Note: Descriptions are shown in the official language in which they were submitted.
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
HYDROGEN GENERATION SYSTEM AND METHOD
RELATED APPLICATION
[0001] This invention claims priority to United States Provisional Application
Serial
Number 60/647,392, filed January 28, 2005, which is hereby incorporated herein
in its
entirety.
STATEMENT REGARDING FEDERALLY SPONSORED
RESEARCH OR DEVELOPMENT
[0002] This invention was made with Government support under Technology
Investment Agreement FA8650-04-3-2411 awarded by the United States Air Force.
FIELD OF THE INVENTION
[0003] The invention relates to generating hydrogen gas using fuel solutions
of
borohydride compounds. More particularly, the invention relates to a fuel
cartridge
and hydrogen generation apparatus having a volume-exchange configuration for
the
storage of fuel solution, hydrogen gas, and a hydrogen separation region.
BACKGROUND OF THE INVENTION
[0004] Hydrogen is the fuel of choice for fuel cells; however, its widespread
use is
complicated by the difficulties in storing the gas. Many hydrogen carriers,
including
hydrocarbons, metal hydrides, and chemical hydrides are being considered as
hydrogen storage and supply systems. In each case, specific systems need to be
developed in order to release the hydrogen from its carrier, either by
reformation as in
the case of hydrocarbons, desorption from metal hydrides, or catalyzed
hydrolysis of
chemical hydrides.
1
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
[0005] One of the more promising systems for hydrogen storage and generation
utilizes borohydride compounds as the hydrogen storage media. Sodium
borohydride
(NaBH4) is of particular interest because it can be dissolved in alkaline
water solutions
with virtually no reaction; in this case, the stabilized alkaline solution of
sodium
borohydride is referred to as fuel. Furthermore, the aqueous borohydride fuel
solutions are non-volatile and will not burn. These traits impart handling and
transport
ease both in the bulk sense and within the hydrogen generator itself.
[0006] Various hydrogen generation systems have been developed for the
production of hydrogen gas from aqueous sodium borohydride fuel solutions.
Such
generators typically require chambers to store fuel, borate product, and a
catalyst or
other reagent to promote hydrolysis of the borohydride. Hydrogen generation
systems
can also incorporate additional components such as hydrogen ballast tanks,
heat
exchangers, condensers, and gas-liquid separators.
[0007] The development of fuel cells as replacements for batteries is
dependent on
finding a convenient and safe hydrogen source. A fuel cell power system for
small
applications needs to be compact and lightweight, have a high gravimetric
hydrogen
storage density, and preferably be operable in any orientation. Additionally,
it should
be easy to match the control of the system's hydrogen flow rate and pressure
to the
operating demands of the fuel cell.
BRIEF SUMMARY OF THE INVENTION
[0008] The invention relates to apparatus and methods for generating hydrogen
gas
using a catalyst or reagent and a boron hydride compound.
[0009] One embodiment of the present invention provides a hydrogen gas
generator
with a housing having a fuel storage chamber, a hydrogen storage chamber, and
a
2
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
hydrogen separation chamber wherein both of the hydrogen separation and fuel
storage chambers include at least one gas permeable membrane to transport
hydrogen
out of the respective chambers. Another preferred embodiment of the present
invention utilizes a volume exchanging configuration having a fuel storage
chamber
enclosed within both a hydrogen storage chamber and a hydrogen separation
chamber,
wherein both the hydrogen separation and fuel storage chambers have at least
one gas
permeable membrane located therein.
[0010] Another embodiment provides a hydrogen generator capable of forming
hydrogen gas, comprising a fuel storage chamber; a hydrogen storage region;
and a
pump for removing fuel from the fuel storage chamber; wherein the fuel storage
chamber comprises a fuel outlet for removing the fuel and at least one gas
permeable
membrane to allow hydrogen gas generated by the fuel to pass through the gas
permeable membrane to the hydrogen storage region; and a hydrogen outlet to
allow
hydrogen gas to pass from the hydrogen storage region to outside of the
system.
[0011] In a further embodiment the invention provides a hydrogen gas
generator,
comprising a hydrogen separation chamber; a hydrogen storage chamber; a fuel
storage
chamber at least partially enclosed within the hydrogen storage chamber; a
first conduit
for conveying a fuel solution from the fuel storage chamber to a reaction
chamber to
promote reaction of the fuel solution to produce hydrogen and product
material, and a
second conduit for conveying the hydrogen and product material from the
reaction
chamber to the hydrogen separation chamber; a hydrogen gas outlet for
discharging
hydrogen from the hydrogen separation chamber; and at least one gas permeable
membrane in contact with each of the fuel storage chamber and the hydrogen
separation chamber to allow hydrogen gas to pass through the gas permeable
3
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
membrane while substantially preventing solid and liquid materials from
passing
through the gas permeable membrane.
[0012] The present invention further provides methods for hydrogen gas
generation,
comprising providing a fuel storage chamber, a fuel solution, a hydrogen
storage
chamber, and a hydrogen separation chamber. The fuel chamber is located at
least
partially within the hydrogen storage chamber, and the hydrogen storage
chamber is
located at least partially within the hydrogen separation chamber. At least a
first gas
permeable membrane is provided in contact with the fuel solution storage
chamber,
and at least a second gas permeable membrane in contact with the hydrogen
separation
chamber, to allow hydrogen to pass through the first and second gas permeable
membranes. The fuel solution is conveyed from the fuel solution storage
chamber to a
reaction chamber for generating hydrogen gas and a product material. The
product
material and hydrogen gas are conveyed from the reaction chamber to the
hydrogen
separation chamber. During operation of the preferred method, the hydrogen
storage
chamber is maintained at a lower pressure than the fuel solution chamber.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] A complete understanding of the present invention may be obtained by
reference to the accompanying drawings when considered in conjunction with the
following detailed description, in which:
[0014] Figures 1A and 1B are schematic illustrations of a fuel container for a
hydrogen gas generation system in accordance with the invention;
[0015] Figure 2 is a schematic illustration of an alternative configuration of
a fuel
container for a hydrogen gas generation system in accordance with the
invention;
4
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
[0016] Figures 3A and 3B are schematic illustrations of an arrangement for a
fuel
cartridge for a hydrogen gas generation system in accordance with the
invention;
[0017] Figures 4A and 4B are schematic illustrations of an alternative
arrangement
for a fuel cartridge for a hydrogen gas generation system; and
[0018] Figure 5 is a schematic illustration of an arrangement for a preferred
hydrogen gas generation system in accordance with the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0019] In U.S. Patent Application Serial No. 10/359,104 entitled "Hydrogen Gas
Generation System," the content of which is hereby incorporated herein by
reference in
its entirety, a hydrogen gas generation system is described that comprises a
housing
that includes a volume exchanging configuration having a fuel storage chamber
containing a first flexible bag and a hydrogen separation chamber containing a
second
flexible bag where either or both of these flexible bags may have a gas
permeable
membrane located therein.
[0020] Such systems meter the flow of the hydrogen generation fuel primarily
through a passive pressure system, wherein applied mechanical pressure from a
spring
or the like or applied gas pressureforces the fuel through a valve into a
reaction
chamber. Control of hydrogen generation is imparted by pressure regulation.
The
liquid hydrogen generation fuel is stable (i.e., little to no hydrogen
generation is
observed) at temperatures below about 40 C, but hydrogen can evolve as the
temperature increases. In such a system, hydrogen gas that is produced
spontaneously
from the fuel solution in the fuel storage chamber can be driven though the
membranes
in the fuel storage chamber and into the main body of the housing by the same
pressure
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
differential that is used to push the fuel through the control valve to the
reaction
chamber.
[0021] While passive systems are useful, it may often be desirable to include
a pump
for the ability to variably control fuel delivery. Pumps are often smaller
volumetrically
and gravimetrically than a spring mechanism, and pumps offer the potential to
reverse
fuel flow to actively withdraw fuel from the reaction chamber. However, if a
pump
were to be incorporated in the same system to transport the fuel to the
reaction
chamber, no pressure differential would be available to remove hydrogen gas
from the
fuel solution and the fuel storage chamber. The presence of gas bubbles in the
fuel
solution is undesirable; for instance, bubbles can cause the pump to cavitate.
In
addition, any hydrogen trapped in the fuel solution is potentially unavailable
for
delivery to the hydrogen device or for conversion to electrical power by a
fuel cell.
[0022] In one aspect of the present invention, a system and method is provided
to
create a pressure differential in a pumped system in order to remove hydrogen
from
the hydrogen generation fuel solution and the fuel storage chamber.
[0023] The hydrogen generation fuel useful in these and the following aspects
of the
present invention is preferably a boron hydride compound that is a liquid or
that can be
formulated as a flowable fuel. Many of the boron hydride compounds are water
soluble and aqueous flowable fuel solutions may be prepared as aqueous
mixtures
which may contain a stabilizer component, such as a metal hydroxide having the
general formula M(OH)n, wherein M is a cation selected from the group
consisting of
alkali metal cations such as sodium, potassium or lithium, alkaline earth
metal cations
such as calcium, aluminum cation, and ammonium cation, and n is equal to the
charge
of the cation. Nonaqueous flowable fuels also can be prepared as dispersions
or
6
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
emulsions in nonaqueous solvents, for example, as dispersions in mineral oil,
or as a
solution in, for example, toluene, glymes, or acetonitrile.
[0024] Boron hydrides as used herein include boranes, polyhedral boranes, and
anions of borohydrides or polyhedral boranes, such as those provided in co-
pending
U.S. Patent Application Serial No. 10/741,199, entitled "Fuel Blends for
Hydrogen
Generators," the content of which is hereby incorporated herein by reference
in its
entirety. Suitable boron hydrides include, without intended limitation,
neutral borane
compounds such as decaborane(14) (BioH14); ammonia borane compounds of formula
NHXBHYand NHXRBHY, wherein x and y independently = 1 to 4 and do not have to
be
the same, and R is a methyl or ethyl group; borazane (NH3BH3); borohydride
salts
M(BH4)n, triborohydride salts M(B3H8)n, decahydrodecaborate salts Mz(BioHlo)n,
tridecahydrodecaborate salts M(BloHls)n, dodecahydrododecaborate salts
M2(Bl2Hl2)n,
and octadecahydroicosaborate salts M2(B2oHi8)n, where M is a cation selected
from the
group consisting of alkali metal cations, alkaline earth metal cations,
aluminum cation,
zinc cation, and ammonium cation, and n is equal to the charge of the cation.
M is
preferably sodium, potassium, lithium, or calcium. The boron hydride fuels may
be
prepared as aqueous mixtures and may contain a stabilizer component, such as a
metal
hydroxide having the general formula M(OH)., wherein M is a cation selected
from the
_group consisting of alkali metal cations such as sodium, potassium or
lithium, alkaline
earth metal cations such as calcium, aluminum cation, and ammonium cation, and
n is
equal to the charge of the cation.
[0025] The hydrogen generation fuel is preferably a stabilized metal
borohydride
solution such as described in U.S. Patent No. 6,534,033, entitled "A System
for
Hydrogen Generation," the content of which is hereby incorporated herein by
reference
in its entirety, from which hydrogen is produced as shown in Equation 1, where
MBH4
and MB(OH)4, respectively, represent an alkali metal borohydride and an alkali
metal
7
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
metaborate:
MBH4 + 4 H2O ---> MB(OH)4 + 4 H2+ heat Equation 1
[0026] Referring to Figure 1A, a fuel container 100 for a hydrogen gas
generation
system includes an outer housing 102 which can be of any suitable material as
appropriate to construct a fuel cartridge of the present invention. Such
materials
include, but are not limited to, metals and plastics. Within the housing are a
fuel
storage chamber 104 separated from a hydrogen storage chamber 106 by a movable
or
flexible partition 108, wherein the partition includes at least one gas
permeable
membrane 110. Examples of suitable gas permeable membranes include materials
that
are more permeable to hydrogen than to a liquid, for example water, such as
silicon
rubber, polyethylene, polypropylene, polyurethane, fluoropolymers or any
hydrogen-
permeable metal membranes such as palladium-gold alloys. Suitable gas
permeable
membranes may be microporous and hydrophobic and/or oleophobic. The flexible
or
movable nature of the partition accommodates volume expansion and reduction
and
thus pressure changes within the two storage regions. The terms "chamber" and
"region" are used interchangeably herein.
[0027] The hydrogen storage chamber is maintained at a lower pressure than the
fuel storage chamber such that a pressure differential is maintained between
the two
chambers. Such pressure differentials can be realized by maintaining regions
within
the system at different pressures. The fuel reservoir may be under pressure
due to the
compression of elastic walls or the application of applied force by a spring
plate, for
example. Hydrogen produced from the fuel solution contained within fuel
storage
chamber 104 can be forced though the gas permeable membrane into the hydrogen
storage chamber by the higher pressure in the fuel storage chamber. The
pressure
differential between the two chambers can be maintained by the removal of
hydrogen
8
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
from the hydrogen storage chamber, such as via a pressure relieve valve that
vents at a
preset pressure below the pressure in the fuel storage chamber, or the
consumption of
hydrogen by a hydrogen device or removal of hydrogen from chamber 106 via
hydrogen outlet 112. For example, when a fuel cell is coupled to the hydrogen
storage
chamber of the fuel container, a region of lower pressure may be created by
the
operation of the fuel cell to help ensure the pressure differential. This
arrangement
allows chamber 106 to be vented to a lower pressure to create the pressure
differential
needed for efficient removal of hydrogen from the fuel solution.
[0028] The hydrogen storage chamber can be vented directly to the atmosphere
through hydrogen outlet 112 which can include a check valve to prevent the
backflow
of air. Preferably, hydrogen outlet 112 can be connected to a hydrogen outlet
line 120
downstream from the pressure drop in order to capture the off-gassed hydrogen
gas for
delivery to the hydrogen device, such as a power module. An illustrative
example of
such a connection is shown in Figure 1B, wherein hydrogen outlet 112 is
connected to
hydrogen line 120 downstream of regulator 124, which receives hydrogen
generated
from the reaction of the hydrogen generation fuel in reaction chamber 116. The
regulator 124 may be replaced by the use of an orifice or other flow
restrictor that
would impart a pressure drop in line 120 One or more pressure relief valves
that vent
to a lower pressure such as the atmosphere may be incorporated in the system
to
remove accumulated hydrogen gas for those instances when the system is
inactive for
extended periods.
[0029] As shown in Figure 1B, a fuel regulator controller 122 such as a fuel
pump
causes the fuel solution to be transported from the fuel storage chamber 104
through
fuel conduit 114 to a reaction chamber 116 which contains a catalyst to
enhance the
reaction of the fuel solution to produce hydrogen gas as shown in Equation 1
for
9
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
borohydrides. The product stream comprising a boron product material and
hydrogen
gas is transported to a hydrogen separation chamber 118 to separate the gas
from liquid
and solid components of the product stream and deliver the gas. The gas may be
delivered for use by a power module comprising a fuel cell or hydrogen-burning
engine for conversion to energy, or any other hydrogen device, including
balloons or
hydrogen storage devices such as a hydrogen cylinders or metal hydrides. At
least one
pressure relief valve may be included in chamber 118 or in the conduit line
120 to vent
hydrogen.
[0030] The reaction chamber used with this embodiment preferably contains a
reagent, such as a catalyst metal supported on a substrate. The preparation of
such
supported catalysts is taught, for example, in U.S. Patent No. 6,534,033
entitled "System
for Hydrogen Generation." Other suitable catalysts or reagents that are known
to
promote the reaction of boron hydride compounds such as unsupported metals,
acids,
or heat can alternatively be present in the reaction chamber. These catalysts
and
reagents can be combined to work in concert for the production of hydrogen;
for
example, heat may be used with a supported metal catalyst system.
[0031] Figure 2 illustrates another configuration of a fuel container in
accordance
with the present invention, wherein features that are the same as those shown
in Figure
1 have like numbering. In this configuration, the fuel storage chamber 104 is
a flexible
liquid-tight material, such as, but not limited to: nylon; polyurethane;
polyvinylchloride
(PVC); polyethylene polymers, including such as low density polyethylene
(LDPE),
linear low density polyethylene (LLDPE), high density polyethylene (HDPE), and
ethylene-vinyl acetate copolymers (EVA); natural rubber; synthetic rubber;
metal foil or
other material, and which contains at least one gas permeable membrane. The
gas
permeable membrane is preferably substantially impermeable to liquids and
solids, and
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
substantially prevents solid and liquid materials from passing through the gas
permeable membrane while allowing gas flow. By "substantially" in this context
what
is meant is preferentially allowing passage of gases relative to the passage
of solids
and/or liquids or, in preferred cases, allowing passage only of gases. The
flexible fuel
storage chamber 104 is contained within the outer housing as illustrated in
Figure 2; the
region bounded by and between the outer housing and the fuel chamber comprises
the
hydrogen storage chamber 106. The flexible walls of the fuel chamber
accommodate
the pressure changes of the two storage regions.
[0032] In hydrogen generation systems of the present invention, it may be
preferable
to design the hydrogen separation chamber and the fuel storage chamber within
one
outer housing to provide advantages such as minimizing the overall system
volume.
Referring to Figures 3A and 3B, wherein features that are the same as those
shown in
previous figures have like numbering, a hydrogen gas generation system 300
includes
an outer housing 102, which contains a flexible fuel storage chamber 104
enclosed
within a flexible hydrogen storage chamber 106, and a hydrogen separation
chamber
302. The hydrogen separation chamber 302 may be the interior of the housing as
shown in Figure 3A, or may be a separate flexible chamber as shown in Figure
3B. One
or more of the various chambers may be comprised of a flexible, liquid-tight
material,
such as nylon; polyurethane; polyvinylchloride; polyethylene polymers
including, such
as, low density polyethylene (LDPE), linear low density polyethylene (LLDPE),
high
density polyethylene (HDPE), and ethylene-vinyl acetate copolymers (EVA);
natural
rubber; synthetic rubber; metal foil or other material, or may be comprised of
a non-
flexible or rigid material, such as metal or plastic, which contains one or
more movable
partitions telescopically or otherwise to provide for a volume exchanging
configuration.
The fuel storage chamber 104 contains at least one gas permeable membrane.
11
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
[0033] The hydrogen generation reaction results in the generation of hydrogen
gas
and a boron product material which are transported to the hydrogen separation
chamber 302 via conduit 304. For example, in the hydrolysis reaction shown in
Equation 1 for borohydride compounds, a borate salt is included in the product
material.
[0034] In the configuration shown in Figure 3A, the hydrogen and product
materials
collect in the interior of the housing, i.e., the hydrogen separation chamber
302, and the
hydrogen is delivered through at least one hydrogen separation membrane 110
present
in the inlet of hydrogen line 120 while maintaining any solid and liquid
components of
the product mixture within the hydrogen separation chamber 302. The hydrogen
can
be delivered for use by a power module, comprising a fuel cell or hydrogen-
burning
engine for conversion to energy, or other hydrogen device.
[0035] Referring now to Figure 3B, the hydrogen and boron product materials
collect in the flexible hydrogen separation chamber 302. The hydrogen is
delivered
through a hydrogen separation membrane 110 present in the wall of chamber 302
while
maintaining any solid and liquid components of the product mixture within the
hydrogen separation chamber 302. The hydrogen collects in the interior of the
housing
and can be drawn off through hydrogen gas outlet 306 for use by a power
module,
comprising a fuel cell or hydrogen-burning engine for conversion to energy, or
other
hydrogen device.
[0036] The hydrogen generation system of Figures 3A and 3B are preferably
operated in a volume exchanging manner, such that initially a full fuel
storage chamber
surrounded by the hydrogen storage bag occupies the majority of the housing's
interior
volume. As fuel is fed to the reaction chamber the hydrogen gas and boron
reaction
products such as borate compounds are transferred to the hydrogen separation
12
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
chamber 302. The reaction products will occupy the volume once occupied by
fuel.
When all fuel is consumed, the hydrogen separation chamber or bag may
constitute a
majority of the interior volume.
[0037] In another embodiment illustrated in Figures 4A and 4B, the hydrogen
separation chamber 302 encloses the fuel storage chamber and hydrogen storage
chamber, wherein features that are the same as those shown in previous figures
have
like numbering. Such a system maximizes volumetric efficiency, operates in a
volume
exchanging manner, and can be operated in an orientation independent manner.
The
hydrogen separation chamber may wholly, as shown in Figure 4A, or partially,
as
shown in Figure 4B, enclose the fuel storage and hydrogen storage chambers.
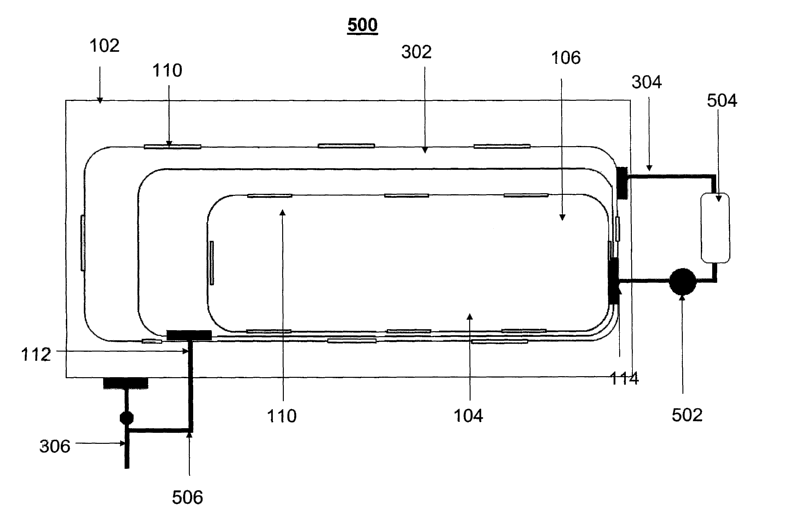
[0038] A complete system for generating hydrogen gas using the fuel container
of
the present invention is illustrated in Figure 5, wherein features that are
the same as
those shown in previous figures have like numbering. Fuel pump 502 conveys
fuel
from the fuel storage chamber 104 via fuel conduit 114 to reaction chamber
504. The
product stream, comprising hydrogen gas and boron reaction products such as
borate
compounds, is transported from the outlet of the reaction chamber to hydrogen
separation chamber 302 via conduit 304. The hydrogen delivered through a
hydrogen
separation membrane 110 iin the wall of chamber 302 and collected in the
interior of the
housing and can be drawn off through hydrogen gas outlet 306 for use by a
power
module comprising a fuel cell or hydrogen-burning engine for conversion to
energy or
a hydrogen device. Alternatively, hydrogen gas outlet 306 could be connected
directly
to the hydrogen separation chamber 302, and at least one hydrogen separation
membrane 110 present in the inlet of the gas line 306 would maintain any solid
and
liquid components of the product mixture within the hydrogen separation
chamber
302.
13
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
[0039] Any accumulated hydrogen in the hydrogen storage chamber 106 can be
provided to the device or power module via an optional regulator 506 which
connects
hydrogen conduits 112 and 306, or it may simply be vented from the system.
Preferably, the hydrogen is withdrawn from both hydrogen storage chamber 106
and
hydrogen separation chamber 302 at the same time; that is, both regions feed
the
hydrogen consuming device at once. Alternatively, hydrogen may be variably
withdrawn from first one region and then the other.
[0040] The following example further describes and demonstrates features of
the
hydrogen generation system according to the present invention. The example is
given
solely for illustration purposes and is not to be construed as a limitation of
the present
invention.
Example
[0041] The fuel container system of Figure 4 was constructed from a set of
three bags
that were constructed of 2 mil polyurethane (Stevens Urethane P/N ST-1522F3).
The
fuel storage chamber 104 and hydrogen separation chamber 302 each contained
19.2
cmz of polytetrafluoroethylene membrane (Gore, Inc.) capable of allowing the
passage
of hydrogen while providing a barrier to solids and liquids. The three bags
were
contained within a copper-plated aluminum housing 102 that was hermetically
sealed
and fitted with a hydrogen outlet and pressure relief valves.
[0042] The inner bag 104 was charged with a solution of 20% by weight sodium
borohydride and 3% by weight sodium hydroxide in water (the fuel solution).
The
hydrogen storage bag surrounding the inner fuel bag was connected to
atmospheric
pressure. The solution was pumped through a reaction chamber containing a
hydrogen
generation catalyst to produce a product stream comprising borate compounds,
water,
14
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
and hydrogen. The product stream was transferred into outer hydrogen
separation
chamber 302 while the hydrogen generation system was held at a pressure
between 5 to
7 psig.
[0043] Hydrogen was separated from the liquid and solid products by allowing
the
gas to pass through the membrane in bag 302 into the interior of the aluminum
housing. Due to the exothermicity of the hydrogen generation reaction, the
product
stream is at a higher temperature than the fuel solution. As the product
stream filled
bag 302, heat was transferred to the fuel solution in bag 104, and, as the
fuel solution
warmed, a portion of the fuel underwent hydrolysis, releasing hydrogen into
inner bag
104. This hydrogen passed from the bag 104 through its membrane to hydrogen
storage bag 106 and vented from the box at atmospheric pressure via outlet
112. The
hydrogen produced through reaction with the hydrogen generation catalyst in
the
reaction chamber was monitored with a mass flow controller external to the
hydrogen
generation system. From 800 mL of fuel solution, 425 cc/min of hydrogen were
produced over continuous operation of the system for 17 hours.
[0044] While the present invention has been described with respect to
particular
disclosed embodiments, it should be understood that numerous other embodiments
are
within the scope of the present invention. For example, while the preceding
figures
and embodiments have shown the reaction chamber as external to the housing,
the
reaction chamber may be incorporated within the outer housing; in such cases,
the
appropriate fuel and product conduit lines would not exit the outer housing.
Additional components of the exemplary hydrogen generation systems such as
regulators and fuel pumps may also be incorporated within the outer housing.
The
outer rigid housing 102 can be replaced with a flexible housing to eliminate
the weight
of an outer container, and increase the energy density of the system. Elements
such as
CA 02600920 2007-07-27
WO 2007/084142 PCT/US2006/002895
pistons or springs that apply pressure mechanically may be incorporated into
some
aspects to push against one or both of chambers 104 and 106 to assist in
maintaining a
pressure differential and/or drive the fuel into the reactor.
16