Note: Descriptions are shown in the official language in which they were submitted.
CA 02593367 2007-07-17
- 1 -
AUTOMATED MICROBIOLOGICAL TESTING
APPARATUS AND METHODS THEREFOR
BACKGROUND OF THE INVENTION
This invention relates to the field of microbiological
testing.
Many conventional systems exist for performing tests on
microbiological samples related to patient diagnosis
and therapy. The microorganism samples may come from a
variety of sources, including infected wounds, genital
infections, cerebro-spinal fluids, blood and abscesses.
From those microorganism samples an inoculum is
prepared in accordance with established procedures
which produce a bacterial or cellular suspension of a
predetermined concentration. Further processing of the
suspension may depend on the testing method employed.
CA 02593367 2007-07-17
- 2 -
These systems are used, for example, for identification
of which microorganisms are present in a patient's
sample. Typically, in such systems, reagents are
placed into cupules, or test wells, of identification
trays, which in the presence of an actively growing
culture of microorganisms change color. Based on the
color change, or lack thereof, the microorganism can be
identified by the use of reference tables.
Other systems have been developed for susceptibility
testing of microorganisms. These systems are used to
determine the susceptibility of a microorganism in a
sample to various therapeutics, such as antibiotics.
Based on these test results, physicians can then, for
example, prescribe an antimicrobial product which will
be successful in killing or inhibiting the
microorganism. In particular, qualitative
susceptibility testing produces an indication of
whether a microorganism is resistant or sensitive to a
particular antibiotic, but does not provide an
indication on the degree of sensitivity or resistance
of the microorganism. On the other hand, quantitative
susceptibility testing, provides an indication of the
concentration of the antimicrobial agent needed to
inhibit growth of the microorganism. The term minimum
inhibitory concentration (MIC) is used to refer to the
minimum concentration of the antimicrobial agent that
is required to inhibit the growth of the microorganism.
The systems have certain drawbacks. For example, when
performing identification and susceptibility testing,
the test trays are incubated at a controlled
temperature for an extended period of time. At
predetermined time intervals, the wells of the test
trays are individually examined for an indication of
color change or other test criteria. This can be a
long and tedious process if done manually by a
CA 02593367 2007-07-17
- 3 -
technician. In addition, the incubation times for
identification and susceptibility test trays may
differ, or the optimal time to read a test result from
the test tray may not be known in advance. Thus, a
technician would need to read and record results for a
specimen at several different times, sometimes long
apart, which may cause assignment or correlation
errors.
Automated systems are desirable in performing these
tests to minimize the technician handling time, as well
as to minimize the possibility of human error. In
addition, automated systems that obtain results rapidly
and accurately are preferred.
In this regard, a microbiological testing apparatus for
the automatic incubation and reading of microbiological
test trays is known. The test trays of this apparatus
have a plurality of wells which contain the samples or
agents to be tested. The trays are first placed in an
incubator for a predetermined amount of time. The test
trays are then moved to an inspection station. A light
source is disposed above the tray and a pair of video
cameras are disposed below the tray at the inspection
station. Each video camera takes a video image of an
entire tray. The video image signal of the entire tray
is sent to an image processor to be analyzed.
The image processor requires uniform lighting over the
inspection station. Consequently, the processor
records the background light level of each pixel within
an area of interest corresponding to each well of the
tray to account for variability in the light source.
The image processor processes the video image of the
tray and determines the number of pixels, for a
particular well, whose intensity exceeds a
predetermined threshold for that area of interest. if
CA 02593367 2007-07-17
- 4 -
the number of pixels exceeds a predetermined number, a
positive result is assigned to that well. The image
processor analyzes the binary partial results from the
wells to determine the possible identity of the
microorganisms. The binary partial results are
compared to prerecorded patterns of results for each
type of test tray to identify the sample in question.
A microbiological testing apparatus for detecting the
presence of a fluorescence emitting reaction resulting
from the interaction of a reacting agent and a sample
for detection, susceptibility, and identification
testing, is also known. In this apparatus, multiple
trays having a plurality of test chambers are contained
within a carousel. This carousel is rotated to move
one of the trays close to a detection area. A
positioning mechanism radially then moves that tray out
of the carousel and into the detection area. A high-
energy light source is disposed proximately to the thus
positioned tray. The light source provides narrow-band
light sufficient to produce an emission fluorescence
from the reaction within test chambers, which in turn
is detected by a video mechanism disposed opposite to
the light source and behind the positioned tray. The
video mechanism produces an image based on the emission
wavelength.
Another test system is known for identifying bacteria
using signals based on the intensity of monochromatic
light reflected from specimens placed in a culture
plate having a plurality of cells. A rotary disk
containing six interference filters is interposed
between a lamp and a group of optical fibers. The
light from the lamp passes through a particular
interference filter to produce monochromatic light of a
certain wavelength. The filtered monochromatic light
is guided by the optical fibers to be incident on
CA 02593367 2007-07-17
-
respective cells of the culture plate. The disk is
rotated so that the six different wavelength
monochromatic lights are caused to be incident on the
cells sequentially. The light reflected from the
5 specimens is guided by additional optical fibers to
corresponding phototransistors. A signal is derived
for each specimen based on the intensity of the
reflected monochromatic light. These signals are then
analyzed to determine the identity of the specimen by
calculating the difference, or ratio, between the
signals and comparing that result with a reference
value.
However, the above-described apparatuses fail to
address all the requirements of a fully automated
microbiological testing system. In particular, they
are not capable of simultaneously performing both
colorimetric- and fluorometric-type testing on
multiple-well test panels that is needed to obtain more
accurate test results. Further, these apparatuses are
generally not designed to continuously gather test data
from a plurality of multiple-well test panels in a
quick and reliable manner. Moreover, the automated
processing of these systems is limited-
SUMMP.RY OF THE INVENTION
The present invention provides a system that overcomes
the above-described problems. In particular, the
present invention provides an automated microbiological
testing system that tests a plurality of multiple-well
test panels, for identification and susceptibility,
with a minimal amount of human intervention during the
testing process. In addition, this system performs
both colorimetric- and fluorometric-type testing.
Moreover, this system quickly analyzes the gathered
CA 02593367 2007-07-17
- 6 -
test data to produce accurate identification and/or
susceptibility testing results.
In particular, one aspect of the present invention is
directed to a diagnostic microbiological testing
apparatus that has a carousel assembly on which is
mounted a plurality of test panels. Each test panel
has a plurality of wells, each of which is inoculated
with a test inoculum fluid for producing a reaction. A
plurality of light sources direct light of a
predetermined range of wavelengths toward the wells of
the test panels to cause the wells to emit or absorb
light based on the reaction of the test inoculum fluid.
A light detection unit, which may include a linear CCD,
is disposed opposite to the light sources with at least
one test panel being positioned between the light
sources and the light detection unit. The light
detection unit detects the light emitted from, or
absorbed by, the wells of the test panels as the
carousel assembly continuously rotates each of the test
panels between the light sources and the light
detection unit to permit light emitted from, or
absorbed by, the wells of the test panels to be
detected by the light detection unit. A controller
receives a plurality of signals generated by the light
detection unit, which correspond, respectively, to the
light, which can be fluorescent or non-fluorescent,
detected from each well. The controller then
determines a test result for each well based on the
received signals.
In another aspect of the present invention, an
incubation chamber for a diagnostic microbiological
testing apparatus is provided. This chamber includes a
carousel assembly on which is mounted a plurality of
test panels, each test panel having a plurality of
wells for receiving a test inoculum fluid for producing
CA 02593367 2007-07-17
- 7 -
a reaction. An enclosure surrounding the carousel
assembly prevents intrusion of ambient light into the
incubation chamber. The enclosure has a door for
providing access to carousel assembly. A drive system
continuously rotates the carousel assembly to directly
position the test panels for testing by the diagnostic
microbiological testing apparatus. A heating unit
heats the incubation chamber and a temperature
controller controls the heating unit to maintain the
temperature within a predetermined temperature range.
In yet another aspect of the present invention, methods
of operating, and computer mediums which include
instructions for controlling, a diagnostic
microbiological testing apparatus are provided. For
example, one method includes the steps of (a) rotating
a carousel of the testing apparatus to position a test
panel mounted thereon between a light source and a
light detection unit of the testing apparatus, (b)
directing light from the light source toward the test
panels, (c) detecting with the light detection unit the
light transmitted or emitted from, or absorbed by, each
of the wells of the test panels due to the test
reaction, (d) generating with the light detection unit
a signal corresponding to the light detected from each
of the wells, and (e) determining a test result for
each of the wells based on the generated signal.
In yet another aspect of the present invention, an
apparatus is provided including a light source capable
of producing a composite light signal having light
elements of variable intensity, and a controller
adapted to control the light source using an
illumination profile. The apparatus may also include a
light detection unit, and an optics system capable of
directing the composite light signal toward the light
detection unit. The illumination profile may be used
CA 02593367 2007-07-17
- 8 -
to correct optical inefficiency in the optics system or
changes in the illumination output of the light source_
In yet another aspect of the present invention, a light
source including a plurality of LEDs arranged in a
linear array is provided. The junction current of each
LED is controllable to produce a predetermined
illumination profile.
In yet another aspect of the present invention, a light
source including a plurality of LEDs arranged in a
linear array having two ends, each end having a group
of LEDs of the plurality of LEDs. The group of LEDs is
geometrically compressed to produce a greater intensity
of light. The LEDs may include red, green and blue
LEDs arranged in a predetermined order in the linear
array.
In one further aspect of the present invention, an
optics system is provided for a microbiological testing
apparatus.
BRIEF DESCRIPTION OF THE DRAWINGS
These and other aspects, features and advantages of the
present invention can best be understood by reference
to the detailed description of the preferred
embodiments set forth below taken with the drawings in
which:
FIG. 1 is a front perspective view of the test
apparatus of the present invention with the enclosure
door closed.
CA 02593367 2007-07-17
- 9 -
FIG. 2 is a front perspective view of the test
apparatus of the present invention with the enclosure
door open.
FIG. 3A is a perspective view of an ID/AST test panel
of the present invention.
FIG. 3B is a top view of an ID/AST test panel of the
present invention.
FIG. 3C is a bottom view of an ID/AST test panel of the
present invention.
FIG. 4 is a schematic top view of the internal
components of the apparatus of FIG. 1.
FIG. 5 is a schematic perspective view of the carousel
assembly of the present invention.
FIG. 6 is a perspective view of the panel carrier of
the present invention.
FIG. 7 is a schematic perspective view of the
measurement system tower of the present invention.
FIG. 8 is a schematic perspective view of the CCD
detection module of the present invention.
FIG. 9 shows one embodiment in which solid-state LEDs
and dichroic color separation filters are used.
FIGS. 10A and lOB are respectively front and side views
of another embodiment in which surface mount LEDs and
color separation filters are used.
FIG. 11 is a schematic view of the configuration of the
light source assemblies of the present invention.
CA 02593367 2007-07-17
- 10 -
FIG. 12A shows a graph of an illumination output from a
light source module.
FIG. 12B shows graph of a illumination profile used to
drive the light source module of the present invention.
FIG. 12C shows a graph of an illumination output from
the light source module of the present invention
resulting from the illumination profile of FIG. 12B.
FIG. 13 shows a circuit for controlling a light source
module of the present invention.
FIG. 14 shows one embodiment of a light source module
of the present invention.
FIG. 15 is a schematic perspective view of a portion of
the panel carrier and test apparatus of the present
invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention provides a system and method for
performing highly reliable microorganism identification
(ID) and antimicrobial susceptibility determinations
(AST). The present invention determines identification
and susceptibility based on readings from wells 31
contained in ID/AST panels 30 (see FIGS. 3A and 3B).
For example, in one embodiment, the wells 31 contain
different reagent substrates and/or different
antimicrobic dilutions which change optical character
sometime after being inoculated with the organism. The
detection method described below measures changes in
absorption, scattering, and/or fluorescence. It may
also measure luminescence. These changes are processed
CA 02593367 2007-07-17
- 11 -
to determine the identification and susceptibility of
the microorganism.
The present invention allows a technician, for example,
after having inoculated the wells 31 of the ID/AST
panel 30 with an unknown microorganism, to place that
panel into an instrument 20 (shown in FIG. 1) where it
is incubated at a set temperature, periodically
interrogated for changes and analyzed for microorganism
identification and antimicrobic susceptibility. The
apparatus 20 holds a plurality of ID/AST panels 30 and
provides positivity analysis results to the technician,
as described below.
As shown in FIGS. 3A-3C, the ID/AST panels 30 are
disposable devices which are inoculated with reagents
needed for both ID and AST testing. The testing is
performed on reactions generated by the samples and
reagents placed in individual wells 31 on each ID/AST
panel 30. The wells 31 are arranged on the ID/AST
panels 30 as a two-dimensional array having rows and
columns.
The instrument 20 is self-contained and sufficiently
autonomous to test the ID/AST panels 30 and supply the
appropriate test results. The instrument 20 stores,
incubates and reads the ID/AST panels 30. The
instrument 20 has a door 21 shown closed in FIG. 1 and
open in FIG. 2 to allow for access to the interior of
the instrument 20.
In one embodiment, as also shown in FIG. 1, a personal
computer (PC) workstation 40 is communicatively
connected to the instrument 20. The PC workstation
complements the instrument's 20 microbiology
information system reporting and data management
features, which are discussed below. The PC
CA 02593367 2007-07-17
- 12 -
workstation 40 provides tools to improve empiric
therapy decision and identify therapy intervention
instances. The PC workstation 40 also incorporates
reporting tools to assist infection control and
epidemiology.
Additionally, the PC Workstation 40 incorporates a
relational database (not shown) on a hard drive.
Finalized AST and ID test results are retained in the
database for a minimum of 52 weeks. Statistically
summarized data is retained for a greater time period.
Patient and specimen information may be gathered
through an electronic interface with the instrument 20
(not shown) or manually entered into the PC workstation
40.
The instrument 20 includes a carousel 50, as shown in
FIG. 2. The carousel 50 includes an assembly 51
comprised of rings and ribs bolted to a drive ring 52
to form a cylindrical cage as shown in FIG. 5. The
carousel 50 is mounted vertically in an instrument
enclosure 60 (shown in FIG. 1). The instrument
enclosure 60 defines the carousel compartment 61 and an
electronics compartment 62 (shown in FIG. 4). The
carousel compartment 61 is insulated to provide a
substantially uniform temperature incubation
environment, and is light-tight under normal operation
to prevent ambient light from entering.
Panel carriers 53 (shown in FIG. 6) are mounted in the
assembly 51 which form four horizontal tiers with each
tier having twenty-six panel positions. A total of
one-hundred and four panel positions are provided. Of
course, these numbers of tiers and panel positions are
merely an example and may be changed to suit the
requirements of any specified application as will be
appreciated by one skilled in the art. The panel
CA 02593367 2007-07-17
- 13 -
carriers 53 are used to mount the ID/AST panels 30 as
well as other types of panels discussed below. The
panel carriers 53 are designed such that improperly
seated panels will not be retained by the panel
carriers 53. When the ID/AST panels 30 are mounted in
the four tiers of assembly 51, they are arranged to
form substantially circular rows and vertical columns
of wells 31. Within each tier, panel positions are
numbered zero through twenty-five. Panel position zero
is reserved for a normalization panel and is not
accessible by an operator during normal operation of
the instrument 20.
As shown in Fig. 15, indicator LEDs 54 are used to
indicate which ID/AST panels 30 should be removed
(i.e., when testing is complete), and which panel
positions are available for new, untested ID/AST panels
30. The indicator LEDs 54 may be located in front of
or behind each panel carrier 53. For example, as shown
in Fig. 15, the indicator LED 54 is mounted in a
printed circuit board 57 behind the panel carrier 53,
which is positioned against a carousel rib 58. A light
guide 59 may also be used to focus the light from the
indicator LEDs 54 through a convex indicator surface.
The indicator LED 54 may be a three-color LED wherein
different colors are used to indicate status/testing
information. For example, red may indicate that a test
is in progress; green may indicate that testing is
complete; and yellow may indicate that a panel position
is available for a new, untested ID/AST panel 30.
The carousel 50 also includes a drive system 56. The
drive system 56 is mounted within the instrument
enclosure 60 and exterior to the cylindrical cage
formed by the assembly 51, as shown in FIG. 4. The
drive system 56 drives the assembly 51, via the drive
CA 02593367 2007-07-17
- 14 -
ring 52, at a predetermined and controllable angular
velocity. One complete rotation of the carousel 50 is
used to acquire and accumulate test data from only
light frequency from each ID/AST panel 30 mounted
within the assembly 51 (i.e., one data accumulation
cycle).
A precision stepper motor is preferably used to provide
accurate rotational control of the assembly 51. Of
course, other types of motors can used which include
servo-motors, synchronous motors and DC motors, for
example.
An oil-treated felt pad is disposed against the drive
ring 52 to ensure it remains properly lubricated. A
poly-alpha-olefin oil, or similar oil, may be used to
minimize oil spray and migration. In a preferred
embodiment, a lubrication free bearing system can be
used.
A home position flag magnet is affixed to the inner
surface of the drive ring 52 corresponding to position
zero of the assembly 51. As the assembly 51 rotates, a
signal is generated by a Hall-effect sensor 55 mounted
within the carousel 50 each time the home position flag
magnet passes. This signal is used by the instrument
20 to keep track of the panel position as the assembly
51 is rotated. Of course, other types of sensors may
be used for this purpose. For example, infrared and
optical sensors may be used instead.
The temperature within the carousel compartment 61 is
tightly controlled by means of an incubation heater,
blower, and associated ductwork (none of which are
shown) which distribute and recirculate the incubation
air. The incubation heater includes one or more
CA 02593367 2007-07-17
- 15 -
sensors 63 (shown in FIG. 11) to monitor the
temperature within the carousel compartment 61.
= The incubation heater includes two heating elements
wired in a three lead arrangement (not shown). An
auto-resetting thermal circuit breaker is provided in
the third, common lead to protect against heater
over-temperature conditions. Should the carousel
compartment 61 temperature rise above a first
predetermined set point, power to the heat is
interrupted. Power is reapplied when the temperature
falls below a second predetermined set-point. The
power supplied to the heater is controlled by a control
processor 70.
Preferably, the carousel compartment 61 is continuously
maintained at a temperature of 35 C with the first and
second predetermined set points being set at 39 C and
33 C, respectively. However, as will be appreciated by
one skilled in the art, other temperature settings may
be used to achieve the particular testing requirements.
In one embodiment, four barcode scanners (not shown)
are mounted on a scanner tower (not shown) located
within the carousel compartment 61, either within or
exterior to the circumference of the assembly 51. One
barcode scanner is provided for each tier of the
assembly 51. The barcode scanners are capable of
reading barcoded labels (not shown) affixed to each
ID/AST panel 30 as the panels are rotated via the
assembly 51. The barcode scanners are supported in
proper relation to the ID/AST panels 30 mounted on the
assembly 51 and are held at a proper scanning distance
by the scanner tower.
The information read by the barcode scanners is used by
the instrument 20 to correlate specific panel sequence
CA 02593367 2007-07-17
- 16 -
numbers to test data gathered from the panels.
Preferably, the barcode scanners are capable of reading
Code-128 Numeric information. However, other known
conventional barcode formats may be used instead to
label the ID/AST panels 30.
In another embodiment, a barcode reader (not shown) is
installed behind the instrument front panel 71. The
barcode reader is used to scan barcode labels affixed
to either side of the ID/AST panels 30 before the
ID/AST panels 30 are mounted in the carousel
compartment 61. This allows, for example, the operator
to affix supplemental barcoded information on each
ID/AST panel 30. The supplemental barcoded information
could be, for example, a hospital-applied assession
label. In this embodiment, the barcoded labels can be
scanned, and the particular ID/AST panel 30 can then
turned over to scan the supplemental barcoded
information, this then links the ID/AST panel 30 to the
supplemental barcoded information. Conventional
barcode formats are supported by the barcode reader.
In another embodiment, a hand-held scanning barcode
wand 72, as shown in FIG. 1, is operatively connected
to the instrument 20. The barcode wand 72 may be used
in the same manner as the barcode reader (e.g., to scan
operator generated accession linkage, or to scan
barcodes too large to be affixed to the ID/AST panels
30). Conventional barcode formats are supported by the
barcode wand 72.
A panel position sensor for each tier is also mounted
on the scanner tower. Panel position flags, integrated
with the panel carriers 53, are read by the panel
position sensors. Upon scanning the leading edge of
the panel flag position flags, the panel position
CA 02593367 2007-07-17
- 17 -
sensors generate a signal used to provide test data
acquisition timing for each ID/AST panel 30.
= As shown in FIG. 4, a plurality of light source
assemblies are mounted within the carousel compartment
61 and exterior to the circumference of the assembly
51. In a preferred embodiment of the present
invention, the light source assemblies comprise a
visible light source assembly 80 and an Ultra-Violet
(UV) light source assembly 81 (shown in FIG. 11).
The visible light source assembly 80 includes four
visible light source modules and a supporting tower.
The supporting tower aligns one visible light source
modules with each tier of the assembly 51. At any
given time, one column of wells from the ID/AST panels
30 can be illuminated by the visible light source
modules.
In one embodiment, each visible light source module
includes three parallel vertical columns of sixteen
light-emitting diodes (LEDs) each. The first column
consists of red LEDs, the second green LEDs and the
third blue LEDs_ A holographic diffuser plate 82 is
located in close proximity to the ID/AST panels 30
mounted in the assembly 51. The holographic diffuser
plate 82 diffuse the illumination energy from each
column of LEDs (when energized). Each column of LEDs
is mounted in the visible light source modules to
maintain a fixed distance from the diffuser plate 82.
Cylindrical lenses may be used to focus the
illumination energy from each column of LEDs onto the
vertical well columns of the ID/AST panels 30. The
illumination axis for each column of LEDs is made
coincident for the red, green and blue illumination.
Thus, each well column sees a uniform stripe of either
CA 02593367 2007-07-17
- 18 -
red, green or blue illumination, depending upon which
column of LEDs is energized.
Each visible light source module may also be fitted
with a partially reflecting beamsplitter. The
beamsplitter would cause a portion of the illumination
energy from the LEDs to be incident on a source monitor
photodiode 84. The signal from the source monitor
photodiode 84 is then used to correct the light
intensity of each of the LED columns as necessary. For
example, the signal from the source monitor photodiode
84 may be used to compensate for fluctuations in
illumination output during LED warm-up at start time,
via an illumination profile discussed below. This
allows the instrument 20 to begin testing more quickly
because the testing would not have to wait for the LEDs
to warm-up (i.e., to reach a steady-state illumination
output).
The visible light source modules are spaced vertically
and positioned properly with respect to the ID/AST
panels 30 mounted in each tier of the assembly 51 by
the supporting tower. The supporting tower can also
include mounts for the beamsplitters, the holographic
diffuser plates 82 and the cylindrical lenses.
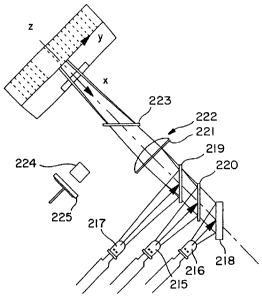
In another embodiment, shown in FIG. 9. other
arrangements are possible for the visible light source
used in the present invention. Figure 9 shows an
overhead view of a tri-spectrum arrangement (e.g., red,
green and blue) using three LEDs (215, 216, 217). A
bank of more or less than three LEDs can be used at any
single Z-axis location. Groups of the LED banks can be
stacked as deep as needed in the Z-axis direction so as
to cover the entire length of the ID/AST panels 30.
CA 02593367 2007-07-17
- 19 -
Light from the LED 216 is reflected 90 by a first
surface mirror 218 along the illumination axis. Some
of this light passes through the dichroic filters 220
and 219, diverging to a holographic diffuser 221 in the
X direction. Some of the light is rejected by each
filter and continues through in the Y direction. The
holographic diffuser 221 acts to homogenize the light
in a defined manner. The filtered, homogenized light
passes through a cylindrical lens 222 which
concentrates it into a homogenous light stripe of a
prescribed width at the ID/AST panel 30.
A portion of the light focused at the ID/AST panel 30
is redirected 90 by a planar glass optical flat 223
into a light pipe 224 that concentrates it to a source
monitor 225. A signal generated by the source monitor
225 is used to correct the light intensity from each of
the groups of the LED banks as necessary.
Similarly, some of the light from the LED 217 is
reflected 90 by the dichroic filter 219 and this
filtered energy is optically processed in the manner
described above. Again, some light from the LED 215 is
reflected 90 by dichroic filter 220 and this filtered
energy is optically processed by the remaining
components in the optical train as described above.
In another embodiment, a solid-state visible light
source assembly is shown in FIG 10A. A plurality of
surface mount LEDs 300 (SMLEDs) are placed in an array
which coincides with a column of wells 31 of the ID/AST
panels 30. The SMLEDs are arranged in a repeated
pattern in the array. For example, the first SMLED can
be red, the second a green SMLED and the third a blue
SMLED. This pattern is then repeated the length of the
array. As many banks of SMLEDs as needed can be
arranged to properly illuminate the area desired.
CA 02593367 2007-07-17
- 20 -
In this embodiment, the illumination axis of the array
of SMLEDs is arranged in the same line with the wells
31. Consequently, the SMLEDs 300 are energized based
on their respective spectral content (i.e., red, green
or blue illumination). As before, this light is
further conditioned to homogenize and concentrate it
onto the target using a holographic diffuser 301 and a
focusing lens 302, as shown in FIG. lOB. As described
above, a beamsplitter and source monitor are also used
in this embodiment.
Because the illumination intensity tends to fall off at
the ends, each column of LEDs, the holographic diffuser
plate 82, and cylindrical lenses can be made physically
longer than the active area of the ID/AST panels 30.
In order to compensate for the light fall-off at the
ID/AST panel 30 extremes caused by the optical
inefficiencies, the intensity of illumination near the
ends of each LED column is boosted to improve
uniformity. One way this may be accomplished is by
driving the LEDs near the ends of each column with
higher currents, which increases the intensity of the
light at those ends.
The junction current used to drive the LED or SMLEDs,
discussed above, can be controlled using a computer
program stored in the control processor 70, as shown in
Fig. 13. Illumination profiles can be used to
dynamically drive the LEDs to compensate for optical
inefficiency. As shown in FIG. 12A, when the LEDs of
one column are all driven with the same drive current,
the illumination output from the LEDs at the ends
(i.e., top and bottom) of the columns, as measured by
an optical detection system 100, is less than the
illumination output from the LEDs near the center of
the column. Figure 12B shows an illumination profile
in which the LEDs at the ends of the column are driven
CA 02593367 2007-07-17
- 21 -
with a higher current. This produces a greater
illumination output from the LEDs at the ends of the
column as compared to the LEDs near the center of the
column. FIG. 12C shows the resulting illumination
output, as measured by the optical detection system
100, of the LED column when driven by the illumination
profile of FIG. 12B.
While the illumination profile of FIG. 12B shows a
complementary profile used to produce a uniform
illumination output, a variety of illumination profiles
can be used. These profiles may be selected based
different criteria, such as, the type of test panel
used, the type of test to be performed, or feedback
signals. For example, a proportional feedback control
loop using signals from the source monitor photodiode
84 can correct for light intensity changes during
testing, during LED warm-up, or for long term
degradation of the LED junction current. Other types
of feedback correction systems may be based on
temperature changes within the instrument 20 or on
signals from a normalizer panel, discussed below.
Another way of compensating for the intensity fall-off
at the ends of the column is to geometrically compress
the spacing of LEDs or SMLEDs at the ends of each
column, i.e., a stacked LED configuration at the ends.
As shown in Fig. 15, LEDs 90 at the ends of the linear
array are geometrically compressed. This type of
configuration compensates for degradation of optical
efficiency at the ends of the columns. When the LEDs
are placed closer together, the intensity of the
illumination increases. Preferably, the LEDs should be
compressed to produce an intensity inverse to the fall-
off. For example, an optical coupling roll-off of 90t
at the column ends (relative to the column center) may
CA 02593367 2007-07-17
- 22 -
be compensated by decreasing the LED center-to-center
distance at the column ends by a factor of ten.
Returning to FIG. 11, the W Light Source assembly 81
includes two tubular UV cold-cathode lamps. Hot-
cathode lamps may also be used. Suitable lamps may be
obtained from Voltarc (VTI, Waterbury, CT 06705). The
radiation passes through excitation filters 85. The
excitation filters 85 eliminate unwanted spectral
components present in the output of the lamps.
As shown in FIG. 11, the lamps are disposed on either
side of the primary illumination axis so that one
column of vertically-aligned ID/AST panels 30 are
illuminated simultaneously. There is no need to align
the W light sources along the primary illumination
axis. Adjustment of the illumination intensity is
performed by altering the high-frequency power applied
to the lamp and its series inductance. This is
controlled by the control processor 70. Only one lamp
is illuminated at a time. The other lamp is held in
reserve.
In this embodiment, the fluorescence reaction is
stimulated via a direct transmission mode of the light
through the wells 31. However, a reflective mode may
also be used which would require repositioning of the
UV light sources.
A UV source monitor photodiode 86 for each lamp is
positioned to intercept a small portion of the
radiation leaving the lamp. The resulting signal is
used to monitor the lamp's intensity. This signal also
allows the control processor 70 to detect a decrease in
the lamp's intensity so that the other reserve lamp can
be activated if necessary.
CA 02593367 2007-07-17
- 23 -
The active lamp is operated at full power only when W
excitation measurements are being taken. Otherwise,
the lamp is dimmed to low power to conserve lamp life,
or turned off, to prevent optical interference with
readings using the visible excitation light sources
discussed above. In addition, an emission filter 83
(shown in Fig. 11) is used eliminate any unwanted
spectral components that may be introduced by the
lamps. For example, the emission filter 83 filters out
the UV light wavelengths of the lamp. The lamp current
is raised to operating level for test data acquisition
by means of a signal line controlled by the control
processor 70 (i.e., switching the lamp from low- to
high-intensity operation).
A W source power supply 92 powers the active lamp. As
discussed above, adjustment of the lamp's intensity is
accomplished by varying the frequency of high voltage
excitation applied to the lamp and its series -
inductance. An increase in frequency causes a decrease
in lamp current as the inductive reactance increases,
which in turn causes a decrease in lamp intensity.
The UV source power supply 92 also includes high
voltage reed relays (not shown) to transfer power from
the active lamp to the reserve lamp as directed by the
control processor 70. As discussed above, lamp
transfer occurs when the source monitor photodiode
detects a significant decrease in the intensity of the
active lamp.
In operation, the visible light source assembly 80 and
the UV light source assembly 81 are sequentially
energized. After one complete rotation of the carousel
50 (i.e., one data accumulation cycle), another type of
wavelength illumination is energized. For example, in
one arrangement, each column of LEDs (i.e., red, green
CA 02593367 2007-07-17
- 24 -
and blue) contained in the visible light source modules
are energized sequentially, then the UV light source
module is switched to full power, each light source
being active for one complete of carousel 50 rotation.
This enables the instrument 20 to gather test data from
each ID/AST panel 30 based on different types of
wavelength light. In a preferred embodiment, the
sequence is UV warm up, W reading, followed by red,
green and blue readings.
As shown in FIGS. 4, the optical measurement system 100
is disposed approximately within the center of the
assembly 51 such that it is aligned to the visible
light transmitted through each well 31 of the ID/AST
panels 30 during excitation with red, green or blue
illumination from the visible light source modules.
Visible fluorescent radiation is similarly detected
from the wells 31 excited by the UV light. As
discussed above, the emission filter 83 eliminates
unwanted spectral components that may be present in the
output signal before detection by the optical
measurement system 100. In another embodiment, near-
Infrared (IR) light can be used to perform the optical
test scans.
As will be appreciated by one skilled in the art, a
variety of means may be used to measure changes in
optical characteristics. For example, photodiodes or
an array of photosensors may be used.
In a preferred embodiment, a plurality of CCD detector
modules 101 and lens assemblies 105 (shown in Figs. 7
and 8) are provided, one for each assembly 51 tier.
The CCD detector modules 101 and lens assemblies 105
are supported on a measurement system tower 103. The
measurement tower 103 supports the lens assemblies 105
and the CCD detector modules 101 so that they are
CA 02593367 2007-07-17
- 25 -
oriented in alignment with the optical axis of one well
column of the ID/AST panels 30.
The lens assemblies 105 include objective lens 102.
The light from of each panel well column is focused
onto the CCD arrays 104 by the objective lens 102.
Each CCD detector module 101 includes a CCD array 104.
A 2048-pixel linear CCD array, for example, may be
used. The CCD arrays 104 detect and measure the
intensity of light transmitted through each well 31
when illuminated by the red, green and blue LEDs.
Visible fluorescent light is similarly detected by the
CCD array 104 under UV light excitation.
Alternatively, the UV light excitation can be
positioned such that the CCD arrays 104 detect the
reflected, or absorbed, visible fluorescent light from
the wells 31.
The CCD arrays 104 are positioned relative to each tier
to provide ample illumination over-scan of all the
locations of the wells 31 in any column of the ID/AST
panels 30. The only light detectable by the CCD arrays
104 is the monochromatic light passing through, or the
visible fluorescence emissions from the wells 31.
Thus, the CCD arrays 104 detect and measure the light
intensity of the wells but not of any other object
illuminated by the light source assemblies. Y-axis
column information, which represents one vertical slice
of information, is scanned electronically by the CCD
arrays 104. Multiple slices of information are
required to detect and measure the light intensity from
one column of wells. X-axis information is accumulated
with the rotation of the assembly 51 (i.e., the ID/AST
panels 30 are rotated so that the next vertical slice
of information can be scanned).
CA 02593367 2007-07-17
- 26 -
Sensitivity of the CCD detection modules 101 is
governed by the integration time selected for each CCD
array 104. As understood by one skilled in the art,
light is composed of individual photons. Each photon
has an extremely small amount of energy associated
therewith. The amount of time necessary to charge the
pixels is called the integration time. Varying amounts
of photons emitted from, or absorbed by, the individual
wells 31 are incident on individual pixels within each
CCD array 104 and charge the pixels to different levels
proportional to the incident light.
The integration time for the CCD arrays 104 in the
present invention is variable. This gives the present
invention the flexibility of having ID/AST panels 30
that contain substrates with a variety of optical
properties (i.e., transparent or optically dense).
From information gathered from the barcode label, the
integration time is set to control the gain for each
ID/AST panel 30. The integration time for the next
ID/AST panel 30 is set before it is illuminated by the
light sources. In one embodiment, a default
integration time is selected to be approximately 4.0
milliseconds. Other integration times.may be selected
by the control processor 70 as needed during testing of
the ID/AST panels 30.
Data processing of the accumulated pixel information is
accomplished by four detector microcontrollers (and
supporting circuitry) 106, one for each CCD detector
module 101. Each detector microcontroller 106 receives
and processes data from the associated CCD arrays 104.
This data is collected from each well 31 when
illuminated by the red, green and blue LEDs and excited
by the IIV light during the rotation of the ID/AST panel
30 via the assembly 51.
CA 02593367 2007-07-17
- 27 -
In operation, the detector microcontrollers 106 use the
panel flag signal generated by the panel position
sensors to initiate panel data acquisition via the CCD
arrays 104. As mentioned above, the panel flag signal
is generated as the panel position flags pass the panel
position sensors during rotation of the assembly 51.
This signal is used as a timing start point for test
data gathering.
The carousel 50 rotates continuously while the detector
microcontrollers 106 receive test data gathered by the
CCD arrays 104. In a this embodiment, the CCD arrays
104 measure more than one variable in parallel
(absorption, turbidity and/or fluorescence) from
essentially the same spatial location. The
measurements are taken by the CCD linear arrays as the
ID/AST panels 30 "fly by.,' All the detector
microcontrollers 106 simultaneously receive the test
data from the CCD arrays 104 as a well column of the
ID/AST panels 30 is illuminated by the light from the
visible light source assembly 80, or excited by the UV
light source assembly 81.
A registration mark (not shown) on each of the ID/AST
panels 30 is located by performing an algorithmic
search on the series of linear array data scans.
Knowing how many steps the step motor of the drive
system 56 have occurred between the timing start point
and the registration mark, in addition to the first CCD
array 104 pixel where the registration mark starts,
gives the information needed to locate precisely any
well 31 on the scanned ID/AST panel 30.
There are two light source normalization processes that
occur during the test data acquisition process. The
first reduces the effects of spatial inhomogeneities
from well to well. The second normalization process
CA 02593367 2007-07-17
- 28 -
involves monitoring the instantaneous source intensity
simultaneous with the CCD array 104 test data
acquisition.
A normalizer panel serves as a reference panel for
instrumental correction of the optical measurement
system 100. Each tier of the assembly 51 contains one
normalizer panel which resides in position location
zero on each tier. The normalizer panel contains a
matrix of absorbers in ID/AST panel-well format. The
normalizer panel is constructed such that it has a
nominal geometry equivalent to the ID/AST panels 30.
The readings from the normalizer panel do not change
overtime and transmits the same light intensity when
illuminated uniformly. By measuring each normalizer
panel well's output, a correction factor for each well
is created to eliminate any nonuniformities in well to
well source intensity, to correct individual well
signals for losses occurring in the optical system, and
to compensate for the reduction in LED output over
time. Test data collected from each ID/AST panel 30 in
a tier of the assembly 51 is corrected (normalized) for
any changes in the optical system since the normalizer
panel for that tier was last read.
In one embodiment, a selectably energized monochromatic
light source provides linear illumination for a column
of uniform wells of the normalizer panel. The profile
of illumination intensity along the column is piecewise
adjusted to provide uniform detector response for all
the wells in the column of the normalizer panel. The
columns of all the ID/AST panels 30 are then
illuminated with this profile. The normalizer optical
response of each well in the ID/AST panel 30 is thereby
measured with uniform sensitivity for all the well
locations within each column.
CA 02593367 2007-07-17
- 29 -
As mentioned above, the signal from the source monitor
photodiode 84 is used to determine any changes in
visible light source assembly's 81 light intensity as
the carousel assembly 51 rotates. While the normalizer
panel is used to monitor relative spatial variations of
intensity, the source monitor photodiode 84 enables the
present invention to monitor quasi-absolute intensity
as it fluctuates throughout a single rotation of the
carousel assembly 51 or varies over a long period of
time. The source monitor photodiode 84 is monitored
simultaneously with each CCD array acquisition. The
detector microcontroller 106 has two correction factors
to apply to each set of test data gathered so that any
differences between test data scans are due only to the
optical properties of the reagents in the wells 31.
Each detector microcontroller 106 also receives data
from a CCD dark current scan. Dark current correction
is applied to the data on a per-pixel basis.
In an alternative embodiment, a fluorescent visible
light source and a filter wheel (not shown) can be used
instead of the visible light sources discussed above.
The filter wheel contains a plurality of spectral
filters. In this embodiment, for example, absorption
and turbidity measurements are acquired in three
consecutive rotations of the assembly 51, while
fluorescence measurements are acquired during a fourth
rotation. Upon completing the first rotation for
normalization and registration mark locations (this is
done for each of the panels per tier), the filter wheel
indexes to it's first spectral filter. Upon reaching
the normalizer panel, the filter wheel indexes the
second spectral filter. Test data acquisition,
normalization and the computation process, as described
above, are repeated for each spectral filter within the
filter wheel. After colorimetric measurements are
CA 02593367 2007-07-17
- 30 -
performed, the visible fluorescent source is turned
off. The filter wheel indexes an emission filter and
fluorescence measurements are taken in a similar
manner.
In order to reduce the post-processing burden, all
pixel information not associated with ID/AST panel
wells are eliminated. For example, the analog signal
from the CCD array 104 can be digitized and the
detector microcontroller 106 can then process the
digitized signal accordingly. The test data for each
well 31 (i.e., the light intensity information) is then
averaged. The averaging is performed based on a per-
pixel value received from the CCD array 104 for each
well 31. The averaging produces a single integer
numerical value for each well 31. One numerical value
is produced for each data accumulation cycle (i.e.,
red, green and blue illumination and UV light
excitation). This information is then sent via a
multi-drop serial data transmission protocol to the
control processor 70.
In one embodiment, spatially averaging of an analog
signal from the CCD arrays 104 is performed by the
detection microcontroller 106 so as to eliminate
unwanted optical and electrical artifacts from sample
column data. The spatial averaging is performed using
partial analog decommutation of the pixel intensity of
the analog signal.
As shown in FIG. 4, the control processor 70 is mounted
in the electronic compartment 62 of the instrument
enclosure 60. The control processor 70 includes the
instrument front panel 71, a keyboard 72, a computer
readable medium drive 73 (e.g., floppy disk or CD-ROM
drive), and a loudspeaker/audible alarm. The control
processor 70 also includes an I/0 interface board, a
CA 02593367 2007-07-17
- 31 -
CPU, memory, an Ethernet interface circuitry, display
driver circuitry (none of which are shown). The
control processor 70 can also be provided with a mouse.
In operation, the control processor 70 performs the
following functions by executing instructions stored in
a computer readable medium. The control processor 70
senses the home flag magnet on the drive ring 52 via
the hall-effect sensor 55. This is done in order to
properly index the ID/AST panels 30 which are mounted
on the assembly 51 while being rotated. High-level
commands are sent to the detector microcontrollers 106
to initiate or stop testing of the ID/AST panels 30.
The intensity of the UV light source assembly 81 is
controlled based on the signal from the UV source
monitor photodiode 86. The control processor 70
illuminates the status indicator LEDs 54 on the panel
carriers 53. The indicator LEDs 54, as discussed
above, identify which ID/AST panels 30 have been tested
and can be removed from the assembly 51. The
incubation temperature is also controlled by the
control processor 70 via signal/control lines
operatively connected to the incubation heater.
The control processor 70 also receives the data
generated from the barcode scanners, the barcode reader
and the barcode wand 72. As discussed above, the data
from the barcode scanners is used to correlate the test
data gathered to a particular ID/AST panel 30. Each
data accumulation cycle (i.e., one rotation of the
assembly 51), the control processor 70 expects to
receive data related to the barcode labels of each
ID/AST panels 30 in assembly 51 and test data for each
ID/AST panel 30. If either is received, the control
processor 70 determines that an ID/AST panel 30 is
logically present in that panel location. However, if
both types of data are not received, the control
CA 02593367 2007-07-17
32 -
processor 70 discards the data for that accumulation
cycle.
Upon the completion of one data accumulation cycle, the
control processor 70 receives serially the data from
the detector microcontrollers 106. This data is stored
in the memory. The control processor 70 then
interprets the data from the ID wells 31 (i.e., from
the wells associated with the ID portion of the ID/AST
panels 30 as discussed below) to produce an organism
identification. The control processor 70 also
interprets the data from AST wells 31 to produce either
MIC results or, via National Committee for Laboratory
Standard (NCCLS) guidelines, produces an Susceptible,
Intermediate, or Resistant (SIR) result which refers to
breakpoint for AST categories. The final results for
the ID/AST panels 30 are stored memory and may be
downloaded to a floppy disk, for example, to conserve
storage space within the memory.
Other functions performed by the control processor 70
include communicating with externally connected network
devices (e.g., a local area network (LAN) and the
like), providing a printer port, performing start-up
and diagnostic-self tests to ensure that the instrument
20 is operating properly, and generating appropriate
alarm signals. The control processor 70 also provides
the operator with a graphical-user interface (not
shown) via the instrument front panel 71, and accepts
user commands and input via the keyboard 72.
Returning to FIGS. 3A-3C, the ID/AST panels 30 are
supplied in a combination format. Each ID/AST panel 30
has reagent well positions capable of performing ID and
AST testing on the same panel. As discussed above, the
ID/AST panels 30 include the wells 31 and the barcode
labels. The wells 31 are segregated into an ID section
CA 02593367 2007-07-17
- 33 -
33 and an AST section 34. The ID section 33 of the
ID/AST panel 30 consists of fifty-one wells 31. The
AST section 34 of the ID/AST panel 30 consists of
eight-five wells 31. For example, the wells 31 of the
AST section 34 may contain dried antibiotics therein.
The ID/AST panels 30 also includes a base 35, a chassis
36, a lid 37, and a cellulose acetate pad 38. Each
ID/AST panel 30 also includes a panel label (not shown)
which includes information to identify the complete
manufacturing history of the particular ID/AST panel
30.
The barcode label provides information related to the
ID/AST panel type and also has a unique sequence number
for identification purposes. The barcode label can be
provided in Code 128, numeric format or any other
suitable barcode format.
Each ID/AST panel 30 is inoculated with an
broth-suspended organism before being placed into the
instrument 20. In practice, the microorganism is a
processed and resuspended dilution of microbiological
growth from primary culture in either an ID inoculum
fluid or an AST inoculum fluid which is then poured
into the test panel. The ID/AST panels 30 are inclined
with the inoculation ports 39 at the top for filling.
Separate inocula are added manually to the ID and AST
ports 39. Each well 31 in the ID section 33 is
inoculated with the ID inoculum fluid as the inoculum
flows down the panel toward the pad 38. Each well 31
in the AST section 34 is inoculated with the AST
inoculum fluid. The inocula flow down the ID/AST panel
30 in a serpentine fashion, filling the wells 31 as the
liquid front progresses toward the pad 38. Each well
31 is vented, permitting liquid to fill the well 31.
Each well 31 has a sharp, circular rim to separate a
CA 02593367 2007-07-17
- 34 -
consistent quantity of liquid from the excess and to
isolate each well 31 from liquid in adjacent wells 31.
The pad 38 absorbs excess liquid.
The ID/AST panels 30 are inoculated with the inoculum
fluids at a panel inoculation station (not shown).
Each station holds two tubes of inoculum fluid (i.e.,
the ID inoculum fluid and the AST inoculum fluid) and
supports one ID/AST panel 30. Gravity drives the
inoculum fluids through the ID/AST panels 30.
The ID inoculum fluid and AST inoculum fluid comprise
the reagent subsystem which includes all reagents
required to process isolated bacterial colonies into
prepared inocula for addition to the ID section 33 and
the AST section 34 of the ID/AST panels 30.
The ID inoculum fluid is used for organism
identification. A variety of ID inoculum fluids can be
used, although a saline solution is preferred. A
detergent may be added to enhance ID/AST panel 30
filling in the panel inoculation station. Preferably,
the inoculum density for ID panel inoculation is at
least 1 x 105 cfu/ml. A variety of identification
reagents may be used which include Phenol Red and
Iodo-Nitro-Tetrazolium (INT). A variety of substrates
may also be used which include 4-Methyl Umbelliferrone
(4-MU) derivatives, Methyl-Amino-Coumarin (4-AMC)
derivatives, para-Nitrophenol derivatives, and Esculin.
The AST inoculum fluid used for AST determination is a
modified formulation of Mueller-Hinton broth.
Preferably, the inoculum density for AST panel
inoculation is at least 1 X 105 cfu/ml. Different
inoculum densities may be used for other embodiments of
the present invention such as "rapid" AST test results.
CA 02593367 2007-07-17
- 35 -
These are AST test results obtained within sixteen
hours of ID/AST panel 30 inoculation.
A variety of AST indicators may be used. The preferred
indicator for AST determinations in the present
invention is alamarBlueTM , a redox-buffered
oxidation-reduction indicator. The indicator is added
to the AST inoculum fluid and mixed just prior to
addition of the microorganism sample to be tested by
the instrument 20.
As mentioned above, the control processor 70 interprets
the data from the wells 31 for the purpose of
detection, identification and susceptibility testing.
The control processor uses three variable threshold
levels to interpret this data: an absolute, a dynamic
and relative threshold. When using the absolute
threshold, a positivity assessment made by determining
if the normalized well 31 reading is above (positive)
or below (negative) a given predetermined value. When
using the dynamic threshold, a reagent reaction
determination is calculated using first- and
second-differences or other mathematical manipulations
of detection data related to the rate-of-change of
signal increase as a function of time by determining
when certain parameters of the calculated first- and/or
second-differences have been exceeded. When using the
relative threshold, a reagent reaction determination is
made by setting a threshold a predetermined percentage
above the starting signal level of the well 31 in
question.
In operation, the ID/AST panels 30 are mounted and
incubated in the carousel 50 of the instrument 20. As
the visible light source assembly 80 and the UV light
source assembly 81 are energized sequentially, one
CA 02593367 2007-07-17
- 36 -
reading is taken corresponding to the red, green, blue
and fluorescent wavelengths of light. Based on the
rotation speed of the carousel 50, light intensity
readings are taken at predetermined intervals by the
optical measurement system 100.
For example, when the carousel 50 is driven by the
drive system 56 at an angular velocity of 2.0
revolutions per minute (RPM), one rotation of the
carousel 50 requires 30 seconds. Thus, to accumulate
data for red, green, blue and W wavelengths, two
minutes are required. Accordingly, in this example, a
complete set of data can be taken by the present
invention every two minutes, Since it is possible to
vary the angular velocity, different angular velocity
may be used for different tests. For example, it may
be desirable to accumulate UV data at 1.0 RPM (while
other test data is accumulated at 2.0 RPM). In this
case, a complete data set would require two and a half
minutes to complete.
In the present invention, AST end-point results based
on the well 31 readings can be obtained after 18-24
hours of incubation. In an alterative embodiment, AST
results can be obtained within 16 hours of panel
inoculation.
With regard to identification accuracy, the control
processor 70 includes an ID taxa database that includes
greater than 126 species for gram-negative organisms,
and 103 species for gram-positive organisms. The
control processor 70 also includes an AST taxa database
equivalent to the ID taxa database for both gram-
positive and -negatives. For the purposes of AST
testing, the present invention also includes a database
with all human and veterinary antimicrobics currently
known_
CA 02593367 2007-07-17
- 37 -
While the present invention has been described above in
terms of specific embodiments, it is to be understood
that the invention is not intended to be confined or
limited to the embodiments disclosed herein. On the
contrary, the present invention is intended to cover
various methods, structures and modifications thereof
included within the spirit and scope of the appended
claims.