Note: Descriptions are shown in the official language in which they were submitted.
CA 02589389 2007-06-05
1
IMPROVEMENTS IN AND RELATING TO DRUG DELIVERY APPARATUS
This invention relates to drug delivery apparatus, particularly, but not
exclusively,
nebulisers and dosimetric spacers.
Many different types of nebulisers are known for delivering medication
directly into the
lungs of a patient, usually for treatment of respiratory diseases. Nebulisers
normally
deliver medication in the form of droplets or a dry powder. In most
nebulisers,
atomisation of the medicament into a stream of air occurs continuously,
regardless of
whether the patient is inspiring or expiring. However, the effect of
continuous
atomisation is that a significant proportion of the medication is lost during
expiration.
Commonly known nebulisers are either pneumatically operated from a compressed
air
source connected to the nebuliser which atomises the liquid, or are ultrasonic
nebulisers
which use a piezo-electric crystal to atomise the liquid. More recently, a
mesh-type
nebuliser has been developed in which the medication is forced through a fine
mesh in
order to create droplets of the medication. A further type of nebuliser, or
inhaler, is one
which uses a piezo-electric vibrator together with an electro-static charge
plate to
fluidise and disperse a dry powder aerosol into an airstream. Such a nebuliser
is
disclosed in US 5694920.
The optimum diameter of medication particles or droplets is about 1-5 microns.
If the
particles or droplets are bigger than this, they are likely to be impacted in
the airway
before they reach the lungs, but if they are smaller than one micron, they
tend to be
carried out of the lungs again on exhalation without sedimenting in the lungs.
Nebulisers and inhalers disperse the small particles of medication into an air
stream, or
stream of other gas, leading to a patient. References to the air which carries
the
medication entrained in it includes other gases suitable for carrying the
medication.
One known nebuliser analyses the pressure changes within the device during the
first
three breaths to determine an average shape of the breathing pattern. A timed
pulse of
CA 02589389 2007-06-05
2
atomisation is commenced when starting subsequent inspirations such that
atomisation
occurs for the first 50% of the inspiration. This is illustrated in Figure 1
where the
breathing pattern and pulse are superimposed. This is effective in reducing
the loss of
medication during exhalation to about 3%. Figure 1 shows the breaths in a
graph of
flow rate against time. When the treatment is commenced, a patient breathes in
and out
three times through the nebuliser before treatment commences. The first three
breaths
are measured so that the timed pulse of atomisation occurs for 50% of the
average time
of inhalation. The duration of inhalation is indicated as T1, T2 and T3. These
timed
periods are averaged, and divided by two in order to determine the pulse
length for the
next fourth breath where treatment starts. For each subsequent breath, the
duration of
the pulse of atomisation is determined by summing the time period of
inhalation of the
previous three breaths, dividing by three to obtain an average and dividing by
two. The
dose administered to the patient is directly proportional to the duration of
the pulse of
atomisation, and so the period of atomisation is summed, and the atomiser is
switched
off, or indicates that the patient should stop once the dose administered to
the patient
reaches the amount of medication prescribed for that treatment.
Other nebulisers are known in which the timed pulse of atomisation is fixed to
be other
than 50% of the duration of inspiration. However, in these other nebulisers,
the pulse
length must be set for each patient by the clinician. Many of the nebulisers
are,
therefore, suitable only for use in a controlled environment, such as a
hospital. The
setting of the pulse length for each patient means that most nebulisers are
not suitable
for a patient to use at home.
DE 3636669 discloses an apparatus for delivering aerosol to the airways of a
patient
which includes separate inspiration and expiration lines. An aerosol generator
is
located in the inspiration line which generates an aerosol only during
expiration of the
patient so that the aerosol fills a region in the inspiration line. On
inspiration by the
patient, the aerosol from the inspiration line is drawn into the lungs of the
patient.
However, the volume of the aerosol is fixed. The volume of aerosol is fixed in
order to
be appropriate for the assumed tidal volume when a patient breathes in a
specific
manner.
CA 02589389 2007-06-05
3
GB 2077444 discloses an apparatus suitable for use in determining at least two
parameters of a human or animal respiratory system. The apparatus includes a
pressure
sensor, a flow sensor, a volume sensor, a monitoring unit and a calculating
unit whereby during each respiratory cycle, the monitoring unit supplies the
calculating
unit with at least two sets of sensed values of the pressure the flow rate and
the volume
and the calculating unit calculates the required parameters of the respiratory
system
from the sets of sensed values. The volume is calculated conventionally by
integrating
the rate of flow over time.
Reference is made to our co-pending International Patent Publication No. WO
97/48431. Figures 2 and 3 of this application show the nebuliser which is
disclosed in
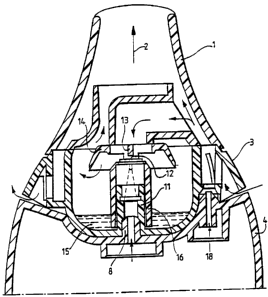
the above co- pending Patent application. Referring to Figure 2, a mouthpiece
1 is
shown through which a patient inhales in the direction of arrow 2. Below the
mouthpiece 1 is a removable atomising section 3 which, in turn, rests on a
base 4.
The base 4 is shown in more detail in Figure 3. Referring to Figure 3, the
base 4
includes an inlet 5 through which air is supplied under pressure from a
compressor (not
shown). The pressurized air is led via a tube 6 to a manifold 7 which controls
the flow
of pressurized air to an air outlet 8 which directs air into the atomising
section 3 shown
in Figure 2. The base 4 also includes a pressure sensor 9 which detects the
pressure
within the atomising section 3 via a port 10.
Referring again to Figure 2, air under pressure passes through the air outlet
8 of the
base 4 and is conducted through a tubular post 11 to an atomiser nozzle 12 out
of which
the air issues under pressure. A deflector 13 is located in the path of the
pressurised air
issuing from the nozzle 12 so that the pressurized air is deflected laterally
so as to pass
beneath a baffle 14. The passage of the pressurized air across the top of the
tubular
post 11 causes medication 15 to be drawn up between the outer surface of the
tubular
post 11 and the inner surface of a sleeve 16 which surrounds the tubular post
11. The
medication 15 is atomised in the stream of air, and carried away in the stream
of air
below the rim of the baffle 14 and up through the mouthpiece 1 to a patient.
CA 02589389 2007-06-05
4
The pressure sensor 9 in the base 4 monitors the breathing pattern of a
patient, and on
the basis of the breathing pattern, the manifold 7 is controlled to supply
pressurized air
to the atomising section 3 only during the first 50% of an inhalation phase.
Whilst a particular type of nebuliser is described above, the present
application is
suitable for application to any type of nebuliser.
The invention also relates to other drug delivery apparatus, such as spacers
in which a
dose of a drug in droplet or powder form is released into a spacer chamber or
holding
chamber from which the patient inhales. These are most appropriate for elderly
patients or children who have difficulty in using a multi-dose inhaler or dry
powder
inhaler, for example, because they have trouble coordinating the release of
the drug
with the beginning of inhalation, or because their inhalation flow rates are
too small.
For example, spacers are disclosed in International patent publication number
WO
96/13294.
According to a first aspect of the present invention, a drug delivery
apparatus comprises
delivery means for delivering medication-laden air and air not carrying any
medication
to a patient for inspiration; monitoring means for monitoring a patient's
breathing
pattern; and control means for controlling the said delivery means to
selectively deliver
the medication-laden air and air not carrying any medication, characterised in
that the
control means is arranged to control the delivery means to deliver the
medication-laden
air in pulses, the length of which, and their proportion of the inspiratory
phase of the
breathing pattern being varied by the control means depending on the breathing
pattern
monitored by the monitoring means.
According to a second aspect of the invention, a method for determining the
duration of
a pulse during which medication-laden air is delivered to a patient during
inspiration
comprises:
(i) measurement of the tidal volume of a patient;
(ii) measuring the duration of inspiration of a patient;
(iii) storing an estimate of the volume of a patient's upper airway; and
CA 02589389 2007-06-05
(iv) calculating the duration of the pulse on the basis of the measured tidal
volume of the patient, the measured duration of inspiration and the stored
estimated volume of the patient's upper airway.
5 In this document, the upper airways of a patient are the mouth and trachea,
and where a
nebuliser is used, preferably include the volume of the nebuliser chamber.
The determination of the length of pulse enables the proportion of the
inhalation time
during which atomisation occurs to be extended above 50% towards 100%. This
will
result in the patient receiving their treatment in a shorter time, since it
will take fewer
breaths to deliver the required dose of medication. However, there is no point
in
continuing delivering the medication into air which is inhaled by the patient
at the end
of his or her inspiratory phase (the 'end volume'), since it will remain in
the upper
airways. The medication which does not go beyond the upper airways will be
wasted
when the patient exhales.
Thus, the invention according to the first and second aspects enables a pulse
of to be
generated which is longer than 50% but which stops before the end volume of
inspiration begins. Another advantage of this invention is that a patient's
adherence to
the treatment regime will be much improved if the length of treatment is
reduced.
In addition, the invention allows automatic optimisation of the pulse length
without it
needing to be set by a clinician. This means that the pulse length will
automatically be
adapted to each patient on the basis of the patient's breathing pattern at the
time the
medication is being administered. Thus, a nebuliser or other drug delivery
apparatus
may be used by the patient outside of the controlled environment of a
hospital, and may
be used at home. In addition, it is possible for the apparatus to indicate
when a dose
has been administered without the patient needing to count the number of
breaths which
he or she has taken.
According to the preferred embodiment, the means for measuring the tidal
volume of a
patient includes means for measuring a patient's peak flow, and tidal volume
prediction
CA 02589389 2007-06-05
6
means for calculating the tidal volume on the basis of the peak flow from the
peak flow
measuring means, and the duration of inspiration measured by the timing means.
Some or all of the values used in the calculations are mean values derived
from a
number of earlier measurements of each breathing pattern of the patient. For
example,
the patient will start inspiration through the apparatus, and the medication
will not be
delivered during the first three breaths. The first three breaths are analysed
by
recording the duration of inspiration, and the peak flows during inhalation as
are
required to determine the duration of a pulse of atomisation. Delivery of the
medication takes place on the fourth and subsequent breaths, in each case the
values in
the calculations are derived from a number of earlier measurements of the
inspiration
phase of a patient, in this case the previous three inspiratory phases.
Preferably, where the apparatus is a nebuliser, the atomisation is caused by a
stream of
gas under pressure passing through the nebuliser and sourced from a gas supply
means.
This gas is normaily air, and the source is preferably a compressor operating
together
with an accumulator. During atomisation, gas from the accumulator is used to
atomise
the medication, and the compressor generates air under pressure to fill the
accumulator.
If a patient's inspiration is very long, the accumulator may be caused to be
emptied,
thereby disrupting atomisation. The atomiser, therefore, preferably includes a
means
for limiting the duration of the pulse so as to maintain the accumulator in a
state where
it is always under some pressure. In addition, the accumulator may include a
valve
which, when the accumulator is full, vents gas to atmosphere thereby
preventing it from
becoming dangerously full. It is often preferable to maintain the compressor
in
operation all the time and to vent excess air to atmosphere rather than to
switch the
compressor on and off.
According to a third aspect of the present invention, a drug delivery
apparatus
comprises means for predicting the tidal volume of a patient comprising means
for
measuring a patient's peak flow, timing means for measuring the duration of
inspiration, and tidal volume prediction means for calculating the tidal
volume on the
CA 02589389 2007-06-05
7
basis of the peak flow from the peak flow measuring means, and the duration of
inspiration measured by the timing means.
According to a fourth aspect of the invention, a method of predicting the
tidal volume
of a patient comprises: (i) measuring a patient's peak flow;
(ii) measuring the duration of inspiration of the patient;
(iii) calculating the tidal volume on the basis of the measured peak flow, and
the
measured duration of inspiration of the patient.
Measuring the patient's respiratory volume (tidal volume) has previously
involved
continually monitoring the patient's inspiratory flow, typically every ten
milliseconds.
The flow rate is integrated over the duration of inspiration to determine the
inspiratory
volume. However, the third and fourth aspects of the invention determine the
tidal
volume of a patient much more simply. This invention reduces the amount of
data
processing which is required, thereby reducing the cost of the overall
nebuliser. The
peak flow is much simpler to measure, and can be used more simply in a
calculation to
determine the tidal volume.
According to a fifth aspect of the invention, a nebuliser comprises means for
determining the duration of a pulse of atomisation during inspiration, the
determination
means including means for measuring the tidal volume of a patient, timing
means for
measuring the duration of inspiration, means for storing an estimate of the
volume of a
patient's upper airway, and means for calculating the duration of the pulse on
the basis
of the tidal volume measured by the tidal volume measuring means, the duration
of
inspiration measured by the timing means, and the stored estimated volume of a
patient's upper airway from the storage means.
Embodiments of the present invention are described below by way of example,
and
with reference to the drawings in which:
Figure 1 is a graph showing the inhalation pattern of a patient over time, and
indicating
when the pulse of atomisation occurs in the first 50% of inspiration, as
occurs in one
known nebuliser;
CA 02589389 2007-06-05
8
Figures 2 and 3 show a known nebuliser which generates pulses of atomisation
during
the first 50% of inspiration;
Figure 4 is a flow diagram showing how the pulse of atomisation during
inspiration is
determined;
Figure 5 is a graph showing the predicted tidal volume against measured tidal
volume;
Figure 6 is a flow diagram showing the limitation of the pulse length
depending on the
supply of pressurised gas;
Figure 7 shows the nebuliser together with a source of pressurised gas;
Figure 8 shows an air accumulator within the air supply;
Figure 9 is a schematic drawing showing the way in which the nebuliser is
controlled;
and
Figure 10 is a schematic drawing of a dosimetric spacer according to the
present
invention.
This invention applies, amongst other things, to nebulisers of the type which
generate
pulses of atomisation, as in the prior art nebuliser described above. The
invention is
not, however, limited to the exact nebuliser described above, but may be
applied to
other nebulisers. For convenience, the description below of the present
invention will
refer to components of the prior art device shown in Figures 2 and 3, and
because many
of the components, for example, the manifold, may be used in the present
invention.
The nebuliser may be one of a jet nebuliser, ultrasonic nebuliser or a
pressure mesh
nebuliser.
Jet nebulisers are of two kinds, these being air-jet nebulisers and liquid-jet
nebulisers.
An example of an air-jet nebuliser, which uses a source of compressed air to
nebulise a
liquid, is disclosed in EP 0627266 (Medic-Aid Limited). An example of a liquid-
jet
CA 02589389 2007-06-05
9
nebuliser, which drives a liquid through one or more nozzle outlets to produce
a spray
of fine droplets is disclosed in WO 94/07607 (Boehringer Ingelheim
International
GmbH et ao.
Ultrasonic nebulisers which nebulise a liquid using ultrasonic waves usually
developed
with an oscillating piezo-electric element, take many forms, these including
nebulisers
where liquid is in direct contact with the piezo-electric element, where there
is an
amplifying interface, typically an enclosed fluid, between the piezo-electric
element
and the liquid, and where the piezo-electric element vibrates a mesh from
which an
aerosol is generated. Examples of ultrasonic nebulisers are disclosed in US
4533082
(Maehara et al) and US 5261601 (Ross et ao. The nebulisers described in those
documents include a housing that has a reservoir which holds a quantity of
liquid to be
dispensed, which housing has a perforated membrane in contact with the
reservoir and
an ultrasonic vibrator connected to the housing to vibrate the perforated
membrane.
Another example of an ultrasonic nebuliser is described in WO 97/29851 (Fluid
Propulsion Technologies, Inc). An example of a pressure mesh nebuliser, which
may
or may not include a piezo-electric element, is disclosed in WO 96/13292
(Aradigm
Corporation).
Extending the proportion of the inhalation of the patient in which atomisation
takes
place above 50% results in the patient receiving their treatment faster since
it will take
fewer breaths to deliver the required volume of medication. However, to avoid
wastage
of the medication which is atomised in the end volume of patient's inspiratory
volume,
the pulse of atomisation must be stopped before the end volume is reached. The
end
volume is the volume of air inhaled by a patient at the end of the inspiratory
volume
which remains in the upper airways (the mouth and trachea) and does not enter
into the
lower parts of the lungs. Medication which is atomised into the end volume is
wasted
when the patient exhales, together with any air atomised medication left in
the
nebuliser, since it does not reach the lungs.
The end volume is the volume of the patient's upper airway, and is
proportional to the
size of the patient. Clearly, the end volume will vary as a percentage of the
inspiratory
tidal volume since the tidal volume changes significantly depending on the
type and
CA 02589389 2007-06-05
extent of the respiratory disease suffered by the patient. The optimum
duration of
atomisation pulse would, therefore, be from the start of inhalation up to the
point during
inspiration when the volume remaining to be inspired equals the end volume.
Atomisation would then be stopped and the remaining end volume would clear the
5 atomised medication from the device and the upper airways of the patient and
into the
lungs. Thus, the percentage of inspiration in which atomised medication is
delivered is
maximised, thereby minimising treatment time and still avoiding wastage of
medication. The length of the atomisation pulse is dependent upon the
patient's
inspiratory tidal volume. The nebuliser must therefore measure the patient's
tidal
10 volume, preferably on a breath by breath basis so as to calculate, for
example from the
previous three breaths, an average inhalation volume for the next breath.
Thus, the
atomisation pulse time will be calculated as follows:
Pulse time = mean inspiratory time x (mean tidal volume - end volume)
mean tidal volume
Timing means are included in the nebuliser connected to the pressure sensor 9
(shown
in Figure 3) in order to measure the duration of inspiration. Storage means
are also
included in the nebuliser in which an estimate of the end volume of a
particular patient
is stored. Since this figure is a constant value for a particular patient,
this can be
entered at the beginning of a course of treatment, and is estimated on the
basis of the
size of the patient. The nebuliser includes a means for measuring the tidal
volume of a
patient. According to one form of the invention, the patient's inspiratory
flow is
monitored continuously, typically every ten milliseconds, and this is
integrated over the
inspiratory duration. Another, simpler, way of measuring the tidal volume of a
patient
is described later in this specification.
The nebuliser also includes means for calculating the atomisation pulse time
on the
basis of the duration of inspiration, the tidal volume and the end volume. The
calculation means carries out the calculation outlined above.
In view of the fact that the nebuliser adapts to the breathing pattern of a
patient, when
the patient starts breathing, no atomisation takes place during the first
three breaths.
CA 02589389 2007-06-05
11
Those first three breaths are used to analyse the breathing pattern of the
patient. The
flow rate of the first three breaths are measured, and from this, the duration
of the
inhalation phase of the first three breaths are calculated, and an average
found. The
average duration of inhalation is then used in the calculation to determine
the pulse
length of atomisation during the fourth breath. In addition, as the patient
continues to
breathe in and out, the previous three breathing patterns are measured and
used to
calculate the next pulse duration. Thus, if a patient's breathing pattern
improves during
treatment, the nebuliser will adapt to this change in order to optimise the
dose
administered during each breath.
Referring now to Figure 4, the steps taken by the nebuliser, and by the
patient are
described. The first operation, box 30 represents the patient starting to
inhale. The
timing means records the time at which inhalation starts as shown in box 31,
and during
inhalation, a calculation is performed to predict the tidal volume of the
patient as shown
in box 33. This step will be described in more detail later in a
specification, but it will
be noted that the calculation requires data to be included in the calculation
which is the
inhalation time and peak flow as an average from the last three breaths, as
shown in box
32. The pulse time is then calculated by the calculation means as shown in box
34, and
the pulse time is adjusted, as shown in box 35 in the event that the pulse
length would
exhaust an accumulator from which is pressurised air is supplied to the
nebuliser. This
step, shown in box 35 is also described in more detail later in this
specification. The
pulse of atomisation occurs during inhalation, and after it has stopped, a
calculation is
carried out to determine what dose has been atomised. At the end of the breath
as
shown in box 38, details of the peak flow of the patient inhalation, and the
duration of
inhalation are recorded so that calculations determining pulse length may be
made for
subsequent breaths. This is shown in box 39.
Reference is made above to the simpler prediction of tidal volume. As will be
appreciated, measuring tidal volume by integrating measured flow rate over the
time of
inspiration requires considerable processing power and is relatively
expensive. A
simpler method of determining the tidal volume is proposed which requires much
simpler calculations and much simpler measurements to be made for use in such
a
CA 02589389 2007-06-05
12
calculation. To carry out the measurement, the nebuliser includes a peak flow
detector
for detecting the peak flow rate of inspiration.
The calculated, or predicted tidal volume is derived from the peak flow
measured by
the peak flow detector, and the duration of inspiration measured by the timer.
The tidal
volume calculation means carries out the following calculation:
Predicted tidal volume = C x Mean Peak Flow x Inspiratory Time
10
C is a constant and it is found that C = 0.7
Figure 5 is a graph of the predicted tidal volume against measured tidal
volume . Each
point on the graph represents a patient whose tidal volume has been measured
by a
15 complex tidal volume calculation by integration of the patient's
inspiratory flow over
the duration of inhalation, and the predicted tidal volume according to the
new, simpler
method of calculation. It will be seen that the predicted tidal volumes are
extremely
accurate, and so the predicted tidal volume may be included in the calculation
of
atomisation pulse time.
The use of a low flow rate compressor together with a accumulator to supply
compressed air to the nebuliser is disclosed in our earlier Patent application
published
as WO 97/48431, which is referred to above. In the past, the size of the
compressor
and accumulator are selected so that the maximum pulse that can be delivered
by the
device (currently 50% of inspiratory time) does not exceed the accumulator
volume for
any given pulse or the mean output of the compressor. Now that the pulse time
is
variable, it is preferable to calculate the maximum pulse time available from
the air
supply system. For patients who have a slightly higher inspiratory demand, the
pulse
time of atomisation will be reduced so that the supply capability of the air
supply
system is not exceeded. The calculations are carried out on a breath by breath
basis,
assuming that the accumulator is filled at constant flow rate from the
compressor. The
volume of air added to the accumulator from the end of previous pulse to the
start of the
CA 02589389 2007-06-05
13
next pulse is calculated and then added to the volume remaining at the end of
the
previous pulse.
Figure 6 is a flow diagram showing the calculations carried out in ensuring
that the
volume of air used does not exceed the volume of the accumulator. If the air
in the
accumulator is calculated to be above the maximum volume of the accumulator,
the
volume is set to be at its maximum V = V,,,a,,. This is because there is an
automatic
vent valve which limits the volume of air stored in the accumulator. The
maximum
pulse time can then be calculated on the basis of the rate of flow of air out
of the
accumulator which is the flow to the atomiser jet, minus the flow rate to the
compressor. If this exceeds the volume available in the accumulator, then the
pulse
time is limited to the current accumulator volume. The volume of accumulator
at the
end of the pulse is then calculated to be used at the beginning of the next
calculation
occurring at the beginning of the next inhalation of the patient. Thus, the
maximum
pulse time for individual breaths is calculated without exceeding the capacity
of the air
supply system. The compressor has a constant output flow rate, typically 1.5
litres per
minute and the nebuliser jet has a flow rate of 6 litres per minute during
pulsing. The
accumulator has a volume of approximately 150 millilitres at NTP.
Figure 7 shows the nebuliser 50 connected to the air supply 51 by a flexible
tube 52.
Referring to Figure 8, the accumulator is shown which has a vent 63, thereby
limiting
the maximum expansion of the accumulator. As each pulse is delivered to the
nebuliser, the diameter of the accumulator is reduced, and the vent 63 is
closed.
The compressor may be mains powered or battery powered. The pump, especially a
mains powered pump, operates continually during use, and operates to inflate
the
accumulator. When the pressure in the accumulator reaches the required level,
a
pressure switch in the hand-held part of the nebuliser is activated as is
described in an
earlier Patent application, WO 97/48431, referred above. That switches the
nebuliser
ON. Once the treatment has been completed, the compressor is switched OFF. The
accumulator deflates and the pressure switch in the hand-held part of the
nebuliser
deactivates the unit.
CA 02589389 2007-06-05
14
Referring to Figure 8, the pump supplies air to the accumulator via a port 64.
Inflation
of the diaphragm 61 of the accumulator is controlled by an assembly including
an arm
62 which is connected to a vent valve 63. When the diaphragm 61 of the
accumulator
reaches the maximum desired extension, it contacts the arm 62 to open the vent
valve
63. This releases to atmosphere the flow of air from the compressor, and
maintains the
accumulator at a fixed extension. During use, air is removed from the
accumulator via
port 65 and the diaphragm 61 shrinks and loses contact with the vent arm 62
which
closes the valve 63 allowing the compressor to recharge the accumulator until
the vent
arm 62 again operates the vent valve 63.
It is also advantageous to vent the accumulator to atmosphere when the
compressor is
switched off, and this is achieved by mounting the main power switch 66 on top
of the
accumulator with a rotary knob 67. The bottom of the knob 67 includes a cam 68
which contacts the vent arm 62 to open the vent 63 thereby releasing pressure
from the
accumulator. Simultaneously, the compressor is switched off. When the
compressor is
switched back on again, the cam 68 is disengaged from the vent arm 62, thereby
closing
the vent valve 63.
Figure 9 illustrates a simplified form of the way in which all of the
components of the
nebuliser are connected together. The compressor and accumulator 70 are shown
as
being separate from the hand-held part of the nebuliser 71, but connected by a
tube 72
carrying the pressurised air into the nebuliser 71. In the compressor and
accumulator
part 70, the pump is shown to supply the accumulator 70 with compressed air.
In the
nebuliser part 71, the nebuliser is switched on at the pressure switch 73 by
the presence
of pressurised air in tube 72. The nebulising part 74 of the nebuliser is
controlled by a
valve or manifold 75 which controls the pulses of pressurised air. The
breathing pattern
of a patient is detected by a sensor 76 which delivers information regarding
the
breathing pattern to the micro-controller 77 which, in turn, controls the
manifold 75.
Once a dose of medication has been delivered, indication means, such as a LED
or
buzzer 78 is activated by the micro-controller to indicate to the patient that
treatment is
complete.
CA 02589389 2007-06-05
A further embodiment of the invention is shown in Figure 10 which is a
dosimetric
spacer 80 including a holding chamber 81 having a port 82 towards one end
thereof to
which is connected a mouthpiece 83. An air pressure sensor 84 is located
between the
mouthpiece 83 and holding chamber 81. This sensor 84 measures the pressure
within
5 the mouthpiece, from which the rate of flow of air inspired and exhaled by
the patient
can be measured air. The mouthpiece 83 also includes a vent 85 which allows a
patient
to exhale through the mouthpiece 83 without filling the holding chamber 81.
More is
disclosed on the vent below.
10 Within the holding chamber, a piston 86 is disclosed to move longitudinally
to vary the
volume of air available in the holding chamber 81 to a patient during
inspiration. The
piston includes a toothed connecting rod 87 which extends through the end of
the
holding chamber 81 such that the teeth may be engaged by the finger of
solonoid 88.
An air inlet 89 is located at the left hand end of the holding chamber in
order to allow
15 air to enter or leave the space behind the piston as the piston moves to
the right or left.
In use, the piston 86 is pulled back so as to fill the holding chamber 81 with
air. The air
within the holding chamber 81 is then loaded with medication, either in the
form of
liquid droplets, or in the form of a cloud of powder. This is delivered into
the holding
chamber 81 through the port 82, and normally requires the removal of the
mouthpiece
83 to do so. A mouthpiece 83 then can be replaced, and a patient breathes in
and out
through the mouthpiece 83. During inspiration, a patient inspires the
medication-laden
air from the holding chamber 81, and during exhalation, the exhaled air is
vented to
atmosphere by the vent 85. During exhalation, the solonoid 88 locks the
connecting
rod 87 of the piston 86 so that it will not move and so that the holding
chamber will not
fill with exhaled air. However, in accordance with the invention, the piston
86 is only
free to move during a proportion of the inhalation phase, and it will be
locked
stationary by the solonoid 88 during the inspiration by the patient of the end
volume.
Once the piston locks, the vent 85 is arranged such that the drop in pressure
in the
mouthpiece 83 caused by the locking of the piston 86 opens the vent 85 such
that
ambient air may be drawn into the mouthpiece. Of course, a separate vent might
be
included in the mouthpiece to carry out this function as appropriate.
CA 02589389 2007-06-05
16
The calculation of the pulse length during which the piston 86 is free to move
to allow
the dispensing of medication to the patient is determined in the same way as
is
described above in relation to the nebuliser. The inspiration of the patient
during the
previous three breaths is monitored by the sensor 84 such that the same
calculations can
be made as described above. On the subsequent breath, the sensor detects the
commencement of a breath, and after the duration of the pulse, the piston is
locked..
Such an arrangement reduces the wastage of medication which is present in the
end
volume of the air normally breathed in by the patient.
This invention is applicable to other types of medical inhalers. For example,
as
described in the introduction to this specification, a dry powder inhaler is
disclosed in
US 5694920 which uses a piezo-electric vibrator and an electrostatic charge
plate to
fluidise and disperse a dry powder into the air stream of the patient. The
electrostatic
charge plate can be operated in response to the patient's breathing pattern so
as to
produce pulses of the powder medication into the air stream leading to the
patient. The
length of the pulses can be determined in exactly the same way as in the
embodiments
previously described such that the dry powder is not dispersed into the end
volume of
the air stream leading to the patient.