Note: Descriptions are shown in the official language in which they were submitted.
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
PATHOGEN DETECTION USING COHERENT ANTI-STOKES
RAMAN SCATTERING MICROSCOPY
TECHNICAL FIELD
The present invention relates generally to water contamination testing, and in
particular to
assessing occurrences of biological pathogens in aqueous samples using
nonlinear microscopy.
BACKGROUND OF THE INVENTION
Cryptosporidium parvum, a protozoan microorganism, is one of principle
contributors to water
contamination and represents a major threat to human health. Ingestion of just
a few oocysts can
cause diarrhea and can be especially fatal in immuno-compromised individuals.
There is no
specific drug therapy proven to be effective to treat cryptosporidial
infections. For these reasons,
detection of cryptosporidia in water supplies is important. It is also
important to be able to
distinguish viable and non-viable cryptosporidia and infectious and non-
infectious
cryptosporidia.
1 5 Cryptosporidia occur outside the body of an animal primarily in the
form of oocysts, which are
environmentally stable and resistant particles having a diameter that is
typically in the range
from about 3 to about 6 micrometers. The oocysts are known to remain viable
for extended
periods of time and are resistant to conventional water disinfection methods.
Due to massive
shedding of oocysts in the feces of infected animals or individuals and the
robust nature of the
2 0 oocysts, they are frequently present in raw surface water and even in
finished drinking water.
Each oocyst typically contains four sporozoites, each of which can
independently infect a host
upon ingestion by the host of the oocyst. Extended exposure to the
environment, treatment with
certain chemicals, exposure to ultraviolet radiation, and other unknown
factors can render
sporozoites within an oocyst non-viable, i.e., unable to infect a host upon
ingestion of the oocyst.
Current methods used in the water quality testing industry for detection of
cryptosporidium
oocysts are time-consuming, labor intensive and require highly trained
microscopists. These
methods rely on microscopic examination of samples that are stained with
fluorescent antibodies
for the presence of cryptosporidium oocysts. The cross reaction of the
antibodies with targets in
1
1
CA 02594444 2011-07-27
Doc No 103-8 CA
Patent
the sample other than the specific pathogen, often gives false positive
results. In the particular
case of parasitic protozoa such as cryptosporidium and giardia, if the
antibody only reacts with
certain variants of the protozoa, but not with the variant present in the
water sample being tested,
the immunological test can fail to detect the pathogen even when it is
present.
In contrast, vibrational spectroscopic techniques such as spontaneous Raman
scattering provide
specific molecular information on samples. Pathogens can be "fingerprinted" by
means of
characteristic vibrational frequencies of the molecular species, even in a
complex multi-
component mixture as disclosed for example in U.S. Patent No: US 6,950,184.
In Raman spectroscopy, incident light having frequency cop is absorbed by a
sample and is re-
radiated at a shifted frequency ws =o-Q, where Q corresponds to a transition
between two
vibrational states of molecules in the sample, also referred to as a vibration
frequency. The
difference between the frequencies of the incident and re-radiated light is
known as the Raman
1 5 shift (RS), and is typically measured in units of wavenumber (inverse
length). If the incident
light is substantially monochromatic (single wavelength) as it is when using a
laser source, the
scattered light which differs in frequency can be more easily distinguished by
filtering.
As an example, FIG. 2 , which is reproduced here from an article by S. Stewart
et al, Proc. of
2 0 SPIE , Vol. 5692, 341 ¨ 350 (2005), illustrates a typical Raman
spectrum of a cryptosporidium
oocysts (A) in comparison with a Raman spectrum from river water (B). As seen
from the
figure, the spontaneous Raman spectrum (A) of cryptosporidium oocysts is
dominated by the
presence of a strong peak around Raman shift of 2930 cm-1 corresponding to
stretching
vibrations of a C-H bond, which can be used as an indicator of the presence of
cryptosporidium
2 5 oocysts in water.
One disadvantage of using the aforedescribed spontaneous Raman scattering for
water testing
relates to low characteristic cross-sections of spontaneous Raman scattering,
which resulting in
low signal levels and hence considerable amount of time needed to record a
Raman spectrum.
3 0 Additionally, the application of conventional Raman spectroscopy can be
disadvantageously
2
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
affected by a background fluorescence signal, which often limits the
sensitivity of detection.
Furthermore, the Raman spectra analysis for the detection of cryptosporidium
oocysts disclosed
in the prior art U.S. Patent 6,950,184 is not capable of discerning between
individual organisms
and how many oocysts are present in a sample, and is therefore not well suited
for quantitative
analysis of the oocysts concentration in water.
There is another optical analysis method based on probing vibrational energies
of molecules in a
sample, namely - a coherent anti-Stokes Raman scattering (CARS) microscopy.
CARS is a third
order nonlinear optical process and involves simultaneous excitation of a
sample under test with
two light beams - a pump laser beam at a frequency wp and a Stokes laser beam
at a frequency
wõ resulting in a signal at the anti-Stokes frequency of was = 2o)p - cos
being generated in a phase
matching direction, provided that the frequency difference between the pump
and Stokes beams
corresponds to a transition between two vibration energy levels of sample
molecules, i.e. Q
(0s; an energy diagram for this process is shown in FIG. 1. In CARS
spectrography, the intensity
of the signal at the anti-Stokes frequency was is typically plotted as a
function of the frequency
shift Q between the pump and Stokes signals and is referred to as the CARS
spectrum, with the
frequency shift Q referred to as the anti-Stokes frequency shift or CARS
frequency shift and is
typically expressed in units of cm-1. Although the CARS microscopy has been
applied recently to
imaging of live cells in laboratory conditions, see for example U.S. Patent
6,108,081 issued to
Holtom et al, it has been largely unknown in the water testing industry.
Therefore the water testing industry currently lacks a method that can provide
a fast and reliable
detection of water-borne pathogens such as cryptosporidium oocysts and can be
used for real-
time automated water testing.
An object of the present invention is to overcome the shortcomings of the
prior art by providing
a method for assessing the presence of individual pathogen organisms in a
sample utilizing
CARS microscopy for fast pathogen detection and identification.
3
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
Another object of the present invention is to provide a method for assessing
the presence of
individual pathogen organisms in a sample that can be used for automated water
monitoring in
real-time.
SUMMARY OF THE INVENTION
In accordance with the invention, there is provided a method of assessing the
presence of a
pathogen in a sample comprising the steps of: a) irradiating the sample with
first radiation having
a spectrum centered at a first frequency and second radiation having a
spectrum including a
second frequency, wherein the first frequency exceeds the second frequency by
a pre-determined
non-zero frequency shift characteristic to the pathogen; b) detecting third
radiation scattered
1 0 from or transmitted through the sample at a third frequency that is
different from the first and
second frequencies, so as to form an image of at least a portion of the
sample; and, c) analyzing
the image to assess occurrence of one or more image artifacts each having one
or more pre-
determined features characteristic of the pathogen.
The method may further comprise the step of obtaining a spectrum of the third
radiation if the
1 5 presence of an image artifact having one or more pre-determined
features characteristic for the
pathogen is detected in step (c), for performing pathogen identification by
comparing the
spectrum to one or more stored reference spectra characteristic to one or more
pathogens.
According to one aspect of the invention, the third radiation results from a
coherent anti-Stokes
Raman scattering (CARS) of the first and second radiation within the pathogen,
so that the third
2 0 frequency exceeds the first frequency by an anti-Stokes frequency shift
equal to the pre-
determined non-zero frequency shift and corresponds to a molecular vibration
frequency in the
pathogen.
According to another aspect of the invention, the method further comprises the
step of flowing
water through a trap medium for accumulating the pathogen therein to form the
sample, so as to
2 5 continuously monitor the water for the presence of a pathogen.
Another aspect of the present invention provides a system for automatic real-
time monitoring of
the presence of a pathogen in water. The system comprises a trap medium, water
directing means
for directing the water through the trap medium for trapping the pathogen in
the trap medium for
forming a sample, means for moving the trap medium carrying the sample out of
the water, a
4
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
CARS optical source for generating a pump beam at a pump optical frequency and
a Stokes
beam at a Stokes optical frequency, a CARS imaging system for obtaining an
image of the
sample, and a processor programmed for analyzing the image to assess
occurrence of one or
more image artifacts having a shape, size or intensity pattern that is
characteristic to the
pathogen.
The CARS imaging system comprises optical means for directing the pump and
Stokes beams
coaxially onto a portion of the trap medium comprising the sample, and an
optical detector for
detecting light from the aqueous sample at a frequency that is shifted from
the pump optical
frequency by a CARS frequency shift for forming an image of a portion of the
sample;
1 0 According to one aspect of the invention, the CARS imaging system
further comprises a
microlens array means for focusing the pump and Stokes beams into a plurality
of focal locations
in the sample, and a photodetector array for detecting optical radiation
generated at each of the
plurality of focal locations. In one embodiment, the spinning micro-lens array
disk for raster
scanning the sample for forming the image.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be described in greater detail with reference to the
accompanying drawings
which represent preferred embodiments thereof and in which like reference
labels are used to
indicate like elements, wherein:
2 0 FIG. 1 is an energy diagram of a CARS process according to prior art;
FIG. 2 is a prior art plot showing Raman spectra of cryptosporidium parvum
oocysts.
FIG. 3 is a schematic block diagram of a CARS apparatus for detecting the
presence of a
pathogen in a sample according to the present invention;
FIGs. 4 and 5 are schematic block diagrams of two alternative embodiments of a
CARS optical
2 5 source for emitting pump and Stokes beams for use in the apparatus of
FIG. 3;
FIGs. 6A and 6B are two exemplary CARS images of live cryptosporidium parvum
oocysts
obtained using an embodiment of the CARS optical source shown in FIG. 4;
5
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
FIG. 6C is a plot showing intensity profile of the CARS image of the live
cryptosporidium
parvum oocysts corresponding to the image cross-section AA in FIG. 6B;
FIG. 7 is a CARS image showing a non-viable cysts of Giardia lamblia.
FIG. 8 is a prior art CARS image of six live, unstained bacteria of the type
Shewanella
putrefaciens, strain CN-32.
FIG. 9 is a prior art CARS image of three live, unstained HeLa cells in
aqueous HEPES buffer
solution.
FIG. 10 is an image of live cryptosporidium parvum oocyst in aqueous organic
trapping medium.
FIG. 11 is a block diagram of a system for automated real-time water
monitoring according to
1 0 the present invention;
FIG. 12 is a schematic illustration of one embodiment of a sample cell that
can be used in the
system of FIG. 11;
FIG. 13 is a schematic diagram illustrating the use of a spinning micro-lens
disk array in the
system shown in FIG. 11;
FIG. 14 is a flowchart showing general steps of the method of water monitoring
for the presence
of a pathogen according to one embodiment of the present invention.
FIG. 15 is a prior art plot showing Raman spectra of viable and non-viable
cryptosporidium
parvum oocysts.
DETAILED DESCRIPTION
2 0 The invention includes a method for detecting Cryptosporidium parvum
organisms, in particular
Cryptosporidium oocysts, and other waterborne pathogens using CARS imaging
and/or
spectroscopy in a variety of aqueous or non-aqueous samples, including but not
limited to,
environmental raw water samples, backwash water samples, process water
samples, finished
water samples, and samples carried by a pathogen trapping medium. The
invention also includes
6
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
a method and system for real time water monitoring for the presence of a
particular pathogen
such as the Cryptosporidium parvum in water reservoirs and flowing water.
Reference will now be made in detail to the embodiments of the invention,
examples of which
are illustrated in the accompanying drawings. While the invention will be
described in
conjunction with the preferred embodiments, it will be understood that they
are not intended to
limit the invention to these embodiments. On the contrary, the invention is
intended to cover
alternatives, modifications and equivalents, which may be included within the
spirit and scope of
the invention as defined by the appended claims. Furthermore, in the following
detailed
description of the present invention, numerous specific details are set forth
in order to provide a
thorough understanding of the present invention. However, it will be obvious
to one of ordinary
skill in the art that the present invention may be practiced without these
specific details. In other
instances, well known methods, procedures, components, and circuits have not
been described in
detail as not to obscure aspects of the present invention unnecessarily.
One aspect of the invention relates to an application of CARS microscopy to
detect occurrence
of cryptosporidium oocysts from water samples, and can also be used to detect
other pathogens
in contaminated water. The present invention overcomes the shortcomings of
prior art methods
and enables speed, sensitivity and chemical selectivity in the detection of
the oocysts, and
enables automated real-time monitoring of water supply. In general, pathogens
are micro-
organisms that cause disease in humans. The term "pathogen" will be used
herein to refer to a
particular pathogen species, such as the cryptosporidium parvum or Guardia,
while the terms
"pathogen organism" or "individual pathogen" will be used to refer to
individual pathogen
organisms such as individual cryptosporidium parvum oocysts.
Each particular pathogen has it own distinct spectrum of vibration
frequencies. By tuning the
difference between the pump and the Stokes beams frequency, i.e. the CARS
frequency shift in a
frequency range containing the molecular vibration frequencies of a particular
pathogen, a CARS
spectrum is obtained. This spectrum is hence characteristic to the particular
pathogen.
In general, the molecular vibration frequencies of most pathogens occur in the
range of 500 ¨
3250 cm-1 giving rise to peaks in the CARS spectrum at the respective
frequencies. The residual
body consisting of the lipid vacuole inside the cryptosporidium oocyst has
large concentrations
7
CA 02594444 2011-07-27
Doc No 103-8 CA
Patent
of C-H vibration bonds with characteristic frequencies in the range of 2810 ¨
2870 cm-I, giving
rise to a strong CARS signal that is used in the invention for imaging the
oocysts in a water
sample. Other peaks in the CARS spectrum such as those due to amide vibrations
that occur in
the range of 1650+\-25 cm-1, can also be used for imaging the oocysts as well
as to distinguish
between various pathogens.
A specific example of an application of the CARS microscopy to detect the
presence of
cryptosporidium oocysts is described herein below. As shown in FIG. 2, a
spontaneous Raman
spectrum of cryptosporidium oocysts is dominated by the presence of a strong
peak around
Raman shift of 2930 cm' corresponding to the C-H stretching vibrations in this
particular
1 0 pathogen. In the CARS spectrum a corresponding peak occurs at around
2840 cm-I; the ¨ 90cm-I
difference between the Raman shift and the CARS shift includes a contribution
from non-
resonant CARS background signal stemming from optical nonlinearities in the
pathogen
excitation process unrelated to the C-H bond vibrations.
In one embodiment of the present invention the frequency difference between
the pump and
1 5 anti-Stokes beams is tuned to this CARS frequency of 2840 cm -I +\-
60cm-1, preferably +\- 25
cm', and most preferably +\- 10cm4 , which corresponds to a peak in the CARS
spectrum
associated with the C-H vibrations in cryptosporidium oocysts. Alternatively,
the frequency
difference between the pump and anti-Stokes beams is tuned to 1650+\-25 cm-I,
or preferably to
1650+\-10 cm-I. Alternatively, the frequency difference between the pump and
anti-Stokes
2 0 beams is tuned to 2950+\-50 cm-1, or preferably to 2950+\-10 cm-1. The
pump and Stokes beams
overlapped in a small focal volume, preferably less than 111m3, within the
sample, are scanned
across the sample in a same focal plane. In this manner, CARS images of a
scanned portion of
the sample are obtained, for example in forward and/or epi-direction of
detection, where the epi-
direction is the direction of back-scattering and is opposite to the forward
direction.
2 5 An exemplary embodiment of an apparatus for detecting the presence of a
pathogen in a sample
using the CARS technique in accordance with the present invention is
illustrated in FIG. 3 and
will now be described.
In this particular implementation of the CARS technique, a single femtosecond
optical source is
used to obtain both the pump and Stokes beams, which significantly simplifies
the apparatus and
8
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
reduces its cost. Advantageously, the apparatus of FIG. 3 enables image and
spectrum
acquisition from a same single CARS signal without the need to tune the
wavelength of the
optical source, and provides good quality images wherein characteristic
features of a particular
pathogen are easily discernable. The apparatus of FIG. 3 utilizes an
improvement to the
conventional CARS known as multiplexed CARS spectroscopy, wherein spectral
width of the
Stokes pulse determines a range of molecular vibrational energies that are
probed, while the
spectral width of the pump pulse determines the spectral resolution of the
technique. In order to
achieve a high spectral resolution, the pump pulses have to be spectrally
narrow and the Stokes
pulses have to be spectrally broad. To achieve a spectrally broad Stokes
pulse, one embodiment
of the apparatus may utilize a photonic crystal fiber (PCF) having suitable
chromatic dispersion
and nonlinear characteristics so as to generate a so called superconinuum
spectrum from the
pump pulses to form stable broadband Stokes radiation so as to enable
multiplexed CARS as
described hereinbelow.
More particularly, an optical pulse source 30, embodied herein as a self mode-
locked Ti:sapphire
femtosecond pulsed laser such as the Spectra Physics Tsunami Laser, and
hereinafter referred
to as the laser 30, emits a sequence of short optical pulses forming a laser
beam 31. The term
"femtosecond" in relation to a pulse is used to mean herein that the pulse
duration is less than
about 0.2 ps, and when used herein in relation to an optical source such as a
laser means a source
which in operation emits femtosecond pulses, i.e. pulses of duration less than
about 0.2 ps. By
way of example, the sequence of short optical pulses emitted by the laser 30
can have the
following parameters: central wavelength ko ¨800nm, repetition frequency F=80
MHz, pulse
duration to=60 fs, pulse power P up to 0.5W or less as required; in other
embodiments, the
pulsed laser 30 can emit pulses having other suitable values of ?A, F, P, and
to as will be evident
to those skilled in the art.
A beamsplitter 15 splits the laser beam 31 into two beams propagating along
two different paths
20 and 21, which are referred to herein as Stokes and pump arms, respectively.
A first beam is
coupled into a first photonic crystal fiber (PCF) 14 that combines desired
dispersive and
nonlinear characteristics so as to form from received optical pulses an
optical signal having a
broad optical spectrum with a spectral lobe centered close to a desired Stokes
wavelength ks.
9
CA 02594444 2013-05-21
--,
Doc No 103-8 CA
Patent
In one embodiment, the PCF 14 has two zero dispersion wavelengths, i.e.
wavelengths at which
the chromatic dispersion of the PCF 14 is equal to zero, in the vicinity of
the Stokes wavelengths
ks, as described in a paper entitled "Optimization of coherent anti-Stokes
Raman scattering
microscopy using photonic crystal fiber", by S. Murugkar et al, presented at
the Photonics North
Conference, Ottawa, June 2007. By way of example, the PCF 14 is a photonic
crystal fiber NL-
1.4.775-945 available from Crystal Fiber, Inc of about 12.5 cm length, which
has two zero
dispersion wavelengths at 775nm and 945nm.
The PCF 14 is followed in the Stokes arm 20 by an optical spectral filter 34
having a passband
centered at the Stokes beam wavelength ks, which produces a spectrally broad
Stokes beam 9
1 0 centered at the Stokes wavelength ks and formed by femtosecond optical
pulses; this Stokes
beam is then directed by a mirror 23 towards a beam combiner 11 in the form of
a dichroic
mirror for combining with a pump beam 10. The choice of the filter 34 depends
upon which
particular chemical bonds in sample molecules is to be imaged. By way of
example, a filter 34
having a narrow passband of about 53nm and centered at ks ¨ 1040nm will enable
obtaining a
1 5 CARS signal from C-H bonds in cryptosporidium parvum lipids, and
therefore imaging of the
lipid distribution in a sample. A more broadband filter, for example with a
passband of about 200
cm-I, will enable multiplexed CARS wherein a CARS spectrum is obtained without
laser or filter
tuning. In one embodiment, the filter 34 can be tunable, for example it can be
in the form of an
adjustable interference filter disclosed in U.S. Pat. No. 5,194,912 "Raman
analysis apparatus".
2 0 The second part of the laser beam 31 from the beamsplitter 15 is
directed along the pump
arm 21 by a mirror 22, first to a chirp inducing element 13, which is embodied
as a prism
pair configured to impose a large negative chirp on the received optical
pulses as known in
the art; alternatively, other chirp inducing elements 13 can be used, such as
a suitable grating
stretcher as described in the paper "Optimization of coherent anti-Stokes
Raman scattering
25
microscopy using photonic crystal fiber", by S. Murugkar et al. The prism
pair 13 is
followed in the pump arm 21 by an optical element 12 having a suitably high
chromatic
dispersion, for example - another PCF. The PCF 12 receives chirped optical
pulses from the
prism pair 13 and generates therefrom spectrally narrow transform limited pump
pulses of a
picosecond duration; these spectrally squeezed pulses form the pump beam 10
having the
30 pump wavelength kp, which in the exemplary embodiment described herein
is equal to about
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
800nm, and may have a spectral width which in one embodiment is at least 5
times less than the
spectral width of the Stokes pulses to enable simultaneous detection of anti-
Stokes signals at
multiple frequencies. The pump arm 21 or the Stokes arm 20 may include a
variable optical
delay line to align the pump and Stokes pulses in time. The term "picosecond"
in relation to a
pulse is used herein to mean that the pulse duration is between about 1 ps and
about 200 ps. The
term "sub-picosecond" in relation to a pulse is used herein to mean that the
pulse duration is less
than 1 ps. By way of example, the Stokes pulses produced in the Stokes arm 20
can be of about
100 fs (femtosecond) duration and have a spectral width of about 200cm-1,
while the pump
pulses produced in the pump arm 21 can be of about 2 ps duration and have a
spectral width of
0 about 10cm- I .
The Stokes beam 9 propagating from the filter 34 and the pump beam 10
propagating from the
PCF 12 are then directed onto a test sample 2 by optical means 11, 8, 27, and
3. In the shown
embodiment the optical means for directing the pump and Stokes beams is formed
by a beam
combiner 11, an optional collimating lens or lens system 8, a scanning mirror
assembly 27, and a
first microscope objective 3. The beam combiner 11 may be embodied as a
dichroic mirror and is
disposed to combine the Stokes beam 9 and the pump beam 10 into a combined
beam 111, which
is also referred to herein as the combined CARS beam or CARS excitation beam,
and is formed
by the substantially overlapping Stokes and pump beams propagating coaxially.
The scanning
mirror assembly 27, for example utilizing a pair of galvanometer mirrors or a
rotating micro-lens
2 0 array disk such as those described in Microscopy and Microanalysis,
Vol. 9 (Suppl. 2), 1090-
1091, (2003), directs the combined beam towards the sample 2. The first
microscope objective 3
is disposed for focusing the pump and Stokes beams into a small focal volume,
preferably of the
order of 1 [tin3 or less, at a particular location within the sample 2.
Alternatively, a commercial
microscope having beam scanning capability can be used in place of the
elements 8, 27 and 3. In
2 5 other embodiments, the apparatus can include means for moving the test
sample 2 in two
directions in a plane normal to the incident pump and Stokes beams as
schematically illustrated
by an arrow 25, so as to obtain a three-dimensional image of a portion of the
sample 2, with a
third dimension provided by varying a focusing depth of the microscope
objective 3.
The CARS radiation, also referred to herein as the third radiation or anti-
Stokes radiation, is
30 generated due to nonlinear four-wave mixing in a location in the sample
cell where the Stokes
11
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
and pump beams are focused. Part of the CARS radiation propagates in the
forward direction, i.e.
in the direction of propagation of the Stokes and pump beams incident on the
sample 2, and is
collected by a second microscope objective 4, and is directed by a second
beamsplitter 29
towards a first photodetector 61 and, optionally, to a spectrometer 71. An
optical filter 51 is
disposed in an optical path of the CARS radiation that passed through the
sample 2, hereinafter
also referred to as the forward detection path, to separate the CARS radiation
from the radiation
of the pump and Stokes beams, which is blocked by the second optical filter
51. Optionally
CARS radiation propagating from the sample 2 in the reverse, i.e. epi-
direction, is collected by
the objective 3, and is then directed by an optional dichroic mirror 18 that
separates the back-
scattered CARS radiation from the pump and Stokes beams, to a second
photodetector 62; the
optical path of the back-scattered CARS radiation will be referred to herein
as the epi detection
path. A second optical spectral filtered 52 can be disposed to filter out
remaining Stokes and
pump radiation and prevent it from reaching the second photodetector 62. The
CARS radiation
generated in the epi-direction may have a significantly higher signal to
background ratio, but
may also be smaller in intensity than that generated in the forward direction.
In one experimental embodiment of the apparatus shown in FIG. 3, a 700nm short-
pass optical
filter from Chroma Technology Corp was used as the second optical filter 51,
an Olympus 40x,
0.8 NA water immersion microscope objective was used as the microscope
objective 4, and two
different microscope objectives: Zeiss Plan Neofluar 16 x, 0.5 NA and Zeiss
Plan Neofluar 40x,
1.3 NA were used as the microscope objective 3 for low and high magnification
images,
respectively.
The photodetector 61, such as a Photo-Multiplier Tube (PMT) or an intensified
CCD camera, is
positioned for detecting the intensity of the CARS radiation generated at a
particular location in
the sample cell 2 for the purpose of generating one pixel of a CARS image. An
optional narrow-
band filter 53 centered at a desired anti-Stokes frequency can be provided
before the detector 61
if a broadband Stokes signal is used, such as in the broadband multiplexed
CARS. Electrical
signals from the photodetector 61 are received and processed by a processor
33, which stores
processed signals for a plurality of scanned locations in the sample 2 so as
to form a CARS
image of said sample or of a selected area therein. The processor 33 can be
embodied as a
general purpose processor equipped with a parallel data acquisition card, or
as a suitable
12
CA 02594444 2011-07-27
Doc No 103-8 CA
Patent
microprocessor, a DSP (digital signal processor), an FPGA (field programmable
gate array), any
combination thereof, or any other digital processing means as would be known
to those skilled in
the art.
In the embodiment shown in FIG. 3, hereinafter referred to as a first
embodiment, the pump
beam 10 and the Stokes beam 9 are provided by a CARS optical source 32, which
utilizes a
single femtosecond pulse source 30 and two PCFs 14, and 12. Advantageously,
this
configuration enables an instantaneous detection of a CARS spectrum using a
spectrometer 71 as
described hereinbelow. However, the CARS optical source 30 can also have
alternative
embodiments.
A second alternative embodiments of the CARS optical source is illustrated in
FIG. 4 wherein it
is indicated as 301. In this embodiment, a smaller fraction of optical
radiation 131 generated by a
single picosecond laser 130 is split-off by a beam splitter 22 and a folding
mirror 115 and used to
form the Stokes beam 9, while a larger fraction of the Laser 130 output is
used to drive an OPO
(optical parametric oscillator) 140 for producing the pump beam 10. Such a
source is described,
1 5 for example, in an article by F. Ganikhanov, S. Carrasco, X. S. Xie, M.
Katz, W. Seitz and D.
Kopf, "Broadly tunable dual-wavelength light source for coherent anti-Stokes
Raman scattering
microscopy", Optics Letters 31, 1292-1294 (2006).
A third alternative embodiment of the CARS optical source is illustrated in
FIG. 5 wherein it is
indicated as 302. In this embodiment, two distinct pulsed lasers 241 and 242
are phase locked
2 0 using a phase locker 211, and are used for generating the Stokes beam 9
and the pump beam 10,
respectively.
EXAMPLE 1
Experiments were performed to illustrate the invention. The experimental setup
was similar to
the apparatus shown in FIG. 3, and utilized the CARS optical sources as
illustrated in FIGs. 4
25 and 5.
A first experiment involved a Nd:vanadate laser 130 from High-Q Laser
(Hohenems, Austria)
disposed as illustrated in FIG. 4; in operation it emits radiation 131 with an
output power of 10W
13
CA 02594444 2011-07-27
Doc No 103-8 CA
Patent
at 1064 nm with a pulse duration of 7 ps and a repetition rate of 76 MHz. A
portion of the output
131 is split off using a power splitter 22 and used as the Stokes beam 9. The
remaining 9 W is
13a
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
used for synchronously pumping an optical parametric oscillator (OPO) 140
(Levante, APE,
Berlin) to generate the pump beam 10, which is then combined with the Stokes
beam 9 to form a
combined CARS excitation beam 111. The OPO signal is intracavity doubled to
produce narrow
spectral bandwidth radiation wavelength tunable between 780 nm and 930 nm in
the form of a
stream of optical pulses having a spectral bandwidth of about 3.5 cm-1 with
pulse duration of 5
ps and an average output power of ¨ 1.5 W at 76 MHz pulse repetition rate.
These parameters
assure a suitably high spectral resolution and a high signal to background
ratio.
An alternate light source for pump and Stokes beams as illustrated in FIG. 5
was available in the
experimental setup as well. This CARS optical source 302 incorporated two
Ti:sapphire lasers
Tsunami 241, 242, which are available from Spectra-Physics, Mountain View,
CA, emitting
two 5 ps optical pulse trains that are synchronized to an 80 MHz clock using a
"Lok-to-Clock"
feature from Spectra Physics schematically illustrated in FIG. 5 at 211, said
optical pulse trains
forming the pump beam 10 and the Stokes beam 9. The timing jitter between the
pulses of the
pump and Stokes beams was about 0.5 ps. The pump beam 10 is tunable from 700
to 840 nm and
the Stokes beam 9 from 780 nm to 900 nm, each with a maximum time-averaged
output power
of ¨1 W.
The divergence of the pump and Stokes beams is controlled by a telescope 209
in each beam
path, while a delay line, which is not shown, is used to provide temporal
overlap of the two
pulse trains. The pump and Stokes beams are coaxially combined using the
dichroic mirror 11,
and the combined beam 111 directed to a laser-scanning microscope (Olympus
FV300/ IX70)
that is modified for CARS microscopy. A pair of galvanometer mirrors in the
microscope
controls the scanning of the two beams on the sample surface. The pump and
Stokes laser beams
are focused onto the sample using a water objective lens (UPlan / APO, 60x,
Olympus America,
Inc.) with a numerical aperture (NA) of 1.2 as the microscope objective 3
illustrated in FIG. 3.
The forward CARS radiation is collected with an air condenser lens (NA = 0.55)
as the
microscope objective 4, is separated from the excitation pump and Stokes beams
using a dichroic
mirror (Chroma, Brattleboro, VT) as the optical filter 51, and is further
filtered using the filter 53
to reject the residual excitation beams and finally detected using a
photomultiplier tube (PMT)
(model R3896, Hamamatsu) as the photodetector 61. The set-up provided a
spatial resolution of
about 0.2 [tm which benefited from the optically non-linear character of the
CARS effect.
14
CA 02594444 2011-07-27
Doc No 103-8 CA
Patent
The frequency difference between the pump and Stokes beams 10, 9 is set so
that it matches the
molecular vibration frequency of the aliphatic C-H vibrations at 2845 cm-1 of
lipid molecules.
This requires tuning the pump beam 10 in the case of the OPO setup 301 to
816.9 nm when the
Stokes beam 9 is at 1064 nm. In the case of the setup 302 with the two
synchronized Ti:sapphire
lasers 241, 242, the wavelengths of the pump and Stokes beams 10, 9 are 716.8
nm and 900.4
nm, respectively. The optical power of the pump and Stokes beams radiation at
the sample,
hereinafter also referred to as the first and second radiation respectively,
was ¨ 24 mW for the
pump beam and ¨ 28 mW for the Stokes beam, respectively when using the
synchronized
Ti:sapphire lasers system 302, and were about 75 mW for the pump beam and ¨ 38
mW for the
Stokes beam when using the OPO based system 301.
Samples of live (viable) Cryptosporidium parvum oocysts originating from
experimentally
infected calves (Iowa isolate) were obtained from Waterborne, Inc. of New
Orleans, LA, U.S.A.
The oocysts were suspended in a solution of phosphate-buffered saline (PBS)
with antibiotics
and a nonionic surfactant and emulsifier Tween 20. Due to the hazardous
nature of the sample,
all sample preparations and imaging experiments were performed in a bio-safety
level 2
accredited laboratory environment. A couple of drops of the PBS solution
containing the
cryptosporidium parvum oocysts were placed on top of a microscope slide and
covered with a
thin coverslip.
FIGs. 6A and 6B show close-up views of typical CARS images of small areas in
the sample
2 0 where live cryptosporidium parvum oocysts were found. The image in FIG.
6A was obtained in
the forward direction (F-CARS) using the CARS optical source 302 based on
synchronized
Ti:sapphire lasers. This image, averaged over two frames, is cropped out of a
bigger image that
is 512 x 512 pixels in size corresponding to an area of ¨125 tm x 125 pm. The
acquisition time
for this image was about 2 second. An image artifact 610 is clearly visible on
a dark background
2 5 in the center of FIG. 6A as a dim diffuse circular feature of about 5
!AM in diameter with a bright
1um spot 615 inside; it has a pattern, size a shape that is characteristic to
a CARS image of a
cryptosporidium parvum oocyst and indicates the presence thereof.
The image in FIG. 6B was obtained in the forward direction (F-CARS) using the
OPO based
CARS optical source 301. The shorter wavelength of the pump in FIG. 6A results
in a higher
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
resolution image. A similar image artifact 620 clearly visible on a dark
background in the center
of FIG. 6B as a dim diffuse elliptical feature of about 5 gm in a large
diameter with a bright ¨
1gm spot 625 inside; it has the same pattern, size a shape that the image
artifact shown in FIG.
6A which is characteristic to a CARS image of a cryptosporidium parvum oocyst,
and therefore
indicates the presence thereof.
It is clearly evident from FIGs. 6A,B that there is a strong CARS intensity
associated with a
spherical structure of about 1 gm in diameter within each of the image
artifacts, indicating a high
lipid density. The circular or slightly elliptical area of about 5 gm in
diameter surrounding this
feature contributes a weaker CARS signal. The intensity profile along a line
AA drawn across
the image in FIG. 6B is shown in FIG. 6C; it is characterized by a strong peak
of about 1 pm +\--
0.5 j_tm in width on a pedestal of about 5i_im +\- 1.5 gm width. This
morphology is consistent
with the structure of cryptosporidium parvum oocyst obtained by electron
microscopy, see for
example F. Petry, Microscopy and Microanalysis, 10, 586-601, (2004). The high
lipid density
seen as the 1 gm bright spots 615, 625 corresponds to a lipid vacuole inside
the residual body.
Along with the amylopectin granules, this lipid vacuole acts as the source of
nutrition for the
sporozoites inside the oocyst.
This feature consisting of the 1 gm bright spot in the 5 micron circular area
is used in one
embodiment of the present invention as an identifying pattern in an algorithm
for image
recognition of cryptosporidium parvum oocyst. When the frequency difference of
the excitation
beams is tuned to be off-resonance, for example at 2750 cm-1, the contrast in
the CARS image
disappears and not much signal is obtained.
FIG. 7 shows a close-up views of an F-CARS image of a small area in a sample
comprising non-
viable (dead) cysts of Giardia lamblia dried on top of microscope well slides.
The sample was
obtained from GAP EnviroMicrobial Services (London, ON, Canada) with the OPO
based
system with the pump-Stokes frequency difference tuned to 2845 cm-1. A bright
image artifact
710 with a characteristic size of about 10-15 gm clearly visible in the
central are of the CARS
image in the figure corresponds to a single cyst of Giardia lamblia. The shape
and pattern of the
image artifact in FIG. 7 indicates that the lipid distribution in a Giardia
cyst is very different
from that of crypto oocyst. The size of 14 micron of the Giardia cyst and the
observed structure
16
CA 02594444 2011-07-27
Doc No 103-8 CA
Patent
is consistent with reports in literature, see for example Microscopy and
Microanalysis 10, 513-
527, 2004.
To illustrate that the characteristic pattern, size and shape of a CARS image
of a cryptosporidium
oocyst is easily discernable from other microorganisms, CARS images of six
live bacteria of the
type Shewanella putrefaciens, strain CN-32, in D20 is shown in FIG. 8, which
reproduces FIG. 8
of U.S. Patent 6,108,081, while FIG. 9 shows CARS images of three live,
unstained HeLa cells
in aqueous HEPES buffer solution reproduced from FIG. 7 of U.S. Patent
6,108,081. Clearly,
CARS imaging of these microorganisms yield image artifacts that differ in
shape, pattern and
size from image artifacts produced by the CARS interaction in the
cryptosporidium oocyst. Note
that in the context of this specification, the term "image artifact" is a
compact discernable feature
in a CARS image of a sample that may be related to a micro-object in the
sample.
REAL-TIME TRAPPING AND AUTOMATED IDENTIFICATION
One important advantage of the system and method for a pathogen detection of
the present
invention is that the CARS signal is generally several orders of magnitude
stronger under similar
conditions than the spontaneous Raman signal used in the prior art. This is
due to the coherent
nature of the CARS process, wherein the frequency-shifted anti-Stokes signal
is a result of a
constructive interference of the Stokes and pump radiation, which gives rise
to a significantly
higher intensity of the CARS radiation compared to the Raman radiation.
Additionally, the
collection efficiency of the CARS radiation is also much higher due to the
directional nature of
2 0 the CARS signal as defined by the phase matching requirement for the
four-wave mixing process
that produces the CARS radiation.
Accordingly, the acquisition time for a typical image in the CARS-based system
of FIG. 3 is
reduced to about a second or less as compared to many hours that are typically
required to
acquire a comparable image in Raman microscopy; according to the present
invention, it can be
2 5 reduced even further using parallel acquisition of a plurality of
pixels of on CARS image as
described hereinabove.
Another significant advantage of using CARS microscopy for detection of
waterborne pathogens
in water samples is that the sample does not need any extra or complicated
preparation. The
17
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
sample for assessment of pathogens may contain water or any physical or
chemical medium used
for the concentration of pathogens without destroying them. This enables to
use the CARS-based
method of the present invention for real-time automated detection of pathogens
in water supplies,
as described hereinbelow. If the CARS spectrum of the medium is known, a
significant
improvement in the signal to noise ratio is obtained by avoiding tuning to the
vibration
frequencies of the medium that may overlap with those of the pathogen, and/or
by subtracting the
known CARS signal of the medium from the measured CARS signal.
Accordingly, the present invention enables a rapid detection of a single
pathogen organism, such
as a single oocysts, without any complicated sample preparation. This for the
first time enables
real-time or almost real time water monitoring for the presence of water-borne
pathogens and
automated identification of the detected pathogens while resolving individual
organisms. One
exemplary embodiment of such a water monitoring apparatus in shown in FIG. 11
and is
hereinafter described.
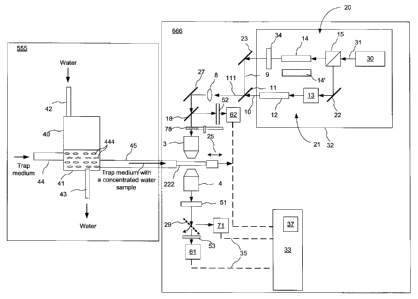
The system shown in FIG. 11 includes a pathogen accumulator 555 wherein a
water sample is
prepared, and a CARS apparatus 666 for pathogen detection, which may be
similar or identical
to that shown in FIG. 3, but as shown includes also an optional spinning micro-
lens array disk 78
as described hereinbelow. The CARS apparatus 666 is also referred to herein as
the CARS
imager. A water container 40 has a water inlet pipe 42 and a water outlet pipe
43 for directing
water under test in and out of a container 40 which has a lower container
section 41 supporting a
trapping medium 444. The container 40 also has trapping medium guides 44 and
45 for slowly
guiding the trapping medium through the container 40 and for directing the
trapping medium 444
out of the water and into a sample cell 222. The pathogen accumulator 555 can
have other
means for automatically directing the trapping medium 444 out of the water
container 40 and
into the sample cell 22 of the CARS imager without human intervention, such as
suitable pumps,
flow meters, flow regulators and the like, which may depend on a particular
implementation of
the trapping medium and would be evident to those skilled in the art. The
water continuously
flows through the trapping medium 444 contained in the lower section 41 of the
water container
40 at a water flow rate that is controlled for example by a peristaltic pump
and a flow meter with
a valve that are not shown. The water flow rate depends on the trapping medium
and by way of
example can be in the range of 0.4 - 4 L/minute. The trapping medium 444 flows
through the
18
CA 02594444 2011-07-27
Doc No 103-8 CA
Patent
container 40 into a sampling cell 222 at a much slower rate, for example about
10 pt/second.
The sampling cell 222 may be made of transparent quartz glass and may be for
example as
shown in FIG. 12.
The trapping medium functions to trap waterborne pathogens such as
cryptosporidium parvum
oocysts so that they can be accumulated therein, forming a sample that may
have pathogen
concentrations exceeding the pathogen concentration in the water by up to 106
times. One
example of a suitable trapping medium is Diatomaceous earth (DE), which is an
organic
microporous material that is commonly used in water filtration methods for
trapping
contaminants in water. Certain products such as chemically treated DE
manufactured by EcoVu
1 0 Analytics (Ottawa, ON, Canada) can enhance the trapping efficiency of
DE by up to 10,000
times; this type of trapping medium is described in US Patent 5,512,491. In
one embodiment that
will be described hereinbelow, the trapping medium 444 is a slurry made of
such a chemically
treated DE and reagent water. An example of reagent water is de-ionized (DI)
water which is
known to be free of pathogens such as oocysts and cysts and other interfering
materials so as not
1 5 to introduce contaminants in the water being tested.
In one embodiment, the trapping medium 444 continuously flows through the
sample cell 222
while the CARS images and spectra are taken as described hereinabove with
reference to FIG.3.
In another embodiment, once the cell 22 is filled with the trapping medium
carrying a sample to
be tested, the flow of the trapping medium is stopped while the sample cell
volume is imaged.
2 0 Once all the CARS measurements on the trapping medium within the cell
222 are finished, the
flow of the trapping medium is resumed until the cell 222 is filled with a new
portion of the
trapping medium. By way of example, the cell 222 may have internal dimensions
of 200 pm x
200 ilm x 100 ?Am, with the last dimension being the cell height in the
direction of pump and
Stokes beams propagation, which corresponds to the cell volume to be imaged of
about 4x10-6
25 cm3.
EXAMPLE 2
An experiment was conducted to demonstrate the feasibility of detecting a
pathogen using CARS
microscopy in the presence of a trapping medium. For this purpose, a sample of
the organic
trapping medium was obtained from EcoVu Analytics (Ottawa, ON, Canada). A
small amount of
19
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
=
this trapping medium was mixed with water and the slurry placed on a
microscope glass slide. A
drop of the PBS solution containing live cryptosporidium parvum oocysts was
added to this.
This sample was covered with a thin glass coverslip and imaged in the forward
direction.
Resulting CARS image is shown in FIG. 10. An image artifact 810 having the
characteristic
pattern, shape and size of an individual cryptosporidium oocyst is clearly
discernable in the left
hand bottom corner of the image. This image artifact visualizes the same lipid
density pattern
corresponding to the cryptosporidium parvum oocyst as described in EXAMPLE 1
and shown
in FIGs. 6A,B. This pattern consists of the 1 wn bright spot in the 5 micron
circular area. A
weaker non-resonant CARS signal, which is not related to the lipid molecular
vibrations and
I O arises mainly due to electronic excitations from the surrounding
trapping medium, is seen in the
CARS image of FIG. 10. This non-resonant background signal can be mostly
suppressed using,
for example, frequency modulation CARS (FM-CARS) as described for example by
F.
Ganikhanov et al, Optics Letters, 31, No. 12, 1872 ¨ 1874 (2006), leaving only
a resonant
CARS signal from the pathogen. Therefore, a pathogen trapped in the aqueous
trapping medium
can be successfully identified using CARS imaging of the trapping medium.
Turning back to FIG. 11, the CARS apparatus 666 may use the pump and Stokes
radiation to
illuminate the trapping medium within the cell 222, which in this case serves
as the sample, at a
single focal location at a time; in such an embodiment it forms CARS images
sequentially pixel
by pixel, changing the focal location within the sample between individual
pixel acquisitions by
means of scanning the combined beam across the cell 222. This method may
provide imaging
time of about 1 image frame per second. However, commercially available
optical sources that
can be used in CARS often have maximum output power that far exceeds power
requirements to
the pump and stokes beams for CARS signal generation.
Therefore, in a preferred embodiment, the CARS apparatus 666 includes means
for parallel
CARS signal acquisition, when several pixels of a CARS image are
simultaneously acquired.
This means may include utilizing an array of micro lenses and a matching
photodetector array
having at least as many elements as the array of micro-lenses as the
photodetector 61. In the
embodiment shown in FIG. 12, a spinning micro-lens array disk (MLAD) 78, which
is available
commercially from, for example, Yokogawa Electric Corporation, Japan, is
disposed between
the objective 3 and the dichroic mirror 18, and is complemented with a CCD
camera as the
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
photodetector 61. This optical arrangement is schematically illustrated in
FIG. 13, which shows a
sample illumination arrangement of the CARS apparatus 666 of FIG. 11, from the
spinning
MLAD 78 down to the photodetector 61.
The MLAD 78 includes a plurality of microlenses 781 arranged in a spiral
pattern to raster scan
the sample cell with multiple light beams focused by the micro-lenses into a
plurality of small,
preferably about 1pm3 or less, focal volumes in a plane that is imaged by the
objective 3 onto a
focal plane within the sample cell 222. When the MLAD 78 is spinning, the
plurality of small
focal volumes whereupon portions of the combined excitation beam 111 are
focused scan the
trapping medium within the sample cell 222. Resulting CARS radiation is
collected and focused
1 0 onto a plurality of pixels of the photodetector 61 embodied herein as a
CCD array, for example ¨
as an electron multiplying CCD camera (EMCCD) having 1024 x 1024 detector
pixels, which is
available for example from Andor Technology, Belfast, Northern Ireland.
By way of example, the sample cell has a square 200 p.m x 200 m cross-section
in a plane
normal to the combined beam 111 direction, and has a thickness of 100 pm. The
combined
5 excitation beam 111, optical power 1 W, illuminates a spot on the MLAD 78
of about 200 pm in
diameter, while the diameter of each microlens of the MLAD 78 is 40 Jim,
illuminating up to
about 18 distinct focal locations within the sample cell 2 simultaneously and
providing about
40mW of excitation power at each of these focal locations. In operation, the
MLAD 78 rotates at
a rotation speed of about 5000 rpm or about 83.3 rps (rotations per second),
and raster scans a
2 0 2001tm x 2001tm field of view within the sample cell 2 with a 0.5 pm
spatial resolution when
rotated at about 30 degrees, so that there are 12scans/rotation, providing an
imaging speed of up
to 1000 frames per second.
In this example, total time required to image the full volume of the sample
cell 222 is about 100
millisecond, including 100 depth scans. If the time it takes for the trapping
medium to fill the
2 5 sample cell 222 is sufficiently short, this embodiment of the system of
the present invention
provides real-time testing of the trapping medium flowing through the sample
cell 222 at an
average flow rate of about 4x10-5 cm3 per second. Accordingly, it will take
about 42 minutes to
test 0.01 cc of the trapping medium, about the amount contained in one drop,
corresponding to a
water volume of about 1 cc to 100 cc assuming the pathogen concentration
factor provided by
21
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
the trapping medium is 100 to 10000. This advantageously compares to several
days that are
typically required to fully analyze this amount of water for the presence of
water-borne
pathogens using conventional anti-gene labeling methods, and tens of hours
that would take to
analyze the same amount of sample water using the prior-art Raman method. As
an example,
USEPA method 1623, that requires that a test sample of 100 mL (=100cc) be
analyzed for e-coli
bacteria by mean of a visual analysis of a specially prepared sample by a
skilled technician,
typically takes up to one week to perform.
According to another aspect of the present invention, the exceptionally fast
image acquisition
provided by the CARS apparatus 666 is supported by automated assessment of
acquired images
for the presence of pathogen signatures. This assessment is performed by the
processor 33 as
described hereinbelow.
Turning back to FIG. 11, electrical signals representing individual image
pixels are provided by
the CCD 61 to the processor 33 during or at the end of each raster scan. The
processor 33 forms
from said signals individual images, and automatically analyzes each obtained
image in real time
for the presence of image artifacts characteristic to a particular pathogen.
The processor 33 can
also be programmed to first perform suitable image processing to reduce image
noise and/or
remove non-resonant CARS background and/or image features related to CARS
signals from
the trapping medium or other known non-pathogenic water contaminants.
In one embodiment, the processor 33 includes a memory 37 for storing a
database of reference
CARS images taken at one or more specific CARS frequencies for a plurality of
pathogens
and/or other waterborne microorganisms, and is programmed to compare obtained
CARS images
with the reference images stored in the database.
According to a preferred embodiment of the invention, the processor 33 is
programmed to
analyze the image to assess occurrence of one or more image artifacts having a
shape, size or
2 5 intensity pattern that is characteristic to a CARS image of a
particular pathogen, and if more that
one such artifact is identified, to count the artifacts matching the pre-
determined criteria to
determine the number of the pathogens in the sample.
22
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
In one embodiment, the method of pathogen identification according to the
present invention
includes the following steps:
a) A CARS image of a calibration sample of the trapping medium without
water-borne
pathogens and other contaminants is obtained and stored in memory 37 of the
processor 33 as a
calibration image;
b) the sample cell 222 is filled with the trapping medium 44 carrying a
concentrated water
sample as described hereinabove;
c) A CARS image of the trapping medium within the sample cell is obtained as
described
hereinabove;
d) The stored calibration image obtained in step (a) is subtracted from the
CARS image obtained
in step (c) to obtain a calibrated CARS image;
e)
The calibrated CARS image is analyzed for the presence of image artifacts
having pre-
determined features characteristic to a specific pathogen. Standard methods in
image recognition
such as image segmentation may be used in this step to distinguish pathogens
based on the
CARS intensity profiles. For example, as seen in the images in FIGs. 6A, 6B
and 10, features of
a CARS intensity profile of a single cryptosporidium parvum oocyst at 2845 cm-
1 CARS shift
include a peak with FWHM of 1 IIM -F\-0.5iim on a pedestal of about 5 in -F\-
2 i_im wide; this
intensity pattern is unique to cryptosporidium parvum oocyst and are used in
the invention to
identify this pathogen. Different pathogens have different CARS intensity
profiles/ patterns as
seen in FIG. 7-9 described hereinabove. A library of such CARS images of
various pathogens is
collected and stored in the processor memory.
0
If the calibrated image is determined to contain an artifact matching one
or more pre-
defined criteria and/or one of the stored reference CARS images, a CARS
spectrum of the
pathogen in a "fingerprint region" of CARS frequency shifts is automatically
collected; in a
2 5 preferred embodiment this fingerprint region is between 600 cm-1 and
1800 cm-1, but may differ
therefrom, for example depending on particular pathogens being analyzed. In
one embodiment, if
an image artifact matching a reference image or other pre-determined criteria
for a given
pathogen is detected, the CARS radiation from a sample location corresponding
to the artifact is
23
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
re-directed to a spectrometer 71 using a flip mirror 29, and a CARS spectrum
is detected. In an
embodiment wherein the pump and/or stokes beams are generated using tunable
lasers, for
example as illustrated in FIGs. 4 and 5, this CARS spectrum can be obtained by
varying one of
the pump or Stokes wavelengths in response to a control signal generated by
the processor 33.
Alternatively, the CARS apparatus 666 may utilize the broadband multiplexed
CARS as
described hereinabove, which employs broad bandwidth Stokes pulses and
spectrally narrow
pump pulses to obtain broadband anti-Stokes radiation, herein termed CARS
radiation, which
spectrum can then be directly analyzed for the presence of molecular
vibrational resonances
within the bandwidth of the Stokes radiation. This approach not only reduces
the time for
obtaining the CARS spectrum, but also gives more accurate information on ratio
of intensities of
CARS signal at two or more frequency shifts in the spectrum, and provides a
greater accuracy in
pathogen identification based on multivariate analysis methods. In one
embodiment, optional
optical switches within the Stokes arm 20, which are not shown, can be used to
direct light from
the laser 30 to pass through an alternative PCF 14' instead of the PCF 14
during the CARS
1 5 spectrum acquisition, in order to spectrally broaden the Stokes pulses
so as to generate the
required "fingerprint" spectral region, e.g. from 600 cnil to 1800 cnii, in
the CARS signal.
g) The detected CARS spectrum is then compared to a stored reference spectrum
for the
respective pathogen or, to a library of stored spectra for a plurality of
known pathogens or other
contaminants. In another embodiment, the Raman spectrum is first retrieved
from the CARS
2 r; spectrum, for example using a method described in E. M. Vartianen et
al, "Direct extraction of
Raman line-shapes from congested CARS spectra", Optics Express 14, 3622
(2006), and is
compared to a library of Raman spectra from various known waterborne
pathogens; this method
can be initially preferred since reference Raman spectra are currently more
readily available than
reference CARS spectra of pathogens. Determining whether the recorded CARS
spectrum
25 matches any of the stored reference spectra can be performed using a
variety of known
mathematical algorithms implemented as computer instructions, which would be
apparent to a
skilled practitioner; for example, this step can utilize well-known
multivariate analysis
techniques. By way of example, FIG. 15 illustrates spontaneous Raman spectra
that could be
contained in the computer memory. It shows Raman spectra of live (bottom
curve) and dead (top
30 curve) cryptosporidium parvum oocysts in the "fingerprint region" of 600
cm-1 to 1800 cm-I,
reproduced from US patent 6,950,184. An intensity ratio of peaks at about 1000
cm-1 and 1050
24
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
cm-1, the former corresponding to DNA backbone stretching vibrations, is
different in the live
cryptosporidium parvum oocysts as compared to the dead oocyst, and can be used
for a positive
identification whether a detected image artifact matching cryptosporidium
parvum oocyst is
produced by a live or dead cryptosporidium parvum oocyst. Accordingly, this
step could be
used as an indicator of oocyst viability, in addition to identifying the
cryptosporidium parvum
oocyst.
h) If a match is found between a Raman spectrum stored in memory and the CARS
spectrum
measured from the location of the suspect pathogen in the sample, a pathogen
report and/ or
alarm is generated.
According to the invention, the aforedescribed steps (d) ¨ (h) are performed
or coordinated by
the processor 33, which is also referred to herein as the computer and
includes stored computer
instructions for performing these tasks automatically in real time without
human intervention,
preferably for each generated image while the images are generated by the CARS
apparatus 666,
thereby advantageously enabling automated real-time pathogen detection and
identification.
1 5 Accordingly, the CARS-based pathogen detection system and method of the
present invention
enables real-time water monitoring for the presence of water-borne pathogens,
while
simultaneously enabling automated detection and authentication of the
pathogens.
Note that the exemplary embodiments of the system and method of the present
invention
described are by way of example only , and alternative embodiments of many
elements and
2 0 steps can be employed in particular applications of the invention as
would be evident for those
skilled in the art.
For example, the trapping medium 444 can include various trapping materials
and can be
chemical trapping medium or physical trapping medium, liquid or solid, for
example based on
microporous materials capable of trapping pathogens preferably without
destroying them. In one
embodiment, the trapping medium is liquid and continuously flows through the
sample cell
during the CARS imaging at a known flow rate, and the processor 33 can be
programmed to
account for the sample movement between successive pixel acquisitions so as to
correct for
image distortions due to the sample flow.
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
In another embodiment, the flow of the trapping medium 444 is stopped while
the CARS images
of the sample volume are acquired, after which the sample cell 222 is
refilled.
In yet another embodiment, the trapping medium 444 is a substantially solid
microporous filter,
for example in the form of a continuous sheet that is slowly pulled through
the water container
40, where it acquires a concentrated water sample, i.e. water contaminants
such as pathogens and
microscopic amount of water trapped by the trapping medium, and is then
provided at a
predetermined rate or at predetermined intervals to the CARS apparatus 666. In
an embodiment
wherein the trapping medium is solid, it may have a specially prepared surface
such as that used
in surface enhanced Raman scattering (SERS) for enhancing the CARS signal.
1 0 General steps of the method of the present invention for assessing the
presence of a pathogen in
water according to a preferred embodiment thereof are summarized in a
flowchart shown in FIG.
14. As shown, the method includes:
Flowing water to be analyzed through a trap medium in a first step 310 to form
a water sample
wherein pathogens may be concentrated;
'15 In a next step 320, continuously or sequentially moving the trap medium
carrying the test sample
out of the water to a CARS imager;
In a step 330, irradiating the sample with first, i.e. pump, radiation having
a spectrum centered at
a first frequency and second, i.e. Stokes, radiation having a spectrum
including a second
frequency, wherein the first frequency exceeds the second frequency by a pre-
determined non-
2 0 zero frequency shift characteristic to the pathogen;
In a step 340, detecting third, i.e. anti-Stokes or CARS, radiation scattered
from or transmitted
through the sample at a third frequency that is different from the first and
second frequencies, so
as to form an image of at least a portion of the sample;
In a step 350, analyzing the image to assess occurrence of at least one image
artifact having one
25 or more pre-determined features characteristic of the pathogen;
If an artifact with the pathogen-specific features is found, performing the
following steps:
26
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
in a step 360 obtaining the spectrum of the third radiation from a location
within the sample
corresponding to the image artifact, for example by performing a CARS
frequency scan as
described hereinabove, or utilizing broadband multiplexed CARS; in a step 370
comparing one
of the spectrum of the third radiation, i.e. the CARS spectrum, or a
corresponding Raman
spectrum obtained therefrom with a saved reference spectrum; if a match is
detected, in a step
380 generating a pathogen report or an alarm.
The steps 330-380 are repeated for a next test sample or a next location in
the test sample, as
schematically shown by a block 390.
The apparatus and methods described herein can be used in water treatment
facilities, centralized
11 0 water testing facilities for testing water samples from various
locations and water reservoirs, and
the likes, to assess occurrence in water or water samples of substantially any
water-borne
pathogen that exhibits identifiable CARS spectrum and CARS image
characteristics. Examples
of pathogens that can be detected in water samples using the methods described
herein include
protozoa such as those of the genus Cryptosporidium and the genus Giardia;
bacteria such as
Escherichia coli, Yersinia pestis, Francisella tularensis, Brucella species,
Clostridium
perfringens, Burkholderia mallei, Burkholderia pseudomallei, Chlamydia
psittaci, Coxiella
burnetii, Rickettsia prowazekii, Vibrio species; Enterococcus faecalis;
Staphylococcus
epidermidis; Staphylococcus aureus; Enterobacter aerogenes; Corynebacterium
diphtheriae;
Pseudomonas aeruginosa; Acinetobacter calcoaceticus; Klebsiella pneumoniae;
Serratia
) 3 marcescens; yeasts such as Candida albicans; and viruses, including
filoviruses such as Ebola
and Marburg viruses, naviruses such as Lassa fever and Machupo viruses,
alphaviruses such as
Venezuelan equine encephalitis, eastern equine encephalitis, and western
equine encephalitis,
rotoviruses, calciviruses such as Norwalk virus, and hepatitis (A, B, and C)
viruses, and
biological warfare agents such as smallpox (i.e., variola major virus). The
methods described
2 5 herein can be used to distinguish between viable and non-viable forms
of these organisms and
between infectious and non-infectious forms.
Although the invention has been described hereinabove with reference to
particular embodiments
thereof, it should be understood that theses embodiments are examples only and
should not be
27
CA 02594444 2007-07-23
Doc No: 103-8 CA
Patent
construed as limiting the invention. It should also be understood that each of
the preceding
embodiments of the present invention may utilize a portion of another
embodiment.
Of course numerous other embodiments may be envisioned without departing from
the spirit and
scope of the invention.
28