Note: Descriptions are shown in the official language in which they were submitted.
CA 02596295 2007-08-15
-1-
METHOD AND APPARATUS FOR STERILIZING OR DISINFECTING
A REGION THROUGH A BANDAGE
Field of the Invention
The present invention relates generally to the field of sterilization or
disinfection systems
and methods.
Background of the Invention
Infection is a primary concern in health care settings. Bacteria and other
potentially
harmful microbes can generate infections when they enter the body through
wounds, catheter
entrance sites, and other openings in the body, thereby bypassing the body's
natural defenses.
Infections, often absent at the time of admission to a hospital, are a serious
source of morbidity,
mortality, and excess cost in health care settings.
Catheters, a frequent conduit into the body for microorganisms, are typically
sterilized
before insertion into the body. Further, regions of skin that are or will be
breached are typically
treated with antiseptic or germicidal chemicals. As evidenced by the continued
high rate of
infection of catheter entrance sites and/or wounds, it is clear that the
present techniques for
sterilizing these regions are inadequate.
While ultraviolet radiation has been used for the sterilization of
disinfection of objects in
some applications, ultraviolet light has long been associated with skin
cancer,
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-2-
sunbums, and other harmful skin effects. Common wisdom and practice has
encouraged
the non-exposure of skin to ultraviolet radiation.
Summary of the Invention
One embodiment of the invention is directed to a method of sterilizing or
disinfecting a region undemeath a bandage on a patient. The method comprises
an act of
applying ultraviolet light to the region through the bandage.
Another embodiment of the invention is directed to an apparatus for
sterilizing or
disinfecting a region of tissue of a patient. The apparatus comprises an
ultraviolet light-
emitting lamp and a bandage adapted to transmit at least some of the
ultraviolet light
emitted by the lamp. The bandage covers at least a portion of the region of
tissue.
A further embodiment of the invention is directed to a method, comprising acts
of
determining the transmissivity of at least a portion of a bandage to
ultraviolet light, and
selecting an intensity of ultraviolet light to be applied through at least a
portion of the
bandage. Another embodiment of the invention is directed to a bandage,
comprising
an ultraviolet light-transmissive film and a color-changing material coupled
to the
film to indicate an exposure of the film to ultraviolet light.
A further embodiment of the invention is directed to a device for use with a
catheter inserted at an entrance site through skin of a patient. The device
comprises a
component having a conduit to retain the catheter and space the catheter from
the skin of
the patient near the entrance site, wherein the component is located and
shaped such that
the component assists in forming a substantially air-tight seal between the
skin and a
bandage adhered to at least a part of the component.
--Anottier emGo(cimeff -o-f ttie mven ion is d'iftTed-fo--a-Tevice or use
witfi a
catheter inserted at an entrance site through skin of a patient. The device
comprises a
component having a conduit to retain the catheter and space the catheter from
the skin of
the patient near the entrance site, wherein the component is located and
shaped such that
the component assists in forming a substantially light-tight seal between the
skin and a
bandage adhered to at least a part of the component.
CA 02596295 2007-08-15
-3-
A further embodiment of the invention is directed to a method of using an
ultraviolet-
transmissive bandage. The method comprises acts of applying the bandage over
skin of a
patient, and applying ultraviolet light through the bandage to the skin.
Brief Description of the Drawino
Figure 1 illustrates a method for sterilizing or disinfecting a region of skin
or tissue with a
light source;
Figure 2 illustrates a method for sterilizing or disinfecting a catheter
entrance site with a
light source;
Figures 3 and 4A-4E illustrate an instantaneous sterilization/disinfection
unit;
Figures5A-5C illustrate a continuous process sterilization/disinfection unit;
Figures 6A-6B illustrate a light directing component for use with a
sterilization/disinfection unit;
Figures 7A-7C illustrate the light directing component of Figures 6A-6B used
with the
instantaneous sterilization/disinfection unit of Figures 3 and 4A-4E;
Figure 8 illustrates a first embodiment of a UV-transmissive bandage;
Figures 9A-9B illustrate another embodiment of a UV-transmissive bandage;
Figures 10A-lOC illustrate a further embodiment of a UV-transmissive bandage;
Figures 11A-11B illustrate another embodiment of a UV-transmissive bandage;
Figure 12 illustrates the instantaneous sterilization/disinfection unit of
Figures 3 and
4A-4E used with a UV-transmissive bandage;
Figure 13 illustrates the continuous process sterilization/disinfection unit
of Figures 5A-
5C used with a UV-transmissive bandage;
Figures 14A-14C illustrate the instantaneous sterilization/disinfection unit
of Figures 3
and 4A-4E used with the light directing component of Figures 6A-6B and a UV
transmissive
bandage;
Figure 15 illustrates a self-sterilizing attachment coupled to the
instantaneous
sterilization/disinfection unit of Figures 3 and 4A-4E;
Figure 16 illustrates a block diagram of exemplary circuitry for use in the
instantaneous
sterilization/disinfection unit of Figures 3 and 4A-4E; and
CA 02596295 2007-08-15
WO 02/102419 PCT/L7S02/19147
-4-
Figure 17 illustrates a schematic diagram of exemplary circuitry for use in
the
instantaneous sterilization/disinfection unit of Figures 3 and 4A-4E.
Detailed Description
As mentioned above, ultraviolet light is potentially harmful to the sldn.
Consequently, many individuals take precautions against exposure. Because of
its
perceived dangerous nature, ultraviolet light has not been contemplated for
the
sterilization or disinfection of slcin, including wounded skin and healthy
skin, or catheter
entrance sites.
In view of the foregoing, one aspect of the present invention is directed to a
method and apparatus for sterilizing or disinfecting a region of tissue and/or
a catheter
entrance site of a patient using ultraviolet (UV) light. A region of tissue to
be sterilized
or disinfected may include unbreached slan, such as a region where a surgical
incision is
to be made, or breaclZed skin, such as a wound site or a catheter entrance
site. In the case
where a catheter entrance site is being sterilized or disinfected, a portion
of the catheter
in the vicinity of the entrance site may also be sterilized. Another aspect of
the invention
is directed to a method and apparatus for sterilizing or disinfecting a region
of tissue
and/or a catheter entrance site of a patient using W light transmitted through
a bandage.
It should be appreciated that while the terms "sterilize" and disinfect" are
used
generally herein, the methods and apparatus described may be used to achieve a
desired
level (e.g., low or high) of sterilization or disinfection. The sterilization
or disinfection
may occur by killing microorganisms, inactivating microorganisms (i.e.,
rendering the
microorganisms unable to reproduce), or any combination thereof. It should
furkher be
appreciaited tliat, accoraing~ the presenfinvention, a region ofI'issue or a
ca~e er
entrance site to be sterilized or disinfected may be that of either a person
or an animal.
Sterilization or Disinfection of Tissue and/or an Inserted Catheter
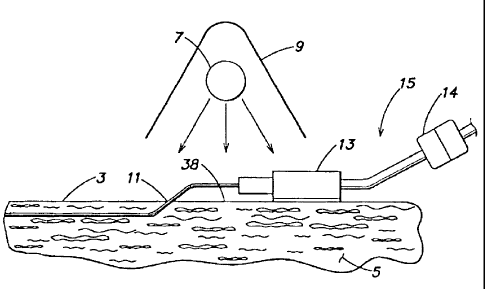
Figure 1 illustrates a method for sterilizing or disinfecting a region of skin
or
tissue of a patient using sterilizing or disinfecting light, in accordance
with one
embodiment of the invention. Sterilizing or disinfecting light is emitted by a
light source
7 and exposed to wound 1 and/or surrounding tissue 5. Tissue 5 includes skin 3
and
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-5-
tissue below the surface of skin 3. While slti.n 3 is highly attenuating to
sterilizing or
disinfecting light, some light may permeate to the tissue below slcin 3, for
example
exposing pores of skin 3. A reflector 9 is disposed near light source 7 to aid
in directing
light emitted by light source 7 towards wound I and surrounding sldn 3. While
reflector
9 is shown as disposed above light source 7, it maybe located on either side
of the light
source 7 or may be eliminated entirely. Further, additional reflectors may be
included
around light source 7 in accordance with the invention.
Light source 7 may be any light source that emits light capable of
sterilization or
disinfection. For example, light source 7 may be an ultraviolet (UV) light
source such as
a mercury vapor lamp, a xenon flash lamp, a continuous arc lamp, LTV light
emitting
diodes (LEDs), a UV laser, or any other solid state or non-solid state UV
light-emitting
device. The lamp may emit narrow spectrum light (e.g., a line spectrum) or
broad
spectrum light. Broad spectrum light may include, e.g., WA, LTVB, and UVC
light, or
UV light accompanied by light from another portion of the electromagnetic
spectrum.
For example, the emission of both UV and visible light from light source 7 may
enhance
the effectiveness of the light source, as the sensitivity of different
microorganisms to
light varies with the wavelength of the light. It should be appreciated that
though a single
light source 7 is described and illustrated, one or more light sources may be
used.
Light may be generated by light source 7 in one or more flashes. If multiple
flashes are generated, the flashes may be applied at specified intervals that
may occur, for
example, one or more times per day. A flash lamp or other non-continuous lamp
may be
used to generate light in one or more flashes. The lamp may be a high
intensity source of
sterilizing or disinfecting light where the sterilization dosage may be
applied in less than
a-&w-minutes-0r-seconds.---T-he-~nerg-y-oÃa single#lash-maybg-,sufficient-ta-
deltver a --
sterilizing or disinfecting dosage, e.g., greater than 10 mJ/cm2 of UVC, to
all surfaces to
be sterilized or disinfected.
Light may also be generated by light source 7 as continuous radiation over a
period of time. To generate continuous radiation, a lower intensity source
capable of
emitting sterilizing or disinfecting light continuously over a period of time
may be used.
3o The intensity of the light emitted by light source 7 may be adjusted for
use on skin of
varying sensitivity to ultraviolet light. For example, the light emitted by
light source 7
CA 02596295 2007-08-15
WO 02/102419 PCTIUS02/19147
-6-
may be controlled at a lower intensity if the sterilization of disinfection
method is
performed on an infant, for whom a lower intensity may be more appropriate.
Wound 1 may be a lesion, cut, abrasion, or sore sustained by the patient.
Alternatively, wound 1 may be an incision or puncture created by a healthcare
professional. The method described-above may also be applied to unbreached
skin, in
accordance with the invention. For example, the method for sterilizing skin 3
and/or
tissue 5 of a patient using sterilizing or disinfecting light may be used to
sterilize or
disinfect the skin at a penetration site prior to a medical procedure that
breaches the skin.
Thus, the method described in connection with Figure 1 may be employed by
medical
professionals prior to or after medical procedures that breach the skin. The
method may
also be employed by consumers or medical professionals to treat the skin after
accidental
breach of the skin.
Figure 2 illustrates a method for sterilizing an installed catheter and/or
surrounding skin of a patient using sterilizing or disinfecting light.
Sterilizing or
disinfecting light is emitted by a light source 7, which directs light towards
an entrance
site 11 of a catheter 15 and/or the catheter itself in the vicinity of
entrance site 11.
Entrance site 11 includes the opening in sldn 3 through which the catheter
passes.
Entrance site 11 may also include slcin 3 and tissue 5 surrounding the
opening. Reflector
9 may have any of the configurations described in connection with Figure 1.
Further,
light source 7 may have any of the configurations described in connection with
Figure 1,
and may be operated in any of the descnbed modes.
As shown in Figure 2, catheter 15 includes a hub 13 and a connector 14. Hub
13,
which is external to the patient, may be any junction where two or more
lumens, each
..having-separ-ate -tubing,-merga into-a-single- -multi<lumen-tube,-Canne6tar-
1-4-rnay be-a -
mechanism for attaching and detaching catheter 15 from external catheter
equipment
(e.g., a bag containing intravenous fluid). It should be appreciated that the
catheter
illustrated in Figure 2 is just one example of a catheter that may be
sterilized or
disinfected in accordance with the invention. As described herein, a catheter
may include
any conduit through which fluids or mechanical devices pass into or out of the
body. For
example, a standard injection needle, a blood sample needle, a cannula, a
trocar sheath,
an introducer, or a shunt may be considered a catheter. A device that breaches
the skin
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-7-
may also be considered a catheter. For example, a heart catheter, an
endoscope, or a
laparoscope may be considered a catheter. The catheter need not pass through
an
opening in the slcin; instead the catheter may pass through a natural opening,
as is the
case with Foley catheters or other urinary catheters. In the above cases, the
catheter
passes through the body's natural barrier to microorganisms, and thus renders
it
susceptible to infection.
Instantaneous Sterilization or Disinfection
Figures 3 and 4A-4E illustrate an instantaneous sterilization/disinfection
unit 16a
i0 adapted to generate one or more light flashes, in accordance with one
embodiment of the
invention. As shown in Figure 3, a housing 17 encloses a flash light source 7a
and
reflector 9. Reflector 9, disposed about flash light source 7a, causes light
emitted by
flash light source 7a to be reflected at range of angles, thereby m;n;miz;,,g
shadowing of
the skin under catheter 15.
Flash light source 7a and reflector 9 are optionally protected by a UV
transmissive window or screen (not shown) in an opening 26 at the bottom of
the unit.
The window may be made from quartz, fused silica, a UV transmissive glass or a
screen,
or a perforated sheet of metal or other material. In some applications, it is
desirable to
limit the amount of UVA, iTVB, visible, infrared light, and/or portions of the
WC
spectrum emitted, for example for use on sensitive skin or on infants
susceptible to
sunburn or local overheating. In this case, an optical filter may be
incorporated into the
window or the light source envelope to absorb or block undesired wavelengths.
Alternatively, a dichroic mirror, which passes, rather than reflects the
undesired
waveleng-ths, mayJbe used'.-A window or mirror may arso inc7ude a tertured
surface or
other diffusing mechanism to alter the exit angle of light and thereby reduce
shadowing:
A light seal 19 is disposed around opening 26 in instantaneous
sterilization/disinfection unit 16a. When light seal 19 is pressed against a
patient or an
object, it creates a substantially light-tight chamber to contain the light
emitted by flash
light source 7a and prevent injury or discomfort to the user or others nearby.
Thus, the
light emitted by flash light source 7a is substantially confined to housing 17
and the
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-8-
region on the patient surrounded by light seal 19. This region may include a
region of
ski.n 3 or tissue 5 and a region of catheter 15 near entrance site 11.
Light seal 19 may be formed from a complaint material. For example, light seal
19 may be formed from a convoluted and/or foamed opaque elastomeric material
such as
neoprene, inatural rubber, silicone rubber, or a thermoplastic elastomer
(TPE). The use of
a compliant material allows a substantially light-tight chamber to be formed
when light
seal 19 of instantaneous sterilization/disinfection unit 16a is placed over an
irregularly
shaped surface. For example, light sea119 may conform to a body, a bandage,
tape, or a
catheter and its components. In Figure 3, a portion of light seal 19 conforms
to the shape
of hub 13 of catheter 15. The compliance of light sea119 also allows
instantaneous
sterilization/disinfection unit 16a to be placed over catheter 15 for
sterilization/disinfection without disconnecting catheter 15 at connector 14
from external
catheter equipment. However, the external catheter equipment may be
disconnected at
connector 14 to allow hub 13 and connector 14 of catheter 15 to fit under
instantaneous
sterilization/disinfection unit 16a, within the confines of light seal 19,
during sterilization
or disinfection.
Instantaneous sterilization/disinfection unit 16a may be used to sterilize or
disinfect entrance site 11 prior to insertion of catheter 15 to prevent the
transport of
microorganisms from skin 3 to tissue 5 during insertion of the catheter, or
may be used
while catheter 15 is in place. Instantaneous sterilization/disinfection unit
16a may also
be used prior to penetration of skin 3 at the location of entrance site 11.
Instantaneous
sterilization/disinfection unit 16a may be used in addition to, or instead of,
chemical
treatment of slcin 3 with a chemical sterilizer or disinfectant, e.g., prior
to incision of slcin
3_atentrance-site_ 1L-Sterilization -or_disinfe.ctant-chemicals xnayincludr-
genni.cidal or
antiseptic chemicals such as alcohol, iodine, or betadine.
Instantaneous sterilization/disinfection unit I6a may contain safety interlock
actuators 21 coupled to light seal 19 to prevent accidental activation of
flash light source =
7a when the unit is not properly positioned. Safety interlock actuators 21
detect the
compression of light seal 19 at one or more locations (e.g., six as shown in
Figure 4C) to
verify that light seal 19 is placed against a surface before flash light
source 7a is allowed
to trigger. An alternate or additional safety interlock may be included to
prevent flash
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-9-
light source 7a from triggering unless the interior of housing 17 contains
substantially no
light, indicating that the light seal between the interior and exterior of
housing 17 is
substantially complete. A photodetector (not shown) in housing 17 may be used
to detect
the presence of light in housing 17.
As noted previously, instantaneous sterilization/disinfection unit 16a is
adapted to
generate light flashes. To generate light flashes, light source 7 may be a
xenon flash
lamp, and may be made with an envelope of quartz, fused silica, or UV
transparent glass
to maximize the output of UV light in the flash. Flash light source 7a may be
driven with
a high current density, e.g., 3,000 to 6,000 amps/cm2, and a short flash
duration, e.g., less
than 200 microseconds for a small flash unit, for maximum UVC light
production. The
energy required by flash light source 7a to generate a flash sufficient for
sterilization or
disinfection is deternzined by the amount of area to be illuminated, the
minimum
sterilizing light dosage desired, the uniformity of the illumination, and the
spectrum of
flash light source 7a. For example, a flash light source made from UV glass
used to
illuminate 25 square centimeters (about 4 square inches) produces a UVC energy
intensity of about 20 mJ/cm2 and a total flash input energy of about 20
joules. Flash light
source 7a may also generate UVA, LJVB, infrared, and visible light.
Instantaneous sterilization/disinfection unit 16a includes a circuit board 29
enclosed within housing 17. Circuit board 29 may include a capacitor 31 for
storing a
charge used by flash light source 7a to generate a flash, and circuitry to
charge the
capacitor and control the charging and flashing. Circuit board 29 is also
coupled to a
power source and safety interlock circuitry to prevent accidental triggering
at
inappropriate times. The circuitry required to charge the capacitor and
trigger the flash
may be-the-same-as that-used_in, ty-pical._photographic_flash units, w-hich is
well-knotun-in
the industry. One example of circuitry that may be included on circuit board
29 will be
discussed in connection with Figures 16 and 17.
Housing 17 includes a power switch 23 to initiate the charging of capacitor
31.
Power switch 23 may be a simple on-off power switch or pushbutton to control
the
power to circuit board 29 to charge capacitor 31. Power switch 23 is coupled
to a power
source, which is shown as batteries 33 in Figures 4A, 4B, and 4E. Batteries
advantageously allow instantaneous sterilization/disinfection unit 16a to be
portable and
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-10-
hand-held. Further, the power requirement for a typical
sterilization/disinfection unit is
such that several hundred of more sterilization/disinfection operations may be
performed
using a single set of batteries. However, external power from an AC power
source may
also be used. Housing 17 also includes a trigger switch 27 to control
activation of flash
light souroe 7a when safety interlock actuators, when present, are activated.
Power
switch 23 and/or trigger switch 27 may be manipulated manually (e.g., by
pressing a
button), or may be coupled to one or more actuators 21 in light sea119 to
trigger upon
depression of light seal 19. The inclusion of power switch 23 and trigger
switch 27
enhances the safety of instantaneous sterilization/disinfection unit 16a and
reduces its
power consumption. However, either of power switch 23 or trigger switch 27 may
be
eliminated, as they are not necessary to the operation of the unit.
A UV dosage control mechanism may also be included to vary the intensity of
the
UV light generated by flash light source 7a. For example, the UV light
intensity may be
varied to compensate for the application of W light through a bandage, which
will be
discussed in connection with Figure 12, or to account for the sensitivity of
the patient's
skin. The UV dosage control may be continuously variable or variable in
discrete steps
deterrnluied by a switch. The sterilizing light output is controlled by
altering the energy
stored in capacitor 31 by changing the voltage to which capacitor 31 is
charged, or by
switching one or more capacitors into the circuit to change the total
capacitance value.
A ready indicator 25, such as a light emitting diode (LED) may be included on
the
external surface of housing 17 to alert an operator when the charging of
capacitor 31 is
complete, and hence when a flash may be generated by flash light source 7a. A
second
indicator (not shown), or a color change or flashing of a light of indicator
25, may be
_included.-tQalert_atLagerat-Qr that-safety- interlockactuators211iave b.cen-
activated,._and-
hence that instantaneous sterilization/disinfection unit 16a unit may be
operated. A third
indicator (not shown), or a change in color or flashing of other indicators,
may be used to
indicate that a successful flash has occurred.
Instantaneous sterilization/disinfection unit 16a, described above, is just
one
exemplary apparatus for sterilizi.ng or disinfecting a catheter, a catheter
entrance site, a
wound, and/or a region of skin using one or more light flashes. Those skilled
in the art
will readily see many possible variations on the physical configuration,
electronic
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-11-
circuitry, and controls of instantaneous sterilization/disinfection unit 16a
described
above, which are intended to fall within the scope of the invention.
Continuous Process Sterilization or Disinfection
Figures 5A-5C illustrate a continuous process sterilization/disinfection unit
16b
adapted to generate continuous radiation for a period of time, in accordance
with one
embodiment of the invention. Continuous process sterilization/disinfection
unit 16b
operates on the same principles as instantaneous sterilization/disinfection
unit 16a,
except that light is generated by a continuous light source 7b at a lower
intensity and over
lo a longer period of time.
As shown in Figure 5A, continuous process sterilization/disinfection unit 16b
operates by positioning the unit over catheter 15 near entrance site 11, such
that it
illuminates entrance site 11 and surrounding skin 3 and/or tissue 5, as well
as a portion of
catheter 15 near entrance site 11. Continuous process
sterilization/disinfection unit 16b
is maintained in this position for a time sufficient to provide a sterilizing
or disinfecting
dosage of UV light. The sterilization may be completely continuous, or it may
be
intermittent and repeated at regular intervals as desired.
For convenience, continuous process sterilization/disinfection unit 16b may
include a mechanism for attaching the unit to a site to be
sterilized/disinfected or a
location near to the site, although the unit may be hand-held. For example,
adhesive tape
or straps with fasteners such as hook-and-loop fasteners (i.e., Velcro) may be
used. The
straps with fasteners may be looped around a portion of the body or fastened
to bandages,
etc. that are already attached to the body. Housing 17 may include receptacles
or
fastening point5-f -or-ffe sfraps:-Altemativ7y, adhesive tapE,- straps,-or
another attaclvnent
mechanism may be used to attach continuous process sterilization/disinfection
unit 16b
to catheter 15. Since the light seal for continuous process
sterilization/disinfection unit
16b is not critical, a primary advantage of attaching the unit is to hold the
unit in the
proper position for sterilization or disinfection.
If tape or bandages are used over entrance site 11, they may be removed before
sterilization or disinfection. If UV-transmissive tape and bandages are used,
they may be
left in place with the sterilization/disinfection unit placed over them, as
will be discussed
CA 02596295 2007-08-15
WO 021102419 PCT/US02/19147
-12-
in connection with Figure 13. Continuous process sterilization/disinfection
unit 16b is
designed to allow for its use over catheter 15 without disconnecting the
catheter from the
external circuit. Alternatively, the external catheter circuit may be
disconnected to allow
hub 13 and connector 14 of catheter 15 to fit beneath continuous process
sterilization/disinfection unit 16b.
As shown, a housing 17 of continuous process steriiization/disinfection unit
16b
encloses continuous light source 7b and reflector 9, and is coupled to a power
cord 35.
Reflector 9 reflects light from continuous light source 7b to the surfaces and
objects to be
sterilized or disinfected. Reflector 9 also serves to redirect the light so
that it strikes the
io surfaces and objects from a multitude of angles, thereby minimizing shadows
and
providing more uniform illumination.
Because the overall power requirement for continuous process
sterilization/disinfection unit 16b tends to be higher than for instantaneous
sterilization/disinfection unit 16a, it is.preferable to power the unit using
AC power
transmitted via a power cord 35, although in some applications batteries maybe
appropriate. To minimize the size and weight of the unit when batteries are
used, it is
preferable, but not necessary, to locate the batteries in a remote location
connected by a
power cord. Operator controls, such as an on-off switch and controls for a
timer are
preferably small and light-weight enough to be included in housing 17,
although they
may be remotely located at the other end of the power cord. Further, in the
example of
Figures 5A-5C, continuous process sterilization/disinfection unit 16b includes
a base 36
rather than a compliant light seal because the lower intensity of the light
generated by
instantaneous sterilization/disinfection unit 16a does not present as much of
a safety
concem,_although-ptecautions may-stilLbe appxopriate -to minimize exposure
.o.f-the_Eye,s
to the UV light.
Because a lower intensity of sterilizing or disinfecting light is required for
continuous process sterilization/disinfection unit 16b, as discussed above,
continuous
light source 7b may be a standard germicidal mercury vapor lamp. These lamps
produce
most of their energy at a wavelength of approximately 253.7 nanometers, in the
middle of
the UVC sterilizing band. With a mercury vapor lamp, continuous process
sterilization/disinfection unit 16b may require several minutes or more for
sterilization or
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-13-
disinfection. Mercury vapor lamps produce a small amount of energy at UV
wavelengths
outside of the UVC band, as well as energy in the visible spectrum. The
intensity of
WA and UVB light produced by these lamps is low and typically does not present
a
hazard for others neazby at the dosage level required for periodic
sterilizations or low-
level, long-term, continuous sterilization.
If the intensity of the UV light at skin 3 is low enough, continuous light
source 7b
may be illuminated for long periods of time (e.g., hours or days) without
damage to skin
3. Commonly available mercury vapor lamps typically produce an intensity
incompatible
with continuous operation, unless the light level is attenuated with an
optical filter or the
lo electrical drive to continuous light source 7b is controlled to reduce the
intensity of the
emitted light. A reduction in the intensity of the light output may be
accomplished by
turning continuous light source 7b alternately on and off. The alternation may
be
performed at a low frequency (e.g., with a period of a few seconds or
minutes), or at a
high frequency (e.g., with a period of less than a second). The alternation
may also be
performed at a low (less than 50%) or high (greater than 50%) duty cycle. The
switching
of power to continuous light source 7b may be performed with an electmnic
circuit, a
mechanical timer, or electromechanically, all of which are well known to those
skilled in
the art.
Alternatively, sterilization or disinfection operations may be performed once
a
2o day or a few times a day, and continuous light source 7b may be turned on
for long
enough to perform a complete sterilization or disinfection operation for each
instance.
The timing for each operation may be preformed by a standard timer or with a
light
sensor that measures light exposure and turns continuous light source 7b off
when a
desir-ed dosage-is-rEached_ -Continuous -p.r-ocess
sterilization/disinfection..unit 16b may. -
also be tumed on and off manually by an operator.
A UV dosage control may be included in continuous process
sterilization/disinfection unit 16b, to compensate for the application of W
light through
a bandage, which will be discussed in connection with Figure 13, or to account
for the
sensitivity of the patient's skin. The TJV dosage control may be continuously
variable or
variable in discrete steps determined by a switch. As discussed above, the
sterilizing
light output is controlled by altering the intensity of light emitted by
continuous light
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-14-
source 7b, the duty cycle of continuous light source 7b, or the total on-time
for each
sterilization or disinfection.
A UV transparent window (not shown), made of a material such as quartz, fased
silica, or UV transparent glass, may be included at opening 26 to protect
continuous light
source 7b while allowing light to reach the target surfaces. The window could
include an
optical filter to alter the spectrum of the emitted light. This may result in
a spectrum
having greater efficacy and/or less damaging light. The window could also
include a
textured surface or other diffusing mechanism to alter the exit angle of the
emitted light
and thereby reduce shadowing of the targets.
The drive circuitry for continuous light source 7b of continuous process
sterilization/disinfection unit 16b is included in housing 17. The circuitry
is not shown
here, as it is typically the same as that used for standard visible
fluorescent lamps and is
well known to those skilled in the art.
Continuous process sterilization/disinfection unit 1 6b, described above, is
just
one exemplary apparatus for sterilizing or disinfecting a catheter, a catheter
entrance site,
a wound, or a region of skin using a continuous application of radiation.
Those skilled in
the art will readily see many possible variations on the physical
configuration, electronic
circuitry, and controls of continuous process sterilization/disinfection utut
16b described
above, which are intended to fall within the scope of the invention. For
example,
continuous light source 7 may be replaced by a pulse light source that
requires a number
of pulses over a period of time to provide the required dosage. Continuous
light source 7
may be replaced by a broad-spectrum light source to provide other wavelengths
of light
along with W light. Optical filters or dichroic mirrors may be incorporated
into
continuous-process .sterilizati-onLdisinfEctian-unit -16b_to-alter -the-
spectrutn-0f the .- - - - -
outputted light by reducing the intensity of damaging wavelengths of light.
Sterilization or Disinfection Using a LiQht Directing Component
For complete sterilization of catheter 15 near entrance site 11, it is
desirable for
all points on catheter 15 near entrance site 11 to be exposed to the
appropriate dosage of
sterilizing light. Further, to prevent microorganisms from entering the body
at entrance
site 11, it is desirable that entrance site 11 and surrounding ski.n 3 be
sterilized or
CA 02596295 2007-08-15
WO 02/102419 PCT/LTS02/19147
-15-
disi.nfected. The shape of some of the catheter components makes it difficult
for light to
reach aIl points on the surface of catheter 15 and sldn 3 near entrance site
11, where the
catheter is placed against the slcin. The catheter may create a partially
shadowed area that
receives less light than other areas. The effects of shadowing may be
mitigated if the
total dosage of sterilizing light is high enough. However, a higher dosage
requires a
more powerful W light source and/or a greater exposure time, which may cause a
greater UV exposure to the skin than desired. Accordingly, in one embodiment
of the
invention, the components of the catheter are shaped to reduce shadowing
and/or include
light reflecting or refracting components to direct light to areas that might
otherwise be
partially or fully shadowed.
Referring again to Figure 2, catheter 15 is shown illuminated with light
source 7.
As shown, an area 38 of skin 3 under the portion of catheter 15 is ordinarily
not exposed
to light from light source 7 due to shadowing by catheter 15. Reflector 9
causes light
emitted by light source 7 to approach the target surfaces and objects from a
multitude of
different angles. Thus, some light will reach partially shadowed area 38, but
the total
intensity of the light striking area 38 will be less than that of the
surrounding areas.
Additional reflectors or diffusers may be used to further increase the
intensity of the light
strildng area 38.
Figure 6B illustrates an example of how catheter components maybe shaped to
2o direct light to partially shadowed area 38 for more uniform light
distribution. In this
example, a reflective surface 37 is included on a light directing component 41
to reflect
light from light source 7 to partially shadowed area 38. Light directing
component 41
may be the hub of catheter 15, as shown in Figure 6B, or may be an additional
compnnent, as. will be. describ_ed in connection with Figure_ 7A._ Thus,.light
dirEcting.
component 41 may be an existing portion of catheter 15 or a component added to
catheter
15. Reflective surface 37 may be a sloped and/or mirrored, as shown in Figures
6A and
6B. Although a curved mirror is shown, one or more planar mirrors or
refractive optics
such as a cylindrical lens made of a UV transparent material, may be used to
direct the
light from light source 7 to area 38 under catheter 15.
Tabs 39 may be provided on either side of light directing component 41 to
provide a mechanism for attaching light directing component 41 to the patient.
For
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-16-
example, tabs may be affixed to tissue 5 using sutures or an adhesive. The
upper surface
of light directing component 41 may be shaped in a smooth arch to provide a
better light
seal with instantaneous sterilization/disinfection unit 16a, as shown in
Figures 7A, 7B,
and 7C.
Figures 7A, 713, and 7C illustrate light directing component 41 used with
instantaneous sterilization/disinfection unit 16a. It should be appreeiated
that while
instantaneous sterilization/disinfection unit 16a is illustrated, other
sterilization/disinfection devices such as continuous process
sterilization/disinfection unit
16b may alternatively be used in this embodiment. In some catheter
installations, hub 13
is not positioned close enough to entrance site 11 for reflective surface 37
to perform the
desired function of directing light to area 38 if reflective surface 37 is
attached to hub 13.
Thus, in this embodiment,light directing component 41 is separate from hub 13.
Light-
directing component 14 may attach to tube 12 of catheter 15 and may be movable
along
tube 12 so that it may be positioned near entrance site 11 after catheter 15
is installed.
Further, light directing component 41 may have adhesive to hold light
directing
component 41 in place once it is positioned on sltin 3. Preferably, light
directing
component 41 holds tube 12 of catheter 15 slightly above the surface of slcin
3 to allow
sterilizing light to reach the slcin under tube 12. Figures 7A, 7B, and 7C
show catheter
15 passing through a hole 40 in light directing component 41, but altematively
the
component could have a groove to accoinmodate tube 12 of catheter 15. Light
directing
component 41 may be molded from plastic, an elastomer, or a photochromic
plastic or
elastomer. Alternatively, light directing component 41 may include a color-
changing
additive that changes color upon exposure to UV light. A color-changing effect
may
provide verification to an operator that the target site has been exposed to W
h_ght.
Light directing component 41 may not include reflective surface 37. In this
case,
the light directing component 41 may still hold tube 12 of catheter 15 away
from skin 3
to allow sterilizing light to reach area 38. If the dispersion of the light
from
instantaneous sterilization/disinfection unit 16a is high enough, partially
shadowed area
38 may receive enough sterilizing light from the unit without the use of a
specific
reflective surface. As above, light directing component 41 without reflective
surface 37
may include photochromic indicators to indicate an exposure to LTV light.
CA 02596295 2007-08-15
, = r
WO 02/102419 PCT/iTS02/19147
-17-
Sterilization/disinfection units 16a and 16b are designed to have a beneficial
effect when used with the standard catheters and installation techniques
currently in
common use. However, alterations to the physical configuration of the catheter
and the
positioning of external catheter components may improve the ease of use and
efficacy of
sterilization/disinfection units 16. These alterations include adding to or
changing the
shape of the external catheter components to mininuze shadowing and/or to
enhance the
light seal of the sterilization/disinfection unit, or adding color-changing
materials to
indicate UV light exposure.
io UV-Transmissive Bandage
The sterilization/disinfection units previously described are also designed to
have
a beneficial effect when used on bare skin, and they maybe used with
traditional
bandages if the bandage is temporarily removed for the exposure to the
sterilizing light.
However, in accordance with an embodiment of the invention, the method for
sterilization or disinfection described herein may be implemented with a UV-
transmissive bandage in place over the region to be sterilized/disinfected.
The term
bandage is intended to include any dressing, medical tape, pad, gauze, film,
ointment, or
paint-on wound covering, or any combination of features thereof.
Bandages that transmit sterilizing or disinfecting light may be made by
choosing
appropriate materials and configurations. For example, materials that are
typically
considered opaque to UVC light may transmit a significant percentage of UVC
light
when fabricated as a thin film. For example, a thin fihn of polyethylene (a
common
material used for medical applications) having a thickness of .002 inches (.05
mm)
#ransrnits up to -$0% -of sterilizing-light from -a xenon-#lash-having-a
wavelength-in the
range of 220 to 310 nm. Even films up to .01 inches (.25 mm) thick may
transmit over
50% of the sterilizing light. Adhesive tapes including a structural film and
adhesive with
a total thickness of .006 inches (.15 mm) may have a transmission of
sterilizing light of
greater than 60%. A typical eight-layer thick medical gauze pad transmits
about 30% of
the sterilizing light.
Medical bandages for use with catheters often consist only of a layer of
visually
transparent tape with a layer of adhesive added. Many of the visually
transparent films
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-18-
currently used are nearly opaque to light with a wavelength shorter than 310
nm and are
unsuitable for UV light transmission. However, bandages may be fabricated from
a
specific material in an appropriate thickness to enhance UV transmission. For
example,
bandages fabricated from hydrophilic polyurethane sheet material with a
thiclrness of
approximately.001 inch (025 mm) and with a film of acrylic based adhesive with
a
thiclrness of approximately .001 inch (.025 mm), as described in U.S. Pat. No.
4,595,001,
may have a transmission of sterilizing light that is greater than 500/o. This
transmissivity
is acceptable for sterilization of disinfection through the bandage. A bandage
for use
with a sterilization/disinfection unit may be manufactured to have a lcnown
and
controlled transmissivity to LJV light. Thus, the light output of the
sterilization/disinfection unit may be adjusted to deliver the correct dosage
of sterilizing
light to the skin and catheter components to be sterilized or disinfected.
Figure 8 illustrates a first con.figuration of a bandage 51 designed for use
with a
sterilization/disinfection unit, as described herein. As shown, bandage 51 has
an
adhesive 53 coupled to the periphery of a fihn 55 of bandage 51. Adhesive 53
may
attenuate UV light and therefore reduce the amount of light that reaches the
skin. To
minimize this attenuation, adhesive 53 in bandage 51 of Figure 8 is
selectively applied
such that the portion of film 55 that is placed above the entrance site of the
catheter is
free of adhesive 53. Adhesive 53 forms a seal around the periphery of bandage
51, which
will provide a barrier to microbes. Since UV light applied to the bandage may
pass
through region 57 of bandage 51, which does not contain adhesive 53, the UV
transmission characteristics of adhesive 53 are not critical and do not need
to be
controlled in manufacture.
--- -.$U-Qf the-bandages.deacritzedhereinlnay-lzeenhanced vvith-
additional.featutesio
facilitate their use with a sterilization/disinfection unit. In one example, a
radiant heat
attenuating material may be added to film 55 of bandage 51 to attenuate any
heat
generated by the UV light source. In another example, a color-changing
material, such as
a photochromic or fluorescent ink or dye may be added to adhesive 53 or film
55 of
bandage 51. The color-changing material may change color or emit light when
exposed
to LTV light. Alternatively, the color-changing material may change color or
emit light
when exposed to light from another portion of the spectrum. A color change
resulting
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-19-
from liglit from another portion of the spectnzm may still provide an
indication of UV
light exposure if the proportion of W light to the light from the other
portion of the
spectrum is known.
Since color-changing material may absorb some of the UV light applied, and
therefore reduce UV transmission, color-changing material may be included only
in a
portion or portions of bandage 51, as desired. For example, color-changing
material may
be applied to adhesive 53 or film 55 discontinuously, e.g., in a pattern. The
pattern may
be an array of lines, dots, or other small shapes, to allow the UV light to
sterilize or
disinfect the areas between the color-changing material. Alternatively, color-
changing
l0 material may be applied along the edge of bandage 51 so as to not interfere
with the
application of W light. In yet another alternative, for bandages that are
larger than the
illuminated area of the sterilization/disinfection unit, a small amount of
color-changing
material may be added to the entire bandage. While the addition of the color-
changing
material to the entire bandage may decrease the UV light transmission of
bandage 51 by a
small amount, the bandage will transmit a sufficient arnount of UV light as
long as the
total transmission of the bandage is known and the light output is adjusted
accordingly.
As discussed above, color-changing material may be added to adhesive 53. For
example, color-changing material may be included in adhesive 53 to make
adhesive-free
region 57 more obvious and, hence, easier to position. Another additive, other
than a
color-changing material, may alternatively be included to achieve easier
positioning.
Color-changing material may also be included in adhesive 53 to indicate that a
sterilization/disinfection operation has successfully occurred.
Also as discussed above, color-changing material may be added to film 55. For
example,_color-chang;ing materialmay-also be includeda.n_ar.-printed-onto-
film_55 of-
bandage 51 to indicate a region or level of exposure of bandage 51 to W light.
In
another example, color-changing material may be included in or printed onto
film 55 of
= bandage 51 in a meaningful pattern to convey iuiformation. As shown in
Figure 8, color-
changing material may be printed to form a logo 58, or other word or icon, or
a barcode
60. Color-changing material may also be printed to provide additional
information or
instructions to a user or indicate a manufacturer of the product.
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-20-
A color-changing material having a long time constant (i.e., a slow color
response) may also be added to film 55 ofbandage 51. The relaxation time
constant for
the color-changing material may be chosen to match the desired time between
doses of
UV light from a sterilization/disinfection unit. For example, when exposed to
a UV light
dose, the color-changing material may change to match a background color,
making the
color-changing material nearly invisible. As the color-changing material
changes back to
its original color, the material becomes more visible. When a user is able to
detect the
color-changing material, or a pattern formed by the material, the user may
determine that
reapplication of UV light is appropriate. Altematively, an optical detection
device (e.g.,
a photodetector) may be included in a sterilization/disinfection unit to
detect a pattern or
hue of the color-changing material, where a hue detected may include a color,
brightness,
saturation, or presence or absence of coloration of the color-changing
material. For
exainple, a pattern of color-changing material may form barcode 60, detectable
by an
optical detection device. The sterilization/disinfection unit may be designed
to operate
only when the barcode, or other pattern or hue, is readable.
Sterilization/disinfection unit may include a sensor to detect if it is being
used on
bare skin or a bandage. One way of sensing the material is to measure the
electrical
conductivity of its surface by making electrical connection with two or more
contact
points of the surface and measuring the resistance between the points. Human
skin will
typically have a resistance of less than a few megaohms, whereas the materials
used for a
bandage will typically be hundreds of times higher. The conductivity may also
be
measured using capacitive coupling and an alternating current sense signal to
measure
the coupling between the contact points. If a sterilization/disinfection unit
detects that it
is applied tabar.e_skin,_tlle-autput level -of its light source-maybPa
adjusted-to.a level
appropriate for bare skin.
The bandage detection feature may be used alone, or in combination with a
feature that automatically detects and adjusts the output of the light source
for different :
bandage types. For example, if the unit detects the presence of a bandage, a
photosensor
or other sensor may be activated to detect a code that appears on the bandage.
The code
may be, for example, a barcode printed on the edge of the bandage. The barcode
may
indicate the UV light transmission characteristics of the bandage so that the
CA 02596295 2007-08-15
WO 02/102419 PCT/LTS02/19147
-21-
sterilization/disinfection unit may adjust its output accordingly. A
sterilization/disinfection unit with this feature would need to be positioned
properly to be
operated, which would encourage proper use. The sterilization/disinfection
unit may
include operator indicators to inform the operator when the unit is properly
positioned
and the code may be read. Indicators may also be provided to inform the
operator as to
what intensity is being selected, or if more than one application is required
for proper
sterilization or disinfection through the bandage. This feature may be
combined with the
long time constant color-changing material used for the barcode to prevent the
application of W light more frequently than is required.
Bandages that include pads (like those sold commercially under the tradename
"Band-Aid," and larger varieties used in professional medicine) may also be
constructed
in a manner that allows sufficient sterilizing light transmission for use with
a
sterilization/disinfection unit. The pad provides greater flexibility, which
results in more
comfortable bandages and improved adhesion to the body. The pad may be made
from a
foamed polyethylene or similar material with significant transmission of UV
light. For
best transmission of UV light, the material would not have colorants added,
but would be
a clear or milky color. However, colorants that do not significantly degrade
the
transniission of UV light, versus visible light, may be used.
Figures 9A and 9B illustrate another configuration of a bandage designed for
use
with a sterilization/disinfection unit, as described herein. In this
configuration, bandage
51 has a pad 59 coupled to film 55 of the bandage, and a pad liner 61 coupled
to pad 59.
Pad 59 and pad liner 61 are sufficiently transmissive to UV Iight. The
adhesive on film
55 also preferably is sufficiently transmissive to W light in the thickness
used. A
variety-of adhesiue.s rneet-this-cond.ition,-inclucling some-cun-ently
usecLforanedical_
bandages and dressings. The adhesive on pad liner 61 holds pad 59 in position
and
adheres film 55 to the user's skin.
For sufficient W light transmission, pad 59 should be made from an appropriate
TJV transmissive material and be made in an appropriate thickness. The pads of
typical
prefabricated bandages are in the range of .02 inch (0.5 mm) to .06 inch (1.5
mm) thick
and are fabricated of medical gauze or a non-woven (felt-like) fabric. Some
bandages
include a perforated polymeric sheet liner on the pad, as shown in Figure 9B.
CA 02596295 2007-08-15
-22-
A UV transmissive bandage may be made with traditional materials if no
colorants are used in the film (as is typical). For example, the pad may be
made from 8
layers of medical gauze (approximately. 04 inch (1 mm) thick), and the pad
liner may be
made from a .002 inch (.05 mm) thick polyethylene sheet. In an exemplary
bandage, the
film with adhesive may have a sterilizing light transmission of 75%, the pad
may have a
sterilizing light transmission of 30%, and the liner may have a sterilizing
light
transmission of about 80%. This would result in a total sterilizing light
transmission of
about 18%. Although a higher transmission of sterilizing light is desirable,
it is still
possible to use a bandage of this construction in connection with the
sterilization and
disinfection methods described herein. Sterilizing or disinfecting the surface
of the skin
through this bandage would require a total sterilizing/disinfecting light
dosage of about
5.5 times that required for bare skin.
One exemplary alternative for the bandage described above is to substitute a
foamed polyethylene pad for a gauze pad. A foamed polyethylene pad with a
thickness
of .04 inch (1 mm) may have a sterilizing light transmission of 70%. The
foamed pad
presents a polymeric surface to the wound, so a pad liner is not required. A
bandage
made in this configuration has a total sterilizing light transmission of about
50%,
requiring only twice the sterilizing light intensity required by bare skin.
This
configuration has the advantage of requiring less energy from the
sterilization/disinfection unit, though there may be medical reasons why a
configuration
with a fabric pad is preferable. Both configurations may be used with an
appropriately
designed sterilization/disinfection unit.
FigureslOA, IOB, and lOC illustrate a further configuration of a bandage
designed for use with a sterilization/disinfection unit, as described herein.
In this
configuration, film 55 is used in place of pad liner 61 of the configuration
of Figure 9B.
Film 55 may be perforated at the position of pad 59 if it is desired for
fluids to flow into
the pad. Pad 59 is attached to film 55 with a movable fastening 63. The
fastening may be
an adhesive fastener or hook and loop fasteners, commonly know as VELCROTM.
Pad 59
may be completely removed or folded to one side. Thus, UV light from a
sterilization/disinfection unit may reach the skin of the user without
traversing pad 59.
Since the UV transmission characteristics of pad 59 are not critical in this
configuration,
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-23-
the pad thiclaaess and material may be determined based on medical
considerations.
Hence, thick gauze pads are possible. Further, in this configuration, the
underside of pad
59 may be sterilized or disinfected when the pad is totally or partially
disengaged from
film 55. Thus, pad 59 may be sterilized or disinfected during use to created a
sterile
surface.
The materials and/or colorants used in the bandages described herein may be
chosen and positioned such that the attenuation of the sterilizing light
through the
bandage is similar for all poraons of the bandage. This allows a sterilizing
dose of UV
light to be applied to the bandage without having some areas of the skin
underneath the
lo bandage overdosed, which could cause damage to the skin. For example, to
achieve
uniform attenuation of the sterilizing light, the section of film 55 of
bandage 51 that does
not cover pad 59 may be made to provide greater attenuation of the sterilizing
light than
film 55 covering pad 59 to compensate for the extra attenuation of pad 59. The
light
attenuation of the film may be controlled by printing film 55 with a colored
ink or dye
that absorbs, blocks or reflects the sterilizing light. Altematively, the
adhesive will
normally provide some attenuation to the sterilizing light and its thickness
and/or
composition may be controlled so the attenuation matches that of the pad. If
the pad is as
large or larger than the illununated area of the wound sterilizer/disinfector,
then the UV
transmission characteristics of the adhesive tape beyond the extent of the pad
may not be
relevant.
Since one of the side effects of the application of UV light to the skin is a
suntan,
it may be desirable to fabricate the bandage to make the tanned spot less
obvious by
feathering the edges. This may be done by grading the UV transmissivity of the
bandage
to successively_lo_tiv.er values-towards the. edges Df theilluminated area-
This vtould cause
any suntan to have a gradual edge, rather that a shape edge that would be more
noticeable
and displeasing. The plastic film in the tape or bandage could also include an
additive
that selectively absorbs or blocks the transmission of some wavelengths of
light to alter
the spectrum of light that reaches the skin to filter out harmful or undesired
wavelengths.
This filter could also reduce the suntan effect.
The bandages described herein may be used for many different professional and
consumer health care applications. Figures 11A and 1 1B illustrate another
configuration
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-24-
of a bandage designed for use with a sterilization/disinfection unit, as
descnbed herein.
The bandage of this configuration is typically a]arger bandage for use in
professional
applications. Bandage 51 includes a substantially square film 55 with a
substantially
square pad 59 attached thereto. This configuration has the advantage that a
secure
airtight seal may be formed on the complete periphery of bandage 51, which may
create a
complete barrier to external infection by microorganisms. Bandages with this
property
may be manufactured in a variety of sizes and shapes for professional medical
use,
consumer use, and veterinary medical use. Catheters and regions of sltin may
be
sterilized or disinfected with one of the described sterilization/disinfection
units before
and/or after the bandage is applied, and periodically with the bandage in
place, either by
medical professionals or by consumers.
Sterilization or Disinfection Using a UV-Transmissive Bandage
Figure 12 illustrates the instantaneous sterilization/disinfection unit 16a of
Figures 3-4 used with a UV-transmissive bandage 51. For illustrative purposes,
bandage
51 is shown covering wound 1. However, bandage 51 may alternatively or
additionally
cover a catheter, a catheter entrance site, or healthy skin. Figure 13
illustrates the
continuous process sterilization/disinfection unit 16b of Figures 5A, 5B, and
5C used
with bandage 51. Similarly, while bandage 51 is shown covering wound 1, it may
alternatively or additionally cover a catheter, a catheter entrance site, or
healthy skin.
Bandage 51 of Figures 12 and 13 may include any of the features or materials
described herein, and is not limited to any of the particular configurations
descnbed. As
discussed, sterilization/disinfection units 16a and 16b may generate UV light
at an
-intensify matclied to tlie IJV-trmns-m- igsivity of 15andage-51.~ The-
lightintensity-gelrerated
by sterilization/disinfection units 16a and 16b may be variable by means of a
knob,
switch, or other mechanism on the units. The UV transmissivity of bandage 51
may be
measured by a user or may be indicated, e.g., on the bandage itself. An
indication on
bandage 51 may be detectable by a sensor, e.g., a photosensor, within
sterilization/disinfection units 16a and 16b. Color-changing material coupled
to the
underside of bandage 51 may indicate an absorption of UV light and, hence, a
transmissivity of bandage 51.
CA 02596295 2007-08-15
WO 02/102419 PCTIUS02/19147
-25-
Although it is not necessary, bandage 51 may form a seal to prevent
contamination of the bandaged site. For example, the bandage may be formed of
a
continuous film that is impervious to microorganisms, such as bacteria and
viruses.
Existing commercially available bandages may have UV-transmissive properties,
although they are not intended to be used in sterilization or disinfection
operations that
use ultraviolet light. Thus, this incidental property of commercially
available bandages
makes them suitable for use with the described sterilization/disinfection
units 16a and
16b.
It is preferable to use bandages with controlled ITV transmission
characteristics so
as to achieve consistent results. Bandages with controlled UV transmission
characteristics may be made using conventional manufacturing processes with
additional
quality control of the materials and thickness used. As discussed previously,
additives
may be used on or in the film, pad, or adhesive of the bandage used with
sterilization/disinfection units 16a and 16b to control UV transmission or
block harmful
or undesirable wavelengths of light.
Sterilization or Disinfection Using a Ligbt Directing Component and a Bandaee
Figures 14A, 14B, and 14C illustrate light directing component 41 and
instantaneous sterilization/disinfection unit 16a of Figures 7A, 7B, and 7C
used with
2o bandage 51. It should be appreciated that while instantaneous
sterilization/disinfection
unit 16a is illustrated, other sterilization/disinfection devices such as
continuous process
sterilization/disinfection unit 16b may alternatively be used in this
embodiment. When
used with bandage 51, light directing component 41 may form an air seal with
tube 12 of
catheter I3 aridbandage 3 T-to prevei~ confaminafion o1'en-france siX 11 ty ex-
temg --
microbes carried by air. Light directing component 41 may assist in forming
this air seal
by providing a smooth convex curved surface over catheter 15, as shown in
Figure 14C,
to which bandage 51 is easily adhered. Without light directing component 41,
it would
be difficult for bandage 51 to form a complete seal between skin 3 and the
underside of
tube 12 of catheter 15.
Figure 14B illustrates a bandage 51 having a film 55 partially coated with an
adhesive 53. A region 57 of film 55 above catheter entrance site 11 is not
coated with
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-26-
adhesive 53. Region 57 without adhesive 53 is used to secure catheter 15 while
providing an ability to sterilize or disinfect entrance site 11 and the
surrounding region by
transmitting UV light through bandage 51. In the example of Figure 14B, region
57
without adhesive 53 is large enough to allow the UV light to sterilize or
disinfect an area
around entrance site 11 and allow the UV light to reach light directing
component 41 to
assure proper illumination under tube 12 of catheter 15. Adhesive 53 forms a
seal with
slrin 3 and light directing component 41 to prevent entrance site 11 from
being infected
from external microbes.
It should be appreciated that adhesive 53 need not be applied to bandage 51 of
l0 Figure 14B in the illustrated way, according to the invention. For example,
region 57,
which does not contain adhesive 53, may be larger or smaller, or shaped
differently.
Further, region 57 may be eliminated altogether so that adhesive 53 is applied
continuously, intermittently, in rows, in dots, or in any other type of
pattern.
To form a complete air seal, light directing component 41 is designed to have
intimate contact with tube 12 of catheter 15. This may be achieved in a
variety of ways,
such as molding the light directing component 41 from an elastomer so that it
forms a
tight fit over tube 12, forming a groove in light directing component 41 that
has a hinged
or separate piece to fill at least part of the groove, using a rigid light
directing component
41 with an inserted elastomeric seal, using the elastomeric properties of the
catheter 15 to
seal against a rigid light directing component 41, or forming a seal with the
addition of
an adhesive material around tube 12 of catheter 15.
Reflective surface 37 of light directing component 41 may be a separate
attached
component or it may be integral with light directing component 41. The light
directing
function-of lightdirec.ting- component 4-1.-may be-separated from the
lightand/ur air.
sealing function of light directing component 41 and one or more separate
components
may be used. It should be appreciated that while a number of example
configurations are
described to perform the functions of light directing, light sealing and air
sealing, those
skilled in the art will readily see a variety of other configurations that
mayperform these
function in various combinations.
Because the underside of hub 13 and/or light-directing component 41 is not
exposed to light, it is not sterilized once in position. However, is not
necessary to
CA 02596295 2007-08-15
i
WO 02/102419 PCT/US02/19147
-27-
repeatedly sterilize this area as the skin under hub 13 and/or light directing
component 41
is intact and provides an appropriate barrier to microorganisms. Skin 3 and
tube 12 of
catheter 15 in the vicinity of entrance site 11 need to be periodically
sterilized/disinfected
to prevent microorganisms from entering the body at entrance site 11. If the
area around
entrance site 11 is periodically sterilized or disinfected, and non-sterile
objects or air do
not come in contact with this area, the body is protected from infection
entering through
the entrance site 11.
Sterilization or Disinfection of a Sterilization/Disinfection Unit
The sterilization/disinfection units described herein may be used for multiple
patients in a professional medical environment. The sterilization/disinfection
unit itself
could become a vector to transmit microorganisms for one patient to another.
In
particular, a bottom surface 48 of light seal 19, which is not normally
exposed to LN
light, may come in contact with a patient, a catheter, or a bandage.
Figure 15 illustrates an example embodiment of a self-sterilizer attachment 42
for
instantaneous sterilization/disinfection unit 16a. Self-sterilizer attachment
42 includes a
housing 43, into which instantaneous sterilization/disinfection unit 16a may
be placed. A
light seal 47, disposed on the inner rim of housing 43, forms a seal with
instantaneous
sterilization/disinfection unit 16a when the unit is positioned within housing
43. Light
seal 47 may be compliant, and substantially prevents light from escaping from
housing
43 when instantaneous sterilization/disinfection unit 16a is in use. Self-
sterilizer
attachment 42 includes pins 45 at the base of housing 43. Pins 45 may be UV-
transmissive to allow the region on light seal 19 that contacts the pins to be
sterilized or
disirifected. When-light-sea119 6f iristantaneous sterilizatibnydisinfectiori
uiiit 16a
contacts and/or depresses pins 19, safety interlock actuators in light seal 19
of
instantaneous sterilization/disinfection unit 16a are activated. The
activation may engage
instantaneous sterilization/disinfection unit 16a in a "ready mode," which
allows an
operator to trigger generation of light by light source 7, e.g., by pressing a
trigger switch
on the unit. Alternatively, activation of the actuators may automatically
cause
instantaneous sterilization/disinfection unit 16a to emit light.
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-28-
Housing 43 includes one ore more reflective surfaces 49. Reflective surfaces
49
direct light to bottom surface 48 of light seal 19, the underside of unit 16a,
and/or the
exterior of housing 17 of unit 16a. Reflective surfaces 49 maybe formed of
aluminum,
mirrors, or another W-light reflective surface. When light is emitted by
instantaneous
sterilizatiob/disinfection unit 16a, reflective surfaces 49 direct light back
towards the unit
to cause sterilization or disinfection of its surfaces. More than one flash or
dose may be
applied for an increased UV light dosage to ensure complete sterilization, as
there are
typically no objects present within housing 43 that would be damaged by a
higher
exposure.
It should be appreciated that although instantaneous
sterilization/disinfection unit
16a is shown, self-sterilizer may be used with any of the
sterilization/disinfection units
described herein. Further, although self-sterilizer attachment 42 is shown as
an
attachment to the sterilization/disinfection unit, alternatively it may be
integrated
therewith. Although pins 45 are shown and descnbed as activating the
actuators, a
I S number of alternative configurations may be used to perform the same
function (e.g., a
light detector, a mechanical lever, a magnetic field detector, or a pressure
sensor).
Electrical Configuration of an Instantaneous Sterilization/Disinfection Unit
According to one embodiment of the invention, electrical circuitry associated
with a flash lamp of an instantaneous sterilization/disinfection unit 16a may
be
implemented as shown by electrical circuit 65 in Figure 16. Electrical circuit
65 may be
used in a sterilization/disinfection unit according to any of the embodiments
described
above. Electrical circuit 65 uses a high voltage power supply 69 that contains
a capacitor
to store tlie energy necessary-to-pawer a flasmp 67. - A power souico 71,-
wliicli may
be an AC line or a battery, typically supplies a voltage in the range of 200V
to 1000V
depending characteristics of the flash lamp used, although the voltage
supplied may be
smaller than 200V or greater than 1000V. Small linear flash lamps typically
operate with
voltages of 200V to 500V; small short-arc flash lamps may require 1000V or
more. The
voltage is selected based on the flash lamp specifications: the total energy
desired per
flash and the maximum flash current desired. A higher voltage will provide a
higher
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
- 29 -
flash current for the same energy, resulting in a greater percentage of the
flash light
output in the ultraviolet spectrum. The energy per flash is determined by
Equation 1:
E =1/2 CV2 [1]
where E is the energy per flash in Joules, C is the value of the energy
storage capacitor in
Farads and V is the voltage in volts. For a sterilizer/disinfector
application, the selected
voltage should be as high as possible so that the flash lamp produces the
greatest amount
of ultraviolet light. The value of the capacitor is then chosen to provide the
desired
amount of energy per flash. The energy required by the flash to perform the
sterilization/disinfection is determined by the amount of area to be
illuminated, the
minimum sterilizing light dosage desired, the uniformity of the illumination,
and the
spectrum of flash lamp 67. For example, a flash lamp made from UV glass used
to
illuminate 25 square centimeters (about 4 square inches) produces a UVC energy
intensity of about 20 mJ/cm2 with a total flash input energy of about 20
joules.
The sterilizer/disinfector circuitry also includes a flash lamp trigger 73
which is
very similar to the trigger circuit in a camera flash. The flash lamp trigger
provides a
very high voltage pulse, typically in the range of 4 kV to 15 kV depending on
the
specifications of the flash lamp, to initiate the flash. According to one
embodiment of
the sterilizer/disinfector, a charge storage capacitor is kept charged to the
appropriate
voltage whenever the unit is powered on. Safety interlock switch 75 may
prevent
triggering of flash lamp 67 when the a light seal formed by
sterilization/disinfection unit
16a is incomplete. Thus, flash lamp trigger 73 may be initiated when a trigger
switch
and a safety interlock switch 75 have been activated. Alternatively, either
trigger switch
(e.g., a pushbutton) or safety interlock switch 75 (e.g., mechanical
actuators) may
individually initiate flash lamp trigger 73.
Figure 17 shows one example of a typical battery powered xenon flash lamp
driver circuit with trigger circuitry for activating flash lamp 67. Circuits
of this nature
are commonly used in camera flash units. For simplicity, the diagram does not
show the
details of an AC power supply or user indicators. A power transistor 77 and
its related
components form a low voltage oscillator, typically in the range of 15 to 20
kHz.
Current from a high voltage transformer 79 passes through a high voltage diode
81 and
charges an energy storage capacitor 83 to a voltage that will drive flash lamp
67. A
CA 02596295 2007-08-15
WO 02/102419 PCT/US02/19147
-30-
resistor 85 charges a trigger capacitor 87 to the flash lamp voltage. When a
diac 92 and a
safety interlock switch 93 are turned-on, trigger capacitor 87 is discharged
through a
trigger transformer 89 which creates a very high voltage pulse to a trigger
electrode 91 on
flash lamp 67 This causes flash lamp 67 to flash using the stored energy in
energy
storage capacitor 83.
It should be appreciated that the above-descnbed circuitry is merely intended
to
illustrate one possible implementation, and many such circuits are possible
and known in
the art. For example, there exists in the art many circuits for driving flash
lamps that may
be suitably applied to the sterilizers/disinfectors described herein. Thus,
the invention is
not limited in this respect.
Having described several embodiments of the invention in detail, various
modifications and improvements will readily occur to those sldlled in the art.
Such
modifications and improvements are ultended to be within the spirit and scope
of the
invention. Accordingly, the foregoing description is by way of example only,
and is not
intended as limiting. The invention is limited only as defned by the following
claims
and equivalents thereto.
What is claimed is: