Note: Descriptions are shown in the official language in which they were submitted.
CA 02595013 2016-01-06
FUSION PROTEINS COMPRISING GM-CSF AND FC FRAGMENT
WITH MODIFIED HINGE REGION
Related Application
[0001] This application is related to US Patent Application No.
11/340,661 filed 27
January 2006 and PCT Patent Application No. PCT/CA2007/000107 filed 25 January
2007.
Technical Field
[0002] This application relates to fusion proteins.
Background
[0003] Recombinant human proteins corresponding to their natural amino
acid sequences
have been used for the treatment and diagnosis of a broad range of human
diseases since the
1980s. However, most recombinant human proteins do not survive long enough in
vivo and are
rapidly cleared from circulation. For example, proteins with a molecular mass
less than 20 kDa
have been reported to be filtered at the level of renal tubules, often leading
to a dose-dependent
nephrotoxicity. The short in vivo half-life of these proteins compromises
their natural biological
functions, requiring higher doses or more frequent administration, which in
turn impairs patient
1
CA 02595013 2007-07-26
compliance and increases the burden on health care providers. These clinical
demands merit the
search and development of therapeutic proteins with longer circulation half-
life.
[0004] In addition to the direct mutations of individual protein
structure for achieving
longer half-life (e.g. ARANESPTM by Amgen and TNI(nase by Genentech), two
systemic
approaches have been used for the creation of therapeutic proteins with longer
half-life. One is
"PEGylation", which refers to chemical cross-linking of polyethylene glycol
(PEG) compounds
to target proteins. PEG-bound proteins have larger molecular sizes and are
more slowly cleared
from the circulation. PEGylation has been clinically demonstrated and
recognized by the biotech
industry as a standard method of extending the half-life of various target
proteins. A
shortcoming of PEGylation is the significant impairment of the biological
activity of target
proteins. The altered structure of PEGylated proteins also risks generating an
immunogenic
response in the human body.
[0005] Another systemic approach is the genetic fusion of target
therapeutic protein(s)
with another human carrier protein to stabilize the target protein in
circulation in the form of a
fusion protein complex. Two ideal human carrier protein candidates for fusion
with therapeutic
proteins are human immunoglobulin and albumin. Both immunoglobulin and albumin
are very
stable and abundant in blood. Fusion proteins comprising a therapeutic protein
and either
immunoglobulin or albumin would theoretically retain the biological activity
of the therapeutic
protein, be more stable in circulation than the therapeutic protein alone, and
be completely
homologous to natural human proteins, minimizing the risk of an immunogenic
responses [1,2].
2
CA 02595013 2007-07-26
,
,
,
[0006] One practical strategy with this approach is to
genetically fuse a therapeutic
protein with an Fc fragment of a human immunoglobulin [1, 3, 4]. Modern
bioengineering
technology has successfully created fusion proteins consisting of a
therapeutic protein, such as
cytokines and soluble receptors, and an Fc fragment of immunoglobulin G (IgG)
[5-26]. For
example, IL-10, an anti-inflammatory and anti-rejection agent, has been fused
to the N-terminal
of murine Fc.gamma.2a to increase IL-10's short circulating half-life [9]. In
another example,
the N-terminal of human IL-2 has been fused to the Fc portion of human IgG 1
or IgG 3 to
overcome the short half life of IL-2 and its systemic toxicity [26]. Two
fusion proteins
comprising an Fc fragment have been successfully developed as biomedicines and
approved by
FDA for the treatment of rheumatoid arthritis and chronic plaque psoriasis
[27, 28, 29].
[0007] Human IgG is composed of four polypeptides (two identical
copies of light chain
and heavy chain) covalently linked by disulfide bonds. The proteolysis of IgG
by papain
generates two Fab fragments and one Fc fragment. The Fc fragment consists of
two
polypeptides linked by disulfide bonds. Each polypeptide, from the N-terminal
to C-terminal, is
composed of a hinge region, a CH2 domain and a CH3 domain. The structure of
the Fc fragment
is nearly identical across all subtypes of human immunoglobulin. IgG is one of
the most
abundant proteins in the human blood and makes up 70 to 75 % of the total
immunoglobulin in
human serum. The half-life of IgG in circulation is the longest among all five
types of
immunoglobulin and may reach 21 days.
[0008] Disulfide bonds formed between thiol groups of cysteine
residues play an
important role in the folding and stability of proteins, usually when proteins
are secreted to an
3
CA 02595013 2007-07-26
extracellular medium. The disulfide bond stabilizes the folded form of a
protein in several ways.
First, it holds two portions of the protein together, biasing the protein
towards the folded state.
Second, the disulfide bond may form the nucleus of a hydrophobic core of the
folded protein, i.e.,
local hydrophobic residues may condense around the disulfide bond and onto
each other through
hydrophobic interactions. Third, and related to the first and second points,
by linking two
segments of the protein chain and increasing the effective local concentration
of protein residues,
the effective local concentration of water molecules is lowered. Since water
molecules attack
amide-amide hydrogen bonds and break up secondary structures, disulfide bonds
stabilize
secondary structure in their vicinity. For example, researchers have
identified several pairs of
peptides that are unstructured in isolation, but adopt stable secondary and
tertiary structure upon
forming a disulfide bond between them. The native form of a protein is usually
a single disulfide
species, although some proteins may cycle between a few disulfide states as
part of their function.
In proteins with more than two cysteines, non-native disulfide species, which
are almost always
unfolded, may be formed.
[0009] A flexible junction region of the fusion protein which allows the
two ends of the
molecule to move independently plays a very important role in retaining each
of the two
moieties' functions separate and efficient. Therefore, the junction region
should act as a linker
which combines the two parts together, and as a spacer which allows each of
the two parts to
form its own biological structure and not interfere with the other part.
Furthermore, in order to
avoid the induction of immunogenicity, the junction region should be native to
the human body
and simple in structure [5, 25].
4
CA 02595013 2007-07-26
[00010] The primary structure of the hinge region of immunoglobulin
includes three
cysteines, such as cys223, cys229 and cys232 in the case of the human IgG 1
structure used by the
present inventors. While the cys229 and cy5232 form two interchain disulfide
bonds by binding
between counterparts of the two chains, the cys223 remains free. Therefore, it
is highly possible
that this free cysteine may bind with another intrachain or interchain
cysteine, to form a non-
native disulfide bond in the protein maturation process upon secretion from
host cells or during
subsequent purification. This non-native disulfide bond may not only alter the
structure and
conformation of the therapeutic protein, but may also interfere with the
biological activity of the
therapeutic protein or induce harmful immunogenicity when the fusion protein
is administrated
into the human body.
[00011] Many therapeutic proteins such as erythropoietin (EPO) and
granulocyte
macrophage colony-stimulating factor (GM-CSF) have a cysteine near their C-
terminal. The
role of this cysteine in maintaining proper structure and function has yet to
be well-defined. The
cysteine proximal to the C-terminal may be essential for maintaining proper
structure, facilitating
correct folding or retaining normal biological activity. The inventors
hypothesize that if proteins
with a cysteine near its C-terminal are fused to the natural sequence of the
hinge region of a Fc
fragment, the very limited space between the last cysteine of the C-terminal
of the fused protein
and the first cysteine of the N-terminal of the Fc fragment (cys223) may lead
to the formation of
an unexpected disulfide bond between these two cysteines. The formation of the
unexpected
disulfide bond may alter the structure and/or the folding of the fused protein
component as well
as alter the flexibility of the hinge region. As a result, normal functions of
the fused therapeutic
protein in the fusion protein complex may be impaired.
CA 02595013 2007-07-26
,
,
,
[00012] Even if the target therapeutic protein does not contain a
cysteine near its C-
terminal, another cysteine in its structure may, after three dimensional
folding, become
sufficiently close to the free cysteine (cys223) of the hinge region to form a
non-natural disulfide
bond that may alter the structure and biological activity of the fused target
protein. The
inventors' hypothesis may partially explain why there has yet to be any
clinically-proven success
in attempts to create functional fusion proteins with widely-used growth
factors such as EPO, G-
CSF and GM-CSF, etc.
[00013] Previous reports have used various methods to create
fusion proteins between a
therapeutic protein and an Fc fragment/immunoglobulin molecule. In most of
these reports,
researchers changed amino acid sequences of the target protein, added a linker
peptide between
the C-terminal of the target protein and the N-terminal of the hinge region of
Fc fragment, or
truncated the hinge region of the Fc fragment of the hinge region (resulting
in the removal of the
free cysteine (cys223)).
[00014] In U.S. Patent No. 5,908,626, a fusion protein of IFN 0
with a human
immunoglobulin Fc fragment is described which was linked by a synthetic
oligopeptide
(GGS)2(GGGS)2 [6]. The inventors in that patent believe this linker can
"reduce the possibility
of generating a new immunogenic epitope (a neoantigen) at what would otherwise
be the fusion
point of the IFN13 and the immunoglobulin Fc fragment". In U.S. Patent Nos.
6,797,493,
6,900,292, 7,030,226, 7,226,759, and 7,232,668, the hinge region was replaced
by a 16-amino
acid peptide linker GS(GGGS)3GS [10, 12, 13, 20, 21]. In addition to the
genetic approach,
6
CA 02595013 2007-07-26
chemical manipulation has also been used to address the problem of non-native
disulfide bonds.
For example, the inventors in U.S. Patent No. 6,808,902 developed a process
for treating an IL-
lra-Fc fusion protein with a copper (II) halide in order to prevent or correct
a non-native
disulfide bond which caused misfolding of that fusion protein [12]. An Fc-EPO
fusion protein
(rather than the conventional EPO-Fc fusion) has shown poor pharmacokinetics
and little EPO
efficacy in mice; mutation of four amino acids of the EPO molecule is required
to obtain a
functional Fc-EPO fusion protein [30].
[00015] As mentioned above, the hinge region plays the role of the
flexible junction
region between the fused therapeutic protein and the Fc fragment (CH2 and
CH3). Truncation or
significant changes of the hinge region may have undesirable effects on
ability of the hinge
region to act as flexible junction. The addition of peptide linkers may not
only impair the natural
conformation of the fusion protein but also greatly increase the risk of
immunogenecity by
introducing a non-native structure.
[00016] The need exists for therapeutic protein/Fc fragment fusion
proteins that have a
prolonged half-life and/or enhanced activity without increasing the risk of an
immunogic
response.
Summary of the Invention
[00017] According to one aspect of the present invention, a fusion protein
having a non-
immunoglobulin polypeptide and a human immunoglobulin component is provided.
The fusion
7
CA 02595013 2007-07-26
protein may have a prolonged half-life in vivo in comparison to naturally
occurring or
recombinant native non-immunoglobulin protein. The fusion protein may also
have enhanced
biological activity in comparison to the native non-immunoglobulin protein.
[00018] In one aspect of the invention, the C-terminal of the non-
immunoglobulin
polypeptide may be directly linked to the N-terminal of the human
immunoglobulin component.
The non-immunoglobulin polypeptide portion of the fusion protein may, for
example, be a
cytokine, a ligand-binding protein, a hormone, a neurotrophin, a neutrophin
receptor, a body-
weight regulator, a serum protein, a clotting factor, a protease, an
extracellular matrix component,
an angiogenic factor, an anti-angiogenic factor, an immunoglobulin receptor, a
blood factor, a
cancer antigen, a statin, a growth factor, a non-human protein, a non-
mammalian protein or a
protein toxin.
[00019] In one aspect of the invention, the human immunoglobulin component
of the
fusion protein may be a FC fragment derived from IgG 1. The FC fragment may
have a hinge
region and CH2 and CH3 domains. The hinge region may be at least 9 amino
acids, and may be
between 10 and 20 amino acids. The first cysteine from the N-terminal of the
hinge region may
be substituted with a non-cysteine amino acid such as glycine or alanine.
[00020] According to one aspect of the present invention, a fusion protein
having a non-
immunoglobulin polypeptide having a cysteine residue proximal to the C
terminal thereof, and a
immunoglobulin component with a hinge region is provided. The cysteine residue
of the hinge
region located nearest the non-immunoglobulin polypeptide is spaced at a
distance from the
8
CA 02595013 2007-07-26
cysteine residue of the non-immunoglobulin polypeptide. The distance is
sufficient to prevent a
disulphide bond from forming between the proximal cysteine residue of the
hinge region and the
cysteine residue of the non-immunoglobulin polypeptide.
Brief Description of the Drawings
[00021] In drawings intended to illustrate various embodiments of the
invention but which
are not intended to be constructed in a limiting manner.
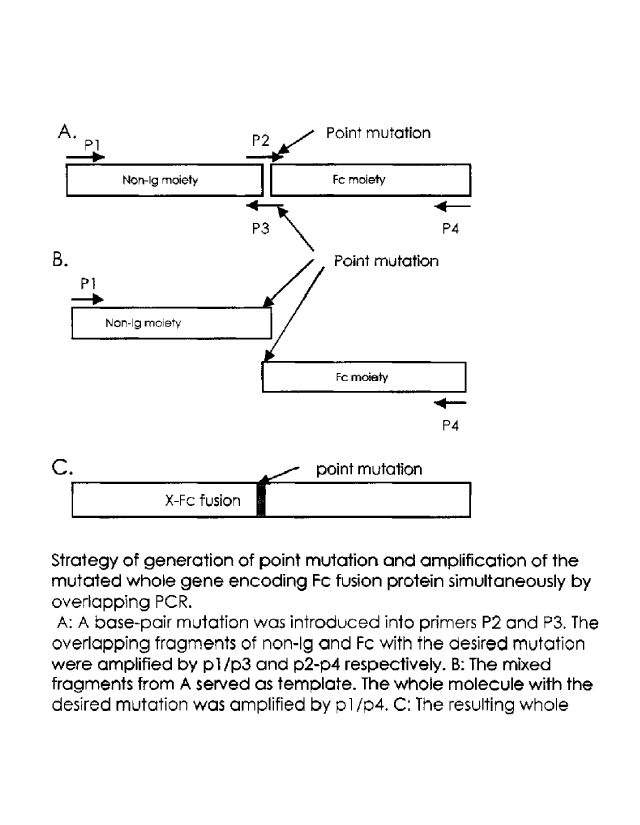
[00022] Figures 1 A to 1C are schematic diagrams illustrating the strategy
for generating
point mutations and the amplification of the mutated whole gene encoding a Fc
fusion protein
simultaneously by overlapping PCR. A: A base-pair mutation was introduced into
primers P2
and P3. The overlapping fragments of non-Ig and Fc with the desired mutation
were amplified by
pl/p3 and p2/p4 respectively. B: The mixed fragments from A served as a
template. The whole
molecule with the desired mutation was amplified by pi/p4. C: The resulting
whole molecule
with the desired mutation.
[00023] Figure 2A is a schematic diagram showing the general structure of
the DNA
molecule encoding the recombinant human EPO-FcG fusion protein (rHuEPO-FcG) in
which the
first cysteine from the N-terminal of the hinge region (cys223) is substituted
by glycine and used
as the mutant hinge region for the construction of an EPO-Fc fusion protein.
This mutant hinge
region-containing Fc fragment is referred to as FcG, and the Fc fragment
containing the hinge
region with the native cysteine at the sixth residue from its N-terminal as
FcC respectively.
9
CA 02595013 2007-07-26
[00024] Figure 2B is a sequence listing showing the nucleotide sequence
[SEQ ID NO:1]
and the deduced amino acid (aa) sequence [SEQ ID NO:2] of rHuEPO-FcG protein.
The total
length of DNA is 1281 bp. The 426 amino acids in the deduced protein sequence
include 27 an
for the signal peptide and 399 an for the complete rHuEPO-FcG protein. The
complete rHuEPO-
FcG protein consists of human EPO domain (166 aa), hinge region (16 aa,
underlined), and CH2
and CH3 domains (217 aa) of the Fc fragment of human IgGl. The calculated
molecular weight
of the polypeptide of the mature rHuEPO-FcG fusion protein is 44.6 kDa,
composed of 18.5 kDa
(41.4%) of EPO fragment and 26.1 kDa (58.6%) of IgG 1 Fc fragment. A homodimer
is formed
by two disulfide bonds via the two cysteine residues (boxed) within the hinge
region. At residue
172 of the mature fusion protein (i.e. the 6th amino acid of hinge region) the
native cysteine
residue has been substituted by glycine (bold).
[00025] Figure 3A is a schematic diagram showing the general structure of
the DNA
molecule encoding the wild type human EPO-FcC fusion protein (rHuEPO-FcC) in
which the
first cysteine from the N-terminal of the hinge region (cys223) is maintained.
[00026] Figure 3B is a sequence listing showing the nucleotide sequence
[SEQ ID NO:3]
and the deduced amino acid (aa) sequence [SEQ ID NO:4] of a wild type rHuEPO-
FcC protein.
The sequence particulars are the same as shown in Figure 2B except that the
native, wild type
cysteine residue is maintained at residue 172 of the mature fusion protein
(i.e. the 6th amino acid
of the hinge region).
CA 02595013 2014-02-20
[00027] Figure 4A is a schematic diagram showing the general structure of
the DNA
molecule encoding the fusion protein between native GM-CSF molecule and the
FcG fragment
(HuGMCSF-FcG).
[00028] Figure 4B is a sequence listing showing the nucleotide sequence
[SEQ ID NO:5]
and the deduced amino acid (ha) sequence [SEQ ID NO:6] of rHuGMCSF-FcG fusion
protein.
The total length of DNA is 1131 bp. The 377 amino acids in the deduced protein
sequence
include 17 an for the signal peptide and 360 aa for the complete HuGMCSF-FcG
fusion protein.
The complete rHuGMCSF-FcG fusion protein consists of complete GM-CSF molecule
(127 aa),
mutant hinge fragment (16 an, underlined), and CH2 and CH3 domains (217 aa) of
the Fe
fragment of human IgG 1. The calculated molecular weight of mature rHuGMCSF-
FcG fusion
protein is 40.6 kDa, composed of 14.5 kDa (35.7%) of GM-CSF fragment and 26.1
kDa (64.3%)
of I.g.G 1 Fe fragment. A homodimer is folmed by two disulfide bonds via the
two cysteine
residues (boxed) within the hinge region. At residue 150 of the fusion protein
(i.e. the 6th amino
acid of hinge region) the native cysteine residue has been substituted by
glycine (bold).
[00029] Figure 5 is an image showing the sizes of the dimeric form of pure
rHuEPO-FcG
protein in non-reduced condition (column A) and monomeric form of pure rHuEPO-
FcG protein
in reduced condition (column B) by SDS-PAGE analysis. The purified rHuEPO-Fc
protein from
the supernatants of the cultured CHO cell-line expressing rHuEPO-FcG exists
mainly as the
dimeric form and has a molecular weight of about 180 kDa on 8% bis-tris gel in
non-reduced
condition. In reduced condition (100 mM dithiothreitol (DTT)) to break
disulfide bonds, the
dimer is separated into two identical monomeric units with a molecular weight
of 75 kDa.
11
CA 02595013 2007-07-26
[00030] Figures 6A and 6B are graphs showing the dose-dependent increase
of
hemoglobin (Hb) levels in normal mice treated three times per week with a
subcutaneous
injection (s.c.) of rHuEPO-FcG or rHuEPO. Each point represents the mean Hb
level of the
group (6 mice). Day 0 levels represent the Hb levels before treatment. A: Mice
treated with
rHuEPO-FcG. B: Mice treated with native rHuEPO.
[00031] Figure 7A and 7B are graphs showing the dose-dependent increase of
hemoglobin
(Hb) levels in normal mice treated with once per week s.c. of rHuEPO-FcG or
rHuEPO. Each
point represents the mean Hb level of the group (6 mice). Day 0 levels
represent the Hb levels
before treatment. A: Mice treated with rHuEPO-FcG. B: Mice treated with native
rHuEPO.
[00032] Figure 8A and 8B are graphs showing the increase of hemoglobin
(Hb) levels in
normal mice treated with intravenously injection (i.v.) of 12.5 p.g/kg of
rHuEPO-FcG or rHuEPO.
Each point represents the mean Hb level of the group (6 mice). Day 0 levels
represent the Hb
levels before treatment. A: Mice with treatment once a week. B: Mice with
treatment 3 times a
week.
[00033] Figure 9 is a graph showing the dose-dependent increase of
hemoglobin (Hb)
levels in 5/6 nephrectomized rats treated with once per week s.c. of rHuEPO-
FcG, rHuEPO or
darbepoetin-alfa (abbreviated Darbe.). Each point represents the mean Hb level
of the group.
Normal controls were normal rats with injection of carrier solution. Model
controls were the 5/6
12
CA 02595013 2007-07-26
nephrectomized rats with injection of carrier solution. Week 0 levels
represent the Hb levels
before treatment. *: week(s) post treatment.
[00034] Figure 10 is a graph showing the dose-dependent increase of
hemoglobin (Hb)
levels in 5/6 nephrectomized rats treated once every two weeks s.c. with
rHuEPO-FcG, rHuEPO
or darbepoetin-alfa (abbreviated Darbe.). Each point represents the mean Hb
level of the group.
Normal controls were normal rats with injection of carrier solution. Model
controls were the 5/6
nephrectomized rats with injection of carrier solution. Week 0 levels
represent the Hb levels
before treatment. *: week(s) post treatment.
[00035] Figure 11 is a graph showing the dose-dependent increase of
hemoglobin (Hb)
levels in 5/6 nephrectomized rats treated once every two weeks i.v. with 62.5p
g/kg of rHuEPO-
FcG, or darbepoetin-alfa (abbreviated Darbe.). Each point represents the mean
Hb level of the
group. Normal controls were normal rats with injection of carrier solution.
Model controls were
the 5/6 nephrectomized rats with injection of carrier solution. Week 0 levels
represent the Hb
levels before treatment. *: week(s) post treatment.
[00036] Figures 12A to 12C are graphs comparing the potency of rHuEPO-FcG,
rHuEPO
and darbepoetin-alfa in stimulating the colony formation of CFU-E and BFU-E in
5/6
nephrectomized rats treated with different doses and schedules. rHuEPO-FcG and
darbepoietin-
alpha (abbreviated Darbe.) treatment showed similar dose-dependent potencies
for stimulating
the CFU-E and BFU-E colony formation, while rHuEPO was less potent. A: s.c.
once every
week. B: s.c. once every 2 weeks. C: i.v. once every two weeks.
13
CA 02595013 2014-02-20
[00037] Figure 13 is a graph showing the serum levels of rHuEPO-FcG and
rHuEPO after
the intravenous injection of 5 ug:/kg of rHuEPO-FcG or rHuEPO to Rhesus
monkeys (mean
levels of 5 monkeys).
[00038] Figure 14 is a graph showing the dose-dependent increase of
hemoglobin (Hb)
levels in normal mice treated three times per week with subcutaneous injection
(s.c.) of rHuEPO-
FcG, rHuEPO-FcC and rELIFPO. Each point represents the mean Hb level of the
_group (8).
Normal control was normal mice with injection of carrier solution. Day 0
levels represent the
Hb levels before treatment.
[00039] Figure 15 is a graph showing the dose-dependent increase of
hemoglobin (Hb)
levels in normal mice treated once per week with subcutaneous injection (s.c.)
of rHuEPO-FcG,
rHuEPO-FcC and rHuEPO. Each point represents the mean Hb level of the group
(8). Normal
control was normal mice with injection of carrier solution. Day 0 represents
the Hb levels before
treatment.
[00040] Figure 16 is a graph comparing,- the growth of white blood cells
(WBC) in dogs
with experimental neutropenia by rHuGMCSF-FcG or by rHuGMCSF. 60Co y-ray
irradiated
dogs were treated s.c. with 10 ug-lka of rHuGMCSF-FcG every other day, 20 ug_-
/kg of
rHuGMCSF-FcG every other day, or 20 ug/kg of rHuGMCSF every day. Day -1 and
day 1
represent the day before irradiation and the next day after irradiation
respectively. Treatment
started from day 1 and lasted for 10 days. Clinical observation and
examination of WBC counts
14
CA 02595013 2007-07-26
lasted for 28 days. Each point represents the mean WBC counts of the group.
Model controls
were the irradiated dogs with injection of carrier solution only.
Detailed Description of the Invention
[00041] Throughout the following description specific details are set
forth in order to
provide a more thorough understanding of the invention. However, the invention
may be
practiced without these particulars. In other instances, well known elements
have not been
shown or described in detail to avoid unnecessarily obscuring the present
invention.
Accordingly, the specification and drawings are to be regarded in an
illustrative, rather than a
restrictive sense.
[00042] This invention relates to recombinant human fusion proteins
combining a target
protein or polypeptide, such as a non-immunoglobulin polypeptide, with a Fc
fragment or
immunoglobulin molecule. The Fc fragment or Ig molecule includes a mutant
hinge region
wherein the first cysteine from the N-terminal of the hinge region (sixth
amino acid, cys223) is
replaced by a non-cysteine residue, such as a non-charged, non-polar amino
acid (neutral amino
acid). The result is a mutant hinge region which maintains the natural length
and flexibility of
the hinge region without the free cysteine that may lead to the formation of a
non-natural
disulfide bond with a cysteine in the non-immunoglobulin polypeptide. The
mutant hinge region
may comprise the whole or part of the human Fc fragment, or the whole or part
of a human
immunoglobulin molecule.
CA 02595013 2007-07-26
[00043] When any non-immunoglobulin polypeptide is directly fused to the
hinge region
of the Fe fragment or Ig molecule to form a protein-Fe or protein-Ig fusion
protein, respectively,
it is believed the mutant hinge region lacking the N-terminal free cysteine
allows the fused target
polypeptide to maintain its structure, folding and biological functions. The
fusion proteins
created with this mutant hinge region have near 100% sequence identity to
natural sequences of
both the target protein and hinge region, and therefore possess minimum
immunogenecity risks.
[00044] The first cysteine from the N-terminal of the hinge region of human
Fe
fragment/immunoglobulin (sixth amino acid, cys223) may be substituted with any
non-charged,
non-polar amino acid (neutral amino acid). In the example disclosed herein,
glycine was used to
replace the free cysteine. A person skilled in the art would appreciate that
other amino acids
could also replace the free cysteine.
[00045] The mutant hinge region that is formed by substituting the free
cysteine near its
N-terminal as part of the Fe fragment or Ig molecule provides a method or
platform for
generically producing fusion proteins between any non-irnmunoglobulin
polypeptide and Fc/Ig
to prevent non-naturally occurring disulfide bonds, and thus retain the
biological functions of the
fused target polypeptide.
[00046] Several methods can be used to make the desired point mutation. One
method,
described in Example 1 of this invention, adopts overlapping PCR to amplify
the whole nucleic
acid sequence of the fusion protein. With specifically-designed oligo primers,
the desired point-
mutation can be introduced into the resulting nucleic acid sequence following
gene amplification.
16
CA 02595013 2007-07-26
Other methods, such as the Quick-ChangeTM mutagenesis method from Invitrogen
or artificial
gene synthesis can also be used to produce the point mutation. A person
skilled in the art would
appreciate that any number of different methods could be used to substitute
the free cysteine of
the hinge region (cys223).
[00047] The fusion protein created by using the mutant hinge region
according to an
embodiment of the present invention comprises a non-Ig moiety linked to an Fc
fragment of IgG
expressed by the formula "X- hinge region-CH2-CH3", wherein X represents the
non-Ig moiety,
and CH2 and CH3 represent two heavy chain domains of the Fc fragment of IgG.
[00048] The hinge region refers to the region between the CH1 and CH2
heavy chain
domains that contains the interchain disulfide bonds. Flexibility in this
region allows the
molecules on both sides of hinge region to move independently. The heavy
chains are also
glycosylated in this region, which helps protect this relatively exposed area
against degradation.
In one embodiment, the non-Ig moiety of the fusion protein links to the hinge
region directly, i.e.
the C-terminal of the non-Ig moiety is directly fused to the N-terminal of
hinge region. The first
cysteine (Cys223) from the N-terminal of the hinge region may be substituted
by a non-charged,
non-polar amino acid, such as glycine. In some embodiments, a synthetic
linker, such as (G4S)3
or G4SG5S, may be inserted between the non-Ig moiety and Fc fragment to ensure
each part
folds properly. In other embodiments, one or more of the amino acid residues
upstream of the
first cysteine (Cys223) site may be removed.
17
CA 02595013 2007-07-26
[00049] The non-immunoglobulin polypeptide may be, but is not limited to,
any
polypeptide sequence with human or non-human origin, having complete or non-
complete amino
acid sequences corresponding to any defined human and non-human proteins,
exhibiting
biological functions or non-biological functions, made artificially or
obtained naturally. The
non-immunoglobulin polypeptide may also be a variant of any proteins defined
or non-defined
before. These variants include but are not limited to polypeptide sequences
modified from a
native protein sequence but still partially or completely retaining its
biological functions.
Modifications include but are not limited to substitution, addition,
insertion, deletion, or
rearrangement of the amino acids of the native polypeptide sequences.
[00050] The non-immunoglobulin polypeptide may, for example, be a
cytokine.
"Cytokine" is used herein to describe proteins, analogs thereof, and fragments
thereof which are
produced by and excreted from a cell, and which elicit the biological response
by binding with
corresponding receptors. Cytokines include but are not limited to
hematopoietic factors such as
EPO, GM-CSF and granulocyte colony stimulating factor (G-CSF), interferons
such as IFN a,
IFN I3 and IFN y, interleukins such as IL-2, IL-4, IL-5, IL-6, IL- 7, IL-10,
IL-11, IL-13, IL-14,
IL-15, IL-16 and IL-18, tumor necrosis factors such as TNF a, and lymphokines
such as
lymphotoxin.
[00051] The non-immunoglobulin polypeptide may also be a ligand-binding
protein that
may block a receptor-ligand interaction at the cell surface, or neutralize the
biological activity of
another molecule in the body fluids. Ligand-binding proteins include but are
not limited to CDs
molecules, CTLA-4, TNF receptors, and interleukin receptors.
18
CA 02595013 2007-07-26
[00052] The non-immunoglobulin polypeptide may also be a hormone, a
neurotrophin, a
neutrophin receptor (e.g. Trk A), a body-weight regulator, a serum protein, a
clotting factor, a
protease, an extracellular matrix component, an angiogenic factor, an anti-
angiogenic factor, an
immunoglobulin receptor(e.g. IgG receptor), a blood factor (e.g. Factor VIII,
Factor IX, Factor
X), a cancer antigen (e.g. PSA, PSMA), a statin (e.g. endostatin, angiostatin)
or a growth-factor
(e.g. Flt-3).
[00053] The non-immunoglobulin polypeptide may also be a non-human or non-
mammalian protein, or even a protein toxin. Examples include gp120, HIV
transactivators,
surface proteins from other viruses such as HBV, HCV and RSV, and parasitic
surface proteins
such as malarial antigens.
[00054] A recombinant vector with the nucleic acid sequence encoding the
Fc fragment
containing a mutant hinge region may be constructed. This vector, which
possesses all the
elements needed for its propagation, selection, and screening in either
prokaryotic cells (such as
E. coli) or eukaryotic cells (such as CHO cells), can serve as a platform to
express Fc fusion
proteins described in this invention. By use this platform, the nucleic acid
sequence encoding
the non-Ig moiety of the fusion protein is conveniently inserted in-frame into
the vector at the 5'-
end of nucleic acid sequence encoding the mutated Fc moiety by molecular
cloning techniques.
[00055] As a specific example, a novel fusion protein having enhanced
erythropoietic
properties was produced according to the present invention. The fusion
protein, referred to
19
CA 02595013 2007-07-26
,
,
,
herein as rHuEPO-FcG, comprises a human EPO molecule genetically linked to an
immunoglobulin Fc fragment containing a mutant hinge region of the present
invention. The
nucleic acid sequence of the rHuEPO-FcG fusion protein of the present
invention is shown in
SEQ ID No: 1, and the corresponding deduced amino acid sequence is shown in
SEQ ID No: 2.
As discussed further below, the fusion protein may be in the form of a dimer
comprising two
identical polypeptide subunits. Each polypeptide subunit, from the N-terminal
to C-terminal,
includes the polypeptide sequence of the human EPO molecule, and the
polypeptide sequence of
the hinge region, CH2 domain and CH3 domains of the Fc fragment of human
immunoglobulin
IgG 1. The two polypeptide subunits are joined by disulfide bonds between the
respective hinge
regions to form the dimer structure. The dimer has the same general shape as
an IgG molecule
and exhibits better stability than free EPO molecules as demonstrated in the
examples below.
[00056] As will be apparent to a person skilled in the art, the
hinge region of an intact
immunoglobulin provides the protein sufficient flexibility for effective
antigen-antibody binding.
Similarly, in the present invention, the hinge region in which the free
cysteine (cys223) is
substituted by glycine is included in the design of the rHuEPO-FcG fusion
protein to maintain its
flexibility, particularly when the fusion protein is in the dimer form. As
described below, this
likely allows the normal binding of the EPO portion of the rHuEPO-FcG fusion
protein to EPO
receptors to effect the biological functions of EPO. It is believed that the
dimer form of the
rHuEPO-FcG fusion protein, by providing two EPO molecules, is capable of
inducing optimal
activation of EPO receptors (for example, by facilitating receptor cross-
linking).
CA 02595013 2007-07-26
[00057] As demonstrated in the examples set forth below, the rHuEPO-FcG
fusion protein
has been successfully synthesized using recombinant DNA techniques. The fusion
protein has
been shown in mice, rat and primate studies to exhibit a prolonged in vivo
half-life and enhanced
erythropoietic properties in comparison to naturally occurring or recombinant
native human EPO.
The rHuEPO-FcG fusion protein containing the mutant hinge region exhibits
normal or even
enhanced erythropoietic functions in normal animals and animals with
experimental anemia.
The half-life of this fusion protein in circulation in primate studies reached
37 hours in
comparison to 8 hours for native human erythropoietin and 24 hours for ARANESP
from Amgen.
As used in this patent application, the terms "native human erythropoietin"
and "native human
EPO" mean EPO having an identical and complete amino acid sequence of the wild
type EPO
molecule. As will be appreciated by a person skilled in the art, native human
EPO may be
naturally occurring or recombinantly produced (e.g. rHuEPO alpha). The term
"native human
EPO" does not include rHuEPO analogs, such as darbepoetin alpha where the EPO
structure has
been significantly modified, such as by hyperglycosylation.
[00058] The nucleic acid sequence of the rHuEPO-FcG fusion protein of the
present
invention is shown in Figure 2B. The complete rHuEPO-FcG fusion protein is 399
amino acids
in length. As shown in Figure 2B, the complete rHuEPO-FcG fusion protein
consists of the EPO
domain (166 amino acids), the hinge region (16 amino acids, underlined) and
the CH2 and CH3
domains (217 amino acids). A signal or leader peptide sequence consisting of
27 amino acids is
also shown in Figure 1B. The signal peptide is cleaved during synthesis of
rHuEPO-FcG.
21
CA 02595013 2007-07-26
[00059] As shown best in Figure 2B, the EPO domain has a cysteine residue
near its C-
terminal (amino acid number 161). The mutant hinge region includes 2 cysteine
residues, at
amino acid numbers 178 and 181 which are boxed in Figure 2B. The hinge region
cysteine
residues form the disulphide bonds between the polypeptide subunits of the
homodimer as
discussed above. The naturally occurring hinge region of a human IgG 1
fragment also has a
cysteine at residue number 6 of the hinge region portion (measured from the N-
terminal).
According to an embodiment of the present invention, the cysteine residue 6 of
the hinge portion
has been substituted by a non-cysteine residue. In particular, in the
embodiment of Figure 2B,
the cysteine has been substituted by glycine (at amino acid residue 172 of
rHuEPO-FcG, which
corresponds to residue 6 of the hinge region). As will be apparent to a person
skilled in the art,
other non-cysteine residues could also be substituted for cysteine at this
location to avoid
formation of a disulfide bond.
[00060] As a result of the amino acid substitution at residue 172, the
first cysteine residue
of the hinge region (at residue 178) is spaced 17 amino acids from the above-
described cysteine
residue of the EPO domain (at residue 161). The inventors believe that the
minimum spacing
between the cysteine residue 161 of the EPO domain and the first cysteine
residue of the hinge
region should be at least 12 amino acids to enable successful assembly and/or
EPO receptor
binding of a homodimer of rHuEPO-FcG. That is, if residue 172 is a cysteine
residue, an
undesirable disulfide bond may potentially be formed, such as between cysteine
residues 161 and
172. This may alter the three dimensional structure of the EPO molecule,
resulting in the
impairment and/or the loss of the biological functions of EPO.
22
CA 02595013 2007-07-26
[00061] In one embodiment of the invention, the EPO domain is linked
directly to the Fc
fragment portion of the fusion protein. By avoiding an external linker
peptide, the preferred
three dimensional structure of the rHuEPO-FcG fusion protein is maintained and
the risk of
triggering an undesirable immunogenic response is minimized. The hinge region
of the Fc
fragment is preferably at least 9 amino acids in length and is preferably in
the range of about 10
to 20 amino acids in length.
[00062] As another specific example, a fusion protein combining human GM-
CSF and the
mutated Fc fragment was also produced genetically (rHuGMCSF-FcG). The nucleic
acid
sequence of the rHuEPO-Fc fusion protein of the present invention is shown in
SEQ ID No: 5.
The corresponding deduced amino acid sequence is shown in SEQ ID No: 6. In
vivo
experiments in animals with experimental neutropenia demonstrate that rHuGMCSF-
FcG
exhibits enhanced biological functions in terms of stimulating the growth of
white blood cells
(WBC) as compared to rHuGMCSF.
[00063] Accordingly, two fusion proteins formed by direct linking of the
target proteins,
human EPO and GM-CSF, to the mutant hinge region in which the free cysteine
(sixth amino
acid, cys223) was substituted by glycine, showed at least full biological
functions of the fused
target proteins, as compared to their natural molecules. These results
strongly suggest that the
mutant hinge region of the present invention allows the direct fusion of a
target protein to an Fc
fragment/Ig molecule while retaining biological functions of the fused target
protein. The
resulting fusion protein of target protein-Fc/Ig complex, in addition to
retaining natural biologic
functions, exhibits significantly prolonged half-life in vivo.
23
CA 02595013 2007-07-26
[00064] In a further embodiment, the mutant hinge region without the free
cysteine (sixth
amino acid from the N-terminal, cys223) could be used as a standard platform
for generating
fusion proteins that have longer circulation half-life and/or exhibit full or
enhanced biological
functions compared to the target protein. Unlike PEGylation, an advantage of
making fusion
proteins of the present invented is that the biological activity of the target
protein will not be
impaired and the potential immunogenic responses are minimized since the
fusion protein has
almost 100% sequence identity to natural human proteins (i.e., only one amino
acid is different).
Examples
[00065] The
following examples will further illustrate the invention in greater detail
although it will be appreciated that the invention is not limited to the
specific examples.
1. Generation and amplification of mutated nucleic acid sequences encoding
the fusion
proteins rHuEPO-FcG and rHuGMCSF-FcG
[00066] Full length DNA molecules encoding the amino-acid sequence of the
polypeptide
of rHuEPO-FcG, rHuEPI-FcC (wild type) and rHuGMCSF-FcG were generated by
overlapping
PCR using the following oligo primers (QIAGEN Inc., US), respectively:
EF5: 5'-ccggaattcgccaccatgggggtgcacgaatgtectgcct-3' [SEQ
ID NO:7]
EF3: 5'-ttaccttttgeggccgcttatttacceggagacagggagag-3' [SEQ
ID NO:8]
EFL5: 5'-aggcctgcaggacaggggacagagttgagcccaaatctggtgaca-3' [SEQ
ID NO :9]
24
CA 02595013 2007-07-26
=
EFL3: 5'-tgtcaccagatttgggctcaactctgteccctgtectgcaggcct-3'
[SEQ ID NO:10]
EFL5w: 5'-aggcctgcaggacaggggacagagttgagcccaaatcttgtgaca-3'
[SEQ ID NO:11]
EFL3w: 5'-tgtcacaagatttgggctcaactctgtcccctgtcctgcaggcct-3'
[SEQ ID NO:12]
GF5:
5'-actgaattcgccaccatgtggctgcagagcctgctgctcttgggcactgtggcctgc-3'[SEQ ID
NO:13]
GF3: 5'-ttttccttttgcggccgcttatttacccggagacagggagag-3'
[SEQ ID NO:14]
GFL5: 5'-gactgctgggagccagtccaggaggttgagcccaaatctggtgacaaaac-3'
[SEQ ID NO:15]
GFL3: 5'-gttttgtcaccagatttgggctcaacctectggactggctcccagcagtc-3'
[SEQ ID NO:16]
[00067] An EcoR I site was introduced in EF5 and GF5, and a Not I
site was introduced in
EF3 and GF3. For optimal expression of the proteins in mammalian cells, the
Kozak sequence
(GCCACCATGG) was also introduced in EF5 and GF5. The pairs EFL5/EFL3 and
EFL3w/EFL5w are complementary sequences consisting of the 3'-terminal DNA
sequence of
EPO (23 bp) and the 5'-terminal DNA sequence of mutated and wild IgG 1 hinge
(22 bp),
respectively. The pair of GFL5/GFL3 are complementary sequences consisting of
the 3'-
terminal DNA sequence of GM-CSF (24bp) and the 5'-terminal DNA sequence of
mutated IgG 1
hinge (26bp).
A. Generation of wild and mutated sequences of rHuEPO-FcG
[00068]
First, an EPO DNA fragment of 0.6 kb was amplified by PCR (Platinum Taq
DNA Polymerase High Fidelity) with primers EF5 and EFL3 or EFL3w from plasmid
p9E
containing the full length of human EPO cDNA, mutated and wild Fc fragment of
0.7 kb with
CA 02595013 2007-07-26
primers EF3/ EFL5, and EF3/EFL5w from plasmid pD containing the full length of
human IgG1
cDNA sequence, respectively (p9E and pD are from the inventors' own lab). The
two fragments
were then purified and mixed in equal amount. Using the mix as a template, the
full length
mutated and wild rHuEPO-FcG DNA of 1.3 kb was amplified by primers EF5 and
EF3,
respectively.
B. Generation of mutated sequence of rHuGMCSF-FcG
[00069] First, a GM-CSF DNA fragment of 0.4 kb was amplified by PCR
(Platinum Taq
DNA Polymerase High Fidelity) with primers GF5 and GFL3 from plasmid pSS-GM
containing
the full length of human GMCSF cDNA, mutated Fc fragment of 0.7 kb with
primers GF3/GFL5
from plasmid pD containing the full length of human IgG1 cDNA sequence,
respectively (pSS-
GM and pD are from the inventors' own lab). The two fragments were then
purified and mixed
in equal amount. Using the mix as a template, the full length of mutated
rHuGMCSF-FcG DNA
of 1.1 kb was amplified by primers GF5 and GF3.
2. Construction of the recombinant plasmids
[00070] Recombinant plasmids pCdEpo-FcG, pCdEpo-FcC and pCdGMCSF-FcG
encoding the fusion protein of of rHuEPO-FcG , rHuEPO-FcC and rHuGMCSF-FcG
were
constructed by cloning the amplified nucleic acid sequences from Example 1
into expression
vector pCD1, respectively.
26
CA 02595013 2007-07-26
[00071] The purified three fragments were digested by EocR I and Not I
(New England
Biolab Inc., USA) and then cloned into EcoR I/Not I-digested mammalian
expression vector
pCD1 (Figure 2). The resulting recombinant vectors were named pCdEpo-FcG,
pCdEpo-FcC
and pCdGMCSF-FcG. The inserted nucleic acid sequences encoding the amino-acid
sequence
of the rHuEPO-FcG, rHuEPO-FcC and rHuGMCSF-FcG were confirmed by DNA
sequencing.
3. Establishment of rHuEPO-FcG , rHuEPO-FcC, and rHuGMCSF-FcG expression
cell
lines
[00072] Chinese hamster ovary (CHO) cell with dihydrofolate
reductase(dhfr) deficiency
(CHO/dhfr-, ATCC No.CRL-9096), which has been approved by the FDA for
biological
substance production, was used as the host cell for recombinant expressions of
rHuEPO-FcG,
rHuEPO-FcC, and rHuGMCSF-FcG.
[00073] The CHO-dhfr- cells were transfected with the recombinant vectors
pCdEpo-FcG,
pCdEpo-FcC and pCdGMCSF-FcG using Lipofectamine (Gibco, Cat.No:18292-037,
USA). The
supernatants from the culture of selected clones were assayed by ELISA (Roche,
Cat.No:1-693
417 and Cat. No: HSGMO, Canada) for EPO and GM-CSF activity respectively.
Positive clones
were further screened under increasing methotrexate (MTX) pressures. Cell
lines with highest
protein expression were selected, and gradually adapted to serum-free media
(CD CHO Medium,
Gibco, and Cat.No:10743-029, USA). These selected CHO cell lines were used for
the
production of rHuEPO-FcG , rHuEPO-FcC, and rHuGMCSF-FcG, respectively.
27
CA 02595013 2007-07-26
4. Purification of rHuEPO-FcG , rHuEPO-FcC, and rHuGMCSF-FcG Proteins
[00074] rHuEPO-FcG , rHuEPO-FcC, and rHuGMCSF-FcG protein molecules
contained
in the supernatants collected from the serum-free media culturing the selected
CHO cells were
isolated at first by Protein A affinity chromatography (Amersham, Cat.No:17-
0402-01, Canada).
The isolated proteins were further purified by gel filtration in HiLoad 16/60
Superdex 200pg
columns (Amersham, Cat.No:17-1069-01, Canada). The purity of the rHuEPO-FcG ,
rHuEPO-
FcC, and rHuGMCSF-FcG proteins was more than 98% as determined by
electrophoresis.
5. Determination of the Sizes of the Purified rHuEPO-FcG Protein
[00075] First, SDS-PAGE was carried out to determine the sizes of the
purified rHuEPO-
FcG, rHuEPO-FcC, and rHuGMCSF-FcG proteins. A single band with molecular
weight of
about 180 kDa for rHuEPO-FcG and rHuEPO-FcC, and 140 kDa for rHuGMCSF-FcG was
seen
on an 8% Bis-Tris gel under non-reducing conditions, which measures the
overall size of the
protein with the existence of disulfide bonds. This indicated that most of
these recombinant
protein molecules were produced in their dimeric form, as expected from the
design of the fusion
protein. When SDS-PAGE analysis was conducted under reducing conditions (100
mM
dithiothreitol (DTT)) to break the disulfide bonds, only single bands with
molecular weight of 75
kDa and 60 kDa were identified, consistent with the estimated molecular weight
of single
polypeptide chains of HuEPO-hinge region-CH2-CH3 and HuGMCSF-hinge region-CH2-
CI3,
respectively.
28
CA 02595013 2007-07-26
[00076] The molecular weight of the purified rHuEPO-FcG fusion protein
with
glycosylation was determined by mass spectrometry (MALDI-TOF-MS) to be 111099
daltons
(111.1 I(Da). In this assay, only a single peak of protein was observed,
indicating that the
purified rHuEPO-FcG protein was nearly 100% pure. The 15 amino acids of the N-
terminal of
the pure rHuEPO-FcG protein were determined by protein sequence analysis as:
APPRLICDSRVLERY. This was consistent with the sequence of the first 15 amino
acids of the
native human EPO polypeptide, and confirms that the purified rHuEPO-FcG G
protein does have
the correct and complete EPO molecule sequence as predicted by the DNA
sequence encoding
the amino acid sequences of the rHuEPO-FcG G fusion protein.
6. Enhanced erythropoietic activities of rHuEPO-FcG in normal mice
[00077] In vivo experiments in mice were conducted to confirm the
retention of
erythropoietic activity of the rHuEPO-FcG protein and to determine its
efficacy compared to
rHuEPO and darbepoetin-alpha. For comparison purposes, the doses of EPO used
in the
described animal experiments of the invention, namely rHuEPO-FcG, rHuEPO
(i.e., native
human EPO) and darbepoetin-alpha, were compared according to the amount of EPO
molecule
portion alone. In respect of rHuEPO-FcG protein, the EPO portion contributes
to 41.4% of the
total rHuEPO-FcG molecular weight as calculated by the ratio of the weight of
amino acids of
EPO to weight of the total amino acids of the whole rHuEPO-FcG molecule (166
of 399 amino
acids). The EPO amount for rHuEPO-FcG was thus determined to be 41.4% of the
total amount
of rHuEPO-FcG protein.
29
CA 02595013 2014-02-20
[00078] rHuEPO-FcG (stock concentration: 0.5 mg/ml, purity of 98.6%) and
native
human rHuEPO (i.e. with natural human EPO structure) (6000 111/0.5 ml,
manufactured by Kirin
Brewery Co., Japan) were diluted in carrier solution (2.5 mg/ml of human serum
albumin, 5.8
mg/ml of sodium citrate, 0.06 mg/ml of citric acid and 5.8 mg/m1 of sodium
chloride, pH 5.5-
5.6). The dose of rHuEPO was calculated according to its activity/amount
ratio. BALB/c mice
(6 to 8 weeks old, weighing 18-22 g, equal numbers of male and female,
purchased from
Experiment Animal Center, AVMS, China) were grouped randomly with 6 in each
group. Each
group of mice was treated with one combination of one dose (0.1, 0.5, 2.5,
12.5, 62.5 rig/kg), one
injection route (i.v. through the tail vein or s.c.) and one injection
schedule (three times per week
or once per week). The control group of mice was injected with an equal volume
of carrier
solution. The treatment lasted for 3 weeks and the total observation time was
5 weeks. Peripheral
blood samples (tail vein) were taken before treatment, on the 4th day and 7th
day of every week
for 5 weeks. Hemoglobin (Hb) was measured as the index by absorptiometry. Mean
SD was
calculated from the data of each group and a t test was conducted among
different groups.
[00079] The administration of EPO three times per week to mice, provided
that the EPOs
have normal erythropoietic activity, would induce saturated stimulation of
erythropoiesis. As
shown in Figure 6, both groups treated 3 times per week s.c. had significant
elevation of Hb
levels even at the dose of 2.5pg/kg. This experiment demonstrated that rHuEPO-
FcG exhibited
an in vivo erythropoietic activity as effective as rHuEPO. The elevation of Hb
levels in the
treated group was dose-dependent. However, saturated elevation of the Hb
levels was induced in
mice at the dose of 12.5uu/kg of rHuEPO-FcG , whereas the similar saturated
elevation of the
CA 02595013 2014-02-20
Hb levels was only achieved at the dose of 62.5p.a/kg of rHuEPO. The elevation
of Hb levels
induced by 2.5 a/kg of rHuEPO-FcG was also greater than that by 2.5n/kg of
rHuEPO. These
results suggest more potent erythropoietic stimulation by rHuEPO-FcG compared
to rliiiFPO.
[00080] The erythropoietic potency of rHuEPO-FcG was further explored by
reducing the
injection times to once per week subcutaneously. As shown in Figure 7, the
rHuEPO-FcG-
treated groups showed dose-dependent elevation of Hb levels at the doses of
12.5, or 62.5ua/ka.
Both doses of 12.5 and 62.5 ,a/ka of rHuEPO also induced the elevation of Hb
levels to a
similar extent, which was much lower than that by 62.5p.g/kg of rHuEPO-FcG.
This strongly
indicates that rHuEPO-FcG has enhanced erythropoietic activity in vivo,
presumably due to
either the prolonged half-life of the rHuEPO-FcG in vivo or improved EPO
receptor
binding/activation by the dimer EPO molecules in the rHuEPO-FcG protein, or by
the combined
effects of both.
[00081] When the same doses (12.51.1a/ka) of rHilFPO-FcG or rHuFP0 were
administrated intravenously either three times per week or once per week,
elevation of the Hb
levels was observed for all the treated groups (Figure 8). However, i.v.
administration once per
week of rHuEPO-FcG induced greater, more persistent elevation of the Hb
levels, which
continued longer after the treatment was over. This data provides further
support for the
enhanced erythropoietic properties of the rHuEPO-FcG protein in comparison
with rHuEPO
having the structure of naturally occurring EPO protein.
7. Enhanced erythropoietic activities of rHuEPO-FcG in 5/6 nephrectomized
rats
31
CA 02595013 2007-07-26
[00082] Experiments in normal mice proved the enhanced erythropoietic
activities of
rHuEPO-FcG in vivo. To further observe the efficacy of rHuEPO-FcG in
stimulating
erythropoiesis, pharmacodynamic studies were conducted in rats with
experimental renal anemia
induced by 5/6 nephrectomy. The efficacy of rHuEPO-FcG was compared with those
of
rHuEPO and darbepoetin-alpha (60p.g/ml, lot. No.N079, manufactured by Kirin
Brewery Co.,
Japan).
[00083] Wistar rats (male and female in equal number, weighing 160-180g,
purchased
from Vitalriver Experiment Animal Inc., Beijing, China. Licence No. SCXKl 1-00-
0008) were
used to create the anaemia model due to the renal functional failure by a two-
step nephrectomy
[27]. 5/6 nephrectomy was performed on rats with general anaesthesia by two
separate
operations under sterile conditions. After 2/3 of the left kidney was
resected, the rats were
allowed to recover for twenty days. The right kidney was then resected.
Antibiotics were
administrated to prevent infection after each operation. In total, 5/6 of the
kidney tissue from
each rat was resected. The nephrectomized rats gradually developed renal
function
insufficiency and anaemia. Anaemia stabilized 50 days after the nephrectomy,
and the rats were
then randomly grouped (9 per group) to begin administration of the EPOs. Each
group of rats
was treated with one combination of one dose (2.5, 12.5, 62.5 g/kg), one
injection route ( i.v.
through the tail vein or s.c.) and one injection schedule (once per week or
once every 2 weeks).
The control group and model group of rats were injected with an equal volume
of carrier solution.
Treatment lasted for 4 weeks and the total observation time was 6 weeks.
32
CA 02595013 2014-02-20
[00084] All doses (2.5, 12.5, 62.5 g/kg) of rHuEPO-FcG, administrated
subcutaneously
once per week, induced dose-dependent elevation of Hb levels compared to the
model control
group that did not receive EPO treatment. Both 12.5 and 62.5 g/kg of rHuEPO
or darbepoetin,
administrated subcutaneously once per week also induced elevation of Hb
levels. The increased
levels of Hb in both groups treated with 12.5 or 62.5 p.a./kg of rHuEPO-FcG
were significantly
higher than those in groups treated with 12.5 or 62.5 g/kg of rHuEPO
respectively. The Hb
levels in 62.5 jig/kg of rHuFPO-FcG-treated group were also slightly higher
than that in 62.5
g/kg of darbepoetin-treated group. After stopping treatment, the decrease of
Hb levels in the
62.5 ,a/kg of rHuEPO-FcG-treated group was much slower and the Bib levels
remained higher
than those of both normal control and model control groups until the end of
observation (two
weeks after treatment), indicating a stronger and/or a prolonged erythopoietic
stimulation
(summarized in Figure 9).
[00085] For treatment with subcutaneous injection once every two weeks,
only 12.5 or
62.5 a/kg of the three EPOs were administrated (Figure 10). 12.5 g/kg of
rHuEPO barely
increased Hb levels compared to the model control group, and the weak
erythropoietic response
in the 62.5 g/kg of rHuEPO-treated group failed to bring the Hb levels to
normal in comparison
with the normal control group. Treatments of either rHuEPO-FcG or darbepoetin
at the doses of
12.5 or 62.5 jig/kg induced significant elevation of Hb levels that was higher
than that of the
normal control group, indicating the effective correction of anaemia by both
rHPFPO-FcG and
darbepoetin. No significant differences were observed between same doses of
rHuEPO-FcG and
darbepoetin in tettns of efficacy. The high dose of 62.5 kg/kg resulted in the
persistent increase
of erytlu-opoiesis until the termination of the observation (two weeks post
treatment). This
33
CA 02595013 2007-07-26
further suggested that rHuEPO-FcG and darbepoetin exhibit the property of long-
lasting
stimulation of erythropoiesis in vivo, which in turn could be translated to
the reduction of
administration frequency to patients clinically.
[00086] While darbepoetin has been approved for clinical application with
less-frequent
injections to increase patient compliance and reduce the work burden on health
care providers,
these experimental data strongly indicate that rHuEPO-FcG disclosed herein has
at least
comparable potential benefits. As discussed above, darbepoetin, as a mutant
analog of the
human EPO molecule containing additional sugar compounds (increased
glycosylation), may
have an increased risk of inducing immunogenesis in vivo due to alteration of
native three
dimensional structures. Only long-term observation of patients undergoing
treatment with
darbepoetin will reveal the immunogenic risks of darbepoetin. In contrast,
rHuEPO-FcG, which
does not modify the EPO molecule portion, has a carbohydrate content identical
or closely
similar to that of native human EPO. The amount of sialic acids in the
inventors' pure rHuEPO-
FcG protein were approximately 10.0 mmol/mmol EPO, consistent with the
reported parameters
of rHuEPO. The Fc portion of rHuEPO-FcG, with no external amino
acid(s)/linking peptide,
represents the general structure of human IgG 1, and should not generate an
immunogenic
response. If approved clinically, rHuEPO-FcG may provide a better choice for
patients than
currently available rHuEPO and EPO analogs, especially those in need of long-
term
administration.
[00087] Intravenous injection once every two weeks, both rHuEPO-FcG and
darbepoetin
(62.5 ps/kg) induced identical increases of Hb levels in the rats with renal
anaemia far above the
34
CA 02595013 2014-02-20
natmal Hb levels in the normal control rats (Figure 11). This further
demonstrates the persistent
stimulation of erythropoiesis by rHuEPO-FcG.
[00088] Data derived from cell culturing experiments of bone marrow cells
collected from
the 5/6 nephrectomized rats after treatments (once per week or per two weeks,
s.c. or i.v.)
showed that rHuFPO-FcG , rHuEPO and darbepoetin all stimulated the formation
of CFU-E and
BFU-E. The potencies of rHuEPO-FcG and darbepoetin were similar and stronger
than that of
rHuEPO (Figures 12A-12C).
[00089] Blood urinonitrogen (BUN) and creatinine levels were similar in
the treated
groups and model control group. The levels of serum Fe in all the treated
õgroups were higher
than that of the model control group. Pathological examinations revealed
increased distribution
of red blood cell (RBC)-related cells in bone marrow and spleen of all EPO-
treated rats.
8. Pharmaeokinetic studies of rHuEPO-FcG in Rhesus monkeys
[00090] As discussed above, the inventors have designed rHuEPO-FcG in such
a way that
the EPO portion of the fusion protein retains the functional properties of
natural EPO, such as
stimulating erythropoiesis, and the Fc fragment of human IgG 1 allows the
retention of the fusion
protein in circulation, thus extending its half-life in vivo. The above animal
studies have
demonstrated the ery-thropoietic activities of rHuEPO-FcG are enhanced in
comparison with
rHuEPO. The inventors have also conducted pharmacokinetic studies to determine
the in vivo
CA 02595013 2014-02-20
half-life of rHuEPO-FcG in comparison to that of rHuEPO. Primates were used to
generate data
as they are biologically very similar to human beings.
[00091] Study design was based on literature reports and the experiments
were conducted
according to the general guidelines of pharmacokinetics. Two groups of Rhesus
monkeys with 5
monkeys in each group (3-5kg, purchased from the Experiment Animal Center,
AMMS, China)
were injected intravenously with 5 1.g./kg, of rHuEPO-FcG or rHuEPO,
respectively. Blood
samples were taken before and at 0.017, 0.167, 0.5, 1, 2, 4, 8, 12, 24, 48,
96, 168 and 240 h after
injection. Sera were collected by centrifugation and the serum rHuEPO-FcG or
rHuEPO levels
were deteiiiiined by using human erythropoietin enzyme-linked immunosorbent
assay (ELISA)
kits (purchased from R&D Systems, Minneapolis, USA). The average half-life of
rHuEPO-FcG
and rHuPPO injected intravenously was 35.24 +/- 5.15 hand 8.72 +/- 1.69 h
respectively.
(summarized in Figure 13).
[00092] To observe the bioavailability of rHuEPO-FcG, 5u2/1a of rHuFPO-FcG
was
injected subcutaneously to 5 Rhesus monkeys. Blood samples were taken before
and 1, 2, 5, 8,
10, 12, 15, 24, 48, 72, 96, 168 and 240 h after the injection, and the serum
levels of rHuEPO-
FcG were determined by the R&D kits. The bioavailability index was calculated
as 35.71 +1-
5.37% with the subcutaneous injection. This is essentially identical to the
reported
bioavailability figures of darbepoetin-alpha (ARANESP in patients with chronic
renal failure.
[00093] This data demonstrates that rHuEPO-FcG has a significantly
prolonged half-life
in primates, and the in vivo half-life of rHuFPO-FcG is at least four fold
longer than that of
36
CA 02595013 2014-02-20
rHuEPO manufactured by Kirin Beer Brewing Co. of Japan. The prolonged half-
life in vivo
likely contributes to the enhanced erythropoietic activity of rHuEPO-FcG.
9. Immunogenicity of rHuEPO-FcG in ltlacaca faseicularis
[00094] As indicated above, the design of rHuEPO-FcG fusion protein
intentionally
avoids or minimizes changes of the immunogenic properties of the rHuEPO-FcG
fusion protein.
The inventors avoided including/adding any external amino acid(s) or linking
peptide sequences
in the fusion protein. According_ to one embodiment of the invention, the
HuEPO-Fc fusion
protein shown in Figure 2B only contains the polypeptide sequences of the
natural EPO protein
and the Fc fragment (hinge region, CH2, CH3) of human IgG 1, and thus should
not induce an
immunogenic response nor the production of antibodies against rHuEPO-FcG
protein.
[00095] Primate studies were conducted to observe the immunogenicity of
rHuEPO-FcG
protein. Ten crab-eating macaques (Illacaca fascicularis) (male/female=5/5, ¨
5 years old,
average weight of male 4.0 =1- 0.3kg., female is 2.9 0.4ka, purchased from
Laboratory Animal
Center, A.MMS, China) were injected subcutaneously with 5 ga/kg of purified
rHuEPO-FcG
three times per week for four weeks, and two were injected with an equal
volume of carrier
solution as the control animals. Sera were collected once a week for 5 weeks
(1 week post-
treatment) and tested for the specific antibodies against rHuEPO-FcG by ELISA
using purified
rHuEPO-FcG (51.1g/m1) as the coating antigen. In addition, RBC counts and Hb
levels in the
peripheral blood were also deterniined within the experimental period. The
resulting data shows
that, while enhancement of erythropoiesis in the rHuEPO-FcG -treated macaques
was observed
37
CA 02595013 2007-07-26
(the mean RBC numbers increased from 4.74 x 109/m1 to 6.67 x 109/m1 and the
mean Hb levels
increased from 12.2 g/dl to 13.7 g/dl), rHuEPO-FcG did not induce the
production of any
detectable specific antibodies against the fusion protein. These results
indicate that rHuEPO-
FcG fusion protein does not cause immunogenicity in primates.
10. Acute toxicity studies of rHuEPO-FcG in normal mice
[00096] To assess the safety of rHuEPO-FcG fusion protein, acute toxic
studies were
conducted in animals.
[00097] Two groups of BALB/c mice (n = 20, equal numbers of male and
female, 5-6
weeks old, the average weight of female is 15.8 0.4g, male is 15.9 0.6g,
purchased from
Chinese Academy of Medicine, China) were injected intravenously once with an
excessive
amount of purified rHuEPO-FcG (male = 13.3 mg/kg, female = 13.2 mg/kg) or
equal volume of
the carrier solution via their tail veins. In addition to observing the
instant reaction following
injection, general behaviour and status, activities, eating and defecation
patterns and changes
were monitored and recorded daily for 14 days. All mice were also weighed at
day 7 and day 14.
At day 15 post-injection, anatomical examination of the main organs of the
mice were conducted.
Pathologic examination were to be conducted if any unusual changes or
suspicious changes of
the organs were observed.
[00098] All mice in the 2 groups had no obvious instant reaction following
injection.
Within the period of 14 days, no obvious changes of behaviour, activities,
eating and defecation
38
CA 02595013 2016-12-13
patterns were observed. Moreover, the weight of the mice in both groups
increased steadily
during the testing period, and no apparent differences were found between the
2 groups on day 7
or day 14 post injection. No abnormal or pathologic changes were detected in
the tissues of
brain, lung, heart, liver and kidney. These results indicate that
administration of an excessive
amount of rHuEPO-FcG, far more than required for exhibiting the normal
erythropoiesis
function, is safe and had no apparent toxic effects.
11. Comparison of wild type and mutated fusion proteins of rHuEPO-FcC and
rHuEPO-FcG
[00099] Investigations were also conducted to compare wild type and mutated
versions of
proteins of HuEPO-Fc. As described above, in one embodiment the invention
includes a single
amino acid mutation at amino acid residue 172 (C172G).
[000100] In vivo experiments in mice were conducted to compare the
erythropoietic activity
of the wild type fusion protein rHuEPO-FcC with the mutated fusion protein
rHuEPO-FcG and
with recombinant human EPO (rHuEPO). For comparison purpose, all the doses of
the three
proteins used in this example, namely rHuEPO-FcG , rHuEPO-FcC and rHuEPO, were
the
amounts of the EPO molecule portion alone on a molar basis. In respect of the
rHuEPO-FcG
and rHuEPO-FcC proteins, the EPO portion contributes 41.4% of the total rHuEPO-
FcG
molecular weight as calculated by the ratio of the weight of amino acids of
EPO to the weight of
the total amino acids of the whole rHuEPO-FcG and rHuEPO-FcC molecules
(i.e.,166 of 399
amino acids).
39
CA 02595013 2016-12-13
[000101] rHuEPO-FcG (stock concentration: 300 mime, rHuEPO-FcC (stock
concentration: 90 ig/m1) and rHuEPO with the natural human EPO structure (6000
1U/0.5 ml,
manufactured by Kirin Brewery Co., Japan) were diluted in carrier solution
(2.5 mg/ml of human
serum albumin, 5.8 mg/ml of sodium citrate, 0.06 mg/ml of citric acid and 5.8
mg/ml of sodium
chloride , pH 5.5-5.6). The dose of rHuEPO was calculated according to its
activity/amount ratio.
BALB/c mice (9 to 10 weeks old, weighing 18-22 g, equal numbers of male and
female,
purchased from Experiment Animal Center, AMMS, China) were grouped randomly
with 8 in
each group. Each group of mice was treated with one combination of one dose
(2.5, 12.5, 62.5
lig/kg), one injection route (s.c.) and one injection schedule (three times
per week or once per
week). The control group of mice was injected with an equal volume of carrier
solution.
Treatment lasted for 26 days. Peripheral blood samples (tail vein) for
measurement were taken
before treatment, on the 2nd, 6th, 9th, 13th, 16th, 19th, 22nd
and 26th days of treatment. Hb was
measured as the index by absorptiometry. Mean + SD was calculated from the
data of each
group and a t test was conducted among different groups.
[000102] The administration of EPO three times per week to mice induced
saturated
stimulation of erythropoiesis. As shown in Figure 14, the mice treated by
rHuEPO-FcG 3 times
per week s.c. had significant elevation of Hb levels even at the dose of 2.5
ug/kg at the 9th day
after treatment. The elevation of Hb levels in the treated group was dose-
dependent. However,
saturated elevation of the Hb levels was induced in mice at the dose of 12.5
ug/kg of rHuEPO-
FcG. The elevation of Hb levels induced by 2.5 ug/kg of rHuEPO-FcG was also
greater than that
by 2.5 ug/kg of rHuEPO-FcC and rHuEPO. These results suggested more potent
erythropoietie
stimulation by rHuEPO-FcG.
CA 02595013 2016-12-13
,
[000103] The erythropoietic potency of rHuEPO-FcG was further explored by
reducing the
injection times to once per week subcutaneously. As shown in Figure 15, the
rHuEPO-FcG-
treated groups showed dose-dependent elevation of Hb levels at the doses of
12.5, or 62.5ug/kg.
Both doses of 12.5 and 62.5ug/kg of rHuEPO also induced the elevation of Hb
levels to the
similar extent, which was much lower than that by 62.5ug/kg of rHuEPO-FcG. The
rHuEPO-
FcC-treated groups showed significantly lower Hb levels. This strongly
indicates that rHuEPO-
FcG has enhanced erythropoietic activity in vivo. It is presumably due to
either improved EPO
receptor binding/activation by the dimer EPO molecules in rHuEPO-FcG protein
or by the
possible prolonged half-life of rHuEPO-FcG in vivo, or due to the combined
effects of both.
[000104] The results demonstrated that rHuEPO-FcG exhibited an enhanced
in vivo
erythropoietic activity compared to rHuEPO-FcC and rHuEPO. This enhanced
activity appears
to be attributable to the single amino acid mutation in the hinge region of
the recombinant
molecule at residue 172 (Figure 2B). The results show that rHuEPO-FcC
exhibited very little
erythropoietic activity in normal mice and much less erythropoietic activity
compared to
rHuEPO-FcG and rHuEPO.
12. Enhanced therapeutic effects of rHuGMCSF-FcG for neutro enia in 60Co-
irradiated dogs
41
CA 02595013 2007-07-26
,
,
,
[000105] The enhanced biological activity of rHuGMCSF-FcG was
observed in model dogs
with neutropenia induced by 60Co -y-ray irradiation. The efficacy of rHuGMCSF-
FcG was also
compared with that of native GM-CSF.
[000106] rHuGMCSF-FcG (stock concentration: 1.8 mg/ml, purity 98%)
and native human
rHuGM-CSF (150 g/vial, manufactured by NCPC GeneTech Biotechnology
Development Co.,
Ltd., China) were diluted in carrier solution (1% of HSA, 1.1% of benzyl
alcohol, 40 mg/ml of
mannitol, 10 mg/ml of sucrose, and 1.2 mg/ml of tromethamine).
[000107] Beagle dogs (male and female in equal number, weighing 8-
10 kg, 12-15 months
old, purchased from Beijing Xieerxin Institute of BioResources. Licence No.
SOCK 2005-0005,
China) were divided into 4 groups (4 in each group) randomly: model control,
low dose of
rHuGMCSF-FcG (10 g/kg), high dose of rHuGMCSF-FcG (20 g/kg), and high dose
of
rHuGMCSF(20 g/kg). Each dog, with the pelvis shielded by lead blocks, was
irradiated by 6.5
Gy of 60Co y-ray at a rate of 295.54 rad/min. The treatment started from the
next day of
irradiation by subcutaneous injection, once every other day for rHuGMCSF-FcG,
or once every
day for rHuGMCSF. The dogs from control group were injected with an equal
volume of carrier
solution. Injections were given over 10 days and the total observation time
was 28 days. After
irradiation, the dogs were examined each day for their general clinical
conditions. Every other
day, peripheral blood was collected for examinations of WBC count, platelet
count, RBC count,
HB, and hematocrit (Hct).
42
CA 02595013 2007-07-26
[000108] As shown in Figure 16, all the irradiated dogs developed
neutropenia immediately
after irradiation. Since dogs from control group were only injected with
carrier solution, their
clinical courses reflected natural recovery with no treatment. WBC counts
decreased
dramatically the day following irradiation and reached a nadir by day 4. The
low WBC count
lasted for another 4-5 days. After that, it began increasing slowly,
indicating the recovery from
neutropenia.
[000109] Dogs from the three administrated groups also developed similar
neutropenia after
irradiation, but their WBC counts showed rapid and sustained increases during
the course of
treatment. All the nadirs of WBC counts were raised compared with control
group. Furthermore,
the duration of low WBC counts was also shorter. The increasing of WBC reached
its peak on
the day following the last injection. These results indicate that both
rHuGMCSF-FcG and
rHuGMCSF induced hematopoietic recovery.
[000110] Different levels of activity were observed within the three
administrated groups.
rHuGMCSF-FcG demonstrated enhanced hematopoietic recovery compared with
rHuGMCSF.
Despite only being administrated every other day, the dogs injected with
rHuGMCSF-FcG
showed stronger recovery potency than dogs with rHuGMCSF administrated daily.
While the
dogs injected with low doses of rHuGMCSF-FcG (10 g/kg) and high doses of
rHuGMCSF (20
[ig/kg ) showed similar WBC counts and recovery tendency, the WBC counts from
dogs with
high doses of rHuGMCSF-FcG (20 mg/kg) were twice as high as the other two
groups during the
entire course of recovery. This dose-dependent, persistent elevation further
indicates that
43
CA 02595013 2007-07-26
rHuGMCSF-FcG possesses enhanced biological activity compared to its native
counterpart
(Figure 16).
[000111] The enhanced biological activity of rHuGMCSF-FcG in vivo, similar
to that
discussed in rHuEPO-FcG experiments, is also presumably due to improved GM-CSF
receptor
binding/activation by the dimer GM-CSF molecules in rHuGMCSF-FcG protein, the
possible
prolonged half-life of rHuGMCSF-FcG in vivo, or due to the combined effects of
both.
[000112] As will be apparent to those skilled in the art in the light of
the foregoing
disclosure, many alterations and modifications are possible in the practice of
this invention
without departing from the spirit or scope thereof.
44
CA 02595013 2007-07-26
,
References
1. Capon, et al., Designing CD4 immunoadhesins for AIDS therapy, Nature
337:525-531, 1989.
2. Yeh et al., Design of yeast-secreted albumin derivatives for human therapy:
Biological and
antiviral properties of a serum albumin-CD4 genetic conjugate, PNAS 89:1904-
1908, 1992.
3. Jones, et al., The development of a modified human IFN-a2b linked to the Fc
portion of
human IgG1 as a novel potential therapeutic for the treatment of hepatitis C
virus infection,
Journal of Interferon and Cytokine Research 24:560-572, 2004.
4. Lo et al., High level expression and secretion of Fc-X fusion proteins in
mammalian cells,
Protein engineering 11(6), 495-500, 1998.
5. United States Patent No. 5,723,125 Chang et al., Hybrid with interferon-
alpha and an
immunoglobulin Fe linked through a non-immunogenic peptide.
6. United States Patent No. 5,908,626, Chang et al., Hybrid with interferon-
.beta. and an
immunoglobulin Fe joined by a peptide linker.
7. United States Patent No. 5,914,111, Wallner et al., CD2-binding domain of
lymphocyte
function associated antigen-3.
8. United States Patent No. 6,165,476, Strom et al., Fusion proteins with an
immunoglobulin
hinge region linker.
9. United States Patent No. 6,403,077, Strom et al., Treatment regimes
featuring an IL-10-
containing chimeric polypeptide.
10. United States Patent No. 6,797,493, Sun et al., Fe fusion proteins of
human granulocyte
colony-stimulating factor with increased biological activities.
11. United States Patent No. 6,808,902, Treuheit et al., Process for
correction of a disulfide
misfold in IL-lra FC fusion molecules
12. United States Patent No. 6,900,292, Sun et al., Fe fusion proteins of
human erythropoietin
with increased biological activities.
13. United States Patent No. 7,030,226, Sun et al., Fe fusion proteins of
human erythropoietin
with increased biological activities.
CA 02595013 2007-07-26
14. United States Patent No. 7,067,110, Gillies et al., Fc fusion proteins for
enhancing the
immunogenicity of protein and peptide antigens.
15. United States Patent No. 7,091,321, Gilles et al., Enhancing the
circulating half-life of
antibody-based fusion proteins.
16. United States Patent No. 7,112,659, Mann et al., OB fusion protein
compositions and
methods.
17. United States Patent No. 7,189,827, Feige, Modified peptides as
therapeutic agents.
18. United States Patent No. 7,211,253, Way, Erythropoietin forms with
improved properties.
19. United States Patent No. 7,217,798, Hinton et al., Alteration of Fc-fusion
protein serum half-
lives by mutagenesis.
20. United States Patent No. 7,226,759, Sun et al., Fc fusion proteins of
human granulocyte
colony-stimulating factor with increased biological activities.
21. United States Patent No. 7,232,668, Sun et al., Fc fusion proteins of
human granulocyte
colony-stimulating factor with increased biological activities.
22. United States Patent Application No. 20010053539. Lauffer et al., Fusion
proteins with
immunoglobulin proteins, the preparation and use thereof.
23. United States Patent Application No. 20020081664, Lo et al., Expression
and export of
interferon-alpha proteins as Fc fusion proteins.
24. United States Patent Application No. 20030105294, Gillies et al.,
Enhancing the circulating
half life of antibody-based fusion proteins.
25. United States Patent Application No. 20030166877, Gillies et al., Reducing
the
immunogenicity of fusion proteins.
26. United States Patent Application No. 20050069521, Gillies et al.,
Enhancing the circulating
half-life of interleukin-2 proteins.
27. Mohler, et al., Soluble tumor necrosis factor (TNF) receptors are
effective therapeutic agents
in lethal endotoxemia and function simultaneously as both TNF carriers and TNF
antagonists,
The Journal of Immunology 151(3):1548-1561, 1993.
28. Goldenberg, Etanercept, a novel drug for the treatment of patients with
severe, active
rheumatoid arthritis, Clin. Ther. 21(1):75-87, 1999.
46
CA 02595013 2007-07-26
29. Wong et al., The use of alefacept in the treatment of psoriasis. Skin
Therapy Letter 8(6):1-
2(2003)
30. Way et al., Improvement of Fc-erythropoietin structure and
pharmacokinetics by
modification at a disulfide bond, Protein Engineering, Design & Selection
18(3):111-118, 2005.
47