Note: Descriptions are shown in the official language in which they were submitted.
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
SECONDARY ELECTROCHEMICAL CELL
This Application claims the benefit of Provisional Application Serial No.
60/666,132 filed March 28, 2005, and also claims the benefit of Provisional
Application Serial No.60/729,932 filed October 25, 2005.
FIELD OF THE INVENTION
[0001] This invention relates to an eiectrochemical cell employing an
electrolyte containing a charge-carrier, and a positive electrode active
material
containing a charge-carrier, wherein in the electrochemical cell's nascent
state,
the charge carrier present in the electrolyte differs from the charge carrier
present in the positive electrode active material.
BACKGROUND OF THE INVENTION
[0002] A battery pack consists of one or more electrochemicai cells or
batteries, wherein each cell typically includes a positive electrode, a
negative
electrode, and an electrolyte or other material for facilitating movement of
ionic
charge carriers between the negative electrode and positive electrode. As the
cell is charged, cations migrate from the positive electrode to the
electrolyte
and, concurrently, from the electrolyte to the negative electrode. During
discharge, cations migrate from the negative electrode to the electrolyte and,
concurrently, from the electrolyte to the positive electrode.
CA 02601912 2007-09-21
Zoo6i1f~f~~,~a
. ~{~p$rQvhemica{ cells employing an alkali me~ai paseas~l
electrode active material employed an electrolyte having a salt of a
corresponding alkali metal dissolved therein. Stated differently, the alkali
metal
of the electrode active material and the alkali metal were the same (e.g. use
of
LiPF6 as an electrolyte salt in a cell containing LiCoO2). Conventional wisdom
has held that this was necessary in order to form a functional secondary
electrochemical cell. Because lithium (Li) is best suited for intercalation
with
graphite-based electrodes (primarily because lithium forms a stable SE{ layer
on the graphite upon cycling), this necessitated use of a lithium-based
electrolyte which, in turn necessitated used of a lithium-based intercalation
active material for the positive electrode (cathode). This necessity has
eliminated numerous lithium-based intercalation materials from actual and
potential use in an electrochemical cell, due to the difficulty or high
production
cost associated with the synthesis of such lithium-based electrode materials.
[0004] However, analogs of many of such intercalation materials can be
synthesized, and often with fewer synthesis steps and at a lesser material and
production cost. Unfortunately, due to conventional wisdom, use of such
analog electrode active materials in a positive electrode (cathode) has been
attempted, because those skilled in the art were operating under the
misconception that a{ithium-based electrolyte could not be employed in a cell
containing non-lithium based positive electrode active material. However, the
inventors of the present invention have now proven that a non-lithium alkali
or
alkaline-based electrode active materials can be employed in a secondary
electrochemical cell in conjunction with a lithium-based electrolyte.
2
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
SUMMARY OF THE INVENTION
[0005] The present invention provides a novel secondary electrochemical
cell employing an electrolyte containing one or more (i.e. at least one)
charge-
carriers, and a positive electrode active material containing one or more
(i.e. at
least one) charge-carriers, wherein in the electrochemical cell's nascent
state,
the charge carrier(s) present in the electrolyte differ from the charge
carrier(s)
present in the positive electrode active material.
[0006] In one embodiment, the electrode active material (in its nascent
state) is represented by the general formula:
AaMb(M'O)c(XY4)dOeZf;
wherein:
(i) A contains at least one element capable of forming a positive ion
and undergoing deintercalation or deinsertion from the active
material upon charge of the electrochemical cell, and 0< a_ 9;
(ii) M and M' are each selected from the group consisting of transition
metals, non-transition metals and mixtures thereof, wherein M and
M' includes at least one redox active element, and 1< b s 6 and 0
_c<1;
(iii) XY4 is selected from the group consisting of X'[O4_x,Y'J, X'[04_
Y,Y'2y], X"S4, [XZ"',X'1_z]04, W04, and mixtures thereof, wherein:
(a) X' and X"' are each independently selected from the group
consisting of P, As, Sb, Si, Ge, V, S, and mixtures thereof;
3
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
from the group consisting of P, As, Sb, Si, Ge,
V, and mixtures thereof;
(c) W is selected from the group consisting of V, Hf, Zr, Ti and
mixtures thereof;
(d) Y' is selected from the group consisting of a halogen
selected from Group 17 of the Periodic Table, S, N, and
mixtures thereof; and
(e) 05 x:5 3,0:5 ys2,0:5 z:5 1,and05 d:5 3,whereinwhene
> 0, c and d (c,d) = 0, and when d > 0, e = 0;
(iv) 0 is oxygen, and 05 e<_ 15, wherein when d > 0, e = 0; and
(v) Z is selected from the group consisting of a hydroxyl (OH), a
halogen selected from Group 17 of the Periodic Table, nitrogen
(N), and mixtures thereof, and 0 s f<_ 4; and
wherein M, X, Y, Z, a, b, c, x, y, z, d, e and f are selected so as to
maintain electroneutrality of the material in its nascent or as-synthesized
state.
[0007] In one embodiment, the secondary electrochemical cell is a
cylindrical cell having a spirally coiled or wound electrode assembly enclosed
in
a cylindrical casing. In an alternate embodiment, the secondary
electrochemical cell is a prismatic cell having a jellyroll-type electrode
assembly
enclosed in a cylindrical casing having a substantially rectangular cross-
section. In yet another embodiment, the secondary electrochemical cell is a
laminate-type cell.
[0008] In each embodiment described herein, the electrode assembly
includes a separator interposed between a first electrode (positive electrode)
4
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
material described above and a counter second
electrode (negative electrode), for electrically insulating the first
electrode from
the second electrode.
[0009] The electrochemical cell further includes a non-aqueous
electrolyte. In the electrochemical cell's nascent state (namely, before the
cell
undergoes cycling), the non-aqueous electrolyte contains one or more charge
carriers (e.g. Li+) that differ from the element(s) selected for moiety A of
the
positive electrode active material. In one preferred embodiment, the
electrolyte
is a lithium-based non-aqueous electrolyte. Stated differently, the positive
electrode active materials is lithium-free in its nascent state.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] Figure 1 is a schematic cross-sectional diagram illustrating the
structure of a non-aqueous electrolyte cylindrical electrochemical cell of the
present invention.
[0011] Figure 2 is a plot of cathode specific capacity vs. cell voltage for a
graphite / 1 M LiPF6 (EC/DMC) / Na3V2(PO4)2F3 rocking chair cell and a.
[0012] Figure 3 is a plot of differential capacity for a graphite / 1 M LiPF6
(EC/DMC) / Na3V2(PO4)2F3 rocking chair cell.
[0013] Figure 4 is a cathode specific capacity plot for multiple cycles for a
graphite / 1 M LiPF6 (EC/DMC) / Na3V2(PO4)2F3 rocking chair cell.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
;lVfiI'ase.bddhJdd'nd that the novel electrochemical cells of this
invention afford benefits over such materials and devices among those known
in the art. Such benefits include, without limitation, one or more of
increased
capacity, enhanced cycling capability, enhanced reversibility, enhanced ionic
conductivity, enhanced electrical conductivity, enhanced rate capability, and
reduced costs. Specific benefits and embodiments of the present invention are
apparent from the detailed description set forth herein below. It should be
understood, however, that the detailed description and specific examples,
while
indicating embodiments among those preferred, are intended for purposes of
illustration only and are not intended to limit the scope of the invention.
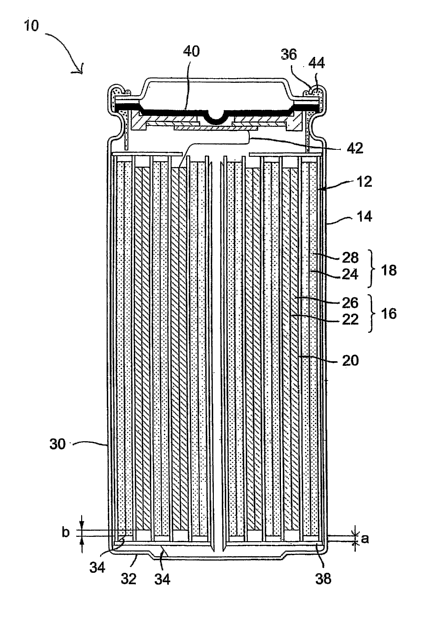
[0015] Referring to Figure 1, a secondary electrochemical cell 10 having
an electrode active material, preferably one described herein below as general
formula (I), is illustrated. The cell 10 includes a spirally coiled or wound
electrode assembly 12 enclosed in a sealed container, preferably a rigid
cylindrical casing 14. The electrode assembly 12 includes: a first or positive
electrode 16 consisting of, among other things, an electrode active material
described herein below; a counter second or negative electrode 18; and a
separator 20 interposed between the first and second electrodes 16,18. The
separator 20 is preferably an electrically insulating, ionically conductive
microporous film, and composed of a polymeric material selected from the
group consisting of polyethylene, polyethylene oxide, polyacrylonitrile and
polyvinylidene fluoride, polymethyl methacrylate, polysiloxane, copolymers
thereof, and admixtures thereof.
6
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
16jul6y ;iE&chmbttdfi'r16,18 includes a current collector 22 and 24,
respectively, for providing electrical communication between the electrodes
16,18 and an external load. Each current collector 22,24 is a foil or grid of
an
electrically conductive metal such as iron, copper, aluminum, titanium,
nickel,
stainless steel, or the like, having a thickness of between 5 pm and 100 pm,
preferably 5 pm and 20 pm. Optionally, the current collector may be treated
with an oxide-removing agent such as a mild acid and the like, and coated with
an electrically conductive coating for inhibiting the formation of
electrically
insulating oxides on the surface of the current collector 22,24. Examples of
suitable coatings include polymeric materials comprising a homogenously
dispersed electrically conductive material (e.g. carbon), such polymeric
materials including: acrylics including acrylic acid and methacrylic acids and
esters, including poly (ethylene-co-acrylic acid); vinylic materials including
poly(vinyl acetate) and poly(vinylidene fluoride-co-hexafluoropropylene);
polyesters including poly(adipic acid-co-ethylene glycol); polyurethanes;
fluoroelastomers; and mixtures thereof.
[0017] The positive electrode 16 further includes a positive electrode film
26 formed on at least one side of the positive electrode current collector 22,
preferably both sides of the positive electrode current collector 22, each
film 26
having a thickness of between 10 pm and 150 pm, preferably between 25 pm
an 125 pm, in order to realize the optimal capacity for the cell 10. The
positive
electrode film 26 is preferably composed of between 80% and 99% by weight of
an electrode active material described herein below as general formula (I),
7
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
~~aefwe~~i' :~~'~b~~~n'c~:~s~l C~ ~~j1,,~y:irveight binder, and between 1% and
10% by weight
electrically conductive agent.
[0018] Suitable binders include: polyacrylic acid; carboxymethylcellulose;
diacetylcellulose; hydroxypropylcellulose; polyethylene; polypropylene;
ethylene-propylene-diene copolymer; polytetrafluoroethylene; polyvinylidene
fluoride; styrene-butadiene rubber; tetrafluoroethylene-hexafluoropropylene
copolymer; polyvinyl alcohol; polyvinyl chloride; polyvinyl pyrrolidone;
tetrafluoroethylene-perfluoroalkylvinyl ether copolymer; vinylidene fluoride-
hexafluoropropylene copolymer; vinylidene fluoride-chlorotrifluoroethylene
copolymer; ethylenetetrafluoroethylene copolymer; polychlorotrifluoroethylene;
vinylidene fluoride-pentafluoropropylene copolymer; propylene-
tetrafluoroethylene copolymer; ethylene-chlorotrifluoroethylene copolymer;
vinylidene fluoride-hexafluoropropylene-tetrafluoroethylene copolymer;
vinylidene fluoride-perfluoromethylvinyl ether-tetrafluoroethylene copolymer;
ethylene-acrylic acid copolymer; ethylene-methacrylic acid copolymer;
ethylene-methyl acrylate copolymer; ethylene-methyl methacrylate copolymer;
styrene-butadiene rubber; fluorinated rubber; polybutadiene; and admixtures
thereof. Of these materials, most preferred are polyvinylidene fluoride and
polytetrafluoroethylene.
[0019] Suitable electrically conductive agents include: natural graphite
(e.g. flaky graphite, and the like); manufactured graphite; carbon blacks such
as
acetylene black, Ketzen black, channel black, furnace black, lamp black,
thermal black, and the like; conductive fibers such as carbon fibers and
metallic
8
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
41b'~r~;+!rneta~!pow,de~~s~cl~t,as carbon fluoride, copper, nickel, and the
like; and
organic conductive materials such as polyphenylene derivatives.
[0020] The negative electrode 18 is formed of a negative electrode film 28
formed on at least one side of the negative electrode current collector 24,
preferably both sides of the negative electrode current collector 24. The
negative electrode film 28 is composed of between 80% and 95% of an
intercalation material, between 2% and 10% by weight binder, and (optionally)
between 1 % and 10% by of an weight electrically conductive agent.
[0021] Intercalation materials suitable herein include: transition metal
oxides, metal chalcogenides, carbons (e.g. graphite), and mixtures thereof
capable of intercalating the alkali metal-ions present in the electrolyte in
the
electrochemical cell's nascent state.
[0022] In one embodiment, the intercalation material is selected from the
group consisting of crystalline graphite and amorphous graphite, and mixtures
thereof, each such graphite having one or more of the following properties: a
lattice interplane (002) d-value (d(002)) obtained by X-ray diffraction of
between
3.35 A to 3.34 A, inclusive (3.35 A:5 d(002)< 3.34 A), preferably 3.354 A to
3.370 A, inclusive (3.354 A s d(002)s 3.370 A; a crystallite size (Lj in the c-
axis
direction obtained by X-ray diffraction of at least 200 A, inclusive (Lc ? 200
A),
preferably between 200 A and 1,000 A, inclusive (200 A_ Lc<_ 1,000 A); an
average particle diameter (Pd) of between 1 pm to 30 pm, inclusive (1 pm <_ Pd
s 30 pm); a specific surface (SA) area of between 0.5 m2/g to 50 m2/g,
inclusive
(0.5 m2/g <_ SA <_ 50 m2/g); and a true density (p) of between 1.9 g/cm3 to
2.25
glcm3, inclusive (1.9 g/cm3 _< p 5 2.25 g/cm3).
9
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
Sefe10h, 0560ai,n to Figure 1, to ensure that the electrodes 16,18 do
not come into electrical contact with one another, in the event the electrodes
16,18 become offset during the winding operation during manufacture, the
separator 20 "overhangs" or extends a width "a" beyond each edge of the
negative electrode 18. In one embodiment, 50 pm < a< 2,000 pm. To ensure
alkali metal does not plate on the edges of the negative electrode 18 during
charging, the negative electrode 18 "overhangs" or extends a width "b" beyond
each edge of the positive electrode 16. In one embodiment, 50 pm < b< 2,000
pm,
[0024] The cylindrical casing 14 includes a cylindrical body member 30
having a closed end 32 in electrical communication with the negative electrode
18 via a negative electrode lead 34, and an open end defined by crimped edge
36. In operation, the cylindrical body member 30, and more particularly the
closed end 32, is electrically conductive and provides electrical
communication
between the negative electrode 18 and an external load (not illustrated). An
insulating member 38 is interposed between the spiraliy coiled or wound
electrode assembly 12 and the closed end 32.
[0025] A positive terminal subassembly 40 in electrical communication
with the positive eiectrode 16 via a positive electrode lead 42 provides
electrical
communication between the positive electrode 16 and the external load (not
illustrated). Preferably, the positive terminal subassembly 40 is adapted to
sever electrical communication between the positive electrode 16 and an
external load/charging device in the event of an overcharge condition (e.g. by
way of positive temperature coefficient (PTC) element), elevated temperature
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
gas generation within the cylindrical casing 14.
Suitable positive terminal assemblies 40 are disclosed in U.S. Patent No.
6,632,572 to Iwaizono, et al., issued October 14, 2003; and U.S. Patent No.
6,667,132 to Okochi, et a1., issued December 23, 2003. A gasket member 42
sealingly engages the upper portion of the cylindrical body member 30 to the
positive terminal subassembly 40.
[0026] A non-aqueous electrolyte (not shown) is provided for transferring
ionic charge carriers between the positive electrode 16 and the negative
electrode 18 during charge and discharge of the electrochemical cell 10. The
electrolyte includes a non-aqueous solvent and an alkali metal salt dissolved
therein capable of forming a stable SEI layer on the negative electrode (most
preferably, a lithium salt). In the electrochemical cell's nascent state
(namely,
before the cell undergoes cycling), the non-aqueous electrolyte contains a
charge carrier other than the element(s) selected for moiety A of the
electrode
active material.
[0027] Suitable solvents include: a cyclic carbonate such as ethylene
carbonate, propylene carbonate, butylene carbonate or vinylene carbonate; a
non-cyclic carbonate such as dimethyl carbonate, diethyl carbonate, ethyl
methyl carbonate or dipropyl carbonate; an aliphatic carboxylic acid ester
such
as methyl formate, methyl acetate, methyl propionate or ethyl propionate; a
.gamma.-lactone such as y-butyrolactone; a non-cyclic ether such as 1,2-
dimethoxyethane, 1,2-diethoxyethane or ethoxymethoxyethane; a cyclic ether
such as tetrahydrofuran or 2-methyltetrahydrofuran; an organic aprotic solvent
such as dimethylsulfoxide, 1,3-dioxolane, formamide, acetamide,
11
11
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
hdirn'e'fhj~l.fdFMr&!ih idey, dicco'~atne, acetonitrile, propyinitrile,
nitromethane, ethyl
monoglyme, phospheric acid triester, trimethoxymethane, a dioxolane
derivative, sulfolane, methylsulfolane, 1,3-dimethyl-2-imidazolidinone, 3-
methyl-
2-oxazolidinone a propylene carbonate derivative, a tetrahydrofuran
derivative,
ethyl ether, 1,3-propanesultone, anisole, dimethylsulfoxide and N-
methylpyrrolidone; and mixtures thereof. A mixture of a cyclic carbonate and a
non-cyclic carbonate or a mixture of a cyclic carbonate, a non-cyclic
carbonate
and an aliphatic carboxylic acid ester, are preferred.
[0028] Suitable alkali metal salts, particularly lithium salts, include (along
with their sodium analogues): LiCIO4; LiBF4; LiPF6; LiAICI4; LiSbF6; LiSCN;
LiCF3SO3; LiCF3CO2i Li(CF3SO2)2; LiAsF6; LiN(CF3SO2)2; LIB10CI10; a lithium
lower aliphatic carboxylate; LiCI; LiBr; Lil; a chloroboran of lithium;
lithium
tetraphenylborate; lithium imides; and mixtures thereof. Preferably, the
electrolyte contains at least LiPF6.
[0029] As noted herein above, the positive electrode film 26 contains a
positive electrode active material wherein, in the electrochemical cell's
nascent
state, the charge carrier(s) present in the positive electrode active material
differs from the charge carrier(s) present in the electrolyte. As used herein,
a
"positive electrode active material charge carrier" refers to an element
capable
of forming a positive ion and undergoing deintercalation (or deinsertion) from
the active material upon the first charge of an electrochemical cell
containing
the same. As used herein, an "electrolyte charge carrier" refers to an ion
present in the electrolyte in the electrochemical cell's nascent state.
12
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
P0309 -r Pinione ernbcrdiMbnt, the positive electrode active material, in its
nascent state, is represented by the general formula (I):
AaMb(M'o)c(XY4)dOeZt- (I)
[0031] For all embodiments described herein, the electrode active
materials described herein are in their nascent or as-synthesized state, prior
to
undergoing cycling in an electrochemical cell. The components of the
electrode active material are selected so as to maintain electroneutrality of
the
electrode active material. The stoichiometric values of one or more elements
of
the composition may take on non-integer values.
[0032] For all embodiments described herein, moiety A contains at least
one positive electrode active material charge carrier. Stated differently, A
contains at least one element capable of forming a positive ion and undergoing
deintercalation (or deinsertion) from the active material upon the first
charge of
an electrochemical cell containing the same, wherein 0 < a<_ 9. In one
embodiment, A is selected from the group consisting of elements from Groups I
and II of the Periodic Table, and mixtures thereof (e.g. Aa = Aa_A'a', wherein
A
and A' are each selected from the group consisting of elements from Groups I
and II of the Periodic Table and are different from one another, and a' < a).
In
one subembodiment, in the material's as-synthesized or nascent state, A does
not include lithium (Li). In another subembodiment, in the material's as-
synthesized or nascent state, A does not include lithium (Li) or sodium (Na).
[0033] As referred to herein, "Group" refers to the Group numbers (i.e.,
columns) of the Periodic Table as defined in the current IUPAC Periodic Table.
(See, e.g., U.S. Patent 6,136,472, Barker et al., issued October 24, 2000,
13
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
herein.) In addition, the recitation of a genus of
elements, materials or other components, from which an individual component
or mixture of components can be selected, is intended to include all possible
sub-generic combinations of the listed components, and mixtures thereof.
[0034] Preferably, a sufficient quantity (a) of moiety A should be present
so as to allow all of the "redox active" elements of moiety M (as defined
herein
below) to undergo oxidation/reduction. Removal of an amount (a) of moiety A
from the electrode active material is accompanied by a change in oxidation
state of at least one of the "redox active" elements in the active material,
as
defined herein below. The amount of redox active material available for
oxidation/reduction in the active material determines the amount (a) of moiety
A
that may be removed. Such concepts are, in general application, well known in
the art, e.g., as disclosed in U.S. Patent 4,477,541, Fraioli, issued October
16,
1984; and U.S. Patent 6,136,472, Barker, et al., issued October 24, 2000, both
of which are incorporated by reference herein.
[0035] For all embodiments described herein, moiety A may be partially
substituted by moiety D by aliovalent or isocharge substitution, in equal or
unequal stoichiometric amounts, wherein:
(a) Aa - [Aa-9' Dh
V
(b) D is an eiement other than the alkali metal charge carrier
present in the electrolyte in the electrochemical cell's
nascent state;
(c) V is the oxidation state of moiety D;
(d) VA = V or VA # V ;
14
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
te ,g='hOP f#h;and
(f) g,h>Oandgsa.
[0036] "Isocharge substitution" refers to a substitution of one element on
a given crystallographic site with an element having the same oxidation state
(e.g. substitution of Ca2+ with Mg2+). "Aliovalent substitution" refers to a
substitution of one element on a given crystallographic site with an element
of a
different oxidation state (e.g. substitution of Na+ with Mg2+).
[0037] Preferably, moiety D is at least one element preferably having an
atomic radius substantially comparable to that of moiety A. In one embodiment,
D is at least one transition metal. Examples of transition metals useful
herein
with respect to moiety D include, without limitation, Nb (Niobium), Zr
(Zirconium), Ti (Titanium), Ta (Tantalum), Mo (Molybdenum), W (Tungsten),
and mixtures thereof. In another embodiment, moiety D is at least one element
characterized as having a valence state of _ 2+ and an atomic radius that is
substantially comparable to that of the moiety being substituted (e.g. M
and/or
A). Unless otherwise specified, a variable described herein aigebraically as
equal to ("="), less than or equal to or greater than or equal to ("?") a
number is intended to subsume values or ranges of values about equal or
functionally equivalent to said number.
[0038] With respect to moiety A, examples of such elements include,
without limitation, Nb (Niobium), Mg (Magnesium) and Zr (Zirconium).
Preferably, the valence or oxidation state of D(V ) is greater than the
valence
or oxidation state of the moiety (or sum of oxidation states of the elements
CA 02601912 2007-09-21
wWO 2006/105253 , PCT/US2006/011571
b~ansislfr~g::~i1Tr~e~n'~~O,i,'~~~)'~ifiding substituted for by moiety D (e.g.
moieiy ivi
and/or moiety A).
[0039] For all embodiments described herein where moiety A is partially
substituted by moiety D by isocharge substitution, A may be substituted by an
equai stoichiometric amount of moiety D, wherein g,h > 0, g s a, and g = h.
[0040] Where moiety A is partially substituted by moiety D by isocharge
substitution and g0 h, then the stoichiometric amount of one or more of the
other components (e.g. A, M, XY4 and Z) in the active material must be
adjusted in order to maintain electroneutrality.
[0041] For all embodiments described herein where moiety A is partially
substituted by moiety D by aliovalent substitution, moiety A may be
substituted
by an "oxidatively" equivalent amount of moiety D, wherein: g = h; g,h > 0;
and
g<a.
[0042] Where moiety is partially substituted by moiety D by aliovalent
substitution and d0 f, then the stoichiometric amount of one or more of the
other components (e.g. A, M, (M'O), XY4, 0 and Z) in the active material must
be adjusted in order to maintain electroneutrality.
[0043] Referring again to general formula (I), in all embodiments
described herein, at least one of M and M' includes at least one redox active
element, and 1:5 b<_ 6. In one embodiment, moieties M and M' are
independently selected from the group consisting of transition metals, non-
transition metals, and mixtures thereof, wherein. As used herein, the term
"redox active element" includes those elements characterized as being capable
of undergoing oxidation/reduction to another oxidation state when the
16
CA 02601912 2007-09-21
~~el~ct W i 6i1 52S3eunder normal operating condit ons SHS6used 1
herein, the term "normal operating conditions" refers to the intended voltage
at
which the cell is charged, which, in turn, depends on the materials used to
construct the cell.
[0044] Redox active elements useful herein with respect to moieties M
and M' include, without limitation, elements from Groups 4 through 11 of the
Periodic Table, as well as select non-transition metals, including, without
limitation, Ti (Titanium), V (Vanadium), Cr (Chromium), Mn (Manganese), Fe
(iron), Co (Cobalt), Ni (Nickel), Cu (Copper), Nb (Niobium), Mo (Molybdenum),
Ru (Ruthenium), Rh (Rhodium), Pd (Palladium), Os (Osmium), Ir (Iridium), Pt
(Platinum), Au (Gold), Si (Silicon), Sn (Tin), Pb (Lead), and mixtures
thereof.
For each embodiment described herein, M and/or M' may comprise a mixture of
oxidation states for the selected element (e.g., M/M' = Mn2+Mn4+).
[0045] In one embodiment, moiety M and/or M' is a redox active element.
In one subembodiment, M is a redox active element selected from the group
consisting of Ti2+, V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Mo2+, Si2+, Sn2+,
and
Pb2+. In another subembodiment, M is a redox active element selected from
the group consisting of Ti3+, V3+, Cr3+, Mn3+, Fe3+, Co3+, Ni3+, Mo3+, and
Nb3+
[0046] In another embodiment, moieties M and/or M' include one or more
redox active elements and (optionally) one or more non-redox active elements.
As referred to herein, "non-redox active elements" include elements that are
capable of forming stable active materials, and do not undergo
oxidation/reduction when the electrode active material is operating under
normal operating conditions.
17
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
If~~6047] ~A':h onO,th& h"bi&,redox active elements useful herein include,
without limitation, those selected from Group 2 elements, particularly Be
(Beryllium), Mg (Magnesium), Ca (Calcium), Sr (Strontium), Ba (Barium); Group
3 elements, particularly Sc (Scandium), Y (Yttrium), and the lanthanides,
particularly La (Lanthanum), Ce (Cerium), Pr (Praseodymium), Nd
(Neodymium), Sm (Samarium); Group 12 elements, particularly Zn (Zinc) and
Cd (Cadmium); Group 13 elements, particularly B (Boron), Al (Aluminum), Ga
(Gallium), In (Indium), TI (Thailium); Group 14 elements, particularly C
(Carbon) and Ge (Germanium), Group 15 elements, particularly As (Arsenic),
Sb (Antimony), and Bi (Bismuth); Group 16 elements, particularly Te
(Tellurium); and mixtures thereof.
[0048] In one embodiment, M and/or M' = MInMIlo, wherein 0< o + n<_ b
and each of o and n is greater than zero (0 < o,n), wherein MI and MII are
each
independently selected from the group consisting of redox active elements and
non-redox active elements, wherein at least one of MI and Mll is redox active.
MI may be partially substituted with MII by isocharge or aliovalent
substitution,
in equal or unequal stoichiometric amounts.
[0049] For all embodiments described herein where MI is partially
substituted by MII by isocharge substitution, MI may be substituted by an
equal
stoichiometric amount of Mll, whereby M= Mln_oMllo. Where MI is partially
substituted by MII by isocharge substitution and the stoichiometric amount of
Ml
is not equal to the amount of M(l, whereby M = Mlõ_oMllp and o0- p, then the
stoichiometric amount of one or more of the other components (e.g. A, D, XY4,
18
CA 02601912 2007-09-21
W 06/1 5?acti;u6AMMial must be adjusted in order to main ains2oo6io11s71
electroneutrality.
[0050] For all embodiments described herein where MI is partially
substituted by MIl by aliovalent substitution and an equal amount of MI is
substituted by an equal amount of MII, whereby M= MIn_oMllo, then the
stoichiometric amount of one or more of the other components (e.g. A, D, XY4,
O and Z) in the active material must be adjusted in order to maintain
electroneutrality. However, MI may be partially substituted by MII by
aliovalent
substitution by substituting an "oxidatively" equivalent amount of Mil for MI
(e.g.
whereby M= MI o MII o, wherein VMl is the oxidation state of MI, and V""" is
the
n-~Mi ~mu
oxidation state of MII).
[0051] In one subembodiment, MI is selected from the group consisting of
Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Mo, Si, Pb, Mo, Nb, and mixtures thereof, and
MII
is selected from the group consisting of Be, Mg, Ca, Sr, Ba, Sc, Y, Zn, Cd, B,
Al, Ga, In, C, Ge, and mixtures thereof. In this subembodiment, MI may be
substituted by MII by isocharge substitution or aliovalent substitution.
[0052] In another subembodiment, MI is partially substituted by MII by
isocharge substitution. In one aspect of this subembodiment, MI is selected
from the group consisting of Ti2+, V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+,
Mo2+,
Si2+, Sn2+, Pb2+, and mixtures thereof, and Mll is selected from the group
consisting of Be2+, Mg2+, Ca2+, Sr2+, Ba2+, Zn2+, Cd2+, Ge2+, and mixtures
thereof. In another aspect of this subembodiment, MI is selected from the
group specified immediately above, and MII is selected from the group
consisting of Be2+, Mg2+, Ca2+, Sr2+, Ba2+, and mixtures thereof. In another
19
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
as~ec~ ~d#f~tf~~iu'e ~~i~io:dithent, MI is selected from the group specified
above,
and MII is selected from the group consisting of Zn2+, Cd2+, and mixtures
thereof. In yet another aspect of this subembodiment, MI is selected from the
group consisting of Ti3+, V3+, Cr3+, Mn3+, Fe3+, Co3+1 Ni3+, Mo3+, Nb3+, and
mixtures thereof, and MII is selected from the group consisting of Sc3+, Y3+,
B3+,
AI3+, Ga3+, In3+, and mixtures thereof.
[0053] In another embodiment, MI is partially substituted by MII by
aliovalent substitution. In one aspect of this subembodiment, MI is selected
from the group consisting of Ti2+, V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+,
Mo2+,
Si2+, Sn2+, Pb2+, and mixtures thereof, and MII is selected from the group
consisting of Sc3+1 Y3+, B3+' A13+, Ga3+, In3+, and mixtures thereof. In
another
aspect of this subembodiment, MI is a 2+ oxidation state redox active element
selected from the group specified immediately above, and MII is selected from
the group consisting of alkali metals, Cu1+, Ag1+ and mixtures thereof. In
another aspect of this subembodiment, MI is selected from the group consisting
of Ti3+, v3+, Cr3+, Mn3+, Fe3+, Co3+, Ni3+, Mo3+, Nb3+, and mixtures thereof,
and
MII is selected from the group consisting of Be2+, Mg2+, Ca2+, Sr2+, Ba2+,
Zn2+,
Cd2+, Ge2+, and mixtures thereof. In another aspect of this subembodiment, MI
is a 3+ oxidation state redox active element selected from the group specified
immediately above, and MII is selected from the group consisting of alkali
metals, Cul+, Ag'+ and mixtures thereof.
[0054] In another embodiment, M and/or M' = MlqM2rM3s, wherein:
(i) Ml is a redox active element with a 2+ oxidation state;
CA 02601912 2007-09-21
WO 2006/105253 ,. }õ .. PCT/US2006/011571
~{ih)~ 1~h~!r~-1se[ected from the group consisting of redox and non-
redox active elements with a 1+ oxidation state;
(iii) M3 is selected from the group consisting of redox and non-
redox active elements with a 3+ or greater oxidation state;
and
(iv) at least one of q, r and s is greater than 0, and at least one
of Ml, M2, and M3 is redox active.
[0055] In one subembodiment, Ml is substituted by an equal amount of
M2 and/or M3, whereby q = q - (r + s). In this subembodiment, then the
stoichiometric amount of one or more of the other components (e.g. A, XY4, Z)
in the active material must be adjusted in order to maintain
electroneutrality.
[0056] In another subembodiment, M' is substituted by an "oxidatively"
equivalent amount of M2and/or M3 (e.g. whereby M=M1 r s M2 r M3 s
q VM7 vM7 VM2 vM3
wherein VM' is the oxidation state of Ml, VM2 is the oxidation state of M2,
and
VM3 is the oxidation state of M3).
[0057] In one subembodiment, Ml is selected from the group consisting of
Ti2+, V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Mo2+, Si2+, Sn2+, Pb2+, and
mixtures
thereof; M2 is selected from the group consisting of Cu'+, Ag'+ and mixtures
thereof; and M3 is selected from the group consisting of Ti3+, V3+, Cra+,
Mn3+,
Fe3+, Co3+, Ni3+, Mo3+, Nb3+, and mixtures thereof. In another subembodiment,
Ml and M3 are selected from their respective preceding groups, and M2 is
selected from the group consisting of Li'+, K1+, Na1+, Rul+, Cs 1+, and
mixtures
thereof,
21
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
1005'97 16,:&6'ib~~er gubembodiment, Ml is selected from the group
consisting of Be2+, Mg2+, Ca2+, Sr2+, Ba2+, Zn2+, Cd2+, Ge2+, and mixtures
thereof; M2 is selected from the group consisting of Cu'+, Ag'+ and mixtures
thereof; and M3 is selected from the group consisting of Ti3+, V3+, Crs+,
Mn3+,
Fe3+, Cos+, Nia+, Mo3+, Nb3+, and mixtures thereof. In another subembodiment,
Ml and M3 are selected from their respective preceding groups, and M2 is
selected from the group consisting of Lil+, K'+, Nai+, Ru'+, Cs '+, and
mixtures
thereof.
[0059] In another subembodiment, Ml is selected from the group
consisting of Ti2+, V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Mo2+, Si2+, Sn2+,
Pb2+,
and mixtures thereof; M2 is selected from the group consisting of Cul+, Ag'+,
and mixtures thereof; and M3 is selected from the group consisting of Sc3+,
Y3+,
B3+, AI3+, Ga3+, In3+, and mixtures thereof. In another subembodiment, Ml and
M3 are selected from their respective preceding groups, and M2 is selected
from the group consisting of Li'+, K'+, Na'+, Ru'+, Cs1+, and mixtures
thereof.
[0060] In all embodiments described herein, moiety XY4 is a polyanion
selected from the group consisting of X'[O4_X,Y'x], X'[O4_y,Y'2y], X"S4,
[XZ"',X',_
Z]04, W04, and mixtures thereof, wherein:
(a) X' and X"' are each independently selected from the group
consisting of P, As, Sb, Si, Ge, V, S, and mixtures thereof;
(b) X" is selected from the group consisting of P, As, Sb, Si, Ge,
V, and mixtures thereof;
(c) W is selected from the group consisting of V, Hf, Zr, Ti and
mixtures thereof;
22
CA 02601912 2007-09-21
WO 2006/105253 ~G :== :== 6( PCT/US2006/011571
~toj =:=a8,s.Eected from the group consisting of a halogen, S, N,
and mixtures thereof; and
(e) 0<x<3,0<y<2,0<z_<1,and05 d<3,whene>0,c
and d (c,d) = 0, and when d> 0, e = 0.
[0061] In one subembodiment, XY4 is selected from the group consisting
of X'04_,eY'X, X'04_yY'2Y, and mixtures thereof, and x and y are both 0 (x,y =
0).
Stated otherwise, XY4 is a polyanion selected from the group consisting of
P04,
Si04, Ge04, V04, AsO4, Sb04, SO4, and mixtures thereof. Preferably, XY4 is
P04 (a phosphate group) or a mixture of P04 with another anion of the above-
noted group (i.e., where X' is not P, Y' is not 0, or both, as defined above).
In
one embodiment, XY4 includes about 80% or more phosphate and up to about
20% of one or more of the above-noted anions.
[0062] In another subembodiment, XY4 is selected from the group
consisting of X'[04_X,Y'x], X'[04_y,Y'2y], and mixtures thereof, and 0< x< 3
and 0
< y< 2, wherein a portion of the oxygen (0) in the XY4 moiety is substituted
with a halogen, S, N, or a mixture thereof.
[0063] In another subembodiment, XY4 = W04 wherein W is selected from
the group consisting of V, Hf, Zr, Ti and mixtures thereof. In another
subembodiment, W is selected from the group consisting of Zr and Ti.
[0064] In all embodiments described herein, moiety Z (when provided) is
selected from the group consisting of OH (Hydroxyl), nitrogen (N), a halogen,
or
mixtures thereof, wherein 0 < f< 4. In one embodiment, Z is selected from the
group consisting of OH, F (Fluorine), CI (Chlorine), Br (Bromine), and
mixtures
23
CA 02601912 2007-09-21
WO 2006/105253 ~, 4 PCT/US2006/011571
th~ere'i~]:'~w~lfi-l~ir~otkle~.~rnbddiment, Z is OH. In another embodiment, L
is I-, or a
mixture of F with OH, Cl, or Br.
[0065] The composition of the electrode active material, as well as the
stoichiometric values of the elements of the composition, are selected so as
to
maintain electroneutrality of the electrode active material. The
stoichiometric
values of one or more elements of the composition may take on non-integer
values. Preferably, the XY4 moiety is, as a unit moiety, an anion having a
charge of -2, -3, or -4, depending on the selection of X', X", X"', Y', and x
and y.
When XY4 is a mixture of polyanions such as the preferred
phosphate/phosphate substitutes discussed above, the net charge on the XY4
anion may take on non-integer values, depending on the charge and
composition of the individual groups XY4 in the mixture.
[0066] In one particular subembodiment, the positive electrode film 26
contains an electrode active material represented by the general formula (II):
AaMb(XY4)dZf, (Il)
wherein:
(i) moieties A, M, and Z are as described herein above, wherein 0 < a
:5 9, 1 sb:5 3,andOsfs4;and
(ii) XY4 is selected from the group consisting of X'[O4_x,Y'X], X'[O4_
y,Y'2Y], X"S4, [XZ"',X'1_Z]O4, and mixtures thereof, wherein:
(a) X' and X"' are each independently selected from the group
consisting of P, As, Sb, Si, Ge, V, S, and mixtures thereof;
(b) X" is selected from the group consisting of P, As, Sb, Si, Ge,
V, and mixtures thereof;
24
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
~Va%' efbcted from the group consisting of a naiogen, 5, N,
and mixtures thereof; and
(d) 0<x:5 3,0_<y<2,0:5 z<1,and1 <d_3;and
wherein A, M, X, Y, Z, a, b, x, y, z, and f are selected so as to maintain
electroneutrality of the material in its nascent or as-synthesized state.
[0067] In one particular subembodiment, M of general formula (II) is
selected from the group consisting of Ti3+, V3+, Crs+, Mns+, Fe3+, Co3+, Ni3+,
Mo3+, Nb3+, and mixtures thereof (preferably V3+), XY4= P04, d = 3 and f = 0.
In
another subembodiment, M of general formula (li) is selected from the group
consisting of Ti2+, V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Mo2+, Si2+, Sn2+,
Pb2+,
and mixtures thereof (preferably Fe2+), XY4 = P04, d = 1 and f = 0.
[0068] In one particular subembodiment, M of general formula (II) is
selected from the group consisting of Ti3+, V3+, Cr3+, Mn3+, Fe3+, Co3+, Ni3+,
Mo3+, Nb3+, and mixtures thereof (preferably V3+), XY4 = P04, and d = 2.
[0069] Methods of making the electrode active materials described by
general formula (II) are well known in the art, and are described in: WO
01/54212 to Barker et al., published July 26, 2001; International Publication
No.
WO 98/12761 to Barker et al., published March 26, 1998; WO 00/01024 to
Barker et al., published January 6, 2000; WO 00/31812 to Barker et al.,
published June 2, 2000; WO 00/57505 to Barker et al., published September
28, 2000; WO 02/44084 to Barker et al., published June 6, 2002; WO
03/085757 to Saidi et al., published October 16, 2003; WO 03/085771 to Saidi
et ai., published October 16, 2003; WO 03/088383 to Saidi et al., published
October 23, 2003; U.S. Patent No. 6,528,033 to Barker et al., issued March 4,
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
l~141erit_'WJ~r-9817,568 to Barker et al., issued May 14, 2002; U.S.
Publication No. 2003/0027049 to Barker et al., published February 2, 2003;
U.S. Publication No. 2002/0192553 to Barker et al., published December 19,
2002; U.S. Publication No. 2003/0170542 to Barker at al., published September
11, 2003; and U.S. Publication No. 2003/1029492 to Barker et al., published
July 10, 2003; the teachings of all of which are incorporated herein by
reference.
[0070] Non-limiting examples of active materials of this subembodiment
and represented by general formulas (I) and (II) include the following:
Na0.95CO0.8Fe0.15Al0.05P04, Na1.025C00.85Fe0.05A10.025Mg0.05P04a
Na1.025C00.80Fe0.10Al0.025Mg0.05PO4, Na1.025CO0.45Fe0.45A10.025Mg0.05PC4j
Na1.025CO0.75Fe0.15A10.025Mg0.05PO4r
Na1.025CO0.7(Fe0.4Mn0.6)0.2AI0.025M90.05P04,
Na1.025CO0.75Fe0.15A10.025Mg0.05P04e Na1.025CO0.85Fe0.05A10.025Mg0.05PO4,
Na1,025CO0.7Fe0.08Mn0.12Al0.025Mg0.05PO4,
NaCO0.75Fe0.15AI0.025Ca0.05PC3.975F0.025,
NaCo0.80Fe0.10Al0.025Ca0.05PO3.975F0.025, Na1.25CO0.6FL'0.1
Mn0.075Mg0.025A10.05PO4,
Na1.oNao.25Coo.6Feo.1 Cuo.075M90.025A10.05P04,
Na1.025Coo.8Feo.1A10.025Mg0.075P44,
N a1.025Coo.6Fe0.05A10.12Mg0.0325PO3.75F0.25)
Na1.025Co0.7Feo.1 M90.o025A10.04PO3.75F0.25,
Nao.75Coo.5Feo.05M9o.015Al0.04PO3F,
Na0.75CO0.5 Fe0.025Cu0.025Be0.015A10.04PO3F,
Na0.75Co0.5Fe0.025Mn0.025Ca0.015A10.04P03Fe
Na1.025CO0.6Fe0.05B0.12Ca0.0325P 3.75F0.25,
Na1.025CO0.65Fe0.05Mg0.0125Al0.1 P03.75F0.25e
Na1.025CD0.65Fe0.05Mg0.065Al0.14PO3.975F0.025Y
Na1.075CO088Fe0_05Mg0.025Al0.05PO3.975F0.025, NaCo0,8Fe0,1
AI0.025Mg0.05PO3.975F0.025r
26
CA 02601912 2007-09-21
WO 2006/105253 õ~ . ~CT/US2006/011571
NdO4i"I~aM~iAI0.067(PO4)0.8(SiO4)0.2, Na0.95C00.9A'0.05Mg0.05HU4,
Na0.95Fe0.8Ca0.15A10.05PO4, Na0.25MnBe0.425Ca0.3SIO4, NaMn0.6Ca0.375AI0.1 PO4,
Na0.25A10.25Mg0.25CO0.75PO4, Na0.55B0.15Ni0.75Ba0.25PO4,
Na1.025CO09gAI0.025Mg0.05PO4, Nca0995CO0,9AI0.05Mg0.05P 4,
Nao.95Feo.8Cao.15AI0.05PO4, Na1.025CO0.7(Fe0.4Mn0.6)0.2AIo.025Mgo.05PO4,,
Na10025COo.8FL'o.1A10.025Mg0.05P04, Na1.025C00.9Al0.025Mg0.05PO4,
Na1.025CO0.75Fe0.15AI0.025Mg0.025PO4,
NaCO0.75Fe0.15AIo.025Ca0.05PO3.975F0.025, NaCo0.9AI0.025Mg0.o5PO3.975F0.025,
Na0.75CO0.625AI0.25PO3.75F0.25, Na1.075CO0.8Cu0.05Mg0.025AI0.05PO3.975F0.025,
Na1.075Fe0.8Mg0.075AI0.05PO3.975F0.025,
Na1.075CO0.8Mg0.075A10.05PO3.975F0.025,
Na1.025CO0.8Mgo.1AIo.05PO3.975F0.025, NaCo0.7Fe0.2AI0.025Mg0.05PO3.975F0.025,
Na2Feo.$Mgo22PO4F; Na2Feo55Co0.5PO4F; Na3COPO4F2; KFe(PO3F)F;
Na2Co(PO3F)Br2; Na2Fe(PO3F2)F; Na2FePO4CI; Na2MnPO4OH; Na2COPO4F;
Na2Feo55Coo55PO4F; Na2Feo.9Mgo.1 PO4F; Na2Feo.8M9o.2PO4F;
Na1225Feo.9MJo.1PO4Fo.25; Na2MnPO4F; Na2CoPO4F; K2Feo.9Mgo.1Po.5Aso.504F;
Na2MnSbO4OH; Na2Feo66Co044SbO4Br; Na3CoAsO4F2; NaFe(AsO3F)CI;
Na2Co(Aso55Sbo55O3F)F2; K2Fe(AsO3F2)F; Na2NiSbO4F; Na2FeAsO4OH;
Na4Mn2(PO4)3F; Na4FeMn(PO4)30H; Na4FeV(PO4)3Br; Na3VAI(PO4)3F;
K3VAI(P04)3CI; Na2KTiFe(PO4)3F; Na4Ti2(PO4)3Br; Na3V2(PO4)3F2;
Na6FeMg(PO4)30H; Na4Mn2(AsO4)3F; K4FeMn(AsO4)30H;
Na4FeV(Po55Sbo55O4)3Br; Na2KAIV(AsO4)3F; K3VAI(SbO4)3C1; Na3TiV(Sb04)3F;
Na2FeMn(Po.5Aso.503F)3; Na4Ti2(PO4)3F; Nca3.25v2(PO4)3F0.25;
Na4Fe2(PO4)3F0.75;
Na6.5Fe2(PO4)3(OH)CI0.5; K$Ti2(PO4)3F3Br2, K8Ti2(PO4)3F5; Na4Ti2(PO4)3F;
Na2.25U2(PO4)3F0.5CI0.75; K3.25Mn2(PO4)3OH0.25, Na2.25KTiV(PO4)3(OH)1.25CI,
27
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
Ijtj!4T~2-~~';i ~~YR3C~~,~ Fe2(PO4)3F2, Na$FeMg(PO4)3F2.25CI0.75,
Na555TiMn(P04)3(OH)2CIo.5; Na3K4.5MnCa(PO4)3(OH)1.5Br; K9FeBa(P04)3F2C12;
Na7Ti2(SiO4)2(PO4)F2; Na$Mn2(SiO4)2(PO4)F2CI; Na3K2V2(SiO4)2(PO4)(OH)CI;
Na4Ti2(SiO4)2(PO4)(OH); Na3KV2(SiO4)2(PO4)F; Na5TiFe(PO4)3F;
Na4K2VMg(PO4)3FCI; Na4NaAINi(PO4)3(OH); Na4K3FeMg(PO4)3F2;
Na4K2CrMn(PO4)3(OH)Br; Na5TiCa(PO4)3F; Na4Tio775Fe155(PO4)3F;
Na4SnFe(PO4)3(OH); Na3NaGe055Ni2(PO4)3(OH); Na3K2VCo(PO4)3(OH)CI;
Na4Na2MnCa(PO4)3F(OH); Na4KTiFe(PO4)3F; Na7FeCo(SiO4)2(PO4)F;
Na6TiV(SiO4)2(PO4) F, K5.5CrMn(SIO4)2(PO4)CI0.5; Na5.5V2(S104)2(PO4)(OH)0.5;
Na5.25FeMn(SI04)2(P04)Br0.25; Na6.5VCO(5104)2.5(PO4)0.5F,
Na7.25v2(S1O4)2.25(PO4)0.75F2; Na5VTi(SiO4)3F0.5CI0.5; Na2K2.5ZrV(S104)3F0.5;
Na4K2MnV(SiO4) 3(OH)2; Na3Na3KTi2(SiO4)3F; K6V2(SiO4)3(OH)Br;
Na$FeMn(SiO4)3F2, Na7.5MnNi(SiO4)3(OH)1,5; Na5K2TiV(SiO4)3(OH)0.5CI0.5;
K9VCr(Si04)3F2CI; Na8V2(Si04)3FBr; Na4FeMg(SO4)3F2;
Na2KNiCo(SO4)3(OH); Na5MnCa(SO4)3F2CI; Na4CoBa(SO4)3FBr;
Na2,5Ko.5FeZn(SO4)3F; Na3MgFe(SO4)3F2; Na3CaV(SO4)3FCI;
Na4NiMn(SO4)3(OH)2; Na2KBaFe(SO4)3F; Na2KCuV(SO4)3(OH)Br;
Nay55COP04F0.5; Na1.25COPO4F0.25; Na1.75FePO4F0.75; Na1.66MnPO4F0.66;
Na1.5CO0.75Ca0.25PO4F0.5; Na1.75CO0.sMno.2PO4F0.75;
Na1.25Fe0.75Mg0.25PO4F0.25;
Na1.66CO0.6Zn0.4PO4F0.66; KMn2SiO4CI; Na2VSiO4(OH)2; Na3CoGeO4F;
NaMnSO4F; NaFeo.9Mgo11SO4CI; NaFeSO4F; NaMnSO4OH; KMnSO4F;
Nay.75Mno.sMgo.2P04Fo.75; Na3FeZn(PO4)F2; Nao.5Vo.75N1go.5(P04)Fo.75;
Na3V0.5A10.5(PO4)F3.5; Na0.75VCa(PO4)F1.75; Na4CuBa(PO4)F4;
Na0.5Vo55Ca(PO4)(OH)155; Na1.5FeMg(PO4)(OH)CI; NaFeCoCa(P04)(OH)3F;
28
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
Rl~~(;c~~a~~~~~~~~H)2~~,",nl(1~{'~ 195Mn1.5AI(PO4)\ H)3.75~
Na2C00.75Mg0.25WU4)F;
Na2Coo.8M9o.2(PO4)F; NaKCoo.5Mgo.5(PO4)F; Na1.5K0.5Fe0.75Mg0.25(PO4)F;
Na1.5K0.5V0.5Zn0.5(PO4)F2; Na6Fe2Mg(PS4)3(OH2)CI;
Na4Mn1,5Co0,5(PO3F)3(OH)3.5; K8FeMg(PO3F)3F3CI3 Na5Fe2Mg(SO4)3CI5,
NaTi2(SO4)3C1, NaMn2(SO4)3F, Na3Ni2(SO4)3CI, Na3Co2(SO4)3F,
Na3Fe2(SO4)3Br, Na3Mn2(SO4)3F, Na3MnFe(SO4)3F, Na3NiCo(SO4)3CI;
NaMnSO4F; NaFeSO4CI; NaNiSO4F; NaCoSO4Cl; NaMn1_XFeXSO4F, NaFe1_
XMgXSO4F; Na7ZrMn(SiO4)3F; Na7MnCo(SiO4)3F; Na7MnNi(SiO4)3F;
Na7VAI(SiO4)3F; Na5MnCo(PO4)2(SiO4)F; Na4VAI(PO4)2(SiO4)F;
Na4MnV(PO4)2(SiO4)F; Na4VFe(PO4)2(SiO4)F; Nao66VP04F066; Nao88VP04Fo,8;
NaVPO4F; Na3V2(PO4)2F3; NaVPO4Cl; NaVPO4OH; NaVPO4F;
Na3V2(PO4)2F3; NaV099AI011PO4F; NaFePO4F; NaTiPO4F; NaCrPO4F; NaFePO4,
NaCoPO4, NaMnPO4; NaFe0.9Mg0,1P04; NaFeo,sMg0,2P04; NaFeo.95Mgo.o5P04;
NaFe0.9Ca0.1P04i NaFeo.$Cao,2P04; NaFeo.$Zno22PO4; NaMno,$Fe0.2PO4;
NaMno99Feo8$PO4; Na3V2(PO4)3; Na3Fe2(PO4)3a Na3Mn2(PO4)3; Na3FeTi(PO4)3;
Na3CoMn(PO4)3; Na3FeV(P04)3; Na3VTi(PO4)3; Na3FeCr(PO4)3;
Na3FeMo(PO4)3; Na3FeNi(P04)3a Na3FeMn(PO4)3; Na3FeAI(PO4)3;
Na3FeCo(PO4)3; Na3Ti2(PO4)3; Na3TiCr(PO4)3i Na3TiMn(PO4)3i Na3TiMo(PO4)3;
Na3TiCo(P04)3; Na3TiAI(PO4)3; Na3TiNi(PO4)3; Na3ZrMnSiP2O12; Na3V2SiP2O12;
Na3MnVSiP2O12; Na3TiVSiP2O12i Na3TiCrSiP2O12i Na355AIVS10.5P2.5O12:
Na355V2Si0.5P2.5O12; Na2.5AICrSi0.5P2.5O12; Na2.5U2P3O11.5F0.5; Na2V2P3011Fr
Na255VMnP3O11.5F0.5; Na2V0.5Fe1.5P3O11 Fa Na3V0.5U1.5P3O11.5F0.5v Na3V2P3011
F,
Na3Mn0.5V1.5P3O11 F0.5; NaCo0.8Feo.lTio.o25M9o.o5PO4;
Na1.025C00.8Fe0.1T10.025A10.025P04;
Na1.025CO0.8Fe0.1Tio.025Mg0.025PO3.975F0.025,
29
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
11"N'aGo0",8~5F ~s0iilst~ts~b.02s~~g0.'d~5~"'04; NaCoo.85FE-
'o.075Tio.025Mg0.025PO4;
NaCoo.8Feo.1Ti0.025Al0.025Mg0.025PO4, Na1.025Co0.8Feo.lTio.025Mg0.05PO4,
Na1.025Co0.8Feo.lTio.025Al0.025Mg0.025PO4, NaCo0.8Feo.yTio,05Mgo.o5PO4, as
wel, as
lithium analogues of the same.
[0071] Preferred active materials of this subembodiment include
NaFePO4; NaCoPO4, NaMnPO4; NaMno.$Feo22PO4; NaMno99Feo88PO4;
NaFe0,9Mg ,1PO4, NaFeo,$Mgo,2P04; NaFeo.95Mgo.o5PO4;
Na10025Co0.85Feo.o5Al0.025N1g0.05PO4, Na1.025Co0.8oFeo.10A10.025Mg0.05PO4,
Na1.025CO0.75Fe0.15A10.025Mg0.05PO4,
Na1.025CO0.7(Fe0.4Mn0.6)o.2Al0.025Mg0.05PO4,
NaCoo.8Feo.1Al0.025Cao.05PO3.975F0.025,
NaCoo.8Feo.lAlo.025Mg0.05P03.975F0.025,
NaCo0.8Feo.lTio.o25Mgo.o5PO4; Na1.025CO0.8Fe0.1Ti0.025Al0.025PO4;
Na1.025CO0.8Fe0.1Ti0.025Mg0.025PO3.975F0.025; NaCO0.825Fe0.1Ti0.025Mg0.025PO4;
NaCo0.85Feo.075Tio.025Mg0.025PO4. A particularly preferred active materials
are
NaFePO4 and Na3V2(PO4)3.
[0072] In another particular subembodiment, the positive electrode film 26
contains an electrode active material represented by the general formula
(111):
AaMbOe, (lll)
wherein:
(i) moieties A and M are as described herein above, wherein 0< a<_ 6
and 1:5 b<_ 6; and
(iii) 0 < e <_ 15;
wherein M, a, b and e are selected so as to maintain electroneutrality of
the material in its nascent or as-synthesized state.
[0073] Preferably 2:5 e<_ 13, and even more preferably 2:5 e<_ 8.
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
I:ji~07'~}~~' ~;;~;~pr6firte41ebtrode active material of the present
subembodiment comprises a compound of the formula (IV):
AaNitCouM4A, (IV)
wherein 0<(t + u) _ 1, and 0<_ t< 1. In one embodiment t=(1- u), where t=
0. In another embodiment t = (1 - u - v), wherein v> 0. M4 is at least one
metal
selected from Group 2, 12, 13, or 14 of the Periodic Table, more preferably M4
is selected from the group consisting of Mg, Ca, Al, and mixtures thereof.
[0075] Methods of making the electrode active materials described by
general formulas (III) and (IV) are well known in the art, and are described
in:
U.S. Patent No. 5,225,297 to Garcia-Alvarado et al., issued July 6, 1993; U.S.
Patent No. 5,340,671 to Koksbang, issued August 23, 1994; U.S. Patent No.
5,366,830 to Koksbang, issued November 22, 1994; U.S. Patent No. 5,587,133
to Amatucci et al., issued December 24, 1996; U.S. Patent No. 5,630,993 to
Amatucci et al., issued May 20, 1997; U.S. Patent No. 5,670,277 to Barker et
al., issued September 23, 1997; U.S. Patent No. 5,693,435 to Amatucci et al.,
issued December 2, 1997; U.S. Patent No. 5,698,338 to Barker et al., issued
December 16, 1997; and U.S. Patent No. 5,744,265 to Barker et al., issued
April 28, 1998.
[0076] Non-limiting examples of active materials of this subembodiment
and represented by general formulas (I), (III) and (IV) include the following:
NaMn2O4, NaNio.75AI0.25O2, Na2CuO2, y-NaV2O5,LiCo0,5Nio,5O2, NaCoO2,
NaNiO2, NaNiCoO2, NaNio.75Coo,2502, NaNio,$Coo,202, NaNio,6Coo,4O2, NaMnO2i
NaMoO2, NaNio,8Coo.15A10.0502, NaFeO3i a-NaFe508, R-NaFe5O8, Na2Fe3O4,
NaFe2O3, NaNio66Co0.2A10.202, NaNio.sCooASMg0.0502, NaNio.sCoo.,5Cao.o502s
31
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
0:"i5 ' IbrO6O6e;]N ''rO~COo.15A1p.05o2, Na0.5Nap.5CQ02, NaN10.6L'00.4U2,
KNio.75COo.2502, NaFeo.75COo.2502, NaCuo.sCOo.2 2, NaTi0.9Nio.102,
NaVp,$Cop,202, Na3V2Cop,5A105505, Na2NaVNip,5Mgp,505, Na5CrFe1.5CaO7,
NaCrO2, NaVO2, NaTiO2, NaVO2, NaTiO2, Na2FeV2O5, Na5Ni2.5Co3O8a
Na6V2Fe1.5CaO9, potassium (K) and lithium (Li) analogs thereof, and mixtures
thereof. Preferred materials include, NaNiO2, NaCoO2, NaNiy_XCox02, y-NaV2O5,
and Na2CuO2.
[0077] In another particular subembodiment, the positive electrode film 26
contains an electrode active material represented by the general formula (V):
AaMnbO4, (V)
(herein "modified manganese oxide") having an inner and an outer region,
wherein the inner region comprises a cubic spinel manganese oxide, and the
outer region is enriched with Mn}4 relative to the inner region, moiety A is
as
described herein above, and a and b are selected so as to maintain
electroneutrality of the material in its nascent or as-synthesized state.
[0078] Preferably 0< a< 2.0, more preferably 0.8 < a< 1.5, and even
more preferably 0.8 _ a< 1.2.
[0079] In a preferred embodiment, such modified manganese oxide active
materials are characterized as particles having a core or bulk structure of
cubic
spinel manganese oxide and a surface region which is enriched in Mn+4 relative
to the bulk. X-ray diffraction data and x-ray photoelectron spectroscopy data
are consistent with the structure of the stabilized manganese oxide being a
central bulk of cubic spinel lithium manganese oxide with a surface layer or
region comprising A2MnO3i where A is an alkali metal.
32
CA 02601912 2007-09-21
WO 2006/105253 õ PCT/US2006/011571
~t1' , mi~tUrb:Fiffeferably contains less than 50% by weignt ot tne aikali
metal compound, preferably less than about 20%. The mixture contains at least
about 0.1 % by weight of the alkali metal compound, and preferably 1% by
weight
or more. In a preferred embodiment, the mixture contains from about 0.1% to
about 20%, preferably from about 0.1 % to about 10%, and more preferably from
about 0.4% to about 6% by weight of the alkali metal compound.
[0081] The alkaii metal compound is a compound of lithium, sodium,
potassium, rubidium or cesium. The alkali metal compound serves as a source
of alkali metal ion in particulate form. Preferred alkali metal compounds are
sodium compounds and lithium compounds. Examples of compounds include,
without limitation, carbonates, metal oxides, hydroxides, sulfates,
aluminates,
phosphates and silicates. Examples of lithium compounds thus include, without
limitation, lithium carbonates, lithium metal oxides, lithium mixed metal
oxides,
lithium hydroxides, lithium aluminates, and lithium silicates, while analogous
sodium compounds are also preferred. A preferred lithium compound is lithium
carbonate. Sodium carbonate and sodium hydroxide are preferred sodium
compounds. The modified manganese oxide is preferably characterized by
reduced surface area and increased alkali metal content compared to an
unmodified spinel lithium manganese oxide. In one alternative, essentially all
of
a lithium or sodium compound is decomposed or reacted with the lithium
manganese oxide.
[0082] In one aspect, the decomposition product is a reaction product of the
LMO particles and the alkali metal compound. For the case where the alkali
metal is lithium, a lithium-rich spinel is prepared. A preferred electrode
active
33
CA 02601912 2007-09-21
WO 2006/105253,,d~ i PCT/US2006/011571
~~- '8t0ri,8P1,6i~nbbdir~ht ~bbMDf,ises a compound of the formula
A,+p1v1n2_pv4, wriere
0_ p< 0.2. Preferably p is greater than or equal to about 0.081.
[0083] In many embodiments, the modified manganese oxide material of
the invention is red in color. Without being bound by theory, the red color
may
be due to a deposit or nucleation of Li2MnO3 (or Na2MnO3i which is also red in
color) at the surface or at the grain boundaries. Without being bound by
theory,
one way to envision the formation of the "red" modified manganese oxide is as
follows. Mn13 at the surface of a cubic spinel lithiated manganese oxide
particle
loses an electron to combine with added alkali metal from the alkali metal
compound. Advantageously, the alkali metal compound is lithium carbonate.
Thus, the cubic spinel lithiated manganese oxide becomes enriched in lithium.
Charge balance is maintained by combination with oxygen from the available
atmosphere, air, during the solid state synthesis. The oxidation of Mn+3 to
Mn+4
at the surface of the particle results in a loss of available capacity and a
contraction of the unit cell. Thus a surface region of the particle relatively
enhanced in Mn+4 forms during the reaction of the cubic spinel lithiated
manganese oxide with the lithium compound in air or in the presence of
oxygen. At least in the early stages of the reaction, a surface layer or
coating
of Li2MnO3 is formed on the surface of the particle. It is believed that
formation
of the red colored Li2MnO3 (or Na2MnO3) at the surface of the particle is
responsible for the red color observed in some samples of the treated LMO of
the invention.
[0084] Methods of making the electrode active materials described by
general formula (V) are well known in the art, and are described in: U.S.
Patent
34
CA 02601912 2007-09-21
WO 2006/105253 al., issued February 9, 1999; U.S. PalefuS V0~6/Olls71
6,183,718 to Barker et al., issued February 6, 2001; U.S. Patent No. 6,869,547
to Barker et al., issued March 22, 2005; and U.S. Patent No. 6,596,435 to
Barker et al., issued July 22, 2003.
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
~~~95f r inranbtk~d~~pb.t~icular subembodiment, the positive c+cLA+Uuc ++++++
~6
contair 3lectrode active material represented by the general formula (VI):
Aa\M'O)cX044, (VI)
wherein:
(i) moieties A, M' and Z are as described herein above, wherein 0< a
s9,0<c:5 1,andOsfs4;and
(ii) X is selected from the group consisting of P, As, Sb, Si, Ge, V, S,
and mixtures thereof;
wherein A, M', X, a, c, and f are selected so as to maintain
electroneutrality of the material in its nascent or as-synthesized state.
[0086] In one particular subembodiment, moiety (M'O) of general formula
(VI) is a 2+ ion containing a metal (M') in the 4+ oxidation state.
Preferably, M'
is vanadium (V), and XY4 = P04.
[0087] Methods of making the electrode active materials described by
general formula (VI) are well known in the art, and are described in U.S.
Publication No. 2002/0262571 to Barker et al., published December 30, 2004.
[0088] Non-limiting examples of active materials of this subembodiment
and represented by general formulas (I) and (VI) include the following:
NaVOPO4, Na(VO)0.75Mn0.25PO4a NaVOPO4, NaVOPO4: Na(VO)o.5Alo55PO4,
Na(VO)0.75Fe0.25PO4r Nao.5Nao.5VOP04, Na(VO)0.75Co0.25PO4,
Na(VO)o,75Moo.25PO4, and NaVOSO4. Particularly preferred are NaVOPO4 and
Na(VO)o.75Mno.25PO4-
[0089] In another particular subembodiment, the positive electrode film 26
contains an electrode active material represented by the general formula
(VII):
36
CA 02601912 2007-09-21
WO 2006/105253 AaMbW04, (VI1) PCT/US2006/011571
wherein:
(i) moieties A and M are as described herein above, wherein 0 < a_ 2
and0<bs1;and
(ii) W is selected from the group consisting of Hf, Ti, Zr, and mixtures
thereof;
wherein A, M, W, a and b are selected so as to maintain electroneutrality
of the material in its nascent or as-synthesized state.
[0090] In one particular subembodiment, moiety M is selected from the
group consisting of Ni, Co, Fe, Mn, V, Cr and mixtures thereof.
[0091] Methods of making the electrode active materials described by
general formula (VI) are well known in the art, and are described in U.S.
Patent
No. 6,103,419 to Saidi et al., issued August 15, 2000.
[0092] Non-limiting examples of active materials of this subembodiment
and represented by general formulas (I) and (VII) include the following:
Na2FeTiO4, Na2FeZrO4, Na2VTiO4, Na2VZrO4, Na2NiTiO4, and Na2NiZrO4.
[0093] The following non-limiting examples illustrate the compositions and
methods of the present invention.
EXAMPLE 1
[0094] An electrode active material of formula Na,.025Coo.9Alo.025M9o.o5P04,
is made as follows. The following sources of Li, Co, Al, Mg, and phosphate are
provided containing the respective elements in a molar ratio of
1.025:0.9:0.025:0.05:1.
37
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
u:t~~l V-'brrnoHeS~~1ma2t03 (moL wt. 105.99 g/mol) ,., y
0.03 moles Co304 (240.8 g/mol) 7.2 g
0.0025 moles Al (OH)3 (78 g/mol) 0.195 g
0.005 moles Mg(OH)2 (58 g/mol) 0.29 g
0.1 moles (NH4)2HP04 (132 g/mol) 13.2 g
0.2 moles elemental carbon (12 g/mol) (> 100% excess) 2.4 g
[0095] The above starting materials are combined and ball milled to mix
the particles. Thereafter, the particle mixture is pelletized. The pelletized
mixture is heated for 4-20 hours at 750 C in an oven in an argon atmosphere.
The sample is removed from the oven and cooled. An x-ray diffraction pattern
shows that the material has an olivine type crystal structure. An electrode is
made with 80% of the active material, 10% of Super P conductive carbon, and
10% poly vinylidene difluoride. A cell with that electrode as cathode and
carbon intercalation anode is constructed with an electrolyte comprising 1 M
LiPF6 dissolved in 2:1 by weight mixture of ethylene carbonate:dimethyl
carbonate.
EXAMPLE 2
[0096] An electrode active material of the formula
Na1,025Coo8$Feo.1Al0.025Mg0.05PO4 is made as follows. The following sources of
Na, Co, Fe, Al, Mg, and phosphate are provided containing the respective
elements in a molar ratio of 1.025:0.8:0.1:0.025:0.05:1.
0.05125 moles Na2CO3 (mol. wt. 105.99 g/mol) 7.7 g
0.02667 moles Co3 04 (240.8 g/mol) 6.42 g
38
CA 02601912 2007-09-21
woiuu~~~1~oles;f;F e~,~~"~ (1 59.7 g/mol) rCTius2oo6roiis7i
,,I~õ v.o y
0.0025 moles Al (OH)3 (78 g/mol) 0.195 g
0.005 moles Mg(OH)2 (58 g/mol) 0.29 g
0.1 moles (NH4)2HP04 (132 g/mol) 13.2 g
0.2 moles elemental carbon (12 g/mol) (> 100% excess) 2.4 g
The above starting materials are combined and ball milled to mix the
particles.
Thereafter, the particle mixture is pelletized. The pelletized mixture is
heated
for 4-20 hours at 750 C in an oven in an argon atmosphere. The sample is
removed from the oven and cooled. An x-ray diffraction pattern shows that the
material has an olivine type crystal structure. An electrode is made with 80%
of
the active material, 10% of Super P conductive carbon, and 10% poly
vinylidene difluoride. A cell with that electrode as cathode and a carbon
intercalation anode is constructed with an electrolyte comprising 1 M LiPF6
dissolved in a 3:1 by weight mixture of y-butyrolactone:ethylene carbonate.
EXAMPLE 3
[0097] An electrode active material comprising Na2NiPO4F, representative
of the formula Na1+XNiPO4Fx, is made as follows. First, a NaNiPO4 precursor is
made according to the following reaction scheme.
0.5 Na2CO3 + 0.334 Ni3(PO4)2.7H20 + 0.334 (NH4)2HP04 --- >
LiNiPO4 + 2.833 H2O + 0.667 NH3 + 0.5 CO2
A mixture of 52.995 g (0.5 mol) of Na2CO3, 164.01 (0.334 mol) of
Ni3(P04)2.7H20, and 44.11 g (0.334 mol) of (NH4)2HPO4 is made, using a
mortar and pestle. The mixture is pelletized, and transferred to a box oven
39
CA 02601912 2007-09-21
1~G WOu00V{E0525a CT/US2006/011571
q pN t. p"e~ic air gas flow. The mixture is heate~u, dL a+dil+N
rate of about 2 C minute to an ultimate temperature of about 800 C, and
maintained at this temperature for 16 hours. The product is then cooled to
ambient temperature (about 21 C).
[0098] Na1+XNiPO4Fx is then made from the NaNiPO4 precursor. In the
Example that follows, x is 1.0, so that the active material produced is
represented by the formula Na2NiPO4F. The material is made according to the
following reaction scheme.
NaNiPO4 + x NaF ---> Nay+xNiPO4FX
For x equal to 1.0, a mixture of 1 mol LiNiPO4 and 1 mol NaF is made, using a
mortar and pestle. The mixture is pelletized, and transferred to a temperature-
controlled tube furnace equipped with a argon gas flow. The mixture is heated
at a ramp rate of about 2 /minute to an ultimate temperature of about 850 C.
The product is then cooled to ambient temperature (about 20 C). An electrode
is made with 80% of the active material, 10% of Super P conductive carbon,
and 10% poly vinylidene difluoride. A cell with that electrode as cathode and
a
carbon intercalation anode is constructed with an electrolyte comprising 1 M
LiPF6 dissolved in a 3:1 by weight mixture of y-butyrolactone:ethylene
carbonate.
EXAMPLE 4
[0099] An electrode active material comprising Na1.2VP04F1,2 is made as
follows. In a first step, a metal phosphate is made by carbothermal reduction
of
CA 02601912 2007-09-21
"""" CT/US2006/011571
ca ~,cwo 2oo6ilos2s3r6'cri~plified by vanadium pentoxide. Th~ ~~c,d~~ cdL~~un
scheme of the carbothermal reduction is as follows.
0.5V205 + NH4H2PO4 + C-4 VPO4 + NH3+ 1.5H20 + CO
9.1 grams of V205, 11.5 grams of NH4H2PO4 and 1.2 grams of carbon (10%
excess) are used. The precursors are premixed using a mortar and pestle and
then pelletized. The pellet is transferred to an oven equipped with a flowing
argon atmosphere. The sample is heated at a ramp rate of 2 per minute to an
ultimate temperature of 300 C and maintained at this temperature for three
hours. The sample is cooled to room temperature, removed from the oven,
recovered, re-mixed and repelletized. The pellet is transferred to a furnace
with
an argon atmosphere. The sample is heated at a ramp rate of 2 per minute to
an ultimate temperature 750 C and maintained at this temperature for 8 hours.
[00100] In a second step, the vanadium phosphate made in the first step is
reacted with an alkali metal halide, exemplified by sodium fluoride, according
to
the following reaction scheme.
xNaF + VPO4 ---> NaXVPO4FX
14.6 grams of VPO4 and 4.2 grams of NaF are used. The precursors are pre-
mixed using a mortar and pestle and then pelletized. The pellet is transferred
to an oven equipped with a flowing argon atmosphere, the sample is heated at
a ramp rate of 2 per minute to an ultimate temperature of 750 C and
maintained at this temperature for an hour. The sample is cooled and removed
from the furnace.
[00101] To make Na122VPO4F1.2, the reaction is repeated with a 20% mass
excess of sodium fluoride over the previous reaction. The precursors are pre-
41
CA 02601912 2007-09-21
ikeW0 2006y105253ionar,,a~!d pestle and pelletized as before.
PCT/US20Q6/ON~~711s
~heated to an ultimate temperature of 700 C and maintained at this temperature
for 15 minutes. The sample is cooled and removed from the oven. There is
only a small weight loss during reaction, indicating almost full incorporation
of
the NaF. To make an active material of formula Nay55VPO4F15 5 the reaction is
repeated with an approximate 50% mass excess of sodium fluoride over the
first reaction. The sample is heated at 700 C for 15 minutes, cooled, and
removed from the oven.
An electrode is made with 80% of the active material, 10% of Super P
conductive carbon, and 10% poly vinylidene difluoride. A cell with that
electrode as cathode and graphite as anode is constructed with an electrolyte
comprising 1 M LiPF6 dissolved in 2:1 by weight mixture of ethylene
carbonate:dimethyl carbonate.
EXAMPLE 5
[00102] An electrode active material comprising NaCoPO4F is made
according to the following reaction scheme.
0.33 Co304.,_ NH4H2PO4 + NaF + 0.083 02 --- > NaCoPO4F + NH3 + 1.5H20
[00103] This active material is made under oxidizing conditions where the
metal in the final product has a higher oxidation state than the metal in the
starting material. 3 grams of Co304, 1.57 grams of NaF, and 4.31 grams of
NH4H2PO4 are mixed, pelletized, and heated to an ultimate temperature of
300 C and maintained at the temperature for three hours. This sample is
cooled, removed from the oven, repelletized, and returned to the oven where it
42
CA 02601912 2007-09-21
raeWO 2006105253;i'rh'ate'!.,teMperature of 800 C and maintained
u~Tius2006i011571
temperature for eight hours. An electrode is made with 80% of the active
material, 10% of Super P conductive carbon, and 10% poly vinylidene
difluoride. A cell with that electrode as cathode and carbon intercalation
anode
is constructed with an electrolyte comprising 1 M LiPF6 dissolved in 2:1 by
weight mixture of ethylene carbonate:dimethyl carbonate.
EXAMPLE 6
[00104] An electrode active material comprising Li011Na0.9VPO4F is made
according to the following reaction scheme.
xLiF + (1 -x)NaF + VPO4 --> LixNa1_xVPO4F
As an alternative to using alkaline fluorides, a reaction between VPO4 and
NH4F and a mixture of Li2CO3 and Na2CO3 may also be used.
[00105] To make Lio,1Na0.9VPO4F, 1.459 grams VPO4, 0.026 grams of LiF,
and 0.378 grams of NaF are premixed, pelletized, placed in an oven and
heated to an ultimate temperature of 700 C. The temperature is maintained for
fifty minutes after which the sample is cooled to room temperature and
removed from the oven. To make Lio.95Nao.osVP04F, 1.459 grams of VPO4,
0.246 grams of LiF, and 0.021 grams of NaF are mixed together and heated in
an oven as in the previous step. An electrode is made with 80% of the active
material, 10% of Super P conductive carbon, and 10% poly vinylidene
difluoride. A cell with that electrode as cathode and carbon intercalation
anode
is constructed with an electrolyte comprising 1 M LiPF6 dissolved in 2:1 by
weight mixture of ethylene carbonate:dimethyl carbonate.
43
CA 02601912 2007-09-21
WO 2006/105253 EXAMPLE 7 PCT/US2006/011571
[00106] An electrode active material comprising NaVPO4F is made
hydrothermally according to the following reaction scheme.
NaF + VPO4-), NaVPO4F
[00107] 1.49 grams of VPO4 and 1.42 grams of NaF are premixed with
approximately 20 milliliters of deionized water, transferred and sealed in a
Parr
Model 4744 acid digestion bomb, which is a Teflon lined stainless steel
hydrothermal reaction vessel. The bomb is placed in an oven and heated at a
ramp rate of 5 per minute to an ultimate temperature of 250 C to create an
internal pressure and maintained at this temperature for forty-eight hours.
The
sample is slowly cooled to room temperature and removed from the furnace for
analysis. The product sample is washed repeatedly with deionized water to
remove unreacted impurities. Then the sample is dried in an oven equipped
with argon gas flow at 250 C for one hour. An electrode is made with 80% of
the active material, 10% of Super P conductive carbon, and 10% poly
vinylidene difluoride. A cell with that electrode as cathode and carbon
intercalation anode is constructed with an electrolyte comprising 1 M LiPF6
dissolved in 2:1 by weight mixture of ethylene carbonate:dimethyl carbonate.
EXAMPLE 8
[00108] An electrode active material of formula NaVPO4OH is made
according to the following alternative reaction scheme.
NaOH + VPO4 --> NaVPO4OH
44
CA 02601912 2007-09-21
WO 2006/105253 PCT/US2006/011571
~061 d9]' Fr} Ithis -Exhfb*pie; -the reaction of the Example 14 is repeaieu,
except
that an appropriate molar amount of sodium hydroxide is used instead of
sodium fluoride. The reaction is carried out hydrothermally as in Example 14.
The hydroxyl group is incorporated into the active material at the relatively
low
temperature of reaction. An electrode is made with 80% of the active material,
10% of Super P conductive carbon, and 10% poly vinylidene difluoride. A cell
with that electrode as cathode and carbon intercalation anode is constructed
with an electrolyte comprising 1 M LiPF6 dissolved in 2:1 by weight mixture of
ethylene carbonate:dimethyl carbonate.
EXAM P LE 9
[00110] An electrode active material comprising NaVPO4F is made
according to the following reaction scheme.
0.5Na2C03 + NH4F + VPO4 ---y NaVPO4F + NH3 + 0.5C02 + 0.5H20
[00111] 1.23 grams of VPO4, 0.31 grams of NH4F, and 0.45 grams Na2CO3
are premixed with approximately 20 milliliters of deionized water and
transferred and sealed in a Parr Model 4744 acid digestion bomb, which is a
Teflon lined stainless steel reaction vessel. The bomb is placed in an oven
and
heated to an ultimate temperature of 250 C and maintained at this temperature
for forty-eight hours. The sample is cooled to room temperature and removed
for analysis. The sample is washed repeatedly with the deionized water to
remove unreacted impurities and thereafter is dried in an argon atmosphere at
250 C for an hour. An electrode is made with 80% of the active material, 10%
of Super P conductive carbon, and 10% poly vinylidene difluoride. A cell with
CA 02601912 2007-09-21
gidt Owo Zo06u1cs~53'%a;~hddi~-Anb carbon intercalation anode is
Cor1LCT/1s2oo6/011571p
electrolyte comprising 1 M LiPF6 dissolved in 2:1 by weight mixture of
ethylene
carbonate:dimethyl carbonate.
EXAM P LE 10
[00112] An electrode active material comprising Li4Fe2(PO4)3F,
representative of materials of the general formula AaMb(PO4)3Zd, is made
according to the following reaction scheme.
2 Li2CO3 + Fe203+ 3NH4H2(P04) + NH4F -> Li4Fe2(P04)3F + 2C02 + 4NH3 +
5H20
[00113] Here, M203 represents a +3 metal oxide or mixture of +3 metal
oxides. Instead of 2 lithium carbonates, a mixture of lithium sodium and
potassium carbonates totaling two moles may be used to prepare an analogous
compound having lithium, sodium and potassium as alkaii metals. The starting
material alkali metal carbonate, the metal or mixed metal +3 oxidation state
oxides, the ammonium dihydrogen phosphate, and the ammonium fluoride are
combined in stoichiometric ratios indicated in the form of powders, and the
powders are mixed and pelletized as in the previous examples. The pellet is
transferred to an oven and is heated up to an ultimate temperature of about
800 C and maintained at that temperature for 8 hours. The reaction mixture is
then cooled and removed from the oven. An electrode is made with 80% of
the active material, 10% of Super P conductive carbon, and 10% poly
vinylidene difluoride. A cell with that electrode as cathode and carbon
46
CA 02601912 2007-09-21
~rn~erWO Zoo6i1os2~~e~=lts=(c~ns't~i'bcted with an electrolyte
comprisin~c;rius2oo6io~~s~~
dissolved in 2:1 by weight mixture of ethylene carbonate:dimethyl carbonate.
EXAMPLE 11
[00114] An electrode active material comprising Na2Li2M2(PO4)3F is made
according to the following reaction scheme.
Li2CO3 + Na2CO3 + 2MP04 + NH4H2PO4 + NH4F ~
Na2Li2M2(P04)3F + 2CO2 + 2NH3 + 2H20
[00115] The starting materials are combined in the stoichiometric ratios
indicated and are reacted according to the general procedure of Example 10.
Here, MPO4 represents a metal +3 phosphate or mixture of metal +3
phosphates. An electrode is made with 80% of the active material, 10% of
Super P conductive carbon, and 10% poly vinylidene difluoride. A cell with
that
electrode as cathode and carbon intercalation anode is constructed with an
electrolyte comprising 1 M LiPF6 dissolved in 2:1 by weight mixture of
ethylene
carbonate:dimethyl carbonate.
EXAMPLE 12
[00116] An electrode active material comprising Na3V2(P04)2F3 is made as
follows. First, a VPO4 precursor is made according to the following reaction
scheme.
V205 + 2 (NH4)2HP04 + C -~ VPO4
A mixture of 18.2 g (0.1 mol) of V205, 26.4 g (0.2 mol) of (NH4)2HP04, and 2.4
g (0.2 mol) of elemental carbon is made, using a mortar and pestle. The
47
CA 02601912 2007-09-21
f;WO 2006/105253 ' T/US2006/011571
mi~ctUlu~~ ~t~~~~ec~; anc!transferred to a box oven equipped wP.. -. ,~.
y.,., y_s
flow. The mixture is heated to a temperature of about 350 C, and maintained
at this temperature for 3 hours. The mixture is then heated to a temperature
of
about 750 C, and maintained at this temperature for 8 hours. The product is
then cooled to ambient temperature (about 21 C).
[00117] Na3V2(PO4)2F3 is then made from the VPO4 precursor. The
material is made according to the following reaction scheme.
2 VPO4 + 3 NaF -> Na3V2(PO4)2F3
A mixture of 2 moI VPO4 and 3 mol NaF is made, using a mortar and pestle.
The mixture is pelletized, and transferred to a temperature-controlled tube
furnace equipped with an argon gas flow. The mixture is heated at a ramp rate
of about 2 /minute to an ultimate temperature of about 750 C for 1 hour. The
product is then cooled to ambient temperature (about 20 C). X-ray powder
diffraction analysis for the Na3V2(PO4)2F3 material indicated the material to
be
single phase with a tetragonal structure (space group P42/mnm). The unit cell
parameters (a = 9.0304(5) A, c= 10.6891(9) A) were calculated from a least
squares refinement procedure, in fair agreement with the structural analysis
for
Na3V2(PO4)2F3 described by Meins et al., J. Solid State Chem., 148, 260,
(1999). (i.e. a = 9.047(2) A, c = 10.705(2) A).
[00118] An electrode is made with 84% of the active material, 5% of Super
P conductive carbon, and 11 -wt % PVdF-HFP co-polymer (Elf Atochem) binder.
The electrolyte comprised a 1 M LiPF6 solution in ethyiene carbonate/dimethyl
carbonate (2:1 by weight) while a dried glass fiber filter (Whatman, Grade
GF/A) was used as electrode separator. A commercial crystalline graphite was
48
CA 02601912 2007-09-21
,;useqwo2006/105253,,ac~tiuia~ rri!aterial. High-resolution
electrochemPCTius2006i011571
measurements were performed using the Electrochemical Voltage
Spectroscopy (EVS) technique. EVS is a voltage step method, which provides
a high-resolution approximation to the open circuit voltage curve for the
electrochemical system under investigation. Such technique is known in the art
as described by J. Barker in Synth. Met 28, D217 (1989); Synth. Met. 32, 43
(1989); J. Power Sources, 52, 185 (1994); and Electrochemica Acta, Vol. 40,
No.
11, at 1603 (1995).
[00119] Figures 2 arid 3 show the voltage profile and differential capacity
plots for the first cycle EVS response for the Graphite / 1 M LiPF6 (EC/DMC) /
Na3V2(PO4)2F3 rocking chair cell. In this configuration the only available Li
in
the system originates from the LiPF6-based electrolyte phase. Based on a
cathode limited system, the actual volume of electrolyte used was carefully
controlled so as to allow charging of the graphite active material to an
approximate utilization limit of 300 mAh/g or Lio881C6.
[00120] Figure 4 shows the cycling behavior of representative
graphite/INa3V2(PO4)2F3 cells. The data was collected at approximate
charge/discharge rates of C/2 and 2C. The initial cathode reversible capacity
is
in the range 115-120 mAh/g and the cells cycle with relatively low capacity
fade
behavior. The minor decrease in discharge capacity recorded at the two
discharge rates is indicative of the excellent rate characteristics of this
system.
[00121] The examples and other embodiments described herein are
exemplary and not intended to be limiting in describing the full scope of
compositions and methods of this invention. Equivalent changes, modifications
49
CA 02601912 2007-09-21
;ah~;4Qo2006i105253'3 ~;e0,ifrc~~~Ombodiments, materials,
compositionPCTiuoo6io~~s?~
may be made within the scope of the present invention, with substantially
similar results.