Note: Descriptions are shown in the official language in which they were submitted.
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
PHOTOSENSITIVE MATERIAL
BACKGROUND OF THE INVENTION
Field of the invention
This invention relates to materials for manufacturing of write-once-rmd many
(WORM)
recording media suitable for holographic data recording and storage. The
invention can be
used for manufacturing of recording media including, but not limiting to CD
write-once
(CDR), DVD write-once (DVDR) discs, cards and tapes. The invention can be used
not
only in holographic data recording and storage but also in other applications,
like
producing of holograms, sculpturing of artistic works, sculpturing of
industrial articles, etc.
Description of the prior art
Materials for writing permanent volume holograms generally involve
irreversible
photochemical or photo thermal reactions within a photo recording material.
The bright
regions of the optical interference pattern trigger these reactions. Since
these reactions are
accompanied by change of refraction index the relevant recording materials are
known in
the art as photo refractive materials. In the future disclosure the material
of the invention in
its different embodiments will be referred-to as photo refractive material.
Among recording mediums employing photo refractive materials onc; can mention
for
example holographic data memory described in US 2003/0161018. This memory has
a
polymer film, which is set up as a storage layer whose refractive index can be
changed
locally by heating.
Materials containing inorganic particles dispersed in a transparent media are
of great
interest for recoding because these materials show very high photochemical
stability, very
small shrinkage after recording and they do not need any treatment after
recording since
those areas of the media, which were not exposed to the recording radiation
are not
sensitive to light.
Recently materials based on inorganic particles dispersed in a transparent
dielectric
material like organic polymers, oxides B2O3, Sb2O3, Bi2O3, PbO etc. were
proposed for
holographic date storage. For example, in JP61053090 is described optical disc
provided
with recording layer that contains pulverous semiconductor particles of Ge, Te
and InSb
dispersed uniformly within bulk of chemically stable dielectric mate-ial.
I
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
The materials containing inorganic particles show excellent durability, they
enable
recording and erasing over a long period of time and enable obtaining a high
signal level.
Recording in these materials occurs due to the transition between a
crystalline phase and an
amorphous phase that is peculiar to dielectrics or due to the dissociation of
nanoparticles as
in the case of Gold particles dispersed in polymers under heating by a laser
pulse.
Materials for manufacturing of recording medium containing nanoparticles have
particular -
advantage since these materials do not scatter light and this property is a
precondition for
the volume hologram recording.
Properties of material provided with nanoparticles incorporated in a glassy
matrix or in
polymers also attract attention of researchers. These properties are discussed
for example
in the following publications: K.V.Yumashev, N.N.Posnov, I.A.Denisov,
P.V.Prokoshin,
V.P.Mikhailov, V.S.Gurin, V.B.Prokopenko, A.A.Alexeenko. Nonlinear optical
properties
of sol-gel-derived glasses doped with copper selenide nanoparticles.
J.Opt.Soc.Am.B, 17,
572 (2000); F. M. Pavel and R.A. Mackay, Reverse Micellar Synthesis of a
Nanoparticle/Polymer Composite, Langmuir, 16, 8568 (2000); S. W Lu, U.
Sohling, M.
Mennig and H. Schmidt, Nonlinear optical properties of lead sulfide
nanocrystals in
polymeric coatings, Nanotechnology 13, 669 (2002).
However, these publications are of purely scientific character and do not
relate to the
described materials as potential candidates for optical data storage in
general, or for
holographic data storage in particular.
Recently, it has been found that amorphous solid chalcogenide systems can
undergo
photoinduced crystallization. This phenomenon is discussed in the following
publication
K.Tanaka, Photoinduced structural changes in amorphous semiconductors, Physics
and
Technics of Semiconductors. 32, 964 (1998). It is also mentioned in the
literature that
thermal process can be utilized in phase-change based memories, see for
example T.Ohta,
N.Akahira, S.Ohara, I.Satoh. Optoelectronics, 10, 361 (1995)].
However, these publications deal with phase transformation, which is induced
in bulk of a
solid, continuous material and not within a photo refractive material
consisting of discrete
nanoparticles.
2
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
SUMMARY OF THE INVENTION
The main object of the present invention is to provide a new and improved
photo refractive
material for manufacturing of recording media.
Another object of the invention is provide a new and improved photo refractive
material
and recording media, which is capable to record information upon pulse laser
irradiation,
while the photo refractive material contains nanoparticles of chalcogenide
containing
compounds dispersed within a matrix consisting of organic polymers or within a
matrix
consisting of inorganic glassy materials.
The further object of the invention is to provide a new and improved material
and
recording media, which is defined by the following properties:
- High photosensitivity;
- Threshold dependence of recording parameters like diffraction efficiency on
the energy
of recording light, while the threshold energy is low so as to provide high
photosensitivity
and fast recording;
- Insensibility of the material to continuous light radiation;
- Long shelf life (>15 years) for the initial media for recorded information;
- Convenience and simplicity in material preparation;
- Very small shrinkage (< 0.01 %) after recording.
Still further object of the invention is to provide a new photo refractive
material employing .,:.
nanoparticles, which exhibit fast and irreversible transformation from
amorphous or
metastable crystalline state to a stable crystalline state upon exposure to
pulsed laser
radiation with energy < 3 mJ/mm2 and duration of the pulse from I up to 50 ns.
Yet another object of the invention is to provide a new and improved photo
refractive
material employing nanoparticles, distributed within a hosting matrix, while
the particles
are defined by a core portion, which substantially remains in a stable
crystalline state
during recording and by a shell portion, which covers the core portion and
which is
substantially either in an amorphous or in a metastable crystalline state,
wherein this shell
portion is capable to undergo irreversible transformation into stable
crystalline state upon
exposure to pulsed laser radiation.
The present invention can be implemented in its various embodiments, which
comprise the
photo refractive material as such, method of preparation of the photo
refractive material
and various recording media manufactured froni the new photo refractive
material. This
3
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
media comprises but is not limited to optical CD and DVD disks including
multilayer
disks.
The present invention has only been summarized briefly.
For better understanding of the present invention as well of its advantages,
reference will
now be made to the following description of its various embodiments with
reference to the
accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
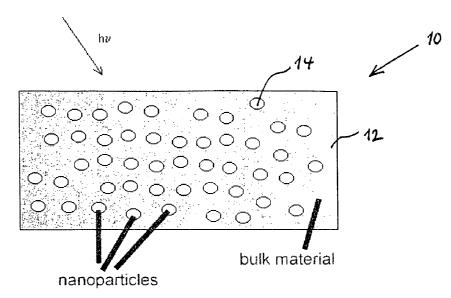
Fig. 1 a shows schematically the structure of the photo refractive material of
the present
invention.
Fig. lb shows enlarged shell layer of Bi2S3 formed on core portion consisting
of Sb2S3.
The shell layer has the same structure as the initial core portion and it is
obtained by
substitution of Sb3+ ions by Bi3+ ions.
Fig. lc shows schematically shell layer of Bi2S3 formed upon laser pulse
irradiation of a
core portion consisting of mixed sulfide Bi2S3/ Sb2S3.
Fig. 2 is an example of threshold dependence of diffraction efficiency on
recording energy.
for nanoparticles consisting of Sb2S3 core portion covered by Bi2S3 shell
portion.
Concentration of the particles in polyvinyl alcohol is 3.75x10"3 M.
Fig. 3 shows how diffraction efficiency depends on concentration of
nanoparticles
composed of core portion consisting of sulfide of antimony and shell portion
consisting of
sulfide of bismuth.
DESCRIPTION OF THE PREFERED EMBODIMENTS
Referring to Fig. I a it shown a photo refractive material 10 of the
invention, which
comprises a polymeric or inorganic glasslike matrix 12 and nanoparticles 14,
distributed
within the bulk of the matrix. The nanoparticles absorb recording pulsed laser
radiation and
upon heating undergo phase transition accompanied by change of their
structure, which, in
its turn, results in essential change in refraction index. Temperature of
nanoparticle
increases with increasing of the laser pulse energy and with decreasi;ig of
the laser pulse
duration. The phase transition occurs when temperature of nanoparticle is
equal or exceeds
the temperature of the phase transition. Temperature of nanoparticles depends
on the
heating rate, which is inversely proportional to the laser pulse duration and
to the heat
diffusion rate. Besides, temperature of nanoparticles decreases due to some
other processes
4
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
like dissociation of nanoparticles and of the surrounding matrix. These
processes are
associated with absorption of energy and loss of heat and therefore with
retardation of the
phase transition.
It has been unexpectedly revealed that in the photo refractive material of the
invention the
phase transition induced by the pulse laser radiation is associated with
producing of heat
assisting to maintain the phase transition in spite of the above mentioned
heat losses. Since
this heat partially compensates for the heat losses the photo refractive
material has
improved holographic sensitivity, because it requires less energy to initiate
and sustain the
phase transition.
There are two principal types of the structural change, namely the transition
from
metastable crystalline phase into stable crystalline phase or the transition
from amorphous
phase into stable crystalline phase. Both structural changes are employed in
the photo
refractive material of the present invention.
In accordance with the present invention the nanoparticles can be synthesized
in such a
manner that they are defined by a crystalline nucleous, which is covered by a
non-
crystalline amorphous or metastable shell. In the present disclosure the
nucleous is referred
to as core portion and the shell as shell portion.
An example of the shell portion is schenlatically depicted in Fig. 1 b, which
shows very
enlarged outer layer (shell portion) obtained on a nucleus (core portion)
consisting of
Sb2S3. The shell portion is comprised of Bi2S3 that is obtained by partial
substitution of
Sb3+ ions by Bi3+ in Sb2S3.
Fig. lc demonstrates the stable structure of Bi2S3 formed upon laser pulse
radiation of
Sb2S3/Bi2S3 mixed sulfide.
In practice diameter of the nanoparticles varies from 5 to 50 nm. The
thickness of the shell
portion lies between 10 and 30 % of the nanoparticle diameter. The
concentration of the
nanoparticles in the polymer matrix lies between 5x10"3 and 5x10-2 M being
preferably
1x10"2 M. The nanoparticles are substantially homogeneously distributed within
the matrix
and center-to-center distance between adjacent particles lies between 20 and
100 nm. The
nanoparticles have round shape or slightly elliptical shape.
In accordance with the invention either the core portion or the shell portion
consists of a
compound, which is capable to produce heat upon exposure to pulsed laser
radiation and to
undergo phase transformation. Among such compounds are for example chalcogene-
containing compounds. The particular chemical composition of the core portion
and the
5
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
shell portion usually is dissimilar, but might be also identical. By virtue of
this provision
the structure of the shell portion is similar to the structure of the core
portion. Under laser
heating the metastable crystalline phase and amorphous phase changes its
structure and
refraction index. This change is accompanied by producing of heat and
therefore the
nanoparticles temperature depends less on the heating rate and the rate of
heat diffusion.
Inasmuch recording occurs due to the phase transition, the photo refractive
material of the
invention exhibits threshold properties, namely recording occurs when the
energy of the
laser pulse exceeds some value. Due to the heat, which accompanies the phase
transition,
this threshold value is significantly lowered. It can be readily apprec:ated
that by virtue of
this provision lasers with less power would be required for recording. On the
other hand,
light beams with smaller energy could be used for reading of the recorded
information,
which means that photo refractive material of the invention would be not
sensitive to
prolonged exposure to daylight and therefore the media manufactured from this
material
would not need any treatment after recording.
Concentration of nanoparticles within the matrix, their chemical composition
and size are
chosen to obtain recording medium with the necessary resolution, the highest
number of
pages in the hologram, and with the high photosensitivity.
In the present invention the nanoparticles are composed from chemical
compounds, which
produce heat upon pulsed laser irradiation. It has been found that it is
especially
advantageous for this purpose if the nanoparticles are composed of sulfides,
selenides or
tellurides as well of chemical compounds containing two or more metallic
chalcogene, e.g.
sulfur and selenium, sulfur and tellurium, selenium and tellurium.
It should be born in mind, however, that the nanoparticles could be composed
also from
other compounds if these compounds are capable to produce heat upon laser
irradiation
and undergo phase transformation from metastable form to stable crystalline
form
accompanied by the change of refraction index. For example the nanoparticles
may contain
the core portion comprised of azides, like Cu(N3)2 or Cd(N3)2 covered by the
shell portion
composed of sulfide. Under laser heating the azide decomposes while producing
energy
that maintains phase transformation of sulfide in the shell portion.
The nanoparticles with required chemical composition are produced by virtue of
chemical
reaction between soluble salts of transition or non-transition metals and
chalcogene
containing compounds, i.e. compounds containing sulfur, selenium and
tellurium. This
reaction is carried out in a solution containing a stabilizer, polymer or a
hardening
6
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
compound. The reaction can be conducted at ambient temperature, however it is
advantageous if this reaction is conducted at elevated temperature.
The obtained photorefractive materials can be used for holographic data
recording and
storage.
It has been found that photo refractive materials of the invention exhibit
very small
shrinkage, which is about 0.01%. This can be explained by the fact that the
phase transition
cannot essentially change the small size of nanoparticles in the matrix.
Non-limiting list of suitable chalcogene containing compounds comprises
hydrides of
sulfur, selenium, and tellurium, soluble sulfides of alkaline metals,
selenosulfites, tellurides
etc. The chemical reaction can be conducted in the presence of stabilizers
like sodium
polyphosphate, trioctyl phosphine oxide or mercaptoacetic acid, which prevent
the
produced nanoparticles from aggregation during precipitation. Polymers like
polyvinyl
alcohol or gelatin can also be used as stabilizers.
.
In accordance with the invention the chemical reaction can be conducted in
such a manner,
that the produced nanoparticles consist of compounds containing a single
chalcogene
element or more than one chalcogene element. This is achieved by precipitation
or by
substitution reactions. In the first case a source of sulfur, selenium or
tellurium is added to
the solution containing salts of two or more metals. An example of s,.iitable
reaction can be ~
interaction of sodium sulfide with acidic solution of bismuth and antimony
chlorides. In
the second case ions capable to form insoluble chalcogene containing compounds
on the
surface of the core portion are added to dispersion of nanoparticles in water
and in the
presence of a stabilizer. This reaction results in the nanoparticles having
the core portion
and the shell portion, which differ in chemical composition. For example, if
bismuth
chloride is added to dispersion of Sb2S3 nanoparticles, bismuth replaces
antimony in the
surface layer of the partciles and mixed sulfide is formed. Eventually the
core portion of
the nanoparticles is composed of Sb2S3 and the shell portion is composed of
Bi2S3. This
reaction can take place if the second sulfide has much smaller solubility
product as
compared with the sulfide constituent of the core portion.
For manufacturing of the recording medium solid holographic films can be
produced by
evaporation of the solvent from the dispersion with nanoparticles distributed
within a
vehicle, which is a polymer or a polymerizable compound. In the lat'er case
the solid film
is hardened photochemically or thermally. Hardening of polymerizable compound
can be
7
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
carried out with the aim of a photoinitiator upon irradiation by the UV light
or by visible
light or with the aim of a proper thermoinitiator.
Non-limiting list of polymers suitable for manufacturing of the present photo
refractive
material and the holographic media of the invention comprises acrylic and
vinyl polymers,
alkyd, coumarone-indene, epoxy and phenolic resins, fluoropolymers,
aminoplasts,
polyacetals, polyacrylics, polyalkylenes, polyalkenyles, polyalkynyles,
polyamicacids,
polyamides, polyanhydrides, polyarylenealkenyles, polyarylenealkylenes,
polyarylenes,
polyazomethynes, polybenzimidazoles, polybenzothiazoles, polybenzoxazinones,
polybenzoxazoles,
polybenzyls, polycarbodiimides, polycarbonates, polycarboranes,
polycarbosilanes,
polycyaurates, polydienes, polyesters, polyurethanes, polyethereketones,
polyethers,
polyuretanes, polyhydrazides, polyimidazoles, polyimides, polyimines,
polyisocyanurates,
polyketones, polyolefins, polyoxadiazoles, polyoxides, polyoxyalkylenes,
polyoxyarylenes, polyoxymethylenes, polyoxyphenylenes, polyoxyphenyls,
polyphosphazenes, polyquinolines, polyquinooxalines, polysilanes,
polysilazanes,
polysiloxazanes, polysilsesquioxanes, polythioethers, polysulfonamides,
polysulfones,
polythiazoles, polythoalkylenes, polythioarylenes, polythiomethylenes,
polyureas,
polyurethanes, polyvinyl acetals, polyvinyl butyrals and polyvinyl formals
etc.
Non-limiting limiting list of suitable polymerizable compounds comprises
acrylates,
metacrylates or epoxides. Examples of suitable commercially available products
are
polyether or polyester urethane acrylates like BR-200, BR- 300, BR- 400
manufactured by
Aldrich Inc. or bifunctional polyester or polyether urethane acrylates or
metacrylates
manufactured by Bomar Specialties Inc
To improve optical characteristics of the recording medium plasticizers can be
introduced
in the photo refractive material. Non-limiting list of suitable plasticizers
comprises alkyl
phthalates, phosphates, adipates and sebacates, polyethers, epoxides, etc.
As suitable substrate one can use transparent inorganic glasses,
polycarbonate,
polymethylmethacrylate, polymethylacrylate, and polystyrene etc.
The non-limiting examples below illustrate preparation of the photo refractive
material of
the present invention.
8
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
Example 1.
ml of Na2S in 10% solution of polyvinyl alcohol (PVA) with typical Mw 9,000 -
10,000
was first prepared (0.15 ml of 1 M aqueous Na2S solution to 10 ml of PVA
solution). Then
to this solution 0.1 ml of I M SbC13 acidic (HCl) aqueous solution was added
successively.
5 Temperature of the Na2S in PVA solution was 25 C.
The reaction resulted in formation of nanoparticles having shell portion
composed of
amorphous Sb2S3.
Resulting solution was deposited on hydroxylized glass plate and then the
plate was dried
in a closed box at room temperature for 4 days.
Example 2.
10 ml of Na2S in 20% PVA solution was first prepared (0.15 ml of I M aqueous
Na2S
solution to 10 ml of PVA solution). Then to this solution 0.3 ml of 0.2 M
aqueous -
thioglycolic acid solution, 0.1 ml of I M BiCl3 acidic (HC1) aqueous solution
was added
successively. Temperature of the Na2S in PVA solution was 25 C.
The reaction resulted in formation of nanoparticles having shell portion
composed of
amorphous Bi2S3.
Resulting solution was deposited on hydroxylized glass plate and then the
plate was dried
in a closed box at room temperature for 4 days.
Example 3.
10 ml of Na2S in 10% PVA solution was first prepared (0.15 ml of 1 M aqueous
Na2S
solution to 10 ml of PVA solution). Then to this solution 0.05 ml of I M SbC13
acidic
(HCl) aqueous solution and 0,05 ml of I M BiCl3 acidic (HCl) aqueous solution
were
added successively. Temperature of the Na2S in PVA solution was 22 C.
The reaction resulted in formation of nanoparticles with core portion
consisting of Sb2S3
and shell portion consisting of Bi2S3.
Resulting solution was deposited on hydroxylized glass plate and then the
plate was dried
in a closed box at room temperature for 4 days.
Example 4.
10 ml of Na2S in 10% PVA solution was first prepared (0.15 ml of I M aqueous
Na2S
solution to 10 ml of PVA solution). Then to this solution 0.1 ml of I M SbC13
acidic (HCI)
9
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
aqueous solution was added successively. Temperature of the Na2S in PVA
solution was
70 C. Then 0.01 ml of I M BiC13 acidic (HC1) aqueous solution wa', added
successively.
The reaction resulted in formation of nanoparticles with core portion
consisting of Sb2S3
and shell portion consisting of Bi2S3.
Resulting solution was deposited on hydroxylized glass plate and then the
plate was drying
in a closed box at room temperature for 4 days.
Example 5.
0.038 ml of 0.2 M solution of Na2SeSO3 was added to 5m] of 15% PVA solution
under
stirring. Than 0.07 ml of 0.1 M aqueous solution of Cu(C104)2 was added drop
wise at 70
C. Solution was mixed during 30 min.
The reaction resulted in formation of nanoparticles having both the core
portion and the
shell portion consisting of copper selenide
Resulting solution was deposited on hydroxylized glass plate and then the
plate was dried
in a closed box at room temperature for 4 days.
Example 6.
10 ml of 1 M aqueous solution of Cu(C104)2 was added dropwise to I M solution
of NaN3
in aqueous 15% solution of polyvinylpyrrolidone under intense stirring. After
that 1 ml of
0.1 M solution of Na2S was added to dispersion of Cu(N3)2 nanoparticles.
The reaction resulted in formation of nanoparticles with core portion
consisting of Cu(N3)2
and shell portion consisting of CuS.
Resulting solution was deposited on hydroxylized glass plate and then the
plate was dried
in a closed box at room temperature for 4 days.
Holograms were recorded with the aim of the medium prepared from the photo
refractive
material of the invention. In Fig.2 is shown dependence of diffraction
effrciency rl of the
holograms on the energy of laser pulse. Diffraction efficiency is the ratio of
the power in
the diffracted beam to the power in the incident beam. It is clearly seen that
this
dependence has a threshold, i.e. the recording is achieved after the laser
pulse energy
reaches certain minimum value and this efficiency increases until soine
maximum value.
With reference to Fig.3 it is shown that diffraction efficiency increases when
concentration
of the nanoparticles rises.
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
With the above dependences it is possible to find optimal condition in terms
of minimum
recording energy and concentration, which would be required for recording a
hologram
with highest diffraction efficiency.
It has been empirically revealed that maximum value of diffraction efficiency
rl can range
up to 80%. The maximum value of diffraction efficiency corresponds to change
of the
refraction index by about 0.005.
Dynamic range, photosensitivity and diffraction efficiency of the photo
refractive materials
of the present invention are summarized in the non-limiting Tables I and 2
below. This
data is presented along with the factors, which influence on these properties.
Among these
factors are concentration of nanoparticles (as shown in Fig. 3), temperature
of synthesis of
nanoparticles and pH of the solution.
A measure for the data-storage capacity of holographic media is defined by so-
called M-
number (M#), which was determined using the following formula
M#= M M 2Tr On. * d 2TC "4 On.
77; _ =d-
~ - ~ ~, * cos B; ~. ~ cos e;
where:
d- thickness of photosensitive layer
9- angle between reference and object beams
~ - wavelength of recording light
r7; - diffraction efficiency of each hologram.
The photo sensitivity S was calculated according to the following formula:
S = q0-5/Eod in cm/mJ,
where:
Eo - the flux energy EP/A where Ep is the pulse energy and A is the spot area
(A=0.5 mmZ )
d- Thickness of the sample
q - Diffraction efficiency of the hologram
11
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
Table 1.
Properties ofphoto refractive materials containing nanoparticles composed
ofSb2S3 and
CuSe.
Sample Composition of Writing Energy [J] M# at standard Photosensitivity
designation nanoparticles Ep 300 thickness of the material,
cm/mJ
14.04.3 Sb2S3 170 3.215 1.18
14.04.3 Sb2S3 150 2.782 1.27
11.2 CuSe 170 5.205 2.78
11.2 CuSe 150 4.920 2.33
Photosensitivity of the present photo refractive materials is not worse than
photosensitivity
of the known in the art photo polymerizable materials. For example, photo
refractive
material developed by Aprilis Ventrures demonstrates photosensitivity between
2.5 and 4.5
cm/mJ.
At the same time the present photo refractive materials have significant
advantage over the
known in the art materials since they do not shrink and have much shorter
recording time.
Non-limiting Table 2 displays how photosensitivity of the present photo
refractive'material
depends on the temperature of the synthesis.
Table 2.
Diffraction efficiency ofphoto ref'ractive material comprising nanoparticles
composed of
Sb2S3 core portion and SbZS3/Bi A shell portion
Sample [Sb2S3] [BizS3] Temperature of ri
designation M M synthesis
2.16.4 5x10" - 25 C 40.5
2.16.5 5x10- - 25 C 44.3
2.16.8 2.5 x10" - 25 C 18
2.17.10 5 x10 2.5 x10" 25 C 36
2.17.11 5x10 2.5x]0" 25 C 41
12
CA 02582440 2007-03-30
WO 2006/040772 PCT/IL2005/001089
2.17.12 5x10" 5x10" 25 C 47
2.17.1 4x10" - 25 C 20
A08.4.1.1 5x 10" 60 C 66
A08.4.1.1 5x10" 50 C 53
A08.4.1.1 5x10- 50 C 57
A08.4.1.2 0-3 65 C 74
A08.4.1.3 5x10 15 C 26
A08.4.1.3 5x10" 50 C 52
A08.4.1.3 5x10" 60 C 62
The present invention has been described using non-limiting detailed
description of
various embodiments thereof. It should be appreciated that the present
invention is not
limited by the above-described embodiments and that one ordinarily skilled in
the art can
make changes and modifications without deviation from the scope of the
invention as will
be defined below in the appended claims.
It should also be appreciated that features disclosed in the foregoing
description, and/or in
the foregoing drawings and/or following claims both separately and in any
combination
thereof, be material for realizing the present invention in diverse forms
thereof.
When used in the following claims, the terms "comprise", "include", "have" and
their
conjugates means "including but not limited to".
20
13