Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 146
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 146
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FORMULATIONS, METHODS OF PRODUCTION AND USES OF FGF-20
1. FIELD OF THE INVENTION
The present invention relates to improved formulations comprising FGF-20, its
fragments,
derivatives, variants, homologs, analogs, or a combination thereof, improved
methods for
production, and methods of use thereof.
2. BACKGROUND OF THE INVENTION
The fibroblast growth factor ("FGF") family consists of more than 20 members,
each
containing a conserved amino acid core (see, e.g., Powers et al., Endocr.
Relat. Cancer, 7(3):65-
197 (2000)). FGFs regulate diverse cellular functions such as growth,
survival, apoptosis, motility,
and differentiation (see, e.g., Szebenyi et aL, lnt. Rev. Cytol., 185:45-106
(1999)). Members of the
FGF family are also involved in various physiological and pathological
processes during
embryogenesis and adult life, including morphogenesis, limb development,
tissue repair,
inflammation, angiogenesis, and tumor growth and invasion (see, e.g., Powers
et al., Endocr. Relat.
Cancer, 7(3):165-197 (2000); and Szebenyi et aL, Int. Rev. Cytol. 185:45-106
(1999)).
Through a homology-based genomic mining process, a novel human FGF, FGF-20,
was
discovered. See U.S. Patent Application Nos. 09/494,585, filed January 13,
2000, and 09/609,543,
filed July 3, 2000, the disclosure of each references is incorporated herein
by reference. The amino
acid sequence of FGF-20 shows close homology with human FGF-9 (70% identity)
and FGF16
(64% identity).
Recombinant full length FGF-20 has been shown to induce a proliferative
response in
mesenchymal and epithelial cells, but not in human smooth muscle, erythroid,
or endothelial cells
(see, e.g., Jeffers et al., Cancer Res. 61(7):3131-3138 (2001)). FGF-20 and
its variants or
derivatives have also been shown to be effective in preventing and/or treating
certain diseases, such
as oral mucositis (see International Patent Application Publication No. WO
2003/099201, filed May
9, 2003), inflammatory bowel disease ("IBD") (see International Patent
Application Publication No.
WO 2002/058716, filed November 6, 2001), and certain diseases related to
central nerve system,
such as Parkinson's Disease, and certain diseases related to cardiovascular
system, such as stroke
(see International Patent Application Publication No. 2004/100892, filed May
10, 2004). FGF-20
and its variants or derivatives have also been shown to be effective in
preventing and/or treating
symptoms associated with radiation exposure (see International Patent
Application Publication No.
WO 2005/025489, filed May 10, 2004). The disclosure of each reference is
incorporated herein by
reference in its entirety.
Therefore, there is a great need for pharmaceutical formulations comprising
FGF-20 and/or
its variants or derivatives that are suitable for clinical uses, of which
formulations are stable and can
be produced at a commercial scale.
1
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Citation or discussion of a reference herein shall not be construed as an
admission that
such is prior art to the present invention.
3. SUMMARY OF THE INVENTION
The present invention provides improved formulations comprising a fibroblast
growth factor,
preferably FGF-20, or its fragments, derivatives, variants, homologs, analogs,
or a combination
thereof. The present invention also provides improved production methods for
isolating one or more
CG53135 proteins. The present invention further provides methods of use of
CG53135 proteins and
the improved formulations comprising one or more CG53135 proteins.
In one embodiment, the present invention provides a formulation comprising
about 0.1-1 M
arginine in a salt form, sulfobutyl ether Beta-cyclodextrin sodium, or
sucrose, about 0.01-0.1 M
sodium phosphate monobasic (NaH2PO4=H2O), about 0.01 %-0.1 % weight/volume
("w/v")
polysorbate 80 or polysorbate 20, and an isolated fibroblast growth factor
("FGF"). In a specific
embodiment, the concentration of the FGF in the formulations of the invention
is about 0.005 mg/mI
to about 50 mg/mI. The FGF protein is preferably a CG53135 protein. In a
specific embodiment,
the formulations of the present invention comprise one or more isolated
proteins selected from the
group consisting of: (a) a protein comprising an amino acid sequence of SEQ ID
NOs:2, 4, 7, 10, 22,
24, 26, 28, 30, 32, 34, 36, 38, or 40; (b) a protein with one or more amino
acid substitutions to the
protein of (a), wherein said substitutions are no more than 15% of the amino
acid sequence of SEQ
ID NOs:2, 4, 7, 10, 22, 24, 26, 28, 30, 32, 34, 36, 38, or 40, and wherein
said protein with one or
more amino acid substitutions retains cell proliferation stimulatory activity;
and (c) a fragment of the
protein of (a) or (b), which fragment retains cell proliferation stimulatory
activity. In some
embodiments, the formulations of the invention comprise one or more isolated
proteins, where the
concentration of the proteins is of 0.5-30 mg/mI. In a specific embodiment,
the concentration of the
proteins is 10 mg/ml. In some embodiments, the formulations of the invention
are lyophilized or
spray dried.
In a specific embodiment, the formulations of the invention comprise an
arginine in a salt
form, which is selected from the group consisting of arginine, arginine
sulfate, arginine phosphate,
and arginine hydrochloride. In one embodiment, the arginine in a salt form,
sulfobutyl ether Beta-
cyclodextrin sodium, or sucrose in the formulations of the invention has a
concentration of 0.01-0.7
M, preferably 0.5 M.
In another embodiment, the sodium phosphate monobasic in the formulations of
the
invention has a concentration of 0.05 M. In another embodiment, polysorbate 80
or polysorbate 20
of the formulations of the invention is 0.01 % (w/v). In one embodiment, the
formulations of the
invention comprise polysorbate 80. In another embodiment, the formulations of
the invention
comprise polysorbate 20.
In a specific embodiment, a formulation of the invention comprises about 10
mg/mI of an
isolated protein comprising an amino acid sequence of SEQ ID NO:24, 0.5 M
arginine sulfate, 0.05
2
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
M sodium phosphate monobasic, and 0.01 % (w/v) polysorbate 80. In another
specific embodiment,
a formulation of the invention comprises about 10 mg/ml of an isolated protein
comprising an amino
acid sequence of SEQ ID NO:2, 0.5 M arginine sulfate, 0.05 M sodium phosphate
monobasic, and
0.01 %(w/v) polysorbate 80. In another embodiment, a formulation of the
invention comprises 0.5 M
arginine sulfate, 0.05 M sodium phosphate monobasic, 0.01 %(w/v) polysorbate
80, and about 10
mg/mi of a mixture of isolated proteins, wherein said proteins comprise a
first protein comprising an
amino acid sequence of SEQ ID NO:24, and a second protein comprising an amino
acid sequence
of SEQ ID NO:2. In a specific embodiment, a formulation of the invention
further comprises one or
more isolated proteins, wherein said proteins comprise an amino acid sequence
selected from the
group consisting of SEQ ID NOs:26, 28, 30 and 32. In some embodiments, the
formulations of the
invention comprise one or more isolated proteins that are carbamylated.
In another embodiment, the present invention provides methods of increasing
solubility of a
fibroblast growth factor ("FGF") in an aqueous solution by adding arginine in
a salt form, sulfobutyl
ether Beta-cyclodextrin sodium, or sucrose, or a combination thereof to said
solution to a final
concentration of 0.01 - 1 M. In some embodiment, the fibroblast growth factor
is an isolated
CG53135 protein. In a specific embodiment, the fibroblast growth factor is an
isolated protein
comprising an amino acid sequence selected from the group consisting of SEQ ID
NOs:2, 4, 7, 10,
22, 24, 26, 28, 30, 32, 34, 36, 38, and 40.
In some embodiments, an arginine in a salt form is selected from the group
consisting of
arginine, arginine sulfate, arginine phosphate, and arginine hydrochloride. In
one embodiment, the
final concentration of arginine in a salt form is 0.01 - 0.7 M, preferably 0.5
M. In some
embodiments, the methods of the invention further comprise adding acetate,
succinate, tartrate, or a
combination thereof to the solution to increase the solubility of the protein.
Preferably, the acetate,
succinate, tartrate, or a combination thereof has a final concentration of
0.01-0.2 M in the solution.
In another embodiment, the present invention provides a method of producing an
isolated
protein comprising the steps of: (1) fermenting an E. coli cell containing a
vector comprising SEQ ID
NO:8; (2) chilling the fermented culture to 10-15 C; (3) diluting the chilled
culture with a lysis buffer
comprising 50-100 mM sodium phosphate, 60 mM ethylene diamine tetraacetic
acid, 7.5 mM DTT,
and 3.5-5 M urea; (4) lysing the cells in the diluted culture; (5) loading the
resultant cell lysate onto a
pre-equilibrated cation exchange column, and flushing the column with a buffer
comprising 50-100
mM sodium phosphate, 40 mM EDTA, 10 mM sodium sulfate, and 3-5 M urea; (6)
washing the
flushed column with a buffer comprising 50-100 mM sodium phosphate, 5 mM EDTA,
10-25 mM
sodium sulfate, and 2.22 mM dextrose; (7) washing the column again with an
elution buffer
comprising 50-100 mM sodium phosphate, 5 mM EDTA, 150-250 mM sodium sulfate,
and 0.5-1 M
L-arginine; (8) loading the resultant eluate onto a hydrophobic interaction
chromatography column
pre-equilibrated with 50-100 mM sodium phosphate, 150-250 mM sodium sulfate, 5
mM EDTA, and
1 M arginine; (9) washing the resulting column with a solution comprising 100-
250 mM sodium
phosphate, 5 mM EDTA, and 0.8-1 M arginine; and (10) washing the column again
with a solution
comprising 50-100 mM sodium phosphate, 5 mM EDTA, and 0.1-0.3 M arginine to
elute the protein.
3
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In a specific embodiment, the method further comprises the steps of: (11)
concentrating the
resultant eluate; (12) filtering the retentate obtained together with a
solution comprising 50 mM
sodium phosphate, 0.5 M arginine; (13) concentrating the filtered retentate;
and (14) filtering the
concentrated retentate.
In some embodiments, the cells are fermented by a method comprising the steps
of: (a)
culturing E. coli cells containing a vector comprising SEQ ID N0:8 to
exponential growth phase with
2.5 to 4.5 OD600 units in a chemically defined seed medium; (b) inoculating
cells of step (a) to a
seed medium and culturing the cells to an exponential growth phase with 3.0 to
5.0 OD600 units; (c)
transferring the cells of step (b) to a chemically defined batch medium; (d)
culturing the cells of step
(c) to 25-35 units OD600, and adding additional chemically defined medium with
a feeding rate of
0.7 g/kg broth/minute; (e) culturing the cells of step (d) to 135 to 165 units
OD600; and (f) culturing
the cells of step (e) for about four hours.
The present invention also provides isolated proteins produced by the methods
of the
invention.
Uses of the compositions and formulations of the invention for preventing
and/or treating a
disease, e.g., alimentary mucositis, arthritis, a disorder or symptom
associated with radiation
exposure, a disorder of central nerve system or cardiovascular system, are
also provided.
3.1 TERMINOLOGY
As used herein, the term "about" in the context of a given numerate value or
range refers to
a value or range that is within 20%, preferably within 10%, and more
preferably within 5% of the
given value or range.
As used herein, the term "CG53135", refers to a class of proteins (including
peptides and
polypeptides) or nucleic acids encoding such proteins or their complementary
strands, where the
proteins comprise an amino acid sequence of SEQ ID N0:2 (211 amino acids), or
its fragments,
derivatives, variants, homologs, or analogs. In a preferred embodiment, a
CG53135 protein retains
at least some biological activity of FGF-20. As used herein, the term
"biological activity" means that
a CG53135 protein possesses some but not necessarily all the same properties
of (and not
necessarily to the same degree as) FGF-20.
A member (e.g., a protein and/or a nucleic acid encoding the protein) of the
CG53135 family
may further be given an identification name. For example, CG53135-01 (SEQ ID
NOs:1 and 2)
represents the first identified FGF-20 (see U.S. Patent Application No.
09/494,585); CG53135-05
(SEQ ID NOs:8 and 2) represents a codon-optimized, full length FGF-20 (i.e.,
the nucleic acid
sequence encoding FGF-20 has been codon optimized, but the amino acid sequence
has not been
changed from the originally identified FGF-20); CG53135-12 (SEQ ID NOs:21 and
22) represent a
single nucleotide polymorphism ("SNP") of FGF-20 where one amino acid in
CG53135-12 is
different from SEQ ID N0:2 (the aspartic acid at position 206 is changed to
asparagine, c206D->N").
Some members of the CG53135 family may differ in their nucleic acid sequences
but encode the
4
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
same CG53135 protein, e.g., CG53135-01, CG53135-03, and CG53135-05 all encode
the same
CG53135 protein. An identification name may also be an in-frame clone ("IFC")
number, for
example, IFC 250059629 (SEQ ID NOs:33 and 34) represents amino acids 63-196 of
the full length
FGF-20 (cloned in frame in a vector). Table 1A shows a summary of some of the
CG53135 family
members. In one embodiment, the invention includes a variant of FGF-20
protein, in which some
amino acids residues, e.g., no more than 1%, 2%, 3%, 5%, 10% or 15% of the
amino acid sequence
of FGF-20 (SEQ ID NO:2), are changed. In another embodiment, the invention
includes nucleic acid
molecules that can hybridize to FGF-20 under stringent hybridization
conditions.
Table 1A. Summary of some of the CG53135 family members
Name SEQ ID NO Brief Description
(DNA/Protein)
CG53135-01 1 and 2 FGF-20 wild type, stop codon removed
CG53135-02 3 and 4 Codon optimized, amino acids 2-54 (as numbered in SEQ ID
NO:2) were removed
CG53135-03 5 and 2 FGF-20 wild type
CG53135-04 6 and 7 Amino acids 20-51 (as numbered in SEQ ID NO:2 were removed,
also valine at position 85 is changed to alanine ("8 V->A")
CG53135-05 8 and 2 Codon optimized, full length FGF-20
CG53135-06 9 and 10 Amino acids 20-51 (as numbered in SEQ ID NO:2) were
removed
CG53135-07 11 and 12 Protein consisting of amino acids 1-18 (as numbered in
SEQ ID
NO:2)
CG53135-08 13 and 14 Protein consisting of amino acids 32-52 (as numbered in
SEQ ID
NO:2)
CG53135-09 15 and 16 Protein consisting of amino acids 173-183 (as numbered in
SEQ
ID NO:2)
CG53135-10 17 and 18 Protein consisting of amino acids 192-211 (as numbered in
SEQ
ID NO:2)
CG53135-11 19 and 20 Protein consisting of amino acids 121-137 (as numbered in
SEQ
ID NO:2)
CG53135-12 21 and 22 FGF-20 SNP, aspartic acid at position 206 is changed to
asparagines (i206D--).N") as compared to CG53135-01
CG53135-13 23 and 24 CG53135-05 minus first 2 amino acids at the N-terminus
CG53135-14 25 and 26 CG53135-05 minus first 8 amino acids at the N-terminus
CG53135-15 27 and 28 CG53135-05 minus first 11 amino acids at the N-terminus
CG53135-16 29 and 30 CG53135-05 minus first 14 amino acids at the N-terminus
CG53135-17 31 and 32 CG53135-05 minus first 23 amino acids at the N-terminus
IFC 250059629 33 and 34 In frame clone, open reading frame comprising a
nucleotide
sequence encoding amino acids 63-196 of FGF-20 (SEQ ID NO:2)
IFC 250059669 35 and 36 In frame clone, open reading frame comprising a
nucleotide
sequence encoding amino acids 63-211 of FGF-20 (SEQ ID NO:2)
IFC 317459553 37 and 38 In frame clone, open reading frame comprising a
nucleotide
sequence encoding amino acids 63-194 of FGF-20 (SEQ ID NO:2)
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
with 1G,E
IFC 317459571 39 and 40 In frame clone, open reading frame comprising a
nucleotide
sequence encoding amino acids 63-194 of FGF-20 (SEQ ID NO:2)
IFC 250059596 41 and 10 In frame clone, open reading frame comprising a
nucleotide
sequence encoding amino acids 1-19 and 52-211 of FGF-20 (SEQ
ID NO:2)
IFC 316351224 41 and 10 In frame clone, open reading frame comprising a
nucleotide
sequence encoding amino acids 1-19 and 52-211 of FGF-20 (SEQ
ID NO:2).
As used herein, the term "effective amount" refers to the amount of a therapy
(e.g., a
formulation comprising a CG53135 protein) which is sufficient to reduce and/or
ameliorate the
severity and/or duration of a disease (e.g., alimentary mucositis, IBD,
Irritable Bowel Syndrome,
arthritis, stroke, radiation-related diseases) or one or more symptoms
thereof, prevent the
advancement of a disease, cause regression of a disease, prevent the
recurrence, development, or
onset of one or more symptoms associated with a disease, or enhance or improve
the prophylactic
or therapeutic effect(s) of another therapy.
As used herein, the term "FGF-20" refers to a protein comprising an amino acid
sequence of
SEQ ID NO:2, or a nucleic acid sequence encoding such a protein or the
complementary strand
thereof.
As used herein, the term "hybridizes under stringent conditions" describes
conditions for
hybridization and washing under which nucleotide sequences at least 30%
(preferably, 35%, 40%,
45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95% or 98%) identical to
each other
typically remain hybridized to each other. Such stringent conditions are known
to those skilled in the
art and can be found in Current Protocols in Molecular Biology, John Wiley &
Sons, N.Y. (1989),
6.3.1-6.3.6. In one, non limiting example, stringent hybridization conditions
comprise a salt
concentration from about 0.1 M to about 1.0 M sodium ion, a pH from about 7.0
to about 8.3, a
temperature is at least about 60 C, and at least one wash in 0.2 X sodium
chloride/sodium citrate
(SSC), 0.01% bovine serum albumin (BSA). In another non-limiting example,
stringent hybridization
conditions are hybridization at 6X SSC at about 45 C, followed by one or more
washes in 0.1X SSC,
0.2% sodium dodecyl sulfate (SDS) at about 68 C. In yet another non-limiting
example, stringent
hybridization conditions are hybridization in 6XSSC at about 45 C, followed by
one or more washes
in 0.2 X SSC, 0.1% SDS at 50-65 C (i.e., one or more washes at 50 C, 55 C, 60
C or 65 C). It is
understood that the nucleic acids of the invention do not include nucleic acid
molecules that
hybridize under these conditions solely to a nucleotide sequence consisting of
only A or T
nucleotides.
As used herein, the term "isolated" in the context of a protein agent refers
to a protein agent
that is substantially free of cellular material or contaminating proteins from
the cell or tissue source
from which it is derived, or substantially free of chemical precursors or
other chemicals when
chemically synthesized. The language "substantially free of cellular material"
includes preparations
of a protein agent in which the protein agent is separated from cellular
components of the cells from
6
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
which it is isolated or recombinantly produced. Thus, a protein agent that is
substantially free of
cellular material includes preparations of a protein agent having less than
about 30%, 20%, 10%, or
5% (by dry weight) of host cell proteins (also referred to as a "contaminating
proteins"). When the
protein agent is recombinantly produced, it is also preferably substantially
free of culture medium,
f.e., culture medium represents less than about 20%, 10%, or 5% of the volume
of the protein agent
preparation. When the protein agent is produced by chemical synthesis, it is
preferably substantially
free of chemical precursors or other chemicals, i.e., it is separated from
chemical precursors or other
chemicals that are involved in the synthesis of the protein agent.
Accordingly, such preparations of
a protein agent have less than about 30%, 20%, 10%, 5% (by dry weight) of
chemical precursors or
compounds other than the protein agent of interest. In a specific embodiment,
protein agents
disclosed herein are isolated.
As used herein, the term "isolated" in the context of nucleic acid molecules
refers to a
nucleic acid molecule which is separated from other nucleic acid molecules
which are present in the
natural source of the nucleic acid molecule. Moreover, an "isolated" nucleic
acid molecule, such as
a cDNA molecule, can be substantially free of other cellular material or
culture medium when
produced by recombinant techniques, or substantially free of chemical
precursors or other chemicals
when chemically synthesized. In a specific embodiment, nucleic acid molecules
are isolated.
As used herein, the terms "prevent," "preventing," and "prevention" refer to
the prevention of
the recurrence, onset, or development of a disease (e.g., alimentary
mucositis, IBD, Irritable Bowel
Syndrome, arthritis, stroke, radiation-related diseases) or one or more
symptoms thereof in a subject
resulting from the administration of a therapy (e.g., a composition comprising
a CG53135 protein), or
the administration of a combination of therapies.
As used herein, the term "prophylactically effective amount" refers to the
amount of a
therapy (e.g., a composition comprising a CG53135 protein) which is sufficient
to result in the
prevention of the development, recurrence, or onset of a disease (e.g.,
alimentary mucositis, IBD,
Irritable Bowel Syndrome, arthritis, stroke, radiation-related diseases) or
one or more symptoms
thereof, or to enhance or improve the prophylactic effect(s) of another
therapy.
As used herein, the term "stability" in the context of a protein formulation,
refers to the ability
of a particular protein formulation to maintain the native, active structure
of a protein as the protein is
exposed to thermo-mechanical stresses over time. In some embodiments,
stability of a protein
formulation generally refers to the tendency of a protein formulation to form
biologically inactive
and/or insoluble aggregates of the protein as a result of exposure of the
protein to thermo-
mechanical stresses, as well as the tendency of a protein formulation to form
biologically inactive
and/or insoluble aggregates of the protein as a result of interaction with
interfaces and surfaces that
are destabilizing, such as hydrophobic surfaces and interfaces. A related
parameter to the "stability"
of a protein formulation is its solubility in that higher molecular weight
aggregates and denatured
forms of a protein, including partially denatured forms of a protein, which
are generally less soluble
than their non-aggregated, lower molecular weight counterparts and native
forms of the protein.
7
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Another related parameter to the "stability" of a protein formulation is the
protein concentration in
that physically stable formulations may become less physically stable as the
concentration of the
protein is increased or decreased.
As used herein, the terms "subject" and "subjects" refer to an animal,
preferably a mammal,
including a non-primate (e.g., a cow, pig, horse, cat, or dog), a primate
(e.g., a monkey,
chimpanzee, or human), and more preferably a human. The term "subject" is used
interchangeably
with "patient" in the present invention.
As used herein, the terms "treat," "treatment," and "treating" refer to the
reduction of the
progression, severity, and/or duration of a disease (e.g., alimentary
mucositis, IBD, Irritable Bowel
Syndrome, arthritis, stroke, radiation-related diseases) or amelioration of
one or more symptoms
thereof, wherein such reduction and/or amelioration result from the
administration of one or more
therapies (e.g., a composition comprising a CG53135 protein).
As used herein, the term "therapeutically effective amount" refers to the
amount of a therapy
(e.g., a composition comprising a CG53135 protein), which is sufficient to
reduce the severity or
duration of a disease (e.g., alimentary mucositis, IBD, Irritable Bowel
Syndrome, arthritis, stroke,
radiation-related diseases), prevent the advancement of a disease (e.g.,
alimentary mucositis, IBD,
Irritable Bowel Syndrome, arthritis, stroke, radiation-related diseases),
cause regression of a
disease (e.g., alimentary mucositis, IBD, Irritable Bowel Syndrome, arthritis,
stroke, radiation-related
diseases), ameliorate one or more symptoms associated with a disease (e.g.,
alimentary mucositis,
IBD, Irritable Bowel Syndrome, arthritis, stroke, radiation-related diseases),
or enhance or improve
the therapeutic effect(s) of another therapy.
4. BRIEF DESCRIPTION OF THE DRAWINGS
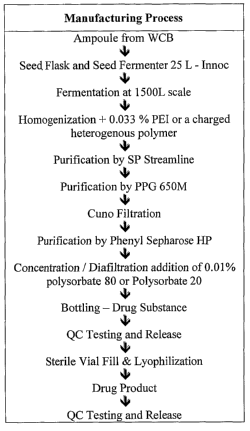
FIG. 1 shows an example of manufacturing a drug product comprising one or more
CG53135 proteins.
FIG. 2 (A) shows SDS-PAGE analysis (gel code blue) of CG53135 produced by
Process I
and Process 2 (as described in Section 6), respectively. Lane 1: molecular
weight markers (kDa);
lane 2-4: purified CG53135 (10 pg), reduced. Lane 5-8: Process 1 reference
standard DEV10 (720,
380, 45, and 28 ng) reduced. (B) shows SDS-PAGE analysis (silver stain) of
CG53135. Lane 1:
molecular weight markers (kDa) are shown on the left; lane 3: purified CG53135
by Process 2 (5
pg); lane 5: purified CG53135 reference standard Process 1(5 pg); lane 7:
purified CG53135
Process 2 (10 pg); lane 9: purified CG53135 reference standard Process 1 (10
pg).
FIG. 3 shows RP-HPLC analysis of CG53135 purified by Process 1 and Process 2,
respectively (Process 1 is represented by the solid line).
FIG. 4 shows SEC-HPLC analysis of CG53135 purified by Process I and Process 2,
respectively.
FIG. 5 shows host cell protein analysis of CG53135 E. coli purified product
(by Process 1
and Process 2, respectively).
8
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FIG. 6 shows Western Blot analysis of CG53135 purified by Process I and
Process 2,
respectively. Western blot was probed with anti-CG53135-05 antibody. Lane 1:
molecular weight
marker (kDa); lane 3: CG53135 purified by Process 2 (10 pg); lane 5: CG53135
purified by Process
1 (10 pg).
FIG. 7 shows RP-HPLC identification analysis of CG53135 purified by Process 1
and
Process 2, respectively. Process 2 is represented by the dashed line.
FIG. 8 shows tryptic map of CG53135 purifled by Process 1 and Process 2,
respectively.
FIG. 9 shows circular dichroism spectroscopy analysis of CG53135 produced by
Process 1
and Process 2, respectively. The lower (grey) trace represents Process 1, and
the upper (black)
trace represents Process 2.
FIG. 10 shows near UV circular dichroism spectroscopy analysis of CG53135
purified by
Process 1 and Process 2, respectively. The upper (grey) trace is the near UV
CD spectrum of
Process I and the black trace (lower) is the near UV CD spectrum of Process 2.
FIG. 11 shows second derivative absorbance spectra for Process 1 (grey trace)
and
Process 2 (black trace).
FIG. 12 shows temperature melting curves for Process 1 and Process 2,
respectively, by
differential scanning calorimetry.
FIG. 13 displays the biological activity of a truncated form of recombinant
FGF-20
(CG53135-17, denoted by (d1-23)FGF20 in the Figure) as represented by its
effects on DNA
synthesis, compared to that of full length FGF-20 (denoted FGF20 in the
Figure). NIH 3T3 mouse
fibroblasts were serum-starved, incubated with the indicated factor for 18
hours, and analyzed by a
BrdU incorporation assay.
FIG. 14 (A) shows dose response of CG53135-induced DNA synthesis in NIH 3T3
Fibroblasts. Serum starved NIH 3T3 cells were treated with purified CG53135-01
(CG53135 in
figure), 10% serum or vehicle only (control). DNA synthesis was measured in
triplicate for each
sample, using a BrdU incorporation assay. Data points represent average BrdU
incorporation and
bars represent standard error (SE). (B) CG53135 stimulates Growth of NIH 3T3
Fibroblasts.
Duplicate wells of serum starved NIH 3T3 cells were treated for 1 day with
purified CG53135-01 (I
iag) or vehicle control. Cell counts for each well were determined in
duplicate. Y- axis identifies cell
number, which is the average of 4 cell counts (treatment duplicates x
duplicate counts) and standard
error (SE). (C) CG53135 induces DNA synthesis in 786-0 Kidney Epithelial
cells. Serum starved
786-0 cells were left untreated or treated with partially purified CG531 35-01
(from 5 ng/pL stock), or
with vehicle control (mock). DNA synthesis was measured in triplicate for each
sample, using a
BrdU incorporation assay. Data points represent average BrdU incorporation and
bars represent
standard error (SE).
9
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FIG. 15 shows effect of Mucositis on the duration of mucositis induced by
chemotherapy.
The number of days with mucositis scores > 3 was evaluated. To examine the
levels of clinically
significant mucositis as defined by presentation with open ulcers (score > 3),
the total number of
days in which an animal exhibited an elevated score was summed and expressed
as a percentage
of the total number of days scored for each group. Statistical significance of
observed differences
was calculated using Chi-square analysis. Vehicle control=disease control.
FIG. 16 shows the cell positions in the crypt.
FIG. 17 (A) shows the crypt survival curve comparing prophylactic
administration of
CG53135-05 E. coli purified product treatment to PBS control group following
different radiation
dosages. (B) Shows the effect of prophylactic administration of CG53135-05 E.
coli purified product
on mice intestinal crypt survival after radiation insult.
FIG. 18 (A) and (B) show the mean daily mucositis scores following treatment
with
CG53135-05 E. coli purified product. Mean group mucositis scores were
obtained. Error bars
represent the standard error of the means (SEM). A comparison of the untreated
control group and
the groups that received CG53135-05 12 mg/kg IP on days 1 and 2, with the
groups that received
CG53135-05 on day -1 only was performed. (A) shows groups that received
CG53135-05 at 6
mg/kg or 12 mg/kg; and (B) shows groups that received CG53135-05 at 24 mg/kg
or 48 mg/kg.
FIG. 19 shows mean daily mucositis scores following treatment with CG53135-05
E. coli
purified product once, twice, thrice or four times. Mean group mucositis
scores were obtained.
Error bars represent the standard error of the means (SEM). A comparison of
the untreated and
vehicle control groups with the groups that received CG53135-05 E. coli
purified product 12 mg/kg
IP was performed. (A) Groups that received CG53135-05 E. coli purified product
for one or two
days; (B) Groups that received CG53135-05 purified product for three or four
days.
FIG. 20 shows percent weight gain in animals with mucositis treated with
CG53135-05
purified product. Animals were weighed daily, the percent weight change from
day -4 was
calculated, and group means and standard errors of the mean (SEM) calculated
for each day. A
comparison of the untreated control group and the groups receiving CG53135-05
E. coli purified
product 12 mg/kg IP on days 1 and 2, with the groups receiving CG53135-05 E.
coli purified product
on day -1 only was performed. (A) Groups that received CG53135-05 E. coli
purified product at 6
mg/kg or 12 mg/kg; (B) Groups that received CG53135-05 E. coli purified
product at 24 mg/kg or 48
mg/kg.
FIG. 21 shows Weight change represented as Area Under the Curve (AUC) gain in
animals
with mucositis treated with CG53135-05 E. coli purified product. The area
under the curve (AUC)
was calculated for the percent weight change exhibited by each animal in the
study. This calculation
was made using the trapezoidal rule transformation. Group means were
calculated and are shown
with error bars representing SEM for each group. A One Way ANOVA was performed
to compare
groups.
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FIG. 22 shows mean daily mucositis scores following treatment with CG53135-05
E. coli
purified product once, twice, thrice or four times. Mean group mucositis
scores were obtained.
Error bars represent the standard error of the means (SEM). A comparison of
the untreated and
vehicle control groups with the groups that received CG53135-05 E. coli
purified product 12 mg/kg
IP was performed. (A) Groups that received CG53135-05 E. coli purified product
for one or two
days; (B) Groups that received CG53135-05 purified product for three or four
days.
FIG. 23 shows weight change represented as Area Under the Curve (AUC) for
animals
treated with single dose of CG53135-05 E. coli purified product for one, two,
three or four days. The
area under the curve (AUC) was calculated for the percent weight change
exhibited by each animal
in the study. This calculation was made using the trapezoidal rule
transformation. Group means
were calculated and are shown with error bars representing SEM for each group.
A One Way
ANOVA was performed to compare groups.
FIG. 24 (A) and (B) show effects of CG53135 on body weight in animals with
gastrointestinal injury induced by whole body irradiation as analyzed by one-
way ANOVA and
Dunnett's Multiple Comparison Test, respectively.
FIG. 25 (A) and (B) show effects of CG53135 on diarrhea score in mice with
gastrointestinal
injury induced by whole body irradiation as analyzed by one-way ANOVA and
Tukey's Multiple
Comparison Test, respectively.
FIG. 26 shows analysis of diarrhea score for each day of observation.
FIG. 27 shows the effect of Phosphate Buffered Saline (PBS) control on mice
survival after
exposure to radiation doses of 484 cGy, 534 cGy, 570 cGy, 606 cGy, or 641 cGy.
FIG. 28 (A) shows the effect of prophylactic administration of CG53135 (day-1)
on survival
of mice after exposure to radiation doses of 484 cGy, 534 cGy, 570 cGy, 606
cGy, or 641 cGy. (B)
shows Kaplan-Meier plots for survival at 570 cGy and 606 cGy, with
statistically significant
differences between CG53135-treated and PBS-treated control animals. (C)
Probit analysis for
survival over the range of radiation doses.
FIG. 29 shows the effect of prophylactic administration of CG53135 (day-2 and -
1) on
survival of mice after exposure to radiation doses of 484 cGy, 534 cGy, 570
cGy, 606 cGy, or 641
cGy.
FIG. 30 shows the effect of CG53135 multiple-dose administration prior to
irradiation on
crypt survival curves. Animals (n = 6/group) were administered PBS or CG53135-
05 E. coli purified
product (12 mg/kg) by intraperitoneal (IP) injection once daily for 4
consecutive days prior to a single
10, 11, 12, 13, or 14 Gy dose of X-ray whole-body irradiation on Day 0. The
plot represents the
radiation dose-response for crypt survival. Data points represent crypt
survival in individual animals
analyzed using a multi-target (Puck) analysis model, DRFIT.
11
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FIG. 31 shows effect of CG53135 multiple-dose administration on crypt survival
curves.
Animals (n = 6/group) were administered PBS or CG53135-05 E. coli (4 mg/kg) by
intraperitoneal
(IP) injection once daily for either for 1, 2, 3, 4 or 5 consecutive days
prior to, or post a single dose
(13 Gy) whole body irradiation on Day 0. The plot represents the level of
protection of crypt cells in
response to treatment schedule. Protection factor value indicates the number
of surviving crypts per
circumference in the CG53135-05-treated animals compared to PBS, expressed as
a ratio.
FIG. 32 (A)and (B) show CG53135 induced expression of scavengers,
cycloxegenase,
trefoil factor, and transcription factors in NIH 3T3 cells. (C) shows CG53135
induced expression of
scavengers, cycloxegenase, trefoil factor, and transcription factors in
CCD1070sk cells. (D) shows
CG53135 induced expression of scavengers, cycloxegenase, trefoil factor, and
transcription factors
in CCD18Co cells. (E) shows activation of ERK and AKT kinases by CG53135. (F)
shows
CG53135 induced expression of scavengers, cycloxegenase, trefoil factor, and
transcription factors
in human umbilical vein endothelial cells (HUVEC).
FIG. 33 (A) shows the effect of CG53135 on the survival of IEC 18 cells
irradiated with
different X-ray doses. (B) shows the effect of CG53135 on the survival of NIH
3T3 cells irradiated
with different X-ray doses.
FIG. 34 shows the effect of CG53135 on the survival of HUVEC irradiated with
different X-
ray doses.
FIG. 35 shows survival curves for irradiated cells. Cells of various types
(hematopoietic -
32D; mesenchymal - CCD18-Co and NIH3T3; epithelial - IEC18, IEC6 and bone -
U2OS and
Saos-2) were irradiated at the indicated doses then plated in complete growth
media either with or
without (untreated) 100 ng/ml CG53135-05 E. coli purified product and allowed
to form colonies for
10-14 days until the colonies grew to an average diameter of 2 mm. The
colonies were stained with
crystal violet and counted. The natural log (Ln) of the surviving fraction is
represented on the Y axis,
and bars represent standard error.
FIG. 36 (A) shows the effect of CG53135 on the release of cytokine in NIH 3T3
cells. (B)
shows IL-6 and IL-11 expression in response to CG53135.
FIG. 37 (A) shows dose response of CM-H2DCFDA fluorescence from IEC18 cells
treated
with CG53135 after 4Gy irradiation. (B) shows response of CM-H2DCFDA
fluorescence from IEC18
cells treated with CG53135 after 2Gy and 4Gy irradiation. (C) shows dose
response of CM-
H2DCFDA fluorescence from CCD-1 8Co cells treated with CG53135 after 4Gy
irradiation.
FIG. 38 (A) shows dose response of Red CC-1 fluorescence from IEC18 cells
treated with
CG53135 after 4Gy irradiation. (B) shows response of Red CC-1 fluorescence
from IEC18 cells
treated with CG53135 after 4Gy and 6Gy irradiation. (C) shows response of Red
CC-1 fluorescence
from CCD-18Co cells treated with CG53135 before and after 10Gy irradiation.
FIG. 39 shows in vitro radioprotection of the myeloid cell line 32D by
CG53135.
12
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FIG. 40 shows effect of CG53135 on repopulation of thymus following bone
marrow ablation
and subsequent bone marrow transplant.
FIG. 41 shows the relative loss in body weight per group for study 439.
FIG. 42 (A) shows the average diarrhea score over 3 days. (B) shows the mean
diarrhea
severity.
FIG. 43 shows effect of CG53135 in in vitro wound repair in CaCo2, HT-29, IEC-
6 human
cell lines.
FIG. 44 shows effect of CG53135 on COX-2 gene expression in HT-29 cells. RT-
PCR
analysis was carried out to detect the expression of COX-2 gene in HT-29 cell
line, in the presence
of various concentration of CG53135 (0.1, 1.0, 10, 100ng/ml). COX-2 expression
was also
analyzed at various time points (1, 3, 6, 24 hrs) after the addition of
100ng/ml of CG53135.
FIG. 45 shows effect of CG53135 on COX-2 gene expression in Caco2 cells. RT-
PCR
analysis was carried out to detect the expression of COX-2 gene in Caco2 cell
line, in the presence
of various concentration of CG53135 (0.1, 1.0, 10, 100ng/mi). COX-2 expression
was also
analyzed at various time points (1, 3, 6, 24hrs) after the addition of
100ng/mI of CG53135.
FIG. 46 shows effect of CG53135 on COX-2 gene expression in IEC-6 cells. RT-
PCR
analysis was carried out to detect the expression of COX-2 gene in I EC-6 cell
line, in the presence
of various concentration of CG53135 (0.1, 1.0, 10, 100ng/ml). COX-2 expression
was also
analyzed at various time points (1, 3, 6, 24hrs) after the addition of
100ng/mI of CG53135.
FIG. 47 shows effect of CG53135 on ITF gene expression in HT-29 and Caco2
cells. ITF
gene was detected by mRNA expression in HT-29 and Caco2 cells, in the presence
of various
concentration of CG53135 (0.1, 1.0, 10, 100ng/mI). ITF gene expression was
also analyzed at
various time points (1, 3, 6, 24hrs) after the addition of 100ng/ml of
CG53135.
FIG. 48 shows effect of CG53135 in inducing COX-2, TGF-R, ITF, PPAR-y in HT-29
mRNA
expression analysis revealed that, upon induction with 100ng/ml of CG53135 for
48hours,
mammalian cells expressed COX-2, TGF-E3, ITF, PPAR-y genes.
FIG. 49 shows mechanism of Epithelial Restitution by CG53135. To assess
whether TGF-
(3 mediates epithelial restitution by FGF-20, wound repair test was performed.
Caco2 cells were
incubated with CG531 35-01 E. coli purified product (100ng/ml) and anti TGF-
(3 (20 pg/mI) and
percent closure was measured.
FIG. 50 (A) shows Effect of CG53135 in stimulation of kinases in Caco2 cells.
Expression
of signal transducing kinases was analyzed after incubation of Caco2 cells
with CG53135 E. coli
purified product (100ng/ml) for different time points (10, 30, 60 minutes).
(B) Effect of kinase
inhibitors in the expression of COX-2 gene in Caco2 cells. Caco2 cells were
incubated with
CG53135-01 E. coli purified product (100ng/mI) in the presence of 40 pM of
PD098059 and 20 pM
of SB203580 and COX-2 expression was analyzed. (C) Effect of CG53135 in
stimulation of kinases
13
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
in THP-1 cells. Macrophage cell line, THP-1 was cultured with CG53135-01 E.
coli purified product
(100ng/ml) for various time periods. Expression of signal transducing kinases
was analyzed at
different time points (10, 30, 60 mins). (D) Effect of CG53135 on expression
of kinases in intestinal
epithelial cells. Caco2 cells were incubated with FGF-20 (100ng/ml) for 10,
30, 60 minutes and
expression of p-Elk-1, p-ATF-2 and p-PKC was analyzed. Similarly, HT-29 cells
were incubated
with FGF-20 (100ng/mi) for 10, 30, 60 minutes and expression of C-Fos and C-
Jun was analyzed.
(E) Effect of CG53135 in activating ITF Transcription in HT-29 cells. ITF
promoter activity in HT-29
cells was measured by reporter assay in the presence of FGF-20 at a
concentration of 100ng/ml.
FIG. 17 also shows ITF expression in HT-29 cells in the presence of CG53135-01
E. coli purified
product.
FIG. 51 (A) shows presents the change in mean body weight from day 0 upon
treating mice
with varying doses of AB020258 (CG53135). (B) presents the percent change in
mean body weight
from day 0 upon treating mice with varying doses of AB020258. (C) presents
mean colon blood
content score upon treating mice with varying doses of AB020258.
FIG. 52 (A) presents mean distal colon inflammation score upon treating mice
with varying
doses of AB020258. (B) presents mean distal colon gland loss score upon
treating mice with
varying doses of AB020258. (C) presents mean distal colon erosion score upon
treating mice with
varying doses of AB020258. (D) presents mean sums of histopathology scores
upon treating mice
with varying doses of AB020258.
FIG. 53 presents mean spienic lymphoid atrophy score upon treating mice with
varying
doses of AB020258.
FIG. 54 presents mean spienic extramedullary hematopoiesis score upon treating
mice with
varying doses of AB020258.
FIG. 55 (A) presents the effect of CG53135 Treatment on Small Intestine Weight
in
Indomethacin-treated rats. (B) presents effect of CG53135 Treatment on
absolute neutrophil and
lymphocyte counts in indomethacin-treated rats. Blood was collected on Day 5
at necropsy and the
cell counts were determined.
FIG. 56 presents effect of CG53135 Treatment on Histopathology Scores in
Indomethacin-
treated rats. Five sections of affected intestine were evaluated and scored
for necrosis and
inflammation as described in the methods.
FIG. 57 presents images showing the protective effect of CG53135 on intestinal
architecture. Panel A: Small intestine from normal control animal treated iv
with vehicle (BSA).
Panel. B: Small intestine from indomethacin- treated rat, further treated with
vehicle (BSA) iv. Panel
C: Small intestine from indomethacin-treated rat further treated with CG53135,
0.2 mg/kg iv.
Sections were stained with H&E and visualized at a magnification of 25). FIG.
60 shows the
protective Effect of CG53135 on Intestinal Architecture in indomethacin
treated rats. Panel A,
normal control; Panel B, disease control (indomethacin treated); Panel C,
disease model animal
14
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
treated with 0.2 mg/kg iv CG53135. Photomicrographs were obtained on sections
stained with
hemotoxylin and eosin, at 25X magnification.
FIG. 58 shows the effect of CG53135 treatment on BrdU Labeling in the
Intestine. BrdU
incorporation was detected by Immunoperoxidase staining. Panel A: Small
intestine from normal
control animal (100X). Panel B: Small intestine from indomethacin + vehicle
(BSA) treated animal
(50X). Panel C: Small intestine from indomethacin + CG53135 0.2 mg/kg iv
treated rat (50X).
FIG. 59 shows effect of therapeutically-administered CG53135 on survival in
the DSS model
of colitis. Female Balb/c mice were exposed to 4% DSS in drinking water for 7
days (day 0 to day 6)
and then switched to normal drinking water for 4 additional days (day 7 to day
10). CG53135 is
identified as FGF-20 in FIG. 64. Disease control animals (n = 9) received
daily SC injections of
vehicle solution on day 4 to day 9. CG53135 groups (n = 9) received daily SC
injections of the
indicated concentrations of CG53135 on day 4 to day 9. Normal control animals
(n = 3) were not
exposed to DSS, but did receive daily SC injections of vehicle solution on day
4 to day 9. Animal
survival was recorded on a daily basis and the experiment was concluded on day
10. Note that the
disease control and the 0.2 mg/kg CG53135 groups overlap.
FIG. 60 (A) shows weight change and histopathology in prophylactic group (IL-
10KO mice).
IL-10 KO mice were treated with various concentrations of CG53135 E. coli
purified product (0.2, 1,
5mg/kg) and weight change and histopathology was assessed. (B) shows Total
Cecal Histologic
Score in prophylactic group (IL-10 KO mice). IL-10 KO mice were treated with
various
concentrations of FGF-20 (0.2, 1, 5mg/kg) and total cecal histology was scored
as described as
described in the Example.
FIG. 61 (A) shows IL-12 production in prophylactic group. IL-12 production was
assayed by
ELISA as described in Example 40, in MLN and colonic strip culture
established. (B) shows IFN-y
production in prophylactic group. IFN-y production was assayed by ELISA, in
MLN and colonic strip
culture established. (C) shows PGE2 production in prophylactic group. PGE2
production was
assayed by ELISA in MLN prepared.
FIG. 62 shows FACS analysis (prophylactic group). FACS analysis was performed
to get
the total MLN number as well as number of CD4+, CD8+ and CD4+CD69+ cells.
FIG. 63 shows weight change in treatment group. Weight change in the treatment
group
was assessed.
FIG. 64 shows Histology of Cecum (treatment) was analyzed in vehicle control
as well as
CG53135 treated animals.
FIG. 65 shows Histology of Rectum (treatment) was analyzed in vehicle control
as well as
CG53135 treated animals.
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FIG. 66 shows Total Cecal Histologic Score in treatment group (IL-10 KO mice).
IL-10 KO
mice were treated with CG53135 E. coli purified product (5mg/kg) and total
cecal histology was
scored.
FIG. 67 shows PGE2 and TNF-a production in treatment group. PGE2 and TNF- a
production was assayed by ELISA, gut culture and unseparated spienocytes of
CG53135 treated IL-
KO mice.
FIG. 68 (A) shows the results of Forelimb Placing Test. The mean and standard
error of the
score for groups receiving vehicle (diamonds), 1.0 pg/injection CG53135-05
(square), and 2.5
pg/injection CG53135-05 (triangles) are represented overtime. Asterisks
indicate significant
difference from vehicle control as assessed by one-way ANOVA. (B) shows the
results of Hindlimb
Placing Test. The mean and standard error of the score for groups receiving
vehicle (diamonds),
1.0 pg/injection CG53135 (square), and 2.5 pg/injection CG53135 (triangles)
are represented over
time. Asterisks indicate significant difference from vehicle control as
assessed by one-way ANOVA.
(C) shows the results of Body Swing Test. The mean and standard error of the
score for groups
receiving vehicle (diamonds), 1.0 pg/injection CG53135 (square), and 2.5
pg/injection CG53135
(triangles) are represented over time. A score range of -50% swings to the
right indicates no
impairment, whereas 0% swings to the right swing indicates maximal impairment.
Asterisks indicate
significant difference from vehicle control as assessed by one-way ANOVA. (D)
shows the results of
Cylinder Test. The mean and standard error of the score for groups receiving
vehicle (diamonds),
1.0 pg/injection CG53135 (square), and 2.5 pg/injection CG53135 (triangles)
are represented over
time. (E) shows the results of Body Weight. The mean and standard errors of
the weights for
groups receiving vehicle (diamonds), 1.0 pg/injection CG53135 (square), and
2.5 pg/injection
CG53135 (triangles) is represented over time.
FIG. 69 (A) shows the effect of CG53135 on Pro-MMP production in SW1353 cells
in the
presence of IL-1 beta. (B) shows the effect of CG53135 on Pro-MMP production
in SW1353 cells in
the presence of TNF-alpha. (C) shows the effect of CG53135 on TIMP production
in SW1353 cells.
FIG. 70 (A) shows the effect of intra-articular injection of CG53135 in the
Meniscal Tear
Model of Rat Osteoarthritis (Prophylactic Dosing): Mean Tibial Cartilage
Degeneration. (B) shows
results of intra-articular injection of CG53135 in the Meniscal Tear Model of
Rat Osteoarthritis:
(Prophylactic Dosing): Total Cartilage Degeneration Width. (C) shows results
of intra-articular
injection of CG53135 in the Meniscal Tear Model of Rat Osteoarthritis:
(Prophylactic Dosing):
Significant Tibial Cartilage Degeneration Width.
FIG. 71 (A) shows results of intra-articular injection CG53135 in the Meniscal
Tear Model of
Rat Osteoarthritis (Therapeutic Dosing): Mean Tibial Degeneration. (B) shows
results of intra-
articular injection of CG53135 in the Meniscal Tear Model of Rat
Osteoarthritis (Therapeutic
Dosing): Total Cartilage Degeneration Width. (C) shows results of intra-
articular injection of
CG53135 in Meniscal Tear Model of Rat Osteoarthritis (therapeutic Dosing):
Significant Tibial
Cartilage Degeneration Width.
16
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
FIG. 72 (A) shows trophic action of EGF, NGF and CG53135; (B) shows the time
course of
CG53135-inhibited serum withdrawal-induced apoptosis.
FIG. 73 shows CG53135 inhibits serum withdrawal-induced caspase activation.
FIG. 74 shows neuritogenic action of CG53135 as compared to NGF.
FIG. 75 shows activation of MAPK by NGF and CG53135, and the inhibition of
activity by
PD98059, a MAPKK inhibitor.
5. DETAILED DESCRIPTION OF THE INVENTION
The present invention provides improved formulations comprising one or more
CG53135
proteins, which are more stable and soluble, and can be easily lyophilized by
commercial
equipments. The improved formulations comprise 0.01-1 M of a stabilizer, such
as arginine in a salt
form, sulfobutyl ether Beta-cyclodextrin sodium, or sucrose, 0.01-0.1 M sodium
phosphate
monobasic (NaH2PO4=H2O), 0.01 %-0.1 % weight/volume ("w/v") polysorbate 80 or
polysorbate 20,
and one or more isolated CG53135 proteins. In a specific embodiment, the
concentration of
CG53135 protein(s) in the improved formulations of the invention is less than
50 mg/ml, less than 30
mg/mi, less than 10 mg/mI, less than 5 mg/mI, or less than 1 mg/mI. In another
embodiment, the
concentration of CG53135 protein(s) in the improved formulations of the
invention is between 0.005-
50 mg/mI. In a preferred embodiment, the formulation is lyophilized.
The present invention also provides methods for increasing solubility of a FGF
protein in a
solution (e.g., an aqueous solution). In one embodiment, the present invention
provides a method
for increasing solubility of a FGF protein in a solution by adding arginine in
a salt form, sulfobutyl
ether Beta-cyclodextrin sodium, or sucrose to the solution. In another
embodiment, the present
invention provides a method for increasing solubility or stability of a FGF
protein in a solution by
adding buffering salts such as acetate, succinate, tartrate, phosphate, or a
combination thereof to
the solution. In yet another embodiment, buffering salts such as acetate,
succinate, tartrate,
phosphate, or a combination thereof is added in combination with arginine in a
salt form, sulfobutyl
ether Beta-cyclodextrin sodium, or sucrose to the solution to increase the
solubility of a FGF protein.
The arginine in a salt form can be, but is not limited to, arginine, arginine
sulfate, arginine
phosphate, and arginine hydrochloride. In a preferred embodiment, arginine
sulfate is used. In
some embodiments, the final concentration of the arginine in a salt form,
sulfobutyl ether Beta-
cyclodextrin sodium, or sucrose is between 0.01 M to 1 M. In one embodiment,
the final
concentration of the arginine in a salt form, sulfobutyl ether Beta-
cyclodextrin sodium, or sucrose is
0.5 M. In some embodiment, the final concentration of the buffering salts such
as acetate,
succinate, tartrate, phosphate, or a combination thereof is 0.05 M. In a
preferred embodiment, the
FGF protein is a FGF-20 protein, a fragment, a derivative, a variant, a
homolog, or an analog of
FGF-20, or a combination thereof.
The present invention further provides improved production methods for CG53135
proteins
and/or formulations comprising one or more CG53135 proteins. The improved
production methods
17
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
allow for commercial scale production of CG53135 proteins and/or formulations
comprising one or
more CG53135 proteins. The improved production methods also allow for
purifying CG53135
proteins to a high degree of purity. In some embodiments, the purity of the
CG53135 purified by the
improved production methods is at least 97%, at least 98%, at least 99%. In a
preferred
embodiment, the purity of CG53135 purified by the improved production methods
is from 99% up to
100% (including 100%).
The present invention also provides methods of use of the improved
formulations and
compositions of the invention for preventing or treating a disease (e.g.,
alimentary mucositis, IBD,
Irritable Bowel Syndrome, arthritis, stroke, radiation-related diseases) or
one or more symptoms
thereof, and dosage regiments.
For clarity of disclosure, and not by way of limitation, the detailed
description of the invention
is divided into the following subsections:
(i) CG53135
(ii) Methods of Preparing CG53135
(iii) Characterization of CG53135
(iv) Prophylactic and Therapeutic Uses
(v) Dosage Regimens
(vi) Pharmaceutical Compositions and Formulations
5.1 CG53135
The present invention provides for improved formulations comprising one or
more CG53135
proteins and improved production methods. As used herein, the term "CG53135"
refers to a class of
proteins (including peptides and polypeptides) or nucleic acids encoding such
proteins or their
complementary strands, where the proteins comprise an amino acid sequence of
SEQ ID NO:2 (211
amino acids, "FGF-20"), or its fragments, derivatives, variants, homologs, or
analogs.
In one embodiment, a CG53135 protein is a variant of FGF-20. It will be
appreciated by
those skilled in the art that DNA sequence polymorphisms that lead to changes
in the amino acid
sequences of the FGF-20 protein may exist within a population (e.g., the human
population). Such
genetic polymorphism in the FGF-20 gene may exist among individuals within a
population due to
natural allelic variation. Such natural allelic variations can typically
result in 1-5% variance in the
nucleotide sequence of the FGF-20 gene. Any and all such nucleotide variations
and resulting
amino acid polymorphisms in the FGF-20 protein, which are the result of
natural allelic variation of
the FGF-20 protein, are intended to be within the scope of the invention. In
one embodiment, a
CG53135 is CG53135-12 (SEQ ID NOs:21 and 22), which is a single nucleotide
polymorphism
("SNP") of FGF-20 (i.e., 206D->N). (For more detailed description of CG53135-
12, see e.g., U.S.
Patent Application No. 10/702,126, filed November 4, 2003, the disclosure of
which is incorporated
18
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
herein by reference in its entirety.) Other examples of SNPs of FGF-20 are
also described in U.S.
Patent Application No. 10/435,087, the content of which is incorporated herein
by reference.
In another embodiment, CG53135 refers to a nucleic acid molecule encoding a
FGF-20
protein from other species or the protein encoded thereby, and thus has a
nucleotide or amino acid
sequence that differs from the human sequence of FGF-20. Nucleic acid
molecules corresponding
to natural allelic variants and homologues of the FGF-20 cDNAs of the
invention can be isolated
based on their homology to the human FGF-20 nucleic acids disclosed herein
using the human
cDNAs, or a portion thereof, as a hybridization probe according to standard
hybridization techniques
under stringent hybridization conditions.
In another embodiment, CG53135 refers to a fragment of an FGF-20 protein,
including
fragments of variant FGF-20 proteins, mature FGF-20 proteins, and variants of
mature FGF-20
proteins, as well as FGF-20 proteins encoded by allelic variants and single
nucleotide
polymorphisms of FGF-20 nucleic acids. An example of an FGF-20 protein
fragment includes, but is
not limited to, residues 2-211, 3-211, 9-211, 12-211, 15-211, 24-211, 54-211,
or 55-211 of FGF-20
(SEQ ID NO:2). In one embodiment, CG53135 refers to a nucleic acid encodes a
protein fragment
that includes residues 2-211, 3-211, 9-211, 12-211, 15-211, 24-211, 54-211, or
55-211 of SEQ ID
NO:2.
The invention also encompasses derivatives and analogs of FGF-20. The
production and
use of derivatives and analogs related to FGF-20 are within the scope of the
present invention.
In a specific embodiment, the derivative or analog is functionally active,
i.e., capable of
exhibiting one or more functional activities associated with a full-length,
wild-type FGF-20.
Derivatives or analogs of FGF-20 can be tested for the desired activity by
procedures known in the
art, including but not limited to, using appropriate cell lines, animal
models, and clinical trials.
In particular, FGF-20 derivatives can be made via altering FGF-20 sequences by
substitutions, insertions or deletions that provide for functionally
equivalent molecules. In one
embodiment, such alteration of an FGF-20 sequence is done in a region that is
not conserved in the
FGF protein family. Due to the degeneracy of nucleotide coding sequences,
other DNA sequences
which encode substantially the same amino acid sequence as FGF-20 may be used
in the practice
of the present invention. These include, but are not limited to, nucleic acid
sequences comprising all
or portions of FGF-20 which are altered by the substitution of different
codons that encode a
functionally equivalent amino acid residue within the sequence, thus producing
a silent change. In a
preferred embodiment, a wild-type FGF-20 nucleic acid sequence is codon
optimized to the nucleic
acid sequence of SEQ ID NO:8 (CG53135-05). Likewise, the FGF-20 derivatives of
the invention
include, but are not limited to, those containing, as a primary amino acid
sequence, all or part of the
amino acid sequence of FGF-20 including altered sequences in which
functionally equivalent amino
acid residues are substituted for residues within the sequence resulting in a
silent change. For
example, one or more amino acid residues within the sequence can be
substituted by another amino
acid of a similar polarity which acts as a functional equivalent, resulting in
a silent alteration.
19
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Substitutes for an amino acid within the sequence may be selected from other
members of the class
to which the amino acid belongs. For example, the nonpolar (hydrophobic) amino
acids include
alanine, leucine, isoleucine, valine, proline, phenylalanine, tryptophan and
methionine. The polar
neutral amino acids include glycine, serine, threonine, cysteine, tyrosine,
asparagine, and glutamine.
The positively charged (basic) amino acids include arginine, lysine and
histidine. The negatively
charged (acidic) amino acids include aspartic acid and glutamic acid. FGF-20
derivatives of the
invention also include, but are not limited to, those containing, as a primary
amino acid sequence, all
or part of the amino acid sequence of FGF-20 including altered sequences in
which amino acid
residues are substituted for residues with similar chemical properties. In a
specific embodiment, 1,
2, 3, 4, or 5 amino acids are substituted.
Derivatives or analogs of FGF-20 include, but are not limited to, those
proteins which are
substantially homologous to FGF-20 or fragments thereof, or whose encoding
nucleic acid is
capable of hybridizing to the FGF-20 nucleic acid sequence.
The FGF-20 derivatives and analogs of the invention can be produced by various
methods
known in the art. The manipulations which result in their production can occur
at the gene or protein
level. For example, the cloned FGF-20 gene sequence can be modified by any of
numerous
strategies known in the art (e.g., Maniatis, T., 1989, Molecular Cloning, A
Laboratory Manual, 2d
ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.). The sequence
can be cleaved at
appropriate sites with restriction endonuclease(s), followed by further
enzymatic modification if
desired, isolated, and ligated in vitro. In the production of the gene
encoding a derivative or analog
of FGF-20, care should be taken to ensure that the modified gene remains
within the same
translational reading frame as FGF-20, uninterrupted by translational stop
signals, in the gene
region where the desired FGF-20 activity is encoded.
Additionally, the FGF-20-encoding nucleic acid sequence can be mutated in
vitro or in vivo,
to create and/or destroy translation, initiation, and/or termination
sequences, or to create variations
in coding regions and/or form new restriction endonuclease sites or destroy
preexisting ones, to
facilitate further in vitro modification. Any technique for mutagenesis known
in the art can be used,
including but not limited to, in vitro site-directed mutagenesis (Hutchinson,
C. et aL, 1978, J. Biol.
Chem 253:6551), use of TAB® linkers (Pharmacia), etc.
Manipulations of the FGF-20 sequence may also be made at the protein level.
Included
within the scope of the invention are FGF-20 fragments or other derivatives or
analogs which are
differentially modified during or after translation, e.g., by glycosylation,
acetylation, phosphorylation,
amidation, derivatization by known protecting/blocking groups, proteolytic
cleavage, linkage to an
antibody molecule or other cellular ligand, etc. Any of numerous chemical
modifications may be
carried out by known techniques, including but not limited to, reagents useful
for protection or
modification of free NH2- groups, free COOH- groups, OH- groups, side groups
of Trp-, Tyr-, Phe-,
His-, Arg-, or Lys-; specific chemical cleavage by cyanogen bromide,
hydroxylamine, BNPS-Skatole,
acid, or alkali hydrolysis; enzymatic cleavage by trypsin, chymotrypsin,
papain, V8 protease,
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
NaBH4; acetylation, formylation, oxidation, reduction; metabolic synthesis in
the presence of
tunicamycin; etc.
In addition, analogs and derivatives of FGF-20 can be chemically synthesized.
For
example, a protein corresponding to a portion of FGF-20 which comprises the
desired domain, or
which mediates the desired aggregation activity in vitro, or binding to a
receptor, can be synthesized
by use of a peptide synthesizer. Furthermore, if desired, nonclassical amino
acids or chemical
amino acid analogs can be introduced as a substitution or addition into the
FGF-20 sequence. Non-
classical amino acids include, but are not limited to, the D-isomers of the
common amino acids, a-
amino isobutyric acid, 4-aminobutyric acid, hydroxyproline, sarcosine,
citrulline, cysteic acid, t-
butylglycine, t-butylalanine, phenylglycine, cyclohexylalanine, R-alanine,
designer amino acids such
as (3-methyl amino acids, Ca-methyl amino acids, and Na-methyl amino acids.
In a specific embodiment, the FGF-20 derivative is a chimeric or fusion
protein comprising
FGF-20 or a fragment thereof fused via a peptide bond at its amino- and/or
carboxy-terminus to a
non-FGF-20 amino acid sequence. In one embodiment, the non-FGF-20 amino acid
sequence is
fused at the amino-terminus of an FGF-20 or a fragment thereof. In another
embodiment, such a
chimeric protein is produced by recombinant expression of a nucleic acid
encoding the protein
(comprising an FGF-20-coding sequence joined in-frame to a non-FGF-20 coding
sequence). Such
a chimeric product can be custom made by a variety of companies (e.g.,
Retrogen, Operon, etc.) or
made by ligating the appropriate nucleic acid sequences encoding the desired
amino acid
sequences to each other by methods known in the art, in the proper coding
frame, and expressing
the chimeric product by methods commonly known in the art. Alternatively, such
a chimeric product
may be made by protein synthetic techniques, e.g., by use of a peptide
synthesizer. In a specific
embodiment, a chimeric nucleic acid encoding FGF-20 with a heterologous signal
sequence is
expressed such that the chimeric protein is expressed and processed by the
cell to the mature FGF-
20 protein. The primary sequence of FGF-20 and non-FGF-20 gene may also be
used to predict
tertiary structure of the molecules using computer simulation (Hopp and Woods,
1981, Proc. Nati.
Acad. Sci. U.S.A. 78:3824-3828); the chimeric recombinant genes could be
designed in light of
correlations between tertiary structure and biological function. Likewise,
chimeric genes comprising
an essential portion of FGF-20 molecule fused to a heterologous (non-FGF-20)
protein-encoding
sequence may be constructed. In a specific embodiment, such chimeric
construction can be used to
enhance one or more desired properties of an FGF-20, including but not limited
to, FGF-20 stability,
solubility, or resistance to proteases. In another embodiment, chimeric
construction can be used to
target FGF-20 to a specific site. In yet another embodiment, chimeric
construction can be used to
identify or purify an FGF-20 of the invention, such as a His-tag, a FLAG tag,
a green fluorescence
protein (GFP), 0-galactosidase, a maltose binding protein (MaIE), a cellulose
binding protein (CenA)
or a mannose protein, etc. In one embodiment, a CG53135 protein is
carbamylated.
In some embodiment, a CG53135 protein can be modified so that it has improved
solubility
and/or an extended half-life in vivo using any methods known in the art. For
example, Fc fragment
of human IgG, or inert polymer molecules such as high molecular weight
polyethyleneglycol (PEG)
21
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
can be attached to a CG53135 protein with or without a multifunctional linker
either through site-
specific conjugation of the PEG to the N- or C-terminus of the protein or via
epsilon-amino groups
present on lysine residues. Linear or branched polymer derivatization that
results in minimal loss of
biological activity will be used. The degree of conjugation can be closely
monitored by SDS-PAGE
and mass spectrometry to ensure proper conjugation of PEG molecules to the
CG53135 protein.
Unreacted PEG can be separated from CG53135-PEG conjugates by size-exclusion
or by ion-
exchange chromatography. PEG-derivatized conjugates can be tested for in vivo
efficacy using
methods known to those of skill in the art.
A CG53135 protein can also be conjugated to albumin in order to make the
protein more
stable in vivo or have a longer half life in vivo. The techniques are well
known in the art, see e.g.,
International Publication Nos. WO 93/15199, WO 93/15200, and WO 01/77137; and
European
Patent No. EP 413, 622, all of which are incorporated herein by reference.
In some embodiments, CG53135 refers to CG53135-01 (SEQ ID NOs:1 and 2),
CG53135-
02 (SEQ ID NOs:3 and 4), CG53135-03 (SEQ ID NOs:5 and 2), CG53135-04 (SEQ ID
NOs:6 and
7), CG53135-05 (SEQ ID NOs:8 and 2), CG53135-06 (SEQ ID NOs:9 and 10), CG53135-
07 (SEQ
ID NOs:11 and 12), CG53135-08 (SEQ ID NOs:13 and 14), CG53135-09 (SEQ ID
NOs:15 and 16),
CG53135-10 (SEQ ID NOs:17 and 18), CG53135-11 (SEQ ID NOs:19 and 20), CG53135-
12 (SEQ
ID NOs:21 and 22), CG53135-13 (SEQ ID NOs:23 and 24), CG53135-14 (SEQ ID
NOs:25 and 26),
CG53135-15 (SEQ ID NOs:27 and 28), CG53135-16 (SEQ ID NOs:29 and 30), CG53135-
17 (SEQ
ID NOs:31 and 32), IFC 250059629 (SEQ ID NOs:33 and 34), IFC 20059669 (SEQ ID
NOs:35 and
36), IFC 317459553 (SEQ ID NOs:37 and 38), IFC 317459571 (SEQ ID NOs:39 and
40), IFC
250059596 (SEQ ID NOs:41 and 10), IFC316351224 (SEQ ID NOs:41 and 10), or a
combination
thereof. In a specific embodiment, a CG53135 is carbamylated, for example, a
carbamylated
CG53135-13 protein or a carbamylated CG53135-05 protein.
Examples of prophylactic and/or therapeutic uses of CG53135 have been
described in
previously filed patent applications (see e.g., U.S. Patent Application Nos.
09/992,840, 10/011,364,
10/321,962, 10/435,087, 10/842,206, 10/842,179, and U.S. Patent No.
6,797,695). The disclosure
of each reference is incorporated by reference herein in its entirety.
5.2 METHODS OF PREPARING CG53135
The present invention provides for formulations comprising one or more
isolated CG53135
proteins and improved methods of production. In accordance with the methods
described herein,
CG53135 proteins employed in a formulation of the invention or produced by the
production
methods of the invention can have a purity in the range of 80 to 100 percent,
or at least 80%, at
least 85%, at least 90%, at least 95%, or at least 98%. In one embodiment, one
or more CG53135
proteins employed in a formulation of the invention or produced by the
production methods of the
invention have a purity of at least 99%. In another embodiment, CG53135 is
purified to apparent
homogeneity, as assayed, e.g., by sodium dodecyl sulfate polyacrylamide gel
electrophoresis. Any
techniques known in the art can be used in purifying a CG53135 protein,
including but are not
22
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
limited to, separation by precipitation, separation by adsorption (e.g.,
column chromatography,
membrane adsorbents, radial flow columns, batch adsorption, high-performance
liquid
chromatography, ion exchange chromatography, inorganic adsorbents, hydrophobic
adsorbents,
immobilized metal affinity chromatography, affinity chromatography), or
separation in solution (e.g.,
gel filtration, electrophoresis, liquid phase partitioning, detergent
partitioning, organic solvent
extraction, and ultrafiltration). See e.g., Scopes, PROTEIN PURIFICATION,
PRINCIPLES AND
PRACTICE, 3rd ed., Springer (1994). During the purification, the biological
activity of CG53135 may
be monitored by one or more in vitro or in vivo assays as described in Section
5.3, infra. The purity
of CG53135 can be assayed by any methods known in the art, such as but not
limited to, gel
electrophoresis. See Scopes, supra.
Methods known in the art can be utilized to recombinantly produce CG53135
proteins. A
nucleic acid sequence encoding a CG53135 protein can be inserted into an
expression vector for
propagation and expression in host cells.
An expression construct, as used herein, refers to a nucleic acid sequence
encoding a
CG53135 protein operably associated with one or more regulatory regions which
enable expression
of a CG53135 protein in an appropriate host cell. "Operably-associated" refers
to an association in
which the regulatory regions and the CG53135 sequence to be expressed are
joined and positioned
in such a way as to permit transcription, and ultimately, translation.
The regulatory regions necessary for transcription of CG53135 can be provided
by the
expression vector. A translation initiation codon (ATG) may also be provided
if a CG53135 gene
sequence lacking its cognate initiation codon is to be expressed. In a
compatible host-construct
system, cellular transcriptional factors, such as RNA polymerase, will bind to
the regulatory regions
on the expression construct to effect transcription of the modified CG53135
sequence in the host
organism. The precise nature of the regulatory regions needed for gene
expression may vary from
host cell to host cell. Generally, a promoter is required which is capable of
binding RNA polymerase
and promoting the transcription of an operably-associated nucleic acid
sequence. Such regulatory
regions may include those 5' non-coding sequences involved with initiation of
transcription and
translation, such as the TATA box, capping sequence, CAAT sequence, and the
like. The non-
coding region 3' to the coding sequence may contain transcriptional
termination regulatory
sequences, such as terminators and polyadenylation sites.
In order to attach DNA sequences with regulatory functions, such as promoters,
to a
CG53135 gene sequence or to insert a CG53135 gene sequence into the cloning
site of a vector,
linkers or adapters providing the appropriate compatible restriction sites may
be ligated to the ends
of the cDNAs by techniques well known in the art (see e.g., Wu et al., 1987,
Methods in Enzymol,
152:343-349). Cleavage with a restriction enzyme can be followed by
modification to create blunt
ends by digesting back or filling in single-stranded DNA termini before
ligation. Alternatively, a
desired restriction enzyme site can be introduced into a fragment of DNA by
amplification of the
DNA using PCR with primers containing the desired restriction enzyme site.
23
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
An expression construct comprising a CG53135 sequence operably associated with
regulatory regions can be directly introduced into appropriate host cells for
expression and
production of a CG53135 protein without further cloning. See, e.g., U.S.
Patent No. 5,580,859. The
expression constructs can also contain DNA sequences that facilitate
integration of a CG53135
sequence into the genome of the host cell, e.g., via homologous recombination.
In this instance, it is
not necessary to employ an expression vector comprising a replication origin
suitable for appropriate
host cells in order to propagate and express CG53135 in the host cells.
A variety of expression vectors may be used, including but are not limited to,
plasmids,
cosmids, phage, phagemids or modified viruses. Such host-expression systems
represent vehicles
by which the coding sequences of a CG53135 gene may be produced and
subsequently purified,
but also represent cells which may, when transformed or transfected with the
appropriate nucleotide
coding sequences, express CG53135 in situ. These include, but are not limited
to, microorganisms
such as bacteria (e.g., E.coli and B. subtilis) transformed with recombinant
bacteriophage DNA,
plasmid DNA or cosmid DNA expression vectors containing CG53135 coding
sequences; yeast
(e.g., Saccharomyces, Pichia) transformed with recombinant yeast expression
vectors containing
CG53135 coding sequences; insect cell systems infected with recombinant virus
expression vectors
(e.g., baculovirus) containing CG53135 coding sequences; plant cell systems
infected with
recombinant virus expression vectors (e.g., cauliflower mosaic virus, CaMV;
tobacco mosaic virus,
TMV) or transformed with recombinant plasmid expression vectors (e.g., Ti
plasmid) containing
CG53135 coding sequences; or mammalian cell systems (e.g., COS, CHO, BHK, 293,
NSO, and
3T3 cells) harboring recombinant expression constructs containing promoters
derived from the
genome of mammalian cells (e.g., metallothionein promoter) or from mammalian
viruses (e.g., the
adenovirus late promoter; the vaccinia virus 7.5K promoter). Preferably,
bacterial cells such as
Escherichia coli and eukaryotic cells are used for the expression of a
recombinant CG531 35
molecule. For example, mammalian cells such as Chinese hamster ovary cells
(CHO) can be used
with a vector bearing promoter element from major intermediate early gene of
cytomegalovirus for
effective expression of a CG53135 sequence (Foecking et al., 1986, Gene
45:101; and Cockett et
a/., 1990, Bio/Technology 8:2).
In bacterial systems, a number of expression vectors may be advantageously
selected
depending upon the use intended for the CG53135 molecule being expressed. For
example, when
a large quantity of a CG531 35 is to be produced, for the generation of
pharmaceutical compositions
of a CG53135 molecule, vectors which direct the expression of high levels of
fusion protein products
that are readily purified may be desirable. Such vectors include, but are not
limited to, the E.coli
expression vector pCR2.1 TOPO (Invitrogen); pIN vectors (Inouye & Inouye,
1985, Nucleic Acids
Res. 13:3101-3109; Van Heeke & Schuster, 1989, J. Biol. Chem. 24:5503-5509)
and the like.
Series of vectors like pFLAG (Sigma), pMAL (NEB), and pET (Novagen) may also
be used to
express the foreign proteins as fusion proteins with FLAG peptide, malE-, or
CBD- protein. These
recombinant proteins may be directed into periplasmic space for correct
folding and maturation. The
fused part can be used for affinity purification of the expressed protein.
Presence of cleavage sites
24
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
for specific protease like enterokinase allows to cleave off the CG53135
protein. The pGEX vectors
may also be used to express foreign proteins as fusion proteins with
glutathione 5-transferase
(GST). In general, such fusion proteins are soluble and can easily be purified
from lysed cells by
adsorption and binding to matrix glutathione agarose beads followed by elution
in the presence of
free glutathione. The pGEX vectors are designed to include thrombin or factor
Xa protease
cleavage sites so that the cloned target gene product can be released from the
GST moiety.
In an insect system, many vectors to express foreign genes can be used, e.g.,
Autographa
californica nuclear polyhedrosis virus (AcNPV) can be used as a vector to
express foreign genes.
The virus grows in cells like Spodoptera frugiperda cells. A CG53135 coding
sequence may be
cloned individually into non-essential regions (e.g., the polyhedrin gene) of
the virus and placed
under control of an AcNPV promoter (e.g., the polyhedrin promoter).
In mammalian host cells, a number of viral-based expression systems may be
utilized. In
cases where an adenovirus is used as an expression vector, a CG53135 coding
sequence of
interest may be ligated to an adenovirus transcription/translation control
complex, e.g., the late
promoter and tripartite leader sequence. This chimeric gene may then be
inserted in the adenovirus
genome by in vitro or in vivo recombination. Insertion in a non-essential
region of the viral genome
(e.g., region El or E3) will result in a recombinant virus that is viable and
capable of expressing
CG53135 in infected hosts (see, e.g., Logan & Shenk, 1984, Proc. Natl. Acad.
Sci. USA 8 1:355-
359). Specific initiation signals may also be required for efficient
translation of inserted CG53135
coding sequences. These signals include the ATG initiation codon and adjacent
sequences.
Furthermore, the initiation codon must be in phase with the reading frame of
the desired coding
sequence to ensure translation of the entire insert. These exogenous
translational control signals
and initiation codons can be of a variety of origins, both natural and
synthetic. The efficiency of
expression may be enhanced by the inclusion of appropriate transcription
enhancer elements,
transcription terminators, etc. (see, e.g., Bittner et al., 1987, Methods in
Enzymol. 153:51-544).
In addition, a host cell strain may be chosen which modulates the expression
of the inserted
sequences, or modifies and processes the gene product in the specific fashion
desired. Such
modifications (e.g., glycosylation) and processing (e.g., cleavage) of protein
products may be
important for the function of the protein. Different host cells have
characteristic and specific
mechanisms for the post-translational processing and modification of proteins
and gene products.
Appropriate cell lines or host systems can be chosen to ensure the correct
modification and
processing of the foreign protein expressed. To this end, eukaryotic host
cells which possess the
cellular machinery for proper processing of the primary transcript and post-
translational modification
of the gene product, e.g., glycosylation and phosphorylation of the gene
product, may be used.
Such mammalian host cells include, but are not limited to, PC12, CHO, VERY,
BHK, HeLa, COS,
MDCK, 293, 3T3, W138, BT483, Hs578T, HTB2, BT2O and T47D, NSO (a murine
myeloma cell line
that does not endogenously produce any immunoglobulin chains), CRL7O3O and
HsS78Bst cells.
Expression in a bacterial or yeast system can be used if post-translational
modifications turn to be
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
non-essential for a desired activity of CG53135. In a preferred embodiment, E.
coli is used to
express a CG53135 sequence.
For long term, high yield production of properly processed CG53135, stable
expression in
cells is preferred. Cell lines that stably express CG53135 may be engineered
by using a vector that
contains a selectable marker. By way of example but not limitation, following
the introduction of the
expression constructs, engineered cells may be allowed to grow for 1-2 days in
an enriched media,
and then are switched to a selective media. The selectable marker in the
expression construct
confers resistance to the selection and optimally allows cells to stably
integrate the expression
construct into their chromosomes and to grow in culture and to be expanded
into cell lines. Such
cells can be cultured for a long period of time while CG53135 is expressed
continuously.
A number of selection systems may be used, including but not limited to,
antibiotic
resistance (markers like Neo, which confers resistance to geneticine, or G-418
(Wu and Wu, 1991,
Biotherapy 3:87-95; Tolstoshev, 1993, Ann. Rev. Pharmacol. Toxicol. 32:573-
596; Mulligan, 1993,
Science 260:926-932; and Morgan and Anderson, 1993, Ann. Rev. Biochem. 62: 191-
217; May,
1993, TIB TECH 11(5):155-2 15); Zeo, for resistance to Zeocin; Bsd, for
resistance to blasticidin,
etc.); antimetabolite resistance (markers like Dhfr, which confers resistance
to methotrexate, Wigler
et al., 1980, Nati. Acad. Sci. USA 77:357; O'Hare et al., 1981, Proc. Natl.
Acad. Sci. USA 78:1527);
gpt, which confers resistance to mycophenolic acid (Mulligan & Berg, 1981,
Proc. Nati. Acad. Sci.
USA 78:2072); and hygro, which confers resistance to hygromycin (Santerre et
al., 1984, Gene
30:147). In addition, mutant cell lines including, but not limited to, tk-,
hgprt- or aprt- cells, can be
used in combination with vectors bearing the corresponding genes for thymidine
kinase,
hypoxanthine, guanine- or adenine phosphoribosyltransferase. Methods commonly
known in the art
of recombinant DNA technology may be routinely applied to select the desired
recombinant clone,
and such methods are described, for example, in Ausubel et al. (eds.), Current
Protocols in
Molecular Biology, John Wiley & Sons, NY (1993); Kriegier, Gene Transfer and
Expression, A
Laboratory Manual, Stockton Press, NY (1990); and in Chapters 12 and 13,
Dracopoli et al. (eds),
Current Protocols in Human Genetics, John Wiley & Sons, NY (1994); Colberre-
Garapin et al., 1981,
J. Mol. Biol. 150:1.
The recombinant cells may be cultured under standard conditions of
temperature,
incubation time, optical density and media composition. However, conditions
for growth of
recombinant cells may be different from those for expression of CG53135.
Modified culture
conditions and media may also be used to enhance production of CG53135. Any
techniques known
in the art may be applied to establish the optimal conditions for producing
CG53135.
An alternative to producing CG53135 or a fragment thereof by recombinant
techniques is
peptide synthesis. For example, an entire CG53135, or a protein corresponding
to a portion of
CG53135, can be synthesized by use of a peptide synthesizer. Conventional
peptide synthesis or
other synthetic protocols well known in the art may be used.
26
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Proteins having the amino acid sequence of CG53135 or a portion thereof may be
synthesized by solid-phase peptide synthesis using procedures similar to those
described by
Merrifield, 1963, J. Am. Chem. Soc., 85:2149. During synthesis, N-a-protected
amino acids having
protected side chains are added stepwise to a growing polypeptide chain linked
by its C-terminal
and to an insoluble polymeric support, i.e., polystyrene beads. The proteins
are synthesized by
linking an amino group of an N-a-deprotected amino acid to an a-carboxyl group
of an N-a-protected
amino acid that has been activated by reacting it with a reagent such as
dicyclohexylcarbodiimide.
The attachment of a free amino group to the activated carboxyl leads to
peptide bond formation.
The most commonly used N-a-protecting groups include Boc which is acid labile
and Fmoc which is
base labile. Details of appropriate chemistries, resins, protecting groups,
protected amino acids and
reagents are well known in the art and so are not discussed in detail herein
(See, Atherton et al.,
1989, Solid Phase Peptide Synthesis: A Practical Approach, IRL Press, and
Bodanszky, 1993,
Peptide Chemistry, A Practical Textbook, 2nd Ed., Springer-Verlag).
Purification of the resulting CG53135 is accomplished using conventional
procedures, such
as preparative HPLC using gel permeation, partition and/or ion exchange
chromatography. The
choice of appropriate matrices and buffers are well known in the art and so
are not described in
detail herein.
5.2.1 Improved Production Methods
The present invention provides improved manufacturing processes for producing
compositions comprising one or more CG53135 proteins. The improved
manufacturing processes
provide benefits such as more stable and more pure drug product, and are also
suitable for
commercial scale production of a composition comprising one or more CG53135
proteins.
The present invention provides methods of isolating a protein, where the
methods
comprises the steps of: (1) fermenting a host cell, such as E. coli, that
containing a vector, where the
vector comprises a nucleotide sequence encoding a CG53135 protein. In a
preferred embodiment,
the vector comprises a codon-optimized, full length CG53135-05 (SEQ ID NO:8);
(2) lysing the
cultured cells. Cells may be lysed by any methods known in the art. In one
embodiment, cells are
lysed by homogenization. In another embodiment, the fermented cultured cells
are chilled, and
diluted with cell lysis buffer comprising 50-100 mM sodium phosphate, 60 mM
EDTA, 7.5 mM DTT,
3.5-5 M urea, pH 7.2, and then lysed by, e.g., homogenization. In a preferred
embodiment,
polyethyleneimine ("PEI") is added to the fermentation broth before
homogenization; (3) purification
by a cation exchange column. In a preferred embodiment, a pre-equilibrated
expanded bed cation
exchanger, such as STREAMLINE SPT"' is used. In one embodiment, after the
cation exchange
column is loaded with the protein to be isolated, the column is flushed with
additional equilibration
buffer comprising 50-100 mM sodium phosphate, 40 mM EDTA, 10 mM sodium
sulfate, 3-5 M urea,
pH 7Ø The column may be further washed with a buffer comprising 50-100 mM
sodium phosphate,
mM EDTA, 10-25 mM sodium sulfate, 2.22 M dextrose, pH 7Ø The elution buffer
to elute the
protein from the cation exchange column comprises, e.g., 50-100 mM sodium
phosphate, 5 mM
27
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
EDTA, 150-250 mM sodium sulfate, 0.5-1 M L-arginine, pH 7.0; and (4) further
purification by using
a hydrophobic interaction chromatography column (e.g., PPG 650M). In one
embodiment, the
hydrophobic interaction chromatography column, e.g., PPG 650M, is equilibrated
and washed with
50-100 mM sodium phosphate, 150-250 mM sodium sulfate, 5 mM EDTA, 1 M
arginine, pH 7Ø In
another embodiment, the column is further washed with 100-250 mM sodium
phosphate, 5 mM
EDTA, 0.8-1 M arginine, pH 7Ø In another embodiment, the protein is eluted
with 50-100 mM
sodium phosphate, 5 mM EDTA, and 0.1-0.3 M arginine, pH 7Ø
In a preferred embodiment, the eluted protein from step (4) described above
may be further
purified by either one or both of the following steps: (5) further
purification by filtering the eluted
protein. In a preferred embodiment, a charged endotoxin binding filter (e.g.,
CUNOTM 30 ZA depth
filter) is used. In one embodiment, the filter is first flushed with water for
injection, and then with 50-
100 mM sodium phosphate, 5 mM EDTA, 0.1-03 M arginine, pH 7.0; and (6) further
purification by
using a hydrophobic interaction chromatography column (e.g., Phenyl Sepharose
HP
Chromatography). In one embodiment, the column is equilibrated and washed with
50-100 mM
sodium phosphate, 10-100 mM ammonium sulfate, 800-1000 mM sodium chloride, 0.5-
1 M arginine,
pH 7Ø In another embodiment, the protein is eluted with 50-100 mM sodium
phosphate, 0.5-1 M
arginine, pH 7Ø
A protein isolated by the methods of the present invention may be further
concentrated and
filtered to produce a drug product. Pharmaceutical carriers may be added to
produce a desired
formulation, such as formulations provided by the present invention.
5.3 CHARACTERIZATION OF CG53135
The characteristics of the protein(s) purified by the production methods of
the instant
invention or the immediate products of the production methods of the instant
invention (e.g., purity,
various characters including the biological activity of CG53135) may be
determined by methods
known in the art. Compositions for use in therapy can be tested in suitable
animal model systems
prior to testing in humans, including but not limited to in rats, mice,
chicken, cows, monkeys, rabbits,
etc (examples of such tests can be found in U.S. Patent Application Nos.
09/992,840, 10/011,364,
10/321,962, 10/435,087, 10/842,206, and 10/842,179, the disclosure of each is
incorporated herein
by reference).
For examples, methods known in the art, such as but not limited to, sodium
dodecyl
sulphate polyacrylamide gel electrophoresis ("SDS-PAGE"), reversed phase high-
performance liquid
chromatography ("RP-HPLC"), size exclusion high-performance liquid
chromatography ("SEC-
HPLC"), Western Blot (e.g., host cell protein Western Blot), can be used to
analyze the purity of the
product of the manufacturing processes of the instant invention. In a
preferred embodiment, a
product of the manufacturing processes of the instant invention is at least
97%, at least 98%, or at
least 99% pure by densitometry. In another preferred embodiment, a product of
the manufacturing
processes of the instant invention is more than 97%, more than 98%, or more
than 99% pure by
densitometry.
28
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Methods known in the art, such as but not limited to, Western Blot, sequencing
(e.g., N-
terminal Edman sequencing), liquid chromatography (e.g., HPLC, RP-HPLC with
both UV and
electrospray mass spectrometric detection), mass spectrometry, total amino
acid analysis, peptide
mapping, SDS-PAGE, can be used to determine the identity of the product of the
manufacturing
processes of the instant invention. The secondary, tertiary and/or quaternary
structure of a product
of the manufacturing processes of the instant invention can analyzed by any
method know in the art,
for example, far UV circular dichroism spectrum can be used to analyze the
secondary structure,
near UV circular dichroism spectroscopy and second derivative UV absorbance
spectroscopy can
be used to analyze the tertiary structure, and light scattering SEC-HPLC can
be used to analyze
quaternary structure.
Potency of a product of the manufacturing processes of the instant invention
can be
measured by methods known in the art or any bioassays that measuring one or
more biological
activities of a CG53135 protein. In one embodiment, potency of a product of
the manufacturing
processes of the instant invention is measured by the ability of the product
to stimulate cell growth of
NIH 3T3 cells (for an example of such assay, see Section 6.5).
Other characters of a product of the manufacturing processes of the instant
invention, such
as safety (e.g., residual DNA, endotoxin, bioburden), pH, osmolarity,
suifyhydryl content, can also be
analyzed by any method known in the art (for examples of some of these
methods, see Section 6).
Such methods are well known in the art and are not described in detail herein.
In one embodiment, a CG53135 protein reference standard, which is
representative of the
bulk drug substance from the improved manufacturing process, is prepared and
characterized.
Preferably, this reference standard is characterized for its purity, identity
(e.g., molecular weight,
amino acid sequence), potency, structure (e.g., secondary, tertiary and
quaternary structures),
safety (e.g., endotoxin, bioburden), and other characters, such as but not
limited to, pH, osmolality,
and suifyhydryl content. Upon visual inspection, a CG53245 protein reference
standard should be
clear and colorless. Once a CG53135 protein reference is established, it can
be used for, e.g.,
quality control of the manufacturing process, and as a positive control for
other assays related to
CG53135.
5.4 PROPHYLACTIC AND THERAPEUTIC USES
The present invention provides methods of preventing and/or treating a disease
(e.g.,
alimentary mucositis, IBD, Irritable Bowel Syndrome, arthritis, stroke,
radiation-related diseases) or
one or more symptoms thereof comprising administering to a subject in need
thereof an effective
amount of a composition comprising one or more isolated CG53135 proteins.
5.4.1. Alimentary Mucositis
In one embodiment, the present invention provides methods of preventing and/or
treating
alimentary mucositis. Alimentary mucositis that can be prevented and/or
treated by the methods of
the invention includes, but is not limited to, oral mucositis, esophagitis,
stomatitis, enteritis, and
29
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
proctitis. In some embodiments, the methods of the invention comprise
administering an effective
amount of a composition comprising one or more isolated CG53135 proteins to a
subject with
mucositis at more than one area in the alimentary canal (e.g., a subject with
both oral mucositis and
enteritis). In some embodiments, the methods of the invention comprise
administering an effective
amount of a composition comprising one or more isolated CG53135 proteins to a
subject with
mucositis at only one area in the alimentary canal (e.g., a subject with only
oral mucositis, or a
subject with only enteritis). In a preferred embodiment, the alimentary
mucositis that can be
prevented and/or treated by the methods of the invention is oral mucositis. In
some embodiments,
the alimentary mucositis that can be prevented and/or treated by the methods
of the invention is not
an oral mucositis. Alimentary mucositis may be induced by, e.g., chemical
insult, radiation insult,
biological insult (e.g., bacteria), or a combination thereof.
In some embodiments, the present invention provides methods of preventing
and/or treating
alimentary mucositis in patient populations with alimentary mucositis and
populations at risk to
develop alimentary mucositis. In one embodiment, the present invention
provides methods of
preventing and/or treating alimentary mucositis in a subject who has been
treated with radiation
therapy and/or chemotherapy. In another embodiment, the present invention
provides methods of
preventing alimentary mucositis by administering a composition comprising one
or more CG53135
proteins to a subject who is going to be treated with radiation therapy and/or
chemotherapy. In a
specific embodiment, the present invention provides methods of preventing
and/or treating
alimentary mucositis in a subject who has been treated with conditioning
myeloablative radiation
therapy and /or chemotherapy in preparation for autologous or allogenic
hematopoietic stem cell
transplant. In another specific embodiment, the present invention provides
methods of preventing
and/or treating alimentary mucositis in a subject who has received or is
receiving mucosatoxic
chemotherapy with mucositis-inducing agents (e.g., leukemia patients treated
with cytarabine). In
yet another specific embodiment, the present invention provides methods of
preventing and/or
treating alimentary mucositis in a subject who has head and/or neck cancer
treated with radiation
therapy with or without adjuvant chemotherapy.
In one embodiment, the present invention provides a method of preventing
alimentary
mucositis comprising administering a composition comprising one or more
CG53135 proteins prior
to an insult (e.g., a chemical insult, a radiation insult, a biological
insult, or a combination thereof)
that may induce alimentary mucositis occurs to a subject. In another
embodiment, the present
invention provides a method of preventing alimentary mucositis comprising
administering a
composition comprising one or more CG53135 proteins after an insult (e.g., a
chemical insult, a
radiation insult, a biological insult, or a combination thereof) that may
induce alimentary mucositis
occurs to a subject, but prior to the development of alimentary mucositis in
the subject. In yet
another embodiment, the present invention provides a method of treating
alimentary mucositis
comprising administering a composition comprising one or more CG53135 proteins
after alimentary
mucositis developed in a subject.
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In some embodiments, the present invention provides a method of preventing
and/or
treating alimentary mucositis comprising cyclically administering a
composition comprising one or
more CG53135 proteins. In one embodiment, cycling therapy involves the
administration of a first
therapy for a period of time, followed by the administration of a second
therapy for a period of time
and repeating this sequential administration, f.e., the cycle, in order to,
e.g., to avoid or reduce the
side effects of one of the therapies and/or to improve the efficacy of the
therapies. In another
embodiment, cycling therapy involves the administration of a therapy for a
period of time, stop the
therapy for a period of time, and repeat the administration of the therapy. In
accordance to the
present invention, a composition comprising one or more CG53135 proteins can
be administered to
a subject prior to, during, or after the administration of a radiation therapy
and/or chemotherapy,
where such radiation therapy and/or chemotherapy is a cycling therapy.
In accordance to the instant invention, a composition comprising one or more
isolated
CG53135 proteins can also be used in combination with other therapies to
prevent and/or treat
alimentary mucositis. In one embodiment, a composition comprising one or more
isolated CG53135
proteins is administered in combination with one or more other agents that
have prophylactic and/or
therapeutic effect(s) on alimentary mucositis and/or have amelioration
effect(s) on one or more
symptoms associated with alimentary mucositis to a subject to prevent and/or
treat alimentary
mucositis. Non-limiting examples of such agents are: mucosal protective agents
(e.g., sucralfate,
colloidal bismuth), antibiotics, antifungal agents (e.g., fluconazole,
amphotericin B), antiviral agents
(e.g., acyclovir), antiemetic agents (e.g., phenothiazines, butyrophenones,
benzodiazepines,
corticosteroids, cannabinoids, 5-HT3 serotonin receptor blockers),
antidiarrhea agents (e.g.,
diphenoxylate, loperamide, kaolin, pectin, methylacellulose, activated
attapulgite, magnesium
aluminum silicate, non-steroidal anti-inflammatory agents (NSAIDs)),
transforming growth factor
(TGF), interieukin-11 (IL-11), granulocyte-macrophage colony stimulating
factor (GM-CSF),
keratinocyte growth factor (KGF), L-glutamine, Amifostene, and Granulocyte
colony stimulating
factor (G-CSF). In another embodiment, a composition comprising one or more
isolated CG53135
proteins is administered in combination with one or more other therapies that
have palliative effect
on alimentary mucositis. Non-limiting examples of such therapies are:
application of topical
analgesics such as lidocaine and/or systemic administration of narcotics and
antibiotics, topical
fluoride application with or without calcium phosphate, mechanical plaque
removal, tooth sponges,
sucking ice chips resulting in oral cooling, oral rinses with various anti-
infective agents, oral
mouthwashes with local anesthetics.
5.4.2. Radiation Protection and Stimulatory Effects on Stem Cells
In one embodiment, the present invention provides methods of preventing and/or
treating
one or more disorders associated with (e.g., caused by) radiation exposure,
chemotherapy,
chemical/biological warfare agents, and/or any other insults affecting rapidly
proliferating tissues in
the body, or one or more symptoms thereof by administering to a subject a
prophylactically or
therapeutically effective amount of a composition comprising one or more
isolated CG53135
proteins.
31
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In some embodiments, the present invention provides methods of preventing
and/or treating
a pathology of epithelial cells and/or mesenchymal cells comprising
administering to a subject in
need thereof a composition comprising one or more CG53135 proteins. In another
embodiment, the
present invention provides methods of stimulating proliferation,
differentiation or migration of
epithelial cells and/or mesenchymal cells comprising administering to a
subject in need thereof an
effective amount of a composition comprising one or more CG53135 proteins.
Epithelial membranes are continuous sheets of cells with contiguous cell
borders that have
characteristic specialized sites of close contact called cell junction. Such
membrane, which can be
one or more cells thick, contain no capillaries. Epithelia are attached to the
underlying connective
tissue by a component known as a basement membrane, which is a layer of
intercellular material of
complex composition that is distributed as a thin layer between the epithelium
and the connective
tissue.
Stratified squamous nonkeratinizing epithelium is common on wet surfaces that
are subject
to considerable wear and tear at sites where absorptive function is not
required. The secretions
necessary to keep such surfaces wet have to come from appropriately situated
glands. Sites lined
by this type of epithelium include the esophagus and the floor and sides of
the oral cavity.
Simple columnar epithelium is made up of a single layer of tall cells that
again fit together in
a hexagonal pattern. In simple secretory columnar epithelium, the columnar
cells are all specialized
to secret mucus in addition to being protective. Sites of this type of
epithelium is present include the
lining of the stomach.
A simple columnar epithelium that is made up of absorptive cells as well as
secretory cells
lines the intestine. To facilitate absorption, this membrane is only one cell
thick. Interspersed with
cells that are specialized for absorption, there are many goblet cells that
secrete protective mucus.
Mesenchymal cells are stem cells that can differentiate into, e.g.,
osteoblasts, chondrocytes,
myocytes, and adipocytes. Mesenchymal-epithelial interactions play an
important role in the
physiology and pathology of epithelial tissues. Mesenchymal cells may
associate with epithelium
basement membrane (e.g., pericytes and perivascular monocyte-derived cells
(MDCs)), or reside
within epithelium (MDCs and T cells). The nature of the interactions between
mesenchymal cells
and tissue-specific cells may depend on the tissue type (e.g., brain versus
epidermis), or on the
prevention or allowance/stimulation of differentiation of cells into the
suicidal state (apoptosis) by
mesenchymal cells in a given epithelium. Specialized mesenchymal cells, such
as pericytes, MDCs,
and T lymphocytes, may significantly influence the differentiation and aging
of epithelial cells.
The stromal compartment of the cavities of bone is composed of a net-like
structure of
interconnected mesenchymal cells. Stromal cells are closely associated with
bone cortex, bone
trabecule and to the hemopoietic cells. The bone marrow-stromal micro-
environment, is a complex
of cells, extracellular matrix (ECM) with growth factors and cytokines that
regulate osteogenesis and
hemopoiesis locally throughout the life of the individual. The role of the
marrow stroma in creating
32
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
the microenvironment for bone physiology and hemopoiesis lies in a specific
subpopulation of the
stroma cells. They differentiate from a common stem cell to the specific
lineage each of which has a
different role. Their combined function results in orchestration of a 3-D-
architecture that maintains
the active bone marrow within the bone.
In adults, blood cells are produced by the bone marrow, the spongy material
filling the
body's bones. The bone marrow produces two blood cell groups, myeloid and
lymphoid. The
myeloid cell line includes, e.g., the following: (1) Immature cells called
erythrocytes that later
develop into red blood cells; (2) Blood clotting agents ( platelets); (3) Some
white blood cells,
including macrophages (which act as scavengers for foreign particles),
eosinophils (which trigger
allergies and also defend against parasites), and neutrophils (the main
defenders against bacterial
infections). The lymphoid cell line includes, e.g., the lymphocytes, which are
the body's primary
infection fighters. Among other vital functions, certain lymphocytes are
responsible for producing
antibodies, factors that can target and attack specific foreign agents
(antigens). Lymphocytes
develop in the thymus gland or bone marrow and are therefore categorized as
either B-cells (bone
marrow-derived cells) or T-cells (thymus gland-derived cells).
According to the present invention, CG53135 can regulate proliferation,
differentiation,
and/or migration of epithelial cells and/or mesenchymal cells, and thus have
prophylactic and/or
therapeutic effects on a disorder associated with a pathology of epithelial
cells and/or mesenchymal
cells.
In some embodiments, a composition used in accordance to the methods of the
invention
comprises a FGF-20 protein, a fragment, a derivative, a variant, a homolog, or
an analog of FGF-20,
or a combination thereof. In some embodiments, a composition used in
accordance to the methods
of the invention comprises CG53135-01 (SEQ ID NO:2), CG53135-02 (SEQ ID NO:
4), CG53135-03
(SEQ ID NO:2), CG53135-04 (SEQ ID N0:7), CG53135-05 (SEQ ID NO: 2), CG53135-06
(SEQ ID
NO: 10), CG53135-07 (SEQ ID NO:12), CG53135-08 (SEQ ID NO:14), CG53135-09 (SEQ
ID
NO:16), CG53135-10 (SEQ ID NO:18), CG53135-11 (SEQ ID NO:20), CG53135-12 (SEQ
ID
NO:22), CG53135-13 (SEQ ID NO:24), CG53135-14 (SEQ ID NO:26), CG53135-15 (SEQ
ID
NO:28), CG53135-16 (SEQ ID NO:30), CG53135-17 (SEQ ID NO:32), IFC 250059629
(SEQ ID
NO:34), IFC 20059669 (SEQ ID NO:36), IFC 317459553 (SEQ ID NO:38), IFC
317459571 (SEQ ID
NO:40), IFC 250059596 (SEQ ID N0:10), or IFC316351224 (SEQ ID NO:10), or any
two or more
combinations of CG53135 proteins. In one embodiment, the compositions used in
accordance to
the methods of the present invention comprise (1) a protein comprising an
amino acid sequence of
SEQ ID NO:2, and (2) a protein comprising an amino acid sequence of SEQ ID
NO:24. In another
embodiment, the compositions used in accordance to the methods of the present
invention comprise
(1) a protein comprising an amino acid sequence of SEQ ID NO:2, (2) a protein
comprising an
amino acid sequence of SEQ ID NO:24, (3) a protein comprising an amino acid
sequence of SEQ ID
NO:26, (4) a protein comprising an amino acid sequence of SEQ ID NO:28, (5) a
protein comprising
an amino acid sequence of SEQ ID NO:30, and (6) a protein comprising an amino
acid sequence of
SEQ ID NO:32. In another embodiment, the compositions used in accordance to
the methods of the
33
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
present invention comprise (1) a protein comprising an amino acid sequence of
SEQ ID NO:2, (2) a
protein comprising an amino acid sequence of SEQ ID NO:24, (3) a protein
comprising an amino
acid sequence of SEQ ID NO:28, (4) a protein comprising an amino acid sequence
of SEQ ID
NO:30, and (5) a protein comprising an amino acid sequence of SEQ ID NO:32. In
another
embodiment, the compositions used in accordance to the methods of the present
invention comprise
(1) a protein comprising an amino acid sequence of SEQ ID NO:32; (2) a protein
comprising an
amino acid sequence of SEQ ID NO:30, (3) a protein comprising an amino acid
sequence of SEQ ID
NO:28; and (4) a protein comprising an amino acid sequence of SEQ ID NO:24. In
yet another
embodiment, the compositions used in accordance to the methods of the present
invention comprise
(1) a protein comprising an amino acid sequence of SEQ ID NO:2, (2) a protein
comprising an
amino acid sequence of SEQ ID NO:24, (3) a protein comprising an amino acid
sequence of SEQ ID
NO:28, (4) a protein comprising an amino acid sequence of SEQ ID NO:30, (5) a
protein comprising
an amino acid sequence of SEQ ID NO:32, (6) a carbamylated protein comprising
an amino acid
sequence of SEQ ID NO:24, and (7) a carbamylated protein comprising an amino
acid sequence of
SEQ ID NO:2.
In some embodiments, an insult affecting rapidly proliferating tissues is
radiation exposure.
In a specific embodiment, the insult is ionizing radiation. In another
embodiment, the insult may be
one or more chemotherapies or one or more chemical/biological warfare agents
(such as a vesicant
agent or bacteria), or a combination thereof. Non-limiting examples of
chemotherapy and
chemical/biological warfare agent are alkylating agents, vesicant agents
(e.g., mustard agents) and
microorganisms. In some embodiments, an insult affecting rapidly proliferating
tissues is one or
more radiation exposures, one or more chemotherapies, one or more
chemical/biological warfare
agents, or a combination thereof.
Organs and body systems most sensitive to the effects of insult such as
ionizing radiation
include, but are not limited to, skin, hematopoietic and lymphatic systems,
gonads, lungs, nerve
tissues, and the GI tract. In one embodiment, the insult are particularly
damaging to hematopoietic
and/or gastrointestinal tissues of a subject. In a specific embodiment, the
disorder to be prevented
or treated is a disorder of hematopoiesis, including but not limited to,
anemia, leukopenia (e.g.,
neutropenia), thrombocytopenia, pancytopenia, and a clotting disorder. In
another embodiment, the
disorder to be prevented or treated is alimentary mucositis, including but not
limited to, oral
mucositis, esophagitis, stomatitis, enteritis, and proctitis. In another
embodiment, the disorder to be
prevented or treated is a cerebrovascular syndrome. In some embodiments, the
symptoms
associated with an insult affecting rapidly proliferating tissues (such as
radiation, chemotherapy, and
chemical/biological warfare agents) include, but are not limited to, diarrhea,
skin burn, sores, fatigue,
dehydration, inflammation, hair loss, ulceration of oral mucosa, xerostomia,
and bleeding (e.g., from
the nose, mouth or rectum).
The present invention also provides methods of upregulating oxygen scavenging
pathways
in a subject, where the methods comprise administering to the subject a
composition comprising one
or more CG53135 proteins. In one embodiment, the oxygen scavenging pathways
comprise one or
34
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
more superoxide dismutases ("SOD"), including but are not limited to,
intracellular CuZnSOD and
MnSOD, and extracellular-SOD ("EC-SOD"). In another embodiment, the oxygen
scavenging
pathways comprise genes selected from the group consisting of ERK, AKT, a
superoxide dismutase,
cyclooxygenase-2 ("COX-2"), and Nrf-2. Cells exposed to radiation must be able
to deal with the
detrimental effects of ionized radicals (reactive oxygen species or ROS), of
which the most reactive
species within the cell are generated by the ionization of H20. Administering
one or more CG53135
proteins to a subject increases transcription of enzymes, like superoxide,
that scavenge ROS and
convert them to less reactive intermediates, like hydrogen peroxide.
Administering one or more
CG53135 proteins to a subject also reduces the load of reactive oxygen species
induced by an
insult, such as radiation.
The present invention further provides methods of stimulating secretion of one
or more
endogenous cytokines and/or endogenous chemokines from cells of a subject
comprising
administering to the subject a composition comprising one or more CG53135
proteins. The
endogenous cytokines secreted can be, but are not limited to, interleukin
("IL") - 1 b, IL-6, IL-7, IL-8,
IL-11, and granulocyte-colony forming factor ("G-CSF"). The endogenous
chemokines secreted can
be, but are not limited to, chemokine (C-X-C motif) ligand 1("CXCL1") and
monocyte
chemoattractant protein ("MCP-1"). Some of these endogenous cytokines and
chemokines have
been shown to be involved in endogenous radioprotective responses.
The present invention provides methods of stimulating proliferation of
hematopoietic stem
cells and/or gastrointestinal stem cells of a subject, where the methods
comprise administering to
the subject a composition comprising one or more CG53135 proteins. In one
embodiment,
administration of one or more CG53135 proteins to a subject stimulates
fibroblast cells within the
bone marrow stroma to secret factors that facilitate the health and
proliferative capacity of
hematopoietic stem cells. In another embodiment, administration of one or more
CG53135 proteins
to a subject leads to a rapid proliferative burst of gastrointestinal stem
cells, which is followed by a
counter-regulatory inhibition in proliferation 24 hours later. This leads to a
synchronization of the cell
cycle at the tissue level, which is more radio-resistant.
The present invention also provides methods of optimizing engraftment of
hematopoietic
stem cells in a subject, where the methods comprise administering to the
subject a composition
comprising one or more CG53135 proteins. In one embodiment, administration of
one or more
CG53135 proteins improves successful engraftment or repopulation of T-cells
following a bone
marrow transplant after marrow radioablation. In another embodiment,
administration of one or
more CG53135 proteins increases the speed of T-cell reconstitution within the
thymus after bone
marrow transplant.
The patient population that can be targeted using the methods of the present
invention
include, but are not limited to, subjects who have been exposed to an insult
affecting rapidly
proliferating tissues (such as radiation, chemotherapy, and
chemical/biological warfare agents),
subjects who are suspected to have been exposed an insult affecting rapidly
proliferating tissues
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
(such as radiation, chemotherapy, and chemical/biological warfare agents),
subjects who will be
exposed to an insult affecting rapidly proliferating tissues (such as
radiation, chemotherapy, and
chemical/biological warfare agents), and subjects who are at risk to be
exposed to an insult affecting
rapidly proliferating tissues (such as radiation, chemotherapy, and
chemical/biological warfare
agents).
In one embodiment, a composition comprising one or more CG53135 proteins is
administered to a subject prior to the subject's exposure to an insult
affecting rapidly proliferating
tissue in a body. In another embodiment, a composition comprising one or more
CG53135 proteins
is administered to a subject after the subject's exposure to an insult
affecting rapidly proliferating
tissue in a body but prior to a disorder associated with the insult or a
symptom thereof developed in
the subject. In another embodiment, a composition comprising one or more
CG53135 proteins is
administered to a subject after a disorder associated with an insult affecting
rapidly proliferating
tissue in a body or a symptom thereof developed in the subject. In yet another
embodiment, a
composition comprising one or more CG53135 proteins is administered to a
subject who is at risk for
exposure to an insult affecting rapidly proliferating tissues.
Compositions comprising one or more CG53135 proteins can also be administered
in
combination with one or more other therapies to prevent, treat, or ameliorate
a disorder or one or
more symptoms associated with an insult affecting rapidly proliferating
tissues (such as radiation,
chemotherapy, and chemical/biological warfare agents). In a preferred
embodiment, a composition
comprising one or more CG53135 proteins is administered in combination with
one or more other
therapies known to be used in preventing, treating, or ameliorating a disorder
or one or more
symptoms associated with an insult affecting rapidly proliferating tissues
(such as radiation,
chemotherapy, and chemical/biological warfare agents). Examples of such other
therapies include,
but are not limited to, Mesna (sodium 2-mercaptoethene sulfonate) and other
analogues with free
thiol moieties, dimesna (disodium 2,2'-dithiobis ethane sulfonate) and other
disulfides, and
compounds such as, for example, described in U.S. Application Publication No.
20030092681, and
KGF (see e.g., U.S. Patent No. 6,743,422). Examples of other agents that can
be used in
combination with a composition comprising CG53135 is shown in Table 1 B.
36
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 1 B.
Trade name, Company / Administration Mechanism Treatment Mode
common or Supplier Method(s)
chemical name
Amifostine Medimmune IV (200 mg/m2) Free radical Possible prophylaxis
Ethyol, WR-2721 Inc. approved scavenger for first responders
phosphorylated SC (500 mg/m2) Protection from 15-30 minutes prior to
aminothiol possible reactive oxygen exposure to radiation
species
Sodium Pharmacies 4 g initial Alkalinization of Treatment following
bicarbonate followed by 2 g urine leading to in~estion of uranium
every 4 h rapid secretion (2 U)
of uranium
carbonate
complex
Ca-DTPA HEYL Chemical 1 g IV or Metal chelation Treatment following
Zn-DTPA Pharmaceutical nebulizer (Calcium is ingestion or inhalation
calcium or zinc Factory (Berlin) substituted by of plutonium,
salt of diethylene Distributed by: other metals) americium
triamine penta- Oak Ridge Promotes
acetate Associated increased renal
Universities / clearance
DOE
Potassium iodide Pharmacies 16-130 mg oral Thyroid blocker Prophylaxis prior
to
depending on Prevents exposure, or
exposure and accumulation of treatment
age radioiodines immediately following
(hrs)
Radiogardase HEYL Chemical 1 g orally three Complex with Treatment following
Prussian Blue Pharmaceutical times per day Cesium (137Cs) ingestion or other
ferric Factory (Berlin) or Thallium internal contamination
hexacyanoferrate Administered leading to of the gut
by: Oak Ridge enhanced
Inst. for secretion
Science and
Industry / DOE
G-CSF Amgen Inc. SC or IV Stimulates the Off label use following
filgrastim, 5 g/kg/day proliferation, radiation exposure to
Neupogen filgrastim differentiation, limit life threatening
pegfilgrastim, SC 6mg and function of infections
Neulasta pegfilgrastim neutrophils Prophylaxis
GM-CSF Berlex SC or IV Stimulates the Off label use following
sargramostim, (Schering AG) 250 mg/m2/day proliferation, radiation exposure to
Leukine differentiation, limit life threatening
and function of infections
neutrophils Prophylaxis
In one embodiment, during a combination therapy, a CG53135 protein and/or
another
therapy are administered in a sub-optimal amount, e.g., an amount that does
not manifest
detectable therapeutic benefits when administered alone, as determined by
methods known in the
art. In such methods, co-administration of a CG53135 protein and another
therapy results in an
overall improvement in effectiveness of treatment.
37
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In one embodiment, a composition comprising one or more CG53135 proteins and
one or
more other therapies are administered within the same patient visit. In
another embodiment, a
composition comprising one or more CG53135 proteins is administered prior to
the administration of
one or more other therapies. In yet another embodiment, a composition
comprising one or more
CG53135 proteins is administered subsequent to the administration of one or
more other therapies.
In a specific embodiment, a composition comprising one or more CG53135
proteins and one or
more other therapies are cyclically administered to a subject. Cycling therapy
involves the
administration of a composition comprising one or more CG53135 proteins for a
period of time,
followed by the administration of one or more other therapies for a period of
time and repeating this
sequential administration. Cycling therapy can reduce the development of
resistance to one or more
of the therapies, avoid or reduce the side effects of one of the therapies,
and/or improve the efficacy
of the treatment.
5.4.3. Inflammatory Bowel Disease and Irritable Bowel Syndrome
In one embodiment, the present invention provides methods of preventing and/or
treating
inflammatory bowel disease or irritable bowel syndrome comprising
administering to a subject in
need thereof an effective amount of a composition comprising one or more
isolated CG53135
proteins. Inflammatory bowel disease that can be prevented and/or treated by
the methods of the
invention includes, but is not limited to, ulcerative colitis and Crohn's
disease.
The present invention provides methods of preventing and/or treating
inflammatory bowel
disease in patient populations with inflammatory bowel disease and populations
at risk to develop
inflammatory bowel disease. The present invention also provides methods of
preventing and/or
treating irritable bowel syndrome in patient populations with irritable bowel
syndrome and
populations at risk to develop irritable bowel syndrome.
In one embodiment, the present invention provides a method of preventing or
treating
inflammatory bowel disease or irritable bowel syndrome comprising
administering to a subject in
need thereof a prophylactically or therapeutically effective amount of an
isolated protein selected
from the group consisting of: (a) a protein comprising an amino acid sequence
of SEQ ID NOs:2, 4,
7, 10, 22, 24, 26, 28, 30, 32, 34, 36, 38, or 40; (b) a protein with one or
more amino acid
substitutions to the protein of (a), wherein said substitutions are no more
than 15% of the amino acid
sequence of SEQ ID NOs:2, 4, 7, 10, 22, 24, 26, 28, 30, 32, 34, 36, 38, or 40,
and wherein said
protein with one or more amino acid substitutions retains cell proliferation
stimulatory activity; and (c)
a fragment of the protein of (a) or (b), which fragment retains cell
proliferation stimulatory activity.
In some embodiments, the present invention provides a method of preventing
and/or
treating inflammatory bowel disease or irritable bowel syndrome comprising
cyclically administering
a composition comprising one or more CG53135 proteins. In one embodiment,
cycling therapy
involves the administration of a first therapy for a period of time, followed
by the administration of a
second therapy for a period of time and repeating this sequential
administration, i.e., the cycle, in
order to, e.g., to avoid or reduce the side effects of one of the therapies
and/or to improve the
38
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
efficacy of the therapies. In another embodiment, cycling therapy involves the
administration of a
therapy for a period of time, stop the therapy for a period of time, and
repeat the administration of
the therapy.
In accordance to the instant invention, a composition comprising one or more
isolated
CG53135 proteins can also be used in combination with other therapies to
prevent and/or treat
inflammatory bowel disease or irritable bowel syndrome. In one embodiment, a
composition
comprising one or more isolated CG53135 proteins is administered in
combination with one or more
other therapies (e.g., therapeutic agents) that have prophylactic and/or
therapeutic effect(s) on
inflammatory bowel disease and/or have amelioration effect(s) on one or more
symptoms
associated with inflammatory bowel disease to a subject to prevent and/or
treat inflammatory bowel
disease. Non-limiting examples of such therapies are: 5-aminosalicylates,
antibiotics,
corticosteroids, immunomodulators (e.g., 6-mercaptoputine, azathioprine,
methotrexate,
cyclosporine), and biological response modifiers (e.g., infliximab). In
another embodiment, a
composition comprising one or more isolated CG53135 proteins is administered
in combination with
one or more other therapies (e.g., therapeutic agents) that have prophylactic
and/or therapeutic
effect(s) on irritable bowel syndrome and/or have amelioration effect(s) on
one or more symptoms
associated with irritable bowel syndrome to a subject to prevent and/or treat
inflammatory bowel
disease. Non-limiting examples of such therapies are: laxatives;
antidiarrheals (e.g., diphenoxylate
(e.g., Lomotil, Lomocot); loperamide (e.g., Imodium, Pepto Diarrhea),
cholestyramine (e.g.,
Questran, Cholybar)); antispasmodics (e.g., dicyclomine, hyoscyamine, and
clidinium (in
combination with chlordiazepoxide hydrochloride)); peppermint oil; direct
smooth muscle relaxants;
antidepressants; 5-HT3 antagonists (e.g., Alosetron (Lotronex), cilansetron);
5-HT4 agonists (e.g.,
tegaserod (Zelnorm/Zelmac) and prucalopride); M3 receptor antagonists (e.g.,
zamifenacin and
darifenacin).
5.4.4. Arthritis and Diseases Associated with CNS System or Cardiovascular
System
In one embodiment, the present invention provides methods of preventing and/or
treating a
disease (e.g., a joint disease, ischemic stroke, hemorrhagic stroke, trauma,
spinal cord damage,
heavy metal or toxin poisoning, or neurodegenerative diseases) comprising
administering to a
subject in need thereof an effective amount of a composition comprising one or
more isolated
CG53135 proteins.
In one embodiment, the present invention provides methods of preventing and/or
treating
arthritis (e.g., osteoarthritis or rheumatic arthritis) comprising
administering to a subject in need
thereof a composition comprising one or more CG53135 proteins.
In another embodiment, the present invention provides methods of reducing
cartilage
degeneration comprising administering to a subject in need thereof a
composition comprising one or
more CG53135 proteins. In another embodiment, the present invention provides
methods of
stimulating cartilage repair comprising administering to a subject in need
thereof a composition
39
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
comprising one or more CG53135 proteins. In a specific embodiment, the present
invention
provides methods of stimulating cartilage healing after surgery in a subject
comprising administering
to a subject a composition comprising one or more CG53135 proteins.
In another embodiment, the present invention provides methods of preventing
and/or
treating a cardiovascular disease, such as stroke (e.g., ischemic stroke,
hemorrhagic stroke),
comprising administering to a subject a composition comprising one or more
CG53135 proteins. In
a specific embodiment, the present invention provides methods of preventing
and/or treating a
cardiovascular disease, such as stroke, comprising administering to a subject
a composition
comprising an isolated protein comprising an amino acid sequence of SEQ ID NO:
4, 7, 10, 22, 24,
26, 28, 30, 32, 34, 36, 38, or 40, or a combination thereof.
In another embodiment, the present invention provides methods of preventing
and/or
treating a neurodegenerative disease (e.g., Alzheimer's disease, Parkinson's
disease, Amyotrophic
Lateral Sclerosis, Huntington's disease) comprising administering to a subject
in need thereof a
composition comprising one or more CG53135 proteins. In a specific embodiment,
the present
invention provides methods of preventing and/or treating a neurodegenerative
disease comprising
administering to a subject in need thereof a composition comprising an
isolated protein comprising
an amino acid sequence of SEQ ID NO: 4, 7, 10, 22, 24, 26, 28, 30, 32, 34, 36,
38, or 40, or a
combination thereof.
In some embodiments, the present invention provides a method of preventing
and/or
treating a disease (e.g., a joint disease, ischemic stroke, hemorrhagic
stroke, trauma, spinal cord
damage, heavy metal or toxin poisoning, or neurodegenerative diseases)
comprising cyclically
administering a composition comprising one or more CG53135 proteins. In one
embodiment,
cycling therapy involves the administration of a first therapy for a period of
time, followed by the
administration of a second therapy for a period of time and repeating this
sequential administration,
i.e., the cycle, in order to, e.g., to avoid or reduce the side effects of one
of the therapies and/or to
improve the efficacy of the therapies. In another embodiment, cycling therapy
involves the
administration of a therapy for a period of time, stop the therapy for a
period of time, and repeat the
administration of the therapy. In accordance to the present invention, a
composition comprising one
or more CG53135 proteins can be administered to a subject prior to, during, or
after the
administration of a radiation therapy and/or chemotherapy, where such
radiation therapy and/or
chemotherapy is a cycling therapy.
In accordance to the instant invention, a composition comprising one or more
isolated
CG53135 proteins can also be used in combination with other therapies to
prevent and/or treat a
disease (e.g., a joint disease, ischemic stroke, hemorrhagic stroke, trauma,
spinal cord damage,
heavy metal or toxin poisoning, or neurodegenerative diseases). In one
embodiment, a composition
comprising one or more isolated CG53135 proteins is administered in
combination with one or more
other agents that have prophylactic and/or therapeutic effect(s) on a disease
(e.g., a joint disease,
ischemic stroke, hemorrhagic stroke, trauma, spinal cord damage, heavy metal
or toxin poisoning,
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
or neurodegenerative diseases) and/or have amelioration effect(s) on one or
more symptoms
associated with the disease to a subject to prevent and/or treat the disease.
Any other agents or
therapies that are known in the art that can be used to prevent and/or treat a
disease, such as a joint
disease, ischemic stroke, hemorrhagic stroke, trauma, spinal cord damage,
heavy metal or toxin
poisoning, or neurodegenerative diseases, can be used in combination with a
composition
comprising one or more CG53135 proteins in accordance to the methods of the
present invention.
In a specific embodiment, the present invention provides methods of
stimulating cartilage healing
after surgery in a subject comprising administering to a subject a composition
comprising one or
more CG53135 proteins.
5.5 DOSAGE REGIMENS
Toxicity and efficacy of the prophylactic and/or therapeutic protocols of the
present invention
can be determined by standard pharmaceutical procedures in cell cultures or
experimental animals,
e.g., for determining the LD50 (the dose lethal to 50% of the population) and
the ED50 (the dose
therapeutically effective in 50% of the population). The dose ratio between
toxic and therapeutic
effects is the therapeutic index and it can be expressed as the ratio
LD50/ED50. Prophylactic and/or
therapeutic agents that exhibit large therapeutic indices are preferred. While
prophylactic and/or
therapeutic agents that exhibit toxic side effects may be used, care should be
taken to design a
delivery system that targets such agents to the site of affected tissue in
order to minimize potential
damage to uninfected cells and, thereby, reduce side effects.
The data obtained from the cell culture assays and animal studies can be used
in
formulating a range of dosage of the prophylactic and/or therapeutic agents
for use in humans. The
dosage of such agents lies preferably within a range of circulating
concentrations that include the
ED50 with little or no toxicity. The dosage may vary within this range
depending upon the dosage
form employed and the route of administration utilized. For any agent used in
the method of the
invention, the therapeutically effective dose can be estimated initially from
cell culture assays. A
dose may be formulated in animal models to achieve a circulating plasma
concentration range that
includes the IC50 (i.e., the concentration of the test compound that achieves
a half-maximal inhibition
of symptoms) as determined in cell culture. Such information can be used to
more accurately
determine useful doses in humans. Levels in plasma may be measured, for
example, by high
performance liquid chromatography.
The amount of the composition of the invention which will be effective in the
treatment of a
particular disorder or condition will depend on the nature of the disorder or
condition, and can be
determined by standard clinical techniques. The precise dose to be employed in
the formulation will
also depend on the route of administration, and the seriousness of the disease
or disorder, and
should be decided according to the judgment of the practitioner and each
patient's circumstances.
In one embodiment, the dosage of a composition comprising one or more G53135
proteins
for administration in a human patient provided by the present invention is at
least 0.001 mg/kg, at
least 0.005 mg/kg, at least 0.01 mg/kg, at least 0.03 mg/kg, at least 0.05
mg/kg, at least 0.1 mg/kg,
41
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
at least 0.2 mg/kg, at least 0.3 mg/kg, at least 0.4 mg/kg, at least 0.5
mg/kg, at least 0.6 mg/kg, at
least 0.7 mg/kg, at least 0.8 mg/kg, at least 0.9 mg/kg, at least 1 mg/kg, at
least 2 mg/kg, at least 3
mg/kg, at least 4 mg/kg, at least 5 mg/kg, at least 6 mg/kg, at least 7 mg/kg,
at least 8 mg/kg, at
least 9 mg/kg, or at least 10 mg/kg (as measured by UV assay). In another
embodiment, the
dosage of a composition comprising one or more CG53135 proteins for
administration in a human
patient provided by the present invention is between 0.001-10 mg/kg, between
0.005-5 mg/kg,
between 0.005-1 mg/kg, between 0.005-0.9 mg/kg, between 0.005-0.8 mg/kg,
between 0.005-0.7
mg/kg, between 0.005-0.6 mg/kg, between 0.005-0.5 mg/kg, or between 0.005-0.3
mg/kg, between
0.01-1 mg/kg, between 0.01-0.9 mg/kg, between 0.01-0.8 mg/kg, between 0.01-0.7
mg/kg, between
0.01-0.6 mg/kg, between 0.01-0.5 mg/kg, or between 0.01-0.3 mg/kg (as measured
by UV assay).
Protein concentration can be measured by methods known in the art, such as
Bradford
assay or UV assay, and the concentration may vary depending on what assay is
being used. In a
non-limiting example, the protein concentration in a pharmaceutical
composition of the instant
invention is measured by a UV assay that uses a direct measurement of the UV
absorption at a
wavelength of 280 nm, and calibration with a well characterized reference
standard of CG53135
protein (instead of IgG). Test results obtained with this UV method (using
CG53135 reference
standard) are three times lower than test results for the same sample(s)
tested with the Bradford
method (using IgG as calibrator). For example, if a dosage of a composition
comprising one or
more CG53135 proteins for administration in a human patient provided by the
present invention is
between 0.001-10 mg/kg measured by UV assay, then the dosage is 0.003-30 mg/kg
as measured
by Bradford assay.
In one embodiment, prior to administering the first full dose, each patient
preferably receives
a subcutaneous injection of a small amount (e.g., 1/100 to 1/10 of the
prescribed dose) of a
composition of the invention to detect any acute intolerance. The injection
site is examined one and
two hours after the test. If no reaction is detected, then the full dose is
administered.
5.6 PHARMACEUTICA COMPOSITIONS AND FORMULATIONS
The present invention encompasses pharmaceutical compositions (including
formulations)
comprising one or more CG53135 proteins. The pharmaceutical compositions can
be administered
to a subject at a prophylactically or therapeutically effective amount to
prevent and/or treat one or
more diseases, such as inflammatory bowel disease ("IBD"), irritable bowel
syndrome, alimentary
mucositis (including oral mucositis), arthritis, diseases associated with the
central nerve system or
cardiovascular system, and symptoms associated with radiation exposure.
Various delivery
systems are known and can be used to administer a composition comprising one
or more CG53135
proteins. Such delivery systems include, but are not limited to, encapsulation
in liposomes,
microparticies, microcapsuies, expression by recombinant cells, receptor-
mediated endocytosis,
construction of the nucleic acids of the invention as part of a retroviral or
other vectors, etc.
Methods of introduction include, but are not limited to, intradermal,
intramuscular, intraperitoneal,
intrathecal, intracerebroventricular, epidural, intravenous, subcutaneous,
intranasal, intratumoral,
42
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
transdermal, rectal, and oral routes. The compositions of the invention may be
administered by any
convenient route, for example, by infusion or bolus injection, by absorption
through epithelial or
mucocutaneous linings (e.g., oral mucosa, virginal mucosa, rectal and
intestinal mucosa, etc.), and
may be administered together with other biologically active agents.
Administration can be systemic
or local. In a specific embodiment, the present invention comprises using
single or double
chambered syringes, preferably equipped with a needle-safety device and a
sharper needle, that are
pre-filled with a composition comprising one or more CG53135 proteins. In one
embodiment, dual
chambered syringes (e.g., Vetter Lyo-Ject dual-chambered syringe by Vetter
Pharmar-Fertigung)
are used. Such systems are desirable for lyophilized formulations, and are
especially useful in an
emergency setting.
In some embodiments, it may be desirable to administer the pharmaceutical
compositions of
the invention locally to the area in need of treatment. This may be achieved
by, for example, topical
application, by injection, by infusion pump, by means of a suppository, or by
means of an implant
(the implant being of a reservoir with a porous, non-porous, or gelatinous
material, including
membranes, such as sialastic membranes, or fibers).
In some embodiments, a CG53135 nucleic acid can be administered in vivo to
promote
expression of their encoded proteins, by constructing the nucleic acid as part
of an appropriate
nucleic acid expression vector and administering it so that it becomes
intracellular, e.g., by use of a
retroviral vector, or by direct intramuscular or intradermal injection, or by
use of microparticle
bombardment (e.g., a gene gun), or coating with lipids or cell-surface
receptors or transfecting
agents, or by administering it in linkage to a homeobox-like peptide which is
known to enter the
nucleus, etc. Alternatively, a CG53135 nucleic acid can be introduced
intracellularly and
incorporated within host cell DNA for expression, by homologous recombination.
The instant invention encompasses bulk drug compositions useful in the
manufacture of
pharmaceutical compositions that can be used in the preparation of unit dosage
forms. In a
preferred embodiment, a composition of the invention is a pharmaceutical
composition. Such
compositions comprise a prophylactically or therapeutically effective amount
of CG53135, and a
pharmaceutically acceptable carrier. Preferably, the pharmaceutical
compositions are formulated to
be suitable for the route of administration to a subject.
In one embodiment, the term "pharmaceutically acceptable" means approved by a
regulatory agency of the Federal or a state government or listed in the U.S.
Pharmacopeia or other
generally regarded as safe for use in humans (GRAS). The term "carrier" refers
to a diluent,
adjuvant, bulking agent (e.g., arginine in a salt form, sulfobutyl ether Beta-
cyclodextrin sodium, or
sucrose), excipient, or vehicle with which CG53135 is administered. Such
pharmaceutical carriers
can be sterile liquids, such as water and oils (e.g., oils of petroleum,
animal, vegetable or synthetic
origins, such as peanut oil, soybean oil, mineral oil, sesame oil and the
like), or solid carriers, such
as one or more substances which may also act as diluents, flavoring agents,
solubilizers, lubricants,
suspending agents, or encapsulating material. Water is a preferred carrier
when the pharmaceutical
43
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
composition is administered intravenously. Saline solutions and aqueous
dextrose and glycerol
solutions can also be employed as liquid carriers, particularly for injectable
solutions. Suitable
pharmaceutical excipients include, but are not limited to, starch or its
synthetically modified
derivatives such as hydroxyethyl starch, stearate salts, glycerol, glucose,
lactose, sucrose,
trehalose, gelatin, sulfobutyl ether Beta-cyclodextrin sodium, sodium
chloride, glycerol, propylene,
glycol, water, ethanol, or a combination thereof. The composition, if desired,
can also contain minor
amounts of wetting or emulsifying agents, or pH buffering agents.
The compositions comprising CG53135 may be formulated into any of many
possible
dosage forms such as, but not limited to, liquid, suspension, microemulsion,
microcapsules, tablets,
capsules, gel capsules, soft gels, pills, powders, enemas, sustained-release
formulations and the
like. The compositions comprising CG53135 may also be formulated as
suspensions in aqueous,
non-aqueous or mixed media. Aqueous suspensions may further contain substances
that increase
the viscosity of the suspension including, for example, sodium
carboxymethylcellulose, sorbitol
and/or dextran. The suspension may also contain stabilizers. The composition
can also be
formulated as a suppository, with traditional binders and carriers such as
triglycerides. Oral
formulation can include standard carriers, such as pharmaceutical grades of
mannitol, lactose,
starch or its synthetically modified derivatives such as hydroxyethyl starch,
stearate salts, sodium
saccharine, cellulose, magnesium carbonate, etc.
A pharmaceutical composition comprising CG53135 is formulated to be compatible
with its
intended route of administration. In a specific embodiment, the composition is
formulated in
accordance with routine procedures as a pharmaceutical composition adapted for
intravenous,
subcutaneous, intramuscular, oral, intranasal, intratumoral or topical
administration to human
beings. Typically, compositions for intravenous administration are solutions
in sterile isotonic or
hypertonic aqueous buffer. Where necessary, the composition may also include a
solubilizing agent
and a local anesthetic such as benzyl alcohol or lidocaine to ease pain at the
site of the injection.
If a composition comprising CG53135 is to be administered topically, the
composition can
be formulated in the form of transdermal patches, ointments, lotions, creams,
gels, drops,
suppositories, sprays, liquids and powders. Conventional pharmaceutical
carriers, aqueous, powder
or oily bases, thickeners and the like may be necessary or desirable. Coated
condoms, gloves and
the like may also be useful. Preferred topical formulations include those in
which the compositions
of the invention are in admixture with a topical delivery agent, such as but
not limited to, lipids,
liposomes, micelles, emulsions, sphingomyelins, lipid-protein or lipid peptide
complexes, fatty acids,
fatty acid esters, steroids, chelating agents and surfactants. The
compositions comprising CG53135
may be encapsulated within liposomes or may form complexes thereto, in
particular to cationic
liposomes. Alternatively, the compositions comprising CG53135 may be complexed
to lipids, in
particular to cationic lipids. For non-sprayable topical dosage forms, viscous
to semi-solid or solid
forms comprising a carrier or one or more excipients compatible with topical
application and having
a dynamic viscosity preferably greater than water are typically employed.
Other suitable topical
dosage forms include sprayable aerosol preparations wherein the active
ingredient, preferably in
44
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
combination with a solid or liquid inert carrier, is packaged in a mixture
with a pressurized volatile
(e.g., a gaseous propellant, such as Freon or hydrofluorocarbons) or in a
squeeze bottle.
Moisturizers or humectants can also be added to pharmaceutical compositions
and dosage forms if
desired. Examples of such additional ingredients are well-known in the art.
A composition comprising CG53135 can be formulated in an aerosol form, spray,
mist or in
the form of drops or powder if intranasal administration is preferred. In
particular, a composition
comprising CG53135 can be conveniently delivered in the form of an aerosol
spray presentation
from pressurized packs or a nebulizer, with the use of a suitable propellant
(e.g.,
dichiorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane,
other
hydrofluorocarbons, carbon dioxide or other suitable gas). In the case of a
pressurized aerosol the
dosage unit may be determined by providing a valve to deliver a metered
amount. Microcapsules
(composed of, e.g., polymerized surface) for use in an inhaler or insufflator
may be formulated
containing a powder mix of the compound and a suitable powder base such as
dissacharides or
starch.
One or more CG53135 proteins may also be formulated into a microcapsule with
one or
more polymers (e.g., hydroxyethyl starch) form the surface of the
microcapsule. Such formulations
have benefits such as slow-release.
A composition comprising CG53135 can be formulated in the form of powders,
granules,
microparticulates, nanoparticulates, suspensions or solutions in water or non-
aqueous media,
capsules, gel capsules, sachets, tablets or minitablets if oral administration
is preferred. Thickeners,
flavoring agents, diluents, emulsifiers, dispersing aids or binders may be
desirable. Tablets or
capsules can be prepared by conventional means with pharmaceutically
acceptable excipients such
as binding agents (e.g., pregelatinised maize starch, polyvinylpyrrolidone, or
hydroxypropyl
methylcellulose); fillers (e.g., lactose, microcrystalline cellulose, or
calcium hydrogen phosphate);
lubricants (e.g., magnesium stearate, talc, or silica); disintegrants (e.g.,
potato starch or sodium
starch glycolate); or wetting agents (e.g., sodium lauryl sulphate). The
tablets may be coated by
methods well-known in the art. Liquid preparations for oral administration may
be prepared by
conventional means with pharmaceutically acceptable additives such as
suspending agents (e.g.,
sorbitol syrup, cellulose derivatives, or hydrogenated edible fats);
emulsifying agents (e.g., lecithin or
acacia); non-aqueous vehicles (e.g., almond oil, oily esters, ethyl alcohol,
or fractionated vegetable
oils); and preservatives (e.g., methyl or propyl-p-hydroxybenzoates or sorbic
acid). The
preparations may also contain buffer salts, flavoring, coloring, and
sweetening agents as
appropriate. Preparations for oral administration may be suitably formulated
for slow release,
controlled release, or sustained release of a prophylactic or therapeutic
agent(s).
In one embodiment, the compositions of the invention are orally administered
in conjunction
with one or more penetration enhancers, e.g., alcohols, surfactants and
chelators. Preferred
surfactants include, but are not limited to, fatty acids and esters or salts
thereof, bile acids and salts
thereof. In some embodiments, combinations of penetration enhancers are used,
e.g., alcohols,
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
fatty acids/salts in combination with bile acids/salts. In a specific
embodiment, sodium salt of lauric
acid, capric acid is used in combination with UDCA. Further penetration
enhancers include, but are
not limited to, polyoxyethylene-9-lauryl ether, polyoxyethylene-20-cetyl
ether. Compositions of the
invention may be delivered orally in granular form including, but is not
limited to, sprayed dried
particles, or complexed to form micro or nanoparticles. Complexing agents that
can be used for
complexing with the compositions of the invention include, but are not limited
to, poly-amino acids,
polyimines, polyacrylates, polyalkylacrylates, polyoxethanes,
polyalkylcyanoacrylates, cationized
gelatins, albumins, acrylates, polyethyleneglycols (PEG), DEAE-derivatized
polyimines, pollulans,
celluloses, and starches. Particularly preferred complexing agents include,
but are not limited to,
chitosan, N-trimethylchitosan, poly-L-lysine, polyhistidine, polyornithine,
polyspermines, protamine,
polyvinylpyridine, polythiodiethylamino-methylethylene P(TDAE),
polyaminostyrene (e.g. p-amino),
poly(methylcyanoacrylate), poly(ethylcyanoacrylate), poly(butylcyanoacrylate),
poly(isobutylcyanoacrylate), poly(isohexylcynaoacrylate), DEAE-methacrylate,
DEAE-hexylacrylate,
DEAE-acrylamide, DEAE-albumin and DEAE-dextran, polymethylacrylate,
polyhexylacrylate,
poly(D,L-lactic acid), poly(DL-lactic-co-glycolic acid (PLGA), alginate, and
polyethyleneglycol (PEG).
A composition comprising CG53135 can be delivered to a subject by pulmonary
administration, e.g., by use of an inhaler or nebulizer, of a composition
formulated with an
aerosolizing agent.
In a preferred embodiment, a composition comprising CG53135 is formulated for
parenteral
administration by injection (e.g., by bolus injection or continuous infusion).
Formulations for injection
may be presented in unit dosage form (e.g., in ampoules or in multi-dose
containers) with an added
,preservative. The compositions may take such forms as suspensions, solutions
or emulsions in oily
or aqueous vehicles, and may contain formulatory agents such as suspending,
stabilizing and/or
dispersing agents. Alternatively, the active ingredient may be in powder form
for constitution with a
suitable vehicle (e.g., sterile pyrogen-free water) before use.
In a preferred embodiment, the composition is formulated in accordance with
routine
procedures as a pharmaceutical composition adapted for intravenous
administration to human
beings. Typically, compositions for intravenous administration are solutions
in sterile isotonic
aqueous buffer. Where necessary, the composition may also include a
solubilizing agent and a
local anesthetic such as benzyl alcohol or lidocaine to ease pain at the site
of the injection.
Generally, the ingredients are supplied either separately or mixed together in
unit dosage form, for
example, as a dry lyophilized powder or water free concentrate in a sealed
container, such as a vial,
ampoule or sachette, indicating the quantity of active agent. Where the
composition is to be
administered by infusion, it can be dispensed with an infusion container
containing sterile
pharmaceutical grade water or saline. Where the composition is administered by
injection, an
ampoule or vial of sterile water for injection or saline can be provided so
that the ingredients may be
mixed prior to administration.
46
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
A composition comprising CG53135 can be formulated as neutral or salt forms.
Pharmaceutically acceptable salts include, but are not limited to, those
formed with free amino
groups such as those derived from hydrochloric, phosphoric, acetic, oxalic,
tartaric acids, etc., and
those formed with free carboxyl groups such as those derived from sodium,
potassium, ammonium,
calcium, ferric hydroxides, isopropylamine, triethylamine, 2-ethylamino
ethanol, histidine, procaine,
etc.
In addition to the formulations described previously, a composition comprising
CG53135
may also be formulated as a depot preparation. Such long acting formulations
may be administered
by implantation (for example, subcutaneously or intramuscularly) or by
intramuscular injection.
Thus, for example, the compositions may be formulated with suitable polymeric
or hydrophobic
materials (for example, as an emulsion in an acceptable oil) or ion exchange
resins, or as sparingly
soluble derivatives, for example, as a sparingly soluble salt. Liposomes and
emulsions are well
known examples of delivery vehicles or carriers for hydrophilic drugs.
Toxicity and efficacy of the prophylactic and/or therapeutic protocols of the
present invention
can be determined by standard pharmaceutical procedures in cell cultures or
experimental animals,
e.g., for determining the LD50 (the dose lethal to 50% of the population) and
the ED50 (the dose
therapeutically effective in 50% of the population). The dose ratio between
toxic and therapeutic
effects is the therapeutic index and it can be expressed as the ratio
LD50/ED50. Prophylactic and/or
therapeutic agents that exhibit large therapeutic indices are preferred. While
prophylactic and/or
therapeutic agents that exhibit toxic side effects may be used, care should be
taken to design a
delivery system that targets such agents to the site of affected tissue in
order to minimize potential
damage to uninfected cells and, thereby, reduce side effects.
The data obtained from the cell culture assays and animal studies can be used
in
formulating a range of dosage of the prophylactic and/or therapeutic agents
for use in humans. The
dosage of such agents lies preferably within a range of circulating
concentrations that include the
ED50 with little or no toxicity. The dosage may vary within this range
depending upon the dosage
form employed and the route of administration utilized. For any agent used in
the method of the
invention, the therapeutically effective dose can be estimated initially from
cell culture assays. A
dose may be formulated in animal models to achieve a circulating plasma
concentration range that
includes the IC50 (i.e., the concentration of the test compound that achieves
a half-maximal inhibition
of symptoms) as determined in cell culture. Such information can be used to
more accurately
determine useful doses in humans. Levels in plasma may be measured, for
example, by high
performance liquid chromatography.
The amount of the composition of the invention which will be effective in the
treatment of a
particular disorder or condition will depend on the nature of the disorder or
condition, and can be
determined by standard clinical techniques. The precise dose to be employed in
the formulation will
also depend on the route of administration, and the seriousness of the disease
or disorder, and
should be decided according to the judgment of the practitioner and each
patient's circumstances.
47
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In one embodiments, the dosage of a composition comprising one or more G53135
proteins
for administration in a human patient provided by the present invention is at
least 0.001 mg/kg, at
least 0.01 mg/kg, at least 0.1 mg/kg, at least 0.5 mg/kg, at least 1mg/kg, at
least 2 mg/kg, at least 3
mg/kg, at least 4 mg/kg, at least 5 mg/kg, at least 6 mg/kg, at least 7 mg/kg,
at least 8 mg/kg, at
least 9 mg/kg, at least 10 mg/kg, at least 15 mg/kg, at least 20 mg/kg, at
least 25 mg/kg, at least 30
mg/kg, at least 35 mg/kg, at least 40 mg/kg, at least 45 mg/kg, at least 50
mg/kg, at least 60 mg/kg,
at least 70 mg/kg, at least 80 mg/kg, at least 90 mg/kg, at least 100 mg/kg,
at least 150 mg/kg, or at
least 200 mg/kg (as measured by Bradford assay). In another embodiment, the
dosage of a
composition comprising one or more CG53135 proteins for administration in a
human patient
provided by the present invention is between 0.001-300 mg/kg, between 0.01-300
mg/kg, between
0.1-300 mg/kg, between 0.5-250 mg/kg, between 1-200 mg/kg, between 1-150
mg/kg, between 1-
125 mg/kg, between 1-100 mg/kg, between 1-90 mg/kg, between 1-80 mg/kg,
between 1-70 mg/kg,
between 1-60 mg/kg, between 1-50 mg/kg, between 1-40 mg/kg, between 1-35
mg/kg, between 1-
30 mg/kg, between 1-25 mg/kg, between 1-20 mg/kg, between 1-15 mg/kg, between
1-10 mg/kg, or
between 1-5 mg/kg (as measured by Bradford assay).
Protein concentration can be measured by methods known in the art, such as
Bradford
assay or UV assay, and the concentration may vary depending on what assay is
being used. In a
non-limiting example, the protein concentration in a pharmaceutical
composition of the instant
invention is measured by a UV assay that uses a direct measurement of the UV
absorption at a
wavelength of 280 nm, and calibration with a well characterized reference
standard of CG53135
protein (instead of IgG). Test results obtained with this UV method (using
CG53135 reference
standard) are three times lower than test results for the same sample(s)
tested with the Bradford
method (using IgG as calibrator). For example, if a dosage of a composition
comprising one or
more CG53135 proteins for administration in a human patient provided by the
present invention is
between 0.1-300 mg/kg measured by Bradford assay, then the dosage is 0.033 -
100 mg/kg as
measured by a UV assay.
In one embodiment, prior to administering the first full dose, each patient
preferably receives
a bolus injection of a small amount (e.g., 1/100 to 1/10 of the prescribed
dose) of a composition of
the invention to detect any acute intolerance. The injection site is examined
one and two hours after
the test. If no reaction is detected, then the full dose is administered.
The invention also provides kits for carrying out the therapeutic regimens of
the invention.
Such kits comprise in one or more containers prophylactically or
therapeutically effective amounts of
the composition of the invention in pharmaceutically acceptable form. The
composition in a vial of a
kit of the invention may be in the form of a pharmaceutically acceptable
solution, e.g., in combination
with sterile saline, dextrose solution, or buffered solution, or other
pharmaceutically acceptable
sterile fluid. Alternatively, the composition may be lyophilized or
desiccated; in this instance, the kit
optionally further comprises in a container a pharmaceutically acceptable
solution (e.g., saline,
dextrose solution, etc.), preferably sterile, to reconstitute the composition
to form a solution for
injection purposes.
48
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In another embodiment, a kit of the invention further comprises a needle or
syringe (single
or dual chambered), preferably packaged in sterile form, for injecting the
formulation, and/or a
packaged alcohol pad. In a specific embodiment, a kit of the invention
comprises pre-filled needles
or syringes (single or dual chambered) that are pre-filled with a composition
comprising one or more
CG53135 proteins. Instructions are optionally included for administration of
the formulations of the
invention by a clinician or by the patient.
In some embodiments, the present invention provides kits comprising a
plurality of
containers each comprising a pharmaceutical formulation or composition
comprising a dose of the
composition of the invention sufficient for a single administration.
5.6.1 Improved Formulations and Methods to Increase Solubility of a FGF
Protein
The present invention provides improved formulations comprising one or more
FGFs,
preferably one or more CG53135 proteins, and methods for increasing solubility
of FGF proteins.
The improved formulations are more stable and more favorable for commercial
scale productions.
While not limited by any theory, the improved formulations are based partially
on the
discovery that high concentrations of arginine in a salt form, sulfobutyl
ether Beta-cyclodextrin
sodium, sucrose, acetate, succinate, or tartrate or a combination thereof can
increase solubility of a
growth factor, including FGF proteins. Accordingly, in one embodiment, the
present invention
provides a method of increasing solubility of a FGF protein in a solution
(e.g., an aqueous solution)
by adding arginine in a salt form, sulfobutyl ether Beta-cyclodextrin sodium,
or sucrose to the
solution. In another embodiment, the present invention provides a method for
increasing solubility of
a FGF protein in a solution by adding acetate, succinate, tartrate, or a
combination thereof to the
solution. In yet another embodiment, acetate, succinate, tartrate or a
combination thereof is added
in combination with arginine in a salt form, sulfobutyl ether Beta-
cyclodextrin sodium, or sucrose to
the solution to increase the solubility of a FGF protein. The arginine in a
salt form can be, but is not
limited to, arginine, arginine sulfate, arginine phosphate, and arginine
hydrochloride. In a preferred
embodiment, arginine sulfate is used. In some embodiments, the final
concentration of the arginine
in a salt form, sulfobutyl ether Beta-cyclodextrin sodium, or sucrose is
between 0.01 M to 1 M. In
one embodiment, the final concentration of the arginine in a salt form is 0.5
M. In some
embodiment, the final concentration of the acetate, succinate, tartrate or a
combination thereof is
0.01 to 0.2 M.
In a preferred embodiment, the FGF protein in the formulation is a FGF-20
protein, a
fragment, a derivative, a variant, a homolog, or an analog of FGF-20, or a
combination thereof. In
some embodiments, the FGF protein in the formulation is CG53135-01 (SEQ ID
NO:2), CG53135-
02 (SEQ ID NO: 4), CG53135-03 (SEQ ID NO:2), CG53135-04 (SEQ ID NO:7), CG53135-
05 (SEQ
ID NO: 2), CG53135-06 (SEQ ID NO: 10), CG53135-07 (SEQ ID NO:12), CG53135-08
(SEQ ID
NO:14), CG53135-09 (SEQ ID NO:16), CG53135-10 (SEQ ID NO:18), CG53135-11 (SEQ
ID
NO:20), CG53135-12 (SEQ ID NO:22), CG53135-13 (SEQ ID NO:24), CG53135-14 (SEQ
ID
49
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
NO:26), CG53135-15 (SEQ ID NO:28), CG53135-16 (SEQ ID NO:30), CG53135-17 (SEQ
ID
NO:32), IFC 250059629 (SEQ ID NO:34), IFC 20059669 (SEQ ID NO:36), IFC
317459553 (SEQ ID
NO:38), IFC 317459571 (SEQ ID NO:40), IFC 250059596 (SEQ ID NO:10), or
IFC316351224 (SEQ
ID NO:10), or any two or more combinations of CG53135 proteins. In one
embodiment, the FGF
proteins in the formulation comprise (1) a protein comprising an amino acid
sequence of SEQ ID
NO:2, and (2) a protein comprising an amino acid sequence of SEQ ID NO:24. In
another
embodiment, the FGF proteins in the formulation comprise (1) a protein
comprising an amino acid
sequence of SEQ ID NO:2, (2) a protein comprising an amino acid sequence of
SEQ ID NO:24, (3)
a protein comprising an amino acid sequence of SEQ ID NO:26, (4) a protein
comprising an amino
acid sequence of SEQ ID NO:28, (5) a protein comprising an amino acid sequence
of SEQ ID
NO:30, and (6) a protein comprising an amino acid sequence of SEQ ID NO:32. In
another
embodiment, the FGF proteins in the formulation comprise (1) a protein
comprising an amino acid
sequence of SEQ ID NO:2, (2) a protein comprising an amino acid sequence of
SEQ ID NO:24, (3)
a protein comprising an amino acid sequence of SEQ ID NO:28, (4) a protein
comprising an amino
acid sequence of SEQ ID NO:30, and (5) a protein comprising an amino acid
sequence of SEQ ID
NO:32. In another embodiment, a formulation of the invention comprises (1) a
protein comprising an
amino acid sequence of SEQ ID NO:32; (2) a protein comprising an amino acid
sequence of SEQ ID
NO:30, (3) a protein comprising an amino acid sequence of SEQ ID NO:28; and
(4) a protein
comprising an amino acid sequence of SEQ ID NO:24. In yet another embodiment,
the FGF
proteins in the formulation comprise (1) a protein comprising an amino acid
sequence of SEQ ID
NO:2, (2) a protein comprising an amino acid sequence of SEQ ID NO:24, (3) a
protein comprising
an amino acid sequence of SEQ ID NO:28, (4) a protein comprising an amino acid
sequence of
SEQ ID NO:30, (5) a protein comprising an amino acid sequence of SEQ ID NO:32,
(6) a
carbamylated protein comprising an amino acid sequence of SEQ ID NO:24, and
(7) a carbamylated
protein comprising an amino acid sequence of SEQ ID NO:2.
The present invention provides improved formulations comprising arginine in a
salt form,
sodium phosphate monobasic (NaH2PO4=H2O), a surfactant, and one or more
CG53135 proteins. In
one embodiment, the present invention provides improved formulations
comprising 0.1-1 M arginine
in a salt form, 0.01-0.1 M sodium phosphate monobasic (NaH2PO4=H2O), 0.01 %-
0.1 %
weight/volume ("w/v") polysorbate 80 or polysorbate 20, and 0.005-50 mg/ml of
one or more
CG53135 proteins. The arginine in a salt form, sulfobutyl ether Beta-
cyclodextrin sodium, or
sucrose thereof can be, but is not limited to, arginine, arginine sulfate,
arginine phosphate, and
arginine hydrochloride. In a preferred embodiment, arginine sulfate is used.
In some embodiments,
the final concentration of the arginine in a salt form, sulfobutyl ether Beta-
cyclodextrin sodium, or
sucrose thereof is 0.01-0.7 M. In one embodiment, the final concentration of
the arginine in a salt
form, sulfobutyl ether Beta-cyclodextrin sodium, or sucrose thereof is 0.5 M.
In some embodiments,
the concentration of sodium phosphate monobasic in the formulations is between
0.02-0.09 M, 0.03-
0.08 M, or 0.04-0.06M. In a specific embodiment, the sodium phosphate
monobasic is 0.05M. In
one embodiment, the improved formulations comprise a surfactant, which may be
added, e.g.,
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
during the diafiltration and/or ultrafiltration step, to minimize the
formation of aggregates. The
surfactant can be, but is not limited to, polysorbate 80 and polysorbate 20.
In a specific
embodiment, the concentration of polysorbate 80 or polysorbate 20 is 0.01%
(weight/volume).
The improved formulations of the present invention comprise one or more
CG53135
proteins: In one embodiment, the improved formulations of the invention
comprise one or more
proteins comprising an amino acid sequence selected from the group consisting
of SEQ ID NOs:2,
4, 7, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38 and 40. In
another embodiment, the
improved formulations of the invention comprise one or more proteins
comprising an amino acid
sequence selected from the group consisting of SEQ ID NOs:2, 4, 7, 10, 22, 24,
26, 28, 30, 32, 34,
36, 38 and 40. In a specific embodiment, the improved formulations of the
invention comprise a
protein comprising an amino acid sequence of SEQ ID NO:2. In another specific
embodiment, the
improved formulations of the invention comprise a protein comprising an amino
acid sequence of
SEQ ID NO:24. In yet another specific embodiment, the improved formulations of
the invention
comprise (1) a protein comprising an amino acid sequence of SEQ ID NO:2, and
(2) a protein
comprising an amino acid sequence of SEQ ID NO:24. In a specific embodiment,
the improved
formulations of the invention comprise (1) a protein comprising an amino acid
sequence of SEQ ID
NO:2, (2) a protein comprising an amino acid sequence of SEQ ID NO:24, (3) a
protein comprising
an amino acid sequence of SEQ ID NO:26, (4) a protein comprising an amino acid
sequence of
SEQ ID NO:28, (5) a protein comprising an amino acid sequence of SEQ ID NO:30,
and (6) a
protein comprising an amino acid sequence of SEQ ID NO:32. In one embodiment,
the improved
formulations of the invention comprise one or more proteins produced by any of
the processes
described in Section 5.2, supra. In some embodiments, the concentration of one
or more CG53135
proteins in the improved formulations of the instant invention is at least 2
mg/ml, at least 10 mg/mI,
at least 15 mg/ml, at least 20 mg/ml, at least 25 mg/ml, at least 30 mg/mI, at
least 35 mg/mi, at least
40 mg/mI, at least 45 mg/ml, or at least 50 mg/ml. In some embodiments, the
concentration of one
or more CG53135 proteins in the improved formulations of the instant invention
is no more than 50
mg/mi, no more than 30 mg/mI, no more than 10 mg/ml, no more than 5 mg/ml, no
more than 1
mg/mi, or no more than 0.5 mg/ml. In some embodiments, the concentration of
one or more
CG53135 proteins in the improved formulations of the instant invention is
0.0005- 60 mg/mi, 0.005-
50 mg/mI, 0.05-50 mg/mi, 0.5-50 mg/mI, 1-60 mg/mi, 1-50 mg/mI, 5-40 mg/mI, 5-
30 mg/ml, or 5-20
mg/mi. In a specific embodiment, the concentration of one or more CG53135
proteins in the
improved formulations of the instant invention is 10 mg/mI.
The improved formulations of the invention can be lyophilized or spray dried,
which results
more stable products with longer shelf life and the ease of handling and
shipment. The process of
lyophilization is very well known in the art and is not described in detail
herein. Briefly, lyophilization
is the process by which the moisture content of the product is reduced by
freezing and subsequent
sublimation under vacuum. The lyophilization process primarily consists of
three stages. The first
stage involves freezing the product and creating a frozen matrix suitable for
drying. This step
impacts the drying characteristics in the next two stages. The second stage is
primary drying.
51
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Primary drying involves the removal of the ice by sublimation by reducing the
pressure (to typically
around 50-500 pm Hg) of the product's environment while maintaining the
product temperature at a
low, desirable level. The third stage in the process is called secondary
drying where the bound
water is removed until the residual moisture content reaches below the target
level. Any
lyophilization process known in the art can be used to lyophilize the
formulations of the invention.
The objective of a lyophilization process is to achieve a freeze-dried protein
cake with
acceptable appearance, biological potency, ease of reconstitution, and long-
term storage stability. A
prudently designed lyophilization cycle is one that is robust, consumes less
time and energy, and
maintains product quality. Both formulation-related and cycle-related factors
contribute to achieving
this goal.
The addition of a lyophilization excipient in the processes described herein
may be
necessary. One or more excipients may be added. The lyophilization excipients
contemplated for
use in the present processes include, but are not limited to, sucrose,
lactose, mannitol, dextran,
sucrose, heparin, glycine, glucose, glutamic acid, gelatin, sorbitol,
histidine, dextrose, trehalose,
methocel, hydroxy ethyl cellulose, hydroxy ethyl starch, poly(ethylene
glycol), poly(vinyl pyrolidone),
sulfobutyl ether Beta-cyclodextrin sodium and polyvinyl alcohol, or various
combinations thereof, as
well as other buffers, protein stabilizers, cryoprotectants, and
cryopreservatives commonly used by
those skilled in the art.
Since the active ingredient of the improved formulations of the invention is a
FGF protein,
preferably one or more CG53135 protein, the improved formulations of the
invention can be used
accordingly in any situation that a FGF protein, preferably a CG53135 protein,
is known to be
effective. For example, the improved formulations of the invention can be used
in prevention and/or
treatment of disorders such as alimentary mucositis, inflammatory bowel
disease, osteoarthritis,
disorders of the central nerve system or cardiovascular system, and disorders
associated with
radiation exposure or symptoms thereof.
6. EXAMPLE
Certain embodiments of the invention are illustrated by the following non-
limiting examples.
6.1 EXAMPLE 1: EXPRESSION OF CG53135
Several different expression constructs were generated to express a CG53135
protein
(Table 2).
Table 2: Constructs Generated to Express CG53135
Construct Construct Description Construct Diagram
1a NIH 3T3 cells were transfected with pFGF-20,
which incorporates an epitope tag (V5) and a CG53135-01 V5 His
polyhistidine tag into the carboxy-terminus of the
CG53135-01 protein in the pcDNA3.1 vector (Invitroge
52
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
lb Human 293-EBNA embryonic kidney cells or
NIH 3T3 cells were transfected with CG53135-01
using pCEP4 vector (Invitrogen) containing an IgK CG53135- V5 His
IgK signal sequence, multiple cloning sites, a V5 01
epitope tag, and a polyhistidine tag
2 E.coli BL21 cells were transformed with
CG53135-01 using pETMY vector (CuraGen His T7 CG53135-01
Corporation) containing a polyhistidine tag and a T7
epitope tag (this construct is also referred to as
E.coli/pRSET)
3 E.coli BLR (DE3) cells (NovaGen) were
transformed with CG53135-05 (full-length, CG53135-05
codon-optimized) using pET24a vector (NovaGen)
4 E.coli BLR (DE3) cells (NovaGen) were
transformed with CG53135 (deletion of amino
acids 2-54, codon-optimized) using pET24a CG53135-02 (deletion mutant)
vector (NovaGen)
In one construct, CG53135-01 (the full-length CG53135 gene) was cloned as a
Bgl II-Xho I
fragment into the Bam HI-Xho I sites in mammalian expression vector,
pcDNA3.lV5His (invitrogen
Corporation, Carlsbad, CA). The resultant construct, pFGF-20 (construct 1 a)
has a 9 amino acid V5
tag and a 6 amino acid histidine tag (His) fused in-frame to the carboxy-
terminus of CG53135-01.
These tags aid in the purification and detection of CG53135-01 protein. After
transfection of pFGF-
20 into murine NIH 3T3 cells, CG53135-01 protein was detected in the
conditioned medium using an
anti-V5 antibody (Invitrogen, Carlsbad, CA).
The full-length CG53135-01 gene was also cloned as a Bgl II-Xho I fragment
into the Bam
HI-Xho I sites of mammalian expression vector pCEP4/Sec (CuraGen Corporation).
The resultant
construct, plgK-FGF-20 (construct 1 b) has a heterologous immunoglobulin kappa
(IgK) signal
sequence that could aid in secretion of CG53135-01. After transfection of plgK-
FGF-20 into human
293 EBNA cells (Invitrogen, Carlsbad, CA; catalog # R620-07), CG53135-01 was
detected in the
conditioned medium using an anti-V5 antibody.
In order to increase the yield of CG53135 protein, a Bgl II-Xho I fragment
encoding the full-
length CG53135-01 gene was cloned into the Bam HI-Xho I sites of E.coli
expression vector,
pETMY (CuraGen Corporation). The resultant construct, pETMY-FGF-20 (construct
2) has a 6
amino acid histidine tag and a T7 tag fused in-frame to the amino terminus of
CG53135. After
transformation of pETMY-FGF-20 into BL21 E.coli (Novagen, Madison, WI),
followed by T7 RNA
polymerase induction, CG53135-01 protein was detected in the soluble fraction
of the cells.
In order to express FGF-20 without tags, CG53135-05 (a codon-optimized, full-
length FGF-
20 gene) and CG53135-02 (a codon-optimized deletion construct of FGF-20, with
the N-terminal
amino acids 2-54 removed) were synthesized. For the full-length construct
(CG53135-05), an Nde I
restriction site (CATATG) containing the initiator codon was placed at the 5'
end of the coding
53
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
sequence. At the 3' end, the coding sequence was followed by 2 consecutive
stop codons (TAA)
and a Xho restriction site (CTCGAG). The synthesized gene was cloned into
pCRScript
(Stratagene, La Jolla, CA) to generate pCRScript-CG53135-05. An Nde I-Xho I
fragment containing
the codon-optimized CG53135-05 gene was isolated from the pCRscript-CG53135-05
and
subcloned into Nde I-Xho I-digested pET24a to generate pET24a-CG53135-05
(construct 3). The
full-length, codon-optimized version of FGF-20 is referred to as CG53135-05.
To generate a codon-optimized deletion construct for CG53135, oligonucleotide
primers
were designed to amplify the truncated FGF-20 gene from pCRScript-CG53135-05.
The forward
primer contained an Nde I site (CATATG) followed by coding sequence starting
at amino acid 55.
The reverse primer contained a Hindlll restriction site. A single PCR product
of approximately 480
base pairs was obtained and cloned into pCR2.1 vector (Invitrogen) to generate
pCR2.1-
CG53135del. An Nde I-Hind III fragment was isolated from pCR2.1-53135del and
subcloned into
Nde I-Hind III-digested pET24a to generate pET24a-CG53135-02 (construct 4).
The plasmids, pET24a-CG53135-05 (construct 3) and pET24a-CG53135-02 (construct
4)
have no tags. Each vector was transformed into E.coli BLR (DE3), induced with
isopropyl
thiogalactopyranoside. Both the full-length and the N-terminally truncated
CG53135 protein was
detected in the soluble fraction of cells.
6.2 EXAMPLE 2: MANUFACTURE OF CG53135-05 AND PHARMACEUTICAL
FORMULATIONS
Aiming for a construct that would be suitable for clinical development,
untagged molecules
were generated in a phage-free bacterial host. The codon-optimized, full-
length, untagged molecule
(CG53135-05) has the most favorable pharmacology profile and was used to
prepare product for the
safety studies and clinical trial.
6.2.1 PRODUCTION PROCESS AND PHARMACEUTICAL FORMULATIONS
(PROCESS 1)
CG53135-05 was expressed in Escherichia coli BLR (DE3) using a codon-optimized
construct, purified to homogeneity, and characterized by standard protein
chemistry techniques.
The isolated CG53135-05 protein migrated as a single band (23 kilodalton)
using standard SDS-
PAGE techniques and stained with Coommassie blue. The CG53135-05 protein was
electrophoretically transferred to a polyvinylidenefluoride membrane and the
stained 23 kD band
was excised from the membrane and analyzed by an automated Edman sequencer
(Procise,
Applied Biosystems, Foster City, CA); the N-terminal amino acid sequence of
the first 10 amino
acids was confirmed as identical to the predicted protein sequence.
Fermentation and Primary Recovery Recombinant
CG53135-05 was expressed using Escherichia co/i BLR (DE3) cells (Novagen).
These cells
were transformed with full length, codon optimized CG53135-05 using pET24a
vector (Novagen). A
Manufacturing Master Cell Bank (MMCB) of these cells was produced and
qualified. The
54
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
fermentation and primary recovery processes were performed at the 100 L (i.e.,
working volume)
scale reproducibly.
Seed preparation was started by thawing and pooling of 1- 6 vials of the MMCB
and
inoculating 4 - 7 shake flasks each containing 750 mL of seed medium. At this
point, 3-6 L of
inoculum was transferred to a production fermentor containing 60-80 L of start-
up medium. The
production fermentor was operated at a temperature of 37 C and pH of 7.1.
Dissolved oxygen was
controlled at 30% of saturation concentration or above by manipulating
agitation speed, air sparging
rate and enrichment of air with pure oxygen. Addition of feed medium was
initiated at a cell density
of 30-40 AU (600 nm) and maintained until end of fermentation. The cells were
induced at a cell
density of 40-50 AU (600 nm) using 1 mM isopropyl-beta-D-thiogalactoside
(IPTG) and CG53135-05
protein was produced for 4 hours post-induction. The fermentation was
completed in 10-14 hours
and about 100-110 L of cell broth was concentrated using a continuous
centrifuge. The resulting
cell paste was stored frozen at -70 C.
The frozen cell paste was suspended in lysis buffer (containing 3M urea, final
concentration)
and disrupted by high-pressure homogenization. The cell lysate was clarified
using continuous flow
centrifugation. The resulting clarified lysate was directly loaded onto a SP-
sepharose Fast Flow
column equilibrated with SP equilibration buffer (3 M urea, 100 mM sodium
phosphate, 20 mM
sodium chloride, 5 mM EDTA, pH 7.4). CG53135-05 protein was eluted from the
column using SP
elution buffer (100 mM sodium citrate, 1 M arginine, 5 mM EDTA, pH 6.0). The
collected material
was then diluted with an equal volume of SP elution buffer. After thorough
mixing, the SP
Sepharose FF pool was filtered through a 0.2 pm PES filter and frozen at -80
C.
Purification of the Drug Substance
The SP-sepharose Fast Flow pool was precipitated with ammonium sulfate. After
overnight
incubation at 4 C, the precipitate was collected by bottle centrifugation and
subsequently solubilized
in Phenyl loading buffer (100 mM sodium citrate, 500 mM L-arginine, 750 mM
NaCI, 5 mM EDTA,
pH 6.0). The resulting solution was filtered through a 0.45 uM PES filter and
loaded onto a Phenyl-
sepharose HP column. After washing the column, the protein was eluted with a
linear gradient with
Phenyl elution buffer (100 mM sodium citrate, 500 mM L-arginine, 5 mM EDTA, pH
6.0). The
Phenyl-sepharose HP pool was filtered through a 0.2 pm PES filter and frozen
at -80 C in 1.8 L
aliquots.
Formulation and Fill/Finish
Four batches of purified drug substance were thawed for 24 - 48 hours at 2 - 8
C and
pooled into the collection tank of tangential flow ultrafiltration (TFF)
equipment. The pooled drug
substance was concentrated -5-fold via TFF, followed by about 5-fold
diafiltration with the
formulation buffer (40 mM sodium acetate, 0.2 M L-arginine, 3% glycerol). This
buffer-exchanged
drug substance was concentrated further to a target concentration of >10
mg/mL. Upon transfer to
a collection tank, the concentration was adjusted to - 10 mg/mL with
formulation buffer. The
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
formulated drug product was sterile-filtered into a sterile tank and
aseptically filled (at 10.5 mL per
20 mL vial) and sealed. The filled and sealed vials were inspected for fill
accuracy and visual
defects. A specified number of vials were drawn and labeled for release
assays, stability studies,
safety studies, and retained samples. The remaining vials were labeled for the
clinical study, and
finished drug product was stored at -80 15 C.
The finished drug product is a sterile, clear, colorless solution in single-
use sterile vials for
injection. CG53135-05 E. coli purified product was formulated at a final
concentration of 8.2 mg/mL
(Table 3).
Table 3: Composition of Drug Product
Component Grade Final concentration Amount per Liter
CG53135-05 E. coli NA 8.2 mg/ml 8.2 g
purified product
Formulation Buffer
Sodium acetate USP 40 mM 5.44 g
(trihydrate)
L-arginine HCI USP 200 mM 42.132
Glycerol USP 3% v/v 30 mL
Acetic acid USP NA QS to H 5.3
Water for injection USP NA QS to 1 L
The pharmacokinetics of optimally-formulated CG53135-05 E. coli purified
product was
assessed in rats following intravenous, subcutaneous, and intraperitoneal
administration to compare
exposure at active doses in animal models and predict exposure in humans.
Intravenous
administration of CG53135-05 E. coli purified product resulted in high plasma
levels (maximum
plasma level = 19,680-47,252 ng/mL), which rapidly declined within the first 2
hours to 30-70 ng/mL;
decreased exposure was observed following the third daily dose (maximum plasma
level = 5373-
7453 ng/mL). Subcutaneous administration of CG53135-05 E. coli purified
product resulted in slow
absorption (maximum plasma level at 10 hours) and plasma levels of 40-80 ng/mL
up to 48 hours
after dosing; some accumulation in plasma was seen following the third daily
dose. Intraperitoneal
administration of CG53135-05 E. coli purified product resulted in slow
absorption (maximum plasma
level at 2-4 hours) and plasma levels of 40-70 ng/mL up to 10 hours after
dosing; decreased
exposure was seen following third daily dose. No significant gender
differences were observed by
any route of administration.
Safety of intravenous administration of CG53135-05 E. coli purified product
(0.05, 5 or 50
mg/kg/day (Bradford) for 14 consecutive days) was assessed in a pivotal
toxicology study in rats.
There were no treatment-related findings in rats administered 0.05 mg/mL
(Bradford) CG53135-05
E. coli purified product for 14 days. In rats administered 5 mg/kg (Bradford)
CG53135 for 14 days,
food consumption was reduced and body weight was decreased; while there were
no treatment-
related changes in organ weights, urinalysis, ophthalmology, or histopathology
parameters in this
dose group, there were treatment-related changes in hematology and clinical
chemistry parameters
56
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
in this treatment group. In rats administered 50 mg/kg (Bradford) CG53135-05
E. coli purified
product for 12 days (estimated maximum plasma level of 20-30 fold higher than
active dose), food
consumption was reduced and body weight was markedly decreased; while there
were no
treatment-related changes in ophthalmology, there were significant treatment-
related changes in
organ weights, urinalysis, hematology, clinical chemistry, and histopathology
in this treatment group.
Safety of intravenous administration of CG53135-05 E. coli purified product (0
or 10
mg/kg/day (Bradford) for 7 consecutive days) was further assessed in a safety
pharmacology study
in rhesus monkeys. There were no treatment-related clinical observations in
animals administered 1
mg/kg (Bradford) CG53135-05 E. coli purified product for 7 days. In animals
administered 10 mg/kg
(Bradford) CG53135-05 E. coli purified product for 7 days, minor effects on
body weight were noted
and associated with qualitative observations of lower food consumption. There
were no apparent
treatment-related effects on hematology, clinical chemistry, ophthalmology, or
electrophysiology in
either dose group.
Stability of CG53135-05 Drug Substances
Stability studies on the CG53135-05 E. coli purified product produced during
cGMP
manufacturing were performed. The analytical methods used as stability
indicating assays for
purified drug substance are listed in Table 4.
Table 4. Stability Assays for Drug Substance
Assay Stability Criteria
SDS-PAGE (Neuhoff stain) >98% pure by densitometry (reduced and
nonreduced)
RP-HPLC Peak at 5.5 t 1.0 min relative retention time
SEC-HPLC >90% mono-disperse peak
Total protein by Bradford method >0.2 mg/mL
Bioassay (BrdU) P1200 > 0.5 ng/mL and <20 ng/mL
pH 5.8 0.4
Visual appearance Clear and colorless
P1200 = concentration of CG53135-05 that results in incorporation of BrdU at 2
times the background
The SDS-PAGE, RP-HPLC, and Bradford assays are indicative of protein
degradation or
gross aggregation. The SEC-HPLC assay detects aggregation of the protein or
changes in
oligomerization, and the bioassay detects loss of biological activity of the
protein. The stability
studies for the purified drug substance were conducted at -80 to 15 C with
samples tested at
intervals of 3, 6, 9, 12, and 24 months.
In one experiment, stability studies of finished drug product were conducted
by Cambrex at
-80 t15 C and -20 t 5 C with samples tested at intervals of 1, 3, 6, 9, 12,
and 24 months. Stability
data collected after 1 month indicate that finished drug product is stable for
at least 1 month when
stored at -80 t15 C or at -20 5 C (Table 5).
57
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 5. Stability Data for Drug Product after 1-month interval
Assay Stability Criteria Initial -80 t15 C -20 t5 C
RP-HPLC Major peak Major peak Major peak Major peak
retention time 0.2 retention time t retention time t retention time t
min relative to 0.2 min relative 0.2 min relative 0.2 min relative
Reference Standard to Reference to Reference to Reference
Standard Standard Standard
SDS-PAGE Major band Pass Pass Pass
migrates at about
23 kDa;
nonreduced minor
band below major
band
SEC-HPLC >90% mono-disperse 100% 100% 100%
peak
Bradford 10 t 0.2 m/mL 8.2 8.6 8.3
Bioassay Pl2oo > 0.5 ng/mL 4.14 ng/mL 2.98 ng/mL 1/45 ng/mL
and <20 ng/mL
Sterility Pass (ie., no Pass NT NT
growth)
pH 5.3t0.3 5.4 5.5 5.4
Visual Clear and colorless Pass Pass Pass
ap earance solution
Lot # 02502001 was stored at -80 t15 C or at -20 t 5 C at Cambrex and tested
after 1
month; P1200 = concentration of CG53135-05 that results in incorporation of
BrdU at 2 times the
background; Pass = results met stability criterion; NT = not tested
In another experiment, samples of finished drug product were stored at -80 t15
C or
stressed at 5 t3 C, 25 t2 C, or 37 t2 C and tested at various intervals for 1
month. Stability data
indicate that finished drug product showed no significant instability after 1
month of storage at -80
t150C or 5 t3 C. When stressed at 25 t2 C, finished drug product was stable
for at least 48 hours;
degradation was apparent after I week at this temperature. When stressed at 37
t2 C, degradation
of finished drug product was apparent within 4 hours.
6.2.2 IMPROVED PHARMACEUTICAL FORMULATIONS AND PRODUCTION
PROCESS OF CG53135-05 (PROCESS 2)
An improved manufacture process for producing a drug product comprising one or
more
CG53135 proteins for clinical uses has been developed. FIG. I shows the steps
involved in the
improved manufacturing process of CG53135. The codon-optimized, full-length,
untagged molecule
of CG53135-05 (construct 3 in Example 1) was used. The process steps for the
improved
manufacture process are described below.
Cell Bank: a Manufacturing Master Cell Bank (MMCB) in animal component free
complex
medium was used in an earlier Process. A second Manufacturing Master Cell Bank
(MMCB) in
animal component free chemically defined medium was derived from the first
MMCB and a
58
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Manufacturing Working Cell Bank (MWCB) was made from the second MMCB. This
MWCB was
used in the manufacturing process as described in FIG. 1.
Innoculum Preparation: the initial cell expansion occurs in shake flasks. Seed
preparation
is done by thawing and pooling 2 -3 vials of the MWCB in chemically defined
medium and
inoculating 3 - 4 shake flasks each containing 500 mL of chemically defined
seed medium.
Seed and Final Fermentation: the shake flasks with cells in exponential growth
phase (2.5
- 4.5 OD600 units) are used to inoculate a single 25 L (i.e., working volume)
seed fermenter
containing the seed medium. The cells upon reaching exponential growth phase
(3.0 - 5.0 OD600
units) in the 25 L seed fermenter are transferred to a 1500 L production
fermenter with 780 - 820 L
of chemically defined batch medium. During fermentation, the temperature is
controlled at 37 2 C,
pH at 7.1 0.1, agitation at 150 - 250 rpm and sparging with 0.5 - 1.5 (vvm)
of air or oxygen-
enriched air to control dissolved oxygen at 25% or above. Antifoam agent
(Fermax adjuvant 27) is
used as needed to control foaming in the fermenter. When the OD (at 600 nm) of
culture reaches
25-35 units, additional chemically defined medium is fed at 0.7 g/kg broth/min
initially and then with
feed rate adjustment as needed. The induction for expression of the CG53135-05
protein is started
when OD at 600 nm reaches 135 - 165 units. After 4 hours post-induction the
fermentation is
completed. The final fermentation broth volume is approximately 1500 L. The
culture is then chilled
to 10 - 15 C.
Homogenization: the chilled culture is diluted with cell lysis buffer at the
ratio of one part of
fermentation broth to two parts of cell lysis buffer (50 mM sodium phosphate,
60 mM EDTA, 7.5 mM
DTT, 4.5 M urea, pH 7.2. Polyethyleneimine (PEI), a flocculating agent is
added to the diluted
fermentation broth to a final PEI concentration at 0.033% (WN). The cells are
lysed at 10 - 15 C
with 3 passages through a high-pressure homogenizer at 750 - 850 bar.
Capture and Recovery: the chilled cell lysate is directly loaded in the upflow
direction onto
a pre-equilibrated Streamline SP expanded bed cation exchange column. During
the loading, the
bed expansion factor is maintained between 2.5 - 3.0 times the packed bed
column volume. After
loading, the column is flushed with additional Streamline SP equilibration
buffer (100 mM sodium
phosphate, 40 mM EDTA, 10 mM sodium sulfate, 3 M urea, pH 7.0) in the upflow
direction. The
column is then washed further with SP Streamline wash buffer (100 mM sodium
phosphate, 5 mM
EDTA, 25 mM sodium sulfate, 2.22 M dextrose, pH 7.0) in the downflow
direction. The protein is
eluted from the column with Streamline SP elution buffer (100 mM sodium
phosphate, 5 mM EDTA,
200 mM sodium sulfate, 1 M L-arginine, pH 7.0) in the downflow direction.
PPG 650M Chromatography: the SP Streamline eluate is loaded on to a pre-
equilibrated
PPG 650 M, hydrophobic interaction chromatography column. The column is
equilibrated and
washed with 100 mM sodium phosphate, 200 mM sodium sulfate, 5 mM EDTA, 1 M
Arginine pH 7Ø
The column is further washed with 100 mM sodium phosphate, 5 mM EDTA, 0.9 M
Arginine, pH 7Ø
The product is eluted with 100 mM sodium phosphate, 5 mM EDTA, 0.2 M Arginine,
pH 7Ø
59
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
CUNO Filtration: the PPG eluate is passed through an endotoxin binding CUNO
30ZA
depth filter. The filter is flushed first with water for injection (WFI) and
then with 100 mM sodium
phosphate, 5 mM EDTA, 0.2 M Arginine, pH 7.0 (PPG eluate buffer). After
flushing, the PPG eluate
is passed through the filter. Air pressure is used to push the final liquid
through the filter and its
housing.
Phenyl Sepharose Chromatography: the CUNO filtrate is then loaded on to a pre-
equilibrated Phenyl Sepharose hydrophobic interaction chromatography column.
The column is
equilibrated and washed with 100 mM sodium phosphate, 50 mM ammonium sulfate,
800 mM
sodium chloride, 0.5 M Arginine, pH 7Ø The product is eluted with 50 mM
sodium phosphate, 0.5
M Arginine, pH 7Ø
Concentration and Diafiltration: a 1% Polysorbate 80 is added to the Phenyl
Sepharose
eluate so that the final concentration in the drug substance is 0.01 %(w/v).
The eluate is then
concentrated in an ultrafiltration system to about 2 - 3 g/L. The retentate is
then diafiltered with 7
diafiltration volumes of 50 mM sodium phosphate, 0.5 M Arginine, pH 7.0
(Phenyl Sepharose elution
buffer). After diafiltration the retentate is concentrated between 12 - 15
g/L. The retentate is filtered
through a 0.22 pm filter and subsequently diluted to 10 g/L.
Bulk Bottling: the retentate from the concentration and diafiltration step is
filtered through a
0.22 pm pore size filter into 2 L single use teflon bottles. The bottles are
frozen at -70 C.
Drug Product / Vial: the Frozen Drug Substance is used for the manufacture of
the Drug
Product. The bottles of frozen Drug Substance are thawed at ambient
temperature. After the Drug
Substance is completely thawed, it is pooled in a sterile container, filtered,
filled into vials, partially
stoppered, and lyophilized. After completion of the freeze-drying process, the
vials are stoppered
and capped. The lyophilized Drug Product is stored at 2-8 C.
6.3 EXAMPLE 3: CHARATERIZATION OF REFERENCE STANDARD OF THE DRUG
PRODUCT
A protein reference standard was prepared using a 140L scale manufacturing
process that
was representative of the bulk drug substance manufacturing process as
described in Section 6.2.2
(Example 2). The reference standard was stored as I mL aliquots in 2 mL
cryovials at -80 C t
15 C. The proposed specifications for the reference standard are listed in
Table 6.
Table 6: Proposed Specifications of CG53135-05 Reference Standard
Property Assay Description of Expected Results
Purity = SDS-PAGE (reduced, colloidal => 98% pure by densitometry
Coomassie stain)
= SDS-PAGE (reduced, silver stain) = At 10 g load, less than 100 ng of
impurities are detectable
= SEC-HPLC = FIO*
= RP-HPLC = > 90 % main peak
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Property Assay Description of Expected Results
= Host cell protein Western Blot = As found
Identity = Western Blot = Major band - 23 kDa
= N-Terminal amino acid sequencing = Consistent with predicted primary N-
terminal amino acid sequence
= LC and MS = HPLC profile shows 1 major peak
which is confirmed by MS to be
= Total amino acid analysis CG53135-related
= Consistent with predicted primary
= Peptide mapping amino acid composition
= Fragment pattern consistent with
predicted primary amino acid
sequence
Strength = Total protein by A280 = 10 1 mg/mL
Potency = Bioassay (Relative Potency) = 60 - 140 % relative to reference
standard
Secondary = Far-UV CD spectroscopy = As found
Structure
Tertiary = UV derivative spectroscopy = As found
Structure = Near-UV CD spectroscopy = As found
Quartenary . Differential Scanning Calorimetry = As found
Structure = Light Scattering (SEC-HPLC) = As found
Safety . Residual DNA =< 100 pg/mg
= Endotoxin (USP <85> gel clot) = 2 EU/mg
= Bioburden = < 1 cfu/mL
Other . pH = 7.0 0.5
= Osmolarity = As found
= Sulfyhydryl content = As found
= Visual inspection = Clear and colorless solution
*FIO: for information only.
6.4 EXAMPLE 4: PURITY ANALYSIS OF THE DRUG PRODUCT
The drug products produced by the manufacturing process as described in
Section 6.2
(Example 2) are analyzed for purity by the experiments described in this
section.
6.4.1 Purity by SDS-PAGE Analyses
Purified protein using the improved manufacturing process as described in
Section 6.2.2
("Process 2") and the purified protein using manufacturing process as
described in Section 6.2.1
("Process 1") were analyzed by loading increasing amounts of protein on a 4-
12% gradient Bis-Tris
NuPAGE gel and stained with the gel code blue stain to detect trace impurities
(FIG. 2A). Purified
protein (from both Process 1 and Process 2, respectively) migrated as a single
major band under
reducing conditions (-23 kDa). No impurities above the LOD (<28 ng) were
detected.
61
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Purified protein of Process 1 and Process 2 was also analyzed by loading
increasing
amounts of protein on a 4-12% gradient Bis-Tris NuPAGE gel and using silver
stain to detect trace
impurities (FIG. 2B). Purified protein (from both Process 1 and Process 2,
respectively) migrated as
a single major band (-23 kDa) at all loads under reducing conditions.
6.4.2 Purity by RP-HPLC
Purified drug product was analyzed by reversed-phase high-performance liquid
chromatography (RP-HPLC). Purified protein from Process 1 and Process 2 was
loaded onto a
Protein C4 column (Vydac, 5 pm, 150 mm X 4.6 mm) using a standard HPLC system
in a mobile
phase containing water, acetonitrile and trifluoroacetic acid. Purified
protein from Process 1 elutes
as a major peak at 24.0 min and additional peaks at 24.3 and 24.7 minutes.
These represent
isoforms of CG53135-05. CG53135-05 obtained using Process 2 elutes as a major
peak with a
retention time of 24.0 minutes (FIG. 3). Characterization of these peaks is
discussed further below
in Section 6.7 (Example 7).
6.4.3 Purity by Size Exclusion-HPLC
Purified protein (from both Process 1 and Process 2, respectively) was
analyzed by size
exclusion chromatography (SEC-HPLC) with UV detection at 280 nm. Analysis was
performed by
injecting the protein onto a size exclusion HPLC column (Bio-Sil SEC-250, 0.78
cm X 30 cm, Bio-
Rad) using a standard HPLC system with a mobile phase containing 100 mM sodium
phosphate, 1
M arginine-HCI, pH 7Ø Purified protein eluted isocratically as a single mono-
disperse peak with a
retention time of 20.5 minutes (FIG. 4) for Process 1 and 2. This retention
time corresponds to an
apparent molecular weight of approximately 45 kilodaltons (when compared
against a set of
calibration standards run under identical conditions), which suggests that FGF-
20 exists as a non-
covalently linked dimer.
6.4.4 Host Cell Protein Determination via Western Blot
The levels of host cell protein impurities in purified drug product were
assessed qualitatively
by Western blot analysis. The purified CG53135 protein was resolved by SDS-
PAGE and
electrophoretically transferred to a nitrocellulose membrane. The membrane was
incubated with a
primary antibody (rabbit anti-E. coli, Dako Systems) followed by a secondary
antibody (goat anti-
rabbit Alkaline Phosphatase conjugated, Bio-Rad) and developed using standard
techniques. No
host cell protein impurities were visible for Process 1 and only one band (-
70kDa) is apparent from
Process 2 (FIG. 5).
6.4.5 Identity of CG53135 via Western Blot
Purified protein (from both Process 1 and Process 2, respectively) was
identified by Western
blot using rabbit polyclonal anti-CG53135 sera (FIG. 6). Purified CG53135-05
was resolved by
loading 10 pg of protein on a 4-12% gradient Bis-Tris NuPAGE gel and
electrophoretically
transferred to a nitrocellulose membrane. The membrane was incubated with a
primary antibody
62
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
(polyclonal anti-CG53135 sera) followed by a secondary antibody (goat anti-
rabbit Alkaline
Phosphatase conjugated, Bio-Rad) and then developed using standard techniques.
Purified protein
(from both Process 1 and Process 2, respectively), which migrates as a single
band of the expected
molecular weight (molecular weight of FGF-20) under reducing and nonreducing
conditions, is
immunoreactive with CG53135-specific antiserum.
6.5 EXAMPLE 5: POTENCY OF CG53135 PRODUCT
The potency was measured by cell growth of NIH 3T3 cells in response to the
purified
protein from Process 1 and Process 2. Cell growth was measured indirectly
using fluorescence by
the conversion of resazurin (CeIlTiter Blue Reagent) into resorufin. Using DEV-
10 (Process 1) as
the reference standard, the Process 2 interim reference standard was found to
have comparable
potency at 101 %. Several lots manufactured by Process 2 were analyzed. These
results are shown
in Table 7.
Table 7: Potency of Lots from Process 2 using DEV-1 0 (Process 1) as Reference
Standard
Lot Number Potency Result (%)
PHP 020904-1 113
PHP 020904-2 123
CUNO-0104-1 102
CUNO-0204-1 95
PT 0504A 114
PT 0504B 98
DS 1002-01 100
The average potency for all of the lots tested is 106.4 t 10.3. This indicates
that the
potency of lots from Process 2 are equivalent to lot DEV-1 0 made with Process
1. Residual DNA,
endotoxin and bioburden in the drug substance can also be tested using
qualified assays.
The biological activity of CG53135-05 related species collected from the 4
peaks identified
by LC and MS was measured by treatment of serum-starved cultured NIH 3T3
murine embryonic
fibroblast cells with various doses of the isolated CG53135-05 related species
and measurement of
incorporation of bromodeoxyuridine (BrdU) during DNA synthesis. For this
assay, cells were
cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum. Cells
were grown in 96-well plates to confluence at 37 C in 10% C02/air and then
starved in Dulbecco's
modified Eagle's medium for 24 - 72 hours. CG53135-05-related species were
added and
incubated for 18 hours at 37 C in 10% C02/air. BrdU (10 mM final
concentration) was added and
incubated with the cells for 2 hours at 37 C in 10% C02/air. Incorporation of
BrdU was measured by
enzyme-linked immunosorbent assay according to the manufacturer's
specifications (Roche
Molecular Biochemicals, Indianapolis, IN).
Peak 4 was not included in this assay since insufficient material was
collected (Peak 4 is
less than 3% of the total peak area for CG53135-05). CG53135-05 and material
collected from all 3
remaining fractions (i.e., Peak 1, 2, and 3) induced DNA synthesis in NIH 3T3
mouse fibroblasts in a
dose-dependent manner (Table 4). The P1200 was defined as the concentration of
protein that
resulted in incorporation of BrdU at 2 times the background. CG53135-05 and
CG53135-05 related
63
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
species recovered from all 3 measurable peaks demonstrated similar biological
activity with a P1200
of 0.7 - 11 ng/mL (Table 8).
Table 8: Biological Activity of CG53135-05 E. coli purified product (DEV10):
Induction of DNA
Synthesis
Pi200 (ng/mL) CG53135-05 (DEV 10) Peak 1 Peak 2 Peak 3
1.0 0.7 11 8.6
6.6 EXAMPLE 6: CHARACTERIZATION STUDIES FOR BIOCHEMICAL
COMPARABILITY
Characterization studies for comparison of the primary, secondary, tertiary
and quaternary
structure as stated below in Table 9 may also be done. The side-by-side
results of reference
standard (designated DEV10) from the Process 1 with reference standard
obtained using Process 2
(interim reference material) can be used to further demonstrate the
biochemical properties of the
purified CG53135 protein.
Table 9: Biochemical Characterization of CG53135-05 Reference Materials
Attributes Process 1 Process 2
SDS-PAGE (silver) SDS-PAGE (silver)
Purity HCP Western (HCP Western - release assay)
Western RP HPLC - peak identification
Identity (Primary N-Terminal sequencing N-Terminal sequencing
Structure) Total Amino acid analysis Total Amino acid analysis
Peptide mapping Peptide mapping / LC-MS
MALDI-TOF MS
Far UV CD spectroscopy
Secondary Structure
UV-derivative spectroscopy
Tertiary Structure Near UV CD spectroscopy
Light Scattering (SEC-HPLC)
Quartenary Structure Diff. scanning calorimetry
pH pH
Osmolality Osmolality
Other Sulfhydryl content Sulfhydryl content
6.7 EXAMPLE 7: CHARACTERIZATION OF PRIMARY STRUCTURE OF THE PURIFIED
PROTEIN
6.7.1 RP-HPLC Assay: Peak Identification
Purified drug substance (by both Process 1 and Process 2, respectively) was
further
analyzed by reversed-phase high-performance liquid chromatography (RP-HPLC)
with both UV and
electrospray mass spectrometric detection. Purified protein from either
Process 1 or Process 2 was
64
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
loaded onto a Protein C4 column (Vydac, 5 pm, 150 mm X 4.6 mm) using a
standard HPLC system
in a mobile phase containing water, acetonitrile and trifluoroacetic acid. The
elution gradient for this
method was modified to resolve four distinct chromatographic peaks eluting at
26.6, 27.3, 28.5 and
30.0 min respectively (FIG. 7). These peaks were characterized by electrospray
mass
spectrometry. As can be observed from the chromatograms, the four equipotent
peaks are present
in the purified final product from Process 1 and 2. However, the proportion of
these peaks (1, 3 and
4) is much lower in the final product purified by Process 2 with the
predominant form being Peak 2.
The identities of each peak from the RP-HPLC separation are indicated in Table
10.
Table 10: Identity of peaks from the RP-HPLC separation of CG53135-05 based
upon accurate
molecular weight determination.
Peak # Retention Molecular Assignment (residue #) ID Number Predicted
Time (min) Weight Molecular
Observed Weight
1 26.6 21329.2 24-211 CG53135-17 21329.2
1 26.6 22185.1 15-211 CG53135-16 22185.1
1 26.6 22412.4 12-211 CG53135-15 22412.4
2 27.3 23296.5 3-211 CG53135-13 23296.4
3 28.5 23498.9 1-211 CG53135-05 23498.7
4 30.0 23339.3 3-211(carbamylated) CG53135-13 23339.4
(carbamylated)
4 30.0 23539.7 1-211(carbamylated) CG53135-05 23539.7
(carbamylated)
All the variants/fragments in the final purified product have high activity in
the proliferation
assays. Thus these variants/fragments are expected to have same utility as
that of FGF-20. For the
purpose of convenience, the term "CG53135-05 E. coli purified product" is used
hereon to refer to a
purified protein product from E. coli expressing a CG53135-05 construct. For
example, a CG53135-
05 E. coli purified product may contain a mixture of the full length CG53135-
05 protein (SEQ ID
NO:2), CG53135-13 (SEQ ID NO:24), CG53135-15 (SEQ ID NO:28), CG53135-16 (SEQ
ID NO:30),
and CG53135-17 (SEQ ID NO:32), with the majority of the content being CG53135-
13 (SEQ ID
NO:24).
6.7.2 Edman Sequencing and Total Amino Acid Analysis
The experimental N-terminal amino acid sequence of the Process 1 reference
standard,
DEV10, and the Process 2 interim reference standard were determined
qualitatively. The reference
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
standards were resolved by SDS-PAGE and electrophoretically transferred to a
polyvinylidenefluoride membrane; the Coomassie-stained -23 kDa major band
corresponding to
each reference standard was excised from the membrane and analyzed by an
automated Edman
sequencer (Procise, Applied Biosystems, Foster City, CA). A comparison of the
two major
sequences is shown in Table 11 below. The predominant sequence for each
reference standard
were identical and corresponded to residues 3-20 in the theoretical N-terminal
sequence of
CG53135-05.
Table 11: Edman sequencing data for the first 20 amino acids of CG53135-05 for
Process 1 and 2.
Theoretical Amino Acid Residue
Residue
Position Process 1 Process 2
3 Pro Pro
4 Leu Leu
Ala Ala
6 Glu Glu
7 Val Val
8 Gly Gly
9 Gly Gly
Phe Phe
11 Leu Leu
12 Gly Gly
13 Gly Gly
14 Leu Leu
Glu Glu
16 Gly Gly
17 Leu Leu
18 Gly Gly
19 GIn GIn
Gln Gln
The experimental amino acid composition of the DEV10 reference standard and
the
PX3536G001-H reference standard were determined in parallel. Quadruplicate
samples of each
reference standard were hydrolyzed for 16 hours at 115 C in 100 pL of 6 N HCI,
0.2% phenol
containing 2 nmol norleucine as an internal standard. Samples were dried in a
Speed Vac
Concentrator and dissolved in 100 NL sample buffer containing 2 nmol
homoserine as an internal
standard. The amino acids in each sample were separated on a Beckman Model
7300 amino acid
analyzer. The amino acid composition of both reference standards showed no
significant
differences as shown in Table 8 below. Note that Cys and trp are destroyed
during acid hydrolysis
of the protein. Asn and gln are converted to asp and glu, respectively, during
acid hydrolysis and
thus their respective totals are reported as asx and glx. Met and his were
both unresolved in this
procedure.
66
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 12: Quantitive amino acid analysis for final purified protein from
Process 1 and Process 2
Amino Acid Mole Percent
Residue DEV10 PX3536G001-H
asx 7.1 7.0
thr 4.0 4.0
ser 6.3 6.1
glx 12.2 12.2
pro 6.0 6.0
gly 14.4 14.3
ala 5.8 5.6
val 5.3 5.3
ile 3.5 3.5
leu 13.6 13.6
tyr 4.6 4.6
phe 5.2 5.2
lys 3.7 3.7
arg 8.5 9.1
6.7.3 Tryptic Mapping by RP-HPLC
Purified drug substance from Process 1 and 2 was reduced and alklated with
iodoacetic
acid and then digested with sequencing grade trypsin. The tryptic peptides
were separated by
reversed-phase high-performance liquid chromatography (RP-HPLC) using both UV
and
electrospray mass spectrometric detection. The tryptic digest from either
Process 1 or Process 2
was loaded onto an ODS-1 nonporous silica column (Micra, 1.5 pm; 53 x 4.6 mm)
using a standard
HPLC system in a mobile phase containing water, acetonitrile and
trifluoroacetic acid. The eluting
peptides were detected by UV at 214 nm (FIG. 8) and by positive-ion
electrospray mass
spectrometry. The major difference between the two chromatograms for Process 1
and Process 2 is
the reduction in peak area of a peak obvious in the Process I trace ( peak at
8.2 min; FIG. 8). This
peak corresponds to the T1 peptide, residues 1-40. This observation is
expected since the source
of this peptide if from the intact CG53135-05, which is in greater abundance
in the Process I
material (peak 3, FIG. 7).
6.8 EXAMPLE 8: CHARACTERIZATION OF SECONDARY STRUCTURE: CIRCULAR
DICHOROISM SPECTROSCOPY OF THE PURIFIED PROTEIN
The far UV circular dichroism spectrum of the purified protein (from both
Process 1 and
Process 2, respectively) is characterized by a broad maximum at 226-227 nm and
a sharp minimum
at approximately 206 nm. Both features are common in other fibroblast growth
factors and suggest
a secondary structure dominated by R-sheet and R-turns. The far UV circular
dichroism spectra of
the DEV10 reference standard and the PX3536G001-H reference standard both
display these
features and are nearly identical (FIG. 9). The small differences in the
spectra are attributable to
experimental error.
67
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
6.9 EXAMPLE 9: CHARACTERIZATION OF TERTIARY STRUCTURE
6.9.1 Near UV Circular Dichroism Spectroscopy of the Purified Protein
The near UV Circular Dichroism (CD) spectrum of a protein reflects the number
and
orientation of the protein's aromatic amino acids. For proteins having
identical numbers of aromatic
amino acids any differences in their near UV CD spectra represent differences
in the position and
orientation of the aromatic amino acids. The position and orientation of the
aromatic amino acids
are a measure of a protein's tertiary structure. Hence, differences in the
near UV CD spectra for
proteins represent differences in tertiary structure.
The near UV CD spectra of the DEV10 reference standard and the PX3536G001-H
reference standard are shown in FIG. 10. There are no significant differences
between these two
spectra and suggest that both reference standards have no significant
differences in their tertiary
structure.
6.9.2 Second Derivative UV Absorbance Spectroscopy of the Purified Protein
The UV absorbance of aromatic amino acids is influenced by the amino acid's
microenvironment. Aromatic amino acids embedded within a protein are in a less
polar
microenvironment than surface exposed residues. This difference in polarity
has a profound effect
on the UV absorbance of an aromatic amino acid. Different microenvironments
can shift the spectra
of aromatic amino acids 4-6 nm in extreme cases. Monitoring these changes for
individual proteins
is done by calculating the second derivative of the protein's UV absorbance
spectrum. The second
derivative UV absorbance spectrum of a protein contains a number of minima
that correspond to the
individual aromatic amino acids. The wavelengths of these minima reflect the
microenvironment of
the amino acid. Therefore, changes in these minima are indicative of
conformational (tertiary)
changes in the protein.
The second derivative UV absorbance spectrum of the purified protein (from
both Process 1
and Process 2, respectively) is characterized by seven minima between 250 and
300 nm. As shown
in Table 13 below, and qualitatively in FIG. 11, the wavelengths of all seven
minima for both the
DEV10 reference standard and the PX3536G001-H reference standard are not
significantly
different. These data demonstrate that the microenvironment around the
individual aromatic amino
acids in both standards are highly similar and suggests significant tertiary
differences do not exist
between these two reference standards.
Table 13: Second derivative UV absorbance spectral data for Process 1 and 2
Sample Result Peak 1 Peak 2 Peak 3 Peak 4 Peak 5 Peak 6 Peak 7
(Phe) (Phe) (Phe) (Phe/T r) (Tyr) (T r/Tr ) (Trp)
DEV10 Average 252.70 258.60 264.60 268.90 277.95 285.38 293.50
DEV10 SD 0.00 0.00 0.00 0.00 0.05 0.04 0.07
PX3536G001-H Average 252.70 258.60 264.60 268.90 277.98 285.40 293.53
PX3536G001-H SD 0.00 0.00 0.00 0.00 0.04 0.00 0.08
Results represent the average of 5 replicates for each reference standard.
68
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
6.10 EXAMPLE 10: CHARACTERIZATION OF QUARTERNARY STRUCTURE
6.10.1 Differential Scanning Calorimetry of the Purified Protein
Differential scanning calorimetric analysis is based upon the detection of
changes in the
heat content (enthalpy) or the specific heat of a sample with temperature. As
thermal energy is
supplied to the sample its enthalpy increases and its temperature rises by an
amount determined,
for a given energy input, by the specific heat of the sample. The specific
heat of a protein changes
slowly with temperature in a particular physical state, but alters
discontinuously at a change of state,
e.g. melting or denaturation of the protein.
As can be seen in FIG. 12 the melting curves for the purified protein are
similar and the
average Tm (melting temperature) is 62.25 C for Process 1 and 62.02 C for
Process 2. These
differences are within the experimental error of the instrument.
6.11 EXAMPLE 11: MEASURING SULFHYDRYL CONTATENT OF THE PURIFIED
PROTEIN
The sulfhydryl content of the purified protein from Process 1 and Process 2
were measured
(Table 14). The purified protein (from Process I and Process 2, respectively)
was analyzed for total
sulfhydryl content spectrophotometrically using 5, 5'-dithio-bis (2-
nitrobenzoic acid), commonly
referred to as Eliman's reagent. The results indicate that the total number of
measurable sulfhydryis
in the final product is the same for Process 1 and Process 2 and are
sufficient to account for all the
theoretical sulfhydryls in the purified protein.
Table 14: Results from Other Characterization Assays Conducted on the purified
protein (Process 1
and Process 2, respectively)
Assay Process1 Process 2
Sulfh dr I(SH content 108.0 3.6 101.4 4.9
6.12 EXAMPLE 12: IMPROVED FORMULATIONS COMPRISING CG53135
A new formulation was developed to meet the three requirements for a
commercial product:
(1) the minimal storage temperature should be 2-8 C for ease of distribution;
(2) product should be
stable at the storage temperature for at least 18 months for a commercial
distribution system; and
(3) product should be manufactured by commercial scale equipment, and
processes should be
transferable to various commercial contract manufacturers.
The new formulation consists 10 mg/mL of the protein product produced by the
process
described in Section 6.2 ("Process 2 protein") in 0.5 M arginine as sulfate
salt, 0.05 M sodium
phosphate monobasic, and 0.01% (w/v) polysorbate 80. The lyophilized product
is projected to be
stable for at least 18 months at 2-8 C based on accelerated stability data. In
contrast to the new
formulation, the previous formulation as described in U.S. Application No.
10/435,087 is not possible
to be lyophilized for the following reasons: firstly, the acidic component of
the acetate buffer is acetic
acid, which sublimes during lyophilization. This loss of acetic acid to
lyophilization increases the pH
to > 7.5, which is far from the target pH of 5.3. Secondly, the glycerol has a
collapse temperature of
69
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
<-45 C, which renders this formulation not be able to be lyophilized
commercially. Most of the
commercial lyophilizers have a shelf temperature ranged from -45 C to -50 C
with temperature
variation of 3 C.
Four unexpected properties of CG53135 were discovered and used to develop the
new
formulation: (1) high concentration of arginine, >0.4 M, increases the
solubility to > 30 mg/mL; (2)
the use of sulfate salt of arginine increases the solubility by at least 2-6
folds; (3) the optimal
concentration of sodium phosphate as a buffering salt is 50 mM, with a
solubility of at least 1-2 fold
increase in comparison with concentrations at 25, 75, and 100 mM; and (4)
adding a surfactant
during the diafiltration/ultrafiltration step minimizes the formation of
aggregates. In development the
lyophilized formulation, each component of the new formulation was evaluated
for solubility
individually. CG53135-05 was precipitated using the precipitate buffer (50 mM
NaPi, 5 mM EDTA, 1
M L-Arginine HCI, 2.5 M(NH4)2SO4). The precipitate was washed with 25 mM
sodium phosphate
buffer at pH 6.5 to remove the residual arginine and ammonium sulfate. The
washed precipitate
was then re-dissolved in the following respective buffers listed in the
tables. The following are
examples of data.
Table 15. High concentration of arginine, >0.4 M, increases the solubility to
> 30 mg/mL
Concenctration of Solubilit of Process 2 protein in mg/mL
Arginine (M) Batch #1 Batch #2 Batch #3 Batch #4 Batch #5
0.05 0.7 0.6 0.5 ND ND
0.10 1.4 0.6 1.2 ND ND
0.15 2.2 1.6 2.2 ND ND
0.20 3.0 4.7 4.3 ND ND
0.30 ND ND ND 5.8 ND
0.35 ND ND ND 10.1 ND
0.40 ND ND ND 9.8 ND
0.45 ND ND ND 32.3 ND
0.50 ND ND ND 23.8* 37
*: The solubility was lower as there was not sufficient protein in the
experiment to be dissolved.
Table 16. The optimal concentration of sodium phosphate as a buffering salt is
50 mM
Concentration of Solubility of Process 2 protein in mg/mL
sodium phosphate
monobasic* Batch #A Batch #B Batch #C Batch #D Batch #E
100 mM 3.78 2.8 2.4 2.9 2.47
75 mM 4.06 2.5 2.6 3.0 2.38
50 mM 5.47 4.7 3.3 4.3 4.81
25 mM 4.01 2.4 2.6 2.4 3.59
All formulation contains 0.2 M arginine.
An optimal concentration of the sodium phosphate as a buffering salt was
observed (Table
16). The optimal concentration of sodium phosphate is 50 mM with a solubility
of at least 1-2 fold
increase in comparison with concentrations at 25, 75, and 100 Mm.
Table 17. The use of sulfate salt of arginine increases the solubility by at
least 1-3 folds
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Solubility Increament of Process 2 protein in
using Arginine Sulfate vs Arginine Phosphate
in mg/mL
Formulation Batch #K Batch # J
50mM sodium phosphate monobasic and
0.15M Arginine at pH 7 4.4 2.3
50mM sodium phosphate monobasic and
0.15M Arginine at pH 7 6.5 5.2
Table 18 shows a need to add a surfactant during the
diafiltration/ultrafiltration step to
minimize the formation of aggregates. The experiment was conducted by
performing the
ultrafiltration/diafiltration at 2.5 mg/mL CG53135-05 in 0.2M arginine and
0.05 M sodium phosphate
buffer at pH 7Ø After exchanging with 7 volumes of the final buffer (0.5M
arginine and 0.05 M
sodium phosphate buffer at pH 7.0), the diafiltrate is concentrated to -20
mg/mL. The diafiltrate is
then diluted with the final buffer to -12.5 mg/mL and lyophilized. Polysorbate
80 is added either
before or after the diafiltration to a final concentration of 0.01%.
Table 18. Adding a surfactant during the diafiltration/ultrafiltration step
minimizes the formation of
aggregates.
Polysorbate added
during Process 2 protein Concentration
ultrafiitration/diafiltration (m /mL) Turbidity (NTU)
Yes 12.5 20.9
No 13.0 4.6
All formulation contains 0.5 M arginine, 0.05 M sodium phosphate monobasic,
and 0.01%
polysorbate 80.
The new formulation has the following advantages: (1) a lyophilized product
with a storage
temperature of 2-8 C; (2) a lyophilized product with a projected shelf-life of
at least 18 months when
stored at 2-8 C achieve the solubility of > 30 mg/mL; and (3) The lyophilized
product has a collapse
temperature of -30 C which can be easily lyophilized by the commercial
equipment. The
interactions between arginine, sulfate, phosphate, and surfactant and CG53135
were unexpected.
6.13. EXAMPLE 13: IDENTIFICATION OF SINGLE NUCLEOTIDE POLYMORPHISMS
IN FGF-20 NUCLEIC ACID SEQUENCES
This example demonstrated how some of the single nucleotide polymorphisms
(SNPs) of
FGF-20 were identified. A SNP can, in some instances, be referred to as a
"cSNP" to denote that
the nucleotide sequence containing the SNP originates as a cDNA. SNPs
occurring within a gene
may result in an alteration of the amino acid encoded by the gene at the
position of the SNP.
Intragenic SNPs may also be silent, when a codon including a SNP encodes the
same amino acid
as a result of the redundancy of the genetic code. SNPs occurring outside the
region of a gene, or
in an intron within a gene, do not result in changes in any amino acid
sequence of a protein but may
result in altered regulation of the expression pattern. Non-limiting examples
include alteration in
temporal expression, physiological response regulation, cell type expression
regulation, intensity of
expression, and stability of transcribed message.
71
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
SeqCallingTM assemblies produced by the exon linking process were selected and
extended
using the following criteria: genomic clones having regions with 98% identity
to all or part of the
initial or extended sequence were identified by BLASTN searches using the
relevant sequence to
query human genomic databases. The genomic clones that resulted were selected
for further
analysis because this identity indicates that these clones contain the genomic
locus for these
SeqCallingTM assemblies. These sequences were analyzed for putative coding
regions as well as
for similarity to the known DNA and protein sequences. Programs used for these
analyses include
Grail, Genscan, BLAST, HMMER, FASTA, Hybrid and other relevant programs.
Some additional genomic regions may have also been identified because selected
SeqCallingTM assemblies map to those regions. Such SeqCallingTM sequences may
have
overlapped with regions defined by homology or exon prediction. They may also
be included
because the location of the fragment was in the vicinity of genomic regions
identified by similarity or
exon prediction that had been included in the original predicted sequence. The
sequence so
identified was manually assembled and then may have been extended using one or
more additional
sequences taken from CuraGen Corporation's human SeqCallingTM database.
SeqCallingTM
fragments suitable for inclusion were identified by the CuraToolsTM program
SeqExtend or by
identifying SeqCalling fragments mapping to the appropriate regions of the
genomic clones
analyzed.
The regions defined by the procedures described above were then manually
integrated and
corrected for apparent inconsistencies that may have arisen, for example, from
miscalled bases in
the original fragments or from discrepancies between predicted exon junctions,
EST locations and
regions of sequence similarity, to derive the final sequence disclosed herein.
When necessary, the
process to identify and analyze SeqCallingTM assemblies and genomic clones was
reiterated to
derive the full length sequence (Alderborn et al., Genome Research 10 (8) 1249-
1265 (2000)).
Variants are reported individually in Table 19, but any combination of all or
select subset of
the variants is also encompassed by the present invention.
Table 19. SNPs of CG53135-01 (SEQ ID NOs: I and 2)
Nucleotides Amino Acids
Variant
Position Initial Modified Position Initial Modified
13377871 301 A G 101 IIe Val
13375519 361 A G 121 Met Val
13375518 517 G A 173 Gly Arg
13375516 523 C G 175 Pro Ala
13381791 616 G A 206 Asp Asn
72
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
6.14. EXAMPLE 14: STIMULATION OF BROMODEOXYURIDINE INCORPORATION
INTO NIH 3T3 CELLS IN RESPONSE TO A TRUNCATED FORM OF FGF-20
A vector expressing residues 24-211 of FGF-20 ((d1-23)FGF-20; See Table 1 and
SEQ ID
NO:32 (CG53135-17) was prepared. The incorporation of BrdU by NIH 3T3 cells
treated with
conditioned medium obtained using the vector incorporating this truncated form
was compared to
the incorporation in response to treatment with conditioned medium using a
vector encoding full
length FGF-20. This experiment was carried out as following:
293-EBNA cells (Invitrogen) were transfected using Lipofectamine 2000
according to the
manufacturer's protocol (Life Technologies, Gaithersburg, MD). Cells were
supplemented with 10%
fetal bovine serum (FBS; Life Technologies) 5 hours post-transfection. To
generate protein for BrdU
and growth assays, cells were washed and fed with Dulbecco's modified Eagle
medium (DMEM;
Life Technologies) 18 hours post-transfection. After 48 hours, the media was
discarded and the cell
monolayer was incubated with 100 pM suramin (Sigma, St. Louis, MO) in 0.5 ml
DMEM for 30 min
at 4 C. The suramin-extracted conditioned media was then removed, clarified by
centrifugation (5
min; 2000 X g), and subjected to TALON metal affinity chromatography according
to the
manufacturer's instructions (Clontech, Palo Alto, CA) taking advantage of the
carboxy-terminal
polyhistidine tag. Retained fusion protein was released by washing the column
with imidazole.
FGF-20 protein concentrations were estimated by Western analysis using a
standard curve
generated with a V5-tagged protein of known concentration. For Western
analysis, conditioned
media was harvested 48 hours post transfection, and the cell monolayer was
then incubated with
0.5 ml DMEM containing 100 pM suramin for 30 min at 4 C. The suramin-
containing conditioned
media was then harvested.
Recombinant FGF-20 and (d1-23)FGF-20 were tested for their ability to induce
DNA
synthesis in a bromodeoxyuridine (BrdU) incorporation assay. NIH 3T3 cells
(ATCC number CRL-
1658, American Type Culture Collection, Manassas, VA), CCD-1070Sk cells (ATCC
Number CRL-
2091) or MG-63 cells (ATCC Number CRL-1427) were cultured in 96-well plates to
-100%
confluence, washed with DMEM, and serum-starved in DMEM for 24 hours (NIH 3T3)
or 48 hours
(CCD-1070Sk and MG-63). Recombinant FGF-20 or (dl-23)FGF-20 was then added to
the cells for
18 hours. The BrdU assay was performed according to the manufacturer's
specifications (Roche
Molecular Biochemicals, Indianapolis, IN) using a 5 hour BrdU incorporation
time.
The results are shown in FIG. 13. It indicates that (d1-23)FGF-20 retains high
activity at the
lowest concentration tested, 10 ng/mL. At this concentration, the activity of
full length FGF-20 has
fallen considerably, approaching the level of the control. It is estimated
that (dl-23)FGF-20 may be
at least 5-fold more active than full length FGF-20.
73
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
6.15. EXAMPLE 15: CELLULAR PROLIFERATION RESPONSES WITH CG53135
(STUDIES L-117.01 AND L-117.02)
Experiments were performed to evaluate the proliferative response of
representative cell
types to CG53135, e.g., a full-length tagged variant (CG53135-01), a deletion
variant (CG53135-02),
and a full-length codon-optimized untagged variant (CG53135-05).
Materials and Methods:
Heterologous Protein Expression: CG53135-01 (batch 4A and 6) was used in these
experiments. Protein was expressed using Escherichia coli (E. coli), BL21
(Novagen, Madison, WI),
transformed with full-length CG53135-01 in a pETMY-hFGF20X/BL21 expression
vector. Cells were
harvested and disrupted, and then the soluble protein fraction was clarified
by filtration and passed
through a metal chelation column. The final protein fraction was dialyzed
against phosphate
buffered saline (PBS) plus 1 M L-arginine. Protein samples were stored at -70
C.
CG53135-02 (batch 1 and 13) was also used in these experiments. Protein was
expressed
in E. coli, BLR (DE3) (Novagen), transformed with the deletion variant CG53135-
02 inserted into a
pET24a vector (Novagen). A research cell bank (RCB) was produced and cell
paste containing
CG53135-02 was produced by fermentation of cells originating from the RCB.
Cell membranes
were disrupted by high-pressure homogenization, and lysate was clarified by
centrifugation.
CG53135-02 was purified by ion exchange chromatography. The final protein
fraction was dialyzed
against the formulation buffer (100 mM citrate, 1 mM
ethylenediaminetetraacetic acid (EDTA), and 1
M L-arginine).
CG53135-05, DEV10, which were also used in these experiments, was prepared by
Cambrex Biosciences (Hopkinton, MA) according to Process 1 as described in
Section 6.18.1, infra.
BrdU Incorporation: proliferative activity was measured by treatment of serum-
starved
cultured cells with a given agent and measurement of BrdU incorporation during
DNA synthesis.
Cells were cultured in respective manufacturer recommended basal growth medium
supplemented
with 10% fetal bovine serum or 10% calf serum as per manufacturer
recommendations. Cells were
grown in 96-well plates to confluence at 37 C in 10% C02/air (to subconfluence
at 5% CO2 for
dedifferentiated chondrocytes and NHOst). Cells were then starved in
respective basal growth
medium for 24-72 hours. CG53135 protein purified from E. coli or pCEP4/Sec or
pCEP4/Sec-FGF
20X enriched conditioned medium was added (10 pL/1 00 pL of culture) for 18
hours. BrdU (10 pM
final concentration) was then added and incubated with the cells for 5 hours.
BrdU incorporation
was assayed according to the manufacturer's specifications (Roche Molecular
Biochemicals,
Indianapolis, IN).
Growth Assay: growth activity was obtained by measuring cell number following
treatment of
cultured cells with a given agent for a specified period of time. In general,
cells grown to -20%
confluency in 6-well dishes were treated with basal medium supplemented with
CG53135 or control,
incubated for several days, trypsinized and counted using a Coulter Z1
Particle Counter.
74
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Results:
Proliferation in Mesenchymal Cells: to determine if recombinant CG53135 could
stimulate
DNA synthesis in fibroblasts, a BrdU incorporation assay was performed using
CG53135-01 treated
NIH 3T3 murine embryonic lung fibroblasts. Recombinant CG53135-01 induced DNA
synthesis in
NIH 3T3 mouse fibroblasts in a dose-dependent manner (FIG. 14(A)). DNA
synthesis was generally
induced at a half maximal concentration of -10 ng/mL. In contrast, treatment
with vehicle control
purified from cells did not induce any DNA synthesis.
CG53135-01 also induced DNA synthesis in other cells of mesenchymal origin,
including
CCD-1070Sk normal human foreskin fibroblasts, MG-63 osteosarcoma cell line,
and rabbit
synoviocyte cell line, HIG-82. In contrast, CG53135-01 did not induce any
significant increase in
DNA synthesis in primary human osteoblasts (NHOst), human pulmonary artery
smooth muscle
cells, human coronary artery smooth muscle cells, human aorta smooth muscle
cells (HSMC), or in
mouse skeletal muscle cells.
To determine if recombinant CG53135-01 sustained cell growth, NIH 3T3 cells
were
cultured with I lag CG53135-01 or control for 48 hours and then counted (FIG.
14(B)). CG531 35
induced an approximately 2-fold increase in cell number relative to control in
this assay. These
results show that CG53135 acts as a growth factor.
Proliferation of Epithelial Cells: to determine if recombinant CG53135 can
stimulate DNA
synthesis and sustain cell growth in epithelial cells, a BrdU incorporation
assay was performed in
representative epithelial cell lines treated with CG53135. Cell counts
following protein treatment
were also determined for some cell lines.
CG53135 was found to induce DNA synthesis in the 786-0 human renal carcinoma
cell line
in a dose-dependent manner (FIG. 14(C)). In addition, CG53135-01 induced DNA
synthesis in other
cells of epithelial origin, including CCD 1106 KERTr human keratinocytes, Balb
MK mouse
keratinocytes, and breast epithelial cell line, B5589.
Proliferation of Hematopoietic Cells: no stimulatory effect on DNA synthesis
was
observed upon treatment of TF-1, an erythroblastic leukemia cell line with
CG53135-01. These data
suggest that CG53135-01 does not induce proliferation in cells of erythroid
origin. In addition,
Jurkat, an acute T-lymphoblastic leukemia cell line, did not show any response
when treated with
CG53135-01, whereas a robust stimulation of BrdU incorporation was observed
with serum
treatment.
Effects of CG53135 on Endothelial Cells: protein therapeutic agents may
inhibit or
promote angiogenesis, the process through which endothelial cells
differentiate into capillaries.
Because CG53135 belongs to the fibroblast growth factor family, some members
of which have
angiogenic properties, the antiangiogenic or pro-angiogenic effects of CG53135
on endothelial cell
lines were evaluated. The following cell lines were chosen because they are
cell types used in
understanding angiogenesis in cancer: HUVEC (human umbilical vein endothelial
cells), BAEC
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
(bovine aortic endothelial cells), HMVEC-d (human endothelial, dermal
capillary). These endothelial
cell types undergo morphogenic differentiation and are representative of large
vessel (HUVEC,
BAEC) as well as capillary endothelial cells (HMVEC-d).
CG53135-01 treatment did not alter cell survival or have stimulatory effects
on BrdU
incorporation in human umbilical vein endothelial cells, human dermal
microvascular endothelial
cells or bovine aortic endothelial cells. Furthermore, CG53135-01 treatment
did not inhibit tube
formation, an important event in formation of new blood vessels, in HUVECS.
This result suggests
that CG53135 does not have anti-angiogenic properties. Finally, CG53135-01 had
no effect on
VEGF induced cell migration in HUVECs, suggesting that it does no play a role
in metastasis.
The above described experiments were also performed using CG53135-02 and
CG53135-
05 protein products, and the results are summarized in the Conclusion section
below.
Conclusions
Recombinant CG53135-01 (which encode the same protein as CG53135-05) induces a
proliferative response in mesenchymal and epithelial cells in vitro (i.e., NIH
3T3 mouse fibroblasts,
CCD-1070 normal human skin fibroblasts, CCD-1106 human keratinocytes, 786-0
human renal
carcinoma cells, MG-63 human osteosarcoma cells and human breast epithelial
cells), but not in
human smooth muscle, erythroid, or endothelial cells. Like CG53135-01 and
CG53135-05,
CG53135-02 also induces proliferation of mesenchymal and epithelial cells. In
addition, CG53135-
02 induces proliferation of endothelial cells.
6.16. EXAMPLE 16: ACTIVITY OF CG53135 IN HAMSTER MODEL OF
CHEMOTHERAPY-INDUCED ORAL MUCOSITIS (N-212 STUDY)
CG53135 was evaluated for the treatment of chemotherapy-induced oral mucositis
in male
Golden Syrian hamsters (protein concentrations in this Example were measured
by Bradford assay).
Materials and Methods
CG53135-05 used in this study (batch 29-NB849:76) was expressed and purified
as
described in Section 6.5, with the exception that the final protein fraction
was dialyzed against
formulation buffer containing 30 mM sodium citrate, 2 mM EDTA, 200 mM
sorbitol, 50 mM KCI, 20%
glycerol (pH 6.1).
Male golden Syrian hamsters (Charles River Laboratories) age 5 to 6 weeks and
with similar
body weight in all groups at study commencement were used in this study. Sixty
male hamsters
were randomized into 6 groups of 10 animals each prior to irradiation. The
treatment groups are
outlined in Table 20.
76
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 20. Treatment Groups
Group No. Treatment (0.1 mL, IP) Dosing Schedule
I Vehicle (Disease control) Day 1 to Day 18
2 CG53135-05 E. coli purified product, 12 Day 1 to Day 18
m /k /da
3 CG53135-05 E. coli purified product, 12 Day 6 to Day 14
m/k/da
4 CG53135-05 E. coli purified product, 12 Day I to Day 9
m /k /da 5 CG53135-05 E. coli purified product, 12 Day 1 to Day 6
m /k /da 6 CG53135-05 E. coli purified product, 12 Day 1 to Day 2
m/k/da
Mucositis was induced using 5-fluorouracil, delivered as single bolus (60
mg/kg, IP) on Days
-4 and -2. A single submucosatoxic dose of radiation (40 Gy/dose) was locally
administered to all
animals on Day 0. Animals were treated once daily with 0.1 mL vehicle or 12
mg/kg CG53135-05 IP
following mucosa toxic insult according to the schedule shown in Table 11.
Mucositis was scored
visually as described in Section 6.5 (Table 9) on alternate days beginning on
Day 6 and every
second day until the conclusion of the experiment on Day 30 (i.e., Days 8, 10,
12, 14, 16, 18, 20, 22,
24, 26, 28, and 30). Each hamster was weighed daily for the period of the
study (i.e., Day 0 to Day
30). Weight and survival were monitored as indices for severity of mucositis
or possible toxicity
resulting from treatment.
The effect of each treatment on mucositis compared with the control group was
assessed
according to the parameters listed in Table 21. Statistical differences
between treatment groups
were determined using the Student's t-test, Mann-Whitney U test, and chi-
square analysis, with a
critical value of 0.05.
77
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 21. Parameters for evaluation of Activity
Parameter Description
The difference in the number of days On each evaluation day, the number of
hamsters in each group have severe animals with a blinded mucositis score of
mucositis (score _3). _ 3 in each drug treatment group was
compared to the vehicle control group.
Differences were analyzed on a
cumulative basis. Treatment success
was considered a statistically significant
lower number of hamsters with this score
in a drug treatment group, versus the
vehicle control value, as determined by
chi-square analysis.
The rank sum differences in daily mucositis For each evaluation day the scores
of the
scores. vehicle control group was compared to
those of the treated group using the non-
parametric rank sum analysis. Treatment
success was considered as a statistically
significant lowering of scores in the
treated group on 2 or more days from
Day 6 to Day 30.
Results
There were no statistically significant differences in weight or survival over
time between the
vehicle control group (Group 1) and CG53135-05 E. coli purified product
treatment groups (Groups
2-6).
In this model of mucositis primarily induced by chemotherapy, dosing schedule
was
important in the treatment of oral mucositis. Administration of CG53135-05 E.
coli purified product
(12 mg/kg/day) from Day 6 to Day 14 or Day 1 to Day 9 did not result in
significant improvement in
the course or severity of mucositis (FIG. 15). Administration of CG53135-05 E.
coli purified product
(12 mg/kg/day) from Day 1 to Day 18 or Day 1 to Day 6 resulted in significant
improvement of the
duration of severe mucositis (Chi-square analysis). However, these treatments
did not result in
significant improvement of daily mucositis scores (rank sum analysis).
Treatment with 12 mg/kg/day
CG53135-05 E. coli purified product (Day 1 to Day 2) had a significant effect
on both the course and
severity of mucositis in this study (FIG. 15). These results suggest that a
short-course of treatment
with a CG53135-05 E. coll purified product immediately after a combined
chemotherapy and
radiation regimen improves the outcome of the disease in this model of
mucositis.
In another experiment, treatment of hamsters with 12 mg/kg/day CG53135-05 E.
coli
purified product starting after radiation (Day 1 to Day 18) resulted in a
significant reduction of
ulceration (p < 0.001) combined with 7 days of significant reduction in
mucositis scores, as
determined by rank sum analysis (N-198 study). This suggests that the
administration of a
CG53135-05 E. coli purified product results in a significantly beneficial
treatment of radiation-
induced oral mucositis when administered after mucosa toxic insult.
78
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In yet another experiment, administration of 12 mg/kg/day of CG53135-05 E.
coli purified
product (formulated in 40 mM sodium acetate, 0.2 M L-arginine, and 3%
glycerol) on Days I to 2
significantly reduced the severity of mucositis (N-237 study). These results
confirm the findings
presented above.
Conclusions
The activity of CG53135 was evaluated in a model of mucositis induced in
hamsters treated
with 60 mg/kg 5-flourouracil on Days -4 and -2, followed by a single sub-
mucosatoxic dose of
radiation (- 30 Gy) on Day 0. Clinically relevant oral mucositis (mucositis
score of _3) developed -
Day 15. Intraperitoneal administration of CG53135 for 2, 6, or 18 days
significantly reduced severity
of mucositis.
6.17. EXAMPLE 17: EFFECT OF CG53135-05 ADMINISTRATION ON HAMSTER
EPITHELIAL PROLIFERATION IN VIVO (N-225 STUDY)
The experiment described herein evaluated in vivo incorporation of BrdU into
the
gastrointestinal epithelium and bone marrow after a single dose of a CG53135-
05 E. coli purified
product (protein concentrations in this example were measured by Bradford
assay).
Materials and Methods
Male Golden Syrian hamsters (Charles River Laboratories or Harlan Sprague
Dawley), aged
to 6 weeks, with a mean body weight of 82 g at study commencement were used.
Twenty-five
male hamsters were randomized into 5 groups of 5 animals each as outlined in
Table 22.
Table 22. Treatment Groups
Group No. of Euthanasia/ Volume (mL);
No. Animals Treatment Treatment
Necropsy
1 5 males BrdU 50 mg/kg, IP, (0 hrs) 2 hrs Adjust by body
weight
2 5 males 12 mg/kg CG53135-05 E. coli 2 hrs Adjust by body
purified product, IP (0 hrs) + weight
BrdU 50 mg/kg, IP, (0 hrs)
3 5 males 12 mg/kg CG53135-05 E. coli 4 hrs Adjust by body
purified product, IP (0 hrs) + weight
BrdU 50 mg/kg, IP, 2 hrs)
4 5 males 12 mg/kg CG53135-05 E. coli 8 hrs Adjust by body
purified product, IP (0 hrs) + weight
BrdU 50 mg/kg, IP, 6 hrs)
5 5 males 12 mg/kg CG53135-05 E. coli 24 hrs Adjust by body
purified product, IP (0 hrs) + weight
BrdU 50 mg/kg, IP, (22 hrs)
A single dose of a CG53135-05 E. coli purified product at 12 mg/kg IP was
administered
and hamsters were sacrificed at 2, 4, 8 and 24 hours post-administration.
BrdU Administration and /mmunohistochemistrY: all animals received BrdU 50
mg/kg IP two
hours before sacrifice, allowing for uptake of the reagent into proliferating
tissues. At euthanasia,
the following tissues were harvested: cheek pouch mucosa, esophagus, stomach,
duodenum,
79
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
jejunum, ileum, cecum, colon, rectum and sternum. All tissue samples were
fixed in 10% neutral
buffered formalin for 24 hours and then transferred to 70% ethanol. Samples
were trimmed, paraffin
embedded, sectioned and mounted. Epithelial tissues were stained for
incorporation of BrdU by
immunohistochemistry using Oncogene Research products BrdU
Immunohistochemistry kit Catalog
# HCS24 in accordance with the manufacturer's instructions.
Results
The effect of CG53135-05 E. coli purified product on the incorporation of BrdU
into all
tissues was essentially the same: a relatively small increase in the number of
BrdU labeled nuclei
was observed 2 hours after the administration of CG53135-05 E. coli purified
product. This was
followed by a decrease in the number of labeled nuclei at 4 hours after the
administration of
CG53135-05 E. coli purified product. All tissues showed a dramatic increase in
BrdU labeling at 8
hours post administration. At 24 hours, all tissues except rectum showed a
decrease in the number
of labeled nuclei compared with the untreated controls, while the rectal
tissue showed a slight
increase over the controls. Since no labeled cells were seen in the rectal
tissue samples from the
untreated animals, the observation of 2 labeled cells in the 24 hour time
point has to be regarded as
observational error, or data scatter, since there must be a low level of cell
replication in the tissue.
Conclusions
The in vivo mechanistic activity of CG53135 was evaluated using
bromodexoyuridine
labeling in vivo to evaluate the effect of a single bolus dose (12 mg/kg) of
CG53135-05 E. coli
purified product on mucosal tissue over a 24-hour period. CG53135-05 E. coli
purified product
stimulated the division of the epithelial cells of the cheek pouch, jejunum
and rectum as well as the
hematopoietic cells of the bone marrow. Peak increases in BrdU incorporation
in these tissues were
seen at 8 hours after the administration of CG53135-05. All tissues showed the
same time
response to the administration of CG53135-05 E. coli purified product.
6.18. EXAMPLE 18: MODULATION OF INTESTINAL CRYPT CELL PROLIFERATION
AND APOPTOSIS BY CG53135-05 ADMINISTRATION TO MICE (N-342)
This study evaluated the effect of CG53135 on small intestinal crypt cell
turnover in order to
discriminate stem cell versus daughter cell effects, and to draw insights
regarding the mode of
action of CG53135 in syndromes associated with gastrointestinal stem cell
damage (e.g., mucositis).
Furthermore, the effect of CG53135 on stem cell radiosensitivity was also
assessed. Protein
concentrations in this example were measured by Bradford assay.
A"crypt" is a hierarchical structure with the stem cells towards the crypt
base. As cells
become more mature, they move progressively from the bottom of the crypt
towards the top of the
crypt. Therefore, changes that may be affecting stem cells versus their
transit amplifying daughter
cells can be detected by looking at changes in event frequency at each cell
position. The cell
positions are marked in FIG. 16. Thus, the effects of CG53135 on the crypt
microarchitecture were
analyzed in the context of crypt cellularity.
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Experimental Design
Animals were sacrificed at various times after a single 12 mg/kg (I P) dose of
a CG53135-05
E. coli purified product. Just prior to sacrifice the mice were labeled with a
single injection of
bromodeoxyuridine to label S-phase cells and determine the effect of the drug
on crypt cell
proliferation / apoptosis. Mice were weighed and then dosed with a CG53135-05
E. coli purified
product (12 mg/kg, single injection, ip). Groups of 6 animals were sacrificed
0, 3, 6, 9, 12, 24, 48
hours post injection with a CG53135-05 E. coli purified product. All received
a single injection of
bromodeoxyuridine 40 minutes prior to sacrifice (see Table 23).
An additional two groups of 6 mice were used to assess the effects of CG53135-
05 E. coli
purified product on stem cell radiosensitivity (groups 8 and 9, see Table 23).
One group was treated
with a CG53135-05 E. coli purified product (12 mg/kg, single injection, ip)
and another group was
injected with a placebo control. Twenty-four hours post injection, animals
were irradiated with 1 Gy
X-ray (specifically to induce stem cell apoptosis) followed by routine in vivo
BrdU labeling. Animals
were sacrificed 4.5 hours later (at time of peak apoptosis).
Table 23. Study Design
Group Number of Treatment Treatment
Number Animals Schedule*
1 6 males CG53135-05 E. coli Injected and euthanize 3 hr later
purified product, 40mg/kg BrdU 40 min prior to sacrifice
12 m/k ,IP
2 6 males CG53135-05 E. coli Injected and euthanize 6 hr later
purified product,
40mg/kg BrdU 40 min prior to sacrifice
mg /k ,IP
3 6 males CG53135-05 E. coli Injected and euthanize 9 hr later
purified product, 40mg/kg BrdU 40 min prior to sacrifice
12 m/k ,IP
4 6 males CG53135-05 E. coli Injected and euthanize 12 hr later
purified product, 40mg/kg BrdU 40 min prior to sacrifice
12m /k ,IP
6 males CG53135-05 E. coli Injected and euthanize 24 hr later
purified product, 40mg/kg BrdU 40 min prior to sacrifice
12 m/k ,IP
6 6 males CG53135-05 E. coli Injected and euthanize 48 hr later
purified product, 40mg/kg BrdU 40 min prior to sacrifice
12m /k ,IP
7 6 males Untreated 40m /k BrdU 40 min prior to sacrifice
8 6 males CG53135-05 E. coli Dose 24 hr prior to irradiation
purified product, Euthanize 4.5 hr post irradiation
12 mg/kg, IP 40mg/kg BrdU 40min prior to sacrifice
1 Gy X ray
81
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
9 6 males PBS, IP Dose 24 hr prior to irradiation
I Gy X-ray Euthanize 4.5 hr post irradiation
40mg/kg BrdU 40 min prior to sacrifice
Intestinal Crypt Cell Proliferation and Apoptosis Modulation: Procedure
All S-phase dividing cells incorporate the injected bromodeoxyuridine (BrdU)
and hence are
marked as cycling cells. Animals that were irradiated were placed,
unanaesthetised, in a perspex jig
and subjected to whole body radiation of 1 Gy X-ray at a dose rate of
0.7Gy/min. This low level of
radiation induced apoptosis in the small intestinal stem cell population, but
not in the more mature
cells.
The small intestine was removed, fixed in Carnoy's fixative, and processed for
histological
analysis (paraffin embedded). One set of 3 mm sections were immunolabeled for
BrdU and one set
of sections were stained with H&E. Longitudinal sections of small intestinal
crypts were analyzed for
the presence of either BrdU or apoptotic/mitotic nuclei. Fifty half crypts
were scored per animal.
Groups 1-7 (Group A in the results) were tested to determine the effect of
CG53135-05 E.
coli purified product over a 48 hour period. Groups 8-9 (Group B in the
results) were tested to
determine whether CG53135-05 E. coli purified product changes the number of
apoptotic cells
generated after low dose irradiation, i.e., whether CG53135-05 E. coli
purified product influences the
radiosensitive stem cell population.
The results generated show a frequency distribution for the crypts in each
group of animals
that were further analyzed for statistical differences. Tissue samples were
harvested at 3, 6, 9, 12,
24, and 48 hours after treatment with CG53135-05 E. coli purified product.
Apoptosis, mitotic index,
and proliferation were the end points for this study.
Results:
Group A.
In groups 1-7 (Table 23), CG53135-05 E. coli purified product had no
significant effect on
spontaneous apoptosis. Similar results were obtained with the mitotic index
(Table 24). However,
results of BrdU uptake as in Table 24, revealed the following:
a) At 3 hour, there was extension/increase of proliferative region (positions
12-22).
b) By 9 hours, large proliferative effects were noted in many positions.
c) By 12 hours, only positions 4-8 showed increase in uptake (stem cells).
d) By 24 hours, there was a significant inhibition of proliferation.
e) By 48 hours, the uptake was comparable to control levels.
82
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 24. Summary of significant cell positions in the crypt after assessment
of apoptosis, mitosis,
and proliferation
Sample time Significant Cell Positions
(hours)
After treatment
BrdU labeling Index A optotic Index Mitotic Index
3 12 to 22 None None
6 None None None
9 5 to 9& 11 to 20 to 21 None None
12 4 to 8 None None
24 4 to 8 None None
48 None None None
The comparisons shown in Table 24 are between treated groups versus the
untreated
group. The cell positions shown are the ones that are significantly different
from the untreated
control (P<0.05).
Group B:
In Groups 8 and 9 (Table 23), stem cell radiosensitivity was assessed. As
shown in Table
23, CG53135-05 E. coli purified product or PBS was administered one day before
dosing with 1 Gy
radiation. Tissues were harvested 4.5 hours after radiation dosing. There was
no significant effect
on both radiation-induced apoptosis and the mitotic index. However, increased
uptake in positions
4-8 by 12 hours and significant inhibition of proliferation were seen in mice
pretreated with
CG53135-05 E. coli purified product and irradiated, consistent with the Group
A results (Table 24).
6.19. EXAMPLE 19: EFFECT OF CG53135-05 PROPHYLACTIC ADMINISTRATION ON
MICE INTESTINAL CRYPT SURVIVAL AFTER RADIATION INJURY (N-343)
The purpose of this study was to evaluate the efficacy of CG53135 against
radiation-
induced crypt cell mortality in vivo using the ClonoquantT"" assay. Protein
concentrations in this
example were measured by Bradford assay.
Mice were weighed and then dosed with a CG53135-05 E. coli purified product
(12 mg/kg)
or placebo. A single injection was given, intraperitoneally (ip), 24 hours
prior to irradiation. Each
group of 6 animals was irradiated as per table below. For each radiation dose,
the response of a
drug treated group and a placebo treated group was compared.
The small intestine was removed, fixed in Carnoy's fixative, and processed for
histological
analysis (paraffin embedded). H&E sections were prepared following
conventional protocols. For
each animal, ten intestinal circumferences were analyzed, the number of
surviving crypts per
circumference was scored, and the average per group was determined. Only
crypts containing 10
or more strongly H&E stained cells (excluding Paneth cells) and only intact
circumferences, not
containing Peyers patches, were scored.
The average crypt width (measured at its widest point) was also measured in
order to
correct for scoring errors due to crypt size difference. The correction was
applied as follows:
83
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Corrected number of crypts per circumference = Mean number of surviving crypts
per
circumference in treatment group X (Mean crypt width in untreated control /
Mean crypt width in
treated animal).
Table 25: Study design
Group Number of Induction Treatment Treatment
Number Animals Schedule*
1 6 males 10 Gy PBS Day -1
Da 0
2 6 males 11 Gy, PBS Day -1
Day 0
3 6 males 12 Gy, PBS Day -1
Day 0
4 6 males 13 Gy, PBS Day -1
Da 0
6 males 14 Gy, PBS Day -1
Da 0
6 6 males 10 Gy CG53135-05 E. Day -1
Day 0 coli purified
product,
12 m/k ,IP
7 6 males 11 Gy, CG53135-05 E. Day -1
Day 0 coli purified
product,
12 m /k , I P
8 6 males 12 Gy, CG53135-05 E. Day -1
Day 0 coli purified
product,
12m /k ,IP
9 6 males 13 Gy, CG53135-05 E. Day -1
Day 0 coli purified
product,
12m /k ,IP
6 males 14 Gy, CG53135-05 E. Day -1
Day 0 coli purified
product,
12m /k ,IP
11 6 males Untreated
Results:
The crypt survival following prophylactic CG53135-05 E. coli purified product
administration
showed inverse correlation to the irradiation dose, the lesser the irradiation
dose, the higher was the
crypt survival (FIGs. 17(A) and (B)). Prophylactic administration of CG53135-
05 E. coli purified
84
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
product significantly increased the number of crypts (P<0.001). Table 26 shows
the protection
factor achieved for the radiation doses following prophylactic administration
of the protein
(CG53135-05 E. coli purified product). Protection factor (Table 26) represents
the ratio between
treated and untreated cells. On average, 1.55 times as many cells survived
irradiation dose of 12
Gy, when animals were administered with CG53135-05 E. coli purified product
prior to the radiation
insult.
Table 26:
Radiation dose (Gy) Protection Factor
1.29
11 1.21
12 1.55
13 1.71
14 1.73
6.20. EXAMPLE 20: EFFECT OF CG53135 ADMINISTRATION ON CHEMOTHERAPY-
RADIATION MODEL OF ORAL MUCOSITIS (N-346 STUDY)
Material and Methods
Escherichia coli BLR (DE3) cells (Novagen, Madison, WI) were transformed with
full-length,
codon-optimized CG53135-05 using pET24a vector (Novagen), and a manufacturing
master cell
bank (MMCB) of these cells was produced. Cell paste containing CG53135-05
produced by
fermentation of cells originating from the MMCB was lysed with high-pressure
homogenization in
lysis buffer and clarified by centrifugation. CG53135-05 was purified from
clarified cell lysate by 2
cycles of ion exchange chromatography and ammonium sulfate precipitation. The
final protein
fraction was dialyzed against the formulation buffer (30 mM citrate, pH 6.0, 2
mM
ethylenediaminetetraacetic acid (EDTA), 200 mM sorbitol, 50 mM KCI, 20%
glycerol). Vehicle
contains 30mM sodium citrate, pH 6.1, 2mM EDTA, 200mM sorbitol, 50mM KCI, 20%
glycerol.
Protein concentrations in this example were measured by Bradford assay.
Golden Syrian hamsters (Charles River Laboratories or Harlan), of age 5 to 6
weeks, and
with an average body weight of 84 g at study commencement, were used in this
study. Animals
were individually numbered using an ear punch and housed in small groups of up
to 7 animals per
cage. Animals were acclimated prior to study commencement. During this period,
the animals were
observed daily in order to reject animals in poor condition.
Sixty (60) hamsters were randomized into six groups of ten animals each, prior
to
irradiation. Each group was assigned a different treatment as listed in Table
27. Animals were
dosed with 60 mg/kg 5-FU on days -4 and -2 and were acutely irradiated on the
left buccal mucosa
on day 0. Animals were treated once daily with CG53135-05 E. coli purified
product IP 6, 12, 24 or
48 mg/kg/day, on day I only or 12 mg/kg/day on days 1 and 2, following acute
radiation. Mucositis
was evaluated on alternate days beginning on day 6 and continued until the
conclusion of the
experiment on day 28 (i.e., days 8, 10, 12, 14, 16, 18, 20, 22, 24, 26 & 28).
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 27. Treatment Groups
Group Number of Treatment Treatment Volume
Number Animals Schedule*
1 10 males Untreated Control Day 1
2 10 males 6 mg/kg CG53135-05 E. coli Day I
purified product, ip
3 10 males 12 mg/kg CG53135-05 E. co/i Day 1
purified product ip
4 10 males 24 mg/kg CG53135-05 E. coli Day 1
purified product, ip
10 males 48 mg/kg CG53135-05 E. coli Day 1
purified product ip
6 10 males 12 mg/kg CG53135-05 E. co/i Day 1& 2
purified product, ip
*Mucositis was evaluated on alternate days beginning on day 6 and every second
day until the
conclusion of the experiment on day 28 (i.e., days 8, 10, 12, 14, 16, 18, 20,
22, 24, 26, & 28).
Chemotherapy/Radiation Model of Oral Mucositis: the 5-FU/acute irradiation
model for
oral mucositis in hamsters is an experimental model designed to extend the
clinical observations
made with the acute radiation model for mucositis (Oral Surg Oral Med Oral
Pathol 69(4):437
(1990)). The earlier acute radiation model has proven to be an accurate,
efficient and cost-effective
technique to provide a preliminary evaluation of anti-mucositis compounds
including growth factors
and cytokines (see e.g., Oral Oncol 36(4):373-381 (2000), Cytokine 9(8):605-
612 (1997); Oral Oncol
33(1):47-54 (1997)).
Mucositis was induced using 5-fluorouracil, delivered as intraperitoneal (IP)
doses (60
mg/kg) on days -4 and -2. A single dose of radiation (30 Gy/dose) was
administered to all animals
on day 0. Radiation was generated with a 160 kilovolt potential (18.75-ma)
source at a focal
distance of 21 cm, hardened with a 3.0 mm Al filtration system. Irradiation
targeted the left buccal
pouch mucosa at a rate of 1.32 Gy/minute. Prior to irradiation, animals were
anesthetized with an IP
injection of ketamine (160mg/kg) and xylazine (8mg/kg). The left buccal pouch
was everted, fixed
and isolated using a lead shield. This resulted in ulcerative oral mucositis
that peaked around day
14.
Evaluation of Mucositis: for the evaluation of mucositis, the animals were
anesthetized
with an inhalation anesthetic, and the left pouch was everted. Mucositis was
scored visually by
comparison to a validated photographic scale, ranging from 0 for normal, to 5
for severe ulceration.
The scale is described in Table 28.
86
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 28. Description of Mucositis Score Values
Score Description:
0 Pouch completely healthy. No erythema or vasodilation.
1 Light to severe erythema and vasodilation. No erosion of mucosa.
2 Severe erythema and vasodilation. Erosion of superficial aspects of mucosa
leaving
denuded areas. Decreased stippling of mucosa.
3 Formation of off-white ulcers in one or more places. Ulcers may have a
yellow/gray
due to pseudomembrane. Cumulative size of ulcers should equal about'/ of the
pouch. Severe erythema and vasodilation.
4 Cumulative size of ulcers should equal about'/ of the pouch. Loss of
pliability.
Severe erythema and vasodilation.
Virtually all of pouch is ulcerated. Loss of pliability (pouch can only
partially be
extracted from mouth)
A score of 1-2 is considered to represent a mild stage of the disease, whereas
a score of 3-
5 is considered to indicate moderate to severe mucositis. Following clinical
scoring, a photograph
was taken of each animal's mucosa using a standardized technique. At the
conclusion of the
experiment, all film was developed and the photographs randomly numbered for
blinded scoring.
Two independent, trained evaluators graded the photographs in blinded fashion
using the above-
described scale. For each photograph, the final blinded score was the average
of the score
assigned by the two independent evaluators. The scores from the blinded
photographic evaluation
were statistically analyzed.
Weights and Survival: each hamster was weighed daily for the period of the
study (i.e.,
day -4 to day 28). Weight and survival was monitored and recorded in order to
assess possible
differences amongst treatment groups as an indication for mucositis severity
and/or possible toxicity
resulting from the treatments. If appropriate, survival was analyzed using a
Kaplan Meier log-rank
analysis. Differences in weight gain were assessed using a One-Way ANOVA
analysis of the area
under the curve (AUC) values for the percentage weight gain for individual
animals, with a critical
value of 0.05.
Evaluation of Activity: the effect of each treatment on mucositis compared to
the control
group was assessed using a Chi-squared (X2) analysis of the number of animal
days with a score of
three or higher, and by using the Mann-Whitney Rank Sum test to compare the
blinded mucositis
scores for each group on each day the evaluations were performed. In each
case, treatment groups
were compared to the control group, with a critical value of 0.05. For the
Mann-Whitney Rank Sum
test, two days of statistically significant improvement are generally regarded
as the minimum
improvement necessary for a positive result.
Results
Mucositis: the mean daily mucositis scores were calculated for each group and
are shown
in Figure 18. The peak of mucositis in the control group was on day 14 when
the mean score for
87
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
this group reached 3.2. All of the groups treated with CG53135-05 E. coli
purified product had their
peak scores on day 16, which ranged from a high of 3.0 in the groups treated
with CG53135-05 E.
co/i purified product at 24 mg/kg or 48 mg/kg on day I to a low of 2.63 in the
group treated with
CG53135-05 E. coli purified product at 12 mg/kg on day 1. To evaluate the
mucositis scores, an
analysis of the number of days with a score of 3 or higher was performed,
using the Chi-squared
test. The results of this analysis are shown in Table 29 and Figure 18.
Further, Figure 19 depicts
the duration of severe mucositis in animals with a mucositis score of >3 as
calculated by the chi-
square analysis. Both groups treated with CG53135-05 E. coli purified product
at 12 mg/kg showed
a significant reduction in the number of days with a score of 3 or higher,
with the group treated on
day 1 only having slightly more significance (P=0.003) than the group treated
on days 1 and 2
(P=0.01 8). The group treated with CG53135-05 E. coli purified product at 6
mg/kg on day 1 showed
some improvement, but failed to reach significance (P=0.092). The groups
treated with CG53135-
05 E. coli purified product at 24 mg/kg and 48 mg/kg were essentially the same
as controls in this
test.
Table 29. Chi Squared analysis of number of days animals had a mucositis score
of 3 or higher
Group Days>=3 Days<3 Total Days % Days Chi Sq. P
>=3 vs control Value
Untreated control 94 140 234 40.2 - -
CG53135-05 E. coli 70 148 218 32.1 2.8330 0.092
purified product 6mg/kg
IPDay1
CG53135-05 E. coli 52 148 200 26.0 9.0760 0.003
purified product 12mg/kg
IP Day 1
CG53135-05 E. coli 80 136 216 37.0 0.3420 0.558
purified product 24mg/kg
IP Day 1
CG53135-05 E. coli 94 126 220 42.7 0.2090 0.647
purified product 48mg/kg
IPDay1
CG53135-05 E. coli 70 168 238 29.4 5.5590 0.018
purified product 12mg/kg
IP Day 1 and 2
To examine the levels of clinically significant mucositis, as defined by
presentation with
open ulcers (score >3), the total number of days in which an animal exhibited
an elevated score was
summed and expressed as a percentage of the total number of days scored for
each group.
Statistical significance of observed differences was calculated using chi-
square analysis.
Further analysis of the significance of the differences in the mucositis
scores was performed
by using the Mann-Whitney Rank-Sum test to compare the test groups with the
control group on
each day of evaluation. The results of this analysis are shown in Table 30
which indicates that the
group treated with CG53135-05 E. coli purified product at 6 mg/kg on day 1
only showed significant
improvement relative to controls on days 14 (P=0.010) and 26 (P=0.031). The
group treated with
CG53135-05 E. coli purified product at 12 mg/kg on day 1 only showed
significant improvement
88
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
relative to controls on days 14 (P=0.01 1), 16 (P=0.031), 18 (P=0.005), and 20
(P=0.037). The group
treated with CG53135-05 E. coli purified product at 24 mg/kg did not show any
significant
improvement relative to controls. The group treated with CG53135-05 E. coli
purified product at 48
mg/kg showed significant improvement on day 12 (P=0.035) but also showed
significant worsening
on days 26 (P=0.036) and 28 (P=0.006). The group treated with CG53135-05 E.
coli purified
product at 12 mg/kg/day on days 1 and 2 showed significant improvements
relative to controls on
days 14 (P=0.010) and 18 (P=0.045). Since the standard for meaningful
improvement in this test is
2 days of statistically significant improvement in the mucositis score
relative to controls, the groups
treated on day 1 with CG53135-05 E. coli purified product at either 6 mg/kg or
12 mg/kg, and the
group treated with CG53135-05 E. coli purified product at 12 mg/kg/day on days
I and 2 showed
meaningful improvements.
Table 30. Mucositis scores as performed by using the Mann-Whitney Rank-Sum
test
Da
Group 6 8 10 12 14 16 18 20 22 24 26 28
Comparison
CG53135-05 0.597 0.735 0.826 0.298 0.010 0.606 0.324 0.164 0.224 0.736 0.03
0.202
E. coli purified
product
6 mg/kg day I
vs control
CG53135-05 0.989 0.595 0.042 0.164 0.011 0.031 0.005 0.037 0.232 0.762 0.57
0.347
E. coli purified
product
12 mg/kg day 1
vs control
CG53135-05 0.781 0.129 0.736 0.104 0.104 0.553 0.115 0.988 0.298 0.356 0.29
0.388
E. coli purified
product
24 mg/kg day 1
vs control
CG53135-05 0.797 0.989 0.393 0.035 0.101 0.553 0.295 0.224 0.780 0.164 0.03
0.006
E. coli purified
product
48 mg/kg day 1
vs control
CG53135-05 0.284 0.161 0.284 0.106 0.010 0.144 0.045 0.260 0.163 0.424 0.98
0.456
E. coli purified
product
12 mg/kg day 1
and 2 vs
control
Significance of Group Differences Observed in Daily Mucositis Scores (Rank Sum
Test).
This nonparametric statistic is appropriate for the visual mucositis scoring
scale. The p values for
each calculation are shown. Significant improvement is highlighted.
Survival: five animal deaths occurred during the study. The deaths occurred on
day 4 in the
group receiving CG53135-05 E. coli purified product at 24 mg/kg, day 7 in the
6 mg/kg group, days 9
and 11 in the 12 mg/kg on day 1 only group and day 11 in the 48 mg/kg group.
No deaths were
observed in either the control group or in the group receiving CG53135-05 E.
coli purified product at
89
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
12 mg/kg/day on days 1 and 2. Survival was consistent with the death rate
usually observed in the
chemotherapy/radiation model.
Weight Change: the mean daily percentage weight change for each group is shown
in FIG.
20. The overall increase in weight during the course of this study for animals
in the untreated
control group was 47.5%, compared with 45.9% in the group treated with CG53135-
05 at 6 mg/kg in
day 1, 53.8% in the group treated with CG53135-05 E. coli purified product at
12 mg/kg in day 1,
41.2% in the group treated with CG53135-05 E. coli purified product at 24
mg/kg in day 1, 49.7% in
the group treated with CG53135-05 E. coli purified product at 48 mg/kg in day
1, and 46.9% in the
group treated with CG53135-05 E. coli purified product at 12 mg/kg on days 1
and 2. Analysis of
group weight gain was done by calculation of the area under the curve (AUC)
for each animal. One-
Way ANOVA analysis of group AUC values for weight among all study groups
indicated that there
were no significant differences between any groups in the study (P=0.687). A
mean comparison of
AUC values for each group in this study is shown in FIG. 21. This result
indicates that the animals
in groups treated with CG53135-05 E. coli purified product gained weight in a
manner that was
equivalent to those in the untreated control group.
6.21. EXAMPLE 21: EFFECT OF CG53135 ON TREATMENT OF ESTABLISHED ORAL
MUCOSITIS IN HAMSTER CHEMO/RADIATION MODEL (N-318)
Animal, type and age, and the chemotherapy/radiation model are same as
described in
Section 6.20. Protein concentrations in this example were measured by Bradford
assay.
Sixty (60) hamsters were randomized into six (6) groups of ten (10) animals
each, prior to
irradiation. Each group was assigned a different treatment as listed and
treated with CG53135-05
E. coli purified product,12 mg/kg IP as indicated in Table 31. In this study,
animals were dosed with
60 mg/kg 5-FU on days -4 and -2, followed by an acute radiation dose of
approximately 30 Gy on
Day 0 in order to produce severe mucositis around Day 15. The duration of this
study was 35 days.
The treatment schedule and dosing started after animals reach an oral
mucositis score of 2. In
addition to the mucositis scoring, this study evaluated the occurrence of
diarrhea, weight loss and
death for each animal in the experimental groups.
Table 31. Treatment Groups
Group Number of Treatment Treatment Schedule
Number Animals
1 8 males Untreated Control None
2 8 males Vehicle control Once Daily (3x) on OM score of 2
3 8 males 12 mg/kg CG53135-05 E. coli Once Daily (lx) on OM score of 2
purified product, ip, once
4 8 males 12 mg/kg CG53135-05 E. coli Once Daily (2x) on OM score of 2
purified product, ip, twice
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
8 males 12 mg/kg CG53135-05 E. coli Once Daily (3x) on OM score of 2
purified product, ip, thrice
6 8 males 12 mg/kg CG53135-05 E. coli Once Daily (4x) on OM score of 2
purified product, ip, four times
Mucositis was evaluated on alternate days beginning on day 6 and every second
day until
the conclusion of the experiment on day 28 (i.e., days 8, 10, 12, 14, 16, 18,
20, 22, 24, 26, & 28).
Mucositis was induced in hamsters. The end points, mucositis, weights and
survival, were
evaluated. Statistics applied were Chi-squared analysis and Mann-Whitney Rank
Sum test. All the
three parameters are described in Section 6.20.
Results
Mucositis: in the untreated control group, the peak of mucositis occurred on
day 14 with a
mean score of 3. In the vehicle control group the peak of mucositis occurred
on day 16 with a mean
score of 3.4. The groups receiving CG53135-05 E. coll purified product 12
mg/kg IP on the first and
second days after reaching a score of 2 showed similar patterns of mucositis
scores to the control
groups (FIG. 22(A)). The groups that received CG53135-05 E. co/i purified
product 12 mg/kg IP on
the third and fourth days after reaching a score of 2 showed a reduction in
mucositis scores relative
to the control groups, predominantly after the peak of mucositis (FIG. 22(B)).
The differences in mucositis scores between the groups were evaluated by
comparing the
number of days with a score of 3 or higher using a Chi-Squared test. In the
untreated control group,
32.3% of the animals days evaluated had a score of 3 or higher, compared with
41.1 % of animals
days in the vehicle control group. As a result of this difference between the
two control groups, two
treated groups (groups receiving CG53135-05 E. coli purified product on 2 and
3 days after reaching
a score of 2) showed a significant improvement when compared with the vehicle
control, but not
when compared to the untreated controls. For the group treated with CG53135-05
E. coli purified
product for 2 days, the P values were 0.347 compared to untreated controls and
0.007 when
compared to the vehicle controls, and for the group treated with CG53135-05 E.
coli purified product
for 3 days the P values were 0.580 compared to untreated controls and 0.020
when compared to
vehicle controls. The group treated with CG53135-05 E. coli purified product
for four days after
reaching a score of 2 showed significant improvement when compared with both
the untreated
(P=0.003) and vehicle (P<0.001) controls. The group treated with CG53135-05 E.
coli purified
product on only one day after reaching a score of 2 did not show significance
when compared with
either control group.
Further evaluation of the significance of the differences seen between the
control and
treated groups was performed by using a Mann-Whitney rank sum test to evaluate
the mucositis
scores for each group on each day that scores were obtained. In this analysis,
the different
treatment groups were compared with either the untreated control group or the
vehicle control
group. The results of this comparison with the untreated control group showed
that there was a
91
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
statistically significant difference between the group treated for 2 days and
the untreated control
group on day 10 only (P=0.011). There were statistically significant
differences between the group
treated for 3 days and the untreated control group on days 14 (P=0.036) and 22
(P=0.013).
Statistically significant differences between the group treated for 4 days and
the untreated control
were seen on days 10 (P=0.009), 12 (P=0.029), 14 (P=0.002) 22 (P=0.021) and 24
(P=0.032). No
statistically significant differences were seen between the group treated with
CG53135 on a single
day and the untreated control group.
The results of the rank sum comparison between the vehicle control group
showed that
there was a statistically significant difference between the group treated on
3 days and the vehicle
control group on days 14 (P=0.020) and 22 (P=0.020). Statistically significant
differences between
the group treated on 4 days and the vehicle control were seen on days 10
(P=0.036), 14 (P<0.001),
18 (P=0.024), 22 (P=0.048), 24 (P=0.021), 26 (P=0.048) and 28 (P=0.004). No
statistically
significant differences were seen between the groups treated with CG53135 on a
single day, or 2
days and the vehicle control group.
Weight Change: animals in the untreated control group gained an average of
50.5% of their
starting body weight by the end of the study. The vehicle control group had
the lowest mean gain in
weight during the study, gaining an average of 41.1 %. The group that received
four daily doses of
CG53135-05 E. coli purified product had the largest gain in weight during the
study at 53.4%, while
the group that received one, two and three daily doses gained an average of
48.1%, 46.8% and
44.4% respectively. These differences were evaluated by calculating the area
under the curve
(AUC) for the percent daily weight gain for each animal and then evaluating
the AUC values using a
one way ANOVA test. No significant differences were seen between the groups
(P=0.266). The
mean AUC data is shown in FIG.23.
6.22. EXAMPLE 22: CG53135 CAN BE USED SAFELY AS A SINGLE DOSE THERAPY
FOR MUCOSITIS IN HUMAN PATIENTS (STUDIES C-214 AND C-325)
Safety, tolerability, and pharmacokinetic assessment of a CG53135-05 E. coli
purified
product (in the formulation as described in Section 6.2.1, referred as
"CG53135-05 drug substance"
in this example) administered intravenously in human patients with advanced
(Stage 4) cancer
(single rising-dose tolerance) was conducted. The goal of this dose-escalating
tolerance study was
to assess the safety, tolerability and pharmacokinetics of CG53135-05 drug
substance in cohorts of
four patients at 0.03, 0.1, 0.33, and 1 mg/kg (UV). Dose escalation was
stopped due to tolerability
information at 0.33 mg/kg delivered in 15 minutes (Phase I study C-325) and
the protocol was
amended to add a 0.2 mg/kg dose.
During the trial, oral mucosa was examined by experienced study staff and
assigned a
mucositis score by both the World Health Organization (WHO) OM scoring system
and the Oral
Mucositis Assessment Scale (OMAS). See, WHO Handbook 1979 WHO. WHO Handbook
for
Reporting Results for Cancer Treatment. In: WHO Offset Publication No. 48.
Geneva, Switzerland:
World Health Organization; 1979. The OMAS provides a more quantitative
assessment of injury.
92
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
After discharge, patients were provided with diaries where they noted a single
WHO score for each
day. Study staff instructed patients on how to self-assess and assign a score
for oral mucositis.
Table 32. WHO Scoring System:
Grade 0 Grade 1 Grade 2 Grade 3 Grade 4
Normal Erythema and Ulceration, but Ulceration, diet Ulceration of
soreness can eat solid limited to liquids such severity
foods that patient
requires
parenteral
feeding
A value of the OMAS system is obtained by summing the erythema and
ulceration/pseudomembrane scores.
Table 33. OMAS Scoring System
0 1 2 3
Erythema None Mild/ moderate Severe
erythema erythema
Ulceration/ No lesions Cumulative Cumulative Cumulative surface
psuedomembrane surface area of surface area of area of lesion is > 3
lesion < 1 cm2 lesion > 1 cm2 cm2
and < 3 cm2
Eleven patients have received the CG53135-05 drug substance at 0.03 mg/kg
(n=4), 0.1
mg/kg (n=6), and 0.2mg/kg (UV) (n=1) as a single 100-m1 intravenous infusion
administered 3 days
after completion of the CT. Tolerability information is available for all 11
patients. Full clinical data
from nine patients are available.
Preliminary pharmacokinetic data demonstrated plasma exposure with an average
Cmax of
564.3 ng/ml at the 0.03 mg/kg (UV) dose level (n=3; range 175.6- 1192.6 ng/ml)
and 564.7 ng/ml at
the 0.1 mg/kg (UV) dose level (n=3; range 420.9 - 797.5 ng/ml). After
infusion, the CG53135-05
drug substance reached maximum plasma concentration within 1 hour (15 to 35
minutes after
completion of infusion). The mean terminal exponential half-life was 49
minutes (range: 16.2-87
minutes, n=5). No patients discontinued the trial due to adverse experiences.
Adverse events
(number of patients) that may be related to the study drug include: nausea
(2); chills (2); fever (2);
vomiting (1); dizziness (1); photopsia (1) (vision- lights flashing" on day
15) and astigmatism (1)
(mild astigmatism on day 28); neuropathy (1) (on soles of the feet on day 15);
tachycardia (1);
headache (1); and asymptomatic, single premature atrial complex noted on ECG
(1). All reported
incidences were mild to moderate. No Grade 3 or 4 laboratory toxicity
associated with the study
drug was noted. Among the 11 patients who have received the drug through
September 3, 2004,
six serious adverse events determined to be unrelated to study drug were noted
from 3 patients.
These events included cancer progression (n=2), catheter infection, small
intestinal obstruction,
esophagitis/mucositis and neutropenic fever.
93
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Among the 11 patients that completed the study, six patients did not develop
oral mucositis.
Four patients developed Grade 1(n=1) or Grade 2 (n=3) oral mucositis. One
patient with a Grade 3
oral mucositis was observed. No patients required total parenteral nutrition.
Patients receiving
CPT-1 1 typically have a high incidence of diarrhea. In this trial, 7 of the
patients received CPT-11
as part of CT and only two patients (both received 0.03 mg/kg (UV) of CG53135-
05 drug substance)
experienced mild to moderate diarrhea and only I patient developed diarrhea
immediately after
receiving CG53135-05 drug substance treatment. We concluded that the CG53135-
05 drug
substance was well-tolerated with single dose administration at 0.03, 0.1,and
0.2 mg/kg (UV).
A concurrent single rising-dose, phase I trial (study C-325) in autologous
stem cell
transplant patients is ongoing and 27 patients have been treated with CG53135-
05 drug substance.
22 patients (pts) (ages 25-75) undergoing HDCT with PBSCT have completed the
study with
escalating doses of CG53135-05 drug substance, including (number of patients):
0.03 mg/kg (2), 0.1
mg/kg (10), 0.2 mg/kg (8), and 0.33 mg/kg (2). Patients were treated for:
multiple myeloma (n=11),
non-Hodgkin's lymphoma (n=9), acute myelogenous leukemia (n=1), and
desmoplastic round cell
tumor (n=1) and were treated with conditioning regimens including melphalan
(Mel 200),
cyclophosphamide, carmustine and etoposide (CBV), carboplatin and thiotepa
(CT), and
busulfan/cyclophosphamide (targeted BuCy). The primary objective of the trial
was to evaluate
safety, tolerability and pharmacokinetics of the CG53135-05 drug substance.
Patients were also
scored daily for presence of OM using both the WHO and OMAS grading scales
(Tables 32 and 33,
supra). Among the 22 patients that completed the study, 8 patients experienced
no OM (including 4
Mel 200 pts); 10 patients experienced only WHO grade 1(n=7) or grade 2(n=3)
OM, while 4
patients experienced severe OM of Grade 3 (n=3) or Grade 4(n=1). I patient
experiencing grade 4
OM required TPN for 4 days. Patients tolerated the study drug well with no
significant side effects
up to a dose of 0.33 mg/kg. At that dose, 2 patients experienced an infusional
reaction consisting of
fevers, nausea, and mild hypotension. Preliminary Pharmacokinetic results from
13 patients
confirmed dose dependent plasma exposure with an average Cmax of 135.5 ng/ml,
343.3ng/ml, and
658.3ng/ml at dose levels of 0.1, 0.2, and 0.33 mg/kg, respectively. The
median day of neutrophil
engraftment (as determined by ANC>500/uL) occurred on day 13 after stem cell
infusion.
Preliminary data suggest that CG53135-05 drug substance is well tolerated in
PBSCT patients at
doses up to 0.33 mg/kg with apparent clinical effects in ameliorating or
preventing OM. 18/22
patients, thus, avoided (WHO Grades 3-4) mucositis following HDCT. A larger
Phase II clinical trial
will be initiated to evaluate the efficacy of CG53135-05 drug substance in
preventing HDCT- induced
OM.
In conclusion, the CG53135-05 drug substance was generally well tolerated
among the 38
patients that were administered in the two phase I trials to date. The doses
tested were 0.03, 0.1,
0.2 and 0.33 mg/kg (UV). Infusional reactions when the 0.33 mg/kg of drug was
administered over
15 minutes coupled with apparent activity observed at lower doses led to a
discontinuation of this
dose level. No other consistent drug-related or apparent dose-related adverse
events or laboratory
abnormals have been observed. No study drug-related serious adverse events
were observed.
94
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Sufficient information on tolerability and preliminary activity is considered
to be present to utilize the
0.03, 0.1 and 0.2 mg/kg (UV) doses in Phase II testing.
6.23. EXAMPLE 23: CG53135 REDUCES THE INCIDENCE. LENGTH AND SEVERITY OF
RADIATION-INDUCED DIARRHEA (N-438)
This study was performed to evaluate the activity of CG53135 against
gastrointestinal injury
induced by whole body irradiation as measured by diarrhea incidence and gut
morphology. Protein
concentrations in this example were measured by UV absorbance.
Materials and methods:
Dosing: Mice were weighed and then dosed with CG53135-05 E. coli purified
product (4 or
16 mg/kg) or untreated. Dosing occurred as described in Tables 34 & 35. Each
group of 20 animals
was irradiated as per table below. All dosing of CG53135-05 E. coli purified
product on day 0 was
immediately after irradiation. No anesthesia was administered.
Intestinal Crypt Cell Damage Induction: Mice underwent whole body irradiation
at a dose of
14 or 14.5 Gy delivered at a dose rate of 0.7Gy/min. Animals were followed for
diarrhea incidence
throughout the study period. After 6 days, animals were sacrificed, and the
intestinal tract of the
mice was harvested for histological analysis.
Body Weight: Every day for the period of the study, each animal was weighed
and its
survival recorded, in order to assess possible differences in animal weight
among treatment groups
as an indication of response to exposure to ionizing radiation.
Animals Found Dead or Moribund: Animals were assessed 2x/day from Day 3
onwards in
order to accurately assess diarrhea onset / progression and detect moribund
animals prior to death.
Such moribund animals were sacrificed by cervical dislocation. The ileum and
mid-colon were
removed and fixed in formalin, embedded in paraffin (1 animal per block, two
tissues per block) for
storage and future analysis/IHC if required. No tissue was removed from
animals found dead.
Table 34. Study Design
Group Number of Induction Treatment Treatment
Volume
Number Animals Schedule* (mL)
1 20 males 14 Gy None None Adjust per body
Day 0 weight
2 20 males 14 Gy CG53135-05 E. coli Day -1, 0, 1 Adjust per body
Day 0 purified product, weight
4 mg/kg, IP
( dx3
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
3 20 males 14 Gy CG53135-05 E. coli Day -1, 0, 1 Adjust per body
Day 0 purified product, weight
16 mg/kg, IP
( dx3
4 20 males 14 Gy CG53135-05 E. coli Day 1 Adjust per body
Day 0 purified product, weight
4 mg/kg, IP
(q6h x 4)
20 males 14.5 Gy None None Adjust per body
Day 0 weight
6 20 males 14.5 Gy CG53135-05 E. coli Day -1, 0, 1 Adjust per body
Day 0 purified product, weight
4 mg/kg, IP
( dx3
7 20 males 14.5 Gy CG53135-05 E. coli Day -1, 0, 1 Adjust per body
Day 0 purified product, weight
16 mg/kg, IP
(qd x 3
Table 35. Test Article Requirements
Conc. of Desired Volume Volume of
Dose stock Conc of of dosing stock
(mg/kg) Mass of solution Dosing solution solution
Type of from #of # of Animal Admin vol by AZBO solution required required for
Solution Group# Conc Animals doses (kg) (mUkg) (mg/mL) (mg/mL) (mL)
dilution(mL)
CG53135 1 0 20 0 0.025 10 10.2 0.000 0.000 0.000
CG53135 2 4 20 3 0.025 10 10.2 0.400 18.750 0.735
CG53135 3 16 20 3 0.025 10 10.2 1.600 18.750 2.941
CG53135 4 4 20 4 0.025 10 10.2 0.400 25.000 0.980
CG53135 5 0 20 0 0.025 10 10.2 0.000 0.000 0.000
CG53135 6 4 20 3 0.025 10 10.2 0.400 18.750 0.735
CG53135 7 16 20 3 0.025 10 10.2 1.600 18.750 2.941
140 ~ Total 8.333
Results:
Excel spreadsheet attached with diarrhea scores and weights for animals
irradiated with 14
or 14.5Gy. Because the data from each radiation dose were very similar, only
the analysis of the
animals irradiated with 14Gy is provided.
Weights: Mass specific growth rate was calculated by:
in(Nff)-hiqvl;)
= ].1'ISGR
TrTi
96
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Significance was calculated using One-way ANOVA and Dunnett's Multiple
Comparison
Test. No significant differences were seen between the changes in weight
during the study between
the groups (FIGs. 24 (A) and 24(B)).
Diarrhea score: Mice were scored for severity of diarrhea on a scale of 0-3
twice a day for
three days beginning at 4 days after irradiation. Average diarrhea score over
three days as well as
the sum of the diarrhea score over three days was measured and graphed.
Significance was
obtained by one-way ANOVA and Tukey's Multiple Comparison Test. (FIGs. 25(A)
and 25(B))
An analysis of for each day of observation was also made to determine
differences at days
of peak diarrhea. Significance was determined as described above (* - P<0.05,
** - P<0.01, *** -
P<0.001). (FIG. 26)
Conclusions:
Dosing animals with 16mg/kg CG53135 at days -1, 0 and +1 respective to
radiation resulted
in a highly significant reduction in the incidence, length and severity of
radiation-induced diarrhea.
Dosing animals every 6 hours on day 1 with 4mg/kg CG53135 also resulted in
significant decrease
in diarrhea incidence. The day of peak diarrhea was 5 days after radiation, at
which point only the
16mg/kg dose of CG53135 provided a significant decrease in diarrhea. There
were no significant
differences between the treatment groups in weight loss over the course of the
study.
6.24. EXAMPLE 24: CG53135 REDUCES CPT-11 INDUCED DIARRHEA IN RATS
(STUDY N-392)
Irinotecan (CPT-11) is a chemotherapeutic agent which is commonly used against
solid
tumors which causes gastrointestinal (GI) mucositis manifest by severe
diarrhea. The primary aim
of this study was to investigate whether CG53135 reduces CPT-1 1 -induced GI
mucositis in an in
vivo animal model. The secondary aim was to test varying schedules of
administration of CG53135.
Methodology:
Diarrhea was induced in tumor-bearing rats with a single intraperitoneal dose
of CPT-11
(200 mg/kg). Animals were treated with 16 mg/kg CG53135-05 E. coli purified
product according to
Process 2 described in Section 6.18.2 below (in a vehicle of 0.5M arginine,
0.05M sodium
phosphate monobasic, 0.01 % polysorbate 80, and pH adjusted with sulfuric acid
to pH 7.0)
intraperitoneally either prior to, prior to and during, or post chemotherapy
treatment. Rats were
monitored closely for the incidence, and severity of diarrhea as well as mo
l'Laiity. Animals were
euthanized 168 hours following diarrhea induction. At euthanasia, tissues were
harvested for
histopathological evaluation of the gastrointestinal tract.
97
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
esults:
Severe or moderate diarrhea occurred in approximately 40% of rats treated with
CPT-11
lone. This was associated with a 50% mortality rate at day 4 following
chemotherapy. Rats that
:ceived CG53135 prior to, or prior to and during CPT-11 treatment developed
severe or moderate
iarrhea, however, it occurred with a lower incidence and was not associated
with mortality. Other
osing regimens were not as effective.
onclusions:
CG53135 pre-treated animals (16 mg/kg) demonstrated an improvement in
gastrointestinal
iucositis as measured by a reduction in the incidence of diarrhea. A reduction
in overall mortality
ras also noted in this group. This has important implications for the use of
CG53135 in GI
lucositis in humans, and should be further studied.
6.25. Example 25: Prophylactic Effect of the CG53135-05 E. colf Purified
Product on
Mice After Exposure to Acute Ionizing Radiation (Study N-308)
This study was performed to investigate the effect of the CG53135-05 E. coli
purified
roduct administered prophylactically to mice that later were exposed to
various doses of total body
anizing radiation. Male C3H/He mice with an average weight of 22.1 gram at
study initiation were
sed for treatment groups. Animals were fed with a standard commercial mouse
diet. Food and
iater were provided ad libitum.
Protein concentration was measured by Bradford assay. Mice were exposed to
ionizing
adiation without anesthesia at a dose range of 484 to 641 cGy on day 0.
Animals were dosed with
BS (control) or the CG53135-05 E. coli purified product (12 mg/kg, Bradford,
daily IP) on day -1, or
ays -2 and -1 before radiation. The schedule is represented in Table 36. The
endpoints for the.
tudy were survival and weight changes. Survival was followed for 30 days post-
irradiation.
Table 36. Stud y Design
Group Number of Induction Treatment Treatment
Number Animals Schedule*
1 16 males 484 cGy Day 0 PBS Day -2, -1
2 16 males 534 cGy Day 0 PBS Day -2, -1
3 16 males 570 cGy Day 0 PBS Day -2, -1
4 16 males 606 cGy Day 0 PBS Day -2, -1
16 males 641 cGy Day 0 PBS Day -2, -1
6 16 males 484 cGy Day 0 CG53135-05 E. coli purified Day-I
product, 12 mg/kg, Bradford,
IP
Day -1
7 16 males 534 cGy Day 0 CG53135-05 E. coli purified
product, 12 mg/kg, Bradford,
IP
98
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
8 16 males 570 cGy Day 0 CG53135-05 E. coli purified Day -1
product, 12 mg/kg, Bradford,
IP
9 16 males 606 cGy Day 0 CG53135-05 E. coli purified Day -1
product, 12 mg/kg, Bradford,
IP
16 males 641 cGy Day 0 CG53135-05 E. coli purified Day -1
product, 12 mg/kg, Bradford,
IP
11 16 males 484 cGy Day 0 CG53135-05 E. coli purified Day -2, -1
product, 12 mg/kg, Bradford,
IP
12 16 males 534 cGy Day 0 CG53135-05 E. coli purified Day -2, -1
product, 12 mg/kg, Bradford,
IP
13 16 males 570 oGy Day 0 CG53135-05 E. coli purified Day -2, -1
product, 12 mg/kg, Bradford,
IP
14 16 males 606 cGy Day 0 CG53135-05 E. coli purified Day -2, -1
product, 12 mg/kg, Bradford,
IP
16 males 641 cGy Day 0 CG53135-05 E. coli purified Day -2, -1
product, 12 mg/kg, Bradford,
IP
Results:
Survival decreased as radiation dose increased in all treatment groups. In
animals
receiving PBS, 30-day survival at the lowest dose of radiation (484 cGy) was
93.75%, and
decreased to 50.0% at 534 cGy, 31.25% at 570 cGy, 12.5% at 606 cGy, and 6.25%
at 641 cGy
(Figure 27). In animals receiving CG53135, 12 mg/kg IP on Day -1 only, the 30-
day survival at the
owest dose of radiation (484 cGy) was 87.5%, compared to 87.5% at 534 cGy,
81.25% at 570 cGy,
43.75% at 606 cGy, and 31.25% at 641 cGy (Figure 28). In animals receiving
GC53135, 12 mg/kg
P on Days -2 and -1, the 30-day survival at the lowest dose of radiation (484
cGy) was 87.5%,
-ompared to 75.0% at 534 cGy, 37.5% at 570 cGy, 31.25% at 606 cGy, and zero at
641 cGy (Figure
29).
A multiple comparison test demonstrated a 4.8-fold increase in the odds of
survival in
animals treated on day -1 versus control animals (p=0.00016). LD5oi3o values
were calculated using
a probit plot of survivorship with 95% confidence intervals calculated by
bootstrapping. However,
he odds of survival in animals treated on day -2 and -1 versus control animals
were not significant
(p=0.4162). The results are indicative of the therapeutic effect of
prophylactic administration of
CG53135-05 in radioprotection. Further, one day treatment before radiation
(day -1) also protected
animals from weight loss in all but the highest radiation level (641 cGy). In
this particular system,
Mngle dose (on day -1) was significantly more effective than the two-dose
regimen (on days -2 and -
1, respectively) especially in higher radiation levels.
99
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
In addition to the above results, the invention could be extended to
additional dose regimens
of the CG53135-05 E. coli purified product, such as prophylactically and/or
therapeutically
administer the CG53135-05 E. coli purified product prior and/or after the
radiation exposure, which
could be tested in the same animal model following the same procedures as
described herein, in
order to define the range of therapeutic efficacy of this compound. The dose
regimen for therapeutic
treatment may include, but is not limited to, +1, +1, and +2 days after
radiation exposure. For
example, in another experiment, mice were dosed IP with 4 mg/kg (UV) CG53135-
05 E. coli purified
product 24 hours prior to whole-body irradiation at the indicated doses. The
survival of the mice was
then followed for 30 days. Figure 28B shows the Kaplan-Meier plots for
survival at 570 cGy and 606
-Gy with statistically significant differences between the group treated with
the CG53135-05 E. coli
purified product and the control group, i.e., p=0.008 and p=0.015,
respectively. Figure 28C shows
probit analysis for survival over the range of doses. The LD5oi3o for control
and animals treated with
he CG53135-05 E. coli purified product is 552.4 cGy and 607.4 cGy,
respectively, with a dose
nodification factor (DMF) of 1.10.
6.26. Example 26: Effects of CG53135 Prophylactic Dose Schedule on Survival of
Irradiated Intestinal Crypt Cells (N-375)
The objective of this study was to evaluate the ability of CG53135 to protect
against
~adiation-induced intestinal crypt cell mortality in vivo when administered
once daily for 4 days prior
:o irradiation. CG53135-05 E. coli purified product (12 mg/kg) or PBS was
administered to BDF1
nice intraperitoneally (IP) once daily for 4 consecutive days prior to
exposure to lethal radiation
loses from 10-14 Gy on Day 0. The number of surviving regenerating crypt foci
was measured 4
lays after irradiation. Protein concentrations in this example were measured
by Bradford assay.
When animals received CG53135 once daily for 4 days, an overall increase in
crypt cell
survival was noted when compared to PBS-treated, irradiated animals (Table
37).
i'able 37: Intestinal Crypt Protection Factors Resulting from CG53135-05 E.
coli purified product
Vlultiple-Dose Administration Prior to Irradiation
Mean Crypt Mean Crypt Survival (#)
Radiation Survival (#) CG53135-05 Protection
Dose PBS (12 mg/kg) Factora
Gy 32.7 32.2 0.98
11 Gy 13.8 19.8 1.43
12 Gy 6.6 8.9 1.35
13 Gy 2.3 4.8* 2.09
14 Gy 1.7 1.3 0.76
Protection factor value indicates the number of surviving crypts per
circumference in the CG53135-
)5-treated animals compared to PBS, expressed as a ratio. *P:50.05 versus
corresponding value
rom PBS-treated control animals by ANOVA. # = number of crypts.
The greatest level of radioprotection occurred following 13 Gy of radiation,
with a protection
actor of 2.09 (e.g., a 2-fold increase in the number of surviving crypt
cells). The crypt survival
urves indicated a significantly reduced sensitivity to the radiation following
CG53135-05 treatment
'Figure 30). Thus, pretreatment with CG53135 for 4 consecutive days increased
the overall crypt
100
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
-ell survival. This study indicates the use of multiple-day prophylactic
dosing with CG53135-05 as a
3chedule with radioprotective properties.
6.27 Example 27: Evaluation of Radioprotection Window (N-382)
Having established the effect of CG53135 on crypt cell radioprotection after a
single day
lose or multiple once daily dosing, this study evaluated the activity of
CG53135 when dosed in
ntervals other than 24 hours prior to irradiation. CG53135-05 E. coli purified
product (12 mg/kg) or
'BS was administered to BDF1 mice by IP injection 6, 12, 24, 36, or 48 hours
prior to exposure to a
3ingle bolus radiation dose of 13 Gy, respectively. The number of surviving
regenerating crypt foci
roas measured 4 days after irradiation. Protein concentrations in this example
were measured by
3radford assay.
Administration of a single dose of CG53135-05 E. coli purified product at 24
or 36 hours
)rior to irradiation offered the highest level of the intestinal crypt cell
protection. These schedules
-esulted in increased crypt survival by 80% and 31 %, respectively (Table 38).
Dosing at 6, 12, or 48
i prior to irradiation resulted in dose modification factors of 0.78, 0.40, or
0.84 respectively.
1'able 38: Intestinal Crypt Protection Factors Resulting from CG53135-05
Administration 6-48 h
'rior to Irradiation
Treatment Mean Crypt Mean Crypt Survival (#)
Schedule Radiation Survival (#) CG53135-05 Protection
Dose PBS (12 m/k ) Factor a
-6 h 13 G4.5 3.5 0.78
-12 h 13 G6.3 2.5* 0.40
-24 h 13 Gy 4.1 7.4" 1.80
-36 h 13 Gy 4.9 6.4 1.31
-48 h 13 Gy 4.4 3.7 0.84
' Protection factor value indicates the number of surviving crypts per
circumference in the CG53135-
)5-treated animals compared to PBS-treated control animals, expressed as a
ratio. *P < 0.001
/ersus value from corresponding PBS-treated control animals by ANOVA. # =
number of crypts.
These results suggest that an optimal window for administration of a single
dose of
:;G53135-05 E. coli purified product occurs from 24 to 36 hours prior to
irradiation.
6.28. Example 28: Effects of CG53135 Dose Schedule on Survival of Irradiated
Intestinal Crypt Cells (N-416)
The objective of this study was to establishing an optimal dosing schedule of
CG53135-05
administration to establish the levels of protection against radiation-induced
crypt cell mortality.
'rotein concentrations in this example were measured by UV absorbance. CG53135-
05 E. coli
)urified product (4 mg/kg) or phosphate-buffered saline (PBS) was administered
to BDFI male mice
)y intraperitoneally (IP) once daily either for 1, 2, 3, 4 or 5 consecutive
days (Days -1, 0, 1, 2 and/or
3) prior to, or post-irradiation (13 Gy). The number of surviving regenerating
crypt foci was
-neasured 4 days after irradiation and the dose modification factor (DMF) were
calculated.
Single dose administration of CG53135-05 on Day -1 resulted in a DMF of 2.3
(Figure 31).
4dministration of CG53135-05 on Days -1, 0 and 1 relative to TBI on Day 0
resulted in a DMF of 3.0
101
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
(e.g., a 3-fold increase in the number of surviving crypt cells). These data
suggest that CG53135 is
a GI crypt cell radioprotectant with prophylactic and intervention (treatment)
properties.
6.29. Example 29: Radioprotective Mechanisms of CG53135
Among the many changes that occur in a cell upon an attack of ionizing
radiation, an
increase of reactive oxygen species occurs via the ionization of H20. As this
process produces the
most reactive molecules within the cell, in order to reduce cellular damage,
the nucleus increases
transcription of enzymes that scavenge these radicals to less reactive
intermediates. As CG53135
has been shown to be a radioprotectant, it is relevant to determine if
treatment of cells with
CG53135 upregulates any of the genes known to be involved in radioprotection
in the interest of
"pre-loading" the cells with oxygen radial scavenging pathways. Evaluation of
the effect of CG53135
on the intricate pathways involving ROS scavengers and transcription factors
will mechanistically
describe the observed in vivo radioprotective effects of this agent. Thus,
expression studies and
survival studies were carried out at the cellular level.
Expression Studies:
To delineate the mechanism of radioprotection by CG53135, expression profile
of free
oxygen radical scavengers and transcription factor(s) were studied in
fibroblast and endothelial cells.
NIH3T3 (murine fibroblast), CCD-1070sk (human foreskin fibroblast), CCD-18Co
(human colonic
Finbroblast), and HUVEC (human umbilical cord vascular endothelial cells)
cells were transferred to
basal medium containing 0.1 % FBS and the indicated concentration of the
CG53135-05 E. coli
purified product. After 18 hours incubation, cells were harvested for total
RNA using RNEasy
;Qiagen, Valencia, CA). RNA was reverse transcribed using SuperScript First
Strand Synthesis
System for RT-PCR (Invitrogen, Carlsbad, CA) and amplified for the gene of
interest using the
arimers and cycles indicated below.
Table 39: Primers for RT-PCR (Human)
Gene Name Primer SEQ ID NO No. of
cycles
COX2 5'- TTCAAATGAGATTGTGGGAAAATTGCT -3' 42 30
5'- AGATCATCTCTGCCTGAGTATCTT -3'
43
TFF3 5'- GTGCCGGCCAAGGACAG -3' 44 40
5'- CGTTAAGACATCAGGCTCCAG -3' 45
SOD1/CuZn SOD 5'- TGGCCGATGTGTCTATTGAA -3' 46 30
5'- GGGCCTCAGACTACATCCAA -3' 47
SOD2/MnSOD 5'- CTGGACAAACCTCAGCCCTA -3' 48 28
5'- CTGATTTGGACAAGCAGCAA -3' 49
SOD3/ECSOD 5'- TCCATTTGTACCGAAACACCCCGCTCAC -3' 50 30
5'- CAAACATTCCCCCAAAGGAGCAGCTCTCAG 51
-3'
102
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Nrf2 5'- ATGGATTTGATTGACATACTT -3' 52 40
5'- CTAGTTTTTCTTAACATCTGG -3' 53
GPXI 5'- AAGGTACTACTTATCGAGAATGTG -3' 54 28
5'- GTCAGGCTCGATGTCAATGGTCTG -3' 55
Actin 5'- GGACTTCGAGCAAGAGATGG -3' 56
5'- AGCACTGTGTTGGCGTACAG -3' 57
Table 15: Primers for RT-PCR (Mouse)
Gene Name Primer SEQ ID NO No. of
cycles
MnSOD 5'- GGGAATTCAGCGTGACTTTGGTCTTTT -3' 58 26
5'- GCGGATCCGAGCAGGCGGCAATCTGTAA -3' 59
Actin 5'- GCATCCATGAAACTACATT -3' 5'- 60
CACTTGCGGTGCACGATGG -3' 61
Results
Expression results are summarized in Table 40 and shown in FIGs. 32 (A) - (F).
Table 40
Gene NIH 3T3 CCD 1070 CCD 18Co HUVEC
MnSOD Increased Moderately Increased No change
Increased
ECSOD Increased No change Moderately Absent
Increased
Cu, Zn-SOD No change No change Increased No change
Nrf2 Increased Increased Increased Increased-
repressed at
higher doses
COX2 No change No change Increased Moderately
Increased
Tff3 No expression Increased Increased Increased
GPX1 No change Moderately No change No change
Increased
Among the radioprotective superoxide dismutases, MnSOD, the most
radioprotective one,
Nas found to be induced by the CG53135-05 E. coli purified product
consistently among cell lines
(Table 40, FIGs. 32(A) - (F)).
The Nrf2 transcription factor, which is involved in regulation of several
antioxidants that were
thought to be the "antioxidant response element" (also termed as ARE), was
induced by CG53135 in
all cell lines studied (Table 40, FIGs. 32(A) -(F)). The ERK and Akt kinases
are also activated by
CG53135. CCD18Co human colonic fibroblasts were starved for 18 hours in basal
media containing
D.1 % BSA or left in complete serum ("Comp") then stimulated with 100ng/ml FGF-
20 and harvested
103
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
at the time points indicated. Lysates were immunoblotted for human ERK or Akt
or their indicated
phosphorylated counterparts. Both ERK and Akt kinases were active by 2 minutes
of treatment with
the CG53135-05 E. coli purified product (FIGs. 32 (D) and (E)). The activation
of these kinases,
particularly Akt, has been associated with radioprotective events.
It is well established that one mechanism by which cells are protected from
ionizing
radiation is through the induction of oxygen radical scavenging pathways. The
gene expression
studies detailed herein indicate that this may be one of the pathways by which
CG53135 modulates
radioprotection. Furthermore, one of the primary target organs of total body
irradiation (TBI) is the
gastrointestinal tract. The data disclosed herein show that (1) the most
responsive of all cell lines
studied was a gastrointestinal fibroblast (CCD-18co); and (2) a well-
characterized intestinal
radioprotectant, Tff3, was strongly upregulated by CG53135. Considering that
other
radioprotectants known in the art strictly affect bone marrow survival and no
other compartments, it
is important to note that CG53135 is active in a tissue that is as equally
affected as the
hematopoietic stem cells, but no less important to the survival of the animal.
Survival Studies:
Cells that receive a certain dose of radiation will have to brace against the
onslaught of
ionized radicals, repair the damage that the radicals perform or delay the
onset of apoptosis in the
face of irreparable harm, or, likely, a combination of all these mechanisms.
Each of these pathways
is thus important for the ultimate survival of a cell and its ability to
proliferate. Cell survival was
assessed by a clonogenic assay in which surviving cells can form colonies in
vitro after irradiation.
Clonogenic assays were performed using CCD-18co cells, FaDu human squamous
cell
carcinoma cells, IEC6 and IEC18 rat colon crypt cells, and NIH 3T3 mouse
fibroblast cells, to assess
the effect of CG53135 on radiation protection. Cell culture conditions were as
follows: NIH 3T3
cells were grown in DMEM + 10% Bovine serum + 50 lag/ml
Pennicilin/Streptomycin; IEC6 and
IEC18 cells were grown in DMEM + 10% FBS + 0.1 U/ml Insulin + 50 Ng/ml
Pennicilin/Streptomycin;
FaDu cells were grown in MEM + 10% FBS + 1 mM Sodium Pyruvate + 50 pg/ml
Pennicilin/Streptomycin + Non-essential amino acids. Cells were plated at a
density of 5x105 per 10
cm dish (NIH3T3) or 5x105 per well of a 6-well dish (IEC18, IEC6, FaDu) and
allowed to attach.
Cells were then treated with the CG53135-05 E. coli purified product at doses
of 10 or 100 ng/ml
(IEC18, IEC6, FaDu) or at 50 and 200 ng/ml (NIH 3T3) in basal media containing
0.1% serum
(IEC18, IEC6, FaDu) or 1% serum (NIH 3T3) and incubated for 16 hours (IEC18,
IEC6, FaDu) or I
hour (NIH 3T3). Cells were then irradiated using a Faxitron X-ray irradiator
(Wheeling, IL) fitted with
a 0.5 mm aluminum filter at 2.5, 5, 7.5, 10, 12.5 and 15 Gy at 130kVp,
resulting in a radiation rate of
50 cGy/min. Immediately after irradiation, cells were trypsinized and plated
in duplicate at densities
of NIH 3T3: 250, 500, 1000, 2000, 5000 and 10,000 cells per 60mm dish; FaDu,
IEC18 and IEC6:
500, 2500 cells per well of a 6-well dish. Cells were grown in complete growth
medium for 1-2
weeks until colonies of average diameter of 2 mm, after which the colonies
were stained with crystal
violet and counted.
104
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Results:
The number of surviving untreated or CG53135-treated cells was plotted as a
function of
radiation dose, giving rise to survival curves. The slopes of different parts
of the survival curves
describe different properties of radiation cell killing and can be described
as follows:
DO is the slope of the curve between the final two points, indicating speed of
cell killing at
the higher doses of radiation. The value is interpreted to indicate the amount
of radiation required to
reduce the fraction of surviving cells by 37% of the previous value on the
graph. A smaller number
indicates a more rapid rate of cell killing.
Dl is the slope of the curve between the first two points, indicating the
speed of cell killing at
the lower doses of radiation. The value is interpreted as the amount of
radiation required to reduce
the fraction of surviving cells by 37% of the previous value on the graph. A
smaller number
indicates a more rapid rate of cell killing.
Dq is the width of the shoulder of the curve before an exponential decrease in
cell survival is
seen. This is essentially the threshold amount of irradiation required before
an incidence of cell
killing is seen. A larger Dq value indicates that the cells are completely
protected at the lower doses
of radiation.
The effect of CG53135 treatment on survival of irradiated IEC18 cells is shown
in Figure
33(A). While the DO and D1 values showed no obvious treatment-related trends,
the Dq value
indicates that IEC18 cells treated with CG53135 are more protected from
killing at the lower doses
of radiation compared to untreated cells (shoulder of survival curves of cells
treated with 10ng/ml
and 100ng/ml CG53135 is broader). Thus, treatment of IEC18 cells with the
CG53135-05 E. coli
purified product results in cell killing at a higher dose of radiation
compared to untreated cells,
indicating that CG531 35 acts as a radioprotectant in these cells.
The effect of CG53135 treatment on survival of irradiated NIH 3T3 cells is
shown in Figure
33(B). The DO values for NIH 3T3 cells treated with 50 ng/ml or 200 ng/ml
CG53135 appear larger
than the DO value for untreated cells, and the difference approaches
significance for the 50 ng/ml
dose. These results suggest that CG53135 may act as a radioprotectant,
promoting survival of NIH
3T3 cells at the higher doses of radiation. Furthermore, the D1 value for
cells treated with 100
ng/mL CG53135 is smaller than for untreated cells, indicating a slower rate of
the cell death at lower
doses of radiation. No trend in Dq values of the survival curves could be
determined, largely due to
the variation of survival in untreated cells.
The effect of CG53135 treatment on survival of irradiated HUVEC cells is shown
in Figure
34. The DO value for cells treated with 100 ng/mL CG53135 is higher than that
for untreated
HUVEC cells or cells treated with 10 ng/mi CG53135, indicating a slower rate
of cell death at higher
radiation doses. In addition, the Dq value for cells treated with 100 ng/mL
CG53135 suggests that
there is a slower rate of cell death at low doses of radiation compared to
untreated HUVEC cells or
cells treated with 10 ng/ml CG53135. No obvious effects of treatment of
CG53135 on Dl values
105
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
were observed. Thus, treatment of HUVEC cells with 100 ng/ml CG53135-05 E.
coli purified
product provides a significant decrease in the speed of cell killing at the
high dose of radiation. The
HUVEC cells treated with 100 ng/ml CG53135-05 E. coli purified product also
appeared to be more
protected from killing at low doses of radiation compared to untreated cells.
In contrast, irradiated FaDu and IEC6 cells did not show any obvious trends in
DO, Dq or D1
values as a result of CG53135 administration.
In another study (L-411 and L-432), post-radiation cell survival was examined
in 7 cell lines
that were representative of different cell types in each layer of intestinal
mucosa: epithelium (IEC6
and IEC18, rat intestinal epithelia), mesenchyme (NIH3T3, mouse fibroblast;
CCD-18Co, human
colonic fibroblast), and hematopoietic (32D, murine hematopoietic cell line)
using a clonogenic
assay (as described above). Cells were irradiated, plated in complete growth
media with or without
100 ng/ml CG53135-05 E. coli purified product, and allowed to form colonies
for 10-14 days. The
data were plotted and analyzed for rate of cell killing at high doses (DO) and
low doses of radiation
(D1, Dq) (Figure 35, Table 41).
Table 41: Survival Response Parameters for Cells
Cell Type Treatment Do D D,
32D Cells Untreated 0.60 2.60 20.38
(Hematopoietic) 100 ng/mL CG53135-05 0.71 3.06 21.45
Post-irradiation
IEC6 Cells Untreated 1.03 1.98 2.50
(Epithelial) 100 ng/mL CG53135-05
Post-irradiation 1.03 3.32 5.89
IEC18 Cells Untreated 1.27 0.68 1.61
(Epithelial) 100 ng/mL CG53135-05
0.95 3.15 5.88
Untreated 0.88 2.87 5.22
NIH3T3 Cells 100 ng/mL CG53135-05
(Mesenchymal) Prior to irradiation 1.18 2.15 2.72
100 ng/mL CG53135-05
Post-irradiation 0.75 3.46 ----
CCD-18Co Untreated 1.23 0.13 1.29
(Mesenchymal) 100 ng/mL CG53135-05
Post-irradiation 0.95 1.68 2.13
Untreated 0.77 0.49 1.02
U2OS 100 ng/mL CG53135-05
(Bone) Prior to irradiation 0.79 0.76 1.21
100 ng/mL CG53135-05
Post-irradiation 0.80 1.60 2.03
Saos2 Untreated 0.74 0.95 1.32
(Bone) 100 ng/mL CG53135-05
Post-irradiation 0.74 1.37 1.73
106
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
I"he D, and Dq parameters are indicative of the rate of cell killing at low
doses of radiation, whereas
he Do parameter reflects the rate of killing at high doses of radiation. An
increase in these
)arameters in CG53135-05-treated cells as compared to untreated indicates a
protective effect.
Significant protection was observed the 32D, NIH3T3, IEC18, IEC6 and U2OS cell
lines,
vhile more modest protection was seen in the CCD18Co and Saos lines (Figure
35, Table 41).
freatment of U2OS and NIH3T3 cells with FGF-20 after irradiation was more
efficacious than
)retreatment.
In summary, these results indicate that the CG531 35-05 E. coli purified
product has a
arotective effect against radiation in vitro.
6.30. Example 30: Effect of CG53135 on Cytokine Release
Cytokines are important cell signaling proteins mediating a wide range of
physiological
*esponses. Ionizing radiation can trigger a series of changes in gene
expression and cytokine
)rofiles. The aim of this study was to evaluate the cytokine profile upon
CG53135 treatment in cell
ulture over a time course.
BioPlex cytokine assays, which are multiplex bead based assays designed to
quantitate
nultiple cytokines from tissue culture supernatants, were used for detecting
the cytokines. The
Drinciple of the assay is similar to a capture sandwich immunoassay. NIH 3T3
cells were plated in a
a6 well plate. The cells were washed with DMEM+0.1 % Calf Serum (SFM). The
CG53135-05 E.
>oli purified product, at 10 ng/ml or 100 ng/ml, was added to the cells. The
cell supernatant was
ollected after 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, and 24 hours.
Fifty ng of TNF was
ised as a positive control. Bioplex 18-Plex Cytokine Assay (BioRad
Laboratories Inc, CA) was
oerformed following the procedure of the manufacturer.
Results:
Figure 36 shows the effect of the CG53135-05 E. coli purified product on Mo KC
release.
Ulo KC is also known as the chemokine CXCL1 (which also has been described as
Grol, Melanoma
growth stimulatory activity (MSGA) or neutrophil-activating protein-3 (NAP3)).
It functions as a
ahemoattractant for neutrophils, signalling through the CXCRI receptor. It has
also been implicated
n the response to whole body irradiation, raising the possibility that it
possesses radioprotective
qualities of its own (see Radiat. Res. 160:637-46, 2003). Figure 36 shows a
consistent dose (p =
D.0085) and time dependent increase (p = 4.6x10"6) in the measured response.
In addition both the
aoncentrations of the CG53135-05 E. coli purified product showed significantly
higher response than
the control (no CG53135).
IL-6 and IL-11 expression in response to CG53135 treatment was also examined.
Both IL-6
and IL-11 have recently been implicated in the response to total body
irradiation. In addition, IL-11
has been used as an agent to combat thrombocytopenia following chemo- or
radiotherapy.
CCD18Co cells were incubated with 100 ng/ml CG53135 in basal media containing
0.1% BSA for
the indicated time periods. Conditioned media was removed and analyzed for IL-
6 and IL-11
107
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
oncentration by Luminex or ELISA respectively. Figure 36B shows that IL-6 and
IL-11 cytokines
are induced upon exposure to the CG53135-05 E. coli purified product in vitro.
Additional experiments can be performed to determine CG53135-05 in combination
with
XCL1 acts synergistically in radioprotection, both in vitro and in vivo.
Furthermore, induction of
)ther cytokines (e.g., IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, MCP-1, GM-CSF,
RANTES) can also be
ested, as a skilled person in art would recognize, in the presence of CG53135-
05 in different cell
ines (e.g., HUVEC, CCD-18, NIH3T3).
6. 31. Example 10: Measurement of Scavengers of Reactive Oxygen Intermediates
After Radiation Exposure
M-H,DCFDA method
Cells with increased reactive oxygen species, as a first step, upregulate the
Superoxide
)ismutases - Cu, Zn-SOD, Mn-SOD, and extracellular-SOD to scavenge the
superoxide radical to
iydrogen peroxide. Activity of these enzymes can be indirectly measured by
their byproduct of
-izO2 using an acetoxymethyl ester. A derivative of this class, 5-(and-6)-
chloromethyl-2',7'-
iichlorodihydrofluorescein diacetate, also known as CM-H2DCFDA, is efficiently
retained within the
ell and fluoresces green when oxidized by H202.
Cu, ZnSOD
Dze- H2O2 + CM-H2DCFDA po Fluorescein
MnSOD
ECSOD
IEC1 8 (rat intestinal epithelial) and CCD-18Co (human colonic fibroblast)
cells were plated
o 60 mm dishes at a density of 1x105 cells per dish. After attachment, the
cells were switched to
nedium containing 0.1% serum and the indicated dose of CG53135. After 18 hours
of incubation,
he cells were then irradiated at 2 or 4 Gy with X-rays using a Faxitron X-
irradiator (Wheeling, IL),
ollowed by incubation with 5 mM CM-H2DCFDA (Molecular Probes, Eugene, OR) for
15 minutes.
rhe cells were then washed, trypsinized, and analyzed on a Becton Dickinson
FACSCalibur (San
)ose, CA) on the FL1 channel.
Results indicate that IEC18 and CCD18Co cells possess increased intracellular
HZOZ after
reatment with the CG53135-05 E. coli purified product in a dose responsive
manner (FIGs. 37 (A)-
C)). This is believed to be due to enhanced expression of Superoxide
dismutases, predominantly
vInSOD induced by the CG53135-05 E. coli purified product.
Production of intracellular H2O2 by the CG53135-05 E. coli purified product in
IEC18 cells is
:nhanced with increasing dose of ionizing radiation (FIG. 37(B)). This result
reflects the increased
)roduction of more reactive oxygen species such as superoxide and hydroxyl by
radiation, thus
ncreasing substrate for the Superoxide Dismutases induced by the CG53135-05 E.
coli purified
)roduct.
2ed CC-1 method
108
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Upon ionizing radiation exposure, cells accumulate reactive oxygen species as
a result of
radiation ionizing H20 to the hydroxyl radical (OH), superoxide (02 ) or
hydrogen peroxide (H202).
As these ions in abundance can have deleterious effects on the cell, pathways
are upregulated to
scavenge these molecules to more stable variants. It is hypothesized that
CG531 35 may protect the
-ell from ionizing radiation damage by upregulating pathways that reduce the
redox capacity of the
-ytosol. A dye called Redox Sensor 1(Red CC-1) can monitor the redox level of
the cytosol upon
:)xidation by changing to a red fluorescent agent that can be measured by FACS
on the FL2
:;hannel.
Cytosolic Reactive Oxygen Species + Red CC-1 --> Oxidized Red CC-1
IEC18 (rat intestinal epithelial) and CCD-18Co (human colonic fibroblast)
cells were plated
:o 60mm dishes at a density of 1x105 cells per dish. After attachment, the
cells were switched to
medium containing 0.1 % serum and the indicated dose of the CG53135-05 E. coli
purified product.
4fter 18 hours of incubation, the cells were then irradiated at 2 or 4 Gy with
X-rays using a Faxitron
K-irradiator, followed by incubation with 5 mM Red CC-1 (Molecular Probes,
Eugene, OR) for 15
,ninutes. The cells were then washed, trypsinized, and analyzed on a Becton
Dickinson
FACSCalibur on the FL2 channel.
Results: IEC18 and CCD18Co cells were found to possess decreased cytosolic
redox
aotential after treatment with CG53135-05 in a dose responsive manner as shown
in FIGs. 38 (A)-
;C). The data shown herein is believed to be the result of enhanced expression
of superoxide
lismutases induced by the CG53135-05 E. coli purified product, which scavenge
the more reactive
3pecies of superoxide and hydroxyl radicals. Also, the CG53135-05 E. coli
purified product is shown
:o increase expression of a key antioxidant-controlling transcription factor,
Nrf2, which may
ontribute to this reduction in reactivity in the cytosol in other ways.
6.32. Example 32: In vitro Radioprotection of the Myeloid Cell Line 32D
The radioprotection effect of CG53135 in myeloid cells was also studied by in
vitro
Bxperiment using the myeloid cell line 32D. 32D cells were irradiated at 0, 1,
2, 3, 4 or 5 Gy then
Aated in methylcellulose-containing growth media including 10 ng/ml IL-3 with
or without 100 ng/ml
-G53135-05 E. coli purified product. Cells were allowed to form colonies for
10 days and were then
Bcored. FIG. 39 shows increased survival of 32D cells upon exposure to the
CG53135-05 E. coli
:)urified product. The cell survival is plotted by the natural Log of the
surviving fraction, and a linear
uadratic equation was used to obtain the curve. The qualities of the curve,
i.e., Dl, Dq and DO,
ndicating at what points radioprotection are observed, were derived using the
methods described in
-lall et al., Radiat. Res. 114(3):415-424 (1988), the description of which is
incorporated herein by
'eference in its entirety. An increase in the Dq value, indicating the amount
of radiation required for
ell death to start exponentially (or the width of the "shoulder" of the curve
at low radiation doses)
'rom 2.10 Gy to 2.79 Gy was observed.
109
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
6.33. Example 33: Effect of CG53135 on Repopulation of Thymus Following Bone
Marrow Ablation and Subseguent Bone Marrow Transplant
Long-term effects of CG53135 specifically in the thymus microenvironment on
reconstitution
of the immune system were also examined. Protein concentrations in this
example were measured
by UV absorbance. The CG53135 E. coli purified product was tested in a bone
marrow ablation and
transplantation model and repopulation of the thymus with thymocytes was
examined. Mice were
irradiated with 9Gy to ablate the bone marrow, and subsequently underwent bone
marrow
transplantation. Prior to this, one group of mice was dosed with 16 mg/kg (UV)
CG53135 (IP), once
daily on days -3, -2, -1, 0 and +1 relative to the day of bone marrow
ablation. Thirty days after bone
marrow transplantation, the thymi of both untreated and treated mice were
harvested and
thymocytes collected. Cells were counted (A) as well as stained (B) for the T-
cell specific markers
CD4 and CD8.
FIG. 40 shows that the total thymocyte cell population, as well as mature
CD4/CD8 positive
T-cells within the thymus, was significantly increased in animals treated with
the CG53135-05 E. coli
purified product (p=0.00003).
6.34. EXAMPLE 34: EFFECTS OF CG53135 ON RADIATION INDUCED DIARRHEA
(STUDY N439)
This study examined the ability of CG53135 to reduce the level and severity of
diarrhea in
mice exposed to a lethal dose of irradiation. A previous study (N-438)
indicated that 16mg/kg
CG53135 given in a triple dosing schedule on Day-1, 0 and +1 significantly
reduced the diarrhea
severity in mice exposed to 14Gy X- irradiation. Conversely, dosing 4mg/kg on
Day 0, at 0, 6, 12,
18 hours post irradiation increased diarrhea severity. This study further
tested these observations,
and assessed whether other therapeutic CG53135 treatment schedules, when the
drug was only
given post radiation exposure, could reduce diarrhea severity.
Materials and Methods:
Test materials: The test material used in this study was CG53135-05 E. coli
purified
product, batch number PT0504A. This was supplied as a frozen stock at 9.9mg/mI
and each vial
thawed from -80 C and diluted as required in Aminosyn to 1.6 or 0.4mg/mI in a
laminar flow hood,
and the Aminosyn was sterile filtered prior to the dilution. Animals were then
injected ip with
0.1 ml/10g body weight. CG53135 was freshly prepared (diluted) each day.
Test animals and husbandry: 140 male BDF1 mice (Harlan, UK) aged 10-12 weeks
at study
initiation were individually numbered using an ear punch. Each treatment group
consisted of 20
mice, housed in 4 cages of 5 mice. Animals were housed in individually
ventilated cages with 27 air
changes/hour. Animals were allowed to acclimatize for 14 days prior to study
commencement. The
rooms were on an automatic timer for a 12 hour light/dark cycle with no
twilight. All cages were
labeled with the appropriate information necessary to identify the study,
dose, animal number, and
treatment group. Animals were fed a standard rodent maintenance diet. Food and
filtered water
were provided ad libitum.
110
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Experimental Procedures:
Mice were divided into 7 treatment groups of 20 animals/ group and ear punched
for
identification. Animals were then dosed with drug (CG53135) by IP injection or
remained untreated
according to the schedule in Table xx. Mice were exposed to a single dose of
14Gy X ray whole
body irradiation using a Pantak PMC 1000, Model HF320 machine with radiation
delivered at
0.7Gy/min. All irradiations were performed during 13:00-17:00 hours. Animals
were restrained but
unanaesthetised for the duration of the irradiation.
Table 42: Study Design:
Group Number Induction on Treatment Treatment
Number of Day 0 Schedule
Male
Animals
1 20 14 Gy Day 0 Untreated Untreated
2 20 14 Gy Day 0 CG53135-05 E. coli purified Day-1, 0*, +1
product, 16m /k IP
3 20 14 Gy Day 0 CG53135-05 E. coli purified Day 0*
product, 16mg/kg IP
4 20 14 Gy Day 0 CG53135-05 E. coli purified Day 0*, +1
product, 16mg/kg IP
20 14 Gy Day 0 CG53135-05 E. coli purified Day 0*, +1, +2
product, 16m /k IP
6 20 14 Gy Day 0 CG53135-05 E. coli purified Day 0*, +1, +2, +3
product, 16mg/kg IP
20 14 Gy Day 0 CG53135-05 E. coli purified Day 0*, and +6,
product, 16mg/kg IP +12, +18 hours
Body weights and diarrhea: Animals were weighed daily to assess possible
changes in
animal weight among treatment groups. Animals losing more than 20% of their
body weight and
showing signs of distress were considered moribund and sacrificed. Animals
were observed for
diarrhea incidence and severity twice a day from Day 3 onwards. In addition to
checking incidence,
the severity of the diarrhea was recorded on a scale of 0-3 for each treatment
group:
0 = no sign of diarrhea
1= loose stools/ mild diarrhea
2= diarrhoea, peri-anal staining/matting of fur
3= severe watery diarrhea with wide area of staining/matting of fur.
Individual animal diarrhea scores were recorded.
Histopathology: At the time of sacrifice the ileum and mid colon was removed
and fixed in
formal saline, prior to paraffin embedding. These embedded samples were then
cut to produce 3pm
111
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
sections that were stained with H&E and the level of intestinal damage
evaluated by eye. The
sections from each mouse were evaluated and assigned a score from 1-5:
1: Normal Histology
2: Regenerating epithelium, almost repaired
3: Ulceration
4: Severe ulceration, few crypts remaining
5: Totally Denuded
Results:
The relative loss in body weight per group is shown in FIG. 41. The raw data
for the
diarrhea severity scores are summarized in FIGs. 42 (A) and (B).
Although all the animals lost weight, the CG53135-treated animals initially
lost weight at a
greater rate than the controls. This lost difference was greatest mid-term
(Days 2 and 3) and was
directly related to the dosing schedule (animals receiving the most doses
losing the greatest weight).
However, by the latter days of the study there was no difference between the
levels of weight loss
among the groups. As previously, mice were deemed moribund and were culled if
they had lost
more than 20% of their body weight and were also displaying signs of distress.
If mice had lost
more than 20% of their body weight but were active, they were spared and re-
evaluated at the next
time point. Occasionally mice that had been spared were found dead, and thus
tissue was not taken
and there are no histology scores.
Diarrhea incidence was measured twice daily from Day 3 onwards, although no
diarrhea
was observed until Day 4. It is immediately obvious that 16mg/kg CG53135-05
reduced diarrhea
severity. Dosing using the previously efficacious protocol on Day-1, 0, +1
with respect to irradiation,
again was the most effective protocol, with dosing 0, 6, 12 and 18 hours post
irradiation the least
effective protocol. A single therapeutic injection immediately following
radiation exposure, or dosing
immediately following radiation exposure and 24 hours later was also very
effective. The former
appeared to be have a lesser effect at the earlier time points, but reducing
diarrhea severity by the
evening of Day 5. During this mid-term phase, there was a correlation between
dose frequency and
severity, with animals receiving the more daily doses experiencing the more
severe diarrhea.
If the total diarrhea scores per group are examined, the effect of dosing on
Day -1, 0, and
Day +1 is to reduce diarrhea severity to 60% of the untreated level (49% if
moribund animals are
assigned a severity score of 3). This increases to 68% for those given CG53135
immediately post
irradiation (70% if moribund animals are given a severity score of 3). Animals
given further doses
have severities of 69 (dose Day 0, 1), 80 (dose Day 0, 1, 2) and 77% (dose Day
0, 1, 2, 3) of control
(60, 56 and 63% if moribund animals are included).
The histology scores in the small intestine confirm the diarrhea data.
Examination of the
total and average scores, with moribund mice assigned a score of 4, reveals
the best response in
112
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
mice dosed on day 0 only, and mice dosed -1, 0, +1. Again, CG53135 dosed 0, 6,
12 and 18 hours
later was least effective.
An additional anecdotal observation during this study was that most of the
mice receiving
16mg/kg CG53135 ejaculated immediately on injection. This is a new
observation, but we have
performed most of our previous work using 4mg/kg, so it may be related to the
higher dose of drug.
In conclusion, 16mg/kg CG53135-05 consistently reduced the diarrhea severity
in mice
exposed to 14Gy X irradiation. Dosing day 0 only or days -1, 0, +1 yielded the
best protection when
examined by a variety of parameters. Further dosing (Days 0, 1; Day 0, +1, +2;
and Day 0, +1, +2,
+3) provided no extra benefit. Dosing 0, 6, 12 and 18 hours post irradiation
also failed to reduce the
total diarrhea severity.
6.35. EXAMPLE 35: WOUND REPAIR TEST
In vitro cell culture: The human colon cancer cell line Caco2, HT29 and THP-1
cells were
obtained from the American Type Culture Collection (Rockville, MD), HT-29 MTX
were provided by
Dr. Lesuffler, INSERM, Dillejuis, France. These cell lines (Caco2, HT-29 and
HT-29MTX) were
grown as described previously. THP-1 cell lines were grown in RPMI-1640 medium
(Life
Technologies, Gaithersburg, MD) with 10% fetal bovine serum, 100 units /ml of
antibiotics/antimycotics (Life Technologies, Gaithersburg, MD).
An in vitro healing assay was performed using a modified method. Briefly,
reference lines
were drawn horizontally across the outer bottom of 24-well plates. HT-29 and
Caco-2 cells were
seeded and grown to confluence, then incubated with media containing 0.1 % FBS
for 24 hours.
Linear wounds were made with a sterile plastic pipette tip perpendicular to
the lines on the
bottom of the well. Isolated CG53135-05 E. coli purified product (100ng/ml)
was then added. The
size of the wound was measured at three predetermined locations at various
times after wounding
(0, 6, 20 and 24 hours). The closure of the wounds was measured
microscopically at 20x
magnification over time, and the mean percentage of wound closure was
calculated relative to
baseline values (time 0). To investigate whether the effect of FGF-20 on cell
restitution is involved
with TGF-R and ITF pathway, anti-TGFR antibody (R&Dsystem, Minneapolis, MN)
and polyclonal
anti-ITF antibody (a gift from D K Podolsky, Harvard Medical School, Boston,
MA) were used.
FIG. 43 shows the effect of FGF-20 in the closure of wounds in various human
cell lines.
There is a dose dependent increase in the effectiveness of FGF-20 in the
closure of wounds in all
the cell lines tested, demonstrating the role of FGF-20 in wound repair.
6.36. EXAMPLE 36: REVERSE TRANSCRIPTASE-POLYMERASE CHAIN REACTION
(RT-PCR)
Total RNA from cell lines and the colonic tissue was prepared using TRlzol
reagent
(Invitrogen) according to the manufacture's instructions. RNA was reverse
transcribed using 2pg of
total RNA, 15U of RNA inhibitors, lx first strand buffer (Life technologies,
Long Island, NY), 5mM
113
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
dNTP (Pharmacia, Uppsal, Sweden), 125 pmol random hexamer primers (Pharmacia),
and 125 U of
Moloney murine leukemia virus RT (Life Technologies) in a final volume of
25u1. The reaction was
carried out for 1 hour at 39 C followed by 7 minutes at 93 C and 1 minutes at
24 C and then slowly
cooled to 4 C for 20 minutes. PCR was carried out in a volume of 50 NI
containing 5 pl of RT
mixture, 1X Thermos aquaticus (Taq) buffer, 5 pmol of each primer, 2.5mM dNTP,
and 1 U of Taq
polymerase.
The sequence of primers used were as follows:
human COX-2 sense; 5'-AGATCATCTCTGCCTGAGTATCTT-3' (SEQ ID NO: 62),
human COX-2 antisense: 5'-TTCAAATGAGATTGTGGGAAAATTGCT-3' (SEQ ID NO: 63),
human Intestinal trefoil factor (ITF) sense: 5'-GTGCCAGCCAAGGACAG-3', (SEQ ID
NO: 64),
human ITF antisense: 5'-CGTTAAGACATCAGCCTCCAG-3', (SEQ ID NO: 65),
human PPAR-y sense: 5'-TCTCTCCGTAATGGAAGACC-3' (SEQ ID NO: 66),
human PPAR-y antisense: 5'-GCATTATGAGACATCCCCAC-3' (SEQ ID NO: 67),
human (3-actin sense: 5'-CCAACCGCAAGAAGATGA-3' (SEQ ID NO: 68),
human R-actin antisense: 5'-GATCTTCATGAGGTAGTCAGT-3' (SEQ ID NO: 69),
mouse COX-2 sense: 5'-GCAAATCCTTGCTGTTCCAATC-3' (SEQ ID NO: 70),
mouse COX-2 antisense: 5'-GGAGAAGGCTTCCCAGCTTTTG-3' (SEQ ID NO: 71),
mouse ITF sense: 5'-GAAGTTTGCGTGCTGCCATGGAG-3' (SEQ ID NO: 72),
mouse ITF antisense: 5'-CCGCAATTAGAACAGCCTTGTG-3' (SEQ ID NO: 73),
mouse IL-10 sense: 5'-CTCTTACTGACTGGCATGAGGATC-3' (SEQ ID NO: 74),
mouse IL-10antisense: 5'-CTATGCAGTTGATGAAGATGTCAAATT-3' (SEQ ID NO: 75),
mouse G3PDH sense: 5'-CGGTGCTGAGTATGTCGTGGAGTCT-3' (SEQ ID NO: 76),
mouse G3PDH antisense: 5'-GTTATTATGGGGGTCTGGGATGGAA-3' (SEQ ID NO: 77).
PCR was carried out in a Perkin-Elmer 9600 cycler set for 20- 40 cycles to
assess linearity
of the amplification. The PCR products were electrophoresed on 2% tris-acetate
and EDTA agarose
gels containing gel star fluorescent dye (FMC Corporation, Philadelphia, PA).
A negative from the
gels was taken with Alphalmager 2000 (Alpha Innotech Corporation, CA) and
relative abundance of
RT-PCR transcript was assessed by Adobe photoshop 3Ø4 soft ware, normalized
to the density of
(3-actin and G3PDH transcript.
Expression of some protective genes was also detected by mRNA expression in
cell lines or
cells isolated from mice (C57BL6) using standard procedures.
COX-2 gene expression in HT29 cell line in the presence of CG53135 was dose
dependent,
showing highest expression when induced by 100ng/ml of CG53135-05 E. coli
purified product (FIG.
44). At this concentration, the gene expression was higher at 1 hour and 3
hour time periods of
incubation and decreased thereafter at 6 hour and 24 hour.
114
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
COX-2 gene expression in Caco2 cell line was high when stimulated with
100ng/ml of
CG53135 as seen in FIG. 45. Increased expression of COX2 was detected at 1, 3
and 6 hours
after incubation with 100ng/ml of CG53135 E. coli purified product.
Expression of COX-2 in IEC-6 cell line showed a dose dependent increase in the
presence
of CG53135 (FIG. 46). Increased expression of COX-2 was detected at 1 hour
after incubation with
100ng/ml of CG53135-05 E. coli purified product.
Expression of Intestinal Trefoil factor (ITF) in HT-29 and Caco2 cell lines,
in the presence of
CG53135, is shown in FIG. 47. Results show dose and time dependent increase in
expression of
ITF in both HT-29 and Caco2 cells when stimulated by FGF-20. FIG. 48
reiterates that COX-2 is
expressed in HT-29 cells. In addition, TGF-(3, ITF, PPAR-y expression is also
shown in FIG. 48.
The results presented suggest that CG53135 plays a key role in mucosal repair
possibly by
inducing COX-2 and ITF genes. Based on the data, that FGF-20 induces TGF-(3
expression, wound
repair in Caco-2 cells was tested as described in Example 35, in the presence
of anti-TGF-(3
antibody (20 pg/ml). FIG. 49 shows that Epithelial Restitution by XG53135 is
mediated in part by
TGF-P pathway (p<0.05 vs CG53135 E. coli purified product + anti-TGF-(3).
6.37. EXAMPLE 37: TRANSCRIPTION PATHWAY ASSAYS
Signal transduction was considered a possible mechanism for inducing COX-2
expression
in epithelial cells, upon stimulation with CG53135. Various kinases were
tested for their expression
in the presence of 100ng/ml of CG53135 E. coli purified product, in Caco2
cells. The results
indicated that phosphorylated MAPK (p-p38MAPK) was induced in the presence of
CG53135, while
no other kinase tested, showed any significant induction (FIG. 50(A)). Also
IkBa expression
demonstrated moderate degradation in the presence of CG53135. In addition,
FIG. 50(B)
demonstrates that inhibitors of Erk and MAPK decreased COX-2 expression in the
presence of
FGF-20, in Caco2 cells. Furthermore, expression of kinases was analyzed in THP-
1 macrophage
cell line, in the presence of 100ng/ml of CG53135-05 E. coli purified product
(FIG. 50(C)). The
results demonstrated increased expression of phosphorylated STAT3, p-p38MAPK
and SOCS-3
genes. Also FIGs. 50(D)-(E) show increased expression of phosphorylated Elk-1,
ATF-2 and
minimal induction of phosphorylated Protein Kinase C in Caco-2 cells in the
presence of CG53135.
In HT-29 cells, C-Fos and C-Jun were induced, when cultured with CG53135 (FIG.
50(D)-(E)).
6.38. EXAMPLE 38: DOSE RESPONSIVE EFFECTS OF CG53135 IN FEMALE SWISS
WEBSTER MICE WITH DEXTRAN SULFATE-INDUCED COLITIS
The experiments reported in this Example report the results of dose titration
experiments in
an animal model of inflammatory bowel disease.
Introduction and General Methods
Colitis Study Design: Normal female Swiss-Webster mice (Harlan Labs), 6 - 8
weeks old
weighing approximately 20 g, were acclimated for 4 days (Day -4 through Day -
1) and then given
water orally (po) ad libitum containing 5% dextran sulfate sodium (DSS) or
control water ad libitum
115
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
for 7 days (Day 0 through Day 6). DSS (Spectrum Chemicals, Gardena CA) was
made as a 5%
solution in tap water; DSS was made every other day and stored at 4 C. Mice
were divided into 8
treatment groups including QD doses of 0.3, 1, 3 and 10 mg/kg, and a BID dose
regimen of 5 mg/kg
per dose (Table 43). On Day 0, daily intraperitoneal (ip) treatments with
vehicle (1 M L-arginine in
phosphate buffered saline) or CG53135 protein in vehicle were initiated and
continued through Day
6. On Day 7, mice were sacrificed with COa.
Table 43: Treatment Groups
Treatment Normal Disease CG53135 CG53135 CG53135 CG53135 Disease CG53135
Group Controla ControlbQD QD QD QD QD BiDtrol BID
Group # 1 2 3 4 5 6 7 8
CG 53135 0 0 10 3 1 0.3 0 5
(mg/kg)
Number
of Test 4 10 10 10 10 10 10 10
Animals
a normal control = vehicle only; b disease control = 5% DSS + vehicle
Protein production: The CG53135 protein was produced in E. coli as follows:
The cDNA for
CG53135-01 was identified and cloned into the pRSET vector (Invitrogen) to
provide the vector
pETMY-CG53135-01. The gene product of this construct provides a polypeptide
incorporating
(His)6-(enterokinase cleavage site)-(multicloning site) at the N-terminal end
of the polypeptide; in
addition, in this construct, the CG53135 sequence begins with the Ala at
position 2 FGF-20 (SEQ ID
NO:2). This vector was transformed into Escherichia co/i. The E. coli cells
were grown up to 10 L
scale and infected with CE6 phage to produce the recombinant CG53135. The
recombinant protein
was purified by disrupting the E. coli cells (resuspended in a 1 M L-arginine
solution) in a
microfluidizer, followed by multiple metal affinity chromatography steps. The
final purified protein
was dialyzed into phosphate buffered saline containing 1 M L-arginine.
Colon content was scored at necropsy according to the following criteria:
0 = normal to semi-solid stool, no blood observed
1= normal to semi-solid stool, blood tinged
2 = semi-solid to fluid stool with definite evidence of blood
3 = bloody fluid
Pathology Methods: Three sections equidistant apart from the distal one third
of the colon
(area that is most severely affected in this model) were processed for
paraffin embedding, sectioned
and stained with hematoxylin and eosin for pathologic evaluation.
For each section, submucosal edema was quantitated by measuring the distance
from the
muscularis mucosa to the internal border of the outer muscle layer.
Inflammation (foamy
116
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
macrophage, lymphocyte and PMN infiltrate) was assigned severity scores
according to the
following: Normal = 0; Minimal = 1; Mild = 2; Moderate = 3; Marked = 4; and
Severe = 5. Splenic
lymphoid atrophy was also scored by the above criteria. The parameters
reflecting epithelial cell
loss/damage were scored individually using a % area involved scoring method:
None = 0; 1-10% of
the mucosa affected = 1; 11-25% of the mucosa affected = 2; 26-50% of the
mucosa affected = 3;
51-75% of the mucosa affected = 4; and 76-100% of the mucosa affected = 5.
. Parameters that were scored using % involvement included: (1) Colon
glandular epithelial
loss - this includes crypt epithelial as well as remaining gland epithelial
loss and would equate to
crypt damage score; and (2) Colon Erosion - this reflects loss of surface
epithelium and generally
was associated with mucosal hemorrhage (reflective of the bleeding seen
clinically and at
necropsy). For each animal, 3 proximal (less severe lesions) and 3 distal
(most severe lesion) areas
were scored and the mean of the scores for each of the regions was determined.
Group means and
% inhibition from disease control were determined. By doing it this way
(rather than summing the
scores from various sections) one can look at the mean SE for in individual
parameter
(represented by 3 sections) and equate it to a delineated severity. As an
example, if the mean is 4
for gland epithelial loss one knows that 51-75% of the mucosa was devoid of
epithelium.
The three important scored parameters (inflammation, glandular epithelial
loss, erosion)
were ultimately summed to arrive at a sum of histopathology score which
indicates the overall
damage and would have a maximum score of 15.
One final summation of proximal + distal summed scores was done to reflect the
overall
total colonic severity score.
Statistics: The mean and standard error (SE) for each treatment group was
determined for
each parameter scored; the data were compared to the data for the disease
controls (Group 2)
using a 2-tailed Student's t test with significance at p<_ 0.05.
Live Phase, Necropsy and Organ Weight Results
Four animals died during the course of the study (#10 in vehicle control group
2 on day 7,
#3 in group 6, 0.3 mg/kg on day 6, #5 in group 8 vehicle control BID on day 7,
and #6 in group 7 5
mg/kg BID on day 6).
DSS treatment-related changes in body weight were obvious by day 5 in all DSS
treated
mice and ultimately were most severe in animals treated with vehicle (FIG.
51(A)). At study
termination, DSS+vehicle controls had a 28% decrease in body weight. A
significant beneficial
effect on DSS induced weight loss was seen in mice given AB020858 (CG53135) QD
at all doses
(FIG. 51(B)).
Clinical evidence of bloody diarrhea was evident in all DSS+vehicle animals.
At necropsy
all DSS controls had blood or blood tinged fluid in the colon. In contrast,
mice treated QD with 10
mg/kg AB020858 (CG53135) generally had semi-solid stool and less blood (except
animals #5).
Clinical benefit was also evident but less impressive in those given doses of
3 or 1 mg/kg QD and
117
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
absent in those treated with 0.3 mg/kg (FIG. 51(C)). Mice treated BID with 5
mg/kg had the most
impressive clinical benefit (68% inhibition) and clinically these mice had the
best overall
improvement.
Absolute colon lengths were decreased 41 % in mice treated with vehicle.
Treatment with
AB020858 (CG53135) QD at 10 mg/kg resulted in significant (21 %) inhibition of
the DSS-induced
changes in colon length. Treatment with AB020858 (CG53135) BID at 5 mg/kg
reduced the colon
length decrease 36%.
Absolute colon weights were decreased approximately 26% in mice treated with
DSS in
vehicle. Treatment with AB020858 (CG53135) at 10 mg/kg QD or 5 mg/kg BID
resulted in significant
reduction of the DSS-induced changes in colon weights.
Absolute spleen weights were increased approximately 40% in mice treated with
DSS+vehicle (due to extreme extramedullary hematopoiesis). Spleen weights were
significantly
greater in all DSS treated animals vs. normal.
Histopathology Findings
Significant reduction of colonic inflammation, gland loss, erosion and total
histopathology
scores occurred in mice treated with AB020858 (CG53135) QD (10 mg/kg) and BID
(5 mg/kg) and
was of approximately equal magnitude (FIGS. 52 (A) - (D)).
Splenic lymphoid atrophy (an indication of stress) was inhibited in these same
animals 47%
and 46% respectively (FIG. 53). Inhibition of induction of splenic
extramedullary hematopoiesis was
greater in mice treated BID vs. QD and occurred in all treatment groups (FIG.
54).
Discussion and Conclusions
The experiments reported in this Example provide dose-response information for
the
administration of AB020258 (CG53135). The results indicate that simultaneous
administration of
AB020258 (CG53135) is effective in inhibiting the appearance of markers of DSS-
induced
inflammatory bowel disease, especially with the highest doses used.
An additional experiment was carried out in which mice were also treated
subcutaneously
with CG53135. These studies demonstrate that prophylactic administration of
CG53135 at doses of
or 10 mg/kg ip and 5 or 1 mg/kg sc significantly reduce the extent and
severity of mucosal damage
induced by dextran sulfate sodium in a murine model of colitis.
6.39. EXAMPLE 39: EFFECTS OF ADMINISTERING CG53135 TO INDOMETHACIN-
TREATED RATS
Treatment of rats with indomethacin results in gross and histopathologic
intestinal
alterations that are similar to those occurring in Crohn's Disease. The
experiments provided in this
Example report on the efficacy of CG53135 in treating the rat model of
indomethacin-induced
intestinal injury. The efficacy of this protein in an alternate model of
intestinal injury adds support to
the therapeutic potential of CG53135 in treatment of inflammatory bowel
disease.
118
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Materials & Methods
Protein production: Preparation of CG53135 protein was the same as described
in Example
38.
Study Design: Female Lewis rats (Harlan, Indianapolis, IN) weighing 175-200 g
were
acclimated for 8 days (Day -8 through Day -1). Rats were divided into 8
treatment groups: four
groups receiving CG53135 (three groups iv and one group sc), two iv controls
for normal and the
disease model, and two sc controls for normal and the disease model. On Day -
1, treatments with
CG53135 or vehicle were initiated and continued through Day 4. CG53135 was
injected iv (tail vein)
at doses of 5, 1 or 0.2 mg/kg, or 1 mg/kg sc; vehicle controls were injected
with BSA (5 mg/mL in
PBS + 1 M L-arginine). On Days 0 and 1 rats were treated with indomethacin
(Sigma Chemical Co.,
St. Louis, MO; 7.5 mg/kg doses) in order to induce gross and histopathologic
intestinal alterations
similar to those occurring in Crohn's Disease. Indomethacin was prepared in 5%
sodium
bicarbonate. On Day 5, rats were injected with a single ip dose of 50 mg/kg 5-
bromo-2'deoxyuridine
(BrdU, Calbiochem, LaJolla, CA) 1 hour prior to necropsy in order to pulse
label proliferating cells in
the intestine and spleen. Following termination, a 10 cm section of jejunum in
the area at risk for
lesions was weighed, given a gross pathology score, and then collected into
formalin for
histopathologic evaluation and scoring of necrosis and inflammation. Blood was
collected for CBC
analysis.
Observations and Analysis of Markers of Pathology
Gross Observations: Body weight was measured daily beginning on Day 0. At
necropsy,
liver and spleen weights were measured, and a 10 cm section of jejunum in the
area at risk was
weighed, scored for gross pathology, and collected into formalin for
histopathologic evaluation and
scoring of necrosis and inflammation. The area at risk for indomethacin-
induced injury was scored
at necropsy according to the following criteria: 0 = normal; 1= minimal
thickening of the
mesentery/mesenteric border of the intestine; 2 = mild to moderate thickening
of the
mesentery/mesenteric border of intestine, but no adhesions; 3 = moderate
thickening with 1 or more
definite adhesions that are easily separated; 4 = marked thickening with 1 to
numerous hard to
separate adhesions; and 5 = severe intestinal lesions resulting in death.
Histopathology: Five sections (approximately equally spaced) taken from the
weighed 10cm
area at risk of small intestine for indomethacin-induced lesions were fixed in
10% neutral buffered
formalin, processed for paraffin embedding, sectioned at 5 pm and stained with
hematoxylin and
eosin for histopathologic evaluation. Necrosis was scored according to the
percent area of the
section affected in the same way as described in Example 38 for scoring
epithelial cell loss.
Inflammation was scored according to the following criteria: 0 = none; 1=
minimal
inflammation in mesentery and muscle or lesion; 2= mild inflammation in
mesentery and muscle or
lesion; 3 = moderate inflammation in mesentery and muscle or lesion; 4 =
marked inflammation in
lesion; and 5 = severe inflammation in lesion.
119
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
The means for inflammation and necrosis were determined for each animal, and
then the
means for each group were calculated.
Statistics. The mean and standard error (SE) for each treatment group were
determined for
each parameter scored; the data were compared to the data for the disease
controls using a 2-tailed
Student's T test with significance at p < 0.05.
Results
Weight loss was observed in all animals treated with indomethacin. A slight,
but significant
reduction in weight loss was observed in animals treated with CG53135 (0.2
mg/kg iv) as compared
with disease controls (iv). Other doses of CG53135 (both iv and sc routes of
administration)
provided diminished, but not statistically significant, indomethacin-induced
weight loss.
At necropsy, a 10 cro section of jejunum in the area at risk from each animal
was weighed.
Indomethacin treatment resulted in an elevation in small intestine weight as
compared with normal iv
and sc controls, consistent with edema and inflammation associated with this
model of intestinal
injury. Treatment with CG53135 (1 mg/kg or 0.2 mg/kg iv) resulted in
significant reductions in small
intestine weight as compared with disease controls (FIG. 55(A)). A slight
reduction in the small
intestine clinical score was observed, with the greatest benefit occurring
with the 1.0 mg/kg iv dose
(38%) and the 0.2 mg/kg iv dose (25%); these benefits, however, were not
statistically significant.
Relative spleen and liver weights were increased in animals treated with
indomethacin.
Administration of CG53135 produced moderate additional increases in these
weights.
Hematology: Administration of 2 doses of indomethacin to rats increased the
total white
blood cell count as a result of increased neutrophils and lymphocytes.
Reductions in red blood cell
count, hematocrit, and hemoglobin concentration were also observed. Treatment
with CG53135 (5
mg/kg and 0.2 mg/kg iv) resulted in significant reductions in absolute
neutrophil counts as compared
with disease controls (FIG. 55(B)). Hemoglobin concentration was diminished in
the indomethacin
controls compared to normal controls, and slightly further diminished in rates
treated with CG53135.
Histopathology: Evaluation and scoring of 5 sections of intestine were
conducted for each
animal. Histologic evidence of a protective effect on the intestine was
observed only in animals
treated with CG53135 (0.2 mg/kg iv). A 53% reduction in jejunal necrosis and
38% reduction in
inflammation score were observed for the 0.2 mg/kg iv CG53135 dose as compared
with disease
controls iv (FIG. 56). Photomicrographs of affected small intestine are shown
in FIG. 57 for a
normal and disease control, and a rat treated with 0.2 mg/kg CG53135. Panel A
shows the small
intestine from a normal control animal treated iv with vehicle (BSA). Normal
villous architecture and
mesentery (arrow) are apparent. Panel B presents a photomicrograph of the
small intestine from an
indomethacin- treated rat, with vehicle (BSA) iv. Focal mucosal necrosis
extending across most of
the area associated with attachment of the mesentery is apparent (see, for
example, the asterisks at
upper right intestinal wall and lower right intestinal wall). Marked
inflammatory cell infiltrate is
present in the mesentery (arrow). Panel C shows the image of the small
intestine from an
indomethacin-treated rat further treated with CG53135, 0.2 mg/kg iv. There is
no apparent necrosis,
120
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
in contrast to the disease control shown in Panel B. There is a focal area of
attenuated villi and
cellular infiltration into muscle layer (see, for example, the three asterisks
at the upper right, right
and lower right of the intestinal wall). Mesentery (arrow) is infiltrated by
inflammatory cells. The
photomicrographs in FIG. 57 provide further support for the protective effect
of 0.2 mg/kg iv
CG53135.
BrdU labeling was carried out by injecting 50 mg/kg 1 hour prior to necropsy.
In the small
intestine from a normal control animal, normal pattern of crypt labeling is
seen at 100X (FIG. 58,
Panel A). BrdU incorporation in the disease model was decreased or absent in
epithelial cells in
mucosal areas of necrosis, but increased in subajacent inflammatory tissue in
which fibroblast
labeling was prominent (FIG. 58, Panel B, visualized at 50X). Focal mucosal
necrosis (arrow) is
delineated by an absence of BrdU immunostaining as well as severe infiltration
of inflammatory cells
and fibroblast proliferation. Small intestine from a rat treated with
indomethacin + CG53135 0.2
mg/kg iv shows an absence of crypt labeling, but relatively intact mucosa
(arrow in FIG. 58, Panel C,
visualized at 50X). Subadjacent smooth muscle and mesentery is only mildly
infiltrated with
inflammatory cells, compared with that seen in the disease control (Panel B).
In certain animals
treated with CG53135, in which preservation of mucosal integrity occurred,
increased crypt labeling
was also observed; this is in the direction found in the normal control.
The results of the experiments in this Example may be summarized as follows:
treatment of
rats with indomethacin results in gross and histopathologic intestinal
alterations that are similar to
those occurring in Crohn's Disease. Administration of CG53135 (0.2 mg/kg iv)
to indomethacin-
treated rats resulted in significant reductions in weight loss, small
intestine weight, absolute
neutrophil counts, and jejunal necrosis and inflammation scores. Higher doses
of CG53135 (5, 1
mg/kg iv and 1 mg/kg sc) were less efficacious. The morphological appearance
of tissues collected
from animals injected with BrdU 1 hour prior to necropsy suggested that the
beneficial effects of
CG53135 in this model of intestinal injury were the result of mucosal
protection rather than a
proliferative effect on target cells.
6.40. EXAMPLE 40: THERAPEUTIC ADMINISTRATION OF CG53135 ENHANCES
SURVIVAL IN THE MURINE DSS MODEL.
In the experiments described above, DSS exposure and CG53135 administration
were
initiated simultaneously on day 0. In the present Example, the effect of
CG53135 administered after
the initiation of DSS treatment was examined. CG53135 was prepared as
described in Example 38.
Balb/c mice were exposed to DSS for 7 days (day 0 to day 6). The mice were
injected daily
subcutaneously with various concentrations of CG53135 (5, 1 and 0.2 mg/kg)
beginning on the fifth
day of DSS exposure (i.e. day 4) and ending 3 days after the termination of
DSS exposure (i.e. day
9), or with vehicle only. Animal survival was recorded on a daily basis and
the experiment was
concluded on day 10.
As shown in FIG. 59, therapeutic administration of CG53135 at 5 mg/kg enhanced
survival
relative to the disease control group. Thus, while only 44% (4 of 9) of the
animals in the disease
121
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
control group survived until the end of the study, 89% (8 of 9) of the animals
treated with CG53135
at 5 mg/kg survived.
6.41. EXAMPLE 41: EFFECTS OF CG53135 IN AN IMMUNE-MEDIATED MODEL OF
INFLAMMATORY BOWEL DISEASE IN IL-10 DEFICIENT MICE (IL-10 KNOCK-OUT
MICE)
The objective of the study was to assess the ability of CG53135 to
therapeutically inhibit the
inflammation that occurs in IL-10 deficient mice when transferred from a germ
free to a specific
pathogen free environment. As inflammatory bowel disease is thought to have an
immune
component, this study evaluated the efficacy and safety of CG53135 in this
immune-mediated model
of IBD when dosed therapeutically at the time of significant disease.
Table 44: Materials and Methods
Species/strain: IL-10 Knock-out Mice (mixed C57BL X 129 Ola
background)
Ph siolo ical state: Germ-free
Age/weight range at start of stud : -8-12 weeks old weighing a proximatel 20-
25 g
Test Article: CG53135-05 (FGF-20) protein (purity >97%) in 20% glycerol
buffer.
Storage Conditions of test article: All tubes were stored at -70 C until ready
for use.
Vehicle: Glycerol buffer: 20% Glycerol, 200mM Sorbitol, 1 mM EDTA, 100mM
Citrate, 50mM KCL
Storage Conditions of vehicle: All tubes were stored at -70 C until ready for
use.
Table 45: Administration of Test Article
Route and method of administration: Intraperitoneal (ip)
Justification for route of administration: This route and dose has been used
in previous studies
with CG53135 in other murine models of colitis.
Frequency and duration of dosin : Once daily
Administered doses of CG53135-05: 5.0 mg/kg in 20 % glycerol buffer
Administered volume: 0.3 mL per mouse
Justification for dose levels: Similar doses have been used in other efficacy
models.
Table 46: Experimental Study Design
Group Number of Animals Dose Volume
Number Treatment Females Males (mg/kg) mUk
1 Normal controla 4 4 0 10
2 Disease controlb 4 4 0 10
3 5 mg/kg CG53135 (14d 4 4 5 mg/kg 10
therapeutic)
a Normal control: mice are untreated and maintained in germ free conditions
throughout study
b Disease control: vehicle administered ip, once daily using the therapeutic
dosing regimen.
122
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Table 47: Study Schedule
Stucdy Schedule
Event Day of Study
1 2 3 4 5 6 7 8 4 10 11 12 13 1415 16 17 18 14 20 21 22 23 24
Transfer from germ free cages x
Fecal slurry~ ~o x
Therapeutic CG53135 (iR) x x x x x x x x x
Therapeutic Vehicle (io x x x x x x x x x
Body weight x x xx x x x x x x xx x xx xxx
Serum collected for CG53135 x x
antibodies
Tissues collected (including x x
colon assessments)
Scheduled terminations x x
a Mice are dosed orally with a slurry of fecal contents solubilized in PBS
from donor SPF
documented free of Heliobacter.
b CG53135-05 or vehicle will be administered daily through to day prior to
scheduled termination.
Experimental Procedures
Mice were acclimated for 2 days before bacterial colonization and given
autoclaved food
and tap water ad libitum during this time. Mice will be treated with CG53135-
05 E. coli purified
product or buffer for 2 days beginning the day of transfer, then colonized
with specific pathogen free
bacteria by swabbing their mouth and rectum with solubilized fecal material.
Animals were
examined prior to initiation of the study to assure adequate health and
suitability. Animals that were
found to be diseased or unsuitable will not be assigned to the study.
This study was performed in two segments of approximately 20 animals each due
to animal
availability and the tedious collections of cells and tissues at necropsy for
T cell stimulation and
colonic strip culture. The two study segments had mice evenly assigned to all
dose groups (e.g., 2
animals per sex per treatment group). If the number of available animals at
the time of initiation is
not evenly divided between males and females, animals were assigned to groups
to balance males
and females as best as possible.
Clinical Observations/Signs
Mice were observed daily for significant clinical signs of toxicity,
moribundity and mortality
approximately 60 minutes after dosing.
123
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Body Weight: Individual body weights of all mice were recorded pretest (for
randomization)
and daily through Day 10. Body weights taken on the day of necropsy for
animals scheduled for
termination was used for determination of organ to body weight ratios.
Following are the
organs/tissues measured.
Table 48: Organs/Tissues For Weight Measurement
Cecum Kidneys Rectum
Colon Liver Spleen
Histopathology: All animals surviving to scheduled termination (Day 10) will
be terminated
using CO2 with assessment of gross observations, organ weights and collection
of all scheduled
tissues into 10% neutral buffered formalin for histopathologic evaluation.
Special colon assessments: From the areas at risk (cecal tip, transverse colon
and rectum),
3 sections approximately 1 cm apart in length will be collected, preserved in
formalin, and stained to
quantitate inflammation (hematoxylin & eosin), mucin (periodic acid schiff),
and collagen (trichrome).
All 3 sections should be representative of the affected area.
Tissues taken from the colon, will be collected and processed for paraffin
embedding,
sectioned and stained as noted above. Histopathology will be performed in a
blinded manner on the
tissue samples of cecum, transverse colon and rectum with assignment of an
inflammation score
ranging from 0 to 4, where: 0 = no inflammation; 1= mild inflammation with
increased
mononuclear cells infiltrating, mild crypt hyperplasia; 2 = more active
inflammation with increased
infiltrating mononuclear cells, mild goblet cell depletion, and mild crypt
hyperplasia; 3 = active
inflammation with crypt hyperplasia, goblet cell depletion and marked
mononuclear cell infiltration;
and 4 = severe active inflammation characterized by widespread infiltrate of
neutrophils, ulceration,
crypt abscesses and marked mucosal hyperplasia.
Following were the organs/tissues considered for macroscopic examination and
histopathology.
Table 49: Organs/Tissues For Histopathology Evaluation*
Cecum Eyes Rectum
Colon Kidneys Spleen
Liver
*AII tissues except eyes to be fixed in 10% neutral buffered formalin, eyes to
be fixed in 6%
glutaraldehyde.
Preparation of cells and cell cultures: Colonic strip cultures were
established from the
remaining colon fragments, pooled from segments of proximal, middle and distal
colon. Colon
segments were flushed with phosphate-buffered saline (PBS) to remove fecal
contents, opened
124
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
lengthwise and cut into 0.5 to1.0 cm pieces and shaken vigorously in PBS.
Approximately 50 to100
mg of tissue was then distributed per well of a 24 well tissue culture plate
in duplicate and cultured in
1 mL of complete medium containing antibiotics and an antimycotic agent
(Veltkamp et al, 2001,
Gastroenterology, Vol 120(4):900-913). After incubation at 37 C for 18 - 24
hours, culture
supernatants were collected in aliquots and frozen at -70 C for cytokines and
possibly
immunoglobulin measurements. IgG2a and IL-12 in the supernatant were measured
by ELISA.
Mesenteric lymph nodes (MLN) was mechanically dispersed, washed, counted and
used for
cecal bacterial lysate-stimulated interferon gamma measurement as described by
Veltkamp et al,
2001, Gastroenterology, Vol 120(4):900-913. Briefly, CD4+ T lymphocytes were
isolated by
negative MACS selection and incubated with antigen-pulsed antigen presenting
cells derived from
wild type mice splenocytes after T cell removal. Alternatively, unfractionated
MLN cells were
incubated with antigen.
Cytokine assays: IL-12 (Pharmingen, San Diego, CA), TNF-a and IFN-y (R&D
systems,
Minneapolis, MN) was measured in MNL cell and splenocyte culture supernatants
by ELISA.
Moreover, IL-12 and PGE2 (Assay Design, Ann Arbor, MI) was measured in
supernatants of colon
cultures using standard ELISA protocol. Concentrations of these cytokines and
PGE2 were
measured in duplicate culture supernatants by comparison with standard curves
generated using
recombinant cytokines.
Results
Body Weight and Histopathology - Prophylactic: Weight change in the
prophylactic group
was assessed. FIG. 60(A) shows weight change when challenged with FGF-20 in IL-
10 knock-out
(KO). FIG. 60(A) also shows the histopathology of the colon when the mice are
challenged with
different concentrations of FGF-20 (0.2, 1, 5 mg/kg). The results indicate
that, administration of
FGF-20 had a protective effect as compared to the vehicle control. FIG. 60(B)
further demonstrates
that, upon administration of FGF-20, there is a dose-dependent decrease in the
total Cecal
Histologic score, as compared to the vehicle (12.2 2.3 vs. 2.5 0.6;
p<0.001).
Cytokine Production - Prophylactic: Cytokine production was assayed by ELISA.
FIGS.
61(A) (IL-12), 61(B) (IFNy) and 61(C) (PGE2) indicate that FGF-20 altered
cytokine production in
MLN, colonic strip culture, Spleen cell culture, which were prepared from the
IL-10KO mice as
described above. FIG. 62 also shows FACS analysis of total MLN number (32 3.4
vs. 23 2.5;
p<0.05), CD4+ and CD8+ and CD4+CD69+ cells (3.2 0.3 vs. 1.67 t 0.1 ; p<0.05).
Body Weight and Histopatho%gy- Treatment: Study protocol for the treatment
group was
established by treatment of established colitis in ex-germ free IL-10 -/- mice
colonized with SPF
bacteria on day 1. On day 10, treatment was started by intraperitoneally
administering either
Vehicle or FGF-20 (5mg/kg) and necropsy was performed on day 17.
FIG. 63 shows the Weight change in the treatment study, where FGF-20 (5mg/kg)
was
administered to IL-10 KO mice. Histology of the cecum and rectum are
respectively shown in FIGS.
125
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
64 and 685, that demonstrates protective effect of FGF-20. Cecal histologic
score shows that FGF-
20 decreased as compared to the vehicle control (13.1 1.8 vs. 5.9 1.4;
p<0.006, FIG. 66).
Cytokine production in treatment group as assayed by ELISA demonstrated that
FGF-20
administration did not significantly alter the cytokine production in Gut
culture and unseparated
splenocytes of IL-10 fCO mice. IL-12, IFN-y, TNF-a and PGE2 were the cytokines
that were
assayed. FIG. 67 shows FACS analysis of MLN number, CD4+ and CD8+ and CD69+
cells, all of
which were decreased in FGF-20 treated group as compared to the vehicle
treatment.
Results in normal mice: Expression of COX-2, IL-10, ITF, TGF-(3 were analyzed
in normal
wild type (WT) C57BL6 mice following 7 days of injection FGF-20 (5mg/kg). RT-
PCR was
performed (as described in Example 36) in colonic tissue and unseparated MLN
to study the
expression of the above list of genes. COX-2, IL-10, ITF and TGF-(3 are
upregulated in the colonic
tissue of WT mice, upon administering FGF-20. In unseparated MLN, IL-10
expression is found to
be upregulated as compared to vehicle.
6.42. EXAMPLE 42: EFFECT OF CG53135-05 IN A CHRONIC 2 WEEK MURINE MODEL
OF DSS-INDUCED ULCERATIVE COLITIS (N-404)
Female Swiss Webster mice exposed to dextran sulfate sodium (DSS) for 7 days
develop
inflammation and gland loss with erosion in the colon. Gross and
histopathologic changes resulting
from this treatment resemble those occurring in human ulcerative colitis (UC),
a subset of
inflammatory bowel disease (IBD) (see Animal Models of Intestinal Erosion in
"Inflammatory Bowel
Disease" ed. MacDermott RP and Stenson WF. Elsevier, New York (1992); Okayasu
et al.,
Gastroenterology 98:694-702 (1990); and Cooper et al., Lab Invest. 69:238-249
(1993)).
Compounds that are effective in the treatment of human IBD have activity in
this model and it is
being used to investigate potential new therapies (see Axelsson et al.,
Aliment Pharmacol Ther.
12:925-934 (1998); Egger et al., Digestive Dis Sci. 44:436-444 (1999); Miceli
et al., J Pharmacol Exp
Ther. 290:464-471 (1999); and Jeffers et al., Gastroenterology 123 (4):1151-62
(2002)). However,
the 7-day version of the model, in which exposure to DSS is continuous, is
only useful for evaluating
the effects of agents on the acute phase of mucosal inflammation and damage. A
more chronic
model of ulcerative colitis, with less potential for lethality, was developed
to test CG53135-05 for its
capacity to enhance mucosal repair. The objective of this study was to assess
the potential
therapeutic activity of CG53135-05 in the chronic 2 week murine model of
ulcerative colitis induced
by administration of 3% (DSS) for 5 days and 1% DSS for 9 days.
Materials and Methods:
Colitis was induced in female Swiss Webster mice by exposure to 3% DSS in the
drinking
water (ad libitum) on study Days 1-5 and maintained by exposure to 1% DSS in
the drinking water
(ad libitum) on study Days 6-15. Mice were randomly assigned to 4 groups of 15
animals (Table
50). Mice were exposed to 3% DSS in drinking water on test Days 1 through 5
and 1% DSS on test
Days 6-15, with concurrent intraperitoneal (IP) treatments of vehicle or
CG53135-05 at 0.33, 1.67
and 3.33 mg/kg (UV) Days 6-14 after disease was established. Mice were weighed
daily. On Day
126
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
15,animals were terminated by cervical dislocation, the colon length was
measured, and colon
content was scored. Tissues were collected into 10% neutral buffered formalin
and processed
routinely for histopathology.
Table 50:Study Design
3% DSS 1% DSS
Treatment Treatment CG53135-05
Group Number of (water po), (water po), Treatment CG53135-05 Treatment
Number Animals* Days 1-5 Days 6-15 (mg/kg, UV) Schedule (IP)
1 15 females Yes Yes 0 Vehicle only, Days 6-14
2 15 females Yes Yes 0.33 Once daily, Days 6-14
3 15 females Yes Yes 1.67 Once daily, Days 6-14
4 15 females Yes Yes 3.33 Once dail , Days 6-14
*Acclimation of animals at least I week prior to randomization and start of
treatment
Clinical Parameters and Gross Pathology: Only animals that survived the
duration of the
study were included in the analysis of body weight change, terminal colon
lengths, and colon
content scores. At necropsy, the colon length was measured and assessed for
evidence of stool
consistency changes. Colon content was scored at necropsy according to the
following criteria: 0
Normal (firm, well formed stool); 1= Semi-solid stool; 2 = Semi-solid to fluid
stool; and 3 = Semi-
solid with definite evidence of blood, bloody fluid or no content.
Histopathology: At necropsy, the colon was harvested and divided into 2
approximately
equal segments, proximal and distal, to assess regional changes induced in
this model. Distal ends
were marked to maintain orientation. These colon segments were collected,
preserved in 10%
neutral buffered formalin, and routinely processed for histopathologic
evaluation. During processing
for histology, the proximal and distal colon segments were each trimmed to
obtain 4 equally spaced
segments, and hematoxylin and eosin (H&E) stained slides were prepared for
each. Both proximal
and distal tissues were examined as these tissues are affected to different
degrees of severity in this
model (more severe and less variable symptoms predominate in the distal
colon).
For each colon section, submucosal edema was quantified by measuring the
distance (mm)
from the muscularis mucosa to the internal border of the outer muscle layer.
The extent of
inflammation (foamy macrophages, lymphocytes and polymorphonuclear cell
infiltrate) was assigned
severity scores according to the following ranking: 0 = Normal; 1 = Minimal; 2
= Mild; 3 = Moderate;
4 = Marked; and 5 = Severe.
The parameters reflecting epithelial cell loss/damage were scored individually
using a
percent area involved scoring method. Parameters that were scored using the
percent involvement
scale included colon glandular loss and colon erosion.
0 = None
1= 1-10% of the mucosa affected
127
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
2 = 11-25% of the mucosa affected
3 = 26-50% of the mucosa affected
4 = 51-75% of the mucosa affected
5= 76-100% of the mucosa affected
Mucosal epithelial hyperplasia (basophilia, mitotic figures, multilayered on
basement
membranes, absence of goblet cells) was scored 0-5 based on the following
criteria
O=Normal
1=Minimal-small foci generally adjacent to the inflammatory changes
2=Mild-11-25 /a of mucosa affected
3=Moderate-26-50% of mucosa affected
4=Marked-51-100% of mucosa affected
5=Severe-51-100% of mucosa affected plus papillary proliferation into lumen
Mucosal thickness (an indicator of proliferative changes or edematous
inflammatory
mucosal expansion) was measured by placing an ocular micrometer at the base of
the glands and
the overlying surface epithelium in a non-tangential area of section
representative of the thickest
areas of mucosa.
The scores for proximal tissues and distal colon were averaged to determine a
score for the
entire colon for each parameter evaluated. The 3 scored parameters (i.e.,
inflammation, glandular
loss, and surface epithelial erosion) were combined to arrive at a sum of
overall histopathology
scores for proximal or distal sections of the colon, and then proximal and
distal overall scores were
averaged to arrive at an overall histopathology score for the entire colon.
These summations
indicate the overall damage in the distal, proximal, or entire colon and would
have a maximum
severity score of 15.
Additional tissues (cheek, tongue, esophagus, mid colon and rectum) were
collected and
divided into two equal sections. One section was snap frozen in liquid
nitrogen, the other was
placed in 10% neutral buffered formalin for paraffin embedding and
immunohistochemical staining
with Ki67 antibody. The formalin preserved tissues were trimmed into
approximately 0.5 cm
sections (1 section/tissue), processed through graded alcohols and a clearing
agent, infiltrated,
embedded in paraffin and sectioned. Slides were then re-hydrated and stained
with mouse Ki67
antibody (Dako) at 1:70 dilution followed by streptavidin/HRP detection system
with a DAB
peroxidase indicator and counterstained with Gill's Hematoxylin. The slides
were exposed to
diaminobenzadine (DAB) for four minutes to provide optimal specific staining
with minimal
background nonspecific staining.
Statistics: Differences in body weight changes between treatment groups were
analyzed
with repeated measures ANOVA with a Greenhouse-Geisser correction for
sphericity, followed by
pair-wise repeated measures analysis to identify the source of any variation.
These pair-wise
measures were performed between each day and the normal control. Quantitative
measurements
of pathology (colon length, edema) were analyzed with a one-way analysis of
variance (ANOVA)
128
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
followed by a linear contrast. Qualitative measures of pathology (histology
scores) were analyzed
as follows: Pathological evaluations were done at several points along the
proximal and distal
colon. These replicates were converted to a mean pathology score and a maximal
pathology score
for each individual to correct for problems of pseudo-replication. The mean
and maximal scores
were analyzed with a Kruskal-Wallis test followed by a Dunn's multiple
comparison test between
each day and normal control animals. In all other cases, multiple comparison
test results were
adjusted for the number of comparisons being performed using a Bonferonni
correction. In the case
of pathology scores, the comparisons were effectively doubled, by analyzing
the mean and the
maximum values.
Results:
Fifty-nine of the sixty mice survived the duration of the study and were
included in the
analysis of body weight loss, terminal colon lengths, and colon content.
Decreased body weight
gain was seen in all groups with the most severe effects occurring on Day 9
and recovery of gain
beginning on Day 10. A dose-dependent trend in inhibition of DSS-induced body
weight loss was
observed, corresponding to 19, 35 and 67% reduction in weight loss for groups
receiving IP
injections of 0.33, 1.67 or 3.33 mg/kg (UV) CG53135-05, respectively. Gross
pathology evaluation
indicated that colon length decreases and colon content score increases were
greatest in the
vehicle-treated disease control group. Dose-responsive increases in colon
length were seen in all
groups treated with CG53135-05 IP, with the greatest effect (29% inhibition,
statistically significant)
occurring in mice administered 3.33 mg/kg (UV) CG53135-05. Colon scores were
also significantly
improved (21-29%) in mice treated with 1.67 or 3.33 mg/kg (UV) CG53135-05.
Disease vehicle-treated control mice all had lesions of colitis with minimal
to severe
inflammation and gland loss. Erosion and gland hyperplasia were evident in 14
of 15 mice with
these changes being greatest in the distal colon. Edema was sporadically seen,
primarily in the
distal colon. Distal colon mucosal thickness was increased approximately 130%
in diseased
controls as compared to normal controls, as a result of inflammation and
hyperplasia. In contrast,
dose-responsive inhibition of proximal, distal, and total colon inflammation
and gland loss as well as
summed total colon histopathologic scores was seen. Furthermore, mice that
received 3.33 mg/kg
(UV) CG53135-05 showed significant improvements in on (35%) and total (34%)
inflammation, distal
(38%) and total (37%) gland loss as well as distal (38%) and total (37%)
summed histopathologic
scores. Protective effects (46-62% inhibition, non-significant) of CG53135-05
were also seen on
proximal, distal and total colon erosion in the mid and high dose groups.
Beneficial effects (dose
responsive, non-significant) on inhibition of mucosal thickness changes and
hyperplasia scores
occurred with 39% inhibition of the total mucosal thickness in mice treated
with 3.33 mg/kg (UV) and
31 % improvement in the hyperplasia score.
Conclusions:
In summary, treatment with CG53135-05 IP, once daily at doses of 1.67 or 3.33
mg/kg (UV)
resulted in mild to moderate, dose-responsive, inhibitory effects on gross and
histopathologic
129
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
parameters in the 15-day chronic model of DSS-induced colitis, when treatment
was initiated after
disease was established. Colon length, mean total gland loss, distal colon
mean histology summed
score, max distal and total inflammation, and max distal and total gland loss
showed a significant
linear dose response and were significantly different between animals in the
vehicle-treated control
group and the group treated with 3.33 mg/kg (UV) CG53135-05.
In another experiment, administration of 3.33 mg/kg (UV) of CG53135-05 IP,
once every
other day on Days 6, 8, 10, 12, and 14 (q2d) significantly reduced the
severity of chronic DSS-
induced ulcerative colitis in female Swiss Webster mice (N-405 study). These
results confirm the
findings presented above.
6.43. EXAMPLE 43: TREATMENT OF STROKE
Thirty male Sprague Dawley rats were allocated to treatment groups as
indicated in the
study design Table 51 below.
Table 51: Experimental Design
F__7 Number of Dose * Volume *
Animals
Treatment Males (pg) (uL)
Vehicle 1 10 0 50
CG53135-05 E. 10 1 50
coli purified
product
CG53135-05 E. 10 2.5 50
coli purified
product
*Administered dose and volume is based on an average bodyweight of 330 g.
Experimental Procedures
Middle cerebral artery (MCA) Surgery and Intracisternal Injections: Animals
were handled
for 7 days prior to surgery. Cefazolin sodium (40 mg/kg, i.p) was administered
on the day before
surgery and just after surgery. At the time of surgery, the rats were
anesthetized with 2% halothane
in a 2:1 N20 :02 mixture. Body temperature was maintained at 37 0.50 C. The
proximal right
MCA was electrocoagulated from just proximal to the olfactory tract to the
inferior cerebral vein and
was then transected. For intracisternal injections, animals were re-
anesthetized as above and
placed in a stereotaxic frame. Rats were given CG53135-05 E. coli purified
product or vehicle
(40mM acetate, 200 mM mannitol (pH 5.3)) by percutaneous injection into the
cisterna magna, once
at 1 day, (approximately 24 hours) and once at 3 days, (approximately 72
hours) after MCA.
Animals were given test article (2 dose groups) or vehicle treatment according
to the study design.
Clinical Observations/Signs
130
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Animals were observed immediately over a 1 hour period following injections
for signs of
seizure (indicated by tremor and violent motion about the cage), pain
(indicated by loud
vocalization), and lethargy. Animals were also observed daily for mortality
and moribundity.
Body Weight: Animals were weighed on Days 1, 3, 7, 14 and 21.
Limb Placing Test: limb placing tests were carried out on all animals on Day -
1 (pre-
operation), Day 1 Qust prior to injection), Day 3 and then every 7 days
thereafter (Days 7, 14, 21).
Forelimb Placing TestAssessment Score: The forelimb placing test measures
sensorimotor
function in each forelimb as the animal places the limb on a table top in
response to visual, tactile,
and proprioceptive stimuli. The forelimb placing test consists of the
following evaluations and
scoring, where the combined total score for the forelimb placing test reflects
a range from 0 (no
impairment) to 10 (maximal impairment):
visual placing (forward, sideways): 0 - 4
tactile placing (dorsal, lateral): 0 - 4
proprioceptive placing: 0 - 2
Total score for all forelimb tests: 0 - 10
Hindlimb Placing TestAssessment Score: Similarly, the hindlimb placing test
measures
sensorimotor function of the hindlimb as the animal places it on a tabletop in
response to tactile and
proprioceptive stimuli. The hindlimb placing test consists of the following
evaluations and scoring,
where the combined total score for the hindlimb placing test reflects a range
from 0 (no impairment)
to 6 (maximal impairment):
tactile placing (dorsal, lateral): 0 - 4
proprioceptive placing: 0 - 2
Total score for all hindlimb tests: 0 - 6
Body Swing Test the body swing test was carried out on all animals on Day -1
(pre-
operation), Day I Qust prior to injection), Day 3 and then every 7 days
thereafter (Days 7, 14, 21).
The body swing test examines side preference as the animal is held
approximately one inch
above the surface of the table, and swings to the right or the left side.
Thirty swings are counted,
and the score is then calculated based on the percentage of swings to the
right. (score range =
-50% right swing (no impairment) - 0% right swing (maximal impairment))
Cylinder Test: the cylinder test was carried out on all animals on Day -1 (pre-
operation)
and 7 days thereafter (Days 7, 14, 21). The cylinder test measures spontaneous
motor activity of
the forelimbs. Animals are placed in a narrow glass cylinder (16.5 x 25 cm)
and videotaped for 5
min on the day before stroke surgery and at weekly intervals thereafter.
Videotapes are then scored
independently by one experienced observer and up to 50 spontaneous movements
will be counted
min per rat per day). Spontaneous movements include those made by each
forelimb to initiate
131
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
rearing, to land on or to move laterally along the wall of the cylinder, or to
land on the floor after
rearing.
Macroscopic and Histomorphology. on the day of scheduled termination (Day 3),
animals
were euthanized by an intraperitoneal injection of Chloral hydrate (500mg/Kg).
Brains were
examined grossly and removed, postfixed in formalin, dehydrated and embedded
in paraffin.
Coronal sections (5 mm) will be cut on a microtome mounted on to glass slides,
and stained with
hematoxylin/eosin (H&E). The area of cerebral infarcts on each of seven slices
(+4.7, +2.7, +0.7, -
1.3, -3.3, -5.3, and -7.3 mm compared with Bregma) was determined using a
computer interface
imaging system using the indirect method (area of the intact contralateral
hemisphere - area of the
intact ipsilateral hemisphere) to correct for brain shrinkage during
processing. Infarct volume was
then expressed as a percentage of the intact contralateral hemispheric volume.
Volumes of the
infarction in the cortex and striatum were also determined separately using
these same methods.
H&E stained section was examined for histological changes such as hemorrhage,
abscess or tumor
formation.
Statistical Analysis: all intracisternal injections, behavioral testing, and
subsequent
histological analyses were done by investigators blinded to the treatment
assignment of each
animal. Data are then expressed as means +/- SEM, and will be analyzed by one
or two way
(ANOVA) followed by appropriate pairwise post hoc tests with correction for
multiple comparisons.
Results
Forelimb Placing Test: on days-1, 1, 3, 7, 14, and 21 relative to MCA
occlusion, animals
were examined by using a limb placing test to assess sensorimotor function in
the forelimb in
response to visual, tactile and proprioceptive stimuli (Kawamata, T.,
Dietrich, W. D., Schallert, T.,
Gotts, E., Cocke, R. R., Benowitz, L. I. & Finklestein, S. P. (1997) Proc.
Natl. Acad. Sci. USA 94,
8179-8184; De Ryck, M., Van Reempts, J., Duytschaever, H., Van Deuren, B. &
Clincke, G.
(1992) Brain Res. 573, 44-60.) Visual placing (scored 0-4), tactile placing
(scored 0-4), and
proprioceptive placing (scored 0-2) were summed to generate a range of
potential total scores from
0 to 12, with 12 representing maximal impairment (FIG. 68 (A)).
Hindlimb Placing: on days -1, 1, 3, 7, 14, and 21 relative to MCA occlusion,
animals were
examined by using a limb placing test to assess sensorimotor function in the
hindlimb in response to
tactile and proprioceptive stimuli (Kawamata, T., Dietrich, W. D., Schallert,
T., Gotts, E., Cocke, R.
R., Benowitz, L. I. & Finklestein, S. P. (1997) Proc. Natl. Acad. Sci. USA 94,
8179-8184; De Ryck,
M., Van Reempts, J., Duytschaever, H., Van Deuren, B. & Clincke, G. (1992)
Brain Res. 573, 44-
60). Tactile placing (scored 0-4), and proprioceptive placing (scored 0-2)
were summed to generate
a range of potential total scores from 0 to 6, with 6 representing maximal
impairment (FIG. 68(B)).
Body Swing Test. On days -1, 1, 3, 7, 14, and 21 relative to MCA occlusion,
animals were
examined by using a body swing test to assess side preference as the animal is
held approximately
one inch above the surface of the table, and swings to the right or the left
side. (Kawamata, T.,
132
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Dietrich, W. D., Schallert, T., Gotts, E., Cocke, R. R., Benowitz, L. I. &
Finklestein, S. P. (1997) Proc.
Natl. Acad. Sci. USA 94, 8179-8184; De Ryck, M., Van Reempts, J.,
Duytschaever, H., Van
Deuren, B. & Clincke, G. (1992) Brain Res. 573, 44-60.) Thirty swings were
counted, and the
score calculated based on the percentage of swings to the right (FIG. 68(C)).
Cylinder Tesfi. On days -1, 1, 3, 7, 14, and 21 relative to MCA occlusion,
animals were
examined by cylinder test to assess spontaneous motor activity of the
forelimbs (Kawamata, T.,
Dietrich, W. D., Schallert, T., Gotts, E., Cocke, R. R., Benowitz, L. I. &
Finklestein, S. P. (1997) Proc.
Natl. Acad. Sci. USA 94, 8179-8184; De Ryck, M., Van Reempts, J.,
Duytschaever, H., Van
Deuren, B. & Clincke, G. (1992) Brain Res. 573, 44-60.) Briefly, animals are
placed in a narrow
glass cylinder (16.5 x 25 cm) and videotaped for 5 min on the day before
stroke surgery and at
weekly intervals thereafter. Videotapes are then scored independently by one
experienced observer
and up to 50 spontaneous movements will be counted (- 5 min per rat per day).
Spontaneous
movements include those made by each forelimb to initiate rearing, to land on
or to move laterally
along the wall of the cylinder, or to land on the floor after rearing (FIG. 68
(D)).
Body Weight: animals were weighed on days -1, 1, 3, 7, 14, and 21 relative to
MCA
occlusion and the results indicate no significant difference between the
vehicle and CG53135-05
treatment (FIG. 68(E)).
Conclusion
Administering CG53135 following MCA occlusion suggested that both the low and
high
doses produced a significant enhancement of recovery on forelimb (FIG. 68(A))
and hindlimb
placing tests (FIG. 68(B)) for the contralateral (affected) limbs, and
improvement on the body swing
test (FIG. 68(C)). This pattern of activity with other therapeutics in this
model has generally been
shown to reflect improvement in cerebrocortical and subcortical (striatal)
function, respectively
(Dijkhuizen RM, Ren J, Mandeville JB, Wu 0, Ozdag FM, Moskowitz MA, Rosen BR,
Finklestein SP.
2001, Proc Nati Acad Sci U S A 98(22):12766-71). No apparent differences were
seen on the
cylinder test (FIG. 68(D)) of spontaneous limb use or on animal body weight
(FIG. 68(E)).
Therefore, CG53135 administration will be useful in the treatment of
pathological conditions
including ischemic stroke, hemorrhagic stroke, trauma, spinal cord damage,
heavy metal or toxin
poisoning and neurodegenerative diseases (such as Alzheimer's, Parkinson's
Disease, Amyotrophic
Lateral Sclerosis, Huntington's Disease).
6.44. EXAMPLE 44: MATRIX METALLOPROTEINASE PRODUCTION ASSAY
The matrix metalloproteinases (MMPs) are a family of related enzymes that
degrade the
extracellular matrix in bone and cartilage. These enzymes operate during
normal development in
tissues differentiation and remodeling. In arthritic diseases, such as
Osteoarthritis (OA) and
Rheumatoid Arthritis (RA), elevated expression of these enzymes contributes to
irreversible matrix
degradation. Thus, effect of CG53135 on MMP production was assayed.
133
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
The activity of CG53135 on matrix metalloproteinase (MMP) production was
assessed using
the SW1353 chondrosarcoma cell line (ATCC HTB-94). This cell line is a well-
established
chondrocytic cellular model for matrix metalloproteinases (MMP) production.
SW1353 cells were
plated in a 24-well plate at I x105 cells/mI (1 ml) in DMEM medium-10 % FBS.
Following overnight
incubation, the medium was replaced with DMEM + 0.2 % Lactabulmin serum.
CG53135-05 E. coli
purified product was added to the wells at doses ranging from 10 to 5000
ng/ml, in the absence or
presence of IL-1 beta (0.1 to I ng/ml, R&D systems Minneapolis, MN), TNF-alpha
(10 ng/ml, R&D
systems) or vehicle control to a final volume of 0.5 ml. IL-1 beta and TNF-
alpha are both potent
stimulators of MMP activity. All treatments were done in triplicate wells.
After 24 hours, the
supernatants were collected and Pro- MMP-1, and -13, as well as TIMP-1 (tissue
inhibitor of matrix
metalloproteinase), a natural inhibitor of MMP activity, was measured by ELISA
(R&D systems). The
measurements were normalized to the number of cells by an MTS assay.
Results
CG53135 significantly decreased MMP-13 production in the presence of either IL-
1 beta or
TNF-alpha as demonstrated in FIG. 69(A) and FIG. 69(B), respectively. IL-1
beta and TNF-alpha
are both potent stimulators of MMP activity. MMP-13 affinity for type II
collagen, the main collagen
that is degraded in OA, is ten times higher that of MMP-1. Since MMP-1 3
expression increases in
OA and RA, the decrease of MMP-1 3 observed with addition of CG53135 indicates
that the protein
can be used as an OA and RA therapeutic. Furthermore, CG53135 up-regulated the
production of
TIMP-1, a natural inhibitor of MMP activity (FIG. 69(C)). This enhancement of
TIMP-1 production by
CG53135 is beneficial in reducing the matrix breakdown by MMP-1 and -13
observed in OA and
RA. In addition, CG53135 had no effect on MMP-3 production constitutively or
after IL-1 induction.
Similarly, CG53135-05 E. coli purified product showed increase in basal
expression of MMP-1 in
SW1353 cells.
6.45. EXAMPLE 45: EFFECT OF CG53135 ON NORMAL RATS: PROOF OF PRINCIPLE
TO THE MENISCAL TEAR MODEL
The effect of CG53135 on the normal rats was studied as a proof of principle
to drive further
studies in disease model (ex: meniscal tear model of osteoarthritis in rats).
The effect of CG53135
on synovium and cartilage was assessed by injecting the protein into normal
male Lewis rats.
Effects of Intra-articular iniection of CG53135-05 E. coil Purified Product in
Normal Rats
The rats were injected intra-articularly three times per week for 2 weeks with
vehicle solution
(8mM acetate, 40 mM arginine, and 0.6% glycerol (pH 5.3) in approximately 1%
hyaluronic acid), 10
pg CG 53135-05 E. coli purified product or 100 pg CG 53135-05 E. coli purified
product.
Study Design: Male Lewis rats weighing 293-325 grams on day 0 were obtained
from
Harlan Sprague Dawley (Indianapolis, Indiana) and acclimated for 8 days. The
rats were divided
into three treatment groups with three animals in each group: two groups
received CG53135 and
one received only the vehicle control. The rats were anesthetized with
isoflurane and injected
through the patellar tendon into the area of the cruciate attachments of both
knees. CG53135 was
134
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
injected at doses of 0.1 mg/ml (0.01 mg/joint) or 1.0 mg/ml (0.1 mg/joint).
Controls were injected
with the vehicle solution as described above. Injections were done Monday,
Wednesday and Friday
for 2 weeks. The animals were terminated on day 15 at which time they were
injected ip with BRDU
(100 mg/kg) in order to pulse label proliferating cells.
Observations and Analysis of Markers of Pathology
Gross observations: Rats were observed daily for abnormal swelling or gait
alterations and
were weighed weekly.
Histopathology: Preserved and decalcified (5% formic acid) knees were trimmed
into 2
approximately equal longitudinal (ankles) or frontal (knees) halves, processed
through graded
alcohols and a clearing agent, infiltrated and embedded in paraffin,
sectioned, and stained with
toluidine blue (knees). Multiple sections (3 levels) of right knee were
analyzed microscopically with
attention to the parameters of interest listed below. Each parameter was
graded as normal, minimal,
mild, moderate, marked or severe. Evaluation of the cartilage was done using
descriptive
parameters rather than the scoring criteria generally used in the
osteoarthritis model because of the
type of alterations generated by the repetitive injection of the protein.
Although animals were
injected with BRDU prior to termination, the proliferative changes were
readily observed in toluidine
blue stained sections.
Results
Table 52: Microscopically Monitored Parameters
Synovial Alterations Cartilage Central Cruciate Chondrogenesis
Alterations Attachment Area
Alterations
-hyperplasia -cartilage -inflammation and -marginal zone or
-infiltration of synovium with proteoglycan fibroplasia periosteal
macrophages loss -bone or cartilage chondrogenesis
-fibroplasia -cartilage damage
-matrix (proteoglycan fibrillation
deposition in fibrotic
synovium)
Live Phase Parameters Body weights were similar in vehicle and protein
injected animals
throughout the study (Table 52). Knees injected with 100 pg of protein had
some evidence of
fibrosis clinically during the injection process beginning with the 3rd
injection.
Morphologic Pathology: Vehicle injected rats had minimal to mild synovial
hyperplasia,
inflammation and fibroplasia with none to minimal matrix deposition in
fibrotic synovium. Articular
cartilage had no proteoglycan loss or fibrillation. The central area of the
joint where the cruciates
attach and in which the intra-articular injections are made had none to
minimal fibroplasia and
cartilage/bone damage. No marginal zone chondrogenesis was present.
135
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Knees injected with 10 pg CG 53135-05 E. coli purified product had mild to
moderate
synovial hyperplasia, inflammation and fibroplasia with minimal to moderate
matrix deposition in
fibrotic synovium. Articular cartilage had no proteoglycan loss or
fibrillation. The central area of the
joint where the cruciates attach and in which the intra-articular injections
are made had none to
minimal fibroplasia and cartilage/bone damage. One knee had minimal marginal
zone
chondrogenesis.
Knees injected with 100 pg CG 53135-05 E. coli purified product had moderate
to marked
synovial hyperplasia, inflammation and fibroplasia with moderate matrix
deposition in fibrotic
synovium. Articular cartilage had none to minimal proteoglycan loss or
fibrillation. The central area
of the joint where the cruciates attach and in which the intra-articular
injections are made had
minimal to marked fibroplasia and cartilage/bone damage. All knees had mild to
moderate marginal
zone chondrogenesis. One animal had chondrogenesis in areas associated with
articular cartilage.
Conclusion
These results demonstrate that repetitive intra-articular injection of CG53135
induces
synovial fibroplasia and chondrogenesis. Vehicle injections resulted in mild
inflammation and
fibroplasia thus suggesting that this vehicle has some irritant potential.
Concentration responsive
increases in synovial proliferative response as well as marginal zone
chondrogenesis occurred in
animals injected with protein. The area of the cruciate attachment where
injections occurred had
areas of bone resorption and fibroplasia which also increased in severity with
increasing
concentrations of the protein as did the synovial inflammation. The
potentially adverse effects of
observed synovial fibroplasia and bone resorption could have been due to
either FGF-20 activity or
endotoxin levels within the non-clinical grade hyaluronic acid used to
formulate the protein. In
addition, inflammation in the joint can induce bone resorption and marginal
zone chondrogenesis so
these results need to be interpreted in light of the possibility that the
inflammatory response to the
protein injection contributed to the proliferative response. The morphologic
appearance of the
proliferative changes and chondrogenesis clearly indicates that the biological
activity of this protein
(CG53135) is important in generating the response.
The results of the experiments reported herein indicate repetitive intra-
articular injection of
CG53135 induces synovial fibroplasia and chondrogenesis.
6.46. EXAMPLE 46: INTRA-ARTICULAR INJECTION OF CG53135-051N MENISCAL
TEAR MODEL OF RAT OSTEOARTHRITIS: PROPHYLACTIC AND THERAPEUTIC
DOSING
Example 45 utilized CG53135 administration into the joints of normal rats to
identify effects on
relevant cell populations by histomorphometric analysis. At the dose of 100
ug/joint, CG53135 induced
significant marginal zone chondrogenesis similar to that seen with other
growth factors such as TGF-
beta, suggesting an effect on pluripotent stem cells within the marginal zone.
There was no apparent
effect on mature chondrocytes as evidenced by the lack of a response in the
mature cartilage areas of
the joints. The potentially adverse effects of observed synovial fibroplasia
and bone resorption could
136
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
have been due to either FGF-20 activity or endotoxin levels within the non-
clinical grade hyaluronic
acid used to formulate the protein.
Further studies in osteoarthritic animals performed addressed the following:
(1) synergy with
an anti-inflammatory drug (standard approach for osteoarthritis patients); (2)
whether CG53135 can
induce functional repair or protection of joint cartilage layers; and (3)
whether synovial fibroplasia and
bone resorption were CG53135-induced or due to contaminating endotoxin within
the formulation.
Thus one aspect of this study was to evaluate the protective and therapeutic
effects of intra-
articular injection of CG53135 on joint damage in osteoarthritis in the
meniscal tear model of rat
osteoarthritis. This relatively new model of OA has been shown to have
morphologic alterations of
cartilage degeneration and osteophyte formation that resemble changes
occurring in spontaneous
disease and surgically induced disease in other species (Bendele, A.M., Animal
Models of
Osteoarthritis. J. Musculoskel. Neuron Interact. 2001; 1:363-376, Bendele, A.
M. and Hulman, J. F.
Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988;
31:561-565). The model
can be used to evaluate potential beneficial effects of anti-degenerative as
well as regenerative
therapies.
Experimental Design
Animals (10/group), housed 2/cage, were anesthetized with isoflurane and the
right knee area
is prepared for surgery. A skin incision was made over the medial aspect of
the knee and the medial
collateral ligament was exposed by blunt dissection, and then transected. The
medial meniscus was
then reflected medially with a fine scissor and a cut was made through the
full thickness to simulate a
complete tear. The skin was closed with suture.
Prophylactic Dosing: intra-articular dosing (CG53135-05 E. coli purified
product) of the right
knee joint was initiated on the day of surgery and is continued for 2 weeks
post-surgery with intra-
articular injections given Thursday, Saturday, and Monday (day 0, 2, 4, 7, 9,
and 11) with rats under
Isoflurane anesthesia. Indomethacin, a nonsteroidal anti-inflammatory drug,
was dosed (1 mg/kg/day)
daily by the oral route starting on the day of surgery to reduce any potential
inflammation due to the
injection. Body weights were recorded on days 0, 7 and 14. After animal
termination on day 14 post-
surgery, both knees were collected for histopathologic evaluation. The study
design is shown in Table
53.
Table 53. Prophylactic Dosing Study Design
Group CG53135-05 Co-therap~ Number of Animals
Treatmenta Treatment Males
Vehicle Vehicle 10
I intra-articular)
2 Vehicle T___Ifndomethacin 10
(intra-articular)
137
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
3 CG53135-05 E. coli Vehicle 10
purified product
(intra-articular)
4 CG53135-05 E. coli Indomethacin 10
purified product
(intra-articu lar)
None None 10
aAdministration 3 times per week for 2 weeks (100 pg/joint, intra-articular)
bAdministration daily for 2 weeks (0.5 mg/kg, PO)
Therapeutic Dosing: intra-articular dosing (CG53135-05 E. coli purified
product) of the right
knee joint is initiated on day 21 of post-surgery and is continued for 2 weeks
with intra-articular
injections given Friday, Sunday, and Tuesday (day 22, 25, 27, 29, 32, and 34)
with rats under
isoflurane anesthesia. Indomethacin is dosed daily by the oral route starting
on the day of surgery.
Body weights are recorded on days 0, 7, 14, 21, 28, and 35. On day 35, both
knees are collected for
histopathologic evaluation. The study design is shown in Table 54.
Table 54. Therapeutic Dosing Study Design
CG53135-05 E. coli b Number of Animals
Group purified product Co-therapy Treatment Males
Treatmenta
1 Vehicle Vehicle 10
(intra-articular)
2 Vehicle Indomethacin 10
(intra-articular)
3 CG53135-05 E. coli Vehicle 10
purified product
(intra-articular)
4 CG53135-05 E. coli Indomethacin 10
purified product
(intra-articular)
5 None None 10
aAdministration 3 times per week for 2 weeks (100 pg/joint, intra-articular)
bAdministration daily for 2 weeks (0.5 mg/kg, PO)
Results of prophylactic dosing study. Observations made include the standards
followed for
this model. Multiple sections (3 levels) of right knee were analyzed
microscopically and scored
according to the following methods. In scoring the 3 sections, the worst case
scenario for the 2
halves on each of the 3 slides representing 3 levels was determined for
cartilage degeneration and
osteophyte formation. This value for each parameter for each slide was then
averaged to determine
overall subjective cartilage degeneration scores for tibia and femur and
osteophyte scores for tibia.
Cartilage degeneration was scored none to severe (numerical values 0-5) for
depth and
area (surface divided into thirds) using the following criteria:
0=no degeneration
138
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
1=minimal degeneration, chondrocyte and proteoglycan loss with or without
fibrillation involving the
superficial zone
2= mild degeneration, chondrocyte and proteoglycan loss with or without
fibrillation involving the
upper 1/3
3=moderate degeneration, chondrocyte and proteoglycan loss with fibrillation
extending well into the
midzone and generally affecting 1/2 of the total cartilage thickness
4=marked degeneration, chondrocyte and proteoglycan loss with fibrillation
extending well into the
deep zone but without complete (to the tidemark) loss of matrix
5=severe degeneration, matrix loss to tidemark
Strict attention to zones (outside, middle, inside thirds) was adhered to in
this scoring
method and the summed scores reflect a global summation of severity of tibial
degeneration.
In addition to this overall subjective analysis of cartilage degeneration, an
additional
subjective assessment was done using similar criteria to evaluate severity of
degeneration but with
attention to specific regional differences across the tibial plateau. In this
OA model, generally the
outside 1/3 of the tibia is most severely affected by the meniscal tear injury
with lesions often
extending to the tidemark by 3 weeks post-surgery. The middle 1/3 is usually a
transition zone
where severe or marked change becomes moderate or mild and the inner 1/3
seldom has changes
greater than mild or minimal. In an attempt to determine potential differences
of treatment on the
severe lesion of the outside 1/3 vs. the milder lesions of the middle 1/3 and
inside 1/3, these regions
were each scored separately. The sum of the regional values was calculated and
expressed as sum
of 3 zones.
In addition to the above subjective scoring, a micrometer measurement of total
extent of
tibial plateau affected by any severity of degeneration (Total Tibial
Cartilage Degeneration Width
pm) extended from the origination of the osteophyte or marginal zone if no
osteophyte was present
with adjacent cartilage degeneration (outside 1/3) across the surface to the
point where tangential
layer and underlying cartilage appeared histologically normal.
An additional measurement (Significant Cartilage Degeneration Width pm)
reflected areas of
tibial cartilage degeneration in which chondrocyte and matrix loss extended
through greater than
50% of the cartilage thickness.
Finally, a micrometer depth of any type of lesion (cell/proteoglycan loss,
change in
metachromasia, but may have good retention of collagenous matrix and no
fibrillation) expressed as
a ratio of depth of changed area vs. depth to tidemark was included and taken
over 4 equally
spaced points on the tibial surface. These measurements were taken (1 st)
matrix adjacent to
osteophyte (2nd) 1/4 of the distance across the tibial plateau (3rd) 1/2 of
the distance across the
tibial plateau (4th) 3/4 of the distance across the tibial plateau. This
measurement was the most
critical analysis of any type of microscopic change present. The depth to
tidemark measurement
139
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
(denominator) also gives an indication of cartilage thickness across the
tibial plateau and therefore
allows comparisons across groups when trying to determine if hypertrophy or
hyperplasia has
occurred.
A single tibial growth plate measurement was taken for each section in an area
thought to
best represent the overall width in the non tangential plane of the section.
Scoring of the osteophytes and categorization into small, medium and large was
done with
an ocular micrometer.
None = 0 no measurable proliferative response at marginal zone
Small osteophytes =1 (up to 299 pm)
Medium osteophytes=2 (300-399 pm)
Large osteophytes=3 (>400pm)
The score (0-3) was included in the overall joint score. In addition, the mean
SE for the
actual osteophyte measurement (average for 3 sections) was also determined.
Generally, in doing the surgery, attempts were made to transect the collateral
ligament at a
location that results in the meniscus being reflected proximally toward the
femur. The cut was then
made by inserting the scissors tip toward the femur rather than the tibia.
Some mechanical damage
may then be detected in the femoral condylar cartilage but is rarely
encountered on the tibia, thus
making the tibia the most appropriate site for assessment of
chondroprotection.
Focal small areas of proteoglycan and cell loss that were likely a result of
physical trauma to
the femoral cartilage were described but not included in the score with larger
more diffuse areas
receiving subjective scores according to methods described for the tibia.
These larger areas were
more consistent with non traumatic degeneration. Because of the possibility of
iatrogenic lesions on
the femur, overall joint scores were expressed both with and without femoral
cartilage degeneration
scores.
Damage to the calcified cartilage layer and subchondral bone was scored using
the
following criteria:
O=No changes
1=lncreased basophilia at tidemark: no fragmentation of tidemark or marrow
changes
2=lncreased basophilia at tidemark: minimal to mild fragmentation of calcified
cartilage of tidemark,
mesenchymal change in marrow involves 1/4 of total area but generally is
restricted to subchondral
region under lesion
3=lncreased basophilia at tidemark: Mild to marked fragmentation of calcified
cartilage,
Mesenchymal change in marrow is up to 3/4 of total area, Areas of marrow
chondrogenesis may be
evident but no collapse of articular cartilage into epiphyseal bone
140
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
4=lncreased basophilia at tidemark: Marked to severe fragmentation of
calcified cartilage, Marrow
mesenchymal change involves up to 3/4 of area and articular cartilage has
collapsed into the
epiphysis to a depth of 250pm or less from tidemark
5=lncreased basophilia at tidemark: Marked to severe fragmentation of
calcified cartilage, Marrow
mesenchymal change involves up to 3/4 of area and Articular cartilage has
collapsed into the
epiphysis to a depth of greater than 250pm from tidemark
Descriptive comments were made on degree of synovial inflammation, synovial
fibrosis,
marginal zone chondrogenesis, bone resorption, fibrous overgrowth with or
without
chondrogenesis/incorporation into existing cartilage
Statistical Analysis: statistical analysis of histopathologic parameters was
done by
comparing group means using the Student's two-tailed t-test with significance
set at p:50.05.
Because of the nature of the data, a non Parametric ANOVA (Kruskal-Wallis
test) was used to
analyze the scored parameters and a parametric ANOVA was used to analyze the
measurements.
The appropriate post test used was Dunnett's multiple comparisons test on the
parametric data and
a Dunn's test was used on the non parametric data. Significance was set at
p:50.05 for all
parameters.
Results: intra-articular injection of 100 pg CG53135-05 E. coli purified
product with or
without concurrent indomethacin administration resulted in significant
inhibition (39%) of tibial
cartilage degeneration on the middle 1/3 (40-43% for zone 1) and an overall
insignificant inhibition of
the summed 3 zones of 41% (FIG. 70(A)). Total cartilage degeneration width was
significantly
decreased 35-37% (FIG. 70(B)) and significant degeneration was reduced 70-89%
with this
inhibition being significant only in the group treated with protein and
indomethacin (FIG. 70(C)).
Results of the prophylactic dosing study: the data described indicate that
intra-articular
injection of 100 pg of CG53135-05 E. coli purified product in knee joints of
rats with medial meniscal
tear results in chondroprotective effects as a result of both inhibition of
cartilage degeneration and
stimulation of cartilage repair. Some joints had layering of proliferated new
cartilage over existing
normal appearing or damaged cartilage. This observation is particularly
exciting as it demonstrates
the potential for resurfacing to occur.
These beneficial effects were always associated with diffuse synovial
fibroplasia, bone
resorption and increased synovial inflammation. Concurrent indomethacin
treatment (1 mg/kg/day)
had minimal if any effect on the disease process in knees injected with
Synvisc alone or the disease
process and reaction to the protein in knees injected with Synvisc containing
protein. The single
exception to this statement is reflected in the data for osteophyte
measurements where all groups
had similar measurements except the group treated with protein and vehicle po.
This group had
greater measurements thus suggesting greater marginal zone stimulation, not an
uncommon
occurrence in inflamed joints.
141
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
The morphologic changes induced by injection of 100 pg of this protein
demonstrate the
potential for CG53135 to be effective in cartilage repair processes. It has
the capacity to induce
fibrous tissue proliferation with differentiation to cartilage and
importantly, integration of that newly
proliferated tissue. The proliferative processes are somewhat disorganized and
counter productive
in areas such as the marginal zone and subchondral bone. However, rodents
definitely have much
greater propensity to exhibit marginal zone, periosteal and marrow
proliferation from a variety of
stimuli including inflammatory mediators so some of the excessive and counter
productive
responses seen in rats might not occur in dogs or primates. Also, there may
have been some
induction of an antibody response thus leading to enhanced knee inflammation
that would not occur
in humans or other animals that did not have an antibody response.
Additional studies that are useful in delineating the potential efficacy of
CG53135 in
osteoarthritis include: (1) evaluation in an animal model, e.g., a rabbit or a
dog model of OA-this
would allow evaluation in a larger joint with cartilage and bone structure
that is more similar to
humans and species that has less tendency to exhibit hyperproliferative
responses such as those
that occur in rodents; and (2) evaluation of ia injections for 3-4 weeks,
possibly with more aggressive
anti-inflammatory systemic therapy followed by a recovery period to see how
the new tissue
remodels would be interesting. It may be that allowing the joint to remodel
with no further
proliferative stimulus would result in a more pleasing morphologic endpoint.
Alternatively, the time
point of which the immune response that would clear may result in a endpoint.
Cycles of treatment
with periods of remodeling might be the way to achieve the most satisfactory
repair. Studies such as
these would also answer the question of whether the repair tissue will hold up
long term. Generally
fibrocartilage has less of a tendency to do this.
Results: Intra-articular injection of 100 pg CG531 35-05 E. coli purified
product with or
without concurrent oral indomethacin administration did not result in
significant inhibition of tibial
cartilage degeneration scores (FIG. 71(A)). Total or significant cartilage
degeneration width was not
decreased (FIGS. 71(B) and (C)).
Results of the therapeutic dosing study: The data described demonstrated the
potential
chondroproliferative activities of CG53135 administered intra-articularly.
However, protein injected
joints had markedly increased inflammation, fibroplasia and connective tissue
resorptive process. -
The most important difference between the prophylactic and therapeutic dosing
studies was
the nature of the OA lesion at the time of initiation of dosing. Rats in the
therapeutic dosing study
had an area of severe matrix loss in the outer to middle 1/3 of the cartilage
thus exposing the
calcified cartilage/subchondral bone to the protein. Effective repair thus
required filling of this defect
with newly proliferated tissue coming from the marginal zone or exposed marrow
pleuripotential
cells. In the prophylactic dosing study, beneficial effects required
inhibition of matrix degradation and
stimulation of repair on a degenerating scaffold with repair tissue
originating from the marginal zone
only. Since the filling of a defect would be much more difficult than
repairing a damaged scaffold, it
142
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
may be that a longer duration of treatment would be required in a therapeutic
model in order to see
beneficial effects.
Indomethacin treatment was not effective in reducing the inflammatory changes
and it had
no beneficial effects on inhibiting the resorptive processes occurring in
bone. In order to achieve
effective proliferation and differentiation to cartilage in the absence of
inflammation and tissue
destruction, following modification to the therapeutic dosing study can be
attempted: Increasing the
dosing interval to once or twice weekly and/or increasing the study duration
to allow time for the
proliferative tissue to fill the large cartilage defects induced by this
disease process. Another
possibility is to investigate the effects of CG53135 in a larger species such
as the dog as dogs have
less of a tendency to proliferate connective tissue and resorb bone in
response to various stimuli
than rodents.
The results detailed herein (both prophylactic and therapeutic dosing studies)
indicate that
CG53135 has specific utility in severely osteoarthritic joints that are
destined for joint replacement.
These types of agents would be injected into joints that have little or no
normal cartilage remaining
and are in need of resurfacing. In this situation, repair could originate from
pleuripotential cells in the
marginal zones or bone marrow. Repair originating from these locations will
likely result in
production of fibrocartilage rather than hyaline cartilage. However, some
cartilage would be
preferable to no cartilage and it may be that an injectable method of
sustaining a cartilage surface
would be acceptable even though treatments would likely have to be repeated
over time to sustain
the repair. Treatments with injectable anabolic agents will likely require
some kind of cyclical
process in conjunction with continuous passive motion rather than sustained
active load bearing
motion.
6.47. EXAMPLE 47: CG53135 RESCUES NEURONAL PC12 CELLS FROM SERUM-
STARVATION INDUCED CELL DEATH
To assess the trophic (neuroprotective) qualities of CG53135 and compare to
the action of
Nerve Growth Factor (NGF) and Epidermal Growth factor (EGF), the following
experiment was
performed.
Materials and Method:
Materials: PC12 cells, tissue culture plates and medium (DMEM +/- 10% FBS),
NGF, EGF,
CG53135-05 E. coli purified product (by Process 1).
Method: Plate PC12 cells at low density on poly-lysine coated tissue culture
plates in DMEM
+ 10% FBS. Culture 24 hours. Administer serum-free media containing NGF or
CG53135 at a
range of doses or no growth factor supplements. Photograph at 72 hours to
visualize cell survival
and proliferation.
Results:
CG53135 prevented cell death in a dose-dependent fashion. The maximal trophic
activity
was achieved at 50 ng/ml. The potency of CG531345 was approximately 20% of the
potency of
143
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
NGF, however the maximal extent of trophic action by both growth factors was
equivalent. EGF also
exhibited trophic activity. Cell death (apoptosis) was measured by LDH assay
and visually. (FIG.
72)
Conclusion:
CG53135 acts similarly to the neurotrophin NGF and to the growth factor EGF to
the extent
that CG53135 is capable of rescuing PC12 cells from serum starvation-induced
cell death. Thus,
CG53135 possesses trophic activity. Trophic activity is recognized to have
value in the treatment of
numerous disorders of the central nervous system. In particular, the ability
to protect neuronal cells
is important to diseases where neurodegeneration is involved, such as
Alzheimer's disease,
Parkinson's disease and diseases with catastrophic cell death such as stroke
and traumatic brain
injury.
6.48. EXAMPLE 48: CG53135 INHIBITS SERUM-WITHDRAWAL INDUCED CASPASE
ACTIVATION IN NEURONAL PC12 CELLS
This experiment was performed to assess the ability of CG53135 to inhibit the
activation of
pro-apoptotic caspace enzymes, and compare to the action of Nerve Growth
Factor (NGF).
Materials and Method:
Materials: PC12 cells, tissue culture plates and medium (DMEM +/- 10% FBS),
NGF,
CG53135-05 E. coli purified product (by Process 1).
Method: Plate PC12 cells at medium density on poly-lysine coated tissue
culture plates in
DMEM + 10% FBS. Culture 24 hrs. Administer serum-free media containing NGF
(100 ng/ml) or
CG53135 (1000 ng/mi) or no growth factor supplements. Collect cell lysates at
various time points
(0, 3, 6 and 20 hrs.). Evaluate caspase2, 3, 8, 9 activation by ELISA.
Results:
Serum withdrawal induced caspase activity time-dependently. Both CG53135 and
NGF
blocked caspase induction. (FIG. 73)
Conclusion:
CG53135 acts similarly to the neurotrophin NGF to the extent that both
proteins are able to
prevent the activation of apoptosis promoting caspase enzymes upon apoptotic
stimuli. Caspases
have been implicated in a number of diseases of the CNS involving neuronal
death, including
Alzheimer's disease, Parkinson's disease, stroke and traumatic brain injury.
Therefore, CG53135
has value in the treatment of these diseases.
6.49. EXAMPLE 49: CG53135 INDUCES NEURITE OUTGROWTH BY NEURONAL PC12
CELLS
This experiment was performed to assess the neuritogenic qualities of CG53135-
05 and
compare to the action of Nerve Growth Factor (NGF)
Materials and Method:
144
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Materials: PC12 cells, tissue culture plates and medium (DMEM +/- 10% FBS),
NGF,
CG53135-05 E. coli purified product (by Process 1).
Method: Plate PC12 cells at low density on poly-lysine coated tissue culture
plates in DMEM
+ 10% FBS. Culture 24 hrs. Administer serum-free media containing NGF or
CG53135 at 100 ng/ml
or no growth factor supplements. Photograph at 72 hrs to visualize neurite
outgrowth.
Results:
CG53135 induced neurite outgrowth in a dose-dependent fashion. The maximal
extent of
neurite outgrowth was achieved at 1000 ng/ml. The maximal extent of neurite
outgrowth induced by
both growth factors was equivalent. EGF did not induce neurite outgrowth.
(FIG. 74)
Conclusion:
CG53135 acts similarly to the neurotrophin NGF to the extent that CG53135 is
capable of
inducing similar neurite outgrowth. The capability of NGF to induce neurite
outgrowth is an
important feature of this growth factor that distinguishes it from other
factors such as EGF which do
not possess such neurotrophic activity. Thus, CG53135 possesses neurotrophic
activity.
Neurotrophic activity is recognized to have value in the treatment of numerous
disorders of the
central nervous system. In particular, the ability to induce neurite outgrowth
is important to diseases
where neurodegeneration is involved, such as Alzheimer's disease, Parkinson's
disease and
diseases with pathological structural changes or neural architecture are
involved such as stroke and
traumatic brain injury.
6.50. EXAMPLE 50: CG53135 ACTIVATES MAP KINASE IN NEURONAL PC12 CELLS
This experiment was performed to assess the MAPK activating action of CG53135
and
compare to the action of Nerve Growth Factor (NGF), Epidermal Growth Factor
(EGF) and Basic
FGF (bFGF).
Materials and Method:
Materials: PC12 cells, tissue culture plates and medium (DMEM +/- 10% FBS),
NGF, EGF,
bFGF, CG53135-05 E. coli purified product (by Process 1), EGF, MAPKK inhibitor
PD98059
Method: Plate PC12 cells at medium density on poly-lysine coated tissue
culture plates in
DMEM + 10% FBS. Culture 24 hours. Administer serum-free media containing NGF
(100 ng/ml),
EGF (100 ng/ml) or CG53135 (100 ng/ml) or no growth factor supplements. Pre-
treat separate
cultures with PD98059 before treating with CG53135 or NGF. Lyse cells 10 min
post treatment,
perform western blot with anti-phospho MAPK antibody to assess MAPK
activation. Also, evaluate
time course of MAPK activation by CG53135 and bFGF in human cortical neuronal
cell line HCNIA
at 0, 10 min, 1 hour and 3 hours.
Results:
CG53135 induced robust MAPK activation in a MAPKK-dependent manner. CG53135
exhibits gradual and sustained MAPK activation timecourse, superior to bFGF.
(FIG. 75)
145
CA 02586213 2007-05-02
WO 2006/055264 PCT/US2005/039833
Conclusion:
CG53135 acts similarly to the neurotrophin NGF in the induction of MAPK, a key
intracellular signaling molecule involved in cell survival and neuronal
differentiation. The activation of
this trophic pathway, which also is involved in processes underlying learning
and memory, is
recognized to have value in the treatment of numerous disorders of the central
nervous system. In
particular, the ability to protect neuronal cells is important to diseases
where neurodegeneration is
involved, such as Alzheimer's disease, Parkinson's disease and diseases with
catastrophic cell
death such as stroke and traumatic brain injury. The ability to stimulate
intracellular pathways
involved in learning and memory also is likely to have relevance to disorders
involving memory
dysfunction, such as Alzheimer's and age-related memory loss.
7. EQUIVALENTS
Those skilled in the art will recognize, or be able to ascertain using no more
than routine
experimentation, many equivalents to the specific embodiments of the invention
described herein.
146
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 146
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 146
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: