Note: Descriptions are shown in the official language in which they were submitted.
CA 02596278 2007-08-21
65902-72E
-1-
NEBULIZING CATHETER SYSTEM AND
METHODS OF USE AND MANUFACTURE
This is a divisional of application
number 2,152,002 filed June 16, 1995.
BACKGROUND OF THE INVENTION
The present invention relates to aerosol delivery
of medication to the lungs and more particularly, the
present invention relates to delivery systems for
application of nebulized medication to the lungs with
improved delivery rates, efficiencies, and control.
Many types of medication can be administered to a
patient via the respiratory tract. Medication delivered
through the respiratory tract may be carried with a
patient's inhalation breath as airborne particles (e.g. an
aerosol or nebula) into the lungs where the medication can
cross through the thin membrane of the alveoli and enter the
patient's bloodstream. Delivery of medication via the
respiratory tract may be preferred in many circumstances
because medication delivered this way enters the bloodstream
very rapidly. Delivery of medication to the lungs may also
be preferred when the medication is used in a treatment of a
disease or condition affecting the lungs in order to apply
or target the medication as close as physically possible to
the diseased area.
Although delivery of medication via the
respiratory tract has been used for many years, there are
difficulties associated with prior systems that have
CA 02596278 2007-08-21
-2-
1 limited their use and application. For example,
2 conventional methods have provided for only limited
3 medication delivery rates, efficiency, and control.
4 Conventional methods for aerosol delivery result in a
substantial portion of the medicine failing to be
6 delivered to the lungs, and thereby possibly being
7 wasted, or possibly being delivered to other parts of the
8 body, e.g. the trachea.
9 Aerosols in general are relatively short-lived
and can settle out into larger particles or droplets
11 relatively quickly. Aerosols can also impact each other
12 or other objects, settle out as sediment, diffuse, or
13 coalesce. Aerosol particles can also be subject to
14 hydroscopic growth as they travel. Delivery of medicine
as airborne particles requires conversion of the
16 medicine, which may be in liquid form, to an aerosol
17 followed relatively quickly by application of the aerosol
18 to the respiratory tract. One such device that has been
19 utilized for this purpose is an inhaler. Inhalers may
atomize a liquid to form an aerosol which a person
21 inhales via the mouth or nose. Inhalers typically
22 provide only limited delivery of medication to the lungs
23 since most of the medication is deposited on the linings
24 of the respiratory tract. It is estimated that as little
as 10-15% of an aerosol inhaled in this way reaches the
26 alveoli.
27 Aerosol delivery of a medication to a patient's
28 respiratory tract also may be performed while the patient
29 is intubated, i.e. when an endotracheal tube is
positioned in the patient's trachea to assist in
31 breathing. When an endotracheal tube is positioned in a
32 patient, a proximal end of the endotracheal tube may be
33 connected to a mechanical ventilator and the distal end
34 is located in the trachea. An aerosol may be added to
the airflow in the ventilator circuit of the endotracheal
36 tube and carried by the patient's inhalation to the
37 lungs. A significant amount of the aerosolized
CA 02596278 2007-08-21
-3-
1 medication may be deposited inside the endotracheal tube
2 and the delivery rate of the medicine to the lungs also
3 remains relatively low and unpredictable.
4 The low and unpredictable delivery rates of
prior aerosol delivery systems have limited the types of
6 medications that are delivered via the respiratory tract.
7 For new medications that are relatively expensive, the
8 amount of wasted medicine may be a significant cost
9 factor in the price of the therapy. Therefore, it would
be advantageous to increase the delivery rate or
11 efficiency of a medicine delivered to the lungs.
12 Another consideration is that some aerosols
13 delivered to the lungs may have adverse side effects,
14 e.g. radioactive tracers used for lung scans. Therefore,
it would be advantageous to minimize the overall amount
16 of medication delivered while maintaining the efficacy of
17 the medication by providing the same or a greater amount
18 of the medication to the desired site in the respiratory
19 tract.
Further, some medications may be more effective
21 when delivered in certain particle sizes. Accordingly,
22 an improved aerosol delivery system may provide for
23 improved rates and efficiencies of delivery also taking
24 into account the aerosol particle size.
It may also be important to administer certain
26 medications in specific, controlled dosages. The prior
27 methods of aerosol delivery not only were inefficient,
28 but also did not provide a reliable means to control
29 precisely the dosage being delivered.
It may also be advantageous to be able to
31 target medication to a specific bronchus, or specific
32 groups of bronchia, as desired, while avoiding delivery
33 of medication to other portions of the lungs.
34 Taking into account these and other
considerations, aerosol delivery via the respiratory
36 tract could become an even more widely used and effective
CA 02596278 2007-08-21
65902-72E
-4-
means of medication delivery if the delivery rate and
efficiency of the delivery could be improved.
SUMMARY OF THE INVENTION
A method and apparatus are disclosed for
delivering a drug with control and efficiency to a patient
via the patient's respiratory system. A nebulization
catheter is positioned in the patient's respiratory system
so that a distal end of the nebulization catheter is in the
respiratory system and a proximal end is outside the body.
According to a first aspect, the nebulization catheter may
be used in conjunction with an endotracheal tube and
preferably is removable from the endotracheal tube. The
nebulization catheter conveys medicine in liquid form to the
distal end at which location the medicine is nebulized by a
pressurized gas or other nebulizing agent. The nebulized
medicine is conveyed to the patient's lungs by the patient's
respiration which may be assisted by a ventilator. The
nebulizing catheter incorporates alternative constructions
taking into account anatomical considerations and the
properties of the medicine being nebulized to provide
delivery of medicine with control and efficiency.
According to one aspect the invention provides a
catheter for delivering an aerosol of medicine to a patient
comprising: a catheter shaft having a proximal end and a
distal end; a lumen through the catheter shaft and
communicating at the proximal end with a port for receiving
a medicine in a liquid form and communicating at the distal
end with a distal orifice from which the medicine can be
discharged; means for nebulizing the medicine discharged at
the distal orifice into an aerosol plume of particles of the
medicine; and means for modifying the aerosol plume of
particles of medicine, wherein the modifying means comprises
CA 02596278 2007-08-21
65902-72E
-4a-
a vacuum orifice located close to the distal orifice from
which the medicine is discharged for scavenging air from the
nebulized aerosol.
According to another aspect the invention provides
a catheter system for delivering an aerosol of medicine to a
patient comprising: a catheter shaft having a proximal end
and a distal end; a lumen through the catheter shaft and
communicating at the proximal end with a port for receiving
a medicine in a liquid form and communicating at the distal
end with a distal orifice from which the medicine can be
discharged; means for nebulizing the medicine discharged at
the distal orifice; and a flow control apparatus connected
to the port, said flow control apparatus comprising: a flow
line communicating with the port, said flow line occupied by
the medicine; a valve associated with the flow line to cause
pulsed delivery of medicine through the flow line; and a
draw back area associated with the flow line, said draw back
area adapted to cause a reversal of flow of medicine through
the flow line controller synchronized with the pulsed
delivery.
According to another aspect the invention provides
a catheter for delivering an aerosol to a patient
comprising: a shaft comprised of: an outer tubular member
defining a first lumen and terminating at a distal end in a
first distal orifice; an inner tubular member defining a
second lumen, said inner tubular member located in the first
lumen and terminating at a distal end in a second distal
orifice; a manifold connected to a proximal portion of said
shaft, said manifold having: a first port communicating
with the first lumen for conveyance of a pressurized gas in
an annular region between the inner and outer tubular
members; and a second port communicating with the second
lumen for conveyance of a medicine; said second distal
CA 02596278 2007-08-21
65902-72E
-4b-
orifice aligned with said first distal orifice to nebulize
the medicine from a distal tip of the catheter; and a
retractable pin located in said second lumen.
According to another aspect the invention provides
a catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
drug delivery orifice located in proximity to the distal gas
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice; and a centering apparatus located on said
catheter shaft close to the distal end.
According to another aspect the invention provides
a catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
drug delivery orifice located in proximity to the distal gas
CA 02596278 2007-08-21
65902-72E
-4c-
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice; and a valve located in at least one of the
lumens.
According to another aspect the invention provides
a catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
drug delivery orifice located in proximity to the distal gas
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice; and a retractable pin located in at least
one of said lumens.
According to another aspect the invention provides
catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
CA 02596278 2007-08-21
65902-72E
-4d-
drug delivery orifice located in proximity to the distal gas
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice; and a baffle located at the distal end of
the nebulization catheter in front of the orifices.
According to another aspect the invention provides
a catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
drug delivery orifice located in proximity to the distal gas
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice; and wherein said catheter shaft includes a
third lumen extending therethrough and a fiber optic scope
extending through said third lumen.
According to another aspect the invention provides
a catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
CA 02596278 2007-08-21
65902-72E
-4e-
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
drug delivery orifice located in proximity to the distal gas
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice; and wherein at least a portion of said
shaft surrounding said drug delivery lumen is formed of a
low compliance material so that flow control at said distal
drug delivery orifice of a fluid delivered through said drug
delivery lumen is more responsive to flow control at a
location proximal thereto.
According to another aspect the invention provides
a catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
drug delivery orifice located in proximity to the distal gas
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice; and a vibrating material located close to
said distal orifices.
According to another aspect the invention provides
a catheter system for delivering an aerosol therapy to a
patient comprising: a stand-alone nebulization catheter
having a distal end for insertion into the patient and a
proximal end, said nebulization catheter having: a catheter
CA 02596278 2007-08-21
65902-72E
-4f-
shaft; a gas pressurization lumen extending through said
catheter shaft; a distal gas exit orifice communicating with
said gas pressurization lumen, said distal gas exit orifice
located at the distal end of said nebulization catheter; a
drug delivery lumen extending along at least a portion of
said catheter shaft; a distal drug delivery orifice
communicating with said drug delivery channel, said distal
drug delivery orifice located in proximity to the distal gas
exit orifice so that gas exiting from said distal gas exit
orifice nebulizes a drug delivered from said distal drug
delivery orifice wherein the distal gas orifice comprises a
distal plug disposed at the distal end of the catheter
shaft, the distal plug comprising a plurality of apertures,
and wherein the apertures define gas orifices in
communication with the gas lumen.
According to another aspect the invention provides
a catheter for delivering an aerosol of medicine to a
patient comprising: a catheter shaft; a liquid lumen
centrally located in said shaft and adapted for conveying a
medicine in liquid form; a plurality of gas lumens
peripherally located around said liquid lumen and adapted
for conveying a gas; a distal liquid orifice communicating
with said liquid lumen; and a plurality of distal gas
orifices communicating with said plurality of gas lumens,
said plurality of distal gas orifices being aligned with
respect to said distal liquid orifice so as to nebulize a
liquid medicine discharged from the liquid orifice.
According to another aspect the invention provides
a catheter system comprising: a catheter shaft having: a
liquid lumen centrally located in said shaft and adapted for
conveying a medicine in liquid form; a plurality of gas
lumens peripherally located around said liquid lumen and
adapted for conveying a gas; a distal liquid orifice
CA 02596278 2008-09-22
65902-72E
-4g-
communicating with said liquid lumen; a plurality of distal
gas orifices communicating with said plurality of gas
lumens, said plurality of distal gas orifices being aligned
with respect to said distal liquid orifice so as to nebulize
a liquid medicine discharged from the liquid orifice;
wherein the catheter shaft has a proximal end and a distal
end and the liquid lumen communicates at the proximal end
with a port for receiving a medicine in a liquid form; and a
flow control apparatus connected to the port, said flow
control apparatus comprising: a flow line communicating
with the port, said flow line occupied by the medicine; and
a valve associated with the flow line to cause pulsed
delivery of medicine through the flow line.
According to another aspect of the invention,
there is provided a catheter for delivering an aerosol of
medicine to a patient comprising: a catheter shaft having a
proximal end and a distal end; a liquid lumen located in the
shaft and adapted for conveying a medicine in liquid form; a
gas lumen located adjacent the liquid lumen and adapted for
conveying a gas; a distal liquid orifice communicating with
the liquid lumen; and a distal gas orifice communicating
with the gas lumen, wherein the distal gas orificP an(i t.nP
distal liquid orifice are aligned to generate a discharge of
nebulized liquid; wherein the distal end of the catheter
shaft is maintained in a j-shape orientation having the
distal liquid orifice and the distal gas orifice pointing
substantially towards a proximal end of the catheter, the
j-shaped orientation maintained by a support member attached
to the catheter shaft.
According to another aspect of the invention,
there is provided a catheter system for delivering an
aerosol to a patient comprising: a catheter shaft having a
proximal end and a distal end, the distal end for insertion
CA 02596278 2008-09-22
65902-72E
-4h-
into the patient; a gas lumen extending through the catheter
shaft, the gas lumen defining a distal gas orifice in
communication with the gas lumen, the distal gas orifice
located at the distal end of the catheter shaft; a medicine
lumen extending along at least a portion of the catheter
shaft, the medicine lumen defining a distal medicine orifice
in communication with the medicine lumen, the distal
medicine orifice located at the distal end of the catheter
shaft; and wherein the distal gas orifice and the distal
medicine orifice are aligned to generate the discharge of
nebulized medicine in the direction toward the proximal end
of the catheter shaft.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. i shows an exploded view of a first
embodiment of the present invention.
FIG. 2 shows an assembled view of the embodiment
of FIG. 1.
FIG. 2A is a sectional view of the nebulization
catheter of FIGS. 1 and 2.
FIG. 3 is a plan view of an alternative embodiment
of the endotracheal tube shown in FIGS. 1 and 2.
CA 02596278 2007-08-21
-5-
1 FIG. 4 is a cross sectional view taken along
2 the line a-a' of the alternative embodiment of the
3 endotracheal tube shown in FIG. 3 without the nebulizing
4 catheter in place.
FIG. 5 is a cross sectional view taken along
6 the line b-b' of the alternative embodiment of the
7 endotracheal tube shown in FIG. 3 with the nebulizing
8 catheter in place.
9 FIG. 6 is a plan view of an embodiment of the
nebulizing catheter of FIGS. 1 and 2 shown in place in
11 the trachea of a patient who is not intubated.
12 FIG. 7 is a view similar to that of FIG. 6
13 showing an alternative embodiment of the nebulization
14 catheter.
FIG. 8 is a cross section taken along lines
16 a-a' of the nebulization catheter of FIG. 7.
17 FIG. 9 is a view similar to that of FIG. 7
18 showing an alternative embodiment of the nebulizing
19 catheter shown in FIG. 7.
FIG. 10 is a perspective view of a distal end
21 of an alternative embodiment of the nebulization catheter
22 shown in FIG. 1.
23 FIG. 11 is a perspective view of a distal end
24 of an alternative embodiment of the nebulization catheter
shown in FIG. 1.
26 FIG. 12 is a perspective view of an alternative
27 embodiment of FIG. 11 with the liquid lumen shown in a
28 closed condition.
29 FIG. 13 is a perspective view of the embodiment
of FIG. 12 with the liquid lumen shown in an open
31 condition.
32 FIG. 14 is a perspective view of a distal end
33 of an alternative embodiment of the nebulization catheter
34 shown in FIG. 1.
FIG. 15 is a perspective view of a distal end
36 of an alternative embodiment of the nebulization catheter
37 shown in FIG. 1.
CA 02596278 2007-08-21
-6-
1 FIG. 16 is a perspective view of a distal end
2 of an alternative embodiment of the nebulization catheter
3 shown in FIG. 1.
4 FIG. 17 is a perspective view of a distal end
of an alternative embodiment of the nebulization catheter
6 shown in FIG. 10.
7 FIG. 18 is a perspective view of a distal end
8 of an alternative embodiment of the nebulization catheter
9 shown in FIG. 1.
FIG. 19 is a sectional view of the distal end
11 of the embodiment of the nebulization catheter shown in
12 FIG. 18.
13 FIG. 20 is a sectional view of a distal end of
14 an alternative embodiment of the nebulization catheter
shown in FIG. 1.
16 FIG. 21 is a sectional view similar to that of
17 FIG. 20 showing an alternative embodiment of the
18 nebulization catheter shown in FIG. 20.
19 FIG. 22 is a perspective view partially in
section of a distal end of an alternative embodiment of
21 the nebulization catheter shown in FIG. 1.
22 FIG. 23 is a view similar to that of FIG. 22,
23 showing an alternative embodiment of the nebulization
24 catheter shown in FIG. 22.
FIG. 24 is a perspective view partially in
26 section of a distal end of an alternative embodiment of
27 the nebulization catheter shown in FIG. 1.
28 FIG. 25 is sectional view of a distal end of an
29 alternative embodiment of the nebulization catheter shown
in FIG. 25.
31 FIG. 26 is sectional view similar to that of
32 FIG. 25 showing the embodiment of FIG. 25 during an
33 exhalation stage of the patient.
34 FIG. 27 is a perspective view of alternative
embodiments of the nebulization catheter and endotracheal
36 tube shown in FIG. 1.
CA 02596278 2007-08-21
-7-
1 FIG. 28 is a perspective view of alternative
2 embodiments of the nebulization catheter and endotracheal
3 tube shown in FIG. 27.
4 FIG. 29 is a perspective view of an alternative
embodiment of the nebulization catheter shown in FIGS. 27
6 and 28.
7 FIG. 30 is a perspective view of the embodiment
8 of the nebulization catheter shown in FIG. 29 shown with
9 an endotracheal tube in a patient's trachea.
FIG. 31 is sectional view of a distal end and a
11 diagrammatic view of a proximal end of an alternative
12 embodiment of the nebulization catheter shown in FIG. 1.
13 FIG. 32 is a cross section view of the
14 embodiment of the nebulization catheter shown in FIG. 31
taken along the line a-a'.
16 FIG. 33 is sectional view of a distal end of an
17 alternative embodiment of the nebulization catheter shown
18 in FIG. 1.
19 FIG. 34 is sectional perspective view of a
distal end of an alternative embodiment of the
21 nebulization catheter and endotracheal tube shown in
22 FIG. 2.
23 FIG. 35 is sectional view of a distal end of an
24 alternative embodiment of the nebulization catheter shown
in FIG. 1.
26 FIG. 37 is a cross section view of the
27 embodiment of the nebulization catheter shown in FIG. 36
28 taken along the line a-a'.
29 FIG. 38 is a perspective view of a distal end
of an alternative embodiment of the nebulization catheter
31 shown in FIGS. 36 and 37.
32 FIG. 39 is a perspective view of alternative
33 embodiments of the nebulization catheter and endotracheal
34 tube shown in FIGS. 37 and 38.
FIG. 40 is sectional perspective view of a
36 distal end of an alternative embodiment of the
37 nebulization catheter shown in FIG. 1.
CA 02596278 2007-08-21
-8-
1 FIG. 41 is a perspective view of alternative
2 embodiments of the nebulization catheter and endotracheal
3 tube of FIG. 1 shown in a patient's trachea.
4 FIG. 42 is a side view of an another embodiment
of the nebulization catheter of FIG. 1 showing an
6 alternative centering device.
7 FIG. 43 is a side view of an another embodiment
8 of the nebulization catheter of FIG. 1 showing another
9 alternative centering device.
FIG. 44 is a side view of an another embodiment
11 of the nebulization catheter of FIG. 1 showing yet
12 another alternative centering device.
13 FIG. 45 is a side view of the embodiment of
14 FIG. 44 shown in another stage of operation.
FIG. 46 is a side view of a distal end of a
16 nebulization catheter positioned in a patient's trachea
17 illustrating an undesirable condition.
18 FIG. 47 is a perspective view similar to that
19 of FIG. 40 of alternative embodiments of the nebulization
catheter and endotracheal addressing the condition shown
21 in FIG. 46.
22 FIG. 48 shows an alternative embodiment of the
23 nebulizing catheter and endotracheal tube of FIG. 47
24 positioned in a patient's trachea.
FIG. 49 shows an alternative embodiment of the
26 nebulizing catheter of FIG. 6.
27 FIG. 50 is a diagram illustrating an embodiment
28 of a drug reservoir and pressurization assembly that can
29 be utilized in connection with the embodiment of the
nebulization catheter of FIG. 1.
31 FIG. 51 is a diagram similar to that of FIG. 50
32 illustrating an alternative embodiment of the drug
33 reservoir and pressurization assembly.
34 FIG. 52 is a sectional view along line c-c' of
FIG. 51.
CA 02596278 2007-08-21
-9-
1 FIG. 53 is a side view of an alternative
2 embodiment of FIG. 1 including an optional humidification
3 and heating arrangement.
4 FIG. 54 is a side view of a flow control system
used in connection with the embodiment of FIG. 1 used for
6 pressuring the liquid flow lumen.
7 FIG. 55 is a view similar to that of FIG. 54
8 showing the flow control system of FIG. 54 in another
9 stage of operation.
FIG. 56 is a perspective view of an alternative
11 embodiment of the present invention illustrating an
12 alternative method of use.
13 FIG. 57 is a perspective view illustrating an
14 entire nebulization catheter system including sensors.
FIG. 58 shows a sectional view of an embodiment
16 of a nebulizing catheter including a sensor.
17 FIG. 59 shows an alternative embodiment of the
18 nebulizing catheter shown in FIG. 58.
19 FIG. 60 is a sectional view of a distal end of
an alternative embodiment of the nebulizing catheter of
21 FIG. 1.
22 FIG. 61 is a sectional view of an embodiment of
23 the present invention that incorporates a baffle to
24 generate a secondary aerosol.
FIG. 62 is a sectional view of another
26 embodiment of the present invention that incorporates a
27 baffle to generate a secondary aerosol.
28 FIG. 63 is a sectional view of yet another
29 embodiment of the present invention that incorporates a
baffle to generate a secondary aerosol.
31 FIG. 64 is a sectional view of still another
32 embodiment of the present invention that incorporates a
33 baffle to generate a secondary aerosol.
34 FIG. 65 is a diagram illustrating an embodiment
of the present invention that incorporates a pressurized
36 drug/propellant mixture canister.
CA 02596278 2007-08-21
-10-
1 FIG. 66 is a side view of an embodiment of a
2 nebulizing catheter incorporated into of a suction
3 catheter.
4 FIG. 67 is a detailed sectional view of the tip
portion of the suction catheter - nebulizing catheter
6 embodiment of FIG. 66.
7 FIG. 68 is a perspective view of the embodiment
8 of FIG. 66 positioned in an endotracheal tube in a
9 patient's respiratory system.
FIG. 69 is cross sectional view of the
11 embodiment of FIG. 66 taken along lines a-a'.
12 FIG. 70 is a perspective view similar to FIG.
13 68 showing the suction catheter advanced during an
14 further stage of operation.
FIG. 71 is a side view of a proximal end of an
16 endotracheal tube illustrating an arrangement of
17 receiving a suction catheter and a nebulization catheter
18 into the endotracheal tube.
19 FIG. 72 is an alternative embodiment of the
arrangement shown in FIG. 71.
21 FIG. 73 is another alternative embodiment of
22 the arrangement shown in FIG. 71.
23 FIG. 74 is another embodiment of a suction
24 catheter incorporating aerosol delivery by nebulization.
FIG. 75 is still another embodiment of a
26 suction catheter incorporating aerosol delivery by
27 nebulization.
28 FIG. 76 is a sectional view of a distal end of
29 an embodiment of a nebulizing catheter also incorporating
a vibrating tip.
31 FIG. 77 is a sectional view of another
32 embodiment of the nebulizing catheter incorporating
33 micropulsation of the liquid supply.
34 DETAILED DESCRIPTION OF THE PRESENTLY PREFERRED
EMBODIMENTS
CA 02596278 2007-08-21
65902-72E
-11-
The present invention provides for the controlled
and efficient delivery of an aerosolized medication to the
lungs of a patient by nebulization of a medication at a
distal end of a catheter positioned in the respiratory
tract. Throughout this specification and these claims, the
nebulization catheter is described as used for the delivery
of medicine or medication. It is intended that the terms
"medication", "medicine", and "drug" should be understood to
include other agents that can be delivered to the lungs for
diagnostic or therapeutic purposes, such as tracers, or for
humidification.
I. Nebulizing Catheter - Basic Configuration
Referring to FIGS. 1 and 2, there is depicted a
first embodiment of the present invention. FIGS. 1 and 2
show an endotracheal tube 10 which may be a conventional
endotracheal tube. The endotracheal tube 10 may have an
inflatable cuff 12 located close to its distal end to
facilitate positioning the tube 10 in the patient's trachea,
or alternatively the endotracheal tube 10 may be of a type
without an inflatable cuff. The inflatable cuff 12 is
connected via a separate inflation lumen in the endotracheal
tube 10 to a proximal fitting 13 for connection to a source
of inflating gas (not shown). The endotracheal tube 10 has
a proximal end connected to a manifold fitting 14. The
fitting 14 has a port 15 suitably adapted for connection to
a ventilator circuit (not shown). The fitting 14 also
includes another port 16 that permits the introduction of a
separate catheter into the endotracheal tube from the
proximal end. The fitting 14 may be similar in construction
to the elbow fitting described in U.S. Pat. No. 5,078,131
(Foley). In FIG. 1, a nebulizing catheter 20 is located in
a position ready to be inserted into a ventilation lumen 22
of the endotracheal tube 10 via the
CA 02596278 2007-08-21
-12-
1 proximal fitting 14. In FIG. 2, the nebulizing catheter
2 20 is positioned fully in the endotracheal tube 10 with a
3 proximal end extending out of the port 16 of the proximal
4 fitting 14.
At a proximal end of the nebulizing catheter 20
6 is a manifold 24. The manifold 24 includes at least a
7 gas port 28 and a liquid (medicine) port 32. These ports
8 28 and 32 may include conventional attaching means, such
9 as luer lock type fittings. In addition, these ports 28
and 32 may also include closure caps 31 that may be used
11 to close the ports when not in use and may be popped open
12 when connection to a gas source or a liquid source is
13 desired. Optionally, the manifold 24 may also include a
14 filter located in-line with either the gas port 28 or the
liquid port 32 or both ports to prevent lumen blockages
16 by particulate matter. The nebulization catheter 20
17 includes at least two separate lumens (as shown in FIG.
18 2A). A first lumen 33 is used for conveyance of a liquid
19 medicine and communicates with the port 32 on the
manifold 24. The other lumen 34 is used for conveyance
21 of a pressurized gas and communicates with the port 28 on
22 the manifold 24. The liquid lumen 33 communicates with a
23 distal liquid orifice 35 and the gas lumen 34
24 communicates with a distal gas orifice 36 near a distal
end 37 of the nebulization catheter 20. The distal
26 opening 36 of the pressurized gas lumen 34 directs
27 pressurized gas across the distal liquid lumen opening 35
28 thereby nebulizing the liquid medication so that it can
29 be delivered to the patient's lungs. The distal liquid
orifice 35 may be open or may be provided with a porous
31 material plug or a sponge-like or felt-like material plug
32 which may extend slightly from the distal orifice and
33 that allows liquid to flow from the orifice yet reduces
34 the likelihood of liquid drooling from the tip.
The length of the nebulization catheter 20
36 should be sufficient so that the distal end 37 can be
37 located in the desired location in the respiratory system
CA 02596278 2007-08-21
-13-
1 while the proximal end (i.e., including the manifold 24)
2 is accessible to the physician or other medical personnel
3 for connection to suitable gas and liquid supplies
4 external of the patient's body. Accordingly, the length
of the nebulization catheter is dependant upon the size
6 of the patient in which it is being used. A shorter
7 nebulization catheter may be preferred in smaller
8 patients, such as infants or children, and a longer
9 nebulization catheter may be needed for adults. For
example, a nebulization catheter suitable for adults may
11 have a length of approximately 45 cm. In one embodiment,
12 approximately 30 cm of the nebulizing catheter 20 is in
13 the endotracheal tube 10. The nebulization catheter may
14 be introduced into the respiratory system through a
patient's mouth or via a tracheostomy tube or through the
16 nasal passages. The nebulization catheter may also be
17 used to deliver an aerosol to a patient's nasal passages
18 in which case the length may be correspondingly shorter.
19 As explained in more detail below, the
generation of an aerosol plume with the desired geometry,
21 particle size, velocity, etc., requires that the distal
22 gas and liquid orifices have small dimensions. Also as
23 explained below, the distal gas orifice 36 and the distal
24 liquid orifice 35 should be in close proximity to each
other in order to produce an aerosol with the desired
26 characteristics and efficiency. Further, in order to
27 provide the desired medicine,delivery rates and to
28 operate with reasonably available pressure sources, the
29 liquid and gas lumens in the nebulizing catheter should
be as large as possible, consistent with anatomical
31 requirements. Accordingly, the nebulization catheter 20
32 has a multiple stage construction with a larger shaft
33 size and larger lumens in a main shaft section and a
34 smaller shaft size and smaller lumens in a distal shaft
section.
36 As shown in FIG. 2A, the nebulizing catheter 20
37 is composed of a shaft 38 having a main section 39 and a
CA 02596278 2007-08-21
-14-
1 distal section 40. In the main shaft section 39 of the
2 nebulization catheter, the liquid and gas lumens 33 and
3 34 have a larger size than in the distal shaft section
4 40. For example, in the main shaft section 39, the
liquid and gas lumens each may have an I.D. of
6 approximately .010 to .030 inches. At a most proximal
7 end where the main shaft section 39 connects to the
8 manifold 24, the lumens may be even larger. In the
9 distal shaft section 40, the liquid and gas lumens taper
to a much smaller I.D. with the liquid lumen
11 approximately .002 to .008 inches or even smaller and the
12 gas lumen .002 to .020 inches. In a preferred
13 embodiment, the liquid and gas orifices 35 and 36 are
14 less than .125 inches apart, and more preferably less
than .030 inches apart, and in a most preferred
16 embodiment less than .001 inches apart-. In a nebulizing
17 catheter having an overall length of 45 cm, the main
18 shaft section 39 may be approximately 25 cm and the
19 distal shaft section 40 may be approximately 20 cm.
Also, although the liquid and gas lumens are shown to be
21 side by side in FIG. 2A, they may also be constructed to
22 have an coaxial or other arrangement. Further, although
23 the main shaft section 39 is shown to be of a uniform
24 diameter and profile, alternatively it may also have a
tapered diameter and profile such that the entire shaft
26 38-is tapered along its length.
27 In a first preferred embodiment of the
28 invention, as shown in FIGS. 1 and 2, the nebulizing
29 catheter 20 is removable, and replaceable with respect to
the endotracheal tube 10. This provides several
31 significant advantages. First, the nebulizing catheter
32 20 may be specifically adapted and chosen to have the
33 desired operating characteristics suitable for delivery
34 of the particular medication being administered to the
patient. In addition, the fact that the nebulizing tube
36 20 is removable and replaceable provides versatility and
37 flexibility regarding the therapy and dosage regime that
CA 02596278 2007-08-21
-15-
1 can be chosen by the physician. For example, a decision
2 by the physician whether to deliver a medication to the
3 respiratory tract, and the selection of the type and
4 dosage of the medication to be delivered, need not be
made by the physician until after the endotracheal tube
6 is already in place in the patient. When the physician
7 determines the proper type of medication to the delivered
8 to the patient via the respiratory tract, the appropriate
9 nebulization catheter can be selected and inserted into
the endotracheal tube. Further, the nebulizing catheter
11 20 can be removed after it is used and therefore it is
12 not necessary for the nebulization catheter to be left in
13 the patient and occupy space in the patient's respiratory
14 tract or in the endotracheal tube 10 when it is no longer
needed. In addition, the decision regarding the proper
16 type of medication can be revisited again at any time
17 after the endotracheal tube is in place. If a different
18 type of nebulizing catheter is required, such as for
19 sterility purposes, the endotracheal tube need not be
replaced as well.
21 Another advantage of providing the nebulization
22 catheter as a separate, removable device is that it can
23 be accommodated in a variety of other instruments and/or
24 devices. For example, the nebulization catheter of FIGS.
1 - 5 is shown used in an endotracheal tube; however, the
26 nebulization catheter could also be positioned inside of
27 a bronchoscope, such as in a working channel of a
28 bronchoscope. The nebulizing catheter could be
29 positioned in any instrument that is positioned in the
respiratory tract and that can accommodate the nebulizing
31 catheter size.
32 The nebulizing catheter may be provided with
33 radiopaque markings 41 to facilitate positioning and
34 placement. The radiopaque markings 41 may be provided by
radiopaque bands of metal or heat shrunk bands of doped
36 radiopaque plastic that are attached to the nebulizing
37 catheter, or alternatively the markings may be provided
CA 02596278 2007-08-21
-16-
1 by doping the plastic material of the nebulizing catheter
2 with a radiopaque material. Alternatively, a radiopaque
3 dye may be added to the liquid being delivered by the
4 nebulization catheter to assist observation. The
markings 41 may be graduated to facilitate recognition,
6 or alternatively may extend over a portion or all of the
7 nebulizing catheter. In still a further embodiment, the
8 markings may be formed of a ultrasonic reflectors, e.g.
9 textured material, that are visible by means of
ultrasonic imaging. The nebulization catheter may also
11 include a stripe 43 extending along a side of the shaft
12 (as shown in FIGS. 5 and 6). The stripe 43 may be
13 radiopaque or ultrasonically visible and may be used to
14 determine the rotational orientation of the shaft. The
stripe may be formed by a coextrusion process or by
16 embedding a wire in the wall of the nebulization
17 catheter.
18 One method that may be employed to facilitate
19 positioning of the nebulization catheter is to monitor
the pressure at the distal end of the endotracheal tube
21 as the nebulization catheter is being advanced.
22 Monitoring the pressure at the end of the endotracheal
23 tube may be accomplished through one of the endotracheal
24 tube lumens. The gas source connected to the proximal
end of the nebulization catheter may be operated so as to
26 expel a gas from the distal end of the nebulization
27 catheter as it is being advanced. The gas being expelled
28 from the distal end of the nebulization catheter affects
29 the pressure being detected through the endotracheal
tube. When the distal end of the nebulization catheter
31 passes the distal end of the endotracheal tube, the
32 pressure being measured through the endotracheal tube
33 abruptly changes thereby providing a clear indication of
34 the location of the distal end of the nebulization
catheter relative to the endotracheal tube.
36= The nebulizing catheter may also include a
37 safety stop 44 located along a proximal portion that
CA 02596278 2007-08-21
-17-
1 engages a portion of the endotracheal tube proximal
2 portion or a fitting thereon, as shown in FIG. 2. The
3 safety stop 44 ensures that the distal end of the
4 nebulizing catheter 20 is correctly positioned with
respect to the distal end 46 of the endotracheal tube 10
6 and prevents the distal end 37 of the nebulizing catheter
7 from extending too far into the trachea. In addition to
8 the safety stop 44, the proximal portion of the
9 nebulizing catheter 20 may also have graduated markings
48 that would be visible to the physician handling the
11 proximal end of the nebulizing catheter to enable a
12 determination of the position of the distal end 37 of the
13 nebulizing catheter 20 relative to a distal end 46 of the
14 endotracheal tube 10.
The nebulizing catheter 20 may also include a
16 critical orifice 49 located at a proximal portion of the
17 nebulizing catheter. The critical orifice 49 may be
18 formed by a small critical opening located in line with
19 the gas pressurization lumen 34 of the nebulizing
catheter shaft close to the manifold 24. The critical
21 orifice 49 is sized so that if the nebulization catheter
22 is supplied with a flow in excess of its designed
23 operating flow, the critical orifice will allow only the
24 designed operating flow to pass through to the distal gas
orifice. Alternatively, a safety valve may be located
26 in the proximal portion of the catheter shaft. The
27 safety valve would be designed to open if supplied with
28 an excess of pressure.
29 In addition, the nebulizing catheter may
include a centering device 50. The centering device 50
31 is located close to a distal end of the nebulizing
32 catheter shaft and helps to center and align the distal
33 end of the nebulizing catheter for improved performance,
34 as explained in more detail below.
According to one embodiment, the removable
36 nebulization catheter 20 is enclosed in a storage sheath
37 51. The storage sheath 51 may be similar to the type of
CA 02596278 2007-08-21
-18-
1 storage sheaths used in conjunction with suction
2 catheters. The storage sheath is preferably flexible,
3 collapsible, or extendable to accommodate insertion of
4 the catheter. The storage sheath 51 may be connected to
the fitting 14. The storage sheath 51 can be used to
6 receive the nebulizing catheter 20 when it is being
7 withdrawn from the endotracheal tube 10. The storage
8 sheath 51 is sealed and can maintain the withdrawn
9 nebulizing catheter in an isolated condition when it is
temporarily removed from the patient's respiratory
11 system. The storage sheath 51 also allows the physician
12 to re-insert the nebulization catheter into the patient.
13 In this manner, the nebulization catheter can be reused
14 in a limited way with respect to a patient and can be
maintained in a sterile condition while withdrawn from
16 the patient. The storage sheath 51 may have a distal
17 sleeve 53 that can slide along the shaft of the
18 nebulization catheter so that the nebulization catheter
19 may be advanced into the ventilation lumen of the
endotracheal tube or withdrawn into the storage sheath
21 51. The sleeve 53 may have a close fitting seal 55
22 located therein which is designed to clean and/or wash
23 the nebulization catheter when it is withdrawn into the
24 sheath. Alternatively, a cleaning seal 55 may be located
in the port 16 of manifold fitting 14.
26 Another feature that may be used in conjunction
27 with certain procedures is radiation shielding. Some
28 procedures for which the nebulization catheter may be
29 used may involve the delivery of radioactive agents, e.g.
tracers to the lungs. To minimize exposure to
31 radioactive materials, the nebulizing catheter may be
32 provided with shielding over all or a significant portion
33 of the overall length of the catheter. Shielding may
34 also be provided at the liquid source reservoir.
The nebulizing catheter is preferably
36 constructed of a biocompatible, chemically resistant
37 polymer in order that it is suitable for use with a wide
CA 02596278 2007-08-21
-19-
1 variety of drugs. The catheter shaft is preferably clear
2 to allow visualization of contaminants or blockages of
3 the interior lumens. Also, the portion of the catheter
4 shaft that forms the liquid lumen 33 is preferably
composed of a relatively non-compliant material. In a
6 present embodiment, the catheter shaft is composed of a
7 polymer such as polyethylene or nylon. A polymer tubing
8 is extruded with multiple lumens to be used for the
9 separate gas and liquid lumens. In order to produce a
nebulization catheter with the tapered distal section 40,
11 a multi-lumen extruded tubing may be drawn down in a
12 portion thereof to form the tapered distal section 40.
13 The draw down ratio may be selected to provide a
14 nebulization catheter shaft with the desired dimensions.
The draw down process serves to make the lumens smaller
16 in size distally as well as closer together while
17 maintaining the proximal cross sectional profile of the
18 multi-lumen tubing. The larger proximal profile provides
19 for greater pushability in the catheter shaft and
facilitates manufacturing by making the manifold
21 connection easier. The draw down ratio used on the
22 extruded polymer tubing may be on the order of 2-to-1, 5-
23 to-1, or even as high as 20-to-i or higher. Prior to
24 drawing down, the extruded polymer tubing is preferably
exposed to high energy radiation to crosslink the polymer
26 molecules to provide for favorable material properties,
27 such as the ability to maintain orifice dimensions and
28 tolerances. The radiation may have an energy of
29 approximately 10-700 kgy. After the crosslinking step,
the tubing is heated to its transition temperature
31 between its melt and glass states, and is drawn down by
32 the desired ratio.
33 As an alternative to drawing down the extruded
34 tubing, the multi-stage nebulization catheter shaft may
be formed by a bubble extrusion process wherein the
36 desired tapered distal section is formed directly in the
37 shaft as it is being extruded. Again, this process may
CA 02596278 2007-08-21
-20-
1 be used for manufacturing efficiency and convenience. As
2 another alternative, the multi-stage shaft may be formed
3 by a combination of both bubble extrusion and drawing
4 down. Still another alternative for forming the desired
tapered profile for the nebulizing catheter shaft is to
6 use a material that can be cold drawn in order to cause a
7 sharp neck down in diameter, such as a linear low density
8 polyethylene. Although the process for forming the
9 tubing is particularly suited for producing a
nebulization catheter shaft for use in delivering
11 medicine to the respiratory tract, it should be
12 understood that the process could be used to produce
13 aerosol nozzles for non-medical purposes as well.
14 Alternatively, all or part of the nebulization
catheter shaft can be molded, especially at locations
16 where close tolerances are preferred such as at the tip.
17 After the shaft is formed with the desired
18 stages, it is cut and assembled with the other components
19 of the nebulizing catheter. Although the nebulization
catheter is preferably constructed of a polymer, in an
21 alternative embodiment it could be formed of other
22 materials such as a metal, especially a malleable metal
23 to facilitate drawing, shaping or forming orifices.
24 During the manufacturing process, the nebulizing catheter
may be pre-sterilized by means of a conventional process,
26 such as a gamma ray or electron beam. The nebulizing
27 catheter is preferably disposable after use with a single
28 patient, but may be reused to a limited extent with a
29 single patient provided that contamination can be
prevented such as through the use of the sheath 51,
31 described above. The nebulizing catheter shaft
32 preferably possesses torsional rigidity so that rotation
33 of the proximal end is transmitted at a 1:1 ratio to the
34 distal end. The nebulizing catheter may also be provided
with an antiseptic coating.
36 Drug delivery rates are closely related to the
37 particle size with larger particles providing greater
CA 02596278 2007-08-21
-21-
1 delivery rates. The embodiments of the nebulization
2 catheter described herein have the capability of
3 generating particle distributions with a GSD between 2
4 and 2.5. Drug delivery rates in a range between
approximately 5 and 1000 mg (.005 - 1.0 ml) per minute
6 may be obtained. A variety of particle size
7 distributions can be generated at most flow rates through
8 selection of the catheter type and aerosol volume output.
9 An aerosol of this type can be generated with the
nebulization catheter using a gas flow rate as low as 0.1
11 liter/minute.
12 There are a number of factors that affect the
13 particle size generated. These factors include: (1) the
14 gas orifice diameter, (2) the liquid orifice diameter,
(3) the liquid delivery tube outer diameter and geometry,
16 (4) the distance between the gas and liquid orifices, (5)
17 the rate of gas delivery, and (6) the pressure of the
18 liquid. Of course, the size of the solid particles in
19 suspension, if present, in the liquid are a defining
aspect of the aerosol particle size generated. In
21 addition, there are other factors that affect the aerosol
22 particle size such as the characteristics of the liquid,
23 e.g. viscosity, suspension, surface tension and the
24 composition of the driving gas, however, these factors
affect the particle size of the aerosol generated to a
26 lesser degree. By selectively varying these parameters,
27 the size and size distribution of the aerosol particles
28 can be changed from less than a micron to at least 10
29 microns.
The embodiments of the present invention,
31 described herein are suitable for delivery of an aerosol
32 by nebulization with a volumetric particle size
33 distribution comparable to other nebulization systems.
34 Further, by generating an aerosol at a location in the
trachea or even deeper in the bronchi, impaction losses
36 in tract can be avoided. By reducing impaction losses,
37 it may be acceptable to use larger particle sizes (e.g.
CA 02596278 2007-08-21
-22-
1 greater than 5 microns). The combination of lower
2 impaction losses and larger particle sizes may provide
3 higher effective delivery rates than prior systems.
4 Reducing impaction losses would enable an embodiment of
the nebulization catheter to provide acceptable delivery
6 rates.with aerosol particle sizes greater than 5 microns.
7 Referring to FIGS. 3 - 5, there is depicted a
8 further embodiment of the present invention. According
9 to the embodiment of FIGS. 3 - 5, there is provided an
endotracheal tube 52 and a nebulizing catheter. The
11 nebulizing catheter may be similar to the nebulizing
12 catheter 20 shown in FIGS. 1 through 3. In the
13 embodiment of FIGS. 3 - 5, the endotracheal tube 52 has
14 an auxiliary lumen 56 in addition to its main ventilation
lumen 60. Some endotracheal tubes provide auxiliary
16 lumens through the shaft wall. The auxiliary lumen 56 is
17 preferably sized and adapted to receive the separate
18 nebulization catheter 20. This embodiment provides many
19 of the same advantages as the embodiment of FIGS. 1
through 3. In addition, in this embodiment, the
21 auxiliary lumen 56 may be provided with a distal aperture
22 64 that facilitates locating and aligning the distal end
23 of 37 the nebulizing catheter 20 at a desired location
24 for nebulization purposes.
In the embodiments of the invention shown in
26 FIGS. 1-5, the nebulizing catheter 20 is shown used in
27 conjunction with an endotracheal tube either of a
28 conventional type 10, as in FIGS. 1 and 2, or of a type
29 especially designed for use with the nebulizing catheter
such as endotracheal tube 52 of FIGS. 3 - 5. The
31 nebulizing catheter 20 according to an embodiment of the
32 present invention may also be used without a separate
33 endotracheal tube, i.e. the nebulizing catheter may be
34 used on a patient who is not intubated, as shown in FIG.
6. If used on a spontaneously breathing patient (without
36 an endotracheal tube), the patient should be properly
CA 02596278 2007-08-21
-23-
1 anesthetized and/or that the airway passage of the
2 patient be topically anesthetized. The nebulizing
3 catheter 20 is positioned in the respiratory system of a
4 patient directed past the carina 68 into one of the
bronchi 72 of the lungs. Alternatively, the nebulizing
6 catheter 20 may also be positioned proximal of the carina
7 in the trachea, as desired. Embodiments of the
8 nebulizing catheter may also be used on patients who have
9 had tracheotomies or who have tracheotomy tubes.
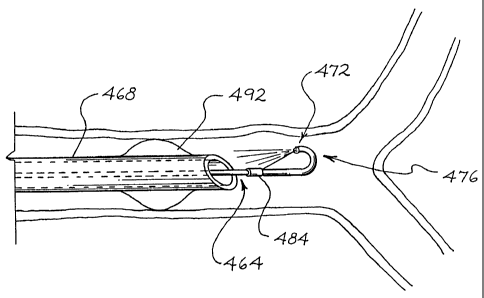
In the embodiment of FIG. 6, a guiding sheath
11 73 is used. The guiding sheath 73 is used to help
12 position the nebulizing catheter 20 in the respiratory
13 system of the patient. The guiding sheath 73 includes a
14 lumen through which the nebulization catheter 20 can be
advanced into a desired bronchi site. To facilitate
16 positioning the nebulization catheter, the guiding sheath
17 73 may have a pre-shaped distal end to facilitate
18 locating the sheath in the desired airway passage.
19 Alternatively, the guiding sheath 73 may have a distal
end that can be formed into a desired shape by the
21 physician just prior to insertion. The guiding sheath 73
22 differs from the endotracheal tube 10 of FIGS. 1-5 in
23 that it may have a smaller outside diameter so that it
24 can be advanced into smaller airway passages deep in the
patientfs bronchi past the carina 68. The inside
26 diameter of the sheath 73 is large enough to advance the
27 nebulization catheter. The guiding sheath 73 is
28 particularly useful when the nebulization catheter 20 is
29 being located deep in the patient's lungs, or when the
nebulization catheter is used without an endotracheal
31 tube. The guiding sheath 73 may also be used with an
32 endotracheal tube through the ventilation lumen thereof.
33 The guiding sheath is preferably composed of a
34 torsionally rigid material so that the distal end of the
guiding sheath is responsive to rotation at the proximal
36 end.
CA 02596278 2007-08-21
-24-
1 Referring to FIGS. 7 and 8, there is shown
2 another embodiment of the nebulizing catheter. In the
3 embodiment of FIG. 7, a nebulizing catheter 76 includes
4 an occlusion balloon 80 located on a distal exterior
surface of the nebulizing catheter shaft body 84. The
6 nebulizing catheter 76 may include an additional lumen
7 88, as shown in FIG. 8, located therethrough and
8 communicating with the interior of the balloon 80 for
9 providing inflation fluid, i.e. preferably gas, to expand
the occlusion balloon 80. This lumen 88 for inflation
11 fluid is in addition to the lumens 92 and 96 in the
12 catheter shaft 84 used for conveyance of the liquid
13 medicine and pressurized gas, respectively. The
14 occlusion balloon 80 may be used to position the
nebulizing catheter in the appropriate respiratory branch
16 100, center the nebulizing catheter tip for proper
17 orientation, and isolate a particular bronchus, as
18 needed. The embodiment of the nebulizing catheter 76
19 shown in FIG. 7 may be used with an endotracheal tube in
a manner similar to that shown in FIGS. 1-3, or
21 alternatively it may be used without a separated
22 endotracheal tube, similar to the embodiment of FIG. 6.
23 When used without a separate endotracheal tube, the
24 nebulizing catheter 76 of FIG. 7 could be used for the
purpose of selective ventilation of one of the bronchi of
26 the lungs even without providing aerosolization.
27 Alternatively, the nebulization catheter 76 could provide
28 aerosolization on an intermittent basis with continuous
29 ventilation. If the nebulization catheter is used to
provide ventilation as well as aerosolized medication,
31 the ventilation regime can be tailored to maximize
32 aerosol transport.
33 In addition, to further facilitate positioning
34 and placement, the nebulizing catheter 76 may be used
with a guide wire 104. The nebulizing catheter may be
36 provided with a separate guide wire lumen 108 to receive
37 the guide wire 104, or alternatively, the guide wire may
CA 02596278 2007-08-21
-25-
1 use one of the existing lumens that is also used for
2 either the pressurized gas or the liquid or alternatively
3 the guide wire may be incorporated and fixed into the
4 nebulizing catheter so that it is non-removable. The
guide wire, whether of the removable type of the type
6 that-is fixed to the nebulizing catheter, may also be
7 steerable, i.e. so that it can be guided from a proximal
8 end to access the appropriate location in the lungs. The
9 steering apparatus may utilize selective tensioning of a
pull wire, etc. from a proximal end. If the guide wire
11 is of the separate removable type, it may be withdrawn
12 after it has been used to position the distal tip of the
13 nebulizing catheter so as to avoid interfering with
14 aerosol delivery. In addition, the distal tip of the
guide wire or nebulization catheter may be pre-shaped or
16 shapable by the physician so as to impart an appropriate
17 curve or bend to facilitate access to the desired airway.
18 Referring to FIG. 9, there is shown another
19 embodiment of a nebulizing catheter of FIG. 7. The
embodiment of FIG. 9 is similar to the embodiment of FIG.
21 7 with the exception that the separate guide wire 104 is
22 received in a loop 106 located close to a distal end of
23 the nebulizing catheter 76. Proximal of the loop 106,
24 the guide wire 104 is positioned adjacent to the shaft 84
of the nebulizing catheter 76. Instead of a loop 106,
26 the guide wire may be received in a short lumen located
27 in the distal end of the nebulizing catheter.
28 II. Generation of Aerosol Plume
29 It has been discovered that the shape of the
aerosol plume can be a significant factor affecting the
31 rate and efficacy of the delivery of medication by an
32 aerosol. In general, it is preferable to generate an
33 aerosol that has a shape that minimizes particle
34 impaction near the distal tip of the nebulizing catheter,
given the location of the tip and the airflow conditions
36 around it. For example, if the aerosol plume is wide, a
CA 02596278 2007-08-21
-26-
1 portion of the drug may be wasted in the end of the
2 endotracheal tube or on the wall of the trachea or other
3 airway passage. On the other hand, if the plume is too
4 narrow or the velocity too high, a portion of the drug
may impact excessively on the patient's carina. In
6 general, a low aerosol particle velocity is desirable.
7 One of the reasons for this is to avoid impacting the
8 carina with the discharge of high velocity aerosol
9 particles. In addition, it is also generally desirable
to have as wide an aerosol plume as possible while
il avoiding significant impact with the walls of either the
12 endotracheal tube or the respiratory airway passage. The
13 effects of aerosol plume velocity and geometry are
14 related to anatomical factors. In some circumstances,
e.g. away from the carina, a narrow, fast aerosol plume
16 may be preferable to a slower, wider plume.
17 Regarding the embodiments described below,
18 certain of the embodiments may be preferable from the
19 standpoint of versatility, i.e. they may be able to
deliver a variety of medications having different
21 viscosities, suspensions, surface tensions, etc. Others
22 of the embodiments may be more suitable for the delivery
23 of specific types of medications or the delivery of
24 particles of certain sizes.
Referring to FIG. 10, there is shown a tip
26 configuration for a nebulizing catheter 112. The
27 nebulizing catheter 112 may be either a stand alone-type
28 of nebulizing catheter, similar to the catheters shown in
29 FIGS. 6 and 10, or may be incorporated into an
endotracheal tube either removably, as in FIGS. 1 - 5, or
31 non-removably. In the embodiment of FIG. 10, the
32 nebulizing catheter 112 has a coaxial configuration.
33 Specifically, the nebulizing catheter 112 includes an
34 outer tubular member 116 defining a lumen 120 and an
inner tubular member 124 also defining a lumen 128. The
36 inner tubular member 124 is located in the lumen 120 of
37 the outer tubular member 116. According to the
CA 02596278 2007-08-21
-27-
1 embodiment shown FIG. 6, pressurized gas is conveyed in
2 the annular region defined between the inner and outer
3 tubular members. Liquid medication is conveyed in the
4 lumen 128 of the inner member 124. As shown in the
embodiment of FIG. 10, a distal end of the outer tubular
6 member 116 is approximately adjacent to a distal.end of
7 the inner tubular member 124. In the embodiment of FIG.
8 10, the outer tubular member 116 has an O.D. of
9 approximately .008 inches and an I.D. of approximately
.006 inches. The inner tubular member 124 has an O.D. of
11 approximately .003 inches and I.D. of approximately .0015
12 inches. Both the inner tubular member 124 and the outer
13 tubular member 116 have larger dimensions proximal of the
14 distal tip portion. Along a main shaft portion proximal
of the distal tip, the outer tubular member 116 has an
16 O.D. of approximately .115 inches and an I.D. of .080
17 inches and the inner tubular member 124 has an O.D. of
18 approximately .060 inches and an I.D. of .050 inches.
19 The embodiment of FIG. 11 shows a tip of a
nebulizing catheter 132. This embodiment is similar to
21 the embodiment of FIG. 10. The tip 133 is formed with a
22 plurality of lumens terminating in a plurality of
23 orifices. An inner lumen 134 is used to convey the
24 liquid medication and the surrounding lumens 135 convey
the pressurized gas used to nebulize the liquid. This
26 embodiment has the advantage that the orifice of the
27 liquid lumen 134 is centered with a fixed spacing
28 relative to the orifices of the gas lumens 135 around it.
29 In the embodiment of FIG. 11, the multiple lumen
construction may extend all the way back to the proximal
31 end of the nebulizing catheter 132 or alternatively, only
32 a distal segment may have the multiple gas lumen
33 configuration in which case the pressurized gas may be
34 conveyed through a single proximal lumen that connects to
the multiple distal lumens.
CA 02596278 2007-08-21
-28-
1 FIGS. 12 and 13 show an alternative embodiment
2 136 of the multiple lumen nebulization catheter in FIG.
3 11. The embodiment in FIGS. 12 and 13 is useful when it
4 is desired to provide the aerosol medicine with a pulsed
delivery. The pulsed delivery may be timed to coincide
6 with the inhalation of the patient so that aerosol is not
7 wasted when the patient is exhaling. A potential
8 drawback with pulsed delivery is that the aerosol may
9 drool from the tip of the nebulizing catheter when the
pressure being applied to the liquid is reduced to effect
11 the pulsation. To avoid this potential problem, the
12 nebulizing catheter 136 provides for closure of the
13 liquid lumen when the pressure being applied to it is
14 reduced. As in the previously described embodiment, the
nebulization catheter 136 in FIGS. 13 and 14, has a
16 centrally located lumen 137 for delivery of a liquid
17 medicine and a plurality of lumens 138 surrounding the
18 central lumen 137 for conveyance of a pressurized gas to
19 nebulize the liquid at the distal orifice 139. In this
embodiment, the catheter 137 is formed of a low
21 compliance material in the outer wall area 140 and a
22 relatively high compliance material in the area 141
23 surrounding the centrally located liquid lumen 137.
24 These differing compliance characteristics may be formed
in the catheter shaft by coextruding a single tube with
26 different materials. When using the embodiment of FIGS.
27 12 and 13, a constant, relatively high pressure is
28 applied to the gas in the lumens 138. Liquid medicine is
29 delivered via the lumen 137 and pressure pulses are
applied to the liquid from an external delivery source,
31 such as a pump. When the pressure in the liquid lumen
32 137 is low, the high pressure in the gas lumens 138
33 deform the compliant inner material 141 thereby
34 compressing the liquid lumen 137 and closing it off, as
shown in FIG. 12. When a pressure pulse is applied to
36 the liquid in the lumen 137, it overcomes the compressive
37 forces from the gas lumens 138 allowing the lumen 137 to
CA 02596278 2007-08-21
-29-
1 open and permitting the liquid medicine to be delivered
2 to the distal orifice 139 to be nebulized, as shown in
3 FIG. 13. In this manner, the embodiment of FIGS. 12 and
4 13 provides for pulsed liquid nebulization with reduced
possibility of drooling.
6 Another feature shown in FIGS. 11 and 12 is a
7 porous plug 142 located in the liquid orifice 139. This
8 porous plug may be made of a felt-like material and may
9 assist in the production of fine aerosol particles.
The embodiment of FIG. 14 shows a distal tip of
11 another embodiment of the nebulizing catheter. In this
12 embodiment, a nebulizing catheter 148 includes a main
13 shaft section 152 and a distal shaft section 156. The
14 distal shaft section 156 is tapered to a tip 160. At the
tip 160, a liquid orifice 164 is surrounded by a
16 plurality of gas orifices 168. In a preferred
17 embodiment, there are six gas lumens terminating in the
18 six orifices 168. In this embodiment, the liquid orifice
19 164 has a diameter of approximately .002 inches and the
gas orifices 168 each have a diameter of approximately
21 .002 inches. This embodiment is similar to the
22 embodiment of FIG. 11 except that the distal section 156
23 provides for a reduction in the tip size and
24 corresponding modification of the nebulization plume
properties. This reduction is preferable as it provides
26 a smaller orifice size.
27 The embodiment of FIG. 15 shows a distal
28 portion of a nebulizing catheter 172. In this
29 embodiment, the nebulizing catheter includes a proximal
shaft section 176 and a distal shaft section 180. The
31 proximal shaft section 176 includes a plurality of lumens
32 184. A central one 188 of the plurality of lumens 184 is
33 used to convey liquid medicine and the remainder of the
34 lumens surrounding it are used to convey gas. The distal
shaft section 180 connects to the distal end of the
CA 02596278 2007-08-21
-30-
1 proximal shaft section 176 and defines a tapered cavity
2 192 between the distal end of the proximal shaft section
3 176 and a distal orifice 196. At least one of the
4 plurality of lumens 184 is used to convey a pressurized
gas that is discharged into the cavity 192. A tubular
6 extension 200 extends the liquid lumen through the cavity
7 192 and distally out the orifice 196. The orifice 196 is
8 sized to provide an annular region around the tubular
9 extension 200 to permit the pressurized gas to flow
through to nebulize the liquid medication that exits a
11 distal orifice 204 of the tubular extension 200. In a
12 preferred embodiment, the distal shaft section 180 is
13 composed of stainless steel and the orifice has an I.D.
14 of .025 inches. The tubular extension 200 has an O.D. of
.012 inches and an I.D. of .007 inches. This embodiment
16 has the advantage of combining a relatively small distal
17 profile with a relatively large proximal flow channel.
18 Another advantage of this embodiment it that it provides
19 for a balanced airflow around the liquid orifice 204.
FIG. 16 shows yet another embodiment for a tip
21 for a nebulizing catheter. In FIG. 16, a nebulizing
22 catheter 208 has a coaxial configuration similar to the
23 embodiment of FIG. 10 (although it could also have a
24 configuration similar to that of other coaxial
embodiments, e.g. FIGS. 11, 14, or 15). In FIG. 16, a
26 thin solid wire or filament 212 is located at a distal
27 end of a liquid orifice 216 located at a distal end of an
28 inner tubular member 220. The tapered wire 212 extends a
29 short distance distally from the distal end of the inner
tubular member 220. The tapered wire 212 is located with
31 respect to the liquid orifice 212 so that liquid being
32 conveyed through the inner member 220 continues to flow
33 distally of the distal orifice 216 along the wire 212,
34 i.e. adhering to it by surface tension. Of course, once
the liquid reaches a distal tip 224 of the wire 212, it
36 is entrained and nebulized by the gas flow from the
CA 02596278 2007-08-21
-31-
1 annular region 228 defined between the inner tubular
2 member 220 and an outer tubular member 232. As mentioned
3 above, one of the factors that affects the nebulization
4 plume particle size and geometry is the size of the
distal liquid orifice. In general, a smaller liquid
6 orifice produces smaller particles and a narrow aerosol
7 plume cone. In the embodiment of FIG. 16, the thin wire
8 212 carries only a small amount of liquid along it so
9 that it functions similarly to an orifice of a very small
size. Accordingly, the embodiment of FIG. 16 has the
11 potential for producing an aerosol of very fine
12 particles. In the embodiment of FIG. 16, the outer
13 tubular member has an I.D. of approximately .020 inches.
14 The inner tubular member has an I.D. of approximately
.006 inches. The thin wire has an O.D. of approximately
16 .002 inches. The wire or filament 212 may be composed of
17 a metal wire or a polymer wire, such as a polyolefin
18 fiber like Spectra fiber. Alternatively, the filament
19 212 may be composed of a porous or felt-like material,
such as nylon or Porex, in which case it may be wider in
21 diameter than if made of a solid material.
22 FIG. 17 shows an alternative embodiment of the
23 embodiment of FIG. 16. In FIG. 17, there is a distal end
24 of a nebulizing catheter 236 having a tapered wire or
filament 240 located at the distal end of a lumen of an
26 inner tubular member 244. The tapered wire 240 in this
27 embodiment has a curved shape that is designed to whip in
28 a spiral when it is in a flow of air. In the embodiment
29 of FIG. 17, when pressurized gas flows through the
annular region 248, it causes the tapered wire 240 to
31 whip around with a spiral motion. The length of the wire
32 240 is chosen so that it does not impact the wall of the
33 trachea or other airway passage when it moves in a spiral
34 whipping motion. In one embodiment, the wire 240 has a
length of approximately 1 - 2 mm. The tapered wire 240
36 carries the liquid out to its tip for entrainment, and
CA 02596278 2007-08-21
-32-
1 the nebulization plume is formed with a conical shape.
2 The width of the plume may be changed by changing the
3 length of the filament 240. The speed of the spiral
4 motion can be controlled by appropriate selection of wire
stiffness and air foil shape. In general, the spiral
6 plume produced by the embodiment of FIG. 17 will be wider
7 than the embodiment of FIG. 16 and have less forward
8 velocity. Both these characteristics may be favored in a
9 nebulization catheter.
FIGS. 18 and 19 show another embodiment of the
11 nebulization catheter. In this embodiment, a
12 nebulization catheter 252 has a coaxial configuration
13 formed of an outer tubular member 256 and an inner
14 tubular member 260. A distal plug 264 fits into a distal
end of the annular region 268 forming the gas lumen. A
16 plurality of apertures 272 extend through the plug 264 to
17 form distal gas orifices. Located in a lumen 276 defined
18 by the inner tubular member 260 is a retractable wire or
19 pin 280. The wire 280 is preferably a solid wire of a
rigid material. For example, the wire may be composed of
21 a metal, such as stainless steel, a polymer, or a
22 radiopaque material. A distal end 284 of the inner
23 member 260 is tapered and may extend distally of the plug
24 264 or alternatively may extend only to the distal end of
the inner tubular member 260 or even proximally thereof.
26 The distal end of the inner member 260 terminates in a
27 distal liquid orifice 285. A distal end 286 of the wire
28 280 may also be tapered. The wire 280 is sized with
29 respect to the inner tubular member 260 so that the
tapered distal portion 286 of the wire 280 seats against
31 the tapered distal portion 284 of the inner tubular
32 member 260 and thereby seals a distal end of the liquid
33 lumen 276 in a manner similar to a needle valve. The
34 wire 280 is retractable and in a preferred embodiment is
operated to reciprocate back and forth to pulse the
36 delivery of liquid out the distal end of the nebulizing
CA 02596278 2007-08-21
-33-
1 catheter 252. The pulsing of aerosol delivery may be
2 adjusted to any suitable time period. In one preferred
3 mode of operation, the aerosol may be delivered only
4 during inhalation by the patient. If the nebulizing
catheter 252 is being used with an endotracheal tube and
6 a ventilator, the pulsing of the aerosol delivery may be
7 timed to coincide with the patient's inhalation by an
8 appropriate connection with the ventilator. By limiting
9 the delivery of medicine to only the period of time when
1D the patient is inhaling, the medicine can be delivered
11 more efficiently and with less waste.
12 One preferred way to generate the pulsed
13 aerosol plume with the embodiment of FIGS. 18 and 19 is
14 with a manifold arrangement 287. A proximal end of the
wire 280 is fixed to an extendable section 288 of the
16 manifold 287. The wire 280 may be fixed by means of an
17 elastomeric seal 289. Pressurized gas is delivered to a
18 port 290 of the manifold that communicates with the outer
19 tubular member 256 and liquid medicine to be nebulized is
delivered to a second port 291 that communicates with the
21 inner tubular member 260. The liquid medicine also fills
22 the volume 292 proximal of the port 291 in the expandable
23 section 288. The wire 280 is connected to the manifold
24 so that the distal end of the wire is biased against the
distal end of the inner tubular member by the resilience
26 of the inner tubular member 260 and/or the expandable
27 section 288. Pulsed pressurization of the liquid
28 medicine from the source causes the extendable section
29 288 to reciprocate back and forth as shown by the arrow
3D 293. Since the proximal end of the wire 280 is attached
31 to the expandable section 288 proximal of the port 291,
32 application of pressure pulses to the liquid causes the
33 proximal end of the wire 280 to reciprocate back and
34 forth as well. This causes the distal end of the wire
280 to reciprocate back and forth in the seat 284.
36 Application of pressure pulses to the liquid medicine can
37 be timed to coincide with the patient's inhalation.
CA 02596278 2007-08-21
-34-
1 Alternatively, instead of forming an expandable or
2 compressible section at the manifold, the shaft of the
3 inner tubular member 260 may be formed of a stretchable
4 material so that pressurization of the liquid causes
retraction of the wire as the entire shaft elongates.
6 Other alternatives for effecting reciprocating operation
7 of the wire 280 are use of an electro- mechanical,
8 mechanical, hydraulic, or pneumatic actuator to drive the
9 wire. Aside from providing for pulsed delivery of the
aerosol, this embodiment of the nebulization catheter has
11 the further advantage that the reciprocating action of
12 the wire may assist in keeping the orifice free of any
13 blockages which may occur, especially with some viscous
14 solutions or suspensions.
In a manner similar to the embodiments 208 and
16 236 of FIGS. 16 and 17, in the embodiment 252 of FIGS. 18
17 and 19, the distal tip of the retractable wire 292 can
18 extend distally from the distal liquid orifice 288 in
19 order to minimize particle size, or alternatively may not
extend distally of the distal liquid orifice 292. In one
21 embodiment, the distal tip of the retractable wire may
22 extend distally of the liquid orifice 288 by
23 approximately .2 mm.
24 FIG. 20 shows another embodiment of a
nebulizing catheter. In this embodiment, a nebulizing
26 catheter 296 has a main shaft portion 300 with a gas
27 lumen 304 adjacent to a liquid lumen 308. The gas and
28 liquid lumens 304 and 308 flow into a distal cavity 312.
29 The distal cavity 312 is formed by an outer tubular
extension 316 that extends distally over and past a
31 distal end 320 of the main shaft portion 300. A filter
32 324 is located in the liquid lumen 308 to filter out any
33 particles in the liquid. The liquid lumen 308 has a step
34 down in diameter immediately distal of the location of
the filter 324. An insert plug 328 is located in a
36 distal end of the outer tubular extension 316. The
CA 02596278 2007-08-21
-35-
1 insert plug 328 (which may be a sapphire jewel, for
2 example) has an aperture 332 through it that forms an
3 exit orifice from the cavity 312. The insert plug 328
4 has a conical shaped proximal profile facing the cavity
312. An inner tubular extension 336 fits into the
6 stepped down portion of the liquid lumen 308 and extends
7 the liquid lumen 308 into the cavity 312. A distal end
8 340 of the inner tubular extension 336 terminates in the
9 cavity 312. Since the gas lumen 304 exits into the
cavity 312, nebulization of the liquid takes place at the
11 tip of the inner tubular extension 336 inside the cavity
12 312. This region of the cavity 312 is a positive
13 pressure region due to the relative sizes and locations
14 of the apertures. The positive pressure in this region
may have the effect of reducing drooling of the liquid
16 medicine as it leaves the orifice of the tubular
17 extension 336. The aerosol exits the catheter 296 via
18 the aperture 332 and the aerosol plume is defined in part
19 by the positive pressure in the cavity 312 and the
aperture size. In this embodiment, the main shaft
21 portion and the tubular extension are composed of a
22 suitable plastic such as polyethylene. The filter is
23 composed of multiple 50 m I.D. tubes or similar course
24 filter material. The gas and liquid lumens may each have
an I.D. of .010 to .015 inches The inner tubular
26 extension 336 may be formed of polyimide tubing with an
27 I.D. of .004 inches and an O.D. of .010 inches. The
28 outer tubular extension 316 may be formed of a heat
29 shrunk tubing, such as polyethylene. The plug 328 may
have an O.D. .087 inches and the aperture 332 in the plug
31 328 may have a diameter of .007 inches.
32 FIG. 21 shows another embodiment of the
33 nebulizing catheter. This embodiment is similar to the
34 embodiment 296 shown in FIG. 20 and accordingly the
components are numbered similarly. The embodiment of
36 FIG. 21 differs from the embodiment of FIG. 20 in that
CA 02596278 2007-08-21
-36-
1 the distal end of the inner tubular extension 312 is
2 located in the aperture 332 of the insert plug 328. In
3 the embodiment of FIG. 21, the orifice 332 at the distal
4 end of the tubular extension 336 is in a low pressure,
high velocity region as compared to the embodiment of
6 FIG. 20. This has a corresponding effect on plume size
7 and shape as well as possible particle size.
8 FIG. 22 shows yet another embodiment for the
9 nebulizing catheter. In this embodiment, a nebulizing
catheter 340 has a main shaft portion 344 that has a gas
11 lumen 348 and a liquid lumen 352. The gas lumen 348
12 terminates distally in a gas orifice 356. Located in the
13 distal end of the liquid lumen 352 is a liquid tubular
14 extension 360. The liquid tubular extension 360 forms an
angle so that a distal liquid orifice 364 is in alignment
16 with the flow of gas out the distal gas orifice 356. In
17 this embodiment, the liquid lumen 352 has an I.D. in the
18 range of .010 to .020 inches. The gas lumen 348 has an
19 I.D. of approximately 0.10 to .020 inches. The liquid
tubular extension 360 is formed of a stainless steel tube
21 with an O.D. of .018 inches and an I.D. of .012 inches.
22 The distal gas orifice 356 has an I.D. of .010 inches.
23 The stainless steel extension tube 360 forms a right
24 angle so that the distal liquid orifice 364 is at a right
angle and aligned with the distal gas orifice 356. The
26 distal gas orifice 356 and the distal liquid orifice 364
27 are positioned as close together as possible, and in one
28 embodiment, these orifices are approximately .010 inches
29 apart.
FIG. 23 shows an alternative embodiment of the
31 nebulization catheter shown in FIG. 22. In this
32 embodiment, the nebulization catheter 340 has an
33 additional lumen 365. This additional lumen 365 may have
34 an I.D. of approximately .020 inches. This additional
lumen 365 may be used for an optical fiber viewing scope
CA 02596278 2007-08-21
-37-
1 366 for illumination and visualization of the distal end
2 of the nebulization catheter 340. The optical viewing
3 scope 366 may be permanently installed in the catheter
4 340 or preferably may be removable. A distal end 367 of
the lumen 365 is open or covered with a transparent lens
6 so that the area distal of the catheter 340 can be
7 observed via an optical viewing device connected to a
8 proximal end of the optical fiber 366. This enables a
9 physician to observe the alignment of the distal end of
the nebulization catheter and also observe the
11 nebulization when it occurs. The gas orifice 356 may be
12 located so that the pressurized gas that is expelled
13 helps to keep the distal end of the viewing lumen 365
14 clear. An optical fiber viewing channel may be
incorporated into any of the embodiments of the
16 nebulization catheter disclosed herein. When the
17 additional lumen 365 is occupied by a removable viewing
18 scope, it may be used for other purposes such as pressure
19 sensing, gas sampling, over pressure relief, or other
diagnostic or therapeutic purposes. Alternatively,
21 another lumen may be provided for these purposes.
22 The embodiment of FIG. 23 also shows opposing
23 orifices. As in the embodiment of FIG. 22, a tubular
24 extension 360 extends distally of the end of the catheter
shaft and is oriented at an angle, e.g. 90 degrees, to
26 the direction of the axis if the catheter shaft. The
27 tubular extension 360 opens to a distal liquid orifice
28 364 from which the liquid being conveyed in the lumen 352
29 exits. In this embodiment, a second tubular extension
363 communicates with the gas lumen 348 and opens to a
31 distal gas orifice 367. The second tubular extension 363
32 is also oriented relative to the axis of the catheter
33 shaft, e.g. by 90 degrees, so that is aimed toward the
34 distal liquid orifice 364 in order to nebulize the liquid
exiting from the liquid orifice 367.
CA 02596278 2007-08-21
-38-
1 FIG. 24 shows still another embodiment of the
2 nebulizing catheter. In this embodiment, a nebulizing
3 catheter 368 has a main shaft section 372 with a gas
4 lumen 376 and a liquid lumen 380. Tubular extensions 384
and 388 extend the gas and liquid lumens 376 and 380 from
6 the main shaft section 372 to a distal tip of the
7 catheter 368. The distal portion of the shaft forms a
8 tapered region 392 that surrounds the tubular extensions
9 384 and 388 and causes them to be angled toward each
other. The tubular extension 388 for the liquid lumen
11 380 extends slightly distally of the distal end of the
12 tubular extension 384 of the gas lumen 376 so that a
13 distal liquid orifice 396 is in alignment with the flow
14 of gas from a distal gas orifice 400. In this
embodiment, the distal liquid orifice 396 has an O.D. of
16 150 microns and an I.D. of 20 microns. The gas orifice
17 400 has an I.D. of approximately .018 inches.
18 FIGS. 25 and 26 show an alternative embodiment
19 of the nebulizing catheter 368 shown in FIG. 24. In
FIGS. 25 and 26, the tubular extensions 384 and 388 of
21 the gas lumen 376 and the liquid lumen 380 are formed
22 with sealable tips. Specifically, the gas tubular
23 extension 384 has a sealable tip 408 and the liquid
24 tubular extension 388 has a sealable tip 412.
Alternatively, only the liquid lumen 380 has the sealed
26 tip 412 and the gas lumen 376 has an open distal orifice.
27 The sealable tips may be formed by heating the material
28 from which the tubular extensions are made to reform the
29 walls of the plastic material so as to form a closed
slit. This is represented in FIG. 26. When pressurized
31 gas and liquid are conveyed through the lumens 376 and
32 380, the slits forming the tips 408 and 412 dilate
33 thereby permitting the gas and liquid to exit to from the
34 aerosol, as illustrated in FIG. 25. However, when the
pressure in the lumens 376 and 380 falls below a
36 threshold, the tips 408 and 412 close thereby sealing off
CA 02596278 2007-08-21
-39-
1 the lumens, as illustrated in FIG. 26. The embodiment
2 404 of the nebulizing catheter is used with pulsation of
3 the gas and/or liquid supplies. In order to pulse the
4 generation of aerosol to coincide with a patient's
inhalation, the pressure to the gas and/or the liquid
6 lumens can also be pulsed. When the pressure in either
7 of the lumens falls below a threshold, the tips 408 or
8 412 close. By closing off the flow of liquid at the tip
9 412 during the period when the aerosol is not being
generated, it is possible to reduce any drooling from the
11 tip of the catheter.
12 III. Nebulization With Counterflow
13 As mentioned above, control of nebulized
14 particle size and plume shape are important
considerations affecting the efficacy of the therapy. In
16 many applications, it is preferable to have as small a
17 particle size as possible combined with as little forward
18 velocity as possible. Some of the embodiments described
19 below accomplish these objectives through use of
counterflow arrangements.
21 FIG. 27 shows a nebulization catheter 416 that
22 can be located inside of an endotracheal tube as in the
23 previously described embodiments. The nebulization
24 catheter 416 has a coaxial tubular arrangement with an
outer tube 417 surrounding an inner tube 418 so that a
26 liquid delivered from a distal liquid orifice 419 of the
27 inner tube 418 is nebulized by the flow of a pressurized
28 gas delivered in a distal direction from the annular
29 region between the inner and outer tubes at the distal
orifice 420 of the outer tube 417. In addition, another
31 lumen 428 extends through the shaft of the nebulization
32 catheter 416. This additional lumen 428 connects to a
33 distal tubular extension 432. The tubular extension 432
34 extends distally of the distal end of the nebulization
catheter 416. A distal end 436 of the distal tubular
36 extension 432 curves back on itself so that a distal
CA 02596278 2007-08-21
-40-
1 orifice 440 of the tubular extension 432 is oriented in a
2 proximal direction back at the orifices 419 and 420 of
3 the inner and outer tubes. The additional lumen 428 also
4 carries a pressurized gas which is directed in a proximal
direction by the orifice 440 against the direction of the
6 aerosol plume generated by the gas and liquid exiting the
7 orifices 419 and 420. The gas from the additional lumen
8 428 presents a counterflow to the gas from these orifices
9 thereby slowing down the velocity of the particles
generated from these orifices. In a preferred
11 embodiment, the distal tubular extension 432 may be
12 formed of a suitable material such as stainless steel
13 needle stock. The O.D. of the nebulization catheter in
14 this embodiment may be similar to the other nebulization
catheter embodiments described above, e.g. O.D of
16 approximately .038 inches. The distal tubular extension
17 432 may have an O.D. of approximately .013 inches and an
18 I.D. of approximately .009 inches. In this embodiment,
19 the outer tubular member of the nebulization catheter may
have an O.D. of approximately .013 inches and an I.D. of
21 approximately .009 inches and the inner tubular member
22 may have an O.D. of approximately .003 inches and an I.D.
23 of approximately .0015 inches.
24 FIG. 28 shows another embodiment of the present
invention for a nebulizing catheter 448 that incorporates
26 a counterflow arrangement. Like the embodiments
27 described above, in this embodiment the nebulizing
28 catheter 448 may be located in an endotracheal tube (not
29 shown). The nebulization catheter 448 has a distal
section 452 that curves back on itself. The nebulization
31 catheter 448 has distal orifices 453 and 454 that
32 generate a plume of nebulized particles in a reverse,
33 i.e. proximal, direction. Also located in the
34 nebulization catheter 448 is another lumen 456 for
carrying a pressurized gas. The additional lumen 456 has
36 a distal orifice 460 oriented in a distal direction. The
CA 02596278 2007-08-21
-41-
1 distal orifice 460 of the additional lumen 456 is aligned
2 with respect to the distal orifices 452 and 453 of the
3 nebulization catheter 448 so that the flow of gas from
4 the additional lumen 456 slows down the velocity of the
nebulization plume generated from the nebulization
6 catheter 448. The aerosol plume generated by the
7 nebulization catheter reverses direction and is delivered
8 to the lungs carried by the inhalation of air through the
9 endotracheal tube or by the flow of gas from the
additional lumen 456 or a combination thereof.
11 FIGS. 29 and 30 show another embodiment of a
12 counterflow nebulization catheter arrangement. In FIGS.
13 29 and 30, a nebulizing catheter 464 is used with an
14 endotracheal tube 468. A nebulization catheter 464 has a
distal tip 472 from which a liquid medicine delivered
16 from a distal liquid orifice is nebulized by a flow of
17 pressurized gas from a gas orifice located adjacent to
18 the liquid orifice. The nebulizing catheter 464 shown in
19 FIG. 30 extends distally of the endotracheal tube 468 and
has a distal section 476 that curves back on itself. The
21 nebulization catheter 464 has distal orifices that
22 generate a plume of nebulized particles in a reverse,
23 i.e. proximal, direction back toward the distal opening
24 of the endotracheal tube 464. In order to maintain a
proper reverse orientation and to prevent snagging, the
26 nebulization catheter 464 includes a wire 480 that
27 extends from the tip 472 of the nebulization catheter
28 464. The wire 480 is secured to a portion of the shaft
29 of the nebulization catheter proximal of the tip. The
wire 480 can be secured by means of a heat shrunk tube
31 484 located on a shaft 488 of the catheter to hold the
32 end of the wire 480. Although some aerosol may impact
33 the wire 480, a wire having a small diameter is used to
34 minimize losses due to such impaction. Moreover, the
overall improved efficiency due to reduction in aerosol
36 impaction on the walls of the trachea or other airway
CA 02596278 2007-08-21
-42-
1 passage is expected to more than compensate for any
2 losses due to impaction on the wire 488.
3 In the embodiment shown in FIG. 30, the
4 nebulization catheter 464 directs a nebulization plume in
a reverse direction back toward the distal opening of the
6 endotracheal tube 468. The nebulization plume from the
7 nebulization catheter encounters the flow of air from the
8 endotracheal tube 468 during the inhalation phase of the
9 patient. The inhalation of air through the endotracheal
tube 468 causes the nebulized medicine to reverse
11 direction and carries it to the lungs. It is noted that
12 the reversal of direction of the nebulization plume has
13 the effect of minimizing the aerosol particle velocity.
14 It is also noted in the embodiment shown in FIG. 30 that
the endotracheal tube 468 is provided with an inflatable
16 cuff 492 located around the distal portion.
17 IV. Other Nebulization Catheter Embodiments
18 In the embodiments described above, the
19 velocity of the nebulization plume was reduced by use of
a counterflow of gas in an opposite direction. In the
21 embodiment of FIGS. 31 and 32, the velocity of the
22 nebulization particles is reduced in another manner. In
23 FIG. 31 a nebulization catheter 496 has a liquid lumen
24 500 terminating in a distal liquid orifice 504 and a one
or more gas lumens 508 terminating in one or more distal
26 gas orifices 512. The liquid delivered through the
27 liquid lumen 500 is nebulized by the pressurized gas
28 flowing out the plurality of gas orifices 512. The
29 nebulization catheter 496 also includes one or more
additional lumens 516 that terminate in additional distal
31 orifices 520. These lumens 516 are used to deliver a
32 vacuum (negative pressure) at the distal orifices 520.
33 The vacuum is provided by a suitable vacuum source (not
34 shown) connected to proximal ends of the additional
lumens 516. The vacuum delivered by the additional
36 lumens 516 helps withdraw the pressurized gas delivered
CA 02596278 2007-08-21
-43-
1 by the lumen 508 after it has nebulized the liquid
2 delivered by the liquid lumen 500. Without the vacuum
3 provided by the additional lumens 516, the pressurized
4 gas delivered by the distal gas orifices 512 may continue
to impart energy to the nebulized liquid particles
6 delivered by the distal liquid orifice 504 thereby
7 causing them to be propelled with a forward velocity.
8 Instead, the vacuum scavenges at least some of the
9 pressurized gas after it has nebulized the liquid so that
the forward velocity of the liquid particles can be
11 reduced. in order to facilitate scavenging of the
12 pressurized gas, the distal liquid orifice 504, the
13 distal gas orifices 512, and the distal vacuum orifices
14 520 all open into a distal cavity 524 formed by an outer
tubular extension 528 of the nebulizing catheter 496.
16 The distal extension 528 has a closed distal end 532 with
17 a small aperture 536 located therein to emit the
18 nebulized liquid particles with a low forward velocity.
19 With the nebulizing gas removed, the aerosol particles
are carried forward primarily only by their inertia.
21 The embodiment of the nebulization catheter 496
22 shown in FIG. 31 includes a vacuum line 516 as a means to
23 reduce the forward velocity of the nebulization plume.
24 Provision of vacuum line 516 to the tip of a nebulization
catheter 496 can serve an additional function of
26 balancing the gas flow and pressure delivered to the
27 airway in which the nebulization catheter is located.
28 This may be useful to prevent excess airway pressure
29 generated by the catheter flow particularly in smaller
airways or where a neutral flow balance may be desired.
31 This may particularly be desired when the nebulization
32 catheter is provided with an inflatable cuff that
33 occludes the airway passage at the distal end of the
34 nebulization catheter. The flow balance may be
controlled with a closed or partially closed pumping
36 system where a gas pump 537 with a single intake and
CA 02596278 2007-08-21
-44-
1 outlet would be connected to the respective vacuum and
2 gas supply lumens 516 and 508 of the catheter. Both the
3 driving gas and vacuum would be balanced and regulated by
4 the pump speed. A vacuum or pressure vent port 538 could
be incorporated into the respective vacuum or pressure
6 lines if a positive or negative flow balance was desired.
7 If flow balance is a concern, but not velocity reduction,
8 it is not important where the air flow is removed at the
9 distal tip of the catheter and accordingly, the distal
end extension 532 may not be needed. Alternatively, a
11 flow balance may be maintained with separate a pressure
12 and vacuum source through the use of regulators,
13 restrictive capillary tubes or orifices, or flow sensors
14 and flow control valves incorporated into the pressure
and vacuum supply lines.
16 FIG. 33 shows another embodiment of a
17 nebulization catheter 540 that incorporates a feature to
18 reduce the forward velocity of the nebulized liquid
19 particles. The nebulization catheter 540 has a main
shaft portion 544 having a liquid lumen 548 and a
21 pressurized gas lumen 552. The lumens 548 and 552
22 terminate in distal orifices 556 and 560. The
23 pressurized gas flow from the orifice 560 nebulizes the
24 liquid exiting from the orifice 556. The nebulization
catheter 540 includes a distal spacer tube 564. The
26 spacer tube 564 has a length of approximately 2-3 mm and
27 an inside diameter larger than the outside diameter of
28 the nebulization catheter shaft 544. Because the inside
29 diameter of the spacer tube 564 is larger than the
airflow lumen and orifice, the velocity of air and
31 entrained particles is reduced as they pass through the
32 spacer tube 564 and out a distal opening 568 thereof.
33 In addition, the spacer tube 564 may have one or more
34 apertures or holes 572 through a wall thereof close to
the proximal end of the spacer tube at its connection to
36 the main shaft 544. These holes 572 draw in air to the
CA 02596278 2007-08-21
-45-
1 inside of the spacer tube 564 thereby causing drag due to
2 turbulence and reducing the velocity of the aerosol as it
3 exits the spacer tube. The holes 572 may also slow the
4 flow of particles through the spacer tube by causing drag
turbulence.
6 The spacer tube 564 also serves to protect the
7 distal orifices 556 and 560 of the nebulization catheter
8 from coming into contact with any part of the
9 endotracheal tube, trachea, or other airway passage
thereby helping to maintain optimum tip operation and to
11 prevent damage to it during handling and insertion. In
12 an alternative embodiment, if only the tip protection
13 feature is desired, the spacer tube 564 of FIG. 33 may be
14 provided without the apertures 572. In such an
alternative embodiment, the spacer tube 564 may be
16 provided in a shorter length, e.g. 1 mm.
17 FIG. 34 shows another embodiment of a
18 nebulization catheter 576 used with an endotracheal tube
19 580. The endotracheal tube 580 may be a conventional
endotracheal tube. The nebulization catheter 576
21 provides for a nebulization plume with.a reduced forward
22 velocity by imparting a spiral component to the liquid
23 particle flow. The nebulization catheter 576 has a
24 distal tip 584 from which a liquid medicine delivered
from a distal liquid orifice is nebulized by a flow of
26 pressurized gas from a gas orifice located adjacent to
27 the liquid orifice. The nebulization catheter 576 is
28 positioned coaxially in the endotracheal tube 580. A
29 centering device 585 may be used to aid in centering the
nebulization catheter 576. Located along a portion of
31 the nebulization catheter 576 proximal from the tip 584
32 is a second gas orifice 588. This second gas orifice 588
33 may open to the same gas lumen that communicates with the
34 nebulizing gas orifice at the distal tip 584 or
alternatively, the second gas orifice 588 may connect to
36 another, separate gas lumen. The second gas orifice 588
CA 02596278 2007-08-21
-46-
1 is oriented to direct a pressurized flow of gas in a
2 spiral, distal direction along the distal end of the
3 nebulization catheter 576. To accomplish this, the
4 second gas orifice 588 may be formed by an inclined
opening or with a deflection foil to direct the flow of
6 gas in the appropriate spiral direction. The spiral flow
7 of pressurized gas travels along the distal portion of
8 the nebulizing catheter 576 inside the endotracheal tube
9 580. The spiral flow of gas entrains the aerosol
generated from the distal end 584 of the nebulizing
11 catheter imparting a spiral flow component to the aerosol
12 plume. This has the effect of reducing the forward
13 velocity component of the liquid particle flow as it
14 leaves the endotracheal tube 580.
FIG. 35 shows an alternative method for using
16 the nebulization catheter 576 of FIG. 34. In FIG. 35,
17 the nebulization catheter 576 is shown extended distally
18 of the distal end of the endotracheal tube 580 so that
19 the distal portion of the nebulization catheter 576
including the second gas orifice 588 is located in an
21 airway passage. Taking into account the size of the
22 airway passage, the nebulization catheter 576 with the
23 second gas orifice 588 would operate similarly to the
24 method shown in FIG. 34 and generate a spiral gas flow to
reduce the forward velocity of the aerosol plume.
26 Another embodiment of a nebulizing catheter 592
27 is shown in FIGS. 36 and 37. This embodiment of the
28 nebulizing catheter 592 can be used with a separate
29 endotracheal tube (not shown). The nebulizing catheter
592 includes a main shaft 596 having a central lumen 600
31 and one or more additional lumens 604 located around the
32 central lumen 600. In this embodiment, the central lumen
33 600 is used for the flow of a pressurized gas and the
34 additional peripheral lumens 604 are used for the
delivery of the liquid medicine. The lumens 600 and 604
CA 02596278 2007-08-21
-47-
1 terminate distally in orifices 608 and 612, respectively.
2 Located at a distal end of the nebulizing catheter 592
3 and immediately adjacent the orifices 608 and 612 is a
4 diffuser 616. In one embodiment, the diffuser 616 is
composed of a generally disk-shaped body that is sized to
6 deflect the flow of gas from the orifice 608 of the
7 central lumen 600 past the liquid orifices 612 thereby
8 nebulizing the liquid medicine. A small gap (or venturi
9 area) 620 between the diffuser 616 and the distal end of
the main shaft section 596 of the catheter 92 provides
11 favorable flow characteristics for generating the
12 aerosol. The diffuser 616 may be connected to a
13 retaining wire 624 that is located in the central lumen
14 600. The retaining wire 624 may be used to secure the
diffuser 616 to the distal end of the nebulizing catheter
16 592. Also, the retaining wire 624 may be used to pulse
17 the generation of aerosol by reciprocation of the
18 diffuser. It is noted that the aerosol produced by this
19 embodiment has a substantially radial velocity component
and may have only a small forward velocity component. In
21 addition, a centering device, such as wings 625, may be
22 attached to the diffuser 616.
23 FIG. 38 shows an alternative embodiment of the
24 diffuser 616. In FIG. 38, the diffuser 616 is formed of
a loop that has its ends located in two apertures in the
26 nebulization catheter shaft tip and a middle portion
27 directly in front of the distal gas orifice 608. The
28 loop may be formed of a metal or polymer wire or other
29 material. The loop could be formed by an extrusion
method or molded.
31 Referring to FIG. 39, there is an alternative
32 embodiment of the nebulization catheter system. A
33 nebulization catheter 627 is located in an endotracheal
34 tube 628. The nebulization catheter 627 includes a
coaxially arranged outer tube 629, a middle tube 630, and
CA 02596278 2007-08-21
-48-
1 an inner tube 631. Liquid delivered through a lumen of
2 the inner tube 631 is nebulized by pressurized gas
3 delivered in the annular region 632 between the inner
4 tube 631 and the middle tube 630. In addition,
pressurized gas is also delivered from a secondary gas
6 supply that communicates with the annular region 633
7 between the middle tube 630 and the outer tube 629. The
8 secondary gas supply may be used to help provide the
9 desired plume shape and velocity. For example, the
secondary gas supply delivered from the outer tube 629
11 can be used to provide a coaxial sheath of air that helps
12 minimize impaction of the nebulized aerosol on the walls
13 of the trachea or other airway passage. Alternatively,
14 the secondary air supply may be used to impart additional
forward velocity to the aerosol plume. With the
16 embodiment of FIG. 39, the additional air flow can be
17 provided by the secondary gas supply via region 633.
18 In the embodiments discussed above,
19 nebulization is provided at a distal tip of a catheter by
directing a pressurized gas from a distal orifice across
21 another distal orifice from which the liquid medicine is
22 delivered. As shown in several of the embodiments above,
23 one way to deliver the liquid from the distal orifice is
24 via a lumen that extends through the catheter to a
proximal end. This construction provides efficient
26 operation for many types of medication delivery. In many
27 cases, the distal liquid medicine orifice is subject to a
28 negative pressure due to the pressurized gas flow across
29 it. This negative pressure may in many applications be
sufficient to draw the liquid out of the orifice in order
31 to nebulize it. If pulsing of the aerosol is desired,
32 the pressure of the gas lumen can be pulsed thereby
33 resulting in pulsed generation of the aerosol. By
34 increasing the gas pressure, it may be possible to also
increase the aerosol output.
CA 02596278 2007-08-21
-49-
1 In other situations, it may be preferable to
2 apply a positive pressure to the liquid, such as at the
3 proximal end of the liquid lumen, in order to deliver
4 liquid from the distal liquid orifice, it is necessary.
This positive pressure applied to the liquid lumen may be
6 the same as that applied to the gas lumen (e.g. 35-50
7 psi) or alternatively may be different (less than the gas
8 lumen). If it is desired to pulse the nebulization of
9 the liquid, this can be accomplished by applying pulses
of pressure to the column of liquid via the proximal end
11 of the liquid lumen or reservoir. It may also be
12 preferred to synchronize the pressurization of the gas in
13 the gas lumen with the pressurization of the liquid
14 lumen. In addition to applying the positive pressure to
the liquid lumen in pulses to generate a pulsed aerosol
16 from the distal orifice, if it may be preferred in an
17 alternative embodiment to apply a small negative pressure
18 immediately after each positive pressure pulse in order
19 to draw the liquid at the distal orifice back into the
liquid lumen to thereby avoid drooling. In a preferred
21 embodiment, the portion of the nebulization catheter in
22 which the liquid lumen is formed may be composed of a
23 relatively low compliance material to transmit pressure
24 pulses to the distal end with minimum attenuation.
A full length liquid lumen may have
26 disadvantages in certain situations. For example,
27 pulsing of the liquid from the distal orifice may not
28 correspond to or follow closely with the application of
29 pressure to the proximal end due to attenuation of the
pressure pulse over the length of the catheter. In
31 addition, applying pressure to the proximal end of the
32 liquid lumen in order to transmit pressure to discharge
33 the liquid from a distal orifice requires that the lumen
34 be filled with the liquid. In some situations, this is
more medicine than would be required by the patient and
36 might result in waste.
CA 02596278 2007-08-21
-50-
1 The embodiment in FIG. 40 addresses these
2 concerns by controlling the pressurization of the liquid
3 as close as possible to the distal liquid orifice,
4 thereby reducing the effects of catheter compliance and
attenuation. In FIG. 40, a nebulization catheter 652 has
6 a main body 656 having a gas lumen 660 that extends from
7 a proximal end (not shown) to a distal gas orifice 664.
8 The main body 656 also includes a distal liquid medicine
9 reservoir 668. In the embodiment shown in FIG. 40, the
liquid reservoir 668 is located in a distal portion of
11 the main shaft 656 of the catheter 652. The liquid
12 reservoir 668 is preferably close to the distal tip of
13 the nebulizing catheter 652. The liquid reservoir 668 is
14 filled with the medicine to be delivered. I the amount
of medicine is small in volume, the liquid reservoir may
16 also be correspondingly small. This embodiment is
17 especially suitable for the delivery of small volumes of
18 medicine such as .1 to .5 ml, e.g. single use. The
19 reservoir 668 may be pre-filled during the manufacturing
stage of the catheter. The reservoir 668 may be formed
21 by plugging a lumen of the catheter at a distal location.
22 Alternatively, the liquid reservoir 668 may also extend
23 back to the proximal end of the catheter, thereby forming
24 a liquid lumen, and communicate with a proximal port as
described with respect to the other embodiments discussed
26 herein. This may be required if the lumen is made of a
27 non-compliant material. In yet another alternative
28 embodiment, the liquid reservoir may be formed in a
29 balloon located externally of the catheter shaft 656.
A filter 672 and plug 676 occupy positions in
31 the distal end of the liquid lumen/reservoir 668. A
32 distal tubular extension 680 extends from the plug 676
33 and communicates with the liquid lumen/reservoir 668.
34 The tubular extension 680 has a distal orifice 684
aligned with the distal gas orifice 664 so that a
36 pressurized gas exiting the gas orifice 664 nebulizes the
37 liquid exiting the liquid orifice 684. The distal liquid
CA 02596278 2007-08-21
-51-
1 orifice may have a sealable cap or wax-like covering
2 associated therewith that can be opened when the
3 nebulization catheter is put into use. In a distal
4 section of the main shaft 656 of the catheter 652, the
gas lumen 660 and the liquid lumen 668 are separated by a
6 flexible, distendable wall or membrane 688. In the
7 embodiment of FIG. 40, pulsing of the aerosol is
8 accomplished by pulsing of the gas pressure in the gas
9 lumen 660. When the pressure in the gas lumen 660 is
high, it causes the flexible wall 688 between the gas and
11 liquids lumens 660 and 668 to distend into the liquid
12 lumen 668. This is represented by the dashed line in
13 FIG. 40. When this occurs, the pressure from the gas
14 lumen 660 is transmitted to the liquid lumen 668 and
liquid medicine is forced out the distal liquid orifice
16 684. When the pressure applied to the gas lumen 660 is
17 low, the distendable wall 688 recovers its original
18 position. It is noted that when the distendable wall 680
19 recovers its original position, it may cause a negative
pressure at the distal liquid orifice 684 which may cause
21 the liquid to withdraw slightly into the tubular
22 extension 680 thereby reducing the occurrence of liquid
23 drooling at the tip. In addition, it is noted that the
24 delivery of liquid from the distal liquid orifice 680 may
not occur immediately upon application of a high gas
26 pressure to the gas orifice since it will take some time
27 for the bladder 688 to distend. This means that gas will
28 be flowing steadily at a high pressure from the distal
29 gas orifice when the liquid begins to flow from the
distal liquid orifice. This also may provide cleaner
31 aerosol delivery and reduce the occurrence of drooling of
32 liquid at the tip.
33 An alternative embodiment of the nebulization
34 catheter 652 shown in FIG. 40 may be made using a
flexible, but inelastic material for the bladder wall
36 688. If the bladder wall 688 were flexible, but
37 inelastic, the pressurized gas passing past the liquid
CA 02596278 2007-08-21
-52-
1 orifice 684 would create a negative pressure (venturi
2 effect) thereby drawing out the liquid and nebulizing it.
3 A continuous or preferably an intermittent gas supply to
4 the venturi area would provide this negative pressure.
The bladder wall may be provided with a vent to
6 facilitate discharge.
7 In order to manufacture a nebulization catheter
8 with compliant and non-compliant regions, as described
9 above, the catheter may be co-extruded using different
compounds or polymers to optimize the physical properties
11 of the different wall sections. It may be preferred to
12 use high energy radiation to crosslink the polymer
13 material in the formation of the bladder wall.
14 V. Alignment of the Aerosol Plume
The embodiments described above are directed to
16 developing an optimum nebulization plume. It is further
17 recognized that another factor that contributes to the
18 efficiency of the nebulization is the position of the
19 nebulization catheter relative to the anatomical
environment. For example, even if the nebulization
21 catheter being used develops an optimal plume, the
22 delivery efficiency of the catheter may be significantly
23 impaired if the plume is directed into the wall of the
24 endotracheal tube, the trachea or other airway passage.
Accordingly, proper location, orientation, and alignment
26 of the nebulization catheter in the anatomy can be an
27 important factor contributing the delivery of medicine
28 via a nebulization catheter. In general, it is
29 preferable to align the catheter coaxially in the airway
passage in which it is located.
31 It is also noted that an endotracheal tube, if
32 present, can adversely effect delivery of aerosol from a
33 separate nebulization catheter. For example, an
34 endotracheal tube has an inner diameter that is smaller
than the diameter of the trachea so that if the
36 nebulization takes place inside the endotracheal tube, a
CA 02596278 2007-08-21
-53-
1 portion of the aerosol may impact the inner wall of the
2 endotracheal tube and thereby be wasted. Most
3 conventional endotracheal tubes have a curved distal end
4 that is relatively rigid so that when it is in place in
the trachea of a patient, the distal end of the
6 endotracheal tube is oriented off center. This can
7 affect the orientation of a nebulization catheter located
8 in the endotracheal tube causing it direct its aerosol
9 into the trachea wall even if the nebulization catheter
is positioned so that its distal end is located distally
11 of the endotracheal tube. In general, it is desirable to
12 allow the aerosol particles to avoid impaction for
13 several centimeters after the aerosol is produced so that
14 the aerosol particles can lose their velocity and become
entrained in the inspiratory airflow.
16 The embodiment of the invention in FIG. 41 is
17 directed at providing improved alignment of a
18 nebulization catheter in a patient's trachea. In FIG.
19 41, an endotracheal tube 700 is positioned in a trachea
704 of a patient. The endotracheal tube 700 is of a type
21 that has an inflatable cuff 708 located around a distal
22 exterior side to facilitate positioning and alignment of
23 the endotracheal tube 700 in the trachea 696. Extending
24 through and out of a distal end of the endotracheal tube
700 is a nebulization catheter 712. The nebulization
26 catheter 712 may be similar to any of the embodiments of
27 the nebulization catheter described above. Located
28 around a distal portion 716 of the nebulization catheter
29 712 is a spring centering apparatus 720. The spring
centering apparatus 720 includes a retainer ring 724
31 fixed to the shaft of the nebulization catheter 712 and a
32 plurality of arms 728 connected to the ring 724. In one
33 embodiment, there are three arms 726. The arms 726 are
34 flexible and resilient. The arms 726 may be made of a
spring tempered metal or a suitable plastic. Located at
36 the end of each of the arms 726 opposite its connection
37 to the ring 724 is a ball 727. The spring centering
CA 02596278 2007-08-21
-54-
1 apparatus 720 is deployed by first positioning the
2 nebulizing catheter 712 including the spring centering
3 apparatus in the lumen 728 of the endotracheal tube 700.
4 The arms 726 are formed so that they assume a size larger
than the diameter of the trachea or airway passage.
6 Accordingly, when the centering device is positioned in
7 the endotracheal tube 700, the arms are resiliently
8 deformed into a compressed configuration with the balls
9 727 close to the shaft of the nebulizing catheter 712.
To deploy the centering device, the nebulizing catheter
11 712 is advanced out the distal end of the endotracheal
12 tube 700. When the balls 727 are advanced out the
13 endotracheal tube 700, they spring out to an expanded
14 size and engage the walls of the trachea or other airway
passage. The balls 727 provide a relatively smooth
16 surface to limit irritation or injury to the trachea
17 walls or other airway passage. With the arms expanded,
18 the nebulizing catheter is centered in the trachea or
19 other airway passage so that a plume discharged from a
distal end of the nebulizing catheter has minimal contact
21 with the walls of the trachea or other airway passage.
22 When it is necessary to remove the nebulizing catheter
23 712, it can be withdrawn in a proximal direction back
24 into the endotracheal tube 700. In a preferred
embodiment, the arms are formed of a thin resilient wire
26 or polymer, preferably less than approximately .015
27 inches in diameter. The arms and/or the balls may be
28 made of, or coated with, a radiopaque material. It is an
29 advantage of the embodiment of the centering device shown
in FIG. 41 that it is located somewhat in advance of the
31 distal end of the nebulization catheter. This positions
32 the arms 726 of the centering device in the portion of
33 the trachea or other airway passage into which the
34 aerosol will be initially flowing. Thus, the centering
device orients the distal tip of the nebulization
36 catheter relative to the portion of the trachea or other
CA 02596278 2007-08-21
-55-
1 airway passage beyond the distal tip thereby helping to
2 reduce impaction along this portion.
3 FIG. 42 shows an alternative embodiment of the
4 nebulization catheter. A nebulization catheter 729 is
used with an endotracheal tube as described above. The
6 nebulization catheter 729 includes a centering device
7 730. The centering device 730 includes a plurality of
8 arms 731 that are formed to resiliently extend outward
9 from the axis of the catheter shaft to engage the wall of
the patient's trachea or airway passage or the interior
11 of an endotracheal tube depending upon the desired
12 location of the distal end of the nebulization catheter.
13 At the ends of each of the arms 731 are balls 732. The
14 proximal ends of the arms 731 are formed of wires 733
that extend through lumens 734 in the shaft of the
16 catheter 729. Each of the lumens 734 has a distal
17 opening 735 from which an arm can extend. The distal
18 openings are approximately .10 - 1 cm from the distal end
19 of the catheter shaft. The proximal ends of the wires
733 exit the lumens 734 of the nebulization catheter via
21 openings 736 that are close to the proximal end of the
22 catheter in a portion of the catheter that would normally
23 be outside the patient's body during use. Thus, the
24 proximal ends of the wires 733 are accessible to the
physician during use. By pulling and pushing on the
26 proximal ends of the wires 733, the portion of the arms
27 731 that extend from the openings 735 can be adjusted.
28 Thus, the arms 731 can be adjusted from a fully retracted
29 to a fully advanced position by pulling or pushing on the
proximal ends of the wires 733. In addition, since the
31 proximal ends can of the wires 733 be adjusted in any
32 intermediate position between the fully retracted and
33 fully advanced positions, the physician can adjust the
34 size of the centering device 730 to any appropriate size,
as desired. Because the wires 733 should assume a
36 desired shape when advanced out of the lumens in which
37 they are contained during positioning, it is preferable
CA 02596278 2007-08-21
-56-
1 that they be formed of a material that has shape memory
2 properties so that the desired expanded shape can be
3 imparted to the wires during manufacture. In one
4 embodiment, the wires may be formed of nitinol.
In one preferred embodiment, a second centering
6 device 737 is also provided. The second centering device
7 737 is located on the shaft of the nebulization catheter
8 729 proximally from the first centering device 730. The
9 second centering device 737 may be formed of resilient
wings formed of a material such as plastic or metal that
11 extend radially outward from the shaft. The second (or
12 proximal) centering device 737 helps keep the distal
13 portion of the catheter 729 in alignment.
14 FIG. 43 shows another alternative embodiment of
the present invention. A nebulizing catheter 738 is
16 shown which may be similar to the catheter 20 of FIG. 1.
17 The nebulizing catheter 738 includes a centering device
18 739. The centering device 739 includes a wire loop 740
19 located at a distal end of the catheter. One end 741 of
the loop 740 connects to the distal end of the nebulizing
21 catheter shaft. The other end 742 of the wire loop 740
22 enters an opening 743 in the shaft that communicates with
23 a lumen 744 that extends to a proximal end of the
24 catheter 738. A proximal end 745 of the wire exits the
lumen 744 via an opening 746 in a proximal portion of the
26 nebulizing catheter which is normally outside the
27 patient's body during use. The size of the wire loop 740
28 can be adjusted by advancing or withdrawing the proximal
29 end 745 of the wire. In this embodiment, it can be
determined that the centering device is fully retracted
31 when the wire 745 cannot be withdrawn any further. The
32 position of the distal end of the nebulization catheter
33 can also be determined by the resistance to further
34 retraction caused when the loops or arms engage the
distal end of the endotracheal tube. When in an expanded
36 size, the wire loop 740 engages the walls of the trachea
CA 02596278 2007-08-21
-57-
1 or airway passage or the interior of the endotracheal
2 tube depending upon where the distal end of the
3 nebulizing catheter is positioned. The size of the wire
4 loop 740 can be adjusted from a fully reduced size to a
fully expanded size as well as intermediate sizes. With
6 the embodiment of FIG. 43, the size of the loop can be
7 adjusted to different size airway passages in different
8 patients or alternatively the size of the loops can be
9 adjusted to different airway passages in the same patient
if the physician desires relocating the nebulizing
11 catheter to different locations in a patient's
12 respiratory tract. In a one preferred embodiment, more
13 than one wire loop may be provided at the distal end of
14 the nebulizing catheter. It is noted that the wire loop
740 of this embodiment may also be used in for
16 facilitating positioning over a guide wire in a manner
17 similar to loop 106 shown in FIG. 9.
18 FIGS. 44 and 45 show another alternative
19 embodiment of the present invention. A nebulizing
catheter 747 has a shaft portion 748 and a wire loop 749
21 extending from a distal end of the shaft 748. In this
22 embodiment, the wire loop 749 is connected at each end
23 750 and 751 to the distal end of the catheter shaft 748.
24 A retractable sheath 752 is positioned over the
nebulizing catheter shaft 748. The sheath 752 can be
26 advanced and withdrawn relative to the catheter shaft
27 748. When it is desired to maneuver the nebulizing
28 catheter into a desired position in the respiratory tract
29 of a patient, the sheath 752 is advanced over the loop
749 to maintain a low profile, as shown in FIG. 45. When
31 the distal end of the nebulizing catheter is suitably
32 positioned, the sheath 752 is then retracted, as shown in
33 FIG. 44, allowing the loop 749 to expand to its expanded
34 size to center and align the distal end of the nebulizing
catheter in the respiratory tract. In one embodiment,
CA 02596278 2007-08-21
-58-
1 the loop 749 is formed of a superelastic material such as
2 nitinol.
3 As noted above, proper positioning and
4 alignment of the nebulization catheter can be an
important factor affecting drug delivery efficiency. In
6 general, it is preferable to position the tip of the
7 nebulizing catheter as closely to the central region of
8 the trachea (or other respiratory passage, such as the
9 bronchi) as possible. It is further noted that even if
the catheter can be centered relative to the trachea, if
11 a section proximal to a centering device is misaligned,
12 it can affect the directional orientation of the tip.
13 This situation is represented in FIG. 46 in which a
14 nebulizing catheter 753 is centered, but the tip is not
properly aimed to provide an optimum plume. This
16 potential problem can be overcome by using an embodiment
17 of the invention shown in FIG. 47. In FIG. 47, a
18 nebulizing catheter 754 is located in a trachea 755 of a
19 patient. The nebulizing catheter 754 extends out the end
of an endotracheal tube 756. A first centering apparatus
21 757 is located on a main shaft 760 of the nebulizing
22 catheter 754 close to the distal end 764. The first
23 centering device 757 may be similar to the centering
24 devices shown in FIGS. 41 - 45. A second centering
device 768 is located axially along the nebulizing
26 catheter shaft 760 proximally from the first centering
27 device 757. The second centering device 768 may be the
28 same as the first centering device 757. As shown in FIG.
29 47, the two centering devices 757 and 768 not only serve
to position the nebulization catheter 7754 centrally in
31 the trachea, but also serve to align the nebulizing
32 catheter tip to expel the plume along a central axis of
33 the trachea.
34 The proximal centering device 768 may be
substituted by another type of centering device or may
36 employ the endotracheal tube 756 for this purpose, as
37 shown in FIG. 48. If the endotracheal tube is used to
CA 02596278 2007-08-21
-59-
1 assist in centering the nebulization catheter, it may
2 incorporate a distal, elongated occlusion cuff 772 or
3 balloon to coaxially align it accurately in the trachea.
4 Most conventional endotracheal tubes are provided with a
curvature to facilitate positioning the trachea of a
6 patient. In addition, most conventional endotracheal
7 tubes are relatively stiff. These factors may result in
8 the misalignment of the distal end of the endotracheal
9 tube relative to a patient's trachea as illustrated in
FIGS. 46 and 47. In order to use the endotracheal tube
11 for centering of the nebulization catheter, it is
12 preferable to make the tip of the endotracheal tube
13 straighter andJor more flexible than in conventional
14 endotracheal tubes to ensure proper concentricity with
the occlusion balloon and the trachea. An endotracheal
16 tube with a straighter and more flexible tip is shown in
17 FIG. 48. In addition, the endotracheal tube may be
18 provided with a centering or aiming device 776 for
19 aligning the nebulization catheter 754. In the
embodiment of FIG. 48, the aiming device 776 is formed by
21 a plurality of flexible or resilient wings the extend
22 from the wall of the endotracheal tube 756 toward an
23 axially central position.
24 Appropriate centering and aiming of the
nebulization catheter can be affected by anatomical
26 factors. It is noted that in some circumstances, it is
27 preferable to position the distal tip of the nebulization
28 catheter into either bronchus of the lungs or even into
29 separate bronchia. Positioning of the nebulizing tip
closer to the alveoli may enhance drug delivery
31 efficiency. In a situation in which it is desired to
32 place the nebulizer tip in both bronchi of the lungs, a
33 nebulizing catheter 780 with dual tips can be employed,
34 as shown in FIG. 49. When using a dual tip catheter such
as shown in FIG. 49, centering and aiming can be
36 important considerations because of the narrower air
37 passages in each of the bronchi. To provide for
CA 02596278 2007-08-21
-60-
1 centering and aiming of a dual tip nebulizing catheter,
2 each of the tips 784 and 788 may be provided with its own
3 centering apparatus, such as 792 and 796. These
4 centering devices may be similar to the centering devices
described above. Alternatively, the centering devices
6 792 and 796 may be formed of arms or struts, made of a
7 flexible or resilient material, that bow out from the
8 shafts of each of the tips 784 and 788, as shown. These
9 struts may be formed with a shorter length in order to
fit into smaller airway passages or alternatively they
11 may be made to provide a range of deployment sizes to
12 accommodate different airway passages.
13 As an alternative to providing a nebulizing
14 catheter with dual tips 784 and 788 as shown in FIG. 49,
if delivery of aerosolized medicine into separate
16 branches of the lungs is desired, it may be preferred to
17 use a nebulizing catheter with a single nozzle tip that
18 has multiple orifices or jets aimed toward the desired
19 branches.
With respect to all the centering devices
21 described above, it is noted that some aerosol may impact
22 the wires or loops that form the centering devices and
23 accordingly, the centering devices are preferably
24 constructed of wires or other materials having a small
diameter or cross section to minimize losses due to such
26 impaction. Moreover, the overall improved efficiency due
27 to the reduction in aerosol impaction on the walls of the
28 trachea or'other airway passage is expected to more than
29 compensate for amy losses due to impaction on the
centering device.
31 Another alternative means for centering the
32 distal end of a nebulization catheter in the air passage
33 is to use part of the pressurized gas for a pneumatic
34 centering device. Air jets generated from two or more
outward directed orifices spaced evenly around the outer
36 circumference of the nebulizing catheter near the tip can
37 be used to center the catheter in the airway. This
CA 02596278 2007-08-21
-61-
1 alternative may help avoid irritation and provide
2 additional advantages compared to physical centering
3 devices.
4 Another alternative way to help center the
nebulizing catheter in the patient's airway passage is to
6 use a balloon or wire centering device placed near the
7 nebulizing catheter tip. The balloon or wire centering
8 device can be temporarily inflated to double check the
9 placement of the nebulizing catheter tip in relation to
the endotracheal tube tip. To use this feature the
il nebulizing catheter is advanced beyond the endotracheal
12 tube tip using markings on the proximal shaft to judge
13 the distance. The centering device or balloon would then
14 be expanded to a diameter larger than the endotracheal
tube and the catheter retracted until the centering
16 device or balloon could be felt engaging with the
17 endotracheal tube tip or until the endotracheal airflow
18 was obstructed.
19 VI. Operation and Flow Control
As mentioned above, the driving gas used to
21 pressurize the gas lumen may be pure (e.g. 100%) oxygen
22 at a pressure of 35-50 psi. Other gases and pressures
23 may be used with suitable adjustments to provide for the
24 desired particle size. The pressurized gas also may be
humidified by a bubbler or other suitable means and
26 warmed, if necessary.
27 Regarding the liquid lumen, one way to deliver
28 the liquid drug through the nebulizing catheter is by a
29 manually operated syringe. To delivery a liquid drug in
this manner, a syringe containing the liquid medicine to
31 be nebulized is connected to the liquid port on the
32 manifold connected to a proximal end of the nebulizing
33 catheter. Then, the liquid is injected while the
34 pressuring gas is being supplied to the nebulizing
catheter via the gas inlet port on the nebulizing
36 catheter manifold. Using a manually operated syringe is
CA 02596278 2007-08-21
-62-
1 reliable, easy to use, and may be preferred when it is
2 desired to deliver only a small amount of medication.
3 In a preferred embodiment, the liquid drug is
4 delivered to the nebulizing catheter from a pressurized
source. A pressurized source for the liquid medicine can
6 provide for a generally higher and more uniform pressure.
7 A high pressure assists in clearing any blockages that
8 may occlude the liquid lumen. Pressurization of the
9 liquid lumen also can ensure that all the liquid drug is
evacuated from the catheter tip. In addition, use of a
11 liquid pressurization source can provide for drug
12 delivery for a longer period of time or a drug delivery
13 that is timed or pulsed to coincide with operation of a
14 ventilator, if used. In a preferred embodiment, the same
pressure source (at 50 psi) that is used to provide the
16 gas pressurization can also be used to provide for
17 pressurization of the liquid. Some ventilators have an
18 auxiliary port that are used for externally located
19 nebulizers. The pressure flow from this auxiliary port
may be used as a pressure source to drive the liquid and
21 gas supplies of the embodiments of the nebulizing
22 catheter considered herein. Alternatively, a sensor
23 located in the flow from this auxiliary port may be used
24 to trigger another control device that operates the
pressurized liquid and gas supplies.
26 _ In a preferred embodiment, the generation of
27 the aerosol can be synchronized with the inhalation of
28 the patient. In one embodiment, this can be accomplished
29 with a manually operable control gas valve on the gas
pressure line to the liquid input port. This may be
31 suitable when the medicine can be delivered in a short
32 period of time, e.g. a few respiratory cycles.
33 Alternatively, when it is preferred to deliver the
34 medicine for an extended period of time, it may be
preferred to employ a system that can automatically
36 deliver medicine via the nebulizer from a source of
37 liquid medicine. In such a system, the gas and/or liquid
CA 02596278 2007-08-21
-63-
1 flow are triggered by the patient's respiratory cycle
2 with the use of an electronic pressure sensor and relay
3 actuator.
4 An important factor relating to effective
delivery of medication via a nebulizing catheter is the
6 flow control system for pressurizing and supplying the
7 gas and liquid to the proximal end of the nebulization
8 catheter. In many circumstances, it is envisioned that
9 medication will be delivered to the patient via a
nebulization catheter that is in place in the patient
11 over an extended period of time, such as several hours or
12 days. In such circumstances, it would be preferred to
13 use a system that automatically delivers the proper
14 dosage of inedication from a supply of the medicine to the
patient at the proper rate, and further that can operate
16 automatically and unattended. Further, it would be
17 preferred to provide a means to detect when the supply is
18 running low so that either the nebulization catheter can
19 be disconnected or a new supply provided. FIGS. 50 and
51 show several embodiments of a reservoir and
21 pressurization system for use with a nebulizing catheter.
22 Referring to FIG. 50, a reservoir and
23 pressurization assembly 800 is connected to a proximal
24 end of a nebulization catheter. The nebulization
catheter may be similar to any of the embodiments
26 described above. The assembly 800 has a gas inlet port
27 804 that can connect to an external pressurized gas
28 supply. The external pressurized gas supply may be the
29 main gas supply of the hospital or may be provided by
another unit. The external gas supply may provide oxygen
31 at 50 psi. The gas inlet port 804 communicates with an
32 airflow passageway 808 defined by and extending through
33 the assembly 800. The assembly 800 includes a gas output
34 port 812 that communicates with the fluid flow passageway
808 and which connects to a gas inlet port of the
36 nebulization catheter (not shown). The gas output port
37 812 is located immediately downstream of the gas inlet
CA 02596278 2007-08-21
-64-
1 port 804. Located in the fluid flow passageway 808
2 downstream of the gas outlet port 812 is a filter 816.
3 The filter 816 is preferably a hydrophobic filter that
4 allows the passage of gas but which would prevent the
backflow of any liquid. Located downstream of the filter
6 816 in the fluid flow passageway 808 is an injection port
7 and reservoir 820. This port 820 communicates with a
8 supply of the liquid fluid medication to be supplied to
9 the nebulizing catheter. Located next in the fluid flow
passageway 808 is a capillary tube drug reservoir 824.
11 The capillary tube reservoir 824 is comprised of a length
12 of plastic tubing adapted to hold a supply of the liquid
13 medication to be delivered. In the embodiment shown, the
14 capillary tube reservoir consists of a helical coil of
transparent tubing. Located downstream of the capillary
16 tubing reservoir 824 is a liquid outlet 828 that connects
17 to a liquid inlet port of the nebulization catheter (not
18 shown). With the embodiment shown in FIG. 50, the
19 transparent capillary tubing 824 provides a convenient
and reliable way to ascertain the supply of medication to
21 the nebulizing catheter. The capillary tubing because of
22 its length is capable of containing a suitable supply of
23 the medication. When the attending medical personnel
24 observe that the medication is about to run out, a new
supply can be readily provided. The clear capillary tube
26 allows easy visualization of the drug flow by watching
27 the gas-drug meniscus travel down the tube. Instead of
28 relying on direct observation by medical personnel, the
29 capillary tubing may be used with an automatic detection
device, e.g. a photocell, that provides an alarm to the
31 medical personnel upon detection that the medication is
32 running out in the capillary tubing or that the meniscus
33 has ceased moving due to a blockage. A blockage may also
34 be detected by detection of an increase in pressure.
FIGS. 51 and 52 show another embodiment of a
36 fluid reservoir and pressurization assembly 832. This
CA 02596278 2007-08-21
-65-
1 embodiment includes a gas inlet 836, a fluid flow
2 passageway 840, a liquid medicine supply vent 844, a
3 filter 848, a capillary channel section 852, and an
4 outlet port 856. In this embodiment, the filter 848 is
located downstream of the filling vent 844. The filter
6 848 allows the pressurized gas to push the liquid drug
7 during use but prevents the liquid drug from backing up
8 to the vent during filling. In this embodiment, a second
9 injection port 860 is provided downstream of the
capillary section 852 and a second filter 864 is located
11 downstream of the second injection port 860. The second
12 filter 864 is preferably a filter having approximately a
13 20 m retention. Also, in this embodiment, the capillary
24 section 852 may be composed of a planar section 865. The
planar section 865 may be a piece of plastic having a
16 winding channel molded, routed or otherwise formed
17 therein. The planar section 868 is preferably colored to
18 provide suitable contrast with the liquid solution
19 flowing therethrough. A transparent flat plastic cover
is positioned over the winding channel of the planar
21 section 865 to form the closed channel of the capillary
22 section. The fluid channel in the capillary section
23 preferably has an I.D. of approximately 2 mm. The second
24 inlet port 864 provides an additional means to add
medication to the nebulizing catheter liquid flow. When
26 the capillary channel in the section 852 has been filled,
27 the gas is used to pressurize the tube and force the
28 fluid to the catheter tip. The second filter 864 acts as
29 a restrictive orifice to precisely meter the flow to the
nebulizing catheter. The clear capillary channel allows
31 easy visualization of the drug flow by watching the gas-
32 drug meniscus travel down the tube. The narrow tube
33 makes the flow appear to move quickly even at slow
34 delivery rates. Thus, any flow interruption can be
easily observed. The capillary tubing sectiori also
36 ensures that almost l00% of the drug is delivered to the
CA 02596278 2007-08-21
-66-
1 catheter tip since there is no dead space in the line
2 except at the injection port 860.
3 During ventilation of a patient with an
4 endotracheal tube, especially when intubation that takes
place for a long period of time, it is considered
6 desirable to humidify the air being delivered. When a
7 nebulization catheter is used for delivery of medicine,
8 either in conjunction with an endotracheal tube or even
9 without an endotracheal tube, it is possible to utilize
the nebulization catheter for providing humidification in
11 addition to medicine delivery. An embodiment of a flow
12 delivery system for a nebulizing catheter incorporating
13 humidification is shown in FIG. 53. A suitably large
14 reservoir 866 holds sterile water or saline. The
reservoir 866 is connected to the liquid supply lumen 867
16 of a nebulization catheter 868. Solution is drawn into
17 the nebulization catheter 868 from the reservoir 866 by
18 negative pressure at the catheter tip, gravity, a pump in
19 the solution supply line distal of the reservoir, or by
pressurizing the reservoir by a suitable means.
21 Medicine may be added to the humidification
22 water in at the following ways. In a first alternative,
23 the medicine is added to the isotonic saline in the
24 solution reservoir 866 thereby providing for high
dilution and slow, continuous delivery of the medicine
26 along with the water. In second alternative, the
27 medicine is introduced into the solution supply line 867
28 via an injection port 869 between the reservoir 866 and
29 the liquid lumen of the catheter 868. The medicine may
be delivered to the injection port of the solution supply
31 line from a solution reservoir system such as system 800
32 of FIG. 50. Using this latter alternative, a more
33 concentrated dose of the medicine can be delivered at the
34 specific time preferred by the physician. It may also be
preferable to include a molecular sieve, check valve or
36 air trap 870 between the reservoir 866 and the injection
CA 02596278 2007-08-21
-67-
1 port to the to ensure that the medicine cannot flow or
2 diffuse backwards into the reservoir 866.
3 When delivering medicine to the lungs or when
4 delivering water for humidification, it may be desired to
heat the liquid prior to delivery. This may especially
6 be appropriate since expanding gases which are associated
7 with the nebulization of liquids may remove heat from the
8 body. In order to address this concern, a heating
9 element 871 may be associated with the liquid supply line
867 to the nebulizing catheter 868. This heating element
11 871 may include an electric coil wound around the supply
12 line 867 or may use a heated water flow in a tubing wound
13 around the supply line 867. The heating element 871 may
14 be used in embodiments that provide for humidification as
well as those that do not. Alternatively, the heating
16 element 871 may be associated with the gas supply line or
17 with the liquid reservoir 866,
18 It is generally considered preferable to
19 operate the nebulizing catheter so as to generate an
aerosol that is carried by a patient's inhalation to the
21 lungs. This requires a pulsing of the aerosol generation
22 so that it is timed to coincide with the inhalation of
23 the patient. If the patient is intubated, the timing of
24 the nebulization can be~synchronized with the operation
of the ventilator. It is recognized that it may be
26 preferable to begin the nebulization slightly in advance
27 of, at, or slightly after, the beginning of the
28 inhalation period in order to account for the distance
29 between the nebulization tip and the alveoli. Also, it
may be preferable to stop the nebulization slightly
31 before the end of the inhalation period in order to avoid
32 wasting aerosol after the inhalation flow has stopped.
33 This continuous pulsing of the aerosol can be
34 accomplished by a system 872 as shown in FIGS. 54 and 55.
FIGS. 54 and 55 show a portion of the flow control system
36 for a nebulizing catheter. A flow line 876 has an inlet
37 880 and an outlet 884. The flow line 876 may be formed
CA 02596278 2007-08-21
-68-
1 of a soft (e.g. compliant) tube. The inlet 880 connects
2 to the source of liquid medicine and in particular may
3 attach to the liquid outlet (828 or 856) of the liquid
4 reservoirs shown in FIGS. 50 - 52. The flow line outlet
884 in FIGS. 54 and 55 connects to the liquid inlet port
6 on the manifold of the nebulizing catheter, such as port
7 32 in FIGS. 1 and 2. Located around a portion of the
8 flow line 876 is an actuator piston 888. The actuator
9 piston 888 includes a solenoid pinch valve 892 that can
impinge upon the portion of the liquid flow line 876
11 extending therethrough thereby pinching it off. The
12 actuator piston 888 is connected to and operated by a
13 controller that receives input from the ventilator (such
14 as from the auxiliary port used for an external
nebulizer) so that the actuator piston 888 is operated to
16 open and close the flow line synchronous with the
17 inhalation and exhalation phases of the ventilator.
18 Instead of a solenoid piston, a metering valve or
19 reversible syringe pump may be used.
In a preferred embodiment, the flow control
21 system 872 uses a dual solenoid arrangement to provide a
22 draw-back feature. Pulsing of the liquid flow by
23 actuation of the actuator piston 888 may result in some
24 liquid being left at the distal nebulizer liquid orifice
when the pressure is turned off. This may result in
26 small amounts of liquid drooling from the distal liquid
27 orifice tip since the liquid is not being expelled under
28 controlled pressure conditions. In order to limit the
29 occurrence of such drooling, a draw back feature is
provided in the flow control system. The draw back
31 feature is provided by a second solenoid 896 which is
32 associated with a bladder 900 that communicates with the
33 flow line 876. The bladder 900 communicates with the
34 flow control line 876 downstream of the actuator piston
888. A small amount of fluid (liquid/air) occupies the
36 bladder 900. The bladder is composed of an elastic
37 material that is formed with a tendency to recover to an
CA 02596278 2007-08-21
-69-
1 expanded size. When the actuator piston 888 opens to
2 allow the flow of fluid to the distal end of the
3 nebulizing catheter, the second solenoid 896 moves to a
4 closed position thereby compressing the bladder 900 and
squeezing fluid out of it into the fluid flow line 876,
6 as shown in FIG. 54. During the exhalation stage of the
7 ventilation cycle, the actuator piston 888 closes to shut
8 off the flow of fluid to the distal end of the nebulizing
9 catheter. When the actuator piston 888 closes, the
second solenoid 896 opens, as shown in FIG. 55. This
11 allows the bladder 900 to resiliently recover to its
12 expanded size, and when it does, it draws fluid into it
13 from the fluid flow line 876. Because the fluid flow
14 line 876 is closed proximally at the actuator piston 888,
when the bladder draws fluid into it from the fluid flow
16 line 876, it draws fluid from the distal end of the fluid
17 flow line that connects to the nebulizing catheter liquid
18 lumen. This causes the entire column of liquid in the
19 liquid lumen of the nebulizing catheter to move slightly
in a reverse direction (i.e. proximally) thereby moving
21 the liquid away from the distal orifice. In this manner,
22 the flow control system of FIGS. 54 and 55 allows the
23 draw back of liquid in the flow line in a reverse
24 direction during the exhalation phase of the ventilator
when the liquid flow line is shut off.
26 VII. Selective Nebulization Therapy Delivery
27 When delivering medication with a nebulizing
28 catheter, it may be desirable to deliver the medication
29 to only one of the bronchi of the lungs and not the other
or to only certain bronchia and not others. A reason for
31 this type of selective therapy may be that only one area
32 of the lungs requires medication. An embodiment of the
33 invention shown in FIG. 56 facilitates selective delivery
34 of a medication via a nebulizing catheter to only one
bronchus. In FIG. 56, an endotracheal tube 904 is
36 positioned in a trachea 908 of a patient. A nebulizing
CA 02596278 2007-08-21
-70-
1 catheter 912 is positioned in the endotracheal tube 904.
2 This nebulizing catheter 904 may be similar to the
3 embodiments described above. This nebulizing catheter
4 904 may even be of the type that is non-removably
incorporated into the endotracheal tube. A second
6 catheter 916 extends distally of the endotracheal tube
7 904. The second catheter 916 may be positioned in the
8 ventilation lumen 920 of the endotracheal tube 904. The
9 second catheter 916 includes a lumen through which a low
flow pressurized gas can be conveyed. A proximal end of
11 the second catheter 916 extends out of the proximal end
12 of the endotracheal tube 904 through a suitable fitting,
13 such as the fitting described in U.S. Pat. No. 5,078,131
14 (Foley). A suitable source of pressurized gas is
attached to a proximal end of the second catheter 916.
16 This gas source may be the same gas source used for the
17 pressurized gas lumen of the nebulization catheter 912.
18 A distal end 928 of the second catheter 916 is positioned
19 in the bronchus 932 other than the bronchus to which it
is desired to deliver nebulized medication. Pressurized
21 gas is delivered through the second catheter 916 out an
22 orifice 936 in the distal end thereof. The delivery of
23 pressurized gas out the distal end 936 of the second
24 catheter 916 causes the pressure level in the bronchus
932 to be slightly greater than in the other bronchus.
26 Accordingly, when the nebulizing catheter 912 generates
27 an aerosol of liquid medicine, it will tend to flow with
28 the inhalation stream from the endotracheal tube 904 to
29 the bronchus other than the one with the second catheter
916. In this manner, one of the bronchi of the lungs, or
31 even selected bronchia, can be selectively medicated
32 using a single nebulization catheter positioned in the
33 endotracheal tube.
34 VIII. Timing of Nebulization
As mentioned before, in order to deliver the
36 nebulized medicine to the lungs, it is preferred that the
CA 02596278 2007-08-21
-71-
1 medicine is carried by the inhalation of the patient. A
2 number of factors affect the efficiency of the medicine
3 delivered this way. The following embodiments are
4 directed to improving drug delivery efficiency taking
into account some of these factors.
6 If the patient is intubated, it may be possible
7 to synchronize the timing of the nebulization pulse with
8 the patient's ventilation. In one embodiment, this may
9 be accomplished by providing an interface between the
ventilator and the nebulizer. In some circumstances it
11 may be preferred to provide other means for triggering
12 the nebulization. For example, the ventilator being used
13 may not provide a suitable interface. Also, the
14 ventilator may not provide sufficiently accurate
information concerning the patient's respiration to
16 enable the nebulization catheter to operate with highest
17 efficiency. In such situations, it may be preferred to
18 utilize one or more separate sensors to obtain
19 information that can be used to trigger and operate the
nebulization catheter.
21 Referring to FIG. 57, there is a nebulizing
22 catheter 944 positioned in an endotracheal tube 948
23 located in the trachea 952 of a patient. A proximal end
24 of the endotracheal tube 948 is connected to a ventilator
956. In order to obtain physiological information
26 concerning the patient's respiration for use in timing
27 the generation of nebulization pulses by the nebulization
28 catheter 944, one or more sensors may be used. For
29 example, a first sensor 960 may be located on a distal
end of the endotracheal tube 948. In addition, a sensor
31 964 may be positioned on the nebulization catheter 944.
32 Another sensor 968 may be positioned on a separate
33 device, such as a separate catheter 972 which is located
34 further distally in the respiratory system. In addition,
a sensor 976 may be positioned in the ventilator circuit
36 of the ventilator 956 or in a ventilator auxiliary port,
37 if available, or elsewhere on the patient. These sensors
CA 02596278 2007-08-21
-72-
1 960, 964, 968, and 976 may be types of sensors that
2 measure pressure, flow or a physiological parameter of
3 the patient, such as muscle contraction,
4 electophysiological activity, etc. In alternative
embodiments, one or more of these sensors may be used.
6 FIGS. 58 and 59 show alternative embodiments of
7 nebulization catheters that incorporate sensors. In FIG.
8 58, a nebulization catheter 980 is shown. This
9 nebulization catheter 980 may be similar to the
nebulization catheter in FIG. 11. In FIG. 58, a main
11 shaft 984 includes a plurality of lumens with a centrally
12 located lumen 988 used to deliver a liquid medicine and a
13 plurality of lumens 992 located peripherally around it
14 used to deliver a pressurized gas. One of the peripheral
lumens 996 is not used for pressurized gas delivery, but
16 instead is used for sensing purposes. This may be
17 accomplished by forming an aperture 1000 through a wall
18 of the main shaft 984. The aperture communicates with
19 the sensing lumen 996. The aperture 1000 may be open or
may be covered with a flexible diaphragm that permits
21 transmission of pressure across it. A pressure sensing
22 device may be located at a proximal end of the nebulizing
23 catheter. The pressure at the distal end of the
24 nebulizing catheter can be sensed by the proximally
located sensing device via the sensing lumen 996. This
26 could rely on pneumatic sensing of the distal air
27 pressure. Because of the effect of the distal gas
28 pressurization orifice, pressure sensing through the
29 sensing lumen 996 may be used for purposes of gross
overpressure for safety purposes. Alternatively, the
31 pressure sensing lumen 996 may be used during periods of
32 time when a pressurizing gas is not being delivered to
33 sense the patient's physiological airway pressure.
34 FIG. 59 shows another embodiment of a pressure
sensing nebulization catheter. This embodiment is
36 similar to the embodiment of FIG. 58 except that a sensor
37 1004 is located at a distal end of the catheter 980,
CA 02596278 2007-08-21
-73-
1 specifically in the aperture 1000. In this embodiment,
2 the sensor 1004 is a pressure transducer. Wire leads
3 1008 extend proximally from the sensor 1004 via the lumen
4 996. Instead of measuring pressure, the sensor 1004
could measure the flow at the distal end of the catheter.
6 This may be accomplished by piezoelectric, optical, Hall
7 effect, or other types of sensor technologies. The
8 sensor may also be of a fiber optic type.
9 Although the embodiments of FIGS. 58 and 59
show sensing apparatuses associated with a nebulization
11 catheter, these same types of sensors could also be used
12 in the endotracheal tube 948, the separate catheter 972,
13 or the ventilator 956 of FIG. 57 or the ventilator
14 circuit.
The sensor outputs information to a controller
16 1012 that operates the flow control portion 1013 of the
17 nebulization catheter system. The flow control portion
18 may include the flow control assembly 872 (shown in FIG.
19 55) as well as include the control functions for gas
pressurization. The controller 1012 may have preset
21 triggering parameters or may be user adjustable. The
22 controller 1012 may use airway flow, pressure, or
23 physiological activity as a basis for controlling the
24 flow control assembly 1013. The controller 1012 may
provide for pulsing based upon any one of the following
26 modes: (1) a controlled volume (bolus) of medicine is
27 delivered with each pulse; (2) medicine is delivered
28 until a physiological condition is sensed, e.g.
29 exhalation; or (3) medicine is delivered for a fixable
time interval, e.g. 2 seconds. These modes of operation
31 may be selectable by the physician based upon the
32 preferred treatment taking into account the patient's
33 condition, the type of medicine being delivered, etc.
34 It may also be desired to regulate the delivery
of aerosol so that it is not delivered with every
36 inhalation. As mentioned above, one concern with
37 delivery of an expanding gas is the cooling effect that
CA 02596278 2007-08-21
65902-72E
-74-
it may have on the body. This can be a factor with high gas
flow rates. Accordingly, it may be preferable to deliver
aerosol on every other inhalation or every third inhalation,
and so on. Alternatively, it may be preferred to deliver
aerosol for certain periods of time, e.g. 5 minutes every
hour. Therefore, by alternating aerosol delivery, the
cooling effect associated with it can be reduced.
IX. Alternative Embodiments
A. Nebulizing Catheter Incorporated in
Endotracheal Tube
The various embodiments of nebulizing catheters,
disclosed above, have been described as being either adapted
for use in conjunction with a separate endotracheal tube, or
adapted to be used without an endotracheal tube. If used
with an endotracheal tube, the embodiments of the nebulizing
catheter disclosed above are preferably removable from the
endotracheal tube if one is present. It is noted that many
of the embodiments of the present invention disclosed herein
may also be used in conjunction with a nebulization catheter
that is non-removable from an endotracheal tube, i.e. in
which the nebulizing catheter is incorporated into and forms
part of the endotracheal tube. An endotracheal tube that
provides for nebulized medication delivery is described in
U.S. Patent No. 5,438,982 of Dr. Neil R. MacIntyre.
According to a system developed by Dr. MacIntyre, there is
provided an endotracheal tube that provides for nebulization
of a medication at a distal end thereof. According to
Dr. MacIntyre's system, an endotracheal tube includes two
additional, separate lumens, in addition to its main
ventilation lumen used for the patient's breathing airflow.
A medication in a
CA 02596278 2007-08-21
-75-
1 liquid form is conveyed through one of the additional
2 lumens and a pressurized gas is conveyed through the
3 other lumen. The two additional lumens have distal
4 openings near the distal end of the endotracheal tube
airflow lumen. The distal opening of the pressurized gas
6 lumen directs the pressurized gas across the distal
7 medication lumen opening thereby nebulizing the liquid
8 medication so that it can be delivered to the patient's
9 lungs. It is intended that the present invention covers
embodiments of nebulization catheters that are non-
11 removable relative to an endotracheal tube.
12 B. Aerosol Generation with Porous Material
13 FIG. 60 shows another catheter 1060 for
14 producing an aerosol. The catheter 1060 generates an
aerosol, or aerosol-like plume by use of a porous
16 material or sponge located in a lumen of the catheter.
17 The catheter 1060 has a main shaft 1064 with a lumen 1068
18 through which liquid medicine is conveyed under pressure
19 and a lumen 1072 through which a gas is conveyed under
pressure. A porous material 1076 is located in a distal
21 end of the shaft 1064 so that both lumens 1068 and 1072
22 convey their contents into the porous material 1076. The
23 porous material 1076 may be a porous polyethylene made by
24 Porex. Alternatively, the porous material may be a
polymer sponge or other polymer material. Located in the
26 main shaft 1064 distal of the porous 1076 is an end cap
27 1080 with an orifice 1084 located therein. The orifice
28 is small and maintains a positive back pressure in the
29 catheter shaft and porous material area. The end cap
1080 is separated from the distal side of the porous
31 material 1076 by a small gap 1082. The liquid and gas
32 delivered under pressure to the porous material 1076
33 migrate through the porous across the gap 1082 toward the
34 aperture 1084. The liquid and gas become intermixed
under pressure and as they are expelled from the fine tip
36 orifice the gas expands and disperses the liquid
CA 02596278 2007-08-21
-76-
1 particles into fine droplets. Upon discharging through
2 the aperture 1084, the medicine forms tiny droplets, e.g.
3 an aerosol. The aerosol is conveyed to the lungs of the
4 patient in a manner similar to that described in the
embodiments above. An advantage of using a porous
6 material or sponge at the distal liquid orifice is that
7 it reduces drooling of the liquid.
8 C. Secondary Aerosol Generation
9 In some situations it may be desirable to
modify the primary aerosol spray generated by a
11 nebulization catheter. One way that this can be
12 accomplished is by causing the primary aerosol spray to
13 impact upon a baffle placed in its path, the velocity and
14 direction of the spray can be altered and the size of the
distribution of the aerosol can be modified creating a
16 secondary aerosol. Impaction upon a properly located
17 baffle can break up large aerosol particles creating a
18 finer aerosol mist. The baffle also deflects or diffuses
19 the airstream carrying the particles reducing their
forward velocity and altering their direction. This can
21 lessen impaction on the carina or airways and enhance the
22 entrainment of the particles into the inspiratory flow.
23 Embodiments of nebulizing catheters incorporating an
24 impaction baffle to provide a secondary aerosol are shown
in FIGS. 61-64.
26 Referring to FIG. 61, a nebulization catheter
27 1140 has a gas lumen 1142 and a liquid lumen 1144 located
28 in a shaft 1146 of the catheter. The gas lumen 1142
29 conveys a pressurized gas to a distal gas orifice 1148
and the liquid lumen 1144 conveys liquid to a distal
31 liquid orifice 1150. A baffle 1152 connects to a baffle
32 extension tube 1154 so that the baffle 1152 is located
33 distally of the liquid orifice 1150. The baffle 1152 is
34 preferably located as close to the solution orifice 1150
as possible without interfering with the generation of
36 the primary aerosol.
CA 02596278 2007-08-21
-77-
1 Some of the primary aerosol that is not broken
2 into fine particles may remain on the baffle 1152 and
3 build up over time forming a thin liquid film on the
4 surface of the baffle 1152. If this film is left to
build up, it will form droplets that either fall or are
6 blown off the baffle. These droplets may become quite
7 large and of little or no therapeutic value representing
8 a waste of the solution.
9 In order to recirculate this film of solution,
the baffle 1152 may be used to collect and return the
11 liquid solution to a liquid supply lumen 1144 To achieve
12 this, the baffle may have with one or more orifices 1158
13 or porous material on its surface of the baffle 1152 for
14 the collection of the film of solution. The orifices
1158 drain into or through the baffle, and are in fluid
16 communication with the solution supply lumen 1144 via a
17 lumen located inside of the extension tube 1154. The
18 lumen inside the extension tube 1154 may communicate
19 directly with the solution lumen 1144 or extension
thereof.
21 In the embodiment of FIG. 61, the negative
22 pressure generated at the nebulization orifice 1150 by
23 the gas flow over it is used to draw the recirculated
24 solution from the baffle recirculation orifice 1158 via
the lumen in the extension 1154 and out the liquid
26 orifice 1150 again. In this case, the recirculation
27 orifices 1158 or surface should be in an area of higher
28 ambient pressure than the solution orifice 1150 to cause
29 the recirculation of the fluid. This may be accomplished
by locating the collection orifices 1158 on a distal side
31 of the baffle 1152 opposite the solution and gas orifices
32 1150 and 1148. The flow of new solution (from the
33 proximally located solution reservoir) pumped into the
34 solution lumen 1144 should be less than the flow drawn
from the solution orifice 1150 to ensure that least some
36 of the solution from the baffle 1152 is recirculated to
37 the orifice 1150.
CA 02596278 2007-08-21
-78-
1 FIG. 62 shows another embodiment of a
2 nebulization catheter that incorporates a baffle for the
3 purpose of generating a secondary aerosol. This
4 embodiment is similar to the nebulization catheter in
FIG. 61 with the exception that the recirculated fluid is
6 drawn back into a recirculation lumen 1160 in the
7 catheter shaft 1146. The recirculation lumen 1160
8 communicates with the liquid lumen 1144 at a junction
9 1162 at which location the recirculated solution is mixed
with newly supplied liquid in the solution lumen 1144.
11 FIG. 63 shows another alternative embodiment.
12 This embodiment is similar to the embodiment of FIG. 62
13 except that the recirculated solution is routed from the
14 baffle 1152 to the recirculation lumen 1160 and then to a
separate solution orifice for re-nebulization. This
16 dedicated solution orifice 1161 is also located at the
17 catheter tip near a gas orifice 1148 to produce
18 nebulization. The aerosol generated from this separate
19 orifice 1161 is directed into the common baffle 1158 to
break it into smaller particles and a portion of the
21 solution will again remain on the baffle and be
22 recirculated. This approach can eliminate the
23 difficulties of balancing the flow of new and
24 recirculated solution to a single solution orifice.
Referring to FIG. 64, there is another
26 embodiment of a nebulizing catheter incorporating a
27 baffle for the generation of a secondary aerosol. In
28 this embodiment, a nebulizing catheter 1170 has a shaft
29 1172 with a liquid lumen 1174 connected to a liquid
supply 1176. A gas lumen 1178 connects to a pressured
31 gas source 1180. The liquid lumen 1174 communicates with
32 a distal liquid orifice 1182 and the gas lumen
33 communicates with a distal gas orifice 1184. A baffle
34 1186 is located in front of the liquid orifice 1182.
Aerosol impacting on the baffle 1186 produces a secondary
36 aerosol that flows around the baffle 1186. A residue
37 film of liquid migrates around the baffle 1186 and enters
CA 02596278 2007-08-21
-79-
1 into baffle orifices 1190 located on the distal side of
2 the baffle 1186. The baffle orifices 1190 communicate
3 with a recirculation lumen 1192 that extends through the
4 catheter shaft to a reservoir 1194 located outside of the
body where the recirculated solution is combined with
6 non-recirculated solution pumped from a proximal drug
7 reservoir. The flow of recirculated and non-recirculated
8 solution into the system should be carefully balanced to
9 match the amount of aerosol generated. To achieve this,
flow metering and pumping strategies can be employed.
11 D. Nebulization Catheter with
12 Pressurized Propellant-Drug Canister
13 In the embodiments described above, medicine is
14 delivered in liquid form to the distal liquid orifice.
In another embodiment, illustrated in the diagram of FIG.
16 65, the medicine may be mixed with a propellant and
17 maintained under pressure and delivered under pressure to
18 the distal tip of a nebulizing catheter 1198. A
19 pressured medicine-liquid propellant mixture could be
supplied from a pressurized canister 1200 such as those
21 used as a component of a metered or non-metered dose
22 inhaler. By using a propellant, an aerosol could be
23 generated from the distal end 1202 of the catheter even
24 without the addition of the pressurized nebulizing gas.
However, the delivery of pressurized gas 1204 from the
26 distal end of the nebulization catheter would be used to
27 assist in breaking up any larger medicine particles and
28 also assist dispersing the aerosolized drug solution
29 delivered through the catheter as well as help shape the
aerosol plume. For example, the delivery of the
31 pressurized, nebulizing gas may assist in shielding the
32 aerosol generated by the medicine-liquid propellant
33 mixture and help avoid losses due to impaction.
34 E. Nebulizing Function Incorporated in
Suction Catheter
CA 02596278 2007-08-21
-80-
1 As mentioned above, the nebulizing catheter can
2 be incorporated into another device, such as an
3 endotracheal tube, either removably or non-removably.
4 Another such device into which a nebulizing catheter can
be adapted is a suction or aspiration catheter. A
6 suction catheter is sometimes used in conjunction with
7 patients who are intubated. A suction catheter has an
8 O.D. and a length such that it can be inserted through
9 the ventilation lumen of an endotracheal tube. The
suction catheter is used to aspirate fluids and mucin
11 secretions that collect in the respiratory tract of in
12 the endotracheal tube of a patient who is intubated. A
13 conventional suction catheter is inserted down the
14 ventilation lumen of the endotracheal tube and out the
distal end. A mucolytic agent may be instilled as a
16 liquid via a lumen of the suction catheter to help in the
17 withdrawal of mucin from the trachea or bronchi. The
18 suction catheter may then be withdrawn froi the
19 endotracheal tube and either disposed or retained in a
sterile sheath connected to a proximal end of the
21 endotracheal tube so that it can be reinserted into the
22 endotracheal tube again.
23 A nebulizing catheter can be incorporated into
24 a suction catheter so that a single device can perform
both the functions of aspiration and nebulization for
26 aerosol delivery. In an alternative embodiment of the
27 present invention, the nebulizing catheter, such as
28 described above, could be incorporated into a suction
29 catheter so that a single catheter can provide both
functions. This could be accomplished by provided any of
31 the embodiments of the nebulization catheter described
32 above with a separate lumen for the purpose of providing
33 a suction to withdraw fluid from a patient's respiratory
34 tract. Combining the functions of a suction catheter and
nebulization catheter into a single device has the
36 advantages of avoiding the expense of separate products
CA 02596278 2007-08-21
-81-
1 as well as avoiding the inconvenience of inserting and
2 withdrawing separate devices.
3 Embodiments of a suction catheter combined with
4 a nebulization catheter are shown catheter is FIGS. 66-
73. FIGS. 66-70 show a suction catheter assembly 1220.
6 The suction catheter assembly 1220 includes a suction
7 catheter shaft 1222 slidably located inside of a flexible
8 sheath 1224. A suction lumen 1225 extends through the
9 suction catheter shaft 1222. A proximal manifold 1226
includes a port 1228 for connecting a vacuum source to
11 the suction catheter lumen 1225. A valve 1230 operates
12 to open and close the port 1228. A distal sleeve 1232
13 provides for connecting to an endotracheal tube such that
14 the suction catheter shaft 1222 can be inserted into the
endotracheal tube by pushing the proximal manifold 1226
16 toward the distal sleeve 1232. The distal sleeve 1232
17 may include a manifold for connection to a flush port
18 1233. A seal 1235 located in the sleeve 1232 closely
19 bears on the suction catheter shaft to remove mucous or
other unwanted materials that can be removed via the
21 flush port 1233. The shaft of the suction catheter may
22 be provided with a low friction, e.g. hydrophilic,
23 coating to reduce adhesion of mucous.
24 The suction catheter assembly 1220 includes two
additional lumens 1234 and 1236. These lumens 1234 and
26 1236 are located in a wall of the suction catheter shaft
27 1222. These lumens 1234 and 1236 communicate with distal
28 orifices 1238 and 1240 located at a distal end of the
29 suction catheter shaft 1222. These lumens 1234 and 1236
are used to deliver a liquid medicine and a pressurized
31 gas for nebulizing the liquid medicine, as described
32 above. Also located at a distal end of the suction
33 catheter shaft 1222 are suction openings 1242.
34 The suction catheter assembly 1220 can be used
in a conventional manner to remove mucin from the trachea
36 and from the bronchi. The suction catheter assembly 1220
37 can also be used to deliver medicines to the lungs as an
CA 02596278 2007-08-21
-82-
1 aerosol by means of the nebulizing lumens 1234 and 1236.
2 The nebulizing lumens can also be used to deliver
3 mucolytic agents as an aerosol. Because the fine aerosol
4 delivered by the nebulizing lumens can be carried by a
patient's inspiratory airflow into the bronchi, the
6 mucolytic agent can be delivered further into bronchi
7 compared to a suction catheter that merely instills or
8 generates a coarse spray of a mucolytic agent. In
9 addition, the flow velocity produced by the gas
pressurization lumen may be used to assist in breaking up
11 mucous at the end of the suction catheter.
12 When using the suction catheter assembly 1220,
13 it can be positioned so that a distal end of the suction
14 catheter shaft 1222 is close to the distal end of the
endotracheal tube 1250 as shown in FIG. 68 or
16 alternatively the suction catheter shaft 1222 can be
17 positioned so that it extends past the distal end of the
18 endotracheal tube 1250 as shown in FIG. 70. As shown in
19 FIG. 70, the suction catheter shaft 1220 may be formed
with a distal curvature so that the distal end can be
21 brought into proximity with the tracheal wall.
22 Rather than incorporate the nebulizing lumens
23 into the wall of the suction catheter, it may be
24 preferably in many situations to use a conventional
suction catheter with a stand-alone nebulizing catheter.
26 The stand-alone nebulizing catheter may be similar to any
27 of the embodiments described above. A suction catheter
28 and a nebulizing catheter can readily be used together
29 with the alternative versions of the manifolds shown in
FIGS. 71-73.
31 Referring to FIG. 71, an endotracheal tube 1252
32 has a proximal end with a single port 1254. A suction
33 catheter 1256 has a distal manifold 1258. The distal
34 manifold 1256 could be formed as part of the suction
catheter 1256 or could be provided as a separate
36 component. The suction catheter manifold 1258 connects
37 to the single port 1254 of the endotracheal tube 1252.
CA 02596278 2007-08-21
-83-
1 The manifold 1258 has a first port 1260 for connecting to
2 a ventilator and a second port 1264 for connecting to a
3 proximal end of a nebulizing catheter 1266. As shown in
4 FIG. 71, the nebulizing catheter 1266 includes a sterile
sheath 1268 which is similar to the sheath included on
6 the suction catheter 1262. In the embodiment of FIG. 71,
7 the suction catheter 1256 and the nebulizing catheter
8 1266 are positioned alternately inside the ventilation
9 lumen of the endotracheal tube 1252. The suction
catheter or the nebulizing catheter can be withdrawn
11 temporarily and maintained in its sterile sheath while
12 the other is being used.
13 Referring to FIG. 72 there is another
14 arrangement for connecting a suction catheter and
nebulizing catheter to an endotracheal tube. In this
16 embodiment, a manifold 1270 connects to the proximal end
17 of the endotracheal tube 1252. The manifold 1270 has
18 port 1274 for receiving the nebulizing catheter 1266 and
19 a second port 1276. A distal manifold 1278 of a suction
catheter 1280 connects to the second port 1276. The
21 suction catheter manifold 1278 has a port 1282 for
22 connecting to the ventilator. This arrangement can be
23 used similarly to the arrangement of FIG. 71.
24 FIG. 73 shows still another arrangement for
connecting a suction catheter and a nebulizing catheter
26 to an endotracheal tube. In this embodiment, the
27 endotracheal tube 1252 is provided with a proximal end
28 that includes dual ports. A first port 1284 receives the
29 nebulizing catheter 1266. The second port 1286 may be
connected to either directly to a ventilator or may be
31 connected to a distal end of a suction catheter (not
32 shown) in a conventional manner.
33 In another alternative embodiment (not shown),
34 the nebulizing catheter could be positioned down the
suction lumen of the suction catheter.
36 FIG. 74 shows another embodiment of a suction
37 catheter also incorporating a nebulization of an aerosol.
CA 02596278 2007-08-21
-84-
1 In FIG. 74, a suction catheter 1400 is extends from the
2 ventilation lumen of an endotracheal tube 1250. The
3 suction catheter 1400 includes distal suction orifices
4 1402 located close to the distal end of the suction
catheter shaft. Located along the suction catheter shaft
6 proximally of the suction orifices 1402 are one or more
7 pairs of liquid and gas orifices 1404. The liquid and
8 gas orifice pairs 1404 are located with respect to each
9 other to produce an aerosol of the liquid being delivered
to the liquid orifice as in the previous embodiments.
11 The nebulization orifices 1404 are oriented radially from
12 the suction catheter shaft to direct the aerosol
13 delivered from the nebulization orifices 1404 toward the
14 airway passage wall. In one embodiment, the aerosol
being delivered is a mucolytic agent. The suction
16 provided by the suction orifices draws the mucolytic
17 agent delivered from the nebulization orifices as well as
18 mucous treated by the mucolytic agent in a distal
19 direction into the suction orifices 1402.
Another embodiment of the suction catheter with
21 aerosol delivery is shown in FIG. 75. A suction catheter
22 1410 is located in a ventilation lumen of the
23 endotracheal tube 1250. As in the previous embodiment,
24 the suction catheter 1410 has a distal suction orifice
1412 for removing mucous from the airway passage. In
26 addition, the suction catheter 1410 also includes distal
27 gas and liquid orifices 1414 located in proximity to each
28 other to produce a aerosol. The liquid and gas orifices
29 are located in a distal extension 1416 of the suction
catheter shaft so that they are distal of the suction
31 orifice 1412. The liquid and gas nebulization orifices
32 1414 are oriented in a proximal direction toward the
33 suction orifice 1412. The distal extension 1416 is
34 formed to bring the nebulization orifices 1414 close to
the wall of airway passage so that the aerosol delivered
36 from the nebulization orifices 1414 washes the airway
37 passage wall. As in the previous embodiment, the aerosol
CA 02596278 2007-08-21
-85-
1 delivered may be a mucolytic agent to facilitate
2 suctioning of the mucous out of the airway passage. The
3 pressurized gas flow may be used to contribute to the
4 dislodgement of mucous from the airway passage walls.
F. Nebulization with Vibration
6 A vibrating orifice, a screen with multiple
7 orifices or perforations, or a vibrating wire located at
8 the distal tip of the nebulizing catheter may also be
9 employed to assist in the generation of fine aerosol
particles. The vibration may be generated by
11 electromechanical, hydraulic, pneumatic, or piezoelectric
12 means. The vibrations may be generated at the tip of the
13 catheter, in the shaft, or extracorporeally.
14 One embodiment of a nebulizing catheter
incorporating a vibrating tip is shown in FIG. 76. A
16 nebulizing catheter 1300 includes a shaft 1302 through
17 which extend a lumen 1304 for the delivery of a liquid
18 medicine and a lumen 1306 for the delivery of a
19 pressurized gas. The liquid lumen 1304 communicates with
a distal liquid orifice 1308 and the gas lumen 1306
21 communicates with a distal gas orifice 1310 located at a
22 distal end of the nebulizing catheter shaft. At the tip
23 the orifices 1308 and 1310 may be drilled or formed in a
24 piezoelectric material 1314 or may be drilled or formed
in an orifice insert, plate, tube or screen mechanically
26 attached to the tip such that the vibrations of the
27 material are transferred to orifices. Although both the
28 gas and liquid orifices may be vibrated, alternatively
29 only the liquid orifice may be vibrated. In still a
further embodiment, the entire shaft of the catheter may
31 be vibrated so that the vibrations are transferred to the
32 tip. The vibrations may be amplified by mechanical means
33 to increase the amplitude of the orifice oscillation.
34 Two electrical lead wires 1316 and 1318 may be used to
conduct bipolar or unipolar pulses from an extracorporeal
36 generator and control circuit to the piezoelectric
CA 02596278 2007-08-21
-86-
1 material 1314. The amplitude and frequency of the
2 orifice vibrations may be adjusted to optimize aerosol
3 production based on the gas and solution flow rates, the
4 orifice configuration, and the desired size of the
aerosol particles. The generation device would be
6 provided with a current leakage sensor to terminate its
7 operation in the event it detects current leakage in the
8 system. The vibrations can be pulsed to coincide with
9 inspiration and also to control heat generated by
vibration at the tip. One or more gas supply lumens and
11 orifices at the catheter tip can be used to assist in the
12 dispersion and transport of the particles produced at the
13 vibrating orifice.
14 In a further alternative embodiment, the
orifice may be vibrated by means of a vibrating wire
16 connected to the orifice that is caused to vibrate from a
17 generator connected to the proximal end. In still a
18 further embodiment, a vibrating wire, similar to the wire
19 tip shown in FIGS. 16-19, may extend distally past a non-
vibrating orifice to cause aerosolization of a liquid
21 delivered from the orifice that impinges onto the
22 vibrating wire. In a still further embodiment, the tip
23 may be vibrated remotely, e.g. from a source outside the
24 body, by means of a magnetic field.
A liquid supply to the catheter tip can also be
26 rapidly pulsed to cause small droplets to be ejected at
27 the solution orifice. This may cause a finer aerosol to
28 be developed than by feeding a continuous stream of
29 solution to the orifice. The pulsation can be
accomplished by rapidly expanding and contracting all or
31 part of the solution reservoir (including the lumen).
32 The expansion and contraction of the reservoir can be
33 caused by electromechanical, hydraulic, pneumatic, or
34 piezoelectric actuators forming the reservoir, within the
reservoir or moving flexible portions of the reservoir.
36 Such an embodiment is shown in FIG. 76. A nebulizing
37 catheter 1500 includes a shaft 1502 having a gas lumen
CA 02596278 2007-08-21
-87-
1 1502 connected to a source of pressurized gas 1504 and a
2 liquid medicine lumen 1506 connected to a source of
3 liquid medicine 1508. Included in the liquid medicine
4 source 1508 is a means for imparting compression waves or
pulsation into the liquid. The waves are indicated in
6 the liquid at 1509. The wave imparting means may be a
7 transducer 1510 or other similar device. The vibration
8 inducing device 1510 may be driven by a frequency
9 generator 1513 at a frequency greater than 100 hertz.
The vibrations induced in the liquid may be focussed or
11 directed toward the distal liquid orifice 1514.
12 In the case where the vibrations are generated
13 at a location proximal of the tip, the nebulizing
14 catheter shaft may incorporate a mechanical means in the
shaft or near the orifices capable of transmitting or
16 amplifying the pulsations. In the embodiment of FIG. 77,
17 a wire 1516 may extend from the pulsation generating
18 means 1510 into the liquid lumen 1506 to help convey the
19 vibrations 1509 to the distal orifice 1514. The
pulsations imparted to the liquid may be used to generate
21 an aerosol from the distal liquid orifice 1514 or
22 alternatively may be used in conjunction with the
23 pressurized gas delivered through the gas lumen 1502 for
24 enhanced aerosolization. The amplitude and frequency of
the orifice vibrations may be adjusted to optimize
26 aerosol production based on the gas and solution flow
27 rates, the orifice configuration, and the desired size of
28 the aerosol particles.
29 The volume dispersed from the liquid orifice
1514 by each pulse should be less than approximately 10
31 microliters and the pulsation should occur at a frequency
32 greater than 100 Hertz, although smaller volumes and
33 faster frequencies may be used to produce a finer
34 aerosol. It is preferable that the reservoir and lumens
be constructed or a material of minimal compliance to
36 ensure minimal attenuation of the pulsation. The gas
37 supply orifice at the catheter tip can be used to assist
CA 02596278 2007-08-21
-88-
1 in the dispersion and transport of the particles produced
2 at the solution orifice. These micro pulsations can be
3 incorporated into a series with pauses between them to
4 coincide with the patient's inspiratory phase.
G. Other Method for Aerosol Generation
6 The above embodiments describe a nebulization
7 catheter in which an aerosol is generated by directing a
8 pressurized gas through a catheter near an orifice from
9 which the liquid to be nebulized exits. It is considered
to be within the scope of the invention described herein
11 to use other means or agents to generate an aerosol for
12 delivery of a medication to the respiratory tract. For
13 example, the above embodiments may be used in conjunction
14 with devices that utilize other means to generate an
aerosol of a liquid medication. A liquid delivered by a
16 single liquid lumen may be nebulized by applying
17 ultrasonic energy to the liquid, electrospray, steam, or
18 a micropump similar to those used in ink jet type
19 printers. These alternative approaches to nebulization
may be substituted for the use of a pressurized gas for
21 some of the embodiments described above, or may be
22 combined with pressurized gas or with each other to
23 produce an aerosol of the liquid medication.
24 The nebulization catheter embodiments described
herein could also be used in other types of nebulizers
26 that are used externally of a patient's respiratory
27 system, such as small volume nebulizers (SVN),
28 humidification nebulizers, or nebulizers used for ocular
29 or nasal drug administration. When used in such other
types of nebulizers, the embodiments of the nebulization
31 catheter disclosed herein provide for a fine aerosol
32 without the potential disadvantages of impacting the
33 liquid on a baffle or recirculating the liquid medicine
34 on a continuous basis which are common in such
nebulizers.
CA 02596278 2007-08-21
65902-72D
-89-
It is i_ntended that thz f oregoing de tailed
2 descr.iption be regaxcied as illustrative rather than
1i.mitIng and that it is understood that the following
d claims including all equivalents are int.ended to def i ne
~ trie scope of the i.rivention_