Note: Descriptions are shown in the official language in which they were submitted.
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
METHOD AND APPARATUS FOR CONTROLLED PRODUCTION OF A GAS
CROSS-REFERENCED APPLICATIONS
This application is a continuation of U.S. Patent
Application No. 11/045,805 entitled "METHOD AND APPARATUS FOR
CONTROLLED PRODUCTION OF A GAS" (Docket No. ROSS 3050000)
filed January 28, 2005, which relates to and claims priority
from co-pending U.S. Patent Application No. 10/718,131
entitled "METHOD AND APPARATUS FOR GENERATING OXYGEN" (Docket
No. ROSS 2864000), filed November 20, 2003, and co-pending
U.S. Patent Application No. 10/856,591, entitled "APPARATUS
AND DELIVERY OF MEDICALLY PURE OXYGEN" (Docket No. ROSS
2934000), filed May 28, 2004, the contents of each of which
are hereby incorporated by reference for all purposes. This
application further claims priority to the following U.S.
Patent Applications filed June 22, 2005: Serial no.
11/045,805; Serial no. 11/158,993; Serial no. 11/159,016;
Serial no. 11/158,377; Serial no. 11/158,362; Serial no.
11/158,618; Serial no. 11/158,989; Serial no. 11/158,696;
Serial no. 11/158,648; Serial no. 11/159,079; Serial no.
11/158,763; Serial no. 11/158,865; Serial no. 11/158,958; and
Serial no. 11/158,867; all entitled Method and Apparatus for
Controlled Production of a Gas, and filed June 22, 2005.
1
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
FIELD OF THE INVENTION
The present invention relates generally to a gas delivery
system and, more particularly, to a system that provides an
activation method and apparatus as well as a method and
apparatus for improving and controlling the gas yield, flow
rates and gas production duration.
DESCRIPTION OF THE RELATED ART
Oxygen and other gas generators using chemical reactions
have been known for some time. However, none of the
conventional devices relating to chemical gas generators have
resulted in variable control of the gas generation, while
providing higher outputs of gas volume and flow rate, and
simultaneously maintaining or improving control of pressure,
temperature, and so forth. Gas volume and flow rate are
particularly important in emergency oxygen markets. For
example, institutions such as the Food & Drug Administration,
the American Heart Association and the American Medical
Association have required or recommended, as the case may be,
a delivery of 90 liters over a 15 minute period, or
alternatively an average or minimum flow rate of 6 liters per
minute over a 15 minute period. Some attempts to control the
flow rate of oxygen have included a catalyst with a gum Arabic
solution. The resultant reaction reaches a flow rate of 2
2
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
liters per minute after 30 minutes. Other devices create a
tablet out of an oxygen generating agent, which similarly
produces a low reaction onset (the flow rate at which the
reaction commences) and low flow rates over the reaction
period. These prior attempted solutions may not be suitable
for emergency applications, usually medical in nature or
situations where life-threatening factors are present where
high flow rates of at least 2 liters per minute to 6 liters
per minute or higher are required almost instantly.
In addition, conventional generators have had limited
adoption in commerce and in industry. There are several
possible factors contributing to this lack of adoption. These
factors may include one or a'combination of unfavorable
characteristics relating to reusability, safety, ease of
use/operation, speed of use, heat management, cost, weight,
aesthetic design, environmental impact, manufacturability,
portability, medical efficacy, effectiveness, flow rate, gas
yield, reaction stability, and purity of the gas. Some or all
of these characteristics are not addressed, or are
inadequately addressed, by the designs in the prior art.
Designs in the prior art have not adequately addressed
flow rate and total gas yield. Depending on the situation,
such as for oxygen production in emergency situations, high
flow rates may be required. For example, the United States
Food and Drug Administration (FDA) has long required a flow
3
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
rate performance for oxygen generators of at least 6 liters
per minute over 15 minutes in order to-obtain market clearance
for over the counter purchase, resulting in at least a total
oxygen yield requirement of 90 liters.
High pressures generated inside the reaction chamber
generally accompany higher flow rate outputs or requirements.
High pressure, such as can be created by confined gases can be
particularly dangerous.
Therefore, a need exists for a method and/or apparatus for
activating gas production and controlling gas production from
a chemical reaction that addresses at least some of the
problems associated with conventional methods and apparatus
for producing gases, and more specifically medically pure
oxygen.
SUMMARY OF THE INVENTION
The present invention provides an apparatus for
generating gas from a plurality of separated chemicals. In
one embodiment, a plurality of reaction chambers operate
cooperatively when the separated chemicals are combined to
generate the gas. The flow rate and the total yield can then
be varied based on the proportion of separated chemicals in
each reaction chamber.
4
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
BRIEF DESCRIPTION OF THE DRAWINGS
For a more complete understanding of the present
invention and the advantages thereof, reference is now made to
the following descriptions taken in conjunction with the
accompanying drawings, in which:
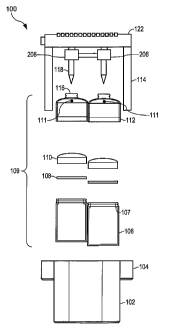
FIGURE 1 is a diagram, partly in section, depicting an
exploded side view of gas activation, production, dispensing
and control vessel in accordance with an embodiment of the
present invention;
FIGURE 2 is a diagram, partly in section, depicting a
side view of a primed gas activation, production, dispensing
and control vessel;
FIGURE 3 is a schematic sectional view of the gas
activation, production, dispensing and control vessel, in use,
with the spiked plungers inserted;
FIGURE 4A is a plan view of an example of a screen;
FIGURE 4B is a sectional view of the screen depicted in
FIGURE 4A;
FIGURE 5A depicts a plan view of a foam breaker, taken
along the lines 5B, 5C;
FIGURE 5B depicts a cross sectional view of the foam
breaker of FIGURE 5A;
FIGURE 5C depicts a cross sectional view of the foam
breaker of FIGURES 5A and 5B when compressed;
5
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
FIGURE 6A depicts a plan cross sectional view of a handle
useful in connection with the present invention;
FIGURE 6B depicts a side cross sectional view of a handle
useful in connection with the present invention, taken along
the line 6B;
FIGURE 7 of the drawings is a partially cross sectioned
view of a female connector useful in connection with the
present invention;
FIGURE 8 depicts a cross sectional view of a male
connector adapted to fit with the female connector depicted in
FIGURE 7;
FIGURE 9 depicts a side view, partly in cross section, of
one embodiment of the connectable spiked plunger, as connected
to the female connector depicted in FIGURE 7;
FIGURE 10A depicts a side cross sectional view of a
spiked plunger;
FIGURE 1 B depicts a side cross sectional view of a
spiked plunger in its female connector housing, with the
spiked plunger disconnected;
FIGURE 10C depicts a side cross sectional view of a
spiked plunger in its female connector housing, with the
spiked plunger connected to it;
FIGURE 11 depicts a side cross sectional view of a spring
loaded spiked plunger and release mechanism;
6
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
FIGURE 12 depicts a side cross sectional view of a
cartridge filled with initially separated chemicals and having
a pressure relief system;
FIGURE 13A depicts a side cross sectional view of an
activation system for one reaction chamber of the gas
activation, production, dispensing and control vessel depicted
in FIGURES 1 and 2, having a spike, with the spike withdrawn
for clarity;
FIGURE 13B depicts a side cross sectional view of an
activation system for one reaction chamber of the gas
activation, production, dispensing and control vessel depicted
in FIGURES 1 and 2, having a spike inserted into the container
holding the water to rupture it and allow mixing the the other
chemicals to create a flow of gas, with the flow of gas
produced indicated by arrows;
FIGURE 14 depicts a side cross sectional view of an
activation system with dual reaction chambers having spikes as
depicted in FIGURES 10A, 10B and lOC, and having a hanging
catalyst bag, with the spike withdrawn and primed for
activation;
FIGURE 15 depicts a side cross sectional view of an
another embodiment of an activation system with dual reaction
chambers having spikes as depicted in FIGURE 9, the male
connectors depicted in FIGURE 8, and compartments for
7
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
retaining the catalyst and water as depicted in FIGURE 16Awith
the spike withdrawn and primed for activation;
FIGURE 16A depicts a cross-sectional side view of the
water containment housing and an adjacent catalyst dispersal
housing depicted in FIGURE 15;
FIGURE 16B depicts cross-sectional side view of a
modified version of the catalyst dispersal housing depicted in
FIGURE 16A;
FIGURE 17A depicts a side cross sectional view of another
embodiment of an activation system for one reaction chamber,
having a fixed activation member, in the primed position;
FIGURE 17B depicts a side cross sectional view of the
embodiment of an activation system for one reaction chamber
depicted in FIGURE 17A, after activation, the arrows
indicating flow of the water and catalyst;
FIGURE 18A depicts a front view, partly in phantom, of a
powder release pouch cartridge assembly;
FIGURE 18B is a sectional side view of the powder release
pouch cartridge assembly depicted in FIGURE 18A, taken along
line 18A-A;
FIGURE 19 is a partially diagrammatic side view of a
bubbler;
FIGURE 20 is a diagram depicting a heat exchanger/
radiator;
8
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
FIGURE 21 depicts a side cross sectional view of an
embodiment of a cartridge for one reaction chamber, showing
different locations for the catalyst and gas/oxygen producing
agent;
FIGURE 22 depicts a side cross sectional view of another
embodiment of a cartridge for one reaction chamber;
FIGURE 23A depicts a cross-sectional front view of a
container for containing pouch-type reaction chambers as
depicted in FIGURES 26A and 26B, utilizing a mechanical lever
to initiate the gas-generating reaction;
FIGURE 23B depicts a cross-sectional side view of the
container depicted in FIGURE 23A, taken along the line 23A-
23A.
FIGURE 24A is a diagram contrasting the flow rate of two
gas producing reactions;
FIGURE 24B is a diagram showing the combined flow rate of
two gas producing reactions of FIGURE 24A;
FIGURE 25A is a diagram contrasting the flow rate of two
gas producing reactions initiated at different times; and
FIGURE 25B is a diagram showing the combined flow rate of
two gas producing reactions of FIGURE 25A.
FIGURE 26A depicts a pouch-type, self-contained, reaction
chamber including separate compartments for the catalyst,
gas/oxygen producing agent and water; and
9
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
FIGURE 26B depicts another embodiment of a pouch-type,
self-contained, reaction chamber including differently shaped,
separate compartments for the catalyst, gas/oxygen producing
agent and water.
DETAILED DESCRIPTION
In the following discussion, numerous specific details
are set forth to provide a thorough understanding of the
present invention. However, those skilled in the art will
appreciate that the present invention may be practiced without
such specific details. In other instances, well-known
elements have been illustrated in schematic or block diagram
form in order not= to obscure the present invention in
unnecessary detail. Additionally, for the most part, details
concerning network communications, electro-magnetic signaling
techniques, and the like, have been omitted inasmuch as such
details are not considered necessary to obtain a complete
understanding of the present invention, and are considered to
be within the understanding of persons of ordinary skill in
the relevant art.
Referring to FIGURE 1 of the drawings, the reference
numeral 100 generally designates an exploded view of a gas
activation, production, dispensing and control assembly using
a manual reaction activation method in accordance with an
embodiment of the present invention. The assembly 100
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
comprises support housing 102, removable reaction chambers
106, screens 108, filters 110, lids 112, and a handle 122.
The main body of the assembly 100 is the support housing
102. There are a number of configurations that can be
employed, but a convenient design is a vessel having
vertically extending side walls and a bottom surface
connecting the side walls. The support housing 102 also has
an opening in the top where other members can be inserted.
The support housing 102 can also be a smooth, continuous
surface or it can be several joined, flat surfaces. For
example, the support housing has a compartment for each
reaction chamber and can have curved surfaces such that it
curves around the reaction chambers 106 in approximately the
shape of a figure eight, as viewed from above. In such a
configuration the gas activation, production, dispensing and
control assembly 100 can be- conveniently worn on the hip, by
clip-on or otherwise of say, a miner, construction worker or
emergency service personnel. Additionally, the support housing
102 can employ two guides 104 that protrude outwardly from the
side walls of the support housing 102 to interface with and/or
slidably receive the guided members 114 of the handle 122. In
the manual activation device shown in FIGURE 1, the two guide
members 104 allow the user to activate the chemical reaction
producing the oxygen or other gas, by pushing the handle 122
in a direction toward the housing 102. The two guide members
11
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
104 allow for this to be a smooth and easy process. Upon
completion of the chemical reaction, the two guide members 104
similarly allow for a smooth and easy disengagement of the
handle 122 in a direction away from the housing 102 utilizing
a quick release mechanism 720 (depicted in FIGURE 7, but not
shown in FIGURE 1). The support housing 102 can also act as
an additional insulating material to act as a heat shield for
any excess heat being generated in the reaction chambers.
Each of the reaction chambers 106 can be placed within
the support housing 102 such that access can be gained to each
reaction chamber 106. The reaction chambers 106 can be made
of a durable thermoplastic with high tensile strength, high
resistance to chemical reactions and high resistance to heat.
For example, the reaction chambers 106 can be made of
polycarbonate or polytetrafluoroethylene. The lids 112 can be
attached to the reaction chambers 106. For example, reaction
chambers 106 can have internal female threads and the lids 112
can have corresponding external male threads. Alternatively,
the lids 112 can be attached to the reaction chambers 106 by
clip in, lock in or click in designs. Screens 108 and filters
110 can be seated on a flange 107 inside reaction chambers
106, but such is not essential to the design. For example,
screens 108 and filters 110 can also simply be maintained in
position by mechanical pressure, or glued, as depicted in
FIGURE 3. The reaction chambers 106 are typically
12
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
cylindrically shaped, but can be any other shape. The
reaction chambers 106, however, can be coupled to the lids 112
prior to insertion into the support housing 102.
Referring to FIGURE 2 of the drawings, the reference
numeral 200 generally designates a primed gas production
control vessel.
When the vessel 200 is in the primed position, gas
production can be initiated by engaging the handle 122. The
guide members 104 (of support housing 102) can contain and
guide the arms 114 of the handle 122. By allowing the arms
114 to freely slide within the guides 104 a user would simply
place pressure on the handle 122 in a direction toward the
support housing 102.
From the primed position, it is evident that alignment
can be an advantageous feature. Each of the spiked plungers
118 can be aligned with an opening 116 of a lid 112.
Therefore, when engaged, each of the spiked plungers 118 can
be slidably inserted into each of the reaction chambers 106 to
initiate the reaction and carry out the resultant gas.
Referring to FIGURE 3 of the drawings, the reference
numeral 300 generally designates a cut-away of a gas
activation, production, dispensing and control vessel in use.
When fully assembled, control of the gas production is
achieved through the use of multiple reaction chambers 106.
Two reaction chambers are depicted, but there can be more
13
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
reaction chambers depending on the desired flow rate and
yield. One reaction chamber can also be used. Chemical
reactions occur in the lower portions 210 of the reaction
chambers 106. By varying the proportion, amounts and/or
composition of the reactants within the vessel, two different
reaction rates (and yields) can be maintained independently in
each of the reaction chambers 106. Hence, each reaction
chamber 106 can contribute a fractional gas output of the
total gas output of the vessel, allowing for a variety of gas
yields and flow rates. Moreover, the reactants in each
reaction chamber 106 can vary, as well, to achieve a desired
gas yield and gas flow rate.
Each of the reaction chambers 106 rests within the
support housing 102. Each of two guided members 114 of the
handle 122 are inserted through one of two guide members 104.
Each of the reaction chambers 106 are then coupled to the
handle 122 by mechanical couplers 206. The mechanical
couplers 206 can be a variety of mechanical coupler types,
such as threaded couplers or couplers employing snapping
edges. Thus, the combination of use of the guide members 104
and the couplers 206 allow for a good mechanical connection
during use.
Also while in use, spiked plungers 118 can be employed to
allow gas transmission from the reaction chambers 106 to the
gas transmission channel 202 of the handle 122. The spiked
14
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
plungers 118 can each be coupled to the handle 122 within the
gas transmission channel 202 of the handle 122 and can each be
inserted into a reaction chamber 106. Each spiked plunger 118
can contact both the filter 110 and the screen 108. The
screens 108 can be located at positions adjacent to the lower
portions 210, which allow gas to pass and provide mechanical
support for the filters 110. Because of the mechanical
constraints of the mechanical couplers 206 and the guide
members 104, the spiked plungers 118 can each maintain
mechanical contact between the filter 110 and the screen 108.
Gas produced within the lower portions of the reaction chamber
106 can then pass around the tip of the plunger 118, through
the screens 108, the filters 110, and into transmission
openings 224 in spiked plungers 118.
Once closed, each of the reaction chambers 106 and the
lids 112, along with the reaction chambers' contents such as
the gas/oxygen generating material, catalyst, water, screen
and filter forms a self-contained cartridge 109 that can be
disposable. Each self-contained cartridge 109 is therefore
easily replaceable if a user requires additional oxygen or gas
(as the case may be) upon completion of a use. For example,
the gas activation, production, dispensing and control
assembly 300 can be designed to produce 15 minutes of oxygen
for emergency or short-duration purposes. If the user
requires additional oxygen at the end of that 15-minute
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
period, he/she can simply replace one or both the cartridges
109 to have an additional 15 minutes of oxygen availability.
Each used cartridge 109 is simply discarded or recycled (if
applicable) after use, allowing for simplicity and ease of
use. Self-contained cartridges can be attached to each other
to form one removable, self-contained cartridge. The lids 112
can each have a cap to close the respective openings 116,
after the completion of the reaction. Closing the openings
116 facilitates the prevention of any leakage of the reaction
residue and thereby facilitates convenient disposal of the
cartridges.
In reference to the self-contained cartridges 109 there
are various configurations possible in regards to the relative
locations of the gas/oxygen releasing agent, the catalyst and
the water, comprising the ingredients used to make the
reaction in the current invention work. The gas/oxygen
releasing agent, the catalyst and the water remain separated
until a reaction is required. The gas/oxygen releasing agent
and the catalyst can remain inert and can have an indefinite
shelf life if they are kept dry and moisture free. One
configuration example is to have the gas/oxygen releasing
agent located at the base of the cartridge (in reaction
chambers 106), the catalyst located above the gas/oxygen
releasing agent, and the water located above the catalyst,
such as for example in the plenums 111 of the lids 112. Upon
16
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
activation, the water is released and can flow in toward the
lower portion of the reaction chamber 106, where the
gas/oxygen producing agent (not shown) is disposed, carrying
the catalyst along with it through a flushing action, to mix
with the gas/oxygen releasing agent at the base of the
cartridge. We refer to this cartridge configuration as a
water releasing cartridge. In this invention we will discuss
different designs for water releasing cartridges. A different
cartridge configuration, however, is one where the gas/oxygen
releasing agent is located above the water and the catalyst.
In this cartridge configuration, the gas/oxygen releasing
agent and/or the catalyst is/are released to mix with the
water in order to activate the reaction. We refer to this
cartridge configuration as a chemical releasing cartridge.
In either cartridge configuration, once a chemical
reaction is initiated, the resultant gas can carry small
airborne droplets of the gas production solution, or can carry
small particles from the reactants. These airborne particles
can be undesirable to the equipment attached to the gas
generator or to the lungs of an individual. Therefore, there
is a need to filter these undesirable particles. There are
several methods that can be used to filter such undesirable
particles. Methods that can be used include selecting
appropriate materials to capture the undesirable particles,
and to select an appropriate configuration by locating the
17
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
selected materials in an appropriate location, relative to
other components in the invention. Therefore, material
selection and placement can be important factors. However,
the filter material employed depends on the gas produced, the
composition of the solution, and the usage of the gas. In
reference to FIGURE 1, the filters 110 can be sponge-like
materials to capture the undesirable particles, while allowing
the gas to flow through at desirable flow rates. Other
effective filter materials can be polytetrafluoroethylene or
can be Nylon , which is available from DuPont. In addition to
absorbing or filtering out undesirable particles, filters can
also be useful in extracting some heat out of the gas being
produced, either in their untreated form, or by being treated
with various substances.
FIGURE 4 depicts an example of a screen that can be used.
The screens 108 can serve to support the filters 110, while
allowing the water to rapidly and evenly disperse into the
reaction chambers 106, in order to activate the chemical
reaction that produces the oxygen or gas, as the case may be.
In order to allow fluid transfer through the screen 108,
several opening can be provided. The edges of the screen 108
would rest against the inner walls of a reaction chamber 106
or on a surface within the reaction chamber 106. Fluids would
then be allowed to pass through the openings 404, 402, and
18
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
406. Additionally, when engaged, the spiked plungers 118
would at least partially reside within the opening 402.
Referring to FIGURES 5A, 5B, and 5C of the drawings the
reference numeral 500 generally designates a foam breaker.
FIGURE 5A depicts a cross sectional view of the foam breaker
500, where the opening 502 would allow the spiked plunger 118
to reside when engaged. FIGURE 5B depicts a side view of the
foam breaker 500, and FIGURE 5C depicts a side view of the
foam breaker 500 when compressed.
Chemical reactions can produce foam, and a foam breaker
500 can counteract this effect. For example, a steel mesh with
an appropriate mesh size can be used. Another material that
can be used as a foam breaker is a commonly used pot scourer
or scrub sponge material, or durable foam material. The foam
breaker can be optionally placed within the same fluid
transmission path in which both the screens 108 and the
filters 110 reside. The screens 108 can also act as foam
breakers, and the filters 110 can also act as foam breakers.
The screens 108 and filters 110, acting together can also act
as foam breakers.
Another method is to apply a defoaming agent or
surfactant to the walls and/or the screen and/or the lid and
filter. Defoaming agents that can be used include silicone
based, polymer based or mineral oil based agents, as well as
other surfactants. Regardless of where the foam breaker or
19
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
defoaming agent is placed in the device, the filter should
follow the foam breaker or defoaming agent (as considered in
the direction of the gas flow).
Referring to FIGURE 6 of the drawings, the reference
numeral 122 generally designates the handle. The handle 122
effectively operates as a manifold. Especially in situations
where multiple reaction chambers are used, it is desirable to
have a manifold or similar method of combining the gas flow
from each individual reaction chamber 106. The manifold gas
transmission channel 202 performs the function of combining
gases, and the gas flows from each reaction chamber 106 into
the ports 602. The gases are then combined in the manifold
gas transmission channel 202.
Upon activation, however, the spiked plungers 118 should
provide a continuous gas transmission to the manifold gas
transmission channel 202. The mechanical coupler 206 can
secure lids 112 in such a manner as to seal off the opening
116 of the lids 112 and maintain the connection between the
spiked plunger 118 and the handle 122. Specifically, the
mechanical coupler 206 can be a simple coupler 206 to which
the nozzle 116 of the self-contained cartridge 109 is
inserted, as depicted in FIGURE 3
In another embodiment, the couple 206 or can comprise a
cooperatively designed male connector adapted to fit over the
nozzle 116, as depicted in FIGURE 8, and a female connector
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
adapted to fit into the male connection, as depicted in
FIGURES 7, 9, 10B and 10C..
With initial reference to FIGURE 7, e the reference
numeral 700 refers to the female connector. The female
connector 700 is typically attached to the spiked plunger 118,
where the spiked plunger 118 is inserted into the opening 704
of the female connector 700. Additionally, as depicted in
FIGURES 14 and 15, the female connector couples to the ports
602 of the handle 122. When engaged, the female connector 700
snaps into place. The female connector 700 comprises an arm
702 that possesses an engagement edge that allows for coupling
to a male connector. Additionally, the female connector 700
can be made of various materials, including, without
limitation polypropylene, polyethylene, polycarbonate, HDPE,
ABS, Acetal, or Polysulfone.
Referring to FIGURE 8 of the drawings, the reference
numeral depicts a male connector. FIGURE 8 is a side view of
the male connector 800, with the 0-ring seal shown in cross-
section for clarity.
The male connector 800 is a cylindrical tube that is able
to engage the female connector 700. The male connector can
comprise an 0-ring 802, an upper edge 804, and a lower edge
806. The 0-ring 802 is responsible for providing a gas seal
between the male connector 800 to the female connector 700 the
male connector 800 is inserted into the female connector
21
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
during use. The 0-ring can be made of various materials,
including, without limitation, silicone or platinum-cured
silicone. Platinum-cured silicone can allow for repeated
usage of more than one thousand times. The lower edge 806 can
engage the edge of the arm 702 by a clicking action. To more
conveniently allow for the clicking action to take place, a
slanted engaging face 808 is employed. Additionally, the
upper edge 804 prevents excessive play by providing a stop for
the edge of the arm 702. The male connector can also be made
of various materials, including, without limitation
polypropylene, polyethylene, polycarbonate, HDPE, ABS, acetal,
or polysulfone.
The male connector 800 can then be secured to the lid 112
by using threads. Typically, the lid 112 is coupled to the
male connector through the opening 810. Therefore, female
threads would be contained on the inner walls of the male
connector 800 while the male threads would be contained on the
lid 112.
Once the reaction is completed, the female connector 700
and the male connector 800 can be easily and quickly
disengaged. The quick release mechanism 720 can be coupled to
the arm 702 of the female coupler 700. By pressing the quick
release mechanism 720 in the direction toward the plane
created by the azimuthal axes of the spiked plungers 118, the
male connector and the female connector can be disengaged.
22
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
Additionally, the quick release mechanism 720 can be
configured to disengage the female connectors 700 from the
male connectors 800 by simply gripping the quick release lever
128 in a direction toward the handle 122.
For applications such as emergency applications it is
desirable to have an efficient and easy activation method,
which is simultaneously manufacturable and economical. For
such emergency applications, the activation method should be
such as to commence the chemical reaction instantaneously or
near instantaneously with typically one easy step. For
example, activation can be achieved by a single push-down
action that applies pressure to the handle 122. A system can
also be electronic or a sensor, such as for example a system
used to detect decompression in aircraft, thereby triggering
the deployment of emergency oxygen in the aircraft cabin.
In one embodiment, during activation of the chemical
reaction, the spiked plungers 118' are each inserted into lids
112. The spiked plunger 118 and 118' are typically hollow
cylindrically-shaped members that have a tip that is suitable
for and utilized to puncture a material. Referring to FIGURE 9
of the drawings, the reference numeral 900 generally
designates one embodiment of the connectable spiked plunger.
Specifically, the connectable spiked plunger 900
comprises a female connector 700 and a spiked plunger 118.
The spiked plunger 118 can comprise a cylindrically-shaped
23
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
shaft 906 with a spiked end 904. Within the spiked plunger
118 is a gas transmission channel 902 along the azimuthal axis
of the spiked plunger 118 that allows gas to travel through
the plunger 900. Additionally, transmission openings 224 are
employed to allow the gas transmission channel 902 to be in
fluid contact with gas outside of the spiked plunger 118.
In particular the plunger 900 is designed to puncture a
material container or containment bag to initiate a chemical
reaction. For example, the spiked plunger 118 can puncture a
container or bag that contains water, or the spiked plunger
118 can be used to puncture a membrane or other material,
causing the release of water or chemicals, as the case may be.
The spiked plungers 118 can be made of durable thermoplastic
with high tensile strength, high resistance to chemical
reactions and high resistance to heat. For example, the
spiked plungers 118 can be made of polycarbonate.
In another embodiment, an extended spiked plunger can be
employed. Referring to FIGURES 10A, 10B, and 10C, the
reference numeral 1000 generally designates an extended spiked
plunger 118'.
Specifically, the plunger 118' can comprise a female
connector 700 and a spiked plunger 118'. However, the spiked
plunger 1181 is different in that it is extended. The spiked
plunger 118 comprises a torso 1002 and an extension shaft 1004
with a sharp tip 1006. The torso 1002 can be cylindrically
24
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
shaped and employ a gas transmission channel 902 along the
azimuthal axis of the torso 1002 that allows gas to travel
through the plunger 118'. Additionally, transmission openings
224 can be employed to allow the gas transmission channel 902
to be in fluid contact with gas outside of the spiked plunger
1181.
Attached at the end of the torso 1002 is the extension
shaft 1004. The extension shaft 1004 can be cylindrically-
shaped with one end inserted into the female receptive
aperture 1008 at the end of the torso 1002. The sharp tip
1006 can then be attached to the other end of the extension
shaft 1004.
In particular, the plunger 1000 is designed to puncture a
material containment container 'or bag to initiate a chemical
reaction. For example, the spiked plunger 118 can puncture a
container or bag that contains water, or the spiked plunger
118 can be used to puncture a membrane or other material,
causing the release of water or chemicals, as the case may be.
The spiked plungers 118 can be made of durable thermoplastic
with high tensile strength, high resistance to chemical
reactions and high resistance to heat. For example, the
spiked plungers 118 can be made of polycarbonate.
In yet another embodiment, an initiator can be employed
as a push-button, lever or pin. An initiation system can also
be electronic or a sensor, such as for example a system used
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
to detect decompression in aircraft, thereby triggering the
deployment of emergency oxygen in the aircraft cabin.
Referring to FIGURE 11 of the drawings, the reference numeral
1100 depicts a spring loaded spiked plunger 1118.
The spring loaded spiked plunger 1118 then can utilize
potential energy stored in a spring to extend its sharp tip
1110 into the containers of water and/or chemicals to begin
the chemical reaction that produces the gas. The spring 1106
can be maintained within the spring housing 1114 and held in
place by a retainer 1104. The process of initiating the
chemical reaction would involve the utilization of an actuator
1102, which is shown as a push-button actuator. The actuator
1102 causes the retainer 1106 a lever arm 1107 to pivot about
pivot 1109, pulling out pin 1104 to release the spring 1106.
The spring 1106 then exerts a force on the spiked plunger
1118.
The spiked plunger 1118 can comprise a cylindrically
shaped shaft with a spiked end 1110. Within the spiked
plunger 1118 is a gas transmission channel 902 along the
azimuthal axis of the spiked plunger 1118 that allows gas to
travel through the plunger 1118. Additionally, transmission
openings 224 can be employed to allow the gas transmission
channel 902 to be in fluid contact with gas outside of the
spiked plunger 118.
26
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
In particular, the plunger 1118 is designed to puncture a
material containment container or bag to initiate a chemical
reaction. For example, the spiked plunger 1118 can puncture a
container or bag that contains water, or the spiked plunger
1118 can be used to puncture a membrane or other material,
causing the release of water or chemicals, as the case may be.
The spiked plungers 1118 can be made of durable thermoplastic
with high tensile strength, high resistance to chemical
reactions and high resistance to heat. For example, the spiked
plungers 1118 can be made of polycarbonate.
There are several other types of systems that can be
employed to initiate a gas generating chemical reaction. An
actuator can utilize the pressure associated with a chemical
release cartridge. A pressure supply can also be achieved by
supplying air pressure to the activation system. Another type
can be a mechanical or electro-mechanical source, such as can
be provided by a mechanical or electro-mechanical pump or
motor. Yet another type can be a pneumatic source, such as
for example a pneumatic pump or motor, or a hydraulic source.
Depending on the type of gas producing reaction,
pressures in the reaction chamber 106 can be high and
dangerous. Referring to FIGURE 12 of the drawings the
reference numeral 1200 generally designates a cartridge with a
relief system. The cartridge 1200 comprises a reaction
27
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
chamber 106, a screen 108, a containment bag 1202, a filter
110, and a lid 112.
When in storage or not in use, the reaction chamber 106
contains "dry" reactants. The "dry" reactants typically
include an oxygen rich powder reactant, such as sodium
carbonate or sodium percarbonate, as the gas/oxygen generating
agent. However, the dry reactants can be liquid reactants
that require an additional solvent, such as water, or other
"wet" chemical to initiate a gas producing reaction. These
"dry" reactants can also contain "dry" catalysts that can
assist in reducing heat or increase the reaction rate, such as
manganese dioxide. There are also be a number of other
catalysts that can be employed for a variety of other
purposes. In addition, it should be noted that the water can
include an additive to depress the freezing point of the
water, but need not do so. Inserted into the reaction chamber
106 is the screen 108. The screen 108 is mechanically
supported in a position adjacent to the cavity containing the
"dry" reactants. The screen 506 can be mechanically supported
in a number of ways, such as by use of threading, snapping
edges, and/or taper of the inner walls of the reaction chamber
106.
The screen 108 can provide mechanical support for the
remaining components contained within the cartridge 1200.
28
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
A containment bag 1202 is positioned adjacent to the
screen 108, so that, when pierced, the contents of the bag
1202 can be transmitted through the screen to the "dry"
chemicals to begin the reaction. The filter 110 is also
supported by the screen 108, so that when gas is produced and
transmitted through the screen 506, the gas can be filtered.
A variety of filter types can be employed that can be
comprised of a variety of materials including, but not limited
to, polytetrafluoroethylene.
The final component of the cartridge 1200 is the lid 112.
The lid 512 can be coupled to the reaction chamber 106. There
are a number of ways to couple the lid 112 to the reaction
chamber 106, such as threading and an adhesive.
An additional feature of the cartridge 1200, however, is
the presence of a pressure relief valve 1214. In cases where
high pressure, volatile gases are produced, such as oxygen or
hydrogen, high pressures can be dangerous. Even in situations
where gases do not present a fire hazard, such as nitrogen,
high pressures can be an undesirable because the high pressure
gas can exploit defects or fractures in the cartridge 1200 to
cause the cartridge to rupture. To relieve pressure within
the cartridge 1200, a relief valve 1214 can be employed to
relieve pressure within the chamber at a calibrated level.
For example, pressure relief can occur at 300 psig. There are
a wide variety of pressure relief systems available, such as
29
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
pop-off valves and rupture discs that can be adequately
calibrated to relieve pressure at a desired level.
There are also alternative arrangements for containing
the materials employed to sustain the chemical reaction.
Referring to FIGURES 13A and 13B of the drawings, the
reference numerals 1300 and 1350 depict an activation system
primed for activation and the system in use, respectively.
The system 1300 comprises a cartridge 1301, a spiked
plunger 118, and a female connector 700. The cartridge 1301
then comprises a filter 110, water-filled bag 1304, a screen
108, a catalyst filled bag 1306, and a gas releasing agent
1308 contained within a reaction chamber 106 and a lid 112.
The bag housing the catalyst 1306 can be made of any
number of materials, but can also be made of a water-soluble
material. The bag 1304 housing the water can be made of any
number of air impermeable and water/moisture impermeable
materials, but can also be made of a laminate material
consisting of aluminum, polypropylene and woven mesh.
The cartridge 1301 typically also has an air-impermeable
and water-impermeable seal 1302. The air-impermeable and
water-impermeable seal 1302 can be made of various materi'als,
including, without limitation materials such as Mylar,
polytetrafluoroethylene or Nylon . The purpose of the seal
1302 is to maintain an hermetic seal so that the cartridge can
have an extended or indefinite shelf life.
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
Upon activation, the spike tip 904 punctures or ruptures
the seal 1302, and the spiked plunger 118 enters the filter
aperture 1320. At that point, the spike tip 904 punctures or
ruptures the water bag 1304, causing the water to flow into
the reaction chamber 106. The spiked plunger 1130 completes
the piercing of the water bag 1172 and proceeds through the
screen aperture 402 such that the spike tip 1142 protrudes
just slightly beyond the screen 108. Once the spiked plunger
1130 has penetrated the water bag 1172 and traversed all the
way through, spiked plunger and connector assembly 1140 is
secured to the cartridge and sealed by the connector 1180.
Once released, the water creates an aqueous environment
for the reaction to take place. The water dissolves the bag
containing the catalyst 1306. The gas generated as a result
of the reaction can then be released from the cartridge 1301
through the spiked plunger 118.
Another embodiment of the cartridge 1301 includes a
hanging catalyst bag. Referring to FIGURE 14 of the drawings,
the reference 1400 generally designates a release system with
a hanging catalyst. The system 1400 comprises cartridges
1401, a handle 122, and cutting members such as spiked
plungers 118. Within the cartridges 1401, there is an upper
assembly 1402, a hanging catalyst 1404, and a gas generating
chemical 1308.
31
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
Upon activation, the spiked plunger 118 engages the upper
assembly 1402. Water then flows into the reaction chamber
106. The water creates an aqueous environment for the
reaction to take place, while dissolving or permeating the bag
containing the catalyst 1404. The gas generated as a result
of the reaction can then be released from the cartridge 1401
through the spiked plunger 118 to the gas transmission channel
202 of the handle 122. The bag housing the catalyst 1404 is
suspended slightly above the gas generating material 1308,
which facilitates faster dissolution of the bag if the bag is
a water-soluble bag, or faster permeation through the bag if
the bag is permeable.
Referring to FIGURE 15, the reference number 1500 depicts
another system primed for activation. The system 1500 is
different in that the catalyst is contained in a catalyst
dispersal housing 1502, located just below the water
containment housing 1504. The water containment housing 1504
can contain a bag with water, or can have water contained
inside of it.
The system 1500 can comprise self-contained water
releasing cartridge 1501, a spiked plunger 118, and a
connector assembly 700 coupled to the handle 122. The
cartridge 1501 comprises a gas or oxygen releasing agent 1308,
the catalyst dispersal housing 1502, the screen 108, and the
water containment housing 1504. If the water is contained in a
32
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
bag, the bag can be made of any number of impermeable
materials, but can also be made of a laminate material
consisting of aluminum, polypropylene and woven mesh.
Upon activation, the spiked plunger 118 engages the water
containment housing 1504 and the catalyst dispersal housing
1502. Water then flows into the reaction chamber 106. The
water creates an aqueous environment for the reaction to take
place. The gas generated as a result of the reaction can then
be released from the cartridge 1301 through the spiked plunger
118 to the gas transmission channel 202 of the handle 122.
A desirable feature of the system 1500 is the
construction of the water containment housing 1504 and the
catalyst dispersal housing 1502. Referring to FIGURE 16A of
the drawings, the reference numerals 1504 and 1502 generally
designate the water containment housing and the catalyst
dispersal housing, respectively. Specifically, water
containment housing 1504 and catalyst dispersal housing 1502
assembly can be made as one piece, and can be made of any
material. Without limitation, the water containment housing
and catalyst dispersal housing assembly can be made of plastic
or thermoplastic, including polypropylene, polyethylene,
polycarbonate, HDPE, ABS, acetal, polysulfone, or poly vinyl
chloride (PVC) .
The water containment housing 1504 and the catalyst
dispersal housing are designed such that it can be a self-
33
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
contained unit. The water containment housing 1504 has an
upper aperture 1602 covered by an upper sealing membrane 1604
and has a lower aperture 1606 covered by a lower sealing
membrane 1608. A spiked plunger can be inserted through the
seals 1604 and 1608 and the apertures 1602 and 1606 upon
activation. The catalyst dispersal housing 1502 also has an
aperture 1612 covered by a catalyst housing seal 1610, which
allows the spiked plunger 118 to finally exit the catalyst
dispersal housing 1502 during the activation process.
Prior to activation, the water is sealed into the water
containment housing 1504 by upper seal 1604 and lower seal
1608. While the upper seal 1604 and the lower seal 1608 are
shown as having been placed on top of each respective adhesion
surface, each can be also be placed on the bottom side of each
respective adhesion surface. Catalyst housing seal 1610 can
also be placed on either side of the adhesion surface. Each of
the seals 1604, 1608, and 1610 can be made of air-impermeable
and water-impermeable materials, including, without limitation
materials such as polytetrafluoroethylene, Mylar , or Nylon
(both available from DuPont).
During activation, the water is released from the water
containment housing 1504 and proceeds in a direction towards
the reaction chamber 106, flushing the catalyst with it.
Referring to FIGURE 16B, the catalyst dispersal housing 1502
can have an angled or beveled surface 1614, which facilitates
34
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
faster and more efficient dispersal of the catalyst and/or
water. Additionally, the water containment housing 1504 can
also have contain an angled or beveled surface in order to
facilitate faster and more efficient dispersal of the water
upon activation. The angled or beveled surface 1614 can
facilitate better flushing of the catalyst, and/or facilitate
faster and more efficient dispersal of the catalyst.
The self-contained housings can also include an in-place
spike. Referring to FIGURES 17A and 17B of the drawings, the
reference numeral 1700 generally designates an alternative
design of the self-contained housings. Specifically, a
plunger 1702 with an upper seal 1704, a lower seal 1706, and
catalyst housing seal 1708 is employed. The seals 1704, 1706,
and 1708 are attached to the plunger 1702 such that the seals
1704, 1706, and 708 do not break away from or separate from
the plunger 1702 during normal use. The seals 1704, 1706, and
1708 are attached to the water containment housing 1504 and
catalyst dispersal housing 1502 such that the seals 1704,
1706, and 1708 are breakable, detachable, or removable upon
activation.
FIGURE 17A depicts the self-contained housings 1700 in a
primed position. Upon activation, the downward force
transferred by the pressure source rips, tears, dislodges or
otherwise detaches the seals 1704, 1706, and 1708, causing the
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
contents to flow into the reaction chamber 106. Stoppers 1710
allow the plunger 1702 to travel only a specified distance.
An alternative activation method can involve a chemical
release cartridge bag configuration. Referring to FIGURES 18A
and 18B, the reference numeral 1800 generally designates a
pouch that employs a method for storing the gas/oxygen
releasing agent and the catalyst.
Accordingly, there is a planar sealed pouch 1800 formed
of air- and water-impermeable sheet material 1802 which is
resistant to the basic chemicals commonly used. The sheet
material 1802 supports the gas/oxygen releasing agent 1804 and
has a web seam 1806 whose apex points upwardly towards the
gas/oxygen releasing agent 1804. The sheet material 1802 has
a base seam 1808 parallel to and below the web seam 1806. The
base seam 1808 then seals the pouch 1800. The region between
the web seam 1806 and the base seam 1810 forms a compartment
1810 into which catalyst 1809 is disposed.
The entire contents of the pouch 1800 are designed to be
released in a rapid fashion into water contained in an outer
container in which the pouch 1800 is contained, such as
container 106. Therefore, it is thought that the web material
1810 is to be a non-permeable laminar sheet so that none of
the chemical material escapes into the volume below the web
material. Additionally, the web seam 1806 is formed with a
36
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
pressure sensitive seal which is broken when pressure is
applied.
The pouch 1800 is constructed using a continuous sheet of
water- and air-impermeable sheet material. 1802 folded such
that the fold, situated in the middle of the sheet, fits over
and advantageously accommodates the nozzle element 1812. The
water- and air-impermeable sheet material 1802 is welded
together at side seams 1816 and bottom seam 1808, and the
sheet material 1802 can be a multi-layer laminate such as
(from inside to outside) polyester, aluminum foil, polyester
and polypropylene. It should be noted that side seams 1816
can also be frangible during use, like seam 1808, but need not
be.
During use, water or air is introd'uced into the pouch
cartridge by means of a hollow injector inserted into the
delivery channel 1814 through membrane 1805. The pressure
causes the web material to evert inside-out to vent by
rupturing the pressure-sensitive seal at 1806. Thus, the
gas/oxygen releasing agent 1804 is released through an opening
made in the web seam 1806. The catalyst is simultaneously
released through the web seam 1806. Because of the geometrical
shape of area 1810, the rupturing of seal 1806 occurs in a
predictable and reproducible manner. Once the gas has been
produced, humidification and/or cooling/warming of the gas may
be required.
37
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
Referring to FIGURE 19 of the drawings, the reference
numeral 1900 generally designates a bubbler. The bubbler 1900
comprises a liquid holding tank 1902, an intake tube 1904, an
exhaust tube 1906, and a liquid 1908.
During the operation, the gas is bubbled through the
liquid. Because gas input pressure into the bubbler 1900 is
higher than atmospheric pressure, the gas can be forced
through the intake tube 1904. Part of the intake tube 1904 is
submerged within the fluid 1908, the exhaust gas bubbles
through the liquid 1908. The effect of traveling through the
liquid 1908 is that the gas will transfer heat to the liquid
1908 (cooling) or receive heat from the liquid 1908 (warming).
Once the gas has bubbled to the surface, the gas can then
exit through the exhaust tube 1906. When the gas exists, it
is likely that small droplets of the liquid can be carried
with the gas. Additionally, vapors of the liquid can also be
carried. In the case of oxygen production, the oxygen can be
cooled or warmed through water. Once bubbled, the oxygen
would carry water vapor, thus, producing humidified oxygen.
Another design to cool or warm a gas is by use of a
radiator. Referring to FIGURE 20 of the drawings, the
reference numeral 2000 generally designates a radiator. The
radiator comprises fins 2004 and a radiator tube 2002.
As gas is output, a heat sink is employed to transfer
heat. The gas is input into the radiator tube 2002 to snake
38
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
through the radiator 2000. As the gas progresses through the
radiator 2000, heat is transferred to the fins 2004. The fins
2004 then transfer heat to a larger heat sink. The larger
heat sink can be a variety of heat sinks which includes, but
is not limited to, the atmosphere.
One of the features of the above referenced devices is
the ability to utilize multiple reactions chambers. Having
multiple reaction chambers creates the ability to increase the
performance of the gas dispenser, without the associated
increase in pressure and temperature if only one reaction
chamber is used. For example, a reaction that produces 90
liters of oxygen in 15 minutes can experience an exponential
increase in pressure, especially after a certain internal (to
the reaction) temperature is reached. By splitting this same
reaction into two reactions, completely isolated from each
other in separate chambers (say, of each producing 45 liters
over 15 minutes), a stable delivery of gas is produced without
the exponential increase in pressure and/or temperature that
can result from the same 90 liter reaction over 15 minutes
contained in one chamber with one reaction.
Similarly, a much higher degree of control is possible
over the increase in temperature of the gas by splitting the
reaction into multiple reactions. Normally, reactions such as
the exothermic reactions that generate oxygen, create heat and
a concomitant increase in pressure in a static volume (i.e.
39
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
there is a direct correlation between temperature and
pressure). A further benefit of using multiple reaction
chambers is that a higher reaction onset can be achieved.
Specifically, any multiple of reaction chambers can be
combined to create any desired output of volume, flow rate
and/or delivery time. For example, 3 reaction chambers, each
producing 30 liters of oxygen can be combined to produce the
same 90 liter reaction, but with lowered pressure inside each
reaction chamber and reduced temperature increase of the
generated gas, relative to using the same quantity of
reactants and catalyst in only one or two chambers, for
example.
Variations in both flow rate and yield can also be varied
or dictated by the compositions of the contents in the
reaction chambers 106. For example, by varying the amount of
a limiting reactant in each chamber and/or by varying the
amount and/or composition of the catalyst contained in each
cartridge, different flow rates and gas yields can be
achieved. For example, by varying the amount of the sodium
percarbonate in an oxygen generation reaction in each of the
chambers, a yield of 90 liters with a flow rate of 6 liters
per minute for 15 minutes or a yield of 30 liters and a flow
rate of 3 liters per minute for 10 minutes can be achieved.
The flow rates and yields can be varied depending on the
desired usage and can be for different situations, such as
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
emergency oxygen for aircraft or mines. While there are many
possible or acceptable flow rate profiles applicable to the
aviation industry, one example may be to have a reaction that
produces approximately 4 liters per minute for 4 minutes and
then drops to 1 liter per minute for 8 minutes. Using 2
reaction chambers can achieve this general performance
profile.
Additionally, there are several other configurations that
can be employed to store the chemicals. Referring to FIGURE
21 of the drawings, the reference numeral 2100 generally
designates a cartridge 2100. The cartridge 2100 comprises a
lid 1126 and a reaction chamber 106.
When combined, the reaction chamber 106 and the lid 112
contain a filter 110, a foam breaker 500, a screen 108, water
2104, a gas producing agent 2102, and a catalyst 2106. The
filter 110 and the foam breaker 500 are layered on top of the
screen 108, and the chemicals 2106, 2102, and 2104 are
contained within the lower portion of the reaction chamber
106. The water 2104 rests at the bottom of the reaction
chamber 106, being held in place by frangible seal 2108. The
catalyst 2106 and the gas producing agent 2102 are each
contained on a side of the reaction chamber, held in place by
a frangible seal 108.
Upon activation, the frangible seals 2108 are broken.
The chemicals 2102, 2104, and 2106 then mix to create a gas
41
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
generating reaction. The gas produced traverses the screen
108, the foam breaker 500, and the filter 110 to exit the
cartridge 2100.
Referring to FIGURE 22 of the drawings, the reference
numeral 2200 generally designates a cartridge. The cartridge
2200 comprises a lid 112 and a reaction chamber 106.
When combined, the reaction chamber 106 and the lid 112
contain a filter 110, a foam breaker 500, a screen 108, water
2204, a gas producing agent 2202, and a catalyst 2206. The
filter 110 and the foam breaker 500 are layered on top the
screen 108, and the chemicals 2206, 2202, and 2204 are
contained within the lower portion of the reaction chamber
106. The water 2204, the catalyst 2206, and the gas producing
agent 2202 each rest at the bottom of the reaction chamber
106. Each of the chemicals 2202, 2204, and 2206 are separated
from one another and held in place by a frangible seals 2208.
Upon activation, the frangible seals 2208 are broken.
The chemicals 2202, 2204, and 2206 then mix to create a gas
generating reaction. The gas produced traverses the screen
108, the foam breaker 500, and the filter 110 to exit the
cartridge 2200.
Referring to FIGURES 23A and 23B of the drawings, the
reference numeral 2300 generally designates a self-contained
activation system. The system 2300 comprises a container 2302
and an activation handle 2304. The sealed unit 2302 is
42
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
particularly adapted for containing one or more pouches 26000
or 2600', depicted in FIGURES 26A and 26B. However, sealed
unit 2302 can also contain a multitude of devices, such as the
configurations of FIGURES 1-3, 12-18, and 21-22, capable of
releasing a gas. To initiate the release of a gas, the
activation handle 2304 is displaced downwardly into an
activation position to apply mechanical pressure to any of the
multitude of devices to break any seals and initiate the
chemical reaction(s). Additionally, the activation position
of the handle 2304 can be reached by being displaced into
either an upward or a downward position relative to the
container 2302.
Referring to FIGURE 24A of the drawing, the reference
numeral 2400 generally designates a diagram contrasting two
gas producing reactions. The first reaction (REACTION 1) is
set up to produce a short reaction that starts high but is
only maintained for a short period. The second reaction
(REACTION 2) is set up to start slow but to be maintained for
a longer period.
Considered individually, neither REACTION 1 in the first
reaction chamber nor REACTION 2 in the second reaction chamber
produce the desired flow rate profile. However, referring to
FIGURE 23B of the drawings, the reference numeral 2450
generally the combined output of REACTION 1 and REACTION 2.
The combined output 2450 shows the sum of the combined
43
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
reactions 1 and 2, and illustrates how the desired profile is
achieved using 2 reaction chambers instead of one.
Similarly, other profiles can be achieved by two reaction
chambers or multiple reaction chambers. For mining
applications, for example, one possible flow rate profile is
to simply maintain a reaction at an average of 2 liters per
minute for 60 minutes.
Another advantage of multiple reaction chambers is that
the reactions can be staged to commence at different times in
order to achieve a desired output. Referring to FIGURE 25A of
the drawings, the reference numeral 2500 generally designates
a diagram showing two contrasted reactions. The diagram 2500
shows two identical reactions, REACTION 3 and REACTION 4, each
with a reaction onset of 1.75 liters per minute. Each of
REACTION 3 and REACTION 4 can take place in respective
reaction chambers. In this case, the reactions are staged
such that Reaction 3 commences at time=0 and runs for 12
minutes, while Reaction 4 commences at time=10 minutes.
Referring to FIGURE 25B of the drawings, the reference
numeral 2550 shows a diagram depicting the combined outputs of
REACTIONS 3 and 4. Considered individually, neither REACTION
3 in the first reaction chamber nor REACTION 3 in the second
reaction chamber may produce the desired flow rate profile.
However, the output of the combined reactions, shown in the
44
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
diagram 2550 shows a 20-minute production with flow rates in a
relatively narrow range, as the trend-line indicates.
By using multiple reaction chambers and/or staging
reactions to commence at different times, a wide variety of
flow rates, volume, time periods and performance profiles can
be achieved, which allows for superior performance
flexibility. This makes it possible for the current invention
to cater effectively to a very broad range of applications,
such as mining, aviation, emergency medical services, the
military, emergency home use or any number of other
applications on a worldwide basis, and to customize the flow
rate profile that is optimum for the particular application.
FIGURE 26A depicts an embodiment of a planar sealed pouch
that employs a method for storing the gas/oxygen releasing
agent, the catalyst and the water all in one pouch. Planar
sealed pouch 2600 is formed of a pair of sheets 2602 of air-
and water-impermeable sheet material which is resistant to the
basic chemicals commonly used (only the top sheet 2602 being
visible in FIGURE 26A). The sheet material 2602 supports the
catalyst in compartment 2604, the gas/oxygen releasing agent
in compartment 2606 and the water in compartment 2608. The
sheet material must be resistant to the chemicals of the
catalyst, gas/oxygen releasing agent and the water. In one
embodiment, the sheet material is a laminate material that can
be any combination of aluminum, polypropylene, polyethylene
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
terephthalate, polyethylene, high density polyethylene, and
any number of materials. The laminate material can also
include a layer of insulating material. The pouch 2600 has a
peripheral border 2611 which is sealed by convenient means,
such as adhesive, ultrasonic welding, or heat sealing and is
able to retain the pressures encountered without bursting.
Each of the compartments 2604, 2606 and 2608 also have
internal sealed borders 2612 to retain their respective
chemicals so that they stay initially separated. Unlike
peripheral border 2611, sealed borders 2612 are sealed with a
pressure-frangible adhesive to create "peel areas" between the
top and bottom sheet material 2610. In this embodiment, the
compartments 2604, 2606 and 2608 do not take up all of the
area of the sheet material 2602, thus also defining an
initially empty compartment 2607. For reasons to be
explained, empty compartment 2607 may also be initially filled
with air at ambient pressure.
The pouch 2600 accommodates nozzle element 2614, which
can be made of suitable plastic such as polypropylene, to
permit the release of the oxygen or other gas produced.
Because the gas produced may include entrained droplets of
water or particulates from the catalyst and gas/oxygen
producing agent, the pouch also includes self-contained
permeable membrane/screen 2616 and a foam breaker 2618 that is
retained by the membrane/screen 2616. When the gas/oxygen is
46
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
produced, it will pass through the membrane/screen 2616 and
the foam breaker 2618, where is effectively filtered, removing
any entrained water droplets, bubbles or particulates before
being exhausted from nozzle 2614 and directed through an
appropriate conduit (not shown) to the user.
To use pouch 2600, force is applied to the outside of the
pouch 2600, either directly or by means of the mechanism
depicted in FIGURE 23A and 23B. This force causes internal
pressure in the pouch, much like attempting to pop a balloon.
Because the peripheral seal 2611 is pressure-resistant, seal
2611 does not burst. However, this internal pressure tends to
cause sealed borders 2612 to peel apart, allowing the top and
bottom sheets of the sheet material 2602 to separate and
allowing the initially separated catalyst, gas/oxygen
releasing agent and water to combine to create gas. It is
believed that having some degree of air in initially empty
compartment 2607 will tend to facilitate the peeling apart of
these sealed borders 2612 by more evenly distributing the
pressure, but this is not necessary to the invention.
FIGURE 26B depicts another embodiment of a pouch having
compartments for initially separating the catalyst, oxygen
producing agent and water. In FIGURE 26B, pouch 2600' is
similar to pouch 2600, the compartments 2604', 2606' and 2608'
containing, respectively, the catalyst, oxygen producing agent
and water, and the initially empty compartment 2607'
47
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
containing air. In pouch 2600', however, each of the
compartments have different shapes and locations. As in pouch
2600, each of the compartments is separated by pressure-
frangible sealed borders 2612', constructed in the same
manner.
The pouch 2600' accommodates nozzle element 2614, which
can also be made of suitable plastic such as polypropylene, to
permit the release of the oxygen or other gas produced.
Because the gas produced may include entrained droplets of
water or particulates from the catalyst and gas/oxygen
producing agent, the pouch also includes self-contained
permeable membrane/screen 2616 and a foam breaker 2618 that is
retained by the membrane/screen 2616, to filter the gas
generated. Otherwise, the construction and operation of the
pouch 2600' is the same as pouch 2600 and need not be further
described.
It should be noted that, as is the case with the multiple
reaction chambers 106 depicted in FIGURE 1, for example,
multiple ones of pouches 2600 and/or 2600' may be connected to
a common conduit and used together. Each of the pouches 2600
and/or 2600' can contain different compositions or proportions
of the water, catalyst and gas/oxygen producing agent, as
previously described, in order to create various flow profiles
such as are depicted in FIGURES 24B and 25B.
48
CA 02596247 2007-07-27
WO 2006/083663 PCT/US2006/002749
It is understood that the present invention can take many
forms and embodiments. Accordingly, several variations may be
made in the foregoing without departing from the spirit or the
scope of the invention. The capabilities outlined herein
allow for the possibility of a variety of implementations.
This disclosure should not be read as preferring any
particular embodiments, but is instead directed to the
underlying mechanisms on which these embodiments can be built.
Having thus described the present invention by reference to
certain of its preferred embodiments, it is noted that the
embodiments disclosed are illustrative rather than limiting in
nature and that a wide range of variations, modifications,
changes, and substitutions are contemplated in the foregoing
disclosure and, in some instances, some features of the
present invention may be employed without a corresponding use
of the other features. Many such variations and modifications
may be considered desirable by those skilled in the art based
upon a review of the foregoing description of preferred
embodiments. Accordingly, it is appropriate that the appended
claims be construed broadly and in a manner consistent with
the scope of the invention.
49