Note: Descriptions are shown in the official language in which they were submitted.
CA 02597179 2007-08-27
WO 96/31435 pCTroS9610407
PROCESS FOR THE PRODUCTION OF CESIUM COMPOUNDS
This application is a division of Canadian Patent Application
Serial No. 2,217,343, filed Apri15, 1996.
Field Of The Invention
The present invention relates to processes for purifying cesium compounds.
The present invention also relates to proeesses for the production or recovery
of
cesium from cesium-including materials, preferably in the fotm of a desired
cesium
compound such as a cesium salt.
Background of the Invention
Proorsses for recovering cesium, in the form of a cesium compound, from
cesium-induding materials such as pollucite and other cesium-including
minerals have
been reported in the technical literature.
One process which is reported involves leaching ground pollucite ore with
strong sulfuric acid to obtain an extract including cesium alum, which is
recovered by
crystallization.
Cesium alum is cesium aluminum sulfate hydrate. Its formula can be
empirically expressed as CsAI(SO4)2-12HZ0, or Cs2SO4-A12(SOa)3=24H2O. The
cesium alum contained in or crystallized from the sulfuric acid extracts of
pollucite is
typically contaminated with other metal ions such as rubidium, sodium,
potassium,
magnesium and iron.
The cesium alum is then redissolved in water at an elevated temperature and
reacted with an alkaline earth metal hydroxide, sudh as barium hydroxide or
calcium
hydroxide, to form an aluminum hydroxide precipitate together with
precipitated
barium sulfate or calcium sulfate. The cesium alum mav alternatively be
reacted with
ammonia to precipitate the aluminum as aluminum hydroxide. The cesium sulfate
remains in the supernatant solution. The cesium can be recovered from the
supernatant
solution and converted into other cesium compounds.
U.S. Patent No. 3,207,571 to Berthold discloses a process for producing
cesium compounds from afuminosilicate ore. German Patent DE 43 13 480 of
Hoffmann et al. discloses a process which avoids the use of barium compounds
in the
production of cesium salts from cesium alum. This process results in a product
including calcium sulfate and magnesium.
One reported use for cesium compounds, such as cesium formate, is in high
specific gravity drilling fluids for oil and gas wells. Bore hole tutnings are
known to
slow or stop the drilling process, and in some cases, plug the porous strata
of the bore
hole. Feedback data on the bore hole condition is limited in the regions of
plugged
sttata thereby reducing the effectiveness of the drilling operation. High
density fluids
CA 02597179 2007-08-27
2
having a specific gravity of about 1.8 and above have been used to convey the
turnings to the surface. For wells having a depth greater than one mile, zinc
bromide and mixtures with other salts have been utilized to improve the
performance of the fluids. However, the nature of these materials renders them
somewhat undesirable. One material which has been mentioned as a replacement
for zinc bromide is cesium formate. Blends of cesium formate with other alkali
metal formates are also mentioned. See European Patent No. 572 113.
A problem which may occur is the incompatibility of impurities found in
cesium compounds such as cesium formate, with the various solutions,
viscosifiers, and additives used in drilling fluids. For example, the presence
of
divalent impurities like calcium in cesium compounds may degrade the polymers
present in the viscosifiers. The presence of divalent impurities is
particularly
harmful in high temperature and high pressure applications commonly found in
deep well drilling where the viscosifier functions to suspend the bore hole
turnings
and act as a drilling lubricant.
Cesium compounds produced by the above described processes, however,
do not avoid the problem of side reaction precipitates forming between
divalent
and multivalent cationic impurities and the carbonates present in the drilling
environment or the corrosion effect of drilling equipment materials caused by
sulphate ion impurities. Therefore, it would be advantageous to have a process
for
purifying cesium compounds produced by commercial processes.
Further, there has been a recognized need for a cesium compound having a
substantially reduced level of divalent and multivalent cation impurities and
sulphate ions and an improved process for its preparation.
CA 02597179 2007-08-27
2a
Summary of the Invention
The aforementioned advantages, and others, are achieved by the processes
of the present invention.
In accordance with the invention there is provided a process for producing
a cesium salt comprising:
(a) treating cesium alum with slaked lime or calcium carbonate and an
acid to produce a cesium salt of said acid and an undissolved solid comprising
aluminum hydroxide, wherein said cesium salt includes calcium ions and sulfate
ions as impurities;
(b) separating the solubilized cesium salt solution from the undissolved
solid; and
(c) adding barium hydroxide to the solution of said cesium salt
containing said impurities in an amount sufficient to precipitate sulfate ions
to
provide said cesium salt with less than 1000 ppm of sulfate ion.
The present invention also provides processes for purifying cesium
compounds produced by heretofore known cesium production processes, and the
processes of the present invention described herein. The purifying processes
of
the present invention may also be useful in purifying reclaimed cesium
compounds, for example, drilling/well servicing fluids comprising cesium
formate.
The purifying processes of the present invention may be utilized to produce
a cesium compound, including but not limited to cesium formate, cesium
nitrate,
cesium
CA 02597179 2007-08-27
PCT/US96104687
WO 96131435
3
cbloride, c,esiium iodide, cesium bromide and omum acetate comprising, on a
dry
weight basis:
less than 0.50% of a sulfate group, less than 03% of barium, calcium, or
magnesium including compounds, and less than 0.2% of other multivalent
cationic
impurities_ Preferably the cesium compound further comprises, on a dry weight
basis,
less than 0.50% a chloride group and less than 03% of aluminum. The purifyina
processes of the present invention may also be utilized to produce a compound,
including but not limited to cesium formate, cesium nitrate, cesium chloride,
cesium
iodide, cesium bromide and cesium acetate eomprising, on a dry weight basis:
less than 1000 parts per million (ppm), preferably less than 500 ppm,
more preferably less tl,an.3 0 ppm sulfate;
less than 1000 ppm, preferably less than 500 ppm, more preferabl~y
less than 30 ppm calcium;
less than 1000 ppm, preferably less than 500 ppm, more preferably
less than 30 ppm barium; and
less than 1000 ppm, preferably less than 500 ppm, more preferably
less than 30 ppm magnesium.
In a preferred embodiment, the low impurity levels of purified cesium formate
render
the material particularly advantageous for use in drilling fluids.
In addition the purifying process of the present invention may be utilized to
produce a cesium sulfate compound comprising:
less than 0.3% of barium, calcium, or mamesiurn including compounds, and
less than 0.2% of otbermultivalent cationic impurities. Preferably the cesium
sulfate
further comprises, on a dry wei,ht basis, less than 0.50% a chloride stroup
and less
than 03% of aluminum.'I'he puri~ying processes of the present invention mav
also be
utilized to produce a purif ed cesium sulfate compound comprising:
less than 1000 ppm, preferably less than 500 ppm, more preferably
less than 30 ppm calcium;
less than 1000 ppm, preferably less t[zan 500 ppm, more preferably
less than 30 ppm batium; and
less thaa 1000 ppm, preferably less than 500 ppm, more preferablv
less than 30 ppm magnesium.
The present invention also provides processes for producing a predetermined
cesium compound comprising: treating a eesium-including material with a
suitable
reazent to dissolve at least a portion of the cesium contained in the matezial
and form a
CA 02597179 2007-08-27
4
slurry ; adding a base comprising slaked lime or calcium carbonate and a
quantity
of an acid including the anion of the predetermined cesium compound to the
slurry, if necessary to produce the predetermined cesium compound; and
separating the predetermined cesium compound wherein the separation occurs in
the presence of the remainder of the starting cesium-including material (the
starting cesium-including material residues). The predetermined cesium
compound may be further purified by the process for purifying cesium compounds
of the present invention.
As used herein, the term "predetermined cesium compound" means a
compound produced by combination of free cesium ion and an anion. Examples
of cesium compounds which may be produced by the processes of the present
invention include but are not limited to cesium formate, cesium sulphate,
cesium
chloride, cesium iodide and cesium nitrate. As explained in more detail below,
in
embodiments of the processes of the present invention, cesium sulphate may be
produced directly from cesium alum without the need to add additional anion.
In
embodiments of the processes of the present invention utilized to produce
cesium
formate, cesium chloride, cesium iodide, cesium nitrate and other
predetermined
cesium compounds (other than cesium sulfate) a quantity of an acid including
the
anion of the predetermined cesium compound is utilized.
In accordance with still another aspect of the invention, there is provided a
drilling fluid comprising:
a cesium compound;
less than 0.5%, by weight, of chloride and sulfate; and
less than 0.3%, by weight, of aluminum, barium, calcium and magnesium,
wherein the drilling fluid has a specific gravity between about 1.2 g/cm3
and about 2.5 g/cm3.
CA 02597179 2007-08-27
4a
In accordance with yet another aspect of the present invention, there is
provided a fluid comprising a cesium compound and having a specific gravity of
between about 1.2 g/cm3 and about 2.5 g/cm3 and having 10% to 100% by weight
of the cesium compound on a dry salt basis, and less than 85% by weight of the
cesium compound on a solution basis. Preferably the cesium compound comprises
on a dry weight basis:
less than 0.50% of a chloride or sulfate group, less than 0.3% of aluminum,
io barium, calcium, or magnesium including compounds, and less than 0.2% of
other
multivalent cationic impurities. In an alternate embodiment of the fluid, the
cesium compound may comprise, on a dry weight basis:
less than 1000 ppm, preferably less than 500 ppm, more preferably less
than 30 ppm sulfate;
less than 1000 ppm, preferably less than 500 ppm, more preferably less
than 30 ppm calcium;
less than 1000 ppm, preferably less than 500 ppm, more preferably less
than 30 ppm barium; and
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
less than 1000 ppm, preferably less than 500 ppm, more preferably
less than 30 ppm magnesium.
The purifying process of the present invention may be advantageously utilized
to purify cesium compounds produced by heretofore known cesium production
5 processes, and the cesium production processes of the present invention.
The cesium production processes of the present invention may be
advantageously utilized to produce cesium compounds in an economic and
efficient
manner.
Further details relating to the present invention are described in the
following
Detailed Description of the Invention.
Brief Description of the Drawings
In the Drawings:
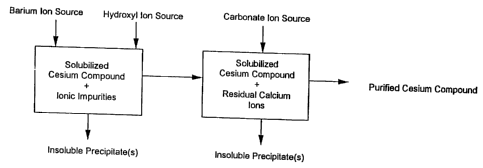
Figure 1 is a block schematic diagram of an embodiment of the process for
purifying cesium compounds of the present invention.
Figure 2 is a block schematic diagram of another embodiment of the process
for purifying cesium compounds of the present invention.
Figure 3 is a block schematic representation of an embodiment of a cesium
production process of the invention.
Figures 4A - 4C illustrate block schematic representations of alternative
embodiments of various aspects of a cesium production process of the
invention.
Figure 5 is a block schematic representation of another embodiment of a
cesium production process of the invention.
Detailed Description of the Invention
The present inventors have found an improved process for purifying cesium
compounds. The purifying process is particularly advantageous for use in
purifying
cesium compounds produced by a process utilizing lime. The purifying processes
of
the present invention mav be carried out on a commercial scale utilizing
conventional
industrial scale mixing vessels and equipment for handling the cesium-
including
materials (e.g., ores) and strong acid and base solutions. The choice of the
particular
equipment utilized to practice the processes of the present invention is
believed to be
within the skill of one of ordinary skill in the art and therefore is not
described below.
According to the present invention, an embodiment of a process for purifying
cesium compounds from a starting cesium compound which includes an ionic
impurity
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
6
comprising: calcium, sulfate, magnesium or mixtures thereof comprises:
reacting
impurities comprising calcium, sulfate, magnesium or mixtures thereof present
in a
solution including the solubilized starting cesium compound with suitable
precipitating
agents to form an insoluble precipitate including the impurity or impurities.
Preferred
precipitating agents include barium ion to precipitate sulfate ionic
impurities (SO42 ) as
barium sulfate; hydroxyl ion to precipitate magnesium ionic impurities as
magnesium
hydroxide and to precipitate calcium ionic impurities as calcium hydroxide;
and carbon
dioxide or carbonate ion to precipitate calcium ionic impurities as calcium
carbonate.
The insoluble precipitates may be separated from the purified cesium compound
by
conventional techniques such as filtering and/or other suitable physical
separation
techniques, for example centrifugation. In an embodiment of the process of the
present invention depicted schematically in Figure 1, the impurities in the
solution
including the solubilized starting cesium compound are first reacted with
barium ion
and hydroxyl ion precipitating agents and the resulting solution is reacted
with carbon
dioxide or carbonate ion to precipitate any remaining calcium ionic
impurities.
The source of barium ions and the source of hydroxyl ions may be the same or
different. Suitable sources of barium ions include: barium hydroxide and
soluble
barium salts having an ion in common with the cesium compound being purified,
for
example barium formate in a process for purifying cesium formate. A preferred
source
of barium ions is barium hydroxide. The barium ion source is employed in an
amount
sufficient, and reacted under conditions sufficient to precipitate at least a
portion of the
impurities. Preferably the barium ion source is employed in an amount, and
reacted
under conditions, sufficient to precipitate all or substantially all of the
impurities. In a
more preferred embodiment of the purifying process of the present invention,
barium
ions are added in an amount approximately equal to the stoichometric amount of
sulfate
ions determined to be in the solution. When barium hydroxide is utilized as
the barium
ion source, the insoluble precipitates may include barium sulfate, calcium
hydroxide
and/or magnesium hydroxide, depending on whether sulfate, calcium and
magnesium
ions are present in the starting cesium compound. The inventors note that it
is possible
to form insoluble precipitates utilizing less than 0.12 kilogram of barium
hydroxide is
added to the solution per 1 kilogram of starting cesium compound contained in
the
solution.
Suitable sources of hydroxyl ions include: barium hydroxide, alkali hydroxides
and calcium hydroxide with barium hydroxide being preferred. The hydroxyl ion
source is employed in an amount sufficient, and reacted under conditions
sufficient to
CA 02597179 2007-08-27
WO 96/31435 PCT/1JS96/04687
7
precipitate at least a portion of the impurities. Preferably the hydroxyl ion
source is
employed in an amount, and reacted under conditions, sufficient to precipitate
all or
substantially all of the impurities. In a preferred embodiment of the
purifying process
of the present invention, hydroxyl ions are added in an amount sufficient to
raise the
pH of the resulting solution to 11.5 or greater. In accordance with the
process of the
present invention, when the pH of the resulting solution is raised to 11.5 or
greater,
magnesium ions in the solution will precipitate, when the pH of the resulting
solution
is raised to greater than 13, calcium ions in the solution will precipitate.
As indicated above, the purifying process of the present invention may further
comprise reacting carbonate ions or carbon dioxide with the solution including
the
solubilized starting cesium compound to form an insoluble precipitate
including at least
a portion of any calcium ions remaining in the solution. Suitable carbonate
ion sources
include, but are not limited to, alkali carbonates such as cesium carbonate,
potassium
carbonate or sodium carbonate. The carbonate ion source is employed in an
amount
sufficient, and reacted under conditions sufficient to precipitate at least a
portion of the
calcium ions remaining in the solution. Preferably the carbonate ion source is
employed in an amount, and reacted under conditions, sufficient to precipitate
all or
substantially all of the calcium ions remaining in the solution.
In general, the extent to which the purification of the cesium compound is
carried out is dependent on the end use application for the purified cesium
compourid.
The foregoing process steps of the process for purifying a cesium compound
of the present invention are particularly well suited to purifying cesium
compounds
such as cesium formate, cesium chloride, cesium iodide, cesium nitrate, cesium
bromide or cesium acetate. These cesium compounds, and others such as cesium
sulfate, may be produced from cesium-including materials, including naturally
occurring minerals or ores, such as pollucite, solutions including cesium
aluminum
sulfate, and other materials, e.g., spent catalysts or residues comprising
cesium
fluoride or cesium sulfate.
A solution including solubilized cesium foitnate, cesium chloride, cesium
iodide, cesium nitrate, cesium bromide or cesium acetate may be produced by a
process of the present invention comprising:
treating a cesium-including material with a suitable reagent to dissolve
at least a portion of the cesium contained in the material and form a slurry
comprising
cesium alum, cesium sulfate or cesium fluoride;
CA 02597179 2007-08-27
WO 96/31435 PCTIUS96/04687
8
adding a base comprising slaked lime or calcium carbonate and an acid
including an anion of cesium formate, cesium chloride, cesium iodide, cesium
nitrate,
cesium bromide or cesium acetate to the sluny to form solubilized cesium
formate,
cesium chloride, cesium iodide, cesium nitrate, cesium bromide or cesium
acetate; and
separating the solubilized cesium compound solution in the presence of
the remainder of the starting cesium-including material.
An alternate embodiment of the purifying process of the present invention is
preferred for purifying a cesium sulfate compound. This alternate embodiment
is
depicted schematically in Figure 2.
According to the present invention, a process for purifying cesium sulfate
from
a starting cesium sulfate compound which includes an ionic impurity
comprising:
calcium, magnesium or mixtures thereof comprises: reacting impurities
comprising
calcium, magnesium or mixtures thereof present in a solution including the
solubilized
starting cesium sulfate compound with suitable precipitating agents to form an
insoluble precipitate including the impurity or impurities. Preferred
precipitating
agents include hydroxyl ion to precipitate magnesium ionic impurities as
magnesium
hydroxide and to precipitate calcium ionic impurities as calcium hydroxide;
and carbon
dioxide or carbonate ion to precipitate calcium ionic impurities as calcium
carbonate.
The insoluble precipitates may be separated from the purified cesium compound
by
conventional techniques such as filtering and/or other suitable physical
separation
techniques, for example centrifugation. In an embodiment of the process of the
present invention depicted schematically in Figure 2, the impurities in the
solution
including the solubilized starting cesium compound are first reacted with an
hydroxyl
ion precipitating agent and the resulting solution is reacted with carbon
dioxide or
carbon ion to precipitate any remaining calcium ionic impurities.
Suitable sources of hydroxyl ion (bases) include hydroxides of a metal
selected
from group 1A and 2A of the Periodic Table of the Elements and mixtures
thereof.
For example the source of hvdroxyl ion (base) may comprise lime, slaked lime,
potassium hydroxide, sodium hydroxide, cesium hNldroxide or a mixture thereof,
with
slaked lime being preferred. The hydroxyl ion source is employed in an amount
sufficient, and reacted under conditions sufficient to adjust the pH of the
solution to an
extent so as to precipitate at least a portion of the impurities. In
accordance with the
process of the present invention, when the pH of the resulting solution is
raised to
11.5 or greater, magnesium ions in the solution will precipitate.
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
9
As indicated above, this embodiment of the purifying process of the present
invention may further comprise reacting carbonate ions, or carbon dioxide,
with the
solution including the solubilized starting cesium sulfate to form an
insoluble
precipitate including at least a portion of any calcium ions remaining in the
solution.
Suitable carbonate ion sources include, but are not limited to, alkali
carbonates such as
cesium carbonate, potassium carbonate or sodium carbonate. The carbonate ion
source is employed in an amount sufficient, and reacted under conditions
sufficient to
precipitate at least a portion of the calcium ions remaining in the solution.
Preferably
the carbonate ion source is employed in an amount, and reacted under
conditions,
sufficient to precipitate all or substantially all of the calcium ions
remaining in the
solution.
A solution including solubilized cesium sulfate may be produced by a proce.ss
of the present invention comprising:
treating a cesium-including material with a suitable reagent to dissolve
at least a portion of the cesium contained in the material and form a slurry
comprising
cesium alum;
adding a base comprising slaked lime or calcium carbonate to the slurry
comprising dissolved cesium to form a solubilized cesium sulfate compound; and
separating the solubilized cesium sulfate compound solution in the
presence of the remainder of the starting cesium-including material.
The purifying processes of the present invention may be utilized to purify
cesium compounds produced by a process of the present invention or by other
cesium
compound production processes. In many cesium production processes the
solution
comprising the solubilized cesium compound will exist at a stage in the
production
process prior to separation and recovery of the cesium compond. A purifying
process
of the present invention may be performed at this stage as part of the cesium
production process. If necessary, in other applications of a purifying process
of the
present invention, a solution comprising the solubilized cesium compound may
be
formed by solubilizing the cesium compound utilizing known techniques.
The purifying processes of the present invention may be utilized to produce a
cesium compound, including but not limited to cesium formate, cesium nitrate,
cesium
chloride, cesium iodide, cesium bromide and cesium acetate comprising, on a
dry
weight basis:
less than 0.50% of a sulfate group, less than 0.3% of barium, calcium, or
magnesium including compounds, and less than 0.2% of other multivalent
cationic
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
impurities. Preferably the cesium compound further comprises, on a dry weight
basis,
less than 0.50% a chloride group and less than 0.3% of aluminum.
The purifying processes of the present invention may also be utilized to
produce a cesium compound, including but not limited to cesium formate, cesium
5 nitrate, cesium chloride, cesium iodide, cesium bromide and cesium acetate
comprising, on a dry weight basis:
less than 1000 parts per million (ppm), preferably less than 500 ppm, more
preferably less than 30 ppm sulfate;
less than 1000 ppm, preferably less than 500 ppm, more preferably less than
10 30 ppm calcium;
less than 1000 ppm, preferably less than 500 ppm, more preferably less than
30 ppm barium; and
less than 1000 ppm, preferably less than 500 ppm, more preferably less than
30 ppm magnesium.
In addition, the purifying process of the present invention may be utilized to
produce a cesium sulfate compound comprising:
less than 0.3% of barium, calcium, or magnesium including compounds, and
less than 0.2% of other multivalent cationic impurities. Preferably the cesium
sulfate
further comprises, on a dry weight basis, less than 0.50% a chloride group and
less
than 0.3% of aluminum. The purifying process of the present invention may also
be
utilized to produce a cesium sulfate compound comprising:
less than 1000 ppm, preferably less than 500 ppm, more preferably less than
ppm calcium;
less than 1000 ppm, preferably less than 500 ppm, more preferably less than
25 30 ppm barium; and
less than 1000 ppm, preferably less than 500 ppm, more preferably less than
30 ppm magnesium.
In accordance with another aspect of the invention there is provided a high
specific gravity fluid which comprises an aqueous mixture on a dry salt basis
of
30 between 10 and 100% of a cesium compound which has been purified in
accordance
with one of the processes of the present invention. The high specific gravity
fluid
produced has varied applications including use as a drilling fluid or in
mineral
extraction processes. The high specific gravity fluid contemplated by this
invention
has a specific gravity of between 1.2 g/cm3 and about 2.5 g/cm3 and on a dry
salt
basis and comprises less than 0.50% (by weight) of chloride or sulfate anions;
less
CA 02597179 2007-08-27
WO 96/31435 PCTIUS96/04687
11
than 0.3% (by weight) of materials such as aluminum, barium, calcium, or
magnesium
including compounds; and less than 0.2% (by weight) total of other multivalent
cationic impurities. In a preferred embodiment the cesium compound comprises:
less than 1000 ppm, more preferably less than 500 ppm, even more preferably
less than 30 ppm sulfate;
less than 1000 ppm, more preferably less than 500 ppm, even more preferably
less than 30 ppm calcium;
less than 1000 ppm, more preferably less than 500 ppm, even more preferably
less than 30 ppm barium; and
less than 1000 ppm, more preferably less than 500 ppm, even more preferably
less than 30 ppm magnesium.
In another aspect of the present invention, the present inventors have also
found an improved process for prepaiing cesium compounds from cesium-including
materials, including naturally occurring minerals or ores, such as pollucite,
solutions
including cesium aluminum sulfate, and other materials, e.g., spent catalysts
or
residues comprising cesium fluoride or cesium sulfate.
The cesium production processes of the present invention may be carried out
utilizing conventional industrial scale mixing vessels and equipment for
handling the
cesium-including materials (e.g., ores) and strong acid and base solutions.
The choice
of the particular equipment utilized to practice the processes of the present
invention is
believed to be within the skill of one of ordinary skill in the art and
therefore is not
described below.
One embodiment of a process of the present invention comprises treating a
cesium-including material with a suitable reagent to dissolve at least a
portion, and
preferably all or nearly all, of the cesium contained therein and form a
slurry, adding a
base comprising slal:ed lime or calcium carbonate, adding an acid includin-
the anion
of a predetermined cesium compound to the slurry comprising dissolved cesium
if
necessary to produce the desired cesium compound, reacting the mixture to
produce
the predetermined cesium compound and separating the predetermined cesium
compound from the mixture in the presence of the remainder of the starting
cesium-
including material. Preferably, as part of the separation step or steps, the
predetermined cesium compound is further purified to remove at least a portion
of any
remaining trace impurities.
With reference to an embodiment of the invention illustrated in Figure 3, a
cesium-including material, such as pollucite ore, and an acid suitable for
digesting the
CA 02597179 2007-08-27
WO 96131435 PCT/US96/04687
12
ore and dissolving at least the cesium present therein are combined to form a
slurry.
Suitable acids include, but are not limited to, mineral acids (e.g., sulfuric
acid) and
hydrofluoric, hydrobromic, and hydrochloric acids. Water may also be added to
assist
in the dissolution of the cesium and any aluminum and other alkali metals that
may be
present in the ore. To further assist in dissolving the cesium and any other
alkali
metals and aluminum in the ore, the ore may be comminuted prior to its being
combined with the acid. In a preferred embodiment, the ore is ball milled to
approximately -200 mesh particle size.
In one embodiment the amount of acid mixed with the ore is equal to or in
excess, preferably greater than 110%, of the stoichiometric amount of acid
theoretically required to dissolve all of the cesium and any aluminum and/or
other
alkali metal(s) present in the ore. (The cesium, aluminum, and alkali metal
content of
the ore can be adequately determined by assaying the ore.) In another
embodiment of
the process of the present invention, a 45% (by weight) solution using 93% (by
weight) sulfuric acid is employed in a ratio of between 0.2 to 0.8 in kilos of
ore per
liters of acid solution.
As will be appreciated by those skilled in the art, the acid used to form the
slurn, may be a single acid or a mixture of acids. The amount of acid and/or
the choice
of the acid or acid mixture is dependent on the composition of the ore or
residue
material from which cesium is being extracted. While the following examples
and
discussions refer to pollucite ore, as used herein, the term "cesium-including
materials" shall include any naturally occurring cesium-including minerals or
ores, as
well as other solids or liquid materials comprising cesium, including process
residues
such as spent catalyst material.
In a preferred embodiment, cesium alum is formed as an intermediate in the
process. Formation of the cesium alum intermediate requires the presence of
sulfate
ions and aluminum ions. If the acid or acid mixture does not include sulfuric
acid, a
source of sulfate ions can be added to facilitate the formation of a cesium
alum
intermediate. If the cesium-including material does not include aluminum, a
source of
aluminum ions can be added to facilitate cesium alum formation.
As shown in Figure 4A, the acid may be recycled into the ore digestion vessel
which Nvill reduce the amount of acid that is used.
The digestion of the ore and acid mixture is preferably conducted under
conditions and for a time period sufficient to extract a sufficient amount of
cesium
from the ore to render the overall process commercially efficient. More
preferably, the
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
13
reaction is permitted to continue until at least approximately 90% of the
cesium is
dissolved from the ore, as may be determined from analysis of the spent ore.
In one
embodiment of the invention, the reaction of the ore and acid is conducted
with hot
sulfuric acid at a temperature of from about 115' C to about 200' C, and
preferably at a
temperature of approximately 120 C. The reaction (or digesdon) period is
preferably
at leasc4 hours, and more preferably approximately 16 hours. When a shorter
digestion period or a lower sulfuric acid temperature is employed, cesium
dissolution
from the ore is less complete. During the reaction, the hot digestion liquor
becomes
increasingly more paste-like in consistency. Additional water may be added to
maintain the original volume of the mixture. If the evaporated water is not
replaced the
slurry may eventually solidify. Optionally, the original volume of the mixture
can be
maintained by refluxing. When aluminum is present in the ore, the orelsulfuric
ac:d
slurry comprises solubilized cesium aluminum sulfate (also referred to herein
as
cesium alum), formed from the cesium dissolved from the ore. When an excess of
15, acid is present after achieving the desired level of digestion, the slurry
may optionally
be diluted with water and cooled to approximately 30 C to crystallize cesium
alum.
The remaining sulfuric acid in the mixture is preferably decanted and
recycled; and the
remaining spent ore and cesium alum can optionally be reslurried. (See again
Figure
4A).
Reslurrying may be accomplished by adding water to the spent ore and cesium
alum. The solubility of the cesium slum in the reslurry is primarily a
function of water
volume and temperature employed and therefore the conditions for
recrystallizing the
cesium alum may be readily determined by those skilled in the art. In a
preferred
embodiment, the temperature of the reslurry after water addition is
approximately 100'
C.
Referring to Figure 4B, those of ordinary sltill in the art will recognize
that
cesium alum and ultimately the predetermined cesium compound may be further
purified at this point in the process by recrystallizing the solubilized
cesium aluminum
sulfate in the slurry for further processing. The recrystallization process
may be
repeated as many times as desired to further purify the cesium alum.
Referring again to Figure 3, a base comprising slaked lime or calcium
carbonate and optionally, an acid including the anion of the predetermined
cesium
compound are added to the slurry and spent ore, either together or
sequentially in
either order, to adjust the pH to about 4 to about 9. If a cesium sulfate
compound is
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
14
the desired product of the process, the addition of acid is not necessary as
cesium
sulfate may be separated directly from the cesium alum.
The slaked lime is prepared by contacting lime (calcium oxide) with water
("slaking"). The "slaking" reaction is provided by equation (1).
(1) CaO + H20 ---> Ca(OH)2
By preslaking the lime, the pH can be controlled so that the level of aluminum
and
calcium impurities in the solubilized cesium compound are minimized.
In a preferred embodiment, the base comprises slaked lime. The slaked lime is
allowed to react with the slurry and acid under conditions sufficient, and for
a
sufficient time period, to allow precipitation of aluminum, any silica and/or
iron
dissolved in the liquid component of the slurry. As provided above, to achieve
the
precipitation of the aluminum hydroxide, sufficient base is added to the
mixture to
achieve a pH in the range of about 4 to about 9. In a more preferred
embodiment, base
is added to achieve a pH of about 7 to about 8. In this more preferred pH
range,
substantially complete precipitation of solubilized aluminum is obtained.
After the slaked lime is added, the spent ore, precipitated aluminum
hydroxide,
and precipitated calcium sulfate are separated from the mixture including the
solubilized cesium ions. The separation may be accomplished by any known
means,
such as by filtering.
In accordance with the invention, the spent ore or undissolved portion of the
cesium-including material is utilized as a filtration aid for separation of
aluminum
hydroxide which is formed by the addition of base to the acid digested ore or
treated
cesium-including material. The use of the spent ore or undissolved material
improves
the filtration rate of the aluminum hydroxide that is formed as well as easing
the
washability of the solids to maximize cesium recovery. Inclusion of the spent
ore also
improves compressibility and dewatering of solids.
In another preferred embodiment, slaked lime and calcium carbonate are
employed together. The slaked lime and calcium carbonate, whether used alone
or in
combination, may also be used with one or more additional bases comprising an
ion of
a metal selected from groups 1A (alkali metals) and 2A (alkaline earth metals)
of the
Periodic Table of the Elements and mixtures thereof. Examples of such
additional
bases include KOH, NaOH, K2CO3, Na2CO3, RbOH, Rb2CO3, LiOH, LizCO3,
Mg(OH)2, MgCO3, Cs,CO3, and CsOH.
The selection of the acid used to produce the predetermined cesium compound
which is added to the slurry (and any optional reslurry) depends on the
particular
CA 02597179 2007-08-27
WO 96/31435 PCTIUS96/04687
cesium compound(s) desired. For example, if one desires to produce cesium
nitrate, a
combination of slaked lime and nitric acid are added in an amount sufficient
to adjust
the pH of the mixture to approximately 7 to 8. It is believed that the
reaction proceeds
according to equation (2) and that similar reactions will occur with other
acids:
5
(2) CsA1(SO4)2 + 2Ca(OH)2 + HNO3 + 3H20 ------> CsNO3 +
A1(OI-i)3 + 2CaSO4.2H20
As discussed above, the addition of a sulfate anion in acid form, is not
necessary to
produce cesium sulfate since the sulfate ion will already exist in the cesium
alum
10 including solution.
Examples of acids suitable for use in preparing a predetermined cesium
compound (or cesium salt), include but are not limited to the acids set forth
in Table 1:
Table 1: Acids/Cesium Compounds
Acid Added Cesium Compound Product
Nitric Acid (HNO3) Cesium Nitrate (CsNO3)
Formic Acid (HCOOH) Cesium Formate (CsCOOH)
Formic Acid (as calcium formate) Cesium Formate (CsCOOH)
(Ca(OOCH)2)
Hvdrochloric Acid (HC1) Cesium Chloride (CsC1)
Hvdrobromic Acid (HBr) Cesium Bromide (CsBr)
Acetic Acid (HC2H302) Cesium Acetate (CsC2H3Oz)
Hvdroiodic Acid (HI) Cesium Iodide (CsI)
As will be recognized by those of ordinary, skill in the art, Table 1 provides
a
list of examples of acids that can be used and is not to be construed as a
complete or
exhaustive list of suitable acids. Rather, suitable acids include any acids
which will
react with the cesium ions to yield the cesium compound desired as the end
product.
As will also be recognized by those of ordinary skill in the art from Table 1,
it
is possible to substitute certain salts for the acid. For example, as shown,
calcium
formate may be added instead of formic acid to produce a cesium formate end
product.
Referring to Figure 4C, as part of the separation and recovery step, the
solubilized cesium compound may be purified or "polished" to remove trace
impurities
according to an embodiment of the purifying process of the present invention.
As
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
16
depicted in Figure 4C, soluble compounds of barium and soluble compounds of
carbonate (or carbon dioxide) may be added to the solution mixture including
solubilized ions of cesium and the anion of the predetermined cesium compound.
Typically, for purposes of polishing, less than 0.12 kilogram of barium
hydroxide is
added per 1 kilogram of cesium compound contained in the solution. Insoluble
barium
sulfate, calcium hydroxide, and magnesium hydroxide formed as a result of the
polishing step may be removed by filtration. Residual calcium ions in solution
may be
removed through the addition of alkali carbonates such as cesium carbonate,
potassium
carbonate, or sodium carbonate, or by treatment with carbon dioxide, to
precipitate
insoluble calcium carbonate. The alkali carbonate is employed in an amount
sufficient
to precipitate all calcium ioins present in the solution mixture. The extent
to which the
purification of the predetermined cesium compound is carried out is dependent
on the
end use application for the cesium compound.
After polishing, the solution including the dissolved cesium compound has an
elevated pH of greater than 11. In order to improve the recovery of the cesium
compound, an additional quantity of acid (of the type employed to form the
predetermined cesium compound) is added to adjust the pH of the solution to a
desired
pH. The desired pH is dependent upon intended use or application. The cesium
compound may then be recovered or separated, e.g., by driving off the %vater
through
heating.
In the process of the invention, the predetermined cesium compound can be
recovered as a solid or in solution, or as a solid or solution mixture
including the
predetermined cesium compound and one or more compounds comprising a different
metal (e.g., alkali metals) and the anion of the predetermined cesium
compound.
Referring now to Figure 5, there is illustrated an embodiment of the present
invention wherein the base comprising slaked lime or calcium carbonate and
acid
including the anion of the predetermined cesium compound are added after the
ore/acid
digestion slurry has been treated with a first quantity of base. As discussed
above and
illustrated in Figure 3, ore which has been preferably ground to a mesh of -
200, is
mixed or contacted with a suitable acid (e.g., sulfuric acid) and water in a
process tank
for dissolution of cesium and aluminum from the ore. The quantity of acid
utilized in
this step is preferably at least a stoichiometr-ic quantity with respect to
group 1A
elements of the Periodic Table of Elements and aluminum contained in the ore.
Water
may be added to maintain the original volume. While not shown, the embodiment
of
the invention illustrated in Figure 5 may further include cooling the hot
digestion slurry
CA 02597179 2007-08-27
WO 96/31435 PCT/US96104687
17
to obtain cesium alum and spent ore to crystallize the cesium alum, decanting
the
supernatant liquid which may include excess unreacted acid, and reslurrying
the
crystallized cesium alum and spent ore in water. Even if no excess acid is
present, the
crystallization and resluny steps may be perfonmed.
Referring again to Figure 5, the first quantity of base is mixed with the hot
digestion slurry or alternatively, with the reslurry of cesium alum and spent
ore, to
adjust the pH to about 4 to about 9. The base comprises an ion of a metal
selected
from groups 1A and 2A of the Periodic Table of the Elements (e.g., slaked
lime,
calcium carbonate, lime, potassium hydroxide, sodium hydroxide, potassium
carbonate, sodium carbonate) and mixtures thereof. The base is allowed to
react with
the slurry or alternatively, the reslurry under conditions sufficient, and for
a sufficient
time period, to allow precipitation of the aluminum as aluminum hydroxide
(A1(OH)3); to allow precipitation of any silica and iron dissolved in the
slurry or
resluiry and to allow the formation of solubilized cesium sulfate. It is
believed that the
precipitation generally proceeds according to the reaction illustrated in
equation (3):
(3) 2CsA 1(SO4)2 + 3Ca(OH)2 + 6H20 ------->
2A 1(OH)3 + 3 CaSO4 - 2H2O + Cs2SO4
After the addition of slaked lime and the formation of solubilized cesium
sulfate, the principal undissolved solids, e.g., precipitated aluminum
hydroxide,
precipitated calcium sulfate, and spent ore, are separated from the liquid
component of
the mixture. The liquid component includes crude cesium sulfate. The
separation may
be accomplished by any means known to the art, such as by filtering.
The inventors have discovered that the spent ore facilitates the filtering,
washing, and dewatering characteristics of the precipitated A 1(OH)3 and
CaSO42H2O
cake much like the enhanced filter throughout achieved by adding granular
silica as a
filtering aid.
A second base comprising slaked lime or calcium carbonate and an acid
including an anion of the predetermined cesium compound are then added to the
solubilized cesium sulfate. The reaction mechanism proceeds in accordance with
the
mechanism identified in equation (4) below:
(4) Cs~SO4 + 2HCOOH + CaO + H20 ----> 2CsCOOH +
CA 02597179 2007-08-27
WO 96/31435 PCTIUS96/04687
18
CaSO4*2H2O
A slight excess of slaked lime can be added to achieve a pH sufficient to
precipitate as magnesium hydroxide at least a portion, and preferably all or
nearly all,
of any trace quantities of soluble magnesium present in the mixture to
facilitate its
removal by known separation techniques.
The acid is selected to contain the anion of the cesium compound desired as an
end product. Examples are set forth in Table 1.
The second base may further include base(s) comprising an ion of a metal
selected from groups lA and 2A of the Periodic Table of the Elements and
mixtures
thereof. For example, the second base may comprise slaked lime or calcium
carbonate, or slaked lime and/or calcium carbonate and one or more of the
following
bases: potassium hydroxide, sodium hydroxide, potassium carbonate and sodium
carbonate.
To further purify the cesium compounds obtained by this embodiment, an
embodiment of the purifNir.g process of the present invention may be utilized
in the
same manner as discussed above.
After polishing (purifying), the solution including the dissolved cesium
compound has an elevated pH of greater than 11. In order to improve the
recovery of
the cesium compound, an additional quantity of acid (of the type employed to
form the
predetermined cesium compound) is added to adjust pH of the solution to a
desired
pH. The desired pH is dependent upon intended use or application. The cesium
compound may then be recovered or separated, e.g., by driving off the water
through
heating.
In the process of the invention, the predetermined cesium compound can be
recovered as a solid or in solution, or as a solid or solution mixture
including the
predetermined cesium compound and one or more compounds comprising a different
metal (e.g., alkali metals) and the anion of the predetermined cesium
compound.
A range of cesium compounds of varying composition and purity which have
been purified or produced and purified in accordance with the present
invention are
suitable for use as drilling fluids or heavy medium separation fluids.
Alternatively,
salts of other metals such as sodium or potassium can be coformed with the
predetermined cesium compounds by adding such ions to the solution mixtures
comprising solubilized cesium at any step of the process. For example, in one
embodiment, a cesium formate is produced by the process of the invention and
sodium
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
19
formate or potassium formate are co-formed therewith in order to produce a
mixed salt
product. The composition of the salt or salt mixture produced is dependent on
the
anion of the acid and cation(s) of the base(s) utilized and the amounts
thereof which
are reacted with the solubilized cesium sulfate or with the solubilized cesium
alum.
The features of the invention are further disclosed and represented by the
following non-limiting Examples. The high specific gravity fluid may further
comprise compounds of sodium or potassium where the anion of the compound is
the
same as that of the cesium compound included in the fluid.
Chemical analysis of the cesium compounds was performed using
conventional gravimetric analysis, emissions spectrographic analysis and
atomic
absorption techniques, readily known to those skilled in the art.
Example 1
This example illustrates the production of cesium formate via a one step
reaction and the purifying of the cesium formate utilizing a process of the
present
invention.
A 4 liter glass beaker was loaded with 444 grams of ground pollucite ore of
nominally -200 mesh, 670 ml water, and 310 ml 98% by weight H2SO4. This
represents about an 82% excess of acid above the stoichiometric requirements
for
dissolution of alkali metals and aluminum from the ore. The mixture was
continually
mixed while heating at approximately 115 C for 16 hours. The leach volume was
maintained by adding water.
After 16 hours, the slurry was diluted to a volume of 2200 mi with water,
reheated to about 80-90 C, then cooled to room temperature. A decant of 940
ml was
taken to remove most of the remaining unreacted H2SO4 acid. Nine hundred (900)
ml
of water were than added to reslutry the spent ore and crystallized cesium
alum and the
reslurry mixture was then heated to 80 C with stirring.
A slurry of slaked lime, made from 185 grams of calcium oxide and 700 ml
water, was added to the heated reslurry mixture of cesium alum and spent ore
along
with 30 ml of 88% (by weight) formic acid. After these additions, the pH of
the
resulting mixture was 7.5. The mixture was heated to about 70' C and stirred
for 1
hour.
The liquid component of the mixture (which contains the solubilized cesium
formate) was then separated from the spent ore and the A 1(OH)3 and CaSO4
precipitates by filtration. The filtered residue weighed 736 grams on a dry
weight
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
basis. A wash of 600 ml of boiling water was applied to the filtered solids.
The
filtrates including the solubilized cesium formate were combined and mixed
first with
38 grams Ba(OH)2 - 8 H20 to remove residual SO4-2; then with 15 grams Cs2CO3
to
remove residual calcium ions. The solubilized cesium formate product was then
5 filtered to separate out barium sulfate, calcium carbonate, calcium
hydroxide, and
magnesium hydroxide. The filtrate was then analyzed and found to contain the
following chemical make-up. (Values are recorded on a part per million on a
dry
weight basis of cesium formate product.)
10 Rb 9500 ppm
K 500 ppm
Na 7900 ppm
Li 90 ppm
Ca 20 ppm
15 Cl 500 ppm
Sv~y <100 ppm
Al 50 ppm
Ba 50 ppm
Fe 4 ppm
20 Mg 1 ppm
The overall extraction yield was approximately 85%.
The cesium formate including filtrate was next mixed with a minimal amount of
88% (by weight) formic acid (less than 1 ml) to adjust the solution to a pH of
from
about 6 to about 7. The cesium formate filtrate was then evaporated to a final
volume
of 53 ml; which had a density of 2.20 g/ml (approximately 79% CsCOOH).
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
21
Example 2
This example also illustrates the production of cesium formate and the
purifying of the cesium formate utilizing a process of the present invention.
A 4 liter glass beaker was loaded with 444 grams of pollucite ground to -200
mesh, 670 milliliters (ml) water, and 310 m198% H2SO4. The mixture was mixed
and heated to approximately 115' C for 16 hours. The leach volume was
maintained
with added water.
After 16 hours, the slurry was diluted to a volume of 2200-2500 ml with
water, reheated, then cooled to room temperature. A decant of 1135 ml was
taken to
remove most of the remaining H2SO4 acid. The remaining cesium alum plus spent
ore
was reslurried with approximately 800 ml water, and heated to approximately 70
with
stirring.
A slurry of slaked lime made from 150 grams of calcium oxide in
approximately 500 ml water was added and a pH of 7-8 was obtained. The slurry
was
mixed for 1 and 1/2 hours at.90 C, cooled to 60 C, and then filtered to
separate the
insoluble solids including aluminum hydroxide, calcium sulfate, and spent ore.
On a
dry basis, the insolubles separated from the slurry weighed 675 grams.
The resulting CS2SO4 filtrate plus wash water was heated to 70 C, and a
mixture of 20 grams of calcium oxide in 100 ml of water, and 28 ml 88% (by
weight)
formic acid was added with mixing. An additional slurry of 2 grams calcium
oxide in
minimal water was added to raise the pH to above 11.5 to precipitate magnesium
hydroxide.
The mixture was heated to 70' C and mixed for 1.5 hours, followed by
filtering and %vashing of the collected solids with water. The cesium formate
filtrate
was then purified by the following steps:
The cesium formate filtrate was mixed with 20 grams Ba(OH)2 = 8 H~O to
remove residual S04-2 ions as BaSo4, and then with 20 grams Cs2CO3 to remove
residual calcium as CaCO3. The BaSO4 precipitate was filtered out prior to the
treatment with CSZCO3. After the CaCO3 precipitate was filtered out; the final
purified
or polished CsCOOH filtrate was analyzed and determined to have the following
chemical make-up:
Rb 6000 ppm
K 270 ppm
Na 4500 ppm
CA 02597179 2007-08-27
WO 96/31435 PCTIUS96/04687
22
Li 25 ppm
Ca 45 ppm
Cl 415 ppm
SO4 < 80 ppm
Al 25 ppm
Fe 5 ppm
Ba 100 ppm
Mg 3 ppm
The overall extraction yield was approximately 80%.
The cesium formate filtrate was mixed with a minimal amount of 88% formic
acid (by weight) (less than 1 ml) to adjust the solution to a pH of between 6
and 7.
The cesium formate filtrate was evaporated to a final volume of 42 mis; which
had a
density of 2.34 g/ml (approximately 83% CsCOOH).
Example 3
This example illustrates the production of cesium sulfate and the purifying of
the cesium sulfate utilizing a process of the present invention.
A 4 liter glass beaker was loaded with 444 grams of pollucite ground to -200
mesh, 670 mis water, and 310 ml 98% H2SO4. The mixture was mixed and heated to
approximately 115 C for 16 hours. The leach volume was maintained with added
water so that an acceptable solids to liquids ratio was maintained. After 16
hours, the
slurry was diluted to a volume of approximately 1800 mis with water, reheated,
and
then cooled to room temperature. A decant of 960 mis was taken to remove most
of
the remaining H2SO4 acid. The remaining cesium alum plus spent ore was
reslunied
with approximately 1000 mis water, and heated to about -80 C with stirring. A
slurry
of slaked lime composed of 160 grams of calcium oxide in approximately 300 mis
water was added to heated solution of cesium alum and spent ore to achieve a
pH of
7.5. The slurry was mixed for 2 hrs. at 80 C, cooled to 60 C, and filtered.
The
aluminum hydroxide, calcium sulfate and spent ore, on a dry basis, weighted
723
grams. The resulting Cs2SO4 filtrate plus wash water was heated to 70 C, and
mixed
with 25 grams Cs~CO3 was added to remove residual calcium as CaCO3. CsOH was
added in lieu of calcium hydroxide to raise the solution pH to 12 in order to
precipitate
Mg as Mg(OH)2. After the CaCO3 and Mg(OH)2 precipitates were filtered out, the
final Cs2SO4 filtrate was analyzed. The overall extraction yield was
approximately
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
23
80%. The final Cs2SO4 liquor analyzed on a dry cesium sulfate basis had the
following chemical make-up:
Rb 7350 ppm
K 1020 ppm
Na 5640 ppm
Li 85 ppm
Ca 17 ppm
Al 5 ppm
Fe 1 ppm
Mg 170 ppm
Si 75 ppm
Ba <10 ppm
The final cesium sulfate filtrate was further treated by adding a few drops of
a
cesium hydroxide solution (50% by weight) to the filtrate and then refiltering
the
treated filtrate using Whatman fine filter paper. This additional treatment of
the filtrate
further reduced the magnesium content from 170 ppm to less than 10 ppm.
Example 4
This example illustrates the production of cesium nitrate and the purifying of
the cesium nitrate utilizing a process of the present invention.
A 2500 gallon process tank was loaded with 350 gallons water and 175 gallons
93% technical grade H2SO4. Two thousand (2000) pounds of pollucite ore ground
to
-200 mesh were added with mixing. The mixture was reacted at approximately 115
-
120 C for 16 hours. The leach volume was maintained with added water. After 16
hours, the slurrywas diluted to a volume of about 2000 gallons with water,
reheated
to 90 C, then cooled to room temperature. A decant of about 1500 gallons was
taken
to remove most of the remaining H2SO4. The remaining cesium alum plus spent
ore
was reslurried with about 1400 gallons of water, heated to 90 C with
agitation, and
filtered through a filter press to remove the spent ore. 200 gallons of water
was also
sent through the filter press was a washing step. The hot cesium alum solution
including wash water was evaporated to a volume of about 1300 gallons, and
allowed
to cool to room temperature. A decant of about 1000 gallons was tal:en. The
cesium
alum was recrvstallized a second time for further purification. The purified
cesium
alum reslurried in 1000 gallons water and heated. A slurry of 266 pounds of
calcium
hydroxide slaked in approximately 125 gallons water was added to the purified
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
24
reslurry to achieve a pH of 8.1. The slurry was mixed for approximately 1 hour
at
80'C, cooled to about 60 C, and filtered. The result CS2SO4 filtrate plus
water was
heated to about 80 C, and a slurry of slaked lime comprising 80 pounds of
calcium
hydroxide in 125 gallons of water, and 199 pounds of 70% HNO3 was added with
mixing. the pH of the mixture was measured to be >11.5. The mixture was
stirred 2
hours, followed by filtering to remove insolubles such as calcium sulfate,
calcium
hydroxide, and magnesium hydroxide. The CsZNO3 filtrate was evaporated to
about
400 gallons. About 65 pounds of Ba(OH)2 -8H20 was added to remove residual
SO4 2 as BaSO4. Then, 30 pounds Cs2CO3 was added to remove residual calcium as
CaCO3. After barium sulfate, calcium hydroxide, and calcium carbonate were
filtered
out as precipitate, the CsNO3 filtrate was pH adjusted to about 7 with HNO3,
and
heated to evaporate water. The result product was 312 pounds of CsNO3
crystals.
The dried CsNO3 contained the following chemical make-up:
Rb 225 ppm
K l ppm
Na 2ppm
L <1 ppm
Al <1 ppm
Ba 25 ppm
Ca 8 ppm
Mg <1 ppm
Si 1 ppm
SO4 <100 ppm
Cl c50 ppm
Example 5
This example illustrates the production of cesium sulfate and the purifying of
the cesium sulfate utilizing a process of the present invention.
A 2500 gallon process tank was loaded with 350 gallons water and 175 gallons
93% technical grade H2SO4. This amounted to an 80% excess of H2SO4 over the
stoichiometric requirements. 2000 pounds of pollucite ore, ground to -200 mesh
was
added with mixing. the mixture was reacted at approximately 115 C-120 C for 16
CA 02597179 2007-08-27
WO 96/31435 PCT1US96/04687
hours. The leach volume was maintained with added water.'After 16 hours, the
slurry was diluted to a volume of 2000 gallons with water, heated to 90'C,
then cooled
to room temperature.. A decant of 1500 gallons was taken to remove most of the
remaining H2SO4. The remaining cesium alum plus spent ore was reslurried with
5 1400 gallons of water, heated to 90'C with agitation, and filtered through a
filter press
to remove the spent ore. A 300 gallon quantity of water at about 100'C was
also sent
through the filter press as a wash. The hot solution of cesium alum sulfate
including
was water was evaporated to a volume of about 1300 gallons, and allowed to
cool to
room temperature. A decant of about 1000 gallons was taken. (first
recrystallization
10 purification). The cesium alum was recrystallized a second time for further
purification. The purified cesium alum was mixed and heated to between 80'C
and
90 C with 1000 gallons water. A slurry of slaked lime comprising 264 pounds of
calcium hydroxide in approximately 125 gallons of water was added to purified
and
heated cesium alum to raise the pH to greater than 9. Two liters of reagent
H2SO4 was
15 added to adjust the pH to 8.5. The slurrywas mixed approximately 1 hour at
80'C,
cooled to about 50 C, and filtered. A second quantitv of approximatel;v 4
pounds of
lime were added to obtain a pH >12. The Cs2SO41iquor was evaporated to a
approximately 300-400 gallons, and 15 pounds of Cs2CO3 was added to remove
residual calcium as calcium carbonate. After the insoluble were filtered out
the
20 Cs2SO4 solution was evaporated to a 50% solution. The overall yield was
approximately 70%. The Cs,SO4 analyzed on a dry cesium sulfate basis
contained:
Rb 475 ppm
K 38 ppm
Na 165 ppm
25 li 4 ppm
Al 10 ppm
Ca 7 ppm
Cr 20 ppm
Fe 5 ppm
Mg <1 ppm
Si 20 ppm
Examnle 6
This example illustrates the purification of cesium sulfate solution including
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
26
approximately 0.6 grams per liter calcium and approximately 0.1 grams per
liter
magnesium according to a process of the present invention. On a dry cesium
sulfate
basis, this represents approximately 7000 ppm calcium and approximately 1000
ppm
magnesium.
Approximately 1600 gallons of dilute cesium sulfate solution (5-10% Cs2SO4)
were mixed with 8 pounds of lime (slaked) to raise the pH from 7.4 to 12.8.
The
mixture was evaporated to a volume of 300-400 gallons, and the cesium sulfate
liquor
was decanted from settled precipitated solids. 18 pounds of cesium carbonate
were
added to precipitate residual calcium ions as calcium carbonate. The purified
cesium
sulfate solution was filtered to remove residual Mg(OH)2 and CaCO3. The cesium
sulfate was evaporated to a final volume of approximately 150 gallons. The
final
cesium sulfate liquor analyzed on a dry cesium sulfate basis had the following
chemical make-up:
Ca 16ppm
MCr Ippm
Example 7
This example illustrates the purification of cesium formate solution including
>5 grams per liter Sulfate, >1 grams per liter calcium, and approximatelv 0.05
grams
per liter magnesium according to a process of the present invention. On a dry
cesium
formate basis, this represents >5% Sulfate, >1% Calcium, and approximately 600
ppm magnesium.
Approximately 1300 gallons of dilute cesium formate solution (5-10%
CsCOOH) were mixed with 30 pounds of lime (slaked) to raise the pH from 7.1 to
>12. The mixture was evaporated to a volume of approximatelv 500 gallons, and
the
cesium formate liquor was filtered to remove precipitated Mg(OH)2 and CaSO4.
The
cesium formate filtrate was heated to >60 C and 110 pounds of Ba(OH)2H20 were
added. The precipitated BaSO4 and Ca(OH)2 were removed by filtration. Residual
soluble calcium ions were precipitated from the cesium formate filtrate as
calcium
carbonate by addition of 2 pounds potassium carbonate. The precipitated
calcium
carbonate was removed by filtration, and the cesium formate was evaporated to
a
specific gravity of approximately 2.3 g/ml (-82% cesium formate). A small
amount of
90% formic acid was added to adjust the pH of the final cesium formate liquor
to
CA 02597179 2007-08-27
WO 96/31435 PCT/US96/04687
27
between 8 and 9. The final cesium formate liquor analyzed on a dry cesium
formate
basis had the following chemical make-up:
Ca <10 ppm
Mg <1 ppm
SO4 200 ppm.
It should be clearly understood that the forms of the present invention herein
described are illustrative only and are not intended to limit the scope of the
invention.