Note: Descriptions are shown in the official language in which they were submitted.
CA 02596421 2008-06-05
PROCESS FOR MAKING AND USE OF
ANIONIC CLAY MATERIALS
This application is a divisional
application of co-pending application Serial
No. 2,308,748, filed September 15, 1998.
BACKGROUND OF THE INVENTION
This invention is generally concerned with
methods of making anionic clays. Such clays are
to characterized by crystalline structures that consist
of positively charged layers that are separated by
interstitial anions and/or water molecules. The
positively charged layers are often comprised of
metal hydroxides of divalent metal cations (e.g.,
Mgt', Cat+, Zn2+, Mn2a, Coe+, Nis+, Sr2+, Bata and Cut')
and trivalent metal cations (e.g., A13% Mn3+, Fe 3-,
Co3+, Ni3+, Cr3+, Ga3+, and Lai+) . The interstitial
anions are usually NO3-, OH-, Cl-, I-, C032 ,
5042-, Si032 , HP032 , Mn04-, HGa032-, HV042-, 0104-,
B032-, monocarboxylates (e.g., acetate) and
dicarboxylates (e.g., oxalate), alkyl
sulphonates (e.g. lauryl sulphonate) and
various combinations thereof.
Therefore, anionic clays are further subdivided
according to the identity of the atoms that make up
their crystalline structures. For example, anionic
clays in the pyroaurite-sjogrenite-hydrotalcite
group are based upon brucite-like layers (wherein
magnesium cations are octahedrally surrounded by
hydroxyl groups) which alternate with interstitial
CA 02596421 2007-08-14
-2-
layers of water molecules and/or various anions
(e.g., carbonate ions). When some of the magnesium
in a brucite-like layer is isomorphously replaced by
a higher charged cation, e.g., A13+, then the
resulting Mg2'-Ala`-OH layer gains in positive
charge. Hence-r- an appropriate number of
interstitial anions, such as those noted above, are
needed to render the overall compound electrically
neutral.
The literature also teaches that as the
concentration of A13+ increases in a Brucite-type
lattice, a reduction of the lattice parameter known
as "a", takes place. The lattice parameter known as
"c" also is reduced. The reduction in lattice
parameter, a, is due to the smaller, plus three
charged, Al'* ions substituting for the larger, plus
two charged Mgt' ions. This higher charge causes
increased coulombic forces of attraction between the
positive charged Brucite-type layer and the negative
interlayer ions - thus giving rise to a decrease in
the size of the interlayer itself.
Natural minerals that exhibit such crystalline
structures include, but by no means are limited to,
pyroaurite, sjogrenite, hydrotalcite, stichtite,
reevesite, eardleyite, mannaseite, barbertonite and
hydrocalumite. The chemical formulas for some of
the more common synthetic forms of anionic clays
would include: [Mg6Fe2 (OH) 16] CO3.4H2O1 [Mg6A12-
(OH) 16] CO3 - 4H2O, [Mg6Cr2 (OH) 16] CO3 -4H20, [Ni6-
Fee (OH) 16] C03. 4H201 [Ni6A12 (OH) 16] C03. 4H201
[Fe4Fe2 (OH) 12] CO3 = #H201 [Ca2Al (OH) 6] (OH) p,75-
CA 02596421 2007-08-14
-3-
(COs) 0.125.2 .5H2O610H= 6H2O1 (Ca2Al- (OH) 6] OR - 3H2O1
[Ca2Al (OH) 6] OH = 2h20, [Ca2A1- (OH) 6] OH,
[Ca2Al (OH) 6] C1.2H20, [Ca2Al (OH) 6] 0 . 5C03. 2 . 5H2O1
[Ca2Al (OH) 6] 0 .5SO4.3H2O, [Ca2-Fe (OH) 610. 5 S04.3H20,
[ (Ni, Zn) 6A12 (OH) 16] C03.4H20,
(Mg6 (Ni, Fe) 2 (OR) 161 (9H) 2.2H20, [Mg6A12 (OH)16-1 (OH) 2.4H201
((Mg3Zn3) a12 (OR)161 C03.4H20, [Mg6A12 (OH) 16] SO4 = XH2O,
[Mg6A12 (OH) 161 (NO3) 2 =x-H20, [Zn6A12 (OH)16] CO3 =xH2O,
[Cu6Al2 (OR)16-1 CO3 = XH20, [Cu6Al2 (OH) 16] SO4 = XH2O and
[Mn6A12- (OH)16] CO3 =xH20, wherein x has a value of from
1 to 6.
Those skilled in this art also will appreciate
that anionic clays are often referred to as "mixed
metal hydroxides." This expression derives from the
fact that, as noted above, positively charged metal
hydroxide sheets of anionic clays may contain two
metal cations in different oxidation states (e.g.,
Mgt' and Al") . Moreover, because the XRD patterns
for so many anionic clays are similar to that of the
mineral known as Hydrotalcite, Mg6A12 (OH) 16 (CO,) = 4H2O,
anionic clays also are very commonly referred to as
"hydrotalcite-like compounds." This term has been
widely used throughout the literature for many years
(see for example: Pausch, "Synthesis of Disordered
and Al-Rich Hydrotalcite-Like Compounds," Clay and
Clay Minerals, Vol. 14, No. 5, 507-510 (1986). Such
compounds also are often referred to as "anionic
clays." Indeed, the expressions "anionic clay,"
"mixed metal hydroxides" and "hydrotalcite-like
compounds" are often found very closely linked
together. For example, in: Reichle, "Synthesis of
CA 02596421 2007-08-14
-4-
Anionic Clay Minerals (Mixed Metal Hydroxides,
Hydrotalcite)," Solid State Ionics, 22, 135-141
(1986) (at Paragraph 1, page 135) the author states:
"The anionic clays are also called mixed metal
hydroxides since the positively charged metal
hydroxide sheets-must contain two metals in
different oxidation states. Crystallographically
they have diffraction patterns which are very
similar or identical to that of hydrotalcite
(Mg6Al2 (OH)16 (C0,) = 4H2O) ; hence they have also been
referred to as hydrotalcites or hydrotalcite -like."
(emphasis added). U.S. Patent 5,399,329 (see col.l,
lines 60-63) contains the statement: "The term
`hydrotalcite-like ' is recognized in this art. It
is defined and used in a manner consistent with
usage herein in the comprehensive literature survey
of the above-referenced Cavani et al. article."
Hence, for the purposes of the present patent
disclosure, applicant will (unless otherwise stated)
use the term "hydrotalcite-like" compound(s) with
the understanding that this term should be taken to
include anionic clays, hydrotalcite itself as well
as any member of that class of materials generally
known as "hydrotalcite-like compounds." Moreover,
because of its frequent use herein, applicant will
often abbreviate the term "hydrotalcite-like" with
The methods by which HTL compounds have been
made are found throughout the academic and the
patent literature. For example, such methods have
been reviewed by Reichle, "Synthesis of Anionic Clay
CA 02596421 2007-08-14
-5-
Minerals (Mixed Metal Hydroxides, Hydrotalcite),"
Solid States Ionics, 22 (1986), 135-141, and by
Cavani et al., CATALYSIS TODAY, Vol. 11, No. 2,
(1991). In the case of hydrotalcite-like compounds,
the most commonly used production methods usually
involve use of concentrated solutions of magnesium
and aluminum which are often reacted with each other
through use of strong reagents such as sodium
hydroxide, and various acetates and carbonates.
Such chemical reactions produce hydrotalcite or
hydrotalcite-like compounds which are then filtered,
washed, and dried. The resulting HTL compounds have
been used in many ways - but their use as
hydrocarbon cracking catalysts, sorbents, binder
materials for catalysts and water softener agents is
of particular relevance to this patent disclosure.
It also is well known that HTL compounds will
decompose in a predictable manner upon heating and
that, if the heating does not exceed certain
hereinafter more fully discussed temperatures, the
resulting decomposed materials can be rehydrated
(and, optionally, resupplied with various anions,
e.g., CO,-, that were driven off by the heating
process) and thereby reproduce the original, or a
very similar, HTL compound. The decomposition
products of such heating are often referred to as
"collapsed," or "metastable," hydrotalcite-like
compounds. If, however, these collapsed or
metastable materials are heated beyond certain
temperatures (e.g., 900 C), then the resulting
decomposition products of such hydrotalcite-like
CA 02596421 2007-08-14
-6-
compounds can no longer be rehydrated and, hence,
are no longer capable of forming the original
hydrotalcite-like compound.
Such thermal decomposition of hydrotalcite-like
compounds has been carefully studied and fully
described in the-academic and patent literature.
For example, Miyata, "Physico-Chemical Properties of
Synthetic Hydrotalcites in Relation to Composition,"
Clays and Clay Minerals, Vol. 28, No. 1, 50-56
(1980), describes the temperature relationships and
chemical identity of the thermal decomposition
products of hydrotalcite in the face of a rising
temperature regime in the following terms:
"It is of interest to know the form
in which the Al occurs after thermal
decomposition of the hydrotalcite
structure. A sample with x = 0.287,
hydrothermally treated at 200 C for 24 hr,
was calcined at 300 -1000 C in air for 2
hr. After calcination at 300 C, both
hydrotalcite and MgO were detected by X-
ray diffraction, but after calcination at
400 -800 C only MgO could be detected. At
900 C MgO, MgAl~Q,-,, and a trace of y-A1201
were detected." (emphasis added, for
reasons to be explained in the ensuing
portions of this patent disclosure)
Miyata then goes on to note that:
"The crystallite size was smaller
than 50 A when the sample was calcined
below 800C. This value was much smaller
than that for MgO obtained from pure
Mg(OH)2. On calcination above 800 C, the
crystallite size rapidly increased. The
changes of the crystallite size and
lattice parameter a have the same
tendency. Consequently, Al substituting
in MgO acts to inhibit crystal growth. If
Al-containing MgO is reacted with water,
it should first form hydrotalcite.
Hydrotalcite calcined at 400-800 C with x
CA 02596421 2007-08-14
-7-
0.287 was hydrated at 80 C for 24 hr,
and the products were examined by X-ray
powder diffraction. According to Table 7,
hydrotalcite was the only hydrated product
detected in samples calcined at 400-700 C.
The lattice parameter a is the same as
that of the original sample. The samples
calcined at 800 C also formed only
hydrotalcite but their lattice parameters
are larger than that of the original
sample. According to Figure 1, the molar
ratio of this product is x = 0.235. On
the other hand, A1203 does not react with
water under the above-mentioned
conditions. Therefore, the results
suggest that Al enters product MgO when
hydrotalcite is calcined between 400 and
700 C." (emphasis added)
U.S. Patent 5,459,118 ("the '118 patent")
describes the character of the materials that result
from progressively heating hydrotalcite-like
compositions (HTlc's) in a passage running from col.
4, line 67 to col. 5, line 14. It reads as follows:
"The natural products of calcination
or activation in inert gas of a HTlc is
believed to be a spinel. In the range
between the temperature at which HTlc
decomposition commences (between 572 and
752 F.) (i.e., between 300 C and 400 C) and
that of spinel formation (1652 F.) (i.e.,
at 900 C), a series of metastable phases
form, both crystalline and amorphous.
Therefore, the surface area, pore volume,
and structure depend on the temperature of
calcination. Upon calcination, the
crystal structure of DHT-4A is decomposed
at about 660 F. (i.e.. 349 C) when water
and carbon dioxide evolved from the
structure, and a MgO-Al2O3 solid solution
of formula 4.5 MgO-Al2O3 is formed. This
solid solution is stable up to 1472 F.
(i.e.. 800 C) MgO and MgA12O4 are formed at
about 652 F. (i.e.. 900 C). On the other
hand, the solid solution calcined at less
than 1472 F. (i.e.. 800 C) can be restored
in the original structure by hydration."
(The underlined portions of this passage
have been added to convert OF to C in
order to more directly compare the
CA 02596421 2007-08-14
-8-
teachings of this reference with other
relevant references wherein temperatures
are expressed in C, again such
comparisons will be made in the next few
paragraphs of this patent disclosure)
It might also be noted here that this quotation
from the 1118 patent is a precise statement of the
temperatures at which certain hydrotalcite
decomposition products are described (e.g., spinel,
MgA12O41 formation taking place at 900 C when
hydrotalcite is thermally decomposed). This more
exact knowledge of the temperatures at which certain
aspects of the decomposition of hydrotalcite take
place, clarifies many other, more general,
statements found in the literature concerning the
temperatures at which certain decomposition products
are formed (e.g., statements concerned with the
temperature at which spinel, MgA12O41 is formed from
a hydrotalcite starting material). That is to say
that many, more general, statements concerning the
temperatures at which various hydrotalcite thermal
decomposition products (e.g., spinel, MgAl2O4) are
formed must be carefully interpreted. For example,
in U.S. Patent 4,889,615 ("the `615 patent") at col.
6, lines 36-43, we find the statement:
"Calcining the Mg/Al hydrotalcites at
temperatures greater than 500 C. gives a
mixture of MgO and MgA12O4, a magnesium
aluminate spinel, a material which has
been reported to reduce FCC regenerator
SO,, emissions (see U.S. Pat. Nos.
4,469,589 (Yoo) and 4,472,267 (Yoo)). The
activity of the dehydrated hydrotalcite
is, however, significantly different than
that observed for the spinel, MgO, or
mixtures of both. No evidence of
MgA13812O4 (sic) is observed in the
CA 02596421 2007-08-14
-9-
regenerated hydrotalcite indicating that
the spinel is not the active component."
(emphasis added)
Thus, in view of the previous, more precise,
descriptions of the temperature of spinel formation
(i.e., 900 C) in the 1118 patent, it seems that the
more general expression "temperatures greater than
500 C" used in the 1615 patent should not be taken
to mean something like 501 C, but rather should be
taken to mean 900 C, a temperature which is indeed
"greater than 500 C. " It also should be noted that
the above-quoted passage recognizes that "spinel is
not the active component" of the materials described
in the `615. We note this point here because it is
consistent with applicant's hereinafter described
goal of not making spinel - so that applicant's heat
treated, intermediate products can in fact be
hydrated (or rehydrated) to form hydrotalcite-like
compositions.
A similar general statement concerning spinel
formation from a hydrotalcite precursor appears in
U.S. Patent 4,458,026. There (at col. 3, lines 54-
56) we find the statement:
"Above 600 C. the resulting metal oxide
mixture begins to sinter and lose surface
area, pore volume, as well as form a
catalytically inactive phase (spinel-
MgA120,) ." (emphasis added)
Here again, applicant is of the opinion that
the general expression "Above 600 C" should not be
taken to mean something like 601 C, but rather
should be taken to mean far enough above 600 C to
CA 02596421 2007-08-14
-10-
form spinel - MgA12O4 that is to say 900 C, the
temperature at which spinel formation from a
hydrotalcite-like compound has been more precisely
determined. This quotation also notes that spinel
is "catalytically inactive".
Indeed, one--can even find generalized
statements about the temperature of spinel formation
that are better interpreted to mean lower
temperature levels. For example, in U.S. Patent
5,114,898 (at col. 4, lines 43-51) we find the
statement:
"Reichle in J. Catal. 101, 352 to 359
(1986) has shown that this heating of
hydrotalcite was accompanied by an
increase in the surface area from about
120 to about 230 m2/g (N2/BET) and a
doubling of pore volume (0.6 to 1.0 cm3/g,
Hg intrusion). Further heating to higher
temperatures causes lowering of surface
area as well as reactivity. At 1000 C. ,
the formation of MgO and the s n phase,
MgA12O4 has been observed." (emphasis
added)
In this case, applicant thinks that the
statement "At 1000 C the formation of MgO and spinel
phase has been observed", is better taken to mean:
spinel is observed at 1000 C because spinel (MgA12O4)
forms at 900 C - rather than taken to mean: 1000 C
is the temperature of formation of spinel. Indeed,
applicant has by his own experimental work confirmed
that spinel begins to from in HTL compounds at
900 C.
The prior art also has noted that when various
anionic clay-forming ingredients such as
hydrotalcite-forming ingredients (e.g., magnesium-
CA 02596421 2007-08-14
-11-
containing compositions and aluminum-containing
compositions) are mixed under certain prescribed
conditions (e.g., certain aging times, pH
conditions, temperatures, etc.), the resulting
slurry or precipitate materials (e.g., hydrotalcite-
like materials) rill exhibit distinct catalytic
properties. Hence, many such production processes
are based upon fine tuning of such time,
temperature, pH, etc. conditions in order to obtain
maximum amounts of a given kind of hydrotalcite-like
precipitate product.
The slurry and/or precipitate products of such
initial chemical reactions also have been heat
treated to obtain various "collapsed" or
"metastable" hydrotalcite materials that have
specific catalytic properties. Such collapsed
materials have, for example, been used as sorbents
(and especially SO, sorbents for fluid catalytic and
fixed hydrocarbon cracking processes), hydrocarbon
cracking catalysts, catalyst binders, anion
exchangers, acid residue scavengers and stabilizers
for polymers, and even as antacids intended for use
in the context of human medicine.
The prior art also has long recognized that
other ingredients such as compounds containing Ce,
V, Fe and Pt can be added to the original
hydrotalcite-forming reaction mixtures so they will
appear as a distinct phase of various solid products
created by such reactions. Dried forms of such
anionic clays (e.g., microspheroidal particles of
such hydrotalcite-like compounds used as so.,
CA 02596421 2007-08-14
-12-
sorbents in fluid catalytic conversion (FCC)
processes) also have been impregnated with solutions
of such metals. Moreover, such metals have even
been made a integral part of the crystalline
S structure of hydrotalcite-like materials (see, for
example, U.S. Patent 5,114,691 and U.S. Patent
5,114,898 which teach use of sulfur oxidizing
catalysts made of layered double hydroxide (LDH)
sorbents, e.g., hydrotalcite-like materials that
contain metal ions (e.g., those of vanadium) that
replace some or all of the divalent metals (Mg'') or
trivalent metals (Al'') that form the layers of the
LDH).
Hydrotalcite-like compounds that are used as
catalysts also have been both heat treated and
associated with various catalyst binder or matrix
materials. For example, U.S. Patent 4,866,019 (the
1019 patent) discloses that hydrotalcite can be heat
treated and used in association with various binder
materials. U.S. Patent 5,153,156 teaches a method
for making magnesium/aluminum synthetic anionic clay
catalysts by (1) spray drying a slurry of a
magnesium aluminum synthetic clay, (2) making a
plasticized mixture of the spray dried clay with
diatomaceous earth and (3) forming, drying and
calcining the resulting plasticized mixture.
The prior art also has long recognized that
anionic clay materials can be used to catalyze
certain specific chemical reactions. For example,
U.S. Patent 4,458,026 teaches use of certain heat
treated anionic clay materials as catalysts for
CA 02596421 2007-08-14
-13-
converting acetone to mesityl oxide and isophorone.
The anionic clays are given this catalytic activity
by heating them to temperatures ranging from about
300 to 600 C.
U.S. Patent 4,952,382 teaches a hydrocarbon
conversion process that employs a catalyst
composition containing an anionic clay wherein the
anionic clay serves as a sulfur oxides binding
material.
U.S. Patent 4,970,191 teaches use of
polymorphic magnesium-aluminum oxide compositions as
catalysts in various base catalyzed reactions such
as alcohol condensation, isomerization of olefins,
etc.
U.S. Patent 4,889,615 discloses a vanadium trap
catalyst additive comprising a dehydrated magnesium-
aluminum hydrotalcite.
U.S. Patent 5,358,701 teaches the use of
layered double hydroxide (LDH) sorbents such as
hydrotalcite-like materials as SO2 sorption agents.
This reference postulates that the sulfur-containing
gas absorbs into the hydrotalcite structure as SO,2-
anions by replacing the gallery Co,'- anions. The
absorbed sulfur is thereafter driven off by
calcination at elevated temperatures (500 C). The
LDH sorbents are regenerated by hydrolyzing the
calcined product, particularly in the presence of
CO2 or CO,2' .
U.S. Patent 5,114,691 teaches removing sulfur
oxide from gas streams using heated layered double
hydroxide (LDH) sorbents having metal-containing:
CA 02596421 2007-08-14
-14-
oxoanions incorporated into the galleries of the LDH
structures.
U.S. Patent 4,465,779 teaches catalytic
cracking composition comprising a solid, cracking
catalyst and a diluent containing a magnesium
compound in combination with a heat-stable metal
compound.
U.S. Patent 5,426,083 teaches catalytic use of
a collapsed composition of microcrystallites
comprised of divalent metal ions, trivalent ions,
vanadium, tungsten or molybdenum.
U.S. Patent 5,399,329 teaches making
hydrotalcite-like materials by preparing a mixture
of magnesium (divalent cation) to aluminum
(trivalent cation) in a molar ratio between 1:1 and
10:1, and in a mono carboxylic anion to aluminum
(trivalent cation) molar ratio between 0.1:1 to
1.2:1. The process involves reacting a mixture
comprising magnesium and aluminum cations and mono
carboxylic anions in an aqueous slurry having a
temperature of at least 40 C. and a pH of at least
7. Generally speaking, a given synthesis of a HTL
compound by any of the methods taught in these
patents was considered a success when the product of
its chemical synthesis reaction (slurries typically
were heated and/or pressured to form a final dry
product or precipitate) produces a given HTL
compound having an x-ray diffraction pattern which
reasonably resembles that of a given card in the
files of the international Center for Diffraction
Data ("ICDD").
CA 02596421 2007-08-14
-15-
In summarizing the prior art, it might be said
that most methods that have been employed to produce
anionic clay compounds, and especially hydrotalcite-
like, anionic clay compounds, usually involve
precipitation or slurry drying of a hydrotalcite-
like product, -washing and, optionally, heat
treatment of the resulting dried slurry, or
precipitated, composition. Once made, these HTL
compounds, or their thermal decomposition products,
have been employed as catalysts (e.g., as vanadium
passivators, SOx additives, aldol condensation
catalysts, water softening agents, and even
medicines).
CA 02596421 2007-08-14
-16-
SU1(ARY OF THE INVENTION
Applicant's contribution to this art has been
to discover certain hereinafter described methods,
whereby HTL compounds can be produced from compounds
that do not exhibit HTL structures (e.g., as
determined by their XRD patterns), but which do
exhibit HTL structures upon being activated by the
processes of this patent disclosure. Applicant also
has discovered certain novel methods whereby anionic
clays in general and hydrotalcite-like compounds in
particular can be given certain attributes
(increased hardness, density, etc.) that make such
compounds better suited for uses where these
attributes are desirable, e.g., as sorbents for
various chemical species - but especially SO,
sorbents - and especially those SO, sorbents (and
binder materials) used in FCC units, as hydrocarbon
catalysts, as water softening agents, etc.
Again, those compounds generally described as
"anionic clays" in the literature, and especially
hydrotalcite, and HTL anionic clay compounds, will
be collectively referred to as "HTL compounds" for
the purposes of this patent disclosure. More
specifically this invention involves formation of
hydrotalcite-like compounds by certain novel
production methods and the use of certain formed
shapes (microspheroidal particles, extrudates,
pellets) containing those hydrotalcite-like
compounds produced by applicant's processing
CA 02596421 2007-08-14
-17-
techniques. For example, these formed shapes (e.g.,
microspheroidal particles, pellets, extrudates,
etc.) for certain specific catalytic uses (e.g., FCC
operations, sox sorption, water softener
regeneration agents, etc.). Hence, the HTL
compounds of this-patent disclosure may constitute
part of (or even all of) a given catalyst particle,
pellet, extrudate, etc. By way of example the HTL
compounds of this patent disclosure may be
associated with various binder or matrix forming
materials known to the catalyst making art. Indeed,
the HTL compounds of this patent disclosure may be
used as catalysts per se (e.g., as hydrocarbon
cracking catalysts), as SOX binding agents, or as
catalyst binder materials for other catalyst
materials. Hence, for the purposes of this patent
disclosure the term "catalyst" should be taken to
mean not only those HTL compounds that have
catalytic or SOX binding activity in their own
right, but also those HTL compounds that are used as
binders, matrices and/or carriers for other
catalytically active compounds (e.g., binders for
metallic, SOX oxidation catalysts such as compounds
containing platinum, cerium and vanadium). These
applications are all related to the fact that the
HTL compounds produced by applicant's methods can,
among other ways, be characterized by their
resistance to mechanical stresses and, hence, by
their ability to function in the severe environments
associated with many chemical reactions.
CA 02596421 2007-08-14
-18-
Applicant's overall invention is primarily
based upon a two step "activation" procedure that is
generally comprised of heat treating and then
hydrating certain hereinafter described
hydrotalcite-producing, precursor compounds. This
two step process may, in some cases, be augmented by
an additional, but purely optional, heat treatment
step (which may be referred to as Step 3 of
applicant's process). These heat treated compounds
may be thought of "collapsed" or "metastable," HTL
compound-forming materials.
Applicant's invention has two general
embodiments. The first embodiment is a method for
producing HTL compounds (e.g., anionic clay
compounds, hydrotalcite per se, and various
hydrotalcite-like compounds) from compounds that do
not possess the structural characteristics of HTL
compounds. The manner by which this first
embodiment of applicant's invention differs from
prior art methods for making similar HTL compounds
is that applicant's initial HTL synthesis is carried
out using those ingredients and those reaction
conditions which are such that they do not directly
produce compounds having a HTL structure, but rather
produce compounds that exhibit a HTL structure only
after experiencing applicant's hereinafter described
activation process. Hence, in the first embodiment
of this invention, an actual XRD determination that
the product of applicant's initial slurry or
precipitation synthesis reaction does not produce a
compound having an XRD pattern that reasonably
CA 02596421 2007-08-14
-19-
resembles that of a compound having the proper
ingredient atoms (e.g., those of magnesium,
aluminum, oxygen and hydrogen in the case of HTL
compounds) on file with the ICDD could be an
optional step in applicant's overall process.
It also should be specially noted, however,
that applicant's synthesis products may well include
"amorphous" (non-crystalline) materials as well as
non-HTL, crystalline phases - and combinations
thereof. Indeed, the term "amorphous" as used
herein could include (1) crystalline phases which
have crystallite sizes below the detection limits of
conventional x-ray diffraction techniques, (2)
crystalline phases which have some significant
degree of ordering, but which lack a crystalline
diffraction pattern due to dehydration or
dehydroxilization (such as in layered
aluminosilicates), and (3) true amorphous materials
which may exhibit short range order, but no long-
range order, such as, for example,. silica and borate
glasses.
Whatever their physical form (crystalline or
amorphous), these precursor, synthesis reaction
products may be subjected to some form of "low
temperature" (i.e., "low temperature" may be taken
to mean less than about 250 C, for the purposes of
this patent disclosure) drying process before they
undergo the heat treatment aspect of applicant's
activation process. Such a low temperature drying
process also may include the physical formation of
those powders, pellets, beads, extrudates,
CA 02596421 2007-08-14
-20-
microspheroidal spheres or granule forms of these
reaction product materials that may be required (or
desired) for use of these materials as catalysts,
sorbents, ion exchange agents, etc. This drying
step should, however, be considered "optional"
because the most--fundamental version of the first
embodiment of applicant's invention could go
directly to its heat treatment step.
This heat treatment step involves heating
applicant's synthesis reaction products to a "medium
temperature" (i.e., a temperature in the range of
about 300 C to about 850 C). This heat treatment
may be carried out for widely varying periods of
time (e.g., from for about 0.1 to about 24.0 hours.
This 300 C-850 C heat treatment step may generally
be referred to as Step 1 of applicant's overall
"activation" process. It is more preferred,
however, that Step 1 be conducted at a temperature
on the low-end of this 300 C-850 C range. This
treatment may be carried out at some preferred
temperature (e.g., 450 C) or at different
temperatures in this 300 C to 850 C range. Step 1,
medium temperature, heat treatments in the range of
about 400 C to about 500 C are, however, highly
preferred. Temperatures at the upper end of
applicant's 300 -850 C range, such as temperatures
ranging from about 700 -850 C, are less preferred
since various less desirable phases (hereinafter
more fully described) may result from heating
applicant's precursor, synthesis reaction products
CA 02596421 2007-08-14
-21-
to such levels. The formation of these less
desirable phases may diminish the precursor
material's potential to form maximum amounts of the
HTL-containing phases that are the object of
applicant's processes.
These higher-- temperatures also are less
preferred because they come dangerously close to the
900 C temperature at which a particularly
undesirable material - namely, spinel (MgAl2O4)
begins to form. Again, applicant regards spinel
formation as "anathema" to this process because
spinel can not be rehydrated. This is not to say
however that any other material, e.g., MgO, that be
present in such a system at temperatures at or above
900 C, can not be employed for applicant's purposes.
For example, if applicant's hydrotalcite-like
starting material is converted into spinel (MgAl2O4)
it becomes useless for applicant's purposes; if,
however, applicant's starting material is converted
into MgO, it still may be useful (e.g., as an SOX
sorbent agent).
In any event, temperatures of 900 C or higher
can be regarded as "high temperatures" for the
purpose of this patent disclosure and they are to be
avoided as far as possible. This admonition also is
consistent with the teachings and spirit of the
literature. That is to say that nowhere does the
literature even remotely suggests that spinel can be
reversibly hydrated into any other phase at ambient
temperatures. By way of sharp contrast with this,
the literature teaches that HTL compounds such as
CA 02596421 2007-08-14
-22-
applicant's, very decidedly possess the
characteristic of rehydratability.
The literature also teaches that the basic
structural building block of HTL, the brucite
structure, Mg(OH)2, also possess this
"rehydratability" -eharacteristic. It is also known
that, if the crystal size of such materials grows
significantly (as it does with increasingly higher
thermal treatment temperatures), then such
"reversibility" is eventually lost. Consequently,
the brucite layer no longer forms upon rehydration.
This is the same situation applicant expected, and
in fact observed, for various HTL compounds made by
the teachings of this patent disclosure. Indeed,
applicant found that as temperature increases beyond
certain levels, an increase in a MgO-like material's
crystallite size, as well as alumina and magnesium
aluminate (spinel) formation, eventually do take
place. Consequently, for maximum SO, activity of
applicant's HTL compounds, it is preferred that all
the MgO in a given system remain with the HTL phase
as opposed to reacting with other phases and thereby
rendering the MgO "inactive" e.g., inactive as a SO,
"pickup agent." Again, this is best achieved by not
using temperatures above about 850 C.
In any case, the heat treated product of Step 1
of applicant's "activation" process is then
subjected to a hydration step. This hydration step
might be termed Step 2 of applicant's activation
procedure. It generally entails mixing the heat
treated product of Step 1 with a quantity of
CA 02596421 2007-08-14
-23-
moisture which is such that heat is evolved from the
heat treated precursor material/liquid (e.g., water)
mixture. The method or manner of hydration to
effect applicant's Step 2 will include, but not
limited to such methods as spraying, impregnating
and blunging.
In any case, the heat release produced by this
hydration is indicative of the heat of formation of
a HTL compound. Additionally, this heat release
signifies the occurrence of the chemical reaction
which is presumed to be the cause of the greatly
improved physical properties of HTL compounds
prepared by the methods of this patent disclosure.
It also should be noted here that in order to
maximize the amount of HTL compound produced by this
hydration step, the amount of water added should be
substantial in quantity (on the order of 30-50
weight percent of the dry, precursor material).
Such amounts of water are required in order to fully
form HTL phases although less water will still
result in a material that exhibits a HTL phase; such
a phase will not, however, be "pure," i.e., other
collapsed HTL phases will be present (i.e., a MgO-
like phase and/or a MgAl solid solution phase).
Again, depending on the hydration method to be
employed, the previously noted "low temperature,"
optional drying step also may be employed in order
to render a material having a desired amount of
physical water. And, once again, this low
temperature drying should not exceed about 250 C
because applicant has found that temperatures in
CA 02596421 2007-08-14
-24-
excess of this may result in a premature release of
various interlayer ions, water, crystalline water,
or certain carbonates. In any case, the HTL
compound product produced by applicant's hydration
step will possess a crystalline structure which
exhibits an x-ray--diffraction pattern that may, and
probably will, reasonably resemble a ICDD "card" for
some HTL compound that has a similar crystalline
structure.
In some cases this hydrated product may again
be "collapsed" by a second heat treatment step which
might be called Step 3 of applicant's process (e.g.,
Step 3 heat treatments at temperatures ranging from
about 300 C to 850 C and preferably at 400 C to
500 C) in order to remove its interstitial water so
that the resulting material is better suited to
certain uses such as a so,, sorbent in a FCC unit.
Compounds created by this third step may be used for
any of the purposes for which the HTL compounds
created by applicant's Step 1 and Step 2 materials
may be used.
From a broad conceptual point of view, the most
fundamental version of the first embodiment of
applicant's invention might be thought of as being
based upon: (1) a "delay" in the production of a
hydrotalcite-like compound end product relative to
the point at which analogous hydrotalcite-like
compounds have been made by prior art production
methods, (2) heat treatment (single stage or
multiple stage) of these "not yet" (e.g., with this
"not yet" quality or state being determined by XRD
CA 02596421 2007-08-14
-25-
methods) hydrotalcite-like materials and (3)
hydration of the these heat treated materials to
form hydrotalcite-like compounds. Stated another
way, it might be said that the goal of applicant's
initial synthesis or chemical reaction step is to
ng make as much-of a subject, end product, HTL
compound as possible (e.g., not to make as much
hydrotalcite as possible), but rather to make as
little of the desired end product compound, (e.g.,
to make as little hydrotalcite) as possible.
In any event, applicant's first general process
may generally employ any combination of those HTL
compound creating starting ingredients (e.g.,
magnesium-containing compounds having less reactive
anions and aluminum-containing compounds having less
reactive anions) and any of those reaction
conditions (e.g., short reaction aging times,
neutral pH levels, and ambient temperatures reaction
conditions) that may serve to - and, indeed, strive
to - produce a resulting slurry or precipitate
material that does not exhibit the crystalline
structure of the HTL compound that ultimately will
be exhibited by applicant's end product
hydrotalcite-like compound. In fact, the precursor
compounds obtained by the initial chemical reaction
step of applicant's first process may well be
entirely amorphous materials having no HTL structure
whatsoever.
In the second embodiment of applicant's
invention, however, a hydrotalcite-like compound is
purposely used as the starting material, or as a
CA 02596421 2007-08-14
-26-
precursor compound. That is to say that a
hydrotalcite-like starting material can be purchased
commercially - or it can be synthesized by use of
any of the many methods known to this art and then
be employed according to the teachings of this
patent disclosure In either case, however,
applicant's process calls for heat treatment of the
hydrotalcite-like compound (however obtained) to
form a "collapsed" or "metastable" material. This
heat treatment also may be thought of as Step 1 of
this second embodiment of applicant's invention.
The collapsed or metastable material of this second
embodiment is then hydrated to again form a
hydrotalcite-like compound. This hydration may be
thought of as Step 2 of this second embodiment of
applicant's invention. Applicant has found that
this "roundabout" method of producing a
hydrotalcite-like compound (from a hydrotalcite-like
compound) is well worth the extra effort because the
resulting hydrotalcite-like compound will be harder
and/or more dense than the original hydrotalcite-
like compound from which the resulting HTL compound
was made.
Stated another way, the starting ingredient in
the second embodiment of applicant's invention
already will be a rehydratable hydrotalcite-like
compound. This may be evidenced, for example, by
the fact that it already generally displays XRD
peaks that resemble those of a known HTL compound
having the same ingredients (e . g . , Mg and Al) . In
any case, this hydrotalcite-like compound starting
CA 02596421 2007-08-14
-27-
material is then heat treated to convert it into a
"collapsed" or "metastable" compound such as those
described in the Miyata reference, or in the 1118
patent. The Step 2, heat treatment of the second
embodiment of this invention can be conducted at a
single preferred t-emperature (e.g., 450 C) or at two
or more distinct temperatures in the general
temperature range of 300 C to 850 C, e.g., at a
first, lower temperature, (e.g., at 300 C) followed
by a second temperature heat treatment (e.g., at
400 C to 500 C). Here again, however, temperatures
greater than about 850 C are to be avoided in this
second embodiment of applicant's process for the
same reasons they were to be avoided in the first
embodiment. For example, if the original synthesis
compound were hydrotalcite, and it experienced a
900 C heat treatment temperature for any significant
period of time in the second embodiment of
applicant's invention, it too would in fact be
converted it into spinel (MgAl2O4) and, thus, would
be rendered useless for the purposes of practicing
this invention. Here again, any hydrotalcite
converted to MgO by such high temperatures would,
however, still be potentially useful in carrying out
functions for applicant's end product materials.
In any case, after the hydrotalcite-like
compound of this second embodiment is heat treated
to an extent that it takes on a "collapsed" or
"metastable" form, it can then hydrated (e.g., in a
water system at 20-100 C for at least 0.1 hours) in
the same manner employed in the first embodiment of
CA 02596421 2007-08-14
-28-
this invention to again form a similar hydrotalcite-
like compound. Again, this "hydrotalcite-like
compound - to hydrotalcite-like compound"
production process is not a useless, redundant or
roundabout journey because the hydrotalcite-like
compounds resulting- from this second embodiment of
applicant's invention will, in fact, have certain
improved physical and/or chemical properties (e.g.,
greater density, attrition resistance, catalytic
activity, etc.) relative to those comparable
properties possessed by the original hydrotalcite-
like compound from which the resulting or end
product hydrotalcite-like compound was derived. And
as in the case of the first embodiment of this
invention, the resulting HTL compound of this second
embodiment can be once again heat treated (this may
be thought of as Step 3 of this second embodiment)
at temperatures ranging from about 300 C to 850 C in
order to obtain a yet harder material whose loss of
water due to this second heat treatment may render
the resulting material better suited to certain uses
(e.g., as a SOX absorbent in a FCC unit) . That is
to say that Step 3 can be employed to give the
resulting material (here again, a "collapsed" or
"metastable" HTL compound-forming material) improved
physical properties relative to those HTL compounds
that are not subjected to this additional heat
treatment process.
The anionic compounds that can be produced by
the hereindescribed processes will most preferably
have a chemical structure:
CA 02596421 2008-06-05
-29-
[Mm2 Nn3+ (OH) 2m+2n1 An/a. - bHZO
wherein M2 and N3+ are cations, m and n are selected
such that the ratio of m/n is about 1 to about 10, a
will have a value of 1, 2 or 3, A is an anion with
charge of -1, -2 or -3, and b will range between 0
and 10, are highly preferred. The most preferred
elements for "M" in the above structure will be Mg,
Ca, Zn, Mn, Co, Ni, Sr. Ba, Fe and Cu . The most
preferred element for "N" will be Al, Mn, Fe, Co,
Ni, Cr, Ga, B, La and Ce. The most preferred
elements for "A" with charge a- will be CO32 NO3",
5042-, Cl and OH , I-, 5042 , S1032-, HPO32 , MnO42
HGaO32 , HVO42- . C1O4" and BO32 and mixtures thereof.
Applicant generally has found that HTL
compounds made by either of the two general
embodiments of this invention are usually at least
about 10% harder and/or 10t more dense than
comparable HTL compounds made from the same
ingredients by prior art production methods. These
physical attribute(s), e.g., of hardness and/or
greater density, makes those catalysts, sorbents,
catalyst binders and ion exchange agents (e.g.,
water softener agents) made from applicant's
hydrotalcite-like compounds more attrition resistant
- and hence longer lasting - especially in a fluid
catalytic converter ("FCC") environment.
Applicant's resulting compounds also have an
improved ability to be regenerated (e.g., with
respect to their ability to continue to serve as SO330 sorbents, hydrocarbon
cracking (or hydrocarbon
CA 02596421 2007-08-14
-30-
forming) catalysts, ion exchange agents, etc.) after
having experienced temperatures which would
permanently deactivate analogous anionic clays (such
as analogous hydrotalcite-like compounds) made by
prior art manufacturing methods. Indeed, these
improved physical--attributes can be thought of as
even further helping to define applicant's materials
and distinguish them from analogus HTL compounds
made by prior art methods. That is to say that, if
applicant's "activation" procedures (using Steps 1
and 2 or using Steps 1, 2 and 3) produce, say, a
hydrotalcite-like compound exhibiting greater
hardness and/or greater density than a comparable
hydrotalcite-like compound made by other methods,
then these qualities may help to distinguish
applicant's "hydrotalcite-like compounds" from those
made by prior art methods.
Expressed in patent claim language, there are
several general embodiments of applicant's processes
for making and using the HTL compounds of this
patent disclosure. The differences between them
generally revolve around: (1) whether or not the
material produced by the original synthesis reaction
is a non-anionic clay compound (e.g., a non-
hydrotalcite-like compound) or an anionic clay
compound (e.g., a hydrotalcite-like compound), (2)
whether or not the non-anionic clay compound or the
anionic clay compound is dried and heat treated in
one or more stages before it is eventually hydrated
and (3) whether or not the resulting HTL compound is
used as a catalyst (or catalyst binder), a SO,
CA 02596421 2007-08-14
-31-
sorbent, an ion exchange agent, etc. Some of these
embodiments may be expressed in patent claim
language as follows:
1. A process for making an anionic clay
compound, said process comprising:
(1) preparing a reaction mixture
comprising a divalent metal-containing compound and
a trivalent metal-containing compound under
conditions such that a product obtained from the
reaction mixture is a non-anionic clay compound;
(2) heat treating the non-anionic clay
compound to create a heat treated, non-anionic clay
compound;
(3) hydrating the heat treated, non-
anionic clay compound to obtain an anionic clay
compound.
2. A process for making an anionic clay
compound, said process comprising:
(1) preparing a reaction mixture
comprising a divalent metal-containing compound and
a trivalent metal-containing compound under
conditions such that a product obtained from the
reaction mixture is a non-anionic clay compound;
(2) low temperature treating the non-
anionic clay compound to obtain a low temperature
treated, non-anionic clay compound;
(3) medium temperature treating the non-
anionic clay compound to create a precursor for an
anionic clay compound;
CA 02596421 2007-08-14
-32-
(4) hydrating the precursor for an
anionic clay compound to obtain an anionic clay
compound.
3. A process for making an anionic clay
compound, said process comprising:
(1) preparing a reaction mixture
comprising a divalent metal-containing compound and
a trivalent metal-containing compound under
conditions such that a product obtained from the
reaction mixture is a non-anionic clay compound;
(2) converting the non-anionic clay
compound into a desired physical form;
(3) heat treating the non-anionic clay
compound to obtain a collapsed, heat treated, non-
anionic clay compound;
(4) hydrating the collapsed, heat
treated, non-anionic clay compound to obtain an
anionic clay compound.
4. A process for making a hydrotalcite-like
compound, said process comprising:
(1) preparing a reaction mixture
comprising an aluminum-containing compound and a
magnesium-containing compound under conditions such
that a product obtained from the reaction mixture is
a non-hydrotalcite-like compound;
(2) heat treating the non-hydrotalcite-
like compound to create a heat treated, non-
hydrotalcite-like compound;
(3) hydrating the heat treated, non-
hydrotalcite-like compound to obtain a hydrotalcite-
like compound.
CA 02596421 2007-08-14
_.1
-33-
5. A process for making a hydrotalcite- like
compound, said process comprising:
(1) preparing a reaction mixture
comprising an aluminum-containing compound and a
magnesium-containing compound under conditions such
that a product obtained from the reaction mixture is
a non-hydrotalcite-like compound;
(2) low temperature treating the non-
hydrotalcite-like compound to obtain a low
temperature treated, non-hydrotalcite-like compound;
(3) medium temperature treating the non-
hydrotalcite-like compound to create a precursor for
a hydrotalcite-like compound;
(4) hydrating the precursor for a
hydrotalcite-like compound to obtain a hydrotalcite-
like compound.
6. A process for making a hydrotalcite-like
compound, said process comprising:
(1) preparing a reaction mixture
comprising an aluminum-containing compound and a
magnesium-containing compound under conditions such
that a product obtained from the reaction mixture is
a non-hydrotalcite-like compound;
(2) converting the non-hydrotalcite-like
compound into a desired physical form;
(3) heat treating the non-hydrotalcite-
like compound to obtain a collapsed, heat treated,
non-hydrotalcite-like compound;
(4) hydrating the collapsed, heat
treated, non-hydrotalcite-like compound to obtain a
hydrotalcite-like compound.
CA 02596421 2007-08-14
-34-
7. A process for making a relatively hard,
hydrotalcite-like catalyst, said process comprising:
(1) preparing a reaction mixture
comprising an aluminum-containing material and a
magnesium-containing compound under conditions such
that a product obt-a4.ned from the reaction mixture is
a relatively soft, hydrotalcite-like compound;
(2) converting the relatively soft,
hydrotalcite-like compound into a form suitable for
use as a catalyst;
(3) heat treating the relatively soft,
hydrotalcite-like compound to obtain a heat treated,
precursor for a relatively hard, hydrotalcite-like
catalyst; and
(4) hydrating the heat treated, precursor
for a relatively hard, hydrotalcite-like catalyst to
obtain a relatively hard, hydrotalcite-like
catalyst.
8. A process for making a relatively hard,
hydrotalcite-like catalyst, said process comprising:
(1) preparing a reaction mixture
comprising an aluminum-containing compound and a
magnesium-containing compound under conditions such
that a product obtained from the reaction mixture
is a relatively soft, hydrotalcite-like compound;
(2) converting the relatively soft,
hydrotalcite-like compound into a form suitable for
use as a catalyst;
(3) low temperature treating the
relatively soft, hydrotalcite-like compound to
CA 02596421 2007-08-14
-35-
obtain a low temperature treated, relatively soft,
hydrotalcite-like compound;
(4) medium temperature treating the
relatively soft, hydrotalcite-like compound to
create a precursor for a relatively hard,
hydrotalcite-like-catalyst; and
(5) hydrating the precursor for a
relatively hard, hydrotalcite-like catalyst to
obtain a relatively hard, hydrotalcite-like
catalyst.
9. A process for making a relatively hard,
hydrotalcite-like sox sorbent, said process
comprising:
(1) preparing a reaction mixture
comprising an aluminum-containing compound and a
magnesium-containing compound under conditions such
that a product obtained from the reaction mixture is
a relatively soft, hydrotalcite-like compound;
(2) converting the relatively soft,
hydrotalcite-like compound into a physical form
suitable for use as a SOX sorbent;
(3) heat treating the relatively soft,
hydrotalcite-like compound to obtain a precursor for
a relatively hard, hydrotalcite-like SOX sorbent;
and
(4) hydrating the precursor for a
relatively hard, hydrotalcite-like SOX sorbent to
obtain a relatively hard, hydrotalcite-like SOX
sorbent.
Because the HTL compounds of this patent
disclosure are harder than HTL compounds made by
CA 02596421 2007-08-14
-36-
prior art processes, they present a method whereby
the useful life of a catalyst or sorbent system
(such as those employed in FCC units or fixed bed
units) can be extended. This extension of a
catalyst's (or sorbent's) useful life will take
place when. the -HTL compounds of this patent
disclosure are used in their own right, e.g., as
hydrocarbon cracking or forming catalysts, sox
sorbents, etc., or when these HTL compounds are used
as binders, matrices, supports, or carriers for
other catalytically active materials (e.g., when
they are used as binders for SO, -). SO, oxidant
metals).
Thus, using SOX sorption in a FCC unit used to
refine petroleum as an example, the method of
extending the useful life of an SOX sorbent (or
catalyst) may be expressed in patent claim language
in the following manner:
A method for extending the useful life of a SOX
sorbent system used in a FCC unit being employed to
refine a petroleum feedstock, said method
comprising: employing a HTL compound made by use of
a process of this patent disclosure as a SOX sorbent
system in the FCC unit and wherein the HTL compound
is in the form of a microspheroidal particle species
whose primary function is sorbing SO, produced by
refining a sulfur-containing petroleum.
Such a HTL compound-containing particle species
may further comprise a binder agent selected group
consisting of magnesium aluminate, hydrous magnesium
silicate, magnesium calcium silicate, calcium
CA 02596421 2007-08-14
-37-
silicate, alumina, calcium oxide and calcium
aluminate.
Expressed in patent claim language, such a
method for extending the useful life of a SO,,
additive system comprised of a SO2 SO3 oxidation
catalyst and a SO,-sorbent may comprise:
(1) employing the SO, additive system in the
form of at least two physically distinct particle
species wherein a first particle species contains
the SO2 -* SO3 oxidation catalyst component and
carries out a primary function of oxidizing sulfur
dioxide to sulfur trioxide and the second particle
species is physically separate and distinct from the
first particle species and carries out a primary
function of sorbing the SO3 produced by the SO2 -+
SO3 oxidation catalyst;
(2) employing the SO2 -* SO3 oxidation catalyst
in the form of a particle species that comprises:
(a) a sulfur SO2 --) S03 oxidation catalyst comprised
of a metal selected from the group consisting of
cerium, vanadium, platinum, palladium, rhodium,
molybdenum, tungsten, copper, chromium, nickel,
iridium, manganese, cobalt, iron, ytterbium, and
uranium; and (b) a binder made from a material
selected from the group of metal-containing
compounds consisting of hydrotalcite-like compounds,
calcium aluminate, aluminum silicate, aluminum
titanate, zinc titanate, aluminum zirconate,
magnesium aluminate, alumina (A1203), aluminum
hydroxide, an aluminum-containing metal oxide
CA 02596421 2007-08-14
-38-
compound (other than alumina (A1203)), clay,
zirconia, titania, silica, clay and clay/phosphate
material; and
(3) using the S0, absorbent component in the
form of a second particle that comprises a
hydrotalcite-like -compound made by use of an
"activation process" of this patent disclosure.
This activation process may involve use of Step
1 and Step 2 (or Steps 1, 2 and 3) upon a non-
hydrotalcite-like starting material or a
hydrotalcite-like starting material. Any of the HTL
compounds may be used in FCC systems wherein the SO,
sorbent particle species comprises from about 10 to
about 90 weight percent of the overall SO, additive
system (i.e., the SO, sorbent particle species and
the SO2 -4 SO3 oxidant particle species) . Such an
overall, SO, additive system will, in turn, normally
comprise from about 0.5 to about 10.0 weight percent
of a bulk hydrocarbon cracking catalyst (e.g.,
zeolite) SO, additive system.
Next, it should be understood that the HTL
compounds made by any of these methods may be used
in any way that the prior art has used hydrotalcite-
like compounds made by any prior art method (e.g.,
they may be used as sorbents and especially SO,,
sorbents, hydrocarbon cracking catalysts, e.g., for
use in fixed bed or fluid bed systems, catalyst
carrier or binder materials, anion exchangers (e.g.,
water softener agents, etc.) acid residue
scavengers, stabilizers for polymers, medicines,
etc.). Applicant's HTL compounds are, however,
CA 02596421 2007-08-14
-39-
particularly useful where the attributes of physical
hardness, toughness, or greater density are
especially desired (e.g., when they are used in FCC
units as SO, sorbents, catalysts and catalyst
binders or carriers).
CA 02596421 2007-08-14
-40-
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a XRD pattern for a 2Mg/lAl ratio
HTL precursor slurry.
Figure 2 is a XRD pattern for a 2Mg/1A1 ratio
HTL precursor slurry in which the slurry has been
heat aged at about 80-85 C.
Figure 3 is a XRD pattern for a 2Mg/]Al ratio
HTL precursor slurry in which the slurry has been
heat aged at about 80-85 C for a longer duration
than the material whose XRD is depicted in Figure 2.
Figure 4 is the XRD for a 2Mg/lAl ratio
precursor material that has been heat age treated;
and wherein the effects of an amorphous phase
associated with that crystalline phase 2Mg/1A1 ratio
HTL precursor have been subtracted from the XRD
pattern.
Figure 5 depicts (via XRD pattern changes) the
various phase changes that take place as a result of
the activation process of this patent disclosure.
Figure 6 gives the XRD pattern for a 2Mg/1A1
ratio HTL phase produced using applicant's
activation process in a case where the starting
2Mg/1A1 ratio HTL precursor slurry was not heated.
Figure 7 depicts the XRD for a 2Mg/1A1 ratio
HTL phase produced by applicant's activation process
using a heated 2Mg/1A1 HTL precursor slurry.
Figure 8 shows the XRD pattern for a 2Mg/lAl
ratio phase material plus oxidants made by
applicant's process.
CA 02596421 2007-08-14
-41-
Figure 9 shows the XRD for a Mg/Al system
activated by applicant's process and wherein the
system has a 5Mg/lAl molar ratio.
Figure 10 depicts the effects of a one hour,
500 C heat treatment on precursor phase with
oxidants.
Figure 11 shows XRD for a 3Mg/lAl/oxidant (Ce,
V) system where the oxidants were added to the
precursor slurry after a one hour, 732 C calcination
and wherein the oxidants were added to a precursor
slurry.
Figure 12 shows XRD for a 3Mg/lAl oxidant (Ce,
V) system wherein the oxidants were added to a
precursor slurry and wherein the system was
activated through use of applicant's methods at
732 C.
Figure 13 is a TGA/SOX Sorption and Release
trace for a 3Mg/lAl HTL system prepared by
applicant's process.
CA 02596421 2007-08-14
-42-
DETAILED DESCRIPTION OF THE INVENTION
Even though this invention is broadly concerned
with anionic clays in general, it is mostly
illustrated through discussions, data and working
examples that focus-on those anionic clays known as
hydrotalcite-like ("HTL") compounds. Applicant does
this because (1) HTL compounds are perhaps the most
readily formulated anionic clay compounds, (2) they
are the most well studied and reported anionic clay
compounds in the literature and because (3) they
are, in fact, the most preferred compounds for
actual practice of applicant's invention. The
crystalline structures of some of the more preferred
forms of HTL compounds for the practice of this
invention reasonably resemble those of: (1)
magnesium aluminum hydroxides, (2) magnesium
aluminum hydroxide hydrates and (3) magnesium
aluminum hydroxide carbonate hydrates. They are
preferably made from compositions primarily
comprised of (1) a magnesium-containing compound and
(2) an aluminum-containing compound (e.g., an
alumina sol, alumina gels or crystalline alumina)
and, optionally, (3) other ingredients such as metal
oxidants and binder materials commonly used to make
certain end product forms of such HTL compounds (FCC
catalysts, SOX sorbents, anion exchange pellets,
etc.).
Some particularly useful magnesium-based
compounds for creating applicant's HTL compounds
will include magnesium hydroxy acetate, magnesium
CA 02596421 2007-08-14
-43-
acetate, magnesium hydroxide, magnesium nitrate,
magnesium hydroxide, magnesium carbonate, magnesium
formate, magnesium chloride, magnesium aluminate,
hydrous magnesium silicate and magnesium calcium
silicate.
Some partiGularly useful aluminum-based
compounds for creating applicant's HTL compounds
will include aluminum acetate, aluminum nitrate,
aluminum hydroxide, aluminum carbonate, aluminum
formate, aluminum chloride, hydrous aluminum
silicate n
and aluminum calcium silicate. In the case
of the first embodiment of this invention, these
magnesium-containing compounds and aluminum-
containing compounds should be employed such that
the product of their initial reaction does not
produce the HTL compound that will ultimately be
produced by applicant's invention. By way of
example only, HTL compound formation by this initial
reaction can be thwarted at this point in the
production process by employing any synthesis-
influencing factor selected from the group
consisting of (1) use of less reactive magnesium-
containing and/or less reactive aluminum-containing
compounds (e.g., use of hydroxides instead of
acetate forms of magnesium), (2) use of particulate
ingredients rather than those in true solution, (3)
use of relatively short reaction periods (e.g., less
than 0.1 hours), (4) use of "neutral" pH levels
(e.g., 6-8 pH levels) and (5) use of relatively low
temperature reaction conditions (e.g., less than
30 C)
CA 02596421 2007-08-14
-44-
Additionally, for use in those applications
where other functions (e.g., oxidation of SO2 --* SO3)
is a part of the proposed end usage of applicant's
HTL compounds (e.g., when they are to be used as FCC
catalyst, or SO, sorbent particles), any number of
well known oxidants- may be employed in conjunction
with applicant's HTL compounds. Such oxidants would
include, for example, platinum, those compounds
which form oxides of the rare earth metals, oxides
of transition metals, etc. Such oxidants can also
be associated with the HTL compounds of this patent
disclosure by impregnating dried forms of these HTL
compounds with solutions containing ions of such
oxidant metals.
Ingredient Proportions
TABLE I illustrates some representative
relative concentrations of several HTL compositions
that can be made by the teachings of this patent
disclosure that are especially useful as SO, sorbent
formulations. They are given in Table I, on a dry
oxide basis, both with and without oxidants.
CA 02596421 2007-08-14
-45-
TAM "I
Mg/Al (molar) 2/1 3/1 5/1
(ratio)
MgO, w% 61.3 70.4 79.8
A1203, w% 38.7 29.6 20.2
Mg/Al (molar) 2/1 3/1 5/1
(ratio)
MgO, w% 52.1 59.8 67.8
A1203, w% 32.9 25.2 17.2
Ce02, w% 12.0 12.0 12.0
V205 , w% 3.0 3.0 3.0
It also should be appreciated that the HTL
compounds of this patent disclosure can be used
alone (that is to say that they can act
catalytically, as sorbents, etc. and serve as their
own binder or matrix material) or they can be
associated with various catalyst binder or matrix-
forming materials that are well known to those
skilled in the catalyst and/or sorbent making arts.
Indeed, such binder or matrix-forming materials may
constitute up to about 99 weight percent of an
overall catalyst or sorbent material (be it a
microspheroidal particle, pellet, extrudate, etc.)
in which the HTL compounds of this patent disclosure
are employed. By way of example only, such
catalyst, SO, binder or matrix-forming materials may
be magnesia, alumina, aluminum-containing metal
oxide compounds, aluminum hydroxide, clay, zirconia,
CA 02596421 2007-08-14
-46-
titania, silica, clay and/or clay/phosphate
materials.
This all goes to say that, even thought the HTL
compounds of this patent disclosure may serve as
both an SO sorbent and as its own binder material in
the practice of -this invention, applicant's SO
sorbent catalysts (as well as any other solid forms
of these HTL compounds) may, more preferably,
comprise at least one HTL compound made by the
processes of this invention and at least one,
chemically different, binder, matrix, support, etc.
material for that HTL compound. For example, a SO,
additive catalyst intended for use in a FCC unit may
be comprised of a hydrotalcite-like compound
supported by, say, a calcium aluminate binder.
Next it should be again noted that when
applicant's HTL compounds are used as SO sorbent
components, or catalysts or anion exchange agents,
etc., they may be so used alone - e.g., as separate
and distinct SO sorbent particles or they may be
used with other active materials which may be
present as different particle species or as
components of the particle species that employ the
HTL compounds of this patent disclosure. By way of
example only, such particles may be provided with
their own SO2 -* SO3 oxidation catalyst
ingredient(s). Moreover, one or more particle
species that make up applicant's SO sorbent
component(s) may be - as an option, and not a
requirement -provided with SO2 -+ SO3 oxidation
catalysts selected from the group consisting of
CA 02596421 2007-08-14
-47-
cerium, vanadium, platinum, .palladium, rhodium,
iridium, molybdenum, tungsten, copper, chromium,
nickel, manganese, cobalt, iron, ytterbium, and
uranium. Of these possible SO2 -5 SO3 oxidation
catalysts, ceria and vanadia have proven to be a
particularly effective SO2 oxidation catalyst when
an SO2 oxidant is used in conjunction with
applicant's HTL compound based, SO3 absorbents. it
also should be understood, however, that SO2 - 503
oxidation catalysts of this kind also could be
placed upon an entirely separate and distinct
particle species that is admixed with those
particles that are made with applicant's HTL
compounds.
Preparation and Processing
As previously discussed, the first embodiment
of this invention, among other things, requires that
an amorphous and/or non-crystalline HTL phase be
present at the end of the slurry or precipitate
preparation step. A diffraction pattern for a
representative material of this kind is shown in
Figure 1. Figures 2-3 show the effects of "low
temperature" (i.e., less than about 100 C) heat
aging the material whose XRD pattern is shown in
Figure 1. These figures show the presence of
significant amorphous phases, as well as non-HTL
crystalline phases. The particular materials
associated with these figures were prepared using a
2Mg/1A1 molar ratio. In one case, illustrated in
Figure 1, the slurry was not heat aged, while the
CA 02596421 2007-08-14
-48-
material whose XRD pattern is shown in Figures 2 and
3 was heat aged at about 80-85 C. Figures 2 and 3
show that upon being subjected to such low
temperature heating, a new crystalline phase
nucleates and grows with increasing aging time.
Figure 4 shows the-crystalline portion of the phase
that was shown in Figure 2. That is to say that the
effects of the presence of the amorphous material
that are present in Figure 2 are "subtracted out" of
the XRD pattern shown in Figure 4.
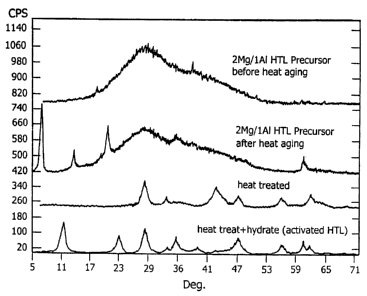
Figure 5 shows the changes in crystal structure
at various steps in applicant's "activation"
process. The top two curves in this plot
(respectively labeled "2Mg/lAl Precursor before heat
aging" and "2Mg/lAl Precursor after heat aging")
already have been discussed as part of the previous
discussion of Figures 1 to 4. The trace in Figure 5
labeled "heat treated" is representative of the
observed phases of HTL structures following Step 1
of applicant's activation process- The trace
labeled "heat treat+hydrate (activated HTL)" depicts
the results of Step 2 of applicant's activation
process. Clearly, an HTL structure has been
created. This is evidenced by the presence of all
major peaks of an HTL compound, including peaks at
about 11.271 degrees, 22.700 degrees and 34.358
degrees manifesting their presence. It also should
be noted that Figure 5 includes the effects of the
CeOZ component that was added during the synthesis
reaction and whose most prominent peaks manifest
CA 02596421 2007-08-14
-49-
themselves at 28.555 degrees, 47.479 degrees and
56.335 degrees.
Figures 6 and 7 plot the XRD pattern for a
2Mg/1A1 HTL compound that is produced using
applicant's activation process. The compound that
generated Figure -6--was derived from an un-heated
slurry, while that for Figure 7 was heat aged. The
"stick diagram" (vertical lines of different heights
at the appropriate 20 positions) for the "best
matching" ICDD card is superimposed on each of these
two plots. In this case the "best match" was with
ICDD "card" 35-965 for Mg6Alz (OH)18-4 .5H7O. It also
should be emphasized here that certain other ICDD
cards (e.g., ICDD card 22-700 for hydrotalcite)
reasonably "matched" the peak positions and
intensities to give reasonably close correlations,
but this particular HTL compound has 20 peak
positions that are nearly identical to those of the
35-965 card. This HTL compound also displayed XRD
intensities which had the fewest inconsistencies
relative to those of the several candidate cards
that were considered. The lattice parameters of
both the aged and non-aged slurry example are
compared in TABLE II with the "best matching" card,
namely, ICDD card 35-965.
CA 02596421 2007-08-14
-s0-
TABLE II
ICDD Card 2Mg/]Al HTL 2Mg/1A1 HTL
35-965 (not aged) (aged slurry)
a, Angstrom 3.054 3.057 3.060
c, Angstrom 23.40 23.05 23.08
alpha, degrees -90 90 90
beta, degrees 90 90 90
gamma, degrees 120 120 120
It can be seen that the lattice parameter, "a,"
of applicant's 2Mg/1A1 HTL compound is nearly
identical to that of the card, while lattice
parameter "c" is substantially lower. Applicant
believes that this signifies that the amount of A13'
substituted into the brucite-like structure is
nearly identical to that of the 35-965 card
material, while the variation in lattice parameter c
is due to the nature and amount of interlayer water
and charge-balancing anions located in the
interlayer.
Figure 8 shows the XRD patterns for the same
2Mg/lA] HTL compound used to generate Figures 6 and
7, except that 12 weight percent CeO2 and 3 weight
percent V2O5 components are present by virtue of
cerium-containing compound (e.g., cerium nitrate)
being added to the slurry formulation after reacting
the magnesium and aluminum containing components
together. It also should be noted that, with the
exception of the effects of the CeO2 present (ICDD
Card 34-394) in this system, the pattern is very
CA 02596421 2007-08-14
-51-
similar to those samples containing no oxidants
(again, see Figures 6 and 7).
The following TABLE III compares XRD patterns
for HTL compounds with, and without, oxidants as
compared to the patterns of the "most closely
matching" ICDD card-.- This comparison shows that the
presence of the oxidants in no way affects the
structure of the HTL compound.
TABLE III
ICDD Card no with oxidants
35-965 oxidants CeO2 and V2O
5
a, Angstrom 3.054 3.057 3.046
c, Angstrom 23.40 23.05 23.07
alpha, degrees 90 90 90
beta, degrees 90 90 90
gamma, degrees 120 120 120
Thus, based upon these and other findings,
applicant has concluded that, within a reasonable
experimental error allowance for this kind of
analysis, no appreciable difference in crystal
structure can be observed between HTL compounds
associated with CeO2 and V2O, oxidants and those
without such oxidants.
Diffraction patterns showing the effect of a
higher Mg/Al ratio (i.e., 5:1) in the HTL structural
formation are shown in Figures 9. In addition to
the HTL compound and oxidant Ce02, a small amount of
magnesium hydroxide (ICDD Card 7-239) was observed
in the pattern shown in Figure 10. This result is
CA 02596421 2007-08-14
-52-
consistent with results published in the literature
in that the maximum HTL formation has been
determined by other workers in this art (e.g.,
Miyata) to be in the Mg/Al ratio range of 2-4.
Since the sample that generated Figure 10 was
prepared at a 5Mg/-1A1 ratio, the amount of magnesium
ions present in such a system exceeded the limit of
their solubility in the brucite layer - hence a
magnesium hydroxide phase was formed and manifested
itself in this way.
Figure 10 shows the crystal structure present
for HTL precursors materials having differing Mg/Al
ratios just prior to Step 2 of applicant's
activation process. Of particular interest here are
the "shoulders" on the 43 degree and 62 degree "MgO-
like" peaks of these diffractograms. It can be seen
that, as the Mg/Al ratio increases from 2:1 to 5:1,
the magnitude of these peaks diminishes to a level
where they become undetectable. This is indicative
of a metastable alumina phase, with or without a
small amount of magnesium oxide dissolved in the
lattice. Additionally, this result shows that
alumina is present, primarily within the lattice of
the MgO, and hence the term "MgO-like" compounds
also might be applied to those HTL compounds that
have undergone applicant's Step 2 heat treatment -
but no hydration. The metastable alumina phase is a
direct corollary to the 5Mg/lAl material previously
discussed wherein the presence of "too many" Mg ions
resulted in an excess that manifested itself as a
magnesium hydroxide phase. In this case, "too low"
CA 02596421 2007-08-14
-53-
a Mg/Al ratio results in an excess of alumina which
can be regarded as a slightly magnesia-rich alumina
phase. This observation has been made in the
literature as well; see, for example, Gastuche et
al, Mixed Magnesium-Aluminum Hydroxides, Clay
Minerals 7, 7 (1967), particularly noting Figure 1
therein. See also the previously noted Miyata
reference, (and especially page 52 thereof).
Thus, for maximum HTL formation by applicant's
processes, hydrotalcite-like compounds having a
Mg/Al ratio in the 2-4 range are highly preferred.
When the Mg/Al ratio drops below 2, a HTL structure
can result, but it will be mixed with alumina and or
a magnesium-aluminum solid solution phase. The
lattice parameter, a, of such a system, however,
generally, will remain unchanged at about 3.04
Angstroms. In a system having a Mg/Al ratio in the
range of 2-4, the lattice parameter, a, will
increases with a linear relationship toward an end-
point associated with magnesium hydroxide of 3.14-
3.15 Angstroms. Above a Mg/Ai ratio of 4, the
lattice parameter continues to increase further, but
magnesium hydroxide will accompany the HTL phase
formation. See again, for example, the previously
cited Miyata reference and the Gastuche et al.
reference (and especially Figure 1, on pg 182
thereof).
The effects of increased temperature of
applicant's activation process with respect to
crystal structure was also studied. This study
verified the literature's pronouncements with
CA 02596421 2007-08-14
-54-
respect to the temperature at which spinel is formed
from hydrotalcite. For example, applicant subjected
a commercially available hydrotalcite compound to
such a rising temperature regime in order to verify
the temperature at which spinel is formed from
hydrotalcite. --The commercially available
hydrotalcite was Alcoa's HTC-30 product (which has
a 3:1 Mg/Al molar ratio and is therefore well suited
to use in the second embodiment of applicant's
invention), and it was subjected to temperatures
that ranged from 250 C to 1200 C. This test showed
MgO-like phase formation commencing at 400 C and
spinel formation commencing at 900 C.
The results of some other analogous heat
treatments, carried out at higher heat treatment
temperatures, is presented in Figures 11 and 12. In
these increased temperature studies, a 732 C
temperature was used for one hour as the heat
treatment aspect of applicant's overall "activation"
step since the literature states that this is near
the upper-end of preferred temperature for maximum
HTL phase formation. In any case, no appreciable
differences in structure are noted beyond those
already noted for lower temperature activations
(e.g., at 450-500 C) .
TGA-S testing of Sorbents
A modified Thermal Gravimetric Analysis (TGA)
technique is used by many laboratories worldwide to
evaluate the relative SO, sorbent performance of
different compositions. This modified technique
CA 02596421 2007-08-14
-55-
employs two tests, which are carried out at
different temperatures. The first test is a SOX
"pickup." In this aspect of the TGA test, a furnace
is ramped up to about 700 C in an inert gas and
allowed to equilibrate. A gas mixture containing
SO2 and 02 is then-introduced into the reactor for
some duration. It should be understood that two
distinct reactions are simultaneously occurring at
this point: Oxidation of SO2-4SO3 and a subsequent
reaction of the S0, thus formed with MgO to form
MgSO4. Typically, the reaction is allowed to
continue until the sample is saturated (meaning all
the possible Mgo is reacted to form the sulfate).
The second aspect of the TGA is to regenerate the
sorbent. This is achieved through use of a lower
temperature (typically 590 C) and a reducing
atmosphere (typically H2), so that the sorbent being
studied releases the sorbed SOX as H.S. The TGA-SOX
plot for one cycle of such a test on one of
applicant's HTL compounds is shown in Figure 13.
Methods for Forming HTL Compositions which are
Particularly Resistant to Mechanical Stress.
The HTL compounds of this invention can be
formed into various shapes (particles,
microspheroidal particles, extrudates, pellets)
which are harder and more dense than HTL compounds
made by prior art processes. These qualities make
applicant's HTL compounds more useful for certain
applications e.g., catalysts, sorbents in general
(and SOX sorbents in particular), ion exchange
pellets (e.g., for water softeners). If made
CA 02596421 2007-08-14
-56-
according to the teachings of this patent
disclosure, such physical forms will display greater
resistance to wear, attrition, or impact, as well as
improvement (i.e., increase) in the bulk density of
the HTL compounds formed by applicant's methods.
The following-Table IV summarizes improvements
in two physical properties (i.e., attrition index
and apparent bulk density ("ABD")) for various
samples that were spray dried into particle forms
and activated according to the teachings of this
patent disclosure. This activation method would
also be applicable to other physical forms of such
HTL compounds, e.g., extrudates, pellets or beads.
TABLE IV
HTL Composition Attrition Index *ABD (g/cc)
(ASTM)
2Mg/1A1 (Step 1 Activation) 3.9 0.39
2Mg/1A1 (Step 2 Activation) 0.54 0.96
SMg/1A1 (Step 1 Activation) 15 0.36
5Mg/1A1 (Step 2 Activation) 0.65 0.75
*Apparent Bulk Density
The 2Mg/1A1 sample described in Table IV also
was subjected to an additional heat treatment step
at 732 C/1 hr. This additional heat treatment has
been described as an optional, "Step 3" in previous
parts of this patent disclosure. This additional
heating was performed to show that applicant's
"activation" was of irreversible nature, meaning the
physical properties do not revert to the original
activation values. Therefore, applicant considers
CA 02596421 2007-08-14
-57-
the products of this "activation" to be new
compositions of matter. In any case, the results of
this Step 3 process are shown in Table V.
TABLE V
HTL Composition- Attrition Index ABD (g/cc)
(ASTM)
2Mg/lA] (Step 1 Activation) 3.9 0.96
2Mg/lAl (Step 2 Activation) 0.54 0.96
2Mg/lA] (Additional heat to 0.81 0.80
732C/lhr.)
Applicability of Activation Process toward HTL
Compounds which Crystallize with RTL Structure
during Slurry Synthesis.
The aforementioned activation process (i.e.,
heat treatment followed by hydration) can be applied
to HTL compounds that are used as starting materials
in the second embodiment of applicant's invention.
That is to say that a HTL compound can be heat
treated to form a "collapsed" or "metastable"
material that can be rehydrated in the same manner
that the heat treated material of the first
embodiment of applicant's invention was hydrated.
If so heat treated, the hydration process of the
second embodiment of this invention will result in
formation of a HTL phase. Here again, applicant's
activation process improves the physical
characteristics of the HTL materials produced by
said process. Again, these improvements include,
but are not limited to, improved mechanical strength
and density of the formed shapes (e.g., FCC
particles, fixed bed pellets, anion exchange beads,
CA 02596421 2007-08-14
-58-
etc.) relative to comparable compounds that do not
experience applicant's activation process. It also
should be noted that, for those materials which form
HTL compounds from HTL starting materials (i.e., the
second embodiment of applicant's invention), the
resulting HTL phase may or may not be exactly
identical to the starting HTL phase (in terms of
exact identity of peak position and intensity), but
which will nonetheless display clearly identifiable
HTL compound peaks and possess the above-noted
improved physical characteristics.
To show this advance, an example is given of a
HTL containing SO, sorbent (marketed by Akzo Nobel
under the trade name "KDESOX(D") that was subjected
to applicant's activation process. The following
TABLE VI summarizes the physical characteristics of
the HTL-containing composition, before and after
Applicant's activation process.
TALE VI
HTL Composition KDESOX KDESOX
(as received) "Activated"
Attrition Index, ASTM 1.8 0.77
Bulk Density, g/cc 0.81 0.97
Thus, the physical properties (shown here as
attrition index and bulk density) of a commercial
available HTL compound made into FCC particles can
be improved markedly by subjecting them to
applicant's Activation process.
While this invention has been described with
respect to various theories, specific examples and a
CA 02596421 2007-08-14
awl
-59-
spirit which is committed to the concept of the use
of an "activation process" that is-based upon heat
treatment and hydration of collapsed, HTL-forming,
compounds, the full scope of this invention relates
to such activation of anionic clays in general;
hence the full seepe of this invention should be
regarded as being limited only by the claims that
follow.