Note: Descriptions are shown in the official language in which they were submitted.
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
LIQUID EGG YOLK PRODUCT COMPRISING
LYSOPHOSPHOLIPOPROTEIN
FIELD OF THE INVENTION
This invention pertains to a novel liquid egg yolk product which contains
lysophospholipoprotein. More particularly, this invention pertains to a novel
liquid
egg yolk product containing lysophospholipoprotein from a phospholipoprotein
modified using a non-animal derived phospholipase A, and a process therefor,
which is kosher, does not have a porcine or bovine source, and does not
contain
appreciable levels of amylase. The product is useful as an emulsifier in
foodstuffs
such as sauces, spreads, mayonnaise, dressings, salad dressings, and the like.
BACKGROUND OF THE INVENTION
Phospholipases are enzymes which act on phospholipids which are found in
animal
and vegetable cells. Phospholipases are selective enzymes which are classified
according to their site of action in the phospholipid molecule. Thus, a
phospholipase Al hydrolyzes the bond between the fatty acid and the glycerine
residue at the 1-position of the phospholipid.
The hydrolysis of a phospholipid by a phospholipase results in the production
of a
"lysophospholipid". Although phospholipids have many industrial uses,
lysophospholipids have been shown to be particularly suitable for certain
industrial
applications. Lysophospholipids have a high solubility in water and this
property
gives them enhanced emulsification properties in oil/water emulsions.
Lysophospholipids have an ability to form emulsions which are reasonably
stable to
changing pH conditions, including acid conditions, and they are resistant to
chang-
ing temperatures. The ability of the lysophospholipid to form an oil-water or
water-
oil emulsion is not reduced by the presence of ions, such as magnesium or
calcium
ions.
The foregoing properties of the lysophospholipids make them particularly
desirable
for use in the food, cosmetics and pharmaceutical industries. It has been
demon-
strated that the conversion of a phospholipid to a lysophospholipid in a
phospholipid
containing substance, such as a food product, generally leads to an
improvement in
the stability of that substance.
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-2-
The most commonly used phospholipase in the industrial hydrolysis of
phospholipids is pancreatin, which is an enzyme prepared from the pancreas of
pigs.
Enzymatic hydrolysis of a phospholipid, using a phospholipase isolated from a
micro-organism is, however, known. Such hydrolysis using a phospholipase A is
described, for example, in Japanese Unexamined Patent Publication No. Sho-58-
212783, and the hydrolysis using a lipase is described in Japanese Unexamined
Patent Publication No. Sho-63-42691. Furthermore, the enzyme Taka-Diastasel,
which was isolated from a species of Aspergillus, A. oryzae, [Biochem. Z., 261
(1933) 275], has demonstrated a lipase activity which is capable of
hydrolyzing a
phospholipid. The enzymes isolated from microorganisms have been shown to have
less activity than porcine pancreatic enzyme. Moreover, the microorganisms
produce amylase and protease as by-products, which are undesirable because
they
break down starch and proteins and lead to emulsion instability.
Although pancreatin has better properties than enzymes isolated from
microorgan-
isms, hydrolysis of a phospholipid using pancreatin has many disadvantages.
Firstly, it may be necessary to make continual adjustments to the pH of the
reaction
mixture during hydrolysis of a phospholipid substrate with porcine pancreatin.
The
optimum pH for activity of pancreatin is in the range from neutral to weakly
alkaline. During the hydrolysis reaction, however, the release of free fatty
acids
causes the pH to drop, that is, it increases the acidity of the reaction
mixture, so that
unless counter action is taken, the mixture will become acidic, and therefore
outside
the pH range for optimum activity of the enzyme.
Traditionally, heat treatment has been used to deactivate the residual enzyme
in
processes involving the use of enzymes. However, porcine pancreatin has
another
disadvantage because it is not fully deactivated by heat treatment, and even
treat-
ment of the enzyme at a temperature of 95 C for 30 minutes may not
sufficiently
deactivate the residual enzyme. The use of a higher temperature is impossible
in
view of the sensitivity of the phospholipid and free fatty acids to heat.
FEMS Microbiol. Lett. 3(2), 85-7, Vol. 3, No. 2, 1978 discloses the detection
of
phospholipase Al activity in various filamentous fungi, including Aspergillus
strains, but there is no disclosure of the isolation and purification of the
enzyme.
Biological Abstracts, vol. 72, Philadelphia, PA, Abstract No. 012592,
discloses the
purification and characterization of phospholipids by various phospholipases.
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-3-
It is known from British patent specification GB-B-1,525,929 (Unilever) to
treat
phospholipoproteins or phospholipoprotein containing materials, such as egg
yolk,
whole egg, blood serum, wheat protein, soybean, and the like, with
phospholipase
A. The phospholipase A is also active when the phospholipid is complexed with
protein. After the treatment with the phospholipase, the
lysophospholipoprotein is
formed. The lysophospholipid is complexed with a protein. The
lysophospholipoprotein containing material disclosed in GB-B-1,525,929 has
achieved considerable commercial success as an emulsion stabilizer,
particularly in
oil-in-water emulsions. They enabled the manufacture of sterilizable
emulsions,
which in practice turned out to be commercially very successful, because they
had a
long shelf life and an excellent creamy taste.
The following patents disclose subject matter which is related to or relevant
to the
subject invention.
Japanese Abstract No. 58212783 A2, Kyowa Hakko Kogyo Co. Ltd., discloses a
process whereby a microorganism, e.g. Streptomyces scabies ATCC15485 or
Streptomyces achromogenes variety streptozoticus NRRL2697, belonging to the
genus Streptomyces, and having the ability to produce phospholipase A, is
cultivated
in a culture medium at 22 C to 40 C and a neutral or slightly alkaline pH for
about
2 to 6 days. The phospholipase A is collected mainly from the culture fluid.
Japanese Abstract No. 06153939 A2, Snow Brand Milk Prod. Co. Ltd., discloses a
process whereby an alga of the genus Euglena (preferably Euglena gracilis)
having
the ability to produce phospholipase A is cultured in a culture medium
containing a
carbon source (preferably glucose), a nitrogen source (preferably glutamic
acid or
diammonium hydrogenphosphate) at 4-35 ratio (C/N) under conditions of
preferably
pH 3.0-4.5, 20-32 C culture temperature and irradiation with light or in the
dark for
3-7 days, to produce and accumulate phospholipase A in the organism. The
resultant phospholipase A is then separated and collected to provide the
objective
phospholipase A.
U.S. Patent No. 5,521,080, Hattori et al., discloses a method for preparing a
phospholipase Al which comprises (a) culturing a phospholipase Al producing
strain of Aspergillus under conditions which allow for the production of the
phospholipase Al; (b) after culturing, diluting the culture with water or an
appropri-
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-4-
ate buffer solution; (c) filtering the resulting solution under pressure to
remove any
insoluble matter; and optionally (d) purifying the enzyme.
U.S. Patent Nos. 5,378,623 and 5,538,874, Hattori et al., are related and
disclose a
phospholipase Al which is capable of hydrolyzing a phospholipid to produce a 2-
acyl lysophospholipid and is obtainable from species of the fungus
Aspergillus.
EP 0 575 133 B1, Sankyo Company Limited, discloses a phospholipase Al obtain-
able from fungus selected from Aspergillus niger and Aspergillus oryzae
character-
ized in that said phospholipase Al: (a) hydrolyzes phospholipid between about
pH
2.5 and about pH 6.0; (b) has a molecular weight of between about 30,000 and
about 40,000 daltons, as determined by sodium dodecyl sulphate polyacrylamide
gel
electrophoresis; (c) has a stability to temperature with an upper limit of
between
about 45 and about 90 C; (d) has a pI under isoelectric point electrophoresis
at
about pH 2.8 to about pH 4.5; and (e) has an optimum temperature for activity
of
from about 30 to about 65 C.
U.S. Patent No. 4,119,564, van Dam, discloses a process whereby oil-in-water
emulsions with an increased viscosity are produced by incorporating an
effective
amount of phospholipase A-treated phospholipoprotein.
Related U.S. Patent No. 4,034,124, van Dam, discloses emulsions comprising an
oil phase, an aqueous phase and a phospholipoprotein which has been subjected
to a
treatment with phospholipase A as an emulsion stabilizer. These emulsions have
an
increased stability, especially heat stability, compared with emulsions which
do not
contain such a stabilizer.
U.S. Patent No. 5,028,447, Schenk, discloses a process whereby oil and water
emulsions which contain a phospholipoprotein material which has been modified
by
phospholipase A, and at least one native starch based thickening agent, are
prepared
by subsequently gelatinizing the thickening agent, incorporating the modified
phospholipoprotein containing material into the gelatinized thickening agent,
then
incorporating the oil (which may at least partially be replaced by a low-
calorie fat
substitute) and finally homogenizing the mixture obtained. Canadian Patent No.
1,210,224 is related.
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-5-
EP 0 319 064 B1, Unilever NV, discloses a process for the preparation of a
water
and oil emulsion comprising a phospholipoprotein containing material, which
has
been modified by phospholipase A, and at least one native starch based
thickening
agent, which comprises: (a) at least partly gelatinizing the native starch
based
thickening agent; (b) incorporating the phospholipoprotein containing
material,
which has been modified by phospholipase A, into the gelatinized native starch
based thickening agent; (c) incorporating from 5 % to 85 % by weight of oil or
fat
containing oil into the mixture obtained in step (b); and (d) homogenizing the
final
mixture obtained.
U.K. Patent No. 1,585,105, Unilever Limited, discloses an oil-in-water
emulsion
which contains a phospholipase A-treated phospholipoprotein having a degree of
conversion of at least 55 % and at least one thickening agent in a proportion
which is
less than that required for obtaining an emulsion of the same composition and
viscosity but containing phospholipoprotein of a lower degree of conversion.
U.S. Patent No. 5,082,674 and Canadian Patent No. 2,026,447, Carrell et al.,
disclose a process for the manufacture of a lysophospholipoprotein-comprising
foodstuff. The dried lysophospholipoprotein or dried lysophospholipoprotein-
comprising material, preferably having a moisture content of at most 10 wt%.,
at a
level of 0.1-90 wt. % therein, is used as a texture-modifying agent, a
glossing agent,
a freeze-thaw stabilizing agent, a heat-stabilizing agent and a syneresis-
inhibiting
agent.
U.S. Patent No. 5,314,706, Colarow et al., discloses an egg yolk fortified
with
exogenous soybean lysophosphatidylcholine contained in exogenous soybean
lysophospholipids which is employed as an emulsification agent in oil and
water
emulsions, particularly in emulsions which are sterilized. The agent may be ob-
tained by hydrolyzing phospholipids derived from soybeans with phospholipase
A2,
deactivating the phospholipase A2 with a proteolytic enzyme and then
inactivating
the proteolytic enzyme by heat-treatment at a temperature of from 800 C to 900
C.
Egg yolk is fortified by combining and homogenizing the so-obtained
lysophospholipids, or exogenous phospholipids containing
lysophosphatidylcholine.
U.S. Patent No. 5,750,164, Saito et al., discloses a method of decreasing
choles-
terol concentration in eggs, processed egg foodstuffs, meat, fish meat, dairy
products and processed foodstuffs thereof, which includes hydrolyzing
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-6-
phospholipids in the eggs or processed egg foodstuffs or other products with
one
member selected from the group of phospholipase A,, Az, B, D,
lysophospholipase
and a mixture thereof, and subjecting the phospholipid-hydrolyzed eggs or pro-
cessed egg foodstuffs or other products to a conventional cholesterol-
decreasing
treatment.
Japanese patent Abstract No. 62262998 A2, QP Corp., discloses a process
whereby
a natural phospholipid-containing substance such as egg yolk is added with a
phospholipase A2 preparation-containing material) (e.g. purified phospholipase
A2
preparation originated from animal pancreas) to effect enzymatic reaction. The
phospholipid in the substance is decomposed by the reaction to obtain a
lysophospholipid-containing material. The obtained lysophospholipid-containing
material is dried at about < 80 C by spray drying, etc., to powder having a
water
content of < 10%. The powder is extracted with a polar solvent such as ethanol
to
extract lysophospholipid. The solvent is distilled from the extract under
reduced
pressure to obtain the objective lysophospholipid-containing material composed
of
68 wt. % neutral lipid and 32 wt. % phospholipid (30 wt. % thereof is
lysophospholipid).
Japanese patent Abstract No. 63209742 A2, QP Corp., discloses a method whereby
an emulsifier is prepared by mixing phospholipid, protein, phospholipase A2
and
clean water uniformly in a mixer or a colloid mill, etc., treating at ca. 40 C
and
drying if necessary. Suitable protein is albumin, globulin, gelatin, etc., and
suitable
proportion of protein to be present in aqueous suspension of phospholipid is
1: (ca
0.5 to 2) phospholipid to protein. The phospholipase A2 is an enzyme for
hydrolyz-
ing the fatty acid ester moiety at the middle part of a glyceride constituting
the
phospholipid, and the amount thereof to be used is ca. 0.1 to 5 wt. % based on
the
amount of the phospholipid.
U.S. Patent No. 5,213,968, Castle et al., discloses a process whereby
emulsifying
agents are prepared by sequentially treating a biological material with a
protease and
a lipase. The enzymatically treated biological material may be pasteurized
during or
following the enzymatic treatment.
EP 0 414 024 B1, Societe des Produits Nestle S.A., discloses a process for the
preparation of an emulsifying agent which comprises treating a biological
material
containing a lipid as well as a lipoprotein and/or a protein with a protease
and a
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-7-
lipase and pasteurizing the product. The treatment with the protease and the
lipase
is carried out sequentially in any order.
SUMMARY OF INVENTION
The present invention relates to a process for the manufacture of
lysophospholipoprotein-comprising foodstuffs, such as sauces, spreads,
mayonnaise,
dressings, soups, bakery products, creamers, creamer-thickeners, ice cream,
drinks,
dairy products, desserts, sherbets, meals, and combinations thereof, with no
detectable amylase (and protease) activity.
The invention is directed to a process for the manufacture of a liquid egg
yolk
product containing lysophospholipoprotein comprising: (a) processing a
phospholipase A-containing microbial fermentate to remove amylase and protease
co-products of the fermentation to produce a refined phospholipase A-
containing
microbial product; and (b) combining a liquid egg yolk with the refined
phospholipase A-containing microbial product of step (a) to produce a modified
liquid egg yolk product containing lysophospholipoprotein, said modified
liquid egg
yolk product having (i) a degree of conversion of phospholipoprotein to
lysophospholipoprotein of at least 10%; and (ii) an amylase activity of less
than 50
units/litre.
The phospholipase A-containing fermentate of step (a) can be produced by a
fermentation of a phospholipase A-producing microorganism in a nutrient
medium.
Suitable prior art phospholipase A producing microorganisms can be used in the
invention but a preferred microorganism can be Streptomyces violaceoruber or a
genetically modified Thermomyces lanuginous/Fusarium oxysporum. Step (a) can
be conducted at a temperature below about 20 C and at a pH between about 4.9
and
5.2.
The undesirable amylase and protease co-products can be removed in step (a) by
passing the fermentate through a cross-flow dialysis apparatus having a PES
50K
membrane.
The liquid egg yolk product containing lysophosphoprotein can have a
phospholipase activity of less than 250 units/litre. The liquid egg yolk
product
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-8-
containing lysophospholipoprotein can have a protease activity of less than
0.01
fluorescence units/ml/min.
The invention is also directed to a product prepared according to the process
of the
invention. The product can contain up to 20 % added salts, and up to 50 %
added
carbohydrates, including sugar, malto-dextrin, glucose or corn syrup solids.
The
product can contain up to 75 % added liquid egg albumin or concentrated liquid
egg
albumin.
The product can be spray dried at an inlet temperature of from about 200 C to
about
250 C and an air outlet temperature from about 75 C to about 100 C so that the
final moisture of the dried product is less than about 10% by weight.
The product can be incorporated into an emulsion of oil, water, vinegar,
starch,
sugar and salt. The emulsion can also include egg yolk.
DRAWINGS
In drawings which illustrate specific embodiments of the invention, but which
should not be construed as restricting the spirit or scope of the invention in
any
way:
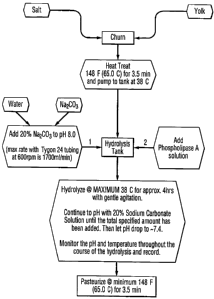
Figure 1 illustrates a schematic flow sheet of a process for modifying liquid
egg
yolk with refined phospholipase A to produce a modified egg yolk product
contain-
ing lysophospholipoprotein.
Figure 2 illustrates a plot of the amount of phospholipase A passing in the
permeate
and amylase concentrating in the retentate after passing a phospholipase A and
amylase fermentator through a dialysis filter having a PES 50K membrane.
Figure 3 illustrates a plot of effect of mayonnaise pH on mayonnaise
viscosity.
Figure 4 illustrates a plot of the effect of enzyme modified yolk pH on
mayonnaise
viscosity.
Figure 5 illustrates a combination of Figures 3 and 4 and represents a plot of
the
effect of enzyme modified yolk pH on mayonnaise pH.
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-9-
Figure 6 illustrates a plot of volume over time of soda ash added to yolk to
maintain
constant pH at different temperatures.
Figure 7 illustrates a plot of hydrolysis of starch samples after different
incubation
times with porcine pancreatic 2-amylase.
Figure 8 illustrates a plot of time course of saccharide formation of A.
pullulans
amylase preparation from maltodextrin DE.
Figures 9A and 9B illustrates plots of time course of glucose production of A.
pullulans amylase preparation from maltodextrin (A) and cornstarch (B).
Figure 10 illustrates plots of production of soluble carbohydrate in white
bread with
Aspergillus intermediate temperature stability enzyme.
Figure 11 illustrates a plot of the relationship between the rate of starch
viscosity
decrease with honey.
Figure 12 illustrates a plot of the effect of pH on honey amylase catalyzed
degrada-
tion of unmodified waxy maize starch at different pH values.
DETAILED DESCRIPTION OF SPECIFIC
EMBODIMENTS OF THE INVENTION
Throughout the following description, specific details are set forth in order
to
provide a more thorough understanding of the invention. However, the invention
may be practiced without these particulars. In other instances, well known
elements
have not been shown or described in detail to avoid unnecessarily obscuring
the
invention. Accordingly, the specification and drawings are to be regarded in
an
illustrative, rather than a restrictive, sense. Applicable knowledge in the
prior art is
incorporated herein by reference.
Over the years, the food industry has evolved to a point where all ingredients
used
in common food products such as sauces, spreads, mayonnaise, starch-based
salad
dressings, oil-water based salad dressings, soups, bakery products, creamers,
creamer-thickeners, ice cream, drinks, dairy products, desserts, sherbets, and
the
like, must be of kosher quality, that is, not derived from porcine sources.
Further-
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-10-
more, with the frequent recurrence of foot and mouth disease in cattle and the
"mad
cow" disease problem prevalent in cattle in Great Britain, a strong phobia has
developed in the food industry to the use of components that are derived from
bovine sources. There is a strong need in the food industry for an emulsifier
which
is not derived from porcine or bovine sources, which can be used in oil-water
and
water-oil emulsions, which can withstand changes in pH level, which has long
shelf
life, and which can withstand a wide variation in temperatures.
The emulsions prepared according to the invention can have either the oil
phase or
the aqueous phase as the continuous phase, and are in this specification
referred to
as water-in-oil emulsions and oil-in-water emulsions, respectively. Oil-in-
water
emulsions are preferred. The latter type of emulsion means for the purpose of
this
specification continuous aqueous phases containing any amount of fat and/or
oil in
dispersed form. Examples of emulsions which come under this definition are
edible
products like phase inversion margarines, soups or sauces, natural or
artificial fruit
juices, mayonnaise, dressings or spreads.
Examples of phospholipoprotein-containing substances are casein, skim milk,
buttermilk, whey, cream, soyabean, yeast, egg yolk, whole egg, blood serum and
wheat proteins. Egg yolk is used preferably as source of the
phospholipoprotein.
Egg yolk or other sources of phospholipoprotein can be subjected to the action
of
phospholipase A and the modified product is then incorporated in the products
according to the invention.
The inventors herein have invented a novel liquid amylase-free egg yolk
product
containing lysophospholipoprotein, which is not derived from animal sources,
notably porcine or bovine sources. The inventors have also invented a process
for
manufacturing a liquid egg yolk product containing lysophospholipoprotein.
The inventors have prepared a process for the manufacture of a liquid egg yolk
product comprising lysophospholipoprotein which comprises: (a) processing a
fermentation of a microorganism in a nutrient medium which produces microbial
phospholipase A to remove undesirable amylase and protease co-products of the
fermentation to produce a refined microbial phospholipase A product; and (b)
modifying a liquid egg yolk with the refined microbial phospholipase A to
produce a
modified liquid egg yolk product which contains lysophospholipoprotein, the
modified liquid egg yolk product comprising lysophospholipoprotein having a
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-11-
degree of conversion of phospholipoprotein to lysophospholipoprotein of at
least
10% and an amylase activity of less than 50 units per litre. Advantageously,
the
process produces a modified liquid egg yolk product containing
lysophospholipoprotein which has a degree of conversion of phospholipoprotein
to
lysophospholipoprotein of at least 50%.
A problem with most methods of producing phospholipase A by microbial fermenta-
tion is that high levels of amylase are obtained as a by-product. Protease is
also
produced as a byproduct. High levels of amylase are undesirable because the
amylase breaks down starch into undesirable products. Many food products are
starch-based and hence significant levels of amylase in a food additive cannot
be
tolerated because the amylase leads to breakdown of the starch base.
Significant
protease levels are also to be discouraged since the protease tends to react
with egg
white and other proteins and cause water in the oil-water emulsions to begin
to
separate.
The inventors have discovered a method and processing equipment which enables
the amylase to be separated from the microorganism produced phospholipase A
fermentation medium so that the amylase activity in the end product is
basically
non-existent. Amylase activities of less than 50 units per litre have been
obtained.
The system according to the invention uses a specific dialysis procedure for
separat-
ing the amylase and the protease from the crude phospholipase A medium. The
inventors have tested and rejected a large number of different dialysis
machines in
an effort to discover a feasible method for separating amylase from the crude
phospholipase A fermentation medium. None were found to be satisfactory for
purposes of the invention. The inventors have now found a dialysis system
avail-
able from North Carolina SRT under the model number NCSRT UF with a PES
50K membrane, or smaller, capable of separating the amylase and protease from
the
phospholipase A.
For purposes of the invention, the inventors have found that a suitable
microbial
fermentation product containing phospholipase A is available from Genencor.
One
type of phospholipase A microorganism fermentation product that can be used in
the
process according to the invention is a Streptomyces viola ceoruber derived
product
available from Genencor. Another microorganism that can be used in the process
is
a genetically modified thermomyces lanuginous/ Fusarium oxysporum produced by
submerged fermentation and available from Novozymes. Other suitable microbial
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-12-
phospholipase A products may be suitable for purposes of the invention,
including
one or more of the phospholipase A-containing products referred to in the Back-
ground. The key to the subject invention is that the inventors have discovered
a
method and apparatus for reducing the amylase and protease concentrations of
the
crude microbial enzyme products available from commercial sources so that the
amylase and protease activity are reduced to insignificant levels.
The following is a detailed discussion of the apparatus and steps the
inventors have
discovered which enable them to successfully separate amylase and protease
from
the phospholipid A-containing material.
Standard Operation Procedure for Separating Amylase and Protease
from Crude Microorganism Produced Phospholipase A
Prefiltration of the crude enzyme solution: The crude enzyme solution should
be
prefiltered through a 5 micron or less filter.
Stage One Dialysis
The following ingredients are used: kosher sodium citrate; kosher citric acid;
crude
enzyme (obtained from Genencor) approx. 100 lbs. (45L); and RO purified water.
The following equipment is used: an NCSRT UF system with PES 50K membrane
module for dialysis stage. Any other type of suitable tangential flow or
spiral
wound membrane system with a similar membrane type can be used.
Method of Preparation:
(1) Prepare approximately 2 to 6 volumes of citrate buffer at pH 4.8 to
5.2.
(2) Load the crude enzyme into feed tank. Collect a sample from the
tank for testing.
(3) Start the dialysis system by setting flow rates so that the flux
(litres/m2/hour) is approximately 2 to 10 LMH and adjust the back
pressure valve to between 0 to 20 psi on the retentate exit line of the
membrane. Collect the permeate in pail(s) or a suitable receiver tank
(150 to 200L). The permeate line flows should not be blocked or
build up significant back pressure.
(4) Start the citrate buffer flow and set it to match the permeate flow into
the feed tank. For a 1 meter2 membrane at 7 LMH, approximately
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
- 13 -
150 (7*1) or 21.4 hours will be required to dialyse the enzyme
through the membranes and into the permeate.
(5) Once all the buffer has been pumped into the feed tank, the system
should be allowed to continue to operate until the volume in the feed
tank has been reduced to approximately 10 to 20L.
(6) Shut the process down and collect from the system and feed tank all
remaining material into containers.
Sta eg Two
A stage two procedure is used to concentrate the product, when required. The
following ingredients are used: 150 to 200L of collected PA2 Permeate from
Stage
One; and RO purified water. The following equipment is required: NCSRT UF
system with a 10K or 5K membrane module for the concentration stage (or other
suitable tangential flow or spiral wound membrane systems).
Method of Preparation:
(1) Provide sufficient cooling to the unit to keep the enzyme in the feed
tank below 20 C, preferably at 10 C, if possible.
(2) Load the dialysed enzyme (PA2 Permeate from Stage One) into the
feed tank. Collect a sample from the tank for analysis.
(3) Start the concentration system by setting flow rates (cross flow of 20
to 100 L/min.) so that the flux is in the range of 5 to 60 LMH and
adjust the back pressure valve to generate between 10 to 100 psi on
the retentate exit line of the membrane.
(4) Reduce the volume in the feed tank to approximately 10 to 20 L (or
less if care is taken to prevent air entrainment and turbulence). This
will take approximately 5 to 20 hours to complete.
(5) Shut the system down and collect the remaining material from the
system and feed tank.
Figure 2 illustrates a plot of the amount of phospholipase A passing in the
permeate
and amylase concentrating in the retentate after passing a phospholipase A and
amylase fermentator through a dialysis filter having a PES 50K membrane.
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-14-
Standard Operating Procedure for Producingti Modified Liquid Egg Yolk
Products Containing Lysophospholipoprotein from Refined Amylase and
Protease-Free enzyme Product Obtained from the Stage One Dialysis
or the Stage Two Concentration
(1) Prepare liquid salt yolk in churn.
(2) Prepare alkaline base solution.
(3) Adjust batch initial pH.
(4) Adjust yolk temperature.
(5) Add enzyme.
(6) Perform hydrolysis step and add alkaline base as necessary.
(7) Terminate hydrolysis.
(8) Pasteurize.
(9) Package.
Figure 1 illustrates a schematic flow sheet of a process for modifying liquid
egg
yolk with refined phospholipase A to produce a modified egg yolk product
contain-
ing lysophospholipoprotein.
Figure 6 illustrates a plot of volume over time of soda ash added to yolk to
maintain
constant pH at different temperatures.
The following is a recipe of a typical starch thickened oil-water emulsion
salad
dressing incorporating a liquid egg yolk product according to the invention.
Ingredients (/g)
Water 159.9
Vinegar 170.0
Starch 30.0
Sugar 105.5
Salt 11.0
Water 190.2
Salt 8.5
Enzyme modified yolk 25.0
Vegetable oil 300.0
Total 1000.0
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
- 15-
Figure 3 illustrates a plot of effect of mayonnaise pH on mayonnaise
viscosity.
Figure 4 illustrates a plot of the effect of enzyme modified yolk pH on
mayonnaise
viscosity. Figure 5 illustrates a combination of Figures 3 and 4 and
represents a
plot of the effect of yolk pH on mayonnaise pH.
Figure 7 illustrates a plot of hydrolysis of starch samples after different
incubation
times with porcine pancreatic 2-amylase.
Figure 8 illustrates a plot of time course of saccharide formation of A.
pullulans
amylase preparation from maltodextrin DE. Figures 9A and 9B illustrate plots
of
time course of glucose production of A. pullulans amylase preparation from
maltodextrin (A) and cornstarch (B). Figure 10 illustrates plots of production
of
soluble carbohydrate in white bread with Aspergillus intermediate temperature
stability enzyme.
Figure 11 illustrates a plot of the relationship between the rate of starch
viscosity
decrease with honey. Figure 12 illustrates a plot of the effect of pH on honey
amylase catalyzed degradation of unmodified waxy maize starch at different pH
values.
The following table illustrates recommended amylase activity for starch
thickened
dressing shelf stability.
O
~
StaP'zh Thickened Dressing Shelf Stability
Novo HI Recommendation Novo LO Recorrimendation MFI Current Use IEP LO Level
Use Purified En me Novo Porcine Control
Amylase Activity 20 20 20 20 3.33 0
PA2 Activity 300 300 300 300 1800 11175 K ,
Units Activity per kg 50000 10000 1000 500 500 500
Yolk 1000 1000 1000 1000 1000 1000
N
0
I
Rw8 11 me 166.667 0.140 33.333 0.028 3.333 0.003 1.667 0.001 0.278 0.000 0.045
0.000 O
20/o Salt Yolk 1020.408 0.860 1020.408 0.860 1020.408 0.860 1020.408 0.860
1020.408 0.860 1020.408 0.860 rn o
suQs Total 1187.075 1053.741 1023.741 1022.075 1020.686 1020.453 L'
Fft9m4er 0.000 0.000 133.333 0.112 163.333 0.138 165.000 0.139 166.389 0.140
166.622 0.140
70tai 1187.075 1.000 1187.075 1.000 1187.075 1.000 1187.075 1.000 1187.075
1.000 1187.075 1.000
To~al Units Amylase 2808.02 561.60 56.16 28.08 0.78 0.00
Amylase Testing 0.392063 0.381954 0.37968 0.379553 0.379448 0.37943
Yolk/Enzyme/Water Mix 10 10 10 10 10 10
Water 50 50 50 50 50 50
Solids 0.0653438 0.063659 0.06328 0.063259 0.063241 0.063238 n
~
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-17-
The following table illustrates a recipe for a typical mayonnaise
incorporating the
liquid egg yolk product according to the invention.
Ingredients (/g) %)
Whole Egg 35 % solids
Enzyme modified egg yolk product 42.9 5.72
Sugar 4.0 0.53
Salt 6.9 0.91
5% Vinegar 56.3 7.51
Canola Oil #1 127.5 17.00
Canola Oil #2 450.0 60.00
Water 62.5 8.33
Total 750.0 100.0
Colorimetric Method for Testing for Presence of a-Am,ylase in Egg
Application and Principle
This method of testing for presence of amylase in egg has been developed by
the
inventors. The reducing groups, maltose and glucose, liberated from starch
hydrolysis reduce 3,5-dinitrosalicylic acid, resulting in the formation of a
colored
product which can be measured spectrophotometrically at 560 nm.
Reagents
(1) 0.02 M sodium phosphate buffer, pH 6.9 containing 0.006 M sodium
chloride.
(2) 2.0 M sodium hydroxide.
(3) Dinitrosalicylic acid color reragent. Dissolve l.Og 3,5-
dinitrosalicylic acid in 20m12M NaOH. Add slowly 30.Og sodium
potassium tartrate tetrahydrate. Dilute to a final volume of 100m1
with distilled water. Store in a tightly sealed container and protect
from CO2. Stable for 2 weeks.
(4) 1 % starch. Dissolve 1.0g of soluble starch in 100m10.02M sodium
phosphate buffer, pH 6.9, containing 0.006M NaC1. Bring to a
gentle boil to dissolve. Cool and make volume up to 100m1, with
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
- 18-
distilled water, if necessary. Incubate at 25 C for 5 minutes prior to
assay.
(5) Enzyme (a-amylase) standards. Dilute to a concentration of 0.01 to
u/ml. Prepare 10 different concentrations within this range for a
5 standard curve (eg. 10, 5, 2.5, 1.25, 0.625, 0.3125, 0,1563, 0.0781,
0.0391 u/ml).
Egg Samples
(1) Measure 0.500g of liquid egg yolk (or 0.250g dried egg yolk, 0.500g
10 liquid egg white, 0.100g dried egg white) into a 1.5m1 Eppendorf
tube.
(2) Bring to 1.00g with NaP buffer (prepared above).
(3) Vortex mixture until the egg and buffer are sufficiently mixed.
(4) Prepare dilutions of this mixture in 1.5m] Eppendorf tubes. Dilute
2x, lOx, 20x and 50x.
(5) Centrifuge all tubes at 10,000rpm for 20 minutes. Do not resuspend.
Supernatant will be used as sample.
Procedure
(1) Pipette 0.030m1 of each of the standard solutions in triplicate as
shown on the plate layout. Use 0.030m1 of NaP buffer as a blank.
(2) Pipette 0.030m1 of each sample dilution supernatant, in duplicate,
into the plate.
(3) Add 0.030m1 of starch solution to all wells and place lid on microtitre
plate. Tap gently to mix.
(4) Incubate plate at 37 C for 25 minutes on Elisa plate incubator.
(5) Turn on hot plate and boil water (use boiling chips!!) about 0.5 -
0.75cm dep in a Pyrex dish.
(6) After 25 minutes of incubation, add 0.060m1 of 2,5-dinitrosalicylic
acid color reagent to all wells. Tap gently to mix.
(7) Cover plate with stick-on type microtitre plate cover and place in
Pyrex dish with boiling water.
(8) Heat for 5 minutes to develop color.
(9) Let plate cool to room temperature in a shallow dish of cold water.
(10) Remove stick-on cover and add 0.120m1 dHzO to each well in plate.
Tap gently to mix.
(11) Read plate in Multiskan using amylase protocol (measure @ 560nm).
CA 02587488 2007-05-14
WO 2005/099487 PCT/CA2004/000572
-19-
Calculations
(1) Plot standard curve in Excel.
(2) Remove data points that are out of range (on high end the absorbance
values will plateau, generally at ABS > 3.1) and add a linear best-fit
trend line.
(3) Average duplicate data for samples and calculate a-amylase activity
based on value from standard curve.
(4) Multiply by dilution factor to obtain result in u/n-d. Multiply again
by w/w dilution factor 0.500g/1.000g (for liquid yolk) to obtain
result in u/g.
As will be apparent to those skilled in the art in the light of the foregoing
disclosure,
many alterations and modifications are possible in the practice of this
invention
without departing from the spirit or scope thereof. Accordingly, the scope of
the
invention is to be construed in accordance with the substance defined by the
following claims.