Note: Descriptions are shown in the official language in which they were submitted.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
ANTI-AGING METHODS AND COMPOSITION
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority to each of provisional application No.
60/635,915, filed December 13, 2004 and No. 60/596,170, filed September 6,
2005,
and is a continuation-in-part of application Serial No. 10/420,280, filed
April 21,
2003; which is a continuation-in-part of application Serial No. 10/301,416
filed
November 21, 2002, which is a continuation-in-part of application Serial No.
09/698,537, filed October 26, 2000, now issued as Patent No. 6,521,248, which
claims priority to provisional application No. 60/161,546, filed October 26,
1999.
This application is also related to provisional applications No. 60/594,612
filed April 22, 2005 and No. 60/594,540, filed April 15, 2005. Each of the
above-
identified applications is incorporated by reference in its entirety.
BACKGROUND OF THE INVENTION
Aging is an inevitable biological process generally characterized by decline
in
physiological function that leads to morbidity and mortality. The aging
process
occurres gradually over a person's lifetime. During this gradual process the
decline in
physiological function is exemplified as a general decrease in physical and
mental
ability. Moreover there is a progressive decline in strength of the immune
system,
with decreased ability for the aging body to heal.
Aging involves death of cells or cell dysfunction due to production of free
radicals, oxidative damage and energy depletion due to mitochondrial
dysfunction.
Harman (1988) linked senescence or death to the injurious effects of free
radicals
arising from the one-electron reduction of oxygen during metabolism. There has
been
an inverse relationship between auto-oxidation rate in different animal
species and life
expectancy in the same species (Cutler 1985; Sohal 1995). Mitochondria are the
major source of oxygen radicals through the respiratory chain and are also
deeply
affected by reactive oxygen species (ROS), resulting in serious risks to their
function.
Mitochondrial dysfunction could result in defects in electron transport,
oxidative
phosphorylation and energy production resulting in cell damage and ultimately
cell
death.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-2-
Although the exact cause for these declining propensities is not known, it has
been proposed that damage to one or more of; cell membranes, electron
transfer, brain
tissue and disruption to energy related metabolic pathways which is caused by
free-
radicals and oxidants plays a significant causative role. An increase in
oxidative
lesions in mitochondrial DNA is observed on older subj_ects as compared to
younger.
This increase is observed in numerous tissue and cell types including, brain,
muscle,
nerve and diaphragm. Importantly, these increases are a comparative along the
continuum of aging.
Damage to the mitochondrial DNA is of particular importance because of their
ubiquitous involvement in energy production. Likewise, mitochondria are a
ubiquitous organelle with a function of paramount importance. Oxidative damage
to
the brain mitochondrial DNA has been linked to increased incidence of
neurodegenerative diseases with aging. Oxidative damage to muscular
mitochondria
has been linked to increased lethargy. Toxicity by oxygen radicals has also
been
suggested as a major cause of cancer, heart disease and aging in general.
A marked increase in life span has occurred within human evolution over the
past 60 million years. At the same time an enormous decrease in the age-
specific
cancer rate has occurred in humans. It is likely that a major factor in
lengthening life
span and decreasing age-specific cancer rates may have been the evolution of
effective mechanisms against free radicals and other sources of oxidative
damage.
Increasing the plasma concentration of radical and oxidant quenching
compositions
has been proposed. Compositions such as uric acid, vitamins A, E and C have
been
extensively studied in this regard, with limited success. One property, shared
by the
vitamins is their lack of water solubility. Their plasma concentration is
limited
because of this lack of solubility in (water) blood. Moreover, these all share
the
property of undergoing digestive degradation, further complicating efforts
towards
increasing plasma concentration. Further problems involve transport of these
anti-
oxidants into the cells, where the oxidative damage is problematic.
One important attribute of an anti-oxidant or a free-radical quenching agent
is
the ability to chemically react with the oxidant or free-radical, in a
biologically non-
destructive manner. Several of the biologically destructive oxidants and free-
radicals
involved include superoxide (02 ), H202, hydroxyl radicals (=OH) and singlet
oxygen
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-3-
i
( 02). The need exists for an anti-oxidant and free-radical quenching
composition
having desired solubility and cell uptalce properties. The present micro-
cluster water
provides these and other beneficial properties.
BRIEF SUMMARY OF THE INVENTION
One aspect of this invention is directed to a method and composition for
quenching free-radicals and oxidants in intracellular fluids.
Another aspect of this invention is directed to a method and composition for
quenching free-radicals and oxidants in extra-cellular fluids.
Another aspect of this invention is directed to a method and composition for
quenching free-radicals and oxidants in intercellular fluids.
A still further aspect of the present invention is directed to a method and
composition for the delivery of cosmeceuticals.
A further aspect of the present invention is a micro-cluster liquid having
anti-
oxidant and free-radical quenching properties.
The micro-cluster liquid, such as micro-cluster water, of the present
invention
further provides at least one property selected from the group comprising,
increased
potential energy, enhanced bioavailability, transdermal migration and
transdermal
facilitator.
The term transdermal migration shall mean having the ability to migrate
through or across the dermis. Whereas the term transdermal facilitator shall
mean the
property of facilitating the transdermal passage of another substance across
the dermal
membrane often coincident with its own migration.
The term cosmaceutical shall mean a cosmetic formulation which includes at
least one nutritional and/or pharmaceutical agent. A cosmaceutical may, for
example,
incorporate titanium dioxide (as the physical sunblock) and creatine pyruvate
(as the
cellular repair pharmaceutical) in a micro-cluster water vehicle. This
composition
may further include permeation enhancers to further facilitate delivery of the
pharmaceutical deep into the dermis. The anti-oxidant and anti-free radical
properties
of the present micro-cluster water further provide the un-expected result of
increased
cell-longevity, decreased DNA mutation rates, increased mitochondria cell
membrane
longevity and increased cellular membrane longevity, in general.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-4-
BRIEF DESCRIPTION OF THE DRAWINGS
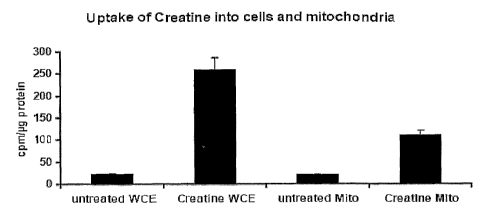
Figure 1 is a bar graph comparing the uptake of creatine into cells and
mitochondria.
Figure 2 is a graph depicting thegel and bar graphs of creatine and normal
cells response to UVA-induced mtDNA mutagenesis.
Figure 3 is a graphic of the chronic oxidative stress cycle.
Figure 4 is a graphic of the defective powerhouse model of cutaneous aging.
Figure 5 is a graph comparing the number of mutations between cells grown in
media made with micro-cluster water (Penta) or lab water (a.d.).
Figure 6 is a set of bar graphs comparing the number of common deletion
mutations between cells grown media made with micro-cluster water (Penta) or
lab
water (a.d.).
Figure 7 is a set of bar graphs comparing the number of common deletion
mutations between cells grown media made with micro-cluster water (Penta) or
lab
water (a.d.).
Figures 8a and 8b are graphs showing the differences between double distilled
water ("DDW") and inicro-cluster water (Micro-cluster) regarding changes to
intracellular pH under standard incubation conditions.
Figure 9 is a bar graph representation of the intracellular pH change in
macrophages under standard incubation conditions.
Figure 10 is a bar graph of the differences in propensity for damage to
cellular
membranes under standard incubation conditions, where the incubation medium
was
made from DDW or Micro-cluster water.
Figure 11 is a bar graph comparing the occurrence of common deletion
mutations in mtDNA between UV exposed skin and un-exposed skin.
DETAILED DESCRIPTION OF THE INVENTION
The methods of the present invention generally comprise administering to a
subject an amount of a micro-cluster fluid, topically, orally, transdermally
or other
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-5-
routes of administration known in the art. It is thought that the micro-
cluster liquids
modulate one or more of the ROS andlor free radicals responsible for oxidative
tissue
damage associated with pre-mature aging. The present invention further
generally
comprises incorporating neutriceutical and pharmaceutical agents in the micro-
cluster
fluid, together with a transdermal enhancer. It is thought that the micro-
cluster liquids
in association with creatine compounds modulate one or more of the structural
or
functional components of mtDNA mutagenesis and/or the creatine
kinase/phosphocreatine system sufficient to prevent, reduce or ameliorate
symptoms
of aging and damage to the skin. Components of the systems which can be
modulated
include the rate of mtDNA mutation, intracellular pH, intercellular pH, ROS
concentration, cell longevity, the enzyme creatine kinase, the substrates
creatine and
creatine phosphate, and the transporter of creatine. The term "modulate,"
"modulation" or "modulating" includes any increase or decrease in the activity
of any
coinponent of the creatine kinase/phosphocreatine system.
In one embodiment, the invention pertains to a method for treating a subject
(e.g., a mammal, preferably, a human) for skin disorders by administering to
the
subject an effective amount of a cosmaceutical coinprising a creatine compound
in a
micro-cluster liquid such that the skin damage is treated.
Creatine compounds are predicted to preserve tissue by boosting up energy
reserves in the skin and also by arresting mechanisms involved in oxidative
damage
and cell death. The micro-cluster liquids are predicted to independently
preserve
tissue by arresting deleterious oxidative mechanisms involved in oxidative
damage
and cell death. The combination of creatine compounds and micro-cluster
liquids
provides a synergistic composition predicted to preserve tissue by boosting,
up energy
reserves in the skin, by arresting deleterious oxidative mechanisms involved
in
oxidative damage and cel-1 death and by enhanced transdermal penetration and
migration of active agents. Compotuzds, which are particularly effective for
this
purpose, include micro-cluster liquids, micro-cluster water, chelated
minerals,
chelated vitamins, creatine, creatine phosphate, taurine, osmolytes, ectoin
and analogs
thereof, which are described in detail below.
The term "creatine compounds" includes creatine, creatine phosphate, and
compounds, which are structtirally similar to creatine or creatine phosphate,
and
analogs of creatine and creatine phosphate, including salts thereof such as
creatine
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-6-
pyruvate. The term "creatine compounds" also includes compounds, which
"miinic"
the activity of creatine, creatine phosphate or creatine analogs. The term
"mimics" is
intended to include compounds, which may not be structurally similar to
creatine but
mimic the therapeutic activity of creatine, creatine phosphate or structurally
similar
compounds. Also the term creatine compound includes "modulators of the
creatine
kinase system," for example, compounds which modulate the activity of the
enzyme,
or the activity of the transporter of creatine or the ability of other
proteins or enzymes
or lipids to interact with the system.
The term "treatment" includes the diminishment or alleviation of at least one
symptom associated or caused by the disorder being treated. For example,
treatment
can be diminislunent of several symptoms of a disorder or coinplete
eradication of a
disorder.
The language "treating for skin disorders" includes both prevention of
disorders, asnelioration and/or arrest of the disorder process. Examples of
skin
disorders include, but are not limited to aging and damage resulting from sun
radiation, stress, fatigue and/or free radicals. Although not wishing to be
bound by
theory, The micro-cluster liquids in association with creatine compounds
described
herein are thought to have both curative and prophylactic effects on
development of
damage and aging of the skin and other tissue. The language also includes any
amelioration or arrest of any, symptoms associated with the disorder process
(e.g.,
wrinkles). For example, treating wrinkles may include preventing, retarding,
arresting,
or reversing the process of wrinkle fonnation in skin, e.g., mammalian skin,
preferably, human skin.
This invention is directed to reducing free-radical and oxidative damage
secondary to free-radicals and other oxidants contacting cells, cellular
components or
tissues. The micro-cluster water of the present invention has free-radical and
oxidant
quenching properties. Contacting cells, cellular components or tissues with
the present
micro-cluster water results in increased cellular longevity, decreased rates
of DNA
mutation, improved mitochondrial efficiency, decreased mitochondrial DNA
mutation, increased collagen and fibroblast growth, amelioration of wrinkles
and
other skin damage; and thus anti-aging. Administration of the compositions of
the
present invention is accomplished by several methods, including ingestion
topical
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-7-
application such as by a cosmetic product, or any other method whereby the
tissue or
system in need of treatment is sufficiently contacted.
Conventional means for administering therapeutic or cosmetics agents ("active
agents") to a human or animal are usually limited to some degree by
biological,
chemical, and physical barriers. Examples of physical barriers are the skin
and various
organ membranes that must be traversed before the agent reaches a target.
Chemical
barriers include pH variations, lipid bi-layers, and degrading enzymes. Both
biologically and chemically active agents are particularly vulnerable to such
barriers.
Many active agents can be applied topically and this provides a convenient
mode of administration, particularly for cosmetics agents that are typically
applied to
an area of the skin that is affected by a skin condition. However, the
effectiveness of
topical application of an active agent depends on two major factors: a)
percutaneous
absorption and penetration; and b) bioavailability of the penetrated active
agent to the
target site in the skin.
For active agents to be effectively applied topically, the agents need to
penetrate the stratum comeum (the outer layer of the skin that includes layers
of
terminally differentiated keratinocytes) into the epidermal layers, and then
be
distributed and bioavailable to the target sites to provide an effect. This
transdermal
migration of active agents shall be termed transdermal administration.
Many cosmetics agents require routine application over an extended time, and
for this reason topical application is advantageous because the administration
regime
is relatively simple and can be achieved with a minimum of inconvenience.
However,
to maximize the effectiveness of the treatment, as much of the cosmetic agent
as
possible needs to be absorbed into the skin and when the agent is applied
topically by
applying a cream or lotion to the skin it is common for least some of the
active agent
to be lost by rubbing off or evaporation. The inventors have discovered that
generation of nanometer sized particles of the active agents when combined
with
micro-cluster water, decreases this rub-off problem and improves absorption of
active
agents.
By way of example, for the purposes of the present invention a cosmetic agent
may preferably be selected from one or more of: anti-aging agents, anti-
wrinkle
agents, antioxidants, anti-scarring agents, phytoestrogens, isoflavones,
coumarines, lip
balms, free radical quenching agents, and antiseptic anti-acne agents.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-8-
As used herein, the term "cosmetic agent" means any compound, mixture of
compounds, or preparations derived theirfrom that are intended to be placed in
contact
with external parts or with mucosal membranes of an animal body. (Especially a
human body) with a view to cleaning, changing the appearance, protecting
and/or
keeping the body parts to wliich the agent is applied in good condition.
Preferably, the cosmetics agent is capable of diminishing, reducing or
preventing the effects of one or more skin conditions including: the visible
effects of
aging, wrinkles, acne, age spots, scars (keloids) broken capillaries and,
includes
compositions which also optionally cleanse the skin, preferably in the form of
liquid
compositions such as liquid soaps, lotions and solutions both additives and
compositions for application to skin, hair, scalp, nails, eyes or teeth.
As used herein, the term "cosmaceutical" means a cosmetic agent according to
the present invention, which is adapted to facilitate delivery of
neutriceutical
compositions comprising vitamins, minerals and osmolytes.
The term "minerals" as used herein means the inorganic compounds normally
part of the class, as known in the art. Examples of metabolically important
minerals
are well documented in numerous health, wellness and medical texts including
for
example, Sb, As, B, Br, Yb, Pd, Re, F, Ir, La, W, Cs, C, Pt, Tm, N, Ni, Ta,
Tb, Fe, K,
I, Co, Mo, V, Ag, Mg, Cr, Cu, Zn, Ca, Si, Sn, Ni, P and S. A chelating matrix
delivery system is preferably used to facilitate transdermal delivery of these
minerals,
as part of the present compositions. Such a chelating matrix delivery system
is
described in patent 6,716,458 to Tarbet, filed August 7, 2000, which is
incorporated
herein by reference. The incorporation of these minerals in a chelating
matrix,
As used herein, the term "osmolyte" means organic solutes accumulated by
cells/tissues in response to osmotic stress. In general, osmolytes increase
thermodynamic stability of folded proteins and provide protection against
denaturing
stresses. Examples of osmolytes includes, but is not limited to, creatines,
taurins,
ectoins, their derivatives and corresponding biologically compatible salts.
Another form of the present invention provides a method of enhancing
penetration of the cosmetic and/or neutriceutic agent through the skin, the
method
including the steps of applying to the skin a composition containing: at least
one
cosmetic or neutriceutic agent, and a dermal penetration enhancer. The topical
application of this composition results in the delivery of the active agents
into the
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-9-
stratum corneum as well as delivery of the active agents into the epidermis
and
dermis.
Although not bound by any proposed theory, the present micro-cluster water
has increased potential energy as compared with double distilled water.
Perhaps
because of this increased energy, the micro-cluster water of the present
invention is
able to quench free-radicals and function as an anti-oxidant.
The term "micro-clustered coynposition" as used herein refers to a
cornposition
which comprises micro-cluster water. The adjective "micro-clustered " which
modifies any of the compositions of bio-affecting agents, body-treating
agents,
adjuvant or carriers, or ingredients thereof refers to micro-clustered water
in that
composition, i.e. which is dissolved in, mixed with, or otherwise combined
with
micro-cluster water. A micro-cluster liquid is any liquid, mixture or
combination of
liquids, whether or not miscible, which have been processed according to the
device
described and claimed in U.S. Patent 6,521,248, which is incorporated herein
by
reference.
The interaction of water and modified water media with various biological
structures and processes is mainly determined by the unique role water plays
in all
biological systems. Water is a major constituent in most biological processes,
as well
as the fluid medium through which proteins and nucleic acids interact. Apart
from
being known as the main medium for biological reactions, water also plays a
role in
determining and stabilizing hydrophilic and lipophyllic structures. Due to
water's
unique capabilities, it is able to influence the efficacy of various
processes. However,
many aspects related to the biological function of water remain unclear. There
are
facts, which indicate that the biological activity of water is due to a change
in
physical/chemical parameters. One of the important aspects in gaining an
understanding of the mechanism controlling water's biological activity is to
study it at
the cell level. Water is highly related to the internal regulation system,
including
intracellular pH and cell membrane status. Macrophage response and viability
is
therefore a useful indicator in this analysis.
The creatine kinase/creatine phosphate energy system is only one component
of an elaborate energy-generating system found in tissue with high and
fluctuating
energy requirements. The components of the creatine energy system include the
enzyme creatine kinase, the substrates creatine and creatine phosphate, and
the
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-10-
transporter of creatine. Some of the functions associated with this system
include
efficient regeneration of energy in cells with fluctuating and high energy
demands,
energy transport to different parts of the cell, phosphoryl transfer activity,
ion
transport regulation, and involvement in signal transduction pathways.
The present invention relates to methods for protecting skin tissue against
age
related damage or insults such as harmfiil UV radiation, stress and fatigue by
preserving energy pools and protecting against free radical production and
oxidative
stress. This is achieved by adininistering an amount of a creatine compound or
compounds together with a micro-cluster fluid, which modulates one or more of
the
biological pathways involved in energy and aging sufficient to prevent, reduce
or
aineliorate skin damage or skin aging. Compounds which are effective for this
purpose include, micro-cluster liquids, such as micro-cluster water, osmolytes
such as
taurine and ectoin and the natural compound creatine in its different
hydration or salt
and analogs of and combinations thereof. The compounds can be mixed in with
creams, oils, emulsions and the like to be spread readily on skin surfaces.
Alternatively, the compounds also can be packaged in a supplement form for
ingestion.
The present invention also provides micro-cluster liquid based compositions
containing creatine compounds in combination with a pharmaceutically or
cosmetically acceptable carrier, and effective amounts of other agents which
act on
tissue preservation such as antioxidants (e.g., CoQ10), vitamins such as C,
B5, B6, B9,
E, energy enhancing agents (for example creatine, chelated minerals, pyruvate,
nicotinamide) osmolytes and skin softeners to slow the process of aging.
The term "modulate," "modulation" or "modulating" includes any increase or
decrease in the activity of any component of an affected biological pathway or
system.
Micro-cluster liquids in combination with creatine compounds are predicted to
preserve tissue by boosting up energy reserves in the skin and also by
arresting
mechanisms involved in oxidative damage and cell death. Compounds which are
particularly effective for this purpose include micro-cluster water in
combination with
creatine, creatine phosphate, and analogs thereof which are described in
detail below.
The term "creatine compounds" includes creatine, creatine phosphate, and
compounds
which are structurally similar to creatine or creatine phosphate, and analogs
of
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
=11-
creatine and creatine phosphate. The term "creatine compounds" also includes
compounds, which "mimic" the activity of creatine, creatine phosphate or
creatine
analogs. The term "mimics" is intended to include compounds, which may not be
structurally similar to creatine but mimic the therapeutic activity of
creatine, creatine
phosphate or structurally similar compounds. Also the term creatine compound
includes "modulators of the creatine kinase system," for example, compounds
which
modulate the activity of the enzyme, or the activity of the transporter of
creatine or the
ability of other proteins or enzymes or lipids to interact with the system.
The term "treatment" includes the diminishment or alleviation of at least one
symptom associated or caused by the disorder being treated. For example,
treatment
can be diminislunent of several symptoms of a disorder or complete eradication
of a
disorder.
The language "treating for skin disorders" includes both prevention of
disorders, amelioration and/or arrest of the disorder process. Examples of
skin
disorders include, but are not limited to aging and damage resulting from sun
radiation, stress, fatigue and/or free radicals. Although not wishing to be
bound by
theory, The micro-cluster liquids in association with creatine compounds
described
herein are thought to have both curative and prophylactic effects on
development of
damage and aging of the skin and other tissue. The language also includes any
amelioration or arrest of any symptoms associated with the disorder process
(e.g.,
wrinkles). For example, treating wrinkles may include preventing, retarding,
arresting,
or reversing the process of wrinkle formation in skin.
The term "topical administration" includes methods of delivery such as laying
on or spreading on the skin. It involves any form of administration, which
involves
the skin. Examples of compositions suitable for topical administration include
but are
not limited to, ointments, lotions, creams, cosmetic formulations, and skin
cleansing
formulations. Additional examples include aerosols, solids (such as bar soaps)
and
gels.
The term "pharmaceutically acceptable" includes drugs, medicaments or inert
ingredients which are suitable for use in contact with the tissues of humans
and lower
animals without undue toxicity, incompatibility, instability, irritation,
allergic
response, and the like, commensurate with a reasonable benefit/risk ratio. The
term
also encompasses cosmetically acceptable ingredients.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-12-
The language "therapeutically or cosmetically effective amount" is intended to
include the amount of the compound sufficient to prevent onset of aging or
damage to
the skin or significantly reduce progression of damage in the subject being
treated. A
therapeutically or cosmetically effective amount can be determined on an
individual
basis and will be based, at least in part, on consideration of the severity of
the
symptoms to be treated and the activity of the specific analog selected if an
analog is
being used. Further, the effective amounts of the compound may vary according
to the
age of the subject being treated. Thus, a therapeutically or cosmetically,
effective
amount of the compound can be determined by one of ordinary skill in the art
employing such factors as described above using no more than routine
experimentation in health care management.
The topical pharmaceutical compositions of the present invention may be
made into a wide variety of product types. These include, but are not limited
to
solutions, lotions, creams, beach products, gels, sticks, sprays, pads,
ointments, pastes,
mousses and cosmetics. These product types may comprise several types of
carrier
systems including, but not limited to solutions, emulsions, gels and solids.
If the topical pharmaceutical coinpositions of the present invention are
formulated as an aerosol and applied to the skin as a spray-on, a propellant
is added to
a solution composition. A more complete disclosure of propellants useful
herein can
be found in Sagarin, Cosmetics Science and Technology, 2nd Edition, Vol. 2,
pp.
443-465 (1972).
The topical pharmaceutical compositions of the present invention may also be
formulated as makeup products such as foundations.
The topical pharmaceutical compositions of the present invention may also be
formulated as medicated pads. Suitable examples of these pads are fully
disclosed in
U.S. Pat. Nos. 4,891,227 and 4,891,228, to Thaman et al., both issued Jan. 2,
1990 the
disclosures of which are incorporated herein.
The topical pharmaceutical compositions of the present invention may contain,
in addition to the aforementioned components, a wide variety of additional oil-
soluble
materials and/or water-soluble materials conventionally used in topical
compositions,
at their art-established levels.
Various water-soluble materials may also be present in the compositions of
this invention. These include humectants, proteins and polypeptides,
preservatives and
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-13-
an alkaline agent. In addition, the topical compositions herein can contain
conventional cosmetic adjuvants, such as dyes, pigments and perfumes.
The topical pharmaceutical compositions of the present invention may also
include a safe and effective amount of a dermal penetration enhancing agent. A
preferred amount of penetration enhancing agent is from about 1% to about 5%
of the
composition. Another useful penetration enhancer for the present invention is
the non-
ionic polymer under the CTFA designation: polyacrylamide and isoparrafin and
laureth-7, available as Sepigel from Seppic Corporation. Also useful is
polyquaternium-32 and mineral oil known as SalCare SC92 available from Allied
Colloids, Suffolk, Va. This is a class of cationic polymers which are
generally
described in U.S. Pat. No. 4,628,078 to Glover et al. issued Dec. 9, 1986 and
U.S. Pat.
No. 4,599,379 to Flesher et al. issued Jul. 8, 1986 both of which are
incorporated by
reference herein.
Examples of useful penetration enhaiicers, among others, are disclosed in U.S.
Pat. No. 4,537,776, Cooper, issued Aug. 27, 1985; U.S. Pat. No. 4,552,872,
Cooper et
al., issued Nov. 12, 1985; U.S. Pat. No. 4,557,934, Cooper, issued Dec. 10,
1985; U.S.
Pat. No. 4,130,667, Smith, issued Dec. 19, 1978; U.S. Pat. No. 3,989,816,
Rhaadhyaksha, issued Nov. 2, 1976; U.S. Pat. No. 4,017,641, DiGiulio, issued
Apr.
12, 1977; and European Patent Application 0043738, Cooper et al., published
Jan. 13,
1982.
Other conventional skin care product additives may also be included in the
compositions of the present invention. For example, collagen, hyaluronic acid,
elastin,
hydrolysates, primrose oil, jojoba oil, epidermal growth factor, soybean
saponins,
mucopolysaccharides, and mixtures thereof may be used.
Various vitamins and minerals may also be included in the compositions of the
present invention. For example, Vitamin A, ascorbic acid, Vitamin B, biotin,
panthothenic acid, Vitamin D, Vitamin E and mixtures thereof and derivatives
thereof
are contemplated.
Also contemplated are skin cleaning compositions comprising both active
compounds of the present invention and a cosmetically-acceptable surfactant.
The
term "cosmetically-acceptable surfactant" refers to a surfactant, which is not
only an
effective skin cleanser, but also can be used without undue toxicity,
irritation, allergic
response, and the like. Furthermore, the surfactant must be capable of being
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-14-
commingled with the active compound in a manner such that there is no
interaction,
which would substantially reduce the efficacy of the composition for
regulating skin
damage, e.g., wrinkles.
The skin cleaning compositions of the present invention preferably contain
from about 0.1% to about 20%, preferably from about 1% to about 5%, of the
creatine
compound (e.g., creatine, cyclocreatine or another creatine compound) and from
about 1% to about 90% micro-cluster liquid, and from about 0.1 % to about 10%,
of a
cosmetically-acceptable surfactant.
The physical form of the skin cleansing compositions is not critical. The
compositions can be, for exainple, formulated as toilet bars, liquids, pastes,
mousses,
or pads.
The cleaning compositions of the present invention can optionally contain, at
their art-established levels, materials, which are conventionally used in skin
cleansing
coinpositions.
Sunblocks and sunscreens incorporating micro-cluster liquids and creatine
compounds are also contemplated. The tenn "sun block" or "sun screen" includes
compositions, which block UV light. Examples of sunblocks include, for
exainple,
zinc oxide and titanium dioxide.
Sun radiation is one major cause of skin damage, e.g., wrinkles. Thus, for
purposes of wrinkle treatment or prevention, the combination of a micro-
cluster liquid
and a creatine compound with a UVA and/or UVB sunscreen would be advantageous.
The inclusion of sunscreens in compositions of the present invention will
provide
immediate protection against acute UV damage. Thus, the sunscreen will prevent
further skin damage caused by UV radiation, while the compounds of the
invention
modulates existing skin damage.
A wide variety of conventional sunscreening agents are suitable for use in
combination with the active compound. Segarin, et al., at Chapter VIII, pages
189 et
seq., of Cosmetics Science and Technology, disclose numerous suitable agents.
Specific suitable sunscreening agents include, for example: p-aminobenzoic
acid, its
salts and its derivatives (ethyl, isobutyl, glyceryl esters; p-
dimethylaminobenzoic
acid); anthranilates (i.e., o-aminobenzoates; methyl, menthyl, phenyl, benzyl,
phenylethyl, linalyl, terpinyl, and cyclohexenyl esters); salicylates (amyl,
phenyl,
benzyl, menthyl, glyceryl, and dipropyleneglycol esters); cinnamic acid
derivatives
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-15-
(methyl and benzyl esters, .alpha.-phenyl cinnamonitrile; butyl cinnamoyl
pyruvate);
Dihydroxyciimamic acid derivatives (umbelliferone, methyluinbelliferone,
methylaceto-umbelliferone); trihydroxycinnamic acid derivatives (esculetin,
methylesculetin, daphnetin, and the glucosides, esculin and daphnin);
hydrocarbons
(diphenylbutadiene, stilbene); dibenzalacetone and benzalacetophenone;
Naphtholsulfonates (sodium salts of 2-naphthol-3,6-disulfonic and of 2-
naphthol-6,8-
disulfonic acids); Dihydroxy-naphthoic acid and its salts; o- and p-
Hydroxybiphenyldisulfonates; Coumarin derivatives (7-hydroxy, 7-methyl, 3-
phenyl);
Diazoles (2-acetyl-3-broinoindazole, phenyl benzoxazole, methyl naphthoxazole,
various aryl benzothiazoles); Quinine salts (bisulfate, sulfate, chloride,
oleate, and
tannate); Quinoline derivatives (8-hydroxyquinoline salts, 2-phenylquinoline);
Hydroxy- or methoxy-substituted benzophenones; Uric and vilouric acids; Tannic
acid and its derivatives (e.g., hexaethylether); (Butyl carbotol) (6-propyl
piperonyl)
ether; Hydroquinone; Benzophenones (Oxybenzene, Sulisobenzone, Dioxybenzone,
Benzoresorcinol, 2,2',4,4'-Tetrahydroxybenzophenone, 2,2'-Dihydroxy-4,4'-
dimethoxybenzophenone, Octabenzone; 4-Iso-propyldibenzoylmethane;
Butylmethoxydibenzoylmethane; Etocrylene; and 4-isopropyl-di-benzoylmethane.
Preferred sunscreens useful in the compositions of the present invention are
nanometer particles of Ti02, ZnO, dispersed in a micro-cluster liquid and
mixtures
thereof.
A safe and effective amount of sunscreen may be used in the compositions of
the present invention. The sunscreening agent must be compatible with the
active
compound. Generally the composition may comprise from about 1% to about 20%,
preferably from about 2% to about 10%, of a sunscreening agent. Exact amounts
will
vary depending upon the sunscreen chosen and the desired Sun Protection Factor
(SPF).
An agent may also be added to any of the compositions of the present
invention to improve the skin substantivity of those compositions,
particularly to
enhance their resistance to being washed off by water, or rubbed off. A
preferred
agent, which will provide this benefit is a copolymer of ethylene and acrylic
acid.
Compositions comprising this copolymer are disclosed in U.S. Pat. No.
4,663,157,
Brock, issued May 5, 1987, which is incorporated herein by reference.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-16-
In another embodiment of the present invention, an anti-inflammatory agent is
included as an active agent along with the micro-cluster liquids in
association with
creatine compounds of the invention. The anti-inflammatory agent protects
strongly in
the WA radiation range (though it also provides some UVB protection as well)
thereby preventing further skin damage caused by UV radiation, while The micro-
cluster liquids in association with creatine compounds of the invention treat
existing
damage. Thus the combination provides broad protection against further damage
while facilitating repair of pre-existing damage. The topical use of anti-
inflammatory
agents reduces photo-aging of the skin resulting from chronic exposure to UV
radiation. (See U.S. Pat. No. 4,847,071, Bissett, Bush, and Chatterjee, issued
Jul. 11,
1989, incorporated herein by reference; and U.S. Pat. No. 4,847,069, Bissett
and
Chatterjee, issued Jul. 11, 1989, incorporated herein by reference.)
A safe and effective amount of an anti-inflammatory agent may be added to
the compositions of the present invention, preferably from about 0.1% to about
10%,
more preferably from about 0.5% to about 5%, of the composition. The exact
amount
of anti-inflammatory agent to be used in the compositions will depend on the
particular anti-inflammatory agent utilized since such agents vary widely in
potency.
In another embodiment, the cosmaceutical further comprises a safe and
effective amount of a skin protectant. The skin protectant preferably
comprises from
about 0.001% to about 2%, more preferably from about 0.01% to about 1% of the
composition. Useful skin protectants are disclosed in the Federal Register
Vol. 48,
No. 32 and include allantoin, aluminum hydroxide gel, bismuth subnitrate,
boric acid,
calamine, cocoa butter, corn starch, dimethicone, glycerin, kaolin, live yeast
cell
derivative, petrolatum, shark liver oil, sodium bicarbonate, sulfur, tannic
acid, white
petrolatum, zinc acetate, zinc carbonate and zinc oxide and mixtures thereof.
Formulations of the present invention include those suitable for topical,
oral,
nasal, transdermal, buccal, sublingual, rectal, vaginal and/or parenteral
administration.
The formulations may conveniently be presented in unit dosage form and may be
prepared by any methods well known in the art of pharmacy..
Methods of preparing these formulations or compositions include the step of
bringing into association all components of the formulation, including
accessory
ingredients. This mixture of ingredients is processed through the device as
described
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-17-
in U.S. patent 6,521,248 until desired nanometer particle size and/or
dissolution of
hydrophobics is accomplished.
Suspensions, in addition to the active compounds, may contain suspending
agents as, for example, ethoxylated isostearyl alcohols, polyoxyethylene
sorbitol and
sorbitan esters, microcrystalline cellulose, aluminum metahydroxide,
bentonite, agar-
agar and tragacanth, and mixtures thereof.
Dosage forms for the topical or transdermal administration of a compound of
this invention include powders, sprays, ointments, pastes, creams, lotions,
gels,
solutions, patches and inhalants. The active compound may be mixed under
sterile
conditions with a pharmaceutically acceptable carrier, and with any
preservatives,
buffers, or propellants which may be required.
The ointments, pastes, creams and gels may contain, in addition to an active
compound of this invention, excipients, such as animal and vegetable fats,
oils,
waxes, paraffins, starch, tragacanth, cellulose derivatives, polyethylene
glycols,
silicones, bentonites, silicic acid, talc and zinc oxide, or mixtures thereof.
,
Transdermal patches have the added advantage of providing controlled
delivery of a compound of the present invention to the body. Such dosage forms
can
be made by dissolving or dispersing the compounds in the proper medium.
Absorption enhancers can also be used to increase the flux of the compound
across
the skin. The rate of such flux can be controlled by either providing a rate
controlling
membrane or dispersing the active compounds in a polymer matrix or gel.
These compositions may, also contain adjuvants such as preservatives, wetting
agents, emulsifying agents and dispersing agents. Prevention of the action of
microorganisms may, be ensured by the inclusion of various antibacterial and
antifingal agents, for example, paraben, chlorobutanol, phenol sorbic acid,
and the
like. It may also be desirable to include isotonic agents, such as sugars,
sodium
chloride, and the like into the compositions. In addition, prolonged
absorption of the
injectable phannaceutical form maybe brought about by the inclusion of agents
which delay absorption such as aluminum monostearate and gelatin.
In a further embodiment, the skin disorder is associated with free radicals,
aging, sun radiation, stress or fatigue. In another embodiment, the subject is
afflicted
with wrinkles or is at risk for a skin disorder.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-18-
The term "associated with free radicals" includes any disorders or damage to
the skin resulting directly or indirectly from free radicals. The free
radicals may be
initiated by, for example, sun radiation (e.g., UV radiation) or pollution.
The term "aging" includes processes where there is oxidative damage, energy
depletion or mitochondrial dysfunction where onset, amelioration, arrest, or
elimination is effectuated by The micro-cluster liquids in association with
creatine
compounds described herein. Symptoms of aging include, but are not limited to,
wrinkles, loss of elasticity of the skin and uneven pigmentation of the skin.
The invention also features a composition for the treatment of the slcin of a
subject. The composition comprises an effective amount of a micro-cluster
liquid
combined with a creatine, creatine phosphate, a creatine compound or a salt
thereof.
Preferably, the effective amount is effective to treat or prevent a skin
disorder.
Preferably, the composition is suitable for topical administration. The
composition
may be formulated as a lotion, cream, or ointment, gel or solid. In one
advantageous
embodiment, the composition also contains a sunblock or sunscreen (e.g., zinc
oxide
or titanium dioxide).
In another further embodiment, the composition may be formulated as a
cosmetic foundation or as a skin cleansing agent. Advantageously, the
composition
may contain a penetration agent. Examples of compounds which may be
incorporated
into the coinposition of the invention include, but are not limited to,
hydroxyacids,
retinols, Aloe, Chamomile, or mixtures thereof.
In a fiu-ther embodiment, the skin disorder is associated with free-radicals,
aging, sun radiation, stress or fatigue. ,
In a further embodiment, the invention contemplates co-administering to the
subject an effective aiuount of a skin preserving agent. Examples of skin
preserving
agents include antioxidants, such as micro-cluster water, ascorbic acid,
vitamins,
coenzyme Q10 (CoQ 10) and its derivatives, cysteine hydrochloride, sodium
bisulfate,
sodium metabisulfite, sodium sulfite and the like; oil-soluble antioxidants,
such as
ascorbyl palmitate, butylated hydroxyanisole (BHA), butylated hydroxytoluene
(BHT), lecithin, propyl gallate, alpha-tocopherol, and the like; and metal
chelating
agents, such as citric acid, ethylenediamine tetraacetic acid (EDTA),
sorbitol, tartaric
acid, phosphoric acid, and the like. Preferred anti-oxidants include, CoQ10
and
vitamin E. Other examples of skin preserving agents include energy-enhancing
agents
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-19-
(e.g., ATP, nicotinamide or pyruvate), vitamins (e.g., E, C, B5, B6, and B9)
and
vitamin precursors.
The term "energy enhancing agents" also includes stimulants of mitochondrial
function or ATP production elsewhere in the cell. Examples include
intermediates
such as, for example, pyruvate, nicotinamide and CoQ10.
Aging Oxidative Stress and Mitochondrial Dysfunction:
A common feature of the life cycle of virtually all multicellular organisms is
the progressive decline in efficiency of various physiological processes once
the
productive phase of life is over. Data has supported the hypothesis that
senescence
cell death secondary to loss of functional capacity is due to accumulation of
molecular
oxidative damage (Harman 1956; Stadtman 1992; Ames et. al., 1993; Sohal 1995).
The hypothesis is based on the fact that oxygen is potentially a toxic
substance, and its
use by aerobes, although necessary for their immediate survival, also may be
hazardous to their long term existence. Molecular oxygen is the precursor of
superoxide, hydrogen peroxide and liydroxyl radicals. Upon further reactions
these
could generate reactive oxygen species that cause extensive oxidative damage
to
macromolecules. Lipid peroxidation, DNA damage and carbonylation of proteins
are
some of the devastating effects. During aging there is an increase in the
amount of
oxidative stress which could be a result of increase in the rate of generation
of
reactive oxygen species, or the decline in anti-oxidative defenses or the
decline in the
efficiency of repair or removal of damaged molecules (Sohal et. al., 1996).
With
aging there is an increase in the production of ROS (Reactive Oxygen Species)
from
mitochondria which results in damage to the inner mitochondrial membrane. By
positive feedback mechanisms this results in further increase in ROS. Among
flies,
those with a longer life expectancy were shown to exhibit a lower rate of
mitochondrial superoxide, hydrogen peroxide generation, a lower rate of
protein
oxidative damage, less DNA oxidative damage, higher activities of SOD and
catalase,
increased glutathione a versatile intracellular reductant. Variations in
maximum life
span among different species are often associated with differences in the
metabolic
rate (rate of oxygen consumption), metabolic potential (total amount of energy
consumed per gram of body weight during life span) and level of oxidative
stress. The
highest degree of oxidative damage occur in tissues such as brain, heart and
skeletal
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-20-
muscle which are composed primarily of long lived postmitotic cells. These
tissues
are also the targets of several age related degenerative disorders in which
oxidative
stress has been implicated (Davies 1995; Weindruch et al., 1993). Agents that
minimize the production of reactive oxygen species are predicted to be
protective.
Creatine Kinase Skin Aging and Skin Damage
The creatine content and the efficiency of the creatine kinase system
decreases
with aging. Aging and several insults result in oxidative stress state and
energy
compromise. Minimizing the rate of production of molecules associated with
oxidative damage correlates well with a decrease in oxidative damage. Such
minimization combined with energy boosting effects should slow damage to
tissue
during aging or exposure to insults. Creatine and analogs of creatine that
modify the
rate of ATP synthesis through creatine kinase could sustain energy production,
mitochondrial function, and protect against free radical production. Such
effects could
have positive impact against aging or insult related skin damage.
Without wishing to be bound by theory, it is thought that modulating the
creatine kinase activity would modulate energy flow and affect skin cell
function,
integrity and survival. An activated energy state should minimizes oxidative
damage
and enable cells to withstand insult secondary to aging or insults such as UV
radiation.
Creatine is taken by athletes to boost muscle function during burst activity
(for
review see Wyss and Kaddurah-Daouk 1999) and during competitions. Creatine was
shown to have neuroprotective properties, in several animal models of
neurodegenerative diseases (Matthews et al., 1988; Kliveny et al 1999;
Matthews et.
al., 1999).
Ingestion of creatine analogs has been shown to result in replacement of
tissue
phosphocreatine pools by synthetic phosphagens with different kinetic and
thermodynamic properties. This results in subtle changes of intracellular
energy
metabolism, including the increase of total reserves of high energy phosphate
(see
refs. Roberts, J. J. and J. B. Walker, Arch Biochem. Biophys 220(2): 563-571
(1983)).
The replacement of phosphocreatine pools with slower acting synthetic
phosphagens,
such as creatine analogs might benefit neurological disorders by providing a
longer
lasting source of energy. One such analog, cyclocreatine (1-carboxymethyl-2-
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-21-
aminoimidazolidine) modifies the flow of energy of cells in stress and may
interfere
with ATP utilization at sites of cellular work.
Similarly, ingestion of micro-cluster water has been shown to improve cellular
energy metabolism. Moreover, the topical application of micro-cluster water
has been
shown to provide a significant decrease in mtDNA mutation rates.
Creatine Compounds Useful in Skin tare
Creatine compounds useful in the present invention include compounds which
modulate one or more of the structural or functional components of the
creatine
kinase/phosphocreatine system. Compounds which are effective for this purpose
include creatine, creatine phosphate and analogs thereof, compounds which
mimic
their activity, and salts of these compounds as defined above. Exemplary
creatine
compounds are described below.
Creatine (also known as N-(aminoiminomethyl)-N-methylglycine;
methylglycosamine or N-methyl-guanido acetic acid) is a well-known substance.
(See, The Merck Index, Eleventh Edition, No. 2570 (1989).
Cyclocreatine is an essentially planar cyclic analog of creatine. Although
cyclocreatine is structurally similar to creatine, the two compounds are
distinguishable both kinetically and thermodynamically. Cyclocreatine is
phosphorylated efficiently by creatine kinase in the forward reaction both in
vitro and
in vivo. Rowley, G. L., J. Am. Chem. Soc. 93: 5542-5551 (1971); McLaughlin, A.
C.
et. al., J. Biol. Chem. 247, 4382-4388 (1972).
The phosphorylated compound phosphocyclocreatine is structurally similar to
phosphocreatine; however, the phosphorous-nitrogen (P--N) bond of
cyclocreatine
phosphate is more stable than that of phosphocreatine. LoPresti, P. and M.
Cohn,
Biochem. Biophys. Acta 998: 317-320 (1989); Annesley, T. M. and J. B. Walker,
J.
Biol. Chem. 253; 8120-8125, (1978); Annesley, T. M. and J. B. Walker, Biochem.
Biophys. Res. Commun. 74:185-190 (1977).
Guanidino acetate is yet another analog of creatine and is a precursor of
creatine in its biosynthetic pathway. Guanidino benzoic acids are structurally
related
to creatine. Also compounds that attach amino acid like molecules covalently
to
creatine are creatine compounds of interest. Examples are creatine-ascorbate
and
creatine-pyruvate. Other types of molecules could be covalently attached.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-21-
aminoimidazolidine) modifies the flow of energy of cells in stress and may
interfere
with ATP utilization at sites of cellular work.
Similarly, ingestion of micro-cluster water has been shown to improve cellular
energy metabolism. Moreover, the topical application of micro-cluster water
has been
shown to provide a significant decrease in mtDNA mutation rates.
Creatine Coinpounds Useful in Skin Care
Creatine compounds useful in the present invention include compounds which
modulate one or more of the structural or functional components of the
creatine
kinase/phosphocreatine system. Compounds which are effective for this purpose
include creatine, creatine phosphate and analogs thereof, compounds which
mimic
their activity, and salts of these compounds as defined above. Exemplary
creatine
compounds are described below.
Creatine (also known as N-(aminoiminomethyl)-N-methylglycine;
methylglycosainine or N-methyl-guanido acetic acid) is a well-known substance.
(See, The Merck Index, Eleventh Edition, No. 2570 (1989).
Cyclocreatine is an essentially planar cyclic analog of creatine. Although
cyclocreatine is structurally similar to creatine, the two coinpounds are
distinguishable both kinetically and'thermodynamically. Cyclocreatine is
phosphorylated efficiently by creatine kinase in the forward reaction both in
vitro and
in vivo. Rowley, G. L., J. Am. Chem. Soc. 93: 5542-5551 (1971); McLaughlin, A.
C.
et. al., J. Biol. Chem. 247, 4382-4388 (1972).
The phosphorylated compound phosphocyclocreatine is structurally similar to
phosphocreatine; however, the phosphorous-nitrogen (P--N) bond of
cyclocreatine
phosphate is more stable than that of phosphocreatine. LoPresti, P. and M.
Cohn,
Biochem. Biophys. Acta 998: 317-320 (1989); Annesley, T. M. and J. B. Walker,
J.
Biol. Chem. 253; 8120-8125, (1978); Annesley, T. M. and J. B. Walker, Biochem.
Biophys. Res. Commun. 74:185-190 (1977).
Guanidino acetate is yet another analog of creatine and is a precursor of
creatine in its biosynthetic pathway. Guanidino benzoic acids are structurally
related
to creatine. Also compounds that attach amino acid like molecules covalently
to
creatine are creatine compounds of interest. Examples are creatine-ascorbate
and
creatine-pyruvate. Other types of molecules could be covalently attached.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-22-
Creatine analogs and other agents which act to interfere with the activity of
creatine biosynthetic enzymes or with the creatine transporter are useful in
the present
method of treating or preventing age related damage. Thus the effects of such
compounds can be direct or indirect, operating by mechanisms including, but
not
limited to, influencing the uptake or biosynthesis of creatine, the function
of the
creatine phosphate shuttle, enzyme activity, or the activity of associated
enzymes, or
altering the levels of substrates or products of a reaction to alter the
velocity of the
reaction.
Compounds which modify the structure or function of the creatine
kinase/creatine phosphate system directly or indirectly are useful in
preventing and/or
treating age related damage to tissue such as skin.
Molecules that regulate the transporter of creatine, or the association of
creatine kinase with other protein or lipid molecules in the membrane, the
substrates
concentration creatine and creatine phosphate also are useful in preventing
and/or
treating age related damage to tissue such as skin.
Compounds which are useful in the present invention can be substrates,
enzyme activity modifiers or substrate analogs of creatine kinase. In
addition,
modulators of the enzymes that work in conjunction with creatine kinase now
can be
designed and used, individually, in combination or in addition to creatine
compounds.
Combinations of creatine compounds with other supplements or other drugs is
proposed.
The pathways of biosynthesis and metabolism of creatine and creatine
phosphate can be targeted in selecting and designing compounds which may
modify
energy production or high energy phosphoryl transfer through the creatine
kinase
system. Compounds targeted to specific steps may rely on structural analogies
with
either creatine or its precursors. Novel creatine analogs differing from
creatine by
substitution, chain extension, and/or cyclization may be designed. The
substrates of
multisubstrate enzymes may be covalently linked, or analogs which mimic
portions of
the different substrates may be designed. Non-hydrolyzable phosphorylated
analogs
can also be designed to mimic creatine phosphate without sustaining ATP
production.
Creatine, creatine phosphate and many creatine analogs are commercially
available. Additionally, analogs of creatine may be synthesized using
conventional
techniques.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-23-
Creatine compounds which currently are available or have been synthesized
include, for example, creatine, b-guanidinopropionic acid, guanidinoacetic
acid,
creatine phosphate disodium salt, cyclocreatine, homocyclocreatine, phosphinic
creatine, homocreatine, ethylcreatine, cyclocreatine phosphate dilithium salt
and
guanidinoacetic acid phosphate disodium salt, 4 guanidino benzoic acid and
derivatives, creatine-pyruvate, creatine-ascorbate among others.
The term "administration" is intended to include routes of administration
which allow the inventive compositions to perform their intended function(s).
EXAMPLES
Numerous types of non-mineralized drinking waters have potential mutagenic
effects because of various free radical components that may be present
secondary to a
multi-step water purification and processing system. The cytogenetic method
represents one approach for evaluating the potential mutagenic effects of
water. This
approach is based on detennination of the frequency of chromosome aberrations,
sister chromatid exchange (SCE), and cell cycle duration. The method was used
in
testing the cytogenetic effects of Micro-cluster research water having the
trade name
AQUA RXTMin the U.S. market. This water was provided by Bio-Hydration Research
Lab (USA). Micro-cluster water is produced through a multistep process
according to
U.S. patent NO. 6,521,248 which provides research water with unique attributes
and a
purity of less than 0.5 ppm of total dissolved substances (TDS). Medicinal
grade
oxygen is added to the water in a final step to pressurize the plastic bottles
for
shipment. Cell culture medium was prepared by dissolving RPMI 1640 (Gibco)
powder in standard deionized water - 18 Mohm (control) or Micro-cluster water.
Experiments were conducted with human lymphocytes, which were cultured in
accordance with standard protocol. Cells are fixed after 48 hours of culturing
in order
to determine the frequency of chromosome aberrations. Upon determining the SCE
frequency, after 48 hours 5-BDU (10 mg/ml) is added to the cell culture. Cells
are
fixed after 80 hours of culturing. Specimen preparation and staining are done
according to procedures known in the art. Experiments were performed twice for
each
of the 3 donors. 1200 metaphases are analyzed to determine the chromosome
aberrations in the control and in Micro-cluster water.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-24-
Example 1. Effects of 1% Creatine Supplementation on 3-Nitrotyrosine/Tyrosine
Concentration in FALS Mice.
Oxidative injury involves the activation of nitric oxide production, and
peroxynitrite which results in nitration of proteins. The nitration of
proteins could be
determined by measuring the ratio of 3-nitrotryrosine to tyrosine. The FALS
mice are
transgenic animals that express a mutant form of Cu/Zn superoxide dismutase
found
in patients with familial ALS (Amyotrophic Lateral Sclerosis). These animals
develop
ALS symptoms with gradual motor neuron loss, muscle weakness, and die within
135
days. Oxidative stress has been associated with the death of motor neurons.
Levels of
3-nitrotyrosine are significantly increased in the spinal cords of these mice
(Ferrante
1997). The transgenic mice with the G93A mutation and the littermate controls
(eight
mice per group) were fed 1% creatine or unsupplemented diets at days 70 of age
and
then killed at 120 days of age for measurements of 3-nitrotyrosine as
described
(Ferrante 1997). Creatine ingestion can significantly inhibit the higher
levels of 3
nitrotyrosine/tyrosine levels in lower spinal cords of transgenic FALS mice.
Example 2. Effect of 1% Creatine Supplementation on Hydroxyl Radical
Production
as Measured by Rate of Conversion of Salicylate to its by Products in FALS
Mice.
The level of free radical production in vivo can be determined using the
microdialysis technique (Matthews et al 1998). Administration of the
mitochondrial
toxin 3-nitropropionic acid results in a significant increase in the
conversion of
salicylate to 2,3-DHBA in the striatum, which is blocked in mice over
expressing Cu,
Zn SOD (Bogdanov et. al., 1998). Here we demonstrate that systemic
administration
of 3-nitropropionic acid (3-NP) resulted in a significant increase in the
conversion of
4-HBA to 3,4-DHBA in G93 A transgenic mice fed unsupplemented diets. In
animals
fed 1% creatine supplemented diets, there was no significant increase in 3,4
DHBA/4HBA after 3-NP administration. This demonstrates that creatine can
minimize the production of hydroxyl radicals that are implicated in aging
related
damage.
Example 3. Production of 2,3 and 2,5 DHBA and 3 Nitrotyrosine (Indicators of
Oxidative Stress) after Intrastriatal Injection of Malonate in Control Animals
Fed with
Creatine and Those Fed with Cyclocreatine
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-25-
The salicylate hydroxyl radical-trapping method was used for measuring
levels of hydroxyl radicals in striatal tissue after malonate injections.
Eight animals in
each group were fed either a normal diet or a diet enriched with 1% creatine
or 1%
cyclocreatine for two weeks before intrastriatal malonate injections. Animals
were
injected with 200 mg/kg salicylate intraperitoneally just before the malonate
injections and were killed 1 hour later. The striata were then dissected
rapidly from a
2-mm thick slice and placed in 0.25 ml of chilled 0.1 M perchloric acid.
Samples were
subsequently sonicated, frozen rapidly and thawed and centrifuged twice. An
aliquot
of supernatant was analyzed by HPLC with the 16-electrode electrochemical
detection
(Beal et. al., 1990). Salicylate, 2,3 and 2,5 DHBA, tyrosine, 3-nitrotyrosine
were
measured electrochemically by oxidation at 840, 240, 120, 600 and 840 mV
respectively with retention times of 20.5, 9.4, 6.3, 10.5, 18.2 min
respectively. The
data were expressed as the ratio of 2,3 and 2,5 DHBA to salicylate to
normalize the
DHBA concentrations for differing brain concentrations of salicylate.
Similarly, 3-
nitrotyrosine levels were normalized to tyrosine levels. We also examined the
effects
of 1% creatine supplementation for 2 weeks on 3-NP induced increases in 3-
nitrotyrosine levels. Male Sprague Dawley rats were treated with 3-NP at a
dose of 20
mg/kg intraperotoneally and then killed at 3 hours. Ten animals were examined
in
each group. The striata were dissected and placed in chilled 0.1 M perchloric
acid. 3-
Nitrotyrosine and tyrosine concentrations were measured by HPLC with
electrochemical detection (Matthews 1998). Statistical comparisons were made
by
unpaired Student's t test or by one way ANOVA followed by Fisher's protected
least
significant difference test for post hoc comparisons.
These Examples demonstrate that both creatine and cyclocreatine can protect
against increases in levels of salisylate derivatives 2,3 DHBA and 2,5 DHBA
after
injection of the mitochondrial toxin malonate. This confirms that creatine
compounds
can indeed protect against production of hydroxyl radicals implicated in
oxidative
stress and mitochondrial dysfunction. These Examples further demonstrate that
creatine and cyclocreatine have protective effects against nitration of
proteins induced
by the mitochondrial toxin malonate. Production of nitric oxide and
peroxynitrite are
part of the cascade of oxidative damage.
Example 4.
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-26-
The frequency of aberrant metaphases is effectively lower for MICRO-
CLUSTER (0.92%) in comparison with standard deionized water (2.50%) (df-~-l;
x2=8,96; P=0.0028). To evaluate the average number of SCE per cell, 300
metaphases
are analyzed in each case. It was shown that the SCE number is lower
(3.38W.120)
compared to standard deionized water (4,01 0,145) (df=598; t=3,311;
P=0,000985)
when MICRO-CLUSTER water is used as a solvent for RPMI 1640 dry mediuni.
Chromosome staining is also performed to determine the cell cycle duration by
counting the number of 1 st, 2nd, and 3rd mitoses. The average number of
divisions in
i-i i-I
cell culture is determined using the formula: (sigina ~ni/2 )/(sigma ni/2 ),
where i
mitosis number, and ni = cell number of i-mitosis after 32 hours in presence
of BDU.
The duration of the cell cycle is calculated as 32 hours divided by the
average number
of divisions. The cell cycle duration is 21.2 hours for both types of water,
which is in
agreement with literature data.
Thus, micro-cluster water doesn't result in mutagenic effects compared to
standard deionized water. Based on the data obtained, it appears that Micro-
cluster
water has a stabilizing effect, which results in both a lower frequency of
sister
chromatid exchanges and chromosome aberrations compared to standard deionized
water.
Example 5.
This example examines the influence of Micro-cluster water on intracellular
pH of mice peritoneal macrophages and to also assess the cell membrane status
under
short and long term exposure to the water.
Procedure: Determine intracellular pH of macrophages after 15 and 240 minutes
of
incubation time in standard lab medium prepared with both double distilled and
Micro-cluster water. Study the kinetics of pHi values during first 15 minutes
in
standard lab medium prepared using either double distilled or Micro-cluster
waters
and estimate the number of cells with damaged plasma membranes in macrophage
population after 15 and 240 minutes of incubation time.
Reagents:
Fluoresceindiacetate (FscDA, Sigma)
Ethidium bromide (EthBr, Sigma)
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-27-
Hanks balanced salt solution
standard lab Powder medium (standard lab media)
HEPES (Sigma)
Double Distilled Water (DDW)
Micro-cluster Water
Nigericin (Sigma)
Mouse Peritoneal Macrophages.
Mice were sacrificed for macrophages isolation. Hanks solution 2 ml (10 mM
HEPES, pH 7.2) had been injected into peritoneurn. After the injection, the
liquid
enriched with macrophages was collected from the peritoneuin. By double
staining
with EthBr and FSCDA, the integrity of the plasma membrane in the collected
cells
6
was controlled. Hanks solution was used to achieve a final cell concentration
of 10
cells/ml in suspension. Small amounts of cell suspension (20 ml) were placed
on the
glass cover slips, incubated for 45 minutes in the wet chamber and then washed
with
Hanks solution for removal of the cells attached to the glass surface. This
was the
method used for the entire experiment, in regards to cell suspension and cover
slips.
Taking Counts of Cells With DamaRed Membranes:
A double staining procedure with EthBr (5ing/ml) and FSCDA (5mg/ml) was
used to count cells with damaged cell membranes. The method is based on the
ability
of EthBr to enter the cells with damaged membranes and bind with DNA. EthBr
has a
bright red fluorescence when bound to DNA. FSCDA easily penetrates into the
cells
from the medium and is structurally transformed to fluorescein with bright
green
fluorescence. Therefore, intact cells accumulate fluorescein and easily leave
the cells
with damaged cell membranes. As a result of this double staining, one can see
intact
cells with green fluorescence and red fluorescent cells with damaged cell
membranes
within 5 minutes of incubation time with the dyes.
Intracellular pH Measurements.
Macrophage intracellular pH measurements were done based on the
microspectrofluorimetric method and using a fluorescent microscope of type
LUMAM 13 (LOMO, Russia). This particular microscope has a modified system of
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-28-
fluorescence excitation and emission. Fluorescence excitation was performed by
using
a blue (kmax=435 nm) photodiode. Fluorescence was measured simultaneously at
two different; k=520 mn and k=567 nm interference filters respectively.
Fluorescence
excitation and synchronous emission measurements were done using a built-in
microcontroller (LA-70M4). Macrophages were incubated with pH indicator FSCDA
dye (5 mg/ml) for 15 minutes. Free dye was washed out of the medium after the
incubation period. Microscopic measurements were accomplished by using the
water
iinmersion objective (x40). A pH calibration curve was used in order to
determine the
value of intracellular pH. The calibration curve was represented as a ratio
K=(I520/I570) (where 1520,1570 - fluorescence intensity at 520 nm and 570 nm
respectively) depending upon macrophage intracellular pHi. To obtain different
pHi
values, macrophages were incubated in 140 MM KCI; 1MM CaC12; 0.5 MM MgC12;
and 20 MM HEPES medium. Intracellular pH values have varied within a range of
6.8
to 7.6. Intracellular pH has been adjusted to the pH of the medium by adding
the
ionophore antibiotic nigericine (Sigma) 5 mg/ml, which has the ability to
exchange
+
OH- for H+ in just 3 minutes. Nigericine has a high affinity for K . This
property
allows it to stabilize to the following transmembrane equilibriuin: Where i
and o
designate internal and external concentrations respectively. Hence
intracellular pH
will be close to the extracellular pH in the medium, having the same K+
concentration
outside and inside the cells.
Incubation media: Hanks solution (10MM Hepes) pH 7.2;
standard lab medium prepared on double distilled water witll 10MM Hepes pH
7.2;
standard lab medium prepared on Micro-cluster water with 10MM Hepes pH
7.2;
Series 1.
Cells were incubated for 15 minutes in media containing EthBr and FSCDA.
They were thoroughly washed in the extracellular media. Cell media was
replaced by
standard lab medium prepared either on double distilled water or on Micro-
cluster
water. A dead cell count was produced and observed over 30 times under the
microscope and experiments with both types of medium replacement were repeated
three times. Cells were incubated with FSCDA dye for 15 minutes and then
washed
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-29-
away from the free dye in the surrounding cell medium. Cell medium was
exchanged
either on standard lab medium prepared on double distilled or on Micro-cluster
water.
Kinetics measurements of intracellular pH was done with at least 30
microscopic
observations which were repeated a total of three times.
Series 2.
Cells were incubated for 230 minutes in standard lab cell media prepared with
either double distilled or Micro-cluster water. Cells were consequently
stained with
FSCDA dye for intracellular pH and EthBr dye for dead cells. Quantification
measurements were performed in a similar way, as described above and as known
in
the art.
Intracellular pH (delta pHi) change in macrophages after 15 minutes, 190
minutes, and 230 minutes of incubation in standard lab-cell medium prepared
using
Micro-cluster or double distilled water. Macrophage incubation in cell media
prepared using Micro-cluster water for 230 minutes resulted in a 0.43 increase
in pHi.
A statistically insignificant increase in intracellular pH of macrophages was
also
observed when medium prepared with double distilled water was used.
The increase in pH is the result of quenching metobolic oxidants. The
quenching of a hydroxyl radical would result in formation of hydroxide,
causing an
increase in pH. Likewise, the quenching of other free-radicals and oxidants
results in
chemical species which increase the pH. Importantly, the reduction of these
free-
radicals and oxidants, prevents their deleterious interaction with
mitochondria or other
biological components. This results in decreased mutation to mitochondria DNA
and
other DNA and cell membranes. This reduction in free-radicals, oxidants and
the
damage they cause provides increased health, blood oxygen levels and more
efficient
oxygen use. Also, the decreased rate of mitochondrial decay and destruction
results
in increased energy secondary to increased population of healthy,
mitochondria.
In general then, increasing the blood concentration of micro-cluster water
results in decreased oxidative damage. Indeed, the inventive water provides
anti-
aging results through decreased damage to mitochondrial DNA, and increased
efficiency in related energetics.
The conclusion of the experimental results discussed above confirms the
importance of the present micro-cluster liquids in association with
cosmaceutical
compounds in protecting against cascades of oxidative stress. The process of
aging is
CA 02590119 2007-06-12
WO 2006/065920 PCT/US2005/045271
-30-
believed to involve mitochondrial dysfunction and oxidative damage resulting
from
the production of molecules like hydroxyl radicals, nitric oxide and
peroxynitrite.
Our results strongly suggest that micro-cluster liquids could indeed affect
the process
of aging and are therefore properly labeled as anti-aging.
Although the present invention has been described herein with reference to
particular means, materials, and embodiments, the present invention is not
intended to
be limited to the particulars disclosed herein; rather, the present invention
extends to
all functionally equivalent structures, methods and uses, such as are within
the scope
of the appended claims.