Note: Descriptions are shown in the official language in which they were submitted.
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
AQUEOUS-BASED METHOD FOR PRODUCING ULTRA-F1NE METAL POWDERS
FIELD OF THE INVENTION
[0001] The present invention relates generally to ultra-fine metallic
compositions and
methods of making the same. The present invention further relates to methods
of coating various
substrates with ultra-fine metallic compositions.
BACKGROUND OF THE INVENTION
[0002] Ultra-fine metallic particles have many unique physical and chemical
characteristics, which make them ideal materials for a variety of
applications, such as electronics,
catalysis, metallurgy, and decorations. Compared to the various particle-
producing techniques
used in the art, the methods based on the chemical precipitation in solutions
provide several
advantages, e.g., low manufacturing cost and a very good control of the
mechanism of metal
particles formation. Others in the art have successfully prepared micron and
submicron-size
metallic powders of Co, Cu, Ni, Pb, and Ag using chemical-based techniques,
such as the ones
based on the reduction in alcohols or polyols. For example, U.S. Patent No.
4,539,041 discusses
a method for producing micrometer-size metallic particles by using polyols to
convert various
metallic compounds into metal powders.
[0003] U.S. Pat. Nos. 3,620,713 and3,620,714 to Short disclose a method for
making
platinum and platinum alloy powders for electronic components, having an
average particle size
of from 0.5 to 2 m, in which a platinum ammonia complex is freshly
precipitated with
ammonium hydroxide and then reduced with hydrazine to produce the metal. U.S.
Pat. Nos.
4,456,473 and 4,456,474 to Jost disclose a similar process in which a silver
ammonium complex
is reduced in water with hydrazine, to produce particles with diameters
averaging 0.6 to 5 m.
U.S. Pat. No. 5,413,617 to Lin et al. also discloses reduction of a silver
ammonium complex with
aqueous hydrazine under a particular temperature regime to give high surface
area powder of
undisclosed particle size. According to U.S. Pat. No. 4,039,317 (Montino et
al.), reduction of a
silver ammonium complex with hydrogen in an autoclave provides silver
particles of 0.5 to 3 m
diameter, while similar reduction of silver oxide suspensions without ammonia
reportedly yields
particles as small as 0.1 m. A gold-ammonia complex has been reduced to 4 m
gold particles
with aqueous bisulfite (U.S. Pat. No. 5,413,617 to Fraioli).
Express Mail No. EV 446 920 045
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
[0004] The polyol procedures, require complex equipment and the metallic
powders
produced are generally more expensive because of the cost of the organic
solvents used. The
aqueous methods are less costly, but except for the Montini process, which
requires an autoclave,
they do not produce particles smaller than about 0.5 m. The present invention
provides a
process capable of cost-effectively generating low-dispersion, crystalline,
ultra-fine metallic
particles in an aqueous medium. Such particles are highly desirable in many
practical
applications, especially in electronics.
SUMMARY OF THE INVENTION
[0005] The present invention provides a method for forming compositions having
a
plurality of ultra-fine metallic particles, and the metallic composition
produced therewith, where
the plurality of ultra-fine metallic particles is obtained in accordance with
a process including:
(a) providing a reducing solution comprising a reducing agent and a
stabilizing agent;
(b) providing a metal-ammonia solution containing a metal-ammonia
complex;
(c) forming a reaction mixture containing the reducing solution and the
metal-ammonia solution;
(d) maintaining the reaction mixture under a condition sufficient to reduce
the
metal-ammonia complex to metallic particles, thereby producing the plurality
of ultra-
fine metallic particles; and optionally,
(e) isolating the metallic particles.
[0006] In one embodiment of the present invention, the metal-ammonia complex
is the
complex of ammonia with a transitional metal or a noble metal, e.g., Cu, Pd,
and Ag, formed by
reacting a solution comprising a metal salt with ammonium hydroxide or
ammonia. In certain
embodiments, the reducing agent is a saccharide, such as D-glucose. In certain
embodiments,
the stabilizing agent is a water-soluble resin (e.g., a natural occurring,
synthetic, or semi-
synthetic water-soluble resin) or gum arabic. The gum arabic may be removed
during the
isolation of the metallic particles through hydrolysis. The plurality of ultra-
fine metallic
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
particles may have at least one desirable feature, such as tight size
distribution, low degree of
agglomeration, high degree of crystallinity, and the ability to be fully re-
dispersed into a liquid
(e.g. an aqueous solution) to form a stable dispersion.
[0007] In another aspect, the present invention provides a substrate coated
with the
plurality of ultra-fine metallic particles obtained in accordance with the
method disclosed herein.
[0008] Other features and advantages of the present invention will become
apparent from
the following detailed description. It should be understood, however, that the
detailed
description and the specific examples, while indicating the preferred
embodiments of the
invention, are given by way of illustration only, since various changes and
modifications within
the spirit and scope of the invention will become apparent to those skilled in
the art from this
detailed description.
BRIEF DESCRIPTION OF THE FIGURES
[0009] Figure 1 depicts an experimental set-up used in the synthesis of ultra-
fine silver
particles.
[0010] Figure 2 shows the FE-SEM images of ultra-fine silver particles
produced using
the method of the present invention. (a) 198.7 g AgNO3 and flow rate at 8
ml/min; (b) 382 g
AgNO3 and flow rate at 8 ml/min; and (c) 382 g AgNO3 and flow rate at 30
ml/min. Images
were acquired using a FE-SEM at two magnifications (25,000 and 100,000).
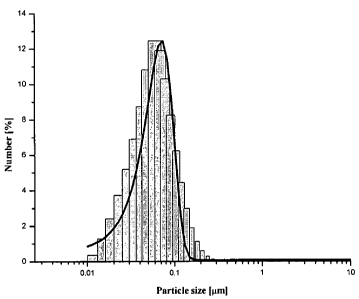
[0011] Figure 3 illustrates the particle size distribution (PSD) of silver
particles as
number (%) (a) and volume (%) (b), obtained from 382 g AgNO3 at a flow rate of
the metallic
precursor solution of 30 ml/min.
[0012] Figure 4 shows the X-ray diffraction patterns of silver particles shown
in Figure
2a.
DETAILED DESCRIPTION OF THE 1NVENTION
[0013] As used herein and in the appended claims, the singular forms "a,"
"an," and "the"
include plural references unless the content clearly dictates otherwise. Thus,
for example,
reference to "a particle" includes a plurality of such particles and
equivalents thereof known to
those skilled in the art, and reference to "the reducing agent" is a reference
to one or more
reducing agent and equivalents thereof known to those skilled in the art, and
so forth. All
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
publications, patent applications, patents, and other references mentioned
herein are incorporated
by reference in their entirety.
[0014] The present invention generally provides a simple and cost-effective
chemical-
based method for producing highly dispersed ultra-fine metallic powders. The
present invention
also provides ultra-fine metallic particles having at least one desirable
feature, such as tight size
distribution, low degree of agglomeration, high degree of crystallinity, and
the ability to re-
disperse fully into a liquid (e.g. an aqueous solution) to form stable
dispersions.
[0015] The present invention provides a method for producing metallic powders,
and
also the metallic powders produced thereby, that comprises the steps of (a)
providing a reducing
solution containing a reducing agent and a stabilizing agent; (b) providing an
aqueous solution
containing a metal-ammonia complex; (c) forming a reaction mixture containing
the reducing
solution and the aqueous solution; (d) maintaining the reaction mixture under
a suitable
conditions (e.g. pH and temperature) for a time sufficient to reduce the metal-
ammonia complex
to metallic particles; and optionally, (e) isolating the metallic particles.
[0016] The process of the present invention may be used to manufacture ultra-
fine
particles of various metals, such as Ag, Au, Co, Cr, Cu, Fe, Ir, Mo, Ni, Nb,
Os, Pd, Pt, Re, Rh,
Ru, Sn, Ta, Ti, V, and W, and alloys or composites containing these metals.
The metal-ammonia
complex may be mixed with a reducing composition or agent, which converts the
metal ions to
ultra-fine metal particles under various reaction conditions.
[0017] The metal-ammonia complex used in the process of the present invention
may be
the complex of ammonium with those transition metals and noble metals, such
as, Ag, Au, Co,
Cu, In, Ir, Ni, Nb, Os, Pd, Pt, Re, Rh, and Ru, and combinations thereof, that
are amenable to
being produced by reduction of a precursor compound with a reducing sugar. In
one
embodiment, the metal-ammonia complex may be obtained by reacting a solution
containing a
metal salt with ammonium hydroxide or ammonia. For example, 198.7 g AgNO3 may
be
dissolved in 234 ml deionized water. After the silver nitrate is completely
dissolved, 195 ml
ammonium hydroxide is added into the silver nitrate solution with stirring.
Deionized water
(291 ml) is then added to bring the overall volume of the silver ammonia
solution to 720 ml.
This solution should be sealed (to prevent ammonia evaporation) and protected
from light with
aluminum foil.
[0018] The term "reducing composition" or "reducing agent," as used herein and
in the
appended claims, generally includes any reducing substance, and a combination
thereof, which is
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
capable of reducing metal ions to metallic particles, such as, without
limitation, aldehydes,
aldose, hydrazine hydrate, and especially reducing saccharides (including
monosaccharides,
disaccharides, oligosaccharides, and polysaccarides). Examples of reducing
saccharides include,
but are not limited to, ascorbic acid, glyceraldehyde, erythrose, threose,
ribose, arabinose, xylose,
lyxose, allose, altrose, glucose, dextrose, mannose, gulose, idose, galactose,
talose, lactose,
maltose, isomaltose, cellobiose, and starch. The nature of the reducing
species and their
composition in the process of the present invention may be dependent upon the
particular metal
being produced.
[0019] The term "stabilizing composition" or "stabilizing agent," as used
herein and in
the appended claims, generally includes any stabilizing substance, such as,
without limitation,
water soluble resins (including, e.g., naturally occurring, synthetic, and
semi-synthetic water
soluble resins), gum arabic, polymers, polysaccharides, glycoproteins, nucleic
acids, various
salts of naphthalene sulphonic-formaldehyde co-polymers, and a combination
thereof, which is
capable of dispersing and stabilizing the newly formed ultra-fine metallic
particles in the reaction
mixture and thus preventing undesirable aggregation of these particles such
that the size of the
resulting metallic particles is less than about 10 m, preferably, less than
about I m, and more
preferably, less than about 100 nm. As used herein and in the appended claims,
the term "ultra-
fine particles" generally includes particles having diameters of less than
about 10 m, preferably,
less than about 1,000 nm, and more preferably, less than about 500 nm, and
even more
preferably, less than about 100 nm.
[0020] The stabilizing composition used in the process of the present
invention may be
commanded by the particular reaction. Examples of suitable stabilizing agents
include, without
limitation, gum arabic, cellulose derivatives (e.g., carboxymethyl cellulose,
carboxyethyl
cellulose, methyl cellulose, etc.) and modified products thereof, polyvinyl
alcohol and
derivatives thereof, polyvinyl pyrrolidone, polyacrylamide and copolymers
thereof, acrylic acid
copolymers, vinylmethyl ether-maleic anhydride copolymers, vinyl acetate-
maleic anhydride
copolymers, various salts of naphthalene sulphonic-formaldehyde co-polymers,
styrene-maleic
anhydride copolymers, calcined dextrin, acid-decomposed dextrin, acid-
decomposed etherified
dextrin, agarose, and salmon sperm DNA. In one embodiment of the present
invention, the
stabilizing agent may be gum arabic. In another embodiment of the present
invention, the
stabilizing agent may be a salt of naphthalene sulphonic-formaldehyde co-
polymer.
[0021] The stabilizing agent, such as gum arabic, may be removed after the
reaction. A
number of protocols for removing the stabilizing agent are known in the art,
such as, acid,
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
alkaline, and/or enzymatic hydrolysis. In one embodiment, gum arabic may be
removed from
the reaction mixture after the reaction through alkaline hydrolysis. For
example, the hydrolysis
may be performed for extended time at high temperature (e.g. between about 70
C and about
100 C, or between about 80 C and about 90 C, or between about 82 C and
about 88 C) and
high pH (e.g. pH 11.5). It is generally desirable to maintain the pH of the
mixture during the
hydrolysis at between about 9 and about 14, or between about 10 and about 12,
or between about
10.5 and about 11.5. The duration of the hydrolysis may be dictated by a
number of facts, such
as the amount of stabilizing agent (e.g. gum arabic) used. In various
embodiments, the
hydrolysis of the gum may generally be performed for about 0.2 to 10 hours, or
about 1 to 5
hours, or about 2 to 3 hours.
[0022] The resulting ultra-fine metal particles may be isolated following
standard
protocols known in the art, such as by precipitation, filtration, and
centrifugation. The particles
may further be washed, such as by using methanol or ethanol, and dried, such
as by air, N2, or
vacuum.
[0023] The ultra-fine metallic particles may also have at least one desirable
feature, such
as, tight size distribution, low degree of agglomeration, high degree of
crystallinity, ability to re-
disperse fully into a liquid (e.g. an aqueous solution) to form stable
dispersion, or a combination
thereof.
[0024] Unlike other metallic powders appearing in the art, the system of the
present
invention produces metallic powders that include ultra-fine metallic particles
that have a tight
size distribution. The breadth of the size distribution, as used herein,
generally refers to the
degree of variation in the diameter of the ultra-fine metallic particles in a
metallic composition.
The ultra-fine metallic particles are deemed to have a tight size distribution
when the diameters
of at least about 80%, preferably, at least about 85%, more preferably, at
least about 95%, and
most preferably 99-100% of the ultra-fine metallic particles of the present
invention are within
the range of N 15% N, where N is the average diameter of the ultra-fine
metallic particles. The
diameters of the ultra-fine metallic particles may be measured by a number of
techniques, such
as by electron microscopy with a scanning electron microscope (e.g. field
emission scanning
electron microscope).
[0025] The metallic powders produced in accordance with the present invention
may also
include ultra-fine metallic particles that have a low degree of agglomeration,
as illustrated in
Figure 3. The degree of agglomeration may be expressed using the index of
agglomeration 1aggi,
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
which is the ratio between the average particle size distribution of the
metallic particles
("PSD50%") and the average diameter of the particles. The average particle
size distribution
may be determined by any methods known in the art, including, but not limited
to, dynamic light
scattering (DLS), laser diffraction, and sedimentation methods, while the
average particle size
may be determined by averaging the diameter of the individual ultra-fine
metallic particles
obtained by, e.g., electron microscopy. An Iaggi value of 1.0 indicates a
complete lack of
agglomeration, while an increase in Iaggi value indicates an increase in the
degree of aggregation.
In one embodiment, the powders of ultra-fine metallic particles of the present
invention have an
Iagg, value of about 1.2 or less.
[00261 The metallic powders produced in accordance with the present invention
may also
include ultra-fine metallic particles that have a high degree of
crystallinity. The term "degree of
crystallinity," as used herein and in the appended claims, generally refers to
the ratio between the
size of the crystallites in the metallic powder and the diameter of the
metallic particles. The size
of the constituent crystallites may be deduced from XRD measurements using the
Sherrer's
equation, while the particle size may be determined by electron microscopy. A
larger ratio of the
size of the crystallites in comparison to the diameter of the metallic
particles indicates an
increased degree of crystallinity and a lower internal grain boundary surface.
In one
embodiment, the ultra-fine metallic particles have a high degree of
crystallinity if at least about
80%, preferably, at least about 85%, more preferably, at least about 90-95%,
and even more
preferably, about 100% of the ultra-fine metallic particles of the present
invention are highly
crystalline. The high degree of crystallinity is reflected by the visible
splitting of the peaks
corresponding to the (220), (311), and (222) reflections in the XRD spectrum
(see Figure 4).
[00271 The ultra-fine metallic particles produced in accordance with the
present
invention may form a free flowing dry powder in which the majority of the
individual particles
may not be strongly attached to each other and may be readily re-dispersed in
a liquid of choice.
[00281 In another embodiment of the present invention, the ultra-fine metallic
particles
forms stable dispersion when re-dispersed into a liquid, such as water, or an
aqueous solution,
where the majority of the individual particles may move substantially freely
in the liquid in
which they are dispersed. In one embodiment, the particle dispersion may be
stable for at least
one week. In another embodiment, the particle dispersion may be stable for
about 12 weeks.
[00291 The present invention further provides a substrate coated with a
plurality of ultra-
fine metallic particles, where the plurality of ultra-fine metallic particles
have at least one desired
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
feature, such as, tight size distribution, a low degree of agglomeration, a
high degree of
crystallinity, and oxidation resistance and are prepared by the methods
described herein.
Preferably the substrate is coated by immersion in the reaction mixture in
which the ultra-fine
metallic particles are produced. The term "substrate" as used herein includes,
without limitation,
metallic subjects (e.g., metallic particles, flakes, tubes, and sheets),
plastic materials, ceramic
subjects, fibers, films, glasses, polymers, organic materials (e.g. resins),
inorganic materials (e.g.,
amorphous carbon and carbon nanotubes), and any other object capable of being
coated with the
ultra-fine metallic particles produced in accordance with the present
invention. The ultra-fine
metallic particles may be the metallic particles of various metals,
preferably, Cu, Pd, and Ag.
EXAMPLES
[0030] The following examples illustrate the present invention, which are set
forth to aid
in the understanding of the invention, and should not be construed to limit in
any way the scope
of the invention as defined in the claims which follow thereafter.
[0031] The ultra-fine silver, palladium and copper particles were prepared by
reducing
the metallic ammonium complex with D-glucose in the presence of gum arabic.
The
experimental set-up for these experiments is illustrated in Figure 1.
[0032] Materials: Silver nitrate (AgNO3) was obtained from Ames Goldsmith
Corp.
(Glens Falls, NY). Gum arabic was obtained from Frutarom Incorporated (North
Bergen, NJ).
Ammonium hydroxide (NH40H, 28% in water) was purchased from Fischer Scientific
Co. (Fair
Lawn, NJ). Acetone, ethanol, and sodium hydroxide (NaOH) solution (10 N) were
supplied by
Alfa Aesar (Ward Hill, MA). D-glucose was purchased from Avocado Research
Chemicals Ltd.
(Shore Road, Heyshane, Lancs.). Cupric nitrate hydrate [Cu(N03)2 2'/z H20] was
obtained from
J.T. Baker Chemical Co. (Phillipsburg, NJ), and palladium nitrate solution
9.0% was obtained
from Umicore (South Plainfield, NJ).
EXAMPLE 1- PREPARATION OF ULTRA-FINE SILVER PARTICLES
(A) PREPARATION OF THE REDUCING SOLUTION
[0033] 3 liters deionised ("DI") water was heated to 55 C in an 8-1 stainless
steel beaker.
When the temperature reached 55 C, 62.5 g gum arabic was slowly added into
the water and
dissolved by stirring the solution with a stirring propeller at low speed for
55 minutes. 36 g of
D-glucose were then added to the solution. The mixture was stirred at 1700 rpm
for 5 minutes.
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
(B) PREPARATION OF SILVER AMMONIUM COMPLEX SOLUTION
[0034] AgNO3 (198.7 g) was dissolved in 234 ml DI water in a 2-1 glass beaker.
After
the silver nitrate was completely dissolved, 195 ml ammonium hydroxide was
added with
stirring, followed by the addition of 291 ml DI water to reach a final volume
of 720 ml.
(C) PREPARATION OF ULTRA-FINE SILVER PARTICLES
'[0035] METHOD A: The reduction process was conducted by pumping the silver
ammonium solution into the reducing solution at a flow rate of 8 mI/min using
a peristaltic
pump. When the addition of the silver complex solution was completed, the
temperature was
brought to 80 C. The entire process was conducted under continuous stirring
(1700 rpm).
[0036] METHOD B: The D-glucose was separately dissolved in 720 ml water. The
volume of the gum arabic solution was correspondingly reduced, and adjusted to
a pH between 9
and 13 with sodium hydroxide. The reduction process was conducted by
simultaneously
pumping the silver ammonium solution and the D-glucose solution into the gum
arabic solution
at 8 ml/min, while maintaining the pH by the addition of sodium hydroxide
solution as needed.
When the addition of the silver and D-glucose solutions was completed, the
temperature was
brought to 80 C. The entire process was conducted under continuous stirring
(1700 rpm).
(D) HYDROLYSIS OF GUM ARABIC
[0037] The excess of gum arabic was removed by increasing the pH of the
dispersion to
11.5 with 10.0 N sodium hydroxide at the temperature of about 85 C. The
dispersion was
maintained in the condition for 2.5 hours.
(D) PROCESSING THE SILVER POWDER
[0038] When the hydrolysis of the gum was complete, the dispersion was allowed
to cool
and the silver particles to settle. The supernatant was then discarded and the
silver particles were
washed with water through 3 successive decantations. During the last wash, 50%
ethanol (in DI
water) was added to the settled metallic deposit instead of DI water. Two more
washes with
pure alcohol were performed. The powder was then dried overnight on filter
paper at room
temperature.
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
EXAMPLE 2- PREPARATION OF ULTRA-FINE PALLADIUM PARTICLES
(A) PREPARATION OF THE REDUCING SOLUTION
[0039] A volume of 500 ml DI water was heated to 70 C in 2-1 glass beaker.
When the
temperature reached 70 C, 10 g gum arabic was slowly added into the water and
dissolved by
stirring the solution. 100 g of D-glucose were then added to the solution and
the mixture was
stirred at 1700 rpm for 5 minutes. The pH of solution was adjusted to 10.5
with 10.0 N NaOH.
(B) PREPARATION OF PALLADIUM AMMONIUM COMPLEX SOLUTION
[0040] Ammonium hydroxide (80 ml) was added quickly with stirring to 50 ml
Pd(NO3)2
solution (9.0%) in a 200 ml glass beaker, followed by the addition of 50 ml DI
water (final
volume: 180 ml).
(C) PREPARATION OF ULTRA-FINE PALLADIUM PARTICLES
[0041] The reducing reaction was conducted by pumping the palladium ammonium
solution into the reducing solution at a flow rate of 5 ml/min using a
peristaltic pump. When the
addition of the palladium complex solution was complete, the temperature was
brought to 80 C.
The process was conducted under continuous stirring (1700 rpm).
[0042] The hydrolysis of gum arabic and the processing of palladium powder
were
carried out in a similar manner as in Example 1(steps D and E).
EXAMPLE 3 - PREPARATION OF ULTRA-FINE COPPER PARTICLES
(A) PREPARATION OF THE REDUCING SOLUTION
[0043] A volume of 500 ml DI water was heated to 70 C in 2-I glass beaker.
When the
temperature reached 70 C, 25 g gum Arabic was slowly added into the water and
dissolved by
stirring the solution with a stirring propeller at 1700 rpm for 55 minutes.
100 g of D-glucose
were then added to the solution and the mixture was stirred at 1700 rpm for 5
minutes. The pH
of solution was adjusted at 10.5 with 10.0 N NaOH.
(B) PREPARATION OF COPPER AMMONIUM COMPLEX SOLUTION
[0044] 18.2 g cupric nitrate (Cu(NO3)2 2'/2 H2O) were dissolved in 50 ml DI
water in a
200 mI glass beaker. After the cupric nitrate was completely dissolved, 100 ml
ammonium
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
hydroxide was added quickly with stirring, followed by the addition of 50 ml
DI water (overall
volume: 200 ml).
(C) PREPARATION OF ULTRA-FINE COPPER PARTICLES
[0045] The reduction process was conducted by pumping the cupric ammonium
solution
into the reducing solution at a flow rate of 5 ml/min using a peristaltic
pump. When the addition
of the cupric complex solution was complete, the temperature was brought to 80
C. The
process was conducted with continuous stirring (1700 rpm). The remaining steps
are similar to
those of Example 1.
[0046] Discussed below are results obtained by the inventors in connection
with the
experiments of Examples 1-3:
[0047] The size of the silver particles obtained did not undergo a substantial
change
when the process was scaled up by a factor of two or when the flow rate of the
silver ammonium
complex solution was raised to 30 ml/min from 8 mI/min, suggesting a minor
impact of both
parameters on the size of the particles formed. For all experiments the
processing yield was
>97%.
[0048] The experimental conditions and results of Example I (Method A) are
summarized in Table I. Method B gave somewhat smaller particles; at pH 12
particles with an
average diameter of 40 nm were obtained.
Table I
Reducing solution Silver ammonium solution Average
Bach size Flow rate
LXp' Ag (g) Water Gum Glucose Water AgNO3 NH4OH (ml/min) size (L) arabic (g)
(g) (ml) (g) (ml) (nm)
1 125 3 62.5 36 525 198.7 195 8 -70
2 245 4 120 69 775 382 390 8 -65
3 245 4 120 69 775 382 390 30 -65
[0049] While the foregoing invention has been described in some detail for
purposes of
clarity and understanding, it will be appreciated by one skilled in the art,
from a reading of the
CA 02585644 2007-04-26
WO 2006/050248 PCT/US2005/039237
disclosure, that various changes in form and detail can be made without
departing from the true
scope of the invention in the appended claims.