained are dried if
required and passed through a mill and lubricated. The granules are
compressed into a shaped form in a rotary tablet press using a conventional
method.
In certain examples granules, tablets, capsules, spheroids, pellets,
microspheres, nanospheres, microcapsules, or crystals comprising an active
ingredient can be prepared by wet or dry granulation, by extrusion
spheronization, by powder or solution layering, by microencapsulation
techniques, by milling and compression techniques. The transition coating
may be carried out using fluid bed coating techniques or by coating using
perforated side vented pan coating technique or by microencapsulation
technique. These methods have been previously taught in the prior art.
In a particular example, an approach taught by the present inventors, is
a multiple wet granulation and drying technique. This involves the granulation
of the active ingredient with or without excipients with a first film forming
polymer solution or dispersion and drying the granulation in an oven or fluid
bed or vacuum drying. The wet granules may be milled or screened before
drying. The first polymeric coated dried granules may be milled. The process
is repeated using the dried or dried milled granules as starting material and
a
second film forming polymer solution or dispersion as granulation liquid. This
process is repeated as many times as necessary to obtain a desired number
-13-
CA 02579382 2007-02-22
of polymeric coats and desired number of transition zones with bordering
polymeric coats. The capsules used in this invention may be hard or soft
gelatin type or made from cellulose ethers.
In certain examples, different populations of coated particles can be
packed together, for example, in a capsule or compressed into a tablet.
Methods of polymeric coating are well known in the art. For example, a
core particle may be coated in a fluidized bed or pan, or by spraying or
painting a polymeric coat onto a core particle. Another known option is a
fluid
bed bottom spray coater by having particles suspended in an air stream, and
an aqueous dispersion of a polymeric coating composition is sprayed on to
the particles. Various conventional coating apparatuses may be employed to
facilitate this including, for example, a centrifugal fluidized bed coating
apparatus, a pan coating apparatus, or a fluidized bed granulating coating
apparatus.
In the preparation of the device, the device may be cured at a
predetermined temperature and relative humidity for a predetermined period
of time in order to decrease or increase the rate of release of active
ingredient(s) from the device. . A curing process may also be carried out to
simply remove a desired amount of solvent from the polymeric coat.
A controlled release delivery device of the present invention, typically,
but not always, comprises two or more polymeric coats of water insoluble
polymers and at least one transition zone between the polymeric coats of
water insoluble polymers.
Water insoluble polymers which are used in the present invention may
be any polymers which are insoluble in water and can preferably retard the
release of active pharmaceutical ingredients. Specific examples of water
insoluble polymers are, ethylcellulose, chitin, chitosan, cellulose esters,
aminoalkyl methacrylate polymer, anionic polymers of methacrylic acid and
methacrylates, copolymers of acrylate and methacrylates with quaternary
ammonium groups, ethylacrylate methylmethacrylate copolymers with a
neutral ester group, polymethacrylates, surfactants, aliphatic polyesters,
zein,
polyvinyl acetate, polyvinyl chloride, and the like. Preferred water insoluble
- 14-
CA 02579382 2007-02-22
polymers are, ethylcellulose, cellulose acetate, polymethacrylates and
aminoalkyl methacrylate copolymer.
In further specific examples, the acrylic polymer, includes, but is not
limited to, acrylic acid and methacrylic acid copolymers, methyl methacrylate
copolymers, ethoxyethyl methacrylates, cyanoethyl methacrylate, aminoalkyl
methacrylate copolymer, poly(acrylic acid), poly(methacrylic acid),
methacrylic
acid alkylamide copolyer, poly(methyl methacrylate), poly(methyl
methacrylate) copolymer, polyacrylamide, aminoalkyl methacrylate
copolymer, poly(methacrylic acid anhydride), and glycidyl methacrylate
copolymers. Additionally, the acrylic polymers may be cationic, anionic, or
non-ionic polymers and may be acrylates, methacrylates, formed of
methacrylic acid or methacrylic acid esters.
The polymers used in the present invention may also be pH insensitive
or pH sensitive.
For a delivery device designed to be orally administered to the
digestive tract, polymers that are known to be orally ingestible can be used
and include, for example, polyvinyl alcohol, hydroxypropyl methyl cellulose,
and other cellulose-based polymers. Other known polymers useful for enteral
delivery include polymer materials which preferentially dissolve or
disintegrate
at different points in the digestive tract. Such polymers include, for
example,
the known acrylic and/or methacrylic acid-based polymers which are soluble
in intestinal fluids, e.g. the EudragitTM series of commercially available
polymers. Examples of these include Eudragit ETM, such as Eudragit E 100TM
which preferentially dissolves in the more acid pH of the stomach, or enteric
polymers such as Eudragit LTM and/or Eudragit STM which preferentially
dissolve in the more alkaline pH of the intestine, or polymers which dissolve
slowly, e.g. a predetermined rate in the digestive tract, such as Eudragit
RLTM,
e.g. Eudragit RL 1 00Tm, and/or Eudragit RSTM e.g. Eudragit R100TM, and/or
blends of such EudragitTM polymers.
While the controlled release delivery device of the present invention
typically comprises two or more polymeric coats comprised of water insoluble
polymers, optional coats or components within a coat may be comprised of
-15-
CA 02579382 2007-02-22
water soluble polymers or enteric polymers or any other polymer known to be
useful for controlled release.
Water soluble polymers which may be used in the present invention
may be any polymers which are soluble in water and can preferably retard the
release of active pharmaceutical ingredients when made into shapes by
press-molding. Preferred water soluble polymers are those which can form
hydrocolloid when molded into shape, thereby retarding release of
pharmaceutically active components. They include naturally occurring or
synthetic, anionic or nonionic, hydrophilic rubbers, starch derivatives,
cellulose derivatives, proteins, and the like. Specific examples are acacia,
tragacanth, xanthan gum, locust bean gum, guar-gum, karaya gum, pectin,
arginic acid, polyethylene oxide, Carbomer, polyethylene glycol, propylene
glycol arginate, hydroxypropyl methylcellulose, methylcellulose, hydroxypropyl
cellulose, hydroxyethyl cellulose, carboxymethylcellulose sodium,
polyvinylpyrrolidone, carboxyvinyl polymer, sodium polyacrylate, alpha starch,
sodium carboxymethyl starch, albumin, dextrin, dextran sulfate, agar, gelatin,
casein, sodium casein, pullulan, polyvinyl alcohol, deacetylated chitosan,
polyethyoxazoline, poloxamers and the like. Of these, preferable are
hydroxyethyl cellulose, xanthan gum, hydroxypropyl methylcellulose,
methylcellulose, hydroxypropyl cellulose, carbomer, polyethylene glycol,
poloxamers, polyethylene oxide, starch derivatives and polyvinylpyrrolidone.
These water soluble polymers can be used either singly or in combinations of
two or more.
Oil components which can be used in the present invention include oils
and fats, waxes, hydrocarbons, higher fatty acids, higher alcohols, esters,
metal salts of higher fatty acids, and the like. Specific examples of oils and
fats include plant oils, such as cacao butter, palm oil, Japan wax (wood wax),
coconut oil, etc.; animal oils, such as beef tallow, lard, horse fat, mutton
tallow, etc.; hydrogenated oils of animal origin, such as hydrogenated fish
oil,
hydrogenated whale oil, hydrogenated beef tallow, etc.; hydrogenated oils of
plant origin, such as hydrogenated rape seed oil, hydrogenated castor oil,
hydrogenated coconut oil, hydrogenated soybean oil, etc.; and the like. Of
- 16-
CA 02579382 2007-02-22
these hydrogenated oils are preferred as an oil component of the present
invention. Specific examples of waxes include plant waxes, such as camauba
wax, candelilla wax, bayberry wax, auricurry wax, espalt wax, etc.; animal
waxes, such as bees wax, breached bees wax, insect wax, spermaceti,
shellac, lanolin, etc.; and the like. Of these preferred are carnauba wax,
white
beeswax and yellow beeswax. Paraffin, petrolatum, microcrystalline wax, and
the like, are given as specific examples of hydrocarbons, with preferable
hydrocarbons being paraffin and microcrystalline wax. Given as examples of
higher fatty acids are caprilic acid, undecanoic acid, lauric acid, tridecanic
acid, myristic acid, pentadecanoic acid, palmitic acid, malgaric acid, stearic
acid, nonadecanic acid, arachic acid, heneicosanic acid, behenic acid,
tricosanic acid, lignoceric acid, pentacosanic acid, cerotic acid,
heptacosanic
acid, montanic acid, nonacosanic acid, melissic acid, hentriacontanic acid,
dotriacontanic acid, and the like. Of these, preferable are myristic acid,
palmitic acid, stearic acid, and behenic acid. Specific examples of higher
alcohols are lauryl alcohol, tridecyl alcohol, myristyl alcohol, pentadecyl
alcohol, cetyl alcohol, heptadecyl alcohol, stearyl alcohol, nonadecyl
alcohol,
arachyl alcohol, behenyl alcohol, carnaubic alcohol, corianyl alcohol, ceryl
alcohol, and myricyl alcohol. Particularly preferable alcohols are cetyl
alcohol,
stearyl alcohol, and the like. Specific examples of esters are fatty acid
esters,
such as myristyl palmitate, stearyl stearate, myristyl myristate, behenyl
behenate, ceryl lignocerate, lacceryl cerotate, lacceryl laccerate, etc.;
glycerine fatty acid esters, such as lauric monoglyceride, myristic
monoglyceride, stearic monoglyceride, behenic monoglyceride, oleic
monoglyceride, oleic stearic diglyceride, lauric diglyceride, myristic
diglyceride, stearic diglyceride, lauric triglyceride, myristic triglyceride,
stearic
triglyceride, acetylstearic glyceride, hydoxystearic triglyceride, etc.; and
the
like. Glycerine fatty acid esters are more preferable. Specific examples of
metal salts of higher fatty acid are calcium stearate, magnesium stearate,
aluminum stearate, zinc stearate, zinc palmitate, zinc myristate, magnesium
myristate, and the like, with preferable higher fatty acid salts being calcium
stearate and magnesium stearate.
-17-
CA 02579382 2007-02-22
The oil components and water insoluble polymers can be used either
singly or in combinations of two or more.
As used herein, the term "active pharmaceutical ingredient" or "active
pharmaceutical ingredients" refers to chemical or biological molecules
providing a therapeutic, diagnostic, or prophylactic effect in vivo. Non-
limiting
active pharmaceutical ingredients contemplated for use in the compositions
described herein include the following categories and examples of drugs and
alternative forms of these drugs such as alternative salt forms, free acid
forms, free base forms, and hydrates: analgesics/antipyretics (e.g., aspirin,
acetaminophen, ibuprofen, naproxen sodium, buprenorphine, propoxyphene
hydrochloride, propoxyphene napsylate, meperidine hydrochloride,
hydromorphone hydrochloride, morphine, oxycodone, codeine,
dihydrocodeine bitartrate, pentazocine, hydrocodone bitartrate, levorphanol,
diflunisal, trolamine salicylate, nalbuphine hydrochloride, mefenamic acid,
butorphanol, choline salicylate, butalbital, phenyltoloxamine citrate,
diphenhydramine citrate, methotrimeprazine, cinnamedrine hydrochloride, and
meprobamate); antiasthamatics (e.g., ketotifen and traxanox); antibiotics
(e.g.,
neomycin, streptomycin, chloramphenicol, cephalosporin, ampicillin,
penicillin,
tetracycline, and ciprofloxacin); antidepressants (e.g., nefopam, oxypertine,
doxepin, amoxapine, trazodone, amitriptyline, maprotiline, pheneizine,
desipramine, nortriptyline, tranylcypromine, fluoxetine, doxepin, imipramine,
imipramine pamoate, isocarboxazid, trimipramine, venlafaxine, paroxetine,
and protriptyline); antidiabetics (e.g., sulfonylurea derivatives); antifungal
agents (e.g., griseofulvin, amphotericin B, nystatin, and candicidin);
antihypertensive agents (e.g., propanolol, propafenone, oxyprenolol,
reserpine, trimethaphan, phenoxybenzamine, pargyline hydrochloride,
deserpidine, diazoxide, guanethidine monosulfate, minoxidil, rescinnamine,
sodium nitroprusside, rauwolfia serpentina, alseroxylon, and phentolamine);
anti-inflammatories (e.g., (non-steroidal) indomethacin, flurbiprofen,
naproxen,
ibuprofen, ramifenazone, piroxicam, (steroidal) cortisone, dexamethasone,
fluazacort, celecoxib, rofecoxib, hydrocortisone, prednisolone, and
prednisone); antiteoplastics (e.g., cyclophosphamide, actinomycin, bleomycin,
- 18-
CA 02579382 2007-02-22
daunorubicin, doxorubicin, epirubicin, mitomycin, methotrexate, fluorouracil,
carboplatin, carmustine (BCNU), methyl-CCNU, cisplatin, etoposide,
camptothecin and derivatives thereof, phenesterine, paclitaxel and derivatives
thereof, docetaxel and derivatives thereof, vinblastine, vincristine,
tamoxifen,
and piposulfan); antianxiety agents (e.g., lorazepam, prazepam,
chlordiazepoxide, oxazepam, clorazepate dipotassium, diazepam,
hydroxyzine pamoate, hydroxyzine hydrochloride, alprazolam, droperidol,
halazepam, chlormezanone, and dantrolene); immunosuppressive agents
(e.g., cyclosporine, azathioprine, mizoribine, and FK506 (tacrolimus));
antimigraine agents (e.g., ergotamine, divalproex, isometheptene mucate, and
dichloralphenazone); sedatives/hypnotics (e.g., barbiturates such as
pentobarbital, pentobarbital, and secobarbital; and benzodiazapines such as
flurazepam hydrochloride, triazolam, and midazolam); antianginal agents
(e.g., beta-adrenergic blockers; calcium channel blockers such as nisoldipine;
and nitrates such as nitroglycerin, isosorbide dinitrate, pentaerythritol
tetranitrate, and erythrityl tetranitrate); antipsychotic agents (e.g.,
haloperidol,
loxapine succinate, loxapine hydrochloride, thioridazine, thioridazine
hydrochloride, thiothixene, fluphenazine, fluphenazine decanoate,
fluphenazine enanthate, trifluoperazine, chlorpromazine, perphenazine,
lithium citrate, respiridone, and prochlorperazine); antimanic agents (e.g.,
lithium carbonate); antiarrhythmics (e.g., bretylium tosylate, esmolol,
amiodarone, encainide, digoxin, digitoxin, mexiletine, disopyramide
phosphate, procainamide, quinidine sulfate, quinidine gluconate, quinidine
polygalacturonate, flecainide acetate, tocainide, and lidocaine);
antiarthritic
agents (e.g., phenylbutazone, sulindac, penicillamine, salsalate, piroxicam,
azathioprine, indomethacin, meclofenamate, gold sodium thiomalate,
auranofin, aurothioglucose, and tolmetin sodium); antigout agents (e.g.,
colchicine, and allopurinol); anticoagulants (e.g., heparin, heparin sodium,
and
warfarin sodium); thrombolytic agents (e.g., urokinase, streptokinase, and
alteplase); antifibriolytic agents (e.g., aminocaproic acid); hemorheologic
agents (e.g., pentoxifylline): antiplatelet agents (e.g., aspirin);
anticonvulsants
(e.g., valproic acid, divalproex sodium, phenyloin, phenyloin sodium,
- 19-
CA 02579382 2007-02-22
clonazepam, primidone, phenobarbitol, amobarbital sodium, methsuximide,
metharbital, mephobarbital, mephenyloin, phensuximide, paramethadione,
ethotoin, phenacemide, secobarbitol sodium, clorazepate dipotassium, and
trimethadione); antiparkinson agents (e.g., ethosuximide);
antihistamines/antipruritics (e.g., hydroxyzine, diphenhydramine,
chlorpheniramine, brompheniramine maleate, cyproheptadine hydrochloride,
terfenadine, clemastine fumarate, triprolidine, carbinoxamine,
diphenylpyraline, phenindamine, azatadine, tripelennamine,
dexchlorpheniramine maleate, methdilazine, loratadine, and); agents useful
for calcium regulation (e.g., calcitonin, and parathyroid hormone);
antibacterial
agents (e.g., amikacin sulfate, aztreonam, chloramphenicol, chloramphenicol
palmitate, ciprofloxacin, clindamycin, clindamycin palmitate, clindamycin
phosphate, metronidazole, metronidazole hydrochloride, gentamicin sulfate,
lincomycin hydrochloride, tobramycin sulfate, vancomycin hydrochloride,
polymyxin B sulfate, colistimethate sodium, and colistin sulfate); antiviral
agents (e.g., interferon alpha, beta or gamma, zidovudine, amantadine
hydrochloride, ribavirin, and acyclovir); antimicrobials (e.g., cephalosporins
such as cefazolin sodium, cephradine, cefaclor, cephapirin sodium,
ceftizoxime sodium, cefoperazone sodium, cefotetan disodium, cefuroxime e
azotil, cefotaxime sodium, cefadroxil monohydrate, cephalexin, cephalothin
sodium, cephalexin hydrochloride monohydrate, cefamandole nafate, cefoxitin
sodium, cefonicid sodium, ceforanide, ceftriaxone sodium, ceftazidime,
cefadroxil, cephradine, and cefuroxime sodium; penicillins such as ampicillin,
amoxicillin, penicillin G benzathine, cyclacillin, ampicillin sodium,
penicillin G
potassium, penicillin V potassium, piperacillin sodium, oxacillin sodium,
bacampicillin hydrochloride. cloxacillin sodium, ticarcillin disodium,
aziocillin '
sodium, carbenicillin indanyl sodium, penicillin G procaine, methicillin
sodium,
and nafcillin sodium; erythromycins such as erythromycin ethylsuccinate,
erythromycin, erythromycin estolate, erythromycin lactobionate, erythromycin
stearate, and erythromycin ethylsuccinate; and tetracyclines such as
tetracycline hydrochloride, doxycycline hyclate, and minocycline
hydrochloride, azithromycin, clarithromycin) anti-infectives (e.g., GM-CSF);
-20-
CA 02579382 2007-02-22
bronchodilators (e.g., sympathomimetics such as epinephrine hydrochloride,
metaproterenol sulfate, terbutaline sulfate, isoetharine, isoetharine
mesylate,
isoetharine hydrochloride, albuterol sulfate, albuterol, bitolterolmesylate,
isoproterenol hydrochloride, terbutaline sulfate, epinephrine bitartrate,
metaproterenol sulfate, epinephrine, and epinephrine bitartrate;
anticholinergic agents such as ipratropium bromide; xanthines such as
aminophylline, dyphylline, metaproterenol sulfate, and aminophylline; mast
cell stabilizers such as cromolyn sodium; inhalant corticosteroids such as
beclomethasone dipropionate (BDP), and beclomethasone dipropionate
monohydrate; salbutamol; ipratropium bromide; budesonide; ketotifen;
salmeterol; xinafoate; terbutaline sulfate; triamcinolone; theophylline;
nedocromil sodium; metaproterenol sulfate; albuterol; flunisolide; fluticasone
proprionate, steroidal compounds and hormones (e.g., androgens such as
danazol, testosterone cypionate, fluoxymesterone, ethyltestosterone,
testosterone enathate, methyltestosterone, fluoxymesterone, and testosterone
cypionate; estrogens such as estradiol, estropipate, and conjugated
estrogens; progestins such as methoxyprogesterone acetate, and
norethindrone acetate; corticosteroids such as triamcinolone, betamethasone,
betamethasone sodium phosphate, dexamethasone, dexamethasone sodium
phosphate, dexamethasone acetate prednisone, methylprednisolone acetate
suspension, triamcinolone acetonide, methylprednisolone, prednisolone
sodium phosphate, methylprednisolone sodium succinate, hydrocortisone
sodium succinate, triamcinolone hexacetonide, hydrocortisone,
hydrocortisone cypionate, prednisolone, fludrocortisone acetate,
paramethasone acetate, prednisolone tebutate, prednisolone acetate,
prednisolone sodium phosphate, and hydrocortisone sodium succinate; and
thyroid hormones such as levothyroxine sodium); hypoglycemic agents (e.g.,
human insulin, purified beef insulin, purified pork insulin, glyburide,
chlorpropamide, tolbutamide, and tolazamide); hypolipidemic agents (e.g.,
clofibrate, dextrothyroxine sodium, probucol, simvastatin, pravastatin,
atorvastatin, lovastatin, and niacin); proteins (e.g., DNase, alginase,
superoxide dismutase, and lipase); nucleic acids (e.g., sense or anti-sense
-21-
CA 02579382 2007-02-22
nucleic acids encoding any therapeutically useful protein, including any of
the
proteins described herein); agents useful for erythropoiesis stimulation
(e.g.,
erythropoietin); antiulcer/antireflux agents (e.g., famotidine, cimetidine,
and
ranitidine hydrochloride); antinauseants/antiemetics (e.g., meclizine
hydrochloride, nabilone, prochlorperazine, dimenhydrinate, promethazine
hydrochloride, thiethylperazine, and scopolamine); oil-soluble vitamins (e.g.,
vitamins A, D, E, K, and the like); as well as other drugs such as mitotane,
halonitrosoureas,,anthrocyclines, and ellipticine.
A description of these and other classes of useful drugs and a listing of
species within each class can be found in Martindale, The Extra
Pharmacopoeia, 30th Ed. (The Pharmaceutical Press, London 1993).
Examples of other drugs useful in the compositions and methods
described herein include ceftriaxone, ceftazidime, oxaprozin, albuterol,
valacyclovir, urofollitropin, famciclovir, flutamide, enalapril, fosinopril,
acarbose, lorazepan, follitropin, fluoxetine, lisinopril, tramsdol,
levofloxacin,
zafirlukast, interferon, growth hormone, interleukin, erythropoietin,
granulocyte
stimulating factor, nizatidine, perindopril, erbumine, adenosine, alendronate,
alprostadil, benazepril, betaxolol, bleomycin sulfate, dexfenfluramine,
fentanyl,
flecainid, gemcitabine, glatiramer acetate, granisetron, lamivudine,
mangafodipir trisodium, mesalamine, metoprolol fumarate, metronidazole,
miglitol, moexipril, monteleukast, octreotide acetate, olopatadine,
paricalcitol,
somatropin, sumatriptan succinate, tacrine, nabumetone, trovafloxacin,
dolasetron, zidovudine, finasteride, tobramycin, isradipine, tolcapone,
enoxaparin, fluconazole, terbinafine, pamidronate, didanosine, cisapride,
venlafaxine, troglitazone, fluvastatin, losartan, imiglucerase, donepezil,
olanzapine, valsartan, fexofenadine, calcitonin, and ipratropium bromide.
These drugs are generally considered to be water soluble.
Other drugs include albuterol, adapalene, doxazosin mesylate,
mometasone furoate, ursodiol, amphotericin, enalapril maleate, felodipine,
nefazodone hydrochloride, valrubicin, albendazole, conjugated estrogens,
medroxyprogesterone acetate, nicardipine hydrochloride, zolpidem tartrate,
amlodipine besylate, ethinyl estradiol, rubitecan, amlodipine
- 22 -
CA 02579382 2007-02-22
besylate/benazepril hydrochloride, paroxetine hydrochloride, paclitaxel,
atovaquone, felodipine, podofilox, paricalcitol, betamethasone dipropionate,
fentanyl, pramipexole dihydrochloride, Vitamin D3 and related analogues,
finasteride, quetiapine fumarate, alprostadil, candesartan, cilexetil,
fluconazole, ritonavir, busulfan, carbamazepine, flumazenil, risperidone,
carbidopa, levodopa, ganciclovir, saquinavir, amprenavir, carboplatin,
glyburide, sertraline hydrochloride, rofecoxib carvedilol,
halobetasolproprionate, sildenafil citrate, celecoxib, chlorthalidone,
imiquimod,
simvastatin, citalopram, ciprofloxacin, irinotecan hydrochloride,
sparfloxacin,
efavirenz, cisapride monohydrate, lansoprazole, tamsulosin hydrochloride,
mofafinil, clarithromycin, letrozole, terbinafine hydrochloride, rosiglitazone
maleate, lomefloxacin hydrochloride, tirofiban hydrochloride, telmisartan,
diazapam, loratadine, toremifene citrate, thalidomide, dinoprostone,
mefloquine hydrochloride, chloroquine, trandolapril, docetaxel, mitoxantrone
hydrochloride, tretinoin, etodolac, triamcinolone acetate, estradiol.
ursodiol,
nelfinavir mesylate, indinavir, beclomethasone dipropionate, oxaprozin,
flutamide, famotidine, prednisone, cefuroxime, lorazepam, digoxin, lovastatin,
griseofulvin, naproxen, ibuprofen, isotretinoin, tamoxifen citrate,
nimodipine,
amiodarone, and alprazolam.
A controlled release delivery device of the present invention may be
used for treatment of a patient, for example, an animal and more particularly,
a mammal. By mammal, is meant any member of the class of mammalia that
is characterized by being a vertebrate having hair and mammary glands.
Examples include, without limitation, dog, cat, rabbit, horse, pig, goat, cow,
human being. The delivery device of the present invention may be
administered to any animal patient or mammalian patient that is in need of
treatment with a site specific, timed, pulsed, chronotherapeutic, extended, or
controlled release of an active ingredient. In one example, a delivery device
of
the present invention is used for treating a horse. In another example, a
delivery device of the present invention is used for treating a human being.
The controlled release delivery device of the present invention may be
used for the treatment of many human diseases, for example, without
- 23 -
CA 02579382 2007-02-22
limitation, hypertension, angina, diabetes, HIV AIDS, pain, depression,
psychosis, microbial infections, gastro esophageal reflux disease, impotence,
cancer, cardiovascular diseases, gastric/stomach ulcers, blood disorders,
nausea, epilepsy, Parkinson's disease, obesity, malaria, gout, asthma,
erectile
dysfunction, impotence, urinary incontinence, irritable bowel syndrome,
ulcerative colitis, smoking, arthritis, rhinitis, Alzheimer's disease,
attention
deficit disorder, cystic fibrosis, anxiety, insomnia, headache, fungal
infection,
herpes, hyperglycemia, hyperlipidemia, hypotension, high cholesterol,
hypothyroidism, infection, inflammation, mania, menopause, multiple
sclerosis, osteoporosis, transplant rejection, schizophrenia, neurological
disorders.
Excipients may also be included in the composition or device of the
present invention. Excipients may be selected from diluents, compression
agents, extrusion agents, glidants, lubricants, solubilizers, wetting agents,
surfactants, penetration enhancers, pigments, colorants, flavoring agents,
sweetners, antioxidants, acidulants, stabilizers, antimicrobial preservatives
and binders.
These excipients may be chosen from:
(1) diluents such as microcrystalline cellulose, calcium phosphate,
=
mannitol, sorbitol, xylitol, glucitol, ducitol, inositiol, arabinitol;
arabitol,
galactitol, iditol, allitol, fructose, sorbose, glucose, xylose, trehalose, al
lose,
dextrose, altrose, gulose, idose, galactose, talose, ribose, arabinose,
xylose,
lyxose, sucrose, maltose, lactose, lactulose, fucose, rhamnose, melezitose,
maltotriose, and raffinose. Preferred sugars include mannitol, lactose,
sucrose, sorbitol, trehalose, glucose,
(2) surfactants, wetting agents and solubilisers such as glycerol
monostearate, cetostearyl alcohol, cetomacrogol emulsifying wax, sorbitan
esters, polyoxyethylene alkyl ethers (e.g., macrogol ethers such as
cetomacrogol 1000), polyoxyethlylene castor oil derivatives, polyoxyethylene
sorbitan fatty acid esters (e.g., TWEENTm), polyoxyethylene stearates, sodium
dodecylsulfate, Tyloxapol (a nonionic liquid polymer of the alkyl aryl
polyether
alcohol type, also known as superinone or triton) is another useful
solubilisers.
-24 -
CA 02579382 2007-02-22
=
Most of these solubilisers, wetting agents and surfactants are known
pharmaceutical excipients and are described in detail in the Handbook of
Pharmaceutical Excipients, published jointly by the American Pharmaceutical
Association and The Pharmaceutical Society of Great Britain (The
Pharmaceutical Press, 1986).
Preferred wetting agents include tyloxapol, poloxamers such as
PLURONICTM F68, F127, and F108, which are block copolymers of ethylene
oxide and propylene oxide, and polyxamines such as TETRONICTm 908 (also
known as POLOXAMINETm 908), which is a tetrafunctional block copolymer
derived from sequential addition of propylene oxide and ethylene oxide to
ethylenediamine (available from BASF), dextran, lecithin, dialkylesters of
sodium sulfosuccinic acid such as AEROSOLTM OT, which is a dioctyl ester of
sodium sulfosuccinic acid (available from American Cyanimid), DUPONOLTM
P, which is a sodium lauryl sulfate (available from DuPont), TRITONTm X-200,
which is an alkyl aryl polyether sulfonate (available from Rohm and Haas),
TVVEENTm 20 and TINEENTm 80, which are polyoxyethylene sorbitan fatty acid
esters (available from ICI Specialty Chemicals), Carbowax 3550 and 934,
which are polyethylene glycols (available from Union Carbide), Crodesta F-
110, which is a mixture of sucrose stearate and sucrose distearate, and
Crodesta SL-40 (both available from Croda Inc.), and SA9OHCO, which is
Cg18H37-CH2 (CON(CH3)CH2(CHOH)4 CF201-02.
Wetting agents which have been found to be particularly useful include
Tetronic 908, the Tweens, Pluronic F-68 and polyvinylpyrrolidone. Other
useful wetting agents include decanoyl-N-methylglucamide; n-decyl-.beta.-D-
glucopyranoside; n-decyl-.beta.-D-maltopyranoside; n-dodecyl-.beta.-D-
glucopyranoside; n-dodecyl.beta.-D-maltoside; heptanoyl-N-methylglucamide;
n-heptyl-.beta.-D-glucopyranoside; n-heptyl-.beta.-D-thioglucoside; n-hexyl-
.beta.-D-glucopyranoside; nonanoyl-N-methylglucamide; n-octyl-.beta.-D-
glucopyranoside; octanoyl-N-methylglucamide; n-octyl-.beta.-D-
glucopyranoside; and octyl-.beta.-D-thioglucopyranoside. Another preferred
wetting agent is p-isononylphenoxypoly(glycidol), also known as Olin-10G or
- 25 -
CA 02579382 2007-02-22
Surfactant 10-G (commercially available as 10G from Olin Chemicals). Two or
more wetting agents can be used in combination.
The pharmaceutical composition or device may further include a
pegylated excipient. Such pegylated excipients include, but are not limited
to,
pegylated phospholipids, pegylated proteins, pegylated peptides, pegylated
sugars, pegylated polysaccharides, pegylated block-co-polymers with one of
the blocks being PEG, and pegylated hydrophobic compounds such as
pegylated cholesterol. Representative examples of pegylated phospholipids
include 1,2-diacyl 1-sn-glycero-3-phosphoethanolamine-N-[Poly(ethylene
glycol) 2000] ("PEG 2000 PE") and 1,2-diacyl-sn-glycero-3-
phosphoethanolamine-N-[- Poly(ethylene glycol) 5000]("PEG 5000 PE"),
where the acyl group is selected, for example, from dimyristoyl, dipalmitoyl,
distearoyl, diolcoyl, and 1-palmitoy1-2-oleoyl.
One skilled in the art can select appropriate excipients for use in the
composition of the present invention.
In an embodiment, the device is coated with a non-disintegrating and
non-semi-permeable coat. Materials useful for forming the non-disintegrating
non-semi-permeable coat are ethylcellulose, polymethylmethacrylates,
methacrylic acid copolymers and mixtures thereof.
In yet another embodiment, the device is coated with a non-
disintegrating semipermeable coat. Materials useful for forming the non-
disintegrating semipermeable coat are cellulose esters, cellulose diesters,
cellulose triesters, cellulose ethers, cellulose ester-ether, cellulose
acylate,
cellulose diacylate, cellulose triacylate, cellulose acetate, cellulose
diacetate,
cellulose triacetate, cellulose acetate propionate, and cellulose acetate
butyrate. Other suitable polymers are described in U.S. Pat. Nos. 3,845,770,
3,916,899, 4,008,719, 4,036,228 and 4,612,008. The most preferred non-
disintegrating semipermeable coating material is cellulose acetate comprising
an acetyl content of 39.3 to 40.3%, commercially available from Eastman Fine
Chemicals.
In an alternative embodiment, the non-disintegrating semipermeable or
non-disintegrating non-semi-permeable coat can be formed from the above-
- 26 -
CA 02579382 2007-02-22
described polymers and materials that will form passage ways or channels in
the coat. The passage way forming agents or channeling agents dissolve on
contact with fluid and form passages through which fluid and active
pharmaceutical ingredient(s) can move through the coat. The passage way
forming agent or channeling agent can be a water soluble material or an
enteric material. Some general examples of passageway forming agents or
channeling agents are watersoluble materials and/or wicking agents such as
cellulose ethers, polyethylene glycols or microcrystalline cellulose. Some
further examples of passageway forming agents or channeling agents are
sodium chloride, potassium chloride, lactose, sucrose, sorbitol, mannitol,
polyethylene glycol (PEG), for example PEG 600, polyvinyl pyrolidone,
propylene glycol, hydroxypropyl cellulose, hydroxypropyl methycellulose,
hydroxypropyl methycellulose phthalate, cellulose acetate phthalate, polyvinyl
alcohols, methacrylic acid copolymers and mixtures thereof.
The active pharmaceutical ingredient(s) that are water soluble or that
are soluble under intestinal conditions may also be used to create passage
ways in the coat.
The passageway creating agent comprises approximately 0 to about
75% of the total weight of the coating, most preferably about 0.5% to about
25% of the total weight of the coating. The passage way creating agent
dissolves or leaches from the coat to form passage ways in the coat for the
fluid to enter the core and dissolve the active ingredient.
As used herein the term passageway includes an aperture, orifice,
bore, channel, hole, weaken area or as created by soluble or leachable
materials.
Polymeric coats may also be formed with commonly known excipients
such as plasticizers and anti-tacking agents. Some commonly known
plasticizers include adipate, azelate, enzoate, citrate, stearate, isoebucate,
sebacate, dibutyl sebacate, triethyl citrate, tri-n-butyl citrate, acetyl tri-
n-butyl
citrate, citric acid esters, and those described in the Encyclopedia of
Polymer
Science and Technology, Vol. 10 (1969), published by John Wiley & Sons.
The preferred plasticizers are triacetin, acetylated monoglyceride, grape seed
-27-
CA 02579382 2007-02-22
oil, olive oil, sesame oil, acetyltributylcitrate, acetyltriethylcitrate,
glycerin
sorbitol, diethyloxalate, diethylmalate, diethylfumarate, dibutylsuccinate,
diethylmalonate, dioctylphthalate, dibutylsebacate, triethylcitrate,
tributylcitrate, glyceroltributyrate, and the like. Depending on the
particular
plasticizer, amounts of from 0 to about 25%, and preferably about 2% to about
20% of the plasticizer can be used based upon the total weight of the coating
polymer. It will be understood that some polymeric coats may be formed
without the use of a plasticizer, for example, without limitation, a polymeric
coat of Eudragit NE3ODTM (methacrylate copolymer).
An example of an anti tacking agent is talc. Depending on the coating
polymer, amounts of from 0 to about 70%, and preferably about 10% to about
50% of talc can be used based upon the total weight of the coating polymer.
Generally, the coat around the device will comprise from about 0.5% to
about 70% and preferably about 0.5% to about 50% based on the total weight
of the device with the coating.
In an alternative embodiment, the dosage form of the device may also
comprise an effective amount of the active pharmaceutical ingredient that is
available for immediate release as a loading dose. This may be coated onto
the coat of the device or it may be incorporated into the coat or it may be
press coated into the coated device.
In the preparation of coated device, various conventional well known
solvents may be used to prepare the device and apply the external coating to
the device. In addition, various diluents, excipients, lubricants, dyes,
pigments, dispersants etc. which are disclosed in Remington's
Pharmaceutical Sciences, 1995 Edition may be used in the device.
In order to illustrate, and without limitation several typical forms of
controlled release devices can be considered, for example, granules, tablets,
capsules, spheroids, pellets, microspheres, nanospheres, microcapsules,
crystals or other types of particles known to the skilled person comprising
one
or more of the following; active pharmaceutical ingredient; biological,
chemical, nutraceutical, agricultural or nutritional materials; surrounded by
two
or more polymer coats in which: (I) polymers are chosen from the group
- 28 -
CA 02579382 2007-02-22
comprised of ethylcellulose polymer, polyvinyl acetate polymer, ammonio
methacrylate copolymer, ethyl acrylate methyl methacrylate copolymer, or
their derivatives (II) polymeric coats are deposited in a such a way as to
transition from one or more layers of coat of one polymer type to one or more
layers coat of another polymer type moving from the inside to the outside of
the controlled release device(III) a transition zone or boundary in the region
of
first contact between the respective polymers of thickness of about 1
angstrom to about 25 millimeter (IV) polymers are applied separately and not
in the same admixture. In certain examples there may be granules, tablets,
capsules, spheroids, pellets, microspheres, nanospheres, microcapsules,
crystals or particles other types of particles known to the skilled person in
which there is one transition zone or boundary, while in other examples there
are more than one transition zones or boundaries. When considering some
examples of the ordering of polymeric coats in more detail: coats may be
deposited in a such a way as to transition from one or more layers of coat of
ethylcellulose to one or more layers of coat of methacrylate copolymer to one
or more layers of coat of polyvinyl acetate polymer moving from the inside to
the outside of the controlled release device; coats may be deposited in a such
a way as to transition from one or more layers of coat of polyvinyl acetate to
one or more layers of coat of methacrylate copolymer to one or more layers of
coat of ethylcellulose moving from the inside to the outside of the device;
coats may be deposited in a such a way as to transition from one or more
layers of coat of polyvinyl acetate to one or more layers of coat of
ethylcellulose to one or more layers of coat of methacrylates copolymer
moving from the inside to the outside of the device; coats may be deposited in
a such a way as to transition from one or more layers of coat of methacrylate
copolymer to one or more layers of coat of ethylcellulose to one or more
layers of coat of polyvinyl acetate moving from the inside to the outside of
the
device; or coats may be deposited in a such a way as to transition from one or
more layers of coat of methacrytate copolymer to one or more layers of coat
of polyvinyl acetate to one or more layers of coat of ethylcellulose moving
from the inside to the outside of the device. When considering examples, of
- 29 -
CA 02579382 2007-02-22
polymers that may be used to deposit coats a polymer may be selected from
one or a mixture of, methyl methacrylate copolymers, ethoxyethyl
methacrylates, cyanoethyl methacrylate, aminoalkyl methacrylate copolymer,
poly(acrylic acid), poly(methacrylic acid), methacrylic acid alkylamide
copolyer, poly(methyl methacrylate), poly(methyl methacrylate) copolymer,
polyacrylamide, aminoalkyl methacrylate copolymer, poly(methacrylic acid
anhydride), and glycidyl methacrylate copolymers. In certain examples, the
polymers are thermoplastic polymers, while in other examples the polymer
may be a pH insensitive water insoluble polymer, while in still other examples
polymeric coats may comprises pH insensitive polymers, pH sensitive
polymers, or both pH insensitive polymersand pH sensitive polymers.
In certain examples, a pharmaceutical dosage form prepared according
to a controlled release delivery device may be usedfor treatment of medical
conditions or disease states. In other examples, the pharmaceutical dosage
form may be for providing a patient with site specific, timed, pulsed,
chronotherapeutic, extended, or controlled release of active pharmaceutical
ingredients.
In other examples, of controlled release delivery devices according to
the present invention, there may be granules, tablets, capsules, spheroids,
pellets, microspheres, nanospheres, microcapsules, crystals or any other
suitable particle known to the skilled person containing one or more of the
following; active pharmaceutical ingredients; biological, chemical,
nutraceutical, agricultural or nutritional materials; surrounded by two or
more
polymer coats in which: (I) coats are made from water insoluble film forming
polymers and or their derivatives (II) coats are deposited in a such a way as
to
transition from one or more layers of coat of one type of polymer to one or
more layers of coat of another type of polymer moving from the inside to the
outside (III) a transition zone or boundary is formed in the region of first
contact between the respective polymers of thickness of about 1 angstrom to
about 25 millimetre (IV), optionally channeling agents are present.
Channeling agents may optionally be present in one or more of the transition
-30 -
CA 02579382 2007-02-22
coats. Other optional components include, without limitation, filler,
lubricant,
antioxidant, anti-tacky or plasticizer agent,
The above disclosure generally describes the present invention. A
more complete understanding can be obtained by reference to the following
specific Examples. The Examples are described solely for purposes of
illustration and are not intended to limit the scope of the invention. Changes
in form and substitution of equivalents are contemplated as circumstances
may suggest or render expedient. Although specific terms have been
employed herein, such terms are intended in a descriptive sense and not for
purposes of limitation.
- 31 -
CA 02579382 2007-02-22
EXAMPLE 1
Controlled Release Venlafaxine HCI Tablets
This is a two step process. In the first step, immediate release tablets are
manufactured by dry granulation process followed by direct compression into
tablets. In step two, three coats consisting of one or more layers of Aquacoat
30ECD (ethylcellulose polymer), Kolicoat SR 30D (polyvinyl acetate) and
Eudragit NE 30D (acrylic polymer) are applied one after the other such that
there is a transition from one coat to another. Note that the film forming
polymers are administered separately and not as an admixture.
(1) Manufacture of Tablet TABLE-1 Venlafaxine formulation (%) Venlafaxine
HCI 20, Lactose 59, Microcrystalline cellulose 20, Silicone dioxide 0.5,
Magnesium stearate 0.5.
% by weight
Venlafaxine hydrochloride 20
Lactose 59
Microcrystalline cellulose 20
Silicone dioxide 0.5
Magnesium stearate 0.5
The materials with exception of the magnesium stearate were charged
into a planetary mixer and blended for 5 minutes. The homogeneous blend
was charged into a V-Blender. Magnesium stearate was added and the
content blended for about 5 minutes. The blended materials were compressed
into tablets in a rotary press.
(2) Coating of Tablets
The tablets were coated with an aqueous dispersion composed of
ethylcellulose (Aquacoat 30ECD) plasticized with dibutyl sebacate to a 2%
weight gain. This was immediately followed with a coat of polyvinyl acetate
- 32 -
CA 02579382 2007-02-22
(Kolicoat SR 30D) plasticized with triethyl citrate to a weight gain of 2%.
Finally a coat of Eudragit NE 30D (methacrylate copolymer) was applied to a
weight of 2%. Coating was carried out in a side vented coating pan. The inlet
and outlet temperature was 62 and 40 degrees Centigrade respectively.
Relative humidity of the coating room was 45%. The transition coated tablets
were cured by drying in a tray dryer oven for 2 hours at 60 degree Centigrade.
- 33 -
CA 02579382 2007-02-22
EXAMPLE 2
Controlled Release Metoprolol Succinate Tablets
This is a two step process. In the first step, immediate release tablets are
manufactured by dry granulation process followed by direct compression into
tablets. In step two, three coats consisting of one or more layers of Aquacoat
30ECD (ethylcellulose polymer), Kolicoat SR 30D (polyvinyl acetate) and
Eudragit NE 30D (methacrylate copolymer) are applied one after the other
such that there is a transition from one coat to another.
(1) Manufacture of Tablet TABLE-2 Metoprolol formulation (%) Metoprolol
succinate 30 Lactose 49 Microcrystalline 20 cellulose Silicone dioxide 0.5
Magnesium stearate 0.5
c1/0 by weight
Metoprolol succinate 30
Lactose 49
Microcrystalline cellulose 20
Silicon dioxide 0.5
Magnesium stearate 0.5
The materials with exception of the magnesium stearate were charged into a
planetary mixer and blended for 5 minutes. The homogeneous blend was
charged into a V-Blender. Magnesium stearate was added and the content
blended for about 5 minutes. The blended materials were compressed into
tablets in a rotary press.
(2) Coating of Tablets
The tablets were coated with Eudragit NE 30D to a 2% weight gain.
This was immediately followed with a coat of polyvinyl acetate (Kolicoat SR
30D) plasticized with triethyl citrate to a weight gain of 2%. Finally a coat
of an
aqueous dispersion composed of ethylcellulose (Aquacoat 30ECD) plasticized
- 34 -
CA 02579382 2007-02-22
with dibutyl sebacate was applied to a weight of 3%. Coating was carried out
in a side vented coating pan. The inlet and outlet temperature was 62 and 40
degrees Centigrade respectively. Relative humidity of the coating room was
45%. The transition coated tablets were cured by drying in a tray dryer oven
for 2 hours at 60 degree Centigrade.
- 35 -
CA 02579382 2007-02-22
EXAMPLE 3
Chrontherapeutic Paroxetine HCI Tablets
This is as in Example 2 except for the following; paroxetine is substituted
for
metoprolol, and hydroxypropylmethyl cellulose 5% by weight of polymer is
added to the transition coat. The tablets are cured by drying in a tray dryer
oven for 2 hours at 60 degree Centigrade. To obtain chronotherapeutic
release a final coat of methacrylic acid copolymer type A (Eudragit L) is
applied to 4% weight gain.
- 36 -
CA 02579382 2007-02-22
EXAMPLE 4
Venlafaxine HCI Granules
(1) Manufacture of Tablet TABLE-3 Venlafaxine formulation ( /0) Venlafaxine
HCI 20 Lactose 59 Microcrystalline 20 cellulose Silicone dioxide 1.0
% by weight
Venlafaxine hydrochloride 20
Lactose 59
Microcrystalline cellulose 20
Silicone dioxide 1.0
The materials were charged into a high shear granulator and blended for 5
minutes. The homogeneous blend was granulated using Eudragit NE30D.
The wet granules were screened through a 1.4 millimetre sieve using a co-mill
and dried in a tray dryer oven. The dried granules were wet granulated in a
planetary mixer using polyvinyl acetate (Kolicoat SR 30D) plasticized with
triethyl citrate. The wet granules were dried and milled. The milled granules
were filled into capsules.
EXAMPLE 5
Venlafaxine granules with a transition zone were prepared according to the
process described in Example 4 and tested in a dissolution experiment
against a comparable Venlafaxine preparation without a transition zone.
The dissolution experiment was done in a USP dissolution apparatus at a acid
media of pH 1.5 for 3 hours and the media was changed to phosphate buffer
pH 7.5 until an asymptote was reached or 24 hours had elapsed. Results
shown in Figure 3 indicate that the Venlafaxine granules with a transition
zone
are more effective at controlling release than the comparable Venlafaxine
preparation without a transition zone.
- 37 -