Note: Descriptions are shown in the official language in which they were submitted.
CA 02585722 2009-11-18
METHOD FOR REPROCESSING A MEDICAL DEVICE WITH A PLURALITY OF
INTERNAL PASSAGEWAYS
SPECIFICATION
This application is a division of Canadian Patent Application No. 2,510,491
for A Method For Determining The Existence Of Obstructions In The Passageways
Of A Medical Instrument filed January 7, 2004.
BACKGROUND OF THE INVENTION
1. FIELD OF INVENTION
This invention relates generally to a method for the reprocessing of a
contaminated device having internal passageways before such a device is reused
in
a clean environment. The term "reprocessing," as used herein constitutes the
washing, disinfecting, sterilizing and/or pasteurizing of such a device. The
term
"device" as used herein constitutes any devices having internal passageways
that
require such reprocessing, including, but not limited to, medical instruments
and
medical devices. The terms "medical instrument" and "medical device" are
understood to constitute devices having one passageway or a plurality of
passageways, including, but not limited to endoscopes, colonoscopes, and other
flexible and rigid medical instruments.
Some automated systems for reprocessing devices having internal
passageways for reuse are generally available and are commonly relied upon.
For
example, systems for reprocessing medical instruments having passageways are
used by hospitals to safeguard patients and hospitals employees from exposure
to
infection and cross-contamination. Such prior art reprocessing units are
manufactured by several different companies including, Custom Ultrasonics,
Inc., of
Ivyland, Pennsylvania, the assignee of the present invention and application.
For-
example there are reprocessing units in the prior art adapted for cleaning,
disinfecting and sterilizing flexible scopes, e.g., upper and lower
gastrointestinal
scopes, colonoscopes and duodescopes.
Prior art reprocessing systems, suitable in particular for reprocessing
medical
instruments, operate in accordance with a predetermined protocol of
reprocessing
steps. The protocol is based upon the specific cleaning requirements of the
particular instruments being cleaned. The reprocessing steps are precisely
timed
and sequenced in order to assure optimal results, based upon the correct
combination of water temperature, detergent and chemical agents. Thus,
parameters such as wash and rinse cycle time, chemical immersion cycle time
and
water temperature and pressure were preset by the reprocessing unit
manufacturer
-1-
CA 02585722 2007-04-23
and could not be altered by an end user of the system. U.S. Patent No.
5,761,069,
issued to Weber, et. al. teaches a system for cleaning medical instruments
having
a database of protocols corresponding to differing medical instruments for
permitting
a user to load and execute the protocol corresponding to the instrument being
reprocessed.
An exemplary protocol for cleaning a medical instrument could include the
following reprocessing steps, after the instrument has been placed in the
cleaning
basin of the reprocessing unit: (1) wash the internal and external surfaces of
the
instrument with a measured detergent water mixture for a preset period of
time; (2)
activate ultrasonic crystals while washing; (3) drain the detergent-water
mixture after
the wash cycle is completed; (4) after draining, rinse the internal and
external
surfaces of the instrument with water at a preset temperature for a preset
period of
time; (5) introduce and circulate disinfectant over and through the instrument
for a
preset period of time; (6) drain the disinfectant from the wash basin; and (7)
after
draining of the disinfectant is complete, rinse the instrument with water; and
(8) re-
rinse the instrument with water.
Prior art reprocessing units adapted, in particular, for reprocessing medical
equipment, typically comprise a variety of mechanical components, e.g., pumps,
tubes, solenoid valves, ultrasonic transducers, heaters and probes that
perform the
various reprocessing steps. The pumps used in these units must be very precise
and reliable over extended periods of time. Thus, pumps that are suitable for
these
units can be quite expensive.
In many cases it is necessary to reprocess devices having passageways of
differing diameters. The differing diameters can occur in a single device
having
passageways of differing diameters, or in multiple devices, each having a
single
differing diameter. The presence of differing diameter passageways creates a
need
for fluid flows of corresponding differing pressures, 'because more narrow
passageways require a higher pressure to force fluid therethrough. Prior art
reprocessing units suitable for reprocessing devices having passageways of
differing diameters included a plurality of pumps and associated tubing
systems,
wherein each pump provided one of the differing pressures required to
reprocess
the differing passageways of the devices.
-2-
I M ell
CA 02585722 2007-04-23
Furthermore, some devices can have extremely narrow passageways,
requiring dedicated high-pressure pumps that are capable of providing
extremely
high pressures. Pumps for such extremely narrow, high-pressure passageways
have very low flow rates. Flow rates that are this low are difficult to
monitor. For
example, the flow rates of fluids through the passageways of some devices can
be
on the order of a drop a minute. Passageways this narrow can be found, for
example, in flexible medical instruments, such as endoscopes.
Known reprocessing units are typically equipped with a pressure sensor for
measuring the overall flow of fluid through the pump for the purpose of
detecting
obstructions in the passageways of the devices. However, is possible for an
obstruction preventing flow of in one of the passageways to go undetected by
the
pressure sensor since the flow can continue through the remaining passageways
and only the overall pressure of the liquid is determined.
Several governmental and independent agencies have issued guidelines for
reprocessing particular types of medical instruments. For example, such
guidelines
often require that certain types of medical instruments be washed and
sterilized
using a chemical disinfectant, while other types of instruments need only be
washed. The design of reprocessing units and the reprocessing steps they
perform
must conform to such guidelines. Additionally, guidelines have been created to
reliably prevent instruments from being reused if an obstruction occurs in a
single
passageway of a plurality of passageways during reprocessing. Prior art
reprocessing units are not reliably able to meet these guidelines.
SUMMARY OF THE INVENTION
A method for the reprocessing of a device having internal passageways by
applying a fluid at a plurality of pressures to the internal passageways of
the device
to permit reuse of the device in a clean environment or patient safe
environment
includes applying a fluid having a single input pressure to a pressure
differentiation
device having first and second pressure control fittings for providing first
and second
differing pressure outputs in accordance with the single input pressure and
transmitting the fluid at the first and second differing pressures from the
pressure
differentiation device to the internal passageways. The internal passageways
are
reprocessed with the transmitted fluid at the first and second differing
pressures,
-3-
I , I . Ir,
CA 02585722 2007-04-23
whereby the internal passageways are reprocessed at differing pressures in
accordance with the single input pressure. The first and second control
fittings can
be pressure fittings having respective first and second openings. The first
and
second control openings can have differing diameters. The pressure
differentiation
device can be a T-manifold.
A method for the reprocessing of a device having a plurality of internal
passageways by applying a plurality of fluid channel flows to the internal
passageways of the device to permit reuse of the device in a clean environment
includes applying a pressurized fluid flow to the input of a manifold having a
plurality
of manifold outputs for forming a plurality of manifold output channel flows
and
transmitting the output channel flows of the plurality of output channel flows
through
respective flowmeters for measuring an individual flow rate for each of the
output
channel flows. Transmitting the measured output-channel flows to the plurality
of
internal passageways and reprocessing the internal passageways using the
output
' channel flows are also included. An obstruction in an internal passageway of
the
plurality of internal passageways is determined in accordance with a measured
individual flow rate. The reprocessing of the device is aborted in accordance
with
the determining of the obstruction. An indication of which internal passageway
of
the plurality of internal passageways is obstructed is provided in accordance
with
a measured individual flow rate.
DESCRIPTION OF THE DRAWINGS
The features of this invention will become readily appreciated as the same
becomes better understood by reference to the following detailed description
when
considered in connection with the accompanying drawings wherein:
Fig. 1 is a top plan view of a prior art reprocessing unit wherein the cover
of
the reprocessing unit is disposed in an opened position to permit a view of a
reprocessing basin containing devices to be reprocessed.
Fig. 2 is an elevational view of a reprocessing unit suitable for use with the
system and method of the present invention.
Fig. 3 shows a top view of the reprocessing basin of the reprocessing unit of
Fig. 2 including a device to be reprocessed.
Figs. 4A-C show top, front and plan views of the pressure differentiation
-4-
CA 02585722 2009-06-04
device of the reprocessing unit of Fig. 2. device of the reprocessing unit of
Fig. 2.
Figs. 5A-D are front and side views of the pressure control devices of the
pressure differentiation manifold of Figs. 4A-C.
Figs. 6A-C show top, front and plan views of the pressure distribution
manifold
of the present invention.
Fig. 7 shows a schematic block diagram illustrating the process flow of the
operations performed by the reprocessing unit of Fig. 2.
Figs. 8A-B show top and front views of a flowmeter of the present invention.
Figs. 9A-C show top, front and plan views of a pressure sensor of the present
invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now to the drawings, wherein like reference numerals refer to like
parts, there are shown representations of reprocessing systems and methods
suitable for using conventional reprocessing protocols to reprocess devices
having
internal passageways, such as medical instruments. An example of such a
reprocessing protocol is disclosed in U.S. Patent No. 5,761,069 , issued to
Weber, et
al.
Fig. 1 shows a top view of a prior art reprocessing unit 10, wherein a cover
(not shown) is disposed in an open position. The reprocessing unit 10 includes
a
reprocessing basin 12, the instrument carrier 14, and a chemical disinfectant
reservoir 16. The instrument carrier 14 is shown seated within the
reprocessing
basin 12. The instrument carrier 14 can be generally rectangular in shape and
comprises a mesh-like bottom 18 which is arranged to hold the surgical
instruments
15 during reprocessing, wherein the surgical instruments 15 each include a
single
passageway therethrough requiring reprocessing. The reprocessing basin 12 is
also
provided with a plurality of spray nozzles 26 for use during the rinse cycle.
The instrument carrier 14 includes a manifold assembly 20 having a plurality
of ports 20a-f, each of which is shown applied to an internal passageway of a
respective surgical instrument 15. In order to reprocess the surgical
instruments 15
having a single passageway within the reprocessing unit 10, the surgical
instruments
15 are disposed on the instrument carrier 14 for coupling to the ports 20a-f.
Since
the surgical instruments 15 have a single passageway, only a single
- 5 -
A
CA 02585722 2007-04-23
one of the ports 20a-f is required for each surgical instrument 15. The
manifold
assembly 20 is connected to a port 22 by means of a tubing segment 24, which
conducts fluid flow from the port 22 to the manifold assembly 20 for
distribution by
way of the ports 20a-f.
The fluid flow of the port 22 is driven by an oscillating pump (not shown).
The
oscillating pump operates to draw fluid, e.g., wash water, rinse water or
chemical
disinfectant, from the reprocessing basin 12, circulate that fluid through the
ports
20a-f and the manifold assembly 20, and through the respective passageways of
the surgical instruments 15 disposed on the instrument carrier 14, to effect
the
decontamination process during the wash, rinse and chemical immersion phases
of the reprocessing protocol.
In this manner, the pressure delivered to each of the passageways of the
surgical instruments 15 can be substantially equal in the reprocessing unit
10.
Reprocessing unit 10 is thus suitable for reprocessing a plurality of surgical
instruments 15 requiring such a single pressure to be applied to all of the
passageways of the surgical instruments 15. However, many surgical instruments
are provided with passageways of differing diameters. Such surgical
instruments
require differing pressures, corresponding to the differing diameters, for
providing
the required circulation of wash water, rinse water and chemical disinfectants
through the passageways.
Referring now to Figs. 2, 3, there is shown a reprocessing unit 80 suitable
for
use with the system and method of the present invention, and a view of a
reprocessing basin 12 within the reprocessing unit 80. The reprocessing basin
12
holds a device 96 having internal passageways 98a-e for reprocessing of the
device
96 by the reprocessing unit 80. In a preferred embodiment of the invention,
the
device 96 being reprocessed by the reprocessing unit 80 can be a medical
instrument 96. In particular, the system and method of the invention are well
suited
for application to medical instruments including flexible scopes such as
endoscopes
that are used for upper and lower gastrointestinal studies.
The reprocessing unit 80 includes a keyboard 40, a monitor 28, a printer 32,
and an associated personal computer (not shown) for permitting a user of the
reprocessing unit 80 to communicate with and control the reprocessing unit 80.
The
-6-
CA 02585722 2007-04-23
reservoir 16 of the reprocessing unit 80 includes the sensors 34, 36, 38 for
controlling devices such as a heater, a pump and a vacuum device (not shown)
in
order to protect against failure conditions such as overflow conditions in the
reservoir 16. A removable door 42 within the reprocessing basin 12 covers
additional sensors (not shown) for providing further operational capability
and safety
protection during the operation of the reprocessing unit 80. The door stops 30
are
provided to stop the motion of the rotatable doors 31 covering the reservoir
16 and
the reprocessing basin 12 when they are opened.
In the preferred embodiment, the reprocessing basin 12 can hold more than
one device 96 upon a mesh for reprocessing of the internal passageways 98a-e
thereof according to conventional reprocessing protocols. The reprocessing
unit 80
is adapted to provide fluid flows of differing pressures to the device 96 or
devices
96 being reprocessed when the internal passageways 98a-e have differing
diameters. The reprocessing unit 80 is adapted to perform the multi-pressure
reprocessing operations using a single pump (not shown), and to provide an
indication of an obstruction in any of the internal passageways 98a-e of the
device
or devices 96 as described in more detail below. The single pump of the
reprocessing unit 80 can be a diaphragm pump, an oscillating pump, or any
other
type of pump known to those skilled in the art.
The reprocessing basin 12 includes the supply ports 123a-I that can be
selectively used to applyfluids at different fluid flow rates to the medical
instruments
96 for reprocessing of the medical instruments 96. For example, the supply
port
123j can be capped and reserved for use when needed. The supply port 123a can
be used to blow off a fluid flow which is unusable due to difficulty in
regulating and
measuring their flow rates, as described in more detail below. In this
example, at
least the supply ports 123a-I that are not capped or blown off can be vented
into the
reprocessing basin 12 or coupled to the internal passageways 98a-e of a
medical
instrument 96 as needed.
For example, an internal biopsy passageway 98a of the medical instrument
96 can be coupled to the supply port 123b byway of the tubing segment 132b,
and
an internal water channel passageway 98b of the medical instrument 96 can be
coupled to the supply port 123c by way of the tubing segment 132c. The
internal
-7-
1 I uVIY
CA 02585722 2007-04-23
passageway 98c can be coupled to the supply port 123d by way of the tubing
segment 132d, and the internal suction passageway 98d can be coupled to the
supply port 123e by way of the tubing segment 132e. The internal elevator
water
channel passageway 98e can be coupled to the supply port 1231 by way of the
tubing segment 1321.
The disk filters 94 and theirtubing extensions can be disposed in line with
the
selected passageways 98a-e for preventing debris from reaching the medical
instrument 96. For example, the disk filters 94 can be provided in the tubing
segments 132c,d,e. The device for coupling the selected tubing segments 132a-I
to the tubing extensions of the disc filters as shown can be the well known
lure lock
type of coupling: Typical diameters for some of the passageways 98a-e can be
.508 millimeters to 4.8 millimeters.
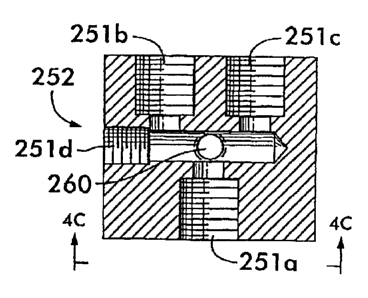
Referring now to Figs. 4A-C, there is shown a pressure differentiation device
252 for providing fluid flows of differing pressures from the output of a
single
conventional pump that provides a single pump output pressure. It is the
different
output pressures at the output of the pressure differentiation device 252 that
are
applied by way of the selected supply ports 123a-I to the internal passageways
98a-
e of the medical instrument 96 for reprocessing the medical instrument 96 or
any
other device 96 having such passageways 98a-e. The single pump applied to the
pressure differentiation device 252 can be a conventional diaphragm type pump,
an
oscillating pump, or any other type of pump known to those skilled in the art.
The
pressure differentiation device 252 can be a conventional T-manifold that is
known
to those skilled in the art.
The single pump output pressure is applied to the pressure differentiation
device 252 at an input port 251 a for application to the two output ports 251
b,c of the
pressure differentiation device 252. The output ports 251 b,cthreadably
receive and
secure different pressure control devices which can have openings of different
diameters, as described in more detail below. The pressure control devices
secured
in the output ports 251 b,c permit the pressure differentiation device 252 to
provide
two different pressures for the internal passageways 98a-e of the medical
instruments 96. In the preferred embodiment the outport port 251 b can be a
high
pressure output port and the output port 251c can be a low pressure output
port.
-8-
I I I I I M VV,
rrr
CA 02585722 2007-04-23
In a typical embodiment of the invention, the higher pressure of the high
pressure output port 251 b of the pressure differentiation device 252 can be
approximately 25 to 50 pounds per square inch. The lower pressure of the low
pressure output port 251 c can be approximately 2 to 20 pounds per square
inch.
The pressures at the output ports 251 b,c can fluctuate within these ranges
depending on factors such as the number of medical instruments 96 coupled to
the
reprocessing unit 80. It will be understood by those skilled in the art that a
pressure
differentiation device 252 having additional output ports with different
pressure
control devices can be used for reprocessing systems 80 requiring more than
two
differing pressures.
Referring now to Figs. 5A-D, there are shown the pressure control devices
257, 259 of the pressure differentiation device 252 for providing the two
different
pressures to the internal passageways 98a-e of the medical instrument 96. The
pressure control devices 257, 259 can be conventional pressure control orifice
fittings 257, 259 that are threadably received and secured in the output ports
251 b,c of the pressure differentiation device 252. The two different
pressures are
provided at the output ports 251 b,c when a single pressure is applied to the
input
port 251a of the pressure differentiation device 252 because of the different
diameters of the openings within the pressure control orifice fittings 257,
259. The
pressure control orifice fitting 257 is a high pressure orifice fitting and
the pressure
control orifice fitting 259 is a low pressure orifice fitting.
In the preferred embodiment of the invention, the pressure differentiation
device 252 can be formed with an entrance 260 for permitting an FDA approved
liquid chemical sterilant as well as alcohol to be injected into the fluid
stream
passing through the device 252 for transmission through the selected supply
ports
123a-I of the reprocessing basin 12 to the medical instruments 96. A
disinfectant
injection bulkhead communicating with the entrance 260 can be located on the
exterior of the reprocessing unit 80 for convenience. Additionally, a filter
(not
shown) can be disposed in a conduit from the pump to the input port 251a of
the
device 252 for filtering fluid in transit to the internal passageways 98a-e.
The filter
can be, for example, a one-hundredth micron filter.
Referring now to Figs. 6A-C, there are shown representations. of the
-9-
I r 1 1 I. III
CA 02585722 2007-04-23
pressure distribution manifold 250 of the reprocessing unit 80, including the
manifold input ports 253, 255, and the manifold output ports 121a-I. The
pressure
distribution manifold 250 can be a conventional air manifold understood by
those
skilled in the art. It is adapted to receive the fluid flows of the two
different
pressures from the output ports 251 b,c of the pressure differentiation device
252 by
way of the manifold input ports 253, 255. The fluid flows from the pressure
distribution manifold 250 are applied by way of the manifold output ports 121
a-I
directly to the corresponding supply ports 123a-I of the reprocessing unit
basin 12.
Therefrom, they are selectively applied to the devices 96 such as the medical
instruments 96. In the preferred embodiment, the manifold output ports 121a j
are
low pressure ports and the manifold output ports 121 k,l are high pressure
ports.
A high pressure fluid flow is received at the high pressure manifold input
port
253 of the pressure distribution manifold 250 from the orifice port 251 b of
the
pressure differentiation device 252. A short longitudinal bore hole 140,
opening at
the high pressure manifold input port 253, is provided at one end of the
pressure
distribution manifold 250. The pressure distribution manifold 250 is bored
transversely from each of the high pressure manifold output ports 121 k,l to
the
longitudinal high pressure bore hole 140 in order to permit the high pressure
output
ports 121k,l to communicate with the high pressure bore hole 140. Thus, a high
pressure fluid flow applied to the input port 253 of the pressure distribution
manifold
250 is distributed to the high pressure, or narrower inner diameter,
passageways of
the medical instruments 96 by way of the high pressure bore hole140 and the
manifold output ports 121 k,l.
A low pressure fluid flow is received at the low pressure input port 255 of
the
pressure distribution manifold 250 from the output port 251-c of the pressure
differentiation device 252. A long longitudinal bore hole 142, opening at the
low
pressure manifold input port 255, is provided within the pressure distribution
manifold 250. Substantially as described with respect to the high pressure
output
ports 121 k,l, transverse bore holes extending from the low pressure output
ports
121 a -j to the longitudinal low pressure bore hole 142 are provided. Thus,
the low
pressure manifold output ports 121 a -j communicate with the low pressure bore
hole
142. In this manner, a low pressure fluid flow applied to the low pressure
input port
-10-
I1 11 I M rl~ii'
1 II M
CA 02585722 2007-04-23
255 of the pressure distribution manifold 250 is distributed to the low
pressure
passageways of the medical instruments 96 by way of the low pressure bore hole
142 and the manifold output ports 121 a+
Those skilled in the art will understand that possible turbulence at the
distal
end of the pressure distribution manifold 250, in the region of the manifold
output
port 121a can make the flow rates difficult to measure and/or difficult to
control.
Therefore, in the preferred embodiment of the invention, the fluid flows
provided by
way of the supply port 123a can be blown off into the reprocessing basin 12,
rather
than applied to a medical instrument 96.
The pressure measurement openings 144 - on the side of the pressure
distribution manifold 250 individually communicate with the longitudinal bore
holes
140, 142. The presence of the pressure measurement openings 144 on the
pressure distribution manifold 250 permits measurement of the pressures within
the
bore holes 140, 142, as described in more detail below.
15, Referring now to Fig. 7, there is shown a block diagram representation of
a
process flow 95 for performing a reprocessing protocol within the reprocessing
unit
80 suitable for reprocessing devices such as the medical instruments 96.
During
a fill step of the process flow 95, a solenoid-type water valve 230 is placed
in an
open position to enable water to flow from an outside hot/cold water-source
232
through a water line 234, into the reprocessing basin 12 to immerse the
medical
instrument 96. The reprocessing basin 12 is provided with a drain 44 (shown in
Fig.
2) located in the bottom of the reprocessing basin 12. The drain 44 is
connected
to a drain line 212. During the fill step, as wash water flows into the
reprocessing
basin 12 it begins to drain through the drain line 212. A drain valve 164,
provided
below the drain line 212 is normally in a closed state to prevent the draining
of the
water out of the system. This action enables the filling of the reprocessing
basin 12.
A flow probe 220 is located adjacent the drain line 212 and is operative to
detect the presence of liquid as wash water begins to fill the drain line 212
during
filling of the reprocessing basin 12. Once the probe 220 detects the presence
of
moisture, the probe 220 sends a signal indicative thereof to a system
controller
which provides an indication to the user that the reprocessing basin 12 is
filling with
water. Additionally, an operational float (not shown) is located within the
-11-
I 1 1 w 11.
CA 02585722 2007-04-23
reprocessing basin 12. During filling, the operational float is buoyed
upwardly and
eventually reaches a predetermined height corresponding to a particular volume
of
wash water being present in the reprocessing basin 12. When the operational
float
reaches this predetermined level, the reprocessing unit 80 indicates to the
user that
the reprocessing basin 12 has been filled and that the washing step can begin.
Thereafter, the water valve 230 is closed so that no additional wash water
enters the
reprocessing basin 12.
As wash water fills into the reprocessing basin 12 over the immersed medical
instruments 96, a solenoid-type detergent valve 262 and a detergent pump 266
operate to withdraw a predetermined amount, e.g., three ounces, of detergent
254
from a detergent container 258 located adjacent the reprocessing unit 80 and
inject
the predetermined amount of detergent into the reprocessing basin 12 through a
detergent line 264. The detergent 254 may be of any suitable composition. One
particularly effective type of detergent is sold under the trademark TERGAL
800 by
Custom Ultrasonics, Inc.
During the wash step, a pump 246, such as a diaphragm pump, is activated
to draw the water/detergent mixture contained in the reprocessing basin 12
through
an intake valve 240 and to circulate the mixture through the circular
reprocessing
basin 12, the output ports 121 a-1 of the pressure distribution manifold 250,
the
tubing segments 132a-I, and through the internal passageways 98a-e of the
immersed medical instrument 96. Any unused output ports 121 a-I can be blown
off
into the basin 12. The pump 246 is a single output pressure pump. In this
manner
fluid is recirculated through the immersed medical instrument 96 for a
predetermined period of time in order to reprocess the internal passageways of
the
internal medical instrument 96 in accordance with a predetermined reprocessing
protocol.
Referring now to Figs. 8A-B, there is shown a flowmeter 256 for selectively
coupling to the manifold output ports 121a-1 and individually measuring the
flow
rates of the fluids within the manifold output ports 121 a-I of the
reprocessing unit 80
coupled thereto. The flowmeter 256 can be any conventional flow sensor
suitable
for measuring the flow rate through the ports 121 a-l, and thereby through the
tubing
segments 132a-I. For example, the flowmeter 256 can be an in line straight
through
-12-
CA 02585722 2007-04-23
flow tube sensorthat uses ultrasonic sensing technologyto measure the rate of
flow
of a fluid passing therethrough, such as the M-1500 Series provided by Malema
Flow Sensors. The flowmeter 256 can be omitted from any unselected output
ports
121 a-I not supplying fluid to any internal passageways, for example the
output ports
121a which is blown off into the reprocessing basin 12.
An ultrasonic sensing flowmeter 256 is preferred because it is non intrusive,
thereby permitting the fluid flow to the internal passageways 98a-e of the
medical
instruments 96 to be measured without interference by the flowmeter 256.
Ultrasonic sensing flowmeters 256 of this type are believed to be accurate
from one-
half cubic centimeter per minute to infinity for a multiple number of outputs.
The flowmeter 256 provides a flow rate signal according to the measured flow
rate, for example by tripping a switch within the flowmeter 256 when the flow
rate
falls below a predetermined value.
In another embodiment of the invention, the flowmeters 256 can be of the
well know piston type, wherein the force of the fluid flow through the
flowmeter 256
raises and suspends a piston therein, until the flow rate falls below a
predetermined
value. When the flow rate falls below the predetermined value, the piston
falls and
a switch within the flowmeter 256 is tripped. The tripping of the switch
within the
flowmeter 256 indicates that the predetermined flow rate through the flowmeter
256
has not been maintained. It is believed that a flowmeter 256 of this type is
not as
accurate the ultrasonic type since it can interfere with the fluid flow being
measured.
In one preferred embodiment of the invention, the minimum flow rate through
the high pressure ports 121 k,I can be approximately one cubic centimeter per
minute. The minimum flow rate through the two lower pressure ports 121 a,b at
the
distal end of the pressure distribution manifold 250 can be approximately
fifty cubic
centimeters per minute. The minimum flow rate through the remaining low
pressure
ports 121 c -j can be .05 gallons per minute.
Thus, the flowmeters 256 disposed in line with the internal passageways 98a-
e provide an indication to the user of the reprocessing system 80 when the
flow
through any of the passageways 98a-e of the surgical instruments 96 coupled to
the
reprocessing unit 80 is obstructed. When any of the internal passageways 98a-e
is determined to be obstructed in this manner, the reprocessing operation set
forth
-13-
I , Y 1 Y ,IY
CA 02585722 2007-04-23
in the process flow 95 is aborted, and the abort condition is communicated to
the
user of the reprocessing unit 80. This feature prevents the inadvertent reuse
of any
device 96 that has not been completely reprocessed due to an obstruction in
any
of the internal passageways 98a-e being reprocessed. Without such a feature
the
operator can be left with a false sense of security regarding the success of
the
reprocessing operation.
In the preferred embodiment of the invention, individual indicator lights (not
shown) corresponding to each flowmeter 256 coupled to the pressure
distribution
manifold 250 are mounted on the exterior of the reprocessing unit 80. The
indicator
lights permit an easy visual determination of which internal passageway 98a-e
is
obstructed when the reprocessing operation is aborted. Additionally, in one
preferred embodiment of the invention, a lag time of approximately ten seconds
can
be provided between the detection of an obstruction by a flowmeter 256 and the
abort of the reprocessing operation to allow for the breaking up of an
obstruction
due to back pressure provided by the pump.
Referring now to Figs. 9A-C, there are shown representations of the pressure
sensing switch 320 of the reprocessing unit 80. The pressure sensing switch
320
is adapted to measure the pressure of the longitudinal bore holes 140, 142
within
the pressure distribution manifold 250, and to provide an electrical pressure
signal
according to the measured pressure of the bore holes 140, 142.
In an alternate embodiment of the invention (not shown) a flowmeter 256
coupled to a manifold output port 121 a-I of the pressure distribution
manifold 250
can be omitted. In such an embodiment, the pressure sensing switch 230 is
mounted in a pressure measurement opening 144 communicating with a
longitudinal bore 140,142 of the pressure distribution manifold 250. For
example,
the flowmeters 256 can be removed from the manifold output ports 121 k,l, and
the
high pressure flow rate can be measured by a pressure sensing switch 320
mounted in the pressure measurement opening 144 disposed in communication
with the longitudinal bore hole 140.
Thus, the pressure of the manifold output ports 121 k,l is monitored using the
pressure sensing switch 320 rather than measuring the fluid flow rate using a
flowmeter 256_ In this alternate embodiment, an obstruction within a high
pressure
-14-
4 I . iII
CA 02585722 2007-04-23
passageway of the medical instrument 96 is detected by sensing a change in
pressure rather than a change in flow rate. Thus, the reprocessing of the
instrument
96 is aborted according to the pressure measured by the pressure sensing
switch
320 rather than a direct measurement of flow rate. In one embodiment of the
invention the pressure sensing switch 320 can be adapted to provide an
electrical
pressure signal when the measured pressure is at a level in the range of 1.5
to 15
psi.
In another alternate embodiment (not shown) of the reprocessing unit 80 an
ultrasonic flow sensor such as the flowmeter 256 can be mounted on the
pressure
distribution manifold 250, for example, at the input end of the pressure
distribution
manifold 250. This type of ultrasonic measurement of flow rate is extremely
sensitive, allowing the detection of changes in flow rate as small as a few
drops per
second. The reprocessing operations of the process flow 95 are aborted when
the
flow detected by such an ultrasonic measurement device mounted on the pressure
distribution manifold 250 in this manner is below the predetermined level.
Once the water/detergent mixture has passed through the internal
passageways 98a-e of the immersed medical instrument 96., it flows back into
the
reprocessing basin 12 where it is again recirculated by the pump 246 for a
predetermined minimum period of time based upon guidelines provided by the
detergent manufacturer, e.g., one-hundred eighty seconds. During the wash
step,
the ultrasonic crystals 282 located below the reprocessing basin are
activated.
When activated, the ultrasonic crystals 282 generate ultrasonic vibrations
that act
in combination with the detergent-water mixture to cause a cleansing action
that
breaks down, loosens and removes contaminants from the exterior and interior
surfaces of the flexible medical instrument 96 to provide enhanced cleaning.
Once the predetermined time period forthe wash step has elapsed, the drain
step begins. During the drain step, the drain valve 164 is opened and the
drain
pump 216 is activated. While the pump 246 continues to pump the
water/detergent
mixture through the medical instrument 96, the mixture begins to drain out of
the
reprocessing basin 12 by means of the drain pump 216 which pumps the
waterldetergent mixture down the drain line 212 and into a T-assembly 217. The
mixture travels through drain valve 164, through a standpipe 165 and into.a
sewer
-15-
1 1 1 U"N,
11, CA 02585722 2007-04-23
drain 167. Once the flow probe 220 detects the absence of moisture in the
drain
line 212, the drain pump 216 is shut off and the drain valve 164 is returned
to its
closed position.
After the drain pump 216 is shut off, an air pump 224 is activated and a
solenoid-type air valve 226 is opened. By use of the air pump 224 forced air
is
directed through the pump 246, the manifold assembly 250, the tubing segments
132a-e, and through the internal channels of the medical instrument 96. The
forced
air acts to purge and clear away any residual water/detergent mixture
remaining in
the interior channels of the medical instrument 96. The purged residual
water/detergent mixture flows down the drain line 212 located below the
reprocessing basin 12 and collects in the bottom of the T-assembly 217 located
below the drain line 212. The purged residual water/detergent mixture is
removed
from the bottom of the T-assembly 217 by means of a residual drain line 310
and
a residual drain pump 314 that is activated simultaneously with the air pump
224.
The first rinse cycle comprises the steps of fill, rinse and drain steps.
During
the fill step, water is introduced into the reprocessing basin 12 from the
outside
source 232. by means of water valve 230 and water line 234. Since this is a
rinse
cycle, as opposed to a wash cycle, no detergent 254 is introduced during the
fill
step. During the rinse step of the process flow 95, the pump 246 draws the
rinse
water contained in the reprocessing basin 12 through the intake valve 240 and-
recirculates the rinse water for a predetermined minimum period of time in a
manner
as previously described above in connection with the wash step. Also, during
the
rinse step, the ultrasonic crystals 282 are activated.
Thereafter, the drain step begins. During the drain step, rinse water is
pumped out of the reprocessing basin 12 by the drain pump 216. The
watertravels
down the drain line 212 through the drain pump 216 and into the T-assembly
217.
Because the drain valve 164 is in the opened position, the water travels
through
drain valve 164 and through standpipe 165 and into a sewer drain 167.
Once the flow probe 220 detects the absence of moisture in the drain line
212, the drain pump 216 is shut off. Some residual water remains in the bottom
of
the T-assembly 217 that cannot be removed by the drain pump 216. This residual
rinse water is removed from the bottom of the T-assembly 217 by means of the
-16-
I I a M.,
CA 02585722 2007-04-23
residual drain line 310 and the residual drain pump 314 in the manner
previously
described. By removing all residual rinse water from the T-assembly 217,
chemical
disinfectant introduced in the next step of the protocol will not become
diluted with
any residual rinse water.
Once the drain step 141 is complete and all residual rinse water has been
removed from the T-assembly 217, the next fill step begins and a chemical
disinfectant 288 is introduced into the reprocessing basin 12. One
particularly
effective type of chemical disinfectant is 2% or3% glutaraldehyde which is
marketed
by a number of different companies under various brand names such as Cidex
manufactured by Johnson & Johnson. The introduction of the disinfectant 288 is
effected by opening a reservoir feed valve 298 to cause a reservoir pump 294
to
pump the chemical disinfectant 288 from a chemical disinfectant reservoir 290
through a chemical line 306 into the reprocessing basin 12. The chemical
disinfectant 288 enters and fills the reprocessing basin 12 to a predetermined
height
as previously described. Once the reprocessing basin 12 has been filled with
the
chemical disinfectant 288 to the predetermined level, the pump 246 is
activated to
draw the chemical disinfectant 288 contained in the reprocessing basin 12
through
the intake valve 240. This action circulates the chemical disinfectant 288
through
the ports of the manifold 250, the tubing segments 132a-e and through the
internal
passageways 98a-e of the immersed medical instrument 96. Once the chemical
disinfectant 288 has passed through the internal passageways of 98a-I of the
immersed medical instrument 96, it flows back into the reprocessing basin 12
where
it is recirculated by the pump 246 for a predetermined minimum period of time
based upon guidelines provided by the manufacturer of the chemical
disinfectant
288. Once the predetermined minimum time period forthe chemical immersion step
has elapsed, the pump 246 is turned off.
'hereafter, the chemical disinfectant 288 is returned to the chemical
disinfectant reservoir 290 for reuse. To enable the return of the chemical
disinfectant 288 to the reservoir 290, the drain valve 164 is closed and the
reservoir
return valve 302 is opened. The drain pump 216 is activated and the chemical
disinfectant 288 is pumped through the chemical line 306, through the
reservoir
return valve 302 and back into the chemical reservoir 290. Once the flow probe
220
-17-
1 1 I U s1Y 11 1
CA 02585722 2007-04-23
detects the absence of moisture in the drain line 212, the drain pump 216 is
tuned
off. Thereafter, two additional rinse cycles are performed. The first rinse
cycle
comprises a first rinse and a drain phase. The rinse cycle is performed in a
manner
similar to the rinse cycle previously described. However, this rinse cycle
does not
include use of the residual drain line 310 and residual drain pump 314. The
ultrasonic crystals 282 are activated during the rinse step of this rinse
cycle.
The second rinse cycle comprises fill, second rinse and drain phases. This
rinse cycle is performed in a manner similar to the rinse cycle previously
described,
i.e., fill, rinse and drain phases, and includes use of the residual drain
line 310 and
residual drain pump 314. The ultrasonic crystals 282 are activated during the
rinse
step of this rinse cycle. Once this rinse cycle has been completed, the
reprocessing
protocol is complete and the instrument may be removed from the reprocessing
chamber for reuse.
Without further elaboration, the foregoing will so fully illustrate the
invention
that others may, by applying current or future knowledge, readily adapt the
same for
use under the various conditions of service.
-18-
I