Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 26
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 26
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
COMPOSITIONS AND METHODS FOR MODULATING TISSUE REGENERATION
AND CHEMOTACTIC RESPONSES
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Application No.
60/615,213, filed
September 28, 2004, the contents of which are incorporated herein by
reference.
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0002] The present invention relates generally to tissue regeneration and
chemotactic responses.
More specifically, the present invention relates to extracellular matrix
signaling molecules such
as CCN3-related polypeptides.
2. Description of Related Art
[0003] In a normal wound healing response to physical disruption of tissue
such as trauma or
surgery, tissue is regenerated through a complex process requiring the
orchestration of many
different types of cells. After hemostatis, inflammation and cell
proliferation and migration
follow. Inflammation is characterized by vasodilation, increased vascular
permeability,
leukocyte infiltration, bacterial killing, and macrophage-based stimulation of
cellular
proliferation and protein synthesis. In cell proliferation and migration,
fibroblasts appear within
2-3 days and dominate wound cell population during the first week. For the
initial 2-3 days,
their activity is confined to fibroblast replication and migration. Cell
migration is based on
chemotaxis, which is the movement of cells with or against a chemical
gradient. At days 4-5
fibroblasts- begin to synthesize and secrete extracellular collagen. The
collagen is polymerized
and cross-linked to increase the tensile strength of the tissue. Granulation
tissue forms at days 5-
7 and contains numerous capillaries. Granulation tissue has a support matrix
rich in fibroblasts,
inflammatory cells, endothelial cells, myofibroblasts, and periocytes. Later
stages of wound
healing may continue for years, depending on the severity of the wound.
[0004] Angiogenesis is essential to wound repair and scar formation. Capillary
proliferation is
required to support fibroblast migration into the wound and to fulfill
fibroblast metabolic
requirements. In the absence of angiogenesis, such as in ischemic ulcers or
arteriosclerosis
-1-
SUBSTITUTE SHEET (RULE 26)
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
cib'littrark; fibromast r~nigrarion -arrests and fails to proceed.
Angiogenesis has the steps of cell
attachment, basement membrane degradation and migration, proliferation and
differentiation,
and is associated with epithelial cell migration.
[0005] If a patient's injury is severe, or if the patient suffers from a
condition in which the
wound healing response is delayed or inhibited, a treatment that would improve
or accelerate
tissue regeneration would be indicated. For example, in patients with
diabetes, liver failure,
renal impairment, peripheral vascular disease, or in patients taking drugs
that inhibit healing such
as corticosteroids or immunosuppressive agents, a therapy to accelerate wound
healing could
forestall serious consequences of persistent wounds such as infection or
tissue necrosis, and
could reduce the need for amputation.
[0006] Under other circumstances, suppression or inhibition of the wound
healing response
would be appropriate. For example, over-expression of the wound healing
response could result
in conditions such as arthrofibrosis, scleroderma, Dupuytren's contracture,
peritoneal adhesions,
frozen shoulder, and keloid formation. For other therapeutic reasons, it may
also be desirable to
suppress or inhibit a normal wound healing response in some patients.
SUMMARY OF THE INVENTION
[0007] Compositions and methods are provided for monitoring the progression of
wound healing
and modulating tissue regeneration responses. Tissue regeneration may be
stimulated by
administering to a patient compositions comprising a CCN3 polypeptide or a
nucleic acid
encoding CCN3 that is operably linked to a control element allowing expression
of the
polypeptide. Tissue regeneration may be inhibited by administering to a
patient a composition
comprising an inhibitory polypeptide that selectively interferes with binding
of CCN3 to its
target receptors. Tissue regeneration may also be inhibited in a patient by
administering to the
patient a composition comprising an antibody that selectively binds to CCN3.
[0008] Compositions and methods are provided for monitoring wound repair in a
patient by
measuring the level of expression of CCN31nRNA and/or CCN3 protein in a sample
obtained
from the patient, and comparing the level of expression to the level of
expression in a control.
[0009] Methods are also provided for inducing chemotaxis of stem cells to a
desired location,
such as a wound site, in which stem cells are administered to a patient in
combination with a
CCN3 polypeptide.
-2-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
[b01'0]' 1VIbtMdds 'Arid etirihpel'siti ts are also provided for aiding in
tissue construction in vitro.
BRIEF DESCRIPTION OF THE DRAWINGS
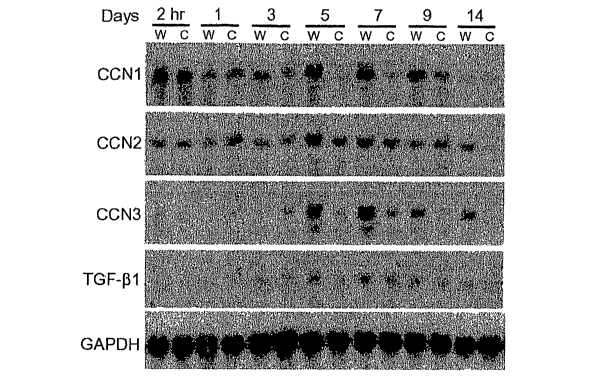
[0011] Figure 1. Expression of CCNI, CCN2, and CCN3 during cutaneous wound
healing. A,
RNA blot of wounded skin. Full-thickness cutaneous excisional wounds were
created on the
back of 2 month old CD-1 mice using a biopsy instrurnent. Mice were sacrificed
from 2 hours
through 15 days post injury as indicated. Total RNA was isolated from wounded
(W) and
control (C, uninjured skin adjacent to the wound site) tissue and analyzed by
RNA blotting with
radiolabeled probes of indicated genes. B, immuostaining of skin wounds. Mice
treated with
cutaneous wounding as described above were sacrificed at indicated days post-
injury.
Cryosections of frozen tissues embedded in OCT were analyzed by immunostaining
affuiity-
purified polyclonal anti-CCN3 antibodies. For negative controls, no primary
antibody was added
(PBS), or anti-CCN3 antibodies (1:1000) were blocked with the CCN3 antigen
(0.5 g/ml; Anti-
CCN3 +GST-CCN3) prior to application on tissue sections. Micrographs were
taken at 100 x
(days 0, 1, and 7) or at 200X magnification (day 5).
[0012] Figure 2. Fibroblast adhesion to CCN3. A, 1064SK skin fibroblasts were
plated in
0
microtiter wells pre-coated with the indicated amount of CCN3. After
incubation at 37 C for 30
min., adherent cells were fixed, stained with methylene blue, and extracted
dye was quantified by
absorbance at 620 nm. B, cells were incubated with GRGDSP, GRGESP polypeptides
(2.0
mM), 0.1 g/ml heparin or in combination prior to plating on microtiter wells
coated with
CCN3, FN. Data shown are mean SD of triplicate determinations and are
representative of
three experiments.
[0013] Figure 3. CCN3 supports fibroblast adhesion through integrins a6(31 and
a5(31.
Fibroblast adhesion was performed as described in Figure 2. Microtiter wells
were coated with
either 3 g/ml CCN3, 0.5 g/ml VN, 2 g/ml FN, 5 g/ml LN, or 0.5 g/ml Type 1
collagen as
indicated. Cells were incubated with 50 g/ml anti-oc5(31 mAb JBS5 (A), 50
g/ml anti-(43
mAb LM609 (B), 20 g/ml anti-a6 mAbs (C), or 40 g/ml anti-(31 mAb P4C10 for 1
hour prior
to plating. Data shown are mean ~: SD of triplicate determinations and are
representative of three
experiments.
[0014] Figure 4. CCN3 induces chemotaxis in fibroblasts. 1064SK fibroblast
migration was
monitored using a modified Boyden chamber assay. Test protein was added to the
bottom
-3-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
"~~ cNiarnl'3~r (ti~nle~s'othei~Vi~e"ihcÃicated) and covered by a gelatinized
polycarbonate filter. Cells
were added to wells in the upper chamber and allowed to migrate for 6 hours at
37~C before
being fixed and stained. Cells that migrated into the lower chamber were
counted in ten random
high power fields. A, cell migration in response to varying concentrations of
CCN3 as indicated.
B, migration of fibroblasts was measured in a checkerboard-type analysis. CCN3
or bFGF (10
ng/ml) was added to the lower chamber, the upper chamber, neither chamber, or
both chambers
as indicated. Data shown are mean SD of triplicate determinations and are
representative of
three experiments.
[0015] Figure 5. Fibroblast migration to CCN3 is a,(35-dependent_ Migration
assays were
performed using a modified Boyden chamber. As chemoattractants, CCN3 (2
g/ml),
vitronectin (10 g/m1), and FN (10 g/ml) were placed in the bottorn chamber.
Cells were
treated with anti-aõ mAb AV1 (50 g/ml), anti-av(35 mAb P1F6 (50 g/ml), anti-
a43 mAb
LM609 (40 g/ml) or anti-integrin (35 mAb (P1D6, 40 g/ml) for 1 hour prior to
chamber
loading. Data shown are mean SD of triplicate determinations and are
representative of three
experiments.
[0016] Figure 6. Direct binding of CCN3 to integrin av(35. A, microtiter wells
were coated with
purified integrin a,,(35 (1 g/ml) and blocked with 1% BSA. Binding of varying
concentrations
of CCN3 was detected using anti-CCN3 antibodies by ELISA. B-D, microtiter
wells were coated
with CCN3 (10 g/ml) or VN (1 g/ml) and blocked with BSA, and binding of
purified av(35 (1
g/ml) was observed by ELISA using anti-a,, antibodies. Effects o f pre-
incubation of coated
proteins with anti-CCN3 antibodies, anti-av(35 mAb P1F6 (50 g/rnl), or 20
g/mi normal mouse
IgG prior to addition and binding of integrin av(35 was observed. In panel C,
integrin ar,(35 was
incubated with 5 mM EDTA, EDTA + 10 mM Mg2+, 0.2 mM GRGDSP polypeptide, or 0.2
mM
GRGESP polypeptide for 30 min. at 4 C prior to addition into microtiter wells.
Data shown are
from three separate experiments and represented as mean SD of triplicate
determinations in
each experiment.
[0017] Figure 7. CCN3 enhances bFGF-induced DNA synthesis. The effect of
soluble CCN3
on bFGF-induced mitogenesis under serum-free conditions was ass essed on
fibroblasts attached
to 24-well plates. Serum-starved cells were treated with 10 ng/ml bFGF, the
indicated
concentration of CCN3, and [3H]thymidine for 21 hours before inc rporation was
measured.
-4-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
Cbritr61s"rlit2ntle smiipti'1e'g'treated with either BSA or 20 g/ml CCN3 in
the absence of bFGF.
Data shown for all panels are mean S.D. of triplicate determinations and are
representative of
duplicate experiments.
[0018] Figure 8. CCN3 induced gene expression in fibroblasts is modulated by
TGF-(31.
Serum-starved human skin fibroblasts were treated for 24 hours with various
concentration (from
0 to 10 g/ml) of CCN3, either in the presence or absence of 20 ng/ml of TGF-
(31. Total RNA
was isolated and analyzed by RNA blotting. Expression level of glyceraldehyde-
3-phosphate
dehydrogenase (GAPDH) was analyzed and served as sample loading control. Serum-
starved
fibroblasts were treated with 10 ng/ml TGF-[31 alone or in combination with
varying
concentrations of CCN3 for 24 hours.
DETAILED DESCRIPTION
[0019] Before the present compounds, products and compositions and methods are
disclosed and
described, it is to be understood that the terminology used herein is for the
purpose of describing
particular embodiments only and is not intended to be limiting. It must be
noted that, as used in
the specification and the appended claims, the singular forms "a," "an" and
"the" include plural
references unless the context clearly dictates otherwise.
1. Definitions
[0020] As used herein, the term "analog", when used in the context of a
peptide or polypeptide,
means a peptide or polypeptide comprising one or more non-standard amino acids
or other
structural variations from the conventional set of amino acids.
[0021] As used herein, the term "antibody" means an antibody of class IgG,
IgM, IgA, IgD or
IgE, or fragments or derivatives thereof, including Fab, F(ab')2, Fd, and
single chain andibodies,
diabodies, bispecific antibodies, bifunctional antibodies, and derivatives
thereof. The antibody
may be a monoclonal antibody, polyclonal antibody, affinity purified antibody,
"huina.nized"
antibody products, CDR-grafted antibody products, or mixtures thereof which
exhibit sufficient
binding specificity to a desired epitope, or a sequence derived therefrom. The
antibody may also
be a chimeric antibody. Also contemplated are antibody fragments. The antibody
products
include the aforementioned types of antibody products used as isolated
antibodies or as
antibodies attached to labels. Labels can be signal-generating enzymes,
antigens, other
antibodies, lectins, carbohydrates, biotin, avidin, radioisotopes, toxins,
heavy metals, and other
-5-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
cbmposittbiftg 'MiWn im Iffe art:' 1me antibody may be derivatized by the
attachment of one or
more chemical, polypeptide, or polypeptide moieties known in the art. The
antibody may be
conjugated with a chemical moiety.
[0022] As used herein "biological activity of CCN3" includes, but is not
limited to, the activities
of full-length CCN3 described herein, and the ability to be bound by an
antibody specific for
CCN3.
[0023] As used herein, the term "derivative", when used in the context of a
peptide or
polypeptide, means a peptide or polypeptide different other than in primary
structure (amino
acids and amino acid analogs). By way of illustration, derivatives may differ
by being
glycosylated, one form of post-translational modification. For example,
peptides or polypeptides
may exhibit glycosylation patterns due to expression in heterologous systems.
If at least one
biological activity is retained, then these peptides or polypeptides are
derivatives according to the
invention. Other derivatives include, but are not limited to, immunogenic
carriers such as
Keyhole Limpet Hemocyanin, radiolabelled peptides or polypeptides, fusion
peptides or fusion
polypeptides having a covalently modified N- or C-terminus, PEGylated peptides
or
polypeptides, peptides or polypeptides associated with lipid moieties,
alkylated peptides or
polypeptides, peptides or polypeptides linked via an amino acid side-chain
ftuictional group to
other peptides, polypeptides or chemicals, and additional modifications as
would be understood
in the art.
[0024] As used herein, the term "fragment", when used in the context of a
peptide or
polypeptide, means a peptide of from about 8 to about 50 amino acids in
length. The fragment
may be 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,
26, 27, 28, 29, 30, 31,
32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 or 50
amino acids in length.
[0025] As used herein, the term "homolog", when used in the context of a
peptide or
polypeptide, means a peptide or polypeptide sharing a common evolutionary
ancestor.
[0026] As used herein, "tissue regeneration" means the growth or regrowth of
tissue, either in
vivo, ex vivo, or in vitro.
[0027] As used herein, the term "treat" or "treating" when referring to
protection of a mammal
from a condition, means preventing, suppressing, repressing, or eliminating
the condition.
Preventing the condition involves administering a composition of the present
invention to a
mammal prior to onset of the condition. Suppressing the condition involves
administering a
-6-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
to a mammal after induction of the condition but before its
clinical appearances. Repressing the condition involves administering a
composition o f the
present invention to a mammal after clinical appearance of the condition such
that the condition
of is reduced or maintained. Elimination of the condition involves
administering a composition
of the present invention to a mammal after clinical appearance of the
condition such that the
mammal no longer suffers the condition.
[0028] As used herein, the term "stem cells" refers to highly proliferative
cells that can give rise
to daughter cells with more than one fate, that is they are pluripotent. Stem
cells may be
autologous or non-autologous.
[0029] As used herein, the term "variant", when used in the context of a
peptide or polypeptide,
means a peptide or polypeptide that differs in amino acid sequence by the
insertion, deletion, or
conservative substitution of amino acids, but retains at least one biological
activity.
2. Modulation of Tissue Regeneration Using CCN3
[0030] CCN3 is shown herein to promote tissue regeneration. As a result, CCN3
polypeptides
may be used to increase tissue regeneration. CCN3 inhibitory polypeptides may
be used to
decrease tissue regeneration. CCN3 polypeptides and inhibitory polypeptides
may be used
alternatively or in combination, depending on the patient's indications and
whether an increase
or decrease of tissue regeneration is desired.
[0031] Modulators of tissue regeneration, such as CCN3 polypeptides, may be
used for treating
wound healing disorders including, but not limited to, diabetic foot and leg
ulcerations, including
neuropathic ulcerations, decubitus lesions, and necrobiosis lipoidica
diabeticorum; vascular
ulcerations, including venous stasis ulceration, arterial ulcerations,
varicose vein ulcerations,
post-thrombotic ulcerations, atrophie blanche ulcerations, congenital absence
of
veins/ulcerations, congenital or traumatic arteriovenous anastomosis, temporal
arteritis,
atherosclerosis, hypertension (Martorell's ulcerations), thrombosis, embolism,
platelet
agglutination, ankle blow-out syndrome, or hemangiomas; decubitus ulcers or
pressure sores
(e.g., with bed rest); traumatic ulcerations, such as those caused by external
injuries, burns,
scalds, chemical injuries, post-surgical injuries, self-inflicted injuries,
lesions at an injection site,
neonatal or perinatal trauma, or sucking blisters; infestations and bites,
such as those caused by
spiders, scorpions, snakes, or fly larvae; cold injury, such as perniosis
(erythrocyanosis frigida),
or cryoglobulinemic ulcerations; neoplastic ulceration, such as those caused
by basal cell
-7-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
1 icartino}tlnag; ,squhriioN1a11 6'dreknomas, malignant melanomas, lymphoma,
leukemia, Kaposi's
sarcoma, tumor erosion, midline lethal granuloma, or Wegener's granulomatosis;
blood diseases
with ulcerations, such as polycythemia, spherocytosis, or sickle cell anemia;
skin diseases with
ulcerations, such as tinea, psoriasis, pemphigoid, pemphigus, neurotic
excoriations,
trichotillomania, erosive lichen planus, or chronic bullous dermatosis of
childhood; metabolic
disease ulcerations, such as those associated with diabetes mellitus or gout
(hyperuricemia);
neuropathic ulcerations, such as those associated with diabetes mellitus,
tabes dorsalis, or
syringomyelia; ischemic ulcerations, such as those associated with scars,
fibrosis, or radiation
dermatitis; vasculitis ulcerations, such as those associated with lupus
erytheinatosus, rheumatoid
arthritis, scleroderma, immune complex disease, pyoderma gangrenosum, or
ulceration
associated with lipodermatosclerosis; infectious ulcerations, such as: viral
ulcerations, e.g. those
associated with Herpes simplex or Herpes zoster in an immunocompromised or
normal
individual; bacterial infections with ulcerations, such as those associated
with tuberculosis,
leprosy, swimming pool granuloma, ulceration over osteomyelitis, Buruli ulcer,
gas gangrene,
Meleny's ulcer, bacterial gangrene associated with other bacterial infection
(e.g., streptococcal
infection), scalded skin syndrome, ecthyma gangrenosum (such as can occur in
children infected
with Pseudomonas aeruginosa), and toxic epidermal necrolysis; mycotic
ulcerations, such as
those associated with superficial fungal infection or deep fungal infection;
spirochetal
ulcerations, such as those associated with syphilis or yaws; leishmaniasis;
mydriasis; or cellulitis;
surgical ulcerations, such as those associated with closed incisions or
excisions, open incisions or
excisions, stab wounds, necrotic incisions or excisions, skin grafts, or donor
sites; or other
ulcerations, such as those associated with skin tears (traumatic ulcerations),
fistula, peristomal
ulcerations, ulcerations associated with aplasia cutis congenita, ulcerations
associated with
epidermolysis bullosa, ulcerations associated with ectodermal dysplasias,
ulcerations associated
with congenital protein C or S deficiency, ulcerations associated with
congenital erosive and
vesicular dermatosis, ulcerations associated with acrodermatitis
enteropathica, and amputation
stump ulcerations.
[0032] Modulators of tissue regeneration, for example, CCN3 inhibitory
polypeptides, may be
used in treatment of disorders such as arthrofibrosis, Dupuytren's
contracture, peritoneal
adhesions, frozen shoulder, scleroderma, and keloid formation.
-8-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
" [0033]''-NIcidV2dtbts ,oi'ti stid~~tegeneration may also be used in tissue
and organ construction in
vitro.
a. CCN3
[0034] During embryonic development, CCN3 is widely expressed in derivatives
of all three
germ layers, with high levels of expression in skeletal muscle, smooth muscle
of vessel walls, the
nervous system, adrenal cortex, and differentiating chondrocytes. Consistent
with a role in
development, CCN3 interacts with the epidermal growth factor-like domain of
Notchl and
regulates Notch signaling. CCN3 is associated with the ECM upon secretion,
interacts with
fibulin in a yeast two-hybrid system and may regulate calcium signaling.
Aberrant expression of
CCN3 has been identified in a variety of tumors and in vascular injury. CCN3
can induce
angiogenesis in vivo and promote pro-angiogenic activities in endothelial
cells in culture. Thus,
CCN3 supports cell adhesion, induces chemotaxis, enhances growth factor-
induced DNA
synthesis, and promotes cell survival in vascular endothelial cells.
Mechanistically, these
activities are mediated through interactions with integrin receptors in a
context-dependent
manner. Endothelial cell adhesion to CCN3 is mediated through integrins
a,,(33, a6(31, and a5(31,
with HSPGs serving as coreceptors of a6(31. CCN3-induced endothelial cell
chemotaxis is
mediated through av(33 and a5(31. Although CCN3 does not contain an RGD
sequence, it is a
direct ligand of integrins a,,(33 and a5[31 as demonstrated by solid phase
binding assays.
[0035] Since CCN3 is expressed in hypertrophic cartilage, where vessel growth
is required for
the formation of a scaffold onto which the osteoblasts settle and deposit bone
matrix, CCN3-
induced angiogenesis may be important in endochondral ossification. During
nephrogenesis,
CCN3 is localized to the metanephric mesenchyme into which endothelial cells
are recruited.
These endothelial cells then proliferate and form a capillary network as the
metanephric
mesenchyme develops to form the glomeruli, the basic units of filtration.
[0036] CCN3 expression is also detected in various tumors, including Wilm's
tumors, and
benign adrenocortical tumors. It is well established that tumor growth beyond -
1 mm in size
requires the growth of new vessels to provide the necessary blood supply.
Furthermore, the
Wilms' Tumor suppressor gene (WT1) negatively regulates CCN3 expression. As
part of its
tumor suppressing function, WT1 down-regulates the angiogenic inducer CCN3.
[0037] In contrast to CCNI and CCN2, which are transcriptionally activated by
mitogenic
growth factors and repressed under conditions of growth arrest, CCN3 is
induced by growth
-9-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
a5rrestanct etown=regutatect tj'y-growtn iactors. TGF-(31 strongly induces
CCNI and CCN2, while
it represses CCN3. Thus, CCN3 is regulated in an antithetical manner compared
to CCNI and
CCN2, suggesting that they may serve opposing functions.
[0038] As shown in the examples, CCN3 regulates angiogenesis and fibroblast
functions during
wound healing. Specifically, the expression of CCN3 is upregulated during
wound healing, with
peak levels observed 5-7 days after wounding. Furthermore, CCN3 functions
through specific
integrins to enhance growth factor-induced DNA synthesis, support fibroblast
adhesion, and
induce fibroblast chemotaxis.
[0039] Additionally, CCN3 cooperates with TGF-(31 in an antagonistic or
synergistic manner in
the regulation of specific genes. CCN3 induces neovascularization in vivo, and
promotes pro-
angiogenic activities in endothelial cells. In contrast to CCN1 and CCN2, CCN3
does not
upregulate VEGF-A expression in human skin fibroblasts. While the potential
role of VEGF-A
upregulation in the angiogenic function of CCN1 and CCN2 has not been
established, the
angiogenic function of CCN3 is unlikely to be mediated through VEGF-A.
However, CCN3 is
able to'upregulate MMP-1 (Figure 8) and MMP-3 proteases that play a role in
matrix remodeling
and angiogenesis during wound healing. The ability of TGF-(3 to repress MMP-1
is dominant
over the upregulation of MMP-1 by CCN3, and CCN3 works synergistically with
TGF-(3 to
upregulate PAI-1 expression (Figure 8). Thus, the bioavailability of CCN3
and/or TGF-(3 in the
cellular microenvironment may profoundly influence the pattern of gene
expression in various
cell types that participate in wound healing. This microenvironment may also
be dynamic, thus
allowing for finely tuned up- or down-regulation of genes such as MMP-1.
[0040] Many of the cellular processes that occur in wound healing are also
observed in the tumor
microenvironment, leading to the proposed notion that tumor stroma is "normal
wound healing
gone awry". Consistently, CCN3 is not only expressed during wound healing, but
is associated
with adrenocortical tumors, cartilage neoplasia, hepatocellular carcinomas,
musculoskeletal
tumors, prostate cancer, and Wilm's tumors. It is likely that both the
angiogenic activity of
CCN3 and its activities upon stromal fibroblasts play a role in tumor growth
as well as wound
healing. CCN3 also interacts with Notchl through its CT domain and modulate
Notch signaling.
Notchl plays a critical role in cell fate determination and lymphocyte
development, and is
implicated in keratinocyte differentiation during wound healing. Thus, CCN3
may regulate
Notch signaling in the context of both embryonic development and tissue
repair.
-10-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
(01' CCNYPW~[i,6~tides and Inhibitory Polypeptides
[0041] CCN3 polypeptides include, but are not limited to, polypeptides
comprising SEQ ID
NO: 1, as well as analogs, derivatives, fragments, homologs and variants
thereof that are at least
75%, 80%, 85%, 90%, 95%, 97%, 98% or 99% identical to SEQ ID NO: 1. The CCN3
polypeptides may comprise the amino acid sequence GQKCIVQTTSWSQCSKS (SEQ ID
NO: 3). The polypeptides may be natural, recombinant or synthetic. The
polypeptides may have
at least one biological activity of CCN3. The polypeptides may also be
inhibitory polypeptides
that are inhibitors or antagonists of CCN3 activity. The CCN3 polypeptides may
also be
antibodies that specifically bind to CCN3.
[0042] The CCN3 polypeptides may comprise one or more of the following CCN3
domains:
insulin growth factor-binding protein (amino acids 47-94), von Willebrand
(vWE) type C
domain (amino acids 110-170) or C-terminal cysteine knot-like domain (amino
acids 269-338).
[0043] The polypeptide may comprise amino acids from SEQ ID NO: 1 selected
from the group
consisting of 1-5, 3-7, 6-10, 8-12, 11-15, 13-17, 16-20, 18-22, 21-25, 23-27,
26-30, 28-32, 31-35,
33-37, 36-40, 38-42, 41-45, 43-47, 46-50, 48-52, 51-55, 53-57, 56-60, 58-62,
61-65, 63-67, 66-
70, 68-72, 71-75, 73-77, 76-80, 78-82, 81-85, 83-87, 86-90, 88-92, 91-95, 93-
97, 96-100, 98-
102, 101-105, 103-107, 106-110, 108-112, 111-115, 113-117, 116-120, 118-122,
121-125, 123-
127, 126-130, 128-132, 131-135, 133-137, 136-140, 138-142, 141-145, 143-147,
146-150, 148-
152, 151-155, 153-157, 156-160, 158-162, 161-165, 163-167, 166-170, 168-172,
171-175, 173-
177, 176-180, 178-182, 181-185, 183-187, 186-190, 188-192, 191-195, 193-197,
196-200, 198-
202, 201-205, 203-207, 206-210, 208-212, 211-215, 213-217, 216-220, 218-222,
221-225, 223-
227, 226-230, 228-232, 231-235, 233-237, 236-240, 238-242, 241-245, 243-247,
246-250, 248-
252, 251-255, 253-257, 256-260, 258-262, 261-265, 263-267, 266-270, 268-272,
271-275, 273-
277, 276-280, 278-282, 281-285, 283-287, 286-290, 288-292, 291-295, 293-297,
296-300, 298-
302, 301-305, 303-307, 306-310, 308-312, 311-315, 313-317, 316-320, 318-322,
321-325, 323-
327, 326-330, 328-332, 331-335, 333-337, 336-340, 338-342, 341-345, 343-347,
346-350, 348-
352, 351-355, and 353-357.
[0044] The polypeptide may also comprise amino acids from SEQ ID NO: 1
selected from the
group consisting of 1-10, 6-19, 11-20, 16-29, 21-30, 26-39, 31-40, 36-49, 41-
50, 46-59, 51-60,
56-69, 61-70, 66-79, 71-80, 76-89, 81-90, 86-99, 91-100, 96-109, 101-110, 106-
119, 111-120,
116-129, 121-130, 126-139, 131-140, 136-149, 141-150, 146-159, 151-160, 156-
169, 161-170,
-11-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
O9, 11 1 f ="1'$~ "176u ~i'$~~;:::'1 kl-~'90, 186-199, 191-200, 196-209, 201-
210, 206-219, 211-220,
216-229, 221-230, 226-239, 231-240, 236-249, 241-250, 246-259, 251-260, 256-
269, 261-270,
266-279, 271-280, 276-289, 281-290, 286-299, 291-300, 296-309, 301-310, 306-
319, 311-320,
316-329, 321-330, 326-339, 331-340, 336-349, 341-350, and 346-357.
[0045] The polypeptide may also comprise amino acids from SEQ ID NO: 1
selected from the
group consisting of 1-15, 8-22, 16-30, 23-37, 31-45, 38-52, 46-60, 53-67, 61-
75, 68-82, 76-90,
83-97, 9 1-105, 98-112, 106-120, 113-127, 121-135, 128-142, 136-150, 143-157,
151-165, 158-
172,166-180, 173-187, 181-195, 188-202, 196-210, 203-217, 211-225, 218-232,
226-240, 233-
247, 241-255, 248-262, 256-270, 263-277, 271-285, 278-292, 286-300, 293-307,
301-315, 308-
322, 316-330, 323-337, 331-345, 338-352, and 346-357.
(2) Formulations of CCN3 polypeptides
[0046] Compositions comprising a CCN3 polypeptide may further comprise one or
more
pharmaceutically acceptable additional ingredients such as carriers,
excipients, diluents such as
water or nonaqueous vehicles, antimicrobial agents, and the like. The
compositions may contain
between about 5% and 60% of an active component by weight.
[0047] Compositions may be in the form of tablets, capsules, dispersible
powders, granules, or
lozenges formulated in a conventional manner. For example, tablets and
capsules for oral
administration may contain conventional excipients including, but not limited
to, binding agents,
fillers, lubricants, disintegrants, wetting agents, buffers, flavoring agents,
and coloring agents.
Binding agents include, but are not limited to, syrup, acacia, gelatin,
sorbitol, tragacanth,
mucilage of starch and polyvinylpyrrolidone. Fillers include, but are not
limited to lactose,
sugar, microcrystalline cellulose, maizestarch, calcium phosphate, and
sorbitol. Lubricants
include, but are not limited to, magnesium stearate, stearic acid, talc,
polyethylene glycol, and
silica. Disintegrants include, but are not limited to, potato starch and
sodium starch glycolate.
Wetting agents include, but are not limited to, sodium lauryl sulfate. Tablets
may be coated
according to methods known in the art.
[0048] Compositions may also be liquid formulations including, but not limited
to, aqueous or
oily suspensions, solutions, emulsions, syrups, and elixirs. The compositions
may also be
formulated as a dry product for constitution with water or other suitable
vehicle before use. Such
liquid preparations may contain additives including, but not limited to,
suspending agents,
emulsifying agents, buffers, flavoring agents, coloring agents, nonaqueous
vehicles and
-12-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
Lnclude, but are not limited to, sorbitol syrup, methyl cellulose,
glucose/sugar syrup, gelatin, hydroxyethylcellulose, carboxymethyl cellulose,
aluminum stearate
gel, and hydrogenated edible fats. Suspensions may contain, for example, from
about 0.05% to
5% of suspending agents. Syrups may contain, for example, from about 10% to
50% of sugar.
Emulsifying agents include, but are not limited to, lecithin, sorbitan
monooleate, and acacia.
Nonaqueous vehicles include, but are not limited to, edible oils, almond oil,
fractionated coconut
oil, oily esters, propylene glycol, and ethyl alcohol. Elixirs may contain,
for example, from
about 20% to 50% of ethanol. Preservatives include, but are not limited to,
methyl or propyl p-
hydroxybenzoate and sorbic acid.
[0049] Compositions may also be formulated as suppositories which may contain
suppository
bases including, but not limited to, cocoa butter or glycerides. Compositions
may also be
formulated for inhalation, which may be in a form including, but not limited
to, a solution,
suspension, or emulsion that may be administered as a dry powder or in the
form of an aerosol
using a propellant, such as dichlorodifluoromethane or trichlorofluoromethane.
Compositions
may also be formulated in transdermal formulations comprising aqueous or
nonaqueous vehicles
including, but not limited to, creams, ointments, lotions, pastes, medicated
plaster, patch, or
membranes.
[0050] Compositions may also be formulated for parenteral administration
including, but not
limited to, by injection or continuous infusion. Formulations for injection
may be in the form of
suspensions, solutions, or emulsions in oily or aqueous vehicles, and may
contain buffers or
formulation agents including, but not limited to, suspending, stabilizing, and
dispersing agents.
Suspensions may contain from about 0.05% to about 5% suspending agent in an
isotonic
medium. Formulations for injection may also include adjuvants. The composition
may also be
provided in a powder form for reconstitution with a suitable vehicle
including, but not limited to,
sterile, pyrogen-free water.
[00511 Compositions may also be formulated as a depot preparation. The
liposome preparation
may comprise liposomes which penetrate the cells of interest, and fuse with
the cell membrane,
resulting in delivery of t][ae contents of the liposome into the cell. For
example, liposomes such
as those described in U.S. Pat. No. 5,077,211 to Yarosh, U.S. Pat. No.
4,621,023 to Redziniak, et
al., or U.S. Pat. No. 4,508,703 to Redziniak, et al. can be used. Other
suitable formulations can
employ niosomes. Niosomes are lipid vesicles similar to liposomes with
membranes consisting
-13-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
rargeiy or Monsionic iipIas;"sorne iorms of which are effective for
transporting compounds across
the stratum corneum.
b. Treatment
(1) Inducing tissue regeneration.
[0052] Tissue regeneration may be induced or accelerated by the administration
of CCN3
polypeptides to a patient whose symptoms so indicate. Upon administration, the
CCN3
polypeptide may replace or augment the patient's native CCN3 with respect to
the fibroblast
interactions and angiogenesis required for wound healing. The CCN3 polypeptide
may be
administered locally or systemically in any of the formulations or by any of
the methods of
administration described above.
[0053] In addition, tissue regeneration can be stimulated by administering to
a patient a nucleic
acid encoding a CCN3 polypeptide. The nucleic acid may be operably linked to a
promoter
which controls expression of CCN3 polypeptide directly in patient's cells at a
wound site. The
promoter may be selected from promoters that are either specific for a
particular type of human
cells (e.g., K14 promoter) or promoters that ensure expression of transgenic
CCN3 in all cell
types found at a wound site (e.g., thymidine kinase promoter).
[0054] Tissue regeneration may also be stimulated by the administration of
stem cells to a
patient in combination with the administration of a CCN3 polypeptide. The CCN3
polypeptide
may be administered in any manner that leads to delivery of the CCN3
polypeptide to the desired
site. For example, the CCN3 polypeptide may be injected directly to the
desired site or may be
conjugated with a targeting agent that allows targeting of the CCN3
polypeptide to the desired
site. Upon delivery of the CCN3 to the desired site, the CCN3 polypeptide may
stimulate the
stem cells to chemotax toward the site.
[0055] Cells such as fibroblasts or endothelial cells may be retained at a
wound site or desired
site by the delivery of CCN3 polypeptide to the site. Other types of cells,
including but not
limited to stem cells, may also be retained at a site by the delivery of CCN3
polypeptides.
(2) Inhibiting tissue regeneration by blocking CCN3 activities.
[0056] CCN3 inhibitory polypeptides may be administered to a patient whose
symptoms indicate
the need to inhibit tissue regeneration. Upon the administration of CCN3
inhibitory
polypeptides, the polypeptides may compete with the patient's native CCN3 for
receptor binding
sites. The angiogenesis and cell migration activities of CCN3 may be decreased
accordingly,
-14-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
arYtt rne patient' s"GLivs- trssn:e regeneration activity may thereby be
diminished or appropriately
modulated. Inhibitory polypeptides may be administered directly, or DNA
encoding the
inhibitory polypeptides may be administered as gene therapy.
[0057] Tissue regeneration may also be inhibited by blocking CCN3 activity
with a
pharmaceutical composition comprising an antibody that specifically binds to
CCN3 and blocks
its activity.
c. Methods of diagnosis/screening of CCN3 levels in monitoring wound healing
[0058] Levels of CCN3 may be screened or monitored to evaluate a patient's
need for
intervention in treating a wound or an area of tissue regeneration. A biopsy
may be taken from a
wound site or another site of interest. Levels of the CCN3 protein may be
measured in the
sample and compared with CCN3 expression in a control, such as a sample from a
healthy
patient. Protein levels may be measured by Western blot, histological
immunostaining, ELISA,
or by another suitable method known to those of skill in the art.
Alternatively, levels of n1RNA
encoding CCN3 from the patient's biopsy may be measured against those of a
control. Levels of
mRNA may be measured by Northern hybridization, RNA dot-blot, RT-PCR, in situ
hybridization, or by another suitable method known to those of skill in the
art.
d. Tissue Construction in vitro.
[00591 In an in vitro tissue culture, CCN3 polypeptide rrnay be added to
induce or accelerate one
or more CCN3 activities such as angiogenesis or cell migration. Cultured
tissue for use in
treatment of wounds may require cells to mature and segregate properly to form
the tissue
components that exist at the wound site. To this end, adrninistration of a
CCN3 polypeptide may
facilitate angiogenesis. Likewise, CCN3 may also be used to facilitate cell
migration in cultures
where the localization of cells (e.g., fibroblasts) is desirable, or for the
production of tissue
having a particular shape. The addition of a CCN3 poly]peptide may also
prevent cells from
migrating uncontrollably during tissue construction. Tissue cultured by this
method may be used
to treat wounds, including but not limited to surgical wounds, burns, or other
injuries, or to treat
patients in need of tissue replacement such as joint replacement or heart
valve replacement.
(3) Administration
[0060] Compositions may be administered in any manner including, but not
limited to, orally,
parenterally, sublingually, transdermally, rectally, transmucosally,
topically, via inhalation, via
buccal administration, or combinations thereof. Parenteral administration
includes but is not
-15-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
limiteel to,"iht'r'a~e~i'61ts;"Ynt~~a~t~rr'ztl, intraperitoneal, subcutaneous,
intra.muscular, intrathecal,
and intraarticular. The compositions may also be administered in the form of
an implant, which
allows slow release of the compositions as well as a slow, controlled
intravenous infusion.
(4) Dosage
[0061] The effective dosage of an active ingredient employed may vary
depending on the
particular composition employed, the mode of administration and the severity
of the condition
being treated, and is ultimately determined by the attendant physician.
However, in general,
satisfactory results are obtained when the compositions of the invention are
administered at a
daily dosage from about 0.5 to about 500 mg/kg of animal body weight. Dosage
forms suitable
for internal use comprise from about 0.5 to 500 mg of the active composition
in admixture with a
solid or liquid pharmaceutically acceptable carrier. This dosage regimen may
be adjusted to
provide the optimal therapeutic response. For example, several divided doses
may be
administered daily or the dose may be proportionally reduced as indicated by
the exigencies of
the therapeutic situation.
[0062] A number of factors may lead to the polypeptides being administered at
a wide range of
dosages. When given in combination with other therapeutics, the dosage of the
composition may
be given at relatively lower dosages. In addition, the use of targeting agents
may allow the
necessary dosage to be relatively low.
[0063] The present invention has multiple aspects, illustrated by the
following non-limiting
examples. Example 1 teaches cloning, expression and purification of
recombinant CCN3.
Example 2 discloses the production of anti-CCN3 antibodies. Example 3
demonstrates that
CCN3 induces neovasculization. Example 4 discloses kinetics of CCN3 expression
during
wound healing. Examples 5 discloses that CCN3 mediates fibroblast adhesion
through integrins
a5(31, a6(31 and HSPGs. Example 6 teaches how CCN3 controls chemotactic
responses.
Example 7 provides a method for CCN3 dependent control of chemotactic
responses. Example 8
teaches that CCN3 enhances bFGF-induced DNA synthesis in fibroblasts- Example
9 teaches
that CCN3 can regulate genes that control matrix remodeling. These exarnples
are intended to be
illustrative and should not be construed to limit the scope of the invention.
-16-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
Example 1
Purification of Recombinant CCN3
[0064] Human CCN3 cDNA was constructed by ligation of a 5' (nt 72-654, Genbank
X96584)
and a 3' (nt 654-1653) fragments, and the resulting full-length cDNA was
cloned into pKS+ and
verified by sequencing. The 5' fragment (nt 72-654) was obtained by reverse
transcriptase-
polymerase chain reaction using total RNA isolated from serum-starved human
skin fibroblasts
using the primer set 5'-AGCAGTGCCAATCTACAGC-3' (SEQ ID NO: 4) and 5'-
CAGCATCTCACATTGACGG-3' (SEQ ID NO: 5). The RT-PCR product was digested with
Sphl and Styl to yield a fragment containing nt 72-654. The 3' fragment (nt
654-1653) was
generated by restriction digestion of IMAGE clone #49415 (human neonatal
brain, nt 590-1653)
with StyI and XbaI. To produce recombinant CCN3 protein, the full-length CCN3
eDNA was
cloned into the baculovirus expression vector pBlueBac 4.5 (Invitrogen,
Carlsbad, CA). The
vector was modified to encode an enterokinase histidine tag linked to the C-
terminus of CCN3.
[0065] CCN3 was produced in serum-free baculovirus expression system using
High Five insect
cells as described. Briefly, High Five cells were maintained in serum-free EX-
CELL 400
medium (JRH Bioscience, Lenexa, KS) at 27 C and infected at a multiplicity of
infection of 10.
Conditioned medium was collected at 38 h post-infection, adjusted to 20 mM
sodium phosphate
and applied to a Sepharose SP (Sigma-Aldrich, St. Louis, MO) column at 4 C.
After washing
with a 20 mM sodium phosphate buffer (pH 6.0) containing 300 mM NaCI, bound
proteins w ere
eluted with a linear gradient of NaCI (0.4 -1 M) in phosphate buffer.
Fractions containing CCN3
as judged by SDS-PAGE were pooled and further purified on a nickel-agarose
column.
Fractions were analyzed by SDS-PAGE followed by Coomassie brilliant blue
staining and
immunoblotting (Figure 1).
Example 2
Preparation of anti-CCN3 Antibodies
[0066] The second domain of CCN3 (von Willebrand type C repeat) and the
central variable
region were cloned separately as glutathione S-transferase (GST) fusion
proteins and used as
antigens to immunize New Zealand white rabbits. DNA fragments were generated
by
polymerase chain reactions using primer sets 5'-
CGCGGATCCGCGGTAGAGGGAGATAACTGTG-3' (SEQ ID NO: 6) and 5'-
-17-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
1',CC'GOAA't'T'CA(jCT6t'AAVj'Cj,(ITAAGGCCTCC-3' (SEQ ID NO: 7) (encoding a.a.
104-188),
and 5'-GATGAGGAGGATTCACTGGGA-3' (SEQ ID NO: 8) and 5'-
AATGCAGTTGACACTTGAG-3' (SEQ ID NO: 9) (encoding a.a. 176-207). To facilitate
cloning, the forward primers start with a BamHI site and the reverse primers
end with an EcoRI
site. The resulting cDNA fragments were cloned directionally into the PGEX-2T
vector
(Amersham Pharmacia Biotech, Inc., Piscataway, NJ) and confirmed by sequence
analysis. The
GST-fusion proteins were purified on a glutathione-S sepharose column and used
as antigens.
[0067] Antisera and affinity-purified antibodies were produced according to
standard protocol
(40). IgG was purified from antisera using protein A column chromatography
(Pierce
Biotechnology, Rockford, IL). For affinity purification, antisera were first
passed through a GST
column to remove antibodies against GST, and then purified through a GST-CCN3
(VWC
domain)-affinity column. Anti-CCN3 antibodies did not cross react with CCN1 or
VN (data not
shown) by ELISA.
Example 3
Cornea Assay
[0068] CCN3 induces neovascularization in vivo. The ability of CCN3 to promote
endothelial
cell adhesion, migration, and survival are consistent with properties of an
angiogenic inducer.
CCN3-induced neovascularization was examined in vivo by implanting Hydron
pellets,
formulated with test substances, into rat corneas essentially as described.
Briefly, male Sprague-
Dawley rats were anesthetized and Hydron pellets (Interferon Sciences, Inc.,
New Brunswick,
NJ) containing test substances were implanted into micropockets made in the
comeal stroma 1 to
1.5 mm from the corneal limbus. Where indicated, CCN3 and bFGF were incubated
with anti-
CCN3 antibodies for 1 hour at RT prior to being incorporated into the Hydron
pellet. 7 days post
implantation, rats were perfused with India ink with heparin (100-U bolus) and
neovascularization was examined and scored.
[0069] As shown in Figure 9, CCN3 induced neovascularization when implanted
into rat cornea,
whereas the vehicle did not induce any response (Table 1). Neovascularization
was also
observed in corneas implanted with Hydron pellets containing bFGF, a known
potent angiogenic
inducer. Pre-incubation of CCN3 with anti-CCN3 antibodies obliterated CCN3-
induced
-18-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
angiogenic activities observed can be ascribed to the
CCN3 polypeptide.
Example 4
Kinetics of CCN3 Expression During Wound Healing
[0070] Excisional Wounds and Immunohistochemistry. Female CD-1 mice at 2
months of
age were anesthetized using I.P.-injected Nembutal, and two standard, full-
thickness, round
wounds of 6 mm diameter were created on the back of each animal using a
disposable biopsy
instrument (Miltex Inc., Bethpage, NY). Mice were sacrificed, and normal skin
and wounds
were harvested at different time points throughout the experiment, ranging
from 2 hours to 15
days. Tissues were rinsed in cold PBS and fixed in 4% paraformaldehyde
overnight at 4 C,
followed by rinses in PBS and incubation in 0.5M sucrose overnight at 4 C. The
tissues were
frozen and embedded in OCT media and cryosectioned at 16 m.
Immunohistochemistry was
performed on cryosectioned tissue using protein A purified antibodies
generated against the
central variable domain of CCN3. The histostain-SAP kit (Broad Spectrum,
BCIP/NBT) kit
(Zymed) was used as described by the supplier with minor modifications. To
avoid non-specific
staining due to addition of streptavidin conjugated to alkaline phosphatase, a
pre-conjugated goat
anti-rabbit secondary antibody (Zymed) was used in place of the antibody
supplied by Zymed.
[0071] RNA Isolation and RNA Blot Analysis. To study the effects of CCN3 and
TGF-(31 on
fibroblast gene expression, 1064SK fibroblasts were serum-starved for 24
hours, then treated
with soluble CCN3 (from 0.1 to 10 g/ml) or TGF-(31 (20 ng/ml) in serum-free
media for 24
hours. Total cellular RNA was isolated, resolved on agarose-formaldehyde gel,
blotted and
probed with 3aP-dCTP-incorporated cDNA according to standard protocol (44).
Blots were
washed at high stringency (0.1 x SSC, 0.1% SDS at 65 C) and analyzed by a
Phosphorlmager
(Molecular Dynamics, Inc., Sunnyvale, CA).
[0072] To study gene expression in skin wounds, full thickness skin excisional
wounds were
created on mice and the wound tissue harvested as described. The tissue was
immediately lysed
by grinding in lysis buffer on ice (4 M guanidine thiocyanate, 25 mM sodium
citrate, 0.5%
sodium N-lauroylsarcosine, 0.1 M 2-mercaptoethanol ) and RNA was extracted by
acid-phenol
protocol (45). The cDNA clones of human glyceraldehyde-3 -phosphate
dehydrogenase
(GAPDH) and matrix metalloproteinase-1 (MMP-1) were obtained from ATCC.
Partial cDNAs
-19-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
c6'i'rdspoifidtrig t'o ,riumah"''~E,(:"r't;'y,A,1'AI-1 and mouse TGF-(31 were
generated by RT-PCR
reactions.
[0073] In reverse transcription reactions total RNAs isolated from human skin
fibroblasts or
mouse embryonic fibroblasts were used as templates and oligo-dT nucleotide as
primer. The
primer set for VEGF-A in the PCR reaction corresponds to nucleotide position
198-226 and 622-
590 in the human VEGF-A cDNA sequence (GenBank #M32977). PCR primers for human
PAI-1 cDNA correspond to nucleotide position 359-381 and 1121-1098 (GenBank
#X04429).
Primers for mouse TGF-(31 cDNA correspond to nucleotide position 873-899 and
1072-1049
(GenBank #M13177).
[0074] Expression of CCN family members during cutaneous wound healing. CCN3
has
been shown to be regulated in an antithetical manner compared to CCNI and CCN2
in
fibroblasts under conditions of mitogen stimulation or growth arrest (31,33-
36). Whereas TGF-
(31 strongly induces CCN1 and CCN2, it potently represses CCN3 in fibroblasts
(34,36). The
effects of TGF,61 on CCNl and CCN2, as opposed to CCN3, may represent a
broader pattern of
expression. Based on this observation of the pattern of expression, CCN3 may
play different or
opposing roles to those of CCN1 and CCN2 in biological processes regulated by
TGF-1, such as
cutaneous wound healing.
[0075] To compare the expression of CCN3 in wound healing against other CCN
family
members, full-thickness excisional cutaneous wounds were made on female CD-1
mice at 2
months of age. The wound tissues were isolated and analyzed at various times
post injury. Skin
tissues adjacent to the wound were used as control, and were thus subjected to
similar
mechanical trauma experienced by the wound tissue but without the actual
injury.
[0076] As shown in Figure 1A, CCNI expression was induced 2 hours post-
wounding, but
declined to basal level one day after injury. CCN1 expression became elevated
again 5 days
post-injury, and remained high until a gradual decline was noted beyond 9
days. CCN2
expression was not elevated until 5 days after injury, and declined to near
basal level after 9
days. CCN3 mRNA level was sharply elevated 5 days post-wounding, peaked on day
7, and
declined after 9 days but remained above the control level for at least 14
days. TGF-(31
expression was also elevated between day 5-7 post injury.
[0077] These results surprisingly show that all three members of the CCN
family are induced
during skin wound repair. Althougli each of these genes has a slightly
different expression
-20-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
iI'pfofihe'thMukh'tir11d, tllVif"eRpfeAion overlaps during days 5-7 post-
injury during granulation
tissue growth, coincident with the expression of TGF-(3.
[0078] The pattern of CCN3 message expression was substantiated by
immunostaining for
CCN3 protein in cryosectioned skin wound tissues. CCN3 was prominently
localized in
fibroblasts and endothelial cells in the granulation tissue 5-7 days post-
injury (Figure 1B).
CCN3 was also detected in migrating keratinocytes that re-epithelialized the
wounded skin, but
not in quiescent keratinocytes in the adjacent uninjured skin. Thus, both CCN3
mRNA and
protein accumulate in the skin wound 5-7 days post-injury.
Example 5
CCN3 Mediates Fibroblast Adhesion Through Integrins 01, 01 and HSPGs.
[0079] Antibodies, polypeptides and reagents. Function-blocking monoclonal Abs
against
various integrins (P1D6, anti-a5i AV1, anti-a,,; LM609, anti-a,,(33; JBS5,
anti-(X5(31) and purified
integrin a,,(35 were from Chemicon (Temecula, CA). GoH3 (anti-a6) was from
Beckman-Coulter,
Inc. (Fullerton, CA) and P4C10 (anti-(31) was from Invitrogen (Carlsbad, CA).
Normal mouse
IgG was from Zymed Laboratories, Inc. (South San Francisco, CA), and normal
rabbit serum
was from Sigma-Aldrich (St. Louis, MO). GRGDSP and GRGESP polypeptides were
purchased
from Invitrogen (Carlsbad, CA). Heparin (sodium salt, from porcine intestinal
mucosa) was
from Sigma-Aldrich (St. Louis, MO). FN, VN, LN, and bFGF were from Invitrogen
(Carlsbad,
CA); type I collagen was from Becton Dickinson (Franklin Lakes, NJ).
[0080] Cell Culture and Adhesion Assay. Normal human skin fibroblasts (1064SK)
were
obtained from American Type Culture Collection (ATCC #CRL-2076), and
maintained in
Iscove's modified Dulbecco's medium (Invitrogen/GIBCO-BRL) with 10% fetal
bovine serum
(Intergen, Purchase, NY) at 37 C with 5% CO2 and used for experiments before
passage 8.
Briefly, test proteins were diluted in PBS and coated onto 96-well microtiter
plates (50 l per
well) with incubation at 4 C for 16 hours. Wells were rinsed with PBS and
blocked with 1%
BSA at RT for 1 hour. Skin fibroblasts were harvested in PBS containing 2.5 mM
EDTA,
washed and resuspended at 2.5 x 105 cells/ml in serum-free Iscove's modified
Dulbecco's
medium containing 1% BSA. Where indicated, cells were mixed with EDTA, Ca2+,
Mg2+,
polypeptides, or heparin before plating, or incubated with antibodies for 1 h
at RT prior to
plating. Cell suspension (50 l) was added to each well, and adherent cells
were fixed in 10%
-21-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
Izormaiin alter.Iv 11n11 IncuflUTIuPUL J7 C. Cells were stained with methylene
blue, and adhesion
was quantified by dye extraction and measurement of absorbance at 620 nm.
[0081] Solid phase integrin binding assay. The binding of CCN3 to purified
integrin aV(35 was
measured by solid-phase receptor binding assay as described previously with
modifications (43).
For CCN3 binding to immobilized integrin, microtiter wells (Immulon 2,
Dynatech Laboratories,
Chantilly, VA) were coated with purified integrin (1 g/ml) and incubated at 4
C overnight. The
wells were washed and blocked with 1% heat-inactivated BSA for 2 hours at room
temperature.
Soluble CCN3 was added and allowed to bind at 4 C for 16 hours; bound ligand
was detected
using affinity-purified anti-CCN3 antibodies (1:1000). For integrin binding to
immobilized
ligands, microtiter wells were coated with 10 g/ml CCN3 or 1 g/ml VN as
above. Where
indicated, coated wells were pre-incubated with affinity-purified anti-CCN3
antibodies or normal
rabbit serum for 2 hours at RT. After washing, purified integrin av(35 (1
g/ml in buffer with 25
mM octylglucoside) was added and incubated overnight at 4 C. Where indicated,
soluble
integrin was either mixed with EDTA, Mgz+, or polypeptides prior to plating,
or incubated with
function-blocking monoclonal antibodies for 30 minutes at 4 C prior to
plating. After washing,
bound integrins were detected with polyclonal antibodies (AB 1930) or mAb
(P3G8) against
integrin a,, (Chemicon, Teinecula, CA). After washing, wells were incubated
with horseradish
peroxidase-conjugated secondary antibody (1:2500), and color reaction was
developed using a
horseradish peroxidase immunoassay kit (Zymed Laboratories, Inc., South San
Francisco, CA)
with absorbance measured at 420 nm.
[0082] CCN3 mediates fibroblast adhesion through integrins a5(31, a6(31, and
HSPGs.
Immobilized CCN3 supports fibroblast adhesion in a dose-dependent manner, with
maximal
adhesion achieved at a coating concentration of 1-2 g/ml (Figure 2A). Cell
adhesion to CCN3
was blocked by the presence of EDTA (5 mM) and restored by the addition of 10
mM Mg2+ or
Ca2+, consistent with the involvement of integrins. To assess which integrins
are involved in
fibroblast adhesion, we investigated the inhibitory effect of RGD-containing
polypeptides. The
addition of GRGDSP polypeptide (2 mM), but not the control polypeptide GRGESP,
significantly blocked cell adhesion to CCN3 (Figure 2B). As expected,
fibroblast adhesion to
FN was completely abrogated. These results suggest that fibroblasts adhere to
CCN3 in part
through RGD-sensitive integrins, such as aV integrins and a5(31 integrins.
-22-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
[0'083]1 To''rd&i{ithtY:th e iiit~gAh9-;1nVo1ved in CCN3-supported fibroblast
adhesion, cells were
incubated with anti-a,,(33 mAb LM609 prior to plating. Whereas LM609 partially
blocked
adhesion to vitronectin, an av(33 ligand, it did not inhibit adhesion to CCN3
(Figure 3B). As
expected, cell adhesion to FN, which binds a5(31, was unaffected by LM609
(Figure 3B).
However, the anti-a5(31 niAb JBS5 partially blocked adhesion to CCN3 and FN,
but not VN
(Figure 3A), indicating that fibroblast adhesion to CCN3 is mediated in part
through integrin
a5[ji.
[0084] Incubation of cells with mAbs against integrin a6 (GoH3) or (31 (P4C10)
blocked
fibroblast adhesion to CCN3, indicating that integrin a6(31 is an adhesion
receptor for CCN3. As
expected, control experiments showed that anti-a6 mAb blocked adhesion to
laminin but not
vitronectin, and anti-(31 mAb blocked adhesion to type 1 collagen but not
vitronectin (Figure 3C,
D). Likewise, when added to cells prior to plating, as little as 50 ng/ml of
soluble low molecular
weight heparin partially but significantly inhibited adhesion of fibroblasts
to CCN3 but had no
effect on cell adhesion to fibronectin (Figure 2B). These results show the
involvement of
integrin a6(31 and HSPGs in fibroblast adhesion to CCN3. The addition of
GRGDSP polypeptide
and soluble heparin (50 ng/ml) together abolished cell adhesion CCN3
completely, suggesting
that the combination of the a5(31 and a6(31-HSPG coreceptors can account for
fibroblast adhesion
to CCN3.
Example 6
CCN3 Induces a Positive Chemotactic Response in Fibroblasts.
[0085] Cell migration assay. A 48-well modified Boyden chamber (Neuro Probe,
Inc.,
Gaithersburg, MD) was used to assay cell migration as described with
modifications (5).
Fibroblasts were harvested with trypsin, washed and resuspended at 5 x 105
cells/ml in DMEM
containing 0.1% BSA. Test proteins were loaded into wells of the lower
chamber; the wells
were then covered with a gelatinized polycarbonate filter (5 m pore diameter,
Nuclepore,
Newton, MA) followed by the upper chamber. Cells were then loaded into the
upper chamber.
Where indicated, cells were either mixed with polypeptides or incubated with
antibodies (lhour
at RT) prior to loading. After a 6 hour incubation at 37 C, the membrane was
removed and
stained using a Diff-Quik Kit (Dade-Behring, Deerfield, IL). Cell migration
was monitored by
- 23 -
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
couniimg ine certar nurnner or~-celss=migrated in 10 randomly selected
microscope fields at 400 X
magnification.
[0086] Fibroblasts migrate to CCN3 through integrin a,,(35. As shown in Figure
4A, CCN3
stimulates migration of neonatal primary human foreskin fibroblasts in a dose-
dependent
manner, reaching maximal level at 2 g/ml. Higher concentrations of CCN3 were
less effective
in promoting cell migration, such that the dose response curve formed a bell
shaped curve typical
of many chemotactic factors. In order to determine whether CCN3 induces
chemotaxis (directed
migration) or chemokinesis (random cell movement) in fibroblasts, a
checkerboard type analysis
was performed. Cell migration was assessed with CCN3 placed in the upper
chamber (with
cells), the lower chamber (opposite side to cells), in both chambers, or in
neither. As shown in
Figure 4B, addition of CCN3 to the lower chamber induces maximal level of cell
migration,
while addition of CCN3 with cells to the upper chamber produced no effect,
indicating that
CCN3 does not induce a chemokinetic response. Addition of CCN3 to both
chambers reduces
the level of fibroblast migration, suggesting that fibroblasts are sensitive
to the gradient of CCN3
protein. These results show that CCN3 is a chemotactic factor in fibroblasts.
[0087] Incubation of cells with either anti-av niAb (AV1) or anti-integrin
a,,(35 mAb (P1F6)
blocked fibroblast migration to CCN3 completely (Figure 5A). As expected,
these antibodies
blocked migration of fibroblasts to VN but not to FN. Neither LM609 nor P1D6
(anti-a5mAb)
inhibited CCN3-induced migration, whereas LM609 blocked cell migration to
vitronectin and
P1D6 inhibited migration to FN (Figure 5B, C). These results indicate that
CCN3 mediates
migration of fibroblasts specifically through integrin av(35.
Example 7
CCN3-induced Chemotaxis is Mediated Through Direct Interaction of CCN3 With
a,#5.
[0088] Purified integrin a,,(35 was immobilized on microtiter wells, onto
which CCN3 was added
in varying concentrations and binding was detected using anti-CCN3 antibodies.
As shown in
Figure 6A, CCN3 binds immobilized integrin av(35 in a dose-dependent and
saturable manner,
with half maximal binding occurring at 1.5 g/ml (40 nM) CCN3. Binding can
also be observed
when CCN3 was immobilized on microtiter wells and integrin aõ(35 added in
soluble form,
detected using antibodies against integrin av subunits (Figures 6B-6D). To
probe the specificity
of CCN3 binding to aV(35i a variety of antagonists was used. The binding of
a,,(35 to immobilized
-24-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
CCN3'; bii't'if6't-"td:VN;::Wg"s~:bleiEl~6d by anti-CCN3 antibodies (Figure
6B). EDTA completely
abrogated the binding of integrin a,(35 to CCN3 and to VN, and as expected,
binding was
restored by the addition of MgClz (Figure 6C). GRGDSP polypeptide (SEQ ID NO:
2), but not
GRGESP polypeptide (SEQ ID NO: 10), was able to inhibit the binding of a,,[i5
to CCN3 and to
positive control VN. Finally, mAb against integrin aV(35 (P1F6) blocked
binding of integrin a,,(35
to CCN3 and VN (Figure 6D). Taken together, these results show that CCN3 is a
novel ligand of
and binds directly and specifically to integrin aõ(35.
Example 8
CCN3 Enhances bFGF-Induced DNA Synthesis in Fibroblasts.
[0089] Thymidine Incorporation Assay. 1064SK fibroblasts were plated on 24-
well plates at
1 x 104 cells/well and grown in Iscove's modified Dulbecco's medium with 10%
fetal bovine
serum for 48 hours, rinsed with PBS, and incubated with serum-free medium
containing 0.5%
BSA for an additional 48 hours. Fresh serum-free medium containing designated
proteins and
1 Ci/well [3H)thymidine were added simultaneously, and after incubation for
21 hours, cells
were washed with phosphate-buffered saline and fixed with 10% trichloroacetic
acid. DNA was
dissolved in 0.1 N NaOH and incorporated thymidine measured using a
scintillation counter.
[0090] Enhancement of bFGF-induced DNA synthesis. Both CCNl and CCN2 have been
shown to potentiate the activities of mitogenic growth factors and enhance DNA
synthesis
without being mitogenic on their own. Likewise, CCN3 enhanced bFGF-induced
fibroblast
DNA synthesis in a dose-dependent manner as judged by a[3H]thymidine
incorporation assay,
whereas CCN3 by itself had no effect (Figure 7). Thus, while CCN3 is not
mitogenic on its own,
it can enhance bFGF-induced DNA synthesis in fibroblasts.
Example 9
CCN3 Can Regulate Genes That Control Matrix Remodeling And Its Effects May Be
Modulated By TGF-(31.
[0091] As described above, CCN3 supports fibroblast adhesion, induces
chemotaxis, and
enhances mitogenesis, consistent with its expression in granulation tissue and
a potential role in
wound repair. Thus, CCN3 may regulate the expression of genes controlling
processes such as
angiogenesis and matrix remodeling.
-25-
CA 02582224 2007-03-28
WO 2006/036962 PCT/US2005/034593
il'f'0092] ' V'a~io~i's an~e~unrs' df C~N3 were added in a soluble form to
serum-starved fibroblasts for
24 hours, both in the presence or absence of TGF-(31 (Figure 8). CCN3 strongly
upregulated
MMP-1 expression at 10 g/ml, but had only a modest effect on PAI-1. VEGF-A
was not
regulated by CCN3 under these conditions. TGF-P 1 strongly represses MMP-l
expression, and
its effect is dominant over that of CCN3 when presented in combination,
completely negating
CCN3-dependent upregulation of MMP-1. However, the effects of TGF-P 1 and CCN3
on PAI-1
were synergistic, leading to a higher level of expression than detected with
either inducer alone.
Thus, CCN3 can regulate genes involved in matrix remodeling, and its effect
may be modulated
by TGF-(31.
-26-
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 26
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 26
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: