Note: Descriptions are shown in the official language in which they were submitted.
CA 02595485 2007-08-01
LIPOSOMAL COMPOSITIONS FOR PARENTERAL DELIVERY OF STATINS
FIELD OF THE INVENTION
The invention is, in general, in the field of drug delivery. More
specifically,
the invention provides methods and compositions for parenteral delivery of a
statin,
using a liposome delivery vehicle.
BACKGROUND OF THE INVENTION
Liposomes are microscopic particles that are made up of one or more lipid
bilayers enclosing an internal compartment. Liposomes have been widely studied
and
used as carriers for a variety of agents such as drugs, cosmetics, diagnostic
reagents,
and genetic material. Since liposomes consist of non-toxic lipids, they
generally have
low toxicity and therefore are useful in a variety of pharmaceutical
applications. In
particular, liposomes are useful for increasing the circulation lifetime of
agents that
have a short half-life in the bloodstream. Liposome-encapsulated agents often
have
biodistributions and toxicities which differ greatly from those of free agent.
For
specific in vivo delivery, the sizes, charges and surface properties of these
carriers can
be changed by varying the preparation methods and by tailoring the lipid
makeup of
the carrier. For instance, liposomes may be made to release an agent more
quickly by
decreasing the acyl chain length of a lipid making up the carrier.
Agents can be encapsulated in liposomes using a variety of methods and
include membrane partitioning, passive encapsulation and active encapsulation.
Agents that have hydrophobic attributes can intercalate into the lipid bilayer
and this
can be achieved by adding the agent during the liposome manufacturing process
or by
adding the agent to pre-formed liposomes. Agent encapsulation is often limited
due
to the ability of the liposome membrane to stably incorporate the agent. In
addition
the agent may adversely impact the physical properties of the liposomes. This
method
is also limited because the associated agent can rapidly transfer out of the
membrane.
Passive loading involves the use of water-soluble or lipid soluble agents
which
are added to liposome components during the liposome manufacturing process.
Some
of the added agent will be encapsulated in the aqueous core or lipid bilayer
of the
liposomes and free agent (agent that has not been trapped within the liposome)
can be
removed by several standard separation methods. This procedure typically
results in
low trapping efficiencies and low agent to lipid ratios and is, therefore, not
ideal.
1
CA 02595485 2007-08-01
Active loading techniques have been used to achieve high concentrations of
agent in liposomes. Active loading involves the creation of pH gradients (ApH)
or
metal ion gradients (OMZ) across the liposomal bilayer. For example, a OpH
generated by preparing liposomes in citrate buffer pH 4.0, followed by
exchange of
external buffer with buffered-saline pH 7.5, can promote the liposomal
accumulation
of weakly basic agent. The neutral form of the agent passively diffuses across
the
lipid bilayer and becomes trapped upon protonation in the low pH environment
of the
liposome interior. This process can result in >98% agent encapsulation and
high
agent-to-lipid ratios (e.g. vinorelbine, doxorubicin, vincristine,
daunorubicin,
mitoxantrone, to name a few). However, successful loading and retention using
a
transmembrane pH gradient is realized while the internal pH of the liposome is
maintained. Since the pH gradient can dissipate following agent loading and
since
maintenance of the pH gradient is critical to achieve optimal agent retention,
clinical
formulation of pH gradient loaded agents requires the generation of a pH
gradient
across the liposomes just prior to agent loading or the use of formulations
that
maintain the pH gradient effectively after loading (e.g. use of strong buffers
or
ionophores that induce pH gradient formation). A second disadvantage of this
method
results from instability of lipid, and some agents, at acidic pH which
decreases the
long-term storage potential of the agent loaded liposomes. Freezing of
liposomal
formulations slows the rate of hydrolysis but conventional liposomal
formulations
often aggregate and leak contents upon thawing unless appropriately selected
cryoprotectants are used.
Agent loading via AM2+ follows a process analogous to the pH gradient
process, with agent accumulation being driven by metal ion-complexation (e.g.
doxorubicin-Mn2) . Agent loading efficiencies are comparable to those
described for
the OpH process. However, loading efficiency is dependent on the choice of
metal
ion and agent.
Statins are a class of compounds that act as competitive inhibitors of
hydroxymethyl-glutaryl (HMG)-CoA reductase. HMG-CoA reductase catalyzes the
conversion of HMG-CoA to mevalonate, a rate limiting step in cholesterol
biosynthesis. Inhibition of this enzyme decreases de novo cholesterol
synthesis,
increasing expression of low-density lipoprotein (LDL) receptors on
hepatocytes.
This increases LDL uptake by the hepatocytes, decreasing the amount of LDL
cholesterol in the blood. Statins also reduce the blood levels of
triglycerides and
2
CA 02595485 2007-08-01
slightly increase levels of HDL-cholesterol. Accordingly, statins are known
hypolipidemic agents and are used to lower cholesterol and prevent
cardiovascular
disease. Statins also appear to have anti-inflanunatory effects that cannot be
accounted for by their lipid lowering abilities. These include suppression of
proinflammatory cytokine and chemokine production, immunomodulation and down-
regulation of endothelial cell activation (Blanco-Colio et al. [2003] Kidney
Int. 63:12;
Leung et al. [2003] J. Immunol. 170:1524). As a consequence of these
properties,
statin therapy has been examined in a number of chronic immune mediated
inflammatory diseases including experimental autoimmune encephalomyelitis and
arthritis, in particular rheumatoid arthritis (RA). The statin simvastatin has
been
shown to exhibit a therapeutic effect in the collagen induced arthritis (CIA)
model of
RA (Leung et al. [2003] J. Immunol. 170:1524). Atorvastatin was found to have
a
therapeutic effect in patients with RA as well as beneficially influencing
inflammatory markers (McCarey et al. [2004] Nature, 363:2015). Other recent
studies provide further support for the therapeutic effect of statins in
patients with RA
as well as other inflanunatory disorders or conditions such as
transplantation, multiple
sclerosis, and chronic kidney disease (see for example: Connor et al. [2006]
Arthritis
Res. Ther. 8:R94; Kinderlerer et al. [2006] Arthritis Res. Ther. 8:R130;
Steffens et al.
[2006] Nat. Clin. Pract. Nephrol. 2:378; Jansen [2006] Rheumatology, 45:1577;
Xu et
al. [2006] Arthritis Rheum. 54:3441; Yamagata et al. [2007] Rheumatol. Int.
27:63 1;
Davignon et al. [2005] Vasc. Health Risk Manag. 1:29; Gazi et al. [2007] Clin.
Exp.
Rheumatol. 25:102; Okamoto et al. [2007] J. Rheumatol. 34:964; Haruna [2007]
Arthritis Rheum. 56:1827). Other recent reports have suggested the use of
statins in
the prophylaxis and treatment of influenza (see for example: Enserink [2005]
Science,
309:1976; Fedson [2006] Clin. Infect. Dis. 43:199; Rainsford [2006]
Inflammopharmacology, 14:2; Terblanche [2006] Crit. Care, 10:168; Frost et al.
[2007] Chest, 131:1006).
SUMMARY OF THE INVENTION
The invention provides methods for loading a statin onto a liposome for
parenteral delivery, compositions prepared using the methods, and uses
thereof.
In one aspect, the invention provides a method for loading a statin, including
pharmaceutically acceptable salts, solvates and prodrugs thereof, into a
liposome by
combining the statin with a micelle-forming compound to form a micelle
including
3
CA 02595485 2007-08-01
the statin, where the statin is releasable from the micelle-forming compound,
and
adding the micelle to the liposome, where the micelle combines with the
liposome
such that the statin is loaded into the liposome to form a loaded liposome.
In alternative embodiments, the micelle may combine with the lipid bilayer of
the liposome such that the micelle components, including the statin is
incorporated
into the outer lipid bilayer of the liposome; the micelle-forming compound may
include a hydrophilic or amphipathic.moiety such as a PEG-lipid conjugate
(e.g.,
DSPE-PEG2000)
In alternative embodiments, the statin may be dissolved in a solvent, such as
ethanol.
In alternative embodiments, the loaded liposome may be about 100 nm to
about 200 nm in diameter. In alternative embodiments, the loaded liposome may
be a
unilamellar liposome. In alternative embodiments, the loaded liposome may
include
one or more of a lipid selected from DMPC or DPPC. In alternative embodiments,
the loaded liposome may include a targeting agent.
In alternative aspects, the invention provides a composition produced by a
method of the invention. In alternative embodiments, the composition may
further
include a pharmaceutically acceptable carrier.
In alternative aspects, the invention provides a liposomal composition
including a statin, where the composition is formulated for parenteral
delivery. In
alternative embodiments, the composition may further include a lipid selected
from
DMPC or DPPC. In alternative embodiments, the composition may further include
DSPE-PEG2000.
In alternative aspects, the invention provides a method of treating a disease
or
condition that benefits from administration of a statin comprising
administering a
composition of the invention to a subject in need thereof. In alternative
aspects, the
invention provides the use of a composition of the invention for preparation
of a
medicament for treating a disease or condition that benefits from
administration of a
statin in a subject in need thereof. In alternative aspects, the invention
provides the
use of a composition of the invention for treating a disease or condition that
benefits
from administration of a statin in a subject in need thereof.
In alternative aspects, the invention provides a kit for preparing a loaded
liposome including a first container including a statin solubilized in a
micelle and a
second container including a liposome of the desired composition, together
with
4
CA 02595485 2007-08-01
instructions for combining the contents of the first and second containers to
prepare a
loaded liposome.
In alternative aspects, the invention provides a kit for preparing a loaded
liposome including a first container including a statin; a second container
including a
micelle-forming compound; and a third container including a liposome of the
desired
composition, together with instructions for combining the contents of the
first and
second containers to form a micelle including the statin, and for combining
the
micelle with the contents of the third container to prepare a loaded liposome.
In
alternative embodiments, the micelle may include DSPE-PEG2ooo; and/or the
liposome may include a lipid selected from DMPC or DPPC.
BRIEF DESCRIPTION OF THE DRAWINGS
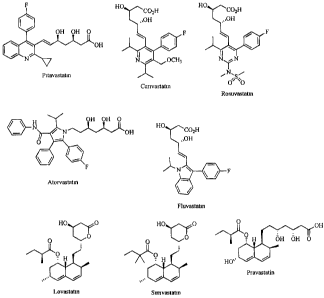
FIGURE 1 depicts the chemical structure of simvastatin, atorvastatin,
cerivastatin, fluvastatin, lovastatin, mevastatin, pitavastatin, pravastatin
and
rosuvastin.
FIGURE 2 A-B are graphs showing the stability of MLV simvastatin and ML
liposomal simvastatin in HBS buffer.
FIGURE 3 A-B are graphs showing the stability of MLV simvastatin and ML
liposomal simvastatin in human plasma.
FIGURE 4 A-B are graphs showing the stability of MLV simvastatin and ML
liposomal simvastatin in human RA synovial fluid.
FIGURE 5 is a bar graph showing the drug:lipid raio following of incubation
of MLV simvastatin or ML liposomal simvastatin in HBS buffer and human
synovial
fluid.
FIGURES 6 A-I are bar graphs showing the drug content of simvastatin-
loaded liposomes with varying lipid compositions prepared using the standard
thin
film extrusion method (TFE) compared with the micelle loading method (ML)
after
incubation in HBS buffer
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides, in part, liposomal compositions for parenteral
delivery of statins and methods of preparation thereof. In some embodiments,
the
invention provides methods for increasing the concentration of statins that
can be
achieved in liposomes. In some embodiments, the invention provides methods for
5
CA 02595485 2007-08-01
increased incorporation of statins into liposomes. In some embodiments, such
methods may: reduce the amount of a solvent required to solubilize a statin;
or may
extend the stability of liposomes containing statins in the bloodstream of a
subject; or
may extend the stability of liposomes containing a statin during storage; or
may
increase the retention of a statin within a liposome during storage or in
circulation in
the bloodstream of a subject; or may otherwise improve the properties of a
liposome
containing a statin generally, either in vitro or in vivo. In some
embodiments, use of a
micelle as a means to solubilize a statin to be incorporated into a liposome
increases
the amount of the statin that can be stably incorporated into the liposome
bilayer.
Statins, when taken in oral form, undergo extensive hepatic metabolism,
reducing the amount of active agent that can get to the areas where they are
needed
(for example an arthritic joint). Further, a well-known side effect of statins
is
myopathy, or muscular weakness. Encapsulation of liposomes can reduce hepatic
metabolism and penetration into muscle cells thus improving the therapeutic
effects of
this important class of compounds.
Liposomes
The term "liposome" as used herein means a vesicle including one or more
concentrically ordered lipid bilayer(s) encapsulating an aqueous phase, when
in an
aqueous environment. Formation of such vesicles requires the presence of
"vesicle-
forming lipids" which are defined herein as amphipathic lipids capable of
either
forming or being incorporated into a bilayer structure. The term includes
lipids that
are capable of forming a bilayer by themselves or when in combination with
another
lipid or lipids. An amphipathic lipid is incorporated into a lipid bilayer by
having its
hydrophobic moiety in contact with the interior, hydrophobic region of the
bilayer
membrane and its polar head moiety oriented towards an outer, polar surface of
the
membrane. Hydrophilicity arises from the presence of functional groups such as
hydroxyl, phosphate, carboxyl, sulfate, amino or sulfhydryl groups.
Hydrophobicity
results from the presence of a long chain of aliphatic hydrocarbon groups.
Liposomes can be categorized into multilamellar vesicles, multivesicular
liposomes, unilamellar vesicles and giant liposomes. Multilamellar liposomes
(also
known as multilamellar vesicles or "MLV") contain multiple concentric bilayers
within each liposome particle, resembling the "layers of an onion".
Multivesicular
liposomes consist of lipid membranes enclosing multiple non-concentric aqueous
6
CA 02595485 2007-08-01
chambers. Unilamellar liposomes enclose a single internal aqueous compartment.
Single bilayer (or substantially single bilayer) liposomes include small
unilamellar
vesicles (SUV) and large unilamellar vesicles (LUV). LUVs and SUVs range in
size
from about 50 to 500 nm and 20 to 50 nm respectively. Giant liposomes
typically
range in size from 5000 nm to 50,000 nm and are used mainly for studying
mechanochemical and interactive features of lipid bilayer vesicles in vitro
(Needham
et al. [2000] Colloids and Surfaces B: Biointerfaces, 18: 183-195).
Any suitable vesicle-forming lipid may be utilized in the practice of this
invention as judged by one of skill in the art. This includes phospholipids
such as
phosphatidylcholine (PC), phosphatidylglycerol (PG), phosphatidylinositol
(PI),
phosphatidic acid (PA), phosphatidyethanolamine (PE) and phosphatidylserine
(PS);
sterols such as cholesterol; glycolipids; sphingolipids such as sphingosine,
ceramides,
sphingomyelin, and glycosphingolipids (such as cerebrosides and gangliosides).
Suitable phospholipids may include one or two acyl chains having any number of
carbon atoms, between about 6 to about 24 carbon atoms, selected independently
of
one another and with varying degrees of unsaturation. Thus, combinations of
phospholipid of different species and different chain lengths in varying
ratios may be
selected. Mixtures of lipids in suitable ratios, as judged by one of skill in
the art, may
also be used.
Liposomes for use in the present invention may be generated using a variety of
conventional techniques. These techniques include: the ether injection method
(Deamer et al., Acad. Sci.[1978] 308:250); the surfactant method (Brunner et
al.,
[1976] Biochim. Biophys. Acta, 455:322); the Ca2+ fusion method
(Paphadjopoulos et
al., [1975] Biochim. Biphys. Acta, 394:483); the freeze-thaw method (Pick et
al.,
[1981] Arach. Biochim. Biophys., 212:186); the reverse-phase evaporation
method
(Szoka et al., [1980] Biochim. Biophys. Acta, 601:559); the ultrasonic
treatment
method (Huang et al. [1969] Biochemistry, 8:344); the ethanol injection method
(Kremer et al. [1977] Biochemistry, 16:3932); the extrusion method (Hope et
al.,
[1985] Biochimica et Biophysica Acta, 812:55); the French press method
(Barenholz
et al., [1979] FEBS Lett., 99:210); or any other technique described herein or
known
in the art.
Different techniques may be appropriate depending on the type of liposome
desired. For example, small unilamellar vesicles (SUVs) can be prepared by the
ultrasonic treatment method, the ethanol injection method, or the French press
7
CA 02595485 2007-08-01
method, while multilamellar vesicles (MLVs) can be prepared by the reverse-
phase
evaporation method or by the simple addition of water to a lipid film followed
by
dispersal by mechanical agitation (Bangham et al., [1965] J. Mol. Biol. 13:238-
252).
LUVs may be prepared by the ether injection method, the surfactant method, the
Ca2+ fusion method, the freeze-thaw method, the reverse-phase evaporation
method,
the French press method or the extrusion method. In some embodiments, LUVs are
prepared according to the extrusion method. The extrusion method involves
first
combining lipids in chloroform to give a desired molar ratio. A lipid marker
may
optionally be added to the lipid preparation. The resulting mixture is dried
under a
stream of nitrogen gas and placed in a vacuum pump until the solvent is
substantially
removed. The samples are then hydrated in an appropriate buffer or mixture of
therapeutic agent or agents. The mixture is then passed through an extrusion
apparatus
(e.g. Extruder, Northern Lipids, Vancouver, BC) to obtain liposomes of a
defined
size. Average liposome size can be determined by quasi-elastic light
scattering or
photon correlation spectroscopy or dynamic light scattering or various
electron
microscopy techniques (such as negative staining transmission electron
microscopy,
freeze fracture electron microscopy or cryo-transmission electron microscopy).
If
desired, the resulting liposomes may be run down a SephadexTM CL4B column or
similar size exclusion chromatography column equilibrated with an appropriate
buffer
in order to remove unencapsulated drug or to create an ion gradient by
exchange of
the exterior buffer. Subsequent to generation of an ion gradient, LUVs may
encapsulate therapeutic agents as set forth herein.
In some aspects, liposomes are prepared to be "cholesterol free", meaning that
such lipid-based vehicles contain "substantially no cholesterol," or contain
"essentially no cholesterol." The term "cholesterol-free" as used herein with
reference
to a liposome means that the liposome is prepared in the absence of
cholesterol, or
contains substantially no cholesterol, or that the vehicle contains
essentially no
cholesterol. The term "substantially no cholesterol" allows for the presence
of an
amount of cholesterol that is insufficient to significantly alter the phase
transition
characteristics of the liposome (typically less than 20 mol % cholesterol). 20
mol % or
more of cholesterol broadens the range of temperatures at which phase
transition
occurs, with phase transition disappearing at higher cholesterol levels. A
liposome
having substantially no cholesterol may have about 15 or less, or about 10 or
less mol
% cholesterol. The term "essentially no cholesterol" means about 5 or less mol
%, or
8
CA 02595485 2007-08-01
about 2 or less mol %, or about 1 or less mol % cholesterol. In some
embodiments, no
cholesterol will be present or added when preparing "cholesterol-free"
liposomes. The
presence or absence of cholesterol may influence the ability of the micelle-
solubilized
compound that can be stably incorporated into the liposome bilayer and may
influence retention of that compound after incorporation.
Liposomes may range from any value between about 50 nm to about 1 m in
diameter. For example, liposomes in a liposomal composition according to the
invention may range from any value between about 100 to about 140 nm in
diameter.
In some embodiments, liposomes in a liposomal composition according to the
invention may be less than about 200 nm in diameter, or less than about 160 nm
in
diameter, or less than about 140 nm in diameter. In some embodiments,
liposomes in
a liposomal composition according to the invention may be substantially
uniform in
size, for example, 10% to 100%, or more generally at least 10%, 20%, 30%, 40%,
50,
55% or 60%, or at least 65%, 75%, 80%, 85%, 90%, or 95%, or as much as 96%,
97%, 98%, 99%, or 100% of the liposomes in the liposomal composition may be
between the size values indicated herein. Liposomes may be sized by extrusion
through a filter (e.g. a polycarbonate filter) having pores or passages of the
desired
diameter.
Liposomes may include a targeting agent (such as a sugar moiety, a cell
receptor ligand, an antibody specific to a target cell, such as a cancer cell,
a
hepatocyte etc.) to achieve enhanced targeting to a specific cell population.
In some embodiments, liposomes may include a hydrophilic moiety. Grafting
a hydrophilic moiety to the surface of liposomes can "sterically stabilize"
liposomes
thereby maximizing the circulation longevity of the liposome. This results in
enhanced blood stability and increased circulation time, reduced uptake into
healthy
tissues, and increased delivery to disease sites (see U.S. Pat. Nos. 5,013,556
and
5,593,622; and Patel et al., [1992] Crit Rev Ther Drug Carrier Syst, 9:39).
Typically,
the hydrophilic moiety is conjugated to a lipid component of the liposome,
forming a
hydrophilic polymer-lipid conjugate. The term "hydrophilic polymer-lipid
conjugate"
refers to a lipid, e.g., a vesicle-forming lipid, covalently joined at its
polar head
moiety to a hydrophilic polymer, and is typically made from a lipid that has a
reactive
functional group at the polar head moiety in order to attach the polymer. The
covalent linkage may be releasable such that the polymer may dissociate from
the
lipid at for example physiological pH after a variable length of time, such as
over
9
CA 02595485 2007-08-01
several to many hours (Adlakha-Hutcheon et al. [1999] Nat Biotechnol.
17(8):775-9).
Suitable reactive functional groups are for example, amino, hydroxyl, carboxyl
or
formyl groups. The lipid may be any lipid described in the art for use in such
conjugates. The lipid may be a phospholipid having one or two acyl chains
including
between about 6 to about 24 carbon atoms in length with varying degrees of
unsaturation.
In some embodiments, the lipid in the conjugate may be a PE, such as of the
distearoyl form. The polymer may be a biocompatible polymer characterized by a
solubility in water that permits polymer chains to effectively extend away
from a
1 o liposome surface with sufficient flexibility that produces uniform surface
coverage of
a liposome. Such a polymer may be a polyalkylether, including polyethylene
glycol
(PEG), polymethylene glycol, polyhydroxy propylene glycol, polypropylene
glycol,
polylactic acid, polyglycolic acid, polyacrylic acid and copolymers thereof,
as well as
those disclosed in U.S. Pat. Nos. 5,013,556 and 5,395,619. The polymer may
have an
average molecular weight of any value between about 350 and about 10,000
daltons.
In alternative embodiments, the phospholipids may be selected from
poly(ethylene glycol) (PEG) modified phospholipids. The average molecular
weight
of the PEG may be any value between about 500 to about 10,000 Daltons.
Combinations of PEG phospholipid of different species and different chain
lengths in
varying ratios may be selected. Combinations of phospholipids and PEG
phospholipids may also be selected. The conjugate may be prepared to include a
releasable lipid-polymer linkage such as a peptide, ester, or disulfide
linkage. The
conjugate may also include a targeting agent. Mixtures of conjugates may be
incorporated into liposomes for use in this invention.
In some embodiments, liposomes may include a statin, prepared by
conventional "active" or "passive" loading methods. For example, a statin can
be
mixed with vesicle-forming lipids and be incorporated within a lipid film,
such that
when the liposome is generated, the statin is incorporated or encapsulated
into the
liposome. Thus, if the statin is substantially hydrophobic, it will be
encapsulated in
the bilayer of the liposome. Alternatively, if the statin is substantially
hydrophilic, it
will be encapsulated in the aqueous interior of the liposome. The statin may
be
soluble in aqueous buffer or aided with the use of detergents or ethanol. The
liposomes can subsequently be purified, for example, through column
chromatography or dialysis to remove any unincorporated therapeutic agent.
CA 02595485 2007-08-01
Liposomes may be prepared and formed in advance i.e., be "pre-formed"
liposomes. Pre-formed liposomes may be used to prepare the liposomal
formulations
according to the invention. Such pre-formed liposomes may include an agent,
such as
a therapeutic agent, or an agent may be added to pre-formed liposomes prior to
preparation of liposomal compositions according to the invention e.g., prior
to
combination with a micelle containing an agent. In some embodiments, pre-
formed
liposomes do not include a hydrophilic moiety. Pre-formed liposomes are
available
from various commercial contract pharmaceutical companies with expertise in
the art
of preparing liposomes.
Micelles
The term "micelle" as used herein means a self-assembled lipid arrangement
without an aqueous phase and are generally <50nm in mean diameter. Micelles
may
be spherical or tubular or wormlike and form spontaneously at or above the
critical
micelle concentration (CMC). In general, micelles are in equilibrium with the
monomers under a given set of physical conditions such as temperature, ionic
environment, concentration, etc.
Formation of a micelle requires the presence of "micelle-forming
compounds," which include amphipathic lipids (e.g., a vesicle-forming lipid as
2o described herein or known in the art), lipoproteins, detergents, non-lipid
polymers, or
any other compound capable of either forming or being incorporated into a
micellar
structure. Thus, a micelle-forming compound includes compounds that are
capable of
forming a micelle by themselves or when in combination with another compound,
and
may be polymer micelles, block co-polymer micelles, polymer-lipid mixed
micelles,
or lipid micelles. A micelle-forming compound, in an aqueous environment,
generally has a hydrophobic moiety in the interior of the micelle, and a polar
head
moiety oriented outwards into the aqueous environment. Hydrophilicity
generally
arises from the presence of functional groups such as hydroxyl, phosphate,
carboxyl,
sulfate, amino or sulfhydryl groups. Hydrophobicity generally results from the
presence of a long chain of aliphatic hydrocarbon groups.
A micelle may be prepared from lipoproteins or artificial lipoproteins
including low density lipoproteins, chylomicrons and high density
lipoproteins.
Artificial lipoproteins may also comprise lipidized protein with targeting
capabilities.
11
CA 02595485 2007-08-01
Uptake of lipoproteins into cell populations may be facilitated by receptors
present on
the target cells.
Micelles for use in the present invention may be generated using a variety of
conventional techniques. These techniques include: simple dispersion by mixing
in
aqueous or buffered or hydroalcoholic media or media containing surfactants or
ionic
substances; sonication, solvent dispersion or any other technique described
herein or
known in the art. Different techniques may be appropriate depending on the
type of
micelle desired and the physicochemical properties of the micelle-forming
components, such as solubility, hydrophobicity and behaviour in ionic or
surfactant-
containing solutions.
Micelles for use in the present invention may range from any value between
about 5 nm to about 50 nm in diameter. In some embodiments, micelles may be
less
than about 50 nm in diameter, or less than about 30 nm in diameter, or less
than about
nm in diameter.
15 In some embodiments, micelles for use in the present invention may include
a
hydrophilic polymer-lipid conjugate, as described herein or known in the art.
As
indicated herein, the term "hydrophilic polymer-lipid conjugate" refers to a
lipid, e.g.,
a vesicle-forming lipid, covalently joined at its polar head moiety to a
hydrophilic
polymer, and is typically made from a lipid that has a reactive functional
group at the
20 polar head moiety in order to attach the polymer. The covalent linkage may
be
releasable such that the polymer may dissociate from the lipid at for example
physiological pH after a variable length of time, such as over several to many
hours
(Adlakha-Hutcheon et al. [1999] Nat Biotechnol. 17(8):775-9). Such conjugates
may
include any compounds known and routinely utilized in the art of sterically
stabilized
liposome technology and technologies which are useful for increasing
circulatory
half-life for proteins, including for example polyethylene glycol (PEG),
polyvinyl
alcohol, polylactic acid, polyglycolic acid, polyvinylpyrrolidone,
polyacrylamide,
polyglycerol, or synthetic lipids with polymeric head groups. For example, a
distearoyl-phosphatidylethanolamine covalently bonded to a PEG alone, or in
further
combination with phosphatidylcholine (PC), may be used to produce a micelle
according to the invention. The molecular weight of the PEG may be any value
between about 500 Daltons to about 10,000 Daltons, inclusive, for example,
1000,
2000, 4000, 6000, 8000, etc. The CMC of the hydrophilic polymer-lipid
conjugate
will be dependent on the molecular weight of the PEG as well as the lipid
anchor and
12
CA 02595485 2007-08-01
the added components used when preparing mixed micelles (e.g. PEG modified
distearoyl-phosphatidylethanolamine and PC).
Statins
The term "statin" as used herein refers to a chemical moiety that acts as a
competitive inhibitor of hydroxymethyl-glutaryl (HMG)-CoA reductase and
includes
any natural or synthetic biologically active statin, and pharmaceutically
acceptable
salts, solvates and prodrugs, thereof.
The term "pharmaceutically acceptable" means compatible with the treatment
of patients.
The term "solvate" as used herein means a statin, or a salt of a statin,
wherein
molecules of a suitable solvent are incorporated in the crystal lattice. A
suitable
solvent is physiologically tolerable at the dosage administered. Examples of
suitable
solvents are ethanol, water and the like. When water is the solvent, the
molecule is
referred to as a "hydrate". The formation of solvates of statins will vary
depending on
the statin and the solvate. In general, solvates are formed by dissolving a
compound
in the appropriate solvent and isolating the solvate by cooling or using an
antisolvent.
The solvate is typically dried or azeotroped under ambient conditions.
The term "pharmaceutically acceptable salt" includes both pharmaceutically
acceptable acid addition salts and base addition salts.
The term "pharmaceutically acceptable acid addition salt" as used herein
means any non-toxic organic or inorganic salt of any basic statin. Basic
statins that
may form an acid addition salt include, for example, those substituted with
NH2,
NHCI-Czoalkyl or N(CI-C2oalkyl)(CI-C2oalkyl). Illustrative inorganic acids
which
form suitable salts include hydrochloric, hydrobromic, sulfuric and phosphoric
acids,
as well as metal salts such as sodium monohydrogen orthophosphate and
potassium
hydrogen sulfate. Illustrative organic acids that form suitable salts include
mono-, di-
, and tricarboxylic acids such as glycolic, lactic, pyruvic, malonic,
succinic, glutaric,
fumaric, malic, tartaric, citric, ascorbic, maleic, benzoic, phenylacetic,
cinnamic and
salicylic acids, as well as sulfonic acids such as p-toluene sulfonic and
methanesulfonic acids. Either the mono or di-acid salts can be formed, and
such salts
may exist in either a hydrated, solvated or substantially anhydrous form. In
general,
the acid addition salts of statins are more soluble in water and various
hydrophilic
organic solvents, and generally demonstrate higher melting points in
comparison to
13
CA 02595485 2007-08-01
their free base forms. The selection of the appropriate salt will be known to
one
skilled in the art. Other non-pharmaceutically acceptable acid addition salts,
e.g.
oxalates, may be used, for example, in the isolation of the statins, for
laboratory use,
or for subsequent conversion to a pharmaceutically acceptable acid addition
salt.
The term "pharmaceutically acceptable basic addition salt" as used herein
means any non-toxic organic or inorganic base addition salt of any acid statin
compound. Acidic statins that may form a basic addition salt include, for
example,
those possessing a carboxylic acid moiety. Illustrative inorganic bases which
form
suitable salts include lithium, sodium, potassium, calcium, magnesium or
barium
1 o hydroxide. Illustrative organic bases which form suitable salts include
aliphatic,
alicyclic or aromatic organic amines such as methylamine, trimethylamine and
picoline, alkylammonias or ammonia. The selection of the appropriate salt will
be
known to a person skilled in the art. Other non-pharmaceutically acceptable
basic
addition salts, may be used, for example, in the isolation of the statins, for
laboratory
use, or for subsequent conversion to a pharmaceutically acceptable basic
addition salt.
The formation of a desired compound salt is achieved using standard
techniques. For example, the neutral statin is treated with an acid or base in
a suitable
solvent and the formed salt is isolated by filtration, extraction or any other
suitable
method.
The term "prodrug" as used herein refers to any compound that has less
intrinsic activity than the corresponding "drug," but when administered to a
biological
system, generates the "drug" substance, either as a result of spontaneous
chemical
reaction or by enzyme catalyzed or metabolic reaction. Prodrugs include,
without
limitation, acyl esters, carbonates, phosphates, and urethanes. These groups
are
exemplary, and not exhaustive, and one skilled in the art could prepare other
known
varieties of prodrugs. Prodrugs may be, for example, formed with available
hydroxy,
thiol, amino or carboxyl groups. For example, available hydroxy or amino
groups
may be acylated using an activated acid in the presence of a base, and
optionally, in
inert solvent (e.g. an acid chloride in pyridine). Some common esters which
have
been utilized as prodrugs are phenyl esters, aliphatic (C1-C24) esters,
acyloxymethyl
esters, carbamates and amino acid esters.
In an embodiment, the statin is selected from simvastatin, atorvastatin,
cerivastatin, fluvastatin, lovastatin, mevastatin, pitavastatin, pravastatin
and
rosuvastin, mixtures thereof, pharmaceutically acceptable salts, solvates and
prodrugs
14
CA 02595485 2007-08-01
thereof and mixtures thereof. In an embodiment the statin is simvastatin or
atorvastatin. In a further embodiment the statin is simvastatin. The chemical
structure of simvastatin, atorvastatin, cerivastatin, fluvastatin, lovastatin,
mevastatin,
pitavastatin, pravastatin and rosuvastin is shown in Figure 1.
Methods of Preparing Liposomal Statin Compositions
The invention provides a method of preparing a liposomal composition
including a statin by incorporating statin into a micelle. The micelle may
include a
PEG-phospholipid, such as DSPE-PEG2000. The micelle is then combined with a
liposome, such as a pre-formed liposome, thus incorporating the statin into
the
liposome. In alternative embodiments, the statin is a poorly soluble compound
that
can be solubilized in a micelle.
In some embodiments, the statin may be solubilized in a solvent, such as
ethanol or hydroalcoholic solutions of ethanol in aqueous media, prior to
incorporation into the micelle. In some embodiments, the final concentration
of
solvent in the phospholipid-containing liposomes, for example those composed
primarily of DPPC, DMPC, DSPC, DOPC or similar compositions, may be limited to
a concentration that does not induce significant toxicity when administered to
a
subject and/or does not disrupt the integrity or performance of the micelle or
liposome. For example, for ethanol, the final concentration may be any value
between about 1 to about 30% (v/v), although lower or higher values are also
contemplated. In some embodiments, the incorporation of statins into liposomes
can
be achieved while minimizing solvent concentrations or the presence of bio-
incompatible solvents. For statins to be encapsulated within the liposomal
bilayer
which are directly soluble in aqueous dispersions of the micelle-forming
components,
solvents such as ethanol may not be necessary.
A statin may be incorporated into a micelle during preparation of a micelle as
described herein or known in the art. For example the statin may be dissolved
in, for
example, aqueous buffer/alcoholic media and combined with a buffer solution
comprising the micelle forming compound and the resulting combination mixed
and
optionally warmed, for example to a temperature of about 30 C to about 70 C,
suitably about 55 C, until a substantially clear solution is obtained.
In alternative embodiments, the statin is not covalently coupled to a micelle
forming compound.
CA 02595485 2007-08-01
The statin-containing micelles are then incorporated into the liposomes, by,
for example, combining the solution of the statin-containing micelles with a
buffered
solution containing the preformed liposomes and optionally warming to a
temperature
of about 30 C to about 70 C, suitably about 35 C to about 55 C, for up to
90
minutes. In an embodiment, the components of the micelles, including the
statin, are
incorporated into the lipid bilayer of the liposomes. The liposomes may
include but
are not limited to one or more of the following lipids: DMPC, DPPC or DSPE,
and
the ratios of the lipids may vary according to embodiments visualized by
persons
skilled in the art of liposome preparation. In some embodiments, the liposome
may
be a pre-formed liposome that may or may not contain the statin or one or more
second or additional agent(s) (e.g., a small molecule, a protein, antibody, or
polypeptide or a nucleic acid, e.g., having membrane localization properties
such as
juxtamembrane localization or transmembrane domains) incorporated or
encapsulated
in it. The second or additional agent may be loaded into the liposome using
conventional loading techniques as described herein or known in the art.
Alternatively, or additionally, more than one statin may be loaded into a
liposome
using the methods of the invention, by for example incorporating one or more
micelles containing one or more statins into the liposome. In an alternative
embodiment, small molecules (chemical compounds), proteins, antibodies or
peptides
or pharmaceutically acceptable salt thereof, may be encapsulated into a
liposome by
prior solubilization, active loading or passive entrapment and incorporation
into a
polymer micelles, polymer-lipid mixed micelles or lipid micelles.
Liposomal compositions according to the invention may be stored in any
suitable form that may vary according to mode of administration. For example,
a
liposomal composition may be a liquid at room temperature (e.g., a sterile
single-vial
liquid), a frozen product, or a dehydrated product (e.g., a powder or a
lyophilized cake
to be reconstituted prior to use). Different storage forms may be prepared
using
methods known to a person skilled in the art. For example, a cryoprotectant
such as a
disaccharide, may be added to a liposomal composition prior to lyophilization
to
enable storage of a liposomal composition as a dehydrated product.
In alternative embodiments, the statin is releasable from a liposome prepared
according to the invention, to facilitate transfer of statin into a target
cell. Thus, a
releasable statin is a statin that is capable of transferring out of a
liposome according
to the invention and exerting its biological action inside, or in the vicinity
of, a cell in
16
CA 02595485 2007-08-01
a subject. In alternative embodiments, the statin is generally stable during
storage of
a liposomal composition. In alternative embodiments, the statin is generally
stable
during circulation in the bloodstream of a subject i.e., the statin is not
substantially
released from the liposome prior to its delivery inside, or in the vicinity
of, a cell in a
subject.
As described herein, simvastatin PEG-lipid micelles including DSPE-PEG2ooo
were added to pre-formed liposomes including DMPC or DPPC. Simvastatin was
rapidly loaded, and remained stably incorporated, into the outer layer of the
liposomes.
Therapeutic Indications
Liposomal compositions according to this invention may be used for delivery
of a statin, for example a poorly soluble therapeutic statin, for treatment of
a variety
of diseases and conditions in a subject in need thereof, or for bringing about
a desired
biological effect such as an immune response in such a subject. Such diseases
and
conditions include those that would benefit from liposomes which increase
retention
or stability of the statin in storage or in circulation in a subject, enabling
therapeutic
drug interventions with superior ADMET (absorption, distribution, metabolism,
excretion and toxicity) properties. In an aspect of the invention the
liposomal
compositions are useful for the treatment of a disease or condition that
benefits from
administration of a statin. In embodiments of the invention, the disease or
condition
that benefits from administration of a statin is selected from dyslipidemia,
hypercholesterolemia, hypertriglyceridemia, cardiovascular disease, acute
coronary
syndrome, experimental autoimmune encephalomyelitis, rheumatoid arthritis,
osteoarthritis, transplantation, multiple sclerosis, chronic kidney disease
and
influenza. In an embodiment, the disease or condition is selected from
dyslipidemia,
hypercholesterolemia, hypertriglyceridemia, cardiovascular disease, acute
coronary
syndrome, rheumatoid arthritis and influenza. In a further embodiment, the
disease or
condition is selected from dyslipidemia, hypercholesterolemia,
hypertriglyceridemia,
cardiovascular disease and rheumatoid arthritis.
As used herein, and as well understood in the art, "treatment" is an approach
for obtaining beneficial or desired results, including clinical results.
Beneficial or
desired clinical results can include, but are not limited to, alleviation or
amelioration
of one or more symptoms or conditions, diminishment of extent of disease,
stabilized
17
CA 02595485 2007-08-01
(i.e. not worsening) state of disease, preventing spread of disease, delay or
slowing of
disease progression, amelioration or palliation of the disease state, and
remission
(whether partial or total), whether detectable or undetectable. "Treatment"
can also
mean prolonging survival as compared to expected survival if not receiving
treatment.
"Treating" or "treatment" as used herein includes prevention of a condition or
disease,
and accordingly, prophylactic uses of the liposomal compositions of the
invention are
also included within the scope of the invention.
Pharmaceutical & Veterinary Compositions, Dosages, And Administration
In some embodiments, the compositions of the invention are particularly
useful for the delivery of poorly soluble statins. Statins in the liposomal
compositions
of the invention can be provided alone or in combination with other compounds
or
agents (for example, nucleic acid molecules, small molecules, peptides, or
peptide
analogues), in the presence any pharmaceutically acceptable carrier, in a form
suitable
for administration to mammals, for example, humans, pigs, horses, cattle,
sheep, etc.
In some embodiments, the compositions may include an adjuvant. In some
embodiments, the liposomal compositions may include a targeting agent to
localize or
direct the liposomes to the region or tissue requiring exposure to therapeutic
doses of
the statin. In some embodiments, the targeting agent may be an antibody or
component that selectively recognizes a diseased cell or tissue. If desired,
treatment
with a liposomal composition according to the invention may be combined with
more
traditional and existing therapies for the condition to be treated. Statins
according to
the invention may be provided chronically or intermittently. "Chronic"
administration refers to administration of the agent(s) in a continuous mode
as
opposed to an acute mode, so as to maintain the initial therapeutic effect
(activity) for
an extended period of time. "Intermittent" administration is treatment that is
not
consecutively done without interruption, but rather is cyclic in nature.
Conventional pharmaceutical practice may be employed to provide suitable
formulations or compositions to administer the compositions to subjects
suffering
from or at risk for a disease or condition that benefits from the
administration of a
statin. In some embodiments, the pharmaceutical compositions are administered
parenterally, i.e. intraarticularly, intravenously, subcutaneously, or
intramuscularly or
via aerosol. Aerosol administration methods include intranasal and pulmonary
administration. In some embodiments, the pharmaceutical compositions are
18
CA 02595485 2007-08-01
administered intravenously, intramuscularly or intraperitoneally by a bolus
injection.
For example, see Rahman et al., U.S. Pat. No. 3,993,754; Sears, U.S. Pat. No.
4,145,410; Papahadjopoulos et al., U.S. Pat. No. 4,235,871; Schneider, U.S.
Pat. No.
4,224,179; Lenk et al., U.S. Pat. No. 4,522,803; or Fountain et al., U.S. Pat.
No.
4,588,578.
Methods well known in the art for making formulations are found in, for
example, "Remington's Pharmaceutical Sciences (2003 - 20th edition) and in The
United States Pharmacopeia: The National Formulary (USP 24 NF 19) published in
1999. Formulations for parenteral administration may, for example, contain
excipients, sterile water, or saline, polyalkylene glycols such as
polyethylene glycol,
oils of vegetable origin, or hydrogenated napthalenes. Formulations for
inhalation
may contain excipients, for example, lactose, or may be aqueous solutions
containing,
for example, polyoxyethylene-9-lauryl ether, glycocholate and deoxycholate, or
may
be oily solutions for administration in the form of nasal drops, or as a gel.
In some
embodiments, a liposomal composition according to the invention is not
suitable for
topical administration. In some embodiments, a liposomal composition according
to
the invention is particularly suitable for parenteral administration, e.g., by
injection.
The liposomal compositions according to the invention are in general capable
of delivering an effective amount of a statin to a cell in a subject. An
"effective
amount" of a statin according to the invention includes a therapeutically
effective
amount or a prophylactically effective amount. A "therapeutically effective
amount"
refers to an amount effective, at dosages and for periods of time necessary,
to achieve
the desired therapeutic result. A therapeutically effective amount of a statin
may vary
according to factors such as the disease state, age, sex, and weight of the
individual,
and the ability of the statin to elicit a desired response in the individual.
Dosage
regimens may be adjusted to provide the optimum therapeutic response. A
therapeutically effective amount is also one in which any toxic or detrimental
effects
of the statin are outweighed by the therapeutically beneficial effects. A
"prophylactically effective amount" refers to an amount effective, at dosages
and for
periods of time necessary, to achieve the desired prophylactic result.
Typically, a
prophylactic dose is used in subjects prior to or at an earlier stage of
disease, so that a
prophylactically effective amount may be less than a therapeutically effective
amount.
A suitable range for therapeutically or prophylactically effective amounts of
a
19
CA 02595485 2007-08-01
compound may be any integer from 0.1 nM-0.1M, 0.1 nM-0.05M, 0.05 nM-15gM or
0.01 nM-10 M.
It is to be noted that dosage values may vary with the severity of the
condition
to be alleviated. For any particular subject, specific dosage regimens may be
adjusted
over time according to the individual need and the professional judgement of
the
person administering or supervising the administration of the compositions.
Dosage
ranges set forth herein are exemplary only and do not limit the dosage ranges
that may
be selected by medical practitioners. The amount of active statin(s) in the
composition may vary according to factors such as the disease state, age, sex,
and
1o weight of the individual. Dosage regimens may be adjusted to provide the
optimum
therapeutic response. For example, a single bolus may be administered, several
divided doses may be administered over time or the dose may be proportionally
reduced or increased as indicated by the exigencies of the therapeutic
situation. It may
be advantageous to formulate parenteral compositions in unit dose form for
ease of
administration and uniformity of dosage.
In the case of vaccine formulations, an immunogenically effective amount of a
statin can be provided, alone or in combination with other compounds or
agents, with
an immunological adjuvant, for example, Freund's incomplete adjuvant,
dimethyldioctadecylammonium hydroxide, or aluminum hydroxide. The statin may
also be linked with a carrier molecule, such as bovine serum albumin or
keyhole
limpet hemocyanin to enhance immunogenicity.
In general, compositions of the invention should be used without causing
substantial toxicity. Toxicity of the statins and compositions of the
invention can be
determined using standard techniques, for example, by testing in cell cultures
or
experimental animals and determining the therapeutic index, i.e., the ratio
between the
LD50 (the dose lethal to 50% of the population) and the LDlpo (the dose lethal
to
100% of the population). In some circumstances however, such as in severe
disease
conditions, it may be necessary to administer substantial excesses of the
compositions.
The compositions may be administered to any suitable subject. As used
herein, a subject may be a human, non-human primate, rat, mouse, cow, horse,
pig,
sheep, goat, dog, cat, etc. The subject may be a clinical patient, a clinical
trial
volunteer, an experimental animal, etc. The subject may be suspected of having
or at
CA 02595485 2007-08-01
risk for having a disorder, be diagnosed with a disorder or be a control
subject that is
confirmed to not have the specific disorder of interest.
Kits
The liposomal compositions of the invention may be provided in a kit,
together with instructions for use. The kit may include a first container
including a
statin solubilized in a micelle, a second container including a liposome of a
desired
composition, and instructions for mixing the contents of the first and second
containers at a desired ratio to provide a liposomal composition containing
the statin
(i.e., to provide a loaded liposome).
In alternative embodiments, the kit may include a first container including an
statin; a second container including a micelle-forming compound; and a third
container including a liposome of the desired composition, together with
instructions
for combining the contents of the first and second containers to form a
micelle loaded
with the statin, and for combining the micelle with the contents of the third
container
to prepare a loaded liposome containing the statin.
In some embodiments, the kit may include a second agent to be loaded into the
liposome using convention techniques, prior to combining the liposome with a
micelle.
The kit components may be stored at suitable temperatures or forms, e.g.,
room temperature, refrigerated (e.g., 4 C), frozen (e.g., -20 C),
cryopreserved,
dehydrated, etc., for suitable lengths of time.
Although various embodiments of the invention are disclosed herein, many
adaptations and modifications may be made within the scope of the invention in
accordance with the common general knowledge of those skilled in this art.
Such
modifications include the substitution of known equivalents for any aspect of
the
invention in order to achieve the same result in substantially the same way.
Numeric
ranges are inclusive of the numbers defining the range. In the specification,
the word
"comprising" is used as an open-ended term, substantially equivalent to the
phrase
"including, but not limited to", and the word "comprises" has a corresponding
meaning. Citation of references herein shall not be construed as an admission
that
such references are prior art to the present invention. All publications are
incorporated
herein by reference as if each individual publication were specifically and
21
CA 02595485 2007-08-01
individually indicated to be incorporated by reference herein and as though
fully set
forth herein. The invention includes all embodiments and variations
substantially as
hereinbefore described and with reference to the examples and drawings.
Various alternative embodiments and examples of the invention are described
herein. These embodiments and examples are illustrative and should not be
construed
as limiting the scope of the invention.
EXAMPLE 1: Liposomal formulation with simvastatin
Simvastatin (Toronto Research Chemicals, North York, Ontario Canada) was
prepared at 2mg/mL in liposomes composed of dipalmitoyl phosphatidyl
choline/distearoyl phosphatidylethanolamine-poly(ethylene glycol)2000
(DPPC/DSPE-
PEG2000 95:5 mol:mol) by passively entrapping simvastatin in multilamellar
vesicles
(MLVs) or by incorporating the drug into large unilamellar vesicles (LUVs,) by
the
micelle loading (micelle exchange or ML) method as previously described (Wasan
et
al. [2006] PCT Patent Application Publication No. W02006/079216, August 3,
2006).
EXAMPLE 2: Stability studies for liuosomal simvastatin in HBS buffer, human
plasma and human rheumatoid arthritis (RA) synovial fluid
Following incubation in HEPES-buffered saline (HBS), plasma or human
synovial fluid from patients with rheumatoid arthritis (RA) for 2h at 37 C,
liposomal
samples were passed down a CL4B gel filtration column and fractions were
collected
to separate the liposomal fractions (2-6) from the micelle and protein
fractions (10-
15). The results are shown in Figure 2A (MLV-simvastatin in HBS buffer), 2B
(ML
liposomal simvastatin in HBS buffer), Figure 3A (MLV-simvastatin in human
plasma), 3B (ML liposomal simvastatin in human plasma), Figure 4A (MLV-
simvastatin in human RA synovial fluid) and 4B (ML liposomal simvastatin in
human
RA synovial fluid). Data represent mean of 3 sets of independently prepared
samples,
shown as % of total added to the fractionation column that was recovered in
each
fraction. The results indicate that simvastatin remains liposome-associated in
HBS
and only a minor amount separates from the liposomes in plasma or synovial
fluid to
become associated with lipid micelles or proteins. Figure 5 presents a bar
graph
showing that the original drug:lipid ratio of 0.02 (w/w) was not reduced
following
22
CA 02595485 2007-08-01
buffer incubation of MLV-simvastatin and ML liposomal simvastatin and
following
synovial fluid incubation of MLV-simvastatin and only slightly reduced for
synovial
fluid incubation of ML liposomal simvastatin.
EXAMPLE 8: Effect of lipid composition on stability of drug loading for
liposomal simvastatin
Liposomal simvastatin was prepared by a standard thin-film extrusion method
(TFE) and the micelle-loading method (ML). Liposomes were stored in HEPES-
buffered saline (pH 7.2) for 1, 3 or 4 and 7 days followed by analysis of drug
content
in the liposomes. The results are shown in the graphs in Figures 6A-I. Lipid
composition is indicated on each graph, with molar ratios of components
indicated.
DPPC: dipalmitoyl phosphatidylcholine; chol: cholesterol; DSPE-PEG: distearoyl
phosphatidylethanolamine-poly(ethylene glycol); DC-chol: 30-[N-
(Dimethylaminoethane)carbamoyl]cholesterol; DMPC: dimyristroyl
pho sphatidylcho line; DMPG: dimyristoyl phosphatidyl glycerol. Data represent
meanfSD for 3 independently prepared liposomal samples. The micelle loading
method results in more stable drug loading for liposomal simvastatin than the
standard TFE method regardless of lipid composition, and regardless of whether
simvastatin is in the lactone or carboxylic acid form ("activated
simvastatin")
indicating the flexibility of this drug loading method.
23