Note: Descriptions are shown in the official language in which they were submitted.
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
ANTIMICROBIAL WATER SOFTENER SALT AND SOLUTIONS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of a) US Application No.
10/460,769,
filed 12 June 2003 entitled ANTIMICROBIAL SALT SOLUTIONS FOR FOOD-
SAFETY APPLICATIONS and b) US Provisional Application No. 60/636,337, filed 15
December 2004 entitled ANTIMICROBIAL WATER SOFTENER SALT AND
SOLUTIONS and c) US Provisional Application No. 60/637,674, filed 16 December
2004 entitled ANTIMICROBIAL WATER SOFTENER SALT AND SOLUTIONS. The
entirety of each of these applications is incorporated herein by reference.
BACKGROUND
[0002] This invention generally relates to salt-based_ formulations having
antimicrobial activity and antimicrobial solutions made therefrom. Aspects of
the
invention have particular utility in connection with water softening and other
applications in which ions in a solution may be removed or exchanged.
[0003] So-called "hard" water contains excess mineral salts, e.g., calcium and
magnesium salts. A variety of techniques have been used to remove or replace
ions
of these mineral salts to "soften" the water. These techniques include
distillation,
adding water softening compounds to the water, membrane filtration, and ion
exchange, e.g., cation exchange. Ion exchange-based water softening is used in
a
variety of industrial applications and is particularly prevalent in smaller-
scale water
softening systems used to treat water coming into individual homes. Ion
exchange is
also used to remove ions from water and other solutions in other applications.
[0004] Generally, ion exchange-based water treatment systems pass water
through an ion exchange medium, which exchanges ions in the water with
substitute
ions, e.g., by exchanging mineral cations with cations of sodium or potassium.
A
wide variety of such media are known in the art, including resins, which may
be
strong or weak acid or strong or weak base ion exchange resins, and
microporous
minerals such as zeolites. For example, U.S. Patent Application Publication
-1-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
No. 2002/0072545 (published 13 June 2002, the entirety of which is
incorporated
herein by reference), suggests a synthetic ion exchange resin that may
comprise a
styrene-divinylbenzene copolymer or an acrylic-divinylbenzene copolymer. From
time to time, such ion exchange media must be regenerated by exchanging
preferred cations for the cations accumulated in the media from treating the
hard
water. This is typically accomplished by delivering brine to the ion exchange
resin
during a regeneration cycle that typically lasts on the order of 30 minutes.
After
passing through the ion exchange medium, the brine is commonly discharged into
the environment.
[0005] Bacteria may become attached to the surface of the ion exchange medium
and proliferate. Over time, the bacteria can create a biofilm on the medium,
reducing
efficacy of the medium. In some circumstances, the water exiting the ion
exchange
medium may have a bacteria population that is higher than the bacteria content
of
the water entering the ion exchange resin. Some opportunistic and disease-
causing
bacteria that have been found to flourish on ion exchange media may present
health
risks for the elderly and those with weakened immune systems if present in
significant enough concentration. As a consequence, some European countries
forbid the use of water softeners without a mechanism for disinfecting the
water
exiting the water softening system.
[0006] Reducing bacterial contamination of ion exchange media by delivering
antimicrobial agents to the resin presents a number of difficulties. Calcium
hypochlorite is inexpensive and is commonly recognized as a highly effective
antimicrobial agent useful in a variety of applications. Unfortunately,
calcium
hypochlorite is known to reduce the useful life of many common ion exchange
resins
and manufacturers of such resins caution against its use. A variety of other
common
antimicrobial agents are ill-suited for applications in which they may be
ingested,
such as in softening potable water, because of health concerns and/or sensory
degradation, e.g., adversely affecting the taste or odor of treated water.
Adequately
flushing the resin prior to reuse can ameliorate these effects, but consumers
may still
resist adopting approaches that employ chemicals they deem undesirable. Still
other
known antimicrobial agents are cost-prohibitive or present environmental waste
disposal challenges.
-2-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
BRIEF DESCRIPTION OF THE DRAWING
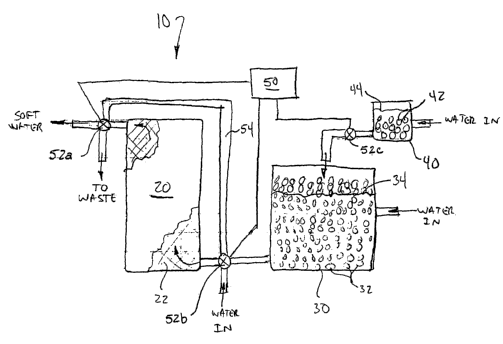
[0007] Figure 1 is a schematic overview of a water softener system in
accordance
with an embodiment of the invention.
DETAILED DESCRIPTION
A. Overview
[0008] Salt has been used in water softener systems to rejuvenate ion exchange
media. Acids and surfactants have been used as antibacterial agents in other
applications. However, it has been discovered that inorganic saits act
synergistically
with acid and surfactant ingredients to provide a significant and unexpected
increase
in their antibacterial effectiveness. More particularly, initial tests
revealed an
unexpected, synergistic effect between sodium chloride and an acid/SLS (sodium
lauryl sulfate) antimicrobial additive. Replicate tests were run to determine
if this
effect was significant. Solutions were also prepared containing an identical
concentration of acid and SLS but no sodium chloride. Results of these tests,
run
with multiple replicates, illustrate that salt formulations including SLS and
either citric
acid or malic acid yield many fewer living microorganisms after 30 minutes
than
either plain NaCI or salt-free compositions of the same levels of SLS and
citric or
malic acid. Further testing suggests that the combination of at least select
inorganic
salts with surfactants can also yield surprising increases in the kill rates
of bacteria
when compared to either the salt alone or the surfactant alone.
[0009] Aspects of the invention described herein variously provide
antimicrobial
additives for a brine solution, salt-based formulations and solutions and
water
softener systems. One embodiment of the invention provides a mixture of a
salt,
such as sodium chloride, with an acid and a surfactant, such as sodium lauryl
sulfate. Suitable acids include citric, malic, acetic, propionic, lactic,
benzoic,
ascorbic, isoascorbic, sorbic, phosphoric, hydrochloric, malic, tartaric,
adipic,
succinic, glutaric, salicylic, and sulfuric acids as well as sodium bisulfate.
The salt
can be selected from inorganic salts including the chloride, sulfate, nitrate,
phosphate, carbonate, and hydroxide salts of sodium, potassium, magnesium,
calcium, iron, and ammonium. Suitable surfactants include sodium lauryl
sulfate,
-3-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
linear alkylbenzene sulfonates, alcohol sulfates, alpha-olefin sulfonates,
alcohol
ethoxylates, nonylphenyl ethoxylates, alkylpolygiucosides, fatty alkanoamides,
fatty
amine oxides, sodium dioctylsulfosuccinate, dodecylbenzene sulfonic acid and
its
salts, the sodium salt of sulfonated oleic acid, sodium dodecylbenzene
sulfonate,
and dodecyldiphenyloxide disulfonic acid and its salts.
[0010] As used herein, a "food-safe" substance, e.g., a "food-safe"
surfactant, is a
substance that is safe for human consumption at the levels anticipated to be
present
in water (for example) treated in accordance with embodiments of the
invention.
Although a "food-safe" substance may be classified by the US Food and Drug
Administration as "generally recognized as safe" (GRAS), many food-safe
substances are not GRAS and their use in some food-related applications may
require further regulatory approval in some countries.
B. Water Softener Systems
[0011] Figure 1 schematically illustrates a water softening system in
accordance
with one embodiment of the invention. The water softening system 10 generally
includes an ion exchange tank 20, a regenerating solution reservoir 30, and an
antimicrobial agent reservoir 40. A controller 50 may be operatively coupled
to one
or more valves 52 (identified as valves 52a, 52b, and 52c in Figure 1) to
control
operation of the water softening system 10.
[0012] The ion exchange tank 20 includes an ion exchange medium 22 through
which water passes for treatment. Any suitable ion exchange medium 22 known in
the art may be employed, including ion exchange resins and suitable mineral-
based
media such as zeolites. In normal operation, incoming water, e.g., water from
a
municipal water supply, is directed by valve 52b into the ion exchange tank
20.
Treated or "softened" water exiting the ion exchange tank 20' may pass through
valve 52a to deliver soft water for use, e.g., as potable water for a
household.
[0013] From time to time, the ion exchange medium 22 may need to be
regenerated by exchanging built-up ions removed from the incoming water supply
with alternate ions, such as sodium or potassium ions. The controller 50 may
be
programmed to initiate a regenerating cycle at fixed intervals or on any other
suitable
basis; a variety of programmable controllers 50 for use in water softening
systems 10
are commercially available. At the beginning of a regenerating cycle, the
valve 52b
-4-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
may be changed to incoming water into a bypass line 54 for delivery to the
first valve
52a. During the regenerating cycle, the valve 52a may direct this bypass water
into
the conduit conventionally used to deliver the softened water. In this
configuration,
the second valve 52b may also direct fluid from the regenerating solution tank
30 into
the ion exchange tank 20. (Each of the valves 52 may comprise a single valve
or a
set of vaives.) The solution passing through the ion exchange tank 20 may then
be
diverted by the first valve 52a to waste, e.g., to be discharged to the
environment.
[0014] The regenerating solution reservoir 30 may include a ready supply of a
regenerating solution 34, which may comprise a dissolved fraction of a salt-
based
formulation in accordance with other embodiments of the invention or a
conventional
water-softening salt. The salt formulation may be provided in the form of
pellets 32
or in other suitable particulate form, such as pieces that are broken from a
large,
thick sheet of the salt. The solution 34 typically is about 10 weight percent
of the
salt.
[0015] The water softening system 10 of Figure 1 also includes an
antibacterial
agent reservoir 40 that includes an antimicrobial solution 44. The
antimicrobial
solution 44 may comprise water in which a portion of the pellets 42 or a block
of a
water-soluble antimicrobial formulation in accordance with select embodiments
is
dissolved. A quantity of the antimicrobial solution 44 may be delivered to the
ion
exchange tank 20 during regeneration. In the schematic illustration of Figure
1, the
controller 50 would open the valve 52c to deliver the antimicrobial solution
44 to the
regenerating solution tank 30. This antimicrobial solution 44 can mix with the
regenerating solution 34 for delivery to the ion exchange tank 20.
Alternatively, the
antimicrobial solution 44 may bypass the regenerating solution reservoir 30,
e.g., by
being delivered to the second control valve 52b. Alternatively, the
regenerating
solution 34 and the antimicrobial solution 44 may be delivered to and pass
through
the ion exchange tank 20 sequentially rather than together. For example, the
antimicrobial solution 44 may be delivered to the tank 20, followed either
immediately
or at a later time by the regenerating solution 34.
[0016] After the regenerating solution 34 and/or antimicrobial solution 44 has
passed -rhrough the ion exchange medium 22, the valves 52a-c may be returned
to
their first configuration and operation can return to normal, i.e., with
incoming water
passing through the ion exchange tank 20 to deliver softened water for use.
-5-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
C. Antimicrobial Formulations and Solutions
[0017] Substantial concentrations of salt make antimicrobial salt formulations
and
solutions in embodiments of the invention well-suited for a variety of
applications,
including use in regenerating ion exchange media such as those employed in
water
softener systems. For ease of understanding, the following discussion refers
to the
water softener system 10 shown in Figure 1. Antimicrobial formulations and
solutions in accordance with aspects of the invention may be used in the water
softener system 10, but are also well-suited for use in conventional water
softening
systems and in other ion exchange-based treatment systems. Further, although
the
following discussion focuses on use of antimicrobial salt formulations and
solutions
for such regeneration, they are useful for other purposes, as well, such as in
chilled
brine processing of meat and other food-safety applications.
[0018] One useful formulation contains about 25-25,000 ppm surfactant, about
0.1-25 weight percent (wt. %.) acid, and about 72.5-99.9 wt. % salt. This
formulation
can be dissolved in water in the regenerating solution reservoir 30 to make a
regenerating solution ranging in concentration from about 1% total solids by
weight
(i.e., about 1 wt. % of the salt-based formulation) up to the saturation
point.
Typically, brine solutions used in regenerating ion exchange media have a salt
concentration of about 10 wt. %; regenerating solutions having about 8-11 wt.
% of
an antimicrobial salt formulation of the invention are expected to work well
for the
same purpose.
[0019] Suitable surfactants include sodium lauryl sulfate (SLS), linear
alkylbenzene sulfonates, alcohol sulfates, alpha-olefin sulfonates, alcohol
ethoxylates, nonylphenyl ethoxylates, alkylpolyglucosides, fatty alkanoamides,
fatty
amine oxides, sodium dioctylsuifosuccinate, dodecylbenzene sulfonic acid and
salts
thereof. The sodium salt of sulfonated oleic acid, sodium dodecylbenzene
sulfonate,
and dodecyidiphenyloxide disulfonic acid and salts thereof may also be
employed as
the surfactant. In one embodiment, the surfactant is soluble in a concentrated
aqueous salt solution, e.g., an aqueous solution containing 10 wt. % or more
of the
salt used in the formulation. Salt-based formulations in accordance with
aspects of
the invention may include about 50 - 25,000 ppm of at least one, possibly two
or
more, of these surfactants. SLS at a level of about 100- 1000 ppm, e.g., about
100
- 500 ppm, is deemed particularly useful for ion exchange media regeneration.
-6-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
[0020] Antimicrobial salt formulations in aspects of the invention also
include
about 0.3 - 25 wt. % of at least one acid selected from the group consisting
of citric,
malic, acetic, propionic, lactic, benzoic, ascorbic, isoascorbic, sorbic,
phosphoric,
hydrochloric, nitric, malic, tartaric, adipic, succinic, glutaric, salicylic,
and sulfuric
acids and sodium bisulfate. Of these, citric and malic acids are generally
preferred,
with contents of about 0.4 - 6 wt. %, e.g., about 0.5 - 2 wt. %, being
expected to
work well for a variety of applications.
[0021] The balance, e.g., about 75 - 99.7 wt. %, of the antimicrobial salt
formulations may comprise at least one inorganic salt selected from the group
consisting of sodium, potassium, magnesium, calcium, iron, and ammonium salts
of
chloride, sulfate, nitrate, phosphate, carbonate, and hydroxide. Salts of
monovalent
cations may be more desirable than polyvalent cation salts, with calcium and
potassium salts, e.g., NaCI and KCI, being generally preferred. For anion
exchange
applications, salts of monovalent anions, e.g., a chloride or nitrate salt,
are desirable.
[0022] In some embodiments, though, the balance of the antimicrobial salt
formulation may include at least one component other than the salt. For
example,
the formulation may include a scenting agent to improve the odor of the
regenerating
solution 34 in the regeneration solution reservoir 30.
.[0023] As explained below in connection with the data in Tables 1 to 11,
testing
suggests that at least certain surfactant/salt combinations can function as a
much
more effective antimicrobial agent than either the salt or the surfactant
alone.
[0024] Formulations in accordance with the invention may be provided in a
variety
of forms. In one embodiment, the formulations are blended to yield a
relatively
homogeneous mixture and this mixture is compressed into pellets of a suitable
size.
Procedures and apparatus for pelletizing water softener salt are well known in
the art
and need not be detailed here. The formulations may instead be compressed into
larger blocks for more convenient storage and handling. As is also known in
the art,
the formulation may be formed as a large, thick sheet and broken into suitably
sized
chunks. If so desired, the ingredients of the formulation may be mixed dry and
compressed to form the sheet or the sheet can be formed by mixing the
ingredients
with a solvent and drying.
-7-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
[0025] As noted above, one embodiment of the invention provides a water
softener system 10 that includes a regenerating solution reservoir 30 and an
antimicrobial agent reservoir 40. In one embodiment, the pellets 32 in the
regenerating solution reservoir 30 may comprise a formulation in accordance
with
embodiments of the invention and the antimicrobial agent solution 44 can be
used in
the regenerating cycle on only intermittentiy or on an as-needed basis. In
another
embodiment, the pellets 32 may comprise a conventional water softening salt.
in
either embodiment, the pellets 42 of the antimicrobial agent may include at
least one
of the acids and/or at least one of the surfactants enumerated above, but at
higher
levels than in the preceding embodiments. The level of acid and surfactant in
the
pellets 42 may be selected so that the combination of the regenerating
solution 34
and the antimicrobial agent solution 44 in desired proportions will yield a
concentration of salt, acid and surfactant analogous to solutions made with
the
formulations outlined above.
[0026] In one particular embodiment, the antimicrobial formulation 42 in the
antimicrobial agent reservoir 40 comprises about 20-35 wt. % of a food-grade
acid,
e.g., citric or malic acid, and about 0.1-1 wt.% of a food-grade surfactant;
one
exemplary formulation inciudes about 25-30 wt. %, e.g., about 28 wt. %, citric
acid,
and about 0.4 wt. % SLS. The balance of the antimicrobial formulation 42 may
comprise an inorganic salt. If deemed necessary, the antimicrobial formulation
42
may also include an encapsulating agent or other additive that can slow
dissolving of
the formulation to a desirable rate. In an alternative approach useful for
conventional
water softening systems that do not employ a separate antimicrobial agent
reservoir
40, a block or pellets 42 of such a more concentrated antimicrobial
formulation 42
may be added directly to the regenerating solution reservoir 30 with
conventional salt
pellets 32, e.g., commercially available water softener salt. In one
illustrative
embodiment, a one-pound block of the antimicrobial formulation may be added to
the
regenerating solution reservoir 30 each time the supply of salt pellets 32 is
replenished.
[0027] In another embodiment, the antimicrobial solution 44 may include an
acid
and a surfactant, but have little or no added salt. An acid/surfactant
formulation in
this embodiment may be formed into pellets 42 or a block and held in the
antimicrobial agent reservoir to make an aqueous solution with added water.
-8-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
Alternatively, the formulation may be in liquid form instead of a solid pellet
42 or the
like. This liquid may comprise a concentrate that is mixed with added water in
the
reservoir 40 or may be delivered in the final desired concentration for
addition to the
regenerating solution reservoir 30 or directly to the ion exchange tank 20, as
described above. One exemplary composition contains about 5 wt. % of an acid,
e.g., citric or malic acid, and about 700 ppm of a surfactant, e.g., SLS, and
about one
eighth of a gallon may be added to the ion exchange medium 22 (either directly
or
with the regenerating solution 34) in a regenerating cycle for the medium 22.
[0028] Early screening tests indicated that there was an unexpected
synergistic
effect between sodium chloride and an acid/SLS antibacterial additive.
Replicate
tests were run to determine if this effect was statistically significant. Ten
percent by
weight solutions were prepared of a formulation of 0.6 wt. % citric or malic
acid, 100
ppm SLS, and 99.4 wt. % sodium chloride. Solutions were also prepared
containing
an identical concentration of acid and SLS but no sodium chloride. A bacterial
culture suspension (Escherichia coli ATCC 11229) that had been incubated for
24
hours in Brain Heart Infusion (BHI) broth and had an initial inoculum count of
about
109 CFU/ml was serially diluted in cold Butterfield's Phosphate Buffered Water
(BPBW) to 105 CFU/ml. A 1.0 ml aliquot of this suspension was added to 100 ml
of
test solution at room temperature and mixed well, providing an initial
inoculum of 103
CFU/ml. After 30 minutes, the E. coli populations were enumerated by plating
on
tryptic soy agar (TSA), making serial dilutions as necessary in BPBW. Plates
were
incubated at 35 C +/- 2 C for approximately 24 hours. Colonies were then
counted
and compared to the initial inoculum counts. Results of these tests run on 16
replicates of each test solution are given in Table 1.
Table 1. Effectiveness of Acid/SLS Solutions with and without Salt on E. coli
Test Solution Average E. Coli Conc. (CFU/ml)
citric acid, SLS, with salt 540
citric acid, SLS, without salt 1054
malic acid, SLS, with salt 141
malic acid, SLS, without salt 2419
[0029] For both the citric acid/SLS and malic acid/SLS additives, the number
of
bacteria remaining alive after 30 minutes is much lower when salt is present
than
-9-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
when there is no salt present. Analysis of the data indicates that there is a
statistically significant increase in kill in the presence of salt (p<0.05).
In contrast, a
10% solution of pure sodium chloride does not provide any significant kill of
the test
microorganisms.
[0030] Although the preceding test was carried out in deionized water, the
tests
reflected in Tables 2 and 3 were carried out in hard water to better simulate
actual
operation conditions for a water softening application. In general, the anti-
bacterial
effectiveness of the formulations decreased significantly in hard water,
requiring
higher acid and/or surfactant contents to achieve the same efficacy. Formulas
containing sodium lauryl sulfate and either citric or malic acid, though, were
effective
in killing both gram negative and gram positive bacteria.
[0031] Replicate tests were next run on formulas containing sodium lauryl
sulfate
and either citric or malic acid to estimate the acid and/or surfactant content
required
to consistently provide at least about 65%, desirably at least about 90%, kill
rates of
the more resistant gram negative bacteria, E. coli. Salt formulations
including at
least about 100 ppm SLS were deemed more effective than formulations
containing
lower SLS levels. SLS will cause a small amount of foam formation in saturated
brine. Other commercially available surfactants (such as Triton QS44 sold by
The
Dow Chemical Company of Midland, Michigan, USA) could be substituted for
sodium
lauryl sulfate to avoid foaming, but many of those surfactants may not be food-
safe
(as is SLS) and may raise environmental concerns. A summary of the performance
of formulations containing 100 ppm sodium Iauryl sulfate and various amounts
of
citric acid or malic acid is given in Table 2, in which runs C1-C10 employed
the
stated citric acid content in the salt-based formulation and runs M1-M5 used
malic
acid instead of citric acid. (Again, the SLS and acid concentrations are in
the salt-
based formulation, not in the resultant solution formed with the formulation.)
Table 2. Percent Kill of E. coli After 30 Minutes in Hard Water*
Run 0.6 wt. % 0.7 wt. % 0.8 wt. %
C1 85.4% 1 - -
C2 89.4% 1 - -
C3 65.7% 10 - -
C4 99.6% 1 99.8% 1 99.8% 1
C5 80.2% 6 99.2% 1 100.0% 1
-10-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
C6 82.4% 3 98.5% 3 99.9% 3
C7 94.8% 3 99.9% 3 99.9% 3
C8 93.3% 3 - 99.4% 3
C9 98.5%(3) 95.1%(3) 93.2% 3
C 10 - 99.6% 3 99.9% 3
M 1 95.5% 1 - -
M2 95.3% 1 - -
M3 100.0% 10 99.8% 1 99.8% 1
M4 68.6% 6 - -
M5 99.8%(3) 99.6%(3)
*The number of replicates in a given experiment is listed parenthetically.
[0032] S. aureus was grown in a fashion directly analogous to that set forth
above
for growth of the E. coli culture, except that the S. aureus plates were
incubated for
about 48 hours instead of 24 hours. Results of tests on S. aureus are given in
Table
3, in which runs C1-C3 employed the stated citric acid content in the salt-
based
formulation and runs Ml and M2 used malic acid instead of citric acid. (The
tested
formulations were generally more effective toward S. aureus than E. coli, so
fewer
replicates were carried out on S. aureus.)
Table 3. Percent Kill of S. aureus After 30 Minutes in Hard Water*
Run 0.4 wt. % 0.5 wt. % 0.6 wt. % 0.7 wt. %
C1 70.6%(1) 90.4%(l) 99.1 %(1) 99.9% (3)
C2 99.9%(l) - 100.0%(1) -
C3 - - 99.9%(3) -
M1 86.3%(l) 95.6%(l) 99.9%(1) -
M2 - - 100.0%(1) -
*The number of replicates in a given experiment is listed parenthetically.
[0033] Based upon the results in Tables 2 and 3, a salt formulation including
about
100 ppm sodium lauryl sulfate, about 0.6 wt. % citric acid or malic acid, and
about
99.4 wt. % salt significantly decreases (e.g., at least a 65% reduction) both
gram
negative and gram positive bacteria populations. Increasing the acid content
slightly
to about 0.7 wt. % consistently killed at least about 90% of both gram
negative and
gram positive bacteria. As a matter of fact, the 0.7% acid formulas
consistently killed
-11-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
about 95% or more of the tested gram negative bacteria and regularly killed at
least
about 99% of such bacteria.
[0034] The following examples further illustrate the synergistic and
unexpected
results from combining acid/surfactant with salt. An experiment was run to
determine if solutions containing sodium chloride, sodium lauryl sulfate, and
various
acids would kill L. monocytogenes at cold temperatures. The following test
procedure was used: A bacterial culture suspension (L. monocytogenes H2446
[CDC Global Standard]; Scott A-serotype 4b; 12243-serotype 1/2a; and a recent
cooked meat and poultry facility isolate, WP4) that had been incubated for at
least 5
days in BHI broth and had an initial inoculum count of about 109 CFU/ml was
serially
diluted in cold BPBW to 105 CFU/ml. A 1.0 ml aliquot of this suspension was
added
to 100 ml of cold (-7 C + 2 C) test solution and mixed well, providing an
initial
inoculum of 103 CFU/ml. The test solutions were incubated at -7 C +/2 C for
the
duration of the experiment. At intervais of 0, 4, and 24 hours the L.
monocytogenes
populations in the test solutions were determined on Modified Oxford agar
(MOX).
MOX plates were incubated at 35 C +/- 2 C for approximately 48 hours. Colonies
were then counted and compared to the initial inoculum counts.
[0035] Results are given in Table 4. Each test solution was a 17% by weight
solution of the listed formula prepared in soft water.
Table 4. Effect of Solutions of NaCI, SLS and various acids on L.
monocytogenes
Sample CFU/ml pH Water
time 4 hr 24 hr Activity
0
100% NaCI 1550 1250 1170 7.88 0.88
2.0% Malic Acid, 500 ppm SLS, 98.0% NaCI 0 0 0 1.21 ND
5.0% Na Bisulfate, 500 ppm SLS, 95.0% NaCI 0 0 0 0.81 0.883
Water Control 1270 400 0 9.34 0.999
0.3% Malic Acid, 100 ppm SLS, 99.7% NaCI 480 5 0 4.1 ND
0.5% Malic Acid, 100 ppm SLS, 99.5% NaCI 176 0 0 3.31 ND
0.7% Malic Acid, 100 ppm SLS, 99.3% NaCI 117 0 0 2.99 0.88
0.3% Citric Acid, 500 ppm SLS, 99.7% NaCI 5 0 0 4.14 ND
0:5% Citric Acid, 500 ppm SLS, 99.5% NaCi 0 0 0 3.37 ND
0.7% Citric Acid, 500 ppm SLS, 99.3% NaCi 0 0 0 2.98 0.88
0.3% Malic Acid, 500 ppm SLS, 99.7% NaCI 11 0 0 4.15 ND
0.5% Malic Acid, 500 ppm SLS, 99.5% NaCI 3 0 0 3.39 ND
0.7% Malic Acid, 500 ppm SLS, 99.3% NaCI 0 0 0 3.06 0.879
1.0% Citric Acid, 500 ppm SLS, 99.0% NaCI 0 0 0 2.69 ND
1.0% Malic Acid, 500 ppm SLS, 99.0% NaCI 0 0 0 2.81 ND
2.0% Lactic Acid, 500 ppm SLS, 98.0% NaCI 0 0 0 2.65 0.885
-12-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
~ _._._. _ ................ .....
2.0% Phosphoric Acid (75%), 500 ppm SLS, 98.0% 0 0 0 1.52 0.884
NaCI
1.0% Benzoic Acid, 500 ppm SLS, 99.0% NaCI 0 0 0 3.93 0.879
2.0% Citric Acid, 500 ppm SLS, 98.0% NaCI 0 0 0 2.3 0.884
2.0% Malic Acid, 500 ppm SLS, 98.0% NaCI 0 0 0 2.46 0.882
[0036] In another experiment, two sets of solutions were tested. The first set
(samples 1-12 in Table 5 below) was prepared in hard tap water and contained
about 17.0% by mass of the identified formulation. These samples were
inoculated
with 103 CFU/ml L. monocytogenes by the same procedure described above. A
second set of samples was prepared from brine taken from a ready-to-eat meat
processing operation. The recirculated brine had been used to chill packaged
meat
for one week. After a week of use the brine typically contains various types
of
aerobic psychrotrophic and mesophilic bacteria. This experiment was done in
order
to determine if the additives would kill the microorganisms naturally
occurring in
actual process brine from a plant. Since the spent chill brine samples already
contained NaCi, citric acid and/or SLS was added to provide an effective
concentration of additive. One set of these samples (samples 13 -17) were
inoculated with 103 L. monocytogenes and the other set (samples 18-22)
contained
only the naturally occurring organisms in the spent chill brine. Results are
given in
Table 5 below. The data indicate that at lower acid levels, the SLS increases
the
effectiveness of the mixture, but at higher acid levels, the SLS is not
necessary. The
results show the formulations are effective in hard water. The results also
demonstrate that the formulations effectively kill L. monocytogenes as well as
the
naturally occurring microorganisms in spent chill brine from an actual meat
processing plant.
Table 5. Effects of Antimicrobial Salt Formulas in Hard Water and in Spent
Chill
Brine
Sample CFU/ml
0 2 hr 24 hr
1 100% NaCl 760 1100 1100
2 0.3% Citric Acid, 100 ppm SLS, 99.7% NaCI 730 670 29
3 0.3% Citric Acid, 99.7% NaCI 1460 1330 830
4. 0.5% Citric Acid, 100 ppm SLS, 99.5% NaCI 890 240 0
0.5% Citric Acid, 99.5% NaCI 1060 1170 330
6 0.7% Citric Acid, 100 ppm SLS, 99.3% NaCl 1010 14 0
7 0.7% Citric Acid, 99.3% NaCI 1040 1030 3
-13-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
8 1.0% Citric Acid, 100 ppm SLS, 99.0% NaCI 840 0 0
9 1.0% Citric Acid, 99.0% NaCI 990 340 0
2.0% Citric Acid, 98.0% NaCI 910 0 0
11 4.0% Citric Acid, 96.0% NaCI 1110 0 0
12 6.0% Citric Acid, 94.0% NaCi 950 0 0
13 Brine Control with L. mono 1260 1290 600
14 1% Citric Acid in Brine with L. mono 1050 0 0
2% Citric Acid in Brine with L. mono 1140 0 0
16 1% Citric Acid + 50 ppm SLS in Brine with L. 1090 0 0
mono
17 2% Citric Acid + 50 ppm SLS in Brine with L. 1070 0 0
mono
18 Brine Control 6000 3100 2000
19 1% Citric Acid in Brine 2490 190 4
2% Citric Acid in Brine 1670 6 0
21 1% Citric Acid + 50 ppm SLS in Brine 2520 122 0
22 2% Citric Acid + 50 ppm SLS in Brine 1480 6 0
[0037] A test was run to determine if salts other than sodium chloride would
show
a synergistic antimicrobial effect with an acid and sodium lauryl sulfate.
Solutions
containing 0.6409 grams malic acid and 0.0107 grams sodium lauryl sulfate per
liter
were prepared with and without 107.0 grams of various salts (added on an
anhydrous basis). Solutions were inoculated with E. coli described above and
the
amount of bacterial kill was measured to determine if the added salt caused an
iricrease in the effectiveness of the acid/surfactant active ingredients.
Results are
shown in Table 6.
Table 6. Effect of Different Salts on the Antimicrobial Action of Malic
Acid/SLS
Solution (salt added) % Kill of E. Coli
No salt addition 4.4%
Sodium sulfate 87%
Magnesium chloride 56%
Potassium chloride 18%
Sodium chloride 78%
Potassium sulfate 34%
Calcium chloride 55%
Magnesium sulfate 93%
[0038] Tests run on solutions containing only the salt and no other ingredient
indicate that sodium sulfate, potassium chloride, and potassium sulfate alone
provide
no bacterial kill. Magnesium chloride provided 61% kill, calcium chloride
provided
-14-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
26% kill, and magnesium sulfate provided 10% kill. Thus, based on the data
developed thus far, sodium sulfate, sodium chloride, and magnesium sulfate
appear
to significantly increase the effectiveness of the acid/surfactant
antimicrobial agent,
even though the salts provide little kill on their own.
[0039] The effectiveness of antimicrobial salt formulas was tested against L.
monocytogenes in a biofilm. Stainless steel coupons (2 x 5 cm, type 302
stainless
steel, 2B finish) were cleaned in acetone followed by an alkaline detergent
and
distilled water and then dried in an autoclave at 121 C for 15 minutes. A
culture of L.
monocytogenes (Scott A - serotype 4b) was prepared by inoculating 10 mL of TSA
and incubating overnight at 35 C. 50 mL of sterile TSA + 0.6% yeast extract
(YE)
was aseptically dispensed into sterile disposable conical shaped plastic tubes
and
one drop of overnight grown L. mono culture was added to each tube. Inoculated
tubes were incubated at 25 C for approximately 48 hours. After the biofilm had
formed on the coupons, a coupon was aseptically removed from the tube and
gently
rinsed with distilled water to remove unattached cells. Coupons were then
immersed
in cold antimicrobial test solution (-6.7 C) and incubated over different time
intervals
(1 hour, 24 hours, and 5 days). After incubation period, the coupon was shaken
in a
tube containing 40 mL of sterile PBW and 10 sterile glass beads (4 mm) for 2
minutes two remove the cells attached to the coupon biofilm. The cells were
plated
in the PBW on TSA + 0.6% YE using appropriate dilutions and incubated at 35 C
for
48 hours.
[0040] Results on triplicate samples of antimicrobial test solutions are given
in
Table 7 below. Each solution contained 17% by weight of a formula consisting
of the
percentages of citric acid and SLS listed in Table 7 with the balance of the
formula
being NaCI in each case. The data indicate that not only are the antimicrobial
salt
solutions effective at killing bacteria suspended in solution, they are also
effective at
killing bacteria within a biofilm.
Table 7. Log Concentration of L. mono in Antimicrobial Salt Solutions
Sample 1 Hour 24 Hours 5 days
0.3% citric acid, 100 ppm SLS -5.08 4.59 1.38
0.3% citric acid, 100 ppm SLS -4.90 3.85 1.79
0.3% citric acid, 100 ppm SLS -4.81 3.48 1.92
0.3% citric acid, 500 ppm SLS 4.81 4.76 2.23
-15-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
0.3% citric acid, 500 ppm SLS 4.90 3.48 2.18
0.3% citric acid, 500 ppm SLS -5.18 3.48 2.36
0.7% citric acid, 100 ppm SLS 1.88 0 0
0.7% citric acid, 100 ppm SLS 2.02 0 0
0.7% citric acid, 100 ppm SLS 1.28 0 0
0.7% citric acid, 500 ppm SLS 0.70 1.00 0.90
0.7% citric acid, 500 ppm SLS 0.90 0.70 0.30
0.7% citric acid, 500 ppm SLS 0.85 0 0
2.0% citric acid, 100 ppm SLS 0 0 ND
2.0% citric acid, 100 ppm SLS 0 0 ND
2.0% citric acid, 100 ppm SLS 0 0 ND
2.0% citric acid, 500 ppm SLS 0 0 ND
2.0% citric acid, 500 ppm SLS 0 0 ND
2.0% citric acid, 500 ppm SLS 0 0 ND
6.0% citric acid, 100 ppm SLS 0 0 ND
6.0% citric acid, 100 ppm SLS 0 0 ND
6.0% citric acid, 100 ppm SLS 0 0 ND
6.0% citric acid, 500 ppm SLS 0 0 ND
6.0% citric acid, 500 ppm SLS 0 0 ND
6.0% citric acid, 500 ppm SLS 0 0 ND
12.0% citric acid, 100 ppm SLS 0 0 ND
12.0% citric acid, 100 ppm SLS 0 0 ND
12.0% citric acid, 100 ppm SLS 0 0 ND
12.0% citric acid, 500 ppm SLS 0 0 ND
12.0% citric acid, 500 ppm SLS 0 0 ND
12.0% citric acid, 500 ppm SLS 0 0 ND
Salt Control A -5.04 -7.15 7.65
Salt Control B -5.48 -7.15 7.42
Salt Control C -5.48 -7.11 7.65
Water Control -5.18 -7.18 7.54
[0041] Another set of experiments was done in order to determine the
efFectiveness of different acids and different types of surfactants in the
antimicrobial
salt formulations. In one experiment, test solutions containing 17% by weight
of
formulas containing various levels of sodium chloride, 100 ppm sodium lauryl
sulfate,
and various levels of different acids were tested for effectiveness in killing
L.
monocytogenes at cold temperatures. The same test procedure was used as
described above; test solutions were plated on MOX TAL (Modified Oxford medium
with a Thin Agar Layer) with TSA. Results are given in Table 8.
-16-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
Table 8. Effect of Different Acids on the Antimicrobial Action of
NaCI/Acid/SLS
Sample Time 0 4 Hours
(CFU/mI) (CFU/ml)
Salt control 850 1380
Salt + 100 ppm SLS, 0.5% citric acid 980 890
100 ppm SLS, 99.5% NaCI, 0.9% succinic acid 1230 18
100 ppm SLS, 99.1 % NaCi 1070 69
1.5% isoascorbic acid, 100 ppm SLS, 98.5% NaCI 1140 59
1.8% adipic acid, 100 ppm SLS, 98.2% NaCI 900 4
1.6% sorbic acid, 100 ppm SLS, 98.4% NaCl 820 500
1.3% acetic acid, 100 ppm SLS, 98.7% NaCI 1070 230
2.1 % propionic acid, 100 ppm SLS, 97.9% NaCi 1440 6
0.6% lactic acid, 100 ppm SLS, 99.4% NaCI 1050 220
1.6% ascorbic acid, 100 ppm SLS, 98.4% NaCi 1230 54
0.3% formic acid, 100 ppm SLS, 99.7% NaCi 1930 38
0.3% phosphoric acid, 100 ppm SLS, 99.7% NaCI 1050 17
0.1 % hydrochloric acid, 100 ppm SLS, 99.9% NaCI 1100 44
0.3% tartaric acid, 100 ppm SLS, 99.7% NaCI 1180 410
1.1 lo glutaric acid, 100 ppm SLS, 98.9% NaCI 610 180
1.2% benzoic acid, 100 ppm SLS, 98.8% NaCl 1020 17
0.5% salicylic acid, 100 ppm SLS, 99.5% NaCi 1100 5
0.2% sulfuric acid, 100 ppm SLS, 99.8% NaCI 830 0
[0042] In another experiment, test solutions containing 17% by weight of
formulas
containing 99.7% sodium chloride, 0.3% citric acid, and 500 ppm of various
types of
surfactants were tested for effectiveness in killing L. monocytogenes at cold
temperatures. The same test procedure was used as described above; test
solutions were plated on MOX TAL (Modified Oxford Medium with a Thin Agar
Layer)
with TSA. Results are given in Table 9.
Table 9. Effect of Different Acids on the Antimicrobial Action of NaCI
Surfactant
Tested
Surfactant Tested Time 0 4 Hours
(CFU/ml) (CFU/ml)
Salt Control (no additive) 880 610
polyoxyethylene-polyoxypropylene block polymer 820 610
sodium salt of sulfonated oleic acid 240 0
sodium xylene sulfonate 910 820
dodecyl diphenyl oxide disulfonate 0 0
-17-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
Surfactant Tested Time 0 4 Hours
(CFU/ml) (CFU/ml)
sodium linear alkyl-benzene sulfonate 490 0
alpha-olefin sulfonate 370 0
Alkylpolyglucoside 280 0
nonylphenol ethoxylate 460 0
fatty alkanolamide 470 0
alcohol ethoxylate 1080 1
lauramine oxide 2 0
[0043] Thus, the data indicate that solutions of salt, acid, and surfactant
provide
efficient kill of bacteria even at temperatures below the freezing point of
water. Salts
such as sodium sulfate, sodium chloride, and magnesium sulfate act
synergistically
with the acid and surfactant to enhance the antimicrobial effectiveness. The
formulations are shown to be effective in killing pathogenic bacteria such as
L.
monocytogenes. The formulas were shown to be effective both in freshly
prepared
brines and in spent process chill brine from a ready-to-eat meat plant. The
levels of
acid and surfactant may be varied to suit the particular application; reducing
the
concentration of the acid may be compensated by raising the concentration of
surfactant and vice versa. In addition to effectively killing bacteria
suspended in
solutions, the formulas are also shown to be effective at killing bacteria
within a
biofilm.
[0044] Further tests were conducted to determine the antimicrobial efficacy of
a
salt formulation containing a surfactant but no added acid. The effect of an
aqueous
solution comprising about 20 wt.% of various salt and salt/surfactant
formulations on
L. monocytogenes were tested in a manner directly analogous to that set forth
above
in connection with the data in Table 4. Table 10 sets forth the compositions
and the
L. monocytogenes population (stated as the log of the concentration of the
bacteria)
found after 4 hours of incubation. [Note that the compositions in Table 10 and
in
Table 11, below, state the concentration in the solution, not in the salt
concentrate.
Since the solutions are 20 wt. % of the salt formulation, the concentration of
surfactant in the salt formulation would be about five times the stated
concentration
in the solution.]
-18-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
Table 10. Effect of Solutions of NaCi and SLS on L. monocytogenes
Solution Composition Population at 4 hours
Water Control 4.61
20% NaCI (control) 4.32
50 ppm SLS (no NaCI) 3.90*
50 ppm SLS, 20% NaCI 0.85**
* The 3.90 value is an average of two runs that yielded values of 3.84 and
3.95.
**The 0.85 value is an average of two runs that yielded values of 0.70 and
1.00
[0045] Further tests were run on a variety of different surfactants,
demonstrating
that a variety of different types of surfactants show a strong synergistic
effect in
combination with sodium chloride:
Table 11. Effect of Solutions of NaCI and various surfactants on L.
monocytogenes
Solution Composition Population(
4 hours)
Water Control 4.85
20% NaCI (control) 5.01
50 ppm sodium salt of sulfonated oleic acid (no NaCI) 5.11
50 ppm sodium salt of sulfonated oleic acid, 20% NaCI 3.89
50 ppm lauramine oxide (no NaCI) 3.56
50 ppm lauramine oxide, 20% NaCI 0
50 ppm fatty alkanolamide (no NaCi) 4.02
50 ppm fatty alkanolamide, 20% NaCI 0
50 ppm nonylphenol ethoxylate (no NaCI) 5.12
50 ppm nonylphenol ethoxylate, 20% NaCI 1.60
50 ppm sodium linear alkly-benzenesulfonate (no NaCI) 3.77
50 ppm sodium linear alkly-benzenesulfonate, 20% NaCi 0
50 ppm alkylpolyglucosides (no NaCI) 4.98
50 ppm alkylpolyglucosides, 20% NaCI 1.00
[0046] Hence, formulations in other embodiments of the invention may comprise
ah inorganic salt and at least about 200 ppm of a surfactant, e.g., about 250-
5000
-19-
CA 02589517 2007-05-29
WO 2006/066253 PCT/US2005/046150
ppm, e.g., 500-1500 ppm, of the surfactant. Such formulations are deemed
particularly useful in food safety applications.
[0047] The foregoing specification describes this invention in relation to
certain
preferred embodiments, and many details have been set forth for purpose of
illustration. As those skilled in the art will appreciate, however, the
invention is
susceptible to additional embodiments and that some of the details mentioned
above
can be varied considerably without departing from the basic principles of the
invention.
-20-