Note: Descriptions are shown in the official language in which they were submitted.
CA 02579520 2007-03-13
~ -- ~-
~D 2004/000326 ~ ~ - PCT/DK20031000412
Combination therapy wherein a serotonin reuptake inhibitor is used
The present invention relates to the combination of a serotonin reuptake
inhibitor and
a GABAB receptor antagonist. Accordingly, the present invention relates to the
use of
certain compounds, and to compositions of compounds having serotonin reuptake
inhibiting activity and GABAB antagonistic, partial agonistic or inverse
agonistic
activity for the treatment of depression and other affective disorders. The
combined
serotonin reuptake inhibiting effect and the GABAB antagonistic, partial
agonistic or
inverse agonistic effect may reside within the same chemical entity or in two
separate
chemical entities.
Background
Selective serotonin reuptake inhibitors (hereinafter referred to as SSRIs)
have become
first choice therapeutics in the treatment of depression, certain forms of
anxiety and
social phobias, because they are effective, well tolerated and have a
favourable safety
profile compared to the classic tricyclic antidepressants.
However, clinical studies on depression and anxiety disorders indicate that
non-
response to SSRIs is substantial, up to 30%. Another, often neglected, factor
in
antidepressant treatment is compliance, which has a rather profound effect on
the
patient's motivation to continue pharmacotherapy.
First of all, there is the delay in therapeutic effect of SSRIs. Sometimes
symptoms
even worsen during the first weeks of treatment. Secondly, sexual dysfunction
is a
side effect common to all SSRIs. Without addressing these problems, real
progress in
the pharmacotherapy of depression and anxiety disorders is not likely to
happen.
In order to cope with non-response, psychiatrists sometimes make use of
augmentation strategies. Augmentation of antidepressant therapy may be
accomplished through the co-administration of mood stabilizers such as lithium
carbonate or triiodothyronin or by the use of electroshock.
CA 02579520 2007-03-13
WO 20 326 PCT/DR2003/000412.
2
In 1993, an augmentation strategy with pindolol was described by Artigas et
al, in
Trends Pharmacol. Sci. 1993, 14, p 262-263. Artigas' idea is based on
intracerebral
microdialysis experiments in animals. In fact, later neurochemical studies
built on the
desensitization hypothesis by Blier and co-workers stated that the delay in
therapeutic
effect of antidepressants is related to a gradual desensitization of 5-HT
autoreceptors
(Blier et al. J. Clin. Psycipharmacol. 1987, 7 suppl. 6, 24S-35S). A key point
in their
hypothesis is that the effects of SSRIs on the release-controlling
somatodendritic
autoreceptors (5-HT,A) limit the release of 5-HT in tenminal areas and thus
the effect of
5-HT uptake inhibition in those regions. This is supported by microdialysis
experiments
in rats, showing that the increase in extracellular 5-HT elicited by a single
dose of an
SSRI is augmented by co-administration of a 5-HTiA autoreceptor antagonist
(Invernizzi
et al. Brain Res, 1992, 584, p 322-324 and Hjorth, S., J. Neurochem, 1993, 60,
p 776-
779).
The effect of combined administration of a compound that inhibits serotonin
reuptake
and a 5-HTiA receptor antagonist has been evaluated in several studies (Innis,
R.B. et
al. Eur. J. Pharmacol. 1987, 143, p. 1095-204 and Gartside, S.E., Br. J.
Pharmacol,
1995, 115, p 1064-1070, Blier, P. et al. Trends in Pharmacol. Science 1994,
15, 220).
In these studies it was found that 5-HTIA receptor antagonists would abolish
the initial
brake on 5-HT neurotransmission induced by the serotonin reuptake inhibitors
and
thus produce an immediate boost of 5-HT transmission and a rapid onset of
therapeutic action.
Several patent applications have been filed which cover the use of a
combination of a
5-HTIA antagonist and a serotonin reuptake inhibitor for the treatment of
depression
(see e.g. EP-A2-687 472 and EP-A2-714 663).
Another approach to increase terminal 5-HT would be through blockade of the
5-HTi B autoreceptor. Microdialysis experiments in rats have indeed shown that
increase of hippocampal 5-HT by citalopram is potentiated by GMC 2-29, an
experimental 5-HT I B receptor antagonist.
CA 02579520 2007-03-13
' = ~- - -~'-
WO 200-1100032V.=-,OW.-,~ - p -003/00-04 12
3
Several patent applications covering the combination of an SSRI and a 5-HT113
antagonist or partial agonist have also been filed (WO 97/28141, WO 96/03400,
EP-
A-701819 and WO 99/13877).
y-aminobutyric acid (GABA) is the most important inhibitory neurotransmitter
in the
brain; up to 50% of all central synapses are GABA-ergic (Paredes R.G. & Agmo
A.,
Neuroscience and Biobehavioural Reviews, vol 16: pp 145-170 (1992)).
Noradrenaline, dopamine and serotonin (5-HT) are all under inhibitory control
of
GABA (Haefely W. The role of GABA in anxiolytic/antidepressant drug action.
Elliott M.M., Heal D.J. & Marsden C.A. (eds), pp 151-168, John Wiley & Sons,
New
York (1992)). There are two subtypes of GABA receptors, GABAA and GABAe,
which have been extensively studied and their influence on 5HT nerve function
and
release have been performed.
Thus stimulation of the GABAA receptor with the agonist muscimol reduced 5-HT
cell activity & 5-HT release in the raphe nuclei (Tao R. & Auerbach S.B. J.
Psychopharmacology vol 14(2): pp 100-113 (2000)) and blockade of GABAA
receptors increases firing and subsequently elevates levels of extracellular 5-
HT (see
below - bicuculline & Tao R. et al., British Journal of Pharmacology vol 119:
pp
1375-1384 (1996)).
The GABAB agonist baclofen produced a decrease in 5-HT in the raphe and
reduction
in the forebrain (Tao R. et al., British Journal of Pharmacology vol 119: pp
1375-1384
(1996)) and when administered by itself the GABAB antagonist, phaclofen, is
devoid
of effect on 5-HT levels in either the raphe (Abellan M.T. et al., Neuroreport
vol 11:
pp941-945 (2000); Tao R. et al., British Journal of Pharmacology vol 119: pp
1375-
1384 (1996)) or in the forebrain (see below & Tao R. et al., British Journal
of
Pharmacology vol 119: pp 1375-1384 (1996)). However, phaclofen, administered
either centrally into the hippocampus or systemically was shown to
significantly
increase the effects of citalopram on extracellular 5-HT levels, as
demonstrated in the
findings reported here.
CA 02579520 2007-03-13
- ~~ -- ~ -
WO 2004/000326 PCT/DK200310tI0412
4
The invention
It has now surprisingly been found that a GABAB antagonist will augment the
effect
of an SSRI on extracellular 5-HT levels.
It is therefore suggested that the combination of an SSRI and a GABAB
antagonist or
a molecule, which has both 5-HT reuptake inhibitory and GABAB antagonistic
properties, would have a better efficacy and faster onset than an SSRI alone.
Antagonism at any GABAB splice variants, including but not limited to GABABRia
and GA.BABRIb is claimed.
This invention covers both SSRI + GABAB antagonist in separate or the same
molecule.
The present invention thus provides:
The use of a GABAB receptor antagonist, inverse agonist or partial agonist for
the
preparation of a pharmaceutical composition to be used in combination with a
serotonin reuptake inhibitor (SRI).
The present invention relates to the use of a compound, which is a serotonin
reuptake
inhibitor, and another compound, which is a GABAB receptor antagonist, inverse
agonist or partial agonist for the preparation of a pharmaceutical composition
for the
treatment of depression, anxiety disorders and other affective disorders, such
as
generalized anxiety disorder, panic anxiety, obsessive compulsive disorder,
acute
stress disorder, post traumatic stress disorder and social anxiety disorder,
eating
disorders such as bulimia, anorexia and obesity, phobias, dysthymia,
premenstrual
syndrome, cognitive disorders, impulse control disorders, attention deficit
hyperactivity disorder, drug abuse or any other disorder responsive to
serotonin
reuptake inhibitors.
CA 02579520 2007-03-13
---
WO 2004/000326 ~ PCT/DK2003/0004
The present invention also relates to the use of a GABAB receptor antagonist,
inverse
agonist or partial agonist for the preparation of a phannaceutical composition
useful
for augnienting and/or providing faster onset of the therapeutic effect of a
serotonin
reuptake inhibitor.
5
In a preferrcd embodinient, the invention relates to the use as above wherein
the
serotonin reupiakc inliibi(or is used for the treatment of depression, anxiety
disorders
and other affective disordcrs, including generalized anxiety disorder, panic
anxiety,
obsessive compulsive disorder, acute stress disorder, post traumatic stress
disorder or
social anxiety disordcr, eating disorders such as bulimia, anorexia and
obesity,
phobias, dystliyniia, premcnstrual syndrome, cognitive disorders, impulse
control
disorders, attention deficit hyperactivity disorder, drug abuse and any other
disorder
responsive to a SRI.
In another enibo(linient, the invention relates to the use of
a) a compound which is a scrotonin reuptake inhibitor and a GABAB receptor
antagonist, iuverse agonist or partial agonist, or
b) a combination of a compound, which is a serotonin reuptake inhibitor, and a
compound, which is a GABAB receptor antagonist, inverse agonist or partial
agonist,
for the preparation of a pharmaceutical composition or kit (kit-of-parts)
useful for the
treatment of depression, anxiety disorders and other affective disorders, such
as
generalized anxiety disorder, panic anxiety, obsessive compulsive disorder,
acute
stress disorder, post traumatic stress disorder and social anxiety disorder,
eating
disorders such as bulimia, anorexia and obesity, phobias, dysthymia,
premenstrual
syndrome, cognitive disorders, impulse control disorders, attention deficit
hyperactivity disorder, drug abuse or any other disorder responsive to
serotonin
reuptake inhibitors.
In two independent embodiments, the invention relates to the use of a
compound,
which is a serotonin reuptake inhibitor, and a compound, which is a GABAB
receptor
antagonist, inverse agonist or partial agonist, for the preparation of a:
(a) pharmaceutical composition, or
CA 02579520 2007-03-13
WO 2004/000326
PCT/DK2003/000412
6
(b) kit
useful for the treatnlent of depression, anxiety disorders and other affective
disorders,
such as generalized anxiety disorder, panic anxiety, obsessive compulsive
disorder,
acute stress disorder, post traumatic stress disorder and social anxiety
disorder, eating
disorders such as bulimia, anorexia and obesity, phobias, dysthymia,
premenstrual
syndrome, cognitive disorders, impulse control disorders, attention deficit
hyperactivity disorder, drug abuse or any other disorder responsive to
serotonin
reuptake inhibitors.
In a further embodiment, the invention relates to a pharmaceutical composition
or kit
comprising:
a) a compound, which is a serotonin reuptake inhibitor, and a GABAB receptor
antagonist, inverse agonist or partial agonist, or
b) a combination of a compound, which is a serotonin reuptake inhibitor, and
another compound, which is a GABAB receptor antagonist, inverse agonist or
partial agonist,
and optionally pharmaceutically acceptable carriers or diluents.
In two further individual embodiments, the invention relates to either a
phannaceutical composition or a kit comprising a compound, which is a
serotonin
reuptake inhibitor, and another compound, which is a GABAB receptor
antagonist,
inverse agonist or partial agonist, and optionally pharmaceutically acceptable
carriers
or diluents.
In yet another embodiment, the invention relates to a method for the treatment
of
depression, anxiety disorders and other affective disorders, such as
generalized
anxiety disorder, panic anxiety, obsessive compulsive disorder, acute stress
disorder,
post traumatic stress disorder and social anxiety disorder, eating disorders
such as
bulimia, anorexia and obesity, phobias, dysthymia, premenstrual syndrome,
cognitive
disorders, impulse control disorders, attention deficit hyperactivity
disorder, drug
abuse or any other disorder responsive to serotonin reuptake inhibitors
comprising
administering to a person in need thereof a therapeutically effective amount
of
CA 02579520 2007-03-13
71%=W, 2004/000326 PCT/DK2003/000412
7
a) a compound, which is a serotonin reuptake inhibitor, and a GABAB receptor
antagonist, inverse agonist or partial agonist, or
b) a combination of a compound, which is a serotonin reuptake inhibitor and a
compound, which is a GABAB receptor antagonist, inverse agonist or partial
agonist.
Whenever mentioned, each of the options
a) a compound, which is a serotonin reuptake inhibitor, and a GABAB receptor
antagonist, inverse agonist or partial agonist, and
b) a combination of a compound, which is a serotonin reuptake inhibitor and a
(or
another) compound, which is a GABAB receptor antagonist, inverse agonist or
partial agonist
are intended to be individual embodiments. Accordingly, each of them may be
claimed individually.
Each of the medical indications depression, anxiety disorders and other
affective
disorders, including generalized anxiety disorder, panic anxiety, obsessive
compulsive
disorder, acute stress disorder, post traumatic stress disorder or social
anxiety
disorder, eating disorders such as bulimia, anorexia and obesity, phobias,
dysthymia,
premenstrual syndrome, cognitive disorders, impulse control disorders,
attention
deficit hyperactivity disorder, drug abuse and any other disorder responsive
to a SRI
is intended to be an individual embodiment. Accordingly, whenever mentioned in
the
present description, each of the indications specified above may be claimed
individually.
Whenever the indications depression, anxiety disorders and other affective
disorders,
including generalized anxiety disorder, panic anxiety, obsessive compulsive
disorder,
acute stress disorder, post traumatic stress disorder or social anxiety
disorder, eating
disorders such as bulimia, anorexia and obesity, phobias, dysthymia,
premenstrual
syndrome, cognitive disorders, impulse control disorders, attention deficit
hyperactivity disorder, drug abuse and any other disorder responsive to a SRI
are
mentioned in relation to use of a GABAB receptor antagonist, inverse agonist
or
partial agonist and an SRI, a pharrnaceutical composition, a kit, a method of
CA 02579520 2007-03-13
WO 20041M26 pCT/DK2D03/000412
8
treatnient and a method for the identification of compounds useful for
treatment it is
intended to be an individual embodiment. Accordingly, each of the indications
specified above niay individually be claimed together with said use of a GABAB
receptor antagonist, inverse agonist or partial agonist and an SRI,
pharmaceutical
compositioti, kit, mcthod of treatment and method for the identification of
compounds
useful for trcatnicnt.
In a particular cmbodinient, a selective serotonin reuptake inhibitor is used
according
to the invention.
In another particular enibodiment, a compound, which is selective for the
GABAB
receptor is used according to the invention.
In a further embodinient, a compound, which is an antagonist, an inverse
agonist at
the GABA1, receptor is used according to the invention.
The pharmaceutical coniposition or kit according to the invention may be
administered by simultaneous administration. The term "simultaneous
administration"
as used herein means, that the GABAB receptor antagonist, inverse agonist or
partial
agonist an(. .,e SRI are administered with a time separation of no more than
15
minutes, such as at most 10 niinutes, such as at most 5 minutes or such as at
most 2
minutes. The GABAB receptor antagonist, inverse agonist or partial agonist and
the
SRI may be contained in the "same unit dosage form" or in "discrete dosage
forms".
As used herein, the term "same unit dosage form" means a dosage form
comprising
both the SRI and the GABAa receptor antagonist, inverse agonist or partial
agonist.
As used herein, the term "discrete dosage form" means that the GABAB receptor
antagonist, inverse agonist or partial agonist is comprised in one dosage fonn
and that
the SRI is comprised in another dosage fonm.
Simultaneous administration of GABAB receptor antagonist, inverse agonist or
partial
agonist and the SRI is optionally combined with administration of
supplementary
doses of GABAB receptor antagonist, inverse agonist or partial agonist. The
supplementary doses of GABAB receptor antagonist, inverse agonist or partial
agonist
CA 02579520 2007-03-13
WO 2004/0003a"-W-MMW- -1FMffffiK2003/OO04i2
9
may be given for instance 1, 2, 3 or 4 times a day whereas the SRI and the
GABAB
receptor antagonist, inverse agonist or partial agonist which are administered
by
"simultaneous administration" may be given one or more times a day, e.g. once
daily
or e.g. twice daily. Accordingly:
= the GABAB receptor antagonist, inverse agonist or partial agonist and the
SRI
may be administered by simultaneous administration once daily and
supplementary doses of GABAB receptor antagonist, inverse agonist or partial
agonist may be administered 1, 2, 3 or 4 times a day, such as 1, 2 or 3 times
a
day, such as once or twice daily, such as twice daily or such as once daily,
or
= the GABAB receptor antagonist, inverse agonist or partial agonist and the
SRI
may be administered by simultaneous administration twice daily and
supplementary doses of GABAB receptor antagonist, inverse agonist or partial
agonist may be administered 1, 2, 3 or 4 times a day, such as 1, 2 or 3 times
a
day, such as once or twice daily, such as twice daily or such as once daily.
Alternatively, the pharmaceutical composition or kit according to the
invention is
administered by sequential administration. The term "sequential
administration" as
used herein means that I or more daily doses of GABAB receptor antagonist,
inverse
agonist or partial agonist and 1 or more daily doses of SRI are administered
with a
time separation between two administered doses of more than 15 minutes and
less
than 4 hours, such as more than 2 hours and less than 4 hours, such as more
than 15
minutes and less than 2 hours, such as more than 1 hour and less than 2 hours,
such as
more than 30 minutes and less than 1 hour, such as more than 15 minutes and
less
than 30 minutes. Either the SRI or the GABAB receptor antagonist, inverse
agonist or
partial agonist may be administered first. The GABAB receptor antagonist,
inverse
agonist or partial agonist and the SRI are contained in discrete dosage forms,
optionally contained in the same container or package. Typically, 1, 2, 3, 4
or 5 daily
doses of GABAB receptor antagonist, inverse agonist or partial agonist and 1
or 2
daily doses of SRI may be administered. Accordingly:
= the GABAB receptor antagonist, inverse agonist or partial agonist and the
SRI
may be administered once daily and the GABAB receptor antagonist, inverse
agonist or partial agonist may be administered 1, 2, 3, 4 or 5 times a day,
such
CA 02579520 2007-03-13
WO 2004/000326 PCT/DK20031M2
as 1, 2, 3 or 4 times a day, such as 1, 2 or 3 times a day, such as once or
twice
daily, such as twice daily or such as once daily,
or
the GABAa receptor antagonist, inverse agonist or partial agonist and the SRI
5 may be administered twice daily and the GABAB receptor antagonist, inverse
agonist or partial agonist may be administered 1, 2, 3, 4 or 5 times a day,
such
as 1, 2, 3 or 4 times a day, such as 1, 2 or 3 times a day, such as once or
twice
daily, such as twice daily or such as once daily.
10 Accordingly, the pharmaceutical composition or kit according to the
invention may be
adapted for simultaneous administration of the active ingredients, or it may
be adapted
for sequential administration of the active ingredients. When the
pharmaceutical
composition or kit is adapted for simultaneous administration, the active
ingredients
may be contained in the same unit dosage form. When the pharmaceutical
composition or kit is adapted for sequential administration, the active
ingredients are
contained in discrete dosage forms, optionally contained in the same container
or
package. As used herein, an "active ingredient" means an SRI or a GABAB
receptor
antagonist, inverse agonist or partial agonist.
A kit (kit-of-parts) comprises a preparation of the GABAB receptor antagonist,
inverse
agonist or partial agonist in a first-unit dosage form, and the SRI in a
second-unit
dosage fonn, and container means for containing said first and second dosage
fonms.
In particular, the present invention relates to the use of, and to
pharmaceutical
compositions or kits comprising the following combinations:
CGP 55845 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, vilazodone, duloxetine,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 62349 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CA 02579520 2007-03-13
- - ~- ~ ~_
WO 2004/000326 ~~~- -- PCT/DK2003/000412
11
CGP 71982 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 76290 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 76291 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 35348 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 36742 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 46381 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 52432 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
CGP 54626 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imiparmin, femoxetine and clomipramine,
CA 02579520 2007-03-13
~ WO 2ii04/000326
PCTIDK2003/000412 12
CGP 55845, CGP 62349, CGP 71982, CGP 76290, CGP 76291, CGP 35348, CGP
36742, CGP 46381, CGP 52432 and CGP 54626 are disclosed in Bowery NG et al.
Pharmacological Reviews 2002, 54 No. 2, p. 247-264.
SCH 50911 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, inzipramin, femoxetine and clomipramine,
Phaclofen and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
Saclofen and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
2-hydroxysaclofen and an SRI selected from citalopram, escitalopram,
fluoxetine,
sertraline, paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine,
vilazodone,
nefazodone, imipramin, femoxetine and clomipramine,
GAS 360 and an SRI selected from citalopram, escitalopram, fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, dapoxetine, duloxetine, vilazodone,
nefazodone, imipramin, femoxetine and clomipramine.
In a final embodiment, the present invention relates to a method for the
identification
of compounds useful for the treatment of depression, anxiety disorders and
other
affective disorders, such as generalized anxiety disorder, panic anxiety,
obsessive
compulsive disorder, acute stress disorder, post traumatic stress disorder and
social
anxiety disorder, eating disorders such as bulimia, anorexia and obesity,
phobias,
3o dysthymia, premenstrual syndrome, cognitive disorders, impulse control
disorders,
attention deficit hyperactivity disorder, drug abuse or any other disorder
responsive to
serotonin reuptake inhibitors, comprising, in any order:
CA 02579520 2007-03-13
2004/000326 ~ PCTIDK2003/000412
13
(a) measuring the ability of test compounds to inhibit serotonin reuptake and
selecting the compounds that have an IC50 value below 20 nM;
(b) measuring the affinity of test compounds to the GABAB receptor and
selecting
the compounds.
and thereafter measuring the efficacy of the selected compounds at the GABAB
receptor and selecting the compounds which are antagonists, inverse agonists
at the
receptor.
Preferred GABAa ligands show affinity of below 1,5 M, whereas other preferred
ligands show affinity of below 1,0 M and yet other preferred ligands show
affinity of
below 500 nM. Even more preferred are compounds with affinity below 100 nM.
Examples of assays for the selection / detection of GABAB antagonists, inverse
agonists or partial agonists are for example the following:
Binding assay for the detection of compounds with affinity for GABAB receptors
are
described in Karla et. al. J. Med. Chem. 1999, 42(i l), 2053-2059; or
Frydenvang et.
al. Chirality 1994; 6(7); 583-589;
Efficacy assay for the detection of antagonists, partial agonists or inverse
agonists at
the GABAB receptors are for example: Kamatchi et. al. Brain Res. 1990, 506(2),
181-
186; or Brauner-Osbome et. al. Br. J. Pharmacol. 1999, 128(7), 1370-1374.
The invention also covers compounds identified according to this method, but
is not
limited to theses assay methods.
According to the invention, it has been found that co-administration of GABAB
receptor antagonist or inverse agonist and a serotonin reuptake inhibitor
produces a
significant increase in the level of serotonin in terminal areas, as measured
in
microdialysis experiments, compared to the administration of the serotonin
reuptake
inhibitor alone.
CA 02579520 2007-03-13
_1111 W_
WO 20041OM6 PCT/DK2ff03/000412
14
According to the invention, animal studies have shown that GABAB receptor
antagonist
or inverse agonist may provide fast onset of therapeutic effect of serotonin
reuptake
inliibitors and potentiate the anxiolytic potential of serotonin reuptake
inhibitors.
The use of a combination of GABAB receptor antagonist, inverse agonist or
partial
agonist and a serotonin reuptake inhibitor may greatly reduce the amount of
serotonin
reuptake inhibitor necessary to treat depression and other affective disorders
and may
thus reduce the side effects caused by the serotonin reuptake inhibitor. In
particular,
the combination of a reduced amount of SRI and a GABAB receptor antagonist,
inverse agonist or partial agonist may reduce the risk of SSRI-induced sexual
dysfunction and sleep disturbances.
Co-administration of a GABAB receptor antagonist, inverse agonist or partial
agonist
and a serotonin reuptake inhibitor may also be useful for the treatment of
refractory
depression, i.e. depression, which cannot be treated appropriately by
administration of a
serotonin reuptake inhibitor alone. Typically, GABAB receptor antagonist,
inverse
agonist or partial agonist may be used as add-on therapy for the augmentation
of the
response to SRIs in patients where at least 40-60% reduction in symptoms has
not been
achieved during the first 6 weeks of treatment with an SRI.
Compounds which are both serotonin reuptake inhibitors and GABAB receptor
antagonists, inverse agonists or partial agonists may have the same
pharmacological
advantages as the combination of a serotonin reuptake inhibitor and a GABAB
receptor
antagonists, inverse agonists or partial agonists, with respect to reduction
of side effects,
fast onset and in the treatment of treatment resistant patients.
Many antidepressants with serotonin reuptake inhibiting effect have been
described in
the literature. Any phannacologically active compound, which primarily or
partly
exert its therapeutic effect via inhibition of serotonin reuptake in the CNS,
may
benefit from augmentation with a GABAB receptor antagonist, inverse agonist or
partial agonist.
CA 02579520 2007-03-13
WO 2004/00032_.~~~ 71 003/00041_2
The following list contains a number of serotonin reuptake inhibitors, which
may
benefit from augmentation with a GABAB receptor antagonist, inverse agonist or
partial agonist: citaloprani, escitalopram, fluoxetine, R-fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, desmethylvenlafaxine, duloxetine,
dapoxetine,
5 vilazodone, nefazodone, imipramine, imipramine N-oxide, desipraniine,
pirandamine,
dazepinil, nefopam, befuraline, fezolamine, femoxetine, clomipramine,
cianoimipramine, litoxetine, cericiamine, seproxetine, WY 27587, WY 27866,
imeldine, ifoxetine, indeloxazine, tiflucarbine, viqualine, milnacipran,
bazinaprine,
YM 922, S 33005, F 98214-TA, Fl 4503, A 80426, EMD 86006, NS 2389, S33005,
lo OPC 14523, alaproclate, cyanodothepine, trimipramine, quinupramine,
dothiepin,
amoxapine, nitroxazepine, McN 5652, McN 5707, VN 2222, L 792339, roxindole,
YM 35992, 0177, Org 6582, Org 6997, Org 6906, amitriptyline, amitriptyline N-
oxide, nortriptyline, CL 255.663, pirlindole, indatraline, LY 280253, LY
285974, LY
113.821, LY 214.281, CGP 6085 A, RU 25.591, napamezole, diclofensine,
trazodone,
15 BMY 42.569, NS 2389, sercloremine, nitroquipazine, ademethionine,
sibutramine,
desmethylsubitramine, didesmethylsubitramine, clovoxamine vilazodone. The
compounds mentioned above may be used in the form of the base or a
pharmaceutically acceptable acid addition salt thereof. Each of the serotonin
reuptake
inhibitors specified above is intended to be an individual embodiment.
Accordingly,
2o each of them and the use thereof may be claimed individually.
Compounds such as citalopram, escitalopram, fluoxetine, R-fluoxetine,
sertraline,
paroxetine, fluvoxamine, venlafaxine, desmethylvenlafaxine, duloxetine,
dapoxetine,
vilazodone, nefazodone, imipramine, imipramine N-oxide, desipramine,
pirandamine,
dazepinil, nefopam, befuraline, fezolamine, femoxetine, clomipramine,
cianoimipramine, litoxetine, cericlamine, seproxetine, imeldine, ifoxetine,
indeloxazine, tiflucarbine, viqualine, milnacipran, bazinaprine, alaproclate,
cyanodothepine, trimipramine, quinupramine, dothiepin, amoxapine,
nitroxazepine,
roxindole, amitriptyline, amitriptyline N-oxide, nortriptyline, pirlindole,
indatraline,
3o napamezole, diclofensine, trazodone, sercloremine, nitroquipazine,
ademethionine,
sibutramine, desmethylsubitramine, didesmethylsubitramine, clovoxamine
vilazodone,
CA 02579520 2007-03-13
_~ --- -
WO 2004/000326 PCT/DK2003/D0Mn
16
N-[( 1-[(6-Fluoro-2-naphthalenyl)methyl]- 4-piperidinyl]amino]carbonyl]-3-
pyridine
carboxamide (WY 27587),
[trans-6-(2-chlorophenyl)-1,2,3,5,6,10b-hexahydropyrrolo- (2,1-a)isoquinoline]
(McN
5707),
(dl-4-exo-amino-8-chloro-benzo-(b)-bicyclo[3.3.1Jnona-2-6 alpha(10 alpha)-
diene
hydrochloride)(Org 6997),
(dl)-(5 alpha,8 alpha,9 alpha)-5,8,9,10-Tetrahydro-5,9- methanobenzocycloocten-
8-
amine hydrochloride (Org 6906),
-[2-[4-(6-fl uoro-1 H-indol-3-yl)-3,6-dihydro-1(2H)-pyridinyl]ethylJ-3-
isopropyl-6-
(methylsulphonyl)-3,4-dihydro-lH-2,1,3-benzothiadiazine-2,2-dioxide
(LY393558),
[4-(5,6-dimethyl-2-benzofiuranyl)-piperidine] (CGP 6085),
di methyl-[5-(4-nitro-phenoxy)-6,7,8,9-tetrahydro-5H-benzocyclohepten-7-yl]-
amine
(RU 25.591),
O
~ (A 80426),
N / o 0
(E1VID 86006),
NO
(S33005),
CA 02579520 2007-03-13
WO 2004/000326 ~ PCT/DK2003/00041
17
ci
cc0
(OPC 14523),
ov
HO I
(McN 5652),
N
H
~
CIH
F (YM 35992),
CA 02579520 2007-03-13
~ WO 204/000326 PCT/DK2003/000412
18
N-OH
ci (Org 6582),
are preferred. The compounds mentioned above may be used in the form of the
base
or a pharmaceutically acceptable acid addition salt thereof. Each of the
serotonin
reuptake inhibitors specified above is intended to be an individual
embodiment.
Accordingly, each of them and the use thereof may be claimed individually.
Other therapeutic compounds, which may benefit from augmentation with GABAB
receptor antagonists, inverse agonist or partial agonists, include compounds,
which
cause an elevation in the extracellular level of 5-HT in the synaptic cleft,
although
they are not serotonin reuptake inhibitors. One such compound is tianeptine.
Accordingly, other compounds than SRIs which cause an elevation in the
extracellular
level of serotonin, may be used instead of SRIs in every aspect of the
invention as
described herein.
The above list of serotonin reuptake inhibitors and other compounds, which
cause an
increase in the extracellular level of serotonin, may not be construed as
limiting.
SRIs, which are particularly preferred according to the present invention,
include
citalopram, escitalopram, fluoxetine, sertraline, paroxetine, fluvoxamine,
venlafaxine,
dapoxetine, duloxetine, vilazodone, nefazodone, imipramin, femoxetine and
clomipramine.
The term selective serotonin reuptake inhibitor (SSRI) means an inhibitor of
the
monoamine transporters, which has stronger inhibitory effect at the serotonin
transporter than the dopamine and the noradrenaline transporters. Particularly
CA 02579520 2007-03-13
_~- -- ~
~"2004/00032,6 ~.'CT/DK2003/000412
19
preferred SSRIs according to the invention are citalopram, escitalopram,
fluoxetine,
fluvoxaniine, sertraline, duloxetine, vilnazodone and paroxetine.
ln particular individual embodiments, citalopram or escitalopram is used.
The following list contains a number of GABAB antagonists, partial agonists or
inverse agonists, which may be used according to the invention: CGP-71982, CGP-
76290, CGP-76291, CGP-35348, CGP-36742, CGP-46381, CGP-52432, CGP-54626,
CGP-55845, CGP-62349, SCH 50911, GAS-360, Phaclofen, Saclofen, 2-
hydroxysaclofen. Each of the GABAB antagonists, partial agonists or inverse
agonists
specified above is intended to be an individual embodiment. Accordingly, each
of
them may be claimed individually.
In a preferred embodiment, a GABAB receptor ligand selected from CGP 71982,
CGP
is 76290, CGP 55845 and CGP 62349.
In particular individual embodiments, Phaclofen, 2-hydroxysaclofen or CGP-
46381 is
used.
Whenever mentioned, each of the terms "GABAB antagonist, partial agonist or
inverse agonist", "GABAB receptor antagonist, partial agonist or inverse
agonist",
"GABAB ligand", and "GABAB receptor ligand" means GABAB receptor antagonist,
partial GABAB receptor agonist and inverse GABAB receptor agonist. Each of
which
is intended to be an individual embodiment. Accordingly, each of these
embodiments
and the use thereof may be claimed individually.
A particular embodiment relates to a GABAB receptor antagonist and the use
thereof.
Pharmaceutical compositions
Each of the active ingredients according to the invention may be administered
alone
or together or in combination with phannaceutically acceptable carriers or
excipients,
in either single or multiple doses. The pharmaceutical compositions according
to the
invention may be fonnulated with pharmaceutically acceptable carriers or
diluents as
CA 02579520 2007-03-13
= ~_ _ _ - ~--- __ . .
WO 2004/0OM -'- CT/DK2003/000412
well as any other known adjuvants and excipients in accordance with
conventional
techniques such as those disclosed in Remington: The Science and Practice of
Phannacy, 19 Edition, Gennaro, Ed., Mack Publishing Co.; Easton, PA, 1995.
5 The pharmaceutical compositions may be specifically formulated for
administration
by any suitable route such as the oral, rectal, nasal, pulmonary, topical
(including
buccal and sublingual), transdermal, intracistemal, intraperitoneal, vaginal
and
parenteral (iiicludinb subcutaneous, intramuscular, intrathecal, intravenous
and
intradermal) routc, thc oral route being preferred. It will be appreciated
that the
10 preferred routc wi I I depend on the general condition and age of the
subject to be
treated, the nature of the condition to be treated and the specific active
ingredient or
active ingredients chosen.
Pharmaceutical compositions for oral administration include solid dosage forms
such
15 as capsules, tablets, dragces, pills, lozenges, powders and granules. Where
appropriate, they may be prepared with coatings such as enteric coatings or
they may
be formulated so as to provide controlled release of one or more active
ingredient
such as sustained or prolonged release according to methods well known in the
art.
20 Liquid dosage fornis for oral administration include solutions, emulsions,
suspensions, syrups and elixirs.
Pharmaceutical compositions for parenteral administration include sterile
aqueous and
nonaqueous injectable solutions, dispersions, suspensions or emulsions as well
as
sterile powders to be reconstituted in sterile injectable solutions or
dispersions prior to
use. Depot injectable formulations are also contemplated as being within the
scope of
the present invention.
Other suitable administration forms include suppositories, sprays, ointments,
cremes,
gels, inhalants, dermal patches, implants etc.
The pharmaceutical compositions of this invention or those which are
manufactured
in accordance with this invention may be administered by any suitable route,
for
CA 02579520 2007-03-13
WO 2004/000326 PC'OM003/000 312
21
example orally in the fonn of tablets, capsules, powders, syrups, etc., or
parenterally
in the fonn of solutions for injection. For preparing such compositions,
methods well
known in the art may be used, and any pharmaceutically acceptable carriers,
diluents,
excipients or other additives normally used in the art may be used.
A typical oral dosage of each of the active ingredients is in the range of
from about
0.001 to about 100 mg/kg body weight per day, preferably from about 0.01 to
about
50 mg/kg body weight per day, and more preferred from about 0.05 to about 10
mg/kg
body weight per day administered in one or more dosages such as 1 to 3
dosages. The
to exact dosage will depend upon the frequency and mode of administration, the
sex,
age, weight and general condition of the subject treated, the nature and
severity of the
condition treated and any concomitant diseases to be treated and other factors
evident
to those skilled in the art.
For parenteral routes such as intravenous, intrathecal, intramuscular and
similar
administration, typically doses are in the order of about half the dose
employed for
oral administration.
The compounds of this invention are generally utilized as the free substance
or as a
phannaceutically acceptable salt thereof. One example is a base addition salt
of a
compound having the utility of a free acid. When an active ingredient contains
a free
acid such salts are prepared in a conventional manner by treating a solution
or
suspension of a free acid of the active ingredient with a chemical equivalent
of a
pharmaceutically acceptable base.
For parenteral administration, solutions of one or more active ingredient in
sterile
aqueous solution, aqueous propylene glycol, aqueous vitamin E or sesame or
peanut
oil may be employed. Such aqueous solutions should be suitably buffered if
necessary
and the liquid diluent first rendered isotonic with sufficient saline or
glucose. The
aqueous solutions are particularly suitable for intravenous, intramuscular,
subcutaneous and intraperitoneal administration. The sterile aqueous media
employed
are all readily available by standard techniques known to those skilled in the
art.
CA 02579520 2007-03-13
WO 2004/000326 PCT/DK2003/000'M
22
Solutions for injections may be prepared by dissolving one or more active
ingredients
and possible additives in a part of the solvent for injection, preferably
sterile water,
adjusting the solution to a desired volume, sterilising the solution and
filling it in
suitable ampules or vials. Any suitable additive conventionally used in the
art may be
added, such as tonicity agents, preservatives, antioxidants, etc.
Suitable pharmaceutical carriers include inert solid diluents or fillers,
sterile aqueous
solution and various organic solvents.
Examples of solid carriers are lactose, terra alba, sucrose, cyclodextrin,
talc, agar,
pectin, acacia, stearic acid and lower alkyl ethers of cellulose corn starch,
potato
starch, talcum, magnesium stearate, gelatine, lactose, gums, and the like.
Any otlier adjuvants or additives usually used for such purposes such as
colourings,
flavourings, preservatives etc. may be used provided that they are compatible
with the
active ingredient or ingredients used.
Examples of liquid carriers are syrup, peanut oil, olive oil, phospho lipids,
fatty acids,
fatty acid amines, polyoxyethylene and water. Similarly, the can-ier or
diluent may
include any sustained release material known in the art, such as glyceryl
monostearate
or glyceryl distearate, alone or mixed with a wax.
The pharmaceutical compositions formed by combining one or more active
ingredients of the invention with the pharmaceutical acceptable carriers are
then
readily administered in a variety of dosage forins suitable for the disclosed
routes of
administration. The formulations may conveniently be presented in unit dosage
fonn
by methods known in the art of pharmacy.
The active ingredients of the invention may be formulated in similar or
dissimilar
pharmaceurical compositions and unit forms thereof.
If a solid carrier is used for oral administration, the preparation may be
tablette,
placed in a hard gelatine capsule in powder or pellet form or it may be in the
fonm of a
CA 02579520 2007-03-13
WO 2004/000326 PCT/DK2003/00041
23
troche or lozenge.
The amount of solid carrier will vary widely but will usually be from about 25
mg to
about 1 g.
If a liquid carrier is used, the preparation may be in the form of a syrup,
emulsion, soft
gelatine capsule or sterile injectable liquid such as an aqueous or non-
aqueous liquid
suspension or solution.
If desired, the pharmaceutical composition of the invention may comprise one
or more
active ingredients in combination with further pharmacologically active
substances
sucli as those described in the foregoing.
Short description of figures
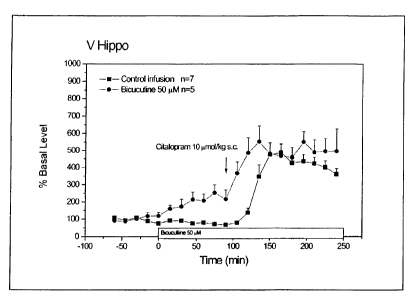
Figure 1. Effect of local administration of bicuculline (50 M), followed by
systemic
administration of citalopram 10 mol/kg s.c.
Figure 2. Effect of local administration of phaclofen (50 M), followed by
systemic
administration of citalopram 10 mol/kg s.c.
Figure 3. Effect of co-administration of phaclofen (2 mg/kg s.c.), followed by
systemic administration of citalopram (10 gmoVkg s.c.).
Figure 4. Effect of administration of GABAB antagonist 2-hydroxysaclofen
(2mg/kg
s.c.) on citalopram induced increase of 5-HT levels in rat ventral
hippocampus.
F(1,172)=3.01, P<0.05. citalopram 10 niol/kg s.c., n=13, citalopram 10
mol/kg s.c.
and 2-hydroxysaclofen 2 mg/kg s.c. n=3.
Figure S. Effect of administration of GABAB antagonist CGP 46381 (mg/kg s.c.)
on
citalopram induced increase of 5-HT levels in rat ventral hippocampus;
citalopram 10
CA 02579520 2007-03-13
-~-~- ~ ~
WO 2004/000326 PCT/DK2003/000412
24
lLmol/kg s.c., n=13, citalopram 10 pmol/kg s.c. and CGP 46381 2 mg/kg s.c.
n=5;
citalopram 10 gmol/kg s.c. and CGP 46381 0.5 mg/kg s.c_n=5; CGP 46381 10
mg/kg s.c. n=2.
Figure 6. Effect of administration of GABAa antagonist Phaclofen (2mg/kg s.c.)
on
citalopram induced increase of 5-HT levels in rat prefrontal cortex.
F(1,198)=3.25,
P<0.05. citalopram 10 mol/kg s.c., n=13, citalopram 10 mol/kg s.c. and
phaclofen 2
mg/kg s.c. n=4.
Materials and Methods
Animals
Male albino rats of a Wistar-derived strain (285-320 g; Harlan, Zeist, The
Netherlands) were used for the experiments. Upon surgery, rats were housed
individually in plastic cages (35 x 35 x 40 cm), and had free access to food
and water.
Animals were kept on a 12 h light schedule (light on 7:00 a.m.). The
experiments are
concordant with the declarations of Helsinki and were approved by the animal
care
committee of the faculty of mathematics and natural science of the University
of
Groningen.
Drugs
The following drugs were used: Citalopram hydrobromide, 2hydroxysaclofen, CGP
46381 (Lundbeck A/S, Copenhagen, Denmark), Phaclofen and (+)-Bicuculline
(Sigma, St Louis, USA).
Surgery
Microdialysis of brain serotonin levels was performed using home made I-shaped
probes, made of polyacrylonitrile / sodium methyl sulfonate copolymer dialysis
fiber
(i.d. 220 gm, o.d. 0.31 m, AN 69, Hospal, Italy). Preceding surgery rats were
CA 02579520 2007-03-13
= . ~- ---~-
~004/000326- PCTIDK2003/000412
anaesthetised using isoflurane (02/N20; 300/300m1/min). Lidocaine-HCI, 10
%(m/v)
was used for local anaesthesia. Rats were placed in a stereotaxic frame (Kopf,
USA),
and probes were inserted into Ventral Hippcampus (V. Hippo, L +4.8 mm, IA:
+3.7
mm, V: -8.0 mm) and median prefrontal cortex (PFC, L -0.9 mm; AP: +3.5 mm
5 relative to bregma; V: -6.0 mm (Paxinos and Watson, 1982). After insertion,
probes
were secured with dental cement.
Microdialysis experiments
10 Rats were allowed to recover for at least 24 h. Probes were perfused with
artificial
cerebrospinal fluid containing 147 mM NaCI, 3.0 mM KCI, 1.2 mM CaCIZ, and 1.2
mM MgClz, at a flow-rate of 1.5 l / min (Harvard apparatus, South Natick,
Ma.,
USA). 15 minute microdialysis samples were collected in HPLC vials containing
7.5
pl 0.02 M acetic acid for serotonin analysis.
Serotonin analysis:
Twenty- l microdialysate samples were injected via an autoinjector (CMA/200
refrigerated microsampler, CMA, Sweden) onto a 100 x 2.0 mm C18 Hypersil 3 m
column (Bester, Amstelveen, the Netherlands) and separated with a mobile phase
consisting of 5 g/L di-ammoniumsulfate, 500 mg/L EDTA, 50 mg/L heptane
sulphonic acid, 4 % methanol v/v, and 30 l/L of triethylamine, pH 4.65 at a
flow of
0.4 ml/min (Shimadzu LC-10 AD). 5-HT was detected amperometrically at a glassy
carbon electrode at 500 mV vs Ag/AgCI (Antec Leyden, Leiden, The Netherlands).
The detection limit was 0.5 fmol 5-HT per 20 gl sample (signal to noise ratio
3).
Data presentation and statistics
Four consecutive microdialysis samples with less then 20 % variation were
taken as
control and set at 100 %. Data are presented as percentages of control level
(mean +
S.E.M.) in time. Statistical analysis was performed using Sigmastat for
Windows
(SPSS, Jandel Corporation). Treatments were compared versus controls using two
CA 02579520 2007-03-13
WO 2004/0 = ' -""-PCT/DK20ff3/000412
26
way analysis of variance (ANOVA) for repeated measurements, followed by
Student
Newman Keuls test. Level of significance level was set at p<0.05.
Results
Local administration of GABAa antagonist bicuculline, followed by systemic
administration of citalopram (fig 1).
Local administration of 50 M bicuculline in ventral hippocampus increased
serotonin levels by about 150 % (treatment vs. time; F(1,79) = 5.20,
P=0.0003). Post-
hoc analysis revealed significance from t=45 to 90 min.
The increase established by systemic administration of 10 mol/kg s.c.
citalopram
was not affected by local application of bicuculline (treatment; F(1,10) =
4.64, P=
0.0567).
Local administration of GABAB antagonist phaclofen, followed by systemic
administration of citalopram (fig 2).
Local infusion of GABAB antagonist Phaclofen did not have any effect on basal
levels
of 5-HT in ventral hippocampus (F(1,9)= 1.44 P=0.26). Systemic administration
of
citalopram during local administration of phaclofen induced augmented levels
of 5-
HT (Treatment F(1,9 )=12.21 P= 0.0068, Treatment vs. Time F(1,112) = 5.03
P<0.0001). Significant differences during post-hoc analysis was attained from
t = 75
to 150 min.
Simultaneous administration ofphaclofen 2 mg/kg s.c. with citalopram 10
,.anol/kg
s. c. (fig 3).
Co-administration of phaclofen 2 mg/kg s.c with citalopram 10 mol/kg s.c.
elicited
enhanced levels of 5-HT when compared to citalopram treatment alone (Treatment
CA 02579520 2007-03-13
WO 2004/000326 ~ NMI T*IUWW031000412-
27
F(1,7)=8.64 P=0.021, Treatment vs. Time, F(1,98)=6.38 P<0.0001). Post-hoc
analysis showed different effects from t=75 to t=135.
Sinzultaneous adnzinistration of 2-hydroxysaclofen (2 mg/kg s. c.) with
citalopram 10
Iunol/kg s.c. on 5-HT levels (fig 4).
Simultaneous administration of citalopram with GABAB antagonist 2-
hydroxysaclofen also induced augmented effects on 5-HT levels. Treatment
F(1,14)=4.80, P= 0.046, treatment versus time F(1,172)=3.01, P=0.0018.
Simultaneous administration qjCGP 46381 with citalopram 10 fanol/kg s.c. on 5-
HT
levels (fig 5).
Administration of a high dose of the GABAB antagonist CGP 46381 (10 mg/kg
s.c.)
did not show any effect on 5-HT levels. However, when CGP 46381 (0.5 and 2
mg/kg) was co-administered with citalopram 10 mol/kg an augmented response on
5-HT levels was observed (0.5 mg CGP; treatment F(1,16)=4.94, p=0.04;
treatment
vs. time (F(1,193)= 3.24, 0.00081); 2 mg cgp treatment F(1,16)=2.94, p=0.10;
treatment vs. time (F(1,192)= 3.79, 0.001)
Simultaneous administration ofphaclofen 2 mg/kg s.c. with citalopram
101.onol/kg
s.c. on 5-HT levels in PFC(fig 6).
Co-administration of phaclofen 2 mg/kg s.c with citalopram 10 mol/kg s.c.
elicited
enhanced levels of 5-HT when compared to citalopram treatment alone (Treatment
F(1,15)=4.61 P=0.048, Treatment vs. Time, F(1,198)=6.3.25 P<0.0008).