Note: Descriptions are shown in the official language in which they were submitted.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
Polyester Polymer and Copolymer Compositions
Containing Metallic Molybdenum Particles
FIELD OF THE INVENTION
The invention relates to polyester compositions that are useful in
packaging, such as in the manufacture of beverage containers by reheat
blow molding, or other hot forming processes in which polyester is
reheated. The compositions exhibit improved reheat, while maintaining
acceptable visual appearance, such as clarity and color.
BACKGROUND OF THE INVENTION
Many plastic packages, such as those made from poly(ethylene
terephthalate) (PET) and used in beverage containers, are formed by
reheat blow-molding, or other operations that require heat softening of
the polymer.
In reheat blow-molding, bottle preforms, which are test-tube shaped
extrusion moldings, are heated above the glass transition temperature of
the polymer, and then positioned in a bottle mold to receive pressurized
air through their open end. This technology is well known in the art, as
shown, for example in U.S. Pat. No. 3,733,309, incorporated herein by
reference. In a typical blow-molding operation, radiation energy from
quartz infrared heaters is generally used to reheat the preforms.
~;ke-~:~ --- ncxr~+io=. ~ ..f r.7!'!~.anino containPr~ using operations that
require
n
~---_ packaging
heat softening of the polymer, the reheat time, or the time required for
the preform to reach the proper temperature for stretch blow
molding(also called the heat-up time), affects both the productivity and
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-2-
the energy required. As processing equipment has improved, it has
become possible to produce more units per unit time. Thus it is
desirable to provide polyester compositions which provide improved
reheat properties, by reheating faster(increased reheat rate), or with less
reheat energy (increased reheat efficiency), or both, compared to
conventional polyester compositions.
The aforementioned reheat properties vary with the absorption
characteristics of the polymer itself. Heat lamps used for reheating
polymer preforms are typically infrared heaters, such as quartz infrared
lamps, having a broad light emission spectrum, with wavelengths
ranging from about 500 nm to greater than 1,500 nm. However,
polyesters, especially PET, absorb poorly in the region from 500 nm, to
1,500 nm. Thus in order to maximize energy absorption from the lamps
and increase the preform's reheat rate, materials that will increase
infrared energy absorption are sometimes added to PET. Unfortunately,
these materials tend to have a negative effect on the visual appearance
of PET containers, for example increasing the haze level and/or causing
the article to have a dark appearance. Further, since compounds with
absorbance in the range of 400 nm to 700 nm appear colored to the
human eye, materials that absorb in this wavelength range will impart
color to the polymer.
A variety of black and gray body absorbing compounds have been used
as reheat agents to improve the reheat characteristics of polyester
preforms under reheat lamps. These reheat additives include carbon
black, graphite, antimony metal, black iron oxide, red iron oxide, inert
iron compounds, spinel pigments, and infrared absorbing dyes. The
amount of absorbing compound that can be added to a polymer is
limited by its impact on the visual properties of the polymer, such as
brightness, which may be expressed as an L* value, and color, which is
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-3-
measured and expressed as an a* value and a b* value, as further
described below.
To retain an acceptable level of brightness and color in the preform and
resulting blown articles, the quantity of reheat additive may be
decreased, which in turn decreases reheat rates. Thus, the type and
amount of reheat additive added to a polyester resin is adjusted to strike
the desired balance between increasing the reheat rate and retaining
acceptable brightness and color levels. It would be ideal to
simultaneously increase the reheat rate and decrease the rate at which
color and brightness degrade as the concentration of the reheat additive
in a thermoplastic composition is increased.
There remains a need in the art for polyester compositions containing
reheat additives that improve reheat without the problems associated
with known reheat additives, such as unacceptable reductions in
brightness, color, and clarity.
SUMMARY OF THE INVENTION
The invention relates to polyester compositions that comprise polyester
polymers or copolymers, and especially thermoplastic polyester
polymers or copolymers, having incorporated therein metallic
molybdenum particles that improve the reheat properties of the
compositions. The molybdenum particles may be incorporated in the
polyester by melt compounding, or may be added at any stage of the
polymerization, such as during the melt-phase of the polymerization. A
range of particle sizes may be used, as well as a range of particle size
distributions.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-4-
The polyester compositions according to the invention are suitable for
use in packaging in which a reheat step is desirable or necessary, and
are provided with metallic molybdenum particles to improve reheat
efficiency. These compositions may be provided as a melt, in solid form,
as preforms such as for blow molding, as sheets suitable for
thermoforming, as concentrates, and as bottles, the compositions
comprising a polyester polymer, with metallic molybdenum particles
dispersed in the polyester. Suitable polyesters include polyalkylene
terephthalates and polyalkylene naphthalates.
The invention relates also to_processes for the manufacture of polyester
compositions, in which metallic molybdenum particles may be added to
any stage of a polyester polymerization process, such as during the melt.
phase for the manufacture of polyester polymers. The metallic
molybdenum particles may also be added to the polyester polymer
which is in the form of solid-stated pellets, or to an injection molding
machine for the manufacture of preforms from the polyester polymers.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 depicts molybdenum particle size distribution of the sample used
in Examples 1 through 3 revealed by scanning electron microscopy.
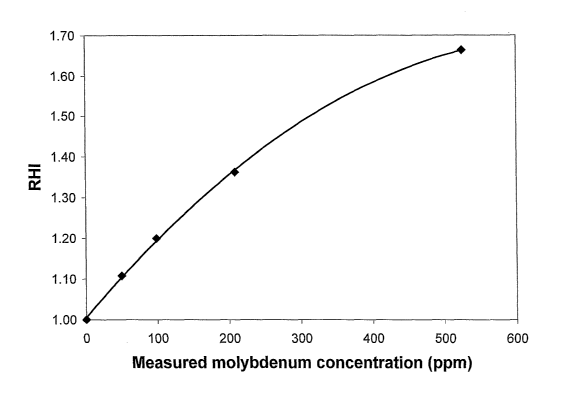
Fig. 2 depicts the relationship between the reheat index and the
concentration of metallic molybdenum particles used as a reheat
additive.
Fig. 3 depicts the impact of the reheat index on the L* value for a
polyester containing metallic moljrbdenum particles.
Fig. 4 depicts the impact of the reheat index on the haze for a polyester
coniaining metallic iilUiybdenurri pariicies.
Fig. 5 depicts the relationship between the reheat index and a* value for
a polyester containing metallic molybdenum particles.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-5-
Fig. 6 depicts the relationship between the reheat index and the b* value
of a polyester containing metallic molybdenum particles.
Fig. 7 depicts the effect of additive concentration on the reheat index for
metallic molybdenum particles added during the polyester
polymerization process.
Fi'g. 8 depicts the relationship between L* value and reheat index for
polyester containing metallic molybdenum particles added during the
polymerization process.
Fig. 9 depicts the relationship between haze and reheat index for
polyesters containing metallic molybdenum particles added to the
polymerization process.
Fig. 10 depicts the relationship between a* value and reheat index for
molybdenum metallic particles added by two different methods: addition
to the polymerization process (pzn) and by melt compounding into a
polyester (cmpd).
Fig. 11 depicts the relationship between b* value and reheat index for
molybdenum metallic particles added by two different methods: addition
to the polymerization process (pzn) and by melt compounding into a
polyester (cmpd).
DETAILED DESCRIPTION OF THE INVENTION
The present invention may be understood more readily by reference to
the following detailed description of the invention, including the
appended figures, and to the examples provided. It is to be understood
that this invention is not limited to the specific processes and conditions
described, because specific processes and process conditions for
processing plastic articles may vary. It is also to be understood that the
terminology used is for the purpose of describing particular
embodiments only and is not intended to be limiting.
A - - A :~ ~-, ~r..~-:':c' ~ õ ,. ~~ .,
~a CS~~ta in ~ ~~ ~i: ~~r~i~a~u~~~.i~: Ã ~r'3:.: tE;a ~;?=.~''!~_ ~..P
singular forms a, an,
and "the" include plural referents unless the context clearly dictates
otherwise. For example, reference to processing a thermoplastic
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-6-
"preform," "container" or "bottle" is intended to include the processing of
a plurality of thermoplastic preforms, articles, containers, or bottles.
By "comprising" or "containing" we mean that at least the named
compound, element, particle, etc. must be present in the composition or
article, but does not exclude the presence of other compounds,
materials, particles, etc., even if the other such compounds, material,
particies, etc. have the same function as what is named.
As used herein, a "d50 particle size" is the median diameter, where 50%
of the volume is composed of particles larger than the stated d50 value,
and 50% of the volume is composed of particles smaller than the stated
d50 value. As used herein, the median particle size is the same as the
d50 particle size.
According to the invention, metallic molybdenum particles are used in
which the molybdenum metal is provided in the elemental state. These
particles are to be distinguished from molybdenum compounds,
including molybdenum (II), molybdenum (III) and molybdenum (IV)
compounds or complexes. Molybdenum compounds are further
described in Kirk-Othmer Encyclopedia of Chemical Technology, Vol 16,
4th ed., (1995) pp. 940 - 962, incorporated herein by reference. Thus
molybdenum compounds which may be used as condensation catalysts
are not intended to fall within the definition of metallic molybdenum
particles. That is, if molybdenum (II) through molybdenum (VI)
compounds are used as condensation catalysts to form the polymer in
the compositions of the claimed invention, such polymers will
additionally contain metallic molybdenum particles in which the
molybdenum is provided in the elemental state, as further described
~r~j-1 r"'~llih!iS~r+l!!~, a!!Cwc ar f ~ hP C ih in
~..._,... -Ã v:iiv:l T _._._? __ f _. . e.L.r#,, r de cr ed
._.~--,_,
Kirk-Othmer Encyclopedia of Chemical Technology, Vol 16, 4th ed.,
(1995) pp. 925-936, incorporated herein by reference,
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-7-
The metallic molybdenum particles useful according to the invention may
predominantly comprise, in terms of weight percent, elemental
molybdenum metal, with typical impurities, in which the molybdenum
metal may be predominantly elemental molybdenum, or a molybdenum
metal alloy in which molybdenum may be alloyed with one or more other
metals, semi-metals, and/or non-metals, so long as the alloys
substantially retain the metallic properties of molybdenum.
Further, the phase or phases present in the metallic molybdenum alloy
particles according to the invention may include amorphous phases,
solid solution phases, or intermetallic compound phase solid solutions,
and may thus be distinguished from compositions comprised
predominantly of molybdenum compounds such as those in which the
molybdenum has a higher oxidation state, although the alloys may, of
course, include compounds of molybdenum that result from the alloying
process, again so long as the alloys substantially retain their metallic
properties.
Alloys useful according to the invention thus include those in which
molybdenum and one or more other metals or nonmetals are intimately
mixed with molybdenum, such as when molten, so that they are fused
together and dissolved with each other to form, at least in part, a solid
solution. We do not mean, of course, to exclude molybdenum alloys that
have measurable amounts of molybdenum compounds present, up to
about 50 wt.%, so iong as such alloys retain substantial metallic
properties, and in any event, the molybdenum present substantially
retains its metallic properties, the presence of molybdenum compounds
in the alloy notwithstanding.
Ailoys are thus suitable for use according to the invention so long as
such ailoys comprise at least 20 wt.% molybdenum metal, or at least 30
wt.% molybdenum, or at least 50 wt.% molybdenum, or at least 60 wt.%
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-8-
molybdenum, or at least 90 wt.% molybdenum, or at least 95 wt.%
molybdenum, as determined, for example, by elemental analysis,
especially when the molybdenum is the major alloying element. Not
wishing to be bound by any theory, we believe that the effectiveness of
molybdenum as a reheat additive may be a function of the absorptive
properties of the molybdenum itself, such as the optical constants in the
wavelength of interest, so that molybdenum alloys are also suitable for
use according to the invention, so long as such alloys have a significant
amount of molybdenum, such as the minimum amounts of molybdenum
as already described.
The metallic molybdenum particles may thus be elemental molybdenum,
or may be a molybdenum metal alloy in which molybdenum is alloy,ed.=
with one or more other materials, such as other metals, so long as such
other materials do not substantially affect the ability of the particles to
increase the reheat properties of the polymer compositions.
We note that molybdenum metal particles can be produced by numerous
techniques, as described in the Powder Metallurgy entry in Kirk-Othmer
Encyclopedia of Chemical Technology, Vol 16, 4th ed., (1995) pp. 353 -
392, incorporated herein by reference. For example, the molybdenum
metal particles according to the invention may be formed by atomization,
reduction, decomposition, electrolytic deposition, precipitation, electrode
spinning, high energy impaction, mechanical comminution,
condensation, decomposition of metal hydrides, or rapid solidification
technology. According to the reference from the Encyclopedia of
Chemical Technology (Kirk-Othmer, Vol. 16, pp. 925 - 936),
molybdenum powder can be produced as follows: technical grade
molybdenum trioxide or ammonium molybdate is reduced to
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-9-
molybdenum powder by hydrogen at 500 C to 1,150 C in a boat-type
or tube-type furnace.
Shapes of metallic molybdenum powder which can be used in this
invention include, but are not limited to, the following: acicular powder,
angular powder, dendritic powder, equi-axed powder, flake powder,
fragmented powder, granular powder, irregular powder, nodular powder,
platelet powder, porous powder, rounded powder, and spherical powder.
The particles may be of a filamentary structure, where the individual
particles may be loose aggregates of smaller particles attached to form a
bead or chain-like structure. The overall size of the particles may be
variable, due to a variation in chain length and degree of,branching.
Metallic molybdenum particles useful according to the invention for the.
improvement of reheat and color in polyester compositions include those
having a range of particle sizes and particle size distributions, although
we have found certain particle sizes and relatively narrow particle size
distributions to be especially suitable in certain applications. For
example, in some embodiments, especially those in which the polyester
comprises PET, metallic molybdenum particles having a median particle
size of approximately 0.15 micrometers (pm), and a relatively narrow
particle size distribution, are advantageous.
The size of the metallic molybdenum particles may thus vary within a
broad range depending on the method of production, and the numerical
values for the particle sizes may vary according to the shape of the
particles and the method of measurement. Particle sizes useful
according to the invention may be from about 0.005 pm to about 10 pm,
or from 0.05 pm to 1 pm, or from 0.05 pm to 0.9 pm. When the
(.,Z: i ~.Ir:isL.~7 E r-~E i -r , 4rS- i i G v.n.. ~E~3t;i ti,: r ,s C i 3
+6v..d t bp i ..6 Ct'cr.-.i!.. '
nn ly G~:.~-1.~r .t
aJ i 1~.3~f3=i;r.-:
-.~t3 poi~tG: i, ite ~J i à ~i C~. 3iGGJ
from 0.08 pm to 1.1 pm are especially suitable.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-10-
The particles useful according to the invention may likewise be
molybdenum hollow spheres or molybdenum-coated spheres, in which
the core is comprised of molybdenum, of mixtures of molybdenum with
other materials, or of other materials in the substantial absence of
molybdenum. Again; not wishing to be bound by any theory, we think it
likely that the effectiveness of molybdenum as a reheat additive is a
function of the absorptive properties of the molybdenum itself, so that
molybdenum-coated particles are suitable for use according to the
invention, so long as the coating thickness is sufficient to provide
adequate reheat properties. Thus, in various embodiments, the
thickness of the coating may be from about 0.005 pm to about 10 pm, or
from 0.01 pm to 5 pm, or from 0.10 pm to 0.5 pm. Such molybdenum
coatings may also comprise molybdenum alloys, as already described..
Metal particles, which have a mean particle size suitable for the
invention, may have irregular shapes and form chain-like structures,
although roughly spherical particles may be preferred. The particle size
and particle size distribution may be measured by methods such as
those described in the Size Measurement of Particles entry of Kirk-
Othmer Encyclopedia of Chemical Technology, 4th ed., vol 22, pp. 256 -
278, incorporated herein by reference. For example, particle size and
particle size distributions may be determined using a Fisher Subsieve
Sizer or a Microtrac Particle-Size Analyzer manufactured by Leeds and
Northrop Company, or by microscopic techniques, such as scanning
electron microscopy or transmission electron microscopy.
The amount of metallic molybdenum particles present in the polyester
compositions according to the invention may vary within a wide range,
for example from about 0.5 ppm to about 1,000 ppm, or from I to 500
ppm, or from 5 to 100 ppm, or from 5 ppm to 50 ppm. Thermoplastic
concentrates according to the invention may, of course, have amounts
greater than these, as further described elsewhere herein.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-11-
The metallic molybdenum particles according to the claimed invention
may be pure molybdenum, or may be particles coated with molybdenum,
or may be molybdenum alloyed with one or more other metals. Metals
that can be alloyed with molybdenum in amounts up to 50 wt.% or more
include nickel, germanium, iron, chromium, tungsten, titanium,
vanadium, carbon, and tantalum. Metals that can be present in minor
amounts, for example up to about 10 wt.% or more include gold, silver,
copper, aluminum, manganese, and silicon.
The metallic molybdenum particles may thus be elemental molybdenum,
or may include other materials, such as other metals, so long as such
other materials do not substantially affect the ability of the particles:to
increase the reheat properties of the polymer compositions.
The molybdenum metal particles can be coated with a fine layer of
molybdenum oxide or other coating, so long as the oxide coating does
not substantially affect the ability of the molybdenum particles to
increase the reheat efficiency of the polymer compositions.
A range of particle size distributions may be useful according to the
invention. The particle size distribution, as used herein, may be
expressed by "span (S)," where S is calculated by the following
equation:
s - d90 - d10
d50
r:....a.. ~yo rc~:;reSei tt , f.~. c -1~~!r. S~ '.: i~- L (..-il10~ %t1L,' ~:;-
;1,-..,,;. :..
vvi ;~t ~: C ~;~cr~it,Ã.: i à u~i ~?~ a iÃ~
composed of particles smaller than the stated d90; and djo represents a
particle size in which 10% of the volume is composed of particles
smaller than the stated djo; and d50 represents a particle size in which
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-12-
50% of the volume is composed of particles larger than the stated d50
value, and 50% of the volume is composed of particles smaller than the
stated d50 value.
Thus, particle size distributions in which the span (S) is from 0 to 10, or
from 0 to 5, or from 0.01 to 2, may be used according to the invention.
In order to obtain a good dispersion of metallic molybdenum particles in
the polyester compositions, a concentrate, containing for example about
500 ppm metallic molybdenum particles, may be prepared using a
polyester such as a commercial grade of PET. The concentrate may
then be let down into a polyester at the desired concentration, ranging,
for example, from 1 ppm to 500 ppm.
The amount of metallic molybdenum particles used in the polyester will
depend upon the particular application, the desired reduction in reheat
time, and the toleration level in the reduction of a* and b* away from
zero along with the movement of L* brightness values away from 100.
Thus, in various embodiments, the quantity of metallic molybdenum
particles may be at least 1 ppm, or at least 5 ppm, or at least 50 ppm. In
many applications, the quantity of metallic molybdenum particles may be
at least 50 ppm, in some cases at least 60 ppm, and even at least 70
ppm. The maximum amount of metallic molybdenum particles may be
limited by one or more of the desired reheat rate, or maintenance in L*,
b* and haze, which may vary among applications or customer
requirements. In some embodiments, the amount may not exceed 500
ppm, or may be at or below 300 ppm, or may not exceed 250 ppm. In
those applications where color, haze, and brightness are not important
features to the application, however, the amount of metallic molybdenum
particles used may be up to 1,000 ppm, or up to 5,000 ppm, or even up
to 10,000 ppm. The amount can exceed 10,000 ppm when formulating
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-13-
a concentrate with metallic molybdenum particles as discussed
elsewhere in this application.
The method by which the metallic molybdenum particles are
incorporated into the polyester composition is not limited. The metallic
molybdenum particles can be added to the polymer reactant system,
during or after polymerization, to the polymer melt, or to the molding
powder or pellets or molten polyester in the injection-molding machine
from which the bottle preforms are made. They may be added at
locations including, but not limited to, proximate the inlet to the
esterification reactor, proximate the outlet of the esterification reactor, at
a point between the inlet and the outlet of the esterification reactor,
anywhere along the recirculation loop, proximate the inlet to the
prepolymer reactor, proximate the outlet to the prepolymer reactor, at a
point between the inlet and the outlet of the prepolymer reactor,
proximate the inlet to the polycondensation reactor, or at a point
between the inlet and the outlet of the polycondensation reactor.
The metallic molybdenum particles may be added to a polyester
polymer, such as PET, and fed to an injection molding machine by any
method, including feeding the metallic molybdenum particles to the
molten polymer in the injection molding machine, or by combining the
metallic molybdenum particles with a feed of PET to the injection
molding machine, either by melt blending or by dry blending pellets.
Alternatively, the metallic molybdenum particles may be added to an
esterification reactor, such as with and through the ethylene glycol feed
optionally combined with phosphoric acid, to a prepolymer reactor; to a
polycondensation reactor, or to solid pellets in a reactor for solid stating,
or at any point in-between any of these stages. In each of these cases,
the metallic molybdenum particles may be combined with PET or its
precursors neat, as a concentrate containing PET, or diluted with a
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-14-
carrier. The carrier may be reactive to PET or may be non-reactive.
The metallic molybdenum particles, whether neat or in a concentrate or
in a carrier, and the bulk polyester, may be dried prior to mixing together.
These may be dried in an atmosphere of dried air or other inert gas,
such as nitrogen, and if desired, under sub-atmospheric pressure.
The impact of a reheat additive on the color of the polymer can be
judged using a tristimulus color scale, such as the CIE L*a*b* scale.
The L* value ranges from 0 to 100 and measures dark to light. The a*
value measures red to green with positive values being red and negative
values green. The b* value measures yellow to blue with yellow having
positive values and blue negative values.
Color measurement theory and practice are discussed in greater detail
in Principles of Color Technology, pp.25-66 by Fred W. Billmeyer, Jr.,
John Wiley & Sons, New York (1981), incorporated herein by reference.
L* values for the polyester compositions as measured on twenty-ounce
bottle preforms discussed herein should generally be greater than 60,
more preferably at least 65, and more preferably yet at least 70.
Specifying a particular L* brightness does not imply that a preform
having a particular sidewall cross-sectional thickness is actually used,
but only that in the event the L* is measured, the polyester composition
actually used is, for purposes of testing and evaluating the L* of the
composition, injection molded to make a preform having a sidewall
cross-sectional thickness of 0.154 inches.
The color of a desirable polyester composition, as measured in twenty-
ounce bottle preforms having a nominal sidewall cross-sectional
_._
~ri
JG Ct i__ici~ .ne5s o_i~~,.=_.(5.~i .i'{l..i iej_, .is .iiy ii it..I~t:~_ . __
_i.~~i _i_i a* __ii._ia't~
= . . i.~~ ci i:i;li~i i.l. _
i~f.~nt-'.t"ci.~ _
value preferably ranging from about minus 2.0 to about plus 1.0, or from
about minus 1.5 to about plus 0.5. With respect to a b* coordinate
value, it is generally desired to make a bottle preform having a b* value
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-15-
coordinate ranging from minus 3.0 to positive value of less than plus 5.0,
or less than plus 4.0, or less than plus 3.8.
Polyesters according to the invention having an acceptable bottle
sidewall haze generally have a haze value, as measured on samples
having a cross-sectional thickness of about 0.0125 inches, of less than
6.0%, or less than 5.0%, or less than 4.0%, or 3.0% or less.
The measurements of L*, a* and b* color values are conducted
according to the following method. The instrument used for measuring
b* color should have the capabilities of a HunterLab UltraScan XE,
model U3350, using the CIE Lab Scale (L*, a*, b*), D65 (ASTM)
illuminant, 100 observer and an integrating sphere geometry. Clear,
plaques, films, preforms, bottles, and are tested in the transmission
mode under ASTM-D1746 "Standard Test Method for Transparency of
Plastic Sheeting." The instrument for measuring color is set up under
ASTM E1164 "Standard Practice for Obtaining Spectrophotometric Data
for Object-Color Evaluation."
More particularly, the following test methods can be used, depending
upon whether the sample is a preform, or a bottle. Color measurements
should be performed using a HunterLab UltraScan XE (Hunter
Associates Laboratory, Inc., Reston VA), which employs diffuse/8
(illumination/view angle) sphere optical geometry, or equivalent
equipment with these same basic capabilities. The color scale
employed is the CIE L*a*b* scale with D65 illuminant and 10 observer
specified.
Preforms having a mean outer diameter of 0.846 inches and a wall 30 thickness
of 0.154 inches, and bottle sidewall sections having a wall
thickness of 0.0115 inches to 0.012 inches are measured in regular
transmission mode using ASTM D1746, "Standard Test Method for
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-16-
Transparency of Plastic Sheeting". Preforms are held in place in the
instrument using a preform holder, available from HunterLab, and
triplicate measurements are averaged, whereby the sample is rotated
90 about its center axis between each measurement.
The intrinsic viscosity (It.V.) values described throughout this description
are set forth in dL/g units as calculated from the inherent viscosity (Ih.V.)
measured at 25 C in 60/40 wt/wt phenol/tetrachloroethane. The
inherent viscosity is calculated from the measured solution viscosity.
The following equations describe these solution viscosity
measurements, and subsequent calculations to Ih.V. and from Ih.V. to
It.V:
'ni.h = [in (ts/to) l /C
where 'n;,,n = Inherent viscosity at 25 C at a polymer
concentration of 0.50 g/ 100 mL of 60% phenol and
40% 1,1,2,2-tetrachloroethane
In = Natural logarithm
ts = Sample flow time through a capillary tube
t = Solvent-blank flow time through a capillary tube
C Concentration of polymer in grams per 100 mL
of solvent (0.50%)
The intrinsic viscosity is the limiting value at infinite dilution of the
specific viscosity of a polymer. It is defined by the following equation:
71int = lim (rlsp/C) = lim in (,qr/C)
r_~n C->0
where rl;.nt = Intrinsic viscosity
r~r = Relative viscosity = ts/to
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-17-
r}sp = Specific viscosity
Instrument calibration involves replicate testing of a standard reference
material and then applying appropriate mathematical equations to
produce the "accepted" I.V. vaTues.
Calibration Factor = Accepted IV of Reference Material /
Average of Replicate Determinations
Corrected IhV = Calculated IhV x Calibration Factor
The intrinsic viscosity (ItV or h;,,t) may be estimated using the
Billmeyer equation as follows:
= 0.5 [e 0.5 x Corrected IhV - 11 + (0.75 x Corrected
11int
IhV)
Thus, a beneficial feature provided by polyester compositions containing
metallic molybdenum particles is that the compositions and preforms
made from these compositions have an improved reheat, as expressed
by twenty-ounce bottle preform surface temperature (PST), rate relative
to a control without a reheat additive. The higher the PST value, the
higher the reheat rate.
In some embodiments, the polyester compositions containing metallic
molybdenum particles, and preforms made from these compositions,
may have a b* color of less than 4.0, or less than 3.8, or less than 3.7,
and in any case greater than minus 3.0, even at loadings ranging from
100 ppm to 200 ppm. Similarly, preforms from the polyester
compositions according to the invention may have an L* brightness of at
least 60.0, or at least 65.0, or at least 70Ø The compositions may also
result in an increase in bottle sidewall percent haze that is much less
than compositions containing other types of reheat additives at the same
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-18-
levels of reheat rate. The sidewall bottle haze value measured at a
thickness of 0.0125 inches (+/- 0.004) may be 6.0% or less, or 5.0% or
less, or even 4.0% or less.
According to the invention, in various embodiments, there are thus
provided concentrate compositions comprising metallic molybdenum
particles in an amount of at least 0.05 wt.%, or at least 2 wt.%, and up to
about 20 wt.%, or up to 35 wt.%, and a thermoplastic polymer normally
solid at 25 C and 1 atm such as a polyester, polyolefin, or polycarbonate
in an amount of at least 65 wt.%, or at least 80 wt.%, or up to 99 wt.% or
more, each based on the weight of the concentrate composition. The
concentrate may be in liquid, molten state, or solid form. The converter
of polymer to preforms has the flexibility of adding metallic molybdenum
particles to bulk polyester at the injection molding stage continuously, or
intermittently, in liquid molten form or as a solid blend, and further
adjusting the amount of metallic molybdenum particles contained in the
preform by metering the amount of concentrate to fit the end use
application and customer requirements.
The concentrate may be made by mixing metallic molybdenum particles
with a polymer such as a polycarbonate, a polyester, a polyolefin, or
mixtures of these, in a single or twin-screw extruder, and optionally
compounding with other reheat additives. A suitable polycarbonate is
bisphenol A polycarbonate. Suitable polyolefins include, but are not
limited to, polyethylene and polypropylene, and copolymers thereof.
Melt temperatures should be at least as high as the melting point of the
polymer. For a polyester, such as PET the melt temperatures are
typically in the range of 250 C to 310 C. Preferably, the melt
compounding temperature is maintained as low as possible. The
extrudate may be withdrawn in any form, such as a strand form, and
recovered according to the usual way such as cutting.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
,....... .. .. ..... .,...,. ...,,,. ,,., .. .n,.,. .,..... ..... ...... -19-
The concentrate may be prepared in a similar polyester as used in the
final article. However, in some cases it may be advantageous to use
another polymer in the concentrate, such as a polyolefin. In the case
where a polyolefin/ metallic molybdenum particles concentrate is
blended with the polyester, the polyolefin can be incorporated as a
nucleator additive for the bulk polyester.
The concentrate may be added to a bulk polyester or anywhere along
the different stages for manufacturing PET, in a manner such that the
concentrate is compatible with the bulk polyester or its precursors. For
example, the point of addition or the It.V. of the concentrate may be
chosen such that the lt.V. of the polyethylene terephthalate and the It.V.
of the concentrate are similar, e.g. +1- 0.2 It.V. measured at 25 C in a
60/40 wt/wt phenol/tetrachloroethane solution. A concentrate can be
made with an lt.V. ranging from 0.3 dL/g to 1.1 dL/g to match the typical
lt.V. of a polyethylene terephthalate under manufacture in the
polycondensation stage. Alternatively, a concentrate can be made with
an It.V. similar to that of solid-stated pellets used at the injection molding
stage (e.g. It.V. from 0.6 dL/g to 1.1 dL/g).
Other components can be added to the polymer compositions of the
present invention to enhance the performance properties of the
polyester composition. For example, crystallization aids, impact
modifiers, surface lubricants, denesting agents, stabilizers, antioxidants,
ultraviolet light absorbing agents, catalyst deactivators, colorants,
nucleating agents, acetaidehyde reducing compounds, other reheat
enhancing aids, fillers, anti-abrasion additives, and the like can be
included. The resin may also contain small amounts of branching
agents such as trifunctional or tetrafunctional comonomers such as
trimellitic anhydride, trimethylol propane, pyromellitic dianhydride,
pentaerythritol, and other polyester forming polyacids or polyols
generally known in the art. All of these additives and many others and
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-20-
their use are well known in the art. Any of these compounds can be
used in the present composition.
The polyester compositions of the present invention may be used to
form preforms used for preparing packaging containers. The preform is
typically heated above the glass transition temperature of the polymer
composition by passing the preform through a bank of quartz infrared
heating lamps, positioning the preform in a bottle mold, and then blowing
pressurized air through the open end of the mold.
A variety of other articles can be made from the polyester compositions
of the invention. Articles include sheet, film, bottles, trays, other
packaging, rods, tubes, lids, and injection molded articles. Any type, of
bottle can be made from the polyester compositions of the invention.,
Thus, in one embodiment, there is provided a beverage bottle made
from PET suitable for holding water. In another embodiment, there is
provided a heat-set beverage bottle suitable for holding beverages or
foods which are hot-filled into the container. In yet another embodiment,
the bottle is suitable for holding carbonated soft drinks.
The metallic molybdenum particle reheat additives used in the invention
affect the reheat rate, brightness, and color of preforms and the haze
value of the bottles made from these preforms. Any one or more of
these performance characteristics can be adjusted by varying the
amount of reheat additive used, or by changing the particle size, or the
particle size distribution.
The invention also provides processes for making polyester preforms
that comprise feeding a liquid or solid bulk polyester and a liquid, molten
or solid polyester concentrate composition to a machine for
manufacturing the preform, the concentrate being as described
elsewhere. According to the invention, not only may the concentrate be
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-21 -
added at the stage for making preforms, but in other embodiments, there
are provided processes for the manufacture of polyester compositions
that comprise adding a concentrate polyester composition to a melt
phase for the manufacture of virgin polyester polymers, the concentrate
comprising metallic molybdenum particles and at least 65 wt.% of a
polyester polymer. Alternatively, the molybdenum particles may be
added to recycled PET.
The polyester compositions according to the invention have a good
reheat rate with improved L* and b* ratings, and low bottle sidewall
haze.
In yet another embodiment of the invention, there is provided a polyester
beverage bottle made from a preform, wherein the preform has a PST of
112 C or more and an L* value of 60.0 or more.
In each of the described embodiments, there are also provided
additional embodiments encompassing the processes for the
manufacture of each, and the preforms and articles, and in particular
bottles, blow-molded from the preforms, as well as their compositions
containing metallic molybdenum particles.
The polyester compositions of this invention may be any thermoplastic
polymers, optionally containing any number of ingredients in any
amounts, provided that the polyester component of the polymer is
present in an amount of at least 30 wt%, or at least 50 wt.%, or at least
80 wt.%, or even 90 wt.% or more, based on the weight of the polymer,
the backbone of the polymer typically including repeating terephthalate
or naphthalate units.
Examples of suitable polyester polymers include one or more of: PET,
polyethylene naphthalate (PEN), poly(1,4-cyclo-hexylenedimethylene)
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-22-
terephthalate (PCT), poly(ethylene-co-1,4-cyclohexanedimethylene
terephthalate) (PETG), copoly(1,4-cyclohexylene dimethylene/ethylene
terephthalate) (PCTG) and their blends or their copolymers. The form of
the polyester composition is not limited, and includes a melt in the
manufacturing process or in the molten state after polymerization, such
as may be found in an injection molding machine, and in the form of a
liquid, pellets, preforms, and/or bottles. Polyester pellets may be
isolated as a solid at 25 C and 1 atm in order for ease of transport and
processing. The shape of the polyester pellet is not limited, and is
typified by regular or irregular shaped discrete particles and may be
distinguished from a sheet, film, or fiber.
It should also be understood that as used herein, the term polyester is
intended to include polyester derivatives, including, but not limited to,
polyether esters, polyester amides, and polyetherester amides.
Therefore, for simplicity, throughout the specification and claims, the
terms polyester, polyether ester, polyester amide, and polyetherester
amide may be used interchangeably and are typically referred to as
polyester, but it is understood that the particular polyester species is
dependant on the starting materials, i.e., polyester precursor reactants
and/or components.
The location of the metallic molybdenum particles within the polyester
compositions is not limited. The metallic molybdenum particles may be
disposed anywhere on or within the polyester polymer, pellet, preform,
or bottle. Preferably, the polyester polymer in the form of a pellet forms
a continuous phase. By being distributed "within" the continuous phase
we mean that the metallic molybdenum particles are found at least within
a portion of a cross-sectional cut of the pellet. The metallic molybdenum
particles may be distributed within the polyester polymer randomly,
distributed within discrete regions, or distributed only within a portion of
the polymer. In a preferred embodiment, the metallic molybdenum
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-23-
particles are disposed randomly throughout the polyester polymer
composition as by way of adding the metallic molybdenum particles to a
melt, or by mixing the metallic molybdenum particles with a solid
polyester composition followed by melting and mixing.
The metallic molybdenum particles may be added in an amount so as to
achieve a preform surface temperature of at least 112 C, or at least
115 C, or at least 120 C, while maintaining an L* brightness of 60 or
more, when measured at a PST of 112 C.
Suitable amounts of metallic molybdenum particles in the polyester
compositions (other than polyester concentrate compositions as
discussed elsewhere), preforms, and containers, may thus range from
about 0.5 ppm to about 500 ppm, based on the weight of the polymer in
the polyester compositions, or as already described. The amount of the
metallic molybdenum particles used may depend on the type and quality
of the metallic molybdenum particles, the particle size, surface area, the
morphology of the particle, and the level of reheat rate improvement
desired.
The particle size may be measured with a laser diffraction type particle
size distribution meter, or scanning or transmission electron microscopy
methods. Alternatively, the particle size can be correlated by a
percentage of particles screened through a mesh. Metallic molybdenum
particles having a particle size distribution in which at least 80%,
preferably at least 90%, more preferably at least 95% of the particles fall
through an ASTM-E11 140 sieve are suitable for use as reheat agents.
Metallic molybdenum particles having a particle size distribution in which
at least 80%, preferably at least 90%, more preferably at least 95% of
the particies fall through a ASTN!-E11 325 sieve are also suitable for
use as reheat agents.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-24-
The metallic molybdenum particles used in the invention not only
enhance the reheat rate of a preform, but have only a minimal impact on
the brightness of the preforms and bottles by not reducing the L* below
acceptable levels. An acceptable L* value of preforms or bottles is
deemed 60 or more when measured at a PST of 112 C.
In various other embodiments, there are provided polyester
compositions, whether in the form of a melt, pellets, sheets, preforms,
and/or bottles, comprising at least 0.5 ppm, or at least 50 ppm, or at
ieast 100 ppm metallic molybdenum particles, having a d50 particle size
of less than 100 m, or less than 50 m, or less than I m or less,
wherein the polyester compositions have an L* value of 60 or more, or
68 or more, or even 70 or more, when measured at a PST of 112 C; or
115 C, or 120 C.
According to various embodiments of the invention, metallic
molybdenum particles may be added at any point during polymerization,
which includes to the esterification zone, to the polycondensation zone
comprised of the prepolymer zone and the finishing zone, to or prior to
the pelletizing or particulating zone, and at any point between or among
these zones. The metallic molybdenum particles may also be added to
solid-stated pellets as they are exiting the solid-stating reactor.
Furthermore, metailic molybdenum particles may be added to the PET
pellets in combination with other feeds to the injection molding machine,
or may be fed separately to the injection molding machine. For
clarification, the metallic molybdenum particles may be added in the melt
phase or to an injection molding machine without solidifying and isolating
the polyester composition into pellets. Thus, the metallic molybdenum
particles can also be added in a melt-to-mold process at any point in the
process for making the preforms. In each instance at a point of addition,
the metallic molybdenum particles can be added as a powder neat, or in
a liquid, or a polymer concentrate, and can be added to virgin or
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-25-
recycled PET, or added as a polymer concentrate using virgin or
recycled PET as the PET polymer carrier.
In other embodiments, the invention relates to processes for the
manufacture of polyester compositions containing metallic molybdenum
particles, such as polyalkylene terephthalate or naphthalate polymers
made by transesterifying a dialkyl terephthalate or dialkyl naphthalate or
by directly esterifying terephthalic acid or naphthalene dicarboxylic acid.
Thus, there are provided processes for making polyalkylene
terephthalate or naphthalate polymer compositions by transesterifying a
dialkyl terephthalate or naphthalate or directly esterifying a terephthalic
acid or naphthalene dicarboxylic acid with a diol, adding metallic
molybdenum particles to the melt phase for the production of a
polyalkylene terephthalate or naphthalate after the prepolymer zone, or
to polyalkylene terephthalate or naphthalate solids, or to an injection
molding machine for the manufacture of bottle preforms.
Each of these process embodiments, along with a description of the
polyester polymers, is now explained in further detail.
The polyester polymer may, for example, be PET, PEN, or copolymers
or mixtures, thereof. A preferred polyester polymer is polyethylene
terephthalate. As used herein, a polyalkylene terephthalate polymer or
polyalkylene naphthalate polymer means a polymer having polyalkylene
terephthalate units or polyalkylene naphthalate units in an amount of at
least 60 mole% based ori the total moles of units in the polymer,
respectively. Thus, the polymer may contain ethylene terephthalate or
naphthalate units in an amount of at least 85 mole%, or at least 90
mole%, or at least 92 mole%, or at least 96 mole%, as measured by the
mole% of ingredients added to the reaction mixture. Thus, a
polyethylene terephthalate polymer may comprise a copolyester of
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-26-
ethylene terephthalate units and other units derived from an alkylene
glycol or aryl glycol with a aliphatic or aryl dicarboxylic acid.
While reference is made in certain instances to polyethylene
terephthalate, it is to be understood that the polymer may also be a
polyalkylene naphthalate polymer or another polyester described herein.
Polyethylene terephthalate can be manufactured by reacting a diacid or
diester component comprising at least 60 mole % terephthalic acid or C,
- C4 dialkylterephthalate, or at least 70 mole %, or at least 85 mole %, or
at least 90 mole %, and for many applications at least 95 mole%,, and a
diol component comprising at least 60 mole % ethylene glycol, or at
least 70 mole %, or at (east 85 mole or at least 90 mole %, and for
many applications, at least 95 mole %. It is preferable that the diacid
component is terephthalic acid and the diol component is ethylene
glycol. The mole percentage for all the diacid component(s) totals 100
mole %, and the mole percentage for all the diol component(s) totals 100
mole %.
The polyester pellet compositions may include admixtures of
polyalkylene terephthalates, PEN, or mixtures thereof, along with other
thermoplastic polymers, such as polycarbonates and polyamides. It is
preferred in many instances that the polyester composition comprise a
majority of a polyalkylene terephthalate polymers or PEN polymers, or in
an amount of at least 80 wt.%, or at least 95 wt.%, based on the weight
of polymers (excluding fillers, compounds, inorganic compounds or
particles, fibers, impact modifiers, or other polymers which may form a
discontinuous phase). In addition to uriits derived from terephthalic acid,
the acid component of the present polyester may be modified with, or
'ju replaced by, units derived from one or more additional dicarboxylic
acids, such as aromatic dicarboxylic acids preferably having from 8 to 14
carbon atoms, aliphatic dicarboxylic acids preferably having 4 to 12
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-27-
carbon atoms, or cycloaliphatic dicarboxylic acids preferably having 8 to
12 carbon atoms.
Examples of dicarboxylic acid units useful for modifying the acid
component are units from phthalic acid, isophthalic acid, naphthalene-
2,6-dicarboxylic acid, cyclohexanedicarboxylic acid, cyclohexanediacetic
acid, diphenyl-4,4'-dicarboxylic acid, succinic acid, glutaric acid, adipic
acid, azelaic acid, sebacic acid, and the like, with isophthalic acid,
naphthalene-2,6-dicarboxylic acid, and cyclohexanedicarboxylic acid
being preferable.
It should be understood that use of the corresponding acid anhydrides,
esters, and acid chlorides of these acids is included in the term
"dicarboxylic acid".
In addition to units derived from ethylene glycol, the diol component of
the present polyester may be modified with, or replaced by, units from
other diols including cycloaliphatic diols preferably having 6 to 20 carbon
atoms and aliphatic diols preferably having 2 to 20 carbon atoms.
Examples of such diols include diethylene glycol; triethylene glycol; 1,4-
cyclohexanedimethanol; propane-l,3-diol; butane-1,4-diol; pentane-1,5-
diol; hexane-1,6-diol; 3-methylpentanediol- (2,4); 2-methylpentanediol-
(1,4); 2,2,4-trimethylpentane-diol-(1,3); 2,5- ethylhexanediol-(1,3); 2,2-
diethyl propane-diol-(1, 3); hexanediol-(1,3); 1,4-di-(hydroxyethoxy)-
benzene; 2,2-bis-(4-hydroxycyclohexyl)-propane; 2,4- dihydroxy-1,1,3,3-
tetramethyl-cyclobutane; 2,2-bis-(3-hydroxyethoxyphenyl)-propane; and
2,2-b is-(4-hyd roxyp ro poxyp he nyl)-p ro pane.
The polyester compositions of the invention may be prepared by
conventional polymerization procedures well-known in the art sufficient
to effect esterification and polycondensation. Polyester melt phase
manufacturing processes include direct condensation of a dicarboxylic
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
- 28 -
acid with a diol optionally in the presence of esterification catalysts in the
esterification zone, followed by polycondensation in the prepolymer and
finishing zones in the presence of a polycondensation catalyst; or else
ester interchange usually in the presence of a transesterification catalyst
in the esterification zone, followed by prepolymerization and finishing in
the presence of a polycondensation catalyst, and each may optionally be
subsequently solid-stated according to known methods. After melt
phase and/or solid-state polycondensation the polyester polymer
compositions typically have an intrinsic viscosity (It.V.) ranging from 0.55
dUg to about 0.70 dL/g as precursor pellets, and an It.V. ranging from
about 0.70 dL/g to about 1.1 dL/g for solid stated pellets.
To further illustrate, a mixture of one or more dicarboxylic acids,
preferably aromatic dicarboxylic acids, or ester forming derivatives
thereof, and one or more diols, are continuously fed to an esterification
reactor operated at a temperature of between about 200 C and 300 C,
typically between 240 C and 290 C, and at a pressure of about 1 psig
up to about 70 psig. The residence time of the reactants typically ranges
from between about one and five hours. Normally, the dicarboxylic, acid
is directly esterified with diol(s) at elevated pressure and at a
temperature of about 240 C to about 270 C. The esterification reaction
is continued until a degree of esterification of at least 60% is achieved,
but more typically until a degree of esterification of at least 85% is
achieved to make the desired monomer. The esterification monomer
reaction is typically uncatalyzed in the direct esterification process and
catalyzed in transesterification processes. Polycondensation catalysts
may optionally be added in the esterification zone along with
esterification/transesterification catalysts.
Typical esterification/transesterification catalysts which may be used
include titanium alkoxides, dibutyl tin dilaurate, used separately or'in
combination, optionally with zinc, manganese, or magnesium acetates or
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-29-
benzoates and/or other such catalyst materials as are well known to
those skilled in the art. Phosphorus-containing compounds and cobalt
compounds may also be present in the esterification zone. The resulting
products formed in the esterification zone include bis(2-hydroxyethyl)
terephthalate (BHET) monomer, low molecular weight oligomers, DEG,
and water as the condensation by-product, along with other trace
impurities formed by the reaction of the catalyst and other compounds
such as colorants or the phosphorus-containing compounds. The
relative amounts of BHET and oligomeric species will vary depending on
whether the process is a direct esterification process, in which case the
amount of oligomeric species are significant and even present as the
major species, or a transesterification process, in which case the relative
quantity of BHET predominates over the oligomeric species. The water.
is removed as the esterification reaction proceeds and excess ethylene
glycol is removed to provide favorable equilibrium conditions. The
esterification zone typically produces the monomer and oligomer
mixture, if any, continuously in a series of one or more reactors.
Alternatively, the monomer and oligomer mixture could be produced in.
one or more batch reactors.
It is understood, however, that in a process for making PEN, the reaction
mixture will contain monomeric species such as bis(2-hydroxyethyl)
naphthalate and its corresponding oligomers. Once the ester monomer
is made to the desired degree of esterification, it is transported from the
esterification reactors in the esterification zone to the polycondensation
zone comprised of a prepolymer zone and a finishing zone.
Polycondensation reactions are initiated and continued in the melt phase
in a prepolymerization zone and finished in the melt phase in a finishing
zone, after which the melt may solidified into precursor solids in the form
of chips, pellets, or any other shape. For convenience, solids are
referred to as pellets, but it is understood that a pellet can have any
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-30-
shape, structure, or consistency. If desired, the polycondensation
reaction may be continued by solid-stating the precursor pellets in a
solid-stating zone. ,
Although reference is made to a prepolymer zone and a finishing zone, it
is to be understood that each zone may comprise a series of one or
more distinct reaction vessels operating at different conditions, or the
zones may be combined into one reaction vessel using one or more sub-
stages operating at different conditions in a single reactor. That is, the
prepolymer stage can involve the use of one or more reactors operated
continuously, one or more batch reactors or even one or more reaction
steps or sub-stages performed in a single reactor vessel. In some
reactor designs, the prepolymerization zone represents the first half.ofõ
polycondensation in terms of reaction time, while the finishing zone
represents the second half of polycondensation. While other reactor
designs may adjust the residence time between the prepolymerization
zone to the finishing zone at about a 2:1 ratio, a common distinction., in
all designs between the prepolymerization zone and the finishing zone is
that the latter zone operates at a higher temperature, lower pressure;
and a higher surface renewal rate than the operating conditions in.the
prepolymerization zone. Generally, each of the prepolymerization and
the finishing zones comprise one or a series of more than one reaction
vessel, and the prepolymerization and finishing reactors are sequenced
in a series as part of a continuous process for the manufacture of the
polyester polymer.
In the prepolymerization zone, also known in the industry as the low
polymerizer, the low molecular weight monomers and minor amounts of
oligomers are polymerized via polycondensation to form polyethylene
terephthalate polyester (or PEN polyester) in the presence of a catalyst.
If the catalyst was not added in the monomer esterification stage, the
catalyst is added at this stage to catalyze the reaction between the
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-31-
monomers and low molecular weight oligomers to form prepolymer and
split off the diol as a by-product. If a polycondensation catalyst was
added to the esterification zone, it is typically blended with the diol and
fed into the esterification reactor as the diol feed. Other compounds
such as phosphorus-containing compounds, cobalt compounds, and
colorants can also be added in the prepolymerization zone. These
compounds may, however, be added in the finishing zone instead of or
in addition to the prepolymerization zone.
In a typical DMT-based process, those skilled in the art recognize that
other catalyst material and points of adding the catalyst material and
other ingredients vary from a typical direct esterification process.
Typical polycondensation catalysts include the compounds of antimony,
titanium, germanium, zinc and tin in an amount ranging from 0.1 to 1,000
ppm based on the weight of resulting polyester polymer. A common
polymerization catalyst added to the prepolymerization zone is an
antimony-based polymerization catalyst. Suitable antimony-based
catalysts include antimony (III) and antimony (V) compounds recognized
in the art, and in particular, diol-soluble antimony (III) and antimony (V)
compounds with antimony (III) being most commonly used. Other
suitable compounds include those antimony compounds that react with,
but are not necessarily soluble in, the diols, with examples of such
compounds including antimony (III) oxide. Specific examples of suitable
antimony catalysts include antimony (III) oxide and antimony (III)
acetate, antimony (III) glycolates, antimony (II1) ethyleneglycoxide and
mixtures thereof, with antimony (111) oxide being preferred. The
preferred amount of antimony catalyst added is that effective to provide
a level of between about 75 ppm and about 400 ppm of antimony by 30 weight of
the resulting polyester.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-32-
This prepolymer polycondensation stage generally employs a series of
two or more vessels and is operated at a temperature of between about
250 C and 305 C for between about one and four hours. During this
stage, the It.V. of the monomers and oligomers is typically increased up
to about no more than 0.35 dUg. The diol byproduct is removed from
the prepolymer melt using an applied vacuum ranging from 15 to 70 torr
to drive the reaction to completion. In this regard, the polymer melt is
typically agitated to promote the escape of the diol from the polymer melt
and to assist the highly viscous polymer melt in moving through the
polymerization vessels. As the polymer melt is fed into successive
vessels, the molecular weight and thus the intrinsic viscosity of the
polymer melt increases. The temperature of each vessel is generally
increased and the pressure decreased to allow for a greater degree of.:
polymerization in each successive vessel. However, to facilitate removal
of glycols, water, alcohols, aldehydes, and other reaction products, the
reactors are typically run under a vacuum or purged with an inert gas.
Inert gas is any gas which does not cause unwanted reaction or product
characteristics at reaction conditions. Suitable gases include, but are
not limited to, carbon dioxide, argon, helium, and nitrogen.
Once an It.V. of typically no greater than 0.35 dL/g is obtained, the
prepolymer is fed from the prepolymer zone to a finishing zone where
the second half of polycondensation is continued in one or more
finishing vessels ramped up to higher temperatures than present in the
prepolymerization zone, to a value within a range of from 280 C to
305 C until the It.V. of the melt is increased from the It.V.of the melt in
the prepolymerization zone (typically 0.30 dL/g but usually not more than
0.35 dL/g) to an It.V in the range of from about 0.50 dL/g to about 0.70
dL/g. The final vessel, generally known in the industry as the "high
polymerizer," "finisher," or "polycondenser," is operated at a pressure
lower than used in the prepolymerization zone, typically within a range of
between about 0.8 torr and 4.0 torr. Although the finishing zone typically
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-33-
involves the same basic chemistry as the prepolymer zone, the fact that
the size of the molecules, and thus the viscosity, differs, means that the
reaction conditions also differ. However, like the prepolymer reactor,
each of the finishing vessel(s) is connected to a flash vessel and each is
typically agitated to facilitate the removal of ethylene glycol.
The residence time in the polycondensation vessels and the feed rate of
the ethylene glycol and terephthalic acid into the esterification zone in a
continuous process is determined in part based on the target molecular
weight of the polyethylene terephthalate polyester. Because the
molecular weight can be readily determined based on the intrinsic
viscosity of the polymer melt, the intrinsic viscosity of the polymer melt is
generally used to determine polymerization conditions, such as ,
temperature, pressure, the feed rate of the reactants, and the residence
time within the polycondensation vessels.
Once the desired It.V. is obtained in the finisher, the melt is fed to a
pelletization zone where it is filtered and extruded into the desired form.
The polyester polymers of the present invention are filtered to remove
particulates over a designated size, followed by extrusion in the melt
phase to form polymer sheets, filaments, or pellets. Although this zone
is termed a "pelletization zone," it is understood that this zone is not
limited to solidifying the melt into the shape of pellets, but includes
solidification into any desired shape. Preferably, the polymer melt is
extruded immediately after polycondensation. After extrusion, the
polymers are quenched, preferably by spraying with water or immersing
in a water trough, to promote solidification. The solidified condensation
polymers are cut into any desired shape, inciuding pellets.
As known to those of ordinary skill in the art, the pellets formed from the
condensation polymers, in some circumstances, may be subjected to a
solid-stating zone wherein the solids are first crystallized followed by
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-34-
solid-state polymerization (SSP) to further increase the It.V. of the
polyester composition solids from the It.V exiting the melt phase to the
desired lt.V. useful for the intended end use. Typically, the It.V. of solid
stated polyester solids ranges from 0.70 dL/g to 1.15 dL/g. In a typical
SSP process, the crystallized pellets are subjected to a countercurrent
flow of nitrogen gas heated'to 180 C to 220 C, over a period of time as
needed to increase the lt.V. to the desired target.
Thereafter, polyester polymer solids, whether solid stated or not, are re-
melted and re-extruded to form items such as containers (e.g., beverage
bottles), filaments, films, or other applications. At this stage, the pellets
are typically fed into an injection molding machine suitable for making
preforms which are stretch blow molded into bottles.
As noted, metallic molybdenum particles may be added at any point in
the melt phase or thereafter, such as to the esterification zone, to the
prepolymerization zone, to the finishing zone, or to the pelletizing zone,
or at any point between each of these zones, such as to metering
devices, pipes, and mixers. The metallic molybdenum particles can also
be added to the pellets in a solid stating zone within the solid stating
zone or as the pellets exit the solid-stating reactor. Furthermore, the
metallic molybdenum particles may be added to the pellets in
combination with other feeds to the injection molding machine or fed
separately to the injection molding machine.
If the metallic molybdenum particles are added to the melt phase, it is
desirable to use particles having a small enough d50 particle size to pass
through the filters in the melt phase, and in particular the pelletization
zone. In this way, the particles will not clog up the filters as seen by an
increase in gear pump pressure needed to drive the melt through the
filters. However, if desired, the metallic molybdenum particles can be
added after the pelletization zone filter and before or to the extruder.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-35-
Thus, according to the invention, metallic molybdenum particles of a
wide range of d50 particle sizes can be added either together with a
phosphorus-containing compound to the esterification zone, the
prepolymer zone, or at any point in between, or after the addition of a
phosphorus compound to the esterification zone prior to completing the
esterification reaction to the desired degree, or after the addition of the
phosphorus compound to any zone and to a reaction mixture containing
an active phosphorus compound. The point at which the metallic
molybdenum particles are added, or the presence or absence of such
other active compounds in the melt, is not limited since the metallic
molybdenum particles function to enhance the rate of reheat. The
function of the metallic molybdenum particles as a reheat enhancing: additive
allows a wide operating window and flexibility to add the metallic
molybdenum particles at any convenient point, even in the presence of
active phosphorus-containing compounds in the melt phase.
Thus, the metallic molybdenum particles may be added together with
phosphorus compounds either as a mixture in a feedstock stream to the
esterification or prepolymer zone, or as separate feeds but added to the
reaction mixture within the zone simultaneously. Alternatively, the
metallic molybdenum particles may be added to a reaction mixture within
the esterification zone after a phosphorus compound has been added to
the same zone and before completion of the esterification reaction.
Typical phosphorus-containing compounds added in the melt phase
include acidic phosphorus-containing compounds recognized in the art.
Suitable examples of such additives include phosphoric acid,
phosphorous acid, polyphosphoric acid, carboxyphosphonic acids, and
each of their derivatives including acidic phosphate esters such as
phosphate mono- and di-esters and non-acidic phosphate esters such
as trimethyl phosphate, triethyl phosphate, tributyl phosphate,
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-36-
tributoxyethyl phosphate, tris(2- ethylhexyl) phosphate, trioctyl
phosphate, triphenyl phosphate, tritolyl phosphate, ethylene glycol
phosphate, triethyl phosphonoacetate, dimethyl methyl phosphonate,
tetraisopropyl methylenediphosphonate, mixtures of mono-, di-, and tri-
esters of phosphoric acid with ethylene glycol, diethylene glycol,,and 2-
ethylhexanol, or mixtures of each, among others.
In addition to adding metallic molybdenum particles to virgin polymer,
whether to make a concentrate or added neat to the melt phase after the
prepolymerization reactors or to an injection molding zone, metallic
molybdenum particles may also be added to post-consumer recycle
(PCR) polymer. PCR containing metallic molybdenum particles is added
to virgin bulk polymers by solid/solid blending or by feeding both,solids.'
to an extruder. Alternatively, PCR polymers containing metallic
molybdenum particles are advantageously added to the melt phase for
making virgin polymer between the prepolymerization zone and the
finishing zone. The It.V. of the virgin melt phase after the
prepolymerization zone is sufficiently high at that point to enable the
PCR to be.melt blended with the virgin melt. Alternatively, PCR may be
added to the finisher. In either case, the PCR added to the virgin melt
phase may contain the metallic molybdenum particles. The metallic
molybdenum particles may be combined with PCR by any of the
methods noted above, or separately fed to and melt blended in a heated
vessel, followed by addition of the PCR melt containing the metallic
molybdenum particles to the virgin melt phase at these addition points.
Other components can be added to the compositions of the present
invention to enhance the performance properties of the polyester
polymers. For example, crystallization aids, impact modifiers, surface
lubricants, denesting agents, compounds, antioxidants, ultraviolet light
absorbing agents, catalyst deactivators, colorants, nucleating agents,
acetaldehyde reducing compounds, other reheat rate enhancing aids,
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-37-
sticky bottle additives such as talc, and fillers and the like can be
included. The polymer may also contain small amounts of branching
agents such as trifunctional or tetrafunctional comonomers such as
trimellitic anhydride, trimethylol propane, pyromellitic dianhydride,
pentaerythritol, and other polyester forming polyacids or diols generally
known in the art. All of these additives and many others and their use
are well known in the art and do not require extensive discussion. Any of
these compounds can be used in the present composition. It is
preferable that the present composition be essentially comprised of a
blend of thermoplastic polymer and metallic molybdenum particles, with
only a modifying amount of other ingredients being present.
Examples of other reheat rate enhancing additives that may be used.in,.
combination with metallic molybdenum particles include carbon black,
antimony metal, tin, copper, silver, gold, palladium, platinum, black iron
oxide, and the like, as well as near infrared absorbing dyes, including,
but not limited to, those disclosed in U.S. Pat. No. 6,197,851,
incorporated herein by reference.
The iron oxide, which is preferably black, may be used in very finely
divided form, e.g., from about 0.01 pm to about 200 pm, or from about
0.1 pm to about 10.0 pm, or from about 0.2 pm to about 5.0 p m.
Suitable forms of black iron oxide include, but are not limited to,
magnetite and maghemite. Red iron oxide is less preferred as it imparts
an undesirable red hue to the resultant polymer. Such oxides are
described, for example, on pages 323-349 of Pigment Handbook, Vol. 1,
(1973), John Wiley & Sons, incorporated herein by reference.
The compositions of the present invention optionally may additionally
contain one or more UV absorbing compounds. One example includes
UV-absorbing compounds which are covalently bound to the polyester
molecule as either a comonomer, a side group, or an end group.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-38-
Suitable UV-absorbing compounds are thermally stable at polyester
processing temperatures, absorb in the range of from about 320 nm to
about 380 nm, and are nonextractable from the polymer. The UV-
absorbing compounds preferably provide less than about 20%, more
preferably less than about 10%, transmittance of UV light having a
wavelength of 370 nm through a bottle wall 305 pm thick. Suitable
chemica4ly reactive UV absorbing compounds may include, for example,
substituted methine compounds.
Suitable compounds, their methods of manufacture and incorporation
into polyesters are further disclosed in U.S. Pat. No. 4,617,374, the,
disclosure of which is incorporated herein by reference. The UV-
absorbing compound(s) may be present in amounts between about 1
ppm to about 5,000 ppm by weight, preferably from about 2 ppm to
about 1,500 ppm, and more preferably between about 10 ppm and about
500 ppm by weight. Dimers of the UV absorbing compounds may also
be used. Mixtures of two or more UV absorbing compounds may be
used. Moreover, because the UV absorbing compounds are reacted
with or copolymerized into the backbone of the polymer, the resulting~
polymers display improved processability including -reduced loss of the
UV absorbing compound due to plateout and/or volatilization and the
like.
The polyester compositions of the present invention are suitable for
forming a variety of shaped articles, including films, sheets, tubes,
preforms, molded articles, containers and the like. Suitable processes
for forming the articles are known and include extrusion, extrusion blow
molding, melt casting, injection molding, stretch blow molding,
thermoforming, and the like.
The polyesters of this invention may also, optionally, contain color
stabilizers, such as certain cobalt compounds. These cobalt compounds
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-39-
can be added as cobalt acetates or cobalt alcoholates (cobalt salts or
higher alcohols). They can be added as solutions in ethylene glycol.
Polyester resins containing high amounts of the cobalt additives can be
prepared as a masterbatch for extruder addition. The addition of the
cobalt additives as color toners is a process used to minimize or
eliminate the yellow color, b*, of the resin. Other cobalt compounds such
as cobalt aluminate, cobalt benzoate, cobalt chloride and the like may
also be used as color stabilizers. It is also possible to add certain
diethylene glycol (DEG) inhibitors to reduce or prevent.the formation of
DEG in the final resin product. Preferably, a specific type of DEG
inhibitor would comprise a sodium acetate-containing composition to
reduce formation of DEG during the esterification and polycondensation
of the applicable diol with the dicarboxylic acid or hydroxyalkyl, or
hydroxyalkoxy substituted carboxylic acid. It is also possible to add
stress crack inhibitors to improve stress crack resistance of bottles, or
sheeting, produced from this resin.
With regard to the type of polyester which can be utilized, any high
clarity, neutral hue polyester, copolyester, etc., in the form of a resin,
powder, sheet, etc., can be utilized to which it is desired to improve the
reheat time or the heat-up time of the resin. Thus, polyesters made from
either the dimethyl terephthalate or the terephthalic acid route or various
homologues thereof. as well known to those skilled in the art along with
conventional catalysts in conventional amounts and utilizing
conventional processes can be utilized according to the present
invention. Moreover, the type of polyester can be made according to
melt polymerization, solid state polymerization, and the like. Moreover,
the present invention can be utilized for making high clarity, low haze
powdered coatings. An example of a preferred type of high clarity
polyester resin is set forth herein below wherein the polyester resin is
produced utilizing specific amounts of antimony catalysts, low amounts
of phosphorus and a bluing agent which can be a cobalt compound.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
- 40 -
As noted above, the polyester is produced in a conventional manner as
from the reaction of a dicarboxylic acid having from 2 to 40 carbon
atoms with polyhydric alcohols such as glycols or diols containing from 2
to about 20 carbon atoms. The dicarboxylic acids can be an alkyl having
from 2 to 20 carbon atoms, or an aryl, or alkyl substituted aryl containing
from 8 to 16 carbon atoms. An alkyl diester having from 4 to 20 carbon
atoms or an alkyl substituted aryl diester having from 10 to 20 carbon
atoms can also be utilized. Desirably, the diols can contain from 2 to 8
carbon atoms and preferably is ethylene glycol. Moreover, glycol ethers
having from 4 to 12 carbon atoms may also be used. Generally, most of
the commonly produced polyesters are made from either dimethyl
terephthalate or terephthalic acid with ethylene glycol. When powder.ed,
resin coatings are made, neopentyl glycol is often used in substantial
amounts.
Specific areas of use of the polyester include situations wherein
preforms exist which then are heated to form a finai product, for
example, as in the use of preforms which are blow-molded to.form a
bottle, for example, a beverage bottle, and the like. Another use is in
preformed trays, preformed cups, and the like, which are heated and
drawn to form the final product. Yet another use relates to polyester yarn
which is forced through a plurality of spinnerets having an infrared
quench collar thereabout. Additionally, the present invention is
applicable to highly transparent, clear and yet low haze powdered
coatings wherein a desired transparent film or the like is desired.
This invention can be further illustrated by the following examples of
preferred embodiments, although it will be understood that these
examples are included merely for purposes of illustration and are not
intended to limit the scope of the invention unless otherwise specifically
indicated.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-41 -
EXAMPLES
Example I 5 In this example, metallic molybdenum particles were purchased from
Alfa
Aesar (Stock number 44599) having a stated average particle size of 0.3
pm. The sample also had a stated purity of 99.95%. The particles were
found to have a d5o of 0.56 pm, with a particle size range from about 0.25
pm to about 1.4 pm, as measured by scanning electron microscopy.
The metallic molybdenum particles were added during melt compounding to
a commercial PET resin, VORIDIAN ''"'' 9921 Polymer (a copolymer PET,'
that has been crystallized and has an It.V. of 0.8 dL/g, available from
Eastman Chemical Company, Kingsport, Tennessee). A concentrate
containing 525 ppm molybdenum was prepared using VORIDIAN 9921-
Polymer as the base resin. The extrusions were performed using a one-
inch single-screw extruder with Saxton and Pineapple mixing head: The.
extruder was also equipped with pelletization capability. The concentrates,
were then let down into 9921 Polymer at different concentrations ranging
from about 50 ppm to 525 ppm. During the compounding process, 9921
Polymer was used to purge the extruder barrel several times to ensure no
cross contamination occurred between different batches.
After melt compounding, discs with a diameter of 3 cm and a thickness of
25. 0.17 cm were molded using a Daca Microcompounder/Microinjector.
Molded discs were also prepared from the 9921 Polymer as a control. The
molded discs were then used for both color (L*, a*, b* and haze) and reheat
measurements.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-42-
Color measurement of the molded discs was conducted in the following
manner. A Hunter Lab UltraScan spectrophotometer was used to measure
L~ , a~ and b* on three discs stacked together (approximately 0.51 cm
thickness). The instrument was operated using a D65 ifluminant light
source with a 10 observation angle and integrating sphere geometry. The
color measurements were made in the total transmission (TTRAN) mode, in
which both light transmitted directly through the sample and the light that is
diffusely scattered is measured. The discs were stacked together using a
holder in front of the light source, with the light normally incident on the
disc
surface. Haze was determined as the ratio of the diffuse light intensity to
the total light intensity transmitted by the specimen. Haze was calculated
according to the following formula:
HdZe _ Y~rlffusetransmission x 100
Ylotaltransmission
where Y represents the intensity of light.
The reheat measurement on molded discs was carried out as follows. The
disc was placed onto a support which was in contact with the sample along
its edges only. An actuator then automatically moved the disc beneath a
pyrometer and measured the initial temperature (Ti). The disc was then
moved to a fixed distance below a lamp housing equipped with a bulb (GE
DYH projection bulb, 250 W, 120 V) operating at 60 V. The sample was
exposed to a radiant light for 20 seconds. The color temperature of the
lamp was approximately 2,200 C. After heating, the disc was automatically
returned to the pyrometer where the surface temperature (Tf) of the center
area of the side which faced the lamp (front side) was recorded two
seconds after the lamp was turned off. A 90 second cooling cycle was used
between consecutive tests, during which,a fan cooled the lamp housing
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-43-
prior to loading the next sample. The reheat index (known as RHI) was
then calculated by comparing the temperature difference of a test sample
with that of the control sample as shown in the foilowing equation:
RHI - CTf - T )sample
~ontrof
As shown in Figure 1, Tables I and 2 below, the average particle size of the
molybdenum powder was in the range of 0.25 pm to 1.4 pm with a median
value of 0.56 pm, and a standard deviation of 0.19 pm.
Table 1. Quantiles of the particle size analysis
Cumulative Statistical Particle
percentage notation diameter (pm)
100.0% maximum 1.39
99.5% 1.39
97.5% 1.28
90.0% 0.81
75.0% quartile 0.65
50.0% median 0.56
25.0% quartile 0.46
10.0% 0.37
2.5% 0.26
0.5% 0.25
0.0% minimum 0.25
Table 2. Moments of the particle size analysis
Mean 0.58
Std Dev 0.19
Std Err Mean 0.028
upper 95% Mean 0.63
lower 95% Mean 0.52
N 49
The final molybdenum concentration in the polymers was determined by
inductively coupled plasma optical emission spectroscopy (ICP-OES) using
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-44-
a Perkin-Elmer Optima 2000 instrument. The levels of loading of
molybdenum and the color and reheat results are shown in Table 3.
Table 3. Reheat and color results of melt compounded samples with
molybdenum as reheat additive
Measured
Sample Reheat Molybdenum RHI L* a* b* haze
additive concentration
(ppm)
1 none 0 1.00 83.6 -0.8 4.2 2.3
2 Mo 49.9 1.11 75.6 -0.9 4.3 13.1
3 Mo 98.5 1.20 68.4 -1.0 4.0 22.9
4 Mo 208.3 1.36 55.0 -1.1 3.6 39.0
5 Mo 524.9 1.66 27.7 -1.3 1.3 66.7
Figure 2 shows the relationship between RHI and the concentration of
molybdenum (note: in this example, RHI is calculated using 9921 Polymer
as the reference). These results show that metallic molybdenum particles
are very effective at increasing the RHI of the base resin.
In Figure 3, the relationship between RHI and L* is illustrated for a
polyester
containing metallic molybdenum particles. The results show that when
compounded into PET, the metallic molybdenum particles provide
satisfactory L* values.
Figure 4 shows the correlation between RHI and haze for 9921 Polymer
containing metallic molybdenum particles.
Figures 5 and 6 show that the addition of metallic molybdenum particles to
9921 Polymer causes only insignificant shifts in color results (a* and b*).
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-45-
Example 2
In this example, the concentrate of 9921 Polymer containing 525 ppm
molybdenum particles as described in Example I was used to prepare
preforms and bottles. The concentrate was combined with Voridian T"'
CMOI Polymer, which is a PET copolymer containing no reheat additive, to
give final molybdenum concentrations of 31 ppm and 48 ppm. Standard
twenty ounce bottle preforms were prepared using a BOY (22D) injection
molding machine operated at a melt temperature of 280 C, and cycle time
of 30s.
Two sets of blow molding experiments were performed using the Sidel .
SB02/3 blow molding unit so as to check the reheat of each composition:,
The first set of experiments was conducted in order to evaluate the reheat
rates, or preform surface temperature (PST), of the preforms containing
molybdenum particles. A series of five preforms was passed in front of the
quartz infrared heaters and the PST of each composition was measured.
The higher the PST value, the higher the reheat rate (or RHI) of the
composition. The infrared lamp settings for the Sidel SB02/3 blow molding
unit are shown in Table 4. The preform heating time in the heaters was 38
seconds, and the power output to the quartz infrared heaters was set at
64%.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-46-
Table 4. Sidel SB02/3 lamp settings. Note lamps in Zones 6 through 8
were not turned on.
Heating Lamps ON=1 OFF=O
zone Lamp power Heater Heater Heater
settin (%) 1 2 3
Zone 8
zone 7
Zone 6
Zone 5 90 1 0 1
Zone 4 90 1 0 1
Zone 3 90 1 0 1
Zone 2 90 1 1 1
Zonel 90 1 1 1
In the second set of experiments, the oven power was changed so as,to,
blow the bottles for different composition at a similar PST to ensue
consistent material distribution in the final bottles with different level of
molybdenum particles. The PST has been controlled to be 115 C in this set
of experiments.
Color measurements on the preforms were performed using a HunterLab
UltraScan XE (Hunter Associates Laboratory, Inc., Reston VA), which
employs diffuse/8 (illumination/view angle) sphere optical geometry. The
color scale employed was the CIE LAB scale with D65 illuminant and 10
observer specified. Twenty ounce preforms, which have a sidewall
thickness of 0.154 inches, overall height of 3.93 inches, and outer diameter
of 0.846 inches, were measured in regular transmission mode using ASTM
D1746, "Standard Test Method for Transparency of Plastic Sheeting".
Preforms were held in place in the instrument using a preform holder,
available from HunterLab, and triplicate measurements were averaged,
whereby the sample was rotated 90 about its center axis between each
measurement.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-47-
Bottie sidewall haze was measured using a BYK-Gardner (Silver Spring,
MD) Haze-Gard Plus according to ASTM D 1003 on sections of the bottle
sidewalls with a sidewall thickness of 0.012 inches.
The results set forth in Table 5 show that the formulations containing
molybdenum particles had high PST compared to CMOI, indicating that the
molybdenum particles were very efficient at absorbing the energy from the
quartz infrared heaters of the blow molding machine.
Table 5. Preform surface temperature (PST) at 64% oven power setting
and preform color results
Measured Preform Color Results.
Sample Resin Reheat molybdenum PST *
additive concentration ( C) L* a b*
(ppm)
6 CM01 none 0 110 81.2 -0.4 2.8
7 CM01 Mo 31 119 74.5 -0.9 2.5
8 CM01 Mo 48 122 69.6 -1.0 2.6
As shown in Table 6, the formulations containing molybdenum particles
(entries 10 and 11) required lower oven power to reach a PST in the range
of 115 C compared to CMOI resin (entry 9). It further illustrates that
molybdenum particles cause only an insignificant increase in bottle sidewall
haze.
Table 6. Sidewall haze for bottles blown at the same preform surface
temperature (PST). Note the oven power needed to reach the same PST in
each sample is also given
Measured Bottle
Sample Resin Reheat molybdenum Oven PST Sidewall
o
additive conc. (ppm) Power ( /o) (,C) Haze
(%)
9 CsEi: i n~nC ~ j9 -i i5, -1.01
10 CM01 Mo 31 61 115 1.71
11 CM01 Mo 48 57 115 2.15
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-48-
Example 3
Molybdenum particles as described in Example I were added to a PET
polymerization process in order to determine their effect on reheat rate and
color. Polymers were prepared in the following manner.
In the first step, a PET oligomer was prepared by charging purified
terephthalic acid (PTA), 'purified isophthalic acid (PIA), ethylene glycol
(EG),
and antimony trioxide (ATO) catalyst to a 2-L autoclave. The formulation.;
was as follows: 651.0 g PTA, 13.0 g PIA, 396.0 g EG and 0.249 g ATO.
The raw materials were reacted at 245 C and 40 psig for 200 minutes. At
the end of the reaction, the resulting oligomer was discharged from the
reactor and allowed to solidify at room temperature and was then pulverized
to a coarse powder.
In the second step, a polymer was prepared from the oligomer in the
following manner. Oligomer (121 g) was charged to a 500 mL-
polymerization flask equipped with a polymer head, an overhead stirrer, a
nitrogen inlet, a dry-ice condensing trap, and a vacuum source. A metal
bath was used as the heating source. Polymerization was carried out in
three stages using the following conditions:
Stage 1 (early prepolymer): 272 C, 140 torr, 70 minutes
Stage 2 (prepolymer): 275 C, 20 torr, 70 minutes
Stage 3 (polycondensation): 285 C, 2.5 torr, 100 minutes
The molybdenum powder was dispersed in EG (to a final concentration of
4.2 wt.% molybdenum in EG) and then a portion of the dispersion was
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-49-
added to the polymerization process during the prepolymer. Phosphorus
was added as a phosphoric acid solution in EG (1 wt.% phosphorus)
immediately following the charge. A series of polymers was prepared with
molybdenum charges of from 0 ppm (control) to 200 ppm. Using this
procedure, polymers were produced with an {t.V. of 0.62 dL/g containing
220 ppm antimony as catalyst, 30 ppm phosphorus and 0 to 116 ppm
molybdenum. The concentrations of Antimony and phosphorus in the
polymer were determined by X-ray fluorescence (XRF), and the final
molybdenum concentration in the polymers was determined by ICP-OES.
Molded discs were prepared, and RHI and color were prepared as
described in Example 1. In the case of the lab polymers, the reheat rate,
was calculated by using a control polymer containing 0 ppm reheat additive.
The results are given in Table 7.
Table 7. Reheat and Color results of lab polymerized samples with
molybdenum as reheat additive
Measured
Sample Reheat Molybdenum RHI L* a* b* haze
additive concentration
(ppm)
11 none 0 1.00 83.1 -0.9 4.3 3.1
12 Mo 20.0 1.02 80.6 -4.2 21.0 7.9
13 Mo 71.6 1.10 73.7 -5.2 30.0 18.8
14 Mo 116.2 1.18 66.4 -4.6 38.0 26.3
Figure 7 shows that on a concentration basis, metallic molybctenum
particles with a median particle size of about 0.56 pm were effective at
increasing the polymer reheat. Figure 8 shows that polymers containing
metallic molybdenum particles have high L* values. Figure 9 shows the
correlation between reheat rate and haze for polymers containing
miolybdenui'n pari:icie5.
CA 02588236 2007-02-28
WO 2007/030090 PCT/US2005/030834
-50-
Figure 10 compares the b* and RHI results obtained when metallic
molybdenum particles are compounded into 9921 Polymer, as described in
Example 1, and the results obtained when molybdenum particles are added
during the polymerization process, as described in Example 2. Figure 11
illustrates the a* and RHI results obtained when metallic molybdenum
particles are compounded into 9921 Polymer, as described in Example 1,
and the results obtained when molybdenum particles are added during the
polymerization process, as described in Example 2 The plots show that a
preferred mode of addition of molybdenum is during the compounding
process, because the impact on a* and b* is less. Without being bound by
any theory, we believe that the reason for the poor b* and a* color in the
polymerization process may be due to the presence of a fine molybdenum-
oxide coating on the metal particles, which then is solubilized in the polymer
during the polymerization process, thus producing poor color in the final
polymer. If the metal did not contain the metal oxide coating, we think it
likely that the polymer would not have poor b* or a* color.
The invention has been described in detail with particular reference to
preferred embodiments, but it will be understood that variations and
modifications can be effected within the spirit and scope of the invention.
Although specific terms are employed, they are used in a generic and
descriptive sense only and not for purposes of limitation, the scope of the
invention being set forth in the following claims.