Note: Descriptions are shown in the official language in which they were submitted.
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
METHODS FOR THE BIOTRANSFORMATION OF THE CLYCLOSPORIN COMPOUND ISA247.
TECHNICAL FIELD
[0001] This invention relates to methods of preparing metabolites of
compounds, and more
particularly to the preparation of such metabolites by biotransformation.
BACKGROUND
[0002] When dosing a patient with a pharmaceutical compound, it is often
necessary to monitor
the serum levels of the drug to ensure that the patient is receiving a
therapeutic dose of the drug.
This is called therapeutic dose monitoring (TDM). TDM may measure the parent
compound
and/or one or more metabolites of the parent compound. Metabolites are formed
when enzymes,
commonly liver enzymes, work to break down or modify a drug so that the drug
can be more
easily eliminated from the body. When the parent compound is rapidly
metabolized, it may be
most convenient to measure the levels of a metabolite for the purposes of TDM.
Frequently,
immunoassays are used for such measurements.
[0003] When a TDM assay for measuring a metabolite is an immunoassay, the
drug, or an
isolated, purified metabolite of the drug, may be used for generating andlor
selecting for an
antibody having the desired specificity for use in that assay. Alternatively,
the purified
metabolite may be used to define an antibody specific for the parent
coinpound, i.e., an antibody
that exhibits minimal cross-reactivity between the parent compound and
metabolites of the
parent compound. Therefore, efficient methods for producing isolated
metabolites are needed in
order to obtain a quantity of metabolite suitable for use in producing
antibodies for TDM.
20, [0004] Metabolites may also have uses that are independent of TDM.
Metabolites may have
pharmaceutically important activities. For example, metabolites may exhibit
beneficial
characteristics such as improved pharmacokinetics, increased pharmacological
activity or
improved bioavailability. A metabolite of a parent drug compound may itself be
a useful
therapeutic. For example, A77 1726 is the active metabolite of leflunomide;
hydroxy-tert-
butylamide is the active metabolite of the HIV drug, nelfmavir; and 4-OH-
tamoxifen is the active
metabolite of tamoxifen. When the metabolite exhibits activity, efficient
methods of producing
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
large quantities of the metabolite may be desired. Alternatively, one or more
of the metabolites
may be toxic. Thus, knowledge about how a drug is metabolized, its resulting
metabolites, and
the activity of these metabolites is important for understanding the activity
of a drug. This
information may also be required prior to drug approval. In order to identify
the metabolites and
their properties, a sufficient quantity of the metabolites must be produced
and isolated.
[0005] One method for producing metabolites is to administer the drug to a
mammal, such as
a human, then collect blood, urine, bile or other body fluids, and extract,
purify, and isolate
metabolites from these fluids. Commonly, biotransformation, the conversion of
a drug to
metabolites of the drug, is achieved in human patients in the liver by the
liver cytochrome P450
enzymes (CYP450 or P450). The P450 enyzme family includes an estimated 70 or
so enzymes
which act to render a compound more soluble for excretion in the bile or
urine. To monitor
metabolite formation by biotransforination, a parent compound may be tagged so
that the
metabolites may be recognized. Alternatively, a drug having a similar
structure may be analyzed
in parallel when the results are monitored by high pressure liquid
chromatography separation and
mass spectral analysis. A second method for biotransformation of a parent
compound is to use a
whole organ, a tissue slice, or cultured cells such as hepatocytes as a
biotransforming system. In
a third method, microsomes prepared from mammalian cells may be used. These
approaches use
animal isolates, thus risking introduction of unwanted contaminants into the
metabolites. These
methods are difficult to scale up when larger quantities of one or more of the
metabolites is
desired. In addition, biotransformations using microorganisms to convert the
parent compound
into metabolites may also be used.
[0006] It may be particularly difficult to produce large quantities of
metabolites when the
xenobiotic agent is highly lipophilic or highly insoluble in the aqueous media
used in large scale
fermentation methods. Therefore, a method for efficiently producing large
quantities of
metabolites of insoluble xenobiotics is needed.
SUIVIlVIARY
[0007] The present inverition provides methods of producing metabolites of
xenobiotic
compounds by biotransformation using a microorganism. The xenobiotic compound
may be
2
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
delivered to the microorganism in a mixture with a surfactant. The method can
be scaled up to
produce large quantities of metabolites by, for example, biotransformation in
a reactor. The
metabolites produced by the present method can be used, for example, for
antibody production,
as standards in therapeutic dose monitoring, or in pha.nnaceutical
applications.
[0008] Accordingly, one aspect of the present invention provides a method for
producing at
least one metabolite of a xenobiotic compound in a microorganism, comprising
the steps of:
(a) providing a mixture of the xenobiotic coinpound and a surfactant;
(b) adding the mixture to a culture of the microorganism; and
(c) incubating the culture for a period of time sufficient to allow the
metabolite to fonn.
[0009] The mixture may comprise the xenobiotic compound, a solvent, and the
surfactant.
Any suitable solvent for the xenobiotic compound can be used. For example, the
solvent may an
alcohol, such as ethanol. The solvents may comprise more than one substance.
In some
embodiments, the solvent comprises both an alcohol and dimetheyl sulfoxide
(DMSO).
[0010] The microorganism may be any microorganism that is capable of
metabolizing the
xenobiotic compound, preferably one that possesses the same metabolizing
pathway for the
xenobiotic coinpound as the human does. In certain embodiments, the
microorganism is selected
from the group consisting of Actinoplanes sp., Streptoynyces griseus,
Streptomyces setonii, and
Saccharopolyspora erthyraea. The microorganism may also be Cunningham
ellaechinulata,
Nerospora crassa, or Actinoplanes sp.
[0011] The surfactant may be any suitable surfactant, wliich can be identified
by a skilled
artisan based on teachirigs of the present disclosure. For example, the
surfactant may be selected
from the group consisting of polyethylene glycol (PEG) 400, castor oil,
isopropyl myristate,
glycerine, Cremophor0 (polyoxyl castor oil), Labrasol0 (caprylocaproyl
macrogolglycerides),
and TWEENO 40.
[0012] The xenobiotic compound is preferably a compound with a low solubility
in aqueous
solutions. In some einbodiments, the xenobiotic compound is selected from the
group consisting
of immunosuppressants and anti-bacterial compounds, preferably a cyclosporin
compound, more
preferably ISA247 or cyclosporin A. The metabolite is preferably selected from
the group
3
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
consisting of IM1-d-1, IMI-d-2, IMI-d-3, IMI-d-4, IM1-c-1, IM1-c-2, IMl-e-1,
IM1-e-2, IM1-
e-3, IM9, IM4, IM4n, IM6, IIVI46, IM69, and IM49.
[0013] The method of the present invention may optionally further comprise the
step of
isolating the metabolite from the culture.
[00141 Another aspect of the present invention provides a method for
identifying a
microorganism suitable for use in a biotransformation system comprising: a)
comparing the
structure of a compound to be metabolized with a known enzyme activity; b)
identifying an
enzyme that expresses the known enzyme activity; c) identifying a
microorganism that expresses
the identified enzyme; and d) using the microorganism that expresses the
identified enzyme in a
biotransformation system to make metabolites of the compound. In certain
embodiments, the
microorganism may be identified by comparing the genomic sequence of various
microorganisms to the sequence of the identified enzyme, thereby identifying
at least one
microorganism that expresses the enzyme.
[0015] The details of one or more embodiments of the invention are set forth
in the accompa-
nying drawings and the description below. Other features, objects, and
advantages of the
invention will be apparent from the description and drawings, and from the
claims.
DESCRIPTION OF DRAWINGS
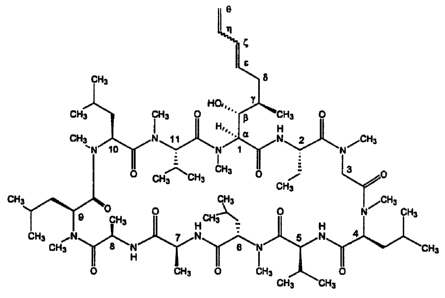
[0016] Figure 1 illustrates the structure of ISA247. The amino acid residues
in ISA247 are
indicated by numbers. Greek letters indicate the carbon positions of amino
acid 1.
[0017] Figures 2A and 2B provide the structures of the trans (E-) and cis (Z-)
isomers of
ISA247 molecule, respectively.
[0018] Figure 3 is an HPLC scan showing the profile of the ISA247 metabolites
isolated from
human whole blood of a subject who had received a 50:50 mixture of cis:trans
ISA247.
[0019] Figure 4 is an HPLC scan illustrating the profile of the ISA247
metabolites isolated
from the biotransformation method described in Example 4.
4
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
[0020] Figure 5 is a graph showing the effects on ISA247 metabolite production
of different
surfactants in a biotransformation system.
[0021] Figure 6 shows the LC-MS profile of the ISA247 metabolites isolated
from human
whole blood of a subject who had received an ISA247 formulation that contained
predominantly
the trans-isomer of ISA247.
[0022] Figures 7 and 8 compare the effects of Media 3 and Media 16 on ISA247
metabolite
production by Actinoplanes sp. (ATCC 53771) and Saccharopolyspora erythraea
(ATCC
11635), respectively.
[0023] Figure 9 demonstrates the effects of different solvents and surfactants
on ISA247
metabolite production by BeaztvaYia bassiana.
DETAILED DESCRIPTION.
[0024] The present invention provides methods of producing metabolites of
xenobiotic
compounds by biotransforrnation using a microorganism. Specifically, the
xenobiotic compound
is delivered to the microorganism in a mixture with a surfactant. The method
can be scaled up to
produce large quantities of metabolites by, for example, biotransformation in
a reactor. The
metabolites produced by the present method can be used, for example, for
antibody production,
as standards in therapeutic dose monitoring, or in pharmaceutical
applications.
[0025] Many pharmaceutically active compounds are poorly soluble in aqueous
solutions. For
example, cyclosporins and certain other immunosuppressive agents (rapamycin,
azathioprine,
mizoribine, and FK506 (tacrolimus)) are known to exhibit poor solubility in an
aqueous
environment. Using a cyclosporin derivative; ISA247, as a test compound, we
discovered that
microbial fermentation can be successfully used to prepare inetabolites of
poorly soluble
compounds. Prior to describing the invention in further detail, the terms used
in this application
are defined as follows unless otherwise indicated.
5
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
Definition
[0026] The term "biotransformation," as used herein, refers to the process of
metabolizing a
compound by a living cell, particularly a cell of a microorganism.
[0027] A "xenobiotic compound," or "xenobiotic," is a compound that is not
native with
respect to a microorganism. A xenobiotic compound may be pharmaceutically
active. The
xenobiotic compounds of this invention are preferably not readily soluble in
water. For example,
the compound may have a water solubility, at 25 C, of 1 mg/ml or less, 0.75
mg/ml or less, 0.5
mg/ml or less, 0.25 mg/ml or less, 0.1 mg/ml or less, 0.08 mg/ml or less, 0.06
mg/ml or less, 0.04
mg/ml or less, or 0.02 mg/ml or less.
[0028] A "cyclosporin compound" is a cyclosporin, or derivative thereof, that
has
immunosuppressive activities. The term encompasses the naturally occurring
cyclosporins,
cyclosporin A to Z, ISA247, synthetic and artificial dihydro- and iso-
cyclosporins such as those
disclosed in US Pat. Nos. 4,108,985; 4,210,581 and 4,220,641; derivatized
cyclosporins such as
shown in US Pat. Nos. 4,384,996; 4,703,033; 4,764,503; 4,771,122; 4,798,823;
4,885,276;
5,525,590; 5,643,870; 5,767,069; and cyclosporine derivative compounds as
provided in
W002069902; W003033010; W003030834; and W004050687.
ISA247 and its metabolites
[0029] ISA247 (ISATX247 or ISA) and its ISA related family members are
illustrated in US
Pat. Nos. 6,613,739 and 6,605,593. Like cyclosporin A, ISA247 is a cyclic
undecapeptide
2o consisting almost entirely of hydrophobic amino acids. Many of these amino
acids are not
normally found in mainmalian proteins. Figure 1 illustrates the structure of
ISA247 and the 11
amino acid residues that comprise the cyclic peptide ring of this molecule. As
shown, the amino
acid residues are numbered in a clockwise direction. As shown in Figure 1,
seven amino acids of
the eleven amino acids of the 11-membered amino acid ring are N-methylated.
The four
remaining protonated nitrogen atoms can form intermolecular hydrogen bonds
with carbonyl
groups, which contribute substantially to the rigidity of the cyclosporin
skeleton for both CsA
and ISA247. CsA has a solubility of about 0.04 mg/ml at 25 C. Due to its low
water-solubility,
6
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
the bioavailability of cyclosporin A is known to be 30% or less when orally
administered to
humans. ISA247 exhibits a similarly low water solubility.
[0030] ISA247 contains a sarcosine residue (whose three letter abbreviation is
Sar; sarcosine is
a methylated glycine residue and may also be abbreviated MeGly), one each of a
D- and an L-
alanine (Ala) residue, an a-amino butyric acid residue (Abu), a valine (Val)
residue, an N-methyl
valine (MeVal) residue, four N-methyl leucine (MeLeu) residues, and an alkene-
containing 9-
carbon, (3-hydroxylated amino acid unique to the cyclosporins called (4R)-4-
[(E)-2-butenyl]-4,N-
dimethyl-L-threonine (MeBmt). The chemical name of ISA247 is cyclo {{(E)- and
(Z)-
(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6,8-nonodienoyl} -L-2-
aminobutyryl-N-
methyl-glycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-
methyl-L-
leucyl-N-methyl-L-leucyl-N-methyl-L-valyl}. Its empirical formula is
C63H111N11012.I. It
has a molecular weight of about 1214.85.
[0031] ISA247 is known to exist in two isomeric forms, cis-ISA247 (or Z-
ISA247) and trans-
ISA247 (or E-ISA247). Figures 2A and 2B illustrates the trans and cis forms of
ISA247. A
mixture of cis and tr-ans forms of the ISA247 compound has been found to be
less toxic and
more potent than CsA (See U.S. Pat. Nos. 6,605,593 and 6,613,739). In
addition, ISA247 has
been found to be less toxic and more potent than CsA as a mixture of cis and
trans fonns when
the mixture contains a predominant proportion of the trans isomer. When
referring to ISA247, it
will be understood by those of skill in the art that ISA247 is a mixture of
the cis and trans
isomers, and that the mixture may be enriched in the trans isomeric form of
the compound. The
isomeric compounds may be present in a mixture, ranging from 1:99 cis:trans to
99:1 cis: trans.
7
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
[0032] ISA247 metabolites can be described as follows: A compound of formula
1:
CH3
R3 RZ R4 RZ R' CH2 R2
I O I I 11 I 11 12 I
RZ-N=C C-N-C-C-N-C-C-N-C-C-N CH2
CO H O H O H O H H O 31
CO
R3-cv I 2 (I)
R2 N-R
-N
OC-C ' N-C-C? N-C-C6 N-C-CS N-C 4-ICH
CH H 0 CH H O R3 Ry 0 R4 H 0 R3
3 3
wherein Rl is selected from the group consisting of:
0 0 I ro
0
HO
I CH3
CH3 HOI CH 3 HO CH3 HO
HO HO HO HO
HO HON'' I
HO HO~~
HO CH3 HO CH3 HO CH3 HO
CH3
OH OH
I OH ~ OH
HO
CH3 HO CH3
I OH ~OH OH LOH
HO HOX\ HO\~ HO
HO CH3 HO CH3 HO CH3 HO
CH3
HO, HO ~
' OH
~- .
O
O
CH3 O CH3 CH3
8
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
HO OH HO
CO2H CHO
HO CH3 HO CH3 HO CH3 HO CH3
> and
Where R2 is selected from the group consisting of CH3 and H; where R3)is
selected
from the group consisting of CH2CH(CH3)2 and CH2C(CH3)20H; and where R4 is
selected
from the group consisting of CH(CH3)2 and C(CH3)20H.
[0034] Structures of ISA247 metabolites which are modifications at amino acid-
1 of the
ISA247 compound are illustrated in Table 1. The boxes represent amino acids 2-
11 which form
the ring portion of the cyclosporin structure with the modified amino acid-l,
see Figure 1. Table
1 is not an exhaustive list of ISA247 metabolites that are modified at amino
acid-1. For
example, amino acid 1 metabolites may include 5, 6, 7 or 8 member rings.
Table 1: Amino Acid 1 Metabolites of ISA
IM1-e-1
1
HO CH3 HO CH3
CH CH
I91 -e-1 IMl-e-1
IM1-e-2
HO
CH3
CH
IM 1-e-2
9
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
IM1-e-3 O
HO
CH3
CH
IM 1-e-3
IM1-d-1 OH H
H/'
HO
+
HO HO
CH3 CH3
IM1Hd-1 IM1 d1
TM 1 -d-2 HO HO
, HopO~' Ho
H HO
cH~ CH~
IMI-J-? IMI-J-?
IM 1-d-3 OH OH
OH OH HO HO
CH3 CH,
IMl d-3 IM1Hd-3
IMl-d-4
I OH I OH I OH onIIOH
HO HO
H CH Ho OH' HO CH~ Ho CH~
IMIHJ-4 IMI-J=A IMICJ-4 IMI-J-4
-_,
IMl-c-1 H H
O
CH~ 9--
F-ICHIMI-ul
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
IM1-c-2
OH
O
CHa
IM 10-2
IM-1 HO CHO
HO
CH3
HO CH3
CH IM-1
1M-1
HO OH
COzH
HO
CH3 HO CH3
IM-1 IM-1
[0035] ISA247 metabolites include N-demethylated metabolites where the N-
demethylation
occurs at at least one methylated nitrogen of the amide linkage of an alnino
acid, for example,
IM4n, (or ISA247 Metabolite, N-demethylation at amino acid-4). N-demethylation
can occur at
amino acid-3 (IM3n), amino acid=4 (IM4n), amino acid-6 (IM6n), amino acid-9
(IM9n), amino
acid- 10 (IM10n) or ainino acid-11 (IM 11 n). ISA247 metabolites also include
hydroxylated
metabolites where the hydroxylation occurs at at least one methyl leucine
amino acid, for
example amino acids 4, 6, 9 or 10 (IM4, IM6, IM9 or IM10), or at valine
residue 5(IM5) or at
methyl valine residue 11 (IMl 1). IM46 are hydroxylated at both ainino acids 4
and 6, IM49 are
hydroxylated at both amino acids 4 and 9, and so on. Combinations of N-
demethylated and
hydroxylated metabolites can occur, as well as combinations of the metabolites
which are
alterations at amino acid-1, as shown in Table 1, with N-demethylations or
hydroxylations.
ISA247 nzetabolites also include metabolites which are glucuronide, sulfonide,
glycosylated and
11
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
phosphorylated derivatives of hydroxylated metabolites of ISA247. U.S. Pat.
Application No.
_(Attorney Docket Number 16593-009001, filed December 19, 2005) co-pending and
commonly assigned to the assignee provides ISA247 metabolites and uses
thereof.
Methods of preparing ISA247 metabolites
[0036] Metabolites of ISA247 were first analyzed using human whole blood of a
subject who
had received a 50:50 mixture of cis:trans ISA247 (Example 1). Figure 3 is an
HPLC scan
illustrating the metabolite profile of metabolites isolated from the whole
blood of this subject.
Using organic extractions on human whole blood, metabolites were extracted,
dried,
reconstituted in methanol and identified using chromatographic techniques
coupled with mass
spectrometry. As shown in Figure 3, at least three diol , two hydroxylated and
three N-
demethylated metabolites were detectable in human whole blood.
[0037] A dog liver microsome preparation was also used to produce 1SA247
metabolites
(Example 2). While ISA247 metabolites can be produced in this manner, the
yield was low and
the cost was high. Therefore, it is not practical to obtain meaningful
quantities of ISA247
metabolites using this approach.
[0038] To our knowledge, prior to the present invention, conventional
biotransformation
methods had not been reported as producing quantities of metabolites of CsA
and ISA247,
perhaps because of the lipophilic nature of these compounds. Without wislung
to be bound by
any theory, we believe the problem is that a hydrophobic compound, such as
ISA247, has a
tendency to adhere to the surfaces of filters, columns, and other hardware
used to carry out the
culture and process the product metabolites. Also, these highly lipophilic
coinpounds do not go
into solution in the aqueous environment of microorganisms in culture.
Cultured
microorganisms may not be able to access these lipophilic compounds to
metabolize them. In
some aspects, providing drug to a microbial growth preparation is not unlike
providing drug to a
mammal. A formulation that increases the bioavailability of the drug may be
necessary.
[0039] In humans, cytochrome P450 enzymes are known to fonn metabolites from
CsA. It has
been found that cytochrome P450 enzymes also act to form ISA247 metabolites.
Specifically,
the cytochrome P450 enzyine CYP3A4 has been identified as the enzyme
responsible for
12
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
cyclosporin and ISA247 metabolism. In order to produce a metabolite profile
that is similar to
that obtained from humans, the biotransformation system must utilize a
microorganism which
has the microbial equivalent of the human cytochrome P450 enzyme, grown in a
medium and
under culture conditions suitable for active growth and metabolism of the
microorganism.
[0040] Biotransformation methods are exemplified in Smith et al. (Arch.
Biochem. Biophys.
(1974) 161: 551-558). Urlacker and Schmid (Curr Opin Biotechnol. (2002)
13(6):557-64)
suggest that biotransformations could be performed using prokaryotic P450
monooxygenase
enzymes. Venisetty andCiddi (Current Pharmaceutical Biotechnology (2003)
4(3):153-167)
have proposed application of microorganisms to natural drugs to find novel
drugs.
[0041] Biotransformation of cyclosporin A solubilized in methanol by Sebekia
beraihana to [y-
hydroxy-MeLeu]4 cyclosporin (AM4) and to [,y-hydroxy-MeLeu]4 [y-hydroxy-
MeLeu]6
cyclosporin (AM46) has been disclosed in US Pat. No. 6,255,100. However, it
should be noted
that when CsA is administered to a human, the predominate metabolites that are
produced and
monitored for TDM are AM 1 (a metabolite of CsA that is hydroxylated at amino
acid-l,
MeBmt), AM9 (a metabolite that is hydroxylated at the MeLeu at amino acid
position 9) and
AM4n (a metabolite of CsA that is demethylated at the Meleu at amino acid
position 4) (LeGatt
et al., Clin Biochem. (1994) 27(1):43-8). Thus, Sebekia benihana exemplifies a
microorganism
that does not provide a metabolic profile that mimics the mainmalian metabolic
profile of CsA.
[0042] We demonstrate herein that biotransformation can be used to produce
metabolites of
ISA247. As shown in Example 3, by delivering a mixture containing ISA247 and
the surfactant
TWEENO 40 (polyoxyethylene sorbitan monopalmitate) to Saccharopolyspora
erytlzey-aea, all
the main categories of ISA247 metabolites found in human blood were produced.
Specifically,
seven ISA247 metabolites were detected: IM4n (ISA247 Metabolite that is N-
demethylated at
amino acid-4), IM9 (ISA247 Metabolite that is hydroxylated at amino acid-9),
IM4 (ISA247
Metabolite that is hydroxylated at amino acid-4), IM1-c-1(See table 1), IM1-d-
1 (Table 1), IMl-
d-2 (Table 1) and IMl-d-3 (Table 1). Different microorganisms produce
different types,
numbers and quantities of ISA247 metabolites, and the production can be
optimized by changing
the media (Example 5). Furthermore, for a given microorganism, the use of
different surfactants
or solvents may result in increased amount of metabolites or an improved
production profile.
13
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
For example, PEG 400 and glycerol led to the production of greater amounts of
metabolites
when Saccharopolyspora erytlzeraea was used (Example 4), while TWEEN 40
significantly
increased the number of different metabolites produced by Beauvaria bassiana
(Example 6).
[0043] Accordingly, one aspect of the present invention provides a method for
producing at
least one metabolite of a xenobiotic compound in a microorganism, comprising
the steps of:
(a) providing a mixture of the xenobiotic compound and a surfactant;
(b) adding the mixture to a culture of the microorganism; and '
(c) incubating the culture for a period of time sufficient to allow the
metabolite to form. The
method may optionally further coinprise the step of isolating the metabolite
from the culture.
[0044] Certain embodiments of the present invention provide an in vitro
biotransformation
system for producing significant quantities of metabolites of poorly soluble
compounds such as
those listed herein, especially immunosuppressive compounds such as
cyclosporins (for example,
ISA247 and CsA), macrolide lactones (for example, FK506), and triene
macrolides (for example,
rapamycin). Suitable xenobiotic compounds are discussed in greater detail
below.
[0045] After addition of the parent compound-surfactant mixture to the
bioreaction mixture
that contains the microorganism in growth medium, the bioreaction is allowed
to proceed for a
time and under conditions which pennit the parent compound to be metabolized.
After the
desired time, the metabolites are extracted from the bioreaction mixture,
purified by separation,
for exainple by chromatography such as high pressure liquid chromatography and
mass spectral
analysis (HPLC-MS). Nuclear magnetic resonance analysis may be used to verify
that the
individual metabolites have been isolated from one another and to verify the
structure thereof.
Individual metabolites that have been verified as separate chemical entities
may be used as
standards in subsequent assays.
[0046] A purified metabolite may be used in a TDM assay. For exainple, ISA247
may be
administered to an organ transplant patient in a dose sufficient to achieve
immunosuppression
and prevent the rejection-of a transplanted organ. In order to ensure that the
patient is
maintaining the proper drug level, and therefore maintaining the proper level
of
immunosuppression to prevent the rejection of a transplanted organ, a blood
sample may be
obtained fiom the patient at intervals. Blood levels of ISA247 may be
measured. In addition,
14
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
blood levels of at least one metabolite may be monitored to ensure that the
patient's body is
metabolizing the drug in a predictable manner. If the patient's own metabolism
is not working to
eliminate the drug from the patient's system, blood levels of the
unmetabolized, active drug may
build up, requiring a change in the patient's dosing regimen. Quantification
may be achieved by,
for example, immunoassay or by HPLC-MS. Similarly, antibodies specific for
ISA247 or one of
its metabolites may be developed.
[0047] In some embodiments of the present invention, ISA247 in ethanol is
mixed with
glycerol and then added to a biotransformation system containing
Saccharopolyspora erytheraea
(e.g., ATCC 11635). In other embodiments PEG 400 is mixed with ISA247 in
ethanol prior to
the addition of ISA247 to the biotransfonnation system. In other embodiments
castor oil is
mixed with ISA247 in ethanol prior to the addition of ISA247 to the
biotransformation system.
In other embodiments isopropyl myristate is mixed with ISA247 in ethanol prior
to the addition
of ISA247 to the biotransformation system. In other embodiinents CremophorOO
is mixed with
ISA247 in ethanol prior to the addition of ISA247 to the biotransfonnation
system. In other
embodiments Labrasol is mixed with ISA247 in ethanol prior to the addition of
ISA247 to the
biotransformation system. In other embodiments TWEEN 40 is mixed with ISA247
in ethanol
prior to the addition of ISA247 to the biotransformation system.
Additional exemplary xenobiotic compounds
[0048] Additional examples of drugs which are poorly soluble in aqueous
solutions include:
analgesics/antipyretics (e.g., aspirin, acetaminophen, ibuprofen, naproxen
sodium,
buprenorphine, propoxyphene hydrochloride, propoxyphene napsylate, meperidine
hydrochloride, hydromorphone hydrochloride, morphine, oxycodone, codeine,
dihydrocodeine
bitartrate, pentazocine, hydrocodone bitartrate, levorphanol, diflunisal,
trolamine salicylate,
nalbuphine hydrochloride, mefenamic acid, butorphanol, choline salicylate,
butalbital,
phenyltoloxamine citrate, diphenhydramine citrate, methotrimeprazine,
cinnamedrine
hydrochloride, and meprobamate); antiasthamatics (e.g., ketotifen and
traxanox); antibiotics
(e.g., neomycin, streptomycin, chloramphenicol, cephalosporin, ampicillin,
peiiicillin,
tetracycline, and ciprofloxacin); antidepressants (e.g., nefopain, oxypertine,
doxepin, amoxapine,
trazodone, amitriptyline, maprotiline, phenelzine, desipramine, nortriptyline,
tranylcypromine,
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
fluoxetine, doxepin, imipramine, imiprainine pamoate, isocarboxazid,
trimipramine, and
protriptyline); antidiabetics (e.g., biguanides and sulfonylurea derivatives);
antifungal agents
(e.g., griseofulvin, ketoconazole, itraconizole, amphotericin B, nystatin, and
candicidin);
antihypertensive agents (e.g., propanolol, propafenone, oxyprenolol,
nifedipine, reserpine,
trimethaphan, phenoxybenzamine, pargyline hydrochloride, deserpidine,
diazoxide, guanethidine
monosulfate, minoxidil, rescinnamine, sodium nitroprusside, rauwolfia
serpentina, alseroxylon,
and phentolamine); anti-inflammatories (e.g., (non-steroidal) indomethacin,
ketoprofen,
flurbiprofen, naproxen, ibuprofen, ramifenazone, piroxicam, (steroidal)
cortisone,
dexamethasone, fluazacort, celecoxib, rofecoxib, hydrocortisone, prednisolone,
and prednisone);
antiteoplastics (e.g., cyclophosphamide, actinomycin, bleomycin, daunorubicin,
doxorubicin,
epirubicin, mitomycin, methotrexate, fluorouracil, carboplatin, carmustine
(BCNU), methyl-
CCNU, cisplatin, etoposide, camptothecin and derivatives thereof,
phenesterine, paclitaxel and
derivatives thereof, docetaxel and derivatives thereof, vinblastine,
vincristine, tamoxifen, and
piposulfan); antianxiety agents (e.g., lorazepam, prazepam, chlordiazepoxide,
oxazepain,
clorazepate dipotassium, diazepam, hydroxyzine pamoate, hydroxyzine
hydrochloride,
alprazolam, droperidol, halazepam, chlormezanone, and dantrolene);
antimigraine agents (e.g.,
ergotamine, propanolol, isometheptene mucate, and dichloralphenazone);
sedatives/hypnotics
(e.g., barbiturates such as pentobarbital, pentobarbital, and secobarbital;
and benzodiazapines
such as flurazepam hydrochloride, triazolam, and midazolam); antianginal
agents (e.g., beta-
adrenergic blockers; calcium channel blockers such as nifedipine, and
diltiazem; and nitrates
such as nitroglycerin, isosorbide dinitrate, pentaerythritol tetranitrate, and
erythrityl tetranitrate);
antipsychotic agents (e.g., haloperidol, loxapine succinate, loxapine
hydrochloride, thioridazine,
thioridazine hydrochloride, thiothixene, fluphenazine, fluphenazine decanoate,
fluphenazine
enanthate, trifluoperazirie, chlorpromazine, perphenazine, lithium citrate,
and prochlorperazine);
antiarrhytlunics (e.g., bretylium tosylate, esmolol, verapainil, amiodarone,
encainide, digoxin,
digitoxin, mexiletine, disopyramide phosphate, procainamide, quinidine
sulfate, quinidine
gluconate, quinidine polygalacturonate, flecainide acetate, tocainide, and
lidocaine); antiarthritic
agents (e.g., phenylbutazone, sulindac, penicillamine, salsalate, piroxicam,
azathioprine,
indomethacin, meclofenainate, gold sodium thiomalate, ketoprofen, auranofin,
aurothioglucose,
and tolmetin sodium); antigout apents (e.g., colchicine, and allopurinol);
anticoagulants (e.g.,
heparin, heparin sodium, and warfarin sodium); thrombolytic agents (e.g.,
urokinase,
16
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
streptokinase, and alteplase); antifibriolytic agents (e.g., aininocaproic
acid); hemorheologic
agents (e.g., pentoxifylline); antiplatelet agents (e.g., aspirin);
anticonvulsants (e.g., valproic
acid, divalproex sodium, phenytoin, phenytoin sodium, clonazepain, primidone,
phenobarbitol,
carbamazepine, amobarbital sodium, methsuximide, metharbital, mephobarbital,
mephenytoin,
phensuximide, paramethadione, ethotoin, phenacemide, secobarbitol sodium,
clorazepate
dipotassium, and trimethadione); antiparkinson agents (e.g., ethosuximide);
antihistamines/antipruritics (e.g., hydroxyzine, diphenhydramine,
chlorpheniramine,
brompheniramine maleate, cyproheptadine hydrochloride, terfenadine, clemastine
fumarate,
triprolidine, carbinoxamine, diphenylpyraline, phenindamine, azatadine,
tripelennamine,
dexchlorpheniramine maleate, methdilazine, and); antibacterial agents (e.g.,
amikacin sulfate,
aztreonam, chloramphenicol, chloramphenicol palmitate, ciprofloxacin,
clindamycin,
clindamycin palmitate, clindamycin phosphate, metronidazole, metronidazole
hydrochloride,
gentamicin sulfate, lincomycin hydrochloride, tobramycin sulfate, vancomycin
hydrochloride,
polymyxin B sulfate, colistimethate sodium, and colistin sulfate); antiviral
agents (e.g., interferon
alpha, beta or gamma, zidovudine, amantadine hydrochloride, ribavirin, and
acyclovir);
antimicrobials (e.g., cephalosporins such as cefazolin sodium, cephradine,
cefaclor, cephapirin
sodium, ceftizoxime sodium, cefoperazone sodium, cefotetan disodium,
cefuroxime e azotil,
cefotaxime sodium, cefadroxil monohydrate, cephalexin, cephalothin sodium,
cephalexin
hydrochloride monohydrate, cefamandole nafate, cefoxitin sodium, cefonicid
sodium,
ceforanide, cefadroxil, cephradine, and cefuroxime sodium; penicillins such as
ampicillin,
amoxicillin, penicillin G benzatliine, cyclacillin, ampicillin sodium,
penicillin G potassium,
penicillin V potassium, piperacillin sodium, oxacillin sodium, bacampicillin
hydrochloride,
cloxacillin sodium, ticarcillin disodium, azlocillin sodium, carbenicillin
indanyl sodium,
penicillin G procaine, methicillin sodium, and nafcillin sodium; erythromycins
such as
erythromycin ethylsuccinate, erythromycin, erythromycin estolate, erythromycin
lactobionate,
erythromycin stearate, and erythroinycin etliylsuccinate; and tetracyclines
such as tetracycline
hydrochloride, doxycycline hyclate, and minocycline hydrochloride,
azithromycin,
clarithromycin); anti-infectives (e.g., GM-CSF); bronchodilators (e.g.,
sympathomimetics such
as epinephrine hydrochloride, metaproterenol sulfate, terbutaline sulfate,
isoetharine, isoetharine
mesylate, isoetharine hydrochloride, bitolterolmesylate, isoproterenol
hydrochloride, terbutaline
sulfate, epinephrine bitartrate, metaproterenol sulfate, epinephrine, and
epinephrine bitartrate;
17
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
anticholinergic agents such as ipratropium bromide; xanthines such as
aminophylline,
dyphylline, metaproterenol sulfate, and aminophylline; mast cell stabilizers
such as cromolyn
sodium; steroidal compounds and hormones (e.g., androgens such as danazol,
testosterone
cypionate, fluoxymesterone, ethyltestosterone, testosterone enathate,
methyltestosterone,
fluoxymesterone, and testosterone cypionate; estrogens such as estradiol,
estropipate, and
conjugated estrogens; progestins such as methoxyprogesterone acetate, and
norethindrone
acetate; corticosteroids such as triamcinolone, betainethasone, betamethasone
sodiuin phosphate,
dexamethasone, dexamethasone sodium phosphate, dexamethasone acetate
prednisone,
methylprednisolone acetate suspension, triamcinolone acetonide,
methylprednisolone,
prednisolone sodium phosphate, methylprednisolone sodium succinate,
hydrocortisone sodium
succinate, triamcinolone hexacetonide, hydrocortisone, hydrocortisone
cypionate, prednisolone,
fludrocortisone acetate, paramethasone acetate, prednisolone tebutate,
prednisolone acetate,
prednisolone sodium phosphate, and hydrocortisone sodium succinate; and
thyroid hormones
such as levothyroxine sodium); hypoglycemic agents (e.g., glyburide,
chlorpropamide,
tolbutamide, and tolazamide); hypolipidemic agents (e.g., clofibrate,
dextrothyroxine sodium,
probucol, pravastitin, atorvastatin, lovastatin, and niacin);
antiulcer/antireflux agents (e.g.,
famotidine, cimetidine, and ranitidine hydrochloride);
antinauseants/antiemetics (e.g., meclizine
hydrochloride, nabilone, prochlorperazine, dimenhydrinate, promethazine
hydrochloride,
thiethylperazine, and scopolamine); and oil-soluble vitamins. Metabolites of
these poorly
soluble compounds may be produced using the methods of the instant invention.
Microorganisms
[0049] Suitable microorganisms for a successful biotransformation may be
chosen based on
the presence of microbial enzymes, such as cytochrome P450 enzymes, having the
capacity to
metabolize the parent compound. Microorganisms that may be useful for
biotransformation
methods include bacteria, fungi and actinomycetes which possess cytochrome
P450 activity.
The organisms having these enzymes can be identified empirically by comparing
the metabolites
found in the blood or urine after ISA247 administration with those found using
a
biotransformation or microbial conversion preparation. For exainple, CYP3A4 is
a human P450
enzyme that can be characterized by its ability to hydroxylate testosterone,
thereby producing 6(3-
hydroxytestosterone. The enzyme is inhibited by such compounds as
clotrimazole, and
18
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
naringenin. It is induced by carbamazipine, phenobarbital, and rifampin. An
organism, growing
in growth media, which expresses an enzyme which has cytochrome P450 activity,
should
produce 6(3-hydroxytestosterone when testosterone is introduced into the
media, and this
production should be affected by the known inhibitors and inducers. Other
substrates
metabolized by CYP3A4 include, for example, acetaminophen, diazepam,
theophylline,
warfarin, taxol, and nifedipine. Similarly, when these compounds are
introduced into media
containing a microorganism, if the microorganism expresses the enzyme, it
should metabolize
the substrate.
[0050] Known and characterized enzymes have known and characterized activity.
By
1'o comparing the structure of the compound to be metabolized with the known
activities of
enzymes, enzyines can be identified that will be active in metabolizing the
compound.
Microorganisms can be screened for the presence of the identified enzyine.
Thus, an aspect of
the present invention provides a method of identifying a microorganism
suitable for use in a
biotransformation systein where the method has the steps: a) comparing the
structure of a
compound to be metabolized with a known enzyme activities; b) identifying an
enzyme that
expresses the desired enzyme activity; c) identifying a microorganism that
expresses the
identified enzyme; and d) using the microorganism that expresses the
identified enzyine in a
biotXansformation process to make metabolites of the compound. By using this
method,
microorganisms which may be useful to produce metabolites of a compound can be
identified.
[0051] Alternatively, genetic sequence data may be used to identify
potentially useful
organisms by comparing the genomic sequence of an organism to the sequence of
a known
mammalian gene which encodes a cytochrome enzyme, for example CYP3A4.
Microorganisms
which have the appropriate genetic sequences, grown in the proper conditions,
should express
the target enzyme. In addition to reference compounds, compounds that inhibit
or induce a
particular human P450 enzyme can be tested in both systems.
[0052] Drugs which are known to be metabolized by specific cytochrome enzymes
include: (1)
acetaminophen, aromatic amines, caffeine, estradiol, imipramine, phenacetin,
theophylline and
warfarin, broken down by CYP 1A2; (2) amitryptiline, bufurolol, captropril,
clozepine,
debrisopuine, flecainide, fluoxetine, haloperidol, metoprolol, mexiletine,
sparteine, timolol,
19
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
tomoxetine, propranolol and codeine, broken down by CYP2D6; (3) acetaminophen,
diazepam,
amiodarone, benzphetamine, carbemazepine, cyclosporine, digitoxin, diltiazem,
erythromycin,
etopiside, flutamide, imipramine, lidocaine, loratidine, nifedipine,
midazolam, retinoic acid,
steroids, tamoxifen, taxol, terfenadine, THC, verapamil and warfarin, broken
down by CYP3A4.
[0053] Microorganisms that express the CYP3A4 enzyme, and that may be useful
for
biotransformation methods include but are not limited to: Actinoplanes sp.
(e.g., ATCC No.
53771), Streptofnyces griseus (e.g.e.g., ATCC 13273), Saccharopolyspora
erythraea (e.g.,
ATCC No. 11635), and Streptoinyces setonii (e.g., ATCC No. 39116). Other
useful
microorganisms that may express cytochrome P450 enzymes include Ainycolata
autotrophica
(e.g., ATCC No. 35204), Streptomyces californica (e.g., ATCC No. 15436),
Saccharopolysora
hirsute (e.g., ATCC No. 20501), Streptomyces lavandulae (e.g., ATCC No.
55209), Stretornyces
aureofaciens (e.g., ATCC No. 10762), Streptorrayces rimosus (e.g., ATCC No.
28893), Bacillus
subtillis (e.g., ATCC No. 55060), and Nocardia asteroids (e.g., ATCC No.
3318),
Sacclzaromyces cerevisiae (e.g., ATCC No. 20137 or ATCC No. 64667) Aspergillus
nidulans
(e.g., ATCC No. 32353) Czcnninghanaella echinulata var. elegans (e.g., ATCC
No.36112),
Rhizopus stolonifer, (e.g., ATCC No. 6227b), Candida apicola (e.g., ATCC No.
96134),
Coprinus cireneus, (e.g., ATCC No. MYA-727, MYA-726, MYA-728, MYA-729, MYA-
730,
MYA-731).
[0054] Selection of appropriate culture time, culture conditions, extraction
and purification
methods is known to those of skill in the art. Growth of the chosen organism
may be achieved
by a skilled artisan, for example, by the use of an appropriate growth medium
containing
nutrients such as carbon and nitrogen, a buffering system, and trace elements
and of conditions
of pH, temperature, and aeration conducive to growtll. Exemplary carbon
sources include
glucose, maltose, dextrin, starch, lactose, sucrose, molasses, soybean oil,
and the like. Suitable
nitrogen sources include soybean meal, cotton seed meal, fish meal,, yeast,
yeast extract, peptone,
rice bran, meat extract, ammonium nitrate, ammoniuin sulfate and the like.
Inorganic salts may
be added such as phosphates, sodium chloride, calcium carbonate and the like.
Different growth
media may be used depending upon the stage of growth of the organism. 20
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
[0055] Exemplary conditions and media for growth of microorganisms suitable
for use in the
bioconversion of cyclosporins and cyclosporine derivatives thereof by
Saccharopolyspora
eiythraea (for example, ATCC 11635), Saccharopolysora hirsute (for example,
ATCC 20501),
Amycolata autotrophica (for example, ATCC 35204), are provided in Corconan,
Methods in
Enzymology 43: 487-498 (1975), US Pat. Nos. 5,124,258; 6,043,064; and
6,331,622. Conditions
for growth of Actinoplanes sp. (for exainple, ATCC No. 53771) are exemplified
in US Pat: No.
5,270,187.
[00561 Exemplary growth conditions for microbial bioconversion of macrolides
to their
hydroxylated and/or demethylated metabolites include: 1) growth conditions as
disclosed for the
demethylation of L-679,934 (FK-506) to its metabolite, L-683,519, using
Actinomycete sp.
(Merck Culture Collection MA 6474; ATCC No. 53828) exemplified in US Pat. No.
5,268,370;
and 2) demethylation of L-679,934 to L-682,993 or L-683,590 to L-683,742) by
Actinoplanes sp.
(ATCC No. 53771) using conditions for growth provided in US Pat. No. 5,202,
258.
Actinoplanes spp. ATCC 53771, Saccharopolyspora erthyreae ATCC 11653,
Streptomyces
lavandulae ATCC 55209, Stretomyces aureofaciens ATCC 10762, Streptoin.yces
rimoszts ATCC
28893, Bacillus subtillis ATCC 55060 and Nocardia asteroids ATCC 3318 may be
used to
produce hydroxylated (for example, 24-OH rapamycin) and/or demethylated
metabolites (for
example, 39-O-demethylrapamycin) of rapamycin (Kuhnt, M., et al., 1997, Enzyme
and
Microbial Technology 21: 405-412).
Surfactants
[0057] Suitable surfactants for use in an embodiment of the inventive method
may be able to
withstand autoclaving prior to being introduced into a microbial growth
environment. Suitable
surfactants are biocompatible surfactants and include but are not limited to
nonionic surfactants
such as polyethylene glycols for example PEG 300, PEG 400, PEG 600 (also known
as Lutrol
E 300, LutrolO E 400, Lutrol E 600 Lutrol F 127, and Lutrol(b F 68 from
BASF);
caprylocaproyl macrogol-8 glycerides such as Labrasol (Gatte Fosse, Cedex
France);
polyoxyethylene sorbitan fatty acid esters such as Tween 20, Tween 21, Tween
40,
Tween 80, TweenO 80K, Tween 81 and Tween 85 (ICI Americas Inc., Bridgewater
NJ,
obtained from Aldrich Chemical Company Inc., Milwaukee Wis.); glycerine (BDH
Fine
21
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
Chemicals, Toronto Ont.); castor oil (Wiler Fine Chemicals Ltd, London Ont.);
Isopropyl
myristate (Wiler Fine Chemicals Ltd, London Ont.); Cremophor EL (Sigma
Chemical, St
Louis MO); and poloxamers such as Pluronics F127 and Pluronics L108 (BASF).
Other
surfactants that may be used include those that can act as lubricants or
emulsifiers such as
tyloxapol [4-(1,1,3,3-tetramethylbutyl)phenol polymer with formaldehyde and
oxirane];
polyethoxylated castor oils such as Cremophor(M A25, Cremophor A6, Cremophor
EL,
Cremophor ELP, Cremophor RH from BASF and Alkamuls EL620 from Rhone Poulenc
Co;
polyethoxylated hydrogenated castor oils, such as HCO-40; and polyethylene 9
castor oil.
[0058] Other surfactants that may be used include; polysorbate 20, polysorbate
60, and
polysorbate 80; Cremophor RH; poloxamers; Pluonics L10, L31, L35, L42, L43,
L44, L61,
L62, L63, L72, L81, L101, L121, L122; PEG 20 almond glyceride; PEG 20 corn
glyceride; and
the like. Suitable surfactants also include alkylglucosides; alkylmaltosides;
alkylthioglucosides;
lauryl macrogolglycerides; polyoxyethylene alkyl ethers; polyoxyethylene
alkylphenols;
polyethylene glycol fatty acids esters; polyethylene glycol glycerol fatty
acid esters;
polyoxyethylene-polyoxypropylene block copolymers; polyglycerol fatty acid
esters;
polyoxyethylene glycerides; polyoxyethylene sterols; polyoxyethylene vegetable
oils;
polyoxyethylene hydrogenated vegetable oils; polyoxyethylene alkylethers;
polyethylene glycol
fatty acids esters; polyethylene glycol glycerol fatty acid esters;
polyoxyethylene sorbitan fatty
acid esters; polyoxyethylene-polyoxypropylene block copolymers; polyglycerol
fatty acid esters;
polyoxyethylene glycerides; polyoxyethylene vegetable oils; polyoxyethylene
hydrogenated
vegetable oils; reaction mixtures of polyols such as PEG- 10 laurate, PEG- 12
laurate, PEG-20
laurate, PEG-32 laurate, PEG-32 dilaurate, PEG-12 oleate, PEG-15 oleate, PEG-
20 oleate, PEG-
20 dioleate, PEG-32 oleate, PEG-200 oleate, PEG-400 oleate, PEG-15 stearate,
PEG-32
distearate, PEG-40 stearate, PEG- 100 stearate, PEG-20 dilaurate, PEG-25
glyceryl:trioleate,
PEG-32 dioleate, PEG-20 glyceryl laurate, PEG-30 glyceryl laurate, PEG-20
glyceryl stearate,
PEG-20 glyceryl oleate, PEG-30 glyceryl oleate, PEG-30 glyceryl laurate, PEG-
40 glyceryl
laurate, PEG-40 palm kernel oil, PEG-50 hydrogenated castor oil, PEG-40 castor
oil, PEG-35
castor oil, PEG-60 castor oil, PEG-40 hydrogenated castor oil, PEG-60
hydrogenated castor oil,
PEG-60 corn oil, PEG-6 caprate/caprylate glycerides, PEG-8 caprate/caprylate
glycerides,
polyglyceryl-101aurate, PEG-30 cholesterol, PEG-25 phyto sterol, PEG-30 soya
sterol, PEG-20
22
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
trioleate, PEG-40 sorbitan oleate, PEG-80 sorbitan laurate, polysorbate 20,
polysorbate 80, POE-
9 lauryl ether, POE-23 lauryl ether, POE-10 oleyl ether, POE-20 oleyl ether,
POE-20 stearyl
ether, tocopheryl PEG- 100 succinate, PEG-24 cholesterol, polyglyceryl- 10
oleate, sucrose
monostearate, sucrose monolaurate, sucrose monopalmitate, PEG 10-100 nonyl
phenol series,
PEG 15-100 octyl phenol series, a poloxamer; PEG-3 5 castor oil, PEG-40
hydrogenated castor
oil, PEG-60 corn oil, PEG-25 glyceryl trioleate, PEG-6 caprate/caprylate
glycerides, PEG-8
caprate/caprylate glycerides, polysorbate 20, polysorbate 80, tocopheryl PEG-
1000 succinate,
and PEG-24 cholesterol, a poloxamer. In addition, oils such as almond oil;
babassu oil; borage
oil; blackcurrant seed oil; canola oil; coconut oil; corn oil; cottonseed oil;
evening primrose oil;
grapeseed oil; groundnut oil; mustard seed oil; olive oil; palm oil; palm
kernel oil; peanut oil;
rapeseed oil; safflower oil; sesame oil; shark liver oil; soybean oil;
sunflower oil; hydrogenated
castor oil; hydrogenated coconut oil; hydrogenated palm oil; hydrogenated
soybean oil;
hydrogenated vegetable oil; hydrogenated cottonseed and castor oil; partially
hydrogenated
soybean oil; soy oil; glyceryl tricaproate; glyceryl tricaprylate; glyceryl
tricaprate; glyceryl
triundecanoate; glyceryl trilaurate; glyceryl trioleate; glyceryl
trilinoleate; glyceryl trilinolenate;
glyceryl tricaprylate/caprate; glyceryl tricaprylate/caprate/laurate; glyceryl
tricaprylate/caprate/linoleate; glyceryl tricaprylate/caprate/stearate;
saturated polyglycolized
glycerides; linoleic glycerides; caprylic/capric glycerides may be used. In
addition, a mixture of
surfactants and/or oils and/or alcohols may be used.
[0059] In some einbodiments of the present invention, the selected lipophilic
xenobiotic is
mixed with an alkanol and a suitable nonionic surfactant before addition to an
actively growing
microbial culture. If the parent coinpound is mixed with an alcohol, the
alcohol may be ethanol.
Additional suitable alcohols include: methanol, isopropanol, 1-propanol, and
other suitable
alcohols well known in the art.
Solvents
[0060] In some embodiments of the present invention, the xenobiotic coinpound
is mixed with
a solvent before being added to a microorganism culture. The solvents may be
sterilized prior to
mixing with the xenobiotic compound. Optionally, a surfactant is also added to
the xenobiotic
compound - solvent mixture. Suitable solvents of the present invention may be
any solvent that
23
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
does not inhibit the growth and metabolism of the microorganism. The solvent
may be non-
polar, polar aprotic, or polar protic. For example, the solvents include the
hydrocarbon series
solvents, such as benzene, toluene, n-hexane, cyclohexane, etc.; ether series
solvents such as
diethyl ether, tetrahydrofuran, 1,4-dioxane, methyl t-butyl ether,
dimethoxyethane, ethylene
glycol dimethyl ether, etc.; alcohols such as methanol, ethanol, 1-propanol,
isopropanol, etc.;
halogen-containing solvents such as methylene chloride, chloroform, 1, 1, 1 -
trichloroethane, etc.;
and other solvents such as dimethylformamide, N-methylpyrrolidone,
hexamethylphosphorotriamide, etc. The solvent is preferably not DMSO.
[0061] The following examples are offered to illustrate this invention and are
not to be
construed in any way as limiting the scope of the present invention. While
this invention is
particularly shown and described with references to preferred embodiments
thereof, it will be
understood by those skilled in the art that various changes in form and
details may be inade
therein without departing from the spirit and scope of the invention as
defined by the appended
claims.
EXAMPLES
[0062] In the examples below, the following abbreviations have the following
meanings.
Abbreviations not defined have their generally accepted meanings.
C = degree Celsius
hr = hour
min = minute
sec or s= second
M = micromolar
mM = millimolar
M = molar
ml = milliliter
l = microliter
mg = milligram
g = microgratn
mol = mole
pmol = picomole
ATCC = American Type Culture Collection
PBS = phosphate buffered saline CSA = cyclosporin A
TDM = therapeutic dose monitoring
LC = liquid chromatography
24
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
MS = mass spectrometry
PEG = polyethylene glycol
General Material and Methods
Liquid Chrofnatographic (LC) Conditions
[0063] For Liquid Chromatography (LC or HPLC) a column having a stationary
phase formed
by chemically bonding a long-chain hydrocarbon group to a porous silica
matrix, a Waters
Symmetry C8, 2.1X50mm, 3.5 m analytical column (Waters cat# WAT 200624) with a
guard column
2 x 20mm (Upchurch Scientific cat# C-130B) packed with Perisorb RP-8 (Upchurch
Scientific cat# C-
601) was used. The solvent percentages and flow rates utilized in the LC
program are given in
Table 2:
0.2 % GAA +
10-5M Na Acetate MeOH:MtBE (9:1) Flow rate
Time (min) (%) (%) (mL/min)
0.00 55 45 0.5
5.00 45 55 0.5
10.00 5 95 0.5
12.00 5 95 0.5
12.01 55 45 0.5
15.00 55 45 0.5
Mass Spectral (MS) Conditions
[0064] For Mass Spectroscopy, an Applied Biosystems / MDS Sciex API3000
(Analyst
software v 1.2) machine was used. Run time was 15 minutes, injection volume
was 5 L, Guard
Column Temperature and Analytical Column Temperatures were 60 C. Manual
settings were as follows:
Turbo Ion Spray was 8000, Turbo Ion Spray horizontal setting was positive 4,
Turbo Ion Spray lateral
setting was 10. The Sciex machine was set with the parameters shown in Table
3.
Table 3 MS Settings
MS Settings:
Scan type: MRM (Multiple Reaction Monitoring)
Polarity: Positive
Period Duration 15.00 min
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
Period Cycle: 1.32 sec
# of Cycles: 692
Advanced MS Settings:
Resolution Q1: Low
Q3: Low
Intensity threshold: 0
Settling time: 50 msec
Pause time: 30 msec
Parameter Settings:
Ion Source: Turbo ion spray
Nebulizer Gas: 12
Curtain Gas: 8
Collision Gas: 12
Ion Spray voltage: 5000 V
Temperature: 550 C
Compound Settings:
Declustering Potential: 60 V
Focusing Potential: 400 V
Collision Energy: 90 V
Table 4 shows ions and ion-specific instrument settings.
Q1 Mass (amu) Q3 Mass (ainu) Time (msec)
1222.8 1098.7 100
1236.8 1112.7 100
1252.8 1128.7 100
1252.8 1224.7 100
1270.8 1112.7 100
1254..8 1130.7 100
1268.8 1128.7 100
1268.8 1144.7 100
1238.8 1114.7 100
1268.8 1240.8 100
Example 1
26
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
Preparation of ISA247 metabolites from whole blood
[0065] Whole blood was taken from humans after administration of ISA247 (a
50:50 mixture
of cis:tr=ans ISA247). ISA247 and its metabolites were extracted from whole
blood using
tertbutyl-methyl-ether (or methyl tertbutyl ether, MTBE), dried and
reconstituted into metilanol.
2mL of MTBE (cat. No. 7001-2; Caledon) were added to 200 uL of blood, shaken
for 10
minutes, and spun down in a table top centrifuge for 2 minutes. The top MTBE
layer was
removed and concentrated under vacuum. That residue was reconstituted in 200
uL of methanol.
Bile and urine extractions can be performed similarly, as can extractions from
microsome
preparations and biotransformation preparations. Once extracted, metabolites
can be
characterized using HPLC-MS, NMR, or other techniques known in the art. Figure
3 shows that
results of LC-MS performed as described in General Materials and Methods. The
ISA247
metabolites from whole blood include three main groups, the diols,
hydroxylated and
demethylated metabolites.
Example 2
ISA247 Metabolite Production by a Dog Liver Microsome Preparation
Preparation of Dog Microsomes
[0066] Dog liver microsomes were prepared in the following manner: after
removing the liver,
it was flushed with 1.15% potassium chloride (KC1); diced into small pieces
(approximately 25
g) and ground until major chunks were disintegrated in chilled grinding buffer
(0.1 M phosphate
buffer pH 7.4; 4 C; 1:1 ratio of buffer to liver) utilizing a Polytron
Homogenizer at 15,000 rpm
for 3 to 5 minutes, thus forming a homogenate, which contained membrane-
bounded organelles,
including liver microsomes. After decantation of supernatant from the
particulate matter, the
supernatant was centrifuged for 90 min. at 100,000 x g to yield a pellet and a
supernatant.
Protein content was determined using the Lowry protein assay. The protein
concentration of this
microsomal preparation was approximately 23.2 mg/mL. To avoid enzyme activity
loss,
microsomes were stored in 4.0 or 6.0 mL aliquots at -80 C to avoid freeze thaw
cycling.
27
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
[0067] The following ingredients were added stepwise into a 257 mL Erlenmeyer
flask: 57.3
mg of NADP, 254 mg of Glucose-6-Phosphate, and 23.0 mg NADPH were added to 6.0
mL of
Phosphate Buffer (adjusted to pH 7.4). Then, 2.0 mL of 5.0 mM MgCI, and 6.0 mL
Glucose-6-
Phosphate Dehydrogenase (10 units/mL, available from CALBIOCHEM, San Diege,
CA, Cat.
No. 346774) were added to the solution. Finally, 10 mL of Phosphate Buffer (pH
7.4) was
added. A 6mL volume of dog liver microsoine, prepared as above, was added into
the flask,
followed by ISA247, and incubated at 37 C for 2 hours at 250 rpm in an
environmentally
controlled incubator/shaker. At 2 hours, the reaction was stopped by adding
500 L of 2M HCI.
[0068] Metabolites produced by this method were then extracted with an organic
solvent, and
further separated using high-pressure liquid chromatography (HPLC). The
metabolites were
further characterized by electrospray mass spectrometry (MS) and NMR. The
resulting
metabolite profile (data not shown) was similar to that from human whole
blood. However, the
diols from the dog microsome were inuch less abundant.
Example 3
ISA247 Metabolite Production by Biotransformation using Saccharopolyspora
erytliraea
[0069] This example illustrates a biotransforination system utilized
microorganisins containing
the microbial equivalent of human cytochrome P450 microsomal enzyme and a
mediuin suitable
for active growth of the microorganism. The parent compound, which is poorly
soluble in water,
was mixed with ethanol and a surfactant prior to addition to the
biotransforination system. In
this exainple, ISA247 in ethanol was mixed with TWEEN 40 and then added to a
biotransformation system containing Saccharopolyspora erytheraea (ATCC 11635).
[0070] Starting cultures were prepared as follows. Fifteen tubes (16 x 26 mm;
6 ml each) of
the ISP2 medium slants were prepared containing 4 g/L yeast extract, 10g/L
malt extract, 4g/L
dextrose and 2g/L agar. These ingredients were mixed in demineralized water up
to liter, pH
neutralized as needed to 7 with NaOH. The medium was sterilized for 30 min. at
100 C. The
tubes were stored at 4 C until use. Each slant was inoculated with
Saccharopolyspora erythraea
28
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
(ATCC number 11635). Innoculated slants were grown for three weeks under
sterile conditions
at room temperature.
[0071] Precultures were transferred to Phase I Media. Phase I Media were
prepared with 10
g/L dextrin, 1 g/L glucose, 3 g/L beef extract, 10 g/L yeast extract, 5 g/L
magnesium sulfate and
400 mg/L potassium phosphate. These ingredients were mixed in deionized water
up to 1 liter,
pH neutralized as needed to 7 with NaOH and 50 mL was aliquoted into each of
two baffled 250
mL culture flasks. The medium was sterilized for 30 min. at 100 C. 5 mL of
the media was
aliquoted into a slant tube containing Sacchar~opolyspora ef ythraea. The
cells were scraped off
the surface of the slant and 2.5 mL of the suspension was placed in each
flask. The flasks were
placed on a Labline Incubator at 27 C and shaken at 250 rpm for 3 days (72
hrs).
[0072] Saccharopolyspora erythraea was transferred to Phase II media from
Phase I media by
centrifuging the contents of a Phase I flask at 3300 rpm for 5 min. and
decanting off the
supernatant to obtain a pellet. 5 mL of Phase II media was added to the pellet
and the tube was
vortexed, then centrifuged at 3300 rpm for 4 min. Again the supernatant was
decanted. The
pellet was resuspended in Phase II media. The subsequent suspension was added
to 50 mL of
Phase II medium in a baffled culture flask.
[0073] Phase II Media contained 10 g/L glucose, 1 g/L yeast extract, 1 g/L
beef extract and
11.6 g/L of 3-N-morpholinopropanesulfonic acid (MOPS) buffer. These
ingredients were mixed
in deionized water to one liter; then 50 mL were dispensed into two baffled
culture flasks (250
mL). After adjustment to pH to 7.0 with 5M NaOH, the medium was autoclaved for
30 min. at
100 C, then cooled. TWEEN 40 was autoclaved before mixing with ISA247 and
ethanol.
[0074] ISA247 (4mg of -50150 mixture of E and Z isomers) was dissolved in 95%
ethanol (0.1
ml), then mixed with 0.4 ml TWEEN 40 (polyoxyethylene sorbitan monopalmitate;
Cat. No.
P1504. Sigma-Aldrich, St. Louis, MO) The parent compound-surfactant mixture
was then added
to Saccharopolyspora efythYaea in the Phase II culture medium. A zero time
sample was
obtained and frozen. Each flask was then capped and placed on an Innova
Incubator at 27 C and
incubated for 120 hrs with shaking at 170 rpm.
29
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
[0075] A second sample was obtained from the Phase II culture medium. The zero
time
sample and the second sample were extracted using tert-butyl-methyl ether
(cat. No. 7001-2;
Caledon). The extracted metabolites were reconstituted in methanol (HPLC
grade) and analyzed
by LC-MS as previously described. As shown in Figure 4, the metabolite profile
obtained by
this method is similar to that obtained from human whole blood (see Example
1). When the
biotransformation mixture was analyzed, seven metabolite compounds were found
to be present:
IM4n (ISA247 Metabolite that is N-demethylated at amino acid-4), IM9 (ISA247
Metabolite that
is hydroxylated at amino acid-9), IM4 (ISA247 Metabolite that is hydroxylated
at amino acid-4),
IM1-c-1(See table 1), IMl-d-1 (Table 1), IM1-d-2 (Table 1) and IM1-d-3 (Table
1). Therefore,
seven out of eight ISA247 metabolites revealed in human blood were produced in
this
biotransformation systein.
EXAMPLE 4
Effect of Varying the Surfactant in Biotransformation using
Saccharopolyspora erythraea
[0076] To test the effects of different surfactants, actively growing Phase II
cultures of
Sacchar opolyspora erythraea (ATCC number 11635) were prepared as above. Seven
tubes each
containing ISA247 (56 mg; 50/50 mixture of E- and Z-isomers) in ethanol (0.33
ml) were
prepared. A surfactant (0.67 ml/tube) was added to each tube as follows:
Tube 1 - PEG 400 (polyethylene glycol 400; Carbowax - Fisher Scientific,
FairLonn NJ);
Tube 2 - castor oil (Wiler Fine Chemicals Ltd, London Ont.);
Tube 3 - isopropyl myristate (Wiler Fine Chemicals Ltd, London Ont.);
Tube 4- glycerine (BDH Fine Chemicals, Toronto Ont. Lot # 120343/73865);
Tube 5- Cremophor EL (Sigma Chemical, St Louis MO);
Tube 6 - Labrasol (Gatte Fosse, Cedex France); and,
Tube 7- TWEEN 40 (Aldrich Chemical Company Inc., Milwaukee Wis.).
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
[0077] The parent compound-surfactant mixture was added to the actively
growing culture of
Saccharopolyspora and a zero time sample was taken. After incubation with
shaking at 27 C for
days, samples were obtained, extracted, and the metabolites were quantified as
described in
Example 4. Area under the curve of HPLC peaks, similar to those shown in
Figures 3 and 4, was
5 measured as an indication of the quantity of metabolite present. The HPLC
peaks corresponded
to one N-demethylated metabolite, which was identified as IM4n; two
hydroxylated metabolites
which were identified as IM4 and IM9; one cyclic metabolite identified as IM 1-
c-1; and three
diol metabolites, diols formed at the 1 amino acid of the ISA247 compound,
identified as IMl-d-
1, IM1-d-2 and IM1-d-3 (See Table 1). The seven surfactants were not all
equivalent in their
activity in increasing the production of metabolites in the biotransformation
preparation. As
shown in Figure 5, the addition of glycerine or PEG 400 to the
biotransformation preparation
resulted in significant increases in the quantity of metabolites produced.
However, the addition
of castor oil, isopropyl myristate, CremophorRO, Labrasol and TWEEN 40 all
resulted in an
increase in the production of metabolites in the biotransformation
preparation, over the
production of metabolites in the preparation without surfactant (not shown).
EXAMPLE 5
Biotransformation Using Various Microorganisms
[0078] A variety of microorganisms were evaluated for production of ISA247
metabolites
from ISA247, including Curvularia lunata (University of Alberta Microfungal
Collection and
Herbarium (UAMH) 9191; ATCC 12017), Cunninghamella echinulata var. elegans
(UAMH
7370; ATCC 36112), Curvularia echinulata var. blakesleena (UAMH 8718; ATCC
8688a),
Cunninghamella echinulata var. elegans (UAMH 7369; ATCC 26269), Beauvaria
bassiana
(UAMH 8717; ATCC 7159), Actinomycetes (ATCC 53828), Actinoplanes sp. (ATCC
53771),
CunnitzghaJnella echinulata (UAMH 4144; ATCC 36190), Cunninghamella echinulata
(UAMH
7368; ATCC 9246), Cunninglaamella bainiere (echinulata) (UAMH 4145; ATCC 9244)
and
Saccharopolyspora eiythraea (ATCC 11635).
[0079] These microorganisms were screened for metabolite conversion yield
(amount of
known ISA247 metabolites produced) as well as metabolic diversity (number of
different
31
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
ISA247 metabolites produced). The microorganisms were grown in Phase I and
incubated with
ISA247 in Phase II. After the addition of ISA247 to the fermentation media,
samples were taken
from the media and analyzed with LC-MS against a huinan standard ISA247
metabolite profile
to identify and quantify the metabolites collected. After primary testing of
each strain via 96
hour biotransformation cycles, the two strains with the highest combination of
metabolite
conversion and metabolic diversity were tested again in Phase III with
different media
compositions, in order to select improved media compositions.
Phase I/Phase II methods
[0080] Each microorganism tested was maintained on culture-specific agar
slants. All slants
were prepared one month in advance to avoid contamination. Prepared agar media
were
autoclaved at 123 C and partial pressure of 360 mmHg for 58 minutes, cooled
slightly, and 6 mL
was pipetted into sterile 16x125 mm culture tubes. After placing the agar into
the tube, the tube
was rested on an incline to create a slant, cooled until the agar set,
labeled, and incubated at 27 C
for 1-2 weeks. ATCC 11635 and ATCC 53771 were sporulated using ISP agar (0.4%
yeast
extract, 1% malt extract, 0.4% dextrose and 2% granulated agar). ATCC 53828,
UAMH 8717
and UAMH 8718 were sporulated using potato dextrose agar (PDA, 3.9% in
distilled water).
UAMH 4145, UAMH 7369, UAMH 7370 and UAMH 9191 were sporulated on cereal slants
(10%% mixed cereal, dry, preferably pabulum for infants; 2% granulated agar;
the cereal was
mixed before and after sterilization to prevent clumping of the cereal and
inadequate distribution
of agar in the slants). Following incubation for two weeks, each microorganism
was inoculated
onto the microorganism-specific agar and returned to 27 C. Once a full lawn of
colonies was
seen on the slants, the slants were preferably used immediately in Phase I or,
if necessary, stored
at 4 C.
[0051] Subsequently, 2mL of sterile Phase I media Phase I media (containing
ISP seed broth:
1% dextrin, 1% glucose, 0.27% beef extract, 1% yeast extract, 0.004% magnesium
sulfate, and
0.036% potassium diphosphate, at pH 7.0) was added to a source slant
containing the '
microorganism to be tested. Using a sterile inoculating loop, the colonies
were removed,
vortexed and then the resulting suspension was added to 50 mL of sterile Phase
I media,
32
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
contained in a sterile, 250-mL baffled culture flask. Each flask was incubated
for 96 hours in to
increase biomass before the addition of ISA247 in Phase II.
[0082] To prepare biomass for transfer to Phase II, the cells were washed
thoroughly to
remove Phase I residue. Phase I contents were aseptically transferred into a
50-mL conical
centrifuge tube, centrifuged at 3300 rpm for five minutes, and decanted to
remove supematant.
The cells were washed with 5mL of excess Phase II media (3.65% MOPS, 0.31%
yeast extract,
3.14% glucose and 0.31% beef extract, at pH 7.0) and centrifuged again for
five minutes at 3300
rpm, after which the supernatant was decanted and 5mL of fresh Phase II media
was added. The
resulting mixture was vortexed thoroughly and quantitatively transferred under
aseptic
conditions to 50 mL of sterile Phase II media in a 250mL baffled culture
flask. A portion of
ISA247 (0.5 mL, 56 mg/mL of ISA247 that was predominantly in trans forin in
33.75% ethanol
(95%):66.25% glycerol) was added to the media and the mixture was shaken
vigorously. Sample
aliquots were taken (0.5mL, 12 hour increments over 96 hours of Phase II
fermentation) and
stored at -80 C until LC-MS analysis. Fermentation was concluded after 96
hours.
Phase III
[0083] In Phase ITI, ATCC 53771 and ATCC 11635 strains were incubated for 96
hours in
Media C (2% glucose, 2% starch, 0.5% yeast extract, 2% soybean protein, 0.32%
CaCO3, 0.25%
NaCI) as seed broth and then aseptically transferred in separate replicate
trials to either Media 3
(2% glycerol, 0.5% peptone, 0.5% yeast extract, 0.2% beef extract, 0.1%
(NH4)2HP04, 0.1%
CaCO3, 0.3% NaCl, 0.03% MgSO4-7H2O, at pH 7.0) or Media 16 (2% glucose, 1%
glycerin, 1%
peptone, 1% meat extract, 2% soybean protein, 0.5% CaCO3, 0.5% NaCI, at pH
7.0). Care was
exercised when taking samples for LC-MS due to the viscous-consistency of the
fennentation
media. -
Table 5: Summary of the media and agar used in Example 5
ATCC ATCC ATCC UAMH UAMH UAMH UAMH UAMH UAMH
11635 53771 53828 4145 7369 7370 8717 8718 9191
Media ISP 2 ISP 2 ISP 2 ISP 2 ISP 2 ISP 2 ISP 2 ISP 2 ISP2
Media 3 Media 3
33
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
ATCC ATCC ATCC UAMH UAMH UAMH UAMH UAMH UAMH
11635 53771 53828 4145 7369 7370 8717 8718 9191
Media Media
16 16
Agar ISP ISP PDA CER CER CER PDA PDA CER
Metabolite analysis by LC-MS
[0084] Samples were thawed from storage at -80 C and 16x10 mm culture tubes
were labeled
to represent the samples to be analyzed. A 200 L aliquot was removed from
each 0.5 mL
sample, and 25uL of a 1 mg/mL solution of CsA (Cyclosporine A) was added as an
internal
standard. 2mL of HPLC-grade methanol was added to each sample and the samples
were capped
and shaken for twenty minutes. The samples were centrifuged at 3300 rpm for
lminute and
45seconds. The supematant was decanted into clean, labeled 16x10 mm culture
tubes and
vacuum concentrated to remove organic solvent. The dried layer, containing
botli the
metabolites and parent drug was re-constituted in 200 L of HPLC-grade
methanol, and the
samples were quantitatively transferred to auto sampler vials. Samples were
run for 15 minutes
in deionized water with 0.01 % acetic acid/sodium acetate, starting with a 12
minute gradient of
increasing m-TBE (methyl tert-butyl ether) and HPLC grade methanol.
[0085] Figure 6 is a graph of mass spec signal versus retention time for
typical metabolites
from a sample pooled from human participants. Table 6 summarizes the ion
masses found,
corresponding quantifiable ISA247 metabolites and approximate retention times.
Ion masses
quantified included 1223, 1237, 1239, 1253, 1255, 1267 and 1271. Note that two
diols (IM1-d-1
and IMl-d-4) were detected here, whereas three diols (IM1-d-1, IM1-d-2, and
IMl-d-3) were
detected in Example.1. The reason for this discrepancy is that the trans-form
of ISA247 is
metabolized to IM1-d-1 and IM1-d-4, while the cis-form of ISA247 is
metabolized to IMl-d-2
and IM1-d-3. Since the parent compound used in Example 1 was a 50:50 mixture
of cis:trans,
and the parent compound in this Example was predominantly trans-ISA247, the
metabolite
profiles were different with respect to the diols.
Table 6
34
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
Ion Mass Metabolite from ISA247 Approximate Retention Time (min)
1223 IM4n 9.789
1237 ISA247 10.206
1239 1239 8.340
1253 IM1-c-1 ; IM9; IM4 8.575;8.939;9.440
1255 1255 8.899
1267 CSA(Internal Standard) 10.678
1271 IM1-d-1; IM1-d-4 7.535; 8.166
[0086] The relative % conversion was calculated from the ratio of the area
under the curve
(AUC) of the peak for each quantifiable metabolite detected and the area under
the curve of the
Internal CsA standard using the following equation:
% Conversion = (Metabolite AUC)/ (CsA Intennal Standard AUC) x 100
Results
[0087] The metabolic diversity after 96 hours of biotransformation is
summarized in Table 7.
A check mark indicates a quantifiable amount of metabolite was produced. Table
8 lists the
amount of each metabolite produced in each microorganism tested.
Table 7
Metabolite ATCC UAMH ATCC ATCC UAMH UAMH UAMH UAMH UAMH
11635 4145 53771 53828 7369 7370 8717 8718 9191
IM1-d-1
IM1-cl-4
1239
1255
IM4n
IM1-c-1
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
Metabolite ATCC UAMH ATCC ATCC UAMH UAMH UAMH UAMH UAMH
11635 4145 53771 53828 7369 7370 8717 8718 9191
IM9
IM4
Rank 1 st 4th 3rd 9th 8th 7th 6th 5th 2nd
Table 8(All weights in nanograms)
Metabolite ATCC UAMH ATCC ATCC UAMH UAMH UAMH UAMH UAMH
11635 4145 53771 53828 7369 7370 8717 8718 9191
IM1-d-1 1120.3 <1.00 <1.00 <1.00 <1.00 <1.00 0.00 <1.00 13.96
IM 1-d-4 4.38 0.00 0.00 0.00 0.00 0.00 2.65 0.00 1.11
1239 6.38 <1.00 <1.00 <1.00 <1.00 <1.00 9.42 <1.00 2.44
1255 23.11 0.00 <1.00 0.00 0.00 0.00 <1.00 0.00 0.00
IM4n 22.02 <1.00 20.99 1.51 0.00 0.00 0.00 <1.00 7.01
IM1-c-1 71.80 <1.00 3.175 <1.00 <1.00 <1.00 0.00 <1.00 6.78
1M9 63.22 <1.00 0.00 <1.00 <1.00 <1.00 0.00 <1.00 11.69
IM4 35.40 <1.00 0.00 1.18 0.00 <1.00 0.00 <1.00 1.66
[0088] Thus, ainong the microorganisms examined in this experiment, ATCC 11635
displayed
the greatest percent conversion and the greatest metabolic diversity. Eight
known human
ISA247 metabolites were detected in ATCC 11635 sainples. UAMH 4145 produced
six of the
eight metabolites. ATCC 53771, often used in the lab because of its inherent
ability to generate
large amounts of IM4n (6.66%), produces five of the eight human metabolites.
ATCC 53828
produced four of the eight metabolites; although each of these metabolites was
produced in small
quantities, the rare metabolite 1239 was produced. UAMH 7369 and UAMH 7370
each
produced four of the metabolites. UAMH 9191 and UAMH 8718 each produced six
metabolites. UAMH 8717 produced three metabolites.
[0089] Another factor considered in microorganism selection was the presence
of "rare"
metabolites of ISA247. "Rare" is defined as metabolites that are not produced
in large amounts
by ATCC 11635, e.g., IM1-d-4, 1239 and 1255. IM1-d-4 was present in ATCC
11635, UAMH
36
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
8717 and UAMH 9191, but was produced in the greatest quantity by ATCC 11635.
The
microbial strains ATCC 11635, ATCC 53771, ATCC 53828, UAMH 7370, UAMH 8717,
UAMH 8718, UAMH 9191 all produced 1239. The microorganism that produced the
greatest
quantity was UAMH 8717. The metabolite corresponding to ion 1255 was
manufactured by
ATCC 11635, UAMH 4145, ATCC 53771, UAMH 8717, with the greatest conversion by
ATCC
11635.
[0090] In Phase III of this experiment, ATCC 11635 and ATCC 53771 were
cultured in Media
3 and Media 16, and the effects of the media compared. Figure 7 shows the
results for ATCC
11635. Media 3 and Media 16 produced similar amounts of each metabolite except
for IM1-d-1,
IM 1-d-4 and IM 1-c-1. IM 1-d-1 production decreased with Media 3 and was not
present in
detectable levels with Media 16. IM1-d-4 production decreased with both Media
3 and Media
16. IM1-c-1 production increased 10% with Media 3. Figure 8 is a graph of the
effect of media
composition on the production of ISA247 metabolites in ATCC 53771. IM1-d-1 was
detected
only when using ISP2 media and IM9 and IM4 were only detected when using Media
3 and
Media 16. Most of the metabolites, with the exception of IM1-d-4 were
increased in quantity by
using Media 3 and Media 16. Therefore, the growth media of the microorganisms
can be altered
to optimize the effect of biotransformation.
EXAMPLE 6
Effect of surfactants and solvents in biotransformation using Beauvaria
bassiana
[0091] In Example 5, Beauvaria bassiana (UAMH 8717) only weakly produced
ISA247
metabolites. To test if different surfactants or solvents can change the
production profile,
dimethyl sulfoxide (DMSO) and TWEEN 40 were coinpared to glycerol (used in
Example 5).
0.5 mL of a 56 mg/mL ISA247 solution (in 33.75% ethanol (95%):66.25% DMSO or
TWEEN
40 instead of glycerol) was added into 50 mL of media. The same Phase I and
Phase II media
and procedure of Example were followed.
[0092] As shown in Figure 9, the use of glycerol as a surfactant led to the
formation of IMl-d-
4, 1239, and 1255; DMSO led to the formation of IM1-d-1, IM4n and IM4; and
TWEENO 40
resulted in IMl-d-1, IM1-d-4, 1255, IM4n, IM9 and IM4. The ainount of
conversion was also
dramatically changed. Consequently, biotransformation results can be optimized
by changing
37
CA 02591781 2007-06-20
WO 2006/066416 PCT/CA2005/001972
the solvents or surfactants used to deliver the parent compound, and a skilled
artisan can choose
a certain solvent, surfactant and media to increase the production of a given
metabolite of
combination of metabolites.
[0093] All of the publications, patents and patent applications cited in this
application are
herein incorporated by reference in their entirety to the same extent as if
the disclosure of each
individual publication, patent application or patent was specifically and
individually indicated to
be incorporated by reference in its entirety.
[0094] A number of einbodiments of the invention have been described.
Nevertheless, it will
be understood that various modifications may be made without departing from
the spirit and
scope of the invention.
38