Note: Descriptions are shown in the official language in which they were submitted.
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
COILED ENDOLUMINAL PROSTHESIS SYSTEM,
DELIVERY CATHETER AND METHOD
BACKGROUND OF THE INVENTION
[0001] Stents, covered stents and other endolurninal prostheses are often
useful for
placement in various hollow body structures, such as blood vessels, including
coronary arteries,
iliac arteries and femoro-popiliteal arteries, the ureter, urethra, bronchus,
biliary tract,
gastrointestinal tract and the like, for the treatment of conditions which may
benefit from the
introduction of a reinforcing or protective structure and/or the introduction
of a therapeutic agent
within the body lumen. The prostheses will typically be placed endoluminally.
As used herein,
"endoluminally" will mean placement by percutaneous or cutdown procedures,
wherein the
prosthesis is transluminally advanced through the body lumen from a remote
location to a target
site in the lumen. In vascular procedures, the prostheses will typically be
introduced
"endovascularly" using a catheter over a guide wire under fluoroscopic, or
other imaging system,
guidance. The catheters and guide wires may be introduced through conventional
access sites to
the vascular system, such as through the femoral artery, or brachial and
subclavian arteries, for
access to the target site.
[0002] An endoluminal prosthesis typically comprises at least one radially
expansible,
usually cylindrical, body segment. By "radially expansible," it is meant that
the body segment
can be converted from a small diameter configuration (used for endoluminal
placement) to a
radially expanded, usually cylindrical, configuration, which is achieved when
the prosthesis is
implanted at the desired target site. The prosthesis may be non-resilient,
e.g., malleable, thus
requiring the application of an internal force to expand it at the target
site. Typically, the
expansive force can be provided by a balloon catheter, such as an angioplasty
balloon for vascular
procedures. Alternatively, the prosthesis can be self-expanding. Such self-
expanding structures
may be provided by a temperature-sensitive superelastic material, such as
Nitinol, which naturally
assumes a radially expanded condition once an appropriate temperature has been
reached. The
appropriate temperature can be, for example, a temperature slightly below
normal body
temperature; if the appropriate temperature is above normal body temperature,
some method of
heating the structure must be used. Another type of self-expanding structure
uses resilient
material, such as a stainless steel or superelastic alloy, such as Nitinol,
and forming the body
segment so that it possesses its desired, radially-expanded diameter when it
is unconstrained, e.g.;
released from radially constraining forces of a sheath. To remain anchored in
the body lumen, the
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
prosthesis will remain partially constrained by the lumen. The self-expanding
prosthesis can be
delivered in its radially constrained configuration, e.g. by placing the
prosthesis within a delivery
sheath or tube and retracting the sheath at the target site. Such general
aspects of construction and
delivery modalities are well known in the'art.
[0003] The dimensions of a typical endoluminal prosthesis will depend on its
intended
use. Typically, the prosthesis will have a length in the range from 0.5 cm to
25 cm, usually being
from about 0.8 cm to 10 cm, for vascular applications. The small (radially
collapsed) diameter of
cylindrical prostheses will usually be in the range from about 1 mm to 10 mm,
more usually being
in the range from 1.5 mm to 6 mm for vascular applications. The expanded
diameter will usually
be in the range from about 2 mm to 50 mm, preferably being in the range from
about 3mm to
15mm for vascular applications and from about 25 mm to 45 mm for aortic
applications.
[0004] One type of endoluminal prosthesis includes both a stent component and
a
covering component. These endoluminal prostheses are often called stent grafts
or covered stents.
A covered stent is typically introduced using a catheter with both the stent
and covering in
contracted, reduced-diameter states. Once at the target site, the stent and
covering are expanded.
After expansion, the catheter is withdrawn from the vessel leaving the covered
stent at the target
site. Coverings may be made of, for example, PTFE, ePTFE or Dacron polyester.
[0005] Grafts are used within the body for various reasons; such as to repair
damaged
or diseased portions of blood vessels such as may be caused by injury,
disease, or an aneurysm. It
has been found effective to introduce pores into the walls of the graft to
provide ingrowth of tissue
onto the walls of the graft. With larger diameter grafts, woven graft material
is often used. In
small and large diameter vessels, porous fluoropolymers, such as ePTFE, have
been found useful.
[0006] Coil-type stents can be wound about the catheter shaft in torqued
compression
for deployment. The coil-type stent can be maintained in this torqued
compression condition by
securing the ends of the coil-type stent in position on a catheter shaft. The
ends are released by,
for example, pulling on wires once at the target site. See, for example, U.S.
Patent Nos.
5,372,600 and 5,476,505. Alternatively, the endoluminal prosthesis can be
maintained in its
reduced-diameter condition by a sleeve; the sleeve can be selectively
retracted to release the
prosthesis. A third approach uses a balloon to expand the prosthesis at the
target site. The stent is
typically extended past its elastic limit so that it remains in its expanded
state after the balloon is
deflated and removed. One balloon expandable stent is the Palmaz-Schatz stent
available from
the Cordis Division of Johnson & Johnson. Stents are also available from
Medtronic AVE of
Santa Rosa, California and Guidant Corporation of Indianapolis, Indiana. A
controlled release
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
catheter assembly, such as disclosed in US Patent numbers 6,238,430 and
6,248,122, may also be
used to deploy a coiled prosthesis. See also US Patent number 6,572,643.
[0007] The following patents may be of interest. U.S. Patent No. 6,660,032
issued
December 9, 2003; U.S. Patent No. 6,645,237 issued November 11, 2003; U.S.
Patent No.
6,572,648 issued June 3, 2003; U.S. Patent No. 6,514,285 issued February 4,
2003; U.S. Patent
No. 6,371,979 issued April 16, 2002; U.S. Patent No. 5,824,053 issued October
20, 1998; U.S.
Patent No. 5,772,668 issued June 30, 1998; U.S. Patent No. 5,443,500 issued
August 22, 1995;
U.S. Patent No. 4,760,849 issued August 2, 1988; and U.S. Patent No. 4,553,545
issued
November 19, 1985. See also PCT Publication Number WO 94/22379 published
October 13,
1994; and PCT Publication Number WO 94/16629 published August 4, 1994.
BRIEF SUMMARY OF THE INVENTION
[0008] A first aspect of invention is directed to a coiled endoluminal
prosthesis delivery
assembly comprising a catheter, a generally helical endoluminal prosthesis
having proximal and
distal portions separated by an intermediate portion, the endoluminal
prosthesis being placeable in
a radially contracted, first state on the catheter. The assembly also
comprises means for engaging
each of the proximal, intermediate and distal portions thereby maintaining the
endoluminal
prosthesis in the first state, and means for controllably releasing the
proximal, distal and
intermediate portions to permit the endoluminal prosthesis to move towards a
radially expanded,
second state.
[0009] A second aspect of invention is directed to a coiled endoluminal
prosthesis delivery
assembly comprising a catheter comprising an outer surface, a lumen, and a
number of openings
extending from the outer surface to the lumen, and a release wire extending
along a release wire
path. The release wire path comprises internal release wire path segments
defined within the
lumen and external release wire path segments external of the catheter. The
assembly also
includes a generally helical endoluminal prosthesis having proximal and distal
portions separated
by an intermediate portion. The endoluminal prosthesis is maintained in a
radially contracted
condition by the release wire engaging the proximal, distal and intermediate
portions of the
endoluminal prosthesis at at least three of the external release wire path
segments. The coiled
endoluminal prosthesis is therefore releasable from the catheter when the
release wire is moved
along the release wire path.
[0010] A third aspect of the invention is directed to coiled endoluminal
prosthesis delivery
assembly comprising a catheter comprising a release wire lumen, the release
wire lumen
comprising a series of axially spaced-apart lumen segments, each lumen segment
comprising an
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
entrance and exit, and a release wire extending along a release wire path. The
release wire path
comprises internal release wire path segments defmed by the lumen segments and
external release
wire path segments extending between the exit of one lumen segment and the
entrance of another
lumen segment. The assembly also includes a generally helical endoluminal
prosthesis having
proximal and distal portions separated by an intermediate portion. The
endoluminal prosthesis is
maintained in a radially contracted condition with the intermediate portion
passing between the
catheter and the release wire at least one of the external release wire path
segments. The proximal
and distal portions of the endoluminal prosthesis have release wire engagement
parts with which
the release wire releasably engages. The coiled endoluminal prosthesis is
therefore releasable
from the catheter when the release wire is moved along the release wire path.
[0011] A fourth aspect of the invention is directed to a coiled endoluminal
prosthesis delivery
assembly comprising a catheter, a release wire and a generally helical
endoluminal prosthesis.
The catheter comprises an outer surface, a main lumen, a release wire lumen,
and a number of
openings extending from the outer surface to the release wire lumen. The
release wire extends
along a release wire path, the release wire path comprising internal release
wire path segments
defined within the release wire lumen and external release wire path segments
external of the
catheter. The generally helical endoluminal prosthesis has proximal and distal
portions separated
by an intermediate portion. The endoluminal prosthesis is maintained in a
radially contracted '
condition by the release wire engaging the endoluminal prosthesis at least two
of the external
release wire path segments. The coiled endoluminal prosthesis is therefore
releasable from the
catheter when the release wire is moved along the release wire path.
[0012] A fifth aspect of the invention is directed to a method for
controllably releasing a
generally helical endoluminal prosthesis from a catheter within a body lumen
of a hollow bo'dy
structure, the endoluminal prosthesis being mounted onto the catheter in a
radially contracted, first
state. The endoluminal prosthesis, carried by the catheter, is placed at a
target location within a
body lumen. The endoluminal prosthesis comprises proximal, distal and
intermediate portions,
each of which is temporarily retained in the radially contracted, first state.
One or more of the
distal, intermediate and proximal portions are released to move towards a
radially expanded,
second state in contact with the hollow body structure while maintaining the
unreleased portions
in the radially contracted, first state. The releasing step comprises
permitting a portion of the
endoluminal prosthesis to move to the radially expanded, second state in
contact with the hollow
body structure. Thereafter, the remaining one or ones of the proximal, distal
and intermediate
portions are selectively released to permit the entire endoluminal prosthesis
to move to the
radially expanded, second state in contact with the hollow body structure. The
catheter is then
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
removed from the body lumen. The releasing step may comprise permitting at
most 50%, and
more preferably at most 25%, of the length of the endoluminal prosthesis to
simultaneously move
to the radially expanded, second state in contact with the hollow body
structure.
[0013] A sixth aspect of the invention directed to a delivery catheter
comprising a first,
flexible, polymer-based tube, having a proximal end, a distal end and a first
tube length, and a
second, metal tube, secured to and extending distally from the distal end of
the first tube. The
second, metal tube has a second tube length, an outside surface and an inside
surface, the inside
surface defining a main lumen, the outside and inside surfaces defining a
tubular wall. The
second tube has flexibility enhancing relief areas along the length of the
second tube. The relief
areas provide localized areas of reduced bending stiffness to enhance the
bending flexibility of the
second, metal tube while retaining greater torsional stiffness relative to the
first tube. In some
embodiments at least some of the relief areas may pass completely through the
tubular wall.
[0014] A seventh aspect of the invention is directed to a notched catheter
comprising a
catheter body having an outer surface and a longitudinally extending lumen.
The outer surface
has a generally cylindrical surface portion and a notched surface portion, the
notched surface
portion extending from the generally cylindrical surface portion to intersect
the lumen. A
filament-containing layer is adjacent to the generally cylindrical surface
portion and the notched
surface portion. A portion of the filament-containing layer is capturable
between the notched
surface portion and an elongate element extendable through the lumen.
[0015] An eighth aspect of the invention is directed to a method for making a
notched
catheter. A catheter body, having an outer surface and a longitudinally
extending lumen, is
obtained. The outer surface has a generally cylindrical surface portion and a
notched surface
portion, the notched surface portion extending from the generally cylindrical
surface portion to
intersect the lumen. A filament-containing layer is placed adjacent to the
generally cylindrical
surface portion and the notched surface portion. An elongate element is
inserted through the
lumen, the elongate element piercing the filament-containing layer at the
notched surface portion
so that a portion of the filament-containing layer is captured between the
notched surface portion
and the elongate element. The filament-containing layer is bonded to the
catheter body. The
elongate element is removed from the lumen.
[0016] A ninth aspect of the invention is directed to mounting a generally
helically
coiled endoluminal prosthesis to a mounting region of a delivery catheter, the
coiled endoluminal
prosthesis extending in a first rotational direction. At least a portion of
the mounting region of the
delivery catheter is placed in a torqued state by rotating one section of the
delivery catheter
relative to another section of the delivery catheter in a second rotational
direction, the second
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
rotational direction being opposite the first rotational direction. The coiled
endoluminal
prosthesis is secured to the mounting region at least two longitudinally
spaced apart positions
along at least a part of the torqued portion of the mounting region of the
delivery catheter. The
torqued portion of the delivery catheter is released from the torqued state,
whereby the delivery
catheter has a tendency to tighten the coiled endoluminal prosthesis onto the
delivery catheter.
According to some embodiments of this method, the placing step may be carried
out to
accommodate variations in at least one of (1) the length of the coiled
endoluminal prosthesis, and
(2) the placement of the coiled endoluminal prosthesis on the mounting region
of the delivery
catheter, so to aid proper alignment of ends of the endoluminal prosthesis
with the delivery
catheter during the securing step.
[0017] A tenth aspect of the invention is directed to a coiled endoluminal
prosthesis
system for use within a target vessel comprising a handle, a delivery catheter
extending from the
handle, a coiled endoluminal prosthesis carried by the delivery catheter, and
means for rotating
the delivery catheter while axially releasing the coiled endoluminal
prosthesis from the delivery
catheter for engagement with a wall of a target vessel. In some embodiment of
the invention the
rotating while axially releasing means comprises at least one of: a release
element engaging the
coiled endoluminal prosthesis at axially spaced apart positions along the
delivery catheter; a
sheath slidably positioned over the coiled endoluminal prosthesis; and
individually releasable
constraining elements releasably securing the coiled prosthesis to the
delivery catheter at axially
spaced apart positions. In other embodiments of the invention the rotating
while axially releasing
means comprises a spool secured to the handle, an elongate release element
extending along the
catheter having a proximal end secured to the spool, a user-actuated rotator
assembly rotatably
mounted to the body, the rotator assembly comprising a rotator and a release
element guide
secured to one another and to the catheter so that rotating the rotator
rotates both the release
element guide and the catheter, and the release element guide engaging the
release element to
wind the release element onto the spool when the rotator is rotated in a
chosen direction so to
axially release the coiled endoluminal prosthesis.
[0018] An eleventh aspect of the invention is directed to a coiled endoluminal
prosthesis delivery assembly comprising a handle, a delivery catheter
extending from the handle
and comprising a proximal end at the handle and a distal end, an elongate,
flexible release
element, having a tip, extending from the handle to the distal end of the
delivery catheter and
movable along the delivery catheter with the tip movable proximally towards
the handle. The
handle comprises a release element retractor constructed to retract the
release element proximally
through the delivery catheter and a delivery catheter rotator constructed to
rotate the delivery
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
catheter, the delivery catheter rotator being operably coupled to the release
element retractor so
that the release element is retracted simultaneously with rotation of the
delivery catheter.
[0019] A twelfth aspect of the invention is directed to a coiled endoluminal
prosthesis
system, for use within a target vessel, comprising a handle, a delivery
catheter extending from the
handle, and a coiled endoluminai prosthesis carried by the delivery catheter.
The coiled
endoluminal prosthesis has a first direction of spiral, the first direction of
spiral being in a first
rotational direction. The handle comprises means for rotating the delivery
catheter in a second
rotational direction while axially releasing the coiled endoluminal prosthesis
from the delivery
catheter for engagement with a wall of a target vessel.
[0020] A thirteenth aspect of the invention is directed to a method for making
a
constant length, generally helical endoluminal prosthesis of the type defining
a generally helical
gap between the turns of the prosthesis when in a relaxed, expanded diameter
state. A first
diameter for an endoluminal prosthesis, when in a reduced diameter state
wrapped down onto a
delivery device, is determined. A second diameter of the endoluminal
prosthesis, when in an
expanded diameter state at a target location, is determined. The endoluminal
prosthesis is
configured to reduce or eliminate any difference between the length of the
endoluminal prosthesis
when in the reduced diameter state and when in the expanded diameter state.
The endoluminal
prosthesis is wrapped onto the delivery device to place the endoluminal
prosthesis in the reduced
diameter state, the endoluminal prosthesis having turns. According to one
embodiment, when the
endoluminal prosthesis is in the expanded diameter state, the endoluminal
prosthesis has a total
area (TA) equal to the external surface area of the turns of the endoluminal
prosthesis (SA) plus
the area of the generally helical gap (GA) between the turns; and the
configuring step is carried
out to reduce or eliminate any difference between the ratio of SA to TA to the
ratio of the first
diameter to the second diameter.
[0021] A fourteeneth aspect of the invention is directed to catheter assembly
comprising
a delivery catheter and a constant length endoluminal prosthesis mounted to a
position along the
delivery catheter. The constant length endoluminal prosthesis comprises a
generally helical body.
The generally helical body comprises generally helically-extending turns, with
adjacent tuxns
being laterally positioned relative to one another when in a reduced diameter
state wrapped down
onto the delivery catheter so adjacent turns do not overlie one another, and a
generally helical gap
between the turns of the body when in a relaxed, expanded diameter state. The
body has a first
diameter when in a reduced diameter state wrapped down onto the delivery
catheter and a second
diameter when in an expanded diameter state at a target site. When in the
expanded diameter
state, the endoluminal prosthesis has a total area (TA) equal to the external
surface area of the
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
turns of the body (SA) plus the area of the generally helical gap (GA) between
the turns. The of
SA to TA is at least substantially equal to the ratio of the first diameter to
the second diameter,
whereby any difference between the length of the endoluminal prosthesis when
in the reduced
diameter state and when in the expanded diameter state is effectively
eliminated. An expandable
and collapsible balloon may be mounted to the delivery catheter between the
delivery catheter and
the endoluminal prosthesis.
[0022] A fifteeneth aspect of the invention is directed to a constant length
endoluminal
prosthesis comprising a generally helical body defining a generally helical
gap between the turns
of the body when in a relaxed, expanded diameter state. The body has a first
length and a first
diameter when in a reduced diameter state wrapped down onto a delivery device
and a second
length and a second diameter when in an expanded diameter state at a target
site. The
endoluminal prosthesis also comprises means for effectively eliminating any
difference between
the first and second lengths when the endoluminal prosthesis is deployed from
the reduced
diameter state to the expanded diameter state. According to one embodiment of
this aspect of the
invention, a delivery catheter may be mounted to a position along the delivery
catheter. Another
embodiment may comprise an expandable and collapsible balloon mounted to the
delivery
catheter between the delivery catheter and the endoluminal prosthesis.
[0023] Various features and advantages of the invention will appear from the
following
description in which the preferred embodiments have been set forth in detail
in conjunction with
the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] Fig. 1 is an overall view of a coiled stent delivery assembly made
according to
the invention;
[0025] Fig. 1 A is an overall view of release wire assembly of Fig. 1;
[0026] Fig. 2 is a side view of the distal portion of the catheter of Fig. 1;
[0027] Fig. 3 is an enlarged overall view of a section of the catheter of Fig.
2;
[0028] Fig. 4 shows the catheter of Fig. 2 with a release wire within a
release wire
lumen;
[0029] Fig. 5 is an enlarged overall view of a section of the catheter and
release wire of
Fig. 4;
[0030] Fig. 6 shows the structure of Fig. 5 with a generally helical covered
stent
mounted thereto in a radially contracted, first state, the structures
constituting the distal portion of
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
the coiled stent delivery assembly of Fig. 1, the target location within the
body lumen being
indicated by dashed lines;
[0031] Fig. 6A is an enlarged view of the covered stent of Fig. 6 showing the
release
wire piercing the distal end of the covered stent;
[00321 Fig. 7 illustrates the assembly of Fig. 6 after the release wire has
begun to be
retracted to release the distal portion of the stent from the catheter;
[0033] Fig. 8 shows the assembly of Fig. 7 after the release wire has been
retracted
further to release part of the intermediate portion of the stent;
[0034] Fig. 9 shows the assembly of Fig. 8 after the release wire has been
completely
retracted and the covered stent is in a radially expanded, second state;
[0035] Fig. 10 shows the covered stent of Fig. 9 within the blood vessel and
after the
catheter has been removed;
[0036] Fig. 11 illustrates an alternative embodiment similar to the catheter
of Fig. 3 in
which the catheter comprises separate tubes connected to one another;
[0037] Fig. 12 is an end of view of the catheter of Fig. 11;
[0038] Fig. 13 is another alternative embodiment similar to the catheter of
Fig. 3 in
which the catheter lacks the cutouts of the Fig. 3 embodiment but rather has
perforations
extending into the release wire lumen, the perforations acting as the
entrances and exits of the
lumen segments;
[0039] Fig. 14 illustrates the embodiment of Fig. 13 in which the release wire
passes
through the perforations in a weaving pattern;
[00401 Fig. 15 illustrates a still further alternative embodiment including a
modified
release wire assembly similar to the release wire assembly of Fig. lA and a
modified catheter
similar to the catheter of Fig. 5;
[0041] Figs. 16 and 17 are cross-sectional views of the catheter taken along
lines 16-16
and 17-17 in Fig. 15 illustrating the presence of a distal release wire in
Fig. 16 and both the distal
and a proximal release wire in Fig. 17;
[0042] Fig. 18 is an overall view of the release wire assembly of Fig. 15
showing the
use of distal and proximal release wires;
[0043] Fig. 19 illustrates the result of initially pulling on the release wire
assembly of
Fig. 15 causing the proximal release wire to disengage from the proximal end
of the covered stent;
[0044] Fig. 20 illustrates the result of continuing to pull on the release
wire assembly of
Fig. 19 causing the distal release wire to disengage from the distal end of
the covered stent, after
which continued pulling on the release wire assembly will cause the distal
release wire to release
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
the intermediate portion of the covered stent to assume the radially expanded,
second state of
Figs. 9 and 10;
[0045] Fig. 21 is a simplified enlarged side elevational view of the catheter
of Fig. 2 at
the distal cut out illustrating the use of a braided material embedded within
a polymer to form the
outer surface of the catheter to provide additional torsional strength;
[0046] Fig. 22 is a simplified cross-sectional view taken along line 22-22 of
Fig. 21;
[0047] Fig. 23 is an overall view of the distal portion of a further
embodiment of the
invention comprising a coiled endoluminal prosthesis delivery assembly having
a coiled
endoluminal prosthesis secured thereto in a radially contracted state;
[0048] Fig. 24 is an enlarged view of the distal and proximal ends of the
assembly of
Fig. 23;
[0049] Fig. 25 illustrates the distal portion of the delivery catheter of the
embodiment of
Fig. 23 in a relaxed state with the coiled endoluminal prosthesis and the
release wire removed to
illustrate a second, metal tube mounted to the distal end of a first,
flexible, polymer based tube,
the Fig. also showing the spiral circumferential offset of the release wire
guide tubes mounted to
the outer surface of the second, metal tube, and flexibility-enhancing relief
areas created along the
length of the second, metal tube;
[0050] Fig. 26 is a simplified cross-sectional view taken along line 26-26 of
Fig. 25
showing a guide tube mounted to the outside surface of the second, metal tube;
[0051] Fig. 27 is a partial cross-sectional view taken along the axis of the
second, metal
tube showing openings formed through the tubular wall of the second, metal
tube to create the
relief areas along the second, metal tube;
[0052] Fig. 28 illustrates the structure of Fig. 25 after the second, metal
tube has been
placed in a torqued state, as indicated by the arrows at either end of the
second, metal tube, so that
the guide tubes become generally axially aligned, whereby after the coiled
endoluminal prosthesis
of Fig. 23 has been mounted to the delivery catheter, the second, metal tube
tends to tighten the
coiled endoluminal prosthesis onto the second, metal tube thereby helping to
create a smaller
placement profile by reducing the cross-sectional size of the delivery
catheter;
[0053] Figs. 29-35 are directed to a further aspect of the invention in which
the coiled
endoluminal prosthesis assembly includes a handle that can be operated to
simultaneously rotate
the delivery catheter and retrieve the release wire;
[0054] Fig. 29 shows a handle from which the delivery catheter extends with a
portion
of the handle body broken away to show how the release element is wound about
a spool as the
delivery catheter is rotated by a rotator knob;
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
[0055] Fig. 30 is an exploded isometric view of the structure of Fig. 29;
[0056] Fig. 31 illustrates an alternative embodiment to the handle of Figs. 29
and 30 in
which the release wire is pulled axially by a grooved follower sleeve as the
delivery catheter is
rotated;
[0057] Fig. 32 is a view of an embodiment similar to that of Fig. 31 but in
which
grooved follower sleeve is much shorter than the embodiment of Fig. 31 by the
use of multiple
spiral groove pins carried by the handle body;
[0058] Figs. 33-35 illustrate a still further alternative embodiment of the
handle of Figs.
29 and 30 similar to the embodiment of Fig. 32 but having a continuous
internal thread engaging
the spiral groove in the grooved follower sleeve and also having a pull ring
assembly that can be
used to rotate the catheter and withdraw the release wire more rapidly than
would typically occur
by turning the rotator knob;
[0059] Figs. 36-40 illustrate a sequence of releasing a constant length
endoluminal
prosthesis from a delivery catheter;
[0060] Fig. 41 illustrates the distal end of a delivery catheter similar to
that of Fig. 4 but
having a balloon mounted along its length between two cutouts; and
[0061] Fig. 42 shows the delivery catheter of Fig. 41 with a constant length
endoluminal prosthesis, similar to that shown in Figs. 36-40, mounted thereto.
DETAILED DESCRIPTION OF THE INVENTION
[0062] The present invention will be described with reference to several
embodiments
with like reference numerals referring to like elements. The following
description of the invention
will typically be with reference to specific structural embodiments and
methods. It is to be
understood that there is no intention to limit the invention to the
specifically disclosed
embodiments but that the invention may be practiced using other features,
elements, methods and
embodiments.
[0063] Fig. 1 is an overall view of a coiled stent delivery assembly 10 made
according
to a first aspect of the invention. Assembly 10 is shown to include a catheter
12 with a generally
helical covered stent 14 mounted to the distal portion 16 of catheter 12.
Covered stent 14 may be
of the type disclosed in US patent numbers 6,572,648 or 6,645,237 including a
ladder type stent
covered by a graft material. Assembly 10 also includes a release wire assembly
13, see Fig. 1 A,
including a flexible release wire 18, extending along catheter 12, connected
to a fmger grip 15 by
a relatively rigid tube 17. Assembly 10 fizrther includes a proximal end
assembly 20 secured to
the proximal end 22 of catheter 12. Proximal and assembly 20 includes various
fittings and ports,
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
such as flush port 19, guide wire port 21 and release wire port 23. The
construction of the various
components of assembly 10 will now be described.
[0064] Fig. 2 is a side view of the distal portion 16 of catheter 10 while
Fig. 3 is an
enlarged overall view of a section of distal portion 16. Distal portion 16 has
a pair of radiopaque
markers 24, 26 used to help properly position covered sent 14 within the body
lumen. Catheter 12
has a main lumen 28 and a release wire lumen 30. Main lumen 28 is typically
used for passage of
a guide wire and may also be used for other purposes, such as irrigation,
aspiration, passage of
tissue extraction devices, and so forth. Figs. 4 and 5 show release wire 18
within release wire
lumen 30.
[0065] Distal portion 16 of catheter 12 has a number of axially spaced apart
cutouts 32,
33, 34 and 35, which create a series of lumen segments 36, 37, 38, 39 and 40
separated by cutouts
32-35. Accordingly, release wire 18 passes along a release wire path including
internal release
wire path segments defined by lumen segments 36-40 and external release wire
path segments
along cutouts 32-35. That is, the external release wire path segments extend
between the exit of
one lumen segment and the entrance of an adjacent lumen segment. Fig. 6 shows
covered stent 14
mounted to distal portion 16 of catheter 12 in a radially contracted, first
state. Note that covered
stent 14 is considered positioned within a body lumen 44 defined by a blood
vesse154, or other
hollow body structure, in Figs. 6-10 but, for simplicity of illustration,
blood vesse154 is only
shown in Fig. 10. The target location 42 within the body lumen 44 of blood
vesse154 is indicated
by dashed lines. The distal and proximal ends 46 and 48 of covered sent 14 are
secured to distal
portion 16 of catheter 12 by release wire 18 passing through distal end 46 at
cutout 32 as shown in
Fig. 6A and through proximal end 48 at cutout 35. The intermediate portion 50
of covered stent
14 is secured to distal portion 16 of catheter 12 at two positions, that is at
intermediate cutouts 33,
34, by passing between release wire 18 and catheter 12 and the intermediate
cutouts.
[0066] The longitudinal or axial length of cutouts 32-35 is oversized with
respect to
covered stent 14 housed therein. It has been found that making cutouts 32-35,
and especially
intermediate cutouts 33 and 34, oversized helps to prevent damage to covered
stent 14 during
assembly and use. In one embodiment catheter 12 has an outside diameter of 1.5
mm (.060 in.),
main lumen 28 has an inside diameter of 1 mm (0.037 in.), release wire 18 has
a diameter of 0.3
mm (0.012 in.), and each turn of covered stent 14 when wrapped down as shown
in Fig. 1 has an
axial or longitudinallength of about 5 or 6 mm. It has been found that making
cutouts 33 and 34
about 8 mm, that is about 2 mm longer than the axial or longitudinal length of
covered stent 14,
helps to eliminate binding of and damage to the covered stent. The proximal
and distal cutouts 35
and 32 are, in one embodiment, each about 4 mm long. The space between the
cutouts is, in this
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
embodiment, 11 mm long to permit two turns of covered stent 14 between each
cutout. Some
overlap of the turns of covered sent 14 over adjacent cutouts does not
compromise the
functionality of the catheter. Cutouts 32-35 are shown in Fig. 3 having a flat
bottom; the cutouts
may also be made with, for example, a convex bottom surface.
[0067] Assembly 10 is positioned at target location 42 while in the wound
down,
radially contracted, first state of Figs. 1 and 6. Proper positioning of
covered stent 14 within
blood vessel 54 is aided by the use of radiopaque markers 24, 26. Fig. 7
illustrates release of
distal end 46 of covered sent 14 by pulling on release wire 18 as indicated by
arrow 52. An
undesirable, uncontrolled expansion of covered stent 14, sometimes referred to
as a "jack-in-the-
box" release, could occur on release of one of the distal and proximal ends
46, 48 of covered stent
14 if it were only secured at its ends. Such a "jack-in-the-box" release is
not desired because it
can adversely affect the proper final positioning of covered stent 14. A "jack-
in-the-box" release
is avoided in this embodiment by the provision of intermediate cutout 33, 34
to permit covered
stent 14 to be placed between release wire 18 and catheter 12 at the
intermediate cutouts.
[0068] Fig. 8 illustrates the result of continuing to pull release wire 18
causing release
wire 18 to be removed from intermediate cutout 33 thereby releasing a part of
intermediate
portion 50. Fig. 9 illustrates covered stent 14 within its radially expanded,
second state after
release wire 18 has been completely removed from catheter 12, that is after
removing release wire
18 from intermediate cutout 34 and from proximal end 48 of covered stent 14 at
proximal cutout
35. The present invention provides a very controlled release of covered stent
14 to help ensure its
proper placement within body lumen 44. Fig. 10 shows covered stent 14 fully
expanded within
body lumen 44 of blood vesse154 and after catheter 12 has been removed from
blood vessel 54.
[0069] Figs. 11 and 12 illustrate an alternative embodiment similar to
catheter 12 of
Fig. 3. Catheter 12A comprises first and second tubes 56, 58 connected to one
another by
adhesive 60 and heat shrink tubing 62. First tube 56 preferably has stainless
steel flat wire braid
filaments 64 to enhance structural integrity.
[0070] Another alternative embodiment, similar to the catheter of Fig. 3, is
shown in
Fig. 13. Catheter 12B lacks the cutouts 32-35 of the catheter of Fig. 3 but
rather has perforations
66 extending into release wire lumen 30, the perforations acting as the
entrances and exits of the
lumen segments. Fig. 14 shows catheter 12B with release wire 18 passing
through perforations 66
in a weaving pattern so that perforations 66 act as the entrances and exits of
lumen segments 36,
37 and 38 in this Fig. In some embodiments it may be desired to provide for a
much greater
number of perforations 66 than would be expected to be used, for example 20 or
40 perforations
instead of 8. This would allow greater flexibility in the placement of the
turns of covered stent 14
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
as well as the number of turns to be captured between release wire 18 and sent
12. If the catheter
were made from a porous material, the pores in the material itself may provide
the perforations.
Also, the perforations could also be formed in the catheter using a tool as
the covered stent is
wound onto the catheter; such a tool could also used to help to guide the
release wire out through
or into the newly formed perforation or both out through and into the newly
formed perforation.
[00711 Fig. 15 illustrates aspects of a still further alternative embodiment
of the present
invention. A modified release wire assembly 13A, shown best in Fig. 18, is
used with a modified
catheter 12C. Figs. 16 and 17 are cross-sectional views of catheter 12C
showing the presence of
an oval or other other-than-round release wire lumen 30A. Release wire lumen
30A is sized and
shaped to house both distal and proximal release wires 18A and 18B. If more
than two release
wires were to be used, the release wire lumen can be appropriately sized and
shaped. Also, and
may be desirable to use an other-than-round cross-sectional shape for the
release wire for greater
space utilization.
[0072] Fig. 18 is an overall view of the release wire assembly of Fig. 15
showing the
use of distal and proximal release wires 18A and 18B. In this embodiment the
release wires are
pulled simultaneously by fmger grip 15. If desired, release wires could be
manipulated
individually. Fig. 19 illustrates the result of initially pulling on the
release wire assembly of Fig.
15. In this embodiment the length of proximal release wire 18B is chosen so
that proximal end 48
of covered sent 14 is released first. Fig. 20 illustrates the result of
continuing to pull on release
wire assembly 13A causing distal release wire 18A to disengage from distal end
46 of the covered
stent. Continued pulling on release wire assembly 13A will cause distal
release wire 18A to
release intermediate portion 50 of covered stent 14 to assume the radially
expanded, second state
of Figs. 9 and 10. Instead of using individual release wires, such as distal
and proximal release
wires 18A and 18B, a single, main release wire can be used having release wire
side branches
welded or otherwise secured to the main release wire; the release side wire
branches would then
be the used to engage covered stent 14 and various positions along the covered
stent. The lengths
of the release wire side branches and the main release wire can be chosen to
permit release of
covered stent 14 from cutouts 32-35 in any order desired, including from
proximal cutout 35 to
intermediate cutout 34, to intermediate cutout 33 and finally to distal cutout
32. Such a release
from the proximal cutout 35 to the distal cutout 32 could also be accomplished
in other manners,
such as my extending the release wire to the distal end of the catheter,
reversing direction, and
then directing the release wire along the release wire lumen back towards the
proximal end of the
catheter.
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
[0073] Instead of the release schemes discussed above, other release schemes
can be
used. For example, release can start simultaneously at proximal end 48 and end
at distal end 46;
also, release of covered stent 14 can be from one or both of intermediate
cutouts 34 and then from
one end and then from the other end. The number and spacing of the cutouts and
perforations can
also be changed. Whatever release scheme is to be used, in some embodiments it
is preferred that
at most 50%, and more preferably at most 25%, of covered sent 14
simultaneously move to the
radially expanded, second state in contact with blood vesse154 or other hollow
body structure. In
one preferred embodiment, using 4 equally spaced cutouts, at most about 33% of
the length of
covered stent moves simultaneously to the radially expanded, second state.
[0074] The present invention has been described as using a release wire. The
release
wire is not limited to structures or materials which are commonly classified
as wire, that is single
or multiple strands of metal. Rather, release wire also includes threads or
strands or other lengths
of material which may or may not have significant flexural strength and may be
nonmetallic or a
combination of metallic and nonmetallic materials. The particular mechanical
characteristics for
the release wire will depend on the operating conditions, including, for
example, the length of the
cutouts, the force expected to be exerted by the covered stent when in the
radially contracted, first
state, and the number of release wires used.
[0075] The release wire and the associated release wire lumen and lumen
openings in
the catheter are used to engage the covered stent and maintain it in the
radially contracted, first
state and then control the subsequent releasing of various portions of the
covered stent to prevent
the sudden, undesirable "jack-in-the-box" deployment of the covered stent.
Instead of a release
wire, the covered stent may be retained in the radially contracted, first
state using a heat
softenable adhesive between the covered stent and the catheter. An appropriate
source of heat can
be used to selectively heat and thus soften the adhesive. The source of heat
could be an RF device
positionable at various locations along the main lumen or a number of
individually operable
resistance heating elements formed in the catheter. Another alternative to a
release wire would be
to tie the covered stent to the catheter using a loop of thread at each
securement point; the loop of
thread would pass into the main lumen, through the wall of the catheter, over
or through the
covered stent, back through the wall of the catheter and into the main lumen
to complete the loop.
The covered stent could then be released by withdrawing a thread cutter
through the main lumen
of the catheter causing the loops of thread to be cut, typically one at a
time. Other structure and
procedures may be used as a substitute for the disclosed release wire
arrangement.
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
[0076] Various embodiments of the invention may and preferably do provide one
or
more of the following advantages: simplicity of design and ease-of-use,
ability to release a coiled
stent gradually, and accuracy of placement.
[0077] Other modification and variation can be made to the disclosed
embodiments
without departing from the subject of the invention as defined in following
claims. For example,
instead of providing a separate release wire lumen, in some embodiments the
delivery catheter
may include a single lumen through which the release wire passes; however, it
is preferred that a
separate release wire lumen be provided because having a separate release wire
lumen helps to
reduce the tendency of the release wire to bend so the release wire holds the
covered stent more
securely. Having a separate release wire lumen helps to prevent any
interference with the passage
of the guide wire or other devices through the catheter. In some situations it
may not be necessary
to provide distal lumen segment 36. For example, the distal end of release
wire 18 could be
releasably secured to distal end 46 of covered stent 14 by, for example,
bending the distal end of
the release wire (which would straighten when pulled), adhering the release
wire to the distal end
using an adhesive (which adhesive bond could be broken when the release wire
was pulled), or a
securing the release wire to the distal end by a breakable thread (which would
break when the
release wire was pulled). In the preferred embodiments the release wire
engages the tips of the
proximal and distal portions of the covered stent; in appropriate cases it may
be possible or
desirable to engage the covered stent at positions spaced apart from the tips
of the proximal and
distal portions. The invention has been described with reference to a covered
stent. However,
other generally helical endoluminal prostheses may also be used. For example,
a bare metal stent
or a metal stent coated with a polymer/drug matrix may be used. In the
preferred embodiments
the release wire passes through or pierces the proximal and distal ends of the
covered stent while
the intermediate portion of the covered stent passes between the release wire
and the catheter; in
some situations it may be desirable to have the release wire pierce one or
more locations along the
intermediate portion of the covered stent. While the stent is typically
released by pulling on the
release wire, release may also be accomplished in appropriate situations by
pushing on the release
wire.
[0078] Use of a braided material including filaments of 64, see Fig. 12, to
increase the
torsional strength of a catheter is well-known. Fig. 21 is a simplified
enlarged side elevational
view of a modification of the notched catheter 12 of Fig. 2. Braid-reinforced
notched catheter 12
of Figs. 21 and 22 has a catheter body 70 including an outer surface including
a generally
cylindrical outer surface portion 72 and a notched surface portion 74. Next, a
filament-containing
layer, typically of braided materia176, is placed adjacent to surface portions
72, 74. Braided
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
material 76 fits snugly against surface portions 72, 74 as indicated in Fig.
21. Next, a mandrel or
other elongate element, not shown, is inserted through release wire lumen 37
to pierce braided
materia176 as the mandrel exits lumen segment 37 and again as it enters lumen
segment 36 to
capture a portion of braided material 76 between notched surface portion 74
and the mandrel.
Next, a polymer sleeve, typically made of nylon or some other polymer 78, is
slid over braided
materia176 and then heated to bond braided materia176 to catheter body 70.
Thereafter polymer
material 78 is removed from the mandrel to permit the mandrel to be removed
from lumen 30.
This results in a notched catheter having an effectively uninterrupted
filament-containing layer 80
at its outer surface, even at cutouts 32-35. In this way the torsional
strength provided by braided
material 76 in filament-containing layer 80 is not compromised as it would be
if the filament-
containing layer were placed over the outer surface of catheter body 70 before
the cutouts were
made. Instead of a separately applied polymer materia178, braided materia176
and polymer
material 78 could be provided as a pre-preg (previously impregnated) material.
[0079] The various coiled stent delivery assemblies 10 discussed above with
reference
to Figs. 1-22 may be used in a manner by which catheter 12 is rotated
simultaneously as release
wire 18 is pulled or otherwise manipulated to release stent 14 from the
catheter. Figs. 23-28
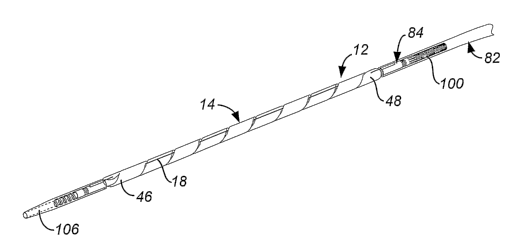
disclose an assembly 10 in which the distal portion of delivery catheter 12 is
flexible but has
superior torsional stiffness. Delivery catheter 12 in this embodiment
comprises a first, polymer-
based tube 82 and a second, metal tube 84 secured to the distal end 86 of
first tube 82. First tube
82 is preferably a braid-reinforced polymer while second tube 84 is typically
a stainless steel
hypotube having flexibility-enhancing relief areas 88 along the length of
second tube 84. In the
disclosed embodiment relief areas 88 comprise a series of angled openings 90
extending between
the inside surface 92 and the outside surface 94 of the tubular wal196 of
second tube 84. While
relief areas 88 are positioned at regular locations along second tube 84, they
could be placed at
irregular intervals or they could be located generally continuously along the
entire length or
portions of the entire length of second tube 84. Also, some or all of relief
areas 88 need not
extend completely through tubular wall 96.
[0080] Fig. 23 is an overall view of coiled endoluminal prosthesis delivery
assembly 10
having a coiled endoluminal prosthesis, such as covered stent 14, secured to
second, metal tube.84
in a radially contracted state through the use of release wire 18. Release
wire 18 passes through a
series of guide tubes 98 with turns of stent 14 passing over the guide tubes.
As shown in Figs. 23
and 24, turns of stent 14 are captured between release wire 18 and second,
metal tube 84 in regions
between adjacent guide tubes 98. The proximal end 100 of second, metal tube 84
is adhered
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
within distal end 86 of first tube 82. The distal end 102 of second tube 84
comprises a highly
flexible coil 104 and is covered by in atraumatic covering 106.
[0081] Fig. 25 illustrates second, metal tube 84. It can be seen that guide
tubes 98 have
a left-hand or counterclockwise spiral circumferential offset when viewed from
proximal end 100
of second tube 84. Prior to mounting stent 14 onto second tube 84, second tube
84 is placed in a
torqued state, as indicated in Fig. 28, by twisting distal end 102 of second
tube 84 in a right hand
or clockwise circumferential direction when viewed from proximal end 100. This
causes guide
tubes 98 to become generally axially aligned. After covered stent 14 has been
mounted to second
tube 84, second, metal tube is released and tends to tighten the covered stent
onto the second,
metal tube thereby helping to create a smaller placement profile by reducing
the cross-sectional
size of the delivery catheter. Pre-torquing second tube 84 accommodates
variations in the length
of stent 14 and in the placement of stent 14 on second tube 84 so to
facilitate proper alignment of
distal end 46 of covered stent 14 with the distal-most guide tube 98.
[0082] Using a flexible metal tube as the portion of catheter 12 on which
covered stent
14 is mounted provides several advantages in addition to enhanced torsional
strength. The wall of
the metal tube can be thinner for the same torsional strength so that main
lumen 28 can be larger
to permit the use of a larger diameter guide wire compared with polymer-based
guide catheters.
Metal tube 84 will typically have a smaller profile (smaller cross-sectional
diameter) than a
polymer-based catheter having an equivalent torsional strength. Also, metal
tube 84 can remain
in a pre-torqued state for a much longer time than an equivalent polymer-based
catheter for
greatly enhanced storage life. This permits assembly 10 to be shipped and
stored in a wound-
down state and not have any appreciable effect on second tube 84.
[0083] When a generally helically wound coiled stent moves from the radially
contracted state to the radially expanded state, see Figs. 6-10, the number of
coils decreases as the
stent expands resulting in circumferential movement of the stent. A further
aspect of the
invention is the recognition that as covered stent 14, or some other
endoluminal prosthesis, is
released into a blood vessel or other hollow body organ, typically starting
from one end of the
covered stent and proceeding towards the other end of the covered stent, it
would be advantageous
to simultaneously rotate catheter 12. Doing so would help keep the coiled
stent in place so that it
rolls out against the wall of the hollow body organ in a controlled manner.
While this could be
accomplished by manually rotating catheter 12 as release wire 18 is pulled,
more control may be
achieved using, for example, one of the handles shown in Figs. 29-35. These
figures illustrate
several embodiments of handles that can be operated to simultaneously rotate
catheter 12 and
retrieve release wire 18.
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
[0084] Figs. 29 and 30 show a handle 110 from which delivery catheter 12
extends.
Handle 110 comprises a handle body 112 to which a nose piece 114 is secured.
Catheter 12
comprises first polymer-based tube 82 to which a stiff metal hypotube
extension 115 is mounted
at its distal end. Handle 110 also includes a release element retractor and
catheter rotator
assembly 116. Assembly 116 includes a spool device 118 secured to handle body
112, device 118
comprising an axially extending spoo1120 to which release wire 18 is wound.
Assembly 116 also
includes a rotator knob 122 mounted adjacent to spool device 118 and secured
to hypotube
extension 115 so that rotating rotator knob 122 causes catheter 12 to rotate.
Rotator knob 122 has
a ring of depressions or detents 124 engageable by a ball 126, ball 126 being
biased toward
detents 124 by a spring 128. Ball 126 and spring 128 are captured between
rotator knob 122 and
spool device 118. Assembly 116 also includes a release element guide 130
affixed to hypotube
extension 115. Release wire 18 is secured to spool device 118 and passes
through an opening (not
shown) in release element guide 130 so that rotating rotator knob 122 relative
to handle body 112
also rotates release element guide 130 and causes release wire 18 to be wound
onto or off of spool
120. The speed of retrieval of release wire 18 relative to the rotation of
delivery catheter 12 can be
changed by changing the diameter of spool 120. If desired, handle 110 could be
modified to
prevent, for example, unwinding release wire 18 from spool 120 or to prevent
inadvertent rotation
of rotator knob 122 relative to handle body 112. While delivery catheter 12 in
this embodiment is
manually rotated, handle 110 could be modified to provide for motorized
rotation of the delivery
catheter with the simultaneous retrieval of release wire 18. The invention may
also be carried out,
in appropriate circumstances, by the simultaneous rotation of delivery
catheter 12 and the
unwinding of release wire 18 from spool 120.
[0085] Fig. 31 illustrates an alternative embodiment to handle 110 of Figs. 29
and 30.
Catheter 12 is fixed to rotator 122 so that rotating rotator 122 causes the
catheter to rotate relative
to handle body 112. A tubular rotator extension 132 extends proximally from
rotator 122 within
handle body 112. A grooved follower sleeve 134 is positioned between the
interior wall of handle
body 112 and rotator extension 132. Sleeve 134 has a spiral groove 136 on its
outer surface. A
spiral groove pin 138 extends from a fixed position along handle body 112 to
engage spiral
groove 136. A pull wire connection element 140 is carried by sleeve 134 and
extends radially
inwardly to pass through an axially-extending slot 142 in rotator extension
132. Release wire 18,
not shown in Fig. 31, passes through the hollow interior of rotator extension
132 and is secured to
pull wire connection element 140. Therefore, rotating rotator extension 132
relative to handle
body 112 causes rotator extension 132 to rotate; the rotation of rotator 122
causes slot 142 to
rotate thus causing sleeve 134 to rotate through the engagement of pull wire
connection element
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
140 within slot 142. However, the engagement of pin 138 within spiral groove
136 causes sleeve
134 to move axially as it rotates. This results in the rotation of catheter 12
and, typically, the
pulling of release wire 18 when rotator 122 is rotated.
[0086] Fig. 32 is a view of an embodiment similar to that of Fig. 31 but in
which
grooved follower sleeve 134 is much shorter than in the embodiment of Fig. 31.
This is possible
through the use of multiple spiral groove pins 138 carried by handle body 112.
[0087] Figs. and 33-35 illustrates a still further alternative embodiment of
the handle of
Figs. 29 and 30 similar to the embodiment of Fig. 32 but differing in several
respects. First, the
embodiment of Figs. 33-35 has a continuous internal thread 144 extending
inwardly from a fixed
(relative to handle body 112) thread structure 145 to engage spiral groove 136
in grooved follower
sleeve 134. The second major distinction is the use of a pull ring assembly
146 that can be used
to rotate catheter 12 and withdraw release wire 18 more rapidly than would
typically occur by
turning rotator knob 122. Assembly 146 comprises a pull ring 148 connected to
a pull wire 150.
Pull wire 150 extends through a slot 152 in handle body 112 and connects to a
cylindrical slider
154. Slider 154 slides along an annular space 156 defined between a
cylindrical inner wall 158 of
handle body 112 and thread structure 145. Slider 154 has a pair of radially
inwardly extending
pins 160, the pins passing through slots 162 formed through opposite sides of
thread structure
145. Pins 160 are secured to a pusher sleeve 164 that passes over rotator
extension 132 and abuts
follower sleeve 134. Therefore, pulling on pull ring 146 causes pusher sleeve
164 to drive
grooved follower sleeve 134 proximally causing connector element 142 to pull
release wire 18.
Pulling on pull ring 148 also causes sleeve 134 to rotate, by the engagement
of internal thread 144
and spiral groove 136, thereby rotating rotator extension 132 and catheter 12.
[0088] It can be appreciated that the embodiment of Figs. 29 and 30 may be
preferred
over the embodiments of Figs. 31-35 when it is useful or important to reduce
the length of handle
110.
[0089] Structure other than release wire 18 could be used to maintain stent 14
in the
wrapped down state. For example, individual constraining elements, such as
loops or bands,
could be used along catheter 12 to secure stent 14 to the catheter. A separate
electric wire could
be connected to each constraining element and energized to release each
constraining element,
and thus a portion of stent 14, in a desired order. Also, a single electric
wire could be connected
to all of the constraining elements with each constraining element being
releasable, such as by
melting a portion of the constraining element, after different periods of
time. In both of these
situations no movement of any release element would necessarily be required.
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
[0090] Another aspect of the invention is the recognition that it would be
desirable if
covered stent 14, or other helically wound endoluminal prosthesis, were to
have the same length
168 when in a radially contracted state, see Fig. 36, as when in a relaxed,
radially expanded state,
see Fig. 40. By doing this, gross axial motions of covered stent 14 when
moving from a radially
contracted to a radially expanded state can being eliminated while minimizing
the axial motion at
any particular position along covered stent 14. Figs. 36-40 illustrate a
sequence of releasing a
constant length covered stent 14 from delivery catheter 12. First, a first
diameter 170 for covered
stent 14 in a reduced diameter state is determined. Next, a second diameter
172, see Fig. 40, for
coiled stent 14 when in an expanded diameter state at a target location 174 is
determined.
Covered stent 14 is configured to reduce or eliminate any difference between
the lengths 168, 172
of covered stent 14 when in the reduced diameter state of Fig. 36 and when in
the expanded
diameter state of Fig. 40. This is achieved, in contrast with the variable
width turns of the stent of
US patent number 5,833,699, without changing the width of the turns of covered
stent 14. One
way to do is as follows. Assume covered stent 14, when in the expanded
diameter state of Fig.
40, has a total area (TA) equal to the external surface area of the turns 176
of the endoluminal
prosthesis (SA) plus the area of the generally helical gap (GA) between the
turns. Covered stent
of 14 is designed to reduce or eliminate any difference between the ratio of
SA to TA to the ratio
of first diameter 170 to second diameter 172. This is achieved by adjusting
how closely turns 176
of covered stent 14 are wound down onto catheter 12.
[0091] One constraint on winding down covered stent is the desire not to have
turns 176
lie on top of one another, and especially not have the solid stent portions of
a covered stent lie on
top of one another, so to limit any increase in cross-sectional area during
stent placement. This
may require adjusting the width 178 of covered stent 14 to prevent overlapping
of turns 176 in a
reduced diameter, wrapped down state, such as in Fig. 36. For example, instead
of using a fixed
width ladder type stent, such as shown in US patent number 6,488,700, the
stent could have two
side rails connected at their ends but without any rung elements to permit the
width of the stent to
be narrowed during wrap down if necessary. Also, a ladder type stent could
have rungs that are
bowed or otherwise act as flexible connectors to permit the side rails to be
oriented closer to one
another during wrap down onto the catheter. After release from the catheter,
the stent would
spring back to its relaxed width when the flexible connectors act as spring
elements. In addition,
width 178 of covered stent 14 could be chosen to prevent overlapping of turns
176.
[0092] Another advantage, in addition to reducing or eliminating gross
movements of
portions of covered stent 14 during deployment, resulting from the use of a
constant length
covered stent 14 is that it permits the use of a balloon 180, see Fig. 41, to
deploy or help deploy a
CA 02590885 2007-06-12
WO 2006/068856 PCT/US2005/044575
helically coiled endoluminal prosthesis, such as covered stent 14. Balloon 180
is mounted to and
carried by catheter 12 between cutouts 33, 34 after which generally helical
covered stent 14 is
mounted over catheter 12 and balloon 180 as shown in Fig. 42. Because of the
minimal axial
shifting of points along covered stent 14 during deployment, balloon 180 may
be expanded before
the entire covered stent 14 has been released while not significantly
affecting any subsequent
expansion movement by covered stent 14. While balloon 180 has been shown as
being centrally
positioned beneath covered stent 14, the balloon could be positioned
elsewhere, such as at one
end. Also, a number of balloons could be used.
[0093] Any and all patents, patent applications and printed publications
referred to
above are incorporated by reference.