Note: Descriptions are shown in the official language in which they were submitted.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
1
DRIED FORMS OF AQUEOUS SOLUBILIZED BILE ACID
DOSAGE FORMULATION: PREPARATION AND USES
THEREOF
BACKGROUND OF THE INVENTION
Bile acids salts, which are organic acids derived from cholesterol are natural
ionic detergents that play a pivotal role in the absorption, transport, and
secretion of
lipids. The tenn, primary bile acid refers to those synthesized de novo by the
liver. In
humans, the primary bile acids include cholic acid (3a, 7a, 12a -trihydroxy-5
(3-cholanic acid) ("CA") and chenodeoxycholic acid (3a, 7a-dihydroxy-5 P-
cholanic
acid) ("CDCA") . Dehydroxylation of these bile acids by intestinal bacteria
produces
the more hydrophobic secondary bile acids, deoxycholic acid (3a, 12a-dihydroxy-
5(3
-cholanic acid) ("DCA") and lithocholic acid (3a-hydroxy-5 (3-cholanic acid)
("LCA"). These four bile acids CA, CDCA, DCA, and LCA, generally constitute
greater than 99 percent of the bile salt pool in humans. Secondary bile acids
that have
been further metabolized by the liver are sometimes denoted as tertiary bile
acids.
Keto-bile acids are produced secondarily in humans as a consequence of
oxidation of bile acid hydroxyl groups, particularly the 7-hydroxyl group, by
colonic
bacteria. However, keto-bile acids are rapidly reduced by the liver to the
corresponding a or 0-hydroxy bile acids. For example, the corresponding keto
bile
acid of a CDCA is 7-keto lithocholic acid and one of its reduction products
with the
corresponding (3-hydroxy bile acid is ursodeoxycholic acid (3a-7j3-dihydroxy-5
(3-cholanic acid) ("UDCA"), a tertiary bile acid.
Bile acids containing a 6p -hydroxyl group, which are found in rats and mice,
are known as muricholic acid; 6a-hydroxy bile acids produced by swine are
termed
hyocholic acid and hyodeoxycholic acids. 23-hydroxy bile acids of aquatic
mammals
are lcnown as phocecholic and phocedeoxycholic acids.
Typically, more than 99 percent of naturally occurring bile salts secreted
into
human bile are conjugated. Conjugates are bile acids in which a second organic
substituent (e.g. glycine, taurine, glucuronate, sulfate or, rarely, other
substituents) is
attached to the side chain carboxylic acid or to one of the ring hydroxyl
groups via an
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
2
ester, ether, or amide linkage. Therefore, the ionization properties of
conjugated bile
acids with glycine or taurine are determined by the acidity of the glycine or
taurine
substituent.
Free, unconjugated, bile acid monomers have pKa values of approximately
5Ø However, pKa values of glycine conjugated bile acids are on average 3.9,
and the
pKa of taurine conjugate bile acids are less than 1Ø The effect of
conjugation,
therefore, is to reduce the pKa of a bile acid so that a large fraction is
ionized at any
given pH. Since the ionized salt form is more water soluble than the
protonated acid
form, conjugation enhances solubility at a low pH. Free bile acid salts
precipitate
from aqueous solution at pH 6.5 to 7. In contrast, precipitation of glycine
conjugated
bile acid occurs only at pH of less than 5. Taurine conjugated bile acids
remain in
aqueous solution under very strongly acidic conditions (lower than pH 1).
However,
in the gastric pH range, certain bile acids such as UDCA and CDCA are no
longer
soluble.
Conjugation of the side chain of a bile acid with glycine or taurine has
little
influence on the hydrophobic activity of fully ionized bile salts. More
hydrophobic
bile salts exhibit greater solubilizing capacity for phospholipid and
cholesterol and are
consequently better detergents. More hydrophobic bile salts are also more
injurious
to various membranes, both in vivo and in vitro.
Natural bile salt pools invariably contain multiple bile acid salts. Mixtures
of
two or more bile salts of differing hydrophobic activity may behave as a
single bile
salt of an intermediate hydrophobic activity. As a result, detergent
properties and the
toxicity of mixtures of two bile acids of differing hydrophobic activity often
are
intermediate between the individual components.
Bile acids have a variety of properties. For example, UDCA may be a useful
immuno-modulating agent. It may also inhibit induction of nitric oxide
synthase
(NOS) in human intestinal epithelial cells and in vivo. Bile acids may act as
pepsin
inhibitors, with UDCA being the most potent. In addition, bile acids may have
membrane stabilizing properties. UDCA is a prototype of a novel and selective
glucocorticoid receptor (GR) modifier and represses NF-kB without induction of
transactivation function of the GR. In addition, UDCA plays a unique role in
modulating the apoptotic threshold to a variety of agents acting through
different
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
3
apoptotic pathways in both hepatic and non-hepatic cells. Finally, UDCA has
specific
antioxidant properties. The OH fiee radical scavenging efficiency of UDCA
appears
remarkable in that its rate constant for reaction with this radical species is
about ten-
fold higher than that of the well known pharmacological scavenger mannitol and
of
the physiological scavengers glucose or histidine. This scavenging activity
may give
rise to the ability of UDCA to inhibit deoxycholic acid-induced apoptosis by
modulating mitochondrial transmembrane potential and reactive oxygen species
production.
Bile flow is generated by the flux of bile salts passing through the liver.
Ursodeoxycholic acid may promote bile flow by inducing hepatocytes to release
ATP
into bile, which then stimulates fluid and electrolyte secretion by bile-duct
epithelia
downstream via changes in cytosolic Ca++. Bile salts in the enterohepatic
circulation
are thought to regulate bile acid synthesis by suppressing or derepressing the
activity
of cholesterol 7-hydroxylase, which is the rate-limiting enzyine in the bile
acid
biosynthesis pathway. Bile formation represents an important pathway for
solubilization and excretion of organic compounds, such as bilirubin,
endogenous
metabolites, such as amphipathic derivatives of steroid hormones, and a
variety of
drugs and other xenobiotics.
Bile acids may play a role in the regulation of hepatic lipoprotein receptors
(apo B.E.) and consequently may modulate the rate of uptake of lipoprotein
cholesterol by the liver. Secretion of bile salts into bile, on the other
hand, is coupled
with the secretion of two other biliary lipids, phosphatidylcholine (lecithin)
and
cholesterol. Coupling bile salt output with the lecithin and cholesterol
output
provides a major pathway for the elimination of hepatic cholesterol. Bile
acids may
also be a factor in the regulation of cholesterol synthesis by acting directly
on the
hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase or indirectly by
modulating the cholesterol absorption in the intestine. Bile salts, along with
lecithin,
solubilize cholesterol in bile in the form of mixed micelles and vesicles. In
the
intestines, bile salts in the form of mixed micelles participate in the
intraluminal
solubilization, transport, and absorption of cholesterol, fat-soluble
vitamins, and other
lipids. Bile salts may be involved in the transport of calcium and iron from
the
intestinal lumen to the brush border.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
4
UDCA, a major component of bear bile, has been used as a major
pharmaceutical agent for the treatment of and protection against many types of
liver
disease. Its medicinal uses include the dissolution of radiolucent gall
stones, the
treatment of biliaiy dyspepsia, primarily biliary cirrhosis, primary
sclerosing
cholangitis, chronic active hepatitis and hepatitis C. High levels of bile
acids
remarkably inhibit the proliferation of hepatitis C virus.
The hydrophilic nature of UDCA may confer cytoprotection in
necroinflammatory diseases of the liver. UDCA also significantly improves
transaminases and cholestatic enzymatic indices of liver injury in chronic
hepatitis
and alleviates alcoholic fatty liver. Bile salt deficiency, and consequently
reduced
cholesterol solubility in bile, may play a role in the pathogenesis of
cholesterol
gallstones.
Bile acids may also have significant therapeutic value in treating a number of
other conditions including those that affect the heart and the
gastrointestinal tract. For
example, UDCA has a vasodilative effect on the systemic vascular bed, but
altered
neither pulmonary vascular function nor cardiac functions. Regarding the
gastrointestinal tract, bile acids substantially inhibit the growth of H.
pyloYi.
In spite of the potentially valuable medical uses of bile acids as
therapeutically
active agents and as carriers and/or adjuvants, commercial use of bile acids
is limited
to pharmaceutical formulations with a solid form of bile acid which are in
tablet,
capsule and suspension. This is due to the insolubility of bile acids in
aqueous media
at pH from approximately 1 to 8. This is also due to bile's extremely bitter
taste and
equally bitter after-taste which lasts several hours. The few aqueous dosage
forms
that are available are unstable, and have very limited uses because of pH
control and
maintenance problems. Moreover, some commercial pharmaceutical dosage forms of
bile acids have been shown to have scant bioavailability. This is even true of
solid
bile acid forms.
Therefore, a need has arisen for an liquid and solid bile acid formulation
that
are (liquids) or form (solids) clear, aqueous solutions.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
SUMMARY OF THE INVENTION
Bile acid compositions may be advantageously stored or administered in a dry
or solid form. Thus, the present invention relates to dry or solid
preparations of bile
acids that form clear or particulate-free solutions upon exposure to water.
Dry or
5 solid forms of the invention may be prepared from clear or particulate-free
solutions
of bile acids ("parent solutions"). The present invention also relates to
methods for
preparing and/or solubilizing such dry or solid forms. Advantages of these
formulations include improved bioavailability, plasma bioavailability, and
absorbability of a bile acid. Additional advantages of formulations of the
invention
include improved bioavailability, plasma bioavailability and absorbability of
one or
more pharmaceutical compounds.
In some preferred embodiments, a dry or solid preparation of the invention
exposed to water results in a solution comprising (1) a bile acid, its
derivative, its salt,
or its conjugate witll an amine, (2) water, and (3) a sufficient quantity of
an aqueous
soluble starch conversion product such that the bile acid and the starch
conversion
product remain in solution at any pH within a selected pH range. According to
some
preferred embodiments, a dry or solid preparation of the invention exposed to
water
results in a solution comprising (1) a bile acid, its derivative, its salt, or
its conjugate
with an amine, (2) water, and (3) a sufficient quantity of an aqueous soluble
non-
starch polysaccharide and an aqueous soluble starch conversion product such
that the
bile acid and the polysaccharide remain in solution at any pH within a
selected pH
range.
Dry or solid forms of the invention exposed to water may result in solutions
further comprising resistant maltodextrin, an aqueous soluble ginseng extract,
a
pharmaceutical compound in a pharmaceutically appropriate amount, an aqueous
soluble bismuth compound, or combinations thereof. Where the solution
comprises
one or more such materials, the solution composition may be adjusted to ensure
that
these materials remain in solution.
Dry or solid preparations of the invention may comprise one or more
disintegrants. In some embodiments of the invention, solution formulations of
bile
acid compositions comprise a disintegrants in order to facilitate breakup or
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
6
disintegration of its dried forms after administration. The pH of solutions of
the
invention may be adjusted with high throughput sonication by acid. In some non-
limiting embodiments of the invention, high throughput sonication accelerates
solubilization of dry or solid bile acid preparations.
Dry or solid forms of the invention are prepared from clear or particulate-
free
parent solutions. In some embodiments of the invention, dried forms derived
from the
solution formulations of bile acid compositions may be granulated by the
method of
granulation evolved from the fluid-bed drying technology. In this system a
soluble
fiber solution (granulating solution) is sprayed into or onto the suspended
dried forms,
which then would be dried rapidly in the suspending air.
In some embodiments of the invention, a composition is provided which
coinprises (1) a bile acid, its derivative, its salt, or its conjugate with an
amine, (2)
water, and (3) a sufficient quantity of carbohydrate such that the bile acid
component
and the carbohydrate remain in solution at any pH within a selected pH range,
wlierein the carbohydrate is a combination of an aqueous soluble starch
conversion
product and an aqueous soluble non-starch polysaccharide. In embodiments
containin.g both soluble non-starch polysaccharide and high molecular weight
starch
conversion product, the amounts of each are such that when combined together
in the
composition they are sufficient to allow the bile acid component, the high
molecular
weight starch conversion product, the soluble non-starch polysaccharide and
the
pharmaceutical compound, if any, to remain in solution at any pH within a
selected
pH range.
In some embodiments of the invention, a combination therapy composition is
provided which may increase the intensity of a response to or efficacy of a
pharmaceutical. Such a composition may permit administration of lower dosages
of a
pharmaceutical compound, attack a disease complex at different points, affect
elimination and/or alter absorption of a pharmaceutical compound. Such a
composition may lead to or contribute to a reduction in toxicity and/or side
effects of
a pharmaceutical.
In some embodiments, bile solutions of the invention are dried. The invention
further relates to dried fozms derived from the solution formulations of bile
acid
compositions by lyophilization, evaporation, or any other means of dehydration
known in the art. The solutions may be partially dried to produce a semi-solid
forms.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
7
The solutions may be thoroughly dried to form a solid, powder and granule.
Dried
forms of the aqueous solutions may be substantially free of water. Dried forms
may
be dried by fluid process, tray process, spray process, and freezing process.
Dried
forms may be administered directly, as solid dosage forms or combined with
water
prior to administration.
The invention further relates to a method of treating or preventing a human or
animal disease comprising administration of a composition of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
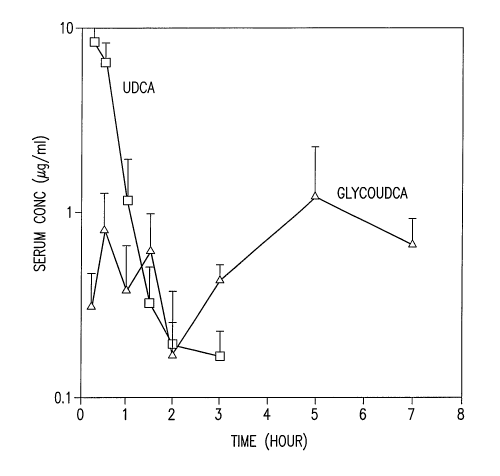
Figure 1: Graph of blood serum - concentration of UDCA (squares) and
GUDCA (triangles) versus time following administration of dosage formulations
according to Examples II and VI and Table 4.
Figure 2: Graph of blood serum concentration of UDCA versus time following
administration of dosage fonnulations of the bile acid according to Examples
III and
VI and Table 4.
Figure 3: Diagram of the mean (n=5) for group I for pharmacokinetic
parameters of UDCA in human after an oral administration of liquid formulation
of
UDCA prepared according to Example IX witliout bismuth.
Figure 4: Diagram of the mean (n=5) for group II for pharmacokinetic
parameters of UDCA in human after an oral adininistration of liquid
formulation of
UDCA prepared according to Example IX.
Figure 5A. Transmission electron micrograph of H. pylof=i cultured from
Columbia medium.
Figure 5B. Transmission electron micrograph of H. pylori 48 hrs after being
treated with UDCA & bismuth citrate prepared according to Example IX.
Figure 5C. Transmission electron micrograph of H. pylori 72 hrs after being
treated with UDCA & bismuth citrate.
Figure 6: NMR data for UDCA in a liquid formulation dosage form prepared
according to Example III without preservatives, flavoring agent, and
sweetener.
Figure 7: HPLC trace of UDCA in a liquid formulation dosage form prepared
according to Example III without preservatives, flavoring agent, and
sweetener.
Figure 8: HPLC trace of a UDCA standard.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
8
Figure 9: H. pyloYi culture method.
Figure 10: H. pylori culture method.
Figure 11: H. pylori culture method.
Figure 12: Plot of pH vs. transparency of secondary aqueous solubilized bile
acid solution dosage formulations prepared according to Example XIX with
regard to
the redissolution of dried form derived from a primary aqueous solubilized
bile acid
formulation within 2 minute. The primary solutions, 100 mL each, comprised 200
mg
UDCA and (A) 4 g, (B) 5 g, (C) 6 g, (D) 7g, (E) 8g or (F) 9 g of maltodextrin
(DE=15)..
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to an aqueous solution comprising (i) one or
more bile acids selected from the group consisting of a soluble bile acid, an
aqueous
soluble bile acid derivative, a bile acid salt, or a bile acid conjugated with
an amine,
(collectively "bile acid"), (ii) water, and (iii) one or more aqueous soluble
starch
conversion products or aqueous soluble non-starch polysaccharide in an amount
sufficient to produce a solution which does not form a precipitate at any pH
within a
desired pH range.
The composition may contain a bile acid or its salt which itself has
pharmaceutical effectiveness. Formulations of the invention may act as a
carrier, an
adjuvant or enhancer for the delivery of a pharmaceutical material which
remains
dissolved in the composition of the invention across the desired pH range. In
some
embodiments of the invention, a non-bile acid pharmaceutical is used though
not
necessarily in solution.
It is an advantage of this invention that the bile acid and the carbohydrate
remain in solution without precipitation at any pH from acidic to alkaline.
These
aqueous solution systems of bile acid are absolutely free of precipitate or
particles. A
further advantage of this invention is that the aqueous solution systems
demonstrate
no changes in physical appearance such as changes in clarity, color or odor
following
the addition of strong acids or alkali even after several months of
observation under
accelerated conditions of storage at 50 C.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
9
In some embodiments of the invention, an aqueous solution system of bile
acid is administered orally whereupon it moves through the gastrointestinal
track
without precipitation of bile acids as solids by exposure to acidic gastric
juices and
into the alkaline environment of the intestine. These formulations demonstrate
that
intact bile acid solution systems in the gastro-intestinal tract can be
effectively and
completely absorbed.
According to the invention, bile acid solubility (e.g. precipitation and
changes
in physical appearance) is unaffected by whether a carboxylic acid side chain
of
certain bile acids can be protonated (non-ionized) or ionized or is a simple
carboxylic
acid. The ionization state of a bile acid carboxylic acid side chain greatly
affects the
ability of micelle formation by the bile acid in these aqueous solution
systems. In
some embodiments of the invention, that ionization state is manipulated by
adjusting
the pH to control the micelle formation of bile acids with a drug in order to
use these
aqueous solution systems as a therapeutically active agent, as an adjuvant of
a drug, as
a carrier of drug or as an enhancer of drug solubility. These aqueous solution
systems
may be prepared for oral consumption, enemas, mouthwashes, gargles, nasal
preparations, otic preparations, injections, douches, topical skin
preparations, other
topical preparations, and cosmetic preparations which have a desired pH
without the
disadvantage of precipitation or deterioration in physical appearance after
long
periods of time.
Soluble bile acids are any type of aqueous soluble bile acids. A bile acid
salt
is any aqueous soluble salt of a bile acid. The soluble bile acid derivatives
of this
invention are those derivatives which are as soluble as or more soluble in
aqueous
solution than is the corresponding underivatized bile acid. Bile acid
derivatives
include, but are not limited to derivatives formed at the hydroxyl and
carboxylic acid
groups of the bile acid with other functional groups including but not limited
to
halogens and amino groups. Aqueous dissolved salts of bile acids may be formed
by
the reaction of bile acids described above and an amine including but not
limited to
aliphatic free amines such as trientine, diethylene triamine, tetraethylene
pentamine,
and basic amino acids such as arginine, lysine, ornithine, and amino sugars
such as
D-glucamine, N-alkylglucamines, and quaternary ammonium derivatives such as
choline, heterocyclic amines such as piperazine, N-alkylpiperazine,
piperidine,
N-alkylpiperidine, morpholine, N-alkylmorphline, pyrrolidine, triethanolamine,
and
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
trimethanolamine. According to the invention, aqueous soluble metal salts of
bile
acids and aqueous soluble 0-sulfonated bile acids are also included as soluble
bile
acid salts.
Bile acids of the invention may be selected from the group consisting of
5 chenodeoxycholic acid, cholic acid, hyodeoxycholic acid, deoxycholic acid,
7-oxolithocholic acid, lithocholic acid, iododeoxycholic acid, iocholic acid,
tauroursodeoxycholic acid, taurochenodeoxycholic acid, taurodeoxycholic acid,
taurolithocholic acid, glycoursodeoxycholic acid, taurocholic acid,
glycocholic acid,
and their derivatives at a hydroxyl or carboxylic acid group on the steroid
nucleus. In
10 addition, bile acids of the invention may be selected from primary,
secondary, and
tertiary bile acids.
UDCA is practically insoluble at pH < 7. The pKa of UDCA is 5.1, and the
solubility of its protonated form is 9 mol/L. The solubility of UDCA in the
solution
formulation is about 100 mg/ml which is equivalent to almost 30,000 folds of
intact
UDCA's solubility. Therefore, the major advantage of the instant invention is
that by
delivery of solubilized bile acid in solution, it achieves high in vivo levels
of bile acids
(bile, blood, etc.) far beyond other preparations. Therefore, the therapeutic
potential
of bile acid may be more fully achieved on the ground of high concentration of
proper
bile acid in each lesion by systemic supply than previous formulations. The in
vivo
levels of bile acids attainable with existing formulations in which bile is
incompletely
and slowly solubilized are lower. Moreover, its low absorption and
enterohepatic
circulatory action result from non-detection of therapeutically active bile
acid such as
UDCA in the blood. Since bile acid is completely dissolved in the inventive
formulations, higher in vivo levels of bile acid may be achieved, even though
lower
doses are administered.
In some embodiments of the invention, pluralities of bile acids are used in a
single formulation. Mixtures of two or more bile salts of differing
hydrophobic
activity may behave as a single bile salt of an intermediate hydrophobic
activity. As a
result, mixtures of two or more bile salts may have the unique physiological
advantages such as increased detergent properties and lowered toxicity than
the
individual bile acids.
Carbohydrates suitable for use in the invention include aqueous soluble starch
conversion products and aqueous soluble non-starch polysaccharides. Aqueous
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
11
soluble starch conversion products may be obtained under various pH conditions
from
the partial or incomplete hydrolysis of starch. They may also have a Dextrose
Equivalent (DE) of from about 4 to about 40. DE is a quantitative measure of
the
degree of starch polymer hydrolysis. It is a measure of reducing power
compared to a
dextrose standard of 100. The higher the DE, the greater the extent of starch
hydrolysis implies. As the product is further hydrolyzed (higher), the average
molecular weight decreases. Non-limiting examples include maltodextrin,
dextrin,
liquid glucose, corn syrup solid (dried powder of liquid glucose), and soluble
starch,
preferably maltodextrin or corn syrup solid, most preferably corn syrup solid.
For the
purpose of this invention, the term "corn syrup" includes both corn syrup and
liquid
glucose. Aqueous soluble non-starch polysaccharides may be aqueous soluble
fiber
such as guar gum, pectin, psyllium, oat gum, soybean fiber, oat bran, corn
bran,
cellulose and wheat bran.
The amount of high molecular weight aqueous soluble starch conversion
product used in the invention is at least the amount needed to render the
chosen bile
acid salt soluble in the concentration desired and in the pH range desired.
The
approximate minimal quantity of maltodextrin required to prevent the
precipitation of
bile acids from the aqueous solution formulations of the invention depended on
its DE
value. In preferred embodiments of the invention, the approximate minimal
quantity
of maltodextrin which has 15-25 DE value such as MaltrinOM150, Maltrin M180,
Maltrin M200, Maltrin M250 (corn syrup solid), liquid glucose, and soluble
starch
required to prevent the precipitation of bile acids from the aqueous solution
formulations of the invention is approximately 30 g for CDCA, approximately 5
g for
UDCA, approximately 12 g for 7-ketolithocholic acid (KLCA), approximately 10 g
for cholic acid, approximately 50 g for deoxycholic acid, approximately 3.5 g
for
hyodeoxycholic acid for every 0.2 g of bile acid. In preferred embodiments of
the
invention, the approximate minimal quantity of a maltodextrin (DE 5-10), such
as
Maltrin M040, Maltrin M100 is approximately 18 g for CDCA, approximately 3 g
for UDCA, approximately 7g for 7-ketolithocholic acid, approximately 6 g for
cholic
acid, approximately 30 g for deoxycholic acid, approximately 2.1 g for
hyodeoxycholic acid for every 0.2 g of bile acid.
Digestion resistant maltodextrin is an aqueous soluble dietary fiber. This
soluble resistant maltodextrin is produced from corn starch (similar to the
process to
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
12
manufacture conventional maltodextrin) to purposefully convert a portion of
the
normal alpha -1,4 glucose linlcages to random 1,2-, 1,3-, and 1,4- alpha or
beta
linkages. The human digestive system effectively digests only alpha 1,4-
linkages;
therefore the other linkages render the molecules resistant to digestion.
Thus, other
linlcages created are not absorbed in the small intestine and passed on to the
large
intestine. This resistant maltodextrin is partially fermented in the large
intestine with
the fractions that aren't utilized excreted. This aqueous soluble maltodextrin
helps
maintain norinal, healthy levels of serum cholesterol, blood triglycerides,
blood
glucose level, intestinal regularity, and intestinal microflora. In some
embodiments of
the invention, a solution formulation may coinprise an aqueous soluble
digestion
resistant maltodextrin.
In some embodiments of the invention, a formulation may comprise
cyclodextrin.
Drugs substances most frequently are administered orally by means of solid
dosage forms such as powder, dried granular mass, tablets and capsules. The
solid
dosage forms can facilitate handling, enhance the physical appearance and
improve
stability. In many cases, it has been shown that a drug substance's solubility
and
other physicochemical characteristics influence its physiological availability
from a
solid dosage form. Dried form contains aqueous soluble bile acid, readily
soluble
high molecular weight starch conversion product, and disintegrants, which are
easy
for solubilization. Increased solubility of bile acid leads to the increased
rate of
dissolution. As a result, the rate of absorption may be increased greatly by
the
increased rate of dissolution.
Compositions of the invention may further comprise a disintegrant to
facilitate
brealcup or disintegration of a dry or solid form after administration.
Disintegrants
may be starches such as Veegum HV, methylcellulose, agar, bentonite, natural
sponge, cation exchange resins, alginic acid, guar gum, citrus pulp, and
carboxymethylcellulose, clays, celluloses, aligns, gums, and cross-linked
polymers
(crospovidone), cross-linked cellulose (Croscarmelose), and cross-linked
starch
(sodium starch glycolate). The disintegrating function is due to capillary
action rather
than swelling. In general, the aqueous soluble disintegrants may be mixed with
the
active ingredients prior to drying. In case of aqueous insoluble
disintegrants, 5%
starch by weight, may be added to the powder blends in the dry state. If more
rapid
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
13
disintegration is desired, this amount may be increased to 10 or 15%. Sodium
starch
glycolate at 2 to 4% swells 7-fold to 12-fold in less than 30 seconds and
Croscarmelose swells 4-fold to 8-fold in less than 10 seconds.
The evolution of carbon dioxide is an effective way to cause fast dissolution
of
dried forms derived from the solution formulations of bile acid compositions.
Dried
forms containing a mixture of sodium bicarbonate and an acidulant such as
tartaric or
citric acid will effervesce when added to water. The amount of sodium
bicarbonate
may be about ten times the amount of bile acid. The amount of acidulant may be
twenty percent more than the amount of sodium bicarbonate. Sufficient acid is
added
to produce a neutral or slightly acidic reaction when dissolution in water is
rapid and
complete.
The invention further relates to the preparation of solution formulations
derived from bile acid compositions. High throughput sonication with or
without
heating at about 60 C may be useful in solubilizing dry or solid preparations
of the
invention. A high throughput sonication system may be used to drive
precipitated
compounds back into solution during preparation of solution formulations. The
effects of sonication time, power, and amplitude have been optimized in order
to drive
compounds back into solution. Sonicator that generate sound, energy at 20 kHz
from
0-1150 watts may be used in forming clear aqueous solutions of the invention.
Dried forms may be prepared from parent solutions by wet granulation, dry
granulation and fluid-bed granulation. When ingredients have sufficient
inherent
binding or cohesive properties, dry granulation method (slugging) may be used
to
make granules. The general steps of wet and dry granulation are weighing,
mixing,
granulation (slugging), and screening. Fluid bed granulation may be performed
by
spraying a granulating solution or solvent into or onto the bed of suspended
particles,
followed by rapid drying in suspending air. In these systems, suspended
particles,
which are dried forms derived from parent solutions, may be coated with
granulating
solution or solvent which contains enteric polymers. The enteric polymers may
comprise cellulose acetate phthalate (CAP), which is capable of functioning
effectively as an enteric coating at pH greater than 6, polyvinyl acetate
phthalate
(PVAP), methacrylic acid-methacylic acid ester copolymers, cellulose acetate
trimellitate (CAT), carboxymethyl ethylcellulose (CMEC), and hydroxylpropyl
methylcellulose acetate succinate (HPMCAS). This granulated form with those
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
14
enteric polymers remains intact in the stomach but will dissolve and release
the active
ingredient once it reaches the intestine and colon.
Spheronization, a form of pelletization, refers to the formation of spherical
particles (spheres) from wet granulation or fluid bed granulation. Rod shaped
cylindrical segments ranging in diameter from 500 microns to 12 millimeters
may be
prepared through an extruding machine. After extrusion the segments are placed
into
the Marumerizer where they are shaped into spheres by centrifugal and
frictional
forces on a rotating plate. The pellets are dried and then coated. In some
embodiments of the invention, dried forms of the solution formulations of bile
acid
compositions may be prepared by spheronization process and then coated with
the
enteric polymers.
The selected pH range for which a formulation will not precipitate its bile
acid, starch conversion product, soluble non-starch polysaccharide or its
pharmaceutical compound may be any range of pH levels obtainable with an
aqueous
system. Preferably this range is between about pH 1 and about pH 14 and more
preferably between about pH 1 and about pH 10. Still more preferably the range
is
any subset of the range of pH levels obtainable in an aqueous system
sufficient for the
pharmaceutical formulation to remain in solution from preparation, to
administration,
to absorption in the body, according to the method of administration. In some
embodiments of the invention, a bile acid remains dissolved under acidic
conditions
as a free bile acid in spite of the general insolubility of bile acids under
acidic
conditions. In some embodiments of the invention, the composition may be used
as a
pharmaceutical formulation wherein the pharmaceutical compound remains in
solution without precipitation at prevailing pH levels in the mouth, stomach
or
intestines.
The invention contemplates the use of a broad range of pharmaceutical
materials. Non-limiting examples include hormones, hormone antagonists,
analgesic,
antipyretics, antiinflammatory drugs, immunoactive drugs, antineoplastic
drugs,
antibiotics, anti-inflammatory agents, sympathomimetic drugs, anti-infective
drugs,
anti-tumor agents, and anesthetics. Further non-limiting examples include
drugs that
target or effect the gastrointestinal tract, liver, cardiovascular system, and
respiratory
system. Further non-limiting examples of pharmaceutical compounds include
insulin,
riluzole, heparin, calcitonin, ampicillin, octreotide, sildenafil citrate,
calcitriol,
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
dihydrotachysterol, ampomorphine, yohimbin, trazodone, acyclovir,
amantadine=HCI,
rimantadine=HCI, cidofovir, delavirdine=mesylate, didanosine, famciclovir,
forscarnet
sodium, fluorouracil, ganciclovir sodium, idoxuridine, interferon-oc,
interferon-(3,
interferon-y, lamivudine, nevirapine, penciclovir, ribavirin, stavudine,
trifluridine,
5 valacyclovir=HCI, zalcitabine, zidovudine, indinavir=H2SO4, ritonavir,
nelfinavir=CH3SO3H, saquinavir=CH3SO3H, d-penicillamine, chloroquine,
hydroxychloroquine, aurothioglucose, gold sodium thiomalate, auranofin
levamisole,
DTC, isoprinosine, methyl inosine monophosphate, muramyl dipeptide, diazoxide,
hydralazine=HCI, minoxidil, dipyridamole, isoxsuprine=HCI, niacin,
nylidrin=HCI,
10 phentolamine, doxazosin=CH3SO3H, prazosin=HCI, terazocin=HCI,
clonidine=HCI,
nifedipine, molsidomine, amiodarone, acetylsalicylic acid, verapainil,
diltiazem,
nisoldipine, isradipine, bepridil, isosorbide=dinitrate,
pentaerythrytol=tetranitrate,
nitroglycerin, cimetidine, famotidine, nizatidine, ranitidine, lansoprazole,
omeprazole,
misoprostol, sucralfate, metoclopramide=HCI, erythromycin, bismuth compound,
15 alprostadil, albuterol, pirbuterol, terbutaline=H2SO4, salmetrol,
aminophylline,
dyphylline, ephedrine, ethylnorepinephrine, isoetllarine, isoproterenol,
metaproterenol, n=docromil, oxy triphylline, theophylline, bitolterol,
fenoterol,
budesonide, flunisolide, beclomethasone=dipropionate, fluticasone=propionate,
codeine, codeine sulfate, codeine phosphate, dextromethorphan=HBr,
triamcinolone=acetonide, montelukast sodium, zafirlukast, zileuton, cromolyn
sodium,
ipratropium bromide, nedocromil sodium benzonate, diphenhydramine=HCI,
hydrocodone=bitartarate, metliadone=HCI, morphine sulfate, acetylcysteine,
guaifenesin, ammonium carbonate, ammonium chloride, antimony potassium
tartarate, glycerin, terpin=hydrate, colfosceril palmitate,
atorvastatin=calcium,
cervastatin=sodium, fluvastatin=sodium, lovastatin, pravastatin=sodium,
simvastatin,
picrorrhazia kurrva, andrographis paniculata, moringa oleifera, albizzia
lebeck, adhata
vasica, curcuma longa, momordica charantia, gymnema sylvestre, terminalia
arjuna,
azadirachta indica, tinosporia cordifolia, metronidazole, amphotericin B,
clotrimazole,
fluconazole, haloprogin, ketoconazole, griseofulvin, itraconazole,
terbinafin=HCl,
econazole=HN03, miconazole, nystatin, oxiconazole=HN03, sulconazole-HNO3,
cetirizine=2HC1, dexamethasone, hydrocortisone, prednisolone, cortisone,
catechin
and its derivatives, glycyrrhizin, glycyrrhizic acid, betamethasone,
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
16
ludrocortisone-acetate, flunisolide, fluticasone-propionate, methyl
prednisolone,
somatostatin, lispro, glucagon, proinsulin, insoluble insulins, acarbose,
chlorpropamide, glipizide, glyburide, metformin-HC1, repaglinide, tolbutamide,
amino
acid, colchicine, sulfinpyrazone, allopurinol, piroxicam, tolmetin sodium,
indomethacin, ibuprofen, diflunisal, mefenamic acid, naproxen, and trientine,
vitamin
E, vitamin C, superoxide dismutase (SOD), N-acetylcysteine, 21-aminosteroid
such as
lazaroids, U74389F and U74006F, catalase (CAT), putrescine-modified catalase
(PUT-CAT), estrogen, alpha-lipoic acid, selegiline, desferrioxanmine, d,l-
penicillamine, alpha and beta-carotene, retinol, selenium, gingko biloba,
riluzole,
flupirtine, pifithrin-alpha, CGP 3466B/TCH346, CPI-1189, CEP-1347, and
coenzyme
Q10.
Bile acid compositions of the invention may also comprise ginseng. Ginseng
contains vitamins A, B-6 and the mineral Zinc, which aids in the production of
thymic
hormones, necessary for the functioning of the defense system. The main active
ingredients of ginseng are the more than 25 saponin triterpenoid glycosides
called
"ginsenosides." These steroid-like ingredients provide the adaptogenic
properties that
enable ginseng to balance and counter the effects of stress. The glycosides
appear to
act on the adrenal glands, helping to prevent adrenal hypertrophy and excess
corticosteroid production in response to physical, chemical or biological
stress.
Pharmacological effects of ginseng have been demonstrated in the CNS and in
cardiovascular, endocrine, and immune systems. In addition, ginseng and its
constituents have been ascribed antineoplastic, antistress, and antioxidant
activity. It
is an herb with many active components, and there is evidence from numerous
studies
that ginseng does have beneficial effects. Ginseng has demonstrated the
combined
effects with various oriental medicines to increase intensity of response or
efficacy, to
decrease individual toxicity, to antagonize untoward actions and to alter
absorption
for long periods.
Pharmacological effects of ginseng have been demonstrated in the CNS and in
cardiovascular, endocrine, and immune systems. In addition, ginseng and its
aqueous
soluble constituents have been ascribed antineoplastic, anti-stress, and
antioxidant
activity. In some embodiments of the invention, solution formulations may
comprise
aqueous soluble ginseng (white and red) extract.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
17
Thus, the invention contemplates the use of a broad range of pharmaceutical
materials. Any pharmaceutical material that becomes and/or remains soluble in
formulations of the invention may be used. With an additional pharmaceutical
compound in the formulation, a bile acid in solution may act as an adjuvant,
carrier, or
enhancer for the solubility of certain therapeutically active agents,
including, but not
limited to, insulin (pH 7.4-7.8), heparin (pH 5-7.5), calcitonin, ampicillin,
amantadine, rimantadine, sildenafil, neomycin sulfate (pH 5-7.5), apomorphine,
yohimbin, trazodone, ribavirin, paclitaxel and its derivatives, retinol, and
tretinoin,
which are soluble and stable in acid and/or alkali and can be added as needed
into
these aqueous solution formulations of certain concentrations of bile acids in
this
invention. Certain therapeutically active agents, including, but not limited
to,
metformin HCl (pH 5-7), ranitidine HC1, cimetidine, lamivudine, cetrizine
2HC1(pH
4-5), amantadine, rimantadine, sildenafil, apomorphine, yohimbine, trazodone,
ribavirin and dexamethasone, hydrocortisone, prednisolone, triamcinolone,
cortisone,
niacin, taurine, vitamins, naturally occurring amino acids, catechin and its
derivatives,
glycyrrhizal extract and its main constituents such as glycyrrhizin and
glycyrrhizic
acid, water soluble bismuth compounds (e.g., bismutll sodium tartrate), and
which are
soluble and stable in acid and/or alkali can be added as needed into these
aqueous
solution dosage formulations containing ursodeoxycholic acid in this
invention.
According to the invention bismuth compounds comprise an aqueous soluble
reaction product between a bismuth ion and a chelator. Non-limiting examples
of
such chelators include citric acid, tartaric acid, malic acid, lactic acid and
eidetic acid.
Non-limiting examples include of bismuth citrate, bismuth sulfate, bismuth
subnitrate,
bismuth subcarbonate, bismuth subsalicylate, and bismuth gallate.
The invention contemplates the use of pH adjustable agents. Non-limiting
examples include HCI, H2SO4, HNO3, CH3COOH, citric acid, malic acid, tartaric
acid, lactic acid, phosphate, eidetic acid and alkalies.
In some embodiments of the invention, the formulations may be used to treat
human and mammalian diseases. The invention contemplates treating
gastrointestinal
disorders, liver diseases, gall stones, and hyperlipidemia. Non-limiting
examples of
liver diseases include alcohol-induced liver diseases and non-alcohol-induced
liver
diseases. Non-limiting examples of gastrointestinal disorders include chronic
gastritis, reflux gastritis, and peptic ulcer disease. Non-limiting examples
of non-
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
18
alcohol-induced liver diseases include primary biliary cirrhosis, acute and
chronic
hepatitis, primary sclerosing cholangitis, chronic active hepatitis, and
excess
accumulation of fat in the liver. The invention further contemplates treating
viral,
bacterial and fungal diseases. In some embodiments of the invention, a
fonnulation is
administered to treat and/or eradicate Helicobacter pylori infection. In some
embodiments of the invention, a formulation is administered to treat and/or
eradicate
hepatitis C virus infection, influenza A, Influenza C, parainfluenza 1,
sendai, rubella,
and pseudorabies virus. In some embodiments of the invention, a formulation is
administered to treat acute or chronic inflammatory diseases. Non-limiting
examples
of inflammatory diseases include bronchitis, chronic pharyngitis, and chronic
tonsillitis. In some embodiments of the invention, a formulation is
administered to
treat hypercholersterolemia.
In some embodiments of the invention, the formulation is modified such that it
may be administered as a liquid, solid, powder or tablet. In some embodiments
of the
invention, the formulation is comprised in a syrup, thick syrup or paste. A
non-
limiting example of a syrup is a solution of maltodextrin wherein the
concentration of
maltodextrin is less than 1.0 kg/L. A non-limiting example of thick syrup is a
solution of maltodextrin wherein the concentration of maltodextrin is between
1.0
kg/L and 1.2 kg/L inclusive. A non-limiting example of a paste is a solution
of
maltodextrin wherein the concentration of maltodextrin is greater than 1.2
kglL.
The aqueous solutions of the invention may be dried. For the purpose of this
disclosure, a "primary" aqueous solution bile acid dosage formulation
according to
the invention is produced by the original combination of a bile acid or its
salts and a
carbohydrate with water. It may be prepared by a simultaneous or stepwise
combination of ingredients. A "secondary" aqueous solution bile acid dosage
formulation, by contrast, is a solution prepared from a powder or solid
comprising
previously co-dissolved bile acid and carbohydrate. Thus, a secondary aqueous
solution bile acid dosage formulation differs at least in that water has been
added,
removed, and added again.
In some embodiments of the invention, a primary aqueous solution bile acid
dosage formulation is dried by spray-drying. Spray-drying consists of bringing
together a highly dispersed liquid and a sufficient volume of hot air to
produce
evaporation and drying of the liquid droplets. The feed liquid may be a
solution,
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
19
slurry, syrup or paste provided it is pumpable and capable of being atomized.
The
liquid feed is sprayed into a current of warm filtered air. The air supplies
the heat for
evaporation and conveys the dried product to the collector; the air is then
exhausted
with the moisture. The spray-dried powder particles are homogeneous,
approximately
spherical in shape, nearly uniform in size, and frequently hollow. The latter
characteristic results in low bulk density with a rapid rate of solution. This
process is
useful in coating one material on another to protect the interior substance or
to control
the rate of its release. For example, dried form of an aqueous solution bile
acid
dosage formulation can be coated with enteric polymers for the colonic
delivery of
solubilized UDCA. Dehydration may also be accomplished with lyophilization,
evaporation or any other dehydration technique known in the art.
The resulting dried fonn, e.g. powder or solid, may be administered directly
or
recombined with water to produce a secondary clear aqueous solution bile acid
dosage
formulation. Secondary aqueous solution bile acid dosage formulations, i.e.
those
produced from dried forms, have substantially the same properties as primary
formulations.
The invention contemplates the addition of additives such as pharmaceuticals
to primary and secondary aqueous bile acid solutions as well as to dried
forms. If
administered in dried form, the dried material may be combined with one or
more
diluents, lubricants, binders, fillers, drugs, disintegrants or other
additives. Thus, the
dried form may be comprised in a powder, granule, a pill, tablet or capsule.
The stability of dosage formulations of the invention were evaluated by
measuring the concentration of the relevant bile acid over time in
preparations
comprising soluble bile acid, a high molecular weight aqueous soluble starch
conversion product, and water at various pH and temperature levels. The
stability
tests were performed with HPLC and microscope light at various pH conditions
under the normal and accelerated conditions. Solution stability tests included
concentration analyses for each bile acid, performed by HPLC as follows: the
elution
solvent was 0.02 M KH2PO4:acetonitrile in a ratio of 55:45, with a pH of 3.01;
the
flow rate was 0.8 milliliters/minute; the injection volume was 20 L, and the
detection wave length was 195 nm. The retention time of each bile acid may be
adjusted as needed to permit individual analysis of each bile acid present in
a sample
having a plurality of bile acids. In the tables, the concentration of the
indicated bile
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
acid salt for each of the three numbered trials and the average thereof is
reported on
each line. The percentage indicates the relative concentration of the bile
acid salt
after incubation for a certain amount of time in comparison with the initial
concentration.
5 Accelerated conditions for testing pharmaceutical compositions have been
described (Remington, The Science and Practice of Pharmacy, 19th ed., p. 640).
All
of these stability test results were satisfactory in that the concentration of
bile acid as
measured by HPLC did not change appreciably over time at various pH levels.
Thus,
the formulations of the examples are suitable for preparing a commercial
liquid
10 dosage form. Particularly, all solution formulations which contained bile
acid showed
excellent results in the stability tests with no precipitation and no physical
appearance
changes over the test period. Some formulations remain stable for over 2
years.
Stability tests were also conducted on the aqueous solution formulations
comprising a mixture of aqueous soluble UDCA, branched chained amino acid
15 (leucine, isoleucine, valine) and maltodextrin according to example IV.
This
formulation may be typical of solution formulations in which bile acid
functions as a
therapeutically active agent, as an adjuvant or carrier, pharmaceutically
active agent,
and a solubility enhancer. According to the test results, there were no
clarity changes,
no discoloration, and no precipitation. Furthermore, there are no detectable
impurities
20 from the deterioration of UDCA or branched chained amino acids when
examined by
HPLC at various pH conditions such as pH 1, 3, 5, 7, 9, and 10 under the
accelerated
conditions (e.g. incubation at 50 C).
The aqueous solution formulations according to this invention did not change
either physically or chemically at various pH conditions under the accelerated
conditions despite the addition of therapeutically and chemically active
agents that are
stable and soluble in hydrochloric acid solution. Therefore, these aqueous
solution
systems may be extremely valuable pharmaceutical dosage forms for delivery of
therapeutically active bile acids, and/or drugs. Without being limited to any
particular
mode of action, in drug (pharmaceutical compound) delivery preparations, bile
acids
may function as an adjuvant, a carrier, or a solubility enhancer (e.g. by
micelle
formation).
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
21
EXAMPLES
Stability tests were conducted on three different aqueous solution systems.
First, a bile acid and a high molecular weight aqueous soluble starch
conversion
product were combined in aqueous solution according to Example I and tested.
Results are shown in Tables IA and 1B. Second, mixed bile acids and high
molecular
weight aqueous soluble starch conversion products were combined in aqueous
solution according to Example II and tested. Results are shown in Table 2.
Third,
bile acids, higll molecular weight aqueous soluble starch conversion products
and
branched chained amino acids (e.g. leucine, isoleucine, valine, or other amino
acid
with a branched side chain) were combined in aqueous solution according to
Example
IV and tested. Results are shown in Tables 3A through 3F.
EXAMPLE I
A first series of solution formulations that were prepared with soluble bile
acids (as
free acids) and high molecular weight aqueous soluble starch conversion
products
according to the following guidelines did not show any precipitation at any pH
tested.
Soluble Bile Acid Starch Conyersion Product (Minimum~
if 200 mg CDCA about 30 g
if 200 mg UDCA about 5 g
if 200 mg KLCA about 12 g
if 200 mg cholic acid about 10 g
if 200 mg deoxycholic acid about 50 g
if 200 mg hyodeoxycholic acid about 3.5 g
Purified water to make 100 mL
Aqueous solutions (100 mL) in which one of the above soluble bile acids is
dissolved were prepared. Maltodextrin, as one high molecular weight aqueous
soluble starch conversion product, was dissolved with agitation about 60-80 C
to
make a clear solution. The pH of this resulting clear solution was adjusted by
acid to
prepare oral dosage forms, topical preparations, and solutions. Purified water
was
added to make the total volume be 100 mL. According to the instant invention
and all
examples, purified water is deionized, distilled deionized-distilled water, or
any grade
commonly used for pharmaceutical preparations.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
22
Based on these formulas, aqueous solution formulations with various
concentrations of certain bile acids (or salts) and their corresponding
minimal
quantities of high molecular weight aqueous soluble starch conversion products
that
have a DE of 15-25 (e.g. MaltrinOM150 (DE = 15), MaltrinOM180 (DE = 18),
MaltrinOM200 (DE = 20), MaltrinOM250 (corn syrup solid; DE = 25), liquid
glucose) or soluble non-starch polysaccharides (e.g. guar gum, pectin, gum
arabic)
were prepared.
EXAMPLE II
A second series of solution formulations that were prepared with soluble bile
acids (as free acids) and high molecular weight aqueous soluble starch
conversion
products according to the following guidelines did not show any precipitation
at any
pH tested.
Soluble Bile Acid Starch Conversion Product (Minimum)
if 200 mg CDCA about 18 g
if 200 mg iJDCA about 3 g
if 200 mg KLCA about 7.2 g
if 200 mg cholic acid about 6 g
if 200 mg deoxycholic acid about 30 g
if 200 mg hyodeoxycholic acid about 2.1 g
Purified water to make 100 mL
Aqueous solutions (100 mL) in which one of the above soluble bile acids is
dissolved were prepared. Maltodextrin, as one high molecular weight aqueous
soluble starch conversion product, was added to the resulting solution and
dissolved
with agitation at room temperature to make a clear solution. The pH of this
resulting
clear solution was adjusted by acid with high throughput sonication to prepare
oral
dosage forms, topical preparations, and solutions. Purified water was added to
make
the total volume be 100 mL.
Based on these formulas, aqueous solution formulations with various
concentrations of certain bile acids (or salts) and their corresponding
minimal
quantities of high molecular weight aqueous soluble starch conversion products
that
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
23
have a DE of 5-10 (e.g. Maltrin(DM040, Maltrin M100) or soluble non-starch
polysaccharides (e.g. guar gum, pectin, gum arabic) were prepared.
EXAMPLE III
A third series of solution formulations that were prepared with soluble bile
acids (as
free acids) and high molecular weight aqueous soluble starch conversion
products
according to the following guidelines did not show any precipitation at pH 6.5-
8.
Soluble Bile Acid Starch Conversion Product (Minimum)
if 200 mg CDCA about 15 g
if 200 mg UDCA about 1.5 g
if 200 mg KLCA about 3.6 g
if 200 mg cholic acid about 3 g
if 200 mg deoxycholic acid about 15 g
if 200 mg hyodeoxycholic acid about 3.5 g
Purified water to make 100 mL
Aqueous solutions (100 mL) in which one of the above soluble bile acids is
dissolved were prepared. Maltodextrin, as one high molecular weight aqueous
soluble starch conversion product, was added to the resulting solution and
dissolved
with agitation at room temperature to make a clear solution. The pH of this
resulting
clear solution was adjusted by acid with high throughput sonication to prepare
injectable, colon-specific, topical, and eye drops dosage forms. Purified
water or
water for injection was added to make the total volume be 100 mL.
Based on these formulas, aqueous solution formulations with various
concentrations of certain bile acids (or salts) and their corresponding
minimal
quantities of high molecular weight aqueous soluble starch conversion products
that
have a DE of 5-10 (e.g. MaltrinOM040, Maltrin M100) or soluble non-starch
polysaccharides (e.g. guar gum, pectin, gum arabic) were prepared.
EXAMPLE I'V
A fourth series of solution formulations that were prepared with soluble bile
acids (as free acids), high molecular weight aqueous soluble starch conversion
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
24
products, and non-starch polysaccharides according to the following guidelines
did
not show any precipitation at any pH tested.
Soluble Bile Acid 200 mg KLCA (10 mg to 3 g)
Starch Conversion Product (Minimum) about 24 g (0.6 g to 75 g)
Soluble Fiber 20 g(5 g to 30 g)
Purified water to make 100 mL
Aqueous solutions (60 mL) in which soluble KLCA is dissolved were
prepared. Maltodextrin, as one high molecular weight aqueous soluble starch
conversion product, was added to the resulting solution and dissolved with
agitation at
room temperature to make a clear solution. The pH of this resulting clear
solution
was adjusted by acid with high throughput sonication to prepare injectable and
topical
dosage forms. Next, soluble non-starch polysaccharide (e.g. guar gum, pectin,
gum
arabic) and soluble resistant maltodextrin was added. Purified water or water
for
injection was added to make the total volume be 100 mL.
Table 1A shows the results of a test of stability over time at pH 7 and 50 C
of
formulations of CA, 7-ketolithocholic acid, CDCA and DCA in solution with
maltodextrin prepared according to Example I. The concentrations of the bile
acids
were measured by HPLC and the concentration of the bile acid as a percentage
of its
concentration on day 0 is reported in the column labeled percentage.
Table 1B shows the results of the test of stability over time at pH 10 and 50
C
of formulations of CA, 7-ketolithocholic acid, CDCA and DCA in solution with
maltodextrin prepared according to Example I.
Table 2 shows results of the test of stability over time at pH 1 and 50 C of
formulations of CA, 7-ketolithocholic acid, CDCA and DCA in solution with
maltodextrin at pH 1 and 50 C prepared according to Example II.
EXAMPLE V
A fifth series of solution formulations that were prepared with soluble bile
acids (as free base), high molecular weight aqueous soluble starch conversion
products, and non-starch polysaccharides according to the following guidelines
did
not show any precipitation at any pH tested.
Soluble Bile Acid 200 mg UDCA (10 mg to
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
3 g)
Starch Conversion Product (Minimum) about 5 g (0.25 g to 75 g)
Resistant maltodextrin 15 g (5 g to 30 g)
Purified water to make 100 mL
5 Aqueous solutions (100 mL) in which soluble UDCA is dissolved were
prepared. Maltodextrin, as one high molecular weight aqueous soluble starch
conversion product, was added to the resulting clear solution and dissolved
with
agitation at room temperature to make a clear solution. The pH of this
resulting clear
solution was adjusted by acid with high throughput sonication to prepare oral
and
10 topical dosage forms. Next, soluble resistant maltodextrin was added.
Purified water
or water for injection was added to make the total volume be 100 mL.
Based on these formulas, aqueous solution formulations with various
concentrations of UDCA (or its salts) and their corresponding minimal
quantities of
high molecular weight aqueous soluble starch conversion products that have a
DE of
15 5-40 (e.g. Maltrin M150, Maltrin M180, MaltrinVM200, Maltrin M250,
Maltrin M040, Maltrin M100) and soluble resistant maltodextrin were prepared.
Example VI
A sixth series of solution formulations that were prepared with soluble bile
acids (as free base), higli molecular weight aqueous soluble starch conversion
20 products, and ginseng extract according to the following guidelines did not
show any
precipitation at any pH tested.
Soluble Bile Acid 200 mg UDCA (10 mg to 3 g)
Starch Conversion Product (Minimum) about 5 g (0.25 g to 75 g)
Aqueous Soluble Ginseng Extract 200 mg (50 mg to 3 g)
25 Purified water to make 100 mL
Aqueous solutions (80 mL) in which soluble UDCA is dissolved were
prepared. Maltodextrin, as one high molecular weight aqueous soluble starch
conversion product, was added to the resulting clear solution and dissolved
with
agitation at room temperature to make a clear solution. The pH of this
resulting clear
solution was adjusted by acid with high throughput sonication to prepare oral
and
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
26
topical dosage forms. Next, soluble ginseng extract was added. Purified water
or
water for injection was added to make the total volume be 100 mL.
Based on these formulas, aqueous solution formulations with various
concentrations of UDCA (or its salts) and its corresponding minimal quantities
of
high molecular weight aqueous soluble starch conversion products that have a
DE of
5-40 were prepared. Aqueous soluble ginseng extract comprises extract from red
ginseng and white ginseng.
EXAMPLE VII
A seventh series of solution formulations that were prepared with soluble bile
acids (as free base), high molecular weight aqueous soluble starch conversion
products, and ginseng extract according to the following guidelines did not
show any
precipitation at any pH tested.
Soluble Bile Acid 200 mg UDCA (10 mg to 3 g)
Starch Conversion Product (Minimum) about 5 g (0.25 g to 75 g)
Aqueous Soluble Ginseng Extract 200 mg (50 mg to 5 g)
Soluble Non-Starch Polysaccharide 5-20 g
Purified water to make 100 mL
Aqueous solutions (80 mL) in which soluble UDCA is dissolved were
prepared. Maltodextrin, as one high molecular weight aqueous soluble starch
conversion product, was added to the resulting clear solution and dissolved
with
agitation at room temperature to make a clear solution. The pH of this
resulting clear
solution was adjusted by acid with high throughput sonication to prepare oral
aiid
topical dosage forms. Then, aqueous soluble ginseng extract and soluble non-
starch
polysaccharide (e.g. guar gum, pectin, gum arabic) or soluble resistant
maltodextrin
were added. Purified water or water for injection was added to make the total
volume
be 100 mL.
Based on these formulas, aqueous solution formulations with various
concentrations of UDCA (or its salts) and its corresponding minimal quantities
of
high molecular weight aqueous soluble starch conversion products that have a
DE of
5-40 were prepared. Aqueous soluble ginseng extract comprises extract from red
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
27
ginseng and white ginseng. Soluble fiber is soluble non-starch polysaccharide
(e.g.
guar gum, pectin, gum arabic) or soluble resistant maltodextrin.
Figure 6 is an NMR spectrum of UDCA illustrating that UDCA, when in a
composition prepared according to Example III, is absolutely free UDCA. That
is, the
carboxylic acid of UDCA at C-24 is the free form (R-COOH) and two hydroxy
group
at C-3 and C-7 are in the free form (R-OH).
In addition, the HPLC profile of UDCA in a composition prepared according
to Example III (Figure 7) is similar to the profile of UDCA dissolved in
methanol
(Figure 8). This data shows that there is no UDCA-complex compound. There is
only free UDCA. A non-aqueous UDCA standard solution was prepared by
dissolving 100 mg UDCA in 100 mL of inethanol. A mixture of acetonitrile (51),
water (49), and acetic acid (1) was used as the mobile phase.
EXAMPLE VIII
An eighth series of solution formulations that were prepared with soluble bile
acids (as free acid), high molecular weight aqueous soluble starch conversion
products, and branched chain amino acids (e.g. leucine, isoleucine, valine)
according
to the following guidelines did not show any precipitation at any pH tested.
Soluble Bile Acid 200 mg UDCA (10 mg to 3 g)
Starch Conversion Product (Minimum) about 5 g (0.25 g to 75 g)
Branched Chained Amino Acid 15 g (1 g to 35 g)
Purified water to make 100 mL
Aqueous solutions (85 mL) in which soluble UDCA is dissolved were
prepared. Maltodextrin, as one high inolecular weight aqueous soluble starch
conversion product, was added to the resulting clear solution and dissolved
with
agitation at room temperature to make a clear solution. The pH of this
resulting clear
solution was adjusted (to from pH 4 to pH 7) by acid with high throughput
sonication
to prepare oral and topical dosage forms. Then, branched chain amino acids
were
added. Purified water or water for injection was added to make the total
volume be
100 mL.
Based on these foimulas, aqueous solution formulations with various
concentrations of UDCA (or its salts) and its corresponding minimal quantities
of
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
28
high molecular weight aqueous soluble starch conversion products that have a
DE of
5-40 were prepared with various quantities of branched amino acid (total
amount of
leucine, isoleucine and valine).
Tables 3A to 3F show stability test results over time of formulation prepared
with amino acids according to Example IV. All stability tests were conducted
at 50
C. Stability test results at pH 1 (Table 3A), pH 3 (Table 3B), pH 5 (Table
3C), pH 7
(Table 3D), pH 9 (Table 3E), and pH 10 (Table 3F) are shown.
Table 3A : Stability of UDCA solution according to Example IV at pH 1, 50 C
Day #1 #2 #3 Average Percentage
0 0.261 0.236 0.249 0.248 100.0
1 0.256 0.275 0.251 0.261 105.0
Ile 2 0.268 0.263 0.251 0.260 104.9
6 0.295 0.268 0.291 0.285 114.6
7 0.249 0.254 0.267 0.257 103.4
8 0.253 0.243 0.240 0.245 98.8
9 0.263 0.268 0.263 0.265 106.6
Day #1 #2 #3 Average Percentage
0 0.485 0.428 0.470 0.461 100.0
1 0.470 0.477 0.456 0.468 101.5
Leu 2 0.485 0.481 0.460 0.475 103.1
6 0.553 0.510 0.529 0.531 115.1
7 0.478 0.473 0.513 0.488 105.8
8 0.474 0.454 0.511 0.480 104.0
9 0.483 0.485 0.476 0.481 104.4
Day #1 #2 #3 Average Percentage
0 0.506 0.448 0.460 0.471 100.0
1 0.438 0.458 0.471 0.456 96.7
Val 2 0.479 0.485 0.513 0.492 104.5
6 0.505 0.536 0.549 0.530 112.4
7 0.494 0.465 0.496 0.485 102.9
8 0.488 0.491 0.459 0.479 101.7
9 0.479 0.496 0.490 0.488 103.6
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
29
Day #1 #2 #3 Average Percentage
0 0.319 0.315 0.322 0.319 100.0
1 0.332 0.344 0.351 0.342 107.4
Sol 2 0.371 0.339 0.403 0.371 116.4
6 0.396 0.409 0.411 0.405 127.2
7 0.365 0.351 0.381 0.366 114.7
8 0.409 0.365 0.331 0.368 115.6
9 0.338 0.391 0.374 0.368 115.4
Day #1 #2 #3 Average Percenta e
0 0.388 0.387 0.389 0.388 100.0
1 0.367 0.370 0.366 0.368 94.8
UDCA 2 0.374 0.388 0.388 0.383 98.9
6 0.371 0.380 0.382 0.377 97.3
7 0.378 0.376 0.379 0.378 97.4
8 0.374 0.382 0.384 0.380 97.9
9 0.370 0.367 0.370 0.369 95.1
Table 3B Stability of UDCA solution according to Example IV at pH 3, 50 C
Day #1 #2 #3 Average Percenta e
0 0.261 0.254 0.253 0.256 100.0
1 0.266 0.268 0.261 0.265 103.3
Ile 2 0.273 0.243 0.247 0.254 99.3
6 0.296 0.306 0.300 0.301 117.4
7 0.247 0.265 0.257 0.256 100.0
8 0.250 0.247 0.247 0.248 96.7
13 0.285 0.240 0.250 0.258 100.9
Day #1 #2 #3 Average Percentage
0 0.495 0.465 0.452 0.471 100.0
1 0.489 0.480 0.470 0.480 101.9
Leu 2 0.495 0.472 0.481 0.483 102.6
6 0.522 0.532 0.556 0.537 114.0
7 0.492 0.482 0.491 0.488 103.7
8 0.543 0.515 0.495 0.517 109.9
13 0.512 0.496 0.543 0.517 109.8
Day #1 #2 #3 Average Percentage
0 0.485 0.491 0.498 0.491 100.0
1 0.467 0.481 0.446 0.465 94.6
Val 2 0.510 0.493 0.527 0.510 103.8
6 0.527 0.491 0.553 0.524 106.6
7 0.485 0.481 0.468 0.478 97.3
8 0.490 0.491 0.544 0.508 103.5
13 0.519 0.498 0.517 0.511 104.1
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
Day #1 #2 #3 Average Percentage
0 0.343 0.355 0.370 0.356 100.0
1 0.340 0.350 0.316 0.335 94.2
Sol 2 0.383 0.371 0.400 0.385 108.0
6 0.378 0.341 0.416 0.378 106.3
7 0.355 0.381 0.315 0.350 98.4
8 0.343 0.350 0.395 0.363 101.9
13 0.377 0.382 0.423 0.394 110.7
Day #1 #2 #3 Average Percentage
0 0.395 0.396 0.393 0.395 100.0
1 0.396 0.401 0.392 0.396 100.4
UDCA 2 0.427 0.421 0.416 0.421 106.8
6 0.407 0.408 0.402 0.405 102.7
7 0.412 0.409 0.411 0.411 104.1
8 0.415 0.418 0.408 0.414 104.9
13 0.415 0.412 0.416 0.414 105.0
Table 3C : Stability of UDCA solution according to Example IV at pH 5, 50 C
Day #1 #2 #3 Average Percentage
0 0.285 0.258 0.295 0.279 100.0
3 0.280 0.275 0.275 0.277 99.0
Ile 6 0.285 0,273 0.270 0.276 98.7
10 0.274 0.276 0.276 0.275 98.4
13 0.273 0.287 0.278 0.279 100.0
17 0.278 0.276 0.270 0.275 98.3
20 0.261 0.275 0.261 0.266 95.0
24 0.267 0.274 0.292 0.277 99.3
5
Day #1 #2 #3 Average Percentage
0 0.495 0.467 0.535 0.499 100.0
3 0.510 0.495 0.494 0.500 100.1
Leu 6 0.489 0.479 0.484 0.484 97.0
10 0.486 0.490 0.499 0.492 98.5
13 0.492 0.509 0.508 0.503 100.8
17 0.514 0.508 0.504 0.509 100.9
20 0.499 0.500 0.499 0.499 101.1
24 0.488 0.509 0.528 0.508 101.9
Day #1 #2 #3 Average Percentage
0 0.483 0.498 0.481 0.487 100.0
3 0.492 0.494 0.526 0.504 103.4
Val 6 0.459 0.475 0.481 0.472 96.8
10 0.500 0.436 0.480 0.472 96.9
13 0.464 0.451 0.474 0.463 95.0
17 0.407 0.491 0.462 0.453 93.0
20 0.471 0.512 0.477 0.487 99.9
24 0.471 0.476 0.458 0.468 96.1
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
31
Day #1 #2 #3 Average Percentage
0 0.341 0.351 0.360 0.351 100.0
3 0.342 0.386 0.371 0.366 104.5
Soi 6 0.316 0.321 0.342 0.326 93.1
0.341 0.299 0.335 0.325 92.7
13 0.355 0.326 0.350 0.344 98.0
17 0.334 0.376 0.353 0.354 101.0
0.347 0.398 0.394 0.380 108.3
24 0.416 0.353 0.378 0.382 109.0
Day #1 #2 #3 Average Percenta e
0 0.407 0.404 0.404 0.405 100.0
3 0.409 0.402 0.403 0.405 99.9
UDCA 6 0.410 0.403 0.409 0.407 100.6
10 0.404 0.405 0.407 0.405 100.1
13 0,408 0.403 0.395 0.402 99.3
17 0.411 0.402 0,404 0.406 100.2
20 0.405 0.394 0.396 0.398 98.4
24 0,399 0.408 0.406 0.404 99.9
Table 3D : Stability of UDCA solution according to Example IV at pH 7, 50 C
Day #1 #2 #3 Average Percentage
0 0.296 0.289 0.281 0.289 100.0
5 0.300 0.282 0.281 0.288 99.7
Ile 8 0.277 0.282 0.268 0.276 95.5
12 0.273 0.278 0.278 0.277 95.8
15 0.271 0.273 0.266 0.270 93.5
19 0.294 0.285 0.281 0.287 99.3
5
Day #1 #2 #3 Average Percentage
0 0.519 0.513 0.495 0.509 100.0
5 0.499 0.499 0.498 0.498 97.9
Leu 8 0.498 0.513 0.480 0.497 97.7
12 0.508 0.516 0.515 0.513 100.9
15 0.503 0.505 0.499 0.502 98.7
19 0.521 0.509 0.516 0.515 101.3
Day #1 #2 #3 Average Percentage
0 0.483 0.530 0.525 0.513 100.0
5 0.502 0.447 0.499 0.483 94.1
Val 8 0.488 0.498 0.493 0.493 96.2
12 0.490 0.469 0.443 0.467 91.2
15 0.492 0.541 0.442 0.492 95.9
19 0.458 0.500 0.482 0.480 93.6
Day #1 #2 #3 Average Percentage
0 0.333 0.352 0.363 0.349 100.0
5 0.344 0.309 0.349 0.334 95.6
Sol 8 0.334 0.379 0.377 0.363 104.0
12 0.345 0.344 0.317 0.335 96.0
15 0.286 0.406 0.321 0.338 96.7
19 0.338 0.416 0.351 0.368 105.4
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
32
Day #1 #2 #3 Average Percentage
0 0.427 0.416 0.428 0.424 100.0
0.406 0.427 0.432 0.422 99.4
UDCA 8 0.419 0.408 0.417 0.414 97.7
12 0.414 0.418 0.419 0.417 98.4
0.413 0.418 0.409 0.414 97.5
19 0.429 0.421 0.424 0.425 100.1
Table 3E : Stability of UDCA solution according to Example IV at pH 9, 50 C
Day #1 #2 #3 Average Percentage
0 0.291 0.286 0.282 0.286 100.0
3 0.266 0.273 0.282 0.273 95.6
Ile 6 0.277 0.274 0.272 0.274 95.9
10 0.243 0.245 0.295 0.261 91.2
13 0.246 0.269 0.236 0.250 87.4
17 0.275 0.280 0.245 0.267 93.1
Day #1 #2 #3 Average Percentage
0 0.509 0.513 0.511 0.511 100.0
3 0.485 0.487 0.492 0.488 95.5
Leu 6 0.495 0.496 0.492 0.494 96.8
10 0.470 0.467 0.528 0.488 95.6
13 0.461 0.491 0.450 0.467 91.5
17 0.468 0.516 0.500 0.495 96.9
Day #1 #2 #3 Average Percentage
0 0.508 0.476 0.484 0.489 100.0
3 0.463 0.487 0.485 0.478 97.8
Val 6 0.493 0.473 0.495 0.487 99.5
10 0.441 0.428 0.471 0.447 91.3
13 0.467 0.483 0.537 0.496 101.3
17 0.499 0.495 0.501 0.498 101.8
5
Day #1 #2 #3 Average Percentage
0 0.341 0.316 0.328 0.328 100.0
3 0.297 0.317 0.317 0.310 94.5
Sol 6 0.313 0.291 0.314 0.306 93.2
10 0.268 0.253 0.324 0.282 85.8
13 0.270 0.266 0.334 0.290 88.3
17 0.337 0.329 0.317 0.328 99.8
Day #1 #2 #3 Average Percentage
0 0.389 0.385 0.389 0.388 100.0
3 0.405 0.400 0.394 0.400 103.2
UDCA 6 0.427 0.411 0.416 0.418 107.9
10 0.420 0.418 0.450 0.429 110.8
13 0.465 0.434 0.441 0.447 115.3
17 0.454 0.457 0.413 0.441 113.9
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
33
Table 3F : Stability of UDCA solution according to Example IV at pH 10, 50 C
Day #1 #2 #3 Average Percentage
0 0.292 0.282 0.287 0.287 100.0
2 0.253 0.237 0.239 0.243 84.7
Ile 5 0.221 0.212 0.221 0.218 76.0
7 0.219 0.215 0.207 0.214 74.5
9 0.206 0.192 0.207 0.202 70.2
Day #1 #2 #3 Average Percentage
0 0.507 0.495 0.509 0.504 100.0
2 0.462 0.442 0.442 0.449 89.1
Leu 5 0.429 0.428 0.427 0.428 85.0
7 0.410 0.417 0.414 0.414 82.1
9 0.417 0.377 0.418 0.404 80.2
Day #1 #2 #3 Average Percentage
0 0.480 0.506 0.471 0.486 100.0
2 0.536 0.478 0.504 0.506 104.2
Val 5 0.371 0.445 0.400 0.405 83.5
7 0.384 0.384 0.424 0.397 81.8
9 0.389 0.354 0.362 0.368 75.8
Day #1 #2 #3 Average Percentage
0 0.368 0.376 0.331 0.358 100.0
2 0.284 0.257 0.266 0.269 75.1
Sol 5 0.053 0.217 0.192 0.154 43.0
7 0.042 0.026 0.156 0.075 20.8
9 0.033 0.019 0.023 0.025 7.0
Day #1 #2 #3 Average Percentage
0 0.416 0.402 0.406 0.408 100.0
2 0.402 0.397 0.400 0.399 97.9
UDCA 5 0.425 0.413 0.423 0.420 103.0
7 0.406 0.402 0.408 0.406 99.4
9 0.424 0.426 0.421 0.423 103.8
ExAMPLE IX
A ninth series of solution formulations that were prepared with soluble bile
acids (as free form) and high molecular weight aqueous soluble starch
conversion
products according to the following guidelines did not show any precipitation
at any
pH within the selected, desired pH range. This formulation is modified based
on the
known analytical data for bear bile.
Soluble Bile Acid 21 g UDCA,
9 g CA, and
9 g CDCA
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
34
Starch Conversion Product about 750 g
Purified water to make 1.0 L
An aqueous solution (400 mL) of soluble UDCA was prepared. Then the high
molecular weight aqueous soluble starch conversion product was added to make a
clear solution. Into the resulting clear solution, soluble CDCA, and soluble
CA were
added. The pH of this solution was adjusted by acid with high throughput
sonication
to prepare oral and topical dosage forms. Purified water or water for
injection was
added to make the total volume be 1.0 L.
EXAMPLE X
A tenth series of solution formulations that were prepared with soluble bile
acids (as free form) and high molecular weight aqueous soluble starch
conversion
products according to the following guidelines did not show any precipitation
at any
pH within the selected, desired pH range. This formulation is modified based
on the
known analytical data for bear bile.
Soluble Bile Acid 21 g UDCA,
9gCA,and
9 g CDCA
Starch Conversion Product about 750 g
Aqueous Soluble Ginseng Extract 20 g
Purified water to make 1.0 L
An aqueous solution (400 mL) of soluble UDCA was prepared. Then the high
molecular weight aqueous soluble starch conversion product was added to make a
clear solution. Into the resulting clear solution, soluble CDCA, soluble CA,
and
aqueous soluble ginseng extract were added. The pH of this solution was
adjusted by
acid with high throughput sonication to prepare oral and topical dosage forms.
Purified water or water for injection was added to make the total volume be
1.0 L.
EXAMPLE XI
Aqueous solution formulations, according to this invention, containing 200 mg
of ursodeoxycholic acid (UDCA), were prepared according to the method
described in
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
the above-described Example III and were administered to three healthy men
having
normal body weight after fasting. The hematic levels of UDCA and glyco UDCA
were evaluated by means of well known analytical methods. After applying
buffered
serum to sep-pak column, methanol eluate was derivatized with phenacyl bromide
at
5 80 C for 45 minutes. These phenacyl bromide derivatives were dissolved in
acetonitrile in preparation for HPLC. The experimental results of the
absorption
measured at certain times after dosage administration include the total
absorption
expressed as the area under the serum concentration-time curve (AUC: g/mL x
hours), the maximum hematic concentration (Cmax; g/mL) that has been
obtained,
10 and the time (Tmax; hour) in which said maximum concentration has been
obtained.
These results are reported in Table 4, Figure 1, and Figure 2.
The experimental pharmacokinetic tests of the aqueous solution formulations
according to this invention carried out on men show substantial improvement in
AUC,
Cmax and Tmax in comparison with the best results from any dosage forms known
15 presently. The maximum hematic concentration (Cmax) in Table 4 shows an
average
of 8.43=L 1.69 g/mL which is at least two times higher than that reported for
use of
enteric coated sodium salt of UDCA preparations and four times higher than
that
obtained using regular UDCA tablet preparations. Moreover, the time of peak
concentration (T,,,ax) which is related closely to the rate of absorption of
UDCA from
20 the aqueous solution formulations is 0.25 hours, at least three times
faster than the
fastest Tmax previously known.
Table 4A and Table 4B show plasma concentration of UDCA and GUDCA
measured in 3 men over time following on oral administration of the UDCA and
GUDCA containing formulations according to Example VI and comparison of
results
25 against results of others employing different pharmaceutical formulations
of UDCA.
Table 5 shows phamacokinetic parameters of UDCA in human after an oral
administration of liquid formulation of UDCA. Cmax is shown.
Taken together, the data in Tables 4 and 5 and Figures 3 and 4 illustrate the
superiority of formulations of the instant invention over conventional
formulations
30 with respect to Cmax and Tmax= the instant The inventive solutions were
effect
without any break-down of the solution system caused by the pH of the
environment
in the stomach and intestines. The therapeutic potential of bile acid and
possibly even
added pharmaceuticals may be more fully realized using the formulations of the
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
36
invention. When the therapeutically active ingredients in aqueous solution
forms are
not precipitated as solid by acidic gastric juices in the stomach and by the
various
alkaline pH levels of the intestine, the formulation overcomes as a natural
consequence, the scarce bioavailability resulted by the unexpected,
undesirable results
for the extent and the rate of release by disintegration, dissolution and/or
diffusion
should be overcome.
Table 4A: Plasma concentration of UDCA and GUDCA after an oral
administration of this invention at a dose of 200 mg to three men
UDCA GUDCA
Time(h) #1 #2 #3 mean #1 #2 #3 mean
0.25 5.1202 10.9171 9.159 8.43~:1.6 0.1419 0.4549 0.3328 0.31 0.0
0.5 4.4528 7.7432 7.4395 6.55 1.0 0.2564 1.2455 0.864 0.79 0.2
1 1.6921 1.546 0.2163 1.15 0.4 0.2162 0.6926 0.2142 0.37 0.1
1.5 0.5256 0.2759 0.168 0.32 0.1 1.1573 0.1929 0.4752 0.61 0.2
2 0.2349 0.2176 0.1227 0.19 0.0 0.4013 0.0312 0.0657 0.17 0.1
3 0.1237 N.D. 0.2074 0.17 0.0 0.5085 0.4303 0.3315 0.42 0.0
5 1,9205 0.0229 1.6311 1.18+0.6
7 0.5328 0.4797 0.91 0.64 0.1
AUC ( g 4.32 6.6 5.47 5.46 0.6 6.26 2.22 4.65 4.38 1.1
Cmax ( g/1nL) 5.21 10.92 9.16 8.43 1.6 1.92 1.25 1.63 1.6
Tmax(h) 0.25 0.25 0.25 0.25 5 0.5 5 3.5 1.5
Table 4B: Pharmacokinetic parameters of UDCA in human after an oral
administration of UDCA (M ~ S.E.)
Cmax ( g/ML) Tmax (hr)
Roda et al. (1994)
UDCA gelatine capsule, 450 mg 2.59 3.8
NaUDC gelatine capsule, 475 mg 3.42 2.4
NaUDC enteric-coated, 475 mg 10 3.4
Nagamatsu et al. (1997)
UDCA 200 mg 1.9 0.25 1.510.4
UDCA 400 mg 7.09 1.43 0.8 0.2
UDCA in this invention, 200 mg 8.43J:1.69 0.25
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
37
Table 5A : Pharmacokinetic parameter (Cmax) of UDCA in human after oral
administration of a liquid solution containing 600 mg UDCA per day.
Time (min) Person #1 Person #2 Person #3 Person #4 Person #5 Average Std. Dev.
0 0.35 1.63 0.40 0.00 0.71 0.618 0.619
2.51 9.79 1.68 2.65 6.26 4.578 3.405
12.50 47.46 8.34 11.84 21.83 20.394 15.933
60 9.72 6.46 7.77 9.81 17.25 10.202 4.183
120 3.77 1.71 1.40 1.15 2.81 2.168 1.097
240 0.65 0.93 0.50 0.48 1.30 0.772 0.346
Table 5B : Pharmacokinetic parameter (Cmax) of UDCA in human after an oral
5 administration of a syrup containing 600 mg UDCA per day.
Time (min) Person #1 Person #2 Person #3 Person #4 Person #5 Average Std. Dev.
0 0.62 0.58 0.38 0.00 0.41 0.398 0.246
5 2.76 2.63 0.83 1.42 2.24 1.976 0.827
15 7.80 4.45 3.54 5.85 14.08 7.144 4.197
60 16.08 20.33 8.76 12.06 17.77 15.000 4.605
120 3.98 4.24 5.09 7.79 3.00 4.820 1.820
240 0.81 0.99 1.47 1.85 1.17 1.258 0.411
EXAMPLE XII
An eleventh series of solution formulations that were prepared with soluble
bile acids (as free acid), high molecular weight aqueous soluble starch
conversion
products, and non-starch polysaccharides (dried powder of liquid glucose, e.g.
10 commercial corn syrup solid) according to the following guidelines did not
show any
precipitation at any pH within the selected, desired range of pH values.
Soluble Bile Acid 200 mg UDCA (10 mg to 3 g)
Dried Powder of Liquid Glucose 25 g (0.25 g to 75 g)
Soluble Non-Starch Polysaccharide 25g (0.25 g to 75 g)
15 Purified water to make 100 mL
An aqueous solution (45 mL) of soluble UDCA was prepared. Then the high
molecular weight aqueous soluble starch conversion product (i. e. dried liquid
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
38
glucose), which has DE of 5-40, was added to make a clear solution. The pH of
this
solution was adjusted by acid with high throughput sonication to prepare oral
and
topical dosage forms. Purified water or water for injection was added to make
the
total volume be 1.0 L.
Into the resulting clear solution, a soluble non starch polysaccharide (guar
gum, pectin, etc.) was added into pH-adjusted clear solution with agitation.
Purified
water was added to make the total volume to 100 mL.
EXAMPLE XIII: MIXTU.RE SOLUTION
The formulations of Examples VIII, IX, and X include bismuth compound. In
each of these examples, solution forrnulations were prepared by adding an
amount of
an ammonium salt of bismuth sulfate sufficient to provide the indicated amount
of
bismuth hydroxide.
A twelfth series of solution formulations that were prepared with soluble bile
acids (as free acid), high molecular weight aqueous soluble starch conversion
products, and bismuth compounds according to the following guidelines did not
show
any precipitation at any pH within the selected, desired range of pH values.
Soluble Bile Acid 20 g UDCA
Bismuth Citrate 5 g
Corn Syrup Solid 500 g
Citric Acid q.s.
Purified water to make 1.0 L
A 3 mL aliquot of 1N NaOH was poured into water (200 mL) followed by
addition of UDCA. The bismuth citrate was added to the resulting clear
solution,
while maintaining pH 9-10, along with 200 mL of water. Next, the corn syrup
solid,
as one high molecular weight aqueous soluble starch conversion product, was
added
portion by portion to the resulting clear solution and dissolved with
agitation to make
a clear solution. The pH of this resulting clear solution was adjusted (to pH
3 to pH
5) by citric acid with high throughput sonication, which may accelerate
solubilization
of the bismuth compound. Purified water was added to adjust the total volume
to 1.0
L.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
39
EXAMPLE XIV: UDCA-THicx SYxuP (30 g UDCA/L
A thirteenth series of solution formulations that were prepared according to
the following guidelines did not show any precipitation at any pH within the
selected,
desired range of pH values.
Soluble Bile Acid 30 g UDCA
1 N NaOH 4 mL
Maltodextrin 750 g
Citric Acid or Lactic Acid q.s.
Purified water to make 1.0 L
The UDCA is first dissolved in NaOH solution and then diluted with 250 mL
of water. Next, the maltodextrin, as one high molecular weight aqueous soluble
starch conversion product that has a lower DE, was added portion by portion
with
vigorous agitation. The pH of this resulting clear solution was adjusted (to
pH 3) by
addition of citric acid with high throughput sonication. Purified water was
added to
adjust the total volume to 1.0 L.
EXAMPLE XV: UDCA-PASTE (45 g UDCA/L)
A fourteenth series of solution formulations that were prepared according to
the following guidelines did not show any precipitation at any pH within the
selected,
desired range of pH values.
Soluble Bile Acid 45 g UDCA
1 N NaOH 135 mL
Maltodextrin 1,575 g
Citric Acid or Lactic Acid q.s.
Purified water to make 1.0 L
The UDCA is first dissolved in 135 mL of a 1N NaOH solution. Next, to the
resulting clear solution were added the bismuth citrate and 200 mL of water.
Then,
1,575 g of maltodextrin was added portion by portion with vigorous agitation.
The
resulting solution was titrated to pH 3 by the addition of citric acid.
Purified water
was added to adjust the total volume to 1.0 L.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
Five human subjects were provided with dosage forms prepared according to
this Example. The results are shown in Tables 5A and 5B and rendered
graphically in
Figures 3 and 4. A comparison of the sharp peak of Figure 3 with the broad
peak of
Figure 4 indicates that, by adjusting the dosage form, a practitioner may
manipulate
5 the bile acid Cmax and Tmax=
H. pylori were cultured on Columbia Blood Agar Base (CRAB) media
containing a preparation of Example IX. 2 L of CRAB plates were prepared which
contained 9.9 g of CRAB, 9.1 g of tryptic soy agar, 50 mL of sheep blood,
vacomycin, amphotericin B, polymixin B, 2 mL of Example IX, and 358 mL
distilled
10 water. After 48 or 72 hours of microaerophillic incubation, bacteria were
fixed using
Karnovsky's fixative and embedded in epon. Electron micrographs of H. pylori
cells
are shown in Figures 5A to 5C.
EXAMPLE XVI: UDCA-PASTE (45 g UDCA/L)
A fifteenth series of solution formulations that were prepared according to
the
15 following guidelines did not show any precipitation at any pH within the
selected,
desired range of pH values.
Soluble Bile Acid 45 g UDCA
1 N NaOH 135 mL
Corn syrup solid 2,300 g
20 Citric acid or lactic acid 50 g
Purified water to make 1.0 L
The UDCA is first dissolved in 135 mL of a 1N NaOH solution. Next, to the
resulting clear solution were added the bismuth citrate and 150 mL of water.
Then,
2,300 g of corn syrup solid was added portion by portion with vigorous
agitation. The
25 resulting solution was titrated to pH 3 by the addition of citric acid.
Purified water
was added to adjust the total volume to 1.0 L.
EXAMPLE XVII: MIXTURE SOLUTION OF UDCA (22 G) AND CDCA (3 G)
A sixteenth series of solution formulations that were prepared according to
the
following guidelines did not show any precipitation at any pH within the
selected,
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
41
desired range of pH values.
UDCA 22g
1 N NaOH 75 mL
CDCA 3 g
Maltodextrin 875 g
Bismuth citrate 4 g
Citric acid or lactic acid q.s.
Purified water to make 1.0 L
The UDCA and CDCA are first dissolved in 75 mL of a 1N NaOH solution.
Next, to the resulting clear solution were added the bismuth citrate and 240
inL of
water. Then, 875 g of maltodextrin was added portion by portion with vigorous
agitation. The resulting solution was titrated to pH 3 by the addition of
citric acid.
Purified water was added to adjust the total volume to 1.0 L.
EXAMPLE XVIII: MIXTURE SOLUTION OF UDCA (22 G) AND
CDCA (3 G)
A seventeenth series of solution formulations that were prepared according to
the following guidelines did not show any precipitation at any pH within the
selected,
desired range of pH values.
UDCA 22 g
1 N NaOH 75 mL
CDCA 3g
Corn syrup solid 1,320 g
Bismuth citrate 4 g
Citric acid or lactic acid q.s.
Purified water to make 1.0 L
The UDCA and CDCA are first dissolved in 75 mL of a 1N NaOH solution.
Next, to the resulting clear solution were added the bismuth citrate and 240
mL of
water. Then, 1,320 g of corn syrup solid was added portion by portion with
vigorous
agitation. The resulting solution was titrated to pH 3 by the addition of
citric acid.
Purified water was added to adjust the total volume to 1.0 L.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
42
EXAMPLE XIX
The effect of treating H. pvlori infected mice with a solution dosage form of
the invention was tested. Six week old C57BL/6 female mice were infected by
feeding a diet comprising 109 CFU/mL H. pylori, SS 1 strain. The animals
consumed
this feed twice, one week apart. Subsequently, 0.2 mL of a solution dosage
form
according to Example XIII was administered to four infected animals once per
day for
one week. Two animals were sacrificed one week following administration of the
last
dose of the inventive solution. The remaining two animals were sacrificed four
weeks
following administration of the last dose of the inventive solution. Whole
stomachs
were washed with saline to remove mucosa and debris. A sample of stomach
tissue
from each animal was subjected to a CLO test using a rapid urease test kit
(Delta
West, Australia). Each residual stomach was fixed with 10% formalin solution
and
embedded with paraffin. Sections (4 m thick) were collected on glass slides
and
stained with H&E staining solution and Warthin staining solution. Tissue was
evaluated for pathological status by conventional light microscopy.
The results, summarized in Table 6, indicate that the urease test results were
negative for mice passed one week after discontinuing administration of the
liquid
dosage form, and H. pylori was not seen in Warthin examination. Of the other
two
mice, one showed a negative urease test and no H. pyloYi were seen by Warthin
examination. The other, however, yielded a positive urease test although only
a few
H. pylori were seen in Warthin examination.
Table 6
Weeks After Animal Urease Test Warthin
Treatment Examination
1 1 Negative No H. pylori
1 2 Negative No H. pylori
4 3 Negative No H. pylori
4 4 Positive A few H. pylori
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
43
EXAMPLE XX
Assays for growth of H. pylof i on media containing UDCA, bismuth citrate or
both UDCA and bismuth citrate were perfoimed. For these assays the following
media was used:
000112B-1 having a pH of 4.0 and comprising 525 g/L maltodextrin
and 15 g/L UDCA.
OSABY having a pH of 3.7 and comprising 1 kg/L corn syrup solid
and 6 g/L bismuth citrate.
Three assays were performed to assess the growth capacity of H. pylori in the
presence of UDCA, bismuth or both wherein the pH, concentration, and length of
exposure was varied.
In the first, Helicobacterpylori was suspended in physiological saline to give
about 109 organisms per milliliter. 50 L of this inoculum was transferred to
tubes
containing 1 mL of citrate-phosphate buffer at pH 3.0, 4.0, and 4.5. Paired
tubes were
prepared with and without 6 mM Urea. Following a 30 minute room temperature
incubation, the suspensions were subcultured on agar plates containing 000112B-
1
using a 1 L loop. Plates were incubated microaerophilically at 37 C for 72
hours.
This procedure is illustrated in Figure 9.
As shown in Table 7, H. pylori grew poorly on pH 3 and pH 4 control media.
Table 7 further shows that H. pylori does not grow on pH 3 and pH 4 media
containing UDCA. The designations "3 ml", "4 ml" and "5 ml" refer to the total
volume of 000112B-1 media per plate. "PBS" is phosphate buffered saline at pH
7Ø
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
44
Table 7
Plate pH Urea Urease Test
1-2 sec. 10 niin. 2 hr. 20 hr.
3.0 Yes FO FO 0 0
No FO FO 0 0
4.0 Yes FO FO 0 0
Control No FO FO 0 p
4.5 Yes FP FP P p
No FO 0 FP P
PBS Yes 0 FP P p
No 0 FP P p
3.0 Yes Y Y y Y
No Y Y y y
000112B-1 Yes Y Y FO 0
(3 mL) 4 0 No Y Y Y FO
4.5 Yes FP FP P p
No FP FP P P
3.0 Yes Y Y Y Y
No Y y y y
000112B-1 Yes Y Y Y FO
(4 mL) 4'0 No Y Y FO FO
4.5 Yes FP FP P P
No FP FP FP P
3.0 Yes Y Y Y Y
No Y Y Y y
000112B-1 Yes Y Y FO FO
(5mL) 40 No Y Y Y Y
4.5 Yes FP FP P P
No FP FP P P
Key
Y FO 0 FP P
Color Yellow Faint Orange Orange Faint Pink Pink
Helicobacter None Very Rare Rare Exist Many
In the second assay, Helicobacterpylori was suspended in physiological saline
to give about 109 organisms per milliliter. 50 L of this inoculum was
transferred to
tubes containing 1 mL of citrate-phosphate buffer at various concentrations of
plating
media such as 1/10, 1/30, 1/50, 1/100, 1/200, 1/500, 1/800, 1/1000, 1/2000.
All tubes
were prepared with 6 mM Urea. Following a 30 minute room temperature
incubation,
the suspensions were subcultured on agar plates using a 1 L loop. These
plates were
substantially free of bismuth and bile acids. Plates were incubated
microaerophilically at 37 C for 72 hours. This procedure is illustrated in
Figure 10.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
Table 8 shows urease test results following 72 hours of growth of H. pylori on
media prepared with dilutions of UDCA (000112B-1) bismuth citrate (OSABY) or
both UDCA and bismuth citrate. Poor growth of H. pylori on media containing
either
UDCA or bismuth citrate was observed (Table 8). Growth of H. pylori was
further
5 attenuated when cultured on media containing both UDCA and bismuth citrate
(Table
8).
Table 8
Plate Urease Test
Immediatel 10 niin. 30 min. 60 min.
000112B-1
Control P p p p
1/10 y FP p p
1/30 Y FP P P
1/50 Y FP P P
1/100 Y FP p p
1/200 Y FP P P
1/500 Y FP P P
1/800 FP P P P
1/1000 FP P P P
1/2000 FP P P P
OSABY
Control P P P P
1/10 Y FP P P
1/30 Y FP P P
1/50 Y FP P P
1/100 FP FP P P
1/200 FP FP P P
1/500 FP FP P P
1/800 FP P P P
1/1000 FP P P P
1/2000 FP P P P
000122B-1 + OSABY
Control P P P P
1/10 Y FP FP FP
1/50 Y Y Y y
1/100 Y Y Y Y
1/500 Y FP FP FP
1/1000 Y FP P P
Key
Y FO 0 FP P
Color Yellow Faint Orange Orange Faint Pink Pink
Helicobacter None Very Rare Rare Exist Man
In the third assay, Helicobacterpylori was suspended in physiological saline
to give about 109 organisms per milliliter. 50 L of this inoculum was
transferred to
tubes containing 1 mL of citrate-phosphate buffer at various concentrations
such as %2,
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
46
1/4, and 1/10 for 15 minutes, l/z, 1/4, and 1/10 for 30 minutes, and %2, 1/4,
and 1/10 for
45 minutes. Paired tubes were inoculated with and without 6 mM Urea. Following
a
30 minute room temperature incubation, the suspensions were subcultured on
agar
plates using a 1 L loop. These plates were substantially free of bismuth and
bile
acids. Plates were incubated microaerophilically at 37 C for 72 hours. This
procedure is illustrated in Figure 11.
Table 9 shows urease test results following 72 hours of growth of H. pylori on
media prepared with dilutions of UDCA (0001 12B-1) bismuth citrate (OSABY) or
both UDCA and bismuth citrate. As indicated, longer exposure times increased
the
adverse effect of the solutions on H. pylori.
Table 9
Dilution Incubation Urease Test min.
Time min. 1 30 60 120 240
000112B-1
Control P P P P P
V2 15 Y Y Y Y Y
30 Y Y Y Y Y
45 Y Y Y Y Y
1/4 15 Y FO FP P P
30 Y Y FO FO FO
45 Y Y Y Y Y
1/10 15 Y FO FO FO FO
30 Y FO FO 0 P
45 Y Y Y FO FO
OSABY
Control P P P P P
~/Z 15 Y Y Y Y Y
30 Y Y Y Y Y
45 Y Y Y Y Y
1/4 15 Y Y Y Y Y
30 Y Y Y Y Y
45 Y Y Y Y Y
1/10 15 Y FO FO FO P
30 Y Y Y Y Y
45 Y Y Y Y Y
000122B-1 + OSABY
Control P P P P P
1/Z 15 Y Y Y Y Y
30 Y Y Y Y Y
45 Y Y Y Y Y
1/4 15 Y Y Y Y Y
30 Y Y Y Y Y
45 Y Y Y Y Y
1/10 15 Y Y Y FO FO
30 Y FO FO FO P
45 Y Y Y Y Y
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
47
EXAMPLE XXI
In the some embodiments of the examples I to XX, dried form may be
prepared by the evaporation under vacuum. Solution formulations of bile acid
compositions were dried in the rotary evaporator at 90-95 C under the vacuum
1.3 x
10 ' Pa.
In the some embodiments of the examples I to XX, spray-dried dried form
may be prepared in the spray-drying equipped with a centrifugal atomizer under
the
following conditions; the feed liquid used in this system is approximate 30-40
%
solution of an aqueous soluble starch conversion product and feed flow rate is
50-70
mL/min, the inlet temperature is 150-180 C and the outlet temperature is 50-
100 C.
The feed liquid was atomized by a centrifugal atomizer which is 30,000 rpm as
rotational speed.
In the some embodiment of the examples I to XX, the granules derived froin
the solution formulations of bile acid compositions were produced in the fluid
bed.
The dried powder of the solution formulations of bile acid compositions (20
kg, 100-
200 mesh) and the corn starch (9 kg) were placed in the fluid bed and were
mixed by
using air. Afterwards the binder solution (700g of hydroxypropylmethyl
cellulose in
22 L of water) was sprayed on the fluidizing powder bed using a peristaltic
pump.
The spraying process was carried out according to the settings of the process
variables
for the specific run. During the spraying process, every 10 min, 10 g
samples were
taken from the powder bed for moisture content determination by the loss on
drying.
Spraying was continued until all the binder solution was used. The wetted
granules
were dried by fluidizing them with an inlet air temperature of 75 C. The
drying cycle
was terininated when an outlet air temperature of 35 C was reached, indicating
that
the granules were dried sufficiently.
In the some embodiment of the examples I to XX, the enteric coated granules
derived from the solution formulations of bile acid compositions were produced
in the
top-spray granulator, bottom-spray granulator or tangential-spray granulator.
Dissolved the ethylcellulose N 100 (1.6 kg) in anhydrous ethanol and spray
this
solution and any additional ethanol into the fluidized dry powder of bile acid
compositions (9 kg). Cease spraying when good granules are produced. Dry to
approximately 3% moisture.
CA 02588168 2007-05-16
WO 2006/057637 PCT/US2004/039507
48
ASSAY. (Table 10 and Figure 12). Increased amounts of maltodextrin
(DE=15) as an aqueous soluble starch conversion product in the primary
solution are
associated with increased solubility of the dried material at low pH in the
secondary
solution, particularly within 2 minute. These results indicate that
maltodextrin is
excellent redissolving agent for the dried form of a primary aqueous
solubilized bile
acid formulation.
Table 10
Amount of maltodextrin in the Solution
4g 5g 6g 7g 8 9g
pH T% pH T% pH T% pH T% pH T% pH T%
4.16 0.88 4.34 1.67 4.09 10.18 4.31 4.79 4.15 91.8 1 100
5.22 1.56 5.3 1.77 4.626 10.16 5.223 5.79 4.81 93.0 3 100
5.8 1.87 5.79 4.83 5.026 10.18 5.69 16.71 5.84 96. 5 100
5.9 1.92 6.02 9.52 5.46 10.21 6 29.44 6.27 10 7 100
6.08 2.6 6.15 13.36 5.78 16.06 6.15 41.59 6.82 10 9 100
6.21 3.77 6.28 17.08 6 34.23 6.342 55.76 7.5 10
6.32 5.96 6.39 26.22 6.14 53.25 6.57 68.36 8.25 10
6.41 12.27 6.48 33.99 6.33 73.6 6.74 79.95 9.25 10
6.54 24.76 6.57 38.26 6.54 87.16 6.89 84.66 9.42 10
6.61 34.99 6.68 44.34 6.71 90.61 7.1 91.55
6.73 49.89 6.78 52.65 6.948 93.5 7.25 95.1
6.93 70.66 6.87 57.73 7.116 94.67 7.41 98.48
7.02 75.67 6.92 61.67 7.35 96.24 7.57 99.65
7.18 80.5 6.98 70.2 7.54 97.11 7.7 99.67
7.24 85 7.06 71.88 7.69 98.15 7.86 100
7.34 88 7.145 75.99 7.87 98.54
7.4 90.9 7.27 85.01 8.21 99
7.62 95.54 7.32 87.88
7.665 96.27 7.38 92.17
7.8 97.15 7.47 93.67
8.07 98.46 7.65 95.79
8.15 99.67 7.82 97.05
8.24 100 7.94 97.75
8.25 100 8.06 97.9
~ 8.18 98.75