Note: Descriptions are shown in the official language in which they were submitted.
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
Fuel and Oil Detergents
Technical Field
This invention relates to a oil-soluble compositions of matter useful as
detergent
components in hydrocarbon oils useful for a wide range of purposes, including
without
limitation general lubricants, lubricating oils for internal combustion
engines, cutting
fluids, emulsions, and dispersions.
More particularly, the invention relates to oil-soluble allcylbenzenes
comprising
alkyl chains having between about 16 and 30 carbon atoms in which the
allcylbenzenes
have a low dialkylate content and unique isomer distribution, including their
sulfonate and
other water-soluble and solubilizable derivatives.
Background
The chemical structure and use of linear allcylbenzenes and their derivatives,
including their sulfonate derivatives, in the manufacture of laundry
detergents is well
known. Generally, linear alkylbenzenes are produced by an alkylation reaction
(according
to one of any well known processes for producing such materials) in which the
net result is
the appendage of a hydrocarbyl radical to a benzene ring. The source of the
hydrocarbyl
radical may be a branched or a linear olefin, either an internal olefin or an
alpha olefin,
and in practice a mixture of substantially linear olefins is typically used,
which mixture
comprises various olefins having different numbers of carbon atoms per
molecule. For the
manufacture of laundry detergents, the range of carbon numbers (the number of
carbon
atoms per molecule of an olefin used) of an olefin mixture used in the
allcylation reaction
is typically in the range of between about 8 and 15 (inclusive) carbon atoms
per molecule,
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
which molecules are sometimes collectively referred to by those in the art as
the
"detergent range".
Alkylation of benzene using olefins in the detergent range leads to a reaction
product mixture which. contains alkylated benzenes having hydrocarbyl radicals
of
different chain lengtll appended to a benzene _ring, and also contains
position isomers of
these alkylation products. Thus, a reaction mixture from the alkylation of
benzene using
detergent range olefins is often complex in makeup.
Of the possible position isomers referred to above, it has been recently
discovered
tliat detergents for use in aqueous systems, which are prepared from
alkylbenzenes having
the benzene ring located at the 2-position on the hydrocarbyl radical possess
enhanced
detergency and other beneficial properties over the other isomers produced in
the
allcylation. This is believed in part to be true because the hydrocarbon chain
that is
appended to the ring extends a greater distance in space in isomers having a
phenyl group
in the 2-position than the other position isomers, thus providing a molecule
having a more
volumetrically exposed hydrocarbon chain poi-tion over other position isomers.
Among
other things, this increased exposure provides increased availability for
interaction with
hydrophobic materials which are sought to be solubilized in an aqueous medium,
when the
alkylbenzene also includes a hydrophilic moiety, such as a sulfonate group
bonded to the
benzene ring.
Detergents useful as components in hydrocarbon oils are often possessive in
general of the same properties as detergents useful in aqueous media, that is,
their
molecules contain both a hydrophilic and a hydrophobic portion. However, in
many
applications it may be beneficial to employ allcylbenzenes having longer
hydrocarbon
chains on the benzene ring than those found in conventional detergents, for
example to
2
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
enhance solubility in hydrocarbon oils, or to provide increased compatibility
and chemical
inertness with respect to other components of the formulation, depending upon
the
intended use.
The production of sulfonates by reaction with, e.g., SO3, is well lcnown to
those
skilled in the art. See, for example, the aiticle "Sulfonates" in Kirk-Othmer
"Encyclopedia
of Chemical Technology", Second Edition, Vol. 19, pp. 291 et seq. published by
John
Wiley & Sons, N.Y. (1969). Other descriptions of neutral and basic sulfonate
salts and
techniques for making them can be found in the following U.S. Pat. Nos.
2,174,110;
2,174,506; 2,174,508; 2,193,824; 2,197,800; 2,202,781; 2,212,786; 2,213,360;
2,228,598;
2,223,676; 2,239,974; 2,263,312; 2,276,090; 2,276,097; 2,315,514; 2,319,121;
2,321,022;
2,333,568; 2,333,788; 2,335,259; 2,337,552; 2,347,568; 2,366,027; 2,374,193;
2,383,319;
3,312,618; 3,471,403; 3,488,284; 3,595,790; and 3,798,012. These and all other
patents,
books, exceipts, articles, and literature cited herein are fully incorporated
by reference.
Although the prior art is replete with prior art concerning the use of
alkylbenzene
based detergents in hydrocarbon based oils such as motor oils, hydraulic
fluids, cutting
fluids, etc., none have thus far provided commercially quantities of an
alkylbenzene based
detergent component in which the hydrocarbon tails of the molecule have carbon
numbers
of any integral value in the range of between about 16 and 30 carbon atoms per
molecule,
in which the 2-phenyl isomer content is in the range of between about 10% and
13%. We
have recognized that a need exists for a method of linear alkylbenzene ("LAB")
production
having high substrate olefin conversion, controlled selectivity to 2-phenyl
isomer LAB, and
employing a catalyst having long lifetimes and easy handling, by which
controllable 2-phenyl
isomer content and low diallcylate content can be achieved in materials having
relatively long
hydrocarbon tails attached to a benzene ring in a linear allcylbenzene based
detergent.
3
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
The present invention employs hydrogen fluoride as a catalyst in the
production of
long-tail linear allcylbenzenes. The processing conditions used in preparing
the materials of
this invention may in one form of the invention provide essentially any
desired percentage
content of 2-phenyl isomer in the range of about 8 to about 27 % (on a weiglit
basis) in the
finished product by adjusting the processing parameters. In this way, LAB may
be produced
having any desired 2-phenyl isomer content in the range of about 10 % to 13%
by weight
based on the total weight of the allcylbenzene.
This invention, in one broad respect, is a process for the production of
linear
alkylbenzenes which comprises contacting benzene and an olefin having about 8,
to about
30 carbons in the presence of an effective catalytic amount of hydrogen
fluoride to form
linear alkylbenzenes, wherein the isomerization of the olefins is conducted in
the same
process step as the alkylation of benzene
In anotller broad respect, this invention is a process for the production of
oil-soluble
alkylbenzene sulfonates suitable for use in fluids used in the transportation
industry,
including without limitation, additives for passenger car engine oils,
additives for diesel
engine oils, driveline lubricants, transmission fluids, or any other
application in which an
oil-soluble sulfonate salt or an overbased sulfonate confers a beneficial
property to the
performance of the lubricant.
For whatever reason, the alkylbenzenes within the prior art which are sold as
a raw
material from which overbased sulfonates may be prepared all contain benzene
at a level
of between about 3 and 10 parts per million. Benzene is notorious for causing
leukemia.
The present invention provides allcylbenzenes from which overbased alkaline
earth metal
sulfonates may be prepared in which benzene is present at a level of less than
100 parts per
billion.
4
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
In addition, the materials of the prior art all contain significant levels of
1-phenyl
(1-aryl) isomer. According to this invention, the 1-phenyl (1-aryl) content is
negligible,
being less than 0.3 % by weight based on the total weight of all allcylaryl
isomers present
in a mixture of the invention.
10
20
5
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
Summary of the Invention
The present invention provides a process for production of allcylaromatic
compounds which comprises the steps of:
a) co-mingling linear alpha olefins, having 16 to 40 carbon atoms per
molecule,
with a non-reactive diluent, such as nonnal or branched paraffin to form a
mixture
of alpha olefins and paraffins;
b) feeding the mixture of alpha olefins and paraffins into a reaction zone
along
with a feed aromatic hydrocarbon and liquid hydrogen fluoride, under
allcylation-
promoting conditions, to produce an effluent stream containing the feed
aromatic
hydrocarbon, hydrogen fluoride, paraffin, and allcylaromatic hydrocarbon
product;
c) separating substantially all of the hydrocarbon mixture in the reaction
zone
effluent stream from the liquid phase hydrogen fluoride present in the
effluent
stream; and
d) and recovering the product alkylaromatic hydrocarbon.
The product alkylaromatic hydrocarbon is conveniently recovered by a series of
steps
involving passage of the hydrocarbon mixture through a plurality of stripping
columns to
remove the feed aromatic hydrocarbon and paraffin diluent components. The
alkylaromatic product is recovered as a net bottom stream.
In another form of the invention is provided a process for producing an
allcylaromatic hydrocarbon which coinprises the steps of:
a) dehydrogenating a paraffin to form an olefin;
b) sending a feed stream of benzene and the olefin through a conduit to a
linear
allcylbenzenes allcylation reactor containing hydrogen fluoride under
conditions
6
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
which enable isomerization of the olefin to occur simultaneously with
alkylation of
benzene by the olefin, to form a crude linear alkylbenzenes stream;
c) distilling the crude linear allcylbenzenes streain in a first distillation
column to
separate benzene that did not react and to form a benzene-free linear
allcylbenzenes
stream;
d) distilling the benzene-free linear allcylbenzenes stream in a second
distillation
coluinn to separate any paraffin present and to form a paraffin-free linear
alkylbenzenes stream;
e) distilling the paraffin-free linear alkylbenzene stream in a third
distillation
colunm to provide an overhead of a purified linear allcylbenzene stream and
removing a bottoms stream containing heavies.
Certain terms and phrases have the following meanings as used herein.
"Conv." and "Conversion" mean the mole percentage of a given reactant
converted to
product. Generally, olefin conversion is about 95 percent or more in the
practice of this
invention.
"Sel." and "Selectivity" mean the mole percentage of a particular component in
the
product. Generally, selectivity to the 2-phenyl isomer is about 70 % or more
in the practice
of this invention.
"LAB" means a mixture linear alkylbenzenes which comprises a benzene ring
appended to any carbon atom of a substantially linear alkyl chain having any
number of
carbon atoms in the range of 16 to 30, inclusive. Hydrogen fluoride is useful
as a catalyst
useful in the production of LAB's in accordance with the process of
manufacturing LAB's of
this invention. LAB is useful as starting material to produce sulfonated LAB,
which is useful
as a surfactant.
7
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
"LAB sulfonates" means LAB which has been sulfonated to include an acidic
sulfonate group appended to the benzene ring (thus forming a "parent acid"),
and
subsequently rendered to a form more soluble to aqueous solution than the
parent acid by
neutralization using any of alkali metal hydroxides, allcaline earth
hydroxides, ammonium
hydroxides, alkylammonium hydroxides, or any chemical agent known by those
skilled in the
art to react with linear alkylbenzene sulfonic acids to foim water-soluble LAB
sulfonates.
"Detergent range" means an olefm, aM:yl group, or molecular species (including
without limitation LAB, LAB sulfonates, and overbased LAB sulfonates) that
comprises
any number of carbon atoms selected from: 16, 17, 18, 19, 20, 21, 22, 23, 24,
25, 26, 27, 28,
29, or 30, as warranted by the context, including mixtures of two or more such
species
having different numbers of carbon atoms appended to the aromatic ring.
"Substantially linear" when referring to a hydrocarbon or allcyl chain that is
part of an
alkylbenzene, whether the allcylbenzene is sulfonated or not, means a
hydrocarbon
comprising between 16 and 30 carbon atoms linked to one anotller to form a
straight chain,
wherein the carbon atoms of said straight chain may have only hydrogen atoms
or a methyl
group bonded to them as appendages.
"Branched alkyl" when referring to a hydrocarbon or allcyl chain that is part
of an
alkylbenzene, whether the alkylbenzene is sulfonated or not, means a
hydrocarbon
comprising between 16 and 30 carbon atoms linlced to one another to form a
straight chain,
wherein one or more of the carbon atoms of said straight chain may have a
hydrogen atom
and any allcyl group other than a methyl group (including without limitation
ethyl groups),
bonded to them as appendages.
"Branched allcylbenzene" means a molecular species which comprises a branched
alkyl chain appended to a benzene ring.
8
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
"Branched alkylbenzene sulfonate" ineans a water-soluble salt of a branched
allcylbenzene that has been sulfonated.
"Overbased sulfonate" means an LAB sulfonate in which an amount of any one or
more alkaline metals selected from the group consisting of Na, K, Mg, Ca, Ba,
Sr are
present in any amount which is greater than the stoichiometric amount of metal
which
would be present if the parent LAB sulfonic acid, R-S(=O) (=O)OH, were fully
neutralized. The exact structure of this type compound has not been
determined.
"2-phenyl alkylbenzenes" means a benzene ring having at least one allcyl group
attached to it, wherein the allcyl group comprises any number of carbon atoms
between 16
and 30 (including every integral number therebetween) linlced to one another
so as to form
a substantially linear chain and wherein the benzene ring is attached the
alkyl group at a
carbon atom that is adjacent to the terminal carbon of the substantially
linear chain. Thus,
the carbon atom that is attached to the benzene ring has a methyl group and an
alkyl group
attached to it in a 2-phenyl alkylbenzene. Thus, the 2-phenyl isomer of
substantially linear
LAB produced in accordance with this invention is of the forinula:
B
I
CH3 CH2 CH3
CH
R1 R5
R2 R4
R3
9
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
in which n is any integer between about 12 and about 30; Rl, R2, R3, R4, and
R5 are each
independently selected from the group consisting of: hydrogen, a methyl group,
an ethyl
group, a propyl group, and a butyl group; B is selected from the group
consisting of:
hydrogen, methyl, or ethyl; B is attached to any single carbon atom along the -
(CH2)n
portion of the allcyl chain; and the total 2-aryl isomer content of said
mixture of
allcylbenzenes is between about 8 % and about 30 % by weight based on the
total weight
of all alkylbenzenes present in said mixture and in which the 1-aryl isomer
content is less
than about 3 % by weight based on the weight of all isomers of alkylbenzene
derivatives
present.
"Alkylbenzenes" means all species containing an alkyl group, whether linear or
branched, appended to a benzene ring. Within this definition are also embraced
monoallcyltoluenes, monoallcylxylenes, allcylethylbenzenes, etc.
"Sulfonated 2-phenyl alkylbenzenes" means 2-phenyl allcylbenzenes as defined
above which further comprise a sulfonate group attached to the benzene ring of
a 2-aryl
allcylbenzene, regardless of the position of the sulfonate group on the ring
with respect to
the location of the alkyl group. However, it is typical, though not always the
case ( as in
ortho isomers invariably present) for the sulfonate group to appear in the
position R3
above with respect to a single alkyl group attached to the benzene ring, as
shown in the
following structure:
10
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
B
CH3 CH2 n CH3
CH
R1 R5
R2 Rq.
0= S =0
I
O
in which n is any integer between about 12 and about 30; Ri, R2, R4, and RS
are each
independently selected from the group consisting of: hydrogen, a methyl group,
an ethyl
group, a propyl group, and a butyl group; B is selected from the group
consisting of:
hydrogen, methyl, or ethyl; B is attached to any single carbon atom along the -
(CH2)n
portion of the alkyl chain; and the total 2-aryl isomer content of said
mixture of
alkylbenzenes is between about 8 % and about 30 % by weight based on the total
weight
of all alkylbenzenes present in said mixture and in which the 1 -aryl isomer
cointent is less
than about 3 % by weight based on the weight of all isomers of alkylbenzene
derivatives
present.
"Motor fuel" means those compositions generally recognized by those in the art
as
liquid hydrocarbon fuels in the gasoline boiling range, including hydrocarbon
base fuels.
Within the meaning of this term is included those fuels often termed as
"petroleum
distillate fuels" by those in the art and which have the above characteristic
boiling points.
The term is, however, not intended to be restricted to straight-run distillate
fractions. The
11
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
distillate fuel can be straight-run distillate fuel, catalytically or
thermally cracked
(including hydrocracked) distillate fuel, or a mixture of straight-run
distillate fuel,
naphthas and the like with cracked distillate stocks. Also, the base fuels
used in the
formulations of the fuel compositions of the present invention can be treated
in accordance
with well-known commercial methods such as acid or caustic treatments,
hydrogen
solvent refining, clay treatment, etc. Gasolines are supplied in a number of
different
grades depending upon the type of service for which they are intended. The
gasolines
useful in the present invention include those designed as motor and aviation
gasolines.
Motor gasolines include those defined by ASTM specification D-439-73 and are
composed of a mixture of various types of hydrocarbons including aromatics,
olefins,
paraffins, isoparaffins, naphthalenes, and occasionally diolefins. Motor
gasolines normally
have a boiling range within the limits of about 20 degrees C to about 230
degrees C., while
aviation gasolines have narrower boiling ranges, usually within the limits of
about 37
degrees C. to 165 degrees C. Also within this definition are the kerosene
range fuels,
whicll include diesel fuels and jet fuel.
"Ashless Dispersants" means any material regarded by those in the motor fuel
arts as
possessive of dispersant characteristics and which upon combustion leaves
substantially no
ash.
"Base Number" or "BN" refers to the amount of base equivalent to milligrams of
KOH in one gram of sample. Thus, higher BN numbers reflect more alkaline
products,
and therefore a greater alkalinity reserve. The BN of a sample can be
determined by
ASTM Test No. D2896 or any other equivalent procedure.
In this specification and the appended claims, unless otherwise specified, all
percentages are in weight percent, all ratios are molar ratios, and all
molecular weights are
number average molecular weights.
12
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
BRIEF DESCRIPTION OF THE DRAWINGS
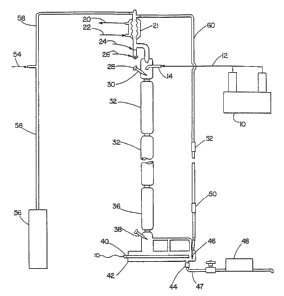
FIG. 1 shows a representation of a first continuous reactive distillation
colurnn
employed in the practice of this invention.
FIG. 2 shows a representation of a second continuous reactive distillation
column
employed in the practice of this invention.
FIG. 3 shows a representative process scheme for one embodiment of this
invention
where a fluorine-containing mordenite is employed with a second, solid
catalyst to achieve
variable 2-phenyl isomer content depending on the relative proportions of the
two catalysts.
20
13
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
Detailed Description
Reactants for LAB Production
In the practice of this invention, benzene or a substituted benzene such as
toluene,
ethylbenzene, propylbenzene, butylbenzene, or one or more xylenes is
allcylated with an
olefinic material to form LAB. Olefins and benzene can be handled and purified
using
standard techniques recognized by those of ordinary slcill in the art. In this
regard, it is
preferred that the reactants are substantially free from hydric compounds such
as water and
alcohols, etc. The olefins employed in the practice of this invention have
from about 16 to
about 30 carbons per molecule, and in one form of the invention preferably
from about 20 to
about 24 carbon atoms. It is most preferred that the olefinic material be a
mono-olefm. It is
most preferred that the mono-olefm be an aipha-olefin, in which the double
bond is located in
a terminal ethylenic unit; however, internal olefms are suitable as well,
since they are
isomeiized in the same step as the alkylation, in one preferred form of the
invention.
Commonly, such olefins would be available from a paraffinic media of the same
carbon range. One route by wliich olefins in the 16 to 30 carbon number range
are available
is from dehydrogenating a mixture of paraffins in the same carbon number
range, namely
C_16 to C_30 paraffms. Such dehydrogenation may be carried out even if such a
paraffm
mixture has any appreciable olefin content, for example, an olefin content in
the range of
about 5 to 20%. It is especially preferred to catry out such a process using
our catalyst and
processes as described in US Patents 6,417,135 and 6,700,028 , both of which
are fully
incorporated herein by reference thereto.
Process Conditions, Procedures, and Apparatus
The process of this invention can be carried out using the continuous reactive
distillation column depicted in FIG. 1. In FIG. 1, a feed mixture of benzene
and olefin,
14
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
generally at a benzene-to-olefni molar ratio range of about 1:1 to 100:1 flows
from feed
pump 10 to feed inlet 14 via line 12. The feed mixture falls to a region
containing hydrogen
fluoride gas 32 where alkylation and isomerization occurs. Altenlatively,
while not depicted
in FIG. 1, the benzene and olefm can be introduced separately into the zone
with mixing
occurring in the HF zone, or the reactants can be mixed via an in-line inixer
prior to
introducing the reactants into the catalyst zone, or the reactants can be
injected separately
above the HF zone with mixing affected by use of standard packing above the
zone, or the
reactants can be sparged into the chamber above the zone. In the catalyst zone
32, the falling
feed mixture also contacts rising vapors of unreacted benzene which has been
heated to
reflux in reboiler 42 by heater 40. Such rising vapors pass over thermocouple
38 which
monitors temperature to provide feedbaclc to heater 40. The rising vapors of
benzene and/or
oleful also pass through standard paclcing 36 (e.g., 7.5 inches of goodloe
paclcing). The rising
vapors heat thermocouple 30 which connects to bottoms temperature controller
28 which
activates heater 40 when teiuperature drops below a set level.
Prior to startup, the system may be flushed with nitrogen which enters via
line 54 and
which flows through line 58. After startup, a nitrogen blanket is maintained
over the system.
Also prior to startup and during nitrogen flush, it may be desirable to heat
catalyst zone 32 so
as to drive off water. In an alternate form of the invention, HF gas may be
admitted to line
54.
Residual water from the feed mixture or whicli otherwise enters the system is
collected in water trap 24 upon being liquefied at condenser 21 (along with
benzene vapor).
If the feed is very diy (free of water) the water trap 24 may not be needed.
Removing water
leads to better product quality. Hence, the water trap 24 is optional. The
same applies to
FIG. 2. Condenser 21 is cooled via coolant sucli as water entering condenser
21 via port 22
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
and exiting via port 20. As needed, water in water trap 24 may be drained by
opening drain
valve 26.
As needed, when LAB content in reboiler 42 rises to a desired level, the
bottoms
LAB product may be removed from the system via line 47, using either gravity
or bottoms
pump 48 to withdraw the product. When product is so withdrawn, valve 44 is
opened.
In FIG. 1, dip tube 46, which is optional, is employed to slightly increase
the pressure
in reboiler 42 to thereby raise the boiling point of benzene a degree or two.
Likewise, a
pressure generator 56 may be optionally employed to raise the pressure of the
system. Other
standard pressure increasing devices can be employed. Pressure can thus be
increased in the
system such that the boiling point of benzene increases up to about 2001 C.
In FIG. 1, control mechanisms for heat shutoff 50 and pump sliutoff 52 are
depicted
which serve to shut off heat and pump if the liquids level in the system rises
to such levels.
These control mechaiiisms are optional and may be included so that the
catalyst zone does
not come into contact with the bottoms of the reboiler.
In the practice of this invention in the alkylation of benzene, a wide variety
of process
conditions can be employed. In this regard, the temperature in the catalyst
zone may vary
depending on reactants, rate of introduction into the catalyst zone, size of
the catalyst zone,
and so forth. Generally, the zone is maintained at the reflux temperature of
benzene
depending on pressure. Typically, the temperature of the catalyst zone is
above about 70 C,
and most lilcely about 78 C or more in order to have reasonable reaction
rates, and about
200 C or less to avoid degradation of reactants and products and to avoid
coke build-up in
the catalyst zone. Preferably, the temperature is in the range from about 80
C to about
140 C. The process may be operated at a variety of pressures during the
contact of HF with
the reactants, with pressures of about atmospheric most typically being
employed. When the
16
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
process is operated using a systein as depicted in FIGS.1 and 2, the reboiler
temperature is
maintained such that benzene and olefin vaporize, the temperature varying
depending on
olefm, and generally being from about 80 C to about 250 C for olefins
having 16 to 24
carbons. The composition of the reboiler will vary over time, but is generally
set initially to
have a benzene olefm ratio of about 10:1, with this ratio being maintained
during the practice
of this invention. The rate of introduction of feed into the catalyst zone may
vary, and is
generally at a liquid hourly space velocity ("LHSV") of about 0.05 hf 1 to
about 10 hr-1, more
typically from about 0.05 hr-1 to about 1 lu-1. The mole ratio of benzene to
olefm introduced
into the catalyst zone is generally from about 1:1 to about 100:1. In
coinmercial benzene
allcylation operations, it is common to run at inole ratios of from about 2:1
to about 20:1,
which can suitably be employed in the practice of this invention, and to
charge said olefms as
an olefm-paraffin mixture comprising 5% to 20% olefin content. Said olefm-
paraffin
inixtures are normally generated commercially through dehydrogenation of the
corresponding paraffin starting material over a noble metal catalyst as
previously specified.
Another continuous reactive distillation apparatus is depicted in FIG. 2. In
FIG. 2,
the feed mixture enters the reactor via feed inlet 114. The feed mixture falls
through the
column into catalyst zone 132, wherein alkylation to form LAB occurs. A
thermowell 133
monitors the temperature of said catalyst zone 132. The catalyst zone 132 may
be optionally '
heated externally and is contained within 1-1/4 inch stainless steel tubing.
Goodloe paclcing
is positioned at paclcing 136 and 137. LAB product, as well as unreacted
benzene and olefin,
fall through packing 136 into reboiler 142. In reboiler 142, electric heater
140 heats the
contents of reboiler 142 such that heated vapors of benzene and olefni rise
fromthe reboiler
142 to at least reach catalyst zone 132. As needed, the bottoms LAB product
may be
removed from reboiler 142 by opening bottoms valve 144 after passing tlirough
line 147 and
filter 145. Residual water from the feed mixture, or which otlierwise enters
the system, may
17
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
be condensed at condenser 121 which is cooled with coolant via inlet line 122
and exit line
120. The condensed water falls to water trap 124, which can be drained as
needed by
opening drain valve 126. Temperature in the system is monitored via
thermocouples 138,
130, and 165. The system includes pressure release valve 166. A nitrogen
blanket over the
system is maintained by introduction of nitrogen gas via inlet line 154. Level
control
activator 150 activates bottoms level control valve 151 to open when the
liquids level in the
reboiler rises to the level control activator 150.
While the systems depicted in FIG.1 and FIG. 2 show single catalyst zone
systeins,
it must be appreciated that multi-catalyst zone reactors are within the scope
of this invention,
as well as multiple ports for inlet feeds, water traps, product removal lines,
and so forth.
Moreover, the process may be run in batch mode, or in other continuous
processes using
plugflow designs, triclde zone designs, and fluidized zone designs.
As average molecular weight of olefins increases, particularly when the
average
number of carbons is greater than about 15, the selectivity to the 2-isomer is
less than for
lower molecular weight olefins. It is thus preferred, although not absolutely
necessary,
that the product of the alkylation using HF is sent to a second, finishing
catalyst zone to
improve yield. An example of such a second catalyst is HF-treated clay such as
montinorillonite clay treated in accordance with the invention to have about
0.5% fluoride
and calcined as stated in Huntsman's earlier US Patent 6,630,430 which is
fully
incorporated herein by reference.
The scheme of FIG. 3 is shown in the context of LAB allcylation based on a
feed
from a paraffin dehydrogenation facility. Thus, in FIG. 3 fresh paraffin is
fed to a
conventional dehydrogenation apparatus 210 via line 211, with recycled
paraffin being
introduced from the paraffin column 250 via line 252. Dehydrogenated paraffm
from the
dehydrogenation apparatus 210 is then pumped into an allcylation reactor (or
reactors) 230
18
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
that contains hydrogen fluoride. The dehydrogenated paraffm feed may of course
be
supplied from any provider. The source of dehydrogenated paraffin (olefin) is
not critical
to the practice of this invention. LAB product from allcylation unit 230 may
thereafter be
purified by a series of distillation towers.
In this regard, allcylation effluent may be delivered to a benzene colunm 240
by
way of line 231. It should be appreciated that the alkylation product may be
sent offsite
for purification. Further, the particular purification scheme used is not
critical to the
practice of this invention. The scheme depicted in FIG. 3 is instead
representative of a
typical commercial operation. In FIG. 3, unreacted benzene is distilled off
from the crude
LAB product. Benzene is then recycled to the allcylation reactor 230. The
benzene-free
LAB crude product from the benzene colunm 240 is pumped through line 241 to
paraffin
column 250 where any paraffin present is distilled off, with the distilled
paraffin being
recycled to paraffin dehydrogenation unit 210 via line 252. Paraffin-free
crude LAB from
the paraffin column 250 is transported to a refining column 260 where purified
LAB is
distilled and removed via line 262. Heavies (e.g., dialkylates and olefin
derivatives) are
withdrawn from refining column 260 via conduit 261.
It should be appreciated that columns 240, 250, and 260 may be maintained at
conditions (e.g., pressure and temperature) well known to those of slcill in
the art and may
be packed with conventional materials, if desired.
Hydrocarbon and other base oils such as the vegetable oils are known to be
rarely
used in their pure forms in any application, but rather contain various
chemical additives
designed to increase the performance of such oils, or to extend the useful
lives of either the
oils themselves or the equipment in which they are designed to function. In
this regard, the
prior art teaches the use of various oil additives which include without
limitation: detergents,
dispersants, anti-wear agents, extreme pressure additives, antioxidants,
corrosion inhibitors,
19
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
viscosity modifiers, pour point depressants, antifoam agents, friction
modifiers, metal
deactivators, water scavengers, free radical scavengers, and compatibilizers.
Although the present invention has been described largely in reference to the
allcylation of benzene using olefins as an allcylating agent, it should be
appreciated that
substituted benzenes are also useful as starting materials within the context
of the present
invention, provided that the chemical groups appended to the benzene ring are
not
prohibitively de-activating of the benzene ring structure. In this regard,
toluene is a
functionally equivalent starting material which may be used in place of all or
part of the
benzene employed. Other substituted benzenes such as xylenes are also useful
in this regard,
1o as well as ethylbenzene, propylbenzene, and butylbenzene.
In cases where a substituted benzene is alkylated in accordance with the
principles of
this invention, the reaction product consists predominantly of para-
substituted reaction
products, witll some oid1o substitution. Subsequent sulfonation of such a
mixture to provide
sulfonate derivatives results in a mixture of sulfonates or their salts or
esters as well. These
materials may be conveniently described by the formula:
B
CH3\ rCHZ n CH3
CH
R1 R5
R2 R4
R3
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
in which n is any integer between about 12 and about 30; Rl, R2, R3, R4, and
R5 are each
independently selected from the group consisting of: hydrogen, a methyl group,
an ethyl
group, a propyl group, a butyl group, a sulfonic acid group, an sulfonate
group, and salts
a.iid esters thereof; B is selected from the group consisting of: hydrogen,
methyl, or ethyl;
B is attached to any single carbon atom along the -(CHZ)n portion of the alkyl
chain; and
the total 2-aryl isomer content of said mixture of allcylbenzenes is between
about 8 % and
about 30 % by weight based on the total weight of all allcylbenzenes present
in said
mixture and in which the 1-aryl isomer content is less than about 3 % by
weight based on
the weight of all isomers of allcylbenzene derivatives present.
Detergents Useful in Hydrocarbon Oils
One popular class of detergents used in lubricating oils, cutting fluids, and
the lilce are
the oil soluble sulfonates. Within this broad class are the aromatic
sulfonates of the type
described in this specification, particularly the LAB sulfonates. These
materials are preferred
because of their effectiveness and compatibility with other components found
in finished oil
products, their widespread availability, and relatively low cost.
Additionally, many of these
detergent materials are anionic in nature, which means that any one of a wide
range of
selected cationic species may accompany the aiiionic detergent, which is of
particular benefit
when it is desired to incorporate other metals into the composition. The most
commonly
used salts of these acids in hydrocarbon oils are the sodiurn, potassium,
lithium, calcium,
magnesium, strontium and barium salts. The "basic salts" are those metal salts
laiown to
the art wherein the metal is present in a stoichiometrically larger amount
than that
necessary to neutralize the acid. The calcium- and barium-overbased
petrosulfonic acids
are typical examples of such basic salts.
21
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
The terms "overbased," "superbased," and "hyperbased," are terms of art which
are
generic to well known classes of the metallic sulfonates and other materials.
These
overbased materials have also been referred to as "complexes," "metal
complexes," "high-
metal containing salts," and the like. Overbased materials are characterized
by a metal
content in excess of that which would be present according to the
stoichiornetry of the
metal and the particular organic compound reacted with the metal, e.g., a
sulfonic acid.
Thus, if a monosulfonic acid such as an LAB sulfonate is neutralized with a
basic metal
coinpound, e.g., calcium hydroxide, the "normal" metal salt produced will
contain one
equivalent of calciuin for each equivalent of acid. However, as is well known
in the art,
various processes are available which result in an inert orga.nic liquid
solution of a product
containing more than the stoichiometric amount of metal. The solutions of
these products
are referred to herein as overbased materials. Following these procedures, the
sulfonic
acid or an alkali or alkaline earth metal salt thereof can be reacted with a
metal base asid
the product will contain an amount of metal in excess of that necessary to
neutralize the
acid, for example, 4.5 times as much metal as present in the normal salt or a
metal excess
of 3.5 equivalents. The actual stoichiometric excess of metal can vary
considerably, for
example, from about 0.1 equivalent to about 30 or more equivalents depending
on the
reactions, the process conditions, and the like. These overbased materials
useful in
preparing the disperse systems will contain from about 3.5 to about 30 or more
equivalents
of metal for each equivalent of material which is overbased. In the present
specification
and claims the term "overbased" is used to designate materials containing a
stoichiometric
excess of metal and is, therefore, inclusive of those materials which have
been referred to
in the art as overbased, superbased, hyperbased, etc., as discussed supra.
22
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
The present invention thus provides LAB from which LAB sulfonates may be
prepared via conventional sulfonation techniques, and fiom which may further
be prepared
overbased sulfonates, using techniques known to those slcilled in the art.
The overbased materials are prepared by treating a reaction mixture comprising
the organic material to be overbased, a reaction medium consisting essentially
of at least
one inert, organic solvent for said organic material, a stoichiometric excess
of a metal
base, and a promoter with an acidic material. The methods for preparing the
overbased
materials as well as an extremely diverse group of overbased materials are
well lcnown in
the prior art and are disclosed for example in the following U.S. Pat. Nos.:
2,616,904;
lo 2,616,905; 2,616,906; 2,616,911; 2,616,924; 2,616,925; 2,617,049;
2,695,910, 2,723,234;
2,723,235; 2,723,236; 2,760,970; 2,767,164; 2,767,209; 2,777,874; 2,798,852;
2,839,470;
2,856,359; 2,859,360; 2,856,361; 2,861,951; 2,883,340; 2,915,517; 2,959,551;
2,968,642;
2,971,014; 2,989,463; 3,001,981; 3,027,325; 3,070,581; 3,108,960; 3,147,232;
3,133,019;
3,146,201; 3,152,991; 3,155,616; 3,170,880; 3,170,881; 3,172,855; 3,194,823;
3,223,630;
3,232,883; 3,242,079; 3,242,080; 3,250,710; 3,256,186; 3,274,135; 3,492,231;
4,230,586.
6,488,725; 6,197,075; 5,944,858; 5,919,276; 4,690,687; 4,505,718; 4,372,862;
4,260,500;
4,253,976; 4,252,659; 4,225,446; 4,179,385; 4,164,474; 4,129,508; 4,104,180;
6015778;
and 4,094,801. The foregoing patents disclose processes, materials which can
be
overbased, suitable metal bases, promoters, and acidic materials, as well as a
variety of
specific overbased products useful in producing the disperse systems of this
invention and
are, accordingly, incorporated herein by reference.
U.S. Pat. No. 4,086,170 (De Clippeleir et al., Apr. 25, 1978) relates to
calcium
sulfonates and concentrated oily solutions thereof that are prepared by
reacting a solution
of allcylbenzene sulfonic acids with an excess of a calcium oxide having a
medium or low
activity towards water and with carbon dioxide. Oily solutions of overbased
calcium
23
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
sulfonate obtained from such a calcium oxide are limpid and filterable. U.S.
Pat. No.
4,604,219 (Whittle, Aug. 5, 1986) is directed to alkaline earth calcium
sulfonates that are
derived from natural or synthetic feedstocks or a mixture of both which can be
overbased
by introducing into a mixture comprising a neutral alkaline earth calcium
sulfonate, a
lower alcohol, a light hydrocarbon diluent carbon dioxide and'water. The water
is
introduced continuously and at a uniform rate over 1-4 hours, preferably 1-3
hours into the
heated mixture with carbon dioxide. Water is added in a molar ratio
water/calcium oxide
of 0.1 to 1.2 preferably 0.4 to 0.8. It has been found that both the water
rate and ainount
are critical. It has been unexpectedly found that a superior product is formed
by adding
water continuously during carbonation rather than all charged in one or
several increments
at the beginning of the carbonation. In this reference, a high calcium
sulfonate product
with improved filterability and high clarity is formed with good lime
utilization. U.S. Pat.
No. 4,775,490 (Nichols et al., Oct. 4, 1988) describes a process for
overbasing a substrate
comprising mixing the substrate, water, a phenol, a source of magnesium and a
carbonating agent, wherein the water is retained throughout the overbasing
reaction and
provided furtller that the weight ratio of the water to the magnesium is in a
10:1 to 1:5
weight ratio, thereby obtaining a magnesium overbased substrate. U.S. Pat. No.
4,954,272
(Jao, et al., Sep. 4, 1990) is directed to a process for producing an
overbased oil soluble
calcium sulfonate having a TBN of 325, said process comprising: (a) diluting a
neutral
calcium sulfonate with a hydrocarbon solvent and a lower alkanol; (b) adding
to the
diluted calcium sulfonate solution, CaO, Ca(OH)2 and H2O in molar ratios
of CaO :
Ca(OH)2 of about 90:10 to about 20:80 and of H20:CaO of about 0.15:1 to about
0.30: 1;
(c) heating the sulfonate mixture to a temperature ranging from about
100° F. to
about 170° F. under a pressure ranging from about 0 to about 50
p.s.i.g.; (d) passing
CO2 into the heated sulfonate mixture for a period of about 50 to out 200
minutes; (c)
24
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
adding a diluent oil to the COZ treated sulfonate mixture; (f) separating the
solids from the
liquid of the sulfonate mixture; and (g) stripping the hydrocarbon solvent
from the
resulting overbased oil soluble sulfonate product having TBN of 325. U.S. Pat.
No.
5,259,966 (Burlce, Jr., et al., Nov. 9, 1993) provides a process for preparing
an overbased
calcium salt, comprising mixing together: (a) an oil-soluble acid material;
(b) a promoter
comprising: (i) an alcohol or alcohol mixture, and (ii) an inorganic calcium
salt other than
chloride which is soluble in the alcohol mixture of (i), or a.n acid or salt
which forins said
inorganic calcium salt when treated with a calcium base; and (c) greater than
1 equivalent
of a calcium base per equivalent of oil-soluble acid material. U.S. Pat. No.
5,534,168
(Cleverley et al., Jul. 9, 1996) relates to the use of magnesium oxide of
specified, low,
reactivity in a process for the production of overbased magnesium sulfonates,
together
witll the introduction of water and an alcohol, into the reaction mixture
during
carbonation, malces it possible to prepare high base number products which
have very low
post carbonation sediments and which can be purified by rapid filtration.
Other detergents known to those skilled in the art are useful as a component
of a
composition according to the invention in addition to the LAB based detergents
described
herein.
Dispersants Useful in Hydrocarbon Oils
Although a dispersant used in a hydrocarbon oil may be a multifunctional
material
that can confer otlier beneficial properties to a base oil, dispersants are
primarily used in
hydrocarbon oils for their ability to maintain small particles of dirt,
combustion products,
metal fines, etc. in the liquid phase, to prevent deposition and accumulation
of sludges in
places where eddy currents exist in various equipment and wares.
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
The use of acylated nitrogen compounds as dispersants in lubricants is
disclosed in
numerous patents, including U.S. Pat. Nos. 3,172,892; 3,219,666; 3,272,746;
3,310,492;
3,341,542; 3,444,170; 3,455,831; 3,455,832; 3,576,743; 3,630,904; 3,632,511;
3,804,763;
and 4,234,435.
The book "Lubricant Additives" by M. W. Ranney, published by Noyes Data
Corporation of Parkridge, N.J. (1973), discloses a number of overbased metal
salts of
various sulfonic acids which are useful as detergent/dispersant in lubricants.
The book also
entitled "lubricant Additives" by C. V. Smallheer and R. K. Smith, published
by the
Lezius-Hiles Co. of Cleveland, Ohio (1967), similarly discloses a number of
overbased
sulfonates which are useful as dispersants. U.S. Pat. No. 4,100,082 discloses
the use of
neutral or overbased metal salts of organic sulfur acids as
detergent/dispersants for use in
fuels and lubricants.
Ashless detergents and dispersants are so called despite the fact that,
depending on
its constitution, the dispersant may upon combustion yield a non-volatile
material such as
boric oxide or phosphorus pentoxide; however, it does not ordinarily contain
metal and
therefore does not yield a metal-containing ash on conibustion. Many types are
known in
the art, and any of them are suitable for use in the lubricant compositions
and functional
fluids of this invention. The following are illustrative of dispersants, not
delimitive of the
term, and are incorporated by reference herein:
(1) Reaction products of carboxylic acids (or derivatives thereof) containing
at
least about 34 and preferably at least about.54 carbon atoms with nitrogen
containing compounds such as amine, organic hydroxy compounds such as phenols
and alcohols, and/or basic inorganic materials. Examples of these "carboxylic
dispersants" are described in many U.S. Pat. Nos., including 3,219,666;
4,234,435;
and 4,938,881. These include the products formed by the reaction of a
26
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
polyisobutenyl succinic anhydride with an amine such as a polyethylene amine.
(2) Reaction products of relatively high molecular weight aliphatic or
alicyclic
halides with amines, preferably oxyalkylene polyamines. These may be
characterized as "amine dispersants" and examples thereof are described for
example, in the following U.S. Pat. Nos.: 3,275,554; 3,438,757; 3,454,555; and
3,565,804.
(3) Reaction products of alkyl phenols in which the allcyl group contains at
least
about 30 carbon atoms with aldehydes (especially formaldehyde) and amines
(especially polyalkylene polyamines), which may be characterized as "Mannich
dispersants." The materials described in the following U.S. Pat. Nos. are
illustrative: 3,649,229; 3,697,574; 3,725,277; 3,725,480; 3,726,882; and
3,980,569.
(4) Products obtained by post-treating the amine or Mannich dispersants with
such
reagents as urea, thiourea, carbon disulfide, aldehydes, ketones, carboxylic
acids,
hydrocarbon-substituted succinic anhydrides, nitriles, epoxides, boron
compounds,
phosphorus compounds or the like. Exemplary materials of this kind are
described
in the following U.S. Pat. Nos. 3,639,242; 3,649,229; 3,649,659; 3,658,836;
3,697,574; 3,702,757; 3,703,536; 3,704,308; and 3,708,422.
(5) Interpolyiners of oil-solubilizing monomers such as decyl methacrylate,
vinyl
decyl ether and high molecular weight olefins with monomers containing polar
substituents, e.g., atninoalkyl acrylates or acrylamides and poly-
(oxyethylene)-
substituted acrylates. These may be characterized as "polyineric dispersants"
and
examples thereof are disclosed in the following U.S. Pat. Nos. 3,329,658;
3,449,250; 3,519,565; 3,666,730; 3,687,849; and 3,702,300.
27
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
Antiwear Agents
A coinposition according to this invention may also include a sulfur-,
phosphorus-,
or sulfur- and phosphorus-containing antiwear agent. The term antiwear agent
is used to
refer to compounds which provide wear protection properties to lubricating
compositions
and functional fluids. Antiwear agents are useful in controlling wear and may
sometimes
also act as extreme pressure agents and as antioxidants. These antiwear agents
include
sulfiuized organic compounds, hydrocarbyl phosphates, phosphorus-containing
amides,
phosphorus-containing carboxylic esters, phosphorus-containing ethers, and
1o dithiocarbamate-containing compounds. Examples of hydrocarbyl phosphates
include
hydrocarbyl thiophosphates. Thiophosphates may contain from one to about three
sulfur
atoms, preferably one or two sulfiu atoms. Thiophosphates are prepared by
reacting one
or more phosplutes with a sulfurizing agent including sulfur, sulfur halides,
and sulfur
containing coinpounds. Salts of thiophosphates include zinc dithiophosphates.
Other
antiwear agents known to those skilled in the art are useful as a component of
a
composition according'to the invention. Other dispersants 1cZown to those
skilled in the
art are useful as a component of a coinposition according to the invention.
Anti-Oxidants
A particularly valuable class of additives known as antioxidants are widely
used in
lubricating oil formulations, cutting oils, and functional fluids.
Antioxidants are materials
which inhibit oxidative decomposition of the oil under consideration. Although
several
examples are given below, these examples should be considered exemplary only
of the
wide variety of antioxidants which may be usefully combined with the detergent
components of this invention.
28
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
In U.S. Pat. No. 2,282,710 to Dietrich issued May 12, 1942 it is lalown that
stabilization of petroleuin hydrocarbons against the deleterious catalytic
action of metals
may be obtained by compositions containing both a nitrogen and a sulfur
functional group.
Various cyclic, aromatic and linear carbon configurations are shown in the
sulfur and
nitrogen containing molecules of Dietrich. Dietrich discloses preparing his
compositions
by the use of ethyleneimine. Dietrich further states that his compounds are
particularly
effective in retarding the formation of products corrosive to inetals,. and
particularly
cadmium, silver, copper, lead and like bearing alloys under normal service
conditions.
German OLS 1,066,019 published Sept. 24, 1959 by Holtschmitt et al describes
various condensation products of thioglycol and nitrogen containing materials.
Holtschmitt shows his compounds as containing free hydroxyl groups.
Holtschinitt further
discloses the use of aromatic amines containing a short aliphatic group on the
aromatic
ring, e.g. toluidine.
It is known from an article entitled=: "Thioglycol Polymers I Hydrochloric
Acid-
Catalysed Auto Condensation of Thiodiglycol" by Woodward, Journal of Polymer
Science
the OL XLI, Pages 219-223 (1959), that the properties of a sulfur and oxygen
containing
compound allow end-to-end condensation. It is further known from the Woodward
article
that multiple sulfur linkages within the molecule, e.g. disulfides,
trisulfides, and the like
may be obtained.
It is further known that various amines may be utilized in antioxidant
compositions. Phenothiazine compounds are known in lubricant products from
U.S. Pat.
No. 2,781,318 issued Feb. 12, 1957 to Cyphers. The alkyl phenothiazines of
Cyphers are
allcylated on the phenylene rings of the phenothiazine structure. Cyphers does
not show or
suggest the allcylation of the amine nitrogen in phenothiazine. The Cyphers
patent is
29
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
directed to the utility of phenothiazine as an antioxidant and corrosion
inhibiting additive
for ester, polyester, polyether and other synthetic lubricallts.
U.S. Pat. No. 3,536,706 issued Oct. 27, 1970 to Randell suggests that
phenothiazines may be used as additives for synthetic lubricants. The
phenothiazines
particularly described by Randell are those containing tertiary alkyl
substituents having
from 4 to 12 carbon atoms on the aryl groups which make up the phenothiazine
structure.
Randell also discloses fused rings on the two phenylene groups which make up
the
phenothiazine structure. Stated otherwise, Randell allows the utilization of
naphthalene for
at least one of the two aryl groups in the phenothiazine structure. U.S. Pat.
No. 3,803,140
issued to Cook et al on Apr. 9, 1974 describes various tertiary alkyl
derivatives of
phenotlliazine. N-alkyl substitution or N-alkenyl substitution is described on
the
phenothiazine structure. Ring allcylation when the phenothiazine is in the
free nitrogen
form is also shown. Cook et al express a preference for non-N substituted
phenothiazine
derivatives.
Cook et al also suggest that organic materials which are susceptible to
oxidative
degradation may benefit through the use of the compounds of their invention.
Such uses
include antioxidants for aliphatic hydrocarbons such as gasoline, lubricating
oils,
lubricating greases, mineral oils, waxes, natural and synthetic polymers such
as rubber,
vinyl, vinylidene, ethers, esters, amides and urethanes. The compounds of Cook
et al are
also suggested for stabilizing aldehydes and unsaturated fatty acids or esters
thereof. Still
= further utilities suggested by Cook et al include the stabilization of
organo-metalloid
substances such as silicone polymers. Another class of uses of the compounds
of Cook et
al include the stabilization of vitamins, essential oils, ketones and ethers.
Normant in U.S. Pat. No. 3,560,531 issued Feb. 2, 1971, describes metallation
of
materials having active hydrogens including phenothiazine. U.S. Pat. No.
3,344,068 issued
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
Sept. 26, 1967, to Waight et al describes antioxidants for ester-based
lubricants. Waight et
al's coinpounds have an N-hydrocarbyl substituted phenothiazine structure. The
N-
substituted phenothiazine compounds of Waight et al are also substituted in at
least one
position on the fused aromatic nuclei. A second required component in the
compositions
of Waight et al is a secondary aromatic amine having two aromatic groups
attached to the
nitrogen atom.
The preparation of alkylthioalkanols wliich are useful as intermediates for
preparing the compounds of the present invention are described in U.S. Pat.
No. 4,031,023
to Musser et al.
U.S. Pat. No. 2,194,527 to Winthrop et al which issued Nov. 24, 1959,
describes
pharmaceutical compounds such as omega-(10-phenothiazinyl)allcyl di-alkyl
sulfonium
salts which are useful as spasmolytics and in particular antihistaminics. U.S.
Pat. No.
3,376,224 issued Apr. 2, 1968 to Elliott et al describes phenothiazine
derivatives which are
stated to be N-substituted methylene compounds which contain an ether linkage
between
the methylene group and an alkyl or cycloalkyl radical. According to Elliott
et al, the alkyl
or cycloalkyl radical may carry an alkoxy or other non-reactive substituent.
U.S. Pat. No. 4,915,858 describes a composition of matter~which is the amine
terminated reaction product obtained from two equivalents of a secondary
aromatic
monoamine with at least two equivalents of a betathiodialkanol. Other
antioxidants known
to those skilled in the art are useful as a component of a composition
according to the
invention.
Corrosion Inhibitors
Corrosion-inhibiting agents are exemplified by chlorinated aliphatic
hydrocarbons
such as chlorinated wax; organic sulfides and polysulfides such as benzyl
disulfide,
bis(chlorobenzyl) disulfide, dibutyl tetrasulfide, sulfurized methyl ester of
oleic acid,
31
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
sulfurized alkylphenol, sulfurized dipentene, and sulfurized terpene;
phosphosulfurized
hydrocarbons such as the reaction product of a phosphorus sulfide with
turpentine or
methyl oleate; phosphorus esters including principally dihydrocarbon and
trihydrocarbon
phosphites such as dibutyl phosphite, diheptyl phosphite, dicyclohexyl
phosphite, pentyl
phenyl phosphite, dipentyl phenyl phosphite, tridecyl phosphite, distearyl
phosphite,
dimethyl naphthyl phosphite, oleyl 4-pentylphenyl phosphite, polypropylene
(molecular
weiglit 500)-substituted phenyl phosphite, diisobutyl-substituted phenyl
phosphite; metal
thiocarbamates, such as zinc dioctyldithiocarbamate, and barium heptylphenyl
dithiocarbamate; Group II metal phosphorodithioates such as zinc
dioctylphosphorodithioate, zinc dicyclohexylphosphorodithioate, barium
di(heptylphenyl)phosphorodithioate, cadinium dinonylphosphorodithioate, and
the zinc
salt of a phosphorodithioic acid produced by the reaction of phosphorus
pentasulfide with
an equimolar mixture of isopropyl alcohol and n-hexyl alcohol. Other corrosion
inhibitors
known to those skilled in the art are useful as a component of a composition
according to
the invention.
Viscosity Modifiers
Viscosity modifiers generally are polymeric materials characterized as being
hydrocarbon-based polymers generally having number average molecular weights
between about 25,000 and 500,000 more often between about 50,000 and 200,000.
Such
materials are typically added to a hydrocarbon based oil and the oil is
heated, with
agitation, until the polymeric material is dissolved.
Polyisobutylene has been used as a viscosity modifier in lubricating oils.
Polymethacrylates (PMA) are prepared from mixtures of methacrylate monomers
having
different allcyl groups. Most PMA's are viscosity-modifiers as well as pour
point
32
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
depressants. The allcyl groups may be either straight chain or branched chain
groups
containing from 1 to about 18 carbon atoms.
Ethylene-propylene copolyiners, generally referred to as OCP can be prepared
by
copolymerizing ethylene and propylene, generally in a solvent, using known
catalysts such
as a Ziegler-Natta initiator. The ratio of ethylene to propylene in the
polymer influences
the oil-solubility, oil-thickening ability, low temperature viscosity, pour
point depressant
capability and engine performance of the product. The common range of ethylene
content
is 45-60% by weight and typically is from 50% to about 55% by weight. Some
commercial OCP's are terpolymers of ethylene, propylene and a small amount of
nonconjugated diene such as 1,4-hexadiene. In the rubber industry, such
terpolymers are
referred to as EPDM (ethylene propylene diene monomer). The use of OCP's as
viscosity
modifiers in lubricating oils has increased rapidly since about 1970, and the
OCP's are
currently one of the most widely used viscosity modifiers for motor oils.
Esters obtained by copolymerizing styrene and maleic anhydride in the presence
of
a free radical initiator and thereafter esterifying the copolyiner with a
mixture of C4_18
alcohols also are useful as viscosity modifying additives in motor oils. The
styrene esters
generally are considered to be multifunctional premium viscosity modifiers.
The styrene
esters in addition to their viscosity modifying properties also are pour point
depressants
and exhibit dispersancy properties when the esterification is terminated
before its
completion leaving some unreacted anhydride or carboxylic acid groups. These
acid
groups can then be converted to imides by reaction with a primary amine.
Hydrogenated styrene-conjugated diene copolymers are another class of
commercially available viscosity modifiers for motor oils. Examples of
styrenes include
styrene, alpha-methyl styrene, ortho-methyl styrene, meta-methyl styrene, para-
methyl
styrene, para-tertiary butyl styrene, etc. Preferably the conjugated diene
contains from
33
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
four to six carbon atoms. Examples of conjugated dienes include piperylene,
2,3-
dimethyl-1,3-butadiene, chloroprene, isoprene and 1,3-butadiene, with isoprene
and
butadiene being particularly preferred. Mixtures of such conjugated dienes are
useful.
The styrene content of these copolymers is in the range of about 20% to about
70%
by weight, preferably about 40% to about 60% by weight. The aliphatic
conjugated diene
content of these copolymers is in the range of about 30% to about 80% by
weight,
preferably about 40% to about 60% by weight.
These copolymers typically have number average molecular weights in the range
of about 30,000 to about 500,000, preferably about 50,000 to about 200,000.
The weight
average molecular weight for these copolymers is generally in the range of
about 50,000 to
about 500,000, preferably about 50,000 to about 300,000.
The above described hydrogenated copolymers have been described in the prior
art
such as in U.S. Pat. Nos. 3,551,336; 3,598,738; 3,554,911; 3,607,749;
3,687,849; and
4,181,618 which are hereby incorporated by reference for their disclosures of
polymers
and copolymers useful as viscosity modifiers in oil compositions according to
this
invention. For example, U.S. Pat. No. 3,554,911 describes a hydrogenated
random
butadiene-styrene copolymer, its preparation and hydrogenation. The disclosure
of this
patent is incorporated herein by reference. Hydrogenated styrene-butadiene
copolymers
useful as viscosity modifiers in the lubricating oil compositions of the
present invention
2o are available cormnercially from, for example, BASF under the general trade
designation
"Glissoviscal". A particular example is a hydrogenated styrene-butadiene
copolymer
available under the designation Glissoviscal 5260 which has a molecular
weight;
determined by gel perineation chromatography, of about 120,000. Hydrogenated
styrene-
isoprene copolymers useful as viscosity modifiers are available from, for
example, The
Shell Chemical Company under the general trade designation "Shellvis".
Shellvis 40 from
34
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
Shell Chemical Company is identified as a diblock copolymer of styrene and
isoprene
having a nuinber average molecular weight of about 155,000, a styrene content
of about
19 mole percent and an isoprene content of about 81 mole percent. Shellvis 50
is available
from Shell Chemical Company and is identified as a diblock copolymer of
styrene and
isoprene having a number average molecular weight of about 100,000, a styrene
content of
about 28 mole percent and an isoprene content of about 72 mole percent. Other
viscosity
modifiers known to those skilled in the art are useful as a component of a
composition
according to the invention.
Pour Point Depressants
Pour point depressants may also be included in a formulation according to the
invention. They are a particularly useful type of additive often included in
the lubricating
oils and functional fluids such as cutting oils or other lubricants, and often
coinprise oil-
soluble polymers. Examples of pour point depressants include those on page 8
of
Lubricant Additives" by C. V. Smalheer and R. Kennedy Smith (Lesius-Hiles
Company
Publishers, Cleveland, Ohio, 1967, which book is incorporated in its entirety
herein by
reference thereto). Other pour point depressants lcnown to those skilled in
the art are useful
as a component of a composition according to the invention.
Antifoafn agents
Anti-foam agents may be used to reduce or prevent the formation of stable foam
and include silicones or organic polymers. Examples of these and additional
anti-foam
compositions are described in "Foam Control Agents", by Henry T. Kerner (Noyes
Data
Corporation, 1976), pages 125-162, which book is incorporated in its entirety
herein by
reference thereto. Other antifoain agents lcnown to those skilled in the art
are useful as a
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
component of a composition according to the invention.
Friction Modifiers
The oil coinpositions of the present invention also may contain at least one
friction
modifier to provide the lubricating oil with the proper frictional
characteristics for a given
application. Various amines, particularly tertiary amines are effective
friction modifiers.
Examples of tei-tiary amine friction modifiers include N-fatty allcyl-N,N-
diethanol amines,
N-fatty alkyl-N,N-diethoxy ethanol amines, etc. Such tertiary amines can be
prepared by
reacting a fatty alkyl ainine witli an appropriate number of moles of ethylene
oxide.
Tertiary amines derived from naturally occurring substances such as coconut
oil and
oleoamine are available from Armour Chemical Coinpany under the trade
designation
"Ethomeen". Particular examples are the Etliomeen-C and the Ethomeen-O series.
Sulfur-
containing compounds such as sulfurized C12_24 fats, alkyl sulfides and
polysulfides
wherein the allcyl groups contain from 1 to 8 carbon atoms, and sulfurized
polyolefins also
may function as friction modifiers in the lubricating oil compositions of the
invention.
Other fiiction modifiers known to those skilled in the at-t are useful as a
component of a
composition according to the invention.
Base Oils
The present invention is broad with respect to the selection of base oil
component
used in its blending. Typically, compositions according to the invention
comprise a base
oil as a major component of the composition. For purposes of this
specification and the
appended claims the term "base oil" as used herein is intended to include
those materials
which are recognized as possessing lubricity characteristics by those of
ordinary skill in
the art. Such materials include, without limitation, materials falling within
the following
classes: 1) lubricity agents such as synthetic polymers (e.g., polyisobutene
having a
36
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
number average molecular weight in the range of about 750 to about 15,000, as
measured
by vapor phase osinometry or gel permeation chromatography); 2) the polyol
ethers (e.g.,
poly(oxyethylene-oxypropylene)ethers); 3) ester oils including natural and
synthetic
triglycerides; 4) natural oil fractions such as mineral oils and those
referred to as bright
stoclcs (including all relatively viscous products formed during conventional
lubricating oil
manufacture from petroleum). Thus, any oil or other material recognized by
those skilled
in the art as possessing lubricity characteristics may be used as a base oil
for purposes of
this invention.
Ashless Dispersants
Witllin the prior art in the realm of motor fuel are a wide range of materials
regarded as ashless dispersants by those of ordinary slkill in such art. There
are a great
many materials capable of functioning in this regard, including various
Mannich bases,
etllyleneamines, polylakylene polyamines, and other primary, secondary and
tertiary
amines known in the art. The following is provided to be exemplary and not
delimitive of
the scope of ashless dispersants which may be employed in the context of the
present
invention.
A large number of such ashless dispersants are derivatives of high molecular
weight carboxylic acid acylating agents. Typically, the acylating agents are
prepared by
reacting an olefin (e.g., a polyalkene such as polybutene) or a derivative
thereof,
containing for example at least about 10 aliphatic carbon atoms or generally
at least 30 to
50 aliphatic carbon atoms, with an unsaturated carboxylic acid or derivative
thereof such
as acrylic acid, methylacrylate, maleic acid, fumaric acid and maleic
anhydride.
Dispersants are prepared from the high molecular weight carboxylic acid
acylating agents
by reaction with, for example, amines characterized by the presence within
their structure
of at least one N-H group, alcohols, reactive metal or reactive metal
compounds, and
37
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
combinations of the above. The prior art relative to the preparation of such
carboxylic acid
derivatives is summarized in U.S. Pat. No. 4,234,435.
It also has been suggested that the carboxylic acid derivative compositions
such as
those described above can be post-treated with various reagents to modify and
improve the
properties of the compositions. Acylated nitrogen compositions prepared by
reacting the
acylating reagents described above with an amine can be post-treated, for
example, by
contacting the acylated nitrogen compositions thus formed with one or more
post-treating
reagents selected from the group consisting of boron oxide, boron oxide
hydrate, boron
halides, boron acids, esters of boron acid, carbon disulfide, sulfur, sulfur
chlorides, alkenyl
cyanides, carboxylic acid acylating agents, aldeliydes, ketones, phosphoric
acid, epoxides,
etc. Lists of the prior art relating to post-treatment of carboxylic ester and
an2ine
dispersants with reagents such as those described above are contained in a
variety of
patents such as U.S. Pat. No. 4,203,855 (Col. 19, lines 16-34) and U.S. Pat.
No. 4,234,435
(Col. 42, lines 33-46). The use of isophthalic and terephthalic acids as
corrosion-
inhibitors is described in U.S. Pat. No. 2,809,160. The corrosion-inhibitors
are used in
combination with detergent additives.
The preparation of lubricating oils containing ashless dispersants obtained by
reaction of aliphatic and aromatic polycarboxylic acids with acylated amines
have been
described previously. For example, U.S. Pat. No. 4,234,435 describes
lubricating oils
containing carboxylic acid derivative compositions prepared by post-treating
acylated
amines with a variety of compositions including carboxylic acid acylating
agents such as
terephthalic acid and maleic acid. U.S. Pat. No. 3,287,271 and French Pat. No.
1,367,939
describe detergent-corrosion inhibitors for lubricating oils prepared by
combining a
polyamine with a high molecular weight-succinic anhydride and thereafter
contacting the
resulting product with an aromatic dicarboxylic acid of fiom 8 to 14 carbon
atoms wherein
38
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
the carboxyl groups are bonded to annular carbon atoms separated by at least
one annular
carbon atom. Illustrative of such aromatic dicarboxylic acids are isophthalic
acid,
terephthalic acid and various derivatives thereof. Lubricating compositions
containing
ainine salts of a phthalic acid are described in U.S. Pat. No. 2,900,339. The
amine salts are
thermally unstable salts of the phthalic acid and a basic tertiary amine. U.S.
Pat. No.
3,692,681 describes dispersions of phthalic acid in hydrocarbon media
containing highly
hindered acylated allcylene polyamines. The polyamines are prepared by
reaction of an
allcenyl succinic anhydride with an alkylene polyamine such as etlzylene
polyamine or
propylene polyamine. The terephthalic acid or its derivative is dissolved in
an auxiliary
solveiit such as a tertiary alcohol or DMSO, and a terephthalic acid solution
is combined
with a hydrocarbon solution containing the hindered acylated amine address
detergent.
The auxiliary solvent then is removed.
U.S. Pat. No. 3,216,936 describes lubricant additives which are coinpositions
derived from the acylation of alkylene polyamines. More specifically, the
compositions
are obtained by reaction of an alkylene amine with an acidic mixture
consisting of a
hydrocarbon-substituted succinic acid having at least about 50 aliphatic
carbon atoms in
the hydrocarbon group and an aliphatic monocarboxylic acid, and thereafter
removing the
water formed by the reaction. The ratio of equivalents of said succinic acid
to the mono-
carboxylic acid in the acidic mixture is from about 1:0.1 to about 1:1. The
aliphatic mono-
carboxylic acids contemplated for use include saturated and unsaturated acids
such as
acetic acid, dodecanoic acid, oleic acid, naphthenic acid, formic acid, etc.
Acids having 12
or more aliphatic carbon atoms, particularly stearic acid and oleic acid, are
especially
useful. The products described in the'936 patent also are useful in oil-fuel
mixtures for
two-cycle internal combustion engines.
39
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
British Pat. No. 1,162,436 describes ashless dispersants useful in lubricating
compositions and fuels. The compositions are prepared by reacting certain
specified
alkenyl substituted succinimides or succinic amides with a hydrocarbon-
substituted
succinic acid or anhydride. The arithmatic mean of the chain lengths of the
two
hydrocarbon substituents is greater than 50 carbon atoms. Formamides of
monoalkenyl
succinimides are described in U.S. Pat. No. 3,185,704. The formamides are
reported to be
useful as additives in lubricating oils and fuels.
U.S. Pat. Nos. 3,639,242 and 3,708,522 describe compositions prepared by post-
treating mono- and polycarboxylic acid esters with mono- or polycarboxylic
acid acylating
agents. The compositions thus obtained are reported to be useful as
dispersants in
lubricants and fuels.
One preferred method for preparing compositions according to the invention is
to
begin with a maj or amount of a base oil material and add the other selected
ingredients to
the base oil, with sufficient agitation to provide a homogeneous mixture
within a
reasonable time. When the viscosity of the additive is much greater than that
of the base
oil, it is beneficial to provide heating to the base oil to facilitate
dissolution and
homogeneity. This is especially true in the cases where polymeric materials
are added to
base oils. However, the dissolution of all of the additives used in the
invention in a base
oil is well known in the art and is thus within the skill level of an ordinary
artisan in the oil
additives field.
The compositions of the present invention may vary widely in composition
depending upon the intended use of the final composition. However, those of
ordinary
skill in formulating lubricating oils, functional fluids, cutting oils,
emulsions, etc., in
which LAB based detergent materials are used as a component readily recognize
that the
detergents prepared from LAB materials provided by the invention may be used
as direct,
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
drop-in substitutes for many detergent components in current formulations,
including
those which are based on linear allcylbenzenes and those which are not.
Compositions
which include detergents based upon the linear alkylbenzenes of the invention
offer
superior detergency over formulations which contain linear allcylbenzene based
detergent
materials of the prior art, on a molar basis, owing to the unique isomer
distribution
provided by the present invention.
Another aspect of the present invention is the use of the LAB surfactants in
fuel
formulations on which various internal combustion engines including diesel,
automobile,
and jet engines may be operated. Since the LAB surfactants of this invention
may be
anionic in nature, such as in the cases when the detergent molecule is a
sulfonate, it is
possible to provide charge balance using a cation which is known to impart
beneficial
properties to motor fuels. Such cations may include the alkali and alkaline
earth metals as
the use of such are well known for the properties they impart to fuel
compositions.
Further, the prior art discloses many ashless dispersants useful as additives
in fuels and
lubricant compositions. Many of these are cationic in nature and are thus
capable of
providing charge balance to chemical compounds in which the anionic portion is
derived
from the LAB according to this invention, to provide a neutral, oil or fuel
soluble material
which possesses both detergent and dispersant characteristics.
One particular and suiprising advantage of using the catalysts of this
invention to
produce alkylbenzenes is that a low content of diallcylbenzene components are
found in
the alkylbenzene product mixture. This is important since diallcylbenzenes are
generally
regarded as undesirable, and the presence of such species tends to raise the
viscosity of the
alkylbenzene reaction product mixture. Thus, using conventional allcylation
technology
known in the art, it is common for allcylbenzenes produced in accordance with
prior art
methods to have a viscosity greater than about 145 SUS viscosity units at a
temperature of
41
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
37.8 degrees centigrade. However, alkylation of benzene with olefins in the
C16 - C30
range in accordance with this invention provides a product having an SUS
viscosity of 85
at 37.8 degrees centigrade. Generally speaking, alkylbenzenes made by
allcylation of
benzene with olefins in the C16 - C3o ra.iige using catalysts an procedures
taught herein
results in the alkylbenzenes containing less than 1 % of dialkylbenzenes.
A material sold by Huntsman LLC of Houston Texas is sold under the trade name
"Alkylate 300" and is available from Huntsman in commercial quantities.
Huntsman's
Allcylate 300 is has a 2-phenyl isomer content in the range of between about
10 and 13%
by weight of all allcylbenzene isomers present, and is substantially fiee of
benzene
inasmuch as we are unable to detect any benzene present down to a level of 100
parts per
billion.
Huntsman's Alkylate 300 is suitable for sulfonation from which allcylbenzene
sulfonates may be produced using means known to those skilled in the art which
may
comprises reacting the Alkylate 300 with sulfuric acid and/or SO3 at elevated
temperatures. A sulfonation method involving an air/sulfur trioxide mixture
whicll is
applicable to providing sulfonated Allcylate 300 is described in U.S. Pat. No.
3,427,342 to
Brooks et al., entitled "Continuous Sulfonation Process," which is
incorporated by
reference herein. In an einbodiment, a sulfur trioxide to alkyl aromatic
product molar ratio
used for sulfonation is 1.03.
The sulfonated Allcylate 300 einerges from the sulfonation reactor as the acid
form
of the sulfonate, which is sometimes referred to as an allcylbenzenesulfonic
acid. This
acid may be neutralized using conventional alkalis such as hydroxides, oxides,
carbonates,
etc. of alkali metals, alkaline earth metals, and combinations thereof. One
popular method
for using Alkylate 300 is to sulfonate the Allcylate 300 to form the
allcylbenzenesul-fonic
acid, and to subsequently neutralize the sulfonic acid so obtained with a
calcium or
42
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
magnesium salt, and subsequently overbasing by successive additions of carbon
dioxide
and magnesium and/or calcium ion until a material liaving a desired base
number is
obtained. The resulting material is an oil-soluble overbased sulfonate, which
is suitable
for use as a detergent additive for lubricants and oils.
Thus, a composition of one form of the present invention is a mixture of two
or
more alkylbenzenes which have a 2-aryl isomer content in the range of between
about 8
and 30% by weight based on the total weight of all of the linear
monoalkylbenzenes
(including 2-aryl and non-2-aryl) present in the mixture. In general,
alkylbenzenes and
detergent-dispersant products derived therefrom according to this invention
are
substantially free from benzene, having a benzene content less than 2 ppm
iri'one form of
the invention, less than 1 ppm in another form of the invention, less than 0.5
ppm in
another fonn of the invention and less than 100 parts per billion in another
form of the
invention. In general, an alkylbenzene and detergent-dispersant products
derived
therefrom according to the invention contain less than about 28 % branching.
Further, the
alkylbenzenes according to the invention are substantially free from 1-aryl
isomers of
allcylbenzenes, which are isomers where the benzene ring is attached to the 1-
position of a
linear alkyl chain having more than about 14 carbon atoms.
Consideration inust be given to the fact that although this invention has been
described and disclosed in relation to certain prefeiTed embodiments, obvious
equivalent
modifications and alterations thereof will become apparent to one of ordinary
skill in this
art upon reading and understanding this specification and the claims appended
hereto. The
present disclosure includes the subject inatter defined by any combination of
any one of
the various claims appended hereto with any one or more of the remaining
claims,
including the incorporation of the features and/or limitations of any
dependent claim,
singly or in combination with features and/or limitations of any one or more
of the other
43
CA 02584152 2007-04-16
WO 2006/047091 PCT/US2005/036684
dependent claims, with features and/or limitations of any one or more of the
independent
claims, with the remaining dependent claims in their original text being read
and applied
to any independent claim so modified. This also includes combination of the
features
and/or limitations of one or more of the independent claims with the features
and/or
limitations of another independent claim to arrive at a modified independent
claim, with
the remaining deperident claims in their original text being read and applied
to any
independent claim so modified.
The present invention includes any overbased sulfonate described herein with
any
one or more conventional additives useful in motor oils and the like specified
herein.
In addition, the 2-aryl isomer content has been described in quantities
relative to
the total weight of a mixture in which such isomers are present. The present
invention
also includes embodiments in which the percentages specified herein relate to
the 2-isomer
content when calculated by taking the weight of the 2-isomer content in a
given sample,
and dividing it by the weight in the same sainple of linear allcylbenzenes,
and multiplying
this quotient by 100 to arrive at a percentage 2-isomers, with all remaining
claim
limitations held constant. A specification sheet for Alkylate 300 is available
from
Huntsman LLC of Houston, Texas.
Thus, the presently disclosed invention is intended to cover all such
modifications
and alterations, and is limited only by the scope of the claims which follow,
in view of the
foregoing and other contents of this specification.
44