Note: Descriptions are shown in the official language in which they were submitted.
CA 02587127 2007-05-10
-1-
METHOD FOR INHIBITING CYCLOOXYGENASE AND INFLAMMATION USING
CHERRY BIOFLAVONOIDS
This application is a divisional application of co-pending application Serial
No., 2,354,042 filed December 10, 1999.
The present invention relates to a method of use of at least one compound
obtained from cherries as cyclooxygenase (COX-1 and COX-2) inhibitors. In
particular, the present invention provides a natural cherry composition
containing a
mixture of anthocyanins, bioflavonoids and phenolics for use as anti-
inflammatory
agents as a result of inhibition of the cyclooxygenase enzymes.
Many plant-derived compounds may impart important positive pharmacological
or "nutraceutical/phytoceutical" traits to foods by way of their abilities to
serve as
antioxidants by maintaining low levels of reactive oxygen intermediates, as
anti-
inflammatory agents by inhibiting prostagiandin synthesis, or as inhibitors of
enzymes
involved in cell proliferation. These activities may be important in
ameliorating chronic
diseases including cancer, arthritis, and cardiovascular disease (Kinsella et
al., Food
Tech. 85-89 (1993)). Thus, with natural products, the dietary supplement/food
industry and nutraceutical/phytoceutical companies have the opportunity to
employ
compounds that can not only enhance food stability as effectively as synthetic
antioxidants, but can also offer significant health benefits to the consumer.
Cherries are thought to have beneficial health properties in general.
Consumption of cherries was reported to alleviate arthritic pain and gout
(Hamel, P.B.,
et al. Cherokee Plants 28: Herald: Raleigh, N.C. (1975)) although there is no
evidence
for its active components or mode of action. These beneficial effects may be
partially
associated with the abundance of anthocyanins, the glycosides of cyanidin.
Prunus Cerasus L. (Rosacease), cv. MONTMORENCY is the major tart cherry
commercially grown in the United States. In order to challenge the MONTMORENCY
monoculture, a new cultivar, BALATON tart cherry (Ujferbertoi furtos), was
introduced into the United States in 1984, and has been tested in Michigan,
Utah, and
Wisconsin. BALATON produces fruits darker than MONTMORENCY does.
Colorants like anthocyanins have been regarded as the index of quality in tart
cherries. Most importantly, recent results showed that anthocyanins such as
cyanidin-3-glucoside have strong antioxidant activities (Tsuda, T., et al, J.
Agric. Food
Chem. 42:2407-2410 (1994)).
CA 02587127 2007-05-10
-2-
Early studies have showed that MONTMORENCY cherry contains the
anthocyanins cyanidin-3-gentiobioside and cyanidin-3-rutinoside (Li, K. C., et
al., J.
Am. Chem. Soc. 78:979-980 (1956)). Cyanidin-3-glucosylrutinoside was also
found in
six out of the seven sour cherry varieties (Harborne, J. B. , et al.,
Phytochemistry
3:453-463 (1964)). Dekazos (Dekazos, E.D., J. Food Sci. 35:237-241 (1970))
reported
anthocyanin pigments in MONTMORENCY cherry as peonidin-3-rutinoside, peonidin
and cyanidin along with cyanidin-3-sophoroside, cyanidin-3-rutinoside and
cyanidin-3-glucoside. However, cyanidin-3-glucosylrutinoside as well as
cyanidin-3-glucoside, cyanidin-3-sophoroside and cyanidin-3-rutinoside were
identified
as main pigments in sour cherries. Using HPLC retention values, Chandra et al.
(Chandra, A., et al., J. Agric. Food Chem. 40:967-969 (1992)) reported that
cyanidin-3-sophoroside and cyanidin-3-glucoside were the major and minor
anthocyanins, respectively, in Michigan grown MONTMORENCY cherry. Similarly,
cyanidin-3-xylosylrutinoside was detected as a minor pigment in MONTMORENCY
cherry (Shrikhande, A. J. and F. J. Francis, J. Food Sci. 38:649-651 (1973)).
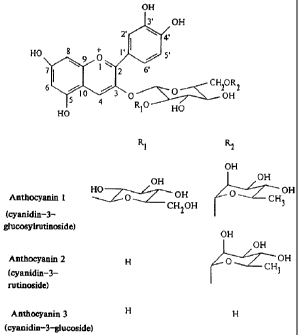
In the prior art, production of pure anthocyanins (compounds 1-3 of Figure 1)
from BALATON and MONTMORENCY cherry juices was carried out first by adsorbing
the pigment on an AMBERLITE XAD-2 (Sigma Chemicals) column (Chandra, A., et
al., J. Agric. Food Chem. 41:1062-1065 (1993)). The column was washed with
water
until the eluant gave a pH of approximately 7Ø The adsorbed pigments along
with
other phenolics were eluted with MeOH. The resulting crude anthocyanins were
fractionated and purified by C-18 MPLC and HPLC, respectively, to afford pure
anthocyanins for spectral studies. Purification of 500 mg crude MONTMORENCY
anthocyanins from AMBERLITE XAD-2 yielded 60 mg of pure anthocyanins 1-3
compared to 391.43 mg from BALATON. This research indicated that crude
anthocyanins from MONTMORENCY obtained from the XAD-2 contained a high
percentage of other organic compounds. There was no attempt to use the crude
mixture of phenolics and anthocyanins for any purpose. U.S. Patent Nos,
5,266,685 to
Garbutt, 5,665,783 to Katzakian et al. and 5,817,354 to Mozaffar describe
various
adsorbent resins and their use for isolating unrelated products. These patents
are only
illustrative of the general state of the art in the use of adsorbent resins.
Cyclooxygenase (COX) or prostaglandin endoperoxide-H synthase (PGHS-1,
PGHS-2 or COX-1/COX-2) enzymes are widely used to measure the anti-
CA 02587127 2007-05-10
-3-
inflammatory effects of plant products (Bayer, T., et al., Phytochemistry 28:
2373-2378
(1989); and Goda, Y., et al. , Chem. Pharm. Bull. 40: 2452-2457 (1992) ). Cox
enzyme is the pharmacological target site for the nonsteroidal anti-
inflammatory drug
discovery (Humes, J., et al., Proc. Natl. Acad. Sci. U.S.A. 78: 2053-2056
(1981); and
Rome, L. H., et al., Proc. Natl. Acad. Sci. U.S.A. 72: 4863-4865 (1975)). Two
isozymes of cyclooxygenase involved in prostaglandin synthesis are
cyclooxygenase-1. (COX-1) and cyclooxygenase-2 (COX-2), respectively (Hemler,
M.,
et al., J. Biol. Chem. 25: 251, 5575-5579 (1976)). It is hypothesized that
selective
COX-2 inhibitors are mainly responsible for anti-inflammatory activity
(Masferrer, J. L.,
et al., Proc. Nati. Acad. Sci. U.S.A. 91: 3228-3232(1994)). Flavonoids are now
being investigated as anti-inflammatory substances as well as their structural
features for cyclooxygenase (COX) activity. The 5,7-dihydroxyflavone, galangin
with
an IC50 of 5.5 M, was found to be the most active cyclooxygenase inhibitory
flavonoid
(Wurm, G., et al., Deutche Apotheker Zeitung 122: 2062-2068 (1982)).
Flavonoids
with an ortho-dihydroxy in ring A or B were stronger inhibitors than those
with a free
3-OH group Murm, G., et al., Deutche Apotheker Zeitung 122: 2062-2068 (1982);
and
Baumann, J.,et al., Prostaglandins 20: 627-640 (1980)). The C2-C3 double bond,
which determines the coplanarity of the hetero rings appears to be a major
determinant of COX activity (Wurm, G., et al., Deutche Apotheker Zeitung 122:
2062-2068 (1982)). Certain prenylated flavonoids, such as morusin, were also
active,
because of their higher lipophilicity (Kimura, Y., et al., Chem. Pharm. Bull.
34: 1223
-1227 (1986)). Also, unsubstituted flavone is a good COX inhibitor (Mower, R.
L., et
al., Biochem. Pharmacol. 33: 357-364 (1984); and Welton, A. F., et al., Prog.
Clin.
Biol. Res. 213: 231-242 (1986)). Most of the flavanones studied in the past
did not
show significant COX inhibition, except for the flavanone-3-ol, silibinin
(Kalkbrenner,
F., et al., Pharmacology 44: 1-12 (1992)). However, the structure-activity
relationships
of isoflavonoids are not reported.
There is a need for natural product derived compositions for use as
cyclooxygenase inhibitors and as anti-inflammatory agents.
CA 02587127 2007-05-10
-4-
SUMMARY OF THE INVENTION
The present invention relates to a method for inhibiting cyclooxygenase or
prostaglandin H synthase enzymes that comprises providing at least one
compound
obtainable from a cherry with at least one of the enzymes to inhibit the
enzymes.
Further, the present invention relates to a method for inhibiting
cyclooxygenase
or prostaglandin H synthase enzymes that comprises providing at least one
bioflavonoid compound obtainable from a cherry with at least one of the
enzymes to
inhibit the enzymes.
Further, the present invention relates to a method for inhibiting inflammation
in
a mammal that comprises administering at least one compound obtainable from a
cherry to the mammal to inhibit inflammation.
Further, the present invention relates to a method for inhibiting inflammation
in
a mammal that comprises administering at least one bioflavonoid, anthocyanin
or
phenolic compound obtained from a cherry to the mammal to inhibit the
inflammation.
The present invention relates to a method for inhibiting inflammation in a
mammal that comprises administering cyanidin to the mammal to inhibit
inflammation.
Also contemplated is a food supplement having anti-inflammatory properties
wherein the food supplement comprises a cherry extract having an anti-
inflammatory
activity greater than the anti-inflammatory activity found in the natural
cherry fruit.
Also provided is a method of inhibiting COX-2 activity in a cell comprising
contacting
the cell with a cherry fruit extract having an anti-inflammatory activity
greater than the
anti-inflammatory activity found in the natural fruit. Certain aspects of the
invention
contemplate the cell being a mammalian cell. Other embodiments contemplate
that
the cell is a human cell. Particular embodiments contact the cell with the
cheny fruit
extract in vitro. Others contemplate contacting the cell in vivo. In preferred
aspects,
these methods use a food or food supplement that is provided in the form of a
capsule, a tablet, a syrup, a beverage, a powder or the like.
Another aspect of the invention provides a method of treating an inflammatory
response in an animal comprising administering to the animal a composition
comprising a cherry fruit extract having an anti-inflammatory activity greater
than the
anti-inflammatory activity found in the natural cherry fruit and a
pharmaceutically
acceptable diluent or excipient.
CA 02587127 2007-05-10
-4a-
The invention provides use of an orally administrable combination
comprising a) at least one bioflavonoid selected from the group consisting of
quercetin, kaempferol, kaempferol 3-rutinoside, 3'-methoxy kaempferol 3-
rutinoside, and 5,8,4'-trihydroxyl-6,7-dimethoxyflavone and; b) at least one
anthocyanin selected from the group consisting of cyanidin-3-glucoside,
cyanidin-
3-glycosylrutinoside, and cyanidin-3-rutinoside in sufficient amounts to
inhibit the
inflammation by inhibiting COX1 and COX2 for inhibiting inflammation in a
mammal.
The invention also provides use of at least one compound anthocyanin
selected from the group consisting of cyanidin-3-glucosylrutinoside, cyanidin-
3-
rutinoside and cyanidin-3-glucoside isolated from the fruit of a cherry for
inhibiting
a cyclooxygenase or a prostagiandin H synthase enzyme.
The invention further provides use of an effective amount of at least one
anthocyanin obtained from the fruit of a cherry, said anthocyanin selected
from the
group consisting of cyanidin-3-glucosylrutinoside, cyanidin-3-rutinoside, and
cyanidin-3-glucoside and mixtures thereof for inhibiting inflammation in a
mammal.
CA 02587127 2007-05-10
-5-
In specific embodiments, the inflammatory response may be selected from the
group consisting of arthritis, pain, an allergic rash, inflammatory bowel
syndrome, and
asthma. Of course, these are exemplary inflammatory diseases and it is
contemplated that the present inventions may provide an excellent herbal
remedy for
any disorder resulting from an inflammatory response. It should be understood
that
the food or food suppiements described herein will be useful when being taken
alone
or in combination with other anti-inflammatory remedies.
In those embodiments where the food supplements are taken in combination
with other remedies, the present method contemplates further administering to
the
animal an anti-inflammatory agent selected from the group consisting of
salicylates,
glucocorticoids, para-aminophenol derivatives, opiods, indomethacin, sulindac,
fenamates, propionic acid derivatives and oxicams. The present supplements may
be
provided in any of the solid or liquid dosage forms, including, but not
limited to, hard or
soft gelatin capsules, other capsules, tablets, solutions, suspensions,
syrups,
beverages or a powders.
Yet another embodiment of the present invention describes a nutraceutical
composition comprising an extract of cherry, wherein the nutraceutical
provides relief
from pain when ingested or otherwise applied to an organism suffering from
pain.
Specifically, the pain may be the pain of arthritis, menstrual cramps,
headaches,
insect bites or an allergic rash.
The term "anthocyanins" includes the color producing compounds contained in
cherries. For the purpose of this application this includes the aglycone
cyanidin.
The term "bioflavonoids" means the isoflavonoid and flavonoid compounds
contained in cherries.
The term "phenolics" refers to compounds with a phenyl group and having one
or more hydroxyl groups.
The compounds isolated from cherries are most useful with living material. The
living material can be in an animal or human. It can also be in tissue
culture.
It is therefore an object of the present invention to provide a cherry
compound
that can be used as cyclooxygenase inhibitors and anti-inflammatory agents.
Further,
it is an object of the present invention to provide a method for isolating the
cherry
compound on a commercial scale. Finally, it is an object of the present
invention to
provide a natural source compound that is economical to prepare and easy to
use.
CA 02587127 2007-05-10
-6-
Other objects, features and advantages of the present invention will become
apparent from the following detailed description. It should be understood,
however,
that the detailed description and the specific examples, while indicating
preferred
embodiments of the invention, are given by way of illustration only, since
various
changes and modifications within the spirit and scope of the invention will
become
apparent to those skilled in the art from the following description and the
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows the structure of select anthocyanins (colorants) that have been
isolated from BALATON and MONTMORENCY cherries. The aglycon cyanidin has a
hydroxyl group at position 3.
Figures 2 and 3 are drawings showing the major bioflavonoids isolated from the
cherries.
Figure 4 shows select phenolics isolated from tart cherries.
Figure 5 shows the steps in a method for isolating anthocyanins,
bioflavonoids,
and phenolics from cherries.
Figure 6 is a schematic drawing showing equipment that may be used in the
method shown in Figure 5.
Figure 7 is a dose-response curve for the inhibition of the human PGHS-1
enzyme by cyanidin. The anti-inflammatory activity of cyanidin was estimated
by its
ability to inhibit the cyclooxygenase activity of the PGHS-1 enzyme. Cyanidin
gave an
IC50 value of 90 pM for PGHS-1 enzyme, while the NSAIDs aspirin, naproxen, and
ibuprofen gave 1C50 values of 1050, 11, and 25 M, respectively.
Figure 8 is a dose-response curve for the inhibition of PGHS-1 and PGHS-2
enzymes by cyanidin. Cyanidin gave 1C50 values of 90 and 60 M for PGHS-1 and
PGHS-2 enzymes, respectively.
Figure 9 is a graph showing the inhibitory effect of PGHS-1 (COX-1) by
flavonoids and isoflavonoids at 200 pm concentrations. Data is expressed as
mean
S.E. of triplicate. Kaempferol 3-rutinoside, 3'-methoxy kaempferol 3-
rutinoside,
5,8,4'-trihydroxy-6,7-dimethoxyflavone and quercetin were not active at 1000
pM
concentrations.
Figure 10 is a graph showing dose response curves for the inhibition of the
PGHS-1 enzyme (COX-1) by flavonoids from BALATON tart cherries compared to the
CA 02587127 2007-05-10
-7-
non-steroidal anti-inflammatory drugs, naproxen, aspirin, and ibuprofen. The
IC50 of
kaempferol, quercetin, luteolin, aspirin, naproxen and ibuprofen are 180, 350,
300,
1050, 11 and 25 M, respectively. Data is expressed as mean S.E. of
triplicate.
Figure 11 are graphs showing dose response curve for the inhibition of the
PGHS-1 enzyme (COX-1) by isoflavonoids from BALATON tart cherries compared to
the non-steroidal anti-inflammatory drugs, naproxen, aspirin and ibuprofen.
The IC5o
of daidzein, biochanin A, genistein, aspirin, naproxen and ibuprofen are 400,
350, 80,
1050, 11 and 25 M, respectively. Data is expressed as mean S.E. of
triplicate.
DESCRIPTION OF PREFERRED EMBODIMENTS
The isolates obtained from a cherry are preferably prepared as a mixture of
anthocyanins, bioflavonoids and phenolics. The isolates may be obtained by a
method for producing a mixture comprising anthocyanins, bioflavonoids and
phenolics
from cherries as a composition that comprises:
(a) providing an aqueous solution containing the anthocyanins,
bioflavonoids and phenolics from the cherries;
(b) removing the anthocyanins, bioflavonoids and phenolics onto a resin
surface from the aqueous solution;
(c) eluting the resin surface with an eluant to remove the anthocyanins,
bioflavonoids and phenolics from the resin surface; and
(d) separating the eluant from the anthocyanins, bioflavonoids and
phenolics.
The cherries used to produce the isolates can be obtained from the genus
Prunus and can be sweet, sour (Prunus avium, Prunus cerasus), and mixtures
thereof. Tart cherries contain high levels of malic acid in addition to other
organic
acids which contributes to the sour taste of tart cherries. The method
isolates malic
acid and other organic acids containing sugars that can be used in foods to
provide
tartness and flavor. Most preferred are the BALATON and MONTMORENCY
cherries. This invention also contemplates the use of anthocyanins,
bioflavonoids and
phenolics from new varieties of cherries, especialiy those that are cultivated
to
maximize their anthocyanin content.
The isolated mixture of anthocyanins, bioflavonoids and phenolics can be
incorporated into any solid or liquid dosage form and used as a natural
nutraceutical,
CA 02587127 2007-05-10
-8-
phytoceutical or dietary supplement. In general, the selected form may provide
a total
daily dose of the anthocyanins, bioflavonoids, and phenolics of about 1 to
1000 mg. -
In one embodiment, a daily dose between 1 to 200 mg is provided. In other
embodiments, the daily dose may be between 1 to 100 mg, 1 to 50 mg, 5 to 50
mg, 5
to 25 mg, and 10 to 25 mg. In a preferred embodiment, at least 1 mg is
provided as
the daily dose, and more preferably, at least 5 mg is provided as the total
daily dose.
As a comparison, one hundred (100) cherries provide about 10 to 100 mg of
anthocyanins and bioflavonoids, depending on the type of cherry consumed.
Similarly, one hundred cherries provide 1-50 mg of phenolics.
The total daily dose may be comprised of a mixture of anthocyanins,
bioflavonoids, and phenoiics. Alternatively, the total daily dose may be
provided by
any one of these three types of compounds or mixtures of any or all of these
compounds. For example, in other embodiments, it may be desirable to provide
between 0.01 to 200 mg of the anthocyanins, and more preferably, between about
0.01 to 100 mg, 0.01 to 50 mg, 1 to 50 mg, 5 to 50 mg, and 5 to 25 mg of one
of more
anthocyanins that have been obtained from cherries. Similarly, the
bioflavonoids and
phenolics individually may be used in same dosage ranges as provided for the
anthocyanins directly above. The amount of the anthocyanins, bioflavonoids and
phenolics can be adjusted by isolating the individual compounds and blending
them
together. In one embodiment, a natural mixture of the anthocyanins,
bioflavonoids
and phenolics is used.
The mixture of anthocyanins, bioflavonoids, and phenolics may be obtained
from an extraction method that uses adsorbent resin. The resin has a surface
to
which the anthocyanins, bioflavonoids and the phenolics are adsorbed. A
preferred
class of adsorptive resins are polymeric crosslinked resins composed of
styrene and
divinylbenzene such as, for example, the AMBERLITE series of resins, e.g.,
AMBERLITE XAD-4 and AMBERLITE XAD-16, which are available commercially from
Rohm & Haas Co., Philadelphia, PA. Other polymeric crosslinked styrene and
divinylbenzene adsorptive resins suitable for use according to the invention
are
XFS-4257, XFS-4022, XUS-40323 and XUS-40322 manufactured by The Dow
Chemical Company, Midland, Michigan, and the like.
It is preferred to use commercially available, govemmentally-approved (where
required), styrene-divinyl-benzene (SDVB) crosslinked copolymer resin, (e.g.,
*Trade-mark
CA 02587127 2007-05-10
-9-
AMBERLITE XAD-16). Thus, in the preferred embodiment, AMBERLITE XAD-16,
commercially available from Rohm and Haas Company, and described in U.S.
Patent
No. 4,297,220, is used as the resin. This resin is a non-ionic hydrophobic,
cross-linked polystyrene divinyl benzene adsorbent resin. AMBERLITE XAD-16 has
a
macroreticular structure, with both a continuous polymer phase and a
continuous pore
phase. In a particulariy preferred embodiment, the resin used in the present
invention
has a particle size ranging from 100-200 microns.
It is contemplated that other adsorbents such as those in the AMBERLITE XAD
adsorbent series, which contain hydrophobic macroreticular resin beads, with
particle
sizes in the range of 100-200 microns, will also be effective in the methods
of the
present invention. Moreover, different variations of the AMBERLITES, such as
the
AMERCHROM CG series of adsorbents, used with particle sizes in the range of
100-200 microns, may also be suitable for use in the present invention. The
AMBERLITE XAD-16 is preferred since it can be re-used many times (over 100
times). However, it is contemplated that for food, the use of govemmentally-
approved
resins in the present invention will be considered important and/or desirable.
Any solvent can be used to remove the adsorbed anthocyanins, bioflavonoids
and phenolics. Preferred are lower alkanols containing 1 to 4 carbon atoms and
most
preferred is ethanol (ethyl alcohol) since it is approved for food use.
Typically the
ethanol is azeotroped with water; however, absolute ethanol can be. used.
Water
containing malic acid and sugars in the cherries pass through the column.
These are
collected and can be used in foods as flavors.
The anthocyanins, bioflavonoids and phenolics are preferably obtained from
the BALATON and the MONTMORENCY cherries. The composition of the cherries is
in part shown in U.S. Patents 5,985,636 and 6,194,469.
As described in these applications, the
Montmorency (Prunus cerasus) variety constitutes more than 95% of tart cherry
cultivations in Michigan and USA. However, Balaton tart cherry (P. cerasus), a
new
tart cherry cultivar, is being planted to replace Montmorency in several
Michigan
orchards. This cherry has higher anthocyanin contents and is regarded as a
better
variety. Anthocyanin contents of Montmorency and Balaton tart cherries have
been
reported (Wang, et al., 1997; Chandra et al., 1993). However, a detailed
investigation
*Trade-mark
CA 02587127 2007-05-10
-10-
of other phenolic compounds in Balaton tart cherry was not carried out before.
Early
studies have shown that MONTMORENCY cherry contains cyanidin-3-gentiobioside
and cyanidin-3-rutinoside (Li, K.C., et al., J. Am. Chem. Soc. 78:979-980
(1956)).
Cyanidin-3-glucosylrutinoside was also found in six out of the seven sour
cherry
varieties (Harbone, J.B., et al., Phytochemistry 3:453-463 (1964)). Dekazos
(Dekazos, E.D., J. Food Sci. 35:237-241 (1970)) reported anthocyanin pigments
in
MONTMORENCY cherry as peonidin-3-rutinoside, peonidin and cyanidin along with
cyanidin-3-sophoroside, cyanidin-s-rutinoside and cyanidin-3-glucoside.
However,
cyanidin-3-glucosylrutinoside as well as cyanidin-3-gluocoside, cyanidin-3-
sophoroside and cyanidin-3-rutinoside were identified as main pigments in sour
cherries. Using HPLC retention values, Chandra et al (Chandra, A., et al., J.
Agric.
Food Chem 40:967-969 (1992)) reported that cyanidin-3-sophoroside and cyanidin-
3-
glucoside were the major and minor anthocyanins, respectively, in Michigan
grown
MONTMORENCY cherry. Similarly, cyanidin-3-xylosylrutinoside was detected as a
minor pigment in MONTMORENCY cherry (Shrikhande, A.J. and F.J. Francis, J.
Food
Sci. 38:649-651 (1973)).
The term "carrier" or "bulking agent" is used to mean a composition, which is
added to increase the volume of the composition of the purified composition
from the
cherry. In one embodiment, dried cherry pulp is used as the carrier. In other
embodiments, edible starch containing material or protein, such as non-fat dry
milk
are used as the carrier. Within this group are flour, sugar, soybean meal,
maltodextrin
and various condiments, such as salt, pepper, spices and herbs, for instance.
The
bulking agent is preferably used in an amount between about 10-6and 106 parts
by
weight of the mixture. The ratio of anthocyanins, bioflavonoids and phenolics
to the
carrier may be between 0.1 to 100 and 100 to 0.1.
The present invention in certain aspects describes the beneficial intake of a
food or food supplement having anti-inflammatory properties wherein the food
or food
supplement comprises a cherry extract having an anti-inflammatory activity
greater
than the anti-inflammatory activity found in the natural fruit. Those of skill
in the art
should understand that such food or food supplement may advantageously be
combined with other anti-inflammatory agents. Such additional anti-
inflammatory
agents will, of course, be secondary to the cherry extracts of the present
invention and
may be any commonly recognized anti-inflammatory agent. If other anti-
inflammatory
CA 02587127 2007-05-10
-11-
agents are used, the ratio of the cherry extracts to the anti-inflammatory
agent(s) may
be between 1:0 to 0:1.
The cherry extract and the additional anti-inflammatory agent may be contacted
with or exposed to a cell either in vivo or in vitro to inhibit the COX-
activity of the cell.
The terms "contacted" and "exposed," when applied to a cell, are used herein
to
describe the process by which a cherry extract and a second anti-inflammatory
agent
are delivered to a target cell or are placed in direct juxtaposition with the
target cell.
To achieve a beneficial effect, both agents may be delivered to a cell in a
combined
amount effective to inhibit COX activity, decrease inflammation, decrease the
production of the inflammation causing prostaglandins or other such effect
that will
decrease the inflammatory response in a cell or an individual subject in which
the cell
is located.
Anti-inflammatory agents are well known to those of skill in the art and
include
agents such as salicylic acid derivatives (e.g. aspirin) paraminophenol
derivative (e.g.
acetaminophen) indole and indene acetic acids (indomethacin, sulindac and
etodalac)
heteroaryl acetic acids (tolmetin diclofenac and ketorolac, aryl propionic
acid
derivatives (ibuprofen, naproxen, ketoprofen, keopren, fenopren, oxaprozine),
anthranilic acids (mefenamic acid, meclofenamic acid) enolic acids (piroxicam,
tenoxicam, phenylbutazone and oxyphenthatrazone). These and other anti-
inflammatory agents are well known to those of skill in the art and additional
description of these agents may be found in Goodman & Gilman's The
Pharmacological Basis of Therapeutics, Ninth Ed. (1996).
The present invention provides a natural food or food supplement made from
cherry extracts wherein the food or food supplement comprises an anti-
inflammatory
activity that is greater than the anti-inflammatory activity found in the
natural cherry.
The present invention provides a cherry extract that can be presented in a
powdered,
liquid, or solid form. Nonlimiting examples include desirable and readily
produced
forms.
The composition may be introduced into a food in an amount between about
0.1 and 10 mg/gm of the active ingredients of the food. The amount is
preferably
selected so as to not affect the taste of the food and to produce the most
beneficial
result. The food can be high (wet) or low moisture (dry) as is well known to
those
skilled in the art.
CA 02587127 2007-05-10
-12-
The cherry extract may be in a reconstitutable powder composition that, when
reconstituted with, for example, water, milk or some other similar liquid will
provide a
drink that may be used to provide an anti-inflammatory activity to a subject
in need
thereof. The powdered composition and drink prepared therefrom are especially
useful as an enterally administered component in a program of pain or
inflammation
management that utilizes a number of carefully designed products in various
forms,
i.e., in shake, soup, fruit drink, snack bar and other solid forms such as
tablets, gel
caps, and the like, which can be mixed and matched over a period of pain
management to provide more attractive and, therefore, more effective support
to a
patient, particulariy those in extended care situations.
In addition to drinks, the cherry extracts of the present invention may be
used in
foodstuffs. Such cherry extracts may be combined with any other foodstuff, for
example, oils containing the extracts of this invention may be used as cooking
oil,
frying oil, or salad oil and may be used in any oil-based food, such as
margarine,
mayonnaise or peanut butter. Grain flour fortified with the compounds of this
invention
may be used in foodstuffs, such as baked goods, cereals, pastas and soups.
Oils
containing the cherry extracts and novel anthocyanins, bioflavonoids, and
phenolics
extracted therefrom can be emulsified and used in a variety of water-based
foodstuffs,
such as drinks, including drink mixes as discussed above. Advantageously, such
foodstuffs may be included in low fat, low cholesterol or otherwise restricted
dietary
regimens.
In addition, other flavorings and additives well known to those of skill in
the art
also, may be added to the formulations to make them more palatable. For
example,
formulations may contain fruit flavoring, coloring, preservatives and the
like.
When formulated as a food supplement (also commonly referred to as dietary
supplements or nutraceuticals) the composition of the invention may
additionally
contain a solid carrier such as a gelatin or an adjuvant. The tablet, capsule,
and
powder may contain from about 0.1 to about 95% by weight of the anti-
inflammatory
composition of the present invention. In one embodiment, the anti-inflammatory
composition comprises about 0.01 to about 95 % by weight of the total weight
of the
supplement. More preferably, the anti-inflammatory composition comprises about
0.1
to about 50 %, about 1 to about 25%, or about 1 to about 10% by weight of the
total
weight of the supplement. When administered in liquid form, a liquid carrier
such as
CA 02587127 2007-05-10
-13-
water, petroleum, oils of animal or plant origin such as peanut oil, mineral
oil, soybean
oil, or sesame oil, or synthetic oils may be added. Similarly, the liquid form
of the
nutraceutical compositions contains from about 0.01 to about 95% by weight of
the
anti-inflammatory compositions of the present invention. More preferably, the
nutraceutical compositions contain from about 0.1 to about 50%, about 1 to
about
25%, or about 1 to about 10% by weight of the anti-inflammatory composition.
The
nutraceutical composition of the present invention may also contain
stabilizers,
preservatives, buffers, antioxidants, or other additives known to those of
skill in the art.
Methods have been developed for extraction and isolation of phytochemicals
(Chandra, A., et al., J. Agric. Food Chem. 41:1062 (1992); Wang, H., et al.,
J. Agric.
Food Chem. 45:2556-2560 (1997)) and for rapid screening of antioxidant
activity
(Arora, A. and G. M., Strasburg, J. Amer. Oil Chem. Soc. 74:1031-1040 (1997)).
These methods are being utilized to identify, characterize and test the
compounds
from BALATON and MONTMORENCY cherries. Juiced cherry tissue was
sequentially extracted with hexane, ethyl acetate and methanol. Both methanol
and
ethyl acetate fractions showed strong antioxidant activity in the screening
assay. The
ethyl acetate fraction was further purified by silica gel vacuum liquid
chromatography
to yield four subfractions; the subfraction was further separated into seven
fractions by
preparative reverse phase HPLC. Figures 2 and 3 show the bioflavonoids
isolated
from the BALATON cherries. There are thus numerous analogous or homologous
compounds in the tart cherries. The anthocyanin components obtained from the
juice
fraction also have been identified and fully characterized (Chandra, A., et
al., J. Agric.
Food Chem. 41:1062 (1992); Wang, H., et al., J. Agric. Food Chem. 45:2556-2560
(1997)).
Two novel phenolic compounds were identified:
I) 1-(3'-4'-dihydroxy cinnamoyl)-2,3-dihydroxy cyclopentane, and
II) 1- (3'-4' -dihydroxy cinnamoyl) -2, 5-dihydroxy cyclopentane.
Other compounds isolated from the ethyl acetate extract of cherry fruits and
characterized by spectral methods include: 1-(3'-methoxy, 4'-hydroxy
cinnamoyl)
quinic acid, 2-hydroxy-3-(2'-hydroxyphenyl) propanoic acid, methyl
2-hydroxy-3-(2'-hydroxyphenyl) propanoate, D(+)-malic acid, (i-sitosterol and
R-sitosterol glucoside. Figure 4 shows some of the phenolics, which were
isolated.
CA 02587127 2007-05-10
-14-
Examoles 1 and 2
As shown in Figure 5, individual quick frozen (IQF) cherries (which had been
pitted) were defrosted and blended in an industrial WARING blender. The
mixture
was centrifuged at 10,000 rpm and the juice was decanted. The residue, pulp,
was
further pressed with cheese cloth to remove any additional juice.
The pulp was lyophilized at 15 C. The juice was processed on AMBERLITE
XAD-16 HP resin to produce cherry sour, anthocyanins, bioflavonoids and
phenolics.
The XAD-16 resin, 1 kg, was washed with ethanol (1-2 L) and then washed with
water
(6 L). The XAD-1 6 resin was allowed to stand in water for 1 hour before
loading into a
glass column (10 ID x 90 cm long) with a cotton plug. The packed column was
washed with water (2 L) before loading the juice for separation. 800 mL juice
was
purified each time. The juice was added onto the surface of the column and
allowed
to settle with no flow. It was then eluted with water and the first 1 L was
discarded.
The next 2L of washing was collected, since it contained the cherry juice that
was sour
since it contained malic acid and sugars from the cherries. The column was
then
washed with an additional 4 L of water in the case of BALATON and 5 L for
MONTMORENCY cherry juice. Once the cherry juice was collected, the remainder
of
the washing with water were discarded. The column was then eluted with ethanol
(1.3-1.5 L) and collected the red solution containing anthocyanins,
bioflavonoids, and
phenolics (700-800 ml). The column was then run dry and washed with 10 L of
water
before repeating the process many of times (over 100).
The red alcoholic solution was then evaporated under a vacuum (20 milliTorr)
to remove ethanol and the aqueous solution, stabilized with 50 ppm ascorbic
acid, and
lyophilized at 10 C. The red powder was collected and stored.
CA 02587127 2007-05-10
-15-
Example 1 results:
BALATON cherry
Weight of IQF cherries 15.74 kg
Weight of dried pulp 605 g
Volume of juice 12.16L
Weight of anthocyanins, bioflavonoids
and phenolics (red powder) 31.35 g
Volume of sour byproduct
(malic acid and sugars) @ 35 L
Example 2 results:
MONTMORENCY cherry
Weight of IQF cherries 30.45 kg
Weight of dried pulp 895 g
Volume of juice 24.03 L
Weight of anthocyanins, bioflavonoids and
phenolics (red powder) 47 g
Volume of cherry by-product
(malic acid and sugars) @ 75 L
The red powders of Examples 1 and 2 were preferably mixed with dried pulp as a
carrier and tableted into 1 to 1000 mg tablets including the carrier (1 adult
daily dose).
Various food grade acids can be added to the isolated anthocyanins,
bioflavonoids and phenolics to prevent decomposition. Preferably, they do not
add
flavor. Ascorbic acid (vitamin C) is preferred. The acid can be added before
or after,
preferably before drying of the cherry compounds.
For small scale processing, lyophilization is used to remove water. For larger
scale production, drying in an air circulating oven is preferred.
Example 3
As shown in Figure 6, an open vessel 10 is provided with an inlet line 11 and
an outlet line 12, with valves 13 and 14, respectively. The resin beads 15 are
provided in the open vessel 10. Water is introduced into the vessel 10 and
then
removed through outlet line 12 and discarded. The cherry juice (without the
pulp or
pits) as in Example 1 is introduced tQ the vessel 10 and allowed to stand for
25
minutes. The temperature of the water and juice is between about 20 and 30
C.
The cherry juice residue containing malic acid and sugars is then removed
through the
CA 02587127 2007-05-10
-16-
outlet line 12 and retained as a food flavoring. The resin 15 in the vessel is
then
washed again with water from inlet line 11 and then removed and discarded
through
outlet line 12. The anthocyanins, bioflavonoids and phenolics on the resin
particles
are then extracted using 95% ethanol introduced through inlet line 11. The
ethanol
containing the anthocyanins, bioflavonoids and phenolics is removed from the
vessel
10. The ethanol is removed from the anthocyanins, bioflavonoids and phenolics
and
dried using flash drying under nitrogen. The resulting powder is preferably
then mixed
with dried cherry pulp or other carrier as in Example 1. The resin particles
are washed
with water and then the resins and ethanol are recycled many times.
Example 4
The anti-inflammatory assays on the anthocyanins and cyanidin were
conducted using prostagiandin endoperoxide H synthase-1 and -2 isozymes
(PGHS-1, and -2) and were based on their ability to convert arachidonic acid
to
prostaglandins (PGs). The positive controls used in this experiment were
aspirin,
naproxen, and ibuprofen. Aspirin gave an IC50 value of 1050 pM each against
PGHS-1 and PGHS-2 enzymes (Figure 7). Naproxen and ibuprofen gave IC50values
of 11 and 25 nM against PGHS-1 enzyme, respectively (Figure 7). A preliminary
experiment with the mixture containing anthocyanins 1-3 (Figure 1) showed PGHS-
1
and PGHS-2 activities at 33 ppm concentration. The aglycon cyanidin showed
good
PGHS-1 and -2 inhibitory activities, with IC50 vaiues of 90 and 60 nM,
respectively
(Figures 7 and 8). The ratio of IC50 values for PGHS-1 to PGHS-2 was about
0.56
(Figure 8). However, pure anthocyanins 1-3 showed little or no activity
against
PGHS-1 and PGHS-2 at 300 -nM test concentrations. Higher concentrations of
anthocyanins I and 2, on the contrary, increased the activity of enzyme. This
is
probably due to the ability of anthocyanins I and 2 to act as oxygen carriers
at high
concentration and enhance the oxygen uptake. It is noted that anthocyanins are
hydrolyzed in the gut of a mammal to cyanidin and other compounds and thus
effective in vivo.
For measurements of time-dependent inhibition of PGHS-2 enzyme activity by
cyanidin, the enzyme was preincubated at 370 C with 15 nM of cyanidin (one-
fourth of
the concentration of 1C50) and added to an oxygen electrode chamber with
arachidonic
acid substrate to initiate the reaction. The results suggest that the rate of
inhibition of
PGHS-2 did not change with time. The specific inhibition of the PGHS-2 enzyme
is a
CA 02587127 2007-05-10
-17-
major advance in anti-inflammatory therapy because it significantly reduces
the
adverse effects of nonsteroidal anti-inflammatory drugs (NSAIDs). It is
generally
believed that ulcerogenic and other adverse properties of NSAIDs result from
the
inhibition of PGHS-1, whereas the therapeutically desirable effects come from
the
inhibition of PGHS-2 enzyme.
Similarly, cyanidin showed better anti-inflammatory activity than aspirin in
the
inflammatory assays. The antioxidant and anti-inflammatory properties of
anthocyanins and cyanidin suggest that consumption of cherries may have the
potential to reduce cardiovascular or chronic diseases in humans.
In particular, arachidonic acid and a microsomal fraction of ram seminal
vesicles containing PGHS-1 enzyme suspended in 100 mM Tris pH 7.8 and 300 pM
diethyldithiocarbamic acid (DDC) as a preservative were purchased from oxford
Biomedical Research (Oxford, MI). Recombinant human PGHS-2 enzyme was
initially obtained from Dr. David Dewitt (Department of Biochemistry, Michigan
State
University, East Lansing, MI) and then purchased from Oxford Biomedical
Research
(Oxford, MI). Naproxen, ibuprofen, and hemoglobin were purchased from Sigma
Chemical Co. (St. Louis, MO). Anthocyanins 1-3 were purified from Balaton tart
cherry by HPLC and were identified by'H and13C NMR spectral data.
To prepare cyanidin, the anthocyanin mixture containing 1-3 (Figure 1; 500 mg)
was stirred with 3N HCI (20 mL) at 80 C for 10 hours. The reaction mixture
was
purified on a XAD-4 column as in the preparation of anthocyanins. The MeOH
solution of cyanidin was evaporated to dryness to yield a red amorphous powder
(190
mg) and stored at -30 C until use.
In the anti-inflammatory assay, cyclooxygenase activities were measured by
using PGHS-1 enzyme (ca. 5 mg protein/mL in 0.1 M TrisHCI, pH 7.8), a
homogeneous protein purified from ram seminal vesicles. Microsomal
preparations
from recombinant human prostaglandin synthase-2 (COX-2) were obtained from
insect cell lysate. Assays were performed at 37 C by monitoring the initial
rate of 02
uptake using an O2 efectrode (Yellow Springs Instrument Inc., Yellow Springs,
OH).
Each assay mixture contained 3 mL of 0.1 M Tris HCI, pH adjusted to 7 by the
addition of 6M HCI, 1 mM phenol, 85 pg hemoglobin, and 10 pM of arachidonic
acid.
Reactions were initiated by the addition of 5-25 pg of microsomal protein in a
volume
of 15-50 pL. Instantaneous inhibition of enzyme activity was determined by
CA 02587127 2007-05-10
-i8-
measuring the cyclooxygenase activity initiated by adding aliquots of
microsomal
suspensions of PGHS-1 or PGHS-2 (10 NM 02/min cyclooxygenase activity/aliquot)
to
assay mixtures containing 10 pM arachidonate and various concentrations of the
test
substances (10-1100 pM). The IC50 values represent the concentrations of the
test
compound that gave half-maximal activity under the standard assay conditions.
Example 5
This is an anti-inflammatory assay for cyclooxygenase inhibition activity of
flavonoids and isoflavonoids. Arachidonic acid and microsomal suspensions of
PGHS-1 (COX-1) and COX-2 (PGHS-2) were purchased from Oxford Biomedical
Research (Oxford, MI, USA). Genistein, genistin, naringenin, quercetin,
5,8,4'-trihydroxy-6,7-dimethoxyflavone, kaempferol-3-rutinoside and 3'-methoxy
kaempferol 3-rutinoside were purified from BALATON tart cherry by HPLC and
were
identified by'H- and13C NMR spectral data. Daidzein and formononetin were
purchased from Research Plus, Inc. (Bayonne, New Jersey, USA). Biochanin A,
kaempferol, quercetin, naproxen, ibuprofen and hemoglobin were purchased from
Sigma Chemical Co. (St. Louis, MO, USA). Luteolin was purchased from Adams
Chemical Co. (Round Lake, IL, USA).
For measuring the COX activity, flavonoids or isoflavonoids were dissolved in
DMSO to yield 40 mM stock solution and was further diluted to the desired
concentration according to the COX-1/COX-2 inhibitory activity of each
compound
assayed.
Anti-inflammatory assay: COX activities were measured using microsomal
suspensions of PGHS-1 and PGHS-2. Microsomal membranes (5 mg protein/mL in
0.1 M Tris HCI, pH 7.4) were prepared and assayed on the same day. COX-1 and
COX-2 assay was performed at 37 C controlled by a circulation bath (Model-
1166,
VWR Scientific Products, Chicago, IL) by monitoring the rate of 02 uptake
using a
5357 Oxygen electrode (INSTECH Laboratory, Plymouth Meeting, PA) (Meade, E.
A.,
et al., J. Biol. Chem. 268 6610-6614 (1993)).
Each assay mixture contained 600 pL of 0.1 M Tris-HCI, pH 8.0, 1 mM phenol,
17 pg hemoglobin and 10 pM arachidonate and were mixed in a microchamber
(INSTECH Laboratory, Plymouth Meeting, PA, USA). For anthocyanins and cyanidin
pH 7 is preferred to prevent decomposition in absence of additives. Reactions
were
initiated by adding 5 pg of microsomal protein (5 L). Instantaneous
inhibition was
CA 02587127 2007-05-10
19-
determined by measuring the cyclooxygenase activity initiated by adding
microsomal
suspensions of PGHS-1 or PGHS-2 in the assay mixtures containing 10 pM
arachidonate and various concentrations of test compounds. The IC50 values
represent the concentrations of inhibitor that gave half-maximal activity
under the
standard assay conditions. The kinetics of the enzyme activity was monitored
by
Biological Oxygen Monitor (YSI model 5300, Yellow Springs Instrument CO.,
Inc.,
Yellow Springs, Ohio) and collected in Quicklog Data Acquisition and Control
computer software (Strawberry Tree Inc., Sunnyvale, CA, USA).
The COX-1/COX-2 activity of BALATON cherry bioflavonoids was determined
by monitoring the 02 uptake. Reactions were initiated by adding PGHS enzyme
preparation. One unit of cyclooxygenase represents oxygenation of 1 nmol of
arachidonate/min under the standard assay condition by the COX enzyme. This
assay was a modification of the assay reported by DeWitt et al. (Dewitt D. L.,
et al., J.
Biol. Chem. 265: 5192-5198 (1990)). 10 pM arachidonate has been used for COX-1
assays, because this substrate concentration was reported to give near-maximal
COX
activity and also permit the detection of enzyme inhibition by lipophilic
inhibitors
(Meade, E. A., et al., Biol. Chem. 268: 6610-6614 (1993)). This methodology
can also
be used for COX-2 assay as well using COX-2 enzyme. Three known COX
inhibitors,
aspirin, ibuprofen and naproxen, were selected as positive controls. COX-1
inhibitory
activities of flavonoids, kaempferol, quercetin, luteolin, quercetin 3-
rhamnoside,
5,8,4'-trihydroxy-6,7-dimethoxyflavone were compared. Kaempferol 3-rutinoside,
3'-methoxy kaempferol 3-rutinoside and naringenin (Figure 9), and five
isoflavonoids,
genistein, genistin, daidzein, formononetin and biochanin A (Figure 8).
COX-11COX-2 inhibitory activities of each compound at different concentrations
was calculated by comparing the tangent of 02 uptake curves of test compounds
with
that of blank control. Each assay was repeated 3 times and the IC50 values (50
inhibitory concentrations) were calculated by linear regression analysis. The
half
-maximal inhibitory concentrations of flavonoids and isoflavonoids are shown
in Figure
11. Dose response curves for the inhibition of the COX-1 enzyme by flavonoids
and
isoflavonoids from BALATON tart cherries compared to the non-steroidal
anti-inflammatory drugs, aspirin, naproxen and ibuprofen are shown in Figures
10 and
11, respectively.
CA 02587127 2007-05-10
-20-
Among the flavonoids tested, kaempferol showed the highest COX-1 inhibition,
followed by luteolin, quercetin, naringenin and quercetin 3-rhamnoside (Figure
9). In
comparing kaempferol with quercetin, it was found that the presence of a
hydroxyl
group at C3 position decreased the COX-1 inhibitory activity (Figure 9). The
COX-1
inhibitory activity of kaempferol and quercetin were reported in other model
systems
(Kalkbrenner, F., et al., Pharmacology 44: 1-12 (1992) ; Hoult, J.R.S., et
al., Agents
and Actions 42: 787-792 (1988) ; and Moroney, M. A., et al., J. Pharrn.
Pharmacol. 40:
787-792 (1988)). The OH group at C3 position is also important for the
activity.
However, the glycosylation of the OH group, at C3 decreased the activity
considerably.
Comparing the COX-1 inhibitory activity of flavones (luteolin) with their
corresponding
flavanols (quercetin), it can be concluded that the absence of an OH group at
C3
enhanced the COX-1 activity slightly. It is important to note that quercetin
3-rhamnoside was not active in the assay, but reported to have in vivo
anti-inflammatory activity (Sanchez De Medina, L. H., et al., J. Pharmacol.
Exp. Ther.
278: 771-779 (1996)). This may be due to the in vivo metabolism of quercetin
3-rhamnoside to quercetin. The C2-C3 double bond, which determines the
coplanarity
of the hetero-rings in flavonoids and isoflavonoids, was essential for a
higher COX
inhibitory activity. If the double bond was saturated, the COX-1 inhibitory
effect was
dramatically decreased as in the case of naringenin (Figure 9). This result is
consistent with previous reports (Wurm, G., et al., Deutche Apotheker Zeitung
122:
2062-2068 (1982); Kalkbrenner, F., et al., Pharmacology 44: 1-12 (1992)).
Also, the
multiple substituents such as OH and OMe groups in the A ring of the
flavonoids
caused little or no COX-1 inhibition as demonstrated by the activity of
5,8,4'-trihydroxy-6,7-dimethoxyflavone.
Among the isoflavonoids (Figures 2 and 3), genistein showed the highest
COX-1 /COX-2 inhibitory activity. The activity was dramatically decreased in
genistin,
when the 7-OH group in ring A of genistein was glycosylated. Also, the
hydroxyl
group at C-4' in isoflavonoids is essential for the COX-1 /COX-2 inhibitory
activity.
When 4' -OH groups in genistein and daidzein were methylated, the activity
decreased considerably. The 5-OH group in isoflavonoids is also important for
COX-1/COX-2 inhibitory effect. These results indicated that Ca', C5 and C7
hydroxyl
groups in isoflavonoids are essential for COX-1 inhibition. Comparison of
genistein
with that of kaempferol indicates that substitutions on ring B and at C3 of
ring C
CA 02587127 2007-05-10
-21-
enhances COX-1 /COX-2 inhibitory effect. In addition to COX-1 /COX-2
inhibition,
these isoflavonoids and flavonoids also showed good antioxidant activity. Both
COX-1 inhibitory and antioxidant activities of these compounds suggests that
tart
cherries may possess significant health benefits to humans. These
bioflavonoids may
be partially responsible for the anecdotal claims associated with tart
cherries of
alleviating pain related to treatment of arthritis and gout.
Thus, several flavonoids and isoflavonoids isolated from BALATON tart cherry
were assayed for prostaglandin H endoperoxide synthase (PGHS-1 or PGHS-2)
enzyme activity. Genistein showed the highest COX-1 inhibitory activity among
the
isoflavonoids studied with an IC50 value of 80 :M. Kaempferol gave the highest
COX-1
inhibitory activity among the flavonoids tested with an IC50 value of 180 :M.
The
structure-activity relationships of flavonoids and isoflavonoids revealed that
hydroxyl
groups at Ca', C5 and C7 in isoflavonoids were essential for appreciable COX-1
inhibitory activity. Also, the C2-C3 double bond in flavonoids is important
for COX-1
inhibitory activity. However, hydroxyl group at C3' position decreased the
COX-1/COX-2 inhibitory activity by flavonoids.
Example 6
The composition of Examples 1 and 2 were tested for anti-inflammatory activity
using cyclooxygenase 1 and 2 (COX-1 and COX-2) in an assay as described in
Wang
et al., J. Nat. Products 62:294-296 (1999); Wang et al., J. of Ag. and Food
Chemistry,
47: 840-844 (1999) and Wang et al., J. of Nat. Products, 62:86-88 (1999) and
Examples 4 and 5. The results were that the compositions exhibited anti-
inflammatory
activities, specifically strong inhibition of COX-1 and COX-2.
It is intended that the foregoing description be only illustrative of the
present
invention and that the present invention be limited only by the hereinafter
appended
claims.