Note: Descriptions are shown in the official language in which they were submitted.
CA 02596305 2007-08-15
CROSS-REFERENCE TO RELATED APPLICATION
This application is a divisional of Canadian Patent Application No. 2,443,841
which is
based on international application No. PCT/US02/12518 filed on April 19, 2002,
which claims
the benefit of priority of U.S. Patent Application No. 09/846,309 filed on
April 30, 2001, the
disclosures of which are incorporated herein by reference.
CANNULA WITH FLOW DIVERSION MECHANISM AND METHODS OF USE
Field of the Invention
The present invention relates generally to medical devices useful for
cannulation of a
vascular tissue, such as the aorta, and for protecting against distal
embolization during
cardiovascular procedures. More particularly, the devices minimize plaque
dislodgement and
damage to a vessel wall during delivery of blood to the vessel.
Backaround of the Invention
Aortic cannuiation is commonly employed during various conventional or
minimally
invasive surgeries, such as coronary artery bypass grafting, heart valve
repair or
replacement, septal defect repair, pulmonary thrombectomy, atherectomy,
aneurysm repair,
aortic dissection repair, and correction of congenital defects, to establish
cardiopulmonary
bypass. After circulatory isolation of the coronary blood flow from the
peripheral vascular
system is established, a cannula is usually inserted in the ascending aorta to
deliver
oxygenated blood from a bypass-oxygenator to maintain blood flow to the
peripheral organs,
e.g., the brain and kidneys. It is well recognized that one of the
complications associated with
cardiovascular procedures is the dislodgement of emboiic materials generated
during
manipulation of the aorta or the heart, thereby causing occlusion of the
vessels downstream
from the aorta causing ischemia or infarct of the organs, e.g., stroke. To
minimize embolic
complication, an arterial filter is often temporarily deployed in the aorta
distal to the aortic
cannula to capture embolic debris.
However, when oxygenated blood is delivered to the aortic cannula through the
bypass-oxygenator, blood exits the cannula with a very high velocity, similar
to a jet-like
profile. When this jet is directed toward the aortic wall, it may damage the
aorta causing
aortic dissection or aneurysm. Furthermore, the jet may dislodge plaque on the
aortic wall,
causing distal embolization and peripheral organ infarction. When oxygenated
blodd is
allowed to flow into a filter, the jet may cause turbulent flow in the filter,
thereby washing out
CA 02596305 2007-08-15
the emboli caught in the filter. As a result of the swirling action by the
jet, the emboli may
escape around the edges of the filter to cause distal embolization and
CA 02596305 2007-08-15
WO 02/087652 ra a~vov~~... ~..
2
result in damage to peripheral organs, or may travel upstream to reach a
coronary artery
and cause myocardial infarction.
New devices and methods are thus needed in aortic cannulation to minimize
embolic dislodgement and vascular wall damage due to delivery of oxygenated
blood to
the aorta during cardiovascular surgeries.
S of the Invention
The invention provides devices and methods for reducing the jet like profile
of
blood delivered through a cannula and the swirling of the blood within a
filter. It will be
understood that, although the present invention is most useful in aortic
cannulation during
cardiovascular surgeries, the devices and methods can be used in any surgeries
where
delivery of fluid or blood through a cannula can potentially damage the body
tissue.
In a first embodiment, the cannula is an elongate tubular member having a
proximal end, a distal end, and a lumen therebetween. A blast plate deployable
from
within the lumen of the elongate tubular member is provided. The blast plate
is retractable
into the lumen of the elongate tubular member after use. In certain cases, the
cannula is
angled at its distal end, generally at a 90 angle to the axis of the lumen at
a proximal end.
In other cases, the cannula will further include a filter deployable from the
distal end of the
cannula. The filter may be mounted on the distal end of the cannula, or the
filter can be
mounted on a separately insertable member, such as a guidewire. In other
cases, the
cannula has more than one lumen extending from its proximal to its distal end.
In still
other cases, the cannula further comprises an occlusion member such as a
balloon
occluder, deployable from the distal end of the cannula. As with the filter,
the occluder
can be mounted on the cannula, or provided on a separately insertable member,
such as an
occlusion catheter.
The blast plate typically comprises a generally flat or curved surface, and
may
comprise a membrane mounted on a flexible wire ring. The membrane generally
comprises a semi-permeable material. In certain cases the member is a mesh
material. In
still other cases, the membrane is made of an impermeable material. While in
certain
cases the blast plate is formed in the shape of a planar surface defined by a
wire ring, in
other cases the blast plate is a cone-shaped sleeve. The sleeve can be made of
an
CA 02596305 2007-08-15
WO 02/087652 rt. i/ u ~uu i~j iLo
3
elastomeric material. The blast plate may also take the form of a
substantially flat surface
mounted at the distal end of a flexible or an inflexible elongate member. For
example, the
blast plate may be fixed to the end of a wire. The blast plate will be angled
relative to the
elongate member, and the angle may be selected from a 45 angle, a 50 angle,
a 55
angle, a 60 angle, a 65 angle, a 70 angle, a 75 angle, an 80 angle, an 85
angle, or a
90 angle.
Iti use, the surgeon inserts the cannula into a body cavity, e.g., a blood
vessel. It
will be understood that the cannula may comprise a standard commercially
available
cannula, or any of the novel cannula described herein. The surgeon will then
advance a
blast plate or dispersion mechanism through the lumen of the cannula and
beyond the
distal end of the cannula. The surgeon then flows a stream of fluid, e.g.,
blood, through
the lumen of the cannula. The blood flow hits the blast plate, and the blood
stream is
diffused and dispersed by the blast plate without jetting against the wall of
the aorta. After
the infusion procedure is complete, the surgeon retracts the blast plate into
the lumen of
the cannula.
It will be understood that the methods of use have particular application
where the
body cavity is a blood vessel, where the blood vessel is an artery, and where
the artery is
the aorta. It will further be understood that there are several advantages to
using the
diffusion-diversion devices and methods described herein. For example, by
dispersing the
stream of blood flow, the devices and methods (1) avoid "sand blasting"
embolic debris
from the lumen of the vessel, (2) avoid the swirling of blood that may carry
embolic debris
upstream during CABG to the coronary arteries, where myocardial ischemia can
occur, (3)
avoid turbulence that can force embolic debris around the periphery of a
deployed filter to
cause distal embolization which can results in stroke, renal failure, or other
organ damage.
Brief Description of the DrawinLzs
Fig. lA depicts a cannula having a blast plate deployed within an artery.
Fig. 1B depicts an end view of the artery and cannula of Fig. 1A.
Fig. 1 C depicts a cannula with blast plate deployed within an artery, and a
separately deployed filter through a second cannula.
Fig. 1D depicts a cannula having a filter and a blast plate deployed through
CA 02596305 2007-08-15
WO 021087652 rl ~/uau~~i~aio
4
separate lumens of the cannula.
Fig. 1E depicts removal of the blast plate of Fig. 1D.
Fig. 2A depicts a blast plate comprising a membrane mounted on a flexible wire
ring.
Fig. 2B depicts an end view of the artery and cannula of Fig. 2A.
Fig. 3A depicts a diverter that comprises a cone-shaped sleeve.
Fig. 3B depicts the diverter of Fig. 3A deployed within a filter.
Fig. 3C depicts an end view of the diverter and filter of Fig. 3B.
Fig. 3D depicts an oblique view of the diverter and filter of Fig. 3B.
Fig. 4A depicts a standard cannula and filter without a diverter.
Fig. 4B depicts a filter and cannula having a windsock embolic trap
incorporated in
the filter.
Fig. 4C depicts the use of the device of Fig. 4B in the ascending aorta.
Detailed Descritption
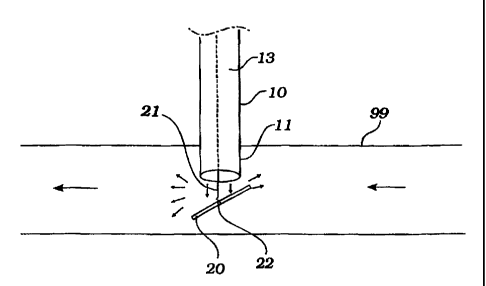
A first cannula with flow diverter is depicted in Fig. 1A. Cannula 10 having
distal
end 11 is deployed through an incision in vessel 99, in certain cases the
aorta. Blast plate
is fixed to elongate wire 21 at bond 22. Blast plate 20 is deployed through
lumen 13 of
cannula 10. Blood flow exits cannula 10, impacts blast plate 20, and is
scattered as shown
by the arrows surrounding blast plate 20. Fig. 1B depicts an end view of the
diverter and
20 cannula of Fig. 1B. As shown in Fig. IA, blast plate 20 is not necessarily
flat but can take
on a curvilinear configuration.
Fig. 1C shows a cannula and diverter deployed within vessel 99, and a separate
filter cannula. Filter cannula 30 carries separately insertable elongate
member 43 having
expansion frame 41 and mesh 40 disposed at a distal end of elongate member 43.
Expansion frame 41 is attached to elongate member 43 through active anchor
wire 42. It
will be understood that anchor wire 42 allows expansion frame 41 to expand to
fill the
lumen of vesse199. Mesh 40 is attached at an edge to expansion frame 41. In
other
devices, expansion frame 41 may be directly connected to elongate member 43.
In this
manner, the filter mechanism is separately insertable through cannula 30,
which is
introduced as a separate stick on vesse199.
CA 02596305 2007-08-15
WO 02/087652 PUTIUauZnaaia
Fig. 1D depicts cannula 10 having first lumen 13 and second lumen 12. First
lumen 13 is adapted for insertion of diverter mechanism 20. Second lumen 12 is
adapted
to receive and pass a separately insertable filter disposed at the distal end
of an elongate
member. Fig. lE depicts blast plate 20 being withdrawn through lumen 13 of
cannula 10.
5 In certain alternative embodiments, diverter 20 or alternately the
filter/diverter may
be stored in lumen 13 through which blood flows, so that the onset of flow
causes diverter
mechanism 20 and/or the filter to move distally and deploy once ejected from
the tip of the
cannula. The mechanism may be tethered to the cannula and may be removed with
the
cannula or withdrawn back into lumen 13 using a wire.
Fig. 2A shows an alternative construction of a diverter mechanism and filter
protection device. The diverter comprises wire ring 23 fixed to elongate
member 21 at
bond 22. An impermeable or semi-permeable material 24 covers wire ring 23 and
acts as
a blast plate for existing blood flow. Filter 40 includes expansion fframe 41
and cantilever
42. The reader is referred to Ambrisco et al., U.S. Patent No. 6,007,557,
incorporated as if
set forth in its entirety herein, for details on the design of a cantilever-
based expansion
frame. Fig. 2B depicts an end view of a membrane blast plate as shown in Fig.
2A.
Fig. 3A shows cannula 10 having angled distal end 11 disposed within vessel
99.
Diverter 20 takes the form of cone-shaped sleeve 25 formed of an impermeable
or semi-
permeable material. Sleeve 25 is open at proximal end 26 for receiving blood
flow from
arterial return cannula 10. Sleeve 25 disperses the jet stream of blood as
shown by the
arrows surrounding sleeve 25. Fig. 3B shows sleeve 25 used with filter 40
mounted on
expansion fiame 41. Fig. 3C depicts an end view of the filter with the cone-
shaped sleeve
of Fig. 3B. Sleeve 25 is connected to elongate member 28 by struts 27.
Elongate member
28 and sleeve 25 are separately insertable through cannula 10. Filter 40 and
expansion
frame 41 may be separately insertable or may be mounted on the distal region
of cannula
10. Fig. 3D shows an oblique view of the cannula, cone-shaped diverter sleeve,
and filter
of Fig. 3B.
Fig. 4A depicts standard cannula 10 and filter 50, without diverter
capabilities.
Unscattered blood flow from cannula 10 creates turbulence within filter 50
that may cause
emboli to escape downstream, and may carry other emboli upstream where they
can
become lodged in the coronary arteries, resulting in myocardial ischemia or
infarct. Fig.
CA 02596305 2007-08-15
WO 021087652 r%. l+ UO..M
6
4B shows a filter construction that traps emboli to prevent movement within
turbulent
blood flow. Expansion frame 41 is attached to filter mesh 60 that includes
reservoir tip 61
(in the shape of a windsock) for retaining captured emboli. This design will
immobilize
emboli and minimize the opportunity for proximal and distal embolization.
Fig. 4C shows the use of a filter with reservoir tip in the ascending aorta.
Expansion frame 41 is deployed through cannula 10 upstream the takeoff for
right
brachiocephalic artery 96, left common carotid artery 97, and left subclavian
artery 98.
Filter 60 includes reservoir tip 61. After filter 60 is deployed, arterial
return is provided
through cannula 10. After termination of arterial return flow, expansion frame
41 and
filter 60 are removed through cannula 10 before removing cannula 10. These
devices will
find application in any surgeries that can make use of arterial cannulation
and/or filter
protection, including coronary artery bypass grafting, heart valve repair or
replacement,
septal defect repair, pulmonary thrombectomy, atherectomy, aneurysm repair,
aortic
dissection repair, and correction of congenital defects.
The length of the cannula will generally be between 15 and 60 centimeters,
preferably approximately between 25 and 40 centimeters. The inner diameter of
the
cannula lumen will generally be between 0.5 and 1.5 centimeters, preferably
between 0.5
and 1.0 centimeters. The diameter of the expanded filter will generally be
between 0.3
and 3.0 centimeters, preferably approximately 2.0 and 2.5 centimeters for use
in the aorta.
The foregoing ranges are set forth solely for the purpose of illustrating
typical device
dimensions. The actual dimensions of a device constructed according to the
pri.nciples of
the present invention may obviously vary outside of the listed ranges without
departing
from those basic principles.
Although the foregoing invention has, for the purposes of clarity and
understanding, been described in some detail by way of illustration and
example, it will be
obvious that certain changes and modifications may be practiced which will
still fall
within the scope of the appended claims. For example, the devices and methods
of each
embodiment can be combined with or used in any of the other embodiments.